Abstract
Vascular interventions are associated with high failure rates from restenosis secondary to negative remodeling and neointimal hyperplasia. Periadventitial delivery of nitric oxide (•NO) inhibits neointimal hyperplasia, preserving lumen patency. With the development of new localized delivery vehicles, •NO-based therapies remain a promising therapeutic avenue for the prevention of restenosis. While the time course of events during neointimal development has been well established, a full characterization of the impact of •NO donors on the cells that comprise the arterial wall has not been performed. Thus, the aim of our study was to perform a detailed assessment of proliferation, cellularity, inflammation, and phenotypic cellular modulation in injured arteries treated with the short-lived •NO donor, PROLI/NO. PROLI/NO provided durable inhibition of neointimal hyperplasia for 6 months after arterial injury. PROLI/NO inhibited proliferation and cellularity in the media and intima at all of the time points studied. However, PROLI/NO caused an increase in adventitial proliferation at 2 weeks, resulting in increased cellularity at 2 and 8 weeks compared to injury alone. PROLI/NO promoted local protein S-nitrosation and increased local tyrosine nitration, without measurable systemic effects. PROLI/NO predominantly inhibited contractile smooth muscle cells in the intima and media, and had little to no effect on vascular smooth muscle cells or myofibroblasts in the adventitia. Finally, PROLI/NO caused a delayed and decreased leukocyte infiltration response after injury. Our results show that a short-lived •NO donor exerts durable effects on proliferation, phenotype modulation, and inflammation that result in long-term inhibition of neointimal hyperplasia.
Keywords: Nitric Oxide, Neointimal Hyperplasia, Proliferation, Inflammation, S-nitrosation, Reactive Nitrogen Species
INTRODUCTION
Atherosclerosis is responsible for the majority of morbidity and mortality associated with cardiovascular disease in the United States. [1] Treatment options for advanced atherosclerosis include angioplasty, stenting, endarterectomy, and bypass grafting. The durability of these interventions is limited due to restenosis, secondary to negative remodeling and neointimal hyperplasia. [2] Negative remodeling is characterized by thickening of the wall, changes of the extracellular matrix, and deposition of hyaluronan and versican. [3–5] Neointimal hyperplasia is a complex process characterized by inflammation, cellular proliferation, and migration. [6–9] Ultimately, this arterial injury response leads to a narrowing of the lumen and the need for revascularization. The time course of cell activation, proliferation, and migration has been extensively studied after balloon injury for vascular smooth muscle cells (VSMC), and more recently for adventitial fibroblasts. [9–14] The importance of the activation of VSMC and their change from a contractile to a migratory and secretory phenotype has been established for both neointimal formation and negative remodeling. [15] In a similar fashion, the contribution of adventitial cells to neointimal formation and their switch to a myofibroblast phenotype has more recently been reviewed. [16] However, most of these studies are concerned with the pathophysiology of restenosis and not with the changes achieved with pharmacological intervention.
•NO donors are a promising therapeutic avenue, given its many vasoprotective properties. [17] Considerable efforts have been made to increase •NO bioavailability in the injured vasculature. [18] Delivery methods have included inhalational, systemic, intraluminal, and periadventitial delivery; however, each of these delivery methods poses challenges for effective clinical use. We have shown that periadventitial delivery of the short-acting •NO donor PROLI/NO (t1/2=2 seconds at pH 7.4 and 37 °C) strongly inhibits neointimal hyperplasia. [19, 20] The mechanisms by which •NO inhibits neointimal growth are diverse: •NO inhibits platelet adherence and aggregation, [21] mitigates leukocyte adhesion, [22] and prevents VSMC proliferation and migration, [23, 24] while simultaneously promoting endothelial cell growth. [23, 25] However, the effect of •NO-based therapies on the temporal profile of inflammation, cellular proliferation, phenotypic marker expression, and inflammation in each layer of the arterial wall has not been established. We have previously compared the efficacy of •NO donors with very different half-lives (ie, short and and long) and established that PROLI/NO is more efficacious than diazeniumdiolates with longer half-lives. [19, 26, 27] Hence, the aim of this study is to characterize the effect of PROLI/NO on cellular proliferation, phenotypic differentiation of vascular cells, and inflammation following arterial injury in each of the three layers of the arterial wall over time. We hypothesize that modulation of cell phenotype within the arterial wall accounts for the durable efficacy of PROLI/NO at inhibiting neointimal hyperplasia.
MATERIALS AND METHODS
Animal Surgery
All animal procedures were performed in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication; National Academy Press, 1996) and approved by the Northwestern University Animal Care and Use Committee. Ten-week-old male Sprague–Dawley rats (Charles River, Wilmington, MA, USA) weighing 300–400 g were anesthetized with inhaled isoflurane (1.0–5.0%). Prior to the procedure, atropine (0.1 mg/kg) and carprofen (Rimadyl TM) (0.15 mg/kg) were administered subcutaneously to decrease airway secretions and for pain control, respectively. Equaline sterile lubricant (Boise, ID) was applied to the animal’s eyes. Following a midline neck incision, the left common, internal and external carotid arteries were identified. After distal ligation of the external carotid artery, the internal and common carotid arteries were occluded with atraumatic clamps and a No. 2 French arterial embolectomy catheter (Edwards Lifesciences, Irvine, CA, USA) was inserted into the external carotid artery and advanced into the common carotid artery. Uniform injury was created by inflating the balloon to 5 atmospheres of pressure for 5 minutes. After removal of the balloon, the external carotid artery was ligated and blood flow restored. Dry PROLI/NO powder (10 mg, 46 μmoles), or proline powder (5.5 mg, 47 μmoles) was immediately applied evenly to the external surface of the injured common carotid artery of the treated rats, as previously published. [19, 20, 28–31] The neck incision was closed in two layers. By the time the first layer was being closed, all the applied powder was clearly dissolved. Rats received an intraperitoneal injection of bromodeoxyuridine (BrdU, 100 mg/kg) at 24 and 1 hour prior to sacrifice. Blood was collected from the tail vein before and 2 hours after surgery. Blood pressure and heart rates were monitored on awake rats before, and 3 hours after surgery, using the non-invasive Volume Pressure Recording CODA System (Kent Scientific, Torrington, CT). Rats were sacrificed at different time points after surgery (2 hours, 1 day, 3 days, 1 week, 2 weeks, 8 weeks, and 6 months) for morphometric and immunohistochemical analysis. Each treatment group included 5–7 animals. Some animals received an intravenous injection of Evans blue dye (0.5 mL of 0.5%, Sigma, St. Louis, MO) immediately before sacrifice to identify denuded endothelial surfaces. Following in situ perfusion with phosphate buffered saline (PBS), carotid arteries were removed and photographed. Areas of denuded endothelium were identified by blue staining, and the portion of the injured area stained blue was quantitated using ImageJ (NIH, Bethesda, MD).
Histology
Carotid arteries were harvested following in situ perfusion/fixation with PBS and 2% paraformaldehyde. Vessels were placed in paraformaldehyde at 4 °C for 1 hour, then overnight in 30% sucrose in PBS at 4 °C for cryo-protection. The tissue was quick-frozen in Optimal Cutting Temperature compound (Tissue Tek, Hatfield, PA) and 5 μm sections were cut throughout the entire injured segment of the common carotid artery using a Microm HM 550 cryostat (Thermo Fisher Scientific Microm International GmbH, Walldorf, Germany). Carotid arteries harvested at 2 weeks, 8 weeks, and 6 months were examined histologically for evidence of neointimal hyperplasia using routine hematoxylin & eosin (H&E) staining. Digital images were collected with light microscopy using an Olympus BHT microscope (Melville, NY) at a total magnification of 40X, 100X, and 400X. Six evenly-spaced sections through each injured carotid artery were analyzed. Intima area, media area, and lumen area were measured. These values were obtained (arbitrary units) using ImageJ software (NIH, Bethesda, MD).
Immunohistochemistry and immunofluorescence
From each animal, three to six evenly spaced carotid sections from the area of injury were stained and examined for evidence of proliferation and presence of various phenotypic markers using immunohistochemical staining. Frozen sections were fixed in 2% paraformaldehyde and permeabilized with 0.3% Triton X-100 in PBS for 10 minutes. Sections were blocked with horse serum 1:20 (Sigma) for BrdU, non-muscle myosin heavy chain (NMMHC), and CD45 staining, or goat serum 1:20 (Sigma) for α-SMA and desmin staining, in 0.5% bovine serum albumin (BSA) for 30 minutes. Samples were incubated with primary and secondary antibodies as follows: anti-BrdU (Cat#ab8955, Abcam, Cambridge, MA) 1:200 in IHC-TEK antibody diluent (IHC World, Woodstock, MD, USA) overnight at 4 °C followed by secondary 1/1000 in PBS for 30 minutes; anti-α-SMA (Cat#ab5694, Abcam) 1/200 in 0.5% BSA for 1 hour, followed by secondary 1/500 in 1x PBS for 30 minutes; anti-desmin (Cat#ab15200, Abcam) 1/200 in 0.5% BSA for 1 hour followed by secondary 1/500 in 1x PBS for 30 minutes; anti-NMMHC (Cat#ab684, Abcam) 1/200 in 0.5% BSA for 1 hour, followed by secondary 1/500 in 1x PBS for 30 minutes; anti-CD45 (Cat#MCA43Ga, AbD Serotec, Kidlington, UK) 1/200 in 0.5% BSA for 1 hour, followed by secondary 1/1000 in 1x PBS for 30 minutes. The sections were incubated in Vectastain ABC reagent for 30 minutes, the chromagen/substrate (DAB peroxidase substrate kit, Vector Labs, Burlingame, CA) for 2 minutes, counterstained with Gill’s hematoxylin solution (Fisher Scientific, Pittsburgh, PA), dehydrated, and cover slipped with mounting medium (Permount, Fisher Scientific). For immunofluorescent staining, carotid artery cross-sections were incubated with an anti-nitrotyrosine antibody (Cat# ab61392, Abcam) at a 1:1000 dilution, or an anti-S-nitrosocysteine antibody (Cat# ab94930, Abcam) at a 1:500 dilution in IHC-Tek antibody diluent (IHC World, Woodstock, MD, USA) for 1 hour at room temperature. After a 2 minute rinse of PBS, the sections were incubated with a F(ab′)2-Goat anti-Mouse IgG (H+L) secondary antibody (Alexa Fluor® 555 conjugate, Cat#A-2145 Thermo Fisher Scientific, Waltham, MA) at 1:500 for the anti-nitrotyrosine primary, and 1:1000 for the anti-S-nitrosocysteine primary, in PBS for 1 hour at room temperature. Nuclei were stained with DAPI 1:500 in PBS for 1 minute (Cat#D3571, Thermo Fisher Scientific). Finally, slides were coverslipped with ProLong Gold (Cat#P36930, Thermo Fisher Scientific). For negative controls, PBS was substituted for the primary antibody. Digital images were taken with light microscopy using an Olympus BHT microscope (Melville, NY) and SPOT camera basic Software (Diagnostic Instruments Inc., Sterling Heights, MI). For nuclear stains, cells with positive staining were counted by a blinded investigator in four high power fields per arterial section and expressed as an average. For cytoplasmic stains, staining was quantified by a blinded investigator using a scale of 0–4. For immunofluorescence, positive red pixels were quantified using ImageJ (NIH, Bethesda, MD).
