Abstract
Objective
To determine whether the ocular anterior segment (aqueous humor and cornea) actively inhibits dendritic cell (DC) maturation.
Methods
Dendritic cells were injected into syngeneic corneas or conjunctivae, and their surface major histocompatibility complex class II expression in response to the local milieu was assessed using confocal microscopy. Immature DCs were cocultured with corneal supernatant or with aqueous humor to evaluate their regulation of DC phenotypic and functional maturity.
Results
In contrast to conjunctivally injected DCs, DCs injected into the cornea resisted up-regulation in expression of surface major histocompatibility complex class II. Corneal supernatant–treated and aqueous humor–treated DCs retained their immaturity, as reflected by high antigen uptake but low costimulatory molecule (CD80 and CD86) expression and poor T-cell stimulation. Anti–transforming growth factor β2 treatment of aqueous humor and of corneal supernatant led to complete and partial blockade of their inhibition of DC maturation, respectively. However, α-melanocyte–stimulating hormone and calcitonin gene-related peptide had no demonstrable effect on DC maturation.
Conclusion
Cornea and aqueous humor, principally through transforming growth factor β2, promote generation of phenotypically and functionally immature DCs.
Clinical Relevance
Our results indicate that relative immune quiescence in the cornea and in the anterior segment is actively maintained in part by the inhibitory effect of transforming growth factor β2 on resident DCs and by their suppression of T-cell–mediated immune and inflammatory responses.
Antigen-presenting cells (APCs), and particularly dendritic cells (DCs), are important sentinels of the immune system.1 The function of DCs is related to their state of maturation. Immature DCs express low levels of major histocompatibility complex (MHC) class II and costimulatory molecules (eg, CD80 and CD86) and are efficient in antigen uptake but are poor in stimulating T cells. To acquire efficient T-cell stimulatory ability, DCs must undergo maturation. This involves the down-regulation of their endocytic activity concomitant with up-regulation in the expression of MHC class II and costimulatory molecules.2 Therefore, maturation of DCs is a fundamental checkpoint in the initiation and shaping of the immune response and needs to be tightly controlled.
For some time, it was widely accepted that the healthy cornea is devoid of any APCs,3 a feature traditionally viewed as one of the main aspects of the immune-privileged status of the cornea and the anterior segment. However, this concept has been questioned by the recent demonstration of resident APCs, including DCs and macrophages, in the rodent and human cornea.4–6 Major histocompatibility complex class II–positive macrophages, albeit in small numbers, have been identified in the cornea. However, DCs, the most potent APCs in peripheral tissues, universally do not express MHC class II, CD80 (B7.1), or CD86 (B7.2) in the central cornea, reflecting an immature state.6,7 In retrospect, these MHC class II DCs were overlooked for a long time because of the erroneous presumption of their constitutive MHC class II expression, a characteristic of resident DCs in many other tissues (eg, skin, gut, and lung).8–10 Given that no other pathogen-contacting tissues in the body exclusively harbor MHC class II DCs, the finding of uniformly immature DCs in the healthy cornea suggests that local tissue–derived factors may play an important role in suppressing the maturation of DCs.
Corneal DCs have the capacity to rapidly up-regulate surface MHC class II and costimulatory factors in response to alteration in their microenvironment induced by inflammation.11,12 Therefore, a central question in corneal immunology is whether these resident DCs are actively down-regulated by locally produced factors or are selectively recruited to the cornea as immature cells. The cornea can produce immunosuppressive cytokines such as transforming growth factor β (TGF-β).13 In addition, the cornea is in direct contact with the aqueous humor, which is composed of many immunosuppressive factors such as TGF-β2, α-melanocyte-stimulating hormone (α-MSH) and calcitonin gene-related peptide (CGRP).14–18 In this study, we demonstrate that the microenvironment of the anterior segment of the eye has a profound effect in defining resident DC phenotype and function and that this is mediated in large part by locally produced TGF-β2.
METHODS
MICE
Six- to 12-week-old C57BL/6, BALB/c and green fluorescent protein (GFP)–positive C57BL/6 mice (Taconic Farms, Germantown, New York, or Jackson Laboratories, Bar Harbor, Maine) were used in these experiments. All procedures were approved by the Schepens Eye Research Institute Institutional Animal Care and Use Committee.
