Abstract
The challenging task of heart regeneration is being pursued in three related directions: derivation of cardiomyocytes from human stem cells, in vitro engineering and maturation of cardiac tissues, and development of methods for controllable cell delivery into the heart. In this review, we focus on tissue engineering methods that recapitulate biophysical signaling found during normal heart development and maturation. We discuss the use of scaffold-bioreactor systems for engineering functional human cardiac tissues, and the methods for delivering stem cells, cardiomyocytes and engineered tissues into the heart.
Keywords: Tissue engineering, human induced pluripotent stem cells, cardiomyocyte, bioreactor, electro-mechanical stimulation, cell and tissue delivery to the heart
Graphical abstract
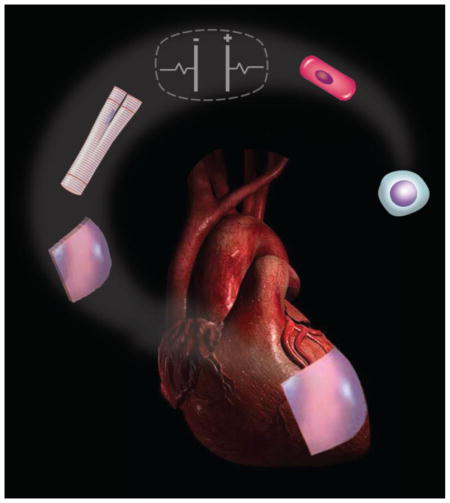
1. Introduction
The burden of cardiovascular disease (CVD) - the leading cause of death worldwide continues to grow, while the treatment modalities remain limited [1, 2]. The fundamental problem with treating CVD is in the minimal ability of the heart to regenerate itself following massive loss of cells to injury or disease. For a failing heart, the only definitive therapy is heart transplantation, with is in turn limited by low supply of matching hearts for the constantly increasing number of patients in need. As a result, there is a pressing need for developing effective methods for regenerating damaged myocardium.
Over the past several decades, new approaches to the repair of myocardium lost to the disease or injury have been explored using combinations of cells, biomaterials, cocktails of factors, and bioengineered cardiac tissues [3–7]. Collectively, the past and ongoing studies suggest that the cell and tissue based therapies can improve heart function by either promoting vascularization of damaged tissue, or regenerating the heart muscle, or a combination of the two. While these studies have advanced our understanding of heart disease and heart regeneration, the actual mechanisms responsible for cardiac tissue formation, signaling, and maturation are only partly known. As a result, current protocols for deriving cardiomyocytes (CMs) from human stem cells by staged molecular induction yield phenotypically immature cells. Likewise, most cardiac tissues engineered in vitro lack the native-like structural anisotropy, gene expression and electromechanical function. Bioengineering approaches are just starting to show success with functional maturation of iPS-CMs [8] and the use of these cells for myocardial regeneration [9].
Interestingly, the quest for cell therapy for heart regeneration had already started a century ago [10]. In their seminal work, Chiu and colleagues injected skeletal muscle satellite cells into the dog heart subjected to myocardial injury [11]. Although these experiments didn’t show much improvement in cardiac functionality, they established an entirely new paradigm for cardiac regeneration. Likewise, engineering of cardiac tissue had started long before the term “tissue engineering” was coined. The first documented effort was already in the 1950s by Moscona and colleagues [12], who formed compact aggregates of chick heart cells in an Erlenmeyer flask under continuous gyration. These three-dimensional (3D) aggregates captured the functionality of the cardiac tissue more closely than any two-dimensional (2D) culture. Although this new approach failed to fully represent the anisotropic structural and functional properties of native heart muscle, it was a significant improvement over all culture methods available at that time.
Since then, there have been numerous efforts to recreate muscle architecture at different length scales. Parker and colleagues showed that physical constraints can be exploited to control heart tissue architecture at the microscale [13]. To this end, neonatal rat cardiomyocytes were aligned by attachment to the patterned strips of fibronectin, towards the formation of 2D structures that captured some features of the heart muscle. Mechanical loading, a factor crucial for heart development, was also shown to control the orientation of the cardiomyocytes and the microarchitecture of engineered cardiac tissue. In pioneering studies, Vandenburgh developed a simple and effective method for applying passive stretch on skeletal or cardiac myocytes encapsulated in hydrogel, and showed that mechanical stimulation promoted cell alignment [14]. Shortly after, Eschenhagen and colleagues established a new approach to form spontaneously contracting, well aligned tissues [15] using auxotonic (passive) loading of cardiac myocytes cultured in collagen gels. In parallel studies, Vunjak-Novakovic and colleagues enhanced the development of electromechanical function in engineered cardiac tissues by stimulating excitation-contraction coupling that orchestrates the contractile function of the native heart [16]. Synchronous contractions of engineered tissues were induced by supra-threshold electrical signals. Over only one week of culture, the engineered tissues exhibited markedly improved morphology, striated ultrastructure, contractile function, and expression of molecular markers when compared to non-stimulated controls.
In this review, we focus on tissue engineering methods that recapitulate biophysical signaling found during heart development and maturation, to grow in vitro functional human myocardium (Figure 1). We also discuss methods for delivering various types of cells (stem cells, committed cardiomyocytes) and engineered tissues into the heart.
Figure 1. Bioengineered approaches to myocardial regeneration.
