Abstract
Working memory (WM) training improves WM ability in Attention-Deficit/Hyperactivity Disorder (ADHD), but its efficacy for non-cognitive ADHD impairments ADHD has been sharply debated. The purpose of this preliminary study was to characterize WM training-related changes in ADHD brain function and see if they were linked to clinical improvement. We examined 18 adolescents diagnosed with DSM-IV Combined-subtype ADHD before and after 25 sessions of WM training using a frequently employed approach (CogmedTM) using a nonverbal Sternberg WM fMRI task, neuropsychological tests, and participant- and parent-reports of ADHD symptom severity and associated functional impairment. Whole brain SPM8 analyses identified ADHD activation deficits compared to 18 non-ADHD control participants, then tested whether impaired ADHD frontoparietal brain activation would increase following WM training. Post hoc tests examined the relationships between neural changes and neurocognitive or clinical improvements. As predicted, WM training increased WM performance, ADHD clinical functioning, and WM-related ADHD brain activity in several frontal, parietal and temporal lobe regions. Increased left inferior frontal sulcus region activity was seen in all Encoding, Maintenance, and Retrieval Sternberg task phases. ADHD symptom severity improvements were most often positively correlated with activation gains in brain regions known to be engaged for WM-related executive processing; improvement of different symptom types had different neural correlates. The responsiveness of both amodal WM frontoparietal circuits and executive process-specific WM brain regions was altered by WM training. The latter might represent a promising, relatively unexplored treatment target for researchers seeking to optimize clinical response in ongoing ADHD WM training development efforts.
Keywords: ADHD, working memory, training, fMRI, brain
Introduction
Working memory (WM) deficits are among the most prominent (Kasper et al., 2012; Martinussen et al., 2005; Willcutt et al., 2005) of the many cognitive impairments often found in patients diagnosed with Attention-Deficit/Hyperactivity Disorder (ADHD) (Frazier et al., 2004; Willcutt et al., 2012). Although WM engages a complex, multi-component, dynamic set of cognitive processes, a useful and effective consensus definition describes WM as active maintenance and flexible updating of goal- or task-relevant information in a form that has limited capacity and resists interference (Baddeley, 1992; RDoC Workgroup, 2010). Poor WM task performance is correlated with symptoms of ADHD inattention (Burgess et al., 2010; Kofler et al., 2010), hyperactivity (Rapport et al., 2009), impulsivity (Raiker et al., 2012) and “real world” ADHD-like behaviors, including multi-tasking (Buhner et al., 2006; Hambrick et al., 2010), mind wandering (Kane et al., 2007), following directions (Engle et al., 1991; Gathercole et al., 2008), as well as indicators of ADHD-related functional deficits such as social problems (Kofler et al., 2011) and educational achievement (Burgess et al., 2010; Rapport et al., 2008; Rapport et al., 2009). This raises the possibility that treatments which improve WM might consequently improve ADHD behaviors.
Because first-line recommended ADHD treatments like stimulant medications (Pliszka, 2007) have inconsistent effects on WM (Bedard et al., 2007; Epstein et al., 2006; Kobel et al., 2009; Pietrzak et al., 2006; Rapport et al., 2013; Rhodes et al., 2006) – typically normalizing neither WM task performance deficits (Biederman et al., 2008; Everett et al., 1991; Gualtieri and Johnson, 2008; Kobel et al., 2009; Risser and Bowers, 1993) nor WM-related brain dysfunction (Kobel et al., 2009; Prehn-Kristensen et al., 2011; Rubia et al., 2014) (but see Cubillo et al., 2014) – the efficacy of other approaches to improve WM have been increasingly explored. WM training is a facilitative intervention (Rapport et al., 2013) that uses intensive repetition of exercises with consistently high WM demands (Morrison and Chein, 2011; Rapport et al., 2013) with an expectation that training alters the brain’s capacity to effectively represent or manipulate information. The most frequently studied WM training regimens involve 4–5 weeks of near daily, computerized WM training sessions that last 30–40 minutes. Using several different types of WM tasks in each session is a strategy believed to enhance a general WM capacity (Backman and Nyberg, 2013; Buschkuehl et al., 2012; Klingberg, 2010, 2012; Morrison and Chein, 2011; Qi and Constantinidis, 2013; Rutledge et al., 2012). This strategy is typical of WM training approaches, and has been the most frequently studied in ADHD (e.g., CogMedTM).
Both qualitative (Chacko et al., 2014; Evans et al., 2013; Morrison and Chein, 2011; Rutledge et al., 2012; Shipstead et al., 2012a; Shipstead et al., 2012b; Toplak et al., 2008) and meta-analytic (Hodgson et al., 2014; Melby-Lervag and Hulme, 2013; Rapport et al., 2013; Sonuga-Barke et al., 2013) reviews find that WM training reliably enhances WM performance on trained and closely-related WM tasks for children, adolescents, and adults with different presentations/subtypes of ADHD. For instance, for Cogmed specifically 13 peer-reviewed clinical trials (including 8 randomized clinical trials) found ADHD patients’ WM ability improves after training (Beck et al., 2010; Chacko et al., 2013; Dahlin, 2013; Egeland et al., 2013; Gibson et al., 2011; Gray et al., 2012; Green et al., 2012; Gropper et al., 2014; Holmes et al., 2010; Hovik et al., 2013; Klingberg et al., 2005; Klingberg et al., 2002; Mezzacappa and Buckner, 2010) and gains persist 2–8 months later (Gropper et al., 2014; Holmes et al., 2010; Hovik et al., 2013) (see also meta-analysis Melby-Lervag and Hulme, 2013). However, the evidence that WM training is effective in reducing ADHD symptom severity or associated clinical dysfunction is mixed. Among published studies measuring ADHD clinical function, 7 studies found improved parent-, teacher-, or self-rated ADHD severity (Beck et al., 2010; Gibson et al., 2011; Gropper et al., 2014; Klingberg et al., 2005; Mezzacappa and Buckner, 2010) or objectively-measured ADHD-like behavior (e.g., actigraph-measured motor restlessness or classroom time-on-task (Green et al., 2012; Klingberg et al., 2005), with evidence that gains persisted months (Beck et al., 2010; Klingberg et al., 2005). Two studies found Cogmed improved academic skills, which also lasted at least 7–8 months (Dahlin, 2013; Egeland et al., 2013). In contrast, some studies had no clinical improvements despite WM gains (Egeland et al., 2013; Gray et al., 2012), and two found ADHD symptom reductions did not surpass that seen with treatment-as-usual or active placebo (Chacko et al., 2013; Green et al., 2012). Several reviews of these trials have criticized the reliance on subjective reports of improvements (particularly in “open” trials without placebo-control). Indeed, the most recent meta-analysis of 6 WM training RCTs failed to find any changes in ratings of ADHD symptom severity (Cortese et al., 2015). Other criticisms that reduce confidence in WM training efficacy for ADHD clinical severity include the inconsistency of specific ADHD dysfunction-related gains across studies and methodological limitations such as failing to take into account psychiatric comorbidity, medication status, practice effects, of pre-training WM ability differences (Evans et al., 2013; Gathercole et al., 2012; Klingberg, 2010; Melby-Lervag and Hulme, 2013; Morrison and Chein, 2011; Rabipour and Raz, 2012; Redick et al., 2013; Shah et al., 2012; Shipstead et al., 2012b; Sonuga-Barke et al., 2013; Takeuchi et al., 2010; Toplak et al., 2008). At best, empirical support for using WM training in ADHD is currently mixed, with sometimes sharp disagreement on the basic question of whether this treatment approach reduces ADHD-associated clinical impairments outside the domain of WM. As a result, an emerging opinion in the field is that WM training ultimately might not be considered a “front-line” treatment for ADHD (Cortese et al., 2015).
Another way to inform the question of whether or not WM training could be effective for ADHD would be to show that any ADHD clinical improvements are linked to WM-induced changes in ADHD brain function during WM task performance. In non-ADHD samples, WM training has been found in several studies to alter brain structure and function, including grey and white matter volume, dopaminergic function, brain activation, and functional connectivity (see review (Buschkuehl et al., 2012). However, specific results differ across studies and WM training techniques. As yet, no single specific, common neurobiological “mechanism” has been identified to explain how WM gains are made (Buschkuehl et al., 2012; Takeuchi et al., 2010), in part likely due to the complexity of WM cognitive processes and their neural correlates. Moreover, neural changes are likely to vary according to disorder-specific pathophysiology in ways that have not yet been clearly characterized. Although some effort has been made to study brain changes following intensive cognitive training in ADHD (e.g., Hoekzema et al., 2011; Hoekzema et al., 2010), the effects of WM training has not yet been examined. Therefore, the neurobiological basis of WM improvements in ADHD following WM training is not understood, despite the NIMH’s recent programmatic emphasis on understanding the neurobiological basis of clinical change to help develop effective new treatments.
There is a need for studies that describe how WM training alters brain function in ADHD-diagnosed patients. This preliminary study sought to characterize for the first time how ADHD brain function is affected by a commonly-used WM training approach that targets WM capacity (Cogmed; Pearson, 2014) and to determine if such changes were associated with clinical improvements. fMRI meta-analyses describe a core frontoparietal WM neural circuit that is engaged across different types of WM tasks regardless of specific cognitive demands (Owen et al., 2005; Rottschy et al., 2012; Wager and Smith, 2003). We first compared ADHD to non-ADHD controls to localize any brain function deficits. Consistent with previous non-ADHD fMRI studies (Hempel et al., 2004; Jolles et al., 2010; Olesen et al., 2004), we predicted WM training would increase and possibly normalize ADHD WM task-related activation in some or most of these WM circuit regions, particularly left caudal superior frontal sulcus (SFS) and left inferior frontal sulcus (SFS) regions which most consistently show hypofunction in ADHD fMRI studies of short term WM storage (Fassbender et al., 2011; Hale et al., 2007; Prehn-Kristensen et al., 2011; Sheridan et al., 2007; Vance et al., 2007; Wolf et al., 2009) or WM updating (i.e., N-back tasks) (Bayerl et al., 2010; Cubillo et al., 2014; Ko et al., 2013; Kobel et al., 2009; Malisza et al., 2012; Massat et al., 2012; Passarotti et al., 2010; Valera et al., 2005). We also asked whether WM training preferentially altered ADHD brain function engaged during encoding, maintenance, or retrieval of information from WM and examined training effects on WM task difficulty. After identifying WM training effects, we conducted post hoc analyses to test the prediction that these neural changes would be associated with improvements in ADHD non-cognitive symptom severity or other, basic clinical characteristics, e.g., age, gender, use of psychostimulant medications.
Material and Methods
Participants
ADHD participants were recruited via community advertisements. Study procedures were explained to potential participants and at least one parent during an informed consent/assent protocol approved by Hartford Hospital’s Institutional Review Board. Participants received monetary compensation for completing study procedures. Thirty-seven ADHD youth were consented, but 6 changed their minds after consent, and 13 were excluded: 5 did not meet diagnostic criteria; 1 had a positive drug urine toxicology result; 1 felt uncomfortable in the MRI; 1 had an MRI-detected brain cyst; and 5 were noncompliant with WM training. The final ADHD sample included 18 adolescents (6 females) ages 12–18. A comparison sample of 18 non-ADHD adolescents was recruited for comparison. This group did not statistically differ from the ADHD sample on sex, age, parental education/SES, WASI-measured IQ, reading level, or reported depression/anxiety-related complaints (Table 1).
Table 1.
