Abstract
Objective
There is considerable evidence that the thalamus is abnormal in psychotic disorders. Resting-state fMRI (RS-fMRI) has revealed an intriguing pattern of thalamic dysconnectivity in psychosis characterized by reduced prefrontal cortex (PFC) connectivity and increased somatomotor-thalamic connectivity. However, critical knowledge gaps remain with respect to the onset, anatomical specificity, and clinical correlates of thalamic dysconnectivity in psychosis.
Method
RS-fMRI was collected on 105 healthy subjects and 148 individuals with psychosis, including 53 early stage psychosis patients. Using all 253 subjects, the thalamus was parceled into functional regions-of-interest (ROIs) on the basis of connectivity with six a-priori defined cortical ROIs covering most of the cortical mantle. Functional connectivity between each cortical ROI and its corresponding thalamic ROI was quantified and compared across groups. Significant differences in the ROI-to-ROI analysis were followed up with voxel-wise seed-based analyses to further localize thalamic dysconnectivity.
Results
ROI analysis revealed reduced PFC-thalamic connectivity and increased somatomotor-thalamic connectivity in both chronic and early stages psychosis patients. PFC hypo-connectivity and motor cortex hyper-connectivity correlated in patients suggesting they result from a common pathophysiological mechanism. Seed-based analyses revealed thalamic hypo-connectivity in psychosis localized to dorsolateral PFC, medial PFC, and cerebellar areas of the well-described ‘executive control’ network. Across all subjects, thalamic connectivity with areas of the fronto-parietal network correlated with cognitive functioning, including verbal learning and memory.
Conclusions
Thalamocortical dysconnectivity is present in both chronic and early stages of psychosis, includes reduced thalamic connectivity with the executive control network, and is related to cognitive impairment.
Keywords: Psychosis, Resting-state fMRI, Thalamus, Cortex, Chronic, Early stage
INTRODUCTION
There is considerable evidence that the thalamus and associated cortical connections are abnormal in psychotic disorders (1–4). However, it has been difficult to localize thalamic pathology and identify dysfunction in specific thalamocortical circuits due to the complex architecture of the thalamus and limitations of traditional neuroimaging methods (5). Furthermore, post mortem investigations have produced conflicting findings or, in the case of bipolar disorder, are few in number (6,7).
Resting-state fMRI (RS-fMRI) is a useful method for mapping functional brain networks and identifying circuit abnormalities in neurological and psychiatric disorders (8,9). Recently, we used RS-fMRI to investigate thalamocortical functional connectivity in a sample of 62 individuals with schizophrenia and 77 healthy subjects (10). Consistent with an earlier small investigation of 10 patients (11), we found that prefrontal cortex (PFC) connectivity with the thalamus was reduced in schizophrenia. Surprisingly, patients also demonstrated increased thalamic connectivity with motor and somatosensory cortex. The combination of reduced prefrontal and increased sensorimotor connectivity was subsequently replicated by several groups and extended to bipolar disorder (12–14).
Despite the consistency of the findings across studies, key questions remain about the onset, anatomical specificity, and clinical correlates of thalamic dysconnectivity. It is unclear if the abnormalities are present early in the illness or emerge as the illness progresses. Based on what is known about the development of thalamocortical functional connectivity, we hypothesized that the combination of reduced PFC connectivity and increased somatomotor connectivity results from a disturbance in brain development during the transition from adolescence to adulthood that prevents PFC-thalamic circuitry from fully developing and derails the normal refinement of somatomotor-thalamic connectivity (10,15). Evidence of similar connectivity disturbances in the early stage of psychosis would support this hypothesis. Alternatively, if the abnormalities are not present in early stage patients it would suggest that thalamocortical dysconnectivity may be progressive and a possible target for treatment intervention.
The anatomical details of thalamocortical functional dysconnectivity in psychotic disorders are not well known. Our prior study used a method initially developed to map anatomical connectivity in which the cortex is divided into large regions-of-interest (ROIs) corresponding to the main targets of specific thalamic nuclei (e.g. prefrontal cortex (PFC), occipital lobe) which are then used as seeds to delineate connectivity within the thalamus (16–18). While this method is excellent for localizing connectivity abnormalities within the thalamus, the use of large cortical ROIs limits anatomical specificity in the cortex and the rest of the brain. Alternatively, other groups have used the whole thalamus as a seed to identify thalamic connectivity abnormalities throughout the brain (12,14). However, by averaging BOLD signals across the entire thalamus, this approach treats the thalamus as a homogenous structure with a unitary connectivity profile, thereby obscuring network specific disturbances.
With these knowledge gaps in mind, the current investigation was undertaken to determine if similar patterns of thalamocortical dysconnectivity are observed in the early and chronic stages of psychotic disorders. Specifically, using a novel approach to better localize thalamocortical network abnormalities, we hypothesized that both chronic and early stage patients with psychosis would exhibit reduced PFC-thalamic connectivity and increased somatomotor-thalamic connectivity. Additionally, we performed exploratory analyses comparing thalamocortical dysconnectivity between schizophrenia and psychotic bipolar disorder, and examined the cognitive correlates of thalamocortical connectivity.
METHODS AND MATERIALS
Study Participants
105 healthy subjects and 148 individuals with a psychotic disorder were included in this investigation (see Table 1). The psychosis group included individuals with schizophrenia/schizoaffective disorder (i.e. non-affective psychosis) and bipolar I disorder with psychotic features (i.e. affective psychosis). 53 patients were within two years of illness onset and considered early stage (19). Most early stage psychosis patients were studied at the time of their first hospitalization for a psychotic disorder, or very shortly thereafter, and had been ill for less than 4 months on average. At the time of study participation, 25 early stage patients were diagnosed with schizophreniform disorder. Follow-up diagnostic data was available on 22 of these patients; 20 converted to a non-affective psychotic disorder, 1 remained diagnosed with schizophreniform disorder, and 1 was subsequently re-classified as affective psychosis. All subjects underwent a structured clinical interview and completed a brief cognitive assessment that included the Wechsler Test of Adult Reading to estimate pre-morbid IQ (20) and the Screen for Cognitive Impairment in Psychiatry (21) which includes tests of verbal learning, working memory, verbal fluency, and processing speed. In addition, patients were also assessed with the Positive and Negative Syndrome Scale (PANSS: 22) to quantify severity of clinical symptoms. Study procedures and exclusion criteria are described in detail in the Supplemental Material. This study was approved by the Vanderbilt University Institutional Review Board.
