Abstract
Anti-DNA antibodies are the serological hallmark of systemic lupus erythematosus, and participate in the pathogenesis of lupus nephritis by cross-reacting with multiple renal antigens. Previously, using a panel of murine anti-DNA IgGs that share identical variable regions but that differ in the constant regions, we demonstrated that the cross-reaction and renal pathogenicity of anti-DNA antibodies are isotype dependent. In this study, we investigated the catalytic potential of this anti-DNA antibody panel, and determined its isotype dependency. The three isotype switch variants (IgG1, IgG2a, IgG2b) and the parent IgG3 PL9-11 anti-DNA antibodies were compared in their catalysis of 500 base pair linear double stranded DNA and a 12-mer peptide (ALWPPNLHAWVP), by gel analysis, MALDI-TOF mass spectrometry, and nuclear magnetic resonance spectroscopy. The binding affinity of anti-DNA antibodies to double stranded DNA and peptide antigens were assessed by ELISA and surface plasmon resonance. We found that the PL9-11 antibody isotypes vary significantly in their potential to catalyze the cleavage of both linear and double stranded DNA and the proteolysis of peptides. The degree of the cleavage and proteolysis increases with the incubation temperature and time. While different PL9-11 isotypes have the same initial attack sites within the ALWPPNLHAWVP peptide, there was no correlation between binding affinity to the peptide and proteolysis rates. In conclusion, the catalytic properties of anti-DNA antibodies are isotype dependent. This finding provides further evidence that antibodies that share the same variable region, but which have different constant regions, are functionally distinct. The catalytic effects modulated by antibody constant regions need to be considered in the design of therapeutic antibodies (abzymes) and peptides designed to block pathogenic autoantibodies.
Keywords: Anti-DNA antibody, Catalysis, Systemic lupus erythematosus
1. Introduction
Systemic lupus erythematosus (SLE) is a potentially severe autoimmune disease characterized by increased titers of serum autoantibodies. Among these pathogenic autoantibodies, anti-DNA IgGs attract particular attention for their pivotal role in the pathogenesis of lupus nephritis, one of the most serious complications of SLE (Yung et al., 2008). The nephritogenicity of anti-DNA antibodies may be caused by their indirect binding to kidney tissue mediated by DNA/nucleosomes, or by direct binding to glomerular antigens mediated by antigenic cross-reactivity (Hanrotel-Saliou et al, 2011). The interactions between anti-DNA antibodies and glomerular resident cells can activate the complement cascade, modulate gene expression, enhance cellular proliferation, and alter cellular phenotypes (Jang et al., 2009; Qing et al., 2008; Yung et al., 2005; Yung et al., 2009; Zhang et al., 2012). Therefore, elucidation of the mechanisms through which anti-DNA antibodies interact with self-antigens is crucial in the development of novel therapeutics for lupus nephritis and other disease manifestations.
Previous studies suggested that the pathogenicity of anti-DNA antibodies in SLE patients and lupus animal models is isotype dependent (Bijl et al., 2002; Baudino et al., 2006; Krishnan et al., 2012). Recently, by using class switching in vitro, we generated a panel of monoclonal antibodies (mAb) from the murine PL9-11 IgG3 anti-DNA antibody. Members of the PL9-11 mAb panel share identical variable regions, but differ from each other in the heavy chain constant region. Immunologic dogma has been that the variable regions in heavy chains and light chains are the sole structures that determine the binding of antibodies to antigens. However, our results showed that both antigenic specificity and renal pathogenicity differ between these PL9-11 derived mAbs (Xia et al., 2012), and that such differences arise from the different constant regions that may alter antibody secondary structure and function (Xia et al., 2013). Thus, anti-DNA antibodies can exhibit isotype dependent properties in binding to DNA or in cross-reaction with non-DNA antigens.
Catalysis of nucleic acids or peptide cleavage is an intrinsic property of certain antibodies associated with antigen binding. Increasing evidence suggests important functional roles for catalytic antibodies in homeostasis, autoimmune disease, and protection against infection (Paul et al., 2012; Tomin et al., 2015; Barrera et al., 2009; Nevinsky et al., 2010). Interestingly, in autoimmunity, natural antibody-enzymes (abzymes) can have both beneficial and detrimental effects, depending on the specific disease and the targets they cleave (for comprehensive review, see Belogurov et al., 2009).
Since DNA-catalyzing antibodies were first described by Shuster et al (1992), there has been increasing interest in the importance of antibodies with this effect in the pathogenesis of SLE. Remarkably, the concentrations of double stranded (ds) and single stranded (ss) DNA-hydrolyzing autoantibodies are elevated in the sera of patients with SLE and in murine lupus models, suggesting a role in disease (Nevinsky et al., 2002; Kostrikina et al., 2011; Ponomarenko et al., 2002). Catalyzing anti-DNA antibodies may share with DNase I several similar, or even identical, amino acid residues, which are necessary for DNA hydrolysis and the binding of magnesium and calcium ions (Kostrikina et al., 2014). Indeed, purified anti-DNA antibodies can enter the nuclei of tumor cells and kill cells through DNA hydrolyzing mechanisms (Kozyr et al., 2002). Moreover, the cytotoxic effect and the DNA hydrolyzing activity of anti-DNA antibodies is enriched in the antibody fractions that display cross-reactivity with nuclear matrix proteins (Kozyr et al., 2000), suggesting an important role of binding specificity in the catalytic potential of these antibodies. However, there has not been a systematic study of the relationship between the catalytic activity and binding affinity or specificity of anti-DNA antibodies. Since we found that heavy chain constant regions affect the binding of anti-DNA antibodies (Xia et al., 2012; Xia et al., 2013), the present study was designed to investigate the influence of such binding alterations on catalytic activity by taking advantage of the PL9-11 anti-DNA mAb panel that share identical variable regions.
2. Methods
2.1 Antibodies and antigens
The murine IgG1, IgG2b, and IgG2a isotype variants were generated from the parent hybridoma clone of PL9-11 (IgG3) by class switching in vitro, as described (Xia et al., 2012). All isotypes share the original PL9-11 heavy and light chain V regions, but differ in the identity of the heavy chain C region. As described previously, PL9-11 mAbs were purified from culture supernatant, and normalized to the same concentration using a goat anti-mouse mAb which bound to the identical kappa chain shared by all members of the panel (Xia et al., 2012). The murine IgG3, IgG1, IgG2b, and IgG2a isotype controls (Southern Biotech, Birmingham, AL) showed no in vitro binding to dsDNA (Xia et al., 2012). The Fab and F(ab′)2 fragments were prepared from the PL9-11 mAb by commercial kits (Thermo Scientific, Rockford, IL).
Double stranded (ds) DNA (500 bp) was obtained from plasmid DNA (pHC-msCu vector) by restriction enzyme digestion, as described previously (Xia et al., 2013). Single stranded (ss) DNA (18 bp, TATAGCGCGCGCTATAT) was synthesized by Invitrogen (Carlsbad, CA).
