Abstract
The human macula uniquely concentrates three carotenoids: lutein, zeaxanthin, and meso-zeaxanthin. Lutein and zeaxanthin must be obtained from dietary sources such as green leafy vegetables and orange and yellow fruits and vegetables, while meso-zeaxanthin is rarely found in diet and is believed to be formed at the macula by metabolic transformations of ingested carotenoids. Epidemiological studies and large-scale clinical trials such as AREDS2 have brought attention to the potential ocular health and functional benefits of these three xanthophyll carotenoids consumed through the diet or supplements, but the basic science and clinical research underlying recommendations for nutritional interventions against age-related macular degeneration and other eye diseases are underappreciated by clinicians and vision researchers alike. In this review article, we first examine the chemistry, biophysics, and physiology of these yellow pigments that are specifically concentrated in the macula lutea through the means of high-affinity binding proteins and specialized transport and metabolic proteins where they play important roles as short-wavelength (blue) light-absorbers and localized, efficient antioxidants in a region at high risk for light-induced oxidative stress. Next, we turn to clinical evidence supporting functional benefits of these carotenoids in normal eyes and for their potential protective actions against ocular disease from infancy to old age.
Keywords: Carotenoid, lutein, zeaxanthin, macular pigment, nutrition, age-related macular degeneration
1. Introduction
Carotenoids are phytochemicals that are classified as carotenes if they are exclusively hydrocarbons, but if they contain oxygen as a result of oxidation or enzymatic addition, they are known as xanthophylls. Carotenes are structurally characterized by a C40H56 conjugated polyene backbone chain that allows electrons in their double-bonds to easily delocalize (Willstätter and Mieg, 1907), lowering the ground state energy of the molecule. This core system of conjugated carbon-carbon double-bonds makes them efficient quenchers of reactive oxygen species (ROS) and absorbers of potentially damaging visible light (Britton, 1995a), and their functions are determined by their physical and chemical properties, functional groups, geometry, and varied structures. In general, carotenoids are present in all organisms of the food chain, but in widely varying amounts (Maoka, 2011), and recent evidence indicates that carotenoid pigments are responsible for brilliant plumage color in birds (Shawkey and Hill, 2005), bright coloration in fish, shrimp, sea sponges and bivalves (Maoka, 2011), as well as essential components of plants' photosynthetic apparatus (Dall'Osto et al., 2006) and for the diverse colors of many fruits and vegetables. In humans, one of their most remarkable and unique functions is as the pigment of the macula lutea, the yellow spot centered on the fovea (Bone et al., 1985; Handelman et al., 1992). The macular pigment carotenoids (MP), lutein, zeaxanthin, and meso-zeaxanthin are widely recommended as dietary supplements for the prevention of visual loss from age-related macular degeneration (AMD) and other ocular diseases, but the basic and clinical science supporting such recommendations is underappreciated by clinicians and vision scientists. Here, we provide a comprehensive review of the chemistry, biochemistry, biophysics, and clinical studies underlying the ocular protective and functional roles of these remarkable pigments throughout the lifespan.
2. Basic Science of the Macular Pigment Carotenoids
2.1. Carotenoid chemistry and analysis
2.1.1. Carotenoid chemistry
Carotenes are hydrophobic, with little or no solubility in water, while the xanthophylls have modestly better aqueous solubility. Hence, these carotenoids are generally restricted to the lipophilic areas in the cell such as the inner core of the cell membranes or else bound to proteins (Britton, 1995a). Polar functional groups alter the polarity and solubility of the carotenoids and affect their interactions with other molecules (Woodall et al., 1997a; Woodall et al., 1997b). The antioxidant properties of different carotenoids vary based on their chemical and physical properties. For example, β-carotene and zeaxanthin have different dynamic behavior in a model membrane system (Cerezo et al., 2013), while lutein and zeaxanthin orient differently in phospholipid bilayers (Sujak and Gruszecki, 2000; Sujak et al., 2000). Of all the carotenoids, a few carotenoids have pro-vitamin A activity which is the ability to yield vitamin A (retinol) as a result of cleavage by the enzymes β-carotene oxygenase 1 (BCO1) and β-carotene oxygenase 2 (BCO2) (Olson, 1989). Depending upon the structure and the point of cleavage, one or two retinol molecules are formed from a carotenoid molecule. β- Carotene, β-cryptoxanthin, and α-carotene are common pro-vitamin A carotenoids, but they are generally not found in the retina. Around 700 carotenoids exist in nature, of which only 15 to 30 enter the human blood stream, but only two dietary carotenoids, lutein and zeaxanthin, ultimately reach the human retina. These two carotenoids, along with their metabolite, meso-zeaxanthin, which is exclusively found in ocular tissues, are called macular pigment (MP) of the human fovea (Bone et al., 1993).
2.1.2. Foveal anatomy and the macular pigment carotenoids
The fovea centralis is a depression located in the middle of the macula of the primate retina (Wolin and Massopust, 1967). The ophthalmoscope invented by Hermann von Helmholtz in 1851, led to the discovery of the foveal pit in live humans (Nussbaum et al., 1981). Later studies showed that this region is responsible for the sharp central vision required for daily activities such as reading, driving, and recognizing faces. The central region of the human macula is free of rod receptors and is composed of tightly packed foveal cone cells (Figure 1). This region, unlike the rest of the macula, has no inner nuclear layer, inner plexiform layer, or ganglion cell layer because the foveal cones' axons are centrifugally directed away from the center. There is a relatively higher concentration of Müller glial cells in this area. The internal limiting membrane, a basal lamina that separates retina from the vitreous, is thinned out at the fovea. The tightly packed cone cells in the central region, the nearly absent basal lamina, and the absence of other cell layers in the fovea are considered to be adaptations to facilitate the passage of light through the retina (Yamada, 1969).
Figure 1.
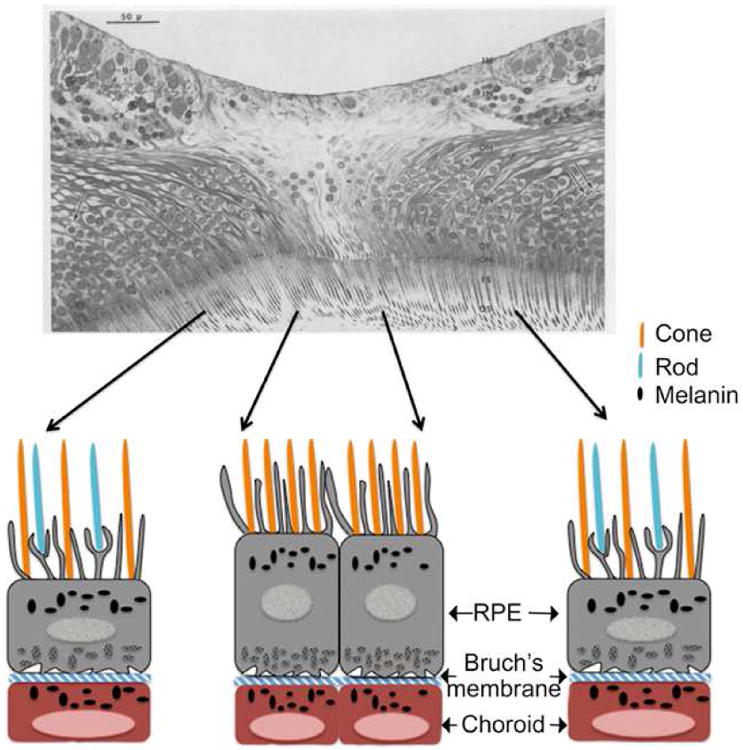
Structure of the human fovea. Upper panel - In this section through the center of the fovea, the tightly packed cone cells in the center are evident. The rod cells are present in the periphery. The central region is devoid of inner limiting membrane, inner nuclear layer, and Henle's fibers. Figure adapted from a light microscopic anatomy of the fovea centralis in the eye of a 45-year-old woman (Yamada, 1969). os-outer segment, is- inner segment, om- outer limiting membrane, of-outer cone fiber, on-outer nuclear layer, oh-outer Henle's layer, in-inner nuclear layer, im-inner limiting membrane, g-ganglion cells, cp-capillary. Lower Panel - Representation of the anatomical details of primate fovea. RPE processes are present in between the photoreceptors. The RPE layer is separated from the choroid by the thin Bruch's membrane. Figure adapted from a schematic diagram by Snodderly to illustrate the anatomic and metabolic relation in the fovea of macaque retina (Snodderly, 1995).
Retina from non-human primates is considered an excellent non-human experimental alternative for high-resolution histological studies. This is because monkey eyes are relatively easier to obtain soon after death compared to human eyes, and perfusion fixation is possible. Careful analysis of the anatomy of primate fovea pointed out that the retinal pigment epithelium (RPE) monolayer is in contact with the tips of the rod and cone photoreceptors in the fovea (Anderson and Fisher, 1979). Beneath the RPE layer is a connective tissue membrane known as Bruch's membrane that separates RPE from choroid (Figure 1). As observed in humans, the center region of the primate fovea is free of rods and is completely made up of cone cells; however, blue cones are absent in this region. Both rod and cone photoreceptors are present at a distance of 0.5 mm away from the foveal center (Yamada, 1969). Long processes arising from the underlying RPE layer are seen in between the photoreceptor cells. These processes cover the outer surface of cones and rods (Yamada, 1969).
The yellow pigmentation of the fovea is the origin of the anatomical term macula lutea, or ‘yellow spot’ (Nussbaum et al., 1981). The absorption spectra of the pigments from this region were recognized to be similar to those of xanthophylls (Wald, 1945), and subsequently, they were chemically identified to be lutein, zeaxanthin, and meso-zeaxanthin (Bone et al., 1988; Bone et al., 1993). Initial studies by Bone et al. quantified the total carotenoid concentrations to range from 0.05 ng/mm2 in the peripheral retina to 13 ng/mm2 at the fovea (Bone et al., 1988). Studies from our laboratory have identified various metabolites of lutein and zeaxanthin such as meso-zeaxanthin, 3′-epilutein, and 3-hydroxy-β,ε-caroten-3′-one in the human retina, lens, and uveal tract (Bernstein et al., 2001). Only trace amounts of carotenoid pigments were identified in the cornea and sclera. The only eye tissue studied that was devoid of carotenoids, was the vitreous (Figure 2) (Bernstein et al., 2001). The carotenoids are highly concentrated near the fovea, and their concentration decreases nearly 100-fold with increasing eccentricity (Snodderly et al., 1984). Near the fovea there is twice as much zeaxanthin and meso-zeaxanthin as lutein; but, in the peripheral retina, this relationship is reversed, and zeaxanthin and meso-zeaxanthin levels are half as much as those of lutein (Bone et al., 1988; Bone et al., 1993). Foveal carotenoids are mainly present in the receptor axons as well as the Henle fiber layer (Bone and Landrum, 1984; Snodderly et al., 1984) (Figure 3a). In the central retina, equal concentrations of lutein, zeaxanthin and meso-zeaxanthin are present; however, the ratio of meso-zeaxanthin to zeaxanthin decreases with the increased eccentricity to the fovea (Bone et al., 1993). Studies from our laboratory have identified and localized the carotenoid-binding proteins, glutathione S-transferase P1 (GSTP1) and steroidogenic acute regulatory domain protein 3 (StARD3) in the photoreceptors of the foveal region and Henle fiber layer (Bhosale et al., 2004; Li et al., 2011) (Figure 3b and c). These proteins facilitate the specific distribution and stability of carotenoids in the foveal region.
Figure 2.
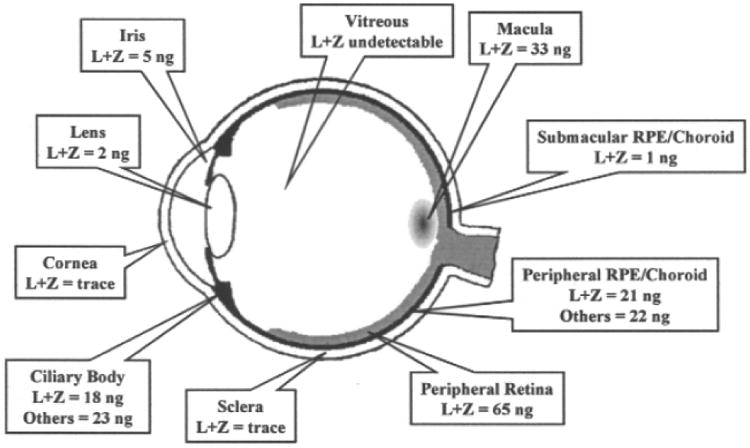
Macular pigment levels in different parts of the eye (Bernstein, 2002).
Figure 3.
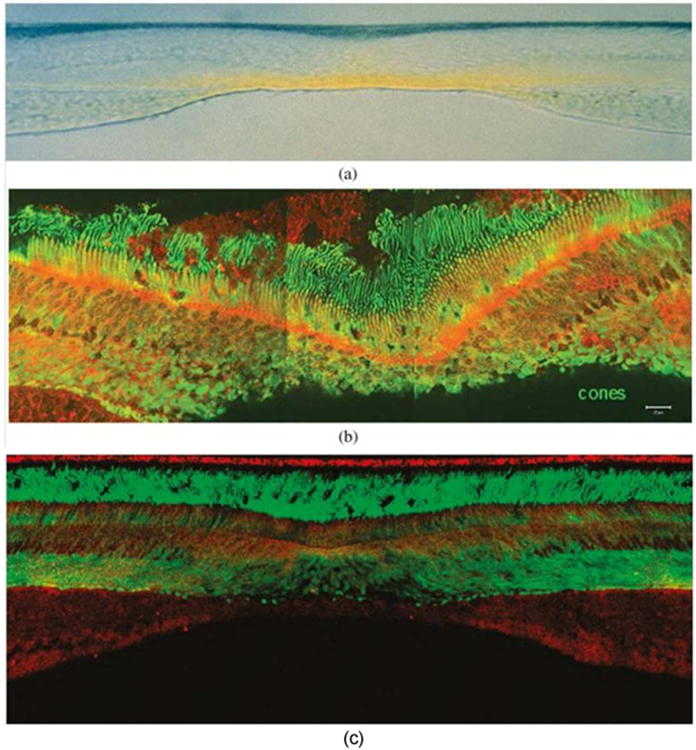
The retinal distribution of macular pigment carotenoids and their binding proteins. (a) Vertical section (vitreous side down) through a monkey fovea showing the distribution of the yellow macular carotenoids. Image courtesy of Dr. Max Snodderly. (b) GSTP1 labeling of foveal cones in the macula of a 3-year-old monkey. This montage shows strongest labeling by antibody against GSTP1 (red) over the myoid and ellipsoid regions of cones identified by monoclonal antibody (7G6, green). (c) A low-magnification view of a near-foveal retina section in which N-62 StAR (red) identifies StARD3, an anti-cone arrestin monoclonal antibody (7G6, green) identifies monkey cones. The sections in (b) and (c) have the same orientation as in (a). Images courtesy of Dr. Jeanne M. Frederick.
Snodderly and co-workers studied the distribution of macular pigment in primates and analyzed the spatial distribution of the pigments in the retina (Snodderly et al., 1984). The highest density of MP was associated with the axons of the cone photoreceptors in the outer plexiform layer. The processes of interneurons present in the inner plexiform layer also contained significant levels of pigment. Similar to the observations made in human retina (Bone et al., 1997), the primate retina also displayed a decrease in MP density with eccentricity to the foveal center.
2.1.3. Stereochemistry of the macular pigment carotenoids
The chemical structures of the macular carotenoids are characterized by the presence of hydroxyl groups attached at the 3 and 3′ positions to each of the terminal ionone rings as shown in Figure 4. Bone and Landrum identified the stereo-isomers of lutein and zeaxanthin in human retina using HPLC-MS (Bone et al., 1993). The lutein component of the MP consists of single stereoisomer of lutein [(3R, 3′R, 6′R)- β, ε-carotene-3,3′-diol] (Figure 4a), whereas the zeaxanthin component consists of three possible stereoisomers which include dietary zeaxanthin itself or RR-zeaxanthin [(3R,3′R)- β,β- carotene-3,3′- diol], SS-zeaxanthin [(3S,3′S)- β, β- carotene-3,3′-diol] (found only in trace amounts), and meso-zeaxanthin [(3R,3′S)- β, β- carotene-3,3′-diol] (Bone et al., 1988; Bone et al., 1993; Bone et al., 1985).
Figure 4.
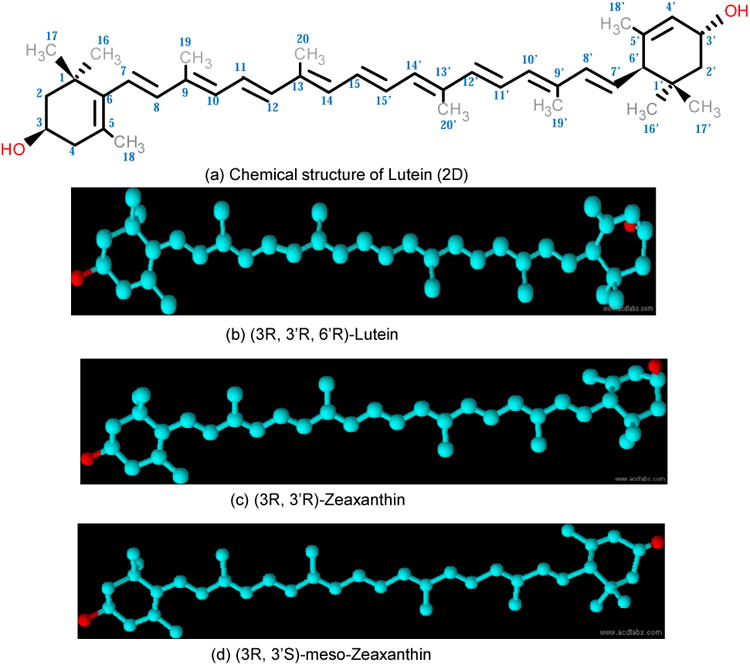
Chemical structure of macular pigment carotenoids. (a),(b) Lutein; (c) zeaxanthin; (d) meso-zeaxanthin.
As shown in Figure 4b and 4c, the hydroxyl group at the C-3′ position in lutein (3R, 3′R, 6′R- β, ε- carotene-3,3′-diol) is configured exactly opposite to that of zeaxanthin (3R, 3′R-β, β -carotene-3,3′-diol), while the C-3 and C-3′ hydroxyl groups in meso-zeaxanthin (3R, 3′S-β, β, -carotene-3,3′-diol) are positioned identically to lutein. The presence of three stereogenic centers at the C-3, C-3′ and C-6′ positions in the lutein molecule can result in eight possible stereoisomers, among which (3R, 3′R, 6′R) lutein is of dietary origin and is predominant in humans (Bone et al., 1993; Khachik and Chang, 2009). The double-bond in lutein at the 4′, 5′ position is shifted to the 5′, 6′ position in zeaxanthin and meso-zeaxanthin (Figure 4c and d). This double-bond position in lutein creates a more allylic hydroxyl end group relative to zeaxanthin. The extra conjugated double bond makes zeaxanthin and meso-zeaxanthin more stable and better antioxidants in comparison to lutein (Chung et al., 2004; Mortensen and Skibsted, 1997).
This conformational similarity of meso-zeaxanthin to lutein makes it more likely that lutein rather than zeaxanthin is the immediate precursor to meso-zeaxanthin because direct stereochemical inversion reactions are rare in nature. In fact, a simple shift of one double-bond will produce meso-zeaxanthin from dietary lutein. Furthermore, an industrial, base-catalyzed reaction at high temperature is known to produce only meso-zeaxanthin from lutein (Karrer and Jucker, 1947), suggesting that a similar enzyme-mediated reaction may occur in the human eye (Bone et al., 1993; Karrer, 1947). meso-Zeaxanthin is not detected in the human plasma and liver but is present in human macula, retina, and RPE/choroid (Khachik et al., 2002), indicating that conversion of lutein to meso-zeaxanthin most likely takes place in the eye. Johnson's laboratory studied the source of meso-zeaxanthin in rhesus monkeys (Johnson et al., 2005). In their studies, primates that had been maintained on a xanthophyll-free diet but then given lutein supplements showed the presence of meso-zeaxanthin in their retina. The control animals that were provided no xanthophylls and the animals supplemented with zeaxanthin alone did not have meso-zeaxanthin in their retina. In another study, Bhosale and coworkers fed deuterated lutein or zeaxanthin to female quails and reported the presence of labelled meso-zeaxanthin only in the retinas of birds fed with deuterated lutein (Bhosale et al., 2007a). Both of the studies described above indicate that lutein is the major precursor of meso-zeaxanthin in the retina.
2.1.4. Proposed orientation of lutein and zeaxanthin in biological membranes
Lutein and zeaxanthin when not bound to proteins easily insert themselves into biological membranes and have been shown to increase the rigidity of the lipid bilayer where they can act as “molecular rivets” because of their orientation within the membrane (Gabrielska and Gruszecki, 1996). Zeaxanthin was found to adopt a roughly perpendicular orientation to the plane of the membrane, while lutein and its isomers follow the perpendicular as well as parallel orientations (Sujak et al., 1999). The direct effect of macular carotenoids on lipid membranes' structural and dynamic properties seems to decrease the lipid bilayer's susceptibility to oxidative degradation (Gruszecki and Strzalka, 2005).
2.1.5. Functional properties
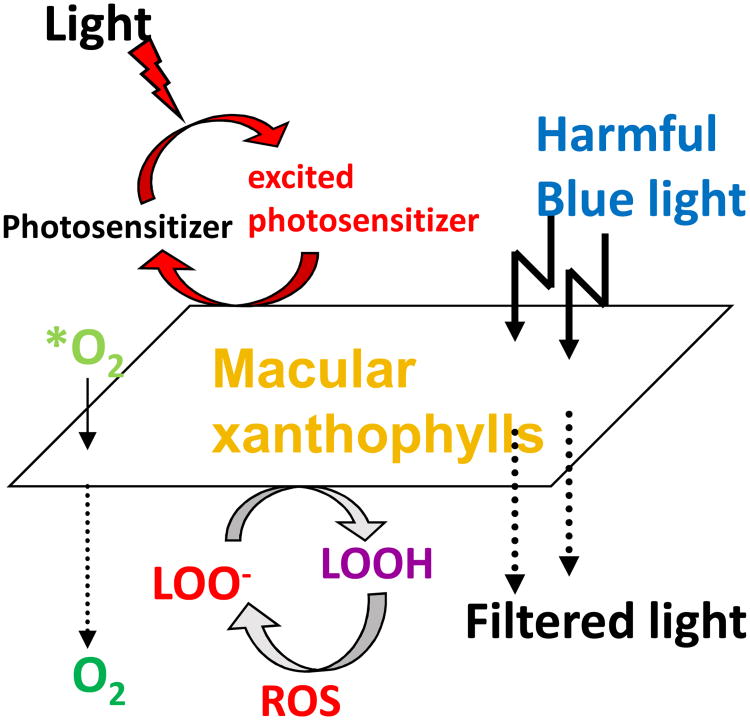
Carotenoids are excellent quenchers of singlet oxygen that react at the limits of diffusion without being consumed in the process (Foote et al., 1970). Reactive oxygen species (ROS) are either radicals such as hydroxyl radical or peroxyl radical, or they are reactive non-radical compounds such as singlet oxygen, peroxynitrite, or hydrogen peroxide (Stahl and Sies, 2002). Singlet-state molecules rapidly form and can create triplet-state molecules via intersystem crossing. These long-lived molecules can then react with oxygen to produce ROS, including superoxide anions, hydroxyl radicals, hydrogen peroxide, and singlet oxygen. These, in turn, can cause lipid peroxidation by attacking polyunsaturated fatty acids, resulting in DNA damage, protein and transmembrane glycoprotein oxidation, and other forms of cellular vandalism (Winkler et al., 1999). Among radicals, hydroxyl radical is the most reactive species (Woodall et al., 1997b). Due to its high reactivity, this radical immediately reacts with surrounding target molecules at the site where it is generated. In general, carotenoids react with ROS in three possible mechanisms oxidation, electron transfer or hydrogen abstraction (Britton, 1995a). Macular carotenoids may neutralize the ROS generated due to various free radical reactions in the eye and other tissues. Lutein and zeaxanthin are very efficient at absorbing and transmitting excited energy when needed, and they can harmlessly release the energy as heat without chemical degradation (Krinsky, 1989). The steps involved in the formation of ROS in the human retina and absorption of ROS by MP are outlined in Figure 5.
Figure 5.
Protective roles of lutein and zeaxanthin, as an absorber of harmful light and as an antioxidant reacting with reactive oxygen species (ROS). *O2, singlet oxygen; LOO-, lipid peroxyl radicals ;LOOH, lipid peroxides.
The potential for generation of ROS in the retina is high. The outer retina, especially membranes of the outer segments of the photoreceptors, has high concentrations of polyunsaturated fatty acids that are susceptible to photo-oxidation (Cai et al., 2000; Conn et al., 1991; Winkler et al., 1999). ROS are produced by absorption of UV and blue light by a photosensitizing compound or molecule (e.g. lipofuscin, protoporphyrin, or cytochrome). Carotenoids are potent scavengers of free radicals (e.g., superoxide anion and hydroxyl radical) and are particularly efficient at neutralizing singlet oxygen (Stahl et al., 1997). These carotenoids molecules have the ability to vibrate away their triplet-state energy as heat (Britton, 1995a; Krinsky, 1989).
Similar to their roles in plants, lutein, zeaxanthin, and meso-zeaxanthin act as protective antioxidants in the eye. These eye protective nutrients undergo oxidation and a series of transformations to protect the macula (Khachik et al., 2002). Khachik and colleagues first identified oxidation products and isomers of lutein in human serum (Khachik et al., 1992). Anhydrolutein and dihydroxylutein were observed as metabolites of lutein in human breast milk using HPLC and liquid chromatography- mass spectrometry (LC-MS) which formed as a result of dehydration and in acidic conditions similar to those of the stomach (Khachik et al., 1997b). 3-Hydroxy-β, ε-caroten-3′-one was identified as the direct oxidation product of lutein present in monkey retinas (Khachik et al., 1997a) and in human eye (Bernstein et al., 2001). 3- Methoxyzeaxanthin was identified in the macula of donor eyes using LC-MS and was present only in older donors, indicating that methylation of carotenoids may be a novel pathway in the eye whose activity increases with age (Bhosale et al., 2007c). The formation of different metabolites of lutein and zeaxanthin are discussed in Figure 6.
Figure 6.
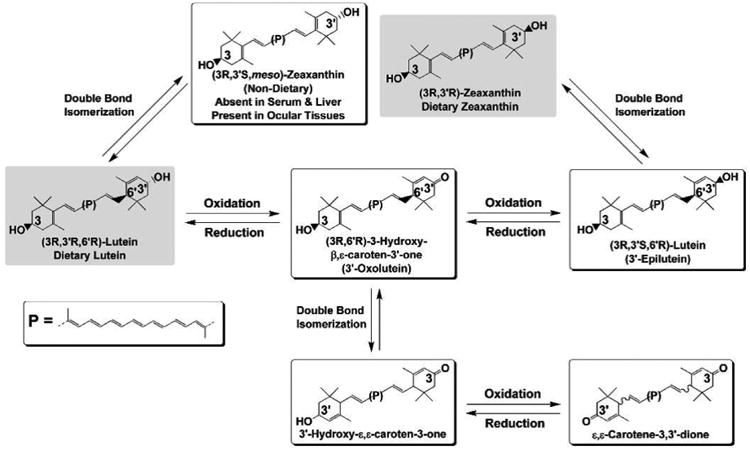
Proposed pathway for formation of oxidative metabolites of lutein and zeaxanthin in human ocular tissues.
