Abstract
Background
Irinotecan has a 20-25% response rate (RR) in patients with previously treated metastatic breast cancer (MBC). Epidermal growth factor receptor (EGFR) is overexpressed in some MBC, especially in triple-negative breast cancer (TNBC). Cetuximab is a monoclonal antibody against EGFR with additive activity preclinical to irinotecan.
Materials and Methods
We report a one-stage phase II study on MBC, measurable disease, and prior anthracycline and/or taxane therapy. Patients received cetuximab 400 mg/m2 on day 1 cycle 1 then 250 mg/m2 weekly thereafter and irinotecan 80 mg/m2 on days 1 and 8 of each 21-day cycle. Primary endpoint was overall response rate (ORR) by RECIST criteria.
Results
19 eligible patients enrolled from February to September 2006; 74% had visceral disease, 37% were hormone receptor positive, 11% HER2+, and 58% triple negative. Patients received a median of 2 cycles (range: 1-37). Confirmed ORR was 11% (95% CI: 1-33%), with 1 PR and 1 CR. One patient had stable disease (SD) for 8 months. RR for TNBC vs non-TNBC was 18% vs 0% (p=0.49). Median time to progression was 1.4 mo (95% CI: 1.0-2.2) and median overall survival was 9.4 mo (95% CI: 2.8-16.1). 12 patients progressed on therapy within 2 cycles. Due to low response rate and rapid progression, the study leadership decided to close the trial early.
Conclusion
The tolerability of the combination of cetuximab and irinotecan is acceptable but demonstrated low overall activity. Potentially promising results were noted in patients with TNBC and further studies of these patients may be considered.
Keywords: breast cancer, metastatic, EGFR, cetuximab, irinotecan
INTRODUCTION
Anthracyclines and taxanes are two of the most active chemotherapeutic agents used for treating breast cancer.1 The widespread use of these agents for the treatment of early-stage breast cancer often results in the emergence of resistant tumor clones at the time of disease-recurrence, thereby reducing the number of treatment options for metastatic breast cancer (MBC). Even when these agents can be used to treat MBC, treatment failure occurs in most cases. As a result, the median survival of MBC from diagnosis is approximately 18 to 29 months (mo) and the 5-year survival rate of MBC is less than 25%.2-5 These data outline the tremendous need for new, effective treatments for MBC, and have led to the investigation of novel agents that target tumor biology in the hope of overcoming the problem of drug resistance and improving overall survival (OS).
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is involved in cellular proliferation, cellular differentiation, motility, survival, and tissue development. EGFR is overexpressed in MBC, especially in triple-negative breast cancer (TNBC) and to lesser degree in HER2-positive breast tumors.6-8 In pre-clinical models, EGFR expression is associated with aggressive phenotypes. In a clinical review of 475 EGFR-overexpressing tumors, it was associated with tumors with high proliferation and in younger, predominantly African American patients, with lower disease-free survival and OS, especially in previously treated patients.9 TNBC lacks the expression of estrogen (ER) and progesterone (PR) receptors as well as HER2, comprises approximately 15-20% of all breast cancers, and tends to occur at higher frequency in younger women, and African American and Hispanic women. Although TNBC is responsive to chemotherapy; it tends to be more aggressive, with early relapse and a shorter median time from relapse to death.10-18 EGFR is overexpressed in 54-91% of the basal-like TNBC.8, 19-22
Cetuximab is a recombinant human-mouse chimeric monoclonal antibody that binds specifically to EGFR, thus competitively inhibiting epidermal growth factor and other ligands.23 This blocks the phosphorylation and activation of receptor-associated tyrosine kinases, leading to tumoricidal effects.24 Preclinical studies have demonstrated growth suppression of EGFR-overexpressing tumors.25-28
Irinotecan is an inhibitor of topoisomerase I, an enzyme necessary for DNA replication. The irinotecan metabolite, SN-38, prevents topoisomerase I enzyme from resealing to the DNA during replication and transcription by binding to the topoisomerase I-DNA complex.29 This causes DNA breaks and induces apoptosis. We have previously reported the efficacy and good tolerability of single agent irinotecan in refractory MBC in a randomized phase II trial, NCCTG 96-32-55.30 The overall response rate (ORR) was 23% (one CR, one PR; 95% CI, 8.0 to 14.2 months). Median duration of response (DR) and OS was 4.9 months and 8.6 months (95% CI, 7.0-12.3 months), respectively. In patients with irinotecan-refractory metastatic colorectal cancer, the combination of cetuximab and irinotecan demonstrated a longer median time to progression (TTP) and survival time as compared to cetuximab alone.31 Thus we hypothesized that a combination of cetuximab plus irinotecan would have synergistic effect in breast cancer, similar to its activity in metastatic colon cancer.31 The results of other trials investigating dual combinations of cetuximab with carboplatin, cisplatin, irinotecan/carboplatin, or paclitaxel had not been reported during the planning and conduction of this study and are briefly outlined in the discussion section.
