Abstract
Background
We conducted a phase II study in men with castration sensitive metastatic prostate cancer to test the hypothesis that AT-101, a small molecule Bcl-2 inhibitor, has clinical activity in patients initiating androgen deprivation therapy (ADT) for metastatic prostate cancer.
Methods
Patients with metastatic prostate cancer scheduled to start, or recently (within 6 weeks) initiated ADT, were enrolled. ADT with a luteinizing hormone-releasing hormone agonist and bicalutamide started 6 weeks prior to initiation of oral AT-101, 20 mg/day for 21 days of 28-day cycle. The primary endpoint of the study was percentage of patients with undetectable PSA (less than or equal to 0.2) after 7.5 months (1.5 months of ADT alone plus 6 months of combined ADT and AT-101). To assess for an association between CHD1 and drug sensitivity, FISH with confocal microscopy was assessed in a subgroup of patients.
Results
Fifty-five patients enrolled, median age 61 years, median PSA of 27.6 ng/dL, and 72% had a Gleason score > 8. Three patients had visceral metastases and the remaining patients had bone or nodal metastasis. An undetectable PSA was achieved in 31% of patients. Twelve patients experienced serious adverse events (SAEs), and seven were considered related to study therapy. The majority of related adverse events were gastrointestinal and nervous system disorders. CHD1 assessment was feasible with a non-significant association with therapeutic sensitivity in a small number of patients.
Conclusion
The combination of ADT and AT-101 did not meet the pre-specified level of activity for further development of this combination
Introduction
Prostate cancer remains a significant source of morbidity and mortality. In 2015, it is estimated that approximately 28,000 men will die from metastatic castration-resistant disease [1]. While androgen deprivation therapy (ADT) is standard initial systemic treatment for advanced prostate cancer, development of resistance is inevitable in the vast majority of patients, generally occurring within 20 months of initial castration therapy [2]. Although newer androgen receptor signaling pathway directed approaches are now available, resistance eventually develops to these agents as well, supporting further efforts at improving the response to initial ADT through the identification and abrogation of mechanisms of drug resistance early in the disease course.
A common mechanism of resistance to various therapeutic agents is overexpression of apoptotic inhibitors, including the Bcl-2 family of proteins. Prior studies in prostate cancer tissue demonstrated that Bcl-2 is over-expressed in most patients with castration-resistant prostate cancer (CRPC) [3, 4]. In animal models, Bcl-2 is responsible for drug resistance to chemotherapy and ADT, and modulation of Bcl-2 improves sensitivity [5, 6]. To date, several strategies have been employed to inhibit Bcl-2 including use of a clinical bcl-2 antisense or modulation of bcl-2 expression with the combination of interferon and cis-retinoic acid [3, 4, 7, 8]. AT-101 [R-(–)-gossypol acetic acid; Ascenta Therapeutics, Inc.] is an enantiomer of racemic gossypol, a natural substance found in cottonseeds. AT-101 binds to the BH3 domain of Bcl-2, Bcl-xL, Mcl-1 and Bcl-w, blocking apoptosis inhibitors while potentially simulating the pro-apoptotic proteins Noxa and Puma [9, 10]. In the VCap human prostate cancer cell line, Bcl-2 expression was significantly upregulated in androgen independent cells in comparison to androgen-dependent cells, suggesting that abrogating Bcl-2 activity in combination with castration may delay onset of castrate resistant prostate cancer [11]. Further studies confirmed that in vitro, and in the VCaP prostate cancer xenograft, AT-101 was synergistic with androgen deprivation leading to decreased tumor volume, and delayed onset of androgen independence [11, 12]. Furthermore, androgen receptor activation by dihydrotestosterone attenuated AT-101-induced apoptosis, by upregulating Bcl-2 and Bcl-Xl, while inhibition of androgen receptor with bicalutamide restored AT-101-induced apoptosis, demonstrating that androgen deprivation and Bcl-2 inhibition act cooperatively to induce apoptosis. Together these studies provide a strong rationale for clinical studies of the synergy between AT-101 and ADT.