Biochemical Analyses
Carotid arteries were harvested 1 or 2 hours after surgery and immediately frozen in liquid nitrogen. Lysates were obtained by homogenization with a glass tapered tissue grinder in HEPES with 0.1 % triton X100 and protease inhibitors. For the biotin switch assay the cysteine residues were immediately blocked with methyl methanethiosulfonate. Cyclic guanosine monophosphate (cGMP) was assayed in carotid lysates and plasma, using an acetylated direct cGMP kit (AbCam) according to the manufacturer’s specifications. Protein S-nitrosation was measured in cell and carotid lysates via a biotin switch assay as previously described. [32]. For the cells 1 × 106 VSMC were plated in 10 cm culture dishes. Cells were serum starved for 24 hours and then treated with DETA/NO, PROLI/NO, or S-nitrosoglutathione (GSNO). PROLI/NO stock (5mM) was prepared in NaOH 10 mM to prevent decomposition before addition to the cell culture. 24 hours after treatment cells were collected, an aliquot was used to determine protein concentration, and the cysteines were immediately blocked with methyl methanethiosulfonate. Protein concentrations in tissue and cell lysates were determined with the BCA assay (Thermo Fisher Scientific, Waltham, MA). Nitrite in plasma was determined using the tri-iodide method, and S-nitrosothiols in plasma were determined using the cysteine/copper method as previously described. [33] Both assays were performed using ozone-based chemiluminescence detection with a Sievers Nitric Oxide Analyzer (GE Analytical Instruments, Boulder, CO).
Data Analysis
Results are expressed as mean ± standard error of the mean. Differences between multiple groups for continuous data (morphometric data, cell counts, proliferation, and CD45+ counts) were analyzed using one way analysis of variance (ANOVA) with the Student–Newman–Keuls post hoc test for all pairwise comparisons. Differences for paired data (nitrite, cGMP, mean arterial blood pressure, heart rate, and biotin switch data) were analyzed using repeated measures ANOVA. Differences between multiple groups for categorical data (phenotypic markers: α-SMA, desmin, and NMMHC), and non-normally distributed data (S-nitrosocysteine staining) were analyzed using a Kruskal-Wallis ANOVA on ranks with the Student–Newman–Keuls post hoc test for pairwise comparisons. All statistical analyses were performed using either SigmaPlot v10.0 (Systat Software Inc. CA, USA) or SAS (SAS Institute Inc., Cary, NC). Statistical significance was assumed when P<0.05.
RESULTS
Periadventitial •NO delivery provides long-term inhibition of neointimal hyperplasia
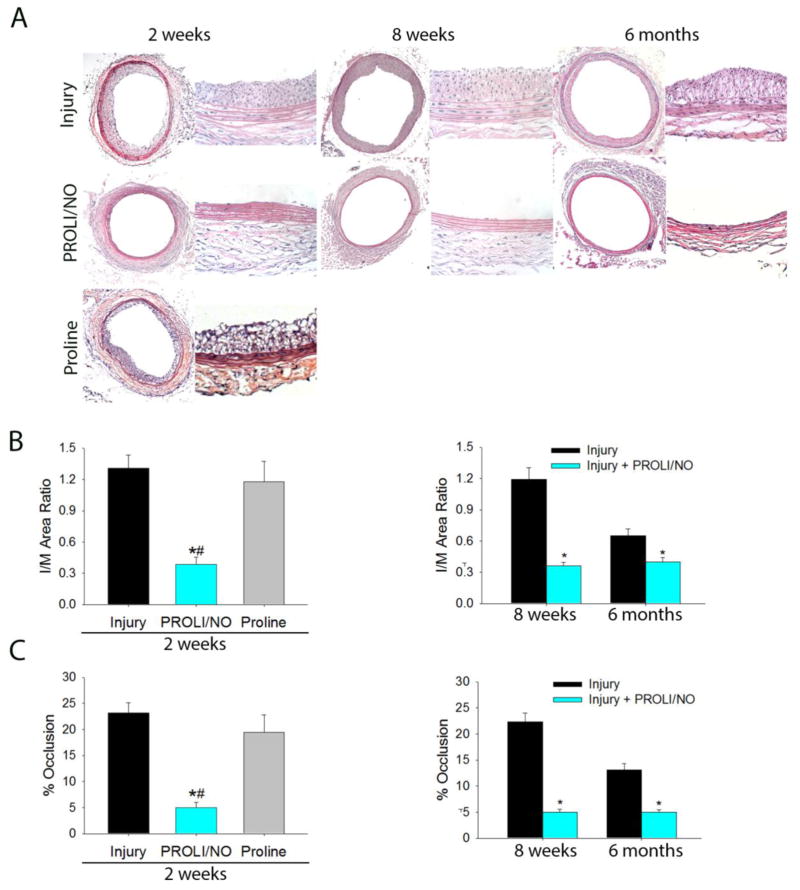
We sought to determine if the short-lived diazeniumdiolate PROLI/NO was capable of providing long-term inhibition of neointimal hyperplasia. Ten-week-old male Sprague Dawley rats underwent carotid balloon injury with or without periadventitial application of the •NO donor PROLI/NO. Rats were sacrificed at different time points up to 6 months. PROLI/NO effectively inhibited neointimal development at all assessed time points (Figure 1A and 1B). PROLI/NO reduced the intima to media (I/M) area ratio by 73%, 69%, and 40%, at 2 weeks, 8 weeks, and 6 months, respectively (Figure 1B, *P<0.05). In a similar fashion, PROLI/NO reduced the percent occlusion by 79%, 77%, and 62%, at 2 weeks, 8 weeks, and 6 months, respectively (Figure 1C, *P< 0.05). Our data show that despite its short half-life, PROLI/NO affords durable inhibition of neointimal hyperplasia. To determine if the effect was due to •NO or the parent compound, proline, an equivalent molar amount of proline was applied to rats undergoing balloon injury. Neointimal hyperplasia was assessed 2 weeks after arterial injury. Proline did not affect any of the morphometric parameters assessed, suggesting that the diazeniumdiolate moiety in PROLI/NO is responsible for its beneficial effects (Figure 1A–C).
Figure 1. PROLI/NO results in durable inhibition of neointimal hyperplasia in the rat carotid artery after balloon injury.
The carotid artery balloon injury model was performed in 10-week-old Sprague Dawley rats with or without periadventitial application of PROLI/NO (10 mg), or proline (5.5 mg). Carotid arteries were harvested at 2 weeks, 8 weeks and 6 months. A) Representative hematoxylin & eosin stained rat carotid artery sections from each treatment group (10X objective and 20X objective). B) Quantification of the intima to media (I/M) area ratio, and C) percent occlusion shows that PROLI/NO provided durable inhibition of neointimal development at all time points evaluated (N= 4 for proline, N=10 for injury alone, N=7 for the rest of the groups, *P<0.05 vs injury, #P<0.05 vs proline).
