Abstract
Objectives
Electrical impedance myography (EIM) measurements of the tongue could provide valuable information about bulbar dysfunction in amyotrophic lateral sclerosis (ALS). A prototype tongue depressor EIM array produced gag reflexes. The objectives of this study were to determine the reliability, mean phase values, and tolerability of tongue EIM measurements using a smaller electrode array.
Methods
Tongue EIM measurements were performed in a total of 31 healthy individuals and four neuromuscular patients with lingual abnormalities. Reliability was assessed by calculating the intraclass correlation coefficient (ICC) and percent difference in addition to performing Bland–Altman analyses. Standard descriptive statistics, including results of a Mann–Whitney test, were also determined.
Results
At the 50 kHz frequency, the ICCs for intra- and inter-rater reliability were 0.76 with 5.17% difference and 0.78 with 5.34% difference respectively. The mean EIM phase values of healthy participants (11.61° ± 1.00°) and patients (9.87° ± 1.28°) were significantly different (p = 0.0051). None of the participants experienced gag reflexes or discomfort.
Conclusions
The small tongue array provided good inter- and intra-rater reliability, could preliminarily distinguish between healthy and diseased muscle, and was well-tolerated.
Significance
Biomarker information about tongue health could be more comfortably obtained with a smaller EIM array.
Keywords: Electrical impedance myography, Tongue, Bulbar, Reliability, Amyotrophic lateral sclerosis, Biomarker
1. Introduction
New biomarkers are essential to help hasten clinical therapeutic trials in amyotrophic lateral sclerosis (ALS) (Wagner, 2009). To that end, imaging, biofluid, and neurophysiological metrics have been proposed as surrogates of disease state and response to treatment (Simon et al., 2014). However, none of these measures are specific to the bulbar system. This is an important gap because bulbar dysfunction inevitably develops in the course of ALS, and approximately 25% of patients have predominantly bulbar symptoms at the time of disease onset (Haverkamp et al., 1995). Craniobulbar muscle health can be assessed by standard needle electromyography (EMG), which often serves a critical role in the diagnosis of ALS (Makki and Benatar, 2007). However, electrodiagnostic tests, including EMG, are sub-optimal tools for following disease progression and response to therapy due to associated discomfort (Gans and Kraft, 1977; Jan et al., 1999). Further, orofacial muscle status is not easily quantified. Quantitative nerve conduction-based techniques used on limbs, such as motor unit number estimation, would be challenging to apply to genioglossus; it would be necessary to stimulate the hypoglossal nerve with needle electrodes and record motor unit potentials with surface electrodes placed on the tongue. As a painless, noninvasive approach that can be used to quantify tongue health, electrical impedance myography (EIM) represents a promising complement to EMG (Table 1). Because EIM is low cost, user-friendly, and quick to perform, it could represent an excellent way to evaluate disease status and therapeutic response longitudinally.
Table 1.
Comparison of standard needle electromyography and electrical impedance myography for assessing tongue health.
| Quantitative | Painless | Non-invasive | Portable | Independent of patient cooperation | User-friendly | Affordable | Safe despite anticoagulation and bleeding diatheses | |
|---|---|---|---|---|---|---|---|---|
| Needle electromyography | ✓ | |||||||
| Electrical impedance myography | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
In EIM, a painless, high frequency, low intensity alternating electrical current is applied at a range of frequencies to a muscle of interest (Rutkove, 2009). Resultant voltages are measured in the form of resistance (R) or reactance (X). From resistance and reactance, the major outcome variable, phase, can be calculated using the equation: phase = arctan(X/R) (Rutkove, 2009). The relative magnitude of impedance values reflect changes in muscle architecture, including atrophy, inflammation, and the replacement of muscle with fat or connective tissue (Rutkove, 2009). EIM has sensitively detected disuse atrophy, myopathy, and ALS progression with limb muscle measurements (Rutkove et al., 2014, 2007; Tarulli et al., 2005, 2009). Interestingly, appendicular EIM 50 kHz phase data correlate with ALSFRS-R limb sub-scores but not ALSFRS-R total or bulbar sub-scores, suggesting that the examination of non-limb (e.g. craniobulbar muscles) with EIM will add valuable information (Rutkove et al., 2014).
