Abstract
It is predicted that Japan and European Union will soon experience appreciable decreases in their populations due to persistently low total fertility rates (TFR) below replacement level (2.1 child per woman). In the United States, where TFR has also declined, there are ethnic differences. Caucasians have rates below replacement, while TFRs among African-Americans and Hispanics are higher. We review possible links between TFR and trends in a range of male reproductive problems, including testicular cancer, disorders of sex development, cryptorchidism, hypospadias, low testosterone levels, poor semen quality, childlessness, changed sex ratio, and increasing demand for assisted reproductive techniques. We present evidence that several adult male reproductive problems arise in utero and are signs of testicular dysgenesis syndrome (TDS). Although TDS might result from genetic mutations, recent evidence suggests that it most often is related to environmental exposures of the fetal testis. However, environmental factors can also affect the adult endocrine system. Based on our review of genetic and environmental factors, we conclude that environmental exposures arising from modern lifestyle, rather than genetics, are the most important factors in the observed trends. These environmental factors might act either directly or via epigenetic mechanisms. In the latter case, the effects of exposures might have an impact for several generations post-exposure. In conclusion, there is an urgent need to prioritize research in reproductive physiology and pathophysiology, particularly in highly industrialized countries facing decreasing populations. We highlight a number of topics that need attention by researchers in human physiology, pathophysiology, environmental health sciences, and demography.
I. INTRODUCTION
During the 20th century, populations of industrialized countries all over the world have experienced a decline in total fertility rates (TFR, average number of live births per woman) far below 2.1, which is the rate considered necessary to sustain a population size at current numbers. At the same time a spectacular rise in testicular germ cell cancer (TGCC) has occurred in all parts of the World. In addition, other male reproductive problems, of which several are linked to testicular cancer, including disorders of spermatogenesis, are widespread and have recently been the focus for many basic and clinical research projects.
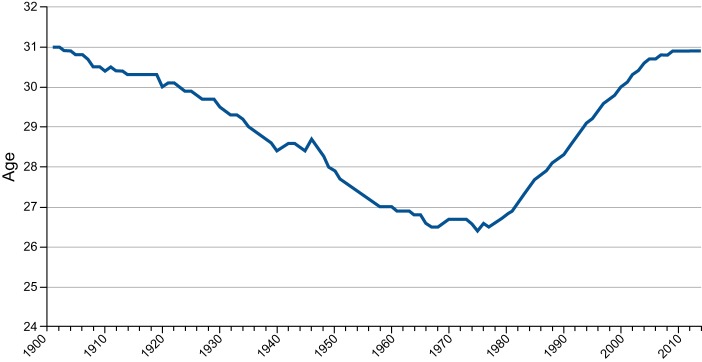
As shown in Figure 1, the total fertility rate has fallen significantly in European Union (EU), Japan, and the United States (US). Also, Hong Kong and Singapore have for decades had TFR significantly below replacement level (now between 1.0 and 1.5). Fertility has therefore become a significant political theme (122). Social and economic factors combined with the advent of effective contraceptive methods and access to induced abortions have contributed substantially to decreasing TFR, although the decline in birth rate started decades before the contraceptive pill and legal abortion were introduced (Figure 2).
Figure 1.

Total Fertility Rates (TFR), European Union, Japan and United States, 1960–2013. Dotted line represents a fertility rate of 2.1, below which a population cannot be sustained. From the World Bank: http://databank.worldbank.org/data/views/variableselection/selectvariables.aspx?source=world-development-indicators.
Figure 2.
Total Fertility Rate (TFR), Denmark 1901–2014. From Statistics Denmark: http://www.statistikbanken.dk/statbank5a/default.asp?w=1600. Dotted line represents a fertility rate of 2.1. Note that a downwards trend in TFR started long before introduction of the contraceptive pill in the 1960s. Apparently the trend was interrupted by the First and Second World Wars.
Surprisingly little is known about biological factors that might have changed human fecundity (capacity to conceive) and thereby influenced the TFR. The lack of knowledge in this area probably reflects the fact that research into human fecundity is difficult. Fecundity depends not only on a number of physiological and pathophysiological factors in both partners of a couple. Studies on rates of conceptions are also confounded by social, economic, and psychological factors that might change over time. In addition, TFR can be a poor marker of fecundity as it can be skewed by multiple factors, including induced abortion rates, availability of contraception, desire for pregnancy, and access to assisted reproduction, for example, before 1990 several Eastern European countries had higher abortion rates than birth rates. Total pregnancy rate, which includes both live births and induced abortions, might be more informative of changes in fecundity in a population than TFR (230).
The aim of this review is to analyze some global trends in male reproductive health problems and their potential effects on male fecundity as well as the possible etiological roles of environmental, epigenetic, and genetic factors for these trends. Besides testicular cancer, we shall review recent studies on infertility, semen quality, cryptorchidism, and hypospadias and how these disorders might be interrelated with testicular cancer and with each other, through a testicular dysgenesis syndrome (TDS) (Figure 3). We shall also review trends in sex ratio and the potential roles of male reproductive disorders for couple fecundity and fertility rates. Finally, we will present some urgent research needs within the field of physiology and pathophysiology of male reproduction.
Figure 3.
The hypothesis of testicular dysgenesis syndrome (TDS) and signs that might be linked to it: poor spermatogenesis, testicular cancer, hypospadias, cryptorchidism, and short ano-genital distance (AGD). The single symptoms and combinations thereof are risk factors for reduced fecundity. [Updated from Skakkebaek et al. (387).]