Two 12 amino acid peptides (ALWPPNLHAWVP, or “ALW”; AHSANNFNVKGI, or “AHS”) used in this study were identified by screening a Ph.D.™-12 phage display library (New England Biolabs, Ipswich, MA) with the PL9-11 mAb panel, and selecting a shared sequence bound by all isotypes (Xia et al., 2015). The 12-mer APNQHTPPWMLK peptide, which is specific for the murine non-DNA binding 3E5 mAb, served as a negative control. Unlabeled peptides were synthesized by the Rockefeller University Proteomics Resource Center (New York, NY). For isotope labeling of ALW, BL21(DE3) competent E. coli (Ipswich, MA) were transformed with the pET-31b vector (EMD Millipore, Billerica, MA) that expresses ketosteroid isomerase-ALW peptide-6×histag fusion protein (GENEWIZ Inc., South Plainfield, NJ). E. coli cells were grown in M9 media supplemented with 15NH4Cl and [13C] D-glucose. After induction with isopropyl-beta-D-thiogalactopyranoside, cells were harvested and then lysed in Tris-HCl buffer (6 M guanidine, pH 7.9). A HisPur Ni-NTA resin kit (Thermo Scientific) was used for the isolation of the fusion protein, followed by cyanogen bromide cleavage (Zerfaß et al., 2014). After the removal of precipitated ketosteroid isomerase, the ALW peptide was lyophilized. The purity and effectiveness of labeling (> 90%) was confirmed by MALDI-TOF mass spectrometry (data not shown).
2.2 Enzyme-linked immunosorbent assay (ELISA)
The dsDNA ELISA was performed as previously described (Gao at al., 2009; Xia et al., 2012), using the prepared linear dsDNA to coat the plates. Alkaline phosphatase-conjugated IgG goat anti-mouse chain was the secondary antibody.
2.3 DNA gel analysis
dsDNA was pre-incubated with PL9-11 mAbs at differential molar ratios and temperatures. Samples were loaded on a 0.8% gel of high-resolution agarose (National Diagnostics, Charlotte, NC). The running conditions were 70 V for 120 minutes in a B1A Mini electrophoresis chamber (Owl Separation Systems, Portsmouth, NH). Ethidium bromide (0.5 μg/ml) was used as a fluorescent indicator.
2.4 Surface plasmon resonance (SPR)
Using a Biacore 3000 instrument (Biacore, Piscataway, NJ), SPR analysis was performed to determine the binding affinity of PL9-11 IgGs to the different antigens (Xia et al., 2012). In brief, the antibodies were immobilized on a CM sensor chip (GE Healthcare, Port Washington, NY) at a concentration of 10 nM in MES buffer. The ALW and AHS peptides and the 18 bp ssDNA fragment (0–250 nM) in HEPES buffer (pH 7.4, with 0.05% Tween 20) were injected over the chip. The simple Langmuir model (A + B ↔ AB) was used for the calculation of binding kinetics, including association (kon) and dissociation (koff) rates. The times associated with the SPR flow are very short (< 5 minutes) compared to the rates of hydrolysis observed (> 30 minutes), and so the binding is assumed to be to unhydrolysed materials.
2.5 Nuclear magnetic resonance (NMR)
NMR was performed as described previously (Janda et al., 2012). In brief, the isotope labeled ALW peptide was dissolved in NMR buffer (10 mM MES, 90 mM NaCl, pH 6.0). ALW (36 to 100 μM) and each of the IgGs (18 to 50 μM) were mixed at molar ratios of 2:1 just before the NMR experiments at 25°C. 15N-1HN heteronuclear single quantum coherence spectra (HSQC) were recorded every 2 hours on a DRX600 Avance spectrometer (Bruker Corporation) at 600 MHz. The running time was 20–22 hours in total. Data processing and analysis used nmrPipe and NMRViewJ. Triple resonance experiments were also recorded to assign the backbone resonances of ALW peptide but the resonances could not be assigned unambiguously, possibly due to proline isomerization.
2.6 MALDI-TOF mass spectrometry
Mass spectrometry analysis was carried out using an ABI 4800 Proteomics TOF/TOF Analyzer (AB Science, Foster City, CA) in positive ion mode. α-Cyano-4-hydroxycinnamic acid in 50% acetonitrile with 0.1% TFA (5 mg/mL) was used as the matrix. A matrix droplet was applied to the top of the sample analyte and air-dried. The samples were diluted 1:100 by distilled water and mixed 1:1 with the matrix. Mixtures (1.5 μL) were spotted onto the MALDI target and then air-dried before analysis. Each sample was prepared in triplicate, and 80 individual spectra of each spot were averaged to produce a mass spectrum. Initial digestion products were not used as substrates for further digestion.
2.7 Statistical analysis
All results were shown as mean ± the standard error of the mean. Analysis of variance (ANOVA) was used for the comparison of more than two groups. A two-tailed t test was used when two groups were compared for statistical differences. P values less than 0.05 were considered significant.
3. Results
3.1 Anti-DNA IgGs catalyze dsDNA hydrolysis in an isotype dependent manner
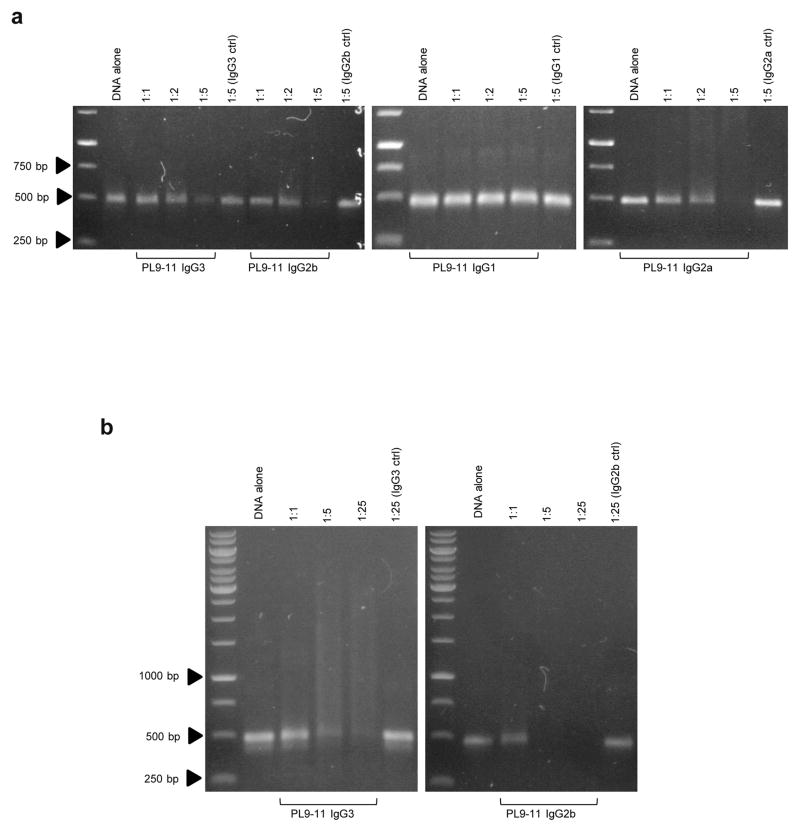
All isotypes of the PL9-11 mAb panel bind specifically to dsDNA (Xia et al., 2012; Xia et al., 2013). To determine if the catalytic effect of anti-DNA antibodies is isotype dependent, we performed gel analysis of the mixtures of each PL9-11 mAb together with dsDNA. The IgG3, IgG2b, and IgG2a isotypes of PL9-11 panel catalyzed linear dsDNA efficiently, while PL9-11 IgG1 exhibited little effect on dsDNA despite identical reaction conditions (Fig. 1a). When keeping incubation times and temperature constant, increasing molar ratios of PL9-11 IgGs to dsDNA enhanced catalysis (Fig. 1a), and even caused the complete disappearance of the dsDNA fragment (Fig. 1b). The isotype controls for each PL9-11 mAb had no detectable effect on dsDNA, even when the IgG to dsDNA ratio was increased to 25:1 (Fig. 1a and b).
