Abstract
Background and objective
Advances in technology are providing new forms of human-computer interaction. The current study examined one form of human-computer interaction, augmented reality (AR), whereby subjects train in the real world workspace with virtual objects projected by the computer. Motor performances were compared with those obtained while subjects used a traditional human-computer interaction, i.e., a personal computer (PC) with a mouse.
Methods
Patients used goal-directed arm movements to play AR and PC versions of the Fruit Ninja video game. The two versions required the same arm movements to control the game but had different cognitive demands. With AR, the game was projected onto the desktop, where subjects viewed the game plus their arm movements simultaneously, in the same visual coordinate space. In the PC version, subjects used the same arm movements but viewed the game by looking up at a computer monitor.
Results
Among 18 patients with chronic hemiparesis after stroke, the AR game was associated with 21% higher game scores (p=0.0001), 19% faster reaching times (p=0.0001), and 15% less movement variability (p=0.0068), as compared to the PC game. Correlations between game score and arm motor status were stronger with the AR version.
Conclusions
Motor performances during the AR game were superior to those during the PC game. This result is due in part to the greater cognitive demands imposed by the PC game, a feature problematic for some patients but preferred for others. Mode of human-computer interface influences rehabilitation therapy demands and can be individualized for patients.
Keywords: stroke, recovery, direct interaction, indirect interaction, augmented reality
Introduction
Computer-assisted technology is being increasingly used to enhance physical and occupational therapy after stroke1, 2. Many current applications rely on a traditional human-computer interaction, in which a person uses a game controller such as a mouse with a desktop personal computer (PC) while looking up at a computer monitor (Figure 1A)3–5. There are potential limitations with this type of human-computer interaction, for example, a subject must perform a visuospatial transform from the coordinates of arm movement space to the coordinates of computer monitor space, and interaction with the computer’s output is indirect.
Figure 1.
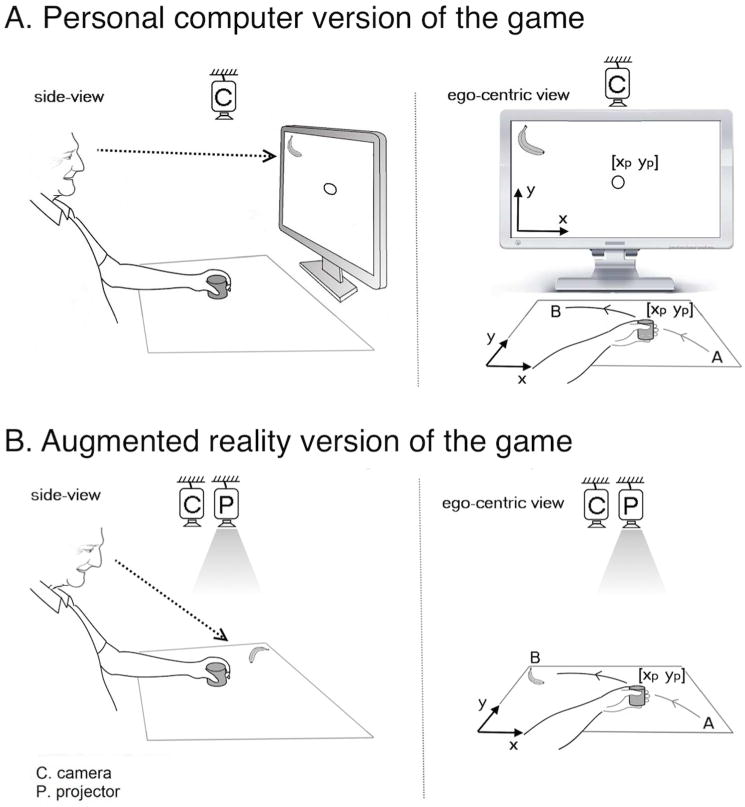
A patient playing the [A] PC and [B] AR versions of the Fruit Ninja game. Game features and movement demands were identical across the two versions of the game. Both required that the subject move the stroke-affected hand across the tabletop in order to control the movement of a cursor that earned points by slicing fruit targets. Both versions of the game leveraged the same camera; however in the PC version, game activity was displayed using a computer monitor, while in the AR version, game activity was displayed on the tabletop using a projector. This difference imposed two key differences. First, during PC play but not AR play, the subject needed to perform an extra spatial transform in order to convert the coordinates of tabletop arm movements to the coordinates of the cursor movements seen on the monitor. Second, during PC play, eye and hand movements were uncoupled, with subjects receiving proprioceptive feedback but no visual feedback (gaze was directed at the monitor not their hand), while during AR play, visual and proprioceptive feedback were coupled. These differences likely account for the significantly poorer motor performances seen with PC play.
