Abstract
In recent times, the study and use of induced pluripotent stem cells (iPSC) have become important in order to avoid the ethical issues surrounding the use of embryonic stem cells. Therapeutic, industrial and research based use of iPSC requires large quantities of cells generated in vitro. Mammalian cells, including pluripotent stem cells, have been expanded using 3D culture, however current limitations have not been overcome to allow a uniform, optimized platform for dynamic culture of pluripotent stem cells to be achieved. In the current work, we have expanded mouse iPSC in a spinner flask using Cytodex 3 microcarriers. We have looked at the effect of agitation on the microcarrier survival and optimized an agitation speed that supports bead suspension and iPS cell expansion without any bead breakage. Under the optimized conditions, the mouse iPSC were able to maintain their growth, pluripotency and differentiation capability. We demonstrate that microcarrier survival and iPS cell expansion in a spinner flask are reliant on a very narrow range of spin rates, highlighting the need for precise control of such set ups and the need for improved design of more robust systems.
Keywords: Microcarrier, Induced pluripotent stem cells, Spinner flask
Introduction
The unique properties of unlimited self renewal and differentiation into all types of cells have made pluripotent stem cells exciting tools for tissue engineering, regenerative medicine and drug screening. The discovery of iPSC has allowed scientists to harness and utilize these cells without the ethical dilemma associated with ESC (Takahashi and Yamanaka 2006). However, practical use in cellular therapy requires cell numbers ranging typically between several thousands to a few billions. For example, it is estimated that on average approximately 4.0 × 1010 cells would be required to treat each patient requiring cardiomyocyte replacement. This roughly equates to 1,144 T-175 flasks per patient, making it an impossible task (Want et al. 2012). The use of large scale culture systems or bioreactors is a necessity for such purposes. Apart from scaling up of culture, the use of bioreactors also allows better aeration, facilitates proper nutrient supply to cells, reduces consumable costs and supports long term culture of cells. Spinner flasks are one of the oldest and most widely used reactor systems and have been used extensively for the expansion of mouse as well as human ESC. Both mouse and human ESC have been expanded as aggregates in spinner flasks (Fok and Zandstra 2005; Cormier et al. 2006; Abbasalizadeh et al. 2012; Krawetz et al. 2010; Steiner et al. 2010). Extensive study in the field of large scale expansion of ES cells has given rise to novel and better systems for these cells but the field of dynamic culture of iPS cells has just started. Recently, efforts have also been made to expand iPSC in spinner flasks as aggregates (Fluri et al. 2012; Olmer et al. 2010, 2012; Shafa et al. 2012). However, expansion as aggregates is associated with some disadvantages. The formation of aggregates may lead to concentration gradients of nutrients and oxygen, resulting in their uneven distribution. In case of larger aggregates, this may even result in improper waste removal along with cell death and necrosis at the centre. The use of microcarriers in conjunction with spinner flasks has been deemed to be a suitable alternative to aggregate expansion of pluripotent cells. A successful microcarrier expansion is dependent on several factors including the type of carrier used, the cell line being expanded, the spinner flask set up, the position of the impeller and the spin rate (revolutions per minute—RPM) used. It is well known that the sensitivity of the cells to the spinning is of utmost importance, but it is equally important to know the tolerance level of the microcarriers themselves. Extensive studies have been conducted for expansion of both mouse and human ESC using microcarriers. But the range of RPM used varies widely across the literature along with the type of carrier used. The success of the carriers also varied with the cell line in question, even for the same cell type. Commercially available microcarriers—such as Cultisphere S, Cytodex 3, Solohill carriers and Hillex II have been widely used for the expansion of ESC, with Cytodex 3 being the most common. The speed used for mouse ESC, varies between 40 and 70 RPM (Fok and Zandstra 2005; Abranches et al. 2007; Alfred et al. 2011; Fernandes et al. 2007; Marinho et al. 2010; Storm et al. 2010; Tielens et al. 2007), while the range for human ESC lies within 24–80 RPM (Storm et al. 2010; Chen et al. 2011; Fernandes et al. 2009; Kehoe et al. 2010; Leung et al. 2011; Lock and Tzanakakis 2009; Marinho et al. 2013; Nie et al. 2009; Oh et al. 2009; Phillips et al. 2008; Serra et al. 2010). iPSC are by nature, delicate, making their large scale culture in spinner flasks using microcarriers a much more difficult proposition. Until now, very few groups have tried to use microcarriers for the expansion of iPSC. Kehoe et al. (2010) have shown some preliminary data in their review article. Recently, Bardy et al. (2013) have expanded iPSC on microcarriers, followed by their differentiation into neural progenitor cells. However, prior to spinner flask culture, they had to adapt the iPSC to expansion on the carriers in static culture. Also, in both these cases, the carriers were coated with Matrigel, which was already well known for supporting the growth of pluripotent stem cells in the absence of feeder cells. Similar to the studies with ESC, the spinner flask spin rate for these two studies varied considerably.
The experimental limitations associated with any type of dynamic culture bring into the forefront the need to integrate engineering aspects such as fluid mechanics with biology. Our group has worked towards this aspect of spinner flask culture for some time now with the earliest work reported in 2006 (Dusting et al. 2006). In this work, a detailed study of the flow dynamics within a prototype reactor system similar to a spinner flask was undertaken for the first time, and it highlighted the need for similar studies to understand the shear stress and flow structures that affect microcarrier breakage, cell attachment and expansion. A similar study involving cell growth in a stirred bioreactor was also carried out, integrating theoretical modeling and experimental results (Thouas et al. 2007). More recently, efforts were also made to understand the mixing properties of flow within a cylindrical system similar to a spinner flask (Meunier and Hourigan 2013). Currently, a new holographic technique for visualization of flow within a spinner flask has been developed in our laboratory (Ismadi et al. 2013). This particular technique can be used for a deeper understanding of fluid flow and carrier dispersement within a spinner flask, providing us with in depth knowledge of the system and design guidelines.
In this current work, we have expanded mouse iPSC in a spinner flask using Cytodex 3, without any prior coating of the carriers. Although widely used for ES cells, this is the first time Cytodex 3 has been used for iPS cells. We have used the cells directly from their 2D feeder dependent culture, thus testing their adaptability to a change in culture condition. We have also studied the effect of different spin rates covering the entire range published till date, related to Cytodex 3 microcarriers and iPSC. Our work highlights the presence of very specific spin rate ranges for: carrier breakage; survival of the carriers but with no cell attachment; short term cell attachment and survival; non suspension of carriers; and finally, an optimized spin rate for carrier suspension without breakage and long term attachment and expansion of iPSC. The results can also be used to corroborate future fluid dynamic modeling and visualization of the current system.
