Abstract
Sex differences in the oxytocin (OT) system in the brain may explain why OT often regulates social behaviors in sex-specific ways. However, a link between sex differences in the OT system and sex-specific regulation of social behavior has not been tested. Here, we determined whether sex differences in the OT receptor (OTR) or in OT release in the posterior bed nucleus of the stria terminalis (pBNST) mediates sex-specific regulation of social recognition in rats. We recently showed that, compared to female rats, male rats have a three-fold higher OTR binding density in the pBNST, a sexually dimorphic area implicated in the regulation of social behaviors. We now demonstrate that OTR antagonist (5 ng/0.5 μl/side) administration into the pBNST impairs social recognition in both sexes, while OT (100 pg/0.5 μl/side) administration into the pBNST prolongs the duration of social recognition in males only. These effects seem specific to social recognition, as neither treatment altered total social investigation time in either sex. Moreover, baseline OT release in the pBNST, as measured with in vivo microdialysis, did not differ between the sexes. However, males showed higher OT release in the pBNST during social recognition compared to females. These findings suggest a sex-specific role of the OT system in the pBNST in the regulation of social recognition.
Keywords: oxytocin, bed nucleus of the stria terminalis, sex differences, social recognition, social investigation, microdialysis
1. Introduction
The neuropeptide oxytocin (OT) is synthesized mainly in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus (Buijs, 1978; Sofroniew, 1983), and regulates a wide variety of social behaviors in rodents and humans (Veenema and Neumann, 2008; Heinrichs et al., 2009; Ross and Young, 2009; Goodson and Thompson, 2010; Guastella and MacLeod, 2012). Importantly, OT has been shown to regulate some of these behaviors in sex-specific ways (reviewed in Dumais and Veenema, 2015; Dumais and Veenema, 2016). This may be due to sex differences in the OT system in the brain. Although OT mRNA expression is similar in the PVN and SON of male and female rats (Dumais et al., 2013), the OT receptor (OTR) is highly sexually dimorphic, with male rats showing higher OTR binding densities in many forebrain regions compared to female rats (Uhl-Bronner et al., 2005; Dumais et al., 2013).
The most robust sex difference in OTR binding density in the rat brain is found in the posterior bed nucleus of the stria terminalis (pBNST), in which males have a three-fold higher OTR binding density compared to females (Dumais et al., 2013). The pBNST has extensive connections with areas involved in social information processing (most notably the accessory olfactory bulb and medial amygdala; Scalia and Winans, 1975; Weller and Smith, 1982; Gu et al., 2003; Dong and Swanson, 2004) and is part of the social decision-making network (O’Connell and Hofmann, 2011). Indeed, the pBNST plays an essential role in transmitting chemosensory social information and modulating olfactory-guided social behaviors (Petrulis, 2013). For example, neuronal activation is increased in the pBNST in male Mandarin voles (He et al., 2014) and in female rats (Hosokawa and Chiba, 2007) in response to opposite-sex odors, and lesioning the pBNST impairs opposite-sex odor preference in male hamsters (Been and Petrulis, 2010). In addition, blocking OTR in the pBNST reduced male odor-induced vaginal marking in female hamsters (Martinez et al., 2010), suggesting a role for the OTR in the pBNST in social odor processing and/or olfactory-guided social behaviors. To the best of our knowledge, there are no comparative studies on the role of OTR in the pBNST in males and females.
We hypothesized that the sex difference in OTR binding density in the pBNST is implicated in the sex-specific regulation of social behavior. To test this, we determined the effects of acute pharmacological manipulations of the OT system in the pBNST on social investigation (reflecting the motivation to approach a conspecific for the assessment of social cues) and social recognition (the ability to discriminate between familiar and unfamiliar conspecifics) in adult male and female rats. Social investigation and social recognition were chosen because these behaviors are modulated by the OT system (Gabor et al., 2012; Lukas et al., 2013; Dumais et al., 2013), require the processing of chemosensory social information, and can be tested with neutral social stimuli (i.e., juvenile rats), allowing focus on social odor processing without interference of sexual or aggressive behaviors.
We further hypothesized that the sex difference in OTR binding density in the pBNST corresponds with a sex difference in local OT release. Higher OTR binding density, as seen in males (Dumais et al., 2013), could be associated with higher OT release or could be a compensatory mechanism for lower OT release. To determine the relationship between sex differences in OTR binding density and OT release in the pBNST, we used in vivo microdialysis to measure extracellular OT release in the pBNST of male and female rats under baseline conditions and during exposure to the social recognition test.
2. Methods
2.1 Animals
Wistar rats were obtained from Charles River at 8–9 weeks of age (Wilmington, MA) and maintained on a 12 h light/dark cycle, lights on at 0700 h, and food and water were available ad libitum. Subjects were adult male and female rats housed in same-sex pairs in standard rat cages (26.7 x 48.3 x 20.3 cm) unless otherwise mentioned, and were given at least one week to acclimate to our facilities. Stimulus male and female rats were 22 days at arrival, were housed four per cage, and were used at 25–30 days of age. All experiments were conducted in accordance with the guidelines of the NIH and approved by the Boston College Institutional Animal Care and Use Committee (IACUC).
2.2 Stereotaxic surgery
2.2.1 Cannulation
After daily handling for one week to familiarize them with the injection procedure, experimental rats were anesthetized using isoflurane and mounted on a stereotaxic frame. A heating pad was used to regulate body temperature of rats while anesthetized. Guide cannulae (22 gauge; Plastics One, Roanoke, VA) were implanted bilaterally 2 mm dorsal to the pBNST (0.8 caudal to bregma, 1.5 and −1.5 lateral to midline, and 4.8 ventral to the skull surface; according to Paxinos and Watson, 1998). Guide cannulae were fixed to the skull with four stainless steel screws and acrylic glue and closed with dummy cannulae (26 gauge; Plastics One, Roanoke, VA). After surgery, rats were individually housed in standard rat cages (26.7 x 48.3 x 20.3 cm). Behavioral testing was performed 3 and 5 days after surgery.
2.2.2 Microdialysis probe placement
A separate set of rats was used for in vivo measurement of extracellular OT release. Handling and surgical procedures were similar to the procedures described above except for the placement of microdialysis probes instead of cannulae. Microdialysis probes (BrainLink, the Netherlands) were implanted unilaterally into the pBNST (0.8 caudal to bregma, −1.5 lateral to midline, and 7.0 ventral to the skull surface). Two inch pieces of polyethylene tubing were fixed to the ends of the microdialysis probes in order for attachment to the microinfusion pumps and eppendorf tubes for sample collection. After surgery, rats were individually housed in standard rat cages (26.7 x 48.3 x 20.3 cm). Microdialysis and behavioral testing were performed 2 days after surgery.
2.3 Behavioral Testing
2.3.1 Social Investigation Test
To test for social investigation, the time rats spent investigating an unfamiliar same-sex juvenile rat was measured according to Dumais et al. (2013). A juvenile rat was used in order to assess general social approach of the experimental rat toward a social stimulus that does not elicit aggressive or sexual behaviors. Indeed, no aggressive or mounting behaviors were observed during the social investigation test. A juvenile rat was placed into the experimental rat’s home cage for 4 min, and time spent investigating the juvenile was measured. Testing was performed during the light phase between 1200 h and 1700 h. Behaviors were video recorded and analyzed using JWatcher (http://www.jwatcher.ucla.edu) by an experimenter blind to treatment groups. Behavior was considered social investigation when the experimental rat was actively sniffing the juvenile, including sniffing the anogenital and head/neck regions.
