Abstract
Dystrophin is a long rod-shaped protein that connects the subsarcolemmal cytoskeleton to a complex of proteins in the surface membrane (dystrophin protein complex, DPC), with further connections via laminin to other extracellular matrix proteins. Initially considered a structural complex that protected the sarcolemma from mechanical damage, the DPC is now known to serve as a scaffold for numerous signaling proteins. Absence or reduced expression of dystrophin or many of the DPC components cause the muscular dystrophies, a group of inherited diseases in which repeated bouts of muscle damage lead to atrophy and fibrosis, and eventually muscle degeneration. The normal function of dystrophin is poorly defined. In its absence a complex series of changes occur with multiple muscle proteins showing reduced or increased expression or being modified in various ways. In this review, we will consider the various proteins whose expression and function is changed in muscular dystrophies, focusing on Ca2+-permeable channels, nitric oxide synthase, NADPH oxidase, and caveolins. Excessive Ca2+ entry, increased membrane permeability, disordered caveolar function, and increased levels of reactive oxygen species are early changes in the disease, and the hypotheses for these phenomena will be critically considered. The aim of the review is to define the early damage pathways in muscular dystrophy which might be appropriate targets for therapy designed to minimize the muscle degeneration and slow the progression of the disease.
I. INTRODUCTION
Dystrophin is a long (110 nm), rod-shaped protein expressed primarily in muscle that connects γ-actin of the subsarcolemmal cytoskeletal system to a group of proteins in the surface membrane, the dystrophin protein complex (DPC). There are further connections via laminin from the DPC to the extracellular basal lamina (Figure 1). Based on this structure, it is generally thought that one function of dystrophin is the transmission of force laterally across the muscle and/or helping to maintain the registration between the membrane and intracellular cytoskeleton matrix and extracellular matrix. However, growing evidence suggests that dystrophin, through its multiple protein connections, also has a major role in regulating signaling pathways, particularly those pathways that activate nitric oxide (NO) production, Ca2+ entry, and the production of reactive oxygen species (ROS).
FIGURE 1.
The dystrophin protein complex (DPC). Shown are the interactions between core components of the DPC, the extracellular matrix, and nNOS. Numbers in dystrophin indicate hinge regions (H1, H2, etc.) and spectrin-like repeat domains (4, 8, 12, etc). nNOS, neuronal nitric oxide synthase; Syn, syntrophin; SSPN, sarcospan; ABD, actin binding domain; DBD, dystroglycan binding domain; SBS, syntrophin binding site; CC, coiled-coil domain; N, amino terminus; C, carboxy terminus.
Most of what we know about the function of dystrophin arises from studying the diseases that result from the absence of dystrophin. The best known of these diseases, Duchenne muscular dystrophy (DMD), occurs through mutations or other genetic rearrangments in the dystrophin gene which lies on the X chromosome. The disease has classical X-linked recessive genetics in which males, who carry the mutation, express the disease while females, who have a single copy of the mutation, do not express the disease but are carriers (for a fuller account of the carrier state, see Ref. 188). As a consequence of these genetic abnormalities, boys with DMD have muscles which largely or completely lack dystrophin. In Becker muscular dystrophy (BMD), which is a later onset and milder disease, the muscles contain reduced quantities of a truncated, partially functional dystrophin protein. Different types of dystrophin mutations occur in DMD/BMD, including large deletions (60-65% of cases) and large duplications (5–10% of cases), with the remaining cases due to point mutations and small deletions or duplications (for a review of dystrophin mutations in DMD and BMD, see Ref. 363).
The purpose of this review is to try to understand the normal function(s) of dystrophin in skeletal muscle and to consider the early stages of the disease process and how these might result from the absence of dystrophin. Despite a large literature on the disease, surprisingly little is known about the earliest stages of the disease, and a coherent set of mechanisms that link the absence of dystrophin to the disease pathogenesis is still unavailable.
A. History of DMD and the Discovery of Dystrophin
DMD is a rare but distinctive disease so there are occasional brief descriptions in the literature before the definitive account by Duchenne in 1868 (103). In this report Duchenne described 13 cases, including 2 girls, and noted the progressive weakness affecting initially the lower limbs, the early hypertrophy followed by gradual atrophy, and the characteristic histology. One of Duchenne's innovations was the use of muscle biopsy to obtain samples during life, and he was able to follow the histological features during the course of the disease and to ascribe the disease to muscle as opposed to the nervous system. Gowers (151) also studied many cases of the disease and described the technique for rising from the ground utilized by patients now known as “Gowers' manoeuvre.” He also noted the low rate of disease in females and the fact that the disease was inherited from the mother rather than the father. This pattern of inheritance, in which females are carriers but do not generally express the disease whereas males are affected, is now recognized as characteristic of recessive mutations on the X chromosome.
For the next 100 years or so, remarkably little was known about the pathogenesis of the disease apart from its X-linked genetics and that skeletal muscle was primarily affected. Gradually other forms of muscular dystrophy were described and characterized clinically and, in particular, Becker (28) described a more benign version of the disease with the same X-linked genetics.
The situation changed dramatically in the 1980s when, within a short period, the location and sequence of the dystrophin gene were discovered and the protein product dystrophin was characterized (for a personal account of these discoveries, see Ref. 225). Prior to the 1980s, it was known only that the mutated gene lay on the X chromosome. Through studies of a series of linked genetic diseases which changed the banding pattern on chromosome X, it gradually became clear that the mutated gene lay in the short arm of the X chromosome (Xp21) and was very large (for review, see Ref. 114). Davies et al. (88) produced a genomic library from the human X chromosome and identified two sequences which genetic studies showed to be linked to DMD. They were able to show that these two sequences lay on either side of the DMD locus identifying the site with new precision. Worton's group (516) focused on a female patient with what appeared to be DMD who had a translocation of part of the X chromosome to an autosome. They assumed that the breakpoint in the X chromosome was at the site of the DMD gene. A nearby group of ribosomal genes was available as a marker, and eventually a clone was identified in close linkage to the DMD site which was capable of detecting deletions in some DMD boys. Kunkel et al. (226) used a subtractive hybridization technique approach which Kunkel had previously used to study the Y chromosome. Their starting clinical material was the DNA from a patient with a large deletion that included the DMD gene. They then used a hybridization technique with normal X chromosome DNA sequences that eventually yielded clones of the sequences of the deleted DNA material in the patient (227). Subsequently they used restriction fragment linked polymorphisms known to be linked to DMD to identify the position and some of the deletions and mutations that can cause DMD (226, 299). The gene was sequenced and proved to be the largest in the human genome spanning 2.5 megabases and containing 79 exons (385). Once the gene sequence was known, antibodies could be produced to epitopes, and the protein product dystrophin was characterized and located on the intracellular side of the cell membrane (184, 492). The discovery of the dystrophin gene and protein has revolutionized our understanding of the disease and led to advances in prenatal diagnosis and carrier detection.
B. Natural History of DMD and BMD
DMD is commonly diagnosed between the ages of 2 and 6 yr because of delay in walking and/or unsteady gait (for details of clinical features, see Refs. 114, 176). Other common features are some delay in intellectual milestones and speech development. Frequently there is muscle hypertrophy, commonly in the calf muscles, but the dominant feature is muscle weakness, starting in the legs and gradually affecting upper limbs and trunk muscles. As the disease proceeds, affected boys have difficulty in rising from the ground, and Gower's sign is the use of the arms to support the legs as the boys slowly rise from the ground to standing. The weakness progresses slowly, but between 7 and 12 yr, most boys are confined to a wheel chair. In the teenage years contractures of many muscles can occur, and atrophy becomes prominent. Curvature of the spine associated with weakness of the postural muscles can also occur. Because of weakness of the thoracic muscles and the diaphragm, respiratory function declines and ventilatory complications are a common cause of death.
While there is currently no specific treatment, many forms of therapy prolong survival. Steroids delay the time to wheel chair use by several years (279) and slow the decline of many aspects of the disease (176) but are associated with many side effects. Surgery for contractures and scoliosis provides symptomatic relief. The use of ventilators, initially at night and eventually during the day, has had a substantial impact on survival. Mean survival in the 1960s was 15 yr, but this has gradually increased with improved therapy of various sorts and is currently in the mid 20s (110, 369). As patients are surviving longer, the cardiac consequences are becoming of greater importance, and currently ∼20% of patients die of cardiac consequences of the disease (428).
Diagnosis rests on the clinical features, the presence of a grossly elevated serum creatine kinase (CK), the histological picture including the absence of dystrophin by antibody staining and, usually, the identification of the mutation in the dystrophin gene. The latter, while not strictly necessary for diagnosis, is of value for carrier studies and prenatal diagnosis and, increasingly in the future, for some types of therapy (see sect. VI).
BMD is also caused by mutations to the dystrophin gene, but while DMD nearly always leads to complete absence of dystrophin expression in muscle, BMD is associated with reduced levels or shortened dystrophin proteins. As a consequence, while the clinical picture is similar, the onset and development are slower and more variable. For instance, the mean age of loss of walking for BMD patients is 37 yr (58).
C. Animal Models: mdx Mouse
Just as DMD occurs in humans through mutations in the dystrophin gene, it is also recognized in a number of animals. The golden retriever dystrophy model has many similarities to the human model (219). For instance, it is X-linked and of comparable severity to the human disease. Serum CK is high and the histological picture of degeneration and regeneration is similar. For these reasons, it is thought to be a good model of the human disease, and treatments that are successful in this model may have a higher probability of success in humans. Unfortunately, the mortality of pups with the disease is high and the cost of maintaining the animals is considerable so that there are relatively few colonies, and consequently, opportunities to use this model for preclinical studies are limited (24, 322).
The mdx mouse was identified in 1984 as a spontaneous, X-linked mutation with elevated serum CK and histological features of muscle damage and regeneration (54). Subsequently, it was shown that the muscles lacked dystrophin, and there was a premature stop codon in exon 23 of the dystrophin gene (for review see Ref. 322). In-breeding has resulted in the mdx line in which both males and females exhibit the disease and all their offspring are affected. Because of the identical genotype and easy availability, mdx mice have become the most widely used model of DMD. However, despite the identical genotype, the disease is much milder than in humans. Affected animals live a near-normal life span and reproduce normally. Muscles appear normal at birth, but a wave of damage occurs around 4–8 wk, which results in substantial regeneration with most fibers showing central nuclei (indicative of regeneration). Thereafter, damage and regeneration remain in balance and the animals remain mobile though voluntary performance on a running wheel is substantially reduced compared with wild-type (WT) animals (167). Respiratory muscles are more affected than limb muscles, possibly reflecting the continuous use of these muscles (432) (see sect. ID). Many studies have shown that eccentric contractions (in which the muscle is stretched during contraction) cause substantially greater damage in mdx mice than in WT (77, 295, 354), and the damage caused by eccentric contractions in the mdx mouse is widely used as a model of the muscle damage in DMD. Chemical mutagenesis programs have produced mutants, such as the mdx3Cv, which is deficient in both full-length dystrophin and the short forms of dystrophin which express in brain and other tissues and may give more insights into the role of dystrophin in other tissues (84).
An important, and only partially resolved, issue is why the phenotype of the mdx mouse is milder than DMD. Perhaps the best established explanation is the degree of utrophin expression. Utrophin is a structurally similar protein to dystrophin which is expressed along the sarcolemma in developing muscle but restricted to the neuromuscular junction and myotendinous junction in normal adults (for review, see Ref. 37). The structural similarities between utrophin and dystrophin have led to the suggestion that utrophin can partially replace dystrophin. In the mouse this idea is supported by a number of findings. One is that in the mdx mouse, sarcolemmal utrophin levels are increased (271). Another is that the double knockout of both dystrophin and utrophin in mice produces a severe phenotype that more closely mimics DMD (96, 154) and may therefore be more suitable for testing possible therapies. In addition, transgenic overexpression of utrophin in the mdx mouse further ameliorates the phenotype (456). These ideas have also led to the search for techniques to upregulate utrophin in humans in the hope that this will be of therapeutic value (see sect. VIA).
A second reason for the milder phenotype of the mdx mouse relates to the ability of the satellite cells to divide repeatedly to allow repair of damaged fibers. Sacco et al. (395) have proposed that the longer telomeres and shorter life span in mouse muscle provide additional capacity for repair and can contribute to the greater resistance to damage. This topic is further explored in section VA2.
A third reason for the milder phenotype is the presence of the cytidine monophosphate sialic acid hydroxylase gene (CMAH) in mice. This gene has been inactivated in humans and appears to exacerbate the consequences of dystrophin absence in humans. The evidence for this is that the insertion of a CMAH inactivating mutation into the mdx mouse produced a much more severe phenotype (65).
A fourth possible reason is that the small size of the mdx mouse is of itself protective. This could arise because forces on muscle fibers are reduced in small animals so that muscle damage would tend to be less (40, 336). In support of this idea, small golden retrievers have a milder phenotype than large (469), and humans with short stature, due to growth hormone deficiency or natural variability, are partially protected (530, 532).
D. Preliminary Ideas About Mechanisms
Even before the site and structure of dystrophin were known, structural evidence suggested that membrane damage and subsequent damage secondary to Ca2+ entry were important elements in the pathology. Mokri and Engel (298) noted focal disruptions of the cell membrane in EM studies of DMD muscle and described contractures of the myofibrils in the vicinity, suggestive of Ca2+ entry through the membrane breach. Bodensteiner and Engel (39) subsequently showed that total Ca2+ was greatly increased in DMD muscle and suggested that raised free intracellular Ca2+ concentration ([Ca2+]i) had a role in the developing pathology. As the site and structural connections of dystrophin became clearer (184), it seemed likely that dystrophin exerted a structural role though the details of that remain unclear and are explored in section iiB. These ideas still have wide support, but the pathway of Ca2+ entry remains under debate.
Evidence has shown that muscle damage in mdx and DMD is dependent on contractile activity. In mdx mice, extensive damage and regeneration of the hindlimb muscles begins within a week after the mice are weaned (3–4 wk of age), and by ∼8 wk of age more than 50% of fibers have undergone a damage-regeneration cycle as quantified by central nuclei. If, however, contractile activity is prevented in 3- to 5-wk-old mdx mice, by immobilizing the EDL and soleus at a fixed length, either short or long, these muscles do not accumulate centrally nucleated fibers (297). These data suggest that the onset of chronic contractile activity in mdx mice is necessary for the initial muscle damage to occur. The elevated resting intracellular Ca2+ concentration in spontaneously contracting mdx myotubes is also dependent on chronic activity, as it was reduced by long-term application of tetrodotoxin (189). In both of these cases, mdx mice running in a cage and myotubes in culture, the activity is chronic over several days. Therefore, dystrophic muscles are susceptible to damage from both acute eccentric contractions as well as chronic, submaximal noneccentric activity. Perhaps this is another important difference between the time courses of muscle damage in mdx and DMD in that mice are very active immediately after weaning (∼21 days old), whereas activity levels in humans are minimal after birth and during the first year or two before the onset of walking.
Thus a key question is how the combination of the loss of dystrophin and contractile activity lead to muscle and membrane damage.
II. THE DYSTROPHIN-ASSOCIATED COMPLEX
A. The Dystrophin Complex: Structural Versus Signaling
In the 25 years since the discovery of the DMD gene, the function of dystrophin and the proteins associated with it (the DPC) has been the subject of intense study. Although skeletal, cardiac, and smooth muscles are the organs most severely affected by abnormalities in the DPC, growing evidence suggests that many cell types, including several in the brain, have some form of the dystrophin complex (161). A full understanding of the DPC in different cells and tissues will likely inform us about its critical role in muscle cells and the mechanistic deficiencies that lead to muscle degeneration in the muscular dystrophies. In this review, we focus primarily on the DPC in skeletal muscle.
Based largely on dystrophin's key role in linking the extracellular matrix to the subsarcolemmal actin cytoskeleton (FIGURES 1 and 2), and functional studies that revealed increased damage to the muscle membrane following eccentric contractions, a structural role for dystrophin became a widely accepted hypothesis. More recently, the signaling component of the DPC has received growing attention. The discovery that signaling molecules of major importance in muscle function, such as nitric oxide synthase (NOS), require an intact DPC for sarcolemmal association has stimulated reconsideration of the function of dystrophin. Furthermore, the demonstration that certain limb girdle muscular dystrophies (LGMDs) are caused by loss of the sarcoglycan complex, while dystrophin remains unperturbed at the sarcolemma (106), prompted reassessment of the hypothesis that loss of the basal lamina-cytoskeletal linkage was the major causative defect in muscular dystrophies. The very mild dystrophic phenotype in mice lacking γ-actin also suggests that the membrane stabilization theory is too simplistic to fully account for the severity of the disease (421).
FIGURE 2.
The dystrophin molecule. Alignment of dystrophin protein structure with gene exons. Top: dystrophin protein domains with binding sites for structural and signaling proteins and lipids. ABD, actin binding domain; DBD, β-dystroglycan binding domain; SBS, syntrophin binding domain; CC, coiled-coiled region that binds α-dystrobrevin. Numbers identify spectrin-like repeats (for clarity, only 4, 8, 12, 16, 20, and 24 are indicated). Par-1b, polarity regulating kinase; AnkB, ankyrin B binding site. Bottom: corresponding exons in the human dystrophin gene. Note that the exons are arranged to correspond with the dystrophin protein structure. Therefore, the widths of the boxes do not correspond to the relative sizes of the exons. Data used to construct this figure are from the Leiden Muscular Dystrophy Pages (http://www.dmd.nl/).
The DPC can be roughly divided into two subcomplexes: the structural complex and the signaling complex. The delineation is not intended to be absolute as some elements of the DPC have roles in both functions.
B. The Structural Complex
For many years (essentially since the identification of dystrophin as the DMD gene product), the DPC has been considered a structural complex that protects the sarcolemma from damage during repeated cycles of muscle fiber contraction. In its simplest form, the structural complex provides a linkage between the submembrane cytoskeleton and components of the extracellular matrix, with dystrophin binding to γ-actin and β-dystroglycan, which is in turn linked to laminin via α-dystroglycan.
1. Dystrophin
The most prominent feature of dystrophin is the large central section of 24 spectrin-like repeats (Figure 1), each comprised of a triple-helical coiled-coil structure (for recent review, see Ref. 238). This presumably flexible repeat region has properties suggestive of a shock absorber that could transmit force laterally during muscle contraction. In fact, atomic force microscopy analyses have demonstrated that the force generated by only a few myosin molecules is sufficient to unfold and extend the molecule (34). However, the spectrin repeats are not merely structural components. Several repeats, or groups of tandem repeats, bind actin, anionic lipids (241), and signaling proteins such as neuronal NOS (nNOS) (6, 49) and polarity regulating kinase, PAR-1b (Figure 2). PAR-1b binds to spectrin repeats 8–9 and phosphorylates sites in this region (520). These results suggest that the spectrin repeats of dystrophin are much more than shock absorbers and likely serve as a scaffold for signaling proteins (for review, see Ref. 239).
Proline-rich hinge regions (labeled H1-H3 in Figure 1) interrupt or border on the repeat region at four sites and are necessary for full dystrophin function (25, 60, 218). The amino- and carboxy-terminal sections of dystrophin contain defined protein motifs that bind other proteins, including actin, β-dystroglycan, syntrophins, and α-dystrobrevin.
2. α/β-Dystroglycan
Dystroglycan has important functions in many different cell types (for review, see Ref. 46). In skeletal muscle, the extracellular glycosylated protein α-dystroglycan and its transmembrane partner, β-dystroglycan, are central to the linkage function of the DPC. Current evidence supports the idea that dystroglycan is both a cell adhesion protein and a signaling receptor (for review, see Ref. 300). α-Dystroglycan interacts directly with extracellular matrix proteins, including laminin, agrin, and perlecan, via their LG domains. Phosphorylated intracellular tyrosine residues on β-dystroglycan recruit signaling proteins that bind to phosphotyrosine. The GTPase dynamin 1 associates with β-dystroglycan, an interaction that appears to regulate endocytosis (533). Cells lacking dystroglycan exhibited greater transferrin uptake, a process that requires dynamin. A large number of congenital muscular dystrophies are linked to dystroglycan abnormalities (285; reviewed in Refs. 405, 501). Many of these abnormalities stem from deficiencies in glycosylation and the subsequent failure to bind extracellular proteins.
Recently, Johnson et al. (204) reported that only a small fraction of α-dystroglycan in normal skeletal muscle is associated with dystrophin. In fact, the dystroglycan to dystrophin molar ratio may exceed 40:1. Dystrophin-free dystroglycan is largely in skeletal muscle fibers (not in vasculature or nerve) and is not associated with utrophin. Dystroglycan remains on the sarcolemma in mdx and dystrophin/utrophin double knockout (DKO) mouse muscle and in DMD muscle, although in lower amounts than in normal muscle. Some sarcoglycans, syntrophins, and α-dystrobrevin remain associated with the core dystroglycan complex in mdx muscle extracts, presumably by association with utrophin.
What then is the function of the dystrophin-independent dystroglycans? Using a proteomics approach, Johnson et al. (204) identified two interesting possibilities. In one case, dystroglycan is associated with cavin-1, a central organizer of caveolae (179). In a separate complex, dystroglycan is associated with the calcium channel regulatory subunit Cavβ2 (55, 56). Although the calcium channel itself was not found, this is may be due to technical limitations in detecting transmembrane proteins by mass spectrometry. However, regulation of Cav 1.1 by cAMP-dependent protein kinase is altered in mdx mouse muscle (203). Based on these findings, Johnson et al. (204) propose a novel model of three distinct dystroglycan complexes in skeletal muscle: 1) the well-known DPC, 2) a dystroglycan complex containing Cavin-1, and 3) a dystroglycan complex with calcium channels. Only the latter is unperturbed by the loss of dystrophin. An intriguing possibility is that the remaining dystroglycan-calcium channel complex is involved in the mechanisms that lead to higher intracellular calcium concentrations in dystrophic muscle.
3. γ-Actin
The three major filamentous structures common to many cell types, F-actin, microtubules, and intermediate filaments, are directly associated with the DPC.
The association of γ-actin with dystrophin has been thoroughly examined and documented (118). The canonical actin-binding domain is necessary but not sufficient for high-affinity binding of dystrophin to filamentous γ-actin. Spectrin repeats in the amino terminus of the repeat region are essential contributors to a strong interaction (15, 392). These results are consistent with a model in which γ-actin lies laterally along the dystrophin rod for approximately half of its length. In view of its predicted critical role in the DPC structural hypothesis, the finding that genetic ablation of γ-actin resulted in a slowly progressive myopathy, similar to the phenotype of centronuclear myopathy (421), was unexpected. Key features of muscular dystrophy in the mdx mouse, including fibrosis, inflammation, and sarcolemmal damage, were not observed. Nevertheless, transgenic overexpression of γ-actin in mdx skeletal muscle, albeit at very high amounts (∼200-fold), attenuated contraction-induced force loss (23). The mild phenotype of γ-actin null muscle might be explained by compensatory effects of other filamentous structures, although further studies are required to test this hypothesis.
4. Microtubules
In skeletal muscle, microtubules are uniquely arrayed in a nontraditional stationary lattice (328). The strands of the lattice are sometimes formed by only a few microtubules, some of which are dynamic and grow along existing static microtubules. Nuclei and Golgi elements serve as nucleation sites for the microtubule lattices, a process that involves proteins that are common in microtubule organizing centers in other types of cells. One model suggests that dystrophin may be an important guide or may restrict the arrangement of the microtubule lattice. At the same time, microtubule lattices appear to be important for localization of intracellular organelles, particularly Golgi elements (113, 344). The microtubule lattice pattern is severely disrupted in dystrophic muscle, but is largely restored by AAV-mediated rescue with a microdystrophin construct (345). Restoration of the lattice is accompanied by partial normalization of Golgi element distribution. These changes in microtubule structure in dystrophic muscle may be explained by the observation that microtubules interact directly with dystrophin (361).
Microtubules likely play a dual structural and signaling role. As discussed in section VD, alterations in microtubules are intimately involved in ROS production in dystrophic muscle (213). Furthermore, microtubule-associate protein 1B (MAP1B) binds α-syntrophin (133) and nNOS (440).
5. Intermediate filaments
Desmin is the most abundant intermediate filament protein in skeletal muscle. Together with keratin 8 and keratin 19, these intermediate filaments link contractile elements to each other and to the sarcolemma. Intermediate filaments that contain keratin 19 associate with the actin-binding domain of dystrophin (437). Genetic ablation of desmin resulted in the loss of costameres, but otherwise had surprisingly mild effects (326). Tibialis anterior muscles in keratin 19 null mice displayed a mild myopathy, characterized by modest decreases in mean fiber diameter and specific tetanic force and mislocalized mitochondria (438).
Several other members of the intermediate filament family of proteins may interact with the DPC. Syncoilin is expressed at highest levels in skeletal and cardiac muscle and binds directly to α-dystrobrevin (321). It is most highly expressed at the neuromuscular junction. Syncoilin does not appear to form intermediate filaments, even when overexpressed. However, it may link desmin filaments to the DPC (359). In contrast to most DPC proteins, syncoilin expression is increased in mdx muscle, and also in muscle from DMD, BMD, and congenital muscular dystrophies, often concentrated in intracellular core structures (52). The structure, interactions and possible functions of syncoilin are discussed in a recent review (301).
Desmuslin (synonymous with β-synemin) is an intermediate filament related protein that was identified as a potential DPC protein based on its interaction with α-dystrobrevin (293). β-Synemin/desmuslin is an A-kinase associated protein (AKAP) in heart (391), but its function in skeletal muscle, both normal and dystrophic, remains unknown.
The plectin cytolinker stabilizes and regulates intermediate filament networks in many cell types (513). As a consequence, mutations in plectin are involved in multiple diseases, including muscular dystrophy, myofibrillar myopathies, skin blistering, and neurological syndromes. Of the eight isoforms generated by alternative splicing, four are found in skeletal muscle. Plectin 1 and 1f are present on the sarcolemma where they interact with dystrophin (the EF-ZZ domains) and multiple sites on β-dystroglycan (for a model of a complex interaction, see Ref. 381). Recent studies of mdx mice lacking plectin 1f implicated this isoform in metabolism. Mice lacking both dystrophin and plectin 1f exhibited a more severe dystrophic phenotype, despite the fact that sarcolemmal integrity (determined by serum CK levels and Evans Blue dye uptake) and glucose uptake were restored to normal levels (368). Increased amounts of glucose transporter 4 at the sarcolemma in the double knockout may result from alterations in the microtubule network in the absence of plectin.
C. The Core Signaling Complex
Six protein groups make up the core DPC signaling complex: sarcoglycans, α-dystrobrevin, syntrophins, sarcospan, integrin, and biglycan (see Figure 1). These core proteins bind numerous other signaling proteins and, in some cases, regulate their interaction with the DPC.
1. Sarcoglycans
The sarcoglycans are transmembrane glycoproteins that form a tetrameric complex. Of the six genes encoding sarcoglycans, four are expressed in skeletal muscle and mutations in each of the genes results in a specific LGMD (for reviews, see Refs. 126, 270). The absence of one of the sarcoglycan subunits usually results in the loss of the entire sarcoglycan complex. In these cases, sarcolemmal dystrophin remains largely unaffected (216). The sarcoglycan complex interacts directly with some of the other core signaling proteins, including biglycan (367), α-dystrobrevin (524), and sarcospan (292), and with other proteins such as filamin 2 (454). Despite its central role in DPC-mediated signaling and its critical importance in muscle health, the molecular function of the sarcoglycan complex remains largely unknown (177; for reviews, see Refs. 333, 402).
2. Dystrobrevins
The two forms of dystrobrevin, a dystrophin-related protein (36, 153, 483), are encoded by distinct genes. α-Dystrobrevin, but not β-dystrobrevin (351), is expressed in skeletal muscle as a component of the sarcolemmal DPC. Five primary splice forms of α-dystrobrevin have been identified (36). α-Dystrobrevin-1 and -2 are the major forms in skeletal muscle and have different carboxy-terminal tail sequences. The longer tail of α-dystrobrevin-1 contains numerous serine, threonine, and tyrosine phosphorylation sites, suggesting that kinases may regulate α-dystrobrevin-mediated interactions of signaling proteins with the DPC (22, 152, 484). Furthermore, these two isoforms are differentially localized in skeletal muscle and preferentially interact with different members of the dystrophin complex and utrophin (352). Mice lacking α-dystrobrevin exhibit a moderate muscular dystrophy (153). Human skeletal muscle diseases due to mutations in the α-dystrobrevin (DTNA) gene have not been reported.
3. Syntrophins
The syntrophins are a family of adapter proteins that contain a PDZ domain and 2 PH domains. Five closely related isoforms are encoded by separate genes (5, 8, 357). Four of the isoforms, α, β1, β2, and γ2, are expressed in skeletal muscle. α-, β1-, and β2-syntrophins are part of the DPC on the sarcolemma (350), whereas γ2-syntrophin is located on the sarcoplasmic/endoplasmic reticulum (12). Each member of the dystrophin protein family has two syntrophin binding domains (9, 320, 444). Thus the potential number and isoform combination of syntrophins associated with the DPC are very large and, in turn, may result in assembly of different functionally interdependent signaling proteins. The list of signaling proteins known to bind syntrophins is large and includes nNOS, ion and water channels, protein and lipid kinases, transporters, G protein receptors, and others (Table 1). Thus the dystrophin-dystrobrevin-syntrophin signaling scaffold has enormous potential for assembling signaling pathways at the muscle sarcolemma. Mutations in the syntrophin genes that result in skeletal muscle disease have not been identified, but changes in syntrophin sarcolemmal expression are associated with numerous myopathies and muscular dystrophies (80). For example, mutations in contactin-1 alter the syntrophin and α-dystrobrevin isoform composition on the sarcolemma and cause a lethal congenital myopathy (79). Mutations that cause single amino acid changes in the PH domains are the basis for one form of human cardiac long QT syndrome (467, 517).
Table 1.
Signaling proteins associated with the dystrophin complex
| Protein | Function | DPC Binding Site | Cell/Tissue | Reference Nos. |
|---|---|---|---|---|
| Signaling enzymes/proteins | ||||
| nNOS/NOS1 | Ca2+/calmodulin-dependent NO synthesis | α-Syntrophin, dystrophin | Skeletal muscle | 6, 49 |
| Tyrosine phosphatase N/ICA 512 | Catalytically inactive receptor tyrosine phosphatase | β2-Syntrophin | Pancreatic β-cells | 408 |
| TAPP1, TAPP2 | Tandem pleckstrin homology domain adapters | Syntrophins (multiple) | B-cells | 83 |
| ARMS | EphA4 receptor-associated protein and a substrate for ephrin receptors | α-Syntrophin | Skeletal muscle | 253 |
| Grb2/P66shc | Involved in many signaling pathways | α-Syntrophin | Human breast cancer cells | 35 |
| Tks5 | Adapter protein involved in many signaling pathways | β-Dystroglycan | Myoblasts | 453 |
| α-Catulin | Multipurpose scaffolding protein for G protein signaling pathways | α-Dystrobrevin-1 | HEK cells, skeletal muscle | 258, 329 |
| Tiam1 | Rac signaling in cell adhesion | β2-Syntrophin (with utrophin and β-dystrobrevin) | Epithelial cells | 259 |
| Mixed-lineage leukemia 5, MLL5 | Regulator of myogenin expression | α-Syntrophin | Skeletal myoblasts | 215 |
| PTEN | Phosphatase and tensin homolog; dephosphorylates PIP3 to PIP2 | α-Syntrophin | Skeletal myoblasts | 215 |
| Channels, receptors, and transporters | ||||
| Nav 1.4, Nav 1.5 | Skeletal and cardiac muscle sodium channels | α-Syntrophin | Skeletal muscle | 142 |
| Kir2.1, Kir4.1 | Inward rectifier potassium channels | Syntrophins (multiple) | Skeletal and cardiac muscle, brain, astrocytes | 81, 242 |
| TRPC1, TRPC4 | Non-voltage-gated cation channels | α-Syntrophin | Skeletal muscle | 394, 472 |
| Aquaporin-4 | Water channel | α-Syntrophin | Skeletal muscle, astrocytic endfeet | 6, 17, 317 |
| ABCA1 | Cholesterol/lipid transporter | Syntrophins (multiple) | Macrophages, Schwann cells, liver | 10, 53, 306, 330 |
| α1D-Adrenergic receptor | G protein receptor regulator of blood pressure | α- and β2-syntrophin | Vascular smooth muscle | 256, 257 |
| Adiponectin receptor | Metabolic regulation | α- and β1-syntrophin | Liver | 318 |
| Kinases | ||||
| Diacylglycerol kinase zeta | Metabolizes diacylglycerol to phosphatidic acid | γ1- and α-syntrophin | Skeletal muscle | 2, 3 |
| Stress-activated kinase 3, ERK6, p38γ | Mitogen-activated protein kinase | α-Syntrophin | Skeletal muscle | 169 |
| MAST205 | Microtubule-associated serine/threonine kinase | β2-Syntrophin | Heart, brain, skeletal muscle | 251 |
| SAST | Syntrophin-associated S/T kinase related to MAST | β2-Syntrophin | Brain, testes | 251, 522 |
| Par-1b | Cell polarity-regulating kinase; maintains dynamic state of microtubules | Dystrophin, utrophin | MDCK, HEK293 cells | 269, 520 |
| Src kinase | Non-receptor tyrosine kinase | β-Dystroglycan | Skeletal muscle | 424, 453 |
| Other | ||||
| Myocilin | Modulator of muscle hypertrophy pathway | α-Syntrophin | Skeletal muscle | 202 |
| MAP1A/MAP1B LC1 | Microtubule-associated proteins (light chain 1) | α-Syntrophin | Schwann cells, brain | 133 |
| Cavin-1 | Central organizer of caveolae | Unknown | Cardiac muscle | 205, 511 |
| Ahnak1 | Large scaffold protein associated with the calcium channel β-subunit | Unknown | Cardiac muscle | 89, 205 |
| Cypher/ZASP | Sarcomeric Z-line AKAP associated with L-type calcium channel | Unknown | Cardiac muscle | 205, 247 |
| CryAB | Heat shock protein | Unknown | Cardiac muscle | 205 |
See text for definitions.
4. Sarcospan
A member of the tetraspanin family of proteins involved in signaling, the role of sarcospan (SSPN) in muscular dystrophy has been elusive (reviewed in Ref. 265). Recently, mice lacking SSPN were shown to have impaired diaphragm contractile function, perhaps resulting from a decreased level of dystrophin and utrophin (265). Furthermore, moderate overexpression of SSPN in skeletal muscle ameliorates dystrophic pathology in the mdx mouse (349). Like other members of the tetraspanin family of proteins, sarcospan forms higher ordered oligomers in the sarcolemma (292). Sarcospan is tightly associated with the sarcoglycan complex (86) and may serve as a chaperone in the assembly of the DGC (266). An alternatively spliced isoform, μSPN, lacking two of the transmembrane domains is localized in the sarcoplasmic reticulum (SR) (291) and may be involved in excitation-contraction coupling.
5. Integrin
The α7β1 isoform of integrin is intimately involved in the pathological development of muscular dystrophy. Although this integrin was initially thought to be a sarcolemmal stabilization structure separate from the DGC, recent results revealed that α7β1 integrin is linked to the DGC by interaction with sarcospan (264). Transgenic and AAV-mediated expression of α7β1 integrin in the mdx mouse ameliorates the dystrophic pathology, increases muscle regenerative capacity, and reduces contraction induced injury (43, 57, 175). Mice that lack both α7 integrin and dystrophin exhibit a severe dystrophy (389). These results suggested that the α7β1 integrin complex could be a therapeutic target for treatment of DMD and other dystrophies and myopathies. Injection of laminin-111, an extracellular ligand for α7 integrin, markedly improves the dystrophic phenotype in the mdx and the merosin-deficient mouse model (388). Laminin-111 and other basement membrane proteins mediate cell adhesion and are linked to signaling platforms (reviewed in Ref. 528). Thus the role of integrin in DMD likely involves perturbations in both structural and signaling functions.
