Abstract
Loss of a partner can have severe effects on mental health. Here we explore the neural mechanisms underlying increased passive stress-coping, indicative of depressive-like behavior, following the loss of the female partner in the monogamous male prairie vole. We demonstrate that corticotropin-releasing factor receptor 2 (CRFR2) in the nucleus accumbens shell mediates social loss-induced passive coping. Further, we show that partner loss compromises the oxytocin system through multiple mechanisms. Finally, we provide evidence for an interaction of the CRFR2 and oxytocin systems in mediating the emotional consequences of partner loss. Our results suggest that chronic activation of CRFR2 and suppression of striatal oxytocin signaling following partner loss result in an aversive emotional state that may share underlying mechanisms with bereavement. We propose that the suppression of oxytocin signaling is likely adaptive during short separations to encourage reunion with the partner and may have evolved to maintain long-term partnerships. Additionally, therapeutic strategies targeting these systems should be considered for treatment of social loss-mediated depression.
Keywords: corticotropin-releasing factor, grieving, partner separation, passive stress-coping, social loss, stresscopin
1. Introduction
Social relationships are vital for mental and physical wellbeing (Berkman, 1995; Biondi and Picardi, 1996; House et al., 1988; Uchino et al., 1996; Zisook et al., 1997). Conversely, social isolation or loneliness is associated with increased risk of general as well as of cardiovascular disease-related mortality (Ramsay et al., 2008; Steptoe et al., 2013). Moreover, the sudden disruption of social relationships is often followed by emotional distress (Resendez and Aragona, 2013), triggering severe physical and psychological sequelae, including depression (Biondi and Picardi, 1996; Zisook et al., 1997). Each year in the US, approximately 8 million individuals suffer from the loss of a close family member (Hensley et al., 2009; Shear and Shair, 2005), which induces depressive symptoms in approximately 40% of these individuals within the first month (Clayton and Darvish, 1979). Therefore, studying the underlying neural mechanisms of social loss-induced depression is essential to understanding the psychiatric consequences associated with these events, and their potential pharmacological treatments. Depression is a heterogeneous disorder with symptoms manifested at the psychological, behavioral and physiological level (Cryan and Mombereau, 2004). Animal models do not typically fully recapitulate depression per se, but rather display behavioral and physiological endophenotypes that may have face, construct and predictive validity relevant to depression. For example, passive coping behavior is commonly used to assess depressive-like behavior in animal models and has predictive validity for identifying drugs that are effective for treating depression (Cryan and Mombereau, 2004; Cryan et al., 2005), but passive coping in animals should not be considered synonymous with depression.
Recently, we characterized the emergence of depressive-like behavioral and physiological phenotypes following social loss using monogamous prairie voles (Microtus ochrogaster), which form selective, enduring pair bonds (Carter and Getz, 1993; Johnson and Young, 2015; Young and Wang, 2004). In male prairie voles, the loss of a bonded female partner, but not of a male sibling, is a stressful event that leads to an increase in basal plasma corticosterone concentration and adrenal hypertrophy (Bosch et al., 2009). Furthermore, separation from the female partner increases passive stress-coping (Bosch et al., 2009; McNeal et al., 2014; Sun et al., 2014). The most common test used to measure passive stress-coping in rodents is the forced swim test (FST) (Slattery and Cryan, 2012). In our previous study, the FST, together with the tail suspension test, was used to demonstrate increased passive stress-coping following partner loss (Bosch et al., 2009). Both of these tests have predictive validity in rodents for identifying pharmacological agents that are effective for treating depression in humans (Cryan et al., 2005). The depressive-like emotional state observed in male prairie voles separated from their female partner is reversed by chronic central blockade of corticotropin-releasing factor (CRF) receptor (CRFR) type 1 and/or 2 (Bosch et al., 2009). When paired with a female partner, but not with a male sibling, CRF synthesis is elevated in the bed nucleus of the stria terminalis (BNST) of male prairie voles (Bosch et al., 2009), and pair bond formation is facilitated by acute CRF signalling in the nucleus accumbens (NAc) (DeVries et al., 2002; Lim et al., 2007). Partner-separation, however, is a severe chronic stressor leading to prolonged brain CRFR activation, which results in increased passive stress-coping (Bosch et al., 2009).
Recent studies in rats demonstrate that severe stress switches the action of CRF from appetitive to aversive within the NAc (Lemos et al., 2012), and that intra-NAc shell CRFR activation causes increased depressive-like behavior (Chen et al., 2012). Interestingly, CRFR2 are more abundant, and CRFR1 less abundant, in the NAc of monogamous compared with non-monogamous vole species (Lim et al., 2005), making CRFR2 a natural focus for our present study. Moreover, prairie vole NAc contains both CRF-immunoreactive fibres (Lim et al., 2007), and oxytocin-(OT) immunoreactive fibres originating primarily from the paraventricular nucleus (PVN) (Ross et al., 2009). OT facilitates pair bond formation, and mediates social buffering against stressors in female prairie voles (Smith and Wang, 2014). These two neuropeptide systems may interact as >99% of OT neurons in the hypothalamus of rats co-express CRFR2 (Dabrowska et al., 2011).
Here, we hypothesized that CRFR2 in the NAc shell mediates increased passive stress-coping after partner loss through interaction with the OT system, and that compromised OT-signalling contributes to partner loss-mediated changes in passive coping. We chronically infused CRFR2 antagonist or agonist into the NAc shell of male prairie voles that were either with or separated from their partner, and tested for passive stress-coping using the FST. Furthermore, we examined hypothalamic OT mRNA expression and intra-NAc OT receptor (OTR) binding in paired and separated males. Next, we locally manipulated OTR in the NAc shell using receptor-selective (ant-)agonist or local RNA interference (RNAi)-mediated OTR knock-down in pair bonded male prairie voles and tested for passive coping. We aimed to determine the extent of colocalization of CRFR2-immunoreactivity on OT fibres in the NAc shell, and to characterize OT-release in the NAc shell after CRFR2-manipulations using in vivo microdialysis. We then investigated the origin of OT fibers in the NAc. Finally, we directly examined the effect of CRFR2 manipulation on PVN OT neuronal excitability using in vitro electrophysiological recordings. Together, our results are consistent with the hypothesis that partner loss compromises OT signalling in the NAc through multiple independent mechanisms, including direct CRFR2-mediated influences on OT fibers in the NAc and potentially indirect CRFR2-mediated modulation of presynaptic drive to OT neurons in the PVN.
2. Material and Methods
2.1 Animals
All animals were sexually naïve adult male and female prairie voles (70–100 days (d) of age) from the laboratory breeding colony originally derived from field-captured voles in Illinois, USA. After weaning at 21d, subjects were housed in same-sex sibling pairs or trios under standard laboratory conditions (14:10h light-dark cycle, lights on at 0600h; 20°C, 60% humidity and free access to water and Purina rabbit chow). All behavioral tests were performed between 0800h and 1200h.
The animal studies were conducted in accordance with the guidelines of the National Institute of Health and were approved by Emory University’s Institutional Animal Care and Use Committee.
2.2 Experimental protocol
Male voles were paired with unfamiliar females or male siblings, and after 5 days, which is more than enough to establish a partner preference (Winslow et al., 1993)), half of the voles of each group were separated from their partner while the remaining half continued to be cohabitated with the partner as described previously (Bosch et al., 2009).
The FST was performed on day 8 after pairing, i.e. three days after separation, according to an established protocol (5min; 4L glass beaker, 15cm diameter, filled to a height of 20cm with tap water, 24±1°C) (Bosch et al., 2009). The behaviors (1) struggling, (2) swimming including diving, and (3) floating were scored by a trained observer blind to treatment using Eventlog (Robert Hendersen, Grand Valley State University, Allendale, MI, USA). In this paradigm, floating is indicative of a passive coping strategy (Bosch et al., 2009; Cryan and Mombereau, 2004).