2.3.2 Social Recognition Test
Social recognition was measured using the social discrimination paradigm, according to Veenema et al. (2012) and adapted from Engelmann et al. (1995). This paradigm consists of two trials. In the first trial (T1), the experimental rat is exposed in its home cage to an unfamiliar same-sex juvenile for 4 min (T1 is the same as the social investigation test described above). After a preset interval, the experimental rat undergoes a second trial (T2) in which the rat is exposed in its home cage to the same (familiar) juvenile along with an unfamiliar same-sex juvenile for 4 min (see Movie 1). To allow the experimenter to distinguish between the two juveniles, juveniles were marked on their backs with either red or black permanent marker 1 h prior to testing. The color of the marker was counterbalanced between novel and familiar juveniles. Using this social discrimination paradigm, previous studies found that adult rats show social recognition after a 1 h interval, but not after a 3 h interval (Veenema et al., 2012; Bernal-Mondragon et al., 2013; Lee et al., 2014). We hypothesized that OTR blockade may impair social recognition, while OT administration may prolong social recognition. Therefore, we used a 1 h interval for the OTR antagonist experiment, and a 3 h interval for the OT experiment. Testing was performed during the light phase between 1200 h and 1700 h. Behaviors were video recorded, and time spent investigating the juvenile rats was measured using JWatcher by an experimenter blind to treatment groups. As expected, no aggressive or mounting behaviors of the experimental rats towards the juvenile rats were observed during the test. The percentage of time investigating the novel juvenile (time investigating novel juvenile/time investigating familiar + novel juvenile x 100) during T2 was calculated as the measure of social recognition. The time spent investigating one juvenile in T1 and two juveniles in T2 was calculated as the measure of total social investigation time (in seconds) in T1 and T2, respectively.
2.4 Experimental Procedures
2.4.1 Experiment 1: Effect of OTR manipulations in the BNST on social investigation and social recognition
The effects of the OTR antagonist desGly-NH2,d(CH2)5-[Tyr(Me)2,Thr4]OVT (5 ng/0.5 μl/side) and synthetic OT (Sigma; 100 pg/0.5 μl/side) were each compared to their own vehicle (Ringer’s solution, 0.5 μl/side) control group. Drug doses were based on prior microinjection studies in rats in which these doses were effective in altering diverse social behaviors (Guzman et al., 2013; Guzman et al., 2014; Bredewold et al., 2014; László et al., 2015). Injection systems were composed of polyethylene tubing connected to an injector cannula which extended 2mm beyond the guide cannula. Injections were made using a 10 μl Hamilton syringe (Hamilton, Reno, NV) and was kept in place for 30 s following injection to allow for tissue uptake. Following the last behavioral test, rats were killed with C02, a small amount of charcoal was injected into the guide cannulae, and proper cannulae placement was verified histologically on Nissl-stained coronal brain sections (see Fig. 1). Rats that did not have proper cannulae placement were removed from analysis (OTR antagonist experiment: males=4, females=8; OT experiment: males=4, females=6). For the OTR blockade experiment, 2 males in the vehicle group were excluded after the social investigation test due to dental cement becoming loose. For the OT experiment, 2 males and 3 females in the vehicle group and 1 female in the OT injection group were excluded after the social investigation test due to clogging of the guide cannulae. The final number of rats per group is indicated below.
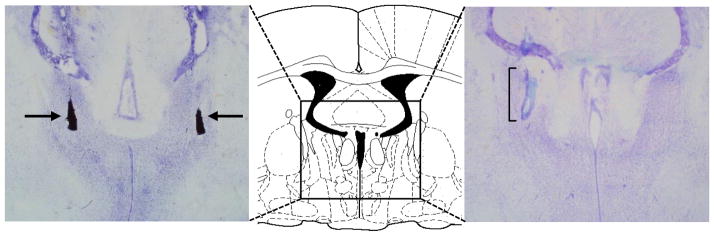
Fig. 1.
Schematic drawing of the posterior bed nucleus of the stria terminalis (pBNST; bregma −0.80 mm; adapted from Paxinos and Watson, 1998) and representative enlargements of photomicrographs of Nissl-stained coronal sections of the rat brain indicating with arrows the bilateral microinjection locations in the pBNST using charcoal as marker (left) and indicating with a bracket the location and extend of the semipermeable membrane of a microdialysis probe placement unilaterally in the pBNST (right). Correct placements were considered at bregma −0.80 mm ± 0.2 mm using the brain atlas by Paxinos and Watson (1998).
2.4.1.1 Experiment 1a: Social investigation
To determine the effects of OTR blockade in the pBNST on social investigation, subjects were injected with either Ringer’s solution (males=15, females=16) or the OTR antagonist (males =10, females=13). To determine the effects of OT administration into the pBNST on social investigation, subjects were injected with either Ringer’s solution (males=9, females=8) or OT (males=7, females=9. Rats were injected 20 min before the start of the social investigation test.
2.4.1.2 Experiment 1b: Social recognition
Rats were exposed to the social discrimination test two days after the social investigation test, and received the same treatment. The time window of two days was chosen to allow the drugs to leave the system (OTR antagonists and OT have a half-life of approx. 20 min; Mens et al., 1983; Goodwin et al., 1995; Ludwig and Leng, 2006) and to prevent any residual effects of drug injections during the social investigation test on social recognition. To determine the effects of OTR blockade in the pBNST on social recognition (1 h interval), subjects were injected with either Ringer’s solution (males=13, females=16) or OTR antagonist (males=10, females=13) in each side. To determine the effects of exogenous OT in the pBNST on social recognition (3 h interval), subjects were injected with either Ringer’s solution (males=7, females=5) or OT (males=7, females=8) in each side. Rats were injected immediately after exposure to the first stimulus juvenile.
2.4.2 Experiment 2: OT release in the BNST during social recognition
OT release in the pBNST was measured in a separate group of male and female rats exposed to the social discrimination test (1 h interval). Two to three days before microdialysis probe implantation, rats were exposed to the social discrimination paradigm to habituate them to the behavioral test. One day after probe implantation, rats were habituated to the sampling procedure for 1 h. Two days after probe implantation, microdialysis probes were connected via polyethylene tubing to Hamilton syringes mounted on a microinfusion pump. Rats were perfused with Ringer’s solution (with 0.25% BSA; 3μl/min) for 2 h to establish an equilibrium between the inside and outside of the microdialysis membrane. Five consecutive 30-min dialysates were then collected in 0.5 ml eppendorf tubes containing 0.1M HCl to inhibit protein degradation. Dialysate 1 was collected before the first juvenile exposure (baseline), dialysate 2 started with the 4-min exposure to the first stimulus juvenile (T1), dialysate 3 started 30 min after T1, dialysate 4 started with the 4-min exposure to the same and novel stimulus juveniles (T2), and dialysate 5 started 30 min after T2. Dialysates were immediately frozen on dry ice, and stored at −45°C until quantification. OT content was measured using radioimmunoassay (RIAgnostics, Munich, Germany). Following the last behavioral test, rats were killed with C02, and proper probe placement was verified histologically on Nissl-stained coronal brain sections (see Fig. 1). Rats that did not have proper probe placement were removed from analysis (males=6, females=4). Six rats (males=1, females=5) were also excluded due technical issues with dialysate sampling. This resulted in a final number of 19 males and 16 females.