We present here the mature results of the North Central Cancer Treatment Group (NCCTG) study (Alliance) N0436, a multi-center phase II clinical trial designed to assess the antitumor activity and toxicity of the combination of the anti-EGFR monoclonal antibody, cetuximab in combination with irinotecan in patients with metastatic breast cancer previously exposed to anthracycline and/or taxane-containing therapy.
PATIENTS AND METHODS
Patient Selection
Patients with histologically confirmed adenocarcinoma of the breast and clinical manifestations of metastatic disease were eligible for this trial. Measurable disease was required. Prior treatment in the metastatic or adjuvant setting must have included an anthracycline and/or a taxane and patients must have had an ECOG performance status of 0, 1 or 2. Laboratory value requirements within 14 days prior to registration included hemoglobin >8.0 g/dL, absolute neutrophil count ≥1500/mL, platelet count ≥ 100,000/mL, creatinine ≤1.5 × upper limit of normal (ULN), total bilirubin ≤ULN, aspartate aminotransferase (AST) ≤5 x ULN and alanine transaminase (ALT) ≤5 × ULN. Other inclusion criteria included age ≥18 years, 0-2 prior chemotherapy regimens for metastatic disease, at least one prior trastuzumab-containing regimen in patients whose tumor was HER2 positive (unless contraindicated), negative pregnancy test within 7 days prior to registration, absence of other invasive non-breast malignancies for ≥3 years, and a life expectancy of >3 months (mo). Patients also had to submit one pretreatment blood sample for UGT polymorphism testing.
Exclusion criteria included prior therapy with irinotecan, an EGFR antagonist, or a dual EGFR/HER2 inhibitor. Major surgery ≤3 weeks, chemotherapy ≤2 weeks, or radiotherapy ≤4 weeks prior to registration was prohibited. Other contraindications included any serious concomitant medical condition that might interfere with treatment, known CNS metastasis unless controlled by prior surgery and/or radiotherapy, New York Heart Association (NYHA) class III or IV cardiovascular disease, history or evidence of Gilbert’s syndrome, and active unresolved infection. Patients with a history of allergy or hypersensitivity to drug product excipients, murine antibodies, or agents chemically similar to irinotecan and/or cetuximab were not enrolled in this study. Women who were pregnant, nursing, or unwilling to use adequate contraception were also excluded.
The study was approved by local institutional review boards, and written informed consent was obtained from all patients before they were enrolled.
Study Treatment
Patients were treated with cetuximab 400 mg/m2 IV on day 1 cycle 1 followed by 250 mg/m2 IV weekly. Irinotecan was dosed at 80 mg/m2 IV on days 1 and 8 of a 21-day cycle. The dose of irinotecan could be escalated to 100 mg/m2 after cycle 1 if the patient experienced minimal adverse events, as judged by the patient and investigator. Pretreatment included diphenhydramine hydrochloride 50 mg IV prior to each dose of cetuximab. Patients were treated until disease progression, unacceptable adverse events, or patient refusal.
Response Assessment
Response assessment was based on RECIST (Response Evaluation Criteria in Solid Tumors). Tumor measurement was completed prior to every other cycle of treatment (i.e., before cycle 3, 5, 7, etc.) if done by CT or MRI, or every cycle if the lesion was clinically measurable. Correlative blood samples were obtained for pre-treatment UGT polymorphism testing.
End Point and Statistical Analysis
This study was a single stage, phase II trial designed to assess the efficacy of concurrent irinotecan and cetuximab in patients with advanced breast cancer with prior exposure to anthracycline and/or taxane containing therapy. The primary endpoint of this trial was confirmed overall response rate (ORR), where a response was defined as either a complete response (CR) or partial response (PR) by the RECIST criteria on two consecutive evaluations at least 6 weeks apart. The study was designed to test the null hypothesis that the ORR was at most 20% versus the alternative that it was at least 40%. With a power of 0.91 and a significance level of 0.09, a total of 36 evaluable patients were required to evaluate the decision criteria. The proportion of responses was estimated by the number of patients who achieved a response divided by the total number of evaluable patients. A 95% binomial confidence interval was calculated to estimate the true ORR.
