Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is implicated in stress regulation and learning and memory. PACAP has neuromodulatory actions on brain structures within the limbic system that could contribute to its acute and persistent effects in animal models of stress and anxiety-like behavior. Here, male Sprague-Dawley rats were implanted with intracerebroventricular (ICV) cannula for infusion of PACAP-38 (0.5, 1, or 1.5 ug) or vehicle followed 30 min later by fear conditioning. Freezing was measured early (1, 4, and 7days) or following a delay (7, 10, and 13 days)after conditioning. PACAP (1.5 μg) produced a bi-phasic response in freezing behavior across test days: relative to controls, PACAP-treated rats showed a reduction in freezing when tested 1 or 7 Days after fear conditioning that evolved into a significant elevation in freezing by the third test session in the early, but not delayed, group. Corticosterone (CORT) levels were significantly elevated in PACAP-treated rats following fear conditioning, but not at the time of testing (Day 1). Brain c-Fos expression revealed PACAP-dependent alterations within, as well as outside of, areas typically implicated in fear conditioning. Our findings raise the possibility that PACAP disrupts fear memory consolidation by altering synaptic plasticity within neurocircuits normally responsible for encoding fear-related cues, producing a type of dissociation or peritraumatic amnesia often seen in people early after exposure to a traumatic event. However, fear memories are retained such that repeated testing and memory reactivation (e.g. re-experiencing) causes the freezing response to emerge and persist at elevated levels. PACAP systems may represent an axis on which stress and exposure to trauma converge to promote maladaptive behavioral responses characteristic of psychiatric illnesses such as post-traumatic stress disorder (PTSD).
Keywords: PACAP, fear conditioning, freezing, corticosterone, c-Fos, PTSD
1. Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) belongs to the secretin/glucagon superfamily of peptides and exists in two biologically active forms (as 38- and 27-amino acid peptides) found in peripheral tissues and in the brain (Vaudry et al., 2009). PACAP-38 is the predominant form in the brain and shares identical amino acid sequence homology in species including mice, rats, sheep, and humans, indicating strong evolutionary conservation (Montero et al., 2000). Although numerous biological and behavioral functions have been ascribed to PACAP actions in the CNS (see Zhou et al., 2002), the presence of high levels of PACAP and its cognate receptor, PACAP-type-I-receptor (PAC1), in limbic brain areas such as the hippocampus, amygdala and bed nucleus of the striaterminalis (BNST) suggests a role in modulating neural activity related to stress and emotional states (Hammack and May 2014; Hannibal 2002; Joo et al., 2004).
Evidence for a functional role of PACAP in anxiety-like behaviors in laboratory animals comes mainly from gene knock-out studies where PAC1 receptors or PACAP itself have been ablated, or studies where exogenous PACAP is delivered directly into the brain. The data generally indicate that increased PACAP function mimics numerous physiological and behavioral responses indicative of a stress or anxiety-like state, including altered coping strategies (Legradi et al., 2007), decreased open arm time in the elevated plus maze (Dore et al., 2013; Missig et al., 2014; Telegdy and Adamik, 2015), and increased expression of the stress peptide corticotropin-releasing factor (CRF) and stress hormone corticosterone (Agarwal et al., 2005; Dore et al., 2013). Conversely, reductions in PACAP-mediated neurotransmission produce anxiolytic-like responses measured in behavioral assays including elevated plus-maze, novel object recognition, light-dark box, and open field test (see Hammack and May 2014). Still other studies have shown that chronic stress increases mRNA for PACAP or the PAC1 receptor in vivo (Hammack et al., 2009), whereas treatment with anxiolytic and/or antidepressant drugs decreases PAC1 mRNA in vitro (Reichenstein et al., 2008). Hence, bi-directional changes in PACAP-mediated neurotransmission may modulate physiological responsiveness to stress and promote, or reduce, anxiety-like states.
We have demonstrated a role for PACAP in modulating synaptic transmission in the central nucleus of the amygdala (CeA; Cho et al., 2012), a brain area that is critically involved in fear learning (Li et al., 2013) and receives heavy PACAPergic innervation from the brainstem (Missig et al., 2014). There is also considerable evidence that PACAP affects synaptic transmission and plasticity in the hippocampus (Otto et al., 2001; Yang et al., 2010), consistent with the finding that high densities of PACAP receptors are expressed throughout this structure (Joo et al., 2004; Vaudry et al., 2009). Direct infusion of PACAP into the CA1 region of the hippocampus immediately after training enhances consolidation of the conditioned freezing response, whereas the PACAP antagonist PACAP6-38 into either the hippocampus or basolateral amygdala reduces conditioned freezing (Schmidt et al., 2015). In Drosophila, mutation of a PACAP homolog (amnesiac mutant) is responsible for deficits in olfactory fear conditioning (see Hashimoto et al., 2002). Together, these findings are consistent with earlier studies suggesting a role for PACAP in modulating learning and memory, particularly in fear conditioning procedures (Otto et al., 2001; Adamik and Telegdy, 2005; Takuma et al., 2014), in a wide variety of species.
Here, we examined the effects of intracerebroventricular (ICV) PACAP on the acquisition of conditioned fear and effects on blood corticosterone (CORT) levels and neuronal activity using the protein product of the immediate early gene c-Fos (Meloni and Davis, 2000). Our rationale for using ICV administration of PACAP rather than localized delivery (e.g. Schmidt et al., 2015) was to investigate how PACAP actions (direct and indirect) in multiple brain areas simultaneously might interact to affect learning of aversive contingencies. The complex interactions of multiple brain areas recruited during fear conditioning (Maren et al., 2013), and modified by the effects of exogenously applied PACAP, may produce a different behavioral outcome to that seen with brain-specific infusions alone (Schmidt et al., 2015). Accordingly, ICV administered PACAP could recapitulate a condition where elevated levels of endogenous PACAP—putatively increased by exposure to chronic stress—affects multiple brain areas thereby sensitizing (or protecting) the brain to the consequences of exposure to atraumatic event. Considering that preclinical fear conditioning paradigms have been useful tools to help understand the neurobiology of psychiatric conditions such as post-traumatic stress disorder (PTSD; Mahan and Ressler, 2012), a disorder where dysfunction within PACAP systems has been implicated (Ressler et al., 2011), the current study may have useful face and construct validity.
Our data indicate that ICV PACAP can induce a profound but temporary amnestic-like effect early after fear conditioning that evolves into a hypermnestic phenotype after multiple re-exposures to the conditioned cues. This behavioral readout, as well as an analysis of the involvement of different brain areas following PACAP plus fear conditioning, may provide a heuristic model to help understand how stress and exposure to trauma converge to produce some of the symptoms commonly seen in PTSD, such as dissociation and peritraumatic amnesia, and may evolve over time with multiple re-experiencing episodes.
2. Methods and Materials
2.1. Animals
The animals were male Sprague-Dawley rats (Charles River; Raleigh, NC) weighing 250 g housed in group cages of four rats each and acclimated to the vivarium for six weeks until surgery. Rats were maintained on 12/12 h light dark cycles and food and water were provided ad libitum. Experiments were performed from 1000h to 1600h. The sample sizes were determined in concordance with our previous work using the conditioned-freezing behavioral assay (Meloni et al., 2014)and c-Fos analyses (Meloni and Davis, 2000). All animal procedures were approved by McLean Hospital’s Institutional Animal Care and Use Committee (Office of Laboratory Animal Welfare Assurance number A3685-01) in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (8th Edition).
