Abstract
Purpose
To estimate the effects of a tablet-based, breast cancer risk education intervention for use in primary care settings (BreastCARE) on patients' breast cancer knowledge, risk perception and concern.
Methods
From June 2011–August 2012, we enrolled women from two clinics, aged 40–74 years with no personal breast cancer history, and randomized them to the BreastCARE intervention group or to the control group. All patients completed a baseline telephone survey and risk assessment (via telephone for controls, via tablet computer in clinic waiting room prior to visit for intervention). All women were categorized as high or average risk based on the Referral Screening Tool, the Gail model or the Breast Cancer Surveillance Consortium model. Intervention patients and their physicians received an individualized risk report to discuss during the visit. All women completed a follow-up telephone survey 1–2 weeks after risk assessment. Post-test comparisons estimated differences at follow-up in breast cancer knowledge, risk perception and concern.
Results
580 intervention and 655 control women completed follow-up interviews. Mean age was 56 years (SD = 9). At follow-up, 73% of controls and 71% of intervention women correctly perceived their breast cancer risk and 22% of controls and 24% of intervention women were very concerned about breast cancer. Intervention patients had greater knowledge (≥75% correct answers) of breast cancer risk factors at follow-up (24% vs. 16%; p = 0.002). In multivariable analysis, there were no differences in correct risk perception or concern, but intervention patients had greater knowledge ([OR] = 1.62; 95% [CI] = 1.19–2.23).
Conclusions
A simple, practical intervention involving physicians at the point of care can improve knowledge of breast cancer without increasing concern.
Trial Registration
ClinicalTrials.gov identifier NCT01830933.
Keywords: Health-related information technology, Breast cancer, Risk reduction, Prevention, RCT
Introduction
Validated breast cancer risk estimation tools are available to physicians [1–4], but are underused [5–7]. As a result, many women may not engage in a discussion of their breast cancer risk with their physician. Lack of knowledge about risk can lead to misperceptions and underuse of prevention and risk reduction therapies for women at high risk for breast and ovarian cancer [8,9].
Effective risk reduction options are available and recommended for high-risk patients including two selective estrogen receptor modulator therapies (SERMS), tamoxifen and raloxifene [10–12]. However, these medications are not without risks. Side effects include increased risk for venous thromboembolic events with use of tamoxifen and raloxifene and increased risk for endometrial cancer with use of tamoxifen. [10–12] Further, because not all women identified as high-risk will go on to develop breast cancer, it is especially important to encourage women to discuss their risk with their physicians who can help them to balance the potential benefits of risk reduction therapies against the potential risks. Therefore, in September 2013, the United States Preventive Services Task Force recommended that clinicians engage in shared decision-making about use of risk reduction medications for women who are at high risk for breast cancer [13].
For women to engage in breast cancer risk reduction practices [14–19], it is helpful for them to have an understanding of their own risk and knowledge of the available options to reduce risk as well as the associated side effects. One potential unintended consequence of informing high-risk women about their risk is that it may increase concern and anxiety [20–22]. As a result, effective communication interventions are needed to educate women about breast cancer risk factors while minimizing anxiety.
Although breast cancer risk assessment tools intended to promote prevention and risk reduction exist [23–28], these have not been well integrated into clinical practice [5,29]. To address this issue, we designed a tablet-based, breast cancer risk education intervention (BreastCARE) to promote patient-physician discussion of breast cancer risk in the primary care setting and evaluated the intervention using a randomized controlled trial (RCT). As reported in a prior publication, we found that BreastCARE increased discussions of family cancer history, personal breast cancer risk, high-risk, and genetic counseling/testing and among high-risk women, all intervention effects were stronger. [30] Here, we assess the impact of BreastCARE on other important outcomes in the delivery of breast cancer risk information including women's knowledge of breast cancer, perceptions of individual risk and concern about breast cancer.
Methods
Study setting and recruitment
The intervention was conducted between June 2011 and August 2012 (when recruitment was met) [30]. Recruitment goals were based on sample size calculations assuming 80% power and α = 0.05 to detect significant differences in main effects between intervention and control groups. Recruitment letters, along with opt-out postcards, were mailed to patients with scheduled upcoming primary care medical appointments at an academic medical center and a safety-net hospital located in San Francisco, California. All patients who did not return a postcard declining to participate were contacted by telephone and those who agreed to participate completed a baseline telephone survey prior to their appointment. The research protocol was approved by the institutional review boards of participating institutions. Informed consent was obtained from all participants.
Patients were eligible to participate if they had an appointment in one of the participating practice sites, were female between the ages of 40 and 74, spoke English, Spanish, or Chinese (Cantonese or Mandarin), had no personal history of breast cancer or ductal carcinoma in situ, were able to complete a telephone interview and if their physicians did not object to their participation.
Study procedures
At completion of a baseline telephone survey, participants were randomized based on random sequence codes designed by a statistician and stratified by race/ethnicity to ensure balance. The intervention and control groups both completed a breast cancer risk assessment questionnaire. Those randomized to the control group completed the risk assessment by telephone two weeks prior to their scheduled appointment. Intervention participants completed a tablet-based version of the same risk assessment at the clinic immediately before their appointment and also received a personalized patient risk report one page in length, which the control patients did not receive. A second version of the report was given to the physician. On average, intervention women completed the tablet-based risk assessment in less than 5 min. One to two weeks after their appointment, all participants were contacted for a follow-up telephone survey to assess study outcomes.