Periadventitial •NO delivery affects proliferation and cellularity throughout the arterial wall after arterial injury
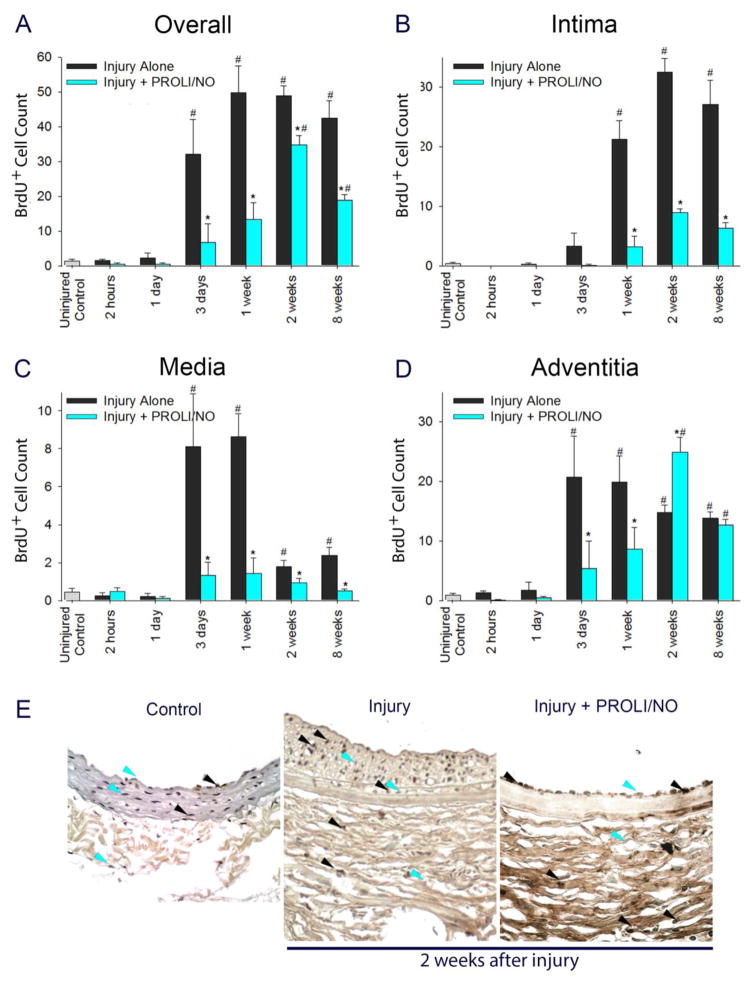
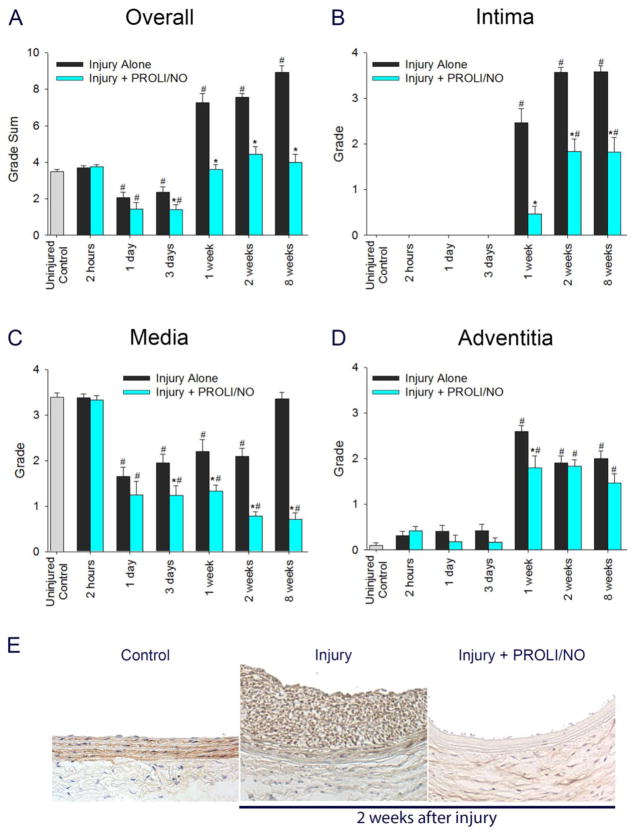
To further evaluate the mechanism by which PROLI/NO inhibits neointimal hyperplasia in a durable manner, we performed a detailed time-course analysis throughout each layer of the arterial wall for proliferation and cellularity. The effect of PROLI/NO on actively proliferating cells was assessed by administering BrdU to animals 24 and 1 hour prior to sacrifice. An interesting pattern emerged. Proliferation was increased compared to uninjured control arteries 3 days after arterial injury (Figure 2A, #P<0.05). PROLI/NO significantly inhibited overall active proliferation 3 days following arterial injury and thereafter compared to injury alone (Figure 2A, *P<0.05). In the intima, cells started to actively proliferate 3 days after balloon injury and PROLI/NO significantly inhibited cellular proliferation by 85% at 1 week, 73% at 2 weeks, and 76% at 8 weeks compared to injury alone (Figure 2B, *P<0.05). In the media, cells started to actively proliferate at 3 days after balloon injury and PROLI/NO inhibited proliferation by 84% at 3 days and 1 week compared to injury alone (Figure 2C, *P<0.05). In the adventitia, cellular proliferation increased at a similar time point after injury as the intima and media. Surprisingly, while PROLI/NO inhibited proliferation in the adventitia by 80% at 3 days, and by 56% at 1 week, it induced proliferation by 68% at 2 weeks compared to injury alone (Figure 2D–E *P<0.05). By 8 weeks, cellular proliferation was similar in the non-•NO and •NO treated arteries, but increased compared to uninjured control arteries (Figure 2D, #P<0.05).
Figure 2. PROLI/NO differentially inhibits proliferation throughout the arterial wall after balloon injury.
The carotid artery balloon injury model was performed in 10-week-old Sprague Dawley rats with or without periadventitial application of PROLI/NO (10 mg). Carotid arteries were harvested at different time points. BrdU (100 mg/kg, intraperitoneal) was administered 24 hours and 1 hour prior to sacrifice; BrdU-positive nuclei were counted in four high power fields per section and averaged. A) Total cell count of BrdU-positive cells throughout the arterial wall. B) BrdU-positive cell count in the intima. C) BrdU-positive cell count in the media. D) BrdU-positive cell count in the adventitia. E) Representative arterial cross sections 2 weeks after balloon injury. The black arrow heads indicate representative BrdU-positive cells, and the blue arrow heads point to representative BrdU-negative cells (only a few representative cells are labeled). #P<0.05 vs. uninjured control, *P<0.05 vs. injury alone, N=7/treatment group.
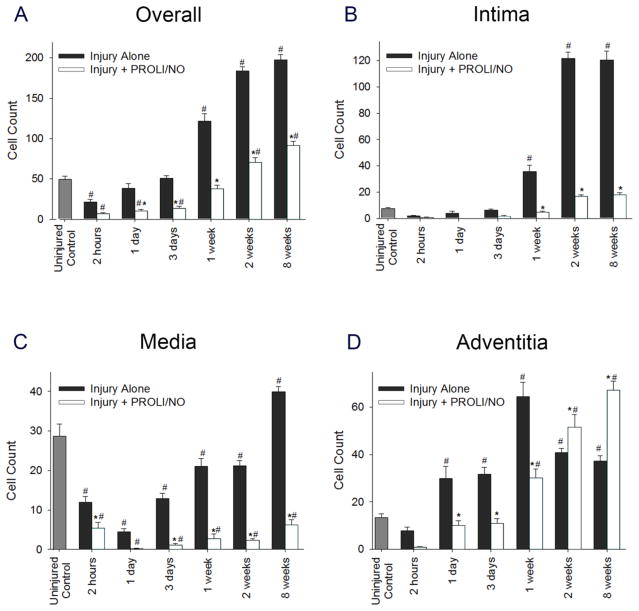
Next, we evaluated the effect of PROLI/NO on cellularity, as cell number can increase independent of proliferation. Nuclei were counted in four high power fields per section and averaged. One week following arterial injury and thereafter, there was increased overall cellularity throughout the vascular wall after balloon injury compared to uninjured control arteries (Figure 3A, #P<0.05). PROLI/NO reduced total cellularity 1 day after arterial injury and thereafter compared to injury alone (Figure 3A, *P<0.05). Evaluation of each layer revealed that PROLI/NO reduced cell number following injury in the intima by 86% at 2 weeks and 85% at 8 weeks compared to injury alone (Figure 3B, *P<0.05) and in the media by 89% at 2 weeks and 84% at 8 weeks compared to injury alone (Figure 3C, *P<0.05). However, while PROLI/NO reduced cellularity in the adventitia at early time points (1 day–1 week), PROLI/NO actually increased cellularity in the adventitia by 20% at 2 weeks, consistent with the proliferation data, and 44% at 8 weeks compared to injury alone (Figure 3D, *P<0.05). Since cellularity can also be affected by different rates of cell death, we evaluated the effect of PROLI/NO on cell apoptosis. We counted TUNEL positive cells relative to total cell number. We found that PROLI/NO did not have a significant effect on apoptosis at any time point throughout the vessel wall (Supplementary Figure S1). Together, these data show a differential effect of PROLI/NO throughout the vascular wall. Whereas PROLI/NO inhibits proliferation and cellularity in the intima and media at all time points without having an effect on cell death, it initially inhibits but then stimulates proliferation and cellularity in the adventitia.
Figure 3. PROLI/NO differentially affects cellularity throughout the arterial wall after balloon injury.
The carotid artery balloon injury model was performed in 10-week-old Sprague Dawley rats with or without periadventitial application of PROLI/NO (10 mg). Carotid arteries were harvested at different time points and nuclei were counted in four high power fields per section and averaged. A) Total cell count throughout the arterial wall. B) Cell count in the intima. C) Cell count in the media. D) Cell count in the adventitia. #P<0.05 vs. uninjured control, *P<0.05 vs. injury alone, N=7/treatment group.