Initial steps in cardiac regeneration: (1) Skin biopsy from the patient, (2) Somatic cell culture and expansion, (3) Derivation of iPS cells by introducing a specific set of pluripotency-associated genes (Oct4, Sox2, cMyc, and Klf4) into the somatic cell, (4) iPS cell expansion; Cardiac tissue engineering: (5a) Differentiation of iPS cells into cardiomyocytes, (5b) Engineering a cardiac patch, (6) Electromechanical conditioning of iPS-CMs and tissue engineered patches within a bioreactor, (7a) Epicardial injection of cells into the infarct zone border, and (7b) implantation of an engineered cardiac patch.
2. Cardiac tissue engineering using cells, scaffolds and bioreactors
2.1 Biophysical signals in cardiac development
The heart is a mechanically active organ, with cardiomyocytes subjected to electrical and mechanical signals throughout the development and adult life. Mechanical signals activate numerous transduction pathways, and regulate reprogramming of numerous genes and synthesis of proteins [17, 18]. Mechanotransduction can profoundly affect cellular organization of the heart [19, 20] and regulate the synthesis and accumulation of extracellular matrix (ECM) components [21]. Cardiomyocytes mature under appropriate mechanical loading, whereas excessive loading can cause pathological hypertrophy and apoptosis [22]. The main types of mechanical loading are the wall shear stress caused by blood flow, and the strain caused by blood pressure and cell contractions (Figure 2A) [23]. Blood flow is present already at the early stages of embryogenesis, initially without a clear role in nourishment and oxygen supply, as evidenced by the lack of hemoglobin-mediated transport of oxygen in chick embryos until Hamburger-Hamilton stage 20 (HH20) [24, 25]. Instead, diffusion is the main mechanism of nutrient and oxygen supply at the early stages of heart development [23, 26].
Figure 2. Bioengineered approaches to myocardial regeneration.
(A) Biophysical stimuli driving cardiomyocyte maturation: In a complex environment, a cardiomyocyte is exposed to electrical, mechanical, and biochemical stimuli, and to cell-cell and cell-matrix interactions. (B) Cardiac tissue engineering: Cardiac tissues formed between two pillars (left) or around a suture (right) in bioreactors imposing either mechanical forces (auxotonic loading at the left) or electrical stimulation (at the right). (C) Hierarchical architecture of cardiac tissues: The assembly of ventricular heart muscle represents a hierarchical arrangement that spans over several orders of spatial magnitude: from the alignment of actin-myosin complex within a sarcomere, to the alignment within myofibrils, the organization of myofibrils in a myocyte, and the coupling between myocytes to form anisotropic, laminar cardiac tissue. (D) Geometric control of cell alignment: in vitro recapitulation of cell alignment at the micrometer scale. Panels B, left and right, are reproduced with permission from references [8] and [68]. Panel D is reproduced with permission from reference [69].
Hemodynamics is an essential epigenetic factor in the development of the electrical conduction (His-Purkinje) system in the heart, with mechanical stimuli playing roles in electrical maturation of cardiomyocytes [27]. Electrical signals are found both in the developing and the adult heart. Endogenous electrical fields in the developing vertebrate embryo have been widely documented [7], with spontaneous action potentials detected in the chick heart primordium as early as at stage HH9, and before detecting myocyte contractions [23, 28]. It is thought that direct current signals mediate cell migration during primitive streak formation and play roles in establishing the left-right asymmetry. Pulsatile signals have been implicated in the development of the cardiac syncytium, and are considered critical for synchronous contractions of heart muscle [7,14].
Because cardiogenesis is regulated by the interplay between biochemical, mechanical and electrical stimuli, all these stimuli are being incorporated into methods for in vitro engineering of cardiac tissues (Figure 2B). It has been shown that mechanical forces regulate cardiac development, and that mutations affecting contractile proteins can cause heart malformations. For example, mutations in cardiac troponin T and sarcomeric actin in zebrafish resulted in defects of endocardial cushion and valves [25, 29], while mutations in myosin heavy chain affected the formation of ventricles [25, 30]. Electrical forces are also required for preserving cardiac chamber morphology, where they act as a key epigenetic factor in cardiac remodeling. Chi and colleagues showed that mutation in dco (cx46) changes the outer curvature of the looped heart tube, and causes defects in the atrial and ventricular cardiomyocytes [31]. The heart experiences significant mechanical and hydrodynamic forces, both of which contribute to cardiogenesis [25].
Complex loading patterns coordinate the form and function of the heart throughout the development: from the linear tube, through the stages of looping, the establishment of chambers, and the initiation of the pumping function [32]. Hove et al clearly demonstrated the importance of mechanical forces in the heart development and function, using the zebrafish model. When the blood flow was blocked in the early zebrafish heart tube, endocardial cushions failed to form [33]. Also, the orderly propagation of electrical signals and force generation are not established if the cardiac microarchitecture is not well aligned. These findings support the notion that the heart function strongly relies on appropriately timed electrical signals, which synchronize mechanical contractions of the muscle and pumping of the blood.