GFP-DC ISOLATION
Spleens were collected from GFP-positive C57BL/6 mice. Single cells were obtained by mincing the tissues through a nylon mesh. Erythrocytes were lysed by hypotonic shock. The remaining cells were incubated using a magnetic-activated cell sorter (MACS CD11c Microbeads; Miltenyi Biotec, Auburn, California) for 15 minutes at 4°C. Dendritic cells were purified by passing the cells twice over a MACS MS-positive cell selection column (Miltenyi Biotec) held in a magnetic field. The DC enrichment was greater than 80% by flow analysis.
INJECTION OF GFP-DCs INTO CORNEA AND CONJUNCTIVA
To evaluate the capacity of the cornea vs the conjunctiva to down-modulate DC maturation, we developed an in vivo assay to analyze MHC class II expression by DCs using confocal microscopy. Mice were anesthetized, and a microsurgical blade was used to make a horizontal 50%-thickness intrastromal incision in the peripheral cornea. After forming a tunnel in the stroma toward the center using a 33-gauge needle, splenic DCs (5×103) were injected into the central cornea in 2 μL of phosphate-buffered saline. Subconjunctival injection was performed to penetrate the superotemporal quadrant of the conjunctiva to deliver 1×104 DCs in 4 μL of phosphate-buffered saline. After 48 hours, the corneas and conjunctivae were excised and were analyzed for MHC class II expression on GFP-DCs by confocal microscopy.
CONFOCAL IMMUNOFLUORESCENCE MICROSCOPY
Corneas and conjunctivae were excised, fixed for 1 hour in 4% paraformaldehyde, and blocked with 2% bovine serum albumin and anti–Fc receptor (BD PharMingen, San Diego, California). Biotin-labeled anti-Iab or isotype-matched antibody (BD PharMingen) was applied to the samples for 2 hours, followed by 1-hour incubation with Cy3-conjugated streptavidin. All procedures were performed at room temperature. Finally, the samples were covered with mounting medium (Vector Laboratories, Burlingame, California) and were examined using a confocal microscope (Leica, Heidelberg, Germany).
GENERATION OF CORNEAL SUPERNATANT
Corneas were removed aseptically and were incubated at 37°C in serum-free medium (20 corneas/mL). Serum-free medium consisted of RPMI-1640, 10mM HEPES, 0.1mM nonessential amino acid, 1mM sodium pyruvate, 100 U/mL of potassium penicillin, and 100 μg/mL of streptomycin sulfate (all from Biowhitaker, Walksville, Maryland), as well as 10−5M 2-ME, 0.1% bovine serum albumin, and ITS (insulin, transferrin, and selenium) (all from Sigma-Aldrich Inc, St Louis, Missouri). The supernatants were collected after 48 hours and were stored at −80°C.
AQUEOUS HUMOR COLLECTION
Many components of aqueous humor are evolutionarily conserved among different species.14 We used rabbit aqueous humor for our experiments, as previously described.15 Briefly, New Zealand rabbits were anesthetized by intramuscular injection of a mixture of ketamine hydrochloride and xylazine hydrocholoride and were managed according to the guidelines of the US Animal Welfare Act. Aqueous humor was aspirated by limbal paracentesis using a 27-gauge needle. Aqueous humor was allowed to flow passively into a siliconized microfuge tube (Fisher Scientific, Fair Lawn, New Jersey) and was stored at −80°C. Rabbits were immediately humanely killed following aqueous humor collection.
GENERATION AND TREATMENT OF BONE MARROW–DERIVED DCs
Bone marrow–derived DCs were generated as described by Lutz et al.19 Briefly, 6- to 8-week-old mice were humanely killed, and femurs were isolated. Bone marrow cells (2×106) collected from femurs were seeded in Petri dishes in 10 mL of culture medium (RPMI-1640 supplemented with 10% fetal calf serum, 2mM L-glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 50mM 2-ME) containing 200 U/mL of granulocyte-macrophage colony-stimulating factor (PeproTech, Rocky Hill, New Jersey). Cultures were fed on days 3 and 6 with granulocyte-macrophage colony-stimulating factor. On day 8, nonadherent and loosely adherent cells were harvested as immature DCs. The purity of the DCs, determined by flow analysis of surface CD11c staining, was greater than 75%. To induce DC maturation, 50 ng/mL of TNF-α (R and D Systems, Minneapolis, Minnesota) was added 24 hours before cell harvesting.