A recent pilot study provided support for the application of EIM technology to the assessment of the tongue muscle in ALS. The lingual EIM 50 kHz phase values of ALS patients were significantly lower than those of healthy volunteers, and correlated with another standard measure of tongue endurance (Shellikeri et al., 2015). However, the tongue depressor electrode array used in the study induced gag reflexes in many ALS patients. Accordingly, the aim of the present study was to determine the reliability of EIM tongue measurements using a new smaller lingual electrode array in a group of healthy individuals and a selection of neuromuscular disease patients (Fig. 1).
Fig. 1.
A comparison of the large, prototype tongue depressor EIM array (left) and the new, smaller EIM array (right).
2. Methods
The Beth Israel Deaconess Medical Center Institutional Review Board approved the study protocol. A total of 31 healthy men and women ranging in age from 22 to 71 years were recruited to the study through an online advertisement. Potential participants were excluded if they reported a history of conditions that could affect the structure or function of orofacial muscles (e.g. cleft-palate, tongue piercings). A total of four neuromuscular patients with bulbar symptoms were referred for enrollment from the Beth Israel Deaconess Medical Center neurology clinic. Subjects provided written consent prior to participation.
2.1. EIM tongue probe
The EIM tongue probe was constructed on a plastic base measuring 15 cm (cm) in length. Aluminum electrodes measuring 0.4–0.5 cm were placed horizontally at one end of the plastic probe. The distance between aluminum electrodes was 0.5 cm. Wires ran from the aluminum electrodes to a commercially-available Imp SFB7 bioimpedance system.
2.2. EIM measurements
For the procedure, participants were asked to open the mouth and let the tongue rest loosely on the floor of the oral cavity. EIM measurements were then obtained with the Imp SFB7 system (Impedimed, Inc, Sydney Australia), using a new custom tongue array. After disinfection with rubbing alcohol, the probe was placed on the top surface of the anterior tongue (i.e. as one would place a tongue depressor) so that all four electrodes made contact with the tongue surface. Midline tongue measurements were performed by evaluator A, then evaluator B, and then evaluator A consecutively.
2.3. Evaluators
The evaluators included a physician trained in clinical neurophysiology and three research assistants, all of whom were taught to perform EIM measurements by the senior author. The senior author provided lessons on how to ensure collected data were technically-sound based on the real-time appearance of the multi-frequency resistance and reactance tracings. To reflect the conditions of a clinical trial, no regular oversight was provided beyond this basic training.
2.4. Data analysis
EIM phase data, expressed in degrees (°), were analyzed at the 50 kHz frequency using R statistical software and MATLAB (Mathworks, Natick MA). Reliability was assessed by calculating the intraclass correlation coefficient (ICC) and percent difference, defined as [|Measure 1 − Measure 2|/((Measure 1 + Measure 2)/2)] * 100%. Bland–Altman plots were also constructed. Mean EIM phase values and standard deviations were calculated. Because the data were non-parametric, a Mann–Whitney test was performed to preliminarily assess the degree of difference between the mean phase values of healthy and patient participants.
3. Results
3.1. Subjects
A total of 31 healthy men and women underwent tongue EIM measurements with the new small array. The age of participants without disease ranged from 22 to 71 years. An additional sample of three men and one woman aged 60–67 years with oculopharyngeal muscular dystrophy (1 patient) and ALS (3 patients) had EIM tongue measurements performed.
3.2. Reliability
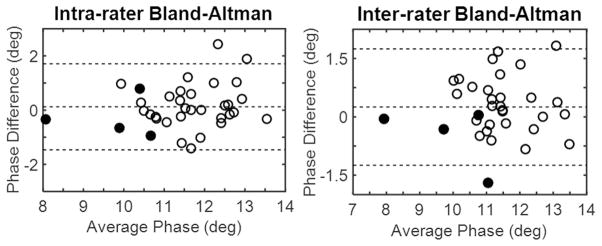
The ICC for intra-rater reliability was 0.76 with 5.17% difference (Fig. 2). The ICC for inter-rater reliability was 0.78 with 5.34% difference (Fig. 2). The data were depicted in Bland–Altman plots (Fig. 3).
Fig. 2.
ICC plots of intra-rater (left) and inter-rater (right) reliability. Data points for healthy participants are depicted as open circles while data points for patients are depicted as black circles.
Fig. 3.
Bland–Altman plots of intra-rater (left) and inter-rater (right) reliability. Data points for healthy participants are depicted as open circles while data points for patients are depicted as black circles.
3.3. Average phase values: differences between healthy and patient participants
The mean EIM phase value was 11.61° ± 1.00° for healthy participants. In the small group of patients, the mean EIM phase value was 9.87° ± 1.28°. There was a significant difference (p = 0.0051) between the mean phase values of the two groups.