Another major proposed mechanism for the protective action of the macular carotenoids is reduction of oxidative stress-induced damage. With aging, the RPE gradually accumulates lipofuscin, a heterogeneous fluorescent mixture rich in lipid-protein complexes. It is composed of by-products of vitamin A metabolism, as well as products of lipid peroxidation (Bernstein et al., 2001; Bhosale et al., 2009; Boulton et al., 1990). It is also a possible photosensitizing source of ROS (Sparrow et al., 2000). There is solid experimental evidence that N-retinyl- N-retinylidene ethanolamine (A2E), a component of lipofuscin, can damage the RPE, is toxic to mitochondria, and induces apoptosis of cultured RPE cells when exposed to blue light (Sparrow and Cai, 2001; Suter et al., 2000). When RPE cells are treated with lutein, this phototoxic effect is reduced greatly (Bian et al., 2012; Shaban and Richter, 2002). The presence of lutein and zeaxanthin has further been shown to reduce the amount of lipofuscin formed in cultured RPE cells and in vivo (Bhosale et al., 2009; Sundelin and Nilsson, 2001; Winkler et al., 1999).
Although the aging process decreases scotopic and shortwave sensitivity, higher levels of MP seem to preserve shortwave and scotopic function to an extent (Hammond et al., 2001). Evidence for the possibility that MP reduces glare and improves photostress recovery may be determined from its optical properties, spectral absorption, and spatial profile (Lien and Hammond, 2011; Stringham and Hammond, 2008). If macular carotenoid molecules are arranged perpendicular to the radially oriented axon, they will preferentially absorb plane polarized light in a direction parallel to the linear carotenoid molecule (perpendicular to the axonal direction), giving rise to the perception of Haidinger's brushes (Bone and Landrum, 1984; Sujak et al., 2000).
2.1.6. Dietary sources
Carotenoids cannot be synthesized in vivo by vertebrates and invertebrates, and they therefore must be obtained from dietary consumption. It has been extensively reported that consumption of lutein-and zeaxanthin-rich green leafy vegetables and orange and yellow fruits and vegetables is associated with lower incidence of cancer, cardiovascular disease, AMD, and cataract formation (Beatty et al., 1999; Krinsky et al., 2003; Landrum and Bone, 2001; Trumbo and Ellwood, 2006). Green leafy vegetables (kale, spinach and broccoli) are rich sources of lutein (Holden, 1999), while corn products are rich sources of zeaxanthin (Perry et al., 2009). Databases of the lutein and zeaxanthin content of fruits and vegetables aid in the assessment of dietary intake of these carotenoids. The data may also provide scientific information directly to consumers and assist public health workers to assess the dietary intake of these carotenoids (Holden, 1999). The carotenoid compositions of foods vary qualitatively and quantitatively (Rodriguez-Amaya, 2003). Factors such as species, cultivation, part of the plant, degree of maturity at harvest, and post-harvest handling practices affect carotenoid levels (Kimura and Rodriguez-Amaya, 1999; Rodriguez-Amaya, 2003). Hence, selection and processing of samples under suitable conditions are essential to retain consistent and maximal levels of carotenoids in the plant materials. The differences in lutein + zeaxanthin among green leafy vegetables studied are often attributed to species variations (Azevedo-Meleiro and Rodriguez-Amaya, 2007; Ismail and Cheah, 2003; Rodriguez-Amaya, 2003). Lutein concentration during maturation differs depending on the vegetable; in some cases, an increase in lutein concentration has been reported, whereas in other cases, a decrease has also been reported (Calvo, 2005; Rodriguez-Amaya, 2003). Recent database development for lutein and zeaxanthin intake (Holden, 1999; O'Neill et al., 2001; Rodriguez-Amaya, 1999) based on the region of origin are likely to become increasingly popular worldwide.
Bio-accessibility of carotenoids from green leafy vegetables is low, and various dietary factors affect their bioavailability (van Het Hof et al., 2000). Given their hydrophobic nature, there is evidence that consuming carotenoid-rich foods in the presence of oils or cholesterol may increase their uptake (Brown et al., 2004). In addition to vegetables, which are less bio-available, egg yolk (Goodrow et al., 2006; Kelly et al., 2014; Krinsky and Johnson, 2005) and fortified milk (Granado-Lorencio et al., 2010), are also good dietary and bioavailable sources of lutein and zeaxanthin. This may explain why some study results suggest a higher bioavailability of lutein from lutein-enriched eggs versus leafy greens such as spinach or other forms of supplementation (Chung et al., 2004). The dietary intake of carotenoids varies widely between individuals, and epidemiological studies have consistently shown that all age groups and ethnicities, as well as both sexes, have overall greater lutein than zeaxanthin consumption (Johnson et al., 2010).
meso-Zeaxanthin is rarely found in the human diet, but it has been detected in shrimp carapace, fish skin, and turtle fat, where all three isomers of zeaxanthin were found (Maoka et al., 1986), and Nolan's group has recently confirmed its presence in fish skin using more modern methods (Nolan et al., 2014; Thurnham et al., 2015). A significant amount of meso-zeaxanthin has been detected in commercially produced chicken eggs in Mexico where it is commonly added to the feed to achieve desirable coloration (Wang et al., 2007).
Carotenoids such as lutein and zeaxanthin are generally recognized as safe (GRAS) for human consumption, which allows food manufacturers to use them as additives. Recently, the European Food Safety Authority (EFSA) Panel on Food Additives and Nutrient Sources added to Food established an acceptable daily intake of 1 mg / kg bodyweight / day for lutein preparations derived from marigold (Tagetes erecta) containing at least 80% carotenoids (Agostoni et al., 2011; EFSA Panel on Food Additives and Nutrient Sources added to Food, 2011). Based on the available data, EFSA concluded that an intake of 0.75 mg / kg bodyweight / day of synthetic zeaxanthin does not raise any safety concerns (Agostoni et al., 2012). These values correspond to a daily intake of 53 mg of zeaxanthin and 70 mg of lutein for a person weighing 70 kg. These numbers are much higher than the earlier claims that 20 mg/day/person was safe in dietary supplements (Agostoni et al., 2012; European Food Safety Authority, 2012). Mutagenic studies have revealed that lutein and zeaxanthin are safe for human consumption (Kruger et al., 2002; Ma and Lin, 2010). The no observed-adverse-effect-level (NOAEL) for lutein/zeaxanthin concentrate was determined to be 400 mg/kg bodyweight/day, the highest dose tested in rats (Ravikrishnan et al., 2011). The safety of supplemental meso-zeaxanthin was recently reviewed (Nolan et al., 2013), and the NOAEL of meso-zeaxanthin in rats is 300 mg/kg bodyweight/day when administered orally for 13 consecutive weeks (Xu et al., 2013).
2.1.7. Chemical synthesis
Recent evidence for the beneficial effects of the macular carotenoids has increased consumer demand for these products. Commercially available lutein preparations such as Lutemax 2020 (OmniActive Health Technologies, Ltd., Mumbai, India), Hi-Fil (Industrial Organica, Topo Chico, Mexico), FloraGlo (Kemin Industries, Inc., Des Moines, USA), and others are made from marigold oleoresin. Lutein produced for human nutritional supplements typically contains 6-16% of waxes, and their zeaxanthin composition varies from one to another. Lutemax 2020 has approximately 13% of zeaxanthin (a mix of (3R, 3′R)-zeaxanthin and (3R, 3′S)-zeaxanthin), while FloraGlo has 2- 9% of zeaxanthin with minimal meso-zeaxanthin.
The commercial purification processes use solvents like hexane, dichloromethane, or propylene glycol, and chemical processes such as saponification (Khachik, 2001). Lutein from green leafy vegetables is uneconomical because of the expensive and time consuming purification steps required to remove chlorophylls and other carotenoids that are also present in green leafy sources (Khachik et al., 1995). Hence, chemical synthesis of lutein has been explored as an option to yield pure crystalline lutein free of isomers and other contaminants, but it is difficult and time-consuming to scale up economically. This is because lutein has eight possible stereoisomers (Khachik and Chang, 2009). The chemical synthesis of lutein could result in any of these stereoisomers, but only a few are naturally found in the eye and in the food supply. The various syntheses of carotenoids employ some key reactions for the formation of the carbon-carbon double-bonds, in particular, aldol condensation, Wittig condensation, Emmons-Horner reaction, Julia's method, and addition of acetylides (Kienzle, 1976). Ito used C-22 intermediates and palladium-based condensation reactions to synthesize carotenoids (Ito et al., 1992), and Lockwood et al. patented the methods for chemical synthesis of different carotenoids using Wittig condensation reactions (Lockwood et al., 2006). In 1980, Mayer and Ruttimann synthesized dietary lutein (3R, 3′R, 6′R-lutein) using a C-25 precursor molecule (R-4-hydroxy- 2,6,6-trimethyl 2-cylcohexen-1-one) with an overall yield of around 1% (Mayer and Rüttimann, 1980). Chemical synthesis of lutein using C-25 hydroxyaldehydes gives up to 74% isomerically pure dietary lutein (Khachik and Chang, 2009). The methodology adapted was a direct reaction and could be improved in the future to yield reasonably pure lutein. The other isomer of lutein naturally found in the eye and in some processed foods, 3′-epilutein (3R, 3′S, 6′R-lutein), can be synthesized from lutein (3R, 3′R, 6′R) (Deli et al., 2004; Eugster et al., 2002; Khachik, 2012).
Natural zeaxanthin occurs mainly in the (3R, 3′R)-configuration and is considered as a symmetrical molecule because it can be synthesized by the condensation of two precursor molecules with identical chemical structure and stereochemistry, whereas lutein and meso-zeaxanthin are asymmetrical molecules. A principal task in the synthesis of zeaxanthin requires introduction of the chiral centers in configurations identical to that of nature (Ernst, 2002). Hoffmann-La Roche has developed a method for the enantioselective reduction of the C=C bond into the (6R) configured levodione by using baker's yeast (Leuenberger, 1976; Paust and Kriegl, 2000). This stereo-center controls the subsequent catalytic hydrogenation of the sterically less shielded carbonyl group, which yields 4:1 epimers of zeaxanthin. The other strategy for chemical synthesis of zeaxanthin is the development of an optically active hydroxyl ketone and use of a double Wittig reaction to yield natural zeaxanthin (Soukup et al., 1990; Widmer et al., 1990). The yield (13-46%) and the purities were lower (Englert et al., 1991) in this entire process, which makes the process unsatisfactory and laborious. Ernst developed a new strategy for synthesis of zeaxanthin using C-15-phosphonium salts (Ernst et al., 2005; Paust et al., 1998) as shown in Figure 7, which is used for commercial synthesis of synthetic zeaxanthin. Khachik patented the use of goji berries to obtain zeaxanthin (Khachik, 2001), while commercially available zeaxanthin can be prepared from red marigold flowers of Tagetus erecta.
Figure 7.
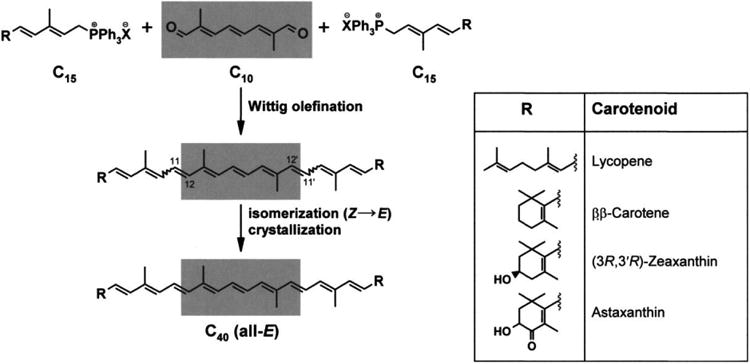
Industrial synthesis of commercial carotenoids.
meso-Zeaxanthin is obtained from partial synthesis by employing a base-catalyzed isomerization of lutein isolated from natural sources (Bernhard and Giger, 1998). Due to elimination of water in the process of isomerization and strong basic conditions, other products are also formed (Ernst, 2002). If chemically and sterically pure meso-zeaxanthin is desired, then this process needs an expensive and time-consuming ultra-purification step. Thus, most commercial meso-zeaxanthin preparations also contain lutein and zeaxanthin in varying amounts.
Recently, microbial sources of carotenoids such as algae are also getting attention as an alternative for supplementation (Nelis and De Leenheer, 1991; Vachali et al., 2012). Microbial carotenoid biosynthesis is a well regulated process dependent on the organism's environmental conditions and cultural stress during growth (Bhosale, 2004; Schnurr et al., 1991). Flavobacterium sp. are well documented in zeaxanthin production (Alcantara and Sanchez, 1999; Masetto et al., 2001; McDermott et al., 1974). Cyanobacteria such as Paracoccus zeaxanthinifaciens and Phormidium laminosum have also been reported to produce zeaxanthin (Fresnedo and Serra, 1992; Sajilata et al., 2010). Other microbial sources include Dunaliella sp, which produce zeaxanthin in stress and altered conditions (Jin et al., 2003; Salguero et al., 2005). Microbial synthesis has an economic niche for those carotenoids which have complex structures that make them difficult to synthesize chemically. Microalgae Chlorella sp. are used to produce lutein in large scale (Bhosale, 2004; Inbaraj et al., 2006; Jeon et al., 2014; Shi et al., 2000). Scenedesmus almeriensis, Chlamydomonas reinhardii, and Muriellopsis sp. also produce lutein (Del Campo et al., 2001; Francis et al., 1975; Sánchez et al., 2008). Commercial potential of carotenoids from microbial sources has been setback recently, however, because industrial extraction of plant oleoresins from marigold and other sources has become predominant in the market.
2.1.8. Absorption spectroscopy
The absorption maximum of lutein is 445 nm, while the maxima of zeaxanthin and meso-zeaxanthin are 450 nm (Britton, 1995b; Krinsky et al., 2003). Wald established that the MP absorbed light between the wavelengths 430 nm and 490 nm, with maximum absorption taking place at ∼460 nm (Wald, 1945). Importantly, he recognized that the absorption spectrum of the pigment was characteristic of the xanthophyll lutein. Wald also noted that the spectrum of the pigment extracted from human retinas agreed quite well with the visual estimate of the MP derived from the differences in the log sensitivity of peripheral and foveal cones. Ruddock used color matching data to deduce the absorption spectra of MP in vivo (Ruddock, 1963). He used trichromatic colorimetry with selected wavelengths, derived an equation, and observed the differences in optical densities of retinal segments, one at the center of retina and another away from the center.
2.1.9. High pressure/performance liquid chromatography (HPLC)
Accurate assessment of the amount of the MP in eyes is necessary to investigate the role of macular carotenoids and their presumed functions. HPLC is the “gold standard” technique for measurement of carotenoids from extracted samples because it can provide unambiguous identification and quantitation, but it requires significant amounts of precious biological tissue. MP was first isolated and characterized using HPLC in 1985 (Bone et al., 1985). In 1988, using HPLC, Bone and Landrum identified that there were actually two xanthophylls present in the human macula, lutein and zeaxanthin (Bone et al., 1988). Reverse-phase columns are the most widely used stationary phases for the analysis of these molecules. C-18 and C-30 stationary phases have provided good resolution for the separation of geometrical isomers and carotenoids with similar polarity (Barua and Olson, 2001; Bhosale et al., 2007b; Khachik et al., 2002). Normal-phase separation is also useful for carotenoids because of their hydrophobic nature, and non-polar solvents can be used for separation. Normal-phase cyano columns have a strong dipole and are suitable for separation of lutein, zeaxanthin and their isomers (Bhosale and Bernstein, 2005a); moreover, they are compatible with LC-MS. Identification of the various carotenoids is confirmed by comparing the retention times to known standards. Furthermore, the identity of individual peaks obtained during HPLC is confirmed from their characteristic ultraviolet / visible absorbance spectra and mass spectra (Barua and Furr, 1992).
Special derivatization methods have been adapted previously to identify the stereosiomers of MP. (Bone et al., 1993; Maoka et al., 1986). Newer HPLC methods have been developed to separate meso-zeaxanthin from dietary zeaxanthin using a chiral column without going through a cumbersome derivatization step (Bhosale et al., 2007a; Khachik et al., 2002). This method is well suited to isolate stereoisomers of MP in human retinal punches. Recently, several reports have shown the successful application of positive ion atmospheric pressure chemical ionization (APCI)-MS in identification of carotenoids isomers and metabolites (de Rosso and Mercadante, 2007; Fang et al., 2003; Khachik et al., 1997a).
2.2. Carotenoid uptake, metabolism, and transport
2.2.1. Proteins involved in macular pigment uptake and transport
Humans require dietary carotenoid intake because the relevant carotenoid synthesis enzymes do not exist in the human body. Most dietary carotenoids are consumed and embedded within a food matrix. When they reach the gut, they will be released from the food matrix through the action of various enzymes including esterases which will cleave xanthophyll esters. The free carotenoids are then solubilized into micelles before being taken up by the intestinal mucosal cells where they are cleaved by the carotenoid cleavage enzymes, BCO1 and/or BCO2, to form vitamin A and other metabolites or packaged into chylomicrons (Erdman et al., 1993). Then carotenoids and their metabolites will be secreted into the lymphatic and portal circulations for transport to the liver, where xanthophyll carotenoids such as lutein and zeaxanthin are loaded to their relevant transporters to be carried to the retina and other tissues through the circulation system. In the human serum, water-soluble lipoproteins are responsible for carrying carotenoids, retinoids, vitamin E, and plasma lipids (Rigotti et al., 2003). Lipoproteins have a polar outer shell of protein and phospholipid and an inner core of neutral lipid, and they can be divided into six groups: chylomicrons, chylomicron remnants, very low-density lipoproteins, low-density lipoproteins, intermediate-density lipoproteins, and high-density lipoproteins (HDL) (Mahley et al., 1984). HDL is the smallest and densest of all plasma lipoproteins, playing a critical function in cholesterol metabolism with an important role in removing cholesterol from peripheral tissues, a process known as reverse cholesterol transport (Trigatti et al., 2000). In the bloodstream, all carotenoids are detectable in all lipoprotein classes to varying degrees, but lutein and zeaxanthin are primarily associated with HDL, consistent with their less hydrophobic nature relative to the carotenes; however, the specific components of HDL responsible for carotenoid binding remain to be identified. The Wisconsin hypoalpha mutant (WHAM) chicken, a natural animal model of HDL deficiency, has a >90% reduction in plasma HDL (Attie et al., 2002). When these chickens are fed a high-lutein diet, lutein levels increase in plasma, heart, and liver, but not in retina, suggesting that HDL is critical for delivery of carotenoids to retinal tissue (Connor et al., 2007).
Scavenger receptor class B member 1 (SR-BI), a cell surface glycoprotein that binds HDL, mediates selective cholesteryl ester uptake from lipoprotein into liver and steroidogenic tissues as well as cholesterol efflux from macrophages (Acton et al., 1996; Pagler et al., 2006). SR-BI is a member of the CD36 superfamily (Oquendo et al., 1989). It has been shown that SR-BI participates in intestinal cholesterol absorption, embryogenesis, and vitamin E transport (During et al., 2005). Recently, there have been several reports that SR-BI is involved in the process of carotenoid uptake and transport to human and fly retina. It was demonstrated that the macular carotenoids lutein and zeaxanthin can be better taken up by RPE cells than β-carotene through an SR-BI–dependent mechanism (During et al., 2008). When macular carotenoids or β-carotene were incubated with fully differentiated ARPE-19 cells, the quantity of the macular carotenoids taken up by the cells was two times higher than β-carotene. Blocking SR-BI by its antibody or knocking down SR-BI expression by small interfering RNA reduced the absorption of carotenoids by RPE cells, especially for zeaxanthin. Similarly, Kiefer showed that the molecular basis for the blindness of a Drosophila mutant, Nina D, is a defect in the cellular uptake of carotenoids caused by a mutation in the Nina D gene which has high similarity to mammalian SR-BI (Kiefer et al., 2002). In the gut, the expression of SR-BI is repressed by the intestine-specific homeobox (ISX) transcription factor which is controlled by retinoic acid, a metabolite of carotenoids (Lobo et al., 2010). Thus, ISX governs the process of carotenoid absorption via SR-BI through a negative feedback regulatory mechanism, but, the retinal regulatory mechanism for SR-BI seems to be different, as there is no current evidence that ISX exists in the human retina or RPE.
CD36, a scavenger receptor relative of SR-BI, is a better match because, unlike SR-BI, it is abundantly expressed in the primate neural retina (Tserentsoodol et al., 2006). CD36 was isolated and identified as a platelet integral membrane glycoprotein (Green et al., 1990; Greenwalt et al., 1992). It also goes by the name of FAT (fatty acid translocase) because it can bind long-chain free fatty acids and transport them into cells (Febbraio et al., 2001; Silverstein and Febbraio, 2009; Silverstein et al., 2010). Interestingly, Cameo2, a CD36 homolog in silkworms, is required for uptake of lutein into the silk gland (Sakudoh et al., 2010). The catabolism of photoreceptor outer segments is mediated by CD36 (Ryeom et al., 1996), and the components of rod outer segments, such as rhodopsin and phospholipids, including anionic phospholipids, are ligands of CD36. More recently, it was reported that genetic variants of CD36 are associated with serum lutein levels and MPOD in AMD patients (Borel et al., 2011), suggesting that CD36 is likely to be involved in the MP uptake process.
2.2.2. Carotenoid cleavage enzymes
BCO1 and BCO2 are the two known carotenoid cleavage enzymes in animals, and they are immunolocalized to both human retina and RPE (Bhatti et al., 2003; Li et al., 2014; Lindqvist and Andersson, 2004; Lindqvist et al., 2005). BCO1 cleaves carotenes symmetrically at the 15-15′ carbon-carbon double bond, an essential step for generation of vitamin A, and it requires its substrates to have at least one non-substituted beta-ionone ring (dela Sena et al., 2013; Lindqvist and Andersson, 2002), which means that BCO1 cannot cleave xanthophylls such as lutein and zeaxanthin that are hydroxylated on both ionone rings. BCO2 catalyzes eccentric cleavage of carotenes at 9′, 10′ carbon-carbon double-bonds, generating 10′-apo-β-carotenal (C27), β-ionone (C13), and C9 dialdehyde as three possible cleavage products (Krinsky et al., 1993; von Lintig et al., 2005). It has been shown that ferret and mouse BCO2 can cleave xanthophylls such as lutein and zeaxanthin in vitro (Mein et al., 2011). More recently, our laboratory has shown that, unlike mouse and many other mammalian BCO2 enzymes, human retinal BCO2 is an inactive xanthophyll cleavage enzyme, possibly as the result of an unusual –GKAA- amino acid insertion near the substrate binding tunnel that appears to be unique to primates and whose insertion into the mouse enzyme leads to its inactivation (Li et al., 2014). This finding can explain why, among mammals, only primates uniquely accumulate lutein and zeaxanthin in their retinas. This discovery has been confirmed by the accumulation of zeaxanthin in the retinas of BCO2 knockout mice (Li et al., 2014). Our novel finding of xanthophyll uptake into the BCO2 knockout mouse retina has been confirmed by a subsequent follow-up study from another group (Babino et al., 2015), but these authors offer a different explanation, in which they propose that human retinal BCO2 cleaves xanthophyll but exists in a cellular compartment different from xanthophyll carotenoids (Babino et al., 2015; Palczewski et al., 2014). When they characterized primate BCO2 using an in vitro enzymatic assay, they could show only a very weak zeaxanthin cleavage activity with a truncated version of macaque BCO2, and they were still unable to demonstrate any xanthophyll cleavage activity with human BCO2 even though these two primate proteins share more than 90% homology. They speculate that their human BCO2 with a His-tag expressed in E. coli is inactive because it is expressed as insoluble protein aggregates in bacterial inclusion bodies. On the other hand, we used a different bacterial expression vector with a GST-tag that generated a soluble BCO2 protein (Li et al., 2014). Our purified human BCO2 protein exhibited much lower binding affinity with lutein and zeaxanthin relative to mouse BCO2 in surface plasmon resonance binding assays, consistent with our hypothesis that human BCO2 is enzymatically inactive due to poor ability to capture substrate carotenoids.
2.2.3. Macular carotenoid-binding proteins
The macular carotenoids lutein, zeaxanthin, and their metabolites, such as meso-zeaxanthin, 3′-oxolutein, and 3′-epilutein are specifically localized in the outer plexiform layers of the human fovea area at extremely high concentrations, which makes the human fovea a visibly yellow spot at the center of the human macula (Figure 3a). In order to understand how these macular carotenoids are specifically delivered and stabilized, our laboratory initiated a long-term project to identify specific, high-affinity macular carotenoid-binding proteins comparable to carotenoid-binding proteins responsible for the accumulation of carotenoids in other organisms such as LHC-II which binds lutein and zeaxanthin in plant chloroplasts and crustacyanin, the astaxanthin-binding protein in the shell of lobster. In our initial approach, tubulin is identified as a carotenoid-binding protein from the total soluble proteins of the human macula using photoaffinity labeling with radioactive canthaxanthin (Bernstein et al., 1997). Biological binding affinity studies showed that human tubulin could bind both lutein and zeaxanthin, but binding specificity and affinity were relatively low which sparked the quest to identify higher affinity, more specific binding proteins. Subsequently, glutathione S-transferase P1 (GSTP1) was identified as the zeaxanthin-binding protein from the total membrane proteins of the human macula (Bhosale et al., 2004). Immunolocalization of GSTP1 in the human and monkey retina revealed that GSTP1 was concentrated in the outer and inner plexiform layers of the fovea and in the photoreceptor inner segment ellipsoid region (Figure 3b). Recombinant human GSTP1 exhibited high affinity for macular zeaxanthins, with an equilibrium two-site average Kd of 0.33 μM for (3R, 3′R)-zeaxanthin and 0.52 μM for (3R, 3′S-meso)-zeaxanthin and only low-affinity interactions with lutein. When closely related human GST proteins were tested, GSTM1 and GSTA1 exhibited no appreciable affinity for lutein or zeaxanthin, further confirming the specificity of interaction between GSTP1 and macular zeaxanthin. It has been reported that GSTP1 can act as a retinoic acid cis-trans isomerase in a glutathione-independent manner (Chen and Juchau, 1997). Our identification of GSTP1 as a zeaxanthin-binding protein in the macula of human eye and our subsequent finding that it can synergistically protect lipid membranes from oxidation assign additional important roles to this well-known protein (Bhosale and Bernstein, 2005b; Bhosale et al., 2004). Three polymorphic GSTP1 genes have been cloned from malignant glioma cells (Ali-Osman et al., 1997). More recently, it has been suggested that certain gene polymorphisms of GSTs including GSTP1 may be associated with the subsequent development of neovascular AMD, cortical cataracts, and MPOD (Juronen et al., 2000; Meyers et al., 2013; Oz et al., 2006).
1n 2011, we identified steroidogenic acute regulatory domain protein 3 (StARD3) as the lutein-binding protein based on its homology to the silkworm lutein-binding protein, CBP (Li et al., 2011). StARD3, also known as MLN64, belongs to a lipid transfer related protein family composed of 15 identified protein members in humans (Alpy and Tomasetto, 2005; Sierra, 2004). StARD3 manifests several properties expected of a lutein-binding protein (Li et al., 2011). Shown macula-enriched by immunoblot analysis, StARD3 binds lutein selectively with high affinity. It induces a spectral shift of lutein's absorption spectrum in a manner that corresponds well with the in vivo MP spectrum, and it reveals an immunolocalization overlapping with our previously measured resonance Raman distribution of MP carotenoids. A specific antibody to StARD3, N-62 StAR, localizes to all neurons of monkey macular retina and is especially present in foveal cone inner segments and axons, but it does not co-localize with the Müller cell marker, glutamine synthetase (Figure 3c). Recombinant StARD3 selectively binds lutein with high affinity (KD= 0.45 μM) when assessed by surface plasmon resonance binding assays. Thus, StARD3 and GSTP1 proteins provide abundant lutein- and zeaxanthin-binding sites, respectively, that account for the unique distribution and stability of carotenoids found in the primate macula lutea. The other functions of StARD3 are still not clear, but it is thought to participate in the transmembrane transport process of cholesterol based on the presence of its StAR domain (Alpy and Tomasetto, 2005; Strauss et al., 2002).