2.2. Scaffolds
Recapitulating the organ-specific structure (including anisotropy) at multiple length scales is necessary for achieving proper tissue function in vitro (Figure 2C, D). Different methods have been developed for providing an appropriate template, known as a scaffold, along with biophysical cues. The scaffolding of the native heart is its collagenous extracellular matrix (ECM), which provides microscale cues for anisotropic alignment, mechanical integrity and support. The scaffolding for cardiac tissue engineering tends to mimic the native ECM, using collagen hydrogels, lyophilized sponges and micropatterned ECM structures. Alternatively, cells can be removed from the native heart tissue (such as porcine) to obtain a scaffold with the composition, architecture and mechanical function closely matching those of the native heart. Decellularized heart tissue, used in vitro, was shown to enhance the vasculogenic capacity of human mesenchymal stem cells (as shown in vivo, in an animal model of heart infarction) [36], and the cardiogenic capacity of cardiomyocytes derived from human embryonic stem cells, as shown by enhanced gene expression and contractile properties [37].
2.3 Bioreactors with medium perfusion
Regulatory signals can be generated by secretion of growth factors and interstitial flow [25], with the 3D cell culture incorporating biomimetic transport and signaling more faithfully than the 2D cell culture (Figure 2A) [26]. Small convective flows driven by cilia can be observed already during early embryo development [27]. In contrast, cell culture plates do not capture the environment to which the cells are exposed in the body: the exchange of metabolites from one cell to another, the extracellular matrix environment that provides chemical and mechanical cues, and the physiological fluid flow are all missing.
Bioengineering is now becoming increasingly successful in reproducing the actual environmental milieu of development, regeneration, and disease, while enabling real-time insights into cellular and morphogenic events. Although it is unreasonable to expect three-dimensional tissue models to exactly match native tissues, bioreactors can recapitulate some of the physiological functions necessary for cardiac maturation and the use of cells for myocardial regeneration (Figure 2B). Additionally, the integration of microfluidics enables effective transport of nutrients and metabolites, beyond the distances limited by diffusional penetration depth for various molecules, and most critically oxygen.
Previous studies have shown that media perfusion supports the engineering of millimeters thick cardiac tissues with increased cell viability and cellular homogeneity (7). Radisic and colleagues have developed perfusion platforms providing convective - diffusive oxygen transport that enabled creation of thicker, more functional cardiac tissues (7). To this end, they developed a scaffold containing a bundle of preformed capillary-like parallel channels that could be arranged to support physiologically relevant cell concentrations, and supplemented an oxygen carrier, perfluorocarbon (PFC) to the culture medium that is perfusing the cell-seeded scaffolds. In this setting, the channels were designed to mimic the capillaries inside the heart muscle, while the PFC was used to mimic the native oxygen carrier hemoglobin by enhancing the carrying capacity of oxygen. A steady-state mathematical model accounting for diffusion, convection and oxygen consumption by the cells was developed to relate oxygen distribution within the tissue to flow parameters, channel diameter, length and spacing, the concentration of PFC, and cell density. This mathematical model helped define conditions for engineering tissues with a specific thickness and cell density. This study established some of the initial principles for “ biomimetic” tissue engineering of cardiac muscle.
Subsequent studies by Maidhof, Tandon and colleagues [38, 39] demonstrated the beneficial effects of combining electrical stimulation with media perfusion for cardiac tissue formation. Protection of cardiomyocytes (localized only in the bulk phase of the porous scaffold) from hydrodynamic shear (associated with the flow that is localized only in perfused channels), and the application of electrical stimulation synergistically enhanced the functionality of tissue constructs. Lower excitation thresholds (indicative of cellular electrical excitability), higher maximum capture rates (indicative of increased intracellular connections), and higher contraction amplitudes (indicative of functional contractile maturation) were measured for young animal cardiomyocytes grown in channeled scaffolds with medium perfusion and electrical stimulation [38, 39].
2.4 Bioreactors with electromechanical stimulation
Current approaches to cardiac tissue engineering provide cardiogenic cells on scaffolds with sequences of biochemical, mechanical and electrical signals, using biomimetic bioreactor settings (Figure 2B, D). In recent years, the engineering designs are increasingly based on biological principles, and attempt recapitulating some of the key developmental events. To this end, a number of bioengineering tools are finding utility in tissue engineering, by subjecting the cultured tissues to gradients of biochemical factors, hydrodynamic forces associated with perfusion, pulsatile electrical stimulation, and mechanical stretch. Advanced imaging modalities and the modeling of heart function are revealing new details of the physiological blueprints of cardiac development [23].
Thus, the in vitro platforms for maturing cardiac tissues will need to demonstrate several distinguishing features of the heart muscle: (1) Anisotropic cell alignment for proper signal propagation, (2) Excitation-contraction (EC) coupling, (3) Synchronous contractions of the tissue at physiologically relevant rates, (4) Ultrastructural and molecular characteristics similar to those in the native tissue, (5) Effective exchange of nutrients and metabolites between the cells and their environment, and (6) Responsiveness of the tissue to electrical pacing stimuli.
2.4.1 Mechanical Stimulation
Because mechanical stimuli are such a critical aspect of the cardiac function, there have been numerous efforts to implement these stimuli in the in vitro cultures of cardiac tissues (Figure 2B, right). Incorporation of passive tension induced anisotropic alignment of the cells that mimicked the cellular arrangement found in vivo, and facilitated electrical conduction. Studies by Zimmerman and colleagues of neonatal rat heart cells in collagen gels demonstrated that mechanical stretch enables engineering of cardiac tissues capable of generating substantial mechanical forces [34, 35].