II. INCIDENCE TRENDS, GENETIC SUSCEPTIBILITY, AND PATHOGENESIS OF REPRODUCTIVE DISORDERS
A. Testicular Germ Cell Cancer
The strongest evidence for adverse trends in male reproductive health comes from epidemiological studies of TGCC, including both seminoma and nonseminoma (231, 481). The increase was first noted in developed countries, where incidence rates have been highest in countries with Northern European ancestry, including Denmark, Norway, and New Zealand (341). In the US, the highest incidences have been found among descendants of Europeans in the Mid- and North West (269). Interestingly, recent studies have shown that countries with previously low rates of TGCC, such as Finland, Italy, and Spain, are now catching up (Figure 4), while high incidence countries such as Denmark and Switzerland now report smaller increases or stabilization of the high rates (231, 481).
Figure 4.
Trends in testicular cancer; age-standardized (world) incidence (regional or national), all ages. [Modified from Znaor et al. (481). Courtesy of Dr. Arinana Znaor and statistician Mathieu Laversanne, M.Sc., WHO, International Agency for Research in Cancer (IARC), Lyon, France.]
1. Genetic aspects
TGCC has a strong genetic component; brothers and sons of TGCC patients have significantly higher rates of TGCC (162). Studies have shown that incidence among Caucasians is much higher than among Afro-Americans living in the same area, confirming a role of genetic disposition to TGCC (Figure 4). In line with these epidemiological studies, several recent genome-wide association studies (GWAS) have identified a number of gene variants that might predispose to TGCC. Interestingly, most of the significantly associated genes are functionally linked to gonadal development and germ cell function, although pathways more typical for any cancer, for example, chromosome aggregation, microtubule assembly, telomerase function, and DNA repair, have also been associated with TGCC risk. The strongest association has been found with the KITLG locus on 12q22 (203, 349), which encodes for the KIT receptor ligand. The KIT/KITLG signaling pathway is indispensable for germ cell migration and survival and is highly expressed in testicular germ cell neoplasia in situ (GCNIS) and malignant TGCC, except somatically differentiated nonseminomas (347, 402). The significance of KIT/KITLG pathway for the TGCC risk, both in the sporadic and familial cases, has been confirmed by subsequent studies in different populations, and by associations with genes functionally linked to this pathway, such as SPRY, BAK1, or PDE11A (32, 86, 116, 215, 233, 337).
Among other biologically interesting gene polymorphisms associated with an increased risk of TGCC, we highlight here four genes, all encoding for proteins involved in sex and germ cell development: DMRT1, PRDM14, DAZL, and HPGDS (77, 204, 215, 367, 432). DMRT1 is a transcription factor needed for sex differentiation and regulation of the onset of germ cell specific meiosis in male and female germ cells (185, 218, 265). Deletions encompassing DMRT1 and DMRT2 loci on chromosome 9p have been found in individuals with gonadoblastoma (244), a germ cell malignancy associated with disorders of sex development and gonadal dysgenesis. On the other hand, amplification of the DMRT1 locus has been detected in spermatocytic tumor, a germ cell tumor of older men not associated with GCNIS (247). PRDM14 is involved in germ cell specification and epigenetic reprogramming (469) and controls expression of the pluripotency genes POU5F1 (OCT4), and NANOG, which are expressed in GCNIS and TGCC (248, 345). Involvement in germ cell specification and embryonic meiosis regulation has also been evidenced for DAZL (206, 237). Of note for the hypothesis linking TGCC and some forms of male infertility to a TDS (see Figure 6, below), some DAZL variants and epigenetic promoter changes (301) have been associated with defective spermatogenesis, but the reports are inconsistent and might depend on the ethnic background of the studied cohort (434, 473). The inconsistent results are likely related to the confounding effect of a variable copy number of DAZ, a gene functionally related to DAZL in postmeiotic germ cells. DAZ copy number variations within partial AZFc deletions (e.g., gr/gr or b2/b3) of this gene are very common in some populations, and gr/gr deletion has been associated with both male subfertility (245, 366) and TGCC (300).
Figure 6.
Examples of testicular dysgenesis in biopsy materials from men with abnormal spermatogenesis. A: specimen showing dysgenetic seminiferous tubules (D) containing numerous undifferentiated Sertoli cells and several microliths (M), but no germ cells. Tubules containing all types of germ cells, including spermatocytes and spermatids, are seen to the right. Hematoxylin-eosin staining was used. B: i) Immunostaining with OCT4, an embryonic marker, of testicular biopsy specimen with a mixture of GCNIS and normal spermatogenesis. ii) Same, higher magnification showing details of tubules with GCNIS and normal spermatogenesis, respectively. Note that OCT4 is only expressed in the nuclei of the GCNIS cells.
The GWAS-detected association of testicular cancer risk with the HPGDS locus is biologically relevant, because this gene encodes for hematopoietic prostaglandin D synthase, an enzyme involved in sex determination in several mammalian species (284, 461). Recent experimental studies provided evidence that this pathway might be a target for endocrine disruption (267), thus giving an example of a possible gene-environment interaction relevant to the pathogenesis of TGCC.
Although a total of 19 genetic polymorphisms associated with TGCC risk have been identified to date, it appears that <25% of TGCC cases, even in the highly susceptible sons of TGCC cases, can be explained by hereditary genetic factors (240, 367). This percentage will likely increase after more genes have been identified in the ongoing large association studies and meta-analyses. Nevertheless, the large increase in sporadic TGCC cases observed worldwide within a generation or two is predominantly caused by an augmented negative influence of yet to be identified environmental factors.