Periadventitial PROLI/NO delivery does not induce •NO-mediated systemic effects
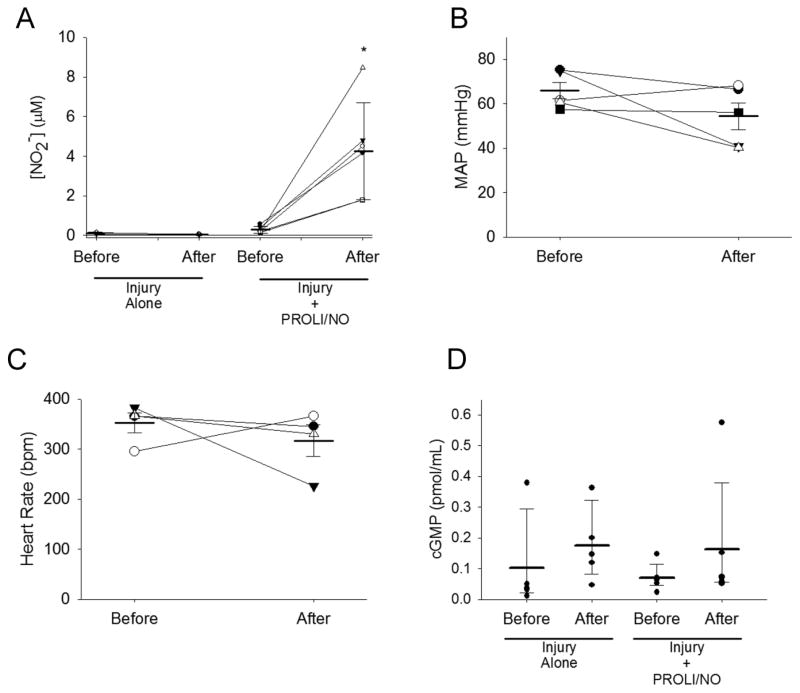
Since PROLI/NO is applied to the outer periadventitial surface of the artery, we assessed if active nitrogen species permeate to the systemic circulation. We measured nitrite (NO2−) concentrations in plasma before and 2 hours after surgery, and found a significant increase in plasma NO2− going from 0.29 μmol/L before surgery to 4.25 μmol/L 2 hours after PROLI/NO application (P<0.05, Figure 4A). These data suggest that •NO-derived species cross the vascular wall. Hence, we proceeded to determine if •NO-mediated systemic effects existed. We measured mean arterial blood pressure (MAP) and heart rate, before and 3 hours after surgery, and found that PROLI/NO had no significant effect on these parameters (MAPbefore=66 mmHg, MAPafter=54 mmHg; HRbefore=352 bpm, HRafter=317 bpm, P=NS, Figure 4B–C). Moreover, we measured plasma levels of cGMP and found that there was no significant change before and 2 hours after surgery, with or without application of PROLI/NO (P=NS, Figure 4D). In addition we evaluated cGMP levels in carotid lysates. Surprisingly, we found that periadventitial PROLI/NO did not cause a significant increase in cGMP 2 hours after application (P=NS, Supplementary Figure S2). Together, these data show that periadventitial application of PROLI/NO increases plasma NO2− concentrations but has little to no systemic effect.
Figure 4. Local application of PROLI/NO does not cause systemic side effects.
The carotid artery balloon injury model was performed in 10-week-old Sprague Dawley rats with or without periadventitial application of PROLI/NO (10 mg). Mean arterial blood pressure (MAP) and heart rate were measured before anesthetizing the rats. Blood was collected from the tail vein before and 2 hours after surgery. After the post-surgery blood draw, rats were allowed to recover from anesthesia and the MAP and heart rate measured again. Nitrite was measured in plasma by the tri-iodide method. cGMP levels were assessed with a colorimetric kit. A) Plasma nitrite. N=5/treatment group, *P<0.05; B) MAP. N=5, P=NS; C) Heart rate. N=4, P=NS; and D) Plasma cGMP concentration. N=5, P=NS.
Periadventitial •NO delivery does not affect re-endothelialization after arterial injury
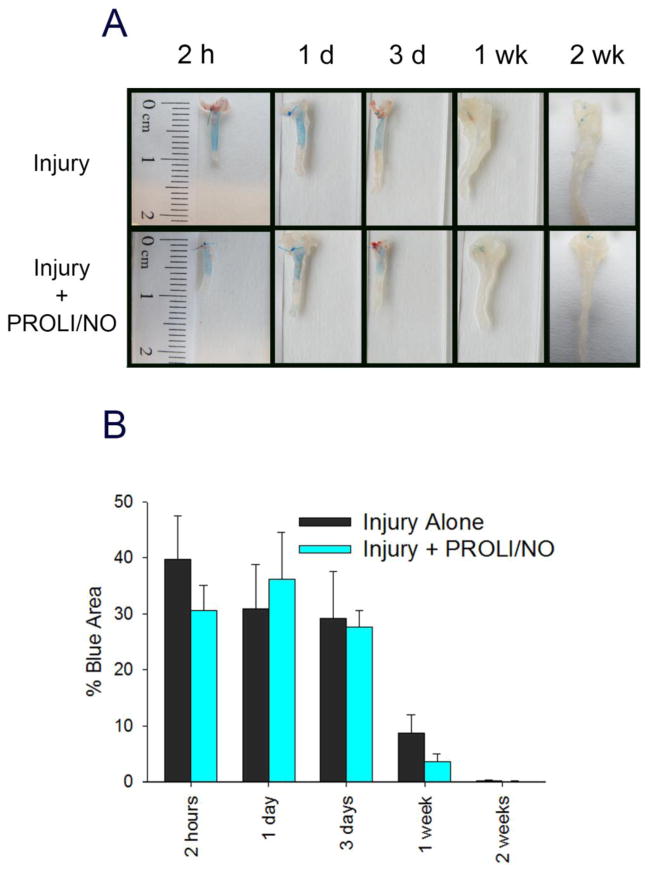
Since •NO is known to have both pro- and anti-proliferative effects on endothelial cells depending on the concentration used, we assessed the effect of PROLI/NO on re-endothelialization after balloon injury. After injury, endothelial denudation is clearly noted by a large area of Evans blue staining, as Evans blue has a high affinity for non-endothelialized surfaces (Figure 5). The stained area starts to decrease after 3 days, and it is minimal by 1 week after injury, indicating near complete re-endothelialization. PROLI/NO had no statistically significant effect on the rate of re-endothelialization (Figure 5, P=NS) following balloon injury.
Figure 5. PROLI/NO does not affect re-endothelialization after carotid artery balloon injury.
The carotid artery balloon injury model was performed in 10-week-old Sprague Dawley rats with or without periadventitial application of PROLI/NO (10 mg). Carotids were harvested at different time points. Evans blue (0.5 ml 0.5%) was administered intravenously immediately before sacrifice. Blue stained area and total injured area was quantified using ImageJ (N=3/treatment group). A) Representative gross images of the harvested carotid arteries showing the Evans blue stain. B) Quantification of the blue area (i.e., denuded endothelium) divided by total injured area, represented as percent.
PROLI/NO affects local oxidative markers
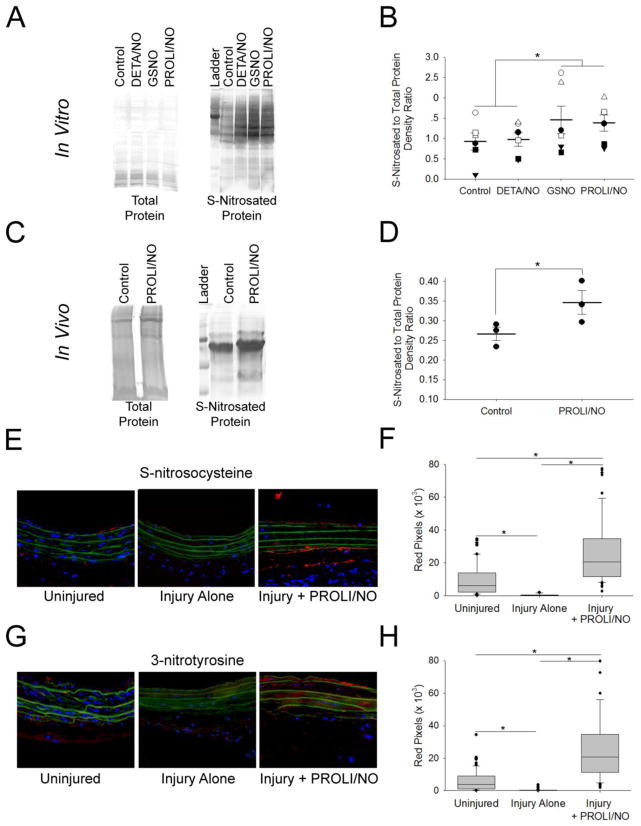
As we showed that plasma nitrite increased after periadventitial PROLI/NO application, it was of interest to assess if reactive nitrogen species are effectively present at the site of vascular injury. Since PROLI/NO has a very short half-life, high concentrations of •NO could be potentially achieved at the application site, favoring formation of secondary reactive nitrogen species. Hence, we first assessed the feasibility of PROLI/NO induced protein S-nitrosation in vitro. VSMC were treated with 100 μM DETA/NO, GSNO, or PROLI/NO. Cells were collected after 24 hours and protein S-nitrosation assessed via a biotin switch assay. As expected, GSNO-treated cells showed evidence of increased protein S-nitrosation (Figure 6A–B). Interestingly, PROLI/NO-treated cells showed S-nitrosation levels comparable to those induced by GSNO. To test if periadventitial application of PROLI/NO promotes S-nitrosation in vivo, we performed a biotin switch on carotid artery lysates 1 hour after surgery with or without PROLI/NO. PROLI/NO-treated arteries showed increased levels of S-nitrosated proteins (Figure 6C–D) compared to control arteries. To further test the hypothesis that PROLI/NO locally increases reactive nitrogen species we evaluated carotid artery sections for S-nitrosocysteine and 3-nitrotyrosine. We found a significant increase in S-nitrosocysteine in histological carotid artery cross sections of animals treated with PROLI/NO (*P<0.05, Figure 6E–F). Similarly, we found a significant increase in 3-nitrotyrosine in histological carotid artery cross sections of animals treated with PROLI/NO (*P<0.05, Figure 6G–H). Next, we tested plasma S-nitrosothiols (RSNO) before and 2 hours after surgery. PROLI/NO did not affect plasma RSNO (Supplementary Figure S3). Basal levels of plasma RSNO before surgery were 25 ± 9 nmol/L, and levels 2 hours after PROLI/NO application were 22 ± 4 nmol/L. Together, our data suggest that •NO-derived species from PROLI/NO reach the site of arterial injury and promote local changes in oxidative markers without affecting systemic RSNO.