We thus conducted a phase II trial in men with castrate sensitive metastatic prostate cancer to test the hypothesis that AT-101, a small molecule Bcl-2 inhibitor, improves clinical outcomes of patients initiating ADT for metastatic prostate cancer. Based on the results from SWOG 9346 [13] demonstrating that PSA nadir after 7 months of combined ADT predicts survival, we utilized a novel phase II trial design, in which the primary endpoint was the percentage of patients with PSA ≤ 0.2 ng/ml at 7 months of treatment initiation. To develop potential predictive biomarkers for future studies, Bcl-2 protein levels were assessed in peripheral blood mononuclear cells and tumor CHD1, a potential predictor of androgen sensitivity, was assessed in tumor tissue.
Materials and Methods
Study Design
The trial (ClinicalTrials.gov: NCT00666666) was conducted with approval from the Institutional Review Boards (IRB) of Rutgers Robert Wood Johnson Medical School (New Brunswick, NJ), University of Michigan (Ann Arbor, MI). University of Chicago (Chicago, IL), and University of Wisconsin (Madison, Wisconsin).
This was an open label, multicenter phase 2 study of AT-101, in patients with newly diagnosed, castrate sensitive metastatic prostate cancer. Subjects were allowed to initiate treatment with standard of care ADT up to 6 weeks prior to starting study therapy with AT-101. ADT was delivered using physician choice of a commercial LHRH agonist. Daily oral bicalutamide 50 mg was required during the first month of LHRH agonist therapy, but continued bicalutamide use was optional. AT-101 was administered orally 20 mg/day on days 1 to 21 of a 28- day cycle. Patients could receive up to 8 cycles of treatment with AT-101, marking the completion of study therapy. Following study treatment, ADT could be continued at the discretion of the treating physician.
Dose modifications of 50% of the AT-101 were allowed in the event of grade 2 persistent or intolerable hematologic and non-hematologic adverse events and grade 3 hematologic events. For grade 4 hematologic and grade 3 and 4 non-hematologic events, the dose was held until recovery and therapy was continued at a 50% reduction.
Patients
Eligible patients had histologically proven adenocarcinoma of the prostate, non-castrate levels of testosterone, soft tissue, visceral and/or bone metastasis, and an elevated PSA ≥ 5 ng/ml within 12 weeks prior to registration. Prior local therapy with radiation or surgery was allowed. Patients could not have received more than 12 months of ADT or anti-androgen therapy in the adjuvant/neoadjuvant setting, which must have been completed at least 12 months prior to registration. No prior ADT for metastatic disease was allowed beyond the 6 week lead in period with ADT prior to registration.
Study assessments
Safety evaluations included all adverse events (AE), clinical laboratory tests, vital sign measurements, physical examinations, and ECOG performance status evaluation during screening and every 4 weeks. ECG was performed at baseline. Complete blood count and complete metabolic panel were performed weekly during cycle 1 and then at the beginning of every cycle. Troponin was measured at baseline and at the beginning of every cycle.
Efficacy evaluation consisted of PSA measurement prior to initiation of ADT, prior to initiating AT-101 and every 4 weeks during treatment. CT scan of the chest abdomen and pelvis and bone scan were performed within 28 days of starting AT-101 and were repeated every 12 weeks, with off study scans completed within 4 weeks of the last cycle of AT-101.
For evaluation of correlative endpoints, paraffin embedded tumor tissue was collected from all patients when available. Peripheral blood mononuclear cells for correlative studies were collected on day 1 prior to starting AT-101 and on day 21 of AT-101.