2.5 Estrus phase measurement
To control for effects of estrus cycle, estrus phase was determined via vaginal smears (according to Dumais et al., 2013) taken from each female immediately following each behavioral test. Using a pipette and a small amount of distilled water, vaginal secretions were taken and assessed for estrus cycle phase via cell characteristics according to Goldman et al. (2007). Females were categorized as being in proestrus/estrus (cells characteristic of proestrus and estrus phases in which females show higher levels of estradiol and progesterone), or non-estrus (cells characteristic of diestrus and metestrus in which females show lower levels of estradiol and progesterone). Number of females in estrus or nonestrus for each experiment were as follows: social investigation (OTR blockade experiment, vehicle: non-estrus=9, estrus=7, OTR antagonist: non-estrus=8, estrus=5; OT injection experiment, vehicle: non-estrus=4, estrus=4, OT injection: non-estrus=7, estrus=2) and social recognition (OTR blockade experiment, vehicle: non-estrus=13, estrus=3, OTR antagonist: non-estrus=9, estrus=4; OT injection experiment, vehicle: non-estrus=4, estrus=1, OT injection: non-estrus=5, estrus=3).
2.6 Statistical Analysis
For experiment 1, social investigation was analyzed using two-way ANOVA (treatment x sex) with social investigation time (in seconds) as the dependent factor. Social recognition was analyzed using two-tailed one-sample T-tests, with the percent novel investigation for each group tested against 50% (chance level) and Bonferroni correction for multiple comparisons (corrected alpha, p=0.0125). To determine differences between groups, social recognition was analyzed using two-way ANOVA (treatment x sex) with percent novel investigation as the dependent factor. Because of main effects of treatment and sex, two-tailed independent sample T-tests were run separately in males and females to test for treatment effects by sex (OTR antagonist and OT) and separately by treatment to test for sex effects by treatment (OT). The effects of estrus phase on social investigation time and on social recognition were analyzed using one-way ANOVAs (treatment) with estrus phase as covariate.
For experiment 2, rats were assigned into two groups based on their ability to show social recognition. Rats were considered to show social recognition if the percent novel investigation was higher than the average percent novel investigation for each sex (males: >57% n=9; females: >62% n=9), and rats were considered to not show social recognition if the percent novel investigation was below the average percent novel investigation for each sex (males: <57% n=10, females: <62% n=7). Four males and 4 females were excluded from the study because they showed higher than 57% investigation of the familiar juvenile.
Effects of sex and social recognition ability on percent novel investigation and total investigation time were measured using two-way ANOVAs. To confirm a significant difference in social recognition ability in each sex, two-tailed independent sample T-tests were run separately in males and females to test for effects of social recognition ability. Effects of sex and social recognition ability on baseline OT concentration (pg/dialysate) were analyzed using the non-parametric Kruskal-Wallis test. OT release was then converted into percent change from baseline OT release for each rat. Effects of sex and social recognition ability on the percentage of OT release was analyzed using a three-way ANOVA for repeated measures (dialysate x sex x social recognition). When main effects were found, differences between groups were analyzed with an LSD post hoc test. Effect of estrus phase on OT release was tested using a two-way ANOVA for repeated measures (dialysate x social recognition) with estrus phase as covariate. Data are presented as mean + SEM, and significance was set at p<0.05.
3. Results
3.1 Experiment 1: Effect of OTR manipulations in the pBNST on social investigation and social recognition
3.1.1 Experiment 1a: Social investigation
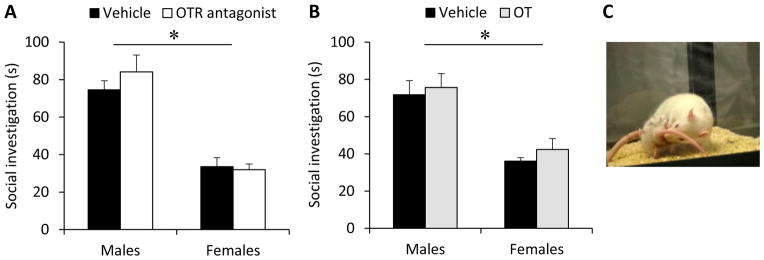
OTR blockade did not alter social investigation time in either sex (treatment effect: F(1,50)=0.91, p=0.35; sex x treatment effect: F(1,50)=1.40, p=0.24). Likewise, OT administration did not alter social investigation time in either sex (treatment: F(1,29)=0.67, p=0.42; sex x treatment: F(1,29)=0.03, p=0.85). However, a sex effect was found in both experiments (OTR antagonist: F(1,50)=85.6, p<0.0001; OT: F(1,29)=30.8, p<0.001), in which males spent more time investigating the social stimulus (Fig. 2A, B). There was no effect of estrus phase on social investigation for either the OTR antagonist experiment (F(1,26)=1.08, p=0.31) or the OT experiment (F(1,14)=0.05, p=0.83).
Fig. 2.
There are no effects of oxytocin (OT) receptor (OTR) antagonist (A) or OT (B) injections into the posterior bed nucleus of the stria terminalis (pBNST) on social investigation in male and female adult rats. However, there is a main effect of sex in both (A) and (B), in which females show lower social investigation compared to males. Social investigation, depicted in (C), represents the total time spent investigating a novel, same-sex juvenile rat placed in the home cage of the experimental rat for a 4-min period. Bars indicate mean + SEM; * p<0.001, two-way ANOVA).
3.1.2 Experiment 1b: Social recognition
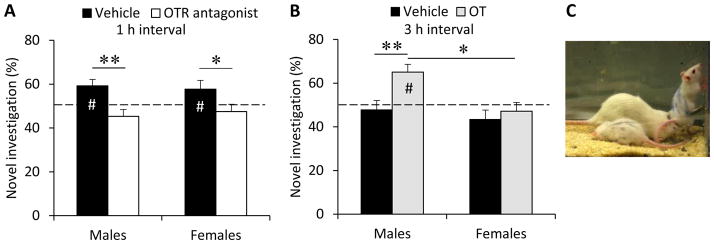
Two days after the social investigation test, and using the same drug treatment, rats were exposed to the social recognition test. OTR blockade impaired social recognition in both sexes. In contrast to vehicle-treated rats (males: t(12)=3.01, p<0.0125; Bonferroni corrected p value); females: t(15)=2.91, p<0.0125), OTR antagonist-treated rats did not investigate the novel rat more than chance level (males: t(9)= −1.52, p=0.16; females: t(12)= −0.75, p= 0.47, Fig. 3A), revealing a lack of social discrimination after OTR blockade. As a result, the percent novel investigation was significantly lower in OTR antagonist-treated rats compared to vehicle-treated rats (F(1,48)=15.5, p<0.0001). Independent samples T-tests showed that OTR blockade decreased the percent novel investigation in both males (t(21)=3.20, p<0.01) and females (t(27)=2.41, p<0.05; Fig. 3A). No main effects of sex (F(1,48)=0.01, p=0.93) or treatment x sex (F(1,48)=0.40, p=0.53) were found for social recognition, nor did estrus phase have an effect on social recognition (F(1,26)=0.96, p=0.34).
Fig. 3.