Secondary endpoints included clinical benefit rate (CBR), time to progression (TTP), overall survival (OS), duration of response (DOR), and assessment of adverse events (AEs) using the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. CBR was defined as the proportion of patients who achieved a response or prolonged stable disease (objective status of SD for >6 months). TTP was defined as the time from registration to documentation of disease progression. If a patient died without progression, the patient was considered to have had progression at the time of their death, unless there was sufficient documented evidence to conclude that no progression occurred prior to death. OS was defined as the time from registration to death due to any cause. DOR was defined as the time from initial response to documentation of disease progression. TTP, OS and DOR were estimated using the Kaplan-Meier method.32 Toxicity was defined as an AE at least possibly related to treatment and rates were estimated by using the highest grade of toxicity observed for each patient during the course of treatment.
Planned monitoring per Alliance guidelines included the data and safety monitoring board review every 6 months. Toxicity was monitored at all patient visits. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on October 17, 2011.
RESULTS
Patient Characteristics
A total of 21 patients were accrued to this study between February 2006 and September 2006. Two patients who did not receive any therapy were excluded from all analyses. These 19 eligible patients had a median age of 49 years (range: 28-76) and 14 (74%) patients had visceral disease. Seven (37%) patients were ER and/or PR positive, 2 (11%) patients were HER2 positive, and 11 (58%) patients were triple-negative. Seventeen (89%) patients had prior neoadjuvant or adjuvant chemotherapy and 15 (79%) patients had prior chemotherapy for metastatic disease. The median follow-up time was 30 mo (28.6-31.5 mo). A summary of patient characteristics is outlined in Table 1.
Table 1.
Patient Characteristics
| N (%)* | |
|---|---|
| Age (yr) Median (Min, Max) |
49 (28, 76) |
| ECOG performance score 0 1 2 |
9 (47%) 8 (42%) 2 (11%) |
| Dominant disease Visceral Non-visceral |
14 (74%) 5 (26%) |
| Prior hormonal therapy in the metastatic setting Yes No |
5 (26%) 14 (74%) |
| Neoadjuvant and/or adjuvant chemotherapy Yes No |
17 (89%) 2 (11%) |
| Number prior metastatic chemotherapy regimens 0 1 2 |
4 (21%) 11 (58%) 4 (21%) |
| ER status Positive Negative |
6 (32%) 13 (68%) |
| PR status Positive Negative |
5 (26%) 14 (74%) |
| HER2 status Positive Negative Not done |
2 (11%) 16 (84%) 1 (5%) |
| HER2 method IHC FISH Both Not done |
9 (47%) 4(21%) 5(26%) 1 (5%) |
| Sites of metastases Nodes (Excluding axillary) Nodes - Axillary Liver Skin Abdomen Bone Brain Lung Chest wall Other (1 pleural fluid, 1 left supraclavicular node) |
8 (42%) 6 (32%) 7 (37%) 4 (21%) 1 (5%) 8 (42%) 1 (5%) 8 (42%) 5(26%) 2 (11%) |
Unless noted otherwise
Efficacy
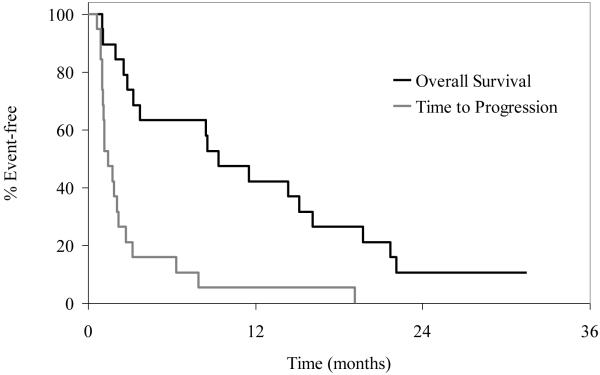
All19 patients were evaluable for response. OS and TTP distributions appear in Figure 1. The confirmed ORR was 11% (95% CI, 1-33), with 1 CR and 1 PR. The patient who achieved a CR had duration of response of 18 mo. The patient who achieved a PR had duration of response of 5 mo. One patient had prolonged SD with duration of 8 mo and was still alive at 31 mo. The clinical benefit rate (CR + PR +SD>6 mo) was 16% (95% CI, 3-40%). RR for TNBC vs non-TNBC was 18% vs 0% (p=0.49). Median OS was 9.4 mo (95% CI: 2.8 -16.1 mo) and the 1-year survival rate was 42%. Median time to progression was 1.4 mo (95% CI: 1.0-2.2 mo). (Figure 1) Patients received a median of 2 cycles (range: 1-37) of treatment. The majority of patients (18 patients, 95%) went off study due to disease progression and 1 patient went off study due to adverse events (persistent dermal and mucosal adverse events). The majority of patients (16/19, 84%) had disease progression within the first 4 cycles (12 patients developed tumor progression within the first 2 cycles). The trial was closely monitored every six months per NCCTG guidelines and upon observation of this high rate of early progression and limited objective response rate; the decision was made by the NCCTG study team to permanently close the study early on January 19, 2007 after 19 evaluable patients were enrolled.
