Abstract
Objective
Clinical trials have shown that serotonin norepinephrine reuptake inhibitors, such as milnacipran, decrease pain in non-inflammatory pain conditions like fibromyalgia and osteoarthritis. We examined the effect of milnacipran on self-reported pain intensity and experimental pain sensitivity among rheumatoid arthritis (RA) patients with widespread pain and stable RA disease activity.
Methods
In this double-blind, crossover study, RA patients with widespread pain, on a stable treatment regimen, were randomized (via a random number generator) to receive milnacipran 50 mg twice daily or placebo for 6 weeks, followed by a 3-week washout and crossed over to the other arm for the remaining 6 weeks. The primary outcome was change in average pain intensity, assessed by the Brief Pain Inventory short form. The sample size was calculated to detect a 30% improvement in pain with power = 0.80 and alpha = 0.05.
Results
Of the 43 randomized subjects, 41 received study drug, and 32 completed the 15-week study per protocol. On a 0–10 scale, average pain intensity decreased by 0.39 (95% CI −1.27, 0.49; P = 0.37) more points during 6 weeks of milnacipran treatment compared to placebo. In the subgroup of subjects with swollen joint count ≤ 1, average pain intensity decreased by 1.14 (95% CI −2.26, −0.01; P= 0.04) more points during 6 weeks of milnacipran compared to placebo. Common adverse events included nausea (26.8%) and loss of appetite (9.7%).
Conclusion
Compared to placebo, milnacipran did not improve overall, self-reported pain intensity among subjects with widespread pain taking stable RA medications. Trial registration: ClinicalTrials.gov NCT01207453
Keywords: Arthritis, Rheumatoid, Pain, Analgesia
INTRODUCTION
Rheumatoid arthritis (RA) patients most frequently seek medical care because of pain (1). Over the past 20 years, the development of strong biologic disease-modifying antirheumatic drugs (DMARDs) has enabled aggressive, early treatment of RA, leading to higher rates of remission. However, despite improvements in disease activity, the majority of early RA patients report incomplete relief of pain (2), and up to 34% of RA patients report chronic widespread pain over a follow-up period of 5 years (3). Pain in this subgroup of RA patients is often related to non-inflammatory factors, such as structural changes, psychological factors and central pain mechanisms (4–9).
Several studies have documented the impact of central pain mechanisms in osteoarthritis (10–12), but data regarding the role of central pain mechanisms in RA is scarce. No studies have examined the effects of serotonin norepinephrine reuptake inhibitors (SNRIs) on pain in RA, although some have suggested that tricyclic antidepressants, which exert their effects via serotonin and norepinephrine, are effective (13–15). In addition, several studies have examined the role of SNRIs in chronic pain conditions associated with defects in central pain processing (e.g., fibromyalgia) (16–19). Milnacipran, the most recent FDA-approved drug for fibromyalgia, improved pain severity in randomized clinical trials of fibromyalgia (20–22).
The objective of this study was to evaluate whether milnacipran improves pain severity among RA patients with pain in a widespread distribution, compared to placebo. We chose to focus on RA patients with widespread pain because these individuals are more likely to have aberrancies in central nervous system pain regulating mechanisms, which may be amenable to treatment with milnacipran.
This study takes advantage of a crossover design to reduce the effects of confounding variables because each subject serves as his/her own control (23). By minimizing the imbalances in covariates between treatment groups, the crossover design enhances statistical power, enabling the use of smaller sample sizes than parallel-group trials (24). The main disadvantages of crossover studies are carryover effects (the first treatment has lingering effects that alters the outcome during the second treatment period) and order effects (the sequence of treatment affects the outcome). To assess the likelihood and adjust for these effects, we use linear mixed models, including covariates for study period and sequence. We hypothesize that subjects will experience greater reductions in pain severity during milnacipran treatment than placebo.
MATERIALS AND METHODS
Study population
RA patients with pain at ≥ 5 body sites were recruited from the Arthritis Center of a large U.S. academic medical center. Inclusion criteria included: 1) age 24 years or older (excluded subjects < 24 years old due to black box warning for increased suicide risk among children, adolescents and young adults), 2) diagnosis of RA as determined by a board-certified rheumatologist, 3) stable RA medication regimen (defined as stable doses of non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids (≤ 20 mg prednisone daily), and/or DMARDs) for ≥ 8 weeks prior to study initiation, 4) ability to maintain stable doses of NSAIDs, corticosteroids and DMARDs for the duration of the study, 5) average pain ≥ 4 on the Brief Pain Inventory – short form at the screening visit (25), 6) ≥ 5 on the Regional Pain Scale at the screening visit (changed after study initiation from a requirement of ≥ 7 due to slow recruitment) (26), and 7) ability to give informed consent. Exclusion criteria included: 1) primary diagnosis of fibromyalgia, 2) cold sensitive conditions (e.g., Raynaud’s syndrome, cryoglobulinemia, paroxysmal cold hemoglobinuria), 3) psychotic disorders (e.g., schizophrenia, schizoaffective disorder, delusional disorder, shared psychotic disorder), 4) treatment with thioridazine, selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors, tricyclic, tetracyclic or atypical antidepressants, 5) treatment with opioid analgesics, 6) hypersensitivity to milnacipran, 7) history of suicide or significant risk of suicide as assessed by the Beck Depression Inventory, 8) pregnant or breast-feeding, 9) actively pending worker’s compensation claim, auto no-fault claim or litigation, 10) myocardial infarction within the past 12 months, active cardiac disease, acute congestive heart failure or clinically significant cardiac rhythm or conduction abnormalities, 11) severe liver impairment, 12) severe or end stage renal disease, 13) recent history of seizures, 14) uncontrolled narrow-angle glaucoma, 15) treatment with an experimental agent within the last 3 months. All subjects provided written informed consent. The Partners Institutional Review Board approved the study.
