Abstract
Rationale
±3,4-methylenedioxymethamphetamine (MDMA) is widely believed to increase sociability. The drug alters speech production and fluency, and may influence speech content. Here, we investigated the effect of MDMA on speech content, which may reveal how this drug affects social interactions.
Method
35 healthy volunteers with prior MDMA experience completed this two-session, within-subjects, double-blind study during which they received 1.5 mg/kg oral MDMA and placebo. Participants completed a 5-min standardized talking task during which they discussed a close personal relationship (e.g., a friend or family member) with a research assistant. The conversations were analyzed for selected content categories (e.g., words pertaining to affect, social interaction, and cognition), using both a standard dictionary method (Pennebaker’s Linguistic Inquiry and Word Count: LIWC) and a machine learning method using random forest classifiers.
Results
Both analytic methods revealed that MDMA altered speech content relative to placebo. Using LIWC scores, the drug increased use of social and sexual words, consistent with reports that MDMA increases willingness to disclose. Using the machine learning algorithm, we found that MDMA increased use of social words and words relating to both positive and negative emotions.
Conclusions
These findings are consistent with reports that MDMA acutely alters speech content, specifically increasing emotional and social content during a brief semistructured dyadic interaction. Studying effects of psychoactive drugs on speech content may offer new insights into drug effects on mental states, and on emotional and psychosocial interaction.
INTRODUCTION
The drug ±3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”, “molly”) is known among drug users for its positive social-emotional effects, such as increased feelings of empathy, interpersonal closeness, and sociability (Bravo 2001; Kelly et al. 2006; Rodgers et al. 2006; Sumnall et al. 2006). In addition, before it was classified in the US as a controlled substance, MDMA was used as an adjunct to psychotherapy by therapists because it appeared to decrease defensiveness and enhance feelings of emotional closeness (Greer and Tolbert 1986; Wolfson 1986). More recently, clinical trials have suggested that the drug may be an effective therapeutic adjunct in patients with post-traumatic stress disorder (Mithoefer et al. 2013; Oehen et al. 2013). Thus, both anecdotal and experimental data indicate that MDMA produces positive acute social-emotional effects that may underlie the drug’s putative therapeutic potential. Analysis of speech content may shed light on the processes by which this drug produces its apparently unique prosocial effects.
Several controlled laboratory studies support the idea that MDMA produces prosocial effects. Single doses of MDMA increase feelings of friendliness and euphoria, and feeling close to others (Bedi et al. 2010; Bedi et al. 2009; Harris et al. 2002; Hysek and Liechti 2012; Kirkpatrick et al. 2012; Kirkpatrick et al. 2014; Tancer and Johanson 2003). On measures of cognitive-emotional function, it increases recognition of positive emotions such as friendliness in others (Hysek et al. 2012) and decreases recognition of negative expressions such as fear (Bedi et al. 2010; Hysek et al. 2012), suggesting that it increases social behavior in part by enhancing sensitivity to positive emotions and reducing sensitivity to negative emotions in others. MDMA also reduces the negative affect produced by simulated social exclusion (Frye et al. 2014). Most of the research to date has utilized standardized, computerized tasks that are typically administered to individual participants, tested in nonsocial contexts. To understand the effects of a drug on social processes, it may be more appropriate to use procedures involving interpersonal interactions.
Speech is a key element of human social interaction. Several drugs, including MDMA, alter speech production and fluency. Amphetamine and alcohol increase speech production (Higgins and Stitzer 1988; Wardle et al. 2012), whereas MDMA (100 mg) disrupted speech fluency (Marrone et al. 2010). Drugs can also alter the content of speech. (Bedi et al. 2014) recently reported that MDMA increased the social content of speech using a machine learning analysis: the drug increased the use of words that were semantically close to “friend”, “support”, “rapport”, and “empathy”. The current study used similar approaches to further investigate the effects of MDMA on speech content, using a separate sample of participants and two different methods of speech content analysis.
Healthy experienced drug users received single doses of MDMA (1.5 mg/kg oral) and placebo. They performed a standardized 5-min dyadic speaking task with a research assistant in which they spoke about their relationship with another person. From the transcriptions we examined speech production and content. We hypothesized that MDMA would increase 1) the amount the talking (i.e., total number of words) and 2) proportion of emotional and social words used and that 3) machine learning methods would be able to distinguish MDMA from placebo based on word usage.
METHODS
Participants
Healthy men and women (N=35; 12 female, 23 male) with light-to-moderate past “ecstasy” experience (i.e., 4–40 times in their lifetime) were recruited via newspaper, community bulletin board, and online advertisements. Potential participants completed an initial telephone and an in-person psychiatric evaluation and medical examination, including an electrocardiogram and physical examination. Inclusion criteria were: age 18 – 35, at least high school education, fluency in English, and BMI 18 – 30. All participants were Caucasian because this was part of a larger genetic study. Exclusion criteria were: smoking more than 10 cigarettes per day, night shift work, any significant medical or psychiatric condition (e.g., cardiovascular, neurological, or major psychiatric illness including all Axis I disorders).