Demographic and clinical characteristics of non-ADHD and in ADHD adolescents.
| ADHD | Non-ADHD | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | p | |
| Age | 15.2 (1.91) | 15.8 (1.40) | ns |
| Sex | 11M/6F | 11M/6F | ns |
| Mean Years of Parental Education | 15.5 (1.84) | 16.0 (2.75) | ns |
| WASI Full Scale IQ Estimate | 108.7 (11.70) | 103.3 (9.51) | ns |
| Wide Range Achievement Test Reading SS | 102.8 (9.33) | 104.8 (9.88) | ns |
| Beck Depression Scale II Total Score | 6.9 (5.31) | 4.6 (3.76) | ns |
| Multidimensional Anxiety Scale for Children Total Score | 30.7 (14.57) | 31.5 (12.55) | ns |
| Brown ADD Scales Total Score (Self-Report) | 65.3 (12.50) | 50.7 (1.61) | <.001 |
| Brown ADD Scales Total Score (Parent-Report) | 67.7 (12.66) | 50.3 (0.97) | <.001 |
Psychiatric diagnoses were evaluated using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) following its recommended interview format of both parent and child (Kaufman et al., 1997), administered by experienced staff under the supervision of a licensed psychologist with over 14 years KSADS-PL clinical research experience. Diagnostic interview information was supplemented with both parent- and child-report ADHD symptom and dysfunction rating scales (see “Clinical Outcome Measures” below). None of the non-ADHD participants met criteria for any Axis I diagnoses. All ADHD participants met DSM-IV criteria for ADHD-Combined Hyperactive/Impulsive and Inattentive subtype (314.01). Their psychiatric comorbidities included: 1 with comorbid Oppositional Defiant Disorder (ODD) and 1 with both ODD and Conduct Disorder. Several participants met past criteria for other disorders that were in remission: 1 Generalized Anxiety Disorder, 1 Dysthymic Disorder, 1 Major Depressive Episode, and 1 Adjustment Disorder with Depressed Mood.
Thirteen of 18 ADHD participants were being prescribed short half-life “psychostimulant” medications: 6 sustained-release methylphenidate and 7 amphetamine-based drugs (5 amphetamine mixed salts, 2 lisdexamfetamine dimesylate). Non-ADHD medications did not preclude participation, as no prescriptions changed during the WM training trial, and participants served as their own controls. Of the medicated participants, 2 also took citalopram, 1 paroxetine, 1 clonidine, and 1 lamotrigine. The medication status for one participant was not reported.
Trial Design
This was an “open label” WM training trial (www.clinicaltrials.gov NCT02151396) using a commercially-available, computer-based program (Cogmed; Pearson, Inc.). Although a blinded, placebo-control study would have permitted stronger inferences about efficacy, the primary study goal was to determine whether any changes in WM brain function following WM training could be linked to neurocognitive and/or clinical improvement. As such, we used the same design as in most previous clinical trials, simply to ensure the WM training was rigorous and could be directly compared to published reports. ADHD participants completed 25 training sessions at home under parental supervision and weekly “coaching” phone calls by study staff to troubleshoot problems and provide motivational encouragement (Pearson, 2014). Performance data was uploaded regularly via internet so the training coach could closely track progress and identify treatment noncompliance. All WM training was completed within 5–6 continuous weeks by all participants. As per the standard Cogmed training approach, individual training item difficulty was adaptively adjusted on a trial-by-trial basis to continually challenge WM capacity throughout all trials. An assessment using fMRI, neuropsychological tests, and parent-/self-reports of ADHD clinical severity (Table 2) was done within 1 week before and within 2 weeks following WM training. Any participants who took psychostimulants underwent a 24-hour medication washout the day prior to these assessments to ensure medications did not influence brain function or cognitive performance during either fMRI/cognitive evaluation. Non-ADHD participants did not undergo WM training or re-assessment.
Table 2.
Neuropsychological test performance in non-ADHD and in ADHD adolescents before and after 5 weeks of working memory training.
| ADHD Pre-Training | ADHD Post-Training | ADHD Pre- vs Post-Training | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | p | |
| Trained WM Tests | |||
| Cogmed Training Index | 85.5 (10.9) | 115.3 (16.4) | 0.0000001 |
| Non-Trained WM Tests | |||
| WISC-IV Digit Span Forwards Scaled Score | 8.4 (2.8) | 11.1 (3.5) | 0.00001 |
| WISC-IV Digit Span Backwards Scaled Score | 8.7 (2.8) | 12.3 (3.2) | 0.0007 |
| WISC-IV Letter-Number Sequencing Scaled Score | 9.3 (2.3) | 11.1 (2.0) | 0.0005 |
| WISC-IV Spatial Span Forwards Scaled Score | 11.2 (2.5) | 13.4 (2.9) | 0.002 |
| WISC-IV Spatial Span Backwards Scaled Score | 10.3 (1.9) | 13.3 (1.9) | 0.0002 |
| Cognitive “Far Transfer” Tests | |||
| Conner’s CPT-II Omissions T-score | 52.5 (13.0) | 50.0 (12.0) | ns |
| Conner’s CPT-II Commissions T-score | 56.9 (10.7) | 58.1 (7.9) | ns |
| Conner’s CPT-II Hit Rate T-score | 46.4 (12.0) | 43.0 (12.2) | ns |
| ADHD Clinical Severity Indicators | |||
| Hyperactive/Impulsive symptom severity (max 27) | 17.9 (7.3) | 11.4 (6.7) | .002 |
| Inattentive symptom severity (max 27) | 21.7 (5.5) | 15.2 (6.5) | .017 |
| Brown ADD Scales Total T (Child) | 65.5 (12.5) | 57.4 (12.3) | .023 |
| Brown ADD Scales Total T (Parent) | 67.7 (12.4) | 62.2 (11.9) | ns |
| Home Situations Questionnaire problem severity | 5.4 (1.3) | 4.1 (1.6) | .015 |
Clinical Outcome Measures
WM training progress was measured using Cogmed’s standard approach to calculating a Training Index, defined as the difference between final and initial training scores. The initial score was each patient’s average performance on two Cogmed exercises practiced during days 2 and 3. The final score was the average of the best two trials throughout training. Although best performance theoretically could occur sporadically and not reflect true gains by treatment endpoint, inspection of patient performance levels throughout treatment showed steadily progressive gains and best task performance at end of training for all participants. Transfer of WM gains to non-trained WM tasks was evaluated using WM subtests of the Wechsler Intelligence Scale for Children (4th edition). Conner’s Continuous Performance Test (2nd Edition; CPT-II) assessed whether WM training produced gains in non-WM cognitive domains. These tests were selected for both their proven construct validity as well as their frequent use in ADHD clinical evaluation and research. Because WM ability is multidimensional and the exact nature of ADHD WM deficits is a subject of ongoing research, it was not possible to predict in advance if any WM sub-factors might be found in the out-of-scanner WM neuropsychological measures examined. Therefore, exploratory principal component analysis (PCA) with Varimax rotation reduced WISC-IV WM treatment-related change scores to explore how training gains on possibly more easily interpretable aspects of WM related to patients’ baseline characteristics.
ADHD symptom severity was evaluated before and after WM training using parent-reported DSM-IV symptoms rated on a 0–3 scale (Barkley and Murphy, 1998). Parent- and Adolescent self-report versions of Brown ADD Scales (Brown, 2001) measured ADHD-associated behavioral problems. The ADHD Home Situations Questionnaire (Barkley and Murphy, 1998) measured ADHD-related functional impairment, asking parents to rate on a 1–9 scale how ADHD symptoms disrupt 16 typical home situations (e.g., meal time or completing chores). Training effects were evaluated using paired t test on post- and pre-training scores. Across the entire sample, there were between 2–6 missing values for each ADHD symptom severity, Brown Parent ADD Scale, Brown Adolescent ADD Scale, and Home Situations scores due to failure for parents/participants to complete either pre- or post-training questionnaires. Little’s MCAR test (χ2(73)=71.244, ns) showed these datapoints were missing randomly. Moreover, bivariate Pearson correlation analysis failed to find any meaningful relationship between missing vs. present status of any datapoint and other measures’ change scores (i.e., only 1 of 33 possible associations was significant at p=.037; not sufficient to survive multiple comparisons correction).
fMRI Task
Stimuli for the nonverbal Sternberg WM fMRI task comprised either 2, 4, or 6 circles in a 4×4 grid which were briefly presented, followed by a cue to actively maintain the locations in WM, then a probe requiring a Yes/No response if it was in one of the locations held in WM (50% of probes were valid). Figure 1 shows a trial layout. Each span was given 6 times during two 7:50 minute sessions, for a total of 36 total trials. Pseudorandom jitter was inserted between trial onsets (5–8 sec) and the three trial periods (3–5 sec) so fMRI modeling could differentiate brain activity change during Encoding, Maintenance, and Retrieval information processing phases.
Figure 1.
Layout of a Sternberg working memory fMRI task trial.
Imaging parameters
Imaging was done on a Siemens Allegra 3T system at the Olin Neuropsychiatry Research Center of the Institute of Living/Hartford Hospital in Hartford, CT. Functional image volumes were collected in axial orientation to the anterior commissure-posterior commissure line using a gradient-echo sequence sensitive to the blood-oxygen-level-dependent (BOLD) signal (TR=1,500 msec, TE=28 msec, flip angle=65°, FOV=24×24 cm, 64×64 matrix, 3.4×3.4 mm in plane resolution, 5 mm slice thickness, 30 slices). Each session acquired 308 volumes. The first 6 volumes where T1 effects stabilized were discarded.
Image processing
Functional images were reconstructed offline and each run was separately realigned using INRIAlign (Freire and Mangin, 2001) and slice-timing corrected via interpolation as implemented in Statistical Parametric Mapping (SPM8). All participants had head motion <1 voxel length in any x,y,z plane. A mean functional image volume was constructed from the realigned image volumes for each session, used to determine parameters for spatial normalization into standardized Montreal Neurological Institute space. Normalized images were smoothed with an 8 mm3 full-width-at-half-maximum Gaussian filter.
fMRI Statistics
Brain activation was estimated using individual participant SPM8 GLM models. For ADHD, all 4 fMRI timeseries (2 pre-training, 2 post-training) were included in a single GLM for each participant. Regressors for each condition/session were derived by extracting stimulus onset timing for Encoding, Maintenance, and Retrieval stimuli and were convolved with a synthetic hemodynamic response function and its temporal derivative. Parametric interaction terms for each task phase identifying the 2, 4, or 6 stimulus conditions quantified greater or lesser brain function according to WM load. Six motion-correction parameter estimates (x, y, and z displacement and roll, pitch, and yaw rotations) were included as covariates-of-no-interest to isolate minor head motion BOLD signal variance. A high-pass filter (128 sec cutoff) removed low-frequency signals. GLM contrast coding produced maps quantifying overall activation, parametric effects of WM difficulty, and differences in activation between post- and pre-training for these effects, separately for Encoding, Maintenance, and Retrieval conditions. Simple activation to each condition was identified using SPM8 one-sample t tests, while non-ADHD vs ADHD differences were evaluated using SPM8 two-sample t tests on activation maps. ADHD treatment effects were evaluated using one-sample t tests on the difference maps constructed via contrast coding across the pre- and post-treatment fMRI sessions. For all group-level tests, “whole brain” statistical significance was evaluated using Monte Carlo simulation (p<.01 entry threshold, p<.05 clusterwise significance).
Supplemental Post Hoc Analyses
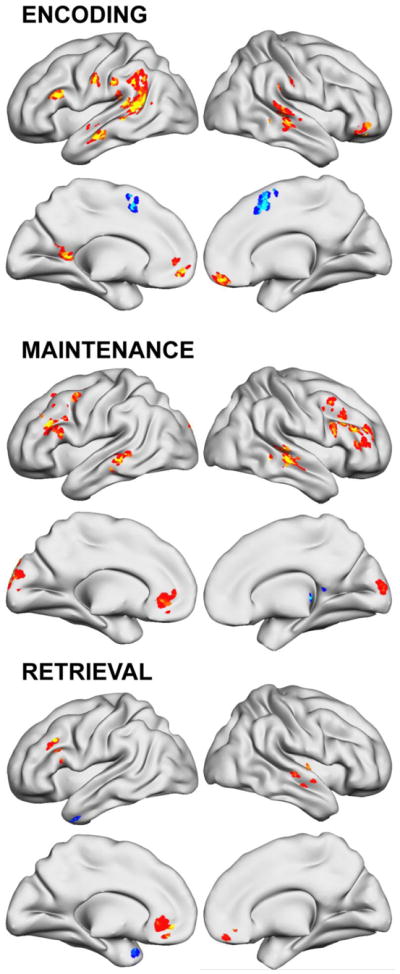
In order to determine if ADHD brain dysfunction was still detectable following WM training, we used an ROI analysis. 5 mm spheres centered on the peak xyz coordinate where pre-training ADHD differed from non-ADHD were interrogated using SPM small volume-corrected (SVC) analyses (p<.05 FWE SVC). Other post hoc analyses included SPM8 correlation to examine the association of condition main effect and parametric activity change maps with the Training Index scores and parent-reported ADHD symptom severity. First, we tested whether any voxels within 5 mm of peak training effects were correlated (p<.05 uncorrected), then examined the whole brain using Monte Carlo multiple comparisons corrections. Because the main effects of task activation to Encoding, Maintenance and Retrieval were not part of the hypothesis-testing but are relevant for fully understanding how the task engaged WM-related brain activity, we included these activation maps for the entire sample in Supplemental Material. Figure S1 shows Sternberg activation to Encoding, Maintenance, and Retrieval conditions. Figure S2 depicts the parametric effect of WM load on activation in each condition.