However, cardiac tissues derived using currently available methods from a variety of cell sources, including the human iPS cells, fail to develop a positive force-frequency relationship, which is a hallmark of adult cardiac tissue. Also, these immature cardiac tissues are unable to achieve functional integration with the host myocardium. Because the contractions of cardiomyocytes are elicited by binding of calcium to cardiac troponin C (cTnT), it is of interest to implement bioreactors with mechanical stimulation to upregulate the expression and assembly of cTnT and thereby enhance the contractile function of engineered cardiac muscle. Additionally, during mechanotransduction, the extracellular matrix (ECM) and cytoskeletal organization sense and respond to mechanical stimuli. Thus, an appropriate structural template and the right types of mechanical forces need to be incorporated into bioengineered approaches to hSC-CM maturation. For mechanical loading, three different modes of stretch have been investigated: (i) static (constant stretch), (ii) phasic (cyclic stretch), and (iii) auxotonic (proportional resistance on a flexible base) [28].
2.4.2 Electrical Stimulation
To pump blood, the heart contracts rhythmically in response to electrical signals that are converted into mechanical responses through a process known as excitation-contraction coupling (ECC). In order to mimic these in vivo conditions, the same signals are being recreated in vitro. An electrical field can be applied within a bioreactor through the use of parallel carbon rods connected to an external stimulator. Studies by Radisic and colleagues pioneered the utilization of bioreactors delivering electrical signals to direct cell maturation and assembly into functional tissue units. Electrical stimulation was shown to upregulate cardiac genes, align CMs, and electrically couple cells for enhanced contractility and function [16]. Electrical field stimulation is less biomimetic than electrical pacing via point stimulation, but is extensively used as it helps overcome the initial spatial heterogeneity and incomplete interconnectivity of cells within engineered tissues, and deliver electrical signals throughout the engineered tissue.
Recently, a study by Nunes et al. [7] demonstrated the use of a “biowire” platform to mature hiPS-CMs through the use of 3D cell culture and electrical stimulation (Figure 2B, right). Aligned cardiac tissues (biowires) were created by encapsulating hiPS-CMs and supporting cells (fibroblasts, endothelial cells, smooth muscle cells) in collagen hydrogel around a suture placed in a small rectangular well. The biowires were about 300μm thick, which is thin enough for maintaining viability by simple diffusional transport of nutrients and oxygen, without convective flow. Upon gel compaction and alignment along the suture, the electrical field stimulation was used to enhance hSC-CM maturation.
While previous studies have shown positive effects of electrical stimulation on CM functionality, this was the first study to explore the effects of electrical stimulation regimens on CM maturation. Ultimately, physiological hypertrophy and maturation of the hSC-CM biowires were demonstrated, and the establishment of these features depended on the stimulation regime. Comparison of low frequency ramp up regimens (1–3Hz) versus high frequency ramp-up regimen (1–6Hz) revealed that cells exposed to the high frequency regimen of stimulation exhibited a greater extent of maturation, as evidenced by physiological hypertrophy, maturation of the contractile apparatus (sarcomeric banding, increased density of mitochondria and desmosomes) and functional properties approaching those of adult cardiac tissue.
This study demonstrated the feasibility of proper excitation-contraction coupling of cultured iPS-CMs. The depolarization of the cell membrane, as a result of the action potential, creates an increased concentration of cytosolic [Ca2+] which activates the calcium induced calcium response (CICR) that leads to contraction. Over time, the buildup of cytosolic [Ca2+] upregulates genes related to calcium handling, effectively enhancing tissue maturation. Synchronization of electrical stimuli results in tightly coupled ECC mechanisms that are important for integration of the engineered tissue into the host myocardium.
The need for biophysical stimulation to mature iPS-CMs comes from the known roles of these cues in cardiac development, and their lasting influence on cardiac function. Native myocardial features include a highly aligned and dense cell network, efficient transport mechanisms, electrical signal propagation and generation of contractile forces. By providing biophysical stimuli using custom designed bioreactors, the culture environment can replicate some of the important cues associated with cardiac development, and most importantly the mechanical stretch and electrical signal propagation. It is expected that the fate of cardiomyocytes – in vitro and in vivo – will be dictated by these cues, providing tools for engineering functional cardiac tissues.
3. Approaches to myocardial regeneration
Clinical translation of cell-based therapies of cardiac regeneration will further depend on the development of minimally invasive and effective methods for delivering engineered tissues/cells into the heart. The key requirements for such methods include the cell retention at the site of delivery, maintenance of cell viability, protection of cells from the harsh environment of cardiac injury, and facilitation of cell integration and coupling with the host tissue. Delivery of the therapeutic cells, alone or within a biomaterial, and engineered tissues are facing different sets of challenges.
3.1 Cell delivery into the heart
Simple cell injections lead to considerable cell death, as well as poor cell retention and engraftment. Numerous factors are associated with cell death in the ischemic environment of the infarct zone.
The first factor is the loss of matrix; when anchorage dependent cells lose their contact with ECM they die through anoikis as survival signals are lost [40].
The second factor is ischemia. Injected cells experience the loss of nutrients and oxygen in the infarct zone due to the lack of blood flow, leading to the depletion of ATP and acidosis associated with the anaerobic glycolysis pathway. Ionic homeostasis is disrupted, and the resulting osmotic loads lead to membrane damage and cell death [22]. Ischemia can also lead to increased mitochondrial permeability secondary to the formation of reactive oxygen species [23].
