Abstract
Adriamycin (ADR) increases the production of reactive oxygen species (ROS), which diminishes mitochondrial function. Angiotensin-II stimulates mitochondrial ROS generation. The aim of the study was to examine whether angiotensin converting enzyme (ACE) or renin inhibitors protect against ADR-induced mitochondrial function impairment. Rats were divided into five groups as control, ADR, co-treatment ADR with captopril, co-treatment ADR with aliskiren, co-treatment ADR with both captopril and aliskiren. Left ventricular function and blood pressures were assessed at the end of treatment period. Mitochondrial membrane potential (MMP) and ATP levels were determined. ADR treatment decreased the left ventricular pressure and increased the left ventricular end-diastolic pressure. ADR decreased MMP and ATP levels in myocyte mitochondria due to increasing oxidative stress. ADR decreased MMP and ATP levels due to increased oxidative stress in the heart. Inhibitors of ACE and renin caused the elevation of the decreased of MMP and ATP levels. The pathologic changes in electrocardiogram, blood pressure and left ventricular function were decreased by inhibition of Ang-II production. We concluded that inhibitors of angiotensin II are effective against ADR cardiotoxicity via the restoration of MMP and ATP production and prevention of mitochondrial damage in vivo.
Keywords: Adriamycin, Aliskiren, Captopril, Mitochondrial ATP, Mitochondrial membrane potential, Oxidative stress index
Introduction
Anthracycline chemotherapeutic drugs [daunorubicin, adriamycin (ADR), and epirubicin] have been among the most effective and commonly used anticancer chemotherapeutics. However, their clinical utilities are limited due to their undesired effects (cardiotoxic), but the mechanism of them to cause cardiac damage is still not fully understood (Arozal et al. 2010).
Causes of ADR-induced cardiotoxicity appear to be multifactorial. A critical component may be the generation of free radicals and the presence of redox-related damage, which occur through both enzymatic and nonenzymatic pathways (Ozdogan et al. 2011). ADR-generated free radicals induce lipid peroxidation, which ultimately produces membrane damage. In addition, mitochondrial damage, increased Ca2+ current along with inhibition of sarcoplasmic reticulum function and decreased activity of Na, K-ATPase, have all been implicated in ADR-induced cardiotoxicity (Harake et al. 2012).
Compared with other organs, the heart is especially susceptible to ADR-induced damage. ADR is shown to accumulate in mitochondria which produce more than 90 % of the ATP utilized by cardiomyocytes (Octavia et al. 2012). Cationic ADR has high affinity binding to cardiolipin, an anionic phospholipid in the inner mitochondrial membrane, which is essential in eukaryotic energy metabolism (Harake et al. 2012). Cardiolipin-bound ADR would induce dissociation of cardiolipin-associated peripheral proteins from the inner mitochondrial membrane, like cytochrome c. This would affect electron transport chain and energy channeling, and may favor initiation of programmed cell death (Harake et al. 2012). Toxicity of mitochondrial, mostly cardiolipin-bound ADR is mediated by oxidative stress, which represents particular threats to cellular energy metabolism in the myocardium and is considered to be the main mediator of ADR induced cardiotoxicity (Iqbal et al. 2008).
An attractive explanation to its cardiotoxic mechanism could be myocyte loss through apoptosis (Montaigne et al. 2012). ADR lead to activate pro-apoptotic signaling pathways through p53 activation (Monti et al. 2013). Furthermore, ADR was reported to impair progenitor cell renewal/cardiac repair as well (Shi et al. 2011). The cardiomyocytes have a limited regenerative capacity which may be an explanation for cumulative toxicity of ADR on cardiac cell due to progressive loss. Cardiac cell stress, e.g. specifically oxidative stress induced by ADR activates apoptosis and necrosis via a mitochondrial pathway. Mitochondria have an important role for both intrinsic apoptotic and necrotic pathways (Montaigne et al. 2012). The induction of free radical production is the best described major mechanism through which adriamycin injures the myocardium (Octavia et al. 2012). The biochemical mechanism of adriamycin induced heart toxicity remains essentially unknown. Until now, ADR’s neurohormonal activation is unclear. ADR administration has been shown to activate the cardiac renin angiotensin system. Previous studies have reported that Angiotensin II (Ang-II) plays a key role in the process of ADR-induced cardiotoxicity (Arozal et al. 2010). However, studies have suggested that angiotensin-converting enzyme (Wallace) inhibitor and angiotensin receptor blocker exert a protective role towards ADR-induced cardiotoxicity (Arozal et al. 2010; Nakamae et al. 2005). A new drug of renin angiotensin system (RAS) inhibitors (aliskiren (AL)) is a direct inhibitor of the renin enzyme (Rashikh et al. 2012) and is reported to be cardioprotective against acute ADR-induced toxicity in heart (Rashikh et al. 2013b) and kidney (Taskin et al. 2014). In other words, the blockade of the renin activity by aliskiren might be a promising approach in the treatment of ADR-induced toxicity (Rashikh et al. 2013a).
Ang-II is the most effective peptide of the renin–angiotensin system. It is well documented that Ang-II is related to the development of some pathophysiologies, e.g. hypertensive and ischemic heart disease in humans. Recent findings have suggested that Ang-II activates intracellular signaling processes, which lead to events that include the generation of reactive oxygen species (ROS), myocardial apoptosis and fibrosis (Arozal et al. 2010). The link between ROS production by Ang-II and mitochondrial function was reported. The interaction might be important for modulating the effect of Ang-II on AP-1 signaling pathway. AP-1 arrange cytochrome c expression. So, Ang-II might be proposed to affect to cytochrome c though these interaction (de Cavanagh et al. 2007). Interestingly, in rats that had undergone short or long-term administration of ADR, coadministration of angiotensin-converting enzyme (ACE) inhibitors were reported almost to restore the cardiac dysfunction. Furthermore, recent evidence has demonstrated that ADR could not cause cardiac dysfunction in Ang-II type I receptor gene knockout mice (Nakamae et al. 2005). In other words, these report pointed out a key role of Ang-II in the pathogenesis of ADR-induced cardiotoxicity.
As a result, both ADR and Ang-II have an induction of ROS via redox cycling on the mitochondrial electron transport chain. This increase in ROS leads to several damaging events in the mitochondrion. Diminished mitochondrial function leads to decreased ATP-production. Thus, the hypothesis of this study was to investigate if ADR cardiotoxicity mechanism is associated with Ang-II.
Materials and methods
Chemicals and reagents
Unless otherwise stated, all reagents were purchased from Sigma Reagents (Interlab A.S., Istanbul, Turkey) used in the experiments were as follows: Adriamycin HCl Pharmacia (Milan, Italy); TAS, TOS kit from Rel Assay Diagnostics (Gaziantep, TURKEY) JC-1 from Cayman Chemical (Ann Arbor, MI, USA) and ATP kit from Cambrex Bio Science (Rockland, ME, USA).
