In vitro, dilated cardiomyopathy (DCM)-causing mutations abolish the change in myofilament Ca2+ sensitivity when troponin I is phosphorylated by PKA. With the use of a mouse model of DCM, we show that this leads to a blunting of the response to dobutamine and under chronic ANG II stress, predisposes to systolic dysfunction characteristic of DCM.
Keywords: dilated cardiomyopathy, heart muscle contractility, dobutamine, angiotensin II stress
Abstract
We investigated cardiac contractility in the ACTC E361G transgenic mouse model of dilated cardiomyopathy (DCM). No differences in cardiac dimensions or systolic function were observed in young mice, whereas young adult mice exhibited only mild diastolic abnormalities. Dobutamine had an inotropic and lusitropic effect on the mouse heart. In papillary muscle at 37°C, dobutamine increased relaxation rates [∼50% increase of peak rate of force decline normalized to force (dF/dtmin/F), 25% reduction of time to 90% relaxation (t90) in nontransgenic (NTG) mice], but in the ACTC E361G mouse, dF/dtmin/F was increased 20–30%, and t90 was only reduced 10% at 10 Hz. Pressure-volume measurements showed increases in maximum rate of pressure decline and decreases in time constant of left ventricular pressure decay in the ACTC E361G mouse that were 25–30% of the changes in the NTG mouse, consistent with blunting of the lusitropic response. The inotropic effect of dobutamine was also blunted in ACTC E361G mice, and the dobutamine-stimulated increase in cardiac output (CO) was reduced from 2,100 to 900 μl/min. Mice were treated with high doses of ANG II for 4 wk. The chronic stress treatment evoked systolic dysfunction in ACTC E361G mice but not in NTG. There was a significant reduction in rates of pressure increase and decrease, as well as reduced end-systolic pressure and increased volume. Ejection fraction and CO were reduced in the ACTC E361G mouse, indicating DCM. In vitro DCM-causing mutations uncouple the relationship between Ca2+ sensitivity and troponin I phosphorylation. We conclude that this leads to the observed, reduced response to β1 agonists and reduced cardiac reserve that predisposes the heart to DCM under conditions of chronic stress.
NEW & NOTEWORTHY
In vitro, dilated cardiomyopathy (DCM)-causing mutations abolish the change in myofilament Ca2+ sensitivity when troponin I is phosphorylated by PKA. With the use of a mouse model of DCM, we show that this leads to a blunting of the response to dobutamine and under chronic ANG II stress, predisposes to systolic dysfunction characteristic of DCM.
dilated cardiomyopathy (DCM) is an important cause of heart failure and is accountable for ∼10,000 deaths annually in the United States. The disease is characterized clinically by severe left ventricular (LV) dilation, with LV end-diastolic dimension in the >95th percentile for age and body surface area and impaired systolic performance, including a shortening fraction <28%, with the exclusion of other known etiologic factors, such as coronary artery disease (12, 14, 21).
Molecular and genetic studies have established that at least 40% of DCM cases are due to genetic mutations. Inheritance is often autosomal dominant with incomplete penetrance, although a case of X-linked inheritance has been reported. More than 100 genes have been reported as causing the disease, including all of the genes coding for sarcomeric proteins [cardiac actin, ACTC, cardiac troponin (cTnI, TNNI3, cTnC, TNNC1, cTnT, TNNT2), tropomyosin (TPM1), myosin heavy and light chains (MYH7, MYL1, MYL2), myosin-binding protein C (MYBPC3), and titin (TTN)] but also including many Z-disk-associated proteins (telethonin, TCAP, muscle LIM protein, CSRP3), cytoskeletal proteins (desmin, DES, dystrophin, DMD), and nuclear envelope proteins (lamin A/C, LMNA) (12). Of particular interest to this study is the fact that cases of idiopathic DCM—those not coupled with other symptoms, such as conduction disease—are most often caused by mutations in contractile proteins, such as actin, myosin, troponin (all three subunits), and tropomyosin (14, 17, 21–23). Such mutations have been investigated extensively by both in vivo and in vitro approaches (3, 17, 20). Since mutations in different contractile proteins lead to a common phenotype, it is possible that they alter the contractile mechanism in the same manner.
The establishment of a molecular defect that accounts for the DCM phenotype has been a complex study, particularly since most transgenic mouse models with DCM-causing mutations exhibit little or no phenotype at rest. Initially, it was proposed that DCM-causing mutations in thin-filament proteins may cause a decrease in the Ca2+ sensitivity of the thin filaments with resultant hypocontractility (3, 20). However, recent work with both recombinant proteins and intact myofibrils has provided results that challenge this simple hypothesis, indicating that decreased Ca2+ sensitivity could not be the prime cause of the DCM phenotype (17, 18).
An alternative hypothesis for the DCM phenotype has been proposed, in which the response of cardiac muscle to β-adrenergic stimulation is blunted. Initial studies on the TNNC1 G159D and ACTC E361G mutations showed that the ability of TnI phosphorylation by PKA to decrease Ca2+ sensitivity was lost as a consequence of the mutations (2, 6, 31). This uncoupling effect was subsequently demonstrated in a wide range of DCM-causing mutations in actin, troponin, and tropomyosin and seems to be a common property of DCM-causing mutations in sarcomeric proteins (18, 19).
These in vitro experiments suggest a disease-causing mechanism for DCM. The PKA-dependent decrease in thin-filament Ca2+ sensitivity and increase in the rate of Ca2+ dissociation from the thin filaments are essential components of the lusitropic element of adrenergic stimulation. The uncoupling of Ca2+ sensitivity from TnI phosphorylation caused by the thin-filament DCM mutations, including ACTC E361G, blunts the response to adrenergic stimulation, compromising the lusitropic response and adversely affecting cardiac reserve. It is predicted that this contractile dysfunction under stress would eventually progress to DCM.
We have been using the ACTC E361G transgenic mouse model of DCM to investigate the physiological consequences of uncoupling. At the molecular level and in single myofibrils, the ACTC E361G mutation causes uncoupling but does not affect length-dependent activation or the effect of Ca2+-sensitizing drugs and has a variable effect on Ca2+ sensitivity (18, 31, 33). At the whole animal level, the phenotype of the ACTC E361G mice appeared benign; the ACTC E361G mice only differed from nontransgenic (NTG) mice with a 15% higher LV internal diameter. No significant differences were found at the cellular level either. Hemodynamic parameters of the whole mouse, obtained by both conductance catheter and MRI, also failed to yield any differences at basal conditions. However, in further cine-MRI experiments involving dobutamine treatment, the ACTC E361G mice showed a blunted response to the catecholamine compared with NTG mice (31). Thus the in vivo results appear to be compatible with the current hypothesis and suggest that the DCM phenotype may only be manifested if the mice were put under chronic cardiac stress.
The aims of this study were to test this hypothesis. Since we have not investigated the ACTC E361G mouse younger than 4 mo and since a study on another transgenic mouse with an ACTC mutation (E99K) showed markedly different contractile phenotypes at 4–6 wk and at 4 mo (32), we concentrated on younger mice. We evaluated the effect of the ACTC E361G mutation on the acute response to stress by increased stimulation frequency and β1 agonists in both whole mouse and isolated papillary muscle and to chronic stress applied by ANG II infusion for 4 wk. We demonstrate that the ACTC E361G mutation blunts responses to dobutamine in intact heart muscle, reduces cardiac reserve, and leads to contractile dysfunction under chronic stress.