Role of the posterior bed nucleus fo the stria terminalis (pBNST)- oxytocin (OT) system in social recognition. (A) OT receptor (OTR) antagonist injected into the pBNST impaired social recognition in both male and female adult rats. (B) OT injected into the pBNST improved social recognition in males, but not in females. Social recognition is expressed as the percentage of time spent investigating a novel same-sex juvenile rat (time investigating novel rat/total time investigating novel + familiar rat x 100). Social recognition was tested after an interval of 1 h (A) or 3 h (B). The picture in (C) shows an experimental rat exposed to a novel and a familiar juvenile rat and investigating one of the juvenile rats. Bars indicate mean + SEM; #: significantly different from chance level (p<0.05, one sample t-test); *p<0.05, **p<0.01, two-way ANOVA followed by T-test.
There was no difference in social investigation time between vehicle- and OTR antagonist-treated rats during T1 (vehicle-treated males: 71.3 ± 5.0 sec, vehicle-treated females: 24.1 ± 2.7 sec, OTR antagonist-treated males: 69.8 ± 7.0 sec, OTR antagonist-treated females: 28.1 ± 3.3 sec; treatment: F(1,48)=0.08, p=0.78; sex x treatment: F(1,48)=0.40, p=0.53; two-way ANOVA) and T2 (vehicle-treated males: 72.7 ± 6.8 sec, vehicle-treated females: 22.2 ± 3.7 sec, OTR antagonist-treated males: 68.3 ± 7.7 sec, OTR antagonist-treated females: 24.7 ± 2.0 sec; treatment: F(1,48)=0.01, p=0.96; sex x treatment: F(1,48)=0.24, p=0.62; two-way ANOVA). However, confirming the sex difference in experiment 1a, males spent more time investigating the juveniles during both T1 (F(1,48)=102, p<0.00001) and T2 (F(1,48)=69.3, p<0.00001). Estrus phase did not have an effect on social investigation time (T1: F(1,26)=0.96, p=0.34; T2: F (1,26)=0.01, p=0.93).
OT treatment prolonged the duration of social recognition in males, but not in females. In detail, OT-treated males investigated the novel juvenile more than chance level (t(6)=4.14, p<0.0125) while vehicle-treated males (t(6)= −0.52, p=0.62), vehicle-treated females (t(4)=−1.53, p=0.20) and OT-treated females (t(7)= −0.71, p=0.50) did not (Fig. 3B). While there was no sex x treatment interaction (F(1,23)=2.60, p=0.12), there were main effects of treatment (F(1,23)=6.35, p<0.05) and sex (F(1,23)=7.10 , p<0.05). To determine whether the effects of OT on social recognition were sex-specific, the data were tested separately by sex and treatment using independent samples T-tests. These tests indicated that OT-treated males spent more time investigating the novel juvenile compared to vehicle-treated males (t(12)=−3.06, p<0.01) and compared to OT-treated females (t(13)=3.25, p<0.01; Fig. 3B). OT-treated females did not investigate the novel juvenile more than vehicle-treated females (t(11)=−0.61, p=0.55) nor was there a difference in percent novel investigation between vehicle treated male and female rats (t(10)=0.70, p=0.50). Finally, estrus phase did not have an effect on social recognition (F(1,10)=0.68, p=0.43).
There was no difference in social investigation time between vehicle- and OT-treated rats during T1 (vehicle-treated males: 53.6 ± 2.5 sec, vehicle-treated females: 41.7 ± 8.7 sec, OT -treated males: 64.3 ± 7.6 sec, OT -treated females: 33.1 ± 7.4 sec; treatment: F(1,23)=4.03, p=0.06; sex x treatment: F(1,23)=0.55, p=0.47; two-way ANOVA) and T2 (vehicle-treated males: 48.4 ± 5.2 sec, vehicle-treated females: 36.5 ± 6.0 sec, OT-treated males: 71.1 ± 7.0 sec, OT-treated females: 38.4 ± 7.4 sec; treatment: F(1,23)=0.02, p=0.88; sex x treatment: F(1,23)=2.00, p=0.17; two-way ANOVA). However, confirming the sex difference in experiment 1a and in the OTR experiment, males showed longer social investigation times during both T1 (F(1,23)=10.1, p<0.01) and T2 (F(1,23)=4.76, p<0.05). Estrus phase did not have an effect on social investigation time (T1: F(1,10)=0.99, p=0.35; T2: F(1,10)=0.31, p=0.59).
3.2 Experiment 2: OT release in the BNST during social recognition
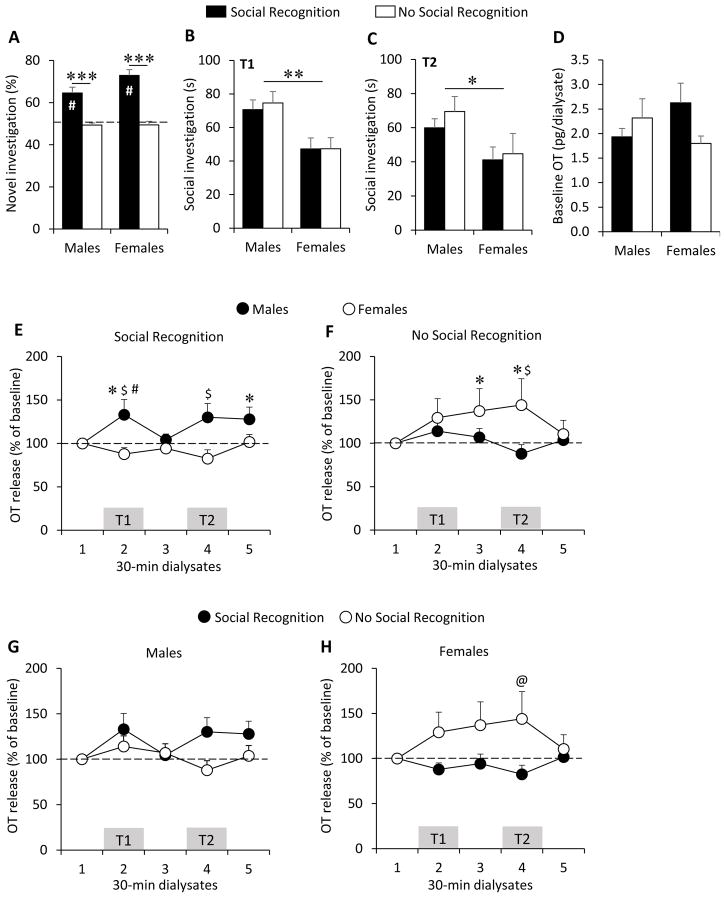
Rats were grouped based on their social recognition ability (see statistics section for selection criteria), and behavioral data for each group is shown in Fig. 4A–C. As expected, the percent novel investigation was significantly different from chance level for rats that showed social recognition (males: t(8)=5.19, p<0.0125; females: t(8)=8.26, p<0.0125; Bonferroni corrected p value), but not for rats that did not show social recognition (males: t(9)=1.81, p=0.11; females: t(6)=0.35, p=0.74; Fig 4A). This was also reflected by a main effect of social recognition ability (F(1,31)=76.1, p<0.0001, two-way ANOVA) and a lack of main effects for sex (F(1,31)=3.63, p=0.07) and sex x social recognition ability (F(1,31)=3.52, p=0.07). Independent samples T-tests were then run separately by sex, which confirmed that the percent novel investigation was higher in males (t(17)=5.33, p<0.0001) and females (t(14)=6.813, p<0.00001) that showed social recognition compared to those that did not (Fig. 4A).
Fig. 4.