2. Fetal origin of TGCC
Basic studies (319, 344, 384) and epidemiological trends (162, 282) favor the hypothesis that TGCC is of fetal origin and should be considered a late-onset disease due to failure of normal fetal programming of the differentiation of primordial germ cells through a gonocyte stage into spermatogonia (Figure 5).
Figure 5.
Model for the pathogenesis of testicular germ cell tumors of young adults, which are derived from germ cell neoplasia in situ (GCNIS), previously known as carcinoma in situ testis (CIS). These tumors are an example of developmental cancer and are thought to be caused by a combination of adverse environmental and genetic factors (multifactorial and polygenic). The key pathogenetic event is insufficient masculinization and impaired function of the testicular somatic cell niche, which in fetal life is mainly composed of Sertoli and Leydig cells. The insufficient stimulation of developing germ cells causes arrest of gonocyte differentiation to spermatogonia and prolonged expression of pluripotency genes (depicted by red nuclei in all pluripotent cell types in the figure). The delayed gonocytes (pre-GCNIS cells) then gradually acquire secondary genomic aberrations (including polyploidization and gain of chromosome 12p), while adapting to the changing niche, especially during and after pubertal hormonal stimulation of the testis. Increased proliferation results in malignant transformation of GCNIS cells into an invasive tumor, either a seminoma or nonseminoma (the latter through the reprogrammed pluripotent stage of embryonal carcinoma, EC). Normal germ cell development is shown in the top part in the figure (PGC, primordial germ cells) on the green background which symbolizes a normal testicular somatic cell niche. [Updated and modified from Rajpert-De Meyts (344).]
The expression pattern of human gonocytes resembles that of embryonic stem cells because of the retention of pluripotency genes, such as POUF5/OCT4 and NANOG, but also includes expression of the KIT receptor, AP-2γ (TFAP2C), podoplanin (PDPN/D2-40), and a number of germ cell specific genes (11, 190, 347, 396). The expression of the pluripotency genes is gradually lost during the second half of pregnancy and is rarely present after birth, although it might occasionally be seen in a few spermatogonia in normal testicles of boys younger than 1 yr (188). However, in individuals with a high risk of developing germ cell cancer, e.g., patients with mutations in SRY or the androgen receptor gene (AR), and in patients with 45X/46 XY mosaicism, undifferentiated gonocytes might persist during childhood and subsequently in adulthood develop into the abnormal intratubular cell pattern termed GCNIS (238, 344, 382, 384) (Figure 5). GCNIS cells are precursors of both seminoma and nonseminoma, although invasive TGCC might not develop in a testis harboring GCNIS until several years after detection of the cells by testicular biopsy (385).
Epidemiological evidence also supports the idea of fetal origin of germ cell cancer. First, the fact that TGCC incidence peaks in young adulthood (between ages 20 and 45 yr) suggests an early onset of the malignant process (40, 79). Second, several epidemiological studies have shown a birth cohort effect in the incidence of TGCC (40, 107) so that men born in later calendar years have higher incidence rates. Third, immigration studies have shown that young men moving from countries with low or high risk of TGCC to a country with an intermediate risk developed TGCC with the same incidence as men of their home countries, while their sons born abroad acquired the risk of the host county (163, 297). Furthermore, the fetal hypothesis is in line with clinical studies of patients with disorders of sex differentiation (DSD) and congenital malformations, such as cryptorchidism and hypospadias, who are at significantly increased risk of developing TGCC (95, 373, 378). In addition, a study has shown that mothers' exposure to persistent chemicals was associated with increased risk of TGCC in their sons (157).
3. Testicular cancer as a sign of TDS
The heterogeneous group of above-mentioned conditions with reported increased risk of TGCC might have one thing in common: compromised development and function of the fetal Leydig and Sertoli cells. Several studies have investigated the role of these cells in the pathogenesis of TGCC and convincingly demonstrated that testicles harboring TGCC, and biopsies from men with cryptorchidism, hypospadias, and men with poor semen quality, often show evidence of dysgenesis in parts of the testicular tissue, including clusters of incompletely differentiated Sertoli cells, microliths, and Leydig cells clumps (sometimes called micro-nodules) (Figure 6) (368, 386, 387).
These histological observations in addition to strong epidemiological evidence that TGCC, impairment of spermatogenesis, cryptorchidism and hypospadias are linked together in a “risk factor network” have prompted us to propose the existence of a TDS of fetal origin (Figure 3) (387).
The disorders that constitute the condition complex of TDS might have one more thing in common, namely, feminization of the ano-genital distance (AGD) which is normally 50-100% longer in males than in females (369). Interestingly, some studies have shown that males with cryptorchidism, low sperm counts, low androgen levels, or hypospadias have decreased AGD (101, 102, 106, 408, 412, 415). It has been confirmed in animal studies that shorter AGD in males with congenital abnormalities of their genitalia reflects decreased androgen levels during the fetal period, as the shorter AGD is already visible after birth. Interestingly, in a large study of boys with cryptorchidism and hypospadias, short AGD was also associated with smaller penis size, confirming the association with neonatal androgen action (415). TGCC, cryptorchidism, hypospadias, and sperm count are not only risk factors for each other at an individual level, but they also seem associated at the population level. Indeed, a French group reviewed international data on TGCC, sperm count, hypospadias, and cryptorchidism and found correlations between the signs of TDS and geographical location, lending support to the unifying concept of the TDS hypothesis (379).
