Abstract
Objective
Prevention of temporary threshold shift (TTS) after laboratory-based exposure to pure-tones, broadband noise, and narrow band noise signals has been achieved, but prevention of TTS under these experimental conditions may not accurately reflect protection against hearing loss following impulse noise. This study used a controlled laboratory-based TTS paradigm that incorporated impulsive stimuli into the exposure protocol; development of this model could provide a novel platform for assessing proposed therapeutics.
Design
Participants played a video game that delivered gunfire-like sound through headphones as part of a target practice game. Effects were measured using audiometric threshold evaluations and distortion product otoacoustic emissions (DPOAEs). The sound level and number of impulses presented were sequentially increased throughout the study.
Study sample
Participants were normal-hearing students at the University of Florida who provided written informed consent prior to participation.
Results
TTS was not reliably induced by any of the exposure conditions assessed here. However, there was significant individual variability, and a subset of subjects showed TTS under some exposure conditions.
Conclusions
A subset of participants demonstrated reliable threshold shifts under some conditions. Additional experiments are needed to better understand and optimize stimulus parameters that influence TTS after simulated impulse noise.
Keywords: gunshot, impulse noise, video game, temporary threshold shift, TTS, hearing
Introduction
Despite the promulgation of the Hearing Conservation Amendment 30 years ago (Code of Federal Regulations, 1983; Suter, 2009), noise-induced hearing loss (NIHL) continues to be one of the top two occupational injuries. Hearing protection devices (HPDs) alone clearly have not proven to be a comprehensive solution to the problem of NIHL. Noise is sometimes unexpected and often occurs when HPDs are not inserted properly or are unavailable. Noise can also exceed the attenuation rating of the HPD, can be transferred via air leaks, or can be transmitted via bone conduction through mechanical stimulation of the skull (for review, see Berger, 2003). In addition, there are military settings under which the use of HPDs can potentially compromise lethality and survival, even with the use of electronically augmented HPDs (Casali et al., 2009).
NIHL, specifically including hearing loss after impulse noise, is a significant medical problem, particularly in the military veteran population (US Department of Veterans Affairs, 2010; Grantham, 2011). Firearm exposure has been identified as a significant contributing factor to the overall problem and prevalence of NIHL (Agrawal et al., 2009; Agrawal et al., 2010). There are many common sources of impulse noise, including not only small arms (either military or civilian) (Tambs et al., 2006; Flamme et al., 2009b; Stewart et al., 2009; Ahroon et al., 2011), but also firecrackers (Flamme et al., 2009a), starter pistols (Meinke et al., 2013), and some workplaces where occupational noise conditions include impulse sounds (Sulkowski et al., 1999; Suvorov et al., 2001; Zera, 2001). Temporary threshold shift (TTS) as a result of impulse noise exposure has been reported (Bapat & Tolley, 2007), as well as changes in otoacoustic emissions (Pawlaczyk-Luszczynska et al., 2004; Balatsouras et al., 2005; Konopka et al., 2005; Olszewski et al., 2007).
Development of therapeutics that can reduce or prevent NIHL is a goal for many research teams, and TTS study designs have been the primary model to date for evaluating proposed otoprotective agents in humans (for review, see Le Prell et al., 2012). The use of TTS models holds appeal for initial studies, given that trial duration and cost are reduced relative to the longer-duration trials needed to assess permanent threshold shift (PTS). With participation time reduced for any given subject, subject attrition and loss to follow-up is less likely to be an issue as well. Benefits have already been shown, in the form of reduced TTS, for participants who are given pre-noise supplements of magnesium (Attias et al., 2004), vitamin B (Quaranta et al., 2004), or alpha-lipoic acid (Quaranta et al., 2012). These studies have used broad-band noise (Attias et al., 2004), narrow band noise centered at 3 kHz (Quaranta et al., 2004), or 3 kHz pure-tone stimuli (Quaranta et al., 2012) as the acoustic insult. While there is little reason to expect that TTS will vary as a function of non-acoustic parameters, listening to these types of continuous signals (i.e., pure-tones, Gaussian noise, shaped noise) would likely be considered unpleasant. More importantly, in both occupational and recreational settings, noise will likely be more variable, reflecting a mix of background noise and impulses and/or impacts (Erdreich, 1986). This variation in spectral amplitude importantly influences the effects of noise on the inner ear, with noise that has more impulsive components and peaks being more hazardous than noise that is more constant (Hamernik et al., 2007; Zhao et al., 2010; Goley et al., 2011).
Otoprotection studies based in real-world acoustic environments provide noise exposures with acoustic signals that include more spectral variation than the continuous signals used in the laboratory studies noted above, but these real-world studies have a number of critical shortcomings as well. One significant shortcoming is the variability of real-world noise exposures across subjects, with up to 10-dB differences in exposure levels across subject cohorts tested on different days reported in a nightclub-based study (see Kramer et al., 2006). In another study, average TTS was minimal in participants recruited from an occupational setting that included prolonged noise exposure during work shifts at a factory, precluding conclusions regarding clinically significant protection (Lin et al., 2010). To reduce the likelihood of “failed studies”, we developed a TTS model that uses a digital music player (Le Prell et al., 2012), in conjunction with a set of tools that allows music presentation level to be fixed from song to song (Le Prell et al., 2011b), to better control noise exposure while maintaining real-world relevance. This model is being used to assess two different proposed therapeutics (NCT00808470; NCT01444846)1. The methodology used in this digital music player model can be readily adapted to be used with simulated impulse noise stimuli that are more relevant to real-world exposures often observed under military or industrial noise conditions. Given the interest in establishing potential otoprotection for military personnel (NCT01345474; Kopke, 2005; Dolgin, 2012), our model could provide a significant framework to evaluate proposed treatments. TTS studies using military personnel participating in weapons training have been challenging in that TTS has not been consistently detected in control subjects (Le Prell et al., 2011a; Lindblad et al., 2011). Although these trials failed to show drug efficacy, the results are important in that they highlight the potential success of HPDs in protecting auditory function during weapons training if HPDs are used properly and consistently.
This report describes the on-going development of a laboratory-based model that incorporates a simulated impulse noise stimulus. Although impulse noise is clearly different from most constant noise with respect to relative risk of mechanical damage, otoprotection studies using impulse noise in animals have been promising (Cassandro et al., 2003; Hight et al., 2003; Kopke et al., 2005; Coleman et al., 2007; Bielefeld et al., 2011; Gavriel et al., 2011; Xiong et al., 2013). A previous attempt at evaluating otoprotection in humans, using c-Jun N-terminal kinase (JNK) inhibitor, suggests potential benefit for human patients exposed to firecracker noise (Suckfuell et al., 2007). However, that study lacked a placebo control against which recovery could be compared; thus, conclusions related to efficacy are limited. Other studies have sought to assess potential protection against impulse noise in military populations, but hearing loss after weapons training has been variable in real-world trials. Given that TTS has not been consistently detected even in control subjects (Le Prell et al., 2011a; Lindblad et al., 2011), and PTS has been more variable than expected in weapons trials (see commentary in Dolgin, 2012) the rationale for human trials using simulated impulse noise is both clear and compelling.
As previously described with respect to the development of music player based TTS models, ensuring participant safety is paramount. Exposures that induce robust TTS in rodents [~40 dB shift in auditory brainstem response (ABR) threshold, measured 24 hours post-noise] produce lasting decrease in ABR amplitude, immediate (and lasting) synaptic deficits, and spiral ganglion loss appears late in the lifespan (Kujawa & Liberman, 2006; Kujawa & Liberman, 2009; Lin et al., 2011; Wang & Ren, 2012). Although these phenomena have not been documented in humans, primary afferent loss in the absence of hair cell loss has been reported in human temporal bones with unknown noise histories (Makary et al., 2011). Assessing the phenomena in human ears will be complicated. This phenomena occurs as a function of age even in animals that have never been exposed to investigational noise (Boettcher et al., 1995; Schmiedt et al., 1996; Sergeyenko et al., 2013). It is reasonable to assume that robust TTS and/or aging alone will have neural consequences in humans similar to those observed in animals; thus, these data increase the urgency of better understanding the phenomena of selective disruption of the inner hair cell/auditory nerve synapse in humans. The safety issue for TTS studies is the critical TTS “threshold” below which there is no lasting synaptic change is not known. The evidence available to date has been interpreted as consistent with a critical boundary of ~20–30 dB TTS measured 24 hours post-noise (Le Prell et al., 2012). This boundary estimation was based on animal data drawn from frequencies where TTS was less robust and no neural consequences were evident (based on inspection of the figures in Kujawa & Liberman, 2006; Kujawa & Liberman, 2009; Lin et al., 2011; Wang & Ren, 2012). Consistent with this interpretation of the animal data, more recent data have confirmed complete recovery of neural response amplitudes in mice that had ~20 dB TTS 24 hours post-noise, with no evidence of TTS-related synaptic changes (Liang et al., 2013).