Figure 1.
Kaplan-Meier curves for OS and TTP
Safety
Overall, 14 (74%) patients had grade 2 or 3 dermatologic toxicities, including the 3 patients who experienced a response (1CR, 1PR and 1SD). Patients with grade 2/3 dermatologic toxicity had a clinical benefit rate of 21% (3/14) vs 0% (0/5) in patients with grade 0/1 (p=0.53). However, the small sample size prevents any meaningful conclusion of this observation. Toxicity data were available for all 19 patients treated on study. Table 2 outlines all grade 3/4 toxicities in the study. One patient (5%) experienced grade 4 toxicities (leukopenia, neutropenia, and febrile neutropenia) and 8 patients had a maximum of grade 3 toxicity. Common grade 3 toxicities included acne/acneiform rash (21%) and rash/desquamation (11%).
Table 2.
Grade 3/4 Toxicities (At least possibly treatment related)
| Body System | Toxicity | Grade | |||
|---|---|---|---|---|---|
| 3 | 4 | ||||
| N | % | N | % | ||
| Hematology | Leukopenia | 1 | 5.3 | ||
| Neutropenia | 1 | 5.3 | |||
| Hepatic | SGPT (ALT) | 1 | 5.3 | ||
| SGOT (AST) | 1 | 5.3 | |||
| Infection | Bladder Infection | 1 | 5.3 | ||
| Febrile Neutropenia | 1 | 5.3 | |||
| Urinary Tract Infection | 1 | 5.3 | |||
| Cellulitis | 1 | 5.3 | |||
| Ungual Infection | 1 | 5.3 | |||
| Metabolic/Laboratory | Hypomagnesemia | 1 | 5.3 | ||
| Musculoskeletal | Muscle Weakness | 1 | 5.3 | ||
| Pain | Headache | 1 | 5.3 | ||
| Pulmonary | Dyspnea | 1 | 5.3 | ||
| Pleural Effusion | 1 | 5.3 | |||
| Constitutional Symptoms | Fatigue | 1 | 5.3 | ||
| Dermatology/Skin | Acne | 4 | 21.1 | ||
| Rash/Desquamation | 2 | 10.5 | |||
| Gastrointestinal | Diarrhea | 1 | 5.3 | ||
| All | 8 | 42.1 | 1 | 5.3 | |
Irinotecan was omitted or held for 4/88 (5%) cycles in 4 patients, primarily due to disease progression. Dose reductions for irinotecan occurred during 1/88 (1%) cycles due to hematologic toxicity. Cetuximab was omitted or held for 18/88 (20%) cycles in 14 patients, with the most common reasons being disease progression and dermatologic adverse events. Dose reductions for cetuximab occurred during 4/88 (5%) cycles in 4 patients, primarily due to dermatologic toxicity. Patients received the full dose of irinotecan on 84/88 (95%) cycles and the full dose of cetuximab on 68/88 (77%) cycles. Table 3 provides a summary of the number of patients who received the full dose of each study drug for each cycle.
Table 3.
Tolerability of Study Regimen
| Cycle | Number of patients treated |
Number (%) of patients receiving full dose of Irinotecan |
Number (%) of patients receiving full dose of Cetuximab |
|---|---|---|---|
| 1 | 19 | 19 (100%) | 15 (79%) |
| 2 | 15 | 13 (87%) | 8 (53%) |
| 3 | 7 | 6 (86%) | 5 (71%) |
| 4 | 5 | 5 (100%) | 3 (60%) |
| 5 | 3 | 3 (100%) | 2 (67%) |
| 6 | 3 | 3 (100%) | 2 (67%) |
Correlative studies
Correlative blood samples were obtained for pre-treatment UGT polymorphism testing. This testing was performed at Mayo Clinic in Rochester, Minnesota in the oncology research lab. The distribution of UGT1A1 alleles is displayed in table 4. Grade 3/4 toxicity was noted in 4/8 (50%) of patients with UGT1A1 allele 66, 5/9(56%) with allele 67 and 77, p=1.00. Grade 2 and 3 rash was noted in 7/8 (88%) for allele 66, 5/9(56%) for allele 67 and 77, p=0.29. Overall, no correlation of UGT1A1 polymorphism was noted with response or toxicity.