Figure 6. PROLI/NO affects local oxidative markers.
VSMC were treated with 100 μM DETA/NO, GSNO, or PROLI/NO. After 24 hours the cells were harvested and the lysates assayed for S-nitrosated proteins with the biotin switch assay. A) Representative silver-stained gels showing total protein input on the left and S-nitrosated proteins on the right. B) Densitometry quantification of 6 separate gels. N=6, *P<0.05. Carotid arteries were harvested 1 h after surgery with or without application of PROLI/NO (10 mg). Carotid lysates were assayed for S-nitrosated proteins with the biotin switch assay. C) Representative silver stained gels showing total protein input on the left, and S-nitrosated proteins on the right. D) Densitometry quantification of 3 separate gels. N=3, *P<0.05. Carotid arteries were harvested at 3 days and sections processed with an antibody to S-nitrosocysteine and an antibody anti nitrotyrosine. Quantification of red pixels was performed by using ImageJ. E) Representative arterial cross sections stained for S-nitrosocysteine. F) Quantification of S-nitrosocysteine. N=5, *P<0.05. G) Representative arterial cross sections stained for 3-nitrotyrosine. H) Quantification of S-nitrotyrosine. N=5, *P<0.05
Periadventitial •NO delivery differentially affects α-smooth muscle actin (α-SMA) positive cells throughout the vascular wall after balloon injury
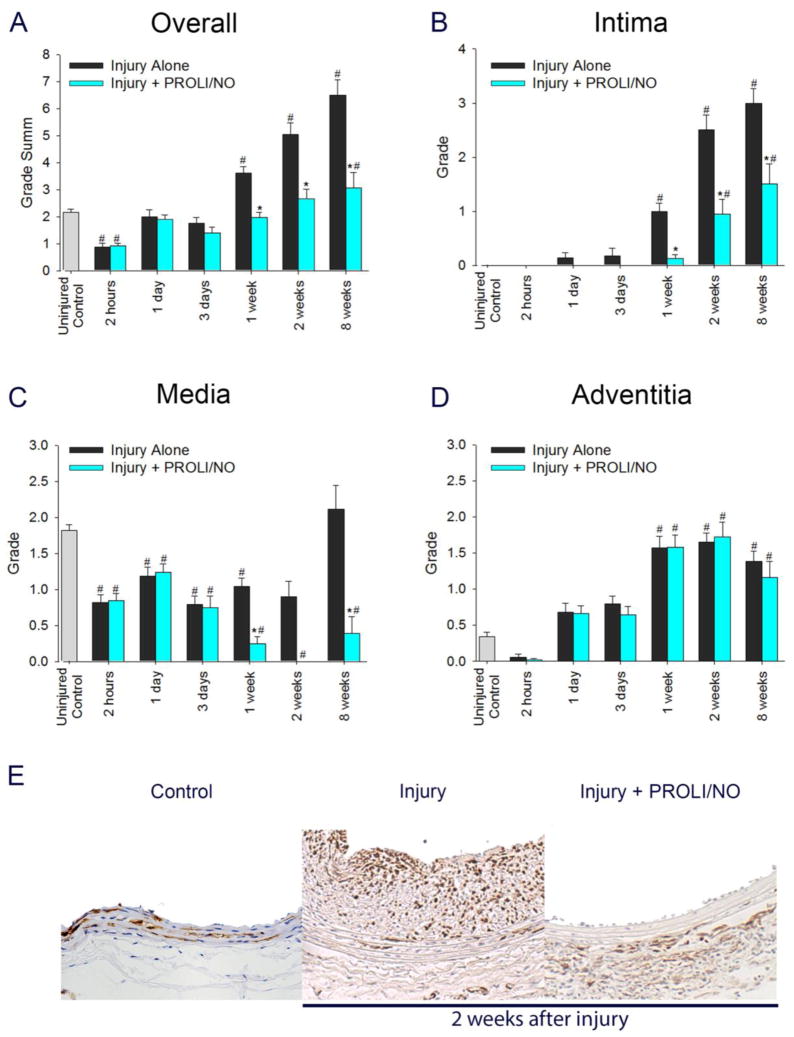
To assess the phenotype of VSMC and myofibroblasts, we evaluated cells that stained positive for α-SMA after balloon injury. Injury caused an overall decrease in α-SMA staining in the arterial wall at 1 and 3 days with a later increase above baseline starting at 1 week compared to uninjured control arteries (Figure 7A, #P<0.05). PROLI/NO further reduced α-SMA positive cells at 3 days after injury and at all time points thereafter compared to injury alone (Figure 7A, *P<0.05). When looking at each layer of the arterial wall separately, injury caused a marked increase in α-SMA staining in the intima starting at 1 week compared to uninjured control arteries (Figure 7B, #P<0.05). PROLI/NO inhibits this increase at all the time points with a 81% decrease at 1 week and a 49% decrease at 2 and 8 weeks compared to injury alone (Figure 7B, 7E, *P<0.05). In the media, injury induced a 51% decrease in α-SMA staining at 1 day that persisted until week 2, returning to baseline levels at 8 weeks, compared to uninjured arteries (Figure 7C, 7E, #P<0.05). However, PROLI/NO decreased α-SMA staining after 1 day, and completely prevented it from returning to baseline levels at all time points evaluated, with a 79% inhibition at 8 weeks compared to injury alone (Figure 7C, *P<0.05). In the adventitia, injury caused a dramatic increase in α-SMA staining at 1, 2, and 8 weeks compared to baseline (Figure 7D, #P<0.05). PROLI/NO caused a slight reduction in α-SMA staining at 1 week. However, it was not significant at any other time point compared to injury alone. Our data suggest that PROLI/NO causes a durable decrease in α-SMA positive VSMC and myofibroblasts in the intima and media, with little effect on the adventitia.
Figure 7. PROLI/NO differentially affects α-smooth muscle actin (α-SMA) positive cells throughout the arterial wall after balloon injury.
The carotid artery balloon injury model was performed in 10-week-old Sprague Dawley rats with or without periadventitial application of PROLI/NO (10 mg). Carotid arteries were harvested at different time points and sections were stained with an antibody to α-SMA. Quantification was performed by grading on a scale of 0–4 by a blinded investigator. A) Total grade sum throughout the arterial wall. B) Grade for the intima. C) Grade for the media. D) Grade for the adventitia. E) Representative arterial cross sections 2 weeks after balloon injury. #P<0.05 vs. uninjured control, *P<0.05 vs injury alone, N=7/treatment group.
Periadventitial •NO delivery differentially affects desmin positive cells throughout the vascular wall after balloon injury
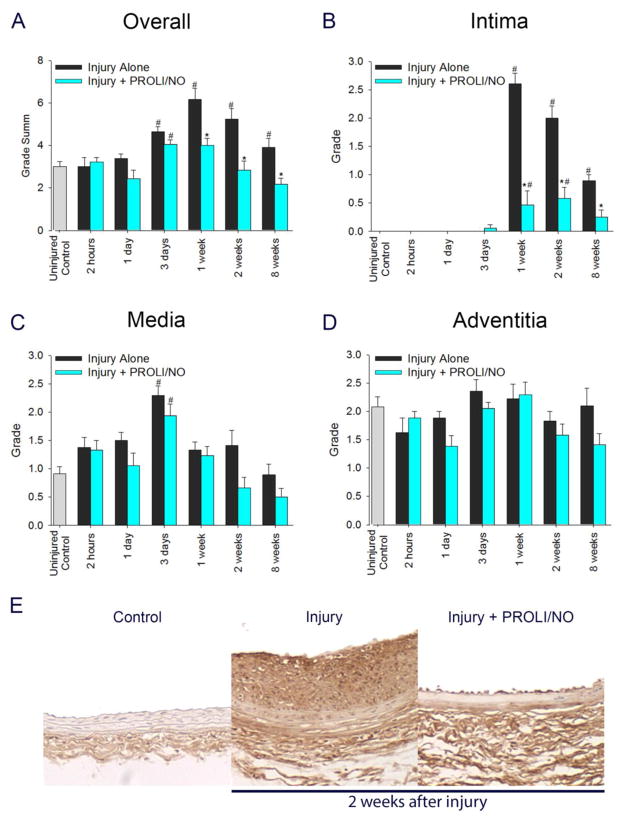
Subsequently, we turned our attention to desmin staining, as a marker for contractile VSMC phenotype. Two hours after balloon injury, there was an overall decrease in desmin staining that normalized after 1 day (Figure 8A, #P<0.05). By 1 week, desmin staining was significantly higher than uninjured control arteries (Figure 8A, #P<0.05). However, PROLI/NO caused a 47% reduction in desmin staining at 1 and 2 weeks, and a 54% reduction at 8 weeks compared to injury alone (Figure 8A, 8E, *P<0.05). When looking at each layer of the arterial wall separately, injury caused a marked increase in desmin staining in the intima starting at 1 week, compared to uninjured arteries (Figure 8B, #P<0.05). PROLI/NO inhibited this increase at all the time points, with an 88% decrease at 1 week, a 62% decrease at 2 weeks, and a 50% decrease at 8 weeks, compared to injury alone (Figure 8B, 8E, *P<0.05). In the media, injury induced a 55% decrease in desmin staining 2 hours after injury compared to uninjured arteries, returning to baseline levels at 8 weeks (Figure 8C, #P<0.05). However, PROLI/NO prevented the return to baseline levels with a persistent inhibition of 82% at 8 weeks compared to injury alone (Figure 8C, *P<0.05). In the adventitia, injury caused a transient trend to decrease in desmin staining at 2 hours that was not statistically significant. By 1 week after injury, desmin staining increased above baseline and persisted through week 8 compared to uninjured arteries (Figure 8D, #P<0.05). PROLI/NO had no effect on adventitial desmin staining. Our data suggest that PROLI/NO exerts a preferential inhibition of desmin-positive cells in the neointima and media, but not the adventitia.