2.3 Chronic local infusion via osmotic minipumps
On the 3rd day of pairing osmotic minipumps (Model 1007D, infusion flow rate: 0.5μl/h; Brain Infusion Kit 2; fixant Loctite 454; Alzet Osmotic Pumps, Cupertino, CA, USA) connected via a PE-20 tubing to a cannula were bilaterally implanted targeting the NAc shell (A/P +1.7mm, ML ±1.0mm, DV −4.5mm) under isoflurane anaesthesia (Novaplus, Hospira Inc., Lake Forest, IL, USA) using a stereotax as previously described (Bosch et al., 2009). The tubing was filled with Ringer’s solution calculated to last for 44h followed by a small air bubble and then the drug to guarantee its delivery on the day of separation. In experiment 1, the osmotic minipumps were filled with Ringer’s solution (Fisher Science Education, Hanover Park, IL, USA; pH adjusted to 7.4; containing 4% DMSO) or Ringer’s solution containing the CRFR2 antagonist astressin-2B (1ng/h; Tocris, Elisville, MO, USA) or the CRFR2 agonist stresscopin (0.1ng/h; Phoenix Pharmaceuticals, Burlingame, CA, USA); both agonist and antagonist are receptor specific in prairie voles (Bosch et al., 2009; Lim et al., 2005). In experiment 2, the osmotic minipumps were filled with Ringer’s solution (pH adjusted to 7.4) or Ringer’s solution containing synthetic OT (0.5ng/h; Sigma-Aldrich, St. Louis, MO, USA) or a selective OT receptor antagonist (OTR-A; (d(CH2)51, Tyr(Me)2, Thr4, Orn8, des-Gly-NH29)-vasotocin; 5ng/h; Bachem, Bubendorf, CH). Doses were chosen based on previous studies (Bosch et al., 2009; Peters et al., 2014)
2.4 Local knock-down of OTR in the NAc shell via shRNA
A prairie vole Oxtr shRNA expressing adeno-associated viral vector (AAV) (2μl/hemisphere (Keebaugh et al., 2015) was infused slowly at 5nl/s bilaterally into the NAc shell of juvenile male prairie voles at 21d according to established protocols (Barrett et al., 2013; Keebaugh et al., 2015). At approximately 60d males were co-housed with an age-matched intact female and 5 days later tested on the the FST. Bilateral OTR knock-down and transfection sites were verified by receptor autoradiography (NAc shell, as well as caudate putamen (CP) and prefrontal cortex (PFC) as control sites) and GFP visualization as previously described (Barrett et al., 2013; Keebaugh et al., 2015).
2.5 Co-localization of CRFR2-and OT-immunoreactive neurons and fibers
Co-expression of OT and CRFR2 was visually assessed in the PVN and NAc using immunohistochemistry in free-floating prairie vole brain tissue. Two male prairie voles (approximately 90d) were transcardially perfused with 50ml of 4% paraformaldehyde (00380, Polysciences, Inc., Warrington, PA) as described previously (Ross et al., 2009). Fixed brains were immediately stored at 4°C in a 30% sucrose solution until serially sectioned at 25μM on a freezing microtome. The sections were stored free-floating in cryoprotectant solution at −20°C until they were assayed for OT and CRFR2 expression. OT and CRFR2 immunofluorescence assays were performed as previously described (Dabrowska et al., 2011), except that 0.014% phenylhydrazine was added to the PBS plus 0.5% Triton-X solution during permeabilization. The antibodies used were anti-CRFR2 (1:500; rabbit host, polyclonal, targeting the extracellular, N-terminal domain of CRFR2; ab12964, Abcam, Cambridge, MA), anti-OT (1:7,500; mouse host, monoclonal; clone 4G11; MAB5296, Chemicon-Millipore, Billerica, MA), or both for dual-labelling. Mounted tissue was air-dried at room temperature overnight and then processed through H2O (5min), followed by 75%, 95%, and 100% ethanol (5min each), and finally cleared in Histoclear (50–899–90147, National Diagnostics, Atlanta, GA) for ≥10min. Slides were coverslipped using Vectashield fluorescence mounting medium (H-1000, Vector Laboratories, Inc., Burlingame, CA) and air-dried.
Immunofluorescent labelling was visualized using spinning disk laser microscopy (CSU10B; Yokagawa Electronic Corporation, Tokyo, Japan), and high-resolution photo-micrographs were obtained using an Orca R2 cooled CCD camera (Hammamatsu, Bridgewater, NJ) attached to a Leica DM5500B microscope (Leica Microsystems, Bannockburn, IL).
2.6 Combined acute intracerebroventricular (icv) manipulation of CRFR2 and intra-NAc shell microdialysis for OT
2.6.1 Implantation of icv guide cannula and local microdialysis probe in the NAc shell
Using isoflurane inhalation anaesthesia, sexually naïve male prairie voles were stereotaxically implanted with a guide cannula (stainless steel; 21G; length: 8mm) 2mm above the left ventricle (A/P −0.6mm; ML +1.0mm; DV −2.0mm) that was capped prior to testing with a dummy cannula. In addition, voles were implanted with a U-shaped microdialysis probe (dialysis membrane: molecular cut-off of 18kDa; Hemophan, Gambro Dialysatoren, Hechingen, Germany; for details see (Neumann et al., 1993)) targeting the right NAc shell (A/P +1.7mm; ML −1.0mm; DV −4.5mm; for details see (Ross et al., 2009)). Prior to the implantation, the microdialysis probe was flushed and filled with sterile Ringer’s solution (pH adjusted to 7.4). The two ends of the probe were then attached to PE-20 polyethylene tubing (adapters, each 3cm long) filled with Ringer’s solution. After surgery, voles were single-housed and allowed to recover for two to three days prior to the start of the experiment.
2.6.2 Acute icv treatment infusion and local microdialysis within the NAc shell
Two days after surgery at 0800h, voles were anaesthetized with urethane i.p. (1.6g/kg) and placed on a heating pad to maintain body temperature. The inflow adapter of the microdialysis probe was connected to a syringe mounted onto a microinfusion pump via a piece of PE-20 tubing. The outflow adapter was attached to a tube holder that allowed sample collection into a 0.5-ml Eppendorf tube. The perfusion rate of the microdialysis probes was 3.3μl/min with sterile Ringer’s solution. Following 2h of initial probe flushing (to establish a local equilibrium between inside/outside the probe), two 30-min basal samples were collected. Afterwards, vehicle (2μl; Ringer’s solution containing 4% DMSO), astressin-2B (200ng/2μl) or stresscopin (20ng/2μl) was infused slowly using a manual Hamilton syringe attached to an icv infusion cannula (27G) inserted into the guide cannula and extending it by 2mm (the astressin-2B dose was chosen based on a previous study (Todorovic et al., 2007) and adjusted for stresscopin by factor of 10). The cannula was kept in place for 2min to allow substance diffusion. Three 30min microdialysate samples were collected following the drug infusion before termination of the experiment. All samples were immediately frozen on dry ice and stored at −80°C.
2.6.3 Radioimmunoassy for OT
The microdialysates were lyophilized and OT was quantified by a highly sensitive and selective radioimmunoassay (RIAgnosis, Munich, Germany). All samples were measured in the same assay and the intra-assay variability was <10%.
2.7 Histology
At the end of the experiments male voles were euthanized via CO2 asphyxiation and the brains were collected, flash-frozen and stored at −80°C until further processing. To verify the correct placement of the infusion cannula or microdialysis probe, the brains were cut into 40μm coronal cryostat sections, and stained with cresyl violet.
2.8 Autoradiography for OT mRNA and OTR binding
In a separate set of animals, male prairie voles were co-housed with either a naïve female or a male sibling for 8 days, or were separated for three days following five days of co-housing. The brains of the males were rapidly removed under basal conditions after short (10s) isoflurane anaesthesia followed by rapid decapitation, flash-frozen and stored at −80°C. Brains were cryosectioned at 16-μm, slide mounted and stored at −80°C until further processing.
One set of a one in six series of slide-mounted brain sections containing the PVN and supraoptic nucleus for each animal was processed for OT in situ hybridization as previously described (Ahern and Young, 2009).
Receptor autoradiography for OTR binding in the Nac was performed as described elsewhere (Lim et al., 2004) using a second set of the six series described above. Expression of mRNA (14 day exposure) or receptor binding (7 day exposure) was analyzed on autoradiographic BioMax MR films using a digital camera setup (Retiga 2000R; QImaging, Surrey, BC, Canada; Sigma 50 mm 1:2.8 DG Macro D; Sigma, Aizu, Japan). The gray density of the brain regions was analysed with a computerized image program (ImageJ 1.31, National Institutes of Health, http://rsb.info.nih.gov/ij/). Bilateral measurements from six slide-mounted brain sections per animal were taken and the mean gray density measurement (arbitrary units) was calculated by subtracting the background activity.
