Abstract
A small subpopulation of pancreatic cancer cells with characteristics of stem cells drive tumor initiation, progression and metastasis. A better understanding of the regulation of cancer stem cells may lead to more effective cancer prevention and therapy. We have shown that the proliferation and migration of pancreatic cancer cell lines is activated by the nicotinic receptor-mediated release of stress neurotransmitters, responses reversed by γ-aminobutyric acid (GABA). However, the observed cancer inhibiting effects of GABA will only succeed clinically if GABA inhibits pancreatic cancer stem cells (PCSCs) in addition to the more differentiated cancer cells that comprise the majority of cancer tissues and cell lines. Using PCSCs isolated from two pancreatic cancer patients by cell sorting and by spheroid formation assay from pancreatic cancer cell line Panc-1, we tested the hypothesis that nicotine induces the self-renewal of PCSCs. Nicotinic acetylcholine receptors (nAChRs) α3, α4, α5 and α7 were expressed and chronic exposure to nicotine increased the protein expression of these receptors. Immunoassays showed that pancreatic cancer stem cells produced the stress neurotransmitters epinephrine and norepinephrine and the inhibitory neurotransmitter GABA. Chronic nicotine significantly increased the production of stress neurotransmitters and sonic hedgehog (SHH) while inducing Gli1 protein and decreasing GABA. GABA treatment inhibited the induction of SHH and Gli1. Spheroid formation and MTT assays showed significant nicotine-induced increases in self renewal and cell proliferation, responses blocked by GABA. Our data suggest that nicotine increases the SHH-mediated malignant potential of pancreatic cancer stem cells and that GABA prevents these effects.
Keywords: Nicotinic receptors, cancer stem cells, GABA, stress neurotransmitters, sonic hedgehog, pancreatic cancer
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer deaths in developed countries due to its high mortality within one year of diagnosis 4. Smoking is a documented risk factor for PDAC 19. However, the mechanisms responsible for this association are poorly understood.
Emerging evidence suggests that a subpopulation of cancer stem cells drives tumor initiation, progression and metastasis of PDAC 17, 23. A better understanding of the regulation of pancreatic cancer stem cells (PCSCs) may thus lead to the development of more effective PDAC prevention and therapy. However, cancer stem cells only constitute up to 5% of cells in pancreatic cancer tissue and pancreatic cancer cell lines 5, 29. Data genrated in cancer cell lines and their xenografts thus represent mostly the reactions of the more differentiated cancer cells while responses of the small stem cell population may remain obscure.
With the discovery of methods for the isolation of PCSCs from tumor tissue and cell lines 5, 29, the sonic hedgehog (SHH) pathway has emerged as a key regulator of PCSCs 12, 14, 22, 27. Overexpression of SHH and its downstream effector, Gli1, is associated with a poor overall survival of PDAC patients 21 and the SHH pathway is among recently explored therapeutic targets for PDAC 16. However, a first pilot clinical trial with an SHH inhibitor alone or in combination with gemcitabine failed to improve clinical outcomes in PDAC patients 15. Similarly, strategies that target signaling pathways overexpressed in more differentiated PDAC cells alone or in combination with conventional cancer therapeutics have disappointed in clinical trials 20. It hence appears that therapeutic strategies need to simultaneously target regulatory pathways in differentiated cancer cells as well as PCSCs to become more successful.
We have shown that pancreatic duct epithelial cells and PDAC cell lines express an autocrine neurotransmitter loop that is regulated by nicotinic acetylcholine receptors (nAChRs) 2, 3. Nicotine increased the proliferation and migration of these cells by stimulating the synthesis and release of the stress neurotransmitters norepinephrine and epinephrine, which in turn activated multiple signaling cascades downstream of beta-adrenergic receptors 2, 3. In addition, beta-adrenergic receptor agonists increased cell proliferation and migration of PDAC cell lines in vitro in a cAMP dependent manner 13, 25, 31. We have shown that nicotine treated mice carrying PDAC xenografts demonstrated increased systemic and tumor levels of norepinephrine, epinephrine and cAMP accompanied by significant increases in xenograft sizes 1. These responses were abrogated by treatments in vitro and in the mouse model with the inhibitory neurotransmitter γ-aminobutyric acid (GABA) via Gαi-mediated inhibition of cAMP formation 1, 2, 25. While the reported tumor inhibiting effects of GABA are promising, they would only translate into successful therapeutic applications in PDAC patients if in addition to the more differentiated cells the self-renewal of PCSCs were also inhibited. However, neither the effects of nicotine nor those of stress neurotransmitters or GABA on PCSCs have been studied to date.
PCSCs have the unique ability to self-renew and form differentiated progeny 17, 23, 29. The maintenance of cancer cells in serum free medium selects for the self-renewal of cancer stem cells as three dimensional floating aggregates (spheroids), a method widely used to generate cell populations enriched in cancer stem cells from cancer cell lines 6, 7, 9. Spheroid formation assays and cell sorting by stem cell markers are both commonly used to isolate cancer stem cells from tumor tissues and cell lines 8, 23, 29. Using PCSCs isolated by cell sorting and PCSCs enriched by spheroid formation assays, the current study has tested the hypothesis that nicotine induces the self-renewal of PCSCs by modulating the autocrine production of regulatory neurotransmitters and that this response can be reversed by treatment with GABA.
