Abstract
Objectives
Gout prevalence is high in older adults and those affected are at risk of physical disability, yet it is unclear whether they have worse physical function.
Methods
We studied gout, hyperuricemia, and physical function in 5,819 older adults (age ≥ 65) attending the 2011–2013 Atherosclerosis Risk in Communities Study visit, a prospective US population-based cohort. Differences in lower extremity [Short Physical Performance Battery (SPPB) and 4 meter walking speed] and upper extremity function (grip strength) by gout status and by hyperuricemia prevalence were estimated in adjusted ordinal logistic regression (SPPB) and linear regression (walking speed and grip strength) models. Lower scores or times signify worse function. The prevalences of poor physical performance (first quartile) by gout and hyperuricemia were estimated using adjusted modified Poisson regression.
Results
10% of participants reported a history of gout and 21% had hyperuricemia. There was no difference in grip strength by history of gout (P=0.77). Participants with gout performed worse on the SPPB test; they had 0.77-times (95%CI:0.65,0.90; P=0.001) the prevalence odds of 1-unit increase in SPPB score and were 1.18-times (95%CI:1.07,1.32; P=0.002) more likely to have poor SPPB performance. Participants with a history of gout had slower walking speed (mean difference = −0.03, 95%CI: −0.05, −0.01; P<0.001) and were 1.19-times (95%CI:1.06,1.34; P=0.003) more likely to have poor walking speed. Similarly, SPPB score and walking speed, but not grip strength, were worse in participants with hyperuricemia.
Conclusion
Older adults with gout and hyperuricemia are more likely to have worse lower but not upper extremity function.
Gout is a common form of inflammatory arthritis in older adults (1) with a prevalence of 9.0% for men and 3.3% for women by age 65 and 13.3% for men and 6.2% for women by age 75 (2). In older adults, there is a greater burden of gout than in younger adults. Older adults with gout more commonly develop tophaceous gout without a prior history of acute arthritis (3), potentially resulting in a delay in the diagnosis and treatment of gout and subsequent development of chronic arthritis leading to functional disability. Previous studies suggest that patients with gout have greater functional disability (4, 5) resulting in lost productivity (6, 7). Among patients with gout, flares are associated with activity impairment (8). Additionally, a small study found that patients with gout have slower walking speeds with longer steps and stride lengths (9). However, little is known about direct measures of lower extremity and upper extremity physical function in older adults with gout. In fact, gout-related physical disability is thought to be an underestimated and understudied problem, especially in older adults (10).
Previous studies have hypothesized that higher levels of serum uric acid may be associated with better muscle function due to its anti-oxidant properties (11, 12). However, the role of uric acid and physical function may be more complicated because several studies suggest that hyperuricemia is associated with inflammation, which may promote muscle weakness (13, 14). The association of urate levels and grip strength has been the subject of two previous studies of Asian populations; there are no US studies of urate level and physical function. In adults of all ages, higher serum urate levels were associated with worse grip strength in one study (11), but better grip strength in the other study (12). No studies have focused on the highest risk groups, namely those with hyperuricemia.
In the Atherosclerosis Risk in Communities (ARIC) study, we sought to better understand the relationship between physical function measured in a sample of older adults (age ≥65) and a self-reported physician-diagnosis of gout as well as a measure of hyperuricemia in older age. We examined the relationship of gout and hyperuricemia on the physical measures of grip strength, the Short Physical Performance Battery (SPPB), and 4-meter walking test. Additionally, we identified which traditional gout risk factors were associated with poor physical function among older adults with gout.
MATERIALS AND METHODS
Study population and study design
ARIC is an ongoing, prospective US population-based cohort, which enrolled 15,792 middle-aged (45–64 years) adults. Participants were selected from four US communities (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland) and took part in 5 examinations: visit 1 (1987–1989), visit 2 (1990–1992), visit 3 (1993–1995), visit 4 (1996–1998), and visit 5 (2011–2013). Participants’ sociodemographic characteristics, smoking status, medication use, and medical history were collected at each visit. Participants have been contacted annually as part of the annual follow-up. Details of the ARIC cohort have been published elsewhere (15). Institutional Review Boards of the participating institutions approved the study protocols. All study participants provided written informed consent. The present study consisted of all white and black ARIC participants who self-reported gout status (see below) and had available physical function measurements (SPPB, 4 meter walk test, and grip strength) at visit 5 (n=5,819) in this cross-sectional analysis.
Assessment of gout
Gout was defined on the basis of self-reported, physician-diagnosed gout at visit 4 or the annual follow-up contact in 2011–2012, the only annual follow-up contact that included the gout query. All participants who reported a physician diagnosis of gout were considered prevalent gout cases. Self-report of a physician diagnosis of gout has been reported to be a reliable and a sensitive measure of gout (16), and has been used in other epidemiologic studies of gout (8, 17–26) including studies from the National Health and Nutrition Examination Survey (27, 28).
Physical function
Lower extremity function was measured at Visit 5 (2011–2013) using the SPPB, a performance-based assessment comprised of 3 tasks: 1) repeated chair stands, 2) standing balance, and 3) a 4-meter usual paced walk in those with and without a walk aid (meters/second [m/s]) (29). Participants received a score of 0 for a task if they were unable to complete the task; otherwise, they received scores of 1–4 based on population-based norms (29). The scores of the three tasks are summed to create the SPPB score. The SPPB score ranges from 0 to 12, with lower scores indicating poorer function (29).
