Abstract
Craving elicited by drug-associated cues intensifies across protracted drug abstinence – a phenomenon termed “incubation of craving” – and drug-craving in human addicts correlates with frontal cortical hyperactivity. Herein, we employed a rat model of cue-elicited cocaine-craving to test the hypothesis that the time-dependent incubation of cue-elicited cocaine-craving is associated with adaptations in dopamine and glutamate neurotransmission within the ventromedial prefrontal cortex (vmPFC). Rats were trained to self-administer intravenous cocaine (6h/day × 10 days) and underwent in vivo microdialysis procedures during 2h-tests for cue-elicited cocaine-craving at either 3 or 30 days withdrawal. Controls rats were trained to either self-administer sucrose pellets or received no primary reinforcer. Cocaine-seeking rats exhibited a withdrawal-dependent increase and decrease, respectively, in cue-elicited glutamate and dopamine release. These patterns of neurochemical change were not observed in either control condition. Thus, cue-hypersensitivity of vmPFC glutamate terminals is a biochemical correlate of incubated cocaine-craving that may stem from dopamine dysregulation in this region.
Keywords: incubation, cocaine craving, ventromedial prefrontal cortex, glutamate, dopamine
1. Introduction
Cocaine addiction is a chronic relapsing disorder, characterized by a high propensity for relapse even during protracted abstinence. Re-exposure to drug-associated cues and contexts are known to trigger drug-craving and can even promote relapse in humans (Childress et al., 1999; Volkow et al., 1999). Following a history of drug-taking, the capacity of drug-associated cues to elicit craving in abstinent humans and operant responding (or drug-seeking) in drug-withdrawn laboratory animals intensifies with the passage of time during abstinence/withdrawal (e.g., Gawin & Kleber, 1986; Grimm et al., 2001). This phenomenon, termed the “incubation of craving”, is theorized to render addicts highly susceptible to cue-elicited relapse, even following prolonged periods of abstinence (Grimm et al., 2001). In rodent models of incubated drug-craving, the amount of operant-responding for drug-associated cues reinforced by the presentation of those cues progressively increases over the first few months into drug withdrawal and can persist at high levels for up to six months following cessation of drug-taking (e.g., Grimm et al., 2001; Kerstetter et al., 2008). As such, incubation of craving currently serves as a model with which to study the time-dependent, as well as enduring, changes in the brain that underpin persistently high levels of drug craving that is a hallmark feature of addiction.
In rodent models, re-exposure to drug-predictive cues increase the firing rate of prelimbic neurons (e.g., Rebec and Sun, 2005; West et al., 2014) and both neuropharmacological and optogenetic inactivation of the ventromedial aspect of the PFC (vmPFC) attenuates the heightened level of cue-reinforced responding exhibited during protracted withdrawal in cocaine-experienced rats (Koya et al., 2009; Ma et al., 2014). We know from prior work that basal and cocaine-elicited changes in extracellular dopamine and glutamate levels are dysregulated within the medial PFC of rats with a chronic history of cocaine-taking, at least during early (24 h) withdrawal (Ben-Shahar et al., 2012). Yet, to the best of our knowledge, no study to date has investigated the neurochemical anomalies within vmPFC that accompany incubated drug-craving in highly cocaine-experienced rats. This being said, incubated cocaine-craving is accompanied by increased levels of the dopamine transporter (DAT) within vmPFC (Grimm et al., 2002; McIntosh et al., 2013), which has been interpreted as reflecting a compensatory response to heightened drug-elicited and/or cue-elicited dopamine release within this region. Furthermore, an intra-medial PFC infusion of dopamine D1 receptor antagonists attenuates cue-induced reinstatement of an extinguished operant response in cocaine-experienced rats, arguing an important role for dopamine-dependent signaling within this region in cue-elicited cocaine-seeking (Ciccocioppo et al., 2001). Interestingly, although re-exposure to cues previously associated with sucrose-taking also elicits cue-reinforced operant responding that can incubate with the passage of time an animal remains sucrose-free (e.g., Grimm et al., 2002), incubated sucrose-seeking is not accompanied by changes in DAT expression within PFC (Grimm et al., 2002). Thus, cocaine appears to elicit changes in dopamine terminals within vmPFC that are distinct from those produced by a non-drug reinforcer. As such, the present study compared the capacity of cocaine- versus sucrose-paired cues to elicit a rise in extracellular dopamine within the vmPFC during early and later withdrawal and their relation to cue-reinforced responding.
Incubated cocaine-craving has also been correlated with changes in the expression of glutamate receptor-related proteins, as well as increased activation of downstream effectors within this subregion (Ben-Shahar et al., 2013; Gould et al., 2014; Koya et al., 2009; Miller et al., in press), suggestive of a hyper-glutamatergic state. While not yet assayed within PFC, the presentation of drug-associated cues after chronic cocaine-taking elicits a rise in extracellular glutamate within both the cell body and terminal regions of the mesolimbic dopamine system, with extracellular glutamate levels varying bi-directionally as a function of cue availability (Suto et al., 2010; Suto et al., 2013; You et al., 2007). Based on the results of neuropharmacological inactivation studies conducted within the confines of the traditional extinction-reinstatement model of drug-seeking, the source of the glutamate regulated within the nucleus accumbens (NAC) by drug-associated cues is likely the PFC (c.f., Kalivas & Volkow, 2005). Given that the activity of corticoaccumbal glutamate projections, and subsequent glutamate release within the NAC, is driven by the activation of somatodendritic glutamate receptors, we hypothesized that the incubation of cue-elicited cocaine-, and perhaps also sucrose-seeking, might reflect heightened glutamate release within the vmPFC.