Materials and Methods
Cell culture
The human PDAC cell line Panc-1 was purchased from the American Type Culture Collection (Manassas, VA, USA) and was authenticated at the end of the experiments by species-specific PCR in March 2015 (IDEXX BioResearch, Columbia, MO, USA). Two batches of PCSCs isolated from different donors by cell sorting were purchased from Celprogen (San Pedro, CA, USA). The purchased PCSCs expressed the stem cell markers CD133, CD44, SSEA3/4, Oct4, alkaline phosphatase, aldehyde dehydrogenase, telomerase and nestin. Cancer stem cells isolated from cell line Panc-1 by spheroid formation assay express the stem cell markers CD133, CD44, Oct4, nestin and CD24 30, 32. Monolayers of Panc-1 cells comprised of mostly differentiated cells were maintained in DMEM medium supplemented with 10 % Fetal Bovine Serum (FBS). Monolayers of the purchased PCSCs comprised of mixed populations of differentiated and cancer stem cells were maintained in complete (containing fetal bovine serum) cancer stem cell growth medium provided by the vendor (Celprogen). Enrichment of stem cells from cell line Panc-1 and the sorted PCSCs was accomplished by maintenance of the cells in serum-free spheroid formation media (Celprogen) consisting of 1:1 proportion of 1X DMEM/F12 and cell specific basal media (without FBS) supplemented with B-27, N-2, glutamax and beta-mercaptoethanol (Invitrogen Life Technologies, Grand Island, NY, USA). All cells were maintained without antibiotics in an atmosphere of 5 % CO2, 99 % relative humidity, and 37 °C.
Spheroid formation assays
Single cell suspensions (1,000 cells/well) were plated in ultra-low adherent 6-well plates (Corning Inc., Corning, NY, USA) in serum-free spheroid formation media to assess PCSC self-renewal. All treatments were started 24 hours after plating of the cells and continued daily for 15 days with subculture of spheroids after 7 days (nicotine: 1 μM/L (−)-nicotine hydrogen tartrate, Sigma-Aldrich, St. Louis, MO, USA; GABA: 30 mM</L, Sigma-Aldrich). This concentration of nicotine is within the range of plasma nicotine levels in smokers 24. The generated spheroids were counted and photographed using a Nexcelom cellometer. The proportion of spheroid-generating cells for each treatment group was calculated by dividing the counted number of spheroids by the number of cells seeded (1000 cells).
MTT assays
Following instructions of the vendor, the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide) colorimetric assay (Sigma-Aldrich) was used to assess the number of viable cells as an indicator of cell proliferation in spheroids. The spheres were either left untreated or treated with 1 μM/L nicotine, 30 μM/L GABA or nicotine + GABA for 7 days. Absorbance values of the collected spheres from all treatment groups were read using an uQuant Bio-Tek Instrument ELISA reader at 570 nm primary and 650 nm reference wavelengths.
Western blotting
The protein expression of nAChR subunits α3, α4, α5 and α7 and of the transcription factor Gli1 was determined by western blots as previously described 2. Protein samples were collected using lysis buffer (RIPA Buffer 1X; Halt Protease Inhibitor Single-Use Cocktail, EDTA-Free (100X); 10 μl of 100 mM phenylmethylsulfonylfluoride / ml RIPA; 10 μl of 100 mM Na3VO4 / ml RIPA; 10 μl of 1 M NaF / ml RIPA; Thermo Scientific, Rockford, IL, USA). After heat denaturation, protein samples were electrophoresed using 12 % SDS gels (Invitrogen) and were then blotted onto membranes. The membranes were blocked (5 % nonfat dry milk solution in 1X TBST) for one hour at room temperature, incubated overnight at 4 °C with the following primary antibodies: anti-nAChR subunits α3 (57 kDA, Abcam, Cambridge, MA, USA), α4 (55 kDa, Millipore, Billerica, MA, USA), α5 (53 kDA, Abcam) and α7 (56 kDA, Abcam) or anti-Gli1 (118 kDa, Santa Cruz Biotechnologies, Dallas, TX, USA). The primary antibody β-actin (42 kDa, Abcam) was used as a loading control. All membranes were then washed (0.5 % Tween 20 / TBS) and incubated with secondary antibodies for two hours. Protein bands were visualized with enhanced chemiluminescence reagent (Pierce ECL Western Blotting Detection Substrate, Thermo Scientific. Following background subtraction, mean densities of 3 rectangular areas of standard size per band from three independent westerns were determined using NIH ImageJ software and mean values and standard deviation (n = 9) of protein expression were calculated.