Figure 8. PROLI/NO differentially affects desmin positive cells throughout the arterial wall after balloon injury.
The carotid artery balloon injury model was performed in 10-week-old Sprague Dawley rats with or without periadventitial application of PROLI/NO (10 mg). Carotids were harvested at different time points and sections were stained with an antibody to desmin. Quantification was performed by grading on a scale of 0–4 by a blinded investigator. A) Total grade sum throughout the arterial wall. B) Grade for the intima. C) Grade for the media. D) Grade for the adventitia. E) Representative arterial cross sections 2 weeks after balloon injury. #P<0.05 vs uninjured control, *P<0.05 vs. injury alone, N=7/treatment group.
Periadventitial •NO delivery differentially affects NMMHC positive cells throughout the vascular wall after balloon injury
Next, we analyzed NMMHC as a marker for fibroblasts in the vascular wall. Injury caused an overall increase in NMMHC staining at 3 days, and extending to 8 weeks, with a peak at 1 week, compared to uninjured arteries (Figure 9A, #P<0.05). PROLI/NO inhibited this increase at 1 week (Figure 9A, *P<0.05) and at all subsequent time points. Upon evaluation of each layer of the arterial wall, injury caused a marked increase in NMMHC staining in the intima starting at 1 week, compared to uninjured arteries (Figure 9B, #P<0.05). PROLI/NO inhibited this increase at all the time points with a maximum inhibition of 82% at 1 week, followed by 71% inhibition at 2 weeks and 70% inhibition at 8 weeks compared to injury alone (Figure 9B, 9E, *P<0.05). In the media, injury induced a 2-fold increase in NMMHC staining 3 days after injury, compared to uninjured arteries (Figure 9C, #P<0.05). NMMHC staining returned to control levels by 1 week. PROLI/NO did not significantly affect NMMHC staining compared to injury alone at any time point. In the adventitia, neither injury nor PROLI/NO treatment caused a statistically significant effect on NMMHC staining (Figure 9D, P=NS). These data suggest that PROLI/NO has a strong inhibitory effect on intimal fibroblast-like cells, but no effect on medial or adventitial fibroblasts.
Figure 9. PROLI/NO differentially affects non-muscle myosin heavy chain (NMMHC) positive cells throughout the arterial wall after balloon injury.
The carotid artery balloon injury model was performed in 10-week-old Sprague Dawley rats with or without periadventitial application of PROLI/NO (10 mg). Carotid arteries were harvested at different time points and sections were stained with an antibody to NMMHC. Quantification was performed by grading on a scale of 0–4 by a blinded investigator. A) Total grade sum throughout the arterial wall. B) Grade for the intima. C) Grade for the media. D) Grade for the adventitia. E) Representative arterial cross sections 2 weeks after balloon injury. #P<0.05 vs. uninjured control, *P<0.05 vs. injury alone, N=7/treatment group.
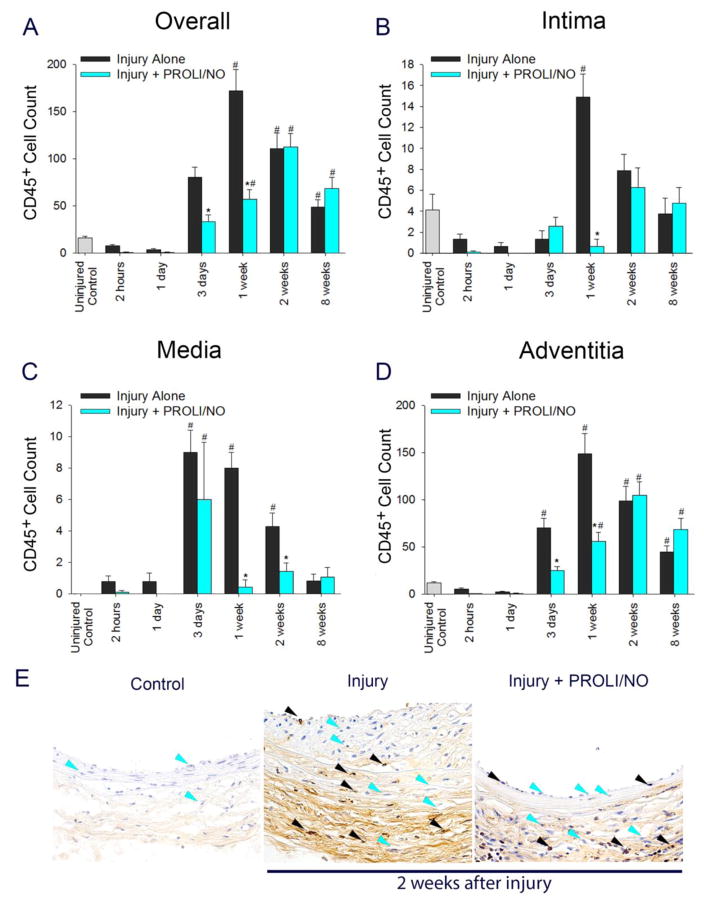
Periadventitial •NO delivery affects CD45+ cell count throughout the arterial wall after arterial injury
To further evaluate the effect of PROLI/NO in the vascular wall after arterial injury, we determined the presence of leukocytes by counting CD45+ cells. Injury caused an increase in the CD45+ cell count 3 days after injury and peaked at 1 week compared to uninjured control arteries (Figure 10A, #P<0.05). Even though the count decreased over the following time points, they remained significantly elevated at 8 weeks compared to uninjured control arteries. PROLI/NO inhibited the CD45+ cell count at 3 days and 1 week compared to injury alone (Figure 10A, *P<0.05). Assessment of each layer revealed the majority of the CD45+ cells localized to the adventitia (Figure 10). In the intima, injury caused an increase in CD45+ cell infiltration that peaked at 1 week compared to uninjured control arteries (Figure 10B, #P<0.05). PROLI/NO dramatically inhibited intimal CD45+ cell numbers by 96% at 1 week compared to injury alone (Figure 10B, *P<0.05). In the media, CD45+ cell count peaked at 3 days after injury; counts remained elevated at 1 and 2 weeks, and returned to baseline by 8 weeks (Figure 10C, 10E #P<0.05). PROLI/NO significantly inhibited the number of CD45+ cell count in the media by 54% at 1 week and 42% at 2 weeks, compared to injury alone (Figure 10C, 10E, *P<0.05). In the adventitia, CD45+ cells started to increase 3 days after injury and peaked at 1 week compared to uninjured arteries (Figure 10D, #P<0.05). The cell count decreased over the subsequent time points. However, the counts remained significantly elevated at 8 weeks compared to control. PROLI/NO inhibited the CD45+ cell count at 3 days and 1 week compared to injury alone (Figure 10D, *P<0.05). Overall, these data suggest that PROLI/NO delays and inhibits leukocyte infiltration following arterial injury, with the greatest effect on the media and adventitia.
Figure 10. PROLI/NO differentially inhibits CD45+ cell numbers throughout the arterial wall after balloon injury.
The carotid artery balloon injury model was performed in 10-week-old Sprague Dawley rats with or without periadventitial application of PROLI/NO (10 mg). Carotids were harvested at different time points and sections were stained with an antibody against CD45. CD45+ cells were counted in four high power fields per section and averaged. A) Total cell count of CD45+ cells throughout the arterial wall. B) CD45+ cell count in the intima. C) CD45+ cell count in the media. D) CD45+ cell count in the adventitia. E) Representative arterial cross sections 2 weeks after balloon injury. The black arrow heads indicate representative CD45+ cells, and the blue arrow heads point to representative CD45-negative cells (only a few representative cells are labeled). #P<0.05 vs. uninjured control, *P<0.05 vs. injury alone, N=7/treatment group.
DISCUSSION
This study shows that the short-lived •NO donor PROLI/NO provides durable inhibition of neointimal hyperplasia up to 6 months after injury. The inhibition of neointimal hyperplasia is accompanied by modulation of cellularity and proliferation, with no changes in apoptosis; an increase in local markers of reactive nitrogen species; modulation of phenotypic differentiation, and inflammatory infiltration in the arterial wall. While PROLI/NO inhibited cellularity and proliferation in the intima and media, it actually increased actively proliferating adventitial cells at 2 and 8 weeks after injury despite an overall inhibition of neointimal hyperplasia, without affecting the rates of apoptosis. Evaluation of phenotypic markers revealed that while PROLI/NO decreased both VSMC and myofibroblast markers in the intima, PROLI/NO predominantly inhibited contractile VSMC in the media, and had no effect on VSMC or myofibroblast in the adventitia. Thus, the increase in proliferation observed in the adventitia is likely due to regulation of cell types other than VSMC and myofibroblasts, such as endothelial cells of the vasa vasorum or resident or circulating progenitor cells. Lastly, PROLI/NO caused a delayed and decreased infiltration of leukocytes into the injured arterial wall.
Clowes et al. first described the time course of cell proliferation in the rat carotid artery after balloon injury. [10] VSMC proliferation peaked between 2 and 4 days in the media, and between 4 days and 1 week in the intima. Cellularity in the carotid artery was maximal at 2 weeks after injury. Hanke et al. described the time course of VSMC proliferation after experimental angioplasty in rabbit carotids, showing that proliferation peaked between 3 and 7 days and cellularity of the intima was maximal at 4 weeks after injury. [11] Angelini et al. showed that VSMC proliferation peaked in the media at 3 days and in the intima at 1 week after balloon angioplasty in rabbit iliac arteries. [12] Similarly, Durand et al showed that proliferation in all three layers was maximal between 3 days and 1 week after angioplasty in a femoral atherosclerotic rabbit model. [13] Our studies are in agreement with these previous reports. In addition, we show for the first time the cellularity and proliferation time course after injury in carotid arteries treated with a •NO donor. •NO is a well-known inhibitor of VSMC proliferation and migration, [23, 24] and a regulator of apoptosis. [34–36] However, whereas we do observe an overall inhibition of cell proliferation, we did not observe any effect on cell apoptosis. Moreover, we report a surprising increase in proliferation in the adventitia with its consequent increase in adventitial cellularity.
