Abstract
Hydrogen peroxide (H2O2), a major reactive oxygen species (ROS) produced during oxidative stress, is toxic to the cells. Hence, H2O2 has been extensively used to study the effects of antioxidant and cytoprotective role of phytochemicals. In the present investigation H2O2 was used to induce oxidative stress via ROS production within PC12 and L132 cells. Cytoprotective propensity of Bacopa monniera extract (BME) was confirmed by cell viability assays, ROS estimation, lipid peroxidation, mitochondria membrane potential assay, comet assay followed by gene expression studies of antioxidant enzymes in PC12 and L132 cells treated with H2O2 for 24 h with or without BME pre-treatment. Our results elucidate that BME possesses radical scavenging activity by scavenging 2,2-diphenyl-1-picrylhydrazyl, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), superoxide radical, and nitric oxide radicals. The IC50 value of BME against these radicals was found to be 226.19, 15.17, 30.07, and 34.55 µg/ml, respectively). The IC50 of BME against ROS, lipid peroxidation and protein carbonylation was found to be 1296.53, 753.22, and 589.04 µg/ml in brain and 1137.08, 1079.65, and 11101.25 µg/ml in lung tissues, respectively. Further cytoprotective potency of the BME ameliorated the mitochondrial and plasma membrane damage induced by H2O2 as evidenced by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and lactate dehydrogenase leakage assays in both PC12 and L132 cells. H2O2 induced cellular, nuclear and mitochondrial membrane damage was restored by BME pre-treatment. H2O2 induced depleted antioxidant status was also replenished by BME pre-treatment. This was confirmed by spectrophotometric analysis, semi-quantitative RT-PCR and western blot studies. These results justify the traditional usage of BME based on its promising antioxidant and cytoprotective property.
Keywords: Bacopa monniera, Hydrogen peroxide, PC12 cells, L132 cells, Oxidative stress, Reactive oxygen species, Cytotoxicity, Antioxidant enzymes
Introduction
Oxidative stress has been implicated in the pathogenesis of several neurodegenerative and lung disorders. Brain tissue is most vulnerable to oxidative stress due to its high glucose metabolism rate, high lipid content, and low antioxidant defense enzyme level. Effect of reactive oxygen species (ROS) is deleterious to cells and contributes to neuronal damage. ROS is known to be involved in various brain related disorders including ischemia–reperfusion, diabetic neuropathy, and neurodegenerative diseases such as Alzheimer’s, Parkinson’s or Huntington’s disease (Packer et al. 1997; Olanow 1993; Zhang and Tang 2000). Apart from the brain, the lung exists in a high-oxygen environment that is susceptible to injury mediated by ROS. Excessive generation of ROS may alter remodeling of extracellular matrix, mitochondrial respiration, cell proliferation, effective alveolar repair response and immune modulation within the lungs (Rahman and MacNee 1999, 2000). These tissues are continuously exposed to oxidants either generated endogenously by metabolic reactions (such as from mitochondrial electron transport during respiration, during activation of phagocytes) or exogenously (by cigarette smoke and other air pollutants). Hydrogen peroxide (H2O2), a major ROS produced during oxidative stress, is toxic to the cells. Oxidative stress-induced production of ROS are known to cause various cellular and sub-cellular damages which include DNA strands break, denaturing cellular proteins and oxidation of biomolecules (Maxwell 1995). Therefore, therapeutic strategies are aimed to prevent or to scavenge ROS production. Supplementation of antioxidants has been considered as an attractive strategy to prevent or attenuate the progression of diseases induced by ROS production.
Phytomedicine obtained from herbal sources provide outstanding contribution to modern therapeutics due to their potent antioxidant activities, no side effects and economic viability. Among the various phytochemicals found in plants, antioxidants are of particular importance because they might serve as leads for the development of novel drugs and neutraceutical products. Bacopa monniera (L.) Wettst (Scrophulariaceae) is a traditional medicinal herb whose major constituent was found to be bacoside-A. Bacoside A is a mixture of bacosaponin isomers. These active components facilitate learning and memory and also possess antiamnesic, antistress (Chowdhuri et al. 2002), anxiolytic (Ernst 2006), antidementic (Dhawan and Singh 1996), antiulcerogenic, antiarthritis, anti-inflammatory (Channa et al. 2006), antifatigue (Anand et al. 2012) and neuroprotective properties (Dhanasekharan et al. 2007; Pandareesh and Anand 2013). We previously demonstrated the antioxidant and plasmid DNA damage preventive properties of hexane, chloroform, ethyl acetate, acetone, methanol and aqueous extracts from B. monniera (Anand et al. 2011). These results are supported by earlier reports (Bhattacharya et al. 2000a, 2000b; Sairam et al. 2001; Tripathi et al. 1996; Russo et al. 2003a; Sumathy et al. 2002). Antioxidant efficiency of B. monniera in brain, support it as a potent therapeutic agent in neurodegenerative pathologies and other age-related cognitive declines. Studies have shown protective efficacy of B. monniera in liver and brain (Sumathy et al. 2001, 2002) against morphine induced inhibition of antioxidant enzyme systems. Anbarasi et al. (2005) demonstrated that isolated bacoside-A protected rat brain tissue from various parameters of oxidative stress induced by chronic cigarette smoke exposure. Russo et al. (2003a, b) and Russo and Borrelli (2005) demonstrated a free radical scavenging activity of Bacopa monniera, which protected against cytotoxicity and DNA damage in human fibroblasts and in cultured rat astrocytes. The present studies have been designed in continuation to earlier studies to elucidate cytoprotective propensity of Bacopa monniera that mitigates oxidative stress-induced neuronal and pulmonary disorder.
Materials and methods
Reagents and chemicals
Nutrient mixture F-12 Ham Kaighn’s modification medium, Dulbecco’s modified Eagle’s medium with high glucose, penicillin–streptomycin antibiotic solution, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2′,7′ dichlorfluorescein-diacetate, rhodamine 123, sodium dodecyl sulfate (SDS), 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS), glutathione standard, acetylthiocholine iodide, 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), bovine serum albumin, protease cocktail inhibitor, low melting agarose and sodium bicarbonate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydrogen peroxide, sodium chloride, disodium hydrogen phosphate, sodium dihydrogen phosphate were purchased from S.D Fine Chemicals (Mumbai, India). RNeasy mini kit was purchased from Qiagen (Valencia, CA, USA). Transcriptor first strand synthesis kit was purchased from Roche (Mannheim, Germany). Fetal bovine serum was procured from Hyclone (Logan, UT, USA), and horse serum from Life Technologies (Carlsbad, CA, USA).
Purification and quantification of Bacoside A
Aerial parts of the plant material were collected from the foothills of Tirumala, Tirupati, Andhra Pradesh, India and identified with the help of Prof. N. Yasodamma, Head, Department of Botany, Sri Venkateswara University, Tirupati, India (Herbarium collection Voucher No. DA-112). The plant material was allowed to dry in shade for 3 days. The shade dried plant material was taken for further studies. The hydroethanolic (90 % ethanol) bacoside rich extract was isolated and quantified as reported in our previous studies. The amount of bacoside A was found to be ~16 % (Anand et al. 2013; Pandareesh and Anand 2014).
Antioxidant capacity assays
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay was carried out as mentioned previously by Eberhardt et al. (2000). 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging activity was estimated using the method published by Re et al. (1999). Superoxide radical scavenging activity was determined using the method published by Sreejayan et al. (1997). Nitric oxide scavenging activity was determined following Beauchamp’s method (Beauchamp and Fridovich 1971). ROS was estimated by oxidation of 2′, 7′ dichlorofluorescein diacetate in rat brain and lung homogenate. Anti-lipoperoxidative activity and protein carbonyl content in rat brain and lung tissue homogenates was determined by the method of Reznick and Packer (1994). Protein oxidation was assayed as described by Kwon et al. (2000) with minor modifications. Briefly, oxidation of bovine serum albumin (5 μg) in phosphate buffer was initiated by 20 mM 2,2′-Azobis(2-methylpropionamidine) dihydrochloride (AAPH) and inhibited by various concentrations of BME (5–40 μg/ml). After incubation for 2 h at 37 °C, butylated hydroxytoluene (BHT) (0.02 %) was added to prevent the formation of further peroxyl radical. The samples were then analyzed with normal SDS-PAGE. The data were expressed in terms of percentage inhibition (Cai et al. 2003).
where Ac is the absorbance of positive control solution and As is the absorbance of test solution. IC50 value, the concentration of sample or extract required to scavenge 50 % of the free radicals in the mixture, was calculated using a linear regression equation derived from the graph of % scavenging activity versus sample concentration.
