The findings of this study provide additional evidence for purinergic glio-endothelial coupling during neuronal activity, highlighting the role of ATP-mediated activation of endothelial nitric oxide synthase via P2Y1 receptors in functional hyperemia. Impairment of this pathway is predicted to play a critical role in the genesis of age-related cognitive impairment.
Keywords: astrocyte, dementia, endothelial dysfunction, endothelium, vascular cognitive impairment, endothelial nitric oxide synthase
Abstract
Impairment of moment-to-moment adjustment of cerebral blood flow (CBF) via neurovascular coupling is thought to play a critical role in the genesis of cognitive impairment associated with aging and pathological conditions associated with accelerated cerebromicrovascular aging (e.g., hypertension, obesity). Although previous studies demonstrate that endothelial dysfunction plays a critical role in neurovascular uncoupling in these conditions, the role of endothelial NO mediation in neurovascular coupling responses is not well understood. To establish the link between endothelial function and functional hyperemia, neurovascular coupling responses were studied in mutant mice overexpressing or deficient in endothelial NO synthase (eNOS), and the role of P2Y1 receptors in purinergic glioendothelial coupling was assessed. We found that genetic depletion of eNOS (eNOS−/−) and pharmacological inhibition of NO synthesis significantly decreased the CBF responses in the somatosensory cortex evoked by whisker stimulation and by administration of ATP. Overexpression of eNOS enhanced NO mediation of functional hyperemia. In control mice, the selective and potent P2Y1 receptor antagonist MRS2179 attenuated both whisker stimulation-induced and ATP-mediated CBF responses, whereas, in eNOS−/− mice, the inhibitory effects of MRS2179 were blunted. Collectively, our findings provide additional evidence for purinergic glio-endothelial coupling during neuronal activity, highlighting the role of ATP-mediated activation of eNOS via P2Y1 receptors in functional hyperemia.
NEW & NOTEWORTHY
The findings of this study provide additional evidence for purinergic glio-endothelial coupling during neuronal activity, highlighting the role of ATP-mediated activation of endothelial nitric oxide synthase via P2Y1 receptors in functional hyperemia. Impairment of this pathway is predicted to play a critical role in the genesis of age-related cognitive impairment.
there is increasing evidence that age-related functional and structural alterations of the cerebral microvascular endothelial cells significantly contribute to the pathogenesis of both vascular cognitive impairment and Alzheimer's disease (30, 39, 50, 56, 65, 75, 87). Neurovascular coupling is a central homeostatic mechanism adjusting regional cerebral blood flow (CBF) to the increased needs of activated neuronal tissue and thus plays a critical role in the preservation of normal neuronal function, including cognition (30, 34). Importantly, endothelial dysfunction, attributable to impaired NO bioavailability, was shown to associate with impaired neurovascular coupling and cognitive decline in aging and in pathophysiological conditions associated with accelerated microvascular aging including hypertension and obesity (22, 40, 55, 56, 79, 83). Despite these advances, the mechanistic role of endothelial NO production in neurovascular coupling is not completely understood.
Previous studies using pharmacological inhibitors of NO synthesis provided solid evidence that production of NO contributes significantly to functional hyperemia upon neuronal activation; however, the relative contribution of neuronal NO synthase (nNOS or NOS1) and endothelial NO synthase (eNOS or NOS3) is a subject of ongoing debate (4, 9, 70). The signaling mechanisms that may be involved in triggering endothelial NO production in the cerebral microcirculation during neuronal activation are also not well understood.
There is growing evidence that astrocytes are intimately involved in neurovascular coupling (21, 44, 47, 52, 72). Following synaptic activation, astrocytes release a number of vasoactive mediators in a glutamate-dependent manner, including arachidonic acid metabolites (e.g., epoxyeicosatrienoic acids and prostaglandins), which act directly on the smooth muscle cells of precapillary arterioles (31, 32, 42, 47) eliciting vasodilation (termed gliovascular coupling) (59). Activated astrocytes also release ATP, which plays an important role in synchronized activation of neighboring astrocytes and is known to cause dilation of cerebral arterioles (2, 54, 89, 93). ATP and adenosine (formed in the extracellular space through hydrolysis of ATP) can bind to purinergic receptors on the smooth muscle cells, exerting direct vasoactive effects (66). Importantly, purinergic receptors, including P2Y1 receptors, are also abundantly expressed on cerebromicrovascular endothelial cells (90). Previous studies in cultured endothelial cells (17) and in isolated cerebral arteries (95) demonstrate that ATP and its degradation products activate eNOS via P2Y1 receptors, triggering endothelial production of NO. Despite these advances, the role of P2Y1 receptors in gliovascular coupling remains elusive (17, 94).
The present study was designed to test the hypothesis that functional eNOS and activation of P2Y1 receptors are needed for normal neurovascular coupling response. To achieve these goals, neurovascular coupling was tested in eNOS−/− mice and in a novel mouse model of eNOS overexpression. ATP-induced cerebrovascular responses were also assessed, and the effects of pharmacological inhibition of P2Y1 receptors on neurovascular coupling and purinergic vasodilation were determined.
METHODS
Experimental animals.
All procedures were approved by the Institutional Animal Use and Care Committees of the participating institutions and in accordance with the ARRIVE guidelines. eNOS-deficient mice (B6.129P2-Nos3tm1Unc/J, developed on a C57BL/6J background; 3 mo old; n = 20) and age-matched wild-type control mice (n = 30) were purchased from the Jackson Laboratories (Bar Harbor, ME).