BreastCARE intervention
BreastCARE was available in English, Spanish and Chinese, designed as a tablet-based, breast cancer risk assessment tool providing individually-tailored print-outs for patients and their physicians. We used measures in our risk assessments and surveys that were validated in multiple languages and used in prior research, or used a group consensus of native speakers to determine adequacy of translations [31].
Patients were queried on breast cancer risk factors in a series of questions written at an eighth-grade reading level [32,33]. Based on patient responses, an individualized risk report was generated that included specific risk reduction recommendations. A consensus panel of experts chose thresholds at which patients would be considered high-risk for each of the measures in our assessment. The rationale for all thresholds was to choose clinically actionable cut-points above which a woman should be referred for genetic counseling or high-risk evaluation for chemoprevention. For each woman identified as high-risk, the patient report stated that her risk was “higher than for other women [her] age” and suggested that she talk with her doctor. The patient message library included 30 potential messages based on risk factors, whereas the physician message library included 45 potential messages to account for all possible scenarios. A research assistant handed the patient her printed individualized report and a second report to be given to her physician who was thereby made aware of intervention status.
Reports for high-risk women indicated the reasons why they were at high risk and gave advice on what should be done to address risk factors (see Appendix). Reports for women who were not at high risk indicated the reasons why they were not at high risk and emphasized behaviors that should be maintained to minimize risk.
Data measures
Descriptive variables
At baseline, we assessed: age, self-reported race/ethnicity, marital status, education, health insurance coverage, self-reported general health, number of primary care visits in the past year and number of self-reported comorbidities [34]. Breast cancer risk assessment indicators included age at menarche, age at first birth, age at menopause, breast biopsy history, tamoxifen or raloxifene use, Jewish ancestry, family history of ovarian and breast cancer and mammographic breast density from medical records when available (73% of sample).
We used three measures to estimate objective risk for breast cancer: the Referral Screening Tool (RST) (personal history of breast or ovarian cancer, Jewish ancestry, history of family breast and ovarian cancer in mother, sister, daughter, grandmother or aunt and history of breast cancer in a male relative; c-statistic = 0.90) [35,36], the Gail Model (personal history of breast cancer, age, age at first menstrual period, age at first birth, number of first-degree relatives with breast cancer, history of breast biopsy and race/ethnicity; c-statistic = 0.67) [1,2] and the Breast Cancer Surveillance Consortium (BCSC) model (age, family history of breast cancer, prior breast biopsy, race/ethnicity and breast density; c-statistic = 0.66) [3,4].
Women were considered to be high-risk if they met at least one of three mutually exclusive criteria, listed in order of priority: (1) family history based on RST [36], (2) BCSC score in top 5% of estimated 5-year risk for her age group when mammographic breast density data was available [3,4], or (3) Gail score in the top 5% estimated 5-year risk for her age group [1]. In addition, women between the ages of 40 and 50 were considered high-risk if their Gail or BCSC score was ≥1.67 [10]. All other women were classified as average risk. The patient risk report for any woman identified as high risk stated that the woman's risk was higher than for other women her age and suggested talking with her doctor about the matter. For intervention women meeting RST high-risk criteria, the physician report contained a message suggesting referral to genetic counseling; for those meeting Gail/BCSC high-risk criteria, the report contained a message suggesting referral to a high-risk clinic.
Knowledge
We asked 10 questions about breast cancer risk factors at follow-up to assess knowledge of whether the following factors increase or decrease breast cancer risk: “Having relatives with breast cancer” (increase); “Being older age” (increase); “Drinking alcohol” (increase); “Using hormone medicine for menopause” (increase); “Being younger when you have your first child” (decrease); “Being younger when you have your first period” (increase); “Regular exercise (decrease); “Not having children” (increase); “Being older when you reach menopause” (increase); “Being overweight” (increase).
We assessed knowledge of breast cancer risk assessment and reduction at follow-up with four questions: “If a woman is at high risk for breast cancer, there is nothing she can do” (false); “Only women with a family history of breast cancer are at risk” (false); “Some women at risk for breast cancer can take medicine to prevent it” (true); “Some women with a strong family history of cancer can take a test to look for the breast cancer gene” (true).
For each set of items, a summary knowledge score was calculated based on the percentage of questions correctly answered and a dichotomous variable was created (≥75% correct answers vs. <75%). We placed the cutoff point for knowledge at 75% correct answers in order to identify differences between the intervention and control groups with respect to “above average” knowledge.
Correct perception of risk
Both at baseline and follow-up, women were asked: “Compared to other women of the same age, do you think your chance of getting breast cancer is: higher, the same, or lower” [37]. We defined correct perception of risk as a match between a woman's objective (high vs. average) and perceived risk of breast cancer (higher vs. same/lower) [38].
Concern about breast cancer
At baseline and follow-up women were asked: “How concerned are you about getting breast cancer? Very, somewhat, a little, not at all” [39]. Responses were dichotomized to “very” vs. “less than very” concerned.