2.2. Intracerebroventricular (ICV) cannulation
Rats were anesthetized with Nembutal (65 mg/kg, IP) and placed in a Kopf stereotaxic instrument (model 900; Kopf Instruments, Tujunga, CA) with blunt ear bars. The skin was retracted, and a hole was drilled in the skull above the lateral ventricle. Stainless-steel guide cannulas (23 gauge; Plastics One, Roanoke, VA) with an internal dummy stylet extending 1.5 mm beyond the guide cannula tip were lowered into the brain using the following coordinates: −0.8 mm caudal to bregma, +1.3 mm lateral to the midline, −3.5 mm ventral to dura according to the Rat Brain Atlas of Paxinos and Watson (2008). Three stainless-steel screws (size 0–80; Small Parts, Miami Lakes, FL) were also placed in the skull to anchor the guide cannula and dental acrylic (Stoelting, Wood Dale, IL) was used to cement the cannula in place. Rats were placed under a heating lamp, and after recovery, the rats were singly housed in plastic Nalgene cages (45 × 24 × 20 cm) with wood-shaving bedding.
2.3. ICV PACAP infusion
The timeline for procedures and tests used in the current study are illustrated in Figure 1. Ten days after surgery, rats were transported in their home cages to a room adjacent to the fear conditioning room, placed in individual plastic cages, and their dummy stylettes were removed and replaced with infusion cannulas (30 gauge, 1.5 mm projection from the tip of the guide cannula; Plastics One) attached to Hamilton microsyringes (10 μl) by polyethylene tubing. A Harvard Apparatus infusion pump (model 22; Harvard Apparatus, Holliston, MA) was used to deliver 3 μl of either vehicle [artificial CSF (aCSF); Harvard Bioscience, Holliston, MA] or PACAP-38 (0.167, 0.333, 0.5 μg/μl; Bachem, Torrance, CA) directly into the lateral ventricle at a rate of 1 μl/min for 3 min. The infusion cannulas were left in place for 2 min after the infusion and then removed and the dummy stylettes replaced. Rats were placed back in their individual home cages for 30 min followed by fear conditioning.
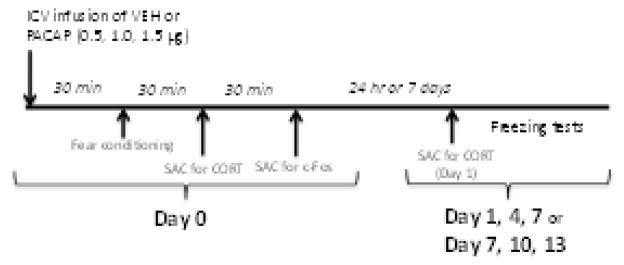
Figure 1. Schematic of the experimental design used in this study.
On Day 0, animals were fear conditioned30 min after ICV infusion of vehicle (VEH) or PACAP (0.5, 1, 1.5 μg). Some animals treated with the highest dose of PACAP (1.5 μg) were sacrificed (SAC) 30 min later for analysis of serum corticosterone (CORT) levels; other animals were sacrificed 60 min later for analysis of c-Fos expression. Twenty-four h or 1 week later (Day 1 and Day 7 respectively) animals were tested for conditioned freezing; some PACAP (1.5 μg)-treated animals were sacrificed immediately after the Day 1 test for analysis of CORT. Other animals from all treatment groups were tested every third day (Days 4 and 7 or 10 and 13) for conditioned freezing.
2.4. Fear-conditioning apparatus
Rats were trained and tested for fear conditioning using procedures adapted from Phillips and LeDoux (1992) as described (Meloni et al., 2014). These procedures enabled us to evaluate the expression of conditioned freezing to both context alone and cues (a tone presented within the same context) that were conditioned while brain PACAP levels were elevated. Conditioning and testing were conducted in four identical 19×9×14-cm Plexiglas behavioral chambers contained in a sound-attenuating cubicle (Med-Associates, Georgia VT). On the conditioning day (Day 0), rats were placed in chambers and after 2 min received two pairings of a 30-s, 5-kHz, 75-dB tone (CS) co-terminating with a 0.6-mA, 0.5-s footshock (US) delivered through the floor bars of the chamber. Shock reactivity (cage movement in response to shock delivery) was measured after each training trial by an accelerometer at the base of the cage. Accelerometer analog output was amplified and digitized on a scale of 0–20 units by an analog-to-digital card interfaced with a PC computer (Med-Associates). The intertrial interval of CS-US pairings was 30 s. After an additional 30 s in the chamber, animals were returned to their home cages. Twenty-four hours, or 7 days, after training rats were returned to the testing chambers and after 2 min animals were exposed to the tone CS (5-kHz, 75-dB) for 60 s. Freezing behavior was video-recorded on each day and scored by an experimenter blind to treatment conditions. Percent freezing was calculated as the % total time that animals remained immobile (frozen), other than breathing, during the first 2 min of re-exposure to the chamber (Context alone) and during the 60-s CS presentation (Context plus tone). Subsequent freezing tests were conducted every third day (Day 4 and 7 or 10 and 13), adapted from previously described procedures (Philips and LeDoux, 1992).
2.5. Corticosterone assay
Serum CORT levels were measured on Day 0 (30 min after fear conditioning) and on Day 1 (immediately after the freezing test) in different cohorts of animals. Rats were overdosed with sodium pentobarbital (115 mg/kg; IP) and upon loss of toe-pinch reflex, the chest cavity was opened. A 5 ml syringe with a 20 gauge needle was used to draw 4–5 ml of blood from the right ventricle of the heart. This procedure took less than 5 min and previous work suggests that it is unlikely that anesthesia significantly impacted CORT levels (Arnold and Langhans, 2010). Blood was transferred to a sterile 10 ml serum blood collection tube (BD Vacutainer; Becton-Dickson, Franklin Lakes, NJ) and allowed to clot at room temperature for 30 minutes before centrifugation for 10 min at 3000 rpm. Serum was removed, aliquoted, and stored at −80°C until assayed by ELISA following the manufacturer’s directions for quantitative determination of CORT levels in rat/mouse serum (Alpco Diagnostics, Salem NH). The interassay and intrassay coefficient of variation was 5.5% and 10.7% respectively, and the sensitivity of the assay was 4.1 ng/ml. Brains from these animals were also removed and placed in 4% paraformaldehyde for 7 days and then stored for 3–4 d in a 30% sucrose/0.1 M PBS solution for subsequent histological verification of accurate cannula placement in the lateral ventricle.
