Abstract
Since α-MSH suppresses endotoxin-induced inflammation by innate immunity, it is possible that α-MSH can suppress the interface between innate and adaptive immunity mediated by TLR4-stimulated macrophages. Endotoxin-stimulated macrophages treated with α-MSH are suppressed in nitric oxide and IL-12p70 production, and cannot enhance antigen-stimulated IFN-γ production by Th1 cells. In macrophages treated with α-MSH, the inhibitory molecule IRAK-M is bound to IRAK-1, the proximal intracellular signal molecule of endotoxin-bound TLR4. These results further demonstrate the dynamic contribution of the nervous system, and the role of α-MSH in modulating the innate and adaptive immune interface in an inflammatory response.
Keywords: Alpha-melanocyte-stimulating hormone, Toll-Like receptor 4, Interleukin-1 receptor-associated kinase-M, Macrophages
1. Introduction
The neuropeptide alpha-melanocyte-stimulating hormone (α-MSH), a 13-amino acid-long peptide derived from the proteolytic cleavage of proopiomelnocotin hormone (Chakraborty et al., 1996; Lee et al., 1961), is well documented to be a potent suppressor of endotoxin-, IL-1-, and TNF-mediated inflammation (Lipton and Catania, 1997). Systemic injections of α-MSH suppress septic shock, and fever. The treatment of mice with α-MSH at the time of haptensensitization causes antigen presenting cells (APC) to prime immunity devoid of a hypersensitivity response (Grabbe et al., 1996). Through melanocortin 1 and melanocortin 3 receptors on macrophages, α-MSH suppresses endotoxin activation of p38 MAPK and NF-κB (Getting et al., 2003; Ichiyama et al., 1999; Yoon et al., 2003). There is a constitutive level of α-MSH in various body tissues and fluids, and in response to a systemic inflammatory response there is an elevation of its concentration in blood (Holdeman et al., 1985; Taylor and Streilein, 1996; Taylor et al., 1992).
For the past decade we have examined the role of α-MSH in ocular regional immunity (Taylor, 2003b). The ocular microenvironment is a tissue site where local mechanisms of suppression barricade the activation of inflammatory immunity. This regional immunity was first experimentally observed by Medawar and his colleagues in the 1940s where they describe the prolong survival of incompatible grafts placed into the anterior chamber of the eye (Medawar, 1948). This immunosuppression is considered an evolutionary adaptation to prevent vision loss caused by the irreversible collateral tissue damage of autoimmune disease and delayed-type hypersensitivity (Streilein, 2003). The unique regulation of immunity in the eye is mediated in part by factors produced and expressed within the ocular microenvironment. The nervous system is a significant contributor of these immunosuppressive factors (Taylor, 2003a). The neurological contribution is characterized by the selective and constitutive presence of immunosuppressive neuropeptides found in aqueous humor, the fluid filling the anterior chamber of the eye.
The prototypical immunosuppressive neuropeptide in aqueous humor is α-MSH, which is maintained at a constitutive level of 30 pg/ml (Taylor et al., 1992). At its physiological concentration, α-MSH suppresses antigen-presenting cell (APC) and mitogen-activated primed Th1 cells, the CD4+ T cells that mediate hypersensitivity and autoimmune disease (Namba et al., 2002; Taylor and Namba, 2001; Taylor et al., 1992). Moreover, we found that α-MSH through the melanocortin 5 receptor mediates the induction of primed CD25+CD4+ regulatory T cells that in turn suppress in an antigen-specific manner other activated Th1 cells (Taylor and Namba, 2001). Most of our studies of α-MSH regulation of immunity has examined the effects of α-MSH on primed Th1 cells. In this manuscript we examine the possibility that α-MSH can affect APC at the interface between innate and adaptive immunity in the activation of Th1 cells.
Innate immunity instructs the activation, induction, and type of adaptive immunity against a specific antigen or pathogen (Medzhitov and Janeway, 1997). This is possible because the effector cells of innate immunity are the same cells (macrophages and dendritic cells) that present antigen to T cells. On the surface of APC are the toll-like receptors (TLR). The TLR are a family of molecules that recognize pathogen associated molecular patterns (PAMP), which are germ-line-encoded molecules of bacteria, yeast, and viruses (Takeda et al., 2003). The most noted PAMPs are the lipopolysaccharides (LPS) of gram-negative bacteria such as Escherichia coli and Salmonella typhimurium. The LPS binds a receptor complex centered on TLR4 (Poltorak et al., 1998; Qureshi et al., 1999). The engagement of TLR4 with LPS induces a series of intracellular signals that result in an intense antimicrobial and inflammatory response. LPS stimulates signaling pathways that activate the synthesis of interferons and IL-12 p70 that polarize the APC to activate Th1 cells (Hirschfeld et al., 2001; Jones et al., 2001; Pulendran et al., 2001; Qi et al., 2003; Re and Strominger, 2001; Toshchakov et al., 2003; Weinmann et al., 2001).