The approach described here is highly conservative in that exposures start at low levels with a limited number of impulse presentations. If there is no evidence of consistent, measurable changes, the exposure level and the number of impulses are increased. Our paradigm uses a modified version of procedures from the Albuquerque studies conducted by the US Army, in which first exposure level and then the number of impulses was systematically increased (Johnson, 1993; Johnson, 1998; see model in Price, 2007). Consistent with the conservative nature of the approach, we did not reach an exposure condition where TTS was consistently observed. Here, we describe the individual TTS variability in normal-hearing listeners after gunshot-like noise. The primary outcome was audiometric assessment of TTS at conventional frequencies (from 0.25 to 8-kHz). In addition, we included extended high frequency (EHF) measurements of hearing sensitivity from 10 to 16-kHz, and repeated measurements of distortion product otoacoustic emission (DPOAE) amplitude within the functional test battery. These data provide important insight into individual differences in vulnerability to noise, and provide guidance regarding sensitivity of different test metrics.
Materials and Methods
Participants
Advertisements posted at multiple locations on the University of Florida campus invited participants with normal hearing to enroll in a study of temporary changes in hearing after video game play. Prospective participants provided written informed consent, and were then required to undergo additional screening to confirm they met the eligibility criteria. As discussed in our previous publication (Le Prell et al., 2012), the informed consent process included disclosure and discussion of the data showing neural loss after robust TTS in animal subjects. All protocols and procedures were approved by the Institutional Review Board at the University of Florida.
Screening Procedures
Participants completed brief health surveys, followed by hearing and tinnitus surveys. The hearing survey included detailed questions about music player use and other sources of noise in the participant’s life (see Le Prell et al., 2013); the tinnitus survey ensured the participants did not currently experience tinnitus on a day-to-day basis. After survey completion, visual examination of the ear canal and tympanic membrane was performed to ensure normal anatomy and the absence of obstructive debris. Tympanometric measures were then collected using a GSI 38 immittance measurement device (Grason-Stadler, Eden Prairie, MN) that complied with ANSI S3.39 and IEC 601-1 criteria. Normal middle ear pressure and compliance was defined as middle ear pressure (MEP) values from −140 to +40 daPa (based on the 90% range for adults, see Margolis & Hunter, 2000), peak compensated static acoustic admittance values from 0.3 to 1.8 ml (Peak Ytm; +200 daPa as the ear canal referent), and acoustic equivalent volume (Vea) values from 0.8 to 2.1 cm3. Conventional pure-tone air conduction thresholds (0.25–8 kHz) were assessed in participants who passed both otoscopy and tympanometry.
Pure-tone air-conduction threshold measurement was conducted using a GSI 61 diagnostic audiometer with EAR 3A insert earphones (calibrated annually according to ANSI 3.6 1996) for test frequencies of 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz. Tests were conducted in a double-walled sound-treated test booth meeting specifications of ANSI/ASA S3.1–1999 (R2008). Thresholds were obtained using a modified Hughson-Westlake procedure. Starting at 30-dB HL stimulus levels, levels were decreased in 10-dB increments. Levels were increased by 2-dB after each missed stimulus, and decreased by 6-dB after each correct detection. Threshold was defined as the lowest level at which two responses were obtained out of three presentations on an ascending run. Reliability was assessed using repeat tests at 2 and 8 kHz in each ear; test and retest threshold differences were required to be ≤ 5 dB (a criterion previously used by Fausti et al., 1999). If the air-conduction threshold at 0.25, 0.5, 1, 2, 3, or 4 kHz was between 15-dB HL and 25-dB HL, bone-conduction pure-tone audiometry was also conducted. Normal threshold assessment was defined as: 1) air-conduction thresholds no worse than 25-dB HL from 0.25 – 8 kHz, 2) threshold asymmetry ≤ 15 dB at all test frequencies, and 3) air-bone gaps ≤ 10 dB if air conduction threshold was ≥15 dB HL but ≤ 25 dB HL. Subjects meeting those inclusion criteria proceeded to the final test metric assessed during the screening session, which was DPOAE measurement.
DPOAE amplitude was measured using the Mimosa HearID system (Mimosa Acoustics Inc., Champaign, IL), in combination with an Etymotic Research microphone-earphone assembly (ER 10C, Etymotic Research Inc., Elk Grove Village, IL) which was coupled to the participant’s ear with a foam ear tip. Responses were elicited by two simultaneously presented ‘primary’ tones (frequencies f1 and f2) at an f2/f1 ratio of 1.2, and with intensity levels (L1 and L2) at L2=L1-10 dB. The f2 frequencies included 2, 3, 4, 6, 8, and 12 kHz and the levels included L1=25 to 65 dB SPL. Stimulus presentation started at L1=65 dB SPL within each frequency, with level decreasing in 5-dB steps within frequencies until complete input-output functions were obtained at each of the six f2 frequencies. The DPOAE protocol specifically followed Goldman et al. (2006) in that DPOAE amplitudes (2f1-f2) and adjacent noise floors were averaged using a simplified stopping rule; i.e., with all tests averaged over 10 seconds.
After completion of the screening procedures, eligible participants were invited to schedule a time to return to the laboratory to participate in study sessions that included audiometric testing, video game play time, and additional audiometric testing post-game play to 1) measure any changes induced by the simulated impulse noise delivered during game play, and 2) track recovery of function if TTS was observed. Participants were only allowed to participate in one exposure session per week, and per the approved protocol, could not participate in additional sessions if a TTS of 10-dB or greater was observed.
Game Play Sessions
Participants who enrolled in the study after completing the screening were compensated $15 per hour for their time. Prior to each game play session, the participants answered a brief series of questions regarding recent noise exposure and current perception of tinnitus. They then underwent conventional pure-tone air-conduction threshold testing at 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10, 12.5, 14 and 16-kHz, to establish pre-game play baseline threshold sensitivity. Thresholds were measured at 10, 12.5, 14, and 16-kHz using the same modified Hughson-Westlake procedure described above, but circum-aural headphones (Sennheiser HDA200; Sennheiser Electronic Corporation, Old Lyme, CT) were used in place of the insert earphones that were used while testing conventional audiometric frequencies. DPOAE tests were completed again prior to game play.
During the game play session, participants played NPPL Championship Paintball (2009) on a Nintendo® Wii™ game console. During game play, practice rounds were completed using a Wii™ Zapper with Link’s Crossbow Training to aim and shoot at the targets on a 25.5″ LCD HDTV (Samsung Touch of Color T260HD). The Wii™ game console was connected to a Tucker-Davis-Technologies RX6 multifunction processor (TDT, Alachua, FL). The RX6 output went to a stereo receiver (Onkyo TX-8555) used to control amplitude. Stimuli were delivered through Sennheiser headphones (HD-280 Professional Headphones used for signals up to 108 dB SPL peak; HD-380 Pro Collapsible High-End headphones used for signals up to 117-dB SPL peak). During game play, the game’s audio was stripped and served only as a trigger for presenting a digital sound file mimicking a gunshot (see Figure 1). The gunshot-like stimuli were presented in real-time as the participant shot targets in the video game; participants were instructed to shoot targets at a rate of approximately once/second.
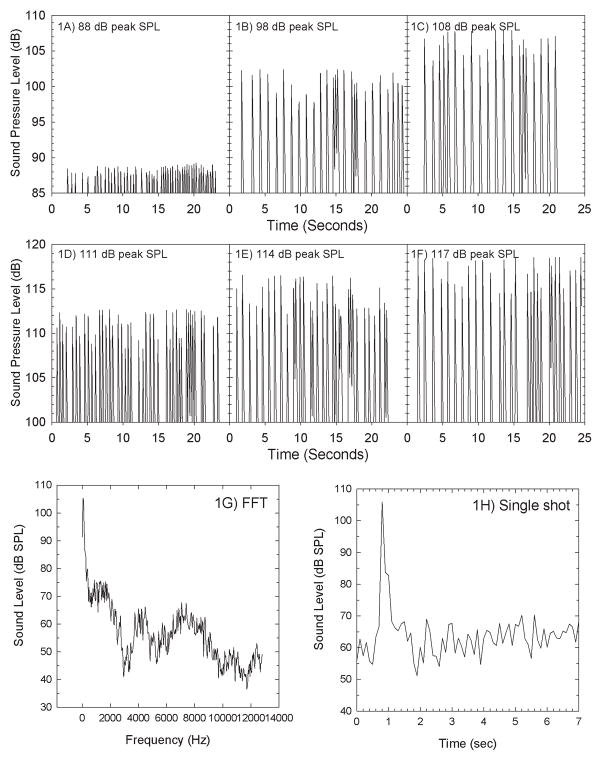
Figure 1.
Sound levels shown here were measured using a Brüel & Kjær Type 4153 Artificial Ear in combination with PULSE spectrum analyzer with levels sampled at 0.125 sec (1/8 sec) intervals. Variability within a given stimulus condition is a function of the challenges inherent to accurately capturing peak SPL for brief impulse-like signals. The individual acoustical signal was routed from a Tucker-Davis-Technology RX6 to a stereo receiver (Onkyo TX-8555) that controlled signal amplitude, which ranged from 88 dB peak SPL (Figure 1A) to 108 dB peak SPL (Figure 1C) using 5 dB increments, and 111 dB peak SPL (Figure 1D) to 117 dB peak SPL (Figure 1F) using 3-dB increments. Approximately 20 seconds are shown for each stimulus level; the presentation rate varied as a function of the rate at which the Wii® zapper was triggered during the acoustic measurements. Presentation was triggered by subject response using the Wii® zapper. The sound spectrum for a single impulse is shown in Figure 1G, and the detailed time sample for a single impulse is shown in Figure 1H.
