Abstract
Dermal white adipose tissue (dWAT) has found little appreciation in the past as a distinct entity from the better recognized subcutaneous white adipose tissue (sWAT). However, recent work has established dWAT as an important contributor to a multitude of processes, including immune response, wound healing and scarring, hair follicle growth and thermoregulation. Unique metabolic contributions are attributed to dWAT as well, at least in part due to thermic insulation properties and its response to cold exposure. Dermal adipocytes can also undergo adipocyte-myofibroblast transition (AMT), a process that is suspected to play an important role in a number of pathophysiological processes within the skin. Here, we discuss emerging concepts regarding dWAT physiology and its significance to a variety of cellular processes.
Keywords: dermal insulation, adipocyte-myofibroblast transition, adipose derived stem cells, scarring, pigmentation
Dermal adipose tissue – a new depot with new properties
During the last few years, adipose tissue morphed from a passive tissue with such well-known functions as energy storage, mechanical protection and heat insulation to a systemically relevant, physiological player with many different features. It transformed from an initial quasi-static structure to a slowly renewing tissue with characteristic half-lives of the embedded adipocytes of about 10 years [1]. Subsequently, adipocytes were discovered to be part of highly dynamic events, such as hair follicle cycling [2], or even more rapidly acting processes, such as wound healing [3]. Over the course of that time, the concept of the common white adipocyte that stands in contrast to the classical brown adipocyte was expanded to the “beige” adipocyte, blurring the lines between the two classical extremes, which is emerging as a new class of “chimeric” adipocytes that can simultaneously display properties of both adipocyte subtypes. Even more complex are the events leading to the differentiation of these types of adipocytes in vivo, with respect to differences for specific fat pads and developmental stages and the precursor cells that are being recruited and activated [4]. At the same time, the description of subcutaneous white adipose tissue (sWAT) was changed from homogeneous to a highly heterogeneous structure with a broad, body area-dependent distribution of adipocyte sizes [5] and with very variable mechanical and electrical properties primarily determined by its peri- and intercellular fibrotic structures [6–10]. There is widespread belief that we must abandon the simple concept of the adipocyte as a uniform cell type, exerting comparable functions independent of its location.
The introduction of a new adipose tissue depot that we refer to as dermal white adipose tissue (dWAT) [11, 12] is a logical consequence of this development. This depot in humans has a very special geometry uniquely distinct from all other known fat depots and demonstrates intriguing spatial correlation with hypertrophic scarring (see Box 1 and Figure I, panel C). Adipocytes from this unique depot within the dermis are involved in various physiological and pathological processes, among them hair follicle (HF) cycling [2], wound healing [3], cutaneous fibrosis [13], skin aging [14], homeostatic temperature regulation [15], protection against skin infection [16], etc.
Box 1. Special geometry of dWAT in humans.
Whereas anatomically dWAT can be classified as a separate adipose tissue depot, in humans it has a very special geometry uniquely distinct from all other known fat depots. It has long been appreciated that two histologically and anatomically distinct layers of adipose tissue exist under the reticular dermis. This anatomical difference is evident in rodents where these layers are separated by the panniculus carnosus, a layer of striated muscle cells (Figure 1 panel A, panel B).
In human skin, where all layers in the dermis have interfaces which run more or less parallel to the skin surface, dWAT is mainly concentrated around the pilosebaceous units that contain hair shafts, hair follicles, the sebaceous glands and the erector pili muscles, and the dWAT has a very special cone geometry [17] (Figure I, panel C). Each dermal cone consists of two parts, whereas its upper part is placed in the dermis and the lower part (referred to as the “fat dome”) transverses the dermis and penetrates into sWAT [18]. From this point of view, single dWAT units build the vertical fractional structures which are connected with each other through the inter-follicular dermis and which have a common reservoir of adipocytes in sWAT. Such geometry can sufficiently influence the metabolic properties and functions of dWAT in humans, especially since the individual units can interact with each other through paracrine signaling producing the clusters of characteristic size.
Analysis of the skin histology in humans reveals that the cone structures in the dermis are present only in those body areas where hypertrophic scars can be produced (e.g. cheek, neck, chest, abdomen, back, buttock, arm, forearm, dorsal hand, thigh, leg, etc.) and is not apparent in body areas which are less prone to scarring (e.g., in early fetus, palm, scalp, forehead, etc.). Also, animals with a more limited propensity for scarring after wounding (such as rats and rabbits) have a reduced number of these structures. Whereas morphological characteristics of dWAT (e.g. cell size distribution) have not been investigated in depth, the very existence of these regional correlations between scarring and dWAT means that dWAT structures and content are different in distinct body areas, which in turn can be reflected in the spatially variable pathways involved in wound healing, hair growth and cutaneous fibrosis in these areas.
