Soluble epoxide hydrolase (sEH)-dependent regulation of arteriolar response to shear stress and control of arteriolar tone, as a function of sex, is discussed. A downregulation of sEH in gonadally intact female mice imitates actions of sEH gene deletion, revealing the possibility of sEH inhibitors being used as substitutes for estrogen replacement therapy in postmenopausal women.
Keywords: sex, soluble epoxide hydrolase, epoxyeicosatrienoic acids, arterioles, flow/shear stress-induced vasodilation
Abstract
We hypothesized that potentiating the bioavailability of endothelial epoxyeicosatrienoic acids (EETs) via deletion of the gene for soluble epoxide hydrolase (sEH), or downregulation of sEH expression, enhances flow/shear stress-induced dilator responses (FID) of arterioles. With the use of male (M) and female (F) wild-type (WT) and sEH-knockout (KO) mice, isolated gracilis muscle arterioles were cannulated and pressurized at 80 mmHg. Basal tone and increases in diameter of arterioles as a function of perfusate flow (5, 10, 15, 20, and 25 μl/min) were recorded. The magnitude of FID was significantly smaller and associated with a greater arteriolar tone in M-WT than F-WT mice, revealing a sex difference in FID. This sex difference was abolished by deletion of the sEH gene, as evidenced by an enhanced FID in M-KO mice to a level comparable with those observed in F-KO and F-WT mice. These three groups of mice coincidentally exhibited an increased endothelial sensitivity to shear stress (smaller WSS50) and were hypotensive. Endothelial EETs participated in the mediation of enhanced FID in M-KO, F-KO, and F-WT mice, without effects on FID of M-WT mice. Protein expression of sEH was downregulated by approximately fourfold in vessels of F-WT compared with M-WT mice, paralleled with greater vascular EET levels that were statistically comparable with those observed in both male and female sEH-KO mice. In conclusion, sex-different regulation of sEH accounts for sex differences in flow-mediated dilation of microvessels in gonadally intact mice.
NEW & NOTEWORTHY
Soluble epoxide hydrolase (sEH)-dependent regulation of arteriolar response to shear stress and control of arteriolar tone, as a function of sex, is discussed. A downregulation of sEH in gonadally intact female mice imitates actions of sEH gene deletion, revealing the possibility of sEH inhibitors being used as substitutes for estrogen replacement therapy in postmenopausal women.
epoxyeicosatrienoic acids (EETs) are metabolites of arachidonic acids processed by cytochrome P-450/epoxygenase. EETs are synthesized in the endothelium and are rapidly incorporated into membrane phospholipids (5), from which they are released in response to any of the stimuli that activates phospholipases, such as shear stress, to initiate vasodilation (5, 11–13, 40) and lower blood pressure (1, 20, 33). Additionally, the vascular endothelium can rapidly convert EETs to dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydroxylase (sEH), an enzyme encoded by the Ephx2 gene (2). Much smaller amounts of DHETs are incorporated into endothelial cell lipids (40), and DHETs themselves are either less active or lacking in bioactivity all together (15, 16). Thus obstructing the hydrolysis of EETs via blocking sEH-mediated signaling to increase EET bioactivities merits consideration as a potential therapeutic option to reduce progression of disease processes. Indeed, mice lacking the Ephx2 gene have significantly lower blood pressure (24, 31, 33), and sEH inhibitors significantly lower blood pressure in various models of hypertension (17, 18, 33, 43). Intriguingly, the disruption of the Ephx2 gene in male mice significantly decreases blood pressure but has minimal effects on blood pressure in females (24). This points to an unaddressed question, which challenges the importance of sEH in females and also reveals an appealing research direction for the sex-specific regulation of sEH.
In addition to the myogenic response, flow/shear stress-induced vasodilation also serves as an important player in the local regulation of arteriolar tone and, consequently, peripheral resistance. Following the activation of endothelial phospholipase by shear tress, EETs are released to hyperpolarize vascular smooth muscle, leading to vasodilation (12). We demonstrated previously that estrogen affords cardiovascular protection in response to nitric oxide (NO) deficiency, via recruitment of EET-dependent signaling as a back-up mechanism to maintain normal arteriolar tone, as evidenced by an EET-mediated potentiation of flow/shear stress-induced vasodilation in the NO-deficient female vessels or male vessels chronically treated with estrogens (10–12, 14, 35, 36, 38, 39, 42). Therefore, we defined a female phonotype of flow-induced responses, characterized as 1) greater vasodilation, which is associated with an EET-mediated portion in female vessels, or male vessels chronically exposed to estrogens; and 2) the presence of an interaction between NO- and EET-mediated portions of responses. We also demonstrated that in male sEH-deficient mice, an attenuated myogenic constriction in skeletal muscle arterioles prevented hypertension (33). Additionally, sEH deficiency reduced coronary resistance to improve cardiac perfusion (29). As such, not only the upregulation of vascular EET synthesis but also reduction of their degradation would increase EET bioavailability in the vasculature. A search of the literature reveals that some studies have indicated anti-inflammatory effects of shear stress on the endothelium via an EETs/sEH-dependent pathway (22, 23), but studies involving the role of sEH in the modulation of shear stress-induced vasodilation remain unknown. Additionally, the issue as to whether the disruption of the Ephx2 gene affects vascular responsiveness in males and females similarly has never been addressed. Given these gaps in the field, we tested the hypothesis that potentiating endothelial EETs, as a function of diminishing their degradation, enhances flow/shear stress-induced dilator responses. We also hypothesized that a downregulation of vascular sEH in female mice is responsible for their minimal hypotensive response to Ephx2 gene knockout (KO).
MATERIALS AND METHODS
Animals.
Twelve to 15-wk-old male (M) and female (F) Ephx2−/− (sEH-KO) and Ephx2+/+ [wild type (WT)] mice, abbreviated as M-KO, F-KO, M-WT, and F-WT, respectively, were used. As described previously (33), cryorecovered heterozygoues (Ephx2+/−, B6.129X-Ephx2tm1Gonz/J) and WT mice were received from the Jackson Laboratory (Bar Harbor, ME), and the homozygous (sEH-KO) mice were developed in the Department of Comparative Medicine, New York Medical College.
All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conform to the guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals.
Measurement of blood pressure and isolation of vessels.