Results
Neuropsychological Training Effects
ADHD participants’ initial working memory Training scores varied from 67 to 102, indicating some participants had marked impairment, while others had near-typical Cogmed trial performance at training start. This was paralleled by non-trained WM test performance, where most patients (12 of 18) had at least a 1 SD weakness (i.e., scaled-score of 7 or less) on one or more WISC-IV WM subtests before WM training. Table 2 shows that ADHD participants’ Cogmed training scores significantly improved after WM training. Relative to normative training scores in youth ages 7–17 (mean start/max scores = 73/100, SD=13; (Pearson, 2014), all ADHD participants improved at least 1 normative SD in WM trial difficulty (range 1–5 SDs, mean 2.3). Post hoc Pearson correlation showed the magnitude of WM training gains were unrelated to baseline Training score, participant age, or use of psychostimulants during the trial. Although ADHD girls on average achieved a slightly higher Training score by trial endpoint (t=2.43, p=.027), intra-individual improvement was not significantly greater than that seen in males.
ADHD performance on all non-trained WM tests significantly improved (Table 2). PCA on these change scores identified two factors with eigenvalues >1.0, accounting for 67% of the variance. Factor loadings >.400 (Table S1 in Supplemental Material) reflected two factors: Factor 1 depicted improvements on tests requiring executive manipulation of WM contents (e.g., Letter-Number Sequencing and Spatial Span Backwards), and Factor 2 showed training-related changes in simple storage WM tests (e.g., Digit Span Forwards and Spatial Span Forwards). Gains in simple WM storage (PCA-derived Factor 2) were inversely correlated with BDI-II and MASC-scores (r=−.506, p=0.077 and r=−.698, p=.003, respectively), suggesting mild mood and anxiety problems constrained these WM gains.
None of the CPT-II indices showed any significant changes with WM training.
Clinical Training Effects
Table 2 also shows that ADHD patients improved on most subjective reports of ADHD symptom severity, ADHD-related problems, and severity of functional impairment at home. Because the handful of missing clinical datapoints was random, these analyses were re-run after replacing missing values with each variable’s mean to ensure information from cases with only a single missing value were utilized in outcome evaluation. Findings were unchanged, except that significant group difference p values were even lower (ranged from p=.003 to .00001), likely due to the increased statistical power. Closer inspection of individual change scores showed a range of clinical responsiveness, with a few patients showing no ADHD symptom severity change or in one case even modestly worse parent-reported symptoms, a larger handful showing modest improvement, and at least half the sample showing marked symptom improvement. Additional post hoc correlations showed clinical changes were unrelated to age or gender. Participants not taking psychostimulants showed significantly greater parent-rated symptom improvement (t=2.91, p=.014; mean/SD 31.3/10.7 vs 10.7/11.5).
The difference in Cogmed Training scores before and after training strongly predicted parent-reported ADHD symptom severity reductions (Inattentive symptoms r = −.913, p=0.000035; Hyperactive/Impulsive symptoms r = −.795, p=.003).
ADHD Deficits in WM-Related Brain Activation
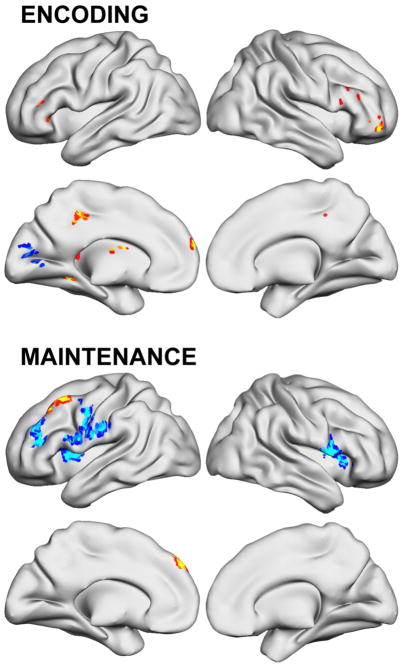
Table 3 lists differences between ADHD and non-ADHD control participants; Figure 2 depicts ADHD activation deficits across the whole brain (p<.05 clusterwise corrected). Significantly lower ADHD activation was found for Maintenance and Retrieval conditions in numerous lateral prefrontal cortex and parietal lobe regions, including in areas within inferior frontal sulcus. No ADHD frontoparietal deficits were found for Encoding, but exploratory analysis at uncorrected statistical thresholds noted left (x,y,z = −39, 17, 22; t=2.39) and right (x,y,z = 27, 47, 22; t=2.10) middle frontal gyri activation deficits in ADHD. There also were regions in each condition where ADHD participants had greater activation than non-ADHD. During Encoding these were found in ventrolateral prefrontal/insular cortex, posterior parietal, putamen and cerebellum. During Maintenance, only insula, right parahippocampal gyrus/amygdala, and a large area of primary visual cortex were greater in ADHD. For information Retrieval, ADHD participants engaged several ventrolateral/ventromedial prefrontal, posterior parietal and cerebellar regions more than non-ADHD.
Table 3.
Significant differences between ADHD and non-ADHD participant’s brain function, using Monte Carlo simulations to set clusterwise control over false positives across the whole brain at p<.05. The Table also lists effect sizes quantifying the magnitude of ADHD Pre-Training and Post-Training differences from non-ADHD controls.
| Non-ADHD vs ADHD Pre-Training | Post-Training Region-of-Interest Analysis | |||||
|---|---|---|---|---|---|---|
| x, y, z | t | Cohen’s d | t | p | Cohen’s d | |
| Encoding | ||||||
|
| ||||||
| Non-ADHD > ADHD | ||||||
| Left middle occipital gyrus/precuneus | −24, −82, 40 | 4.72 | 1.60 | 2.86 | † | 0.97 |
| Right superior parietal lobule/precuneus | 36, −76, 40 | 4.30 | 1.45 | 2.73 | † | 0.92 |
| Left lingual/inferior occipital gyri | −15, −100, −11 | 3.64 | 1.23 | 3.12 | 0.031 | 1.05 |
| Right parahippocampal gyrus | 21, −46, −14 | 3.35 | 1.13 | 2.81 | † | 0.95 |
| Non-ADHD < ADHD | ||||||
| Left middle frontal gyrus (BA 8/9) | −36, 32, 43 | 4.02 | 1.36 | 3.86 | 0.006 | 1.30 |
| Midline superior frontal gyrus (BA 8) | 0, 35, 55 | 4.64 | 1.57 | 3.41 | 0.017 | 1.15 |
| Medial frontal gyrus (BA 9) | −3, 44, 31 | 3.13 | 1.06 | 4.76 | 0.001 | 1.61 |
| Left inferior frontal gyrus (BA 45) | −54, 38, 4 | 3.62 | 1.22 | 4.24 | 0.001 | 1.43 |
| Left inferior frontal gyrus (BA 47) | −36, 32, −20 | 3.36 | 1.14 | 2.98 | 0.041 | 1.01 |
| Right insular cortex (BA 13/47) | 30, 17, −5 | 2.44 | 0.82 | 2.81 | † | 0.95 |
| Left insular cortex (posterior BA 13) | −42, −13, 1 | 3.96 | 1.34 | 3.83 | 0.007 | 1.29 |
| Right sensorimotor cortex (pre/postcentral gyri) | 18, −19, 58 | 4.07 | 1.38 | 3.81 | 0.007 | 1.29 |
| Right supramarginal gyrus (BA 40) | 57, −28, 34 | 3.25 | 1.10 | 4.41 | 0.002 | 1.49 |
| Right fronto-temporal space (BA 45) | 60, 11, 7 | 3.65 | 1.23 | 3.10 | 0.032 | 1.05 |
| Right putamen | 27, −7, −8 | 3.52 | 1.19 | 3.42 | 0.017 | 1.16 |
| Left cerebellum (posterior lobe) | −12, −70, −17 | 3.99 | 1.35 | 3.51 | 0.014 | 1.19 |
|
| ||||||
| Maintenance | ||||||
|
| ||||||
| Non-ADHD > ADHD | ||||||
| Left middle frontal gyrus (BA 8) | −30, 17 52 | 3.86 | 1.30 | 1.45 | ns | 0.49 |
| Left middle/inferior frontal gyri (BA 46) | −42, 14, 22 | 3.38 | 1.14 | 1.55 | ns | 0.52 |
| Right middle/inferior frontal gyri (BA 46) | 42, 32, 25 | 3.96 | 1.34 | 2.69 | † | 0.91 |
| Left inferior parietal lobule (BA 40) | −36, −37, 43 | 3.53 | 1.19 | 2.05 | ns | 0.69 |
| Left precuneus | −24, −85, 40 | 3.73 | 1.26 | 2.32 | ns | 0.78 |
| Right precuneus/superior parietal lobule | 33, −79, 37 | 4.33 | 1.46 | 1.44 | ns | 0.49 |
| Left middle occipital gyrus (BA 19) | −42, −88, 10 | 3.37 | 1.14 | 1.68 | ns | 0.57 |
| Non-ADHD < ADHD | ||||||
| Left insular cortex (BA 13) | −39, 11, 1 | 3.01 | 1.02 | 2.22 | ns | 0.75 |
| Primary visual cortex (L/R cuneus/lingual) | 15, −100, 19 | 4.69 | 1.59 | 4.74 | <0.001 | 1.60 |
| Right parahippocampal gyrus/amygdala | 18, 5, −23 | 4.01 | 1.36 | 2.59 | † | 0.88 |
|
| ||||||
| Retrieval | ||||||
|
| ||||||
| Non-ADHD > ADHD | ||||||
| Midline superior/medial frontal gyri (BA 8) | 3, 26, 52 | 3.31 | 1.12 | 3.48 | 0.015 | 1.18 |
| Left inferior/middle frontal gyri (BA 46) | −48, 17, 19 | 3.17 | 1.07 | 1.76 | ns | 0.59 |
| Right inferior frontal gyrus | 42, 11, 22 | 3.02 | 1.02 | 2.32 | ns | 0.78 |
| Left middle frontal gyrus (BA 10) | −27, 47, −5 | 3.53 | 1.19 | 2.44 | ns | 0.82 |
| Left inferior parietal lobule (BA 40) | −36, −40, 40 | 3.43 | 1.16 | 2.55 | † | 0.86 |
| Right precuneus | 36, −73, 31 | 3.29 | 1.11 | 2.70 | † | 0.91 |
| Left middle occipital gyrus (BA 19) | −42, −88, 4 | 3.35 | 1.13 | 2.78 | † | 0.94 |
| Right parahippocampal/fusiform gyri (BA 37) | 30, −52, −11 | 3.37 | 1.14 | 2.32 | ns | 0.78 |
| Non-ADHD < ADHD | ||||||
| Midline superior/medial frontal gyri (BA 9) | 3, 53, 31 | 3.51 | 1.19 | 3.76 | 0.008 | 1.27 |
| Right precentral/inferior frontal gyri (BA 44) | 60, 11, 7 | 3.64 | 1.23 | 2.75 | † | 0.93 |
| Orbital/medial frontal gyri (BA 11) | 3, 44, −23 | 2.88 | 0.97 | 3.23 | 0.022 | 1.09 |
| Right inferior frontal gyrus (BA 47) | 24, 23, −23 | 3.82 | 1.29 | 2.50 | ns | 0.85 |
| Right paracentral lobule | 6, −28, 52 | 4.22 | 1.43 | 4.80 | 0.001 | 1.62 |
| Left inferior parietal lobule (BA 40) | −57, −40, 52 | 4.45 | 1.50 | 2.01 | ns | 0.68 |
| Right inferior parietal lobule (BA 40) | 48, −25, 28 | 3.24 | 1.10 | 3.88 | 0.006 | 1.31 |
| Left superior temporal gyrus (BA 22) | −51, 11, −14 | 3.09 | 1.04 | 2.40 | ns | 0.81 |
| Left cerebellum (posterior lobe) | −18, −67, −23 | 5.26 | 1.78 | 2.57 | † | 0.87 |
| Right cerebellum (posterior lobe) | 18, −70, −23 | 3.74 | 1.26 | 2.26 | ns | 0.76 |
represents a trend level of FWE small-volume corrected significance (> .05 but <.10). This was included because the p values from the ROI analysis results are not directly comparable to Monte Carlo “whole brain” corrections (although the t statistics at the peak voxel of study group difference are equivalent for head-to-head comparison).