Real-time PCR
Having previously identified the expression of nAChRs with subunits α3, α4, α5 and α7 in PDAC cell lines and in immortalized pancreatic duct epithelial cells 3, the mRNA levels of these receptors were assessed in untreated and nicotine treated (1 μM/L for 7 days) spheroids by real-time PCR as previously described 26 using a Cepheid SmartCycler. RNA samples from each group were isolated using an Absolutely RNA Miniprep kit (Agilent Technologies, Santa Clara, CA, USA). The collected RNA samples along with the QuantiTect Primer assays (Qiagen, Germantown, MS, USA) for genes CHRNA3 (NM_000743), CHRNA4 (NM_000744), CHRNA5 (NM_000745) and CHRNA7 (NM_000746) were used in the QuantiFast SYBR Green PCR kit to quantify mRNA expression levels of nAChR subunits α3, α4, α5 and α7. Detection reagent for 18S rRNA (Eurogenetec, San Diego, CA, USA) served for normalization. Data were analyzed using the 2−ΔΔCT method 18.
Determination of neurotransmitters and SHH
Spheroids were treated for 7 days (nicotine: 1 μM/L; GABA: 30 μM/L). Untreated control and treated spheroids were then harvested into 1.5 ml Eppendorf tubes (5 samples per group) after a one time wash with warm 1X PBS. Norepinephrine, epinephrine (Kat Elisa kits, Rocky Mountain Diagnostics, Colorado Springs, CO, USA), GABA (GABA Research Elisa kit, Rocky Mountain Diagnostics Inc.) or SHH (sonic hedgehog human ELISA kit, Abcam) were determined by ELISA assays per instructions by the vendors. Absorbance of samples was read using an uQuant Bio-Tek Instrument ELISA reader at 450 nm primary wavelength with a 630 nm reference wavelength.
Statistical analysis
GraphPad Instat 3 and Graphpad Prism 6 software (GraphPad Instant Biostatistics, San Diego, CA, USA) was used for the statistical evaluation of data. Statistical tests used included non-parametric one way ANOVA, non-parametric Mann-Whitney test, two-tailed t-test and F-test for comparison of dose-response curves. Densitometry data of western blots are expressed as mean values and standard deviations of 3 density determinations per band from three independent westerns per antibody (n = 9). Dose –response curves of GABA on SHH production in the presence and absence of nicotine (1 μM) are expressed as mean values and standard errors normalized to untreated controls of triplicate samples that were fitted to non-linear regression curves. All other data are expressed as mean values and standard deviations of five samples per treatment group.
Results
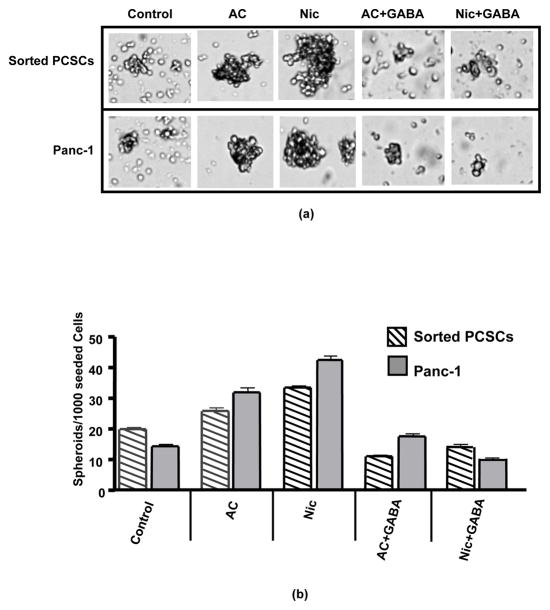
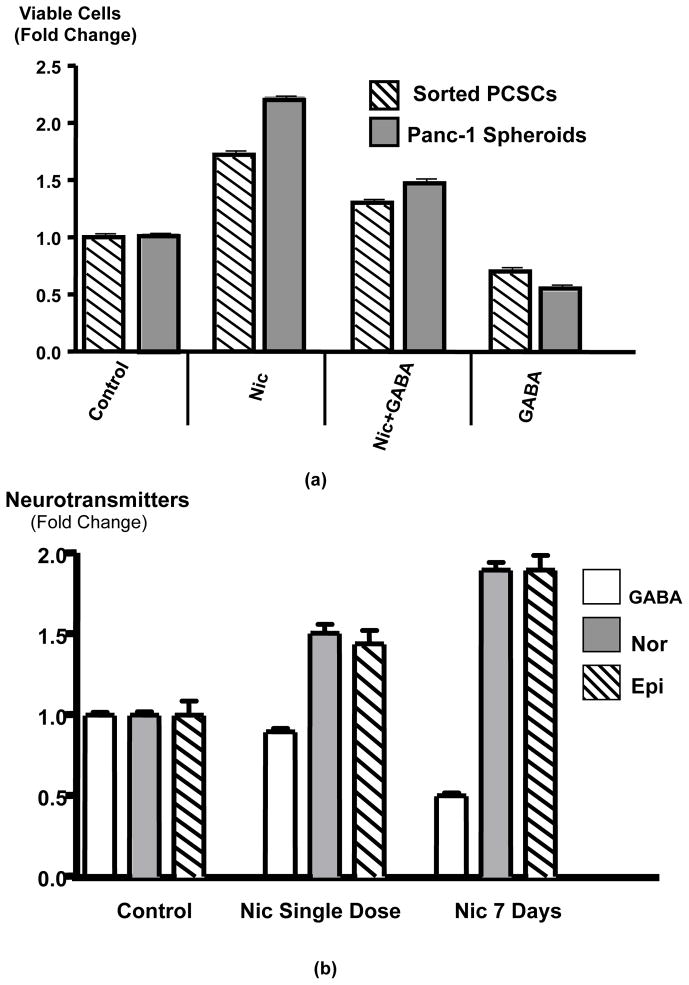