Mice were anesthetized by inhalation of Isothesia (isoflurane). Blood pressure was recorded using a carotid artery catheterization with a controlled heart rate (∼450 beats/min) by adjusting the depth of anesthesia (29). Mesenteric arteries were isolated for LC/MS/MS and Western blot analyses. Gracilis muscles from both legs were dissected, and second-order arterioles were isolated and cannulated in a vessel chamber perfused with physiological salt solution (PSS) for the study of flow-induced dilator responses.
LC/MS/MS-based measurements for EETs and DHETs in isolated arteries.
The measurement was described in detail previously (29, 33). Isolated mesenteric arteries were pulverized in liquid nitrogen. Phospholipids were extracted with the Bligh-Dyer method, and EETs and DHETs were extracted with ethyl acetate following alkali hydrolysis of the phospholipids to release esterified EETs or DHETs and quantified with a Q-trap 3200 linear ion trap quadruple LC/MS/MS (AB ScieX; Qtrap 3200) equipped with a Turbo V ion source operated in negative electrospray mode (Applied Biosystems, Foster City, CA). Protein concentration of samples was determined by the Bradford method (Bio-Rad) and was used to normalize the detected lipids. Data are presented as total EETs and DHETs and expressed as picograms per milligram protein. Mesenteric arteries were selected for the assessment of EETs and DHETs because of their abundance and sufficient length. Also, mesenteric arteries have similar patterns of vascular response and endothelial mediators to shear stress as those found in the gracilis muscle arteries in both rats and mice (12, 14, 33, 38, 39).
Flow/shear stress-induced vasodilation.
The microcirculation of skeletal muscle is responsible for the sizable fraction of peripheral resistance. To this end, isolated gracilis muscle arterioles (90–150 μm in diameter) were cannulated in a vessel chamber and perfused with PSS containing (in mM) 117 NaCl, 4.7 KCl, 1.1 MgSO4, 1.2 KH2PO4, 5.5 glucose, 2.5 CaCl2, 2.0 pyruvate, 0.1 ascorbate, and 0.1 L-arginine. The PSS was gassed in a water-jacketed (37°C) reservoir with 95%O2-5%CO2 and equilibrated with NaHCO3 (∼24 mM) to a pH value of 7.4. The procedure of isolating and cannulating arterioles has been described in detailed previously (11, 14). Isolated arterioles were equilibrated, under 80 mmHg without flow for 1 h, during which all vessels developed spontaneous tone that reached to ∼60–70% of their maximal diameter (passive diameter). The known values of 5, 10, 15, 20, and 25 μl/min flow rates were sequentially applied to vessels via a syringe pump (Harvard Apparatus), and changes in diameter were recorded. Changes in diameter of arterioles in response to each dose of flow rate were kept stable for ∼2 min before second dose was applied. After control experiments, Nω-nitro-l-arginine methyl ester [l-NAME; 3 × 10−4 M; NO synthase (NOS) inhibitor], indomethacin (10−5 M; cyclooxygenase inhibitor), and 6-(2-proparglyoxyphenel) hexanoic acid (PPOH; 5 × 10−5 M, an inhibitor of CYP/epoxygenase) were administered, either alone or in alternating combinations, to the vessels and incubated for 45 min before the flow-induced vasodilation was once again recorded. At the conclusion of the experiments, vessels were incubated in a Ca2+-free solution with 1 mM EGTA for 10 min, followed by recording their passive diameter (PD) at 80 mmHg.
Western blot analysis.
Mesenteric arteries/arterioles isolated from the four groups of mice were pulverized in liquid N2. Equal amounts of total protein (25 μg) extracted from vessels were loaded on a 10% SDS-PAGE gel and transferred to a polyvinylidene difluoride membrane. The membrane was probed with specific primary antibody for sEH or endothelial NOS (Santa Cruz Biotechnology) and secondary antibody conjugated with horseradish peroxidase. Specific bands were visualized with a chemiluminescence kit and normalized to GAPDH. The X-ray film was scanned into a computer, and band densitometry was digitalized with UN-SCAN-IT software.
Calculation and statistical analysis.
The change in diameter of arterioles in response to increase in flow was normalized to their PD and expressed as percentage of PD. The value of shear stress at each flow rate was calculated by the equation of 4ηQ/πr3, where η is the perfusate viscosity (0.007 poise at 37°C), Q is the perfusate flow (μl/min), and r is the vessel radius in centimeters. EC50 of wall shear stress (WSS50) was defined as the value of shear stress required to initiate a half-maximal dilator response of vessels and served as an index of endothelial sensitivity to shear stress. Data are expressed as means ± SE, and n refers to the number of mice. Statistical analysis was performed using one- or two-way ANOVA followed by the Tukey-Kramer post hoc test. Student's t-test or pair t-test was used where appropriate. Statistical significance was accepted at a level of P < 0.05.
RESULTS
Male mice exhibited greater responses to sEH deficiency in the control of blood pressure and arteriolar tone.
The characteristics of gracilis muscle arterioles isolated from M-WT and F-WT and M-KO and F-KO mice, as well as their mean arterial blood (MABP), were summarized in Table 1. Female mice (F-WT and F-KO) had significantly lower blood pressure than their male counterparts (M-WT and M-KO). When compared with M-WT mice, deletion of sEH (M-KO) significantly reduced MABP to a level comparable with that observed in F-WT mice. F-KO mice had the lowest MABP among the four groups of mice. In isolated vessels, active diameter and PD of arterioles were comparable among M-KO, F-WT, and F-KO mice, except for M-WT that exhibited a bigger PD and smaller active diameter. As a result, the basal arteriolar tone expressed as a percentage of the PD was significantly greater (smaller diameter) in M-WT than in the other three groups of mice. sEH deficiency in male mice (M-KO) reversed the greater basal tone to the level of female mice; the latter two (F-WT and F-KO) had similar basal arteriolar tone. These results suggest that outcomes of Ephx2 gene deletion are functionally more observable in male mice.
Table 1.