Experimental animals and protocol
All experimental protocols were approved by the Medical Faculty Ethics Committee on Animal Research at Erciyes University (protocol #08/80). Male Sprague–Dawley rats were housed individually in polypropylene cages under hygienic and standard environmental conditions (24 ± 1 °C, humidity 60–70 %, 12 h light/dark cycle). The rats were randomly assigned into five groups, 14 animals each. Control group was intraperitoneally (i.p.) injected with vehicle (saline) and served as normal control group. ADR group was injected with adriamycin 4 mg/kg i.p. in four equal injections at 48 h intervals for eight consecutive days to achieve accumulative dose of 16 mg/kg, which is well documented to achieve cardiotoxicity and nephrotoxicity, and served as toxic control group. The Captopril group (CAP) received ADR plus captopril 10 mg/kg gavage. The Aliskiren group (AL) received ADR plus Aliskiren 50 mg/kg gavage. Captopril + Aliskiren group (CAP + AL) received ADR plus captopril and aliskiren. Administration of captopril and/or aliskiren were started and stopped at the same day of ADR administration.
At the end of the 8 days treatment period, left ventricular and arterial blood pressures were measured and blood samples were collected. Plasma was separated by centrifugation and used for biochemical assays; then the hearts were also removed from the rats, weighed and kept at −80 °C until use. Body weights were recorded at 2 time points: at the beginning of first injection (BW1) and at 24 h after last injection (BW2). Hearts were explanted and weighed at the end of the measurement of hemodynamic parameters.
Hemodynamic study
Animals were anesthetized with a combination of ketamin (40 mg/kg, i.m.) and of kylasine (5 mg/kg, i.m.). A micromanometer-tipped catheter (SPR 407, Millar Instruments Inc., Houston, TX, USA) was inserted into the right carotid artery up to the left ventricule. The left ventricle systolic pressure (LVSP), left ventricle end-diastolic pressure (LVEDP), the maximal rate of increased pressure (+dP/dt) and decreased pressure (−dP/dt) in the left ventricle were recorded. Left ventricular developed pressure (LVDP) was calculated according to the formula: LVDP = LVSP–LVEDP. During the measurement of heart function, ECG was recorded by using a MP30 (Biopac System, Inc., Santa Barabara, CA, USA).
The direct methods was used for recording blood pressures and heart rate. A polyethylene catheter (PE-50, Intramedic, Clay Adams, MD, USA) was implanted in the femoral artery. 20-30 minutes after the stabilization period, the measurement of blood pressure was started (MP30, Biopac System, Inc.).
Preparation of mitochondria and cytosol
Mitochondria from fresh rat hearts were isolated as described before (Dursun et al. 2011b): The hearts were homogenized in an ice-cold buffer A (250 mM sucrose, 2 mM EGTA, 5 mM Tris HCl) with a homogenizer and centrifuged at 2,000g for 8 min at 4 C. The supernatant (cytosol) was further centrifuged at 12,000g for 10 min at 4 C in a new tube. The pellet of mitochondria was suspended in an ice-cold buffer B (140 mM potas sium, 20 mM Tris HCl). The mitochondria kept at −80 °C until use.
Measurement of mitochondrial membrane potential (MMP)
A commercial fluorescence kit was used to measure MMP. The assessment is based on undergoing potential-dependent accumulation of JC-1 (5,5′,6,6′-tetrachloro- 1,1′,3,3′-tetraethyl benzimidazolyl carbocyanine iodide) in the mitochondria. JC-1 tends to aggregate in mitochondria at normal potential (red fluorescence) and forms monomers in depolarized mitochondria (green fluorescence). MMP was measured according to the manufacturer's protocol by using a fluorescent plate reader (Biotek, Synergy HT, Winooski, VT, USA).
Measurement of mitochondrial ATP content
The kit is based on the bioluminescent methods which use an enzyme, luciferase, which catalyses the formation of light from ATP. ATP level was measured according to manufacturer's protocol by using a fluorescent plate reader (Biotek, Synergy HT).
Biochemical studies
Measurement of total antioxidant status
Total antioxidant status of the plasma, myocytes cytosol and mitochondria was measured using Rel Assay Kit. TAS was measured according to the manufacturer's protocol by using a ELISA reader. The results were expressed in mmol Trolox equiv./L.
Measurement of total oxidant status
Total oxidant status (TOS) of the plasma, myocytes cytosol and mitochondria was measured using Rel Assay Kit. TOS was measured according to the manufacturer's protocol by using a ELISA reader. The results were expressed in μmol H2O2/L.
Calculation of oxidative stress index
Oxidative stress index (OSI) was calculated by using a ratio between TOS to TAS ratio in plasma, cytosol and mitochondria. OSI value was calculated as follows: OSI = [(TOS, μmol/L)/(TAS, μmol Trolox equivalent/L)/100].
Statistical data analysis
Statistical analyses were conducted using SPSS version 16.0. All results were expressed as mean ± SEM. Comparison among different groups were made using multiple analyses of variance (ANOVA), followed by a post hoc protected Tukey test. Nonparametric test including Kruskal–Wallis one-way analysis of variance and Mann–Whitney U test were applied to find the statistical significance. In all cases, p < 0.05 was considered to be significant.
Results
Effect of adriamycin on body and heart rates
Whereas control group gained weight throughout the protocol, adriamycin induced a significant loss of body weight (p < 0.05). Treatment with ACE and renin inhibitions were not able to restore the loss of body weight. ADR also decreased heart weight compared to CONT. However ACE and/or renin inhibition attenuated to decrease heart weight compared to CONT. But heart to body weight ratio was not different between groups (Table 1).
Table 1.