Statistical analysis
Baseline equivalence between intervention and control groups was assessed, comparing demographics, health characteristics and breast cancer risk factors. Intervention outcomes were determined by comparing breast cancer knowledge scores, correct risk perception and breast cancer concern at follow-up. We used generalized estimating equations (GEE) regression in Stata (Version 11.2) to account for clustering of patients within physicians and to estimate differences at follow-up between intervention and control groups with respect to (a) knowledge of breast cancer risk factors; (b) knowledge of breast cancer risk assessment and reduction (high-risk women); (c) correct perception of breast cancer risk; (d) concern about getting breast cancer. We estimated odds ratios (OR) and 95% confidence intervals (CI). We adjusted model (c) for baseline correct perception of risk and model (d) for baseline breast cancer concern.
Results
Description of study population
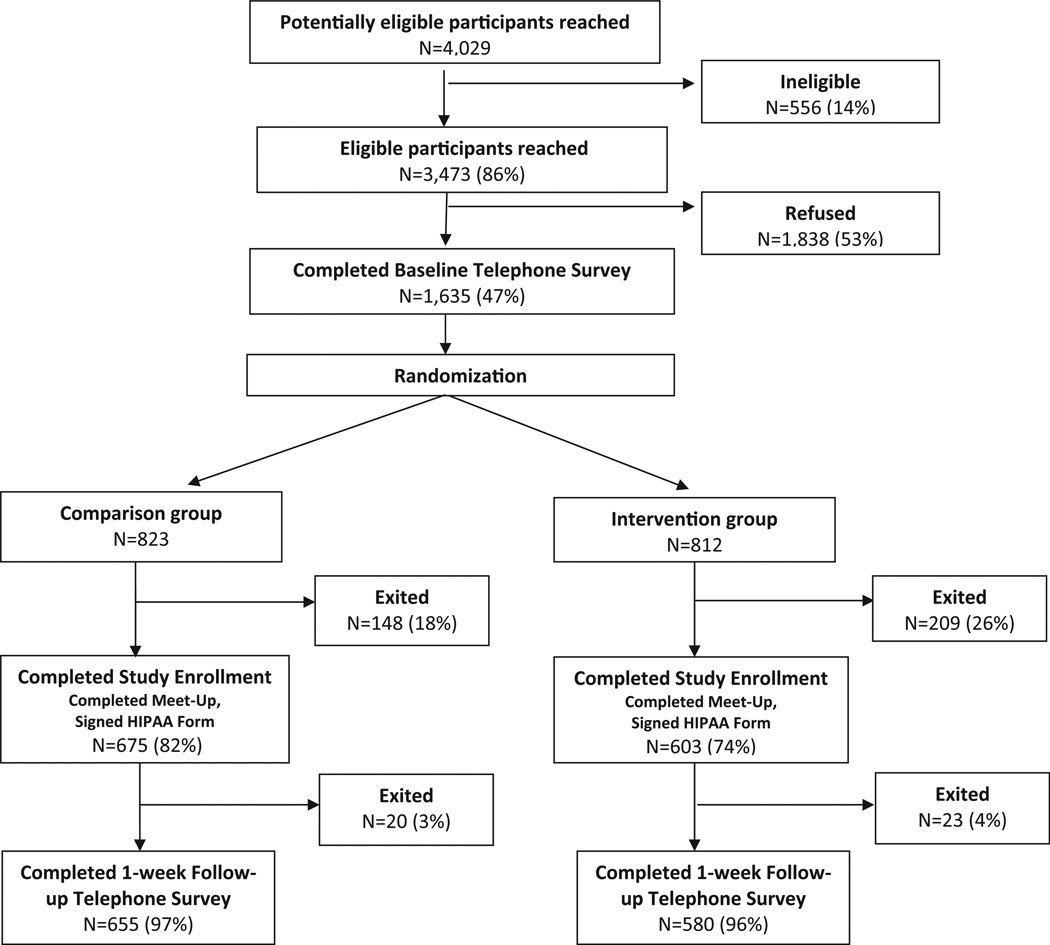
Out of 3473 eligible women who were reached, 1635 (47%) completed the baseline telephone survey. Out of these 1635 women, 812 were randomized to the intervention group and 823 to control group. Among women randomized, 74% in the intervention group and 82% of controls completed study enrollment. Over 95% completed the one-week follow-up telephone survey (Fig. 1) and 192 physicians participated.
Fig. 1.
Flowchart of enrollment/exclusions for BreastCARE population, San Francisco, 2011–2012.
Intervention and control groups were well-balanced at baseline with respect to demographic characteristics (Table 1). The majority of women were at average risk for breast cancer (75%); 15% were average risk but reported at least one blood relative with breast cancer. Twenty-five percent were identified as high-risk; 16% due to their BCSC or Gail score and 9% due to RST score ≥2 checks.
Table 1.