Characteristics of gracilis muscle arterioles and blood pressure of mice
| M-WT | F-WT | M-KO | F-KO | |
|---|---|---|---|---|
| n | 6–8 | 8 | 6–12 | 6–17 |
| Diameter, μm | ||||
| Active | 90.1 ± 2.1+ | 98.2 ± 3.6 | 100.1 ± 3.3 | 100.5 ± 1.7 |
| Passive | 146.3 ± 2.2+ | 132.0 ± 3.3 | 137.9 ± 4.9 | 133.8 ± 3.2 |
| Basal tone, %passive diameter | 61.5 ± 1.1+ | 74.4 ± 1.6 | 72.8 ± 1.4 | 75.4 ± 1.1 |
| Mean arterial blood pressure, mmHg | 93.5 ± 0.2 | 84.5 ± 0.5* | 83.2 ± 0.5* | 78.5 ± 1.1*# |
Values are means ± SE. F-KO, female soluble epoxide hydrolase knockout (sEH-KO) mice. +Significant difference from the other 3 groups of mice;
significant difference from male wild-type (M-WT);
significant difference from female wild-type (F-WT) and male sEH-KO (M-KO) mice.
sEH-deficient mice display increased levels of vascular EETs.
Table 2 shows that M-KO mice exhibited significant increases in vascular EETs, as a function of reduction in their hydrolysis (DHETs), leading to a higher ratio of EETs to DHETs compared with that of M-WT controls, providing functional evidence for Ephx2 gene deficiency. On the other hand, arteries of F-WT mice had significantly higher total level of EETs and lower DHETs to form a greater ratio of EETs to DHETs than M-WT mice, revealing a sex difference in the EET metabolism profile. The sex difference in vascular EET parameters was essentially disappeared by sEH deletion, showing a nonstatistical difference in levels of vascular EETs, DHETs, and the ratio of EETs to DHETs in M-KO and F-KO mice. However, unlike M-KO mice, the deletion of sEH in females (F-KO) failed to initiate statistically significant increases in total EETs and EETs/DHETs in comparison with F-WT mice, revealing a sex-different response to sEH deficiency.
Table 2.
Vascular EET levels in the mesenteric arteries of mice
| M-WT | F-WT | M-KO | F-KO | |
|---|---|---|---|---|
| n | 9 | 8 | 6 | 6 |
| Total EETs, pg/mg protein | 0.285 ± 0.03 | 0.345 ± 0.089* | 2.192 ± 1.802* | 1.601 ± 0.382* |
| Total DHETs | 0.055 ± 0.009 | 0.026 ± 0.01* | 0.119 ± 0.058 | 0.07 ± 0.017 |
| EETs/DHETs | 6.167 ± 1.078 | 16.142 ± 2.644* | 16.043 ± 3.532* | 24.065 ± 5.23* |
Values are means ± SE. EET, epoxyeicosatrienoic acids; DHET, dihydroxyeicosatrienoic acid.
Significant difference from M-WT.
sEH deficiency enhanced flow-induced vasodilation in arterioles of male mice.
Increasing flow from 0 to 25 μl/min elicited significant increases in arteriolar diameter in M-WT and M-KO mice. The magnitude of flow-induced dilation was significantly greater in M-KO than in M-WT controls (Fig. 1A); as a result, the same flow rate elicited significantly smaller increments of shear stress in arterioles of M-KO than in M-WT mice (15.8 ± 2.0 vs. 21.6 ± 1.6 dyne/cm2 at 25 μl/min flow; Fig. 1B). This suggests that sEH deficiency promotes flow-induced vasodilation, as a function of increased endothelial sensitivity to shear stress.
Fig. 1.
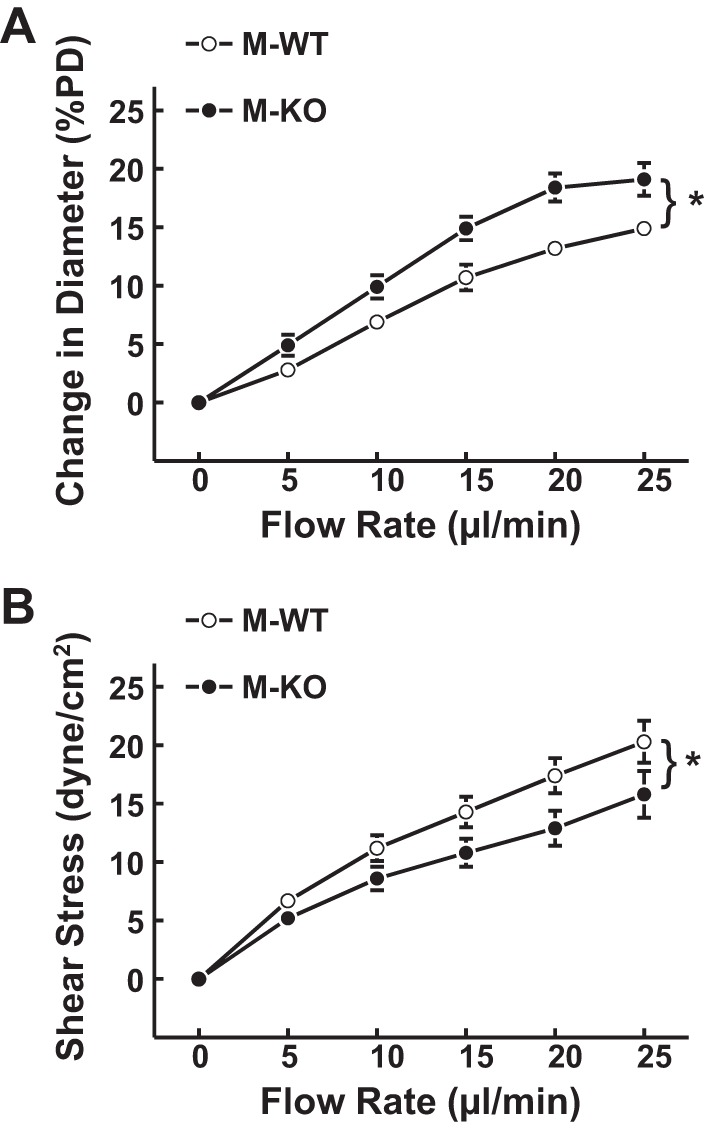
Normalized diameter of gracilis muscle arterioles (A) and calculated shear stress (B) as a function of perfusate flow in male wild-type (M-WT; n = 8) and male soluble epoxide hydrolase (sEH) knockout (M-KO; n = 12) mice. *Significant difference between the 2 curves. PD, passive diameter.
