Abstract
Osteoclasts are responsible for physiological bone remodeling as well as pathological bone destruction in osteoporosis, periodontitis and rheumatoid arthritis, and thus represent a pharmacological target for drug development. We aimed to characterize and compare the cytokine-induced osteoclastogenesis of bone marrow and spleen precursors. Established protocols used to generate osteoclasts from bone marrow were modified to examine osteoclastogenesis of the spleen cells of healthy mice. Osteoclast formation was successfully induced from spleen precursors using receptor activator of nuclear factor κB ligand (50 ng/ml) and macrophage colony stimulating factor (50 ng/ml). Compared to bone marrow cultures, differentiation from spleen required a longer cultivation time (9 days for spleen, as compared to 5 days for marrow cultures) and a higher plating density of non-adherent cells (75,000/cm2 for spleen, as compared to 50,000/cm2 for bone marrow). Osteoclasts generated from spleen precursors expressed osteoclast marker genes calcitonin receptor, cathepsin K and matrix metalloproteinase 9 and were capable of resorbing hydroxyapatite. The differentiation capacity of spleen and bone marrow precursors was comparable for BALB/c, C57BL/6 and FVB mice. We also developed and tested a cryopreservation protocol for the osteoclast precursors. While 70–80 % of cells were lost during the first week of freezing, during the subsequent 5 weeks the losses were within 2–5 % per week. Osteoclastogenesis from the recovered bone marrow precursors was successful up to 5 weeks after freezing. Spleen precursors retained their osteoclastogenic capacity for 1 week after freezing, but not thereafter. The described protocol is useful for the studies of genetically modified animals as well as for screening new osteoclast-targeting therapeutics.
Keywords: Osteoclastogenesis, Spleen, Bone marrow, Cryopreservation, Protocol
Introduction
Osteoclasts are responsible for the degradation of bone during the physiological processes of skeletal modeling and remodeling as well as tooth eruption (Park et al. 2013; Vaananen et al. 2000). Osteoclasts also play a critical role in diseases associated with abnormal bone and joint destruction, such as rheumatoid arthritis, periodontitis and cancer metastasis to bone (Boyle et al. 2003; Feng and Zhou 2005; Park et al. 2013). As mediators of pathological destruction, osteoclasts represent a valuable pharmacological target for drug development. Receptor activator of nuclear factor κB ligand (RANKL) has been identified as a key factor regulating osteoclastogenesis (Boyce 2013; Dimitroulas et al. 2013; Tiedemann et al. 2009). Since this discovery, protocols for differentiating osteoclasts from bone marrow precursors using RANKL and macrophage colony stimulating factor (MCSF) have become standard in pharmacological studies (Boyle et al. 2003; Russell et al. 1999; Tiedemann et al. 2013). However, for studies targeting the earlier stages of osteoclastogenesis, a source of more immature osteoclast precursors is required. Spleen cells have previously been successfully used to generate osteoclasts in co-culture systems (Chen et al. 2011; Lee et al. 2002; Li et al. 2000, 2002; Okada et al. 2000; Spencer et al. 2006). The cytokine-induced osteoclastogenesis from spleen cells has been used in previous studies in genetically modified animal models (Holloway et al. 2002; Li et al. 2000; Okada et al. 2000; Takahashi et al. 1988). However, the osteoclast differentiation protocols used in these studies were only briefly described in the methods section, and detailed validation and comparison of osteoclastogenesis from different precoursor sources has not been previously described. Well-validated protocols for osteoclastogenesis from different sources will be instrumental for the analysis of the contribution of the bone microenvironment to anomalous osteoclastogenesis in genetically modified animal models.
The primary goal of this study was to develop a protocol for the differentiation of osteoclasts from spleen precursors and to compare it to standard bone marrow-derived osteoclastogenesis. Gene modifications are performed on mouse strains of different backgrounds, and it is known that the strain of the mouse affects its bone parameters, including microarchitecture, bone density and mechanical properties (Beamer et al. 1996; Buie et al. 2008; Holguin et al. 2013), which in turn are affected by the differentiation capacity and activity of bone cells, osteoclast and osteoblasts. Therefore, we examined the performance of osteoclast differentiation protocol in three different mous strains: FVB, BALB/c and C57BL/6, which are commonly used in the genetic studies of bone phenotype (Chen et al. 2014; Gowen et al. 1999; Li et al. 2002). Finally, we developed a protocol for the successful cryopreservation of bone marrow and spleen-derived osteoclast precursors.
Materials and methods
Osteoclast culture
Animal studies were conducted in compliance with McGill University guidelines, established by the Canadian Council on Animal Care. Primary mouse bone marrow osteoclast culture was performed as described previously (Tiedemann et al. 2009).
Protocol for bone marrow precursor isolation and culture:
6–17 week-old male and female FVB, C57BL/6 and BALB/c mice (Charles River, St. Constant, QC, Canada) were euthanized, and the femur and tibia were dissected.
The bones were rinsed with 10 ml of phosphate-buffered saline (PBS: 140 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) and cut in half. The halves were placed into longitudinally-cut 1 ml pipette tips (1 cm), which were then placed in eppendorf tubes and centrifuged at 12,000 rpm, 3 times for 30 s, to isolate the bone marrow cells.
