Abstract
Epstein-Barr virus (EBV) is associated with multiple sclerosis (MS), and antibodies to the EBV nuclear antigen-1 (EBNA-1) are consistently increased in MS patients. The hypothesis of this study is that anti-EBNA-1 antibodies cross-react with a self antigen in MS patients. We affinity purified anti-EBNA-1 antibodies from human plasma, used the anti-EBNA-1 to immunoprecipitate antigens from human brain, and identified bound antigens with mass spectrometry. Anti-EBNA-1 consistently bound heterogeneous nuclear ribonucleoprotein L (HNRNPL). We expressed both the long and short isoforms of this protein, and verified with Western blots and ELISA that the long isoform cross-reacts with EBNA-1. Immunohistochemistry demonstrated that anti-EBNA-1 bound to an antigen in the nucleus of cultured rat central nervous system cells. ELISA demonstrated the presence of antibodies to HNRNPL in the plasma of both healthy controls and MS patients, but anti-HNRNPL was not increased in MS patients. We conclude that HNRNPL is an autoantigen which cross-reacts with EBNA-1. The relevance of this autoantigen to MS and other autoimmune diseases remains to be investigated.
Keywords: Epstein-Barr virus, multiple sclerosis, molecular mimicry, antibody cross-reactivity
1. Introduction
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus which infects almost all humans worldwide. Following the initial infection, EBV remains present for the life of the host, and a substantial proportion of circulating T cells and immunoglobulin are specific for EBV.
EBV infection is associated with multiple sclerosis (MS), a putative autoimmune disease of the central nervous system (CNS), through several lines of evidence. Antibodies to EBV antigens are consistently increased in people with MS compared to healthy controls[1, 2], severe initial infection with EBV increases risk of MS[3], and asymptomatic young adults with high levels of anti-EBV IgG have an increased risk for developing MS[4–6]. EBV contains multiple distinct antigens, and the EBV nuclear antigen-1 (EBNA-1) is a major target of the antibody response. An elevated anti-EBNA-1 antibody response is consistently and strongly associated with MS[6–8].
Although EBV is associated with MS, it is not clear what role the virus plays in the pathogenesis of MS. Multiple mechanisms have been suggested by which EBV might contribute to CNS damage. These include active EBV infection in the CNS[9–11] or activation of innate imunity in the CNS by latent EBV infection[12], but the evidence that EBV is present in the CNS is controversial[13]. A less direct mechanism is cross-reactivity between virus antigens and central nervous system proteins through which reactivation of EBV infection outside the CNS could drive the autoimmune process inside the CNS through molecular mimicry[14, 15]. The objective of this study was to identify CNS proteins which cross-react with anti-EBNA-1 antibodies.
2. Materials and Methods
2.1 Specimens
Plasma samples were obtained from MS patients attending clinic and from normal controls recruited from the medical center. The set of plasma used in the ELISA included 62 relapsing-remitting MS subjects and 62 controls, each matched to one MS patient for age, gender, and ethnicity. These 62 samples included 44 females and 18 males; mean age 39.6 years with a standard deviation of 10.4 years; with 44 caucasian, 13 African-American, and 5 other. The MS subjects included 21 on interferon, 21 on glatiramer, 19 untreated, and 1 on dimethyl fumarate.
Human brain tissue was obtained from autopsy specimens. All specimens were stored at −80° C. Specimen collection was approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston, and subjects signed an informed consent.
2.2 Proteins, antigens, and IgG
Full length EBNA-1 protein was purchased from Advanced Biotechnologies (Columbia, MD) and a mouse monoclonal anti-EBNA-1antibody from Virostat (Portland, ME). Recombinant heterogeneous nuclear ribonucleoprotein L (HNRNPL) (both isoforms) and the EBV protein BFRF3 were produced in our laboratory. The DNA for the full length protein was spliced into the pET-45b(+) vector, amplified in NovaBlue cells and then transfected into BL21(DE3)pLysS cells (all from Novagen, San Diego, CA). The plasmid inserts were fully sequenced, and were identical to the reference sequences (NM_001533 for HNRNPL, NC_007605 for BFRF3). Protein expression was induced with IPTG, and protein was purified with Ni-NTA (Sigma, St. Louis, MO), verified on Coomassie stained gels, and quantified with the bicinchonic acid assay (Thermo Scientific, Rockford, IL).
Recombinant proteins extracted from bacteria unavoidably include a small amount of contaminating bacterial proteins. To control for this, we also transfected BL21(DE3)pLysS cells with the original pET-45b(+) vector with no DNA insert. For each batch of recombinant protein, we simultaneously performed an identical culture and extraction on these bacteria.
The reference IgG used was from a single vial of human IgG for medical use (Gammagard, Baxter, Westlake Village, CA). These IgG products are isolated from pooled plasma from large numbers of blood donors, and thus approximate a population average IgG. Brain protein was produced by homogenizing brain tissue in a Dounce homogenizer in 10 mM HEPES with protease inhibitors at 125 mg wet tissue per ml. The homogenate was centrifuged at 14,000 g for 5 minutes, the pellet rehomogenized in the same volume of PBS with 1% Triton X100, and centrifuged again. The combined supernatants were passed through a 0.2 micron filter and then a protein G column to remove IgG.
2.3 Affinity columns
The EBNA-1 protein was coupled to AffiGel 10 (BioRad, Hercules, CA) according to the manufacturer’s instructions. Antigen-specific IgG was obtained by incubating plasma or reference IgG with the AffiGel coupled antigen for 2 hours at room temperature with constant mixing. AffiGel was then washed extensively with PBS followed by PBS with 0.5 M NaCl and 1% Triton to remove weakly bound proteins. Bound IgG was eluted with 0.1 M glycine, pH 2.7. Specificity was verified with ELISA and Western blot on both the purified protein and extract from EBV-infected cells.
