Abstract
Background
The QT interval is a risk marker for cardiac events such as TdP. However, QT measurements obtained from a 12-lead ECG during clinic hours may not capture the full extent of a patient’s daily QT range.
Objective
We evaluated the utility of 24-hour Holter ECG recording in patients with long QT syndrome to identify dynamic changes in the heart-rate corrected QT interval, and we investigated methods of visualizing the resulting data sets.
Methods
Beat-to-beat QTc (Bazett) intervals were automatically measured across 24-hour Holters from 202 LQT1, 89 LQT2, 14 LQT3 genotyped patients and a reference group of 200 healthy individuals. We measured the percentage of beats with QTc greater than the gender-specific threshold (QTc>470ms in women and QTc>450ms in men). The percentage of beats with QTc prolongation was determined across the 24-hour recordings.
Results
Based on the median percentage of heart beats per patient with QTc prolongation, LQT1 patients showed more frequent QTc prolongation during the day (~3PM) than they did at night (~3AM): 97% vs. 48%, p~10−4 for men, 68% vs. 30%, p~10−5 for women. LQT2 patients showed less frequent QTc prolongation during the day, compared to nighttime: 87% vs. 100%, p~10−4 for men, 62% vs. 100%, p~10−3 for women.
Conclusion
In patients with genotype positive LQTS, significant differences exist in the degree of daytime and nocturnal QTc prolongation. Holter monitoring using the “QT clock” concept may provide an easy, fast, and accurate method to assess the true personalized burden of QTc prolongation.
Keywords: Long QT syndrome, electrocardiography, diagnostic, cardiac monitoring
1. Introduction
The congenital Long QT syndrome (LQTS) is an inherited channelopathy that is associated with a prolonged duration of ventricular repolarization, predisposing such patients to the occurrence of life-threatening ventricular tachyarrhythmias such as torsades de pointes and sudden cardiac death.1, 2 Hundreds of mutations have been identified in the LQTS, however mutations in the KCNQ1 and KCNH2 genes are the most commons forms of the disease – LQT1 and LQT2, respectively. LQT1 is associated with a reduction of the slow component of the late repolarizing potassium current (IKs), while LQT2 is associated with a reduction of the rapid component (IKr).3 Heart rate corrected QT prolongation (QTc), type of mutation, gender, history of syncope, and age are recognized modulating risk factors associated with the LQTS.4
In standard clinical practices, the LQTS diagnosis is confirmed by the presence of known LQTS mutation(s), but the detection of the disease is suspected primarily by a history of syncope or cardiac events in the patient or family and the presence of a QTc prolongation measured from the standard ECG at rest. Despite the recognized clinical value of QTc prolongation as a surrogate marker of the presence of the syndrome, Goldenberg et al.5 reported that about 25% of genotyped-confirmed patients have a concealed form of the disease, wherein genotype positive patients have an ECG tracing exhibiting normal range QTc intervals (QTc ≤440 ms5). To address this issue, experts have proposed strategies to unmask the repolarization impairment in LQTS patients to reveal the presence of the syndrome using protocols that include drug (epinephrine) and exercise-based challenges.5, 6
In this work, we investigated the role of 24-hour Holter recordings in LQTS patients to determine the dynamic nature of QTc prolongation. We hypothesize that beat-to-beat analysis of QT intervals from Holter ECG recordings of patients with LQTS may help identify the periods of the day when QTc interval prolongation is exacerbated. We propose a unique and comprehensive solution to quickly assess the presence of QTc prolongation and monitor changes in the QT interval over time.
2. Methods
2.1. Study Population
Holter tracings used to conduct this study were obtained from the Telemetric and Holter ECG Warehouse (THEW) 7, 8, an initiative hosting a warehouse of continuous and fully de-identified digital Holter ECG’s open to the scientific community for the development of ECG technologies. From this database, we accessed 200 Holter recordings from 200 healthy subjects, 294 Holter recordings from 202 genotyped LQT1 patients, 145 Holters from 89 genotyped LQT2 patients, and 35 Holter recordings from 14 genotyped LQT3 patients. All Holter recordings were 24-hour recordings (2 or 3 lead configuration) recorded from different clinical devices. The LQTS data was donated to the THEW8 by the Hospital Lariboisière (Paris, France),9, 10 while the recordings from healthy subjects were recorded during the Intercity Digital Electrocardiology Alliance project (IDEAL).8 In addition to genetic testing, demographic data and cardiac events, i.e. syncope, documented TdP, sudden cardiac death, and treatment (specifically beta-blocker treatment) were available. We did not know whether the Holter recordings were acquired before or after the patient’s cardiac events. We report the gender-specific distribution of QTc intervals using Bazett’s correction formula since this is the most often clinically-used QT correction formula. Finally, we considered subgroups of the LQT1 and LQT2 population by characterizing patients using the specific locations of missense mutations (while ignoring other mutation types, e.g. frameshift). We extracted the Holter recordings of LQT1 and LQT2 patients on and off beta-blockers. Mutations in the cytoplasmic loop (c-loop) in LQT1 patients were defined as the ones in the region between the S2 and S3 transmembrane domains and between S4 and S5, involving amino acid residues 171–195 (S2-S3) and 242–262 (S4-S5) of the KCNQ1 subunit. In LQT2 subjects, the pore region of the hERG channel was defined as the domain extending from the 5th transmembrane region (S5) to the end of S6 from residues 548 to 659.