Participants were told that the purpose of the study was to evaluate individual differences in drug response. They were told they could receive a stimulant (explained as including drugs such as MDMA and amphetamine), sedative, cannabinoid or placebo. Participants were instructed to consume their normal amount of caffeine, but refrain from tobacco use for 9 hrs, and other drug use for 48 hrs, prior to each session. Women who used hormonal contraceptives were tested regardless of menstrual cycle phase, but women not using hormonal contraceptives were tested only during the follicular phase (days 2–14; White et al. 2002). The study was approved by the Institutional Review Board at the University of Chicago in accordance with the Code of Federal Regulations (Title 45, Part 46) adopted by the National Institutes of Health and the Office for Protection from Research Risks of the US Federal Government. Participants provided written informed consent prior to participation and they were debriefed after completion to explain the study.
Design
The current study was a part of a larger study investigating the behavioral and physiological effects of MDMA (0.75 and 1.5 mg/kg; (Kirkpatrick et al. 2014). Because the 0.75 mg/kg dose produced only modest effects it was not included in the present analysis. Participants attended 4.5-hour laboratory sessions during which they ingested capsules containing placebo or MDMA in randomized order. Sessions were separated by at least five days. Subjects’ mood states and physiological measures were monitored at baseline and for 4 hours after drug administration.
Procedure
Sessions were conducted from 9:00 am to 1:30 pm. Upon arrival for the session, participants provided urine and breath samples to confirm abstinence from alcohol (as measured by an Alco-Sensor III Breathalyzer, Intoximeters Inc., St Louis, MO), amphetamine, cocaine and opiates (as measured by urine toxicology: Ontrak TesTstik, Roche Diagnostic Systems Inc., Somerville, NJ), and marijuana (as measured by a saliva test: Oratect, Branan Medical Corp., Irvine, CA), and women were tested for pregnancy. Sessions were rescheduled if the participant tested positive for drugs.
At 9:20 am, baseline (pre-capsule) measures of heart rate and blood pressure were obtained, and participants completed self-report mood and drug effects questionnaires. At 9:30 am, participants ingested capsules containing either MDMA or placebo. Physiological and subjective measures (reported previously, Kirkpatrick et al. 2014) were obtained at 10:00, 10:30, 11:00, 11:30 am, 1:00, and 1:30 pm. The talking task (described below) was completed at 11:00am to coincide with expected peak drug effects. During times when no measures were scheduled the participants were allowed to relax and watch movies or read. At 1:30 pm, participants were discharged provided that their heart rate and blood pressure had returned to baseline levels.
Talking Task
The talking task was a modification (Wardle et al. 2012) of the Interpersonal Perception Task (Janowsky 2003) previously used to study psychoactive drugs. During an initial orientation session before the study began, participants provided the names of three important people in their lives. At each subsequent experimental session, the research assistant randomly selected one name, and asked the participant to talk about this person for 5 min. Research assistants, who were gender matched to participants, were trained in reflective listening, in which they encouraged the participant to speak freely, mirroring the mood of the speaker by reflecting the emotional state with words and nonverbal communication, and minimizing their own input into the conversation. Speech samples were professionally transcribed and any speech of the research assistants was deleted.
Self-report Measures
Participants completed questionnaires before and at regular intervals after ingesting the MDMA/placebo. As fully described elsewhere in Kirkpatrick et al. (2014), eighteen visual analogue items were measured by having participants make a mark along a 100-mm line labeled “not at all” at one end and “extremely” on the other end. The items were: “feel drug,” “feel high,” “like drug,” “dislike drug,” and “want more drug,” “anxious,” “dizzy,” “elated,” “restless,” “sedated,” “stimulated”, “confident,” “friendly,” “insightful,” “loving,” “lonely,” “playful,” and “sociable.”
Drugs
Drug conditions were administered in counter-balanced order, under double-blind conditions. Capsules were prepared by The University of Chicago Hospitals investigational pharmacy. MDMA powder (1.5 mg/kg) was encapsulated in size 00 opaque capsules with lactose filler. Placebo capsules contained only lactose. These MDMA doses were selected based on previous studies indicating that the drug reliably increases positive mood and alters emotional processing at these doses (Bedi et al. 2010; Bedi et al. 2009; Harris et al. 2002). Doses were given as mg per kg body weight to avoid systematic gender differences in dose and differences related to variations in body weight.
Data Analysis
General analysis plan
We analyzed the data in python 2.7 (Python Software Foundation) and R (R Core Team 2014). For statistical testing, we generally used mixed effects models in which participant was a random effect and drug condition was a fixed effect. Results were considered statistically significant at p less than or equal to 0.05.
Analysis of speech
We used two approaches to analyze participants’ transcribed speech: a standardized dictionary approach and a machine learning approach. Dictionary approaches score text based on the count of words from previously validated categories and are straightforward to interpret. However, these analyses may fail to capture changes in areas outside of the validated categories of the dictionary, such as slang or other specialized terminology. Machine learning techniques, such as cross-validation and variable selection, can make classifications based on characteristics of the data set rather than on predetermined categories.
Standardized dictionary approach
For our dictionary-based analysis, we used Pennebaker’s Linguistic Inquiry and Word Count 2007 (LIWC, version 1.11), which has been used extensively to analyze speech and text samples (reviewed in Tausczik and Pennebaker, 2010). LIWC is a word count program that matches text against an extensive dictionary, and provides the percentage of words in a large set of well-validated categories. We a priori chose to examine 43 specific variables, relating to time frame, affect, social interaction, perception and cognition (see Table 1, Results). Reliability and validity of LIWC measures have been reported by Pennebaker and King (1999).
