Abstract
Background
A health utility value represents an individual’s preference for living in a specific health state and is used in cost-utility analyses. This study investigates the impact of continuing medical therapy on health utility outcomes in patients with chronic rhinosinusitis (CRS).
Methods
The Medical Outcomes Study Short Form-6D (SF-6D) was administered to patients prospectively enrolled in a longitudinal study examining treatment outcomes for CRS. Patients were prescribed robust, initial medical therapy and then elected to continue with medical therapy (n=40) or undergo endoscopic sinus surgery (ESS), followed by medical therapy (n=152). Patients observed through treatment crossover to ESS were also evaluated (n=20). Health utility values (SF-6D) were generated at baseline, 6-months, and 12-months follow-up for both cohorts and evaluated using repeated measures ANOVA.
Results
Treatment crossover patients were found to have a significantly higher prevalence of previous sinus surgery compared to medical management (χ2=6.91; p=0.009) and surgical intervention (χ2=8.11; p=0.004) subgroups. Mean baseline utility value for the medical therapy cohort was significantly better compared to the ESS cohort (0.76[0.12] versus 0.70[0.15]; p=0.023). Significant improvement in health utility was reported in the ESS cohort (F(2)=37.69; p<0.001), while values remained stable, without significant improvement, in both the medical therapy cohort (F(2)=0.03; p=0.967) and treatment crossover cohort (F(2)=2.36; p=0.115).
Conclusions
Patients electing continued medical management report better baseline health utility compared to patients electing ESS. Patients electing ESS demonstrate significant improvement in health utility while those electing continued medical management demonstrate stable health utility over 12 months.
Index Medicus Key Words: Sinusitis, endoscopy, chronic disease, quality of life, therapeutics, medication therapy management, utility, health utility, cost-effectiveness
INTRODUCTION
One of the primary factors driving health reform in the United States is the unsustainable yearly increase in healthcare expenditure, currently estimated to be 4% of the gross domestic product.[1] In this climate, health care providers are challenged to critically evaluate the risk and cost effectiveness of medical and surgical interventions. Economic analyses of quality of life (QOL) outcomes can help decision-makers best allocate limited healthcare resources toward those who would most benefit.
A health state utility value quantifies an individual’s perception of his or her current health. These values are used in identifying optimal cost-effective treatments for the management of chronic disease.[2, 3] Utility values are useful because they allow the impacts of different diseases to be compared using a common metric.[4] Prior studies have shown patients with chronic rhinosinusitis (CRS) report baseline utility values similar to patients with end stage renal disease on hemodialysis and moderate asthma.[5, 6]
Up to 50% of patients with CRS will fail to improve after initial medical management and will be faced with a decision: to continue with medical therapy or to pursue endoscopic sinus surgery (ESS).[7, 8] This decision represents a balance of possible benefits, risks and monetary concerns. Previous studies demonstrate improved health utility in patients with refractory CRS after endoscopic sinus surgery (ESS).[6, 9] The literature also supports the long-term cost-effectiveness of ESS over continued medical management in these patients.[1, 5] However, no prior studies have reported the specific trend of health utility values in patients with CRS who elect continued medical management instead of surgical intervention. An improved understanding of the longitudinal health state utility outcomes in patients choosing to continue with medical therapy would aid in decision-making.
The primary purpose of this study is to measure baseline and follow-up utility values using the SF-6D instrument in patients with CRS who elect continued medical management. Data for patients who elected surgical management was also collected for comparison. We hypothesize that patients who elect continued medical management for CRS have higher baseline health utility when compared to patients who elect surgical management for CRS. This study expands on previously published data to characterize health utility in patients electing medical management for CRS and provides a basis for future economic modeling in cost-effectiveness research.
MATERIAL and METHODS
Patient population
Study patients (≥18 years of age) were recruited from the Oregon Sinus Center at Oregon Health & Science University (OHSU, Portland, OR), Stanford University (Palo Alto, CA), the Medical University of South Carolina (MUSC, Charleston, SC) and the University of Calgary (Calgary, Alberta, Canada) as part of a continuing, observational, prospective cohort investigation to assess outcomes of various treatment modalities for CRS. Preliminary findings from this cohort study are readily available through published literature.[10–14] All patients were diagnosed with medically refractory CRS and met criteria endorsed by the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS2012) and the American Academy of Otolaryngology.[15,16] Refractory CRS was defined as patients having persistent symptoms of CRS despite maximal medical therapy and were considered candidates for ESS. For this study, maximal medical therapy included at least one course (>14-days) of broad spectrum or culture directed antibiotic therapy and at least one course of topical corticosteroid (>21-days) or a 5-day course of systemic corticosteroid therapy.
Patients were interviewed during an initial enrollment meeting and considered study participants after providing informed consent in English and agreeing to complete all baseline study evaluations. The Institutional Review Board at each academic enrollment site granted study approval and annual review of protocol safety, potential adverse events, and enrollment progression. Central study coordination was conducted at OHSU (eIRB #7198) by the Principal Investigator (TLS). Participants were assured study involvement was completely voluntary and in no way altered the standard of care for their chosen treatment modality. Study participants were followed for 12-month duration with observational, follow-up evaluations at 6 month intervals, either during routine, physician-directed clinical appointments or via follow-up mailings using the United States Postal Service with self-addressed return envelopes.
Exclusion criteria
Due to differences in disease etiologies and potential variability in medical treatment regimens study participants with exacerbations of other comorbid conditions including: recurrent acute rhinosinusitis, cystic fibrosis / ciliary dyskinesia, autoimmune disorders, or steroid dependency (eg. asthma, sinusitis) were excluded. Study participants were also excluded if they had not yet entered the initial follow-up appointment window (≤6 months) or completed baseline and follow-up evaluations at the appropriate time intervals.
Treatment modality
Prior to any study enrollment meeting and following physician directed counseling patients selfselected subsequent treatment. Patients elected to either continue physician-directed medical management or to pursue endoscopic sinus surgery (ESS) directed by the intraoperative clinical judgment of the enrolling physician at each site. Study patients were categorized into one of three treatment arms including a medical management cohort, surgical treatment cohort, and a treatment crossover cohort of patients initially electing medical therapy who elected to change treatment modality to include ESS at some point during the duration of the study period. Surgical intervention consisted of either unilateral or bilateral maxillary antrostomy, partial or total ethmoidectomy, sphenoidotomy, middle turbinate resection or inferior turbinate reduction, septoplasty, or frontal sinusotomy procedures with judicious use of image guidance.