2.2. Beat-to-beat QTc measurements
The ECG measurements were computed using the open source program developed by Chesnokov et al.11. The software was modified to read the International Society for Holter and Non-invasive Electrocardiology (ISHNE) file format, and applied to Holter ECG signals to all available leads delivering measurement of R peak locations, QRS onset, and T-wave offset on a beat-to-beat basis. Then, the QT intervals were measured as the time between earliest QRS onset and latest T-wave offset amongst all available leads. For RR interval measurements, we used the median value across all leads.
The QT interval measurements were computed in all beats. Non-sinus beats and annotation errors were removed using the following filtering techniques:
RR, QT, and therefore QTc were not calculated in the neighborhood of ventricular ectopic beats.
Values outside expected ranges were discarded, such as values: 40bpm>HR>160bpm, and/or 210ms>QT >700ms.
Beat-to-beat QTc datasets were filtered using a 10 minute moving median to remove any remaining noise or outliers.
The corrected values of QT interval for heart rate (QTc) were computed using Bazett’s formula.
2.3. The “QT Clock”
We developed a novel graphical concept designed to facilitate the review of a large set of QTc measurements and to provide an infographic understanding of 24-hour QTc dynamics. The QT clock is a circular plot representing a 24-hour clock (00:00 to 24:00) with midnight at the top of the clock. The radius of the clock represents the QTc interval values varying from 300ms to 600ms from the center to the perimeter. The clock is used to present information for two different purposes:
Viewing the expected QTc range for a population (i.e. their typical QTc values across the day).
Monitoring changes in QTc for an individual patient. QTc values extracted from a single Holter recording may provide insight about personal daily variation and period of maximum QTc prolongation.
2.4. Statistical analysis
Statistical analysis was performed using the SciPy stats module. The Mann-Whitney U test was used when assessing statistical differences between different populations, and the Wilcoxon signed-rank test was used when comparing results within the same population. A chi-square test was used in the case of binary variables. In all cases, p-values ≤0.05 were considered statistically significant.
3. Results
3.1. Study population
The description of the study population is provided in Table 1. There was no statistical difference in age between the LQT1 and LQT2 populations. The LQT3 cohort, however, was of a younger average age than the other groups, and the healthy cohort was of a higher average age than any of the LQTS cohorts. Differences in gender distribution, symptoms, and beta blocker use were statistically insignificant between the three types of LQTS, though the LQT1 group was slightly skewed towards more female subjects compared to the healthy cohort (p=0.05). Among patients with LQT1, we identified 36 patients with missense mutations in the cytoplasmic loop regions (C-loop) and 42 patients with missense mutation outside the loop region (non C-loop). In the LQT2 group, 31 patients had missense mutation in the pore region while 36 had their mutation outside the pore regions.
Table 1.
Demographics of the study population. Some individuals were monitored several times under different circumstances (e.g. at different ages), so age, beta-blocker use, and symptoms are reported as a percentage of recordings rather than a percentage of patients.
| Healthy (n=200) | LQT1 (n=202) | LQT2 (n=89) | LQT3 (n=14) | |
|---|---|---|---|---|
| Gender (male/female) | 101/99 (51%) | 88/114 (44%) | 42/47 (47%) | 8/6 (57%) |
| Age (years) | 38±16 | 26±18 | 24±18 | 17±15 |
| Beta-blockers (%) | 0 | 43% | 55% | 57% |
| Symptoms | 0 | 48% | 47% | 50% |
In Table 2, we report the gender-specific average values and standard deviations of heart rate (HR), QT and QRS intervals and the heart rate corrected QT values based on Bazett’s formula for each of the groups.
Table 2.