Mice overexpressing eNOS were developed by the Baur laboratory as follows: a constitutively expressing eNOS construct was generated by cloning the murine Nos3 cDNA at the Not1 site between the CAGGS promoter and rabbit globin polyA terminator sequences on the pCAGEN vector backbone (Takahiko Matsuda; Addgene, Cambridge, MA). This was then linearized using PsiI and AccI to remove the ampicillin resistance gene. The linearized construct, named pCAGEN-meNOS, was purified from agarose gel with the QiaQuick Gel Extraction Kit (Qiagen, Valencia, CA) and further purified by ethanol precipitation. Linearized pCAGEN-meNOS was injected into fertilized eggs and implanted into pseudopregnant C57BL6/J female mice by the Transgenic and Chimeric Mouse Core at the University of Pennsylvania Perelman School of Medicine. Resultant weanlings were genotyped for expression of the cDNA construct using the primers F: ATCTCAGGCAGCCTAACTCCT; R: TTCCCAGCTGCTGTGCGTA. A female founding pup was confirmed and subsequently backcrossed and maintained on a C57BL/6 Taconic line. Systolic blood pressure was measured by the tail cuff method (CODA Non-Invasive Blood Pressure System; Kent Scientific, Torrington, CT), as previously reported (81). Genetic depletion of eNOS resulted in a mild but significant increase in systolic blood pressure compared with control mice (control: 103 ± 2 mmHg, eNOS−/−: 112 ± 1 mmHg; P < 0.05). Overexpression of eNOS did not significantly affect systolic blood pressure as measured by the tail cuff method.
Endothelium-dependent dilation of isolated cerebral arteries.
To characterize the effect of overexpression of eNOS on endothelium-dependent vasodilation, responses of isolated cerebral vessels to ATP and acetylcholine (ACh) were obtained, as previously described. In brief, mice were decapitated, the brains were removed, and branches of the middle cerebral artery were isolated using microsurgical technique (67, 68, 79–81). Vascular segments were mounted onto two glass micropipettes in an organ chamber and pressurized to 60 mmHg. The hydrodynamic resistance of the micropipettes was matched. Inflow and outflow pressures were controlled and measured by a pressure servo-control system (Living Systems Instrumentation, Burlington, VT). Inner vascular diameter was measured with a custom-built videomicroscope system and continuously recorded using a computerized data-acquisition system, as reported (77). All vessels were allowed to stabilize for 60 min in oxygenated (5% CO2, balanced with air) Krebs buffer (at 37°C). After a stable, spontaneous pressure-induced myogenic tone developed, changes in vascular diameter were measured in response to administration of ATP (10−7 mol/l) and ACh (10−7 mol/l) (Sigma Aldrich, St. Louis, MO). At the end of each experiment, the passive, maximal diameter was obtained at 60 mmHg in the presence of Ca2+-free Krebs buffer containing the L-type calcium channel inhibitor nifedipine (10−5 mol/l). Dilation is expressed as a percentage and calculated as: [(d − db)/(pd − db)] × 100, where d is the actual diameter, db is baseline diameter, and pd is the passive diameter.
Immunoblotting.
Cerebral tissues of control, eNOS transgene (TG), and eNOS−/− were lysed with the Qiagen TissueLyzer II system (20 Hz for 2 min at 4°C) in lysis buffer (25 mM Tris, pH 7.5; 150 mM NaCl; 1% Triton X-100; 0.5% Na-deoxycholate; 1% Nonidet P-40; 0.5% SDS; 1 mM EDTA) supplemented with complete protease inhibitor mixture (Roche Applied Science, Penzberg, Germany). SDS-PAGE was performed on 4–15% Tris·HCl gels (Bio-Rad, Hercules, CA) using 50 μg of cleared lysate boiled for 5 min in 1× Laemmli buffer. Gels were transferred to PVDF membranes (Millipore, Billerica, MA) and blocked with 5% fatty acid-free BSA (Roche Applied Science). Primary antibody for eNOS (NOS3, c-20, sc-654; Santa Cruz Biotechnology, Dallas, TX) was diluted 1:500 in TBS-Tween 20 according to the manufacturer's instructions. The primary antibody was then removed using a stripping buffer (62.5 mM Tris, pH 6.8; 2% SDS; 100 mM β-mercaptoethanol) for 15 min at 50°C, thoroughly washed, and blocked in 5% BSA. The blot was reprobed with β-actin-horseradish peroxidase (ab49900; Abcam, Cambridge, MA) for the protein loading control. Antibody binding was detected using chemiluminescent horseradish peroxidase substrate (PerkinElmer Life Sciences, Billerica, MA).
Surgical procedures.
Mice were anesthetized (α-chloralose, 50 mg/kg plus urethane, 750 mg/kg ip), endotracheally intubated, and ventilated (MousVent G500; Kent Scientific). Rectal temperature was maintained at 37°C using a thermostatic heating pad (Kent Scientific). End-tidal CO2 (with dead space) was kept between 3.2% and 3.7% to maintain blood gas values within the physiological range, as reported (79–81). Animals were immobilized and placed on a stereotaxic frame (Leica Microsystems, Buffalo Grove, IL). The scalp and periosteum were pulled aside, and a craniotomy was made with a dental drill over the left somatosensory cortex corresponding to the barrel field. The dura was gently removed, and the open cranial window was continuously superfused with artificial cerebrospinal fluid (composition: 119.0 mM NaCl, 26.2 mM NaHCO3, 2.5 mM KCl, 1.0 mM NaH2PO4, 1.3 mM MgCl2, 10.0 mM glucose, 2.5 mM CaCl2; pH 7.3, 37°C). The right femoral artery was cannulated for arterial blood pressure measurement (Living Systems Instrumentation) (79–81) to ensure that the blood pressure was within the physiological range throughout the experiments (90–100 mmHg).
CBF responses to whisker stimulation and pharmacological studies.