The third factor is inflammation. In response to injury, large amounts of leukocytes – neutrophils as a first response, macrophages thereafter – migrate to the infarct area [24], producing oxygen-derived free radicals and cytokines that can damage injected cells or initiate signaling cascades resulting in apoptosis. This effect is most significant during acute myocardial injury, but may also affect cells injected into a chronic infarct, since macrophages preferentially localize to the nonhomogeneous regions of engraftment.
These three factors, individually and in combination, heavily influence the cells’ ability to stay in the infarct region, survive and exert benefit. The best reported values for cell retention are <10% in animals [41–43] and for intracoronary infusions in patients [44]. The cells end in other organs such as liver and lung, carrying various risks, including tumor formation for stem and progenitor cells [45].
To improve survival of delivered cells, various strategies to directly manipulate the cells have been developed. For example, transduction with survival factors like Bcl-2 prior to injection, or inducing pro-survival signals through heat shock have been investigated. [27, 30] However, these methods do not address cell attachment. To this end, alternative biomaterial approaches to cell delivery have been studied. Two interesting modalities are the injection of biocompatible hydrogels loaded with cells into the myocardial wall [46–49], and the implantation of cell-seeded patches onto the epicardial surface [50–52]. In both cases, the extracellular matrix improves cellular retention and survival at the injury region. Biomaterials can also confer some protection against the damaging effects of ischemia and inflammation.
Other methods for cell delivery to the infarct zone include the intracoronary infusion, intravenous administration, retrograde coronary sinus delivery, and intramyocardial administration (Figure 3A) [53]. It has been shown that the cell retention rate within the heart is 11% for intramyocardial, 3% for intracoronary, and 3% for intravenous delivery [54]. Intramyocardial injection has two subtypes: epicardial and transendocardial (guided by fluoroscopy and/or electromechanical mapping), both of which are compatible with the percutaneous catheter-based approach. This method has the highest retention rate, but is limited by the invasive nature of the procedure [53]. In contrast, intracoronary infusion is an easier route that can result in a more uniform cell distribution in the infarct zone. However, this procedure is associated with transpulmunory first-pass attenuation effects on cells [55], and low cell retention. Tissue engineering approaches – such as cell delivery by a cardiac patch - can improve cell retention, but again the patch needs to be surgically implanted onto the heart.
Figure 3. Delivering cells and engineered patches into the heart.
(A) Routes of stem cell delivery: intracoronary infusion, retrograde coronary sinus delivery, epicardial injection, intravenous infusion, and transendocardial injection. (B) Animal models: Comparison of frequently used animal models. Scores are from 1 (low) to 5 (high) in each category. The data in panel B are taken in part from reference [70].
3.2. Tissue engineering of a patch for implantation
Recent approaches have used the patch not only for the delivery and retention of cells but also to recapitulate complex structure of the cardiac tissue [56]. The use of a patch extends to prevention of high cell washout and the provision of some immediate cardiac function. Tissue engineered approaches seek to effectively combine cells, scaffolds/ECM, and biophysical stimuli to increase the maturation and functionality of the patch, and enhance its myocardial regeneration capabilities.
Repairing the myocardial structure and function impaired by cardiovascular disease (CVD) requires the engineering approaches at multiple hierarchical levels. At the nanometer scale, biophysical cues that drive the establishment of proper ion channels and gap junctions are critical for electrical signal propagation. At the micrometer scale, efficient transport of nutrients and wastes is critical for supporting the high metabolic demands of cardiac tissue. The establishment of effective vascular and capillary networks enables larger tissues to be engineered. At the millimeter scale, biophysical cues are used to anisotropically align cardiomyocytes. Here, electromechanical stimulation upregulates the establishment of tight structural and contractile connections, through excitation-contraction coupling.
While scaffolds provide templates for structural organization of the cells, it is expected that they will be degraded over time and gradually replaced with the new matrix upon implantation into the myocardium. Alternatively, scaffold-free tissue constructs, known as cell sheets formed by combining multiple confluent cell monolayers have been developed by Okano and colleagues [57, 58]. Upon implantation into the myocardium, these layered sheets demonstrated increased vascularization and contractile function. These benefits might result from either recovered functionality upon integration of the implanted and host tissue, or the secondary paracrine mechanisms that promote favorable responses. As more studies of tissue engineered myocardial regeneration are conducted, the mechanisms behind their beneficial role can be established.
To select the animal model that is best suited for the heart regeneration studies, anatomical, physiological, and pathophysiological properties of different species must be considered. In the specific case of cardiac tissue engineering, additional functional parameters should be considered, such as the heart rate, heart size (and the numbers of cells needed), and regeneration capability (Figure 3B). The mismatch between the physiology and functionality of human and animal model could skew the data. For example, the mismatch between the native beating frequency of human pluripotent stem cells and rodents may prevent host–graft coupling or arrhythmias that could occur in humans [59].
4. Challenges and future work
The maturity of iPS-CMs represents a major challenge associated with bioengineered treatments of myocardial infarction. Calcium handling and homeostasis are the key regulators of EC coupling within cardiac tissues that are necessary for functional maturation. The development of a functional sarcoplasmic reticulum (SR) enables proper kinetics of Ca2+ storage and release/uptake. The key proteins associated with calcium handling include sarcoplasmic reticulum calcium-ATPase (SERCA), ryanodine receptor-2 (RyR2), junction (Jun), and calsequestrin-2 (CASQ2). The SERCA functions to actively re-uptake calcium from the cytosol into the SR during diastole, and the immature calcium handling associated with hSC-CMs can be due to the underdeveloped SERCA. This kind of immaturity can directly lead to pro-arrhythmic conditions and render the cells unsuitable for myocardial regeneration. Maturation of calcium handling profiles has been reported in studies of electromechanical stimulation within bioreactors. However, the lack of t-tubules in hSC-CMs, necessary for co-localization of L-type Ca channels and RyR’s, may explain the eventual lack of mature calcium handling.