Immature DCs (1×106) were cultured in 24-well plates in serum-free medium supplemented with 50 U/mL of granulocyte-macrophage colony-stimulating factor in the presence or absence of various concentrations of corneal supernatant or aqueous humor. Cells were harvested after 48 hours for phenotyping or functional studies. In separate experiments, CGRP or α-MSH (Peninsula Laboratories, San Carlos, California) was added to DCs at and above aqueous humor physiological concentrations (2 ng/mL and 30 pg/mL, respectively) to determine the effect of these factors on DC phenotype.
FLOW CYTOMETRY
Dendritic cells were blocked with anti–Fc receptor and then stained with fluorescein isothiocyanate (FITC)–conjugated anti-CD11c in combination with PE-conjugated anti-Iab, anti-CD80, anti-CD86, and anti-CD45 (all from BD PharMingen) for 30 minutes at 4°C. Their respective isotype-matched antibodies served as negative controls. The stained cells were analyzed on a flow cytometer (EPICS XL; Beckman Coulter, Fullerton, California).
MIXED LEUKOCYTE REACTION
Splenic T cells from C57BL/6 mice were purified using MACS Thy 1.2 Beads (Miltenyi Biotec) and were stimulated in a 96-well round-bottom plate (105/well) with titrated numbers of mitomycin C–treated (50 μg/mL for 30 minutes at 37°C; Sigma-Aldricht Inc), BALB/c mice–derived DCs (untreated or treated with corneal supernatant or aqueous humor) for 72 hours. Cell proliferation was pulsed with 0.5 μCi/well of 3H-thymidine for the last 18 hours. The plates were harvested, and 3H-thymidine uptake was measured in a scintillation counter. The results are presented as the mean counts per minute of the triplicated cultures.
FITC-DEXTRAN ASSAY
Dendritic cells (2×105) were incubated with 1 mg/mL of FITC-dextran (Sigma-Aldricht Inc) at 37°C in phosphate-buffered saline. After 30 minutes, the cells were washed with ice-cold 0.1% sodium azide and phosphate-buffered saline. Cell fluorescence was analyzed by flow cytometry. Parallel experiments were performed at 4°C (negative control) to show that uptake of FITC-dextran by DCs is suppressed at low temperatures.
IMMUNOMODULATORY FACTOR NEUTRALIZATION
Neutralizing anti-TGF-β2 antibody or normal rabbit IgG (R and D Systems) was added to corneal supernatant or to aqueous humor at 30 μg/mL, a concentration sufficient to neutralize the inhibitory activity of 3 ng/mL of TGF-β2 (the reported normal value of several different species) according to the manufacturer’s instructions.14 Neutralizing anti–TGF-β1 or chicken immunoglobulin (R and D Systems) was used at 0.4 μg/mL, considerably greater than the concentration required to neutralize 3 ng/mL of TGF-β1. They were incubated at room temperature for 1 hour before addition to the DC culture. To examine whether the inhibitory effect of aqueous humor or corneal supernatant is mediated by CGRP, CGRP-(8-37) (Peninsula Laboratories) was used at 100-fold excess to selectively antagonize the effect of CGRP (2 ng/mL). Anti-CGRP and anti-α-MSH antibodies (Peninsula Laboratories) were also added in excess to aqueous humor or to corneal supernatant to assess the neutralization of the suppressive activity on DC maturation.
STATISTICAL ANALYSIS
All statistical analyses were conducted using paired t tests. Values were considered statistically significant at P < .05.
