Abstract
New therapeutic intervention strategies for the treatment of human malignancies are always desired. Approval of bortezomib as a front-line treatment for multiple myeloma highlighted the significance of ubiquitin–proteasome system (UPS) as a promising therapeutic target. However, due to the broad impact of proteasome inhibition, deleterious side effects have been reported with bortezomib treatment. Cullin RING ligases (CRLs)-mediated ubiquitin conjugation process is responsible for the ubiquitin conjugation of 20 % cellular proteins that are designated for degradation through the UPS, most of them are critical proteins involved in cell cycle progression, signaling transduction and apoptosis. Studies have depicted the upstream NEDDylation pathway that controls the CRL activity by regulating the conjugation of an ubiquitin-like-protein NEDD8 to the cullin protein in the complex. A specific pharmaceutical inhibitor of NEDD8 activating enzyme (NAE; E1) MLN4924 was recently developed and has been promoted to Phase I clinical trials for the treatment of several human malignancies. This article summarizes the most recent understanding about the process of NEDD8 conjugation, its relevance for cancer therapy and molecular mechanisms responsible for the potent anti-tumor activity of MLN4924.
Keywords: NEDDylation, NEDD8, Ubiquitin, MLN4924, mTOR, DNA damage
Introduction
The ubiquitin–proteasome system (UPS) does not obviously drive any oncogenic pathways and yet bortezomib (Velcade®)-mediated specific inhibition of proteasome, the system responsible for the final step of protein degradation, turned out to be a paradigm shift therapeutic strategy for multiple myeloma treatment (Field-Smith et al. 2006; Richardson et al. 2005, 2007). Clinical trials of bortezomib in other types of tumors, including mantle cell lymphoma, acute leukemia and non-small cell lung cancer also highlighted the significance of UPS as a novel target for human malignancy management (clinicaltrials.gov). Bortezomib-mediated proteasome inhibition was depicted to exert its potent anti-myeloma activity by inhibiting NFκB signaling, stabilizing pro-apoptotic proteins and triggering endoplasmic reticulum (ER) stress/unfolded protein response (UPR) (Mujtaba and Dou 2011). Mechanistically, however, since proteasome is also an essential system for normal tissue cell survival, bortezomib-mediated proteasome inhibition may cause serious side effects as have been reported in clinical trials. Typical side effects include thrombocytopenia, gastrointestinal toxicities and peripheral neuropathy (Richardson et al. 2005, 2007). Last 35 years’ endeavor uncovered the detailed molecular mechanisms mediating the transferring and conjugation of ubiquitin (Ub) proteins to their substrate targets. Consequently, a new therapeutic intervention strategy that will more specifically and potently impede cancer relevant portion of the UPS will presumably exert potent anti-tumor activity and in the meantime, will be well-tolerated by the patients.
Studies on multiple human malignancies showed that hyper-activated UPS will sustain uncontrolled proliferation and progression of cancer cells by constitutively activation of pro-survival pathways and dysregulation of proteins in cell cycle (Watson et al. 2011). Up-regulation of UPS has been shown in human malignancies like melanoma, lung cancer and squamous-cell carcinoma (Li et al. 2014; Cheng et al. 2014). Proteasome-mediated degradation of proteins starts with conjugation of an ubiquitin (Ub) to substrate proteins. Ub conjugation happens in three successive enzymatic steps (Komander 2009). Ub was first activated in an ATP dependent manner by Ub-activating enzymes (E1s), transferred to Ub-conjugating enzymes (E2s), which will further form a E3 complex to conjugate Ub to substrate proteins (Hershko and Ciechanover 1998). Besides Bortezomib, the significance of UPS for cancer treatment was further highlighted by the development of novel agents that specifically target the components in the Ub conjugation pathway. In vertebrates, two E1 enzymes (Uba1 and Uba6) have been shown to activate ubiquitin and an inhibitor targeting these enzymes, PYR-41, has recently been studied for its antitumor activity (Yang et al. 2007). Like bortezomib, PYR-41 was believed to exert its anti-tumor activity by inhibiting NFκB signaling (Yang et al. 2007). More than 35 E2s have been found in vertebrates and an agent called CC0651 was specifically designed to inhibit one of the E2 called CDC34 (Ceccarelli et al. 2011; Huang et al. 2014). Structural studies indicated that CC0651 can inhibit the spontaneous hydrolysis of the Cdc34A-ubiquitin thioester and thus inhibit the ubiquitin and subsequent degradation of p27(Kip1), accumulation of which will induce cell cycle G1 phase arrest (Huang et al. 2014). The most inspiring story of targeted therapy against UPS system comes from the recent identification of MLN4924 as a mechanism-based specific inhibitor of NAE, impacting a subgroup of E3 ligases called Cullin RING ligases (CRLs) (Soucy et al. 2009a, b). CRLs are responsible for ubiquitin conjugation of 20 % cellular proteins designated for degradation through the UPS system (Soucy et al. 2009a, b). Typical CRL substrates include proteins involved in cell cycle regulation [Cdt1, Orc6, p21(Cip1), p27(Kip1), WEE1], apoptosis (BIM, Mcl1) and signaling transduction pathways (IκBα; β-catenin; HIF1α; REDD1; Deptor) (Genschik et al. 2013; Lee and Zhou 2010; Soucy et al. 2009a, 2010). Promising pre-clinical studies have promoted the Phase I clinical trial of this compound in human malignancies (Lin et al. 2010; Milhollen et al. 2011; Zhao and Sun 2012; Swords et al. 2010; clinicaltrials.gov). Furthermore, hyper-activation of CRL complexes has been reported in melanoma, squamous-cell carcinoma, lung cancer, colon cancer and intrahepatic cholangiocarcinoma (Li et al. 2014; Cheng et al. 2014; Gao et al. 2014; Xie et al. 2014). The up-regulation of CRL activity in these human malignancies further validated CRL complexes as a promising therapeutic target. However, detailed molecular level understanding of how MLN4924 kills the cancer cells remains elusive. In this review, we will introduce the relevance of NEDDylation inhibition for cancer therapy and summarize/review the cytotoxic mechanisms so far proposed underlying the potent anti-tumor activity of MLN4924 as a new generation of compounds that specifically target the cellular protein turnover process (Nawrocki et al. 2012).