2.9 Co-localization of Venus in PVN OT neurons and NAc fibers
Adult male prairie voles (n=6), were injected with an AAV incorporating a mouse OT promoter sequence driving the expression of the green fluorescent protein, Venus (see Knobloch et al., 2012). Bilateral injections (600nl/side at 3nl/s) targeted the PVN (AP −0.4mm, ML ±0.7mm, DV −5.7mm angled medially at 6°). Eighteen days were allowed for viral transfection and expression. All subjects were then transcardially perfused with 4% paraformaldehyde as described above. Brains were sectioned at 40μm and immunohistochemistry was performed using anti-OT (1:10,000; rabbit host; Immunostar 20068) and anti-GFP (1:1,200; chicken host; Abcam ab13970) followed by anti-rabbit (1:500; ab175471 AlexaFluor 568) and anti-chicken (1:500; ab150169 AlexaFluor 488) secondary antibodies. Slides were coverslipped using Vectashield mounting medium with DAPI (Vector H-1200). Slide images were captured using a Nikon Eclipse E800 fluorescent microscope (Nikon Instruments, Inc.; Melville, NY) and MCID Core imaging software (InterFocus Ltd, Cambridge, UK). Extra-PVN co-localization of Venus and OT within the accessory nucleus (AN) and supraoptic nucleus (SON) of the hypothalamus was used as a criterion for exclusion. Two independent scorers quantified co-localization of Venus and OT by first selecting and imaging OT-positive fibers and then imaging Venus within the same frame. The average of the two raters provided the final measure of co-localization. A rate of 50% co-localization of OT and Venus was used to operationally define whether injections were targeted successfully or not. Analysis of co-localization was restricted to the NAc shell and a single section was analysed per subject that reached 50% colocalization in the PVN. The section was always immediately anterior to the first merging of the left and right corpus callosum to control for A/P position across subjects. Multiple images were captured from both left and right hemispheres within each section, yielding an average of approximately 15 fibers analysed per subject.
2.10 In vitro whole cell patch-clamp electrophysiological recordings of Venus-positive, putative OT neurons in the PVN
2.10.1 Stereotaxic infusion of AAV to selectively express Venus in OT neurons
OT neurons were visually identified using the OT-Venus AAV described above in section 2.9. Briefly, male prairie voles (60–90d) were injected into the PVN as describe above (225nl/side at 3nl/s) using a 32G Hamilton Neuros syringe and allowed to recover for two weeks to permit transgene expression.
2.10.2 Electrophysiological experiments
Slices of 350μm containing the PVN were obtained as described previously in rats (Dabrowska et al., 2013a). Briefly, voles were anesthetized with isoflurane (Fisher Scientific, Hanoverpark, IL, USA), decapitated, and brain slices containing the PVN were rapidly cut using a Leica VTS-1000 Vibratome (Leica Microsystems Inc., Bannockburn, IL, USA) as previously described (Dabrowska et al., 2013a).
Individual slices were transferred to a submersion type recording chamber mounted on the fixed stage of a Leica DM6000 microscope (Leica Microsystems Inc., Bannockburn, IL, USA), and continuously perfused by gravity-fed oxygenated 32°C regular artificial cerebrospinal fluid (ACSF) at 1–2ml/min. Whole-cell patch-clamp recordings from the PVN slices were obtained using standard techniques (Dabrowska et al., 2013a).
Individual PVN neurons expressing the Venus fluorescent protein (putative OT neurons), were identified using epifluorescence microscopy and the appropriate fluorescein isothiocyanate (FITC) excitation and emission filter sets. Data acquisition and analysis were performed using a MultiClamp700B amplifier in conjunction with pClamp10.0 software and a Digi-Data 1320A AD/DA interface (Molecular Devices, Sunnyvale, CA, USA).
At the start of each experiment, a series of standardized current clamp protocols were performed to determine the following intrinsic membrane properties of the OT neurons; input resistance (Rin), time constant of membrane charging (tau), first inter-spike-interval (ISI1), last inter-spike-interval (ISIN), fast and slow after-hyperpolarization potential (fAHP and sAHP, respectively), as well as Ih ratio (hyperpolarization-activated non-specific cation current normalized to membrane potential). The effects of stresscopin (200nM, 10min), on intrinsic membrane properties of the putative OT neurons, were examined before, during and after drug application. Briefly, the voltage response of neurons was determined using a series of transient (750ms) outward and inward current steps of 20–50pA based on membrane input resistance (Rm) of neurons, with the maximum hyperpolarizing voltage restricted to −150mV. Next, the action potential threshold and kinetic properties (amplitude, rise time, decay time, half-width) were determined using a transient depolarizing current ramp (250ms) of sufficient magnitude to evoke a single action potential. Finally, to determine the effect of stresscopin on excitatory synaptic transmission in the OT neurons, sEPSCs were measured in voltage-clamp mode in the presence of the specific GABAA receptor antagonist, SR95531 (5μM). All the experiments were performed before, during, and after stresscopin application (200nM). All electrophysiological data were analyzed using MATLAB 2009a with customized scripts (Mathwork, Natick, MA). Spontaneous synaptic events were captured continuously for at least 180 sec during each phase of the experiment. All sEPSCs events were detected offline and their amplitude and frequency calculated using MiniAnalysis 6.0 (Synaptosoft Inc., Decatur, GA, USA).
2.11 Statistics
Experimental subjects were only included in the statistical analysis when the infusion cannulae or microdialysis probes were correctly placed in the brain target region. As the OT content in the microdialysates collected from the NAc shell of male voles showed high individual variation, the microdialysis data of each animal was standardized to the mean of its two basal samples (=100%) and presented as mean percentage of the group. Statistical tests performed were independent t-test (FST; in situ hybridization), Mann-Whitney U-test (sEPSCs), or two-way analysis of variance (ANOVA; FST; in situ hybridization; receptor autoradiography; where main factors are partner type (female/sibling) and factor separation condition (paired/separated)). For microdialysis, the data was analyzed using a two-way ANOVA for repeated measures (factors time relative to drug infusion x treatment) and separate paired t-tests. All ANOVAs with significant main or interaction effects were followed by Sidak post hoc analysis with correction for multiple comparisons. In cases where we a priori predicted specific outcomes, planned comparisons of specific contrasts were also performed. Statistics were performed using GraphPad Prism 4.0 (GraphPad Software, Inc., La Jolla, CA, USA; electrophysiological studies) or SPSS 21 (IBM, Ehningen, Germany; all other data). All data are presented as mean+SEM; significance was accepted at p≤0.05.
3. Results
3.1 CRFR2 in the NAc shell mediates passive stress-coping after loss of a female partner
We first examined the role of CRFR2 in the NAc shell in increased passive stress-coping in the FST after loss of the female partner in male prairie voles (Bosch et al., 2009; McNeal et al., 2014). Here, male prairie voles were chronically infused bilaterally within the NAc shell with vehicle until the day of separation, after which the osmotic minipumps either continued to deliver vehicle or switched to astressin-2B (antagonist) or stresscopin (agonist) continually until testing for passive stress-coping in the FST.
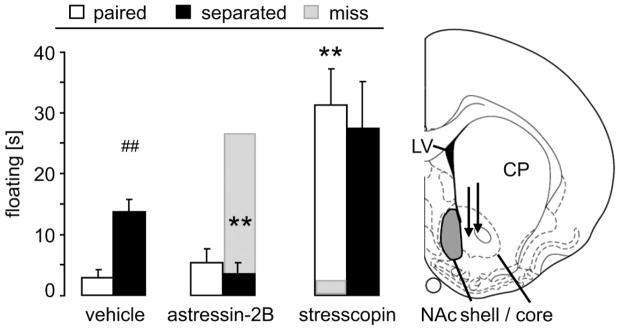
A 2 way ANOVA failed to reveal a significant main effect of separation condition, drug treatment or an interaction for active (factors separation condition x treatment; struggling: F(2,43)=1.75, p=0.19; swimming: F(2,43)=1.68, p=0.20) or passive stress-coping behaviors (floating: F(2,43)=1.39, p=0.26; Fig. 1). However, since we previously identified a role for CRFR2 in mediating passive coping and since CRFR2 are highly concentrated in the NAcc, we performed planned t-tests comparing floating duration in key comparator groups (α=0.01 for 5 comparisons). These planned contrasts revealed that in the vehicle-treated group, 1) floating was increased in separated versus non-separated males (t-test; p=0.001), confirming previous findings (Bosch et al., 2009; McNeal et al., 2014). This separation-induced effect was 2) abolished in males chronically infused with astressin-2B (p=0.001 vs separated vehicle), whereas non-separated males 3) were not affected. Chronic infusion of stresscopin 4) increased floating in non-separated voles (p=0.001 vs non-separated vehicle); 5) stresscopin had no effect on the separated group. Importantly, the behavioral effects of the infusions were site-specific as males with misplaced cannulae outside the NAc (“miss”; Fig. 1 gray bars) did not show altered passive stress-coping.