Cell culture and treatments
The PC12 (rat pheochromocytoma cells) and human embryonic lung epithelial cells (L132) used in the current study were supplied by the National Centre for Cell Science (Pune, India). The PC12 cells were grown in nutrient mixture Ham’s F-12 medium (Kaighn’s modification) containing 2.5 % fetal bovine serum and 15 % horse serum. Whereas L132 cells were grown in Dulbecco’s modified Eagle’s medium with high glucose and supplemented with 5 % fetal bovine serum. Penicillin (100 U/ml) and streptomycin (100 µg/ml) were added to the growth media. Both cell lines were maintained in a humid atmosphere of 5 % CO2 and 95 % air at 37 °C. For all experiments, 80 % confluence with more than 95 % cell viability was considered as optimum. All the experiments were conducted in serum free media. Freshly prepared hydrogen peroxide was added as free radical inducer for 24 h to the cells with or without pretreatment with BME (100 µg/ml) for 1 h before any experiment.
Cell viability assay
Cell viability was assessed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Pandareesh and Anand 2013). The principle of the assay is based on the cleavage of tetrazolium salts by mitochondrial succinate reductase in viable cells to form formazan dye. The PC12 cells were seeded in 96-well plates at a density of 1 × 104 cells/well and grown for 24 h and then subjected to the treatments of interest. After treatments, MTT (0.5 mg/ml) was added to each well and incubated for 2 h at 37 °C and the formed formazan crystals were dissolved in DMSO. The absorbance was then measured at 540 nm using Multi-technology plate reader (Plate Chameleon, Type 425-106 s/n 2090137, Turku, Finland). Cell viability was expressed as a percentage of the value against the control group.
Lactate dehydrogenase (LDH) Leakage
Cytotoxicity was quantified by estimating the LDH activity as per the manufacturer’s instructions (Agappe-11407002, Pattimattom (PO), Dist. Ernakulam, Kerala, India). The assay is based on leakage of cytosolic LDH into media due to plasma membrane damage. The PC12 and L132 cells were plated at a density of 5 × 104 cells/well on 24-well plates, and after 24 h of adherence, the cells were subjected to H2O2 treatment. After 24 h of treatment period, LDH activity was measured using 10 µl of medium from all the samples. Control cells were lysed by adding 10 µl of cell lysis solution (2 % Triton X-100). Cell lysate was centrifuged at 2,650×g for 5 min. Supernatant was used to measure the total LDH activity. LDH activity measured within the cell homogenate was considered as 100 % and compared with other treated samples.
Estimation of intracellular ROS production
The cells were cultured in 24 well plates for fluorimetric analysis and treated as mentioned earlier. After treatments, the oxidation-sensitive dye 2′,7′ dichlorfluorescein-diacetate (0.5 mg/ml) was added to the cells and incubated for 30 min at 37 °C. The cells were then collected after washing twice with phosphate buffer saline (PBS) and the intracellular ROS formation was detected at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using Multi-technology plate reader (Plate Chameleon, Type 425-106 s/n 2090137, Finland). For imaging, the cells were grown on cover slips (Blue star, North Brunswick, NJ, USA) which were pre-coated with poly l-lysine. After experimental treatments, the cells were treated with 2′,7′ dichlorfluorescein-diacetate as mentioned above and excess dye was removed by washing twice with PBS. The cells were imaged using a fluorescence microscope (Olympus, Tokyo, Japan) equipped with a Cool SNAP® Pro color digital camera.
Measurement of lipid peroxidation
Malonyldialdehyde (MDA), a lipid peroxidation product, was measured following the method of Ohkawa et al. (1979) with minor modifications. Both PC12 and L132 cells were seeded in 75 cm2 flasks at a concentration of 1 × 106 cells/ml and incubated at 37 °C. After 80 % confluence was reached, the cells were treated as described earlier. They were collected, washed twice with PBS and lysed in ice-cold 1.15 % KCl with 1 % Triton X-100 by sonication for 5 min. Aliquots (100 µl) of the cell lysates were mixed with 0.2 ml of 8.1 % SDS, 1.5 ml of 20 % acetic acid (pH 3.5), 1.5 ml of 0.8 % thiobarbituric acid and the volume was brought up to 4 ml using distilled water. The content(s) were boiled for 2 h to develop the colour and cooled. The content was centrifuged at 3,000×g for 10 min and the absorbance of supernatants was measured at 532 nm. The MDA content was calculated using the molar extinction coefficient of 1.56 × 105 M−1 cm−1 (Buege and Aust 1978).
Single cell gel electrophoresis (SCGE) assay
DNA damage preventing efficacy of BME against H2O2 induced oxidative stress was assessed by alkaline comet assay with slight modifications (Singh et al. 1988). The cells (1 × 106 cells) were seeded in 75 cm2 flasks and treated as described previously. The cells were collected and equal volume of cell suspension (4 × 105) was mixed with 0.5 % (w/v) low melting agarose (LMA) in 0.01 M of PBS. The mixture was pipetted on to frosted slides pre-coated with 1 % (w/v) normal melting agarose. After solidification of agarose, the slides were covered with another 100 μl of 0.5 % (w/v) LMA and immersed in lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris–HCl buffer, 0.1 % SDS and 1 % Triton X-100 and 10 % DMSO; pH 10.0) for 120 min in dark at 4 °C to lyse the cellular and nuclear membranes. The slides were rinsed with unwinding buffer and transferred into an electrophoresis tank containing unwinding buffer (3 M NaOH, 10 mM EDTA; pH 13.0) for denaturing the DNA followed by electrophoresis for 30 min with an electric current of 25 V. The slides were washed twice with neutralizing buffer (0.4 M Tris–HCl; pH 7.5) for 10 min and treated with ethanol for another 5 min. The slides were stained with 40 μl of ethidium bromide (20 mg/ml) and DNA damage was visualized by using fluorescence microscope (Olympus, equipped with Cool SNAP® Pro color digital camera). The undamaged chromosomal DNA remains compact within the nucleus and when damaged by H2O2 exposure, this organization is disrupted. During electrophoresis damaged fragments move faster and can be visualized in the form of a tail and appear as ‘comet’, whereas undamaged DNA remains compact. The length of the comet tail is measured as an index of DNA damage by Comet Assay IV software to determine the tail movement. The results were expressed as percent tail movement.
Measurement of mitochondrial membrane potential (MMP)
The protective effect of BME on mitochondrial damage induced by H2O2 was determined by measuring the MMP using the fluorescent dye rhodamine 123. The cells were cultured in 24 well plates for fluorimetric analysis. After the treatments, rhodamine 123 (10 mg/ml) was added to the cells and incubated for 1 h at 37 °C. After washing twice with PBS, the cells were collected and the fluorescence was detected at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using Multi-technology plate reader (Plate Chameleon, Type 425-106 s/n 2090137). The fluorescence emitted by control cells was considered as 100 % and compared with the other treated groups.
Estimation of antioxidant status
Both PC12 and L132 cells (1 × 106 cells) were seeded in 75 cm2 flasks and treated as described previously. The cells were collected and lysed by sonication in ice-cold 50 mM potassium phosphate buffer, pH 7.4 containing 2 mM EDTA and 0.1 % Triton X-100. The content was centrifuged at 13,000×g for 10 min at 4 °C to remove cell debris. The resulting supernatants were analyzed for protein contents by Lowry’s method (Lowry et al. 1951). Activities of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidise (GPx) and glutathione reductase (GR) were estimated according to the manufacturer’s instructions (Randox, Cat no. SD. 125, RS 504, GR 2368, Mississauga, ON, Canada) while catalase (CAT) was estimated by measuring the decay of 6 mM H2O2 solution at 240 nm by the spectrophotometric degradation method (Aebi 1984). An extinction coefficient of 43.6 M−1 cm−1 was used to determine the enzyme activity and values were expressed as mmol H2O2 degraded/min/mg of protein. GSH was determined by its reaction with 5, 5′ dithiobis (2-nitrobenzoic acid) (DTNB) to yield a yellow chromophore which was measured using spectrophotometer at 412 nm. GSH concentration was calculated by using a standard curve prepared with reduced glutathione and expressed as μg/mg protein. Total antioxidant capacity was determined by scavenging ABTS radicals. ABTS was dissolved in water to a 7 mM concentration. ABTS radical cation (ABTS•+) was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate (final concentration) and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. Dilutions of ABTS•+ solution was prepared in double distilled water to give it an absorbance value of 0.7 ± 0.02 at 734 nm. PC12 cells were grown in 25 cm2 flask, have received various treatments for 24 h, were washed with ice-cold PBS and homogenized in 50 mM phosphate buffer (pH 7.4). Cell homogenate (10 µl) was added as source of antioxidants to the preformed radical cation. Decolorization of ABTS on a time-scale represents the antioxidant activity of BME. The concentration of the antioxidant and the duration of the reaction were monitored at 734 nm for 3 min at an interval of 1 min. The concentration of total antioxidants was determined using the molar extinction coefficient of ABTS•+ 1.5 × 104 M−1 cm−1 (Roberta et al. 1999).
