Abstract
Viper envenomation results in inflammation at the bitten site as well as target organs. Neutrophils and other polymorphonuclear leukocytes execute inflammation resolving mechanism and will undergo apoptosis after completing the task. However, the target specific toxins induce neutrophil apoptosis at the bitten site and in circulation prior to their function, thus reducing their number. Circulating activated neutrophils are major source of inflammatory cytokines and leakage of reactive oxygen species (ROS)/other toxic intermediates resulting in aggravation of inflammatory response at the bitten/target site. Therefore, neutralization of venom induced neutrophil apoptosis reduces inflammation besides increasing the functional neutrophil population. Therefore, the present study investigates the venom induced perturbances in isolated human neutrophils and its neutralization by crocin (Crocus sativus) a potent antioxidant carotenoid. Human neutrophils on treatment with venom resulted in altered ROS generation, intracellular Ca2+ mobilization, mitochondrial membrane depolarization, cyt-c translocation, caspase activation, phosphatidylserine externalization and DNA damage. On the other hand significant protection against oxidative stress and apoptosis were evidenced in crocin pre-treated groups. In conclusion the viper venom induces neutrophil apoptosis and results in aggravation of inflammation and tissue damage. The present study demands the necessity of an auxiliary therapy in addition to antivenin therapy to treat secondary/overlooked complications of envenomation.
Keywords: Apoptosis, Crocin, Neutrophils, Oxidative stress, Phytochemicals, Vipera russelli
Introduction
Snakebite envenomation is considered as priority health hazard as well as neglected tropical disease as it accounts for about 5 million snakebite incidents resulting in 1.25 million deaths globally each year. About 3.75 million victims survive with permanent physical and secondary complications resulting in a serious medical and social-economic crisis. The most severe cases of envenomation are inflicted by the species of Viperidae family. In Asia, Vipera russelli is the major culprit for the clinical complications of severe local and systemic pathology (Girish and Kemparaju 2011; Ushanandini et al. 2006).
At present intravenous administration of bovine or ovine derived anti-venoms are the mainstay and the only medically approved therapy for snakebite management. The clinical concurrence is that the effectiveness of anti-venoms is limited against local toxicities that develop swiftly after the bite which include edema, hemorrhage, myonecrosis and degradation of basement membrane surrounding the blood capillaries. The local tissue damage will continue even after the administration of anti-venoms and results in irreversible scarring and deformity. The ineffectuality of anti-venom against the local effects has been attributed to the brisk action of venom components and the inability of anti-venom F(ab′)2 and F(ab) fragments of IgG to penetrate blood/tissue barrier. Other limitations could be the inability of anti-venoms against the possible venom induced apoptosis of inflammatory cells at the bitten site (Lalloo and Theakston 2003; Girish and Kemparaju 2011; Leon et al. 2000).
The accumulation of inflammatory phagocytes in tissue is the hallmark of all inflammatory diseases. Macrophages/monocytes, polymorphonuclear neutrophils (PMNs), mast cells, lymphocytes, and dendritic cells participate in the pathogenesis of inflammation. Among these, PMNs are the most abundant type, accumulating in large numbers at the injured tissue site. Several snake venom disintegrins, a group of cysteine rich proteins have been shown to induce the neutrophil chemotaxis. After the completion of the task the activated neutrophils are removed from the system through apoptosis and phagocytosed by macrophages resulting in resolution of inflammation (Coelho et al. 2004; Luo and Loison 2008). However, if neutrophil death occurs before their function, the number of functional neutrophils in the target site will get reduced. In addition, the recruited activated neutrophils trigger the respiratory burst, by releasing a variety of reactive oxygen species (ROS), including superoxide, hydrogen peroxide and hypochlorous acid along with hydrolytic enzymes. Release of ROS from the activated neutrophils as well as leakage of toxic compounds and intermediates may result in oxidative stress on the resident healthy cells and aggravation of inflammatory response at the bitten site. Therefore, neutrophil apoptosis needs to be tightly regulated to ensure the efficient resolution of inflammation at the bitten site.
In view of this, bioactive molecules with an efficacy to neutralize the oxidative stress and venom induced neutrophil apoptosis at the bitten site will certainly be helpful in the management of continued tissue necrosis in viper bites. Although the management of these complications is quite difficult, their magnitude could be reduced by the use of phytochemicals. Fortunately, compounds with antioxidant properties have been shown to attenuate viper venom-induced cellular damage by inhibiting the oxidative cascade and improving membrane stabilization (Mukherjee and Maity 1997; Alam and Gomes 1998; Sebastin et al. 2013). Based on these facts, the present study demonstrates the viper venom induced neutrophil apoptosis and its amelioration by crocin, a water soluble carotenoid from saffron. Saffron is obtained from the dry stigmas of Crocus sativus L (Iridaceae). Studies have shown that crocin is an effective antioxidant with therapeutic properties such as anti-inflammatory, anti-atherosclerotic, anti-depressant, anti-carcinogenic, neuroprotective, anti-hyperlipidemic, and prevent aging and hepatic damage (Montalvo-Hernández et al. 2012).
Materials and methods
Chemicals
Vipera russelli venom was procured from Hindustan Park, Kolkata, India. Histopaque, 2,7-dichlorofluorescein diacetate (DCFDA), purified crocin, 5,5′-6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1), hydrogen peroxide, calcium ionophore (A23187), fluorescein isothiocyanate-labeled annexin V, Nacetyl-Asp-Glu-Val-Asp-7-amido-4-methyl-coumarin (AC-DEVD-AMC), N-(2-Hydroxyethyl) piperazine-N′′-ethanesulfonic acid (HEPES), N′-acetyl-Leu-Glu-His-Asp-7-amido-4-methylcoumarin (AC-LEHD-AMC), CHAPS, Fura 2-AM, homovanillicacid (HVA), sodium orthovanadate (Na3VO4), enhanced chemiluminiscence detection reagent were from Sigma Chemical Co. (St. Louis, MO, USA). Antibodies against cytochrome c and β-actin were purchased from Epitomics (Burlingame, CA, USA). All other reagents were of analytical grade. The graphs were prepared using the software Graphpad prism 5. All the protocols were approved and followed according to the Institutional Human Ethical Committee (IHEC) guidelines.
Isolation of neutrophils
Buffy coats containing neutrophils were extracted from citrated whole blood by differential centrifugation. Briefly, 5 mL whole blood collected from healthy volunteers was layered onto equivolume of histopaque in polypropylene tubes followed by centrifugation at 800×g for 30 min. Buffy coats separated were aspirated into new polypropylene tubes and washed (800×g, 10 min) twice in phosphate buffered saline (10 mM PBS, pH 7.4). Cell density was adjusted to 4 × 106 cells/mL (purity >96 % and viability >98 %) and added to suitable medium.