Other human-computer interfaces6–9 have been studied, such as spatial augmented reality (AR, Figure 1B)10. With an AR-based approach, subjects interact in the real world workspace with virtual objects projected by a computer11. No visuospatial transform by the subject is required due to the fact that the subject interacts directly with the computer’s output. The extent to which a subject’s interaction with computer output is direct vs. indirect may be important at many levels12–15. With an indirect interaction, subjects do not handle the computer’s output medium; with a direct interaction, they do. For instance, using a mouse to play a game is an indirect interaction between the user and the output medium (computer monitor), whereas using a finger on an iPad surface or on a virtual object projected by a computer in order to control a game is a direct interaction between user and computer output.
These differences between a PC and an AR approach provide an opportunity to study how choice of human-computer interface affects motor performance, and ultimately stroke rehabilitation. To examine this issue, motor performance of patients with chronic stroke was measured as each played two different versions of a popular video game, one PC-based and one AR-based. The two versions required the same arm movement kinematics to control the game but differed in level of cognitive demand, which was higher with the PC approach because it uncouples a subject’s eye and hand movements and requires the subject to perform a visuospatial transform16, 17. Increased cognitive demand is an important consideration when designing computerized therapies to promote stroke rehabilitation18–20. The current study hypothesized that the higher level of cognitive demand during the PC-based game would be associated with lower game scores and less efficient movements as compared to the AR-based game.
Methods
Subjects
Patients with chronic stroke were recruited from the community. This study was approved by the Institutional Review Board, and all subjects signed informed consent. Entry criteria were age >18 years, history of stroke with onset >6 months prior, and arm weakness operationally defined as Fugl-Meyer (FM) total arm motor score21 of 15–55 (out of 66) plus at least 5 degrees active range of motion in either wrist or index finger metacarpophalangeal joint on the stroke-affected side. Exclusion criteria were severe cognitive, attentional, or language deficits; a non-stroke diagnosis that affected arm or hand function; and co-existent major neurological or psychiatric disease.
After signing consent, medical history was reviewed, followed by a brief exam that included a measure of loss of body/function, the FM arm motor scale21 as well as a measure of activity limitations, the Box & Blocks test (BBT)22, 23. Subjects then played the PC-based and the AR-based versions of the game with the paretic arm, as below.
Game design
Design of the computerized PC and AR games was modeled after the movements required by the BBT22, 23 which asks subjects to coordinate proximal and distal arm activity during a rapidly repeated task with high movement precision. We applied to this framework a simple version of the popular Fruit Ninja game, which by 2012 had been downloaded to 1 in 3 U.S. iPhones24. Thus, in both our PC and AR versions of this game, subjects were seated at a table and asked to perform precise, rapid, repeated goal-directed reaching tasks requiring coordinated hand/shoulder movements (Figure 1). A small plastic cup was held in the stroke-affected hand. This cup served as a color-marker that was tracked by the camera and guided the fruit-slicing cursor during game play; during the PC game this cup guided the on-screen cursor, while during the AR game, this cup was the cursor.
Subjects were seated and introduced to the two versions of the game. A fruit target would then appear in one of the four corners of the tabletop and start moving around the desktop workspace. The subject was to use the stroke-affected hand to move the cursor onto the fruit (which would slice the fruit in half, make a noise, and score 1 point) before the fruit disappeared (which would score 0 points). Once the fruit was sliced, or disappeared unsliced, a new fruit target would appear in one of the four corners, the sequence of which was pseudo-randomized. Subjects were instructed to slice as many fruits as possible within the allotted time. The score was presented throughout each game. One round of each game was 90 seconds long.