6. Biglycan
Originally defined as a structural protein of the extracellular matrix, biglycan has emerged as a ubiquitously expressed signal modulating protein (for review, see Ref. 316). In part, the evidence for a signaling role is based on biglycan's interaction with cytokines and growth factors implicated in muscular dystrophy pathology, including transforming growth factor (TGF)-β, tumor necrosis factor (TNF)-α, and several forms of bone morphogenetic proteins. Biglycan also interacts with components of the DPC: α-dystroglycan and the α- and γ-sarcoglycans (45, 367). The expression of α- and γ-sarcoglycans are also regulated by biglycan during muscle development (367). Finally, and perhaps most importantly, biglycan regulates nNOSμ association with the DPC (284). This process is mediated by association with the sarcoglycan complex and involves α-dystrobrevin and the syntrophins. The therapeutic potential of biglycan for DMD has been demonstrated in the mdx mouse model (525). Injection of recombinant biglycan improves several key pathological features in skeletal muscle, perhaps by upregulation of utrophin and increased levels of γ-sarcoglycan, β2-syntrophin, and nNOSμ at the sarcolemma (16).
Although not part of the core signaling complex defined above, several other signaling/scaffolding proteins are associated with the DPC. These include Grb2 (327), Ca2+/calmodulin (319), and Gβγ (518). Some of these will be discussed below in their functional context. Also, the physiological importance and therapeutic potential of nNOSμ will be discussed in detail.
D. DPC Diversity and Dynamic Regulation of Protein Composition
The function of the DPC in other tissues and cell types is likely to yield important information for DMD. Cardiac and smooth muscle are especially relevant to DMD. Some form of the DPC, often containing utrophin or shorter forms of dystrophin, is present in many cell types. These include endothelial and epithelial cells, neurons, Schwann cells, astroglial cells, and pancreatic β-cells (10, 69, 161, 252, 332, 449). In these cases, membrane stabilization is not likely to be relevant. Results from studies of numerous tissues cells types have demonstrated that many other signaling proteins can associate with the DPC. These results are summarized in Table 1. Further studies of the dystrophin complex in nonmuscle cells types and in nonmammalian models (32, 62, 146, 209) will be important in understanding the full range of signaling pathways mediated by the DPC and the therapeutic potential of drugs that target these pathways.
Even in muscle cells, the molecular composition of the DPC differs depending on the muscle and fiber type. Within specialized regions of the sarcolemma of a single muscle fiber, individual DPCs may have different syntrophin and α-dystrobrevin isoform compositions. Consequently, the associated signaling proteins may differ from one complex to another. Identification of signaling proteins associated with the DPC in different regions of the sarcolemma (postsynapse, myotendious junction, costameres, extra-costameric membrane, caveolae, etc.) will be very informative in understanding the signaling role of the DPC and related complexes, and how disruption of these signaling pathways contribute to the dystrophic pathology. Finally, dystrophin is also located in the transverse tubules (invaginations of the surface membrane; abbreviated t-tubules) (492). In contrast to the t-tubular network in cardiac myocytes, the composition of the DPC in skeletal muscle t-tubules is relatively unknown. It is possible, perhaps likely, that the signaling function of the t-tubular DPC is very different from the sarcolemmal form. Because of its proximity to the sarcoplasmic reticulum, the t-tubular DPC could have a major impact on calcium homeostasis.
In the context of DMD, differences in the signaling function of the DPC are especially important, since any treatment will need to target both skeletal and cardiac muscle. In fact, improvement in skeletal muscle by targeted expression of microdystrophin heightens cardiac injury in the mdx mouse (463). Proteomic studies conducted on skeletal and cardiac muscle revealed major differences in signaling proteins associated with the DPC. In addition to differences in the syntrophin and dystrobrevin isoform composition, nNOS is not associated with the DPC in cardiac muscle (205). Furthermore, four novel DPC proteins, specific to cardiac muscle, were identified: Cypher, Ahnak1, Cavin-1, and CRYAB.
The protein composition of the DPC is likely to be dynamic, but the regulation of its association with signaling proteins is poorly understood. The phosphorylation state of DPC proteins appears to be important. In the insulin secretory granules in pancreatic β-cells, binding of the granule protein ICA512 to the PDZ domain of β2-syntrophin is thought to link the granules to actin filaments, thereby restricting their mobility (205, 331). This interaction is regulated by phosphorylation of β2-syntrophin (332). Cdk5 phosphorylates a serine residue near the PDZ domain of β2-syntrophin and decreases binding to ICA512. Phosphorylation of a serine residue only 15 residues away (by a yet to be identified kinase) has the opposite effect (408). Similar regulatory mechanisms may apply in skeletal muscle to nNOSμ association with α-syntrophin, for example.
Phosphorylation of β-dystroglycan is important in regulating its interaction with dystrophin, modulating the degradation of dystroglycan and perhaps initiating a signal transduction pathway. The interaction with dystrophin is disrupted by phosphorylation of a tyrosine residue near the carboxy-terminal region of β-dystroglycan (196, 424). Tyrosine 980 (Y980) in the WW binding motif of mouse β-dystroglycan is especially critical in the interaction between dystroglycan and dystrophin. Also, Y980 phosphorylation recruits several SH2 domain proteins, including several tyrosine kinases (424). In addition to disrupting the dystroglycan-dystrophin interaction, Y980 phosphorylation causes translocation of β-dystroglycan from the sarcolemma to an intracellular vesicle population, perhaps a subset of recycling endosomes. Tyrosine phosphorylation of β-dystroglycan is stimulated by ligands for α-dystroglycan, including laminin and agrin.
Using gene knock-in technology, Miller et al. (290) generated a mouse line with a phenylalanine substitution at Y980, a key tyrosine phosphorylation site in dystroglycan. Introduction of this modification into mdx mice improved several dystrophic features, including fewer central nuclei, reduced Evans blue dye uptake, lower serum CK activity, resistance to muscle damage, and force loss due to eccentric contractions. α-Sarcoglycan and sarcospan (but not utrophin) were restored to the sarcolemma and the levels of plectin were also increased.
Recently, global analysis has identified 18 phosphorylated residues in dystrophin. Many of these sites are located in the cysteine-rich region downstream of the spectrin repeats (446). Phosphorylation of serine3059 enhances the interaction with dystroglycan. Clearly, phosphorylation is important in regulation of dystroglycan localization and interaction with dystrophin. Phosphorylation of other DPC proteins, including syntrophins (169, 260, 332, 408, 484, 537) and dystrobrevins (22, 152, 407, 483), is likely to modulate the composition of the DPC in skeletal muscle and the signaling proteins bound to it. Further studies on the regulation of DPC assembly and function by phosphorylation are clearly warranted. Other modes of posttranslational modification, especially nitrosylation, should also be examined.
In summary, we have learned much about the DPC since the discovery of dystrophin, but the full function of the complex and its regulation are not yet known. The shift from the structural hypothesis to greater emphasis on dystrophin as a signaling complex has greatly enhanced the potential for identification of therapeutic targets that slow the progression of muscle degeneration. Additional studies are needed so that informed design of genetic treatments restore as much of the signaling capability of the DPC as possible. Combinatorial approaches involving genetic correction and drug enhancement of signaling pathways will most likely be needed for the most effective treatments.
III. EARLY FUNCTIONAL CHANGES IN THE ABSENCE OF DYSTROPHIN
A. Muscle Weakness
Muscle weakness is an early and progressive feature of DMD. Its most obvious manifestations are the delayed walking, unsteady gait, and difficulty in rising from the ground (Gowers sign) which commonly lead to the diagnosis at the age of 2–6 yr. The magnitude of the weakness is not commonly quantified in humans, partly because it is very variable in different patients and different muscles, but a variety of clinical stagings are used (114). As an example, the maximum voluntary force produced by hand flexors in normal 10-yr-old boys was 15.1 ± 1.7 (SE) kg (n = 10), whereas in DMD boys of the same age it was 4.5 ± 0.5 kg (401). As the disease progresses there is gradual onset of severe atrophy which compounds the muscle weakness.
The 6-min walk test is a useful test of global muscle and cardiovascular/respiratory activity (274) and is becoming the gold standard for assessing therapies. While simple to perform, it has some practical difficulties. In normal boys, the distance increases with age as the boys become bigger and better coordinated. In DMD patients, the distance initially increases with age and then falls off as the disease becomes more debilitating. Thus an increase in distance in a therapeutic trial is only unequivocally the result of the drug if the stage of disease is one of decline. Another important practical issue is that the enthusiasm of the assistant during the test can influence the patient and the outcome so that adequate placebo controls and double blinding of all concerned is critical.
Muscle fatigue during and after exercise is also a prominent symptom in muscular dystrophies (for review, see Ref. 481). Again there are very few quantitative studies, and it is unclear to what extent the fatigue arises simply because the weakened muscles are being used at nearer their maximum capacity and hence fatigue faster. Inactivity will also contribute to fatigue, and the preferential damage to fast rather than slow fibers (494) may partially offset this factor since slow fibers fatigue more slowly than fast. It is possible that some of the muscle weakness derives from a very slow component of recovery from fatigue (217). In normal muscles, fatigue is closely related to the metabolic and ionic changes that accompany intense muscle activity (for review, see Ref. 13). Whether these and/or additional mechanism contribute to fatigue in dystrophic muscle requires further research.
Muscle damage is another likely cause of weakness in DMD. Operationally, muscle damage is defined as weakness which recovers very slowly after activity, typically with a time course similar to repair or regeneration of damaged muscle (4–8 days). Activities in which the muscle is stretched during contraction (eccentric contractions) are particularly prone to cause damage, which is characterized by reduced force, membrane damage leading to loss of soluble muscle proteins, overstretched and understretched sarcomeres, and swelling and tenderness of muscles (for review, see Ref. 129). If a muscle fiber is damaged to the point at which it is unexcitable, then it will not contribute to the overall force until regeneration is complete, and it is likely that such damaged fibers contribute to the muscle weakness that characterizes DMD.
There is good agreement in the literature that the absolute force from isolated mdx muscles is similar to WT but that mdx muscles are somewhat bigger and that specific force (force per unit area) is moderately reduced (for review, see Ref. 489). Most mammalian muscles produce a maximum specific isometric force of ∼200–400 mN/mm2, whereas values for mdx muscles are typically 150–200 mN/mm2 (255), except for the diaphragm where values range from 50–150 mN/mm2 (120).
In considering the many possible causes of muscle weakness, one approach is to consider those factors within the intact fiber, specifically 1) Ca2+ release and 2) Ca2+ sensitivity and maximum force production of the myofibrillar proteins. There are also a range of possible mechanisms associated with 3) muscle damage, 4) impaired lateral transmission of force by cytoskeletal proteins and extracellular collagen, and 5) the possibility that muscle ischemia impairs recovery from fatigue.
1. Ca2+ release
Ca2+ release in skeletal muscle occurs when an action potential, having conducted along the surface membrane and into the t-tubules, excites the voltage sensor in the t-tubules (a modified L-type Ca2+ channel) which through an interaction with the ryanodine receptor (RyR1, the SR Ca2+ release channel in skeletal muscle) releases a quantity of Ca2+ from the store within the SR. In mdx mice, the action potential is little changed and conduction of the AP through the t-tubule network appears to be normal (514). However, a number of studies have shown that the amount of Ca2+ released, judged by the amplitude of the [Ca2+]i increase caused by a single action potential or a train of action potentials, is moderately reduced (174, 187, 515, 523). This could come about either because the amount of Ca2+ stored in the SR is reduced or because the openings of the RyR1s are reduced. There is evidence that calsequestrin, the main buffer for Ca2+ in the SR, is reduced in mdx muscles (100), and this would reduce buffering of Ca2+ in the SR and the amount of Ca2+ released by an action potential.
A long-standing observation in studies of human muscle fatigue is that long and demanding periods of activity lead to a prolonged (2–4 days) reduction in force at low frequencies of stimulation while the maximum force observed at high frequencies is normal (for review, see Refs. 13, 112). This phenomenon can be caused by reduced SR Ca2+ release (502) and can be triggered by a prolonged preceding period of elevated resting [Ca2+]i (73, 232). The mechanism by which elevated [Ca2+]i causes reduced SR Ca2+ release has long been uncertain; calpain inhibitors have only limited effect, and proteolysis products of RyR and the voltage sensor are not detectable (232). Recently, Corona et al. (82) showed that junctophilin, a protein required to stabilize the SR membrane connection to the t-tubule, was disrupted after eccentric contractions and suggested that this might cause the failure of Ca2+ release. Subsequently Murphy et al. (308) showed that μ-calpain, when activated by moderate elevations of [Ca2+]i, was capable of cleaving junctophilin which could then cause a failure of Ca2+ release. Given that the resting [Ca2+]i is elevated in mdx muscle (see sect. IVC), and Murphy et al. (308) showed disruption of junctophilin in mdx muscle, it seems likely that this mechanism contributes to reduced Ca2+ release and the muscle weakness observed in dystrophic muscles.
There is also substantial evidence that the properties of RyR1 are modified in mdx muscle and may contribute to reduced Ca2+ release. A recently proposed mechanism is that nitrosylation of the RyR specifically in the mdx mouse may change the properties of the RyR1 in a way that elevates resting [Ca2+]i and reduces tetanic Ca2+ release (30). In earlier studies, the Marks group established that a variety of posttranslational modifications of the RyR1 lead to loss of an ancillary protein, calstabin1 (also known as FKBP12), which causes increased open probability of isolated RyR1s. This, in turn, might be expected to raise the resting [Ca2+]i, deplete the SR of Ca2+, and reduce tetanic Ca2+ release in intact muscle (31). In their later study (30), a reduction in nNOS was confirmed but there was a substantial increase in expression of iNOS, which was proposed to be the source of the NO leading to nitrosylation. In addition, they showed that a calstabin1 stabilizing agent, rycal or S107, reversed the depletion of calstabin1 and the RyR1 “leakiness” attenuated the eccentric muscle damage in isolated muscles and improved exercise performance in intact animals. Most previous work has suggested that the elevated resting [Ca2+]i in mdx muscle following eccentric contractions arises from extracellular Ca2+ entry (see sect. IIIC); these studies (31, 31) raise the possibility that changes in RyR1 performance contribute to elevated resting [Ca2+]i. Thus these experiments offer a novel and therapeutically reversible pathway of muscle damage, which can help to explain the damage and reduced muscle performance in the mdx mouse.
2. Ca2+ sensitivity and maximum force of the contractile proteins
The Ca2+ sensitivity of the contractile proteins is normally determined after removal of the surface membrane (skinning) and measuring the force at an appropriate range of [Ca2+]. The resulting curve relating force and Ca2+ is characterized by the steepness, or Hill coefficient, the Ca2+ sensitivity and the maximum Ca2+-activated force. Many physiological and pathological factors that might change in muscular dystrophies are known to affect Ca2+ sensitivity, especially ROS and NO (for review, see Ref. 233). However, studies on muscle biopsies from boys with DMD (123) and from mdx mice (174, 509) have not found changes in Ca2+ sensitivity, provided comparisons are made between comparable fiber types. It remains possible that acute production of ROS or NO associated with intense activity or eccentric contractions produce changes in sensitivity that may be persistent (108).
Maximum force was very substantially reduced in the skinned fibers obtained from muscle biopsies of 5- to 8-yr-old DMD boys (123). In fast (IIA) fibers, force from normal boys was 360 mN/mm2, whereas in the DMD boys it was only 67 mN/mm2. Slow fibers showed a lesser reduction, and some gave almost normal force. The reasons for this drastic reduction in force in fast fibers are not clear. It is unlikely to be directly related to the presence or absence of dystrophin since in the mechanical skinning procedure used here, the entire surface membrane and underlying dystrophin complex are removed. The weakness more likely relates to poor alignment (131) or reduced numbers of myofibrils associated with the disease or the regeneration process. Alternatively some other change associated with the disease, such as ROS/RNS or Ca2+-activated proteolysis, may have reduced the force-generating capacity. In contrast, studies on mdx fibers showed reductions of ∼20% mainly in slow fibers while the fast fibers showed no significant reduction (509).
3. Muscle damage
Muscle damage can contribute to weakness in many ways depending on the components of the cell that are affected. In this section we focus on some new studies of the earliest manifestations of damage. An intriguing study of damage mechanisms in mdx muscle (5–13 mo of age) utilized direct observation of isolated lumbrical muscles (76). Muscles were stimulated to give maximal 1-s tetani at 1-min intervals and, in these very small muscles, it was possible to observe the behavior of individual fibers. As expected, the wild-type muscles maintained tetanic force well under these nonfatiguing conditions. However, the mdx muscles showed a progressive reduction in force to ∼50% after 10 tetani. Observation showed localized contractures progressing to “hypercontracted clots” occurring during the interval between tetani. This localized damage was associated with increases in resting intracellular Ca2+ and prevented by removal of extracellular calcium. These experiments suggest that normal tetani in the mdx cause Ca2+ entry that triggers local contracture and then individual cell death. The pathway of Ca2+ entry was not defined but, because the damage occurred between the tetani rather than within a tetanus, it does not seem to be caused by “membrane tears” associated with the stress/strain of contraction. Even extending the tetani to 5 s in the mdx muscle did not cause failure during the tetani. A surprising aspect of these experiments is that single fibers dissected from mdx muscles do not show failure of this type, even after eccentric contractions (523), suggesting that the lateral connections between fibers are needed for this particular pattern of failure. Furthermore, intact muscles of the mdx mouse do not show such rapid decline of force for tetani at this low frequency (295), suggesting that some aspect of isolation worsens the situation. One hypothesis is that the Ca2+ entry that triggers this damage is through a channel whose opening is triggered with a delay after tetani. If ROS are involved in the opening of this channel (145, 507), perhaps the lack of blood perfusion in this preparation exacerbates the accumulation of ROS and drives this very rapid damage pathway.
Head (174) has investigated the effect of branching on muscle damage, using single mdx fibers that frequently exhibit two or more side arms (branches), thought to be a consequence of incomplete or abnormal regeneration. Single fibers were isolated by collagenase treatment from 18- to 25-mo-old mdx mice, and resting [Ca2+]i was found to be the same in both WT and branched or unbranched mdx fibers. However, when stimulated, mdx fibers had reduced tetanic [Ca2+]i compared with WT, and branched fibers showed a further reduction. When stimulated repeatedly, branched mdx fibers frequently failed, becoming inexcitable with raised resting [Ca2+]i. When fibers were skinned, the properties of WT and mdx were similar except that branched fibers frequently ruptured at the branch site. Head (174) suggests a two-stage process leading to damage in the mdx. In young muscles, the absence of dystrophin leads to channel activation that elevates Ca2+ followed by Ca2+-activated damage. This damage then causes splitting of fibers that subsequently leads to further damage and impaired muscle force, as a consequence of the imbalance of forces around the split site (see Figure 6). Head (174) suggests that therapy needs to be implemented in the early phase, before mechanical impairment adds an additional component of instability (see also sect. VC4).
FIGURE 6.
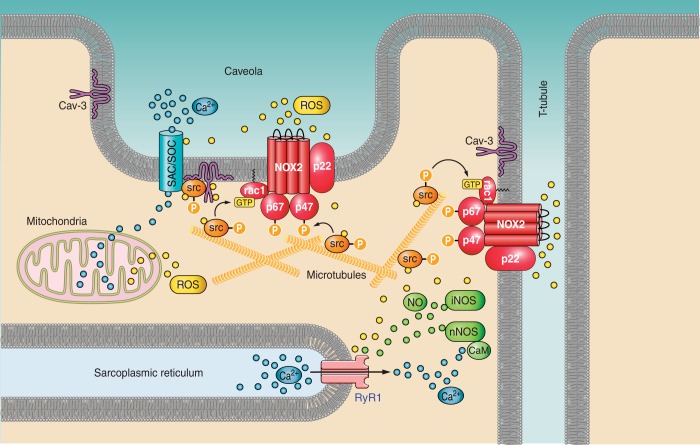
Mechanisms of damage and functional impairment in dystrophic muscle. This schematic highlights some of the key deleterious pathways that lead to impaired muscle function, as a result of the loss of dystrophin. Particular focus is given to the pathways relating to ROS, Ca2+, NO, and fibrosis. See text for details.
4. Lateral transmission of force
Muscles consist of sarcomeres in series and myofibrils and fibers in parallel. During activation, the force produced is developed by crossbridges between the thick and thin filaments and is transmitted by the thick and thin filaments and the Z line proteins between thin filaments; thus the primary route of force transmission is longitudinal along the myofibrils to the tendon via the myotendinous junction. During normal activity, sarcomeres remain in register across a muscle fiber and muscle fibers remain in register across a whole muscle, implying that there is some lateral connection that retains this registration. It is also important that the sarcomeres remain in register with the sarcolemma; otherwise, t-tubules would be subjected to shear forces. The lateral connections between myofibrils occur between the Z lines and are composed principally of desmin (338). At the edge of the cell where myofibrils are close to the surface membrane, these lateral connections continue and insert into a circumferential group of proteins associated with the membrane known as the costamere (360). Many proteins are associated with the costamere including vinculin, a-actinin, γ-actin, dystrophin, integrins, and spectrin (for review, see Ref. 117). Costameres include the DPC and make further connection via laminin to the extracellular matrix and hence to neighboring fibers.
While the principal mode of force transmission is longitudinal, there are a variety of situations in which lateral force transmission is liable to be important. For instance, when a muscle is partially activated, fibers scattered randomly throughout a muscle are activated, and because in large muscles fibers do not run the whole length of a muscle, there would be no force production unless force were capable of lateral transmission as well as longitudinal transmission. Another example would be if a small group of myofibrils is damaged, then longitudinal force transmission through that region may fail, but if lateral force transmission occurs, a damaged region can be bypassed. The first clear demonstration of such lateral transmission in frog skeletal muscle was made by Street (439). She used a preparation of a single fiber with a cuff of damaged fibers surrounding it. Force production was first measured conventionally from tendon to tendon of the single fiber. Then one tendon was detached and connection made to the cuff of damaged fibers surrounding the single fiber. When the fiber was stimulated, it was still possible to measure 85% of the original force between one tendon and the cuff of surrounding fibers demonstrating that lateral force transmission between fibers must occur. This work has recently been extended to mammalian muscles by the use of a “yoke” which was sewed to the fibrous connective tissue (epimysium) surrounding a small muscle (371). Force was first measured from tendon to tendon and then from tendon to yoke with the other tendon detached. This study confirmed that lateral force transmission occurs between fibers and the surrounding connective tissue, but they also extended the study to mdx mice and old WT rats and mice. In both the mdx and the aged groups, lateral transmission was substantially reduced compared with appropriate controls. This important study establishes that in mdx mice, presumably because of loss of dystrophin, lateral force transmission is impaired, and this is likely to contribute to the loss of force production when there are localized regions of damage in an mdx fiber. Another possible cause of impaired lateral force transmission in DMD is the excessive deposition of fibrotic tissue in the endomysium between muscle fibers (97).
5. Blood flow regulation in mdx muscles
Blood flow to active muscles increases by mechanisms that are still only partly understood (399). Sympathetic activity increases in exercise and, while this decreases blood flow in resting muscles, it is relatively ineffective in contracting muscle, an effect know as “functional sympatholysis” (452). The mechanism of this inhibition is not known in detail, but Thomas and Victor (1998) showed that the NOS inhibitor NG-nitro-l-arginine (l-NAME) attenuated this effect. In a later study they showed that functional sympatholysis was also attenuated in mdx muscle, in which nNOSμ is absent from the surface membrane, and in a nNOSμ KO muscle (450). Despite the absence of nNOSμ, the contraction-induced increase in blood flow was similar in l-NAME, mdx muscle, and the nNOSμ KO, suggesting that other mechanisms are capable of maintaining the contraction-induced stimulation of blood flow. Functional sympatholysis is also attenuated in boys with DMD (401), raising the possibility that impaired blood flow to muscles is an element in the disease. Early clinical studies on DMD boys failed to detect differences in blood flow between normal and dystrophic muscles (47). However, a recent study in BMD showed that tadalafil, a phosphodiesterase inhibitor, increased cGMP and NO production and thereby restored functional sympatholysis and increased exercise-induced muscle blood flow (267). Whether tadalafil will affect the pathogenesis and progression of BDM remains to be determined. For further discussion of the role of nNOS, see section IVA.
B. Membrane Properties
As noted in the introduction and Figure 1, the distribution of dystrophin and other cytoskeletal proteins suggests they might have a role in lateral transmission of force (considered in sect. IIIA4) or in strengthening the membrane or maintaining the registration of sarcomeres with the surface membrane. Dystrophin molecules are rod shaped with a length of ∼110 nm (403, 485). They form a network on the intracellular side of the sarcolemma with increased concentration at the level of the Z line contributing to the costamere (117, 512). Because t-tubules run laterally across the fiber, it is critical that the registration of the membrane to the sarcomeres is maintained; otherwise, there is a risk that the t-tubules will be sheared off from the surface membrane during contraction or stretch. In the present section we consider mechanical measurements of the membrane designed to establish which, if any, properties are affected by the absence of dystrophin.
An influential early paper by Menke and Jockusch (282) studied the sensitivity of collagenase-isolated single muscle fibers to hyposmotic solutions. Such solutions cause muscle cells to swell, to produce numerous membrane blebs, and eventually lead to hypercontraction and the leakage of soluble intracellular proteins from the muscle. Menke and Jockusch (282) found that mdx fibers were more susceptible to this type of damage and suggested that dystrophin helped to stabilize the membrane and protect against this type of damage. However, they also pointed out that the mechanism of damage in this model is uncertain and that the elevated intracellular calcium that mdx cells often exhibit might make cells more sensitive to this procedure. In a follow up study, they showed that addition of the Ca2+ ionophore A23187 at the time of hyposmotic shock did not affect the control fibers (283), making this explanation less likely. Furthermore, they showed that preincubation with cytochalasin D, which disrupts the actin cytoskeleton, increased the susceptibility of control fibers so that they were indistinguishable from mdx fibers. These studies suggest that the combination of the actin cytoskeleton and dystrophin contribute in some way to the integrity of the membrane when exposed to osmotic stress.
While it is possible that mechanical properties of dystrophin/actin contribute to the deleterious effects of osmotic stress, recent studies have identified Ca2+ entry and ROS production pathways that are involved in this response (268, 418, 487). These studies will be considered in more detail in section IIIC.
Another approach to investigate membrane properties is to suck the membrane into a glass tube; when the suction pressure is sufficiently great, the membrane fails. In muscle using conventional patch pipettes (tip diameter ∼1 μm), membrane failure occurs with a negative pressure of ∼180 mmHg, equivalent to a membrane stress of ∼6 mN/m (127, 159, 195). When mdx muscles were compared with WT, Franco and Lansman (127) found no difference while Hutter et al. (195) found a small but statistically significant difference (6.0 mN/m for WT vs. 5.2 mN/m for mdx) but did not believe that this difference was responsible for the large differences in osmotic sensitivity discussed above. Instead, they suggested that dystrophin might have an analogous role to spectrin, which maintains the discoid shape of red blood cells, and in whose absence the more spherical red blood cells are very sensitive to osmotic swelling and damage. They suggested that dystrophin, through its role in the costameric connections, might constrain the caveolae and membrane folds of the skeletal muscle membrane. In its absence, lack of this folding might lead to less “spare membrane” so that osmotic swelling would lead the membrane to reach its stretchable limit earlier (194). In later work, Nichol and Hutter (323) studied the membrane vesicles obtained by treatment of muscle fibers with collagenase and hyposmotic solution and showed that increases in the intracellular Ca2+ produced by the ionophore A23187 led to a drastic and rapid decrease in tensile strength. The mechanism of this effect was not explored and the weakening only occurred above 0.8 μM Ca2+, but this mechanism might offer a partial explanation for the greater susceptibility of the mdx muscle to mechanical perturbations.
Studies of membrane deformation have been extended by use of simultaneous microscopy to define the shape of the membrane in the pipette. Suchnya and Sachs (441) found that the shape of the membrane in the patch pipette was slightly curved towards the pipette tip in wild-type muscle in the absence of applied pressure. As negative pressure was applied, the membrane became curved away from the tip as expected. Unexpectedly, in mdx muscles, the resting membrane was more strongly curved towards the tip, and this curvature was eliminated in both wild-type and mdx by cytochalasin D, which disrupts the γ-actin cytoskeleton. These data have important implications for opening of stretch-sensitive channels in the membrane (which are sensitive to membrane curvature) but might also explain the small differences in membrane stress at failure noted by Hutter et al. (195).
Garcia-Pelagio et al. (139) also investigated the deformability of the membrane but used much larger pipettes of ∼25 μm diameter (approximately half the fiber diameter). At low pressures, the membrane and attached contractile machinery were sucked into the pipette. Above the separation pressure (367 kdyn/cm2, 287 mmHg in wild-type muscles), the membrane separated from the contractile proteins presumably by failure of the costameres. At still higher negative pressure (631 kdyn/cm2, 476 mmHg), the sarcolemmal bleb burst. Both the separation pressure (171 kdyn/cm2) and the bursting pressure (321 kdyn/cm2) were significantly lower in mdx mice compared with wild type. These results differ from the patch-clamp studies discussed above, possibly because the patch-clamp studies used cultured myotubes or collagenase-treated myocytes which lack the extracellular matrix. Perhaps when this mechanical support is absent, the structural role of dystrophin is minimized, which could explain why the membrane stiffness only appears smaller in mdx fibers when an intact preparation is tested. Another possibility is that the much larger pipette tips (25 vs. 1 μm) probe different aspects of the mechanical properties of the membrane, cytoskeleton, and myofibrillar complex.
Indentation stiffness has also been measured with glass rods that depress the surface of myotubes grown in culture (337). The values obtained in this way for indentation stiffness were 12 dyn/cm for wild-type myotubes versus 3.4 dyn/cm for mdx fibers. The authors considered the possibility that elevated [Ca2+]i might have influenced the stiffness of mdx fibers, for instance, by activating calpains that proteolyse cytoskeletal proteins, but attempts to modulate [Ca2+]i or inhibit calpains with leupeptin had minimal effect. They concluded that dystrophin contributes to indentation stiffness. Indentation stiffness has also been measured in intact muscles using an atomic force microscope whose conical probe was indented into the muscle. A Young's modulus was calculated as 4.2 kPa for wild-type and 1.4 for mdx fibers. When cytochalasin D was used to disrupt the actin cytoskeleton, stiffness values in wild-type muscles fell to values indistinguishable from mdx fibers while cytochalasin D only produced small falls in stiffness in mdx fibers. When either utrophin or dystrophin was expressed in mdx muscle fibers, stiffness returned to wild-type values.
In summary, the results suggest that under near-normal conditions, when muscles have their normal complement of extracellular matrix and myofibrillar proteins, dystrophin makes some contribution to the radially measured stiffness, probably through connections with the actin cytoskeleton. Whether this reduced stiffness has a broader role in the developing pathology of the disease remains to be established.
C. Increased Membrane Permeability
1. Appearance of CK in the blood
Intense physical activities often result in elevated serum levels of enzymes, such as CK and lactate dehydrogenase (LDH), which are abundantly expressed inside skeletal muscle fibers. For normal individuals, the level of these enzymes can be increased in the blood after strenuous exercises, such as marathon running or resistance training, particularly when these exercises include eccentric contractions (48). Several inherited myopathies, including many forms of muscular dystrophy, are also clinically characterized by elevated serum CK levels, which can reach values 100-fold higher than normal levels in DMD patients (531). On the basis of this evidence, it is widely accepted that high blood CK levels are due to or associated with muscle damage, either as a result of a genetic neuromuscular disease or following damaging eccentric contractions. Nevertheless, studies in animals have shown that the magnitude of CK increase does not directly correlate with the extent of muscle damage after eccentric contractions, as measured by the loss of force (130, 261). Furthermore, mutations in muscle-specific genes, such as caveolin-3, result in hyperCKemia, an increased blood CK level without any measurable muscle weakness, abnormal pathology, or other neuromuscular disease symptoms. HyperCKemia can also occur from certain drugs and toxins, as well as alcoholism and malignant hyperthermia (48). Therefore, while elevated blood CK levels are clearly a very useful clinical marker of myopathy in patients presenting with other symptoms of neuromuscular disease, its use as an accurate, quantitative measure of muscle damage and muscle function in animal and human studies should be evaluated with some caution. On this point, it is known that skeletal muscle CK is susceptible to oxidative modification by ROS and reactive nitrogen species (RNS), which reduces its enzymatic activity and targets it for proteolysis (325). Therefore, in mdx and DMD muscles, under conditions of oxidative stress, only CK not significantly modified by ROS is likely to be detected by the standard CK activity assays. This may further complicate the interpretation of blood CK activity levels and their relationship with muscle damage under conditions of increased ROS production in dystrophic muscles. Quantitative measurements of CK protein levels by western blotting would be a useful complement to measurement of CK activity.
As in humans, rodents also have large increases in serum CK and LDH after eccentric contractions and in animal models of DMD such as the mdx mouse. In addition, animal studies have shown that eccentric contractions can cause an influx of extracellular proteins, such as albumin (275), or membrane-impermeable dyes, such as procion orange and Evans Blue dye (162, 354, 504, 505) into muscle fibers, the magnitude of which is significantly greater in mdx muscle than WT. Intuitively, the apparent free movement of molecules into (albumin, Evans Blue dye) and out of (CK, LDH) muscle fibers would be consistent with one common membrane damage pathway leading to enhanced diffusion of these molecules across the sarcolemma. Over the past 20 years or so, a popular idea to explain the increased membrane permeability, and the subsequent damage and fiber necrosis in dystrophic muscles, was mechanical injury of the sarcolemma as a result of high force eccentric contractions (295, 354) and the subsequent influx of extracellular Ca2+ which stimulated proteolysis and muscle degradation. This idea was based on the rationale that during muscle contraction, dystrophin acts as a mechanical shock absorber, dissipating force laterally from the contractile filaments, via the actin cytoskeleton, across the sarcolemma to the extracellular matrix. Thus dystrophin was thought to provide protection against sarcolemmal tears or lesions developing during high force contractions, particularly eccentric contractions (354). While this theory is plausible, it has, to our knowledge, not been supported by direct experimental evidence. It is important to emphasize that the presence of dye positive fibers and elevated blood CK after eccentric contractions in mdx mice does not per se provide any information about the physiological pathway(s) leading to the increased sarcolemmal permeability. Furthermore, despite the popular theory that molecules diffuse in or out of muscle fibers via mechanically induced sarcolemmal tears, evidence from DMD patients indicates that high force muscle contractions are not necessary for increased membrane permeability, since blood CK rises significantly soon after birth, before the onset of high force muscle activity and peaks between ∼1 and 6 yr of age (531). Thus the dystrophic disease process, independent of high force contractions, is sufficient to raise blood CK levels in DMD.
Based on the current knowledge of membrane repair processes in mammalian cells, including mdx skeletal muscle, mechanically induced membrane holes, even very large laser-induced lesions up to 5 μm (26) are rapidly repaired in seconds, a process that requires extracellular Ca2+ and intracellular vesicles (377). Thus, if membrane micro-tears occurred during eccentric contractions, they would be expected to quickly reseal so that any dye entry would occur during and within seconds after the contractions and then remain trapped inside the fiber after the membrane was rapidly repaired. In other words, the membrane tear theory would predict a constant level of dye inside fibers, regardless of the time at which the muscle was analyzed after the eccentric contractions (see Figure 3). Moreover, the number of tears would be predicted to be greatest during the first eccentric contraction, when peak force is greatest, and least during the last, when peak force is lowest, given that the absolute force produced by the muscle would be the main factor leading to micro-ruptures (354). Thus muscles collected for analysis 30 or 60 min after eccentric contractions would not provide any insight into the very early time course of dye entry. To test this hypothesis, a time course study is required, with the first measurements made immediately after the eccentric contractions. McNeil and Khakee (275) measured the uptake of albumin in eccentrically exercised rats at 0 and 90 min post-exercise and found dye-positive fibers at both time points. However, the eccentric exercise duration was 90 min in this study, so this does not provide any information regarding the early time course or pathway of albumin uptake during the first few contractions. Similarly, studies of dye uptake in mdx mice in vitro are typically measured 60–90 min post-eccentric contractions (295, 325).
FIGURE 3.