The immediate intracellular response to LPS binding by TLR4 is the binding of adaptor molecules such as MAL and MyD88 to the cytoplasmic tail of TLR4 (Akira and Takeda, 2004). Here, the literature describes multiple pathways that are independent or dependent on MyD88. The MyD88-dependent pathway involves the binding and phosphorylation of interleukin-1 receptor-associated kinase (IRAK; Kobayashi et al., 2002; Li et al., 1999; Maschera et al., 1999). The phosphorylated IRAK starts the next step in the signal cascade that ends with the activation of NF-κB. There is a negative regulator of the signal cascade, another member of the IRAK family, IRAK-M (Kobayashi et al., 2002; Rosati and Martin, 2002; Wesche et al., 1999). The negative regulator IRAK-M is presumed to bind other members of the IRAK family to suppress TLR4-dependent responses to LPS. These findings suggest that there are intracellular mechanisms that can regulate the innate immune response, and therefore the type of adaptive immunity induced by a TLR4-stimulated APC. We will demonstrate that α-MSH suppresses TLR4-stimulated activity in APC, and that α-MSH functions early in the LPS/TLR4 response by promoting the association of IRAK-M with IRAK-1.
2. Material and methods
2.1. Mice and reagents
The mice used in these experiments were female 6- to 8-week-old BALB/c mice from Jackson Labs (BarHarbor, ME), and all animal use was approved by the institutional animal care and use committee. The neuropeptide α-MSH (Bachem Bioscience, King of Prussia, PA) was suspended in sterile 0.01 M phosphate-buffered saline (PBS) and stored at -70 °C before use. To stimulate TLR we used pheno-extracted, gel chromatography filtered lipopolysaccharide (LPS) of E. coli O111:B4 (Sigma-Aldrich, St. Louis, MO), heat-killed, formalin-fixed Staphylococcus aureus cells (Pansorbin; Calbiochem-EMD Biosciences, San Diego, CA; Rabehi et al., 2001), and non-viable, desiccated Mycobacterium tuberculosis H37 Ra (Difco Laboratories, Detroit, MI). To immunoprecipitate IRAK-1, we used an anti-IRAK-1 antibody (Upstate, Lake Placid, NY) that was specific to a unique amino acid sequence (amino acids 700–712) of IRAK-1. Primary antibodies for immunoblotting were anti-IRAK-M (ProSci, Poway, CA)), alkaline phosphatase-conjugated anti-phospho-tyrosine Py20 (Calbiochem-EMD Biosciences), alkaline phosphatase-conjugated, anti-phospho-serine (Sigma-Aldrich). Secondary antibodies were alkaline phosphatase-conjugated anti-rabbit IgG and alkaline phosphatase-conjugated anti-mouse IgG from Jackson Immunoresearch Laboratories (West Grove, PA).
2.2. Macrophage-stimulating assays
2.2.1. Nitric oxide assay
The generation of nitric oxide was assayed on stimulated mouse monocyte-macrophage cell line J774A.1 (ATCC, Manassas, VA; Peteroy-Kelly et al., 2001). The J774 cells cultured in pheno-red free DMEM (Cambrex, Walkersville, MD) plus 10% FBS (Hyclone, Logan, UT) were placed into the wells of a flat-bottom 96-well plate at 1.5×105 cells per well. The cells were incubated for 2 h at 37 °C, 5% CO2, and washed twice with pheno-red free, serum-free media: pheno-red free DMEM supplemented with 10 μg/ml gentamicin (Sigma, St. Louis, MO), 0.01 M HEPES, 1× NEAA mixture, 1 mM Sodium pyruvate (BioWhittaker), 1 mg/ml BSA, and 1:500 dilution of “ITS+” culture supplement (Sigma-Aldrich). At its physiological concentration of 30 pg/ml in pheno-red free, serum-free media, α-MSH was added to the wells. This was followed with the addition of LPS (1 μg/ml), S. aureus (200 μg/ml), or M. tuberculosis (15 μg/ml). The bacteria and bacterial products were at their optimal nitric oxide-stimulating concentration. The cultures were incubated for 24 hours at 37 °C, 5% CO2. The culture supernatants were assayed using a Griess reagent kit (Molecular Probes, Eugene, OR) to determine the concentration of the nitrites. The concentration of nitrites over the background is proportional to level of nitric oxide generated by the macrophages.
2.2.2. IL-12 ELISA
Adherent spleen cells from naive BALB/c mice were assayed for LPS induced IL-12 production. The spleen cells were added at 1×106 cells per well of a 96-well flat-bottom culture plate. The cultures were incubated for 90 min at 37 °C in 5% CO2. The wells were washed twice with serum free media: RPMI-1640 supplemented with 10 μg/ml gentamicin (Sigma, St. Louis, MO), 0.01 M HEPES, 1× NEAA mixture, 1 mM Sodium pyruvate (BioWhittaker), 1 mg/ml BSA, and 1:500 dilution of “ITS+” culture supplement (Sigma-Aldrich). The cultures were incubated for an additional 30 min in serum-free media and washed twice more. In serum-free media the adherent spleen cells were treated with α-MSH (30 pg/ml) and 1 ng/ml of LPS and were incubated for 48 h. The supernatants were assayed using the IL-12p40 and the IL-12p70 ELISA kits of R&D Systems (Minneapolis, MN).
2.3. Antigen-specific T cell assay
2.3.1. Antigen pulsed APC
Spleen cells from naive BALB/c mice suspended in RPMI-1640 (Bio Whittaker, Walkersville, MD) plus 5% FBS (Hyclone Laboratories, Logan, UT) and 1×106 cells were seeded into each well of a 96-well flat-bottom culture plate. Adherent cells were cultivated as described above. The adherent cells were washed twice with serum free media and treated in serum-free media with α-MSH (30 pg/ml) followed by the addition of OVA (Sigma-Aldrich; 200 μg/ml). The OVA was purposely contaminated with either LPS (1 μg/ml) or S. aureus (40 or 400 μg/ml). The cultures were incubated for 24 h, washed, and OVA-primed CD3+ T cells were added to the wells.