Subjects played one practice round each of the PC and the AR game, which were not recorded or analyzed. Next, study data were acquired as the subject played three rounds of either the PC or the AR game, the order of which was randomly assigned across subjects, followed by three rounds of the other version of the game. Subjects were provided with a brief break between each round.
PC vs. AR game setup
All game features and arm movement demands were identical across the two versions of the game and required that the subject move the stroke-affected hand around the tabletop to slice fruit. Subjects sat in the same chair at the same table. Both game versions used the same overhead camera (Microsoft LifeCam VX-2000 Webcam, 30 fps, 720p HD capture) to capture the subject’s tabletop hand movements, which moved the game’s fruit-slicing cursor. These camera images were fed to a computer running a program that tracked the color-marker and that provided real time audiovisual feedback.
PC set-up (Figure 1A)
The subject was instructed to gaze straight ahead at a 15-inch computer monitor whose position was fixed throughout the experiments. This monitor displayed the fruit targets as well as the cursor, a circle that represented the real time position of the subject’s hand and moving in a 15-inch tabletop workspace. With the PC setup, the subject’s hand was 45 degrees away from his/her direction of gaze.
AR set-up (Figure 1B)
The subject was instructed to gaze directly at the tabletop, onto which images appeared from a projector (P4-X Pico Projector, AAXA Technologies, Tustin, CA); the monitor was not used and remained blank. The projector displayed (720p HD) fruit targets, and the 15-inch workspace with four corners, directly onto the tabletop. With the AR setup, the subject gazed directly at his/her hand as it moved to slice the virtual fruit targets.
Performance metrics
The primary metric was game score. Four secondary metrics were extracted from game performance to aid interpretation of game scores:
Game score: The number of fruit targets successfully sliced during a 90-second round of game play. The 3 PC rounds were averaged to produce a single score, as were the 3 AR rounds.
Reaching time: The amount of time between reaching one fruit target and reaching the next fruit target.
Movement time consistency: The coefficient of variation of reaching times.
Response latency: The amount of time between display and target reaching, recorded for the first fruit target only in each round of the game.
Movement efficiency: The ratio of the ideal travel distance (the distance that would be travelled if the same number of targets were reached using perfect straight lines) over the actual travel distance (the actual distance travelled by each subject reaching the respective number of targets).
Statistical Data Analysis
Data were analyzed using JMP 10.0 (SAS, Cary, NC) and used two-tailed testing at alpha of 0.05. Parametric methods were used to analyze data that were normally distributed or could be transformed to a normal distribution, otherwise non-parametric methods were used. The primary, plus three secondary, game performance metrics were compared between the PC and AR versions of the game. Next, the relationship between game scores and motor status (BBT score and FM score) were calculated, with secondary analyses considering FM sub-scores. Finally, in an exploratory analysis, reaching times were compared according to the quadrant of visual space in which the fruit target first appeared, separately for the PC and for the AR game versions, and according to side of stroke.
Results
A total of 18 patients were recruited. All completed the study protocol, with no adverse events reported including no fatigue. Enrollees were on average 57 years old, 70 months post-stroke, and had mild-moderate motor deficits (Table 1).
Table 1.
Subject characteristics
| n | 18 |
| Age | 57 ± 14 years |
| Gender (F/M) | 8/10 |
| Dominant hand (R/L) | 12/6 |
| Stroke-affected Side (R/L) | 12/6 |
| Time post-stroke | 70 ± 73 months |
| Hypertension | 61% |
| Diabetes mellitus | 22% |
| Atrial fibrillation | 5% |
| FM total score | 56 ± 11 |
| FM hand/wrist subscore | 21 ± 5 |
| FM proximal subscore | 30 ± 7 |
| BBT score | |
| Affected arm | 38 ± 14 |
| Affected/unaffected arm | 0.66 ± 0.25 |
Values are mean ± SD or percentages.