The pipette tips with the bone pieces were removed and the marrow was thoroughly disintegrated by repeated pipetting into 200 μl of culture medium (αMEM; GIBCO: 12,000-022, Burlington, ON, Canada) supplemented with 10 % FBS (Wisent; 080152, St-Bruno, QC, Canada), 1 % sodium pyruvate (Wisent; 600-110-EL), and 1 % penicillum, streptomycin (Wisent; 450-201-EL) and 0.02 M sodium bicarbonate (Sigma Aldrich Ltd; S5761-500G, Oakville, ON, Canada). The marrow was then transferred to a conical tube containing 10 ml of culture medium.
The bone marrow suspension was centrifuged at 1,000 rpm for 5 min. The resulting cell pellet was redissolved in 1 ml of red blood cell (RBC) lysis buffer (Sigma Aldrich Ltd; R7757), incubated for 10 min on ice and washed two times by centrifugation at 1,000 rpm for 5 min with 10 ml of medium.
The remaining bone marrow cells were resuspended in 10 ml of culture medium supplemented with MCSF (25 ng/ml, Peprotech: 300-25, Rocky Hill, NJ, USA), plated at 20–30 × 106 cells per 75 cm2 flask and incubated overnight.
The cell medium was collected and centrifuged at 1,000 rpm for 5 min. The resulting pellet of non-adherent cells was resuspended in 10 ml of culture medium and viable precursors were counted using trypan blue (Thermo scientific: SV30084.01, Logan, UT, USA) and a hemocytometer. Although Trypan Blue does not account for apoptotic cells with intact membranes (Liegler et al. 1995), it provides sufficient accuracy in identifying live cells to be routinely used in the most osteoclast culture protocols (Huh et al. 2005; Lum et al. 1999).
Precursors were plated at 25–150 × 103 cells/cm2, supplemented with MCSF (50 ng/ml) and RANKL (25–100 ng/m), and incubated at 37 °C and 5 % CO2. The medium was changed on day 3 with culture medium containing fresh MCSF (50 ng/ml) and RANKL (25–100 ng/m), and on day 5 mature osteoclasts are generally observed.
Protocol for spleen precursor isolation and culture:
6–17 week-old male and female FVB, C57BL/6 and BALB/c mice (Charles River) were euthanized and their spleens were dissected. The dissected spleens were then placed in a 1.5 ml eppendorf tube containing 1 ml of PBS, and kept for no longer than 45 min before cell isolation.
The spleens were transferred to a petri dish with 1–2 ml of culture medium, cut into small pieces using surgical sterile scissors, and compressed using the plunger of a 1 ml syringe in circular motion against bottom of the petri dish, until no macroscopic pieces of the spleen were visible.
The suspension of cells was filtrated through a 40 µm nylon mesh (Fisher Scientific; 22363547, Nepean, ON, Canada), and transferred to a 50 ml tube to which 8 ml of culture medium was added.
The spleen cell suspension was centrifuged at 1,000 rpm for 5 min, after which the cell pellet was redissolved in 2 ml of red blood cell (RBC) lysis buffer (Sigma Aldrich Ltd.; R7757). The suspension was then incubated for 10 min on ice and washed two times by centrifugation at 1,000 rpm for 5 min with 10 ml of medium.
The remaining spleen cells were resuspended in 10 ml of culture medium supplemented with MCSF (25 ng/ml), plated at 20–30 × 106 cells per 75 cm2 flask and incubated overnight.
The cell medium was collected and centrifuged at 1,000 rpm for 5 min. The resulting pellet of non-adherent cells was resuspended in 10 ml of culture medium and viable precursors were counted using trypan blue and a hemocytometer.
Precursors were plated at 25–150 × 103 cells/cm2, supplemented with MCSF (50 ng/ml) and RANKL (25–100 ng/m) and incubated at 37 °C and 5 % CO2. The medium was changed on day 3, 5 and 7 with culture medium containing fresh MCSF (50 ng/ml) and RANKL (25–100 ng/m), and on day 9 mature osteoclasts are generally observed.
Quantification of osteoclasts
Mature osteoclast cultures were fixed using 10 % formalin in PBS pH 7.4, for 8–10 min at room temperature and stained for tartrate resistant acid phosphatase (TRAP) using commercial kit (TRAP, Sigma: 387A-KT), 50 μl of Fast Garnet GBC Base Solution and 50 μl of sodium nitrite was mixed for 30 s, left to stand at room temperature for 2 min and added to 4.5 ml of prewarmed deionized water (37 °C). Then, 50 μl of naphthol AS-BI phosphate solution, 200 μl of acetate solution and 200 μl of tartrate solution were added. The samples were incubated with the complete solution for 15–20 min at 37 °C. Osteoclasts were identified as TRAP positive cells with three or more nuclei and a defined membrane.
Quantification of resorption
Calcium phosphate was coated on tissue culture plates and mature osteoclasts derived from the bone marrow or spleen cells were transferred as described previously (Maria et al. 2014). After incubation for 2 days with RANKL (50 ng/ml) at 37 °C and 5 % CO2, the cells were fixed using 10 % formalin, stained for TRAP and osteoclast numbers were quantified. The cells were removed by incubation with 10 % sodium hypochlorite for 3–5 min. The substrates were washed with deionized water three times, and left to dry. Ten images of resorption areas per condition were taken and planar areas of single resorption pits were measured using ImageJ software. The average area of single pit and the average resorption area per 1 mm2 of substrate were assessed.