2.4 Immunoprecipitation
EBNA-1 specific IgG was incubated with Dynabeads protein G (Novex, Oslo, Norway) for 2 hours at room temperature, briefly washed, then added to 12 ml of brain protein, and incubated with rotation for 2 hours. The magnetic beads were extensively washed with PBS with 0.05% Tween 20 and PBS with 0.5 M NaCl and 1% Triton. Following the final wash, as much liquid was removed as possible, and the beads with bound IgG and brain protein were stored at −20.
2.5 Mass spectrometry
Samples were processed at the Proteomics Facility at the University of Texas M. D. Anderson Cancer Center, under the direction of Dr. David Hawke. The entire sample was subjected to trypsin digestion, the Dynabeads were removed from the digest, and peptides were identified with mass spectrometry.
2.6 ELISA
Coating buffer was Na2CO3 50 mM, pH 9.6. Wash buffer was Tris 50 mM, NaCl 140 mM, Tween 20 0.05%, pH 8.0. Blocking buffer was wash buffer with 1% bovine serum albumin. Primary and secondary antibodies were diluted in blocking buffer. MaxiSorp plates (Thermo Scientific) were coated with 0.1 µg protein per well in 100 µl of coating buffer, blocked with 200 µl blocking buffer, then incubated with primary antibody followed by secondary antibody. Each incubation was one hour, and plates were washed 4 times with water and twice with wash buffer between each step. Primary antibody was 0.03 µl plasma per well for EBNA-1 and 0.1 µl plasma per well for HNRNPL. Secondary antibody was goat anti-human IgG coupled to horseradish peroxidase (Southern Biotech, Birmingham, AL). Following the secondary antibody, plates were developed for 4 minutes with a solution of orthophenylenediamine, and optical density was read at 450 nm on a Molecular Devices plate reader.
Two different types of standard curve were used to obtain quantitative results. For quantification of EBNA-1, all wells were coated with antigen, and serial dilutions of the reference IgG were included on each plate. The concentration in the unknowns is then expressed as the µg of reference IgG which gave the same optical density as a µl of plasma. For the HNRNPL isoforms, we included wells coated with anti-human IgG with serial dilutions of reference serum. The unknowns were then expressed as µg IgG bound per well. For experiments with recombinant HNRNPL proteins, we coated equal numbers of wells on the same plate with HNRNPL and the bacteria control. The value for the bacterial control was subtracted from the value for HNRNPL to correct for the small degree of contamination with bacterial proteins.
Samples were run in duplicate, and intra-assay coefficients of variation were <5%. Inter-assay coefficient of variation was 9.2% for HNRNPL and 14.2% for EBNA-1.
2.7 Western blots
Proteins were run on a 4–12% gradient bis/tris polyacrylamide gel (Invitrogen, Carlsbad, CA) in MES buffer, and transferred to nitrocellulose membranes using an Xcell II blot module (Invitrogen). Membranes were blocked with Tris-buffered saline containing 2.5% non-fat dried milk and 0.05% Tween 20 for an hour, and then incubated for 1 hour with primary antibody and then an hour with secondary antibody. Antibodies were diluted in blocking buffer, and the secondary antibody was mouse anti-human IgG Fc conjugated to alkaline phosphatase (Southern Biotechnology) diluted 1:2000. After the secondary antibody, the blot was washed extensively in water, and then bound antibody was visualized with NBT/BCIP.
2.8 Primary neuron-glia co-cultures
We chose to use rat cells for the immunohistochemistry experiments because HNRNPL is highly homologous in rat and human, and we have and established procedure for generation of cultured rat neurons and glia. The primary neuron-glia co-cultures from E-16 pre-natal embryos of Sprague Dawley rats were prepared as described with minor modifications[16]. Briefly, the whole cerebral cortex and sub-cortical basal ganglia were dissected under a dissection microscope after removing the pia, meninges and olfactory bulbs. The selectively dissected brain tissues were triturated with flame-restricted glass pipets and the dissociated cells were suspended in the DMEM with 4.5 mg/ml glucose, DM1 and DM2[17], 5% fetal calf serum, 2 mM L-glutamine, 100 units/ml penicillin and 100 µg/ml streptomycin, and seeded on poly-L-lysine-coated culture plates or German glass inserted in the culture well at a cell density of 4×105 cells/mm2. The cultures were maintained in a CO2 incubator for 12–15 days until the cells were completely confluent. These neuron-glial co-cultures contain about 52% astrocytes, 19% neurons, 12% oligodendroglia and 7% microglia at 15 days in culture[16].
2.9 Immunofluorescence
The immunofluorescence for EBNA-1 or IgG were performed as described[18]. Briefly, the 12d-old neuron-glia co-cultures were fixed in 95% methanol plus 5% acetic acid for 10 minutes at −20°C. After blocking with 1% goat serum in PBS, the cells were incubated with anti-EBNA-1 antibody at 0.08 µg/ml or an equal concentration of unselected human Ig as a negative control overnight at 4°C. Goat anti-human IgG-Alexa Fluor 488 was used to visualize EBNA-1 or Ig immuno-signal. The cell nuclei were labeled with DAPI. A Zeiss Axioskop 2 microscope equipped with CCD camera and operated by MetaMorph 7.4 software was used for image acquisition. The fluorescence-labeled cells were visualized using the filter sets of Ex/Em of 490/520 nm for Alexa 488, and Ex/Em of 365/480 nm for DAPI.