Sex-specific oxytocin (OT) release in the posterior bed nucleus of the stria terminalis (pBNST) as a function of social recognition ability. (A) Males and females that showed social recognition have a significantly higher percent novel investigation compared to males and females that did not show social recognition (see statistics section for selection criteria). Independent of social recognition ability, males show significantly higher total social investigation time during T1 (B) and during T2 (C) compared to females. (D) There is no effect of sex or social recognition ability on baseline OT concentrations in the pBNST. (E) The percentage of OT release in the pBNST of rats that showed social recognition is significantly higher during T1 versus dialysate 1 and 3 and during dialysate 5 versus dialysate 1 in males only. The percentage of OT release in the pBNST of rats that showed social recognition is also significantly higher in males than in females during T1 and T2. (F) In females that did not show social recognition, the percentage of OT release is higher during T2 and during dialysate 3 versus dialysate 1. The percentage of OT release in the pBNST of rats that did not show social recognition is higher in females than in males during T2. (G) The percentage of OT release in the pBNST is not different between males that showed social recognition and males that did not show social recognition. (H) The percentage of OT release is higher in females that did not show social recognition compared to females that showed social recognition during T2. OT release is expressed as percent change from baseline OT concentrations. Data are expressed as mean + SEM. A–D: **p<0.001, *<0.05, two-way ANOVA, ***p<0.0001, two-way ANOVA, followed by T-test, #: significantly different from chance level (p<0.05, one sample t-test). EH: * p<0.05 versus dialysate 1, # p<0.05 versus dialysate 3, $ p<0.05 versus other sex, @p<0.05 versus females that show social recognition, three-way ANOVA for repeated measures followed by LSD post hoc test; T1, 4-min exposure to first juvenile; T2, 4-min exposure to previously-exposed juvenile along with a novel juvenile.
In agreement with the sex difference in experiments 1a and 1b and irrespective of social recognition ability (T1, social recognition ability: F(1,31)=0.09, p=0.76; social recognition ability x sex: F(1,31)=0.09, p=0.77. T2, social recognition ability: F(1,31)=0.61, p=0.44; social recognition ability x sex: F(1,31)=0.13, p=0.72), males showed higher social investigation compared to females during both T1 (F(1,31)=14.8, p<0.001, two-way ANOVA; Fig. 4B) and T2 (F(1,31 )=6.67, p<0.05, two-way ANOVA; Fig. 4C).
There was no difference in baseline OT concentration in the pBNST between males and females that did and did not show social recognition (x2(3)=3.87, p=0.28; Fig. 4D). Effect of social recognition ability was also not significant when analyzing baseline OT concentration independently by sex (males: x2(1)=0.43, p=0.51; females: x2(1)=3.05, p=0.08).
While there were no main effects of dialysate (F(4,120)=1.19, p=0.32), sex (F(1,30)=0.13, p=0.72), or social recognition (F(1,30)=0.93, p=0.34) on the percentage of OT release, a significant dialysate x sex x social recognition interaction effect was found (F(4,120)=2.81, p<0.05). Post hoc testing revealed a sex difference in OT release in rats that showed social recognition. Here, males show a significant increase in the percentage of OT release during T1 (p<0.05 vs dialysate 1 and 3) and a trend toward an increase in the percentage of OT release during T2 (p=0.072 vs dialysate 1), while females do not show a change in the percentage of OT release during T1 (p=0.42 vs dialysate 1; p=0.64 vs dialysate 3) or T2 (p=0.29 vs dialysate 1; Fig. 4E). This sex difference is further reflected by males showing higher OT release compared to females during both T1 (p<0.05) and T2 (p<0.05; Fig. 4E). In addition, the percentage of OT release was higher in dialysate 5 compared to dialysate 1 (p<0.05) in males that showed social recognition (Fig. 4E).
In rats that did not show social recognition, females showed an increase in the percentage of OT release during T1 (trend: p=0.089 vs. dialysate 1), T2 (p<0.05 vs. dialysate 1), and dialysate 3 (p<0.05 vs. dialysate 1), while males did not (T1 vs dialysate 1: p=0.38; T2 vs dialysate 1: p=0.67; Fig. 4F). This sex difference was further reflected by higher OT release in females compared to males during T2 (p<0.05; Fig. 4F).
There was no difference in the percentage of OT release in males that showed social recognition compared to males that did not show social recognition (Fig. 4G). However, females that showed social recognition had lower OT release during T2 compared to females that did not show social recognition (p<0.05; Fig. 4H). No effects of estrus phase were found for the percentage OT release (estrus phase: F(1,13)=0.28, p=0.61; dialysate x estrus phase: F(4,52)=1.2, p=0.34).
4. Discussion
We demonstrated that endogenous activation of the OTR in the pBNST is necessary for social recognition in both males and females and that exogenous OT facilitates social recognition in males only. Specifically, OTR antagonist (5 ng/0.5 μl/side) injected into the pBNST impaired social recognition in both sexes, while OT (100 pg/0.5 μl/side) injected into the pBNST prolonged the duration of social recognition in males, but not females, to 3 h. We speculate that the higher OTR binding density in the pBNST of males compared to females (Dumais et al., 2013) may explain the sex-specific facilitating effects of exogenous OT on social recognition. The higher percentage of OT release in the pBNST of males versus females during successful social recognition further indicates the sex-specific involvement of OT in the pBNST in social recognition. Furthermore, our current microdialysis findings along with our previous receptor autoradiography findings (Dumais et al., 2013) demonstrate that the sex difference in OTR binding density in the pBNST is not associated with a sex difference in extracellular OT concentration in the pBNST. To the best of our knowledge, this is the first report exploring the concomitant dynamics between OTR binding density and OT release in a single brain region.
OT and OTR are well known to play a role in the regulation of social recognition in rats and mice (reviewed in Gabor et al., 2012). For example, male OTR knockout mice (Takayanagi et al., 2005; Lee et al., 2008; Macbeth et al., 2009), male OT knockout mice (Ferguson et al., 2000; Macbeth et al., 2009) and female OT knockout mice (Choleris et al., 2003; Choleris et al, 2006) show impaired social recognition. In rats, intracerebroventricular administration of an OTR antagonist impaired social recognition in both males (Lukas et al., 2013) and females (Engelmann et al., 1998). Moreover, brain-region specific regulation of social recognition by the OTR has been found in which social recognition was impaired after OTR blockade in the medial amygdala in male mice and rats (Ferguson et al., 2001; Lukas et al., 2013), in the lateral septum (Lukas et al., 2013) and ventral hippocampus (Van Wimersma-Greidanus and Maigret, 1996) in male rats, and in the olfactory bulbs in female rats (Larrazolo-Lopez et al., 2008). Our results confirm the involvement of the OT system in social recognition, and add the pBNST as a region important in OTR-mediated regulation of social recognition in both male and female rats.
A role for the OTR in the pBNST in social recognition complements previous findings demonstrating a role for the OTR in the pBNST in male odor-induced vaginal marking in female hamsters (Martinez et al., 2010). Interestingly, our study used juvenile conspecifics as social stimuli, suggesting that the pBNST may be involved in the processing of social odors outside the context of sociosexual behaviors. The processing of social cues could be mediated via direct projections from the accessory olfactory bulb to the pBNST (Halpern, 1987; Guillamon and Segovia, 1997). On the other hand, the pBNST has extensive connections with areas involved in learning, memory, and motivation (i.e., medial amygdala, hippocampus, hypothalamus, midbrain; Krettek and Price, 1978; Georges and Aston-Jones, 2002; Gu et al., 2003; Dong and Swanson, 2004; Krüger et al., 2015). This may indicate the potential of the pBNST to modulate the learning and/or motivational aspects associated with social recognition. Future research is needed to determine whether OTR activation in the pBNST regulates social recognition by modulating the processing of social cues, or by modulating higher order memory and learning processes.