Table 1.
Comparison of word counts (mean ± SEM) used to describe significant others in placebo and 3,4-methylenedioxymethamphetamine (MDMA) sessions.
| LIWC Variable | Placebo | MDMA | F-test | p-value |
|---|---|---|---|---|
| affect | 5.36 ± 0.25 | 5.76 ± 0.36 | F(1, 30) = 3.629 | ns |
| anger | 0.32 ± 0.05 | 0.29 ± 0.07 | F(1, 30) = 0.096 | ns |
| anxiety | 0.23 ± 0.04 | 0.25 ± 0.04 | F(1, 30) = 0.001 | ns |
| negative emotion | 1.04 ± 0.11 | 1.05 ± 0.11 | F(1, 30) = 0.018 | ns |
| positive emotion | 4.53 ± 0.25 | 4.99 ± 0.44 | F(1, 30) = 3.192 | ns |
| sad | 0.11 ± 0.02 | 0.14 ± 0.03 | F(1, 30) = 0.629 | ns |
| causal | 1.34 ± 0.13 | 1.28 ± 0.09 | F(1, 30) = 0.25 | ns |
| cognitive mechanisms | 20.85 ± 0.53 | 20.25 ± 0.40 | F(1, 30) = 2.343 | ns |
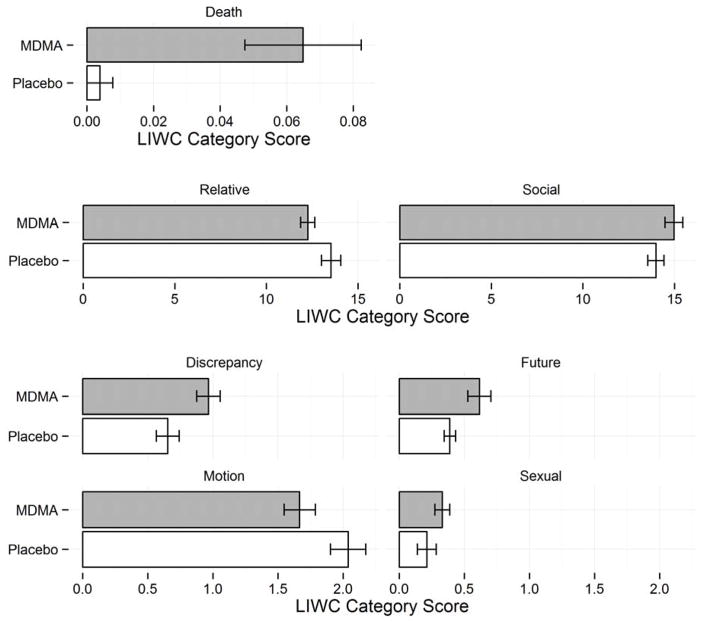
| discrepancy | 0.65 ± 0.09 | 0.96 ± 0.09 | F(1, 30) = 8.108 | 0.008* |
| insight | 1.98 ± 0.16 | 1.68 ± 0.12 | F(1, 30) = 2.344 | ns |
| family | 0.77 ± 0.13 | 0.81 ± 0.13 | F(1, 30) = 0.012 | ns |
| friend | 0.61 ± 0.1 | 0.64 ± 0.1 | F(1, 30) = 0.015 | ns |
| humans | 0.87 ± 0.10 | 0.9 ± 0.08 | F(1, 30) = 0.414 | ns |
| feel | 0.42 ± 0.06 | 0.58 ± 0.06 | F(1, 30) = 3.382 | ns |
| hear | 0.55 ± 0.09 | 0.54 ± 0.09 | F(1, 30) = 0.001 | ns |
| perception | 1.65 ± 0.15 | 1.78 ± 0.14 | F(1, 30) = 0.371 | ns |
| see | 0.62 ± 0.07 | 0.58 ± 0.07 | F(1, 30) = 0.197 | ns |
| social | 13.98 ± 0.44 | 14.97 ± 0.49 | F(1, 30) = 7.967 | 0.008* |
| assent | 0.86 ± 0.11 | 0.91 ± 0.13 | F(1, 30) = 1.064 | ns |
| certainty | 1.66 ± 0.13 | 1.71 ± 0.18 | F(1, 30) = 0.775 | ns |
| exclamation | 4.46 ± 0.32 | 4.25 ± 0.20 | F(1, 30) = 0.817 | ns |
| inclusive | 7.61 ± 0.35 | 7.1 ± 0.28 | F(1, 30) = 2.658 | ns |
| inhibition | 0.27 ± 0.04 | 0.28 ± 0.04 | F(1, 30) = 0.092 | ns |
| tentatative | 3.64 ± 0.21 | 3.48 ± 0.23 | F(1, 30) = 0.428 | ns |
| future | 0.39 ± 0.04 | 0.62 ± 0.09 | F(1, 30) = 4.958 | 0.034* |
| past | 4.47 ± 0.34 | 4.21 ± 0.26 | F(1, 30) = 0.93 | ns |
| present | 10.34 ± 0.47 | 10.49 ± 0.43 | F(1, 30) = 1.658 | ns |
| achieve | 1.04 ± 0.09 | 1.04 ± 0.07 | F(1, 30) = 0 | ns |
| biology | 1.15 ± 0.13 | 1.26 ± 0.12 | F(1, 30) = 1.18 | ns |
| body | 0.29 ± 0.06 | 0.28 ± 0.06 | F(1, 30) = 0.005 | ns |
| death | 0 ± 0.00 | 0.06 ± 0.02 | F(1, 30) = 11.56 | 0.002* |
| health | 0.34 ± 0.05 | 0.28 ± 0.04 | F(1, 30) = 0.286 | ns |
| home | 0.51 ± 0.06 | 0.47 ± 0.07 | F(1, 30) = 0.134 | ns |
| ingest | 0.35 ± 0.09 | 0.4 ± 0.08 | F(1, 30) = 0.237 | ns |
| leisure | 1.23 ± 0.12 | 1.37 ± 0.12 | F(1, 30) = 2.093 | ns |
| money | 0.35 ± 0.06 | 0.34 ± 0.07 | F(1, 30) = 0.078 | ns |
| motion | 2.04 ± 0.14 | 1.67 ± 0.12 | F(1, 30) = 5.854 | 0.022* |
| relative | 13.53 ± 0.53 | 12.26 ± 0.39 | F(1, 30) = 5.782 | 0.023* |
| religion | 0.21 ± 0.05 | 0.25 ± 0.06 | F(1, 30) = 1.535 | ns |
| sexual | 0.21 ± 0.07 | 0.33 ± 0.06 | F(1, 30) = 4.332 | 0.046* |
| space | 5.9 ± 0.24 | 5.63 ± 0.26 | F(1, 30) = 0.213 | ns |
| time | 6.07 ± 0.38 | 5.6 ± 0.23 | F(1, 30) = 1.644 | ns |
| work | 1.29 ± 0.11 | 1.29 ± 0.12 | F(1, 30) = 0.269 | ns |
The talking task required participants to discuss a person who was important to them. However, past investigations have suggested some aspects of speech may be impaired by MDMA, including ability to coherently focus on single topics (Marrone et al. 2010). In order to better detect the influence of MDMA on speech about an emotionally salient person, we isolated and analyzed individual phrases describing the topic person. This was operationalized as phrases beginning with “he is,” “she is,” or a contraction of either phrase (e.g. “he’s”). We edited these phrases by removing filler words (such as “um”) and ungrammatical word repetitions (such as “people” in “he’s one of the people, people I’ve known the longest here”) and analyzed the phrases using the same LIWC categories as the entire text samples. We also categorized whether the phrases were (1) psychological, such as describing an aspect of the topic person’s personality, (2) a non-psychological, such as appearance or profession, or (3) describing the relationship between the participant and topic person. These three categories, which were not mutually exclusive, were rated by individuals who were blinded to study design and condition.