Western blot analysis
Total cellular protein was separated on SDS-PAGE and transferred onto a nitrocellulose membrane using an electro blotting apparatus (Cleaver Scientific Ltd, UK) as per earlier method (Anand et al. 2012). After transfer, the membranes were probed with α-Tubulin (sc-5286), SOD (sc-8637), α-CAT (sc-34280), α-GPx (sc-22146) and α-GR (sc-32408) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1: 1,000 dilutions and incubated at 37 °C for 3 h. The membranes were washed four times in TBST (Tris-Buffered Saline and Tween 20) for 15 min followed by incubation at room temperature for 2 h in horseradish peroxidase conjugated goat anti-mouse and rabbit anti-goat secondary antibodies (DAKO, Denmark) at 1:10,000 dilutions. The membranes were washed again and developed using an enhanced chemiluminescence detection system (ProteoQwest®, Sigma). Developed membranes were exposed to x-ray film and the developed band intensity was captured. The western blot band intensity was measured using NIH image J software.
Total RNA isolation, c-DNA synthesis and semi-quantitative PCR
Total RNA was extracted using RNeasy spin columns (Qiagen, Valencia, CA, USA) as per manufacturer’s instructions. c-DNA synthesis was also performed as per the manufacturer’s instructions (Roche Diagnostics, Mannheim, Germany) and stored at −20 °C for further use. Transcript abundances for the SOD, CAT, GPx and GR genes were examined by semi-quantitative PCR. Primers were designed using GeneTool 1.0 software and synthesis was done at Imperial Life Sciences Pvt. Ltd (Gurgaon, Haryana, India) (Table 1). 18 s RNA was used as housekeeping gene. The PCR reaction mixture (1 µl of c-DNA, 2 × ready mix PCR reagent containing dNTP`s, Taq-Polymerase 10 µl) with 10 pmol forward and reverse primers was added to the final volume of 20 µl with PCR grade water. The PCR reaction was performed with DNA Thermal Cycler (Veriti, Applied Biosystems/Life Technologies, Carlsbad, CA, USA). The cycling programs were as follows: initial denaturation at 94 °C for 5 min, followed by denaturation for 30 s at 94 °C, annealing for 30 s at 55 °C, extension for 50 s at 72 °C and final extension for 5 min at 72 °C. After completion of PCR (22 cycles for 18 s RNA; 35 cycles for SOD, CAT, GPx and GR), the thermal cycler was stopped. The amplified products were loaded onto 1 % agarose containing 0.1 mg/ml ethidium bromide in the gel and run at 100 V for 60 min. The band intensity of control and treated sample in agarose gel was quantified using NIH Image J software.
Table 1.
Primers sequences used for Semi-quantitative PCR
| Gene | Accession No. | Primers | 5′–3′ Product length | Product Size (bp) |
|---|---|---|---|---|
| 18 s RNA | X_01117 | Forward -5′ GCCCGAGCCGCCTGGATA 3′ | 836–853 | 277 |
| Reverse-5′ CCGCCGCATCGCCAGTC 3′ | 1,096–1,112 | |||
| SOD | NM_017050 | Forward-5′ GGCCGTGTGCGTGCTGAA 3′ | 105–122 | 257 |
| Reverse-5′ CAGCCACATTGCCCAGGTCTC 3′ | 341–361 | |||
| CAT | NM_012520 | Forward-5′ TGCCGTCCGATTCTCCACAGT 3′ | 410–430 | 458 |
| Reverse-5′ GGCCATAATCCGGGTCTTCCT 3′ | 847–867 | |||
| GPx | NM_030826 | Forward-5′ CGCTCATGACCGACCCCAAGT 3′ | 425–445 | 221 |
| Reverse-5′ GCCAGCCATCACCAAGCCAATA 3′ | 624–645 | |||
| GR | NM_053906 | Forward-5′ ACGGCTACGCAACATTTCGAGAT 3′ | 359–381 | 230 |
| Reverse-5′ CGCAATCTCCACGGCAATGTAA 3′ | 567–588 |
Statistical analysis
The data are expressed as mean ± SD of the mean (SEM). Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test using SPSS 15.0 for Windows software. Differences at p < 0.05 were considered to be significant.
Results
Antioxidant activity
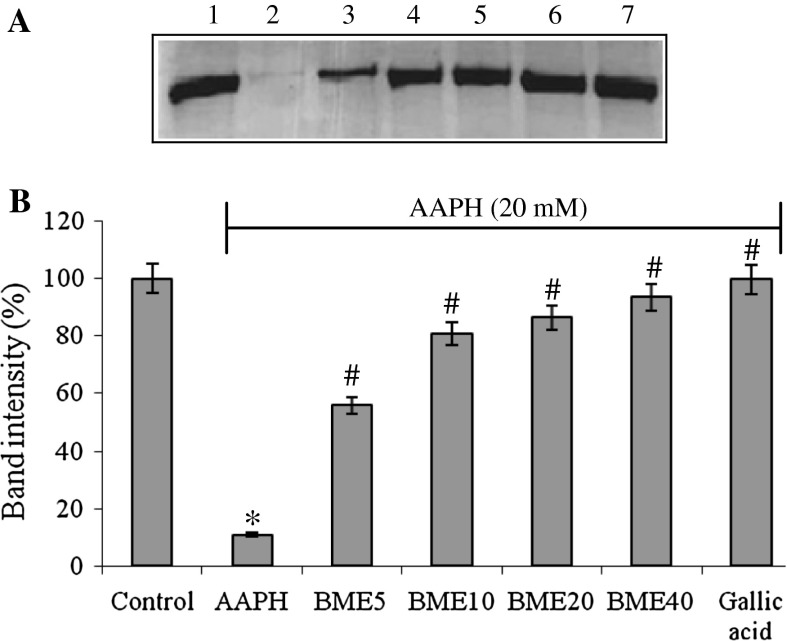
The scavenging activity of DPPH, a stable free radical, is a widely used index and a quick method to evaluate antioxidant activity. The DPPH radical scavenging potency of BME showed the IC50 value at the concentration of 226.19 µg/ml. ABTS is an excellent tool for determining antioxidant activity of hydrogen donating antioxidants and of chain breaking antioxidants. The IC50 value of ABTS radical scavenging potency of BME was found to be 15.17 µg/ml. Superoxide anions are the preliminary radical generated during oxidative stress. The IC50 value of superoxide anion scavenging activity of BME was found to be 30.07 µg/ml. Nitric oxide has an important role in various inflammatory processes and sustained levels of production of this radical are directly toxic to tissues. NO reacts with superoxide anion resulting in formation of highly reactive peroxynitrate anion (ONOO−). The IC50 value of nitric oxide radical scavenging effect of BME was found to be 34.55 µg/ml (Table 2). ROS inhibition, lipid peroxidation and protein carbonyl formation were inhibited by BME. The IC50 value of ROS inhibition, lipid peroxidation and protein carbonyl formation was found to be 1296.53, 753.22 and 589.04 µg/ml, respectively, in rat brain homogenate followed by 1,137.08, 1,079.65 and 1,101.25 µg/ml, respectively, in rat lung homogenate (Table 3). Protein oxidation induced by AAPH was inhibited by BME pretreatment. The band intensity was restored by BME pretreatment at 55.90, 80.88, 86.37, 93.60 % in comparison to control at the concentration of 5, 10, 20 and 40 μg/ml of BME (Fig. 1).
Table 2.
In vitro antioxidant activity of BME
| IC50 value | |
|---|---|
| DPPH radical scavenging activity | 226.19 ± 4.64 |
| BHA | 10.21 ± 1.21 |
| ABTS radical scavenging activity | 15.17 ± 1.34 |
| Ascorbic acid | 13.90 ± 2.02 |
| Superoxide radical scavenging activity | 30.07 ± 2.64 |
| Ascorbic acid | 34.21 ± 1.93 |
| NO radical scavenging activity | 34.55 ± 1.75 |
| Ascorbic acid | 15.24 ± 2.09 |
The results shown are averages of three independent experiments, values are mean ± SEM. BHA was used as standard for DPPH radical scavenging activity and ascorbic acid was used as standard for ABTS, superoxide and nitric oxide radical scavenging activity. Ascorbic acid was used as antioxidant standard
Table 3.