The gunshot stimulus was modeled after a 12-gauge shotgun discharge. To calibrate sound levels, headphone output was measured using a Brüel & Kjær Artificial Ear Simulator (type 4153) connected to a Brüel & Kjær PULSE Analyzer Platform (Type 3560-B-030). The A, B, and D-durations for a single impulse were estimated following the classification scheme described by Smoorenburg (1982; 1992). The A-duration of a single shot stimulus as played through the headphones was approximately 0.4 msec. The B-duration ranges from 102 to 246 ms. D-duration, defined as the duration of time over which the peak SPL decreases 10 dB, was 39–41 msec. These values are consistent with measures from actual shotguns, with B-durations of indoor measurements often being 200–500 ms. Peak sound levels ranged from 88–117 dB peak SPL, and the number of presentations ranged from 50 to 3,200 stimuli.
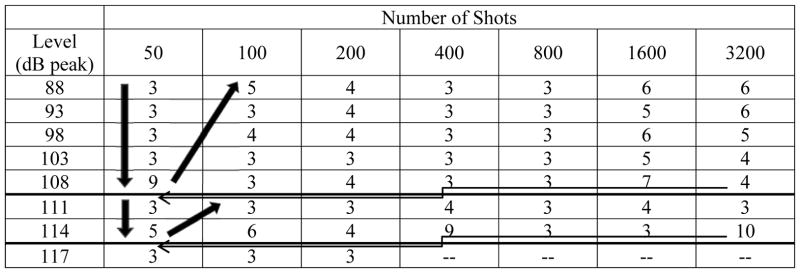
Based on previously reported data, the Albuquerque studies provide a unique data set on TTS after impulse noise exposure during tests sponsored by the US Army Medical Research and Development Command (Johnson, 1993; Johnson, 1998). The protocol was based on systematically increasing sound exposure level (see model in Price, 2007). If no TTS was detected at the highest planned exposure level, the level was dropped “1-step” and the number of impulses was then sequentially doubled. Here, we used a variant of the Albuquerque studies, modified to provide a more conservative approach. Exposures began at 88-dB SPL peak × 1 round of game play (~50 impulses). Following 3 consecutive participants with ≤5-dB TTS, exposure levels for the next participant increased by 5-dB. This process was repeated up to the highest exposure level approved by the IRB, which was initially 108-dB SPL peak. With ≤ 5 dB TTS in 3 consecutive participants at 108-dB SPL peak × 1 round of game play (~50 impulses), levels were dropped to the lowest study level of 88-dB SPL peak, and the number of impulses was doubled. This process was repeated to a maximum of 108-dB SPL peak × 64 rounds of game play (~3200 impulses). With no reliable TTS detected at those levels, we sought (and obtained) approval from the IRB to increase sound levels using a more conservative 3-dB step size. The above iterative process was repeated for 111- and 114-dB SPL peak stimuli. A final series of 117-dB SPL peak stimulus presentations was initiated, and data were collected through the 117-dB SPL peak × 200 impulses condition. A total of 52 different sound level × number of impulse combinations were assessed. To assure that we were not “missing” a rapidly recovering TTS by waiting until 15-min post shooting to assess TTS as we had with the first sets of exposure levels (up to 108-dB peak SPL), we requested (and received) permission to add a 2-min post-shooting audiometric test (limited to 2, 3, 4, 6, and 8 kHz) when we requested permission to present higher stimulus levels. The intent of the design was to quickly identify a set of stimulus parameters resulting in a small but reliable TTS. The “target” TTS was defined as ~6- to 10-dB, based on existing data showing recovery to baseline within hours of the exposure, with virtually no change measured 24 hours post music after music-induced TTS of this magnitude (Le Prell et al., 2012). The majority of the stimulus conditions (i.e., combinations of level and number of impulses) tested in this study included only 3–4 participants as many participants had less than 5-dB change (see Table 1).
Table 1.
Procedure for Selecting Exposure Level and Number of Shots Firedi
The initial series of exposures included 1 round of game play (~50 shots) delivered at 88 dB peak SPL. For every 3 consecutive participants with TTS ≤ 5 dB, the level was increased in 5-dB steps up to a maximum of 108 dB peak SPL. Several participants had TTS exceeding 5 dB at 108 dB peak × 50 shots, resetting the “counter” each time. There was no reliable change in function across subjects however. With 3 consecutive participants with TTS ≤ 5 dB at 50 shots × 108 dB peak SPL, the level was decreased back to 88 dB SPL peak, and the number of shots was doubled. This process was repeated to a maximum of 108 dB peak SPL × 3200 shots. After receiving IRB approval for higher sound level tests, the effect of 111 dB and 114 dB SPL stimuli was assessed following a similar iterative procedure. A final series of tests at 117 dB peak SPL were initiated, ending with a maximum of 200 shots, with no reliable changes in any of the subjects who participated in that level series.
Results
Participants Included
Participants were drawn from an initial pool of 101 volunteers (50 male, 51 female; mean age=22.1+2.7 years; range =18–31). Of these, 6 participants were excluded for obstructive cerumen (earwax), 1 participant was excluded after observations consistent with the presence of middle ear fluid, 3 participants were excluded based on tympanometry outside normal limits (specifically, hypercompliance), 4 participants were excluded on the basis of thresholds greater than 25-dB HL at one or more frequencies, 3 participants were excluded based on right/left hearing asymmetry greater than 15-dB, 1 participant was excluded based on air-bone gaps greater than 10-dB, and 1 participant was excluded based on verbal report of frequent, severe headaches. Sex distribution of the 19 excluded participants was 14 males, and 5 females. In addition to the 19 excluded participants, there were 16 participants who were eligible to enroll after completing the screening, but who chose not to schedule study sessions post-screening (9 males, 7 females).
After exclusions and drop-outs, the final participant cohort included 66 normal-hearing volunteers (27 male, 39 female, mean age=22.1±2.7 years; range =18–29). The average thresholds for right and left ears were equivalent among the eligible participants who did (n=66) and did not (n=16) choose to participate, with higher thresholds measured in those that were excluded (n=9; note that while there were 19 excluded participants, 10 of these 19 were excluded prior to threshold assessment based on obstructive cerumen or middle ear issues precluding participation). For the 66 study participants, there were no statistically reliable differences between right and left ear thresholds (all p values > 0.05). When the average threshold at each frequency was compared in males and females, a small but statistically reliable difference was observed at 4 kHz with males having approximately 3.5-dB worse thresholds than females (t=2.988, df=45.670; p=0.005). Although statistically significant, the measured thresholds were within normal limits for both sexes, with both males and females having average thresholds that were better than 5-dB HL. Data from male and female participants were therefore pooled in subsequent analyses of the effects of simulated impulse noise on hearing. Although the effects of prior noise history on baseline measures of function are of significant interest, we do not attempt to explore these factors as part of this report. The relationship between reported noise sources and auditory function at frequencies from 250-Hz to 16-kHz was recently described in a separate report (Le Prell et al., 2013). Readers are referred to that report for a more complete discussion of various recreational noise sources and their potential impact on hearing.