The mechanism of stretch-induced membrane permeability in dystrophic muscle. A: according to the original “membrane tear” theory, the increased dye entry into dystrophic muscle fibers was attributed to micro-tears in the sarcolemma, caused by eccentric contractions. The membrane tear theory predicts that extracellular dyes would rapidly enter the muscle fiber immediately after the mechanically induced tear was produced, and within seconds the membrane would reseal and trap the dye inside the fiber. Therefore, the amount of dye inside the fiber would remain constant at all times after the eccentric contractions. However, experimental results indicate a different mechanism of dye entry. Extracellular dye uptake is very small immediately after the eccentric contractions and progressively increases up to at least 60 min post eccentric contractions. Moreover, both mechanosensitive channel blockers (streptomycin and Gsmtx-4) and the antioxidant N-acetyl cysteine can prevent almost all of the dye uptake, suggesting pathways involving Ca2+ entry through mechanosensitive channels and ROS produced by NOX2 are responsible for the increased membrane permeability (see text for details). B: experimental results showing the time course of procion orange dye uptake after eccentric contractions in mdx EDL muscle and the inhibitory effect of the MSC blocker streptomycin. [From Whitehead et al. (505).] C: experimental results showing the inhibitory effect of the antioxidant NAC on Evans Blue dye entry in mdx EDL muscle following eccentric contractions caused by downhill treadmill running. [From Whitehead et al. (504).]
2. Role of Ca2+ influx through mechanosensitive channels and ROS on membrane permeability
To our knowledge, the study by Whitehead et al. (505) is the only paper to investigate the early time course of dye uptake in mdx muscle, from immediately after eccentric contractions up to 60 min. In this study, muscle force fell significantly in mdx muscles to ∼35% of the original force at 30 min post-contractions, indicating that considerable muscle damage had occurred. However, despite this, procion orange dye uptake immediately after the eccentric contractions was minimal, only a few percent higher than nonstimulated mdx control muscles. Interestingly, the area of dye-positive fibers increased progressively at 30 min and further at 60 min, to reach a value ∼15% higher than nonstimulated mdx muscles at this time point. These data indicate that dye uptake in severely damaged mdx muscles is a relatively slow process occurring gradually over at least an hour after the eccentric contractions. The data also suggest that up to 60 min, most fibers do not take up dye and those that do have a heterogeneous time course of dye uptake. In the same study, we also investigated if blocking mechano-sensitive channels (MSC) could reduce the number of dye positive fibers. Importantly, the dye uptake that occurred in this study was significantly reduced by two MSC blockers, streptomycin and Gsmtx-4, which also reduced the force loss from stretched contractions. Interestingly, the relatively slow, progressive increase in dye uptake over 60 min is similar to the time course of Ca2+ influx and raised [Ca2+]i in mdx fibers after stretched contractions. Taken together, these data argue against membrane tears as the main route of dye entry after eccentric contractions. Instead, it strongly indicates that MSC-induced Ca2influx triggers a pathway that leads to increased membrane permeability and dye entry.
In considering the pathway(s) causing increased membrane permeability in dystrophic muscle, it should be emphasized that eccentric contractions carried out in vitro generate considerably higher forces and strains than those produced in vivo, where contractions are generated by nonsynchronous, submaximal stimulation, and muscle strains are much lower than those used in vitro. Since the micro-tear theory is based on the concept that mechanically induced membrane lesions are caused by high muscle forces (354), the lack of direct evidence for micro-tears in vitro makes it very unlikely that these lesions occur in vivo. In support of this idea, a recent paper (366) did not find any reduction in Evans Blue dye positive fibers in eccentrically exercised mdx mice injected with Poloxamer 188, a membrane sealant used for stabilizing and repairing membrane breaches. In contrast, Evans Blue uptake in mdx mice could be prevented by administering the MSC blocker streptomycin before the eccentric exercise (505). This result is also supported by a recent study wherein mdx mice expressing a dominant negative TRPV2 mutant protein in skeletal muscle had significantly reduced Evans Blue uptake and blood CK levels in vivo (198). TRPV2 forms a Ca2+-permeable channel, which can be activated by stretch and store depletion (198). Furthermore, lowering [Ca2+]i by overexpression of SERCA1 in skeletal muscles of mdx mice dramatically reduced blood CK levels (149). Therefore, the current data on intact mdx mice also argue against mechanically induced micro-tears as the mechanism for stretch-induced dye uptake, while providing strong evidence that Ca2+ influx through SACs and increased [Ca2+]i is a major mechanism for triggering the increased membrane permeability.
In addition to Ca2+ entry through MSC, ROS also play an essential role in membrane permeability of dystrophic muscles. Following eccentric contractions of mdx EDL muscles in vitro, Evans Blue uptake was significantly reduced by the antioxidant N-acetylcysteine (NAC), with similar levels to WT controls (504). A recent study using the antioxidant ascorbic acid also showed a reduction in Evans Blue uptake in mdx muscle in vivo (460). Moreover, serum CK levels were significantly reduced in mdx mice by intraperitoneal delivery of the antioxidants α-lipoic acid and l-carnitine (182) and oral administration of green tea extract (59). Based on these data, although the precise sequence of events remains to be elucidated, both Ca2+ influx through SACs and excessive ROS production are primary pathways for mediating dye entry and CK efflux from dystrophic muscles.
3. Possible Ca2+- and ROS-mediated membrane permeability pathways
In nondystrophic mouse diaphragm muscles, procion yellow dye uptake was stimulated by BAY K 8644-mediated Ca2+ influx, which was mediated by cytosolic, Ca2+-dependent phospholipase A2 (PLA2) activity and ROS, particularly the hydroxyl radical (OH−). Cytosolic phospholipase A2 (cPLA2) is a Ca2+-dependent enzyme that targets membrane phospholipids, giving rise to lysophospholipids and free fatty acids (FFA), both of which can cause further membrane damage (354). The activity of cPLA2 was found to be 10 times greater in DMD muscles compared with control muscles (248). Moreover, mass spectrometry lipid imaging studies of mdx and DMD muscles using Time-of-Flight Secondary Ion Mass Spectrometry found increased FFA and arachadonic acid levels in degenerating areas of muscle (447). Together these results indicate that increased PLA2 activity occurs in dystrophic muscle and is a likely mechanism of sarcolemmal lipid disruption and increased permeability. ROS such as hydroxyl (OH−) and peroxinitrite (ONOO−) initiate lipid peroxidation, which can also disrupt the chemical structure and fluidity of membranes and increase permeability. A recent study has shown that DMD muscles have threefold greater levels of lipid peroxidation than control muscles, which is accompanied by a 50% reduction of the endogenous antioxidant glutathione (GSH) (379). Studies in mdx mice have also shown evidence of ROS-mediated lipid peroxidation (44, 105), which can be prevented by antioxidants (286). It is also possible that PLA2 might work in conjunction with ROS to permeabilize the membrane, since PLA2 is very effective at removing oxidized lipids (416, 417). With this in mind, it is interesting to note that PLA2 can also directly increase mitochondrial ROS production in muscle, possibly by effects on the electron transport chain (324).
4. Role of increased membrane permeability on muscle function
It is clear that the relatively small number of dye positive fibers (5 to 20%) (295, 505) does not, by itself, explain the large, immediate force drop (often 70% or more) that commonly occurs from eccentric contractions in mdx muscle. Assuming the most extreme case scenario, even if all the dye positive fibers failed to generate any force, which is unlikely, this would contribute up to 20% or so of the force deficit. However, as discussed earlier, the stretch-induced increase in membrane permeability is negligible immediately after the contractions and increases progressively for at least 60 min in mdx muscle (505), whereas the force loss is instantaneous, becoming greater with each successive eccentric contraction. This difference in the time course of development suggests that the immediate loss of force is not directly caused by the increased membrane permeability. However, the same pathways that mediate the increased permeability, ROS and Ca2+ entry through SACs, also contribute to the force loss after eccentric contractions (see below).
A more likely outcome to explain the apparent selectivity of membrane-permeable fibers is that these fibers are the most vulnerable to eccentric contraction-induced damage. This could occur because some fibers are already partially damaged before the eccentric protocol, as a result of the normal, dystrophic process in vivo. For example, if the sarcolemma of these fibers was already undergoing lipid peroxidation or lipid damage due to PLA2 activity, the additional Ca2+ influx and ROS production from the eccentric contractions could accelerate the membrane permeability process, leading to dye uptake within the first 60 min or so following the contractions. Another possibility is that certain fibers may be more susceptible due to anatomical differences such as cross-sectional area, changes in signaling protein expression or activity, or fiber type differences. Some support for this last hypothesis was provided by a study in which mdx EDL (a predominantly fast glycolytic muscle) but not soleus (a slow, oxidative muscle) was susceptible to eccentric contraction-induced membrane permeability and force loss (295). This also supports the finding that fast fibers are preferentially damaged and lost in muscles of DMD patients (494) and in the mdx diaphragm (355), the most severely affected muscle in these mice. Therefore, Evans Blue dye uptake might reflect the normal degenerative process in a subset of susceptible fibers, and this process can be accelerated by eccentric contractions.
Another reason for the selective uptake of dye into a relatively small subset of fibers was provided by the studies of Head and colleagues (see sect. IVA3). They showed that following eccentric contractions, localized Evans Blue accumulation occurred preferentially in branched EDL fibers from mdx mice, at the junction of the normal to branched fiber region (174). Also, they found that nonbranched fibers were mostly protected from Evans Blue uptake. These findings could account for the relatively low numbers of Evans Blue positive fibers found in cross-sections of mdx muscles after eccentric contractions. Interestingly, it was shown that in 2-mo-old mdx mice, the EDL muscle contained 17% branched fibers (64), which is very similar to the percent Evans Blue positive fibers found after eccentric contractions of mdx mice of the same age (505). In older mdx mice, the number of branched fibers increases to 87%, and this was accompanied by a commensurate rise in Evans Blue positive fibers and greater force loss after eccentric contractions (64). Interestingly, branched fibers have reduced SR Ca2+ release and increased Ca2+ spark activity at branch points following osmotic stress than normal, unbranched mdx fibers (250). On the basis of the evidence already presented, it is possible that increased mechanical stress at the branched region activates more ROS and Ca2+ influx through mechanosensitive channels (MSC, also known as stretch-activated channels), which leads to localized membrane permeability and dye uptake in these branched regions.
IV. PROTEIN CHANGES IN THE ABSENCE OF DYSTROPHIN
As described in section II, the loss of dystrophin from the sarcolemma causes significant changes in the expression and localization of many structural and signaling proteins associated with the dystrophin complex. Recent studies using proteomics have also shown protein expression changes dystrophic muscles that involve a wide range of cellular pathways including metabolism, ion channel regulation, extracellular matrix composition, and cellular stress response (107, 312, 375). These studies highlight the complex nature of the disease and are useful for identifying new mechanistic pathways and novel therapeutic targets. In this section, we have focused on the expression changes and localization of proteins relating to NO, ROS, and Ca2+ signaling pathways in dystrophic muscle, and how these proteins contribute to muscle damage in DMD.
A. NOS and NO
Nitric oxide is an important signaling molecule in many aspects of skeletal muscle physiology (431), particularly those relating to adaptation from exercise (442). NO modulates glucose uptake, regulates vascular perfusion during contraction, maintains mitochondrial function, and modulates excitation-contraction coupling (ryanodine receptor function) in normal muscle. It is not surprising, then, that abnormalities in NO bioavailability are associated with muscle diseases.
Skeletal muscle tissue expresses all three distinct gene products of NOS: NOS1 (nNOS), NOS2 (inducible NOS, iNOS), and NOS3 (endothelial NOS, eNOS). All NOS isoforms are active in the dimeric form (Figure 4). The predominant form of NOS in skeletal muscle fibers is one isoform of nNOS. Alternative splicing of the NOS1 gene product yields four major isoforms: nNOSα, nNOSμ, nNOSβ, and nNOSγ (50). nNOSμ is the predominant form in skeletal muscle and differs from nNOSα by the inclusion of a muscle-specific exon that encodes a 34-amino acid insert located in the catalytic domain (419). The function of the μ insert is not known. nNOSβ lacks the amino-terminal PDZ domain present in nNOSμ. nNOSγ lacks part of the catalytic domain and its expression at the protein level has not been convincingly demonstrated. Since nNOSα is not expressed in skeletal muscle, this discussion of nNOS in skeletal muscle will be limited to nNOSμ and nNOSβ.
FIGURE 4.
Nitric oxide synthase (NOS). A: Different isoforms of NOS are generated by 3 genes. All have three domains in common: the oxygenase domain, the reductase domain, and the calcium/calmodulin binding domain (CaM). The muscle-specific form of nNOS contains an insert (μ) that is absent from the brain isoform, nNOSα. The PDZ domain in the NH2-terminal region of nNOSμ binds numerous signaling proteins. The nNOSβ NH2-terminal domain likely targets this isoform to the Golgi elements. eNOS is cotranslationally myristoylated (Myr) on the NH2 terminus and posttranslationally by palmitoylation (Palm) at two nearby sites. B: All isoforms are active as homodimers formed by interaction of the oxygenase domains. Arg, arginine binding site; BH4, tetrahydrobiopterin binding site; FMN, flavin mononucleotide binding site; FAD, flavin adenine dinucleotide binding site; NADPH, binding site for reduced form of nicotinamide adenine dinucleotide phosphate. [The figure of the NOS dimer (B) was kindly provided by Dr. Philip Dash, Univ. of Reading, UK.]
Structurally, nNOS and eNOS differ primarily in the amino-terminal region. eNOS lacks a PDZ domain but is dually acylated by myristoylation and palmitoylation near the amino terminus. In skeletal muscle, eNOS is expressed primarily in endothelial cells in the vasculature, although some reports show low levels of the enzyme on the sarcolemma and in association with mitochondria. Unlike nNOS and eNOS, iNOS is constitutively active, due to its high affinity for calcium/calmodulin. Resting levels of Ca2+ are sufficient to maintain iNOS in the active state. Like eNOS, iNOS is present in only low levels in skeletal muscle fibers, but in dystrophic muscle tissue, iNOS present in inflammatory cells contain high levels of the enzyme. For reviews of NOS in skeletal muscle, see References 90, 272, 273, 372, 431.
1. nNOSμ on the sarcolemma
In skeletal muscle, nNOSμ is largely associated with the sarcolemma and is enriched at costameres and myotendious junctions (66). This localization, and presumably its function, is determined by the interaction of nNOSμ with several proteins, most notably the DPC. For purposes of this review, we focus on those interactions that are relevant to muscular dystrophy.
Association of nNOSμ with the DPC requires α-syntrophin (6, 49). The PDZ domains of nNOSμ and α-syntrophin are both required. Analysis of the structure of this complex revealed an unexpected interaction. The PDZ domain of α-syntrophin binds to a pseudo-SXV sequence in a β-turn structure in nNOSμ that lies just downstream of the nNOSμ PDZ domain (180). In this arrangement, the PDZ domain of nNOSμ is available to bind other proteins.
In studies of muscle from patients with BMD, nNOSμ is absent from the sarcolemma in some cases, despite the presence of α-syntrophin (67). Subsequent studies identified the repeats 16–17 of the dystrophin rod domain as necessary for nNOS association with the DPC (500). These results are consistent with the demonstration that dystrophin constructs used for AAV-mediated expression in mdx muscle need the spectrin-like repeats 16–17 in the rod domain to restore nNOS to the sarcolemma (229). The docking site has been further delineated to certain α-helices within the rod region (230). Some studies suggest that utrophin is unable to restore nNOS to the sarcolemma (244), a potential problem for utrophin replacement therapy. Nevertheless, sarcolemmal localization of nNOS is considered a reliable diagnostic tool for Becker MD (461, 500) and a marker for complete restoration of the dystrophin complex (500).
In addition to α-syntrophin and dystrophin, nNOSμ also interacts with other proteins that are important for muscle function. Phosphofructokinase-M (PFK-M) is an important regulatory enzyme in glycolysis. Humans that are genetically deficient in PFK-M have muscle weakness (172), experience activity-induced fatigue (482), and exhibit reduced glycogen metabolism and increased serum levels of CK (315). PFK-M binds via its carboxy-terminal tail to the PDZ of nNOSμ (125). Further studies are needed to fully understand the metabolic and physiological role of the nNOSμ-PFK-M interaction (496).
The activity of sarcolemmal nNOSμ is modulated by its interaction with several other proteins, including caveolin-3, heat shock protein 90 (HSP90), and protein inhibitor of NOS (PIN). PIN is a small, highly conserved protein, now known to be dynein light chain 8 (181), that blocks nNOS dimer formation (200, 458). Caveolin-3, also an inhibitor of nNOS (475), is upregulated in mdx and DMD muscle (380, 468). Furthermore, mutations in caveolin-3 result in LGMD1C, hyperCKemia, rippling muscle disease, and distal myopathy (141). HSP90 regulates nNOS turnover via ubiquitination (340). These interactions illustrate the complexity of the mechanisms of nNOS regulation in normal and dystrophic muscle.
The loss of nNOS from the sarcolemma is not limited to DMD or BMD. In several other muscle diseases, sarcolemmal nNOS is absent or markedly reduced. These include LGMD 2C, 2D, and 2E and inflammatory myositis (85) and several forms of inherited and acquired myopathic conditions (122). Also, diseases in which denervation occurs, such as amyotrophic lateral sclerosis and spinal muscular atrophy or in which transmission at the neuromuscular junction is defective, such as myasthenia gravis, have reduced sarcolemmal nNOS (122, 277). The loss of sarcolemmal nNOS in these diseases is surprising, since at least in one case, dystrophin and α-syntrophin are present at normal levels (277). Understanding the mechanism of this defect in these diseases may shed new light on the regulation of nNOSμ association with the sarcolemma.
2. nNOSμ in the cytoplasm
In skeletal muscle fibers, ∼50% of nNOSμ is found in the cytosol (450). In mdx muscle, the total amount of nNOSμ is markedly reduced, but the remaining enzyme is almost entirely in the soluble fraction. Muscle from mice lacking α-syntrophin also has a higher level of soluble nNOSμ (451). The function of soluble nNOSμ is not known, although transgenic expression of nNOSα in skeletal muscle resulted in reduced amounts of inflammatory cells, fibrosis, and serum CK in mdx (495) and dystrophin/utrophin DKO mice (497).
Carboxy-terminal binding protein (CtBP), a transcriptional corepressor activated by NADH, may be involved in nNOS-mediated improvements in skeletal muscle function. CtBP binds via its carboxy terminus to the nNOS PDZ domain (383). As NADH levels are depleted by muscle activity, the repressive effects of CtBP are reduced, thereby activating transcriptional modulators of mitochondrial biogenesis (503). Binding of CtBP via its carboxy-terminal tail to nNOSμ shifts the location of CtBP from the nucleus to the cytosol (383), a process that could boost mitochondrial biogenesis.
The association of nNOS with microtubules may have implications for the early events leading to the dystrophic pathology. Recent studies demonstrated that nNOS (440) and α-syntrophin (133) bind to microtubule-associated proteins 1A and/or 1B (MAP1A/1B). Growing evidence suggests that MAPs are involved in signal transduction by modulating microtubules and through interaction with signaling proteins, such as nNOS. Microtubules interact with dystrophin (361), and the highly organized lattice structure characteristic of healthy muscle is disrupted in dystrophic muscle (345). Furthermore, the lattice structure is largely restored by rAAV-microdystrophin (345). Abnormalities in microtubules may be a critical step in dysfunction in calcium and ROS signaling (213 and see sect. IVD6).
3. nNOSβ on the Golgi apparatus
In mice, nNOSβ is sufficient to mediate penile erection, even though it generates only a small fraction of NO production (193). As in other tissues, the nNOSβ isoform is expressed in skeletal muscle at much lower levels than nNOSμ, but its localization is highly restricted. The discovery that nNOSβ is localized on skeletal muscle Golgi elements, along with soluble guanylate cyclase and protein kinase G, is strong evidence that it mediates an NO-cGMP signaling pathway (343). This and other studies prompted examination of phosphodiesterase (PDE) inhibitors as potential therapeutic agents in mdx mouse skeletal and cardiac muscle (341). Inhibition of PDE5 showed improvement in mdx skeletal (18, 217, 347) and cardiac function (4, 19, 212). Very recently, a trial of tadalafil was conducted in men with BMD in which muscle oxygenation was reduced due to impaired modulation of the exercise-induced vasoconstrictor response (267). The results demonstrated that tadalafil alleviates muscle ischemia and restores normal blood flow. These results provide further evidence for nNOS and NO-cGMP as essential components of normal muscle function and as promising therapeutic targets.
The mechanism(s) by which PDE5 inhibitors alleviate the dystrophic phenotype are not fully understood. Defects in mitochondrial localization and ATP synthesis in mdx muscle are important in the development of the dystrophic pathology, but these abnormalities are not corrected by sildenafil administration (346). Vascular dysfunction in mouse models (217) and in humans (267) probably contributes to, but is not the primary cause of, muscular dystrophy.
Two genetically engineered mouse models have proven to be very helpful in understanding nNOS function in skeletal muscle (160, 191). The KN1 mouse lacks nNOSμ but nNOSβ is retained. All isoforms of nNOS are absent in the KN2 mouse. A comparison of these models on the same genetic background (C57BL/6) has provided evidence that nNOSμ and nNOSβ may have different functions in skeletal muscle (343). Tibialis anterior muscle from the KN1 mouse analyzed in situ exhibited normal force output and post exercise fatigue. This is in contrast to earlier studies showing a modest increase in fatigue on the B6129 strain, emphasizing the importance of proper genetic-background controls in these studies (342). The KN2 mouse, which lacks both nNOSμ and nNOSβ, displays a more pronounced functional phenotype. KN2 tibialis anterior muscle exhibited dramatically increased contraction-induced fatigue and force deficits after exercise. Also, the rate of recovery after fatigue was greatly slowed. Finally, KN2 muscle generated markedly reduced maximum isometric force and was significantly weaker, based on reduced mean specific force.
The role of nNOS isoforms in the dystrophic pathology of DMD has received considerable attention, in part because of their potential as therapeutic targets. Two general approaches have been used: 1) elimination of one or both forms of nNOSμ in mdx mice and 2) expression of nNOS as a soluble or membrane-targeted protein in mdx muscle. Interpretation of the studies from different labs is compromised by the use of different genetic strains, methodologies, ex vivo versus in situ physiological tests, muscle type, and the nNOS isoform. Nevertheless, some general conclusions can be gleaned from the studies.
Genetic deletion of nNOSμ from the mdx mouse (dystrophin/nNOSμ DKO) improved force generation by the EDL measured ex vivo (246). This result strongly suggests that the level of cytosolic nNOSμ has a marked impact on muscle function. In a tail suspension model of unloading-induced muscle atrophy, nNOSμ became dislocated from the sarcolemma to the cytosol (445). Atrophy was much milder in nNOSμ null mice subjected to the same treatment. The mechanism appears to involve cytosolic NO production by delocalized nNOSμ resulting in Foxo transcription factor translocation to the nucleus, which then activates muscle atrophy genes.
In addition to causing the loss of nNOSμ from the sarcolemma, dystrophin deficiency also affects nNOSβ associated with skeletal muscle Golgi elements (132). In the mdx mouse gastrocnemius muscle, the Golgi levels of nNOSβ are reduced. Furthermore, the “beads on a string” organization characteristic of Golgi elements in normal skeletal muscle is also disrupted, even in 2-wk-old mdx mice (132, 345). Thus the absence of dystrophin disrupts signaling by nNOSβ by reducing levels of the Golgi-associated enzyme and altering its organized distribution, even before the onset of muscle necrosis.
Complete elimination of nNOS in mdx mouse muscle was accomplished by crossing the mdx mouse with the KN2 mouse that lacks both nNOSμ and nNOSβ (mdx-NOS DKO) (132). These mice have smaller body mass than WT or mdx mice and lack the skeletal muscle hypertrophy characteristic of mdx mice. Central nucleation is also reduced, suggesting that regeneration may be impaired in mdx-NOS DKO muscle. Also, macrophage infiltration was dramatically increased (>10-fold) compared with mdx muscle. Muscle weakness was markedly worse as was susceptibility to eccentric contraction-induced muscle damage in the mdx-NOS DKO mouse. However, mdx muscles lacking both forms of nNOS do not exhibit increased muscle fatigue. TA muscle from mdx and mdx-NOS DKO mice show a similar force decrement during repetitive stimulation in situ. This surprising result suggests that the absence of dystrophin has a compensatory effect on muscle lacking both nNOSμ and nNOSβ, which is very susceptible to fatigue. These results revealed unappreciated complexity in the role of nNOS in dystrophic muscle.
Restoration of nNOSμ to dystrophic muscle could potentially correct physiological defects associated with DMD. Transgenic expression of nNOS in the mdx mouse has been reported to improve the histopathology of skeletal muscle, without targeting of the enzyme to the sarcolemma (495). Immune cell infiltration and fiber central nucleation were both decreased. Similar experiments on the mdx:utrophin double knockout showed a small increase in life span and reduced fibrosis, cellular infiltration, inflammation, and procion red uptake (497). While these results are consistent with a positive impact of nNOS overexpression on the dystrophic histopathology, the approach had several limitations. First, these studies used nNOSα, the brain isoform rather than the muscle-specific nNOSμ. Second, the enzyme was expressed in skeletal muscle at very high levels (455). In addition, the high levels of nNOS resided entirely in the cytosol (or at least, not on the sarcolemma), where excess production of NO could be detrimental. Finally, no contractile physiological studies were performed to determine if nNOSα expression had a functional effect.
To force expression on the sarcolemma, nNOSμ was modified by introduction of a RAS sequence at the carboxy terminus. Earlier studies had shown that α-dystrobrevin containing a RAS sequence is targeted to the sarcolemma when transgenically expressed in the mdx mouse (7). As predicted, nNOSμ-RAS was targeted to sarcolemma in mdx muscle. In situ contractile physiological studies revealed that nNOSμ-RAS markedly reduced both muscle fatigue and eccentric contraction-induced force loss, in mdx TA muscle (Rebolledo et al., unpublished data). Unmodified nNOSμ did not associate with the sarcolemma and failed to improve the contractile properties of mdx TA muscle, highlighting the importance of sarcolemmal localization of nNOS for improving physiological function of mdx muscle. The beneficial effects of nNOSμ-RAS may be due, in part, to increased sarcolemmal expression of utrophin, α-syntrophin, α-dystrobrevin, and β-dystroglycan. Therefore, AAV-mediated expression of this novel, sarcolemmal targeted form of nNOSμ may be therapeutically useful in diseases in which sarcolemmal nNOS is lacking.
4. eNOS
This form of NOS has received little attention in the DMD field. In one interesting study, eNOS levels in resistance arteries were reduced in the mdx mouse, compared with control mice (249). Importantly, NO-dependent vascular function and vascular density were also reduced. Treatment with gentamycin to promote readthrough of the early stop-codon in dystrophin restored both parameters. These results indicate that eNOS may play a significant role in the pathological development. Additional studies of vascular eNOS are warranted.
5. iNOS
iNOS is expressed at low levels in normal muscle fibers, but in dystrophic muscle, infiltration by inflammatory cells containing iNOS leads to much higher levels of this isoform in skeletal muscle tissue. However, cultured mdx myotubes express higher levels of iNOS than wild-type myotubes, a result of Ca2+ induced gene expression (14) and exhibit increased NO production. Reduction of iNOS levels by siRNA treatment markedly reduces the levels of iNOS protein and NO production. As discussed above, iNOS has been proposed as the source of NO that nitrosylates RyRs, thereby increasing calcium leak at the triad (30). This proposal has been challenged by the finding that genetic ablation of the iNOS gene fails to improve the histopathology or contractile function of mdx muscle (245). In addition, the level of nNOS in the cytosol of BMD muscle correlates with RyR1 hypernitrosylation and disease severity (144). Finally, association of nNOS with RyR1 in skeletal muscle has been observed (397, 397, 519). The relative roles of nNOS and iNOS in promoting Ca2+ leak from RyR1 clearly require further study.
B. Caveolae and Caveolin-3 as Signaling Platforms in Muscular Dystrophy
Caveolae are 60- to 80-nm vesicular structures of the plasma membrane, broadly defined by the presence of the structural protein caveolin, and their unique lipid composition, rich in cholesterol and sphingolipids. A number of excellent reviews have described the numerous structural, signaling, and trafficking functions of caveolae and the caveolin proteins in many cell types, under a wide variety of cellular conditions (78, 335). The current review will instead focus on the role of caveolae and caveolin-3 in skeletal muscle and, in particular, recent evidence that caveolae are mechano-signaling platforms for Ca2+, ROS, and NO production that contribute to the pathophysiology of dystrophin-deficient muscles.
There are three caveolin isoforms: 1, 2, and 3, which are differentially expressed in a wide range of cell types. Caveolin-3 (Cav-3) is the muscle-specific isoform, expressed in skeletal, smooth, and cardiac muscle. In addition to caveolin, recent studies have shown that another protein family, the cavins, is required for correct formation and stabilization of caveolae (335). The long-standing view was that caveolae are omega/flask-shaped membrane vesicles; however, recent evidence has shown that they can also be cup-shaped, depending on the method in which the cells are fixed (406). Caveolae formation in muscle requires the oligomerization of caveolin, as highlighted by the lack of caveolae in muscle of Cav-3 KO mice (78). Interestingly, it has been estimated that between 100 and 200 caveolin proteins can oligomerize to form a single caveola. This provides a remarkable repertoire of possible proteins, including ion channels, kinases, and enzymes, which can associate within a single caveola to orchestrate signaling cascades in response to conditions such as muscle contraction, stretch, and neurotransmitter or hormonal stimulation. The scaffolding domain of Cav-1 and Cav-3, a 20-amino acid residue region, is thought to bind and sequester many signaling proteins within caveolae and to maintain them in an inactive conformation until activated by various stimuli. Of particular relevance for DMD, many proteins involved in the pathophysiological production of Ca2+, ROS, and NO in dystrophic muscle have been shown to localize in caveolae, and some can also directly bind to caveolin. These include TRPC1 and 4, NADPH oxidase 2 (NOX2), nNOS, src kinase, and rac1. Thus there is good evidence that caveolae act as a signaling platform for Ca2+, ROS, and NO pathways and therefore may regulate these deleterious pathways in dystrophic muscle.
1. Caveolin-3 expression and localization in muscular dystrophy
During skeletal muscle development, Cav-3 is expressed during the process of myoblast fusion to form myotubes. The loss of Cav-3 interferes with normal myogenesis, leading to abnormal growth of myotubes and abnormal t-tubular structural development (136). In mature muscle, Cav-3 is localized along the sarcolemma but is also highly expressed in t-tubules (310, 370), with “hot spots” of expression at the necks of the t-tubules (310). Cav-3 has a key role in the formation of the t-tubular system, and certain Cav-3 mutations cause improper formation of the t-tubules leading to abnormal conduction of action potentials within muscle fibers. In humans this condition is referred to as rippling muscle disease (78).
While Cav-3 is not considered an integral component of the dystrophin complex (87), it does co-immunoprecipitate with members of the DGC (420), and caveolae are in close proximity to dystrophin and β-dystroglycan in rat ventricular muscle (102).
Regulation of Cav-3 expression and/or localization is associated with many forms of muscular dystrophy. Mutations in Cav-3 result in absence or significant reduction in sarcolemmal Cav-3 expression and cause four different types of muscle disease: limb girdle muscular dystrophy 1C, rippling muscle disease, distal myopathy, and hyperCKemia (for review, see Ref. 140). In contrast, mdx and DMD muscle have significantly increased Cav-3 expression levels compared with normal muscle (145, 380, 468). In addition, muscle freeze-fracture studies of the sarcolemma have demonstrated increased numbers of caveolae in DMD, which are smaller in size and abnormally distributed (42). Interestingly, in mice that transgenically overexpress Cav-3 in skeletal muscle, a “Duchenne-like” dystrophy develops, with many features similar to mdx mice including raised CK, muscle necrosis, and central nuclei (137). A key finding of this study was that Cav-3 transgenic mice had no dystrophin expression and very low levels of β-dystroglycan on the sarcolemma, essentially the same as mdx mice. This result can be explained by the finding that Cav-3 binds to the WW domain of β-dystroglycan and therefore effectively competes with dystrophin for this interaction (425). Thus high expression of Cav-3 competitively inhibits dystrophin from binding to β-dystroglycan and essentially creates a dystrophin-deficient muscle phenotype. The binding of dystrophin and Cav-3 to β-dystroglycan is also regulated by phosphorylation of dystroglycan. As discussed above, phosphorylation of a tyrosine residue (Y890) within the WW domain of β-dystroglycan, by src kinase, prevents dystrophin and utrophin binding to β-dystroglycan but allows Cav-3 binding to the same site (197). Furthermore, β-dystroglycan that is phosphorylated by src is internalized from the sarcolemma to an internal membrane vesicular structure and coimmunoprecipitates with src at this location (423). Recently, it was shown that crossing mdx mice with a β-dystroglycan knock-in mouse, with a phenylalanine residue substituting the tyrosine residue (Y890F), led to reduced muscle damage and provided some protection against eccentric contraction-induced force loss (290). Therefore, increased src expression and activity in dystrophic muscle (145) is likely to play an important role in linking Cav-3, and its associated signaling proteins, with the DPC, through β-dystroglycan. This might be a point of consideration for DMD gene therapy and exon skipping approaches (see sect. VIA), since the high expression of Cav-3 in dystrophic muscles might restrict the amount of dystrophin that can bind β-dystroglycan and therefore limit the effectiveness of these therapies.
2. Caveolin-3 and TRPC channels
TRPC1, a putative MSC responsible for the increased stretch- and/or store-operated Ca2+ influx in mdx muscle, requires Cav-3 for sarcolemmal localization and stretch-induced activity in C2 myoblasts (145). In the same paper, it was shown that src, also a Cav-3 binding protein, triggers Ca2+ influx through TRPC1, in response to H2O2 exposure (145). While the precise mechanism by which src activates TRPC1 channel activity is unclear, there is evidence that all TRPC channel members, including TRPC1, contain a src homology domain and are able to bind src (211), suggesting that src-mediated tyrosine phosphorylation might regulate the activity of TRPC channels. TRPC1 has also been shown to form heterodimers with TRPC4 in dystrophic muscle, and the activity of these SAC/SOCs is reduced by binding to α-syntrophin (394). These findings suggest another possible mechanosignaling link between Cav-3 and the dystrophin complex, via TRPC1/4 and syntrophin. In addition to interactions between TRPC1 and Cav-3, the lipid composition of caveolae, in particular cholesterol, can also influence MSC activity. A recent study showed that MSC activity, measured by patch clamping, was greatly enhanced in WT myotubes following knockdown of Cav-3 or depletion of cholesterol from caveolae by methyl-β-cyclodextrin (190). This result is interesting, since the membrane cholesterol content of myotubes derived from DMD muscle is 35% lower than nondystrophic control cells (237). Further studies will be required to determine if this membrane cholesterol reduction impacts the activation of MSC in dystrophic muscles, particularly during periods of mechanical stretch.
3. Caveolae and Nox2
In some nonmuscle cells, the ROS-producing enzyme NADPH oxidase (Nox2) and its subunits (described in more detail in sect. IVD) have been found to assemble and localize in lipid rafts and caveolae, which can serve as lipid environments for activation or inhibition of Nox2 (165, 480, 521). In skeletal muscle, Nox2 is located along the sarcolemma (507) and in the t-tubules (178), both of which are enriched with Cav-3. Immunostaining of isolated mouse fibers indicates colocalization between Nox2 and Cav-3 (396) and between the Nox2 subunit p22phox and Cav-3 (507). Furthermore, the Nox-2 activator protein rac1 has been shown to localize in caveolae and bind Cav-3 in cardiac muscle (210), thereby providing another link between Nox2 and caveolae. Thus there is some data suggesting that Nox2 and its subunits localize in caveolae in muscle, although the mechanisms regulating Nox2 activation by caveolae and Cav-3 during muscle stretch remain to be studied.