To assess neurovascular coupling, a laser Doppler probe (Transonic Systems, Ithaca, NY) was positioned above the barrel cortex (1.0–1.5 mm posterior and 3.0–3.5 mm lateral to bregma), and the contralateral whiskers were stimulated for 1 min at 5 Hz from side to side. Changes in CBF (n = 7–8 in each group) were assessed in three trials divided by 5–10-min intervals. CBF responses to whisker stimulation were repeated in the presence of the following inhibitors administered topically onto the brain surface of separate groups of animals: Nω-nitro-l-arginine methyl ester (a pharmacological inhibitor of NOS, l-NAME; 10−4 mol/l for 20 min; Sigma Aldrich) (79); the potent, selective, competitive P2Y1 purinergic receptor antagonist MRS2179 (2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate ammonium salt; 6 × 10−5 mol/l for 20 min; Ki = 100 nmol/l; Cayman Chemicals, Ann Arbor, MI) (45, 74); and fluoroacetate (the precursor of fluorocitrate, a metabolic toxin used to inhibit astrocyte function, 3 × 10−4 mol/l for 20 min; Sigma Aldrich) (42). In a separate series of experiments (n = 8 in each group), CBF responses to topical administration of ATP (10−5 mol/l) and ACh (10−5 mol/l) were assessed in the presence and absence of l-NAME (79). Changes in CBF are expressed as percentage changes from baseline (40, 79).
Statistical analysis.
Statistical analysis was carried out by unpaired t-test, one-way ANOVA, or two-way ANOVA for repeated measures followed by Bonferroni multiple-comparison test, as appropriate, using Prism 5.0 for Windows (GraphPad Software, La Jolla, CA). A P value <0.05 was considered statistically significant. Data are expressed as means ± SE.
RESULTS
Endothelial NO contributes to neurovascular coupling.
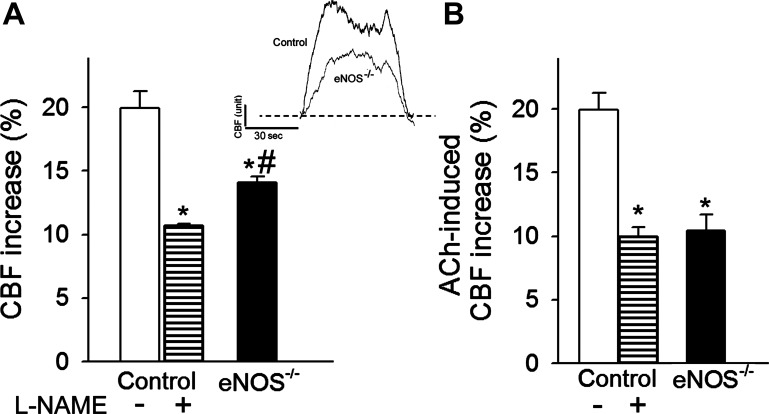
Changes in CBF in the whisker barrel cortex in response to contralateral whisker stimulation were significantly attenuated in eNOS−/− mice (Fig. 1A), indicating that deficiency in endothelial NO synthesis leads to neurovascular uncoupling, mimicking the aging phenotype (79). We found that in control animals administration of the NO synthase inhibitor l-NAME also significantly decreased CBF responses in the barrel cortex elicited by contralateral whisker stimulation (Fig. 1A). Topical application of the endothelium-dependent vasodilator agent ACh (10−5 mol/l) (63) resulted in a significant increase in CBF in the barrel cortex of control mice (Fig. 1B). ACh-induced CBF responses were significantly attenuated both by inhibition of NO synthesis by treatment with l-NAME and by genetic depletion of eNOS (Fig. 1B).
Fig. 1.
Endothelial nitric oxide synthase (eNOS) contributes to neurovascular coupling. A: increases in cerebral blood flow (CBF; expressed as percentage of baseline) measured above the barrel field of the primary somatosensory cortex in response to whisker stimulation in control mice in the presence and absence of the nitric oxide synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) and in mice with genetic depletion of eNOS (eNOS−/−). Data are means ± SE (n = 8, *P < 0.05 vs. control, #P < 0.5 vs. control + l-NAME). Inset: representative traces of CBF measured with a laser Doppler probe above the whisker barrel cortex during contralateral whisker stimulation (5 Hz) in control and eNOS−/− mice. 1 AU corresponds to ∼5% increase in CBF from baseline. B: CBF responses elicited by topical administration of acetylcholine (ACh, 10−5 mol/l) to the barrel field of control and eNOS−/− mice (n = 5 in each group, *P < 0.05 vs. control). Data are means ± SE.
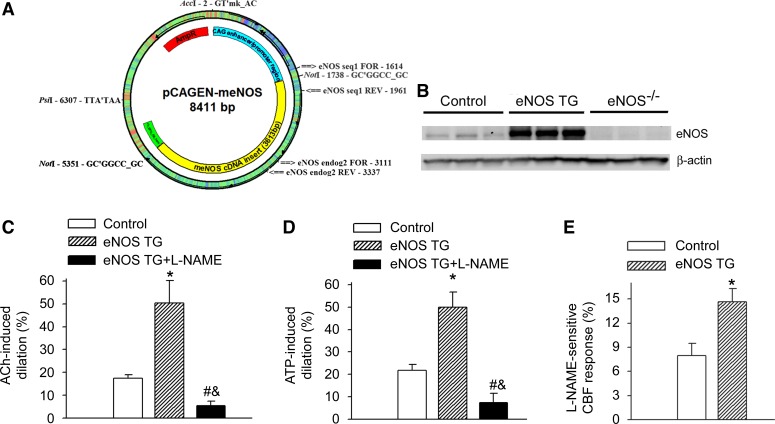
To provide additional evidence for the contribution of eNOS to neurovascular coupling responses, we utilized a novel mouse model of eNOS overexpression, developed by the Baur laboratory (Fig. 2A). In the brains of eNOS TG animals, transgene expression was confirmed by Western blot analysis of eNOS (Fig. 2B). Cerebral vessels isolated from eNOS TG mice exhibited increased dilation in response to the NO-dependent vasodilators ACh and ATP (Fig. 2, C and D, respectively), providing functional validation for the model. We found that overexpression of eNOS significantly enhanced the l-NAME-sensitive, NO-mediated portion of the CBF response (calculated based on the percentage decline in CBF in the presence of l-NAME) measured above the barrel field of the primary somatosensory cortex in response to whisker stimulation (Fig. 2E), providing additional support for the contribution of endothelium-derived NO to neurovascular coupling responses.