Trial design
In this 15-week, cross-over study, 43 subjects were randomized 1:1 to group A vs. B (Supplemental Figure) via a random number generator, with 4 subjects per block. The institution’s Investigational Drug Services generated the random allocation sequence and assigned subjects to treatment groups. Group A received 6 weeks of milnacipran (Savella), followed by a 3-week wash-out and 6 weeks of placebo. Group B received 6 weeks of placebo, followed by a 3-week wash-out and 6 weeks of milnacipran. The 6-week treatment period was chosen based on previous studies of milnacipran in fibromyalgia, showing significant differences between the milnacipran and placebo-treated group as early as one week after starting treatment, with the curve in improvement plateauing at six weeks after initiating treatment (21, 22). The dose was titrated to 50 mg twice daily. Subjects and study assessors were blinded to group allocation. The placebo tablets were identical in appearance to the milnacipran tablets. The dose was titrated according to the following schedule: 1) Days 1–3: milnacipran/placebo 12.5 mg twice daily, 2) Days 4–6: milnacipran/placebo 25 mg twice daily, 3) Days 7–42: milnacipran/placebo 50 mg twice daily. If subjects could not tolerate the full dose, the dose was decreased to the highest tolerated dose. The protocol is accessible on ClinicalTrials.gov (NCT01207453).
Assessment of clinical variables
Following screening, subjects were evaluated at baseline, 6-weeks, 9-weeks and 15-weeks. The disease activity score in 28 joints (DAS28), using C-reactive protein (CRP), was used to assess inflammatory disease activity (27). Pain was quantified using the Brief Pain Inventory – short form (25) and the Symptom Intensity Scale, a 20-item scale that includes the Regional Pain Scale (26) and a visual analog scale (VAS) for fatigue (28). Mental health, sleep, and pain catastrophizing were assessed using the Hospital Anxiety and Depression Scale (29), the Medical Outcomes Study Sleep Scale (30) and the Pain Catastrophizing Scale (31).
Quantitative sensory testing
A Wagner FPK 20 algometer (Wagner Instruments, Greenwich, CT, USA) was used to assess pressure pain thresholds in kg/cm2 (9, 32). This instrument has an accuracy of ± 2 graduations for capacities through 2500 grams and ± 1 graduation over 2500 grams. The order of testing was standardized as follows: 1) right thumbnail, 2) left thumbnail, 3) right wrist, 4) left wrist, 5) right trapezius muscle, 6) left trapezius muscle, 7) right knee, 8) left knee. We increased the pressure at a rate of approximately 1 kg/s from 0 kg to a maximum of 11 kg. The pressure pain threshold was defined as the pressure at which the subjects first felt pain. We performed 2 assessments at each site. As in previous studies (32), the first test was a trial run, to acclimate subjects to testing procedures. The second trial was the test run, from which all reported data were obtained. We averaged pressure pain thresholds at bilateral sites to provide 1 value for each pair of body sites, a method that has been validated in previous studies (33).
Conditioned pain modulation (CPM) was tested using the cold pressor test, with immersion of the right hand in a 6° C water bath as the conditioning stimulus and pressure at the trapezius muscle as the test stimulus (34, 35). The conditioning stimulus is a painful stimulus that activates the descending analgesic pain pathways. The test stimulus is applied to assess changes in pain thresholds after activating the descending analgesic pain pathways. If the descending analgesic pathways are intact, application of the condition stimulus leads to an increase in pain thresholds. In this study, the specific paradigm involved first assessing the pressure pain threshold at the trapezius. Subjects were then instructed to immerse their right hand in the water bath for 30 seconds. At 20 seconds (while the hand was still immersed in water), pressure pain threshold at the trapezius was assessed again. We defined the magnitude of participants’ CPM as the difference in pressure pain threshold between baseline and 20 seconds after cold water immersion. If participants were unable to keep their hand in the cold water bath for at least 20 seconds (due to overwhelming pain), the second measure of pressure pain threshold was assessed immediately after removing the hand from the cold water bath.
Statistical analyses
Both per protocol and intention-to-treat analyses (using a modified last observation carried forward method to handle missing data) were performed. For the intention-to-treat analyses, data from the first period were not carried over to the second period because this was a crossover study, and it was not advisable to apply data obtained during one treatment period to the other treatment period. When data were available for visit 3 but missing for visit 4, these data were carried over from visit 3 to visit 4 since this remained consistent with the intention-to-treat concept of analyzing individuals as they were randomized.
The primary outcome was the change in the Brief Pain Inventory average pain intensity (measured on a 0–10 numeric rating scale) from baseline to week 6 and from week 9 to week 15. Secondary outcomes included changes in the Symptom Intensity Scale score, pressure pain thresholds and CPM from baseline to week 6 and from week 9 to week 15. Effect sizes were calculated using least square means (for changes in pain within treatment groups), the difference of least square means (for differences in changes in pain between treatment groups) and 95% confidence intervals.
Paired t-tests were used for unadjusted comparisons between treatments. To account for potential carryover effects, we fit a linear mixed model, including indicator variables for treatment group, study period and sequence. A significant carryover effect was defined as P < 0.05 for the association between sequence and the dependant variable. Exploratory analyses were conducted in subgroups defined by baseline values of pain and inflammatory disease activity. These analyses were performed using paired t-tests. No corrections for multiple testing were performed due to the exploratory nature of these subgroup analyses. In post hoc analyses, we assessed the characteristics of responders (those with a ≥ 30% decrease in BPI-sf average pain score) versus non-responders. All statistical analyses were performed using the SAS 9.3 software package (SAS Institute, Cary, NC, USA).
Power Calculation
Based on our pilot data, the average Brief Pain Inventory pain score among RA patients with widespread, non-joint pain was 4.77 (SD 2.80). To detect a clinically important improvement in pain intensity of 30% (36) with an alpha level of 0.05, 32 participants were required to achieve 80% power. The trial was ended in November 2013 when 32 participants completed the study.