Experimental design and treatment
The isolated neutrophils were categorized into seven different groups. Group I-control, Group II-H2O2/A23187 alone, Group III-Vipera russelli (VR) (50 µg) alone, Group IV and V—VR venom pre-incubated with crocin for 10 min at 37 °C [1:0.25 (13 nM) and 1:0.5 (25 nM) ratio respectively (venom:crocin; w/w)], Group VI—H2O2 pre-incubated with crocin (25 µg) for 10 min at 37 °C ratio and Group VII-crocin alone (100 µg). 100 mM H2O2 and 2 µM calcium ionophore were used as a positive control (Singh et al. 2007). The dose of the venom used in this study is based on our preliminary studies in which 50 µg of VR venom was able to induce apoptosis in neutrophils. The experiments were done according to the methods described and the percentage increase/decrease in the activity was calculated.
Antioxidant assays in-vitro
Free radical scavenging ability of crocin on 1,1, Diphenyl,2-picryl hydrazyl (DPPH) radical was measured employing the method of Yamaguchi et al. (1998) with slight modifications. Briefly, 1.5 ml of DPPH solution (0.1 mM in 95 % ethanol) was incubated with varying concentrations of crocin (0–10 µM). The reaction mixture was mixed and incubated for 20 min at room temperature, and the absorbance was measured at 517 nm against a blank. The antioxidant quercetin was used as a positive control.
Determination of reducing ability
Reducing ability of crocin was determined according to the method of Hsieh and Yan (2000). Briefly, different concentration of crocin (0–50 µM) were mixed with potassium ferricyanide (1 %), 2.5 ml of sodium phosphate buffer (200 mM, pH 6.6) and the mixture was incubated at 50 °C for 20 min. At the end of incubation, 2.5 ml of 5 % TCA was added and centrifuged at 3,000 rpm for 10 min. The supernatant (2.5 ml) was taken and mixed with 0.5 ml of aqueous ferric chloride (0.1 %). The absorbance was measured at 700 nm.
Lipid peroxidation
The modulatory effect of crocin on FeCl3 induced lipid peroxidation in liver homogenate of mice was determined according to the method of Ohkawa et al. (1979). An aliquot of tissue homogenate (500 mg protein) was added to Locke’s buffer (154 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 5.0 mM HEPES, 2.0 mM CaCl2, 10 mM glucose/dl, pH 7.4) and pre-incubated with different concentrations of crocin (0–50 μg) for 30 min at 37 °C and later challenged with FeCl3 (100 mM). After 1 h, the reaction was stopped by adding 200 µl of SDS (8 %) followed by 1.5 ml acetic acid (20 %, pH 3.5) and 1.5 ml TBA (0.8 %). The samples were vortexed and kept in boiling water bath for 45 min. The pink-colored complex formed was measured at 532 nm and quantified using TMP as the standard and expressed as nmol MDA formed/ mg protein.
Determination of intracellular ROS
Intracellular ROS levels were measured according to the method described by Driver et al. (2000) with slight modifications. Washed neutrophils were suspended in Locke’s buffer and incubated with VR venom (50 μg) for 30 min at 37 °C followed by the addition of 10 μM of DCFDA. The reaction mixture was incubated for 30 min at 37 °C and the resulting fluorescence was measured using Varioskan multimode plate reader (Thermo Fisher Scientific, Waltham, MA, USA) with excitation and emission wavelengths of 480 and 530 nm. ROS levels were quantified from a dichlorofluorescein standard curve and expressed as pmol DCF formed/ mg protein. Calcium ionophore (2 µM) was used as a positive control. For inhibition studies, the venom samples/ calcium ionophore were pre-incubated with different concentrations of crocin for 10 min at 37 °C.
Determination of endogenously generated H2O2
The levels of endogenously generated H2O2 were determined according to the method of Barja (2002) with minor modifications. The levels of H2O2 production in neutrophils can be successfully determined using HVA, a specific H2O2-sensitive fluorescent probe. Neutrophils were incubated with VR venom (50 μg) for 1 h at 37 °C in 200 µl HEPES-buffered saline (HBS, 10 mM HEPES, 145 mM NaCl, 10 mM d-glucose, 5 mM KCl, 1 mM MgSO4, pH 7.45) followed by the addition of 100 µM HVA. The reaction mixture was incubated at 37 °C for 30 min and washed twice with PBS. Samples were excited at 312 nm and the resulting fluorescence was measured at 420 nm using multimode plate reader. Calcium ionophore (2 µM) was used as a positive control. For inhibition studies, the venom samples/ calcium ionophore were pre-incubated with different concentrations of crocin for 10 min at 37 °C.
Myeloperoxidase activity
Myeloperoxidase activity was measured according to the method of Bradley et al. (1982) with slight modifications. Briefly, the reaction mixture consisting of 3 ml of potassium phosphate buffer (50 mM, pH 7.0) containing 100 mM guaiacol and 0.0017 % H2O2 (w/w), 150 µl of cells were added and the reaction was monitored at 470 nm for 3 min. One unit of myeloperoxidase enzyme activity is defined as an increase in absorbance of 1.0 /min at pH 7.0 at 25 °C. H2O2 (100 mM) was used as a positive control. For inhibition studies, the venom samples/H2O2 were pre-incubated with different concentrations of crocin for 10 min at 37 °C.
Preparation of cell lysate
Cell lysate was prepared according to the method of Rosado et al. (2000). Briefly, venom treated (with/without crocin for 30 min) and control cells were lysed with an equal volume of 2× Triton buffer (100 mM Tris-HCl, pH 7.2 containing 2 % TritonX-100, 2 mM EGTA, 100 μg/mL leupeptin, 2 mM PMSF, 10 mM benzamidine, 2 mM Na3VO4) at 4 °C for 30 min. Cell lysates were centrifuged at 8,000×g for 5 min. The pellet thus obtained is the cytoskeleton-rich (Triton-insoluble) fraction, which was subjected to caspase activity and detection of cytochrome c (cyt-c) by western blotting as described below. For inhibition studies, the venom samples/ H2O2 were pre-incubated with different concentrations of crocin for 10 min at 37 °C.
Detection of cyt-c release
Immunobloting was carried out to detect the cyt-c release from cytosolic fractions of the samples (Lopez et al. 2007). Cytosolic proteins were separated on 10 % SDS-PAGE and electrophoretically transferred on to a Polyvinylidene fluoride (PVDF) membrane for 1 h at 50 V using a wet blotter. Blots were then blocked in TBST (10 mM Tris-HCl, pH 8.0 containing 150 mM NaCl and 0.05 % tween-20) containing 5 % non-fat milk powder to block residual protein binding sites. Membrane was incubated with anti-cytochrome c antibody (1:1,000) in TBST for 3 h. After washing membrane was incubated with horseradish-peroxidase (HRP)-conjugated anti-IgG antibody (1:10,000), β-actin was used as loading control and the membranes were incubated with enhanced chemiluminescence (ECL) reagent for 90 s. Finally the blots were exposed to photographic films and developed using radiograph developer.