B. Cryptorchidism
Cryptorchidism is one of the most common birth defects, affecting 2–9% of boys born full term (47). The testes normally descend to the bottom of the scrotum before birth, and if one or both of them fail to do that, the condition is called congenital cryptorchidism. Once fully descended, the testes can later ascend to a cryptorchid position (443), which is called acquired cryptorchidism or ascending testis: its frequency is variable with the highest reported incidence nearly equal to that of congenital cryptorchidism (3). Epidemiological studies that have used registries as a data source usually combine these two groups together, because they are not separated in any International Classification of Diseases (ICD) classification. This adds some confusion to literature. Incidence rates that are based on numbers of orchidopexy typically reflect the frequency of both congenital and acquired cryptorchidism. The most reliable data on the incidence of congenital cryptorchidism come from cohort studies where the boys have been examined with standardized techniques and clear diagnostic criteria. Many clinical cohort studies with a long follow-up have used the classification of Scorer (377) to divide congenital cryptorchidism into subgroups based on the lowest position of the testis by physical examination: nonpalpable, inguinal, supra-scrotal, high scrotal, and normal scrotal. English studies using this classification showed an increase of the incidence of cryptorchidism from 2.7% at the end of the 1950s (377) to 3.8% at the end of the 1980s (184), and further up to 5.0% in the early 2000s in boys with birth weight above 2,500 g (3) (Figure 7). Similarly, studies in Copenhagen showed an increase in the cryptorchidism rate from 1.8% in 1959–1961 to 8.5% in 1997–2001 (47). Interestingly, in Finland, the incidence of cryptorchidism remained at a low level (2.1%) (47) (Figure 7). Some pediatric surgeons have challenged the disease definition by discounting high scrotal cryptorchidism as a defect (81). It is evident, however, that the proper place of the testis is at the fully descended position, although high scrotal testes are usually not treated surgically as are all other, more severe cryptorchid cases (356). Cryptorchid testes are brought down to the scrotum surgically to preserve their spermatogenic capacity and to facilitate cancer surveillance. Spermatogenic cells suffer and start to disappear early in childhood unless the testes are in the proper position (211).
Figure 7.

Incidence of cryptorchidism at birth on the basis of prospective clinical studies from the 1950s to the 2000s in Denmark, Finland, and United Kingdom. The data points are marked on the year of the publication of the study which represents the preceding incidence rate (3, 47, 61, 184, 377).
Cryptorchidism is a risk factor for infertility, testis cancer, and hypospadias (373, 374), suggesting that these conditions share similar causes affecting fetal testicular development. However, although early orchidopexy (surgical treatment) improves the fertility chances, it might not decrease the risk of testicular cancer (296, 452), although this has been suggested in a few studies (330).
1. Causes of cryptorchidism
Testicular descent is hormonally regulated. The key regulatory hormones are testosterone and insulin-like peptide 3 (INSL3), both of which are secreted by Leydig cells in the testis (38). Pituitary luteinizing hormone (LH) stimulates Leydig cell differentiation and hormone secretion. In the presence of a decrease in these hormones, or defects in their receptors, the testes remain incompletely descended. A number of genetic defects in hormone synthesis and receptors have been described over the last 30 years and are often associated with cryptorchidism as part of a syndrome, but they are found very rarely in patients with isolated cryptorchidism (i.e., without other genital abnormalities) (263). It is noteworthy that isolated cryptorchidism might be caused by gene mutations that physically hamper the testicular descent, such as mutations of the AMH gene or its receptor (AMHR2) in the Persistent Müllerian Duct Syndrome (1, 192).
Children with 46,XY karyotype and androgen insensitivity typically have testes either in the abdominal or inguinal position, i.e., they have not undergone inguinoscrotal transfer in utero. Mice with INSL3 deficiency, or with RXFP2/LGR8 (INSL3 receptor) inactivating mutation, have testes in a high abdominal position, which led to a hypothesis that the early trans-abdominal descent would depend on this hormone (302, 479). However, it seems apparent that both androgens and INSL3 act in the whole process. They act on the gubernaculum, which is an actively transforming fetal organ first attaching the testis to the inner opening of the inguinal canal and then guiding it through the canal to the scrotum, and finally dissolving away. Mutations in INSL3 or RXFP2 have been detected in surprisingly few cryptorchid patients (44, 108). Also, although some polymorphisms have also been described, they have appeared only in a heterozygous manner and have also been reported in healthy individuals. In recent years, however, GWA studies have begun to shed some light on possible gene polymorphisms that predispose to problems with testis descent, either as part of TDS or nonsyndromic cryptorchidism. A study of several TDS components, including cryptorchidism, which combined GWAS with systems biology approaches, found weak associations with gene variants within TGFBR3 and BMP7 loci (86). Associations with other SNPs located in or near TGFBR3 locus have recently been confirmed in a larger study, which also found decreased expression of TGFBR3 protein in the gubernaculum of cryptorchid rats (34).
However, until larger studies are performed, the most commonly identified genetic defects associated with cryptorchidism will remain those that affect androgen production or action. Cryptorchidism is clustered in families, which suggests a genetic or intrafamilial environmental cause. This has been analyzed in large registry-based epidemiological studies comparing the incidence in first-degree relatives. Monozygotic and dizygotic twin brothers have similar concordance for cryptorchidism, suggesting a minor role for genetic factors (375). Full brothers have a lower risk than twin brothers if one of the boys is cryptorchid, but their risk is higher than that of half-brothers. Furthermore, maternal half-brothers have a higher risk than paternal ones. All these findings implicate the importance of maternal environment during pregnancy.