In vitro antioxidant activity of BME
| IC50 value | ||
|---|---|---|
| Brain | Lung | |
| ROS inhibition | 1,296.53 ± 25.64 | 1,137.08 ± 23.50 |
| Lipid peroxidation inhibition | 753.22 ± 14.65 | 1,079.65 ± 16.47 |
| Protein carbonyl inhibition | 589.04 ± 17.46 | 1,101.25 ± 21.46 |
The results shown are averages of three independent experiments in rat brain and lung tissue, values are mean ± SEM
Fig. 1.
a Protective role of BME on 20 mM AAPH induced BSA oxidative fragmentation. Lane 1: BSA; lane 2: BSA + AAPH; lane 3: BSA + AAPH + 5 μg/ml BME; lane 4: BSA + AAPH + 10 μg/ml BME; lane 5: BSA + AAPH + 20 μg/ml BME; lane 6: BSA + AAPH + 40 μg/ml extract; lane 7, BSA + AAPH + 10 μg/ml Gallic acid. b The band intensity is quantified by NIH Image J analysis software. The data are presented as mean ± SEM of three independent experiments. *p < 0.05 versus the respective control group and # p < 0.05 versus the respective AAPH treatment
Protective role of BME against H2O2 induced cell death
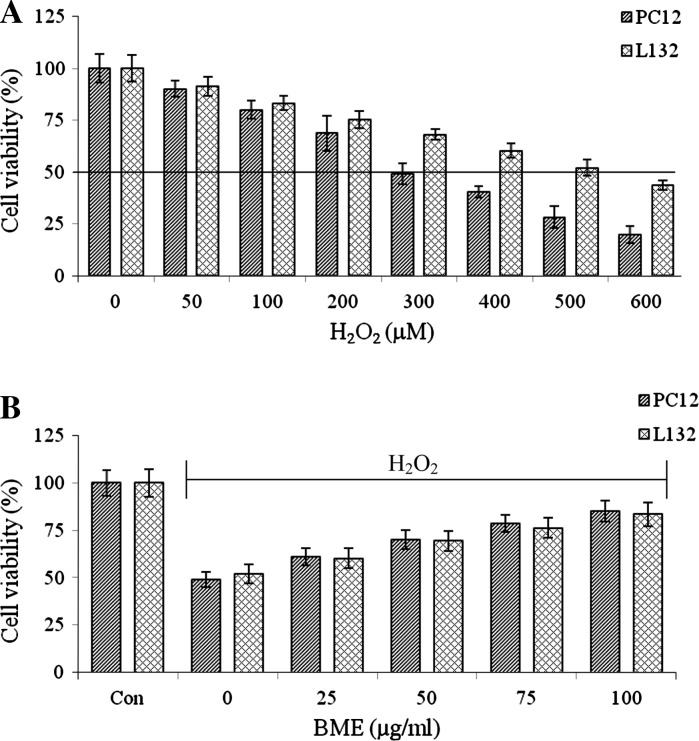
Cytoprotective role of BME was evaluated in PC12 and L132 cells. H2O2 exposure induced toxicity by decreasing cell survival in a dose-dependent manner. H2O2 treatment (300 µM for PC12 cells and 500 µM for L132 cells) reduced the cell viability to 49.12 and 52.14 % respectively. The same concentration of H2O2 was used in subsequent experiments (Fig. 2a). Further, to determine the neuroprotective and lung protective efficacy of BME, the cells were pretreated with 25–100 μg/ml of BME for a period of 1 h, followed by treatment with H2O2 (300 μM for PC12 and 500 μM for L132) for 24 h. As shown in Fig. 2b, H2O2-induced cell death was significantly reduced by BME pretreatment and the viability was restored to the extent of 85.15 % (PC12 cells) and 83.43 % (L132 cells) of control cells. These results were further confirmed by the LDH leakage assay. The PC12 and L132 cells were treated with increasing concentrations of H2O2 (50–600 μM) for 24 h. The H2O2 exposure induced toxicity by releasing LDH into the medium in a dose-dependent manner. A 46.24 and 49.76 % LDH release were observed at 300 and 500 μM H2O2 in PC12 and L132 cells, respectively (Fig. 3a). In contrast, 100 μg/ml BME pretreated cells showed a decreased release of LDH up to 27.82 % (PC12 cells) and 32.56 % (L132 cells) as compared to the H2O2 alone treated group (Fig. 3b).
Fig. 2.
a Dose-dependent effects of H2O2 (0–600 µM) on PC12 and L132 cell viability. b Dose dependent protective effect of BME on H2O2 (300 µM for PC12 and 500 µM for L132 cells) induced cytotoxicity. The data are presented as mean ± SEM of three independent experiments
Fig. 3.
a Dose-dependent effects of H2O2 (0–600 µM) on LDH leakage. b Protective effect of BME (100 µg/ml) on H2O2 (300 µM for PC12 and 500 µM for L132 cells) induced LDH leakage. The data are presented as mean ± SEM of three independent experiments. *p < 0.05 versus the respective control group and # p < 0.05 versus the respective H2O2 treated group
Effect of BME on ROS generation and lipid peroxidation
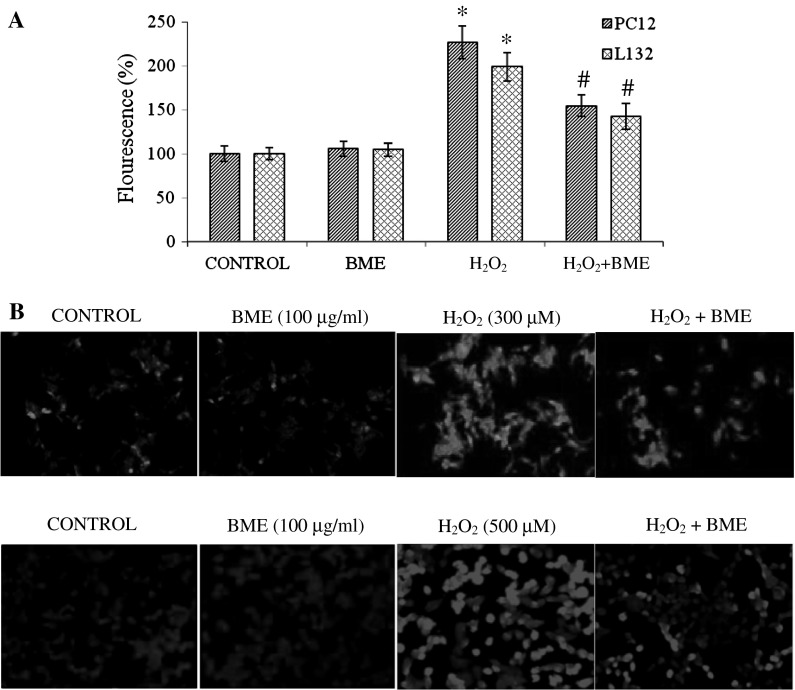
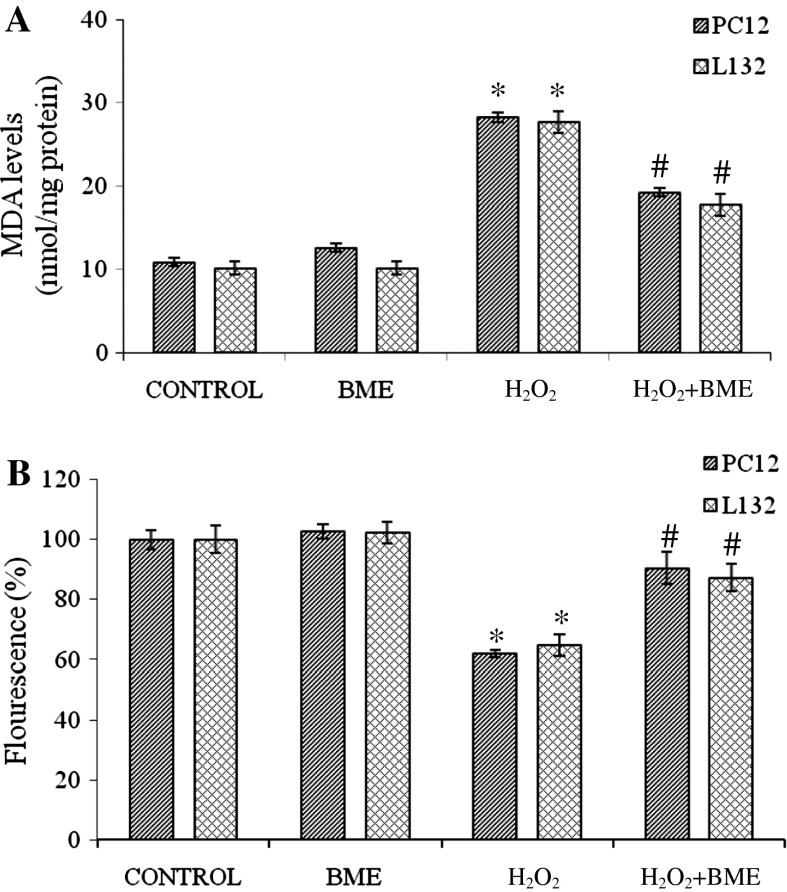
Exposure of PC12 and L132 cells to H2O2 elicited ROS production by ~2 fold increase as compared to their respective control groups. ROS generation was attenuated significantly (p < 0.05) when cells were pretreated with BME followed by H2O2 treatment for 24 h. The fluorescence intensity decreased to 154.38 % (PC12 cells) and 142.56 % (L132 cells) with BME pretreatment indicating the potent antioxidant propensity of BME (Fig. 4a). The ROS production in PC12 and L132 cells was also monitored by fluorescence microscopy. The images captured are shown in Fig. 4b. ROS generated by H2O2 treatment causes oxidation of membrane lipids and releases lipid peroxidation products (MDA). PC12 and L132 cells exposed to H2O2 for 24 h significantly increased the MDA levels by 258.98 and 272.08 % in relation to the control group. Pretreatment with BME had significantly decreased the rate of lipid peroxidation (176.38 % in PC12 and 174.34 % in L132 cells) as compared to the H2O2 treated group (p < 0.05) (Fig. 5a).