METHODS
The generation of ACTC E361G transgenic mice and their phenotypic characterization have been described previously (31). Experiments and animal handling were carried out in accordance with College and Home Office guidelines. Mice were euthanized using humane methods specified under Schedule I of the UK Animals (Scientific Procedures) Act 1986. All other procedures conformed to the guidelines provided and were approved by the Home Office as per the personal and project licenses.
Echocardiography
Mice (6 wk old) were anesthetized by 5% isoflurane vol/vol in 100% O2 (0.5 ml/min) inside an anesthetic chamber (VetTech, Cheshire, UK), then transferred to a temperature-controlled platform (Mouse Handling Platform II; Indus Instruments, Webster, TX) with gold-surface ECG electrodes, and placed in the ventral decubitus position where they received 1–1.5% isoflurane and 0.5 ml/min O2 via a Bain coaxial nose cone connected to a scavenger for the removal of excess anesthetic gases. ECG, respiratory rate, and temperature were recorded continuously throughout the protocol using the THM150 Physiological Controller Unit.
Two-dimensional cardiac echocardiography was carried out using a VisualSonics Vevo 770 system with an RMV-707B probe with a transducer frequency of 30 MHz. LV cavity size and wall thickness were measured in at least three beats from each projection and averaged. LV wall thickness [interventricular septum (IVS) and posterior wall thickness] and internal dimensions at diastole and systole (LVIDd and LVIDs, respectively) were measured. LV fractional shortening [(LVIDd − LVIDs)/LVIDd] is calculated from the M-mode measurements.
Pressure-Volume Relationship in Vivo
In vivo cardiac function was assessed in anesthetized mice (16 wk old) by pressure and volume measurements using a 1.2F Mikro-Tip pressure-volume (PV) catheter (Millar Instruments, Houston, TX) and recorded with the MPVS-300 system (Millar Instruments) coupled to a PowerLab (ADInstruments, San Diego, CA) and a personal computer. Anesthesia was induced with 5% isoflurane. The mouse was placed supine on a thermoregulated surgical table at 37 ± 0.5°C. Invasive surgeries were done under 2.5% isoflurane. Positive end-expiratory pressure (2.5 cmH2O) ventilation via endotracheal catheter was maintained with 100% O2 using a mouse ventilator (Minivent type 845; Hugo Sachs Elektronik, Harvard Apparatus, Holliston, MA).
The subclavian vein was exposed for saline injection. The conductance catheter was advanced into LV through carotid artery. Anesthesia was then maintained with 1–1.5% isoflurane for recording. Calibration of the parallel conductance was performed using injection of 10% hypertonic saline via the subclavian vein. LV blood was collected at the end of the experiment for volume calibration.
Pressure and volume were recorded throughout the entire procedure from the point of insertion of the PV catheter into the carotid artery. Upon hemodynamic parameters reaching a steady state, mice were given a bolus intraperitoneal (ip) injection of the selective β1-adrenoceptor agonist dobutamine (1.5 mg/kg body wt). The effect was assessed at the point where the drug was deemed to have exerted its maximum effect, as judged by the maximum increase in heart rate; this typically occurred 5–10 min after the dobutamine injection. PV loop analysis was made with PVAN 4.0 (Millar Instruments). Heart rate, maximal LV end-systolic pressure (ESP), LV end-diastolic pressure, maximum rate of pressure increase (dP/dtmax) and the maximum rate of pressure decline (dP/dtmin), time constant of LV pressure decay (tau), ejection fraction (EF), stroke volume (SV), end-diastolic volume (EDV), cardiac output (CO), and stroke work were computed.
Contractility of Intact Papillary Muscles
We used muscles from ACTC E361G transgenic mice and control NTG littermates (18–20 wk old). Muscle preparation and conditioning protocol were the same as described in our earlier study (31). The heart was quickly removed and rinsed in standard saline solution plus 30 mmol/l 2,3-butanedione 2-monoxime (BDM) to remove blood. The standard saline solution contained (in mmol/l) 118 NaCl, 4.75 KCl, 1.18 KH2PO4, 1.18 MgSO4, 24.8 NaHCO3, 2.5 CaCl2, 10 glucose, and 0.28 mM ascorbic acid (all Sigma, Dorset, UK) and was equilibrated with 95% O2-5% CO2. The ventricle was opened, and papillary muscle was dissected with BDM present. Platinum foil clips were attached to both ends of the muscle, which was transferred to an Aurora 801C Small Intact Muscle Apparatus setup (Aurora Scientific, Bristol, UK), where one end was attached to a force transducer and the other end to a hook. The organ bath temperature was controlled by a Thermometer/TEC Controller (825A; Aurora Scientific). Standard saline solution (without BDM) flowed continuously through the bath at 27 or 37°C. Muscle length was adjusted to give a passive force of 5 kPa. This degree of passive force was shown corresponding to a sarcomere length of ∼2.1 μm in our previous studies (31, 32). After 1 h conditioning, the muscle was stimulated, and voltage was adjusted to supramaximal strength at a constant pulse duration of 0.5 ms. The muscle was stimulated at 0.2 Hz for a 10-min run-in period before being stimulated at a series of frequencies between 0.1 and 5 Hz. At 27°C, stimulation at increasing frequencies was given in a continuous sequence. At 37°C, to avoid diffusion lag and muscle rundown, we adopted an intermittent protocol, starting at the highest frequency with a short rest between each frequency. Specific force (in kPa) was evaluated by expressing force relative to muscle cross-sectional area (CSA) as follows: CSA = w/(L0 × d), where w is the mass of the blotted muscle after removal of the tissue held within the clips, L0 is the distance between the clips measured on the digital photo of the muscle on the thermopile, and d is muscle density (assumed to be 1.0 mg/mm3). Muscle radius was calculated assuming a circular cross-section.
Chronic ANG II Infusion Using Osmotic Minipump
Anesthesia in mice (8 wk old) was induced by 5% isoflurane vol/vol in 100% O2 (0.5 ml/min). Once mice were fully anesthetized (confirmed by loss of the Labyrinthine righting reflex), they were transferred to a heated surgical table (VetTech), and anesthesia was maintained at 2.5% using a custom-made nose cone. The mice were initially placed in the ventral decubitus to facilitate an ip injection of buprenorphine (0.1 mg/kg body wt; Vetergesic; Alstoe Animal Health, York, UK) to provide postsurgical analgesia. Mice were then moved into the dorsal decubitus position to facilitate access to the scapula region. A roughly 3-cm2 area in the mid-scapular region was shaved and prepared for the surgery. The surgical area was then cleaned using disinfectant (Hydrex Pink Derma Spray; Ecolab, Cheshire, UK). A small (∼1 cm sc) incision was then made. Blunt dissection with hemostats was subsequently used to separate the subcutaneous connective tissues to create a tunnel and subdermal pocket for the minipump in the subscapula region. Alzet 1004 minipumps (Alzet Osmotic Pumps, Kent, UK) were loaded with ANG II to give 1.4 or 2.0 mg·kg−1·day−1 for 28 days, according to the manufacturer's instructions. The minipump was then inserted through the incision and positioned in the pocket using blunt-tipped forceps. The surgical wound was then briefly cleaned before two to three, 7-mm wound clips (Alzet Osmotic Pumps) were applied, using a wound clip applier (Reflex 7 mm wound clip applier; Alzet Osmotic Pumps) to close the incision. The wound was then inspected and cleaned a final time and the anesthesia withdrawn, allowing the animal to recover under oxygen (0.5 ml/min). Mice were then transferred to a heated recovery chamber until sufficiently recovered to return to their original cage. Mice were closely monitored for signs of surgical pain (28) in the postoperative period and given further injections of analgesia (buprenorphine) if required. Mouse body weight was also monitored throughout the infusion period as a general indicator of health status.