Assessment of cancer stem cell self-renewal by spheroid formation assays showed significant (p < 0.0001) increases in the number of spheroids formed during 14 days of daily exposure to nicotine (Figures 1a, b). Simultaneous treatment with GABA completely (p < 0.0001) blocked this response to nicotine (Figure 1a, b). As exemplified in Figure 1a, the increases in spheroid numbers were associated with increases in spheroid sizes while spheroids were smaller when their numbers decreased. In accord with this observation, exposure of spheroids to nicotine for 7 days significantly (p < 0.001) increased the number of viable cells suggesting enhanced cell proliferation in spheroids (Figure 2a). GABA significantly (p < 0.001) inhibited the nicotine-induced increases in viable cells and additionally significantly (p < 0.001) reduced the number of viable cells below that observed in control spheroids when administered as a single agent (Figure 2a).
Figure 1.
(a) Assessment of cancer stem cell self-renewal by spheroid formation assays of 15 days duration with Panc-1 cells and sorted PCSCs showed that daily treatment with acetylcholine (10 μM/L) or nicotine (1 μM/L) during the entire assay period significantly (p < 0.0001) increased the number of spheroids and these responses were completely blocked by simultaneous treatment with GABA (30 μM/L).
(b) Columns in the graph represent mean values and standard deviations of spheroids per 1000 seeded cells from 5 samples per treatment group.
Figure 2.
(a) The number of viable cells in spheroids as an indicator of PCSCs proliferation was determined by MTT assay. In accord with the larger spheroid sizes after nicotine treatment (Figure 1), exposure of spheroids for 7 days to nicotine (1 μM/L) significantly (p < 0.001) increased the number of viable cells, a response significantly (p < 0.001) inhibited by treatment with GABA (30 μM/L). GABA treatment alone significantly (p < 0.001) reduced the number of viable cells below control levels. Data are mean values and standard deviations of 5 samples per treatment group.
(b) Results of ELISA assays for the assessment of total (intracellular plus secreted) norepinephrine (Nor), epinephrine (Epi) and GABA in PCSC spheroids. Daily treatment of spheroids for 7 days with nicotine (1 μM/L) significantly (p < 0.01) increased the levels of both stress neurotransmitters while reducing GABA. Exposure to a single dose of nicotine for 30 minutes was less effective. Data are mean values and standard deviations of 5 samples per treatment group.
As exemplified in Figure 2b, PCSCs enriched in spheroids produced the neurotransmitters norepinephrine, epinephrine and GABA. Nicotine significantly (p = 0.0079) increased the production of both cancer stimulating stress neurotransmitters significantly (p = 0.0079) when administered as a single dose for 30 minutes (Figure 4) and these responses were enhanced further when identical doses of nicotine were administered daily for 7 days (Figure 2b). By contrast, the production of inhibitory GABA was significantly reduced by chronic nicotine (p = 0.0079), whereas a single dose of nicotine did not significantly change GABA levels (Figure 2b).
Figure 4.