The effect of angiotensin II inhibiton on body heart and left weights and ratios in the groups
| CONT | ADR | CAP | AL | CAP + AL | |
|---|---|---|---|---|---|
| BW1 (g) | 334 ± 9 | 320 ± 14 | 361 ± 5 | 366 ± 15 | 382 ± 12 |
| BW2 (g) | 351 ± 23 | 257 ± 14*a | 296 ± 11* | 280 ± 16*a | 325 ± 12*b |
| HW (mg) | 954 ± 51 | 737 ± 8a | 821 ± 59a | 849 ± 55a | 858 ± 37 |
| LVW (mg) | 586 ± 37 | 450 ± 30 | 579 ± 62 | 543 ± 64 | 653 ± 33b |
| HW(mg)/100 g BW2 | 302 ± 19 | 259 ± 7 | 285 ± 18 | 314 ± 12 | 270 ± 4 |
| LVW(mg)/100 g BW2 | 181 ± 11 | 166 ± 10 | 196 ± 18 | 205 ± 16 | 208 ± 2 |
BW1 at the beginning of first injection, BW2 at 24 h after last injection. HW heart weight, LVW left ventricular weight
* p < 0.01 versus BW1
a p < 0.05 versus CONT
b p < 0.05 versus ADR
Effect of angiotensin II inhibition on adriamycin changed hemodynamic and ECG parameters
It is known that under the pathologic condition any of tissues firstly changed electrical activity, secondly function, then thirdly structure. Previous studies demonstrated to be a correlation between the prolongation of ST segment and ADR related cardiac dysfunction. ADR significantly caused a prolongation of ST segment compared to the control (p < 0.01; Table 2). Attenuation of Ang-II production by ACE and renin inhibition decreased prolongation of ST segment when compared to the ADR group (p < 0.01; Table 2). According to these results, prolongation of ST segment by ADR seems to be related with the Ang-II level.
Table 2.
The effect of angiotensin II inhibiton on ECG parameters and heart rate in the groups
| PR (ms) | QRS (ms) | ST (ms) | QT (ms) | R (mV) | HR (beats/min) | |
|---|---|---|---|---|---|---|
| CONT | 49 ± 2.7 | 35 ± 1.1 | 6.5 ± 0.7 | 69 ± 0.8 | 1.5 ± 1.3 | 368 ± 14 |
| ADR | 62 ± 3 | 41 ± 2.3 | 11 ± 1a | 80 ± 10 | 0.18 ± 0.17 | 300 ± 12 |
| CAP | 43 ± 4.3 | 42 ± 1.4 | 6 ± 0.5b | 79 ± 4.5 | 0.17 ± 0.08 | 333 ± 21 |
| AL | 33 ± 3.1b | 41 ± 2.4 | 5 ± 0.1b | 75 ± 3.9 | 0.09 ± 0.02 | 365 ± 31 |
| CAP + AL | 31 ± 0.6b | 39 ± 1.1 | 5 ± 0.1b | 73 ± 1.4 | 0.32 ± 0.07 | 335 ± 8 |
a p < 0.01 versus CONT
b p < 0.01 versus ADR
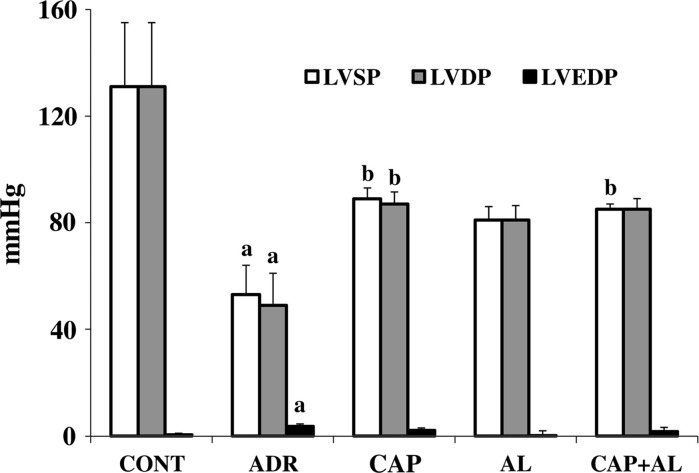
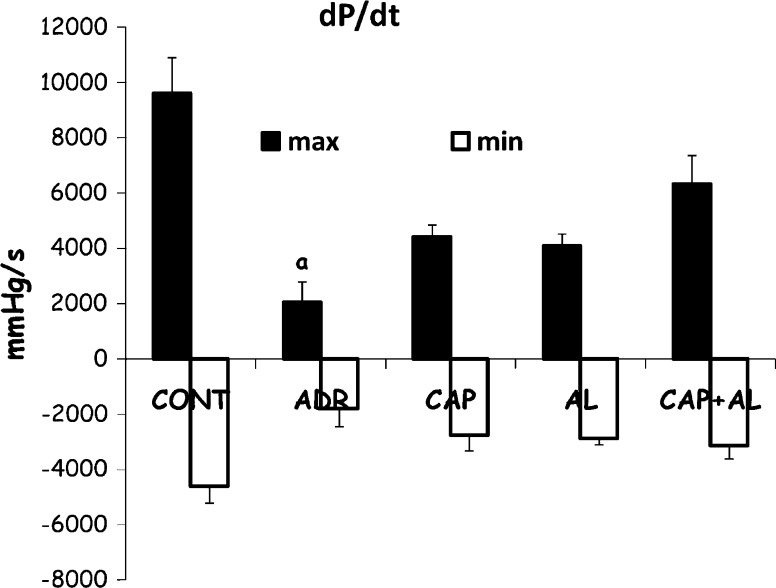
Heart function was evaluated by in vivo pressure measurement. In the present study, left ventricular systolic function was assessed by LVSP and +dP/dt and left ventricular diastolic function was demonstrated by LVEDP and −dP/dt. ADR leads to diminshed heart function when compared to control group (p < 0.01), including systolic (decreased LVSP and +dp/dt; Figs. 1, 2) and diastolic functions (increased LVEDP; Fig. 1). Inhibition of Ang-II production attenuated the ventricular dysfunction-induced by ADR. Specially, ACE and/or renin inhibitions restored systolic dysfunction compared to the ADR group (increased LVSP; p < 0.01; Fig. 1). These data demonstrated that ADR mainly caused a decreased heart systolic and diastolic functions 8 days after the first ADR injection. The decreased Ang-II production gave rise to attenuated ADR’s cardiotoxicity. Having established that the expected ADR’s cardiotoxicity exacerbation by Ang-II was observed, our experiments were focused on understanding the molecular basis of the ADR-dependent development of cardiotoxicity.
Fig. 1.
The effect of angiotensin II inhibition on cardiac functions in ADR-treated rats. LVSP left ventricular systolic pressure, LVDP left ventricular develop pressure, LVEDP left ventricular end diastolic pressure, CAP captopril group, AL aliskiren group, CAP + AL captopril plus aliskiren group. a p < 0.01 versus CONT. b p < 0.01 versus ADR
Fig. 2.