Using in vivo microdialysis procedures, we examined for changes in extracellular levels of glutamate and dopamine within the vmPFC during cue-elicited responding at early versus later withdrawal from a history of excessive cocaine-taking. To determine the reinforcer-specificity of our observed effects, parallel studies were conducted in animals with a history of sucrose-pellet self-administration or in animals allowed to respond for the presentation of neutral cues in the absence of any primary reinforcer.
2. Materials and methods
2.1 Subjects, Lever-Response Training, and Surgery
All procedures were approved by the Institutional Animal Care and Use Committee of the University of California Santa Barbara and were consistent with the guidelines of the NIH Guide for Care and Use of Laboratory Animals. Male Sprague-Dawley rats (275-325g; Charles River Laboratories, Hollister, CA) were pair-housed under standard reverse light-cycle conditions (lights off: 0700 h), with ad libitum food/water except during lever-response training, during which food was restricted (16 g/day), 24 h prior to 16-h overnight operant sessions (FR1 schedule of reinforcement; 45 mg food pellet; Bio Serv, Frenchtown, NJ; acquisition criterion=100 responses on the active lever/session). Self-administration training was conducted in standard 2-lever operant chambers (Med Associates Inc., St. Albans, VT). Under ketamine/xylazine anesthesia (respectively 56.25 and 7.5mg/kg, IM; 2mg/kg banamine analgesic, SC, for post-operative pain), animals were implanted with a unilateral microdialysis guide cannula (20-gauge; 8mm long; Synaptech, Marquette, MI) aimed 2 mm above the vmPFC (AP: +3.0; ML ± 0.75; DV: –3.0, in mm from Bregma), with the placement counterbalanced across hemisphere within each group. Animals slated to self-administer cocaine were also implanted with a chronic indwelling jugular catheter as described previously by our group (see Ben-Shahar et al., 2013; Kersetter et al., 2008). A minimum of 4 days was allowed for recovery, with jugular catheter patency maintained by daily flushing of sterile heparin/timentin/saline (60 IU/ml and 100 mg/ml, respectively; vol=0.1 ml) and confirmed weekly by intravenous infusion of 5 mg/kg brevital (JHP Pharmaceuticals, Parsippany, NJ).
2.2 Self-Administration and In Vivo Microdialysis during Cue-Testing Procedures
Animals were trained to lever-press under an FI20 schedule of reinforcement for intravenous cocaine (0.25 mg in 0.1 ml saline infusion; NIDA, Bethesda, MD) or a 45 mg sucrose pellet (Bio Serv), with delivery of either reinforcer signaled by a 20-second light-tone compound stimulus. For control rats, active lever-presses resulted in the light-tone stimulus only. Depression of the “inactive lever” had no programmed consequences for any group. During training of the initial cohorts of rats, cocaine animals received an average of 102 reinforcer-stimulus pairings/6-hour session. Thus, the total maximum number of reinforcer-stimulus pairings earned by sucrose-trained animals was capped at 102 to equate associative learning across groups. On average, sucrose-trained animals earned 102 reinforcers within 3 hours. Thus, control rats were permitted to respond for the neutral cues for 3 hours/day. Animals were trained under the above conditions once daily across 10 days, and were then left undisturbed in their home cages for either 3 or 30 days, at which time in vivo microdialysis procedures were conducted (e.g., Ben-Shahar et al., 2012) during a 2-h test for cue-elicited responding (Cue Test). For these Cue Tests, active lever-presses resulted in presentation of the light-tone stimulus only. A minimum of 4 hours prior to the Cue Test, a microdialysis probe (8 mm long with 2 mm membrane; Synaptech) was inserted into the guide cannula, the animals were placed into their operant chamber with levers retracted and house lights off, and probes were perfused with artificial cerebral spinal fluid (2.0 μl/min; see Ben-Shahar et al, 2012). Dialysate collection occurred, in 20-min intervals, for 1 hour prior to the Cue Test and then throughout the duration of the 2-hour Cue Test session. l0μl of preservative (4.76 mM citric acid, 150 mM NaH2P04, 50 μM EDTA, 3 mM sodium dodecyl sulfate, 10% methanol (v/v), 15% acetonitrile (v/v), pH 5.6) was added into each dialysate sample to minimize the oxidation of dopamine. Immediately upon collection, the dialysate sample was stored at -80 °C until assay. Upon completion of the Cue Test, probes were removed, animals were anesthetized with 4% isoflurane, brains extracted and then drop-fixed in 4% paraformaldehyde for later determination of probe placement within the PFC by standard histological methods. Only data from rats exhibiting probe placement within the boundaries of the vmPFC (prelimbic and/or infralimbic subregions) were employed in the statistical analyses of the data. Dialysate content of dopamine (27μl) and glutamate (20μl) was determined for each sample using high pressure liquid chromatography with electrochemical detection as described previously (Ben-Shahar et al, 2012).