Table 4.
UGT1A1 Allele Distribution
| UGT1A1 | |
| 66 (negative) | 8 (42%) |
| 67 (positive) | 7 (37%) |
| 77 (positive) | 2 (11%) |
| Not enough sample for DNA extraction at BAP | 2 (11%) |
DISCUSSION
A small number of phase I/II studies have been conducted in an attempt to capitalize on the EGFR-dependent pathway by inhibiting EGFR and thus improving the outcome of patients with MBC (Table 5). EGFR overexpression is commonly observed in TNBC, especially in the basal-like 2 and mesenchymal stem-like subtypes of the Lehmann subclassification.33 Preclinical studies have demonstrated growth suppression of EGFR-overexpressing TNBC cell lines with the EGFR monoclonal antibody cetuximab.25, 26 A retrospective review by Rivera, et al evaluated the association of platinum agents and cetuximab in advanced TNBC.34 Among the 37 evaluable patients there was a 45.9% ORR. In a phase I study of 12 patients with EGFR-positive MBC, of the 10 evaluable patients for response, 2 patients experienced SD and the remaining 8 patients had PD. Additionally, prohibitive dermatologic toxicity led to premature closure of this trial.35 That trial did not show any obvious correlation between tumor regression and EGFR status or rash. A large phase II trial randomized 173 patients with MBC to cetuximab plus cisplatin (n=115) or cisplatin alone (n=58).36 The ORR was 20% (95%, CI 13-29) with cetuximab and cisplatin vs. 10% (95%, CI 4-21%) with cisplatin alone (OR=2.13, 95%, CI 0.81-5.59, P=0.11). Thirty-one patients whose disease progressed on cisplatin alone were allowed to cross over to the cetuximab-containing therapy. A statistically significant median progression-free survival (PFS) of 3.7 mo in the combination arm vs. 1.5 mo in the cisplatin arm was noted. Although statistically significant PFS values were observed, these numbers did not seem clinically meaningful. Corresponding median OS was 12.9 vs. 9.4 mo (HR 0.82, 95% CI 0.56-1.20, P=0.31). In another randomized phase II trial for patients with TNBC (TBCRC 001), Carey et al. demonstrated a response rate (RR) of 17% for the combination of cetuximab and weekly carboplatin (area under curve 2) vs. 6% for cetuximab alone. TTP and OS were 2.1 mo and 10.4 mo, respectively, with the combination. Interestingly, although EGFR pathway activation was seen in most TNBC patients, cetuximab therapy caused pathway inhibition in a minority of patients, suggesting alternate mechanisms for pathway activation. 37 In the randomized phase II US Oncology 25200 clinical trial, the combination of irinotecan and carboplatin plus cetuximab resulted in an ORR of 49% vs. 30% with irinotecan and carboplatin alone in a subset of 72 TNBC patients. However, there was no significant difference in the PFS between the two arms. A higher proportion of patients experienced side effects such as diarrhea, neutropenia and rash.38
Table 5.
Studies of Cetuximab in Breast Cancer
| Author | Trial | Phase | Regimen | Line of Treatment |
Population | Number of Patients |
ORR (%) |
PFS (mo) |
OS (mo) |
|---|---|---|---|---|---|---|---|---|---|
| Baselga et al.36 | BALI-1 | II | Cisplatin ± Cetuximab |
First or Second |
TN MBC | 115 58 |
20 10 |
3.7 1.5 |
12.9 9.4 |
| Carey et al.37 | TBCRC 001 |
II | Cetuximab ± Carboplatin* |
First | TN MBC | 71 54 |
17 6 |
2.1+ | 10.4 |
| O’Shaughnessy et al.38 |
US Oncology 25200 |
II | Irinotecan + Carboplatin ± Cetuximab |
First or Second |
TN MBC | 39 33 |
49 30 |
4.7 5.1 |
15.5 12.3 |
| Modi et al.35 | I | Cetuximab +Paclitaxel |
First or Second |
EGFR+ MBC |
12 | ||||
| Rivera et al.44 | Retrospective | Cisplatin or Carboplatin + Cetuximab |
TN MBC | 2.3 | 7.7 | ||||
| Crozier et al. | N0436 | II | Cetuximab + Irinotecan |
Second | MBC | 19 | 11 | 1.4 | 9.4 |
ORR – Overall Response Rate, PFS – Progression Free Survival, OS – Overall Survival, MBC – metastatic breast cancer, TN – triple negative
Crossover to cetuximab + carboplatin arm after progressive disease
For entire cohort
In our study, the combination of cetuximab plus irinotecan demonstrated a CBR of 16% and ORR of 11%. Sixteen patients (84%) developed tumor progression within 4 cycles, of which 12 (63%) patients progressed within the first 2 cycles. RR and CBR for TNBC versus non-TNBC was 18% vs 0% (p=0.49) and 27% vs 0% (p=0.23), respectively. Although the small sample size of this study prevents any meaningful conclusion of this observation, these data are intriguing.