RESULTS
MHC CLASS II EXPRESSION BY DCs INJECTED INTO THE CORNEA
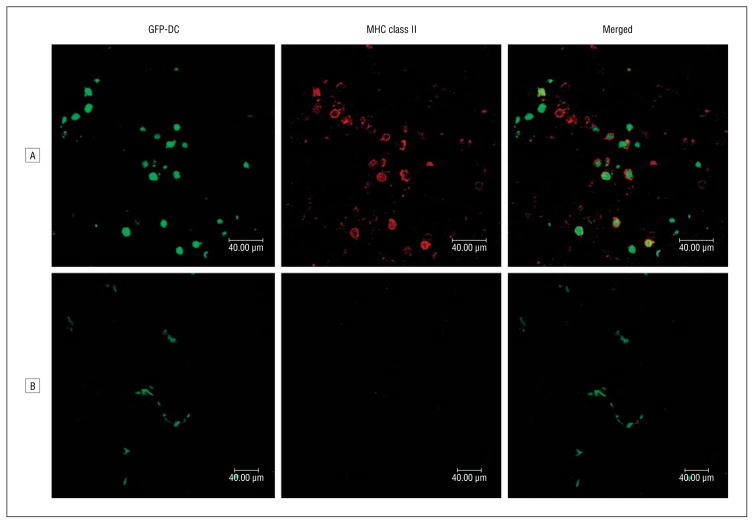
The conjunctiva contains a contiguous network of monocytic cells, including DCs, that generally display an MHC class II–positive phenotype.20 The phenotype of DCs in the cornea differs from that of DCs in the conjunctiva; while MHC class II–bearing DCs can be found in the corneal periphery, the central region of the cornea exclusively harbors MHC class II DCs.5,7 To provide in vivo evidence that the specific microenvironment of the anterior segment of the eye can impose a suppressive effect on DC maturation, we purified DCs from GFP-positive C57BL/6 mice and injected them into the corneas or conjunctivae of wild-type C57BL/6 mice. Surface MHC class II expression of GFP-DCs was monitored after 48 hours using confocal microscopy. As shown in Figure 1, injected DCs were identified by green fluorescence in the corneal or conjunctival specimens. Most GFP-DCs in the conjunctiva acquired a mature phenotype, as revealed by bright surface-encompassing MHC class II staining, whereas the GFP-DCs injected in the cornea had little to no surface MHC class II staining.
Figure 1.
Confocal microscopy of surface major histocompatibility complex (MHC) class II expression (red) on green fluorescent protein dendritic cells (GFP-DC) (green) 48 hours after their injection into the conjunctivae (row A) or corneas (row B) of syngeneic mice. The merged images are shown on the right (original magnification ×400).
EFFECT OF CORNEAL SUPERNATANT AND AQUEOUS HUMOR ON DC PHENOTYPIC MATURATION
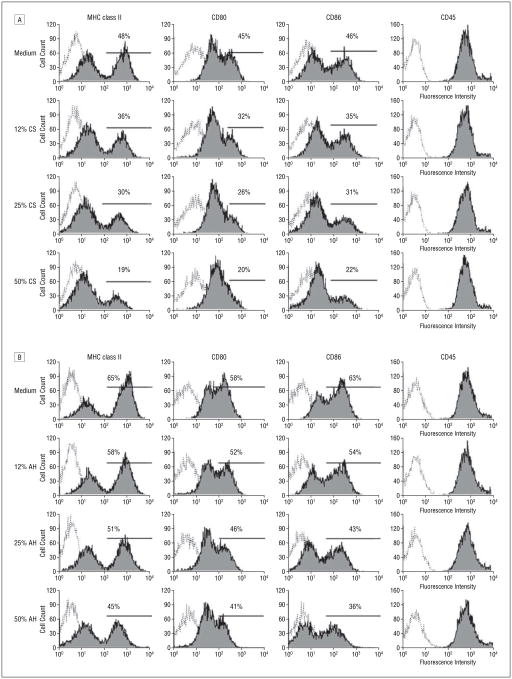
To investigate the potential of locally produced factors within the cornea or aqueous humor in inhibiting DC phenotypic maturation, corneal supernatant or aqueous humor was used in culture media of DCs. Immature DCs were generated from bone marrow by 8-day culture with granulocyte-macrophage colony-stimulating factor as already described, with 90% displaying an MHC class IIlowCD80lowCD86low phenotype; these cells were then cultured for an additional 48 hours with or without various concentrations of corneal supernatant or aqueous humor. As shown in Figure 2, immature DCs in medium alone yielded a distinct mature DC population (MHC class IIhighCD8highCD86high). However, when cultured in the presence of corneal supernatant (Figure 2A) or aqueous humor (Figure 2B), their maturation was compromised, as indicated by a reduction in the yield of mature relative to immature DCs. The suppressive effects seemed to be dose-dependent for corneal supernatant and for aqueous humor. CD45 expression, cell viability, and total cell numbers were unaffected by the treatments, indicating that the observed change in immunophenotype of these cells could not be attributable to a general down-regulation of protein synthesis or to cell death.