Machine learning approach
We took a bag-of-words approach in which we quantified word occurrence but not word order or context. Using the python packages gensim (Řehůřek and Sojka 2010) and Natural Language Tool Kit (nltk; Bird, Loper and Klein 2009), we removed names of individuals and converted (i.e., lemmatized) remaining words in each speech document to their root form using nltk’s morphy function with part of speech identified using a maximum entropy Treebank tagger. We counted the number of occurrences of each word in each document and used word occurrences as predictor variables in statistical machine learning models that predicted dosing condition.
Our modeling approach used random forests. Random forest is an ensemble classifier that generates a group of classification trees based on predictor variables and then uses the majority vote of the trees to determine membership. Each classification tree is fit using a random subset of predictor variables on a random subset of the observations drawn with replacement. Because it is based on decision trees, the random forest algorithm is well suited for capturing nonlinear interactions between predictor variables.
We used individual words as predictors and used recursive feature elimination with random forest ensemble models to select a smaller subset of words that predicted dosing condition. To summarize these models, we estimated variable importance using gini impurity, a standard measure for random forests and other decision trees. When a node in a tree is split using a variable, the two child nodes are more homogeneous in the outcome measure compared to the parent node. This homogeneity is standardly quantified using gini impurity, which measures how often a randomly chosen element in a node would be incorrectly labeled if it were randomly labeled according to the distribution of labels in the node. Gini impurity is computed by summing the probability of each item being chosen times the probability of a mistake in categorizing that item. Summing gini decreases between parent and child nodes for each variable across all trees yields a measure of variable importance in the ensemble model.
Predicting dosing condition from individual words
We used recursive feature elimination (Guyon and Elisseeff 2006; Kuhn and Johnson 2013) to build ensembles with decreasing numbers of predictor variables. We iteratively fit random forests, beginning with 300 variables, fitting models, and discarding the 50 variables with the lowest variable importance. We repeated this process 2000 times. Because each tree uses only a subset of available data, the relative performances of different trees and ensembles can be measured using out-of-bag accuracy, which entails measuring performance by predicting observations that were not used in a tree’s construction. Out-of-bag accuracy is optimistic and poorly predicts accuracy in new datasets, but it is useful for comparing relative performance of models fit with different numbers of variables.
Relating Speech changes to self-report measures
To avoid a large number of comparisons, we focused on the statistically significant results from our dictionary-based speech analysis and reduced their dimensionality using principal components analysis with varimax rotation. We then attempted to predict these components using least angle lasso regression (Tibshirani 1996) with 10-fold cross-validation to select the lambda penalty parameter and the penalized score test of Voorman, Shojaie, and Whitten (2014) to determine statistical significance of associations.