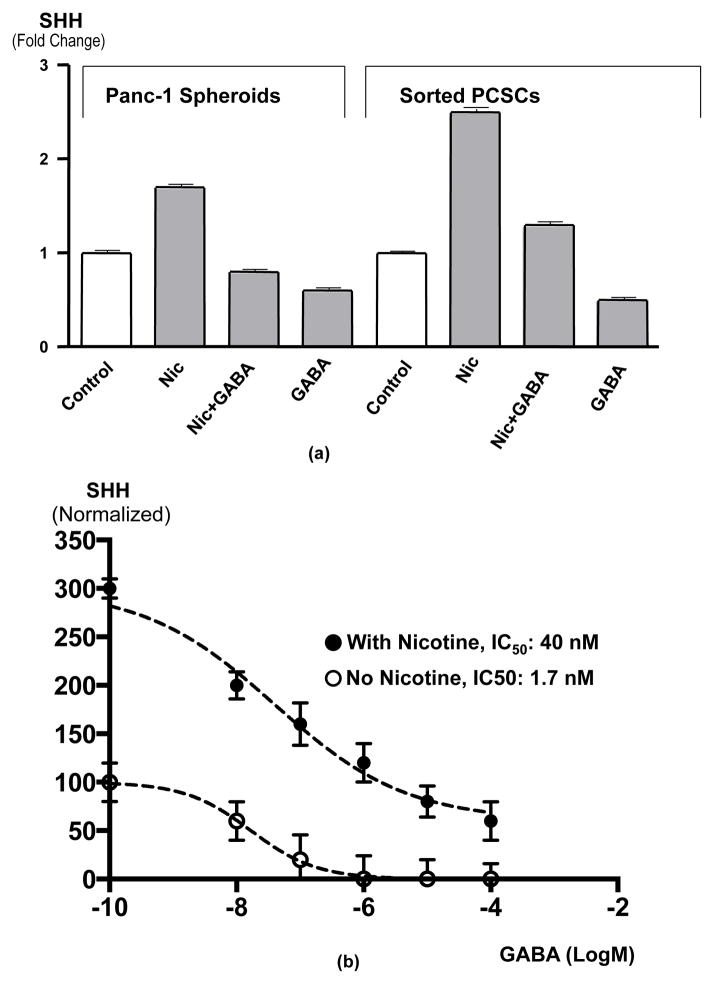
(a) Results of ELISA assays for the detection of total (intracellular plus secreted) sonic hedgehog (SHH) in spheroids from Panc-1 and sorted PCSCs. Exposure of spheroids for 7 days to nicotine (1 μM/L) significantly (p < 0.003) increased SHH levels. This response was completely blocked by simultaneous treatment for 7 days with GABA (30 μM/L) while GABA alone significantly (p < 0.003) reduced SHH below control levels. Data are mean values and standard deviations of 5 samples per treatment group.
(b) Dose-response curves for GABA-induced SHH inhibition in Panc-1 spheroids in the presence and absence of 7-day nicotine (1 μM). The IC50 in the presence of nicotine was 23.5 times higher than in the absence of nicotine, emphasizing the antagonistic effects of nicotine and GABA on SHH production. Data points are mean values and standard deviations of triplicate samples normalized to controls with untreated controls set as 100% and maximum inhibition of non-nicotine exposed samples set as 0%. The curves and IC50 values were determined by nonlinear regression analysis.
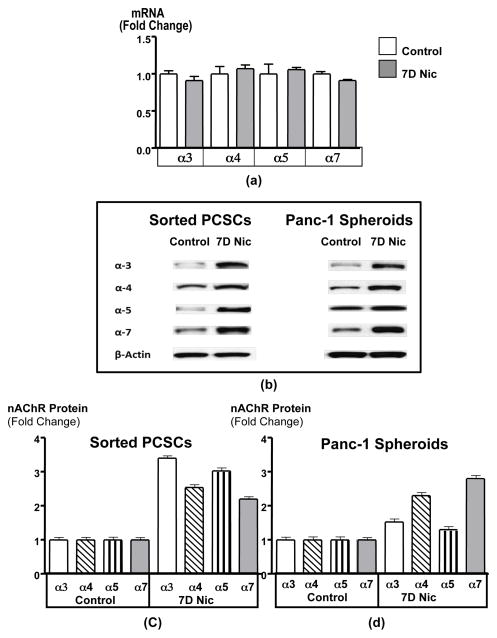
Real-time PCR revealed the expression of mRNA for nAChR subunits α3, α4, α5, and α7 in spheroids (Figure 3d). In accord with the behavior of these receptors in the brain 10 and in the predominantly differentiated cells represented in monolayers of pancreatic cancer cell lines 2, exposure to nicotine did not significantly change the expression levels of their mRNA (Figure 3d). However, as shown in Figures 3a–3c, the protein expression of α3, α4 and α7 nAChRs was significantly (p < 0.0001) increased by chronic nicotine in sorted cells and spheroids.
Figure 3.
(a) Real-time PCR showed that the mRNA levels of nAChR receptor subunits α3, α4, α5 and α7 were expressed in spheroids but did not significantly change in response to treatment for 7 days with nicotine (1 μM/L), suggesting that the upregulations in receptor protein determined by Western blots (b) in these spheroids were caused by post-translational mechanisms. Densitometry values of western blots (c and d) revealed that protein upregulation of nAChR subunits a3, 4 and 7 were significant (p < 0.0001). Columns in graphs c and d represent the mean values and standard deviations of 3 densitometric readings per band from three independent Western blots.
PCSCs in spheroids synthesized and released SHH as detected by ELISA assays. Total SHH (intracellular plus secreted) was significantly (p = 0.0286) increased in spheroids from both sources (Figure 4a) by chronic nicotine. This response was significantly (p = 0.0286) inhibited by simultaneous treatment with GABA (Figure 4a). In addition, exposure to GABA alone significantly (p = 0.0286) decreased SHH production below the levels observed in untreated control spheroids (Figure 4a). As shown in Figure 4b, the inhibition of SHH by GABA was concentration-dependent. Analysis of the data by nonlinear regression revealed that the IC50 of GABA in the presence of nicotine was almost 40 times higher than in the absence of nicotine (Figure 4b).