Regulatory role of NEDD8 conjugation in CRL complexes
Several levels of regulation were evolutionally developed to tightly control the activity of the CRL (Lydeard et al. 2013). CRLs are composed of a scaffold protein called cullin (CUL1, 2, 3, 4A, 4B, 5, 7, 9), substrate receptor (SR) protein and an adaptor protein that mediates the interaction between the cullin N-terminus and the substrate receptors (Lee and Zhou 2010; Sarikas et al. 2011). The C-terminus of cullin interacts with the RING finger protein (Rbx1 and Rbx2), which will mediate the recruitment of the Ub-conjugated E2 enzymes (Bohnsack and Haas 2003). Proposed mechanisms regulating the activity of this multimeric complex include binding of cullin-associated NEDD8-dissociated protein 1 (CAND1) to the cullin-RING complex, substrate-mediated up-regulation of SR proteins and NEDDylation and deNEDDylation of a Ub-like (Ubl) protein NEDD8 to the C-terminal area of cullins (Lydeard et al. 2013). So far, NEDD8 conjugation is one of the best studied mechanisms that can turn ‘on’ and ‘off’ CRL activity in a timely manner to delicately regulation the turn over of cellular proteins. Conjugation of NEDD8 to the cullin protein also happens in three enzymatic steps that involve activating of NEDD8 by NEDD8-activating enzyme (NAE; AppBp1/Uba3), and transfer to one of the E2 enzymes (Ubc12, Ube2F). E3 enzymes will then facilitate the conjugation of NEDD8 to the substrate proteins (Bohnsack and Haas 2003; Parry and Estelle 2004). A more detailed review on the regulatory role of NEDDylation on CRL complex was published by King and Finley (2014), recently. The cullin family of proteins constitutes the major substrates of NEDDylation, accompanied with other recently identified substrates including TGF-β type II receptor, histone H4, p53, p73, ribosomal proteins and L11 (Ma et al. 2013; Zuo et al. 2013; Abida et al. 2007; Watson et al. 2006; Xirodimas 2008; Xirodimas et al. 2004, 2008; Sundqvist et al. 2009). The caveat here is that identification of non-cullin NEDDylation substrates all relied on over-expressed NEDD8 and recent studies have suggested that NEDD8 may serve as a surrogate for ubiquitin when its cellular levels are up-regulated by over-expression (Hjerpe et al. 2012; Leidecker et al. 2012). Conjugation of NEDD8 to the C-terminal area will initiate a profound structural change of the cullin-RING complex and facilitate the recruitment of E2 and SRs and thus, promote Ub conjugation to substrate proteins (Duda et al. 2008). Consequently, inhibition of NEDD8 conjugation will down-regulate CRL activity and induce accumulation of CRL substrates. The intimate link between NEDD8-activated proteolysis and tumorigenesis was substantiated by the development of MLN4924, which is now in a Phase I clinical trial, as a specific inhibitor of NAE (Soucy et al. 2009a, b).
MLN4924 is an adenosine sulfamate analogue that inhibits the NEDD8 activation by forming a NEDD8-MLN4924 adduct (Brownell et al. 2010). The selectivity of MLN4924 in inhibiting NEDD8 activation was established by showing that MLN4924 only inhibits SUMOylation and ubiquitination activating enzymes (Ubc10 and Ubc9, respectively) at much higher dosages and this goes also for the protein kinases, which generally require a concentration of MLN4924 higher than 100 μM to reach their IC50s (Soucy et al. 2009a, b). With the treatment of MLN4924, NEDD8 will not be able to be conjugated to cullin proteins, inducing CRL inhibition. Recent proteomic studies identified hundreds of CRL substrates critical for cell cycle progression, glucose metabolism, signaling pathways and cell death (Emanuele et al. 2011; Harper and Tan 2012; Liao et al. 2011). Consequently, upon MLN4924 treatment, these substrates will be stabilized and may trigger cytotoxic responses.