Table 1.
Sample Demographics
| Psychosis | Post-Hoc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy Subjects | Chronic | Early Stage | Statistics | ||||||
| N=105 | N=95 | N=53 | F/t/x2 | p | |||||
| Gender (male:female) | 61:44 | 50:45 | 39:14 | 6.29 | .043 | CP>ESP (males) | |||
| Ethnicity (White:AA:Other) | 69:29:7 | 57:31:7 | 36:14:3 | 1.17 | .882 | -- | |||
| Affective/Non-Affective Psychosis | -- | 17:78 | 20:33 | 7.14 | .008 | ||||
| Mean | SD | Mean | SD | Mean | SD | ||||
|
|
|
|
|||||||
| Age | 32.5 | 11.3 | 38.1 | 11.8 | 22.0 | 3.7 | 40.28 | <.001 | CP>HS>ESP |
| Premorbid IQ | 109.9 | 12.0 | 96.8 | 16.2 | 101.3 | 13.3 | 21.19 | <.001 | HS>CP, ESP |
| SCIP Global Cognition z-score | 0.0 | 0.68 | −1.53 | 0.94 | −0.68 | 0.87 | 77.06 | <.001 | HS>ESP>CP |
| % of fMRI Volumes Exlcuded | 6.1 | 11.4 | 12.9 | 16.4 | 7.6 | 12.5 | 6.41 | .002 | CP>ESP, HS |
| Duration of Illness (years) | -- | -- | 15.7 | 11.1 | 0.36 | 0.5 | 10.01 | <.001 | -- |
| PANSS Positive | -- | -- | 19.4 | 7.0 | 19.3 | 8.1 | 0.09 | .925 | -- |
| PANSS Negative | -- | -- | 14.6 | 6.7 | 15.3 | 8.3 | 0.53 | .597 | -- |
| PANSS General | -- | -- | 31.7 | 8.0 | 31.5 | 8.6 | 0.14 | .886 | -- |
| CPZ Equivalents | -- | -- | 464.6 | 232.2 | 325.4 | 171.4 | 3.15 | .002 | -- |
Abbreviations: CP=Chronic Psychosis; CPZ=Chlorpromazine; ESP=Early Stage Psychosis; HS=Healthy Subjects; PANSS=Positive and Negative Syndrome Scale; SCIP=Screen for Cognitive Impairment in Psychiatry
Neuroimaging Data Acquisition and Functional Connectivity Analysis
Imaging data acquisition and pre-processing are described in detail in the Supplemental Material. Briefly, a 7-minute echo-planar imaging resting-state scan and a high-resolution T1-weighted structural scan were collected on each subject. Functional images were slice-time corrected, motion corrected, co-registered to native space structural data, and normalized to MNI space. As described earlier, prior investigations of thalamocortical functional dysconnectivity in psychosis have either: 1) parceled the cortex into large, anatomically defined ROIs corresponding to the primary cortical targets of specific thalamic sub-regions (e.g. PFC) and used these as seeds to identify functional connectivity within the thalamus; or 2) used the whole thalamus as a seed to map thalamic connectivity with the rest of the brain (10,12,14). The primary advantage of the first method is that it can map multiple thalamic networks and segment the thalamus according to its functional connectivity (16). However, the use of large cortical ROIs limits spatial specificity within the cortex. Using the whole thalamus as a seed overcomes this limitation, but, by averaging BOLD signals from the entire thalamus, precludes an analysis of specific thalamocortical networks.
To overcome these limitations, we combined elements of both methods. First, we performed a ‘cortical ROI-to-thalamus ROI’ analysis in which connectivity between anatomically defined cortical ROIs and functionally defined thalamic ROIs was calculated and compared across groups (see Figure 1A). This primary analysis was followed up with seed-based analyses examining connectivity of functionally defined thalamic sub-regions with the rest of the brain. The analysis steps were as follows. First, as described previously, the cortex was divided into six, a-priori defined non-overlapping ROIs shown in Figure 1A (10). Functional connectivity maps, restricted to the Harvard-Oxford thalamus probabilistic atlas (thresholded at 10%), were then created for each cortical ROI. Using the entire dataset of 253 individuals, the thalamus was parceled into functional ROIs using the ‘winner take all’ strategy (15); each voxel in the thalamus was assigned to the cortical ROI it was most strongly connected to. Average connectivity of the voxels within each thalamic ROI with its respective cortical ROI was then calculated resulting in 6 thalamocortical ‘network’ values for each subject; one for each cortical anatomical ROI and its corresponding thalamic functional ROI which served as the dependent variables in the statistical analysis. Thalamocortical network values were analyzed using multivariate repeated measures ANOVA with network entered as the within the subjects variable, group a between subjects variable, and age and sex entered as covariates.