2.6. c-Fos immunohistochemistry
Expression of c-Fos protein was measured on Day 0 (60 min after the end fear conditioning; Radulovic et al., 1998). Rats were overdosed with sodium pentobarbital (115 mg/kg; IP) and perfused intracardially with 0.9% saline (200 ml) followed by 2% paraformaldehyde, 0.05% gluteraldehyde, and 0.2% picric acid in 0.1 M PBS (500 ml). After the perfusion, the brains were removed and stored for 3–4 d in a 30% sucrose/0.1 M PBS solution. Brains were then cut serially in 40 μm coronal sections and every third section was placed in a 4-ml borosilicate glass vial (16 sections/vial) for processing of c-Fos immunohistochemistry as previously described (Meloni and Davis, 2000). Sections were mounted on microscope slides and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA) and observed with a Zeiss Axioscope 2 (Zeiss, Oberkochen, Germany). Still frame images were captured with a digital camera (Axiocam, Zeiss) interfaced with a PC using image-acquisition software. To quantify the number of c-Fos positive cells within each brain area, a fixed region-of-interest (ROI) template was transcribed from the atlas of Paxinos and Watson (2008; shaded areas shown in Figure 4A) for that brain area and affixed to the captured images using Adobe Photoshop software (CS6; Adobe System Incorporated, Mountain View, CA, USA) using structural landmarks for placement of the template. Neurons with round/oval nuclei clearly stained dark brown were tagged and counted in each brain area by an observer blind to the treatment conditions using the Adobe Photoshop analysis count tool. Data represent the average of c-Fos counts from the left and right sides of the brain averaged across at least three 40 μm sections for each brain area for both treatment conditions (VEH and PACAP).
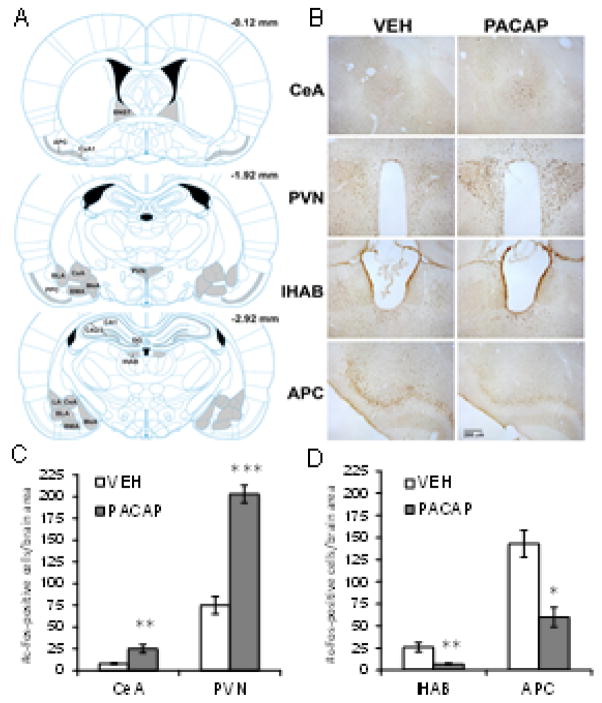
Figure 4. Effects of PACAP plus fear conditioning on c-Fos expression.
(A) Brain atlas plates from the atlas of Paxinos and Watson (2008) showing the brain areas examined (gray shaded) for c-Fos expression after treatment with VEH or PACAP (1.5 μg) followed by fear conditioning on Day 0. Brain section levels are indicated in millimeters posterior to bregma. (B) Representative coronal brain sections through areas showing a significant increase (e.g. central nucleus of the amygdala, CeA; paraventricular nucleus of the hypothalamus, PVN) or decrease (e.g. lateral habenula, lHAB; anterior piriform cortex, APC) in c-Fos expression after treatment with PACAP (1.5 ug) compared to VEH. Quantification of c-Fos expression in brain areas showing an increase (C) or decrease (D) in the number of c-Fos-positive cells after treatment with PACAP (1.5 μg) compared to VEH. VEH, n=5; PACAP, n=5. ***P<0.0005; **P<0.005; *P<0.05. Data are shown as mean±s.e.m.
2.7. ICV cannula placement
At the end of behavioral testing, all animals not used for CORT or c-Fos analyses were overdosed with sodium pentobarbital (115 mg/kg; IP) and perfused intracardially with 0.9% saline (200 ml) followed by 4% paraformaldehyde (500 ml). The brains were removed and stored for 3–4 d in 30% sucrose/0.1 M PBS and subsequently cut in 40 μm coronal sections. Sections were mounted on microscope slides and coverslipped with Permount for verification of cannula placement in the lateral ventricle; all animals used in this study were found to have accurate cannula placement.
2.8. Statistical analysis
Data are presented as means ± standard error. The effect of PACAP on freezing behavior was analyzed using two-way ANOVAs with treatment group (PACAP dose: 0, 0.5, 1, 1.5 μg) as a between-subjects factor and test day as a within-subjects factor. Shock reactivity and CORT levels were analyzed using independent-measures one-way ANOVAs. For measurements yielding significant main effect, subsequent multiple pairwise comparisons were made using Newman-Keuls tests. Differences in c-Fos expression between VEH and PACAP (1.5 μg) treated rats were analyzed with two-tailed t-tests for each brain area examined.
3. Results
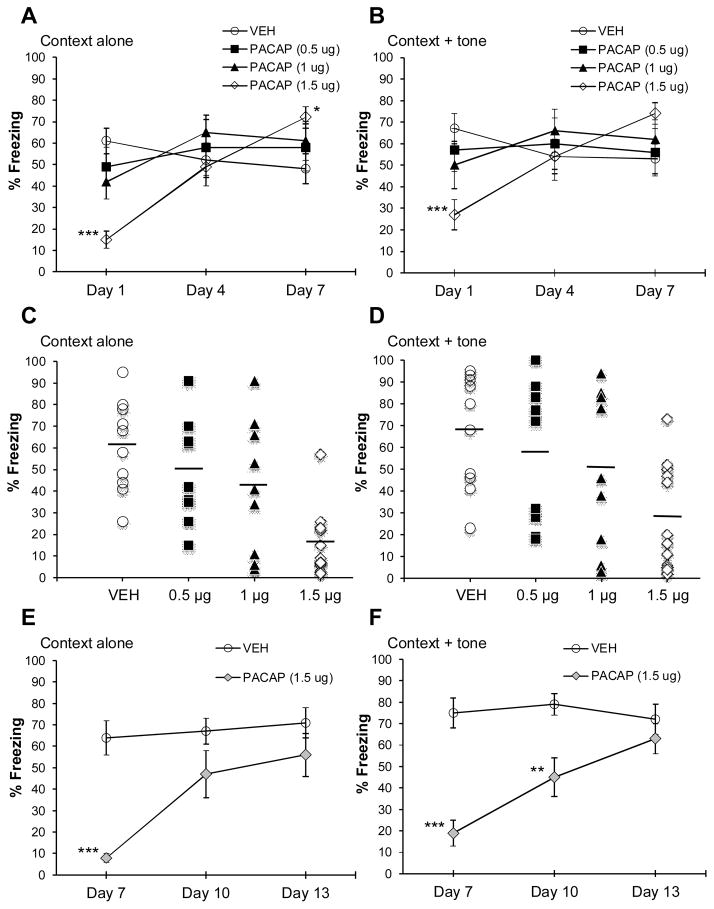
As shown in Figure 2, PACAP given 30 min before fear conditioning had a dose- and test-day dependent effect on conditioned freezing. Significant main effects include: context alone (Figure 2A; test day: F2,72=12.13, P <0.0001; dose x test day interaction: F6,72=7.71, P<0.0001) context + tone (Figure 2B; test day: F2,72=3.13, P<0.05; dose x test day interaction: F6,72=4.60, P<0.0005). Rats that received the highest dose of PACAP (1.5 μg) showed a significant reduction in freezing to context (P<0.0005) and context plus tone (P<0.05) on Day 1 and a significant increase in freezing to context (P<0.05) on Day 7 compared to VEH-treated rats. Rats treated with 0.5 or 1 μg PACAP showed no significant differences in freezing across test days compared to VEH-treated animals. The distribution of individual freezing responses on Day 1 from individual rats in each treatment group is shown in Figure 2C (freezing to context alone) and 2D (freezing to context plus tone). A trend for a reduction in freezing with increasing doses of PACAP was observed for both freezing to the context alone (r2 = .37, P<0.0001) and context plus tone (r2 = .22, P<0.005) on Day 1. Based on these observations, an additional cohort treated with either VEH (n=8) or the high dose of PACAP (1.5 μg; n=9) was included to determine if these phenotypes persisted when the first test day was delayed until 7 days after fear conditioning. As shown in Figure 2 E & F, freezing was significantly reduced on the first test day (Day 7) in PACAP-treated animals but was not significantly different from VEH-treated animals by the third test (Day 13). Significant main effects include: context alone (Figure 2E; group: F1,15=9.76, P < 0.05; test day: F2,30=16.23, P < 0.0001; dose x test day interaction: F2,30=9.48, P<0.005) context + tone (Figure 2F; group: F1,15=18.11, P < 0.005; test day: F2,30=7.27, P < 0.05; dose x test day interaction: F2,30=8.92, P<0.005).