Caspase activity
Caspase activity was determined according to the method described by Ben amor et al. (2006) with slight modification. In brief, cell lysates were incubated with the substrate solution (20 mM HEPES, pH 7.4, 2 mM EDTA, 0.1 % CHAPS, 5 mM DTT and 8.25 µM of caspase substrate) at 37 °C for 2 h. H2O2 (100 mM) was used as a positive control. Substrate cleavage was measured fluorimetrically with an excitation and emission wavelength of 360 and 460 nm for caspase-3 and 400 and 505 nm for caspase-9. The activities of caspase-3 and -9 were calculated from the cleavage of the respective fluorogenic substrates (AC-DEVD-AMC for caspase-3 and AC-LEHD-AFC for caspase-9).
Determination of mitochondrial membrane potential
Changes in the mitochondrial membrane potential were registered using the cationic dye JC-1. In brief, cells were incubated with venom for 1 h at 37 °C followed by the addition of freshly diluted JC-1 (2.5 µg/mL in DMSO). After incubation for 15 min at 37 °C, the cells were washed with HBS. JC-1 accumulates in mitochondria forming red fluorescent aggregates at high membrane potentials and at low membrane potential JC-1 exists mainly in the green fluorescent monomeric form. JC-1 loaded cells were excited at 488 nm, and emission was detected at 585 nm (JC-1 aggregates) and 516 nm (JC-1 monomers) using a spectrofluorimeter (Salvioli et al. 1997). Data are presented as emission ratios (585/516). H2O2 (100 mM) was used as a positive control. For inhibition studies, the venom samples/H2O2 were pre-incubated with different concentrations of crocin at 37 °C for 10 min.
Determination of phosphatidyl serine (PS) externalization
PS externalization was determined according to the method described by Jardin et al. (2007) with minor modifications. Briefly, the cell suspensions (5 × 104) in 500 µl of PBS were transferred to 500 µl ice-cold 1 % (w/v) glutaraldehyde in phosphate-buffered saline (PBS) and incubated with venom for at 37 °C for 1 h. The cells were then incubated for 10 min with annexin V-fluorescein isothiocyanate (FITC) (0.6 µg/ml) in PBS supplemented with 0.5 % (w/v) BSA and 2 mM CaCl2 protected from light. H2O2 (100 mM) was used as a positive control. Cells were collected by centrifugation for 60 s at 3,000×g and re-suspended in PBS. Cell staining was measured using a spectrofluorimeter with excitation and emission wavelength of 496 and 516 nm respectively.
Measurement of intracellular Ca2+ levels
Intracellular Ca2+ concentration in neutrophils was measured according to the method of Lopez et al. (2006) with slight modifications. Briefly, the treated cells were incubated at 25 °C in modified Tyrode’s buffer (10 mM HEPES, pH 7.4 containing 150 mM NaCl, 2.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.0 mM CaCl2, and supplemented with 1 % BSA) with 2 μM Fura 2-AM, a fluorescent Ca2+ indicator. After incubation, cells were washed twice with the HBS to remove the unbound dye and the absorption shift that occurs upon calcium binding was measured spectrofluorimetrically with excitation at 340 and 380 nm [calculated as (340/380) ratio of fluorescence intensity], and emission at 510 nm. Data are presented as percentage increase/decrease in Fura 2-AM fluorescence. For inhibition studies, the venom samples/H2O2 were pre-incubated with different concentrations of crocin at 37 °C for 10 min.
Comet assay
DNA strand damage in neutrophil cell suspension was assayed using Comet assay according to the method of Singh et al. (1988) with slight modifications. The final concentration of neutrophils was adjusted to 1 × 105 cells/ml with RPMI medium. Cells were incubated with VR venom/H2O2 in presence/absence of crocin for 1 h at 37 °C, after incubation Comet assay was performed to assess the DNA damage. A suspension of cells in 0.75 % low melting point (LMP) agarose dissolved in PBS was spread onto microscopic slides which were pre-coated with 0.5 % normal-melting agarose. The slides were then immersed in lysis buffer (10 mM Tris-HCl, pH 10.0 containing 2.5 M NaCl, 100 mM EDTA, 1 % Triton X-100 and 10 % DMSO) and kept at 4 °C for 1 h. After lysis, the slides were placed in an electrophoresis unit, and the DNA was allowed to unwind for 20 min in the electrophoretic running buffer consisting of 300 mM NaOH and 1 mM EDTA. Electrophoresis was carried out at 300 mA for 30 min. The slides were then neutralized with 400 mM Tris-HCl, pH 7.5 followed by dehydration with methanol, dried and stained with 1X ethidium bromide. H2O2 (100 mM) was used as a positive control. All steps after cell lysis were performed in the dark or under dimmed red light to prevent additional DNA damage.
Protein estimation
The protein concentration was determined by the method of Lowry et al. (1951) using BSA as the standard.
Statistical analysis
Results are expressed as mean ± SEM of three independent experiments. The degree of induction, measured as increase or decrease in ROS generation and hydroperoxide formed. Caspase activities were analyzed with respect to the endogenous values. Similarly the release of cyt c, membrane potential, PS externalization (induced or inhibited) was also analyzed between treatment and control groups. The data were analyzed by ANOVA, followed by Duncan’s test for multi-group comparison, with p < 0.05 (*) and p < 0.01 (**) were considered to be statistically significant.
Results
Free radical scavenging potential of crocin in vitro
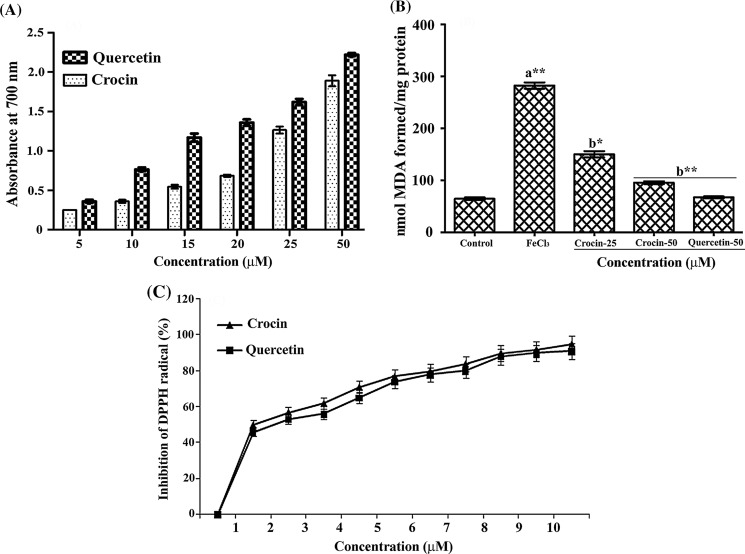
Reducing power assay is based on the ability of the compound to reduce the Fe3+ to Fe2+. The reducing ability of the crocin was found to be significant and dose dependent (Fig. 1A). Further crocin inhibited lipid peroxidation in vitro in a dose depended manner (Fig. 1B). On exposure to FeCl3, the tissue homogenate showed an increased lipid peroxidation as evidenced by the increased MDA levels (3.45 fold) and crocin provided a dose dependent (52 and 79 %) protection at the concentration of 25 and 50 µM, respectively against FeCl3 induced lipid peroxidation. The free radical scavenging potential of crocin was assayed using DPPH. Significant and dose dependent DPPH radical scavenging activity was evident in different concentrations of crocin tested (Fig. 1C). Quercetin, a known antioxidant was used as the positive control.