In the next series of experiments, the endothelial mediators responsible for the flow-induced dilation in arterioles of male WT and sEH-KO mice were assessed. Consistent with our previous findings, corelease of NO and prostaglandins mediated flow-induced dilation in arterioles of M-WT mice (19, 35). As shown in Fig. 2A, l-NAME initiated a reduction of flow-induced dilation by ∼48%, and the remaining portion of dilator responses was completely abolished by additional administration of indomethacin. Interestingly, in arterioles of M-KO mice, l-NAME alone did not affect flow-induced dilation (Fig. 2B) but significantly inhibited the response by ∼55% when combined with an inhibitor of EET synthase (PPOH). The same responsive pattern was observed when vessels were exposed to PPOH alone, followed by PPOH plus l-NAME (data not shown). These results suggest a negative interaction between the two mediators. Inhibition of NO synthesis with l-NAME disinhibited its inhibitory effects on EETs, making the PPOH-sensitive portion of the responses discernable, and vice versa. Additional administration of indomethacin essentially eliminated the residual portion of vasodilation; the percentage of inhibition (∼41%) was similar to that induced by indomethacin alone (data not shown), indicating that the prostaglandin-mediated response was not significantly influenced by NO or EETs.
Fig. 2.
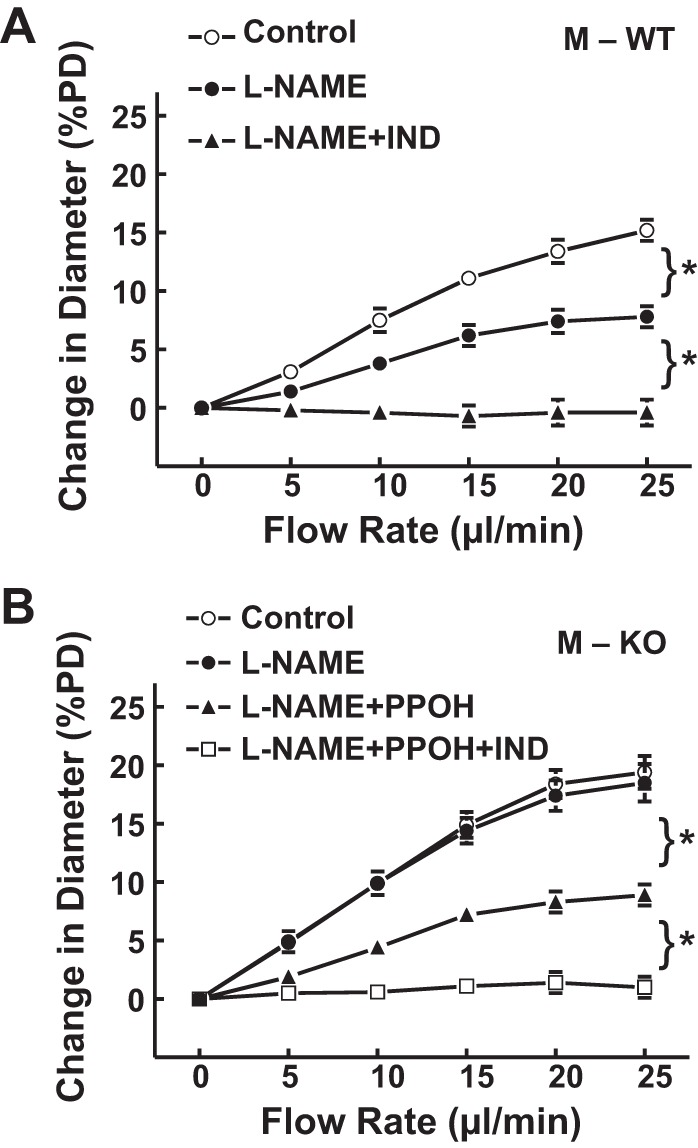
Normalized diameter of gracilis muscle arterioles as a function of perfusate flow, in the control condition, in the presence of Nω-nitro-l-arginine methyl ester (l-NAME; 3 × 10−4) and l-NAME + indomethacin (IND; 10−5 M) or l-NAME + 6-(2-proparglyoxyphenel) hexanoic acid (PPOH; 5 × 10−5 M) and with additional IND in M-WT (A; n = 6) and M-KO (B; n = 6–12) mice. *Significant difference between the 2 curves.
sEH deficiency has minimal effects on flow-induced vasodilation in female mice.
M-WT mice had a smaller magnitude of flow-induced dilation and less endothelial sensitivity to shear stress compared with F-WT mice, responses that were improved significantly in M-KO mice (Fig. 1). Contrastingly, arterioles of F-KO mice did not display significant differences in the magnitude of flow-induced dilation (Fig. 3A) and endothelial sensitivity to shear stress (Fig. 3B) compared with those of F-WT controls. Moreover, both F-WT and F-KO mice exhibited a similar phenotype of endothelial mediators in response to shear stress (Fig. 4). Specifically, in F-WT (Fig. 4A) and F-KO mice (Fig. 4, B and C), neither l-NAME nor PPOH alone was able to inhibit flow-induced dilation. In the presence of both inhibitors, however, the flow-induced vasodilation was significantly inhibited by ∼59% and ∼63%, respectively. This coincides with the phenomenon observed in M-KO mice (Fig. 2B) indicating that the NO-mediated response is revealed only when EET synthase has been blocked by PPOH (Fig. 4C) and vice versa for the EET-mediated portion that emerged in the absence of NO after l-NAME treatment (Fig. 4, A and B). Additionally, the prostaglandin-mediated dilator portion was eliminated by indomethacin.
Fig. 3.