Additionally, we considered walking speed (4-meter walk test in those with and without a walk aid) as a separate measure of physical function as it is strongly associated with mortality and currently considered an important vital sign of health in older adults (30, 31). For walking speed, a slower speed signifies worse function.
Grip strength in the participants’ preferred or best hand using a calibrated dynamometer was measured at Visit 5. The best grip strength of two measures spaced 15–20 seconds apart was used for analysis. Grip strength is measured in kg and lower values signify worse function. Few participants refused (n=19 for SPPB/walk test and n=6 for grip strength) and were excluded from analysis.
Assessment of gout risk factors
Potential confounders were chosen based on reported associations with gout and with physical function; all were assessed using standard protocols (15, 32). At visit 5 the following confounders were measured. Body-mass index (33) and blood pressure (34) were measured according to published methods. Hypertension was defined as measured systolic blood pressure of at least 140 mmHg or a diastolic of at least 90 mmHg, or use of a medication to treat hypertension. Participants reported their educational level and smoking status (never, former, or current). Alcohol intake was quantified as amount of regular intake per week and drinks per week of beer, wine, and liquor. Additional comorbidities, including coronary heart disease, stroke, and diabetes (measured Hemoglobin A1c>6.5% or fasting glucose>140 mg/dL), as well as kidney function (estimated glomerular filtration rate (eGFR) Chronic Kidney Disease Epidemiology Collaboration equation (35, 36)) were ascertained at visit 5. Osteoarthritis was self-reported at visit 4.
Central laboratories performed analyses on fasting specimens using conventional assays to obtain specimens including uric acid (37). At visit 5, plasma urate level was measured using the enzymatic colorimetric method.
Statistical analysis
In this study we first compared grip strength, SPPB score, and walking speed for participants with and without a previous physician-diagnosis of gout using a t-test (grip strength and walking speed) and a Kolmogorov-Smirnov test (SPPB score); this non-parametric test was used because it is a general nonparametric test which takes into account the cumulative distribution function location and shape for both of the samples. The median and the interval from the 25th to the 75th percentile (QR) were estimated for SPPB score due to a non-normal distribution. Additionally, we compared these measures of physical function by hyperuricemia (plasma urate level ≥7.0 mg/dL). We plotted walking speed by urate level stratified by sex using fractional polynomials. Then we assessed the mean difference in grip strength and walking speed by a history of physician-diagnosed gout using linear regression adjusted for age, sex, race, BMI, current smoking status, alcohol intake, hypertension, coronary heart disease, stroke, diabetes, osteoarthritis, and kidney function; comorbidities were separately included in the models. The prevalence odds ratio (POR) of SPPB scores by a history of physician-diagnosed gout was assessed using ordinal logistic regression and adjusted for the same gout risk factors that were considered confounders in the previous analysis. We also tested the association of hyperuricemia and physical function measures using the same models. Additionally, we assessed whether gout and hyperuricemia (separately) were associated with poor physical function (lowest quartile of grip strength, SPPB and walking speed) through prevalence ratios (PR) using a modified Poisson regression (38). Finally, we identified which characteristics of participants with gout were associated with poor physical function using a modified Poisson regression and restricting the study population to those with a history of a physician-diagnosis of gout. As the study was cross-sectional, PRs were estimated using modified Poisson regression.
All analyses were conducted using Stata SE, version 12.1. All reported P values are two-sided.
RESULTS
Study characteristics
Among 5,819 participants at visit 5, 42% were women, 22% were African American and the mean age was 75.5 years. Ten percent had a history of physician-diagnosed gout (7% of women; 15% of men) and hyperuricemia (≥7 mg/dL) was present in 21% of participants (16% of women and 28% of men). The characteristics of the 5,819 older adults by history of physician-diagnosed gout and by hyperuricemia are listed in Table 1; the table lists the percentage of participants with physician-diagnosed gout and hyperuricemia who have each characteristic (column percentages reported).
Table 1.
Characteristics of older adults by a history of physician-diagnosed gout and by hyperuricemia.
| No hyperuricemia (n=4,577) | Hyperuricemia (n=1,242) | No gout (n=5,224) | Gout (n=595) | |
|---|---|---|---|---|
|
| ||||
| Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | |
| Male sex | 38.3 | 56.0 | 40.1 | 60.0 |
| Black race | 19.5 | 30.6 | 20.9 | 29.9 |
| Age | 75.5 (5.1) | 75.5 (5.1) | 75.4 (5.1) | 75.8 (5.2) |
| Body mass index (kg/m2) | 28.1 (5.4) | 30.8 (5.9) | 28.5 (5.6) | 30.4 (5.7) |
| Alcohol (grams/week) | 28.1 (62.9) | 33.0 (72.4) | 27.7 (61.4) | 42.2 (90.4) |
| Current smoker | 5.8 | 5.9 | 5.9 | 5.4 |
| Coronary heart disease | 13.0 | 19.4 | 13.3 | 24.0 |
| Stroke | 3.2 | 4.4 | 3.3 | 5.0 |
| Hypertension | 70.3 | 84.7 | 72.1 | 84.7 |
| Diabetes | 26.8 | 39.5 | 28.2 | 41.3 |
| eGFR (ml/min/1.73m2) | ||||
| ≥ 90 | 11.5 | 5.6 | 10.3 | 9.2 |
| 60–89 | 66.8 | 45.4 | 64.1 | 46.1 |
| <60 | 21.7 | 49.0 | 25.6 | 44.7 |
| Osteoarthritis | 9.4 | 6.6 | 8.7 | 9.8 |
| Urate level (mg/dL) | 5.2 (1.0) | 8.0 (1.0) | 5.7 (1.5) | 6.6 (1.9) |
Grip strength in older adults
The mean grip strength in this cohort of older adults was 29.1 kg (SD=10.4); for women the mean was 23.2 kg (SD=6.4) and for men the mean was 37.2 kg (SD=9.2).