Fig. 1.
Efficacy of crocin measured in terms of (A) reductive potential (B) FeCl3 induced lipid peroxidation and (C) free radical scavenging activity as measured by DPPH radical scavenging assay in comparison with Quercetin. Bars assigned with letter a and b are statistically significant compared to control and FeCl3 treated group respectively. *p < 0.05, **p < 0.01 and each value represents the mean ± SEM of five independent experiments
ROS and H2O2 generation
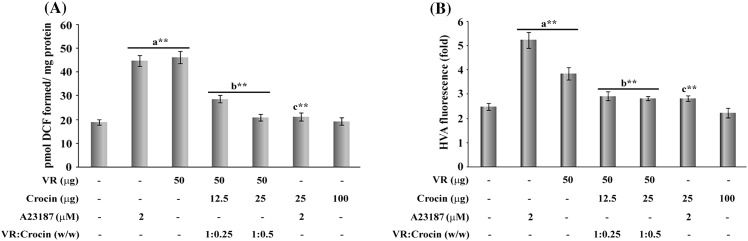
Crocin was evaluated for its efficacy against VR venom induced endogenous generation of ROS and H2O2 levels in neutrophils. A marked enhancement in the levels of oxidative markers was apparent in neutrophils after the treatment with VR venom. The VR venom induced vigorous elevation in the levels of ROS by 144 ± 5 % as well as HP by 67 ± 2 %, against 137 ± 5 % and 110 ± 6.5 in ROS and HP (2 µM calcium ionophore), compared to control cells. Crocin significantly and dose dependently (Fig. 2A) abrogated the ROS by 65 ± 1.7 % and 93 ± 3.5 % and HP (Fig. 2B) levels by 52 ± 2.5 and 67 ± 3.8 % at 67 ± 3.8 % at 1:0.25 and 1:0.5 ratio, respectively, in the crocin treated groups compared to venom treated cells. Further, crocin offered 91.3 ± 6 and 96 ± 5.8 % protection against 2 μM calcium ionophore, respectively, whereas no such alterations were observed in crocin alone treated cells compared to control cells.
Fig. 2.
Effect of crocin treatment on the generation of (A) reactive oxygen species measured in terms of pmol DCF fluorescence and (B) hydroperoxide levels measured in terms of HVA fluorescence in VR venom and A23187 treated neutrophils with indicated doses. Bars assigned with letters a, b and c are statistically significant compared to control, VR venom and A23187 alone respectively. **p < 0.01 and each value represent the mean ± SEM of three independent experiments
Myeloperoxidase activity
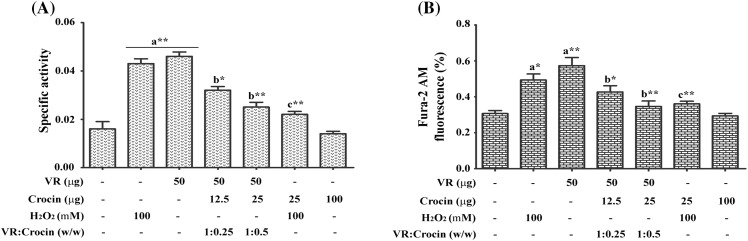
Treatment of cells with VR venom resulted in the robust elevation of myeloperoxidase activity by 187 ± 4.6 % while 168 ± 4 % with 100 mM H2O2 when compared to the control cells. Whereas, in the crocin treated groups protection was observed to an extent of about 47 and 80 % at 1:0.25 and 1:0.5 ratio, respectively. A reduction of 78 ± 5.2 % was observed in the H2O2 treated cells. While, crocin alone did not cause any alteration in the activity level (Fig. 3A).
Fig. 3.
Inhibitory effect of crocin on the VR venom induced (A) myeloperoxidase activity and (B) intracellular Ca2+ level measured in terms of Fura 2-AM fluorescence in VR venom and H2O2 treated neutrophils with indicated doses. Bars assigned with letter a, b and c are statistically significant compared to control, VR venom and H2O2 alone respectively. *p < 0.05, **p < 0.01 and each value represents the mean ± SEM of three independent experiments
Intracellular Ca2+ levels
The fluorescent Ca2+ indicator, Fura 2-AM was used to measure the intracellular Ca2+ concentration. Treatment of neutrophils with VR venom resulted in elevated intracellular Ca2+ levels by 86 ± 4.8 % against 61 % increase with H2O2. In crocin pre-treated groups the intracellular concentration of Ca2+ was reduced by 65.2 ± 2.4 and 85 ± 4 % at 1:0.25 and 1:0.5 ratio, respectively, while it offered 67 ± 3.5 % protection against H2O2. Slight but not significant increase in Ca2+ levels was observed in the crocin treated neutrophils (Fig. 3B).
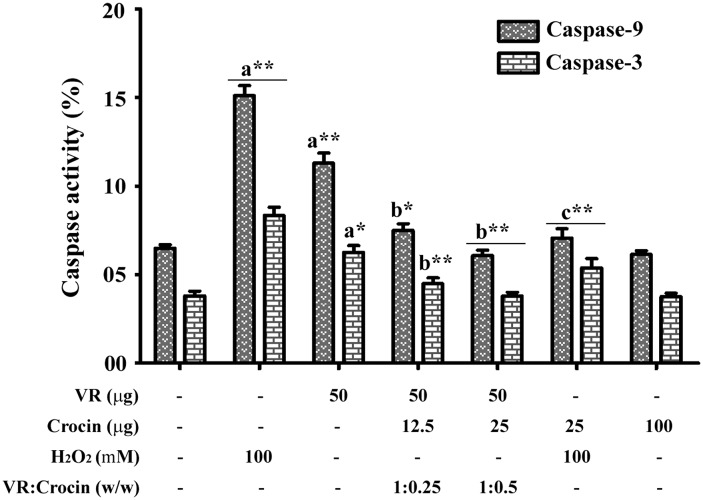
Activation of caspase-3 and -9
Caspase activation was determined using their respective fluorescent substrates. Treatment of neutrophils with VR venom significantly increased the activities of caspase-3 and -9 by 63 ± 3 and 78 ± 3.4 % respectively (Fig. 4), compared to 93 ± 5.6 % increase with H2O2, and though the maximal activation was not reached in the range of time studied. Crocin treatment significantly abrogated the venom induced activation of caspases in a dose dependent manner. Crocin ameliorated the venom induced activation of caspase-3 and -9 by 72 ± 5 and 78 ± 4.5 % at 1:0.25 ratio, respectively, while at 1:0.5 ratio crocin completely inhibited both the venom and H2O2 induced caspase activity. Crocin alone did not alter the caspase levels.