2.2.4. The pathways for macular pigment carotenoid uptake and transport
In Figure 8, we provide a brief schematic to describe our current understanding of the whole process of transport and retinal capture of MP carotenoids. Dietary carotenoids are released from ingested foods after ester cleavage (if necessary) and incorporated into lipid micelles. SR-BI and CD36 located on the surface of intestinal cells facilitate uptake and transport to the lymphatic and portal circulations in the chylomicron fraction. Although it is still not known if carotenoids are modified in the liver before release into the bloodstream, it is clear that supplying carotenoids to animals can increase their content in the liver. Most hydrophobic carotenoids such as lycopene and β-carotene are transported on low-density lipoprotein (LDL), whereas the more hydrophilic xanthophyll carotenoids, such as lutein and zeaxanthin, are primarily carried by HDL. RPE SR-BI facilitates uptake of lutein, zeaxanthin, and other carotenoids into the cell. Interphotoreceptor retinoid binding protein (IRBP) may facilitate transport of lutein and zeaxanthin to the retinal cells via CD36 (Vachali et al., 2013), but specificity and uptake are ultimately driven by selective binding proteins such as GSTP1 and StARD3. Poor cleavage activity of endogenous human retinal BCO2 enzymes assures sustained high levels of macular carotenoids
Figure 8.
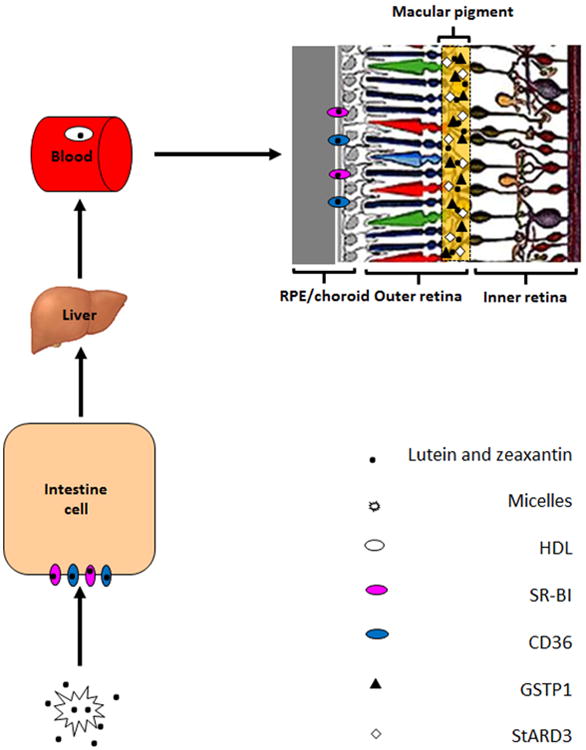
Possible pathway for macular pigment carotenoid uptake, transport, and accumulation in the human retina.
2.3. Non-human models for carotenoid physiology
2.3.1. Non-human primates
A macula lutea similar to humans makes non-human primates a favorable choice to study AMD (Handelman et al., 1992; Snodderly et al., 1991). Khachik and Bernstein identified oxidation products of lutein and zeaxanthin both in human and monkey retinas, which led them to propose that the oxidative-reductive pathways are similar in human and monkey retinas (Khachik et al., 1997a). Leung studied the effect of age and n-3 fatty acids, lutein, and zeaxanthin on the RPE (Leung et al., 2004). In xanthophyll-free monkeys, they observed a dip in the RPE cell density profile at the foveal center. They also observed a difference in the RPE profile depending upon the level of n-3 fatty acids. Thus, they concluded xanthophylls and n-3 fatty acids are essential for the development and maintenance of RPE cells. Neuringer studied the accumulation of lutein and zeaxanthin in rhesus monkeys grown on xanthophyll-free diets to understand the accumulation of serum carotenoids and MP over time (Neuringer et al., 2004). Johnson et al. fed lutein to carotenoid-deficient rhesus monkeys and found the presence of meso-zeaxanthin in the monkey retinas, demonstrating that lutein is the precursor for meso-zeaxanthin (Johnson et al., 2005). Ocular toxicities of lutein and zeaxanthin were assessed using high-dose supplementation of monkeys (Khachik et al., 2006). Eighteen female monkeys were used in this study; five each in the lutein and zeaxanthin treatment groups, five in the lutein and zeaxanthin mixed feeding group, and three controls. They were supplemented 12 months and followed another six months after that. Although the sample size was relatively lower to derive a clinically significant conclusion, the supplementation of lutein or zeaxanthin for one year at a dosage of 10 mg/kg did not cause any ocular toxicity. Despite the fact that non-human primates have proven to be an excellent model to study macular pigment, the relatively high costs of maintenance and management of these animals in a laboratory setting limit their use (Lee et al., 1999).
2.3.2. Rodents
Mice and rats are the most extensively used animal models in carotenoid research. These animals have been used to study the various physiological aspects of carotenoid absorption and distribution. In 1951, High and Day reported one of the earliest animal studies on the impact of carotenoids on vitamin A storage and growth in rats (High and Day, 1951). Several other groups also have reported the absorption and bioavailability of carotenoids in rats and mice (Krinsky et al., 1990; Shapiro et al., 1984). In all of these studies, researchers have used a much higher level of carotenoids than what a weight-normalized human subject would eat in a typical western diet (2-7 mg/day), and every rodent study published prior to 2014 failed to rigorously show that administered carotenoids actually accumulated in the animals' retinas. Although a few rodent studies did show that carotenoids were detectable in whole eyes, it turns out that wild-type mice never take up detectable lutein or zeaxanthin into their retinas unless carotenoid cleavage enzymes have been knocked out (Li et al., 2014), so one must be careful in interpreting data from these previously published studies. Park and colleagues studied the effect of dietary lutein absorption from marigold extracts in BALB/c mice (Park et al., 1998a). Their study showed that mice can absorb lutein from the diet, and it is rapidly taken up by the plasma, liver, and spleen (Park et al., 1998b). Nagao's group reported a possible transformation of lutein to its corresponding keto-carotenoid when lutein ester is supplemented in mice (Yonekura et al., 2010). In recent decades, genetically manipulated mice have become available for exploring the impact of different genes on carotenoid uptake and regulation; much research using these animals has been carried out in this direction.
Mice with genetically mediated knockout of carotenoid oxygenase enzymes (BCO1 and BCO2) have been used as a model system to study carotenoid metabolism. Von Lintig and colleagues studied the biochemical properties of BCO2 enzyme and the effect of BCO2 deficiency in a mouse model (Amengual et al., 2011). Later, using the BCO1 knockout mouse model they showed that genetic disruption of BCO1 would result in β-carotene accumulation and vitamin A deficiency. Also, they observed that mammals employ both BCO1 and BCO2 enzymes to synthesize retinoids from provitamin A carotenoids (Amengual et al., 2013). We have reported that the inactivity of human BCO2 underlies the retinal accumulation of the human macular carotenoid pigment (Li et al., 2014). The BCO2 knockout mice accumulated carotenoids in the retina as opposed to the wild-type mice fed with the same carotenoid supplemented food. Also, surface plasmon resonance binding studies showed that the binding affinities between human BCO2 and lutein, zeaxanthin, and meso-zeaxanthin are 10- to 40-fold weaker than those for mouse BCO2. This results in a less efficient capture of these carotenoids by the human BCO2 enzyme. These results provide a novel explanation for how primates uniquely concentrate xanthophyll carotenoids at high levels in retinal tissue.
Fernandez-Robredo studied the effect of lutein and antioxidant supplementation on vascular endothelial growth factor (VEGF) expression, matrix metalloproteinase 2 (MMP-2) activity, and RPE ultrastructural alterations in apolipoprotein E-deficient mouse models (Fernandez-Robredo et al., 2013). They concluded that supplementation with lutein, glutathione, and a vitamin complex appears to be effective in reducing the ultrastructural RPE changes such as swelling of basal infoldings and opening of intracellular space junction between RPE cells (Fernandez-Robredo et al., 2013). A recent study by Yu investigated whether dietary wolfberry altered carotenoid metabolic gene expression and enhanced mitochondrial biogenesis in the retina of diabetic mice (Yu et al., 2013). It was concluded that dietary wolfberry up-regulated carotenoid metabolic gene expression, attenuated hypoxia, and enhanced mitochondrial biogenesis in the retina, which resulted in the neural protection of diabetic mice retina (Yu et al., 2013). Lutein is known to protect retinal neurons by its anti-oxidative and anti-apoptotic properties in ischemia/reperfusion (I/R) injury (Li et al., 2012; Ozawa et al., 2012). Li studied the anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury in both in vivo and in vitro models (Li et al., 2012). In their study, the effect of lutein on Müller cells was investigated in a murine model of I/R injury and a culture model of hypoxic damage. The lutein-treated groups exhibited reduced gliosis in the I/R retina (Li et al., 2012). They also observed decreased production of pro-inflammatory factors from Müller cells (Li et al., 2012).
2.3.3. Avian species
Birds are also commonly used in MP research, but they differ from the human system in significant ways, including a wider diversity of retinal carotenoids and extensive esterification and deposition of carotenoid esters in photoreceptor oil droplets. Wang studied the selective retention of lutein, meso-zeaxanthin, and zeaxanthin in the retina of chicks fed a xanthophyll-free diet (Wang et al., 2007) and found that lutein and zeaxanthin were selectively retained in their retinas. At the same time, the plasma and other tissues lost up to 90% of their original content of xanthophylls (Wang et al., 2007), confirming a high priority for retention of ocular xanthophylls (Wang et al., 2007). Japanese quail (Coturnix japonica) is another animal model that has been used to study MP metabolism because the cone-rich quail retina is similar to human macula (Lee et al., 1999), and the xanthophyll profiles in quail mimic those in primates. Toyoda studied the effect of zeaxanthin on tissue distribution of xanthophylls in quail (Toyoda et al., 2002). Xanthophyll supplementation increased the zeaxanthin levels in various tissues including retina. Using quail lens as a model, Dorey investigated the effect of zeaxanthin distribution in the lens by dietary supplementation (Dorey et al., 2005) and found that the zeaxanthin levels increase in response to supplementation and reduce the risk of cataract. Thomson et al. studied the effect of photoreceptor cell death in quails supplemented with zeaxanthin (Thomson et al., 2002b) and found that a higher retinal zeaxanthin level reduced light-induced photoreceptor apoptosis. They also did a long-term supplementation study using the same model and found a similar retinal cell protective effect (Thomson et al., 2002a). Our group studied metabolic transformations in the quail retina (Bhosale et al., 2007a). Apart from dietary lutein (2.1%) and zeaxanthin (11.8%), we identified adonirubin (5.4%), 3′-oxolutein (3.8%), meso-zeaxanthin (3.0%), astaxanthin (28.2%), galloxanthin (12.2%), ε,ε-carotene (18.5%), and β-apo-2′-carotenol (9.5%) as major ocular carotenoids in the quail retina (Bhosale et al., 2007a). Deuterium-labeled lutein and zeaxanthin supplements revealed that dietary zeaxanthin is the precursor of 3′-oxolutein, β-apo-2′-carotenol, adonirubin, astaxanthin, galloxanthin, and ε,ε-carotene, while dietary lutein is the precursor for meso-zeaxanthin, confirming Johnson et al.'s previous finding (Johnson et al., 2005). Like non-human primates models, birds are also relatively expensive and difficult to care for in a typical laboratory setting. Also, their carotenoids are mostly present is the esterified form, and care should be taken to perform efficient ester cleavage without generating artifacts during the extraction and analysis process.
2.3.5. Other species
Along with the major laboratory animal models discussed above, other animal species are also used in carotenoid research. Zebrafish were used to demonstrate BCO2's role as an oxidative-stress regulated protein during development (Lobo et al., 2012). They found knockout of this mitochondrial enzyme resulted in anemia at larval stages of the zebrafish. It was concluded that BCO2 was an important enzyme against oxidative stress that has a role in apoptotic pathways (Lobo et al., 2012). Voolstra studied the Drosophila class B scavenger receptor NinaD-I (Voolstra et al., 2006) and identified this protein as a cell-surface receptor mediating carotenoid transport for visual chromophore synthesis (Voolstra et al., 2006). Mein used ferret BCO2 as a model to study the enzymatic formation of apo-carotenoids from lutein, zeaxanthin, and β-cryptoxanthin and identified both volatile and non-volatile apo-carotenoid products including 3-OH-β-ionone, 3-OH-α-ionone, β-ionone, 3-OH-α-apo-10′-carotenal, 3-OH-β-apo-10′-carotenal, and β-apo-10′-carotenal (Mein et al., 2011). Rabbits tissues were used to study the in-vitro toxicity profile of lutein and zeaxanthin-based dye solutions (Casaroli-Marano et al., 2015). They did not see any structural alterations in the neurosensory retina, RPE, or choroidal complex. These xanthophyll-based dye solutions have proven to be safe and can be used to stain intraocular structures in rabbits (Casaroli-Marano et al., 2015). Rabbits have also been used to study the absorption and distribution of carotenoids in plasma, liver, and adrenal glands(Yap et al., 1997). In this study, rabbits were fed with the diet enriched with palm carotenes. Most of the supplemented carotenes were metabolized into retinol and retinyl esters and stored in liver and pancreas. They also found that vitamin E supplementation helps in the absorption of carotenes (Yap et al., 1997).
3. Carotenoids and Eye Disease and Function Throughout The Lifespan
3.1. Measurement of carotenoids in living tissue and evidence for effects of supplementation
There is a growing and evidence-based consensus that MP is important for optimal visual performance because of its blue light-filtering properties and consequential attenuation of chromatic aberration, veiling luminance, and blue haze (Hammond et al., 2014; Loughman et al., 2012), and it has been hypothesized that MP may protect against AMD because of the same optical properties and because of the antioxidant capacity of the three macular carotenoids (Sabour-Pickett et al., 2012). Also, it has been found that MP levels correlate with concentrations of lutein and zeaxanthin in the brain (Vishwanathan et al., 2013b; Vishwanathan et al., 2015). This had led researchers to speculate that the carotenoids that comprise MP may also play a role in the brain (the retina is part of the central nervous system), but the mechanisms whereby carotenoids may play a role in brain health are not known. It has been suggested that carotenoids may be important because of their antioxidant (Khachik et al., 1997a; Li et al., 2010) and anti-inflammatory properties (Ciccone et al., 2013; Kijlstra et al., 2012). It has also been suggested that the carotenoids may play a beneficial role by enhancing gap junctional communication in the brain (Johnson, 2012; Stahl et al., 1997; Stahl and Sies, 2001). For the above reasons, there is a need to be able to measure MP in vivo, especially given these important hypothesized preventative roles of these nutrients in the human macula and brain. Today, valid measurement of MP is confined to the research setting, although there are now many commercially available devices which claim “clinic-friendly” measurement of MP; however, it is important to point out that any device that has been designed and promoted to measure MP quickly (a requirement of any busy ophthalmic clinic) may add more uncertainty about the validity of the measurement.
There are a variety of methods currently in use that claim to measure MP (Table S1). Of note, researchers have been debating the advantages and limitations of these techniques for over 20 years, but it is still not agreed which method is most suitable for measuring MP. This is not surprising, as it is extremely challenging to measure the yellow pigment of the macula lutea in living tissue. Indeed, one must remember that when we measure MP, we are attempting to quantify nutrients located at the macula, and therefore we must take into account all the optical variables (e.g. lens, cornea, vitreous, etc.) that may influence or confound the values yielded with any given instrument. Therefore, it is important for any method attempting to measure MP to disclose and understand the assumptions upon which the method is premised and how these assumptions may relate to the optical properties of the human eye and the visual system. Add to this that, just like people, every eye is different. In other words, no method is perfect or without its limitations, assumptions, or challenges. Below, we discuss the various methods available to measure MP, and we discuss the most commonly used techniques.
The methods that are available to measure MP can be divided into psychophysical (sometimes referred to as “subjective”) and physical (sometimes referred to as “objective”). The psychophysical techniques available include color matching (Davies and Morland, 2002), motion photometry, heterochromatic flicker photometry (Bone and Sparrock, 1971), and customized heterochromatic flicker photometry (cHFP) (Stringham and Hammond, 2008). Of these psychophysical techniques, HFP and cHFP are the most widely used. With HFP, the subject is required to make isoluminance matches between two flickering lights: a green light (not absorbed by MP) and a blue light (maximally absorbed by MP). The log ratio of the amount of blue light absorbed centrally, where MP peaks, to that absorbed at a peripheral retinal locus (the reference point), where MP is assumed to be zero, gives a measure of the subject's MP optical density (MPOD). Customized HFP enhances the HFP technique by customizing the procedure for each subject by optimizing the flicker frequency and brightness of the targets used during each trial. Importantly, scientists have made significant efforts to validate the cHFP technique. For example, the Macular Densitometer™ (a device which uses cHFP) has been validated by comparing MP measurements to known biochemical markers (i.e. serum/plasma concentrations of MP's constituent carotenoids) and by comparing the data it generates with the in vitro spectral absorption curve of the macular carotenoids. Importantly, HFP and cHFP have demonstrated an ability to detect changes in MPOD following supplementation with MP's constituent carotenoids (Landrum et al., 1997; Sabour-Pickett et al., 2014).
Physical techniques currently used for measuring MP include resonance Raman spectroscopy, fundus autofluorescence, and fundus reflectance. Raman spectroscopy is based on Raman effect/shift, which is inelastic scattering of photons by the molecule under investigation. In other words, the wavelength of a small fraction of the radiation scattered by certain molecules differs from that of the incident beam, and the shift in wavelength depends upon the chemical structure of the molecules responsible for the scattering. Of note, the carotenoid pigments, by virtue of their long, conjugated isoprenoid backbones are ideally suited for detection by resonance Raman spectroscopy. Bernstein and Gellermann were the first to develop a method using a laser spectroscopic technique of resonance Raman scattering to measure MP (Bernstein et al., 1998). Blue/green argon laser lines are used to resonantly excite the electronic absorption of the carotenoid pigments, and Raman signals relating to the pigments are recorded on a spectrometer. The Raman scatter corresponds to discrete shifts in the light frequency of photons exactly equal in magnitude to the stretch frequencies of the carbon single- and double- bonds. Raman scattering is a very weak optical effect, which means that Raman spectra obtained from biological tissues can be quite complex, making it challenging to distinguish and quantify the peak(s) of interest from the multitude of other compounds present; however, when a molecule is illuminated with monochromatic light overlapping its absorption band, then the Raman scattered light will exhibit a substantial resonance enhancement. In the case of a carotenoid molecule, a 488 nm argon laser light provides an extraordinarily high resonance enhancement of Raman signals of up to five orders of magnitude, allowing carotenoids to be readily detected and quantified in complex biological samples such as the human macula. Furthermore, Raman peaks are highly specific for a carotenoid molecule because their spectral locations correspond exactly to the vibrational energies of the Raman-active bonds within the molecules and have specific relative intensities. While the principle of using Raman scatter to measure MP is logical, this technique is not routinely used in clinical studies, which is likely due to the cost of the laser and high sensitivity detection equipment and uncertainties around the impact of the lens and pupil size when performing a measurement. The technique, however, has been shown to be reproducible (Neelam et al., 2005), and capable to detect change in MP following supplementation with the macular carotenoids (Beatty et al., 2013).
Fundus autofluorescence imaging (AFI) uses a confocal scanning laser ophthalmoscope (cSLO) or fundus camera. AFI exploits the fluorescent properties of lipofuscin present in the RPE. RPE lipofuscin is a fluorophore that accumulates over time from the phagocytosis of photoreceptor outer segments. Lipofuscin is excited in vivo between 400 and 590 nm (peak excitation at 490-510 nm) and emits autofluorescence at 520-800 nm (peak emission at 590-630 nm). MP, which is located anterior to the RPE, and which maximally absorbs light at 460 nm, attenuates lipofuscin's autofluorescence if the excitation wavelength falls within the absorption spectrum of MP (Figure 9). At the fovea, excitation light within the absorbance range of MP is partially absorbed by the carotenoids, resulting in an area of reduced fluorescence. New devices, such as the Heidelberg Spectralis (Heidelberg Engnieering, Carlsbad, CA, USA), use the two-wavelength autofluorescence technique to measure MP. With this technique, the device captures sets of images at two excitation wavelengths (Figure 10). These images are averaged and aligned and are used to produce a map of MP extending in a 10° radius around the center of the fovea (Figure 11).
Figure 9.
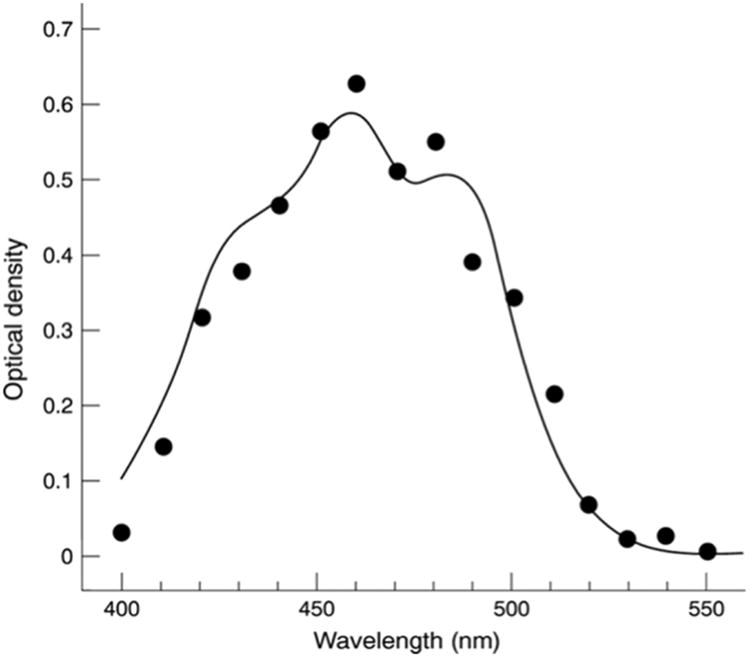
Absorption Spectrum of macular pigment.
Figure 10.
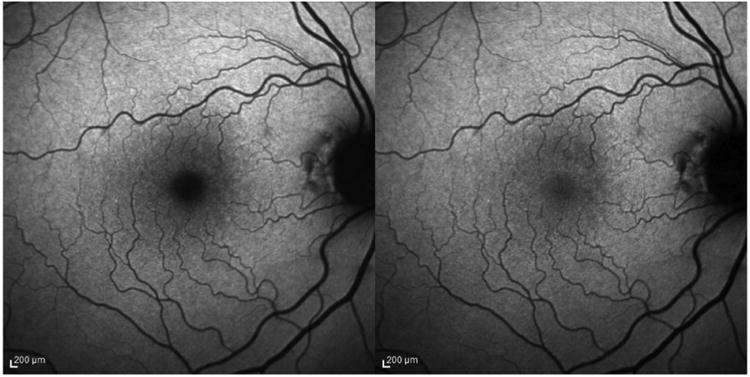
Image of macular pigment measured by Heidelberg Spectralis (Left, excitation wavelength at 488 nm; Right, excitation wavelength at 514 nm).
Figure 11.
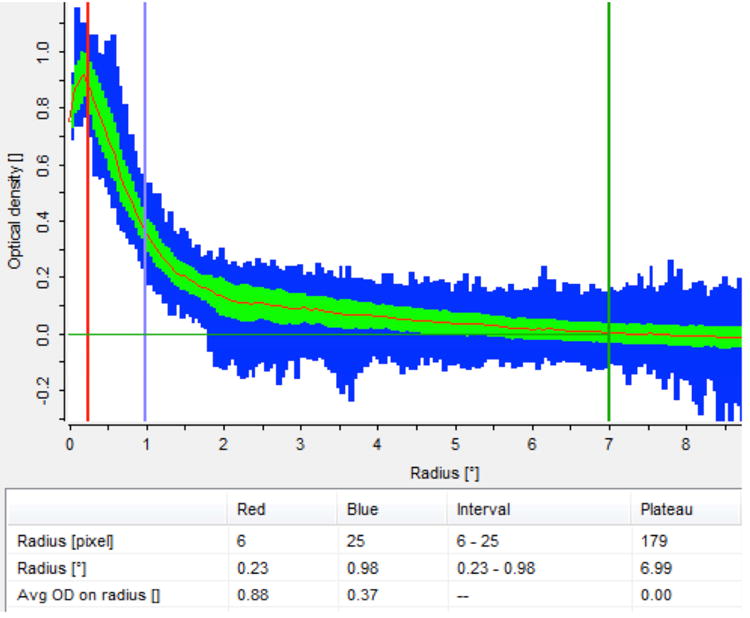
Autofluorescence technique, implemented by the Heidelberg Spectralis® (HRA+OCT MultiColor) to produce a full spatial profile image of macular pigment optical density (MPOD).
Work by Delori et al in 2001 comparing MP obtained using AFI and HFP in the same subjects (mean age = 52± 17 years, with normal retinal status) reported that MP determined by the autofluorescence method were higher than the MP densities measured psychophysically by HFP (Delori et al., 2001). Of note, a recent study has confirmed that measuring MP using AFI displays good concordance with MP measured using cHFP on the same subjects (free of retinal disease) (Dennison et al., 2013), but additional work is needed to confirm its reliability across populations (e.g. patients with AMD and patients with cataracts; this work is currently underway in Waterford, Ireland). This technique has also been successful in detecting augmentation of MP following supplementation with the carotenoids lutein, zeaxanthin, and meso-zeaxanthin in normal subjects and in subjects with Alzheimer's disease (Nolan et al., 2015).
Fundus reflectance, which quantitatively measures the light reflected from the retina and choroid using a reflectometer, a fundus camera, or a cSLO has also been widely used for the measurement of MP. There are currently two methods utilizing the reflectance technique to measure MP. Reflectance methods, in general, are susceptible to image degradation attributable to intra-ocular light scatter. The first technique uses white light to illuminate the retina in conjunction with the spectral analysis of the reflected light, and it requires complex and controversial mathematical models (with many assumed free parameters) to derive a measure of MP. The second technique uses two wavelengths, one substantially absorbed by MP (e.g. 488 nm) and one not absorbed by MP (e.g. 540 nm) for the purpose of normalization (Berendschot et al., 2003; Berendschot and van Norren, 2004). This latter procedure is necessary in order to take account of the absolute difference in reflection between the fovea and parafovea; otherwise the measure would be a compound of MP absorption and absolute reflectance. In contrast to the agreement seen between cHFP and AF discussed above, MP values obtained using fundus reflectance in that study were not comparable with either cHFP or AF values (Dennison et al., 2013).
Other recent developments in MP research include the measurement of MP in infants and children. It is believed that measuring MP distribution in the infant population may be important in our understanding of the role of MP in later life. Bernstein's laboratory recently developed a protocol using a digital video fundus camera (RetCam; Clarity Medical Systems, Inc., Pleasanton, CA) to measure MP distributions in premature infants and in children (Bernstein et al., 2013). In brief, this is a reflectance method where video-captured images centered on the fovea are collected on a digital fundus camera using a blue-light fluorescein angiography light source and an 80° collection lens. Illumination intensity for the blue light source and the detector gain sensitivity are set at midrange on the instrument's dials and adjusted as necessary to produce usable images for MP measurement.
In conclusion, there are many important factors that need to be considered when choosing a device to measure MP, and this will be influenced greatly by the population being studied (e.g. patients with and without retinal disease, infants, patients with Alzheimer's disease, etc.). The main factors to consider include: 1) Is the measurement reliable? For example, has the device been validated? 2) Is the measurement influenced by the optics of the eye and/or external factors? 3) Is the device suitable for use in the research or clinic setting, how much training is required, is it easy to use, are measurement protocols available, and are they standardized? 4) How much does it cost, and is it feasible to obtain? 5) Does it provide a full spatial image of MP or just MP measurements at discrete eccentricities? Of course, it is understood that all methods currently available to measure MP have advantages and limitations. It is our view that cHFP and AFI techniques are the most promising techniques and are suitable to detect change in MP following supplementation with the macular carotenoids, however, we advise that, for clinical trials, the same method should be used throughout the study in order to limit error when measuring MP.