2. Roles of hormonal exposures
Animal experiments show that anti-androgens and estrogens can cause cryptorchidism. Androgen action at a specific male programming window during rat embryonic days 13.5–17.5 is critical for proper masculinization, and failure at this developmental phase causes an irreversible undermasculinization, including cryptorchidism, that becomes apparent much later (454). Human development is of course different in timing, but the same principles seem to work there also. In the human, critical male programming occurs at gestational weeks 7–15, i.e., in early and mid-pregnancy (344, 387).
The list of emerging anti-androgens is growing. The compounds that act at the receptor level as antagonists or partial agonists include widely spread pesticide congeners such as dichlorodiphenyldichloroethylene (DDE) and fungicides such as vinclozolin and procymidone. Even larger groups of compounds perturb androgen synthesis. Phthalates are well-studied examples of these chemicals. Interestingly, the effects of phthalates show variation in effects on different species (148, 228), whereas the effects of receptor antagonists are similar over these species. Since testing is performed with rodent models, some anti-androgens that might disturb human steroidogenesis without affecting rodents could go unnoticed.
A crucial question is whether human exposure to one or a mixture of many of these chemicals is sufficient to cause disruption of male programming. Mixture studies in experimental animals have shown clearly that these chemicals can act in a simple additive manner, rendering even low doses harmful (76). Modeling studies have demonstrated that experimental results from exposing animals to mixtures of chemicals can be predicted on the basis of response curves of the individual chemicals (213). Estrogenic chemicals and dioxins can also cause cryptorchidism. Estrogens can prevent production of INSL3, which might explain its mechanism of action. In humans, exposure to a synthetic estrogen diethylstilbestrol was linked to increased rate of cryptorchidism (321), but environmental estrogens are typically much less potent. However, together with anti-androgens they might act in the same direction. It is uncertain how significant is their impact. Dioxins act via aryl hydrocarbon receptor (AhR). Rodent studies have shown that dioxins can induce cryptorchidism (141), but it is not known how this effect is mediated.
Epidemiological studies on relationships between exposures to endocrine disruptors and cryptorchidism have analyzed single chemicals or chemical groups, and only a few have attempted to integrate these data. Exposures have been measured in blood, urine, placenta, and breast milk that serve as a proxy to mother's load of chemicals during pregnancy. In some cohort studies, careful ascertainment of the diagnosis has been combined with exposure measurements. The results vary according to the matrix that has been used for exposure assessment. The breast milk level of polybrominated flame retardants was associated with an increased risk of cryptorchidism, whereas placental levels were not (256). Similarly dioxin levels in breast milk of Danish women were associated with an increased risk of cryptorchidism (222, 223), whereas placental levels did not show an association (446). In Finland, dioxin levels in neither breast milk nor placenta were associated with cryptorchidism. In American studies of dioxins and DDT, no association was observed with maternal serum values and children's cryptorchidism risk (246). French studies found an association with polychlorinated biphenyls (PCB) levels in breast milk and the incidence of cryptorchidism (55), whereas Danish-Finnish studies did not, or showed the opposite (222).
Mixture effects have been analyzed in only a few studies. After combining data from several pesticide exposures by permutation analysis, an association between the level of chlorinated pesticides in breast milk and risk of cryptorchidism in offspring was found (89). Greenhouse workers exposed to pesticides during pregnancy were also shown to have an increased risk of producing cryptorchid sons (14). Phthalate levels in breast milk were not associated with cryptorchidism risk, but they were linked to an increased LH-to-free testosterone ratio in the son at the age of 3 mo, suggesting testicular impairment during lactation (257). It is apparent that there are large data gaps, because only few exposures have been analyzed thus far. It is also unlikely that any individual chemical would have a major impact on the incidence of cryptorchidism. Combination of data from several exposures, particularly those that are known to affect the same signaling cascade, is needed to assess the whole chemical load, or “exposome” as it is called nowadays.
3. Lifestyle factors
The significance of lifestyle factors, such as smoking and alcohol consumption, as risk factors for cryptorchidism remains contentious. There is evidence that heavy smoking during pregnancy (>10 cigarettes per day) is associated with an increased risk of having a bilaterally cryptorchid son (417), while other studies have not shown a link between smoking during pregnancy and cryptorchidism (88). Registry-based studies did not find an association with mother's alcohol consumption and cryptorchidism in their sons (404), whereas a prospective follow-up study demonstrated a dose-dependent increase in the incidence of cryptorchidism in drinking mothers' offspring (90). The lowest adverse effect dose was five units of alcohol per week, which is considerably lower than expected. However, the number of mothers in the group with the highest consumption was small, which influenced strongly the overall result and might therefore also be a chance finding. Smoking affects the growth of the fetus, and being small for gestational age at birth is a well-established risk factor for cryptorchidism (47). Prematurity is another strong risk factor, because testicular descent occurs normally during the last trimester and therefore might not occur before a premature birth.
Gestational diabetes has become more frequent due to the increasing trends in obesity. In women with gestational diabetes, the risk of delivering a cryptorchid son is increased fourfold compared with non-diabetics (447). The underlying mechanism is not known, although early growth delay of the fetus in the first trimester might play a role. Interestingly, even children of diabetic mothers, who are born large due to compensatory growth in last part of pregnancy, have been found to grow poorly in the first trimester (328). In contrast, no association between gestational diabetes and cryptorchidism was found in a registry-based study from Israel (424).