As noted, due to the observed low response rate and rapid progression, the study leadership decided to close the trial early on January 19, 2007. We continued follow-up for secondary endpoints, completed the pharmacogenomic studies of UGT1A1 polymorphisms, and now have completed all analyses. UGT1A1 enzyme is important in the glucuronidation of the active metabolite of irinotecan, SN-38 and polymorphisms for this enzyme are common. For example, 55% of Caucasians carry the wild-type (6/6/genotype) allele of the gene coding for the enzyme while 35-45% have a single polymorphism (6/7 genotype) and 10% have the (7/7) genotype39. Decreased glucuronidation of SN-38 by the 6/7 or 7/7/genotype has been reported to be associated with increased toxicity related to irinotecan40 such as neutropenia and diarrhea. In our study, no significant correlation was noted between UGT1A1 polymorphisms and toxicity or response, which may be due to the relatively small number of patients enrolled in the study. Median time to progression in the current study was only 1.4 months compared to 2.8 months in the weekly single agent irinotecan trial; median OS was 9.4 months, which is comparable to the median OS of 9.7 months noted in the weekly irinotecan regimen.30 Notwithstanding the comparison across small trials, the results seem to indicate a lack of benefit in this unselected population, although caveats are that we did not extend the studies to patients with TNBC and did not have tumor biopsies to assess whether EGFR measurement had any impact on efficacy or whether molecular analysis could have determined the subtypes of TNBC where activity was suggested.
More research regarding novel formulations of irinotecan have been conducted since we completed this small phase II trial. Etirinotecan pegol (NKTR-102) is a novel long-acting topoisomerase I-inhibitor engineered to concentrate in the tumor tissue, thereby providing sustained drug exposure throughout the chemotherapy cycle.41 In an open label phase II study evaluating the safety and efficacy of two etirinotecan pegol dosing schedules (145 mg/m2 every 14 days or every 21 days) in 70 patients with MBC who had received ≤2 prior chemotherapy regimens, the overall response rate (primary endpoint) was reported to be 29% (95% CI 18.4-40.6).41 This study led to the multicenter, randomized, phase III BEACON study comparing etirinotecan pegol to treatment of physician’s choice per standard of care in advanced breast cancer patients who had previously received an anthracycline, taxane and capecitabine (NCT01492101).42 In this study, blood samples were collected for circulating tumor cell (CTC) evaluation of potential target-specific pharmacodynamic biomarkers that would be assessed for their ability to predict clinical response; these results were presented at the 2014 San Antonio Breast Cancer Symposium.43 Future trials evaluating CTCs for prognostic and predictive biomarkers may facilitate development of tailored treatments for MBC patients.
It is possible that the low activity of our trial may be the result of poor patient selection, small patient cohort, or the inclusion of patients with chemo-refractory disease, although Carey et al. in their study did not find a correlation between response and the number of prior therapies.37 The results of our study, and the less than impressive results of other small phase II published studies exploring EGFR blockade in MBC, lead us to hypothesize that EGFR overexpression may not be a strong driver event in breast cancer or that downstream activation of the EGFR pathway (Ras/Raf/MEK/MAPK/ERK/Fos) occurs through parallel pathways. On the other hand, perhaps evaluation of these agents in more specific subpopulations based on molecular profile may be a good consideration.
CONCLUSION
The tolerability of the combination of cetuximab with irinotecan is acceptable. This regimen has low activity in the overall population studied in this trial, although the data for those with TNBC are intriguing. The role of the EGFR pathway in MBC still needs to be elucidated and validated and thus further studies including TNBC patients based on molecular profiling may be considered.
Clinical Practice Points.
Metastatic breast cancer (particularly triple-negative subtype) is associated with poor survival outcomes, illustrating the need for development of novel effective therapies.
EGFR is overexpressed in 54-71% of triple negative breast cancer (TNBC) and to lesser degree in HER2-positive breast tumors. EGFR positivity is reported to be associated with worse prognosis in TNBC.