Fig. 4.
Protective effect of crocin on the VR venom induced caspase-3 and -9 activation in VR venom and H2O2 treated neutrophils with indicated doses. Bars assigned with letter a, b and c are statistically significant compared to control, VR venom and H2O2 alone respectively. *p < 0.05, **p < 0.01 and each value represents the mean ± SEM of three independent experiments
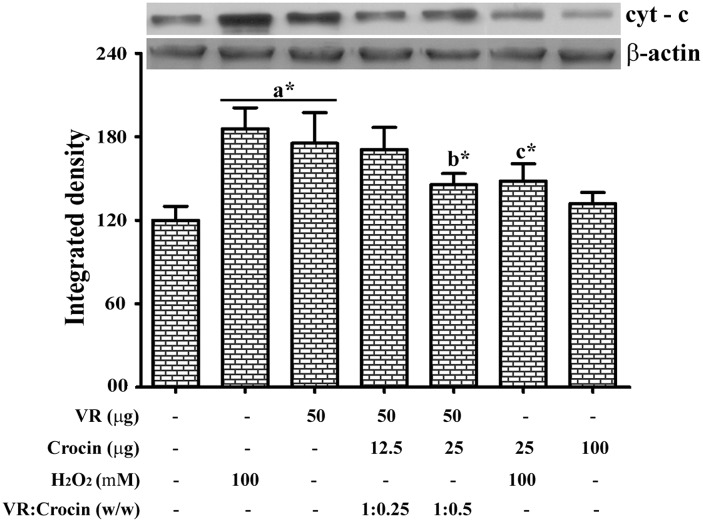
Cytochrome C level
To determine the activation of caspase pathway by VR venom, cyt-c release in neutrophils was assessed by western blotting. Both H2O2 and VR venom promoted the release of cyt-c from the mitochondria as evidenced from the blot (Fig. 5). On the other hand, the cyt-c levels of control cells as well as the crocin treated cells were found to be unaltered. In contrast, in the pre-treatment group crocin markedly reduced the VR venom induced release of cyt-c.
Fig. 5.
Representative immunoblot for cytochrome C and β-actin with corresponding densitogram. The protein expression levels of cytochrome C were determined in VR venom, H2O2 and crocin treated neutrophils with indicated doses. β-actin was used as the loading control. The cytosolic proteins from respective groups were electrophoresed followed by transferred to membranes and blots were further processed as described in materials and methods section. Bars assigned with letter a, b and c are statistically significant compared to control, VR venom and H2O2 alone respectively. *p < 0.05 and each value represent the mean ± SEM
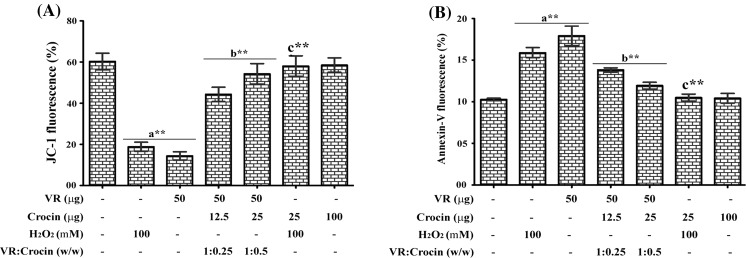
Mitochondrial membrane potential and PS scrambling
The altered mitochondrial membrane depolarization was detected by the decrease in JC-1 fluorescence ratio (585/516 nm). A significant reduction in membrane potential (320 ± 16 and 223 ± 14.5 %) was observed in VR venom and H2O2 treated neutrophils compared to the control. At 1:0.25 and 1:0.5 ratio, crocin restored the changes, respectively, by 65 ± 5.5 and 86 ± 6.4 % compared to venom treated cells and 94 ± 5.2 % compared to H2O2 treated cells. Crocin alone did not alter the membrane potential and values were similar to that of control (Fig. 6A).
Fig. 6.
Ameliorative efficacy of crocin on VR venom induced (A) mitochondrial membrane depolarization and (B) PS externalization in VR venom and H2O2 treated neutrophils with indicated doses. Bars assigned with letter a, b and c are statistically significant compared to control, VR venom and H2O2 alone respectively. **p < 0.01 and each value represent the mean ± SEM of three independent experiments
PS externalization was quantified by annexin V staining. Fig. 6B indicates the levels of PS externalization in neutrophils. VR venom induced the surface PS exposure by 81 ± 6.2 % in neutrophils while H2O2 resulted in 58 ± 3.2 %. In contrast, crocin dose dependently inhibited the PS exposure induced by VR venom by 62 ± 2.5 and 85 ± 3.8 %, respectively, at 1:0.25 and 1:0.5 ratio compared to venom treated neutrophils and completely neutralized the H2O2 induced PS externalization.
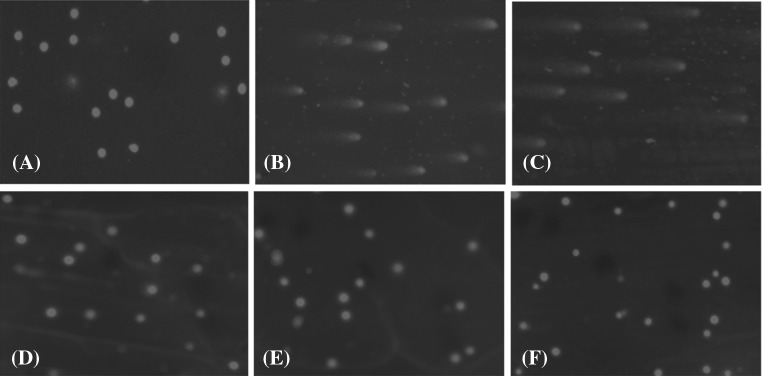
Comet assay
DNA damage was assessed by comet assay in the images of control and crocin treated neutrophils, undamaged DNA remained within the core (Fig. 7A, F). In H2O2 and venom treated group the damaged DNA, however, migrated from the core towards the anode, forming the tail of a comet (Fig. 7B, C). On the other hand, in crocin pre-treated group (1:0.5 ratio) complete inhibition was observed (Fig. 7D). In addition, Crocin completely inhibited H2O2 induced DNA damage (Fig. 7E). Fluorescent microscopy revealed fluorescent structures corresponding to the ethidium bromide stained nuclear DNA of the neutrophils. In undamaged cells, the DNA was tightly compressed and maintained the circular disposition of the normal nucleus.