Temporary Threshold Shift after Game Play
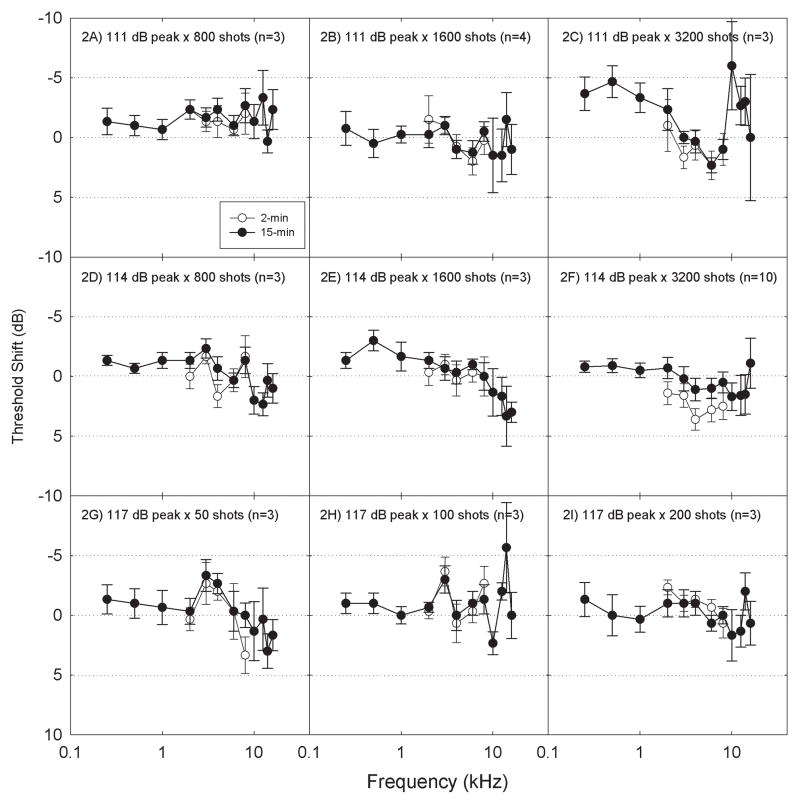
The number of sessions completed by each participant ranged from 1 to 12 (average=3.2±2.7 sessions). As is clearly evident from the number of conditions in which there were only 3–4 participants (Table 1), there was no consistent TTS reaching/exceeding 5-dB in many of the stimulus conditions. Space constraints prohibit detailed presentation of the data from all 52 exposure conditions; in Figure 2 we present the data for stimuli presented at 111-dB peak SPL (2A: 800 shots; 2B: 1600 shots; 2C: 3200 shots), 114-dB peak SPL (2D: 800 shots; 2E: 1600 shots; 2F: 3200 shots), and 117-dB peak SPL (2G: 50 shots; 2H: 100 shots; 2I: 200 shots). For each level, these are the three sessions with the highest number of impulses presented. In addition, these are the stimulus conditions for which both 2-min and 15-min TTS data were collected. These data clearly illustrate three key findings. First, there was no reliable TTS, even with the highest level × greatest number of impulse combinations. Second, comparisons of TTS measured at 2-min with TTS measured at 15-min did not provide any evidence that there was a rapidly reversing TTS, which recovered within the first 15-min. Third, there was no consistent evidence for greater change at EHF frequencies (i.e., frequencies above 8 kHz) than at conventional test frequencies. Although there were a small number of exposure conditions in which some individuals showed a shift at one or more EHF frequencies, thresholds at EHF frequencies are more variable than thresholds at conventional frequencies (Schechter et al., 1986; Green et al., 1987; Stelmachowicz et al., 1989; Frank, 2001; Schmuziger et al., 2004; Le Prell et al., 2013). Given this increased variability, “changes” observed at EHF frequencies for a small number of individuals in a small number of test conditions should be interpreted with caution. Although the current data do not establish a paradigm for inducing TTS in the laboratory using an impulse noise stimulus, they provide useful documentation regarding the significant individual variability in vulnerability.
Figure 2.
There was no reliable effect of game play on pure-tone air conduction thresholds at either 2-min or 15-min post game play; the average change in thresholds was less than 5 dB regardless of exposure condition. Data were collected for 52 different exposure conditions (see Table 1). Exposure conditions shown here include the three highest test levels (2A–2C: 111 dB peak; 2D–2F: 114 dB peak; 2G–2I: 117 dB peak). The highest number of impulses presented within each sound level are shown (2C, 2F: 3200 shots; 2B, 2E: 1600 shots; 2A, 2D: 800 shots; 2I: 200 shots; 2H: 100 shots; 2G: 50 shots). Data are Mean ± S.E., to illustrate confidence with respect to the mean change.
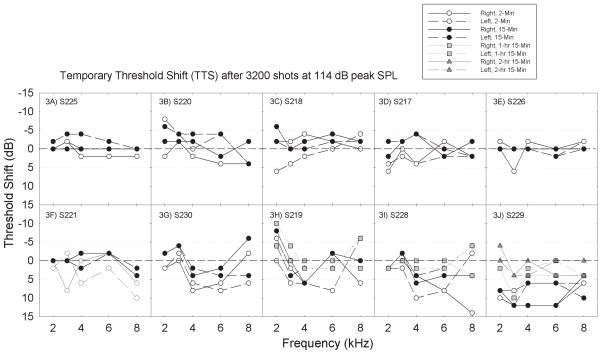
There were three stimulus conditions in which TTS was observed in a subset of participants, and the sample size for those exposure conditions was thus larger. Individual data from the participants who completed the 114-dB peak SPL × 3200 impulse condition (from Figure 2F) are presented in Figure 3. In Figure 3, the panels are organized such that participants with the smallest changes are shown first, with later panels showing participants with increasing magnitude of change. Changes of up to +/− 5 dB are within the range typically attributed to test-retest variability and should not be interpreted as reliable changes in function. Threshold improvements > 5 dB were not common, but were occasionally observed (see for example panel 3H). In cases where a shift was observed at both 2-min and 15-min, the shift can be considered “repeatable.” However, in those cases where a shift of ~5-dB was present at 2-min but absent at the 15-min test, it is not possible to distinguish small and rapidly recovering changes in function from test-retest variability. The strongest evidence for reliable change as a function of the exposure would be > 5 dB change in the group data, and this was not observed here. Other data that can be extracted from the single participant plots include the observation that TTS was sometimes (Subject 230, Figure 3G) but not always (Subject 219, Figure 3H; Subject 229, Figure 3J) symmetrical; there was no consistent difference with respect to the more affected ear. In addition, the frequency at which the maximum TTS was observed varied across subjects. The pattern of change observed here would make it difficult to implement a “simple” TTS metric (such as average change at 4 kHz) on which the potential for otoprotection might be assessed.
Figure 3.
Within subjects exposed to a given noise condition, there was significant individual variability. Individual subjects are plotted in panels 3A–3J, one subject per panel. Panels are sorted such that subjects with smaller changes are followed by subjects with larger changes. Subjects were followed until complete recovery was observed; subjects with larger threshold shifts were followed for longer periods than subjects with smaller shifts, based on time to recovery. The longest post-game monitoring interval was 2 hrs 15 mins (Figure 3J).
DPOAE Input-Output (IO) Data
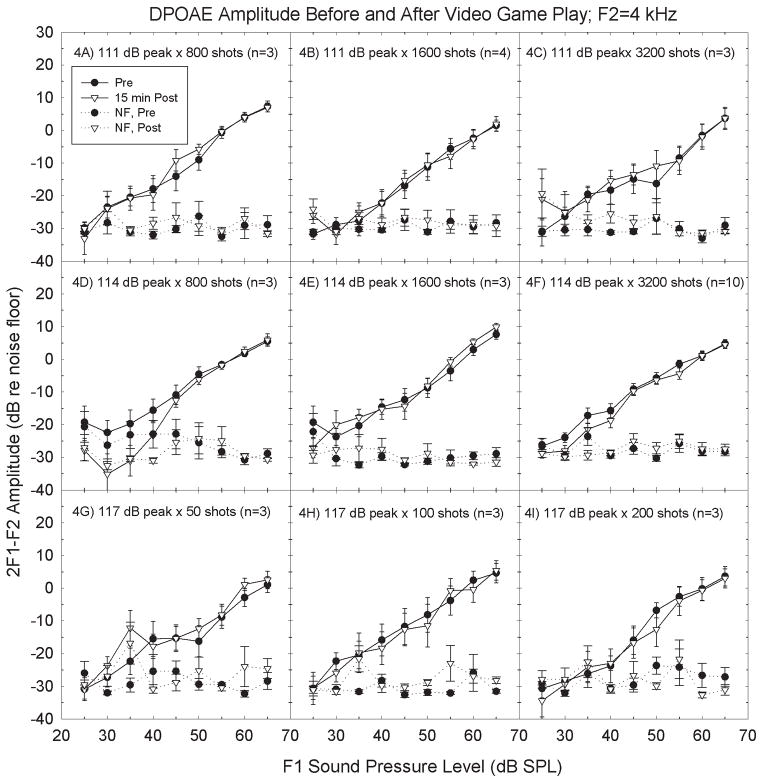
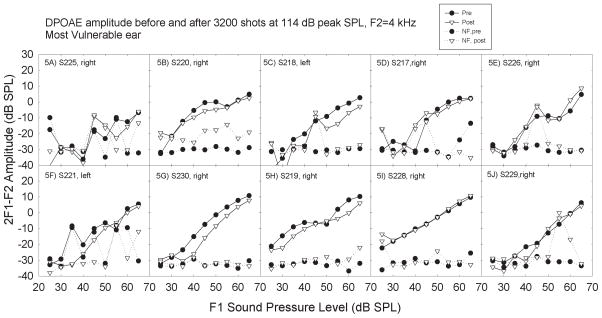
DPOAE amplitudes were measured at 9 different sound levels for 6 different f1/f2 frequency pairs, with tests conducted pre- and post-game play for all 52 exposure conditions. There was no evidence that the OAE metric was more sensitive than the pure-tone threshold metric; DPOAE amplitude was not systematically depressed as a function of any of the exposure parameters during inspection of the input-output functions. DP-grams were generated by extracting responses at a single level across frequencies; no consistent differences emerged with that alternative analysis. Figure 4 illustrates the measured amplitude of the 2f1-f2 DPOAE for F2 = 4 kHz for the same game play signal conditions shown in Figure 2. There were no reliable changes in amplitude at any of the test frequencies. Inspection of the individual responses within participants did not suggest that participants with measurable TTS had any greater change in OAEs than subjects without measureable TTS (see Figure 5).
Figure 4.
There was no reliable effect of game play on distortion product otoacoustic emission (DPOAE) amplitude 15-min post game play. Data were collected for 52 different exposure conditions (see Table 1). Exposure conditions shown here include the three highest test levels (4A–4C: 111 dB peak; 4D–4F: 114 dB peak; 4G–4I: 117 dB peak). The highest number of impulses presented within each sound level are shown (4C, 4F: 3200 shots; 4B, 4E: 1600 shots; 4A, 4D: 800 shots; 4I: 200 shots; 4H: 100 shots; 4G: 50 shots). Stimulus conditions are identical to those in Figure 2; subjects are the same in Figures 2 and 4. Data are Mean ± S.E., to illustrate confidence with respect to the mean change.