Materials and methods
Cell culture
Mouse OG2 iPSC were generated previously in the lab (Tat et al. 2010) and were regularly maintained on mitomycin C inactivated feeder cells using ES media containing DMEM, 15 % fetal bovine serum, 1× Glutamax, 1× non essential amino acid, 1× Pen/Strep Solution, 0.1 mM β-mercaptoethanol and 1,000 U/ml LIF solution (Merck-Millipore, Billerica, MA, USA). The cells were incubated in a humidified incubator at 37 °C with 5 % CO2. Unless otherwise mentioned, all media materials were purchased from Invitrogen (Life Technologies, Carlsbad, CA, USA). The iPSC used have a GFP transgene under the control of the promoter of the pluripotent gene Oct4.
Spinner flask culture
Based on the available literature on expansion of ES cells on carriers, Cytodex 3 and Hillex II were selected for a preliminary study on iPS cell attachment on these two carriers. This study demonstrated that the iPS cell line used for our main study displayed better cell attachment and expansion on Cytodex 3 (data not shown). Hence Cytodex 3 was chosen for the spinner flask culture.
100 ml spinner flasks from Bellco Biotechnology (Bellco Glass, Inc., Vineland, NJ, USA) were used with a final media volume of 50 ml. The spinner flasks were coated with SigmaCote (Sigma Aldrich, St. Louis, MO, USA) prior to usage. Cytodex 3 carriers (GE Health Care, Little Chalfont, UK) equivalent to a surface area of 200 cm2/flask were weighed, hydrated using PBS and sterilized by autoclaving as per manufacturer’s instructions. The carriers were equilibrated prior to use by incubating them in culture medium for around 1 h. A seeding density of 2 × 105 cells/ml was used for each flask. The cells were inoculated with the carriers in a low adherence 10 cm dish for about 24 h in order to facilitate cell attachment. After 24 h, carriers with cells were transferred to the spinner flasks and the culture volume was adjusted to 50 ml. The impeller was adjusted after initial trial and error experiments in such a way that the stirrer was half immersed, in order to minimise mechanical damage to the carriers as well as the cells as far as possible. We observed more carrier breakage if the impeller was submerged too much and if the motor rotation was not smooth. The spinner flask’s impeller was directly connected to a stepper motor using a custom-made coupling shaft (Fig. 1). The speed of the motor was precisely regulated with a motion controller (National Instrument, Clayton, VIC, Australia). The iPSC were expanded for 7 days in the spinner flask with 50 % medium replacement every day.
Fig. 1.

Spinner flask set up with the impeller directly connected to a stepper motor
Control
As control, the iPSC were grown on 0.1 % gelatin coated 24 well plates (Corning (Corning, NY, USA), Sigma-Aldrich, St Louise, USA) in the same seeding density. The medium was changed at the same frequency as the spinner flasks.
Cell count and microscopy
Samples of microcarriers (1 ml) were taken on days 3, 5 and 7 and viewed under a microscope. The cells were then washed with PBS and trypsinized using 1X Tryple Express (Life Technologies, Carlasbad, USA) for cell detachment. Microcarriers with cells were incubated at 37 °C for about 5 minutes to facilitate cell detachment. The suspension was then filtered through a 100 µm strainer to remove the microcarriers. Cell counts and cell viability were recorded using a haemocytometer and trypan blue assay. On each occasion the sampling and counting were done in duplicates.
Spontaneous differentiation
The differentiation capability of the cells was measured by in vitro EB formation. After 7 days of culture, iPSC from the control set up, as well as the spinner flask culture, were seeded on low-adherence 6 well plates (Nunc, Sigma-Aldrich, St. Louis, USA) with mouse embryonic fibroblasts (mEF) medium at a seeding density of 50,000 cells/well. Medium was changed on alternate days. After 10 days of culture, the EBs were collected and the presence of cells of the three germ layers was ascertained by RT-PCR and immunostaining.
RT-PCR analysis
Cells for RT-PCR analysis were snap frozen on dry ice and stored at −80 °C until analysis. Oct 4, Nanog and c-myc genes were anlaysed for pluripotency while Nestin, Brachyury and Fox A2 were analysed for measuring the cells’ differentiation capability. β actin was used as the house keeping gene in all cases (Primers are listed in Table 1). RNA was isolated from the cells using RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Isolated RNA was cleaned using Ambion RNA Turbo DNAfree kit (Invitrogen) to remove any contaminating genomic DNA. Cleaned RNA was quantified using NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA). cDNA synthesis was carried out using Superscript III kit (Invitrgen) following the manufacturer’s instructions. To verify the procedures, RT-PCR for β-actin was carried out after RNA clean up and cDNA synthesis. The PCR amplification included a total 35 cycles of denaturation at 95 °C for 30 s, followed by annealing at appropriate temperature for 30 s and extension at 72 °C for 1 min with an initial denaturation step at 95 °C for 4 min and a final extension step at 72 °C for 10 min. The PCR products were run on a 1 % agarose gel at 80 V. Gels were visualised using the Bio Rad Universal Hood II GelDoc system (Bio Rad Laboratories Inc., Hercules, CA, USA) and images were taken using Quantity One (version 4.6.3) software.
Table 1.
Primer sequences for genes used in pluripotency and differentiation capability analysis
| Gene | Primer Sequence |
|---|---|
| β-actin |
F: GGA ATC CTG TGG CAT CCA TGA AAC R: AAA ACG CAG CTC AGT AAC AGT CCG |
| Oct 3/4 |
F: TCT TTC CAC CAG GCC CCC GGC TC R: TGC GGG CGG ACA TGG GGA GAT CC |
| Nanog |
F: TCA AGG ACA GGT TTC AGA AGC A R: GCT GGG ATA CTC CAC TGG TG |
| c-myc |
F: AAG TTT GAG GCA GCA GTT AAA ATT ATG GCT GAA R: TGA CCT AAC TCG AGG AGG AGC TGG AAT C |
| Nestin |
F: TCT GGA AGT CAA CAG AGG TGG R: ACG GAG TCT TGT TCA CCT GC |
| Brachyury |
F: CAT GTA CTC TTT CTT GCT GG R: GGT CTC GGG AAA GCA GTG GC |
| Fox A2 |
F: TGG TCA CTG GGG ACA AGG GAA R: GCA ACA ACA GCA ATA GAG AAC |
Immunostaining
Cells on microcarriers were fixed using 4 % paraformaldehyde followed by permeabilization and blocking using 0.1 % Triton X and 2 % BSA in PBS, respectively. Subsequently, the cells were incubated overnight with primary antibodies against SSEA-1 and Nanog. Next day, the cells were stained with appropriate secondary antibodies that were rhodamine conjugated for SSEA-1 and Alexa Fluor 594 conjugated for Nanog, following which the cells were subjected to nuclear staining with Hoechst 33342. Cells were also trypsinized for detachment from the microcarriers following the previously mentioned protocol after 7 days of culture and seeded on to 24 well plates for 3 days followed by staining for SSEA 1 and Nanog markers as per the aforementioned protocol.