Clinical measures of disease severity
Standard clinical measures of disease severity, collected during initial clinical evaluations, were used simultaneously for investigational purposes. High resolution computed tomography (CT) with bone and tissue windows was utilized to evaluate sinonasal disease severity using 1.0mm contiguous images in both sagittal and coronal planes. Images were also staged by each enrolling physician in accordance with the semi-quantitative Lund-Mackay bilateral scoring system (score range: 0–24) which quantifies the severity of image opacification in the maxillary, ethmoidal, sphenoidal, ostiomeatal complex, and frontal sinus regions using a Likert scale.[17] Follow-up CT evaluations were not routinely collected per the standard of care.
The paranasal sinuses were also evaluated bilaterally using rigid, fiberoptic endoscopes (SCB Xenon 175, Karl Storz, Tuttlingen, Germany) by each enrolling physician. Endoscopic exams were staged by the enrolling physician using the bilateral Lund-Kennedy scoring system (score range: 0–20) that quantifies pathologic states within the paranasal sinuses including the severity of polyposis, discharge, edema, scarring, and crusting on a Likert scale.[18] Endoscopic examinations were collected during concurrent 6-month intervals when feasible during standard clinic follow-up visitations. Higher scores on both staging systems reflect worse disease severity. Enrolling physicians were blinded to all survey responses during the study duration.
Health state utility values
Study participants completed the Medical Outcomes Study Short Form-6D (SF-6D) during each study evaluation time point as part of a larger total battery of evaluative instruments. The SF-6D is a subset of questions extracted from the longer SF-36 survey and includes general-health survey inquiries measuring physical functioning, role limitations, social functioning, bodily pain, mental health, and vitality using standard Likert scales. Health states measured by SF-6D item scores were transformed into standardized health utility values using a weighted algorithm described by Brazier et al. and used with permission from the Department of Health Economics and Decision Science at the University of Sheffield, Sheffield, United Kingdom.[19] This algorithm determines a normalized value that an individual patient places on their particular health state described using the SF-6D questionnaire. Health utility values range from 0.3 to 1.0 where lower values represent lower/worse valuations of health state and 1.0 representing perfect health. A minimal clinically important difference over time of at least 0.03 for SF-6D values has been previously defined.[20]
Missed productivity
During each study evaluation time point participants in both treatment arms were also asked to recall the number of days (out of the previous 90 days) that were missed or impacted due to CRS related symptoms (eg. missed work days, school days, or volunteer time).
Data management and statistical analyses
Study data was stripped of all patient health information and manually entered into a relational database (Microsoft Access; Microsoft Corp., Redmond, WA). Statistical analyses were completed using SPSS v.22 statistical software (IBM Corp., Armonk, NY) while SF-6D values were estimated using SPSS syntax provided by the University of Sheffield. Baseline study population characteristics, clinical measures of disease severity, disease-specific QOL scores, and SF-6D values were evaluated descriptively and data normality was verified for all continuous measures using graphical analysis. Mean follow-up (months) for the medical management and treatment crossover subgroups was determined from the original enrollment date whereas follow-up from the surgical group was calculated from the date of sinus surgery. All statistical comparisons utilized complete case analysis.
Simple analysis of variance (ANOVA) and Kruskall-Wallis omnibus tests were used to evaluate between treatment group comparisons for all continuous variables with adjustments for pairwise multiple comparisons when significant. Chi-square (χ2) and Fishers exact testing was used to evaluate differences in the prevalence of comorbid conditions and patient characteristics between treatment groups. Two-tailed matched pairs t-tests or Wilcoxon signed rank tests were used to evaluate changes in SF-6D values between study time points. Two-tailed Spearman’s rank correlation coefficients (Rs) were utilized to evaluate correlation between SF-6D values and measures of diseases severity and productivity. Repeated measures ANOVA, with Greenhouse-Geisser corrections to evaluate level III within-subject differences over time, were used to evaluate significant improvement over time across each distinct treatment modality. All statistical comparisons assumed a 0.050 error probability.
RESULTS
Final study cohort and baseline comparisons
The final study cohort was comprised of 212 study participants who met inclusion criteria and were enrolled between March 2011 and November 2013. Baseline characteristics and medical comorbidities are described in Table 1 for the medical management (n=40; 19%), surgical intervention (n=152; 72%), and treatment crossover (n=20; 9%) subgroups. Mean total follow-up times for 6-month and 12-month interval evaluations were 5.7[1.2] and 11.8[1.4] months, respectively. Medical management and surgical intervention subgroups were followed for similar average times at the 6-month (5.8[0.9] vs. 5.7[1.1]; p=0.490) and 12-month (12.1[1.3] vs. 11.8[1.4]; p=0.213) evaluations. Treatment crossover participants were followed for approximately 12 months during which 11 patients (55%) elected ESS within the first 6 months of follow-up and 9 patients (45%) elected ESS between 6 and 12 months of follow-up. Participants electing treatment crossover to ESS were found to have a significantly higher prevalence of previous sinus surgery compared to both the medical management (χ2=6.91; p=0.009) and the surgical intervention (χ2=8.11; p=0.004) subgroups after adjusting for pairwise multiple comparisons. Similarly, treatment crossover participants were found to have a significantly smaller prevalence of deviated septum compared to the surgical intervention group (χ2=4.23; p=0.040).
Table 1.