Gender-based description of the baseline ECG measurements for healthy and LQTS cohorts using 24-hour averages. Values were computed using the process described in Section 2.2, with the addition of J point annotation to compute QRS.
| Healthy | LQT1 | LQT2 | LQT3 | |||||
|---|---|---|---|---|---|---|---|---|
| Men (N=101) | Women (N=99) | Men (N=88) | Women (N=114) | Men (N=42) | Women (N=47) | Men (N=8) | Women (N=6) | |
| HR (bpm) | 77±9 | 81±10* | 72±11 | 77±14* | 77±17 | 76±15 | 72±15 | 77±7 |
| QT (ms) | 358±21 | 365±20* | 430±48 | 435±48 | 433±59 | 450±54 | 463±58 | 453±65 |
| QRS (ms) | 93±5 | 90±4* | 95±10 | 92±8* | 94±9 | 92±8 | 97±11 | 94±6 |
| QTc (ms) | 400±16 | 416±16* | 466±53 | 482±36* | 476±42 | 491±40* | 490±35 | 497±57 |
: P<0.05 vs. men of same genotype.
3.2. QTc prolongation and concealment in patients with the LQTS
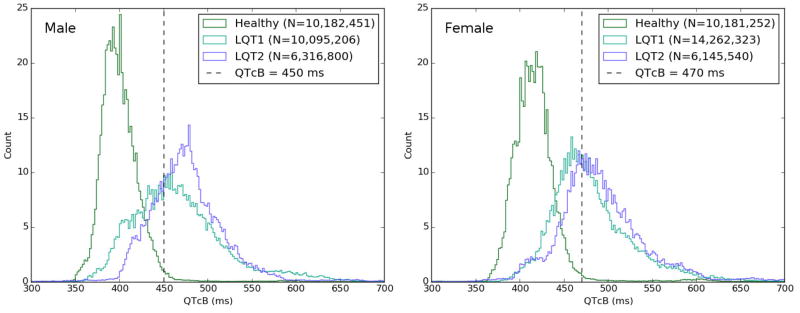
We measured the beat-to-beat RR and QT intervals in 24-hour Holter recordings for healthy patients (n=200) and genotype positive LQTS patients (n=305). The number of QTc intervals measured was around 10 million in each healthy group (male and female), 12 million in each LQT1 group, and 6 million in each LQT2 group. Figure 1 highlights gender-specific QTc distribution for healthy patients compared with LQT1 and LQT2. There is a difference in the distribution of QTc intervals in men versus women. In men, LQT2 is associated with longer median QTc intervals when compared to those with LQT1 (471ms vs. 455ms, p=0.03) while this difference is weaker in females (479ms vs. 470 ms, p=0.04). The QTc distribution plot in LQT1 males shows the largest common area with the distribution of QTc in healthy males, indicating that this group of LQTS patients may have the least QTc prolongation.
Figure 1.
Distribution of beat-to-beat QTc intervals (corrected using Bazett’s formula) in healthy individuals, LQT1 and LQT2 patients. Distributions were normalized (same area), so “Count” is a normalized count of cardiac beats. LQT3 cohort was not plotted because of its small size. Note the overlap of LQT1 and LQT2 groups with the healthy population indicating the level of concealment in these populations.
3.3. Gender-specific QTc prolongation in Holter ECGs from LQTS patients
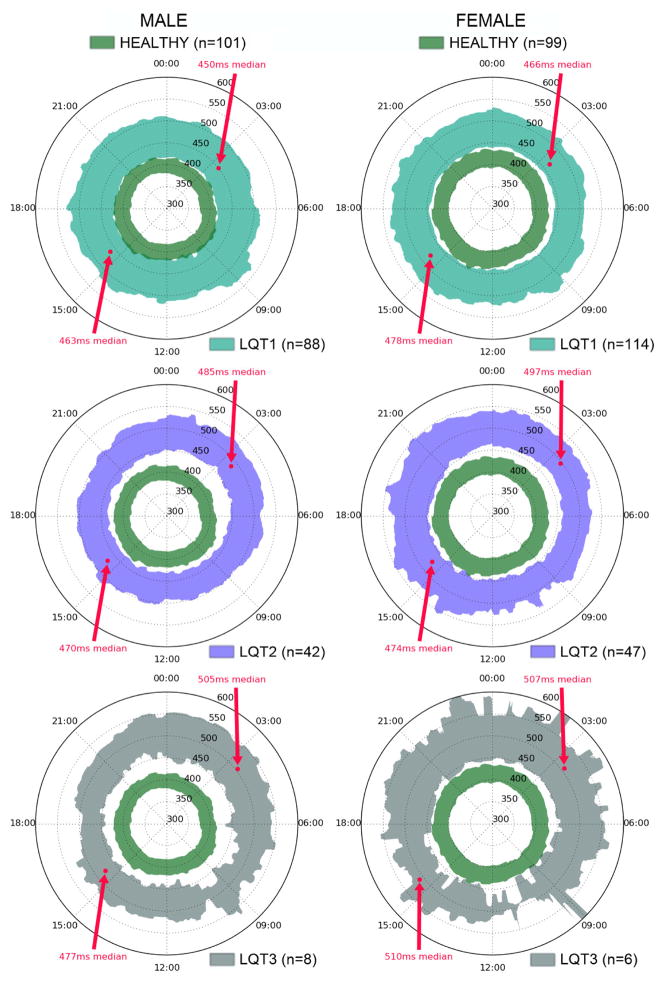
Population and gender-specific distribution of QTc for our study cohorts are plotted on the QT clock in Figure 2. The lower and higher boundaries of these patterns correspond to the 16th and 84th percentile (i.e., inner 68% of patients), with 1-minute resolution. This percentile range was chosen to highlight the equivalent of ±1 standard deviation during each 1-minute epoch, without assuming a normal distribution. Such presentations reveal that there is a very stable, circular, “healthy” range of QTc across 24-hour variations (defined by our healthy cohort, dark green areas in Figure 2). We superimposed on these plots the 24-hour variation of QTc for LQT1 in the upper panels, LQT2 in the middle panels, and LQT3 in the lower panels. This figure reveals different configurations of 24-hour QTc dynamics according to LQTS types. The