Figure 2. PACAP produces a dose- and time-dependent effect on the expression of conditioned freezing.
(A & B) Percent freezing to context alone and context + tone, respectively, in animals treated with different doses of PACAP given prior to fear conditioning. Animals treated with the highest dose of PACAP (1.5 μg) showed a bi-phasic effect on freezing; freezing was significantly reduced on Day 1 and significantly enhanced (context alone only) on Day 7. (C & D)Distribution of percent freezing responses for individual animals in each treatment group to context alone and context + tone, respectively, on Day 1; bar represents mean percent freezing for each treatment group. VEH, n=10; PACAP 0.5, 1, 1.5 μg, n=9, n=9, n=12 respectively.(E & F) Percent freezing to context alone and context + tone, respectively, in animals treated with PACAP (1.5 μg) given prior to fear conditioning and received their first freezing test 1 week later (Day 7). VEH, n=8; n=9. ***P<0.0005; **P<0.005; *P<0.05. Data are shown as mean±s.e.m.
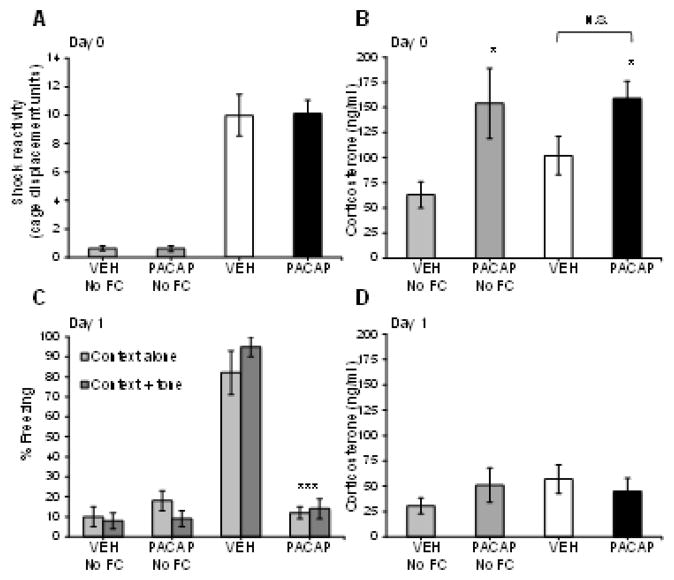
Figure 3A & B illustrates the effect of VEH and PACAP (1.5 μg) on shock reactivity (i.e. cage movement in response to shock delivery) and blood serum CORT levels in animals that did, and did not receive fear conditioning (FC) on Day 0. A one-way ANOVA revealed a main effect of treatment on shock reactivity (F3,19=41.39, P<0.0001) but, there was no significant difference between fear conditioned animals that received either VEH or PACAP. These data suggest that ICV PACAP (1.5 μg) infusion does not affect perception of the shock (as indicated by flinch response to shock delivery and movement of the cage), which could serve as a confound for the degree of fear conditioning and subsequent expression of freezing seen between these treatment groups on Day 1. A one-way ANOVA examining serum CORT levels from these same animals sacrificed 30 min after fear conditioning revealed a significant main effect (F3,19=3.97, P<0.05). Individual pairwise comparisons revealed significant differences between VEH-No FC treated animals and PACAP-No FC (F1,19=7.96, P<0.05) and PACAP rats that were fear conditioned (F1,19=8.87, P<0.05). There was no significant difference between fear conditioned animals that received either VEH or PACAP. We confirm that this was not due to a ceiling effect based on the sensitivity of the assay indicated by the standard curve generated from CORT samples as high as 2250 ng/ml. Also, there was no significant difference between animals that received VEH and either were or were not fear conditioned (VEH No-FC versus VEH groups). This finding may be related to the fact that the ICV infusion itself is slightly stressful and may have elevated CORT in rats that received VEH but no fear conditioning. Although we did not include a control group that did not receive any ICV infusions to test this possibility, a comparison of the CORT response from VEH-No FC animals on Day 0 versus Day 1 (data shown in Figure 3D) raises the possibility that the ICV infusion procedure itself is slightly stressful; an independent-groups t-test revealed a near-significant difference in VEH-No FC rats on Day 0 versus Day 1(P=0.08).
Figure 3. PACAP has acute but not long-term effects on blood corticosterone levels.
(A) Shock-reactivity levels in vehicle (VEH) and PACAP (1.5 μg)-treated animals that were fear conditioned or not (No FC) on Day 0. PACAP had no effect on the animals’ reaction to the shock as measured by cage displacement (arbitrary units) in response to shock delivery. (B) Day 0 corticosterone levels were significantly elevated in PACAP-treated rats, compared to VEH-treated rats that did not receive fear conditioning (VEH No FC), and was independent of whether animals received fear conditioning. VEH-No FC, n=6; PACAP-No FC, n=6; VEH, n=5; PACAP, n=6. (C) Conditioned freezing to context alone and context + tone measured on Day 1 in vehicle (VEH) and PACAP (1.5 μg)-treated animals that were fear conditioned or not (No FC) on Day 0. Conditioned freezing to context alone and context + tone was significantly reduced in PACAP (1.5 μg)-treated rats compared to VEH-treated rats. However, corticosterone levels were not significantly different between any of the treatment groups measured immediately after the freezing test on Day 1 (D). VEH-No FC, n=10; PACAP-No FC, n=11; VEH, n=6; PACAP, n=5. ***P<0.0005; *P<0.05. N.S., not significantly different. Data are shown as mean±s.e.m.