Statistical Analysis
All data are presented as means ± SE, unless indicated otherwise. Statistical analysis was performed between two treatment groups by unpaired Student's t-test and between multiple treatment groups by one-way or two-way ANOVA. P < 0.05 was considered significant.
RESULTS
Young ACTC E361G Mice Do Not Show a DCM Phenotype under Basal Conditions
Echocardiography.
M-Mode recordings were used to make measurements of cardiac dimensions and function in young (6 wk old) ACTC E361G mice and age-matched NTG littermates. Consistent with the findings in older mice (30), young ACTC E361G mice show significantly decreased LVIDd. This is further associated with decreased EDV and SV, leading to a trend toward decreased CO. Taken together, these results hint at LV hypertrophy in ACTC E361G mice; however, no significant differences were observed in systolic performance.
PV relationship in vivo.
Since PV loops are technically challenging to perform in very young mice, we recorded from 16-wk-old ACTC E361G mice and age-matched NTG littermates. Consistent with echocardiography results, there was no significant difference in systolic function between ACTC E361G and NTG mice. In contrast, the relaxation time constant (tau) increased significantly in ACTC E361G mice (16.6 ± 0.7 ms vs. 12.3 ± 0.3 ms, P < 0.0001). Furthermore, ACTC E361G mice had a significantly faster heart rate compared with NTG mice (508.2 ± 3.6 beats/min vs. 453.4 ± 7.2 beats/min, P < 0.0001). No differences were observed in SV, CO, and EF.
Contractility of intact papillary muscles.
Since ACTC E361G mice show only a slightly abnormal contractile phenotype in vivo, we next studied the contractility of isolated mouse heart papillary muscle with ACTC E361G mutation to detect subtle differences due to the mutation. ACTC E361G papillary muscles were initially examined at 27°C, as previous work has shown that at subphysiological temperatures, intact cardiac preparations endure less rundown of performance than at more physiological temperatures (32, 34). Papillary muscles were stimulated at a range of subphysiological frequencies (from 0.1 to 5 Hz), and isometric twitch parameters were recorded and analyzed. The final protocol was based on the methodology used by Redel et al. (27) with the timing adjusted in a series of optimization experiments to ensure twitch force had reached steady state at each frequency tested.
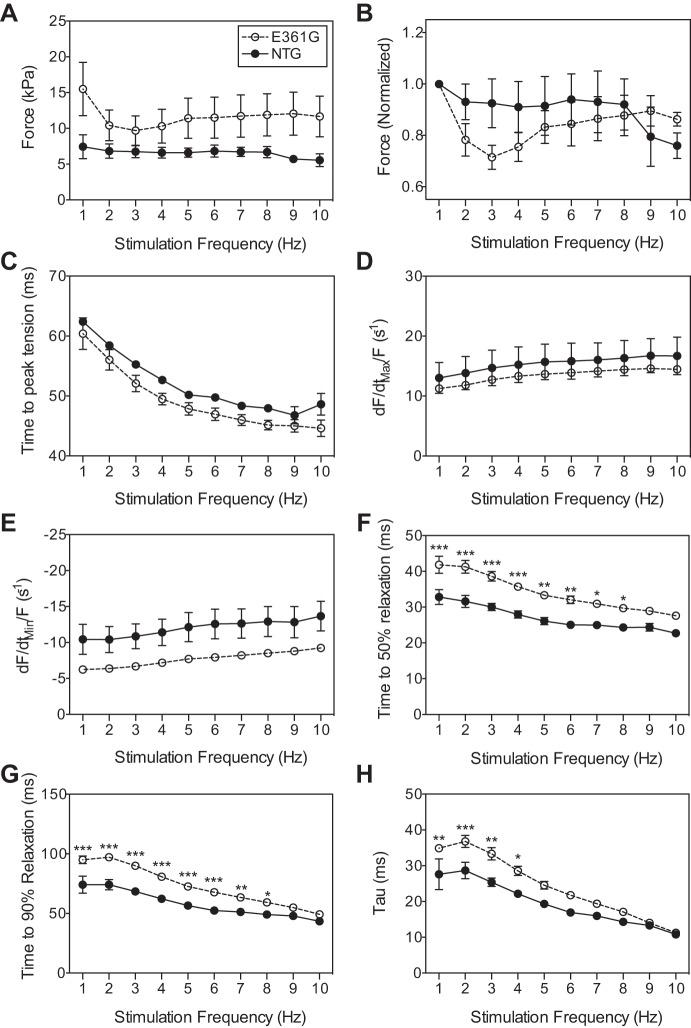
A decline in peak force was observed with increasing stimulation frequency in both ACTC E361G and NTG muscles (P = 0.001), indicating a negative force-frequency relationship. The ACTC E361G mutation produced significantly higher force than NTG at frequencies below 2 Hz (P = 0.0151). The time to peak tension was significantly shorter in ACTC E361G muscles compared with NTG muscles (P = 0.0054). The increase in frequency caused a significant decrease in the time to 50% and 90% relaxation (t50 and t90, respectively) in both ACTC E361G and NTG muscles (P < 0.0001), indicating a frequency-dependent acceleration of relaxation (FDAR) that was not significantly different between the two groups. However, ACTC E361G mouse muscle showed significantly longer t50 and t90 compared with NTG (frequency range 0.1–4 Hz, P < 0.0001). Consistent with the decreased duration of relaxation, increased stimulation frequency also caused a significant decrease in the time constant of relaxation (tau) in both ACTC E361G and NTG muscles (P < 0.0001). The ACTC E361G mutation showed a greater tau across the frequency range studied (0.1–4 Hz, P < 0.0001).
We then measured the papillary muscle contractility at the physiologically relevant temperatures of 37°C to compare with the in vivo measurements. At 37°C, the cellular processes that facilitate muscle relaxation occur faster, and thus twitch duration is shorter. Stimulation frequency can therefore be increased, allowing a range of more physiological frequencies (up to 10 Hz) to be investigated. We used an intermittent stimulation protocol to avoid muscle rundown.
The force-frequency relationship changed from slightly negative, as observed at 27°C, to flat (P > 0.9999; Fig. 1, A and B) in both ACTC E361G and NTG muscles. Maximum force production occurred at 1 Hz, and similar to responses at 27°C, ACTC E361G muscles produced significantly more force than NTG (12.92 ± 3.61 kPa vs. 7.34 ± 1.65 kPa for ACTC E361G and NTG, respectively, P = 0.0026). Time to peak force was again significantly shorter in ACTC E361G muscles (Fig. 1C). The effect of frequency on the peak rate of force development (dF/dtmax/F; Fig. 1D) and decline (dF/dtmin/F; Fig. 1E), normalized to the maximum force produced by each muscle during the force-frequency experiment, showed no significant differences.
Fig. 1.