2.3. Statistical Analyses
As the studies of cocaine-trained, sucrose-trained and control rats were conducted in series, the behavioral and neurochemical data were analyzed separately for each self-administration group. The data for the average number of reinforcers earned, and the average number of lever-presses emitted, during the last 3 days of self-administration training, as well as the average baseline levels of dopamine and glutamate, were analyzed for time-dependent differences using t-tests for independent samples (3 vs. 30 days withdrawal). The time-courses of behavioral and neurochemical responding were analyzed by Withdrawal (3 vs. 30 days) × Time ANOVAs, with repeated measures on the Time factor (6 or 9 levels, depending upon the dependent variable examined). To determine whether or not group differences were withdrawal-dependent, Significant Withdrawal × Time interactions were followed up using simple effects analyses of group differences between 3-day and 30-day withdrawn animals. α=0.05 for all analyses.
3. Results
3.1 Self-administration training
Relative to both sucrose-trained and control rats, cocaine-trained animals exhibited the highest active-lever responding. However, due to our capping procedure, the number of reinforcers/cue presentations earned by cocaine- and sucrose-trained animals was comparable (see Table 1). Importantly, the lever-responding, as well as the number of reinforcers/cue presentations earned, over the last 3 days of self-administration training was equivalent between rats within each self-administration group who were slated to be tested at 3 versus 30 day withdrawal (t-tests, p's>0.05).
Table 1.
Summary of the average number of active lever-presses emitted and number of reinforcer/cue presentations earned (± SEMs) over the last 3 days of self-administration training by rats reinforced by neutral cues (Control), by sucrose pellets paired with neutral cues (Sucrose) or by cocaine infusions paired with neutral cues (Cocaine), slated to be tested for cue-reinforced lever-pressing behavior at either 3 or 30 days withdrawal (WD).
| Active Lever Presses | Reinforcers/Cue Presentations | |||
|---|---|---|---|---|
| Group | 3 days WD | 30 days WD | 3 days WD | 30 days WD |
| Neutral | 25.3 ± 4.6 | 27.3 ± 4.7 | 19.1 ± 3.8 | 18.5 ± 2.6 |
| Sucrose | 135.4 ± 15.2 | 142.0 ± 9.2 | 92.8 ± 6.5 | 96.4 ± 3.8 |
| Cocaine | 182.0 ± 41.3 | 146.5 ± 19.6 | 100.4 ± 6.1 | 95.6 ± 5.2 |
3.2 Neurochemical correlates of incubated cocaine-seeking
When tested for cue-elicited cocaine-seeking at 3 or 30 days withdrawal, cocaine-trained rats exhibited a withdrawal-dependent increase in active lever-pressing that manifested most robustly during the first 20-min of the 2-hour Cue Test session (Fig.1A) [Withdrawal × Time: F(5,80)=3.51, p=0.006; simple main effect for first 20-min bin: F(1,16)=31.91, p<0.05; all other bins, p's>0.05]. Cocaine rats responded primarily on the active lever and the total number of active lever-presses increased as a function of drug withdrawal (Fig.1B) [Lever × Withdrawal: F(1,19)=8.01, p=0.01; simple main effect for active lever: F(1,16)=22.73, p<0.05; for inactive lever: p>0.05], indicative of incubated cue-elicited cocaine-seeking.
Figure 1.
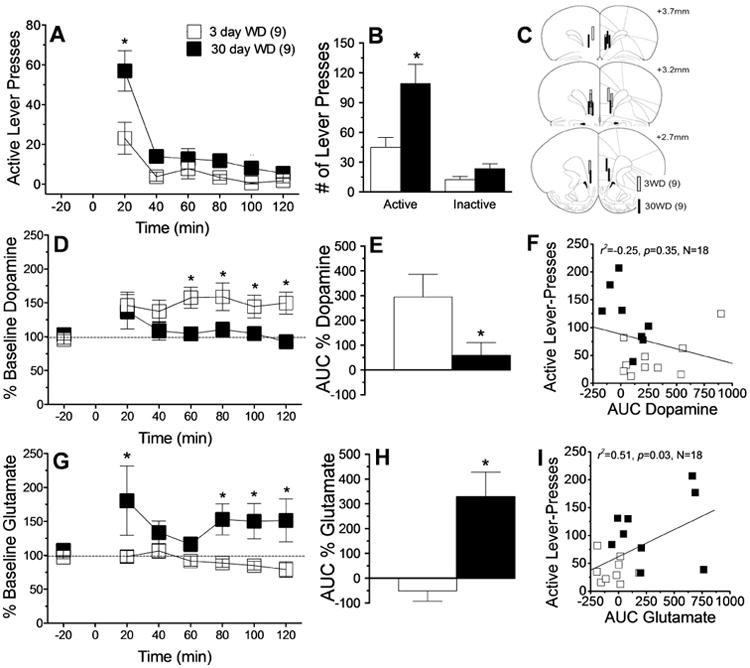
Summary of the effects of short- (3 day; n=9) versus long-term (30 day; n=9) withdrawal (WD) from self-administered cocaine upon cue-elicited behavior and neurochemistry within the vmPFC when animals were tested for 2 h in a cocaine-free state. (A) Time-course of active lever presses (in 20-min bins) emitted by rats during the 2-h session, illustrating greater responding throughout testing at 30 vs. 3 days WD, indicative of incubation. (B) Comparison of the total number of active and inactive lever-presses at 3 vs. 30 days WD, indicating that incubated behavior was goal-directed. (C) Summary of unilateral placements of microdialysis probes within the vmPFC. (D-F) Summary of the time-course and area under the curve (AUC) for vmPFC extracellular dopamine, illustrating a waning of cue-elicited dopamine release at 30 days WD and an inverse relation between cue-elicited dopamine release and cocaine-seeking. (G-I) Comparable results for vmPFC extracellular levels of glutamate, illustrating an incubation of cue-elicited glutamate release and a predictive relation between cue-elicited glutamate release and cocaine-seeking. Data represent the means ± SEMs of the number of rats indicated in parentheses. *p<0.05 vs. 3 days WD (t-tests).