C. Hypospadias
The penile congenital malformation, in which the urethra opens somewhere on the ventral side of the penis instead of the tip, is called hypospadias. The urethra might remain split over a long distance. The severity of the hypospadias is defined by the location of the opening. In the distal, mild form, the urethra opens in the glans or corona (sulcus) which is the border of the glans and the shaft. Registration of this birth defect varies in the malformation registries, because it does not necessarily require any surgical treatment. Physiological phimosis might also hide this defect in the newborn, and it might become apparent only after the foreskin can be easily retracted (45, 46). This should be considered when incidence data between countries are compared, because ascertainment, reporting, and registering practices vary (266, 421). More severe forms of hypospadias require surgical reconstruction of the penile urethra, and hospital discharge registries give a reliable estimate of their prevalence. In middle, or penile hypospadias, the opening of the urethra is located on the shaft of the penis, while in proximal hypospadias the opening can be found in the penoscrotal area. Sometimes both of these are called proximal in contrast to the distal form.
1. Incidence of hypospadias
Increased incidence of hypospadias has been reported in Australia, US, and Europe over different time periods (200, 299, 326, 327, 421). The latest data from Denmark and Sweden also indicate an increasing trend (252, 308, 309). Until the 1990s, many malformation registries suffered from under-reporting; however, after a more active search, the hypospadias rate was found to be much higher than previously reported (161). This was also one reason for the controversies in the debate of incidence rates (5, 66, 97, 118, 334). Prospective clinical studies might be more accurate and therefore show higher rates than registry studies, for example, 1% versus 0.5% in Denmark (46, 253), as more mild cases might be noted in prospective studies. Interestingly, there are great differences between countries, for example, 1% in Denmark versus 0.3% in Finland according to parallel standardized clinical studies (46, 445). A list of incidence data of hypospadias is presented in Table 1.
Table 1.
Incidence of hypospadias in prospective or cross-sectional clinical studies
| Country | Study Type | Rate of Hypospadias | Reference Nos. |
|---|---|---|---|
| USA, Rochester, MN | Prospective cohort study (n = 4,474) | 0.6% (body wt >2,500 g), 0.8% of all boys | 158 |
| USA, New York City, NY | Prospective study on pregnant women and infants | 0.54% of live-born boys | 271 |
| USA, collaborative perinatal project | Prospective study (n = 53,394 consecutive single births) | 0.80% of single-born boys (76% of cases detected at birth) | 295 |
| Korea, 38 hospitals | Prospective study (n = 7,990) | 0.21% of boys | 74 |
| Southern Jordan | Clinical study of 1,748 boys (aged 6 to 12 yr) | 0.74% of boys | 9 |
| Finland,Turku | Prospective cohort study (n = 1,505); total hospital cohort (n = 5,798) | 0.27% of live-born boys, 0.33% of live-born boys | 445 |
| Netherlands, Rotterdam | Prospective study (n = 7,292) | 0.73% of newborn boys | 332 |
| Denmark, Copenhagen | Prospective cohort study (n = 1,072) | 1.03% of live-born boys (at 3 yr: 4.64% including also milder cases detected after physiological phimosis resolved) | 46 |
| Bulgaria, 5 regions | Cross-sectional clinical study (n = 6,200 boys aged 0 to 19 yr) | 0.29% of boys | 224 |
Modified from Toppari et al. (423).
2. Causes of hypospadias
Masculinization of the male is driven by androgens during early fetal development. Penile development is regulated by dihydrotestosterone that is produced locally from testosterone by 5-α-reductase. Several genetic mutations leading to hypospadias are known, and they are typically linked to disorders of testicular differentiation, testosterone synthesis, conversion of testosterone to dihydrotestosterone, or androgen receptor action (199). The same classification that is used for the severity of androgen insensitivity can also be used for hypospadias (342). Despite this knowledge, a genetic cause can be found only in a minority of hypospadias cases, and an endocrine abnormality can be found only in ∼20% of the patients (353). Environmental anti-androgens cause hypospadias in experimental animals in the same manner as they induce cryptorchidism (354), which makes it reasonable to search for common causes of these disorders.
Genetic defects, other than those of androgen receptor and steroidogenic enzymes, include Homeobox genes HOXA and HOXD, fibroblast growth factor (FGF) 8, FGF10, FGF receptor 2, and bone morphogenetic protein 7 (123, 123, 132, 288, 290, 292). Activating transcription factor (ATF) 3 might also be involved, since its transcript level was found to be elevated more often in the foreskin of boys operated on for hypospadias than in those circumcised (242). The gene is estrogen regulated and influences transforming growth factor-β signaling, which might explain why estrogens can also increase the risk of hypospadias (241, 462). HOXA13 mutations can cause the hand-foot-genital syndrome which includes hypospadias (123, 290), and hypospadias can be a part of many other multi-malformation syndromes.
Mutations in MAMLD1 and NR5A1/SF1 can cause testicular dysgenesis, with hypospadias (36, 125). Mutations are rare (313), but the genes can be targets of endocrine disruptors as demonstrated for NR5A1 (407). Androgen and estrogen receptor polymorphisms have been associated with varying risk of hypospadias, but the results are not very consistent and require larger study populations than available so far (27, 39, 440, 451). Diacylglycerol kinase κ polymorphism has also been linked to the risk of hypospadias (439).