3.2. Functional effects of carotenoids in the normal eye
3.2.1. Visual benefits of macular pigment
Over two decades of research has been conducted into the preventative role of MP and its constituent carotenoids for AMD (Sabour-Pickett et al., 2012). Indeed, we know that MP is a short-wavelength (blue) light filter (Bone et al., 1992), and a powerful antioxidant (Khachik et al., 1997a), and because of these properties it is believed to protect against AMD. Beyond its “protective” hypothesis, MP's optical and anatomic properties have prompted the “optical” hypotheses of this pigment. The “optical” hypotheses of MP were originally discussed by Schultze et al in1866 (Schultze, 1866) Reading and Weale in 1974 (Reading and Weale, 1974) and later by Nussbaum, Pruett, & Delori in 1981 (Nussbaum et al., 1981) and include MP's ability to enhance visual function and comfort by attenuation of the effects of chromatic aberration (Figure 12) and light scatter (Figure 13), via its light-filtering properties (Walls and Judd, 1933). In 1866, Schultze et al proposed that that MP could improve acuity by reducing the deleterious effects associated with the aberration of short-wave light (Schultze, 1866). This hypothesis has since been discussed by Werner in 1987 (Werner et al., 1987) and later by Wooten and Hammond in 2002 (Wooten and Hammond, 2002). However, in 2007 Engles et al modelled and evaluated the hypothesis and found that MPOD did not correlate significantly with either gap or hyperacuity measured in the yellow or white conditions, and therefore, their data did not support the predictions of the acuity hypothesis (Engles et al., 2007). It appears that any acuity advantage gained by higher levels of MP is offset by a commensurate reduction in luminance (which correlates positively with acuity). It has also been suggested that MP could enhance the contrast of objects on a background via color filtering (Wooten and Hammond, 2002). This hypothesis was recently tested and found to be tenable, which has important implications for visual function in the non-diseased eye (Renzi and Hammond, 2010).
Figure 12.
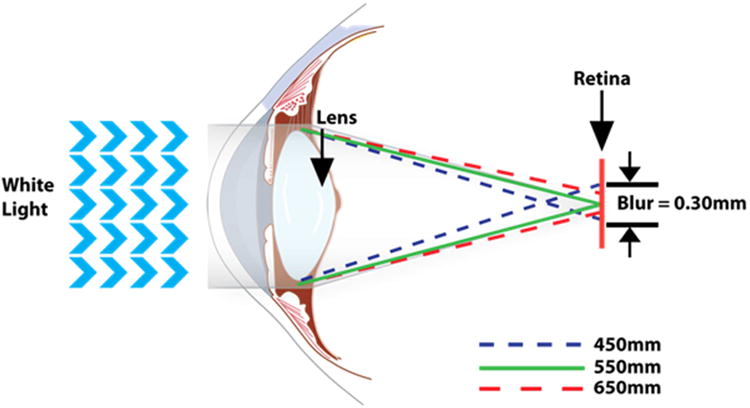
Illustration of chromatic aberration in the normal eye.
Figure 13.
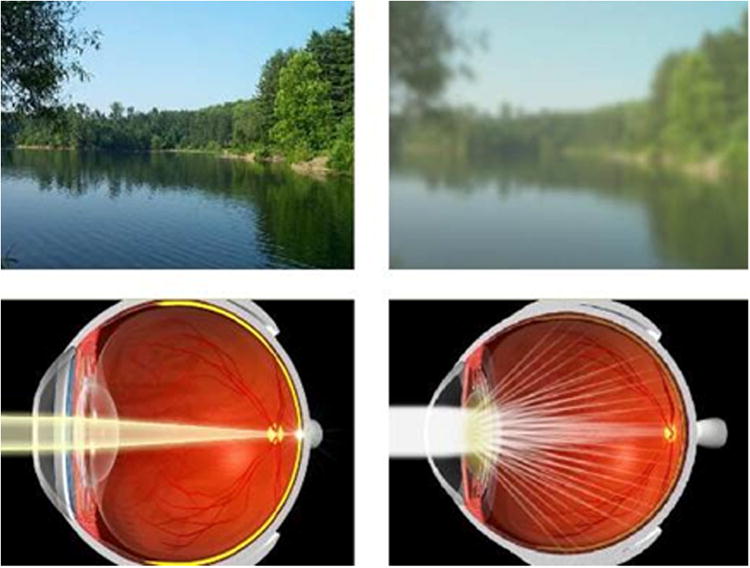
Illustration showing the effect of light scatter on vision.
For example, under natural conditions (e.g. walking outside), objects are often presented on short-wavelength backgrounds, such as a blue sky or green leaves, meaning that the filtering properties of MP is likely to impact positively on real-life vision. MP's pre-receptoral filtration of short-wavelength (blue) light is believed to reduce the adverse impact of glare disability, light scatter and chromatic aberration, thereby optimizing contrast sensitivity (Hammond et al., 2012; Stringham and Hammond, 2008). It follows, therefore, that augmentation of MP would result in enhanced contrast sensitivity and improved glare disability, and studies investigating the impact of carotenoid supplementation in normal subjects are discussed below.
Also, the visual benefits of MP are not restricted to the effects of its optical properties, reflected in a growing body of evidence that the macular carotenoids may have a favorable effect on neuronal processing (Renzi et al., 2013). These carotenoids have been shown to improve communication through cell-to-cell channels, modulate the dynamic instability of microtubules (structural units of neurons), and prevent degradation of synaptic vesicle proteins (Crabtree et al., 2001; Ozawa et al., 2012; Stahl and Sies, 2001).
3.2.2. Clinical trials investigating the macular carotenoids in normal subjects
As discussed above, there exists a biologically plausible hypothesis that MP is important for visual performance in normal subjects (i.e. subjects without ocular disease). Of note, many studies have reported on the cross-sectional relationship between MP and a plethora of visual performance parameters (Loughman et al., 2010; Stringham et al., 2011; Stringham and Hammond, 2008), and a number of trials have investigated the impact of supplementation with the macular carotenoids on visual performance in these subjects, with the majority of these studies exhibiting a positive effect following supplementation with these nutrients (Table 1). Here we discuss some of the published clinical trials assessing the effects of visual performance in normal subjects. We focus on trials conducted in Waterford, Ireland, but present data and findings for all relevant trials in Table 1.
Table 1.
Interventional studies assessing the effects of the macular carotenoids on visual performance in normal subjects.
| Principal author(s) | Year | n | Placebo-control | Carotenoids | Visual performance tests | Study duration (months) | Observed visual benefit following supplementation |
|---|---|---|---|---|---|---|---|
| Monjea | 1948 | 14 | No | L dipalmitate | Dark adaptation & scotopic VA | 2-6 | Yes‡ |
| Wustenberga | 1951 | 7 | No | L dipalmitate | Dark adaptation | - | No |
| Klaes & Riegelb | 1951 | - | No | L dipalmitate | Dark adaptation | - | Yes |
| Andreani & Volpia | 1956 | 10 | No | L dipalmitate | Dark adaptation | - | Yes |
| Moscia | 1956 | - | No | L dipalmitate | Light sensitivity | - | Yes |
| Hayanoa | 1959 | - | No | L dipalmitate | Dark adaptation | - | Yes† |
| Wenzelc | 2006 | 10 | Yes | 30mg L + 2.7mg Z | Photophobia | 3 | Yes |
| Rodriguez-Carmonad | 2006 | 24 | Yes* | 10mg/20mg of L/Z/L+Z | B/Y color discrimination | 12 | No |
| Kvansakule | 2006 | 34 | Yes | 10mg L/10mg Z/combination | Mesopic CS | 6 | Yes |
| Bartlett & Eperjesif | 2008 | 29 | Yes | 6mg L | VA (dist.&near), CS, photostress recovery | 18 | No |
| Stringham & Hammondg | 2008 | 40 | No | 10mg L + 2mg Z | Photostress recovery & grating visibility | 6 | Yes; both |
| Nolanh | 2010 | 121 | Yes | 12mg L + 1mg Z | VA, CS, GD, photostress recovery | 12 | No |
| Loughmani | 2012 | 36 | Yes | 10mg L+2mg Z+10mg MZ/20mg L+2mg Z | VA, CS, GD, photostress recovery | 6 | Yes; VA, CS, GD |
| Yaoj | 2013 | 120 | Yes | 20mg L | VA, CS, GD | 12 | Yes |
Abbreviations: carotenoids=macular carotenoids investigated; L=lutein; VA=visual acuity; Z=zeaxanthin; B/Y=blue/yellow; CS=contrast sensitivity; GD=glare disability; MZ=meso-zeaxanthin; - =data not available.
data obtained respectively from published literature (Nussbaum et al., 1981) (Klaes and Riegel, 1951) (Wenzel et al., 2006) (Rodriguez-Carmona et al., 2006) (Kvansakul et al., 2006) (Bartlett and Eperjesi, 2008) (Stringham and Hammond, 2008) (Nolan et al., 2011) (Loughman et al., 2012) (Yao et al., 2013).
for second 6 months of the study
proportional to serum L
described as having a “transient” benefit
The Collaborative Optical Macular Pigment Assessment Study (COMPASS), a randomized controlled trial, was designed to investigate the impact of supplementation with macular carotenoids versus placebo, on MP and visual performance (Nolan et al., 2011). In COMPASS, one hundred and twenty-one normal subjects were recruited (age range: 18 - 41 years). The active group consumed 12 mg of lutein and 1 mg of zeaxanthin (but no meso-zeaxanthin) every day for 12 months (n=61), and the remainder of the subjects were on placebo. A range of psychophysical tests were used to assess visual performance, including: visual acuity, contrast sensitivity, glare disability, and photostress recovery. Subjective visual function was determined by questionnaire, and MP was measured using customized heterochromatic flicker photometry. The results of this study showed that central MP increased significantly in the active group, whereas no such augmentation was demonstrable in the placebo group. Of note, however, this modest augmentation in MP using a lutein-based formulation was observed only at the 12-month study visit, and the increase is much smaller in comparison with recent studies which include all three macular carotenoids in the formulation (see below). Also, the increase in MP did not correlate with an improvement in visual performance (Nolan et al., 2011).
The meso-zeaxanthin Ocular Supplementation Vision Trial (MOST Vision) investigated the effect of supplemental macular carotenoids, including a formulation containing meso-zeaxanthin, on visual performance in normal subjects (Loughman et al., 2012). The thirty-six recruited subjects were assigned to one of three groups: the first was given a high dose (20 mg) of lutein and 2 mg zeaxanthin (Group 1); the second group was given 10 mg lutein, 10 mg meso-zeaxanthin and 2 mg zeaxanthin (Group 2); and the third group was given placebo (Group 3), every day for six months. A statistically significant increase in MP was observed (determined at three months following commencement of supplementation) only among subjects supplemented with a formulation containing all three macular carotenoids, including meso-zeaxanthin (Group 2). Statistically significant improvements in visual acuity were observed at six months, but only for subjects in Group 2. Contrast sensitivity (under mesopic and photopic conditions) and glare disability under mesopic conditions were assessed using the Functional Acuity Analyzer™ at the following spatial frequencies: 1.5, 3, 6, 12 and 18 cycles per degree (cpd). Statistically significant improvements in CS were noted across a range of spatial frequencies, under photopic (3, 12 and 18 cpd) and mesopic conditions (1.5, 3, 12 and 18 cpd), again only among subjects supplemented with meso-zeaxanthin (with a single exception of improved contrast sensitivity at a single spatial frequency [6 cpd] in the high lutein group [Group 1]). There were no statistically significant improvements in mesopic glare disability between baseline and six months in Groups 1 and 3, however, there was a demonstrable improvement in mesopic glare disability for subjects in Group 2 for all spatial frequencies tested (with the exception of 18 cpd).
A recent randomized, double-blind, placebo-controlled, 1-year interventional study in 120 normal subjects (Chinese drivers) examined the effect of lutein supplementation on visual function (Yao et al., 2013). The active group consumed 20 mg of lutein daily. Participants were assessed at baseline, 1, 3, 6, and 12 months. Assessment included visual acuity, serum lutein concentrations, MP, and visual performance. At the onset and at the end of the intervention, visual-related quality-of-life was measured. Serum lutein and central MP in the active group increased significantly, whereas no change was observed in the placebo group. The authors reported important increases in contrast sensitivity and glare disability, especially in the mesopic condition, and there were significant improvements in the vision-related quality-of-life in the active group. The authors concluded that supplementation with lutein may benefit driving at night and other spatial discrimination tasks conducted under low illumination.
The Central Retinal Enrichment Supplementation Trial (CREST) is currently underway and will further enhance our understanding of the role of the macular carotenoids in normal subjects (Akuffo et al., 2014). CREST has two trial study populations under investigation (Trial 1 = normal subjects with low MP and Trial 2 = subjects with early AMD), and the main objectives of both trials are to investigate the impact of MP enrichment on visual function. The active intervention (in Trial 1) contains 10 mg lutein, 2 mg zeaxanthin, and 10 mg meso-zeaxanthin, which is interesting because recent studies suggest that supplementation with a formulation containing all three macular carotenoids (i.e. lutein, zeaxanthin, and meso-zeaxanthin) offers advantages over formulations not containing all three components of MP (e.g. just lutein and zeaxanthin) (Loughman et al., 2012; Meagher et al., 2013; Nolan et al., 2012; Sabour- Pickett et al., 2014; Thurnham et al., 2015), and it is important to fully test this hypothesis in the context of a correctly powered, well-designed, clinical trial with appropriate outcome measures (i.e. visual function, including CS and GD). The results of CREST Trial 1 are expected to be available in 2015.
In conclusion, there is a biologically plausible rationale, supported by MP's light-filtering properties, which suggests that augmentation of MP will enhance visual function and comfort by attenuation of the effects of chromatic aberration and light scatter. Indeed, clinical trials have repeatedly shown that supplementation with the macular carotenoids lutein, zeaxanthin, and meso-zeaxanthin results in augmentation of MP, and consequential benefits in visual performance such as improved contrast sensitivity and reduced glare diability. The importance of these findings extends to those involved in vision-dependent-specialized activities, such as pilots, vehicle drivers, military personnel, and athletes.
3.3. Carotenoid interventions against age-related macular degeneration (AMD)
Much has changed in ophthalmologists' management and treatment of AMD in the past few decades. A once largely ignored and poorly understood disease of aging now consumes billions of healthcare dollars in the United States and other developed countries, and with longer lifespans, its prevalence is rising in the developing world as well. The exudative or “wet” form of AMD formerly was considered the most devastating manifestation of the disease because of its abrupt onset and inexorable decline of vision toward legal blindness, but the introduction of effective anti-VEGF compounds ten years ago has given hope that vision can even improve with treatment; however these medications come at a steep price both monetarily and in the often monthly returns to the retina specialist's office for repeated intravitreal injections. Moreover, the advanced “dry” form of AMD known as geographic atrophy has proven much more resistant to therapeutic interventions. Even though its rate of progression is much slower than exudative AMD by at least a factor of 10, loss of central vision still occurs, and no medication for dry AMD has successfully cleared the FDA's rigorous clinical testing requirements for approval in the United States. Thus, there is still considerable interest in preventing or delaying the onset of AMD by identifying and modifying risk factors for this devastating blinding condition.
We now understand that AMD is a complex disorder with multiple inherited risk factors including one major genetic risk locus on chromosome 1 in the complement factor H region and a second equally important locus in the HTRA1/ARMS2 region on chromosome 10, along with myriad other minor genetic risk factors identified through genome-wide association studies (GWAS) (Ding et al., 2009; Kanda et al., 2007; Klein et al., 2005; Montezuma et al., 2007; Neale et al., 2010; Yang et al., 2006). Other non-modifiable traits associated with increased risk of AMD include light skin color, light iris color, and possibly female gender, and, of course, increasing age is associated with a nearly exponential rise in incidence and prevalence of clinically significant AMD (Holz et al., 1994; Hyman et al., 2000; West et al., 1989). Although identification of inherited and other non-modifiable risk factors can guide the development of rational novel treatment strategies such as pharmacologic, gene therapy, growth factor, and stem cell treatments, the current reality demands attention to identify and alter more modifiable risk factors.
Modifiable risk factors for AMD are generally identified initially through epidemiological studies conducted in conjunction with logical inferences based on the known pathophysiology of the disease. In case-control studies, cohorts of AMD patients are matched by age and other demographic characteristics with individuals without AMD, and then through the use of sophisticated statistical models, potential risk factors for AMD can be identified after correcting for any confounding influences. Epidemiological studies are best at identifying associations, but they do not prove causality, and confirmation of findings from multiple independent studies generates added confidence that the associations are clinically verifiable. Once these associations are identified, they can be used to guide future, focused small and large interventional studies and to generate scientifically based public health recommendations. AMD is a prime target for epidemiological studies because it is so common, but its late onset and diverse manifestations have made it a challenge. Cigarette smoking has repeatedly been identified as an AMD risk factor, and it is therefore noncontroversial to recommend that all individuals at risk for AMD cease smoking or never start (Cheng et al., 2000; Seddon et al., 1996; Sobrin and Seddon, 2014). Excessive light exposure would seem to be a reasonable risk factor for AMD based on its potential to incite oxidative damage to lipid membranes and proteins under both acute and chronic conditions. Surprisingly, so far, only a few studies have generated positive associations between light exposure and AMD (Evans, 2001; McCarty and Taylor, 1999; West et al., 1989). This is in part due to the difficulty of quantifying long-term light exposure in subjects, which means that most studies have concentrated on extreme conditions encountered by fishermen and other outdoor workers where it is logistically straightforward to match subjects who routinely used hats and sunglasses with subjects who did not employ these sun protection strategies.
Diet has been of particular interest to AMD epidemiologists because multiple laboratory studies have implicated oxidative stress as a major potential mechanism underlying damage generated in cell culture and animal models of AMD (Crabtree et al., 1996; Kelly et al., 2014; Rabin et al., 2013), and diet is the usual source of antioxidants and other protective nutrients for most individuals. Moreover, the general public is often interested in and receptive to dietary and supplement recommendations from physicians and public health authorities as a means to empower themselves to avoid a common and dreaded disease such as AMD. Nutritional epidemiological studies have cast a very wide net based largely on dietary surveys and to a lesser extent on blood levels and other biomarker studies. Dietary surveys can be quite challenging, however, because they may be limited by the quality of nutrient databases and questionnaires, by subject fatigue when faced with burdensome comprehensive food diaries and surveys, and by subject recall bias, while blood and biomarker studies are often invasive and may be of limited value in defining nutritional status. A wide variety of nutrients have been implicated in AMD risk including antioxidant minerals such as zinc and selenium, antioxidant vitamins such as vitamin C and vitamin E, omega-3 fatty acids such as EPA and DHA, and various carotenoids naturally found in the eye such as lutein and zeaxanthin (Age-Related Eye Disease Study Research et al., 2007; Augood et al., 2008; Evans and Lawrenson, 2012; Landrum et al., 1997; Seddon et al., 1994; Seddon et al., 2001; Vishwanathan et al., 2013a).
The association of carotenoids and eye health extends back for centuries based largely on the recognition that consumption of certain foods such as carrots can help to treat and prevent symptoms of night blindness, and Chinese traditional medicine has long recommended consumption of carotenoid-rich bright orange goji berries as healthy for the eyes. Nineteenth and early twentieth century chemists isolated and elucidated the structures of vitamin A and β-carotene and recognized that central cleavage of β-carotene could directly lead to vitamin A aldehyde which could be readily reduced to vitamin A itself, and in the mid-twentieth century, Wald conducted his Nobel-prize winning work that showed that a metabolite of vitamin A, 11-cis-retinaldehyde, was the chromophore for the “visual purple” which later became known as rhodopsin (Wald and Brown, 1958). Meanwhile, anatomists recognized that the macula of the human eye had a distinct yellow spot, the macula lutea, and Wald was able to determine that macular extracts had spectral characteristics typical of carotenoids, but that the MP was not β-carotene because it had chemical properties consistent with xanthophylls, oxygenated carotenoid derivatives of the carotenes (Wald, 1945). Several decades later, Bone and Landrum revisited the chemical composition of the human MP using HPLC, and they preliminarily identified the MP as a mixture of two plant-derived xanthophylls with no vitamin A activity, lutein and zeaxanthin (Bone et al., 1988). A few years later, they completed their chemical characterization of the MP when they found that the zeaxanthin component was actually a mixture of dietary 3R,3′R-zeaxanthin and non-dietary 3R,3′S-meso-zeaxanthin (Bone et al., 1993).
At about the same time that Bone and Landrum and other groups were analyzing the carotenoid composition of the human macula, the first major epidemiological study of nutrition and AMD, the Eye Disease Case-Control (EDCC) Study, noted that blood levels of lutein and zeaxanthin inversely correlated better than blood levels of β-carotene with risk of exudative AMD (Eye Disease Case-Control Study Group, 1993), and a follow up study led by Seddon confirmed with dietary surveys of the EDCC subjects that consumption of foods rich in lutein and zeaxanthin such as dark green leafy vegetables and various orange and yellow fruits and vegetables were associated with a significantly lower risk of advanced AMD, while β-carotene-rich foods such as carrots were not significantly protective (Seddon et al., 1994). Specifically, individuals with the highest quintile of lutein and zeaxanthin consumption (∼6 mg per day) had a 43% lower risk of exudative AMD relative to individuals in the lowest quintile of consumption (<1 mg per day). As mentioned above, single epidemiological studies should not be interpreted in isolation from other evidence unless they can be replicated in independent cohorts. Relative to smoking, this has been much more challenging in the case of lutein and zeaxanthin. First, quantitation of dietary lutein and zeaxanthin intake is limited by the quality of available nutrient databases and dietary surveys. Second, dietary assessment of nutrient intake is confounded by many more factors relative to taking a smoking history. Thus, replication of the EDCC findings has had a decidedly mixed history, with some studies confirming the apparent protective effects of lutein and zeaxanthin and others that did not confirm the association (Mares-Perlman et al., 1995). Many of these non-confirming studies were inadequately powered, however, whereas a similarly sized analysis of the AREDS cohort did come to the same conclusion as the EDCC Study (Age-Related Eye Disease Study 2 Research, 2013). Trials are also limited by their short term nature, poorly modeling the influences on early stages of AMD which develop over a long time period. Therefore, such epidemiologic controversy is best settled by a randomized clinical trial, but no such data were available in the mid-1990s when commercial production and marketing of lutein containing supplements began in the United States. Nevertheless, lutein supplementation was enthusiastically embraced as a low-dose “eye healthy” component of general consumer multivitamins at ∼0.25 mg per day and in supplements marketed to AMD patients at doses ranging from 2-20 mg per day.
Dietary surveys and blood level analyses of lutein and zeaxanthin provide only indirect information on the nutritional status of the tissue of interest, the human macula. Although clinical studies generally confirmed that dietary surveys and blood lutein and zeaxanthin levels significantly correlated with central MPOD, the correlation coefficients were typically rather low. This was not unexpected based on growing knowledge that deposition of lutein and zeaxanthin in the macula is a saturable, regulated process mediated by specific binding proteins and transporters. Therefore, after the publication of the EDCC studies, several research groups embarked on studies to correlate MP levels with risk of AMD (Arend et al., 1995; Beatty et al., 2000; Berendschot et al., 2002; Bernstein et al., 2002; Bone et al., 2001; LaRowe et al., 2008; Nolan et al., 2007; Snodderly et al., 2004; Stringham et al., 2008). As discussed earlier in this article, there are numerous methods to measure MP, each of which has particular strengths and limitations, and all have been used in recent years to probe the relationship of macular carotenoids and AMD risk. Direct HPLC analysis of macular tissue is the most chemically specific method because it can separate all three major components of the macular carotenoid pigment, but it requires the collection of valuable postmortem tissue, and its spatial resolution is limited to the 2-5 millimeter scale by detection sensitivity and by the fact that the tissue must be dissected and trephined by hand. Moreover, clinical histories of control and AMD donor eyes may be very limited. Bone and Landrum published the most comprehensive donor eye study in 2001, a time when lutein and zeaxanthin supplementation was very uncommon, in which 56 donor eyes with AMD and 56 control eyes were analyzed by HPLC using concentric regions of retina from 0-5 degrees, 5-19 degrees, and 19-38 degrees (Bone et al., 2001). Lower levels of lutein and zeaxanthin were found in the AMD eyes at all eccentricities, and there were concerns that AMD pathology could contribute to the lower central levels, but the fact that lower levels persisted to the periphery was felt to be consistent with low levels of retinal xanthophylls as a risk factor for AMD. Unfortunately, 3R, 3′R-zeaxanthin and 3R, 3′S-meso-zeaxanthin were not separable by their HPLC methods. Future replicate case-control autopsy eye studies will be even more challenging because lutein and zeaxanthin use by AMD patients and even-non-AMD controls has become so prevalent in the United States. This was made clear in a 2007 study from our laboratory in which ocular tissues from 228 eyes from 147 Utah donors without known AMD were analyzed using normal phase and chiral chromatography (Bhosale et al., 2007b). Eighteen percent of donors age 48 and older had unusually high levels of lutein and its metabolite meso-zeaxanthin in macula, peripheral retina, and lens, and retrospective questionnaires of selected donors' families confirmed that these high levels could be explained by high-dose lutein supplementation prior to death.
Numerous epidemiological studies have used the most common method of MPOD measurement, heterochromatic flicker photometry (HFP). This psychophysical measurement is well suited to large population studies because the equipment is portable and relatively low cost and can be used without pupil dilation, but it requires a significant commitment to train the subjects to perform the task accurately and reproducibly. HFP can be particularly difficult in elderly individuals with macular pathology which also limits its use in the AMD population. Its spatial resolution is limited, which means that many AMD studies use just a single measurement at ∼0.5 degrees of eccentricity relative to a zero point at ∼7 degrees, but some studies try to map out a rough spatial profile with additional eccentricities which lengthens the measurement time and may lead to subject fatigue. Initial studies examined subjects with intermediate AMD in one eye and advanced exudative AMD in the fellow eye and found that the better eye had lower MPOD relative to unaffected age-matched controls (Beatty et al., 2000), and more recent studies from this same group have correlated low MPOD with various well-known risk factors for AMD such as age, smoking, and a positive history of AMD in a close family member (Nolan et al., 2007), although other groups have failed to confirm these correlations, especially with regard to age (Berendschot and van Norren, 2005), suggesting either methodological differences or subject selection bias (clinic-based versus population-based versus recruited volunteers). The CAREDS study correlated MPOD with various genetic risk factors for AMD and found some interesting correlations with genes such as GSTP1, BCMO1, SCARB1, ABCA1, ABCG5, LIPC, ELOVL2, FADS1, FADS2, ALDH3A2 and RPE65 (Meyers et al., 2013). As with any genetic association study, these findings will need to be confirmed in independent study populations.
Resonance Raman measurement of macular carotenoids was developed as an optical alternative to the psychophysical methods of MP measurement that is best suited to a clinic-based population because it requires pupil dilation. It is an integral method that measures the total amount of macular carotenoids in the 1-mm field illuminated by the low-power blue laser light for less than one second (Bernstein et al., 1998). It does not require a peripheral zero reference point, but small pupils and various media opacities may artifactually lower measured levels (Ermakov et al., 2005). It is typically measured on an externally calibrated scale of Raman counts which makes it difficult to compare results to more commonly reported MPOD levels from other methods. Initial studies on a case-control Utah clinic-based population found that Raman counts were 32% lower in AMD subjects versus age-matched controls unless the AMD subjects had a history of routine lutein supplementation (Bernstein et al., 2002). Follow up studies in Japan using an improved version of the instrument with video monitoring of subject fixation have confirmed these findings (Obana et al., 2014; Obana et al., 2008), but further research elsewhere has been hampered by limited availability of this costly custom-built laser-based equipment. On the other hand, resonance Raman measurement of skin carotenoids is a commercialized noninvasive method to measure systemic carotenoid status that is well correlated with tissue and serum levels and is an excellent biomarker of fruit and vegetable consumption (Mayne et al., 2013; Scarmo et al., 2012). Unfortunately, correlations with macular carotenoid levels are poor in adults (Bernstein et al., 2012), but they do correlate significantly in infants and children (Henriksen et al., 2013).