The mean unadjusted grip strength was greater in those with gout compared to those without gout (31.8 kg vs. 28.8 kg; P<0.001) (Table 2). However, these differences in grip strength can be attributed to the higher prevalence of gout in men: when stratified by sex, there was no difference in grip strength by a history of gout for women (23.9 kg vs. 23.2; P=0.08) or for men (37.0 kg vs. 37.3; P=0.61) (Table 2). Results were similar after adjusting for potential confounders (mean difference = −0.09, 95% CI: −0.70, 0.52; P=0.77) and there was no difference in the risk of having low grip strength (1st quartile of grip strength) by gout (PR=1.04, 95% CI: 0.92, 1.18; P=0.49) (Table 3). The association between gout and grip strength was not different between men and women (continuous grip strength P for interaction=0.19 and low grip strength P for interaction=0.30).
Table 2.
Physical function in older adults, by a history of physician-diagnosed gout and by hyperuricemia.
| Grip strength (kg) | SPPB score | Walking speed (m/s) | |
|---|---|---|---|
|
All participants
a
| |||
| No gout (n=5,224) | 28.8 (10.3) | 9 (7–10) | 0.90 (0.22) |
| Gout (n=595) | 31.8 (10.9) | 8 (6–10) | 0.85 (0.23) |
| P-value | <0.001 | <0.001 | <0.001 |
| No hyperuricemia (n=4,577) | 28.5 (10.1) | 9 (7–10) | 0.91 (0.22) |
| Hyperuricemia (n=1,242) | 31.6 (10.8) | 9 (6–10) | 0.86 (0.21) |
| P-value | <0.001 | <0.001 | <0.001 |
|
| |||
|
Women
b
| |||
| No gout (n=3,132) | 23.2 (6.4) | 9 (7–10) | 0.88 (0.22) |
| Gout (n=238) | 23.9 (6.5) | 8 (5–9) | 0.79 (0.23) |
| P-value | 0.08 | <0.001 | <0.001 |
| No hyperuricemia (n=2,824) | 23.1 (6.4) | 9 (7–10) | 0.88 (0.22) |
| Hyperuricemia (n=546) | 24.0 (6.6) | 8 (6–9) | 0.79 (0.21) |
| P-value | 0.003 | <0.001 | <0.001 |
|
| |||
|
Men
c
| |||
| No gout (n=2,092) | 37.3 (9.0) | 9 (8–10) | 0.95 (0.21) |
| Gout (n=357) | 37.0 (10.1) | 9 (6–10) | 0.89 (0.21) |
| P-value | 0.61 | <0.001 | <0.001 |
| No hyperuricemia (n=1,753) | 37.2 (8.9) | 9 (8–10) | 0.95 (0.21) |
| Hyperuricemia (n=696) | 37.5 (9.8) | 9 (7–10) | 0.91 (0.20) |
| P-value | 0.43 | <0.001 | <0.001 |
Note: For all three measures of physical function greater values are indicative of better function. SPPB values shown are the median (interval from the 25th to the 75th percentile). The sample size for walking speed is 5,769. Hyperuricemia: plasma urate level ≥ 7.0 mg/dL.
Among participants with walking speed measured 5,182 were not diagnosed with gout and 587 participants were diagnosed with gout. 4,545 did not have hyperuricemia at visit 5 and 1224 did have hyperuricemia at visits 5.
Among female participants with walking speed measured 3,105 were not diagnosed with gout and 236 participants were diagnosed with gout. 2,804 did not have hyperuricemia at visit 5 and 537 did have hyperuricemia at visits 5.
Among male participants with walking speed measured 2,077 were not diagnosed with gout and 351 participants were diagnosed with gout. 1,741 did not have hyperuricemia at visit 5 and 687 did have hyperuricemia at visits 5.
Table 3.
Independent association of physical function, gout and hyperuricemia in older adults
| Grip strength (kg) | SPPB score | Walking speed (m/s) | |
|---|---|---|---|
|
Adjusted mean difference in physical function
| |||
| No gout (n=5,224) | Reference | Reference | Reference |
| Gout (n=595) | −0.09 (−0.70, 0.52) | 0.77 (0.65, 0.90) | −0.03 (−0.05, −0.01) |
| P-value | 0.77 | 0.001 | <0.001 |
| No hyperuricemia (n=4,577) | Reference | Reference | Reference |
| Hyperuricemia (n=1,242) | −0.05 (−0.52, 0.43) | 0.87 (0.77, 0.98) | −0.02 (−0.03, −0.005) |
| P-value | 0.85 | 0.02 | 0.01 |
|
| |||
|
Poor physical function
| |||
| No gout (n=5,224) | Reference | Reference | Reference |
| Gout (n=595) | 1.04 (0.92, 1.18) | 1.18 (1.07, 1.32) | 1.19 (1.06, 1.34) |
| P-value | 0.49 | 0.002 | 0.003 |
| No hyperuricemia (n=4,577) | Reference | Reference | Reference |
| Hyperuricemia (n=1,242) | 0.98 (0.89, 1.07) | 1.09 (1.00, 1.19) | 1.11 (1.00, 1.22) |
| P-value | 0.60 | 0.048 | 0.04 |
All models were adjusted for: Age, sex, race, BMI, smoking status, hypertension, stroke, diabetes, CHD, osteoarthritis, kidney function, and alcohol intake. The measure of associations for poor physical function are prevalence ratios.