2.3.2. Stimulated T cell cultures
Primed CD3+ T cells were enriched from draining lymph nodes of BALB/c mice (Jackson Labs) immunized 7 days earlier with OVA (100 μg/mouse) in Freund's complete adjuvant. The enriched CD3+ T cells (4×105 cells/well) were added to cultures of antigen pulsed adherent spleen cells as previously described (Namba et al., 2002). The cultures were incubated for 48 h and the supernatant was assayed for IFN-γ by ELISA.
2.3.3. IFN-γ ELISA
The concentration of IFN-γ in the culture supernatant of stimulated T cells incubated for 48 h was measured by sandwich ELISA. In brief, the wells of a 96-well microtiter plate were coated with capturing monoclonal anti-IFN-γ antibody (BD Pharmagen) and blocked. The culture supernatants or media with known concentrations of recombinant cytokine for a standard curve were added to the wells. After a 3-h incubation at room temperature, the plate was washed and biotinylated-anti-IFN-γ (BD Pharmagen) antibody added and incubated for 1 h. This was followed by a 30-min incubation of streptavidin-β-galactosidase (Gibco BRL) and a 1-h incubation of the substrate chlorophenyl-red-β-d-galactoside (Calbiochem, LaJolla, CA). The optical density of the color change was read on a standard ELISA plate reader at a wavelength of 574 nm, and based on the optical density of the known concentrations of recombinant lymphokine, a standard curve was calculated. The sample's optical density was applied to the standard curve and the concentration of the lymphokine in the sample was calculated. The limit of detection for IFN-γ was 10 pg/ml.
2.4. Immunoprecipitating and immunoblotting assays
The J774 monocyte cells were placed at 2×107 cells per well into each well of a 12-well plate in DMEM plus 10% FBS, and incubated for 2 h at 5% CO2. The cultures were washed with serum-free media, and 30 pg/ml of α-MSH followed by 1 μg/ml of LPS were added to the wells. At 10, 20, and 30 min after the addition of LPS, the cells were washed with room temperature PBS, and lysed using ice-cold buffer (10 mM TBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 100 μg/ml phenylmethylsulfonyl fluoride (PMSF), 60 μg/ml Aprotinin, and 1 mM sodium orthovanadate). The lysate was passed through a 21-gauge needle three times and an additional 20 μg of PMSF was added to the lysate. The lysate was incubated for 30 min on ice then centrifuged for 20 min at 4 °C, 13,000×g. The supernatant was collected and assayed for protein using the BioRad Protein Assay Kit (Hercules, CA). To the same amount of lysate protein (0.5 mg/ml) for each experimental condition was added 4 μg/ml of anti-IRAK1 antibody. After being agitated overnight at 4 °C, a 20 μl solution of protein-A/G agarose beads (Santa Cruz Biologics) was added to the lysates. The lysates were incubated end over end for 1 h at 4 °C. The beads were centrifuged down at 1100×g, 5 min, washed three times with ice-cold lysis buffer, and after the final centrifugation, the beads were resuspended in Nu-PAGE MOPS denaturing and reducing sample-buffer (Novex-Invitrogen, Carlsbad, CA). The processed beads were electrophoresed on a 4–12% Nu-PAGE gel, and the electrophoresed proteins were transferred to nitrocellulose. The membrane was blocked with TBS SuperBlock (Pierce, Rockford, IL) for 1 h at room temperature and placed into a sealable bag containing 5 ml of the appropriate primary antibody diluted in 10% TBS SuperBlock, sealed, and incubated overnight at room temperature. The membrane was washed with 10% TBS SuperBlock. For unconjugated primary antibodies, the membrane was placed into a new sealable bag and incubated for 1 h at room temperature with 5 ml of appropriate secondary alkaline phosphatase conjugated antibody, and washed three times with 10% TBS Super-Block. The membrane was washed once more with distilled water and incubated with substrate BCIP/NBT (Sigma Chemical) until bands appeared. The substrate reaction was stopped by washing the membrane with distilled water and the membrane was digitally photographed. To confirm uniform protein transfer to the membrane, the intensity of the immunoprecipitating antibody's immunoglobulin heavy chain band (50 kDa) was analyzed by NIH Image software.
2.5. Statistical analysis
For the cytokine and nitric oxide assays, the experimental results were compared to the appropriate controls using Student's t-test with significance determined at P≤0.05. All experiments were repeated as indicated in each figure.
3. Results
3.1. The effects of α-MSH on specific TLR-stimulated innate immune activity
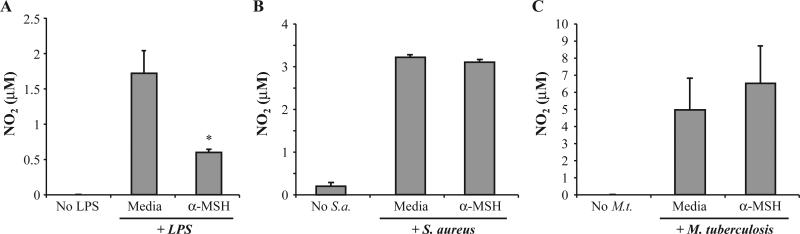
We examined whether α-MSH could suppress innate immune activity in macrophages stimulated through different TLR, TLR2 and TLR4. The cells of the macrophage cell line J774 were treated with 30 pg/ml of α-MSH, the physiological level of α-MSH in the ocular microenvironment. The macrophages were then optimally stimulated through TLR4 with LPS (1 μg/ml), through TLR2 with S aureus (200 μg/ml), or through multiple pathways with M. tuberculosis (Fig. 1). Only the nitric oxide production by macrophages stimulated with LPS, TLR4 (Fig. 1A), but not with S. aureus or M. tuberculosis was suppressed by α-MSH treatment. This suggests that α-MSH modulation of innate immunity is limited, and it is limited to at least subduing a TLR4-associated inflammatory response in macrophages.