most striking one is the change in symmetry of the pattern in LQT2 and LQT3 groups revealing the exacerbation of the QTc interval prolongation during the night period. This phenomenon is stronger in LQT3 male patients while it is not present in LQT1 patients. Note that the “healthy” and “LQTS” regions rarely overlap, in contrast to what we see in Figure 1. This is mainly because the clocks only show the inner 68% of each group – the tails of their respective histograms are omitted. A version of this figure with an expanded percentile range is available as a supplementary download.
Figure 2.
“QTc Clock” plots for the LQT1 males and females (upper left and right panels, respectively), LQT2 males and females (middle left and right panels, respectively), and LQT3 males and females cohorts (lower left and right panels, respectively). Each area is defined by the middle 68 percentile of QTc values – i.e., same percentage as ±1 standard deviation – measured across all Holter recordings, for each 1-minute slice. The dark green area represents the expected range of QTc variations in our healthy cohort. The figure highlights the changes in QT prolongation in LQT2 and LQT3 cohorts during the night. This phenomenon is expressed as graph asymmetry where the area corresponding to LQT2/3 cohort stretches away from the normal value during the night time (22:00 to 08:00). Arrows indicate median QTc at the time periods that were reported in Table 3. Note that the LQT3 ranges are computed from only 6 female and 8 male patients.
Rather than comparing QTc value distributions at different times of day, it can be instructive to compare QTc prolongation percentages – i.e., the chance of QTc exceeding the clinical prolongation thresholds. In Table 3, we report the percentage of heart beats that showed QTc prolongation in the three LQTS cohorts for the same time periods: one nocturnal (3AM-4AM), and one diurnal (3PM-4PM). In the LQT1 and LQT2 cohorts, we observed a significant variation in median QTc prolongation (13–49%) between these two periods, p<0.01 (or p<10−5 if gender is ignored). Specifically, in the LQT1 cohort, prolongation was significantly higher, regardless of gender, during the afternoon than late at night, while in LQT2 the afternoon hours showed significantly lower prolongation than at night. In LQT3, there were no statistically significant differences in prolongation when comparing these two time ranges, most likely due to the small size of our cohort.
Table 3.
Percentage of QTc prolongation (median values) in LQTS subjects between two periods of the day: 3–4AM and 3–4PM. Prolongation percentage is defined as the percentage of heart beats with QTc above the gender-specific threshold during the given hours. Periods starting at 3AM and 3PM were chosen as representative times to illustrate changes in QTc prolongation between sleeping and waking hours. The median QTc values at these times are identified in Figure 2, and prolongation percentage for the full 24 hours is shown in Figure 3. N is reported as the number of patients with Holter recordings containing data during the required hours.