Comparison of baseline characteristics and medical comorbidity for CRS patients electing medical management, surgical intervention, or treatment crossover
| Medical management (n=40) |
Surgical intervention (n=152) |
Treatment crossover (n=20) |
|||||
|---|---|---|---|---|---|---|---|
| Baseline Characteristics: | Mean [SD] | N (%) | Mean [SD] | N (%) | Mean [SD] | N (%) | p-value |
| Age (years) | 54.1 [13.0] | 53.3 [14.6] | 57.0 [15.0] | 0.563 | |||
| Male | 21 (53%) | 76 (50%) | 12 (60%) | ---- | |||
| Female | 19 (48%) | 76 (50%) | 8 (40%) | 0.694 | |||
| Previous sinus surgery | 20 (50%) | 78 (51%) | 17 (85%) | 0.015 | |||
| Nasal polyposis | 17 (43%) | 56 (37%) | 10 (50%) | 0.468 | |||
| Deviated septum | 11 (28%) | 67 (44%) | 4 (20%) | 0.031 | |||
| Turbinate hypertrophy | 3 (8%) | 24 (16%) | 1 (5%) | 0.202 | |||
| Asthma | 12 (30%) | 47 (31%) | 7 (35%) | 0.920 | |||
| Aspirin sensitivity | 5 (13%) | 10 (7%) | 2 (10%) | 0.444 | |||
| Allergies (history) | 8 (20%) | 24 (16%) | 6 (30%) | 0.277 | |||
| Allergies (mRAST confirmed) | 13 (33%) | 56 (37%) | 8 (40%) | 0.824 | |||
| Depression | 4 (10%) | 27 (18%) | 2 (10%) | 0.373 | |||
| Current smoker | 0 (0%) | 3 (2%) | 0 (0%) | 0.546 | |||
| Alcohol consumption | 23 (58%) | 78 (51%) | 8 (40%) | 0.441 | |||
SD, standard deviation; mRAST, modified radioallergosorbent testing;
Mean differences in clinical measure of disease severity, health utility values, and missed days of productivity between subgroups electing medical management, surgical intervention, and treatment crossover are compared in Table 2. Participants initially electing surgical intervention reported significantly worse average utility values (p=0.023) and greater average productivity days lost (p=0.009) due to symptoms of CRS compared to the medical management group after adjusting for pairwise multiple comparisons. Treatment crossover participants also reported significantly greater average productivity days lost compared to the medical management group (p=0.011). Mean baseline SF-6D values were compared across baseline characteristics and comorbid conditions between treatment modality (Table 3).
Table 2.
Comparison of baseline clinical measure of disease severity, health state utility values, missed days of productivity for across treatment modality for chronic rhinosinusitis
| Medical management (n=40) |
Surgical intervention (n=152) |
Treatment crossover (n=20) |
||
|---|---|---|---|---|
| Clinical measures of disease severity: | Mean [SD] | Mean [SD] | Mean [SD] | p-value |
| Computed tomography (CT) score | 13.3 [6.7] | 13.1 [5.9] | 13.0 [7.1] | 0.985 |
| Endoscopy score | 6.6 [3.9] | 6.5 [3.7] | 8.4 [5.1] | 0.293 |
| Health state utility: | ||||
| SF-6D value | 0.76 [0.12] | 0.70 [0.15] | 0.69 [0.14] | 0.069 |
| Productivity: | ||||
| Missed days (out of past 90) | 4.2 [13.7] | 9.6 [20.5] | 8.3 [12.9] | 0.017 |
SD, standard deviation;
Table 3.
Comparison of mean baseline SF-6D health state utility values between treatment modality across patient characteristics
| Medical management (n=40) |
Surgical intervention (n=152) |
Treatment crossover (n=20) |
||
|---|---|---|---|---|
| Baseline Characteristics: | Mean [SD] | Mean [SD] | Mean [SD] | p-value |
| Age: (years) | ||||
| 18–40 (n=38) | 0.76 [0.14] | 0.69 [0.14] | 0.77 [0.04] | 0.318 |
| 41–60 (n=95) | 0.73 [0.13] | 0.69 [0.14] | 0.63 [0.13] | 0.146 |
| 61–86 (n=59) | 0.80 [0.07] | 0.73 [0.15] | 0.73 [0.14] | 0.316 |
| Gender: | ||||
| Male | 0.76 [0.12] | 0.73 [0.15] | 0.73 [0.14] | 0.666 |
| Female | 0.75 [0.13] | 0.67 [0.14] | 0.65 [0.13] | 0.067 |
| Previous sinus surgery: | ||||
| Present | 0.72 [0.12] | 0.69 [0.16] | 0.72 [0.14] | 0.749 |
| Absent | 0.80 [0.11] | 0.71 [0.13] | 0.69 [0.14] | 0.042 |
| Nasal polyposis: | ||||
| Present | 0.75 [0.14] | 0.72 [0.15] | 0.68 [0.15] | 0.563 |
| Absent | 0.77 [0.10] | 0.69 [0.14] | 0.71 [0.12] | 0.036 |
| Deviated septum: | ||||
| Present | 0.72 [0.11] | 0.69 [0.14] | 0.69 [0.14] | 0.680 |
| Absent | 0.77 [0.12] | 0.71 [0.15] | 0.70 [0.14] | 0.122 |
| Turbinate hypertrophy: | ||||
| Present | 0.63 [0.05] | 0.66 [0.14] | 0.8 [--] | 0.573 |
| Absent | 0.77 [0.12] | 0.71 [0.14] | 0.69 [0.14] | 0.054 |
| Asthma: | ||||
| Present | 0.73 [0.16] | 0.69 [0.16] | 0.68 [0.15] | 0.614 |
| Absent | 0.77 [0.10] | 0.71 [0.14] | 0.71 [0.13] | 0.078 |
| Aspirin sensitivity: | ||||
| Present | 0.85 [0.09] | 0.63 [0.11] | 0.76 [0.05] | 0.021 |
| Absent | 0.75 [0.12] | 0.71 [0.15] | 0.69 [0.14] | 0.268 |
| Allergies (history): | ||||
| Present | 0.81 [0.14] | 0.70 [0.14] | 0.72 [0.13] | 0.192 |
| Absent | 0.74 [0.11] | 0.70 [0.15] | 0.69 [0.14] | 0.221 |
| Allergies (mRAST confirmed): | ||||
| Present | 0.79 [0.11] | 0.72 [0.15] | 0.64 [0.16] | 0.061 |
| Absent | 0.74 [0.12] | 0.69 [0.15] | 0.73 [0.11] | 0.271 |
| Depression: | ||||
| Present | 0.68 [0.14] | 0.60 [0.09] | 0.57 [0.07] | 0.283 |
| Absent | 0.77 [0.12] | 0.72 [0.15] | 0.71 [0.13] | 0.263 |
| Current smoker: | ||||
| Present | 0.0 [0.0] | 0.68 [0.10] | ---- | ---- |
| Absent | 0.76 [0.12] | 0.70 [0.15] | 0.70 [0.14] | 0.079 |
| Alcohol consumption: | ||||
| Present | 0.77 [0.11] | 0.74 [0.14] | 0.70 [0.12] | 0.413 |
| Absent | 0.74 [0.14] | 0.66 [0.14] | 0.70 [0.15] | 0.081 |
SD, standard deviation; mRAST, modified radioallergosorbent testing;
Mean baseline SF-6D utility values were significantly worse in the surgical intervention subgroup for patients without a history of previous sinus surgery (p=0.011), without nasal polyposis (p=0.011), and with aspirin sensitivity (p=0.008) compared to medical management after adjusting for multiple comparisons. No significant differences in mean baseline SF-6D utility values for the treatment crossover subgroup across any patient characteristic. Baseline utility values were not found to significantly correlate with either baseline CT or endoscopy scores but were found to significantly correlate with past missed days of productivity in all treatment groups (Table 4).