RESULTS
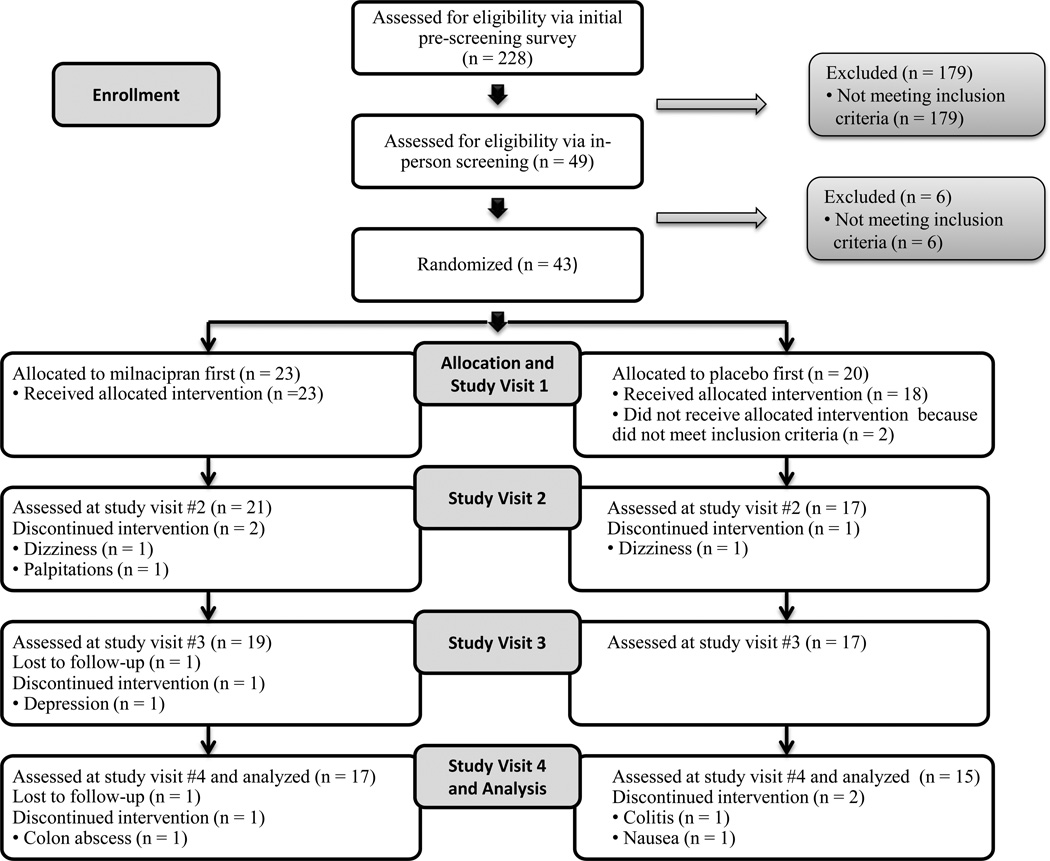
Between January 2011 and July 2013, 228 individuals with RA completed the pre-screening survey. Forty-nine met pre-screening criteria and provided written informed consent (Figure). Forty-three were randomized, and 41 received study drug/placebo (Table 1). Of these 41 participants, 19 (46.3%) correctly identified when they had received study drug vs. placebo. Nine (22.0%) did not correctly identify when they received study drug vs. placebo. Four (9.8%) did not answer this question, and nine (22.0%) were not asked this question because they dropped out of the study before the question was asked. Thirty-two (milnacipran first: 17, placebo first: 15) completed the study and were analyzed by the original assigned groups (Table 1). Of these 32 participants, 31 (96.9%) had pain on both the left and right side of the body. Thirty-one (96.9%) had pain both above and below the waist, and 28 (87.5%) had pain along the axial skeleton.
Figure.
Flow diagram of the process from screening through study completion.
Table 1.
Baseline characteristics of study subjects (N = 32)
| Clinical characteristics | Milnacipran First (N = 17) |
Placebo First (N = 15) |
|---|---|---|
| Age in years, mean (SD) | 54.2 (11.3) | 53.8 (14.1) |
| Rheumatoid arthritis disease duration in years, mean (SD) | 13.2 (11.8) | 9.37 (11.0) |
| Female gender, n (%) | 13 (76.4) | 12 (80.0) |
| Caucasian race, n (%) | 11 (64.7) | 11 (73.3) |
| Rheumatoid factor/Cyclic citrullinated peptide positive, n (%) | 13 (76.5) | 7 (46.7) |
| Disease-modifying antirheumatic drug use, n (%) | ||
| Non-biologic | 11 (64.7) | 10 (66.7) |
| Biologic | 8 (47.1) | 6 (40.0) |
| Oral glucocorticoid use, n (%) | 5 (29.4) | 6 (40.0) |
| Glucocortioid dose in mg of prednisone equivalents, mean (SD) a | 4.4 (3.3) | 6.7 (7.0) |
| Meets American College of Rheumatology 2010 criteria, n (%) | 14 (82.4) | 12 (80.0) |
| Swollen joint count (0–28), median (IQR)b | 0 (1.0) | 1 (4.0) |
| Swollen wrists and/or knees, n (%) | 4 (23.5) | 3 (20.0) |
| Tender joint count (0–28), median (IQR) b | 3 (5.0) | 8 (10.0) |
| Tender wrists and/or knees, n (%) | 11 (64.7) | 10 (66.7) |
| C-reactive protein, median (IQR) | 1.8 (3.1) | 1.2 (2.9) |
| Disease Activity Score in 28 joints – C-reactive protein (1–10), median (IQR) | 3.0 (1.0) | 3.6 (0.8) |
| Tender point count (0–18), median (IQR) | 7 (5.0) | 7 (9.0) |
| Hospital Anxiety and Depression Scale Anxiety score (0–21), mean (SD) | 5.7 (4.7) | 6.3 (4.6) |
| Hospital Anxiety and Depression Scale Depression score (0–21), mean (SD) | 4.0 (4.6) | 4.5 (3.3) |
| Medical Outcomes Study Sleep Problems Index II score (0–100), mean (SD) | 45.4 (20.3) | 47.0 (20.2) |
| Pain Catastrophizing Scale score (0–52), mean (SD) | 33.1 (14.9) | 29.7 (12.8) |
| Regional Pain Scale (0–19), mean (SD) | 9.1 (4.2) | 11.6 (3.4) |
| Symptom Intensity Scale (0–9.75), mean (SD) | 5.2 (4.0) | 6.0 (1.3) |
| Brief Pain Inventory – short form pain intensity (0–10), mean (SD) | 6.2 (1.7) | 5.7 (1.6) |
Abbreviations: SD, standard deviation; IQR, interquartile range.