RESULTS
Sample Characteristics
In total, data from 35 participants (34% female) were used for the current study. They were 24.3±4.3 (mean±SD) years old, had a BMI of 22.7±2.7, and completed 14.6±1.4 years of formal education. They had used “ecstasy” a mean of 13.1±10.2 times (range 4–40 lifetime). Thirty participants currently drank alcohol (9.3±6.4 drinks/week), 31 drank caffeinated beverages (1.7±1.3 cups/day), 30 currently smoked marijuana (7.4±7.2 days/month), and 12 smoked tobacco (6.8±5.1 cigarettes/day).
Talking Task
Standardized Dictionary Approach
When whole transcripts were analyzed, MDMA altered use of words, compared to placebo. As shown in Table 1 and Figure 1, the drug increased words with sexual and social content, language reflecting discrepancies, increased discussion of the future, and decreased tendency to speak in relative terms and use motion language (F1, 30 = 4.33 to 11.562, p = 0.046 to 0.002). Participants also spoke significantly more about death (see Figure 3), although the scale of this increase was low. No gender differences were detected. MDMA also did not significantly affect number of words spoken (mean ± SEM was 692.5 ± 187.08 words after placebo versus 685.7 ± 171.14 words after MDMA). Data from four participants were missing (placebo condition) due to recording failure.
Figure 1.
Significant speech differences between ±3,4-methylenedioxymethamphetamine (MDMA) and placebo sessions detected with a standard dictionary approach (Linguistic Inquiry and Word Count (LIWC)).
MDMA also affected isolated phrases specifically describing the target person. When we categorized phrases about the target person based on psychological, non-psychological, and relationship content, we saw that MDMA decreased proportion (and absolute counts; not shown) of phrases with psychological content and increased proportion of phrases with non-psychological factual content about the target person. Psychological statements decreased from 76.0% ± 3.73% after placebo to 56.7% ± 3.91% of phrases after MDMA (F1,32.86 = 19.49, p < 0.0001). Nonpsychological statements correspondingly increased from 15.4% ± 3.52% after placebo to 30.4% ± 4.03% phrases after MDMA (F1,32.93 = 10.29, p = 0.003). The proportion (and absolute number) of phrases describing the relationship of the speaker with the target person was not significantly changed. Across all phrases describing the target person, MDMA decreased use of words relating to the body (0.53 ± 0.2 words after placebo vs. 0 ± 0.0 words after MDMA, F1, 498 = 5.571, p = 0.019) and increased words in other categories, including cognitive mechanisms (12.2 ± 0.92 words after placebo vs. 16.57 ± 1.03 words after MDMA, F1, 498 = 11.516, p = 0.001) and insight (0.51 ± 0.16 words after placebo vs. 1.31 ± 0.38 words after MDMA, F1, 498 = 4.503, p = 0.034).
Relating speech effects to self-report measures
As fully described in Kirkpatrick et al. (2014), MDMA significantly increased multiple self-report measures. To explore how these related to speech changes, we first reduced the dimensionality of the significant LIWC categories using principal component analysis (PCA). We retained three components from our PCA procedure, which each respectively explaining 29.0, 24.0, and 17.6 % of the variance. The first component, hereafter “Motion/Relative,” correlated highly with the motion and relative scales (r = 0.87 and 0.92, respectively) and had correlations less than ±0.15 with other LIWC variables. The second component, hereafter “Discrepancy/Future,” correlated with discrepancy and future scales (r = 0.91 and 0.91, respectively) and had correlations less than ±0.15 with other LIWC variables. The third component, hereafter “Social,” was correlated with social, sexual, and death scales (0.86, 0.79, and 0.48), and had correlations less than ±0.16 with other LIWC variables.
We then attempted to predict each component using lasso regression and peak self-report visual analog scores (Table 2). All three components were significantly predicted by high, feel drug, elated, stimulated, loving, social, and friendly scores. Relative/Motion was additionally predicted by playful and dislike drug scores. Discrepancy/Future were predicted by playful and want more drug scores. Finally, Social was predicted by confident, insightful, dizzy, and want more drug scores.
Table 2.