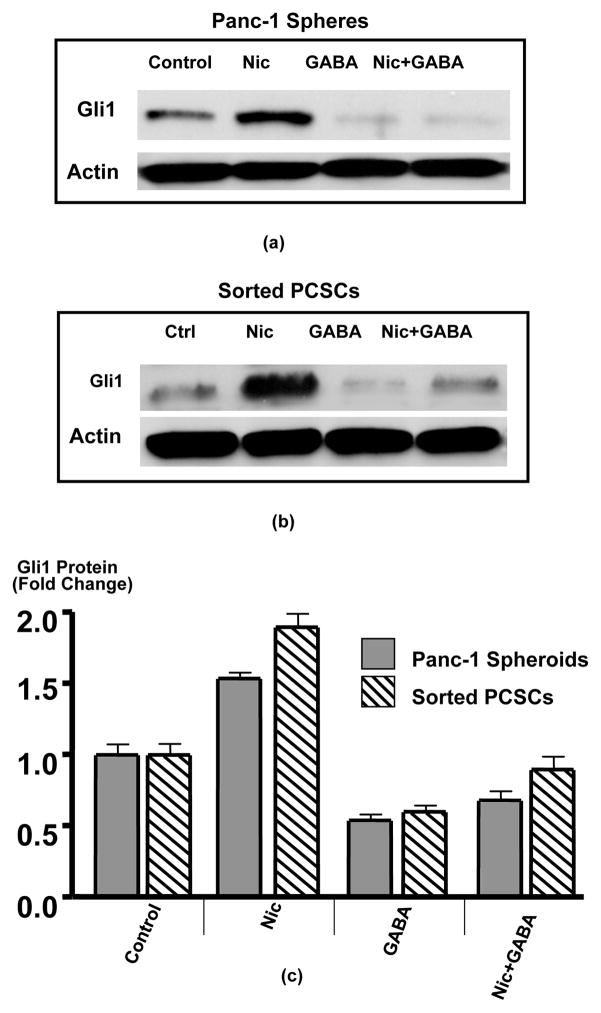
In accord with the documented function of the Gli1 transcription factor as an effector of SHH signaling 14, Western blots revealed significant (p < 0.001) increases in Gli1 protein in spheroids treated for 7 days with nicotine (Figures 5a–c)). This response was completely abrogated (p < 0.001) by simultaneous exposure to GABA while GABA treatment alone significantly (p < 0.001) reduced Gli1 protein below the levels observed in control spheroids (Figures 5a–c).
Figure 5.
Western blots showing protein expression of Gli1 in spheroids from Panc-1 (a) and sorted PCSCs (b). Nicotine (1μM/L) for 7 days significantly (p < 0.001) induced Gli1 protein, a response completely blocked by simultaneous treatment with GABA (30 μM/L) GABA alone significantly (p < 0.001) reduced Gli1 protein below control levels. Columns in the graph (c) are mean values and standard deviations of 3 densitometric readings per band from 3 independent Western blots.
Discussion
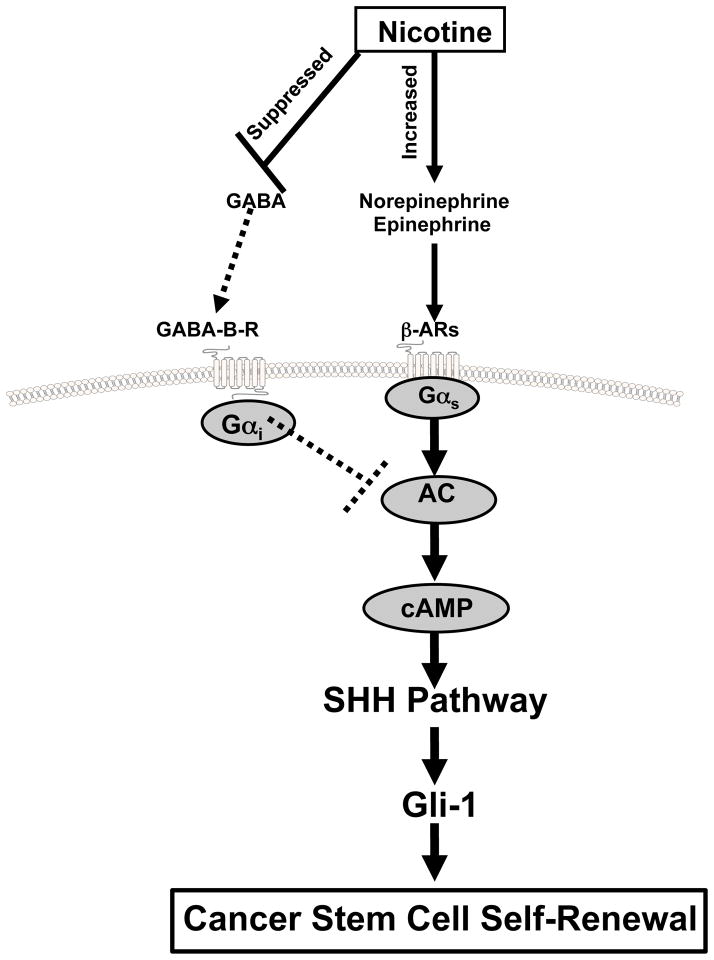
Our data show, for the first time, that nicotine induces the self-renewal of PCSCs derived from pancreatic ductal adenocarcinomas via increased SHH production caused by a simultaneous increase in stress neurotransmitter production and suppression of GABA (Figure 6). These findings add novel mechanistic insights to the recently reported nicotine-induced PCSC activation and acinar dedifferentiation via down-regulation of the GATA6 gene in a Kras mutated mouse model during initiating events in the development of PDAC 11. Exogenous administration of GABA prevented these effects of nicotine in a concentration-dependent manner, emphasizing the antagonistic effects of this inhibitory neurotransmitter on the adverse effects of nicotine on PCSCs.