Fig. 4.
a Estimation of ROS production induced by H2O2 in PC12 and L132 cells. The data are represented as mean ± SEM of three independent experiments. *p < 0.05 versus the respective control group and # p < 0.05 versus the respective H2O2 treated group. b ROS production in PC12 and L132 cells was monitored by fluorescence microscopy. (from left to right) a Control cells without any treatment, b 100 μg/ml BME, c H2O2 (300 µM for PC12 and 500 µM for L132) for 24 h d cells pre-treatment with 100 μg/ml BME for 1 h and then exposed to H2O2 (300 µM for PC12 and 500 µM for L132) for 24 h
Fig. 5.
a Protective effect of BME (100 µg/ml) against H2O2 (300 µM for PC12 and 500 µM for L132 cells) induced lipid peroxidation in PC12 and L132 cells. b Effect of BME (100 µg/ml) on H2O2 (300 µM for PC12 and 500 µM for L132 cells) induced decrease of mitochondrial membrane potential determined by spectrofluorimetric method. The data are presented as mean ± SEM of three independent experiments. *p < 0.05 versus the respective control group and # p < 0.05 versus the respective H2O2 treated group
Protective role of BME on H2O2 induced MMP reduction
Lipid peroxidation caused by the free radicals attack leads to a loss of mitochondrial membrane potential. This was analyzed in terms of accumulation of rhodamine 123, a membrane permeant, cationic fluorescent dye. The rhodamine 123 fluorescence decreased by 62.12 % in PC12 and 64.76 % in L132 cells with H2O2 exposure compared to control indicating depolarization of mitochondrial membrane. However, the cells pretreated with BME, prior to H2O2 exposure, showed a significant regain in the fluorescence intensity to an extent of 90.54 and 87.32 %, respectively, in PC12 and L132 cells as compared to its respective control (Fig. 5b).
Protective role of BME on H2O2 induced DNA damage
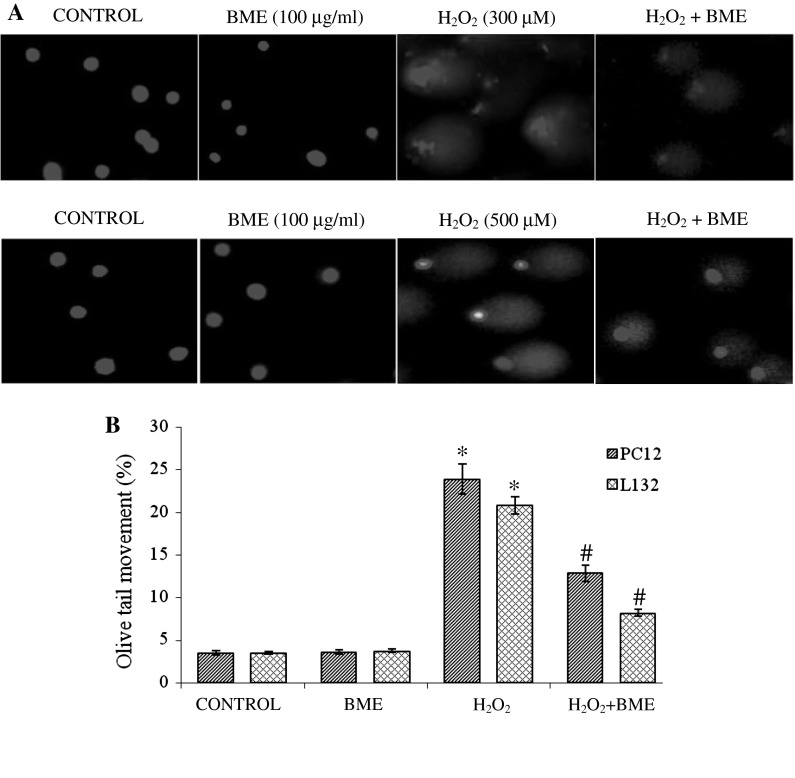
H2O2 treated cells showed definite tail with a consistent amount of fragments indicating DNA damage (Fig. 6a). BME pretreatment was found to decrease the tail movement from 23.9 to 12.9 % in PC12 cells and from 20.8 to 8.2 % in L132 cells, respectively, (Fig. 6b). These pretreated cells appeared with a small tail and a little migration of fragments of DNA indicating protective efficacy of BME against H2O2 induced DNA damage.
Fig. 6.
a Effect of BME on DNA damage induced by H2O2 in PC12 and L132 cells. a Control cells without any treatment, b 100 μg/ml BME, c H2O2 (300 µM for PC12 and 500 µM for L132) for 24 h d cells pre-treatment with 100 μg/ml BME for 1 h and then exposed to H2O2 (300 µM for PC12 and 500 µM for L132) for 24 h and (b). The tail length of the comet was measured in each cell using image pro® plus software and represented as percent tail movement. The data are presented as mean ± SEM of three independent experiments. *p < 0.05 versus the respective control group and # p < 0.05 versus the respective H2O2 treated group
Effect of H2O2 on antioxidant status
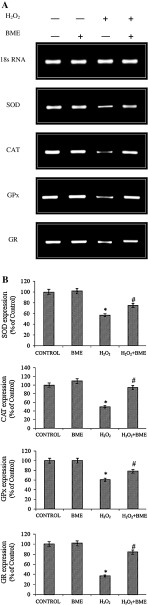
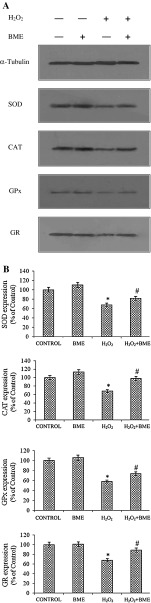
The cellular antioxidant enzymes including SOD, CAT, GPx, GR activity was reduced by H2O2 exposure as compared to that of their respective control cells. However, BME pretreatment restored the H2O2-induced down-regulation of target proteins in both PC12 and L132 cells. These results were confirmed by the activation of SOD, CAT, GPx, and GR by BME pretreatment in terms of gene expression by semi-quantitative PCR analysis in PC12 cells (Fig. 7) and protein expression by Western blot analysis in L132 cells (Fig. 8). The protein levels of all enzymes were found consistent with their enzymatic activities (Table 4). These results indicate that the enhancement in antioxidant enzyme activities by BME pretreatment is associated with the inhibition of ROS production. The cellular reduced GSH levels and total antioxidant capacity was significantly depleted by H2O2-induced toxicity (p < 0.05). The decrease in GSH levels and total antioxidant capacity was improved by pretreatment with BME (70.93 % in PC12 cells and 81.00 % in L132 cells, respectively) (Table 4).