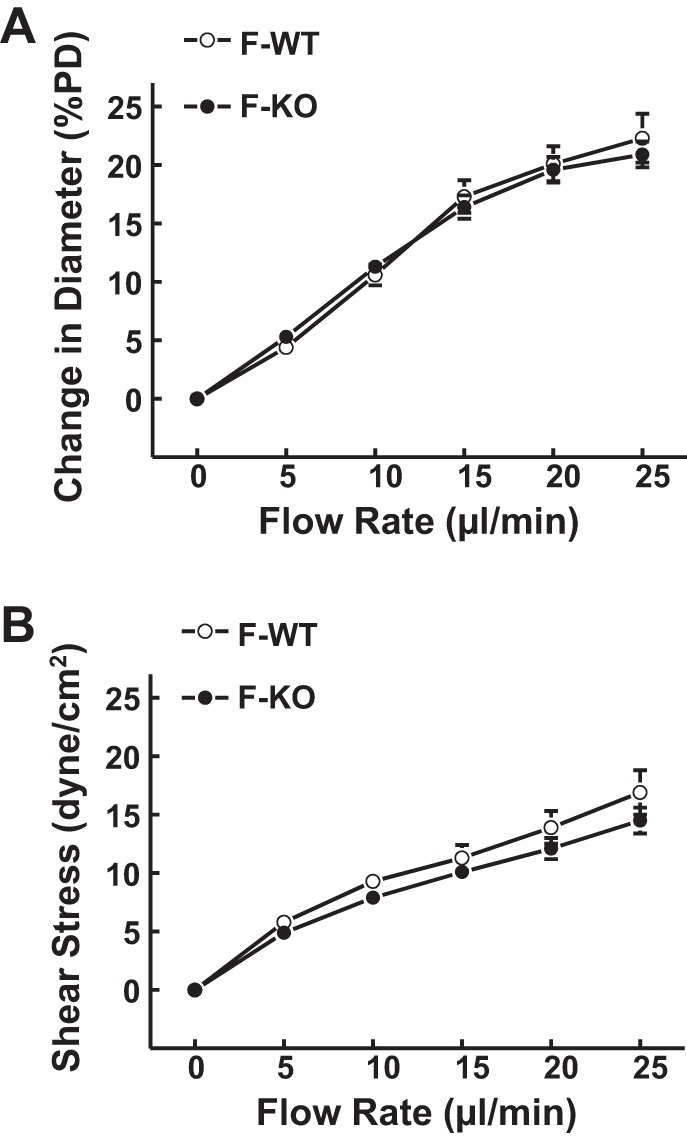
Normalized diameter of gracilis muscle arterioles (A) and calculated shear stress (B) as a function of perfusate flow in female WT (F-WT; n = 8) and female sEH-KO (F-KO; n = 17) mice.
Fig. 4.
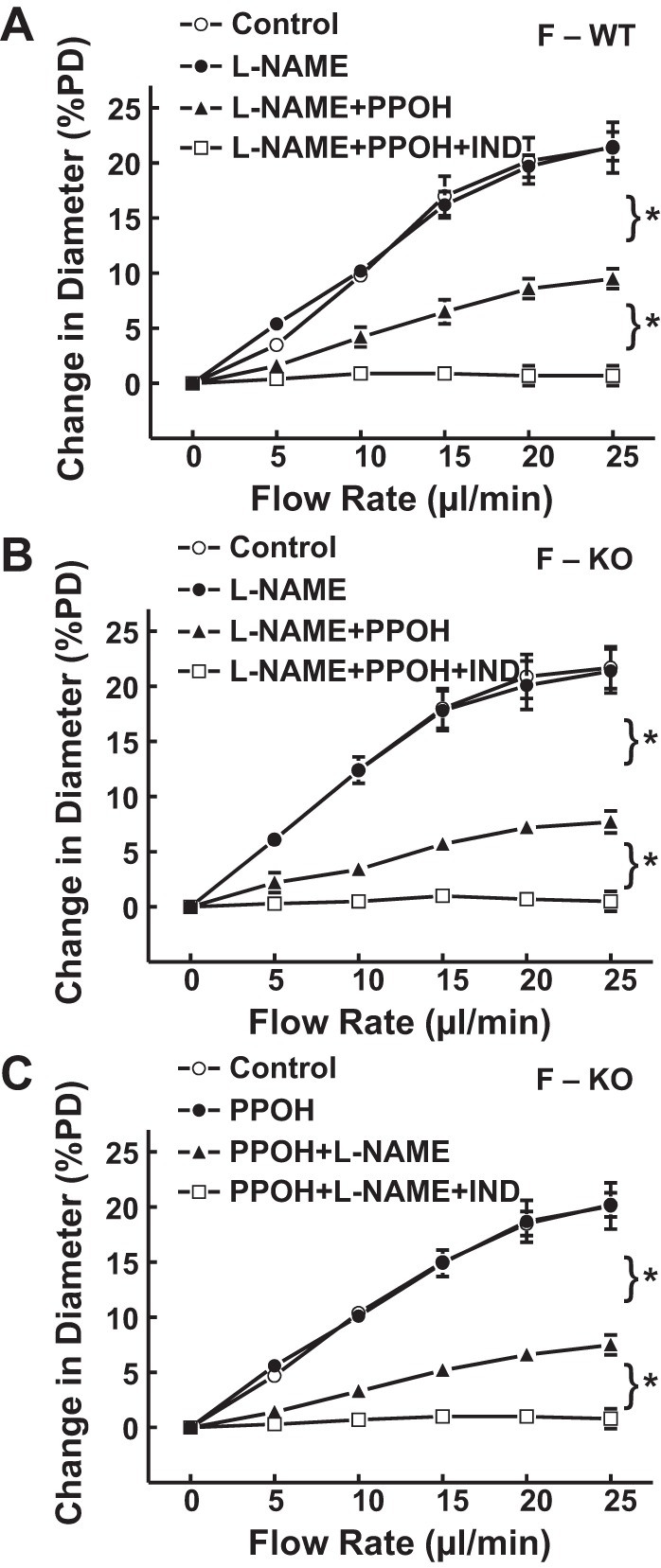
A: normalized diameter of gracilis muscle arterioles in F-WT mice (n = 6), as a function of perfusate flow in the control condition, in the presence of l-NAME, l-NAME + PPOH, and with additional IND. B and C: normalized diameter of gracilis muscle arterioles in F-KO (n = 8 and 9) as a function of perfusate flow, in the control condition, in the presence of l-NAME or PPOH alone, combination of the 2 inhibitors, and with additional IND mice. *Significant difference between the 2 curves.