Fig. 2.
eNOS overexpression increases the NO-mediated part of neurovascular coupling response. A: construct used to develop the transgenic (TG) mouse overexpressing eNOS (see methods). B: representative Western blot image showing expression of eNOS in the cortex of control wild-type mice, eNOS TG mice, and eNOS−/− mice. C and D: overexpression of eNOS (eNOS TG) is associated with significantly enhanced dilation of isolated cerebral vessels to ACh (10−7 mol/l; C) and ATP (10−7 mol/l; D), which are significantly decreased by the NOS inhibitor l-NAME (10−4 mol/l for 30 min). Data are means ± SE. (n = 5 in each group, *P < 0.05 eNOS TG vs. control, #P < 0.05 eNOS TG + l-NAME vs. eNOS TG; &P < 0.05 eNOS TG + l-NAME vs. control). E: overexpression of eNOS significantly enhances the l-NAME-sensitive, NO-mediated portion of the CBF response (calculated based on the percentage decline in CBF in the presence of l-NAME) measured above the barrel field of the primary somatosensory cortex in response to whisker stimulation. Data are means ± SE (n = 5, *P < 0.05 eNOS TG vs. control).
Role of P2Y1 receptors in ATP-mediated and endothelial NO-dependent cerebromicrovascular responses.
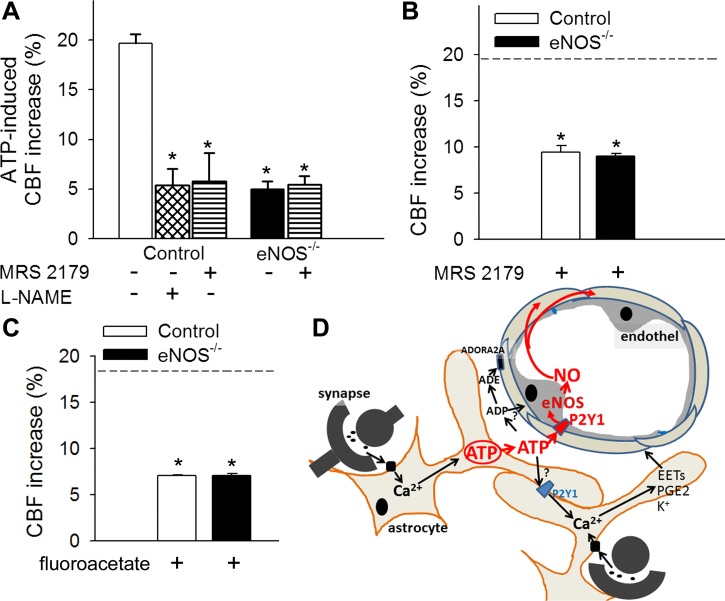
Topical administration of ATP elicited significant increases in CBF in the barrel field of the primary somatosensory cortex, which were inhibited by l-NAME and the potent, selective P2Y1 receptor antagonist MRS2179 (Fig. 3A). In eNOS−/− mice, ATP-induced CBF responses were significantly impaired and were unaffected by MRS2179 (Fig. 3A). These findings indicate that, in the mouse somatosensory cortex, P2Y1 receptor-dependent activation of eNOS mediates ATP-induced cerebromicrovascular responses.
Fig. 3.
ATP-mediated CBF responses, role of P2Y1 receptors in NO mediation of neurovascular coupling. A: increases in CBF (expressed as a percentage of baseline) in the barrel field of the primary somatosensory cortex of control and eNOS−/− mice in response to topical administration of ATP in the presence and absence of the potent, selective, competitive P2Y1 receptor antagonist MRS2179 (6 × 10−5 mol/l for 20 min) or the NOS inhibitor l-NAME (10−4 mol/l for 20 min) (n = 6, *P < 0.05 vs. control). CBF was measured with a laser Doppler probe above the whisker barrel cortex. B and C: increases in CBF in the barrel field of the primary somatosensory cortex of control and eNOS−/− mice induced by contralateral whisker stimulation (5 Hz) in the presence of MRS2179 (6 × 10−5 mol/l for 20 min; B) or the glial-specific metabolic toxin fluoroacetate (3 × 10−4 mol/l for 20 min, C). Dashed line indicates the mean of the control responses (n = 6, *P < 0.05 vs. control). Data are means ± SE. D: scheme depicting purinergic glio-endothelial coupling during neuronal activity, highlighting the role of ATP-mediated activation of eNOS via P2Y1 receptors in functional hyperemia. The model acknowledges that multiple parallel astrocytic pathways exist [including synthesis of epoxyeicosatrienoic acids (EETs), prostaglandins (PGE2), and release of K+, which hyperpolarizes vascular smooth muscle cells] that are activated simultaneously to regulate blood flow and that ATP and its hydrolysis products [ADP and adenosine (ADE)] can evoke arteriolar dilation via adenosine A2 receptors located on the smooth muscle cells, as well.
We found that in control mice topical administration of MRS2179 also significantly attenuated CBF responses induced by contralateral whisker stimulation (Fig. 2B). In the presence of MRS2179, there was no difference between whisker stimulation-induced CBF responses in control and eNOS−/− mice (Fig. 3B), suggesting that P2Y1 receptor-dependent activation of eNOS plays an important role in neurovascular coupling responses in the mouse cortex. We also found that administration of the glial-specific metabolic toxin fluoroacetate eliminated the difference between whisker stimulation-induced CBF responses in control and eNOS−/− mice (Fig. 3C), consistent with the proposed role of eNOS in glio-endothelial coupling.