Motor performance with PC vs. AR game versions
By all measures, motor performance while playing the AR-based version of the Fruit Ninja game was superior to the PC-based version. Game score, the primary metric, averaged 58±10 targets/game (mean±SD; range 42–78) with the AR-based version, 21% higher (p=0.0001) than when the same patients played the same game but using the PC-based version (48±10 targets, range 34–71). All three secondary metrics were also significantly different between AR and PC versions and provided insight into these game scores. Reaching times with AR were 19% faster than with PC (870±350 vs. 1070±510 msec, p=0.0001); consistent with faster movements in the AR-based version, subjects traveled longer distances during 90 seconds of playing the AR-based version as compared to the PC-based version (22.113±0.433 vs. 19.998±0.549 meters, p=0.0002). Movement time consistency was also better with AR, showing 15% less variability compared to PC (0.41 vs. 0.48, p=0.0068). Response latencies were 15% shorter with AR as compared to PC (1.71±0.66 vs. 2.01±0.79 sec, p=0.0249). Movements were more efficient with AR as compared to PC (60.33±14.8% vs. 55.28±14.4%, p=0.0019).Visual inspection was consistent, indicating that movements during the AR-based game were more consistent and less erratic vs. the PC-based game (Figure 2).
Figure 2.
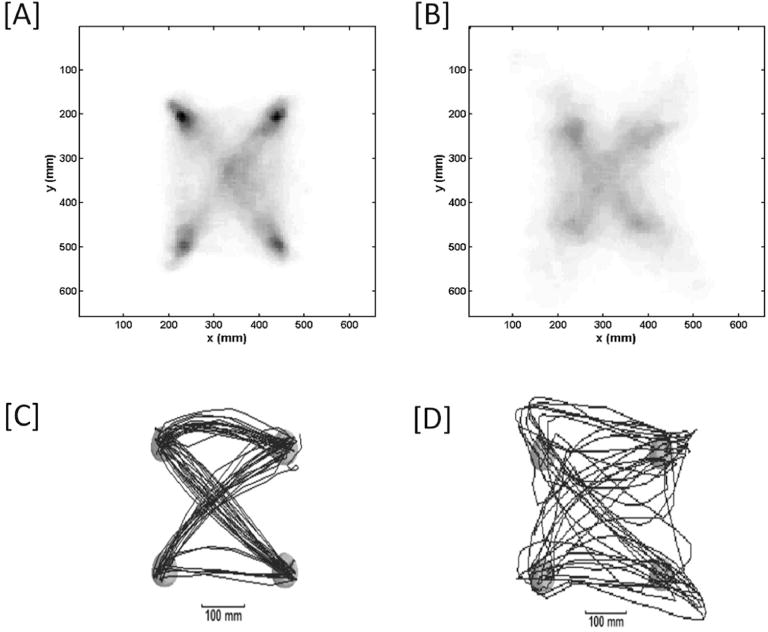
Distribution of hand position for [A] all patients playing the AR version of the game and [B] all patients playing the PC versions of the Fruit Ninja game. The target locations and straight trajectories between targets are more prominent in the AR version, while movements are more inconsistent and erratic during the PC version, consistent with quantitative values for game metrics. Also pictured are the distribution of hand position for a single exemplary subject during the [C] AR version and [D] the PC version of the game.
The rate of task learning was similar between AR and PC based on improvement in scores over time. Analysis of the scores in consecutive rounds within each version shows that in both the AR and PC versions, performances improved significantly from round 1 to 2 (from 54.88±9.24 to 60.28±9.18 in AR, p= 0.0010; and from 45.11±7.78 to 48.72±9.98 in PC, p= 0.0257), but not from round 2 to 3 (from 60.28±9.18 to 61.94±9.66 in AR, p= 0.2795; and from 48.72±9.98 to 50.22±9.47 in PC, p= 0.2991). This suggests similar learning curves for the AR-based and the PC-based versions of the game.
Behavioral correlates of PC and AR scores
AR scores correlated significantly with all four motor assessments of the stroke-affected arm (Table 2 and Figure 3), particularly the BBT score. By contrast PC scores were related to motor status in only one instance, and to a weaker degree as compared to AR scores. If a formal Bonferroni correction was applied to correct for multiple comparisons, only AR scores would remain significantly related to motor status.
Table 2.