Under our conventional microdialysis procedures, no time-dependent differences were apparent for baseline extracellular levels of glutamate (t-test, p>0.10), although baseline dopamine levels were lower in dialysate collected from Cocaine rats tested at 3 vs. 30 days withdrawal [t(16)=4.00, p=0.001] (data not shown). This time-dependent effect did not appear to relate to group differences in probe localization within the vmPFC as histology revealed comparable placements within the prelimbic or infralimbic cortices between rats tested at 3 vs. 30 days withdrawal (Fig.1C). Responding for cocaine-associated cues elicited dopamine release, but the magnitude of this effect was greater in early vs. later withdrawal, most notably during the 2nd hour of testing (Fig.1D) [Withdrawal × Time: F(8,136)=4.33, p<0.0001; simple main effects for 60 min: F(1,16)=10.79, p<0.05; for 80 min: F(1,16)=8.98, p<0.05; for 100 min: F(1,16)=6.04, p<0.05; for 120 min: F(1,16)=12.36, p<0.05; all other bins: p's<0.05]. The time-dependent reduction in the cocaine cue-reactivity of vmPFC dopamine was made even more apparent by an analysis of the area under the curve (AUC) for dopamine release during the Cue Test session (Fig.1E) [t(16)= 2.09, p=0.05], however, there was no significant correlation between the magnitude of cue-elicited dopamine release within vmPFC and cocaine-seeking behavior (Fig.1F). However, opposite dopamine (Fig.1D), responding for cocaine-associated cues induced a rise in vmPFC glutamate only in later withdrawal. This was confirmed by analyses of either the time-course (Fig.1G) [Withdrawal × Time: F(8,128)=2.11, p=0.04; simple main effects for 20 min: F(1,16)=12.23, p<0.05; for 80 min: F(1,16)=7.44, p<0.05; for 100 min: F(1,16)=7.71, p<0.05; for 120 min: F(1,16)=7.43, p<0.05; other bins: p's>0.05] or the magnitude of the glutamate response (Fig.1H) [t(16)= 2.90, p=0.01], the latter of which did predict cocaine-seeking behavior (Fig. 1I).
3.3 Neurochemical correlates of sucrose-seeking
When tested for cue-elicited sucrose-seeking at 3 or 30 days withdrawal, sucrose-trained rats exhibited a withdrawal-dependent increase in active lever-pressing, which manifested only during the 1st 20 min of the 2-hour Cue Test session (Fig.2A) [Withdrawal × Time: F(5,80)=3.24, p=0.01; simple main effect for 20 min bin: F(1,16)=23.36, p<0.05; all other bins: p's>0.05]. Sucrose-trained rats responded selectively on the active lever, but the time-dependent increase in total active lever-responding did not reach statistical significance (Fig.2B) [Lever effect: F(1,15)=32.17,p>0.001; Lever × Withdrawal, p=0.16], suggesting that reinforcer capping, employment of pellets, or allowing ad libitum homecage feeding may blunt incubation (for comparison, see e.g. Grimm et al., 2002; 2011).
Figure 2.