Figure 5.
Within subjects exposed to a given noise condition, there was little individual variability with respect to changes in DPOAE amplitude. Individual subjects are plotted in panels 5A–5J, one subject per panel. Panels are sorted such that subjects with smaller changes in thresholds are followed by subjects with larger changes in thresholds, based on the data shown in Figure 3. Subjects are the same in Figures 3 and 5. However, only the DPOAE data from the most vulnerable ear are shown.
Tinnitus
Participants were asked if they had a sensation of ringing in their ears (tinnitus) post-game play. There were 16 reports of tinnitus immediately after game-play, but there was no consistent reporting of tinnitus within or across exposure conditions (see Table 2). For those participants who reported tinnitus, there were follow-up questions regarding the nature of the tinnitus. In addition, participants were asked to rate their tinnitus on both loudness and objectionable/bothersome scales that ranged from 1 (barely noticeable/not bothersome) to 10 (almost unbearably loud/unbearable). The average loudness score was 2.1±1.3 (range=1–6) and the average objectionable/bothersome score was 1.3±0.9 (range=0–4). The tinnitus was transient and was fully resolved in all cases.
Table 2.
Number of Subjects Reporting Tinnitus Post-Shooting/Number of Subjects Exposed Within Exposure Level and Number of Shots Fired. For subjects that reported tinnitus, “Loudness” and “Bothersomeness” were described using a scale of 1 to 10. These ratings are reported in parentheses for all subjects that reported experiencing tinnitus.
| Number of Shots | |||||||
|---|---|---|---|---|---|---|---|
| Level (dB peak) | 50 | 100 | 200 | 400 | 800 | 1600 | 3200 |
| 88 | 0/3 | 0/5 | 1/4 (1,1) | 0/3 | 0/3 | 1/6 (2.5,1) | 0/6 |
| 93 | 0/3 | 1/3 (2,1) | 0/4 | 0/3 | 1/3 (1,1) | 1/5 (2,1) | 0/6 |
| 98 | 0/3 | 1/4 (1,1) | 0/4 | 0/3 | 1/3 (2.5,1) | 1/6 (2,1) | 0/5 |
| 103 | 1/3 (1,0) | 0/3 | 0/3 | 0/3 | 0/3 | 1/5 (1.5,1) | 0/4 |
| 108 | 1/10 (3,1) | 0/3 | 0/4 | 0/3 | 0/3 | 0/7 | 0/4 |
| 111 | 1/3 (1,1) | 0/3 | 0/4 | 0/4 | 0/3 | 1/4 (6,4*) | 0/3 |
| 114 | 0/5 | 0/6 | 0/4 | 0/9 | 0/3 | 0/3 | 2/10 (1.5,1;2,1) |
| 117 | 0/3 | 0/3 | 1/3 (3,3) | -- | -- | -- | -- |
Subject 225 participated in 6 exposure conditions, including 111 dB peak × 1600 shots, 111 dB peak × 3200 shots, 114 dB peak × 3200 shots, 117 dB peak × 50 shots, 117 dB peak × 100 shots, and 117 dB peak × 200 shots. This subject did not experience TTS in any of the above exposure conditions. Tinnitus was reported in 2 of the conditions, including 111 dB peak × 1600 shots, and 117 dB peak × 200 shots. The tinnitus fully resolved during each session. Tinnitus post-shooting was not defined as a study endpoint; this subject volunteered to participate in additional sessions despite tinnitus being reported at the first (lowest level) session. Tinnitus was not consistently reported after other higher level and/or higher number of impulse exposures.
Discussion
The 1992 CHABA report by Dixon Ward et al. (CHABA, 1992) essentially endorsed an earlier risk criterion (CHABA, 1968), at least for small arms fire. The reports suggested that exposures of 100 impulses would be safe at levels below 157 dB when B-duration is 0.3 msec, such as gunfire in an open field, or 138 dB when B-duration exceeds 200 msec, as might occur in a very reverberant environment, such as gunfire in a small room with hard walls. In that report, “safe” was defined using a more permissive definition of a significant TTS, based on 2-min post-noise TTS reaching 10 dB at 1,000 Hz or below, 15 dB at 2,000 Hz, or 20 dB at 3,000 Hz or above. The B-duration for our stimulus was consistent with the reverberant shooting environment, and sound levels up to 117-dB peak failed to result in consistent TTS, even using our more lenient description of TTS as > 5 dB change at 3, 4, or 6 kHz. The sound levels assessed here did not reliably induce TTS, and are not appropriate for assessing the efficacy of otoprotective agents in human participants.
The most important outcome of the current study is the development of a protocol through which TTS exposure paradigms can be developed using sequentially increasing time and level parameters. To maximize participant safety, the paradigm described here was highly conservative in that the starting levels selected for the initial exposure conditions were not intended to induce robust change. Additionally, because individual variability is significant, the paradigm required that 3 consecutive study participants have less than 5-dB change within a given exposure condition, prior to any increases in exposure level or duration. Using these two key criteria, we were able to identify stimulus conditions that resulted in unilateral TTS > 5-dB in some 25–30% of participants. Presumably, the percent of participants with TTS > 5-dB and the average magnitude of the TTS would grow with increasing stimulus level and/or duration of the exposure (which increased linearly with increasing numbers of shots, as shots were fired at a rate of ~1/second within each round of game play).
The potential for safety concerns with noise exposures that induce robust TTS is clear (for detailed discussion, see Le Prell et al., 2012). Here, we defined target threshold shift as 6 to 10-dB at 15-minutes post-game play. This small targeted TTS clearly contrasts with the robust (40-dB) threshold shifts 24-hours post-noise associated with neural loss in animal studies (Kujawa & Liberman, 2006; Kujawa & Liberman, 2009; Lin et al., 2011). Exposures that induce smaller TTS changes are clearly more conservative than exposures that induce larger TTS changes. Because the critical boundary below which there is no effect of TTS on synaptic density and evoked potential amplitude has not been established, lower exposures should always precede higher exposure levels to assure that the exposure paradigm elicits minimal TTS. Consistent with the notion that small TTS changes are unlikely to have long-term consequences, Liang et al. (2013) recently reported complete recovery of neural response amplitude in mice that had ~20-dB TTS 24-hours post-noise. Those data support previous suggestions that small TTS deficits measured after a single 100% daily noise dose (defined using OSHA standards after converting sound level from coupler-level to free-field equivalent) are not likely to have significant risk of harm for human participants (Le Prell et al., 2012). However, a provocative new report seemingly draws the safety of small TTS changes into question. Small decreases in synaptic density and small decreases in ABR amplitude were reported in mice that had ~15-dB shifts in DPOAE thresholds 1-hour after the conclusion of a more moderate noise exposure (Maison et al., 2013). In that study, mice were exposed to 84-dB SPL noise continuously for 7-days. These findings raise new questions, and require critical analysis.
According to OSHA regulations, the experimental exposure duration of 24 hours per day with a constant level of 84-dB SPL would result in a daily noise dose of ~130% accumulating over each 24-hour period (OSHA, 1983) 2. NIOSH recommended exposure limits are more conservative, and a daily noise dose of ~238% would accumulate within each 24-hour period per NIOSH recommended noise standards (NIOSH, 1998). With a daily OSHA-defined noise dose of 130% per day, a total dose of ~910% accumulates over a 7-day exposure. Using the more conservative NIOSH noise dose guidelines, a daily dose of 238% per day yields a total dose of 1,666% after 7 days. Clearly, these dose levels do not represent typical occupational noise exposures (which require the use of engineering or administrative controls, or the use of HPDs, to reduce noise exposure to 100% dose per day). Given daily noise doses in excess of 100%, and the lack of any daily noise-free recovery periods, it may not be surprising if permanent damage accrued in the mice tested by Maison et al. (2013). However, it is worth note that susceptibility to noise damage varies across strains of mice (Yoshida et al., 2000; Davis et al., 2001), across rodent species (Duan et al., 2008), and human vulnerability likely differs from mice. Pigmented guinea pigs appear to less vulnerable than CBA/CA mice and albino Sprague-Dawley rats (Duan et al., 2008), and humans have been suggested to be more tolerant than guinea pigs (Liang, 1992). Limits for humans have been suggested to be 164 dB peak SPL for impulses of 25 μs, with limits decreasing as impulse duration increases (limit of 138 dB peak SPL for impulses with B-durations of 200–1000 ms (Ward, 1968).
There is little reason to assume that ears experiencing a single daily noise dose of 100% or less, for one day, will be damaged, despite data from mice subjected to continuous 7-day long exposures of ~130% to 238% daily noise dose per day (i.e., the noise doses for human listeners), with no recovery periods. The average sound level for the signals plotted in Figure 1 ranges from 81-dB SPL (Figure 1A) to 91-dB SPL (Figure 1F), over each of the time intervals sampled. Even with the longest game play conditions (~3200 signals over ~45-min), total exposure within a session would be less than 100% daily noise dose, based on both the OSHA regulations, which limit exposure to 91-dBA sound to 7-hours (per Table G-16A in Appendix A; in OSHA, 1983), and the NIOSH recommendations, which limit exposure to 91-dBA sound to 2-hours (per Table 1-1 in NIOSH, 1998). We did not measure A-weighted SPL; for completeness, we note the A-weighted sound level would be lower than the unweighted SPL we report here, and thus the regulations would permit longer exposures after discounting low frequency signal components in the signals we used.