Fig. 7.
a Protective effect of BME (100 μg/ml) against H2O2 treatment on expression of oxidative stress marker SOD, CAT, GPx and GR was analyzed by semi-quantitative PCR analysis. b The band intensity is quantified by NIH Image J software. The data are represented as mean ± SEM of three independent experiments. *p < 0.05 versus the respective control group and # p < 0.05 versus the respective H2O2 treated group
Fig. 8.
a Protective effect of BME (100 μg/ml) against H2O2 treatment on expression of oxidative stress marker proteins SOD, CAT, GPx and GR analyzed by immunobloting. b The band intensity was calculated by NIH Image J software. The data are represented as mean ± SEM of three independent experiments. *p < 0.05 versus the respective control group and # p < 0.05 versus the respective H2O2 treated group
Table 4.
Effect of BME on antioxidant status
| SOD (U/mg of protein) | CAT (mM H2O2 degraded/min/mg of protein) | GPx (U/mg of protein) | GR (U/mg of protein) | GSH (µM/mg of protein) | TAC (nmol/mg of protein) | |
|---|---|---|---|---|---|---|
| PC12 | ||||||
| CON | 2.08 ± 0.18 | 0.94 ± 0.06 | 1,065.89 ± 93.54 | 1,069.36 ± 56.49 | 4.66 ± 0.34 | 51.11 ± 4.40 |
| BME | 2.18 ± 0.08 | 1.06 ± 0.04 | 1,069.51 ± 79.36 | 1,125.58 ± 43.44 | 5.26 ± 0.17 | 52.27 ± 1.75 |
| H2O2 | 1.30 ± 0.17* | 0.56 ± 0.05* | 590.91 ± 55.90* | 650.19 ± 62.40* | 1.96 ± 0.11* | 27.76 ± 4.97* |
| H2O2 + BME | 1.93 ± 0.13# | 0.91 ± 0.03# | 914.69 ± 98.50# | 934.81 ± 47.96# | 3.31 ± 0.12# | 45.11 ± 3.20# |
| L132 | ||||||
| CON | 2.34 ± 0.16 | 0.89 ± 0.03 | 716.12 ± 36.67 | 680.17 ± 33.80 | 5.38 ± 0.22 | 36.25 ± 1.73 |
| BME | 2.51 ± 0.08 | 0.88 ± 0.03 | 716.01 ± 39.51 | 660.63 ± 27.99 | 5.52 ± 0.31 | 37.45 ± 1.59 |
| H2O2 | 1.37 ± 0.22* | 0.49 ± 0.03* | 384.30 ± 42.28* | 360.94 ± 12.47* | 2.08 ± 0.33* | 18.20 ± 1.44* |
| H2O2 + BME | 2.14 ± 0.15# | 0.79 ± 0.04# | 595.57 ± 45.24# | 558.01 ± 22.26# | 4.17 ± 0.35# | 32.67 ± 1.39# |
The data are presented as mean ± SEM of three independent experiments. * p < 0.05 versus respective control group and # p < 0.05 versus respective H2O2 treated group
Discussion
Phytochemicals obtained from plants have become a significant source of nutraceutical and traditional medicines. The therapeutical properties of most of these plants are due to their antioxidant potential. It is obvious that no single method is capable of providing a comprehensive picture of the antioxidant profile of a studied sample. Hence, in the present study, antioxidant role of B. monniera has been evaluated by various phytochemical and biochemical assays (Tables 1, 2). Lipid peroxidation and protein oxidation are reported to be involved in the etiology of several human diseases such as atherosclerosis, ageing and neurodegenerative diseases. Thus, the inhibition of oxidation of these biomolecules by BME may be vital for the alleviation of an array of diseases.
H2O2 has been extensively used to study the effects of antioxidant and cytoprotective role of phytochemicals (Gulden et al. 2010; Brenner et al. 2010). Hence, the cytoprotective propensity of BME has been evaluated against H2O2 induced oxidative stress in PC12 and L132 cells. The possible mechanism of cytoprotection in neuronal and lung epithelial cells by its potent antioxidant propensity was evaluated by estimating the biomarkers known to be induced by oxidative stress. H2O2 is a well known potent inducer of ROS capable of inducing cell injury both in vitro and in vivo (Kim et al. 2012; Terashvili et al. 2012). Studies have shown that H2O2 is known to induce oxidative damage, leading to lipid peroxidation, ROS generation, GSH depletion and reduction in mitochondrial membrane potential, antioxidant enzymes (SOD, catalase and GPx) activity preceding cell death (Chan et al. 2009; Brenner et al. 2010; Gulden et al. 2010).
In the present study, exposure of PC12 and L132 cells to H2O2 induced cell mortality, which is confirmed by MTT and LDH leakage assay (Figs. 2, 3). Recently Li et al. (2013) demonstrated neuroprotective property of sulfiredoxin-1 against oxidative stress mediated cytotoxicity of H2O2 in PC12 cells. In another study Si et al. (2013), demonstrated the antioxidant and neuroprotective property of isocampneoside II against H2O2 induced oxidative injury in PC12 cells. Similarly our present study is in corroboration with above findings showing anti-oxidative and neuroprotective propensity of BME against H2O2 induced oxidative damage in both PC12 and L132 cells (Figs. 2, 3). Cytotoxic effect of H2O2 is due to ROS generation within the cells which were estimated spectrofluorimetrically using a non-ionic, non-polar fluorescent dye 2′,7′ dichlorfluorescein-diacetate. The emitted fluorescence is directly proportional to the ROS generation induced by H2O2. Our results showed BME pretreatment decreases fluorescence, indicating the ROS inhibitory effect of BME (Fig. 4a, b). The generated ROS reacts with the glycophospholipids, fatty acids and other essential lipids present in mitochondrial membrane and cell membrane leading to formation of lipid peroxides and other radicals which can propagate end products such as aldehydes, among which malonyldialdehyde is one. In the present study, we found that BME pretreatment inhibits the H2O2-induced lipid peroxidation (Fig. 5). These results are corroborating with our previous in vivo and in vitro studies (Anand et al. 2012; Pandareesh and Anand 2013).
Lipid peroxidation triggers the arachidonic-acid cascade, with the production of cell proliferation-stimulating eicosanoids. The by-products of the arachidonic-acid pathway such as malondialdehyde and 4-hydroxynonenol are DNA-damaging agents. In the present study, the protective effect of BME against H2O2 induced DNA damage by single cell gel electrophoresis (SCGE) assay was observed. As mentioned earlier, free radical generation causes lipid peroxidation and increases the permeability of the mitochondrial membrane. This decrease in mitochondrial membrane potential was found to be associated with several apoptosis models and its estimation is a biomarker to evaluate stress induced apoptotic cell damage (Ly et al. 2003). Rhodamine 123, a mitochondrial selective, cationic and lipophilic fluorescent dye that partitions into active mitochondria based on its highly negative mitochondrial membrane was used to check mitochondrial membrane potential. The diffusion and accumulation of this dye in mitochondria is proportional to the degree of mitochondrial membrane potential (Ubl et al. 1996). Any damage in mitochondrial membrane will lead rhodamine 123 to leak out into cytosol which subsequently moves out of the plasma membrane and exhibits decreased intracellular fluorescence. However, the present study showed the restoration of fluorescence by BME pre-treatment indicating cytoprotective role of BME by restoring mitochondrial membrane potential against H2O2-induced damage.
Oxidative stress can occur either due to over production of ROS or due to the decrease in cellular antioxidant levels. The predominant enzymatic defense systems against H2O2 induced toxicity are SOD, CAT, GPx and GR which are present in all cells and play a crucial role in ameliorating the oxidative damage within the cells (Li et al. 2012; Halliwell and Gutteridge 2001). In the present study, both PC12 and L132 cells exposed to H2O2 have significantly depleted the levels of these antioxidant enzymes. This perturbation within the system makes the cells more vulnerable to oxidant injury. However, in BME alone treated cells the expression of SOD and CAT was slightly elevated (not significant). This elevation in expression may be due to antioxidant property of BME, which boosts the antioxidant enzyme levels. These findings are in support with the earlier investigation of Iqbal et al. (2003), showing that the dietary supplementation of curcumin enhances antioxidant enzyme levels. The decreased activity of antioxidant enzymes may be due to decreased cellular antioxidant levels viz GSH levels. Hence, the GSH redox cycle plays a major role in protection against severe oxidative stress (Merad-Boudia et al. 1998; Ibi et al. 1999) and GSH may be considered as one of the most prevalent and important free radical scavenger for the antioxidant defense of the cells. Depletion of GSH leads to peroxidation of lipids, proteins, carbohydrates and DNA damage consequently causing cellular damage (Table 4). In an earlier study, Chan et al. (2009) reported that the antioxidant and neuroprotective role of astaxanthin and canthaxanthin protected the differentiated PC12 cells from H2O2-induced cell death, LDH leakage, DNA fragmentation, mitochondrial membrane damage, lipid peroxidation, glutathione depletion and antioxidant enzyme status. Similarly neuro and lung protective property of various novel compounds have been established by many researchers (Gao et al. 2010; Lu et al. 2010; Shen et al. 2011; Liu et al. 2011; Yoon et al. 2011; Luo et al. 2011). In the same way the present results are in support with earlier studies suggesting neuro and lung protective propensity of BME.