3. Roles of prenatal exposures
Being small-for-gestational age is a risk factor for both cryptorchidism and hypospadias (6, 7, 26, 331, 331). Both birth defects can be caused by anti-androgens and estrogens, as shown by epidemiological studies following the children of women who used diethylstilbestrol (DES) during pregnancy (422). DES increases the risk of hypospadias even in the second generation, as the sons of in utero-exposed women have a higher prevalence of hypospadias than other males (54, 199, 210). This might reflect an epigenetic effect by DES. DES-related adverse effects are very similar in human and experimental animals (272), and there is no reason to believe that the anti-androgen-related effects would differ.
Epidemiological studies on hypospadias have largely relied on registries, because the condition is rather rare and it is difficult to collect enough cases in prospective clinical studies to reach statistical power. As presented earlier, the registry studies on hypospadias are problematic due to several sources of error in classification of cases versus controls. A possible association between the risk of hypospadias and pesticide exposure was assessed in a meta-analysis that showed a small, increased risk of hypospadias in sons if parents were exposed to pesticides. Medical charts, parental interviews, occupation, job exposure matrix, or linkage of agricultural census and birth records were used for the assessment of exposure (360). Pooled risk ratios were 1.36 (95% CI 1.04–1.77) and 1.19 (95% CI 1.00–1.41) for maternal and paternal exposures, respectively (360). However, the studies could not assess which chemicals were behind the association, because the pesticides included a large number of different chemicals.
Recent studies using a job exposure matrix as a proxy for pesticide exposure suggested an association of hypospadias with heavy metals, or maternal exposure to any endocrine disrupting chemical (EDC) (134), but did not show a significant association between pesticide exposure and hypospadias (287, 298, 361). Maternal serum samples were collected during pregnancy in the Collaborative Perinatal Project (CPP) conducted in the US in the 1950s and 1960s (246, 333). Several chemicals were analyzed in these samples, and the children were examined many times before they were 7 yr old. There was no linear association of PCB levels with hypospadias, but there was an increased odds ratio for the sum of some PCBs (270). No significant association was found between hypospadias and chlordane-related contaminants, DDE, β-hexachlorocyclohexane, or other pesticides (246, 270, 333, 425). Another study relating the levels of DDT or DDE in pregnancy serum samples from the 1950s and 1960s with hypospadias in the sons also showed no association (42). In a study from the 2000s, an increased risk of hypospadias was associated with above-median level of hexachlorobenzene (HCB) in primiparous women as assessed from serum samples collected several weeks after delivery (134).
No significant associations between hypospadias and mid-pregnancy serum levels of PCBs, PBDEs, HCB, DDT, or DDE were found in the study of Carmichael et al. (66). Serum levels of PBB at the time of conception showed no association with the risk of hypospadias according to a small questionnaire-based study (392). Phthalate metabolite level (mono-4-methyl-7-carboxyheptyl)phthalate (7cx-MMeHP, a DiNP metabolite) in amniotic fluid showed elevated odds ratios for hypospadias (1.69 [0.78 to 3.67]), but was not consistently associated with the amniotic fluid levels of steroid hormones or insulin-like peptide 3 (172). DEHP [di(2-ethylhexyl)phthalate] metabolite levels did not show similar associations (172).
A vegetarian diet of mothers was associated with an increased risk for hypospadias in the British ALSPAC study (310). In contrast, a decreased risk was reported for mothers having fish or meat in their diet during pregnancy (6). Also, a phytoestrogen-rich diet was associated with a reduced risk of hypospadias (65). No difference was found in the hypospadias risk of boys whose mothers used, or did not use, organic food diet, but a frequent concurrent consumption of high-fat dairy products (milk, butter) while rarely or never choosing the organic alternatives during pregnancy was associated with an increased odds ratio of hypospadias (adjusted OR 2.18, 95% CI 1.09–4.36) (75). The need for assisted reproductive techniques (ART) and subfertility are risk factors for hypospadias (83, 201, 209, 413, 455). While fetal exposure to DES increased the risk of hypospadias, the role of other pharmaceutical sex steroids is controversial. Use of progestins was reported to increase the risk of hypospadias (63, 84). However, according to a meta-analysis of 14 studies, no association between exposure to sex steroids (except DES) during the first trimester and external genital malformations could be found (348). It is apparent that epidemiological studies have difficulties in case ascertainment, exposure assessment, and statistical power. Experimental studies have clearly indicated risks of hypospadias associated with anti-androgenic chemicals, such as phthalates and vinclozolin (76, 208), but epidemiological studies have failed to reach any comprehensive measurement of these compounds in large enough study populations of humans to draw conclusions about their role in hypospadias.
D. Onset of Male Puberty
While a clear downward trend in timing of puberty has been documented among girls, this trend has not been clear in males until recently. In fact, American data on male puberty (1940–1994) were reviewed by an expert panel in 2006, which failed to find a significant decline, probably due to insufficient data as well as use of different study designs and study populations (111). However, several data have emerged since then suggesting a significant downward trend in male pubertal timing (Figure 8). For example, a European study of 21,612 boys born 1935–1969 showed a downward trend in age at peak height velocity (PHV) (8), although other European studies did not find such changes (323).
Figure 8.

Recent changes in male pubertal timing. Testicular volume was >3 ml. [From Mouritsen et al. (293).]
Male puberty marks the transitional period during which the infantile boy attains adult reproductive capacity and develops into a mature man. Puberty usually starts at 11.5 yr of age, although with large interindividual variability (9–14 yr). Pubertal onset in a boy before 9 yr or after 14 yr of age is considered pathological and necessitates further evaluation to exclude underlying pathologies. The timing of puberty is determined by genetic as well as environmental factors such as body composition, physical fitness, nutritional and socioeconomic status, ethnicity, residence, foreign adoption, and exposure to endocrine disrupters (325).