2.10 Statistics
The ELISA results were analyzed with the rank sum test using the statistical software in SigmaPlot version 11.0.
3. Results
3.1 Immunoprecipitation and mass spectrometry
EBNA-1-specific IgG was selected from pooled plasma from 11 MS patients and used to immunoprecipitate brain proteins. Bound proteins were identified with mass spectrometry. An equal amount of unselected IgG or a sample with no IgG were included as negative controls. In our first experiment, peptides from a total of 61 human proteins were identified. Six of these were found only with the EBNA-1 specific IgG and not in the negative controls.
We performed two replicate experiments. The first replicate used pooled plasma from 14 MS patients, and identified 10 proteins present in the EBNA-1-specific IgG but not the control samples. Only two of the 10 proteins were also identified in the first experiment. The third replicate compared EBNA-1 specific IgG from 20 MS patients, EBNA-1 specific IgG from 20 controls, and unselected reference IgG as a negative control. The MS sample contained 33 proteins not present in the negative control. The only protein identified in all 3 experiments with pooled plasma was heterogeneous nuclear ribonucleoprotein L (HNRNPL). Multiple peptides from this protein were identified in each experiment (Table 1, complete list of identified proteins in supplemental data).
Table 1.
Selected proteins identified by mass spectrometry
| protein | Pool 1 | Pool 2 | Pool 3 | C1 | C2 | C3 | M1 | M2 | M3 |
|---|---|---|---|---|---|---|---|---|---|
| HNRNPL | 9 | 9 | 7 | 2 | 14 | 6 | 10 | 6 | 2 |
| Present in one pooled and one individual experiment | |||||||||
| ALYREF | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Present in two pooled experiments | |||||||||
| A2M | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Proteins are identified by the gene symbol, ALYREF is a nuclear protein and A2M is alpha-2-macroglobulin. Pool 1, etc. refer to the immunoprecipitation experiments with pooled plasma described in the text. The immunoprecipitation experiments with individual plasma are labeled C for control plasma and M for MS plasma. The numbers indicate how many peptides from that protein were identified in the experiment.
To verify the findings with pooled plasma, we performed similar experiments using plasma from 3 individual controls and 3 individual MS patients. Mass spectrometry identified peptides from multiple proteins in each sample (range 49 to 117). HNRNPL was present in each, but otherwise, there was little overlap with the previous experiments with pooled plasma. The only other finding of interest was that the 60 kDa SS-A/Ro ribonucleoprotein, which is reported to be cross-reactive with EBNA-1[19], was present in a single immunoprecipitate from one of the control subjects (supplemental data).
3.2 Verification of Cross reactivity
The immunoprecipitation results suggest that antibodies against EBNA-1 also bind HNRNPL. HNRNPL has two known splice variants. The longer variant includes 133 amino acids at the N terminal which are absent in the short form. These additional N terminal residues include a stretch rich in glycines, with one 61 residue section which is 45.9% identical to a region in EBNA-1 (amino acids 29 to 89 in HNRNPL and 91 to 151 in EBNA-1). The peptides identified by mass spectrometry included many from the shared C terminal portion of the protein, and a few peptides from the N terminal segment found only in the long isoform. We expressed both isoforms of the protein.
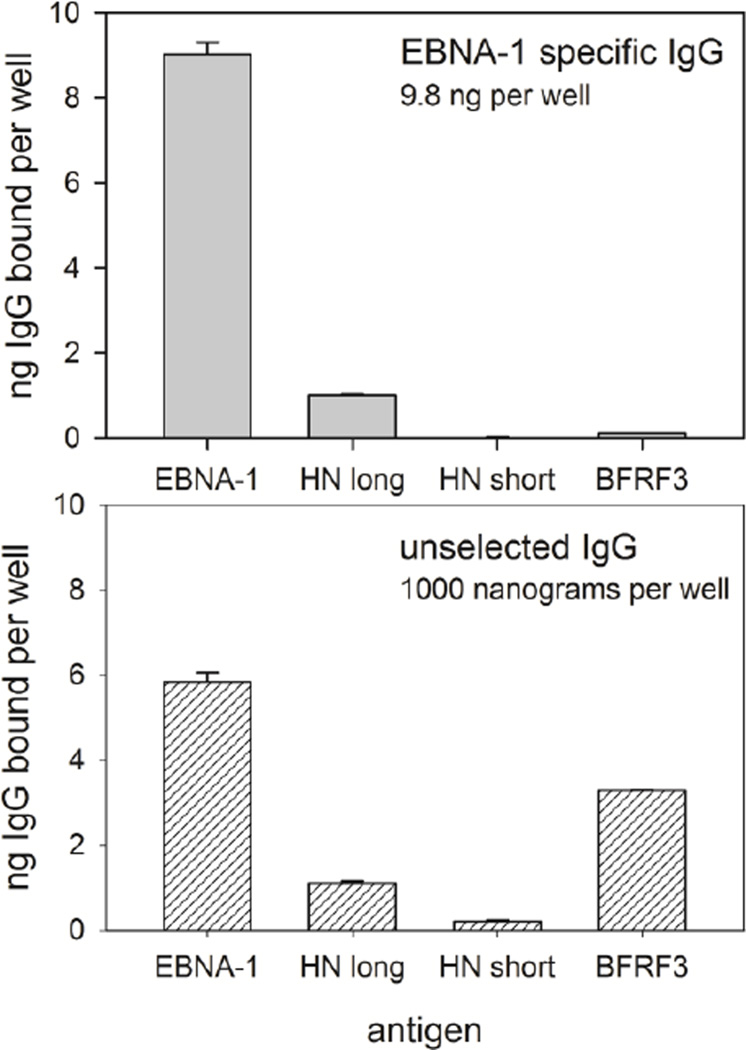
We used ELISA to verify that anti-EBNA-1 antibodies also bound HNRNPL. We took the reference IgG, isolated Ig specific for EBNA-1, and tested the EBNA-1 specific IgG for for binding to EBNA-1 and HNRNPL, with the EBV protein BFRF3 as a negative control (Figure 1). The absolute amount of IgG bound in each well was determined with a standard curve of serial dilutions of reference serum bound to wells coated with mouse anti-human IgG. For the anti-EBNA-1 IgG, 11.2% bound to the long isoform of HNRNPL, while 0.09% bound the short isoform. Binding of EBNA-1 specific IgG to negative controls, including the Epstein-Barr virus protein BFRF3, no antigen, and bacterial proteins was minimal. Similar results were obtained in a replicate experiment, with 6.6% of anti-EBNA-1 binding to HNRNPL. Unselected IgG showed strong binding to EBNA-1 and BFRF3, with weaker binding to HNRNPL.