Behavioral correlates of AR and PC game scores
| Behavioral Measure | AR game | PC game | ||
|---|---|---|---|---|
| r | p | r | p | |
| FM hand/wrist subscore | 0.56 | 0.0149 | 0.36 | 0.148 |
| FM proximal subscore | 0.52 | 0.0266 | 0.32 | 0.1957 |
| BBT score, affected arm | 0.64 | 0.0042 | 0.48 | 0.0433 |
Figure 3.
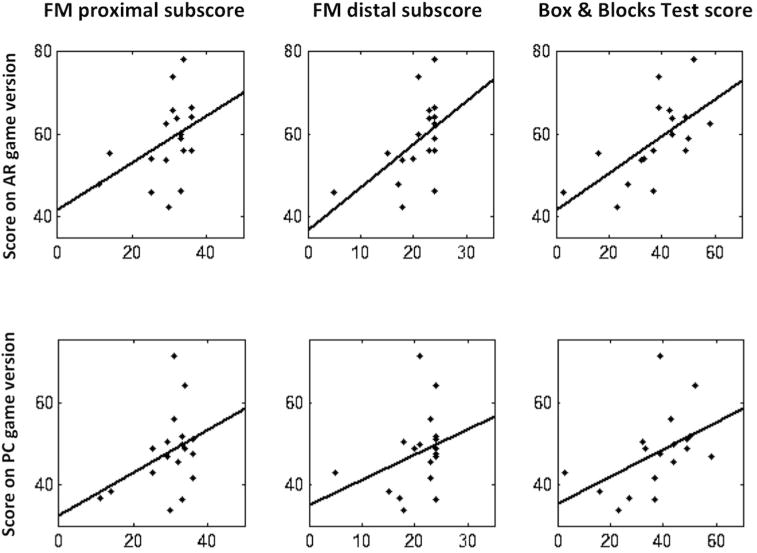
The top row shows correlation of AR game scores with the proximal Fugl-Meyer subscores, distal Fugl-Meyer subscores, and the Box & Blocks Test scores, from left to right; the bottom row shows correlation of PC game scores with the same. FM=Fugl-Meyer.
Motor performances in relation to quadrant of visual space
The quadrant into which each fruit target appeared during game play was classified in egocentric space, i.e., either near or far from the body, plus either ipsilateral or contralateral to the stroke-affected arm. During game play with the AR setting, patients with right arm motor deficits (n=12) showed the fastest reaching times when targets appeared in the near-contralateral quadrant, significantly faster vs. targets appearing in any of the three other quadrants (p = 0.0001–0.0002). The same was true when patients with left arm motor deficits (n=6) played the game with the AR setting, with reaching times being fastest for targets appearing in the near-contralateral quadrant as compared to each of the other three quadrants (p = 0.0001–0.0007). For both right arm and left arm groups, reaching times did not differ in relation to quadrant of space of target origin when the game was played using the PC setting.
Discussion
In the current study, patients with chronic post-stroke hemiparesis used the affected arm to play the Fruit Ninja game using two different human-computer interfaces, one PC and one AR. During the AR-based version of the game, as compared to PC, scores were higher, movements were faster and more consistent, and performances were more tightly linked with arm motor function. These differences are likely due to the additional cognitive demands imposed when playing the PC-based game. The current findings underscore how choice of human-computer interface can influence task demands and thus behavioral performances and is thus likely to be important when designing a stroke rehabilitation protocol.
Although movement demands were identical across the two versions of the game, cognitive demands were not. Both versions of the Fruit Ninja game required subjects to make arm movements that rapidly integrated visual and somatosensory inputs25, 26. During AR version play, subjects looked directly at their hand as it moved to control gameplay, but during PC version play, subjects instead looked at a computer screen, which increased cognitive demand. This modulation of cognitive demand can be understood in terms of a difference in the spatial congruence between movement goal and motor output27, an uncoupling between eye and hand movements16, or the imposition of an added sensorimotor transformation28. Regardless, the higher cognitive demand associated with playing the PC-based version of the game reduced game scores by 21% compared with the AR-based version (p=0.0001), in association with slower and less consistent arm movements. Differences in cognitive requirements are particularly important after stroke, as demand increases having a negligible effect in healthy subjects can exceed cognitive reserve and degrade behavioral performance in subjects with stroke16, 18–20.