Src kinase is another Cav-3 binding protein that is involved in activating Nox2. In other cells, Src regulates the activation of Nox2, via two main mechanisms. First, in smooth muscle it phosphorylates the Nox2 organizer subunit p47phox, stimulating its translocation from the cytosol to the membrane complex (462). Second, it can activate rac1 by regulating the guanylyl exchange factors Vav2 and Tiam1 (415). As discussed in section IVD7, Src phosphorylation of p47phox also occurs in mdx muscle and is an important step in Nox2 activation (324). Given that Src is activated by ROS and can stimulate TRPC1 channel opening, we postulate that this kinase might provide an important link between Nox2-mediated ROS and subsequent TRPC activity and Ca2+ influx.
As discussed in section IVD6, in order for stretch to activate Nox2 in mdx muscles, an intact microtubule network is required (213). Microtubules can associate with caveolae in cardiac muscle cells and regulate the expression of Cav-3 (173). Furthermore, several studies have shown that microtubules are required for the trafficking of caveolae from the Golgi to the plasma membrane (173, 305, 508). Given the abnormal distribution of caveolae on the sarcolemma of dystrophic muscles, it is tempting to speculate that the irregular microtubule lattice structure at the membrane in mdx muscle (345, 361) might contribute to this disorganized spatial arrangement of caveolae. Microtubules can also bind src and rac1 (33, 313), suggesting that muscle stretch might lead to activation and translocation of these proteins to stimulate Nox2 activity. In support of this idea, it has recently been shown that src can be very rapidly activated by an integrin-mediated mechanotransduction pathway (<300 ms) and travel large distances (15–60 μm) along microtubules following activation (313).
4. Cav-3 and nNOS
As discussed previously, nNOS binds to α-syntrophin, which localizes it to the dystrophin complex at the sarcolemma. It has also been shown that nNOS can bind to the Cav-3 scaffolding domain in vitro, which inhibits its activity (475). However, while these two proteins can bind together, it is unclear whether this interaction is very prevalent in dystrophic muscle. For example, the loss of dystrophin or α-syntrophin causes mislocalization of nNOS from the sarcolemma to a cytosolic location. This occurs despite increased sarcolemmal expression of Cav-3 expression in the case of mdx or DMD muscles. Therefore, while Cav-3 might regulate the activity of nNOS, it alone is not sufficient to target nNOS to the sarcolemma. One possibility is that nNOS can simultaneously bind α-syntrophin and Cav-3, through the nNOS β-finger domain binding α-syntrophin (180) and part of the nNOS catalytic domain binding Cav-3 (404). In this way, nNOS activity could potentially be regulated by the interaction between the dystrophin, via α-syntrophin, and Cav-3 mechanosignaling pathways. Another potential reason why nNOS binds to Cav-3 is to regulate the activity of other proteins within the caveolae by NO-mediated S-nitrosylation of cysteine groups. Of particular relevance to dystrophy, it was recently shown that Nox2 activity is inhibited by S-nitrosylation of a cysteine residue (Cys 89), which is evolutionarily conserved in plants and animals (527). Therefore, loss of nNOS from the sarcolemma might enable Nox2 activation to be enhanced in dystrophic muscle due to the absence of this NO-mediated inhibitory mechanism.
C. Mechanosensitive Channels, Store-Operated Channels, SERCA, and Ca2+
It has long been appreciated that total muscle calcium was greatly increased in damaged DMD fibers (39), but there has been very variable literature on whether myoplasmic free [Ca2+] ([Ca2+]i) is elevated in mdx fibers prior to obvious damage (for review, see Ref. 147). Many studies have found increases in resting [Ca2+]i in mdx compared with wild-type muscle (189, 465, 466, 523), but others have failed to detect any such difference (134, 174). Gillis (147) pointed out that collagenase-treated fibers generally did not show an increase in [Ca2+]i, whereas manually dissected fibers did. This is consistent with the concept that mechanical stresses involved in dissection could trigger channel activity leading to the elevated [Ca2+]i. Given that [Ca2+]i can be elevated in mdx fibers, how does this arise? In this section, we review the substantial evidence that activation of Ca2+-permeable channels contribute to this rise in [Ca2+]i. An alternative view, that mechanically induced defects in the membrane (micro-tears) cause increased [Ca2+]i, has been considered in section IIIC. We also briefly review the role of the SR Ca2+ pump (SERCA) in modulating Ca2+ and consider the pathways by which elevated [Ca2+]i contribute to the pathology of DMD.
1. Mechanosensitive channels
Mechanosensitive channels (MSC) were first described by Guharay and Sachs (159) in chick embryonic muscle. They patch-clamped channels on the surface membrane and identified a class of channels whose activity increased when negative pressure was applied to the interior of the patch electrode. The application of negative pressure to the pipette sucks membrane into the patch electrode and is assumed to stretch channels therein (441). These MSC were permeable to a range of cations and thus allow Na+ and Ca2+ entry when activated. MSC were shown to be more prevalent and active in mdx fibers (127, 128) and in DMD myotubes (473). Yeung et al. (523) showed that a series of eccentric contractions caused a large rise in [Ca2+]i in mdx fibers that lasted for at least an hour. This rise in [Ca2+]i could be prevented by the known blockers of MSC (Gd3+, streptomycin, and GsMTx-4) and is therefore likely to be caused by activation of MSC. Mdx mice that were exercised on a treadmill with downhill running, which provokes severe muscle damage, had reduced force loss and membrane permeability when treated before the exercise with streptomycin. This result suggests that elevated [Ca2+]i associated with activation of MSC has a role in the muscle damage of dystrophinopathies.
Unfortunately, the identity of the gene that encodes MSC is still uncertain. Maroto et al. (263) originally suggested that TRPC1 encoded the muscle MSC. This was based on 1) purification of the 80-kDa protein, the size predicted; 2) demonstration that an antibody to TRPC1 bound the protein; 3) expression of mammalian TRPC1 in frog oocytes and mammalian COS cells produced a 10-fold increase in MSC activity; and 4) that siRNA to TRPC1 reduced MSC expression in oocytes. Later, the same group partially retracted this conclusion on the basis that TRPC1 expression in COS cells no longer reliably increased MSC activity and the expressed protein did not traffic to the surface membrane (150). Further doubt about the role of TRPC1 in MSC has been raised by studies of muscles from a TRPC1 KO mouse, which still contained MSC activated by negative pressure in the patch pipette (529). Instead, a smaller conductance and pressure-insensitive channel was absent in the TRPC1 KO muscle. However, a study of cytoskeletal damage induced by eccentric contractions in WT muscle found that the damage was reduced in TRPC1 KO mice, suggesting that TRPC1 has some role in Ca2+ entry associated with damage (534).
Other candidates for the MSC include TRPC6, which appears to be responsible for the shear strain-activated current in ventricular myocytes (109), and TRPV2, which encodes stretch-sensitive channels in vascular smooth muscle (307) and appears to be the source of Ca2+ entry in the δ-sarcoglycan deficient mouse model of muscular dystrophy (198).
Given the possibility that TRPC1 has some role in MSC and in store-operated channels (see next section), several studies have investigated the binding partners of TRPC1. Gervasio et al. (145) showed that TRPC1 and Cav-3 coimmunoprecipitated and appeared to be close binding partners, based on FRET between coexpressed TRPC1-CFP and Cav-3-YFP. TRPC1, Cav-3, and src kinase are all upregulated in mdx mice and may form a complex which allows pathological, unregulated Ca2+ entry. Homer-1, a scaffolding protein, has been shown to bind to TRPC1 (526), and Stiber et al. (436) showed that a Homer 1 KO had abnormal Ca2+ influx and developed a skeletal myopathy. The Ca2+ influx and myopathy was ameliorated by gene silencing of TRPC1. TRPC1 also binds to α-syntrophin, and Vandebrouck et al. (472) showed that dystrophin, α-syntrophin, and TRPC1 form a macromolecular complex based at the costamere. In mdx muscle, the loss of expression of dystrophin and α-syntrophin is associated with abnormal Ca2+ influx that can be restored by expression of a mini-dystrophin and α-syntrophin. Subsequently, this group showed that silencing of α-syntrophin in normal muscle was sufficient to cause enhanced Ca2+ entry. The above studies all suggest that a macromolecular complex including dystrophin, α-syntrophin, Cav-3, TRPC1, and src-kinase is located at the costamere and allows regulated Ca2+ entry. In DMD, dystrophin is absent and α-syntrophin is severely downregulated, but the other members of this complex are upregulated and allow unregulated Ca2+ entry (for review, see Ref. 393).
In theory, MSC can be activated by a variety of pathways (74; for review, see Ref. 224): 1) intrinsic bilayer forces acting upon the channel within the membrane and modulated by curvature of the membrane or insertion of lipids into the bilayer; 2) tethered proteins connecting the channel to the cytoskeleton (the cellular cytoskeletal is ubiquitous and connects to many proteins in the membrane); and 3) indirect activation by cell signaling pathways. Although MSC were discovered by their pressure sensitivity, this is not necessarily the mechanism by which they are activated in vivo. Pathways that cause posttranslational modification of a channel may change the mechanism of activation. Alternatively, expression of proteins involved in the macromolecular complex that regulates the channel may change.
The activation of MSC in mdx mice after eccentric contractions appears to be an example of indirect activation. In patch-clamp experiments, the muscle MSC is turned on and off within 100 ms of the instigating pressure change (163, 523). In contrast, Yeung et al. (523) could detect no change in Ca2+ during the period when the muscle was stretched. However, they observed a persistent (30 min) increase in myoplasmic Ca2+ following eccentric contractions, even though the muscle was no longer being stretched during this period. Thus activation of the MSC appears after a substantial delay, suggesting that activation is indirect. A considerable body of evidence points to the involvement of ROS, produced by NADPH oxidase, which subsequently activate MsC. This will be discussed in section VD6.
The hypothesis that the activation of MSC in dystrophic muscles has a central role in dystrophic pathology has been questioned based on studies in which increased utrophin was expressed at various ages (429). When utrophin expression was increased in utero or at birth, both MSC function and dystrophic pathology were corrected. However, when utrophin expression was increased at 30 days, MSC function was corrected but the pathology was not. An alternative interpretation of these findings is that the pathology caused by elevated intracellular calcium eventually becomes self-sustaining.
2. Store-operated Ca2+ channels
Extracellular Ca2+ entry into cells following store depletion was first described in nonexcitable cells (365) and is now recognized to occur in skeletal muscle (228, 235). The functional role of these channels is not entirely clear. They appear to have roles in enhancing Ca2+ influx during repeated stimulation leading to fatigue, when stores become depleted, and in regulating myotube development and gene expression (236; for recent review, see Ref. 435). Several studies have established that store-operated Ca2+ entry (SOCE) is more active in mdx muscle and may well contribute to the increased [Ca2+]i (104, 111, 474).
There has been a prolonged debate about the identity of genes that encode SOCE. TRP channels have long been candidates for SOCE, and Vandebrouck et al. (474) showed that antisense downregulation of TRPC1 and 4 reduced the SOCE in mdx fibers. In a different approach, Millay et al. (289) overexpressed TRPC3 in mouse skeletal muscle and found an approximately sixfold increase in SOCE and the induction of a dystrophic-like myopathy. Thus these studies are consistent with the idea that TRPC channels contribute to SOCE.
However, since the discovery that Orai1 constitutes the surface membrane channel in lymphocytes (121), studies in muscle have implicated Orai1 as the membrane channel of SOCE. Lyfenko and Dirksen (254) showed that in normal muscle, siRNA knockdown of STIM1, the putative Ca2+ sensor in the SR, or replacement of Orai1 by an impermeant version both eliminated SOCE. These findings led to a model of SOCE in which STIM1, situated in the junctional SR membrane, interacts with Orai1, located in the junctional t-tubular membrane. The implication of this result is that, if a component of SOCE depends on a TRPC channel, it also requires Orai1. In the mdx mouse, both STIM1 and Orai1 are upregulated, which may explain the increased SOCE in mdx muscle (111). Furthermore, siRNA knockdown of Orai1 can return SOCE in the mdx muscle to wild-type levels (535).
While the demonstration of increased SOCE in mdx is of interest, it does not necessarily provide an explanation for the increased [Ca2+]i. SOCE is activated only when Ca2+ within the SR falls below the level required to activate STIM1. Current estimates are that SOCE is first activated when SR Ca2+ falls below 225 μM and is maximally activated below 100 μM (236). Whether these reductions ever occur under physiological conditions is debatable. However, in mdx muscle, these activation levels are higher (111), so a smaller degree of depletion would activate a greater fraction of the available SOC channels. The magnitude of the SOCE would presumably also be increased by the greater number of STIM1 and Orai1 proteins (111). Several studies suggest that Ca2+ leak from the SR is greater in mdx muscle (30, 386), and the combination of reduced SR Ca2+ content and raised activation threshold for SR Ca2+ could provide a stimulus for SOCE. Another class of possibilities is that some other factor in the myoplasm activates the SOCE process without changing the threshold for SR Ca2+. One possibility is that the products of Ca2+-independent PLA2, such as lysophosphatidylcholine, can activate SOCE (41). A further possibility follows from the observation in cell lines that H2O2 can stimulate SOCE through activating inositol trisphosphate (IP3) receptors in the SR and causing SR Ca2+ depletion (158). Given that ROS production is increased in muscular dystrophies (see sect. VD), this could provide another pathway for SOCE activation.
3. SR Ca2+ pump
The primary function of the SR Ca2+ pump (SERCA1) is to return Ca2+ from the myoplasm to the SR and thus cause relaxation. Whether its function is affected in mdx muscle is controversial with increased pump rate (386), unchanged pump rate (358, 448), and decreased pump rate (99, 143, 207) all being reported. Despite these diverse findings, overexpression of SERCA in both the mdx mouse and the δ-sarcoglycan-null mouse produced a robust improvement of the dystrophic phenotype with substantial reductions in serum creatine kinase, central nuclei, fibrosis, and improvements in the histological appearance of muscle and an increase in running time (149). SERCA overexpression also caused a concurrent reduction in resting [Ca2+]i, accelerated decay of the Ca2+ transient, and rescued the dystrophic phenotype caused by TRPC3 overexpression. Goonasekera et al. (149) proposed that accelerated SR uptake reduces resting [Ca2+]i and mitochondrial Ca2+ and that, as a consequence, calpain activation was reduced. These experiments support the concept that elevated [Ca2+]i is the “final common pathway” in several dystrophic diseases and that increased SERCA activity can correct this. While this is an intuitively attractive concept, there is a theoretical difficulty that changes in internal buffering of [Ca2+]i within a cell, such as provided by the SR, should have no long-term effect on the resting [Ca2+]i which is determined by the balance of Ca2+ influx and efflux at the surface membrane (384). To paraphrase the situation, if it is true, as observed experimentally, that enhanced SR leak is associated with increased myoplasmic [Ca2+]i, then there must be a concomitant rise in surface membrane Ca2+ influx or a decrease in surface membrane Ca2+ efflux. The presence of SOCE might conceivably represent this link, but it seems hard to believe that a small SR Ca2+ leak could deplete the SR to the point at which SOCE is activated. Goonasekera et al. (149) report that SR Ca2+, judged by 4-CMC Ca2+ efflux, was unaffected by SERCA overexpression in the disease model. For a recent review of the role of Ca2+ handling in DMD, see Reference 471.
An interesting study by Gehrig et al. (143) provides further support for the benefits of improving SERCA performance in mdx muscle and in the more severe dystrophic model in which both dystrophin and utrophin are not expressed (dystrophin/utrophin DKO). The study focused on Hsp72, a heat shock protein that acts as a molecular chaperone and can prevent the degradation of proteins, including SERCA, under conditions of cellular stress. Gehrig et al. (143) showed that in mdx muscles, SERCA activity was reduced and that this could be improved by overexpression of Hsp72 in the muscles. Simultaneously, a large range of indicators of muscle pathology were also improved. Of course, Hsp72 might be protecting other proteins, but the value of improving SERCA function has been independently demonstrated by Goonasekera et al. (149). An important aspect of this study (143) is that a pharmacological agent, BGP-15, causes increased expression of Hsp-72, and this agent was shown to produce more modest improvements in the dystrophic phenotype. Both Hsp-72 overexpression and BGP-15 treatment were also effective in the mdx/utrophin dko mouse model, which has a much more severe phenotype and arguably is a better model for establishing the efficacy of treatment for DMD. For further discussion of the role of molecular chaperones in DMD, see Reference 51.
The SR, in addition to Ca2+ release and reuptake, has a role in protein folding. In a range of conditions, collectively known as SR stress, Ca2+ homeostasis and protein folding are abnormal and cause an unfolded protein response which can trigger apoptosis. There is some evidence that SR stress occurs in DMD (164, 302) and contributes to the phenotype.
4. Calpains
Calpains are Ca2+-activated proteases of which three forms, μ-calpain, m-calpain, and calpain-3, are expressed in muscle (for comprehensive review, see Ref. 148). Given that resting [Ca2+]i can be elevated in mdx muscle, it might be expected that calpain activity would increase and make a contribution to the pathology. Turner et al. (465) first showed that resting [Ca2+]i was increased in mdx compared with wild-type muscle fibers and that this was associated with an increased rate of protein degradation. In subsequent studies, this group established that the elevated [Ca2+]i arose through an unidentified leak channel and could be blocked by a calpain inhibitor (11). Many groups have subsequently shown that calpain activity is higher in mdx muscles (for example, Ref. 427). Moreover, overexpression of the endogenous calpain inhibitor calpastatin could at least partly ameliorate some of the features of the dystrophic pathology (426).
Recent studies have given more detailed insights into the extent and pathways of calpain activation. Gailly et al. (135) used a membrane-permeant fluorescent calpain substrate and could modulate calpain activity over a 30-fold range by lowering or raising [Ca2+]i. Fast transient changes of [Ca2+]i, such as caused by normal tetani, did not increase activity, whereas slow increases in [Ca2+]i by depleting the SR or by activating store-operated Ca2+ entry produced moderate (50 or 100%) increases in calpain activity. Hyposmotic solutions, which cause swelling and should activate mechanosensitive channels, also produced increased calpain activity, which was blocked by GsMTx-4, a specific inhibitor of stretch-activated channels. The collagenase-isolated fibers used in the present studies did not show an elevated [Ca2+]i in mdx versus WT; nevertheless, they had a 50% higher calpain activity. Gailly et al. (135) showed that ∼10% of the μ-calpain was present in the autolysed form (78 vs. 80 kDa for the un-autolysed form). Autolysis is required for activation, and the autolysed form is more sensitive to [Ca2+]i and is expected to provide the calpain activity measured in their experiments.
Recent experiments on skinned fibers with strongly buffered [Ca2+]i provide further insights into the distribution of calpains and the [Ca2+]i required to activate them. Murphy et al. (311) showed that μ-calpain is mostly inactive at rest and requires a concentration of >2 μM for >2 min to produce significant autolysis and activity. However, the autolysed μ-calpain can have its activity maintained by much lower levels of [Ca2+]i. Thus the elevated Ca2+ levels noted by Gailly et al. (135) (∼300 nM for 10 min) would appear insufficient to cause the autolysis of μ-calpain but might allow the continuing activity of preautolysed calpain. However, the appearance of calpain activity at rest in mdx fibers with normal resting [Ca2+]i is not explicable on this basis and requires some additional explanation. These could include elevated [Ca2+]i near the channels in the membrane or in vivo situations that increase the sensitivity of calpains to calcium, such as phospholipids or phosphorylation (for review, see Ref. 148).
While there is good agreement that calpains, and probably μ-calpain, are activated in mdx and DMD muscle, the targets and possible therapeutic benefits of inhibiting calpain are less clear. Goll et al. (148) list many proteins that are targets for calpain, but this list has to be modified by the knowledge that most calpain-3 is tightly bound to titin or the triad region (309), whereas μ-calpain, while freely diffusible at rest, rapidly binds when activated, much of it to the surface membrane (311). One of the most likely targets is the reduction in Ca2+ release from the SR, which is often a feature of muscles subject to prolonged elevations in [Ca2+]i (13). This is particularly clear in skinned fibers where Ca2+ release from the SR can be shown to decline in a Ca2+- and time-dependent fashion after elevation of [Ca2+]i (476). It has recently been shown that junctophilin, a structural protein that maintains the correct separation between the t-tubules and SR, is degraded after eccentric contractions (82). Murphy et al. (308) subsequently showed that junctophilin is an activated calpain target, and its proteolysis is associated with a decline in SR Ca2+ release. Thus this Ca2+-calpain activated process may explain an important element of the weakness in DMD and mdx muscle.
While the study of Spencer and Mellgren (426) established that elevated calpastatin could reduce the histological features of dystrophy and the number of regenerating fibers in mdx muscle, it required an overexpression of calpastatin of more than 300-fold to achieve this outcome. Subsequent studies with pharmacological inhibitors of calpains have given quite variable results. Badalamente and Stracher (21) used intramuscular injections of the membrane-permeant tripeptide calpain inhibitor leupeptin and showed that calpain activity was reduced while histological signs of dystrophy (cell size, central nuclei, and fibrosis) were all improved. However, several other studies of leupeptin-based agents found minimal benefit either in the canine model of DMD (72) or in mdx mice after either 1 or 6 mo treatment, testing a range of functional, histological, and biochemical markers (410).
Thus current evidence suggests that calpain inhibition is of limited value as a treatment option in DMD. This view is reinforced by the fact that mutations in calpain-3, which lead to lack of proteolytic activity, are the cause of limb girdle muscular dystrophy 2A (382, 422). Thus it seems that the main physiological role of calpain-3 is sarcomeric remodeling (221).
D. ROS and NADPH Oxidase
1. Physiological and deleterious effects of ROS in skeletal muscle
In a biological system, ROS are oxygen-containing molecules that can chemically react with lipids, DNA, and proteins to modulate their structure and function. At low to moderate concentrations, ROS play an important role in many diverse cell signaling pathways but at high levels can cause reversible or irreversible damage. The cellular effects of ROS are complex and depend on a number of factors including the expression, localization, and regulation of the ROS source; the type of ROS produced and its reactivity; and the effectiveness of the antioxidant defense system.
Oxidative stress is a general term that characterizes pathological conditions in which cellular damage is induced by ROS. It is manifest by a complex series of pathways that cause dysregulation of the normal redox state in cells and tissues. Oxidative stress is commonly defined as an elevated ROS concentration due to increased ROS production and/or a reduced capacity for antioxidant pathways to scavenge excessive ROS; however, it has been more recently defined as a disruption of redox signaling and control (206). Oxidative stress can be acute or chronic and is associated with a wide range of common human diseases, including cardiovascular disease, diabetes, neurological disorders, and cancer (470). In skeletal muscle, oxidative stress is produced under pathophysiological conditions such as hypoxia, metabolic disorders, aging, and various myopathies. Oxidative stress can result in damage to muscle leading to impairment of muscle function, reduced regenerative capacity, atrophy, and ultimately muscle weakness. However, in recent years, this view has been modified to incorporate many important physiological functions of ROS in muscle, including muscle regeneration, mitochondrial function, metabolism, and muscle hypertrophy (27, 199). In addition, muscle function can be positively regulated by low levels of ROS during exercise, although at higher levels, ROS can impair force production and contribute to muscle fatigue (199, 378). Therefore, it is important to take into consideration the beneficial functions of ROS under normal physiological conditions when studying the deleterious effects of ROS in muscle undergoing oxidative stress.
2. Antioxidant clinical trials in DMD
Three decades ago, oxidative stress was hypothesized to contribute to muscle damage in DMD (for review, see Ref. 373). Initially, this hypothesis was based on similarities in muscle pathology of DMD and vitamin E-deficient individuals and animals (398, 459). Vitamin E is an abundant and important lipid-soluble antioxidant, and its deficiency in humans produces muscle myopathy with preferential damage to type IIB muscle fibers (459), as is the case in DMD (494). These findings prompted a series of clinical trials in DMD boys using antioxidants such as superoxide dismutase, vitamin E, and selenium (20, 138, 434). At the time, these trials were considered to be unsuccessful. In one trial, a superoxide dismutase mimetic was administered to DMD boys over an 18-mo period and changes in muscle strength were used as the main outcome measure. While muscle force improvement was not statistically significant overall, a wide range of muscles did show a positive improvement trend. This raises some important questions for consideration. First, was the dosing regimen appropriate? A single dose was used and apparently not optimized before the study was designed. Second, superoxide dismutase mimetics act extracellularly, and therefore will not effectively target ROS produced intracellularly. Third, even if most of the ROS is produced extracellularly, for example, by NADPH oxidase (see below), the SOD mimetic will catalyze the reaction of superoxide to H2O2. This would favor the production of H2O2 instead of other superoxide-derived ROS or RNS. While this may provide some benefits, by reducing the levels of damaging radicals such as peroxinitrite or superoxide itself, it would instead favor the accumulation of the H2O2, a relatively stable and membrane permeable ROS, which can modify a wide range of intracellular and extracellular molecules. For example, mdx myotubes are much more susceptible than WT myotubes to cell death by H2O2 (374), and this oxidant also directly increases Ca2+ influx in mdx fibers through SACs (145). In other words, converting one ROS (superoxide) to another (H2O2) may not be an effective antioxidant strategy. In further support of this idea, a recent study in mdx mice showed that overexpression of catalase, which decomposes H2O2, improved muscle function (411). Unfortunately, the lack of observable improvement in these original antioxidant trials has thus far diminished subsequent interest in ROS scavengers as a treatment for DMD. However, with renewed interest in the role of ROS in the pathogenesis of dystrophy from studies in the mdx mouse and DMD (see below), hopefully this will pave the way for established or novel ROS scavengers to be tested in DMD patients.
3. Evidence of ROS-induced damage in dystrophic muscle
Despite the unsuccessful clinical trials in DMD, numerous studies in the intervening years have provided compelling evidence that ROS contribute to the pathophysiology of muscular dystrophy. In DMD muscle biopsies, there is evidence of oxidative stress as measured by the twofold increased levels of protein carbonyl groups, a measure of protein damage by ROS (171). Oxidative damage to lipids has also been found in DMD muscle as determined by the increased levels of isoprostanes, a measure of lipid peroxidation (155). Similar results have been shown in mdx mice, with increased levels of muscle protein oxidation (170) and lipid peroxidation (286) compared with WT mice. In recent years, a plethora of different antioxidants have been administered to mdx mice, and many have been shown to significantly reduce muscle damage and improve muscle function, strongly supporting the role of oxidative stress in muscular dystrophy. These studies have been described in detail in a number of recent review papers (214, 373, 506) and therefore will not be further discussed here. Rather, the next sections will focus on the susceptibility of mdx muscle to ROS-induced damage and the sources of ROS in dystrophic muscles.
4. Increased susceptibility of dystrophic muscle to ROS
An important finding from Rando et al. (374) was that cultured dystrophic muscles have an increased susceptibility to muscle damage and cell death from oxidative stress. They showed that mdx myotubes underwent cell death with much lower concentrations of ROS such as H2O2 and the superoxide generating agent paraquat compared with WT myotubes (374). On the other hand, the rate of cell death was the same for mdx and WT myotubes after exposure to several metabolic toxins, suggesting a selective vulnerability to oxidative and not metabolic stress for mdx myotubes. Interestingly, undifferentiated myoblasts from mdx and WT muscles were equally susceptible to oxidative stress. Since dystrophin is not found in myoblasts but is expressed in WT myotubes, this finding indicated that the absence of dystrophin in mdx myotubes was the primary cause of the increased susceptibility to oxidative stress. This hypothesis was subsequently tested in another study, in which mdx myotubes expressing various truncated isoforms of dystrophin were exposed to oxidants (98). Here, it was shown that myotubes expressing the Dp71 isoform were more susceptible to oxidative stress than those expressing a Becker dystrophy isoform, which, in turn, were more susceptible than myotubes expressing full-length dystrophin. These results suggested that the susceptibility to ROS-induced cell death in myotubes positively correlates with the severity of the dystrophy in transgenic mice expressing these various dystrophin isoforms. This draws a direct link between dystrophin and the sensitivity to oxidative damage in skeletal muscle. Interestingly, this group also showed that overexpression of nNOS in mdx myotubes did not affect the susceptibility to oxidative stress, suggesting an NO-independent mechanism for the enhanced sensitivity to ROS-induced cell death (538). However, whether or not nNOS contributes to the sensitivity of ROS-induced oxidative stress in mature mdx muscle fibers, particularly during muscle contraction, is not known.
The mechanisms underlying the increased susceptibility to oxidative stress in dystrophic muscle have not been fully elucidated. However, as discussed in other sections, there is good evidence that ROS-induced Ca2+-influx through SACs is one pathway that contributes to muscle damage in mdx muscle and that mdx muscles are much more sensitive than WT to activation of this pathway (145, 418, 504, 507). Recent evidence demonstrates that depletion of endogenous antioxidant defense systems, such as glutathione (GSH), increases oxidative stress in DMD muscles (379). Therefore, it is likely that both an increased susceptibility of ROS-activated damage pathways, as well as reduction in endogenous antioxidants, contribute to the greater susceptibility of dystrophic muscles to oxidative stress. Research from our laboratory has shown that ROS enhance the influx of Ca2+ following stretched contractions (145, 507). We also found in resting, uncontracted fibers that H2O2 increased Ca2+ in mdx but not WT, again highlighting the greater susceptibility of dystrophic muscle to ROS-induced Ca2+ influx (145). Moreover, the increased [Ca2+]i in mdx was reduced to baseline levels by addition of streptomycin, suggesting that the exogenous H2O2 was somehow regulating the opening of MSC (145).
5. NOX2 is a major source of ROS in normal muscle
For a number of years, mitochondria were considered to be a major source of ROS production in a many cells and tissues, including skeletal muscle, produced as a byproduct of oxidative metabolism. Early studies estimated that between 2 and 5% of the oxygen consumed by mitochondria produced superoxide; however, subsequent results predict much lower values of 0.1–0.15% (166, 430). This evidence suggests that other sources of ROS might play an important role in muscle under both normal and pathophysiological conditions. Indeed, recent evidence indicates that during repetitive contractions of isolated mouse muscle fibers, mitochondrial ROS production is not increased despite an almost 100% increase in cytosolic ROS levels (288). In addition, this study provided evidence that NADPH oxidase (NOX) was a major source of ROS during contractile activity.
NOX is a ROS-producing (superoxide or H2O2), multiprotein enzyme complex. It was first identified in inflammatory cells, such as neutrophils and macrophages, and plays a key role in the body's host defense system by generating high, most likely molar, concentrations of ROS which kill invading microbes. The importance of NOX to innate immunity is highlighted by the inherited condition chronic granulatomous disease, where NOX mutations lead to reduction or inhibition of NOX activity in inflammatory cells, which compromises the host defense system. Paradoxically, increased NOX activity in other organs and tissues is associated with many common human diseases (234). Therefore, a tight regulation of NOX expression and activity is essential to provide cells with both the beneficial ROS-mediated signaling pathways and protection from the deleterious effects of excessive and chronic ROS production. There are several isoforms of the catalytic subunit of NOX: NOX1, NOX2 (also known as gp91phox), NOX3, NOX4, and NOX5, which are differentially expressed, and regulated, in a wide range of mammalian tissues and organs (for a comprehensive review, see Ref. 29).
Until recently, little was known about NOX expression and activity in skeletal muscle. However, in the last few years several studies have investigated the expression, localization, regulation, and function of NOX in skeletal muscle under various physiological conditions. Skeletal muscle of mice and humans expresses both RNA and protein for the NOX2 and NOX4 isoforms, and their activity is important for regulating myoblast proliferation (296). Both NOX2 and NOX4 constitutively bind to the p22phox subunit within cell membranes. However, unlike NOX4, which is an inducible isoform regulated by increased transcription (412), NOX2 requires several regulatory proteins to become activated; in mouse skeletal muscle, these subunits are p47phox, p67phox, and rac1 (201, 507). An additional subunit, p40phox, found in phagocytes, was not found in rat skeletal muscles but was expressed in muscle of a cancer cachexia mouse model (201). Therefore, this subunit may be differentially expressed depending on the muscle type, species, and biological stimulus. NOX2 and its associated subunits are localized at the sarcolemma and t-tubules in skeletal muscle fibers (178, 507). The t-tubule fraction of NOX2 was found to play a role in regulating increased Ca2+ release from RyR1 via S-glutathionylation in isolated triads from mouse muscle (178). NOX4 is localized at the SR in skeletal muscle and is regulated by the partial O2 pressure (Po2), with higher Po2 increasing its activity (443). This study also showed that H2O2 produced by NOX4 can regulate RyR1 activity and enhance muscle force production.
6. NOX2 is stretch-activated in dystrophic muscle and causes muscle damage and weakness
As discussed earlier, stretched contractions of mdx fibers increased ROS production which triggered Ca2+ entry through SACs and reduced force production (145). Since NOX is rapidly activated in smooth muscle during cyclic stretch (70, 156), it seemed plausible that it might also be stretch-activated in skeletal muscles. To test this hypothesis, mdx fibers were stretched after incubation with the NOX inhibitor DPI. Blocking NOX activity significantly reduced the [Ca2+]i and improved muscle force after stretched contractions compared with control, untreated mdx fibers (507). These data suggested that in mdx fibers, stretch-induced activation of NOX2 increased ROS levels, which subsequently led to the opening of SACs and Ca2+ influx. The same study also demonstrated increased NOX2 activity in mdx muscle compared with WT and two- to threefold higher expression of NOX2 and its subunits, p67phox and rac1, in mdx muscle. Importantly, these proteins were increased in young adult mdx mice but also in muscles of prenecrotic, 19-day-old mdx mice (507), suggesting that increased ROS production from NOX2 might be a key source of the oxidative stress at this time (98) and therefore a mediator of the subsequent muscle fiber damage and necrosis. Similarly, other studies have also reported increased protein expression of gp91phox, p67phox (213, 418), and rac1 (213) in adult mdx muscle. In the first of these studies (418), isolated mdx muscle fibers were subjected to hyposmotic stress, and both intracellular and mitochondrial ROS and Ca2+ were measured with fluorescent indicators. Here, it was shown that both basal ROS and stress-induced ROS levels were much higher in mdx than WT fibers and were inhibited by blocking NOX with DPI. Furthermore, they showed that stress-induced Ca2+ influx was blocked by an antioxidant and DPI. Interestingly, it was also reported that basal mitochondrial ROS production was not higher in mdx muscles; however, stress-induced Ca2+ influx led to increased mitochondrial Ca2+ content, which then triggered increased mitochondrial ROS production. Together, these results suggest that during stretch of dystrophic muscle, NOX2 rapidly produces ROS, which triggers Ca2+ entry through SACs, leading to increased mitochondrial Ca2+ and additional ROS production (Figure 5), causing muscle damage and weakness (Figure 6).
FIGURE 5.
Mechanisms of stretch-induced reactive oxygen species (ROS) production and Ca2+ influx in dystrophic muscle. This schematic highlights some of the key deleterious pathways that lead to impaired muscle function, resulting from the loss of dystrophin. Particular focus is given to the pathways relating to ROS, Ca2+ and NO. During eccentric contractions, NADPH oxidase 2 (NOX2) is stimulated by a pathway involving microtubules and activation of rac1 and src kinase (src). This leads to phosphorylation of Nox subunits and activation of Nox2. The ROS produced by Nox2 further activates src, which triggers the opening of stretch-activated or store-operated channels (SAC/SOC), and influx of Ca2+. Increased Ca2+ uptake by mitochondria can also stimulate additional ROS production. Both ROS and NO, produced by mislocalized nNOS and/or iNOS, increase the opening of the RyR, leading to Ca2+ leak. See text for further details.
Recently, a new insight into the pathway by which stretch increases NOX2 activity in mdx muscle was reported (213). This study found that a dense network of microtubules provided a mechanosensitive pathway for NOX2 activation in mdx muscle. The paper showed that the microtubule lattice is denser and grossly disorganized in mdx muscle, supporting findings of earlier studies in which dystrophin was shown to directly bind microtubules (361) and micro-dystrophin expression in mdx muscle restored the normal microtubule lattice structure (345). In the study of Khairallah et al. (213), muscle fibers were attached to micro-tweezers with an adhesive (MyoTak) and passively stretched by 10% of resting sarcomere length. Intracellular ROS and Ca2+ were measured using standard fluorescent indicators. The role of microtubules on NOX-induced ROS production in mdx fibers was determined by using microtubule depolymerizing agents, which prevented, or a microtubule polymerizing agent, which increased, stretch-induced ROS production. These results were recapitulated by in vivo administration of these agents or a NOX2 inhibitor, which subsequently reduced stretch-induced force loss in vitro. The same effect was found after inhibiting the activity of rac1, a key regulator of the NOX2 complex, which is increased in mdx muscle (507).