Figure 1. ELISA with EBNA-1 specific IgG and unselected IgG.
EBNA-1 specific IgG was affinity purified from the reference IgG. Both the EBNA-1 specific and the unselected IgG were tested for reactivity to EBNA-1, the long and short isoforms of HNRNPL, and the EBV small capsid protein BFRF3. For the affinity purified IgG, 92% bound to EBNA-1, indicating that the affinity purification was effective. Of the EBNA-1 specific IgG, 11.2% also bound the long form of HNRNPL, but only negligible amounts bound the short form of HNRNPL or the unrelated EBV antigen BFRF3. For the unselected IgG, 0.58% bound EBNA-1, 0.33% bound BFRF3, and 0.11% bound the long isoform of HNRNPL. Error bars indicate standard deviations.
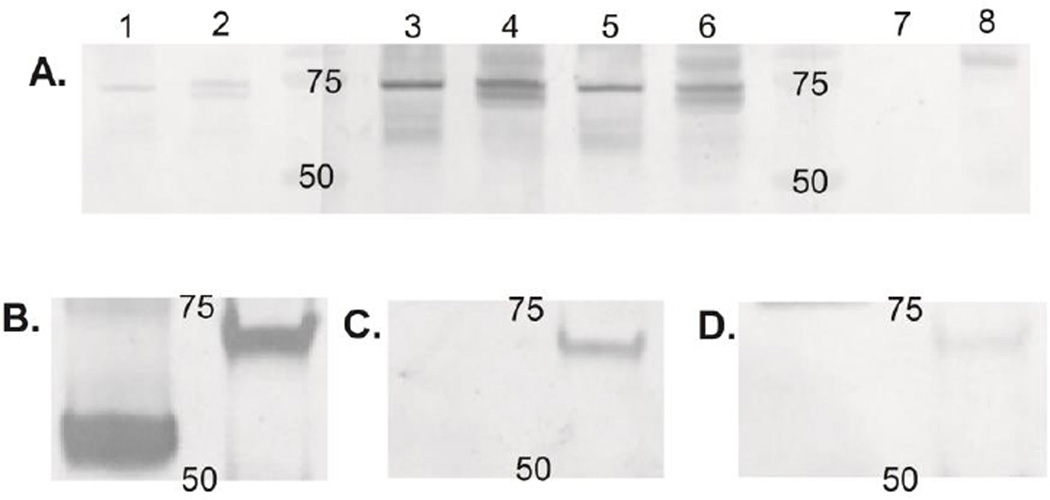
We also verified the EBNA-1 specificity and cross-reactivity with Western blot. The affinity purified anti-EBNA-1 IgG bound as expected to both the recombinant protein used for selection and EBNA-1 expressed in infected human cells (Figure 2A). Anti-EBNA-1 IgG bound to the long isoform of HNRNPL, but not the short isoform (Figure 2B,C,D). Unselected reference IgG also bound the long form of HNRNPL, but not to the short isoform, but the binding is weak compared to anti-EBNA-1.
Figure 2. Western blot with EBNA-1 specific IgG.
A. Western blot to verify EBNA-1 specificity of the selected antibody. Antigen in lanes 1, 3, 5, and 7 is the recombinant EBNA-1 protein used in antibody selection. Antigen in lanes 2, 4, 6, and 8 is protein extracted from human EBV infected lymphoblastoid cell lines (LCL). Primary antibodies are a mouse monoclonal anti-EBNA-1 in lanes 1 and 2, 500 µg of reference IgG in lanes 3 and 4, and 0.5 µg EBNA-1 specific antibody in lanes 5 and 6. Lanes 7 and 8 have no primary antibody. All three primary antibodies bind a protein at the expected molecular weight for EBNA-1 in both the recombinant protein and the LCL extract. The EBNA-1 in the LCL extract is a doublet. The numbers on the blot indicate the position of the 50 and 75 kDa molecular weight markers. B, C, and D: Western blot to demonstrate cross-reactivity of EBNA-1 specific antibody. Antigens are the short isoform of HNRNPL in the left lane and the long isoform in the right lane. B. Primary antibody anti-hexahistidine. The HNRNPL isoforms are present at the expected molecular weights, 54 kDa for the recombinant short isoform and 67 kDa for the long isoform. C. The primary antibody is 3 µg EBNA-1 specific IgG selected from the standard IgG. Only the long isoform is detected. D. Primary antibody is 600 µg unselected reference IgG. The long isoform is faintly detected. Numbers indicate 50 and 75 kDa.