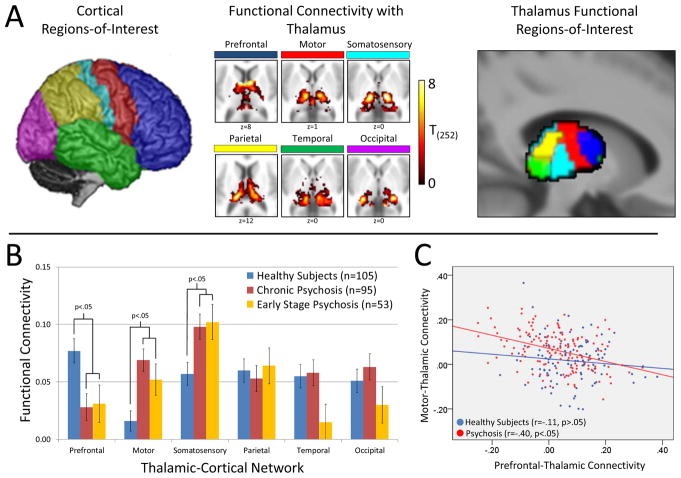
Figure 1.
Thalamocortical functional connectivity in chronic and early stage psychosis. Panel A: Within the entire sample of 253 subjects, connectivity of 6 a-priori defined cortical areas (left) correlated with distinct, largely non-overlapping regions of the thalamus (middle). The thalamus was parceled into functional ROIs using the ‘winner-take-all’ approach in which each voxel in the thalamus is classified according to which cortical ROI it is most strongly connected to (right). Connectivity between each cortical ROI and its corresponding thalamic functional ROI was then calculated for each subject. Panel B: Thalamic-cortical network functional connectivity varied between groups (repeated measures ANOVA network × group interaction: F(10,490)=3.20, p=.001). Follow-up univariate ANOVAs indicated that, compared to healthy subjects, PFC-thalamic network connectivity was reduced in both chronic (p=.002) and early stage (p=.020) psychosis, whereas connectivity in the motor-thalamic and somatosensory-thalamic networks was increased in chronic (p=.00006 and p=.006, respectively) and early stage psychosis (p=.027 and p=.014, respectively). Panel C: PFC-thalamic network connectivity inversely correlated with motor-thalamic network connectivity in patients with psychosis (r=-.40, p=.0000006), but not healthy subjects (r=-.11, p=.264). Direct comparison between groups indicated that the correlation was significantly greater in the psychosis group compared to healthy subjects (Fischer Z=2.42, p=.016).
The ‘cortical ROI-to-thalamic ROI’ analysis described above was followed up with seed-based connectivity analyses using the thalamic functional ROIs as seeds to examine connectivity of specific thalamic sub-regions with the rest of the brain. Briefly, the mean BOLD times series derived from the thalamic ROIs was extracted from each subjects’ unsmoothed functional data and entered into a general linear model to create functional connectivity maps of specific thalamic ROIs. The functional connectivity maps, in beta units, were then smoothed (6 mm) and entered into a one-way ANOVA with group entered as the between subjects variable, and age and sex as covariates. A priori contrasts comparing healthy subjects to psychosis patients and each patient group (i.e. chronic, early stage) to healthy subjects were performed. Results were thresholded at the cluster-level pFWE-corrected=.05 for voxel-wise p=.005, masked to include only voxels that demonstrated significant positive functional connectivity in healthy subjects and/or psychosis patients at the cluster-level pFWE-corrected=.05 for voxel-wise p=.005.
Functional connectivity maps for the thalamic ROIs were created using the CONN-fMRI Functional Connectivity toolbox (24). Briefly, the mean BOLD time series was extracted from an ROI and entered as a predictor in a multiple regression general linear model (GLM). Regressors corresponding to the 6 motion correction parameters and their first temporal derivatives, along with grey matter, white matter, and CSF were included to remove variance related to head motion, the global grey matter signal, white matter, and CSF, respectively. Functional data were band-pass filtered (.01-.10 Hz). We took several steps to limit the effects of head motion. First, resting-state scans underwent “motion scrubbing” as described by Power et al. (25). Volumes with frame-wise displacement greater than 0.5 and BOLD intensity changes between frames greater than 0.5% were identified and excluded from the functional connectivity analysis by including the tagged scans as nuisance regressors in the connectivity GLM. Second, nuisance regressors for white matter and CSF were derived from each subject’s white matter and CSF segmentations using the anatomical component-based noise reduction method (aCompCor), as implemented in the Conn-fMRI toolbox. The aCompCor method has been shown to be effective at reducing the effects of head movement on functional connectivity estimates (26). Finally, motion correction parameters were regressed out before temporal band pass filtering was applied as performing these steps in reverse order (i.e. band-pass filtering before nuisance regression) over-estimates connectivity and exacerbates the effects of head motion due to re-introduction of nuisance related variation (27).
RESULTS
Demographic, cognitive, and clinical data are presented in Table 1. The overall patient cohort was well matched to healthy subjects on sex (healthy subjects; 58.1% male; psychosis: 60.1% male; x2(1)=0.11, p=.745) and age (healthy subjects: 32.5; patients: 32.3; (251)=0.07, p=.941). Importantly, the distribution of ages was virtually identical in healthy subjects and patients (see Supplementary Figure S1). As expected, early stage patients were younger than healthy subjects (p<.001), which, in turn, were younger than chronic patients (p<.001). Average daily dose of antipsychotic, in chlorpromazine equivalents based on Gardner et al., (28), was higher in chronic patients (478.0±247.8 mg vs. 308.4±174.3 mg; t(128)=4.08, p<.001).
Thalamocortical Functional Connectivity: Cortical ROI-to-Thalamic ROI Analysis
As shown in Figure 1A and Supplemental Figure S2, functional sub-divisions of the thalamus were very consistent with prior studies that have parceled the thalamus based on its functional and structural connectivity (e.g. 15,17,18).