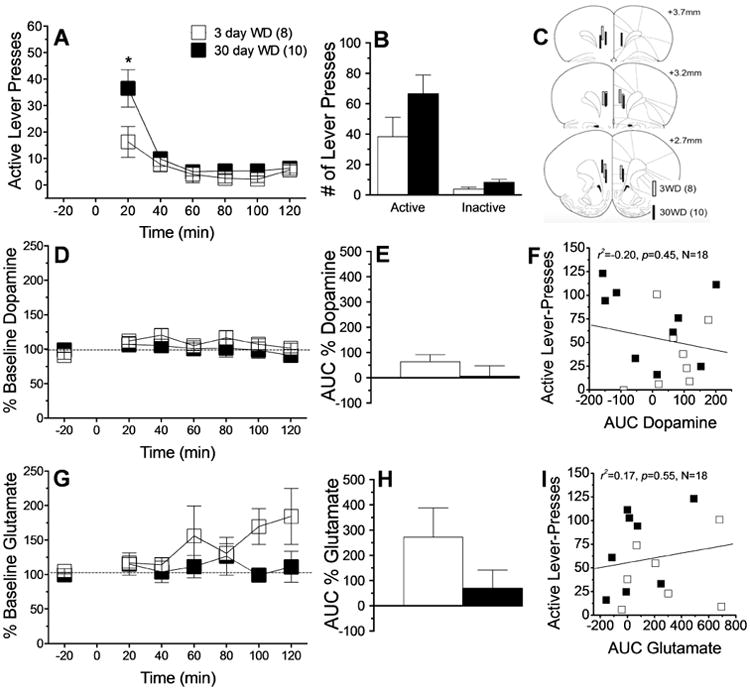
Summary of the effects of short- (3 day; n=8) versus long-term (30 day; n=10) withdrawal (WD) from self-administered sucrose upon cue-elicited behavior and neurochemistry within the vmPFC when animals were tested for 2 h in a sucrose-free state. (A) Time-course of active lever presses (in 20-min bins) emitted by rats during the 2-h session, illustrating greater responding during the first 20min bin at 3 vs. 30 days WD, indicative of a weak incubation. (B) Comparison of the total number of active and inactive lever-presses at 3 vs. 30 days WD, which failed to support an incubation of responding. (C) Summary of unilateral placements of microdialysis probes within the vmPFC. (D-F) Summary of the time-course and area under the curve (AUC) for vmPFC extracellular dopamine, illustrating no cue-elicited dopamine release and no relation to sucrose-seeking. (G-I) Comparable results for vmPFC extracellular levels of glutamate, illustrating no cue-elicited glutamate release and no relation to sucrose-seeking. Data represent the means ± SEMs of the number of rats indicated in parentheses. *p<0.05 vs. 3 days WD (t-tests).
Microdialysis probes were localized to both the prelimbic and infralimbic cortices in sucrose-trained animals (Fig.2C). However, responding for sucrose-paired cues failed to elicit a significant rise in vmPFC dopamine (Fig.2D-E; p's>0.20) and there was no relation between sucrose-seeking and the magnitude of vmPFC dopamine release (Fig.2F). Engaging in sucrose-seeking elevated vmPFC extracellular glutamate [Time effect: F(8,104)= 2.28, p=0.03]; however, this effect did not vary significantly with sucrose withdrawal (Fig.2G, Withdrawal × Time: p=0.10; Fig.2H, t-test: p=0.15) and there was no predictive relation between sucrose-seeking and the magnitude of cue-elicited glutamate release (Fig.2I).
3.4 Neurochemical correlates of neutral cue-seeking
Control rats exhibited very low and stable rates of lever-pressing for the neutral cues across the 2-h Cue Test session (Fig.3A) [Withdrawal × Time ANOVA, all p's>0.06]. Control rats did selectively allocate their responding towards the “active” lever, most likely due to residual learning from lever-response training, however cue-reinforced behavior did not vary significantly between the Cue Tests (Fig.3B) [Lever effect: F(1,15)=30.45, p<0.0001; interaction, p>0.07]. The localization of the microdialysis probes within the vmPFC of the control rats was comparable to that for the other 2 self-administration groups (Fig.3C). Inspection of Fig.3D and 3E suggested that the opportunity to lever-press for neutral cues elevated vmPFC dopamine levels in control rats, particularly at the 3-day time-point. However, the results of the statistical analyses of these data failed to confirm group differences (Fig.3D, Withdrawal × Time ANOVA: p's>0.08; Fig.3E, t-test: p=0.61) and there was no significant relation between cue-elicited responding for neutral cues and the magnitude of dopamine release in control animals (Fig.3F). Lever-pressing for neutral cues did elevate vmPFC glutamate levels, but this effect did not vary across Cue Tests [Fig.3G, Time effect: F(8,88)= 3.68, p=0.001; other p's>0.50; Fig.3H, t-test: p=0.91] and did not predict cue-elicited responding (Fig.3I). These data argue that while the mere presentation of neutral cues is reinforcing and can elicit glutamate release within the vmPFC, neither behavior nor glutamate release incubates with the passage of time since last cue exposure.
Figure 3.