Table 4.
Bivariate correlation coefficients between baseline SF-6D health state utility values, clinical measures of disease severity, and missed days of productivity
| Medical management (n=40) |
Surgical intervention (n=152) |
Treatment crossover (n=20) |
||||
|---|---|---|---|---|---|---|
| Clinical measures of disease severity: | Rs | p-value | Rs | p-value | Rs | p-value |
| Computed tomography (CT) score | 0.173 | 0.336 | 0.069 | 0.400 | −0.055 | 0.824 |
| Endoscopy score | 0.093 | 0.574 | −0.021 | 0.797 | −0.096 | 0.689 |
| Productivity: | ||||||
| Missed days (out of past 90) | −0.470 | 0.003 | −0.510 | <0.001 | −0.510 | 0.022 |
Rs, Spearman’s rank correlation coefficient;
Longitudinal Changes in SF-6D Values per Treatment Modality
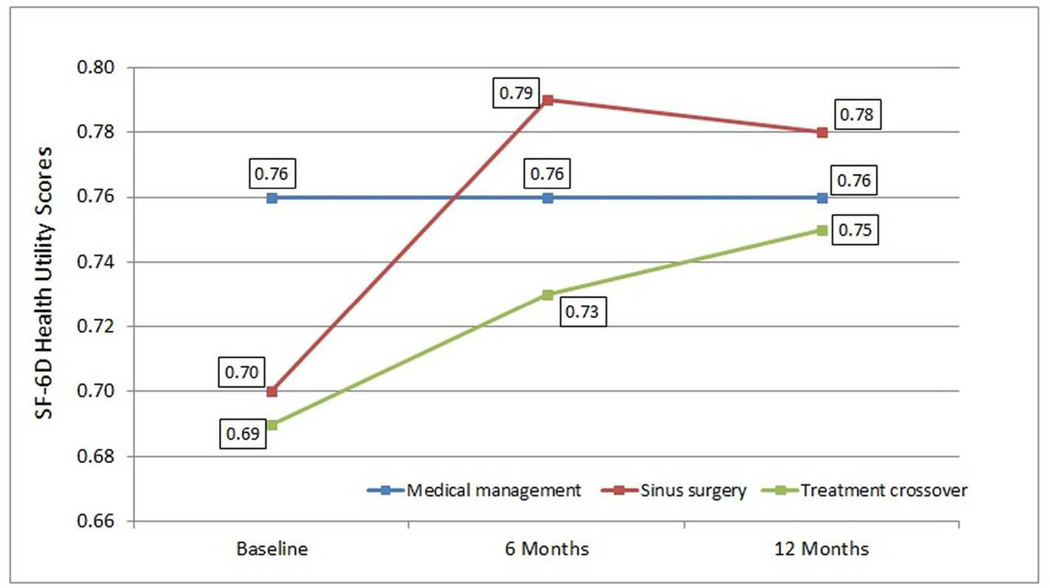
Both statistical and clinically meaningful significant improvement in SF-6D health utility values over time was reported by all participants electing endoscopic sinus surgery (n=152; F(2)=37.69; p<0.001) but not by all participants electing continued medical management for symptoms of CRS (n=40; F(2)=0.03; p=0.967) or participants selecting treatment crossover (n=20; F(2)=2.36; p=0.115; Figure 1) during the study duration. No significant difference in SF-6D values was found between baseline and 6-month evaluations in the medical management group (p=0.746) however significant improvement was reported for the group electing ESS (p<0.001). Mean improvement in SF-6D values was reported by the treatment crossover group between baseline and 6 months, but not to a significant level (p=0.055). No significant differences in mean SF-6D values were found between 6-month and 12-month for any treatment group (p≥0.786).
Figure 1.
Average longitudinal health utility SF-6D health utility values for study participants in the medical management group (n=40), surgical intervention group (n=152), and treatment crossover group (n=20).
Average baseline SF-6D values were similar between the surgical intervention and treatment crossover groups (p=0.826), however due to sample size limitations only the surgical intervention group reported significantly worse average baseline utility values compared to the medical management group (p=0.023). Average SF-6D values were statistically similar between all treatment groups at 6-month follow-up (p≥0.183) and 12-month follow-up (p≥0.269).
Bivariate Correlations
Bivariate correlations between SF-6D values and measures of disease severity were also evaluated at both 6-month (Table 5) and 12-month (Table 6) follow-up. Health utility values were not found to significantly correlate with endoscopy scores for any treatment modality subgroup at either follow-up time point but were found to be significantly correlated again with past missed days of productivity at both follow-up time points for the medical management and surgical intervention treatment groups.
Table 5.