Figure 3C & D illustrates the effect of VEH and PACAP (1.5 μg) on conditioned freezing and blood serum CORT, respectively, on Day 1. In a replication of part of the data presented in Figure 2A & B, PACAP significantly reduced conditioned freezing to both the context alone and context plus tone compared to VEH-treated animals on Day 1 in this cohort. In fact, freezing levels of PACAP-treated rats were indistinguishable from animals that received either VEH or PACAP and were not fear conditioned (No FC) indicating a complete blockade of conditioned freezing by PACAP on Day 1 in this cohort of animals. A two-way ANOVA with treatment as a between subject comparison and exposure condition (context alone and context plus tone) as a within-subjects comparison revealed a main effect of treatment (F3,28=66.9, P<0.0001) and a treatment x exposure condition interaction (F3,28=2.9, P<0.05). Individual pairwise comparisons revealed significant differences in freezing levels between VEH and PACAP treated animals to both the context alone (F1,28=64.7, P<0.0001) and the context plus tone (F1,28=86.9, P<0.0001). A one-way ANOVA examining serum CORT levels from these same animals sacrificed immediately after the freezing test revealed no significant main effect of treatment, indicating that PACAP-induced elevations in corticosterone seen on Day 0 (Figure 3B) return to control levels within 24 hr. Importantly, this finding suggests that the PACAP-induced reduction in conditioned freezing on Day 1 cannot be attributable to elevated CORT levels which could potentially interfere with retrieval of the fear memory.
Figure 4 illustrates the effect of VEH and PACAP (1.5 μg) on c-Fos expression 60 min after fear conditioning on Day 0. Regions (Figure 4A) were selected for analysis based on the known involvement of these brain regions in stress, fear conditioning, and/or PACAP-mediated effects. Figure 4B shows representative coronal brain sections from VEH and PACAP treated rats where a significant increase (e.g. CeA, paraventricular nucleus of the hypothalamus (PVN)) or decrease (e.g. lateral habenula (lHab), anterior piriform cortex (APC)) in c-Fos expression was observed. Averaged c-Fos-positive cell counts for each of these areas from VEH and PACAP-treated animals is illustrated in Figure 4C and 4D, respectively; individual t-tests between treatment groups for each brain area revealed significant differences. For all other brain areas examined, there were no significant differences in c-Fos expression between VEH and PACAP treated rats (Table 1).
Table 1. Quantification of c-Fos expression.
Number of c-Fos positive cells per brain area† x treatment
| Brain area examined | VEH | PACAP (1.5 μg) |
|---|---|---|
| Hippocampus:
|
||
| dentate gyrus | 4 ±1 | 6 ±2 |
| CA1 | 3 ±1 | 2 ±1 |
| CA2/3 | 9 ±3 | 11 ±3 |
| Amygdala:
|
||
| LA | 5 ±2 | 6 ±2 |
| BLA | 8 ±1 | 7 ±1 |
| BMA | 21 ±6 | 13 ±8 |
| MeA | 138 ±26 | 124 ±21 |
| CeA | 9 ±1 | 29 ±4** |
| BNST | 10 ±4 | 21 ±4 |
| PVN | 78 ±6 | 249 ±29*** |
| APC | 120 ±4 | 64 ±7* |
| CxA1 | 98 ±14 | 64 ±10 |
| PPC | 80 ±11 | 46 ±10 |
| lHAB | 23 ±4 | 5 ±1** |
LA, lateral amygdala; BLA, basolateral amygdala; BMA, basomedial amygdala; MeA, medial amygdala; CeA, central nucleus of the amygdala; BNST, bed nucleus of the striaterminalis; PVN, paraventricular nucleus of the hypothalamus; APC, anterior piriform cortex; CxA1, cortex-amygdala transition zone; PPC, posterior piriform cortex; lHAB, lateral habenula.
average of left and right sides across at least three coronal brain sections for each area VEH, n=5; PACAP, n=5.
P<0.0005;
P<0.005;
P<0.05.
Data are shown as mean ± s.e.m.
4. Discussion
The results of the current study extend a growing body of evidence for a role of PACAP in modulating learning and memory mechanisms, especially as it applies to fear conditioning. Here, we demonstrate that ICV infusion of PACAP prior to fear conditioning has a dose- and time-dependent effect on the expression of conditioned freezing tested 1, 4 and 7 days after administration. Our data show that PACAP at the highest dose tested (1.5 μg) significantly reduced conditioned freezing 24 h after it was given in combination with fear conditioning (Figure 2A & 2B). In one replication study focusing on this effect at Day 1 only (Figure 3C), conditioned freezing to the context alone and context plus tone was roughly equivalent to that of rats which did not receive fear conditioning, indicating a near total blockade of conditioned freezing in PACAP- (1.5 μg) treated animals. This effect appeared, however, to be temporary, since the conditioned freezing response re-emerged with subsequent testing and ultimately reached significantly higher levels than those of control rats on the third test day (Day 7; Figure 2A & 2B). Lower doses of PACAP, including a dose frequently used as a standard in many published studies (e.g. 1 μg) did not produce this phenotype, although there was evidence that some animals were sensitive to this dose and showed reductions in freezing similar to the majority of animals treated with the high dose of PACAP (Fig 2C & 2D). As such, there may be individual differences that increase susceptibility to PACAP in this behavioral paradigm. The underlying state of an individual’s stress system could be one potential factor contributing to enhanced sensitivity to PACAP’s effects, consistent with literature suggesting an interaction between PACAP and stress-related neurocircuits (Hammack et al., 2010; Hashimoto et al., 2011; Stroth et al., 2011). PACAP- (1.5 μg) treated rats tested 1 week after fear conditioning also showed reduced freezing (Figure 2E & 2F) on their first test day (Day 7) that re-emerged with repeated testing. However, freezing levels did not exceed those of control rats on the third test day (Day 13) as was previously seen when memory reactivation tests were closer to the conditioning day.
There are numerous interpretations that could potentially explain the reduction of conditioned freezing seen on the first test day, including an amnestic-like effect, temporary retrieval deficits, or other non-specific effects that produce a behavior incompatible with freezing (i.e. increased locomotion or other motor effects) after administration of PACAP plus fear conditioning. Support for an amnestic-like effect rooted in PACAP-dependent neuroadaptations comes from data showing that PACAP knockout mice have reduced conditioned freezing (exhibiting ~10% freezing to context) similar to that seen in the present study (Takuma et al., 2014). Such data suggest that the initial PACAP-dependent deficit in conditioned freezing is related to the underlying neurobiology, rather than non-specific effects per se, because it is completely restored to control levels by environmental enrichment (Takuma et al., 2014). Environmental enrichment is known to enhance neuroplasticity, particularly in the hippocampus (Eckert and Abraham, 2013), which is critically involved in contextual fear conditioning (Maren et al., 2013) and shows PACAP-dependent alterations in synaptic transmission and plasticity (Otto et al., 2001; Yang et al., 2010). Although both studies implicate PACAP in an amnestic-like effect, the question of how acute elevations in PACAP present during fear conditioning (our study) and chronic depletion of PACAP in the knockout mice (Takuma et al., 2014) can produce a nearly identical behavioral phenotype (see also Otto et al., 2001) remains to be reconciled. Adaptive changes in the expression or relative ratios of PACAP-sensitive receptors (e.g. compensatory up-regulation of PAC1, or vasoactive intestinal peptide receptors VPAC1 & VPAC 2 which also bind PACAP) or other receptors modulated by PACAP (e.g. glutamate receptors; Cho et al., 2012; Schmidt et al., 2015)could be the variable between the two studies that produces similar behavioral outcomes despite differences in the animal models.