Among participants taking prednisone.
Swollen and tender joint counts were done according to the guidelines for the DAS28.
Subjects randomized to receive milnacipran first (Group A) had similar clinical characteristics compared to subjects randomized to receive placebo first (Group B). The only statistically significant differences were the median tender joint count (Group A 3 vs. Group B 8, P = 0.04) and median DAS28 (Group A 3.0 vs. Group B 3.6, P = 0.03).
Seven subjects (Group A: 4, Group B: 3) withdrew because of adverse events (lightheadedness, nausea, anxiety, palpitations, colitis, colectomy), and 2 (both in Group A) were lost to follow-up (Table 2). Study completers were more likely to be seropositive for rheumatoid factor and/or cyclic citrullinated peptide antibodies (P = 0.02). Though not statistically significant, study completers were almost ten years younger than excluded individuals. Five subjects could not tolerate the full dose of 50 mg twice daily of study drug and were reduced to 25 mg twice daily. All dose reductions occurred while subjects were taking milnacipran. Analyses did not show a statistically significant cross-over effect.
Table 2.
Baseline characteristics of study subjects who withdrew from the study or were lost to follow-up (N = 9)
| Clinical characteristics | (N=9) | |
|---|---|---|
| Age in years, mean (SD) | 63.2 | (14.5) |
| Rheumatoid arthritis disease duration in years, mean (SD) | 13 | (14.3) |
| Female gender, n (%) | 9 | (100.0) |
| Caucasian race, n (%) | 7 | (77.8) |
| Rheumatoid factor/Cyclic citrullinated peptide positive, n (%) | 2 | (22.2) |
| Disease-modifying antirheumatic drug use, n (%) | ||
| Non-biologic | 3 | (33.3) |
| Biologic | 5 | (55.6) |
| Oral glucocorticoid use, n (%) | 1 | (11.1) |
| Meets American College of Rheumatology 2010 criteria, n (%) | 5 | (55.6) |
| Swollen joint count, median (IQR) | 1 | (1) |
| Tender joint count, median (IQR) | 8 | (14) |
| C-reactive protein, median (IQR) | 1.9 | (3) |
| Disease Activity Score in 28 joints – C-reactive Protein, median (IQR) | 3.3 | (1.6) |
| Tender point count, median (IQR) | 11 | (14) |
| Hospital Anxiety and Depression Scale Anxiety score, mean (SD) | 5.4 | (5) |
| Hospital Anxiety and Depression Scale Depression score, mean (SD) | 3.2 | (2) |
| Medical Outcomes Study Sleep Problems Index II score, mean (SD) | 42.5 | (17.7) |
| Pain Catastrophizing Scale score, mean (SD) | 25.8 | (9.2) |
Abbreviations: SD, standard deviation; IQR, interquartile range.
When subjects were treated with milnacipran, the mean Brief Pain Inventory pain intensity score decreased by 0.67 points (95% CI −1.29, −0.04), or 12.9%, compared to a decrease of 0.28 (95% CI −0.90, 0.35) points, or 4.9%, during placebo treatment. The difference between the decreases in pain intensity during milnacipran vs. placebo treatment was −0.39 points (95% CI −1.27, 0.49) (Table 3). This difference was not statistically significant (P = 0.37). Similarly, the mean Symptom Intensity Scale score decreased by 0.71 points (95% CI −1.33, −0.07) when subjects were treated with milnacipran and by 0.80 (95% CI −1.43, −0.17) during placebo treatment. The difference between the decreases in Symptom Intensity Scale score during milnacipran vs. placebo treatment was 0.10 (95% CI −0.80, 0.99; P = 0.83). At the thumbnails, pain threshold increased by 0.75 (95% CI 0.19, 1.31) when subjects were treated with milnacipran and increased by 0.08 (95% CI −0.49, 0.64) when subjects were treated with placebo. The difference between the changes in thumbnail pain threshold during milnacipran vs. placebo treatment was 0.67 (95% CI 0.02, 1.32; P = 0.04). In intention-to-treat analyses comparing changes in the above outcomes, the results were the same. In secondary analyses using only data from the first period of treatment, the results were the same except the difference in change in thumbnail pain threshold was no longer statistically significant.
Table 3.
Effect sizes and 95% confidence intervals (CIs) for measures of pain in per protocol analyses
| Unadjusted Analyses (N = 32) |
Adjusted Analyses (N = 32) |
|||||
|---|---|---|---|---|---|---|
| Change during Placebo (95% CI) |
Change during Milnacipran (95% CI) |
Difference between Placebo & Milnacipran (95% CI) |
Change during Placebo (95% CI) |
Change during Milnacipran (95% CI) |
Difference between Placebo & Milnacipran (95% CI) |
|
| BPI-sf Pain a | −0.25 (−0.95, 0.45) |
−0.72 (−1.33, −0.11) |
−0.47 (−1.45, 0.51) |
−0.28 (−0.90, 0.35) |
−0.67 (−1.29, −0.04) |
−0.39 (−1.27, 0.49) |
| SIS b | −0.80 (−1.48, −0.11) |
−0.73 (−1.31, −0.16) |
0.06 (−0.90, 1.03) |
−0.80 (−1.43, −0.17) |
−0.71 (−1.33, −0.07) |
0.10 (−0.80, 0.99) |
| Thumbnail PPT c | 0.08 (−0.52, 0.67) |
0.76 (0.25, 1.27) |
0.69 (0.04, 1.34) |
0.08 (−0.49, 0.64) |
0.75 (0.19, 1.31) |
0.67 (0.02, 1.32) |
| Trapezius PPT c |
0.69 (0.13, 1.26) |
0.35 (−0.18, 0.88) |
−0.34 (−1.04, 0.36) |
0.71 (0.16, 1.26) |
0.33 (−0.22, 0.88) |
−0.38 (−1.06, 0.31) |
| Wrist PPT c |
0.81 (0.23, 1.39) |
0.77 (0.19, 1.36) |
0.04 (−0.68, 0.76) |
0.75 (0.17, 1.33) |
0.79 (0.21, 1.37) |
0.04 (−0.69, 0.78) |
| Knee PPT c | 0.21 (−0.47, 0.90) |
0.37 (−0.16, 0.90) |
0.16 (−0.64, 0.95) |
0.20 (−0.42, 0.83) |
0.37 (−0.26, 0.99) |
0.16 (−0.64, 0.97) |
| CPM c | 0.17 (−0.26, 0.59) |
0.09 (−0.54, 0.71) |
−0.08 (−0.88, 0.72) |
0.17 (−0.37, 0.71) |
0.09 (−0.45, 0.64) |
−0.07 (−0.84, 0.69) |
Abbreviations: BPI-sf, Brief Pain Inventory – short form; SIS, Symptom Intensity Scale; PPT, pressure pain threshold; CPM, conditioned pain modulation.