Bivariate correlation coefficients between 6-month SF-6D health state utility values, clinical measures of disease severity, and missed days of productivity.
| Medical management (n=40) |
Surgical intervention (n=152) |
Treatment crossover (n=20) |
||||
|---|---|---|---|---|---|---|
| Clinical measures of disease severity: | Rs | p-value | Rs | p-value | Rs | p-value |
| Endoscopy score | −0.241 | 0.352 | −0.039 | 0.706 | −0.212 | 0.447 |
| Productivity: | ||||||
| Missed days (out of past 90) | −0.336 | 0.039 | −0.421 | <0.001 | −0.504 | 0.028 |
Rs, Spearman’s rank correlation coefficient;
Table 6.
Bivariate correlation coefficients between 12-month SF-6D health state utility values, clinical measures of disease severity, and missed days of productivity.
| Medical management (n=40) |
Surgical intervention (n=152) |
Treatment crossover (n=20) |
||||
|---|---|---|---|---|---|---|
| Clinical measures of disease severity: | Rs | p-value | Rs | p-value | Rs | p-value |
| Endoscopy score | 0.015 | 0.960 | 0.056 | 0.637 | −0.290 | 0.416 |
| Productivity: | ||||||
| Missed days (out of past 90) | −0.412 | 0.010 | −0.546 | <0.001 | 0.115 | 0.651 |
Rs, Spearman’s rank correlation coefficient
DISCUSSION
Health utility values quantify an individual’s preference for his or her current state of health. These values are unique when compared to traditional CRS-specific measures of QOL (SNOT-22, RSDI, CSS) because they allow for comparison across disease states and form the basis for which quality adjusted life years (QALYs) are derived. QALYs are the preferred metric used in cost effectiveness analysis, which can provide valuable information for health care resource allocation. Prior studies have projected that ESS is more cost effective than medical therapy to treat refractory CRS with an estimated cost effectiveness ratio of $5,901.90 per QALY for ESS versus medical therapy.[21]
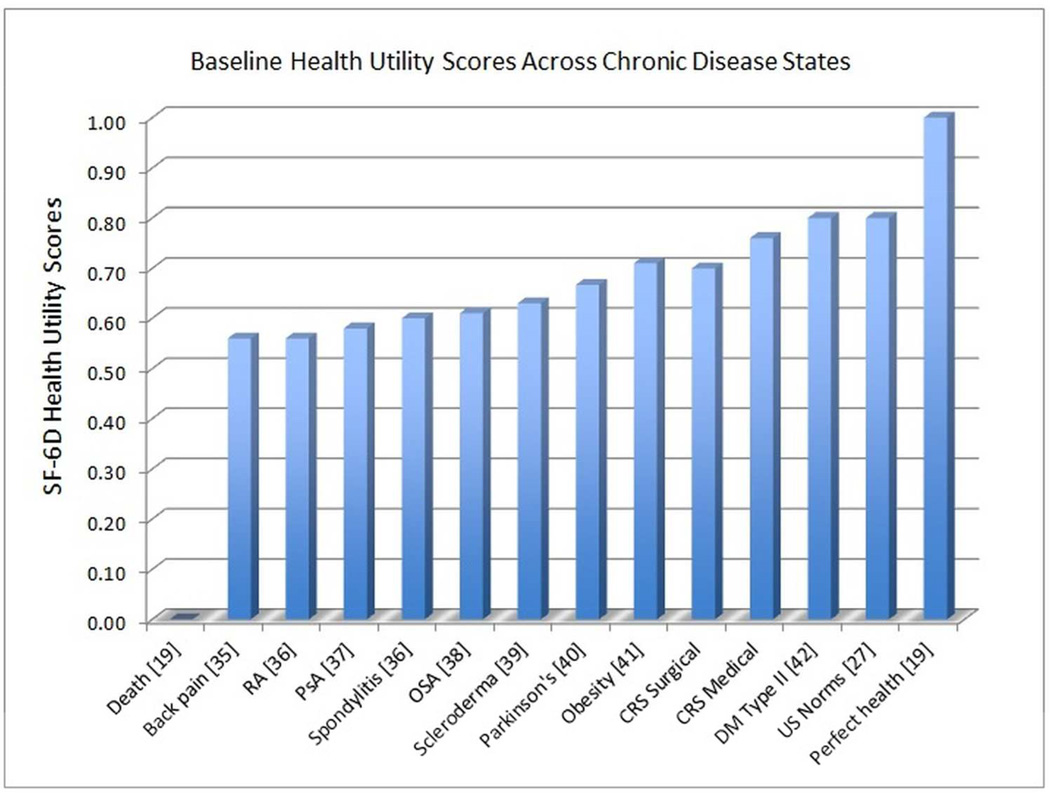
A change in health utility of 0.03 has been validated among many different chronic disease states to represent clinically significant change that alters patient’s subjective well-being by one point on a five point global rating of change scale (5= “much better health”; 4= “somewhat better health”; 3= “no change in health”; 2= “somewhat worse health”; 1= “much worse health”).[20] Baseline health utility values for all CRS patients in this study were significantly less than reported US norms (0.81) and similar to other chronic disease states (Figure 2) in which utility values have been reported.[22]
Figure 2.
Baseline health utility values for a variety of chronic disease processes. AS = ankylosing spondylitis, RA= rheumatoid arthritis, PsA = psoriatic arthritis, OSA=obstructive sleep apnea, CRS= chronic rhinosinusitis, DM=diabetes mellitus, US=United States
Participants electing ESS achieved significant improvement in mean utility from 0.70[0.15] at baseline to 0.79[0.14] at 6 months, with stabilization through 12 months (0.78[0.15], p=0.800). Similarly, the literature supports ESS in improving health utility values for recalcitrant CRS. In 2011, Soler et al. reported clinically significant improvements in baseline disease specific QOL scores as well as utility values (0.087) following ESS.[5] In 2013, Rudmik et al. reported additional long-term improvement in utility values after ESS at five year follow up of a prospective cohort.[23] Most importantly, long-term health utility values reached an average of 0.80, which is comparable to the U.S. norm of 0.81.[6,9,23]
Patients who elected continued medical management reported a significantly better baseline utility as compared to those who elected surgery (0.76[0.12] vs. 0.70[0.15], p=<0.001). Interestingly, there were no significant differences in objective measures such as baseline CT or endoscopy scores between the medical and surgical groups, highlighting the difficulty in stratifying CRS patients and prognosticating outcomes based on imaging and physical exam. However, worse baseline utility values were significantly correlated to increased missed days of productivity, which supports the use of health utility values to determine economic impact of this disease process. The estimated productivity cost associated with refractory CRS is about $10,000 per patient.[24]
In this study, patients who elected continued medical management reported stable mean utility values up to 12 months. Despite lack of improvement of mean utility from baseline in the medical management group, their overall mean health utility was comparable to the surgical group at 6-month (p=0.257) and 12-month follow-up (p=0.269). These findings support prior studies that demonstrate a tendency for patients to self-select appropriate therapy based on their QOL.[25] Patients with a mild reduction in QOL measures chose medical therapy, while those with moderate to severe QOL impairment chose ESS.[6,9,26,27] Further research is needed to further clarify the specific QOL factors that drive patients to choose medical management.