Predicting speech (as rotated components from PCA) using self-report drug effects as predictors
| Predictor | Motion/Relative Component | Discrepancy/Future Component | Social Component | |||
|---|---|---|---|---|---|---|
| Test score | p value | Test score | p value | Test score | p value | |
| High | 2.501 | <0.001* | 2.701 | 0.006* | 3.046 | 0.002* |
| Feel Drug | 2.656 | <0.001* | 2.723 | 0.006* | 2.766 | 0.006* |
| Elated | 2.405 | <0.001* | 2.527 | 0.011* | 2.79 | 0.005* |
| Stimulated | 2.133 | 0.004* | 2.777 | 0.005* | 2.445 | 0.014* |
| Loving | 2.668 | 0.001* | 2.332 | 0.019* | 2.265 | 0.023* |
| Like Drug | 3.059 | 0.002* | 1.972 | 0.047* | 1.895 | ns |
| Social | 2.271 | 0.003* | 1.976 | 0.046* | 2.614 | 0.009* |
| Friendly | 2.05 | 0.007* | 2.331 | 0.019* | 2.262 | 0.024* |
| Dizzy | 1.665 | ns | 1.752 | ns | 2.696 | 0.007* |
| Playful | 1.206 | ns | 2.28 | 0.022* | 2.591 | 0.01* |
| Want More Drug | 1.812 | 0.007* | 1.59 | ns | 2.487 | 0.013* |
| Dislike Drug | 1.102 | ns | 3.145 | 0.002* | 1.135 | ns |
| Insightful | 1.201 | ns | 1.137 | ns | 2.915 | 0.004* |
| Confidence | 0.962 | ns | 0.372 | ns | 3.679 | <0.001* |
| Lonely | 0.878 | ns | 1.908 | ns | 1.318 | ns |
| Restless | 0.355 | ns | 1.34 | ns | 1.725 | ns |
| Anxiety | 0.115 | ns | 0.09 | ns | 1.747 | ns |
| Sedated | 0.387 | ns | 1.009 | ns | −0.078 | ns |
Given our a priori interest in social effects and the lack of interaction terms in lasso models, we further explored the relationships of feelings confidence and insight to the rotated speech components using random effects models. Our goal was to better understand if the relation between self-report confidence and insight and the Social component was truly unusual to that component. We accordingly fit models in which the self-report ratings were predicted by all three rotated speech components and their interactions, treating participant as a random effect. Both models indicated an effect of the Social component (Confidence: F1, 23 = 17.28, p = 0.0004; Insightful: F1, 23 = 9.89, p = 0.0045) and no main effects of the two other components. In addition there was a significant or trend interaction of the Social and Relative/Motion components (Confidence: F1, 23 = 5.35, p = 0.030; Insightful: F1, 23 = 3.86, p = 0.061, ns) in predicting peak Confidence or Insight scores.
Machine Learning Approach
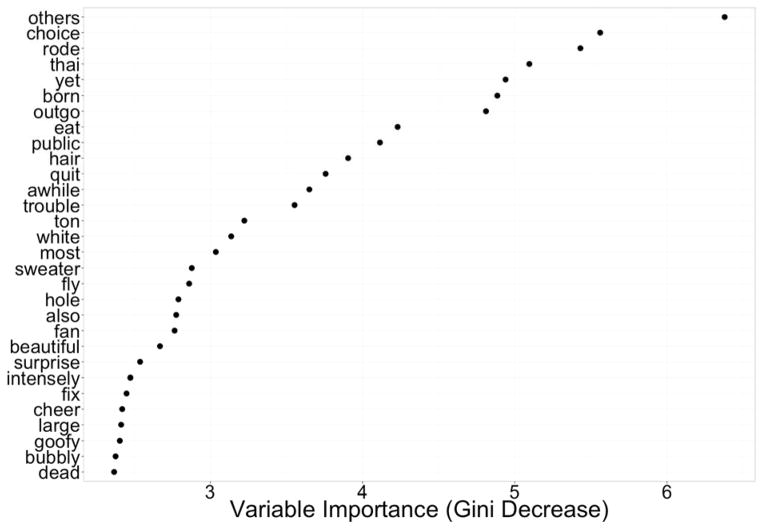
We used recursive feature elimination to build models that used subsets of words to predict participant’s dosing condition. After lemmatizing and removing proper names, there were 1755 unique words in the corpus. We further used a t-test and a threshold of p<.5 to filter half of the words for inclusion in the models. Out-of-bag accuracy for different size models varied based on number of predictor variables (words) used in the model. A model with 300 predictors was selected as the smallest model still performing within 10% accuracy of the absolute best model. We created a final model using 300 words and 2000 repeats of 5-fold cross-validation with results averaged. This final model had an accuracy of 0.72 and sensitivity and specificity of 0.71 and 0.80 in predicting dosing condition. The words identified as important included social words (others, public, camaraderie, outgoing) and words with both positive (goofy, beautiful, cheer, fix) and negative emotional valence (trouble, dead), (Figure 2).
Figure 2.
Most important words (variables) for distinguishing ±3,4-methylenedioxymethamphetamine (MDMA) from placebo sessions in machine learning (random forest) analysis.
DISCUSSION
We used two complementary techniques to investigate the effects of MDMA on the important social behavior of speech. Using a standardized dictionary approach we found that MDMA altered word choice in specific, validated categories. Using an exploratory data mining approach to look for changes relating to social and emotional functioning, we found that specific emotional words were useful for distinguishing speech on MDMA from speech on placebo.
MDMA is thought to have prosocial effects that are unusual or even unique (e.g., Nichols 1986). Our analysis of transcribed speech about emotionally important others showed evidence of these prosocial effects. Both the dictionary and machine learning analyses of the entire transcriptions indicated that MDMA increased use of social words. These findings are generally complementary with those of Bedi et al (2014), who employed a data mining approach to show that MDMA increased use of words that were semantically close to positive social words such as “friend”, “support”, “rapport”, and “empathy”.
MDMA might produce these prosocial effects in part by increasing positive emotional reactions or by blunting anxiety (Bedi et al,, 2010; Hysek et al., 2012; Mithoefer et al., 2013; Kirkpatrick et al., 2014). In this study, we indeed found many of the effects of MDMA on speech were associated with changes in self-report euphoria and sociability. When we predicted rotated principal components using self-report measures, components were predicted by a largely consistent set of self-report measures relating to euphoric and prosocial feelings. Interestingly, a social component — indicating increased use of words relating to social, sexual, and death— was also predicted by changes in self-reported confidence and feelings of insight, while other components were not. This suggests that the putatively unusual effects of MDMA on social-related speech content may not only involve euphoria-related sociability but also another cognitive phenomenon involving feelings of insight and certainty.