The possibility that anamnestic-like effect accounts for our results is also supported by a report describing the effects of PACAP on hippocampal dendritic spine remodeling (Gardoni et al., 2012). In that study, application of PACAP (300 nM) to hippocampal primary cultures produced a significant decrease of dendritic spine-head size and a concomitant reduction of glutamate GluR1 receptors along the same time course (e.g. maximum effects 30 min after PACAP administration) as we used in our PACAP pretreatment prior to fear conditioning. If similar PACAP-dependent effects are happening in the hippocampus in vivo (our study) as that seen in vitro (Gardoni et al., 2012), it is conceivable that fear conditioning-induced synaptic transmission/plasticity would be compromised via a reduction in the available substrates (e.g. dendritic spine heads, glutamate receptors) necessary for hippocampal-dependent learning. One limitation of the current study is that animals were not tested for cue-induced freezing in a different context, which may help to differentiate PACAP effects on hippocampal- versus amygdala-dependent learning. Our intention in these initial studies was to elucidate the basic phenomena using a paradigm similar to that of Phillips and LeDoux (1992), which successfully established a differential role for the hippocampus and amygdala in context versus cued fear conditioning. On the basis of these data, a more comprehensive set of studies is now planned to test PACAP’s effects on cue-induced freezing elicited in a different context, as well as studies involving different fear conditioning paradigms (e.g. fear-potentiated startle, olfactory conditioning) to determine if PACAP effects generalize across tests of fear-learning.
Our data should be considered together with those from a recent report showing that intra-hippocampus (limited to the dorsal CA1 region) infusion of a very low dose of PACAP (40 pg) immediately after fear conditioning produced a small but significant increase in conditioned freezing measured 24 h later (Schmidt et al., 2015). The most parsimonious explanation for the discrepancies between studies lies in the differences in doses of PACAP, coverage (dorsal CA1 versus all PACAP-receptor rich areas of the hippocampus that would be affected by ICV PACAP) and the timing of administration (before or after fear conditioning). Also, it is unclear how local infusion of PACAP directly into hippocampus alone might affect CORT release, which we found to be significantly increased with ICV PACAP administration (Figure 3B). Considering that the hippocampus is one of the primary targets of glucocorticoids (Rodrigues et al., 2009), the influence of PACAP-induced CORT in the hippocampus, plus direct modulatory effects of PACAP on synaptic transmission/plasticity (Yang et al., 2010), could have complex effects on the consolidation of fear learning in this structure (e.g., Kaouane et al., 2012) and account for the differences between the findings of Schmidt et al., (2015) and the current study.
Although we found a significant increase in serum CORT levels by PACAP on Day 0, consistent with other reports (Agarwal et al., 2005; Dore et al., 2013), CORT levels were not significantly different from controls 24 h later (Figure 3D) in a cohort that was also tested for freezing (Figure 3C). This finding indicates that the reduction in freezing seen in PACAP-treated animals on Day 1 is likely not due to the known disruptive effect of CORT on memory retrieval (de Quervain et al., 2009). The exogenous PACAP we administered would be expected to be degraded by 24 h after ICV administration due to its short half-life in blood and brain (Dogrukol-Ak et al., 2004), and therefore not considered to be alingering factor that could influence or interfere with fear memory retrieval on Day 1. Indeed, previous reports have shown that motor- (e.g. locomotion, rearing, grooming) and more complex motivated behaviors (e.g., responding for rewarding brain stimulation, attention) that are acutely increased by ICV administration of PACAP returned to normal 24 h later (Adamik and Telegdy, 2004; Donahue et al., 2015), suggesting that the reduced freezing seen on Day 1 in the current study is not due to a lingering non-specific effect on behavior that is incompatible with the freezing response. Further, our data showing that the Day 1 phenotype is reproduced even when the first freezing test is delayed for 1 week support the hypothesis that the amnestic-like effect is due to underlying neuroadaptations (induced by the combined effects of PACAP+fear conditioning on Day 0) rather than the presence of other PACAP-stimulated hormones/factors (e.g. CRF, somatostatin, growth hormone-releasing hormone, etc.; Vaudry et al., 2009; Zhou et al., 2002) that could impair memory retrieval or have other non-specific effects. However, the possibility that PACAP might affect other functions, such as feeding and sleep (Zhou et al., 2002), that contribute to the conditioned freezing phenotype discovered in the current study cannot be excluded.
Following the Day 1 amnestic-like effect on conditioned freezing, we observed a rebound in the level of freezing over repeated test days in animals treated with the high dose of PACAP (1.5 μg). In fact, by Day 7 PACAP-treated rats had significantly higher levels of freezing compared to controls. One explanation is that the multiple re-exposures to the conditioning cues were able to reshape the rat’s behavior, possibly via an incubation-like process (Elharrar et al., 2013). In this case the fear memory might be retained in the CeA, an area that showed a significant increase in PACAP-induced c-Fos expression on Day 0 (Fig 4B & 4C). With multiple re-exposures, the retained memory may be redistributed to other brain areas (e.g. hippocampus) that were initially occluded by the primary effects of PACAP, thus re-integrating the nodes of the fear neurocircuitry that now allow the fear response to be expressed. Other groups have reported similar re-exposure-dependent, bi-phasic memory effects of PACAP in fear learning paradigms (Adamik and Telegdy, 2005). Freezing also re-emerged in PACAP-treated rats that were not tested until Day 7. Under these conditions, however, freezing levels did not exceed that of controls, perhaps due to the fact that VEH-treated animals showed less between-test extinction of freezing with activation of a remote memory versus one that is reactivated closer to the conditioning day. Unresolved questions regarding the neurobiological mechanisms that underlie the development of these dynamic PACAP-dependent effects and how they interact with phenomena such as incubation and extinction will require a significant amount of additional research to address comprehensively.
An analysis of c-Fos expression after PACAP (1.5 μg) administration and fear conditioning on Day 0 revealed bi-directional changes in expression levels in different brain areas that, taken together, may have influenced the initial consolidation of the fear memory within interconnected neurocircuits. The increase in c-Fos in the CeA is consistent with a role for this structure in the acquisition of fear conditioning (Li et al., 2013) and of enhanced synaptic plasticity in CeA neurons after PACAP application (Cho et al., 2012). Likewise, the increase in c-Fos expression in the PVN—a primary structure of the descending limb of the hypothalamic-pituitary-adrenal axis—is consistent with PACAP’s ability to increase blood CORT levels seen in the present study and others (Agarwal et al., 2005). Interestingly, we identified two brain areas that showed a significant decrease in c-Fos expression after the Day 0 treatment: the lateral habenula (lHab) and the anterior piriform cortex (APC). The lHab is an important node in transmitting input from the limbic system to dopamine neurons in the midbrain (e.g. ventral tegmental area (VTA) and substantianigra) and has been implicated in a number of functions including: behavioral avoidance, prediction error and attention, and stress and anxiety-like behaviors (Hikosaka, 2010; Stamatakis and Stuber, 2012). The ways in which PACAP-induced inhibition of the lHab (inferred from the reduction in c-Fos expression) on Day 0 might influence the consolidation and/or expression of freezing are currently unknown. Possibilities include: attentional disturbances (Lecourtier and Kelly, 2005; Gill et al., 2013), alterations in dopamine-dependent modulation of synaptic plasticity (Pezze and Feldon, 2004) or disruption of motor suppression (i.e. freezing; Hikosaka, 2010).