Fig. 1.
The effects of α-MSH on nitric oxide production by macrophages stimulated by specific TLR. Macrophages (1.5×105 cells) were treated with α-MSH (30 pg/ml) and stimulated through their (A) TLR4 with LPS (1 μg/ml), (B) TLR2 with S. aureus (200 μg/ml), or (C) TLR4 and TLR2 with M. tuberculosis (15 μg/ml). Nitrite levels in the culture supernatant were assayed 24 h later and presented as μM±S.E.M. of four independent experiments each. *Significance (P≤0.05) was determined in comparison with nitrite levels of TLR-stimulated macrophages not treated with α-MSH (Media). No significant difference was found between the nitrite levels of α-MSH-treated and untreated macrophage cultures stimulated with S. aureus or M. tuberculosis.
3.2. The effects of α-MSH on TLR4-influenced APC activity
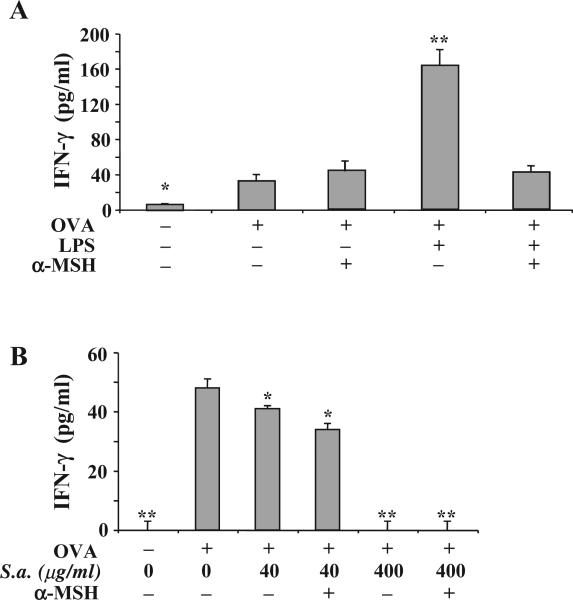
If α-MSH can block TLR4-mediated inflammatory responses in macrophages then it is possible that α-MSH can suppress TLR4-influenced antigen processing and presentation. To examine this possibility it was necessary to use an antigen that did not induce a detectible innate immune response in our cultures. We prescreened preparations of ovalbumin (OVA) for their ability to stimulate nitric oxide production by macrophages, and used only the OVA preparations that could not on its own induce nitric oxide production. Then the OVA preparation was purposely contaminated with either LPS (1 μg/ml) or S. aureus (200 μg/ml), representing antigen that is processed and presented under TLR4- or TLR2-stimulated conditions. The adherent spleen APC was incubated for 24 h with the contaminated OVA and was washed, and OVA-primed Tcells were added to the antigen-pulsed macrophage cultures. The APC that processed and presented OVA contaminated with LPS-enhanced IFN-γ production by the OVA-specific primed T cells. In contrast, the α-MSH-treated APC could not enhance IFN-γ production, but were not suppressed in their stimulatory activity (Fig. 2A). Moreover, α-MSH treatment of macrophages presenting uncontaminated OVA did not affect the stimulation of IFN-γ production by the primed T cells (Fig. 2A). The APC processing S. aureus-contaminated OVA were suppressed in their ability to stimulate IFN-γ production by the OVA-primed Th1 cells (Fig. 2B). Moreover, α-MSH treatment of the APC did not change nor enhance the TLR2-mediated suppression. The results suggest that the suppression mediated by α-MSH is solely on the enhancing effects of LPS stimulation of the macrophages. This further implies that α-MSH is limited to antagonizing a TLR4-associated response in the APC.
Fig. 2.
The effects of α-MSH on antigen-specific APC presentation under the influence of specific TLR-stimulation. Adherent spleen cells were treated with α-MSH (30 pg/ml) and pulsed with OVA (200 μg/ml) that was mixed with (A) LPS (1 μg/ml) or (B) S. aureus (200 μg/ml). The cells were washed and OVA-antigen primed T cells (4×105 cells/well) were added to the cultures. The amount of IFN-γ produce in the cultures 48 h later was assayed by ELISA. The results are presented as the mean pg/ml±S.E.M. of IFN-γ measured in the cultures of 4 independent experiments. Significant differences (*P<0.001, **P<0.0001) were calculated in comparison with the IFN-γ concentration in cultures of T cells activated with APC pulsed with uncontaminated OVA and not treated with α-MSH. The difference in panels (A) and (B) between the IFN-γ concentrations in cultures of T cells activated with APC pulsed with uncontaminated OVA and not treated with α-MSH is insignificant.