Ordinal logistic regression was used for SPPB score
For all three measures of physical function greater values are indicative of better function.
Low physical function was defined as the lowest quartile for grip strength (≤22 kg), SPPB (≤7) and walking speed (≤0.76 m/s).
Sample sizes for walking speed are listed in Table 1 footnotes.
Similarly, participants with hyperuricemia had significantly higher unadjusted mean grip strength (31.6 kg vs. 28.5 kg; P<0.001) (Table 2). When stratified by sex, there was higher unadjusted mean grip strength among women with hyperuricemia (24.0 vs. 23.1; P=0.003) but not for men with hyperuricemia (37.5 vs. 37.2; P=0.43). Additionally, there was no difference in grip strength by hyperuricemia after adjusting for potential confounders including age, sex, BMI, and comorbidities (mean difference = −0.05, 95% CI: −0.52, 0.43; P=0.85) and there was no difference in the risk of having low grip strength by hyperuricemia (PR=0.98, 95% CI: 0.89, 1.07; P=0.60). The association between hyperuricemia and grip strength was not different between men and women (continuous grip strength P for interaction=0.28 and low grip strength P for interaction=0.48).
SPPB in older adults
The median SPPB score was 9 (QR=7–10; mean=8.4, SD=2.3); for women the median was 9 (QR=7–10) and for men the median was 9 (QR=8–10).
The median SPPB score was lower (worse lower extremity function) in those with gout compared to those without gout (median=8, QR=6–10 vs. median=9 QR=7–10; Kolmogorov-Smirnov P<0.001) (Table 2) and the results were similar when stratified by sex: women (median=8, QR=5–9 vs. median=9, QR=7–10; Kolmogorov-Smirnov P<0.001) and men (median=9, QR=6–10 vs. median=9, QR=8–10; Kolmogorov-Smirnov P<0.001) (Table 2). Additionally, participants with gout had 0.77-times (95% CI: 0.65, 0.90; P=0.001) the prevalence odds of 1-unit increase in the SPPB score, such that those with gout had worse performance on the SPPB. Participants with gout were 1.18-times (95% CI: 1.07, 1.32; P=0.002) more likely to have poor performance on SPPB (Table 3). The association between gout and SPPB score was not different between men and women (SPPB score P for interaction=0.64). The association between gout and poor SPPB performance was different between men (PR=1.32, 95% CI: 1.13, 1.54) and women (PR=1.09, 95% CI: 0.95, 1.26) (P for interaction=0.02).
Similarly, participants with hyperuricemia had a significantly lower SPPB, (median=9, QR=6–10 vs. median=9, QR=7–10; Kolmogorov-Smirnov P<0.001) and results were similar when stratified by sex (Table 2). Participants with hyperuricemia had 0.87-times (95% CI: 0.77, 0.98; P=0.02) the prevalence odds of 1-unit increase in the SPPB score, such that those with hyperuricemia had worse performance on the SPPB. Participants with hyperuricemia were 1.09-times (95% CI: 1.00, 1.19; P=0.048) more likely to have poor performance on SPPB (Table 3). The association between hyperuricemia and SPPB score was different between men (POR=0.96, 95% CI: 0.81, 1.13) and women (POR=0.76, 95% CI: 0.64, 0.91) (P for interaction=0.03). However, there was no interaction between sex and hyperuricemia for poor SPPB performance (P for interaction=0.52).
Walking speed in older adults
The mean unadjusted walking speed was 0.90 m/s (0.22); 0.87 m/s (0.22) in women and 0.94 m/s (0.21) in men.
The mean unadjusted walking speed was lower in those with gout compared to those without gout (0.85 vs. 0.90 m/s; P<0.001) (Table 2) and the results were similar for women (0.79 vs. 0.88 m/s; P<0.001) and men (0.89 vs. 0.95 m/s; P<0.001) (Table 2). Additionally, there was a significant difference in walking speed by history of gout after adjusting for potential confounders (mean difference = −0.03 m/s, 95% CI: −0.05, −0.01; P<0.001). Older adults with gout were 1.19-times (95% CI: 1.06, 1.34; P=0.003) more likely to have poor walking speed (Table 3). The association between gout and walking speed was not different between men and women (continuous walking speed P for interaction=0.36). The association between gout and poor walking speed performance was different between men (PR=1.34, 95% CI: 1.12, 1.60) and women (PR=1.10, 95% CI: 0.94, 1.28) (P for interaction=0.008).
Hyperuricemia was also associated with lower walking speed (0.86 vs. 0.91 m/s; P<0.001) (Table 2) and the results were similar for women (0.79 vs. 0.88 m/s; P<0.001) and for men (0.91 vs. 0.95 m/s; P<0.001). Additionally, there was a significant difference in walking speed by hyperuricemia after adjusting for potential confounders (mean difference= −0.02 m/s, 95% CI: −0.03, −0.005; P=0.01). Participants with hyperuricemia were 1.11-times (95% CI: 1.00, 1.22; P=0.04) more likely to have poor walking speed (Table 3). The association between hyperuricemia and walking speed was not different between men and women (continuous walking speed P for interaction=0.29 and poor walking speed P for interaction P=0.21).