| Percentage of cardiac beats with QTc prolongation | LQT1 | LQT2 | LQT3 | |||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| N | 82 | 108 | 40 | 47 | 8 | 5 |
| Period : 3AM–4AM | 48% | 30% | 100% | 100% | 100% | 81% |
| Period : 3PM–4PM | 97% | 68% | 87% | 62% | 100% | 95% |
| p-value | 0.0002 | 0.00001 | 0.0002 | 0.002 | 0.2 | 1.0 |
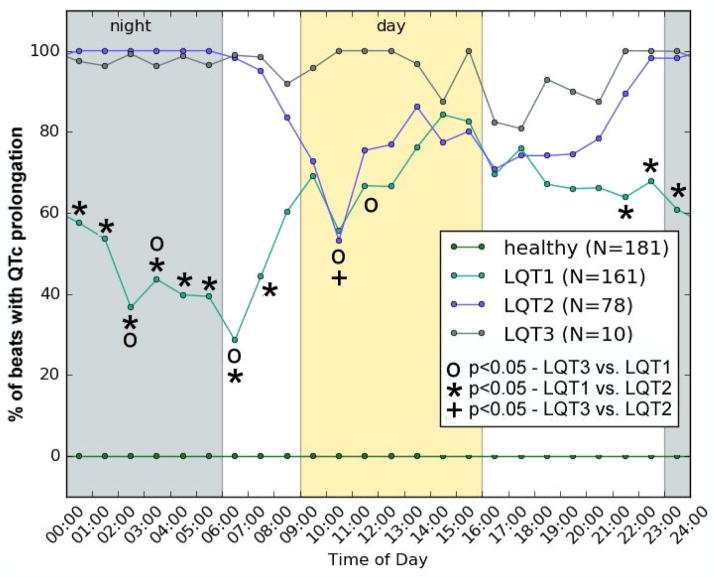
Hourly median prolongation percentages are plotted in Figure 3. LQT1 and LQT2 patients appear to have very similar patterns of QTc prolongation during clinic hours (“day”), but are quite distinct during sleeping hours (“night”). At night, QTc prolongation becomes much more prevalent in LQT2 subjects, and less common in LQT1. For readability, we did not plot separate lines for each gender in this figure. However, when the plot is separated by gender, it reveals a slightly higher QTc prolongation percentage in men than women – about 12% higher, on average. This agrees with our expectations from inspecting Figure 1, in which the threshold for males (450ms) appears to offer higher sensitivity than the threshold for females (470ms). Finally, we identified the two periods of the day with the most significant differences in median percentage of QTc prolongation within each LQTS family. In LQT1, the most significant difference in percentage of beats with QTc prolongation is found when comparing 05:30 (early morning) to 15:30 (afternoon) (39% vs. 83%, p~10−9), while in LQT2 the most significantly different periods of the day are around 00:30 (night) and 18:30 (evening) (100% vs. 74%, p~10−6).
Figure 3.
Median levels of QTc prolongation across 24 hours for the Healthy, LQT1, LQT2 and LQT3 cohorts. This plot can be thought of as the chance to detect prolonged QTc (though, because this is the median, 100% indicates that most, not all, beats show QTc prolongation at a given hour). Prolongation in all LQTS cohorts is significantly higher than in healthy subjects (p<10−6 at all times). Availability of Holter data varied from hour to hour, as patients may have been recorded for (e.g.) 22–23 hours rather than the full 24. We therefore report N values as the minimum across all data points.
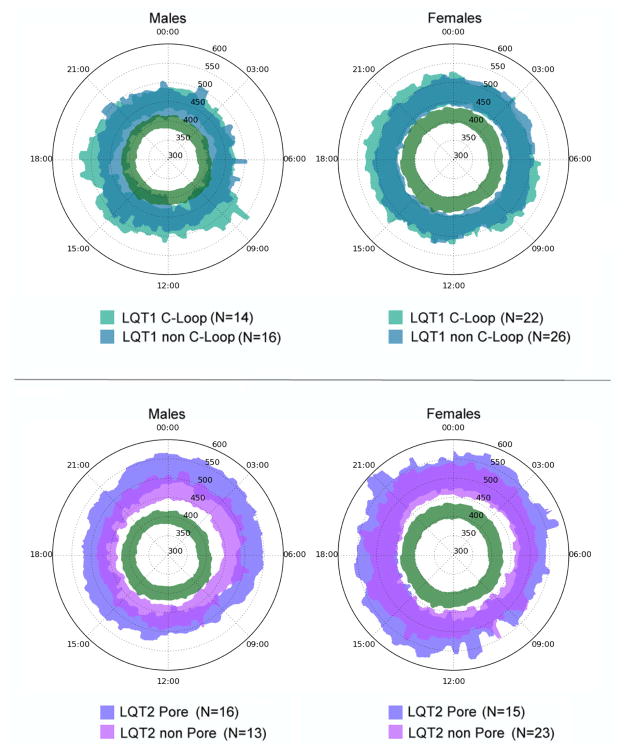
3.4. Mutation location and QT Clock profile in LQT1 and LQT2
Figure 4 presents the gender-specific QT clocks for the groups of LQT1 patients with C-loop versus non-C-loop mutations, and LQT2 patients with pore versus non-pore mutations. In male LQT1 patients, we observed no clear differences in the QTc clock profiles between patients with mutations inside or outside the C-loop regions, though the C-loop region appears slightly worse overall (i.e. more QTc prolongation). In women with LQT1, QTc values remained >450 ms throughout the day and nearly overlapping QTc clock profiles were observed for those with C-loop and non C-loop mutations. However, among male patients with LQT2, those with a pore region mutation consistently had more QTc prolongation when compared to those with non-pore mutations. This observation is consistent with prior report in LQT2 patients which revealed that male LQT2 patients with non-pore mutation were associated with lower risk for events.12 Finally, in female LQT2 patients, the trend for increased QTc prolongation in those with pore mutations also appears to be present, but has more overlap with the non-pore subjects than we see in the male cohort.