Fig 1.
PL9-11 derived autoantibodies catalyze dsDNA antigen in an isotype dependent manner. Different isotypes of the PL9-11 parent anti-DNA antibody were mixed with linear dsDNA (500 bp) before incubation at 4°C overnight. dsDNA fragments were visualized by gel analysis. (a) IgG3, IgG2a, and IgG2b PL9-11, but not the respective isotype controls, cleaved dsDNA. Increasing molar ratios promoted the cleavage. (b) Higher molar ratio (25:1) of IgG3 and IgG2b to dsDNA caused the disappearance of dsDNA, while the isotype controls had little effect. Data were from three to five independent experiments. Representative images are shown.
3.2 Isotype dependence of PL9-11 mAbs is related to properties of the antigen
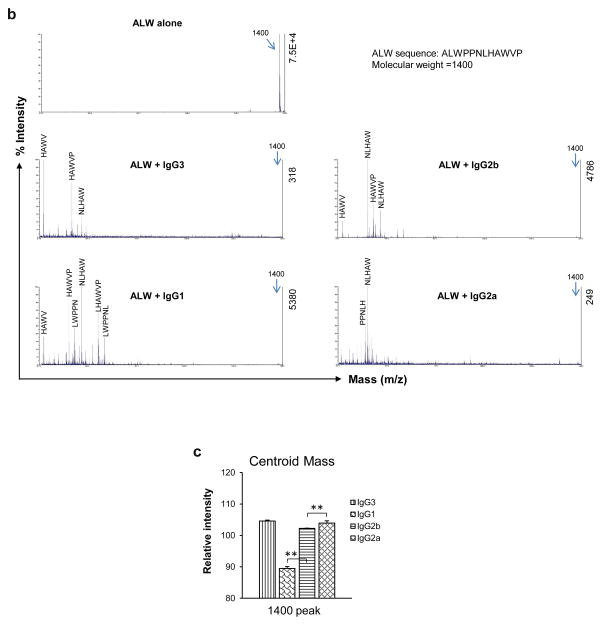
To study the effect of PL9-11 panel antibodies on non-DNA antigens, we used isotope labelled ALW as a substrate for proteolysis. The isotope labeled ALW peptide was subjected to 2 hour HSQC experiments in the presence of the PL9-11 isotypes. The intensities of resonance peaks were observed as a function of time (Fig. 2a). The HSQC spectra displayed different profiles indicating that PL9-11 mAb isotypes have different proteolysis sites on the ALW peptide, while the differences in the chemical shift intensity changes that occur over time point toward differential proteolysis rates. Moreover, the resonance profiles were similar for IgG1, IgG2b and IgG2a but different from IgG3, indicating that IgG3 is exhibiting little peptidase activity under these specific conditions. These results with the ALW peptide were quite different from those found above with the dsDNA antigen.
Fig 2.
The catalysis of peptide antigen by anti-DNA antibodies is isotype dependent. (a) 15N labeled ALW was mixed with different anti-DNA isotypes at a molar ratio of 2:1, and then analyzed by 2D NMR at 25°C. The circles indicate some of the differences between the PL9-11 isotypes. (b and c) ALW and PL9-11 mAbs (molar ratio=2:1) were mixed at 37°C for 4 hours. ALW cleavage was detected by MALDI-TOF mass spectrometry, showing different fragment patterns induced by each of anti-DNA antibodies (b). In (b), the relative (to highest peak) and absolute intensities are shown on the left and right y-axis, respectively. The relative intensity of the 1400 peak in each isotype was measured (n=4), displaying an order of IgG1<IgG2b<IgG2a=IgG3 (c). The centroid mass values reflected the intensities of the main peaks. Representative images are shown. **p < 0.01 in (c).
MALDI-TOF mass spectrometry (MS) verified the proteolytic activities of the PL9-11 isotypes, with profiles compatible with the NMR observations (Fig. 2b). The relative intensities of a centroid mass (indicating the main peak values) after 4 hours were calculated, showing levels from highest to lowest in order of IgG3≥IgG2a>IgG2b>IgG1. This corresponds to relative potency of hydrolysis by the mAbs in the order of IgG1>IgG2b>IgG2a≥IgG3 (Fig. 2c). However, the control peptide (APNQHTPPWMLK) was not cleaved by any of the PL9-11 antibodies, as demonstrated by MALDI-TOF mass spectrometry (data not shown). Moreover, the IgG isotype control mAbs exhibited no proteolytic activity for ALW (data not shown).
3.3 Incubation time and temperature affect the effectiveness of nuclease and peptidase activities
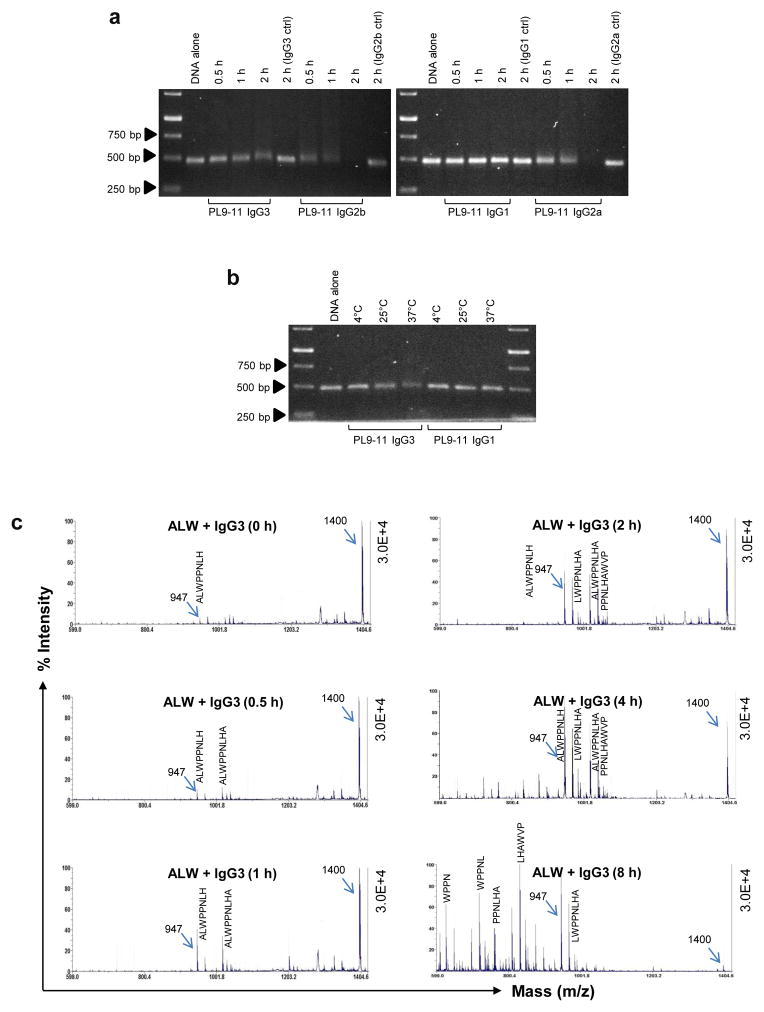
To investigate the factors that might determine the efficiency of anti-DNA antibodies in cleaving self-antigens, we manipulated the incubation time and/or incubation temperature of the antibody/substrate mixtures. As the incubation time increased from 30 minutes to 2 hours, the efficiency of IgG3, IgG2b, and IgG2a PL9-11 mAb nuclease activity increased (Fig. 3a). Similarly, increasing the incubation temperature enhanced IgG3 nuclease cleavage of dsDNA (Fig. 3b). In contrast, the alteration of incubation time and temperature (Fig. 3a and b) did not affect the low nuclease activity of PL9-11 IgG1.