Consistent with the findings of Marrone et al (2010), MDMA did not increase talkativeness, as measured by the number of spoken words. Although MDMA is structurally similar to psychostimulants such as amphetamine and methamphetamine, it produces less psychomotor activation, including speech, compared to these drugs (Marrone et al., 2010; Kirkpatrick et al., 2012). MDMA also appears to differ from psychostimulants in that it can induce the feeling of cognitive impairments (e.g. ratings of inability to concentrate and decreased fluency in a talking task: (Hysek and Liechti 2012; Liechti et al. 2001; Marrone et al. 2010; Verheyden et al. 2003) as well as improvements, while prototypic stimulants produce only feelings of improvements and competence (Ballard et al. 2014; Kirkpatrick et al. 2008).
Whereas Marrone and colleagues (2010) studied the effect of MDMA on talking by asking them to recount the plot of a movie, our task involved speaking about a psychologically important target person. Under the influence of the drug, participants in our study described the target person using proportionally fewer phrases with psychological content and more with factual content. Although this initially appears inconsistent with the purported insight-producing effect of MDMA, a closer examination of the phrases describing target individuals suggests the effect may be due to a shift from stating general abstract opinions to mentioning more specific concrete details and episodes. Levels of linguistic abstraction are known to indicate levels of interpersonal closeness (Reitsma-van Rooijen et al., 2007; IJzerman and Semin, 2009; Douglas and Sutton, 2010) and thus the current data could indicate a deeper and less superficial consideration of the target person. Indeed, LIWC analysis of the descriptive phrases found significant increases in words relating to insights and cognitive mechanisms.
Overall, these data suggest that MDMA does not only selectively blunt availability of negative emotional memories or enhance positive ones, but may also increase willingness or ability to consider emotional memories, at least in the presence of another person. This appears consistent with clinical observations (e.g., Mithoefer et al 2011, Greer and Tolbert 1998), although further research will be needed to confirm analogous results in patient populations.
This study has several limitations. We used a bag-of-words approach in which no attention is paid to word order or context within a document. This is computationally appealing, but unlikely to capture more than a small portion of the nuances of word usage. Further investigations should expand to include bigram and trigrams. Our talking task had participants discuss individuals who were psychologically important to them. This did not control for, and may have limited, the range of emotional memories that were recalled. Future studies could elicit memories with specific emotional content or use behavioral paradigms to create emotional experiences, as in the Trier social stress task (Kirschbaum et al., 1993). These would allow further insights into the effects of MDMA on speech and emotional experience.
In conclusion, we found that MDMA altered social aspects of speech during a brief semistructured dyadic interaction, using two different analytic methods. Combined with natural language processing, studying effects of psychoactive drugs on speech content can offer new insights into drug effects on mental states, as well as emotional and psychosocial interaction.
Acknowledgments
Supported by DA026570, DA02812 (Harriet de Wit PI)
This research was supported by R21 DA026570 and R01 DA02812. The authors thank Jonathan Solamillo for excellent technical assistance, and Emmanuel Semmes for pharmacy support.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Ballard ME, Gallo DA, de Wit H. Amphetamine increases errors during episodic memory retrieval. J Clin Psychopharmacol. 2014;34:85–92. doi: 10.1097/JCP.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Cecchi GA, Fernandez Slezak D, Carrillo F, Sigman M, de Wit H. A Window into the Intoxicated Mind? Speech as an Index of Psychoactive Drug Effects. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of +/−3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol Psychiatry. 2010;68:1134–40. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl) 2009;207:73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird S, Klein E, Loper E. Natural language processing with Python. Sebastopol, CA: O’Reilly Media, Inc; 2009. [Google Scholar]
- Voorman A, Shojaie A, Witten D. Inference in high dimensions with the penalized score test. http://arxiv.org/abs/1401.2678.