Figure 2.
ADHD working memory brain activation deficits before working memory training compared to non-ADHD control participants (p<.05 clusterwise significance threshold).
WM Training Effects on WM-Related Brain Activation
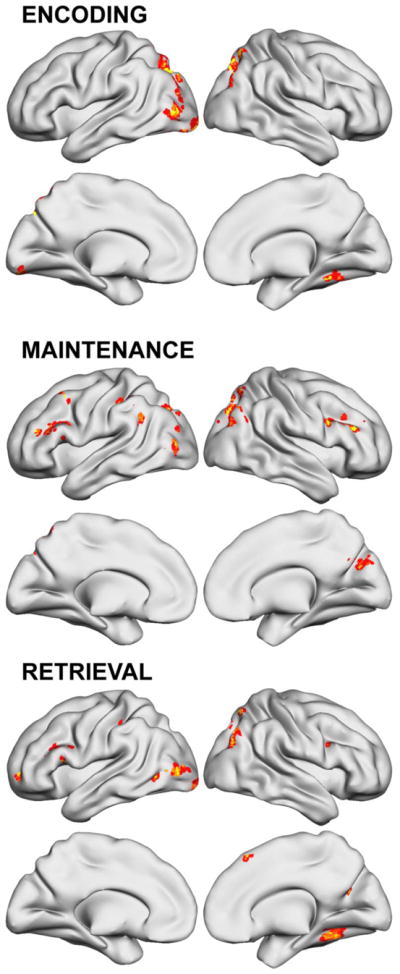
Brain regions with significantly altered activation after WM training are shown in Figure 3 and listed in Table 4. Changes were almost exclusively activation increases. Normal task-elicited activity increased in IFS, caudal SFS, and medial PFC during Encoding and Maintenance. Training effects were predominantly, though not entirely, seen in the left hemisphere. In particular, Maintenance activation was greater after WM training in homologous right PFC structures not engaged in these ADHD participants by this task (refer to Figure S1 for visual comparison). Left IFS regions (i.e., middle/inferior frontal gyri) showed greater activity regardless of Sternberg task phase, although WM training effects were localized to BA 45 for Encoding and Maintenance, but had a slightly more dorsal peak (i.e., including some aspects of BA 9/46) for Retrieval. In the parietal lobe, WM training-related increases were localized to different regions in each hemisphere. Both parietal and posterior temporal lobe changes were only observed during Encoding of stimuli into WM, and comparison to activation main effects (Figure S1) indicates they also represented recruitment of brain regions not normally engaged for task completion. Post hoc ROI analyses examined whether any of these changes normalized ADHD activation abnormalities listed in Table 3. Although there was evidence that some of the occipital lobe ADHD hypoactivation during Encoding changed, in general none of these deficits or ADHD over-activation in frontal, parietal, basal ganglia or cerebellar over-activation resolved. These Encoding differences either still significantly differed, or were detectable as “trend” levels of difference. In contrast, the majority of ADHD activation abnormalities during Maintenance and many during Retrieval Sternberg task phases were non-significantly different from non-ADHD following WM training.
Figure 3.
Working memory brain activation changes after 5 weeks of working memory training (p<.05 clusterwise significance threshold).
Table 4.
Main effect of 5 weeks of working memory training on brain activation during different phases of the Sternberg fMRI task. Analyses are repeated-measures t tests comparing fMRI assessment of Encoding, Maintenance, and Retrieval brain activation at study endpoint to pre-training baseline fMRI, using Monte Carlo simulations to set clusterwise control over false positives across the whole brain at p<.05. For each region showing significant differences, post hoc tests evaluated the Pearson correlation of brain function within 5 mm of the peak and either Cogmed WM task performance improvement, or change in parent-reported ADHD symptom severity separately for Inattentive versus Hyperactive/Impulsive symptoms.
| Cogmed Training Index Change r | Inattentive Symptom Change r | Hyperactive/Impulsive Symptom Change r | |||
|---|---|---|---|---|---|
| Brain region | Peak x, y, z | Peak t | |||
| Encoding | |||||
|
| |||||
| Post-Training Increases | |||||
| Left inferior frontal gyrus (BA 46/45/9) | −57, 23, 22 | 3.44 | ns | ns | ns |
| Medial frontal gyrus | −6, 50, −5 | 3.90 | ns | ns | ns |
| Left postcentral gyrus | −60, −13, 37 | 4.12 | ns | ns | ns |
| Left supramarginal gyrus/inferior parietal lobule | −63, −25, 34 | 3.46 | 0.07 | ns | ns |
| Right inferior parietal lobule | 45, −31, 31 | 4.12 | ns | ns | ns |
| Left angular/superior temporal gyri (posterior) | −60, −49, 37 | 3.68 | 0.02 | ns | ns |
| Left middle temporal gyrus | −63, −16, −14 | 4.69 | ns | ns | ns |
| Right middle/superior temporal gyri | 54, −31, 4 | 3.56 | ns | 0.03 | ns |
| Right posterior cingulate | −15, −52, 13 | 3.37 | ns | ns | ns |
| Brainstem | −6, −31, −38 | 3.90 | ns | ns | 0.05 |
| Post-Training Decreases | |||||
| Superior frontal/medial frontal gyri (pre-SMA) | 6, 11, 46 | 3.64 | 0.06 | 0.01 | ns |
|
| |||||
| Maintenance | |||||
|
| |||||
| Post-Training Increases | |||||
| Left middle/inferior frontal gyri (BA 9/8/46/45) | −27, 17, 52 | 3.45 | ns | ns | ns |
| Right middle/inferior frontal gyri (BA 46/9) | 42, 23, 25 | 5.29 | ns | ns | 0.08 |
| Anterior cingulate/Medial frontal gyrus (BA 32/10) | −6, 35, −8 | 2.91 | ns | ns | ns |
| Left middle temporal gyrus | −63, −37, −2 | 3.62 | 0.006 | 0.01 | ns |
| Right middle/superior temporal gyri | 54, −31, 1 | 4.21 | 0.10 | 0.10 | ns |
| Bilateral cuneus (BA 18/19) | 3, −97, 16 | 2.95 | ns | 0.001 | 0.02 |
|
| |||||
| Retrieval | |||||
|
| |||||
| Post-Training Increases | |||||
| Left middle/inferior frontal gyri (BA 9/46) | −51, 20, 25 | 3.74 | ns | 0.08 | ns |
| Anterior cingulate/medial frontal gyri (BA 32) | −3, 35, −11 | 3.48 | ns | ns | ns |
| Right superior temporal gyrus | 54, −10, −2 | 3.33 | ns | ns | 0.03 |
| Post-Training Decreases | |||||
| Left cerebellum | −36, −55, −38 | 5.29 | ns | ns | 0.004 |
| Right cerebellum | 42, −55, 38 | 4.31 | ns | 0.02 | ns |
Figure 4 and Table 5 report altered parametric effects of WM task difficulty on brain activation after training. The most notable effect was lesser recruitment of several dlPFC and vlPFC regions at greater WM loads during Maintenance. Visual comparison to parametric main effects (Figure S2) localizes most of these effects to activated regions (note, in right vlPFC, change was found at p<.01 that did not survive clusterwise corrections). Midline superior/medial frontal gyri activation was the only parametric WM load effect showing greater activity in a region that did not show a main or parametric effect for Maintenance in this sample.
Figure 4.
Changes to the parametric effect of working memory load on brain activation after 5 weeks of working memory training (p<.05 clusterwise significance threshold).
Table 5.
Main effect of 5 weeks of working memory training on the brain’s response to task difficulty. Analyses are repeated-measures t tests of the parametric effect of working memory load (2, 4, or 6 stimuli to be remembered) comparing study endpoint fMRI and pre-training baseline fMRI. Increases represent a brain region that showed a greater relationship between difficulty and activation amplitude at baseline.
| Brain region | Peak x, y, z | Peak t | Cluster extent |
|---|---|---|---|
| Encoding Parametric | |||
|
| |||
| Post-Training Increases | |||
| Medial frontal gyrus (BA 10) | 0, 65, 22 | 4.93 | 25 |
| Right inferior frontal gyrus | 48, 20, 19 | 3.58 | 26 |
| Left inferior frontal gyrus (anterior insula) | −42, 32, −2 | 3.10 | 37 |
| Right middle frontal gyrus (BA 10/47) | 45, 50, −5 | 4.01 | 33 |
| Precuneus/paracentral lobule (BA 31) | 0, −40, 43 | 3.05 | 57 |
| Post-Training Decreases | |||
| Left cuneus (BA 17/18/23) | −3, −85, 16 | 3.43 | 44 |
|
| |||
| Maintenance Parametric | |||
|
| |||
| Post-Training Increases | |||
| Left middle/superior frontal gyri (BA 8/6) | −33, 26, 52 | 3.16 | 42 |
| Midline superior/medial frontal gyri (BA 9/8) | −6, 56, 37 | 4.06 | 37 |
| Post-Training Decreases | |||
| Left middle/inferior frontal gyri (BA 44) | −42, 38, 31 | 3.71 | 484 |
| Left insula | −36, 11, 10 | 4.36 | |
| Left precentral gyrus | −54, 2, 19 | 3.44 | |
| Left postcentral gyrus | −57, −16 28 | 3.54 | |
| Right insula | 33, 17, 7 | 4.47 | 181 |
|
| |||
| Retrieval Parametric | |||
|
| |||
| None | |||
All fMRI analyses were repeated using either age, gender, or both age and gender together as covariates-of-no-interest. Results remained essentially unchanged.
Relationship of Brain Function Changes to Clinical Improvements
Post hoc analyses examined the relationship of neural change to various cognitive and clinical changes. Table 4 shows that several of the specific peak prefrontal and parietal WM training effects also correlated with WM or clinical gains at trend significance levels or better.
Figure 5 and Table 6 list other brain regions from supplemental analyses where the activation changes following WM training were correlated at a “whole brain” significance threshold with Cogmed Training change score. Greater Maintenance-elicited activity with greater WM change was seen in right IFS and left mid-lateral PFC.
Figure 5.
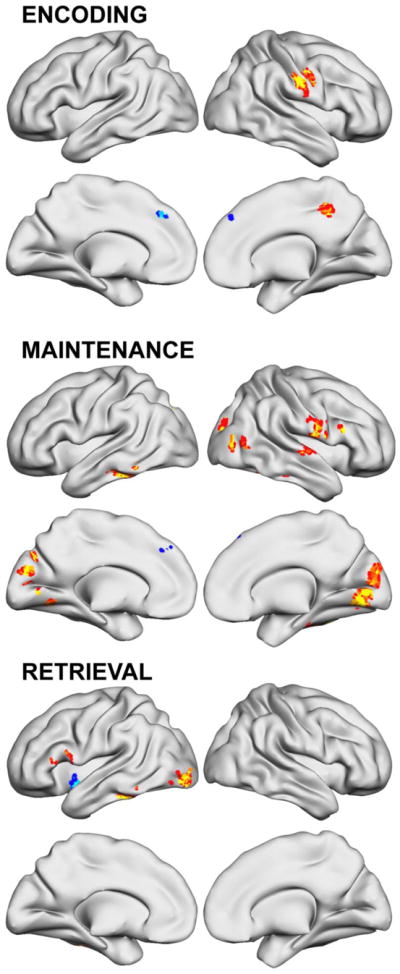
Brain regions where working memory brain activation changes after 5 weeks of working memory training correlated with Cogmed’s Training Index working memory performance improvement score (p<.05 clusterwise significance threshold).
Table 6.