EBs were stained for Brachyury, Nestin and FoxA2 markers following the same protocol. The secondary antibodies for all three markers were conjugated with Alexa Fluor 594.
Scanning electron microscopy
After 7 days of culture, the cells on microcarriers were fixed with 3 % gluteraldehyde, followed by drying using graded ethanol. This was followed by chemical drying using a transition from 100 % ethanol to 100 % Hexamethyldisilazane (HMDS) through a graded series of ethanol–HMDS mixture, ending at 100 % HMDS. The samples were then sputter coated and imaged using Hitachi S570 scanning electron microscope (Hitachi, Tokyo, Japan).
Pluripotency marker fluorescence intensity quantification
At the end of 7 days of culture, the cells were trypsinized using the previously mentioned protocol and re-plated on gelatin coated Optilux opaque wall plates for 6–8 h to allow attachment, following which they were fixed using 4 % paraformaldehyde. The cells were then permeabilized using 0.1 % Triton X followed by blocking with 2 % BSA for 30 min. The cells were then incubated overnight with mouse IgM anti SSEA 1 antibody (Merck-Millipore) at 4 °C. After washing, the cells were incubated with anti mouse—IgM rhodamine (Chemicon International, Merck-Millipore) for 1 h followed by 30 min incubation with 1 µg/ml Hoechst 3342 solution. The fluorescence intensity for the pluripotency gene SSEA 1 was measured using an Array Scan High Content Screening instrument (Thermo Scientific, Waltham, MA, USA).
Effect of spin rate on microcarriers
In order to study the effect of different spin rates on Cytodex 3 microcarriers (1.5 mg/ml), the carriers were suspended in PBS in spinner flasks for 2 days at various RPMs. After 2 days, 1 ml of the suspension was taken out and studied under microscope.
Results
Effect of spin rate on microcarrier breakage
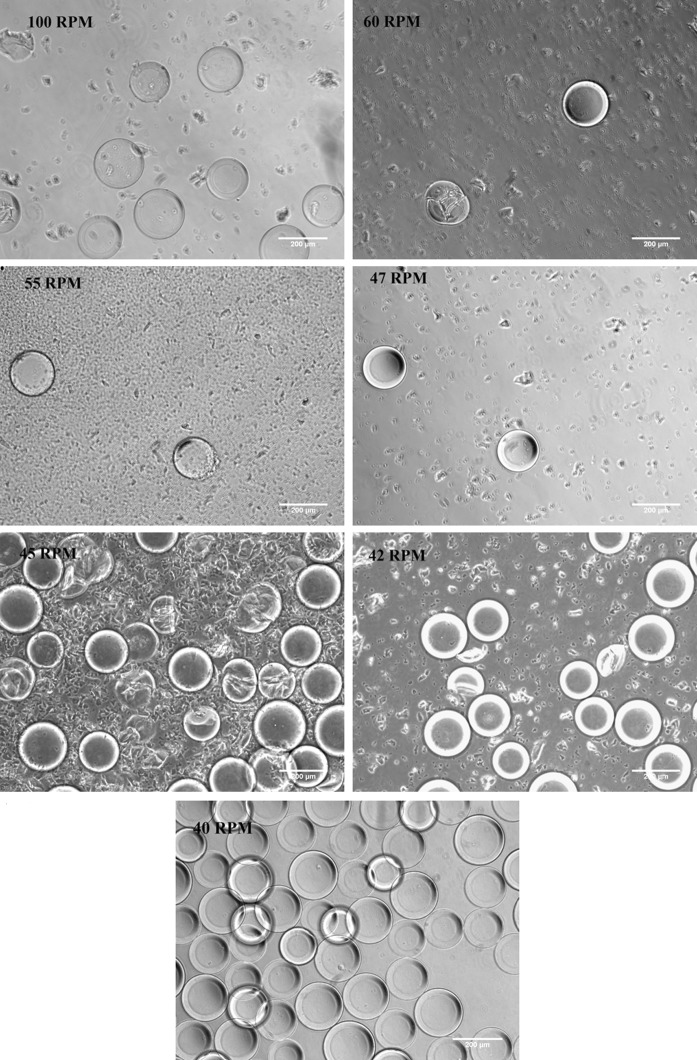
Proper attachment and expansion of iPSC in spinner flasks is dependent on the survival of the microcarriers themselves. We were interested in studying the effect of different speeds on the Cytodex 3 microcarriers. The aim was to find out the maximum speed that the carriers can tolerate within the spinner flask without any breakage. 100 RPM was taken as the upper limit based on literature survey along with set ups at 60, 55, 47, 45, 42 and 40 RPM. As seen in Fig. 2, spin rates between 42 &100 RPM resulted in the breakage of the Cytodex 3 carriers after 2 days in the spinner flask. However, at 40 RPM, no carrier breakage was observed. 40 RPM was thus optimal as far as being the highest RPM that the Cytodex 3 carriers could tolerate.
Fig. 2.
Effect of different spin rates on Cytodex 3 microcarriers. Carriers were put in a spinner flask and kept in dynamic motion for 2 days. Samples were then taken out and observed under a phase contrast microscope. Scale bar 200 µm. The threshold level of tolerance for Cytodex 3 was found to be at 40 RPM
The proper suspension of microcarriers in the spinner flask was also considered to be an important aspect for long term culture of cells in a dynamic environment. For this purpose we also looked at the suspension of Cytodex 3 carriers at lower spin rates (data not shown). It was observed that the suspension of the carriers was negligible at 20 RPM and below.
Based on these results, spin rates between 25 and 40 RPM were tested for attachment and expansion of mouse iPSC in spinner flask using Cytodex 3 microcarriers. The aim was to find a spin rate at which the carriers were properly suspended and at the same time supported long term cell expansion.
Attachment and expansion of iPSC in spinner flask at different spin rates
Similar to any mammalian cell line, the mouse iPSC will have a threshold spin rate in terms of cell attachment, expansion and pluripotency maintenance. Based on the above results, we looked at attachment and expansion of mouse iPSC on Cytodex 3 carriers at 40, 30, 28 and 25 RPM in order find out the optimum spin rate for their long term expansion.
Cells and microcarriers were put in spinner flasks after 18–24 h of static attachment and subjected to 7 days spinning. Samples were drawn and imaged daily while a cell count was performed on days 3, 5 and 7.