RNA isolation, qRT PCR
Total RNA was isolated from bone marrow- and spleen-derived osteoclasts with the RNeasyMini Kit (Qiagen Inc.:74106, Toronto, ON, Canada). RNA and cDNA concentration was quantified with the Quanti-iT™ instrument (Invitrogen: Q32860, Burlington, ON, Canada). Reverse-transcription with 0.75 ng of RNA was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Fisher Scientific; Nepean, ON, Canada). Real-time PCR was performed using a 7500 Applied Biosystems Instrument, the TaqMan Universal PCR Master Mix (Applied Biosystems, Burlington, ON, Canada) and the following TaqMan probes: calcitonin receptor (Mm00432271_m1), cathepsin K (Mm00484039_m1) and matrix metalloproteinase 9 (Mm00600163_m1). Glyceraldehyde 3-phosphate dehydrogenase (Mm99999915_g1) was used as the endogenous control, and untreated bone marrow precursors were used as the calibrator.
Freezing and thawing osteoclast precursors
Freezing protocol:
After step 6 of the isolation protocol, the cells were centrifuged at 1,000 rpm for 5 min.
The pellet was resuspended in 500 μl of FBS (Wisent; 080152, St-Bruno, QC, Canada) and after a freezing solution containing 400 μl of culture medium and 100 μl of DMSO (Fisher Scientific; BP231-100, Nepean, ON, Canada) was added dropwise.
The cryovials were placed in a Cryo 1 °C Mr. Frosty freezing container (Nalgene; 5100-0001, Rochester, NY, USA), at −80 °C for 24 h.
The cryovials were then transferred to liquid nitrogen for 1–7 weeks.
Thawing protocol:
The cryovial was transferred from the liquid nitrogen directly to the 37 °C water bath until 50–75 % of the cells were thawed.
The cells were transferred to a 50 ml tube, 9 ml of pre-warmed medium was added dropwise and the cells were centrifuged at 1,000 rpm for 5 min.
The pellet was resuspended in 10–12 ml of culture medium supplemented with MCSF (50 ng/ml), plated onto a 75 cm2 flask and incubated overnight at 37 °C and 5 % CO2.
The cell medium was collected and centrifuged at 1,000 rpm for 5 min. The resulting pellet of non-adherent cells was resuspended in 10 ml of culture medium and viable precursors were counted using trypan blue and a hemocytometer.
Osteoclastogenesis was induced as described in step 7 of osteoclast culture protocol.
Statistics
Data are presented as mean ± standard error of the mean with n indicating the number of independent experiments. The experiments were run in triplicates. Differences were assessed by two-way ANOVA or Student’s t test, and accepted as statistically significant at p < 0.05.
Results and discussion
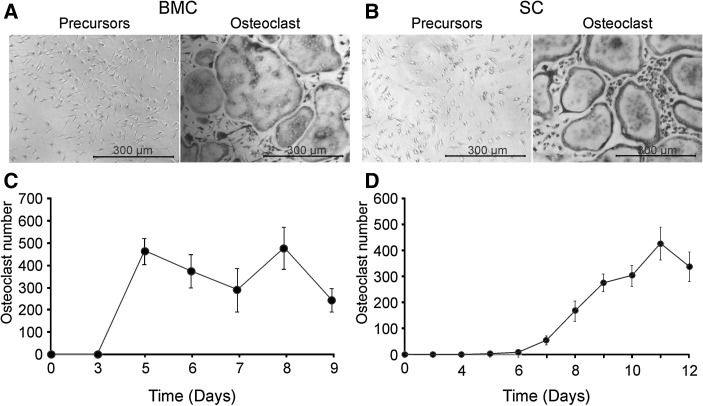
We first compared the time course of osteoclastogenesis from FVB bone marrow and spleen precursors, plated at a density of 50,000 cells/cm2 for bone marrow and 75,000 cells/cm2 for spleen precursors and cultured with MCSF (50 ng/ml) and RANKL (50 ng/ml) (Fig. 1). When bone marrow precursors were used, well-formed osteoclasts were visible by day 5 of culture (Fig. 1a, c). In contrast, osteoclastogenesis from spleen precursors occurred over a longer period of time, resulting in successful osteoclast formation only by day 8–12 (Fig. 1b, d). These data suggest that (1) the frequency of osteoclast precursors in spleen is likely lower than in bone marrow, and (2) the spleen precursors require more time to differentiate, which likely reflects their more immature state. Previous studies demonstrated that osteoclast precursors from bone marrow and spleen are regulated by the same main factors, RANK, RANKL and OPG (Dougall et al. 1999; Kong et al. 1999; Zehnder et al. 2006). However, the differences in osteoclasts generated from different precursor pools were noted both in their gene expression profiles (Hayashi et al. 1997, 2003), and in their responsiveness to different stimuli (Hayashi et al. 2003; Rittling et al. 1998). In addition, the interactions between osteoclast precursors and other cells present in the preparations from spleen and bone marrow can affect the differentiation dynamics of osteoclast precursors.
Fig. 1.