Figure 2.
Flow analysis of major histocompatibility complex (MHC) class II, CD80, CD86, and CD45 on dendritic cells (DCs) (shaded areas) treated for 48 hours with various concentrations of corneal supernatant (CS) (A) or aqueous humor (AH) (B) (serum-free medium alone as a control). Dashed lines indicate isotype controls; percentages, mature DCs with high-fluorescence staining.
EFFECT OF CORNEAL SUPERNATANT AND AQUEOUS HUMOR ON DC FUNCTIONAL MATURATION
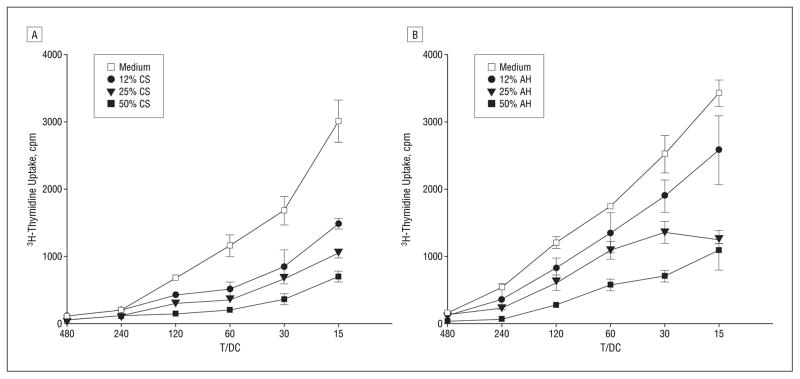
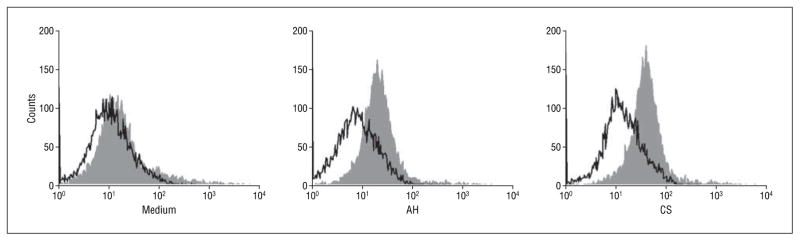
The profound suppression of DC phenotypic maturation by corneal supernatant and by aqueous humor led us to further investigate their effect in modulating DC functions. As demonstrated, 72 hours of mixed leukocyte reaction, corneal supernatant (Figure 3A), or aqueous humor (Figure 3B) pretreatment resulted in a dose-dependent reduction in DC efficiency in stimulating allogeneic T cells. T-cell proliferation was suppressed by 80% and by 70% when the DCs were pretreated with 50% corneal supernatant and with 50% aqueous humor, respectively. Because DC immaturity is characterized by high antigen uptake, DC endocytic activity was assayed using FITC-dextran (Figure 4). Dendritic cells pre-treated with corneal supernatant or aqueous humor displayed significantly higher endocytic activity for FITC-dextran particles than untreated DCs, confirming that these cells, when exposed to the anterior segment milieu, retained functional immaturity.
Figure 3.
T-cell stimulatory ability of dendritic cells (DCs) pretreated for 48 hours with varying concentrations of corneal supernatant (CS) (A) or aqueous humor (AH) (B). Allogeneic T cells were stimulated for 72 hours with titrated numbers of mitomycin C–treated DCs. Cell proliferation was measured by 3H-thymidine uptake and was expressed as the mean counts per minute (cpm) with standard deviations for triplicates. T/CD indicates T cells/DC ratio.