Supplemental analysis showing additional brain regions where post-training versus pre-training fMRI measurements significantly correlated with changes in the Cogmed Training Index score of WM exercise performance. The analysis used using Monte Carlo simulations to set clusterwise control over false positives across the whole brain at p<.05.
| Brain region | Peak x, y, z | Peak t | Cluster extent |
|---|---|---|---|
| Encoding | |||
|
| |||
| Post-Training Increases | |||
| Right pre/postcentral gyri (sensorimotor cortex) | 54, −16, 34 | 5.43 | 119 |
| Left cerebellum | −15, −52, −32 | 4.57 | 388 |
| Post-Training Decreases | |||
| Medial frontal gyrus (BA 6/9/8) | 0, 38, 40 | 3.98 | 32 |
|
| |||
| Maintenance | |||
|
| |||
| Post-Training Increases | |||
| Right inferior frontal gyrus (BA 45/9) | 63, 17, 22 | 3.63 | 39 |
| Right pre/postcentral gyrus (sensorimotor cortex) | 63, −7, 13 | 3.81 | 79 |
| Left middle/inferior temporal gyri | −51, −37, −17 | 3.97 | 44 |
| Bilateral cuneus (BA 18/19/17) | 0, −88, 13 | 3.49 | 381 |
| Right middle temporal/middle occipital gyri | 45, −79, 10 | 3.82 | 41 |
| Bilateral cerebellum (anterior lobe) | −9, −52, −26 | 5.07 | 567 |
| Post-Training Decreases | |||
| Medial frontal gyrus (BA 6/9/8) | 3, 41, 43 | 3.65 | 34 |
|
| |||
| Retrieval | |||
|
| |||
| Post-Training Increases | |||
| Left inferior frontal gyrus (BA 44/45) | −60, 14, 7 | 2.97 | 32 |
| Left middle temporal/fusiform gyri | −48, −37, −20 | 4.27 | 42 |
| Left middle/inferior occipital gyri (BA 18) | −30, −97, −8 | 3.82 | 70 |
| Cerebellum | 6, −58, −23 | 3.78 | 41 |
| Brainstem (pons/midbrain) | −6, −28, −23 | 3.31 | 63 |
| Post-Training Decreases | |||
| Left superior temporal gyrus/insula | −36, 2, −14 | 3.75 | 32 |
Figures 6 and 7 and Table 7 report other regions where training-related changes to brain activity correlated with parent-rated ADHD-Inattentive or ADHD-Hyperactive/Impulsive symptom improvements. Different brain region change were linked to improvement of symptom severity in each symptom domain. Neural change in caudal SFS (bilateral middle frontal gyrus; BA 6) and cingulate/medial frontal gyri during Maintenance were the only associations found for both Inattentive and Hyperactivity symptom change.
Figure 6.
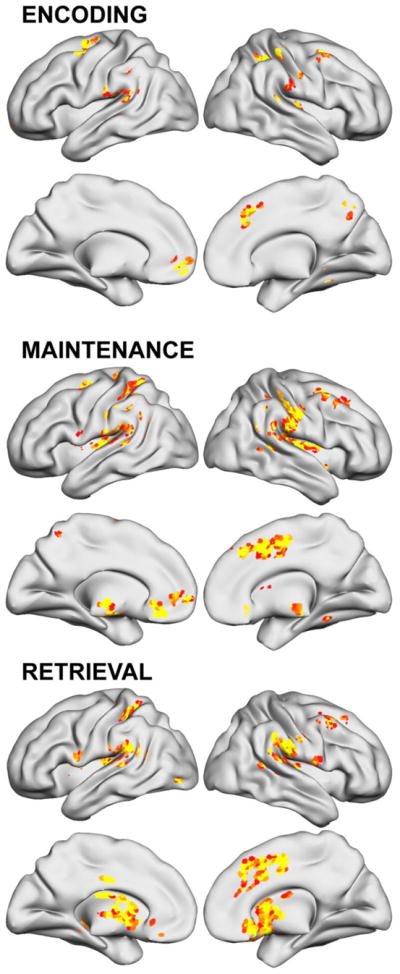
Regions where training-related changes to ADHD brain activity correlated with parent-rated ADHD-Inattentive symptom improvements after 5 weeks of working memory training (p<.05 clusterwise significance threshold).
Figure 7.
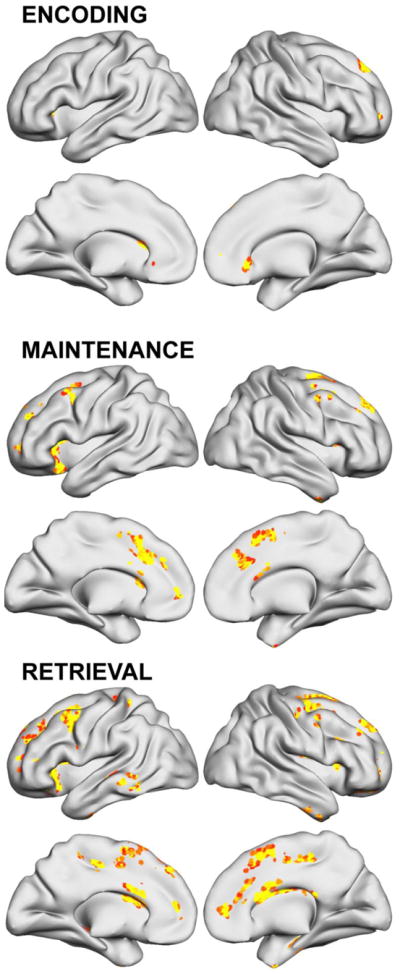
Regions where training-related changes to ADHD brain activity correlated with parent-rated ADHD-Hyperactive/Impulsive symptom improvements after 5 weeks of working memory training (p<.05 clusterwise significance threshold).
Table 7.
Supplemental analysis showing additional brain regions where post-training versus pre-training fMRI measurements significantly correlated with changes in parent-rated DSM-IV ADHD symptom severity. The analysis used using Monte Carlo simulations to set clusterwise control over false positives across the whole brain at p<.05.
| Inattentive Symptom Change Correlation | Hyperactive/Impulsive Symptom Change Correlation | |||
|---|---|---|---|---|
| Brain region | Peak x, y, z | Peak t | Peak x, y, z | Peak t |
| Encoding | ||||
|
| ||||
| Post-Training Increases | ||||
| Left middle frontal gyrus (BA 6) | −36, −1, 58 | 6.49 | ||
| Right middle frontal gyrus (BA 6) | 36, 11, 58 | 3.26 | ||
| Right superior frontal gyrus (BA 8) | 15, 44, 49 | 4.98 | ||
| Right cingulate/medial frontal gyri (BA 32,8) | 12, 23, 40 | 4.41 | ||
| Right anterior cingulate (rostral BA 24) | 3, 23, −2 | 3.93 | ||
| Right superior frontal gyrus (BA 10) | 18, 53, 4 | 4.37 | ||
| Left Medial frontal gyrus | −12, 53, −8 | 5.37 | ||
| Right inferior parietal lobule (BA 40) | 42, −37, 55 | 4.92 | ||
| Right precuneus (BA 7) | 15, −64, 46 | 4.45 | ||
| Left supramarginal gyrus/inferior parietal lobule/posterior insula | −45, −22, 19 | 5.41 | ||
| Right inferior parietal lobule/postcentral gyrus | 69, −34, 19 | 5.04 | ||
| Right putamen/lentiform nucleus | 30, −22 1 | 3.96 | ||
| Anterior cerebellum | 18, −46, −14 | 5.14 | ||
| Right posterior cerebellum | 33, −76, −35 | 4.50 | ||
|
| ||||
| Maintenance | ||||
|
| ||||
| Post-Training Increases | ||||
| Left middle frontal gyrus (BA 6) | −21, −7, 55 | 5.49 | −12, 2, 58 | 3.92 |
| Left middle frontal gyrus (BA 6/8) | −36, 8, 55 | 7.78 | ||
| Right middle frontal gyrus (BA 6) | 33, 8, 55 | 3.52 | 48, 8, 49 | 4.19 |
| Right cingulate/medial frontal gyri (BA 32/24/8) | 9, 11, 49 | 5.26 | 6, 17, 49 | 3.89 |
| Right medial/superior frontal gyri (BA 6) | 15, 8, 49 | 4.02 | ||
| Right superior frontal gyrus (BA 8/9) | 15, 47, 46 | 4.86 | ||
| Right cingulate/medial frontal gyri | −12, 26, 40 | 4.81 | ||
| Right anterior cingulate gyrus (BA 32) | 9, 29, 28 | 3.40 | ||
| Left medial/superior frontal gyri (BA 10) | −9, 56, 1 | 6.22 | −15, 50, 4 | 3.70 |
| Left anterior cingulate (rostral) | −6, 29, −14 | 5.63 | ||
| Left insula (BA 13) | 39, 5, 13 | 4.28 | ||
| Right anterior insula (BA 47) | −30, 23, −14 | 5.17 | ||
| Left postcentral gyrus | −18, −34, 61 | 4.40 | ||
| Left superior parietal lobule/precuneus | −18, −55, 61 | 4.18 | ||
| Right postcentral gyrus/inferior parietal lobule | 45, −40, 49 | 5.01 | ||
| Left supramarginal gyrus/inferior parietal lobule | −45, −43, 34 | 3.56 | ||
| Left posterior insula/lentiform nucleus | −45, −19, 13 | 3.41 | ||
| Right posterior insula/lentiform nucleus | 45, −31, 16 | 4.64 | ||
| Midbrain | −3, −22, −17 | 6.32 | ||
| Anterior cerebellum | −9, −49, −14 | 4.66 | ||
|
| ||||
| Retrieval | ||||
|
| ||||
| Post-Training Increases | ||||
| Left middle frontal gyrus (BA 6) | −36, 5, 49 | 11.85 | ||
| Right middle/superior frontal gyri (BA 8) | 33, 17, 58 | 4.03 | ||
| Left medial frontal/cingulate gyri | −15, 5, 55 | 6.16 | ||
| Right cingulate/medial frontal gyri (BA 24/32) | 9, 14, 49 | 4.51 | 12, 20, 49 | 4.32 |
| Right precentral gyrus | 51, 2, 49 | 4.23 | ||
| Right precentral gryrus | 36, −10, 55 | 8.55 | ||
| Left medial/superior frontal gyri | −12, 32, 43 | 7.41 | ||
| Right insula (BA 13) | 39, 14, 13 | 4.29 | ||
| Left inferior frontal gyrus | −33, 32, 4 | 6.70 | ||
| Right pregenual cingulate (BA 25) | 6, 20, −11 | 5.40 | ||
| Left inferior parietal lobule (BA 40)/superior temporal gyrus | −63, −46, 22 | 4.65 | ||
| Left middle temporal gyrus | −60, −40, −8 | 5.22 | ||
| Right inferior parietal lobule (BA 40)/superior temporal gyrus | 63, −37, 34 | 5.49 | ||
| Left middle occipital gyrus | −30, −85, 1 | 4.62 | ||
| Left caudate | −15, 17, 16 | 2.83 | ||
| Right caudate | 18, 11, 13 | 3.65 | ||
| Right claustrum | 30, 14, 16 | 8.18 | ||
| Right globus pallidus | 21, −13, −2 | 8.09 | ||
| Left thalamus | −15, −13, 13 | 5.36 | ||
| Left anterior cerebellum | −18, −43, −23 | 3.24 | ||
| Pons | −6, −31, −44 | 4.66 | ||
Several additional post hoc analyses of potential interest were included as Supplemental Material. Table S3 lists brain regions where training-related changes in the brain’s response to task difficulty (i.e., parametric effect of WM load) correlated with improvement in the Cogmed Training change score. Table S3 lists regions where training-related change to Encoding, Maintenance, and Retrieval correlated with the PCA-derived Factor 1 (executive WM) or Factor 2 (short term WM storage).