Baseline characteristics of intervention and control group participants (N = 1235).
| Control group | Intervention group |
p-value* | |
|---|---|---|---|
| n (%) | n (%) | ||
| 655 | 580 | ||
| Demographic characteristics | |||
| Age at diagnosis (categories) | |||
| <50 years | 183 (27.9) | 182 (31.4) | 0.331 |
| 51–65 years | 362 (55.3) | 300 (51.7) | |
| >65 years | 110 (16.8) | 98 (16.9) | |
| Race/ethnicity | |||
| Non Latina White | 229 (35.0) | 202 (34.8) | 0.877 |
| Latina | 144 (22.0) | 141 (24.3) | |
| Black or African American | 150 (22.9) | 125 (21.6) | |
| Asian or Pacific Islander | 123 (18.8) | 105 (18.1) | |
| Native American or Other | 9 (1.4) | 7 (1.2) | |
| Marital status | |||
| Married/living with a partner | 288 (44.3) | 261 (45.2) | 0.802 |
| Education | |||
| High school diploma or less | 216 (33.3) | 175 (30.4) | 0.200 |
| Some college | 155 (23.9) | 167 (29.1) | |
| College degree and higher | 278 (42.8) | 233 (40.5) | |
| Language of interview | |||
| English | 572 (87.3) | 507 (87.4) | 0.944 |
| Spanish or Chinese | 83 (12.7) | 73 (12.6) | |
| Health characteristics | |||
| Clinic site | |||
| Site 1 | 435 (66.4) | 411 (70.9) | 0.135 |
| Site 2 | 220 (33.6) | 169 (29.1) | |
| Health insurance | |||
| Any private insurance | 291 (44.4) | 297 (51.2) | 0.022 |
| Only public insurance | 350 (53.4) | 265 (45.7) | |
| No insurance | 14 (2.1) | 18 (3.1) | |
| Primary care visits during last year | |||
| 0 to 1 | 176 (27.2) | 164 (28.6) | 0.097 |
| 2 to 3 | 211 (32.5) | 214 (37.4) | |
| 4+ | 261 (40.3) | 195 (34.0) | |
| Comorbid conditions | |||
| 0 | 45 (6.9) | 39 (6.7) | 0.979 |
| 1 to 2 | 256 (39.1) | 226 (39.0) | |
| 3+ | 354 (54.1) | 315 (54.3) | |
| Perception of health status | |||
| Excellent/very good | 211 (32.4) | 203 (35.2) | 0.399 |
| Good/fair/poor | 441 (67.6) | 373 (64.8) | |
| Assessment of risk for breast cancer | |||
| Risk category for breast cancer | |||
| Average risk, no relatives with BC | 410 (62.6) | 338 (58.3) | 0.097 |
| Average risk, + relatives with BC | 99 (15.1) | 81 (14.0) | |
| High risk, BCSC/Gail | 97 (14.8) | 101 (17.4) | |
| High risk, RST ≥2 | 49 (7.5) | 60 (10.3) | |
Boldface indicates statistical significance (p < 0.05).
P-values from GEE analyses accounting for clustering of observations by physician.
Knowledge, risk perception and concern at follow-up
At follow-up, patients answered 53% of breast cancer risk factor knowledge questions correctly, but scores were higher among intervention women vs. controls (average score = 56% vs. 49%; p < 0.001) (Table 2). Intervention women were significantly more likely to answer that drinking alcohol, younger age at first period and being overweight were associated with higher risk of breast cancer, and that younger age at first child's birth and regular exercise were associated with lower risk (Table 2). Overall, intervention women were more likely than controls to answer at least 75% of questions correctly (24% vs. 16%; p = 0.002).
Table 2.
Follow-up assessment of breast cancer knowledge (N = 1235).
| Knowledge of breast cancer risk factors among all women | Control group | Intervention group | p-value* |
|---|---|---|---|
| n (%) | n (%) | ||
| n = 655 | n = 580 | ||
| Having relatives with breast cancer (Higher Risk) | 563 (86.4) | 510 (87.9) | 0.436 |
| Being older age (Higher Risk) | 348 (53.4) | 339 (58.5) | 0.098 |
| Drinking alcohol (Higher Risk) | 346 (53.1) | 369 (63.7) | <0.001 |
| Using hormone medicine for menopause (Higher Risk) | 409 (62.7) | 369 (63.6) | 0.760 |
| Being younger when having the first child (Lower Risk) | 186 (28.6) | 223 (38.5) | <0.001 |
| Being younger when having first period (Higher Risk) | 133 (20.4) | 177 (30.5) | <0.001 |
| Regular exercise (Lower Risk) | 413 (63.3) | 443 (76.4) | <0.001 |
| Not having children (Higher Risk) | 227 (34.9) | 233 (40.2) | 0.029 |
| Being older when you reach menopause (Higher Risk) | 151 (23.2) | 154 (26.6) | 0.197 |
| Being overweight (Higher Risk) | 412 (63.2) | 455 (78.5) | <0.001 |
| Summary: Risk Factor Knowledge Score (mean, SD) | 48.9 ± 24.3 | 56.4 ± 24.3 | <0.001 |
| ≥75% correct answers | 105 (16.2) | 138 (23.9) | 0.002 |
| Knowledge of breast cancer risk assessment and risk reduction among high-risk women | n = 146 | n = 161 | |
| If a women is at high risk for breast cancer, there is nothing she can do (False) | 133 (91.1) | 146 (90.7) | 0.903 |
| Only women with a family history of breast cancer are at risk (False) | 137 (93.8) | 144 (89.4) | 0.162 |
| Some women at risk for breast cancer can take medicine to prevent breast cancer (True) | 42 (28.8) | 66 (41.3) | 0.033 |
| Some women with a strong family history of cancer can Take a test to look for the breast cancer gene (True) | 127 (87.0) | 144 (89.4) | 0.541 |
| Summary: Breast Cancer Knowledge Score (mean, SD) | 75.2 ± 16.5 | 77.7 ± 18.9 | 0.288 |
| ≥75% correct answers | 115 (78.8) | 132 (82.5) | 0.513 |
Boldface indicates statistical significance (p < 0.05).