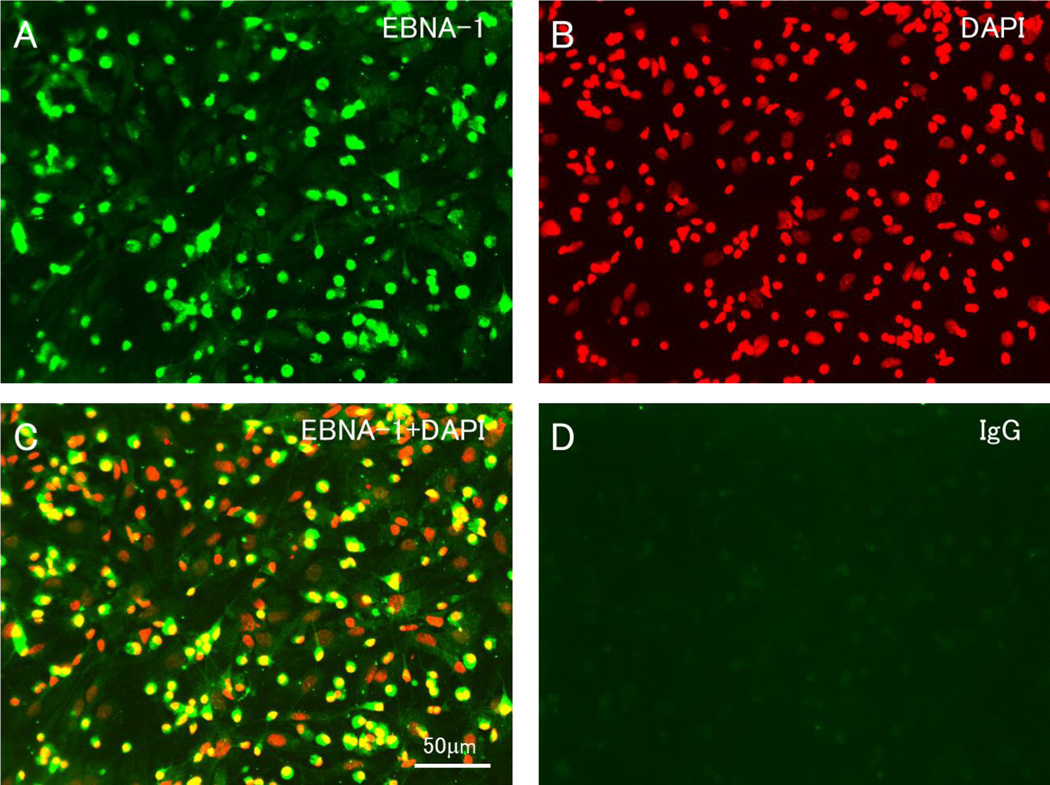
The preceding experiments used HNRNPL protein produced in E. coli, which may not be in its native conformation. HNRNPL in the rat is almost identical to that in human, with the addition of an exon. We investigated the binding of anti-EBNA-1 antibodies isolated from human IgG to cultured cells from rat central nervous system. Although these cells are not infected with EBV, the anti-EBNA-1 antibody bound to an antigen present in the nucleus of most cells (Figure 3). The identity of the nuclear antigen is not known, since anti-EBNA-1 antibody is reported to bind other nuclear antigens in addition to HNRNPL[20–23].
Figure 3. Immunohistochemistry with EBNA-1 specific IgG.
EBNA-1 specific IgG labeled the nucleus of most cells in mixed cell cultures containing neurons, astrocytes, oligodendroglia and microglia derived from prenatal rat brain. This was not seen with equal concentrations of unselected IgG. A. staining with anti-EBNA-1. B. DAPI. C. combined anti-EBNA-1 and DAPI. D. unselected IgG.
3.3 ELISA for HNRNPL and EBNA-1 with MS and control plasma
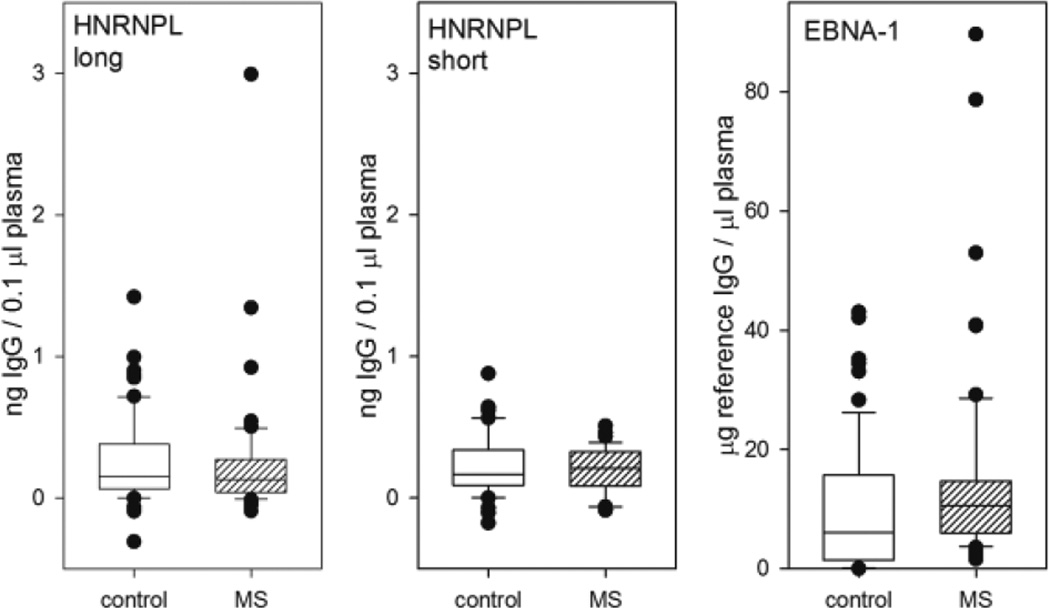
As anti-EBNA-1 antibodies are consistently increased in MS, we tested whether anti-HNRNPL antibodies are also increased in MS. We found that the antibody response to the long isoform of HNRNPL was generally low, with occasional high outliers in both MS and control subjects (Figure 4). There was no significant difference between the groups (p=0.345, rank sum test, n=62 in each group). The antibody response to the short isoform was similarly low, and there was no difference between the groups (p=0.541, t test, n=40 in each group). In contrast, the antibody response to EBNA-1 was vigorous in both groups, and was significantly increased in the MS group (p=0.017, rank sum test, n=62). There was a modest correlation between the response to EBNA-1 and HNRNPL long isoform (r=0.264, p=0.0031, Spearman rank order correlation), but not between EBNA-1 and the short isoform (r=0.103, p=0.363). The response to the long and short isoforms of HNRNPL was not correlated (r=0.0312, p=0.78).