Discussion
The dual purposes of this preliminary study were to characterize WM brain function change after 5 weeks of WM training in ADHD for the first time, and to determine if brain changes were associated with any clinical improvements. ADHD WM training was generally successful and cognitive gains were WM-specific. Gains did not occur for sustained attention, response inhibition or response speed. WM training resulted in greater ADHD activation in several WM-linked frontoparietal brain regions, with the magnitude of neural change in posterior brain regions predicting degree of WM gains. Many of these changes coincided closely with frontoparietal activation deficits localized by comparison to non-ADHD controls, and which were consistent with previous ADHD fMRI WM studies. Indeed, ROI analysis showed many of the ADHD brain activation abnormalities detected before WM training were no longer significantly different after treatment, particularly during Sternberg task Maintenance and many prefrontal regions engaged for Retrieval. This suggests that WM training is normalizing some aspects of ADHD WM-related brain dysfunction. However, it is important to note that many effect sizes of ADHD vs. non-ADHD differences remained meaningful, despite the lack of statistical significance. So it would be misleading to conclude full brain function normalization occurs following a standard course of WM training. Despite some evidence for specificity of brain function benefit, neural changes were seen throughout fMRI task phases in numerous regions, consistent with the assumption that core WM training approaches have a broad, generalized effect (Morrison and Chein, 2011). Importantly, post hoc analyses showed that clinically-meaningful improvement ADHD clinical functioning not only was linked to WM training gains, but also, as hypothesized was linked to neurobiological change in WM-linked brain regions when ADHD-diagnosed participants engaged in WM processing. Interestingly, the training-induced activation increases seen in SMA, cingulate, and parietal lobe regions across all task phases were correlated with improvement in ADHD Inattentive symptoms, while Hyperactive/Impulsive symptom reduction was associated with greater activity in premotor/motor, cerebellar, cingulo-opercular, and caudate regions. The latter finding is consistent with proposals that transfer of WM training benefits to other functions might require intact basal ganglia function (Dahlin et al., 2009; Dahlin et al., 2008). It is possible that individual ADHD patient differences in striatal dysfunction might explain inconsistent clinical improvement in previous ADHD WM training clinical trials (Beck et al., 2010; Chacko et al., 2013; Dahlin, 2013; Egeland et al., 2013; Gibson et al., 2011; Gray et al., 2012; Green et al., 2012; Gropper et al., 2014; Klingberg et al., 2005; Mezzacappa and Buckner, 2010). The lack of corrections for multiple comparisons on these post hoc analyses should be taken into account when evaluating the relevance of these latter findings.
ADHD brain activation changes in this study did not coincide with those found in the one previous fMRI study of ADHD after cognitive training, but this is unsurprising as that study trained neither WM nor evaluated ADHD brain function using a WM task (Hoekzema et al., 2010). The greater activation of ADHD middle/inferior frontal gyri and superior/inferior parietal lobe regions – two well-described WM network regions (Owen et al., 2005; Rottschy et al., 2012; Wager and Smith, 2003) – after WM training overlap with brain function change findings in previous non-ADHD WM training fMRI studies (Hempel et al., 2004; Jolles et al., 2010; Olesen et al., 2004). We also found that WM training increased ADHD activation in other frontoparietal regions linked to WM, particularly left IFS and bilateral IPS regions. This supports the idea that continual, intensive practice of WM tasks in ADHD potentiates activation within a broader network of WM task-specialized brain regions than previously observed in non-ADHD. This observation is tempered by the recognition that most previous ADHD WM fMRI studies employed N-back or simple storage retrieval tasks, not specifically the Sternberg paradigm. Specifically. WM training increased activation in IFS and caudal SFS prefrontal regions that were impaired for ADHD at pre-treatment, suggesting WM training at least partially normalizes some neural deficits. Also, although temporal lobe, angular gyrus, and other TPO junction regions were not normally engaged by ADHD patients while performing this WM task, they were among the only regions both to increase activation following WM training and to correlate with Cogmed task performance. This raises the possibility that in ADHD, WM performance gains might also involve some form of neural compensation. The angular gyrus is a heteromodal information processing hub that supports attention reorientation, semantic concept retrieval, and mental information manipulation (Seghier, 2013), and is directly connected with both dlPFC and vlPFC via superior longitudinal fasciculi (Frey et al., 2008; Makris et al., 2005). The posterior aspects of middle temporal gyrus also are believed to contribute semantic knowledge relevant to current goal-directed behavior (Jefferies, 2013). Therefore, WM training might not simply remediate ADHD neural deficits, but also improve local processing in other brain regions to overcome ADHD neurobiological abnormalities.
Interestingly, WM training effects appear categorically different from published descriptions of ADHD psychostimulant medication effects. Although there is some evidence that drugs like methyphenidate improve WM in ADHD (Mehta et al., 2004), its behavioral effects are minimal or inconsistent at best, mirroring the results of fMRI studies of how such medications alter ADHD WM-related brain dysfunction (Rubia et al., 2014). In pediatric ADHD, methylphenidate generally increases right inferior frontal cortex, insula, superior temporal lobe and putamen activation (Rubia et al., 2014; Schweren et al., 2013). Previous WM fMRI comparisons of medicated and non-medicated ADHD have had mixed results, variably finding PFC decreases (Sheridan et al., 2007), no differences (Kobel et al., 2009; Rubia et al., 2014), effects in some but not all WM contexts (Cubillo et al., 2014; Prehn-Kristensen et al., 2011), or WM-related functional connectivity effects only (Wong and Stevens, 2012). In contrast, WM training clearly targeted ADHD bilateral dlPFC and bilateral parietal cortex in different functional contexts, reflecting alteration to the responsiveness of domain-general, sensory amodal components of the WM circuit (Shallice, 1994, 2004; Stuss, 2006; Stuss and Alexander, 2000) and to posterior temporal lobe regions. Such differences between treatment approaches might be because neural changes induced through WM training are hypothesized to involve microstructural synaptic connectivity alterations induced by neuroplastic mechanisms (Willis and Schaie, 2009), instead of the short-term, neurotransmitter availability-dependent alterations to cellular function seen with medications. Only left mid-lateral PFC (BA 9/46) consistently showed increased activation during encoding, maintenance and retrieval, suggesting it might be a key focus of putatively neuroplastic WM training effects in ADHD. This IFS region not only is robustly engaged for WM (Owen et al., 2005; Rottschy et al., 2012; Wager and Smith, 2003), but also hypofunctional in ADHD across different tasks (Cortese et al., 2012). It is involved in configuring information processing in response to changing task demands, integrating external and internal information, and flexibility in adjusting ongoing cognition or behavior. This brain region also was one of the few affected by WM task difficulty. As seen in Supplemental Figure S2, a greater parametric effect of WM load was found before WM training, which could indicate either reduced need to engage this region at higher loads after training (e.g., greater “efficiency” (Haier et al., 1992; Neubauer and Fink, 2009), or consistently greater activation after training, regardless of task demands.
Although we found clear evidence that WM training was linked to improved ADHD clinical and cognitive functioning in the majority of patients in our sample, we note our findings were somewhat more positive, and cognitive gains more specific to WM than seen in many previous non-fMRI clinical trials of the Cogmed WM training approach. It is possible that clinical outcome was related to expectancy effects, which are especially problematic in open-label, WM training trials when placebo control is not used. It also is possible that outcome was better because our sample was more clinically homogenous than previous studies (all Combined-subtype ADHD, post-pubertal development, minimal comorbid psychiatric symptoms, most with pre-existing WM weaknesses). Alternatively, outcome might reflect greater motivation typical in those who participate in an MRI study, or another uncontrolled sampling characteristic. Several factors argue against (but cannot eliminate without a randomized trial) the idea that the generally positive outcome was due to expectancy effects. First, neuropsychological and brain function changes are objectively measured, and as such, far less likely than subjective symptom severity ratings to be the result of patients’ or their parents’ wishful thinking about treatment success. WM training had a demonstrable effect on both. Second, WM training-related brain function changes did not occur in regions identified by an emerging literature on placebo and expectancy effects (Beauregard, 2009; Wager et al., 2011). Third, clinical and cognitive outcome was generally consistent with prior WM training trials, in that WM performance improved on both trained and untrained tasks, transfer of gains to other cognitive domains typically was limited, and patients showed a range of overall clinical responsiveness (i.e., not everyone improved). Fourth, specific brain function changes following WM training that were linked to symptom reduction were found in many regions that already have been linked to ADHD symptom severity in previous fMRI WM reports (Passarotti et al., 2010; Valera et al., 2005). Finally, many WM training-induced changes occurred in regions that were impaired relative to non-ADHD prior to training, particularly in prefrontal cortex. The latter is taken as evidence that WM training helps to normalize some aspects of abnormal ADHD brain function. It is important to emphasize that this study was not conducted as another test of Cogmed WM training efficacy in ADHD, but rather to show that non-cognitive improvement –when it does occur – is related to the degree of neurobiological improvements in WM brain function. That said, we emphasize the importance of conducting a future randomized-clinical trial to re-examine the questions we ask in this study. We not only need to replicate our preliminary findings, but also provide the strongest possible evidence that they are not due to uncontrolled sampling factors, varying participant expectancies, or other such hazards to experimental inference in the absence of placebo control. Mitigating the study limitations, the sample’s psychiatric comorbidity was reasonably well-controlled, its ADHD sampling clinical homogeneity lowers the likelihood that ADHD clinical or putative etiological diversity obscured meaningful findings, and factors such as minor mood/anxiety symptoms, age, or gender were found to be unrelated to fMRI-measured neural change. Medication status did seem to curtail parent-rated ADHD symptom change scores, but this also could reflect that unmedicated patients had greater room for improvement. Medication status was unrelated to brain activity change.
This initial study leaves several interesting clinical and neuroscientific questions unaddressed. For this reason, it should be viewed as only an initial step in this line of research. It will be important to determine whether ADHD brain changes following WM training predict long-term maintenance of clinical gains. Future studies also should attempt to better describe which brain regions and what precise types of neural activity changes are associated with optimal ADHD symptom reductions by studying larger samples, using different types of WM fMRI paradigms. Given the suspected heterogenous nature of ADHD pathophysiology (Castellanos and Proal, 2012; Cortese et al., 2012; Makris et al., 2009), it also is important to learn if pre-training profiles of ADHD brain dysfunction could predict whether a patient is more or less likely to benefit from treatment. We have taken one step towards this goal by finding that ADHD adolescent cognitive, clinical, and neurobiological outcomes after Cogmed WM training were unrelated to patient age or gender. This suggests inquiry should turn to finding whether neurobiological factors dictate treatment success. However, there might exist other clinical features that predict WM training outcome (e.g., types or severities of WM deficits) that could be easily assessed by paper-and-pencil measures in the clinical setting.
It has been proposed that WM training exercises could be re-developed to more optimally target the brain systems known to be specifically impaired in ADHD, or the various circuits engaged for WM. Although Cogmed exercises do not focus on improving executive WM, we observed that ADHD patients nonetheless showed increased activation after WM training in brain regions known to contribute to executive control, such as bilateral caudal SFS. Indeed, caudal SFS was the only region to show increased engagement in all three Sternberg task conditions, suggesting it had the most generalized contribution to WM improvements. Caudal SFS underlies diverse WM executive demands, including distractor resistance, susceptibility to interference, updating WM, and shifting attention among elements within WM (Nee et al., 2013). During Sternberg retrieval, brain activity changes also were seen in ventral attention network regions, linked to other putatively “executive” WM processing, such as selection of information for WM recall (Badre and Wagner, 2005), resolving proactive interference (Jonides and Nee, 2006), and selective attention. One of the few activation decreases after training was found during Encoding in midline pre-SMA (medial/superior frontal gyri) – a region linked to filtering external distraction during WM stimulus encoding (Nee et al., 2013). The involvement of so many executive WM-linked regions in Cogmed WM training, despite its theoretical focus on simple storage capacity, raises the possibility that directly training executive aspects of WM might produce greater or more consistent clinical effects in ADHD. Moreover, numerous executive WM brain regions were linked with transfer of WM training benefits to the clinical symptom domain. Just as some studies (Gibson et al., 2014; Gibson et al., 2012; Gibson et al., 2013) have criticized WM training for only targeting short-term storage and not “secondary memory” (e.g., a cue-based controlled/strategic search of medial temporal lobe long-term memory stores (Chein et al., 2011) to access information displaced from primary WM) important to dual-process WM theories (Unsworth and Engle, 2007a, b), our results suggest that executive WM processes might also be good targets for WM training. Interestingly, our PCA analyses of WM test performance found that ADHD gains in “executive” WM were not related to anxiety or mood symptoms, thus possibly representing a clearer pathway towards WM ability improvement. Although several studies have attempted to train different types of executive functions in both ADHD and non-ADHD with some encouraging initial success 171, 172, none so far has specifically focused on exercises designed to train the executive aspects of WM (Nee et al., 2013). To guide future model building and neuroimaging research for WM training development, the supplemental material further details which brain regions were specifically associated with the PCA-derived “executive” WM change scores.