Figure 4.
Flow cytometric analysis of endocytic activity of dendritic cells pretreated with 25% corneal supernatant (CS) or 25% aqueous humor (AH). Shaded histograms show the specific fluorescein isothiocyanate–dextran uptake at 37°C; dotted lines represent the background uptake at 4°C.
EFFECT OF BLOCKING TGF-β2 ON SUPPRESSIVE ACTIVITY OF AQUEOUS HUMOR AND CORNEAL SUPERNATANT
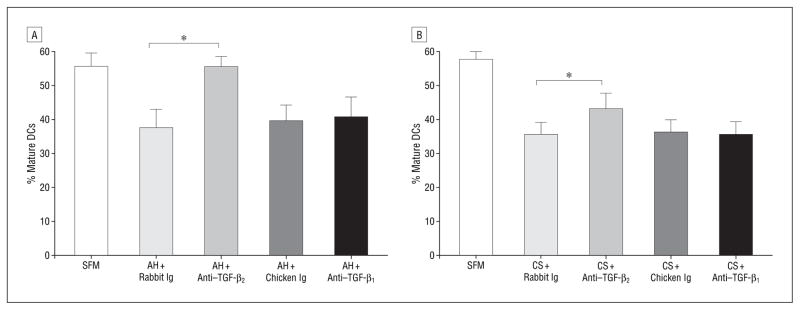
Transforming growth factor β2 is present in high concentration in aqueous humor as TGF-β2 and is produced within the cornea in various isoforms.13,14 To examine whether TGF-β functions as a suppressor of DC maturation in the anterior segment of the eye, corneal supernatant and aqueous humor were treated with TGF-β–neutralizing antibodies. As shown in Figure 5A, aqueous humor markedly decreased the mature DC population. This suppressive activity was completely blocked by incubating aqueous humor with anti–TGF-β2 antibody (P =.01). By contrast, pretreatment with anti–TGF-β1 did not affect its suppression on DCs. The critical role of TGF-β2 in mediating DC suppression in aqueous humor was further supported by the inability of CGRP receptor antagonist or antibodies against CGRP and α-MSH to demonstrate any measurable effect on DC suppression. Moreover, DCs treated with CGRP and α-MSH at aqueous humor physiological and excess concentrations matured normally; no suppression on maturation was demonstrated (data not shown). Parallel experiments were conducted using corneal supernatant (Figure 5B). In contrast to aqueous humor, the suppression of DC maturation by corneal supernatant was only partially (32%) reversed by anti–TGF-β2 (P =.02); no effect was seen with anti–TGF-β1. Taken together, these results suggest that the inhibitory effect of aqueous humor on corneal DC maturation can wholly be accounted for by TGF-β2, whereas TGF-β2, while contributory, cannot alone explain the inhibitory effect of the cornea on DC maturation.
Figure 5.
Capacity of anti–transforming growth factor β (anti–TGF-β1) and anti–TGF-β2 to block the aqueous humor (AH)–mediated (A) and corneal supernatant (CS)–mediated (B) suppression of dendritic cell (DC) maturation. Isotype antibodies were used as controls. The percentages of mature DCs (major histocompatibility complex class IIhigh) in different cultures were measured by flow cytometry and were compared. Ig indicates immunoglobulin; SFM; serum-free medium. *P < .05.
COMMENT
Until recently, the immune-privileged status of the cornea was thought to rely on the putative absence of resident APCs in the central cornea. The recent discovery of a unique DC population with an MHC class II phenotype, in the epithelium and stroma,6,7 significantly revises this notion and begs the question of why corneal DCs are universally of MHC class II. One of the most appealing teleological reasons is the following: the ocular surface constantly comes in contact with numerous antigens that need to be cleared without inciting a protracted T-cell–mediated response, and DCs in the immature stage of their life cycle are ideally situated to sample and capture antigen.1 Therefore, we propose that the exclusive presence of MHC class II DCs in the cornea ensures maximal clearance of foreign antigens without inducing blinding inflammatory damage. As such, induction of T-cell immunity in the cornea is restricted to significant encounters with detrimental pathogens but not trivial antigens, which would otherwise wreak havoc on the clarity of sight. This explanation still begs the question of whether the DCs are recruited to the cornea as immature cells or whether cornea- and aqueous humor–derived factors actively suppress the maturity of resident corneal APCs.