Assessment of response
PSA was the primary endpoint to assess response, but the patient's treatment was governed by objective disease status. If a patient had progressive disease based on evaluation of measurable or non-measurable disease using Response Evaluation Criteria in Solid Tumors (RECIST) criteria [14] while experiencing stable or declining PSA, the patient discontinued protocol treatment. PSA response at the end of 7.5 months of ADT (6 months of combined ADT and AT-101) was evaluated as no response (PSA > 4.0 ng/ml), intermediate response (PSA ≥ 0.2 but < 4 ng/ml), or full response (PSA < 0.2 ng/ml).
Statistical Analysis
The primary endpoint was the percentage of patients with undetectable PSA (less than or equal to 0.2) after 7.5 months of ADT (1.5 months of ADT alone plus 6 months of combined ADT and AT-101). Based on historical data demonstrating the expectation of 48% of patients achieving PSA≤0.2 after 6 months of combined ADT alone, we would consider this a positive study, and worthy of future more definitive larger studies, if we found an undetectable PSA an additional 20% of patients over the expected rate; specifically in ≥ 68% of patients. Patients who do not complete 7.5 months of androgen deprivation therapy due to toxicity, disease progression or voluntary study withdrawal were considered to not meet the primary endpoint.
To detect the aforementioned improvement in the primary endpoint with a significance of 10% and power 90%, and utilizing a single stage design, at least 28 of 48 patients would need to experience a PSA ≤0.2 at the 7.5 month timepoint. To account for potential patient discontinuation prior to starting AT-101, the study was designed to enroll a total of 55 patients.
Results
Study Subjects
A total of 55 patients with a median age of 61 years (45-82 years) were enrolled at four sites. As shown in Table 1, the majority of patients had a Gleason Score greater than 8. Three patients had visceral metastasis and the remaining patients had bone or nodal metastasis. Eighty-eight percent of patients were Caucasian, 11% were African American.
Table 1. Demographic Data.
| (N=55) | N | Percent |
|---|---|---|
| Age | ||
| Mean | 61 | |
| Range | 45-82 | |
| PSA (Median Pre-ADT) | 27.6 range | 5.5- 7141 |
| Sites of Metastasis | ||
| Bone/Nodes | 52 | 94% |
| Visceral | 3 | 6% |
| Gleason Score | ||
| 6 | 2% | |
| 7 | 26% | |
| 8 | 21% | |
| 9 | 45% | |
| 10 | 6% | |
| PSA ≤ 0.2 ng/ml at end of 7.5 mo ADT | 17 | 31% |
| PSA > 0.2 and ≤ 4.0 ng/ml after 7.5 mo ADT | 14 | 25% |
| PSA > 4.0 ng/ml after 7.5 mo ADT | 2 | 4% |
ADT: Androgen Deprivation Therapy
mo: months
Treatment Delivery and Response
There were 23 patients that completed the 8 months of combined ADT and AT-101 allowed by protocol. A total of 33 patients (60%) received at least 7.5 months of ADT (6 months of combined ADT and AT-101). For the patients who discontinued treatment prior to 7.5 months of ADT, 11 patients (20%) had disease progression, 10 patients (18%) discontinued due to toxicity and 1 patient was lost to follow-up.
Analysis of the primary endpoint in 55 patients at the 7.5 month time point from initiation of ADT (and 6 months from initiation of combined ADT and AT-101) demonstrated that 17 (31%) achieved an undetectable PSA (≤ 0.2 ng/ml), 14 (25%) had PSA > 0.2 and ≤ 4.0 ng/ml and 2 (4%) had PSA >4ng/ml. No additional patients developed undetectable PSA after 7.5 months of ADT.