Figure 4.
Gender-specific QTcB clocks for LQT1 and LQT2 patients with missense mutations. The clocks show the 16th–84th percentile of QTc for LQT1 patients with mutations inside or outside the cytoplasmic loop (C-loop), and for LQT2 patients with mutations in pore and non-pore regions. The green area is the healthy region defined in Figure 2. The plots reveal the more pronounced QTc prolongation in males with missense mutations in the C-loop and pore regions for LQT1 and LQT2, respectively. In females, these differences are less pronounced.
The percentage of patients on beta-blockers was not significantly different in LQT1 patients with C-loop mutations vs. non C-loop mutations (33% vs. 24%, p=0.15) or in LQT2 patients with mutations in pore vs. non-pore regions (47% vs. 42%, p=0.47).
4. Discussion
The severity of the functional defects and the phenotypic penetrance in the LQTS is modulated by numerous factors amongst which age, gender, type and location of LQT mutation play crucial roles.13 But this also means that genetic tests do not fully capture individual risk. Further, while the standard 12 lead ECG is an important diagnostic tool used in the investigation, evaluation, and monitoring of patients with LQTS, its utility in predicting risk may be limited by the fact that QT concealment may give the false impression of low risk in certain subsets of patients. The presence of QTc concealment in LQTS has been studied previously using standard 10-second ECG tracings. The limitation of such an approach is that these ECG’s are usually acquired during clinical hours, and they may not provide the most accurate assessment of risk but rather should be considered a snapshot measurement of risk.
The cardiac events in LQTS have been shown to be strongly associated with triggers linked to inappropriate QT adaptation to changes in heart rate. Therefore, challenging the ventricular repolarization process in these patients using protocols to exacerbate the QT prolongation have been proposed such as brisk standing,14 exercise, or epinephrine challenge6, 14, 15. Such protocols exploit the fact that β-adrenergically enhanced potassium currents (Ikr and Iks) are required to counterbalance the larger inward depolarizing current of the L-type Ca2+ current (also enhanced by β-adrenergic stimulation).16 Abnormal regulation of these potassium currents under condition of an adrenergic tone put the LQT1 and LQT2 patients at risk for life-threatening ventricular arrhythmias. The cellular and environmental factors that affect repolarization are of course dynamic, and therefore a static assessment of QTc may be limited in conveying the true extent of abnormal repolarization for any given patient.
In contrast to the methods above, we use Holter data to identify dynamic changes in repolarization during a 24-hour time period. While modern Holter systems may provide basic reporting on the QTc interval – e.g. maximum QTc, and percentage of time that it is prolonged – the QT clock can present the time, duration, and magnitude of all prolongation events in a simple picture. The QT clock was used to display results for populations of patients, and to compare the dynamic QT changes of individuals to larger populations of interest. Our findings show that there are significant differences in the patterns and degrees of QT prolongation in patients with LQT1 and LQT2. We demonstrate that Holter monitoring of LQTS patients (coupled with computerized beat-to-beat QT interval measurement) provides an opportunity to better characterize the extent and duration of QT prolongation, and provides insights as to when maximum and minimum QT prolongation present in the subpopulations of patients with LQTS. Note that the QT clock could also use 12-lead ECG data as input. However, the utility of these clocks in understanding beat-to-beat variations in QTc, and whether they can be used to predict adverse clinical events remain to be proven.
Interestingly in our study, we identified a signature pattern of QT prolongation in patients with LQT1 and LQT2 during a 24-hour period. Patients with LQT1 typically have adverse cardiac events during high sympathetic tone. When looking at the degree of QT prolongation, our results reveal that the LQT1 patients are more likely to have diagnostic QTc prolongation during the day time hours; during the night, when sympathetic withdrawal occurs, a much lower degree of QTc prolongation can be observed. On the other hand, LQT2 patients show QT prolongation during the night time hours when compared to the day time hours. These findings raise the question as to whether these signature patterns of QTc prolongation can be used to identify and separate out subgroups of LQTS. The number of patients with LQT3 in our study were too few to conclude any differences in QT prolongation over time.