Results of the cortical ROI-to-thalamic ROI analysis is presented in Figure 1B. Multivariate repeated measures ANOVA revealed a significant network × group interaction (F(10,490)=3.20, p=.001), but no main effects of network (F(5,244)=1.56, p=.165) and group (F(2,248)=1.22, p=.298). Follow-up univariate ANOVAs indicated that the interaction was due to significant group differences in PFC-thalamic (F(2,248)=5.97, p=.003), motor-thalamic (F(2,248)=8.96, p=.0002), and somatosensory-thalamic (F(2,248)=5.37, p=.005) networks. Compared to healthy subjects, PFC-thalamic network connectivity was reduced in both chronic (p=.002) and early stage (p=.020) psychosis, whereas motor-thalamic and somatosensory-thalamic network connectivity was increased in chronic (p=.00006 and p=.006, respectively) and early stage psychosis (p=.027 and p=.014, respectively). In terms of effect sizes (ES), the reduction in PFC-thalamic hypo-connectivity was comparable in chronic (ES=−0.45) and early stage (ES=−0.43) patient groups. Motor-thalamic hyper-connectivity was somewhat larger chronic compared to early stage patients (ES=0.59 and ES=0.41, respectively); although somatosensory-thalamic hyper-connectivity was virtually identical in chronic and early stage groups (ES=0.40 and 0.43, respectively).
To confirm the results were not affected by head motion, scanner assignment, age, and sex, we performed a series of analysis controlling for these effects as best as possible (see Supplemental Material). In brief, adding scanner or percentage of functional volumes excluded due to head motion as covariates did not affect the results (see Supplemental Figure S3). We also obtained very similar results when we compared sub-groups of patients and healthy subjects matched on age and sex, and age, sex, and head motion.
In light of a previous report linking PFC-hypo-connectivity and somatomotor-thalamic hyper-connectivity in psychosis (12), we examined correlations between PFC-thalamic, motor-thalamic, and somatosensory-thalamic networks. Replicating the results of Anticevic et al. (12), we found a significant inverse correlation between PFC-thalamic network under-connectivity and motor-thalamic network hyper-connectivity in patients with psychosis (r=-.40, p<.001). However, in contrast to Anticevic et al. (2013), this relationship was not present in healthy subjects (r=-.11, p=.264). As shown in Figure 1C, the correlation was significantly greater in the psychosis group compared to healthy subjects (Fisher Z=2.42, p=.016). Somatosensory-thalamic connectivity was unrelated to PFC-thalamic connectivity in both psychosis (r=-.02, p=.816) and healthy subjects (r=.01, p=.906)
Additional analyses examining the relationship between thalamocortical functional connectivity and diagnosis, antipsychotic medication, clinical symptoms, and cognitive functioning were also performed. As shown in Supplemental Figure S4, after adjusting for age, sex, and illness stage, PFC-thalamic network connectivity was lower in non-affective psychosis patients compared to affective psychosis at the trend significance level (F(1,143)=3.11, p=.080). In contrast, there were no differences between affective and non-affective psychosis in motor (F(1,143)=0.03, p=.869) and somatosensory (F1,143)=0.34, p=.562) network connectivity. Among patients, antipsychotic dose, PANSS positive, negative, and general scores, and overall cognitive function (i.e. SCIP global Z-score) did not correlate with functional connectivity of any thalamocortical network (all r values<|.11|, p>.217).
Thalamocortical Functional Connectivity: Thalamus ROI Seed-based Analysis
The ‘cortical ROI-to-thalamus ROI’ analysis was followed up with seed-based analyses using the thalamic PFC, motor, and somatosensory sub-regions as seeds to better localize thalamic dysconnectivity with the rest of the brain. Functional connectivity of the thalamus PFC, motor, and somatosensory seeds in healthy subjects and individuals with psychosis are shown rendered on the cortical surface and cerebellum in Figure 2, and on serial axial slices covering the entire brain in Supplemental Figure S5 (cortical and cerebellum renderings were created using Caret v5.65; http://brainmap.wustl.edu/caret.html).
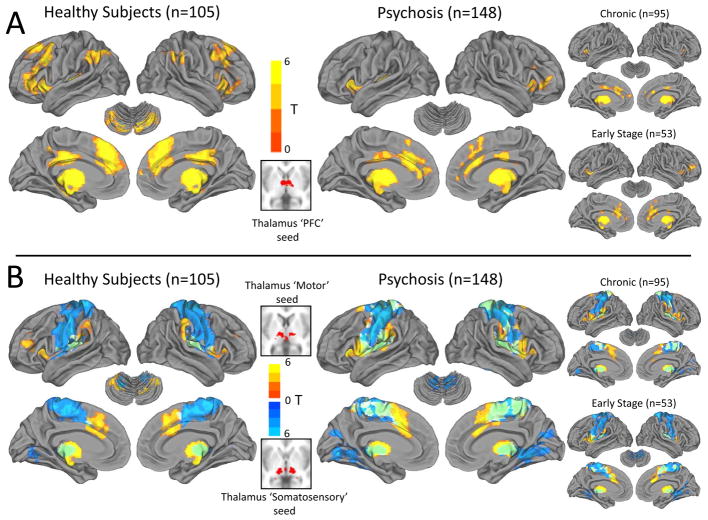
Figure 2.
Functional connectivity of PFC, motor, and somatosensory thalamus seeds in psychosis. Panel A: Functional connectivity of the ‘PFC’ thalamus seed in healthy subjects resembled the well-described “executive control” network (i.e. Niendam et al., 2012) and included the dorsolateral PFC, medial PFC/anterior cingulate, mid-cingulate, inferior parietal lobule, and cerebellum. This pattern was markedly attenuated in psychosis, including both chronic and early stage patients. Panel B: Functional connectivity of the thalamus ‘motor’ seed (warm colors) and ‘somatosensory’ seed (cool colors) included mainly motor and somatosensory areas and cerebellum. Qualitatively, functional connectivity of the thalamus motor and somatosensory seeds appeared more widespread and less segregated in psychosis patients (overlap in motor and somatosensory thalamus seed connectivity is shown in green).