Fig. 7.
Protective efficacy of crocin against the VR venom induced DNA damage in neutrophils analyzed by comet assay as described in materials and methods section. (A) Control cells; (B) neutrophils treated with H2O2 alone (100 mM); (C) neutrophils treated with VR venom alone (50 µg); (D) neutrophils treated with VR venom pre-incubated with crocin at 1:0.5 ratio (venom: crocin; w/w); (E) neutrophils treated with H2O2 pre-incubated with crocin (25 µg) (F) neutrophils treated with crocin alone (50 µg)
Discussion
Viper envenomation results in the physiological insult to the victim leading to a wide range of pharmacological effects including hypotension, coagulopathy, tissue necrosis and local as well as systemic hemorrhage. Owing to its high mortality and morbidity rate and high frequency of envenomation by this species the World Health Organization has designated Vipera russelli venom as one of the International Reference venom and anti-venom against this venom is considered important (Tsai et al. 1996).
Inflammation is a universal defense response of the organism against infection or tissue injury. During envenomation, various enzymatic and non-enzymatic toxins are known to induce inflammation at their target sites. SVMPs, c-type lectins and other toxins are known to induce inflammation by recruiting inflammatory cells and mediators. These actions activate the early events of an acute inflammatory response at bitten site as well as the systemic target sites (Teixeira et al. 2005; Clissa et al. 2006).
Among the inflammatory cells, neutrophils are the most abundant and considered to be the central players in acute inflammation. These cells swiftly reach the injured tissue site and display their action by installing antimicrobial proteins, proteases and reactive oxygen species. During the process, activated neutrophils release intracellular toxic components including lipid mediators, chemokines and cytokines such as IL-1β, IL-6 and TNF-α in order to amplify the inflammatory response by recruiting more cells into the site of injury. In addition, neutrophils are also capable of capturing and killing pathogens extracellularly through the release of neutrophil extracellular traps (NETs). Prolonged or excessive liberation of these toxic substances could intensify the inflammatory process and enhance tissue damage. Therefore, activity of neutrophils declines consecutively by the effect of anti-inflammatory mediators. Several studies have demonstrated that the induction of neutrophil chemotaxis by SVMPs and c-type lectins, a group of cysteine rich proteins are known to induce inflammation locally as well as systemically (Zamuner et al. 2005; El Kebir et al. 2012; Moreira et al. 2012; Santhosh et al. 2013a; Setubal et al. 2013).
Inflammation and oxidative stress are the key events that are interconnected and act in a coordinated fashion. Oxidative stress exerts its destructive action on cells/organs in association with other phenomenon including inflammation and apoptosis. The interplay between oxidative stress and inflammation has been implicated in the pathophysiology of many diseases including cardiovascular diseases, diabetes, cancer, arthritis, chronic kidney and neurological diseases. Several studies have demonstrated that the snake venom and their isolated components such as PLA2 and metalloproteases induce apoptosis in different cell types (Valko et al. 2007; Brenes et al. 2010; Murakami et al. 2011; Park et al. 2012). Venom induced cell death is currently the subject of considerable interest for toxicologists in order to understand the molecular mechanism and to explore the possible therapeutic purposes of venom. Snake venoms can activate apoptosis either through extrinsic pathway by triggering the binding of extracellular death ligand to the transmembrane death receptors, such as the Fas receptors (Díaz et al. 2005) or through intrinsic pathway initiated by cellular stress, specifically mitochondrial oxidative stress caused by factors such as DNA damage and heat shock (Park et al. 2012; Liu and Chang 2009).
We conducted experiments to evaluate various parameters of apoptotic events in venom treated human neutrophils. The incubation of VR venom significantly stimulated human neutrophils to produce elevated levels of endogenous ROS as well as Ca2+ compared to control, which were effectively impaired when the venom was pre-incubated with crocin. The significant increase in the ROS level indicates the ability of the VR venom to stimulate neutrophils to activate the respiratory burst. Recent studies have shown the induction of intracellular ROS generation in macrophages and peritoneal leukocytes upon treatment with venom proving its ability to stir up respiratory burst (Setubal et al. 2011; De Souza et al. 2012). Studies have shown that the neutrophil apoptosis could be triggered by ROS and elevated Ca2+ through intrinsic/mitochondrial pathway by altering the mitochondrial inner transmembrane potential (ΔΨm). Recent studies have reported the apoptotic effect of various venom toxins on different cancer cell lines. The experimental cells were shown to undergo apoptosis via up-regulation of ROS and JNK-mediated death receptor expression on treatment with venom components. Some of the studies have also shown the inhibition of venom induced apoptosis by using antioxidant enzymes. Our previous study has also shown the protective role of crocin against viper venom induced apoptosis of platelets (Torii et al. 1997; Kumar et al. 2002; Park et al. 2012; Liu and Chang 2009; Santhosh et al. 2013b).
Mitochondrial membrane depolarization is the initial and crucial step of the intrinsic/mitochondrial pathway of apoptosis. The current results clearly affirm the drastic reduction in the membrane potential after treating neutrophils with VR venom. Increased ROS along with elevated levels of Ca2+ facilitate the formation of mitochondrial membrane transition pore (MPTP) responsible for depolarization of mitochondrial inner transmembrane potential, permeabilization of inner and outer mitochondrial membrane and subsequent release of pro-apoptotic proteins (such as cyt-c and apoptosis inducing factor) from mitochondria to cytosol (Danial and Korsmeyer 2004). To further ascertain the pro-apoptotic effect of VR venom, other parameters such as cytosolic cyt-c levels, caspase-9 and -3 activation and PS externalization were evaluated. The study clearly suggested the activation of apoptotic cascade in the human neutrophils upon treatment with VR venom and their abrogation by crocin.
Further, the VR venom induced DNA fragmentation was evidenced using comet assay. The assay is more sensitive and rapid for the detection of DNA damage due to strand breaks, open repair sites, cross-links and labile site at the individual cell levels. The extent of nuclear fragmentation clearly demonstrates the intrinsic pathway mediated apoptosis of neutrophil by VR venom. The induction of DNA damage could be associated with VR venom induced oxidative stress. Additionally, the results demonstrated in the present study may be correlated to VR venom induced pharmacological effects and could also contribute to the elucidation of possible mechanism of action of target specific toxins. Several SVMPs (VAP1, VAP2, VLAIP, graminelysin), L-amino acid oxidase and PLA2 have been reported to induce apoptosis in HUVECs and several cancer cell lines including human liver (Bel7402), human leukemia (K-562) and human gastric carcinoma (BGC823) cells (Zhu et al. 2010; Murakami et al. 2011; Teklemariam et al. 2011).