A second more fundamental issue with respect to interpreting the risk of neural loss after a small TTS is that the noise-exposed animals described by Maison et al. (2013), deemed to have decreased synaptic density based on comparisons to control measurements from the ears of 3 animals, in fact have an average synaptic density measurement that is within the range of previous reports using larger control groups (Kujawa & Liberman, 2009; Wang & Ren, 2012). Taken together, new data regarding small TTS changes in animal models are provocative, but suggestions about human risk must be interpreted with caution based on small sample sizes and the multi-day noise exposures that exceed daily noise limits as defined by OSHA, as well as differences in vulnerability and critical limits for exposures across mammalian species. Further research is needed to characterize the dose-response relationship for damage underlying TTS and primary afferent neural loss. In addition, the perceptual consequences of this type of neural loss remain speculative. There are no behavioral data demonstrating evidence of perceptual deficits in any of the studies that are currently available.
Although caution is required in the development and use of laboratory-based TTS paradigms, we maintain that the development of laboratory-based TTS paradigms is useful, as these controlled paradigms resolve many of the shortcomings of previous field-based otoprotection studies. As reviewed above, multiple investigators have sought to determine whether a potential drug agent reduces TTS after a real-world noise exposure, but have failed to obtain conclusive evidence. Study conclusions were limited by the minimal TTS in controls in some cases (Lin et al., 2010; Le Prell et al., 2011a; Lindblad et al., 2011) whereas in other cases, the variability of the exposures across participants limited conclusions (Kramer et al., 2006). To circumvent some of these shortcomings, we have accelerated the incorporation of digital music stimuli into laboratory-based otoprotection studies (NCT00808470; NCT01444846).
The incorporation of digital gunshot-like impulse noise into a TTS paradigm here has carried additional challenges, in that TTS has been smaller and more variable, and has largely been observed to be unilateral (occurring at approximately equal rates in right and left ears). The stimulus used here had significant energy at lower frequencies; a frequency-shifted stimulus with more high-frequency energy might be expected to produce more consistent changes, and also more robust changes. The spectral content of the impulse sounds produced by different weapon systems has been briefly described for some firearms (Kardous et al., 2003; Flamme et al., 2009b; Guida et al., 2011), and differences between A-weighted and unweighted SPL’s allow some inferences about low frequency energy in sounds produced by a subgroup of firearms (Flamme et al., 2011). The available data, while limited, suggest there are differences across weapons. A stimulus “battery” in which several shots are quickly repeated as a “salvo” might also be useful in eliciting more robust, and more reliable, TTS changes across participants (Danielson et al., 1991). However, as noted by Ward (1968), the middle ear muscle reflex contraction will reduce the effect of acoustic energy entering the ear more than 100 to 200 milliseconds subsequent to pulse onset.
The data presented here are highly encouraging with respect to the potential for impulse-noise based TTS models that can be safely implemented in a laboratory setting; however, further research is needed to optimize the parameters essential needed for more consistent and reliable TTS, and models may ultimately require selection of other weapon signatures as well as better understanding of individual vulnerability across subjects. The variability in individual vulnerability to NIHL is well known in both animal models (Maison & Liberman, 2000; Yoshida & Liberman, 2000; Wang et al., 2002) and human participants (Ward, 1970; Mills et al., 2001; Strasser et al., 2003; Le Prell et al., 2012), but biological factors distinguishing “tough” ears from “tender” ears are relatively unknown. These likely include genetics, sex, age, noise and ototoxic drug exposure history, pre-exposure hearing status, and other additional individual risk factors such as smoking, cardiovascular health, and nutrition. Moreover, it is clear that signal characteristics such as A, B, and D durations, inter-pulse interval (which can allow recovery), peak signal level, and total energy of the stimulus (which can be described using dB SPL, A-weighted SPL, C-weighted SPL, or even 8-hr equivalent levels) will all interact, and these interactions require further study as to their effects on noise risk. With the widespread use of firearms by civilians, police officers, and military personnel, improved understanding of the factors influencing noise risk related to these exposures remains an important hearing healthcare goal.
Acknowledgments
Support: The project was supported by R44 DC009106 from the National Institute on Deafness And Other Communication Disorders, National Institutes of Health and the Center for Hearing Research at the University of Florida.
This project was supported by R44 DC009106 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by the University of Florida Hearing Research Center (subject payments) and Sound Pharmaceuticals, Inc. (Graduate Student Assistant stipends). The authors thank Dr.’s D. Clark Bennett and Peter Boxer for technical suggestions during the planning of this study, and we thank Dr. Victor Rush for expert assistance designing stimulus presentation protocols. We thank Mr. Jim Wyatt at Brüel & Kjær for ongoing support for signal level measurements, as well as CAPT William Murphy, who assisted us with A, B, and D duration measurements. We thank Dr. Pat Jeng, Mr. Kyle Rust, and Mr. Adam Le at Mimosa Acoustics for development of HearID protocols. We thank Dr. Danielle Rosier Youngstrom for additional technical assistance, and we also thank Drs. Robert Dobie and Eric Bielefeld for their thoughtful critiques of an earlier version of this manuscript. This project was previously presented at the 38th Annual National Hearing Conservation Conference-The Art of Hearing Conservation [Morgenstein, K., de la Calle, S., Guercio, D., Ledon, V., Griffiths, S., Spankovich, C. and Le Prell, C.G. (2013). Impulse noise paradigm developed for otoprotection trials.; NHCA Spectrum, Volume 30, Supplement IV, page 54].
Footnotes
NCT numbers are the clinical trial identification numbers assigned in the ClinicalTrials.gov registry and results database. This database is a service of the U.S. National Institutes of Health. Complete study references are provided in the References section of this document.
Readers are reminded that OSHA and NIOSH limit noise based on A-weighted SPL. The noise levels reported in these animal studies are unweighted SPL. The A-weighted level for those exposures was not reported; however, because the acoustic signal contained energy from 8–16 kHz, and A-weighting primarily discounts energy below 1 kHz, the effect of A-weighting on signal level would likely be fairly small. However, comments on dose should be interpreted with caution, given the unknown effect A-weighted spectral filtering would have.
References
- Agrawal Y, Niparko JK, Dobie RA. Estimating the Effect of Occupational Noise Exposure on Hearing Thresholds: The Importance of Adjusting for Confounding Variables. Ear Hear. 2010;32:234–237. doi: 10.1097/AUD.0b013e3181c6b9fd. [DOI] [PubMed] [Google Scholar]
- Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol. 2009;30:139–145. doi: 10.1097/MAO.0b013e318192483c. [DOI] [PubMed] [Google Scholar]
- Ahroon WA, Hill ME, Goodes DP. Analysis of army-wide hearing conservation database for hearing profiles related to crew-served and individual weapon systems. Noise Health. 2011;13:76–83. doi: 10.4103/1463-1741.73992. [DOI] [PubMed] [Google Scholar]
- Attias J, Sapir S, Bresloff I, Reshef-Haran I, Ising H. Reduction in noise-induced temporary threshold shift in humans following oral magnesium intake. Clin Otolaryngol. 2004;29:635–641. doi: 10.1111/j.1365-2273.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- Balatsouras DG, Tsimpiris N, Korres S, Karapantzos I, Papadimitriou N, et al. The effect of impulse noise on distortion product otoacoustic emissions. Int J Audiol. 2005;44:540–549. doi: 10.1080/14992020500190201. [DOI] [PubMed] [Google Scholar]
- Bapat U, Tolley N. Temporary threshold shift due to recreational firearm use. Journal of Laryngology and Otology. 2007;121:927–931. doi: 10.1017/S0022215107005087. [DOI] [PubMed] [Google Scholar]
- Berger EH. Hearing Protection Devices. In: Berger EH, Royster LH, Royster JD, Driscoll DP, Layne M, editors. The Noise Manual. 5. Fairfax: American Industrial Hygiene Association; 2003. pp. 379–454. [Google Scholar]
- Bielefeld EC, Wantuck R, Henderson D. Postexposure treatment with a Src-PTK inhibitor in combination with N-L-acetyl cysteine to reduce noise-induced hearing loss. Noise Health. 2011;13:292–298. doi: 10.4103/1463-1741.82962. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, White DR, Mills JH, Schmiedt BN. Age-related changes in auditory evoked potentials of gerbils. III. Low-frequency responses and repetition rate effects. Hear Res. 1995;87:208–219. doi: 10.1016/0378-5955(95)00091-h. [DOI] [PubMed] [Google Scholar]
- Casali JG, Ahroon WA, Lancaster JA. A field investigation of hearing protection and hearing enhancement in one device: for soldiers whose ears and lives depend upon it. Noise Health. 2009;11:69–90. doi: 10.4103/1463-1741.48564. [DOI] [PubMed] [Google Scholar]
- Cassandro E, Sequino L, Mondola P, Attanasio G, Barbara M, et al. Effect of superoxide dismutase and allopurinol on impulse noise-exposed guinea pigs--electrophysiological and biochemical study. Acta Otolaryngol (Stockh) 2003;123:802–807. [PubMed] [Google Scholar]
- CHABA. Proposed damage-risk criterion for impulse noise (gunfire) Washington D.C: National Academy of Sciences; 1968. Committee on Hearing, Bioacoustics, and Biomechanics: Commission on Behavioral and Social Sciences and Education; National Research Council. [Google Scholar]
- CHABA. Hazardous exposure to noise: Working group on hazardous exposure to impulse noise. Washington D.C: National Academy Press; 1992. Committee on Hearing, Bioacoustics, and Biomechanics: Commission on Behavioral and Social Sciences and Education; National Research Council. [Google Scholar]
- Code of Federal Regulations 1983. Department of Labor Occupational Noise Standard. Code of Federal Regulations, Title 29, Chapter XVII, Part 1910, Subpart G, 36 FR 10466, May 29, 1971; Amended 48 FR 9776–9785, March 8, 1983.