Figure 1.
Blocking CRFR 2 in the NAc shell (gray area in schematic drawing) eliminated heightened passive stress-coping after female partner loss.
A three-day separation from the female partner after five days of group housing resulted in increased floating in the forced swim test, indicative of passive stress-coping, in male prairie voles chronically infused with vehicle bilaterally into the NAc shell. Blocking CRFR2 with astressin-2B in the NAc shell diminished this increased passive stress-coping after separation. Activating CRFR2 by stresscopin increased passive stress-coping in the non-separated males. Passive stress-coping of male prairie voles treated with stresscopin or astressin-2B outside the NAc shell (“miss”; infusion site is depicted by the tips of black arrows in schematic drawing (Paxinos and Watson, 1998)) did not differ from vehicle-treated controls.
CP = caudate putamen; LV = lateral ventricle. Numbers of animals included in the statistics were vehicle paired = 6; separated = 5; antagonist paired = 7; separated = 10; agonist paired = 9; separated = 7; miss = 1. Data are expressed as mean + sem. ** p = 0.001 vs corresponding vehicle-treated group; ## p = 0.001 vs corresponding female-paired group.
3.2 Hypothalamic OT mRNA expression and NAc OTR binding in male prairie voles after separation from a female partner or male sibling
As OT synthesized in the PVN plays a role in social buffering (Smith and Wang, 2014), and hypothalamic OT neurons innervate the NAc where OT acts on OTR to facilitate social bonding (Ross et al., 2009; Young et al., 2001), we explored the consequences of pair bond disruption on these systems. In addition to the male-female groups, we also tested male-male groups (sibling) in order to dissect possible effects of the sex of the partner on the OT system.
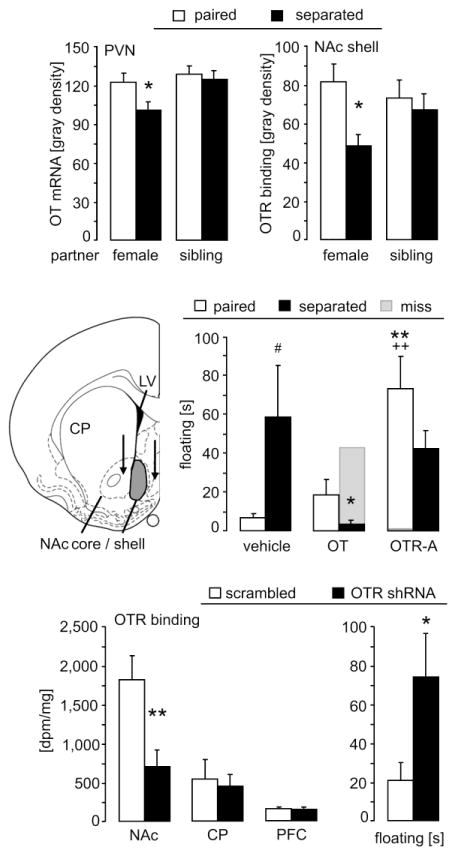
OT mRNA expression within the PVN differed between the groups depending on the partner’s sex (2-way ANOVA; factor partner: F(1,24)=5.26, p=0.03) and tended to differ as a result of the partner presence (factor separation: F(1,24)=3.87, p=0.06; Fig. 2 top); no interaction was found. However, planned comparisons using t-tests revealed that within the pair bonded groups, separated males had lower OT mRNA expression in the PVN compared with non-separated males (t-test; p=0.03). While supporting the hypothesis that separation from the partner decreases OT mRNA in the PVN, this result does not survive correction for multiple contrasts in this experiment (α=0.05/2=0.025) and thus should be interpreted cautiously. OT mRNA expression in the supraoptic nucleus (SON) did not differ between the groups (2-way ANOVA; factors partner x separation: F(1,24)=0.93, p=0.35; data not shown).
Figure 2.
Changes in the OT system of the PVN and NAc in response to separation and altered passive stress-coping following manipulations of OTR in the NAc shell.
Top: Decreased OT mRNA expression in the PVN and decreased OTR binding in the NAc shell after 3-day separation from the pair bonded female partner, but not from the male sibling. Numbers of animals included in the statistics were female paired = 7; separated = 6; sibling paired = 6; separated = 5.
Middle: Separation from the female partner increased floating in the forced swim test, indicative of passive stress-coping, in male prairie voles chronically infused with vehicle bilaterally into the NAc shell. Activating OTR by synthetic OT in the NAc shell abolished the increased passive stress-coping after separation. Blocking OTR by a specific OTR antagonist (OTR-A) increased passive stress-coping in the non-separated males. Floating was higher in both OTR-A treated groups compared to the corresponding OT-treated males. Passive stress-coping of male prairie voles treated with OT or OTR-A outside the NAc shell (“miss”; infusion site is depicted by the tips of black arrows in schematic drawing (Paxinos and Watson, 1998)) did not differ from vehicle-treated controls. Numbers of animals included in the statistics were vehicle = 6/5; OT = 7/9; OTR-A = 11/6 (paired/separated); miss = 1.
Bottom: The viral vector expressing the shRNA targeting the prairie vole OTR resulted in a significant reduction in OTR binding in the NAc shell, but not caudate putamen (CP) or prefrontal cortex (PFC), compared to the scrambled control viral vector. Knock-down of OTR in male prairie voles increased passive stress-coping on day 10 of cohabitation with a female. Numbers of animals included in the statistics were scrambled sequence = 8; OTR shRNA = 7.
Data are expressed as mean + sem. ** p < 0.01, * p < 0.05 vs corresponding control group (top: female paired; middle: vehicle-treated; bottom: scrambled-treated); ## p = 0.01 vs corresponding female-paired group; ++ p≤0.01 vs corresponding OT-treated group.
With respect to OTR binding within the NAc shell there was no main effect on partner, separation, or interaction (2-way ANOVA; factors partner x separation: F(1,22)=2.55, p=0.13; Fig. 2 top). However, planned comparisons using t-tests revealed that in the the pair bonded group, separated males had lower OTR binding compared with the non-separated males (t-test; p=0.02, α=0.025). OTR binding in the NAc core did not differ between the groups (F(1,22)=1.77, p=0.20; data not shown).
3.3 The role of OTR in the NAc shell in passive stress-coping after female partner loss
Given the above results, we wanted to directly test whether the OT system in the NAc shell was involved in the increased passive stress-coping observed following the loss of a female partner. We hypothesized that the partner-loss mediated passive stress-coping behavior could be prevented by increasing extracellular OT concentration locally while reducing OTR signaling by blocking the receptors even in the presence of the parther would facilitate passive coping behavior.
The groups did not differ in active coping behaviors (2-way ANOVA; factors separation condition x treatment; struggling: F(2,43)=0.35, p=0.71; swimming: F(2,43)=1.12, p=0.34) but did in passive stress-coping behavior (floating: F(2,43)=4.60, p=0.02; Fig. 2 middle). The post hoc test revealed that in the vehicle-treated males separation from the female partner increased floating (p=0.02; Fig. 2 middle). This separation-induced effect was eliminated after chronic OT infusion (p=0.03 vs separated vehicle), which did not affect non-separated males. However, in the latter, OTR-A within the NAc shell increased floating (p=0.002 vs non-separated vehicle; p=0.009 vs non-separated OT), mimicking the effect of partner loss, whereas this treatment did not affect the separated males. Importantly, the behavior was not affected when cannulae were placed outside of the NAc (“miss”; Fig. 2 middle, gray bars), thus, demonstrating the region-specificity of the OT system.
To determine the functional role of OTR in the NAc shell, and to exclude possible nonspecific actions of synthetic OT or of the OTR antagonist on other receptors, we selectively reduced OTR density using an RNAi approach. The shRNA reduced OTR binding in the NAc shell and its immediate surroundings by 63 % compared to scrambled control (t-test; p=0.006; Fig. 2 bottom), but never reached other OTR expressing structures like the CP (p=0.73) or PFC (p=0.81). The lack of spread to these regions was confirmed by GFP expression, as previously reported (Keebaugh et al., 2015). Strikingly, consistent with our pharmacological manipulations, such reduced OTR signalling in the NAc shell caused increased floating in males even in the presence of their partner (t-test; p=0.04; Fig. 2 bottom). Active stress-coping behavior was not affected by the treatment (struggling: p=0.15; swimming: p=0.79; data not shown).