Toxicity
Treatment was discontinued in 35% (19/55) of patients due to adverse events occurring anytime on protocol. Ten patients (18%) discontinued treatment due to toxicity prior to evaluation for the efficacy endpoint at 7.5 months of ADT (6 months of combined ADT and AT-101). Nine patients (16%) discontinued treatment due to toxicity in months 7 or 8 of combined ADT and AT-101. Twelve patients (22%) experienced serious adverse events (SAE). Seven patients (13%) experienced SAEs considered related to study therapy. Grade 1/2 toxicities (%) included fatigue (36/11), hot flashes (25/9), nausea (20/9), anorexia (15/2), vomiting (9/9), constipation (15/2), dry skin (11/0), increased AST/ALT (31/4), hypercalcemia (7/0), anemia (18/0), and hyperglycemia (9/2). Sensory neuropathy was an unexpected toxicity that, regardless of attribution, was experienced by 13% (grade 1), 16% (grade 2) and 4% (grade 3) of patients. Other grade 3 toxicities were ileus in two patients, small intestine obstruction in one patient, and syncope in one patient.
Biomarker assessment
Western blots were performed on peripheral blood mononuclear cells at baseline and at cycle 1, day 21. Peripheral blood mononuclear cells were available for evaluation in 21 subjects. Twelve subjects had a >10% increase in Bcl-2 levels while nine subjects had a >10% decrease in Bcl-2 levels. Shown in Figure 1 are representative Bcl-2 levels from 3 patients with decreases in Bcl-2 protein levels by 74%, 47% and 34%, respectively. FISH and confocal microscopy was performed for CHD1 in eight patients for whom initial paraffin tumor blocks were available. As shown in Figure 2, CHD1 is detectable in prostate cancer by FISH assay. Figure 3 demonstrates the pretreatment assessment of CHD1 in patients treated with androgen deprivation and AT-101. Although clearly exploratory given the small number of patients, CHD1 correlated with therapeutic activity defined as highly sensitivity for PSA values that had a nadir ≤ 0.2 ng/ml, moderate sensitivity with PSA 0.2 to ≤ 4.0 ng/ml and resistance with PSA > 4.0 ng/ml.
Figure 1.
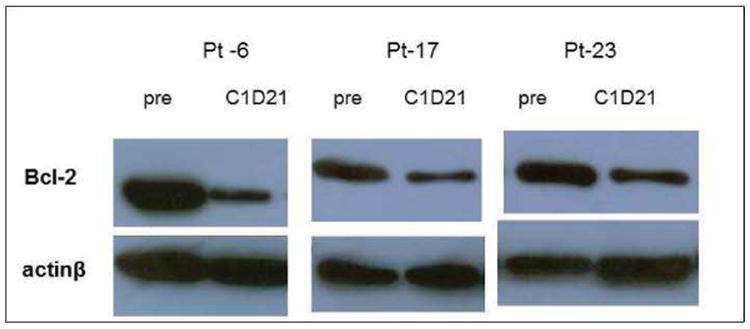
Bcl-2 protein expression in PBMCs: Bcl-2 levels were assessed by Western analysis. Three patients with decreases in Bcl-2 protein levels by 74%, 47% and 34% respectively are shown.
Figure 2.
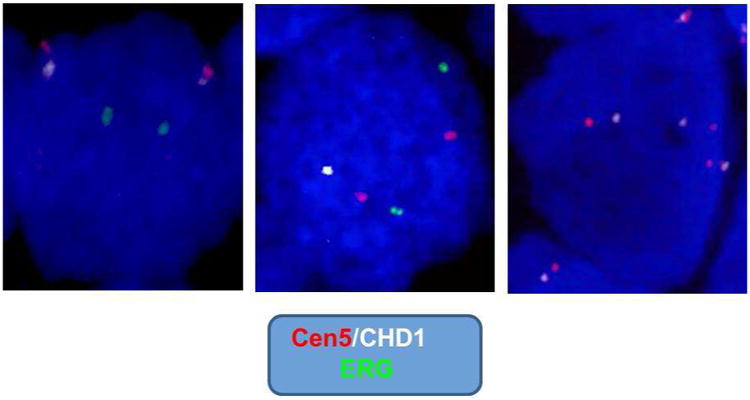
CHD1 rearrangements in prostate cancer: FISH assay for detection of CHD1 combining a specific BAC probe for CHD1 gene (white) and the probe- reporter located on peri-centromeric region of human chromosome 5 (Cen5, red). An ERG gene probe (green) was utilized as an additional reporter. The left panel represents normal CHD1 without deletion; the middle panel represents CHD1 gene mono-allelic deletion; and the right panel represents CHD1 gene copy number gain (three copies of CHD1 gene represented).