The extremely short half-life of this donor signifies that our treatment modality resembles a bolus of •NO. Under these conditions, locally high concentrations of •NO would be expected for a short time. However, we did not detect an increase in tissue cGMP resulting from the activation of guanylate cyclase. One possible explanation is that activation of guanylate cyclase is fast and the levels of cGMP peaked before we performed our measurements. As we harvested the arteries 2 hours after PROLI/NO application, we could have lost our detection window. However, many effects of •NO, including inhibition of VSMC proliferation are cGMP-independent. [35–37] Moreover, we found increased S-nitrosation and increased 3-nitrotyrosine staining after PROLI/NO application. These data show that •NO-derived reactive species reach the site of interest. These results have relevant implications for the protective effects of PROLI/NO. S-nitrosation inhibits VSMC and chondrocyte migration possibly by direct post-translational modification of actin. [38, 39] Similarly, nitration of tyrosines in tubulin inhibits proliferation in VSMC. [40] Moreover, S-nitrosation inhibits VSMC proliferation, independently of cGMP [36] by regulating the polyamine pathway. [35] All these effects could in part account for the inhibition of hyperplasia observed in PROLI/NO-treated arteries.
The arterial injury response is accompanied with a change in phenotype of both VSMC and fibroblasts. [15, 41, 42] VSMC change from a contractile to a secretory phenotype. The proportion of desmin positive staining cells, a marker of contractile SMC, has been shown to decrease with time after injury in the neointima and media. [43] Our results show a decrease in medial desmin staining after injury alone and an increase in desmin positive cells in the neointima after 1 week. Also, the neointima showed the presence of α-SMA staining after 1 week. PROLI/NO caused a decrease in both medial and intimal desmin and α-SMA staining. •NO is necessary to maintain VSMC phenotype and disruption of •NO regulation during injury has been implicated in VSMC phenotype switch. [44] Therefore, an exogenous source of •NO at the time of injury might prevent VSMC activation. Similar to the VSMC phenotype switch after injury, adventitial fibroblasts change to a myofibroblast phenotype expressing α-SMA and have been shown to migrate from the adventitia to the neointima. [45] Our results agree with the literature showing that adventitial staining for α-SMA shows a dramatic increase 1 week after injury, confirming a phenotypic modulation from adventitial fibroblast to myofibroblast. [16] However, PROLI/NO did not have an effect on this change. Similarly, PROLI/NO had no effect on NMMHC staining in the media or adventitia, just the intima. Thus, our data suggest that PROLI/NO preferentially inhibits medial and intimal VSMC, with little to no effect on adventitial fibroblast phenotype modulation.
The time course of CD45+ leukocyte infiltration after injury agrees with previous reports. [46–48] Recruitment of inflammatory cells has been associated with both negative remodeling and neointimal hyperplasia, ultimately contributing to restenosis. [49] Inflammatory cells have been implicated in activation and phenotypic modulation of adventitial fibroblast and VSMC. [15, 41] •NO is capable of modulating several aspects of inflammation, including cytokine release, chemotaxis, and inflammatory cell adhesion. [50] Some of these effects have been shown to occur via S-nitrosation. [51] Inhibition of leukocyte recruitment inhibits neointimal formation and restenosis. [48, 52] Barbato et al. reported that •NO reduces vascular inflammation after rat balloon injury, reducing neointimal hyperplasia. [47] Our results demonstrate that early delivery of pharmacological doses of •NO results in a delayed and less intense inflammatory response, as measured by leukocyte infiltration.
Our study is not without limitations. Whereas our study describes the changes in proliferation, cellularity, phenotypic markers, and leukocyte infiltration after arterial injury observed in rats treated with and without PROLI/NO, we do not provide a conclusive mechanistic explanation of such observations. It is known that activated VSMC and myofibroblasts secrete chemokines that recruit immune cells. It is also known that S-nitrosation decreases expression of proinflammatory cytokines and adhesion molecules. [51] Moreover, we correlate PROLI/NO application with decreased, delayed, and less intense inflammatory infiltration. These results support a redox-mediated anti-inflammatory effect of PROLI/NO. However, we cannot establish causality. In addition, we describe an increase in cellularity and proliferation in the adventitia but we did not identify the cell population that was affected or the functional significance of such increase. These findings will require further study that is outside the scope of this current work
Despite these limitations, we present the first detailed time course of cellularity, proliferation, and phenotypic markers in •NO-treated balloon injured arteries. We also provide evidence of a local PROLI/NO-mediated increase in S-nitrosation and tyrosine nitration, which could in part account for the long-lasting protective effects of this short-lived •NO donor. Our findings contribute to the understanding of the vasoprotective effects of •NO, especially its potent inhibition of neointimal hyperplasia. We have previously shown that •NO-based therapies have an inhibitory effect on neointimal hyperplasia well after the •NO-donating moiety is no longer present in the vasculature. [19, 53] Here, we present further evidence that a short-acting •NO donor has long-lasting effects and provides durable inhibition of neointimal growth. Moreover, the increase in protein S-nitrosation, the increase in adventitial proliferation, the inhibition of neointimal and medial VSMC, as well as the inhibition of leukocyte infiltration, are observed well after all of the PROLI/NO should have decomposed, suggesting that the initial •NO burst inhibits early processes that result in durable effects, maybe by creating a •NO reservoir in the pool of S-nitrosated proteins. Further studies are needed to elucidate the effect of •NO on the adventitia, identify what cell population is stimulated to proliferate after •NO exposure, and the functional relevance of this proliferation in inhibiting neointimal hyperplasia.
Supplementary Material
HIGHLIGHTS.
PROLI/NO provides long-term inhibition of neointimal hyperplasia in the rat carotid balloon injury model for 6 months despite its short half-life.
This inhibition depends upon the diazeniumdiolate moiety as proline has no inhibitory effect. Nonetheless, it appears to be cGMP-independent despite the need of •NO.
Periadventitial delivery of this •NO donor, significantly increased plasma nitrite. However, it caused neither a drop in blood pressure, nor an increase in plasma S-nitrosothiols.
PROLI/NO inhibited proliferation and cellularity in the media and intima at all of the time points studied. Surprisingly, PROLI/NO caused an increase in adventitial proliferation at 2 weeks, resulting in increased cellularity at 2 and 8 weeks compared to injury alone.
PROLI/NO predominantly inhibited contractile smooth muscle cells in the intima and media, and had little to no effect on the phenotype of adventitial cells.
PROLI/NO caused a delayed and decreased leukocyte infiltration response after injury.
Interestigly, PROLI/NO promoted local protein S-nitrosation in vivo without measurable systemic effects. This pool of S-nitrosated protein could provide an •NO reservoir long after all the PROLI/NO has decomposed.
PROLO/NO locally affects S-nitrosation and protein nitration. This local redox change could in part account for the ability of PROLI/NO to exert durable effects.
Acknowledgments
The authors would like to acknowledge Dr. Nick Tsihlis for his critical review of the manuscript, Mrs. Lynnette Dangerfield for her administrative and editorial assistance, and Ms. Emma Gargus and Mr. Wulin Jiang for their assistance performing some experiments. We also would like to acknowledge the generosity of Mrs Hilda Rosenbloom and Mrs. Eleanor Baldwin and Edwards Lifesciences for providing the Fogarty balloon catheters.
GRANTS
Funding from The Department of Veterans Affairs VA Merit Review Grant I01 BX000409; the National Institutes of Health, K08HL084203 and R01HL108118-01; the American Medical Association Foundation Seed Grant program; and the Northwestern Memorial Foundation, Center for Limb Preservation, Collaborative Development Initiative Award in Vascular Surgery.