Collectively, data of flow-induced dilation obtained from the four groups of mice are summarized in Fig. 5, showing an identical enhancement in flow-induced vasodilation associated with EET-mediation (Figs. 2–4) and reduction in WSS50 (an index of endothelial sensitivity to shear stress) in M-KO (WSS50, 8.9 ± 1.3 dyne/cm2), F-WT (WSS50, 8.7 ± 1.1 dyne/cm2), and F-KO mice (WSS50, 7.9 ± 1.0 dyne/cm2) in comparison with M-WT mice (WSS50, 12.4 ± 2.0 dyne/cm2).
Fig. 5.
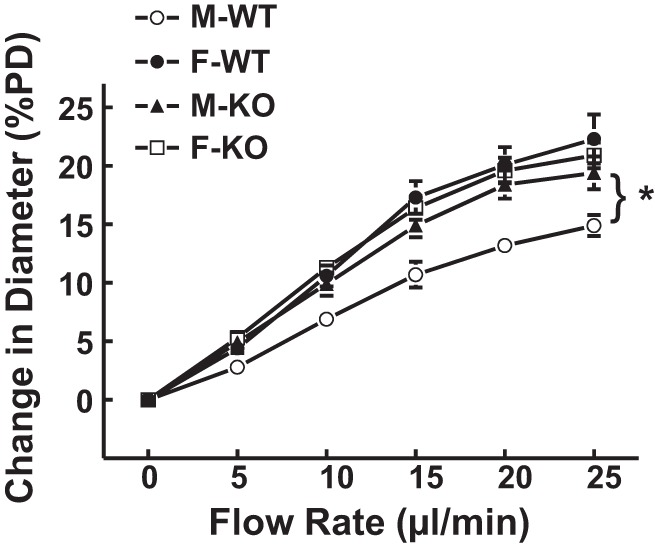
Normalized diameter of gracilis muscle arterioles, as a function of perfusate flow in the 4 groups of mice (n = 8–17 in each group). *Significant difference from M-WT mice.
Downregulation of sEH expression in female mice.
Figure 6 shows the original and summarized data from Western blot analysis, indicating that sEH protein expression in mesenteric arteries was dramatically suppressed by approximately fourfold in F-WT compared with M-WT mice (Fig. 6A). As indicated previously (33), vascular sEH protein was undetectable in sEH-KO mice, and, also, endothelial NOS expression was comparable among the four groups of mice (data not shown).
Fig. 6.
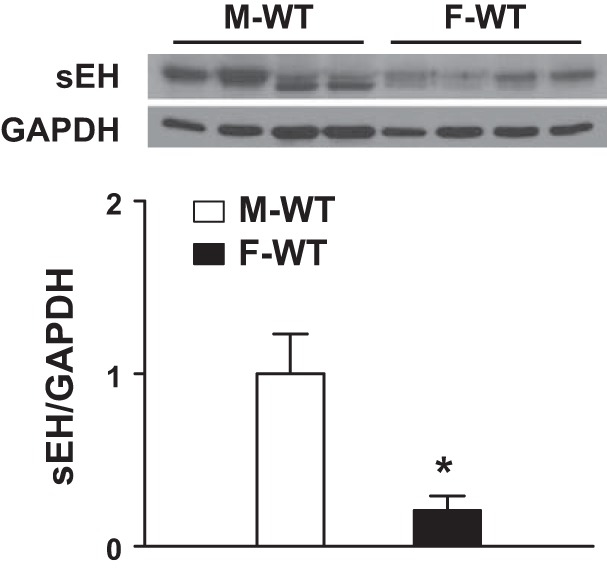
Protein expression of sEH in mesenteric arteries of male and female WT mice. *Significant difference from M-WT mice (n = 3 blots).
DISCUSSION
The salient findings of the present study are that deletion of the sEH gene evokes an enhanced EET-mediated flow-induced vasodilation in skeletal muscle arterioles. Moreover, a downregulation of sEH expression in female mice imitates the action of Ephx2 deletion, resulting in an identical phenotype of arteriolar/endothelial responsiveness to shear stress in M-KO, F-KO, and F-WT mice. To the best of our knowledge, this is the first study that indicates a specific role of sEH in the modulation of endothelial sensitivity to shear stress. During this process, suppression of sEH expression generates a duplicated/overlapping disruption of the sEH gene, a mechanism that may contribute to the sex difference in response to Ephx2 knockout. Thus the present study provides a mechanistically based explanation for the paradoxically compromised hypotensive activity of sEH knockout in females, a phenomenon that was once speculated to be attributed to an upregulation of renal 20-HETE production compensating for the loss of the Ephx2 gene (24).
Sex difference in response to sEH deficiency.
In male mice, Ephx2 gene deletion significantly lowered blood pressure, enhanced flow-induced dilation in an EET-dependent manner (Figs. 1 and 2), and attenuated basal arteriolar tone (Table 1), as a consequence of increased vascular EET levels (Table 2). In contrast, F-KO mice exhibited a similar vascular responsive phenotype to shear stress as that displayed by F-WT controls (Figs. 3 and 4), suggesting a sex difference in the vascular response to sEH deficiency. This finding may challenge the cardioprotective effectiveness of sEH inhibitors in females, since these compounds have been used in clinical trials (3), as well as on healthy human volunteers (4).
Aiming to clarify the nature of the sex-different response to sEH deficiency, we tested the hypothesis that the minimal vascular responses to Ephx2 deletion in females attribute to their inherently compromised sEH activity. We indeed indicated a sex difference in blood pressure and vascular behaviors, which includes the phenotype of flow-induced dilation (Fig. 5) and endothelial sensitivity to shear stress, basal arteriolar tone (Table 1), and vascular profile of EET metabolism (Table 2) between M-WT and F-WT mice. However, knockout of the Ephx2 gene in males (M-KO) switched their vascular responsive phenotype (Fig. 2B) to the same pattern as those observed in both F-WT (Fig. 4A) and F-KO mice (Fig. 4, B and C). This supports our hypothesis that the female sex possesses a specific regulatory mechanism that duplicates the outcome of Ephx2 gene deletion. Consequently, female sex (e.g., F-WT mice), sEH disruption (M-KO mice), or the redundant presence of both (F-KO mice) is able to generate an identical pattern of vascular responsiveness, perhaps, even with some synergistic/additive effects in the case of F-KO mice, which indeed displayed the lowest blood pressure among the four groups of mice. The enhanced vasodilation to shear stress and attenuated basal myogenic tone, two of the most important local regulators in the control of arteriolar diameter and peripheral resistance (34), in arterioles of M-KO, F-KO, and F-WT mice indicate a female-favorable regulation of microcirculation via a sEH-dependent pathway. Notably, the sex-different regulation of blood pressure seems not to be completely dependent on sEH, since sEH deficiency failed to abolish the difference (Table 1). In this regard, we interpret the result to mean that the regulation of blood pressure is a multifactorial and complex process that can be governed by varying female-favorable mechanisms in addition to the EET-mediated local regulation of vascular tone via shear stress- and myogenic-dependent mechanisms (10).