Recent studies have also attempted to clarify the role of medical management for refractory CRS. Smith et al. demonstrated severe reductions in baseline QOL, significant worsening of endoscopy scores, and increased missed days of work in refractory CRS patients treated with medical therapy while waiting to undergo ESS.[28] These patients report worse baseline QOL than the patients in this study who elected medical management and achieved stable QOL. This variation in outcome highlights the importance of accurate assessment of the impact of the chronic disease process in shared patient-provider decision-making.
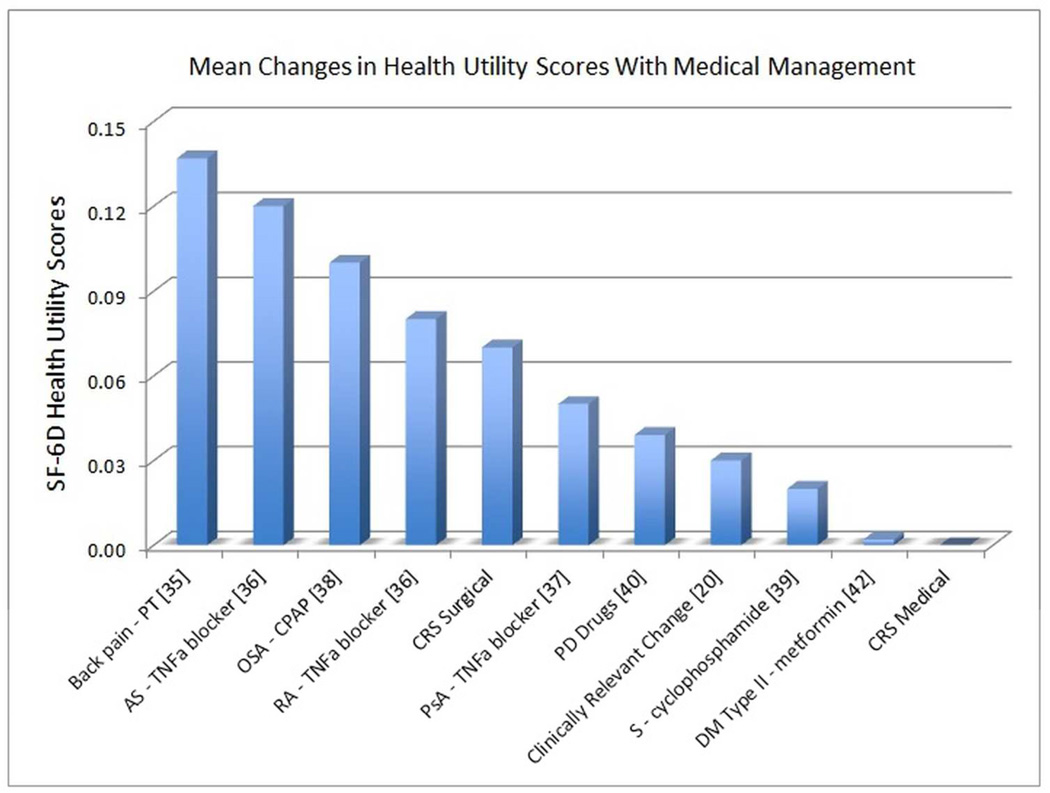
Maintenance of health utility values over time with continued medical management in the current cohort may be interpreted in several ways. First, no improvement in health utility may be interpreted as festering disease burden. In this setting, patients continue to experience detriment to health related QOL despite medical therapy. On the other hand, lack of improvement may also be interpreted as therapeutic control of the chronic disease process at an acceptable health utility state for this patient group. The stabilization of utility with medical management in CRS patients is comparable to medical management of other chronic disease processes such as type 2 diabetes (Figure 3).
Figure 3.
Mean changes in health utility values after medical management. PT=physical therapy, AS = ankylosing spondylitis, RA= rheumatoid arthritis, TNFa = tumor necrosis factor-alpha, PsA = psoriatic arthritis, CRS= chronic rhinosinusitis, OSA = obstructive sleep apnea, CPAP=continuous positive airway pressure; PD = Parkinson’s disease, S =scleroderma, DM=diabetes mellitus.
Average baseline SF-6D values reported in the treatment crossover group (0.69[0.14]) were similar to the surgical group (0.70[0.15]; p=0.826) but lower than the medical group (0.76[0.12]) though this was not statistically significant. In addition, 85% of the crossover group had prior history of ESS. In the setting of prior ESS, lower average baseline utility values suggest that additional continued medical therapy is unlikely to further improve QOL or health utility. Delayed ESS, in appropriate CRS candidates, has been associated with increased health care utilization.[29] The finding that medical management stabilizes health utility may only be applicable to a self-selected group of recalcitrant CRS patients with a relatively high baseline health utility.
There are several caveats to consider when interpreting the results from this study. A small subset of patients (n=20) elected to crossover from the medical management to the surgical intervention cohort, and these patients were analyzed separately. Evaluating this patient subgroup using an intention to treat analysis is not wholly appropriate given that the initial treatment assignment was not randomized. Due to the small sample size of this group and the variations in crossover points, it is difficult to draw definitive conclusions when comparing this crossover group to the medical and surgical groups.