Effect of stimulation frequency on force development and relaxation phase indices in isolated ACTC E361G and nontransgenic (NTG) papillary muscles contracting isometrically at 37°C. Data are presented as means ± SE of n = 5 ACTC E361G (open circles) muscles and n = 3 NTG (solid circles) muscles. Force (A), force normalized to force produced at 1 Hz (B), time to peak force (C), peak rate of force development (dF/dtmax/F; D), peak rate of force decline normalized to force (dF/dtmin/F; E), time to 50% relaxation (F), time to 90% relaxation (G), and relaxation time constant (tau; H). Data were analyzed by 2-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001 E361G vs. NTG.
The increase in frequency caused a significant decrease in the duration of relaxation for both ACTC E361G and NTG muscles, indicating an FDAR. t50 and t90 were prolonged significantly in ACTC E361G at a frequency range compared with NTG (1–8 Hz, P < 0.0001; Fig. 1, F and G). Consistent with the decreased duration of relaxation, increased stimulation frequency also caused a significant decrease in tau, in both ACTC E361G and NTG muscles (P < 0.0001; Fig. 1H). For the ACTC E361G mouse muscle, tau was significantly greater than for NTG across the frequency range (1–4 Hz). Thus even at the more physiological temperature and stimulation frequencies, ACTC E361G mice do not exhibit contractile dysfunction.
The ACTC E361G Mutation Blunts the Response to β1-Adrenergic Stimulation
We tested the hypothesis that the ACTC E361G mutation uncouples the relationship between TnI phosphorylation and myofibril Ca2+ sensitivity and thus blunts the response to adrenergic agonists by examining the effects of dobutamine on cardiac contractile function in ACTC E361G and NTG mice in vitro and in vivo.
Effects of dobutamine on contractility of intact papillary muscles.
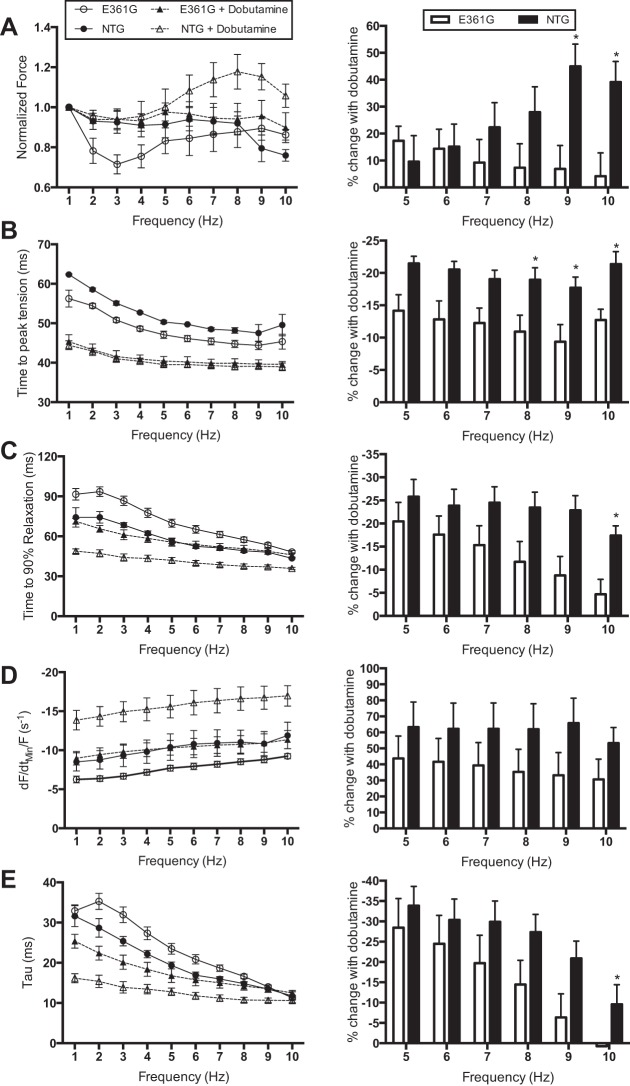
The effect of dobutamine on the force-frequency relationship of mouse papillary muscle was measured at 37°C. To compare the difference in force production in the presence of dobutamine, force was normalized to the force produced at 1 Hz. It is apparent from this data that whereas the effect of dobutamine on normalized force production is minimal in both ACTC E361G and NTG muscles between 1 and 5 Hz, from 6 to 10 Hz, dobutamine increased force in NTG muscle but not in ACTC E361G muscle (Fig. 2A). Furthermore, at these higher and thus more physiologically relevant frequencies, dobutamine changes the force frequency relationship in NTG muscles from slightly negative to positive, whereas in the ACTC E361G muscles, the force-frequency relationship remains flat with or without dobutamine, indicating a blunting of the inotropic response. Dobutamine also reduced time to peak contraction with a significantly smaller change in the ACTC E361G mouse compared with NTG (Fig. 2B).
Fig. 2.
Effect of dobutamine on intact papillary muscle contractility. Papillary muscles, isolated from both ACTC E361G (n = 5) and NTG mice (n = 3–5), were stimulated with the addition of 10 μM dobutamine to the perfusion solution at 37°C. Means ± SE of the muscle function measurements (left); percent change with treatment at physiologically relevant frequencies (right) of the following: force production normalized to force produced at 1 Hz (A), time to peak tension (B), time to 90% relaxation (C), dF/dtmin/F (D), and relaxation rate constant (tau; E). Data were analyzed by 2-way ANOVA; *P < 0.05.
Dobutamine shortened relaxation time and increased the maximum speed of relaxation in both ACTC E361G and NTG papillary muscles, contracting isometrically at 37°C. Dobutamine reduced t90 in ACTC E361G and NTG muscles (P < 0.0001; Fig. 2C), but again, this effect was reduced significantly in ACTC E361G muscles at higher frequencies (8–10 Hz, P < 0.05). Dobutamine also increased dF/dtmin/F, but the difference was not significant (Fig. 2D). Furthermore, dobutamine accelerated the relaxation rate, but this effect was again less in ACTC E361G papillary muscles, reaching 0 at 10 Hz (Fig. 2E). Taken together, these data indicate a substantial blunting of the lusitropic response in ACTC E361G mouse heart muscle.
Effects of dobutamine on PV relationship in vivo.
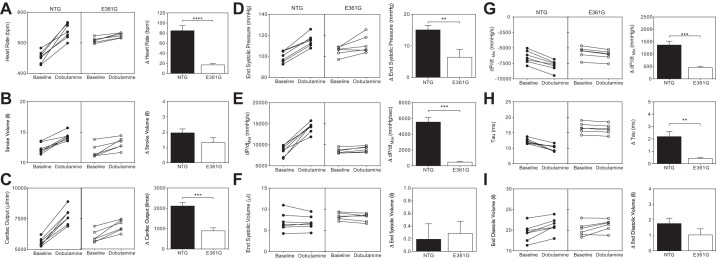
In NTG mice, the dobutamine bolus injection was associated with a 1.2-fold increase in heart rate (P = 0.0001; Fig. 3A), indicating a positive chronotropic response, as expected, with a simultaneous increase in SV (P = 0.0003; Fig. 3B), resulting in increased CO (P < 0.0001; Fig. 3C). The effect of dobutamine was less in the ACTC E361G mice. The trend toward a blunted increase in SV in ACTC E361G mice was not significant (P = 0.1425), but the dobutamine-induced increase in heart rate and consequent increase in CO were significantly smaller in ACTC E361G mice (P < 0.0001 and P < 0.0003, respectively), indicating a blunted chronotropic response, synonymous with a reduced cardiac reserve.
Fig. 3.