Osteoclastogenesis from bone marrow- and spleen-derived precursors. Bone marrow (BMC) and spleen cells (SC) from FVB mice were cultured overnight with MCSF (25 ng/ml). The following day, non-adherent precursors were plated at 50,000 cells/cm2 for bone marrow and 75,000 cell/cm2 for spleen cells and treated with MCSF (50 ng/ml) and RANKL (50 ng/ml) for 3–9 days for bone marrow and 3–12 days for spleen cells. Samples were fixed, and stained for TRAP. a, b Representative images of untreated cultures (left) and RANKL-treated cultures (right) generated from bone marrow precursors on day 5 (a) and from spleen precursors on day 9 (b). c, d Changes in average numbers of osteoclasts with time in cultures from bone marrow (c) and spleen (d) precursors. Data are mean ± SE, n = 5–25 experiments
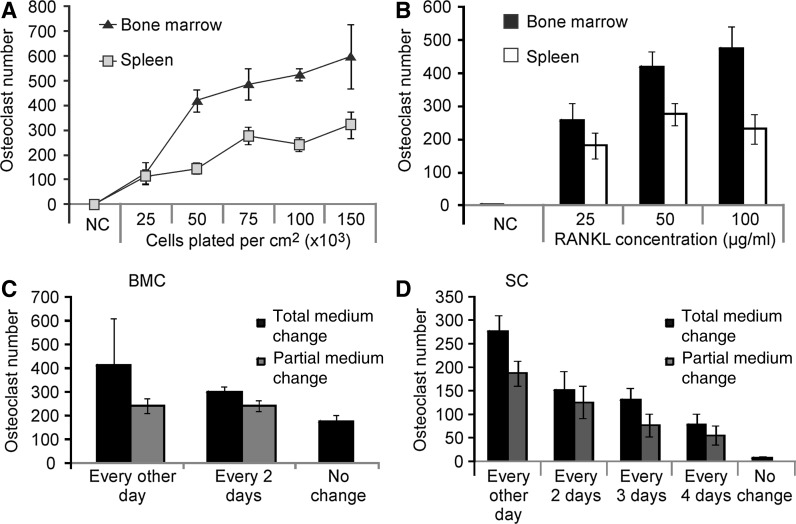
In order to optimize osteoclastogenesis in bone marrow and spleen cultures, we varied the plating density, amount of RANKL added and medium change frequency (Fig. 2). We terminated osteoclastogenesis from spleen cultures on day 9, despite the fact that more osteoclasts are formed by day 12, because prolonged cultures require two additional medium changes, which is not economically practical. The plating density of 50,000 cells/cm2 for bone marrow and 75,000 cells/cm2 for spleen precursors was sufficient to generate maximal levels of osteoclasts (Fig. 2a). Osteoclastogenesis was not significantly affected by RANKL above 50 ng/ml (Fig. 2b). Since the osteoclastogenic cytokines are expensive, we examined if their consumption could be optimized by varying the medium change frequency as well as the fraction of medium being changed. Complete medium changes every other day resulted in the highest numbers of osteoclasts formed from both bone marrow and spleen precursors (Fig. 2c, d). However, when we decreased the medium change frequency to every 2–3 days or employed a partial (50 %) medium change, we still observed prominent osteoclastogenesis (Fig. 2c, d). For bone marrow cultures, even without a single medium change during 5 days of culture, the formation of more than 100 osteoclasts per cm2 was evident (Fig. 2c). Thus, the optimal conditions for osteoclastogenesis from mouse bone marrow and spleen precursors, respectively, are as follows: plating density 50,000 and 75,000 cells/cm2; 50 ng/ml RANKL; 5 days and 9 days of culture; complete medium change every 2–3 days.
Fig. 2.
Optimization of cell culture conditions. a Non-adherent bone marrow or spleen precursors from FVB were plated at the indicated densities, treated with MCSF (50 ng/ml) and RANKL (50 ng/ml) for 5 days for the bone marrow and 9 days for the spleen cells, fixed, and stained for TRAP. b Bone marrow and spleen precursors from FVB mice were treated for 5 and 9 days, respectively, with MCSF (50 ng/ml) and RANKL (0, 25, 50 and 100 ng/ml), fixed, and stained for TRAP. c, d Bone marrow cells (BMC) were plated at 50,000 cells/cm2 (c) and spleen cells (SC) at 75,000 cells/cm2 (d), and treated with MCSF (50 ng/ml) and RANKL (50 ng/ml). The medium was changed either completely (90 %) or partially (50 %) at the indicated frequencies. Data are mean ± SE, n = 3–19 experiments, for a and b bone marrow and spleen groups were significantly different (p < 0.001) by two-way ANOVA
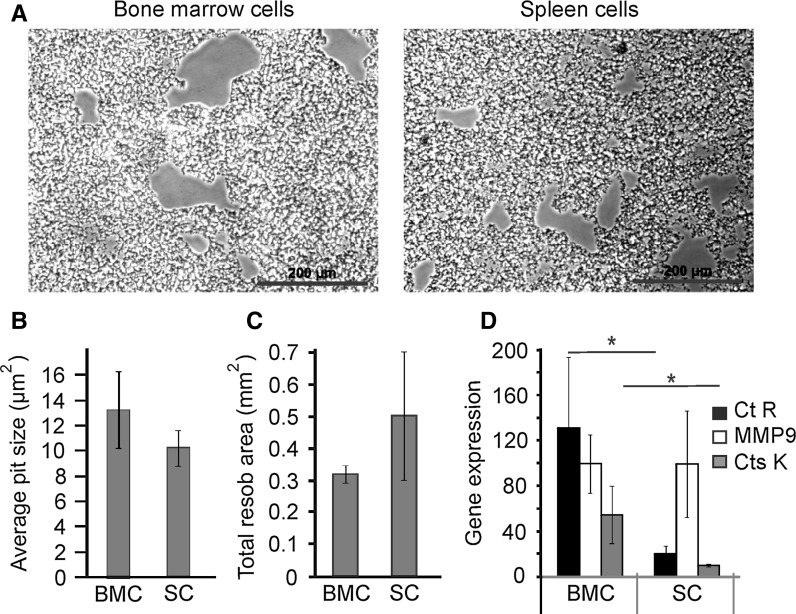