Fig 3.
The catalytic efficiency of anti-DNA antibodies is modulated by incubation time and temperature. (a and b) PL9-11 mAbs and 500 bp dsDNA (molar ratio=2:1) were mixed at 37°C before gel analysis. Increasing incubation times enhanced the dsDNA catalysis by anti-DNA antibodies (except IgG1),while the isotype controls had no effect on dsDNA cleavage (a). The incubation temperature enhanced dsDNA catalysis by PL9-11 IgG3 or IgG1 (b). (c) ALW and anti-DNA IgG3 (molar ratio=2:1) were mixed at 37°C before MALDI-TOF mass spectrometry. The results showed more fragments (peaks) with the increase in incubation time. The 947 peak was seen at each of the time points. Data were from three to five independent experiments. Representative images were shown.
Peptidase activity was also time and temperature dependent. For ALW, cleavage by PL9-11 IgG3 increased with longer incubation times (Fig. 3c). The other isotypes exhibited similar results (data not shown). Moreover, changing the incubation temperature from 4°C to 37°C resulted in increased ALW cleavage at the higher temperature for all isotypes (data not shown).
3.4 Catalytic activities of PL9-11 mAbs are independent of antigen binding affinity
Previously, we demonstrated that PL9-11 IgGs have binding potential to dsDNA in the order of IgG3>IgG2a>IgG1>IgG2b (Xia et al., 2012). In this study, we applied SPR to quantitate the affinity of PL9-11 IgGs by using an 18 bp ssDNA fragment, which exhibits antigenic properties similar to dsDNA in interacting with anti-DNA antibodies (Stollar et al., 1986). The SPR results showed that these antibodies bound to 18 bp ssDNA in the order of IgG3>IgG2a=IgG1>IgG2b (Table 1). The relative apparent affinity for dsDNA of each PL9-11 isotype was discrepant with its nuclease activity (IgG2a=IgG2b>IgG3>IgG1) (Fig. 3a and b), ignoring the effect of cleavage on the measurement. While the absolute cleavage rates are not available from the assay shown in Fig. 1, the SPR measurements were completed in a short time frame time.
Table 1.
SPR analysis for the binding affinities of anti-DNA antibodies to ssDNA antigen
| Isotype | Ka (1/Ms) | Kd (1/s) | KD (M) | Rmax (RU)Δ |
|---|---|---|---|---|
| PL9-11-IgG3 | 9.86 | 2.92 (10−3) | 2.96 (10−4) | 22.85 |
| PL9-11-IgG1 | 0.85 | 6.06 (10−3) | 7.10 (10−3)* | 8.76 |
| PL9-11-IgG2b | 1.33 | 1.84 (10−2) | 1.38 (10−2)* | 18.69 |
| PL9-11-IgG2a | 0.77 | 2.53 (10−3) | 3.29 (10−3)* | 12.14 |
PL9-11 Abs were Immobilized to chip at a concentration of 10nM;
MES (C6H13NO4S) buffer was used for the Ab immobilization;
Lower KD value (=Kd/Ka) means higher binding affinity (n = 3);
Maximum value of response unit at the range of 0 to 250 nM of 18 bp ssDNA;
p<0.05, compared to PL9-11-IgG3 Ab.
The binding affinity of PL9-11 IgGs to ALW was also measured by SPR, showing an order of IgG2b>IgG2a≥IgG3>IgG1 (Xia et al., 2015). However, the NMR analysis determined that PL9-11 IgG3 had less peptidase activity than that seen for the other isotypes (Fig. 2a). Moreover, the MALDI-TOF mass spectrometry similarly displayed differences between the isotypes in peptidase cleavage of ALW (Fig. 2b and c). The binding affinity of the PL9-11 isotypes to the second peptide, AHS, was also measured by SPR, exhibiting an order of IgG3>IgG2a≥IgG2b>IgG1 (data not shown). This apparent discrepancy between peptide catalysis and antigen affinity is comparable to that described above for nuclease activity.
3.5 Constant regions influence the efficacy but not the nature of the first-attack sites in ALW cleavage by PL9-11 mAbs
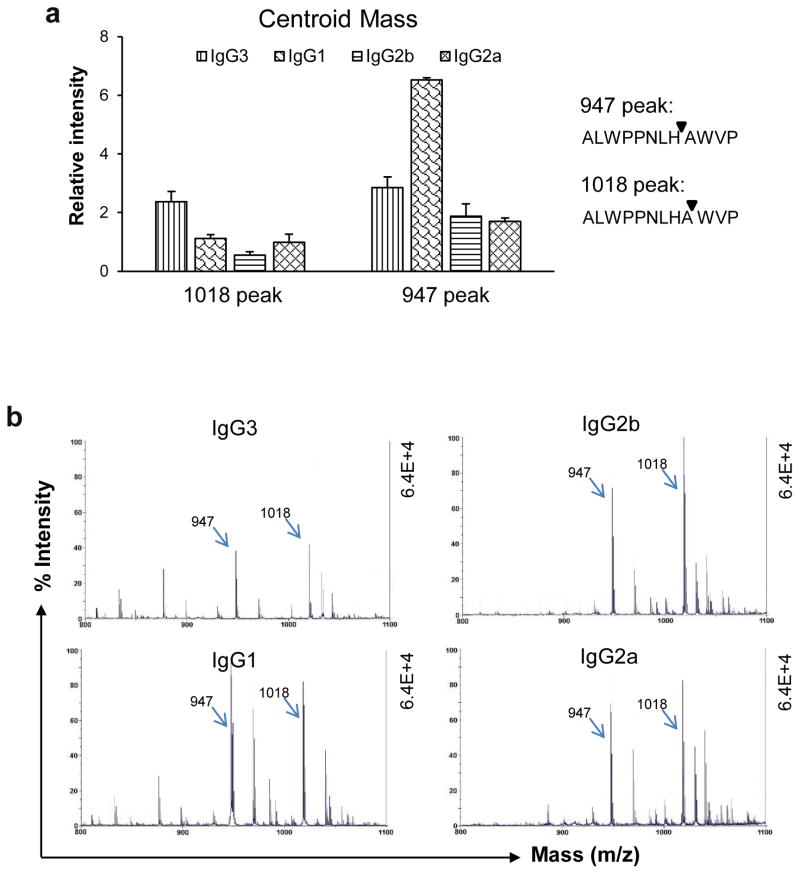
In the above studies, we found significant and unexpected variability between the PL9-11 antibody isotypes in cleavage of both DNA and ALW substrates. To quantify the influence of constant regions on peptidase activity, we further compared these effects by MALDI-TOF mass spectrometry. The four PL9-11 isotypes had similar primary cleavage sites when the incubation time and temperature were identical (Fig. 4b). Besides the 1018 peak, the second most significant peak was at 947 (Fig. 4a and b), which indicated cleavage between the 8th and 9th residues in the ALW peptide.