3.3. The effects of α-MSH on IL-12 production by TLR4-stimulated APC
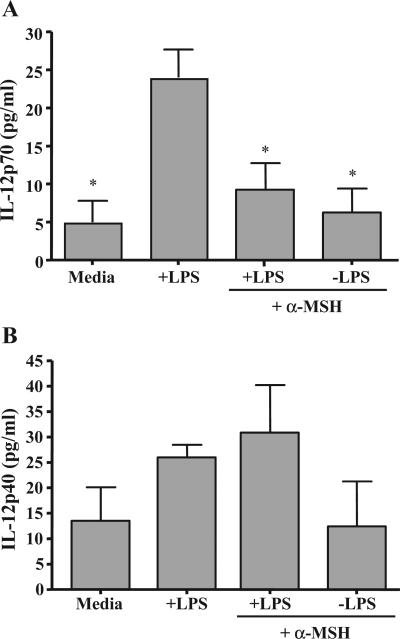
One important TLR4-stimulated response by macrophages is the production of IL-12p70 (Qi et al., 2003), which promotes the production of IFN-γ by Th1 cells (Hill et al., 2003). The adherent spleen macrophages were stimulated with LPS and treated with α-MSH at its physiological concentration. The α-MSH treatment significantly suppressed the production of IL-12p70 by the LPS-stimulated cells (Fig. 3A). However, α-MSH treatment had no significant effect on the constitutive production of IL-12p40 by these adherent spleen cells (Fig. 3B). These results further support the possibility that α-MSH antagonizes the signals from LPS-stimulated TLR4 that are necessary to release the translational regulation of IL-12p35 and the heterodimerization of p35 with p40 to make IL-12p70 (Babik et al., 1999).
Fig. 3.
The effects of α-MSH on IL-12 production by LPS-stimulated macrophages. Adherent spleen macrophages were treated with α-MSH (30 pg/ml) and LPS (1 μg/ml). After a 48-h incubation, the culture supernatants were assayed for (A) IL-12p70 or (B) IL-12p40. The results are the mean ng/ml±S.E.M. of IL-12p70 or IL-12p40 measured in the cultures of 4 independent experiments each. *Significant differences ( P<0.01) were calculated in comparison with the IL-12 concentration in the cultures of macrophages treated with only LPS.
3.4. The effects of α-MSH on TLR4 signaling in macrophages
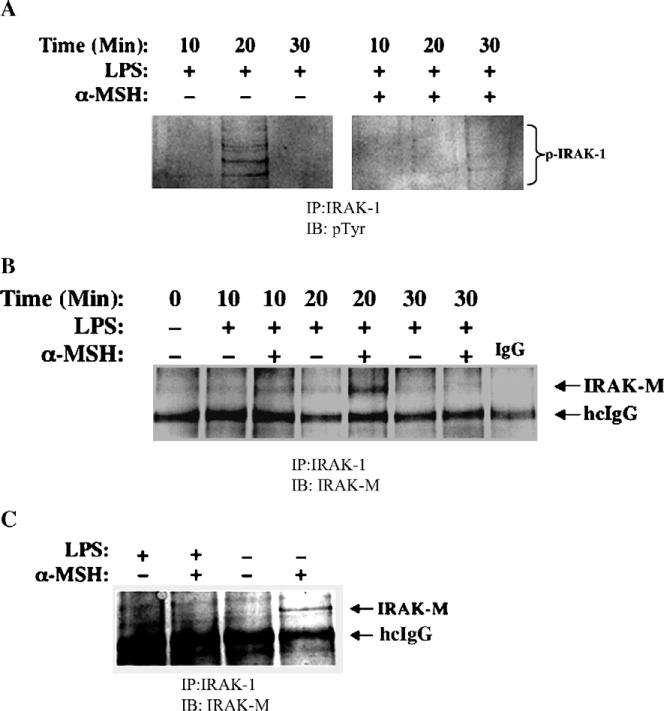
One the most immediate responses to LPS through TLR4 is the hyper-serine phosphorylation of IRAK-1. To detect whether α-MSH prevented the hyper-phosphorylation of IRAK-1, we immunoprecipitated IRAK-1 from LPS-stimulated macrophages treated with α-MSH, and immunoblotted for phospho-serine. We could not detect any change in the presence, intensity, or characterization of the phospho-serine pattern of IRAK-1 (data not shown). However, we did find that there was a suppression of tyrosine phosphorylation of IRAK-1 in LPS-stimulated macrophages treated with α-MSH (Fig. 4A). Tyrosine phosphorylation of IRAK-1 is in a specific region of IRAK-1 that is associated with kinase activity and clearance, although it is not required for IRAK-1 serine auto-phosphorylation (Jensen and Whitehead, 2001). This suggests that α-MSH treatment can stop the response to TLR4-stimulation at the initial steps of the signaling cascade.
Fig. 4.
Effects of α-MSH on intracellular signaling associated with TLR4 stimulation in macrophages. Macrophages (2×107 cells) were treated with α-MSH (30 pg/ml) and stimulated through their TLR4 with LPS (1 μg/ml). The cells were lysed at various times after LPS-stimulation. Equal amounts of lysate protein (300 μg) were immunoprecipitated (IP) with anti-IRAK-1. The immunoprecipitates were electrophoresed, transferred to nitrocellulose filters and immunoblotted (IB) with (A) anti-Phospho-Tyrosine (pTyr), or with (B and C) anti-IRAK-M. The photographic digital images of the immunoblots are presented as an example of three experiments each having the same result. The phosphorylated IRAK-1 (p-IRAK-1) migrated between 80 to 110 kDa and IRAK-M migrated to 60 kDa on the NuPage MOPS gel. The 50-kDa band of the heavy chain of the immunoprecipitating anti-IRAK-1 IgG is identified (hcIgG).