P-values from GEE analyses accounting for clustering of observations by physician.
Most high-risk women were knowledgeable about general breast cancer risk assessment and reduction approaches (average score = 77%), with the exception of SERM availability (Table 2). Intervention women were more likely than controls to know that high-risk women can take medicine to prevent breast cancer (41% vs. 28%; p = 0.03).
At baseline, a high percentage of women in both groups had correct perception of risk (70% control and 66% intervention, p = 0.11) (Table 3) and the change from baseline to follow-up was equivalent for both groups (Δ = 3%).
Table 3.
Correct perception of risk and breast cancer concern (N = 1235).
| Baseline | Follow-up | |||||
|---|---|---|---|---|---|---|
| Control group | Intervention group | p-value* | Control group | Intervention group | p-value* | |
| n (%) | n (%) | n (%) | n (%) | |||
| n = 655 | n = 580 | n = 655 | n = 580 | |||
| All women | ||||||
| Correct perception of risk | ||||||
| Correct | 455 (69.9) | 380 (65.9) | 0.111 | 477 (73.3) | 410 (71.1) | 0.367 |
| Concerned about getting breast cancer | ||||||
| Very concerned | 145 (22.3) | 157 (27.1) | 0.044 | 143 (22.0) | 140 (24.2) | 0.334 |
Boldface indicates statistical significance (p < 0.05).
P-values from GEE analyses accounting for clustering of observations by physician.
At baseline, 22% of controls and 27% of intervention women were very concerned about breast cancer (p = 0.04) (Table 3). There was no change in concern about breast cancer from baseline to follow-up among controls while there was a slight but non-significant decrease in being very concerned among intervention women (Δ = −3%).
GEE analysis
Intervention women had greater knowledge of breast cancer risk factors at follow-up than controls (OR = 1.62; CI = 1.19–2.23) (Table 4). Among high-risk women, there was no statistically significant difference between groups in knowledge of breast cancer risk assessment and reduction at follow-up ([OR] = 1.27; [CI] = 0.70–2.32) (Table 4).
Table 4.
GEE analysis*: Breast cancer knowledge, risk perception and concern (post-intervention).
| Knowledge of breast cancer risk factors (≥75% correct answers) |
Knowledge of breast cancer risk assessment and risk reduction (≥75% correct answers) |
Correct perception of breast cancer risk |
Very concerned about breast cancer |
|
|---|---|---|---|---|
| N = 1227 | N = 306 | N = 1211 | N = 1213 | |
| aOR (95% CI) | aOR (95% CI) | aORa (95% CI) | aORb (95% CI) | |
| Control group | Ref | Ref | Ref | Ref |
| Intervention group | 1.62 (1.19–2.23) | 1.27 (0.70–2.32) | 0.98 (0.72–1.33) | 0.94 (0.69–1.28) |
Boldface indicates statistical significance (p < 0.05).
Analyses account for clustering of patients by physician.
Adjusted for baseline risk perception.
Adjusted for baseline concern about breast cancer.
There was no difference between groups at follow-up in correct perception of risk ([OR] = 0.98; [CI] = 0.72–1.33) or in being very concerned about getting breast cancer ([OR] = 0.94; [CI] = 0.69–1.28) (Table 4).
Discussion
One-quarter of the women in our population were categorized as high-risk by RST, BCSC or Gail score, but only 26% of these women perceived themselves to be high-risk at baseline, emphasizing the need to move the needle of knowledge of risk factors, risk perception and action by clinicians to refer, counsel and/or treat these high-risk women. Clinicians will often rely on family history to signal increased risk for breast cancer but 15% of participants in this study were at average risk despite having a blood relative with breast cancer. We found that our intervention improved knowledge of breast cancer risk factors without increasing concern and identified women at high risk who were not likely to be otherwise found.
Women receiving the BreastCARE intervention had greater overall knowledge of breast cancer risk factors than controls at the time of follow-up, consistent with findings from prior RCTs involving breast cancer educational interventions [40–42]. In our study, women receiving BreastCARE were more likely to recognize lifestyle factors associated with increased breast cancer risk (e.g., alcohol, obesity, lack of physical activity). Among high-risk women, those receiving BreastCARE were more likely to be aware of medications to reduce risk of breast cancer although their overall breast cancer knowledge scores were not significantly greater. Improving knowledge of breast cancer risk factors can signify an important first step in helping women to understand their own risk and encouraging them to engage in screening and modify risk where possible [43–45]. A shared understanding or knowledge of breast cancer between patients and physicians is key to the meaningful discussion of breast cancer risk and risk reduction options. Improved knowledge can facilitate the use of prevention and risk reduction therapies, particularly among high-risk women. Previous work by our group found that women were more willing to consider taking a medication to reduce risk of breast cancer if identified as high-risk [46].