Interestingly, actin cytoskeleton depolymerization had no effect on ROS production, suggesting that the actin cytoskeleton is not directly involved in the mechanosignaling leading to NOX2 activation. These data are supported by a study in which knockout of cytoskeletal γ-actin, which is substantially upregulated in mdx muscle, did not affect the dystrophic phenotype or the susceptibility to stretch-induced damage compared with regular mdx mice (362). Another important result from the study of Khairallah et al. (213) was that a NOX2 inhibitor, a microtubule depolymerizing agent, and the MSC blocker GsMTX-4 all prevented the influx of Ca2+, supporting the idea that mechanical stretch initiates a mechanosignaling pathway involving microtubules that lead to activation of NOX2 and subsequent influx of Ca2+ through MSC (418, 507). Finally, it was shown that several NOX subunits, microtubule proteins, and putative MSC candidates, TRPC1 and TRPV2, were all increased by microarray RNA analysis (213), supporting the involvement of the microtubule-NOX-SAC network in DMD muscle (summarized in Figure 6).
7. Nox2 and impaired autophagy in dystrophic muscle
Autophagy is an important cellular pathway in which damaged intracellular proteins, organelles, and protein aggregates are degraded and then recycled by the cell for energy use. The autophagy process involves several, highly regulated steps, involving two main organelles, the autophagosome and the lysosome (for review, see Refs. 231, 294).
Impaired autophagy can lead to cell death, and there is growing evidence that it is implicated in a number of human diseases, including neuromuscular disorders (for review, see Refs. 222, 294). In skeletal muscle, proper regulation of autophagy is essential for normal cellular and physiological function, with high autophagic activity contributing to muscle atrophy and low activity causing muscle degeneration and weakness (91). Two key protein markers of impaired autophagic flux are protein sequestosome-1 (p62) and microtubule-associated protein 1A/1B light chain 3 (LC3), which has a cytosolic form (LC3A) and a lipidated form (LC3B). When autophagy is reduced, p62 binds ubiquinated protein aggregates which accumulate in the autophagosome membrane and therefore p62 levels increase (294). Impaired autophagy is also measured by the ratio of LC3A to LC3B, with lower LC3B relative to LC3A, indicative of reduced autophagic flux. In both mdx and DMD muscles, p62 levels are increased and LC3B levels reduced, highlighting a significant impairment of autophagy. Also, persistently increased Akt/mTOR signaling in mdx muscle is associated with the impaired autophagic flux (93, 334). Treatment of mdx mice with the AMPK activator AICAR enhanced autophagy and improved diaphragm health and force production (339). In addition, long-term feeding of a low-protein diet also triggered autophagy in mdx muscle, and this reduced inflammation and fibrosis and improved muscle function (93).
Recent evidence shows that impaired autophagy in mdx muscle is mediated by a pathway involving NOX2-derived ROS and Src kinase (334). Here it was shown that ROS produced by NOX2 activates Src, which in turn stimulates the Akt/mTOR pathway, and inhibits autophagolysosome formation, both contributing to impaired autophagy. In addition, Nox2 activity was further enhanced by Src-mediated tyrosine phosphorylation of the Nox2 organizer subunit p47phox, thereby providing a positive feedback loop for NOX2 activation. Importantly, genetic knockout of p47phox in mdx mice led to enhanced autophagy, which was accompanied by inhibition of NOX2 activity and Ca2+ influx, reduced muscle fibrosis, and improved diaphragm force production. Therefore, together these data highlight a pathway by which NOX2 and Src perturb normal autophagic flux, which leads to accumulation of damaged proteins and organelles, and this contributes to muscle damage, fibrosis, and impaired physiological function in dystrophic muscle (see Figure 6).
8. Nox and cardiac muscle dysfunction in mdx mice
While not the focus of this review, it should be mentioned that NOX-induced ROS also plays a role in the pathogenesis of dystrophic cardiac muscle (364, 510). Interestingly, a stretch-induced microtubule signaling pathway underlies the activation of NOX2 in cardiac muscle as well (364). Here, it was shown that NOX2 is localized at the sarcolemma and t-tubule in close proximity to junctional SR in mdx cardiomyocytes and stretch causes increased ROS production from NOX2, which is dependent on an intact microtubule network. The ROS produced by NOX2 sensitizes the RyR2, leading to Ca2+ sparks which trigger arrhythmogenic Ca2+ waves. The authors suggested that this pathway would likely be activated in vivo during each diastolic stretch and could contribute to the progressive cardiomyopathy found in mdx mice and DMD. In support of this idea, administration of the antioxidant NAC to mdx mice improved cardiac function and reduced fibrosis and inflammation (510).
V. IMPAIRED MEMBRANE REPAIR AND MUSCLE REGENERATION
The muscular dystrophies are characterized by activity-induced membrane damage and muscle necrosis which, eventually, exceed the repair capacity of the muscle. Here, we briefly review the substantial evidence that both membrane repair and regenerative capacity can be impaired in muscular dystrophies. For fuller reviews on muscle repair and regeneration in muscular dystrophies, see Wallace and McNally (486) as well as Ciciliot and Schiaffino (75).
Skeletal muscle has two repair processes that might potentially be defective and contribute to the muscular dystrophies.
A. Repair of Small Membrane Breaches
Membrane integrity is critical to the maintenance of cellular function and, consequently, cells have mechanisms for repairing membrane lesions. Membrane breaches can occur in skeletal muscle as reviewed in section IIIC. Much of the early work on membrane repair has been performed on large cells, such as the sea urchin eggs, and the various mechanisms involved in small and large membrane lesions have been defined (for review, see Ref. 276). Lesions in the membrane (>1 μm diameter) are rapidly repaired by an extracellular, Ca2+-dependent process that involves the recruitment and fusion of intracellular membrane vesicles in a process that appears similar to exocytosis and involves the SNARE proteins.
Bansal et al. (26) developed a membrane damage and repair assay for isolated skeletal muscle fibers utilizing a laser which damaged a 5-μm-diameter region of the membrane. Damage and repair were assessed with the dye FM1-43 which is soluble in water and lipids but does not cross membranes. It fluoresces in lipids so that before damage there is minimal fluorescence from the surface membrane and t-tubules. When the membrane is breached, the dye has access to the intracellular membranes (SR and ER), which have much greater area and produce a large spreading fluorescence. In the presence of extracellular Ca2+, normal fibers after damage showed a rapid increase in fluorescence over 1 min and fluorescence then stabilized, showing that the membrane breach had been sealed. In the absence of Ca2+, fluorescence continued to rise. These data are consistent with the membrane vesicle sealing hypothesis. Importantly, the rate of membrane repair was similar in mdx fibers, suggesting that absence of dystrophin and the dystrophin-associated proteins do not markedly influence membrane repair.
In contrast, a dysferlin KO mouse was constructed and exhibited major defects in membrane repair, which were unchanged in the presence or absence of Ca2+ (26). Dysferlin is a synaptotagmin-like protein, which together with the SNARE proteins cause vesicle fusion and release. Mutations in this protein cause the type IIB limb girdle muscular dystrophy and Miyoshi myopathy. The dysferlin KO mouse showed a slowly developing dystrophy with characteristic disrupted sarcolemmal lesions, which showed underlying vesicles. Eccentric muscle exercise caused Evans blue uptake, but the distribution differs from that in the mdx mouse with individual fibers showing uptake whereas in the mdx muscles the damaged fibers are characteristically grouped in bundles. The authors proposed that dysferlin is a Ca2+-sensitive protein necessary for the fusion of membrane vesicles and that in its absence, membrane lesions that occur normally fail to heal leading to necrosis and degeneration. Thus dysferlinopathy appears to be a dystrophy in which the primary mechanism is inability to repair membrane defects.
B. Regeneration of Damaged Muscle
A critical feature of skeletal muscle is its ability to regenerate after major or minor damage. The general features of the regenerative process are well known, and here we provide only a very brief outline as a background to considering how the process might be modified in muscular dystrophies (for review, see Ref. 68).
Regeneration depends on satellite or muscle stem cells located outside the surface membrane but within the basal lamina. Severe muscle damage leads to break-down of the muscle membrane, allowing entry of Ca2+ and activation of proteases with resulting disruption to myofibrils and other cellular structures. This necrotic process triggers an inflammatory response characterized by the entry of neutrophils and macrophages. Some aspect of the inflammatory process, probably involving a range of trophic factors (for review, see Ref. 68), leads to activation of satellite cells and they start to divide (proliferation phase). Importantly, some of the early divisions of satellite cells are asymmetric, meaning that some products of the division remain as satellite cells so that the overall number of satellite cells remains roughly constant, while others become “committed myogenic precursors,” initiating a differentiation phase leading to myoblasts. Myoblasts continue to divide and at some point begin to fuse forming myotubes, characterized by expression of muscle proteins. Finally myotubes fuse with existing, damaged myofibers. The fact that multiple myotubes fuse to form muscle cells explains the central nuclei that are one of the hallmarks of regenerated fibers. Failure of complete fusion of myotubes is thought to explain the frequent appearance of split fibers in dystrophic muscle (63). Details of the marker protein characterizing each of these cell types and the signaling pathways involved in each step have been recently reviewed (75).
It has long been recognized from histopathological studies that very early in the disease (DMD boys aged 2–3 yr) regenerating fibers are evident; however, as the disease progresses (age 6–7), when fibrosis and atrophy are apparent, regenerating fibers are less frequent (490, 493). Early evidence of deficiencies in myoblast activation and proliferation was provided by Blau et al. (38), who studied myoblasts grown in culture from biopsies of normal and DMD muscles. Dystrophic muscles provided only 5% of the number of myoblasts per weight of muscle compared with normal muscles, and these myoblasts grew more slowly and ceased division earlier. Fibroblasts obtained in the same way from dystrophic muscle were present in normal numbers and divided normally, so the defect appears limited to myoblasts. Quantification of satellite cells assessed by histological methods generally shows increased numbers in dystrophic muscle (220, 491), so Blau et al. (38) suggest that many of the cells counted in intact tissue may already be senescent and incapable of proliferation. Later, Webster and Blau (493) extended this work by systematically studying the number of myoblast doublings before replicative senescence occurred. In muscle biopsies from normal 5 yr olds, myoblasts were capable of 56 doublings (providing 1017 cells from the initiating cell), and this declined in older subjects (44 doublings at 32 yr and 22 doublings at 48 yr). In DMD patients, at the age of diagnosis (2–3 yr), myoblasts could only achieve 20–40 doublings, whereas by the age of 14–16, doublings were down to about 10. Age-related falls in doubling capacity have frequently been reported in other cell lines, and as a general biological rule, doubling capacity falls in proportion to the number of previous doublings the cells achieved in vivo. Thus, Webster and Blau (493) proposed that the reduced replicative potential of DMD myoblasts reflected the increased doublings previously expended in fiber regeneration.
In the last decades of the 20th century, the role of telomeres in chromosomal degradation, replicative senesence, and proliferative capacity of cells has gradually emerged. Telomeres are DNA repeats at the end of the chromosome that protect the chromosome at division from abnormal fusion, recombination, etc. At each cell division, telomeres are shortened and thus provide both a record of cellular turnover and an indication of future proliferative capacity. Decary et al. (95) isolated human myoblasts and showed that telomere length was shorter in clones that had undergone greater numbers of divisions. In addition, myoblasts from older subjects had reduced telomere length. Subsequently, Decary et al. (94) extended this approach to dystrophic patients and estimated telomere lengths from muscle biopsies. For this analysis, they used the minimal telomere length because whole muscle includes some nuclei that have undergone very few divisions (quiescent myonuclei), whilst some of the satellite cells may have completed many divisions and thus have shorter telomeres. The main finding was that minimal telomere lengths were shorter at comparable ages in dystrophic patients compared with controls and the rate of reduction of telomere length with age was greater (by a factor of 14) in the dystrophic samples than the normal controls. Thus these data indicate that dystrophic muscle has undergone more rounds of proliferation and regeneration than normal muscle and that consequently its capacity for division is restricted. This was confirmed by the observation that amongst the oldest dystrophic patients (ages 11–13), regeneration was not visible in the biopsies.
Telomere lengths have been further explored by Sacco et al. (395) in a mouse model that is a double KO for dystrophin and telomerase, the enzyme that can restore telomeres by synthesis of the repeated DNA sequences. In-bred strains of mice have longer telomeres (>40 kb) than humans (5–15 kb), and Sacco et al. postulate that this difference contributes to the milder phenotype of the mdx mouse compared with the DMD patients. In keeping with this hypothesis, Sacco et al. (395) show that the phenotype of the dystrophin/telomerase KO is much more severe than the mdx with higher creatine kinase levels, more rapid exhaustion on a treadmill, more central nuclei, more severe histology, greater muscle atrophy, worsened scoliosis, and increased severity of symptoms with age. They showed that these features were caused by telomere shortening by demonstrating more chromosomal free ends and showed that the myoblasts from the double KO had impaired proliferation. Importantly, the proportion of myoblasts that developed muscle specific markers was unchanged, indicating that differentiation of the myoblasts was unimpaired. They also showed that the double KO myoblasts did not proliferate well when engrafted in a host muscle. These data strongly support the concept that excessively shortened telomeres, as a result of repeated regeneration, are an important factor in the failure of regeneration in older DMD patients. Furthermore, they provide an animal model that more closely simulates the human disease and offer an explanation for the mild phenotype in the mdx mouse.
In summary, there is compelling evidence that failure of regeneration contributes to the pathology of muscular dystrophies. The best-explored mechanism is the reduced proliferative potential of myoblasts associated with repeated episodes of regeneration and reflected in the telomere lengths. However, other possible mechanisms including failure of activation of satellite cells and reduced fusion ability, possibly related to the changing muscle environment, remain to be explored in detail.
VI. THERAPEUTIC DIRECTIONS
A. Gene-Based Therapies
After many decades in which steroids were the only approved treatment that slowed progression of the disease, the situation is changing rapidly with a range of treatments in clinical trials (for recent reviews, see Refs. 1, 119, 185, 387). All of these treatments have been extensively trialled in the mdx mouse so that proof of principle is established. Most of these treatments focus on replacement of the dystrophin gene or repair of the mutation and are dependent on the rapid growth in knowledge about manipulation, modification, and repair of genes.
Viral gene therapy has been attempted or considered in many genetic diseases with mixed results. A fundamental problem for the treatment of DMD is that the very large size of the dystrophin mRNA (14 kb) exceeds the carrying capacity of most viral vectors, including the adeno-associated virus with a carrying capacity of ∼5 kb. Based on the observation of a relatively mild clinical phenotype in a Becker MD patient with a deletion of exons 17–48 (115), dystrophin mini genes have been designed, which lack many of the spectrin repeats but retain much of the functionality (168) including the nNOS binding site (229). Such mini-dystrophins can be packaged in an adeno-associated virus (AAV) and have been successfully used in mdx mice, where they induce expression of mini-dystrophin and ameliorate symptoms (168, 488). In a small trial, six boys with DMD were injected with an AAV containing mini-dystrophin into one biceps muscle. Only minimal and transient expression of the mini-dystrophin was detected (278). However, an immune response to the AAV virus was generated in four of the six boys, possibly because a generalized CMV promoter was used as opposed to a muscle-specific promoter (280, 387).
Mutations causing stop codons are present in ∼15% of DMD cases. In principle, if the stop codon can be read through by the ribosome, a full-length dystrophin could be expressed. A drug, ataluren, formerly called PCT124, has this property and in the mdx mouse generated dystrophin expression and protection against eccentric muscle damage (499). Multiple small clinical trials have been published, and a very recent trial (124) demonstrated a small but statistically significant increase in dystrophin expression. Whether the small and variable dystrophin expression (average increase of 11% above pretreatment level) will lead to a clinically useful outcome remains to be seen. Results of the 6-min walk test in a double blind trial with 174 boys over 48 wk have not been published, but a company press release suggests a marginally significant increase present in the low dose but not the high dose. Other drugs in the same class with higher read-through potential are under investigation.
Perhaps the most promising direction under development is the use of drugs that promote exon skipping. 60–80% of DMD patients have a frame-shifting deletion. In such patients, if the exon with the mutation is skipped, then expression of a shortened dystrophin is possible. Drugs in this class include anti-sense oligonucleotides that are designed to hybridize to the complementary section of the premessenger RNA and lead to exclusion of the relevant exon. A difficulty inherent in this approach is that mutations in different exons will require alternative drug designs. The current drugs are targeted to frame-shifting mutations within exon 51 which encompasses ∼13% of DMD boys. One drug in this class, drisapersen, which is in the 2'-O-methyl-phosphorthioate class, has been successfully used in several small scale open-label trials and was shown to induce some dystrophin expression and produce benefit in the 6-min walk test. However, a recent press release from the manufacturer revealed that a phase 3 double blind trial with 186 patients failed to show improvement in the 6-min walk test, the primary endpoint. A second drug, eteplirsen, with morpholino chemistry, has also been successful in small scale open-label trials, but again the dystrophin expression was variable and only reached 20% of normal levels in the best expressing patients (281). For a recent analysis of the current status of exon-skipping as a treatment for DMD, the reader is referred to Hoffman and McNally (186).
A fourth approach, which is in active development, is the use of small molecules that cause increased expression of utrophin. The protective role of utrophin, a dystrophin analog, in mdx mice was introduced in section IC. As a result of these studies, a systematic search was undertaken to find drugs that could increase utrophin expression. In mdx mice, SMT-C1100 increases utrophin expression and reduces the dystrophic phenotype (457). Small-scale toxicity and dose-ranging studies are commencing in humans.
The fact that four different gene-based approaches to treatment have reached the stage of clinical trials in the last few years is an enormously exciting and significant development. None has yet achieved the current gold standard, which is an increase in the 6-min walk time in a double-blind clinical trial. The most obvious alternative marker is the appearance of dystrophin (or additional utrophin) in the muscles, but this is a highly invasive test, difficult to quantify and subject to a large sampling error not suited to large scale use (for discussion, see Ref. 185). Several of these approaches to therapy will lead to the expression of a shortened dystrophin, and this has triggered renewed interest in the clinical history of BMD patients with equivalent mutations (1). It is still unclear how much dystrophin needs to be expressed to improve the disease, and it is likely that all the current methods to produce dystrophin will need to be more effective. Furthermore, the importance of spectrin-like repeats in several aspects of DPC function must be taken into account when large segments of the dystrophin repeat region are deleted. A combinatorial approach of genetic manipulation and drug administration to correct signaling deficiencies caused by the absence of a fully functional dystrophin will likely be required for successful treatment.
B. Targeting Damage Pathways: Nongenetic Therapeutic Approaches
As an alternative or complementary approach to dystrophin or utrophin gene or cell-based therapies for DMD, numerous drugs and recombinant protein therapies have been tested in dystrophic animal models over the last decade or more (262, 390; for recent reviews, see Ref. 536). Some of these drugs have been chosen based on their known improvement of signaling pathways that are deficient in dystrophic muscle. Others have been found by unbiased screens, for example, using the zebrafish model of DMD (209). The next section discusses some of the most promising therapeutic approaches aimed at targeting key damage pathways in DMD (Figure 7).
FIGURE 7.
Therapeutic pathways for Duchenne muscular dystrophy (DMD). This figure highlights some of the key therapeutic targets for DMD, which include both muscle-specific and extracellular pathways. A wide range of therapeutic approaches are being evaluated for DMD (red boxes). These include gene-based therapies, pharmacological agents, and recombinant proteins (see text for details).
1. Drugs targeting ROS, Ca2+, and NO
As discussed in previous sections, antioxidants have shown efficacy in the mdx mouse (214). Of these, NAC is particularly attractive based on its improvement of muscle function and amelioration of muscle damage in mdx mice (92, 223, 504). NAC has been extensively studied in a wide range of human diseases and acute conditions and has only mild side effects (400). The antioxidant properties of NAC involve direct scavenging of ROS, reducing disulfide bonds in proteins and boosting intracellular GSH synthesis, via its cysteine group (400). This later pathway is likely to be particularly important in DMD, since skeletal muscle GSH concentrations are decreased by 50% compared with normal muscle, and this is accompanied by oxidative muscle damage (379). Another compound with antioxidant properties, epigallocatechin gallate (EGCG), a component of green tea, has shown efficacy in ameliorating muscle damage in mdx mice (101, 314). As a result of these preclinical studies, DMD patients are currently being recruited for a phase 2 clinical trial to test the safety and efficacy of EGCG (clinicaltrials.gov). As discussed earlier, antioxidants are also beneficial at improving intracellular Ca2+ homeostasis, by inhibiting Ca2+ influx via MSC activation and preventing increased membrane permeability (145, 504). In addition, compounds that stabilize the binding of the RyR1 to calstabin, termed Rycals, have been shown to reduce RyR1 Ca2+ leak and improve muscle function in mdx mice (30). While still in the preclinical stage, these compounds are being developed for future clinical trials in DMD. The NO-cGMP pathway is another therapeutic target for DMD. Sildenafil and Tadalafil, PDE-5 inhibitors that enhance the NO-cGMP signaling pathway, have both shown efficacy in mdx mice (18, 347). Sildenafil improved diaphragm force production and reduced fibrosis and inflammation (347). Tadalafil was found to reduce muscle damage in mdx mice following muscle stimulation during ischemia (18). Improved muscle blood flow is thought to be one mechanism by which PDE-5 inhibitors improve dystrophic muscle, and Tadalafil was shown to reduce ischemia and improve blood flow in muscles of BMD patients (267). A phase 3 clinical trial to test the efficacy of Tadalafil in DMD is currently in the recruitment stage (clinicaltrials.gov). Sildenafil also increased hemeoxygenase 1, a recently discovered therapeutic target involved in mitigating disease progression (208).
2. Drugs targeting inflammation and fibrosis
Inflammation and fibrosis are major pathogenic processes affecting muscle function in mdx mice and DMD (see Figure 6). They are both regulated by complex interactions involving many different cell types, and as such, a detailed description is beyond the scope of this review. Several recent reviews provide a very good overview of the underlying mechanisms and current therapeutic approaches for targeting inflammation and fibrosis and should be referred to for a more detailed understanding of these processes (262, 413, 414, 536). Here, we will briefly outline the major pathways and the most promising, current therapeutic approaches for targeting inflammation and fibrosis in DMD.
Inflammation has diverse, regulatory effects on the pathophysiology of DMD. On one hand, inflammatory cells such as macrophages are required for effective muscle regeneration following muscle damage (409) and for improved myoblast survival in dystrophic mice (243). However, an excessive, chronic inflammatory response, as occurs in DMD, exacerbates muscle damage, impairs effective regeneration, and promotes excessive extracellular matrix deposition, resulting in fibrosis (for a review, see Ref. 414). Therefore, targeting the deleterious pathways of inflammation, while preserving its beneficial effects, is a key criterion for effective anti-inflammatory approaches for DMD. Proinflammatory macrophages, often termed M1 macrophages, produce cytokines such as TNF-α and IL-1β. Studies in mdx mice have tested two TNF-α inhibitors, Etanercept (183, 356) and Remicade (116, 157), which are both used in humans to treat autoimmune diseases such as rheumatoid arthritis. These drugs have shown some benefits in mdx mice, including reduced muscle necrosis and inflammation (157, 183), improved muscle function and decreased fibrosis (116), and reduced collagen and TGF-β1 levels (356). Therefore, these drugs have potential to attenuate muscle damage and fibrosis in DMD. The transcription factor NF-κB is chronically activated in mdx mice and during the early stages of DMD. It is a key regulator of the inflammatory response to injury, but chronic activation likely contributes to ongoing muscle degeneration. NEMO binding domain (NBD) is a small peptide designed to block activation of NF-κB, and after systemic administration in mdx mice, there is improvement in pathology (376) and diaphragm function (353). NBD is currently in development for clinical studies in DMD. However, one note of caution regarding NF-κB inhibition as a potential treatment for DMD relates to the time course of NF-κB activation. It was shown that NF-κB nuclear activity peaks very early in DMD, up to ∼2 yr of age, and then progressively decreases to low levels by 9–10 yr of age (71, 287). This implies that these drugs would need to be administered very early in the disease to have maximum benefits. Another approach is the use of antioxidants such as NAC, which have been shown to effectively inhibit NF-κB translocation to the nucleus in mdx muscle (219, 490).
Fibrosis also occurs at an early age in DMD patients and is a major cause of progressive muscle weakness (97), as depicted schematically in Figure 6. Therefore, therapeutic approaches aimed at preventing fibrotic deposition and/or reversing preexisting fibrosis are a major priority for effectively treating DMD, at all stages of the disease. Several studies have shown that TGF-β is a key activator of fibrosis in mdx mice, is upregulated in DMD, and its activity is influenced by modifier genes (for review, see Ref. 61). TGF-β is produced by a number of cells associated with muscle repair, including fibroblasts, macrophages, and neutrophils (61). Inactive TGF-β resides in the extracellular matrix as a latent complex; however, upon cleavage by serine proteinases such as MMP2 and 9, active TGF-β can bind to the TGF-β1/2 receptor complex. This initiates phosphorylation of the transcription factors SMAD2 and 3, which translocate to the nucleus and increase expression of profibrotic proteins such as collagen I and fibronectin (240). Recently, the cytokine osteopontin was shown to be an important inducer of TGF-β in mdx and DMD muscle, and its deletion improved mdx muscle function and reduced fibrosis (477). A number of drugs have been tested in mdx mice for their ability to reduce fibrotic deposition by inhibiting the TGF-β pathway (348). Currently, one of the most promising candidates is halofuginone, a natural alkaloid compound with known antifibrotic effects. Halofuginone reduced fibrosis and improved muscle function in mdx mice, via inhibition of the TGF-β/SMAD signaling pathway (192, 464). DMD boys are currently being recruited for a phase II clinical trial to test the safety and pharmacokinetics of Halofuginone (HT-100) (clinicaltrials.gov).
Connective tissue growth factor (CTGF) is another key stimulator of fibrosis in dystrophic muscle. CTGF overexpression in tibialis anterior muscles of WT mice produces a dystrophic-like muscle disease, with muscle damage and regeneration, increased fibrosis, and reduced specific force production (303). The same group more recently showed in mdx mice that genetic reduction of CTGF levels or treatment with a neutralizing monoclonal antibody against CTGF (FG-3019), both reduced muscle fibrosis and increased muscle function (304). These data suggest that FG-3019 might have therapeutic potential in DMD.
Recent studies have identified novel pathways that could lead to new therapeutic approaches for reducing fibrosis in DMD. Genetic ablation of the serum and glucocorticoid-inducible kinase (SGK1) dramatically improved muscle function and reduced fibrosis in mdx muscles (433). Since SGK1 has been shown to increase CTGF levels, this could provide a new therapeutic target for reducing fibrosis. Another recently discovered pro-fibrotic pathway, mediated by increased fibrinogen, is evident in muscles of mdx mice and DMD (478, 479). Fibrotic deposition in dystrophic muscles is accompanied by accumulation of fibrinogen, and its depletion by genetic and pharmacological approaches reduces diaphragm fibrosis in mdx mice. The findings indicate that fibrinogen binds to the Mac-1 receptor on alternatively activated, M2a macrophages, which are considered to play an important role in dystrophic muscle fibrosis (262a). The activation of M2a macrophages by fibrinogen triggers TGF-β1 release that, in turn, stimulates secretion of fibrotic proteins from fibroblasts (478, 479). Therefore, drugs targeted to reduce fibrinogen levels and/or block fibrinogen binding to macrophages could provide a novel therapeutic approach for reducing fibrosis in DMD.
3. Protein therapies
An alternative to replacing dystrophin via gene therapy or exon skipping is to improve the function of the dystrophin complex by exogenous delivery of proteins associated with the dystrophin complex. Perhaps the best example of such an approach is the work of Fallon and colleagues, using biglycan (16, 45, 284, 367). Biglycan is upregulated in the mdx mouse and has been shown to bind to several members of the dystrophin complex, notably α-dystroglycan and α- and γ-sarcoglycan (45, 367). Importantly, systemic delivery of recombinant, nonglycanated biglycan (rNG-BGN) in mdx mice increases sarcolemmal nNOS and utrophin expression, and this is associated with improved muscle health and function (16). Currently, rNG-BGN is under preclinical development as a potential therapeutic approach for DMD (525). Another protein, mitsugumin 53 (MG53), has recently been shown to reduce membrane permeability in mdx mice when injected as a recombinant protein (rhMG53) (498). While these results are encouraging, further preclinical studies, particularly testing muscle function, are required to determine if rhMG53 is a viable protein therapy for DMD.
VII. CONCLUDING REMARKS
Mutations in the dystrophin gene lead to a complex disease affecting many aspect of muscle function. There is still no clear definition of the normal function of dystrophin because absence of dystrophin leads to multiple changes in membrane properties, signaling pathways, and many aspects of muscle function. Almost three decades after the identification of the mutated gene and its protein product, gene-based treatments now appear to be a therapeutic possibility with multiple clinical trials in progress utilizing a variety of approaches. In the mdx mouse, an extraordinary range of interventions have produced some therapeutic benefit, and it seems likely that, if and when gene-based approaches become a possibility, some of these interventions will be helpful in minimizing those aspects of the disease that are not improved by the gene therapy.
The literature on muscular dystrophy continues to grow at an astonishing rate. If we define the literature by papers that include “dystrophin,” “muscular dystrophy,” or “mdx mouse” in their title, abstract, or key words, then prior to 1940 there was on average only 0 or 1 paper per year. By 1960 this had grown to around 100 per year, and by 1980 to 400 per year. Since then, the rate of publications has continued to increase linearly, and in 2013 there were 1,100 papers per year and still steadily growing. This huge interest reflects the still unmet clinical need coupled with the insights into so many aspects of muscle function that have been triggered by trying to understand the role(s) of dystrophin.
It is still widely considered that the initial consequences of dystrophin's absence are the mechanical defects associated with dystrophin's structural role and the membrane damage that results directly from this mechanical role. While this sequence of events may play some minor role, it is increasingly clear that dystrophin and the DPC have multiple signaling roles and disruption of these functions leads to the abnormal regulation of Ca2+, ROS, and NO that seem to cause many of the pathological effects of the disease. We believe that as molecular and pharmacological approaches to therapy start to minimize some of these disruptions, a more sophisticated understanding of the mechanisms regulating these signaling pathways will provide additional therapeutic avenues for DMD and related neuromuscular diseases.
GRANTS
D. G. Allen gratefully acknowledges support from a Program Grant of the National Health and Medical Research Council of Australia. S. C. Froehner and N. P. Whitehead gratefully acknowledge current and past support from the National Institutes of Health (Grants R01 NS33145, R01 AR056221, R21 NS088691, and P01 NS04678), Parent Project Muscular Dystrophy, and the Raymond and Beverly Sackler Foundation. The contents of this review are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
We thank Dr. Philip Dash, University of Reading, UK, for kindly granting us permission to use the figure of the NOS dimer shown in Figure 4.
Address for reprint requests and other correspondence: D. Allen, Sydney Medical School & Bosch Institute, Univ. of Sydney, NSW 2006, Australia (e-mail: david.allen@sydney.edu.au).