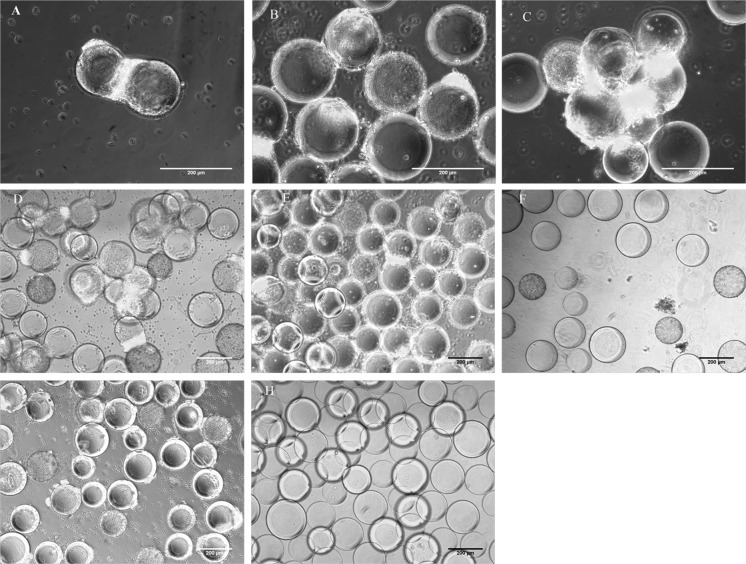
It was observed that at 40 RPM the cells detached within 24 h of spinner flask culture and thus it was deemed unfit for further studies. Microscopic images showed cell attachment at 28 (Fig. 3D–F) and 30 RPM (Fig. 3G, H) in the initial culture days, but they were not able to support long term cell culture.
Fig. 3.
Expansion of mouse iPSC on Cytodex 3 carriers at different spin rates over days 3, 5 and 7. A–C 25RPM, D–F 28 RPM, G, H 30 RPM. A, D, G day 3, B, E, H day 5, C, F day 7. Scale bar 200 µm
On the other hand, at 25 RPM cell expansion was observed till day 7. Cells were able to grow on the surface of microcarriers but they also showed some amount of ‘bead bridging’, due to the tendency of iPSC to form clustered colonies. The small hydrodynamic forces at the low RPMs were not able to prevent bead bridging. The Oct4-GFP expression of the cells on the carriers suggested that the cells were able to maintain their pluripotency (Fig. 3A–C).
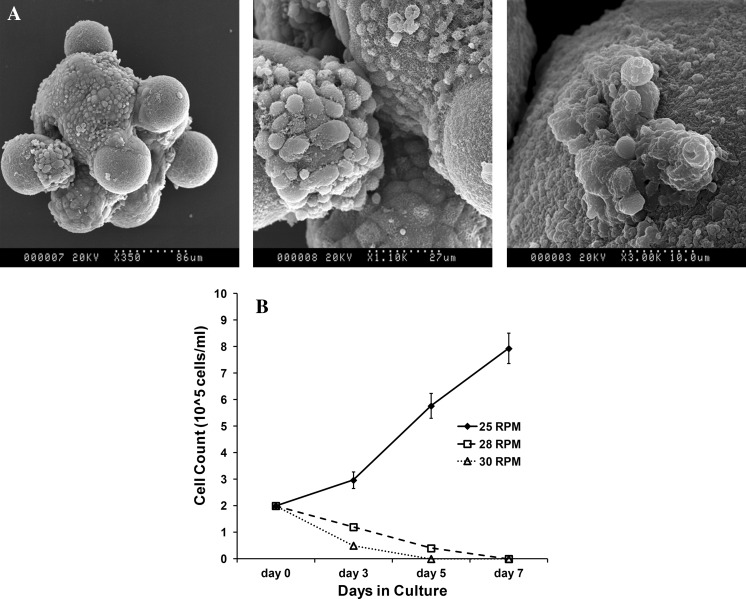
Scanning electron microscopy images of cells on Cytodex 3 carriers at the end of 7 days culture shows cell attachment, spreading and expansion on the microcarriers (Fig. 4A). Cell count data also supported the microscopy observation (Fig. 4B). At 25 RPM, cell density increased from 2 × 105 cells/ml to around 8 × 105 cells/ml at the end of 7 days giving rise to a total cell number of 40 × 106 while the total cell number in the control set up was 6 × 106. However, at 28 and 30 RPM, the cell number decreased over time. Cell count became negligible between days 4 and 6 for both 28 and 30 RPM. The ability of the iPSC to expand in the spinner flasks highlights their adaptability to changed culture conditions.
Fig. 4.
Cell attachment and expansion on microcarriers. A Scanning electron microscopy (SEM) images of cells growing on Cytodex 3 microcarriers at the end of 7 days culture in spinner flask at 25 RPM. B Density of mouse IPSC on days 3, 5 and 7 at RPMs 25 (filled diamond with solid line), 28 (open square with dashed line) and 30 (open triangle with dotted line). Based on the preliminary data from microscopy and cell count, 25 RPM was deemed to be the best possibility and the set up was repeated n = 3 times to validate its reproducibility for all experiments
Cell pluripotency
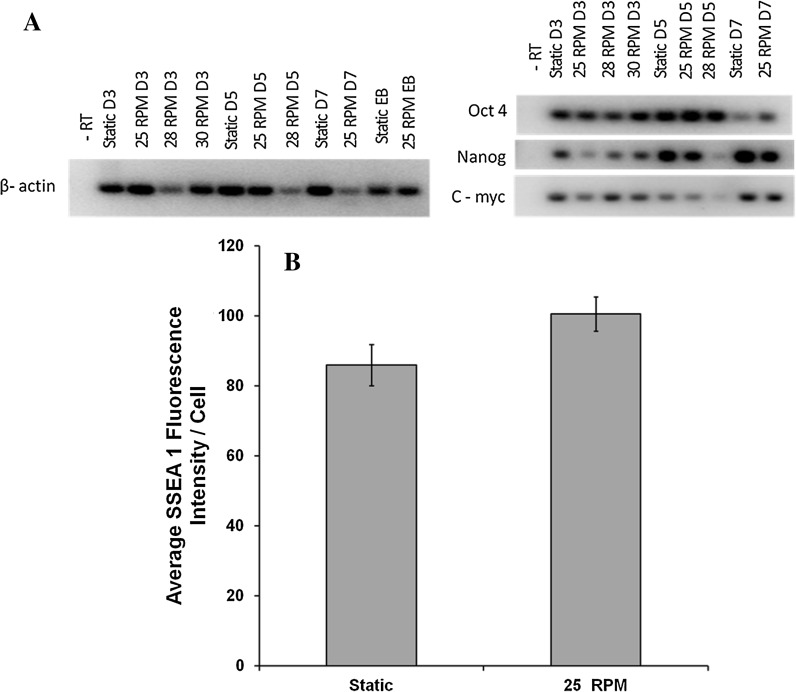
Along with cell attachment and expansion, the maintenance of pluripotency of the mouse iPSC was also considered to be an important aspect for dynamic culture optimization. RT-PCR analysis for cells collected on days 3, 5 and 7 of culture at 25, 28, and 30 RPM was carried out to ascertain their pluripotency. As shown in Fig. 5A, it was observed that Oct 4, Nanog and c-myc expressions were present in all cases although Nanog and c-myc were very low for cells expanded at 28 RPM on day 5. This suggested that although cells were present on day 5 at 28 RPM, their pluripotency was negatively affected.