One of the potential blocks of TLR signaling is the activation of IRAK-M (Kobayashi et al., 2002). IRAK-M functions by binding IRAK-1 and it is uncertain whether IRAK-M keeps IRAK-1 bound to the TLR4/MyD88 complex or that it blocks IRAK-1 kinase activity needed for the next step in the TLR4/MyD88 signaling cascade. When we immunoprecipitated IRAK-1 from LPS-stimulated macrophages treated with α-MSH, we co-precipitated IRAK-M (Fig. 4B). The detection of IRAK-M was maximum 20 min after LPS stimulation of the α-MSH treated macrophages and declined after. Macrophages not stimulated with LPS, but treated with α-MSH had, 24 h later, IRAK-M bound to IRAK-1, suggesting that there is a direct relationship between IRAK-M binding of IRAK-1 and α-MSH treatment (Fig. 4C). In addition, the lack of detecting IRAK-M at 30 min following LPS stimulation is expected because of the natural clearance or degradation of IRAK-1 following LPS stimulation (Hu et al., 2002; Jensen and Whitehead, 2001; Yamin and Miller, 1997). Therefore, α-MSH suppresses LPS-stimulated TLR4 activity through a mechanism that promotes IRAK-M binding of IRAK-1.
4. Discussion
The literature has repeatedly shown that the neuropeptide α-MSH is an important factor in maintaining non-inflammatory homeostasis, in regulating the existent of the inflammatory response, and in establishing the blockade to inflammation in the immune privileged eye (Lipton and Catania, 1997; Taylor et al., 1992). The treatment of LPS-stimulated macrophages with α-MSH prevents the translocation of NF-κB from the cytoplasm to the nucleus resulting from a block in IκB degradation (Deng et al., 2004). In addition, the α-MSH-treated macrophages have deactivated p38 (Yoon et al., 2003). These findings suggested that there must be a more proximal inhibition of the LPS-induced intracellular signaling cascade. Our results demonstrate for the first time that the immunosuppressive neuropeptide α-MSH inhibits LPS stimulation of macrophages by blocking TLR-4 signaling using the intracellular TLR-inhibitor IRAK-M. This is also the first time that a factor has been found that can summon IRAK-M to block TLR activity.
From our results, we demonstrate that α-MSH selectively suppresses the activity associated with stimulating macrophages through TLR-4. This is seen in α-MSH suppression of TLR-4-stimulated nitric oxide generation and IL-12p70 production. The α-MSH-treated APC still present antigen, since only the additional effects of TLR-4-stimulation in promoting Th1 cell activation by the APC was suppressed by α-MSH. In contrast, TLR-2-stimulated activity of macrophages and APC were not affected by α-MSH treatment. Therefore, α-MSH-mediated IRAK-M activity is limited to suppressing the TLR4-signaling cascade. This finding is very interesting since it supports the possibility that there are significant differences between the intracellular signaling cascades of the TLRs that converge on NF-κB.
How α-MSH treatment can lead to IRAK-M binding of IRAK-1 is not addressed by our findings, and how this can happen is complicated by the expression of three melanocortin receptors (MC1r, MC3r, and MC5r) that bind α-MSH on macrophages (Getting et al., 1999; Neumann Andersen et al., 2001; Taherzadeh et al., 1999). In general, the melanocortin receptors are G-protein coupled and are not evenly distributed throughout the body. The most predominate melanocortin receptor on macrophages and dendritic cells is MC1r (Neumann Andersen et al., 2001), although message for MC3r and MC5r can be detected (Taherzadeh et al., 1999). The engagement of α-MSH with MC1r elevates cAMP levels in the macrophages and activates a cAMP-dependent protein kinase A (PKA; Yoon et al., 2003). The activation of PKA is linked to the inhibition of LPS-activated p38 MAPK and NF-κB. However, our results place an additional level of α-MSH suppression at the LPS receptor itself, TLR4. The MC5r works through a different signaling cascade involving the Janus kinase2/signal transducers and activators of transcription1 (JAK2/STAT1) pathway (Buggy, 1998). This means that α-MSH through its various receptors can influence multiple regulatory pathways associated with activated macrophages. It also suggests that the selective distribution and expression of the melanocortin receptors maybe related to whether α-MSH mediates neurological, metabolic, or immunomodulating activity. Based on our findings and others, the immunomodulating activity of α-MSH is to suppress the inflammatory response mediated by LPS, IL-1, TNF-α, and Th1 cells.
The singularity of the ocular microenvironment to subdue the activation of inflammatory immunity has made it possible to understand that ocular immunity is uniquely an intersection of innate, adaptive, and neural immunity. In addition, our research into the immunosuppressive activity of α-MSH and its importance in ocular immune privilege has demonstrated the dynamic contribution of the nervous system to immunoregulation. Further understanding of α-MSH immunomodulating activity will continue to promote the use α-MSH in a therapeutic manner to subdue inflammation and to manipulate immunity to prevent induction of inflammation resulting from specific infections and autoimmune diseases (Casalino-Matsuda et al., 2002; Colombo et al., 2002; Huang et al., 1998; Namba et al., 2002; Nishida et al., 2004; Shiratori et al., 2004).
Acknowledgments
This work is dedicated to the memory of my former postdoctoral mentor J. Wayne Streilein (1936–2004). I am greatly appreciative of the technical assistance of David Yee. This work was supported by a PHS grant through the NEI, EY10752.