Antioxidants obtained from plant source are capable to remove free radicals. Among them, phenolic and polyphenolic compounds, such as flavonoids and catechin in edible plants, exhibit potent antioxidant activities (Fang 2002). Polyphenolic compounds from tea scavenging free radicals like superoxide and hydroxyl radicals and enhance Cu, Zn-SOD activity by decreasing malondialdehyde levels, an indicator of lipid peroxidation (Youdim et al. 2000). Vitamins also directly scavenge ROS and up-regulate the activities of antioxidant enzymes. Among them, vitamin E has been recognized as one of the most important antioxidants. Vitamin E inhibits ROS-induced generation of lipid peroxyl radicals, thereby protecting cells from peroxidation of PUFA in membrane phospholipids, from oxidative damage. It also reduces the activities of hepatic catalase, GPx, and GR (Chow et al. 1973; Topinka et al. 1989). In corroboration with these above studies, BME extract has effectively scavenged free radicals like superoxide radical, nitric oxide radical and ABTS radical. The IC50 value of BME for these radicals has been reported in Table 2. Further, ethyl acetate extract of germinated brown rice has been reported to attenuate hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuroblastoma cells via anti-apoptotic, pro-survival and antioxidant genes (Azmi et al. 2013). Similarly in the present study also BME has effectively scavenged the ROS levels and elevated the levels of antioxidant genes to protect the cells against oxidative damage induced by hydrogen peroxide. Collectively, these studies suggest that phytochemicals may be used as effective antioxidants for improving human health and preventing multiple disorders.
Conclusion
In conclusion, our present study showed neuro and lung protective role of Bacopa monniera by up-regulating the expression of enzymatic antioxidants against H2O2 induced oxidative injury in PC12 and L132 cells. Administration of BME attenuates the oxidative damage induced by H2O2 by improving the antioxidant status, mitochondrial membrane integrity and by preventing DNA fragmentation and lipid peroxidation. Hence, the study supports the traditional usage of BME for its promising antioxidant, neuro and lung protective propensity.
Acknowledgments
The authors are thankful to Dr. HV Batra, Director, Defence Food Research Laboratory and Dr. Farhath Khanum, Head, Biochemistry and Nanosciences Discipline, Defence Food Research Laboratory, India for their constant encouragement and support to carry out the research work.
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126 [DOI] [PubMed]
- Anand T, Naika M, Swamy MSL, Khanum F. Antioxidant and DNA damage preventive properties of Bacopa monniera (L.) Wettst. Free Rad Antiox. 2011;1:84–90. doi: 10.5530/ax.2011.1.13. [DOI] [Google Scholar]
- Anand T, Phani Kumar G, Pandareesh MD, Swamy MSL, Khanum F, Bawa AS. Effect of bacoside extract from Bacopa monniera on physical fatigue induced by forced swimming. Phytother Res. 2012;26:587–593. doi: 10.1002/ptr.3611. [DOI] [PubMed] [Google Scholar]
- Anand T, Bhanu Prakash K, Pandareesh MD, Farhath Khanum (2013) Development of bacoside enriched date syrup juice and its evaluation for physical endurance. J Food Sci Technol. doi:10.1007/s13197-013-0955-5 [DOI] [PMC free article] [PubMed]
- Anbarasi K, Vani G, Balakrishna K, Desai CS. Creatine kinase isoenzyme patterns upon chronic exposure to cigarette smoke: protective effect of bacoside A. Vascul Pharmacol. 2005;42:57–61. doi: 10.1016/j.vph.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Azmi NH, Ismail N, Imam MU, Ismail M. Ethyl acetate extract of germinated brown rice attenuates hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuroblastoma cells: role of anti-apoptotic, pro-survival and antioxidant genes. BMC Complement Altern Med. 2013;1:177. doi: 10.1186/1472-6882-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–277. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res. 2000;14:174–179. doi: 10.1002/(SICI)1099-1573(200005)14:3<174::AID-PTR624>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Kumar A, Ghosal S. Effect of Bacopa monniera on animal models of Alzheimer’s disease and perturbed central cholinergic markers of cognition in rats. In: Siva Sanka DV, editor. Molcular aspects of asian medicine. New York: PJD Publications; 2000. [Google Scholar]
- Brenner S, Gulden M, Maser E, Seibert H. Lasting effect of preceding culture conditions on the susceptibility of C6 cells to peroxide-induced oxidative stress. Toxicol In Vitro. 2010;24:2090–2096. doi: 10.1016/j.tiv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Meth Enzymol. 1978;52:301–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cai YJ, Fang JG, Ma LP, Li Y, Liu ZL. Inhibition of free radical induced peroxidation of rat liver microsomes by resveratrol and its analogues. Biochim Biophys Acta. 2003;1637:1–38. doi: 10.1016/S0925-4439(02)00173-4. [DOI] [PubMed] [Google Scholar]
- Chan KC, Mong MC, Yin MC. Antioxidative and anti-inflammatory neuroprotective effects of astaxanthin and canthaxanthin in nerve growth factor differentiated PC12 cells. J Food Sci. 2009;74:225–231. doi: 10.1111/j.1750-3841.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- Channa S, Dar A, Anjum S, Yaqoob M, Rahman A. Anti-inflammatory activity of Bacopa monniera in rodents. J Ethnopharmacol. 2006;104:286–289. doi: 10.1016/j.jep.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Chow CK, Reddy K, Tappel AL. Effect of dietary vitamin E on the activities of the glutathione peroxidase system in rat tissues. J Nut. 1973;103:618–624. doi: 10.1093/jn/103.4.618. [DOI] [PubMed] [Google Scholar]
- Chowdhuri DK, Parmar D, Kakkar P, Shukla R, Seth PK, Srimal RC. Antistress effects of bacosides of Bacopa monnieri: modulation of HSP70 expression, superoxide dismutase and cytochrome P450 activity in rat brain. Phytother Res. 2002;16:639–645. doi: 10.1002/ptr.1023. [DOI] [PubMed] [Google Scholar]
- Dhanasekharan M, Tharakan B, Holcomb LA, Hitt AR, Young KA, Manyam BV. Neuroprotective mechanisms of Ayurvedic antidementia botanical Bacopa monniera . Phytother Res. 2007;21:965–969. doi: 10.1002/ptr.2195. [DOI] [PubMed] [Google Scholar]
- Dhawan BN, Singh HK. Pharmacological studies on Bacopa monnieri an ayurvedic nootropic agent. Eur Neuropsychopharmacol. 1996;3:144–150. doi: 10.1016/0924-977X(96)87969-7. [DOI] [Google Scholar]
- Eberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apple. Nature. 2000;405:903–904. doi: 10.1038/35016151. [DOI] [PubMed] [Google Scholar]
- Ernst E. Herbal remedies for anxiety—a systematic review of controlled clinical trials. Phytomedicine. 2006;13:205–208. doi: 10.1016/j.phymed.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fang YZ (2002) Free radicals and nutrition. In: Fang YZ, Zheng RL (eds) Theory and application of free radical biology. Scientific Press, Beijing, p 647
- Gao Y, Zhang HW, Qiao HL, Wang W, Chang JB. Protective effect of 3-butyl-6-bromo-1 (3H)-isobenzofuranone on hydrogen peroxide-induced damage in PC12 cells. Brain Res. 2010;1358:239–247. doi: 10.1016/j.brainres.2010.08.043. [DOI] [PubMed] [Google Scholar]