3.4 Co-localization of CRFR2 on OT neuronal fibers extending into the NAc shell
In rats, more than 99% of OT-expressing neurons in the PVN co-express CRFR2, and this co-expression occurs not only in the soma but also along the large fibers that radiate throughout the forebrain, including in the NAc shell (Dabrowska et al., 2011). Thus, we hypothesized that CRFR2 might be present on the OT fibers that extend into the NAc shell from the vole PVN, providing a mechanism for an interaction between the CRFR2 and OT systems. To test our hypothesis, we first assessed double-labeling for OT and CRFR2 using immunofluorescence in the prairie vole PVN.
Similar to rats (Dabrowska et al., 2011), OT and CRFR2 showed nearly complete somatodendritic immunoreactive overlap in PVN neurons and within the sparse large-caliber neuronal fibres that course through the NAc shell (Fig. 3A–F). No other CRFR2 immunoreactivity was detected in the NAc shell, suggesting that the effects of CRFR2 manipulations in the NAc shell are modulating OT fibers.
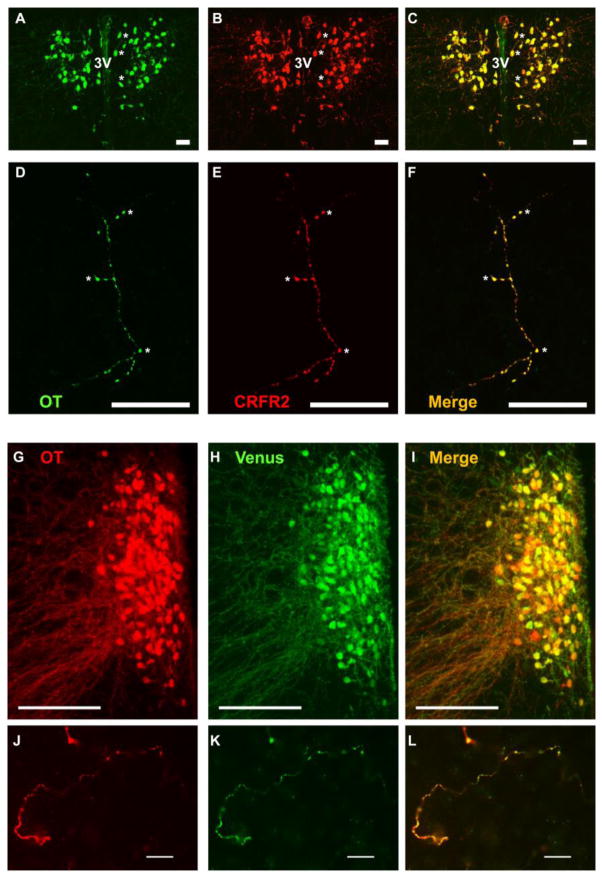
Figure 3.
Co-localization of OT and CRFR2 (A – F) or Venus (G – L) in the prairie vole PVN and NAc shell.
The PVN exhibits high somatodendritic immunoreactivity of OT (A; green) and CRFR2 (B; red) in neurons flanking the 3rd ventricle (3V). Asterisks (*) in A – C mark cells within the right hemisphere that clearly reveal overlapping (C; yellow), but nonidentical, immunoreactivity. This pattern appears equivalent to the double-labelling described in detail in rats (Dabrowska et al., 2011). Sparse large-caliber neuronal fibers that extend into the NAc shell also exhibit strong co-localization of OT (D) and CRFR2 (E; overlap F). Asterisks (*) in D – F mark distinct co-labeled puncta.
In the PVN, both OT (G; red) and Venus (H; green) immunoreactivity were co-localized in one hemisphere of the PVN exhibiting a robust plume of OT neurons (merge: I; yellow). In the NAc shell, both OT (J; red) and Venus (K; green) immunoreactivity were co-localized (merge: L; yellow).
Scale bars = 20 μm (A–F; J – L) and 200 μm (G – I)
3.5 Evidence that NAc shell OT fibers originate from the PVN
In the next step, we aimed to determine whether the PVN is the main source of the OT fibers found in the NAc shell. An average of 75.2±7.5% OT-Venus co-localization was found within the PVN, with no co-localization observed between Venus and OT within the AN or SON (Fig. 3G–L). Within NAc shell fibers, an average of 87.9±3.4% of OT fibers exhibited co-expression of Venus across subjects. Thus, the vast majority of OT fibers innervating the NAc shell originate from PVN OT neurons, as described in rats (Knobloch et al., 2012).
3.6 Central CRFR2 manipulation affects local OT release within the NAc shell
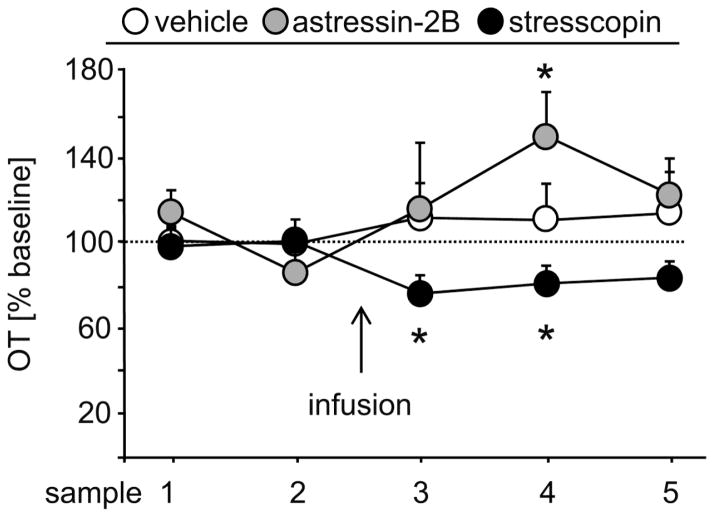
Our data led us to hypothesize that CRFR2 may directly act on the presynaptic OT system to suppress OT release in the NAc shell, thereby increasing passive stress-coping after chronic separation from the partner. Therefore, male prairie voles had been fitted with an icv guide cannula as well as with a microdialysis probe targeting the NAc shell. Before (basal conditions; samples 1, 2) and for 90 min after (samples 3 – 5) an acute icv infusion of vehicle, stresscopin or astressin-2B, 30-min microdialysis samples were collected and later analyzed for OT concentration by radioimmunoassay.
A 2-way repeated measures ANOVA failed to identify any main effect of treatment, time or an interaction in local release of OT within the NAc shell (factors treatment x time: F(8,60)=1.62, p=0.14; Fig. 4). However, given our a priori hypothesis that CRFR2 activity would suppress OT release, we performed planned comparisons using paired t-tests. These analysis revealed that infusion of stresscopin decreased the release of OT immediately by 24% (samples 3 vs. 2; paired t-test; p=0.05) and was still 20% lower in the following sample (samples 4 vs. 2; p=0.04). By contrast, astressin-2B significantly increased the OT release by 48% with a short delay of 30min (samples 4 vs. 2; p=0.05). Infusion of vehicle had no effect on local OT levels within the NAc shell. While these independent planned contrasts between drug treatment and basal conditions are not significant if corrected for multiple comparisons, the consistency and opposing directions in the effects of the agonist and antagonist provide additional support that CRFR2 modulates OT release in the NAc shell. Indeed, OT concentrations were significantly higher relative to baseline in the stresscopin microdialysates compared to the astressin-2b 30 min post drug infusion (α=0.008; p=0.007 at 30 min; unpaired t-test, one tailed).
Figure 4.
Central CRFR2 manipulations affected local OT release within the NAc shell of male prairie voles
Local release of OT within the NAc shell of male prairie voles did not differ between the groups over time, whereas separate statistics for each group revealed that a single acute central infusion of CRFR2 antagonist (astressin-2B) and of CRFR2 agonist (stresscopin) increased and decreased, respectively, intra-NAc shell OT release.
Numbers of animals included in the statistics were n = 6 per group. Data are expressed as percentage of baseline (mean of sample 1 & 2: equal 100%; dotted line) + sem. * p ≤ 0.05 vs sample 2 of same group.
3.7 Electrophysiological evidence for CRFR2 – OT neuron interaction in the PVN
While the previous experiment related central CRFR2 manipulations to potential changes in local OT release in the NAc shell, we now examined the direct effect of CRFR2 signalling on excitability of the OT neurons within the PVN that project to the NAc. Unfortunately it is not feasible to conduct electrophysiological studies on the OT fibers within the NAcc to ascertain how CRFR2 signalling on those fibers influences local OT dynamics; however this experiment provides an opportunity to assess how CRFR2 signalling may influence neural activity in the populations of OT neurons that are the source of OT fibers in the NAc. The PVN is innervated by fibers containing urocortin 2 and 3, endogenous ligands for CRFR2, likely originating from soma in the BNST and/or the lateral septum (Jamieson et al., 2006; Li et al., 2002).