The effect of acute dobutamine treatment on cardiac performance. Data are presented as means ± SE of n = 6 ACTC E361G (open bars) mice and n = 7 NTG (solid bars) mice. Δ, change from the baseline. Heart rate (A), stroke volume (SV; B), cardiac output (CO; C), end-systolic pressure (ESP; D), maximum rate of pressure increase (dP/dtmax; E), end-systolic volume (ESV; F), maximum rate of pressure decline (dP/dtmin; G), tau (H), and end-diastolic volume (EDV; I). **P < 0.01, ***P < 0.001, ****P < 0.0001, unpaired Student's t-test.
A positive inotropic response was also observed in NTG mice, as shown by a significant increase in ESP (P < 0.0001; Fig. 3D) and dP/dtmax (P < 0.0001; Fig. 3E). The dobutamine-induced increases in ESP and dP/dtmax (Fig. 3, D and E, respectively) were significantly smaller in ACTC E361G mice when compared with NTG mice (P = 0.0095 and P < 0.0001, respectively). There was no significant change in end-systolic volume (ESV) with dobutamine in NTG or ACTC E361G mice (P = 0.4662 and P = 0.208, respectively; Fig. 3F) and accordingly, no difference in the effect of dobutamine on this parameter between genotypes (P = 0.7844).
NTG mice showed a positive lusitropic response to dobutamine, characterized by a 1.2-fold increase in dP/dtmin (dP/dtmin was −6,479 ± 376 mmHg/s at baseline vs. −7,844 + 317 mmHg/s after dobutamine, P = 0.0001; Fig. 3G) and a significant abbreviation of the time constant of relaxation (tau was 12.30 ± 0.32 ms baseline vs. 10.10 ± 0.39 ms after dobutamine, P = 0.002; Fig. 3H), indicating an increased speed of relaxation. Conversely, ACTC E361G hearts had a blunted lusitropic response to dobutamine, which increased the maximal rate of relaxation (dP/dtmin was −5,694 ± 374 at baseline vs. −6,151 ± 328 mmHg/s after dobutamine, P = 0.0003) and slightly but significantly decreased the time constant of relaxation (tau was 16.59 ± 0.67 ms at baseline vs. 16.18 ± 0.65 ms after dobutamine, P = 0.01). However, these changes were significantly smaller than in NTG mice (P = 0.0017 and P = 0.0203, respectively). In contrast to our previous measurements (31), EDV increased slightly in both NTG (by 9.1%) and ACTC E361G (by 5.1%) mice, but there was no difference in the dobutamine-induced increase (P = 0.1838) or in the mean postdobutamine EDV (P = 0.9576; Fig. 3I) between NTG and ACTC E361G. This suggests that despite the blunted, lusitropic response to dobutamine in ACTC E361G mice, the ACTC E361G hearts still achieve complete relaxation by the end of diastole, albeit more slowly.
The ACTC E361G Mutation Predisposes Mice to Systolic Dysfunction under Chronic Stress
We next used the potent vasopressor ANG II to induce chronic cardiac stress in 8-wk-old mice. In acute measurements, ANG II increased ESP in a dose-dependent manner, which was similar for NTG and ACTC E361G mice. In our initial experiments, ACTC E361G and NTG mice were implanted with osmotic minipumps, delivering 1.4 mg·kg−1·day−1 ANG II, dissolved in physiological saline for 4 wk. Mice were examined by echocardiography at baseline and at weekly intervals for the duration of the infusion period.
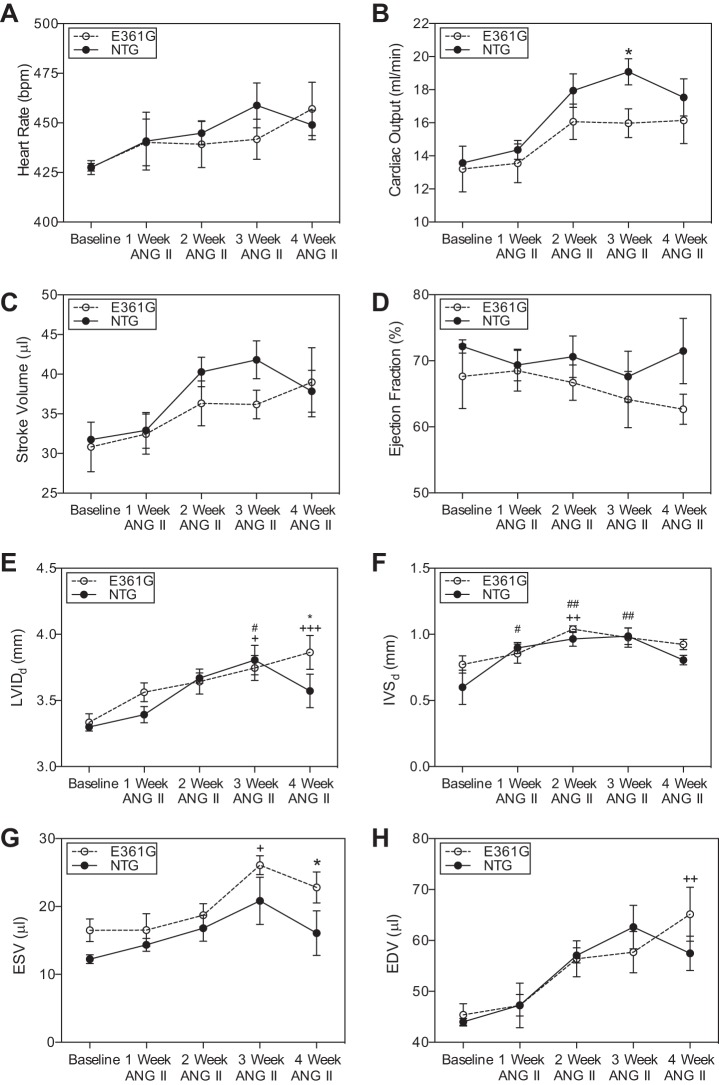
At baseline, heart rate and fractional shortening were not significantly different in NTG and ACTC E361G mice. ANG II infusion caused a modest effect on cardiac function over the period of 4 wk with trends toward a greater increase in heart rate (Fig. 4A), CO (Fig. 4B), and SV (Fig. 4C) in NTG mice. EF (Fig. 4D) increased in NTG mice but decreased in ACTC E361G mice, and by the end of the experiment, ACTC E361G mice had significantly decreased EF (P = 0.021) compared with NTG mice.
Fig. 4.
Effect of 4 wk ANG II (1.4 mg·kg−1·day−1) infusion on cardiac dimensions of ACTC E361G and NTG mouse hearts measured by M-mode echocardiography. Data are presented as means ± SE of n = 8 ACTC E361G (open circles and lines) and n = 6 NTG (solid circles and lines) mice. Heart rate (A), CO (B), SV (C), ejection fraction (EF; D), end-diastolic left ventricle internal dimension at diastole (LVIDd; E), end-diastolic interventricular septum thickness (IVSd; F), ESV (G), and EDV (H). Data were analyzed by 2-way ANOVA (+P < 0.05, ++P < 0.01, +++P < 0.001 ACTC E361G vs. baseline; #P < 0.05, ##P < 0.01 NTG vs. baseline; *P < 0.05 ACTC E361G vs. NTG).