The effect of angiotensin II inhibiton on maximal rate of pressure increase. Maximal rate of pressure decrease in ADR-treated rats. Max maximal rate of pressure increase. Min maximal rate of pressure decrease. a p < 0.01 versus CONT
Effect of angiotensin inhibition on adriamycin changed blood pressures and heart rate
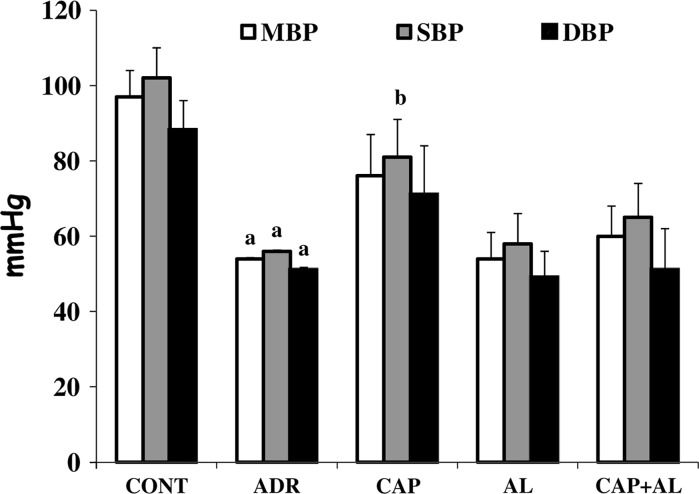
Regulation of blood pressure involves the exercise of a number of different functions in different parts of the body. Their collective task is to maintain blood pressure value within a certain range. Blood pressure is clearly related to cardiac output (the function expressed by the heart in pumping blood) and to peripheral vascular resistance (the resistance of the passage of blood through the precapillary arterioles). Ang-II and sympathetic mechanisms also contribute to the BP. Therefore, we measured the BP as well. As shown in Fig. 3, both systolic and diastolic dysfunction induced by ADR gave rise to lower blood pressure compared to the control group (p < 0.01), but the heart rate was not significantly decreased. The blood pressure of rats treated with ACE and/or renin improved. Increased systolic heart function by ACE inhibition led to a significant rise in the systolic blood pressure compared by the ADR treated group (p < 0.01; Fig. 3). In each group, no differences in the heart rate were observed (Table 2).
Fig. 3.
The effect of angiotensin II inhibiton on arterial blood pressures in ADR-treated rats. MBP mean blood pressure, SBP systolic blood pressure, and DBP diastolic blood pressure. a p < 0.01 versus CONT. b p < 0.01 versus ADR
Effect of angiotensin inhibition on adriamycin changed MMP and ATP production
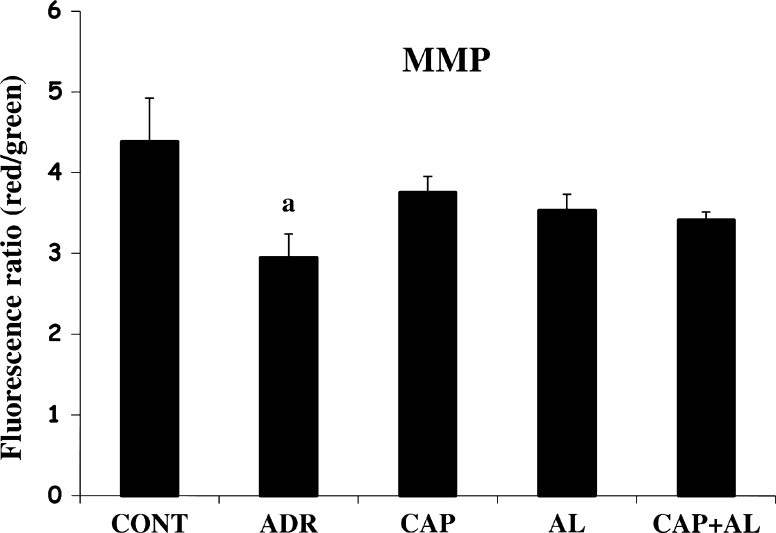
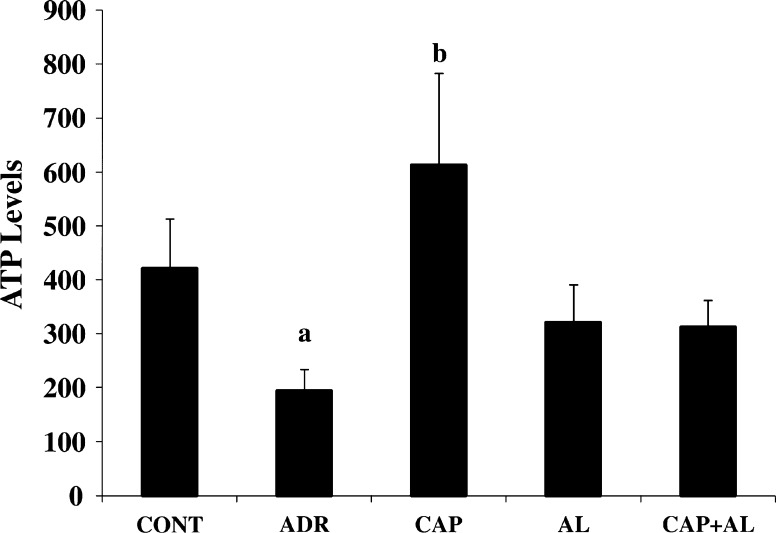
The mitochondria are the most extensively and progressively injured subcellular organelles of ADR-induced cardiotoxicity. Attenuation of mitochondrial dysfunction might prevent myocardial damage and subsequently improve the cardiac function (Octavia et al. 2012). So, we next investigated mitochondrial function impairment by using MMP and ATP production. Using the fluorescent mitochondrial-specific cationic dye, JC-1, MMP was measured. JC-1 agggregates in normal mitochondria and presents red fluorescence. The ratio of red and green fluorescence was used to demonstrate ADR’s toxicity in mitochondria and the effect of angiotensin II inhibition on toxicity of ADR. As expected, ADR lead to the dissipation of MMP (p < 0.05; Fig. 4). Depolarization of MMP by ADR awas ccompanied by decreased ATP production (p < 0.05; Fig. 5). However, inhibition of Ang-II production by CAP and AL did not completely restore the dissipation of MMP (Fig. 4). But ACE inhibition increased ATP level in cardiac mitocondria (p < 0.05; Fig. 5). These data demonstrated that the targets of ADR were mitochondria. ADR diminished ATP production, resulting from dissipation of MMP and, consequently causing mitochondria function impairment. This result could infer that intracellular target of ADR-induced cardiac toxicity could be thought to support a major role in mitochondrial dysfunction and ATP depletion. The selective toxicity of ADR to the heart was attributed to the selective damage to cardiac mitochondria.
Fig. 4.
The effect of angiotensin II inhibiton on mitochondrial membrane potential in ADR-treated rats. MMP mitochondrial membrane potential. a p < 0.05 versus CONT
Fig. 5.