Cetuximab is a recombinant monoclonal antibody that specifically inhibits EGFR and is currently approved for the treatment of colorectal cancer as well as head and neck cancer.
A randomized phase II trial demonstrated efficacy and tolerability of irinotecan, a topoisomerase I inhibitor, in the treatment of refractory metastatic breast cancer.
The combination of cetuximab and irinotecan demonstrated improved outcomes as compared to cetuximab alone in patients with irinotecan-refractory metastatic colorectal cancer, forming the rationale to explore this combination in metastatic breast cancer.
In our multicenter single-arm phase II study, the combination of irinotecan and cetuximab demonstrated acceptable toxicity but had a low response rate. Patients with TNBC had a higher response rate as compared to non-TNBC.
Future research into the biologic role of the EGFR pathway in TNBC and patient selection based on molecular profiling are required.
ACKNOWLEGEMENTS
Funding sources: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), 1U10CA180790, and 1U10CA180799, as well as the following grants to the legacy North Central Cancer Treatment Group (NCCTG): CA-25224, CA-63848, CA-37404, CA-60276. Additional support was provided by Bristol-Myers Squibb and Imclone systems. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
The following institutions participated in this study: Cedar Rapids Oncology Association, Cedar Rapids, IA; Colorado Cancer Research Program CCOP; Mayo Clinic, Rochester, MN; Mayo Clinic, Scottsdale, AZ; Mayo Clinic, Jacksonville, FL; Minnesota Cooperative Group Outreach Program; Rapid City Regional Hospital, Rapid City, SD; Saint Vincent Hospital – CCOP, Green Bay, WI.
REFERENCES
- 1.Crozier JA, Swaika A. Moreno-Aspitia A. Adjuvant chemotherapy in breast cancer: To use or not to use, the anthracyclines. World J Clin Oncol. 2014;5:529–538. doi: 10.5306/wjco.v5.i3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Kuraya K, Schraml P, Torhorst J, et al. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res. 2004;64:8534–8540. doi: 10.1158/0008-5472.CAN-04-1945. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii11–19. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 4.Gluck S, Arteaga CL, Osborne CK. Optimizing chemotherapy-free survival for the ER/HER2-positive metastatic breast cancer patient. Clin Cancer Res. 2011;17:5559–5561. doi: 10.1158/1078-0432.CCR-10-2051. [DOI] [PubMed] [Google Scholar]
- 5.Harris JR. Diseases of the breast. Lippincott-Raven Publishers; Philadelphia: 1996. [Google Scholar]
- 6.Bhargava R, Gerald WL, Li AR, et al. EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol. 2005;18:1027–1033. doi: 10.1038/modpathol.3800438. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S, Ushiro H, Stoscheck C, Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982;257:1523–1531. [PubMed] [Google Scholar]
- 8.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 9.Rimawi MF, Shetty PB, Weiss HL, et al. Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer. 2010;116:1234–1242. doi: 10.1002/cncr.24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 11.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 12.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 13.Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113:357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 14.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 15.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 16.Huo D, Ikpatt F, Khramtsov A, et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol. 2009;27:4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee J, Han SW, Oh DY, et al. The clinicopathologic characteristics and prognostic significance of triple-negativity in node-negative breast cancer. BMC Cancer. 2008;8:307. doi: 10.1186/1471-2407-8-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol. 2010;23:123–133. doi: 10.1038/modpathol.2009.145. [DOI] [PubMed] [Google Scholar]
- 19.Korsching E, Packeisen J, Agelopoulos K, et al. Cytogenetic alterations and cytokeratin expression patterns in breast cancer: integrating a new model of breast differentiation into cytogenetic pathways of breast carcinogenesis. Lab Invest. 2002;82:1525–1533. doi: 10.1097/01.lab.0000038508.86221.b3. [DOI] [PubMed] [Google Scholar]
- 20.Pintens S, Neven P, Drijkoningen M, et al. Triple negative breast cancer: a study from the point of view of basal CK5/6 and HER-1. J Clin Pathol. 2009;62:624–628. doi: 10.1136/jcp.2008.061358. [DOI] [PubMed] [Google Scholar]