REFERENCES
- 1.Aartsma-Rus A, Muntoni F. 194th ENMC International Workshop. 3rd ENMC workshop on exon skipping: towards clinical application of antisense-mediated exon skipping for Duchenne muscular dystrophy 8–10 December 2012, Naarden, The Netherlands. Neuromuscul Disord 23: 934–944, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Abramovici H, Gee SH. Morphological changes and spatial regulation of diacylglycerol kinase-zeta, syntrophins, and Rac1 during myoblast fusion. Cell Motil Cytoskeleton 64: 549–567, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Abramovici H, Hogan AB, Obagi C, Topham MK, Gee SH. Diacylglycerol kinase-zeta localization in skeletal muscle is regulated by phosphorylation and interaction with syntrophins. Mol Biol Cell 14: 4499–4511, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamo CM, Dai DF, Percival JM, Minami E, Willis MS, Patrucco E, Froehner SC, Beavo JA. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA 107: 19079–19083, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams ME, Butler MH, Dwyer TM, Peters MF, Murnane AA, Froehner SC. Two forms of mouse syntrophin, a 58 kd dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron 11: 531–540, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Adams ME, Mueller HA, Froehner SC. In vivo requirement of the alpha-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J Cell Biol 155: 113–122, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams ME, Tesch Y, Percival JM, Albrecht DE, Conhaim JI, Anderson K, Froehner SC. Differential targeting of nNOS and AQP4 to dystrophin-deficient sarcolemma by membrane-directed alpha-dystrobrevin. J Cell Sci 121: 48–54, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Ahn AH, Freener CA, Gussoni E, Yoshida M, Ozawa E, Kunkel LM. The three human syntrophin genes are expressed in diverse tissues, have distinct chromosomal locations, and each bind to dystrophin and its relatives. J Biol Chem 271: 2724–2730, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Ahn AH, Kunkel LM. Syntrophin binds to an alternatively spliced exon of dystrophin. J Cell Biol 128: 363–371, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrecht DE, Sherman DL, Brophy PJ, Froehner SC. The ABCA1 cholesterol transporter associates with one of two distinct dystrophin-based scaffolds in Schwann cells. Glia 56: 611–618, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alderton JM, Steinhardt RA. Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem 275: 9452–9460, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Alessi A, Bragg AD, Percival JM, Yoo J, Albrecht DE, Froehner SC, Adams ME. γ-Syntrophin scaffolding is spatially and functionally distinct from that of the alpha/beta syntrophins. Exp Cell Res 312: 3084–3095, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: Cellular mechanisms. Physiol Rev 88: 287–332, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Altamirano F, Lopez JR, Henriquez C, Molinski T, Allen PD, Jaimovich E. Increased resting intracellular calcium modulates NF-kappaB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes. J Biol Chem 287: 20876–20887, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amann KJ, Renley BA, Ervasti JM. A cluster of basic repeats in the dystrophin rod domain binds F-actin through an electrostatic interaction. J Biol Chem 273: 28419–28423, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Amenta AR, Yilmaz A, Bogdanovich S, McKechnie BA, Abedi M, Khurana TS, Fallon JR. Biglycan recruits utrophin to the sacrolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci USA 108: 762–767, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci USA 100: 2106–2111, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asai A, Sahani N, Kaneki M, Ouchi Y, Martyn JA, Yasuhara SE. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS One 2: e806, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ascah A, Khairallah M, Daussin F, Bourcier-Lucas C, Godin R, Allen BG, Petrof BJ, Des RC, Burelle Y. Stress-induced opening of the permeability transition pore in the dystrophin-deficient heart is attenuated by acute treatment with sildenafil. Am J Physiol Heart Circ Physiol 300: H144–H153, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Backman E, Nylander E, Johansson I, Henriksson KG, Tagesson C. Selenium and vitamin E treatment of Duchenne muscular dystrophy: no effect on muscle function. Acta Neurol Scand 78: 429–435, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Badalamente MA, Stracher A. Delay of muscle degeneration and necrosis in mdx mice by calpain inhibition. Muscle Nerve 23: 106–111, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Balasubramanian S, Fung ET, Huganir RL. Characterization of the tyrosine phosphorylation and distribution of dystrobrevin isoforms. FEBS Lett 432: 133–140, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Baltgalvis KA, Jaeger MA, Fitzsimons DP, Thayer SA, Lowe DA, Ervasti JM. Transgenic overexpression of gamma-cytoplasmic actin protects against eccentric contraction-induced force loss in mdx mice. Skelet Muscle 1: 32, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banks GB, Chamberlain JS. The value of mammalian models for duchenne muscular dystrophy in developing therapeutic strategies. Curr Top Dev Biol 84: 431–453, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Banks GB, Judge LM, Allen JM, Chamberlain JS. The polyproline site in hinge 2 influences the functional capacity of truncated dystrophins. PLoS Genet 6: e1000958, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423: 168–172, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Barbieri E, Sestili P. Reactive oxygen species in skeletal muscle signaling. J Signal Transduct 2012: 982794, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker PE. Two families of benign sex-linked recessive muscular dystrophy. Rev Can Biol 21: 551–566, 1962. [PubMed] [Google Scholar]
- 29.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15: 325–330, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, Lacampagne A, Marks AR. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA 105: 2198–2202, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger J, Berger S, Hall TE, Lieschke GJ, Currie PD. Dystrophin-deficient zebrafish feature aspects of the Duchenne muscular dystrophy pathology. Neuromuscul Disord 20: 826–832, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Best A, Ahmed S, Kozma R, Lim L. The Ras-related GTPase Rac1 binds tubulin. J Biol Chem 271: 3756–3762, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Bhasin N, Law R, Liao G, Safer D, Ellmer J, Discher BM, Sweeney HL, Discher DE. Molecular extensibility of mini-dystrophins and a dystrophin rod construct. J Mol Biol 352: 795–806, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Bhat HF, Baba RA, Adams ME, Khanday FA. Role of SNTA1 in Rac1 activation, modulation of ROS generation, and migratory potential of human breast cancer cells. Br J Cancer 110: 706–714, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blake DJ, Nawrotzki R, Peters MF, Froehner SC, Davies KE. Isoform diversity of dystrobrevin, the murine 87-kDa postsynaptic protein. J Biol Chem 271: 7802–7810, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82: 291–329, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Blau HM, Webster C, Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 80: 4856–4860, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodensteiner JB, Engel AG. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology 28: 439–446, 1978. [DOI] [PubMed] [Google Scholar]
- 40.Bodor M, McDonald CM. Why short stature is beneficial in Duchenne muscular dystrophy. Muscle Nerve 48: 336–342, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Boittin FX, Petermann O, Hirn C, Mittaud P, Dorchies OM, Roulet E, Ruegg UT. Ca2+-independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibers. J Cell Sci 119: 3733–3742, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Bonilla E, Fischbeck K, Schotland DL. Freeze-fracture studies of muscle caveolae in human muscular dystrophy. Am J Pathol 104: 167–173, 1981. [PMC free article] [PubMed] [Google Scholar]
- 43.Boppart MD, Burkin DJ, Kaufman SJ. Alpha7beta1-integrin regulates mechanotransduction and prevents skeletal muscle injury. Am J Physiol Cell Physiol 290: C1660–C1665, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Bornman L, Rossouw H, Gericke GS, Polla BS. Effects of iron deprivation on the pathology and stress protein expression in murine X-linked muscular dystrophy. Biochem Pharmacol 56: 751–757, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Bowe MA, Mendis DB, Fallon JR. The small leucine-rich repeat proteoglycan biglycan binds to alpha-dystroglycan and is upregulated in dystrophic muscle. J Cell Biol 148: 801–810, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bozzi M, Morlacchi S, Bigotti MG, Sciandra F, Brancaccio A. Functional diversity of dystroglycan. Matrix Biol 28: 179–187, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Bradley WG, O'Brien MD, Walder DN, Murchison D, Johnson M, Newell DJ. Failure to confirm a vascular cause of muscular dystrophy. Arch Neurol 32: 466–473, 1975. [DOI] [PubMed] [Google Scholar]
- 48.Brancaccio P, Maffulli N, Limongelli FM. Creatine kinase monitoring in sport medicine. Br Med Bull 81–82: 209–230, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84: 757–767, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Brenman JE, Xia H, Chao DS, Black SM, Bredt DS. Regulation of neuronal nitric oxide synthase through alternative transcripts. Dev Neurosci 19: 224–231, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Brinkmeier H, Ohlendieck K. Chaperoning heat shock proteins: proteomic analysis and relevance for normal and dystrophin-deficient muscle. Proteomics Clin Appl 8: 875–895, 2014. [DOI] [PubMed] [Google Scholar]
- 52.Brown SC, Torelli S, Ugo I, De BF, Howman EV, Poon E, Britton J, Davies KE, Muntoni F. Syncoilin upregulation in muscle of patients with neuromuscular disease. Muscle Nerve 32: 715–725, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Buechler C, Boettcher A, Bared SM, Probst MC, Schmitz G. The carboxy terminus of the ATP-binding cassette transporter A1 interacts with a beta2-syntrophin/utrophin complex. Biochem Biophys Res Commun 293: 759–765, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81: 1189–1192, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buraei Z, Yang J. The ss subunit of voltage-gated Ca2+ channels. Physiol Rev 90: 1461–1506, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buraei Z, Yang J. Structure and function of the beta subunit of voltage-gated Ca2+ channels. Biochim Biophys Acta 1828: 1530–1540, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burkin DJ, Wallace GQ, Milner DJ, Chaney EJ, Mulligan JA, Kaufman SJ. Transgenic expression of α7β1 integrin maintains muscle integrity, increases regenerative capacity, promotes hypertrophy, and reduces cardiomyopathy in dystrophic mice. Am J Pathol 166: 253–263, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bushby KM, Gardner-Medwin D. The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy I. Natural history. J Neurol 240: 98–104, 1993. [DOI] [PubMed] [Google Scholar]
- 59.Call JA, Voelker KA, Wolff AV, McMillan RP, Evans NP, Hulver MW, Talmadge RJ, Grange RW. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J Appl Physiol 105: 923–932, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carsana A, Frisso G, Tremolaterra MR, Lanzillo R, Vitale DF, Santoro L, Salvatore F. Analysis of dystrophin gene deletions indicates that the hinge III region of the protein correlates with disease severity. Ann Hum Genet 69: 253–259, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Ceco E, McNally EM. Modifying muscular dystrophy through transforming growth factor-beta. FEBS J 280: 4198–4209, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chamberlain JS, Benian GM. Muscular dystrophy: the worm turns to genetic disease. Curr Biol 10: R795–R797, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Chan S, Head SI. The role of branched fibres in the pathogenesis of Duchenne muscular dystrophy. Exp Physiol 96: 564–571, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Chan S, Head SI, Morley JW. Branched fibers in dystrophic mdx muscle are associated with a loss of force following lengthening contractions. Am J Physiol Cell Physiol 293: C985–C992, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Chandrasekharan K, Yoon JH, Xu Y, deVries S, Camboni M, Janssen PM, Varki A, Martin PT. A human-specific deletion in mouse Cmah increases disease severity in the mdx model of Duchenne muscular dystrophy. Sci Transl Med 2: 42ra54, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang WJ, Iannaccone ST, Lau KS, Masters BS, McCabe TJ, McMillan K, Padre RC, Spencer MJ, Tidball JG, Stull JT. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci USA 93: 9142–9147, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chao DS, Gorospe JR, Brenman JE, Rafael JA, Peters MF, Froehner SC, Hoffman EP, Chamberlain JS, Bredt DS. Selective loss of sarcolemmal nitric oxide synthase in Becker muscular dystrophy. J Exp Med 184: 609–618, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Chelly J, Kaplan JC, Maire P, Gautron S, Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature 333: 858–860, 1988. [DOI] [PubMed] [Google Scholar]
- 70.Chen Q, Li W, Quan Z, Sumpio BE. Modulation of vascular smooth muscle cell alignment by cyclic strain is dependent on reactive oxygen species and P38 mitogen-activated protein kinase. J Vasc Surg 37: 660–668, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology 65: 826–834, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Childers MK, Bogan JR, Bogan DJ, Greiner H, Holder M, Grange RW, Kornegay JN. Chronic administration of a leupeptin-derived calpain inhibitor fails to ameliorate severe muscle pathology in a canine model of duchenne muscular dystrophy. Front Pharmacol 2: 89, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chin ER, Allen DG. The role of elevations in intracellular Ca2+ concentration in the development of low frequency fatigue in mouse single muscle fibres. J Physiol 491: 813–824, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci 8: 510–521, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Ciciliot S, Schiaffino S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des 16: 906–914, 2010. [DOI] [PubMed] [Google Scholar]
- 76.Claflin DR, Brooks SV. Direct observation of failing fibers in muscles of dystrophic mice provides mechanistic insight into muscular dystrophy. Am J Physiol Cell Physiol 294: C651–C658, 2008. [DOI] [PubMed] [Google Scholar]
- 77.Clarke MS, Khakee R, McNeil PL. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci 106: 121–133, 1993. [DOI] [PubMed] [Google Scholar]
- 78.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev 84: 1341–1379, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Compton AG, Albrecht DE, Seto JT, Cooper ST, Ilkovski B, Jones KJ, Challis D, Mowat D, Ranscht B, Bahlo M, Froehner SC, North KN. Mutations in contactin-1, a neural adhesion and neuromuscular junction protein, cause a familial form of lethal congenital myopathy. Am J Hum Genet 83: 714–724, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Compton AG, Cooper ST, Hill PM, Yang N, Froehner SC, North KN. The syntrophin-dystrobrevin subcomplex in human neuromuscular disorders. J Neuropathol Exp Neurol 64: 350–361, 2005. [DOI] [PubMed] [Google Scholar]
- 81.Connors NC, Adams ME, Froehner SC, Kofuji P. The potassium channel Kir4.1 associates with the dystrophin-glycoprotein complex via alpha-syntrophin in glia. J Biol Chem 279: 28387–28392, 2004. [DOI] [PubMed] [Google Scholar]
- 82.Corona BT, Balog EM, Doyle JA, Rupp JC, Luke RC, Ingalls CP. Junctophilin damage contributes to early strength deficits and EC coupling failure after eccentric contractions. Am J Physiol Cell Physiol 298: C365–C376, 2010. [DOI] [PubMed] [Google Scholar]
- 83.Costantini JL, Cheung SM, Hou S, Li H, Kung SK, Johnston JB, Wilkins JA, Gibson SB, Marshall AJ. TAPP2 links phosphoinositide 3-kinase signaling to B-cell adhesion through interaction with the cytoskeletal protein utrophin: expression of a novel cell adhesion-promoting complex in B-cell leukemia. Blood 114: 4703–4712, 2009. [DOI] [PubMed] [Google Scholar]
- 84.Cox GA, Phelps SF, Chapman VM, Chamberlain JS. New mdx mutation disrupts expression of muscle and nonmuscle isoforms of dystrophin. Nat Genet 4: 87–93, 1993. [DOI] [PubMed] [Google Scholar]
- 85.Crosbie RH, Barresi R, Campbell KP. Loss of sarcolemma nNOS in sarcoglycan-deficient muscle. FASEB J 16: 1786–1791, 2002. [DOI] [PubMed] [Google Scholar]
- 86.Crosbie RH, Lebakken CS, Holt KH, Venzke DP, Straub V, Lee JC, Grady RM, Chamberlain JS, Sanes JR, Campbell KP. Membrane targeting and stabilization of sarcospan is mediated by the sarcoglycan subcomplex. J Cell Biol 145: 153–165, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crosbie RH, Yamada H, Venzke DP, Lisanti MP, Campbell KP. Caveolin-3 is not an integral component of the dystrophin glycoprotein complex. FEBS Lett 427: 279–282, 1998. [DOI] [PubMed] [Google Scholar]
- 88.Davies KE, Pearson PL, Harper PS, Murray JM, O'Brien T, Sarfarazi M, Williamson R. Linkage analysis of two cloned DNA sequences flanking the Duchenne muscular dystrophy locus on the short arm of the human X chromosome. Nucleic Acids Res 11: 2303–2312, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis TA, Loos B, Engelbrecht AM. AHNAK: the giant jack of all trades. Cell Signal 26: 2683–2693, 2014. [DOI] [PubMed] [Google Scholar]
- 90.De Palma C, Clementi E. Nitric oxide in myogenesis and therapeutic muscle repair. Mol Neurobiol 46: 682–692, 2012. [DOI] [PubMed] [Google Scholar]
- 91.De Palma C, Morisi F, Cheli S, Pambianco S, Cappello V, Vezzoli M, Rovere-Querini P, Moggio M, Ripolone M, Francolini M, Sandri M, Clementi E. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis 5: e1363, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Senzi Moraes PR, Ferretti R, Moraes LH, Neto HS, Marques MJ, Minatel E. N-acetylcysteine treatment reduces TNF-alpha levels and myonecrosis in diaphragm muscle of mdx mice. Clin Nutr 32: 472–475, 2013. [DOI] [PubMed] [Google Scholar]
- 93.De PC, Morisi F, Cheli S, Pambianco S, Cappello V, Vezzoli M, Rovere-Querini P, Moggio M, Ripolone M, Francolini M, Sandri M, Clementi E. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis 3: e418, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F, Butler-Browne GS. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord 10: 113–120, 2000. [DOI] [PubMed] [Google Scholar]
- 95.Decary S, Mouly V, Hamida CB, Sautet A, Barbet JP, Butler-Browne GS. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther 8: 1429–1438, 1997. [DOI] [PubMed] [Google Scholar]
- 96.Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 90: 717–727, 1997. [DOI] [PubMed] [Google Scholar]
- 97.Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C. Endomysial fibrosis in Duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol 68: 762–773, 2009. [DOI] [PubMed] [Google Scholar]
- 98.Disatnik MH, Dhawan J, Yu Y, Beal MF, Whirl MM, Franco AA, Rando TA. Evidence of oxidative stress in mdx mouse muscle: studies of the pre-necrotic state. J Neurol Sci 161: 77–84, 1998. [DOI] [PubMed] [Google Scholar]
- 99.Divet A, Huchet-Cadiou C. Sarcoplasmic reticulum function in slow- and fast-twitch skeletal muscles from mdx mice. Pflügers Arch 444: 634–643, 2002. [DOI] [PubMed] [Google Scholar]
- 100.Doran P, Dowling P, Lohan J, McDonnell K, Poetsch S, Ohlendieck K. Subproteomics analysis of Ca2+-binding proteins demonstrates decreased calsequestrin expression in dystrophic mouse skeletal muscle. Eur J Biochem 271: 3943–3952, 2004. [DOI] [PubMed] [Google Scholar]
- 101.Dorchies OM, Wagner S, Vuadens O, Waldhauser K, Buetler TM, Kucera P, Ruegg UT. Green tea extract and its major polyphenol (−)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol 290: C616–C625, 2006. [DOI] [PubMed] [Google Scholar]
- 102.Doyle DD, Goings G, Upshaw-Earley J, Ambler SK, Mondul A, Palfrey HC, Page E. Dystrophin associates with caveolae of rat cardiac myocytes: relationship to dystroglycan. Circ Res 87: 480–488, 2000. [DOI] [PubMed] [Google Scholar]
- 103.Duchenne GBA. Recherches sur la paralysie musculaire pseudohypertrophique ou paralysie myo-sclerosique. Arch Gen Med 11: multiple articles, 1868. [Google Scholar]
- 104.Ducret T, Vandebrouck C, Cao ML, Lebacq J, Gailly P. Functional role of store-operated and stretch-activated channels in murine adult skeletal muscle fibres. J Physiol 575: 913–924, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dudley RW, Danialou G, Govindaraju K, Lands L, Eidelman DE, Petrof BJ. Sarcolemmal damage in dystrophin deficiency is modulated by synergistic interactions between mechanical and oxidative/nitrosative stresses. Am J Pathol 168: 1276–1287, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duggan DJ, Hoffman EP. Autosomal recessive muscular dystrophy and mutations of the sarcoglycan complex. Neuromuscul Disord 6: 475–482, 1996. [DOI] [PubMed] [Google Scholar]
- 107.Duguez S, Duddy W, Johnston H, Laine J, Le Bihan MC, Brown KJ, Bigot A, Hathout Y, Butler-Browne G, Partridge T. Dystrophin deficiency leads to disturbance of LAMP1-vesicle-associated protein secretion. Cell Mol Life Sci 70: 2159–2174, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dutka TL, Mollica JP, Posterino GS, Lamb GD. Modulation of contractile apparatus Ca2+ sensitivity and disruption of excitation-contraction coupling by S-nitrosoglutathione in rat muscle fibres. J Physiol 589: 2181–2196, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dyachenko V, Husse B, Rueckschloss U, Isenberg G. Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium 45: 38–54, 2009. [DOI] [PubMed] [Google Scholar]
- 110.Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord 12: 926–929, 2002. [DOI] [PubMed] [Google Scholar]
- 111.Edwards JN, Friedrich O, Cully TR, von WF, Murphy RM, Launikonis BS. Upregulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle. Am J Physiol Cell Physiol 299: C42–C50, 2010. [DOI] [PubMed] [Google Scholar]
- 112.Edwards RHT, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol 272: 769–778, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Elhanany-Tamir H, Yu YV, Shnayder M, Jain A, Welte M, Volk T. Organelle positioning in muscles requires cooperation between two KASH proteins and microtubules. J Cell Biol 198: 833–846, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Emery AE, Muntoni F. Duchenne Muscular Dystrophy. New York: Oxford Univ. Press, 2003. [Google Scholar]
- 115.England SB, Nicholson LV, Johnson MA, Forrest SM, Love DR, Zubrzycka-Gaarn EE, Bulman DE, Harris JB, Davies KE. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature 343: 180–182, 1990. [DOI] [PubMed] [Google Scholar]
- 116.Ermolova NV, Martinez L, Vetrone SA, Jordan MC, Roos KP, Sweeney HL, Spencer MJ. Long-term administration of the TNF blocking drug Remicade (cV1q) to mdx mice reduces skeletal and cardiac muscle fibrosis, but negatively impacts cardiac function. Neuromuscul Disord 24: 583–595, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem 278: 13591–13594, 2003. [DOI] [PubMed] [Google Scholar]
- 118.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol 122: 809–823, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fairclough RJ, Wood MJ, Davies KE. Therapy for Duchenne muscular dystrophy: renewed optimism from genetic approaches. Nat Rev Genet 14: 373–378, 2013. [DOI] [PubMed] [Google Scholar]
- 120.Faulkner JA, Ng R, Davis CS, Li S, Chamberlain JS. Diaphragm muscle strip preparation for evaluation of gene therapies in mdx mice. Clin Exp Pharmacol Physiol 35: 725–729, 2008. [DOI] [PubMed] [Google Scholar]
- 121.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006. [DOI] [PubMed] [Google Scholar]
- 122.Finanger Hedderick EL, Simmers JL, Soleimani A, Andres-Mateos E, Marx R, Files DC, King L, Crawford TO, Corse AM, Cohn RD. Loss of sarcolemmal nNOS is common in acquired and inherited neuromuscular disorders. Neurology 76: 960–967, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fink RH, Stephenson DG, Williams DA. Physiological properties of skinned fibres from normal and dystrophic (Duchenne) human muscle activated by Ca2+ and Sr2+. J Physiol 420: 337–353, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Finkel RS, Flanigan KM, Wong B, Bonnemann C, Sampson J, Sweeney HL, Reha A, Northcutt VJ, Elfring G, Barth J, Peltz SW. Phase 2a study of ataluren-mediated dystrophin production in patients with nonsense mutation duchenne muscular dystrophy. PLoS One 8: e81302, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Firestein BL, Bredt DS. Interaction of neuronal nitric-oxide synthase and phosphofructokinase-M. J Biol Chem 274: 10545–10550, 1999. [DOI] [PubMed] [Google Scholar]
- 126.Flanigan KM. The muscular dystrophies. Semin Neurol 32: 255–263, 2012. [DOI] [PubMed] [Google Scholar]
- 127.Franco A Jr, Lansman JB. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature 344: 670–673, 1990. [DOI] [PubMed] [Google Scholar]
- 128.Franco-Obregon A, Lansman JB. Changes in mechanosensitive channel gating following mechanical stimulation in skeletal muscle myotubes from the mdx mouse. J Physiol 539: 391–407, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Friden J, Lieber RL. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand 171: 321–326, 2001. [DOI] [PubMed] [Google Scholar]
- 130.Friden J, Lieber RL. Serum creatine kinase level is a poor predictor of muscle function after injury. Scand J Med Sci Sports 11: 126–127, 2001. [DOI] [PubMed] [Google Scholar]
- 131.Friedrich O, Both M, Weber C, Schurmann S, Teichmann MD, von WF, Fink RH, Vogel M, Chamberlain JS, Garbe C. Microarchitecture is severely compromised but motor protein function is preserved in dystrophic mdx skeletal muscle. Biophys J 98: 606–616, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Froehner SC, Reed SM, Anderson KN, Huang PL, Percival JM. Loss of nNOS inhibits compensatory muscle hypertrophy and exacerbates inflammation and eccentric contraction-induced damage in mdx mice. Hum Mol Genet 24: 492–505, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fuhrmann-Stroissnigg H, Noiges R, Descovich L, Fischer I, Albrecht DE, Nothias F, Froehner SC, Propst F. The light chains of microtubule-associated proteins MAP1A and MAP1B interact with alpha1-syntrophin in the central and peripheral nervous system. PLoS One 7: e49722, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gailly P, Boland B, Himpens B, Casteels R, Gillis JM. Critical evaluation of cytosolic calcium determination in resting muscle fibres from normal and dystrophic (mdx) mice. Cell Calcium 14: 473–483, 1993. [DOI] [PubMed] [Google Scholar]
- 135.Gailly P, De Backer F, Van Schoor M, Gillis JM. In situ measurements of calpain activity in isolated muscle fibres from normal and dystrophin-lacking, mdx mice. Ca2+ dependence in various physiological conditions. J Physiol 582: 1261–1275, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem 276: 21425–21433, 2001. [DOI] [PubMed] [Google Scholar]
- 137.Galbiati F, Volonte D, Chu JB, Li M, Fine SW, Fu M, Bermudez J, Pedemonte M, Weidenheim KM, Pestell RG, Minetti C, Lisanti MP. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci USA 97: 9689–9694, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gamstorp I, Gustavson KH, Hellstrom O, Nordgren B. A trial of selenium and vitamin E in boys with muscular dystrophy. J Child Neurol 1: 211–214, 1986. [DOI] [PubMed] [Google Scholar]
- 139.Garcia-Pelagio KP, Bloch RJ, Ortega A, Gonzalez-Serratos H. Biomechanics of the sarcolemma and costameres in single skeletal muscle fibers from normal and dystrophin-null mice. J Muscle Res Cell Motil 31: 323–336, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gazzerro E, Bonetto A, Minetti C. Caveolinopathies: translational implications of caveolin-3 in skeletal and cardiac muscle disorders. Handb Clin Neurol 101: 135–142, 2011. [DOI] [PubMed] [Google Scholar]
- 141.Gazzerro E, Sotgia F, Bruno C, Lisanti MP, Minetti C. Caveolinopathies: from the biology of caveolin-3 to human diseases. Eur J Hum Genet 18: 137–145, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gee SH, Madhavan R, Levinson SR, Caldwell JH, Sealock R, Froehner SC. Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J Neurosci 18: 128–137, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gehrig SM, van der Poel C, Sayer TA, Schertzer JD, Henstridge DC, Church JE, Lamon S, Russell AP, Davies KE, Febbraio MA, Lynch GS. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature 484: 394–398, 2012. [DOI] [PubMed] [Google Scholar]
- 144.Gentil C, Leturcq F, Ben YR, Kaplan JC, Laforet P, Penisson-Besnier I, Espil-Taris C, Voit T, Garcia L, Pietri-Rouxel F. Variable phenotype of del45-55 Becker patients correlated with nNOSmu mislocalization and RYR1 hypernitrosylation. Hum Mol Genet 21: 3449–3460, 2012. [DOI] [PubMed] [Google Scholar]
- 145.Gervasio OL, Whitehead NP, Yeung EW, Phillips WD, Allen DG. TRPC1 binds to caveolin-3 and is regulated by Src kinase: role in Duchenne muscular dystrophy. J Cell Sci 121: 2246–2255, 2008. [DOI] [PubMed] [Google Scholar]
- 146.Giacomotto J, Brouilly N, Walter L, Mariol MC, Berger J, Segalat L, Becker TS, Currie PD, Gieseler K. Chemical genetics unveils a key role of mitochondrial dynamics, cytochrome c release and IP3R activity in muscular dystrophy. Hum Mol Genet 22: 4562–4578, 2013. [DOI] [PubMed] [Google Scholar]
- 147.Gillis JM. Understanding dystrophinopathies: an inventory of the structural and functional consequences of the absence of dystrophin in muscles of the mdx mouse. J Muscle Res Cell Motil 20: 605–625, 1999. [DOI] [PubMed] [Google Scholar]
- 148.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 83: 731–801, 2003. [DOI] [PubMed] [Google Scholar]
- 149.Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG, Molkentin JD. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest 121: 1044–1052, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch 455: 1097–1103, 2008. [DOI] [PubMed] [Google Scholar]
- 151.Gowers WR. Pseudo-Hypertrophic Muscular Paralysis–A Clinical Lecture. London: Churchill, 1879. [Google Scholar]
- 152.Grady RM, Akaaboune M, Cohen AL, Maimone MM, Lichtman JW, Sanes JR. Tyrosine-phosphorylated and nonphosphorylated isoforms of alpha-dystrobrevin: roles in skeletal muscle and its neuromuscular and myotendinous junctions. J Cell Biol 160: 741–752, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grady RM, Grange RW, Lau KS, Maimone MM, Nichol MC, Stull JT, Sanes JR. Role for alpha-dystrobrevin in the pathogenesis of dystrophin-dependent muscular dystrophies. Nat Cell Biol 1: 215–220, 1999. [DOI] [PubMed] [Google Scholar]
- 154.Grady RM, Teng HB, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 90: 729–738, 1997. [DOI] [PubMed] [Google Scholar]
- 155.Grosso S, Perrone S, Longini M, Bruno C, Minetti C, Gazzolo D, Balestri P, Buonocore G. Isoprostanes in dystrophinopathy: evidence of increased oxidative stress. Brain Dev 30: 391–395, 2008. [DOI] [PubMed] [Google Scholar]
- 156.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 92: e80-e86, 2003. [DOI] [PubMed] [Google Scholar]
- 157.Grounds MD, Torrisi J. Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J 18: 676–682, 2004. [DOI] [PubMed] [Google Scholar]
- 158.Grupe M, Myers G, Penner R, Fleig A. Activation of store-operated I(CRAC) by hydrogen peroxide. Cell Calcium 48: 1–9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol 352: 685–701, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology 143: 2767–2774, 2002. [DOI] [PubMed] [Google Scholar]
- 161.Haenggi T, Fritschy JM. Role of dystrophin and utrophin for assembly and function of the dystrophin glycoprotein complex in non-muscle tissue. Cell Mol Life Sci 63: 1614–1631, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat 200: 69–79, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hamill OP. Twenty odd years of stretch-sensitive channels. Pflügers Arch 453: 333–351, 2006. [DOI] [PubMed] [Google Scholar]
- 164.Hammadi M, Oulidi A, Gackiere F, Katsogiannou M, Slomianny C, Roudbaraki M, Dewailly E, Delcourt P, Lepage G, Lotteau S, Ducreux S, Prevarskaya N, Van CF. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: involvement of GRP78. FASEB J 27: 1600–1609, 2013. [DOI] [PubMed] [Google Scholar]
- 165.Han W, Li H, Villar VA, Pascua AM, Dajani MI, Wang X, Natarajan A, Quinn MT, Felder RA, Jose PA, Yu P. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension 51: 481–487, 2008. [DOI] [PubMed] [Google Scholar]
- 166.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr 29: 89–95, 1997. [DOI] [PubMed] [Google Scholar]
- 167.Hara H, Nolan PM, Scott MO, Bucan M, Wakayama Y, Fischbeck KH. Running endurance abnormality in mdx mice. Muscle Nerve 25: 207–211, 2002. [DOI] [PubMed] [Google Scholar]
- 168.Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF, Harper HA, Robinson AS, Engelhardt JF, Brooks SV, Chamberlain JS. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med 8: 253–261, 2002. [DOI] [PubMed] [Google Scholar]
- 169.Hasegawa M, Cuenda A, Spillantini MG, Thomas GM, Buee-Scherrer V, Cohen P, Goedert M. Stress-activated protein kinase-3 interacts with the PDZ domain of alpha1-syntrophin. A mechanism for specific substrate recognition. J Biol Chem 274: 12626–12631, 1999. [DOI] [PubMed] [Google Scholar]
- 170.Hauser E, Hoger H, Bittner R, Widhalm K, Herkner K, Lubec G. Oxyradical damage and mitochondrial enzyme activities in the mdx mouse. Neuropediatrics 26: 260–262, 1995. [DOI] [PubMed] [Google Scholar]
- 171.Haycock JW, MacNeil S, Jones P, Harris JB, Mantle D. Oxidative damage to muscle protein in Duchenne muscular dystrophy. Neuroreport 8: 357–361, 1996. [DOI] [PubMed] [Google Scholar]
- 172.Hays AP, Hallett M, Delfs J, Morris J, Sotrel A, Shevchuk MM, DiMauro S. Muscle phosphofructokinase deficiency: abnormal polysaccharide in a case of late-onset myopathy. Neurology 31: 1077–1086, 1981. [DOI] [PubMed] [Google Scholar]
- 173.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281: 26391–26399, 2006. [DOI] [PubMed] [Google Scholar]
- 174.Head SI. Branched fibres in old dystrophic mdx muscle are associated with mechanical weakening of the sarcolemma, abnormal Ca2+ transients and a breakdown of Ca2+ homeostasis during fatigue. Exp Physiol 95: 641–656, 2010. [DOI] [PubMed] [Google Scholar]
- 175.Heller KN, Montgomery CL, Janssen PM, Clark KR, Mendell JR, Rodino-Klapac LR. AAV-mediated overexpression of human alpha7 integrin leads to histological and functional improvement in dystrophic mice. Mol Ther 21: 520–525, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Henricson EK, Abresch RT, Cnaan A, Hu F, Duong T, Arrieta A, Han J, Escolar DM, Florence JM, Clemens PR, Hoffman EP, McDonald CM. The cooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle Nerve 48: 55–67, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Heydemann A, McNally EM. Consequences of disrupting the dystrophin-sarcoglycan complex in cardiac and skeletal myopathy. Trends Cardiovasc Med 17: 55–59, 2007. [DOI] [PubMed] [Google Scholar]
- 178.Hidalgo C, Sanchez G, Barrientos G, Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J Biol Chem 281: 26473–26482, 2006. [DOI] [PubMed] [Google Scholar]
- 179.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132: 113–124, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hillier BJ, Christopherson KS, Prehoda KE, Bredt DS, Lim WA. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science 284: 812–815, 1999. [PubMed] [Google Scholar]
- 181.Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279: 519–526, 1998. [DOI] [PubMed] [Google Scholar]
- 182.Hnia K, Hugon G, Rivier F, Masmoudi A, Mercier J, Mornet D. Modulation of p38 mitogen-activated protein kinase cascade and metalloproteinase activity in diaphragm muscle in response to free radical scavenger administration in dystrophin-deficient Mdx mice. Am J Pathol 170: 633–643, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hodgetts S, Radley H, Davies M, Grounds MD. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul Disord 16: 591–602, 2006. [DOI] [PubMed] [Google Scholar]
- 184.Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928, 1987. [DOI] [PubMed] [Google Scholar]
- 185.Hoffman EP, Connor EM. Orphan drug development in muscular dystrophy: update on two large clinical trials of dystrophin rescue therapies. Discov Med 16: 233–239, 2013. [PubMed] [Google Scholar]