Fig. 5.
Pluripotency analysis of cells cultured in spinner flask. A Reverse transcription–polymerase chain reaction analysis of pluripotency markers on days 3, 5, 7 for 25, 28 and 30 RPM cultures. Analysis of static culture cells at the same time points was considered as control. β actin was considered as the house keeping gene. B Quantitative analysis of pluripotency by comparing average SSEA 1 fluorescence intensity/cell between static culture and dynamic culture (25 RPM) at the end of 7 days. Experiments were repeated for n = 3 times and data represents mean ± SEM
Quantitative analysis of SSEA-1 fluorescence intensity was carried out after 7 days of culture at 25 RPM and was compared to static culture. Figure 5B shows average fluorescence intensity per/cell due to SSEA 1 marker. Although not significant, the intensity in the control culture was slightly less than that of the 25 RPM culture cells.
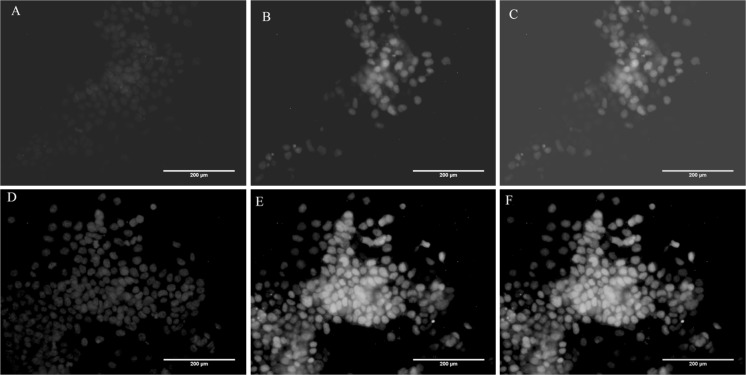
SSEA 1 and Nanog immunostaining was carried out after 7 days of culture at 25 RPM for cells fixed directly on carriers. Figure 6 demonstrates that miPS cell cultured on microcarriers in a spinner flask for 7 days at 25 RPM stained positive for both SSEA 1 and Nanog.
Fig. 6.
Immunostaining of fixed cells on microcarriers at the end of 7 days culture at 25 RPM. (A, D) Nuclei were stained blue with Hoechst, (B) SSEA 1 postive cells stained red, (C) SSEA 1 staining merged image, (E) Nanog positive cells stained red, (E) Nanog staining merged image. Scale bar 200 µm
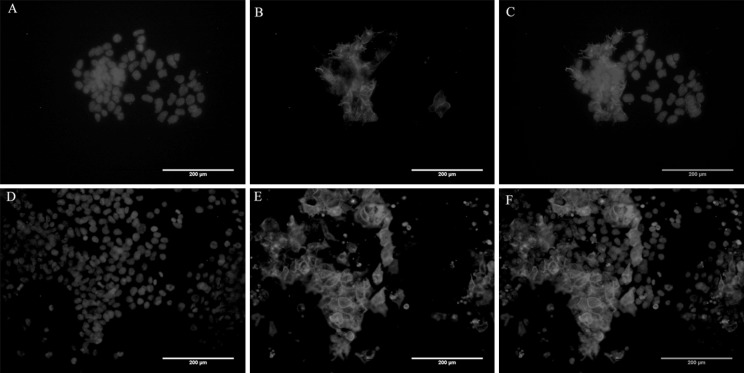
SSEA 1 staining was also carried out for replated cells after 7 days of spinner flask culture, along with Oct4-GFP live cell imaging. Cells from static culture worked as a control for comparison purpose in this case (Figs. 7, 8). Pluripotency was largely maintained by the cells for both the control and 25 RPM cultures although loss of a small fraction of SSEA 1 marker and Oct4-GFP was also observed, suggesting some loss of pluripotency. However, this could be due to the cells being re-plated on 2D gelatin coating and not a direct effect of dynamic culture itself.
Fig. 7.
Oct4-GFP immunofluorescence of cells from day 7 culture at 25 RPM. Cells were replated and imaged after 3 days of culture. (A, D) Hoechst staining for nuclei, (B, E) Oct4-GFP positive cells, (C, F) Merged images. Scale bar 200 µm
Fig. 8.
SSEA 1 immunostaining of cells from day 7 culture at 25 RPM. Cells were replated and imaged after 3 days of culture. (A, D) Hoechst staining for nuclei, (B, E) SSEA-1 positive cells stained red, (C, F) Merged images. Scale bar 200 µm
Flow cytometric analysis of Oct4-GFP showed that around 96 ± 2 % cells for static culture and around 90 ± 2 % cells in case of dynamic culture (25 RPM) maintained the GFP fluorescence at the end of 7 days (data not shown).