Based on a 0–10 scale with 10 being worse pain
Based on 0–9.75 scale with 9.75 being greater intensity of symptoms consistent with fibromyalgia
Units are kg/cm2
Changes in thumbnail pain threshold were inversely correlated with changes in Brief Pain Inventory pain intensity score (Spearman’s r = −0.38, P = 0.008) during milnacipran treatment but were not correlated with changes in pain intensity during placebo (Spearman’s r = 0.004, P = 0.97). Neither changes in pain thresholds at other sites nor changes in CPM differed between milnacipran and placebo treatment.
Among the subgroup of RA patients with ≤ 1 swollen joint at baseline, the mean Brief Pain Inventory pain intensity score decreased by 1.05 points (95% CI −1,78, −0.32), compared to an increase of 0.09 (95% CI −0.76, 0.94) points during placebo treatment. The difference between the decreases in pain intensity during milnacipran vs. placebo treatment was −1.14 points (95% CI −2.26, −0.01) (Table 4). Increases in pressure pain threshold during milnacipran treatment compared to placebo were again noted in the subgroup of RA patients with ≤ 1 swollen joint at baseline and the subgroup with baseline average pain intensity ≥ 4 at baseline. No significant differences were noted in other subgroup analyses.
Table 4.
Effect sizes and 95% confidence intervals for measures of pain in subgroups of interest in per protocol analyses
| Change during Placebo (95% Confidence Interval) |
Change during Milnacipran (95% Confidence Interval) |
Difference between Placebo & Milnacipran (95% Confidence Interval) |
|
|---|---|---|---|
| BPI-sf Average Pain Intensitya≥ 4 at baseline (n=29) | |||
| BPI-sf Pain Intensity a | −0.31 (−1.07, 0.45) | −0.76 (−1.41, −0.10) | −0.45 (−1.51, 0.61) |
| SIS b | −0.87 (−1.62, −0.12) | −0.69 (−1.31, −0.07) | 0.18 (−0.87, 1.23) |
| Thumbnail PPT c | 0.06 (−0.60, 0.72) | 0.78 (0.23, 1.34) | 0.72 (0.03, 1.42) |
| Trapezius PPT c | 0.42 (−0.13, 0.97) | 0.72 (0.10, 1.35) | −0.30 (−1.06, 0.45) |
| Regional Pain Scale ≥ 7 at baseline (n=25) | |||
| BPI-sf Pain Intensity a | −0.08 (−0.96, 0.80) | −0.48 (−1.22, 0.26) | −0.40 (−1.64, 0.84) |
| SIS b | −0.64 (−1.48, 0.20) | −0.91 (−1.60, −0.22) | −0.27 (−1.41, 0.87) |
| Thumbnail PPT c | 0.20 (−0.55, 0.95) | 0.73 (0.13, 1.33) | 0.53 (−0.20, 1.26) |
| Trapezius PPT c | 0.67 (0.10, 1.24) | 0.36 (−0.28, 1.00) | −0.31 (−1.05, 0.44) |
| Swollen joint count ≤ 1 at baseline (n=22) | |||
| BPI-sf Pain Intensity a | 0.09 (−0.76, 0.94) | −1.05 (−1.78, −0.32) | −1.14 (−2.26, −0.01) |
| SIS b | −0.45 (−1.23, 0.32) | −1.02 (−1.71, −0.34) | −0.57 (−1.68, 0.55) |
| Thumbnail PPT c | 0.06 (−0.60, 0.73) | 0.95 (0.28, 1.62) | 0.89 (0.16, 1.61) |
| Trapezius PPT c | 0.65 (0.01, 1.29) | 0.82 (0.17, 1.47) | 0.17 (−0.61, 0.94) |
Abbreviations: BPI-sf, Brief Pain Inventory – short form; SIS, Symptom Intensity Scale; PPT, pressure pain threshold.
Based on a 0–10 scale with 10 being worse pain
Based on 0–9.75 scale with 9.75 being greater intensity of symptoms consistent with fibromyalgia
Units are kg/cm2
In analyses comparing responders to non-responders, no differences were statistically significant (Table 5).
Table 5.