In recent years, imaging-based methods of MP measurement have come into wider use in AMD epidemiological studies. These methods have the advantage of micron spatial resolution over a field that can encompass the entire macula, and they are well adapted to measure both peak MPOD levels and integrated total MPOD measurements (“area under the curve”). They provide spatial maps of MP distributions that are easily appreciated by both ophthalmologists and their patients, and post-processing analysis is readily accomplished if zero-point baselines or foveal centration need to be adjusted. AMD patients can pose a substantial challenge for imaging, however, if they have significant media opacities, small pupils, or macular pathology. Autofluorescence imaging (AFI) is the most commonly used MP imaging technique. It is based on the principle that RPE lipofuscin's fluorescence is attenuated by the MP's absorption of blue excitation light (Delori, 2004). This manifests as a central dark spot centered at the fovea on blue light autofluorescence images that can then be displayed as a MP intensity map. Although it can be done in a single wavelength mode, digitally subtracting a green autofluorescence image taken at a wavelength with minimal MP absorption will improve image quality and reliability in patients with significant AMD pathology (Delori, 2004). Initial AFI studies on AMD patients and normal patients (at high risk of developing AMD) were most remarkable for the recognition that AMD patients were more likely to have MP patterns that deviated from the classically described smoothly rising central peak (Bernstein et al., 2012). Some subjects had ring-shaped shoulders or even central dips. Quantitative differences between normal and AMD subjects were harder to prove because by this time lutein supplementation had become commonplace in the United States and Europe. In fact, when Utah AMD patients were measured at their baseline AREDS2 visit, their average peak MPOD was twice the Utah population average, due presumably to the high rate of prior carotenoid supplementation (70%) in the enrolled population (Bernstein et al., 2012). Some AFI studies have suffered from inconsistencies in the AMD population that have been ascribed to cataracts and other age-related media opacities (Sharifzadeh et al., 2014), but newer dual-wavelength technologies based on modern confocal scanning laser ophthalmoscopes with infrared laser image tracking and high sensitivity detectors with excellent linear response seem particularly promising for future AMD studies. There is much less experience with other MP imaging techniques in the AMD population. Reflectometry may suffer from media opacities and macular pathology that will complicate image acquisition and that may invalidate the assumptions in the underlying mathematical model. Only a few reflectometry studies have been carried out with commercial instrumentation in Europe and Japan (Kazato et al., 2010; van de Kraats et al., 2008), and it is concerning that results do not correlate well when tested head-to-head against AFI or HFP in the same patients (Dennison et al., 2013). Resonance Raman imaging can also be implemented in a chemically specific imaging mode, but required light levels approach ANSI limits, so only a few normal volunteers have been imaged (Sharifzadeh et al., 2008).
Most of the small-scale prospective carotenoid supplementation studies have been carried out in the AMD population with monitoring by HFP, AFI, resonance Raman, or reflectometry, but they have generally been underpowered in terms of subjects, means of supplementation, and duration to detect an actual impact on progression of AMD. Many have therefore substituted various functional endpoints such as visual acuity, reading speed, contrast sensitivity, glare recovery, or multifocal ERG response. In general, these studies have shown increases in MP at varying eccentricities with supplementation, but the time courses have been surprisingly variable with some studies claiming substantial rises within weeks, and others concluding that supplementation may be required for a year or more to see a sustained clinically meaningful increase (Berendschot et al., 2000; Bone et al., 2003; Huang et al., 2015; Johnson et al., 2000; Landrum et al., 1997; Wenzel et al., 2006). Often, 20% or more of the subjects may show no response at all even with substantial rises in serum carotenoid levels, consistent with saturable MP uptake mechanisms. Detected improvements in functional endpoints have been encouraging that carotenoid supplementation is beneficial for the AMD population beyond just prevention of progression of advanced AMD. A few studies have focused on trying to normalize atypical MP distributions with the assumption that this may help lower the risk of future AMD progression. These studies have compared various combinations of lutein, 3R, 3′R-zeaxanthin and 3R, 3′S-meso-zeaxanthin with HFP as the monitoring method. Positive results have been reported that preparations containing all three macular carotenoids may be particularly effective in normalizing distributions (Connolly et al., 2011; Connolly et al., 2010; Stringham and Hammond, 2008).
Many retina specialists are interested in making nutritional recommendations to their patients at risk for visual loss from AMD, but they generally demand high level evidence from randomized, placebo controlled trials before dispensing such advice. The National Eye Institute incorporated the current nutritional knowledge of the 1980s when they initiated the Age-Related Eye Disease Study in 1989 consisting of 80 mg zinc oxide (along 2 mg copper oxide to combat potential zinc-induced anemia), 500 mg vitamin C, 400 IU of vitamin E, and 15 mg (25,000 IU) of β-carotene (Age-Related Eye Disease Study Research, 2001). The study followed more than 4000 high risk AMD patients at 11 centers with large soft drusen and/or advanced AMD in one eye with progression to advanced AMD (choroidal neovascularization or geographic atrophy) or visually significant cataracts as the primary study outcomes at the end of five years. The four major treatments were: (1) zinc; (2) antioxidant vitamins (vitamin, vitamin E, and β-carotene); (3) zinc + antioxidant vitamins; or (4) placebo. All three active treatment arms showed positive results, with the combined intervention group achieving a 25% reduction of progression to advanced AMD relative to placebo. After publication of these results in 2001, the AREDS formulation rapidly became standard-of-care, but the AREDS investigators appreciated that nutritional knowledge of AMD had advanced considerably and should be tested in a next-generation AREDS2 supplementation trial. With regard to the original AREDS formulation's carotenoid content, there was substantial concern about β-carotene's safety and efficacy. First, while AREDS was in progress, two large randomized clinical trials of high-dose β-carotene supplementation noted an unexpected increase in lung cancer risk in smokers, raising substantial concerns for AREDS supplement use in nearly half of the population at high risk for advanced AMD (Albanes et al., 1996; The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group, 1994). Second, it was now appreciated that lutein and zeaxanthin might be the more physiologically relevant carotenoids for AMD supplementation because of the EDCC epidemiology results and the basic science knowledge that β-carotene is not detectable in the human retina and that lutein, zeaxanthin and meso-zeaxanthin form the MP. Third, commercial sources of lutein and zeaxanthin suitable for clinical trials were now available.
The AREDS2 study was designed to test whether the original AREDS supplement formulation could be made safer and more effective. The organizers reviewed the most recent nutritional knowledge for AMD and concluded that inclusion of lutein + zeaxanthin along with omega-3 fatty acids should be examined. It was decided to mimic a typical dietary ratio of 5:1 for lutein:zeaxanthin, and the 10 mg lutein + 2 mg zeaxanthin dose for the carotenoid arm was chosen based on a small-scale, dose-ranging study that showed robust serum response to this dose and evidence that higher doses were approaching the saturation region of the dose-response curve (Rosenthal et al., 2006). This lutein/zeaxanthin daily dose was considerably higher than a typical American diet which is usually in the 1-2 mg range for lutein and 0.2 mg for zeaxanthin, but it was still considered safe because many vegetarians routinely consume these levels without problems. No MPOD measurements were included in the AREDS2 protocol because the image-based instrumentation was not sufficiently standardized at that time, and the psychophysical methods were thought to require too much time and training to be of use during the very busy study visits. Only the Utah site had an ancillary study to measure MP with dual-wavelength autofluorescence imaging. A baseline study from Utah showed that this site's subjects had unexpectedly high peak MPOD at entry into the study even after the 30 day supplement-free washout period, consistent with the observation that 70% of Utah subjects had been taking lutein and zeaxanthin regularly for many years prior to enrollment (Bernstein et al., 2012). Follow up MPOD measurements on these subjects at their scheduled visits every six months during the study proved inconclusive due to the relatively small number of subjects in this ancillary study and the challenges of reproducibly imaging MP in patients with significant macular pathology.
The primary randomization in AREDS2 was between four groups: (1) lutein + zeaxanthin (10 mg and 2 mg, respectively); (2) fish oil containing DHA +EPA (350 mg and 650 mg, respectively); (3) lutein + zeaxanthin + DHA + EPA; and (4) placebo (Age-Related Eye Disease Study 2 Research, 2013). All enrolled subjects who were nonsmokers were offered secondary randomization between the original AREDS formula or to a modified AREDS formula containing no β-carotene and/or lower levels of zinc. All smokers were offered the no β-carotene version of the AREDS supplement. Subject retention and compliance were excellent, especially for a five-year study with over 4000 subjects. The study did not reach its primary endpoint of a 25% incremental improvement upon the original AREDS results when each of the three treatment groups were compared individually with the placebo group, but pre-planned secondary analyses did provide important results that have guided subsequent clinical practice (Age-Related Eye Disease Study 2 Research et al., 2014). Evaluation of the main effects of lutein + zeaxanthin in which the entire study cohort that received lutein + zeaxanthin was compared to the cohort that did not receive lutein + zeaxanthin demonstrated statistically significant reduction of progression to advanced AMD, but similar pre-planned analysis for DHA + EPA did not. The results were even more impressive when the subgroup receiving lutein + zeaxanthin with no β-carotene was compared to the subgroup that received β-carotene alone. This was thought to be due in part to competitive absorption between β-carotene and the macular xanthophylls which was confirmed when serum levels of carotenoids were analyzed. Lutein + zeaxanthin also appeared to be safer than β-carotene because no increase in lung cancer incidence was noted among subjects assigned to the lutein + zeaxanthin arms, while β-carotene was associated with a statistically significant rise in lung cancer incidence in former smokers. Based on the totality of evidence, the AREDS2 investigators concluded that substitution with 10 mg of lutein and 2 mg of zeaxanthin for 15 mg of β-carotene is an appropriate modification of the original AREDS formulation for smokers, former smokers, and non-smokers, and these recommendations have been rapidly incorporated into the consumer marketplace and clinical practice.
Although it is unlikely that there is enough funding and interest to perform a third-generation AREDS3 study, there are a number of interesting questions that can still be addressed with regard to the role of carotenoids in the prevention of AMD. Most of the AREDS2 subjects have been genotyped, so it will be important to assess the impact of various genetic variants on response to the lutein + zeaxanthin intervention. Both AREDS studies were designed to measure the impact of carotenoid supplementation in patients who already had intermediate or unilateral advanced AMD; it is not clear whether lutein + zeaxanthin supplementation would be efficacious in the “worried well” population even if they happen to carry high risk genes for AMD. Supplement formulations containing meso-zeaxanthin have recently become available in the American and European markets, but randomized, controlled studies to demonstrate their superiority to traditional lutein + zeaxanthin supplements for the prevention of AMD remain to be done.
The AREDS studies also considered whether or not carotenoids might be able to slow down cataract progression and age-related cognitive decline. This was based in part on their antioxidant properties and the observation that the human lens and brain are other tissues that accumulate lutein and zeaxanthin selectively (Khachik et al., 2002; Vishwanathan et al., 2015; Yeum et al., 1999; Yeum et al., 1995), although in much lower concentrations than the human macula. Several epidemiological studies have also associated diets rich in carotenoids with decreased risk of cataracts and cognitive decline (Gale et al., 2001; Jacques and Chylack, 1991; Johnson, 2012; Moeller et al., 2008). Small interventional studies have had mixed results, and the AREDS2 study failed to prove a protective effect for β-carotene or for lutein + zeaxanthin with respective to cataracts or cognitive function (Age-Related Eye Disease Study 2 Research et al., 2013; Chew et al., 2015).
3.4. Carotenoid interventions against other eye diseases
Various carotenoid interventions against retinitis pigmentosa (RP) and related inherited retinal and macular degenerations have been studied for many years. β-Carotene has been considered a promising intervention in light of the prominent role of vitamin A metabolism in normal retinal function and the fact that some forms of RP are caused by genetic defects in retinoid function and processing (Bowne et al., 2011; den Hollander et al., 2009; Rando et al., 1991). Moreover, supplementation with vitamin A palmitate has been shown to modestly slow the progression of RP in a large, randomized clinical trial (Berson et al., 1993), but concerns about vitamin A teratogenicity and liver toxicity at the recommended 15,000 IU dose have further dampened enthusiasm for this intervention. Supplementation with β-carotene would certainly be safer because cleavage to retinoids is a regulated process that functions to prevent vitamin A toxicity even when large amounts of this carotenoid are consumed, but this beneficial process will buffer any increase in retinal vitamin in response to β-carotene supplementation, and, so far, no β-carotene supplementation studies have reported an effect except for one study that used the 9-cis isomer of β-carotene whose 9-cis-retinoid cleavage product could potentially bypass retinoid isomerization defects in ocular tissues (Meshi et al., 2015). Cone function in the macula is preserved until very late in the course of RP and related diseases such as choroideremia which has led researchers to consider whether or not supplementation with lutein and zeaxanthin could have a beneficial effect. Measurement of MP in RP and choroideremia patients has generally shown that their levels are comparable to unsupplemented control individuals which argues against a potential role for lutein and zeaxanthin supplementation in these individuals (Aleman et al., 2001; Zhao et al., 2003), and the only large scale randomized trial of lutein in RP patients yielded just subtle positive results for peripheral visual fields when secondary analyses were performed (Berson et al., 2010). When MP has been measured in macular dystrophies, the levels have been lower than normal controls, but the inherent loss of photoreceptors in these diseases complicates interpretation as to whether photoreceptor loss is exacerbated by low levels of macular carotenoids or whether the loss of macular carotenoids is simply a consequence of loss of the rod and cone cells (Zhao et al., 2003). There are no published studies showing a beneficial effect of lutein or zeaxanthin in Stargardt disease or other macular dystrophies, and β-carotene is contraindicated in patients with ABCA4 mutations because retinoid supplementation exacerbates the course of the disease in animal models with ABCA4 defects, and it is assumed that the same problem will likely occur in humans (Charbel Issa et al., 2013a).
A handful of ocular conditions are clearly associated with deficiency of MP or strikingly abnormal distributions. Patients with ocular albinism not only have abnormal low melanin levels, but they also have no detectable MP (Abadi and Cox, 1992). The pathophysiological mechanism for the absence of MP in albinos remains unclear because there are no obvious connections between melanin synthesis and MP accumulation, but the absence of development of an anatomical fovea is almost certainly involved. Likewise, patients with Sjögren-Larsson syndrome, an inherited defect in the ALDH3A2 gene which encodes a fatty aldehyde dehydrogenase enzyme, have no MP (Meyers et al., 2014; Theelen et al., 2014; van der Veen et al., 2010), but there is currently no pathophysiological mechanism to explain why this occurs. One of the earliest manifestations of macular telangiectasia type II (MacTel), an uncommon bilateral maculopathy that features cystic changes of the fovea and abnormal perifoveal vasculature, is a temporal wedge-shaped disruption of the MP which may eventually progress to a ring-shaped redistribution of the macular carotenoids at a radius 1-2 mm from the fovea that was clearly seen with autofluorescence imaging (Figure 14) (Theelen et al., 2014; Wong et al., 2009). We suggest that abnormal localization of the macula's carotenoid-binding proteins may underlie this redistribution, but direct proof of this hypothesis is still lacking. Histopathological analysis of MacTel eyes notes a profound loss of Müller glial cells in the affected regions, and monkey models of acquired Müller cell destruction feature loss of MP (Charbel Issa et al., 2013b; Powner et al., 2010), yet no known binding proteins for lutein or zeaxanthin localize to the Müller cell (Li et al., 2011). Researchers in Europe tried to re-normalize MP distributions in MacTel using lutein supplementation, but they only succeeded in further enhancing the ring without filling in the center (Zeimer et al., 2010). We speculated that zeaxanthin, the more centrally located of the dietary macular carotenoids might be more efficacious than lutein, but our clinical trial with 10-20 mg of zeaxanthin likewise led to ring enhancement only, and in one of the eight subjects, yellowish hypofluorescent crystalline deposits reversibly appeared in the macula in localized patches and rings. These visually asymptomatic crystals are reminiscent of canthaxanthin crystalline maculopathy. The genetic defect(s) underlying MacTel remains undefined, but it is hoped that when its genetic origins are solved, they will provide new insights into carotenoid metabolism in the normal human macula.
Figure 14.
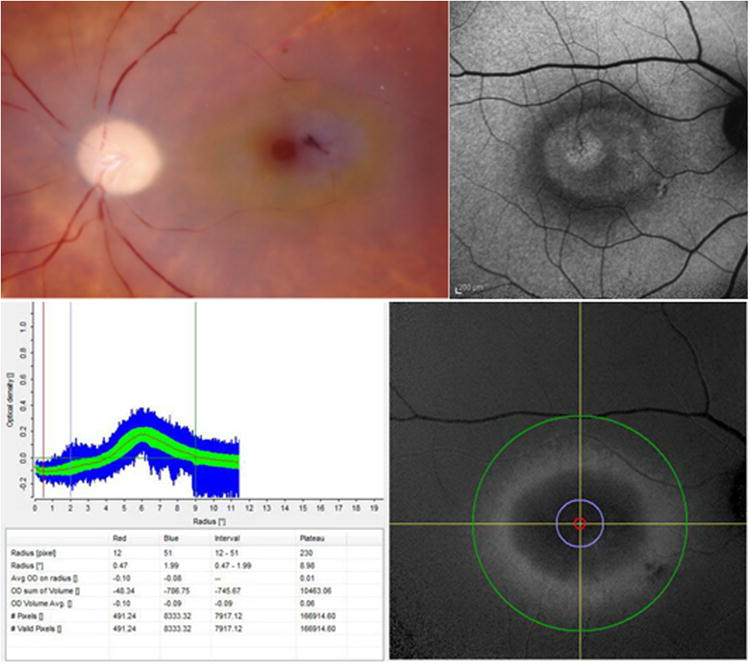
Anomalous macular pigment distributions in MacTel patients. Post-mortem specimen from a MacTel patient showing a circular distribution of yellow carotenoid pigment around the fovea (upper left); autofluorescence image of another MacTel patient with a hypofluorescent ring of macular pigment centered on the fovea (upper right); Heidelberg Spectralis macular pigment output from a third MacTel patient demonstrating absence of macular pigment at the fovea and a ring of macular pigment at 6 degrees eccentricity (approximately 1.7 mm) (bottom images).
3.5. Carotenoid interventions in infancy and childhood
Carotenoids play important roles in macular protection, as has been shown in age-related macular degeneration, but the influence of carotenoids on macular development is not well documented. The rate-limiting step in determining the role of carotenoids in macular development has been quantification of MP in infants due to unique challenges that limit imaging modalities. HFP requires subjective participation and is impossible to perform with infants and children, and autofluourescence imaging (AFI) is dependent on lipofuscin which is not present in infants and children (Bernstein et al., 2010; Bernstein et al., 2013; Howells et al., 2011). A recent blue light reflectometry technique developed by Bernstein and associates has allowed for imaging of infants with the RetCam (Clarity Medical Systems Incorporated, Pleasanton, CA) (Figure 15) (Bernstein et al., 2013). Reflectometry is particularly useful in infants due to clearer ocular media when compared to adults. This technique has been used to document MP in term infants and young children; however, pre-term infants have not had detectable MP (Bernstein et al., 2013; Henriksen et al., 2013)
Figure 15.
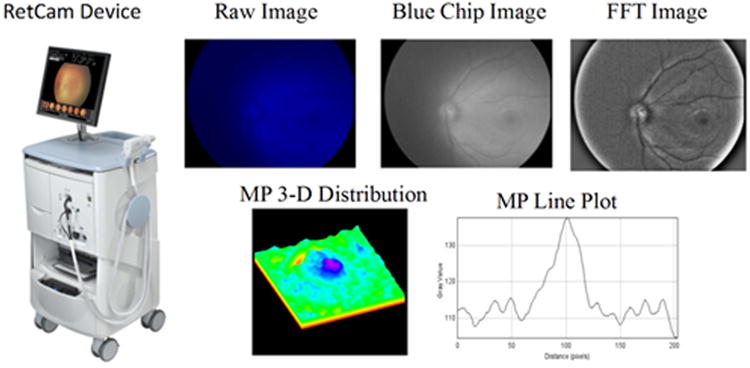
RetCam reflectometry images of macular pigment in an infant eye. The left picture shows the RetCam device; the upper images present the raw blue light reflectance image, the blue channel output from the CCD chip, and the a fast Fourier transform (FFT) digital enhancement of the blue channel output; the bottom images show the digitally processed macular pigment data as a 3-D surface plot and as a line-scan through the fovea.
Placental transfer of carotenoids has yet to be studied in great detail, although preliminary data indicate that a critical gestational period may be involved. In adults, carotenoids have been shown to be absorbed from the diet and deposited in tissues in detectable amounts in a matter of weeks (Johnson et al., 2000), but the transfer of carotenoids from mother to child in utero has not been directly studied through clinical supplementation trials. In 2013, Henriksen et al. reported significant correlations between maternal and infant skin and serum carotenoids within the first 24 to 72 hours after delivery (Henriksen et al., 2013). Specifically, maternal total serum carotenoid levels correlate with infant total serum carotenoid levels (r = 0.43, P = 0.017). Similar correlations between mothers and infants were seen with serum zeaxanthin (r = 0.049, P = 0.006), and serum lutein (r = 0.53, P = 0.003). This relationship also held true with tissue carotenoid levels with maternal skin carotenoids correlating with infant skin carotenoids (r = 0.59, P < 0.001). These findings suggest that maternal carotenoid status, influenced by dietary intake, may have a role in infant carotenoid levels via placental transfer of these vitamins during the gestational period. Additionally, reports indicate that lutein levels are much higher in neural tissues from term infants when compared to pre-term infants (Vishwanathan et al., 2014). This suggests that most lutein deposition occurs during the last trimester via placental transfer (Henriksen and Chan, 2014; Vishwanathan et al., 2014).
The deposition of carotenoids within the eye appears to increase with later gestational stages. Cadaver studies have confirmed the presence of carotenoids within retinal tissue as early as 20 weeks gestation (Bone et al., 1988), with ratios of lutein: zeaxanthin: meso-zeaxanthin differing from the composition of serum (Bone et al., 1993). In 2013, we reported MPOD measured with RetCam imaging and blue light reflectometry increases linearly with age in infants and children (r = 0.36, P = 0.0142) (Bernstein et al., 2013). All pre-term infants in this cohort did not have detectable MPOD. In a companion study, MP was detected in term infants (gestational age of 37 weeks or older) with MPOD values ranging from 0.04 to 0.16 and an average value of 0.087 (SD = 0.032). Furthermore, correlations between infant MPOD and infant serum zeaxanthin (r = 0.68, P = 0.007), and maternal serum zeaxanthin (r = 0.59, P = 0.03), suggest a role of carotenoid status in MP deposition during the final stages of gestation (Henriksen et al., 2013).
Although most clinical studies including carotenoids have focused on the prevention of degeneration or oxidative damage, the influence of carotenoids on normal visual function is also of great interest. Reports from adult populations indicate that carotenoid supplementation may increase normal visual performance (Yao et al., 2013). These suggest a role for carotenoids in enhancing visual function and suggest a potential role for carotenoid supplementation and MP in normal visual development in infants (Hammond, 2008). The carotenoids involved in MP may be involved in foveal and visual development as in conditions associated with hypoplastic foveal development such as albinism which generally lack MP (Abadi and Cox, 1992).
The time-course of foveal development and lutein deposition may provide key insight into the role of carotenoids on normal visual development. Histological studies have shown that while the site of foveal pit development (caused by lateral displacement of the inner retinal layers) can be identified as early as 12 weeks gestation using morphologic and molecular cues, the pit itself does not form until 24-26 weeks gestation (Cornish et al., 2005; Dubis et al., 2012; Yuodelis and Hendrickson, 1986). During the final trimester, photoreceptors at the foveal center are immature compared to parafoveal photoreceptors. The RPE also continues to mature during this time by forming interdigitations with retinal outer segments (Hendrickson and Yuodelis, 1984; Yuodelis and Hendrickson, 1986). The foveal pit contour continues maturation and reaches maturity roughly at 18 months post-term (Dubis et al., 2012).
Lutein deposition appears to begin in the early stages of gestation and continues throughout. A study of donor eyes from preterm infants identified lutein and zeaxanthin by high performance liquid chromatography (HPLC) as early as 20 weeks gestation (Bone et al., 1988). This roughly corresponds to the time course of foveal pit maturation (Dubis et al., 2012). However, MP has not been detected using reflectometry until infants have reached term (Bernstein et al., 2013; Henriksen et al., 2013). Similar findings have been described in brain tissue with pre-term infants having significantly lower carotenoid levels when compared to term infants (Vishwanathan et al., 2014). These findings suggest that while carotenoid deposition and foveal development may start early in gestation, these processes both seem to be of greater significance during the final stages of gestation.
Maternal carotenoid status during the gestational period may impact infant macular development, and prenatal supplementation may play a role in maximizing visual development. As shown by us, maternal serum zeaxanthin levels correlate with infant MPOD in term infants shortly after birth (Henriksen et al., 2013). This suggests a key role for maternal nutrition and macular development in utero. Although one prenatal supplement on the US market contains lutein and zeaxanthin (Similac, Abbott Nutrition, Columbus, OH, USA), the majority of commercially available prenatal supplements do not have any added lutein or zeaxanthin. Interventional trials are needed to confirm the role for prenatal supplementation prior to widespread use or recommendation.
Similar to the prevention of macular degeneration, the role of carotenoids in the prevention of oxidative damage in retinopathy of prematurity (ROP) is also promising. Preliminary studies suggest a potential role for carotenoids in decreasing oxidative stress (Perrone et al., 2010). However, a small cohort study of supplementation with and without carotenoids did not yield definitive results (Manzoni et al., 2013). With suggestive evidence of potential decreases in oxidative stress thought to be a major factor in the pathophysiological mechanism of tissue damage in retinopathy of prematurity (Jewell et al., 2001), further studies are needed to identify the supplementation dosing, delivery, and timing regimens that may have the most impact on ROP. An examination of current literature would suggest that prenatal supplementation starting early in pregnancy would have some impact, due to presence of carotenoids in retinal tissue as early as 20 weeks gestation (Bone et al., 1988), but supplementation during later stages may have even greater clinical impact on the treatment or prevention of ROP (Bernstein et al., 2013; Henriksen et al., 2013; Henriksen and Chan, 2014).
4. Future Directions: Controversies And Frontiers In Ocular Carotenoid Science
Since the preliminary identification of the human MP as a xanthophyll carotenoid by Wald in the 1940s, there has been astounding progress in our knowledge of the roles of lutein, zeaxanthin, and meso-zeaxanthin in promoting ocular health. We now understand their core antioxidant chemistry, and we have identified key specific binding proteins and metabolic enzymes. AREDS2 has shown that lutein and zeaxanthin are appropriate substitutes for β-carotene in nutritional supplement formulations that we recommend to patients at significant risk for visual loss from AMD, and we should all be proud at the rapidity that these important public health recommendations have been adopted by eye care professionals and their patients. In the upcoming years and decades, a number of controversies and frontiers remain to be addressed.
From a basic science standpoint, we still have much to learn about carotenoid physiology and metabolism in humans. The molecular aspects of carotenoid antioxidant effects remain to be elucidated, especially when they are bound to proteins or embedded in lipid bilayers. There are many persistent gaps in our knowledge of carotenoid transport from the gut to the bloodstream and to the eventual target tissues in the eye. We still have a rather rudimentary understanding of enzymes that interact with carotenoids such as BCO1 and BCO2, and we know next to nothing about how meso-zeaxanthin is made from precursor carotenoids. These fundamental biochemical problems must be solved on a molecular level in order to better understand why individuals have widely varying peak levels of MP in the fovea and diverse responses to identical supplement interventions. These questions are particularly challenging to address because the macula lutea is primate-specific, which means that most typical small animal mammalian models such as mice and rats are of limited utility because they do not naturally accumulate carotenoids in their retinas, and vertebrates that do deliver carotenoids to the retina and RPE (e.g. birds, amphibians, reptiles, and fish) utilize markedly different biochemical strategies such as esterification to fatty acids in oil droplets. The recent development of BCO2 knockout “macular pigment mice” raises hope that improved mammalian animal models are at hand, but there will always be limitations of laboratory-engineered models because they never fully reproduce the human system.