Fig 4.
The first-attack sites in ALW are shared by the PL9-11 panel isotypes. ALW and anti-DNA IgGs (molar ratio=2:1) were mixed at 37°C for 4 hours before MALDI-TOF mass spectrometry. (a) The relative intensities of the 947 peak (cleavage between 8th and 9th residues) and the 1018 peak (cleavage between 9th and 10th residues) were measured quantitatively, showing the highest 947 peak with PL9-11 IgG3, and the highest 1018 peak with IgG1 (p<0.05, compared to the other three isotypes accordingly). (b) Representative images (800 to 1010 m/z range) are shown, indicating the significance of the 947 and 1018 peaks. Data were from four independent experiments.
The nuclease and peptidase activities of the different PL9-11 monoclonal antibodies are summarized in Table 2.
Table 2.
Summary of catalytic activity of the PL9-11 isotypes
| Antigen | Method | PL9-11 isotype | |||
|---|---|---|---|---|---|
| IgG3 | IgG1 | IgG2b | IgG2a | ||
| 500 bp dsDNA* | Gel analysis | + | − | ++ | + |
| ALW 12AA peptideΔ | MALDI-TOF | + | +++ | ++ | + |
The catalytic strength was compared based on data shown in Figures 2b and 2c.
3.6 Fab fragments retain the catalytic properties of PL9-11 mAbs
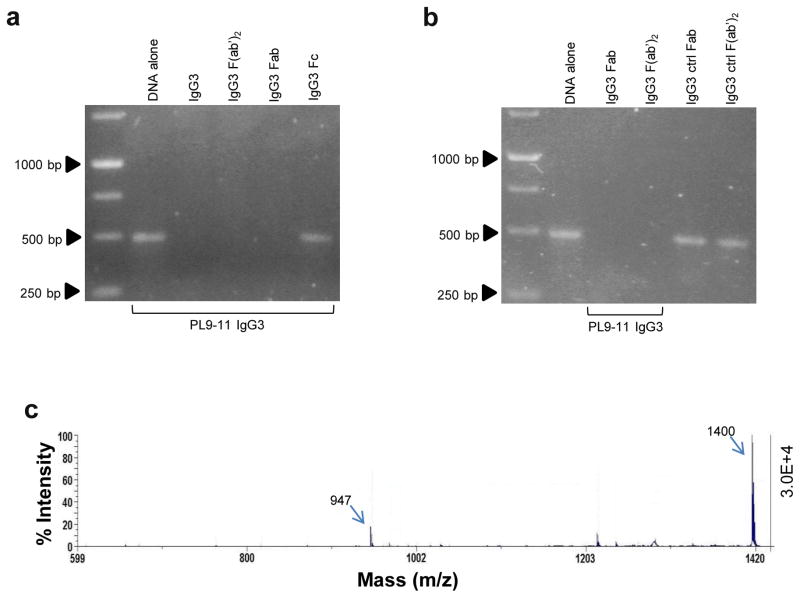
An antibody Fab fragment contains the complete variable regions, but not the CH2 and CH3 constant regions. Binding of the Fab (and F(ab′)2) but not Fc fragments of PL9-11 IgG3 and IgG2a to dsDNA was demonstrated by ELISA (data not shown). Furthermore, although the absence of the Fc may indeed influence catalysis rates as compared to the full length antibody, we found that the nuclease activity was specific to the Fab and F(ab′)2 fragments derived from the parent PL9-11 IgG3 mAb. In contrast, the Fc domain did not cleave dsDNA (Fig. 5a and b). The Fab fragment of PL9-11 IgG3 showed peptidase activity against the ALW peptide as well, characterized by the same 947 mass peak as the parent antibody (Fig. 5c). Similar results were seen with the Fab fragment of PL9-11 IgG2a isotype (data not shown).
Fig 5.
Fab fragments retain the catalytic properties of anti-DNA antibodies. (a and b) Intact anti-DNA IgG3, and Fab and F(ab′)2 fragments, were mixed with 500 bp dsDNA (molar ratio=150:1) at 4°C overnight. The results showed that the Fab and F(ab′)2 fragments, but not the Fc fragment, catalyzed dsDNA (a). The Fab and F(ab′)2 fragments from the control IgG3 mAb had no effect on dsDNA (b). (c) The Fab fragment of PL9-11 IgG3 was mixed with ALW (molar ratio=1:2) at 37°C for 4 hours before MALDI-TOF mass spectrometry, showing cleavage peaks (including the 947 peak) similar to those induced by the parent mAb. Data were from three to five independent experiments. Representative images were shown.
4. Discussion
We demonstrated in this study that anti-DNA antibodies can cleave both dsDNA and peptide antigens, and that this effect is isotype dependent. The efficiency of catalysis depends on the incubation time and temperature, antibody to antigen ratios, and nature of the antigen. Furthermore, the primary cleavage sites in the ALW sequence were similar between the various PL9-11 isotypes, although their constant regions affected catalytic efficiency. Our novel results reveal here the basic features of antigenic catalysis by anti-DNA isotypes that share identical variable regions.
Researchers have previously studied the catalytic properties of polyclonal anti-DNA antibodies obtained from lupus patients and murine lupus models (Shuster et al., 1992; Nevinsky et al., 2002; Kostrikina et al., 2011; Kozyr et al., 2002; Suchkov et al., 2001). These studies indicated an intrinsic relationship between the DNase activity of anti-DNA antibodies and bound metal ions, and suggested that the cytotoxicity of anti-DNA antibodies may be due to their hydrolysis of nuclear matrix proteins (Nevinsky et al., 2002; Kostrikina et al., 2011; Kozyr et al., 2002; Mouratou et al., 2002; Cavallo et al., 2012; Gololobov et al., 1997). In contrast, we used monoclonal anti-DNA antibodies containing both heavy and light chains that have more stable and definable affinity for antigens than polyclonal antibodies isolated from sera. Moreover, we quantitatively compared the catalytic properties of different anti-DNA isotypes. Earlier studies also showed that the DNase activity of antibodies differs from serum DNases by temperature resistance, optimum pH, and hydrolysis rate (Nevzorova et al., 2003). Similarly, we found here that increasing incubation temperature accelerates nuclease activity. Our results in this study therefore encourage further consideration of isotype dependent structural alterations on target binding and potential side effects, as well as provide molecular specificity to the previous observations.