Baseline characteristics of responders to milnacipran vs. non-responders to milnacipran (response ≥ 30% improvement in BPI-sf average pain intensity)
| Clinical characteristics | Non Responders (N = 23) |
Responders (N = 9) |
|---|---|---|
| Age in years, mean (SD) | 53.4 (13.4) | 55.4 (10.2) |
| Rheumatoid arthritis disease duration in years, mean (SD) | 9.4 (10.8) | 16.5 (12) |
| Female gender, n (%) | 20.0 (87.0) | 5 (55.6) |
| Caucasian race, n (%) | 15 (65.2) | 7 (77.8) |
| Rheumatoid factor/Cyclic citrullinated peptide positive, n (%) | 14 (60.9) | 6 (66.7) |
| Disease-modifying antirheumatic drug use, n (%) | ||
| Non-biologic | 15 (65.2) | 6 (66.7) |
| Biologic | 10 (43.5) | 4 (44.4) |
| Oral glucocorticoid use, n (%) | 6 (26.1) | 5 (55.6) |
| Meets American College of Rheumatology 2010 criteria, n (%) | 19 (82.6) | 7 (77.8) |
| Swollen joint count, median (IQR) | 1 (3) | 0 (1) |
| Tender joint count, median (IQR) | 6 (6) | 3 (6) |
| C-reactive protein (CRP), median (IQR) | 1.5 (3) | 1.7 (2.5) |
| Disease Activity Score in 28 joints – C-reactive protein, median (IQR) | 3.6 (0.9) | 3.1 (1.2) |
| Tender point count, median (IQR) | 7 (9) | 6 (6) |
| Hospital Anxiety and Depression Scale Anxiety score, mean (SD) | 6.1 (5.1) | 5.6 (3.3) |
| Hospital Anxiety and Depression Scale Depression score, mean (SD) | 4.7 (4.3) | 3.1 (2.8) |
| Medical Outcomes Study Sleep Problems Index II score, mean (SD) | 46.9 (20.5) | 44.1 (19.4) |
| Pain Catastrophizing Scale score, mean (SD) | 29.9 (13.6) | 35.6 (14.4) |
Abbreviations: BPI-sf, Brief Pain Inventory – short form; SD, standard deviation; IQR, interquartile range.
Of the 41 participants who received at least 1 dose of milnacipran and/or placebo, 24 (58.4%) reported ≥1 adverse effect. When participants were treated with milnacipran, the most common adverse effects were nausea (26.8%), loss of appetite (9.7%), insomnia (7.3%) and vomiting (7.3%). When participants were treated with placebo, the most common adverse effects were nausea (7.3%), insomnia (4.9%), headaches (4.9%) and paresthesias (4.9%). One serious adverse event was reported. A participant developed abdominal pain 1 day after starting the placebo phase of the trial (after completing 6 weeks on milnacipran and 3 weeks of washout). A CT scan showed a colonic abscess, and she underwent partial colectomy.
DISCUSSION
In the overall study population, in both per protocol and intention-to-treat analyses, we found no improvement in Brief Pain Inventory average pain intensity or other pain measures when participants were treated with milnacipran 50 mg twice daily vs. placebo. However, in subgroup analyses including only RA patients with ≤ 1 swollen joint, the difference between changes in pain during milnacipran treatment and changes in pain during placebo treatment was statistically significant, suggesting that milnacipran may be efficacious for RA patients with extremely well-controlled inflammation. The latter was an exploratory analysis, however, performed in a small subgroup, and, in this subgroup, the baseline pain intensity prior to milnacipran treatment was higher than the baseline pain intensity before placebo treatment. Thus, regression towards the mean may mix with the true treatment effect.
The finding of no difference in changes in average pain intensity or other pain measures during milnacipran vs. placebo treatment suggests that central pain mechanisms may not be the predominant cause of pain in RA patients with widespread pain. Among RA patients, many potential causes of pain exist, including pain due to inflammatory joint disease and pain due to structural damage (37, 38). When multiple factors contribute to an individual’s overall pain experience, it is likely that treating just one pathway (e.g., the serotonin-norepinephrine pathways involved in central pain processing) may not yield clinically important improvements in overall pain. Our observation that milnacipran only reduced pain among RA patients with ≤ 1 swollen joint supports this hypothesis, indicating that inflammation needs to be very well-controlled in order for central-acting pain medications to be effective. In a previous study, we reported that the descending inhibitory pain pathways are dysregulated among RA patients, resulting in greater sensitivity to experimental stimuli (32). This phenomenon, known as hyperalgesia, may also be associated with an increased sensitivity to endogenous painful stimuli, such as inflammation at joint sites. Future studies with a larger sample size of RA patients in remission or with low disease activity are necessary to elucidate the role of milnacipran and other central-acting pain medications in this population.
The adverse effects data contribute new knowledge to the published literature because nearly all previous studies of milnacipran excluded individuals with systemic inflammatory diseases, such as RA (39–41). Compared to previous study populations (42–44), which mostly consisted of fibromyalgia patients, this study population was older, less likely to be female and more likely to be taking corticosteroids and DMARDs. Despite these differences, the side effect profile was similar to what has previously been reported (45–47).
Strengths of the study include the randomized, cross-over design. Because subjects served as their own controls, the effects of confounding were minimized. Critical to the crossover design was the 3-week washout phase, which minimized potential residual effects of milnacipran among participants who started the study in the milnacipran treatment group. The half-life of milnacipran is approximately 8 hours (48, 49), and it is recommended that the washout period be at least 5 times the half-life of the active ingredient (50). Thus, 3 weeks should be more than sufficient to allow for drug washout.
A limitation of this study is the generalizability of the results. The inclusion/exclusion criteria were specifically selected to identify a subgroup of RA patients who would be most likely to respond to milnacipran and least likely to suffer serious adverse effects. Thus, the results may not be generalizable to the overall RA population. In addition, the average RA disease duration of individuals in this study was 11.4 (SD 11.4) years, and only 5 (15.6%) had disease duration ≤ 2 years. It is possible that individuals with established disease have more structural damage and are less likely to respond to milnacipran than individuals with early disease. A separate study of individuals with early RA is needed to adequately address this question.
A second limitation is that participants were often able to identify when they were receiving study drug vs. placebo, even though both study investigators and participants were blinded per study design. Based on conversations with study participants, this was most commonly due to the perception of side effects from the active drug. Of the 41 subjects who received study drug, 9 dropped out due to side effects or were lost to follow-up. Although not statistically significant, the average age of subjects who dropped out was nearly 10 years higher than the average age of included subjects. Thus, the effects of milnacipran on older patients with RA need further study.