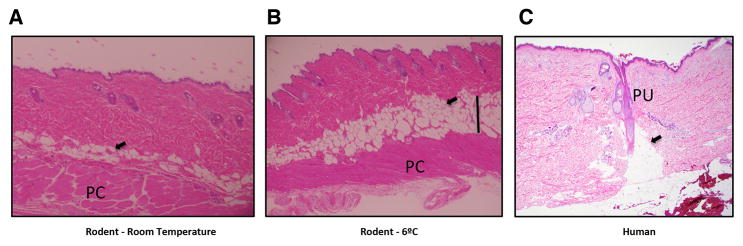
Figure I. Typical layered dWAT structures in rodents and humans.
A) Section of dWAT from a C57/Bl6 mouse maintained at room temperature. The dWAT contains several layers of adipocytes (arrowheads) placed parallel to the panniculus carnosus (PC).
B) Section of dWAT from a C57/Bl6 mouse after cold exposure of 6°C for 4 days; both hypertrophy and hyperplasia can be seen (area indicated with a black line). dWAT can quickly react to different types of physical and pharmacological stimuli with significant modulation of its thickness. Slides A and B courtesy Dr. Min Kim, Touchstone Diabetes Center, UT Southwestern Medical Center, Dallas.
C) Human adipocytes are congregated around the single pilosebaceous units (PU) producing the “dermal cones” (arrowhead). Morphological characteristics of these cones are dependent on the body area and the phase of HF cycle. Slide courtesy Dr. Travis Vandergriff, Department of Dermatology, UT Southwestern Medical Center, Dallas.
Whereas dWAT has been talked about in various settings in the literature during the last years, many important questions are still open. Among them are the possible phenotypic differences between dermal adipocytes and the adipocytes from the underlying sWAT. This is important in the context of the unique extra-cellular matrix (ECM) microenvironment that these cells are embedded in. This also raises the question how this ECM drives the expression of unique dWAT phenotypes in their local spatial and global metabolic phenotypes as well as the role of these cells in inflammation and scarring.
dWAT and myofibroblasts – Are they independent global contributors to wound healing and scarring?
If dWAT is involved in wound healing process [3], one can suppose that dermal adipocytes should be somehow connected with scarring. Appearance of myofibroblasts in an injured tissue is connected with their differentiation from the existing pool of fibroblasts or with epithelial to mesenchymal transdifferentiation of epithelial cells. This differentiation was long believed to be an irreversible process. According to this model, the only possibility to remove the low-motility myofibroblasts from the tissue to avoid scarring seemed to be their death through apoptosis. Defective apoptosis of myofibroblasts was therefore thought to be the main reason for fibrosis and scarring [19], and major efforts were undertaken to understand the nature and possible pathways for the regulation of this process. More recent data argue that this model needs to be revised. First, myofibroblasts are not terminally differentiated cells, and they have a phenotype with a high degree of plasticity, i.e. they can either re- or de-differentiate [20, 21] into other cell types. Second, while the dysfunctional regulation of apoptosis of the myofibroblasts may still play an important role, it is far from being the only reason for scarring upon skin wounding. For example, under excessive scarring conditions, such as the development of keloids, the myofibroblasts completely disappear after the granulation phase and are not present in mature scars. Third, adipose tissue is directly involved in the activation of fibroblasts in the wound [3]. Fourth, the myofibroblasts can emerge from adipose-derived intradermal progenitors; moreover, we appreciate that an entirely new pathway – the “adipocyte-myofibroblast transition” (AMT) - can significantly contribute to fibrosis [13]. This puts the spotlight in the context of wound healing and scarring on adipogenic progenitors and mature adipocytes, an important novel role beyond the direct metabolic role for these cells.
The versatile dermal adipocyte: Immature and mature adipocytes in wound healing and scarring
As every adipose depot, dWAT contains adipose derived stem cells (ADSCs), preadipocytes and mature adipocytes, which can be differentially involved in the above-mentioned processes. Mature dermal adipocytes repopulate the skin wound, and this process is realized via activation of adipogenic precursors and adipogenesis [3]. These cells appear in the wound concurrently with fibroblasts. This suggests a possible crosstalk between these two cell types. At the same time, wounds in lipoatrophic mouse models, such as the A-ZIP/F1 mice that lack mature white adipocytes system-wide and only carry immature adipocyte precursor cells, demonstrate aberrant recruitment of fibroblasts and wound instability [3]. The same is true for mice treated with inhibitors of the master adipogenic factor peroxisome proliferator-activated receptor gamma (PPARγ. Blocking PPARγ produces defective, immature adipocytes, and the absence of functional adipocytes has again a negative impact on wound healing. In contrast, the immature adipocytes are not only necessary, but also sufficient for the proper hair follicle cycling [2], as judged by the fact that an almost normal hair follicle cycling is observed in the A-ZIP/F1 mice.
Whereas the role of ADSCs in tissue regeneration was intensively investigated during the recent past [22], we are far from having a clear picture of their involvement in this process. A recent report indicated that ADSCs can differentiate into myofibroblasts or fibroblast-like cells using growth factors present in the wound bed. In this context, transforming growth factor beta (TGF-β) can stimulate the myofibroblastic phenotype, whereas basic fibroblast growth factor (bFGF) reduces this conversion [20]. Remarkably, these authors also demonstrated that myofibroblasts can re-differentiate into fibroblast-like cells, which could be an important mechanism ensuring for these cells disappear without the need to undergo apoptosis from a wound whose healing process has fully resolved. Application of ADSCs can indeed modulate the processes of early scar formation and remodelling, and improvements in scar formation were shown to correlate with suppression of TGF-β and enhanced expression of matrix metalloproteinases [23]. Altogether, the role of myofibroblasts in hypertrophic scarring might be overestimated in the current models, whereas the role of immature dermal adipocytes is in contrast underestimated (see Box 2).