- Gulden M, Jess A, Kammann J, Maser E, Seibert H. Cytotoxic potency of H2O2 in cell cultures: impact of cell concentration and exposure time. Free Radic Biol Med. 2010;49:1298–1305. doi: 10.1016/j.freeradbiomed.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- Ibi M, Sawada H, Kume T, Katsuki H, Kaneko S, Shimohama S, Akaike A. Depletion of intracellular glutathione increases susceptibility to nitric oxide in mesencephalic dopaminergic neurons. J Neurochem. 1999;73:1696–1703. doi: 10.1046/j.1471-4159.1999.731696.x. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Sharma SD, Okazaki Y, Fujisawa M, Okada S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacol Toxicol. 2003;92:33–38. doi: 10.1034/j.1600-0773.2003.920106.x. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lee K, Kang KA, Lee NH, Hyun JW. Phloroglucinol exerts protective effects against oxidative stress-induced cell damage in SH-SY5Y cells. J Pharmacol Sci. 2012;119:186–192. doi: 10.1254/jphs.12056FP. [DOI] [PubMed] [Google Scholar]
- Kwon HY, Choi SY, Won MH, Kang TC, Kang JH. Oxidative modification and inactivation of Cu, Zn-superoxide dismutase by 2, 2′ azobis(2-amidinopropane) dihydrochloride. Biochem Biophys Acta. 2000;1543:69–76. doi: 10.1016/s0167-4838(00)00197-7. [DOI] [PubMed] [Google Scholar]
- Li JL, Wang QY, Luan HY, Kang ZC, Wang CB. Effects of l-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha. J Biomed Sci. 2012;19:32. doi: 10.1186/1423-0127-19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yu S, Wu J, Zou Y, Zhao Y (2013) Sulfiredoxin-1 protects PC12 cells against oxidative stress induced by hydrogen peroxide. J Neurosci Res 91:861–870 [DOI] [PubMed]
- Liu Q, Kou JP, Yu BY. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation. Neurochem I. 2011;58:119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenberg NJ, Farr AL, Randal RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275 [PubMed]
- Lu YH, Su MY, Huang HY, Yuan CG. Protective effects of the citrus flavanones to PC12 cells against cytotoxicity induced by hydrogen peroxide. Neurosci Lett. 2010;484:6–11. doi: 10.1016/j.neulet.2010.07.078. [DOI] [PubMed] [Google Scholar]
- Luo T, Zhang H, Zhang WW, Huang JT, Song EL, Chen SG, Wang HQ. Neuroprotective effect of jatrorrhizine on hydrogen peroxide-induced cell injury and its potential mechanisms in PC12 cells. Neurosci Lett. 2011;498:227–231. doi: 10.1016/j.neulet.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (Δψm) in apoptosis; an update. Apoptosis. 2003;8:115–128. doi: 10.1023/A:1022945107762. [DOI] [PubMed] [Google Scholar]
- Maxwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- Merad-Boudia M, Nicole A, Santiard-Baron D, Saille C, Ceballos-Picot I. Mitochondrial impairment as an early event in the process of apoptosis induced by glutathione depletion in neuronal cells: relevance to Parkinson’s disease. Biochem Pharmacol. 1998;56:645–655. doi: 10.1016/S0006-2952(97)00647-3. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Olanow CW. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993;16:439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Rad Biol Med. 1997;22:359–378. doi: 10.1016/S0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- Pandareesh MD, Anand T. Neuromodulatory propensity of Bacopa monniera against scopolamine-induced cytotoxicity in PC12 cells via down-regulation of AChE and up-regulation of BDNF and muscarnic-1 receptor expression. Cell Mol Neurobiol. 2013;33:875–884. doi: 10.1007/s10571-013-9952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandareesh MD, Anand T. Neuroprotective propensity of Bacopa monniera extract against sodium nitroprusside induced activation of iNOS, heat shock proteins and apoptotic markers in PC12 cells. Neurochem Res. 2014;39:800–814. doi: 10.1007/s11064-014-1273-7. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airways disease. Am J Physiol. 1999;277:1067–1088. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Oxidative stress and regulation of glutathione synthesis in lung inflammation. Eur Respir J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Meth Enzymol. 1994;233:357–363. doi: 10.1016/S0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- Roberta RE, Nicoletta P, Anna P, Ananth P, Yang M, Catherine RE. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Russo A, Borrelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomed. 2005;12:305–317. doi: 10.1016/j.phymed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Russo A, Borrelli F, Campisi A, Acquaviva R, Raciti G, Vanella A (2003a) Nitric oxide-related toxicity in cultured astrocytes: Effect of Bacopa monnieri. Life Sci 73: 1517-1526 [DOI] [PubMed]
- Russo A, Izzo AA, Borrelli F, Renis M, Vanella A. Free radical scavenging capacity and protective effect on DNA damage of Bacopa monniera . Phytother Res. 2003;17:870–875. doi: 10.1002/ptr.1061. [DOI] [PubMed] [Google Scholar]
- Sairam K, Rao CV, Babu MD, Goel RK. Prophylactic and curative effects of Bacopa monniera in gastric ulcer models. Phytomed. 2001;8:423–430. doi: 10.1078/S0944-7113(04)70060-4. [DOI] [PubMed] [Google Scholar]
- Shen W, Song D, Wu J, Zhang W. Protective effect of a polysaccharide isolated from a cultivated Cordyceps mycelia on hydrogen peroxide-induced oxidative damage in PC12 cells. Phytother Res. 2011;25:675–680. doi: 10.1002/ptr.3320. [DOI] [PubMed] [Google Scholar]
- Si CL, Shen T, Jiang YY, Wu L, Yu GJ, Ren XD, Xu GH, Hu WC. Antioxidant properties and neuroprotective effects of isocampneoside II on hydrogen peroxide-induced oxidative injury in PC12 cells. Food Chem Toxicol. 2013;59:145–152. doi: 10.1016/j.fct.2013.05.051. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Sreejayan Rao MNA. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- Sumathy T, Subramanian S, Govindasamy S, Balakrishna K, Veluchamy G. Protective effect of Bacopa monniera onmorphine induced hepatotoxicity in rats. Phytother Res. 2001;15:643–645. doi: 10.1002/ptr.1007. [DOI] [PubMed] [Google Scholar]
- Sumathy TS, Govindasamy S, Balakrishna K, Veluchamy G. Protective role of Bacopa monniera on morphine-induced brain mitochondrial enzyme activity in rats. Fitoterapia. 2002;73:381–385. doi: 10.1016/S0367-326X(02)00114-4. [DOI] [PubMed] [Google Scholar]
- Terashvili M, Sarkar P, Nostrand MV, Falck JR, Harder DR. The protective effect of astrocyte-derived 14,15-epoxyeicosatrienoic acid on hydrogen peroxide-induced cell injury in astrocyte-dopaminergic neuronal cell line co-culture. Neurosci. 2012;223:68–76. doi: 10.1016/j.neuroscience.2012.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topinka J, Bincova B, Sram RJ, Erin AN. The influence of α-tocopherol and pyritinol on oxidative DNA damage and lipid peroxidation in human lymphocytes. Mutat Res. 1989;225:131–136. doi: 10.1016/0165-7992(89)90130-9. [DOI] [PubMed] [Google Scholar]
- Tripathi YB, Chaurasia S, Tripathi E, Upadhyay A, Dubey GP. Bacopa monniera Linn. as an antioxidant: mechanism of action. Indian J Exp Biol. 1996;34:523–526. [PubMed] [Google Scholar]
- Ubl JJ, Chatton JY, Chen S, Stucki JW. A critical evaluation of in situ measurement of mitochondrial electrical potentials in single hepatocytes. Biochim Biophys Acta. 1996;1276:124–132. doi: 10.1016/0005-2728(96)00067-9. [DOI] [PubMed] [Google Scholar]
- Yoon MY, Hwang JH, Park JH, Lee MR, Kim HJ, Park E, Park HR. Neuroprotective effects of SG-168 against oxidative stress-induced apoptosis in PC12 cells. J Med Food. 2011;14:120–127. doi: 10.1089/jmf.2010.1027. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Shukitt-Hale B, Mackinnon S, Kalt W, Joseph JA. Polyphenols enhance red blood cell resistance to oxidative stress in vitro and in vivo. Biochim Biophys Acta. 2000;1523:117–122. doi: 10.1016/S0304-4165(00)00109-4. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Tang XC. Huperzine B, a novel acetylcholinesterase inhibitor, attenuates H2O2 induced injury in PC12 cells. Neurosci Lett. 2000;292:41–44. doi: 10.1016/S0304-3940(00)01433-6. [DOI] [PubMed] [Google Scholar]