Figure 4. ELISA with HNRNPL and EBNA-1.
Box plots comparing antibody concentration in controls and MS for three antigens. There is no significant difference between MS and controls in the antibody response to either the long or short isoforms of HNRNPL (n=62 in each group for the long form, n=40 for the short form). Antibody response to EBNA-1 is increased in MS, as expected (p=0.017, rank sum test, n=62 in each group). Box encloses 25th to 75th percentile, whiskers indicate 10th and 90th percentile, individual outliers shown by symbols, line in box indicates median.
4. Discussion
EBV is a ubiquitous human virus which has been associated with MS and other autoimmune diseases, including systemic lupus erythematosus and rheumatoid arthritis. Molecular mimicry or cross-reactivity of human and virus proteins is one potential mechanism by which EBV could contribute to autoimmunity. EBNA-1 is an attractive target for investigation of cross-reactivity since it is a major antigen for the antibody response and anti-EBNA-1 antibodies are significantly increased in MS. We identified a human protein, HNRNPL, which cross-reacts with EBNA-1 IgG as demonstrated by immunoprecipitation, ELISA, and Western blot. Heterogeneous nuclear ribonucleoproteins (hnRNP) are a conserved family of proteins that form complexes with RNA transcripts in the nucleus, and play roles in the formation, packaging, and processing of mRNA. A region in the N-terminal exon of the long isoform of HNRNPL has a high level of identity to one of the extensive stretches of glycinealanine repeats in EBNA-1. Based on the ELISA and immunoprecipitation results, the long isoform of this protein appears to be a frequent autoantigen. This finding is in concordance with a previous report that autoantibodies to HNRNPL were common in healthy Chinese individuals[24]. The short form of HNRNPL, which omits the N-terminal, glycine-rich sequences, does not cross-react with EBNA-1 and is not detected on Western blots with human IgG.
Although HNRNPL is a target of autoantibodies, it is not clearly relevant to MS. First, the antibody response is not increased in MS relative to controls. It is possible that the T cell response to HNRNPL is increased in MS, but this remains to be investigated. Second, one would expect an autoantigen relevant to MS would be expressed primarily in the central nervous system, while HNRNPL is expected to be expressed in all tissues. Although reasonable, this expectation may be incorrect, as there are instances in which autoimmunity against a widely expressed antigen results in an organ-specific disease[25, 26].
The current findings have interesting similarities to previous studies of autoimmunity. Antibodies against the human T-lymphotropic virus 1 (HTLV-1) tax protein cross-react with a different human heterogeneous nuclear ribonucleoprotein (hnRNP A1), and this cross-reactivity is proposed to play a pathogenic role in HTLV1 associated myelopathy[27, 28]. Also, autoantibodies to HNRNPL have been previously reported in a small fraction of scleroderma patients[29], and a pathogenic role of anti-HNRNL autoimmunity is more plausible in that disease. Finally, there are reports linking MS and autoimmunity to various hnRNP, including B1, A1, A2/B1, and [30–32].
Cross-reactivity of HNRNPL and EBNA-1 has not previously been reported. But there is an extensive literature on EBNA-1 antibody cross-reactivity with other self proteins. These have been demonstrated in autoimmune diseases or as a transient phenomenon during infectious mononucleosis. In systemic lupus, EBNA-1 antibodies cross-react with multiple antigens, including the 60 kDa Ro protein, the small nuclear ribonucleoprotein SmD1, and the small nuclear ribonucleoprotein-associated proteins Sm B/B’[21, 23, 33]. In rheumatoid arthritis, anti-EBNA-1 antibodies cross-react with collagen[34]. Most relevant to the current work are a series of papers showing cross-reactivity of EBNA-1 antibodies with RALY, a 32.5 kDa heterogeneous nuclear ribonucleoprotein[35–38]. The cross-reactive epitope in RALY was a C-terminal glycine-rich region, and these autoantibodies were identified in subjects with multiple sclerosis, systemic sclerosis, lupus, and inflammatory bowel disease. We immunoprecipitated Ro with anti-EBNA-1 antibodies derived from one of the healthy control samples, but we did not see any of the other proteins.
In summary, we demonstrate that HNRNPL is a novel autoantigen which is crossreactive with EBNA-1. IgG antibodies to HNRNPL can be found in both healthy controls and in patients with MS. The relevance of these autoantibodies to MS or other autoimmune disease remains to be demonstrated.
Supplementary Material
Highlights.