BreastCARE was not associated with greater concern about getting breast cancer. Prior research suggests that awareness of breast cancer risk can increase anxiety, particularly among those at high risk [20–22]. However, in the context of BreastCARE, immediate involvement of physicians who were available to discuss patients' risk and explain the options available for risk reduction likely helped to minimize patients' anxiety. Our results, combined with findings from earlier studies [42,47,48], support the notion that women can be informed about their breast cancer risk without increasing their concern. An alternative explanation for our findings is that concern was not affected by the intervention because risk perceptions did not change. Because the intervention did not make women more aware of their breast cancer risk, it did not increase concern.
Our finding that BreastCARE was not associated with an improvement in correct perception of risk is in contrast to results from three prior interventions [41,42,49]. BreastCARE was similar in intensity and scope to two of these prior interventions [41,49]. The first provided individualized breast cancer risk counseling to women aged 35 years and older with a family history of breast cancer in a first degree relative, by a trained nurse educator [49]. The second provided tailored print materials followed by tailored telephone counseling by trained health advisors for women aged 40–44 years and 50–54 years, enrolled in Blue Cross Blue Shield of North Carolina [41]. BreastCARE was slightly more intensive than a third intervention which provided tailored educational materials to promote genetic testing, without one-on-one counseling, for women aged ≤55 years, estimated to have a high probability of carrying a BRCA1/BRCA2 mutation [42].
Possible explanations for differences in study findings include differences in: definitions of “correct” risk perception; methods used to calculate objective risk [37,38,41,49]; the format of risk presentation [50] or delivery of information by healthcare providers. In addition, 72% of women enrolled in our study already had correct perception of breast cancer risk at baseline, which could explain why a significant improvement in risk perception was not observed. Further, one of the prior interventions found no relationship between risk accuracy and behavior [41], suggesting that there may be more to understanding risk and acting upon risk information than recalling numerical probabilities. Reactions to risk reports or perceived disease severity, may be more important in driving behavior change [51].
Our study has several limitations. Knowledge was only assessed at follow-up so we cannot determine how knowledge differed between groups at baseline, although groups were similar with respect to education and other demographic characteristics. We also used a not-yet-validated BCSC risk assessment tool (vs. a validated tool [52]) to identify candidates for enhanced breast cancer screening. However, our goal was to identify patients suitable for referral to genetic testing and preventive therapy rather than those suitable for enhanced screening. In addition, we categorized risk as a dichotomous variable. Undoubtedly there are different degrees of risk even among women meeting our age-based high-risk cutoffs. However, cutoffs were based on clinically meaningful thresholds where risk reduction options should be discussed and recommended. Further, some women in the control and intervention groups could have been seen by the same physician, leading to potential contamination of the intervention effect. An important strength of our study was the inclusion of three different language groups. However, the education level of the sample was relatively high which may limit the generalizability of the findings.
Conclusion
Our simple, practical primary care clinic-based intervention using a tablet-based breast cancer risk assessment tool to target both patients and their physicians improved women's knowledge of breast cancer risk factors without increasing their concern, irrespective of objective risk. As reported in our prior publication, [30] BreastCARE also improved patient-physician discussions of risk which is likely how the intervention worked to enhance knowledge. The involvement of physicians at the point of care who were available to discuss patients' risk factors and explain the risk reduction options available likely contributed to the intervention's success. Our findings support the use of health-related information technology in the primary care setting to enhance education about breast cancer and associated risk factors.
Acknowledgment
All authors have made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, and the drafting or critical revision of the article for important intellectual content, and all have provided final approval of the version to be published. Drs. J. Livaudais-Toman and C.P. Kaplan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Grant support
This research was funded by the California Breast Cancer Research Program (150B-0158) and Susan G. Komen for the Cure (KG090504). Dr. Kaplan was Principal Investigator of both grants. Both trials were recorded under one NCT identifier (NCT01830933) based on recommendations from NIH ClinicalTrials.gov. Dr. Pérez-Stable's time was supported through a grant from the National Cancer Institute, Special Populations Network Program, Redes en Acción (U01CA86117 and U54CA153511).
Role of the funding source(s)
The funding sources had no role in the study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Appendix
Footnotes
Conflict of interest statement
None of the authors have any financial conflicts of interest to disclose.