The effect of angiotensin II inhibiton on ATP level in ADR-treated rats. a p < 0.05 versus CONT. b p < 0.05 versus ADR
Effect of angiotensin inhibition on adriamycin changed total oxidant (TOS), total antioxidant (TAS) status and the oxidative stress index (OSI)
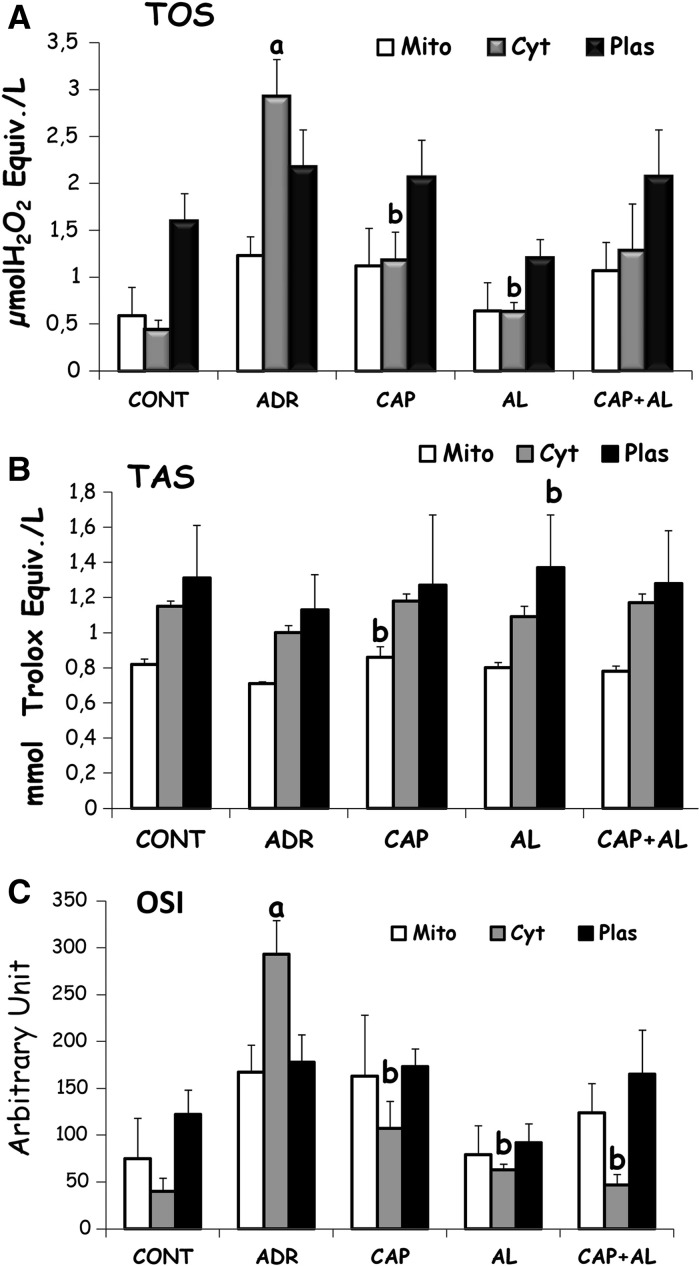
It was well documented that the proposed principal mechanisms of ADR cardiotoxicity have increased oxidative stress, as evidenced from increased levels of reactive oxygen species, and decreased levels of antioxidants. So the level of oxidative stress was estimated by total oxidant status to total antioxidant status ratio. TAS and TOS were assessed in mitochondria, cytosol and plasma. ADR increased cytosolic OSI, resulting from increasing cytosolic TOS (p < 0.01; Fig. 6A and C). It was shown that attenuation of Ang-II production by ACE and/or renin inhibition had a strong effect on cytosol and especially decreased cytosolic TOS, but did not increase TAS. Cap led to increased cytosolic TAS, AL led to increased plasma TAS (p < 0.05; Fig. 6A, B, C).
Fig. 6.
The effect of angiotensin II inhibiton on total oxidant status (TOS; a). Total antioxidant status (TAS; b) and Oxidative stress index (OSI; c) in ADR-treated rats. Mito mitochondrial, Cyt cytosolic and Plas plasma. a p < 0.001 versus CONT. b p < 0.05 versus ADR
Discussion
At the end of the 8-day treatment with ADR, we observed ventricular dysfunction (systolic and diastolic) and abnormal ECG (prolongation of ST and PR). In the current study the reduction of heart function was associated with decreased mitochondrial membrane potential and ATP production due to increased cytosolic OSI. Cardiac electrical function was evaluated by ECG recording. In our previous investigations (Dursun et al. 2011a, b) and also other studies (Xu et al. 2010) ADR gave rise to a prolonged ST interval in only ADR treated rats. A strong correlation was demonstrated between ST interval duration and ADR- cardiotoxicity (Fisher et al. 2005). ADR’s toxicity mainly affects cardiac tissue more than the other tissue. Cardiac tissue is enriched in mitochondria because of energy production based on fatty acid oxidation (Tokarska-Schlattner et al. 2006). ADR has been reported to accumulate in mitochondria so that ADR’s cardiotoxicity is related with mitochondrial dysfunction. It is still not completely understood how ADR can inhibit mitochondrial electron transport and oxidative phosphorylation. However, some evidences have relevted ADR-mitochondrial dysfunction to depolarized MMP (Dursun et al. 2011b; Taskin and Dursun 2012; Taskin et al. 2014; Zhu et al. 2011), decreased ATP production (Dursun et al. 2011b; Taskin and Dursun 2012; Xu et al. 2010; Zhu et al. 2011), and disrupted mitochondrial calcium homeostasis (Wallace 2007). ADR also inhibits the transport of some of the proteins from the cytosol to the mitochondria. (Montaigne et al. 2012; Wallace 2003). ADR has also been demonstrated to cause an attenuation of RyR2 and SERCA2a mRNA levels resulting in contractile failure (Boucek et al. 1999).
ADR could lead to a release of cytochrome-c as a key structural element for contact with cardiolipin. Cardiolipin is an anionic phospholipid and locates on the intramitochondrial membrane. When the ADR ligands with cardiolipin, cardiolipin related peripheral proteins, such as cytochrome-c or mitochondrial creatine kinase, are released to the cytoplasm. Release of these proteins may trigger apoptosis due to disruption of the energy production system. It was well documented that complex-I and complex-III of mitochondria are the key sites for primary production of ROS by ADR-induced cardiotoxicity (Zhu et al. 2011). Singal et al. (1997) demonstrated that both increasing oxidant and decreasing antioxidant play a key role in ADR-induced cardiotoxicity. Antioxidant enzymes such as catalase (Chandran et al. 2009) and rapidly inactivated GPx1 (Simmons and Jamall 1989) have been reported to be less expressed or have lower activity in cardiac tissue. Therefore, cardiac tissue is very vulnerable for oxidative stress damage.