Epstein-Barr virus (EBV) is associated with multiple sclerosis (MS) and other autoimmune diseases.
Antibodies to the EBV nuclear antigen-1 (EBNA-1) are significantly increased in MS compared to healthy controls.
Antibodies specific for EBNA-1 immunoprecipitate heterogeneous nuclear ribonucleoprotein L (HNRNPL) from proteins solubilized from human brain tissue.
Western blots and ELISA with recombinant protein confirmed that anti-EBNA-1 IgG cross-reacts with HNRNPL.
Anti-EBNA-1 IgG also binds the nucleus of cultured rat central nervous system cells.
Acknowledgements
Ms. Pate was generously supported by a student summer research fellowship from the Foundation of the Consortium of Multiple Sclerosis Centers. Dr. Zhao supported in part by NIH-NINDS R01-NS064109.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ascherio A, Munch M. Epstein-Barr virus and multiple sclerosis. Epidemiology. 2000;11:220–224. doi: 10.1097/00001648-200003000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Almohmeed YH, Avenell A, Aucott L, Vickers MA. Systematic review and meta-analysis of the sero-epidemiological association between Epstein Barr virus and multiple sclerosis. PLoS One. 2013;8:e61110. doi: 10.1371/journal.pone.0061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handel AE, Williamson AJ, Disanto G, Handunnetthi L, Giovannoni G, Ramagopalan SV. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One. 2010;5:e12496. doi: 10.1371/journal.pone.0012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundstrom P, Juto P, Wadell G, Hallmans G, Svenningsson A, Nystrom L, et al. An altered immune response to Epstein-Barr virus in multiple sclerosis: a prospective study. Neurology. 2004;62:2277–2282. doi: 10.1212/01.wnl.0000130496.51156.d7. [DOI] [PubMed] [Google Scholar]
- 5.DeLorenze GN, Munger KL, Lennette ET, Orentreich N, Vogelman JH, Ascherio A. Epstein-Barr virus and multiple sclerosis: evidence of association from a prospective study with long-term follow-up. Arch Neurol. 2006;63:839–844. doi: 10.1001/archneur.63.6.noc50328. [DOI] [PubMed] [Google Scholar]
- 6.Munger K, Levin L, O'Reilly E, Falk K, Ascherio A. Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: a prospective study among United States military personnel. Mult Scler. 2011;17:1185–1193. doi: 10.1177/1352458511408991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunemann JD, Tintore M, Messmer B, Strowig T, Rovira A, Perkal H, et al. Elevated Epstein-Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann Neurol. 2010;67:159–169. doi: 10.1002/ana.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsey JW, Hatfield LM, Vu T. Epstein-Barr virus neutralizing and early antigen antibodies in multiple sclerosis. Eur J Neurol. 2010;17:1263–1269. doi: 10.1111/j.1468-1331.2010.03005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serafini B, Rosicarelli B, Franciotta D, Magliozzi R, Reynolds R, Cinque P, et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serafini B, Severa M, Columba-Cabezas S, Rosicarelli B, Veroni C, Chiappetta G, et al. Epstein-Barr Virus Latent Infection and BAFF Expression in B Cells in the Multiple Sclerosis Brain: Implications for Viral Persistence and Intrathecal B-Cell Activation. J Neuropathol Exp Neurol. 2010;69:677–693. doi: 10.1097/NEN.0b013e3181e332ec. [DOI] [PubMed] [Google Scholar]
- 11.Serafini B, Muzio L, Rosicarelli B, Aloisi F. Radioactive in situ hybridization for Epstein-Barr virus-encoded small RNA supports presence of Epstein-Barr virus in the multiple sclerosis brain. Brain. 2013 doi: 10.1093/brain/aws315. [DOI] [PubMed] [Google Scholar]
- 12.Tzartos JS, Khan G, Vossenkamper A, Cruz-Sadaba M, Lonardi S, Sefia E, et al. Association of innate immune activation with latent Epstein-Barr virus in active MS lesions. Neurology. 2012;78:15–23. doi: 10.1212/WNL.0b013e31823ed057. [DOI] [PubMed] [Google Scholar]
- 13.Lassmann H, Niedobitek G, Aloisi F, Middeldorp JM. Epstein-Barr virus in the multiple sclerosis brain: a controversial issue--report on a focused workshop held in the Centre for Brain Research of the Medical University of Vienna, Austria. Brain. 2011;134:2772–2786. doi: 10.1093/brain/awr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito M, Venkatesh V, Otvos L, Weng Z, Vajda S, Banki K, et al. Human transaldolase and cross-reactive viral epitopes identified by autoantibodies of multiple sclerosis patients. J Immunol. 1999;163:4027–4032. [PubMed] [Google Scholar]
- 15.Sospedra M, Martin R. Molecular mimicry in multiple sclerosis. Autoimmunity. 2006;39:3–8. doi: 10.1080/08916930500484922. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Song S, Sun G, Strong R, Zhang J, Grotta JC, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15819–15827. doi: 10.1523/JNEUROSCI.3776-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitson JS, Kampfl A, Zhao X, Hayes RL. Time course of neurofilament protein loss following depolarization-induced injury in CNS culture. Neuroscience letters. 1995;197:159–163. doi: 10.1016/0304-3940(95)11921-i. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 19.Poole BD, Scofield RH, Harley JB, James JA. Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity. 2006;39:63–70. doi: 10.1080/08916930500484849. [DOI] [PubMed] [Google Scholar]