It is of interest to consider the relative catalytic strength of PL9-11 as compared to that of anti-DNA antibodies previously described in the literature. However, previous studies mostly used polyclonal IgG from serum samples (Shuster et al., 1992), or single chain Fv constructs (Kozyr et al., 2012) rather than native, intact antibodies as was the focus in this study, precluding a direct quantitative comparison. Furthermore, our emphasis here was in comparison of the catalytic effects of antibodies with a shared variable region, but different Fc receptors.
Binding affinities to DNA (current paper) and peptide (Xia et al., 2015) of the PL9-11 antibodies were relatively low, as was the in vitro catalytic rate. Nevertheless, these autoantibodies can still exert profound effects, considering in combination the pathogenic potential of antibodies with catalytic activity, potentially amplified effects via a contribution of antibody avidity in vivo, and the typical serum half-life of IgGs (2–3 weeks). Indeed, PL9-11 antibodies demonstrated in vivo pathogenicity (nephrotoxicity) when administrated as hybridomas intraperitoneally (Xia et al., 2012).
One theoretical concern that might arise in the interpretation of our results is a possibility of DNase contamination in the PL9-11 antibody samples. However, Shuster et al found that acetic acid (dissociating noncovalent complexes) does not substantially effect the DNA hydrolyzing activity of anti-DNA antibodies purified from lupus serum (Shuster et al., 1992). Furthermore, in the present study the PL9-11 antibodies were purified from culture supernatants of hybridoma cells rather than from serum, and showed high purity by both Coomassie blue staining and their ability (in the form of Fab) to form crystals (data not shown). Moreover, the antibodies used as isotype controls were purified in a similar way, yet showed no DNA cleaving activity. Therefore, we believe that potential contamination with DNAse is not a significant concern; rather, the most reasonable conclusion is that DNA catalysis is an intrinsic property of the PL9-11 derived antibodies.
Although classically the variable regions of antibodies were believed to be the sole determinant of antigenic specificity, more recent studies have shown that the constant regions are vital contributors to the binding interactions between antibody and antigen (Janda et al., 2012; Casadevall et al., 2012; Tudor et al., 2012; Torres et al., 2008). Indeed, we had found that the heavy chain constant regions affect the interaction of anti-DNA antibodies with self-antigens, possibly through altering the secondary structure of antibody-antigen complexes (Xia et al., 2012; Xia et al., 2013). Since hydrolysis is one of the chemical reactions possible with antibody-antigen interactions, it was interesting to explore whether constant regions can modulate this enzymatic activity. The present study showed that the influence of constant regions is dependent on the properties of the substrate. These findings not only support our previous observation that the differential binding affinity of PL9-11 derived isotypes is also dependent on intrinsic attributes of the antigens bound by these autoantibodies (Xia et al., 2012), but also are consistent with a previous report that V-domain identical IgG isotypes (directed against the polysaccharide capsule of Cryptococcus neoformans capsule) show different rates of catalysis (Janda et al., 2012). Finally, although high level of catalysis were seen with IgM but not IgG anti-viral antibodies containing the same V-domains (Sapparapu et al., 2012), we chose not to include the PL9-11 IgM anti-DNA in our present study since antibodies of this isotype are much less pathogenic than IgG anti-DNA antibodies in SLE, and may even exert a protective effect (Xia et al., 2012; Witte, 2008).
Another intriguing finding in this study was that the catalytic strength of PL9-11 mAbs does not parallel their apparent binding affinity to antigens. The specific affinity of anti-DNA antibodies to antigens presumably determines the possibility of catalysis, but not the catalytic efficiency of the reaction. Affinity between antibody and antigen results from the interaction of the two molecules through electrostatic attraction, van der Waals forces, hydrogen bonding or hydrophobic interactions (Mian et al., 1991). However, catalysis can occur if the antibody lowers the activation energy of the transition pathways, thereby favoring the conversion of reactants to products (Rao et al., 2007). Therefore, the affinity of anti-DNA antibodies is a prerequisite for catalysis, but does not necessarily affect the acceleration of the reaction. It is also important to note that Kozyr et al previously demonstrated that antibodies closely related by variable chain sequence (but from different sources) can have disparate catalytic activity; moreover, in the specific autoantibody system they used in their study, a specific engineered mutation leading to decreased DNA binding actually did also significantly attenuate the catalytic activity (Kozyr et al., 2012).
While the abzymes described here display both nuclease and peptide activity, we do not yet have conclusive evidence that DNA hydrolysis and proteolysis are mediated by the same active site. Nevertheless, the PL9-11 antibodies studied were all isotype switch variants of the same parent IgG3 antibody. Thus they share the same variable region and likely the same paratope in their interactions with antigen, indicating the same or very similar active site(s). However, the constant regions do influence the active site, as evident by differential catalysis rates. We hope to be able to address this interesting question more directly in the future with structure-function analysis of antibodies crystallized in complex with DNA and peptide.
Studying the sites in amino acid sequences where antibody mediated cleavage occurs may assist in planning the structural modifications of peptides that can resist hydrolysis. Our results show that isotype switching in anti-DNA antibodies influences the efficacy but not the primary site of ALW peptide cleavage, likely through influencing the dynamics and/or leading to subtle conformational differences within the antibody paratope. Furthermore, the fact that the Fab fragment possesses antibody catalytic properties (Gololobov et al., 1995), is also consistent with variable region dependent determination of the primary sites. The constancy of primary cleavage sites in ALW is helpful in the design of new, catalysis-resistant peptides intended for non-isotype restricted inhibition of the deposition of pathogenic anti-DNA antibodies in target organs. Furthermore, the identified attack sites in peptide sequences may assist in future elucidation of the tissue-selective pathogenicity of anti-DNA antibodies.
5. Conclusions
Anti-DNA antibodies cleave both dsDNA and peptide antigens in an isotype dependent manner. Incubation conditions (time, temperature, and the molar ratio of antibody to antigen) as well as antigenic properties, affect the catalytic pattern. The binding affinity of anti-DNA antibodies to antigens determines whether catalysis does occur, but not the efficiency of the process. Decreasing the deposition of pathogenic IgG antibodies in lupus target organs is a potential therapeutic approach in this antibody-mediated disease. The ALW peptide had the same first-attack sites for all the tested anti-DNA isotypes, and therefore would potentially be a good candidate to be chemically modified as an inhibitor of the in vivo interactions between autoantibodies and self-antigen.
Highlights.
Catalytic activities of anti-DNA antibodies are not only a function of the variable region, but are also isotype dependent;
DNA cleavage and proteolysis by anti-DNA antibodies increase with incubation time and temperature;
The design of antibodies for therapeutic purposes should include consideration of the catalytic effects contributed by the antibody constant region.