Box 2. The pathophysiology of hypertrophic scars – mesenchymal stem cells or myofibroblasts?
One important marker for myofibroblasts is α smooth muscle actin (α-SMA). High expression of α-SMA in correlation with wound contraction was assumed to be a sign for the presence of fat-derived (myo)fibroblastic cells in the healing wound, since fibroblast-like cells isolated from subcutaneous fat contain a high percentage of (α-SMA)-positive cells [24]. However, α-SMA is also a well-known mesenchymal stem cell marker. It is therefore possible that the adipose-derived cells previously described as myofibroblasts are in fact the adipose-derived stem cells (ADSCs). Indeed, cultured mesenchymal stem cells from sWAT and from hypertrophic scars demonstrate significantly higher expression of α-SMA than the corresponding stem cells taken from dermis [24]. Such expression correlates with higher production of collagen I and collagen cross-linking, in these cultures. This leads to a model in which ADSCs are responsible for hypertrophic scar formation. In a tissue-engineered human scar model, ADSCs isolated from healthy human skin (in contrast to dermal mesenchymal cells) indeed facilitate the hypertrophic scar formation [25].
The transition from the adipocyte to a mesenchymal cell (AMT)
In a very recent paper, Varga and colleagues demonstrated that myofibroblasts can be produced from the adiponectin-positive intradermal progenitors, i.e. these cells are derived from dermal adipocytes [13]. A well-characterized model of dermal fibrosis involves the administration of daily subcutaneous bleomycin injections for 2 weeks. This leads to a progressive increase in dermal thickness and collagen deposition, ultimately leading to dermal fibrosis. This is associated with marked attenuation of the intradermal adipose layer [26]. Remarkably, the loss of intradermal adipocytes after bleomycin treatment could not be explained solely by intradermal apoptosis. The authors proposed that dermal fibrosis might appear through transdifferentiation of dermal adipocytes. Recently, Martins and colleagues [27] reported that resistin-like molecule α(RELMα /FIZZ1) can cause the suppression of adipocyte-specific genes, leading to a de-differentiation, while in parallel inducing α-smooth muscle actin and type I collagen expression, reflecting a transition to myofibroblasts. RELMα is induced in the bleomycin-induced dermal fibrosis model also employed by Varga and colleagues. These observations were further supported by additional ex vivo studies on adipocytes. Whereas ADSCs cultivated for 10 days differentiated in typical adipocytes, application of TGF-β produced an unusually rapid modulation of cell morphology. After 24 hours these cells were in a transition state and expressed both perilipin and α-SMA. Later they lost the adipogenic markers altogether and further increased α-SMA, producing the typical features of myofibroblasts [13]. AMT could be of primary importance for cutaneous fibrogenesis. This has additional implications in the context of adipose tissue loss that frequently correlates with fibrosis in lipodystrophy secondary to panniculitis, in different autoimmune diseases, in cancer cachexia, as well as under some other conditions (see Box 3), and suggests that this phenomenon is of general physiological importance. There are a host of additional factors that may be critical for the process of fibrosis locally. Connective tissue growth factor (CTGF; also known as CCN2) can induce human bone marrow mesenchymal stem/stromal cells (MSCs) to differentiate into fibroblasts. More importantly, CTGF expression levels are correlated with the amount of peri-adipocyte fibrosis in WAT from obese individuals [33], but it is not known whether CTGF plays a role locally in the dWAT.
Box 3. Adipocytes in the involution processes.
Another process that is physiologically important in this context is the restructuring of the mammary fat pad during lactation. During late pregnancy and post-partum, many adipocytes in the mammary gland disappear as the milk producing lobules appear and then reappear during involution of the milk producing structures. The mammary fat pad is considered an subcutaneous fat pas (sWAT). The involution process remains an incompletely understood process. Cinti and colleagues suggested a transition from adipocytes to epithelial cells as a possible mechanism [28]; recently the same was assumed to be important also in fat grafting [29]. Another possibility is that adipocytes simply undergo apoptosis. A third possibility is that they undergo AMT and turn into fibroblasts. Clearly, we have only a limited understanding of this process, and much work still needs to be done to determine the fate of these mammary adipocytes.
Similarly during breast cancer progression, there is a significant reduction in the number of adipocytes in the mammary gland as the transformed ductal epithelial cells break through the basal lamina and invade the stromal compartment. In that situation, it is instrumental as well to determine whether the adipocytes simply apoptose, or whether there is a conversion going on resembling AMT, with the resulting fibroblasts potentially playing a direct role in tumor progression. A very recent paper by Seo and colleagues [30] highlighted the role of these myofibroblasts that are more prominent in obese adipose tissue for the tumor growth-promoting enhanced ECM in this context. With the availability of newer models, such as the “adipochaser” mouse [31, 32], it will be fairly straightforward to determine the fate of the adipocytes in these settings.