In healthy subjects, the thalamic PFC seed was functionally connected to dorsolateral PFC, inferior frontal gyrus/anterior insula, anterior cingulate cortex (ACC), inferior parietal lobule (supramarginal and angular gyri), mid cingulate, left transverse temporal gyrus, and posterior quadrangle of the cerebellum. Sub-cortically, significant connectivity was detected with the caudate, especially the head of the caudate, and putamen (see Supplemental Figure S5). This pattern of connectivity bears a striking resemblance to the fronto-parietal “executive control” network (e.g. 29). Functional connectivity of the motor and somatosensory thalamus seeds was restricted almost exclusively to primary and secondary motor and somatosensory cortex, striatum, and cerebellum. In psychotic disorders, functional connectivity of the thalamus PFC seed was restricted to the medial PFC and ACC; inferior frontal gyrus/anterior insula; mid-cingulate; and left transverse temporal gyrus (see Figure 2 and Supplemental Figure S5). Connectivity with the cerebellum was notably absent. In contrast, connectivity of the motor thalamus seed was more extensive and there was greater overlap between motor and somatosensory seeds in midline cortical areas. Functional connectivity patterns were very similar in chronic and early stage psychosis patients.
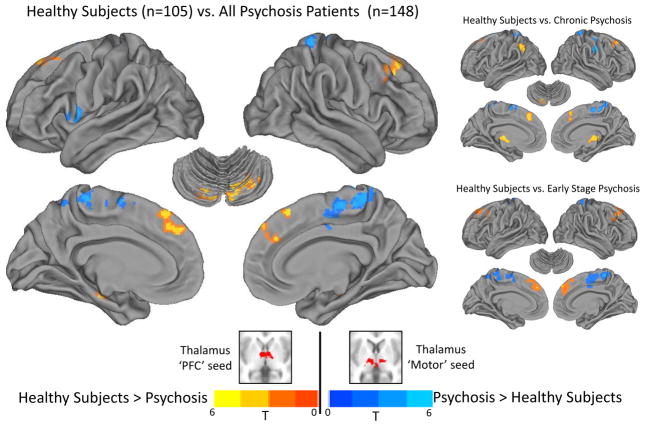
Direct comparison between healthy subjects and psychosis patients confirmed that some of the qualitative differences observed between groups were statistically significant (see Figure 3 and Table 2). As shown in Figure 3, thalamus PFC seed connectivity with medial superior frontal gyrus extending laterally to middle frontal gyrus was reduced in psychosis. Thalamic PFC seed connectivity with cerebellum was reduced bilaterally. Chronic patients demonstrated less connectivity with medial and lateral aspects of the superior frontal gyrus, left cerebellum, and left inferior parietal lobule. No differences between healthy subjects and early stage psychosis patients were observed at the a-priori defined statistical threshold. However, relaxing the voxel-wise threshold to p=.05, while still maintaining a cluster-level corrected threshold of the pFWE-corrected=.05, revealed a very similar pattern of thalamic dysconnectivity in early stage psychosis. In contrast to the thalamus PFC seed, psychosis patients exhibited greater motor thalamus seed connectivity with a cluster that included midline Brodmann’s areas 6 and 4 and left precentral gyrus corresponding to Brodmann’s area 44. In chronic patients, hyper-connectivity of the thalamus motor seed localized to the right lateral precentral gyrus corresponding to Brodmann’s area 6, midline cortical areas corresponding to medial aspects of Brodmann’s areas 4 and 6, and left postcentral gyrus corresponding to Brodmann’s area 5. No significant differences between healthy subjects and early stage psychosis patients were observed at the corrected significance level. However, relaxing the voxel-wise threshold to p=.05, while still maintaining a whole-brain cluster-level corrected threshold of pFWE-corrected=.05, revealed a similar pattern of thalamus motor seed hyper-connectivity in early stage psychosis; elevated connectivity with medial aspects of precentral and postcentral gyrus, and lateral precentral gyrus. With respect to the thalamus somatosensory seed, no differences in functional connectivity were detected between healthy subjects and psychosis patients. In order to better appreciate the similar patterns of thalamic dysconnectivity in chronic and early stage psychosis, the results of the voxel-wise contrasts comparing each illness stage group to healthy subjects are also presented in Supplemental Figure S6 without statistical thresholding.
Figure 3.
Functional dysconnectivity of the thalamus PFC and motor seeds in psychosis. Compared to healthy subjects, functional connectivity of the PFC thalamus seed with lateral PFC (i.e. superior frontal gyrus), anterior medial PFC, and cerebellum was reduced in individuals with a psychotic disorder (warm colors). In contrast, functional connectivity of the thalamus motor seed was increased in patients with a psychotic disorder in medial and ventral-lateral primary motor cortex (cool colors). Chronic and early stage psychosis patients exhibited very similar patterns of functional connectivity abnormalities. All results thresholded at cluster-level corrected pFWE-corrected=.05 for voxel-wise p=.005 (p=.05 for the healthy subjects vs. early stage psychosis patients contrast).
Table 2.