Future applications of the QT Clock
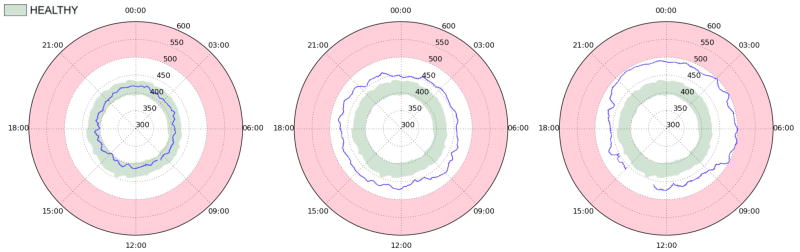
We have proposed a visualization tool to quickly review long term QTc monitoring data and visually assess the presence of abnormal QTc interval prolongation throughout the day. The QT clock concept can be used to display measurements from a single ECG recording for a given patient. Such examples are provided in Figure 5, where we plot the QTc interval across whole recordings of a healthy individual (left panel), a patient with the LQT1 (middle panel), and a patient with the LQT2 (right panel). The blue line is a 10-minute moving median. The light green area is the 16th–84th percentile range for healthy individuals of the same gender as the subject. This percentile range is used to remain consistent with the other figures; however, for diagnostic purposes, it may be desirable to expand this area – to 5%–95%, for example – or to show several percentile tiers/contours in different shades of green. This representation helps to easily pinpoint periods of the day associated with QTc prolongation, and therefore counsel patients about their daily periods/activities associated with risk. It is worth noting that the QT clock may be used to monitor patients receiving known QT-prolonging drugs, specifically during inpatient dofetilide initiation when the dose-dependent effect of the drug needs to be assessed over a couple of days. The QT clock may also be used during the ambulatory outpatient period to assess the long-term safety and efficacy of a QT prolonging drug.
Figure 5.
Example QT clock plots of individual patients (blue line) in reference to the healthy QTc range (green area as defined in Figure 2) for three different subjects: healthy 27 year-old (left panel), 64 year-old LQT1 patient with non-C-loop mutation (middle panel), and 1 year-old LQT2 patient with pore missense mutation (right panel). All subjects are female, and none are on beta blockers. The red area marks the QTc>500ms zone. The individual plottings are median-filtered beat-to-beat QTc values. The plots emphasize how the proposed “QTc clock” enables the physician to quickly identify periods of prolonged QTc, e.g. nighttime for the LQT2 patient.
Limitations
Our analysis is based on Holter recordings acquired using 2 or 3 lead configurations. These leads were not systematically reported but they were V1-, VF-, or V5-like leads. Therefore, in this analysis we do not deliver a lead-based comparison in terms of identifying prolongation but we are limited to assess the presence of prolongation using the longest QT interval measured from non-standard leads in all beats. How the lead selection may affect our analysis, and the accuracy compared to standard 12-lead ECGs remains to be elucidated. We were limited by the low number of recordings available for the LQT3 cohort, making it difficult to draw conclusions from that group. Additionally, in the “population average” QT clocks (Figure 2 and Figure 4) and the QTc histogram (Figure 1), every heart beat is given equal weight; consequently, patients with different numbers of heartbeats on file (due to length/number of Holter recordings, or different average heart rates) will contribute disproportionately to these plots.
Finally, we did not report the comparison of the QT clocks between asymptomatic and symptomatic LQTS patients because we did not have information about the timing of events in relation to when the Holter recordings were acquired. We also opted not to report the QT clocks comparing groups of patients with and without beta-blocker therapies, because the prescription of beta-blocker therapy is dependent on the level of QTc prolongation hence biasing the data. While we do have Holters from a few patients before and after initiation of beta blocker therapy, the number of these patients is too small to draw any conclusions. The QT clocks for the LQTS cohorts 1) with and without beta-blockers, and 2) with and without symptoms have been made available as supplementary figures.
5. Conclusion
In genotype positive LQTS patients, dynamic changes in the QTc can be uncovered using Holter monitoring. The QT clock is a useful illustration of QT dynamics, and can be used to plot QTc measurements for a population or for an individual patient. There are unique patterns of QT prolongation during a 24-hour period in patients with LQT1 and LQT2. Future research is needed to determine if Holter monitoring and QT prolongation patterns can aid in identifying the different subsets of LQTS patients.
Supplementary Material
Clinical Perspectives.
Cardiac repolarization, recorded on the surface ECG using the QT interval, is a dynamic parameter and can be influenced by a variety of cellular and environmental factors. In our study we used Holter monitoring and the concept of a QT clock to display changes in QTc in genotype positive LQTS patients. A computer algorithm that measures QTc in a beat-to-beat manner was developed. Periods of maximum and minimum QTc prolongation over 24 hours were determined for each of the subgroups of LQTS. Unique patterns of QTc prolongation were observed for patients with LQT1 and LQT2. We believe this study will encourage clinicians to make use of standard Holter recordings and the newly developed QT clock concept to better understand an individual patient’s burden of QT prolongation and the potential risk associated with it. Furthermore, the QT clock may prove beneficial as an application to closely and continuously monitor the QTc interval in patients exposed to QT-prolonging drugs, such as for the monitoring of patients with atrial fibrillation treated with dofetilide.