- 186.Hoffman EP, McNally EM. Exon-skipping therapy: a roadblock, detour, or bump in the road? Sci Transl Med 6: 230fs14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hollingworth S, Zeiger U, Baylor SM. Comparison of the myoplasmic calcium transient elicited by an action potential in intact fibres of mdx and normal mice. J Physiol 586: 5063–5075, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hoogerwaard EM, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, Leschot NJ, van Essen AJ, Brunner HG, van der Wouw PA, Wilde AA, de VM. Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in The Netherlands: a cohort study. Lancet 353: 2116–2119, 1999. [DOI] [PubMed] [Google Scholar]
- 189.Hopf FW, Turner PR, Denetclaw WF Jr, Reddy P, Steinhardt RA. A critical evaluation of resting intracellular free calcium regulation in dystrophic mdx muscle. Am J Physiol Cell Physiol 271: C1325–C1339, 1996. [DOI] [PubMed] [Google Scholar]
- 190.Huang H, Bae C, Sachs F, Suchyna TM. Caveolae regulation of mechanosensitive channel function in myotubes. PLoS One 8: e72894, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75: 1273–1286, 1993. [DOI] [PubMed] [Google Scholar]
- 192.Huebner KD, Jassal DS, Halevy O, Pines M, Anderson JE. Functional resolution of fibrosis in mdx mouse dystrophic heart and skeletal muscle by halofuginone. Am J Physiol Heart Circ Physiol 294: H1550–H1561, 2008. [DOI] [PubMed] [Google Scholar]
- 193.Hurt KJ, Sezen SF, Champion HC, Crone JK, Palese MA, Huang PL, Sawa A, Luo X, Musicki B, Snyder SH, Burnett AL. Alternatively spliced neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci USA 103: 3440–3443, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hutter OF. The membrane hypothesis of Duchenne muscular dystrophy: quest for functional evidence. J Inherit Metab Dis 15: 565–577, 1992. [DOI] [PubMed] [Google Scholar]
- 195.Hutter OF, Burton FL, Bovell DL. Mechanical properties of normal and mdx mouse sarcolemma: bearing on function of dystrophin. J Muscle Res Cell Motil 12: 585–589, 1991. [DOI] [PubMed] [Google Scholar]
- 196.Ilsley JL, Sudol M, Winder SJ. The interaction of dystrophin with beta-dystroglycan is regulated by tyrosine phosphorylation. Cell Signal 13: 625–632, 2001. [DOI] [PubMed] [Google Scholar]
- 197.Ilsley JL, Sudol M, Winder SJ. The WW domain: linking cell signalling to the membrane cytoskeleton. Cell Signal 14: 183–189, 2002. [DOI] [PubMed] [Google Scholar]
- 198.Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet 18: 824–834, 2009. [DOI] [PubMed] [Google Scholar]
- 199.Jackson MJ. Redox regulation of adaptive responses in skeletal muscle to contractile activity. Free Radic Biol Med 47: 1267–1275, 2009. [DOI] [PubMed] [Google Scholar]
- 200.Jaffrey SR, Snyder SH. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science 274: 774–777, 1996. [DOI] [PubMed] [Google Scholar]
- 201.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med 165: 412–418, 2002. [DOI] [PubMed] [Google Scholar]
- 202.Joe MK, Kee C, Tomarev SI. Myocilin interacts with syntrophins and is member of dystrophin-associated protein complex. J Biol Chem 287: 13216–13227, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Johnson BD, Scheuer T, Catterall WA. Convergent regulation of skeletal muscle Ca2+ channels by dystrophin, the actin cytoskeleton, and cAMP-dependent protein kinase. Proc Natl Acad Sci USA 102: 4191–4196, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Johnson EK, Li B, Yoon JH, Flanigan KM, Martin PT, Ervasti J, Montanaro F. Identification of new dystroglycan complexes in skeletal muscle. PLoS One 8: e73224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Johnson EK, Zhang L, Adams ME, Phillips A, Freitas MA, Froehner SC, Green-Church KB, Montanaro F. Proteomic analysis reveals new cardiac-specific dystrophin-associated proteins. PLoS One 7: e43515, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jones DP. Redefining oxidative stress. Antioxid Redox Signal 8: 1865–1879, 2006. [DOI] [PubMed] [Google Scholar]
- 207.Kargacin ME, Kargacin GJ. The sarcoplasmic reticulum calcium pump is functionally altered in dystrophic muscle. Biochim Biophys Acta 1290: 4–8, 1996. [DOI] [PubMed] [Google Scholar]
- 208.Kawahara G, Gasperini MJ, Myers JA, Widrick JJ, Eran A, Serafini PR, Alexander MS, Pletcher MT, Morris CA, Kunkel LM. Dystrophic muscle improvement in zebrafish via increased heme oxygenase signaling. Hum Mol Genet 23: 1869–1878, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kawahara G, Kunkel LM. Zebrafish based small molecule screens for novel DMD drugs. Drug Discov Today Technol 10: e91-e96, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem 278: 31111–31117, 2003. [DOI] [PubMed] [Google Scholar]
- 211.Kawasaki BT, Liao Y, Birnbaumer L. Role of Src in C3 transient receptor potential channel function and evidence for a heterogeneous makeup of receptor- and store-operated Ca2+ entry channels. Proc Natl Acad Sci USA 103: 335–340, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Khairallah M, Khairallah RJ, Young ME, Allen BG, Gillis MA, Danialou G, Deschepper CF, Petrof BJ, Des RC. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci USA 105: 7028–7033, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y, Chen YW, Raiteri R, Lederer WJ, Dorsey SG, Ward CW. Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci Signal 5: ra56, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kim JH, Kwak HB, Thompson LV, Lawler JM. Contribution of oxidative stress to pathology in diaphragm and limb muscles with Duchenne muscular dystrophy. J Muscle Res Cell Motil 34: 1–13, 2013. [DOI] [PubMed] [Google Scholar]
- 215.Kim MJ, Hwang SH, Lim JA, Froehner SC, Adams ME, Kim HS. Alpha-syntrophin modulates myogenin expression in differentiating myoblasts. PLoS One 5: e15355, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Klinge L, Dekomien G, Aboumousa A, Charlton R, Epplen JT, Barresi R, Bushby K, Straub V. Sarcoglycanopathies: can muscle immunoanalysis predict the genotype? Neuromuscul Disord 18: 934–941, 2008. [DOI] [PubMed] [Google Scholar]
- 217.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 456: 511–515, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Koenig M, Kunkel LM. Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J Biol Chem 265: 4560–4566, 1990. [PubMed] [Google Scholar]
- 219.Kornegay JN, Bogan JR, Bogan DJ, Childers MK, Li J, Nghiem P, Detwiler DA, Larsen CA, Grange RW, Bhavaraju-Sanka RK, Tou S, Keene BP, Howard JF Jr, Wang J, Fan Z, Schatzberg SJ, Styner MA, Flanigan KM, Xiao X, Hoffman EP. Canine models of Duchenne muscular dystrophy and their use in therapeutic strategies. Mamm Genome 23: 85–108, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kottlors M, Kirschner J. Elevated satellite cell number in Duchenne muscular dystrophy. Cell Tissue Res 340: 541–548, 2010. [DOI] [PubMed] [Google Scholar]
- 221.Kramerova I, Kudryashova E, Venkatraman G, Spencer MJ. Calpain 3 participates in sarcomere remodeling by acting upstream of the ubiquitin-proteasome pathway. Hum Mol Genet 14: 2125–2134, 2005. [DOI] [PubMed] [Google Scholar]
- 222.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 9: 1004–1010, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kumar A, Boriek AM. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J 17: 386–396, 2003. [DOI] [PubMed] [Google Scholar]
- 224.Kung C. A possible unifying principle for mechanosensation. Nature 436: 647–654, 2005. [DOI] [PubMed] [Google Scholar]
- 225.Kunkel LM. 2004 William Allan Award address. Cloning of the DMD gene. Am J Hum Genet 76: 205–214, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kunkel LM, Hejtmancik JF, Caskey CT, Speer A, Monaco AP, Middlesworth W, Colletti CA, Bertelson C, Muller U, Bresnan M, Shapiro F, Tantravahi U, Speer J, Latt SA, Bartlett R, Pericak-Vance MA, Roses AD, Thompson MW, Ray PN, Worton RG, Fischbeck KH, Gallano P, Coulon M, Duros C, Boue J, Junien C, Chelly J, Hamard G, Jeanpierre M, Lambert M, Kaplan JC, Emery A, Dorkins H, McGlade S, Davies KE, Boehm C, Arveiler B, Lemaire C, Morgan GJ, Denton MJ, Amos J, Bobrow M, Benham F, Boswinkel E, Cole C, Dubowitz V, Hart K, Hodgson S, Johnson L, Walker A, Roncuzzi L, Ferlini A, Nobile C, Romeo G, Wilcox DE, Affara NA, Ferguson-Smith MA, Lindolf M, Kaariainen H, de la Chapelle A, Ionasescu V, Searby C, Ionasescu R, Bakker E, van Ommen GJ, Pearson PL, Greenberg CR, Hamerton JL, Wrogemann K, Doherty RA, Polakowska R, Hyser C, Quirk S, Thomas N, Harper JF, Darras BT, Francke U. Analysis of deletions in DNA from patients with Becker and Duchenne muscular dystrophy. Nature 322: 73–77, 1986. [DOI] [PubMed] [Google Scholar]
- 227.Kunkel LM, Monaco AP, Middlesworth W, Ochs HD, Latt SA. Specific cloning of DNA fragments absent from the DNA of a male patient with an X-chromosome deletion. Proc Natl Acad Sci USA 82: 4778–4782, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol 533: 185–199, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest 119: 624–635, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lai Y, Zhao J, Yue Y, Duan D. alpha2 and alpha3 helices of dystrophin R16 and R17 frame a microdomain in the alpha1 helix of dystrophin R17 for neuronal NOS binding. Proc Natl Acad Sci USA 110: 525–530, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol 14: 759–774, 2013. [DOI] [PubMed] [Google Scholar]
- 232.Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol 489: 349–362, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol 589: 2119–2127, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 43: 332–347, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Launikonis BS, Barnes M, Stephenson DG. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc Natl Acad Sci USA 100: 2941–2944, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Launikonis BS, Murphy RM, Edwards JN. Toward the roles of store-operated Ca2+ entry in skeletal muscle. Pflügers Arch 460: 813–823, 2010. [DOI] [PubMed] [Google Scholar]
- 237.Le Borgne F, Guyot S, Logerot M, Beney L, Gervais P, Demarquoy J. Exploration of lipid metabolism in relation with plasma membrane properties of Duchenne muscular dystrophy cells: influence of l-carnitine. PLoS One 7: e49346, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Le Rumeur E, Hubert JF, Winder SJ. A new twist to coiled coil. FEBS Lett 586: 2717–2722, 2012. [DOI] [PubMed] [Google Scholar]
- 239.Le Rumeur E, Winder SJ, Hubert JF. Dystrophin: more than just the sum of its parts. Biochim Biophys Acta 1804: 1713–1722, 2010. [DOI] [PubMed] [Google Scholar]
- 240.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 18: 816–827, 2004. [DOI] [PubMed] [Google Scholar]
- 241.Legardinier S, Raguenes-Nicol C, Tascon C, Rocher C, Hardy S, Hubert JF, Le RE. Mapping of the lipid-binding and stability properties of the central rod domain of human dystrophin. J Mol Biol 389: 546–558, 2009. [DOI] [PubMed] [Google Scholar]
- 242.Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR III, Vandenberg CA. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins. J Biol Chem 279: 22331–22346, 2004. [DOI] [PubMed] [Google Scholar]
- 243.Lesault PF, Theret M, Magnan M, Cuvellier S, Niu Y, Gherardi RK, Tremblay JP, Hittinger L, Chazaud B. Macrophages improve survival, proliferation and migration of engrafted myogenic precursor cells into MDX skeletal muscle. PLoS One 7: e46698, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Li D, Bareja A, Judge L, Yue Y, Lai Y, Fairclough R, Davies KE, Chamberlain JS, Duan D. Sarcolemmal nNOS anchoring reveals a qualitative difference between dystrophin and utrophin. J Cell Sci 123: 2008–2013, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Li D, Shin JH, Duan D. iNOS ablation does not improve specific force of the extensor digitorum longus muscle in dystrophin-deficient mdx4cv mice. PLoS One 6: e21618, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Li D, Yue Y, Lai Y, Hakim CH, Duan D. Nitrosative stress elicited by nNOSmicro delocalization inhibits muscle force in dystrophin-null mice. J Pathol 223: 88–98, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lin C, Guo X, Lange S, Liu J, Ouyang K, Yin X, Jiang L, Cai Y, Mu Y, Sheikh F, Ye S, Chen J, Ke Y, Cheng H. Cypher/ZASP is a novel A-kinase anchoring protein. J Biol Chem 288: 29403–29413, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lindahl M, Backman E, Henriksson KG, Gorospe JR, Hoffman EP. Phospholipase A2 activity in dystrophinopathies. Neuromuscul Disord 5: 193–199, 1995. [DOI] [PubMed] [Google Scholar]
- 249.Loufrani L, Dubroca C, You D, Li Z, Levy B, Paulin D, Henrion D. Absence of dystrophin in mice reduces NO-dependent vascular function and vascular density: total recovery after a treatment with the aminoglycoside gentamicin. Arterioscler Thromb Vasc Biol 24: 671–676, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lovering RM, Michaelson L, Ward CW. Malformed mdx myofibers have normal cytoskeletal architecture yet altered EC coupling and stress-induced Ca2+ signaling. Am J Physiol Cell Physiol 297: C571–C580, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lumeng C, Phelps S, Crawford GE, Walden PD, Barald K, Chamberlain JS. Interactions between beta 2-syntrophin and a family of microtubule-associated serine/threonine kinases. Nat Neurosci 2: 611–617, 1999. [DOI] [PubMed] [Google Scholar]
- 252.Lumeng CN, Hauser M, Brown V, Chamberlain JS. Expression of the 71 kDa dystrophin isoform (Dp71) evaluated by gene targeting. Brain Res 830: 174–178, 1999. [DOI] [PubMed] [Google Scholar]
- 253.Luo S, Chen Y, Lai KO, Arevalo JC, Froehner SC, Adams ME, Chao MV, Ip NY. α-Syntrophin regulates ARMS localization at the neuromuscular junction and enhances EphA4 signaling in an ARMS-dependent manner. J Cell Biol 169: 813–824, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol 586: 4815–4824, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol 535: 591–600, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lyssand JS, DeFino MC, Tang XB, Hertz AL, Feller DB, Wacker JL, Adams ME, Hague C. Blood pressure is regulated by an alpha1D-adrenergic receptor/dystrophin signalosome. J Biol Chem 283: 18792–18800, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lyssand JS, Lee KS, DeFino M, Adams ME, Hague C. Syntrophin isoforms play specific functional roles in the alpha1D-adrenergic receptor/DAPC signalosome. Biochem Biophys Res Commun 412: 596–601, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lyssand JS, Whiting JL, Lee KS, Kastl R, Wacker JL, Bruchas MR, Miyatake M, Langeberg LK, Chavkin C, Scott JD, Gardner RG, Adams ME, Hague C. Alpha-dystrobrevin-1 recruits alpha-catulin to the alpha1D-adrenergic receptor/dystrophin-associated protein complex signalosome. Proc Natl Acad Sci USA 107: 21854–21859, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mack NA, Porter AP, Whalley HJ, Schwarz JP, Jones RC, Khaja AS, Bjartell A, Anderson KI, Malliri A. Beta2-syntrophin and Par-3 promote an apicobasal Rac activity gradient at cell-cell junctions by differentially regulating Tiam1 activity. Nat Cell Biol 14: 1169–1180, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Madhavan R, Jarrett HW. Phosphorylation of dystrophin and alpha-syntrophin by Ca2+-calmodulin dependent protein kinase II. Biochim Biophys Acta 1434: 260–274, 1999. [DOI] [PubMed] [Google Scholar]
- 261.Mair J, Mayr M, Muller E, Koller A, Haid C, Artner-Dworzak E, Calzolari C, Larue C, Puschendorf B. Rapid adaptation to eccentric exercise-induced muscle damage. Int J Sports Med 16: 352–356, 1995. [DOI] [PubMed] [Google Scholar]
- 262.Malik V, Rodino-Klapac LR, Mendell JR. Emerging drugs for Duchenne muscular dystrophy. Expert Opin Emerg Drugs 17: 261–277, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262a.Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Muñoz-Cánoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1: 21, 2011. (doi: 10.1186/2044-5040-1-21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7: 179–185, 2005. [DOI] [PubMed] [Google Scholar]
- 264.Marshall JL, Chou E, Oh J, Kwok A, Burkin DJ, Crosbie-Watson RH. Dystrophin and utrophin expression require sarcospan: loss of alpha7 integrin exacerbates a newly discovered muscle phenotype in sarcospan-null mice. Hum Mol Genet 21: 4378–4393, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Marshall JL, Crosbie-Watson RH. Sarcospan: a small protein with large potential for Duchenne muscular dystrophy. Skeletal Muscle 3: 1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Marshall JL, Holmberg J, Chou E, Ocampo AC, Oh J, Lee J, Peter AK, Martin PT, Crosbie-Watson RH. Sarcospan-dependent Akt activation is required for utrophin expression and muscle regeneration. J Cell Biol 197: 1009–1027, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Martin EA, Barresi R, Byrne BJ, Tsimerinov EI, Scott BL, Walker AE, Gurudevan SV, Anene F, Elashoff RM, Thomas GD, Victor RG. Tadalafil alleviates muscle ischemia in patients with Becker muscular dystrophy. Sci Transl Med 4: 162ra155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Martins AS, Shkryl VM, Nowycky MC, Shirokova N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J Physiol 586: 197–210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Masuda-Hirata M, Suzuki A, Amano Y, Yamashita K, Ide M, Yamanaka T, Sakai M, Imamura M, Ohno S. Intracellular polarity protein PAR-1 regulates extracellular laminin assembly by regulating the dystroglycan complex. Genes Cells 14: 835–850, 2009. [DOI] [PubMed] [Google Scholar]
- 270.Mathews KD, Moore SA. Limb-girdle muscular dystrophy. Curr Neurol Neurosci Rep 3: 78–85, 2003. [DOI] [PubMed] [Google Scholar]
- 271.Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 360: 588–591, 1992. [DOI] [PubMed] [Google Scholar]
- 272.McConell GK, Rattigan S, Lee-Young RS, Wadley GD, Merry TL. Skeletal muscle nitric oxide signaling and exercise: a focus on glucose metabolism. Am J Physiol Endocrinol Metab 303: E301–E307, 2012. [DOI] [PubMed] [Google Scholar]
- 273.McConell GK, Wadley GD. Potential role of nitric oxide in contraction-stimulated glucose uptake and mitochondrial biogenesis in skeletal muscle. Clin Exp Pharmacol Physiol 35: 1488–1492, 2008. [DOI] [PubMed] [Google Scholar]
- 274.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Atkinson L, Elfring GL, Reha A, Miller LL. The 6-minute walk test in Duchenne/Becker muscular dystrophy: longitudinal observations. Muscle Nerve 42: 966–974, 2010. [DOI] [PubMed] [Google Scholar]
- 275.McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol 140: 1097–1109, 1992. [PMC free article] [PubMed] [Google Scholar]
- 276.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol 19: 697–731, 2003. [DOI] [PubMed] [Google Scholar]
- 277.Meinen S, Lin S, Ruegg MA, Punga AR. Fatigue and muscle atrophy in a mouse model of myasthenia gravis is paralleled by loss of sarcolemmal nNOS. PLoS One 7: e44148, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, Bowles D, Gray S, Li C, Galloway G, Malik V, Coley B, Clark KR, Li J, Xiao X, Samulski J, McPhee SW, Samulski RJ, Walker CM. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med 363: 1429–1437, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, King W, Signore L, Pandya S, Florence J. Randomized, double-blind six-month trial of prednisone in Duchenne's muscular dystrophy. N Engl J Med 320: 1592–1597, 1989. [DOI] [PubMed] [Google Scholar]
- 280.Mendell JR, Rodino-Klapac L, Sahenk Z, Malik V, Kaspar BK, Walker CM, Clark KR. Gene therapy for muscular dystrophy: lessons learned and path forward. Neurosci Lett 527: 90–99, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, Lowes LP, Alfano L, Gomez AM, Lewis S, Kota J, Malik V, Shontz K, Walker CM, Flanigan KM, Corridore M, Kean JR, Allen HD, Shilling C, Melia KR, Sazani P, Saoud JB, Kaye EM. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol 74: 637–647, 2013. [DOI] [PubMed] [Google Scholar]
- 282.Menke A, Jockusch H. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature 349: 69–71, 1991. [DOI] [PubMed] [Google Scholar]
- 283.Menke A, Jockusch H. Extent of shock-induced membrane leakage in human and mouse myotubes depends on dystrophin. J Cell Sci 108: 727–733, 1995. [DOI] [PubMed] [Google Scholar]
- 284.Mercado ML, Amenta AR, Hagiwara H, Rafii MS, Lechner BE, Owens RT, McQuillan DJ, Froehner SC, Fallon JR. Biglycan regulates the expression and sarcolemmal localization of dystrobrevin, syntrophin, and nNOS. FASEB J 20: 1724–1726, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mercuri E, Muntoni F. The ever-expanding spectrum of congenital muscular dystrophies. Ann Neurol 72: 9–17, 2012. [DOI] [PubMed] [Google Scholar]
- 286.Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, Monici MC, Bitto A, Mazzeo A, Marini H, Squadrito F, Vita G. Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol 168: 918–926, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Messina S, Vita GL, Aguennouz M, Sframeli M, Romeo S, Rodolico C, Vita G. Activation of NF-kappaB pathway in Duchenne muscular dystrophy: relation to age. Acta Myol 30: 16–23, 2011. [PMC free article] [PubMed] [Google Scholar]
- 288.Michaelson LP, Shi G, Ward CW, Rodney GG. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve 42: 522–529, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Millay DP, Goonasekera SA, Sargent MA, Maillet M, Aronow BJ, Molkentin JD. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc Natl Acad Sci USA 106: 19023–19028, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Miller G, Moore CJ, Terry R, La RT, Mitchell A, Piggott R, Dear TN, Wells DJ, Winder SJ. Preventing phosphorylation of dystroglycan ameliorates the dystrophic phenotype in mdx mouse. Hum Mol Genet 21: 4508–4520, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Miller G, Peter AK, Espinoza E, Heighway J, Crosbie RH. Over-expression of Microspan, a novel component of the sarcoplasmic reticulum, causes severe muscle pathology with triad abnormalities. J Muscle Res Cell Motil 27: 545–558, 2006. [DOI] [PubMed] [Google Scholar]
- 292.Miller G, Wang EL, Nassar KL, Peter AK, Crosbie RH. Structural and functional analysis of the sarcoglycan-sarcospan subcomplex. Exp Cell Res 313: 639–651, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mizuno Y, Thompson TG, Guyon JR, Lidov HG, Brosius M, Imamura M, Ozawa E, Watkins SC, Kunkel LM. Desmuslin, an intermediate filament protein that interacts with alpha-dystrobrevin and desmin. Proc Natl Acad Sci USA 98: 6156–6161, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 147: 728–741, 2011. [DOI] [PubMed] [Google Scholar]
- 295.Moens P, Baatsen PH, Marechal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J Muscle Res Cell Motil 14: 446–451, 1993. [DOI] [PubMed] [Google Scholar]
- 296.Mofarrahi M, Brandes RP, Gorlach A, Hanze J, Terada LS, Quinn MT, Mayaki D, Petrof B, Hussain SN. Regulation of proliferation of skeletal muscle precursor cells by NADPH oxidase. Antioxid Redox Signal 10: 559–574, 2008. [DOI] [PubMed] [Google Scholar]
- 297.Mokhtarian A, Lefaucheur JP, Even PC, Sebille A. Hindlimb immobilization applied to 21-day-old mdx mice prevents the occurrence of muscle degeneration. J Appl Physiol 86: 924–931, 1999. [DOI] [PubMed] [Google Scholar]
- 298.Mokri B, Engel AG. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology 25: 1111–1120, 1975. [DOI] [PubMed] [Google Scholar]
- 299.Monaco AP, Neve RL, Collettifeener C, Bertelson CJ, Kurnit DM, Kunkel LM. Isolation of candidate cDNAs for portions of the Duchenne Muscular-Dystrophy gene. Nature 323: 646–650, 1986. [DOI] [PubMed] [Google Scholar]
- 300.Moore CJ, Winder SJ. The inside and out of dystroglycan post-translational modification. Neuromuscul Disord 22: 959–965, 2012. [DOI] [PubMed] [Google Scholar]
- 301.Moorwood C. Syncoilin, an intermediate filament-like protein linked to the dystrophin associated protein complex in skeletal muscle. Cell Mol Life Sci 65: 2957–2963, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Moorwood C, Barton ER. Caspase-12 ablation preserves muscle function in the mdx mouse. Hum Mol Genet 23: 5325–5341, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Morales MG, Cabello-Verrugio C, Santander C, Cabrera D, Goldschmeding R, Brandan E. CTGF/CCN-2 over-expression can directly induce features of skeletal muscle dystrophy. J Pathol 225: 490–501, 2011. [DOI] [PubMed] [Google Scholar]
- 304.Morales MG, Gutierrez J, Cabello-Verrugio C, Cabrera D, Lipson KE, Goldschmeding R, Brandan E. Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet 22: 4938–4951, 2013. [DOI] [PubMed] [Google Scholar]
- 305.Mundy DI, Machleidt T, Ying YS, Anderson RG, Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci 115: 4327–4339, 2002. [DOI] [PubMed] [Google Scholar]
- 306.Munehira Y, Ohnishi T, Kawamoto S, Furuya A, Shitara K, Imamura M, Yokota T, Takeda S, Amachi T, Matsuo M, Kioka N, Ueda K. Alpha1-syntrophin modulates turnover of ABCA1. J Biol Chem 279: 15091–15095, 2004. [DOI] [PubMed] [Google Scholar]
- 307.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93: 829–838, 2003. [DOI] [PubMed] [Google Scholar]
- 308.Murphy RM, Dutka TL, Horvath D, Bell JR, Delbridge LM, Lamb GD. Ca2+-dependent proteolysis of junctophilin-1 and junctophilin-2 in skeletal and cardiac muscle. J Physiol 591: 719–729, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Murphy RM, Lamb GD. Endogenous calpain-3 activation is primarily governed by small increases in resting cytoplasmic [Ca2+] and is not dependent on stretch. J Biol Chem 284: 7811–7819, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Murphy RM, Mollica JP, Lamb GD. Plasma membrane removal in rat skeletal muscle fibers reveals caveolin-3 hot-spots at the necks of transverse tubules. Exp Cell Res 315: 1015–1028, 2009. [DOI] [PubMed] [Google Scholar]
- 311.Murphy RM, Verburg E, Lamb GD. Ca2+-activation of diffusible and bound pools of μ-calpain in rat skeletal muscle. J Physiol 576: 595–612, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Murphy S, Henry M, Meleady P, Zweyer M, Mundegar RR, Swandulla D, Ohlendieck K. Simultaneous pathoproteomic evaluation of the dystrophin-glycoprotein complex and secondary changes in the mdx-4cv mouse model of Duchenne Muscular Dystrophy. Biology 4: 397–423, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA 105: 6626–6631, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Nakae Y, Dorchies OM, Stoward PJ, Zimmermann BF, Ritter C, Ruegg UT. Quantitative evaluation of the beneficial effects in the mdx mouse of epigallocatechin gallate, an antioxidant polyphenol from green tea. Histochem Cell Biol 137: 811–827, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Nakajima H, Raben N, Hamaguchi T, Yamasaki T. Phosphofructokinase deficiency; past, present and future. Curr Mol Med 2: 197–212, 2002. [DOI] [PubMed] [Google Scholar]
- 316.Nastase MV, Young MF, Schaefer L. Biglycan: a multivalent proteoglycan providing structure and signals. J Histochem Cytochem 60: 963–975, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci USA 98: 14108–14113, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Neumeier M, Krautbauer S, Schmidhofer S, Hader Y, Eisinger K, Eggenhofer E, Froehner SC, Adams ME, Mages W, Buechler C. Adiponectin receptor 1 C-terminus interacts with PDZ-domain proteins such as syntrophins. Exp Mol Pathol 95: 180–186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Newbell BJ, Anderson JT, Jarrett HW. Ca2+-calmodulin binding to mouse alpha1 syntrophin: syntrophin is also a Ca2+-binding protein. Biochemistry 36: 1295–1305, 1997. [DOI] [PubMed] [Google Scholar]
- 320.Newey SE, Benson MA, Ponting CP, Davies KE, Blake DJ. Alternative splicing of dystrobrevin regulates the stoichiometry of syntrophin binding to the dystrophin protein complex. Curr Biol 10: 1295–1298, 2000. [DOI] [PubMed] [Google Scholar]
- 321.Newey SE, Howman EV, Ponting CP, Benson MA, Nawrotzki R, Loh NY, Davies KE, Blake DJ. Syncoilin, a novel member of the intermediate filament superfamily that interacts with alpha-dystrobrevin in skeletal muscle. J Biol Chem 276: 6645–6655, 2001. [DOI] [PubMed] [Google Scholar]
- 322.Ng R, Banks GB, Hall JK, Muir LA, Ramos JN, Wicki J, Odom GL, Konieczny P, Seto J, Chamberlain JR, Chamberlain JS. Animal models of muscular dystrophy. Prog Mol Biol Transl Sci 105: 83–111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Nichol JA, Hutter OF. Ca2+ loading reduces the tensile strength of sarcolemmal vesicles shed from rabbit muscle. J Physiol 493: 199–209, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Nordmann C, Strokin M, Schonfeld P, Reiser G. Putative roles of Ca-independent phospholipase A in respiratory chain-associated ROS production in brain mitochondria: influence of docosahexaenoic acid and bromoenol lactone. J Neurochem 131: 163–176, 2014. [DOI] [PubMed] [Google Scholar]
- 325.Nuss JE, Amaning JK, Bailey CE, DeFord JH, Dimayuga VL, Rabek JP, Papaconstantinou J. Oxidative modification and aggregation of creatine kinase from aged mouse skeletal muscle. Aging 1: 557–572, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.O'Neill A, Williams MW, Resneck WG, Milner DJ, Capetanaki Y, Bloch RJ. Sarcolemmal organization in skeletal muscle lacking desmin: evidence for cytokeratins associated with the membrane skeleton at costameres. Mol Biol Cell 13: 2347–2359, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Oak SA, Russo K, Petrucci TC, Jarrett HW. Mouse alpha1-syntrophin binding to Grb2: further evidence of a role for syntrophin in cell signaling. Biochemistry 40: 11270–11278, 2001. [DOI] [PubMed] [Google Scholar]
- 328.Oddoux S, Zaal KJ, Tate V, Kenea A, Nandkeolyar SA, Reid E, Liu W, Ralston E. Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. J Cell Biol 203: 205–213, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Oh HJ, Abraham LS, van HJ, Stove C, Proszynski TJ, Gevaert K, DiMario JX, Sanes JR, van RF, Kim H. Interaction of alpha-catulin with dystrobrevin contributes to integrity of dystrophin complex in muscle. J Biol Chem 287: 21717–21728, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Okuhira K, Fitzgerald ML, Sarracino DA, Manning JJ, Bell SA, Goss JL, Freeman MW. Purification of ATP-binding cassette transporter A1 and associated binding proteins reveals the importance of beta1-syntrophin in cholesterol efflux. J Biol Chem 280: 39653–39664, 2005. [DOI] [PubMed] [Google Scholar]
- 331.Ort T, Maksimova E, Dirkx R, Kachinsky AM, Berghs S, Froehner SC, Solimena M. The receptor tyrosine phosphatase-like protein ICA512 binds the PDZ domains of beta2-syntrophin and nNOS in pancreatic beta-cells. Eur J Cell Biol 79: 621–630, 2000. [DOI] [PubMed] [Google Scholar]
- 332.Ort T, Voronov S, Guo J, Zawalich K, Froehner SC, Zawalich W, Solimena M. Dephosphorylation of beta2-syntrophin and Ca2+/mu-calpain-mediated cleavage of ICA512 upon stimulation of insulin secretion. EMBO J 20: 4013–4023, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ozawa E, Mizuno Y, Hagiwara Y, Sasaoka T, Yoshida M. Molecular and cell biology of the sarcoglycan complex. Muscle Nerve 32: 563–576, 2005. [DOI] [PubMed] [Google Scholar]
- 334.Pal R, Palmieri M, Loehr JA, Li S, Abo-Zahrah R, Monroe TO, Thakur PB, Sardiello M, Rodney GG. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun 5: 4425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 14: 98–112, 2013. [DOI] [PubMed] [Google Scholar]
- 336.Partridge TA. The mdx mouse model as a surrogate for Duchenne muscular dystrophy. FEBS J 280: 4177–4186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Pasternak C, Wong S, Elson EL. Mechanical function of dystrophin in muscle cells. J Cell Biol 128: 355–361, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res 301: 1–7, 2004. [DOI] [PubMed] [Google Scholar]
- 339.Pauly M, Daussin F, Burelle Y, Li T, Godin R, Fauconnier J, Koechlin-Ramonatxo C, Hugon G, Lacampagne A, Coisy-Quivy M, Liang F, Hussain S, Matecki S, Petrof BJ. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am J Pathol 181: 583–592, 2012. [DOI] [PubMed] [Google Scholar]
- 340.Peng HM, Morishima Y, Pratt WB, Osawa Y. Modulation of heme/substrate binding cleft of neuronal nitric-oxide synthase (nNOS) regulates binding of Hsp90 and Hsp70 proteins and nNOS ubiquitination. J Biol Chem 287: 1556–1565, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Percival JM, Adamo CM, Beavo JA, Froehner SC. Evaluation of the therapeutic utility of phosphodiesterase 5A inhibition in the mdx mouse model of duchenne muscular dystrophy. Handb Exp Pharmacol 323–344, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Percival JM, Anderson KN, Gregorevic P, Chamberlain JS, Froehner SC. Functional deficits in nNOSmu-deficient skeletal muscle: myopathy in nNOS knockout mice. PLoS One 3: e3387, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Percival JM, Anderson KN, Huang P, Adams ME, Froehner SC. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest 120: 816–826, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Percival JM, Froehner SC. Golgi complex organization in skeletal muscle: a role for Golgi-mediated glycosylation in muscular dystrophies? Traffic 8: 184–194, 2007. [DOI] [PubMed] [Google Scholar]
- 345.Percival JM, Gregorevic P, Odom GL, Banks GB, Chamberlain JS, Froehner SC. rAAV6-microdystrophin rescues aberrant Golgi complex organization in mdx skeletal muscles. Traffic 8: 1424–1439, 2007. [DOI] [PubMed] [Google Scholar]
- 346.Percival JM, Siegel MP, Knowels G, Marcinek DJ. Defects in mitochondrial localization and ATP synthesis in the mdx mouse model of Duchenne muscular dystrophy are not alleviated by PDE5 inhibition. Hum Mol Genet 22: 153–167, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol 228: 77–87, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pessina P, Cabrera D, Morales MG, Riquelme CA, Gutierrez J, Serrano AL, Brandan E, Munoz-Canoves P. Novel and optimized strategies for inducing fibrosis in vivo: focus on Duchenne Muscular Dystrophy. Skelet Muscle 4: 7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Peter AK, Marshall JL, Crosbie RH. Sarcospan reduces dystrophic pathology: stabilization of the utrophin-glycoprotein complex. J Cell Biol 183: 419–427, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Peters MF, Adams ME, Froehner SC. Differential association of syntrophin pairs with the dystrophin complex. J Cell Biol 138: 81–93, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Peters MF, O'Brien KF, Sadoulet-Puccio HM, Kunkel LM, Adams ME, Froehner SC. Beta-dystrobrevin, a new member of the dystrophin family. Identification, cloning, and protein associations. J Biol Chem 272: 31561–31569, 1997. [DOI] [PubMed] [Google Scholar]
- 352.Peters MF, Sadoulet-Puccio HM, Grady MR, Kramarcy NR, Kunkel LM, Sanes JR, Sealock R, Froehner SC. Differential membrane localization and intermolecular associations of alpha-dystrobrevin isoforms in skeletal muscle. J Cell Biol 142: 1269–1278, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Peterson JM, Kline W, Canan BD, Ricca DJ, Kaspar B, Delfin DA, DiRienzo K, Clemens PR, Robbins PD, Baldwin AS, Flood P, Kaumaya P, Freitas M, Kornegay JN, Mendell JR, Rafael-Fortney JA, Guttridge DC, Janssen PM. Peptide-based inhibition of NF-kappaB rescues diaphragm muscle contractile dysfunction in a murine model of Duchenne muscular dystrophy. Mol Med 17: 508–515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90: 3710–3714, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Petrof BJ, Stedman HH, Shrager JB, Eby J, Sweeney HL, Kelly AM. Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am J Physiol Cell Physiol 265: C834–C841, 1993. [DOI] [PubMed] [Google Scholar]
- 356.Pierno S, Nico B, Burdi R, Liantonio A, Didonna MP, Cippone V, Fraysse B, Rolland JF, Mangieri D, Andreetta F, Ferro P, Camerino C, Zallone A, Confalonieri P, De Luca A. Role of tumour necrosis factor alpha, but not of cyclo-oxygenase-2-derived eicosanoids, on functional and morphological indices of dystrophic progression in mdx mice: a pharmacological approach. Neuropathol Appl Neurobiol 33: 344–359, 2007. [DOI] [PubMed] [Google Scholar]
- 357.Piluso G, Mirabella M, Ricci E, Belsito A, Abbondanza C, Servidei S, Puca AA, Tonali P, Puca GA, Nigro V. Gamma1- and gamma2-syntrophins, two novel dystrophin-binding proteins localized in neuronal cells. J Biol Chem 275: 15851–15860, 2000. [DOI] [PubMed] [Google Scholar]
- 358.Plant DR, Lynch GS. Depolarization-induced contraction and SR function in mechanically skinned muscle fibers from dystrophic mdx mice. Am J Physiol Cell Physiol 285: C522–C528, 2003. [DOI] [PubMed] [Google Scholar]
- 359.Poon E, Howman EV, Newey SE, Davies KE. Association of syncoilin and desmin: linking intermediate filament proteins to the dystrophin-associated protein complex. J Biol Chem 277: 3433–3439, 2002. [DOI] [PubMed] [Google Scholar]