Cytotoxic mechanisms of NEDDylation inhibition in cancer cells
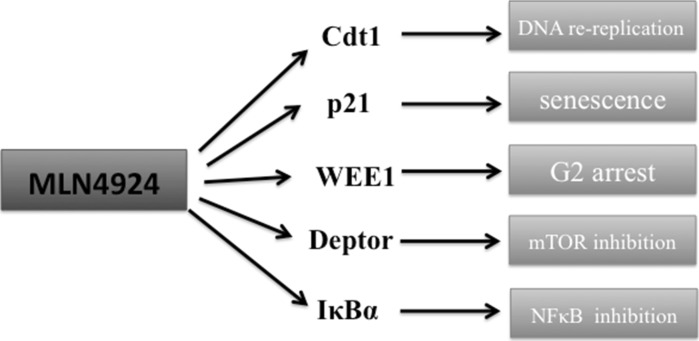
Most recent studies proposed several cytotoxic mechanisms of NEDDylation inhibition towards cancer cells (Fig. 1). However, given the amount of CRL substrates that can be stabilized upon MLN4924 treatment, different cancer types may have distinct mechanisms of vulnerability towards MLN4924. Cytotoxic CRL substrates so far proposed in mediating MLN4924-induced cancer cell death include a cell cycle licensing factor Cdt1, NFκB inhibitor IκBα, mTOR inhibitor Deptor and REDD1 and cell cycle checkpoint proteins p21(Cip1), p27(Kip1) and WEE1 (Lin et al. 2010; Zhao et al. 2012; Swords et al. 2010; Mackintosh et al. 2013; Jia et al. 2011a, b; Gu et al. 2014). New mechanisms are emerging based on different cellular context of cancer types and here we will outline those cytotoxic events so far proposed when NEDDylation is inhibited.
Fig. 1.
Proposed cytotoxic mechanisms of NEDDylaton inhibition in human malignancies. With inhibition of NEDD8 conjugation pathway, certain critical CRL substrates will accumulate and will lead to cancer cell death. Major proteins identified to induce cell death include cell cycle licensing factor Cdt1; cyclin-dependent kinase inhibitor p21; a negative regulator of entry into mitosis WEE1; mTOR pathway inhibitor Deptor 1; NFκB pathway inhibitor IκB. Due to distinct cellular context, different mechanisms were proposed in different cancer cell lines tested with MLN4924
First report regarding the cytotoxicity of MLN4924 highlighted the cell cycle licensing factor Cdt1 as a substrate of CRL that accumulates upon NEDDylation inhibition (Soucy et al. 2009a, b; Lin et al. 2010; Milhollen et al. 2011). Cdt1, coordinated with other replication factors cell division cycle 6 (Cdc6) and origin recognition complex (Orc), will recruit MCM2-7 complexes to the origins of DNA replication to form a pre-replication complex (Pre-RC) (Caillat and Perrakis 2012). After initiation of DNA replication Cdt1 will be either targeted for degradation by CUL4-DDB1Cdt2 and SCFSkp2 or bound by its inhibitor geminin (Ballabeni et al. 2013; Nishitani et al. 2006). With MLN4924 treatment, both E3 ligases (CUL4-DDB1Cdt2 and SCFSkp2) with be inhibited and Cdt1 will not be timely degraded at late S phase. Accumulated Cdt1 will trigger a process called DNA re-replication in which DNA replication origins are repeatedly initiated to replicate, leading to the accumulation of >4N DNA content (Truong and Wu 2011). Supportive evidence of the existence of DNA re-replication came from studies showing that with MLN4924 treatment a cell population of >4n DNA content was observed, indicating DNA replication was fired multiple times (Soucy et al. 2009a, b; Lin et al. 2010; Jia et al. 2011a, b). More recent studies showed that the cell line HCT116 used in those studies has intra-S phase checkpoint defects, whereas other cell lines that do not have such defects will not undergo DNA re-replication to such an extent as in HCT116 (Blank et al. 2013). The intensity of DNA re-replication induced with MLN4924 treatment does not necessarily relate with cancer cell death (Blank et al. 2013). Consequently, depending on the integrity of cell-cycle checkpoints, the relevance of Cdt1 induced DNA re-replication in mediating cancer cell death upon NEDDylation inhibition may depend on the specific cellular context in each cancer types. MLN4924 treatment has been shown to induce cell-cycle arrest in different phases. The specific cell phase arrested with MLN4924 treatment also need to be evaluated on the basis of a different cellular context. Cdt1 accumulation will promote cell cycle S phase entry whereas WEE1 accumulation will trigger G2/M phase arrest (Soucy et al. 2009a, b; Mackintosh et al. 2013). Accumulation of cell cycle regulatory proteins upon MLN4924 treatment was constantly accompanied with DNA damage response, which was partially responsible for the apoptosis and senescence induced upon NEDDylation inhibition (Soucy et al. 2009a, b; Mackintosh et al. 2013; Jia et al. 2011a, b). Given the potency and the unique mechanism of MLN4924 in inducing DNA damage in cancer cells, synergistic interactions between MLN4924 with other DNA-damage-inducing compounds (platinum, cytarabine, Cisplatin, mitomycin C) and radiation will promote its incorporation into current treatment regimens for human malignancies (Nawrocki et al. 2013, 2015; Yang et al. 2012; Wei et al. 2012; Kee et al. 2012; Jazaeri et al. 2013; Garcia et al. 2014). These studies highlighted potential incorporation of MLN4924 into current treatment regimens as addition of MLN4924 was shown to sensitize malignant cells to those traditional therapeutic strategies.