- 20.Yadav P, Tran H, Ebegbe R, Gottlieb P, Wei H, Lewis RH, et al. Antibodies elicited in response to EBNA-1 may cross-react with dsDNA. PLoS One. 2011;6:e14488. doi: 10.1371/journal.pone.0014488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nature medicine. 2005;11:85–89. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 22.Sundar K, Jacques S, Gottlieb P, Villars R, Benito ME, Taylor DK, et al. Expression of the Epstein-Barr virus nuclear antigen-1 (EBNA-1) in the mouse can elicit the production of anti-dsDNA and anti-Sm antibodies. Journal of autoimmunity. 2004;23:127–140. doi: 10.1016/j.jaut.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Sabbatini A, Bombardieri S, Migliorini P. Autoantibodies from patients with systemic lupus erythematosus bind a shared sequence of SmD and Epstein-Barr virus-encoded nuclear antigen EBNA I. Eur J Immunol. 1993;23:1146–1152. doi: 10.1002/eji.1830230525. [DOI] [PubMed] [Google Scholar]
- 24.Li WH, Zhao J, Li HY, Liu H, Li AL, Wang HX, et al. Proteomics-based identification of autoantibodies in the sera of healthy Chinese individuals from Beijing. Proteomics. 2006;6:4781–4789. doi: 10.1002/pmic.200500909. [DOI] [PubMed] [Google Scholar]
- 25.Yeaman SJ, Fussey SP, Danner DJ, James OF, Mutimer DJ, Bassendine MF. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988;1:1067–1070. doi: 10.1016/s0140-6736(88)91894-6. [DOI] [PubMed] [Google Scholar]
- 26.Ito Y, Hashimoto M, Hirota K, Ohkura N, Morikawa H, Nishikawa H, et al. Detection of T cell responses to a ubiquitous cellular protein in autoimmune disease. Science. 2014;346:363–368. doi: 10.1126/science.1259077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SM, Dunnavant FD, Jang H, Zunt J, Levin MC. Autoantibodies that recognize functional domains of hnRNPA1 implicate molecular mimicry in the pathogenesis of neurological disease. Neuroscience letters. 2006;401:188–193. doi: 10.1016/j.neulet.2006.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin MC, Lee SM, Kalume F, Morcos Y, Dohan FC, Jr, Hasty KA, et al. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nature medicine. 2002;8:509–513. doi: 10.1038/nm0502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siapka S, Patrinou-Georgoula M, Vlachoyiannopoulos PG, Guialis A. Multiple specificities of autoantibodies against hnRNP A/B proteins in systemic rheumatic diseases and hnRNP L as an associated novel autoantigen. Autoimmunity. 2007;40:223–233. doi: 10.1080/08916930701352357. [DOI] [PubMed] [Google Scholar]
- 30.Sueoka E, Yukitake M, Iwanaga K, Sueoka N, Aihara T, Kuroda Y. Autoantibodies against heterogeneous nuclear ribonucleoprotein B1 in CSF of MS patients. Ann Neurol. 2004;56:778–786. doi: 10.1002/ana.20276. [DOI] [PubMed] [Google Scholar]
- 31.Yukitake M, Sueoka E, Sueoka-Aragane N, Sato A, Ohashi H, Yakushiji Y, et al. Significantly increased antibody response to heterogeneous nuclear ribonucleoproteins in cerebrospinal fluid of multiple sclerosis patients but not in patients with human Tlymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. J Neurovirol. 2008;14:130–135. doi: 10.1080/13550280701883840. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Xu L, Shin Y, Gardner L, Hartzes A, Dohan FC, et al. A potential link between autoimmunity and neurodegeneration in immune-mediated neurological disease. J Neuroimmunol. 2011;235:56–69. doi: 10.1016/j.jneuroim.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 33.McClain MT, Rapp EC, Harley JB, James JA. Infectious mononucleosis patients temporarily recognize a unique, cross-reactive epitope of Epstein-Barr virus nuclear antigen-1. J Med Virol. 2003;70:253–257. doi: 10.1002/jmv.10385. [DOI] [PubMed] [Google Scholar]
- 34.Birkenfeld P, Haratz N, Klein G, Sulitzeanu D. Cross-reactivity between the EBNA-1 p107 peptide, collagen, and keratin: implications for the pathogenesis of rheumatoid arthritis. Clin Immunol Immunopathol. 1990;54:14–25. doi: 10.1016/0090-1229(90)90002-8. [DOI] [PubMed] [Google Scholar]
- 35.Vaughan JH, Nguyen MD, Valbracht JR, Patrick K, Rhodes GH. Epstein-Barr virus-induced autoimmune responses. II. Immunoglobulin G autoantibodies to mimicking and nonmimicking epitopes. Presence in autoimmune disease. J Clin Invest. 1995;95:1316–1327. doi: 10.1172/JCI117782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaughan JH, Valbracht JR, Nguyen MD, Handley HH, Smith RS, Patrick K, et al. Epstein-Barr virus-induced autoimmune responses. I. Immunoglobulin M autoantibodies to proteins mimicking and not mimicking Epstein-Barr virus nuclear antigen-1. J Clin Invest. 1995;95:1306–1315. doi: 10.1172/JCI117781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaughan JH, Riise T, Rhodes GH, Nguyen MD, Barrett-Connor E, Nyland H. An Epstein Barr virus-related cross reactive autoimmune response in multiple sclerosis in Norway. J Neuroimmunol. 1996;69:95–102. doi: 10.1016/0165-5728(96)00069-0. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes GH, Valbracht JR, Nguyen MD, Vaughan JH. The p542 gene encodes an autoantigen that cross-reacts with EBNA-1 of the Epstein Barr virus and which may be a heterogeneous nuclear ribonucleoprotein. Journal of autoimmunity. 1997;10:447–454. doi: 10.1006/jaut.1997.9996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.