In the current study, increased LVDEP and decreased LVSP were associated with reduction in the left ventricular chamber wall thickness. ADR caused decreased blood pressure (MBP, SBP, and DBP); this was probably responsible for the decreased LV function due to a decreasd ratio of LV to body weight. The decline of LV weight may be explained by myofibrillar loss associated with inducion of apoptosis and due to the inhibition of the expression of some genes specific for cardiac muscles, such as α-actin, troponin by ADR (Boucek et al. 1999; Nakamura et al. 2000). The compensation mechanism e.g. hormonal or neural may active after cardiac output reduces. The initial of this hormonal or neural activation support to maintain function, later to contribute the worse the prognosis of heart failure (Roig Minguell 2004). So, the loss of the cardiomyocytes may be counterbalanced by hypertrophy.
Cooperating of ADR and neurohormonal activation has not been cleared yet. ADR has been suggested to activate the cardiac renin angiotensin system (Boucek et al. 2003; Rashikh et al. 2011). Therefore, we investigated the interaction between RAS and the ADR cardiotoxicity. Cotreatment of ACE and/or renin inhibitors with ADR caused an important recovery in declined heart functions. The recovery of function was likely to be associated with attenuated cytosolic OSI by increased mitochondrial TAS resulting in restoration of MMP and ATP level. Only a few studies also investigated this interaction (Boucek et al. 2003; Ibrahim et al. 2009; Iqbal et al. 2008; Okumura et al. 2002; Rashikh et al. 2011; Sacco et al. 2009; Taskin 2014; Toko et al. 2002).
One of these studies was evaluated by Iqbal et al. (2008) that angiotensin type 1 receptor (AT1) antagonist was administrated pre and post treatment with ADR injection. AT1 mediated angiotensin pathway plays an important role in ADR-induced cardiac dysfunction as pretreatment with AT1 receptor antagonist attenuated ADR-induced biochemical and morphological changes. However, the authors pointed also out to have some evidence to partly eliminate ADR-induced cardiotoxicity when AT1 receptor antagonist was used as post-treatment. Another study was performed by Toko et al. (2002) who used AT1 knock out or AT1 inhibitor in ADR-treated mice. Although cardiac dysfunction was significantly elevated in the ADR-treated WT-mice, cardiac dysfunction in AT1 KO or AT1 receptor inhibitor treated WT mice were significantly lower than in WT mice, by both short and long term ADR treatment. It was also reported that in ADR treated WT mice cardiomyocytes were lost due to an increase in the number of apoptotic cells, but not in AT1 KO mice or WT mice administered with AT1 inhibitor. Altogether, these two studies interestingly seem to indicate that AT1 mediated Ang-II pathway is important in initiating ADR-cardiac dysfunction. Thirdly, Okumura et al. (2002) investigated ACE activity, converting angiotensin I to angiotensin II, on the ADR-treated hamster. At the end of the two weeks treatment, the cardiac ACE activity was found to increase in the adriamycin-treated hamsters. ADR was reported to increase the number of macrophages in cardiac tissues. The macrophages are suggested also to express ACE. Therefore, the increase of ACE activity in the adriamycin-induced cardiac toxicity may be dependent on the accumulation of ACE-expressing from macrophages in the cardiac tissues. These findings suggest that cardiac ACE may participate in the development of cardiotoxicity induced by adriamycin (Okumura et al. 2002).
Most studies have shown that ACE inhibition has a cardioprotective effect against heart dysfunction. Firstly, its direct Ang-II inhibition effect causes a decrease of total peripheral resistant and resulted in decreasing blood pressure. Decreasing afterload elevates stroke volume and also cardiac output. Secondly, bradykinin and NO are increased by ACE inhibition (Dagenais and Jamali 2005). At the same time, ACE inhibition decreases LVEDP, end of diastolic volume, and mean circulation filling pressure (Pfeffer et al. 1985). It was shown in vitro study that ACE inhibition has a reactive radical removal effect and leads to a decreased myocardial oxygen utilization. Increased ROS by ADR was detoxified by ACE inhibition was also shown to relate to reduced ANP expression and myocyte loss by mediating apoptosis (Boucek et al. 2003). Therefore, in the current study the beneficial effects of the ACE inhibitor on heart function was more effective due to a reduction of systemic blood pressure because the MBP, SBP and DBP of the ACE inhibitor-treated group did not significantly differ from that of the control group. SBP was significantly higher in the ACE inhibition group than in the ADR group and we, therefore, evaluated that the recovery effects of ACE inhibition on ADR-cardiotoxicity might be related to inhibition of Ang II because the local renin–angiotensin system in ADR-induced cardiac injury was reported to have a key role (Sacco et al. 2009).
Nakamae et al. (2005) investigated whether valsartan, an angiotensin II blocker, could reduce cyclophosphamide, adriamycin, vincristine and prednisolone (CHOP) based chemotherapy-induced cardiomyopathy in adults with non-Hodgkin's lymphoma. The authors concluded that valsartan reduced CHOP-induced cardiac damage by its role as an angiotensin II blocker.
We measured total oxidant and antioxidant status in cytosol, mitochondria and plasma from cardiac tissue. Cytosolic TOS was increased by ADR, but decreased by ACE or renin inhibitions. According to Iqbal et al. (2008), cardiac glutathione (GSH), the major endogenous antioxidant in cytosol, was decreased by ADR due to its effect on biomembrane disruption and peroxidation. Telmisartan, antagonist of AT1, was reported to increase cardiac GSH level when animals were pretreated before ADR-pretreatment. Otherwise, telmisartan might not have an effect on cardiac GSH level when it was used to posttreatment of ADR. This information indicated that AT1 receptor probably plays an important role to produce oxidant by ADR. Ibrahim et al. (2009) showed that ADR coadministration with telmisartan, or CAP attenuated the induction of free radical production by ADR. These results might indicate that there is a potential relationship between Ang-II and ADR-induced cardiotoxicity for causing oxidative stress.