Neutrophil recruitment and removal of their potentially injurious contents from inflamed tissues determine whether the inflammation has to resolve or to progress into a chronic state. After the completion of task, apoptosis of the inflammatory cells and their subsequent clearance (efferocytosis) by macrophages are key mechanisms orchestrating successful resolution of inflammation (Luo and Loison 2008; Michlewska et al. 2009; Dalli and Serhan 2012). It has been well established that neutrophil accumulation frequently occurs at the site of snakebite as part of the inflammatory response to envenoming (Wei et al. 2010).
In the context of envenomation, locally (SVMPs, myotoxins and c-type lectins) and systemically acting target specific toxins may induce neutrophil apoptosis at the bitten site as well as in the circulation. If neutrophils undergo apoptosis by venom components before their function, the number of functional neutrophils at the site of inflammation will get reduced. Therefore, neutralization of venom induced neutrophil apoptosis not only reduces the inflammation but also increase the number of functional neutrophils. The present study clearly demonstrates the pro-apoptotic effect of VR venom on neutrophils and its amelioration by crocin a known dietary colorant. The antioxidant property of the crocin was confirmed by the in vitro radical scavenging assays. Mechanistically, it is clear that VR venom triggers neutrophils to undergo apoptosis by increasing oxidative stress. However, the possible action of venom components on monocyte differentiation at the site of inflammation cannot be ignored. The neutrophil apoptosis and possible action of venom on differentiation of monocyte to macrophages and dendritic cells would be of particular importance in the management of persistent tissue necrosis at the bitten site and in systemic toxicities.
Acknowledgements
S.S.M thanks the Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi, India, for financial assistance (SRF-Ref: 9/119(0191)2K12EMR-I Dated 28-02-2012). Authors thank Central Instrumentation Facility, University of Mysore, Institute of Excellence Project (IOE), MHRD, Government of India.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Alam MI, Gomes A. Viper venom-induced inflammation and inhibition of free radical formation by pure compound (2-hydroxy-4-methoxy benzoic acid) isolated and purified from anantimul (Hemidesmus indicus R. BR) root extract. Toxicon. 1998;36:207–215. doi: 10.1016/S0041-0101(97)00070-6. [DOI] [PubMed] [Google Scholar]
- Barja G. The quantitative measurement of H2O2 generation in isolated mitochondria. J Bioenerg Biomembr. 2002;34:227–233. doi: 10.1023/A:1016039604958. [DOI] [PubMed] [Google Scholar]
- Ben Amor N, Pariente JA, Salido GM, Bartegi A, Rosado JA. Caspases 3 and 9 are translocated to the cytoskeleton and activated by thrombin in human platelets. Evidence for the involvement of PKC and the actin filament polymerization. Cell Signal. 2006;18:1252–1261. doi: 10.1016/j.cellsig.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618–622. [PubMed] [Google Scholar]
- Brenes O, Munoz E, Roldan-Rodriguez R, Diaz C. Cell death induced by Bothrops asper snake venom metalloproteinase on endothelial and other cell lines. Exp Mol Pathol. 2010;88:424–432. doi: 10.1016/j.yexmp.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Clissa PB, Lopes-Ferreira M, Della-Casa MS, Farsky SH, Moura-da-Silva AM. Importance of jararhagin disintegrin-like and cysteine-rich domains in the early events of local inflammatory response. Toxicon. 2006;47:591–596. doi: 10.1016/j.toxicon.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Coelho AL, De Freitas MS, Mariano-Oliveira A, Rapozo DC, Pinto LF, Niewiarowski S, Zingali RB, Marcinkiewicz C, Barja-Fidalgo JP. RGD- and MLD-disintegrins, jarastatin and EC3, activate integrin-mediated signaling modulating the human neutrophils chemotaxis, apoptosis and IL-8 gene expression. Exp Cell Res. 2004;292:371–384. doi: 10.1016/j.yexcr.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- De Souza CA, Kayano AM, Setubal SS, Pontes AS, Furtado JL, Kwasniewski FH, Zaqueo KD, Soares AM, Stábeli RG, Zuliani JP (2012) Local and systemic biochemical alterations induced by Bothrops atrox snake venom in mice. J Venom Res 3:28–34 [PMC free article] [PubMed]
- Díaz C, Valverde L, Brenes O, Rucavado A, Gutiérrez JM. Characterization of events associated with apoptosis/anoikis induced by snake venom metalloproteinase BaP1 on human endothelial cells. J Cell Biochem. 2005;94:520–528. doi: 10.1002/jcb.20322. [DOI] [PubMed] [Google Scholar]
- Driver AS, Kodavanti PS, Mundy WR. Age related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol. 2000;22:175–181. doi: 10.1016/S0892-0362(99)00069-0. [DOI] [PubMed] [Google Scholar]
- El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci USA. 2012;109:14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girish KS, Kemparaju K. Overlooked issues of snakebite management: time for strategic approach. Curr Top Med Chem. 2011;11:2494–2508. doi: 10.2174/156802611797633393. [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Yan GC. Antioxidant actions of du-zhong (Eucommia ulmoides Oliv.) toward oxidative damage in biomolecules. Life Sci. 2000;66:1387–1400. doi: 10.1016/S0024-3205(00)00450-1. [DOI] [PubMed] [Google Scholar]
- Jardin I, Ben Amor N, Bartegi A, Pariente JA, Salido GM, Rosado JA. Differential involvement of thrombin receptors in Ca2+ release from two different intracellular stores in human platelets. Biochem J. 2007;401:167–174. doi: 10.1042/BJ20060888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz. 2002;27:662–668. doi: 10.1007/s00059-002-2430-3. [DOI] [PubMed] [Google Scholar]
- Lalloo D, Theakston DG. Snake antivenoms. J Toxicol Clin Toxicol. 2003;41:277–290. doi: 10.1081/CLT-120021113. [DOI] [PubMed] [Google Scholar]
- Leon G, Valverde JM, Rojas G, Lomonte B, Gutierrez JM. Comparative study on the ability of IgG and Fab sheep anti-venoms to neutralize local hemorrhage, edema and myonecrosis induced by Bothrops asper (terciopelo) snake venom. Toxicon. 2000;38:233–244. doi: 10.1016/S0041-0101(99)00152-X. [DOI] [PubMed] [Google Scholar]