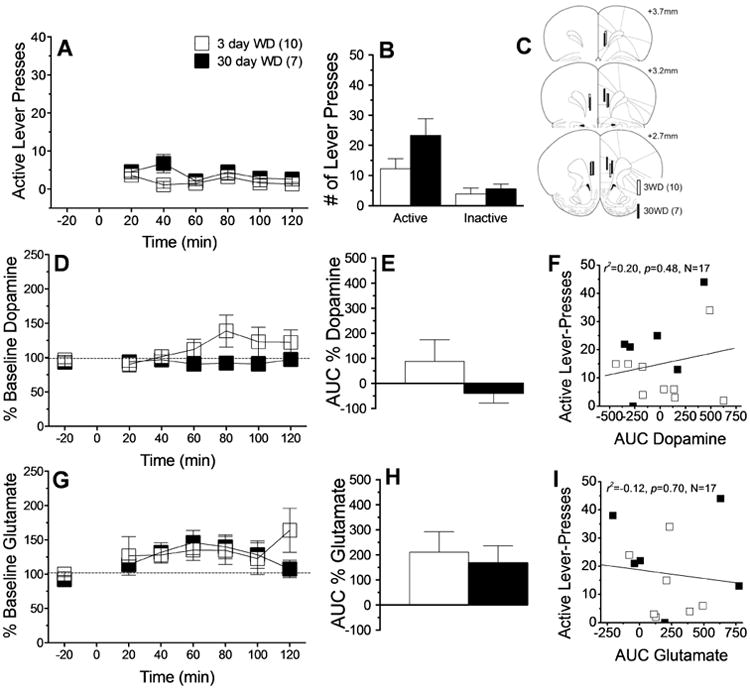
Summary of the effects of short- (3 day; n=10) versus long-term (30 day; n=7) “withdrawal” (WD) from operant sessions in which rats responded for neutral cues in the absence of any primary reinforcer upon cue-elicited behavior and neurochemistry within the vmPFC when animals were tested for 2 h. (A) Time-course of active lever presses (in 20-min bins) emitted by rats during the 2-h session, illustrating stable, low levels of responding at both WD time-points. (B) Comparison of the total number of active and inactive lever-presses at 3 vs. 30 days WD, which failed to support an incubation of responding. (C) Summary of unilateral placements of microdialysis probes within the vmPFC. (D-F) Summary of the time-course and area under the curve (AUC) for vmPFC extracellular dopamine, illustrating no cue-elicited dopamine release and no relation to cue-seeking. (G-I) Comparable results for vmPFC extracellular levels of glutamate, illustrating no cue-elicited glutamate release and no relation to cue-seeking. Data represent the means ± SEMs of the number of rats indicated in parentheses. *p<0.05 vs. 3 days WD (t-tests).
4. Discussion
Neuronal activity within the vmPFC is critical for incubated cocaine-craving as derived from studies of animal models (Koya et al., 2009; Ma et al., 2014). Using in vivo microdialysis procedures, the present results demonstrate that incubated cocaine-seeking is associated with a withdrawal-dependent increase in cue-elicited glutamate release within the vmPFC, concomitant with a waning of dopamine release. To the best of our knowledge, this study is the first to examine by in vivo microdialysis the changes in extracellular neurotransmitter content within vmPFC as cocaine-free animals engage in cue-reinforced responding at different time-points during cocaine withdrawal. The inverse relation between vmPFC extracellular dopamine and glutamate levels is contrary to our original hypothesis, but is in line with the results from an earlier in vivo microdialysis study of the neurochemical effects of cocaine-taking conducted by our group (Ben-Shahar et al., 2012). The diametrically opposed cue-reactivity of vmPFC dopamine and glutamate during both early and later cocaine withdrawal suggest an antagonistic relation between these two neurotransmitter systems in the regulation of cue-elicited cocaine-seeking, with dopamine suppressing and glutamate facilitating behavioral hyper-reactivity to cocaine-paired cues. Moreover, these results point to time-dependent dysregulation of the balance between these two neurotransmitter systems within the vmPFC as a neurochemical correlate of incubated cocaine-craving during protracted withdrawal.
The precise nature of the dopamine-glutamate interaction at play within the vmPFC to regulate cue-elicited drug-seeking is a topic of current investigation in our laboratory. One theory under investigation poses that a time-dependent dysregulation of autoinhibitory mechanisms occur within vmPFC glutamate terminals. The resultant increase in vmPFC glutamate hyper-activates corticofugal afferents to the nucleus accumbens (NAC) and/or amygdala to increase the incentive salience of drug-associated cues and invigorate drug-seeking behavior. Supporting excitatory corticofugal drive in craving, cue/imagery-elicited craving in human psychomotor stimulant addicts is associated with a coordinate increase in metabolic hyperactivity within frontal cortex, striatum and amygdala, which is theorized to reflect cue/imagery-elicited hyper-activation of corticofugal afferents (c.f., Kalivas et al., 2005). More relevant to the incubation of craving phenomenon, (1) optogenetic inhibition of vmPFC glutamate afferents to the NAC prevents incubated cocaine-seeking in rats (Ma et al., 2014), (2) incubated cocaine-seeking is associated with an incubated or sensitized rise in extracellular glutamate within both the vmPFC (Fig.1G,H) and interconnected NAC (Suto et al., 2010) and (3) incubated cocaine-seeking can be inhibited by the local infusion of mGlu2/3 autoreceptor agonists into the central nucleus of the amygdala (Lu et al., 2007). Indeed, mGlu2/3 autoreceptor function is down-regulated within mPFC during protracted withdrawal in rats with a history of repeated cocaine injections (Xie & Steketee, 2009). Although the present observation of heightened cue-elicited glutamate release in cocaine-incubated rats is consistent with a deficit in autoregulatory mechanisms within vmPFC, we do not yet know how mGlu2/3 function and expression is impacted within PFC subregions by a history of cocaine-taking. While it is tempting to generalize across models, our prior immunoblotting research indicates clearly that withdrawal from IV self-administered cocaine produces changes in the expression of certain glutamate receptor proteins that are distinct from those produced by classical, cocaine sensitization, injection protocols (Ary & Szumlinski, 2007; Ben-Shahar et al., 2009, 2013). Moreover, and important for our understanding of the neural substrates of incubated drug-craving, the expression pattern of glutamate receptor-related proteins varies with the opportunity to engage in cue-reinforced cocaine-seeking behavior during protracted withdrawal (e.g., Ben-Shahar et al., 2013). For example, repeated cocaine-injected rodents exhibit increased PFC expression of mGlu1/5 receptors during protracted withdrawal (Ary & Szumlinski, 2007), while mGlu1/5 receptor expression is down-regulated within vmPFC of rats exhibiting incubated cocaine-seeking, but not in similarly cocaine-experienced and –withdrawn rats not afforded the opportunity to drug-seek (Ben-Shahar et al., 2013). mGlu1/5 receptors desensitize rapidly upon stimulation and exhibit slow recovery (e.g., Gereau & Heinemann, 1998), raising the possibility that the reduction in vmPFC mGlu1/5 expression observed in incubated cocaine-seeking rats (Ben-Shahar et al., 2013) results from the incubation of cue-elicited glutamate release within this region. As reduced vmPFC mGlu1/5 function produces cognitive impairments that promote cue-elicited drug-seeking (Ben-Shahar et al., 2013), current research in the laboratory seeks to replicate the results of Xie and Steketee (2009) within the context of incubated cocaine-seeking to test the hypothesis that incubated cue-elicited glutamate release within vmPFC might reflect a down-regulation of autoreceptor function.