Mechanisms responsible for the differential sex-dependent responses to sEH deficiency.
Female sex and/or estrogen favors the contribution of EETs over NO in the mediation of agonist- and shear stress-induced vasodilation (25) and control of blood pressure (30) in both pathological and physiological states. In response to an overconsumption of fructose or chronic insulin-loading, only hyperinsulinemic male rats, not females, develop hypertension (6, 7). Additionally, impaired vascular responses to insulin in male rats with metabolic syndrome are not apparent in arteries of their female littermates (41), despite both sexes exhibiting significantly impaired NO-mediated responses. In the clinically failing human heart, a condition associated with significant impairment of NO synthesis, myocyte necrosis and apoptosis are significantly lower in females than in males, leading to a longer duration of the process and a later onset of cardiac decompensation in women (8). The aforementioned studies allude to the presence of a mechanism(s) by which the sex difference in susceptibility to cardiovascular disease is independent of NO, but perhaps mediated by EETs, based on their properties of preventing cardiomyocyte necrosis and apoptosis, and improving insulin sensitivity (26, 27). We also provide increasing evidence of an estrogen-dependent upregulation of EET synthesis in the compensatory adaptation of endothelial sensitivity to shear stress in response to impaired NO bioavailability (10–12, 14, 36, 39, 42). All of these studies provided support for female-favorable actions via EET-dependent mechanism counteracting pathological processes that are etiologically distinct. However, whether sEH plays a role in the sex-different regulation of signaling remained unknown. In the present study, a downregulation of sEH protein in vessels of female mice (Fig. 6) mimics the effect of Ephx2 deletion in males, leading to a female phenotypic regulation of vascular responsiveness to shear stress and blood pressure in male sEH-KO mice. It also explains the different levels of EETs, DHETs, and their ratio in female (F-WT) versus male (M-WT) vasculatures, whereas these differences became statistically insignificant between sEH-KO mice (Table 2). The sexually dimorphic expression of sEH was reported mostly in the liver and kidneys of rodents (21, 24, 28). A suppression of sEH expression in the brain was responsible for the better cerebral perfusion and smaller infarct size after middle cerebral artery occlusion in female compared with male mice (44). A downregulation of sEH in isolated single vessels of female mice was reported for the first time (Fig. 6). This provides molecular evidence clarifying a differential expression of vascular sEH that contributes to the sex-different regulation of arteriolar responses to shear stress.
Interaction between EETs and NO.
The concept of the female phenotype of flow-induced dilation needs now to be updated to include sEH gene disruption in male vessels (Fig. 2B) (29). In the aforementioned citations (10–14, 36–39, 42), we have provided details pertaining to the interaction between NO and EETs and have discussed the mechanisms involved. Essentially, the phenomenon of negative feedback inhibition of EETs by NO is evidenced by the fact that EET activity and its contribution to the regulation of vascular function are dampened under physiological conditions and become discernable in most instances only with decreased endothelial NO synthesis that coincides with increased EET bioavailability. In male mice/rats, flow/shear stress-induced dilation was mediated by endothelial NO and prostaglandins, without contributions from EETs. As manifested in Fig. 4C, the l-NAME-sensitive portion (NO-mediation) was discerned when EET synthesis was inhibited by PPOH, and vice versa, as evidenced in Fig. 2B and Fig. 4, A and B, showing that the EET-mediated portion was discovered only in the absence of NO.
In conclusion, the present study reveals a female-favorable regulation of shear stress-induced vasodilation, as a function of downregulated vascular sEH, which provides mechanistically based rationale for using sEH inhibitors in the treatment of cardiovascular diseases (4). On the other hand, our findings are able to bridge the gap, if any, between clinically using sEH inhibitors as therapeutic strategies and their effectiveness in female populations. Moreover, the positive relationship between estrogen replacement therapy and improvement of cardiovascular function in postmenopausal women is weak at best (9, 32). In this context, we demonstrate a female-specific downregulation of sEH that is the target of sEH inhibitors, which raises the intriguing possibility that sEH inhibitors may substitute, at least in part, for estrogen replacement therapy to benefit not only menopausal women, but male populations, as well.
GRANTS
This work was supported by National Institutes of Health Grants HL070653 and HL34300 and partially supported by National Natural Science Foundation of China 381370548 and Shanghai Municipal Bureau of Health 201344109.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Q., S.K., G.F., H.J., M.L., D.S., and A.H. performed experiments; J.Q., S.K., D.S., and A.H. analyzed data; J.Q., S.K., D.S., and A.H. interpreted results of experiments; J.Q., S.K., D.S., and A.H. prepared figures; J.Q., S.K., G.F., H.J., M.L., D.S., and A.H. approved final version of manuscript; G.F., D.S., and A.H. edited and revised manuscript; D.S. and A.H. conception and design of research; D.S. and A.H. drafted manuscript.