DISCUSSION
Here we demonstrate that both genetic depletion of eNOS and pharmacological inhibition of NO synthesis lead to profound neurovascular dysregulation, characterized by impaired CBF responses induced by synaptic activity (Fig. 1). Our results extend findings by previous investigations using different pharmacological inhibitors of NO synthesis (29, 70, 79) and disruption of the cerebrovascular endothelium using the light-dye technique (9). Neurovascular uncoupling associated with experimentally induced impairment of endothelial NO mediation mimics impairment of functional hyperemia observed in aging (79) and pathophysiological conditions associated with accelerated cerebromicrovascular aging, including hypertension (40), obesity (82, 83), and Alzheimer's disease (28). The aforementioned cardiovascular risk factors were shown to decrease the bioavailability of NO in the microcirculation by promoting oxidative stress, uncoupling eNOS, and/or by upregulating asymmetric dimethylarginine, an endogenous inhibitor of NO synthase (55, 79, 88).
Impairment of a key homeostatic mechanism matching energy supply with the needs of active neuronal tissue is predicted to have deleterious effects on brain function. Indeed, there is strong evidence that, in elderly patients, cerebromicrovascular dysfunction and impaired neurovascular coupling (69, 76, 96) are associated with decline in higher cortical functions including cognition. Previous studies demonstrate that, compared with wild-type C57BL/6 mice, eNOS−/− mice also exhibit impaired working memory performance (3, 20). Pharmacological inhibition of NO synthesis in experimental animals was also reported to promote cognitive dysfunction (5, 11, 18, 62), mimicking aspects of the aging phenotype (73). Interestingly, previous studies have demonstrated that there are significant strain-dependent differences in eNOS expression and NO-mediated microvascular responses in mice (6). Thus, it is predicted that the relative importance of endothelium-derived NO in neurovascular coupling responses in mice may also be strain specific. This concept is supported by the previous observations (4) that, in the SV-129 mouse model, in which NO-dependent vasodilation (33, 64) and short-term memory (92) are compromised compared with those in C57BL/6 mice, genetic depletion of eNOS has only a marginal effect on functional hyperemia. Furthermore, SJL mice, which exhibit impaired NO-mediated vasodilation due to oxidative stress associated with a spontaneous mutation in superoxide dismutase 2 (10), also show impaired hippocampal learning and memory (8).
Because there is growing evidence that impaired endothelial function associated with aging and various pathophysiological conditions can be rescued, the cerebral microvasculature emerges as a potential therapeutic target for treating elderly patients with different forms of cognitive impairment. This concept is supported by previous findings that treatment of aged mice with resveratrol (3,5,4′-trihydroxy-trans-stilbene), which is known to exert multifaceted endothelial protective effects (12–16, 46, 58, 78, 84–86), rescues neurovascular coupling responses (79) and improves cognitive function (51). Because the microvascular endothelium is directly exposed to the bloodstream, several drugs that do not readily cross the blood-brain barrier yet exert protective effects on the microvascular endothelium could be potentially exploited to improve neurovascular coupling and thereby cognitive function in the elderly.
This is the first study to suggest that upregulation of eNOS increases NO mediation of functional hyperemia in response to neuronal activation (Fig. 2). This observation provides additional support for the concept that endothelium-derived NO plays an important role in neurovascular coupling. The functional consequences of increased NO bioavailability in this model remain unclear. Further studies are needed to determine whether overexpression of eNOS is associated with improved cognitive function and/or whether these mice exhibit resistance to cognitive decline induced by cardiovascular risk factors.
Over the past decade, a number of important studies have identified astrocytes as key intermediaries in neurovascular coupling (25). Although because of methodological challenges there are still many controversies in the field (7, 60), there is substantial evidence supporting the view that neuronal activity-dependent Ca2+ signals in astrocytic processes, which precede the onset of functional hyperemia, trigger the release of a number of astrocyte-derived vasoactive mediators (26, 36, 52). Previous findings that eNOS-deficient mice exhibit impaired cortical vasodilation in response to astrocyte Ca2+ uncaging compared with wild-type mice (70) suggest that mediators released from the astrocytes play a critical role in activation of eNOS in cerebromicrovascular endothelial cells. This finding is also substantiated by the observation that the astrocyte-specific metabolic inhibitor fluoroacetate eliminates the difference between CBF responses in control and eNOS−/− mice (Fig. 3). Interestingly, there are reports that NO may also regulate astrocytic Ca2+ signaling (49), raising the possibility that a bidirectional communication may exist between the vascular endothelium and the astrocytic network during functional hyperemia.
Purinergic signaling represents one of the most important pathways by which astrocytes communicate with other cells, including neurons and neighboring astrocytes (71). There is strong morphological (electron microscopy) and biochemical evidence suggesting that ATP, as an astrocytic transmitter, is released by Ca2+-dependent exocytosis in the extracellular space (reviewed in Ref. 57). Exogenously administered ATP is a potent vasodilator in cerebral vessels (Fig. 2D), and it significantly increases CBF in the somatosensory cortex (Fig. 3A), mimicking functional hyperemia. Following synaptic activation, astrocyte-derived ATP may induce vasodilation by activating multiple cellular pathways. The finding that administration of a potent P2Y1 receptor antagonist significantly attenuates whisker simulation-induced CBF increases in the mouse somatosensory cortex (Fig. 3B) supports the concept that astrocyte-derived ATP contributes to the neurovascular coupling responses (2, 54, 66, 89, 91, 93) and that P2Y1 receptors play an important role in gliovascular coupling. Importantly, P2Y1 receptors are abundantly expressed on cerebromicrovascular endothelial cells (90), and previous studies in cultured endothelial cells (17) and in isolated cerebral arteries (95) demonstrate that ATP activates eNOS via P2Y1 receptors, triggering endothelial production of NO. The observations that in eNOS−/− mice both ATP-induced and whisker stimulation-induced CBF responses are blunted and both responses are unaffected by MRS2179 (Fig. 3, A and B) extend the aforementioned findings, suggesting that predominantly endothelial P2Y1 receptors mediate ATP-induced eNOS activation in the somatosensory cortex. As predicted by this hypothesis, ATP-induced vasodilation is significantly increased in eNOS-overexpressing mice (Fig. 2). Previous studies showed that ATP can be hydrolyzed in the extracellular space into adenosine (23), which can directly dilate cerebral arterioles via adenosine A2A receptors located on smooth muscle cells (2, 89). Although this mechanism could contribute to the residual, P2Y1 receptor-independent portion of ATP-induced CBF responses, recent evidence suggests that astrocyte-derived ATP, rather than its breakdown products, is a primary mediator of neurovascular coupling (91). Additional cellular and molecular mechanisms linking neuronal activation to endothelial synthesis of NO may involve activation of eNOS via adrenergic receptors (1), glutamate receptors (43, 70), and/or eicosanoid receptors. Previous studies showed that arteriolar dilation induced by neuronal activation can propagate proximally in an endothelium-dependent manner (35) and that ATP is capable to initiate propagated dilation in peripheral vessels (24), suggesting that purinergic glio-endothelial coupling mechanisms may contribute to the endothelium-dependent component of conducted vasodilation in the brain.