Figure 3.
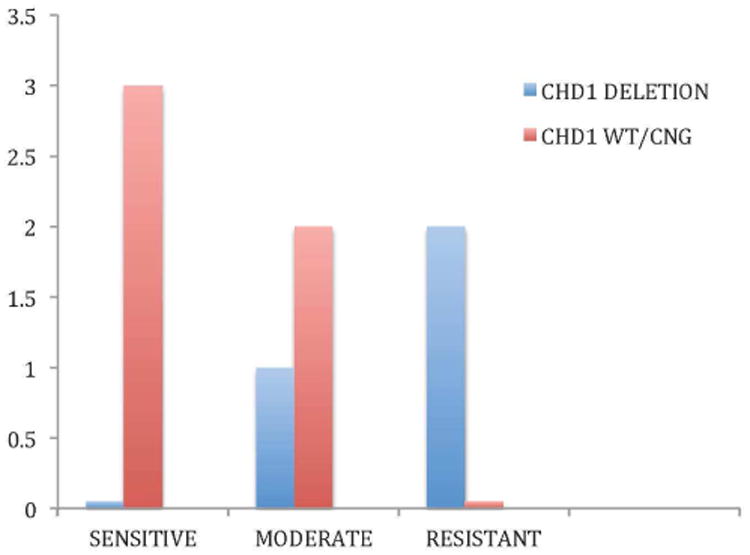
Pretreatment assessment of CHD1: CHD1 status by FISH and confocal microscopy in patients treated with androgen depravation and AT-101. The best nadir responses within 7 months of therapy are shown for patients with high sensitivity, moderate sensitivity and resistance to androgen depravation and AT-101.
Discussion
In men with hormone sensitive metastatic prostate cancer, ADT is the first line of therapy. Unfortunately, progression to castration resistance develops in almost all men treated with hormone therapy, supporting the investigation of new strategies to delay or prevent progression to castration resistance. Recently in a phase III trial comparing ADT plus 6 cycles of docetaxel to ADT alone, a survival benefit was seen when docetaxel was added to ADT (HR 0.61 0.47-0.80 p=0.0003), demonstrating that multi-targeted therapy can improve overall survival of men with newly diagnosed castration sensitive metastatic prostate cancer [15].
Bcl-2 is an antiapoptotic protein that is overexpressed in multiple tumors including prostate cancer, thereby conferring resistance to therapies that induce apoptosis, such as ADT [3, 4]. Based upon preclinical data suggesting that the efficacy of ADT could be enhanced by combing ADT with AT-101, we undertook the current single arm phase II study in men with newly diagnosed hormone sensitive metastatic disease in order to determine if the combination could improve upon a novel clinical endpoint in this disease setting, the rate of patients achieving an undetectable PSA after 7 months of ADT and AT-101.
Recent work from SWOG has demonstrated that the expected duration of benefit from ADT, where overall survival was correlated with rate of undetectable PSA after 7 months of ADT. (12) Patients achieving this benchmark had a median survival of 75 months compared to a median survival of 44 months in patients who had PSA greater than 0.2 and less than 4.0ng/ml at the end of 7 months of ADT. These data from a large intergroup study provides an important benchmark for the development of novel therapies in the patient with castration sensitive metastatic prostate cancer.
In the current trial we hypothesized that adding a Bcl-2 inhibitor to ADT would increase the percent of patients achieving an undetectable after 7 months of combined ADT and AT-101. Patients were started on ADT for 6 weeks prior to starting the combination in order to accommodate those patients initiating ADT prior to being enrolled on study. In SWOG 9346, 48% of men achieved an undetectable PSA after 7 months of ADT. In order for AT-101 to be worthy of further investigation in combination with ADT, we assumed that overcoming a major mechanism of resistance to cell death would increase the percentage of patients attaining undetectable PSA from 48% to 68%. The combination of AT-101 and ADT did not yield that level of activity, however. In an intention to treat analysis, 31% of patients achieved an undetectable PSA during the course of the study with an additional 25% of patients normalizing their PSA to less than 4ng/ml.