LVIDd increased over the period of 4 wk of ANG II infusion in both NTG and ACTC E361G mice (Fig. 4E). However, NTG mice showed a decrease in LVID in the final week of the experiment, whereas in ACTC E361G mice, it remained increased significantly. Similarly, end-diastolic IVS thickness increased significantly in both ACTC E361G and NTG mice over the infusion period (P < 0.001). In the final week of the experiment, NTG mice showed a decrease in IVS thickness, whereas in ACTC E361G mice, it remained increased (Fig. 4F). There was also a significant increase recorded in ESV and EDV from the baseline for both NTG and ACTC E361G mice (Fig. 4, G and H, respectively). At the end of the experiment, ESV and EDV remained increased in ACTC E361G mice compared with NTG mice. Altogether, these data suggest that under chronic angiotensin-induced stress, ACTC E361G mouse hearts develop hypertrophy initially and later dilation. We performed a small number of terminal PV measurements that produce results compatible with this conclusion.
Since the effects of ANG II infusion were surprisingly small, especially in the NTG mice, we increased the dose to 2 mg·kg−1·day−1, and heart function was analyzed by PV catheter at the end of the treatment, since in our experience, this methodology could report small differences more reliably than echocardiography. In this experiment, we also included a sham-operated ACTC E361G mouse control.
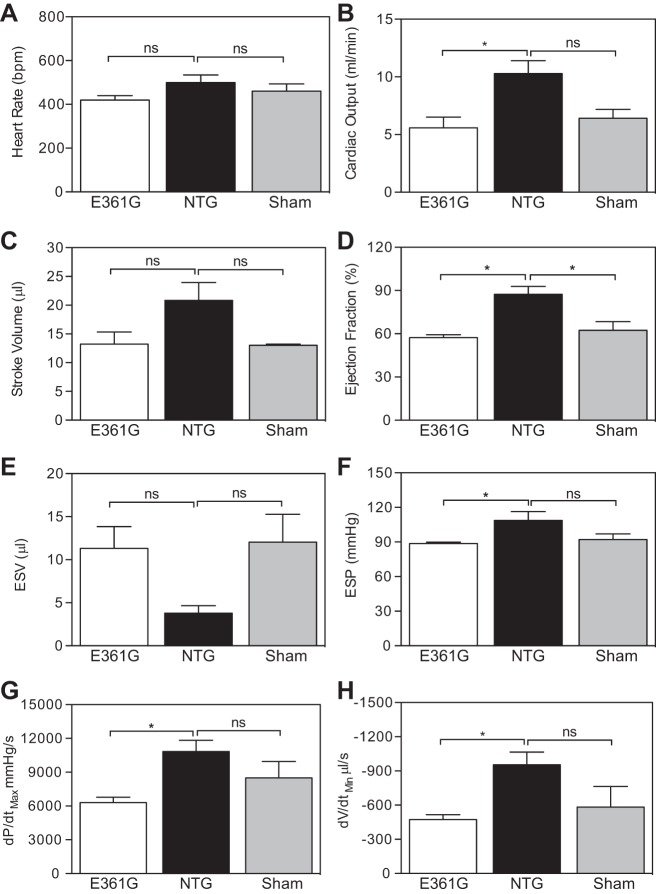
After 4 wk infusion, the heart rate (Fig. 5A) and SV (Fig. 5C) were not affected by the treatment, but the EF (Fig. 5D) and consequently, CO (Fig. 5B) were reduced significantly in ACTC E361G mice, indicative of systolic dysfunction. This was associated further with significantly decreased ESP (Fig. 5F) and a trend toward an increase in ESV (Fig. 5E). ACTC E361G mice also showed significantly reduced rates of pressure rise (Fig. 5G) and volume reduction (Fig. 5H) in systole compared with NTG mice.
Fig. 5.
Effect of 4 wk ANG II (2.0 mg·kg−1·day−1) infusion on myocardial contraction indices of ACTC E361G and NTG mouse hearts measured by pressure-volume catheter. Data are presented as means ± SE of n = 4 ACTC E361G (open bars), n = 3 NTG (solid bars), and n = 4 ACTC E361G sham-operated (shaded bars) mice. Heart rate (A), CO (B), SV (C), EF (D), ESV (E), ESP (F), dP/dtmax (G), and peak rate of volume reduction (dV/dtmin; H). Data were analyzed by 1-way ANOVA (*P < 0.05); ns, not significant.
DISCUSSION
We have investigated the ACTC E361G transgenic mouse model of familial DCM to understand the mechanism by which the sarcomeric protein mutation causes the DCM phenotype. This model is particularly suitable for mechanistic investigation for several reasons. The transgene is expressed in a hybrid strain of mouse C57BL/6xCBA/Ca, which is always backcrossed to certified hybrid stock rather than a pure-bred line. We have previously shown that in the ACTC E361G mouse model, mutant actin is consistently expressed at ∼50% of total actin—the level expected in human heterozygote patients (31)—and it has been demonstrated that the MYH promoter overexpression system used to drive expression of mutant actin does not lead to accumulation of excess mutant in the sarcoplasm (15)—a problem that has been highlighted in another model of DCM (26). Finally, cardiac actin has the same amino acid sequence and isoform distribution in both human and mouse (4). Thus this model avoids the potentially confounding issues of nonhuman isoforms and sequences.
Studies on isolated thin filaments containing mutant actin plus human heart troponin and tropomyosin and in myofibrils from ACTC E361G transgenic mice have demonstrated that the basal myofilament Ca2+ sensitivity is not consistently lower than wild-type, which was initially proposed as an explanation for the DCM phenotype (3, 20). In fact, native ACTC E361G thin filaments have the same Ca2+ sensitivity as wild-type in the in vitro motility assay, whereas if skeletal muscle troponin is used, the mutant Ca2+ sensitivity is 2.5-fold lower (31). In contrast, in ACTC E361G myofibrils, the Ca2+ sensitivity of isometric tension is 2.5-fold higher than wild-type (33). This extreme variability in the effect of the mutation on Ca2+ sensitivity within one mutation under different conditions is mirrored by the variability of the Ca2+-sensitivity change induced by different DCM-causing mutations under the same conditions and indicates that the DCM phenotype cannot be related to the basal Ca2+ sensitivity (17, 18). It is likely that mutations affect the modulation of Ca2+ sensitivity by TnI phosphorylation rather than baseline Ca2+ sensitivity.
In vitro studies have demonstrated that the modulation of Ca2+ sensitivity and relaxation rate by PKA-catalyzed phosphorylation of TnI is abolished by many DCM-causing mutations in thin-filament proteins, including ACTC E361G, and in some hypertrophic cardiomyopathy-causing mutations also (18, 19). Since this is the only consistent abnormality of DCM mutations, we have proposed that this “uncoupling” of Ca2+ sensitivity from TnI phosphorylation could be causative of the DCM phenotype. The increase in the rate of Ca2+ release from troponin when it is phosphorylated is an essential component of the lusitropic response to β1-adrenergic stimulation in the heart; therefore, the loss of this modulation is predicted to lead to a blunted response to β1 stimulation under stress and a reduced cardiac reserve.
The ACTC E361G Mutation Is Associated with Hypercontractility and Prolonged Relaxation Rather Than a DCM Phenotype at Rest
We made a thorough comparison of the contractility of the ACTC E361G heart in isolated papillary muscle by PV catheter and by echocardiography in young adult mice. In good agreement with the preliminary investigation of this mouse model at 12 and 18 mo (31), we found that ACTC E361G mice kept under resting conditions do not have an overt DCM phenotype. No differences in cardiac dimensions or systolic function were observed in young mice, whereas young adult mice exhibited only mild diastolic abnormalities and again, no systolic dysfunction.