An alternative, but not necessarily mutually exclusive, theory under investigation relates to the observation that dopamine activation of D1 receptors, localized to GABAergic interneurons within PFC, inhibits local glutamate release in drug-naïve subjects via GABA-mediated heteroinhibition of glutamate terminals (e.g., Abekawa et al., 2000). Completely hypothetical at this point, we propose that the withdrawal-dependent waning of the cocaine cue-reactivity of presumed mesocortical dopamine projections (Fig.1D,E) relieves inhibitory GABA tone upon glutamate terminals within vmPFC, thereby disinhibiting local glutamate release. The withdrawal-dependent waning of the cue-reactivity of vmPFC dopamine observed herein is a finding in line with clinical evidence for dysregulated frontal cortex dopamine in human cocaine addicts (e.g., Kalivas & Volkow, 2011), and is consistent with earlier work indicating reduced cocaine-cue reactivity of PFC dopamine in rats with a prolonged history of cocaine self-administration (40 days) (Ikegami et al., 2007). In line with prior in vivo microdialysis results for the NAC (e.g., Weiss et al., 1992) and the notion that a history of cocaine impairs dopamine transmission within PFC, baseline dopamine levels were low in cocaine-experienced rats, at least in early withdrawal, but this effect recovered with protracted withdrawal. While it is tempting to generalize findings from the NAC to the PFC, the anatomical architecture of dopamine terminals within the PFC are very distinct from those within other dopamine terminal regions, including the NAC, with dopamine signaling through volumetric versus synaptic transmission (see Bechta and Riegel, 2014). Thus, at the present time, we hesitate to generalize results across brain regions, particularly considering the potential contribution of individual probe recovery to the absolute neurotransmitter values obtained and further work will be required to confirm the reduction in basal extracellular dopamine content in cocaine-experienced animals and whether or not it varies as a function of the time in drug withdrawal.
The molecular underpinnings of the withdrawal-dependent waning of dopamine cue-reactivity are unclear at the present time, but could theoretically relate also to anomalies in autoinhibitory mechanisms. At present, we surmise that blunted cue-elicited dopamine release reflects a progressive hyper-sensitivity of D3 dopamine autoreceptors on vmPFC dopamine terminals. Although D3 receptor expression is relatively low within PFC, the local infusion of D3 receptor antagonists is sufficient to influence different aspects of social behavior in rodents, supporting their relevance in motivated behavior (e.g., Watson et al., 2012). While no study to date has examined directly the role for vmPFC D3 receptors in regulating drug-seeking, systemic pretreatment with D3 receptor antagonists or partial agonists attenuate drug-seeking behavior under various procedures, including the cue-induced reinstatement model of relapse (c.f., Keck et al., 2015). Such findings further the notion that postsynaptic (presumably D1) receptor stimulation within vmPFC normally serves to inhibit drug-seeking behavior, rendering D3 autoreceptors as an intriguing candidate for further exploration as a neural substrate of incubated drug-seeking.
Interestingly, neither dopamine nor glutamate within the vmPFC responded in any significant manner in animals trained to respond for sucrose-paired (Fig. 2) or neutral cues (Fig. 3). However, compared to prior studies of sucrose reinforcement (e.g., Grimm et al., 2002), we observed a relatively modest, albeit significant, incubation of sucrose-seeking; whether the magnitude of these effects were due to capping of the number of reinforcers, differences in sucrose delivery (pellet vs solution), or ad libitum vs restricted homecage feeding is unclear. Nevertheless, it is clear from the present data that when rats are subjected to comparable self-administration training, sucrose-paired cues are less potent than cocaine-paired cues at eliciting both an incubation of reinforcer-seeking (see also Grimm et al., 2002) and dopamine/glutamate release within the vmPFC (Fig. 2). Interestingly, the differential impact of time on the ability of cocaine- vs sucrose-paired cues to elicit behavior and neurochemical changes are consistent with recent data indicating that cocaine generates strong secondary, but not primary, reinforcement relative to sucrose (Turnstall & Kearns, 2014). Such observations are consistent with previous reports indicating that drugs and natural rewards produce different biochemical effects within PFC (e.g., Grimm et al., 2002; Koya et al., 2009) and argue that certain biochemical underpinnings of incubated craving may be reinforcer-specific.