Figure 6.
Working model illustrating the putative regulatory pathway in pancreatic cancer stem cells that is stimulated by nicotine and inhibited by GABA. Nicotine simultaneously suppresses GABA while increasing stress neurotransmitter production, resulting in the cAMP-driven hyperactivity of the SHH pathway. Exogenous supplementation with GABA reverses these effects of nicotine.
It is well established that nAChRs are expressed in embryonic stem cells and adult cancer stem cells 33. However, their function in these cells is poorly understood. By contrast, an important regulatory role of the SHH pathway for self renewal of embryonic stem cells and adult cancer stem cells, including PCSCs, has been well documented 14. Interestingly, a potential functional link between neurotransmitter receptors and the SHH pathway in cancer stem cells has not been investigated to date. Our findings that chronic exposure to nicotine significantly increases SHH signaling, self-renewal and proliferation of PCSCs by augmenting SHH production has important clinical implications and are in accord with the reported strong growth stimulation of PDAC xenografts by chronic nicotine, which increased the systemic levels of stress neurotransmitters while reducing GABA 1. In light of the current data and the observation that PCSCs drive the progression of pancreatic cancer 5, these effects of chronic nicotine on xenograft progression was not only caused by the reported activation of multiple signaling pathways in differentiated cancer cells but also by the activation of the SHH pathway in PCSCs. In vitro studies have additionally shown that pancreatic cancer cell lines and immortalized pancreatic duct epithelial cells have the ability to synthesize and release their own norepinephrine, epinephrine and GABA, with the stress neurotransmitters stimulating cell proliferation and migration whereas GABA inhibited 2, 3. As our current data show, the regulatory functions of these neurotransmitters are not limited to the more differentiated PDAC cell populations but are also fully functional in PCSCs.
Exposure to nicotine by smoking or chronic abuse of nicotine replacement products often persists after cancer diagnosis. In these scenarios, PCSCs and differentiated cancer cells are continuously stimulated by increases in stress neurotransmitters and simultaneous decrease in GABA. Our current data in conjunction with our published in vivo findings 1 indicate that reversal of the nicotine-induced GABA deficiency by exogenous GABA supplementation yields high anti-tumorigenic efficiency in pancreatic cancer by inhibiting differentiated cancer cells and PCSCs simultaneously. GABA has been safely used as a nutritional supplement for many years without detectable adverse effects 28. Accordingly, nutritional GABA supplementation may significantly improve clinical outcomes in pancreatic cancer patients.
Acknowledgments
This work was financially supported by NIH grant RO1CA042829 to Hildegard M. Schuller. The funding agency had no role in the study design, collection, analysis and interpretation of data.