However, a consistent finding across the different methodologies was that the ACTC E361G mutation is associated with a prolongation of cardiac relaxation. Investigation of young adult mice using a PV catheter showed that the ACTC E361G mutation is associated with an increase in the time constant of relaxation—a robust measurement of isovolumic relaxation time. In intact muscle preparations, all analyzed parameters of cardiac relaxation were slower in ACTC E361G muscles. This included parameters used to characterize the early phase of relaxation (t50 and dF/dtmin/F), as well as parameters used to characterize the late phase of relaxation [t90 and the time constant of relaxation (tau) (27)]. These observations are consistent with the enhanced Ca2+ sensitivity and slower relaxation rate observed in isolated ACTC E361G myofibrils (33). However, prolonged relaxation does not appear to be a consistent finding among DCM-causing thin-filament protein mutations, with some mouse models showing diastolic dysfunction (26), whereas others show only systolic impairment (5). In the ACTC E361G mouse, Song et al. (31) reported a 40% increase in tau at 12 mo but not at 18 mo; similarly, heart rate was elevated in ACTC E361G mice at 4 mo but not at 12 or 18 mo, suggesting that age-related secondary changes in phenotype might mask the direct effect of the mutation. Such findings emphasize again that DCM mutations in mouse models do not appear to produce a consistent and potentially disease-causing effect on basal contractility.
The DCM Mutation ACTC E361G Blunts Contractile Response to Dobutamine
It has been demonstrated that myofibril Ca2+ sensitivity is not modulated by TnI phosphorylation in isolated ACTC E361G thin filaments and in single myofibrils (18, 31, 33). When we tested the effect of the β1-specific agonist dobutamine on contractility in young adult mice, we found that dobutamine had an inotropic and lusitropic effect on wild-type mouse heart, both in papillary muscle and in PV measurements, that was substantially attenuated in the ACTC E361G mouse. In wild-type papillary muscle at 37°C, the effects of dobutamine were more marked at frequencies above 6 Hz that correspond to the normal heart rate in mice. As expected, relaxation was faster after dobutamine treatment of wild-type mice. In papillary muscle at 37°C, dobutamine increased relaxation rates (∼50% increase of dF/dtmin/F, 25% reduction t90), but in the ACTC E361G mouse, dF/dtmin/F was increased 20–30%, and t90 was only reduced 10% at 10 Hz. Correspondingly, PV measurements showed increases in dP/dtmin and decreases in tau in the ACTC E361G mouse that were one-fourth to one-third of the changes in the wild-type mouse, consistent with substantial blunting of the lusitropic response, as suggested by Song et al. (31). Somewhat surprisingly, the inotropic effect of dobutamine was also blunted in ACTC E361G mice, as demonstrated by a reduced effect of dobutamine on ESP, dF/dtmax/F, and ESV in PV measurements and 85% reduction of the increase in maximum force in papillary muscle. The combination of these effects is to reduce the dobutamine-stimulated increase in CO (a measure of cardiac reserve) from 2,100 to 900 μl/min. This result is similar to that obtained when TnI was made unphosphorylatable, by substituting slow skeletal TnI (8) or by introducing a mutation where serine 23/24 was converted to aspartic acids (35). Thus as predicted, the uncoupling of the relationship between Ca2+ sensitivity and TnI phosphorylation by the ACTC E361G DCM-causing mutation results in reduced physiological cardiac reserve. This behavior is compatible with the absence of a DCM phenotype in the ACTC E361G mouse at rest and indicates further that it is likely that the mutant mouse hearts will be unable to respond normally to chronic stress, and this may be sufficient to induce a DCM phenotype.
Reduced Cardiac Reserves Predispose ACTC E361G Mice to Symptoms of DCM under Stress
Therefore, we hypothesized that the loss of cardiac reserve would predispose ACTC E361G hearts to failure under chronic stress. Whereas humans are generally exposed to a plethora of external stresses, caged mice are kept under a consistent, controlled environment and live a relatively sedentary and stress-free life (29). To test this hypothesis, we implanted ACTC E361G and NTG mice with osmotic minipumps filled with agents that hemodynamically and pharmacologically stress the heart when applied chronically (24).
To elicit evidence of mutation-related DCM, we needed to treat the mice with high doses of ANG II for 4 wk. The effects of 1.4 mg·kg−1·day−1 ANG II induced quite mild systolic dysfunction in the ACTC E361G mice, with lower fractional shortening and EF developing compared with NTG from wk 2 onward. At the end of a 4-wk infusion period, with 2 mg·kg−1·day−1 ANG II, PV measurements showed that the chronic stress treatment evoked symptoms of contractile dysfunction in ACTC E361G mice but not NTG. Compared with the NTG mice, ACTC E361G mice had significantly lower rates of pressure increase and decrease, as well as reduced ESP and increased volume. As a result, EF and CO were reduced in the ACTC E361G mouse, indicating mutation-induced contractile dysfunction characteristic of the initial stages of DCM.
C57BL/6xCBA/Ca Mouse Strain Is Resistant to Chronic Cardiac Stress
It is interesting to note that the chronic ANG II treatment had very little effect on the wild-type mouse, and throughout our studies of the ACTC E361G transgenic mouse, it has been apparent that the mutation-induced contractile dysfunction is barely detectable, whereas others have reported mouse models of DCM that cause overt dysfunction (5, 13). Moreover, in preliminary studies, we found that both wild-type and ACTC E361G mice proved surprisingly resistant to 1- and 2-wk treatments with isoprenaline + phenylephrine or low doses of ANG II that have been demonstrated to cause heart failure in other mouse models (11, 16, 29); a high dose of ANG II over 4 wk was required to uncover evidence of the DCM phenotype in our ACTC E361G mouse model. There is no reason to believe that the ACTC E361G mutation is especially benign in vivo (23); however, we suspect that the variation in effects of pharmacological stress agents is related to the variety of different mouse strains used in transgenic and knock-in mouse models. A number of studies point to a significant effect of mouse strain, or even substrain, in determining the response to chronic cardiac stress, including chronic isoprenaline or angiotensin infusion (1, 7, 9, 10, 30), and it appears that C57BL/6 mice are particularly resistant to ANG II (25). There do not appear to be any studies on the effect of hybrid backgrounds, such as the C57BL/6xCBA/Ca strain used for this study, but it is reasonable to expect that the compensatory mechanisms of both genotypes may be combined.
Conclusion
We conclude that the DCM-causing mutation ACTC E3621G leads to an uncoupling of the relationship between Ca2+ sensitivity and TnI phosphorylation that results in the observed reduced response to β1 agonists and reduced physiological cardiac reserve and that this predisposes the heart to developing symptoms of DCM under conditions of chronic stress.
GRANTS
Support for this work was provided by grants from the British Heart Foundation (BHF; RG/11/20/29266 and FS/09/024/24014).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.W., W.S., and S.M. conception and design of research; R.W. and W.S. performed experiments; R.W., N.S., and S.M. analyzed data; R.W., W.S., N.S., and S.M. interpreted results of experiments; R.W., N.S., and S.M. prepared figures; W.S., N.S., and S.M. drafted manuscript; W.S., N.S., and S.M. edited and revised manuscript; R.W., W.S., N.S., and S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of W. Song: Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore 637553.