Results from this study may lack generalizability because patients were recruited from academic, tertiary rhinology centers and may represent a specific group of patients with greater burden of disease as compared to average patients with CRS. In addition, to be eligible for this study, many patients failed a course of maximal medical therapy with oral steroids. Prior definitions of maximal medical therapy only included topical nasal spray and antibiotics.[30] Once patients fail oral steroids, continued medical management may be less palatable. As previously reported by Smith et al., lack of improvement or worsening of QOL may be a factor driving patient decision-making to elect ESS.[25] These factors may explain the unbalanced sample size with 40 individuals choosing medical management as opposed to 152 individuals electing ESS and reflect the overall patient populations in these enrollment centers. The prevalence of patients who elected treatment crossover to ESS also reduced the size of the medical management cohort. However, this medical cohort with refractory CRS is comparable in size, baseline characteristics, and clinical measures of disease severity to other medical cohorts in the literature and represents recruitment at four large rhinology centers.[13,30,31] While medical management was not standardized in the current study, the multi-institutional nature of the study reflects current clinical practice and represents real world prescribing practices and outcomes.
Interpretation of published utility values can be challenging as a single best health related QOL construct has not been established for CRS.[32] Rather, there are several different QOL instruments from which health utility values can be derived, including EQ-5D, Health Utilities Index Mark 2, Health Utilities Index Mark 3, SF-6D, Assessment of Quality of Life, and the Quality of Well-Being Index.[33] The SF-6D and EQ-5D are the two most commonly employed constructs within the CRS literature.[3,5,6,9,34] Health utility values are derived from different QOL instruments are not interchangeable due to differing conceptualization, content, size, and methods for computing health utility.[33] The mean baseline health utility value resulting from SF-6D for participants electing ESS in this study was 0.70[0.15]. In contrast, the mean baseline utility value resulting from EQ-5D was 0.81 as reported by Remeschneider et al.[6] Both instruments demonstrate comparable gains in utility (SF-6D: 0.08; EQ-5D: 0.08) following ESS, which supports the use of each instrument in cost-analyses. The health utility values reported herein provide insight into patients’ view of their global health related QOL and will inform future cost-analysis and economic evaluations for medically managed CRS patients. Future studies should confirm our initial results and ideally would include long-term follow-up for more accurate evaluations of economic impact.
CONCLUSION
Patients with recalcitrant CRS electing continued medical management report better baseline health utility compared to patients electing ESS, and their utility values remained stable up to 12 months follow up. Patients electing ESS had lower baseline utility values and demonstrated significant improvement in utility over 12 months after surgery. Outcomes from this study may be used to improve the accuracy of future cost-utility analyses for management of CRS with either medical therapy or ESS. Multi-institutional long–term studies are required to confirm these findings.
Acknowledgments
Timothy L. Smith, Jess C. Mace, and Zachary M. Soler are supported by a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD., USA (R01 DC005805; PI/PD: TL Smith). Public clinical trial registration (www.clinicaltrials.gov) ID# NCT01332136. Zachary M. Soler is also supported by a grant from the NIDCD (R03 DC-013651; PI/PD: ZM Soler). Timothy L. Smith is a consultant for IntersectENT (Menlo Park, CA., USA) which is not affiliated with this investigation. Zachary M. Soler is a consultant for BrainLab (Westchester, IL., USA), which is not affiliated with this investigation.
Footnotes
Potential Conflicts of Interest: None
Financial disclosures: No financial disclosures for Lauren J. Luk, Toby O. Steele, or Luke Rudmik.
Accepted for oral presentation to the American Rhinologic Society at the annual Combined Otolaryngology Spring Meetings (COSM), April 22–26, 2015, Boston, MA, USA.
REFERENCES
- 1.Rudmik L, Soler ZM, Mace JC, Schlosser RJ, Smith TL. Economic evaluation of endoscopic sinus surgery versus continued medical therapy for refractory chronic rhinosinusitis. Laryngoscope. 2015;125:25–32. doi: 10.1002/lary.24916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ. 1986;5:1–30. doi: 10.1016/0167-6296(86)90020-2. [DOI] [PubMed] [Google Scholar]
- 3.Rudmik L, Drummond M. Health economic evaluation: important principles and methodology. Laryngoscope. 2013;123:1341–1347. doi: 10.1002/lary.23943. [DOI] [PubMed] [Google Scholar]
- 4.Petrou S, Hockley C. An investigation into the empirical validity of the EQ-5D and SF-6D based on hypothetical preferences in a general population. Health Econ. 2005;14:1169–1189. doi: 10.1002/hec.1006. [DOI] [PubMed] [Google Scholar]
- 5.Soler ZM, Wittenberg E, Schlosser RJ, Mace JC, Smith TL. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope. 2011;121:2672–2678. doi: 10.1002/lary.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remenschneider AK, Scangas G, Meier JC, et al. EQ-5D-derived health utility values in patients undergoing surgery for chronic rhinosinusitis. Laryngoscope. 2014 doi: 10.1002/lary.25054. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Lal D, Scianna JM, Stankiewicz JA. Efficacy of targeted medical therapy in chronic rhinosinusitis, and predictors of failure. Am J Rhinol Allergy. 2009;23:396–400. doi: 10.2500/ajra.2009.23.3334. [DOI] [PubMed] [Google Scholar]
- 8.Young LC, Stow NW, Zhou L, Douglas RG. Efficacy of medical therapy in treatment of chronic rhinosinusitis. Allergy Rhinol (Providence) 2012;3(1):e8–e12. doi: 10.2500/ar.2012.3.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remenschneider AK, D'Amico L, Gray ST, et al. The EQ-5D: a new tool for studying clinical outcomes in chronic rhinosinusitis. Laryngoscope. 2015;125:7–15. doi: 10.1002/lary.24715. [DOI] [PubMed] [Google Scholar]