Both adipo-epithelial [28] and adipocyte-myofibroblast transitions [13] must play important role in physiological and pathophysiological processes in the skin involving dWAT. For example, processes of epithelial-mesenchymal remodeling are strongly involved in the cycling of hair follicles (HFs), being especially pronounces during mid-anagen (the active growth phase of HFs) at the distal end of growing follicles [34]. This correlates with disappearance of dermal adipocytes during mid-anagen and massive production of these cells in the late anagen sub-phase of the hair follicle cycle [2], during which the epithelial-mesenchymal remodeling processes are strongly suppressed. On the other hand, AMT can be involved in formation of so called “connective tissue streamers” which are the residual fibrovascular tracks representing the transient, lower part of the hair follicle [35]. These tracks are normally developed during the involution phase of the hair follicle cycle and can be considered as a local micro-scarring in the site of hair follicle involution.
Are dermal adipocytes “chimeras”?
Recently, Cinti and colleagues suggested the existence of cells having an intermediate adipo-epithelial phenotype in the mammary gland during and after pregnancy [36]. This suggested process does need to be independently validated though. The same must be the case for the adipocytes from dWAT, which have much higher turnover-rates than typical for sWAT. These transformations in dWAT occur in a short time frame, compared to the hair follicle cycle or even compared to the characteristic times of wound healing. This reflects a much higher propensity of dermal adipocytes to undergo these transitions, likely reflecting a transcriptional machinery that is highly responsive to environmental changes. Whereas from these data we cannot conclude unambiguously that dermal adipocytes are more flexible in their phenotypic adaptations than adipocytes from sWAT, such enhanced flexibility is strongly suspected.
Fetal skin and oral mucosa do not form scars – a reflection of the absence of dermal WAT?
Every theory of cutaneous scarring must be able to explain the scarless wound healing in oral mucosa and in fetal skin (at least in the first trimester of its development) [37, 38]. This property has long been considered to be mainly connected to the special structure and dynamics of the extracellular matrix in these tissues which significantly deviate from those in adult skin. Introduction of adipocytes as new global players in wound healing and scarring demands however the re-analysis of these phenomena which must take into account the adipogenic features of fetal skin and oral mucosa.
It is known that the adipose tissue in the fetus appears and progressively develops from the 14th to 24th week of gestation, e.g. during the second trimester of fetal development [39], whereas the hair follicles appear around 10 weeks gestation. Interestingly, recently it was shown that development of dWAT in the mouse occurs independently of sWAT and must be primarily connected with HFs appearance [40]. If the AMT [13] is involved in dermal scarring by wound healing, this process must be completely excluded in the first trimester of gestation, thereby explaining the lack of scar formation during the healing process in early fetus. On the other hand, mucosa (other areas that do not form scars at any stage of development or in the adult), do not contain any intradermal fat tissue. Instead, mucosa has the lamina propria, a thin layer of loose connective tissue which lies beneath the epithelium and together with the epithelium constitutes the mucosa. Thus, the scarless wound healing in oral mucosa also correlates with the absence of dermal adipocytes in this tissue.
Mosaic skin structure - Is there a link to dWAT clusters that has gone unnoticed?
Different physical characteristics of the skin point to its fractional micro-structure. Spatially inhomogeneous distribution of skin properties (e.g. nicely reflected in the mosaic structure of dermal electrical conductivity) can at least partly be correlated to a fractional structure of dWAT in humans. One example is observed in the HF cycling. Whereas in humans, the phases of the hair cycle are believed to be independent of neighboring HFs, thus producing the spatially random HF structure, rodents clearly demonstrate a spatially coordinated HF growth. Taking into account the essential role of dWAT in HF cycling [2], it can be supposed that this phenomenon is connected with a fractional dWAT structure in humans vs. a continuous character of dWAT in rodents. At the same time, a characteristic correlation radius of a single dWAT unit in humans should be dependent on the inter-follicular distance and thus one can expect some spatial correlation between HFs at least for small inter-follicular intervals. Another phenomenon connected with fractional dWAT structure might be the skin hyperpigmentation (see Box 4).
Box 4. Is skin hyperpigmentation connected with dWAT?
In skin hyperpigmentation (e.g. melasma), the irregular but well demarcated hyperpigmented macules randomly appear over a big surface. Pathogenesis of melasma has been long connected with a local stimulation of melanocytes placed in epidermis, but now there is increasing evidence that other cells have a key role in this process [41]. To influence the melanocytes, these cells must be located in the vicinity of epidermis and their activity must be spatially inhomogeneous, which could mediate the fractional structure of hyperpigmentation. One possible candidate for this might be the dWAT units associated into the clusters that have a characteristic radius that mediate the effects on melanocytes through paracrine signaling. Indeed, ADSCs were shown to suppress melanin production both in vitro [42] and in vivo by intradermal injections [43]. Proposed mechanisms include the secretion of TGF-β1 by ADSCs, which provides significant inhibition of the melanin synthesis through a downregulation of tyrosinase and expression of tyrosinase-related protein 1 (TRP1). In addition, TGF-β1 is a well-known dominant paracrine mediator, which determines the hyaluronic acid and collagen expression profiles [44]. Another group recently confirmed this effect and proposed that inhibition of melanin synthesis can be realized via interleukin-6 [45]. From this point of view, the fractional structure of skin hyperpigmentation can be theoretically connected with the local absence of ADSC paracrine activity.