To identify OT neurons in hypothalamic slice preparations, an AAV vector expressing Venus exclusively in OT neurons was infused into the PVN (Knobloch et al., 2012) (Fig. 5A) enabling visually-guided patch-clamping of putative OT neurons. Therefore our recordings were restricted to neurons expressing Venus that met standard criteria of physiologically responsive neurons.
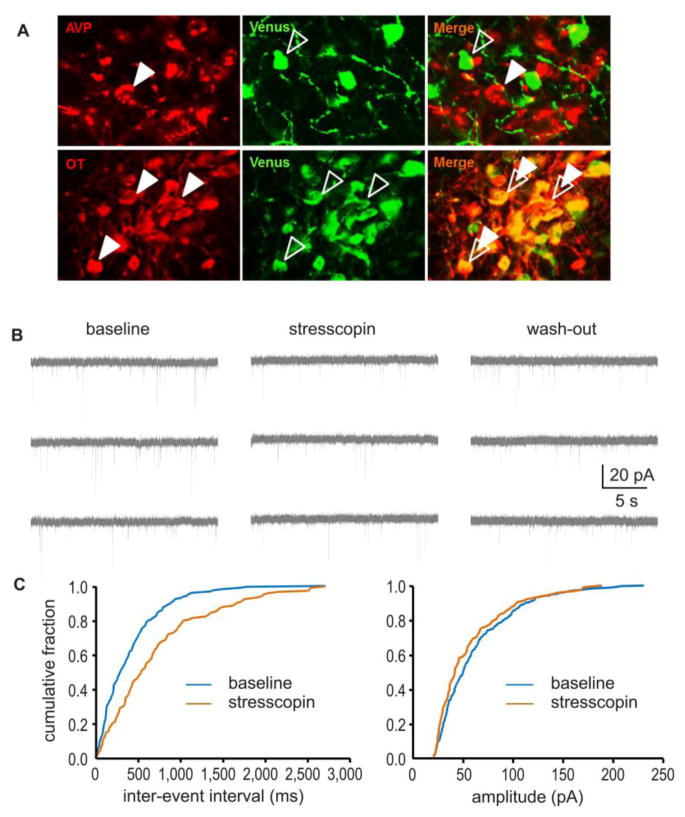
Figure 5.
Central CRFR2 manipulations reduced excitatory drive of oxytocin neurons within the PVN
(A) Photomicrograph showing the selective expression of the fluorescent protein, Venus, exclusively in OT neurons of the PVN. As illustrated by dual-immunofluorescence labelling, Venus-positive neurons (shown in green, open arrows) in the PVN co-localize with OT-positive neurons (top row, shown in red, closed arrows), but not vasopressin (AVP)-positive neurons (bottom row, shown in red, closed arrows). (B): Stresscopin (200 nM) significantly decreased the mean frequency of spontaneous excitatory postsynaptic currents (sEPSCs) in the OT neurons of the PVN, an effect that was partially reversed by wash-out. (C): Quantitative analysis revealed that stresscopin caused a rightward shift of the cumulative probability curve for the inter-event-intervals of the sEPSCs, but had no effect on the cumulative distribution of sEPSC amplitudes.
Stresscopin (200nM) alone did not affect the intrinsic membrane properties of OT neurons, including Rin, tau, ISI1, ISIN, fAHP and sAHP, respectively, and Ih ratio. Stresscopin also did not affect action potential threshold and kinetics, including rise and decay time, half-width, or threshold for action potential generation (Tab. 1), thereby suggesting that stresscopin has no apparent direct postsynaptic effect on putative OT neuron soma in the PVN. Thus this experiment does not provide mechanistic insights into the NAc fiber CRFR2 regulation of OT dynamics.
Table 1.
Intrinsic membrane properties and spike characteristics of Venus positive, putative OT neurons in the vole PVN before, during, and after application of CRFR2 agonist (stresscopin, 200 nM; mean ± SEM).
| IH | IAR | Tau | Rin | ISI1 | ISIN | ISI1/ISIN | fAHP | sAHP | Spike Amplitude |
Half Widths |
Rise Time |
Decay Time |
Threshold | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 0.04 ± 0.01 | 1.45 ± 0.13 | 55.19 ± 11.70 | 747.51 ± 55.99 | 61.36 ± 7.24 | 109.21 ± 11.13 | 0.58 ± 0.04 | −16.02 ± 1.11 | 3.27 ± 0.39 | 66.57 ± 1.92 | 1.38 ± 0.06 | 0.57 ± 0.03 | 1.09 ± 0.04 | −32.75 ± 0.60 |
| Stresscopin | 0.04 ± 0.01 | 1.44 ± 0.18 | 49.63 ± 2.06 | 974.78 ± 44.30 | 56.92 ± 6.74 | 91.68 ± 10.67 | 0.66 ± 0.05 | −16.71 ± 1.18 | 3.75 ± 0.47 | 65.67 ± 2.51 | 1.36 ± 0.07 | 0.57 ± 0.04 | 1.06 ± 0.05 | −32.89 ± 0.80 |
| Wash-out | 0.02 ± 0.01 | 1.18 ± 0.22 | 73.19 ± 15.84 | 1031.53 ± 90.60 | 63.66 ± 12.92 | 85.10 ± 9.67 | 0.71 ± 0.07 | −17.93 ± 2.02 | 2.38 ± 0.48 | 60.09 ± 3.84 | 1.55 ± 0.17 | 0.66 ± 0.07 | 1.17 ± 0.12 | −31.10 ± 1.46 |
Abbreviations: Ih ratio = hyperpolarization-activated non-specific cation current normalized to membrane potential; tau = time constant of membrane charging; Rin = input resistance; ISI1 = first inter-spike-interval; ISIN = last inter-spike-interval; fAHP = fast after-hyperpolarization potential; sAHP = slow after-hyperpolarization potential.
We examined the effect of CRFR2 activation on spontaneous synaptic transmission in OT neurons by recording the frequency and amplitude of sEPSCs in voltage clamp method. Application of stresscopin (200nM, 10min) significantly decreased the mean frequency of sEPSCs (baseline 6.64±1.67Hz; stresscopin 4.76±1.35Hz; Mann–Whitney U-test; p<0.01, n=13), an effect that only partially washed-out (5.83±1.52Hz), but had no effect on sEPSC amplitude (Fig. 5B). Quantitative analysis revealed that stresscopin caused a rightward shift in the cumulative probability curve for the inter-event-intervals of the sEPSCs, further suggesting that CRFR2 activation decreased the frequency of sEPSCs. In contrast, no effect was observed on the cumulative distribution of sEPSC amplitudes (Fig. 5C). Together these data suggest that stresscopin acts primarily presynaptically in the PVNto regulate excitatory drive onto OT neurons. Hence, CRFR2 activation decreases glutamate drive and excitability of the OT neurons. This observation suggest an mechanism independent of CRFR2 action in the NAc by which elevated CRFR2 ligand may compromise OT signaling, potentially decreasing OT release within the PVN.
4. Discussion
In bonded male prairie voles, loss of the female partner increases passive stress-coping (McNeal et al., 2014; Sun et al., 2014), which is indicative of depressive-like behavior (Cryan and Mombereau, 2004; Cryan et al., 2005). Such partner loss-induced passive stress-coping can be prevented by centrally blocking CRFR2 signaling (Bosch et al., 2009). Using a battery of behavioral and physiological tests, the present study further characterized this paradigm and extended it to the OT system. Taken together, our data are consistent with the hypothesis that, in bonded males housed with their partner, CRFR2 signaling in the NAc and possibly other areas is low, and consequently OT signaling in the NAc in response to social interaction is normalized, thereby minimizing passive coping in the FST. In contrast, separation from the female partner results in increased CRFR2 signaling in the NAc shell, which lowers local OT release. In addition, OT synthesis in the PVN and OTR density in the NAc shell is reduced. In parallel, CRFR2 activation possibly in the PVN reduces the glutamatergic drive of PVN OT neurons which could contribute to the reduced OT release in terminal sites. The additive effect of each of these mechanisms leads to compromised OTR signaling in the NAc, thereby increasing passive coping behavior in the FST. Thus the CRF and OT systems interact to maintain a balance between active and passive stress-coping in an opposing manner in response to social stimuli. This homeostasis is, however, disturbed by loss of the bonded female partner, but not by social isolation in non-bonded males.