Several factors could be responsible for the lower than expected observed activity of the ADT and AT-101 combination compared to results of ADT as a single agent. The population in the current study may represent a poorer prognosis population compared to a broad based cooperative group study. In the current trial, 72% of patients had Gleason score of 8 or higher compared to 47 % in SWOG 9346.
AT-101 toxicity may have also contributed to the inferior outcome with the AT-101 and ADT combination compared to historical controls, in this intention to treat analysis. Toxicity of AT-101 was generally consistent with known data and was predominantly related to gastrointestinal motility. However 33% of patients developed neuropathy while on study, due to AT-101, potentially contributing to treatment discontinuation. In studies of AT-101 with chemotherapy using a 3-day pulse-dosing schedule of AT-101, neuropathy was not seen [16],[17] suggesting there is additional toxicity from a 21-day continuous cycle of AT-101. The results in this trial raise the possibility that AT-101 is antagonistic with ADT, AT-101 is not specific and potent enough to modulate Bcl-2 activity in patients; and/or the primary endpoint is the most optimal assessment of clinical activity.
Laboratory correlates performed in this study did not confirm that AT-101 clearly modulated Bcl-2 protein levels in PBMCs and correlations between changes in Bcl-2 levels after 21 days of treatment and outcome could not be made. Future studies with a larger number of subjects could explore the significance of changes in Bcl-2 levels in circulating tumor cells or in cells from tumor biopsies as these tissues may be more representative of changes taking place within the target tissue. As shown in Figure 3, CHD1 status also appeared to correlate with therapeutic sensitivity, although our sample size was too small to be significant or definitive. This finding is in agreement with our prior studies, as well as that of others, which have shown that TMPRSS2-ERG fusions are dependent on the status of CHD1, as a tumor suppressor gene responsible for chromatin-remodeling [18]. Given an association with CHD1 and chromosomal breaks, which may occur in areas of androgen receptor mediated transcriptional activity, we hypothesized that CHD1 status could predict future androgen sensitivity [19]. As shown in Figure 3, a non-significant correlation with CHD1 deletion and resistance occurred, suggesting that further studies in a larger cohort would be helpful.
In summary, the combination of AT-101 and ADT did not meet the pre-specified level of activity for further development of the combination in this high-risk cohort of patients where 72% having a Gleason Score of 8 or higher. Sensory neuropathy was an unexpected toxicity of the combination. CHD1 status is promising as a potential biomarker for future androgen sensitivity but further validation studies in larger cohorts would need to be conducted to test this hypothesis.
Clinical Practice Points.
What is already known?
Androgen deprivation therapy (ADT) is standard initial systemic treatment for advanced prostate cancer and the development of resistance is inevitable in the vast majority of patients, generally occurring within 20 months of initial castration therapy.
Bcl-2 is an antiapoptotic protein that is overexpressed in multiple tumors including prostate cancer, thereby conferring resistance to therapies that induce apoptosis, such as androgen deprivation therapy.
AT-101 binds to the BH3 domain of Bcl-2, Bcl-xL, Mcl-1 and Bcl-w, blocking apoptosis inhibitors while potentially simulating the pro-apoptotic proteins Noxa and Puma
SWOG 9346 demonstrated that PSA nadir after 7 months of combined ADT predicts survival in men with castration sensitive prostate cancer.
What are the new findings?
The combination of ADT and AT-101 did not meet the pre-specified level of activity for further development of this combination.
Toxicity of AT-101 was generally consistent with known data and was predominantly related to gastrointestinal motility. However 33% of patients developed neuropathy while on study due to AT-101.