The APC is part of sensory olfactory cortex that is rich in mRNA for PACAP receptors (Joo et al., 2004) and may play a role in associative encoding of olfactory information (Roesch et al., 2007). The PACAP-induced reduction in c-Fos in this brain area may indicate that olfactory sensory information (i.e. any odors associated with the fear conditioning chambers) is not being integrated in the memory engram, which could present an obvious confound for conditioning of this component of the “context” on Day 0. However, previous studies indicate that PACAP neurotransmission does not play a role in odor discrimination of non-social olfactory cues (Nicot et al., 2004) suggesting that our PACAP-treated rats would still be able to perceive the smells associated with the conditioning context. The posterior piriform cortex (PPC), a secondary associative cortex that does play a role in consolidation of olfactory cues in fear conditioning (Sacco and Sacchetti, 2010), also showed nominal (but intriguing) reductions in c-Fos expression in PACAP-treated rats on Day 0.
There were no significant differences in c-Fos expression between VEH and PACAP-treated rats in numerous other brain areas that were examined. Of these areas, the BNST, an area that has been strongly implicated in mediating many of the stress-like effects of PACAP (Hammack et al., 2010), showed a trend for an increase in c-Fos expression in PACAP-treated rats, but this difference was not significant. In VEH-treated animals it was somewhat surprising that c-Fos expression was low throughout the dorsal hippocampus and basolateral amygdala, areas that usually show robust c-Fos expression after fear-conditioning (e.g. Milanovic et al., 1998). It is possible that the prior experience of the surgical procedure to implant the ICV cannula (e.g. handling, transport, stress) may have reduced the novelty of the fear conditioning stimuli and blunted c-Fos expression in these areas as has been demonstrated (Radulovic et al., 1998). How this putative reduced-novelty effect may have impacted PACAP’s effects on c-Fos expression is not known. An examination of other markers of neuroadaptations, such as ΔFosB, activity-regulated cytoskeletal protein (Arc) or the phosphorylated form of cAMP response element binding protein (pCREB), could provide a more comprehensive picture of PACAP-induced changes that occur after fear conditioning in various brain areas and cell types (e.g., CRF or GABA neurons).
Dissociation is a common symptom of trauma exposure that involves “disruption in and fragmentation of the usually integrated functions of consciousness, memory, identity, body awareness, and perception of the self and the environment” (Lanius et al., 2012; Dorahy and van der Hart, 2015) and may be a major risk factor for the development of PTSD (Breh and Seidler, 2007). While it is clear that environmental stimuli present during traumas can become hyper-consolidated in some patients, thereby contributing to symptoms of avoidance and re-experiencing (e.g. flashbacks, nightmares), amnesia for other details surrounding a traumatic experience is a common feature of dissociation and may reflect fragmentation of normally integrated neural ensembles that code emotionally charged aspects of the traumatic memory (Spiegel 1997). The difficulty in recapitulating both the memory intensification and amnesia for stimuli associated with the trauma has been a limitation in preclinical models of PTSD and is clearly not adequately captured through use of the fear conditioning paradigm alone (Layton and Krikorian, 2002; Desmedt et al., 2015). In the current study, we found that by elevating brain-wide levels of PACAP during fear conditioning we could induce an amnestic-like effect early (24 h) or late (7 days)after fear conditioning that evolved into a hypermnestic-like effect with multiple re-experiencing episodes when memory reactivation was close to the conditioning event. Given accumulating evidence suggesting an association between dys function within brain PACAP systems and PTSD (Ressler et al., 2011; Pohlack et al., 2015), the conditions and behavioral endpoints used in the current study may have especially strong face and construct validity for the study of PTSD, and facilitate the development of more effective strategies to treat or prevent stress-related illness.
Highlights.
PACAP given prior to fear conditioning produces a dose and time-dependent change in the level of conditioned freezing.
PACAP significantly elevates serum corticosterone levels 30 min after administration that returns to normal 24 h later.
PACAP produces significant changes in c-Fos expression in different brain areas.
Acknowledgments
Role of the funding source:
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This research was funded by National Institutes of Health grant MH097860 (WAC).
Footnotes
Contributors:
All authors have materially participated in the research and/or article preparation. Conceived and designed the experiments: EGM. Performed the experiments: EGM, AV, RJD. Analyzed the data: AV. Contributed to the writing of the manuscript: EGM, WAC, AV, RJD.
Conflicts of interest:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamik A, Telegdy G. Involvement of different receptors in pituitary adenylate cyclase activating polypeptide induced open field activity in rats. Neuropeptides. 2004;38:16–20. doi: 10.1007/s11064-014-1337-8. [DOI] [PubMed] [Google Scholar]
- Adamik A, Telegdy G. Effects of pituitary adenylate cyclase polypeptide (PACAP) on extinction of active avoidance learning in rats: involvement of neurotransmitters. Regul Pept. 2005;127:55–62. doi: 10.1016/j.regpep.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M, Langhans W. Effects of anesthesia and blood sampling techniques on plasma metabolites and corticosterone in the rat. Physiol & Behav. 2010;99:592–598. doi: 10.1016/j.physbeh.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Breh DC, Seidler GH. Is peritraumatic dissociation a risk factor for PTSD? J Trauma Dissociation. 2007;8:53–69. doi: 10.1300/J229v08n01_04. [DOI] [PubMed] [Google Scholar]
- Cho JH, Zushida K, Shumyatsky GP, Carlezon WA, Jr, Meloni EG, Bolshakov VY. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. J Neurosci. 2012;32:14165–14177. doi: 10.1523/JNEUROSCI.1402-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt A, Marighetto A, Piazza P-V. Abnormal fear memory as a model for posttraumatic stress disorder. Biol Psych. 2015;78:290–297. doi: 10.1016/j.biopsych.2015.06.017. doi:org/10.1016/j.biopsych.2015.06.017. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Dogrukol-Ak D, Tore F, Tuncel N. Passage of VIP/PACAP/secretin family across the blood-brain barrier: therapeutic effects. Curr Pharm Des. 2004;10:1325–1340. doi: 10.2174/1381612043384934. [DOI] [PubMed] [Google Scholar]
- Donahue RJ, Venkataraman A, Carroll FI, Meloni EG, Carlezon WA. Pituitary adenylate cyclase-activating polypeptide disrupts motivation, social interaction, and attention in male sprague-dawley rats. Biol Psych. 2015 doi: 10.1016/j.biopsych.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorahy MJ, van der Hart O. DSM-5’s posttraumatic stress disorder with dissociative symptoms: challenges and future directions. J Trauma Dissociation. 2015;16:7–28. doi: 10.1080/15299732.2014.908806. [DOI] [PubMed] [Google Scholar]
- Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V. CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology. 2013;38:2160–2169. doi: 10.1038/npp.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MJ, Abraham WC. Effects of environmental enrichment exposure on synaptic transmission and plasticity in the hippocampus. Curr Top Behav Neurosci. 2013;15:165–187. doi: 10.1007/7854_2012_215. [DOI] [PubMed] [Google Scholar]