Group differences in functional connectivity of thalamus seed regions at a Cluster-level Corrected p(FWE)=.05
| Brain Area | All Psychosis Patients | Chronic Psychosis | Early Stage Psychosis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxels | MNI | Peak t | Voxels | MNI | Peak t | Voxels | MNI | Peak t | |||||||
| x | y | z | x | y | z | x | y | z | |||||||
| Thalamus PFC Seed | |||||||||||||||
| Healthy Subjects > Psychosis | |||||||||||||||
| R. Cerebellum, Posterior Lobe | 669 | 10 | −78 | −28 | 5.02 | ||||||||||
| 30 | −70 | −28 | 4.25 | ||||||||||||
| 40 | −66 | −40 | 3.76 | ||||||||||||
| L. Cerebellum, Posterior Lobe | 351 | −10 | −76 | −30 | 4.77 | 352 | −8 | −78 | −28 | 4.66 | |||||
| L./R. Superior/Medial Frontal Gyrus (BA 8/9) | 1507 | −30 | 24 | 54 | 3.95 | 1267 | −30 | 22 | 54 | 3.90 | 2780 | −22 | 34 | 46 | 2.51 |
| 18 | 34 | 50 | 3.49 | 18 | 32 | 50 | 3.79 | 24 | 28 | 40 | 3.61 | ||||
| −6 | 46 | 36 | 3.55 | −2 | 34 | 40 | 3.72 | −6 | 46 | 38 | 3.18 | ||||
| 4 | 52 | 42 | 3.53 | 5 | 52 | 42 | 3.52 | ||||||||
| Thalamus | 471 | 6 | −22 | 2 | 4.76 | ||||||||||
| −2 | −8 | −4 | 3.64 | ||||||||||||
| 18 | −28 | 4 | 3.50 | ||||||||||||
| L. Inferior Parietal Lobule (BA 39/40) | 508 | −46 | −50 | 42 | 4.25 | ||||||||||
| −62 | −60 | 38 | 4.05 | ||||||||||||
| −32 | −62 | 42 | 3.26 | ||||||||||||
| Psychosis > Healthy Subjects | No Significant Clusters | ||||||||||||||
| Thalamus Motor Seed | |||||||||||||||
| Healthy Subjects > Psychosis | No Significant Clusters | ||||||||||||||
| Psychosis > Healthy Subjects | |||||||||||||||
| L. Precentral/Inferior Frontal Gyrus (BA 44) | 335 | −60 | 10 | 6 | 4.9 | ||||||||||
| R./L. Paracentral Lobule (BA 4) | 1320 | 4 | −32 | 62 | 4.12 | 2068 | 4 | −32 | 62 | 3.41 | |||||
| −14 | −30 | 60 | 3.14 | ||||||||||||
| R. Medial Frontal/Postcentral Gyrus (BA 6) | 8 | −26 | 80 | 3.88 | 567 | 8 | −26 | 80 | 4.47 | 8 | −6 | 50 | 2.93 | ||
| 8 | −18 | 58 | 3.72 | ||||||||||||
| 8 | −42 | 72 | 3.86 | ||||||||||||
| L. Superior/Medial Frontal Gyrus (BA 6) | −12 | 6 | 72 | 3.93 | 753 | −12 | 6 | 72 | 4.43 | ||||||
| −14 | 4 | 64 | 4.32 | ||||||||||||
| −2 | −4 | 68 | 3.75 | ||||||||||||
| R. Pre/Postcentral Gyrus (BA 3/4) | 431 | 44 | −16 | 56 | 3.83 | ||||||||||
| 62 | −10 | 36 | 5.19 | ||||||||||||
| 46 | −14 | 42 | 2.83 | ||||||||||||
| L. Postcentral Gyrus (BA 5/7) | 424 | −14 | 50 | 68 | 3.7 | ||||||||||
| −30 | −40 | 64 | 3.63 | ||||||||||||
| −24 | −46 | 64 | 3.47 | ||||||||||||
Abbreviations: BA=Brodmann Area; FWE=Family-wise Error; L=left; MNI=Montreal Neurological Institute; PFC=Prefrontal Cortex; R=right
Exploratory analyses were performed examining the relationship between thalamic functional connectivity and cognition, clinical symptoms, and psychotic disorder diagnosis. Functional connectivity, in beta units, was extracted from the clusters identified in the healthy subjects vs. psychosis contrasts and averaged to create two values per subject; one indicating average PFC thalamic under-connectivity, the other indicating average motor thalamus hyper-connectivity. In ES terms, thalamus PFC seed hypo-connectivity was similar in chronic and early stage patients (ES=−0.94 and −0.87, respectively), as was thalamus motor seed hyper-connectivity (ES=0.78 and 0.85, respectively). After controlling for group, average connectivity in the PFC thalamus seed regions demonstrating reduced connectivity in psychosis correlated with SCIP Global z-score of cognitive functioning across all subjects (partial r=.14, p=.029). This relationship was strongest for the verbal learning sub-test (partial r=.18, p=.006). Scatterplots depicting these correlations are presented in Supplemental Figure S7. Motor thalamus hyper-connectivity was unrelated to cognitive functioning (partial r=-.06, p=.319). Neither PFC hypo-connectivity nor motor thalamus seed hyper-connectivity was related to positive, negative, and general symptoms from the PANSS (all r values<.|14|, p>.101). Consistent with the cortical ROI-to-ROI analysis presented earlier, PFC-thalamic seed connectivity was lower in non-affective psychosis compared to affective psychosis at the trend significance level after controlling for age, sex, and illness stage (F(1,143)=2.86, p=.093) (see Supplementary Figure S8). Motor functional connectivity did not differ between psychotic disorders (F(1,143)=0.12, p=.730).
DISCUSSION
We confirmed that the combination of reduced PFC-thalamic connectivity and somatomotor-thalamic hyper-connectivity observed in prior investigations of chronic patients is present in the early stage of psychosis. Models of the etiology of psychotic disorders have oscillated over time between neurodegenerative and neurodevelopmental models (30). Mounting evidence indicates that both processes are likely involved; some abnormalities are detected early in the course of psychotic illnesses and remain relatively static, whereas other abnormalities emerge over time and progressively worsen (31,32). Based on earlier findings in chronic patients, we hypothesized that reduced PFC-thalamic connectivity and increased somatomotor-thalamic in psychosis may result from abnormal late brain maturation that derails the normal development of PFC-thalamic circuitry and refinement of somatomotor-thalamic connectivity (10). The current results obtained from a sample of early stage patients are consistent with a neurodevelopmental explanation for thalamocortical dysconnectivity. Nonetheless, our results, while consistent with neurodevelopmental models, cannot definitively confirm that thalamocortical network abnormalities result from atypical neurodevelopment. It remains possible that thalamocortical dysconnectivity emerges prior to or at the onset of psychosis. Recent findings from a cross-sectional investigation showing reduced dorsal caudate connectivity with PFC and thalamus in individuals at high risk for psychosis further support a developmental basis for thalamic dysconnectivity (33). Longitudinal investigations of high-risk/prodromal patients will be useful in further pinpointing the timing and clarifying the functional relevance of cortico-striatal-thalamic dysconnectivity.