In summary, this randomized, blinded cross-over trial of milnacipran vs. placebo revealed no overall differences in changes in pain intensity, fibromyalgia symptoms and experimentally assessed pain measures. In exploratory analyses, we found some evidence for an effect of milnacipran in RA patients with ≤ 1 swollen joint, but issues of regression to the mean and small sample size require that this finding be examined in a larger sample.
Supplementary Material
ACKNOWLEDGMENTS
We thank Cassandra Coleman for her assistance in recruiting study subjects, Ajay D. Wasan, MD, MMSc for serving as the independent safety monitor, Kinara S. Yang, PharmD for serving as the study pharmacist, and Zhi Zack Zhang for his assistance in performing additional analyses requested by reviewers. This work was conducted with support from Forest Research Institute, NIH-NIAMS K23AR057578, NIH-NIAMS K24 AR055989, Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers). Study drug and placebo were supplied by Forest Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, the National Institutes of Health, Harvard University, its affiliated academic healthcare centers, or its corporate contributors.
REFERENCES
- 1.Borenstein D, Altman R, Bello A, Chatham W, Clauw DJ, Crofford LJ, et al. Report of the American College of Rheumatology Pain Management Task Force. Arthritis Care Res (Hoboken) 2010;62:590–599. doi: 10.1002/acr.20005. [DOI] [PubMed] [Google Scholar]
- 2.McWilliams DF, Zhang W, Mansell JS, Kiely PD, Young A, Walsh DA. Predictors of change in bodily pain in early rheumatoid arthritis: an inception cohort study. Arthritis Care Res (Hoboken) 2012;64:1505–1513. doi: 10.1002/acr.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson ML, Svensson B, Bergman S. Chronic widespread pain in patients with rheumatoid arthritis and the relation between pain and disease activity measures over the first 5 years. J Rheumatol Suppl. 2013;40:1977–1985. doi: 10.3899/jrheum.130493. [DOI] [PubMed] [Google Scholar]
- 4.Meeus M, Vervisch S, De Clerck LS, Moorkens G, Hans G, Nijs J. Central sensitization in patients with rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum. 2012;41:556–567. doi: 10.1016/j.semarthrit.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Pollard LC, Kingsley GH, Choy EH, Scott DL. Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology (Oxford) 2010;49:924–928. doi: 10.1093/rheumatology/kep458. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg DL, Clauw DJ, Fitzcharles MA. New Concepts in Pain Research and Pain Management of the Rheumatic Diseases. Semin Arthritis Rheum. 2011;41:319–334. doi: 10.1016/j.semarthrit.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Barsky AJ, Ahern DK, Orav EJ, Nestoriuc Y, Liang MH, Berman IT, et al. A randomized trial of three psychosocial treatments for the symptoms of rheumatoid arthritis. Semin Arthritis Rheum. 2010;40:222–232. doi: 10.1016/j.semarthrit.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YC, Lu B, Boire G, Haraoui BP, Hitchon CA, Pope JE, et al. Incidence and predictors of secondary fibromyalgia in an early arthritis cohort. Ann Rheum Dis. 2013;72:949–954. doi: 10.1136/annrheumdis-2012-201506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YC, Chibnik LB, Lu B, Wasan AD, Edwards RR, Fossel AH, et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2009;11:R160. doi: 10.1186/ar2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arendt-Nielsen L, Eskehave TN, Egsgaard LL, Petersen KK, Graven-Nielsen T, Hoeck HC, et al. Association between experimental pain biomarkers and serologic markers in patients with different degrees of painful knee osteoarthritis. Arthritis Rheumatol. 2014;66:3317–3326. doi: 10.1002/art.38856. [DOI] [PubMed] [Google Scholar]
- 11.Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. E J Pain. 2014;18:1367–1375. doi: 10.1002/j.1532-2149.2014.499.x. [DOI] [PubMed] [Google Scholar]
- 12.Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64:2907–2916. doi: 10.1002/art.34466. [DOI] [PubMed] [Google Scholar]
- 13.Richards BL, Whittle SL, Buchbinder R. Antidepressants for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008920.pub2. CD008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards BL, Whittle SL, van der Heijde DM, Buchbinder R. The efficacy and safety of antidepressants in inflammatory arthritis: a Cochrane systematic review. J Rheumatol Suppl. 2012;90:21–27. doi: 10.3899/jrheum.120338. [DOI] [PubMed] [Google Scholar]
- 15.Sarzi Puttini P, Cazzola M, Boccassini L, Ciniselli G, Santandrea S, Caruso I, et al. A comparison of dothiepin versus placebo in the treatment of pain in rheumatoid arthritis and the association of pain with depression. J Int Med Res. 1988;16:331–337. doi: 10.1177/030006058801600502. [DOI] [PubMed] [Google Scholar]
- 16.Choy E, Marshall D, Gabriel ZL, Mitchell SA, Gylee E, Dakin HA. A systematic review and mixed treatment comparison of the efficacy of pharmacological treatments for fibromyalgia. Semin Arthritis Rheum. 2011;41:335–345. doi: 10.1016/j.semarthrit.2011.06.003. e6. [DOI] [PubMed] [Google Scholar]
- 17.Bradley LA, Wohlreich MM, Wang F, Gaynor PJ, Robinson MJ, D’Souza DN, et al. Pain response profile of patients with fibromyalgia treated with duloxetine. Clin J Pain. 2010;26:498–504. doi: 10.1097/AJP.0b013e3181dee80e. [DOI] [PubMed] [Google Scholar]
- 18.Arnold LM, Lu Y, Crofford LJ, Wohlreich M, Detke MJ, Iyengar S, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50:2974–2984. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 19.Carville SF, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, Buskila D, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67:536–541. doi: 10.1136/ard.2007.071522. [DOI] [PubMed] [Google Scholar]