Importantly, the effects of OT system manipulations in the pBNST appear specific to social recognition, as the same dose of OTR antagonist or OT injected into the pBNST did not alter social investigation time in either sex (as tested in the social investigation test and in T2 of the social recognition test). This was surprising given that both the OT system and the pBNST are known to modulate social investigation. For example, chronic intracerebroventricular OT administration in adult male rats (Witt et al., 1992) and acute intranasal OT administration in adult male mice (Huang et al., 2014) increased social investigation of adult females, while intracerebroventricular OTR antagonist injections in adult male rats and mice decreased the investigation time towards adult males (Lukas et al., 2011). In addition, excitotoxic lesions of the pBNST in male hamsters reduced the investigation time towards female hamsters (Been and Petrulis, 2010). However, stimuli in the above-mentioned studies were adults (i.e., highly salient social stimuli), while our study used juveniles (neutral and less salient social stimuli and thus avoiding aggression or sexual behaviors). We therefore cannot exclude that the OT system in the pBNST is involved in the investigation of more salient social stimuli. Overall, our data indicate that the OT system in the pBNST facilitates social recognition without affecting general social approach to investigate neutral social stimuli.
We also provide evidence for the sex-specific involvement of the OT system in the pBNST in social recognition. OT injected bilaterally into the pBNST (100pg/0.5 μl/side) prolonged social recognition to 3 h in adult male rats, but failed to do so in adult female rats. This seems in line with previous studies demonstrating that intracerebroventricular administration of OT (1 ng) prolonged the duration of social recognition in adult male rats (Benelli et al., 1995), but not in adult female rats (Engelmann et al., 1998). It should be noted that it is unclear whether higher doses of OT would prolong social recognition in female rats. Dose dependent effects of OT administration on social recognition have been found in adult male rats, with sometimes higher doses impairing social recognition. However, these effects were seen after either subcutaneous or intracerebroventricular administration of OT (Popik et al., 1996; Popik et al., 1992a; Dantzer et al., 1987; Benelli et al., 1995; Popik and Vetulani, 1991). In contrast, studies using locally administered OT at different doses have reported only facilitating effects (i.e., medial preoptic area, Popik and vanRee, 1991; lateral septum, Popik et al., 1992b). Therefore, the facilitating effect of OT in the pBNST on social recognition in male rats is in line with the effects of OT in other brain regions in males. Moreover, females have much lower OTR binding density in the pBNST compared to males (Dumais et al., 2013), making it less likely that a higher dose of OT were to have an effect in females. Together, this indicates that male rats are more sensitive to the effects of the same dose of OT on social recognition and that the pBNST is a critical component of this effect.
A limitation of the current OTR manipulation study is that rats were not drug naïve during the social recognition test (i.e., the rats received the same drug treatment 2 days prior during the social interest test). However, the half-life of OTR antagonists and of OT is approximately 20 min (Mens et al., 1983; Goodwin et al., 1995; Ludwig and Leng, 2006). Therefore, a washout of 2 days would likely allow for the OTR antagonist and OT to leave the system, making it less likely that the effects of OT or OTR antagonist on social recognition are due to residual drug effects.
Furthermore, recent studies have shown that OT can mediate its behavioral effects via the vasopressin V1a receptor (Schorscher-Petcu et al. 2010; Sala, et al. 2011; Ramos, et al. 2013; Qiu, et al. 2014; Song et al., 2014). Indeed, there is cross-reactivity between OT and vasopressin and their receptors (Manning et al., 2012). However, V1a receptor binding density is virtually absent in the area of the pBNST where OTR binding is very dense (Dumais and Veenema, 2015), making it less likely that the effects of OT on social recognition are mediated through the vasopressin V1a receptor.
Importantly, our in vivo microdialysis data also reveal a sex-specific involvement of OT in the pBNST in successful social recognition. In detail, males, but not females, showed an increase in the percentage of OT release in the pBNST during T1 (p< 0.05; exposure to an unfamiliar juvenile) and T2 (p=0.072; exposure to the same T1 juvenile along with a novel juvenile) compared to baseline OT release. As a result, the percentage of OT release in the pBNST was higher during both T1 and T2 in males than in females that showed social recognition. We further found that males investigated juveniles longer than females did, suggesting that the sex difference in percentage OT release in the pBNST could be associated with the sex difference in social investigation. However, as discussed above, OTR blockade in the pBNST did not alter social investigation in either sex. We therefore propose that higher OT release in males versus females during T1 and T2 of the social recognition test does not play a role in the sex difference to investigate neutral social stimuli, but rather is part of a sex-specific mechanism underlying social recognition.
Interestingly, females that did not show social recognition had higher OT release during T2 compared to baseline OT release. Further, females that did not show social recognition had higher OT release during T2 compared to females that showed social recognition and males that did not show social recognition. These findings, although highly surprising, may provide a rationale as to why exogenous OT (and thus, increasing OT levels) in the pBNST failed to improve social recognition after a 3 h interval in females. Yet, OTR blockade impaired social recognition in females, suggesting that a baseline level of OT release in the pBNST is still necessary for the appropriate expression of social recognition at the 1 h interval, possibly by maintaining baseline neuronal activity. Although it is yet unclear whether the increase in OT release in males and females predetermines their social recognition ability or is an effect of social stimuli exposure, findings from both OT injections and OT release suggest that higher OT signaling in the pBNST facilitates social recognition in males, but not in females.
A limitation of the current microdialysis study is the relatively long dialysate sampling period (30 min) as compared to the short stimulus exposure period (4 min). The 30-min sampling period is required due to detection limits of the radioimmunoassay, but this could have obscured a possible significant rise in OT release during T2 in males that show social recognition and during T1 in females that did not show social recognition.
Although extracellular OT release has been measured in various brain regions of male (Engelmann et al., 1999; Ebner et al., 2000; Waldherr and Neumann, 2007) and female (Nyuyki et al., 2011; Neumann et al., 1993; Bosch et al., 2010; Bosch et al., 2004) rats, we are the first to compare OT release patterns between males and females in the same study. Our data show that extracellular OT concentrations in the pBNST are similar between sexes. This is in line with the absence of a sex difference in OT mRNA expression in the rat PVN and SON (Dumais et al., 2013) of which the PVN is a likely source of OT release in the pBNST (Knobloch et al., 2012). This also suggests that, at least for the pBNST, a sex difference in static OTR binding density (Dumais et al., 2013) does not correspond with a sex difference in static OT release. Instead, the higher OT release in the pBNST seen in males versus females during successful social recognition test may indicate that higher OTR binding density in males serves to accommodate stimulus-induced dynamic increases in local OT release. Similarly, the higher OTR binding density in males may allow exogenous OT injections to have a greater effect in males than in females by binding to unoccupied OTRs in the pBNST of males, while endogenous OT may have already saturated all OTR binding sites in the pBNST of females.