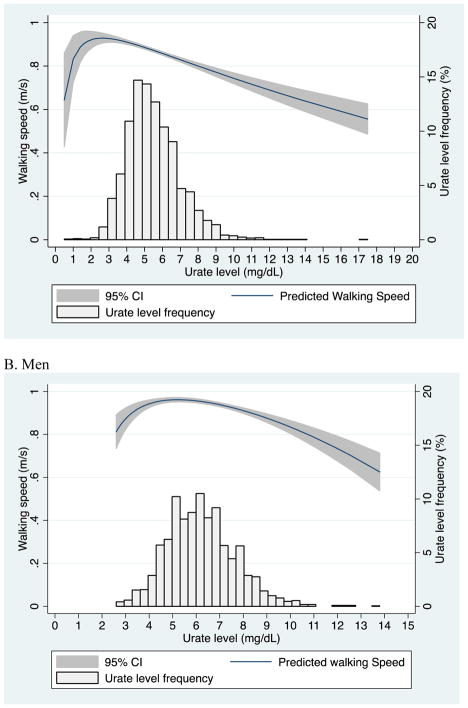
There was an increase in walking speed for increasing urate levels up to 2.5 mg/dL (a clinically uncommon urate level) in women (Figure 1A) and up to 5 mg/dL in men (Figure 1B), then there was a steady decline in walking speed with increasing urate level. For both men and women, the association of urate level and walking speed suggests that there is not a linear association of walking speed across the range of urate levels; however, only 541 participants had a urate level <4.0 mg/dL.
Figure 1.
Walking speed (m/s) by urate level and distribution of urate level: A) Women and B) Men. Predicted walking speed (m/s) estimated by fractional polynomials (unadjusted).
Predictors of poor physical function in older adults with gout
Among older adults with gout, poor grip strength was more likely in participants who were older (for every 5 year increase in age PR=1.36, 95% CI: 1.20, 1.53) and less likely for male participants (PR=0.13, 95% CI: 0.08, 0.19) and black participants (PR=0.42, 95% CI: 0.29, 0.61) (Table 4). Additionally, poor SPPB score was more likely for older participants (for every 5 year increase in age, PR=1.27, 95% CI: 1.16, 1.39), black participants (PR=1.35, 95% CI: 1.09, 1.66), participants with higher BMI (for every 5 kg/m2 increase in BMI, PR=1.10, 95% CI: 1.02, 1.19), participants who were current smokers (PR=1.45, 95% CI: 1.04, 2.04). Participants with gout who also had a history of a stroke (PR=1.47, 95% CI: 1.06, 2.04), diabetes (PR=1.54, 95% CI: 1.27, 1.88), and osteoarthritis (PR=1.60, 95% CI: 1.25, 2.03) were also at risk of poor SPPB score. Similar risk factors for poor walking speed in older adults with gout were identified: older aged participants (for every 5 year increase in age, PR=1.41, 95% CI: 1.27, 1.56), black participants (PR=1.61, 95% CI: 1.26, 2.05), participants with higher BMI (for every 5 kg/m2 increase in BMI, PR=1.14, 95% CI: 1.04, 1.24), as well as participants who also had a history of a stroke (PR=1.67, 95% CI: 1.22, 2.28), diabetes (PR=1.28, 95% CI: 1.03, 1.59), and osteoarthritis (PR=1.56, 95% CI: 1.16, 2.11).
Table 4.
Characteristics of older adults with gout who have low physical function
| Poor Grip strength (n=595) | Poor SPPB score (n=595) | Poor Walking Speed (n=551) | |
|---|---|---|---|
| Age (5 year) | 1.36 (1.20, 1.53) | 1.27 (1.16, 1.39) | 1.41 (1.27, 1.56) |
| Male sex | 0.13 (0.08, 0.19) | 0.92 (0.75, 1.12) | 0.88 (0.71, 1.11) |
| Black race | 0.42 (0.29, 0.61) | 1.35 (1.09, 1.66) | 1.61 (1.26, 2.05) |
| BMI (5 kg/m2) | 1.05 (0.95, 1.17) | 1.10 (1.02, 1.19) | 1.14 (1.04, 1.24) |
| Current smoker | 1.34 (0.86, 2.09) | 1.45 (1.04, 2.04) | 1.38 (0.98, 1.94) |
| Alcohol intake | 1.00 (0.85, 1.18) | 0.91 (0.82, 1.01) | 0.89 (0.76, 1.03) |
| Coronary heart disease | 1.00 (0.85, 1.18) | 1.24 (0.99, 1.54) | 1.18 (0.92, 1.51) |
| Stroke | 1.17 (0.68, 2.01) | 1.47 (1.06, 2.04) | 1.67 (1.22, 2.28) |
| Hypertension | 0.87 (0.63, 1.20) | 0.86 (0.64, 1.15) | 1.03 (0.72, 1.45) |
| Diabetes | 1.10 (0.84, 1.44) | 1.54 (1.27, 1.88) | 1.28 (1.03, 1.59) |
| Osteoarthritis | 1.11 (0.82, 1.50) | 1.60 (1.25, 2.03) | 1.56 (1.16, 2.11) |
| eGFR (ml/min/1.73m2) | |||
| ≥90 | Reference | Reference | Reference |
| 60–89 | 0.74 (0.47, 1.15) | 0.87 (0.60, 1.24) | 0.67 (0.60, 1.25) |
| <60 | 0.90 (0.58, 1.41) | 1.23 (0.87, 1.74) | 0.87 (0.60, 1.25) |
Prevalence ratio and 95% Confidence Intervals were estimated using modified Poisson regression models.