GLOSSARY
- α-SMA
alpha smooth muscle actin
- BrdU
bromodeoxyuridine
- cGMP
cyclic guanosine monophosphate
- MAP
mean arterial pressure
- NMMHC
non-muscle myosin heavy chain
- •NO
nitric oxide
- NO2−
nitrite
- PROLI/NO
1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate
- RSNO
S-nitrosothiols
- VSMC
vascular smooth muscle cells
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007;100:3K–9K. doi: 10.1016/j.amjcard.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Kenagy RD, Plaas AH, Wight TN. Versican degradation and vascular disease. Trends Cardiovasc Med. 2006;16:209–215. doi: 10.1016/j.tcm.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riessen R, Wight TN, Pastore C, Henley C, Isner JM. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation. 1996;93:1141–1147. doi: 10.1161/01.cir.93.6.1141. [DOI] [PubMed] [Google Scholar]
- 5.Wight TN. Arterial remodeling in vascular disease: a key role for hyaluronan and versican. Front Biosci. 2008;13:4933–4937. doi: 10.2741/3052. [DOI] [PubMed] [Google Scholar]
- 6.Clowes AW, Clowes MM, Fingerle J, Reidy MA. Regulation of smooth muscle cell growth in injured artery. J Cardiovasc Pharmacol. 1989;14(Suppl 6):S12–S15. [PubMed] [Google Scholar]
- 7.Clowes AW, Kirkman TR, Clowes MM. Mechanisms of arterial graft failure. II. Chronic endothelial and smooth muscle cell proliferation in healing polytetrafluoroethylene prostheses. J Vasc Surg. 1986;3:877–884. [PubMed] [Google Scholar]
- 8.Lindner V, Reidy MA. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991;88:3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 10.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 11.Hanke H, Strohschneider T, Oberhoff M, Betz E, Karsch KR. Time course of smooth muscle cell proliferation in the intima and media of arteries following experimental angioplasty. Circ Res. 1990;67:651–659. doi: 10.1161/01.res.67.3.651. [DOI] [PubMed] [Google Scholar]
- 12.Angelini A, Visona A, Calabrese F, Pettenazzo E, Yacoub A, Valente M, Bonandini EM, Jori G, Pagnan A, Thiene G. Time Course of apoptosis and Prolideration in vascular Smooth Muscle Cells after Balloon Angioplasty. Basic Appl Myol. 2012;12:33–42. [Google Scholar]
- 13.Durand E, Mallat Z, Addad F, Vilde F, Desnos M, Guerot C, Tedgui A, Lafont A. Time courses of apoptosis and cell proliferation and their relationship to arterial remodeling and restenosis after angioplasty in an atherosclerotic rabbit model. J Am Coll Cardiol. 2002;39:1680–1685. doi: 10.1016/s0735-1097(02)01831-4. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 15.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 16.Havelka GE, Kibbe MR. The vascular adventitia: its role in the arterial injury response. Vasc Endovasc Surg. 2011;45:381–390. doi: 10.1177/1538574411407698. [DOI] [PubMed] [Google Scholar]
- 17.Popowich DA, Varu V, Kibbe MR. Nitric oxide: what a vascular surgeon needs to know. Vascular. 2007;15:324–335. doi: 10.2310/6670.2007.00051. [DOI] [PubMed] [Google Scholar]
- 18.Keefer LK. Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates. Annu Rev Pharmacol Toxicol. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- 19.Pearce CG, Najjar SF, Kapadia MR, Murar J, Eng J, Lyle B, Aalami OO, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Kibbe MR. Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radic Biol Med. 2008;44:73–81. doi: 10.1016/j.freeradbiomed.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vavra AK, Havelka GE, Martinez J, Lee VR, Fu B, Jiang Q, Keefer LK, Kibbe MR. Insights into the effect of nitric oxide and its metabolites nitrite and nitrate at inhibiting neointimal hyperplasia. Nitric Oxide. 2011;25:22–30. doi: 10.1016/j.niox.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 22.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo JP, Panday MM, Consigny PM, Lefer AM. Mechanisms of vascular preservation by a novel NO donor following rat carotid artery intimal injury. Am J Physiol. 1995;269:H1122–H1131. doi: 10.1152/ajpheart.1995.269.3.H1122. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ Res. 1996;78:225–230. doi: 10.1161/01.res.78.2.225. [DOI] [PubMed] [Google Scholar]
- 25.Tzeng E, Kim YM, Pitt BR, Lizonova A, Kovesdi I, Billiar TR. Adenoviral transfer of the inducible nitric oxide synthase gene blocks endothelial cell apoptosis. Surgery. 1997;122:255–263. doi: 10.1016/s0039-6060(97)90016-7. [DOI] [PubMed] [Google Scholar]
- 26.Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Eng JW, Murar J, Martinez J, Popowich DA, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Hulvat JF, Stupp SI, Kibbe MR. Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia. J Vasc Surg. 2008;47:173–182. doi: 10.1016/j.jvs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsihlis ND, Murar J, Kapadia MR, Ahanchi SS, Oustwani CS, Saavedra JE, Keefer LK, Kibbe MR. Isopropylamine NONOate (IPA/NO) moderates neointimal hyperplasia following vascular injury. J Vasc Surg. 2010;51:1248–1259. doi: 10.1016/j.jvs.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapadia MR, Eng JW, Jiang Q, Stoyanovsky DA, Kibbe MR. Nitric oxide regulates the 26S proteasome in vascular smooth muscle cells. Nitric Oxide. 2009;20:279–288. doi: 10.1016/j.niox.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Morales RC, Bahnson ES, Havelka GE, Cantu-Medellin N, Kelley EE, Kibbe MR. Sex-based differential regulation of oxidative stress in the vasculature by nitric oxide. Redox Biol. 2015;4:226–233. doi: 10.1016/j.redox.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsihlis ND, Oustwani CS, Vavra AK, Jiang Q, Keefer LK, Kibbe MR. Nitric oxide inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by increasing the ubiquitination and degradation of UbcH10. Cell Biochem Biophys. 2011;60:89–97. doi: 10.1007/s12013-011-9179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varu VN, Ahanchi SS, Hogg ME, Bhikhapurwala HA, Chen A, Popowich DA, Vavra AK, Martinez J, Jiang Q, Saavedra JE, Hrabie JA, Keefer LK, Kibbe MR. Insulin enhances the effect of nitric oxide at inhibiting neointimal hyperplasia in a rat model of type 1 diabetes. Am J Physiol Heart Circ Physiol. 2010;299:H772–H779. doi: 10.1152/ajpheart.01234.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forrester MT, Foster MW, Benhar M, Stamler JS. Detection of protein S-nitrosylation with the biotin-switch technique. Free Rad Biol Med. 2009;46:119–126. doi: 10.1016/j.freeradbiomed.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogra B Analyt Technol Biomed Life Sci. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res. 2007;75:349–358. doi: 10.1016/j.cardiores.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu G, del Soldato P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young DV, Serebryanik D, Janero DR, Tam SW. Suppression of proliferation of human coronary artery smooth muscle cells by the nitric oxide donor, S-nitrosoglutathione, is cGMP-independent. Mol Cell Biol Res Commun. 2000;4:32–36. doi: 10.1006/mcbr.2000.0254. [DOI] [PubMed] [Google Scholar]
- 37.Huang J, Lin SC, Nadershahi A, Watts SW, Sarkar R. Role of redox signaling and poly (adenosine diphosphate-ribose) polymerase activation in vascular smooth muscle cell growth inhibition by nitric oxide and peroxynitrite. J Vasc Surg. 2008;47:599–607. doi: 10.1016/j.jvs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Frenkel SR, Clancy RM, Ricci JL, Di Cesare PE, Rediske JJ, Abramson SB. Effects of nitric oxide on chondrocyte migration, adhesion, and cytoskeletal assembly. Arthritis Rheum. 1996;39:1905–1912. doi: 10.1002/art.1780391118. [DOI] [PubMed] [Google Scholar]
- 39.Dalle-Donne I, Milzani A, Giustarini D, Di Simplicio P, Colombo R, Rossi R. S-NO-actin: S-nitrosylation kinetics and the effect on isolated vascular smooth muscle. J Muscle Res Cell Motil. 2000;21:171–181. doi: 10.1023/a:1005671319604. [DOI] [PubMed] [Google Scholar]
- 40.Phung AD, Soucek K, Kubala L, Harper RW, Chloe Bulinski J, Eiserich JP. Posttranslational nitrotyrosination of alpha-tubulin induces cell cycle arrest and inhibits proliferation of vascular smooth muscle cells. Eur J Cell Biol. 2006;85:1241–1252. doi: 10.1016/j.ejcb.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arteriosclero Thromb Vasc Biol. 2011;31:2391–2396. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 42.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas AC, Campbell JH. Smooth muscle cells of injured rat and rabbit arteries in culture: contractile and cytoskeletal proteins. Atherosclerosis. 2001;154:291–299. doi: 10.1016/s0021-9150(00)00483-4. [DOI] [PubMed] [Google Scholar]
- 44.Bundy RE, Marczin N, Birks EF, Chester AH, Yacoub MH. Transplant atherosclerosis: role of phenotypic modulation of vascular smooth muscle by nitric oxide. Gen Pharmacol. 2000;34:73–84. doi: 10.1016/s0306-3623(00)00047-1. [DOI] [PubMed] [Google Scholar]
- 45.Wilcox JN, Waksman R, King SB, Scott NA. The role of the adventitia in the arterial response to angioplasty: the effect of intravascular radiation. Int J Radiat Oncol Biol Phys. 1996;36:789–796. doi: 10.1016/s0360-3016(96)00299-4. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 47.Barbato JE, Zuckerbraun BS, Overhaus M, Raman KG, Tzeng E. Nitric oxide modulates vascular inflammation and intimal hyperplasia in insulin resistance and the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2005;289:H228–236. doi: 10.1152/ajpheart.00982.2004. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi S, Watanabe N, Nakazawa K, Suzuki J, Tsushima K, Tamatani T, Sakamoto S, Isobe M. Roles of P-selectin in inflammation, neointimal formation, and vascular remodeling in balloon-injured rat carotid arteries. Circulation. 2000;102:1710–1717. doi: 10.1161/01.cir.102.14.1710. [DOI] [PubMed] [Google Scholar]
- 49.Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31:224–230. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 50.Wallace JL. Nitric oxide as a regulator of inflammatory processes. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):5–9. doi: 10.1590/s0074-02762005000900002. [DOI] [PubMed] [Google Scholar]
- 51.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gotoh R, Suzuki J, Kosuge H, Kakuta T, Sakamoto S, Yoshida M, Isobe M. E-selectin blockade decreases adventitial inflammation and attenuates intimal hyperplasia in rat carotid arteries after balloon injury. Arteriosclero Thromb Vasc Biol. 2004;24:2063–2068. doi: 10.1161/01.ATV.0000145942.31404.20. [DOI] [PubMed] [Google Scholar]
- 53.Havelka GE, Moreira ES, Rodriguez MP, Tsihlis ND, Wang Z, Martinez J, Hrabie JA, Kiefer LK, Kibbe MR. Nitric oxide delivery via a permeable balloon catheter inhibits neointimal growth after arterial injury. J Surg Res. 2013;180:35–42. doi: 10.1016/j.jss.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.