REFERENCES
- 1.Athirakul K, Bradbury JA, Graves JP, DeGraff LM, Ma J, Zhao Y, Couse JF, Quigley R, Harder DR, Zhao X, Imig JD, Pedersen TL, Newman JW, Hammock BD, Conley AJ, Korach KS, Coffman TM, Zeldin DC. Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB J 22: 4096–4108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chacos N, Capdevila J, Falck JR, Manna S, Martin-Wixtrom C, Gill SS, Hammock BD, Estabrook RW. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys 223: 639–648, 1983. [DOI] [PubMed] [Google Scholar]
- 3.Chen D, Whitcomb R, MacIntyre E, Tran V, Do ZN, Sabry J, Patel DV, Anandan SK, Gless R, Webb HK. Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J Clin Pharmacol 52: 319–328, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y, Theken KN, Lee CR. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J Mol Cell Cardiol 48: 331–341, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming I. Cytochrome P450 epoxygenases as EDHF synthase(s). Pharmacol Res 49: 525–533, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Galipeau D, Verma S, McNeill JH. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol 283: H2478–H2484, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Galipeau DM, Yao L, McNeill JH. Relationship among hyperinsulinemia, insulin resistance, and hypertension is dependent on sex. Am J Physiol Heart Circ Physiol 283: H562–H567, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Guerra S, Leri A, Wang X, Finato N, Di LC, Beltrami CA, Kajstura J, Anversa P. Myocyte death in the failing human heart is gender dependent. Circ Res 85: 856–866, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Harman SM. Estrogen replacement in menopausal women: recent and current prospective studies, the WHI and the KEEPS. Gend Med 3: 254–269, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation 11: 9–38, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol 280: H2462–H2469, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res 96: 376–383, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang A, Sun D, Smith CJ, Connetta JA, Shesely EG, Koller A, Kaley G. In eNOS knockout mice skeletal muscle arteriolar dilation to acetylcholine is mediated by EDHF. Am J Physiol Heart Circ Physiol 278: H762–H768, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Huang A, Sun D, Wu Z, Yan C, Carroll MA, Jiang H, Falck JR, Kaley G. Estrogen elicits cytochrome P450-mediated flow-induced dilation of arterioles in NO deficiency: role of PI3K-Akt phosphorylation in genomic regulation. Circ Res 94: 245–252, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev 24: 169–188, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov 8: 794–805, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension 46: 975–981, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Busse R, Fleming I. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension 45: 759–765, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Koller A, Sun D, Huang A, Kaley G. Corelease of nitric oxide and prostaglandins mediates flow-dependent dilation of rat gracilis muscle arterioles. Am J Physiol Heart Circ Physiol 267: H326–H332, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow AL, Lepp AN, Kannon MA, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J 24: 3770–3781, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Dahl M, Grande P, Tybjaerg-Hansen A, Nordestgaard BG. Genetically reduced soluble epoxide hydrolase activity and risk of stroke and other cardiovascular disease. Stroke 41: 27–33, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, Shyy JY. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA 102: 16747–16752, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Zhu Y, Rannou F, Lee TS, Formentin K, Zeng L, Yuan X, Wang N, Chien S, Forman BM, Shyy JY. Laminar flow activates peroxisome proliferator-activated receptor-gamma in vascular endothelial cells. Circulation 110: 1128–1133, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, Jiang H, Gill R, Morisseau C, Newman JW, Hammock BD. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem 282: 2891–2898, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCulloch AI, Randall MD. Sex differences in the relative contributions of nitric oxide and EDHF to agonist-stimulated endothelium-dependent relaxations in the rat isolated mesenteric arterial bed. Br J Pharmacol 123: 1700–1706, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohtoshi K, Kaneto H, Node K, Nakamura Y, Shiraiwa T, Matsuhisa M, Yamasaki Y. Association of soluble epoxide hydrolase gene polymorphism with insulin resistance in type 2 diabetic patients. Biochem Biophys Res Commun 331: 347–350, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Oni-Orisan A, Alsaleh N, Lee CR, Seubert JM. Epoxyeicosatrienoic acids and cardioprotection: the road to translation. J Mol Cell Cardiol 74: 199–208, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinot F, Grant DF, Spearow JL, Parker AG, Hammock BD. Differential regulation of soluble epoxide hydrolase by clofibrate and sexual hormones in the liver and kidneys of mice. Biochem Pharmacol 50: 501–508, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Qin J, Sun D, Jiang H, Kandhi S, Froogh G, Hwang SH, Hammock BD, Wolin MS, Thompson CI, Hintze TH, Huang A. Inhibition of soluble epoxide hydrolase increases coronary perfusion in mice. Physiol Rep 3: e12427, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111: 796–803, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem 275: 40504–40510, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Sun A, Ren J. Estrogen replacement therapy and cardiac function under metabolic syndrome: a treacherous art. Hypertension 59: 552–554, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Sun D, Cuevas AJ, Gotlinger K, Hwang SH, Hammock BD, Schwartzman ML, Huang A. Soluble epoxide hydrolase-dependent regulation of myogenic response and blood pressure. Am J Physiol Heart Circ Physiol 306: H1146–H1153, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun D, Huang A, Koller A, Kaley G. Flow-dependent dilation and myogenic constriction interact to establish the resistance of skeletal muscle arterioles. Microcirculation 2: 289–295, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res 85: 288–293, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Jiang H, Wu H, Yang Y, Kaley G, Huang A. A novel vascular EET synthase: role of CYP2C7. Am J Physiol Regul Integr Comp Physiol 301: R1723–R1730, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun D, Ojaimi C, Wu H, Kaley G, Huang A. CYP2C29 produces superoxide in response to shear stress. Microcirculation 19: 696–704, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun D, Yan C, Jacobson A, Jiang H, Carroll MA, Huang A. Contribution of epoxyeicosatrienoic acids to flow-induced dilation in arteries of male ERalpha knockout mice: role of aromatase. Am J Physiol Regul Integr Comp Physiol 293: R1239–R1246, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun D, Yang YM, Jiang H, Wu H, Ojaimi C, Kaley G, Huang A. Roles of CYP2C29 and RXR gamma in vascular EET synthesis of female mice. Am J Physiol Regul Integr Comp Physiol 298: R862–R869, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanRollins M, Kaduce TL, Fang X, Knapp HR, Spector AA. Arachidonic acid diols produced by cytochrome P-450 monooxygenases are incorporated into phospholipids of vascular endothelial cells. J Biol Chem 271: 14001–14009, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Verma S, Bhanot S, Yao L, McNeill JH. Vascular insulin resistance in fructose-hypertensive rats. Eur J Pharmacol 322: R1–R2, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Huang A, Sun D, Falck JR, Koller A, Kaley G. Gender-specific compensation for the lack of NO in the mediation of flow-induced arteriolar dilation. Am J Physiol Heart Circ Physiol 280: H2456–H2461, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, Alkayed NJ. Role of soluble epoxide hydrolase in the sex-specific vascular response to cerebral ischemia. J Cereb Blood Flow Metab 29: 1475–1481, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