To further confirm the functionality of osteoclasts generated from bone marrow and spleen, we examined their resorptive activity and the expression of osteoclast marker genes (Fig. 3). We transferred mature bone marrow- and spleen-derived osteoclasts onto hydroxyapatite-coated plates and after 48 h of culture evaluated the pits generated by osteoclast resorption (Fig. 3a). The average pit size and total area resorbed by the bone marrow- and spleen-derived osteoclasts was not significantly different (Fig. 3b, c). We next examined the expression of calcitonin receptor (Ct R), matrix metalloproteinase (MMP) 9 and cathepsin K (Cts K). Undifferentiated bone marrow cells express very low levels of Ct R, MMP9 and Cts K, while in undifferentiated spleen cells no detectable expression was observed. In contrast, osteoclasts generated from both bone marrow and spleen precursors exhibited robust expression of these markers (Fig. 3d). While the expression levels of MMP9 were similar in bone marrow- and spleen-derived osteoclasts, the levels of Ct R and Cts K were significantly lower in osteoclasts derived from spleen. However, importantly, these differences did not compromise the resorptive activity of spleen-derived osteoclasts.
Fig. 3.
Bone marrow- and spleen-derived osteoclasts express functional markers. Bone marrow and spleen precursors from FVB mice were treated with MCSF (50 ng/ml) and RANKL (50 ng/ml) for 5 days for bone marrow and 9 days for spleen cells. a–c Mature osteoclasts from FVB mice were transferred to calcium phosphate-coated plates, incubated for 48 h, and removed using 10 % sodium hypochlorite. a Representative images of resorption areas generated on calcium phosphate by bone marrow- and spleen-derived osteoclasts. b, c The average area of a single resorption pit (b) and average resorption area per 1 mm2 of substrate (c) generated by bone marrow- (BMC) and spleen- (SC) derived osteoclasts was assessed. Data are mean ± SE, n = 3–6 experiments. d mRNA was extracted and the expression of calcitonin receptor (CtR), cathepsin K (Cts K), and matrix metalloproteinase-9 (MMP9) was assessed, Data are mean ± SE, n = 3 experiments. *p < 0.05 as assessed by Student t test
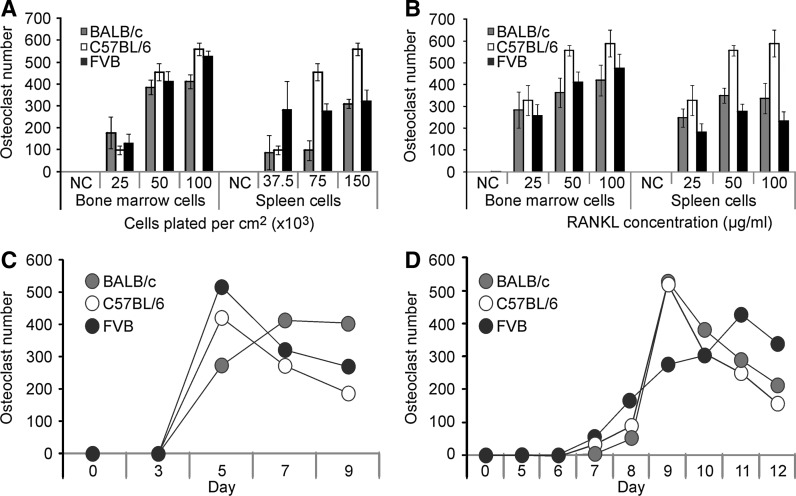
One potential application of a method to assess spleen-derived osteoclastogenesis, is to examine the contribution of the bone microenvironment to altered osteoclastogenesis in genetically modified mice, which can be produced on different background strains. We tested if osteoclastogenesis from bone marrow and spleen precursors is affected by the mouse strain (Fig. 4). We examined the influence of plating density (Fig. 4a) and RANKL (Fig. 4b) on generating osteoclasts from the bone marrow and spleen of BALB/c, C57BL/6 and FVB mice. Bone marrow- and spleen-derived osteoclastogenesis was similar in these three mouse strains, except for a higher capacity of C57BL/6 mice for spleen osteoclastogenesis at higher plating density (150,000 cells/cm2). Similarly, the time course of osteoclastogenesis from BALB/c, C57BL/6 and FVB bone marrow (Fig. 4c) and spleen (Fig. 4d) precursors did not significantly differ when precursors from different mouse strains were used. Thus, the protocol developed in this study can be successfully used with different strains of mice.
Fig. 4.