References
- 1.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 2.Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA, et al. Validation of the Gail. Model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93:358–366. doi: 10.1093/jnci/93.5.358. [DOI] [PubMed] [Google Scholar]
- 3.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tice JA, Cummings SR, Ziv E, Kerlikowske K. Mammographic breast density and the Gail model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat. 2005;94:115–122. doi: 10.1007/s10549-005-5152-4. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan CP, Haas JS, Perez-Stable EJ, Des Jarlais G, Gregorich SE. Factors affecting breast cancer risk reduction practices among California physicians. Prev Med. 2005;41:7–15. doi: 10.1016/j.ypmed.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan CP, Haas JS, Perez-Stable EJ, Gregorich SE, Somkin C, Des Jarlais G, et al. Breast cancer risk reduction options: awareness, discussion, and use among women from four ethnic groups. Cancer Epidemiol Biomarkers Prev. 2006;15:162–166. doi: 10.1158/1055-9965.EPI-04-0758. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong K, Quistberg DA, Micco E, Domchek S, Guerra C. Prescription of tamoxifen for breast cancer prevention by primary care physicians. Arch Intern Med. 2006;166:2260–2265. doi: 10.1001/archinte.166.20.2260. [DOI] [PubMed] [Google Scholar]
- 8.Lerman C, Shields AE. Genetic testing for cancer susceptibility: the promise and the pitfalls. Nat Rev Cancer. 2004;4:235–241. doi: 10.1038/nrc1301. [DOI] [PubMed] [Google Scholar]
- 9.Levy DE, Byfield SD, Comstock CB, Garber JE, Syngal S, Crown WH, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: black and Hispanic women particularly at risk. Genet Med. 2011;13:349–355. doi: 10.1097/GIM.0b013e3182091ba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 11.Chlebowski RT, Col N, Winer EP, Collyar DE, Cummings SR, Vogel VG, 3rd, et al. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. J Clin Oncol. 2002;20:3328–3343. doi: 10.1200/JCO.2002.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Kinsinger LS, Harris R, Woolf SH, Sox HC, Lohr KN. Chemoprevention of breast cancer: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:59–69. doi: 10.7326/0003-4819-137-1-200207020-00017. [DOI] [PubMed] [Google Scholar]
- 13.Moyer VA. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:698–708. doi: 10.7326/0003-4819-159-10-201311190-00717. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann LC, Schaid DJ, Woods JE, Crotty TP, Myers JL, Arnold PG, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 15.Lostumbo L, Carbine N, Wallace J, Ezzo J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2004:CD002748. doi: 10.1002/14651858.CD002748.pub3. [DOI] [PubMed] [Google Scholar]
- 16.McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 17.Terry MB, Zhang FF, Kabat G, Britton JA, Teitelbaum SL, Neugut AI, et al. Lifetime alcohol intake and breast cancer risk. Ann Epidemiol. 2006;16:230–240. doi: 10.1016/j.annepidem.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler RG, Hoover RN, Nomura AM, West DW, Wu AH, Pike MC, et al. Relative weight, weight change, height, and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1996;88:650–660. doi: 10.1093/jnci/88.10.650. [DOI] [PubMed] [Google Scholar]
- 19.Files JA, Stan DL, Allen SV, Pruthi S. Chemoprevention of breast cancer. Womens Health Lond Engl. 2012;8:635–646. doi: 10.2217/whe.12.56. [DOI] [PubMed] [Google Scholar]
- 20.Kash KM, Holland JC, Halper MS, Miller DG. Psychological distress and surveillance behaviors of women with a family history of breast cancer. J Natl Cancer Inst. 1992;84:24–30. doi: 10.1093/jnci/84.1.24. [DOI] [PubMed] [Google Scholar]
- 21.Zakowski SG, Valdimarsdottir HB, Bovbjerg DH, Borgen P, Holland J, Kash K, et al. Predictors of intrusive thoughts and avoidance in women with family histories of breast cancer. Ann Behav Med. 1997;19:362–369. doi: 10.1007/BF02895155. [DOI] [PubMed] [Google Scholar]
- 22.Andersen MR, Smith R, Meischke H, Bowen D, Urban N. Breast cancer worry and mammography use by women with and without a family history in a population-based sample. Cancer Epidemiol Biomarkers Prev. 2003;12:314–320. [PubMed] [Google Scholar]
- 23.James BC. Making it easy to do it right. N Engl J Med. 2001;345:991–993. doi: 10.1056/NEJM200109273451311. [DOI] [PubMed] [Google Scholar]
- 24.Chambers CV, Balaban DJ, Carlson BL, Ungemack JA, Grasberger DM. Microcomputer-generated reminders. Improving the compliance of primary care physicians with mammography screening guidelines. J Fam Pract. 1989;29:273–280. [PubMed] [Google Scholar]
- 25.Roubidoux MA. Breast cancer detective: a computer game to teach breast cancer screening to Native American patients. J Cancer Educ. 2005;20:87–91. doi: 10.1207/s15430154jce2001s_17. [DOI] [PubMed] [Google Scholar]
- 26.Kreuter MW, Black WJ, Friend L, Booker AC, Klump P, Bobra S, et al. Use of computer kiosks for breast cancer education in five community settings. Health Educ Behav. 2006;33:625–642. doi: 10.1177/1090198106290795. [DOI] [PubMed] [Google Scholar]