The idea of clustering and spatial correlation of processes in dWAT units has to our knowledge never been discussed before. Such clustering is also untypical for sWAT. If such clustering units indeed play a functional role, they will not only have an impact on physiological but also on pathological processes in the skin and maybe even impact superficial sWAT layers.
Is dWAT metabolically relevant?
dWAT depots in rodents and in humans have different structures. Our insights into the metabolic properties of dWAT stem predominantly from studies in rodents. Since we lack specific markers for dWAT, our genetic tools to selectively manipulate this depot are nonexistent. However, we have glanced some insights from a number of different knockout mouse models. One of them is the caveolin-1 knockout mouse, wherein the dWAT layer was shown to be completely absent [46]. Caveolin-1 is an important signalling mediator, highly abundant in adipocytes, and it plays a very complex metabolic role system-wide in many different tissues [47]. Deficiency of caveolin-1 leads to increased cells death and fibrosis of sWAT [48]. Another model is the syndecan-1 null mouse where dWAT layer was profoundly depleted [15]. It is also known that syndecan-1 is essential for adipocyte differentiation [15, 49]. The third model is the Collagen VI knockout mouse, which demonstrated significantly increased dWAT [6]. Collagen VI is essential for terminal differentiation of preadipocytes [50]; its absence improves the metabolic phenotype of the mice, in part at least due to the reduced fibrosis prevalent in normal adipose tissue upon exposure to high fat diets [6]. It looks like dWAT and sWAT in the Col VI knockout model demonstrate opposite responses, with the subcutaneous layer potentially being thinner in contrast to the hypertrophic nature of the dWAT. However, overexpression of endotrophin (a cleavage product of the collagen VIα3 chain) selectively in adipocytes, also leads to adipose tissue fibrosis and to a massive hyperproliferation of the dWAT layer [51], suggesting that dWAT is highly responsive to either increases or decreases in local fibrotic microenvironment.
While the main focus of these models was the characterization of conventional adipose tissue depots, the skin modifications in these knockout models were not investigated in depth. Only in the case of the syndecan-1 null mice, the authors suggested an important role of dWAT in homeostatic thermoregulation that includes involution and expansion of the dWAT layer in response to changes in ambient temperature [15]. This example demonstrates that dWAT can respond to other cues and in different ways than sWAT. Whereas sWAT normally responds to mild cold exposure with the release of free fatty acids and the induction of browning/beiging, dWAT reacts to the mild cold exposure with significant expansion of its thickness (up to 4-fold) [15]. This effect is reversible at least in the range of physiological temperatures. Furthermore, this process reaches a maximum response and “saturation”, i.e. it does not progress towards further expansion with further temperature reduction. The authors suggest that the disruption of the intradermal adipose tissue development could result in cold stress and associated complex metabolic changes. Theoretical modeling of thermal conductivity given in [15] has shown that heat loss through a 40 μm intradermal fat layer (typical for syndecan-1 null mice) must be 1.8-times higher than corresponding loss through a 200 μm fat layer typical for the wild mouse. Given the data, this is indeed an attractive hypothesis, though difficult to directly proof, since the layer overall is very thin (consisting of a thickness of only 5 to 10 adipocytes).
Another metabolic effect of dWAT can be connected with hyaluronan (HA), which is abundantly present in the dermis providing a distinct extra-cellular matrix structure, different from that in sWAT. A possible role of HA in conferring resistance of adipocytes to lipolytic stimuli was discussed in [52]. Multiple applications of a hyaluronidase leading to a reduction in local HA content in C57BL/6J mice fed a high-fat diet resulted in significant (up to 35%) reduction of fat mass with simultaneous reduction of the size of the adipocytes [53]. These results were recently confirmed in [54], who also demonstrated inhibition of adipogenesis in 3T3-L1 cells after downregulation of HA levels in vitro. Whereas neither of these efforts focused on intradermal adipocytes, we can assume similarities to the modifications of dWAT observed in Col VI knockout mouse, since purified hyaluronidase is able to disrupt Col VI fibers as well [55].
These examples suggest that dWAT and sWAT have not only different structures, but that they differentially react to extrinsic manipulations.
Dermal adipocytes and hormonal effects in the skin – correlation or causation?
Since dWAT is potently involved in wound healing and HF cycling, we infer an interplay of dermal adipocytes with androgens, on which there is a long-standing separate literature exploring their role in these processes. There is a pronounced sexual dimorphism apparent with respect dWAT: intact female mice have much thicker dWAT than male mice [56]. After gonadectomy, dWAT thickness increases both in male and female mice, whereas treatment with dihydrotestosterone causes its reduction [56], which could be related to an inhibition of adipocyte differentiation [57]. Reduction of dWAT in this study correlated with inhibition of HF growth, thereby further supporting an important role of dWAT in HF cycling [2].