Comparison of osteoclastogenesis from different mouse strains. Osteoclast precursors were isolated from the bone marrow and spleen of BALB/c (grey), C57BL/6 (white) and FVB (black) mice. a The average number of osteoclasts generated from bone marrow or spleen precursors, which were plated at the indicated densities and treated with MCSF (50 ng/ml) and RANKL (50 ng/ml) for 5 days for the bone marrow and 9 days for the spleen cells. b The average number of osteoclasts generated from bone marrow or spleen precursors, which were treated for 5 and 9 days, respectively, with MCSF (50 ng/ml) and RANKL (0, 25, 50 and 100 ng/ml). c, d Bone marrow cells were plated at 50,000 cells/cm2 (c) and spleen cells at 75,000 cells/cm2 (d), and treated with MCSF (50 ng/ml) and RANKL (50 ng/ml). Changes in the average numbers of osteoclasts over time were assessed. For a–d, data are mean ± SE, n = 3–19 experiments
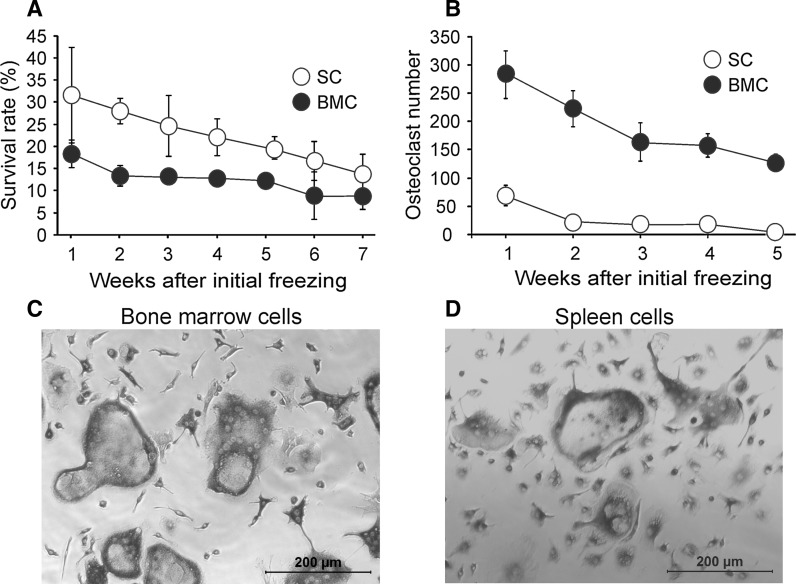
We next examined if bone marrow and spleen-derived precursors are amenable for cryopreservation. Non-adherent precursors were frozen using the protocol adapted from previous studies (Deng et al. 2004; Pagliaro et al. 1998; Risom and Knudsen 1999). The cells were thawed after 1–7 weeks of cryopreservation and their survival and osteoclastogenic capacity were examined (Fig. 5). The precursor survival, estimated as the percentage of recovered cells from the number of frozen cells, demonstrated an initial loss of 70 % of the cells, followed by the progressive decline from 20 to 10 % for bone marrow and from 30 to 15 % for spleen precursors (Fig. 5a). Nevertheless, large numbers of well-formed osteoclasts were obtained from the frozen bone marrow precursors, even 5 weeks after freezing (Fig. 5b). In contrast, frozen spleen precursors demonstrated little osteoclastogenic capacity beyond 1 week of freezing. While the exact reason for the loss of osteoclast precursors during cryopreservation of spleen cells, but not bone marrow cells is unclear, it has been previously documented that similar cells from different sources, such as hematopoietic stem cells and embryonic stem cells, exhibit different tolerance for cryopreservation and require individual optimization of the technique (Hunt 2011). Exposure to low temperatures was demonstrated to induce the production of reactive oxygen species, activation of caspases 8 and 9 and apoptosis (Boer et al. 2002; Heng et al. 2006; Xu et al. 2010). Therefore, our data suggest that spleen-derived osteoclast precursors may be less resistant to apoptosis leading to their cell death following cryopreservation. Nevertheless, the protocol developed in this study allows for the successful long-term cryopreservation of bone marrow osteoclast precursors.
Fig. 5.
Cryopreservation of osteoclast precursors. Bone marrow (BMC) and spleen cells (SC) from FVB mice were cultured overnight with MCSF (25 ng/ml). Non-adherent precursors were counted, washed, re-suspended in a solution containing 50 % FBS, 10 % DMSO and 40 % culture medium, placed at −80 °C for 24 h and then transferred to liquid nitrogen for 1–7 weeks. a, b The vials were thawed at 37 °C degrees in a water bath until 75 % of the vial was thawed, transferred to a 50 ml tube to which 9 ml of culture medium was added drop wise, centrifuged for 5 min at 1,000 rpm, re-suspended in 10–12 ml of culture medium and cultured in 75 mm2 flask with MCSF (50 ng/ml) for 24 h. a Non-adherent cells were collected and the number of viable cells was counted and normalized to the number of frozen cells. b Viable precursors were plated at 50,000 cells/cm2 for bone marrow and at 75,000 cells/cm2 for spleen precursors, treated with MCSF (50 ng/ml) and RANKL (50 ng/ml) during 5 and 9 days for bone marrow and spleen cells, respectively, and osteoclast numbers were assessed. For a, b data are mean ± SE, n = 3–11 experiments, bone marrow and spleen groups were significantly different (p < 0.001) by two-way ANOVA. c, d Representative images of osteoclasts generated from bone marrow (c) and spleen (d) precursors following cryopreservation
Conclusion
We have validated the protocol for generating osteoclasts from spleen cells, and confirmed that it can be successfully used with different mouse strains. In addition, we have optimized the protocol for the cryopreservation of freshly isolated osteoclast precursors, and confirmed successful osteoclastogenesis from frozen bone marrow precursors for up to 5 weeks and spleen precursors for up to 1 week. These protocols are instrumental for the analysis of osteoclast phenotype in genetically modified animals, as well as for performing standardized pharmaceutical research.