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest
References
- 1.Al-Wadei HA, Plummer HK, 3rd, Schuller HM. Nicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter gamma-aminobutyric acid. Carcinogenesis. 2009;30:506–11. doi: 10.1093/carcin/bgp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Wadei MH, Al-Wadei HA, Schuller HM. Effects of chronic nicotine on the autocrine regulation of pancreatic cancer cells and pancreatic duct epithelial cells by stimulatory and inhibitory neurotransmitters. Carcinogenesis. 2012;33:1745–53. doi: 10.1093/carcin/bgs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Wadei MH, Al-Wadei HA, Schuller HM. Pancreatic Cancer Cells and Normal Pancreatic Duct Epithelial Cells Express an Autocrine Catecholamine Loop that Is Activated by Nicotinic Acetylcholine Receptors alpha3, alpha5, and alpha7. Mol Cancer Res. 2012;10:239–49. doi: 10.1158/1541-7786.MCR-11-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almhanna K, Philip PA. Defining new paradigms for the treatment of pancreatic cancer. Curr Treat Options Oncol. 2011;12:111–25. doi: 10.1007/s11864-011-0150-8. [DOI] [PubMed] [Google Scholar]
- 5.Balic A, Dorado J, Alonso-Gomez M, Heeschen C. Stem cells as the root of pancreatic ductal adenocarcinoma. Exp Cell Res. 2012;318:691–704. doi: 10.1016/j.yexcr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72:335–45. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benayoun L, Shaked Y. In vitro enrichment of tumor-initiating cells from human established cell lines. Curr Protoc Stem Cell Biol. 2013;Chapter 3(Unit 3):7. doi: 10.1002/9780470151808.sc0307s24. [DOI] [PubMed] [Google Scholar]
- 8.Benayoun L, Shaked Y. In vitro enrichment of tumor-initiating cells from human established cell lines. Curr Protoc Stem Cell Biol. 2013;Chapter 3(Unit 3):7. doi: 10.1002/9780470151808.sc0307s24. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 10.Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78:756–65. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermann PC, Sancho P, Canamero M, Martinelli P, Madriles F, Michl P, et al. Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice. Gastroenterology. 2014;147:1119–33. e4. doi: 10.1053/j.gastro.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Hidalgo M, Maitra A. The hedgehog pathway and pancreatic cancer. N Engl J Med. 2009;361:2094–6. doi: 10.1056/NEJMcibr0905857. [DOI] [PubMed] [Google Scholar]
- 13.Huang XY, Wang HC, Yuan Z, Huang J, Zheng Q. Norepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via beta-adrenergic receptor-dependent activation of P38/MAPK pathway. Hepatogastroenterology. 2012;59:889–93. doi: 10.5754/hge11476. [DOI] [PubMed] [Google Scholar]
- 14.Kelleher FC. Hedgehog signaling and therapeutics in pancreatic cancer. Carcinogenesis. 2011;32:445–51. doi: 10.1093/carcin/bgq280. [DOI] [PubMed] [Google Scholar]
- 15.Kim EJ, Sahai V, Abel EV, Griffith KA, Greenson JK, Takebe N, et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res. 2014;20:5937–45. doi: 10.1158/1078-0432.CCR-14-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kundranda M, Kachaamy T. Promising new therapies in advanced pancreatic adenocarcinomas. Future Oncol. 2014;10:2629–41. doi: 10.2217/fon.14.197. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Pancreatic cancer stem cells: Emerging target for designing novel therapy. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.03.018. (EPub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Lowenfels AB, Maisonneuve P. Risk factors for pancreatic cancer. J Cell Biochem. 2005;95:649–56. doi: 10.1002/jcb.20461. [DOI] [PubMed] [Google Scholar]
- 20.Mancuso A, Calabro F, Sternberg CN. Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Hematol. 2006;58:231–41. doi: 10.1016/j.critrevonc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Marechal R, Jean-Baptiste B, Annabelle C, Pieter D, Jean Robert D, Magali SM, et al. Sonic Hedgehog and Gli1 expression predict outcome in resected pancreatic adenocarcinoma. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-0667. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar FH, Li Y, Wang Z, Kong D. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir. 2009;64:489–500. [PMC free article] [PubMed] [Google Scholar]
- 24.Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res. 2014;12:14–23. doi: 10.1158/1541-7786.MCR-13-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuller HM, Al-Wadei HA, Majidi M. GABA B receptor is a novel drug target for pancreatic cancer. Cancer. 2008;112:767–78. doi: 10.1002/cncr.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuller HM, Al-Wadei HA, Ullah MF, Plummer HK., 3rd Regulation of pancreatic cancer by neuropsychological stress responses: a novel target for intervention. Carcinogenesis. 2012;33:191–6. doi: 10.1093/carcin/bgr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh BN, Fu J, Srivastava RK, Shankar S. Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PLoS One. 2011;6:e27306. doi: 10.1371/journal.pone.0027306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorne Research I. Gamma-aminobutyric acid. Altern Med Rev. 2007;12:274. [PubMed] [Google Scholar]
- 29.Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, La Noce M, et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J. 2013;27:13–24. doi: 10.1096/fj.12-218222. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Zhu H, Zhu Y, Liu Y, Shen H, Yin R, et al. CD133(+)/CD44(+)/Oct4(+)/Nestin(+) stem-like cells isolated from Panc-1 cell line may contribute to multi-resistance and metastasis of pancreatic cancer. Acta Histochem. 2013;115:349–56. doi: 10.1016/j.acthis.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Liu H, Chen X, Zhang M, Xie K, Ma Q. Immune sculpting of norepinephrine on MHC-I, B7-1, IDO and B7-H1 expression and regulation of proliferation and invasion in pancreatic carcinoma cells. PLoS One. 2012;7:e45491. doi: 10.1371/journal.pone.0045491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei HJ, Yin T, Zhu Z, Shi PF, Tian Y, Wang CY. Expression of CD44, CD24 and ESA in pancreatic adenocarcinoma cell lines varies with local microenvironment. Hepatobiliary Pancreat Dis Int. 2011;10:428–34. doi: 10.1016/s1499-3872(11)60073-8. [DOI] [PubMed] [Google Scholar]
- 33.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–71. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]