In vitro differentiation
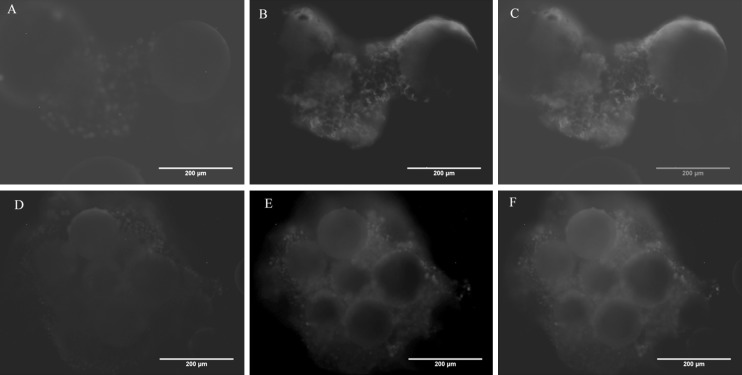
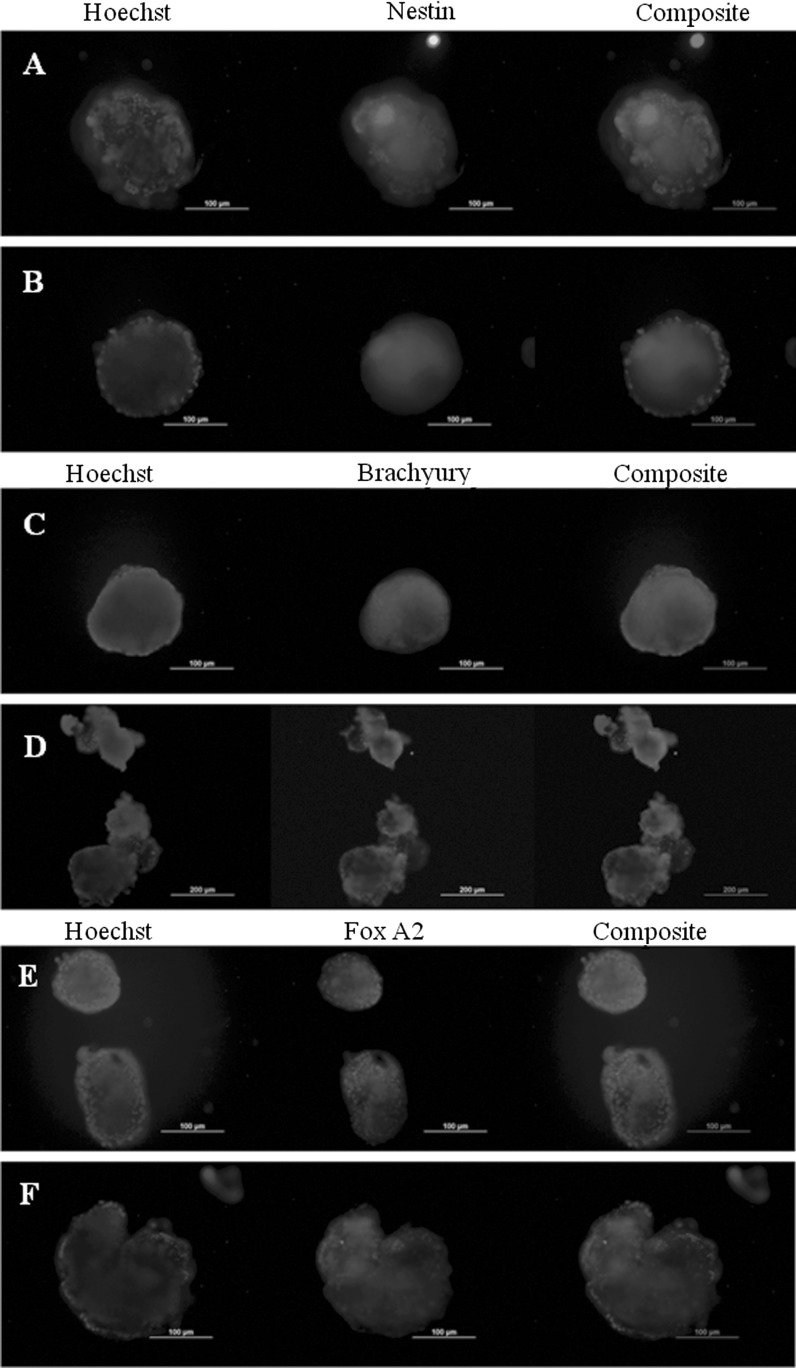
Spontaneous in vitro differentiation capability of the mouse iPSC after dynamic culture was tested by EB formation using suspension culture method. Cells were collected at the end of 7 days of culture and EBs were formed in low adherence culture plates using media without LIF. EBs were collected on day 10 for immunostaining and RT-PCR analysis. Cells from static culture were used as a control. Cells from both static culture and dynamic culture (at 25RPM) were able to form embryoid bodies. RT-PCR analysis (Fig. 9) shows the presence of all three germ layer markers at the end of 10 days. Immunostaining of EBs also shows the presence of 3 germ layer markers (Fig. 10).
Fig. 9.
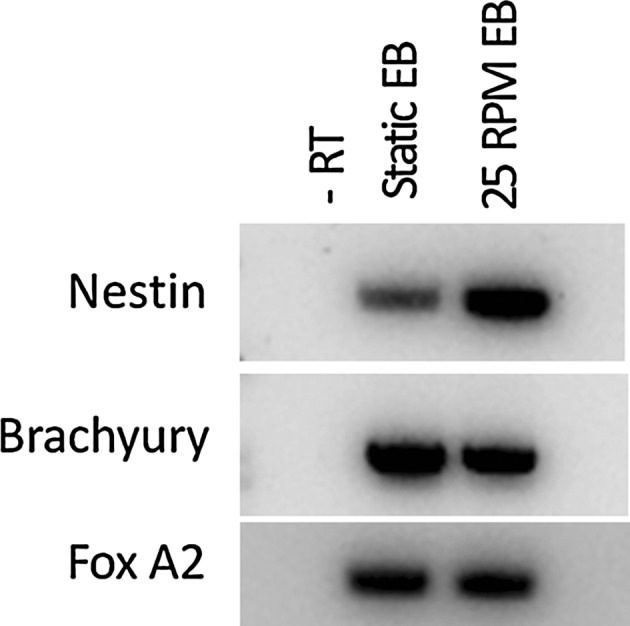
Reverse transcription–polymerase chain reaction for 3 germ layers marker on day 10 EBs; Ectoderm (Nestin), Mesoderm (Brachyury) and Endoderm (FoxA2). Static culture EBs were considered as control. β actin was used as the house keeping gene (data shown in Fig. 5A)
Fig. 10.
Embryoid body immunostaining for detection of 3 germ layers marker. A, C, E EB from static culture, B, D, F EB from 25 RPM dynamic culture. Scale bar 200 µm
Discussion and conclusion
The ethical issues associated with the use of ESC have led to extensive research in the field of iPSC. Scaling up of pluripotent stem cell culture from the bench top to a reactor system is essential before these cells can be used for various applications. A spinner flask in conjunction with microcarriers has been widely used for large scale expansion of pluripotent stem cells. Efforts have been made for culturing of ESC (both mouse and human) in such a set up since 2005 (Fok and Zandstra 2005) but until now, a uniform system with optimized parameters for the expansion of a specific cell type is yet to be achieved. The available reports on large scale expansion of ES cells differ widely in terms of many variables including the type of microcarrier used and the agitation rate. Also, the relevant studies have considered only the effect of a dynamic culture system on the cells and not on the microcarriers being used despite the existence of a threshold tolerance of carriers themselves.