Interestingly, the pBNST is a highly sexually dimorphic structure in volume, cell number, neurochemical expression, and neurocircuitry. For example, the pBNST is larger and contains more cells in males than in females in rats, mice, guinea pigs, and humans (Hines et al., 1985; Guillamon et al., 1988; Del Abril et al., 1987; Hines et al., 1992; Allen and Gorski, 1990; Chung et al., 2002; Forger et al., 2004). In rats, males show higher vasopressin (De Vries and Miller, 1998; Miller et al., 1989), estrogen receptor-alpha (Kelly et al., 2013), substance P (Malsbury and McKay, 1987), and cholecystokinin (Miceevych et al., 1988) immunoreactivity in the pBNST. Moreover, male rats have denser projections of the pBNST to several hypothalamic areas than female rats (Gu et al., 2003). Along with higher OTR binding (Dumais et al., 2013), all these parameters are higher or denser in males than in females. This suggests that the pBNST in males is programmed to respond differently to incoming social olfactory information from afferent brain regions such as the accessory olfactory bulb and medial amygdala (Halpern, 1987; Guillamon and Segovia, 1997) and/or may convey such information differently onto downstream projection areas that mediate behavioral responses. This may allow for the pBNST to promote male and female-specific expression of social behaviors, including male copulatory behavior (Emery and Sach, 1976; Claro et al., 1995), male aggressive behavior (Patil and Brid, 2010; Calcagnoli et al., 2014; Masugi-Tokita et al., 2015), and maternal behavior (Numan and Numan, 1996). Our findings are the first to suggest that the processing of social cues by the pBNST-OT system is different in males and females and may contribute to sex-specific modulation of social recognition.
5. Conclusions
In summary, our results show that the OT system in the pBNST is implicated in sex-specific regulation of social recognition in adult rats. We discussed that this could be due to higher OTR binding density in the pBNST of male versus female rats as was previously reported (Dumais et al., 2013) in combination with higher endogenous OT release in the pBNST of male versus female rats during successful social recognition.
Movie 1: Example video showing an adult male rat in the social recognition test during microdialysis testing. The video shows a clip during T2 of the social recognition test, in which the experimental rat shows more social investigation of the novel juvenile (marked in black) compared to the familiar juvenile (marked in red).
Supplementary Material
Acknowledgments
Role of the funding source This research is financially supported by NRSA Predoctoral Fellowship F31MH100891 to KMD and NIMH R15MH102807 to AHV. These sponsors did not have a role in study design; in collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
We would like to thank the Veenema Lab for technical assistance and for critically reading the manuscript, the animal caretakers at Boston College for excellent animal care, and Dr. Maurice Manning for kindly providing the OTR antagonist. This research is supported by NRSA Predoctoral Fellowship F31MH100891 to KMD and NIMH R15MH102807 to AHV.
Footnotes
Contributions
Authors Kelly M. Dumais and Alexa H. Veenema designed the study and wrote the manuscript. Author Kelly Dumais performed all experiments and analyzed the data. Authors Andrea G. Alonso, Marisa A. Immormino and Remco Bredewold assisted in the experiments. Author Remco Bredewold edited the manuscript. All authors have approved the final manuscript.
Conflict of interest: Conflict of Interest: none
References
- Allen LS, Gorski RA. Sex differences in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Lesions of the posterior bed nucleus of the stria terminalis eliminate opposite-sex odor preference and delay copulation in male Syrian hamsters: role of odor volatility and sexual experience. Eur J Neurosci. 2010;32:483–93. doi: 10.1111/j.1460-9568.2010.07277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli A, Bertolini A, Poggioli R, Menozzi B, Basaglia R, Arletti R. Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides. 1995;28(4):251–5. doi: 10.1016/0143-4179(95)90029-2. [DOI] [PubMed] [Google Scholar]
- Bernal-Mondragon C, Rivas-Arancibia S, Kendrick KM, Guevara-Guzman R. Estradiol prevents olfactory dysfunction induced by A-β 25–35 injection in hippocampus. BMC Neurosci. 2013;14(104):1–14. doi: 10.1186/1471-2202-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Krömer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124(2):439–48. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal beahviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J Neuroendocrinol. 2010;22:420–9. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- Bredewold R, Smith CJ, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci. 2014;8:216. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192(3):423–35. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, de Boer SF, Beiderbeck DI, Althaus M, Koolhaas JM, Neumann ID. Local oxytocin expression and oxytocin receptor binding in the male rat brain is associated with aggressiveness. Behav Brain Res. 2014;261:315–22. doi: 10.1016/j.bbr.2013.12.050. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and beta-knockout mice. Proc Natl Acad Sci USA. 2003;100(10):6192–7. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5(7):528–39. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Chung WCJ, De Vries GJ, Swaab DF. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J Neurosci. 2002;22:1027–33. doi: 10.1523/JNEUROSCI.22-03-01027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claro F, Segovia S, Guilamon A, Del Abril A. Lesions in the medial posterior region of the BST impair sexual behavior in sexually experienced and inexperienced male rats. Brain Res Bull. 1995;36(1):1–10. doi: 10.1016/0361-9230(94)00118-k. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology. 1987;91(3):363–8. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- de Vries GJ, Miller MA. Anatomy and function of extrahypothalamic vasopressin systems in the brain. Prog Brain Res. 1998;119:3–20. doi: 10.1016/s0079-6123(08)61558-7. [DOI] [PubMed] [Google Scholar]
- Del Abril A, Segovia S, Guillamon A. The bed nucleus of the stria terminalis in the rat: Regional sex differences controlled by gonadal steroids early after birth. Dev Brain Res. 1987;32:295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- Dong H, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: Implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neur. 2004;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav. 2013;64(4):693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Presence and absence of sex differences in structure and function of the brain oxytocin system: Implications for understanding social behavior. In: Shansky R, Johnson J, editors. Neuroscience of Sex. Elsevier; 2016. [Google Scholar]
- Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2015 May 4; doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. A single social defeat experience selectively stimulates the release of oxytocin, but not vasopressin, within the septal brain area of male rats. Brain Res. 2000;872(1–2):87–92. doi: 10.1016/s0006-8993(00)02464-1. [DOI] [PubMed] [Google Scholar]
- Emery DE, Sach BD. Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol Behav. 1976;17:803–6. doi: 10.1016/0031-9384(76)90044-5. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol. 1999;11(11):867–72. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Wotjak CT, Landgraf R. Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav Brain Res. 1998;90:89–94. doi: 10.1016/s0166-4328(97)00084-3. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: An alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58(2):315–21. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–85. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25(3):284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, De Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton–Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci. 2012;126(1):97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22(12):5173–87. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Research (Part B) 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiology. 2010;20(6):784–94. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Goodwin TM, Millar L, North L, Abrams LS, Weglein RC, Holland ML. The pharmacokinetics of the oxytocin antagonist atosiban in pregnant women with preterm uterine contractions. Am J Obstet Gynecol. 1995;173(3 Pt 1):913–7. doi: 10.1016/0002-9378(95)90365-8. [DOI] [PubMed] [Google Scholar]