- Liu WH, Chang LS. Reactive oxygen species and p38 mitogen-activated protein kinase induce apoptotic death of U937 cells in response to Naja nigricollis toxin-gamma. J Cell Mol Med. 2009;13:1695–1705. doi: 10.1111/j.1582-4934.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- Lopez JJ, Salido GM, Gómez-Arteta E, Rosado JA, Pariente JA. Thrombin induces apoptotic events through the generation of reactive oxygen species in human platelets. J Thromb Haemost. 2007;5:1283–1291. doi: 10.1111/j.1538-7836.2007.02505.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement using folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83:288–295. doi: 10.1002/ajh.21078. [DOI] [PubMed] [Google Scholar]
- Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB J. 2009;23:844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- Montalvo-Hernández B, Rito-Palomares M, Benavides J. Recovery of crocins from saffron stigmas (Crocus sativus) in aqueous two-phase systems. J Chromatogr A. 2012;1236:7–15. doi: 10.1016/j.chroma.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Moreira V, Dos-Santos MC, Nascimento NG, Borges da Silva H, Fernandes CM, D’Império Lima MR, Teixeira C. Local inflammatory events induced by Bothrops atrox snake venom and the release of distinct classes of inflammatory mediators. Toxicon. 2012;60:12–20. doi: 10.1016/j.toxicon.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Mukherjee AK, Maity CR. Effect of dietary supplementation of vitamin E in partial inhibition of Russell’s viper venom phospholipase A2 induced hepatocellular and microsomal membrane damage in rats. Acta Physiol Hung. 1997;85(4):367–374. [PubMed] [Google Scholar]
- Murakami T, Kamikado N, Fujimoto R, Hamaguchi K, Nakamura H, Chijiwa T, Ohno M, Oda-Ueda N. A [Lys49] phospholipase A2 from Protobothrops flavoviridis venom induces caspase-independent apoptotic cell death accompanied by rapid plasma-membrane rupture in human leukemia cells. Biosci Biotechnol Biochem. 2011;75:864–870. doi: 10.1271/bbb.100783. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohnishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituricacid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Park MH, Jo MR, Won D, Song HS, Han SB, Song MJ, Hong JT. Snake venom toxin from Vipera lebetina turanica induces apoptosis in colon cancer cells via upregulation of ROS- and JNK-mediated death receptor expression. BMC Cancer. 2012;12:228. doi: 10.1186/1471-2407-12-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado JA, Graves D, Sage SO. Tyrosine kinases activate store-mediated Ca2+ entry in human platelets through the reorganization of the actin cytoskeleton. Biochem J. 2000;351:429–437. doi: 10.1042/bj3510429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/S0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- Santhosh MS, Sundaram MS, Sunitha K, Kemparaju K, Girish KS. Viper venom-induced oxidative stress and activation of inflammatory cytokines: a therapeutic approach for overlooked issues of snakebite management. Inflamm Res. 2013;62:721–731. doi: 10.1007/s00011-013-0627-y. [DOI] [PubMed] [Google Scholar]
- Santhosh MS, Thushara RM, Hemshekhar M, Sunitha K, Devaraja S, Kemparaju K, Girish KS. Alleviation of viper venom induced platelet apoptosis by crocin (Crocus sativus): implications for thrombocytopenia in viper bites. J Thromb Thrombolysis. 2013;36:424–432. doi: 10.1007/s11239-013-0888-x. [DOI] [PubMed] [Google Scholar]
- Sebastin Santhosh M, Hemshekhar M, Thushara RM, Devaraja S, Kemparaju K, Girish KS. Vipera russelli venom induced oxidative stress and hematological alterations: amelioration by crocin a dietary colorant. Cell Biochem Funct. 2013;31:41–50. doi: 10.1002/cbf.2858. [DOI] [PubMed] [Google Scholar]
- Setubal Sda S, Pontes AS, Nery NM, Bastos JS, Castro OB, Pires WL, Zaqueo KD, et al. Effect of Bothrops bilineata snake venom on neutrophil function. Toxicon. 2013;76:143–149. doi: 10.1016/j.toxicon.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Setubal SS, Pontes AS, Furtado JL, Kayano AM, Stabeli RG, Zuliani JP. Effect of Bothrops alternatus snake venom on macrophage phagocytosis and superoxide production: participation of protein kinase C. J Venom Anim Toxins Incl Trop Dis. 2011;17:430–441. [Google Scholar]
- Singh NP, McCoy T, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–192. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Singh M, Sharma H, Singh N. Hydrogen peroxide induces apoptosis in HeLa cells through mitochondrial pathway. Mitochondrion. 2007;7:367–373. doi: 10.1016/j.mito.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Teixeira Cde F, Fernandes CM, Zuliani JP, Zamuner SF. Inflammatory effects of snake venom metalloproteinases. Mem Inst Oswaldo Cruz. 2005;1:181–184. doi: 10.1590/S0074-02762005000900031. [DOI] [PubMed] [Google Scholar]
- Teklemariam T, Seoane AI, Ramos CJ, Sanchez EE, Lucena SE, Perez JC, Mandal SA, Soto JG (2011) Functional analysis of a recombinant PIII-SVMP, GST-acocostatin; an apoptotic inducer of HUVEC and HeLa, but not SK-Mel-28 cells. Toxicon 57:646–656 [DOI] [PMC free article] [PubMed]
- Torii S, Naito M, Tsuruo T. Apoxin I, a novel apoptosis-inducing factor with L-amino acid oxidase activity purified from Western diamondback rattlesnake venom. J Biol Chem. 1997;272:9539–9542. doi: 10.1074/jbc.272.14.9539. [DOI] [PubMed] [Google Scholar]
- Tsai IH, Lu PJ, Su JC. Two types of Russell’s viper revealed by variation in phospholipases A2 from venom of the subspecies. Toxicon. 1996;4:99–109. doi: 10.1016/0041-0101(95)00114-X. [DOI] [PubMed] [Google Scholar]
- Ushanandini S, Nagaraju S, Harish Kumar K, Vedavathi M, Machiah DK, Kemparaju K, Vishwanath BS, Gowda TV, Girish KS (2006) The anti-snake venom properties of Tamarindus indica (Leguminosae) seed extract. Phytother Res 20:851–858 [DOI] [PubMed]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wei JF, Wei XL, Chen QY, He SH. Induction of inflammatory cell accumulation by TM-N49 and promutoxin, two novel phospholipase A(2) Toxicon. 2010;56:580–588. doi: 10.1016/j.toxicon.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 1998;62:1201–1204. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- Zamuner SR, Zuliani JP, Fernandes CM, Gutiérrez JM, de Fátima Pereira Teixeira C. Inflammation induced by Bothrops asper venom: release of proinflammatory cytokines and eicosanoids, and role of adhesion molecules in leukocyte infiltration. Toxicon. 2005;46:806–813. doi: 10.1016/j.toxicon.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Zhu L, Yuan C, Chen Z, Wang W, Huang M. Expression, purification and characterization of recombinant Jerdonitin, a P-II class snake venom metalloproteinase comprising metalloproteinase and disintegrin domains. Toxicon. 2010;55:375–380. doi: 10.1016/j.toxicon.2009.08.016. [DOI] [PubMed] [Google Scholar]