The use of dynamic culture systems like spinner flasks for the expansion of iPSC is still in its nascent stage and similar to the ES cells, a versatile culture system is yet to be achieved. To date, three groups have attempted the expansion iPSC as aggregates in spinner flask or stirred bioreactor and all three have used different agitation rates (Fluri et al. 2012; Olmer et al. 2012; Shafa et al. 2012). The use of microcarrier for iPSC expansion has been reported by only two groups: Kehoe et al. (2010) mentioned iPSC expansion in a review article, while recently, Bardy et al. (2013) looked at the expansion of iPSC and its neural differentiation. Surprisingly, both these groups had used the same type of carrier (with Matrigel coating) for the expansion of human iPSC but the agitation rates used were very different. Amongst the commercially available microcarriers, Cytodex 3 is one of the most widely used carriers for the expansion of both human and mouse ESC, but the present study is the first where these have been used for expansion of iPSC.
Specifically, we have attempted to study the expansion of mouse iPSC on Cytodex 3 microcarriers in a spinner flask. We have looked at the effect of different agitation rates not only on the attachment and expansion of iPSC but also on the microcarriers. We were also able to expand these cells directly off the feeder on to Cytodex 3 without any prior coating of the carriers.
As hypothesized, it was seen that the Cytodex 3 indeed has an agitation tolerance threshold above which there was extensive carrier breakage. 40 RPM was found to be the maximum agitation rate that these carriers could tolerate. Breakage was observed upwards of 42 RPM. Since Cytodex 3 has been previously used at higher spin rates for cell expansion, it is possible that the effect of agitation rate on microcarriers is also dependent on the reactor set up itself. Thus, it is evident that similar studies for other carriers and different set-ups are essential to determine their future use in pluripotent stem cell expansion.
Based on the carrier study, spin rates of 40 RPM and below were tested for iPSC expansion. Agitation rates between 28 and 40 RPM were unable to support long term cell attachment and expansion, with cell detachment being directly proportional to the agitation rate. At 40 RPM, cells detached within 24 h while for 30 and 28 RPM, the cell number showed a steady decline with time, with complete detachment taking place between days 4 and 6. The 25 RPM flow, on the other hand, was able to support the attachment and expansion of the cells for 7 days. The shear stress at 25 RPM varies at different points in the spinner flask with a maximum stress of 0.0984 Pa and mean of 0.0520 Pa (Ismadi et al. 2014, Submitted). The above observations are interesting, since they show that the range of agitation speed that can support iPSC expansion is very narrow, and hence requires very precise control in dynamic cultures for future work with such cell lines. Maintenance of pluripotency and differentiation capability of these cells during spinner flask expansion is highly important. Immunostaining, RT-PCR analysis of cells from the dynamic culture system and of EBs formed from the cells prove that the miPSC were able to maintain their pluripotency and differentiation capability after spinner flask culture.
Taken together, 25 RPM was found to be the optimum agitation rate for the expansion of miPSC in the spinner flask while maintaining all their essential characteristics. Our findings are in line with Bardy et al. (2013) who had also used a 25 RPM system for the expansion of hiPSC and their neural differentiation on DE-53 microcarriers. The spinner flask used by this group was the same as ours but it is possible that the impeller height may have been different. Given the remarkably small range of spin rates found to be viable for iPSC proliferation and microcarrier integrity, the present work also highlights the need for precise speed and set up control for spinner flasks and their extensive study prior to being used for dynamic culture of pluripotent stem cells.
Acknowledgement
The authors would like to acknowledge Ms. Joan Clark, Monash Micro Imaging Facility (Monash University), for helping with the SEM imaging, Dr. Trevor Wilson, Medical Genomics Facility, Monash Health and Technology Precinct, for his help in the Array Scan analysis, and Ms. Karla Contreras, Division of Biological Engineering (Monash University) for her assistance. This work was supported by the Australian Research Council Discovery Program (Grant DPDP130100822) and by the Australia India Strategic Research Fund (Grant BF050038).
Conflict of interest
The authors declare that no competing financial interests exist for this work.
Contributor Information
Priyanka Gupta, Phone: +91 22 2576 4247, Email: priyankka.g@gmail.com.
Mohd-Zulhilmi Ismadi, Phone: +61 (0)3 990 51927, Email: zac.ismadi@monash.edu.
Paul J. Verma, Phone: +61 8 8524 9667, Email: paul.verma@monash.edu, Email: paul.verma@sa.gov.au
Andreas Fouras, Phone: + 61 3 990 53493, Email: andreas.fouras@monash.edu.
Sameer Jadhav, Phone: +91 (22) 2576 7219, Email: srjadhav@che.iitb.ac.in.
Jayesh Bellare, Phone: +912225767207, Email: jb@iitb.ac.in.
Kerry Hourigan, Phone: +61 3 9905 9624, Email: kerry.hourigan@monash.edu.
References
- Abbasalizadeh S, Larijani MR, Samadian A, Baharvand H. Bioprocess development for mass production of size-controlled human pluripotent stem cell aggregates in stirred suspension bioreactor. Tissue Eng Part C Methods. 2012;18:831–851. doi: 10.1089/ten.tec.2012.0161. [DOI] [PubMed] [Google Scholar]
- Abranches E, Bekman E, Henrique D, Cabral JM. Expansion of mouse embryonic stem cells on microcarriers. Biotechnol Bioeng. 2007;96:1211–1221. doi: 10.1002/bit.21191. [DOI] [PubMed] [Google Scholar]
- Alfred R, Radford J, Fan J, Boon K, Krawetz R, Rancourt D, Kallos MS. Efficient suspension bioreactor expansion of murine embryonic stem cells on microcarriers in serum-free medium. Biotechnol Prog. 2011;27:811–823. doi: 10.1002/btpr.591. [DOI] [PubMed] [Google Scholar]
- Bardy J, Chen AK, Lim YM, Wu S, Wei S, Weiping H, Chan K, Reuveny S, Oh SK. Microcarrier suspension cultures for high-density expansion and differentiation of human pluripotent stem cells to neural progenitor cells. Tissue Eng Part C Methods. 2013;19:166–180. doi: 10.1089/ten.tec.2012.0146. [DOI] [PubMed] [Google Scholar]
- Chen AK, Chen X, Choo AB, Reuveny S, Oh SK. Critical microcarrier properties affecting the expansion of undifferentiated human embryonic stem cells. Stem Cell Res. 2011;7:97–111. doi: 10.1016/j.scr.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Cormier JT, Nieden NIZ, Rancourt DE, Kallos MS. Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 2006;12:3233–3245. doi: 10.1089/ten.2006.12.3233. [DOI] [PubMed] [Google Scholar]