Low physical function was defined as the lowest quartile for grip strength (≤22 kg), SPPB (≤7), and walking speed (≤0.76 m/s).
Sensitivity analyses
Our findings on the association of hyperuricemia and physical function were similar among participants with and without a history of using a gout medication (allopurinol, probenecid, or colchicine). Additionally, the characteristics of older adults with gout who were at risk of poor physical function did not differ by disease duration; disease duration was not associated with poor grip strength (P=0.67), poor SPPB score (P=0.28) nor poor walking speed (P=0.46).
DISCUSSION
In this US population-based cohort, older adults with gout were more likely to have poor lower extremity function but not upper extremity function, consistent with the most common localization of affected joints. Older adults with gout were 1.18-times more likely to have poor SPPB scores and 1.19-times more likely to have poor walking speed even after accounting for important confounders like age, sex, BMI, and comorbidities. Similarly those with hyperuricemia had worse performance on these two measures of lower extremity function. Interestingly, grip strength was stronger in participants with gout and in participants with hyperuricemia in the unadjusted analyses. However, there was evidence that sex confounded these univariate associations and after adjustment for confounding, there was no association between gout or hyperuricemia and grip strength. We identified a nonlinear association between urate level and walking speed, possibly suggesting that both low and high urate levels are high-risk for slowed walking speed. Finally, we identified a subgroup of older adults with gout who were likely to have poor lower extremity physical function: gout patients who are older, have higher BMI, are of black race, as well as those with a history of stroke, diabetes, and osteoarthritis.
To our knowledge, this is the first US study of physical function in older adults with gout. One small study found that chronic and tophaceous gout was associated with poor hand function among patients with gout (39). Additionally, a radiographic study found that gout patients without visible subcutaneous tophi about the knee but multiple tophaceous deposits within and around the joint (identified by MRI) had limiting knee joint range of motion leading to walking disability in gout patients (40). Another small study of 25 patients with chronic gout (and 25 controls) found that patients with gout have higher levels of foot-specific disabilities, pain and impairment as well as slower walking speeds with longer steps and stride lengths (9). A study of US veterans found that men with gout were more likely to have functional limitations but that gout was not associated with these self-reported functional limitations after adjusting for comorbidities (41). Our study suggests that both older men and women with gout are at increased risk of poor lower extremity function regardless of their comorbidities. Interestingly, older adults with gout and additional comorbidities, like diabetes or osteoarthritis, are at an increased risk of poor lower extremity function.
While both clinically apparent structural changes and the absence of such changes has been associated with worse function in upper and lower extremities, our findings suggest that a clinical diagnosis of gout was associated only with lower extremity function but not upper extremity function. This difference may be due to the extent of gout progression in the ARIC cohort; it is unlikely that most of the participants who reported gout had structural changes. These previous findings of walking disability may help explain the observed association between gout and poor lower extremity function, although in most patients mechanical issues are secondary to chronic inflammation in the knee joint leading to effusion with pain and stiffness.
While no other studies to our knowledge have focused on hyperuricemia per se, previous studies have assessed urate level and physical function and have had inconsistent findings. In one study of adults aged 30 years and older, a continuous measure of urate levels was associated with worse grip strength and leg extension power (11). However, another study linked higher levels of urate to better grip strength and knee extension torque, even after adjusting for important confounders like age, sex and BMI (12). There is some biological support for this hypothesis; oxidative protein damage is independently associated with low grip strength among older women, suggesting that oxidative stress may contribute to loss of muscle strength and mass in older adults (42), and uric acid exerts a protective effect on the oxidative stress generated during physical activity (43). In this study of older adults, there was no association of hyperuricemia and grip strength after adjusting for predictors of hyperuricemia; and knee extension torque was not measured.
Several strengths and potential limitations of the present study deserve comment. In this study, both upper and lower extremity physical function were measured among several thousand older adults from the general population using standardized objective assessments rather than self-report of disability. We were able to demonstrate that the median SPPB score in this population was similar to that observed in the original publication of the SPPB (median=8) (29). This allowed us to assess a broad spectrum of functioning prior to the onset of disability. We were able to demonstrate that gout and hyperuricemia were associated with poor lower extremity function independent of risk factors, including BMI. Furthermore, we identified characteristics of older adults with gout who were likely to have poor physical function.
There are a number of limitations to recognize. Our definition of gout did not require observation of monosodium urate crystals in joint fluid or fulfillment of the American College of Rheumatology criteria (44) for gout. However, in population-based studies, synovial fluid analysis is logistically challenging. Our previous study suggests that self-reported gout is a reliable and valid measurement (16). Furthermore, ARIC does not collect radiographic measures to detect joint damage or gout-specific characteristics such as tophi or number of gout flares; we were unable to test whether gout severity was also associated with physical function. Gout was reported at visit 4 and during the most recent annual follow-up call; therefore, the exclusion criterion with the greatest impact on sample size was the report of gout status, which may introduce selection bias. The ARIC participants who were excluded were primarily those who did not answer gout queries or did not participate at visit 5. As expected, those who did not attend visit 5 were more likely to be male and older at enrollment. Our results are likely to be generalizable to older adults who are healthy enough to participate in research. The majority of African Americans were from a single site; thus associations with race could be due to regional or other factors that are not race-specific.