- 20.Ormseth MJ, Eyler AE, Hammonds CL, Boomershine CS. Milnacipran for the management of fibromyalgia syndrome. J Pain Res. 2010;3:15–24. doi: 10.2147/jpr.s7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mease PJ, Clauw DJ, Gendreau RM, Rao SG, Kranzler J, Chen W, et al. The efficacy safety of milnacipran for treatment of fibromyalgia a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:398–409. doi: 10.3899/jrheum.080734. [DOI] [PubMed] [Google Scholar]
- 22.Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther. 2008;30:1988–2004. doi: 10.1016/j.clinthera.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Harden RN, Bruehl S. Conducting clinical trials to establish drug efficacy in chronic pain. Am J Phys Med Rehab. 2001;80:547–557. doi: 10.1097/00002060-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Richens A. Proof of efficacy trials: cross-over versus parallel-group. Epilepsy Res. 2001;45:43–47. doi: 10.1016/s0920-1211(01)00214-5. [DOI] [PubMed] [Google Scholar]
- 25.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 26.Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatol. 2003;30:369–378. [PubMed] [Google Scholar]
- 27.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe F, Rasker JJ. The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. J Rheumatol. 2006;33:2291–2299. [PubMed] [Google Scholar]
- 29.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 30.Hays RD, Stewart AL. Sleep Measures. In: Ware ALSaJE., editor. Measuring functioning and well-being: The Medical Outcomes Study approach. Durham, NC: Durham University Press; 1992. pp. 235–259. [Google Scholar]
- 31.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 32.Lee YC, Lu B, Edwards RR, Wasan AD, Nassikas NJ, Clauw DJ, et al. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum. 2013;65:59–68. doi: 10.1002/art.37733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petzke F, Khine A, Williams D, Groner K, Clauw DJ, Gracely RH. Dolorimetry performed at 3 paired tender points highly predicts overall tenderness. J Rheumatol. 2001;28:2568–2569. [PubMed] [Google Scholar]
- 34.Biurrun Manresa JA, Fritsche R, Vuilleumier PH, Oehler C, Morch CD, Arendt-Nielsen L, et al. Is the conditioned pain modulation paradigm reliable? A test-retest assessment using the nociceptive withdrawal reflex. PLoS One. 2014;9:e100241. doi: 10.1371/journal.pone.0100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis GN, Heales L, Rice DA, Rome K, McNair PJ. Reliability of the conditioned pain modulation paradigm to assess endogenous inhibitory pain pathways. Pain Res Manag. 2012;17:98–102. doi: 10.1155/2012/610561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88:287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 37.Sarzi-Puttini P, Salaffi F, Di Franco M, Bazzichi L, Cassisi G, Casale R, et al. Pain in rheumatoid arthritis: a critical review. Reumatismo. 2014;66:18–27. doi: 10.4081/reumatismo.2014.760. [DOI] [PubMed] [Google Scholar]
- 38.Walsh DA, McWilliams DF. Pain in rheumatoid arthritis. Curr Pain Headache Rep. 2012;16:509–517. doi: 10.1007/s11916-012-0303-x. [DOI] [PubMed] [Google Scholar]
- 39.Gendreau RM, Thorn MD, Gendreau JF, Kranzler JD, Ribeiro S, Gracely RH, et al. Efficacy of milnacipran in patients with fibromyalgia. J Rheumatol. 2005;32:1975–1985. [PubMed] [Google Scholar]
- 40.Vitton O, Gendreau M, Gendreau J, Kranzler J, Rao SG. A double-blind placebo-controlled trial of milnacipran in the treatment of fibromyalgia. Hum Psychopharmacol. 2004;19(Suppl 1):S27–S35. doi: 10.1002/hup.622. [DOI] [PubMed] [Google Scholar]
- 41.Geisser ME, Palmer RH, Gendreau RM, Wang Y, Clauw DJ. A pooled analysis of two randomized, double-blind, placebo-controlled trials of milnacipran monotherapy in the treatment of fibromyalgia. Pain Pract. 2011;11:120–131. doi: 10.1111/j.1533-2500.2010.00403.x. [DOI] [PubMed] [Google Scholar]
- 42.Uceyler N, Hauser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59:1279–1298. doi: 10.1002/art.24000. [DOI] [PubMed] [Google Scholar]
- 43.Goldenberg DL, Clauw DJ, Palmer RH, Mease P, Chen W, Gendreau RM. Durability of therapeutic response to milnacipran treatment for fibromyalgia Results of a randomized, double-blind, monotherapy 6-month extension study. Pain Med. 2010;11:180–194. doi: 10.1111/j.1526-4637.2009.00755.x. [DOI] [PubMed] [Google Scholar]
- 44.Arnold LM, Gendreau RM, Palmer RH, Gendreau JF, Wang Y. Efficacy and safety of milnacipran 100 mg/day in patients with fibromyalgia: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:2745–2756. doi: 10.1002/art.27559. [DOI] [PubMed] [Google Scholar]
- 45.Branco JC, Zachrisson O, Perrot S, Mainguy Y. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in treatment of fibromyalgia. J Rheumatol Suppl. 2010;37:851–859. doi: 10.3899/jrheum.090884. [DOI] [PubMed] [Google Scholar]
- 46.Hauser W, Urrutia G, Tort S, Uceyler N, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD010292. CD010292. [DOI] [PubMed] [Google Scholar]
- 47.Arnold LM, Palmer RH, Ma Y. A 3-year, open-label, flexible-dosing study of milnacipran for the treatment of fibromyalgia. Clin J Pain. 2013;29:1021–1028. doi: 10.1097/AJP.0b013e31828440ab. [DOI] [PubMed] [Google Scholar]
- 48.Puozzo C, Panconi E, Deprez D. Pharmacology and pharmacokinetics of milnacipran. Int Clin Psychopharmacol. 2002;17(Suppl 1):S25–S35. doi: 10.1097/00004850-200206001-00004. [DOI] [PubMed] [Google Scholar]
- 49.Kyle JA, Dugan BD, Testerman KK. Milnacipran for treatment of fibromyalgia. The Ann Pharmacother. 2010;44:1422–1429. doi: 10.1345/aph.1P218. [DOI] [PubMed] [Google Scholar]
- 50.Chow S-C, Liu J-P. Design and Analysis of Bioavailability and Bioequivalence Studies. 3rd ed. CRC Press; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.