- Coleman JK, Littlesunday C, Jackson R, Meyer T. AM-111 protects against permanent hearing loss from impulse noise trauma. Hear Res. 2007;226:70–78. doi: 10.1016/j.heares.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Danielson R, Henderson D, Gratton MA, Bianchi L, Salvi R. The importance of “temporal pattern” in traumatic impulse noise exposures. J Acoust Soc Am. 1991;90:209–218. doi: 10.1121/1.402361. [DOI] [PubMed] [Google Scholar]
- Davis RR, Newlander JK, Ling X, Cortopassi GA, Krieg EF, et al. Genetic basis for susceptibility to noise-induced hearing loss in mice. Hear Res. 2001;155:82–90. doi: 10.1016/s0378-5955(01)00250-7. [DOI] [PubMed] [Google Scholar]
- Dolgin E. Sound Medicine. Nat Med. 2012;18:642–645. doi: 10.1038/nm0512-642. [DOI] [PubMed] [Google Scholar]
- Duan M, Laurell G, Qiu J, Borg EG. Susceptibility to impulse noise trauma in different species: guinea pig, rat, and mouse. Acta Otolaryngol. 2008;128:277–283. doi: 10.1080/00016480701509941. [DOI] [PubMed] [Google Scholar]
- Erdreich J. A distribution based definition of impulse noise. J Acoust Soc Am. 1986;79:990–998. doi: 10.1121/1.393698. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Henry JA, Helt WJ, Phillips DS, Frey RH, et al. An individualized, sensitive frequency range for early detection of ototoxicity. Ear Hear. 1999;20:497–505. doi: 10.1097/00003446-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Flamme GA, Liebe K, Wong A. Estimates of the auditory risk from outdoor impulse noise. I: Firecrackers. Noise Health. 2009a;11:223–230. doi: 10.4103/1463-1741.56216. [DOI] [PubMed] [Google Scholar]
- Flamme GA, Stewart M, Meinke D, Lankford J, Rasmussen P. Auditory risk to unprotected bystanders exposed to firearm noise. J Am Acad Audiol. 2011;22:93–103. doi: 10.3766/jaaa.22.2.4. [DOI] [PubMed] [Google Scholar]
- Flamme GA, Wong A, Liebe K, Lynd J. Estimates of auditory risk from outdoor impulse noise. II: Civilian firearms. Noise Health. 2009b;11:231–242. doi: 10.4103/1463-1741.56217. [DOI] [PubMed] [Google Scholar]
- Frank T. High frequency (8 to 16 kHz) reference thresholds and intrasubject threshold variability relative to ototoxicity criteria using a sennheiser HAD 200 earphone. Ear Hear. 2001;22:161–168. doi: 10.1097/00003446-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Gavriel H, Shulman A, Stracher A, Sohmer H. Leupeptin reduces impulse noise induced hearing loss. J Occup Med Toxicol. 2011;6:38. doi: 10.1186/1745-6673-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman B, Sheppard L, Kujawa SG, Seixas NS. Modeling distortion product otoacoustic emission input/output functions using segmented regression. J Acoust Soc Am. 2006;120:2764–2776. doi: 10.1121/1.2258871. [DOI] [PubMed] [Google Scholar]
- Goley GS, Song WJ, Kim JH. Kurtosis corrected sound pressure level as a noise metric for risk assessment of occupational noises. J Acoust Soc Am. 2011;129:1475–1481. doi: 10.1121/1.3533691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham MAM. Noise-Induced Hearing Loss and Tinnitus: Challenges for the Military. In: Le Prell CG, Henderson D, Fay RR, Popper AN, editors. Noise-Induced Hearing Loss: Scientific Advances, Springer Handbook of Auditory Research. New York: Springer Science+Business Media, LLC; 2011. [Google Scholar]
- Green DM, Kidd G, Jr, Stevens KN. High-frequency audiometric assessment of a young adult population. J Acoust Soc Am. 1987;81:485–494. doi: 10.1121/1.394914. [DOI] [PubMed] [Google Scholar]
- Guida HL, Diniz TH, Kinoshita SK. Acoustic and psychoacoustic analysis of the noise produced by the police force firearms. Braz J Otorhinolaryngol. 2011;77:163–170. doi: 10.1590/S1808-86942011000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamernik RP, Qiu W, Davis B. Hearing loss from interrupted, intermittent, and time varying non-Gaussian noise exposure: The applicability of the equal energy hypothesis. J Acoust Soc Am. 2007;122:2245–2254. doi: 10.1121/1.2775160. [DOI] [PubMed] [Google Scholar]
- Hight NG, McFadden SL, Henderson D, Burkard RF, Nicotera T. Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA. Hear Res. 2003;179:21–32. doi: 10.1016/s0378-5955(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Johnson D. USAARL Contract Report No. 94-2. Us. Army Aeromedical Research Laboratory; Fort Rucker, AL: 1993. Blast overpressure studies with animals and man: A walk-up study. [Google Scholar]
- Johnson D. USAARL Contract Report No. CR-98-03. Us. Army Aeromedical Research Laboratory; Fort Rucker, AL: 1998. Blast overpressure studies. [Google Scholar]
- Kardous CA, Willson RD, Hayden CS, Szlapa P, Murphy WJ, et al. Noise exposure assessment and abatement strategies at an indoor firing range. Applied occupational and environmental hygiene. 2003;18:629–636. doi: 10.1080/10473220301409. [DOI] [PubMed] [Google Scholar]
- Konopka W, Pawlaczyk-Luszczynska M, Sliwinska-Kowalska M, Grzanka A, Zalewski P. Effects of impulse noise on transiently evoked otoacoustic emission in soldiers. Int J Audiol. 2005;44:3–7. doi: 10.1080/14992020400022561. [DOI] [PubMed] [Google Scholar]
- Kopke R, Bielefeld E, Liu J, Zheng J, Jackson R, et al. Prevention of impulse noise-induced hearing loss with antioxidants. Acta Otolaryngol (Stockh) 2005;125:235–243. doi: 10.1080/00016480410023038. [DOI] [PubMed] [Google Scholar]
- Kopke RD. International Symposium - Pharmacologic Strategies for Prevention and Treatment of Hearing Loss and Tinnitus. Niagra Falls; Ottawa, Canada: 2005. NAC for Noise: From the bench top to the clinic. [Google Scholar]
- Kramer S, Dreisbach L, Lockwood J, Baldwin K, Kopke RD, et al. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J Am Acad Audiol. 2006;17:265–278. doi: 10.3766/jaaa.17.4.5. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Dell S, Hensley BN, Hall JWI, Campbell KCM, et al. Digital music exposure reliably induces temporary threshold shift (TTS) in normal hearing human subjects. Ear Hear. 2012;33:e44–58. doi: 10.1097/AUD.0b013e31825f9d89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Johnson AC, Lindblad AC, Skjönsberg A, Ulfendahl M, et al. Increased vitamin plasma levels in Swedish military personnel treated with nutrients prior to automatic weapon training. Noise Health. 2011a;13:432–443. doi: 10.4103/1463-1741.90317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Spankovich C, Lobarinas E, Griffiths SK. Extended high frequency thresholds in college students: effects of music player use and other recreational noise. J Am Acad Audiol. 2013;24 doi: 10.3766/jaaa.24.8.9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Yang Q, Harris J. Modification of digital music files for use in human temporary threshold shift studies. J Acoust Soc Am Ex Lett. 2011b;130:EL142–146. doi: 10.1121/1.3630017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Zhou H, Zhu G, Lei D, Wu L, et al. One critical subcellular damage that switches noise-induced temporary to permanent threshold shift. Abs Assoc Res Otolaryngol. 2013;36:26–27. [Google Scholar]
- Liang Z. Parametric relation between impulse noise and auditory damage. In: Dancer AL, Henderson D, Salvi RJ, Hamernik RP, editors. Noise-Induced Hearing Loss. St. Louis: Mosby Year Book; 1992. pp. 325–335. [Google Scholar]
- Lin CY, Wu JL, Shih TS, Tsai PJ, Sun YM, et al. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269:42–47. doi: 10.1016/j.heares.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad AC, Rosenhall U, Olofsson A, Hagerman B. The efficacy of N-acetylcysteine to protect the human cochlea from subclinical hearing loss caused by impulse noise: A controlled trial. Noise Health. 2011;13:392–401. doi: 10.4103/1463-1741.90293. [DOI] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci. 2000;20:4701–4707. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Usubuchi H, Liberman MC. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci. 2013;33:5542–5552. doi: 10.1523/JNEUROSCI.5027-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12:711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RH, Hunter LL. Acoustic immittance measurements. In: Roeser RJ, Valente M, Hosford-Dunn H, editors. Audiology Diagnosis. New York: Thieme; 2000. pp. 381–342. [Google Scholar]
- Meinke DK, Finan DS, Soendergaard J, Flamme GA, Murphy WJ, et al. Impulse noise generated by starter pistols. Int J Audiol. 2013;52(Suppl 1):S9–S19. doi: 10.3109/14992027.2012.745650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JH, Schulte BA, Boettcher FA, Dubno JR. A comparison of age-related hearing loss and noise-induced hearing loss. In: Henderson D, Prasher D, Kopke R, Salvi RJ, Hamernik R, editors. Noise induced hearing loss: basic mechanisms, prevention and control. London: Noise Research Network; 2001. pp. 497–511. [Google Scholar]
- NCT00808470. [Accessed on May 8, 2012];Micronutrients to Prevent Noise-induced Hearing Loss. http://clinicaltrials.gov/ct2/show/NCT00808470.