Conclusions
This study takes a meaningful step towards understanding the basis of WM training in ADHD. WM gains and non-cognitive clinical improvement are linked to ADHD brain activity augmentation in regions showing activation deficits and possible compensation-like neurobiological changes. The study sets the stage for future research to more conclusively determine whether clinical improvement not only is directly mediated through WM neural circuit change, but also if improved WM ability is the proximate cause of reduced clinical impairment. Alternatively, WM training might exert its effects on clinical functioning via changes in non-WM systems that might contribute to WM processing, but in actuality improve ADHD symptoms and disorder-related functional impairment in a coincidental fashion. While this might seem a minor, subtle distinction, it actually goes to the heart of WM training efficacy – Are clinical gains the product of having better WM itself, or does WM training somehow provide a means to alter dysfunctional neural systems more directly linked to disorder expression? The current findings also point towards the potential value in training specific executive aspects of WM processes as a likely avenue forward for optimizing outcome or achieving a mechanistic understanding of the neural basis of WM training effects in ADHD. The identification of specific neural correlates of ADHD clinical benefits help to validate WM training as a viable treatment approach, and exemplifies the potential utility of future clinical trials that integrate fMRI measurement as an informative clinical trial outcome measure.
Supplementary Material
Brain regions activated during the Encoding, Maintenance, and Retrieval conditions of the Sternberg working memory fMRI task for all n=36 ADHD and non-ADHD participants (p<.05 uncorrected).
Parametric effect of WM load on activation in Encoding, Maintenance, and Retrieval conditions of the Sternberg working memory fMRI task for all n=36 ADHD and non-ADHD participants (p<.05 uncorrected).
Brain regions where training-related changes in the brain’s response to task difficulty (i.e., parametric effect of WM load) correlated with improvement in the Cogmed Training change (p<.05 clusterwise significance threshold).
Table S1. Principal component analysis (PCA) with Varimax ro results showing two factors that capture 67% of the variance associated with changes between pre-training and training endpoint assessments in non-trained WM neuropsychological test performance.
Table S2. Brain regions where Post-training versus Pre-Training fMRI-measured parametric effects of working memory load significantly correlated with changes in the Cogmed Training Index score reflecting working memory gains on trained exercises
Table S3. Brain regions where Post-training versus Pre-Training fMRI measurements significantly correlated with PCA Factor 1 reflecting changes in non-trained, executive working memory “near transfer” neuropsychological test performance
Acknowledgments
This research was supported by R21HD061915 and by R01MH081969.
Footnotes
Financial Disclosures
The investigators have no conflicts of interest to declare.
Preliminary results were presented at the annual meeting of the Society for Biological Psychiatry in June 2013 in San Francisco, CA.
References
- Backman L, Nyberg L. Dopamine and training-related working-memory improvement. Neuroscience & Biobehavioral Reviews. 2013;37:2209–2219. doi: 10.1016/j.neubiorev.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cerebral Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR. Attention-Deficit Hyperactivity Disorder: A Clinical Workbook. 2. The Guilford Press; New York: 1998. [Google Scholar]
- Bayerl M, Dielentheis TF, Vucurevic G, Gesierich T, Vogel F, Fehr C, Stoeter P, Huss M, Konrad A. Disturbed brain activation during a working memory task in drug-naive adult patients with ADHD. Neuroreport. 2010;21:442–446. doi: 10.1097/WNR.0b013e328338b9be. [DOI] [PubMed] [Google Scholar]
- Beauregard M. Effect of mind on brain activity: evidence from neuroimaging studies of psychotherapy and placebo effect. Nord J Psychiatry. 2009;63:5–16. doi: 10.1080/08039480802421182. [DOI] [PubMed] [Google Scholar]
- Beck SJ, Hanson CA, Puffenberger SS, Benninger KL, Benninger WB. A controlled trial of working memory training for children and adolescents with ADHD. J Clin Child Adolesc Psychol. 2010;39:825–836. doi: 10.1080/15374416.2010.517162. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Jain U, Johnson SH, Tannock R. Effects of methylphenidate on working memory components: influence of measurement. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2007;48:872–880. doi: 10.1111/j.1469-7610.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Seidman LJ, Petty CR, Fried R, Doyle AE, Cohen DR, Kenealy DC, Faraone SV. Effects of stimulant medication on neuropsychological functioning in young adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69:1150–1156. doi: 10.4088/jcp.v69n0715. [DOI] [PubMed] [Google Scholar]
- Brown TE. Brown Attention-Deficit Disorder Scales for Adolescents and Adults. Harcourt Assessment; San Antonio, TX: 2001. [Google Scholar]
- Buhner M, König CJ, Prick M, Krumm S. Working memory dimensions as differential predictors of the speed and error aspect of multitasking performance. Human Performance. 2006:19. [Google Scholar]
- Burgess GC, Depue BE, Ruzic L, Willcutt EG, Du YP, Banich MT. Attentional control activation relates to working memory in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;67:632–640. doi: 10.1016/j.biopsych.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschkuehl M, Jaeggi SM, Jonides J. Neuronal effects following working memory training. Dev Cogn Neurosci. 2012;2(Suppl 1):S167–179. doi: 10.1016/j.dcn.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko A, Bedard AC, Marks DJ, Feirsen N, Uderman JZ, Chimiklis A, Rajwan E, Cornwell M, Anderson L, Zwilling A, Ramon M. A randomized clinical trial of Cogmed Working Memory Training in school-age children with ADHD: a replication in a diverse sample using a control condition. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2014;55:247–255. doi: 10.1111/jcpp.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko A, Feirsen N, Bedard AC, Marks D, Uderman JZ, Chimiklis A. Cogmed Working Memory Training for youth with ADHD: a closer examination of efficacy utilizing evidence-based criteria. J Clin Child Adolesc Psychol. 2013;42:769–783. doi: 10.1080/15374416.2013.787622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Moore AB, Conway AR. Domain-general mechanisms of complex working memory span. Neuroimage. 2011;54:550–559. doi: 10.1016/j.neuroimage.2010.07.067. [DOI] [PubMed] [Google Scholar]
- Cortese S, Ferrin M, Brandeis D, Buitelaar J, Daley D, Dittmann RW, Holtmann M, Santosh P, Stevenson J, Stringaris A, Zuddas A, Sonuga-Barke EJ, European AGG. Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54:164–174. doi: 10.1016/j.jaac.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Smith AB, Barrett N, Giampietro V, Brammer M, Simmons A, Rubia K. Drug-specific laterality effects on frontal lobe activation of atomoxetine and methylphenidate in attention deficit hyperactivity disorder boys during working memory. Psychol Med. 2014;44:633–646. doi: 10.1017/S0033291713000676. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Backman L, Neely AS, Nyberg L. Training of the executive component of working memory: subcortical areas mediate transfer effects. Restor Neurol Neurosci. 2009;27:405–419. doi: 10.3233/RNN-2009-0492. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Dahlin KIE. Working memory training and the effect on mathematical achievement in chidilren with attention deficits and special needs. Journal of Education and Learning. 2013;2:118–133. [Google Scholar]
- Egeland J, Aarlien AK, Saunes BK. Few effects of far transfer of working memory training in ADHD: a randomized controlled trial. PLoS ONE. 2013;8:e75660. doi: 10.1371/journal.pone.0075660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Carullo JJ, Collins KW. Individual differences in the role of working memory in comprehension and following directions. Journal of Educational Research. 1991:84. [Google Scholar]
- Epstein JN, Conners CK, Hervey AS, Tonev ST, Arnold LE, Abikoff HB, Elliott G, Greenhill LL, Hechtman L, Hoagwood K, Hinshaw SP, Hoza B, Jensen PS, March JS, Newcorn JH, Pelham WE, Severe JB, Swanson JM, Wells K, Vitiello B, Wigal T, Group MTACS. Assessing medication effects in the MTA study using neuropsychological outcomes. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2006;47:446–456. doi: 10.1111/j.1469-7610.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- Evans SW, Owens JS, Bunford N. Evidence-Based Psychosocial Treatments for Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. J Clin Child Adolesc Psychol. 2013 doi: 10.1080/15374416.2013.850700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett J, Thomas J, Cote F, Levesque J, Michaud D. Cognitive effects of psychostimulant medication in hyperactive children. Child Psychiatry Hum Dev. 1991;22:79–87. doi: 10.1007/BF00707786. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Schweitzer JB, Cortes CR, Tagamets MA, Windsor TA, Reeves GM, Gullapalli R. Working memory in attention deficit/hyperactivity disorder is characterized by a lack of specialization of brain function. PLoS ONE. 2011;6:e27240. doi: 10.1371/journal.pone.0027240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18:543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Dunning DL, Holmes J. Cogmed training: Let’s be realistic about intervention research. Journal of Applied Research in Memory and Cognition. 2012;1:201–203. [Google Scholar]
- Gathercole SE, Durling E, Evans M, Jeffcock S, Stone S. Working memory deficits in laboratory analogues of activities. Applied Cognitive Psychology. 2008:22. [Google Scholar]
- Gibson BS, Gondoli DM, Johnson AC, Robison MK. Recall initiation strategies must be controlled in training studies that use immediate free recall tasks to measure the components of working memory capacity across time. Child Neuropsychol. 2014;20:539–556. doi: 10.1080/09297049.2013.826185. [DOI] [PubMed] [Google Scholar]
- Gibson BS, Gondoli DM, Johnson AC, Steeger CM, Dobrzenski BA, Morrissey RA. Component analysis of verbal versus spatial working memory training in adolescents with ADHD: a randomized, controlled trial. Child Neuropsychol. 2011;17:546–563. doi: 10.1080/09297049.2010.551186. [DOI] [PubMed] [Google Scholar]
- Gibson BS, Gondoli DM, Johnson AC, Steeger CM, Morrissey RA. The future promise of Cogmed working memory training. Journal of Applied Research in Memory and Cognition. 2012;1:214–216. doi: 10.1016/j.jarmac.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BS, Gondoli DM, Kronenberger WG, Johnson AC, Steeger CM, Morrissey RA. Exploration of an adaptive training regimen that can target the secondary memory component of working memory capacity. Mem Cognit. 2013;41:726–737. doi: 10.3758/s13421-013-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SA, Chaban P, Martinussen R, Goldberg R, Gotlieb H, Kronitz R, Hockenberry M, Tannock R. Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD: a randomized controlled trial. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2012;53:1277–1284. doi: 10.1111/j.1469-7610.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- Green CT, Long DL, Green D, Iosif AM, Dixon JF, Miller MR, Fassbender C, Schweitzer JB. Will working memory training generalize to improve off-task behavior in children with attention-deficit/hyperactivity disorder? Neurotherapeutics. 2012;9:639–648. doi: 10.1007/s13311-012-0124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropper RJ, Gotlieb H, Kronitz R, Tannock R. Working memory training in college students with ADHD or LD. J Atten Disord. 2014;18:331–345. doi: 10.1177/1087054713516490. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Johnson LG. Medications do not necessarily normalize cognition in ADHD patients. J Atten Disord. 2008;11:459–469. doi: 10.1177/1087054707305314. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Jr, MacLachlan A, Soderling E, Lottenberg S, Buchsbaum MS. Regional glucose metabolic changes after learning a complex visuospatial/motor task: a positron emission tomographic study. Brain Res. 1992;570:134–143. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- Hale TS, Bookheimer S, McGough JJ, Phillips JM, McCracken JT. Atypical brain activation during simple & complex levels of processing in adult ADHD: an fMRI study. J Atten Disord. 2007;11:125–140. doi: 10.1177/1087054706294101. [DOI] [PubMed] [Google Scholar]
- Hambrick DZ, Oswald FL, Darowski ES, Rench TA, Brou R. Predictors of multitasking performance in a synthetic work paradigm. Applied Cognitive Psychology. 2010:24. [Google Scholar]
- Hempel A, Giesel FL, Garcia Caraballo NM, Amann M, Meyer H, Wustenberg T, Essig M, Schroder J. Plasticity of cortical activation related to working memory during training. Am J Psychiatry. 2004;161:745–747. doi: 10.1176/appi.ajp.161.4.745. [DOI] [PubMed] [Google Scholar]