The involvement of mTOR pathway in mediating MLN4924-induced cell death was highlighted by the findings that mTOR upstream inhibitors Deptor and REDD1 are CRL substrates (Zhao et al. 2012; Gu et al. 2014). Stabilization/induction of REDD1 was induced upon MLN4924 treatment in multiple myeloma and siRNA-mediated knockdown was shown to alleviate the cytotoxicity of MLN4924 (Gu et al. 2014). Furthermore, MLN4924-mediated mTOR inhibition was shown to trigger pro-survival autophagy in liver cancer cells and simultaneous inhibition of autophagic responses enhances cytotoxic effects (Luo et al. 2012). This is consistent with a previous report with knockdown of RING finger protein Rbx1 in the CRL complex, in which protective autophagic responses were also induced (Yang et al. 2013). Consequently, concomitant inhibition of autophagy and NEDD8 conjugation hold significant therapeutic implications.
Stabilization of NFκB inhibitor IκBα was found to be one major cytotoxic mechanism of MLN4924 in acute myeloid leukemia (AML) and also in B cell-like (ABC) diffuse large B cell lymphoma (DLBCL) (Swords et al. 2010; Milhollen et al. 2010). Stabilization of IκBα was also reported as major cytotoxic mechanism of bortezomib although more recent studies showed contradictory results showing that instead of inhibition NFκB, bortezomib actually activates this pathway by inducing phosphorylation/activation of IκB kinase (IKKβ) (Hideshima et al. 2009). Consequently, the role and relevance of MLN4924-induced IκBα stabilization in mediating NFκB inhibition and cancer cell death warrants further investigation beyond AML and DLBCL. Also, given other aspects of NFκB pathway are strictly regulated by ubiquitination, the detailed impact of MLN4924 on it remains elusive (Chen 2005).
Discussion
Before the development of MLN4924 as a specific inhibitor of NEDD8 activating enzyme, therapeutic significance of CRL complexes was investigated using siRNA-mediated knocking down of certain components in the CRL complex. The RING component of the CRL complex Rbx1 was shown to be up-regulated in multiple cancer cell lines and primary tumor tissues (Jia et al. 2009). Meanwhile, siRNA-mediated Rbx1 knockdown induced apoptosis, senescence and autophagy, indicating the critical role of CRL complexes in sustaining tumor cell growth (Jia et al. 2011a, b; Yang et al. 2013). Further studies on Rbx2 showed the relevance of pro-apoptotic factor NOXA in mediating cytotoxicity of CRL inhibition (Jia et al. 2010). Also siRNA mediated Skp2 knockdown has been well studied to have potent anti-tumor effects (Katagiri et al. 2006). All these studies on the role of CRL complex in tumor cells paved the way for the development and clinical evaluation of MLN4924 as a therapeutic agent targeting CRL complexes. The development of MLN4924 further highlighted the UPS system as a ‘drugable’ target for human malignancy treatment, although the detailed molecular understanding of how MLN4924 exerts its cytotoxicity remains largely unknown. In this article, we summarized/reviewed the most recent cytotoxic mechanisms proposed underlying the potent anti-tumor activity of NEDDylation inhibition.
However, given that CRLs are responsible for about 20 % of UPS-mediated protein turnover, certain oncogenic substrates may also accumulate with MLN4924 treatment. For instance, Hypoxia-inducible factor-1α (HIF-1α) is a well-documented substrate of pVHL-associated SCF ubiquitin ligase complex (Lisztwan et al. 1999), although most recent studies on the impact of MLN4924 on angiogenesis showed that NEDDylation inhibition could efficiently inhibit tumor vascularization process by inducing RhoA accumulation (Yao et al. 2014). Also, components in the tumorigenic Wnt signaling pathway, including the Dishevelled protein that integrates extra-cellular stimulus to activate Wnt pathway and β-catenin a key transcriptional factor activation of which will promote cell proliferation and invasion, are also well-established as substrates of CRLs (Angers et al. 2006; Gao and Chen 2010; Su et al. 2003). Currently, there is no study evaluating the impact of the stabilization of these oncogenic proteins upon MLN4924 treatment. Given the critical role HIF-1α and Wnt pathway plays in tumorigenic vascular remodeling, complex in vivo micro-environment that nourish the tumor cells will also be affected with stabilization of these oncogenic proteins (Semenza 2003; Easwaran et al. 2003). Induction of apoptosis or senescence by MLN4924 results in a permanent change of the tumor cell. Similarly, oncogenic proteins are permanently altered by mutation or amplification in order to promote tumor growth. Whether the transient stabilization of these oncogenic proteins is sufficient to impact tumor cell growth in such a way as to alter the prognosis of patients warrants further study. Consequently, a more detailed mechanistic understanding of the cytotoxicity of MLN4924 is required to fully evaluate the therapeutic significance MLN4924 as a new generation of targeted treatment for human malignancy.