There is strong evidence that depression may be induced by a dysregulated CRF system as shown in both clinical and animal studies (for reviews see (Bale, 2005; Holsboer and Barden, 1996; Keck, 2006; Nemeroff, 1996)). In prairie voles, the NAc shell receives CRF input (Lim et al., 2007), and the CRF-tone in the BNST is elevated upon pair bonding (Bosch et al., 2009). Interestingly, CRF mRNA expression in the BNST remains increased after partner loss as separation from the female partner is a chronic stressor (Bosch et al., 2009). In rats, chronic stress also leads to selective activation of CRF neurons in the BNST (Dabrowska et al., 2013b). Therefore, the increased CRF-tone in prairie voles starting with mating might prime the CRF system to maximally respond after separation from the partner. During short term separation, this may be an adaptive response to create an aversive state, motivating the male to reunite with his partner, whereas longer separation is a chronic stressor, accompanied by increased passive stress-coping (Bosch et al., 2009). Here we show that this effect is mediated in part via CRFR2 localized on OT fibers in the NAc shell; while neither treatment affected locomotor activity, chronic local blockade of CRFR2 abolished the increased passive stress-coping after partner loss. Conversely, chronic CRFR2 activation in the NAc shell of still-paired male prairie voles increased passive stress-coping. Paradoxically, injections of CRF into the NAc of male prairie voles accelerate partner preference formation (Lim et al., 2007), although CRF signaling is not necessary to maintain a pair bond (Bosch et al., 2009). These findings seem to be in contrast to our present study where CRFR2 in the NAc shell mediated passive stress-coping after partner loss. However, a recent study in rats demonstrated that severe stress can change the effect of CRF in the NAc from engagement with the environment to withdrawal, which was demonstrated by increased immobility in the FST, as also observed in major depressive disorders (Lemos et al., 2012). Therefore, we hypothesize a similar mechanism in our present experiment; the initially prosocial CRF activity in the NAc that facilitates partner preference switches to causing increased passivity in the FST, as separation from the partner is a major stressor.
While pair bond-disruption increases passive stress-coping, a healthy social relationship can buffer against stressors with OT as a main mediator (Kikusui et al., 2006; Smith and Wang, 2014). In prairie voles, the NAc has a high density of OTR and OT-immunoreactive fibers originating from the PVN (Lim et al., 2004). Blocking OTR within the NAc shell prevents partner preference-formation, both in female (Keebaugh et al., 2015; Young et al., 2001) and male prairie voles (Keebaugh and Young, unpublished observations). In addition, in female prairie voles intra-NAc OT levels increase during socio-sexual interaction (Ross et al., 2009), and increasing OTR density in the NAc facilitates partner preference formation (Ross et al., 2009), while OTR knock-down in the NAc inhibits partner preference (Keebaugh et al., 2015). In free-living prairie voles, monogamous males express higher OTR density in the NAc than non-monogamous (Ophir et al., 2012). Here, comparison between male prairie voles bonded to a female or co-housed with a sibling revealed no difference in OTR binding in the NAc shell and core or in OT mRNA in the PVN and SON. However, separation from the female partner, but not from a male sibling, tended to decrease both OTR binding in the NAc shell as well as OT mRNA in the PVN, potentially resulting in a dually compromised OT system, which may contribute to social loss-induced passive stress-coping. That said, a decrease in receptor binding could be interpreted as a reduction in receptor synthesis and protein density, or as the result of receptor inactivation due to heighted ligand signaling. Since there is a simultaneous reduction in OT synthesis, and as OT release is triggered by positive social interactions (for review see Neumann and Landgraf, 2012), we hypothesize that partner loss results in a decrease rather than in an increase of OT release in the NAc shell. Therefore, the parallel decrease in OTR binding in the NAc shell after partner loss is unlikely to be due to a down-regulation or desensitization of OTR following increased OT release. In support of this hypothesis, partner loss-induced passive stress-coping was prevented by chronically supplementing OT levels in the NAc shell during the separation period. Furthermore, mimicking reduced receptor availability in the NAc shell as seen after pair bond separation (chronic OTR-A or Oxtr shRNA) increased passive stress-coping in males still with the partner. These data demonstrate that OTR activation in the NAc shell can buffer against the emotional consequences of partner loss.
In order to integrate our findings on the OT and the CRF system, we directly monitored OT release within the NAc shell via microdialysis after central infusion of CRFR2 agonist or antagonist in naïve male prairie voles. Our results suggest that blocking and activating CRFR2, which are present on OT fibres in the NAc shell, increases and decreases, respectively, the local basal release of OT. Therefore, the increased passive stress-coping after partner loss is likely to be mediated by activation of CRFR2 causing decreased release of OT in the NAc shell, thereby potentiating the negative effect of separation. This was prevented by blocking CRFR2, causing increased OT levels in the NAc shell, which buffered against the increased passive stress-coping. It should be noted that in our microdialysis study, CRFR2 (ant-)agonists were injected icv due to technical limitations; placing an infusion cannula along with a microdialysis probe into the NAc shell of a prairie vole brain could damage a lot of the tissue, thereby affecting the experimental outcome. Hence, we cannot exclude that CRFR2 activation outside of the NAc shell contributed to the decreased OT release.
We further showed that stresscopin decreased the frequency of the spontaneous excitatory synaptic activity in OT neurons in the PVN. This suggests a pre-synaptic CRFR2-mediated mechanism, while others have also reported direct postsynaptic effect of stresscopin in PVN neurons (Chu et al., 2013). Nonetheless, as the firing rate of OT neurons fluctuates as a function of their synaptic input (Leng et al., 2005), the stresscopin effect in the PVN might lead to an indirect decrease in OT release in terminal structures, including the NAc shell. Furthermore, the CRFR2-mediated reduction of the excitatory drive onto OT neurons might act to modulate the response of these neurons to any salient sensory input (i.e. grief) and modulate the release in terminal sites. However, there is a possibility that the effect in the PVN is independent from the effects we have observed in NAc, as CRFR2 activation on local OT fibers may have a direct inhibitory effect on OT release in NAc. Nonetheless, taken together, our data clearly demonstrate multiple points of interaction between the CRFR2 and OT systems in mediating the passive stress-coping after partner loss.
In summary, forming a pair bond causes fundamental changes in the brain, which profoundly sensitize the neural and behavioral response to partner loss as we observed in a battery of behavioural and physiological tests. Those changes might be severe enough to increase mortality in nature and even explain why 80% of animals that loose a partner never find a new partner again (Carter et al., 1995). But it is important to mention that while our separation paradigm allows us to study important individual components of depression and bereavement, further characterization is be needed to validate it as model of bereavement or grieving. However, data from us and others suggest that OT not only plays a role in pair bond formation, but continual OT signalling in the presence of the partner facilitates active coping strategies. Brief separations induce negative affect, thereby motivating reuniting with the partner. Chronic partner-separation, however, activates multiple mechanisms that effectively clamp OTR signalling, including decreased OT synthesis in the PVN, downregulation of OTR in the NAc, and CRFR2 mediated reduction in OT release. Within the PVN, CRFR2 activation decreases the excitatory drive to OT neurons and, consequently, the probability of firing action potentials, which presumably reduces OT release in terminal sites. It is unclear how CRFRs act locally within the NAc shell to affect the OT system, but since CRFR2 immunoreactivity in the NAc shell is restricted to OT fibers, we suspect that CRFR agonists (either CRF, urocortin 2 or urocortin 3, the latter being an N-terminally extended analog of stresscopin) inhibit dense core vesicle mobilization and release from the large caliber, unmyelinated OT axons that course through the NAc shell. While we did not detect any evidence that CRFR2 activation has direct postsynaptic effects on OT neuron properties in the PVN, we did discover a presynaptic CRFR2-driven mechanism that leads to reduction of excitatory drive onto OT neurons. Therefore, the CRFR2 and the OT systems play an integral role in maintaining pair bonds by titrating aversive responses to separations from the bonded partner, thus both systems may be viable targets for moderating the debilitating effects of partner loss.
Supplementary Material
Highlights.
Male prairie voles show high passive stress-coping after loss of female partner
This is mediated via activation of CRFR2 in nucleus accumbens shell
Chronic activation of CRFR2 is capable of impairing the oxytocin system
The oxytocin system gets compromised at multiple levels
Suppression of oxytocin signaling is likely to encourage reunion with the partner
Acknowledgments
Funding
This research was supported by NIH Grants R01MH-077776, R01MH096983, and 1P50MH100023 to LJY, R00MH-096746 and start-up funds of the Chicago Medical School, Rosalind Franklin University of Medicine and Science to JD. CEB was supported by a US NSF Graduate Research Fellowship. Additional funding was provided by the NIH Office of Research Infrastructure Programs/OD P51OD11132 to YNPRC and NINDS P30NS055077 to the Emory Neuroscience Viral Vector Core Facility. VG was supported by the German Research Foundation grants GR 3619/2-1, GR 3619/3-1, GR 3619/4-1 and by the Chica & Heinz Schaller Research Foundation.