- Dusting J, Sheridan J, Hourigan K. A fluid dynamics approach to bioreactor design for cell and tissue culture. Biotechnol Bioeng. 2006;94:1196–1208. doi: 10.1002/bit.20960. [DOI] [PubMed] [Google Scholar]
- Fernandes AM, Fernandes TG, Diogo MM, da Silva CL, Henrique D, Cabral JM. Mouse embryonic stem cell expansion in a microcarrier-based stirred culture system. J Biotechnol. 2007;132:227–236. doi: 10.1016/j.jbiotec.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Fernandes AM, Marinho PA, Sartore RC, Paulsen BS, Mariante RM, Castilho LR, Rehen SK. Successful scale-up of human embryonic stem cell production in a stirred microcarrier culture system. Braz J Med Biol Res. 2009;42(6):515–522. doi: 10.1590/S0100-879X2009000600007. [DOI] [PubMed] [Google Scholar]
- Fluri DA, Tonge PD, Song H, Baptista RP, Shakiba N, Shukla S, Clarke G, Nagy A, Zandstra PW. Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nat Methods. 2012;9:509–516. doi: 10.1038/nmeth.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok EY, Zandstra PW. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23:1333–1342. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- Ismadi MZ, Higgins S, Samarage CR, Paganin D, Hourigan K, Fouras A. Optimisation of a stirred bioreactor through the use of a novel holographic correlation velocimetry flow measurement technique. PLoS ONE. 2013;8:e65714. doi: 10.1371/journal.pone.0065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismadi ZM, Gupta P, Fouras A, Verma P, Jadhav S, Bellare J, Hourigan K (2014) Flow characterization of spinner flask for induced pluripotent stem cell culture application. Plos One (Submitted) [DOI] [PMC free article] [PubMed]
- Kehoe DE, Jing D, Lock LT, Tzanakakis ES. Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue Eng Part A. 2010;16:405–421. doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawetz R, Taiani JT, Liu S, Meng G, Li X, Kallos MS, Rancourt DE. Large-scale expansion of pluripotent human embryonic stem cells in stirred-suspension bioreactors. Tissue Eng Part C Methods. 2010;16:573–582. doi: 10.1089/ten.tec.2009.0228. [DOI] [PubMed] [Google Scholar]
- Leung HW, Chen A, Choo AB, Reuveny S, Oh SK. Agitation can induce differentiation of human pluripotent stem cells in microcarrier cultures. Tissue Eng Part C Methods. 2011;17:165–172. doi: 10.1089/ten.tec.2010.0320. [DOI] [PubMed] [Google Scholar]
- Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng Part A. 2009;15:2051–2063. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho PA, Fernandes AM, Cruz JC, Rehen SK, Castilho LR. Maintenance of pluripotency in mouse embryonic stem cells cultivated in stirred microcarrier cultures. Biotechnol Prog. 2010;26:548–555. doi: 10.1002/btpr.328. [DOI] [PubMed] [Google Scholar]
- Marinho PA, Vareschini DT, Gomes IC, Paulsen Bda S, Furtado DR, Castilho Ldos R, Rehen SK. Xeno-free production of human embryonic stem cells in stirred microcarrier systems using a novel animal/human-component-free medium. Tissue Eng Part C Methods. 2013;19:146–155. doi: 10.1089/ten.tec.2012.0141. [DOI] [PubMed] [Google Scholar]
- Meunier P, Hourigan K. Mixing in a vortex breakdown flow. J Fluid Mech. 2013;731:195–222. doi: 10.1017/jfm.2013.226. [DOI] [Google Scholar]
- Nie Y, Bergendahl V, Hei DJ, Jones JM, Palecek SP. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol Prog. 2009;25:20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SK, Chen AK, Mok Y, Chen X, Lim UM, Chin A, Choo AB, Reuveny S. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2009;2:219–230. doi: 10.1016/j.scr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Olmer R, Haase A, Merkert S, Cui W, Palecek J, Ran C, Kirschning A, Scheper T, Glage S, Miller K, Curnow EC, Hayes ES, Martin U. Long term expansion of undifferentiated human iPS and ES cells in suspension culture using a defined medium. Stem Cell Res. 2010;5:51–64. doi: 10.1016/j.scr.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Olmer R, Lange A, Selzer S, Kasper C, Haverich A, Martin U, Zweigerdt R. Suspension culture of human pluripotent stem cells in controlled, stirred bioreactors. Tissue Eng Part C Methods. 2012;18:772–784. doi: 10.1089/ten.tec.2011.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BW, Horne R, Lay TS, Rust WL, Teck TT, Crook JM. Attachment and growth of human embryonic stem cells on microcarriers. J Biotechnol. 2008;138:24–32. doi: 10.1016/j.jbiotec.2008.07.1997. [DOI] [PubMed] [Google Scholar]
- Serra M, Brito C, Sousa MF, Jensen J, Tostoes R, Clemente J, Strehl R, Hyllner J, Carrondo MJ, Alves PM. Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J Biotechnol. 2010;148:208–215. doi: 10.1016/j.jbiotec.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Shafa M, Sjonnesen K, Yamashita A, Liu S, Michalak M, Kallos MS, Rancourt DE. Expansion and long-term maintenance of induced pluripotent stem cells in stirred suspension bioreactors. J Tissue Eng Regen Med. 2012;6:462–472. doi: 10.1002/term.450. [DOI] [PubMed] [Google Scholar]
- Steiner D, Khaner H, Cohen M, Even-Ram S, Gil Y, Itsykson P, Turetsky T, Idelson M, Aizenman E, Ram R, Berman-Zaken Y, Reubinoff B. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat Biotechnol. 2010;28:361–364. doi: 10.1038/nbt.1616. [DOI] [PubMed] [Google Scholar]
- Storm MP, Orchard CB, Bone HK, Chaudhuri JB, Welham MJ. Three-dimensional culture systems for the expansion of pluripotent embryonic stem cells. Biotechnol Bioeng. 2010;107:683–695. doi: 10.1002/bit.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tat PA, Sumer H, Jones KL, Upton K, Verma PJ. The efficient generation of induced pluripotent stem (iPS) cells from adult mouse adipose tissue-derived and neural stem cells. Cell Transplant. 2010;19:525–536. doi: 10.3727/096368910X491374. [DOI] [PubMed] [Google Scholar]
- Thouas GA, Sheridan J, Hourigan K. A bioreactor model of mouse tumor progression. J Biomed Biotechnol. 2007;2007:32754. doi: 10.1155/2007/32754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielens S, Declercq H, Gorski T, Lippens E, Schacht E, Cornelissen M. Gelatin-based microcarriers as embryonic stem cell delivery system in bone tissue engineering: an in vitro study. Biomacromolecules. 2007;8:825–832. doi: 10.1021/bm060870u. [DOI] [PubMed] [Google Scholar]
- Want AJ, Nienow AW, Hewitt CJ, Coopman K. Large-scale expansion and exploitation of pluripotent stem cells for regenerative medicine purposes: beyond the T flask. Regen Med. 2012;7:71–84. doi: 10.2217/rme.11.101. [DOI] [PubMed] [Google Scholar]