Our results fit the paradigm that soluble factors derived from the cornea and aqueous humor prevent DCs from acquiring phenotypic and functional maturation, thereby retaining DCs in an immature state. Using bone marrow–derived DCs, we showed that corneal supernatant–DCs and aqueous humor–pretreated DCs have an impaired ability to acquire maturation-associated molecules, inhibited the mixed leukocyte reaction when co-cultured with allogeneic T cells, and exhibited a higher degree of antigen uptake when incubated with FITC-dextran particles. Indeed, these cells remain maturation resistant, with continued suppressed MHC class II expression, even in the inflammatory milieu created by the addition of proinflammatory cytokines such as tumor necrosis factor α (data not shown). Further supportive evidence came from our in vivo investigations in which exogenously introduced GFP-DCs, when localized in the central cornea, seem to be resistant to the acquisition of surface MHC class II.
Our data demonstrate that corneal supernatant and aqueous humor are sources of soluble factors capable of causing dose-dependent inhibition of DC maturation. The complete blockade of the inhibitory activity of aqueous humor by anti–TGF-β2, but not anti–TGF-β1, further indicates that TGF-β2 accounts for aqueous humor–mediated DC inhibition. This observation is in accord with the fact that TGF-β2 is the prominent isoform of TGF-β in the aqueous humor. On the other hand, the inability of anti–TGF-β2 to completely block the corneal supernatant–mediated DC inhibition suggests that other factors contribute to DC maturity in the corneal environment. The cornea is reported to produce TGF-β1 and TGF-β213; however, the possibility remains that TGF-β2 in the aqueous humor could also diffuse into the cornea through the tight junctions of the corneal endothelium and could contribute in large part to the effect observed in the corneal supernatant. For this reason, we digested corneal tissue into single cells to remove any aqueous humor–derived TGF-β2. The supernatant yielded from corneal cells still gave rise to a comparable level of inhibitory activity, suggesting that the causative factors in the corneal supernatant were indeed produced principally, if not entirely, by the cornea.
Other immunosuppressive molecules in the aqueous humor known to have the potential to affect DCs are the neuropeptides CGRP and α-MSH.17,18 Our results suggest that CGRP and α-MSH do not play a significant role in aqueous humor–mediated DC inhibition. There is increasing evidence that the nervous system modulates immune cell function through neuropeptides.21–24 However, we could not fully address the potential regulation of corneal resident DCs by neuropeptide-containing nerves using our assays because the corneal supernatant was produced by corneal explants deprived of nerves. Further studies are needed to determine whether and how corneal nerves regulate resident DCs in the innervated cornea.
The results of this study demonstrate a critical role of the anterior segment of the eye in promoting corneal immune privilege. Significantly different from many properties that mainly interfere with the T-cell–mediated expression of immunity in the cornea (eg, Fas ligand), this feature targets the APC-dependent induction phase of the immune response. Specifically, these findings indicate that the anterior segment of the eye is active in modulating the maturational status of DCs rather than merely affecting their numbers in the cornea, thereby reducing the vulnerability of corneal cells as targets of effector T cells. Further identification and characterization of the immunoregulatory factors elaborated by the cornea and aqueous humor will have important applications in corneal transplantation and in the treatment of myriad corneal inflammatory and autoimmune disorders.
Acknowledgments
Funding/Support: This study was supported in part by grants EY01752 (Dr Taylor) and EY12963 (Dr Dana) from the US Public Health Service.