- 10.Alt JA, Smith TL, Mace JC, Soler ZM. Sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope. 2013;123:2364–2370. doi: 10.1002/lary.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alt JA, Smith TL, Schlosser RJ, Mace JC, Soler ZM. Sleep and quality of life improvements after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4:693–701. doi: 10.1002/alr.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeConde AS, Bodner TE, Mace JC, Smith TL. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2014;140:712–719. doi: 10.1001/jamaoto.2014.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeConde AS, Mace JC, Alt JA, Schlosser RJ, Smith TL, Soler ZM. Comparative effectiveness of medical and surgical therapy on olfaction in chronic rhinosinusitis: a prospective, multi-institutional study. Int Forum Allergy Rhinol. 2014;4(9):725–733. doi: 10.1002/alr.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeConde AS, Mace JC, Smith TL. The impact of comorbid gastroesophageal reflux disease on endoscopic sinus surgery quality-of-life outcomes. Int Forum Allergy Rhinol. 2014;4:663–669. doi: 10.1002/alr.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;23:3. preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 16.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 17.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 18.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117:S35–S40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 19.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 20.Walters SJ, Brazier JE. What is the relationship between the minimally important difference and health state utility values? The case of the SF-6D. Health Qual Life Outcomes. 2003;1:4. doi: 10.1186/1477-7525-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudmik L, Smith TL. Economic Evaluation of a Steroid-Eluting Sinus Implant following Endoscopic Sinus Surgery for Chronic Rhinosinusitis. Otolaryngol Head Neck Surg. 2014;151:359–366. doi: 10.1177/0194599814533779. [DOI] [PubMed] [Google Scholar]
- 22.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26:391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 23.Rudmik L, Mace J, Soler ZM, Smith TL. Long-term utility outcomes in patients undergoing endoscopic sinus surgery. Laryngoscope. 2014;124:19–23. doi: 10.1002/lary.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudmik L, Smith TL, Schlosser RJ, Hwang PH, Mace JC, Soler ZM. Productivity costs in patients with refractory chronic rhinosinusitis. Laryngoscope. 2014;124(9):2007–2012. doi: 10.1002/lary.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith TL, Kern R, Palmer JN, et al. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study with 1-year follow-up. Int Forum Allergy Rhinol. 2013;3:4–9. doi: 10.1002/alr.21065. [DOI] [PubMed] [Google Scholar]
- 26.DeConde AS, Mace JC, Bodner T, et al. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(12):972–979. doi: 10.1002/alr.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soler ZM, Rudmik L, Hwang PH, Mace JC, Schlosser RJ, Smith TL. Patient-centered decision making in the treatment of chronic rhinosinusitis. Laryngoscope. 2013;123:2341–2346. doi: 10.1002/lary.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith KA, Rudmik L. Impact of continued medical therapy in patients with refractory chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4:34–38. doi: 10.1002/alr.21238. [DOI] [PubMed] [Google Scholar]
- 29.Benninger MS, Sindwani R, Holy CE, Hopkins C. Early versus Delayed Endoscopic Sinus Surgery in Patients with Chronic Rhinosinusitis: Impact on Health Care Utilization. Otolaryngol Head Neck Surg. 2015;152:546–552. doi: 10.1177/0194599814565606. [DOI] [PubMed] [Google Scholar]
- 30.Smith TL, Kern RC, Palmer JN, et al. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study. Int Forum Allergy Rhinol. 2011;1:235–241. doi: 10.1002/alr.20063. [DOI] [PubMed] [Google Scholar]
- 31.Rimmer J, Fokkens W, Chong LY, Hopkins C. Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst Rev. 2014;12:CD006991. doi: 10.1002/14651858.CD006991.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold D, Girling A, Stevens A, Lilford R. Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. BMJ. 2009;339:b2688. doi: 10.1136/bmj.b2688. [DOI] [PubMed] [Google Scholar]
- 33.Richardson J, Khan MA, Iezzi A, Maxwell A. Comparing and Explaining Differences in the Magnitude, Content, and Sensitivity of Utilities Predicted by the EQ-5D, SF-6D, HUI 3, 15D, QWB, and AQoL-8D Multiattribute Utility Instruments. Med Decis Making. 2015;35:276–291. doi: 10.1177/0272989X14543107. [DOI] [PubMed] [Google Scholar]
- 34.Remenschneider AK, D'Amico L, Litvack JR, et al. Long-Term Outcomes in Sinus Surgery: A New Tool for Measuring Health-Related Quality of Life. Otolaryngol Head Neck Surg. 2014;151:164–170. doi: 10.1177/0194599814529536. [DOI] [PubMed] [Google Scholar]
- 35.Johnsen LG, Hellum C, Nygaard OP, et al. Comparison of the SF6D, the EQ5D, and the oswestry disability index in patients with chronic low back pain and degenerative disc disease. BMC Musculoskelet Disord. 2013;14:148. doi: 10.1186/1471-2474-14-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heiberg MS, Nordvag BY, Mikkelsen K, et al. The comparative effectiveness of tumor necrosis factorblocking agents in patients with rheumatoid arthritis and patients with ankylosing spondylitis: a sixmonth, longitudinal, observational, multicenter study. Arthritis Rheum. 2005;52:2506–2512. doi: 10.1002/art.21209. [DOI] [PubMed] [Google Scholar]
- 37.Saad AA, Ashcroft DM, Watson KD, et al. Improvements in quality of life and functional status in patients with psoriatic arthritis receiving anti-tumor necrosis factor therapies. Arthritis Care Res. 2010;62:345–353. doi: 10.1002/acr.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzi CF, Ferraz MB, Poyares D, Tufik S. Quality-adjusted life-years gain and health status in patients with OSAS after one year of continuous positive airway pressure use. Sleep. 2014;37:1963–1968. doi: 10.5665/sleep.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khanna D, Yan X, Tashkin DP, et al. Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheum. 2007;56:1676–1684. doi: 10.1002/art.22580. [DOI] [PubMed] [Google Scholar]
- 40.Vossius C, Nilsen OB, Larsen JP. Health state values during the first year of drug treatment in earlystage Parkinson's disease: a prospective, population-based, cohort study. Drugs Aging. 2009;26:973–980. doi: 10.2165/11318750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Pinto AM, Subak LL, Nakagawa S, et al. The effect of weight loss on changes in health-related quality of life among overweight and obese women with urinary incontinence. Qual Life Res. 2012;21:1685–1694. doi: 10.1007/s11136-011-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Florez H, Pan Q, Ackermann RT, et al. Impact of lifestyle intervention and metformin on health-related quality of life: the diabetes prevention program randomized trial. J Gen Intern Med. 2012;27:1594–1601. doi: 10.1007/s11606-012-2122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]