Although the increased cognitive demand in the PC-based game reduced movement quality (lower scores, slower reaching, less consistent movement), this might nonetheless be the preferred rehabilitation approach for some subjects such as those needing to strengthen visuospatial skills. The brain constructs a stable spatial representation of the workspace environment29, transforming hand and target positions into a common body-centered space30–32. The AR game exists in this same space–spatial transforms for game play are the same as those used during real world activities–but the PC game plays out on the computer monitor, which has its own coordinate space. As such, with an PC-based approach, the patient must perform at least one extra spatial transform to convert the virtual world’s coordinates into body-centered coordinates. A PC approach may thus be useful to promote cognitive recovery after stroke. Furthermore, increased cognitive demand modulates activity in brain motor networks and can enhance motor learning in healthy subjects and in subjects with stroke33–36, and so might have a role in some types of motor rehabilitation. Therefore, future studies might test the hypothesis that sustained practice of the PC game, as compared to the AR game, may be associated with a larger degree of motor improvement after stroke.
Advances in technology are providing many new types of computer interface to promote brain repair after stroke. The current study examined two forms, PC and AR, incorporating the popular Fruit Ninja game into each. A PC approach to gaming substitutes a non-immersive virtual environment in place of the real world11, shifting the subject’s attention towards the virtual world (computer monitor) rather than to the real world (the tabletop upon which the paretic hand is moving)37. An AR approach to gaming overlays computer-generated virtual objects into the real world, thereby offering a direct interaction between user and computer output. Such real world activities may be more intuitive than many PC-based approaches, which might promote massed practice. An AR approach might also allow patients to practice real-life functional tasks safely, for example, a patient might work on being independent in the kitchen by working with computer-generated objects that have zero risk of spilling, burning, or electric shock. It also provides standardization and consistency across repeated trials, features that are difficult to achieve in traditional therapy involving real objects; furthermore, adopting an AR approach allows tasks to be practiced in a structured way, using highly controlled environments, and can be gamified to encourage and intensify practice. Also, feedback (multimedia, error augmentation etc.) and task difficulty are readily adjustable to the level of disability and/or personal preferences of individual patients. A PC-based approach might nonetheless have advantages, for example, to promote recovery of visuospatial skills, or by using modulation of cognitive demands to promote other forms of recovery. The optimal choice of human-computer interface likely varies across individuals, over time, and according to specific goals. The current study provides insights into how choice of human-computer interface affects motor performance and thus might influence stroke rehabilitation and motor recovery.
Acknowledgments
This work was funded by grants from the American Heart Association (13GRNT16990060) and from the National Institutes of Health (R01 NS059909, NIH/NCRR U1 TR000153, and K24 HD074722).
Footnotes
Conflict of interest disclosure
H.M. Hondori: none, M. Khademi: none, A. McKenzie: none, L. Dodakian: none, C.V. Lopes: none, S.C. Cramer: consultant/advisory board; modest; GlaxoSmithKline, Microtransponder, Dart Neuroscience, RAND Corporation, and Roche. Dr. Cramer is a cofounder of personal RN.