- Hodgson K, Hutchinson AD, Denson L. Nonpharmacological treatments for ADHD: a meta-analytic review. J Atten Disord. 2014;18:275–282. doi: 10.1177/1087054712444732. [DOI] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos-Quiroga JA, Barba E, Bielsa A, Tremols V, Rovira M, Soliva JC, Casas M, Bulbena A, Tobena A, Vilarroya O. Training-induced neuroanatomical plasticity in ADHD: a tensor-based morphometric study. Hum Brain Mapp. 2011;32:1741–1749. doi: 10.1002/hbm.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Tremols V, Gispert JD, Guitart M, Fauquet J, Rovira M, Bielsa A, Soliva JC, Tomas X, Bulbena A, Ramos-Quiroga A, Casas M, Tobena A, Vilarroya O. Enhanced neural activity in frontal and cerebellar circuits after cognitive training in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2010;31:1942–1950. doi: 10.1002/hbm.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J, Gathercole SE, Dunning DL. Poor working memory: impact and interventions. Adv Child Dev Behav. 2010;39:1–43. doi: 10.1016/b978-0-12-374748-8.00001-9. [DOI] [PubMed] [Google Scholar]
- Hovik KT, Saunes BK, Aarlien AK, Egeland J. RCT of working memory training in ADHD: long-term near-transfer effects. PLoS ONE. 2013;8:e80561. doi: 10.1371/journal.pone.0080561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E. The neural basis of semantic cognition: converging evidence from neuropsychology, neuroimaging and TMS. Cortex. 2013;49:611–625. doi: 10.1016/j.cortex.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Jolles DD, Grol MJ, Van Buchem MA, Rombouts SA, Crone EA. Practice effects in the brain: Changes in cerebral activation after working memory practice depend on task demands. Neuroimage. 2010;52:658–668. doi: 10.1016/j.neuroimage.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: an experience-sampling study of working memory and executive control in daily life. Psychol Sci. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Kasper LJ, Alderson RM, Hudec KL. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin Psychol Rev. 2012;32:605–617. doi: 10.1016/j.cpr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends Cogn Sci. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Training of Working Memory and Attention. In: Posner MI, editor. Cognitive Neuroscience of Attention. The Guilford Press; New York, NY: 2012. pp. 475–486. [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, Gillberg CG, Forssberg H, Westerberg H. Computerized training of working memory in children with ADHD--a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- Ko CH, Yen JY, Yen CF, Chen CS, Lin WC, Wang PW, Liu GC. Brain activation deficit in increased-load working memory tasks among adults with ADHD using fMRI. European Archives of Psychiatry & Clinical Neuroscience. 2013;263:561–573. doi: 10.1007/s00406-013-0407-2. [DOI] [PubMed] [Google Scholar]
- Kobel M, Bechtel N, Weber P, Specht K, Klarhofer M, Scheffler K, Opwis K, Penner IK. Effects of methylphenidate on working memory functioning in children with attention deficit/hyperactivity disorder. Eur J Paediatr Neurol. 2009;13:516–523. doi: 10.1016/j.ejpn.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS. ADHD and working memory: the impact of central executive deficits and exceeding storage/rehearsal capacity on observed inattentive behavior. J Abnorm Child Psychol. 2010;38:149–161. doi: 10.1007/s10802-009-9357-6. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS, Alderson RM. Working memory deficits and social problems in children with ADHD. J Abnorm Child Psychol. 2011;39:805–817. doi: 10.1007/s10802-011-9492-8. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Monuteaux MC, Seidman LJ. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev Neurosci. 2009;31:36–49. doi: 10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Buss JL, Bolster RB, de Gervai PD, Woods-Frohlich L, Summers R, Clancy CA, Chudley AE, Longstaffe S. Comparison of spatial working memory in children with prenatal alcohol exposure and those diagnosed with ADHD; A functional magnetic resonance imaging study. J Neurodev Disord. 2012;4:12. doi: 10.1186/1866-1955-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Massat I, Slama H, Kavec M, Linotte S, Mary A, Baleriaux D, Metens T, Mendlewicz J, Peigneux P. Working memory-related functional brain patterns in never medicated children with ADHD. PLoS ONE. 2012;7:e49392. doi: 10.1371/journal.pone.0049392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- Melby-Lervag M, Hulme C. Is working memory training effective? A meta-analytic review. Dev Psychol. 2013;49:270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E, Buckner JC. Working memory training for chilidren with attention problems or hyperactivity: A school-based pilot study. School Mental Health. 2010;2:202–208. [Google Scholar]
- Morrison AB, Chein JM. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon Bull Rev. 2011;18:46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J. A meta-analysis of executive components of working memory. Cerebral Cortex. 2013;23:264–282. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer AC, Fink A. Intelligence and neural efficiency. Neuroscience & Biobehavioral Reviews. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, Inc. Research Coaching manual: Cogmed Working Memory Training. Pearson Inc; 2014. [Google Scholar]
- Pietrzak RH, Mollica CM, Maruff P, Snyder PJ. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neuroscience & Biobehavioral Reviews. 2006;30:1225–1245. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Krauel K, Hinrichs H, Fischer J, Malecki U, Schuetze H, Wolff S, Jansen O, Duezel E, Baving L. Methylphenidate does not improve interference control during a working memory task in young patients with attention-deficit hyperactivity disorder. Brain Res. 2011;1388:56–68. doi: 10.1016/j.brainres.2011.02.075. [DOI] [PubMed] [Google Scholar]
- Qi XL, Constantinidis C. Neural changes after training to perform cognitive tasks. Behav Brain Res. 2013;241:235–243. doi: 10.1016/j.bbr.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabipour S, Raz A. Training the brain: fact and fad in cognitive and behavioral remediation. Brain Cogn. 2012;79:159–179. doi: 10.1016/j.bandc.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Raiker JS, Rapport MD, Kofler MJ, Sarver DE. Objectively-measured impulsivity and attention-deficit/hyperactivity disorder (ADHD): testing competing predictions from the working memory and behavioral inhibition models of ADHD. J Abnorm Child Psychol. 2012;40:699–713. doi: 10.1007/s10802-011-9607-2. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Alderson RM, Kofler MJ, Sarver DE, Bolden J, Sims V. Working memory deficits in boys with attention-deficit/hyperactivity disorder (ADHD): the contribution of central executive and subsystem processes. J Abnorm Child Psychol. 2008;36:825–837. doi: 10.1007/s10802-008-9215-y. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, Alderson RM. Hyperactivity in boys with attention-deficit/hyperactivity disorder (ADHD): a ubiquitous core symptom or manifestation of working memory deficits? J Abnorm Child Psychol. 2009;37:521–534. doi: 10.1007/s10802-008-9287-8. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Orban SA, Kofler MJ, Friedman LM. Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clin Psychol Rev. 2013;33:1237–1252. doi: 10.1016/j.cpr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Redick TS, Shipstead Z, Harrison TL, Hicks KL, Fried DE, Hambrick DZ, Kane MJ, Engle RW. No evidence of intelligence improvement after working memory training: a randomized, placebo-controlled study. J Exp Psychol Gen. 2013;142:359–379. doi: 10.1037/a0029082. [DOI] [PubMed] [Google Scholar]
- Rhodes SM, Coghill DR, Matthews K. Acute neuropsychological effects of methylphenidate in stimulant drug-naive boys with ADHD II--broader executive and non-executive domains. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2006;47:1184–1194. doi: 10.1111/j.1469-7610.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- Risser MG, Bowers TG. Cognitive and neuropsychological characteristics of attention deficit hyperactivity disorder children receiving stimulant medications. Percept Mot Skills. 1993;77:1023–1031. doi: 10.2466/pms.1993.77.3.1023. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, Radua J. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry. 2014;76:616–628. doi: 10.1016/j.biopsych.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge KJ, van den Bos W, McClure SM, Schweitzer JB. Training cognition in ADHD: current findings, borrowed concepts, and future directions. Neurotherapeutics. 2012;9:542–558. doi: 10.1007/s13311-012-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweren LJ, de Zeeuw P, Durston S. MR imaging of the effects of methylphenidate on brain structure and function in attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23:1151–1164. doi: 10.1016/j.euroneuro.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PJ, Buschkuehl M, Jaeggi S, Jonides J. Cognitive training for ADHD: The importance of individual differences. Journal of Applied Research in Memory and Cognition. 2012;1:204–205. [Google Scholar]
- Shallice T. Multiple levels of control processes. In: Umilta C, Moscovitch M, editors. Conscious and nonconscious information processing. Attention and Performance. MIT Press; Cambridge, MA: 1994. pp. 395–420. [Google Scholar]
- Shallice T. The fractionation of supervisory control. In: Gazzaniga MS, editor. The cognitive neurosciences. 2004. pp. 943–956. [Google Scholar]
- Sheridan MA, Hinshaw S, D’Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1357–1366. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- Shipstead Z, Hicks KL, Engle RW. Cogmed working memory training: Does the evidence support the claims? Journal of Applied Research in Memory and Cognition. 2012a;1:185–193. [Google Scholar]
- Shipstead Z, Redick TS, Engle RW. Is working memory training effective? Psychol Bull. 2012b;138:628–654. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, Stevenson J, Danckaerts M, van der Oord S, Dopfner M, Dittmann RW, Simonoff E, Zuddas A, Banaschewski T, Buitelaar J, Coghill D, Hollis C, Konofal E, Lecendreux M, Wong IC, Sergeant J, European AGG. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275–289. doi: 10.1176/appi.ajp.2012.12070991. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Frontal lobes and attention: processes and networks, fractionation and integration. J Int Neuropsychol Soc. 2006;12:261–271. doi: 10.1017/S1355617706060358. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Kawashima R. Effects of working memory training on cognitive functions and neural systems. Rev Neurosci. 2010;21:427–449. doi: 10.1515/revneuro.2010.21.6.427. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Connors L, Shuster J, Knezevic B, Parks S. Review of cognitive, cognitive-behavioral, and neural-based interventions for Attention-Deficit/Hyperactivity Disorder (ADHD) Clin Psychol Rev. 2008;28:801–823. doi: 10.1016/j.cpr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: active maintenance in primary memory and controlled search from secondary memory. Psychol Rev. 2007a;114:104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. On the division of short-term and working memory: an examination of simple and complex span and their relation to higher order abilities. Psychol Bull. 2007b;133:1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Vance A, Silk TJ, Casey M, Rinehart NJ, Bradshaw JL, Bellgrove MA, Cunnington R. Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Mol Psychiatry. 2007;12:826–832. 793. doi: 10.1038/sj.mp.4001999. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Loo SK, Carlson CL, McBurnett K, Lahey BB. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Schaie KW. Cognitive training and plasticity: theoretical perspective and methodological consequences. Restor Neurol Neurosci. 2009;27:375–389. doi: 10.3233/RNN-2009-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Plichta MM, Sambataro F, Fallgatter AJ, Jacob C, Lesch KP, Herrmann MJ, Schonfeldt-Lecuona C, Connemann BJ, Gron G, Vasic N. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30:2252–2266. doi: 10.1002/hbm.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CG, Stevens MC. The effects of stimulant medication on working memory functional connectivity in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:458–466. doi: 10.1016/j.biopsych.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workgroup, R.D.C; Health, N.I.o.M, editor. Working Memory: Workshop Proceedings. Bethesda, MD: 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Brain regions activated during the Encoding, Maintenance, and Retrieval conditions of the Sternberg working memory fMRI task for all n=36 ADHD and non-ADHD participants (p<.05 uncorrected).
Parametric effect of WM load on activation in Encoding, Maintenance, and Retrieval conditions of the Sternberg working memory fMRI task for all n=36 ADHD and non-ADHD participants (p<.05 uncorrected).
Brain regions where training-related changes in the brain’s response to task difficulty (i.e., parametric effect of WM load) correlated with improvement in the Cogmed Training change (p<.05 clusterwise significance threshold).
Table S1. Principal component analysis (PCA) with Varimax ro results showing two factors that capture 67% of the variance associated with changes between pre-training and training endpoint assessments in non-trained WM neuropsychological test performance.
Table S2. Brain regions where Post-training versus Pre-Training fMRI-measured parametric effects of working memory load significantly correlated with changes in the Cogmed Training Index score reflecting working memory gains on trained exercises
Table S3. Brain regions where Post-training versus Pre-Training fMRI measurements significantly correlated with PCA Factor 1 reflecting changes in non-trained, executive working memory “near transfer” neuropsychological test performance