As a potential initial biomarker at diagnosis for predicting androgen sensitivity, CHD1 assessment in paraffin tissue is feasible and should be studied further in larger cohorts.
How might it impact on clinical practice in the foreseeable future?
No changes in clinical practice will occur in the foreseeable future based on this study.
Acknowledgments
This study was supported from the National Cancer Institute, National Institutes of Health, CTEP (P30CA072720, U01CA132194, UM1CA186716), and in part by the Department of Defense (W81XWH-09-1-0145). Its content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense or National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberger MA, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339(15):1036–42. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 3.DiPaola RS, et al. A randomized phase II trial of mitoxantrone, estramustine and vinorelbine or bcl-2 modulation with 13-cis retinoic acid, interferon and paclitaxel in patients with metastatic castrate-resistant prostate cancer: ECOG 3899. Journal of translational medicine. 2010;8:20. doi: 10.1186/1479-5876-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thalasila A, et al. A phase I trial of weekly paclitaxel, 13- cis-retinoic acid, and interferon alpha in patients with prostate cancer and other advanced malignancies. Cancer Chemother Pharmacol. 2003;52(2):119–24. doi: 10.1007/s00280-003-0644-6. [DOI] [PubMed] [Google Scholar]
- 5.McDonnell TJ, et al. Expression of bcl-2 oncoprotein and p53 protein accumulation in bone marrow metastases of androgen independent prostate cancer. J Urol. 1997;157(2):569–74. [PubMed] [Google Scholar]
- 6.Raffo AJ, et al. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res. 1995;55(19):4438–45. [PubMed] [Google Scholar]
- 7.Morris MJ, et al. Safety and biologic activity of intravenous BCL-2 antisense oligonucleotide (G3139) and taxane chemotherapy in patients with advanced cancer. Appl Immunohistochem Mol Morphol. 2005;13(1):6–13. doi: 10.1097/00129039-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Morris MJ, et al. Phase I trial of BCL-2 antisense oligonucleotide (G3139) administered by continuous intravenous infusion in patients with advanced cancer. Clin Cancer Res. 2002;8(3):679–83. [PubMed] [Google Scholar]
- 9.Wang G, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. Journal of medicinal chemistry. 2006;49(21):6139–42. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 10.Meng Y, et al. Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Molecular cancer therapeutics. 2008;7(7):2192–202. doi: 10.1158/1535-7163.MCT-08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loberg RD, et al. In vivo evaluation of AT-101 (R-(-)-gossypol acetic acid) in androgen-independent growth of VCaP prostate cancer cells in combination with surgical castration. Neoplasia. 2007;9(12):1030–7. doi: 10.1593/neo.07778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGregor N, et al. AT-101 (R-(-)-gossypol acetic acid) enhances the effectiveness of androgen deprivation therapy in the VCaP prostate cancer model. Journal of cellular biochemistry. 2010;110(5):1187–94. doi: 10.1002/jcb.22633. [DOI] [PubMed] [Google Scholar]
- 13.Hussain M, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24(24):3984–90. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Sweeney C, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. ASCO Meeting Abstracts. 2014;32(18_suppl):LBA2. [Google Scholar]
- 16.Sonpavde G, et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann Oncol. 2012;23(7):1803–8. doi: 10.1093/annonc/mdr555. [DOI] [PubMed] [Google Scholar]
- 17.Schelman WR, et al. A phase I study of AT-101 with cisplatin and etoposide in patients with advanced solid tumors with an expanded cohort in extensive-stage small cell lung cancer. Invest New Drugs. 2014;32(2):295–302. doi: 10.1007/s10637-013-9999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tereshchenko IV, et al. ERG and CHD1 heterogeneity in prostate cancer: use of confocal microscopy in assessment of microscopic foci. Prostate. 2014;74(15):1551–9. doi: 10.1002/pros.22873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baca SC, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153(3):666–77. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