- 27.Gustafson DH, Hawkins R, Pingree S, McTavish F, Arora NK, Mendenhall J, et al. Effect of computer support on younger women with breast cancer. J Gen Intern Med. 2001;16:435–445. doi: 10.1046/j.1525-1497.2001.016007435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green MJ, Peterson SK, Baker MW, Friedman LC, Harper GR, Rubinstein WS, et al. Use of an educational computer program before genetic counseling for breast cancer susceptibility: effects on duration and content of counseling sessions. Genet Med. 2005;7:221–229. doi: 10.1097/01.GIM.0000159905.13125.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suther S, Goodson P. Barriers to the provision of genetic services by primary care physicians: a systematic review of the literature. Genet Med. 2003;5:70–76. doi: 10.1097/01.GIM.0000055201.16487.61. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan CP, Livaudais-Toman J, Tice JA, Kerlikowske K, Gregorich SE, Perez-Stable EJ, et al. A randomized, controlled trial to increase discussion of breast cancer in primary care. Cancer Epidemiol Biomarkers Prev. 2014;23:1245–1253. doi: 10.1158/1055-9965.EPI-13-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brislin RW. Translation: applications and research. New York: Gardner; 1976. [Google Scholar]
- 32.McLaughlin G. SMOG grading: a new readability formula. J Read. 1969;12:639–646. [Google Scholar]
- 33.Crawford AN. A Spanish language fry type readability procedure: elementary level. Los Angeles: Evaluation Dissemination and Assessment Center, California State University; 1984. [Google Scholar]
- 34.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered co-morbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 35.Breast cancer genetics referral screening tool (B-RST) Peachtree Solutions, LLC; 2013. [accessed 2013]. at, https://www.breastcancergenescreen.org/providers.aspx. [Google Scholar]
- 36.Bellcross CA, Lemke AA, Pape LS, Tess AL, Meisner LT. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783–789. doi: 10.1097/GIM.0b013e3181b9b04a. [DOI] [PubMed] [Google Scholar]
- 37.Lipkus IM, Iden D, Terrenoire J, Feaganes JR. Relationships among breast cancer concern, risk perceptions, and interest in genetic testing for breast cancer susceptibility among African-American women with and without a family history of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:533–539. [PubMed] [Google Scholar]
- 38.Fehniger J, Livaudais-Toman J, Karliner L, Kerlikowske K, Tice JA, Quinn J, et al. The relationship between perceived risk of breast cancer, breast cancer concern, and objective breast cancer risk in a diverse sample of women. J Womens Health Larchmt. 2013;23:420–427. doi: 10.1089/jwh.2013.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finney Rutten LJ, Blake KD, Hesse BW, Augustson EM, Evans S. Illness representations of lung cancer, lung cancer worry, and perceptions of risk by smoking status. J Cancer Educ. 2011;26:747–753. doi: 10.1007/s13187-011-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lerman C, Biesecker B, Benkendorf JL, Kerner J, Gomez-Caminero A, Hughes C, et al. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J Natl Cancer Inst. 1997;89:148–157. doi: 10.1093/jnci/89.2.148. [DOI] [PubMed] [Google Scholar]
- 41.Rimer BK, Halabi S, Sugg Skinner C, Lipkus IM, Strigo TS, Kaplan EB, et al. Effects of a mammography decision-making intervention at 12 and 24 months. Am J Prev Med. 2002;22:247–257. doi: 10.1016/s0749-3797(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 42.Skinner CS, Schildkraut JM, Berry D, Calingaert B, Marcom PK, Sugarman J, et al. Pre-counseling education materials for BRCA testing: does tailoring make a difference? Genet Test. 2002;6:93–105. doi: 10.1089/10906570260199348. [DOI] [PubMed] [Google Scholar]
- 43.Bowen DJ, Powers D. Effects of a mail and telephone intervention on breast health behaviors. Health Educ Behav. 2010;37:479–489. doi: 10.1177/1090198109348463. [DOI] [PubMed] [Google Scholar]
- 44.Bowen DJ, Robbins R, Bush N, Meischke H, Ludwig A, Wooldridge J. Effects of a web-based intervention on women's breast healthbehaviors. Transl Behav Med. 2011;1:155–164. doi: 10.1007/s13142-011-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SE, Perez-Stable EJ, Wong S, Gregorich S, Sawaya GF, Walsh JM, et al. Association between cancer risk perception and screening behavior among diverse women. Arch Intern Med. 2008;168:728–734. doi: 10.1001/archinte.168.7.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplan CP, Kim SE, Wong ST, Sawaya GF, Walsh JM, Perez-Stable EJ. Willingness to use tamoxifen to prevent breast cancer among diverse women. Breast Cancer Res Treat. 2012;133:357–366. doi: 10.1007/s10549-012-1960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bloom JR, Stewart SL, Chang S, You M. Effects of a telephone counseling intervention on sisters of young women with breast cancer. Prev Med. 2006;43:379–384. doi: 10.1016/j.ypmed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Helmes AW, Culver JO, Bowen DJ. Results of a randomized study of telephone versus in-person breast cancer risk counseling. Patient Educ Couns. 2006;64:96–103. doi: 10.1016/j.pec.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Lerman C, Lustbader E, Rimer B, Daly M, Miller S, Sands C, et al. Effects of individualized breast cancer risk counseling: a randomized trial. J Natl Cancer Inst. 1995;87:286–292. doi: 10.1093/jnci/87.4.286. [DOI] [PubMed] [Google Scholar]
- 50.Freedman AN, Yu B, Gail MH, Costantino JP, Graubard BI, Vogel VG, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327–2333. doi: 10.1200/JCO.2010.33.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol Bull. 2001;127:267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- 52.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]