Contractile forces should be eventually increased to levels matching those in the native cardiac tissue (50 mN/mm2 under optimized preload). Currently, cell densities within bioengineered tissues are lower than those in the native tissue and thus, provide less contractile “machinery” for generating contractile force. Thus, it is expected that the optimized electromechanical conditioning regimens and increased cell density will result in increased force generation.
Although the lack of mature hiPS-CMs continues to hinder the development of physiologically relevant cardiac tissues, current studies have yielded promising results by the application of multiple biophysical stimuli. During development, the neonatal heart rate undergoes many frequency changes as it matures, with an average rate of 3 Hz [23]. Surprisingly, studies by Nunes and colleagues revealed that the electrical stimulation of the cardiac tissues at higher frequencies was beneficial [8]. This finding suggests that it may be beneficial to adopt the electrical stimulation regime that forces the cells to beat at their highest possible frequency in order to induce maturation. Cyclic mechanical stretch has also shown promising effects on maturation of 3D tissues formed of human embryonic stem cell-derived cardiomyocytes [60]. Overall, an optimal combination of mechanical, electrical, biochemical, and microfluidic stimuli responsible for driving hSC-CM maturation still need to be defined.
To determine if the bioengineered tissue is sufficiently mature for myocardial regenerative approaches, physiologically relevant responses to physiological interventions need to be established. Specifically, the Frank-Starling mechanism should be demonstrated, with the force increasing in response to the increased preload, and the increased force in response to increased stimulation frequency (positive force-frequency relationship). Similarly, the electrical properties responsible for generating contractile forces should be similar to those in the native cardiac tissue, and involve functional SR for [Ca2+] storage that would result in the increase in the force of contraction with an increase in [Ca2+]. Advanced molecular analysis and imaging at high spatiotemporal resolution are needed to link electromechanical stimuli and morphogenesis of heart and understand the exact role of electromechanical loading on cardiac tissue maturation [61].
Vascularization is another major bottleneck in engineering functional cardiac tissue. High metabolic rate of cardiomyocytes necessitates a dense capillary system in the cardiac tissue; even 30 minutes without blood flow may be long enough to induce apoptosis in CMs [62]. Engineered cardiac tissue is either vascularized prior to implantation or conditioned for improved survival until being vascularized following implantation. Numerous methods have been proposed for each of these two approaches. For prevascularization, blood vessels could be microfabricated in the cardiac tissue prior to implantation while bioactive scaffold could be used to promote vascularization post implantation. While larger sized engineered tissues require vasculature to effectively transport nutrients and most critically oxygen, the necessity of establishing functional vascularization before implantation is not clearly defined [71]. Delayed vascularization strategies include implanting engineered tissues containing channels/pores [63] or chemical growth factors to guide angiogenesis. Additional approaches are again based on utilizing paracrine signaling to induce the formation of vasculature [64, 65].
During cardiac development, the initially avascular heart progressively develops an integrated vascular network as the heart matures [66]. Therefore, engineered tissues that are small enough to maintain sufficient transport in vitro could potentially be used for in vivo transplantation, where they will subsequently become vascularized. It is not clear if prevascularization or avascular tissue implantation will lead to better results. Yet, there might be a middle ground solution, where engineering methods could guide the formation of the vascular tissue post implantation [71].
Inflammatory response initiated after myocardial infarction also plays a major role in vascularization and survival of implanted tissues. The inflammatory response is characterized by polarization of steady-state macrophages (M0) into “classical” (M1) macrophages, associated with inflammation, tumor rejection, and insulin resistance [14], followed by “alternative” (M2) macrophages, associated with assist ECM turnover, angiogenesis, and improved insulin sensitivity [15]. These responses result in significant production of cytokines and growth factors and thus, the resulting immune response should considered when determining the proper timing for myocardial regenerative therapies. Studies by Laflamme et al. revealed the necessity of using additional pro-survival factors to inhibit the negative effects of the immune response so that the transplanted cardiac graft was able to survive [67]. Recent studies suggest that the inflammatory cells and cytokines can in fact be harnessed to augment and facilitate vascularization and regenerative processes [72].
In summary, recent advances in the derivation of immature but committed cardiomyocytes from human iPS cells and tissue engineering methods for the assembly of these cells into synchronously contracting cardiac tissue organoids have unprecedented potential to lead to the new and effective modalities for treating cardiac disease. Bioengineering methods are now becoming available for maturing these cardiomyocytes and generating human cardiac tissues of biological fidelity not achieved ever before. The implementation of biophysical signals that are inherent to the development and function of the heart is instrumental for the in vitro cultivation of cardiac cells and tissues and their delivery to the heart.