References
- Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev., Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Babik JM, Adams E, Tone Y, Fairchild PJ, Tone M, Waldmann H. Expression of murine IL-12 is regulated by translational control of the p35 subunit. J. Immunol. 1999;162:4069–4078. [PubMed] [Google Scholar]
- Buggy JJ. Binding of alpha-melanocyte-stimulating hormone to its G-protein-coupled receptor on B-lymphocytes activates the Jak/STAT pathway. Biochem. J. 1998;331(Pt 1):211–216. doi: 10.1042/bj3310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino-Matsuda SM, Durando PE, Celis ME. Effects of alpha-MSH on progesterone and nitric oxide release by cultured ovarian granulosa cells in experimental rat autoimmune oophoritis. J. Physiol. Biochem. 2002;58:25–31. doi: 10.1007/BF03179835. [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim. Biophys. Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- Colombo G, Buffa R, Bardella MT, Garofalo L, Carlin A, Lipton JM, Catania A. Anti-inflammatory effects of alpha-melanocyte-stimulating hormone in celiac intestinal mucosa. Neuroimmuno-modulation. 2002;10:208–216. doi: 10.1159/000068323. [DOI] [PubMed] [Google Scholar]
- Deng J, Hu X, Yuen PS, Star RA. Alpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/reperfusion. Am. J. Respir. Crit. Care Med. 2004;169:749–756. doi: 10.1164/rccm.200303-372OC. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Gibbs L, Clark AJ, Flower RJ, Perretti M. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. J. Immunol. 1999;162:7446–7453. [PubMed] [Google Scholar]
- Getting SJ, Christian HC, Lam CW, Gavins FN, Flower RJ, Schioth HB, Perretti M. Redundancy of a functional melanocortin 1 receptor in the anti-inflammatory actions of melanocortin peptides: studies in the recessive yellow (e/e) mouse suggest an important role for melanocortin 3 receptor. J. Immunol. 2003;170:3323–3330. doi: 10.4049/jimmunol.170.6.3323. [DOI] [PubMed] [Google Scholar]
- Grabbe S, Steinert M, Mahnke K, Schwartz A, Luger TA, Schwarz T. Dissection of antigenic and irritative effects of epicutaneously applied haptens in mice. Evidence that not the antigenic component but nonspecific proinflammatory effects of haptens determine the concentration-dependent elicitation of allergic contact dermatitis. J. Clin. Invest. 1996;98:1158–1164. doi: 10.1172/JCI118899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Ichim TE, Kusznieruk KP, Li M, Huang X, Yan X, Zhong R, Cairns E, Bell DA, Min WP. Immune modulation by silencing IL-12 production in dendritic cells using small interfering RNA. J. Immunol. 2003;171:691–696. doi: 10.4049/jimmunol.171.2.691. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 2001;69:1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdeman M, Khorram O, Samson WK, Lipton JM. Fever-specific changes in central MSH and CRF concentrations. Am. J. Physiol. 1985;248:R125–R129. doi: 10.1152/ajpregu.1985.248.1.R125. [DOI] [PubMed] [Google Scholar]
- Hu J, Jacinto R, McCall C, Li L. Regulation of IL-1 receptor-associated kinases by lipopolysaccharide. J. Immunol. 2002;168:3910–3914. doi: 10.4049/jimmunol.168.8.3910. [DOI] [PubMed] [Google Scholar]
- Huang QH, Hruby VJ, Tatro JB. Systemic alpha-MSH suppresses LPS fever via central melanocortin receptors independently of its suppression of corticosterone and IL-6 release. Am. J. Physiol. 1998;275:R524–R530. doi: 10.1152/ajpregu.1998.275.2.R524. [DOI] [PubMed] [Google Scholar]
- Ichiyama T, Sakai T, Catania A, Barsh GS, Furukawa S, Lipton JM. Inhibition of peripheral NF-kappaB activation by central action of alpha-melanocyte-stimulating hormone. J. Neuroimmunol. 1999;99:211–217. doi: 10.1016/s0165-5728(99)00122-8. [DOI] [PubMed] [Google Scholar]
- Jensen LE, Whitehead AS. IRAK1b, a novel alternative splice variant of interleukin-1 receptor-associated kinase (IRAK), mediates interleukin-1 signaling and has prolonged stability. J. Biol. Chem. 2001;276:29037–29044. doi: 10.1074/jbc.M103815200. [DOI] [PubMed] [Google Scholar]
- Jones BW, Means TK, Heldwein KA, Keen MA, Hill PJ, Belisle JT, Fenton MJ. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 2001;69:1036–1044. [PubMed] [Google Scholar]
- Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr., Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- Lee TH, Lerner AB, Buettner-Janusch V. The isolation and structure of a- and h-melanocyte-stimulating hormones from monkey pituitary glands. J. Biol. Chem. 1961;236:1390–1394. [PubMed] [Google Scholar]
- Li X, Commane M, Burns C, Vithalani K, Cao Z, Stark GR. Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol. Cell. Biol. 1999;19:4643–4652. doi: 10.1128/mcb.19.7.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JM, Catania A. Anti-inflammatory actions of the neuroimmunomodulator alpha-MSH. Immunol. Today. 1997;18:140–145. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- Maschera B, Ray K, Burns K, Volpe F. Overexpression of an enzymically inactive interleukin-1-receptor-associated kinase activates nuclear factor-kappaB. Biochem. J. 1999;339(Pt 2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Medawar P. Immunity to homologous grafted skin: III. The fate of skin homografts transplanted to the brain to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- Namba K, Kitaichi N, Nishida T, Taylor AW. Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2. J. Leukoc. Biol. 2002;72:946–952. [PubMed] [Google Scholar]