The macula lutea contains lutein, zeaxanthin, and meso-zeaxanthin at a 1:1:1 ratio, yet the typical American consumes these carotenoids from the diet in a ratio of 5:1:0. AREDS2 teaches us that xanthophyll carotenoid supplementation has health benefits, but is the current 10 mg lutein and 2 mg zeaxanthin dose optimal? Would higher levels of supplementation and/or altered ratios of these three carotenoids increase MP more efficiently and promote better vision and lessen risk of AMD progression? Now that meso-zeaxanthin is readily available from commercial sources, is it beneficial to bypass the postulated ocular enzymatic metabolism of lutein to meso-zeaxanthin by supplying it pre-formed in a supplement? Are there any long-term consequences to consuming high concentrations of all three macular carotenoids, given that a typical diet contains circa 1.5 mg per day of lutein and zeaxanthin, and little or no meso-zeaxanthin? In light of our current knowledge that known binding proteins cannot discriminate between zeaxanthin and meso-zeaxanthin, should both be administered in equal amounts, or is one preferred over the other? All of these important questions demand high quality, sufficiently powered clinical trials; some are in progress, but unfortunately, definitive large-scale, randomized, controlled clinical trials on the order of AREDS2 are unlikely to be conducted in the foreseeable future. However, it is our view that, in the absence of large-scale, clinical trials, we must view and assess the totality of the scientific data and weight of evidence as currently available, identifying and acknowledging such evidence from respected sources and scientific institutions, to find more suitable alternatives to randomized clinical trials to make important public health recommendations in a timely manner.
There is ongoing debate as to which methods of non-invasive MP measurement are “best” or the “gold standard”. Clearly, this depends on the goals of the researcher or clinician and the characteristics of the subjects, and each method has its particular strengths and weaknesses. Psychophysical methods are the least expensive to implement and are well suited to large-scale studies because the equipment can be portable and does not need pupil dilation, but it requires rigorous subject training and attention for optimal results, and it provides measurements at a limited number of eccentricities. Autofluorescence imaging is currently much more expensive to implement and can prove challenging in the face of anterior and posterior segment pathology, but it does provide rapid, detailed, and reproducible spatial profiles. Resonance Raman spectroscopy and imaging are the most chemically specific methods to quantify and image MP, but laser light levels are high, and expensive research-grade instruments are the only current option. Reflectometry is particularly well suited for infants and children because their media are clear and because the other methods cannot be used due to lack of cooperation, absence of significant lipofuscin, and concerns about laser safety in the infant eye; however, this technique is much more challenging to implement quantitatively and reproducibly in the adult eye. We are indeed fortunate to have an abundance of MP measurement options, most of which correlate reasonably well. It will be interesting to see if and when these techniques can be successfully incorporated into busy optometric and ophthalmological practices to provide authoritative guidance and feedback to patients and clinicians who want to promote optimum ocular health and function.
While AREDS2 has firmly established a clinical benefit for supplements such as lutein and zeaxanthin in AMD patients, other ocular diseases and conditions are truly the next frontier. Disorders such as MacTel feature prominent MP abnormalities early in the course of the disease, so it is likely that further knowledge how and why this happens will certainly provide insights into potential physiological and therapeutic roles in many eye conditions beyond just MacTel. There is growing appreciation that the MP is important much earlier in life as evidenced by its presence at birth and by intriguing new studies that indicate that the macular carotenoids can promote enhanced ocular and cognitive function in normal individuals. Further studies in these fields of inquiry should provide evidence-based guidance on nutritional supplementation with lutein, zeaxanthin, and meso-zeaxanthin throughout a person's lifetime.
Supplementary Material
Acknowledgments
Conflicts of Interest: This work was supported by National Eye Institute grants EY-11600 and EY-14800 and by an unrestricted departmental grant to the Moran Eye Center from Research to Prevent Blindness. The authors gratefully acknowledge the expert critical reading and editorial assistance of Kelly Nelson.
Dr. Bernstein is a consultant for Kemin Health, Kalsec, DSM, and Science Based Health, and he is a co-inventor of methods to measure carotenoids in ocular and other tissues using resonance Raman spectroscopy.
Dr. Nolan is currently funded by the European Research Council (ERC) under the CREST project (code: 281096) and also holds a Howard Chair at Waterford Institute of Technology in Human Nutrition Research. Within his capacity as a director of Nutrasight Consultancy Ltd., Dr Nolan carries out consultancy work for nutraceutical companies, including Bausch + Lomb, Heidelberg Engineering, Alliance Pharma PLC, and MacuHealth.
Abbreviations
- ABCA1
ATP-binding cassette sub-family A member 1
- ABCA4
ATP-binding cassette sub-family A member 4
- A2E
N-retinyl- N-retinylidene ethanolamine
- AFI
Autofluorescence imaging
- AMD
Age-related macular degeneration
- AREDS
Age-Related Eye Disease Study
- AREDS2
Age-Related Eye Disease Study 2
- BCO1
β-carotene oxygenase 1 (also known as β-carotene-15′,15′-monoxygenase)
- BCO2
β-carotene oxygenase 2 (also known as β,β-carotene-9′,10′-dioxygenase)
- cHFP
customized heterochromatic flicker photometry
- cSLO
confocal scanning laser ophthalmoscope
- DHA
Docosahexaenoic acid
- DMAPP
Dimethylallyl pyrophosphate
- EDCC
Eye Disease Case-Control
- EPA
Eicosapentaenoic acid
- EFSA
European Food Safety Authority
- GGPP
Geranylgeranyl pyrophosphate
- GRAS
generally recognized as safe
- GSH
Glutathione
- GSTs
Glutathione-S-transferases
- GSTP1
Glutathione S-transferase P1
- GWAS
Genome-wide association studies
- HDL
High-density lipoproteins
- HFP
Heterochromatic flicker photometry
- HPLC
High pressure/performance liquid chromatography
- I/R
Ischemia/reperfusion
- IRBP
Interphotoreceptor retinoid-binding protein
- ISX
Intestine-specific homeobox
- IPP
Isopentenyl pyrophosphate
- LC-MS
Liquid chromatography- mass spectrometry
- LC–MS/MS
Liquid chromatography–tandem mass spectrometry
- LDL
Low-density lipoprotein
- MacTel
Macular telangiectasia type II
- MP
Macular pigment
- MPOD
Macular pigment optical density
- NOAEL
No observed-adverse-effect-level
- ROS
Reactive oxygen species
- ROP
Retinopathy of prematurity
- RP
Retinitis pigmentosa
- RPE
Retinal pigment epithelium
- SR-BI
Scavenger receptor class B member 1
- StARD3
Steroidogenic acute regulatory domain protein 3
- VEGF
Vascular endothelial growth factor
- WHAM
Wisconsin hypoalpha mutant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Binxing Li, Email: binxing.li@hsc.utah.edu.
Preejith P. Vachali, Email: preejith.vachali@hsc.utah.edu.
Aruna Gorusupudi, Email: aruna.gorusupudi@utah.edu.
Rajalekshmy Shyam, Email: r.shyam@utah.edu.
Bradley S. Henriksen, Email: brad.henriksen@hsc.utah.edu.
John M. Nolan, Email: jmnolan@wit.ie.
References
- Abadi RV, Cox MJ. The distribution of macular pigment in human albinos. Investigative ophthalmology & visual science. 1992;33:494–497. [PubMed] [Google Scholar]
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study 2 Research, G. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL, 3rd, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agron E, Toth CA, Bernstein PS, Sperduto RD Age-Related Eye Disease Study 2 Research, G. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA ophthalmology. 2014;132:142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, SanGiovanni JP, Ferris FL, Wong WT, Agron E, Clemons TE, Sperduto R, Danis R, Chandra SR, Blodi BA, Domalpally A, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Friberg TR, Rosenfeld PJ, Toth CA, Bernstein P Age-Related Eye Disease Study 2 Research, G. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA ophthalmology. 2013;131:843–850. doi: 10.1001/jamaophthalmol.2013.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research, G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of ophthalmology. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD Age-Related Eye Disease Study Research, G. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Archives of ophthalmology. 2007;125:1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- Agostoni C, Bresson J, Fairweather-Tait S, Flynn A, Golly I, Korhonen H, Lagiou P, Løvik M, Marchelli R, Martin A. Scientific Opinion on the substantiation of health claims related to lutein and protection of DNA, proteins and lipids from oxidative damage (ID 3427), protection of the skin from UV-induced (including photo-oxidative) damage (ID 1605, 1779) and maintenance of normal vision (ID 1779, 2080) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA journal. 2011;9:2030–2030. [Google Scholar]
- Agostoni C, Bresson J, Fairweather-Tait S, Flynn A, Golly I, Korhonen H, Lagiou P, Løvik M, Marchelli R, Martin A, Moseley B, Neuhäuser-Berthold M, Przyrembel H, Salminen S, Sanz Y, Strain S, Strobel S, Tetens I, Tome D, van Loveren H, Verhagen H. Scientific Opinion on the substantiation of health claims related to lutein and maintenance of normal vision (ID 1603, 1604, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2012;10 [Google Scholar]
- Akuffo KO, Beatty S, Stack J, Dennison J, O'Regan S, Meagher KA, Peto T, Nolan J. Central Retinal Enrichment Supplementation Trials (CREST): design and methodology of the CREST randomized controlled trials. Ophthalmic epidemiology. 2014;21:111–123. doi: 10.3109/09286586.2014.888085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. Journal of the National Cancer Institute. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Sanchez S. Influence of carbon and nitrogen sources on Flavobacteriumgrowth and zeaxanthin biosynthesis. Journal of Industrial Microbiology and Biotechnology. 1999;23:697–700. doi: 10.1038/sj.jim.2900688. [DOI] [PubMed] [Google Scholar]
- Aleman TS, Duncan JL, Bieber ML, de Castro E, Marks DA, Gardner LM, Steinberg JD, Cideciyan AV, Maguire MG, Jacobson SG. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Investigative ophthalmology & visual science. 2001;42:1873–1881. [PubMed] [Google Scholar]
- Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. The Journal of biological chemistry. 1997;272:10004–10012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. Journal of cell science. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:948–959. doi: 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amengual J, Widjaja-Adhi MA, Rodriguez-Santiago S, Hessel S, Golczak M, Palczewski K, von Lintig J. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. The Journal of biological chemistry. 2013;288:34081–34096. doi: 10.1074/jbc.M113.501049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK. The relationship of primate foveal cones to the pigment epithelium. Journal of Ultrastructure Research. 1979;67:23–32. doi: 10.1016/s0022-5320(79)80014-3. [DOI] [PubMed] [Google Scholar]
- Arend O, Weiter JJ, Goger DG, Delori FC. In vivo fundus fluorescence measurements in patients with age related macular degeneration. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 1995;92:647–653. [PubMed] [Google Scholar]
- Attie AD, Hamon Y, Brooks-Wilson AR, Gray-Keller MP, MacDonald MLE, Rigot V, Tebon A, Zhang LH, Mulligan JD, Singaraja RR, Bitgood JJ, Cook ME, Kastelein JJP, Chimini G, Hayden MR. Identification and functional analysis of a naturally occurring E89K mutation in the ABCA1 gene of the WHAM chicken. Journal of lipid research. 2002;43:1610–1617. doi: 10.1194/jlr.m200223-jlr200. [DOI] [PubMed] [Google Scholar]
- Augood C, Chakravarthy U, Young I, Vioque J, de Jong PT, Bentham G, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling JR, Fletcher AE. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. The American journal of clinical nutrition. 2008;88:398–406. doi: 10.1093/ajcn/88.2.398. [DOI] [PubMed] [Google Scholar]
- Azevedo-Meleiro CH, Rodriguez-Amaya DB. Qualitative and quantitative differences in carotenoid composition among Cucurbita moschata, Cucurbita maxima, and Cucurbita pepo. Journal of agricultural and food chemistry. 2007;55:4027–4033. doi: 10.1021/jf063413d. [DOI] [PubMed] [Google Scholar]
- Babino D, Palczewski G, Widjaja-Adhi MA, Kiser PD, Golczak M, von Lintig J. Characterization of the role of beta;-carotene-9,10-dioxygenase in macular pigment metabolism. J Biol Chem. 2015 doi: 10.1074/jbc.M115.668822. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett HE, Eperjesi F. A randomised controlled trial investigating the effect of lutein and antioxidant dietary supplementation on visual function in healthy eyes. Clinical nutrition (Edinburgh, Scotland) 2008;27:218–227. doi: 10.1016/j.clnu.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Barua AB, Furr HC. Extraction and analysis by high-performance liquid chromatography of carotenoids in human serum. Methods in enzymology. 1992;213:273–281. doi: 10.1016/0076-6879(92)13128-k. [DOI] [PubMed] [Google Scholar]
- Barua AB, Olson JA. Xanthophyll epoxides, unlike beta-carotene monoepoxides, are not detectibly absorbed by humans. The Journal of nutrition. 2001;131:3212–3215. doi: 10.1093/jn/131.12.3212. [DOI] [PubMed] [Google Scholar]
- Beatty S, Boulton M, Henson D, Koh HH, Murray IJ. Macular pigment and age related macular degeneration. The British journal of ophthalmology. 1999;83:867–877. doi: 10.1136/bjo.83.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty S, Chakravarthy U, Nolan JM, Muldrew KA, Woodside JV, Denny F, Stevenson MR. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology. 2013;120:600–606. doi: 10.1016/j.ophtha.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Beatty S, Koh HH, Carden D, Murray IJ. Macular pigment optical density measurement: a novel compact instrument. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians. 2000;20:105–111. [PubMed] [Google Scholar]
- Berendschot TT, DeLint PJ, van Norren D. Fundus reflectance--historical and present ideas. Progress in retinal and eye research. 2003;22:171–200. doi: 10.1016/s1350-9462(02)00060-5. [DOI] [PubMed] [Google Scholar]
- Berendschot TT, Goldbohm RA, Klopping WA, van de Kraats J, van Norel J, van Norren D. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Investigative ophthalmology & visual science. 2000;41:3322–3326. [PubMed] [Google Scholar]
- Berendschot TT, van Norren D. Objective determination of the macular pigment optical density using fundus reflectance spectroscopy. Archives of biochemistry and biophysics. 2004;430:149–155. doi: 10.1016/j.abb.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Berendschot TT, van Norren D. On the age dependency of the macular pigment optical density. Experimental eye research. 2005;81:602–609. doi: 10.1016/j.exer.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Berendschot TT, Willemse-Assink JJ, Bastiaanse M, de Jong PT, van Norren D. Macular pigment and melanin in age-related maculopathy in a general population. Investigative ophthalmology & visual science. 2002;43:1928–1932. [PubMed] [Google Scholar]
- Bernhard K, Giger A. Process for the manufacturing of zeaxanthin from lutein. Google Patents 1998 [Google Scholar]
- Bernstein PS. New insights into the role of the macular carotenoids in age-related macular degeneration. Resonance Raman studies. Pure Appl Chem. 2002;74:1419–1425. [Google Scholar]
- Bernstein PS, Ahmed F, Liu A, Allman S, Sheng X, Sharifzadeh M, Ermakov I, Gellermann W. Macular pigment imaging in AREDS2 participants: an ancillary study of AREDS2 subjects enrolled at the Moran Eye Center. Investigative ophthalmology & visual science. 2012;53:6178–6186. doi: 10.1167/iovs.12-10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein PS, Balashov NA, Tsong ED, Rando RR. Retinal tubulin binds macular carotenoids. Investigative ophthalmology & visual science. 1997;38:167–175. [PubMed] [Google Scholar]
- Bernstein PS, Delori FC, Richer S, van Kuijk FJ, Wenzel AJ. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision research. 2010;50:716–728. doi: 10.1016/j.visres.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Experimental eye research. 2001;72:215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- Bernstein PS, Sharifzadeh M, Liu A, Ermakov I, Nelson K, Sheng X, Panish C, Carlstrom B, Hoffman RO, Gellermann W. Blue-light reflectance imaging of macular pigment in infants and children. Investigative ophthalmology & visual science. 2013;54:4034–4040. doi: 10.1167/iovs.13-11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein PS, Yoshida MD, Katz NB, McClane RW, Gellermann W. Raman detection of macular carotenoid pigments in intact human retina. Investigative ophthalmology & visual science. 1998;39:2003–2011. [PubMed] [Google Scholar]
- Bernstein PS, Zhao DY, Wintch SW, Ermakov IV, McClane RW, Gellermann W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology. 2002;109:1780–1787. doi: 10.1016/s0161-6420(02)01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, Weigel-DiFrano C, Willett W. Vitamin A supplementation for retinitis pigmentosa. Archives of ophthalmology. 1993;111:1456–1459. doi: 10.1001/archopht.1993.01090110014001. [DOI] [PubMed] [Google Scholar]
- Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Brockhurst RJ, Hayes KC, Johnson EJ, Anderson EJ, Johnson CA, Gaudio AR, Willett WC, Schaefer EJ. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Archives of ophthalmology. 2010;128:403–411. doi: 10.1001/archophthalmol.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti RA, Yu S, Boulanger A, Fariss RN, Guo Y, Bernstein SL, Gentleman S, Redmond TM. Expression of beta-carotene 15,15′ monooxygenase in retina and RPE-choroid. Investigative ophthalmology & visual science. 2003;44:44–49. doi: 10.1167/iovs.02-0167. [DOI] [PubMed] [Google Scholar]
- Bhosale P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Applied Microbiology and Biotechnology. 2004;63:351–361. doi: 10.1007/s00253-003-1441-1. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Bernstein PS. Quantitative measurement of 3′-oxolutein from human retina by normal-phase high-performance liquid chromatography coupled to atmospheric pressure chemical ionization mass spectrometry. Analytical biochemistry. 2005a;345:296–301. doi: 10.1016/j.ab.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Bernstein PS. Synergistic effects of zeaxanthin and its binding protein in the prevention of lipid membrane oxidation. Biochimica et biophysica acta. 2005b;1740:116–121. doi: 10.1016/j.bbadis.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. The Journal of biological chemistry. 2004;279:49447–49454. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Serban B, Bernstein PS. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Archives of biochemistry and biophysics. 2009;483:175–181. doi: 10.1016/j.abb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale P, Serban B, Zhao da Y, Bernstein PS. Identification and metabolic transformations of carotenoids in ocular tissues of the Japanese quail Coturnix japonica. Biochemistry. 2007a;46:9050–9057. doi: 10.1021/bi700558f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale P, Zhao da Y, Bernstein PS. HPLC measurement of ocular carotenoid levels in human donor eyes in the lutein supplementation era. Investigative ophthalmology & visual science. 2007b;48:543–549. doi: 10.1167/iovs.06-0558. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Zhao da Y, Serban B, Bernstein PS. Identification of 3-methoxyzeaxanthin as a novel age-related carotenoid metabolite in the human macula. Investigative ophthalmology & visual science. 2007c;48:1435–1440. doi: 10.1167/iovs.06-1046. [DOI] [PubMed] [Google Scholar]
- Bian Q, Gao S, Zhou J, Qin J, Taylor A, Johnson EJ, Tang G, Sparrow JR, Gierhart D, Shang F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free radical biology & medicine. 2012;53:1298–1307. doi: 10.1016/j.freeradbiomed.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RA, Landrum JT. Macular pigment in Henle fiber membranes: a model for Haidinger's brushes. Vision research. 1984;24:103–108. doi: 10.1016/0042-6989(84)90094-4. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vision research. 1992;32:105–110. doi: 10.1016/0042-6989(92)90118-3. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Investigative ophthalmology & visual science. 1988;29:843–849. [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Experimental eye research. 1997;64:211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. The Journal of nutrition. 2003;133:992–998. doi: 10.1093/jn/133.4.992. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Investigative ophthalmology & visual science. 1993;34:2033–2040. [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Investigative ophthalmology & visual science. 2001;42:235–240. [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision research. 1985;25:1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- Bone RA, Sparrock JM. Comparison of macular pigment densities in human eyes. Vision research. 1971;11:1057–1064. doi: 10.1016/0042-6989(71)90112-x. [DOI] [PubMed] [Google Scholar]
- Borel P, de Edelenyi FS, Vincent-Baudry S, Malezet-Desmoulin C, Margotat A, Lyan B, Gorrand JM, Meunier N, Drouault-Holowacz S, Bieuvelet S. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann Med. 2011;43:47–59. doi: 10.3109/07853890.2010.531757. [DOI] [PubMed] [Google Scholar]
- Boulton M, Docchio F, Dayhaw-Barker P, Ramponi R, Cubeddu R. Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vision research. 1990;30:1291–1303. doi: 10.1016/0042-6989(90)90003-4. [DOI] [PubMed] [Google Scholar]
- Bowne SJ, Humphries MM, Sullivan LS, Kenna PF, Tam LC, Kiang AS, Campbell M, Weinstock GM, Koboldt DC, Ding L, Fulton RS, Sodergren EJ, Allman D, Millington-Ward S, Palfi A, McKee A, Blanton SH, Slifer S, Konidari I, Farrar GJ, Daiger SP, Humphries P. A dominant mutation in RPE65 identified by whole-exome sequencing causes retinitis pigmentosa with choroidal involvement. European journal of human genetics : EJHG. 2011;19:1074–1081. doi: 10.1038/ejhg.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G. Structure and properties of carotenoids in relation to function. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1995a;9:1551–1558. [PubMed] [Google Scholar]
- Britton G. UV/visible Spectroscopy. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids Vol 1B: Spectroscopy. Birkhauser Verlag; Basel, Switzerland: 1995b. pp. 13–62. [Google Scholar]
- Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. The American journal of clinical nutrition. 2004;80:396–403. doi: 10.1093/ajcn/80.2.396. [DOI] [PubMed] [Google Scholar]
- Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Progress in retinal and eye research. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Calvo MM. Lutein: a valuable ingredient of fruit and vegetables. Crit Rev Food Sci Nutr. 2005;45:671–696. doi: 10.1080/10408690590957034. [DOI] [PubMed] [Google Scholar]
- Casaroli-Marano RP, Sousa-Martins D, Martinez-Conesa EM, Badaro E, Nunes RP, Lima-Filho AA, Rodrigues EB, Belfort R, Jr, Maia M. Dye solutions based on lutein and zeaxanthin: in vitro and in vivo analysis of ocular toxicity profiles. Current eye research. 2015;40:707–718. doi: 10.3109/02713683.2014.952831. [DOI] [PubMed] [Google Scholar]
- Cerezo J, Zuniga J, Bastida A, Requena A, Ceron-Carrasco JP. Conformational changes of beta-carotene and zeaxanthin immersed in a model membrane through atomistic molecular dynamics simulations. Physical chemistry chemical physics : PCCP. 2013;15:6527–6538. doi: 10.1039/c3cp43947j. [DOI] [PubMed] [Google Scholar]
- Charbel Issa P, Barnard AR, Singh MS, Carter E, Jiang Z, Radu RA, Schraermeyer U, MacLaren RE. Fundus autofluorescence in the Abca4(-/-) mouse model of Stargardt disease--correlation with accumulation of A2E, retinal function, and histology. Investigative ophthalmology & visual science. 2013a;54:5602–5612. doi: 10.1167/iovs.13-11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbel Issa P, Gillies MC, Chew EY, Bird AC, Heeren TF, Peto T, Holz FG, Scholl HP. Macular telangiectasia type 2. Progress in retinal and eye research. 2013b;34:49–77. doi: 10.1016/j.preteyeres.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Juchau MR. Glutathione S-transferases act as isomerases in isomerization of 13-cis-retinoic acid to all-trans-retinoic acid in vitro. The Biochemical journal. 1997;327(Pt 3):721–726. doi: 10.1042/bj3270721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AC, Pang CP, Leung AT, Chua JK, Fan DS, Lam DS. The association between cigarette smoking and ocular diseases. Hong Kong medical journal = Xianggang yi xue za zhi / Hong Kong Academy of Medicine. 2000;6:195–202. [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Agron E, Launer LJ, Grodstein F, Bernstein PS Age-Related Eye Disease Study 2 Research, G. Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function: The AREDS2 Randomized Clinical Trial. Jama. 2015;314:791–801. doi: 10.1001/jama.2015.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. The Journal of nutrition. 2004;134:1887–1893. doi: 10.1093/jn/134.8.1887. [DOI] [PubMed] [Google Scholar]
- Ciccone MM, Cortese F, Gesualdo M, Carbonara S, Zito A, Ricci G, De Pascalis F, Scicchitano P, Riccioni G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators of inflammation. 2013;2013:782137. doi: 10.1155/2013/782137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PF, Schalch W, Truscott TG. The singlet oxygen and carotenoid interaction. Journal of Photochemistry and Photobiology B: Biology. 1991;11:41–47. doi: 10.1016/1011-1344(91)80266-k. [DOI] [PubMed] [Google Scholar]
- Connolly EE, Beatty S, Loughman J, Howard AN, Louw MS, Nolan JM. Supplementation with all three macular carotenoids: response, stability, and safety. Investigative ophthalmology & visual science. 2011;52:9207–9217. doi: 10.1167/iovs.11-8025. [DOI] [PubMed] [Google Scholar]
- Connolly EE, Beatty S, Thurnham DI, Loughman J, Howard AN, Stack J, Nolan JM. Augmentation of macular pigment following supplementation with all three macular carotenoids: an exploratory study. Current eye research. 2010;35:335–351. doi: 10.3109/02713680903521951. [DOI] [PubMed] [Google Scholar]
- Connor WE, Duell PB, Kean R, Wang Y. The prime role of HDL to transport lutein into the retina: evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Investigative ophthalmology & visual science. 2007;48:4226–4231. doi: 10.1167/iovs.06-1275. [DOI] [PubMed] [Google Scholar]
- Cornish EE, Madigan MC, Natoli R, Hales A, Hendrickson AE, Provis JM. Gradients of cone differentiation and FGF expression during development of the foveal depression in macaque retina. Visual neuroscience. 2005;22:447–459. doi: 10.1017/S0952523805224069. [DOI] [PubMed] [Google Scholar]
- Crabtree DV, Adler AJ, Snodderly DM. Vitamin E, retinyl palmitate, and protein in rhesus monkey retina and retinal pigment epithelium-choroid. Investigative ophthalmology & visual science. 1996;37:47–60. [PubMed] [Google Scholar]
- Crabtree DV, Ojima I, Geng X, Adler AJ. Tubulins in the primate retina: evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site. Bioorganic & medicinal chemistry. 2001;9:1967–1976. doi: 10.1016/s0968-0896(01)00103-1. [DOI] [PubMed] [Google Scholar]
- Dall'Osto L, Lico C, Alric J, Giuliano G, Havaux M, Bassi R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006;6:32. doi: 10.1186/1471-2229-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NP, Morland AB. Color matching in diabetes: optical density of the crystalline lens and macular pigments. Investigative ophthalmology & visual science. 2002;43:281–289. [PubMed] [Google Scholar]
- de Rosso VV, Mercadante AZ. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. Journal of agricultural and food chemistry. 2007;55:5062–5072. doi: 10.1021/jf0705421. [DOI] [PubMed] [Google Scholar]