Footnotes
Financial Disclosure: None reported.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Streilein JW, Toews GB, Bergstresser PR. Corneal allografts fail to express Ia antigens. Nature. 1979;282(5736):326–327. doi: 10.1038/282326a0. [DOI] [PubMed] [Google Scholar]
- 4.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, et al. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43(7):2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 5.Yamagami S, Ebihara N, Usui T, et al. Bone marrow–derived cells in normal human corneal stroma. Arch Ophthalmol. 2006;124(1):62–69. doi: 10.1001/archopht.124.1.62. [DOI] [PubMed] [Google Scholar]
- 6.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74(2):172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 7.Williams DL. Major histocompatibility class II expression in the normal canine cornea and in canine chronic superficial keratitis. Vet Ophthalmol. 2005;8(6):395–400. doi: 10.1111/j.1463-5224.2005.00412.x. [DOI] [PubMed] [Google Scholar]
- 8.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt PG, Schon-Hegrad MA, McMenamin PG. Dendritic cells in the respiratory tract. Int Rev Immunol. 1990;6(2–3):139–149. doi: 10.3109/08830189009056625. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190(2):229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II–positive dendritic cells derived from MHC class II–negative grafts. J Exp Med. 2002;195(2):259–268. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamrah P, Liu Y, Zhang Q, Dana MR. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation [published correction appears in Arch Ophthalmol. 2003;121(11):1555] Arch Ophthalmol. 2003;121(8):1132–1140. doi: 10.1001/archopht.121.8.1132. [DOI] [PubMed] [Google Scholar]
- 13.Li DQ, Lee SB, Tseng SC. Differential expression and regulation of TGF-β1, TGF-β2, TGF-β3, TGF-βRI, TGF-βRII and TGF-βRIII in cultured human corneal, limbal, and conjunctival fibroblasts. Curr Eye Res. 1999;19(2):154–161. doi: 10.1076/ceyr.19.2.154.5321. [DOI] [PubMed] [Google Scholar]
- 14.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-β as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32(8):2201–2211. [PubMed] [Google Scholar]
- 15.Taylor AW, Streilein JW, Cousins SW. Identification of α-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. 1992;11(12):1199–1206. doi: 10.3109/02713689208999545. [DOI] [PubMed] [Google Scholar]
- 16.Taylor A. A review of the influence of aqueous humor on immunity. Ocul Immunol Inflamm. 2003;11(4):231–241. doi: 10.1076/ocii.11.4.231.18269. [DOI] [PubMed] [Google Scholar]
- 17.Luger TA, Scholzen TE, Brzoska T, Bohm M. New insights into the functions of α-MSH and related peptides in the immune system. Ann N Y Acad Sci. 2003;994:133–140. doi: 10.1111/j.1749-6632.2003.tb03172.x. [DOI] [PubMed] [Google Scholar]
- 18.Torii H, Tamaki K, Granstein RD. The effect of neuropeptides/hormones on Langerhans cells. J Dermatol Sci. 1998;20(1):21–28. doi: 10.1016/s0923-1811(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 19.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 20.Hingorani M, Metz D, Lightman SL. Characterization of the normal conjunctival leukocyte population. Exp Eye Res. 1997;64(6):905–912. doi: 10.1006/exer.1996.0280. [DOI] [PubMed] [Google Scholar]
- 21.Asahina A, Hosoi J, Grabbe S, et al. Modulation of Langerhans cell function by epidermal nerves. J Allergy Clin Immunol. 1995;96(6 pt 2):1178–1182. doi: 10.1016/s0091-6749(95)70203-2. [DOI] [PubMed] [Google Scholar]
- 22.Luger TA. Neuromediators: a crucial component of the skin immune system. J Dermatol Sci. 2002;30(2):87–93. doi: 10.1016/s0923-1811(02)00103-2. [DOI] [PubMed] [Google Scholar]
- 23.Lighvani S, Huang X, Trivedi PP, Swanborg RH, Hazlett LD. Substance P regulates NK cell IFN-γ production and resistance to Pseudomonas aeruginosa infection. Eur J Immunol. 2005;35(5):1567–1575. doi: 10.1002/eji.200425902. [DOI] [PubMed] [Google Scholar]
- 24.Szliter EA, Lighvani S, Barrett RP, Hazlett LD. Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa–infected cornea and protects against corneal perforation. J Immunol. 2007;178(2):1105–1114. doi: 10.4049/jimmunol.178.2.1105. [DOI] [PubMed] [Google Scholar]