The present investigation also clarifies the anatomical specificity of thalamic circuitry abnormalities. Prior studies investigated either connectivity of large swaths of cortex with the thalamus or connectivity of the whole thalamus with the rest of the brain. The former approach provides excellent anatomical specificity within the thalamus, but at the cost of cortical specificity (e.g. 10), while the second approach provides better specificity within the cortex and rest of the brain, but by treating the thalamus as a homogeneous structure obscures network specific abnormalities (e.g. 12). Using a combination of ROI-to-ROI and seed-to-voxel approaches, we found that the anterior/medial-dorsal region of the thalamus is functionally connected to medial and dorsolateral PFC, mid cingulate, inferior parietal lobule, striatum, primarily the caudate, and cerebellum. The connectivity profile of the anterior/medial-dorsal thalamus bears a striking resemblance to the fronto-parietal or ‘executive control’ network that has been linked to a range of ‘higher’ cognitive functions often impaired in psychotic disorders, including working memory, cognitive flexibility, initiation, and inhibition (29). Consistent with our findings, human lesion and animal electrophysiology studies support a role for the mediodorsal thalamus in executive cognitive functions and memory (34–39). Both task-based and resting-state imaging studies have repeatedly found abnormal executive control network function in psychotic disorders; however, most investigations focused on cortical components and cortico-cortical connectivity of this network (40–42). Recently, reduced PFC-caudate was identified in first episode psychosis (43). The current results deepen our understanding of executive control network dysfunction in psychosis by showing that dysconnectivity within this network extends to the thalamus, is present in the early stage of the illness, and is related, albeit modestly, to cognition. Additionally, reduced thalamic connectivity with the cerebellum is particularly noteworthy as it further supports cortico-thalamo-cerebellum circuitry models of psychosis and is consistent with the growing appreciation of the cerebellum’s role in cognition (1,44).
Consistent with a prior investigation (12), we found that PFC-thalamic hypo-connectivity and motor-thalamic hyper-connectivity were inversely correlated in psychosis suggesting that they are related. Anatomical investigations of non-human primates have found that there is greater connectivity between mediodorsal thalamus and motor cortical areas than is often appreciated (45–47). Direct microinjection of GABA agonists into the mediodorsal nucleus leads to increased motor activity and reduced dopamine metabolism in the PFC (48). Interestingly, a recent rodent electrophysiology investigation found that modest inhibition of mediodorsal nucleus activity disrupts PFC-thalamic functional connectivity and impairs cognition (34). Combined, these findings suggest that disruption of mediodorsal thalamus function may lead to reduced PFC-thalamic functional connectivity and a corresponding increase in motor-thalamic connectivity. Human neuroimaging and animal electrophysiology investigations examining the inter-relationships between thalamic networks and the impact of selective cortical and thalamic lesions on multiple thalamic networks will help clarify the mechanisms underlying thalamocortical deficits in psychosis.
Our investigation has several limitations. First, it is unclear if functional dysconnectivity is a consequence of compromised anatomical connectivity. Altered thalamocortical structural connectivity has been reported in schizophrenia (49). However, brain regions exhibiting strong functional coupling do not always share a direct anatomical pathway suggesting functional connectivity likely represents polysnaptic connectivity (50). Multi-modal investigations will be helpful in clarifying the nature of thalamocortical dysconnectivity. Anatomical connectivity methods may also improve localization of thalamic sub-regions which are difficult to delineate using conventional anatomical imaging (51). The relatively small number of psychotic bipolar patients included in our sample is another limitation. The finding that reduced thalamic-PFC connectivity was more prominent in non-affective psychosis should be considered preliminary, especially given evidence that other brain areas, such as the ventral anterior cingulate, exhibit similar patterns of dysconnectivity in psychotic bipolar disorder and schizophrenia (52). Similarly, our sample sizes were too small to examine diagnosis by illness stage effects. This may prove important given evidence that cognitive impairment is more severe in non-affective psychosis at the early stage of the illness, in contrast to the chronic stage when affective and non-affective psychosis patients exhibit a similar degree of impairment (32).
In conclusion, we confirmed that the combination of PFC-thalamic hypo-connectivity and somatomotor-thalamic hyper-connectivity is present at both the chronic and early stages of psychotic disorders. These two features are related, lower PFC-thalamic connectivity correlates with motor-thalamic hyper-connectivity, suggesting they result from a common pathophysiological mechanism. Moreover, thalamic hypo-connectivity is characterized by reduced connectivity between the anterior/medial-dorsal thalamus and the ‘executive control’ network and correlated with cognitive functioning. Future studies are required to: 1) clarify the relationship between thalamocortical functional dysconnectivity and anatomical connectivity; 2) confirm that thalamic dysconnectivity, especially reduced connectivity with the PFC and executive control network, is more severe in non-affective psychosis; and 3) determine if there is diagnosis by illness stage interactions.
Supplementary Material
Acknowledgments
This research was supported by the NIMH (R01-MH102266 awarded to NDW; R01-MH070560 awarded to SH), the Jack Martin, MD., Research Professorship in Psychopharmacology (held by NDW), the Brain and Behavior Research Fund (NARSAD Young Investigator Award awarded to NDW), and the Vanderbilt Institute for Clinical and Translational Research (1-UL1-RR024975 NCRR/NIH). The authors are indebted to the individuals who participated in the study. We thank Kristan Armstrong, Julia Sheffield and Austin Woolard for their assistance recruiting and screening subjects for participation in the study.