References
- Abida WM, Nikolaev A, Zhao W, Zhang W, Gu W. FBXO11 promotes the Neddylation of p53 and inhibits its transcriptional activity. J Biol Chem. 2007;282:1797–1804. doi: 10.1074/jbc.M609001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, Moon RT. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat Cell Biol. 2006;8:348–357. doi: 10.1038/ncb1381. [DOI] [PubMed] [Google Scholar]
- Ballabeni A, Zamponi R, Moore JK, Helin K, Kirschner MW. Geminin deploys multiple mechanisms to regulate Cdt1 before cell division thus ensuring the proper execution of DNA replication. Proc Natl Acad Sci USA. 2013;110:E2848–E2853. doi: 10.1073/pnas.1310677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank JL, Liu XJ, Cosmopoulos K, Bouck DC, Garcia K, Bernard H, Tayber O, Hather G, Liu R, Narayanan U, Milhollen MA, Lightcap ES. Novel DNA damage checkpoints mediating cell death induced by the NEDD8-activating enzyme inhibitor MLN4924. Cancer Res. 2013;73:225–234. doi: 10.1158/0008-5472.CAN-12-1729. [DOI] [PubMed] [Google Scholar]
- Bohnsack RN, Haas AL. Conservation in the mechanism of Nedd8 activation by the human AppBp1-Uba3 heterodimer. J Biol Chem. 2003;278:26823–26830. doi: 10.1074/jbc.M303177200. [DOI] [PubMed] [Google Scholar]
- Brownell JE, Sintchak MD, Gavin JM, Liao H, Bruzzese FJ, Bump NJ, Soucy TA, Milhollen MA, Yang X, Burkhardt AL, Ma J, Loke HK, Lingaraj T, Wu D, Hamman KB, Spelman JJ, Cullis CA, Langston SP, Vyskocil S, Sells TB, Mallender WD, Visiers I, Li P, Claiborne CF, Rolfe M, Bolen JB, Dick LR. Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol Cell. 2010;37:102–111. doi: 10.1016/j.molcel.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Caillat C, Perrakis A. Cdt1 and geminin in DNA replication initiation. Subcell Biochem. 2012;62:71–87. doi: 10.1007/978-94-007-4572-8_5. [DOI] [PubMed] [Google Scholar]
- Ceccarelli DF, Tang X, Pelletier B, Orlicky S, Xie W, Plantevin V, Neculai D, Chou YC, Ogunjimi A, Al-Hakim A, Varelas X, Koszela J, Wasney GA, Vedadi M, Dhe-Paganon S, Cox S, Xu S, Lopez-Girona A, Mercurio F, Wrana J, Durocher D, Meloche S, Webb DR, Tyers M, Sicheri F. An allosteric inhibitor of the human Cdc34 ubiquitin-conjugating enzyme. Cell. 2011;145:1075–1087. doi: 10.1016/j.cell.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, He R, Zhang L, Li H, Zhang W, Ji X, Kong F, Sun J, Chen S. Expression of neddylation-related proteins in melanoma cell lines and the effect of neddylation on melanoma proliferation. Oncol Lett. 2014;7:1645–1650. doi: 10.3892/ol.2014.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell. 2008;134:995–1006. doi: 10.1016/j.cell.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, Randazzo F, Gundel R, Warren RS, Escobedo J, Aukerman SL, Taylor RN, Fantl WJ. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–3153. [PubMed] [Google Scholar]
- Emanuele MJ, Elia AE, Xu Q, Thoma CR, Izhar L, Leng Y, Guo A, Chen YN, Rush J, Hsu PW, Yen HC, Elledge SJ. Global identification of modular cullin-RING ligase substrates. Cell. 2011;147:459–474. doi: 10.1016/j.cell.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Smith A, Morgan GJ, Davies FE. Bortezomib (Velcadetrade mark) in the treatment of multiple myeloma. Ther Clin Risk Manag. 2006;2:271–279. doi: 10.2147/tcrm.2006.2.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Gao Q, Yu GY, Shi JY, Li LH, Zhang WJ, Wang ZC, Yang LX, Duan M, Zhao H, Wang XY, Zhou J, Qiu SJ, Jeong LS, Jia LJ, Fan J (2014) Neddylation pathway is up-regulated in human intrahepatic cholangiocarcinoma and serves as a potential therapeutic target. Oncotarget 5:7820–7832 [DOI] [PMC free article] [PubMed]
- Garcia K, Blank JL, Bouck DC, Liu XJ, Sappal DS, Hather G, Cosmopoulos K, Thomas MP, Kuranda M, Pickard MD, Liu R, Bandi S, Smith PG, Lightcap ES. Nedd8-activating enzyme inhibitor MLN4924 provides synergy with mitomycin C through interactions with ATR, BRCA1/BRCA2, and chromatin dynamics pathways. Mol Cancer Ther. 2014;13:1625–1635. doi: 10.1158/1535-7163.MCT-13-0634. [DOI] [PubMed] [Google Scholar]
- Genschik P, Sumara I, Lechner E (2013) The emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): cellular functions and disease implications. EMBO J 32:2307–2320 [DOI] [PMC free article] [PubMed]