3.4. Tolerability
None of the healthy or patient participants expressed discomfort in the course of tongue EIM measurements. No gag reflexes were elicited, including in the ALS patients.
4. Discussion
The findings of this study suggest that a new small tongue EIM array provides good intra- and inter-rater reliability. The tongue EIM ICCs are at least on par with the ICCs for test–retest reliability obtained using the Iowa Oral Performance Instrument, a standard measure of anterior tongue strength (Adams et al., 2014, 2015). Perhaps surprisingly, the tongue EIM repeatability data attained with the small array are also comparable to those previously reported for the larger tongue depressor EIM array (Shellikeri et al., 2015). EIM electrical current penetrates more deeply into tissue as the distance between electrodes on an array widens (Rutkove, 2009); therefore, larger EIM arrays are theoretically favored over smaller arrays. However, it appears that the inter-electrode distance on the array tested here is still sufficient. This may be because the epithelial tissue on the surface of the tongue is relatively thin, allowing good penetration of the electrical current into the underlying muscle.
Technical factors are important considerations in both the collection and interpretation of EIM information. In this study, data were analyzed at 50 kHz because most commercially-available bio-impedance devices provide only a single 50 kHz frequency of current (Rutkove, 2009). In addition, healthy muscle is most reactive at 50 kHz (Rutkove, 2009). While beyond the scope of this study, future work will assess data at several different frequencies since disease-associated changes in cellular structure do alter EIM tracing trajectories along the multi-frequency spectrum (Esper et al., 2006).
At the 50 kHz frequency, the new smaller lingual EIM array could distinguish between healthy and diseased tongue muscle. The average EIM phase value was significantly lower in patients than in healthy volunteers, consistent with the results from the pilot tongue EIM study (Shellikeri et al., 2015). This reduction in phase is also observed when taking appendicular measurements in patients with both primary myopathic and denervating diseases such as ALS (Rutkove et al., 2014; Tarulli et al., 2005).
In the pilot study conducted with ALS patients, tolerability of the prototype EIM tongue array was a concern (Yunusova and Shellikeri, personal communication). Forty-seven percent of ALS patients could not complete the protocol because of increasingly vigorous gag reflexes (Yunusova and Shellikeri, personal communication). In contrast, none of the healthy or patient participants experienced discomfort with the new, smaller tongue array tested here.
Comfort is a particularly important consideration in choosing methods of surveillance in clinical practice and research trials. While standard needle EMG can provide invaluable information about craniobulbar muscle dysfunction and help characterize underlying processes, the invasive nature of the procedure can be an understandable deterrent. Alternative testing modalities such as video fluoroscopy and the ALS Functional Rating Scale-revised (ALSFRS-R) help identify oropharyngeal deficits, but can be inaccurate since they depend on patient cooperation and subjectivity. Biomarkers offer a helpful alternative as objective indicators of disease process and response to treatment (Wagner, 2009). By generating surrogate data on early therapeutic effects, ALS biomarkers could allow for smaller and more efficient proof-of-concept drug studies (Wagner, 2009). EIM is well-suited to provide ALS biomarker information because it is appropriate for use in patients with physical and cognitive limitations and is non-invasive (Turner et al., 2009). The results presented here suggest that EIM of the tongue, in particular, could help meet a need for reliable, widely applicable measures of bulbar function.
While these findings are encouraging, the current study has several important limitations. First, no direct comparison was made between the prototype tongue depressor array and the new, smaller EIM array. Second, tongue EIM measurements were performed in a single study session so inter-session variability was not assessed. Third, the number of patients is small. For this reason, the study is underpowered for any definitive comparisons between healthy volunteers and patients. Finally, the neuromuscular patients included have evidence of lower motor neuron dysfunction on examination; whether the tongue EIM electrode array can detect preclinical signs of disease is yet to be determined. Additional work is also needed to learn if tongue EIM can discriminate upper from lower motor neuron deficits.
5. Conclusions
In summary, these data support the potential utility of a new small EIM tongue array. The array is well-tolerated, easy to implement, and quick to manufacture, making it amenable to use in therapeutic trials. Beyond ALS, the technology could play important roles in the investigational and clinical evaluation of a variety of other neurological disorders associated with tongue weakness and dysfunction.
HIGHLIGHTS.
A new, smaller tongue electrical impedance myography (EIM) array is reliable.