- Elharrar E, Warhaftig G, Issler O, Sztainberg Y, Dikshtein Y, Zahut R, Redlus L, Chen A, Yadid G. Overexpression of corticotropin-releasing factor receptor type 2 in the bed nucleus of striaterminalis improves posttraumatic stress disorder-like symptoms in a model of incubation of fear. Biol Psychiatry. 2013;74:827–836. doi: 10.1016/j.biopsych.2013.05.039. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Saraceno C, Malinverno M, Marcello E, Verpelli C, Sala C, Di Luca M. The neuropeptide PACAP38 induces dendritic spine remodeling through ADAM10-N-cadherin signaling pathway. J Cell Sci. 2012;125(Pt 6):1401–1406. doi: 10.1242/jcs.097576. [DOI] [PubMed] [Google Scholar]
- Gill MJ, Ghee SM, Harper SM, See RE. Inactivation of the lateral habenula reduces anxiogenic behavior and cocaine seeking under conditions of heightened stress. Pharmacol Biochem Behav. 2013;111:24–29. doi: 10.1016/j.pbb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the striaterminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–43. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the striaterminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci. 2010;42:327–340. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, May V. Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biol Psychiatry. 2014;78:167–177. doi: 10.1016/j.biopsych.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Baba A. Higher brain functions of PACAP and a homologous Drosophila memory gene amnesiac: insights from knockouts and mutants. Biochem Biophys Res Comm. 2002;297:427–432. doi: 10.1016/s0006-291x(02)02144-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanida M, Hayata A, Hashimoto R, Baba A. PACAP is implicated in the stress axes. Curr Pharm Des. 2011;17:985–989. doi: 10.2174/138161211795589382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- Kaouane N, Porte Y, Vallée M, Brayda-Bruno L, Mons N, Calandreau L, Marighetto A, Piazza PV, Desmedt A. Glucocorticoids can induce PTSD-like memory impairments in mice. Science. 2012;335:1510–1513. doi: 10.1126/science.1207615. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton B, Krikorian R. Memory mechanisms in posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 2002;14:254–261. doi: 10.1176/jnp.14.3.254. Erratum in: J Neuropsychiatry Clin Neurosci. 2003; 15, 121. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. Bilateral lesions of the habenula induce attentional disturbances in rats. Neuropsychopharmacology. 2005;30:484–496. doi: 10.1038/sj.npp.1300595. [DOI] [PubMed] [Google Scholar]
- Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM. Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast. 2007;2007:79102. doi: 10.1155/2007/79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci. 2013;16:332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Davis M. Synergistic enhancement of the acoustic startle reflex by dopamine D1 and 5-HT1A agonists and corresponding changes in c-Fos expression in the dorsal raphe of rats. Psychopharmacology. 2000;151:359–367. doi: 10.1007/s002130000474. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Gillis TE, Manoukian J, Kaufman MJ. Xenon impairs reconsolidation of fear memories in a rat model of post-traumatic stress disorder (PTSD) PLoS One. 2014;9:e106189. doi: 10.1371/journal.pone.0106189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanovic S, Radulovic J, Laban O, Stiedl O, Henn F, Spiess J. Production of the Fos protein after contextual fear conditioning of C57BL/6N mice. Brain Res. 1998;784:37–47. doi: 10.1016/s0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- Missig G, Roman CW, Vizzard MA, Braas KM, Hammack SE, May V. Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology. 2014;86:38–48. doi: 10.1016/j.neuropharm.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M, Yon L, Kikuyama S, Dufour S, Vaudry H. Molecular evolution of the growth hormone-releasing hormone/pituitary adenylate cyclase-activating polypeptide gene family. Functional implication in the regulation of growth hormone secretion. J Mol Endocrinol. 2000;25:157–168. doi: 10.1677/jme.0.0250157. [DOI] [PubMed] [Google Scholar]
- Nicot A, Otto T, Brabet P, Dicicco-Bloom EM. Altered social behavior in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. J Neurosci. 2004;24:8786–8795. doi: 10.1523/JNEUROSCI.1910-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, Gröne HJ, Kellendonk C, Tronche F, Maldonado R, Lipp HP, Konnerth A, Schütz G. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci. 2001;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. New York: Academic Press; 2008. [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. org.ezp-prod1.hul.harvard.edu/10.1037/0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Ruttorf M, Cacciaglia R, Winkelmann T, Schad LR, Witt SH, Rietschel M, Flor H. Neural mechanism of a sex-specific risk variant for posttraumatic stress disorder in the type I receptor of the pituitary adenylate cyclase activating polypeptide. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. Relationship between Fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J Neurosci. 1998;18:452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenstein M, Rehavi M, Pinhasov A. Involvement of pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors in the mechanism of antidepressant action. J Mol Neurosci. 2008;36:330–338. doi: 10.1007/s12031-008-9116-0. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. Erratum in: Nature. 2011; 477, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in anterior piriform cortex versus orbitofrontal cortex during odor discrimination and reversal learning. Cereb Cortex. 2007;17:643–52. doi: 10.1093/cercor/bhk009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco T, Sacchetti B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science. 2010;329:649–56. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- Schmidt SD, Myskiw JC, Furini CR, Schmidt BE, Cavalcante LE, Izquierdo I. PACAP modulates the consolidation and extinction of the contextual fear conditioning through NMDA receptors. Neurobiol Learn Mem. 2015;118:120–124. doi: 10.1016/j.nlm.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Spiegel D. Trauma, dissociation, and memory. Ann N Y Acad Sci. 1997;821:225–37. doi: 10.1111/j.1749-6632.1997.tb48282.x. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Holighaus Y, Ait-Ali D, Eiden LE. PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Ann N Y Acad Sci. 2011;1220:49–59. doi: 10.1111/j.1749-6632.2011.05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Maeda Y, Ago Y, Ishihama T, Takemoto K, Nakagawa A, Shintani N, Hashimoto H, Baba A, Matsuda T. An enriched environment ameliorates memory impairments in PACAP-deficient mice. Behav Brain Res. 2014;272:269–278. doi: 10.1016/j.bbr.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Telegdy G, Adamik A. Neurotransmitter-mediated anxiogenic action of PACAP-38 in rats. Behav Brain Res. 2015;281:333–338. doi: 10.1016/j.bbr.2014.12.039. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Yang K, Lei G, Jackson MF, Macdonald JF. The involvement of PACAP/VIP system in the synaptic transmission in the hippocampus. J Mol Neurosci. 2010;42:319–326. doi: 10.1007/s12031-010-9372-7. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Shioda S, Yada T, Inagaki N, Pleasure SJ, Kikuyama S. PACAP and its receptors exert pleiotropic effects in the nervous system by activating multiple signaling pathways. Curr Protein Pept Sci. 2002;3:423–439. doi: 10.2174/1389203023380576. [DOI] [PubMed] [Google Scholar]