The study findings suggest that older adults with hyperuricemia and gout are more likely to have lower but not upper extremity dysfunction. Additionally, among older adults with gout, those who are African American, current smokers, those with higher BMI, and those with comorbid conditions like diabetes and osteoarthritis may be more likely to have lower extremity dysfunction. While the differences in walking speed and SPPB for patients with gout and hyperuricemia may appear minimal, they are clinically meaningful (45–47). In older adults, small meaningful changes were estimated as 0.05 m/s for walking speed and 0.5 units for SPPB and substantial changes were estimated as 0.1 m/s for walking speed and 1 unit for SPPB (46). A 0.1m/s slower walking speed is associated with a 12% higher mortality (47). Our novel findings suggest that older adults with gout or hyperuricemia are two groups of older adults who are likely to experience poor lower extremity function and these patients may be targeted for interventions to improve lower extremity function.
Significance and Innovations.
Older adults with gout and those with hyperuricemia were more likely to have poor lower extremity function but not upper extremity function.
There is a nonlinear association between urate level and walking speed, possibly suggesting that both low and high urate levels are high-risk for slowed walking speed.
We identified a subgroup of older adults with gout who were likely to have poor lower extremity physical function: gout patients who are older, have higher BMI, are of black race, as well as those with a history of stroke, diabetes, and osteoarthritis.
Acknowledgments
This work was jointly funded by the Arthritis National Research Foundation and the American Federation for Aging Research. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions. AK was supported by the Emmy Noether Programme (KO 3598/2–1) of the German Research Foundation. Mara McAdams-DeMarco was supported by NIH grant K01AG043501–01A1. Dorry Segev was supported by NIH grant K24DK101828.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities study
- BMI
Body Mass Index
- CI
confidence intervals
- POR
prevalence odds ratio
- PR
prevalence ratio
- QR
Quartile Range:25th – 50th Percentile
- SD
standard deviation
- SPPB
Short Physical Performance Battery
Footnotes
Author Contributions: All authors contributed: 1) to the conception and design or acquisition of data or analysis and interpretation of the data; 2) drafting the article or revising it critically for important intellectual content; and 3) final approval of the version to be published.
Sponsor’s Role: The sponsor had no role in the design, methods, recruitment, data collection, analysis, or preparation of the paper.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke B, Köttgen A, Law A, Grams M, Baer A, Coresh J, et al. Gout in Older Adults: The Atherosclerosis Risk in Communities Study. Journal of Gerontology: Series A Under Review. 2014 doi: 10.1093/gerona/glv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. Report of six cases and review of the literature. Arch Intern Med. 1992;152(4):873–6. [PubMed] [Google Scholar]
- 4.Scire CA, Manara M, Cimmino MA, Govoni M, Salaffi F, Punzi L, et al. Gout impacts on function and health-related quality of life beyond associated risk factors and medical conditions: results from the KING observational study of the Italian Society for Rheumatology (SIR) Arthritis Res Ther. 2013;15(5):R101. doi: 10.1186/ar4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker MA, Schumacher HR, Benjamin KL, Gorevic P, Greenwald M, Fessel J, et al. Quality of life and disability in patients with treatment-failure gout. The Journal of rheumatology. 2009;36(5):1041–8. doi: 10.3899/jrheum.071229. [DOI] [PubMed] [Google Scholar]
- 6.Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Current therapeutic research, clinical and experimental. 2013;75:1–4. doi: 10.1016/j.curtheres.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards NL, Sundy JS, Forsythe A, Blume S, Pan F, Becker MA. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. Journal of medical economics. 2011;14(1):10–5. doi: 10.3111/13696998.2010.540874. [DOI] [PubMed] [Google Scholar]
- 8.Khanna PP, Nuki G, Bardin T, Tausche AK, Forsythe A, Goren A, et al. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: Results from a cross-sectional survey. Health and quality of life outcomes. 2012;10:117. doi: 10.1186/1477-7525-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rome K, Survepalli D, Sanders A, Lobo M, McQueen FM, McNair P, et al. Functional and biomechanical characteristics of foot disease in chronic gout: A case-control study. Clinical biomechanics. 2011;26(1):90–4. doi: 10.1016/j.clinbiomech.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.ten Klooster PM, Vonkeman HE, van de Laar MA. Disability due to gouty arthritis. Current opinion in rheumatology. 2012;24(2):139–44. doi: 10.1097/BOR.0b013e32834ff59d. [DOI] [PubMed] [Google Scholar]
- 11.Huang C, Niu K, Kobayashi Y, Guan L, Momma H, Cui Y, et al. An inverted J-shaped association of serum uric acid with muscle strength among Japanese adult men: a cross-sectional study. BMC musculoskeletal disorders. 2013;14:258. doi: 10.1186/1471-2474-14-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Zhang D, Pang Z, Jiang W, Wang S, Tan Q. Association of serum uric acid level with muscle strength and cognitive function among Chinese aged 50–74 years. Geriatrics & gerontology international. 2013;13(3):672–7. doi: 10.1111/j.1447-0594.2012.00962.x. [DOI] [PubMed] [Google Scholar]