References
- 1.Ong S-K, Shen Y, Zhang J, Nee AY. Handbook of augmented reality. Springer; 2011. Augmented reality in assistive technology and rehabilitation engineering; pp. 603–630. [Google Scholar]
- 2.Fluet GG, Deutsch JE. Virtual reality for sensorimotor rehabilitation post-stroke: The promise and current state of the field. Current physical medicine and rehabilitation reports. 2013;1:9–20. doi: 10.1007/s40141-013-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs HI, Ferraro M, Buerger SP, Newbery MJ, Makiyama A, Sandmann M, et al. Rehabilitation robotics: Pilot trial of a spatial extension for mit-manus. J Neuroeng Rehabil. 2004;1:5. doi: 10.1186/1743-0003-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi C, Der-Yeghiaian L, Le V, Motiwala R, Cramer S. Robot-based hand motor therapy after stroke. Brain: a journal of neurology. 2008;131:425. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- 5.Housman S, Scott K, Reinkensmeyer D. A randomized controlled trial of gravity-supported, computer-enhanced arm exercise for individuals with severe hemiparesis. Neurorehabil Neural Repair. 2009;23:505. doi: 10.1177/1545968308331148. [DOI] [PubMed] [Google Scholar]
- 6.Saposnik G, Levin M. Virtual reality in stroke rehabilitation a meta-analysis and implications for clinicians. Stroke. 2011;42:1380–1386. doi: 10.1161/STROKEAHA.110.605451. [DOI] [PubMed] [Google Scholar]
- 7.Viaud-Delmon I, Gaggioli A, Ferscha A, Dunne S. Human computer confluence applied in healthcare and rehabilitation. Stud Health Technol Inform. 2012;181:42–45. [PubMed] [Google Scholar]
- 8.Herz DM, Haagensen BN, Christensen MS, Madsen KH, Rowe JB, Lokkegaard A, et al. The acute brain response to levodopa heralds dyskinesias in parkinson disease. Annals of neurology. 2014;75:829–836. doi: 10.1002/ana.24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paolucci S, Antonucci G, Grasso MG, Bragoni M, Coiro P, De Angelis D, et al. Functional outcome of ischemic and hemorrhagic stroke patients after inpatient rehabilitation: A matched comparison. Stroke. 2003;34:2861–2865. doi: 10.1161/01.STR.0000102902.39759.D3. [DOI] [PubMed] [Google Scholar]
- 10.Mousavi HH, Khademi M, Dodakian L, Cramer S, Lopes C. A spatial augmented reality rehab system for post-stroke hand rehabilitation. Studies in health technology and informatics. 2013;184:279. [PubMed] [Google Scholar]
- 11.Baus O, Bouchard S. Moving from virtual reality exposure-based therapy to augmented reality exposure-based therapy: A review. Front Hum Neurosci. 2014;8:112. doi: 10.3389/fnhum.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoedel S, Hachet M. Multi-touch rst in 2d and 3d spaces: Studying the impact of directness on user performance. 3D User Interfaces (3DUI), 2011 IEEE Symposium on. 2011:75–78. [Google Scholar]
- 13.Schmidt D, Block F, Gellersen H. Human-computer interaction–interact 2009. Springer; 2009. A comparison of direct and indirect multi-touch input for large surfaces; pp. 582–594. [Google Scholar]
- 14.Cheng K, Pulo K. Direct interaction with large-scale display systems using infrared laser tracking devices. Proceedings of the Asia-Pacific symposium on Information visualisation-Volume 24. 2003:67–74. [Google Scholar]
- 15.Forlines C, Balakrishnan R. Evaluating tactile feedback and direct vs. Indirect stylus input in pointing and crossing selection tasks. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. 2008:1563–1572. [Google Scholar]
- 16.Lajoie Y, Teasdale N, Bard C, Fleury M. Attentional demands for static and dynamic equilibrium. Experimental brain research. 1993;97:139–144. doi: 10.1007/BF00228824. [DOI] [PubMed] [Google Scholar]
- 17.Faust-Socher A, Kenett YN, Cohen OS, Hassin-Baer S, Inzelberg R. Enhanced creative thinking under dopaminergic therapy in parkinson disease. Annals of neurology. 2014;75:935–942. doi: 10.1002/ana.24181. [DOI] [PubMed] [Google Scholar]
- 18.Hondori HM, Khademi M, McKenzie A, Dodakian L, Lopes CV, Cramer SC. Abstract t mp43: Utility of augmented reality in relation to virtual reality in stroke rehabilitation. Stroke. 2014;45:ATMP43–ATMP43. [Google Scholar]
- 19.Knaut L, Subramanian S, McFadyen B, Bourbonnais D, Levin M. Kinematics of pointing movements made in a virtual versus a physical 3-dimensional environment in healthy and stroke subjects. Archives of physical medicine and rehabilitation. 2009;90:793. doi: 10.1016/j.apmr.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian S, Knaut LA, Beaudoin C, McFadyen BJ, Feldman AG, Levin MF. Virtual reality environments for post-stroke arm rehabilitation. J Neuroeng Rehabil. 2007;4:20. doi: 10.1186/1743-0003-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.See J, Dodakian L, Chou C, Chan V, McKenzie A, Reinkensmeyer DJ, et al. A standardized approach to the fugl-meyer assessment and its implications for clinical trials. Neurorehabilitation and neural repair. 2013;27:732–741. doi: 10.1177/1545968313491000. [DOI] [PubMed] [Google Scholar]
- 22.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the box and block test of manual dexterity. The American Journal of Occupational Therapy. 1985;39:386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 23.Desrosiers J, Bravo G, Hebert R, Dutil E, Mercier L. Validation of the box and block test as a measure of dexterity of elderly people: Reliability, validity, and norms studies. Archives of physical medicine and rehabilitation. 1994;75:751–755. [PubMed] [Google Scholar]
- 24.Wikipedia Contributers. Fruit ninja. 2014 Last accessed september 26 2014. Http://en.Wikipedia.Org/wiki/fruit_ninja.