- Gu Y, Kaufman JL, Bernal L, Torre C, Matulis SM, Harvey RD, Chen J, Sun SY, Boise LH, Lonial S. MLN4924, an NAE inhibitor, suppresses AKT and mTOR signaling via upregulation of REDD1 in human myeloma cells. Blood. 2014;123:3269–3276. doi: 10.1182/blood-2013-08-521914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Tan MK. Understanding cullin-RING E3 biology through proteomics-based substrate identification. Mol Cell Proteomics. 2012;11:1541–1550. doi: 10.1074/mcp.R112.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Ikeda H, Chauhan D, Okawa Y, Raje N, Podar K, Mitsiades C, Munshi NC, Richardson PG, Carrasco RD, Anderson KC. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood. 2009;114:1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjerpe R, Thomas Y, Chen J, Zemla A, Curran S, Shpiro N, Dick LR, Kurz T. Changes in the ratio of free NEDD8 to ubiquitin triggers NEDDylation by ubiquitin enzymes. Biochem J. 2012;441:927–936. doi: 10.1042/BJ20111671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ceccarelli DF, Orlicky S, St-Cyr DJ, Ziemba A, Garg P, Plamondon S, Auer M, Sidhu S, Marinier A, Kleiger G, Tyers M, Sicheri F. E2 enzyme inhibition by stabilization of a low-affinity interface with ubiquitin. Nat Chem Biol. 2014;10:156–163. doi: 10.1038/nchembio.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazaeri AA, Shibata E, Park J, Bryant JL, Conaway MR, Modesitt SC, Smith PG, Milhollen MA, Berger AJ, Dutta A. Overcoming platinum resistance in preclinical models of ovarian cancer using the neddylation inhibitor MLN4924. Mol Cancer Ther. 2013;12:1958–1967. doi: 10.1158/1535-7163.MCT-12-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Soengas MS, Sun Y. ROC1/RBX1 E3 ubiquitin ligase silencing suppresses tumor cell growth via sequential induction of G2-M arrest, apoptosis, and senescence. Cancer Res. 2009;69:4974–4982. doi: 10.1158/0008-5472.CAN-08-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Yang J, Hao X, Zheng M, He H, Xiong X, Xu L, Sun Y. Validation of SAG/RBX2/ROC2 E3 ubiquitin ligase as an anticancer and radiosensitizing target. Clin Cancer Res. 2010;16:814–824. doi: 10.1158/1078-0432.CCR-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Bickel JS, Wu J, Morgan MA, Li H, Yang J, Yu X, Chan RC, Sun Y. RBX1 (RING box protein 1) E3 ubiquitin ligase is required for genomic integrity by modulating DNA replication licensing proteins. J Biol Chem. 2011;286:3379–3386. doi: 10.1074/jbc.M110.188425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Li H, Sun Y. Induction of p21-dependent senescence by an NAE inhibitor, MLN4924, as a mechanism of growth suppression. Neoplasia. 2011;13:561–569. doi: 10.1593/neo.11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri Y, Hozumi Y, Kondo S. Knockdown of Skp2 by siRNA inhibits melanoma cell growth in vitro and in vivo. J Dermatol Sci. 2006;42:215–224. doi: 10.1016/j.jdermsci.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Kee Y, Huang M, Chang S, Moreau LA, Park E, Smith PG, D’Andrea AD. Inhibition of the Nedd8 system sensitizes cells to DNA interstrand cross-linking agents. Mol Cancer Res. 2012;10:369–377. doi: 10.1158/1541-7786.MCR-11-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Finley D. Sculpting the proteome with small molecules. Nat Chem Biol. 2014;10:870–874. doi: 10.1038/nchembio.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D. The emerging complexity of protein ubiquitination. Biochem Soc Trans. 2009;37:937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- Lee J, Zhou P. Cullins and cancer. Genes. Cancer. 2010;1:690–699. doi: 10.1177/1947601910382899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidecker O, Matic I, Mahata B, Pion E, Xirodimas DP. The ubiquitin E1 enzyme Ube1 mediates NEDD8 activation under diverse stress conditions. Cell Cycle. 2012;11:1142–1150. doi: 10.4161/cc.11.6.19559. [DOI] [PubMed] [Google Scholar]
- Li L, Wang M, Yu G, Chen P, Li H, Wei D, Zhu J, Xie L, Jia H, Shi J, Li C, Yao W, Wang Y, Gao Q, Jeong LS, Lee HW, Yu J, Hu F, Mei J, Wang P, Chu Y, Qi H, Yang M, Dong Z, Sun Y, Hoffman RM, Jia L. Overactivated neddylation pathway as a therapeutic target in lung cancer. J Natl Cancer Inst. 2014 doi: 10.1093/jnci/dju083. [DOI] [PubMed] [Google Scholar]