Another relevant hormone in this context is the thyroid hormone (TH). TH is known as a key regulator of the basic metabolic rate [58]. This hormone can induce uncoupling protein 1 (UCP1) expression in WAT via TRβ [59], highlighting its ability to induce the “beiging” process in white adipocytes. However, TH is also a strong regulator of adipogenesis and it can be involved both in proliferation and differentiation of preadipocytes [60]. This raises the question whether some known effects of thyroid hormone (TH) on the skin (e.g. increase of dermal thickness reported both after topical and intraperitoneal TH administration [61]) can be connected with dWAT.
We assume therefore that at least some established hormonal effects on the skin might be mediated not directly through fibroblasts and keratinocytes, but indirectly through dWAT modulation.
Dermal adipocytes in skin protection – should we talk about skin efflorescences?
Recent observations suggest that dermal adipocytes are critically involved in the protection of skin against infections [16]. Infection of murine skin with S. aureus caused a significant expansion of dWAT and induced high level production of cathelicidin (an anti-microbial peptide) by dermal adipocytes. Moreover, lipodystrophic mice with impaired adipogenesis demonstrated a reduced immune reaction against this pathogen. These results suggest an unexpected link between dermal adipocytes and skin efflorescences.
Cathelicidin is known to be strongly overexpressed in rosacea [62]. Expression of cathelicidin was shown to be significantly disturbed also in atopic dermatitis (AD) and psoriasis [63]. Recently it was also proposed that anti-microbial peptides are critically involved into the pathogenesis of acne [64]. Since cathelicidin exerts pro-inflammatory effects [62], overexpression of this peptide leads a local skin inflammation. On the other side, low-level expression of cathelicidin (as a result of a weak or a complete absence of a dWAT response) can be connected to a suppressed immune response in the skin.
If dermal adipocytes in humans produce anti-microbial peptides comparable to the observations in mice, we can suspect that human dWAT is involved in the development of at least some skin efflorescences.
Concluding remarks
Up to the relatively recent past, the dWAT layer has received minimal attention. In fact, there has been significant confusion as to how we refer to this fat pad, as dermal and intradermal versus subcutaneous fat tissue were considered synonymous. The vast majority of phenotypic characterizations of rodent models does not analyse this layer of adipocytes as a distinct entity, and in fact our own efforts in the past have frequently neglected the description of dWAT. However, there is an emerging picture in the literature that suggests that changes in dWAT could have major significance for a variety of processes, and we increase our appreciation that these adipocytes are highly flexible cell types that despite their “terminal” differentiation phenotype may have the potential to undergo de-lipidation and transitions to fibroblast and myofibroblast-like phenotypes. Future phenotyping efforts should make the examination of dWAT an integral part of the analysis. With more information at hand how this layer of adipocytes responds to genetic manipulations of other fat pads, a better picture may emerge about this interesting structure.
Outstanding questions.
A number of questions remain to reach a better understanding of dWAT. Specifically, these relate to the communalities and differences between the adipocytes in dWAT and the adipocytes in the adjacent sWAT.
Do dWAT adipocytes arise from identical precursor cells as sWAT adipocytes and do they acquire their distinct characteristics as a function of their unique microenvironment?
Do the mature adipocytes between the dWAT and sWAT depots truly reflect functionally distinct cells with unique transcriptional profiles?
Can dWAT adipocytes undergo “beiging” to the same extent as their sWAT counterparts? We see a number of interesting correlations, but are these cells truly indispensable for immune responses, hair follicle growth, wound healing and thermal regulation?
These questions will have to await the availability of genetic tools to selectively manipulate these adipocytes. As we still lack tools to tissue-selectively manipulate any adipocytes, the identification of unique markers for these cells will facilitate study of specific depots.
TRENDS.
Dermal adipocytes are a population of cells that are distinct from subcutaneous adipocytes. Unlike other fat depots, these cells demonstrate high phenotypic flexibility and high turnover rates.
Their ability to undergo adipocyte-myofibroblast transition suggests that they can be involved in scarring.
Dermal adipocytes exhibit insulating action, and are involved in hormonal skin reactions, as well as hair follicle growth.
dWAT can produce anti-microbial peptides and thus is involved in the pathogenesis of some skin efflorescences.
dWAT can be spatially inhomogeneous thus contributing into the mosaic skin structure and being involved in appearance of skin hyperpigmentation.
dWAT is emerging as a critical metabolic tissue that can also be considered a new target in anti-scarring and anti-aging strategies and in hair regrowth.
Acknowledgments
We thank Dr. Min Kim, Touchstone Diabetes Center and Dr. Travis Vandergriff, Department of Dermatology, UT Southwestern Medical Center, Dallas for their generous help with the histological samples. P.E.S. is supported by NIH grants R01-DK55758, P01-DK088761 and R01-DK099110, as well as Cancer Prevention and Research Institute of Texas (CPRIT) grant RP140412.