Angiotensin II can be produced from alternative pathways independent of ACE. The most important alternative pathway to produce Ang-II is the chymase pathway. Chymase is a tissue-specific enzyme originally discovered in mast cells that can also known to convert angiotensin I to angiotensin II. Although the importance of these alternative pathways is still controversial, some researchers recognize that chymase is the major route of angiotensin II synthesis in tissues and may be more important in the pathogenesis of cardiovascular disease than previously thought. Thus, ACE inhibitor therapy may only partially inhibit angiotensin II activity (Dagenais and Jamali 2005). Therefore, in the present study another potent drug-Aliskiren directly inhibiting the renin enzyme - was used for inhibiting the RAS. Because the rate limiting step is renin for Ang-II production, the renin inhibitors have been reported to be more effective for blocking to RAS effect than ACE inhibitors or angiotensin receptor blockers (Westermann et al. 2008). We found that the administration of AL (AL and CAP + AL groups) caused some important recovery in both electrical activities and biochemical parameters of the heart. AL decreased OSI due to decreasing TOS and increasing TAS. This recovery in electrical activities of the heart was observed in ST and PR intervals. Mechanical activities of heart in AL group was ameliorated but not significantly. These discrepancies may reflect that electrical activity can firstly be affected at any tissue before its mechanical activity. Only few studies exist about the effect of Aliskiren on ADR cardiotoxicity. Because renin is a species-specific enzyme human renin inhibitors are only weakly able to inhibit the rodent enzymes (St-Jacques et al. 2011), there has been limited preclinical evaluation of aliskiren in animal models of heart failure (Muller et al. 2008). This interaction was only studied by Rashikh et al. (2011) who found that prophylactic administration of AL for 7 days before ADR-treatment had a cardioprotective effect, as evidenced by biochemical (decreased MDA, LDH, increased GSH) and histopathological changes in the heart. Our findings are in accordance with this cardioprotection. Westermann et al. (2008) have suggested that treatment with aliskiren in coronary ligation mice increased cardiac systolic (LVSP, +dP/dt) and diastolic function (LVEDP, −dP/dt). They were also reported to attenuate cardiac dilatation, and eventually improved cardiac output. Aliskiren was reported to be a potent candidate to decline the cardiosuppresive effects of angiotensin II–induced pathology. Furthermore, renin inhibition was also demonstrated to reduce cardiac hypertrophy, fibrosis and inflammation, and the inducibility of arrhythmias (Muller et al. 2008).
Conclusion
In conclusion, our findings demonstrated that it seems that Ang-II plays a key role in the process of ADR-induced cardiotoxicity. This study has suggested that angiotensin-converting enzyme and renin inhibitors exert a protective role towards ADR-induced cardiotoxicity. The mechanism underlying the beneficial effects of Ang-II inhibition in ADR cardiotoxicity is not only due to decreasing oxidative stress, but also protecting mitochondrial membrane potential and improving cardiac mitochondria energy production. It is well-known that mitochondria play an important role in the pathogenesis of ADR-induced cardiotoxicity. Prevention of mitochondria dysfunction will prevent myocardial alteration and subsequently improve cardiac function. Nevertheless, more studies will need to evaluate these beneficial effects and have to prove the role of Ang-II in the process of ADR-induced cardiotoxicity.
Acknowledgments
The authors wish to thank to Prof. Dr. Cem SUER and Prof. Dr. Yunus DURSUN for helping statistical analysis of data. This work was supported by the Medical Research Council of Erciyes University (Grant number TSD-09-733).
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- Arozal W, Watanabe K, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, Suzuki K, Kodama M, Aizawa Y (2010) Effect of telmisartan in limiting the cardiotoxic effect of daunorubicin in rats. J Pharm Pharmacol 62:1776–1783. doi:10.1111/j.2042-7158.2010.01196.x [DOI] [PubMed]
- Boucek RJ, Jr, Miracle A, Anderson M, Engelman R, Atkinson J, Dodd DA. Persistent effects of doxorubicin on cardiac gene expression. J Mol Cell Cardiol. 1999;31:1435–1446. doi: 10.1006/jmcc.1999.0972. [DOI] [PubMed] [Google Scholar]
- Boucek RJ, Jr, Steele A, Miracle A, Atkinson J. Effects of angiotensin-converting enzyme inhibitor on delayed-onset doxorubicin-induced cardiotoxicity. Cardiovasc Toxicol. 2003;3:319–329. doi: 10.1385/CT:3:4:319. [DOI] [PubMed] [Google Scholar]
- Chandran K, Aggarwal D, Migrino RQ, Joseph J, McAllister D, Konorev EA, Antholine WE, Zielonka J, Srinivasan S, Avadhani NG, Kalyanaraman B (2009) Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys J 96:1388–1398. doi:10.1016/j.bpj.2008.10.042 [DOI] [PMC free article] [PubMed]
- Dagenais NJ, Jamali F. Protective effects of angiotensin II interruption: evidence for antiinflammatory actions. Pharmacotherapy. 2005;25:1213–1229. doi: 10.1592/phco.2005.25.9.1213. [DOI] [PubMed] [Google Scholar]
- de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol. 2007;27:545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- Dursun N, Taskin E, Ozturk F. Protection against adriamycin-induced cardiomyopathy by carnosine in rats: role of endogenous antioxidants. Biol Trace Elem Res. 2011;143:412–424. doi: 10.1007/s12011-010-8875-y. [DOI] [PubMed] [Google Scholar]
- Dursun N, Taskin E, Yerer Aycan MB, Sahin L. Selenium-mediated cardioprotection against adriamycin-induced mitochondrial damage. Drug Chem Toxicol. 2011;34:199–207. doi: 10.3109/01480545.2010.538693. [DOI] [PubMed] [Google Scholar]
- Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- Harake D, Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiotoxicity in childhood cancer survivors: strategies for prevention and management. Future Cardiol. 2012;8:647–670. doi: 10.2217/fca.12.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MA, Ashour OM, Ibrahim YF, El-Bitar HI, Gomaa W, Abdel-Rahim SR. Angiotensin-converting enzyme inhibition and angiotensin AT(1)-receptor antagonism equally improve doxorubicin-induced cardiotoxicity and nephrotoxicity. Pharm Res. 2009;60:373–381. doi: 10.1016/j.phrs.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Dubey K, Anwer T, Ashish A, Pillai KK. Protective effects of telmisartan against acute doxorubicin-induced cardiotoxicity in rats. Pharmacol Rep. 2008;60:382–390. [PubMed] [Google Scholar]