5. Conclusions
The results of the present study indicate that incubated drug-seeking is associated with a time-dependent increase in cue-elicited glutamate elevations within the vmPFC but a blunted dopamine rise within the same region under the same conditions. This neurochemical adaptation is not observed in sucrose-seeking animals or cocaine-naïve controls responding for cues, arguing that it is a pharmacodynamic response produced by withdrawal from cocaine use. These data implicate an incubation of cue-elicited glutamate release and dopamine dysfunction within vmPFC as neurochemical cordons to relapse prevention and addiction recovery. If relevant to humans, these results pose pharmacotherapeutic strategies that curb corticofugal glutamate responsiveness to cocaine-paired cues as a viable strategy for facilitating addiction recovery.
Highlights.
Along with craving, cue-elicited glutamate release incubates in response to cocaine-paired cues.
Dopamine does not exhibit cue-elicited incubation.
The incubation of cue-elicited glutamate release is selective for cocaine-seeking animals
Acknowledgments
The authors declare no competing financial interests. This work was funded by NIH grant DA024038.
Abbreviations
- Cue Test
2-h test for cue-elicited responding
- mPFC
medial prefrontal cortex
- NAC
nucleus accumbens
- vmPFC
ventromedial prefrontal cortex
- WD
withdrawal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abekawa T, Ohmori T, Ito K, Koyama T. D1 dopamine receptor activation reduces extracellular glutamate and GABA concentrations in the medial prefrontal cortex. Brain Res. 2000;867:250–254. doi: 10.1016/s0006-8993(00)02298-8. [DOI] [PubMed] [Google Scholar]
- Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: A two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE, Caruana AL, Gordon EJ, Ploense KL, Ditzhazy J, Kippin TE, Szumlinski KK. Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci. 2013;33:495–506. doi: 10.1523/JNEUROSCI.3710-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar OM, Szumlinski KK, Lominac KD, Cohen A, Gordon E, Ploense KL, DeMartini J, Bernstein N, Rudy NM, Nabhan AN, Sacramento A, Pagano K, Carosso GA, Woodward N. Extended access to cocaine self-administration results in reduced glutamate function within the medial prefrontal cortex. Addict Biol. 2012;17:746–757. doi: 10.1111/j.1369-1600.2011.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proc Natl Acad Sci USA. 2001;98(4):1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Gereau RW, 4th, Heinemann SF. Role of protein kinase C phosphorylation in rapid desensitization of metabotropic glutamate receptor 5. Neuron. 1998;20:143–151. doi: 10.1016/s0896-6273(00)80442-0. [DOI] [PubMed] [Google Scholar]
- Gould AT, Sacramento AD, Wroten MG, Miller BW, Klugmann M, Ben-Shahar O, Szumlinski KK. Extended access to intravenous cocaine imbalances ventromedial prefrontal cortex Homer1 versus Homer2 expression: Implications for relapse. Addiction Biology. 2015;20:148–157. doi: 10.1111/adb.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Barnes J, North K, Collins S, Weber R. A general method for evaluating incubation of sucrose craving in rats. J Vis Exp. 2011;(57):e3335. doi: 10.3791/3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. Neuroadaptation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cueinduced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas of rats. Behav Pharmacol. 2002;13:379–88. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami A, Olsen CM, D'Souza MS, Duvauchelle CL. Experience-dependent effects of cocaine self-administration/conditioning on prefrontal and accumbens dopamine responses. Behav Neurosci. 2007;121(2):389–400. doi: 10.1037/0735-7044.121.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45(5):647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Keck TM, John WS, Czoty PW, Nader MA, Newman AH. Identifying Medication Targets for Psychostimulant Addiction: Unraveling the Dopamine D3 Receptor Hypothesis. J Med Chem. 2015 doi: 10.1021/jm501512b. 2015 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schlüter OM, Huang YH, Dong Y. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh S, Howell L, Hemby SE. Dopaminergic dysregulation in prefrontal cortex of rhesus monkeys following cocaine self-administration. Front Psychiatry. 2013;4:88. doi: 10.3389/fpsyt.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Sun W. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J Exp Anal Behav. 2005;84:653–66. doi: 10.1901/jeab.2005.105-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology. 2010;211:267–275. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnstall BJ, Kearns DN. Cocaine can generate a stronger conditioned reinforcer than food despite being a weaker primary reinforcer. Addict Biol. 2014 doi: 10.1111/adb.12195. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Loiseau F, Ingallinesi M, Millan MJ, Marsden CA, Fone KC. Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacology. 2012;37:770–786. doi: 10.1038/npp.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Paulus MP, Lorang MT, Koob GF. Increases in extracellular dopamine in the nucleus accumbens by cocaine are inversely related to basal levels: effects of acute and repeated administration. J Neurosci. 1992;12:4372–4380. doi: 10.1523/JNEUROSCI.12-11-04372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, Saddoris MP, Kerfoot EC, Carelli RM. Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine-seeking before and following abstinence. Eur J Neurosci. 2014;39:1891–1902. doi: 10.1111/ejn.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Steketee JD. Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology. 2009;203:501–510. doi: 10.1007/s00213-008-1392-4. [DOI] [PubMed] [Google Scholar]