- NCT01345474. [Accessed on May 8, 2012];Phase II Clinical Trial: D-Methionine to Reduce Noise-Induced Hearing Loss (NIHL) http://clinicaltrials.gov/ct2/show/NCT01345474.
- NCT01444846. [Accessed on January 11, 2013];Otoprotection With SPI-1005. http://clinicaltrials.gov/ct2/show/NCT01444846.
- NIOSH. Criteria for a Recommended Standard, Occupational Noise Exposure. 1998. DHHS (NIOSH) Publication No.98–126. [Google Scholar]
- Olszewski J, Milonski J, Olszewski S, Majak J. Hearing threshold shift measured by otoacoustic emissions after shooting noise exposure in soldiers using hearing protectors. Otolaryngol Head Neck Surg. 2007;136:78–81. doi: 10.1016/j.otohns.2006.07.004. [DOI] [PubMed] [Google Scholar]
- OSHA. 29 CFR 1910.95. Occupational Noise Exposure; Hearing Conservation Amendment; Final Rule, effective 8 March 1983. U.S. Department of Labor, Occupational Safety & Health Administration; 1983. [Google Scholar]
- Pawlaczyk-Luszczynska M, Dudarewicz A, Bak M, Fiszer M, Kotylo P, et al. Temporary changes in hearing after exposure to shooting noise. Int J Occup Med Environ Health. 2004;17:285–293. [PubMed] [Google Scholar]
- Price GR. Validation of the auditory hazard assessment algorithm for the human with impulse noise data. J Acoust Soc Am. 2007;122:2786–2802. doi: 10.1121/1.2785810. [DOI] [PubMed] [Google Scholar]
- Quaranta A, Scaringi A, Bartoli R, Margarito MA, Quaranta N. The effects of ‘supra-physiological’ vitamin B12 administration on temporary threshold shift. Int J Audiol. 2004;43:162–165. doi: 10.1080/14992020400050022. [DOI] [PubMed] [Google Scholar]
- Quaranta N, Dicorato A, Matera V, D’Elia A, Quaranta A. The effect of alpha-lipoic acid on temporary threshold shift in humans: a preliminary study. Acta Otorhinolaryngol Ital. 2012;32:380–385. [PMC free article] [PubMed] [Google Scholar]
- Schechter MA, Fausti SA, Rappaport BZ, Frey RH. Age categorization of high-frequency auditory threshold data. J Acoust Soc Am. 1986;79:767–771. doi: 10.1121/1.393466. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol. 1996;76:2799–2803. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Schmuziger N, Probst R, Smurzynski J. Test-retest reliability of pure-tone thresholds from 0.5 to 16 kHz using Sennheiser HDA 200 and Etymotic Research ER-2 earphones. Ear Hear. 2004;25:127–132. doi: 10.1097/01.aud.0000120361.87401.c8. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa S. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoorenburg GF. Damage risk criteria for impulse noise. In: Hamernik RP, Henderson D, Salvi RJ, editors. New Perspectives on Noise-Induced Hearing Loss. New York: Raven Press; 1982. [Google Scholar]
- Smoorenburg GF. Damage risk for low-frequency impulse noise: The spectral factor in noise-induced hearing loss. In: Dancer AL, Henderson D, Salvi RJ, Hamernik RP, editors. Noise-Induced Hearing Loss. St. Louis: Mosby Year Book; 1992. [Google Scholar]
- Stelmachowicz PG, Beauchaine KA, Kalberer A, Jesteadt W. Normative thresholds in the 8- to 20-kHz range as a function of age. J Acoust Soc Am. 1989;86:1384–1391. doi: 10.1121/1.398698. [DOI] [PubMed] [Google Scholar]
- Stewart M, Borer SE, Lehman M. Shooting habits of U.S. waterfowl hunters. Noise Health. 2009;11:8–13. doi: 10.4103/1463-1741.45307. [DOI] [PubMed] [Google Scholar]
- Strasser H, Irle H, Legler R. Temporary hearing threshold shifts and restitution after energy-equivalent exposures to industrial noise and classical music. Noise Health. 2003;5:75–84. [PubMed] [Google Scholar]
- Suckfuell M, Canis M, Strieth S, Scherer H, Haisch A. Intratympanic treatment of acute acoustic trauma with a cell-permeable JNK ligand: a prospective randomized phase I/II study. Acta Otolaryngology. 2007;127:938–942. doi: 10.1080/00016480601110212. [DOI] [PubMed] [Google Scholar]
- Sulkowski W, Kowalska S, Lipowczan A, Prasher D, Raglan E. Tinnitus and impulse noise-induced hearing loss in drop-forge operators. Int J Occup Med Environ Health. 1999;12:177–182. [PubMed] [Google Scholar]
- Suter AH. The hearing conservation amendment: 25 years later. Noise Health. 2009;11:2–7. doi: 10.4103/1463-1741.45306. [DOI] [PubMed] [Google Scholar]
- Suvorov G, Denisov E, Antipin V, Kharitonov V, Starck J, et al. Effects of peak levels and number of impulses to hearing among forge hammering workers. Appl Occup Environ Hyg. 2001;16:816–822. doi: 10.1080/10473220119058. [DOI] [PubMed] [Google Scholar]
- Tambs K, Hoffman HJ, Borchgrevink HM, Holmen J, Engdahl B. Hearing loss induced by occupational and impulse noise: results on threshold shifts by frequencies, age and gender from the Nord-Trondelag Hearing Loss Study. Int J Audiol. 2006;45:309–317. doi: 10.1080/14992020600582166. [DOI] [PubMed] [Google Scholar]
- US Department of Veterans Affairs. 2009 Annual Benefits Report. 2010 http://www.vba.va.gov/REPORTS/abr/index.asp.
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ren C. Effects of repeated “benign” noise exposures in young CBA mice: shedding light on age-related hearing loss. J Assoc Res Otolaryngol. 2012;13:505–515. doi: 10.1007/s10162-012-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WD. Proposed damage-risk criterion for impulse noise (gunfire). Report of Working Group 57, National Academy of Sciences-National Research Council (NAS-NRC) Committee on Hearing, Bioacoustics, and Biomechanics (CHABA).1968. [Google Scholar]
- Ward WD. Temporary threshold shift and damage-risk criteria for intermittent noise exposures. J Acoust Soc Am. 1970;48:561–574. doi: 10.1121/1.1912172. [DOI] [PubMed] [Google Scholar]
- Xiong M, Wang J, Yang C, Lai H. The cochlea magnesium content is negatively correlated with hearing loss induced by impulse noise. Am J Otolaryngol. 2013 doi: 10.1016/j.amjoto.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Hequembourg SJ, Atencio CA, Rosowski JJ, Liberman MC. Acoustic injury in mice: 129/SvEv is exceptionally resistant to noise-induced hearing loss. Hear Res. 2000;141:97–106. doi: 10.1016/s0378-5955(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Liberman MC. Sound conditioning reduces noise-induced permanent threshold shift in mice. Hear Res. 2000;148:213–219. doi: 10.1016/s0378-5955(00)00161-1. [DOI] [PubMed] [Google Scholar]
- Zera J. Impulse noise in industrial plants: statistical distribution of levels. Int J Occup Med Environ Health. 2001;14:127–133. [PubMed] [Google Scholar]
- Zhao YM, Qiu W, Zeng L, Chen SS, Cheng XR, et al. Application of the kurtosis statistic to the evaluation of the risk of hearing loss in workers exposed to high-level complex noise. Ear Hear. 2010;31:527–532. doi: 10.1097/AUD.0b013e3181d94e68. [DOI] [PubMed] [Google Scholar]