- Montaigne D, Hurt C, Neviere R. Mitochondria death/survival signaling pathways in cardiotoxicity induced by anthracyclines and anticancer-targeted therapies. Biochem Res Int. 2012;2012:951539. doi: 10.1155/2012/951539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti M, Terzuoli E, Ziche M, Morbidelli L. The sulphydryl containing ACE inhibitor Zofenoprilat protects coronary endothelium from Doxorubicin-induced apoptosis. Pharmacol Res. 2013;76:171–181. doi: 10.1016/j.phrs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Muller DN, Derer W, Dechend R. Aliskiren-mode of action and preclinical data. J Mol Med (Berl) 2008;86:659–662. doi: 10.1007/s00109-008-0330-6. [DOI] [PubMed] [Google Scholar]
- Nakamae H, Tsumura K, Terada Y, Nakane T, Nakamae M, Ohta K, Yamane T, Hino M. (2005) Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 104:2492–2498. doi:10.1002/cncr.21478 [DOI] [PubMed]
- Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: in vivo study. Circulation. 2000;102:572–578. doi: 10.1161/01.CIR.102.5.572. [DOI] [PubMed] [Google Scholar]
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Okumura K, Jin D, Takai S, Miyazaki M. Beneficial effects of angiotensin-converting enzyme inhibition in adriamycin-induced cardiomyopathy in hamsters. Jpn J Pharmacol. 2002;88:183–188. doi: 10.1254/jjp.88.183. [DOI] [PubMed] [Google Scholar]
- Ozdogan K, Taskin E, Dursun N. Protective effect of carnosine on adriamycin-induced oxidative heart damage in rats. Anadolu Kardiyol Derg. 2011;11:3–10. doi: 10.5152/akd.2011.003. [DOI] [PubMed] [Google Scholar]
- Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res. 1985;57:84–95. doi: 10.1161/01.RES.57.1.84. [DOI] [PubMed] [Google Scholar]
- Rashikh A, Abul Kalam N, Akhtar M, Mahmood D, Pillai KK, Ahmad SJ. Protective effects of aliskiren in doxorubicin-induced acute cardiomyopathy in rats. Hum Exp Toxicol. 2011;30:102–109. doi: 10.1177/0960327110369819. [DOI] [PubMed] [Google Scholar]
- Rashikh A, Ahmad SJ, Pillai KK, Kohli K, Najmi AK. Aliskiren attenuates myocardial apoptosis and oxidative stress in chronic murine model of cardiomyopathy. Biomed Pharmacother. 2012;66:138–143. doi: 10.1016/j.biopha.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Rashikh A, Pillai KK, Ahmad SJ, Akhtar M, Najmi AK. Aliskiren alleviates doxorubicin-induced nephrotoxicity by inhibiting oxidative stress and podocyte injury. J Renin Angiotensin Aldosterone Syst. 2013;14:14–22. doi: 10.1177/1470320312459980. [DOI] [PubMed] [Google Scholar]
- Rashikh A, Pillai KK, Najmi AK. Protective effect of a direct renin inhibitor in acute murine model of cardiotoxicity and nephrotoxicity. Fundam Clin Pharmacol. 2013 doi: 10.1111/fcp.12054. [DOI] [PubMed] [Google Scholar]
- Roig Minguell E. Clinical use of markers of neurohormonal activation in heart failure. Rev Esp Cardiol. 2004;57:347–356. doi: 10.1016/S0300-8932(04)77113-4. [DOI] [PubMed] [Google Scholar]
- Sacco G, Mario B, Lopez G, Evangelista S, Manzini S, Maggi CA. ACE inhibition and protection from doxorubicin-induced cardiotoxicity in the rat. Vasc Pharmacol. 2009;50:166–170. doi: 10.1016/j.vph.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Shi Y, Moon M, Dawood S, McManus B, Liu PP. Mechanisms and management of doxorubicin cardiotoxicity. Herz. 2011;36:296–305. doi: 10.1007/s00059-011-3470-3. [DOI] [PubMed] [Google Scholar]
- Simmons TW, Jamall IS. Relative importance of intracellular glutathione peroxidase and catalase in vivo for prevention of peroxidation to the heart. Cardiovasc Res. 1989;23:774–779. doi: 10.1093/cvr/23.9.774. [DOI] [PubMed] [Google Scholar]
- Singal PK, Iliskovic N, Li T, Kumar D. Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J. 1997;11:931–936. doi: 10.1096/fasebj.11.12.9337145. [DOI] [PubMed] [Google Scholar]
- St-Jacques R, Toulmond S, Auger A, Binkert C, Cromlish W, Fischli W, Harris J, Hess P, Jie Lan, Liu S, Riendeau D, Steiner B, Percival MD (2011) Characterization of a stable, hypertensive rat model suitable for the consecutive evaluation of human renin inhibitors. J Renin Angiotensin Aldosterone Syst 12:133–145. doi:10.1177/1470320310392618 [DOI] [PubMed]
- Taskin EDN. Recovery of adriamycın induced mitochondrıal dysfunctıon in liver by selenium. Cytotechnology. 2014 doi: 10.1007/s10616-014-9736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskin E, Dursun N. The protection of selenium on adriamycin-induced mitochondrial damage in rat. Biol Trace Elem Res. 2012;147:165–171. doi: 10.1007/s12011-011-9273-9. [DOI] [PubMed] [Google Scholar]
- Taskin E, Ozdogan K, Kunduz Kindap E, Dursun N. The restoration of kidney mitochondria function by inhibition of angiotensin-II production in rats with acute adriamycin-induced nephrotoxicity. Ren Fail. 2014;36:606–612. doi: 10.3109/0886022X.2014.882737. [DOI] [PubMed] [Google Scholar]
- Tokarska-Schlattner M, Wallimann T, Schlattner U. Alterations in myocardial energy metabolism induced by the anti-cancer drug doxorubicin. CR Biol. 2006;329:657–668. doi: 10.1016/j.crvi.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Toko H, Oka T, Zou Y, Sakamoto M, Mizukami M, Sano M, Yamamoto R, Sugaya T, Komuro I (2002) Angiotensin II type 1a receptor mediates doxorubicin-induced cardiomyopathy. Hypertens Res 25:597–603 [DOI] [PubMed]
- Venkatesan N, Ramesh CV, Jayakumar R, Chandrakasan G. Angiotensin I converting enzyme activity in adriamycin induced nephrosis in rats. Toxicology. 1993;85:137–148. doi: 10.1016/0300-483X(93)90038-T. [DOI] [PubMed] [Google Scholar]
- Wallace KB. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol Toxicol. 2003;93:105–115. doi: 10.1034/j.1600-0773.2003.930301.x. [DOI] [PubMed] [Google Scholar]
- Wallace KB. Adriamycin-induced interference with cardiac mitochondrial calcium homeostasis. Cardiovasc Toxicol. 2007;7:101–107. doi: 10.1007/s12012-007-0008-2. [DOI] [PubMed] [Google Scholar]
- Westermann D, Riad A, Lettau O, Roks A, Savvatis K, Becher PM, Escher F, Jan Danser AH, Schultheiss HP, Tschöpe C (2008) Renin inhibition improves cardiac function and remodeling after myocardial infarction independent of blood pressure. Hypertension 52:1068–1075. doi:10.1161/HYPERTENSIONAHA.108.116350 [DOI] [PubMed]
- Xu M, Sheng L, Zhu X, Zeng S, Chi D, Zhang GJ. Protective effect of tetrandrine on doxorubicin-induced cardiotoxicity in rats. Tumori. 2010;96:460–464. doi: 10.1177/030089161009600314. [DOI] [PubMed] [Google Scholar]
- Zhu SG, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L. Dietary nitrate supplementation protects against doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol. 2011;57:2181–2189. doi: 10.1016/j.jacc.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]