REFERENCES
- 1.Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 292: H2119–H2130, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Biesiadecki BJ, Kobayashi T, Walker JS, John Solaro R, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res 100: 1486–1493, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev 10: 225–235, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Copeland O, Nowak K, Laing N, Ravenscroft G, Messer AE, Bayliss CR, Marston SB. Investigation of changes in skeletal muscle alpha-actin expression in normal and pathological human and mouse hearts. J Muscle Res Cell Motil 31: 207–214, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, Lu QW, Wang YY, Zhan DY, Mochizuki M, Kita S, Miwa Y, Takahashi-Yanaga F, Iwamoto T, Ohtsuki I, Sasaguri T. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res 101: 185–194, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Dyer E, Jacques A, Hoskins A, Ward D, Gallon C, Messer A, Kaski J, Burch M, Kentish J, Marston S. Functional analysis of a unique troponin C mutation, Gly159Asp that causes familial dilated cardiomyopathy, studied in explanted heart muscle. Circ Heart Fail 2: 456–464, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Faulx MD, Ernsberger P, Vatner D, Hoffman RD, Lewis W, Strachan R, Hoit BD. Strain-dependent beta-adrenergic receptor function influences myocardial responses to isoproterenol stimulation in mice. Am J Physiol Heart Circ Physiol 289: H30–H36, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol 517: 143–157, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Festing MF. Wheel activity in 26 strains of mouse. Lab Anim 11: 257–258, 1977. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Menendez L, Karamanlidis G, Kolwicz S, Tian R. Substrain specific response to cardiac pressure overload in C57BL/6 mice. Am J Physiol Heart Circ Physiol 305: H397–H402, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gramlich M, Michely B, Krohne C, Heuser A, Erdmann B, Klaassen S, Hudson B, Magarin M, Kirchner F, Todiras M, Granzier H, Labeit S, Thierfelder L, Gerull B. Stress-induced dilated cardiomyopathy in a knock-in mouse model mimicking human titin-based disease. J Mol Cell Cardiol 47: 352–358, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol 10: 531–547, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Juan F, Wei D, Xiongzhi Q, Ran D, Chunmei M, Lan H, Chuan Q, Lianfeng Z. The changes of the cardiac structure and function in cTnTR141W transgenic mice. Int J Cardiol 128: 83–90, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, Smoot L, Mullen MP, Woolf PK, Wigle ED, Seidman JG, Seidman CE, Jarcho J, Shapiro LR. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med 343: 1688–1696, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Crawford K, Flick R, Klevitsky R, Lorenz JN, Bove KE, Robbins J, Lessard JL. Transgenic overexpression of cardiac actin in the mouse heart suggests coregulation of cardiac, skeletal and vascular actin expression. Transgenic Res 13: 531–540, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Maass AH, Ikeda K, Oberdorf-Maass S, Maier SK, Leinwand LA. Hypertrophy, fibrosis, and sudden cardiac death in response to pathological stimuli in mice with mutations in cardiac troponin T. Circulation 110: 2102–2109, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Marston SB. How do mutations in contractile proteins cause the primary familial cardiomyopathies? J Cardiovasc Transl Res 4: 245–255, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Memo M, Leung MC, Ward DG, dos Remedios C, Morimoto S, Zhang L, Ravenscroft G, McNamara E, Nowak KJ, Marston SB, Messer AE. Mutations in thin filament proteins that cause familial dilated cardiomyopathy uncouple troponin I phosphorylation from changes in myofibrillar Ca2+-sensitivity. Cardiovasc Res 99: 65–73, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Messer A, Marston S. Investigating the role of uncoupling of troponin I phosphorylation from changes in myofibrillar Ca2+-sensitivity in the pathogenesis of cardiomyopathy. Front Physiol 5: 315, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirza M, Marston S, Willott R, Ashley C, Mogensen J, McKenna W, Robinson P, Redwood C, Watkins H. Dilated cardiomyopathy mutations in three thin filament regulatory proteins result in a common functional phenotype. J Biol Chem 280: 28498–28506, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Mogensen J, Murphy RT, Shaw T, Bahl A, Redwood C, Watkins H, Burke M, Elliott PM, McKenna WJ. Severe disease expression of cardiac troponin C and T mutations in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 44: 2033–2040, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Olson TM, Kishimoto NY, Whitby FG, Michels VV. Mutations that alter the surface charge of alpha-tropomyosin are associated with dilated cardiomyopathy. J Mol Cell Cardiol 33: 723–732, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science 280: 750–752, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Osadchii OE, Norton GR, McKechnie R, Deftereos D, Woodiwiss AJ. Cardiac dilatation and pump dysfunction without intrinsic myocardial systolic failure following chronic beta-adrenoreceptor activation. Am J Physiol Heart Circ Physiol 292: H1898–H1905, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Peng H, Yang XP, Carretero OA, Nakagawa P, D'Ambrosio M, Leung P, Xu J, Peterson EL, Gonzalez GE, Harding P, Rhaleb NE. Angiotensin II-induced dilated cardiomyopathy in Balb/c but not C57BL/6J mice. Exp Physiol 96: 756–764, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajan S, Ahmed RP, Jagatheesan G, Petrashevskaya N, Boivin GP, Urboniene D, Arteaga GM, Wolska BM, Solaro RJ, Liggett SB, Wieczorek DF. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ Res 101: 205–214, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Redel A, Baumgartner W, Golenhofen K, Drenckhahn D, Golenhofen N. Mechanical activity and force-frequency relationship of isolated mouse papillary muscle: effects of extracellular calcium concentration, temperature and contraction type. Pflugers Arch 445: 297–304, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Roughan JV, Flecknell PA. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain 90: 65–74, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Schlossarek S, Schuermann F, Geertz B, Mearini G, Eschenhagen T, Carrier L. Adrenergic stress reveals septal hypertrophy and proteasome impairment in heterozygous Mybpc3-targeted knock-in mice. J Muscle Res Cell Motil 33: 5–15, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Shusterman V, Usiene I, Harrigal C, Lee JS, Kubota T, Feldman AM, London B. Strain-specific patterns of autonomic nervous system activity and heart failure susceptibility in mice. Am J Physiol Heart Circ Physiol 282: H2076–H2083, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Song W, Dyer E, Stuckey D, Leung MC, Memo M, Mansfield C, Ferenczi M, Liu K, Redwood C, Nowak K, Harding S, Clarke K, Wells D, Marston S. Investigation of a transgenic mouse model of familial dilated cardiomyopathy. J Mol Cell Cardiol 49: 380–389, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Song W, Vikhorev PG, Kashyap MN, Rowlands C, Ferenczi MA, Woledge RC, MacLeod K, Marston S, Curtin NA. Mechanical and energetic properties of papillary muscle from ACTC E99K transgenic mouse models of hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 304: H1513–H1524, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vikhorev PG, Song W, Wilkinson R, Copeland O, Messer AE, Ferenczi MA, Marston SB. The dilated cardiomyopathy-causing mutation ACTC E361G in cardiac muscle myofibrils specifically abolishes modulation of Ca2+ regulation by phosphorylation of troponin I. Biophys J 107: 2369–2380, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widen C, Barclay CJ. ATP splitting by half the cross-bridges can explain the twitch energetics of mouse papillary muscle. J Physiol 573: 5–15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasuda S, Coutu P, Sadayappan S, Robbins J, Metzger JM. Cardiac transgenic and gene transfer strategies converge to support an important role for troponin I in regulating relaxation in cardiac myocytes. Circ Res 101: 377–386, 2007. [DOI] [PubMed] [Google Scholar]