Because with advanced age expression of P2Y1 receptors is downregulated (48) and astrocytic ATP generation is decreased (41), future studies should determine the role of purinergic mechanisms in age-related glio-endothelial uncoupling. There are also studies showing that cardiovascular risk factors, including hypertension (27) and diabetes mellitus (37), may also affect vascular P2Y1-dependent pathways. Thus further studies are warranted to elucidate the role of dysregulation of purinergic glio-endothelial coupling responses in these pathophysiological conditions as well.
Limitations of the study.
In the present study, we have not investigated the putative role of purinergic nerves in neurovascular coupling. The P2Y1 receptor is also involved in interastrocytic communication in both physiological (38) and pathological conditions (19). It should be noted that pharmacological blockade of P2Y1 receptors likely also disrupts these astrocytic pathways as well, which may also affect the neurovascular coupling response. Because likely different cellular mechanisms contribute to the different phases of the CBF response upon initiation of neuronal stimulation, further studies could provide additional valuable data by analyzing the effects of inhibitors to the early and later phases of the CBF response separately.
Conclusions.
In conclusion, our results add to the growing evidence that purinergic glio-endothelial coupling mechanisms are activated during neuronal activity, which contribute to functional hyperemia in the mouse cortex. We propose that ATP-mediated activation of P2Y1 receptors plays an important role in eNOS-dependent increases in CBF during neuronal activation (Fig. 3D). It is an important aspect of the proposed model that purinergic glio-endothelial coupling mechanisms provide a physiological link between astrocytic metabolism and endothelium-dependent vasodilation of cerebral microvessels. Our findings, taken together with the results of earlier studies (reviewed in Refs. 28 and 61), point to potential additive benefits of interventions preventing metabolic dysfunction of astrocytes and promoting microvascular health for prevention of cognitive decline in the elderly.
GRANTS
This work was supported by grants from the American Heart Association (to P. Toth, A. Csiszar, Z. Tucsek, S. Tarantini, and Z. Ungvari), the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (P30 AG028718), the Oklahoma Center for the Advancement of Science and Technology (to A. Csiszar, Z. Ungvari, and W. Sonntag), the Bolyai Research Scholarship of the Hungarian Academy of Sciences (to P. Toth), the National Center for Complementary and Alternative Medicine (R01-AT006526 to Z. Ungvari), the National Institute on Aging (R01-AG047879 to A. Csiszar, R01-AG038747 to W. Sonntag, R01-NS056218 to A. Csiszar and W. Sonntag, R00-AG031182 and R01-AG043483 to J. Baur), and the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK098656 to J. Baur). We thank the University of Pennsylvania Diabetes Research Center (DRC) for the use of the Transgenic and Chimeric Mouse Core (P30-DK19525).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.T., S.T., M.N.V.-A., P.B., W.E.S., J.A.B., A.C., and Z.I.U. conception and design of research; P.T., S.T., A.D., B.V., J.A.B., A.C., and Z.I.U. performed experiments; P.T., S.T., A.D., B.V., J.A.B., A.C., and Z.I.U. analyzed data; P.T., S.T., A.D., M.N.V.-A., B.V., P.B., W.E.S., J.A.B., A.C., and Z.I.U. interpreted results of experiments; P.T., A.D., J.A.B., A.C., and Z.I.U. prepared figures; P.T., A.C., and Z.I.U. drafted manuscript; P.T., S.T., A.D., M.N.V.-A., P.B., W.E.S., J.A.B., A.C., and Z.I.U. edited and revised manuscript; P.T., S.T., A.D., M.N.V.-A., B.V., P.B., W.E.S., J.A.B., A.C., and Z.I.U. approved final version of manuscript.