- Del Campo JA, Rodrıguez H, Moreno J, Vargas MÁ, Rivas Jn, Guerrero MG. Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. Journal of biotechnology. 2001;85:289–295. doi: 10.1016/s0168-1656(00)00380-1. [DOI] [PubMed] [Google Scholar]
- dela Sena C, Narayanasamy S, Riedl KM, Curley RW, Jr, Schwartz SJ, Harrison EH. Substrate specificity of purified recombinant human beta-carotene 15,15′-oxygenase (BCO1) The Journal of biological chemistry. 2013;288:37094–37103. doi: 10.1074/jbc.M113.507160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli J, Molnar P, Osz E, Toth G, Zsila F. Epimerisation of lutein to 3′-epilutein in processed foods. Bioorg Med Chem Lett. 2004;14:925–928. doi: 10.1016/j.bmcl.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Delori FC. Autofluorescence method to measure macular pigment optical densities fluorometry and autofluorescence imaging. Archives of biochemistry and biophysics. 2004;430:156–162. doi: 10.1016/j.abb.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Delori FC, Goger DG, Hammond BR, Snodderly DM, Burns SA. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. Journal of the Optical Society of America A, Optics, image science, and vision. 2001;18:1212–1230. doi: 10.1364/josaa.18.001212. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, McGee TL, Ziviello C, Banfi S, Dryja TP, Gonzalez-Fernandez F, Ghosh D, Berson EL. A homozygous missense mutation in the IRBP gene (RBP3) associated with autosomal recessive retinitis pigmentosa. Investigative ophthalmology & visual science. 2009;50:1864–1872. doi: 10.1167/iovs.08-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison JL, Stack J, Beatty S, Nolan JM. Concordance of macular pigment measurements obtained using customized heterochromatic flicker photometry, dual-wavelength autofluorescence, and single-wavelength reflectance. Experimental eye research. 2013;116:190–198. doi: 10.1016/j.exer.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Progress in retinal and eye research. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey CK, Granata L, Nichols CR, Cheng KM, Craft NE. Dietary modulation of lens zeaxanthin in quail. Experimental eye research. 2005;81:464–477. doi: 10.1016/j.exer.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Dubis AM, Costakos DM, Subramaniam CD, Godara P, Wirostko WJ, Carroll J, Provis JM. Evaluation of normal human foveal development using optical coherence tomography and histologic examination. Archives of ophthalmology. 2012;130:1291–1300. doi: 10.1001/archophthalmol.2012.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. The Journal of nutrition. 2005;135:2305–2312. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. Journal of lipid research. 2008;49:1715–1724. doi: 10.1194/jlr.M700580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific Opinion on the re-evaluation of lutein preparations other than lutein with high concentrations of total saponified carotenoids at levels of at least 80% EFSA Journal. 2011;9:2144. [Google Scholar]
- Englert G, Noack K, Broger EA, Glinz E, Vecchi M, Zell R. Synthesis, Isolation, and Full Spectroscopic Characterization of Eleven (Z)-Isomers of (3R,3′R)-Zeaxanthin. Helvetica Chimica Acta. 1991;74:969–982. [Google Scholar]
- Engles M, Wooten B, Hammond B. Macular pigment: a test of the acuity hypothesis. Investigative ophthalmology & visual science. 2007;48:2922–2931. doi: 10.1167/iovs.06-0883. [DOI] [PubMed] [Google Scholar]
- Erdman JW, Jr, Bierer TL, Gugger ET. Absorption and transport of carotenoids. Annals of the New York Academy of Sciences. 1993;691:76–85. doi: 10.1111/j.1749-6632.1993.tb26159.x. [DOI] [PubMed] [Google Scholar]
- Ermakov IV, Sharifzadeh M, Ermakova M, Gellermann W. Resonance Raman detection of carotenoid antioxidants in living human tissue. Journal of biomedical optics. 2005;10:064028. doi: 10.1117/1.2139974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst H. Recent advances in industrial carotenoid synthesis. Pure and applied chemistry. 2002;74:1369–1382. [Google Scholar]
- Ernst H, Henrich K, Keller A. Method for producing carotenoids. Google Patents 2005 [Google Scholar]
- Eugster CH, Montoya-Olvera R, Torres-Quiroga JO. Process for obtaining 3′-epilutein. Google Patents 2002 [Google Scholar]
- European FoodSafetyAuthority. Technical specifications on the harmonised monitoring and reporting of antimicrobial resistance in methicillin-resistant Staphylococcus aureus in food-producing animals and food. EFSA Journal. 2012;10:2897. [Google Scholar]
- Evans JR. Risk factors for age-related macular degeneration. Progress in retinal and eye research. 2001;20:227–253. doi: 10.1016/s1350-9462(00)00023-9. [DOI] [PubMed] [Google Scholar]
- Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. The Cochrane database of systematic reviews. 2012;6:Cd000253. doi: 10.1002/14651858.CD000253.pub3. [DOI] [PubMed] [Google Scholar]
- Eye Disease Case Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Archives of ophthalmology. 1993;111:104–109. doi: 10.1001/archopht.1993.01090010108035. [DOI] [PubMed] [Google Scholar]
- Fang L, Pajkovic N, Wang Y, Gu C, van Breemen RB. Quantitative analysis of lycopene isomers in human plasma using high-performance liquid chromatography-tandem mass spectrometry. Analytical chemistry. 2003;75:812–817. doi: 10.1021/ac026118a. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. The Journal of clinical investigation. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Robredo P, Sadaba LM, Salinas-Alaman A, Recalde S, Rodriguez JA, Garcia-Layana A. Effect of lutein and antioxidant supplementation on VEGF expression, MMP-2 activity, and ultrastructural alterations in apolipoprotein E-deficient mouse. Oxidative medicine and cellular longevity. 2013;2013:213505. doi: 10.1155/2013/213505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote CS, Chang YC, Denny RW. Chemistry of singlet oxygen. X. Carotenoid quenching parallels biological protection. Journal of the American Chemical Society. 1970;92:5216–5218. doi: 10.1021/ja00720a036. [DOI] [PubMed] [Google Scholar]
- Francis GW, Strand LP, Lien T, Knutsen G. Variations in the carotenoid content of Chlamydomonas reinhardii throughout the cell cycle. Archives of microbiology. 1975;104:249–254. doi: 10.1007/BF00447333. [DOI] [PubMed] [Google Scholar]
- Fresnedo O, Serra JL. Effect of nitrogen starvation on the biochemistry of Phormidium laminosum (cyanophyceae) Journal of phycology. 1992;28:786–793. [Google Scholar]
- Gabrielska J, Gruszecki WI. Zeaxanthin (dihydroxy-beta-carotene) but not beta-carotene rigidifies lipid membranes: a 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochimica et biophysica acta. 1996;1285:167–174. doi: 10.1016/s0005-2736(96)00152-6. [DOI] [PubMed] [Google Scholar]
- Gale CR, Hall NF, Phillips DI, Martyn CN. Plasma antioxidant vitamins and carotenoids and age-related cataract. Ophthalmology. 2001;108:1992–1998. doi: 10.1016/s0161-6420(01)00833-8. [DOI] [PubMed] [Google Scholar]
- Goodrow EF, Wilson TA, Houde SC, Vishwanathan R, Scollin PA, Handelman G, Nicolosi RJ. Consumption of one egg per day increases serum lutein and zeaxanthin concentrations in older adults without altering serum lipid and lipoprotein cholesterol concentrations. The Journal of nutrition. 2006;136:2519–2524. doi: 10.1093/jn/136.10.2519. [DOI] [PubMed] [Google Scholar]
- Granado-Lorencio F, Herrero-Barbudo C, Olmedilla-Alonso B, Blanco-Navarro I, Perez-Sacristan B. Lutein bioavailability from lutein ester-fortified fermented milk: in vivo and in vitro study. The Journal of nutritional biochemistry. 2010;21:133–139. doi: 10.1016/j.jnutbio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Green DW, Aykent S, Gierse JK, Zupec ME. Substrate specificity of recombinant human renal renin: effect of histidine in the P2 subsite on pH dependence. Biochemistry. 1990;29:3126–3133. doi: 10.1021/bi00464a032. [DOI] [PubMed] [Google Scholar]
- Greenwalt DE, Lipsky RH, Ockenhouse CF, Ikeda H, Tandon NN, Jamieson GA. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992;80:1105–1115. [PubMed] [Google Scholar]
- Gruszecki WI, Strzalka K. Carotenoids as modulators of lipid membrane physical properties. Biochimica et biophysica acta. 2005;1740:108–115. doi: 10.1016/j.bbadis.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Fletcher LM, Roos F, Wittwer J, Schalch W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Investigative ophthalmology & visual science. 2014;55:8583–8589. doi: 10.1167/iovs.14-15573. [DOI] [PubMed] [Google Scholar]
- Hammond BR., Jr Possible role for dietary lutein and zeaxanthin in visual development. Nutr Rev. 2008;66:695–702. doi: 10.1111/j.1753-4887.2008.00121.x. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Jr, Wooten BR, Curran-Celentano J. Carotenoids in the retina and lens: possible acute and chronic effects on human visual performance. Archives of biochemistry and biophysics. 2001;385:41–46. doi: 10.1006/abbi.2000.2184. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Jr, Wooten BR, Engles M, Wong JC. The influence of filtering by the macular carotenoids on contrast sensitivity measured under simulated blue haze conditions. Vision research. 2012;63:58–62. doi: 10.1016/j.visres.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Handelman GJ, Snodderly DM, Adler AJ, Russett MD, Dratz EA. Measurement of carotenoids in human and monkey retinas. Methods in enzymology. 1992;213:220–230. doi: 10.1016/0076-6879(92)13123-f. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91:603–612. doi: 10.1016/s0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]
- Henriksen BS, Chan G, Hoffman RO, Sharifzadeh M, Ermakov IV, Gellermann W, Bernstein PS. Interrelationships between maternal carotenoid status and newborn infant macular pigment optical density and carotenoid status. Investigative ophthalmology & visual science. 2013;54:5568–5578. doi: 10.1167/iovs.13-12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen BS, Chan GM. Importance of carotenoids in optimizing eye and brain development. Journal of pediatric gastroenterology and nutrition. 2014;59:552. doi: 10.1097/MPG.0000000000000471. [DOI] [PubMed] [Google Scholar]
- High EG, Day HG. Effects of different amounts of lutein, squalene, phytol and related substances on the utilization of carotene and vitamin A for storage and growth in the rat. The Journal of nutrition. 1951;43:245–260. doi: 10.1093/jn/43.2.245. [DOI] [PubMed] [Google Scholar]
- Holden JM, Eldridge AL, Beecher G, Buzzard IM, Bhagwat S, Davis CS, Douglass LW, Gebhardt S, Haytowitz D, Schakel S. Carotenoid Content of U.S. Foods: An Update of the Database. Journal of Food Compositon and Analysis. 1999;12:169–196. [Google Scholar]
- Holz FG, Piguet B, Minassian DC, Bird AC, Weale RA. Decreasing stromal iris pigmentation as a risk factor for age-related macular degeneration. American journal of ophthalmology. 1994;117:19–23. doi: 10.1016/s0002-9394(14)73010-7. [DOI] [PubMed] [Google Scholar]
- Howells O, Eperjesi F, Bartlett H. Measuring macular pigment optical density in vivo: a review of techniques. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011;249:315–347. doi: 10.1007/s00417-010-1577-5. [DOI] [PubMed] [Google Scholar]
- Huang YM, Dou HL, Huang FF, Xu XR, Zou ZY, Lin XM. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. BioMed research international. 2015;2015:564738. doi: 10.1155/2015/564738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Archives of ophthalmology. 2000;118:351–358. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- Inbaraj BS, Chien JT, Chen BH. Improved high performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. Journal of chromatography A. 2006;1102:193–199. doi: 10.1016/j.chroma.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Ismail A, Cheah SF. Determination of Vitamin C, b-carotene and Riboflavin Contents in Five Green Vegetables Organically and Conventionally Grown. Malays J Nutr. 2003;9:31–39. [PubMed] [Google Scholar]
- Ito M, Yamano Y, Shibata Y. Synthesis and characterization of carotenoids by different methods. In: Lester P, editor. Methods in enzymology. Academic Press; 1992. pp. 13–22. [Google Scholar]
- Jacques PF, Chylack LT., Jr Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. The American journal of clinical nutrition. 1991;53:352S–355S. doi: 10.1093/ajcn/53.1.352S. [DOI] [PubMed] [Google Scholar]
- Jeon JY, Kwon JS, Kang ST, Kim BR, Jung Y, Han JG, Park JH, Hwang JK. Optimization of culture media for large-scale lutein production by heterotrophic Chlorella vulgaris. Biotechnology progress. 2014;30:736–743. doi: 10.1002/btpr.1889. [DOI] [PubMed] [Google Scholar]
- Jewell VC, Northrop-Clewes CA, Tubman R, Thurnham DI. Nutritional factors and visual function in premature infants. The Proceedings of the Nutrition Society. 2001;60:171–178. doi: 10.1079/pns200089. [DOI] [PubMed] [Google Scholar]
- Jin E, Feth B, Melis A. A mutant of the green alga Dunaliella salina constitutively accumulates zeaxanthin under all growth conditions. Biotechnology and bioengineering. 2003;81:115–124. doi: 10.1002/bit.10459. [DOI] [PubMed] [Google Scholar]
- Johnson EJ. A possible role for lutein and zeaxanthin in cognitive function in the elderly. The American journal of clinical nutrition. 2012;96:1161s–1165s. doi: 10.3945/ajcn.112.034611. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Hammond BR, Yeum KJ, Qin J, Wang XD, Castaneda C, Snodderly DM, Russell RM. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. The American journal of clinical nutrition. 2000;71:1555–1562. doi: 10.1093/ajcn/71.6.1555. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Maras JE, Rasmussen HM, Tucker KL. Intake of lutein and zeaxanthin differ with age, sex, and ethnicity. J Am Diet Assoc. 2010;110:1357–1362. doi: 10.1016/j.jada.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investigative ophthalmology & visual science. 2005;46:692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- Juronen E, Tasa G, Veromann S, Parts L, Tiidla A, Pulges R, Panov A, Soovere L, Koka K, Mikelsaar AV. Polymorphic glutathione S-transferases as genetic risk factors for senile cortical cataract in Estonians. Investigative ophthalmology & visual science. 2000;41:2262–2267. [PubMed] [Google Scholar]
- Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer P, Jucker E. Umwandlung von a-Carotin in /UCarotin und von Xanthophyll in Zeaxanthin. Helv. Chim. Acta. 1947;30:266–267. [PubMed] [Google Scholar]
- Kazato Y, Shibata N, Hanazono G, Suzuki W, Tanifuji M, Tsunoda K. Novel snapshot imaging of photoreceptor bleaching in macaque and human retinas. Japanese journal of ophthalmology. 2010;54:349–356. doi: 10.1007/s10384-010-0826-9. [DOI] [PubMed] [Google Scholar]
- Kelly ER, Plat J, Haenen GR, Kijlstra A, Berendschot TT. The effect of modified eggs and an egg-yolk based beverage on serum lutein and zeaxanthin concentrations and macular pigment optical density: results from a randomized trial. PloS one. 2014;9:e92659. doi: 10.1371/journal.pone.0092659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachik F. Process for extraction and purification of lutein, zeaxanthin and rare carotenoids from marigold flowers and plants. University of Maryland; College Park, MD: United States Patent 6262284. 2001 http://www.freepatentsonline.com/6262284.html.
- Khachik F. Partial synthesis of serum carotenoids and their metabolites. Acta Biochim Pol. 2012;59:75–78. [PubMed] [Google Scholar]
- Khachik F, Beecher GR, Smith JC., Jr Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J Cell Biochem Suppl. 1995;22:236–246. doi: 10.1002/jcb.240590830. [DOI] [PubMed] [Google Scholar]
- Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investigative ophthalmology & visual science. 1997a;38:1802–1811. [PubMed] [Google Scholar]
- Khachik F, Chang AN. Total synthesis of (3R,3′R,6′R)-lutein and its stereoisomers. J Org Chem. 2009;74:3875–3885. doi: 10.1021/jo900432r. [DOI] [PubMed] [Google Scholar]
- Khachik F, de Moura FF, Zhao DY, Aebischer CP, Bernstein PS. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Investigative ophthalmology & visual science. 2002;43:3383–3392. [PubMed] [Google Scholar]
- Khachik F, Englert G, Daitch CE, Beecher GR, Tonucci LH, Lusby WR. Isolation and structural elucidation of the geometrical isomers of lutein and zeaxanthin in extracts from human plasma. J Chromatogr. 1992;582:153–166. doi: 10.1016/0378-4347(92)80314-g. [DOI] [PubMed] [Google Scholar]
- Khachik F, London E, de Moura FF, Johnson M, Steidl S, Detolla L, Shipley S, Sanchez R, Chen XQ, Flaws J, Lutty G, McLeod S, Fowler B. Chronic ingestion of (3R,3′R,6′R)-lutein and (3R,3′R)-zeaxanthin in the female rhesus macaque. Investigative ophthalmology & visual science. 2006;47:5476–5486. doi: 10.1167/iovs.06-0194. [DOI] [PubMed] [Google Scholar]
- Khachik F, Spangler CJ, Smith JC, Jr, Canfield LM, Steck A, Pfander H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Analytical chemistry. 1997b;69:1873–1881. doi: 10.1021/ac961085i. [DOI] [PubMed] [Google Scholar]
- Kiefer C, Sumser E, Wernet MF, Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzle F. total synthesis of carotenoids. pure and applied chemistry. 1976;47:7. [Google Scholar]
- Kijlstra A, Tian Y, Kelly ER, Berendschot TT. Lutein: more than just a filter for blue light. Progress in retinal and eye research. 2012;31:303–315. doi: 10.1016/j.preteyeres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Kimura M, Rodriguez-Amaya DB. Sources of errors in the quantitative analysis of food carotenoids by HPLC. Archivos latinoamericanos de nutricion. 1999;49:58s–66s. [PubMed] [Google Scholar]
- Klaes H, Riegel H. Effect of adaptinol on dark adaptation of the human eye. Medizinische Monatsschrift. 1951;5:334–337. [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky NI. Antioxidant functions of carotenoids. Free radical biology & medicine. 1989;7:617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Molecular aspects of medicine. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annual review of nutrition. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- Krinsky NI, Mathews-Roth MM, Welankiwar S, Sehgal PK, Lausen NC, Russett M. The metabolism of [14C]beta-carotene and the presence of other carotenoids in rats and monkeys. The Journal of nutrition. 1990;120:81–87. doi: 10.1093/jn/120.1.81. [DOI] [PubMed] [Google Scholar]
- Krinsky NI, Wang XD, Tang G, Russell RM. Mechanism of carotenoid cleavage to retinoids. Annals of the New York Academy of Sciences. 1993;691:167–176. doi: 10.1111/j.1749-6632.1993.tb26168.x. [DOI] [PubMed] [Google Scholar]
- Kruger CL, Murphy M, DeFreitas Z, Pfannkuch F, Heimbach J. An innovative approach to the determination of safety for a dietary ingredient derived from a new source: case study using a crystalline lutein product. Food Chem Toxicol. 2002;40:1535–1549. doi: 10.1016/s0278-6915(02)00131-x. [DOI] [PubMed] [Google Scholar]
- Kvansakul J, Rodriguez-Carmona M, Edgar DF, Barker FM, Kopcke W, Schalch W, Barbur JL. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians. 2006;26:362–371. doi: 10.1111/j.1475-1313.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Archives of biochemistry and biophysics. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Experimental eye research. 1997;65:57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- LaRowe TL, Mares JA, Snodderly DM, Klein ML, Wooten BR, Chappell R, Group CMPS. Macular pigment density and age-related maculopathy in the Carotenoids in Age-Related Eye Disease Study. An ancillary study of the women's health initiative. Ophthalmology. 2008;115:876–883 e871. doi: 10.1016/j.ophtha.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Lee CM, Boileau AC, Boileau TW, Williams AW, Swanson KS, Heintz KA, Erdman JW., Jr Review of animal models in carotenoid research. The Journal of nutrition. 1999;129:2271–2277. doi: 10.1093/jn/129.12.2271. [DOI] [PubMed] [Google Scholar]
- Leuenberger HG, Boguth W, Widmer E, Zell R. Synthesis of optically active natural carotenoids and structurally related compounds. I. Synthesis of chiral key compound 4R,6R)-4-hydroxy-2,2,6-trimethylcyclohexanone. helv. Chim Acta. 1976;59:1832–1849. [Google Scholar]
- Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas, II: effects of age, n-3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Investigative ophthalmology & visual science. 2004;45:3244–3256. doi: 10.1167/iovs.02-1233. [DOI] [PubMed] [Google Scholar]
- Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Archives of biochemistry and biophysics. 2010;504:56–60. doi: 10.1016/j.abb.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry. 2011;50:2541–2549. doi: 10.1021/bi101906y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, Bernstein PS. Inactivity of human beta,beta-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10173–10178. doi: 10.1073/pnas.1402526111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Fung FK, Fu ZJ, Wong D, Chan HH, Lo AC. Anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: in vivo and in vitro studies. Investigative ophthalmology & visual science. 2012;53:5976–5984. doi: 10.1167/iovs.12-10007. [DOI] [PubMed] [Google Scholar]
- Lien EL, Hammond BR. Nutritional influences on visual development and function. Progress in retinal and eye research. 2011;30:188–203. doi: 10.1016/j.preteyeres.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15′-monooxygenase. The Journal of biological chemistry. 2002;277:23942–23948. doi: 10.1074/jbc.M202756200. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Andersson S. Cell type-specific expression of beta-carotene 15,15′-mono-oxygenase in human tissues. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2004;52:491–499. doi: 10.1177/002215540405200407. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9′,10′-monooxygenase in human tissues. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2005;53:1403–1412. doi: 10.1369/jhc.5A6705.2005. [DOI] [PubMed] [Google Scholar]
- Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo GP, Isken A, Hoff S, Babino D, von Lintig J. BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development. 2012;139:2966–2977. doi: 10.1242/dev.079632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood S, Tang P, Nadolski G, Jackson H, Fang Z, Du Y, Yang M, Geiss W, Williams R, Burdick D. Methods for the synthesis of lutein. Google Patents 2006 [Google Scholar]
- Loughman J, Akkali MC, Beatty S, Scanlon G, Davison PA, O'Dwyer V, Cantwell T, Major P, Stack J, Nolan JM. The relationship between macular pigment and visual performance. Vision research. 2010;50:1249–1256. doi: 10.1016/j.visres.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Loughman J, Nolan JM, Howard AN, Connolly E, Meagher K, Beatty S. The impact of macular pigment augmentation on visual performance using different carotenoid formulations. Investigative ophthalmology & visual science. 2012;53:7871–7880. doi: 10.1167/iovs.12-10690. [DOI] [PubMed] [Google Scholar]
- Ma L, Lin XM. Effects of lutein and zeaxanthin on aspects of eye health. J Sci Food Agric. 2010;90:2–12. doi: 10.1002/jsfa.3785. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Innerarity TL, Rall SC, Jr, Weisgraber KH. Plasma lipoproteins: apolipoprotein structure and function. Journal of lipid research. 1984;25:1277–1294. [PubMed] [Google Scholar]
- Manzoni P, Guardione R, Bonetti P, Priolo C, Maestri A, Mansoldo C, Mostert M, Anselmetti G, Sardei D, Bellettato M, Biban P, Farina D. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: a multicenter randomized controlled trial. American journal of perinatology. 2013;30:25–32. doi: 10.1055/s-0032-1321494. [DOI] [PubMed] [Google Scholar]
- Maoka T. Carotenoids in marine animals. Mar Drugs. 2011;9:278–293. doi: 10.3390/md9020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoka T, Arai A, Shimizu M, Matsuno T. The first isolation of enantiomeric and meso-zeaxanthin in nature. Comp Biochem Physiol B. 1986;83:121–124. doi: 10.1016/0305-0491(86)90341-x. [DOI] [PubMed] [Google Scholar]
- Mares-Perlman JA, Brady WE, Klein BE, Klein R, Palta M, Bowen P, Stacewicz-Sapuntzakis M. Serum carotenoids and tocopherols and severity of nuclear and cortical opacities. Investigative ophthalmology & visual science. 1995;36:276–288. [PubMed] [Google Scholar]
- Masetto A, Flores-Cotera LB, Díaz C, Langley E, Sanchez S. Application of a complete factorial design for the production of zeaxanthin by Flavobacterium sp. Journal of bioscience and bioengineering. 2001;92:55–58. doi: 10.1263/jbb.92.55. [DOI] [PubMed] [Google Scholar]
- Mayer H, Rüttimann A. Synthesis of optically active natural carotenoids and structurally related compounds. IV. Synthesis of (3R, 3′R, 6′R)-lutein. Helvetica Chimica Acta. 1980;63:1451–1455. [Google Scholar]
- Mayne ST, Cartmel B, Scarmo S, Jahns L, Ermakov IV, Gellermann W. Resonance Raman spectroscopic evaluation of skin carotenoids as a biomarker of carotenoid status for human studies. Archives of biochemistry and biophysics. 2013;539:163–170. doi: 10.1016/j.abb.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty S, Taylor HR. Light and risk for age-related eye diseases. In: Taylor A, editor. Nutritional and Environmental Influences on the Eye. CRC Press; Boca Raton, FL: 1999. pp. 135–150. [Google Scholar]
- McDermott JC, Brown DJ, Britton G, Goodwin TW. Alternative pathways of zeaxanthin biosynthesis in a Flavobacterium species. Experiments with nicotine as inhibitor. Biochem J. 1974;144:231–243. doi: 10.1042/bj1440231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher KA, Thurnham DI, Beatty S, Howard AN, Connolly E, Cummins W, Nolan JM. Serum response to supplemental macular carotenoids in subjects with and without age-related macular degeneration. The British journal of nutrition. 2013;110:289–300. doi: 10.1017/S0007114512004837. [DOI] [PubMed] [Google Scholar]
- Mein JR, Dolnikowski GG, Ernst H, Russell RM, Wang XD. Enzymatic formation of apocarotenoids from the xanthophyll carotenoids lutein, zeaxanthin and beta-cryptoxanthin by ferret carotene-9′,10′-monooxygenase. Archives of biochemistry and biophysics. 2011;506:109–121. doi: 10.1016/j.abb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi A, Belkin A, Koval T, Kornhouser T, Assia EI, Rotenstreich Y. An experimental treatment of ocular quinine toxicity with high-dose 9-cis Beta-carotene. Retinal cases & brief reports. 2015;9:157–161. doi: 10.1097/ICB.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, Liu Z, Igo RP, Jr, Truitt B, Klein ML, Snodderly DM, Blodi BA, Gehrs KM, Sarto GE, Wallace RB, Robinson J, LeBlanc ES, Hageman G, Tinker L, Mares JA. Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study. Investigative ophthalmology & visual science. 2013;54:2333–2345. doi: 10.1167/iovs.12-10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers KJ, Mares JA, Igo RP, Jr, Truitt B, Liu Z, Millen AE, Klein M, Johnson EJ, Engelman CD, Karki CK, Blodi B, Gehrs K, Tinker L, Wallace R, Robinson J, LeBlanc ES, Sarto G, Bernstein PS, SanGiovanni JP, Iyengar SK. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS) Investigative ophthalmology & visual science. 2014;55:587–599. doi: 10.1167/iovs.13-13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SM, Voland R, Tinker L, Blodi BA, Klein ML, Gehrs KM, Johnson EJ, Snodderly DM, Wallace RB, Chappell RJ, Parekh N, Ritenbaugh C, Mares JA, Group CS, Women's Helath I. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an Ancillary Study of the Women's Health Initiative. Archives of ophthalmology. 2008;126:354–364. doi: 10.1001/archopht.126.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezuma SR, Sobrin L, Seddon JM. Review of genetics in age related macular degeneration. Seminars in ophthalmology. 2007;22:229–240. doi: 10.1080/08820530701745140. [DOI] [PubMed] [Google Scholar]
- Mortensen A, Skibsted LH. Free radical transients in photobleaching of xanthophylls and carotenes. Free radical research. 1997;26:549–563. doi: 10.3109/10715769709097826. [DOI] [PubMed] [Google Scholar]
- Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E, Bernstein PS, Li B, Frederick JM, Zhang K, Brantley MA, Jr, Lee AY, Zack DJ, Campochiaro B, Campochiaro P, Ripke S, Smith RT, Barile GR, Katsanis N, Allikmets R, Daly MJ, Seddon JM. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC) Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelam K, O'Gorman N, Nolan J, O'Donovan O, Wong HB, Au Eong KG, Beatty S. Measurement of macular pigment: Raman spectroscopy versus heterochromatic flicker photometry. Investigative ophthalmology & visual science. 2005;46:1023–1032. doi: 10.1167/iovs.04-1032. [DOI] [PubMed] [Google Scholar]