The array preliminarily distinguishes between healthy and diseased muscle.
Use of the array is well-tolerated.
Acknowledgments
This study was funded by the National Institutes of Health K24NS060951.
Footnotes
Conflict of interest statements: Dr. Rutkove has equity in, and serves a consultant and scientific advisor to, Skulpt, Inc. a company that designs impedance devices for clinical and research use; he is also a member of the company’s Board of Directors. The company also has an option to license patented impedance technology of which Dr. Rutkove is named as an inventor. None of the other authors have potential conflicts of interest to be disclosed.
References
- Adams V, Mathisen B, Baines S, Lazarus C, Callister R. Reliability of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument with healthy adults. Dysphagia. 2014;29(1):83–95. doi: 10.1007/s00455-013-9486-5. [DOI] [PubMed] [Google Scholar]
- Adams V, Mathisen B, Baines S, Lazarus C, Callister R. Reliability of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument with elderly adults. Disabil Rehabil. 2015;37(5):389–95. doi: 10.3109/09638288.2014.921245. [DOI] [PubMed] [Google Scholar]
- Esper GJ, Shiffman CA, Aaron R, Lee KS, Rutkove SB. Assessing neuromuscular disease with multifrequency electrical impedance myography. Muscle Nerve. 2006;34(5):595–602. doi: 10.1002/mus.20626. [DOI] [PubMed] [Google Scholar]
- Gans BM, Kraft GH. Pain perception in clinical electromyography. Arch Phys Med Rehabil. 1977;58(1):13–6. [PubMed] [Google Scholar]
- Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118(Pt 3):707–19. doi: 10.1093/brain/118.3.707. [DOI] [PubMed] [Google Scholar]
- Jan MM, Schwartz M, Benstead TJ. EMG related anxiety and pain: a prospective study. Can J Neurol Sci. 1999;26(4):294–7. doi: 10.1017/s031716710000041x. [DOI] [PubMed] [Google Scholar]
- Makki AA, Benatar M. The electromyographic diagnosis of amyotrophic lateral sclerosis: does the evidence support the El Escorial criteria? Muscle Nerve. 2007;35(5):614–9. doi: 10.1002/mus.20748. [DOI] [PubMed] [Google Scholar]
- Rutkove SB. Electrical impedance myography: background, current state, and future directions. Muscle Nerve. 2009;40(6):936–46. doi: 10.1002/mus.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkove SB, Zhang H, Schoenfeld DA, Raynor EM, Shefner JM, Cudkowicz ME, et al. Electrical impedance myography to assess outcome in amyotrophic lateral sclerosis clinical trials. Clin Neurophysiol. 2007;118(11):2413–8. doi: 10.1016/j.clinph.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography correlates with standard measures of ALS severity. Muscle Nerve. 2014;49(3):441–3. doi: 10.1002/mus.24128. [DOI] [PubMed] [Google Scholar]
- Shellikeri S, Yunusova Y, Green JR, Pattee GL, Berry JD, Rutkove SB, et al. Electrical impedance myography (EIM) in the evaluation of the tongue musculature in amyotrophic lateral sclerosis (ALS) Muscle Nerve. 2015 doi: 10.1002/mus.24565. http://dx.doi.org/10.1002/mus.24565. [DOI] [PMC free article] [PubMed]
- Simon NG, Turner MR, Vucic S, Al-Chalabi A, Shefner J, Lomen-Hoerth C, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol. 2014;76(5):643–57. doi: 10.1002/ana.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarulli A, Esper GJ, Lee KS, Aaron R, Shiffman CA, Rutkove SB. Electrical impedance myography in the bedside assessment of inflammatory myopathy. Neurology. 2005;65(3):451–2. doi: 10.1212/01.wnl.0000172338.95064.cb. [DOI] [PubMed] [Google Scholar]
- Tarulli AW, Duggal N, Esper GJ, Garmirian LP, Fogerson PM, Lin CH, et al. Electrical impedance myography in the assessment of disuse atrophy. Arch Phys Med Rehabil. 2009;90(10):1806–10. doi: 10.1016/j.apmr.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009;8(1):94–109. doi: 10.1016/S1474-4422(08)70293-X. [DOI] [PubMed] [Google Scholar]
- Wagner KR. The need for biomarkers in amyotrophic lateral sclerosis drug development. Neurology. 2009;72(1):11–2. doi: 10.1212/01.wnl.0000338538.18938.9d. [DOI] [PubMed] [Google Scholar]