The authors would like to thank Lorra Mathews, Pravina Fernandez, Desirée De Leon, as well as Kathrin Bohrer, Martina Fuchs and Gabriele Schindler for their excellent technical support. Furthermore, we thank Stefanie Klampfl for expert statistical support and Steve Ryan for help with electrophysiology data analysis. This research was supported by NIH Grants R01MH-077776, R01MH096983, and 1P50MH100023 to LJY, R00MH-096746 and start-up funds of the Chicago Medical School, Rosalind Franklin University of Medicine and Science to JD. CEB was supported by a US NSF Graduate Research Fellowship. Additional funding was provided by the NIH Office of Research Infrastructure Programs/OD P51OD11132 to YNPRC and NINDS P30NS055077 to the Emory Neuroscience Viral Vector Core Facility. VG was supported by the German Research Foundation grants GR 3619/2-1, GR 3619/3-1, GR 3619/4-1 and by the Chica & Heinz Schaller Research Foundation.
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
Contributors
Oliver J. Bosch oliver.bosch@ur.de, Department of Behavioural and Molecular Neurobiology, Institute of Zoology, University of Regensburg, 93053 Regensburg, Germany
Joanna Dabrowska joanna.dabrowska@rosalindfranklin.edu, Department of Cellular and Molecular Pharmacology, Department of Neuroscience, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, IL 60064-3095, USA
Meera E. Modi Meera.Modi@pfizer.com, Center for Translational Social Neuroscience, Department of Psychiatry and Behavioral Sciences, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA. Current address: Pfizer Neuroscience Research Unit, Pfizer Inc. Cambridge, MA 02139, USA
Zachary V. Johnson zjohnso2@gmail.com, Center for Translational Social Neuroscience, Department of Psychiatry and Behavioral Sciences, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA, Silvio O. Conte Center for Oxytocin and Social Cognition, Emory University, Atlanta, GA 30322, USA
Alaine C. Keebaugh akeebaugh@mriinterventions.com, Center for Translational Social Neuroscience, Department of Psychiatry and Behavioral Sciences, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA
Catherine E. Barrett cbarrett27@gmail.com, Center for Translational Social Neuroscience, Department of Psychiatry and Behavioral Sciences, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA, Silvio O. Conte Center for Oxytocin and Social Cognition, Emory University, Atlanta, GA 30322, USA
Todd H. Ahern todd.h.ahern@gmail.com, Center for Translational Social Neuroscience, Department of Psychiatry and Behavioral Sciences, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA. Current address: Center for Behavioral Neuroscience, Department of Psychology, Quinnipiac University, Hamden, CT 06518, USA
JiDong Guo Guo, jguo4@emory.edu, Center for Translational Social Neuroscience, Department of Psychiatry and Behavioral Sciences, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA, Silvio O. Conte Center for Oxytocin and Social Cognition, Emory University, Atlanta, GA 30322, USA
Valery Grinevich v.grinevich@dkfz-heidelberg.de, Schaller Research Group on Neuropeptides, German Cancer Research Center DKFZ, Cell Network Cluster of Excellence, University of Heidelberg, 69120 Heidelberg, Germany
Donald G. Rainnie drainni@emory.edu, Center for Translational Social Neuroscience, Department of Psychiatry and Behavioral Sciences, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA, Silvio O. Conte Center for Oxytocin and Social Cognition, Emory University, Atlanta, GA 30322, USA, Inga D. Neumann, Department of Behavioural and Molecular Neurobiology, Institute of Zoology, University of Regensburg, 93053 Regensburg, Germany
Larry J. Young lyoun03@emory.edu, Center for Translational Social Neuroscience, Department of Psychiatry and Behavioral Sciences, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA, Silvio O. Conte Center for Oxytocin and Social Cognition, Emory University, Atlanta, GA 30322, USA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (microtus ochrogaster) Frontiers in behavioral neuroscience. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm Behav. 2013;63:518–526. doi: 10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF. The role of social relations in health promotion. Psychosom Med. 1995;57:245–254. doi: 10.1097/00006842-199505000-00006. [DOI] [PubMed] [Google Scholar]
- Biondi M, Picardi A. Clinical and biological aspects of bereavement and loss-induced depression: a reappraisal. Psychother Psychosom. 1996;65:229–245. doi: 10.1159/000289082. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL. Monogamy and the prairie vole. Sci Am. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- Chen YW, Rada PV, Butzler BP, Leibowitz SF, Hoebel BG. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–166. doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Chu CP, Jin WZ, Bing YH, Jin QH, Kannan H, Qiu DL. Effects of stresscopin on rat hypothalamic paraventricular nucleus neurons in vitro. PloS one. 2013;8:e53863. doi: 10.1371/journal.pone.0053863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton PJ, Darvish HS. Course of depressive symptoms following the stress of bereavement. In: Barrett J, Rose RM, Kleman GL, editors. Stress and Mental Disorder. Raven Press; New York: 1979. pp. 121–136. [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG. Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Frontiers in neuroscience. 2013a;7:156. doi: 10.3389/fnins.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, Li C, Dewitt S, Xu J, Lombroso PJ, Rainnie DG. Striatal-enriched protein tyrosine phosphatase-STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stria terminalis. Biological psychiatry. 2013b;74:817–826. doi: 10.1016/j.biopsych.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology. 2002;27:705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- Hensley PL, Slonimski CK, Uhlenhuth EH, Clayton PJ. Escitalopram: an open-label study of bereavement-related depression and grief. J Affect Disord. 2009;113:142–149. doi: 10.1016/j.jad.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Barden N. Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocrine reviews. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Li C, Kukura C, Vaughan J, Vale W. Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology. 2006;147:4578–4588. doi: 10.1210/en.2006-0545. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Current Opinion in Behavioral Sciences. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME. Corticotropin-releasing factor, vasopressin and receptor systems in depression and anxiety. Amino acids. 2006;31:241–250. doi: 10.1007/s00726-006-0333-y. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, LaPrairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Social neuroscience. 2015;7:1–10. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Caquineau C, Sabatier N. Regulation of oxytocin secretion. Vitamins and hormones. 2005;71:27–58. doi: 10.1016/S0083-6729(05)71002-5. [DOI] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, Young LJ. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm Behav. 2007;51:508–515. doi: 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) The Journal of comparative neurology. 2004;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Lim MM, Nair HP, Young LJ. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. The Journal of comparative neurology. 2005;487:75–92. doi: 10.1002/cne.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti MA, Wardwell J, Chandler DL, Bates SL, Larocca M, Trahanas DM, Grippo AJ. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Autonomic neuroscience : basic & clinical. 2014;180:9–16. doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1:336–342. [PubMed] [Google Scholar]
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends in neurosciences. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng DJ, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm Behav. 2012;61:445–453. doi: 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 2014;42:225–236. doi: 10.1016/j.psyneuen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Ramsay S, Ebrahim S, Whincup P, Papacosta O, Morris R, Lennon L, Wannamethee SG. Social engagement and the risk of cardiovascular disease mortality: results of a prospective population-based study of older men. Annals of epidemiology. 2008;18:476–483. doi: 10.1016/j.annepidem.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Aragona BJ. Aversive motivation and the maintenance of monogamous pair bonding. Reviews in the neurosciences. 2013;24:51–60. doi: 10.1515/revneuro-2012-0068. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear K, Shair H. Attachment, loss, and complicated grief. Dev Psychobiol. 2005;47:253–267. doi: 10.1002/dev.20091. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nature protocols. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biological psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, Wang Z. Breaking bonds in male prairie vole: Long-term effects on emotional and social behavior, physiology, and neurochemistry. Behavioural brain research. 2014 doi: 10.1016/j.bbr.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic C, Radulovic J, Jahn O, Radulovic M, Sherrin T, Hippel C, Spiess J. Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety. The European journal of neuroscience. 2007;25:3385–3397. doi: 10.1111/j.1460-9568.2007.05592.x. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zisook S, Paulus M, Shuchter SR, Judd LL. The many faces of depression following spousal bereavement. J Affect Disord. 1997;45:85–94. doi: 10.1016/s0165-0327(97)00062-1. discussion 94–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.