- 25.Sober SJ, Sabes PN. Multisensory integration during motor planning. J Neurosci. 2003;23:6982–6992. doi: 10.1523/JNEUROSCI.23-18-06982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shumway-Cook A, Woollacott M. Attentional demands and postural control: The effect of sensory context. The journals of gerontology. Series A, Biological sciences and medical sciences. 2000;55:M10–16. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- 27.Fitts PM, Seeger CM. S-r compatibility: Spatial characteristics of stimulus and response codes. J Exp Psychol. 1953;46:199–210. doi: 10.1037/h0062827. [DOI] [PubMed] [Google Scholar]
- 28.Messier J, Kalaska JF. Differential effect of task conditions on errors of direction and extent of reaching movements. Experimental brain research. 1997;115:469–478. doi: 10.1007/pl00005716. [DOI] [PubMed] [Google Scholar]
- 29.Fiehler K, Rösler F, Henriques DY. Interaction between gaze and visual and proprioceptive position judgements. Exp Brain Res. 2010;203:485–498. doi: 10.1007/s00221-010-2251-1. [DOI] [PubMed] [Google Scholar]
- 30.Crawford JD, Henriques DY, Medendorp WP. Three-dimensional transformations for goal-directed action. Annual review of neuroscience. 2011;34:309–331. doi: 10.1146/annurev-neuro-061010-113749. [DOI] [PubMed] [Google Scholar]
- 31.Flanders M, Tillery SIH, Soechting JF. Early stages in a sensorimotor transformation. Behavioral and Brain Sciences. 1992;15:309–320. [Google Scholar]
- 32.Soechting J, Tillery SH, Flanders M. Transformation from head-to shoulder-centered representation of target direction in arm movements. Journal of Cognitive Neuroscience. 1990;2:32–43. doi: 10.1162/jocn.1990.2.1.32. [DOI] [PubMed] [Google Scholar]
- 33.Turolla A, Dam M, Ventura L, Tonin P, Agostini M, Zucconi C, et al. Virtual reality for the rehabilitation of the upper limb motor function after stroke: A prospective controlled trial. Journal of neuroengineering and rehabilitation. 2013;10:85. doi: 10.1186/1743-0003-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todorov E, Shadmehr R, Bizzi E. Augmented feedback presented in a virtual environment accelerates learning of a difficult motor task. Journal of motor behavior. 1997;29:147–158. doi: 10.1080/00222899709600829. [DOI] [PubMed] [Google Scholar]
- 35.Dennis A, Bosnell R, Dawes H, Howells K, Cockburn J, Kischka U, et al. Cognitive context determines dorsal premotor cortical activity during hand movement in patients after stroke. Stroke. 2011;42:1056–1061. doi: 10.1161/STROKEAHA.110.597880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dodakian L, Sharp KG, See J, Abidi NS, Mai K, Fling BW, et al. Targeted engagement of a dorsal premotor circuit in the treatment of post-stroke paresis. NeuroRehabilitation. 2013;33:13–24. doi: 10.3233/NRE-130923. [DOI] [PubMed] [Google Scholar]
- 37.Imam B, Jarus T. Virtual reality rehabilitation from social cognitive and motor learning theoretical perspectives in stroke population. Rehabilitation research and practice. 2014;2014:594540. doi: 10.1155/2014/594540. [DOI] [PMC free article] [PubMed] [Google Scholar]