- Liao H, Liu XJ, Blank JL, Bouck DC, Bernard H, Garcia K, Lightcap ES. Quantitative proteomic analysis of cellular protein modulation upon inhibition of the NEDD8-activating enzyme by MLN4924. Mol Cell Proteomics. 2011;10:M111.009183. doi: 10.1074/mcp.M111.009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Milhollen MA, Smith PG, Narayanan U, Dutta A. NEDD8-targeting drug MLN4924 elicits DNA rereplication by stabilizing Cdt1 in S phase, triggering checkpoint activation, apoptosis, and senescence in cancer cells. Cancer Res. 2010;70:10310–10320. doi: 10.1158/0008-5472.CAN-10-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. The von Hippel–Lindau tumor suppressor protein is a component of an E3 ubiquitin–protein ligase activity. Genes Dev. 1999;13:1822–1833. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D, Pan Y, Ding C, Qian J, Wu L, Chu Y, Yi J, Wang X, Sun Y, Jeong LS, Liu J, Jia L. The Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and apoptosis to suppress liver cancer cell growth. Cancer Res. 2012;72:3360–3371. doi: 10.1158/0008-5472.CAN-12-0388. [DOI] [PubMed] [Google Scholar]
- Lydeard JR, Schulman BA, Harper JW. Building and remodelling Cullin-RING E3 ubiquitin ligases. EMBO Rep. 2013;14:1050–1061. doi: 10.1038/embor.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Chen Y, Zhang F, Yang CY, Wang S, Yu X. RNF111-dependent neddylation activates DNA damage-induced ubiquitination. Mol Cell. 2013;49:897–907. doi: 10.1016/j.molcel.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh C, García-Domínguez DJ, Ordóñez JL, Ginel-Picardo A, Smith PG, Sacristán MP, de Álava E. WEE1 accumulation and deregulation of S-phase proteins mediate MLN4924 potent inhibitory effect on Ewing sarcoma cells. Oncogene. 2013;32:1441–1451. doi: 10.1038/onc.2012.153. [DOI] [PubMed] [Google Scholar]
- Milhollen MA, Traore T, Adams-Duffy J, Thomas MP, Berger AJ, Dang L, Dick LR, Garnsey JJ, Koenig E, Langston SP, Manfredi M, Narayanan U, Rolfe M, Staudt LM, Soucy TA, Yu J, Zhang J, Bolen JB, Smith PG. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-κB- dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- Milhollen MA, Narayanan U, Soucy TA, Veiby PO, Smith PG, Amidon B. Inhibition of NEDD8-activating enzyme induces rereplication and apoptosis in human tumor cells consistent with deregulating CDT1 turnover. Cancer Res. 2011;71:3042–3051. doi: 10.1158/0008-5472.CAN-10-2122. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Dou QP. Advances in the understanding of mechanisms and therapeutic use of bortezomib. Discov Med. 2011;12:471–480. [PMC free article] [PubMed] [Google Scholar]
- Nawrocki ST, Griffin P, Kelly KR, Carew JS. MLN4924: a novel first-in-class inhibitor of NEDD8-activating enzyme for cancer therapy. Expert Opin Investig Drugs. 2012;21:1563–1573. doi: 10.1517/13543784.2012.707192. [DOI] [PubMed] [Google Scholar]
- Nawrocki ST, Kelly KR, Smith PG, Espitia CM, Possemato A, Beausoleil SA, Milhollen M, Blakemore S, Thomas M, Berger A, Carew JS. Disrupting protein NEDDylation with MLN4924 is a novel strategy to target cisplatin resistance in ovarian cancer. Clin Cancer Res. 2013;19:3577–3590. doi: 10.1158/1078-0432.CCR-12-3212. [DOI] [PubMed] [Google Scholar]
- Nawrocki ST, Kelly KR, Smith PG, Keaton M, Carraway H, Sekeres MA, Maciejewski JP, Carew JS. The NEDD8-activating enzyme inhibitor MLN4924 disrupts nucleotide metabolism and augments the efficacy of cytarabine. Clin Cancer Res. 2015;21:439–447. doi: 10.1158/1078-0432.CCR-14-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani H, Sugimoto N, Roukos V, Nakanishi Y, Saijo M, Obuse C, Tsurimoto T, Nakayama KI, Nakayama K, Fujita M, Lygerou Z, Nishimoto T. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 2006;25:1126–1136. doi: 10.1038/sj.emboj.7601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Estelle M. Regulation of cullin-based ubiquitin ligases by the Nedd8/RUB ubiquitin-like proteins. Semin Cell Dev Biol. 2004;15:221–229. doi: 10.1016/j.semcdb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Bladé J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC, Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, Miguel JS, Bladé J, Boccadoro M, Cavenagh J, Alsina M, Rajkumar SV, Lacy M, Jakubowiak A, Dalton W, Boral A, Esseltine DL, Schenkein D, Anderson KC. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- Sarikas A, Hartmann T, Pan ZQ. The cullin protein family. Genome Biol. 2011;12:220. doi: 10.1186/gb-2011-12-4-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Soucy TA, Smith PG, Rolfe M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin Cancer Res. 2009;15:3912–3916. doi: 10.1158/1078-0432.CCR-09-0343. [DOI] [PubMed] [Google Scholar]
- Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- Soucy TA, Dick LR, Smith PG, Milhollen MA, Brownell JE. The NEDD8 conjugation pathway and its relevance in cancer biology and therapy. Genes Cancer. 2010;1:708–716. doi: 10.1177/1947601910382898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Ishikawa S, Kojima M, Liu B. Eradication of pathogenic beta-catenin by Skp1/Cullin/F box ubiquitination machinery. Proc Natl Acad Sci USA. 2003;100:12729–12734. doi: 10.1073/pnas.2133261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist A, Liu G, Mirsaliotis A, Xirodimas DP. Regulation of nucleolar signalling to p53 through NEDDylation of L11. EMBO Rep. 2009;10:1132–1139. doi: 10.1038/embor.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swords RT, Kelly KR, Smith PG, Garnsey JJ, Mahalingam D, Medina E, Oberheu K, Padmanabhan S, O’Dwyer M, Nawrocki ST, Giles FJ, Carew JS. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood. 2010;115:3796–3800. doi: 10.1182/blood-2009-11-254862. [DOI] [PubMed] [Google Scholar]
- Truong LN, Wu X. Prevention of DNA re-replication in eukaryotic cells. J Mol Cell Biol. 2011;3:13–22. doi: 10.1093/jmcb/mjq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson IR, Blanch A, Lin DC, Ohh M, Irwin MS. Mdm2-mediated NEDD8 modification of TAp73 regulates its transactivation function. J Biol Chem. 2006;281:34096–34103. doi: 10.1074/jbc.M603654200. [DOI] [PubMed] [Google Scholar]
- Watson IR, Irwin MS, Ohh M. NEDD8 pathways in cancer, Sine Quibus Non. Cancer Cell. 2011;19:168–176. doi: 10.1016/j.ccr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Wei D, Li H, Yu J, Sebolt JT, Zhao L, Lawrence TS, Smith PG, Morgan MA, Sun Y. Radiosensitization of human pancreatic cancer cells by MLN4924, an investigational NEDD8-activating enzyme inhibitor. Cancer Res. 2012;72:282–293. doi: 10.1158/0008-5472.CAN-11-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Zhang M, He S, Lu K, Chen Y, Xing G, Lu Y, Liu P, Li Y, Wang S, Chai N, Wu J, Deng H, Wang HR, Cao Y, Zhao F, Cui Y, Wang J, He F, Zhang L. The covalent modifier Nedd8 is critical for the activation of Smurf1 ubiquitin ligase in tumorigenesis. Nat Commun. 2014;5:3733. doi: 10.1038/ncomms4733. [DOI] [PubMed] [Google Scholar]
- Xirodimas DP. Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans. 2008;36:802–806. doi: 10.1042/BST0360802. [DOI] [PubMed] [Google Scholar]
- Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell. 2004;118:83–97. doi: 10.1016/j.cell.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Xirodimas DP, Sundqvist A, Nakamura A, Shen L, Botting C, Hay RT. Ribosomal proteins are targets for the NEDD8 pathway. EMBO Rep. 2008;9:280–286. doi: 10.1038/embor.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kitagaki J, Dai RM, Tsai YC, Lorick KL, Ludwig RL, Pierre SA, Jensen JP, Davydov IV, Oberoi P, Li CC, Kenten JH, Beutler JA, Vousden KH, Weissman AM. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 2007;67:9472–9481. doi: 10.1158/0008-5472.CAN-07-0568. [DOI] [PubMed] [Google Scholar]
- Yang D, Tan M, Wang G, Sun Y. The p21-dependent radiosensitization of human breast cancer cells by MLN4924, an investigational inhibitor of NEDD8 activating enzyme. PLoS One. 2012;7:e34079. doi: 10.1371/journal.pone.0034079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Li L, Liu H, Wu L, Luo Z, Li H, Zheng S, Gao H, Chu Y, Sun Y, Liu J, Jia L. Induction of autophagy and senescence by knockdown of ROC1 E3 ubiquitin ligase to suppress the growth of liver cancer cells. Cell Death Differ. 2013;20:235–247. doi: 10.1038/cdd.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WT, Wu JF, Yu GY, Wang R, Wang K, Li LH, Chen P, Jiang YN, Cheng H, Lee HW, Yu J, Qi H, Yu XJ, Wang P, Chu YW, Yang M, Hua ZC, Ying HQ, Hoffman RM, Jeong LS, Jia LJ. Suppression of tumor angiogenesis by targeting the protein neddylation pathway. Cell Death Dis. 2014;5:e1059. doi: 10.1038/cddis.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun Y. Targeting the mTOR-DEPTOR pathway by CRL E3 ubiquitin ligases: therapeutic application. Neoplasia. 2012;14:360–367. doi: 10.1593/neo.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xiong X, Jia L, Sun Y. Targeting Cullin-RING ligases by MLN4924 induces autophagy via modulating the HIF1-REDD1-TSC1-mTORC1-DEPTOR axis. Cell Death Dis. 2012;3:e386. doi: 10.1038/cddis.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Huang F, Chiang YJ, Li M, Du J, Ding Y, Zhang T, Lee HW, Jeong LS, Chen Y, Deng H, Feng XH, Luo S, Gao C, Chen YG. c-Cbl-mediated neddylation antagonizes ubiquitination and degradation of the TGF-β type II receptor. Mol Cell. 2013;49:499–510. doi: 10.1016/j.molcel.2012.12.002. [DOI] [PubMed] [Google Scholar]