Acknowledgments
This work was supported by grants from the NIH (AR048692 and DK090319 to C. Putterman and OD016305 to D. Cowburn). We thank Dr. Huiyong Cheng (Dept. of Biochemistry, Albert Einstein College of Medicine) for help with the Biacore analysis, and Dr. Jennifer Aguilan (Dept. of Biochemistry, Albert Einstein College of Medicine) for help with MALDI-TOF mass spectrometry.
Abbreviations
- AHS
AHSANNFNVKGI
- ALW
ALWPPNLHAWVP
- ds
double stranded
- ELISA
enzyme-linked immunosorbent assay
- mAb
monoclonal antibody
- NMR
nuclear magnetic resonance
- SLE
systemic lupus erythematosus
- SPR
surface plasmon resonance
Footnotes
Authors’ contributions
CP conceived and supervised this project. YX performed the experiments and statistical analysis. The manuscript was written by YX and CP. EE performed the NMR experiments. QZ and YX carried out the MALDI-TOF mass spectrometry studies. DC directed the NMR experiments and critically revised the manuscript. All authors have read and approved the final manuscript.
Competing interests
The authors declare no financial or commercial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yumin Xia, Email: ymxia@whu.edu.cn.
Ertan Eryilmaz, Email: ertan.eryilmaz@boehringer-ingelheim.com.
Qiuting Zhang, Email: qiuting_zhang@zju.edu.cn.
David Cowburn, Email: david.cowburn@einstein.yu.edu.
Chaim Putterman, Email: chaim.putterman@einstein.yu.edu.
References
- Barrera GJ, Portillo R, Mijares A, Rocafull MA, del Castillo JR, Thomas LE. Immunoglobulin A with protease activity secreted in human milk activates PAR-2 receptors, of intestinal epithelial cells HT-29, and promotes beta-defensin-2 expression. Immunol Lett. 2009;123:52–59. doi: 10.1016/j.imlet.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Baudino L, Azeredo da Silveira S, Nakata M, Izui S. Molecular and cellular basis for pathogenicity of autoantibodies: lessons from murine monoclonal autoantibodies. Sem Immunopathol. 2006;28:175–184. doi: 10.1007/s00281-006-0037-0. [DOI] [PubMed] [Google Scholar]
- Belogurov A, Jr, Kozyr A, Ponomarenko N, Gabibov A. Catalytic antibodies: balancing between Dr. Jekyll and Mr Hyde Bioessays. 2009;31:1161–1171. doi: 10.1002/bies.200900020. [DOI] [PubMed] [Google Scholar]
- Bijl M, Dijstelbloem HM, Oost WW, Bootsma H, Derksen RH, Aten J, Limburg PC, Kallenberg CG. IgG subclass distribution of autoantibodies differs between renal and extra-renal relapses in patients with systemic lupus erythematosus. Rheumatology. 2002;41:62–67. doi: 10.1093/rheumatology/41.1.62. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Janda A. Immunoglobulin isotype influences affinity and specificity. Proc Natl Acad Sci. 2012;109:12272–12273. doi: 10.1073/pnas.1209750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo MF, Kats AM, Chen R, Hartmann JX, Pavlovic M. A novel method for real-Time, continuous, fluorescence-based analysis of anti-DNA abzyme activity in systemic lupus. Autoimmune Dis. 2012;2012:814048. doi: 10.1155/2012/814048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HX, Campbell SR, Cui MH, Zong P, Hwang JH, Gulinello M, Putterman C. Depression is an early disease manifestation in lupus-prone MRL/lpr mice. J Neuroimmunolgy. 2009;207:45–56. doi: 10.1016/j.jneuroim.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gololobov GV, Chernova EA, Schourov DV, Smirnov IV, Kudelina IA, Gabibov AG. Cleavage of supercoiled plasmid DNA by autoantibody Fab fragment: application of the flow linear dichroism technique. Proc Natl Acad Sci. 1995;92:254–257. doi: 10.1073/pnas.92.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gololobov GV, Rumbley CA, Rumbley JN, Schourov DV, Makarevich OI, Gabibov AG, Voss EW, Jr, Rodkey LS. Induction of strain and metal coordination could be constituents of a mechanism by which this antibody catalyzed DNA hydrolysis. Mol Immunology. 1997;34:1083–1093. doi: 10.1016/s0161-5890(97)00129-6. [DOI] [PubMed] [Google Scholar]
- Hanrotel-Saliou C, Segalen I, Le Meur Y, Youinou P, Renaudineau Y. Glomerular antibodies in lupus nephritis. Clin Rev All Immunol. 2011;40:151–158. doi: 10.1007/s12016-010-8204-4. [DOI] [PubMed] [Google Scholar]
- Janda A, Eryilmaz E, Nakouzi A, Cowburn D, Casadevall A. Variable region identical immunoglobulins differing in isotype express different paratopes. J Biol Chem. 2012;287:35409–35417. doi: 10.1074/jbc.M112.404483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang EJ, Nahm DH, Jang YJ. Mouse monoclonal autoantibodies penetrate mouse macrophage cells and stimulate NF-kappaB activation and TNF-alpha release. Immunol Lett. 2009;124:70–76. doi: 10.1016/j.imlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Kostrikina IA, Kolesova ME, Orlovskaya IA, Buneva VN, Nevinsky GA. Diversity of DNA-hydrolyzing antibodies from the sera of autoimmune-prone MRL/MpJ-lpr mice. J Mol Recog. 2011;24:557–569. doi: 10.1002/jmr.1067. [DOI] [PubMed] [Google Scholar]
- Kostrikina IA, Odintsova ES, Buneva VN, Nevinsky GA. Systemic lupus erythematosus: molecular cloning and analysis of recombinant DNase monoclonal κ light chain NGK-1. Int Immunol. 2014;26:439–450. doi: 10.1093/intimm/dxu047. [DOI] [PubMed] [Google Scholar]
- Kozyr AV, Kolesnikov AV, Khlyntseva AE, Bogun AG, Savchenko GA, Shemyakin IG, Gabibov AG. Role of structure-based changes due to somatic mutation in highly homologous DNA-binding and DNA-hydrolyzing autoantibodies exemplified by A23P substitution in the VH domain. Autoimmune Dis. 2012;2012:683829. doi: 10.1155/2012/683829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozyr AV, Kolesnikov AV, Zelenova NA, Sashchenko LP, Mikhalap SV, Bulina ME, Ignatova AN, Favorov PV, Gabibov AG. Autoantibodies to nuclear antigens: correlation between cytotoxicity and DNA-hydrolyzing activity. App Biochem Biotechnol. 2000;83:255–268. doi: 10.1385/abab:83:1-3:255. [DOI] [PubMed] [Google Scholar]
- Kozyr AV, Sashchenko LP, Kolesnikov AV, Zelenova NA, Khaidukov SV, Ignatova AN, Bobik TV, Gabibov AG, Alekberova ZS, Suchkov SV, Gnuchev NV. Anti-DNA autoantibodies reveal toxicity to tumor cell lines. Immunol Lett. 2002;80:41–47. doi: 10.1016/s0165-2478(01)00308-x. [DOI] [PubMed] [Google Scholar]