- 21.Sutton LM, Han JS, Molberg KH, et al. Intratumoral expression level of epidermal growth factor receptor and cytokeratin 5/6 is significantly associated with nodal and distant metastases in patients with basal-like triple-negative breast carcinoma. Am J Clin Pathol. 2010;134:782–787. doi: 10.1309/AJCPRMD3ARUO5WPN. [DOI] [PubMed] [Google Scholar]
- 22.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Normanno N, De Luca A, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Mokhtarieh AA, Cheong S, Kim S, Chung BH, Lee MK. Asymmetric liposome particles with highly efficient encapsulation of siRNA and without nonspecific cell penetration suitable for target-specific delivery. Biochim Biophys Acta. 2012;1818:1633–1641. doi: 10.1016/j.bbamem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, Norton L, Masui H, et al. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993;85:1327–1333. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- 26.Oliveras-Ferraros C, Vazquez-Martin A, Lopez-Bonet E, et al. Growth and molecular interactions of the anti-EGFR antibody cetuximab and the DNA cross-linking agent cisplatin in gefitinib-resistant MDA-MB-468 cells: new prospects in the treatment of triple-negative/basal-like breast cancer. Int J Oncol. 2008;33:1165–1176. [PubMed] [Google Scholar]
- 27.Real PJ, Benito A, Cuevas J, et al. Blockade of epidermal growth factor receptors chemosensitizes breast cancer cells through up-regulation of Bnip3L. Cancer Res. 2005;65:8151–8157. doi: 10.1158/0008-5472.CAN-05-1134. [DOI] [PubMed] [Google Scholar]
- 28.Viale G, Rotmensz N, Maisonneuve P, et al. Invasive ductal carcinoma of the breast with the "triple-negative" phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat. 2009;116:317–328. doi: 10.1007/s10549-008-0206-z. [DOI] [PubMed] [Google Scholar]
- 29.Cao Y, Jin ZX, Tong XP, et al. Synergistic effects of topoisomerase I inhibitor, SN38, on Fas-mediated apoptosis. Anticancer Res. 2010;30:3911–3917. [PubMed] [Google Scholar]
- 30.Perez EA, Hillman DW, Mailliard JA, et al. Randomized phase II study of two irinotecan schedules for patients with metastatic breast cancer refractory to an anthracycline, a taxane, or both. J Clin Oncol. 2004;22:2849–2855. doi: 10.1200/JCO.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 33.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivera PFT, Gladieff L, Delord J, Mery E, Roche H, Dalenc F. Efficacy of cetuximab plus platinum agent in advanced, triple-negative breast carcinoma: Results of a retrospective analysis. 2011;29(suppl) abstr e11581. [Google Scholar]
- 35.Modi S, D'Andrea G, Norton L, et al. A phase I study of cetuximab/paclitaxel in patients with advanced-stage breast cancer. Clin Breast Cancer. 2006;7:270–277. doi: 10.3816/CBC.2006.n.040. [DOI] [PubMed] [Google Scholar]
- 36.Baselga J, Gomez P, Greil R, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31:2586–2592. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30:2615–2623. doi: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Shaughnessy JWD, Vukelja SJ, McIntyre K, Krekow L, Holmes FA, Asmar L, Blum JL. Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer. Breast Cancer Res Treat. 2007;106:S132–S133. [Google Scholar]
- 39.Lampe JW, Bigler J, Horner NK, Potter JD. UDP-glucuronosyltransferase (UGT1A1*28 and UGT1A6*2) polymorphisms in Caucasians and Asians: relationships to serum bilirubin concentrations. Pharmacogenetics. 1999;9:341–349. doi: 10.1097/00008571-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Iyer L, Das S, Janisch L, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2002;2:43–47. doi: 10.1038/sj.tpj.6500072. [DOI] [PubMed] [Google Scholar]
- 41.Awada A, Garcia AA, Chan S, et al. Two schedules of etirinotecan pegol (NKTR-102) in patients with previously treated metastatic breast cancer: a randomised phase 2 study. Lancet Oncol. 2013;14:1216–1225. doi: 10.1016/S1470-2045(13)70429-7. [DOI] [PubMed] [Google Scholar]
- 42.Adams ST, Jr., Miller SC. Beyond D-luciferin: expanding the scope of bioluminescence imaging in vivo. Curr Opin Chem Biol. 2014;21C:112–120. doi: 10.1016/j.cbpa.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez EA, Caygill K, Hannah AL, et al. Etirinotecan pegol target-specific pharmacodynamic biomarkers in circulating tumor cells from patients with metastatic breast cancer in the phase 3 BEACON study. Cancer Res. 2015;75 P3-10-03. [Google Scholar]
- 44.Rivera P, Filleron T, Gladieff L, et al. Efficacy of cetuximab plus platinum agent in advanced, triple-negative breast carcinoma: Results of a retrospective analysis. J Clin Oncol. 2011;29:e11581. [Google Scholar]