Footnotes
Conflict of interests: I. Kruglikov is the managing partner of Wellcomet GmbH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 2.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang QA, et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol. 2015 doi: 10.1038/ncb3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchoukalova YD, et al. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107:18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan T, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruglikov IL. General theory of body contouring: 2. Modulation of mechanical properties of subcutaneous fat tissue. J Cosm Dermatol Sci Appl. 2014;4:117–127. [Google Scholar]
- 8.Kruglikov IL. Microstructural inhomogeneity of electrical conductivity in subcutaneous fat tissue. PLoS One. 2015;10:e0117072. doi: 10.1371/journal.pone.0117072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lackey DE, et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am J Physiol Endocrinol Metab. 2014;306:E233–E246. doi: 10.1152/ajpendo.00476.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun K, et al. Fibrosis and adipose tissue dysfunction. Cell Metab. 18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driskell RR, et al. Defining dermal adipose tissue. Exp Dermatol. 2014;23:629–631. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausman GJ, Martin RJ. The development of adipocytes located around hair follicles in the fetal pig. J Anim Sci. 1982;54:1286–1296. doi: 10.2527/jas1982.5461286x. [DOI] [PubMed] [Google Scholar]
- 13.Marangoni RG, et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67:1062–1073. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivera-Gonzalez G, et al. Adipocytes in skin health and disease. Cold Spring Harb Perspect Med. 2014;4:a015271. doi: 10.1101/cshperspect.a015271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasza I, et al. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet. 2014;10:e1004514. doi: 10.1371/journal.pgen.1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang LJ, et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumura H, et al. Cones of skin occur where hypertrophic scar occurs. Wound Repair Regen. 2001;9:269–277. doi: 10.1046/j.1524-475x.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 18.Engrav LH, et al. Functional genomics unique to week 20 post wounding in the deep cone/fat dome of the Duroc/Yorkshire porcine model of fibroproliferative scarring. PLoS One. 2011;6:e19024. doi: 10.1371/journal.pone.0019024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widgerow AD. Cellular/extracellular matrix cross-talk in scar evolution and control. Wound Repair Regen. 2011;19:117–133. doi: 10.1111/j.1524-475X.2010.00662.x. [DOI] [PubMed] [Google Scholar]
- 20.Desai VD, et al. Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e86865. doi: 10.1371/journal.pone.0086865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, et al. Reversal of myofibroblast differentiation: a review. Eur J Pharmacol. 2014;734:83–90. doi: 10.1016/j.ejphar.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Zuk P. Adipose-derived stem cells in tissue regeneration: a review. ISRN Stem Cells. 2013;2013:1–35. [Google Scholar]
- 23.Yun IS, et al. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatol Surg. 2012;38:1678–1688. doi: 10.1111/j.1524-4725.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- 24.van den Bogaerdt AJ, et al. The suitability of cells from different tissues for use in tissue-engineered skin substitutes. Arch Dermatol Res. 2002;294:135–142. doi: 10.1007/s00403-002-0305-3. [DOI] [PubMed] [Google Scholar]
- 25.van den Broek LJ, et al. Development, validation and testing of a human tissue engineered hypertrophic scar model. ALTEX. 2012;29:389–402. doi: 10.14573/altex.2012.4.389. [DOI] [PubMed] [Google Scholar]
- 26.Wu M, et al. Rosiglitazone abrogates bleomicin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-γ. Am J Pathol. 2009;174:519–533. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins V, et al. FIZZ1-Induced Myofibroblast Transdifferentiation from Adipocytes and Its Potential Role in Dermal Fibrosis and Lipoatrophy. Am J Pathol. 2015;185:2768–2776. doi: 10.1016/j.ajpath.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morroni M, et al. Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland. Proc Natl Acad Sci U S A. 2004;101:16801–16806. doi: 10.1073/pnas.0407647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derby BM, et al. Adipose-derived stem cell to epithelial stem cell transdifferentiation: a mechanism to potentially improve understanding of fat grafting's impact on skin rejuvenation. Aesthet Surg J. 2014;34:142–153. doi: 10.1177/1090820X13515700. [DOI] [PubMed] [Google Scholar]
- 30.Seo BR, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang QA, Scherer PE. The AdipoChaser mouse: A model tracking adipogenesis in vivo. Adipocyte. 2014;3:146–150. doi: 10.4161/adip.27656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang QA, et al. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellegrinelli V, et al. Human adipocyte function is impacted by mechanical cues. J Pathol. 2014;233:183–195. doi: 10.1002/path.4347. [DOI] [PubMed] [Google Scholar]
- 34.Kruglikov IL. Melanin light absorption as the necessary but not sufficient condition for photoepilation: Intra-anagen variability of hair follicle light sensitivity. Am J Cosm Surg. 2012;29:266–272. [Google Scholar]