Acknowledgments
The authors gratefully acknowledge funding by the NIH (grants HL076485, EB002520 and EB 17103) and NYSTEM (grant C028119). Additionally, the authors would like to thank Bryan Z. Wang for his help with the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minino AM. Death in the United States, 2011. NCHS Data Brief. 2013;(115):1–8. [PubMed] [Google Scholar]
- 3.Pedron S, et al. Stimuli Responsive Delivery Vehicles for Cardiac Microtissue Transplantation. Advanced Functional Materials. 2011;21(9):1624–1630. [Google Scholar]
- 4.Wang F, Guan JJ. Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy. Advanced Drug Delivery Reviews. 2010;62(7–8):784–797. doi: 10.1016/j.addr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Chavakis E, Koyanagi M, Dimmeler S. Enhancing the Outcome of Cell Therapy for Cardiac Repair Progress From Bench to Bedside and Back. Circulation. 2010;121(2):325–335. doi: 10.1161/CIRCULATIONAHA.109.901405. [DOI] [PubMed] [Google Scholar]
- 6.Liu JB, et al. Autologous stem cell transplantation for myocardial repair. American Journal of Physiology-Heart and Circulatory Physiology. 2004;287(2):H501–H511. doi: 10.1152/ajpheart.00019.2004. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakaran MP, et al. Biomimetic material strategies for cardiac tissue engineering. Materials Science & Engineering C-Materials for Biological Applications. 2011;31(3):503–513. [Google Scholar]
- 8.Nunes SS, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10(8):781–7. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res. 1998;39(2):280–300. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- 11.Marelli D, et al. Cell transplantation for myocardial repair: an experimental approach. Cell Transplant. 1992;1(6):383–90. doi: 10.1177/096368979200100602. [DOI] [PubMed] [Google Scholar]
- 12.Moscona AA. Tissues from dissociated cells. Sci Am. 1959;200(5):132–4. doi: 10.1038/scientificamerican0559-132. passim. [DOI] [PubMed] [Google Scholar]
- 13.Parker KK, et al. Myofibrillar architecture in engineered cardiac myocytes. Circ Res. 2008;103(4):340–2. doi: 10.1161/CIRCRESAHA.108.182469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenburgh HH, Karlisch P, Farr L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitro Cell Dev Biol. 1988;24(3):166–74. doi: 10.1007/BF02623542. [DOI] [PubMed] [Google Scholar]
- 15.Eschenhagen T, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. Faseb Journal. 1997;11(8):683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 16.Radisic M, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101(52):18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chicurel ME, et al. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature. 1998;392(6677):730–3. doi: 10.1038/33719. [DOI] [PubMed] [Google Scholar]
- 18.Hammond GL, Wieben E, Markert CL. Molecular Signals for Initiating Protein-Synthesis in Organ Hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(5):2455–2459. doi: 10.1073/pnas.76.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda T, et al. N-cadherin-mediated cell adhesion determines the plasticity for cell alignment in response to mechanical stretch in cultured cardiomyocytes. Biochemical and Biophysical Research Communications. 2005;326(1):228–232. doi: 10.1016/j.bbrc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Yamane M, et al. Rac 1 activity is required for cardiac myocyte alignment in response to mechanical stress. Journal of Pharmacological Sciences. 2007;103:98p–98. doi: 10.1016/j.bbrc.2006.12.144. [DOI] [PubMed] [Google Scholar]
- 21.Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res. 2006;72(3):375–83. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Opie LH, et al. Controversies in ventricular remodelling. Lancet. 2006;367(9507):356–67. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 23.Lindsey SE, Butcher JT, Yalcin HC. Mechanical regulation of cardiac development. Front Physiol. 2014;5:318. doi: 10.3389/fphys.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cirotto C, Arangi I. How Do Avian Embryos Breathe - Oxygen-Transport in the Blood of Early Chick-Embryos. Comparative Biochemistry and Physiology a-Physiology. 1989;94(4):607–613. doi: 10.1016/0300-9629(89)90602-6. [DOI] [PubMed] [Google Scholar]
- 25.Granados-Riveron JT, Brook JD. The impact of mechanical forces in heart morphogenesis. Circ Cardiovasc Genet. 2012;5(1):132–42. doi: 10.1161/CIRCGENETICS.111.961086. [DOI] [PubMed] [Google Scholar]
- 26.Burggren W, et al. Body, eye, and chorioallantoic vessel growth are not dependent on cardiac output level in day 3–4 chicken embryos. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2004;287(6):R1399–R1406. doi: 10.1152/ajpregu.00086.2004. [DOI] [PubMed] [Google Scholar]
- 27.Reckova M, et al. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circulation Research. 2003;93(1):77–85. doi: 10.1161/01.RES.0000079488.91342.B7. [DOI] [PubMed] [Google Scholar]
- 28.Kamino K, Hirota A, Fujii S. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature. 1981;290(5807):595–7. doi: 10.1038/290595a0. [DOI] [PubMed] [Google Scholar]
- 29.Bartman T, et al. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004;2(5):E129. doi: 10.1371/journal.pbio.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berdougo E, et al. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development. 2003;130(24):6121–6129. doi: 10.1242/dev.00838. [DOI] [PubMed] [Google Scholar]
- 31.Chi NC, et al. Cardiac conduction is required to preserve cardiac chamber morphology. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14662–14667. doi: 10.1073/pnas.0909432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407(6801):221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 33.Hove JR, et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421(6919):172–7. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 34.Eschenhagen T, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 1997;11(8):683–94. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann WH, et al. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng. 2000;68(1):106–14. [PubMed] [Google Scholar]