- Neumann Andersen G, Nagaeva O, Mandrika I, Petrovska R, Muceniece R, Mincheva-Nilsson L, Wikberg JE. MC(1) receptors are constitutively expressed on leucocyte subpopulations with antigen presenting and cytotoxic functions. Clin. Exp. Immunol. 2001;126:441–446. doi: 10.1046/j.1365-2249.2001.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Miyata S, Itoh Y, Mizuki N, Ohgami K, Shiratori K, Ilieva IB, Ohno S, Taylor AW. Anti-inflammatory effects of alpha-melanocyte-stimulating hormone against rat endotoxin-induced uveitis and the time course of inflammatory agents in aqueous humor. Int. Immunopharmacol. 2004;4:1059–1066. doi: 10.1016/j.intimp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Peteroy-Kelly M, Venketaraman V, Connell ND. Effects of Mycobacterium bovis BCG infection on regulation of l-arginine uptake and synthesis of reactive nitrogen intermediates in J774.1 murine macrophages. Infect. Immun. 2001;69:5823–5831. doi: 10.1128/IAI.69.9.5823-5831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Denning TL, Soong L. Differential induction of interleukin-10 and interleukin-12 in dendritic cells by microbial toll-like receptor activators and skewing of T-cell cytokine profiles. Infect. Immun. 2003;71:3337–3342. doi: 10.1128/IAI.71.6.3337-3342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabehi L, Irinopoulou T, Cholley B, Haeffner-Cavaillon N, Carreno MP. Gram-positive and gram-negative bacteria do not trigger monocytic cytokine production through similar intracellular pathways. Infect. Immun. 2001;69:4590–4599. doi: 10.1128/IAI.69.7.4590-4599.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- Rosati O, Martin MU. Identification and characterization of murine IRAK-M. Biochem. Biophys. Res. Commun. 2002;293:1472–1477. doi: 10.1016/S0006-291X(02)00411-4. [DOI] [PubMed] [Google Scholar]
- Shiratori K, Ohgami K, Ilieva IB, Koyama Y, Yoshida K, Ohno S. Inhibition of endotoxin-induced uveitis and potentiation of cyclooxygenase-2 protein expression by alpha-melanocyte-stimulating hormone. Invest. Ophthalmol. Vis. Sci. 2004;45:159–164. doi: 10.1167/iovs.03-0492. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J. Leukoc. Biol. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- Taherzadeh S, Sharma S, Chhajlani V, Gantz I, Rajora N, Demitri MT, Kelly L, Zhao H, Ichiyama T, Catania A, Lipton JM. alpha-MSH and its receptors in regulation of tumor necrosis factor-alpha production by human monocyte/macrophages. Am. J. Physiol. 1999;276:R1289–R1294. doi: 10.1152/ajpregu.1999.276.5.R1289. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Taylor A. A review of the influence of aqueous humor on immunity. Ocul. Immunol. Inflamm. 2003a;11:231–241. doi: 10.1076/ocii.11.4.231.18269. [DOI] [PubMed] [Google Scholar]
- Taylor AW. Modulation of regulatory T cell immunity by the neuropeptide alpha-melanocyte stimulating hormone. Cell. Mol. Biol. (Noisy-le-grand) 2003b;49:143–149. [PubMed] [Google Scholar]
- Taylor A, Namba K. In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH). Immunol. Cell Biol. 2001;79:358–367. doi: 10.1046/j.1440-1711.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Streilein JW. Inhibition of antigen-stimulated effector T cells by human cerebrospinal fluid. Neuroimmunomodulation. 1996;3:112–118. doi: 10.1159/000097235. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr. Eye Res. 1992;11:1199–1206. doi: 10.3109/02713689208999545. [DOI] [PubMed] [Google Scholar]
- Toshchakov V, Jones BW, Lentschat A, Silva A, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, Vogel SN. TLR2 and TLR4 agonists stimulate unique repertoires of host resistance genes in murine macrophages: interferon-beta-dependent signaling in TLR4-mediated responses. J. Endotoxin Res. 2003;9:169–175. doi: 10.1179/096805103125001577. [DOI] [PubMed] [Google Scholar]
- Weinmann AS, Mitchell DM, Sanjabi S, Bradley MN, Hoffmann A, Liou HC, Smale ST. Nucleosome remodeling at the IL-12 p40 promoter is a TLR-dependent, Rel-independent event. Nat. Immunol. 2001;2:51–57. doi: 10.1038/83168. [DOI] [PubMed] [Google Scholar]
- Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J. Biol. Chem. 1999;274:19403–19410. doi: 10.1074/jbc.274.27.19403. [DOI] [PubMed] [Google Scholar]
- Yamin TT, Miller DK. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J. Biol. Chem. 1997;272:21540–21547. doi: 10.1074/jbc.272.34.21540. [DOI] [PubMed] [Google Scholar]
- Yoon SW, Goh SH, Chun JS, Cho EW, Lee MK, Kim KL, Kim JJ, Kim CJ, Poo H. alpha-Melanocyte-stimulating hormone inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production in leukocytes by modulating protein kinase A, p38 kinase, and nuclear factor kappa B signaling pathways. J. Biol. Chem. 2003;278:32914–32920. doi: 10.1074/jbc.M302444200. [DOI] [PubMed] [Google Scholar]