Acknowledgments
We are grateful to Dr. Morris F. Manolson, University of Toronto, for providing GST-RANKL clones. This work was supported by the Canadian Institutes of Health Research. IBD is supported by the Faculty of Dentistry, McGill University. SVK holds a Canada Research Chair in Osteoclast Biology.
References
- Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- Boer FD, Dräger AM, Pinedo HM, Kessler FL, Monnee-van Muijen M, Weijers G, Westra G, Wall EVD, Netelenbos T, Oberink JW, Huijgens PC, Schuurhuis GJ. Early apoptosis largely accounts for functional impairment of CD34+ cells in frozen-thawed stem cell grafts. J Hematother Stem Cell Res. 2002;11:951–963. doi: 10.1089/152581602321080619. [DOI] [PubMed] [Google Scholar]
- Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92:860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Buie HR, Moore CP, Boyd SK. Postpubertal architectural developmental patterns differ between the L3 vertebra and proximal tibia in three inbred strains of mice. J Bone Miner Res. 2008;23:2048–2059. doi: 10.1359/jbmr.080808. [DOI] [PubMed] [Google Scholar]
- Chen H, Gilbert LC, Lu X, Liu Z, You S, Weitzmann MN, Nanes MS, Adams J. A new regulator of osteoclastogenesis: estrogen response element–binding protein in bone. J Bone Miner Res. 2011;26:2537–2547. doi: 10.1002/jbmr.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Guo R, Itoh S, Moreno L, Rosenthal E, Zappitelli T, Zirngibl RA, Flenniken A, Cole W, Grynpas M, Osborne LR, Vogel W, Adamson L, Rossant J, Aubin JE. First mouse model for combined osteogenesis imperfecta and ehlers–danlos syndrome. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2177. [DOI] [PubMed] [Google Scholar]
- Deng W, Bivalacqua TJ, Chattergoon NN, Jeter JR, Kadowitz PJ. Engineering ex vivo-expanded marrow stromal cells to secrete calcitonin gene-related peptide using adenoviral vector. Stem Cells. 2004;22:1279–1291. doi: 10.1634/stemcells.2004-0032. [DOI] [PubMed] [Google Scholar]
- Dimitroulas T, Nikas SN, Trontzas P, Kitas GD. Biologic therapies and systemic bone loss in rheumatoid arthritis. Autoimmun Rev. 2013;12:958–966. doi: 10.1016/j.autrev.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Zhou H (2005) Osteoclast biology. Curr Top Bone Biol 71–93
- Gowen M, Lazner F, Dodds R, Kapadia R, Feild J, Tavaria M, Bertoncello I, Drake F, Zavarselk S, Tellis I, Hertzog P, Debouck C, Kola I. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res. 1999;14:1654–1663. doi: 10.1359/jbmr.1999.14.10.1654. [DOI] [PubMed] [Google Scholar]
- Hayashi S-I, Miyamoto A, Yamane T, Kataoka H, Ogawa M, Sugawara S, Nishikawa S, Nishikawa S-I, Sudo T, Yamazaki H, Kunisada T. Osteoclast precursors in bone marrow and peritoneal cavity. J Cell Physiol. 1997;170:241–247. doi: 10.1002/(SICI)1097-4652(199703)170:3<241::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Hayashi S-I, Yamada T, Tsuneto M, Yamane T, Takahashi M, Shultz LD, Yamazaki H. Distinct osteoclast precursors in the bone marrow and extramedullary organs characterized by responsiveness to toll-like receptor ligands and TNF-α. J Immunol. 2003;171:5130–5139. doi: 10.4049/jimmunol.171.10.5130. [DOI] [PubMed] [Google Scholar]
- Heng B, Ye C, Liu H, Toh W, Rufaihah A, Yang Z, Bay B, Ge Z, Ouyang H, Lee E, Cao T. Loss of viability during freeze–thaw of intact and adherent human embryonic stem cells with conventional slow-cooling protocols is predominantly due to apoptosis rather than cellular necrosis. J Biomed Sci. 2006;13:433–445. doi: 10.1007/s11373-005-9051-9. [DOI] [PubMed] [Google Scholar]
- Holguin N, Brodt M, Sanchez M, Kotiya A, Silva M. Adaptation of tibial structure and strength to axial compression depends on loading history in both C57BL/6 and BALB/c mice. Calcif Tissue Int. 2013;93:211–221. doi: 10.1007/s00223-013-9744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17:200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- Huh YJ, Kim JM, Kim H, Song H, So H, Lee SY, Kwon SB, Kim HJ, Kim HH, Lee SH, Choi Y, Chung SC, Jeong DW, Min BM. Regulation of osteoclast differentiation by the redox-dependent modulation of nuclear import of transcription factors. Cell Death Differ. 2005;13:1138–1146. doi: 10.1038/sj.cdd.4401793. [DOI] [PubMed] [Google Scholar]