- Nelis HJ, De Leenheer AP. Microbial sources of carotenoid pigments used in foods and feeds. Journal of Applied Bacteriology. 1991;70:181–191. [Google Scholar]
- Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Investigative ophthalmology & visual science. 2004;45:3234–3243. doi: 10.1167/iovs.02-1243. [DOI] [PubMed] [Google Scholar]
- Nolan JM, Akkali MC, Loughman J, Howard AN, Beatty S. Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment. Experimental eye research. 2012;101:9–15. doi: 10.1016/j.exer.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Nolan JM, Beatty S, Meagher KA, Howard AN, Kelly D, Thurnham DI. Verification of -Zeaxanthin in Fish. Journal of food processing & technology. 2014;5:335. doi: 10.4172/2157-7110.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JM, Loskutova E, Howard A, Mulcahy R, Moran R, Stack J, Bolger M, Coen RF, Dennison J, Akuffo KO, Owens N, Power R, Thurnham D, Beatty S. The impact of supplemental macular carotenoids in Alzheimer's disease: a randomized clinical trial. Journal of Alzheimer's disease : JAD. 2015;44:1157–1169. doi: 10.3233/JAD-142265. [DOI] [PubMed] [Google Scholar]
- Nolan JM, Loughman J, Akkali MC, Stack J, Scanlon G, Davison P, Beatty S. The impact of macular pigment augmentation on visual performance in normal subjects: COMPASS. Vision research. 2011;51:459–469. doi: 10.1016/j.visres.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Nolan JM, Meagher K, Kashani S, Beatty S. What is meso-zeaxanthin, and where does it come from? Eye. 2013;27:899–905. doi: 10.1038/eye.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JM, Stack J, OD O, Loane E, Beatty S. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Experimental eye research. 2007;84:61–74. doi: 10.1016/j.exer.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Nussbaum JJ, Pruett RC, Delori FC. Historic perspectives. Macular yellow pigment. The first 200 years. Retina. 1981;1:296–310. [PubMed] [Google Scholar]
- O'Neill ME, Carroll Y, Corridan B, Olmedilla B, Granado F, Blanco I, Van den Berg H, Hininger I, Rousell AM, Chopra M, Southon S, Thurnham DI. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. The British journal of nutrition. 2001;85:499–507. doi: 10.1079/bjn2000284. [DOI] [PubMed] [Google Scholar]
- Obana A, Gohto Y, Tanito M, Okazaki S, Gellermann W, Bernstein PS, Ohira A. Effect of age and other factors on macular pigment optical density measured with resonance Raman spectroscopy. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2014;252:1221–1228. doi: 10.1007/s00417-014-2574-x. [DOI] [PubMed] [Google Scholar]
- Obana A, Hiramitsu T, Gohto Y, Ohira A, Mizuno S, Hirano T, Bernstein PS, Fujii H, Iseki K, Tanito M, Hotta Y. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology. 2008;115:147–157. doi: 10.1016/j.ophtha.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Olson JA. Provitamin A function of carotenoids: the conversion of beta-carotene into vitamin A. The Journal of nutrition. 1989;119:105–108. doi: 10.1093/jn/119.1.105. [DOI] [PubMed] [Google Scholar]
- Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- Oz O, Aras Ates N, Tamer L, Yildirim O, Adiguzel U. Glutathione S-transferase M1, T1, and P1 gene polymorphism in exudative age-related macular degeneration: a preliminary report. European journal of ophthalmology. 2006;16:105–110. doi: 10.5301/EJO.2008.3524. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Sasaki M, Takahashi N, Kamoshita M, Miyake S, Tsubota K. Neuroprotective effects of lutein in the retina. Current pharmaceutical design. 2012;18:51–56. doi: 10.2174/138161212798919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagler TA, Rhode S, Neuhofer A, Laggner H, Strobl W, Hinterndorfer C, Volf I, Pavelka M, Eckhardt ER, van der Westhuyzen DR, Schutz GJ, Stangl H. SR-BI-mediated high density lipoprotein (HDL) endocytosis leads to HDL resecretion facilitating cholesterol efflux. The Journal of biological chemistry. 2006;281:11193–11204. doi: 10.1074/jbc.M510261200. [DOI] [PubMed] [Google Scholar]
- Palczewski G, Amengual J, Hoppel CL, von Lintig J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:4457–4469. doi: 10.1096/fj.14-252411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Chew BP, Wong TS. Dietary lutein absorption from marigold extract is rapid in BALB/c mice. The Journal of nutrition. 1998a;128:1802–1806. doi: 10.1093/jn/128.10.1802. [DOI] [PubMed] [Google Scholar]
- Park JS, Chew BP, Wong TS. Dietary lutein from marigold extract inhibits mammary tumor development in BALB/c mice. The Journal of nutrition. 1998b;128:1650–1656. doi: 10.1093/jn/128.10.1650. [DOI] [PubMed] [Google Scholar]
- Paust J, Kriegl W. Starting from 4-hydroxy-2,2,6-trimethylcyclohexanone. Google Patents 2000 [Google Scholar]
- Paust J, Kriegl W, Hartmann H. Preparation of pure trans- and cis-4-Hydroxy-2,2,6-trimethylcyclohexan-1-one from isomerc mixtures. Google Patents 1998 [Google Scholar]
- Perrone S, Longini M, Marzocchi B, Picardi A, Bellieni CV, Proietti F, Rodriguez A, Turrisi G, Buonocore G. Effects of lutein on oxidative stress in the term newborn: a pilot study. Neonatology. 2010;97:36–40. doi: 10.1159/000227291. [DOI] [PubMed] [Google Scholar]
- Perry A, Rasmussen H, Johnson EJ. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. Journal of Food Composition and Analysis. 2009;22:9–15. [Google Scholar]
- Powner MB, Gillies MC, Tretiach M, Scott A, Guymer RH, Hageman GS, Fruttiger M. Perifoveal muller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010;117:2407–2416. doi: 10.1016/j.ophtha.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin DM, Rabin RL, Blenkinsop TA, Temple S, Stern JH. Chronic oxidative stress upregulates Drusen-related protein expression in adult human RPE stem cell-derived RPE cells: a novel culture model for dry AMD. Aging. 2013;5:51–66. doi: 10.18632/aging.100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando RR, Bernstein PS, Barry RJ. Chapter 7 New insights into the visual cycle. Progress in Retinal Research. 1991;10:161–178. [Google Scholar]
- Ravikrishnan R, Rusia S, Ilamurugan G, Salunkhe U, Deshpande J, Shankaranarayanan J, Shankaranarayana ML, Soni MG. Safety assessment of lutein and zeaxanthin (Lutemax™1 2020): Subchronic toxicity and mutagenicity studies. Food and Chemical Toxicology. 2011;49:2841–2848. doi: 10.1016/j.fct.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Reading VM, Weale RA. Macular pigment and chromatic aberration. Journal of the Optical Society of America. 1974;64:231–234. doi: 10.1364/josa.64.000231. [DOI] [PubMed] [Google Scholar]
- Renzi LM, Bovier ER, Hammond BR., Jr A role for the macular carotenoids in visual motor response. Nutritional neuroscience. 2013;16:262–268. doi: 10.1179/1476830513Y.0000000054. [DOI] [PubMed] [Google Scholar]
- Renzi LM, Hammond BR. The effect of macular pigment on heterochromatic luminance contrast. Experimental eye research. 2010;91:896–900. doi: 10.1016/j.exer.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocrine reviews. 2003;24:357–387. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Amaya DB. Latin American food sources of carotenoids. Archivos latinoamericanos de nutricion. 1999;49:74s–84s. [PubMed] [Google Scholar]
- Rodriguez-Amaya DB. Food carotenoids: analysis, composition and alterations during storage and processing of foods. Forum Nutr. 2003;56:35–37. [PubMed] [Google Scholar]
- Rodriguez-Carmona M, Kvansakul J, Harlow JA, Kopcke W, Schalch W, Barbur JL. The effects of supplementation with lutein and/or zeaxanthin on human macular pigment density and colour vision. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians. 2006;26:137–147. doi: 10.1111/j.1475-1313.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal JM, Kim J, de Monasterio F, Thompson DJ, Bone RA, Landrum JT, de Moura FF, Khachik F, Chen H, Schleicher RL, Ferris FL, 3rd, Chew EY. Dose-ranging study of lutein supplementation in persons aged 60 years or older. Investigative ophthalmology & visual science. 2006;47:5227–5233. doi: 10.1167/iovs.05-1513. [DOI] [PubMed] [Google Scholar]
- Ruddock KH. Evidence for Macular Pigmentation from Colour Matching Data. Vision research. 1963;61:417–429. doi: 10.1016/0042-6989(63)90093-2. [DOI] [PubMed] [Google Scholar]
- Ryeom SW, Sparrow JR, Silverstein RL. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. Journal of cell science. 1996;109(Pt 2):387–395. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- Sabour-Pickett S, Beatty S, Connolly E, Loughman J, Stack J, Howard A, Klein R, Klein BE, Meuer SM, Myers CE, Akuffo KO, Nolan JM. Supplementation with three different macular carotenoid formulations in patients with early age-related macular degeneration. Retina. 2014;34:1757–1766. doi: 10.1097/IAE.0000000000000174. [DOI] [PubMed] [Google Scholar]
- Sabour-Pickett S, Nolan JM, Loughman J, Beatty S. A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Molecular nutrition & food research. 2012;56:270–286. doi: 10.1002/mnfr.201100219. [DOI] [PubMed] [Google Scholar]
- Sajilata MG, Bule MV, Chavan P, Singhal RS, Kamat MY. Development of efficient supercritical carbon dioxide extraction methodology for zeaxanthin from dried biomass of Paracoccus zeaxanthinifaciens. Separation and Purification Technology. 2010;71:173–177. [Google Scholar]
- Sakudoh T, Iizuka T, Narukawa J, Sezutsu H, Kobayashi I, Kuwazaki S, Banno Y, Kitamura A, Sugiyama H, Takada N, Fujimoto H, Kadono-Okuda K, Mita K, Tamura T, Yamamoto K, Tsuchida K. A CD36-related transmembrane protein is coordinated with an intracellular lipid-binding protein in selective carotenoid transport for cocoon coloration. The Journal of biological chemistry. 2010;285:7739–7751. doi: 10.1074/jbc.M109.074435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salguero A, León R, Mariotti A, de la Morena B, Vega JM, Vílchez C. UV-A mediated induction of carotenoid accumulation in Dunaliella bardawil with retention of cell viability. Applied microbiology and biotechnology. 2005;66:506–511. doi: 10.1007/s00253-004-1711-6. [DOI] [PubMed] [Google Scholar]
- Sánchez J, Fernández-Sevilla J, Acién F, Cerón M, Pérez-Parra J, Molina-Grima E. Biomass and lutein productivity of Scenedesmus almeriensis: influence of irradiance, dilution rate and temperature. Applied microbiology and biotechnology. 2008;79:719–729. doi: 10.1007/s00253-008-1494-2. [DOI] [PubMed] [Google Scholar]
- Scarmo S, Henebery K, Peracchio H, Cartmel B, Lin H, Ermakov IV, Gellermann W, Bernstein PS, Duffy VB, Mayne ST. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. European journal of clinical nutrition. 2012;66:555–560. doi: 10.1038/ejcn.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr G, Schmidt A, Sandmann G. Mapping of a carotenogenic gene cluster from Erwinia herbicola and functional identification of six genes. FEMS microbiology letters. 1991;62:157–161. doi: 10.1016/0378-1097(91)90151-y. [DOI] [PubMed] [Google Scholar]
- Schultze M. Ueber den gelben Fleck der Retina, seinen Einfluss auf normales Sehen und auf Farbenblindheit. ohen & Sohn: 1866. [Google Scholar]
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. Jama. 1994;272:1413–1420. [PubMed] [Google Scholar]
- Seddon JM, Rosner B, Sperduto RD, Yannuzzi L, Haller JA, Blair NP, Willett W. Dietary fat and risk for advanced age-related macular degeneration. Archives of ophthalmology. 2001;119:1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. Jama. 1996;276:1141–1146. [PubMed] [Google Scholar]
- Shaban H, Richter C. A2E and blue light in the retina: the paradigm of age-related macular degeneration. Biol Chem. 2002;383:537–545. doi: 10.1515/BC.2002.054. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Mott DJ, Machlin LJ. Kinetic characteristics of beta-carotene uptake and depletion in rat tissue. The Journal of nutrition. 1984;114:1924–1933. doi: 10.1093/jn/114.10.1924. [DOI] [PubMed] [Google Scholar]
- Sharifzadeh M, Obana A, Gohto Y, Seto T, Gellermann W. Autofluorescence imaging of macular pigment: influence and correction of ocular media opacities. Journal of biomedical optics. 2014;19:96010. doi: 10.1117/1.JBO.19.9.096010. [DOI] [PubMed] [Google Scholar]
- Sharifzadeh M, Zhao DY, Bernstein PS, Gellermann W. Resonance Raman imaging of macular pigment distributions in the human retina. Journal of the Optical Society of America. A, Optics, image science, and vision. 2008;25:947–957. doi: 10.1364/josaa.25.000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey MD, Hill GE. Carotenoids need structural colours to shine. Biol Lett. 2005;1:121–124. doi: 10.1098/rsbl.2004.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Zhang X, Chen F. Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzyme and microbial technology. 2000;27:312–318. doi: 10.1016/s0141-0229(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Sierra A. Neurosteroids: the StAR protein in the brain. Journal of neuroendocrinology. 2004;16:787–793. doi: 10.1111/j.1365-2826.2004.01226.x. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Science signaling. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein RL, Li W, Park YM, Rahaman SO. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Transactions of the American Clinical and Climatological Association. 2010;121:206–220. [PMC free article] [PubMed] [Google Scholar]
- Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. The American journal of clinical nutrition. 1995;62:1448S–1461S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Investigative ophthalmology & visual science. 1984;25:674–685. [PubMed] [Google Scholar]
- Snodderly DM, Handelman GJ, Adler AJ. Distribution of individual macular pigment carotenoids in central retina of macaque and squirrel monkeys. Investigative ophthalmology & visual science. 1991;32:268–279. [PubMed] [Google Scholar]
- Snodderly DM, Mares JA, Wooten BR, Oxton L, Gruber M, Ficek T. Macular pigment measurement by heterochromatic flicker photometry in older subjects: the carotenoids and age-related eye disease study. Investigative ophthalmology & visual science. 2004;45:531–538. doi: 10.1167/iovs.03-0762. [DOI] [PubMed] [Google Scholar]
- Sobrin L, Seddon JM. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Progress in retinal and eye research. 2014;40:1–15. doi: 10.1016/j.preteyeres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup M, Widmer E, Lukáč T. Technical Procedures for the Syntheses of Carotenoids and Related Compounds from 6-Oxo-isophorone: Syntheses of (3R, 3′ R)-Zeaxanthin. Part II. Helvetica Chimica Acta. 1990;73:868–873. [Google Scholar]
- Sparrow JR, Cai B. Blue light-induced apoptosis of A2E-containing RPE: involvement of caspase-3 and protection by Bcl-2. Investigative ophthalmology & visual science. 2001;42:1356–1362. [PubMed] [Google Scholar]
- Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investigative ophthalmology & visual science. 2000;41:1981–1989. [PubMed] [Google Scholar]
- Stahl W, Nicolai S, Briviba K, Hanusch M, Broszeit G, Peters M, Martin HD, Sies H. Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis. 1997;18:89–92. doi: 10.1093/carcin/18.1.89. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H. Effects of carotenoids and retinoids on gap junctional communication. BioFactors. 2001;15:95–98. doi: 10.1002/biof.5520150209. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H. Carotenoids and protection against solar UV radiation. Skin Pharmacol Appl Skin Physiol. 2002;15:291–296. doi: 10.1159/000064532. [DOI] [PubMed] [Google Scholar]
- Strauss JF, 3rd, Liu P, Christenson LK, Watari H. Sterols and intracellular vesicular trafficking: lessons from the study of NPC1. Steroids. 2002;67:947–951. doi: 10.1016/s0039-128x(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Stringham JM, Garcia PV, Smith PA, McLin LN, Foutch BK. Macular pigment and visual performance in glare: benefits for photostress recovery, disability glare, and visual discomfort. Investigative ophthalmology & visual science. 2011;52:7406–7415. doi: 10.1167/iovs.10-6699. [DOI] [PubMed] [Google Scholar]
- Stringham JM, Hammond BR. Macular pigment and visual performance under glare conditions. Optometry and vision science : official publication of the American Academy of Optometry. 2008;85:82–88. doi: 10.1097/OPX.0b013e318162266e. [DOI] [PubMed] [Google Scholar]
- Stringham JM, Hammond BR, Nolan JM, Wooten BR, Mammen A, Smollon W, Snodderly DM. The utility of using customized heterochromatic flicker photometry (cHFP) to measure macular pigment in patients with age-related macular degeneration. Experimental eye research. 2008;87:445–453. doi: 10.1016/j.exer.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Sujak A, Gabrielska J, Grudzinski W, Borc R, Mazurek P, Gruszecki WI. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Archives of biochemistry and biophysics. 1999;371:301–307. doi: 10.1006/abbi.1999.1437. [DOI] [PubMed] [Google Scholar]
- Sujak A, Gruszecki WI. Organization of mixed monomolecular layers formed with the xanthophyll pigments lutein or zeaxanthin and dipalmitoylphosphatidylcholine at the argon-water interface. J Photochem Photobiol B. 2000;59:42–47. doi: 10.1016/s1011-1344(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Sujak A, Okulski W, Gruszecki WI. Organisation of xanthophyll pigments lutein and zeaxanthin in lipid membranes formed with dipalmitoylphosphatidylcholine. Biochimica et biophysica acta. 2000;1509:255–263. doi: 10.1016/s0005-2736(00)00299-6. [DOI] [PubMed] [Google Scholar]
- Sundelin SP, Nilsson SE. Lipofuscin-formation in retinal pigment epithelial cells is reduced by antioxidants. Free radical biology & medicine. 2001;31:217–225. doi: 10.1016/s0891-5849(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Suter M, Reme C, Grimm C, Wenzel A, Jaattela M, Esser P, Kociok N, Leist M, Richter C. Age-related macular degeneration. The lipofusion component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells. The Journal of biological chemistry. 2000;275:39625–39630. doi: 10.1074/jbc.M007049200. [DOI] [PubMed] [Google Scholar]
- The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The New England journal of medicine. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- Theelen T, Berendschot TT, Klevering BJ, Fuijkschot J, Hoyng CB, Willemsen MA. Multimodal imaging of the macula in hereditary and acquired lack of macular pigment. Acta ophthalmologica. 2014;92:138–142. doi: 10.1111/aos.12092. [DOI] [PubMed] [Google Scholar]
- Thomson LR, Toyoda Y, Delori FC, Garnett KM, Wong ZY, Nichols CR, Cheng KM, Craft NE, Dorey CK. Long term dietary supplementation with zeaxanthin reduces photoreceptor death in light-damaged Japanese quail. Experimental eye research. 2002a;75:529–542. doi: 10.1006/exer.2002.2050. [DOI] [PubMed] [Google Scholar]
- Thomson LR, Toyoda Y, Langner A, Delori FC, Garnett KM, Craft N, Nichols CR, Cheng KM, Dorey CK. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Investigative ophthalmology & visual science. 2002b;43:3538–3549. [PubMed] [Google Scholar]
- Thurnham DI, Nolan JM, Howard AN, Beatty S. Macular response to supplementation with differing xanthophyll formulations in subjects with and without age-related macular degeneration. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2015;253:1231–1243. doi: 10.1007/s00417-014-2811-3. [DOI] [PubMed] [Google Scholar]
- Toyoda Y, Thomson LR, Langner A, Craft NE, Garnett KM, Nichols CR, Cheng KM, Dorey CK. Effect of dietary zeaxanthin on tissue distribution of zeaxanthin and lutein in quail. Investigative ophthalmology & visual science. 2002;43:1210–1221. [PubMed] [Google Scholar]
- Trigatti BL, Rigotti A, Braun A. Cellular and physiological roles of SR-BI, a lipoprotein receptor which mediates selective lipid uptake. Biochimica et biophysica acta. 2000;1529:276–286. doi: 10.1016/s1388-1981(00)00154-2. [DOI] [PubMed] [Google Scholar]
- Trumbo PR, Ellwood KC. Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: an evaluation using the Food and Drug Administration's evidence-based review system for health claims. The American journal of clinical nutrition. 2006;84:971–974. doi: 10.1093/ajcn/84.5.971. [DOI] [PubMed] [Google Scholar]
- Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Molecular vision. 2006;12:1319–1333. [PubMed] [Google Scholar]
- Vachali P, Bhosale P, Bernstein PS. Microbial carotenoids. Methods Mol Biol. 2012;898:41–59. doi: 10.1007/978-1-61779-918-1_2. [DOI] [PubMed] [Google Scholar]
- Vachali PP, Besch BM, Gonzalez-Fernandez F, Bernstein PS. Carotenoids as possible interphotoreceptor retinoid-binding protein (IRBP) ligands: a surface plasmon resonance (SPR) based study. Archives of biochemistry and biophysics. 2013;539:181–186. doi: 10.1016/j.abb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kraats J, Kanis MJ, Genders SW, van Norren D. Lutein and zeaxanthin measured separately in the living human retina with fundus reflectometry. Investigative ophthalmology & visual science. 2008;49:5568–5573. doi: 10.1167/iovs.08-1939. [DOI] [PubMed] [Google Scholar]
- van der Veen RL, Fuijkschot J, Willemsen MA, Cruysberg JR, Berendschot TT, Theelen T. Patients with Sjogren-Larsson syndrome lack macular pigment. Ophthalmology. 2010;117:966–971. doi: 10.1016/j.ophtha.2009.10.019. [DOI] [PubMed] [Google Scholar]
- van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. The Journal of nutrition. 2000;130:503–506. doi: 10.1093/jn/130.3.503. [DOI] [PubMed] [Google Scholar]
- Vishwanathan R, Chung M, Johnson EJ. A systematic review on zinc for the prevention and treatment of age-related macular degeneration. Investigative ophthalmology & visual science. 2013a;54:3985–3998. doi: 10.1167/iovs.12-11552. [DOI] [PubMed] [Google Scholar]
- Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ. Lutein and preterm infants with decreased concentrations of brain carotenoids. Journal of pediatric gastroenterology and nutrition. 2014;59:659–665. doi: 10.1097/MPG.0000000000000389. [DOI] [PubMed] [Google Scholar]
- Vishwanathan R, Neuringer M, Snodderly DM, Schalch W, Johnson EJ. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutritional neuroscience. 2013b;16:21–29. doi: 10.1179/1476830512Y.0000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanathan R, Schalch W, Johnson EJ. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutritional neuroscience. 2015 doi: 10.1179/1476830514Y.0000000141. in press. [DOI] [PubMed] [Google Scholar]
- von Lintig J, Hessel S, Isken A, Kiefer C, Lampert JM, Voolstra O, Vogt K. Towards a better understanding of carotenoid metabolism in animals. Biochimica et biophysica acta. 2005;1740:122–131. doi: 10.1016/j.bbadis.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, von Lintig J. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry. 2006;45:13429–13437. doi: 10.1021/bi060701u. [DOI] [PubMed] [Google Scholar]
- Wald G. Human Vision and the Spectrum. Science. 1945;101:653–658. doi: 10.1126/science.101.2635.653. [DOI] [PubMed] [Google Scholar]
- Wald G, Brown PK. Human rhodopsin. Science. 1958;127:222–226. doi: 10.1126/science.127.3292.222. [DOI] [PubMed] [Google Scholar]
- Walls GL, Judd HD. The Intra-Ocular Colour-Filters of Vertebrates. The British journal of ophthalmology. 1933;17:705–725. doi: 10.1136/bjo.17.12.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Connor SL, Wang W, Johnson EJ, Connor WE. The selective retention of lutein, meso-zeaxanthin and zeaxanthin in the retina of chicks fed a xanthophyll-free diet. Experimental eye research. 2007;84:591–598. doi: 10.1016/j.exer.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Wenzel AJ, Gerweck C, Barbato D, Nicolosi RJ, Handelman GJ, Curran-Celentano J. A 12-wk egg intervention increases serum zeaxanthin and macular pigment optical density in women. The Journal of nutrition. 2006;136:2568–2573. doi: 10.1093/jn/136.10.2568. [DOI] [PubMed] [Google Scholar]
- Werner JS, Donnelly SK, Kliegl R. Aging and human macular pigment density. Appended with translations from the work of Max Schultze and Ewald Hering. Vision research. 1987;27:257–268. doi: 10.1016/0042-6989(87)90188-x. [DOI] [PubMed] [Google Scholar]
- West SK, Rosenthal FS, Bressler NM, Bressler SB, Munoz B, Fine SL, Taylor HR. Exposure to sunlight and other risk factors for age-related macular degeneration. Archives of ophthalmology. 1989;107:875–879. doi: 10.1001/archopht.1989.01070010897038. [DOI] [PubMed] [Google Scholar]
- Widmer E, Soukup M, Zell R, Broger E, Wagner HP, Imfeld M. Technical Procedures for the Syntheses of Carotenoids and Related Compounds from 6-Oxo-isophorone: Syntheses of (3R,3′R)-Zeaxanthin. Part I. Helvetica Chimica Acta. 1990;73:861–867. [Google Scholar]
- Willstätter R, Mieg W. Ueber die Gelben Begleiter des Chlorophylls. Justus Liebigs Ann Chem. 1907;355:1–28. [Google Scholar]
- Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Molecular vision. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- Wolin LR, Massopust LC., Jr Characteristics of the ocular fundus in primates. Journal of anatomy. 1967;101:693–699. [PMC free article] [PubMed] [Google Scholar]
- Wong WT, Forooghian F, Majumdar Z, Bonner RF, Cunningham D, Chew EY. Fundus autofluorescence in type 2 idiopathic macular telangiectasia: correlation with optical coherence tomography and microperimetry. American journal of ophthalmology. 2009;148:573–583. doi: 10.1016/j.ajo.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall AA, Britton G, Jackson MJ. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: relationship between carotenoid structure and protective ability. Biochimica et biophysica acta. 1997a;1336:575–586. doi: 10.1016/s0304-4165(97)00007-x. [DOI] [PubMed] [Google Scholar]
- Woodall AA, Lee SW, Weesie RJ, Jackson MJ, Britton G. Oxidation of carotenoids by free radicals: relationship between structure and reactivity. Biochimica et biophysica acta. 1997b;1336:33–42. doi: 10.1016/s0304-4165(97)00006-8. [DOI] [PubMed] [Google Scholar]
- Wooten BR, Hammond BR. Macular pigment: influences on visual acuity and visibility. Progress in retinal and eye research. 2002;21:225–240. doi: 10.1016/s1350-9462(02)00003-4. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang L, Shao B, Sun X, Ho CT, Li S. Safety evaluation of meso-zeaxanthin. Food Control. 2013;32:678–686. [Google Scholar]
- Yamada E. Some structural features of the fovea centralis in the human retina. Archives of ophthalmology. 1969;82:151–159. doi: 10.1001/archopht.1969.00990020153002. [DOI] [PubMed] [Google Scholar]
- Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, Dewan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- Yao Y, Qiu QH, Wu XW, Cai ZY, Xu S, Liang XQ. Lutein supplementation improves visual performance in Chinese drivers: 1-year randomized, double-blind, placebo-controlled study. Nutrition. 2013;29:958–964. doi: 10.1016/j.nut.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Yap SC, Choo YM, Hew NF, Goh SH. Distribution of dietary palm carotenes and their metabolites in the rabbit. Nutrition Research. 1997;17:1721–1731. [Google Scholar]
- Yeum KJ, Shang FM, Schalch WM, Russell RM, Taylor A. Fat-soluble nutrient concentrations in different layers of human cataractous lens. Current eye research. 1999;19:502–505. doi: 10.1076/ceyr.19.6.502.5282. [DOI] [PubMed] [Google Scholar]
- Yeum KJ, Taylor A, Tang G, Russell RM. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Investigative ophthalmology & visual science. 1995;36:2756–2761. [PubMed] [Google Scholar]
- Yonekura L, Kobayashi M, Terasaki M, Nagao A. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. The Journal of nutrition. 2010;140:1824–1831. doi: 10.3945/jn.110.126466. [DOI] [PubMed] [Google Scholar]
- Yu H, Wark L, Ji H, Willard L, Jaing Y, Han J, He H, Ortiz E, Zhang Y, Medeiros DM, Lin D. Dietary wolfberry upregulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Molecular nutrition & food research. 2013;57:1158–1169. doi: 10.1002/mnfr.201200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuodelis C, Hendrickson A. A qualitative and quantitative analysis of the human fovea during development. Vision research. 1986;26:847–855. doi: 10.1016/0042-6989(86)90143-4. [DOI] [PubMed] [Google Scholar]
- Zeimer MB, Kromer I, Spital G, Lommatzsch A, Pauleikhoff D. Macular telangiectasia: patterns of distribution of macular pigment and response to supplementation. Retina. 2010;30:1282–1293. doi: 10.1097/IAE.0b013e3181e096dd. [DOI] [PubMed] [Google Scholar]
- Zhao DY, Wintch SW, Ermakov IV, Gellermann W, Bernstein PS. Resonance Raman measurement of macular carotenoids in retinal, choroidal, and macular dystrophies. Archives of ophthalmology. 2003;121:967–972. doi: 10.1001/archopht.121.7.967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.