Acknowledgments
This work was supported in part by the National Heart, Lung, and Blood Institute of the U.S. Department of Health and Human Services grants U24HL096556 and R01HL114944, the National Science Foundation grant CNS-1239423, and a gift from Nvidia Corp.
ABREVIATIONS
- LQTS
Long QT syndrome
- LQT1
type 1 LQTS
- LQT2
type 2 LQTS
- LQT3
type 3 LQTS
- QTcB
heart rate corrected QT based on Bazett’s formula
- IKs
slow component of the late repolarizing potassium current
- IKr
rapid component of the late repolarizing potassium current
- TdP
torsades de pointes
- C-loop
cytoplasmic loop
Footnotes
No conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alex Page, Email: alex.page@rochester.edu.
Mehmet K. Aktas, Email: mehmet_aktas@urmc.rochester.edu.
Tolga Soyata, Email: tolga.soyata@rochester.edu.
Wojciech Zareba, Email: Wojciech_Zareba@urmc.rochester.edu.
Jean-Philippe Couderc, Email: Jean-Philippe.Couderc@heart.rochester.edu.
Reference List
- 1.Moss AJ, Robinson JL. Long QT syndrome. Heart Disease & Stroke. 1992 Sep;1(5):309–14. [PubMed] [Google Scholar]
- 2.Zareba W, Moss AJ, Schwartz PJ, et al. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998 Oct 1;339(14):960–5. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu W, Moss AJ, Wilde AA, et al. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol. 2009 Nov 24;54(22):2052–62. doi: 10.1016/j.jacc.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001 Jan 2;103(1):89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg I, Horr S, Moss AJ, et al. Risk for life-threatening cardiac events in patients with genotype-confirmed long-QT syndrome and normal-range corrected QT intervals. J Am Coll Cardiol. 2011 Jan 4;57(1):51–9. doi: 10.1016/j.jacc.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu W, Noda T, Takaki H, et al. Epinephrine unmasks latent mutation carriers with LQT1 form of congenital long-QT syndrome. J Am Coll Cardiol. 2003 Feb 19;41(4):633–42. doi: 10.1016/s0735-1097(02)02850-4. [DOI] [PubMed] [Google Scholar]
- 7.Couderc JP. The telemetric and holter ECG warehouse initiative (THEW): A data repository for the design, implementation and validation of ECG-related technologies. IEEE 2010 publishing. 2010:6252–5. doi: 10.1109/IEMBS.2010.5628067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couderc JP. The Telemetric and Holter ECG Warehouse (THEW): the first three years of development and research. J Electrocardiol. 2012 Nov;45(6):677–83. doi: 10.1016/j.jelectrocard.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Extramiana F, Tatar C, Maison-Blanche P, et al. Beat-to-beat T-wave amplitude variability in the long QT syndrome. Europace. 2010 Sep;12(9):1302–7. doi: 10.1093/europace/euq137. [DOI] [PubMed] [Google Scholar]
- 10.Extramiana F, Maison-Blanche P, Denjoy I, et al. Gene-specific effect of beta-adrenergic blockade on corrected QT interval in the long QT syndrome. Ann Noninvasive Electrocardiol. 2013 Jul;18(4):399–408. doi: 10.1111/anec.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesnokov YC, Nerukh D, Glen RC. Individually adaptable automatic QT detector. 2006:337–40. [Google Scholar]
- 12.Migdalovich D, Moss AJ, Lopes CM, et al. Mutation and gender-specific risk in type 2 long QT syndrome: Implications for risk stratification for life-threatening cardiac events in patients with long QT syndrome. Heart Rhythm. 2011 Mar 25; doi: 10.1016/j.hrthm.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barsheshet A, Dotsenko O, Goldenberg I. Genotype-specific risk stratification and management of patients with long QT syndrome. Ann Noninvasive Electrocardiol. 2013 Nov;18(6):499–509. doi: 10.1111/anec.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viskin S, Postema PG, Bhuiyan ZA, et al. The response of the QT interval to the brief tachycardia provoked by standing: a bedside test for diagnosing long QT syndrome. J Am Coll Cardiol. 2010 May 4;55(18):1955–61. doi: 10.1016/j.jacc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noda T, Takaki H, Kurita T, et al. Gene-specific response of dynamic ventricular repolarization to sympathetic stimulation in LQT1, LQT2 and LQT3 forms of congenital long QT syndrome. Eur Heart J. 2002 Jun;23(12):975–83. doi: 10.1053/euhj.2001.3079. [DOI] [PubMed] [Google Scholar]
- 16.Choe CU, Schulze-Bahr E, Neu A, et al. C-terminal HERG (LQT2) mutations disrupt IKr channel regulation through 14-3-3epsilon. Hum Mol Genet. 2006 Oct 1;15(19):2888–902. doi: 10.1093/hmg/ddl230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.