REFERENCES
- 1.Aldasoro M, Martinez C, Vila JM, Medina P, Lluch S. Influence of endothelial nitric oxide on adrenergic contractile responses of human cerebral arteries. J Cereb Blood Flow Metab 16: 623–628, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA 99: 9840–9845, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin SA, Santhanam AV, Hinton DJ, Choi DS, Katusic ZS. Endothelial nitric oxide deficiency promotes Alzheimer's disease pathology. J Neurochem 127: 691–700, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayata C, Ma J, Meng W, Huang P, Moskowitz MA. l-NA-sensitive rCBF augmentation during vibrissal stimulation in type III nitric oxide synthase mutant mice. J Cereb Blood Flow Metab 16: 539–541, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Bannerman DM, Chapman PF, Kelly PA, Butcher SP, Morris RG. Inhibition of nitric oxide synthase does not impair spatial learning. J Neurosci 14: 7404–7414, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendall JK, Heymes C, Wright TJ, Wheatcroft S, Grieve DJ, Shah AM, Cave AC. Strain-dependent variation in vascular responses to nitric oxide in the isolated murine heart. J Mol Cell Cardiol 34: 1325–1333, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci 34: 13139–13150, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown RE, Wong AA. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn Mem 14: 134–144, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc 3: e000787, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Korshunov VA, Massett MP, Yan C, Berk BC. Impaired vasorelaxation in inbred mice is associated with alterations in both nitric oxide and super oxide pathways. J Vasc Res 44: 504–512, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Cobb BL, Ryan KL, Frei MR, Guel-Gomez V, Mickley GA. Chronic administration of l-NAME in drinking water alters working memory in rats. Brain Res Bull 38: 203–207, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 54: 668–675, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson KJ, de Cabo R, Pacher P, Zhang C, Ungvari ZI. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297: H13–H20, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721–H2735, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Csiszar A, Pinto JT, Gautam T, Kleusch C, Hoffmann B, Tucsek Z, Toth P, Sonntag WE, Ungvari Z. Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci 70: 303–313, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–H1699, 2006. [DOI] [PubMed] [Google Scholar]
- 17.da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation 119: 871–879, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Torre JC, Aliev G. Inhibition of vascular nitric oxide after rat chronic brain hypoperfusion: spatial memory and immunocytochemical changes. J Cereb Blood Flow Metab 25: 663–672, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Delekate A, Fuchtemeier M, Schumacher T, Ulbrich C, Foddis M, Petzold GC. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat Commun 5: 5422, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Dere E, Frisch C, De Souza Silva MA, Godecke A, Schrader J, Huston JP. Unaltered radial maze performance and brain acetylcholine of the endothelial nitric oxide synthase knockout mouse. Neuroscience 107: 561–570, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Dunn KM, Hill-Eubanks DC, Liedtke WB, Nelson MT. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci USA 110: 6157–6162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn KM, Nelson MT. Neurovascular signaling in the brain and the pathological consequences of hypertension. Am J Physiol Heart Circ Physiol 306: H1–H14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24: 31–55, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Duza T, Sarelius IH. Conducted dilations initiated by purines in arterioles are endothelium dependent and require endothelial Ca2+. Am J Physiol Heart Circ Physiol 285: H26–H37, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Filosa JA, Iddings JA. Astrocyte regulation of cerebral vascular tone. Am J Physiol Heart Circ Physiol 305: H609–H619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filosa JA, Morrison HW, Iddings JA, Du W, Kim KJ. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giachini FR, Leite R, Osmond DA, Lima VV, Inscho EW, Webb RC, Tostes RC. Anti-platelet therapy with clopidogrel prevents endothelial dysfunction and vascular remodeling in aortas from hypertensive rats. PLoS One 9: e91890, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100: 328–335, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol 27: 303–309, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86: 1009–1031, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Higashimori H, Blanco VM, Tuniki VR, Falck JR, Filosa JA. Role of epoxyeicosatrienoic acids as autocrine metabolites in glutamate-mediated K+ signaling in perivascular astrocytes. Am J Physiol Cell Physiol 299: C1068–C1078, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-l-arginine. J Cereb Blood Flow Metab 16: 981–987, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Iadecola C. The pathobiology of vascular dementia. Neuron 80: 844–866, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol 78: 651–659, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Institoris A, Rosenegger DG, Gordon GR. Arteriole dilation to synaptic activation that is sub-threshold to astrocyte endfoot Ca transients. J Cereb Blood Flow Metab 35: 1411–1415, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishida K, Matsumoto T, Taguchi K, Kamata K, Kobayashi T. Mechanisms underlying reduced P2Y(1) -receptor-mediated relaxation in superior mesenteric arteries from long-term streptozotocin-induced diabetic rats. Acta Physiol (Oxf) 207: 130–141, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Jacob PF, Vaz SH, Ribeiro JA, Sebastiao AM. P2Y1 receptor inhibits GABA transport through a calcium signalling-dependent mechanism in rat cortical astrocytes. Glia 62: 1211–1226, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Katusic ZS, Austin SA. Endothelial nitric oxide: protector of a healthy mind. Eur Heart J 35: 888–894, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 95: 1019–1026, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Lalo U, Rasooli-Nejad S, Pankratov Y. Exocytosis of gliotransmitters from cortical astrocytes: implications for synaptic plasticity and aging. Biochem Soc Trans 42: 1275–1281, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Lecrux C, Kocharyan A, Sandoe CH, Tong XK, Hamel E. Pyramidal cells and cytochrome P450 epoxygenase products in the neurovascular coupling response to basal forebrain cholinergic input. J Cereb Blood Flow Metab 32: 896–906, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeMaistre JL, Sanders SA, Stobart MJ, Lu L, Knox JD, Anderson HD, Anderson CM. Coactivation of NMDA receptors by glutamate and D-serine induces dilation of isolated middle cerebral arteries. J Cereb Blood Flow Metab 32: 537–547, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci USA 110: E4678–E4687, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Li C, Falck JR, Roman RJ, Harder DR, Koehler RC. Interaction of nitric oxide, 20-HETE, and EETs during functional hyperemia in whisker barrel cortex. Am J Physiol Heart Circ Physiol 295: H619–H631, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab 20: 183–190, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci 26: 2862–2870, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miao LY, Tang JP, Esposito DP, Zhang JH. Age-related changes in P2 receptor mRNA of rat cerebral arteries. Exp Gerontol 37: 67–79, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Munoz MF, Puebla M, Figueroa XF. Control of the neurovascular coupling by nitric oxide-dependent regulation of astrocytic Ca(2+) signaling. Front Cell Neurosci 9: 59, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Obasi CN, Cruickshanks KJ, Nondahl DM, Klein BE, Klein R, Nieto FJ, Shankar A, Fischer ME, Tsai MY, Chappell R. Association of Biomarkers for Inflammation, Endothelial Dysfunction and Oxidative Stress with Cognitive Impairment. The Epidemiology of Hearing Loss Study (EHLS). Oxid Antioxid Med Sci 1: 169–173, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci 1: 4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otsu Y, Couchman K, Lyons DG, Collot M, Agarwal A, Mallet JM, Pfrieger FW, Bergles DE, Charpak S. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci 18: 210–218, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem 282: 28749–28758, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab 27: 1908–1918, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Park L, Koizumi K, El Jamal S, Zhou P, Previti ML, Van Nostrand WE, Carlson G, Iadecola C. Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke 45: 1815–1821, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Rev 63: 83–92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pelligrino DA, Vetri F, Xu HL. Purinergic mechanisms in gliovascular coupling. Semin Cell Dev Biol 22: 229–236, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petravicz J, Boyt KM, McCarthy KD. Astrocyte IP3R2-dependent Ca(2+) signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci 8: 384, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron 71: 782–797, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Rickard NS, Gibbs ME, Ng KT. Inhibition of the endothelial isoform of nitric oxide synthase impairs long-term memory formation in the chick. Learn Mem 6: 458–466, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rutkai I, Katakam PV, Dutta S, Busija DW. Sustained mitochondrial functioning in cerebral arteries after transient ischemic stress in the rat: a potential target for therapies. Am J Physiol Heart Circ Physiol 307: H958–H966, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ryan MJ, Didion SP, Davis DR, Faraci FM, Sigmund CD. Endothelial dysfunction and blood pressure variability in selected inbred mouse strains. Arterioscler Thromb Vasc Biol 22: 42–48, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Sena CM, Pereira AM, Carvalho C, Fernandes R, Seica RM, Oliveira CR, Moreira PI. Type 2 diabetes aggravates Alzheimer's disease-associated vascular alterations of the aorta in mice. J Alzheimers Dis 45: 127–138, 2014. [DOI] [PubMed] [Google Scholar]
- 66.Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab 28: 111–125, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci 70: 1355–1359, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab 35: 527–530, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stefanova I, Stephan T, Becker-Bense S, Dera T, Brandt T, Dieterich M. Age-related changes of blood-oxygen-level-dependent signal dynamics during optokinetic stimulation. Neurobiol Aging 34: 2277–2286, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Stobart JL, Lu L, Anderson HD, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci USA 110: 3149–3154, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takano T, Oberheim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke 40: S8–S12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9: 260–267, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges E, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thyssen A, Hirnet D, Wolburg H, Schmalzing G, Deitmer JW, Lohr C. Ectopic vesicular neurotransmitter release along sensory axons mediates neurovascular coupling via glial calcium signaling. Proc Natl Acad Sci USA 107: 15258–15263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toda N, Ayajiki K, Okamura T. Obesity-induced cerebral hypoperfusion derived from endothelial dysfunction: one of the risk factors for Alzheimer's disease. Curr Alzheimer Res 11: 733–744, 2014. [DOI] [PubMed] [Google Scholar]
- 76.Topcuoglu MA, Aydin H, Saka E. Occipital cortex activation studied with simultaneous recordings of functional transcranial Doppler ultrasound (fTCD) and visual evoked potential (VEP) in cognitively normal human subjects: effect of healthy aging. Neurosci Lett 452: 17–22, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Toth P, Rozsa B, Springo Z, Doczi T, Koller A. Isolated human and rat cerebral arteries constrict to increases in flow: role of 20-HETE and TP receptors. J Cereb Blood Flow Metab 31: 2096–2105, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell 14: 400–408, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol 306: H299–H308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab 34: 1887–1897, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: Effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease. J Gerontol A Biol Sci Med Sci 69: 1212–1226, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci 69: 1339–1352, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18–H24, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876–H1881, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417–H2424, 2007. [DOI] [PubMed] [Google Scholar]
- 87.Vendemiale G, Romano AD, Dagostino M, de Matthaeis A, Serviddio G. Endothelial dysfunction associated with mild cognitive impairment in elderly population. Aging Clin Exp Res 25: 247–255, 2013. [DOI] [PubMed] [Google Scholar]
- 88.Veresh Z, Racz A, Lotz G, Koller A. ADMA impairs nitric oxide-mediated arteriolar function due to increased superoxide production by angiotensin II-NAD(P)H oxidase pathway. Hypertension 52: 960–966, 2008. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 9: 816–823, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Webb TE, Feolde E, Vigne P, Neary JT, Runberg A, Frelin C, Barnard EA. The P2Y purinoceptor in rat brain microvascular endothelial cells couple to inhibition of adenylate cyclase. Br J Pharmacol 119: 1385–1392, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wells JA, Christie IN, Hosford PS, Huckstepp RT, Angelova PR, Vihko P, Cork SC, Abramov AY, Teschemacher AG, Kasparov S, Lythgoe MF, Gourine AV. A critical role for purinergic signalling in the mechanisms underlying generation of BOLD fMRI responses. J Neurosci 35: 5284–5292, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolff M, Savova M, Malleret G, Segu L, Buhot MC. Differential learning abilities of 129T2/Sv and C57BL/6J mice as assessed in three water maze protocols. Behav Brain Res 136: 463–474, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Xu HL, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp Physiol 92: 647–651, 2007. [DOI] [PubMed] [Google Scholar]
- 94.Xu HL, Ye S, Baughman VL, Feinstein DL, Pelligrino DA. The role of the glia limitans in ADP-induced pial arteriolar relaxation in intact and ovariectomized female rats. Am J Physiol Heart Circ Physiol 288: H382–H388, 2005. [DOI] [PubMed] [Google Scholar]
- 95.You J, Johnson TD, Childres WF, Bryan RM Jr. Endothelial-mediated dilations of rat middle cerebral arteries by ATP and ADP. Am J Physiol Heart Circ Physiol 273: H1472–H1477, 1997. [DOI] [PubMed] [Google Scholar]
- 96.Zaletel M, Strucl M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct Neurol 20: 115–120, 2005. [PubMed] [Google Scholar]