- Gu G, Cornea A, Simerly RB. Sexual differentiation of projections from the principal nucleus of the bed nuclei of the stria terminalis. The Journ of Comp Neurol. 2003;460:542–62. doi: 10.1002/cne.10677. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61(3):410–8. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Segovia S. Sex differences in the vomeronasal system. Brain Res Bull. 1997;44(4):377–82. doi: 10.1016/s0361-9230(97)00217-7. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Segovia S, Del Abril A. Early effects of gonadal steroids on the neuron number in the medial posterior and the lateral divisions of the bed nucleus of the stria terminalis in the rat. Dev Brain Res. 1988;44:281–290. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, Nishimori K, Radulovic J. Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci. 2013;16(9):1185–7. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Sato K, Mesic I, Guedea AL, Nishimori K, Radulovic J. Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacology. 2014;231(10):2097–105. doi: 10.1007/s00213-013-3356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. The organization and function of the vomeronasal system. Ann Rev Neurosci. 1987;10:325–62. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- He F, Wu R, Yu P. Study of Fos, androgen receptor and testosterone expression in the subregions of the medial amygdala, bed nucleus of the stria terminalis, and medial preoptic area in male mandarin voles in response to chemosensory stimulation. Behav Brain Res. 2014;258:65–74. doi: 10.1016/j.bbr.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30(4):548–57. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–6. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Hines M, Davis FC, Coquelin A, Goy RW, Gorski RA. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1985;5:40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N, Chiba A. Effects of sexual experience on conspecific odor preference and male odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in female rats. Brain Res. 2007;1175:66–75. doi: 10.1016/j.brainres.2007.07.071. [DOI] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Manago F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B, Scattoni ML, Papaleo F. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39:1102–14. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DA, Varnum MM, Krentzel AA, Krug S, Forger NG. Differential control of sex differences in estrogen receptor α in the bed nucleus of the stria terminalis and anteroventral periventricular nucleus. Endocrinology. 2013;154(10):3836–46. doi: 10.1210/en.2013-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178(2):225–54. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Krüger O, Shiozawa T, Kreifelts B, Scheffler K, Ethofer T. Three distinct fiber pathways of the bed nucleus of the stria terminalis to the amygdala and prefrontal cortex. Cortex. 2015;66:60–8. doi: 10.1016/j.cortex.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Larrazolo-Lopez A, Kendrick KM, Aburto-Arciniega M, Arriaga-Avila V, Morimoto S, Frias M, Guevara-Guzman R. Vaginocervical stimulation enhances social recognition memory in rats via oxytocin release in the olfactory bulb. Neuroscience. 2008;152(3):585–93. doi: 10.1016/j.neuroscience.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Laszlo K, Kovacs A, Zagoracz O, Ollmann T, Peczely L, Kertes E, Lacy DG, Lenard L. Positive reinforcing effect of oxytocin microinjection in the rat central nucleus of the amygdala. Behav Brain Res. 2015;296:279–85. doi: 10.1016/j.bbr.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mice line of the oxytocin receptor. Endocrinology. 2008;149(7):3256–63. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Chan JT, Hazarika O, Vutskits L, Sall JW. Early exposure to volatile anesthetics impairs long-term associative learning and recognition memory. PLoS One. 2014;9(8):e105340. doi: 10.1371/journal.pone.0105340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neursci. 2006;7(2):126–36. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–68. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M, Toth I, Veenema AH, Neumann ID. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology. 2013;38(6):916–26. doi: 10.1016/j.psyneuen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Lee HJ, Edds J, Young WS., 3rd Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes Brain Behav. 2009;8(5):558–67. doi: 10.1111/j.1601-183X.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsbury CW, McKay K. A sex difference in the pattern of substance P-like immunoreactivity in the bed nucleus of the stria terminalis. Brain Res. 1987;420(2):365–70. doi: 10.1016/0006-8993(87)91258-3. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. J Neuroendocrinol. 2012;24(4):609–28. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Albers HE, Petrulis A. Blocking oxytocin receptors inhibits vaginal marking to male odors in female Syrian hamsters. Physiol Behav. 2010;101(5):685–92. doi: 10.1016/j.physbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masugi-Tokita M, Flor PJ, Kawata M. Metabotropic glutamate receptor subtype 7 in the bed nucleus of the stria terminalis is essential for intermale aggression. Neuropsychopharmacology. 2015:1–10. doi: 10.1038/npp.2015.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262(1):143–9. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Micevych P, Akesson T, Elde R. Distributions of cholecystokinin-immunoreactive cell bodies in the male and female rat: II. Bed nucleus of the stria terminalis and amygdala. J Comp Neurol. 1988;269:381–91. doi: 10.1002/cne.902690306. [DOI] [PubMed] [Google Scholar]
- Miller MA, Vician L, Clifton DK, Dorsa DM. Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Peptides. 1989;10(3):615–9. doi: 10.1016/0196-9781(89)90152-6. [DOI] [PubMed] [Google Scholar]
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: A microdialysis study. Neuroscience. 1993;53(1):65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29(1):23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Nyuyki KD, Waldherr M, Baeumi S, Neumann ID. Yes, I am ready now: differential effects of paced versus unpaced mating on anxiety and central oxytocin release in female rats. Plos One. 2011;6(8):e23599. doi: 10.1371/journal.pone.0023599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519(18):3599–639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Patil SN, Brid SV. Relative role of neural substrates in the aggressive behavior of rats. J Basic Clin Physiol Pharmacol. 2010;21(4):357–67. doi: 10.1515/jbcpp.2010.21.4.357. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Petrulis A. Chemosignals and hormones in the neural control of mammalian sexual behavior. Front Neuroendocrinol. 2013;34(4):255–67. doi: 10.1016/j.yfrne.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol. 1991;1(4):555–60. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- Popik P, Vetulani J. Opposite action of oxytocin and its peptide antagonists on social memory in rats. Neuropeptides. 1991;18(1):23–7. doi: 10.1016/0143-4179(91)90159-g. [DOI] [PubMed] [Google Scholar]
- Popik P, Vetulani J, van Ree JM. Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology. 1992a;106(1):71–4. doi: 10.1007/BF02253591. [DOI] [PubMed] [Google Scholar]
- Popik P, Vetulani J, Van Ree JM. Facilitation and attenuation of social recognition in rats by different oxytocin-related peptides. Eur J Pharmacol. 1996;308(2):113–6. doi: 10.1016/0014-2999(96)00215-4. [DOI] [PubMed] [Google Scholar]
- Popik P, Vos PE, Van Ree JM. Neurohypophyseal hormone receptors in the septum are implicated in social recognition in the rat. Behav Pharmacol. 1992b;3(4):351–358. [PubMed] [Google Scholar]
- Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, McGregor IS. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology. 2013;38(11):2249–59. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Qiu CY, Cai H, Liu TT, Qu ZW, Yang Z, Li JD, Zhou QY, Hu WP. Oxytocin inhibits the activity of acid-sensing ion channels hrough the vasopressin, V1A receptor in primary sensory neurons. Br J Pharmacol. 2014;171(12):3065–76. doi: 10.1111/bph.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacological rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69(9):875–82. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161(1):31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30(24):8274–84. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Morphology of vasopressin and oxytocin neurons and their central and vascular projections. Prog Brain Res. 1983;60:101–14. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill JK, 4th, Larkin TE, 2nd, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–9. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102(44):16096–101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl-Bronner S, Waltisperger E, Martínez-Lorenzana G, Condes Lara M, Freund-Mercier MJ. Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience. 2005;135(1):147–54. doi: 10.1016/j.neuroscience.2005.05.025. [DOI] [PubMed] [Google Scholar]
- van Wimersma Greidanus TB, Maigret C. The role of limbic vasopressin and oxytocin in social recognition. Brain Res. 1996;713:153–9. doi: 10.1016/0006-8993(95)01505-1. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age- specific ways. Horm Behav. 2012;61(1):50–6. doi: 10.1016/j.yhbeh.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–76. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104(42):16681–4. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232(2):255–70. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem and Behav. 1992;43:855–61. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.