- 360.Porter GA, Dmytrenko GM, Winkelmann JC, Bloch RJ. Dystrophin colocalizes with beta-spectrin in distinct subsarcolemmal domains in mammalian skeletal muscle. J Cell Biol 117: 997–1005, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Prins KW, Humston JL, Mehta A, Tate V, Ralston E, Ervasti JM. Dystrophin is a microtubule-associated protein. J Cell Biol 186: 363–369, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Prins KW, Lowe DA, Ervasti JM. Skeletal muscle-specific ablation of gamma(cyto)-actin does not exacerbate the mdx phenotype. PLoS One 3: e2419, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Prior TW, Bridgeman SJ. Experience and strategy for the molecular testing of Duchenne muscular dystrophy. J Mol Diagn 7: 317–326, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333: 1440–1445, 2011. [DOI] [PubMed] [Google Scholar]
- 365.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium 7: 1–12, 1986. [DOI] [PubMed] [Google Scholar]
- 366.Quinlan JG, Wong BL, Niemeier RT, McCullough AS, Levin L, Emanuele M. Poloxamer 188 failed to prevent exercise-induced membrane breakdown in mdx skeletal muscle fibers. Neuromuscular Disorders 16: 855–864, 2006. [DOI] [PubMed] [Google Scholar]
- 367.Rafii MS, Hagiwara H, Mercado ML, Seo NS, Xu T, Dugan T, Owens RT, Hook M, McQuillan DJ, Young MF, Fallon JR. Biglycan binds to alpha- and gamma-sarcoglycan and regulates their expression during development. J Cell Physiol 209: 439–447, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Raith M, Valencia RG, Fischer I, Orthofer M, Penninger JM, Spuler S, Rezniczek GA, Wiche G. Linking cytoarchitecture to metabolism: sarcolemma-associated plectin affects glucose uptake by destabilizing microtubule networks in mdx myofibers. Skeletal Muscle 3: 14, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Rall S, Grimm T. Survival in Duchenne muscular dystrophy. Acta Myol 31: 117–120, 2012. [PMC free article] [PubMed] [Google Scholar]
- 370.Ralston E, Ploug T. Caveolin-3 is associated with the T-tubules of mature skeletal muscle fibers. Exp Cell Res 246: 510–515, 1999. [DOI] [PubMed] [Google Scholar]
- 371.Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 589: 1195–1208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Rando TA. Role of nitric oxide in the pathogenesis of muscular dystrophies: a “two hit” hypothesis of the cause of muscle necrosis. Microsc Res Tech 55: 223–235, 2001. [DOI] [PubMed] [Google Scholar]
- 373.Rando TA. Oxidative stress and the pathogenesis of muscular dystrophies. Am J Phys Med Rehabil 81: S175–S186, 2002. [DOI] [PubMed] [Google Scholar]
- 374.Rando TA, Disatnik MH, Yu Y, Franco A. Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromuscular Disorders 8: 14–21, 1998. [DOI] [PubMed] [Google Scholar]
- 375.Rayavarapu S, Coley W, Cakir E, Jahnke V, Takeda S, Aoki Y, Grodish-Dressman H, Jaiswal JK, Hoffman EP, Brown KJ, Hathout Y, Nagaraju K. Identification of disease specific pathways using in vivo SILAC proteomics in dystrophin deficient mdx mouse. Mol Cell Proteomics 12: 1061–1073, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Reay DP, Yang M, Watchko JF, Daood M, O'Day TL, Rehman KK, Guttridge DC, Robbins PD, Clemens PR. Systemic delivery of NEMO binding domain/IKKgamma inhibitory peptide to young mdx mice improves dystrophic skeletal muscle histopathology. Neurobiol Dis 43: 598–608, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 106: 157–169, 2001. [DOI] [PubMed] [Google Scholar]
- 378.Reid MB. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol 90: 724–731, 2001. [DOI] [PubMed] [Google Scholar]
- 379.Renjini R, Gayathri N, Nalini A, Srinivas Bharath MM. Oxidative damage in muscular dystrophy correlates with the severity of the pathology: role of glutathione metabolism. Neurochem Res 37: 885–898, 2012. [DOI] [PubMed] [Google Scholar]
- 380.Repetto S, Bado M, Broda P, Lucania G, Masetti E, Sotgia F, Carbone I, Pavan A, Bonilla E, Cordone G, Lisanti MP, Minetti C. Increased number of caveolae and caveolin-3 overexpression in Duchenne muscular dystrophy. Biochem Biophys Res Commun 261: 547–550, 1999. [DOI] [PubMed] [Google Scholar]
- 381.Rezniczek GA, Konieczny P, Nikolic B, Reipert S, Schneller D, Abrahamsberg C, Davies KE, Winder SJ, Wiche G. Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with beta-dystroglycan. J Cell Biol 176: 965–977, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudaut C. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 81: 27–40, 1995. [DOI] [PubMed] [Google Scholar]
- 383.Riefler GM, Firestein BL. Binding of neuronal nitric-oxide synthase (nNOS) to carboxyl-terminal-binding protein (CtBP) changes the localization of CtBP from the nucleus to the cytosol: a novel function for targeting by the PDZ domain of nNOS. J Biol Chem 276: 48262–48268, 2001. [DOI] [PubMed] [Google Scholar]
- 384.Rios E. The cell boundary theorem: a simple law of the control of cytosolic calcium concentration. J Physiol Sci 60: 81–84, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Roberts RG, Coffey AJ, Bobrow M, Bentley DR. Exon structure of the human dystrophin gene. Genomics 16: 536–538, 1993. [DOI] [PubMed] [Google Scholar]
- 386.Robin G, Berthier C, Allard B. Sarcoplasmic reticulum Ca2+ permeation explored from the lumen side in mdx muscle fibers under voltage control. J Gen Physiol 139: 209–218, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Rodino-Klapac LR, Mendell JR, Sahenk Z. Update on the treatment of Duchenne muscular dystrophy. Curr Neurol Neurosci Rep 13: 332, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Rooney JE, Gurpur PB, Burkin DJ. Laminin-111 protein therapy prevents muscle disease in the mdx mouse model for Duchenne muscular dystrophy. Proc Natl Acad Sci USA 106: 7991–7996, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and alpha7 integrin. J Cell Sci 119: 2185–2195, 2006. [DOI] [PubMed] [Google Scholar]
- 390.Ruegg UT. Pharmacological prospects in the treatment of Duchenne muscular dystrophy. Curr Opin Neurol 26: 577–584, 2013. [DOI] [PubMed] [Google Scholar]
- 391.Russell MA, Lund LM, Haber R, McKeegan K, Cianciola N, Bond M. The intermediate filament protein, synemin, is an AKAP in the heart. Arch Biochem Biophys 456: 204–215, 2006. [DOI] [PubMed] [Google Scholar]
- 392.Rybakova IN, Ervasti JM. Dystrophin-glycoprotein complex is monomeric and stabilizes actin filaments in vitro through a lateral association. J Biol Chem 272: 28771–28778, 1997. [DOI] [PubMed] [Google Scholar]
- 393.Sabourin J, Cognard C, Constantin B. Regulation by scaffolding proteins of canonical transient receptor potential channels in striated muscle. J Muscle Res Cell Motil 30: 289–297, 2009. [DOI] [PubMed] [Google Scholar]
- 394.Sabourin J, Lamiche C, Vandebrouck A, Magaud C, Rivet J, Cognard C, Bourmeyster N, Constantin B. Regulation of TRPC1 and TRPC4 cation channels requires an alpha1-syntrophin-dependent complex in skeletal mouse myotubes. J Biol Chem 284: 36248–36261, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, Blau HM. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 143: 1059–1071, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18: 603–621, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Salanova M, Schiffl G, Rittweger J, Felsenberg D, Blottner D. Ryanodine receptor type-1 (RyR1) expression and protein S-nitrosylation pattern in human soleus myofibres following bed rest and exercise countermeasure. Histochem Cell Biol 130: 105–118, 2008. [DOI] [PubMed] [Google Scholar]
- 398.Salminen A, Kainulainen H, Arstila AU, Vihko V. Vitamin E deficiency and the susceptibility to lipid peroxidation of mouse cardiac and skeletal muscles. Acta Physiol Scand 122: 565–570, 1984. [DOI] [PubMed] [Google Scholar]
- 399.Saltin B. Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol 583: 819–823, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta 1830: 4117–4129, 2013. [DOI] [PubMed] [Google Scholar]
- 401.Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA 97: 13818–13823, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Sandona D, Betto R. Sarcoglycanopathies: molecular pathogenesis and therapeutic prospects. Expert Rev Mol Med 11: e28, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Sato O, Nonomura Y, Kimura S, Maruyama K. Molecular shape of dystrophin. J Biochem 112: 631–636, 1992. [DOI] [PubMed] [Google Scholar]
- 404.Sato Y, Sagami I, Shimizu T. Identification of caveolin-1-interacting sites in neuronal nitric-oxide synthase. Molecular mechanism for inhibition of NO formation. J Biol Chem 279: 8827–8836, 2004. [DOI] [PubMed] [Google Scholar]
- 405.Schessl J, Zou Y, Bonnemann CG. Congenital muscular dystrophies and the extracellular matrix. Semin Pediatr Neurol 13: 80–89, 2006. [DOI] [PubMed] [Google Scholar]
- 406.Schlormann W, Steiniger F, Richter W, Kaufmann R, Hause G, Lemke C, Westermann M. The shape of caveolae is omega-like after glutaraldehyde fixation and cup-like after cryofixation. Histochem Cell Biol 133: 223–228, 2010. [DOI] [PubMed] [Google Scholar]
- 407.Schmidt N, Akaaboune M, Gajendran N, Martinez P, Wakefield S, Thurnheer R, Brenner HR. Neuregulin/ErbB regulate neuromuscular junction development by phosphorylation of alpha-dystrobrevin. J Cell Biol 195: 1171–1184, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Schubert S, Knoch KP, Ouwendijk J, Mohammed S, Bodrov Y, Jager M, Altkruger A, Wegbrod C, Adams ME, Kim Y, Froehner SC, Jensen ON, Kalaidzidis Y, Solimena M. Beta2-Syntrophin is a Cdk5 substrate that restrains the motility of insulin secretory granules. PLoS One 5: e12929, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Segawa M, Fukada S, Yamamoto Y, Yahagi H, Kanematsu M, Sato M, Ito T, Uezumi A, Hayashi S, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res 314: 3232–3244, 2008. [DOI] [PubMed] [Google Scholar]
- 410.Selsby J, Pendrak K, Zadel M, Tian Z, Pham J, Carver T, Acosta P, Barton E, Sweeney HL. Leupeptin-based inhibitors do not improve the mdx phenotype. Am J Physiol Regul Integr Comp Physiol 299: R1192–R1201, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Selsby JT. Increased catalase expression improves muscle function in mdx mice. Exp Physiol 96: 194–202, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Serrano AL, Mann CJ, Vidal B, Ardite E, Perdiguero E, Munoz-Canoves P. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr Top Dev Biol 96: 167–201, 2011. [DOI] [PubMed] [Google Scholar]
- 414.Serrano AL, Munoz-Canoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316: 3050–3058, 2010. [DOI] [PubMed] [Google Scholar]
- 415.Servitja JM, Marinissen MJ, Sodhi A, Bustelo XR, Gutkind JS. Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J Biol Chem 278: 34339–34346, 2003. [DOI] [PubMed] [Google Scholar]
- 416.Sevanian A, Kim E. Phospholipase A2 dependent release of fatty acids from peroxidized membranes. J Free Radic Biol Med 1: 263–271, 1985. [DOI] [PubMed] [Google Scholar]
- 417.Sevanian A, Wratten ML, McLeod LL, Kim E. Lipid peroxidation and phospholipase A2 activity in liposomes composed of unsaturated phospholipids: a structural basis for enzyme activation. Biochim Biophys Acta 961: 316–327, 1988. [DOI] [PubMed] [Google Scholar]
- 418.Shkryl VM, Martins AS, Ullrich ND, Nowycky MC, Niggli E, Shirokova N. Reciprocal amplification of ROS and Ca2+ signals in stressed mdx dystrophic skeletal muscle fibers. Pflügers Arch 458: 915–928, 2009. [DOI] [PubMed] [Google Scholar]
- 419.Silvagno F, Xia H, Bredt DS. Neuronal nitric-oxide synthase-mu, an alternatively spliced isoform expressed in differentiated skeletal muscle. J Biol Chem 271: 11204–11208, 1996. [DOI] [PubMed] [Google Scholar]
- 420.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 271: 15160–15165, 1996. [DOI] [PubMed] [Google Scholar]
- 421.Sonnemann KJ, Fitzsimons DP, Patel JR, Liu Y, Schneider MF, Moss RL, Ervasti JM. Cytoplasmic gamma-actin is not required for skeletal muscle development but its absence leads to a progressive myopathy. Dev Cell 11: 387–397, 2006. [DOI] [PubMed] [Google Scholar]
- 422.Sorimachi H, Ono Y, Suzuki K. Skeletal muscle-specific calpain, p94, and connectin/titin: their physiological functions and relationship to limb-girdle muscular dystrophy type 2A. Adv Exp Med Biol 481: 383–395, 2000. [DOI] [PubMed] [Google Scholar]
- 423.Sotgia F, Bonuccelli G, Bedford M, Brancaccio A, Mayer U, Wilson MT, Campos-Gonzalez R, Brooks JW, Sudol M, Lisanti MP. Localization of phospho-beta-dystroglycan (pY892) to an intracellular vesicular compartment in cultured cells and skeletal muscle fibers in vivo. Biochemistry 42: 7110–7123, 2003. [DOI] [PubMed] [Google Scholar]
- 424.Sotgia F, Lee H, Bedford MT, Petrucci T, Sudol M, Lisanti MP. Tyrosine phosphorylation of beta-dystroglycan at its WW domain binding motif, PPxY, recruits SH2 domain containing proteins. Biochemistry 40: 14585–14592, 2001. [DOI] [PubMed] [Google Scholar]
- 425.Sotgia F, Lee JK, Das K, Bedford M, Petrucci TC, Macioce P, Sargiacomo M, Bricarelli FD, Minetti C, Sudol M, Lisanti MP. Caveolin-3 directly interacts with the C-terminal tail of beta-dystroglycan. Identification of a central WW-like domain within caveolin family members. J Biol Chem 275: 38048–38058, 2000. [DOI] [PubMed] [Google Scholar]
- 426.Spencer MJ, Mellgren RL. Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum Mol Genet 11: 2645–2655, 2002. [DOI] [PubMed] [Google Scholar]
- 427.Spencer MJ, Tidball JG. Calpain concentration is elevated although net calcium-dependent proteolysis is suppressed in dystrophin-deficient muscle. Exp Cell Res 203: 107–114, 1992. [DOI] [PubMed] [Google Scholar]
- 428.Spurney C, Shimizu R, Hache LP, Kolski H, Gordish-Dressman H, Clemens PR. CINRG Duchenne Natural History Study demonstrates insufficient diagnosis and treatment of cardiomyopathy in Duchenne muscular dystrophy. Muscle Nerve 50: 250–256, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Squire S, Raymackers JM, Vandebrouck C, Potter A, Tinsley J, Fisher R, Gillis JM, Davies KE. Prevention of pathology in mdx mice by expression of utrophin: analysis using an inducible transgenic expression system. Hum Mol Genet 11: 3333–3344, 2002. [DOI] [PubMed] [Google Scholar]
- 430.St Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002. [DOI] [PubMed] [Google Scholar]
- 431.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001. [DOI] [PubMed] [Google Scholar]
- 432.Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352: 536–539, 1991. [DOI] [PubMed] [Google Scholar]
- 433.Steinberger M, Foller M, Vogelgesang S, Krautwald M, Landsberger M, Winkler CK, Kasch J, Fuchtbauer EM, Kuhl D, Voelkl J, Lang F, Brinkmeier H. Lack of the serum- and glucocorticoid-inducible kinase SGK1 improves muscle force characteristics and attenuates fibrosis in dystrophic mdx mouse muscle. Pflügers Arch 467: 1965–1974, 2015. [DOI] [PubMed] [Google Scholar]
- 434.Stern LZ, Ringel SP, Ziter FA, Menander-Huber KB, Ionasescu V, Pellegrino RJ, Snyder RD. Drug trial of superoxide dismutase in Duchenne's muscular dystrophy. Arch Neurol 39: 342–346, 1982. [DOI] [PubMed] [Google Scholar]
- 435.Stiber JA, Rosenberg PB. The role of store-operated calcium influx in skeletal muscle signaling. Cell Calcium 49: 341–349, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Stiber JA, Zhang ZS, Burch J, Eu JP, Zhang S, Truskey GA, Seth M, Yamaguchi N, Meissner G, Shah R, Worley PF, Williams RS, Rosenberg PB. Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol Cell Biol 28: 2637–2647, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Stone MR, O'Neill A, Catino D, Bloch RJ. Specific interaction of the actin-binding domain of dystrophin with intermediate filaments containing keratin 19. Mol Biol Cell 16: 4280–4293, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Stone MR, O'Neill A, Lovering RM, Strong J, Resneck WG, Reed PW, Toivola DM, Ursitti JA, Omary MB, Bloch RJ. Absence of keratin 19 in mice causes skeletal myopathy with mitochondrial and sarcolemmal reorganization. J Cell Sci 120: 3999–4008, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol 114: 346–364, 1983. [DOI] [PubMed] [Google Scholar]
- 440.Stroissnigg H, Trancikova A, Descovich L, Fuhrmann J, Kutschera W, Kostan J, Meixner A, Nothias F, Propst F. S-nitrosylation of microtubule-associated protein 1B mediates nitric-oxide-induced axon retraction. Nat Cell Biol 9: 1035–1045, 2007. [DOI] [PubMed] [Google Scholar]
- 441.Suchyna TM, Sachs F. Mechanosensitive channel properties and membrane mechanics in mouse dystrophic myotubes. J Physiol 581: 369–387, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Suhr F, Gehlert S, Grau M, Bloch W. Skeletal muscle function during exercise-fine-tuning of diverse subsystems by nitric oxide. Int J Mol Sci 14: 7109–7139, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc Natl Acad Sci USA 108: 16098–16103, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Suzuki A, Yoshida M, Ozawa E. Mammalian alpha 1- and beta 1-syntrophin bind to the alternative splice-prone region of the dystrophin COOH terminus. J Cell Biol 128: 373–381, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Suzuki N, Motohashi N, Uezumi A, Fukada S, Yoshimura T, Itoyama Y, Aoki M, Miyagoe-Suzuki Y, Takeda S. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest 117: 2468–2476, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Swiderski K, Shaffer SA, Gallis B, Odom GL, Arnett AL, Scott EJ, Baum DM, Chee A, Naim T, Gregorevic P, Murphy KT, Moody J, Goodlett DR, Lynch GS, Chamberlain JS. Phosphorylation within the cysteine-rich region of dystrophin enhances its association with beta-dystroglycan and identifies a potential novel therapeutic target for skeletal muscle wasting. Hum Mol Genet 23: 6697–6711, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Tahallah N, Brunelle A, De La Porte S, Laprevote O. Lipid mapping in human dystrophic muscle by cluster-time-of-flight secondary ion mass spectrometry imaging. J Lipid Res 49: 438–454, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Takagi A, Kojima S, Ida M, Araki M. Increased leakage of calcium ion from the sarcoplasmic reticulum of the mdx mouse. J Neurol Sci 110: 160–164, 1992. [DOI] [PubMed] [Google Scholar]
- 449.Takahashi J, Itoh Y, Fujimori K, Imamura M, Wakayama Y, Miyagoe-Suzuki Y, Takeda S. The utrophin promoter A drives high expression of the transgenic LacZ gene in liver, testis, colon, submandibular gland, and small intestine. J Gene Med 7: 237–248, 2005. [DOI] [PubMed] [Google Scholar]
- 450.Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA 95: 15090–15095, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires alpha-syntrophin-mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ Res 92: 554–560, 2003. [DOI] [PubMed] [Google Scholar]
- 452.Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Thompson O, Kleino I, Crimaldi L, Gimona M, Saksela K, Winder SJ. Dystroglycan, Tks5 and Src mediated assembly of podosomes in myoblasts. PLoS One 3: e3638, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Thompson TG, Chan YM, Hack AA, Brosius M, Rajala M, Lidov HG, McNally EM, Watkins S, Kunkel LM. Filamin 2 (FLN2): a muscle-specific sarcoglycan interacting protein. J Cell Biol 148: 115–126, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Tidball JG, Wehling-Henricks M. Expression of a NOS transgene in dystrophin-deficient muscle reduces muscle membrane damage without increasing the expression of membrane-associated cytoskeletal proteins. Mol Genet Metab 82: 312–320, 2004. [DOI] [PubMed] [Google Scholar]
- 456.Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, Davies K. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med 4: 1441–1444, 1998. [DOI] [PubMed] [Google Scholar]
- 457.Tinsley JM, Fairclough RJ, Storer R, Wilkes FJ, Potter AC, Squire SE, Powell DS, Cozzoli A, Capogrosso RF, Lambert A, Wilson FX, Wren SP, De LA, Davies KE. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS One 6: e19189, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Tochio H, Ohki S, Zhang Q, Li M, Zhang M. Solution structure of a protein inhibitor of neuronal nitric oxide synthase. Nat Struct Biol 5: 965–969, 1998. [DOI] [PubMed] [Google Scholar]
- 459.Tomasi LG. Reversibility of human myopathy caused by vitamin E deficiency. Neurology 29: 1182–1186, 1979. [DOI] [PubMed] [Google Scholar]
- 460.Tonon E, Ferretti R, Shiratori JH, Santo NH, Marques MJ, Minatel E. Ascorbic acid protects the diaphragm muscle against myonecrosis in mdx mice. Nutrition 28: 686–690, 2012. [DOI] [PubMed] [Google Scholar]
- 461.Torelli S, Brown SC, Jimenez-Mallebrera C, Feng L, Muntoni F, Sewry CA. Absence of neuronal nitric oxide synthase (nNOS) as a pathological marker for the diagnosis of Becker muscular dystrophy with rod domain deletions. Neuropathol Appl Neurobiol 30: 540–545, 2004. [DOI] [PubMed] [Google Scholar]
- 462.Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 23: 981–987, 2003. [DOI] [PubMed] [Google Scholar]
- 463.Townsend D, Yasuda S, Li S, Chamberlain JS, Metzger JM. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther 16: 832–835, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Turgeman T, Hagai Y, Huebner K, Jassal DS, Anderson JE, Genin O, Nagler A, Halevy O, Pines M. Prevention of muscle fibrosis and improvement in muscle performance in the mdx mouse by halofuginone. Neuromuscular Disorders 18: 857–868, 2008. [DOI] [PubMed] [Google Scholar]
- 465.Turner PR, Westwood T, Regen CM, Steinhardt RA. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335: 735–738, 1988. [DOI] [PubMed] [Google Scholar]
- 466.Tutdibi O, Brinkmeier H, Rudel R, Fohr KJ. Increased calcium entry into dystrophin-deficient muscle fibres of MDX and ADR-MDX mice is reduced by ion channel blockers. J Physiol 515: 859–868, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, Ackerman MJ, Makielski JC. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci USA 105: 9355–9360, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Vaghy PL, Fang J, Wu W, Vaghy LP. Increased caveolin-3 levels in mdx mouse muscles. FEBS Lett 431: 125–127, 1998. [DOI] [PubMed] [Google Scholar]
- 469.Valentine BA, Cooper BJ, de LA, O'Quinn R, Blue JT. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: clinical studies. J Neurol Sci 88: 69–81, 1988. [DOI] [PubMed] [Google Scholar]
- 470.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007. [DOI] [PubMed] [Google Scholar]
- 471.Vallejo-Illarramendi A, Toral-Ojeda I, Aldanondo G, Lopez de MA. Dysregulation of calcium homeostasis in muscular dystrophies. Expert Rev Mol Med 16: e16, 2014. [DOI] [PubMed] [Google Scholar]
- 472.Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, Kitzis A, Raymond G, Cognard C, Bourmeyster N, Constantin B. Regulation of capacitative calcium entries by alpha1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of alpha1-syntrophin. FASEB J 21: 608–617, 2007. [DOI] [PubMed] [Google Scholar]
- 473.Vandebrouck C, Duport G, Cognard C, Raymond G. Cationic channels in normal and dystrophic human myotubes. Neuromuscul Disord 11: 72–79, 2001. [DOI] [PubMed] [Google Scholar]
- 474.Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol 158: 1089–1096, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Venema VJ, Ju H, Zou R, Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem 272: 28187–28190, 1997. [DOI] [PubMed] [Google Scholar]
- 476.Verburg E, Murphy RM, Richard I, Lamb GD. Involvement of calpains in Ca2+-induced disruption of excitation-contraction coupling in mammalian skeletal muscle fibers. Am J Physiol Cell Physiol 296: C1115–C1122, 2009. [DOI] [PubMed] [Google Scholar]
- 477.Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, Miceli MC, Spencer MJ. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J Clin Invest 119: 1583–1594, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Vidal B, Ardite E, Suelves M, Ruiz-Bonilla V, Janue A, Flick MJ, Degen JL, Serrano AL, Munoz-Canoves P. Amelioration of Duchenne muscular dystrophy in mdx mice by elimination of matrix-associated fibrin-driven inflammation coupled to the alphaMbeta2 leukocyte integrin receptor. Hum Mol Genet 21: 1989–2004, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Vidal B, Serrano AL, Tjwa M, Suelves M, Ardite E, De MR, Baeza-Raja B, Martinez de LM, Lafuste P, Ruiz-Bonilla V, Jardi M, Gherardi R, Christov C, Dierssen M, Carmeliet P, Degen JL, Dewerchin M, Munoz-Canoves P. Fibrinogen drives dystrophic muscle fibrosis via a TGFbeta/alternative macrophage activation pathway. Genes Dev 22: 1747–1752, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Vilhardt F, van Deurs B. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J 23: 739–748, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Voet NB, Geurts AC, Bleijenberg G, Zwarts MJ, Padberg GW, Van Engelen BG. Muscle fatigue in muscular dystophies. In: Human Muscle Fatigue, edited by Williams CA, Ratel S. London: Routledge, 2009. [Google Scholar]
- 482.Vorgerd M, Karitzky J, Ristow M, Van SE, Tegenthoff M, Jerusalem F, Malin JP. Muscle phosphofructokinase deficiency in two generations. J Neurol Sci 141: 95–99, 1996. [DOI] [PubMed] [Google Scholar]
- 483.Wagner KR, Cohen JB, Huganir RL. The 87K postsynaptic membrane protein from Torpedo is a protein-tyrosine kinase substrate homologous to dystrophin. Neuron 10: 511–522, 1993. [DOI] [PubMed] [Google Scholar]
- 484.Wagner KR, Huganir RL. Tyrosine and serine phosphorylation of dystrophin and the 58-kDa protein in the postsynaptic membrane of Torpedo electric organ. J Neurochem 62: 1947–1952, 1994. [DOI] [PubMed] [Google Scholar]
- 485.Wakayama Y, Shibuya S, Jimi T, Takeda A, Oniki H. Size and localization of dystrophin molecule: immunoelectron microscopic and freeze etching studies of muscle plasma membranes of murine skeletal myofibers. Acta Neuropathol 86: 567–577, 1993. [DOI] [PubMed] [Google Scholar]
- 486.Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol 71: 37–57, 2009. [DOI] [PubMed] [Google Scholar]
- 487.Wang X, Weisleder N, Collet C, Zhou J, Chu Y, Hirata Y, Zhao X, Pan Z, Brotto M, Cheng H, Ma J. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol 7: 525–530, 2005. [DOI] [PubMed] [Google Scholar]
- 488.Watchko J, O'Day T, Wang B, Zhou L, Tang Y, Li J, Xiao X. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum Gene Ther 13: 1451–1460, 2002. [DOI] [PubMed] [Google Scholar]
- 489.Watchko JF, O'Day TL, Hoffman EP. Functional characteristics of dystrophic skeletal muscle: insights from animal models. J Appl Physiol 93: 407–417, 2002. [DOI] [PubMed] [Google Scholar]
- 490.Watkins SC, Cullen MJ. Histochemical fibre typing and ultrastructure of the small fibres in Duchenne muscular dystrophy. Neuropathol Appl Neurobiol 11: 447–460, 1985. [DOI] [PubMed] [Google Scholar]
- 491.Watkins SC, Cullen MJ. A quantitative comparison of satellite cell ultrastructure in Duchenne muscular dystrophy, polymyositis, and normal controls. Muscle Nerve 9: 724–730, 1986. [DOI] [PubMed] [Google Scholar]
- 492.Watkins SC, Hoffman EP, Slayter HS, Kunkel LM. Immunoelectron microscopic localization of dystrophin in myofibres. Nature 333: 863–866, 1988. [DOI] [PubMed] [Google Scholar]
- 493.Webster C, Blau HM. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet 16: 557–565, 1990. [DOI] [PubMed] [Google Scholar]
- 494.Webster C, Silberstein L, Hays AP, Blau HM. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 52: 503–513, 1988. [DOI] [PubMed] [Google Scholar]
- 495.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol 155: 123–131, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Wehling-Henricks M, Oltmann M, Rinaldi C, Myung KH, Tidball JG. Loss of positive allosteric interactions between neuronal nitric oxide synthase and phosphofructokinase contributes to defects in glycolysis and increased fatigability in muscular dystrophy. Hum Mol Genet 18: 3439–3451, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Wehling-Henricks M, Tidball JG. Neuronal nitric oxide synthase-rescue of dystrophin/utrophin double knockout mice does not require nNOS localization to the cell membrane. PLoS ONE 6: e25071, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen PJ, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao RP, Ma J. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med 4: 139ra85, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, Paushkin S, Patel M, Trotta CR, Hwang S, Wilde RG, Karp G, Takasugi J, Chen G, Jones S, Ren H, Moon YC, Corson D, Turpoff AA, Campbell JA, Conn MM, Khan A, Almstead NG, Hedrick J, Mollin A, Risher N, Weetall M, Yeh S, Branstrom AA, Colacino JM, Babiak J, Ju WD, Hirawat S, Northcutt VJ, Miller LL, Spatrick P, He F, Kawana M, Feng H, Jacobson A, Peltz SW, Sweeney HL. PTC124 targets genetic disorders caused by nonsense mutations. Nature 447: 87–91, 2007. [DOI] [PubMed] [Google Scholar]
- 500.Wells KE, Torelli S, Lu Q, Brown SC, Partridge T, Muntoni F, Wells DJ. Relocalization of neuronal nitric oxide synthase (nNOS) as a marker for complete restoration of the dystrophin associated protein complex in skeletal muscle. Neuromuscular Disorders 13: 21–31, 2003. [DOI] [PubMed] [Google Scholar]
- 501.Wells L. The o-mannosylation pathway: glycosyltransferases and proteins implicated in congenital muscular dystrophy. J Biol Chem 288: 6930–6935, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol 75: 382–388, 1993. [DOI] [PubMed] [Google Scholar]
- 503.White AT, Schenk S. NAD+/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab 303: E308–E321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Whitehead NP, Pham C, Gervasio OL, Allen DG. N-acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol 586: 2003–2014, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscular Disorders 16: 845–854, 2006. [DOI] [PubMed] [Google Scholar]
- 506.Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol 33: 657–662, 2006. [DOI] [PubMed] [Google Scholar]
- 507.Whitehead NP, Yeung EW, Froehner SC, Allen DG. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced muscle damage in the mdx mouse. PLoS One 5: e15354, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Wickstrom SA, Lange A, Hess MW, Polleux J, Spatz JP, Kruger M, Pfaller K, Lambacher A, Bloch W, Mann M, Huber LA, Fassler R. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell 19: 574–588, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Williams DA, Head SI, Lynch GS, Stephenson DG. Contractile properties of skinned muscle fibres from young and adult normal and dystrophic (mdx) mice. J Physiol 460: 51–67, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Williams IA, Allen DG. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol 293: H1969–H1977, 2007. [DOI] [PubMed] [Google Scholar]
- 511.Williams JJ, Palmer TM. Cavin-1: caveolae-dependent signalling and cardiovascular disease. Biochem Soc Trans 42: 284–288, 2014. [DOI] [PubMed] [Google Scholar]
- 512.Williams MW, Bloch RJ. Differential distribution of dystrophin and beta-spectrin at the sarcolemma of fast twitch skeletal muscle fibers. J Muscle Res Cell Motil 20: 383–393, 1999. [DOI] [PubMed] [Google Scholar]
- 513.Winter L, Wiche G. The many faces of plectin and plectinopathies: pathology and mechanisms. Acta Neuropathol 125: 77–93, 2013. [DOI] [PubMed] [Google Scholar]
- 514.Woods CE, Novo D, DiFranco M, Capote J, Vergara JL. Propagation in the transverse tubular system and voltage dependence of calcium release in normal and mdx mouse muscle fibres. J Physiol 568: 867–880, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Woods CE, Novo D, DiFranco M, Vergara JL. The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibres. J Physiol 557: 59–75, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Worton RG, Duff C, Sylvester JE, Schmickel RD, Willard HF. Duchenne muscular dystrophy involving translocation of the dmd gene next to ribosomal RNA genes. Science 224: 1447–1449, 1984. [DOI] [PubMed] [Google Scholar]
- 517.Wu G, Ai T, Kim JJ, Mohapatra B, Xi Y, Li Z, Abbasi S, Purevjav E, Samani K, Ackerman MJ, Qi M, Moss AJ, Shimizu W, Towbin JA, Cheng J, Vatta M. Alpha-1-syntrophin mutation and the long-QT syndrome: a disease of sodium channel disruption. Circ Arrhythm Electrophysiol 1: 193–201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Xiong Y, Zhou Y, Jarrett HW. Dystrophin glycoprotein complex-associated Gbetagamma subunits activate phosphatidylinositol-3-kinase/Akt signaling in skeletal muscle in a laminin-dependent manner. J Cell Physiol 219: 402–414, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Yamada T, Fedotovskaya O, Cheng AJ, Cornachione AS, Minozzo FC, Aulin C, Friden C, Turesson C, Andersson DC, Glenmark B, Lundberg IE, Rassier DE, Westerblad H, Lanner JT. Nitrosative modifications of the Ca2+ release complex and actin underlie arthritis-induced muscle weakness. Ann Rheum Dis 74: 1907–1914, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Yamashita K, Suzuki A, Satoh Y, Ide M, Amano Y, Masuda-Hirata M, Hayashi YK, Hamada K, Ogata K, Ohno S. The 8th and 9th tandem spectrin-like repeats of utrophin cooperatively form a functional unit to interact with polarity-regulating kinase PAR-1b. Biochem Biophys Res Commun 391: 812–817, 2010. [DOI] [PubMed] [Google Scholar]
- 521.Yang B, Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol 292: H954–H962, 2007. [DOI] [PubMed] [Google Scholar]
- 522.Yano R, Yap CC, Yamazaki Y, Muto Y, Kishida H, Okada D, Hashikawa T. Sast124, a novel splice variant of syntrophin-associated serine/threonine kinase (SAST), is specifically localized in the restricted brain regions. Neuroscience 117: 373–381, 2003. [DOI] [PubMed] [Google Scholar]
- 523.Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol 562: 367–380, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Yoshida M, Hama H, Ishikawa-Sakurai M, Imamura M, Mizuno Y, Araishi K, Wakabayashi-Takai E, Noguchi S, Sasaoka T, Ozawa E. Biochemical evidence for association of dystrobrevin with the sarcoglycan-sarcospan complex as a basis for understanding sarcoglycanopathy. Hum Mol Genet 9: 1033–1040, 2000. [DOI] [PubMed] [Google Scholar]
- 525.Young MF, Fallon JR. Biglycan: a promising new therapeutic for neuromuscular and musculoskeletal diseases. Curr Opin Genet Dev 22: 398–400, 2012. [DOI] [PubMed] [Google Scholar]
- 526.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114: 777–789, 2003. [DOI] [PubMed] [Google Scholar]
- 527.Yun BW, Feechan A, Yin M, Saidi NB, Le BT, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, Pallas JA, Loake GJ. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478: 264–268, 2011. [DOI] [PubMed] [Google Scholar]
- 528.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 3: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Zanou N, Shapovalov G, Louis M, Tajeddine N, Gallo C, Van SM, Anguish I, Cao ML, Schakman O, Dietrich A, Lebacq J, Ruegg U, Roulet E, Birnbaumer L, Gailly P. Role of TRPC1 channel in skeletal muscle function. Am J Physiol Cell Physiol 298: C149–C162, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Zatz M, Betti RT, Levy JA. Benign Duchenne muscular dystrophy in a patient with growth hormone deficiency. Am J Med Genet 10: 301–304, 1981. [DOI] [PubMed] [Google Scholar]
- 531.Zatz M, Rapaport D, Vainzof M, Passos-Bueno MR, Bortolini ER, Pavanello RC, Peres CA. Serum creatine-kinase (CK) and pyruvate-kinase (PK) activities in Duchenne (DMD) as compared with Becker (BMD) muscular dystrophy. J Neurol Sci 102: 190–196, 1991. [DOI] [PubMed] [Google Scholar]
- 532.Zatz M, Rapaport D, Vainzof M, Rocha JM, Pavanello RC, Colletto GM, Peres CA. Relation between height and clinical course in Duchenne muscular dystrophy. Am J Med Genet 29: 405–410, 1988. [DOI] [PubMed] [Google Scholar]
- 533.Zhan Y, Tremblay MR, Melian N, Carbonetto S. Evidence that dystroglycan is associated with dynamin and regulates endocytosis. J Biol Chem 280: 18015–18024, 2005. [DOI] [PubMed] [Google Scholar]
- 534.Zhang BT, Whitehead NP, Gervasio OL, Reardon TF, Vale M, Fatkin D, Dietrich A, Yeung EW, Allen DG. Pathways of Ca2+ entry and cytoskeletal damage following eccentric contractions in mouse skeletal muscle. J Appl Physiol 112: 2077–2086, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Zhao X, Moloughney JG, Zhang S, Komazaki S, Weisleder N. Orai1 mediates exacerbated Ca2+ entry in dystrophic skeletal muscle. PLoS One 7: e49862, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Zhou L, Lu H. Targeting fibrosis in Duchenne muscular dystrophy. J Neuropathol Exp Neurol 69: 771–776, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Zhou YW, Thomason DB, Gullberg D, Jarrett HW. Binding of laminin alpha1-chain LG4-5 domain to alpha-dystroglycan causes tyrosine phosphorylation of syntrophin to initiate Rac1 signaling. Biochemistry 45: 2042–2052, 2006. [DOI] [PubMed] [Google Scholar]
- 538.Zhuang W, Eby JC, Cheong M, Mohapatra PK, Bredt DS, Disatnik MH, Rando TA. The susceptibility of muscle cells to oxidative stress is independent of nitric oxide synthase expression. Muscle Nerve 24: 502–511, 2001. [DOI] [PubMed] [Google Scholar]