- Krishnan MR, Wang C, Marion TN. Anti-DNA autoantibodies initiate experimental lupus nephritis by binding directly to the glomerular basement membrane in mice. Kid Int. 2012;82:184–192. doi: 10.1038/ki.2011.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian IS, Bradwell AR, Olson AJ. Structure, function and properties of antibody binding sites. J Mol Biol. 1991;217:133–151. doi: 10.1016/0022-2836(91)90617-f. [DOI] [PubMed] [Google Scholar]
- Mouratou B, Rouyre S, Guesdon JL. A method for the detection and screening of catalytic anti-DNA antibodies. J Immunol Methods. 2002;269:147–155. doi: 10.1016/s0022-1759(02)00231-4. [DOI] [PubMed] [Google Scholar]
- Nevinsky GA, Buneva VN. Human catalytic RNA- and DNA-hydrolyzing antibodies. J Immunol Methods. 2002;269:235–249. doi: 10.1016/s0022-1759(02)00234-x. [DOI] [PubMed] [Google Scholar]
- Nevinsky GA, Buneva VN. Natural catalytic antibodies in norm, autoimmune, viral, and bacterial diseases. Sci World J. 2010;10:1203–1233. doi: 10.1100/tsw.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevzorova TA, Temnikov DA, Vinter VG. Special features of the DNA-hydrolyzing activity of the antibodies in systemic lupus erythematosus. Biochem. 2003;68:1300–1306. doi: 10.1023/b:biry.0000011650.48894.7e. [DOI] [PubMed] [Google Scholar]
- Paul S, Planque SA, Nishiyama Y, Hanson CV, Massey RJ. Nature and nurture of catalytic antibodies. Adv Exp Med Biol. 2012;50:56–75. doi: 10.1007/978-1-4614-3461-0_5. [DOI] [PubMed] [Google Scholar]
- Ponomarenko NA, Durova OM, Vorobiev II, Aleksandrova ES, Telegin GB, Chamborant OG, Sidorik LL, Suchkov SV, Alekberova ZS, Gnuchev NV, Gabibov AG. Catalytic antibodies in clinical and experimental pathology: human and mouse models. J Immunol Methods. 2002;269:197–211. doi: 10.1016/s0022-1759(02)00324-1. [DOI] [PubMed] [Google Scholar]
- Qing X, Pitashny M, Thomas DB, Barrat FJ, Hogarth MP, Putterman C. Pathogenic anti-DNA antibodies modulate gene expression in mesangial cells: involvement of HMGB1 in anti-DNA antibody-induced renal injury. Immunol Letters. 2008;121:61–73. doi: 10.1016/j.imlet.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao DN, Wootla B. Catalytic antibodies: concept and promise. Resonance. 2007;12:6–21. [Google Scholar]
- Shuster AM, Gololobov GV, Kvashuk OA, Bogomolova AE, Smirnov IV, Gabibov AG. DNA hydrolyzing autoantibodies. Science. 1992;256:665–667. doi: 10.1126/science.1585181. [DOI] [PubMed] [Google Scholar]
- Sapparapu G, Planque S, Mitsuda Y, McLean G, Nishiyama Y, Paul S. Constant domain-regulated antibody catalysis. J Biol Chem. 2012;287:36096–36104. doi: 10.1074/jbc.M112.401075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar BD, Zon G, Pastor RW. A recognition site on synthetic helical oligonucleotides for monoclonal anti-native DNA autoantibody. Proc Natl Acad Sci. 1986;83:4469–4473. doi: 10.1073/pnas.83.12.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchkov SV. Comparative study of catalytic (DNA-hydrolyzing) and cytotoxic properties of anti-dna autoantibodies. Bull Exp Biol Med. 2001;131:353–355. doi: 10.1023/a:1017904219726. [DOI] [PubMed] [Google Scholar]
- Tomin A, Dumych T, Tolstyak Y, Kril I, Mahorivska I, Bila E, Stoika R, Herrmann M, Kit Y, Bilyy R. Desialylation of dying cells with catalytically active antibodies possessing sialidase activity facilitate their clearance by human macrophages. Clin Exp Immunol. 2015;79:17–23. doi: 10.1111/cei.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Casadevall A. The immunoglobulin constant region contributes to affinity and specificity. Trends Immunol. 2008;29:91–97. doi: 10.1016/j.it.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Tudor D, Yu H, Maupetit J, Drillet AS, Bouceba T, Schwartz-Cornil I, Lopalco L, Tuffery P, Bomsel M. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc Natl Acad Sci. 2012;109:12680–12685. doi: 10.1073/pnas.1200024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte T. IgM antibodies against dsDNA in SLE. Clin Rev Allergy Immunol. 2008;34:345–7. doi: 10.1007/s12016-007-8046-x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Eryilmaz E, Pawar RD, Guo X, Cowburn D, Putterman C. A peptide mimic blocks the cross reaction of anti-DNA antibodies with glomerular antigens. Clin Exp Immunol. 2015 doi: 10.1111/cei.12734. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Janda A, Eryilmaz E, Casadevall A, Putterman C. The constant region affects antigen binding of antibodies to DNA by altering secondary structure. Mol Immunol. 2013;56:28–37. doi: 10.1016/j.molimm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Pawar RD, Nakouzi AS, Herlitz L, Broder A, Liu K, Goilav B, Fan M, Wang L, Li QZ, Casadevall A, Putterman C. The constant region contributes to the antigenic specificity and renal pathogenicity of murine anti-DNA antibodies. J Autoimmunity. 2012;39:398–411. doi: 10.1016/j.jaut.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung S, Chan TM. Anti-DNA antibodies in the pathogenesis of lupus nephritis--the emerging mechanisms. Autoimmunity Rev. 2008;7:317–321. doi: 10.1016/j.autrev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Yung S, Tsang RC, Sun Y, Leung JK, Chan TM. Effect of human anti-DNA antibodies on proximal renal tubular epithelial cell cytokine expression: implications on tubulointerstitial inflammation in lupus nephritis. J Amer Soc Nephrol. 2005;16:3281–3294. doi: 10.1681/ASN.2004110917. [DOI] [PubMed] [Google Scholar]
- Yung S, Zhang Q, Zhang CZ, Chan KW, Lui SL, Chan TM. Anti-DNA antibody induction of protein kinase C phosphorylation and fibronectin synthesis in human and murine lupus and the effect of mycophenolic acid. Arthritis Rheum. 2009;60:2071–2082. doi: 10.1002/art.24573. [DOI] [PubMed] [Google Scholar]
- Zerfaß C, Braukmann S, Nietzsche S, Hobe S, Paulsen H. High yield recombinant production of a self-assembling polycationic peptide for silica biomineralization. Prot Exp Purif. 2014;108C:1–8. doi: 10.1016/j.pep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang J, Jiang S, Fang C, Xiong L, Cheng H, Xia Y. The lupus-derived anti-double-stranded DNA IgG contributes to myofibroblast-like phenotype in mesangial cells. J Clin Immunol. 2012;32:1270–1278. doi: 10.1007/s10875-012-9724-x. [DOI] [PubMed] [Google Scholar]