- 36.Godier-Furnemont AF, et al. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc Natl Acad Sci U S A. 2011;108(19):7974–9. doi: 10.1073/pnas.1104619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan Y, et al. Hybrid Gel Composed of Native Heart Matrix and Collagen Induces Cardiac Differentiation of Human Embryonic Stem Cells without Supplemental Growth Factors. Journal of Cardiovascular Translational Research. 2011;4(5):605–615. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tandon N, et al. Portable bioreactor for perfusion and electrical stimulation of engineered cardiac tissue. Conf Proc IEEE Eng Med Biol Soc. 2013:6219–23. doi: 10.1109/EMBC.2013.6610974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maidhof R, et al. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J Tissue Eng Regen Med. 2012;6(10):e12–23. doi: 10.1002/term.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zvibel I, Smets F, Soriano H. Anoikis: roadblock to cell transplantation? Cell Transplant. 2002;11(7):621–30. doi: 10.3727/000000002783985404. [DOI] [PubMed] [Google Scholar]
- 41.Dow J, et al. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res. 2005;67(2):301–7. doi: 10.1016/j.cardiores.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Qiao H, et al. Death and proliferation time course of stem cells transplanted in the myocardium. Mol Imaging Biol. 2009;11(6):408–14. doi: 10.1007/s11307-009-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, et al. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33(5):907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann M, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111(17):2198–202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 45.Cao F, et al. Spatial and temporal kinetics of teratoma formation from murine embryonic stem cell transplantation. Stem Cells Dev. 2007;16(6):883–91. doi: 10.1089/scd.2007.0160. [DOI] [PubMed] [Google Scholar]
- 46.Roche ET, et al. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials. 2014;35(25):6850–8. doi: 10.1016/j.biomaterials.2014.04.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dib N, et al. Recommendations for successful training on methods of delivery of biologics for cardiac regeneration: a report of the International Society for Cardiovascular Translational Research. JACC Cardiovasc Interv. 2010;3(3):265–75. doi: 10.1016/j.jcin.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Sherman W, et al. Catheter-based delivery of cells to the heart. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S57–64. doi: 10.1038/ncpcardio0446. [DOI] [PubMed] [Google Scholar]
- 49.De La Fuente LM, et al. Transendocardial autologous bone marrow in chronic myocardial infarction using a helical needle catheter, two year follow-up in an open-label, non-randomized, pilot study (the TABMMI study) Circulation. 2008;118(12):E442–E442. doi: 10.1016/j.ahj.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 50.Kochupura PV, et al. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112(9 Suppl):I144–9. doi: 10.1161/CIRCULATIONAHA.104.524355. [DOI] [PubMed] [Google Scholar]
- 51.Wei HJ, et al. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29(26):3547–56. doi: 10.1016/j.biomaterials.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Wendel JS, et al. Functional Consequences of a Tissue-Engineered Myocardial Patch for Cardiac Repair in a Rat Infarct Model. Tissue Engineering Part A. 2014;20(7–8):1325–1335. doi: 10.1089/ten.tea.2013.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dib N, et al. Cell therapy for cardiovascular disease: a comparison of methods of delivery. J Cardiovasc Transl Res. 2011;4(2):177–81. doi: 10.1007/s12265-010-9253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou D, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112(9 Suppl):I150–6. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 55.Strauer BE, Kornowski R. Stem cell therapy in perspective. Circulation. 2003;107(7):929–34. doi: 10.1161/01.cir.0000057525.13182.24. [DOI] [PubMed] [Google Scholar]
- 56.Sarig U, Machluf M. Engineering cell platforms for myocardial regeneration. Expert Opin Biol Ther. 2011;11(8):1055–77. doi: 10.1517/14712598.2011.578574. [DOI] [PubMed] [Google Scholar]
- 57.Sakaguchi K, et al. In vitro engineering of vascularized tissue surrogates. Sci Rep. 2013;3:1316. doi: 10.1038/srep01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sekine H, et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shiba Y, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489(7415):322–5. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mihic A, et al. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials. 2014;35(9):2798–808. doi: 10.1016/j.biomaterials.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 61.Kowalski WJ, et al. Investigating developmental cardiovascular biomechanics and the origins of congenital heart defects. Front Physiol. 2014;5:408. doi: 10.3389/fphys.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye L, et al. Patching the Heart Cardiac Repair From Within and Outside. Circulation Research. 2013;113(7):922–932. doi: 10.1161/CIRCRESAHA.113.300216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radisic M, et al. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12(8):2077–91. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 64.Caspi O, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100(2):263–72. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 65.Narmoneva DA, et al. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110(8):962–8. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rakusan K, et al. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992;86(1):38–46. doi: 10.1161/01.cir.86.1.38. [DOI] [PubMed] [Google Scholar]
- 67.Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 68.Legant WR, et al. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(25):10097–10102. doi: 10.1073/pnas.0900174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang G, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nature Medicine. 2014;20(6):616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meyer U. Fundamentals of tissue engineering and regenerative medicine. Berlin: Springer; 2009. p. xxvi.p. 1049. [Google Scholar]
- 71.Montgomery M, et al. Cardiac tissue vascularization: From angiofenesis to microfluidic blood vessels. J Cardiovasc Pharmacol Ther. 2014;19(4):382–393. doi: 10.1177/1074248414528576. [DOI] [PubMed] [Google Scholar]
- 72.Spiller KL, et al. Macrophages modulate engineered human tissues for enhanced vascularization and healing. Ann Biomed Eng. 2015;43(3):616–27. doi: 10.1007/s10439-014-1156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]