- 13.Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, et al. Uric acid and inflammatory markers. European heart journal. 2006;27(10):1174–81. doi: 10.1093/eurheartj/ehi879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyngdoh T, Marques-Vidal P, Paccaud F, Preisig M, Waeber G, Bochud M, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PloS one. 2011;6(5):e19901. doi: 10.1371/journal.pone.0019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 16.McAdams MA, Maynard JW, Baer AN, Kottgen A, Clipp S, Coresh J, et al. Reliability and sensitivity of the self-report of physician-diagnosed gout in the campaign against cancer and heart disease and the atherosclerosis risk in the community cohorts. J Rheumatol. 2011;38(1):135–41. doi: 10.3899/jrheum.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMarco MA, Maynard JW, Huizinga MM, Baer AN, Kottgen A, Gelber AC, et al. Obesity and younger age at gout onset in a community-based cohort. Arthritis care & research. 2011;63(8):1108–14. doi: 10.1002/acr.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maynard JW, McAdams DeMarco MA, Baer AN, Kottgen A, Folsom AR, Coresh J, et al. Incident gout in women and association with obesity in the Atherosclerosis Risk in Communities (ARIC) Study. The American journal of medicine. 2012;125(7):717 e9–e17. doi: 10.1016/j.amjmed.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maynard JW, McAdams-Demarco MA, Law A, Kao L, Gelber AC, Coresh J, et al. Racial Differences in Gout Incidence in a Population-Based Cohort: Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2013 doi: 10.1093/aje/kwt299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdams DeMarco MA, Maynard JW, Baer AN, Coresh J. Hypertension and the Risk of Incident Gout in a Population-Based Study: The Atherosclerosis Risk in Communities Cohort. The Journal of Clinical Hypertension. 2012;14(10):675–9. doi: 10.1111/j.1751-7176.2012.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams DeMarco MA, Maynard JW, Baer AN, Gelber AC, Young JH, Alonso A, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: the Atherosclerosis Risk in Communities cohort study. Arthritis and rheumatism. 2012;64(1):121–9. doi: 10.1002/art.33315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAdams-Demarco MA, Maynard JW, Baer AN, Kao LW, Kottgen A, Coresh J. A urate gene-by-diuretic interaction and gout risk in participants with hypertension: results from the ARIC study. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-201186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAdams-DeMarco MA, Maynard JW, Coresh J, Baer AN. Anemia and the onset of gout in a population-based cohort of adults: Atherosclerosis Risk in Communities study. Arthritis research & therapy. 2012;14(4):R193. doi: 10.1186/ar4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.See LC, Kuo CF, Yu KH, Luo SF, Chou IJ, Ko YS, et al. Hyperthyroid and hypothyroid status was strongly associated with gout and weakly associated with hyperuricaemia. PloS one. 2014;9(12):e114579. doi: 10.1371/journal.pone.0114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin WY, Lung CC, Liu TS, Jian ZH, Ko PC, Huang JY, et al. The association of anthropometry indices with gout in Taiwanese men. BMC endocrine disorders. 2013;13:30. doi: 10.1186/1472-6823-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan E. Gout and the risk for incident heart failure and systolic dysfunction. BMJ open. 2012;2(1):e000282. doi: 10.1136/bmjopen-2011-000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juraschek SP, Kovell LC, Miller ER, Gelber AC. Dose-response association of uncontrolled blood pressure and cardiovascular disease risk factors with hyperuricemia and gout. PloS one. 2013;8(2):e56546. doi: 10.1371/journal.pone.0056546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juraschek SP, Miller ER, 3rd, Gelber AC. Body mass index, obesity, and prevalent gout in the United States in 1988–1994 and 2007–2010. Arthritis care & research. 2013;65(1):127–32. doi: 10.1002/acr.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 30.Cummings SR, Studenski S, Ferrucci L. A Diagnosis of Dismobility-Giving Mobility Clinical Visibility: A Mobility Working Group Recommendation. JAMA : the journal of the American Medical Association. 2014 doi: 10.1001/jama.2014.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA : the journal of the American Medical Association. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol. 1996;49(12):1441–46. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 33.Idem. Operations manual no. 2: cohort component procedures, version 1.0. Chapel Hill: University of North Carolina School of Public Health; 1987. [Google Scholar]
- 34.Idem. Operations manual no. 11: sitting blood pressure, version 1.0. Chapel Hill: University of North Carolina School of Public Health; 1987. [Google Scholar]
- 35.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Astor BC, Arnett DK, Brown A, Coresh J. Association of kidney function and hemoglobin with left ventricular morphology among African Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2004;43(5):836–45. doi: 10.1053/j.ajkd.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 37.Center ARiCC. Operations manual no. 10: clinical chemistry determinations, version 1.0. Chapel Hill: University of North Carolina School of Public Health; 1987. [Google Scholar]
- 38.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 39.Dalbeth N, Collis J, Gregory K, Clark B, Robinson E, McQueen FM. Tophaceous joint disease strongly predicts hand function in patients with gout. Rheumatology (Oxford) 2007;46(12):1804–7. doi: 10.1093/rheumatology/kem246. [DOI] [PubMed] [Google Scholar]
- 40.Yu KH, Lien LC, Ho HH. Limited knee joint range of motion due to invisible gouty tophi. Rheumatology (Oxford) 2004;43(2):191–4. doi: 10.1093/rheumatology/keg478. [DOI] [PubMed] [Google Scholar]
- 41.Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Annals of the rheumatic diseases. 2008;67(9):1310–6. doi: 10.1136/ard.2007.081604. [DOI] [PubMed] [Google Scholar]
- 42.Howard C, Ferrucci L, Sun K, Fried LP, Walston J, Varadhan R, et al. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol (1985) 2007;103(1):17–20. doi: 10.1152/japplphysiol.00133.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waring WS, McKnight JA, Webb DJ, Maxwell SR. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. 2006;55(11):3127–32. doi: 10.2337/db06-0283. [DOI] [PubMed] [Google Scholar]
- 44.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 45.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. Journal of the American Geriatrics Society. 2007;55(11):1727–34. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 46.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society. 2006;54(5):743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 47.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA : the journal of the American Medical Association. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]