- Bravo GL. What does MDMA feel like? In: Holland J, editor. Ecstasy: The complete guide A comprehensive look at the risks and benefits of MDMA. Rochester, NY: Park Street Press; 2001. pp. 21–28. [Google Scholar]
- Douglas KM, Sutton RM. By their words ye shall know them: Language abstraction and the likeability of describers. European Journal of Social Psychology. 2010;40:366–374. [Google Scholar]
- Frye CG, Wardle MC, Norman GJ, de Wit H. MDMA decreases the effects of simulated social rejection. Pharmacol Biochem Behav. 2014;117:1–6. doi: 10.1016/j.pbb.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. J Psychoactive Drugs. 1986;18:319–27. doi: 10.1080/02791072.1986.10472364. [DOI] [PubMed] [Google Scholar]
- Greer GR, Tolbert R. A method of conducting therapeutic sessions with MDMA. Journal of psychoactive drugs. 1998;30:371–379. doi: 10.1080/02791072.1998.10399713. [DOI] [PubMed] [Google Scholar]
- Guyon I, Elisseeff A. An introduction to feature extraction. In: Guyon I, Gunn S, Nikravesh M, et al., editors. Feature extraction. Berlin: Springer; 2006. pp. 1–25. [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Stitzer ML. Effects of alcohol on speaking in isolated humans. Psychopharmacology (Berl) 1988;95:189–94. doi: 10.1007/BF00174508. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology (Berl) 2012;222:293–302. doi: 10.1007/s00213-012-2645-9. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Liechti ME. Effects of MDMA alone and after pretreatment with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology (Berl) 2012;224:363–76. doi: 10.1007/s00213-012-2761-6. [DOI] [PubMed] [Google Scholar]
- IJzerman H, Semin GR. The thermometer of social relations mapping social proximity on temperature. Psychological science. 2009;20:1214–1220. doi: 10.1111/j.1467-9280.2009.02434.x. [DOI] [PubMed] [Google Scholar]
- Janowsky DS. Depression and dysphoria effects on the interpersonal perception of negative and positive moods and caring relationships: effects of antidepressants, amphetamine, and methylphenidate. Curr Psychiatry Rep. 2003;5:451–9. doi: 10.1007/s11920-003-0084-3. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Parsons JT, Wells BE. Prevalence and predictors of club drug use among club-going young adults in New York city. J Urban Health. 2006;83:884–95. doi: 10.1007/s11524-006-9057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2012;219:109–22. doi: 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, et al. Effects of MDMA and intranasal oxytocin on social and emotional processing. Neuropsychopharmacology. 2014;39:1654–1663. doi: 10.1038/npp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Metcalfe J, Greene MJ, Hart CL. Effects of intranasal methamphetamine on metacognition of agency. Psychopharmacology (Berl) 2008;197:137–44. doi: 10.1007/s00213-007-1018-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Johnson K. Applied predictive modeling. New York: Springer; 2013. [Google Scholar]
- Liechti ME, Geyer MA, Hell D, Vollenweider FX. Effects of MDMA (ecstasy) on prepulse inhibition and habituation of startle in humans after pretreatment with citalopram, haloperidol, or ketanserin. Neuropsychopharmacology. 2001;24:240–52. doi: 10.1016/S0893-133X(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Marrone GF, Pardo JS, Krauss RM, Hart CL. Amphetamine analogs methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) differentially affect speech. Psychopharmacology (Berl) 2010;208:169–77. doi: 10.1007/s00213-009-1715-0. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of±3, 4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. Journal of Psychopharmacology. 2011;25(4):439–452. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Martin SF, Yazar-Klosinski B, Michel Y, Brewerton TD, Doblin R. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. J Psychopharmacol. 2013;27:28–39. doi: 10.1177/0269881112456611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: entactogens. Journal of psychoactive drugs. 1986;18(4):305–313. doi: 10.1080/02791072.1986.10472362. [DOI] [PubMed] [Google Scholar]
- Oehen P, Traber R, Widmer V, Schnyder U. A randomized, controlled pilot study of MDMA (+/− 3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD) J Psychopharmacol. 2013;27:40–52. doi: 10.1177/0269881112464827. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, King LA. Linguistic styles: Language use as an individual difference. J Pers Soc Psychol. 1999;77:1296–1312. doi: 10.1037//0022-3514.77.6.1296. [DOI] [PubMed] [Google Scholar]
- Řehůřek R, Sojka P. Software framework for topic modelling with large corpora. In: Witte R, Cunningham H, Patrick J, et al., editors. Proceedings of LREC 2010 workshop: New challenges for NLP frameworks. Valletta, Malta: University of Malta; 2010. pp. 46–50. [Google Scholar]
- Reitsma-van Rooijen M, Semin GR, Van Leeuwen E. The effect of linguistic abstraction on interpersonal distance. European Journal of Social Psychology. 2007;37:817–823. [Google Scholar]
- Rodgers J, Buchanan T, Pearson C, Parrott AC, Ling J, Hefferman TM, Scholey AB. Differential experiences of the psychobiological sequelae of ecstasy use: quantitative and qualitative data from an internet study. J Psychopharmacol. 2006;20:437–46. doi: 10.1177/0269881105058777. [DOI] [PubMed] [Google Scholar]
- Sumnall HR, Cole JC, Jerome L. The varieties of ecstatic experience: an exploration of the subjective experiences of ecstasy. J Psychopharmacol. 2006;20:670–82. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 2003;72:33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Tausczik YR, Pennebaker JW. The psychological meaning of words: LIWC and computerized text analysis methods. Journal of language and social psychology. 2010;29:24–54. [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society Series B (Methodological) 1996;58(1):267–288. [Google Scholar]
- Verheyden SL, Henry JA, Curran HV. Acute, sub-acute and long-term subjective consequences of ‘ecstasy’ (MDMA) consumption in 430 regular users. Hum Psychopharmacol. 2003;18:507–17. doi: 10.1002/hup.529. [DOI] [PubMed] [Google Scholar]
- Voorman A, Shojaie A, Witten D. [accessed on 1 April 2015];Inference in high dimensions with the penalized score test. http://arxiv.org/abs/1401.2678.
- Wardle MC, Garner MJ, Munafo MR, de Wit H. Amphetamine as a social drug: effects of d-amphetamine on social processing and behavior. Psychopharmacology (Berl) 2012;223:199–210. doi: 10.1007/s00213-012-2708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–41. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wolfson PE. Meetings at the edge with Adam: a man for all seasons? J Psychoactive Drugs. 1986;18:329–33. doi: 10.1080/02791072.1986.10472365. [DOI] [PubMed] [Google Scholar]