- Hunt CJ. Cryopreservation of human stem cells for clinical application: a review. Transfus Med Hemother. 2011;38:107–123. doi: 10.1159/000326623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y-Y, Yoshida H, Sarosi I, Tan H-L, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL Is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Lee S-K, Kalinowski J, Jastrzebski S, Lorenzo JA. 1,25 (OH)2 vitamin D3-stimulated osteoclast formation in spleen-osteoblast cocultures is mediumted in part by enhanced IL-1α and receptor activator of NF-κB ligand production in osteoblasts. J Immunol. 2002;169:2374–2380. doi: 10.4049/jimmunol.169.5.2374. [DOI] [PubMed] [Google Scholar]
- Li X, Okada Y, Pilbeam CC, Lorenzo JA, Kennedy CRJ, Breyer RM, Raisz LG. Knockout of the murine prostaglandin EP2 receptor impairs osteoclastogenesis in vitro. Endocrinology. 2000;141:2054–2061. doi: 10.1210/endo.141.6.7518. [DOI] [PubMed] [Google Scholar]
- Li X, Pilbeam CC, Pan L, Breyer RM, Raisz LG. Effects of prostaglandin E2 on gene expression in primary osteoblastic cells from prostaglandin receptor knockout mice. Bone. 2002;30:567–573. doi: 10.1016/S8756-3282(02)00683-X. [DOI] [PubMed] [Google Scholar]
- Liegler TJ, Hyun W, Yen TS, Stites DP. Detection and quantification of live, apoptotic, and necrotic human peripheral lymphocytes by single-laser flow cytometry. Clin Diagn Lab Immunol. 1995;2:369–376. doi: 10.1128/cdli.2.3.369-376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L, Wong BR, Josien R, Becherer JD, Erdjument-Bromage H, Schlöndorff J, Tempst P, Choi Y, Blobel CP. Evidence for a role of a tumor necrosis factor-α (TNF-α)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J Biol Chem. 1999;274:13613–13618. doi: 10.1074/jbc.274.19.13613. [DOI] [PubMed] [Google Scholar]
- Maria SM, Prukner C, Sheikh Z, Mueller F, Barralet JE, Komarova SV (2014) Reproducible quantification of osteoclastic activity: characterization of a biomimetic calcium phosphate assay. J Biomed Mater Res B Appl Biomater. 102:903–912. doi:10.1002/jbm.b.33071 [DOI] [PubMed]
- Okada Y, Lorenzo JA, Freeman AM, Tomita M, Morham SG, Raisz LG, Pilbeam CC. Prostaglandin G/H synthase-2 is required for maximal formation of osteoclast-like cells in culture. J Clin Invest. 2000;105:823–832. doi: 10.1172/JCI8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaro LC, Liu B, Munker R, Andreeff M, Freireich EJ, Scheinberg DA, Rosenblum MG. Humanized M195 monoclonal antibody conjugated to recombinant gelonin: an anti-CD33 immunotoxin with antileukemic activity. Clin Cancer Res. 1998;4:1971–1976. [PubMed] [Google Scholar]
- Park S-J, Bae H-S, Cho Y-S, Lim S-R, Kang S-A, Park J-C. Apoptosis of the reduced enamel epithelium and its implications for bone resorption during tooth eruption. J Mol Histol. 2013;44:65–73. doi: 10.1007/s10735-012-9465-4. [DOI] [PubMed] [Google Scholar]
- Risom L, Knudsen LE. Use of cryopreserved peripheral mononuclear blood cells in biomonitoring. Mutat Res. 1999;440:131–138. doi: 10.1016/S1383-5718(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Rittling SR, Matsumoto HN, McKee MD, Nanci A, An X-R, Novick KE, Kowalski AJ, Noda M, Denhardt DT. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res. 1998;13:1101–1111. doi: 10.1359/jbmr.1998.13.7.1101. [DOI] [PubMed] [Google Scholar]
- Russell RGG, Rogers MJ, Frith JC, Luckman SP, Coxon FP, Benford HL, Croucher PI, Shipman C, Fleisch HA. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J Bone Miner Res. 1999;14:53–65. doi: 10.1002/jbmr.5650140212. [DOI] [PubMed] [Google Scholar]
- Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFκB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Hussein O, Sadvakassova G, Guo Y, Siegel PM, Komarova SV. Breast cancer-derived factors stimulate osteoclastogenesis through the Ca2+/protein kinase C and transforming growth factor-β/MAPK signaling pathways. J Biol Chem. 2009;284:33662–33670. doi: 10.1074/jbc.M109.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedemann K, Boraschi-Diaz I, Rajakumar I, Kaur J, Roughley P, Reinhardt DP, Komarova SV. Fibrillin-1 directly regulates osteoclast formation and function by a dual mechanism. J Cell Sci. 2013;126:4187–4194. doi: 10.1242/jcs.127571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113:377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- Xu X, Cowley S, Flaim CJ, James W, Seymour L, Cui Z. The roles of apoptotic pathways in the low recovery rate after cryopreservation of dissociated human embryonic stem cells. Biotechnol Prog. 2010;26:827–837. doi: 10.1002/btpr.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder AF, Kristiansen AG, Adams JC, Kujawa SG, Merchant SN, McKenna MJ. Osteoprotegrin knockout mice demonstrate abnormal remodeling of the otic capsule and progressive hearing loss. Laryngoscope. 2006;116:201–206. doi: 10.1097/01.mlg.0000191466.09210.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]