- 35.Kruglikov IL. Melanin light absorption as the necessary but not sufficient condition for photoepilation: miniaturization and eclipse phenomena. Am J Cosm Surg. 2013;29:21–27. [Google Scholar]
- 36.Prokesch A, et al. Molecular aspects of adipoepithelial transdifferentiation in mouse mammary gland. Stem Cells. 2014;32:2756–2766. doi: 10.1002/stem.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coolen NA, et al. Comparison between human fetal and adult skin. Arch Dermatol Res. 2010;302:47–55. doi: 10.1007/s00403-009-0989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mak K, et al. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci. 2009;56:168–180. doi: 10.1016/j.jdermsci.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Poissonnet CM, et al. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev. 1984;10:1–11. doi: 10.1016/0378-3782(84)90106-3. [DOI] [PubMed] [Google Scholar]
- 40.Wojciechowicz K, et al. Development of the mouse dermal adipose layer occurs independently of subcutaneous adipose tissue and is marked by restricted early expression of FABP4. PLoS One. 2013;8:e59811. doi: 10.1371/journal.pone.0059811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passeron T. Melasma pathogenesis and influencing factors - an overview of the latest research. J Eur Acad Dermatol Venereol. 2013;27(Suppl 1):5–6. doi: 10.1111/jdv.12049. [DOI] [PubMed] [Google Scholar]
- 42.Kim WS, et al. Whitening effect of adipose-derived stem cells: a critical role of TGF-beta 1. Biol Pharm Bull. 2008;31:606–610. doi: 10.1248/bpb.31.606. [DOI] [PubMed] [Google Scholar]
- 43.Chang H, et al. Whitening effects of adipose-derived stem cells: a preliminary in vivo study. Aesthetic Plast Surg. 2014;38:230–233. doi: 10.1007/s00266-013-0116-2. [DOI] [PubMed] [Google Scholar]
- 44.Jung H, et al. Transforming growth factor-beta 1 in adipose derived stem cells conditioned medium is a dominant paracrine mediator determines hyaluronic acid and collagen expression profile. Cytotechnology. 2011;63:57–66. doi: 10.1007/s10616-010-9327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim DW, et al. Adipose-derived stem cells inhibit epidermal melanocytes through an interleukin-6-mediated mechanism. Plast Reconstr Surg. 2014;134:470–480. doi: 10.1097/PRS.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 46.Razani B, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 47.Asterholm IW, et al. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab. 2012;15:171–185. doi: 10.1016/j.cmet.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin S, et al. Caveolin-1 deficiency leads to increased susceptibility to cell death and fibrosis in white adipose tissue: characterization of a lipodystrophic model. PLoS One. 2012;7:e46242. doi: 10.1371/journal.pone.0046242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaragosi LE, et al. Syndecan-1 regulates adipogenesis: new insights in dedifferentiated liposarcoma tumorigenesis. Carcinogenesis. 2015;36:32–40. doi: 10.1093/carcin/bgu222. [DOI] [PubMed] [Google Scholar]
- 50.Flynn L, Woodhouse KA. Adipose tissue engineering with cells in engineered matrices. Organogenesis. 2008;4:228–235. doi: 10.4161/org.4.4.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun K, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kruglikov IL. Biophysical basics of body treatments: Is hyaluronan a link that has gone unnoticed? Am J Cosm Surg. 2012;29:121–127. [Google Scholar]
- 53.Kang L, et al. Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance. Diabetes. 2013;62:1888–96. doi: 10.2337/db12-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji E, et al. Inhibition of adipogenesis in 3T3-L1 cells and suppression of abdominal fat accumulation in high-fat diet-feeding C57BL/6J mice after downregulation of hyaluronic acid. Int J Obes (Lond) 2014;38:1035–1043. doi: 10.1038/ijo.2013.202. [DOI] [PubMed] [Google Scholar]
- 55.Kielty CM, et al. Type VI collagen microfibrils: evidence for a structural association with hyaluronan. J Cell Biol. 1992;118:979–990. doi: 10.1083/jcb.118.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azzi L, et al. Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. J Invest Dermatol. 2005;124:22–27. doi: 10.1111/j.0022-202X.2004.23545.x. [DOI] [PubMed] [Google Scholar]
- 57.Blouin K, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf) 2010;72:176–188. doi: 10.1111/j.1365-2265.2009.03645.x. [DOI] [PubMed] [Google Scholar]
- 58.Mullur R, et al. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JY, et al. Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am J Physiol Cell Physiol. 2012;302:C463–C472. doi: 10.1152/ajpcell.00010.2011. [DOI] [PubMed] [Google Scholar]
- 60.Obregon MJ. Adipose tissues and thyroid hormones. Front Physiol. 2014;5:479. doi: 10.3389/fphys.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211–215. doi: 10.4161/derm.3.3.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamasaki K, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nature Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 63.Reinholz M, et al. Cathelicidin LL-37: an antimicrobial peptide with a role in inflammatory skin disease. Ann Dermatol. 2012;24:126–135. doi: 10.5021/ad.2012.24.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harder J, et al. What is the role of antimicrobial peptides (AMP) in acne vulgaris? Exp Dermatol. 2013;22:386–391. doi: 10.1111/exd.12159. [DOI] [PubMed] [Google Scholar]