Footnotes
Disclosures: No commercial support was received for the preparation of this manuscript. All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 2.Sim K, Cullen T, Ongur D, Heckers S. Testing models of thalamic dysfunction in schizophrenia using neuroimaging. J Neural Transm. 2006;113:907–928. doi: 10.1007/s00702-005-0363-8. [DOI] [PubMed] [Google Scholar]
- 3.Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. doi: 10.1007/7854_2010_55. [DOI] [PubMed] [Google Scholar]
- 4.Cerullo MA, Adler CM, DelBello MP, Strakowski SM. The functional neuroanatomy of bipolar disorder. Int Rev Psychiatry. 2009;21:314–322. doi: 10.1080/09540260902962107. [DOI] [PubMed] [Google Scholar]
- 5.Jones EG. The Thalamus. Cambridge, UK: University Press; 2007. [Google Scholar]
- 6.Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117:347–368. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- 7.Gigante AD, Young LT, Yatham LN, Andreazza AC, Nery FG, Grinberg LT, et al. Morphometric post-mortem studies in bipolar disorder: possible association with oxidative stress and apoptosis. Int J Neuropsychopharmacol. 2011;14:1075–1089. doi: 10.1017/S146114571000146X. [DOI] [PubMed] [Google Scholar]
- 8.Karbasforoushan H, Woodward ND. Resting-State Networks In Schizophrenia. Curr Top Med Chem. 2012 doi: 10.2174/156802612805289863. [DOI] [PubMed] [Google Scholar]
- 9.Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol. 2013;34:1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. Characterizing Thalamo-Cortical Disturbances in Schizophrenia and Bipolar Illness. Cereb Cortex. 2013 doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomasi D, Volkow ND. Mapping small-world properties through development in the human brain: disruption in schizophrenia. PLoS One. 2014;9:e96176. doi: 10.1371/journal.pone.0096176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klingner CM, Langbein K, Dietzek M, Smesny S, Witte OW, Sauer H, et al. Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264:111–119. doi: 10.1007/s00406-013-0417-0. [DOI] [PubMed] [Google Scholar]
- 15.Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 19.Williams LE, Avery SN, Woolard AA, Heckers S. Intact relational memory and normal hippocampal structure in the early stage of psychosis. Biol Psychiatry. 2012;71:105–113. doi: 10.1016/j.biopsych.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Test of Adult Reading. Pearson Education; 2001. [Google Scholar]
- 21.Purdon SE. The Screen for Cognitive Impairment in Psychiatry (SCIP): Administration Manual and Normative Data. Edmonton, Alberta: PNL Inc; 2005. [Google Scholar]
- 22.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinical Version (SCID-CV) Washington, D.C: American Psychiatric Press Inc; 1996. [Google Scholar]
- 24.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 25.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage. 2014;96:22–35. doi: 10.1016/j.neuroimage.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 29.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberger DR, McClure RK. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Arch Gen Psychiatry. 2002;59:553–558. doi: 10.1001/archpsyc.59.6.553. [DOI] [PubMed] [Google Scholar]
- 31.Woodward ND. The course of neuropsychological impairment and brain structure abnormalities in psychotic disorders. Neurosci Res. 2014 doi: 10.1016/j.neures.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;41:225–241. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- 33.Dandash O, Fornito A, Lee J, Keefe RS, Chee MW, Adcock RA, et al. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 2014;40:904–913. doi: 10.1093/schbul/sbt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellendonk C. Decreasing activity of the medio-dorsal thalamus in vivo impairs prefrontal-dependent cognitive behaviors. 8th Federation of European Neuroscience Societies Forum of Neuroscience.2012. [Google Scholar]
- 35.De WL, Brouns R, Kavadias D, Engelborghs S, De Deyn PP, Marien P. Cognitive, affective and behavioural disturbances following vascular thalamic lesions: a review. Cortex. 2011;47:273–319. doi: 10.1016/j.cortex.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 37.Kessler J, Markowitsch HJ. Delayed-alternation performance after kainic acid lesions of the thalamic mediodorsal nucleus and the ventral tegmental area in the rat. Behav Brain Res. 1981;3:125–130. doi: 10.1016/0166-4328(81)90033-4. [DOI] [PubMed] [Google Scholar]
- 38.Hunt PR, Aggleton JP. Neurotoxic lesions of the dorsomedial thalamus impair the acquisition but not the performance of delayed matching to place by rats: a deficit in shifting response rules. J Neurosci. 1998;18:10045–10052. doi: 10.1523/JNEUROSCI.18-23-10045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubat-Silman AK, Dagenbach D, Absher JR. Patterns of impaired verbal, spatial, and object working memory after thalamic lesions. Brain Cogn. 2002;50:178–193. doi: 10.1016/s0278-2626(02)00502-x. [DOI] [PubMed] [Google Scholar]
- 40.Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011 doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70:1143–1151. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- 44.Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 45.Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988;277:195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- 46.Kultas-Ilinsky K, Sivan-Loukianova E, Ilinsky IA. Reevaluation of the primary motor cortex connections with the thalamus in primates. J Comp Neurol. 2003;457:133–158. doi: 10.1002/cne.10539. [DOI] [PubMed] [Google Scholar]
- 47.Rouiller EM, Tanne J, Moret V, Boussaoud D. Origin of thalamic inputs to the primary, premotor, and supplementary motor cortical areas and to area 46 in macaque monkeys: a multiple retrograde tracing study. J Comp Neurol. 1999;409:131–152. doi: 10.1002/(sici)1096-9861(19990621)409:1<131::aid-cne10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 48.Churchill L, Zahm DS, Duffy P, Kalivas PW. The mediodorsal nucleus of the thalamus in rats--II. Behavioral and neurochemical effects of GABA agonists. Neuroscience. 1996;70:103–112. doi: 10.1016/0306-4522(95)00352-j. [DOI] [PubMed] [Google Scholar]
- 49.Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL, et al. Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology. 2012;37:499–507. doi: 10.1038/npp.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 52.Anticevic A, Savic A, Repovs G, Yang G, McKay DR, Sprooten E, et al. Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophr Bull. 2015;41:133–143. doi: 10.1093/schbul/sbu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.