The pubertal development of secondary sexual characteristics begins with growth of the testes as a result of follicle stimulating hormone (FSH) stimulation of seminiferous epithelium. When testicular volume exceeds 3–4 ml, it is considered a definite clinical sign of pubertal onset. The stimulation of spermatogenesis involves multiple endocrine and local factors including FSH, LH, testosterone, inhibin B, AMH, etc., and the increasing testicular volume is a marker of spermatogenesis. Leydig cells are stimulated by LH at the onset of puberty, and subsequently start to produce testosterone which influences the growth of the penis (width and length), androgenization of the scrotal sac, and pubic hair development. Alternative pubertal markers include the pubertal growth spurt which is best described by the age at PHV, the point of maximal growth velocity in puberty (8). Age at PHV is a late pubertal marker, and usually occurs when the boy is in genital stages 3–4 when testicular volumes are 10–11 ml. Another late marker of puberty is voice breaking, which occurs at an average age of 14 yr (195). Age at first emission of spermatozoa (spermarche) could be considered the male counterpart of age at menarche in females. Age at first emission of spermatozoa can be recorded in first morning urine samples (307), or by self-reported involuntary or voluntary emissions (420).
1. Endocrine regulation of pubertal onset
The hypothalamic-pituitary-gonadal (HPG) hormone axis has already been activated in the neonatal period, which results in increase in circulating levels of FSH, LH, testosterone, and inhibin B (a period termed “mini-puberty”) (19). The physiological reason for this phenomenon, which lasts a few months, is not known, and the presence of circulating androgens does not result in virilization of genitals, probably because of the relatively short period of androgen exposure, and because androgen receptors are not yet widely expressed. Most of testosterone is bound to sex hormone binding globulin during mini-puberty. Mini-puberty is, however, accompanied by descent of undescended testes (47). Although the factors responsible for this early HPG activation and its subsequent silencing remain unknown, epigenetic factors have been suggested to play a role. After 11 years of postnatal suppression, the HPG axis is reactivated, which results in increases in circulating levels of FSH and LH which in turn stimulates testosterone, Insl3 and inhibin B (18, 183). The mechanisms underlying the pubertal reactivation of the HPG axis are largely unknown, but most likely include physiological and lifestyle factors such as fat mass, physical fitness, nutrition, vitamin D, and psychosocial factors (325). The pubertal period is characterized by very high activity of the growth hormone-insulin-like growth factor axis (194) as well as high insulin levels (397, 398), and resembles in many ways a transient acromegalic state associated with insulin resistance.
Signs of current changes in pubertal timing were evident in the cross-sectional Copenhagen Puberty study, where a significant 3–4 mo downward change in age at pubertal onset (testicular volume >3 ml) during a 15-yr period was reported in the very same area of the capital region (399). These findings were confirmed in a longitudinal followup study in the same Copenhagen area (293). In accordance with these findings, much earlier pubic hair development was found in the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort from the United Kingdom (UK) (data collected 1999–2005) compared with the original UK data collected 1949–1969 by Marshall and Tanner (11.4 vs. 13.4 yr) (285). Likewise, a recent US study reported mean ages of beginning of genital and pubic hair growth 6 mo to 2 yr earlier than in older US studies (165). Altogether, it appears that the age at which the Copenhagen male population reaches puberty and attains adult reproductive capacity is decreasing. We do not know the reasons or long-term consequences for these trends, but suggest that the observed changes in age at pubertal onset might represent early warnings of environmental factors influencing male reproductive health.
E. Changing Testosterone Levels
Testosterone produced by the Leydig cells in the testes is the major male sex steroid. It plays important roles in sex differentiation as well as in male puberty and in adulthood for developing and sustaining the secondary male sex characteristics and spermatogenesis. Production of testosterone is stimulated by LH secreted by the pituitary, which itself is stimulated by gonadotropin releasing hormone (GnRH) from the hypothalamus. On the other hand, circulating testosterone has, together with estrogen, an inhibitory effect on both GnRH and LH secretion. In the adult male, a balance between LH and testosterone level is thus attained through a hormonal negative-feedback loop, which is part of the hypothalamo-pituitary-testis hormone axis (166). Only 1–2% of circulating testosterone occurs free in the bloodstream; the vast majority of circulating sex steroids is bound to serum proteins such as sex steroid binding protein (SHBG), which has a high affinity for binding of both testosterone and estradiol (154). Tissues of the body, including the hypothalamus and pituitary, only “see” the free (unbound) sex steroids, and SHBG serum level is therefore an important coplayer in the GnRH-LH-testosterone hormonal feedback loop.
1. Age-related changes in male testosterone levels
Both total and free testosterone levels decrease in men with increasing age (Figure 9), while SHBG and gonadotropin levels increase. There is no doubt that age-related changes in body composition and lifestyle contribute towards this change as overweight, type 2 diabetes, and decreased exercise all are associated with decreased total testosterone levels. Moreover, obesity or more severe metabolic illnesses are also associated with decreased free testosterone (144, 174, 426). When adjusting for some of these covariates, the age trend in total testosterone is attenuated, while declining free testosterone and increasing LH and SHBG with age are seen even with adjustment for BMI, comorbidity, and smoking (468). Declining testosterone with increasing LH levels in aging men suggest an impairment of testicular function with age, which is further supported by the fact that older men have an attenuated testosterone response to LH stimulation (243).
Figure 9.

Average male serum testosterone levels by age (full drawn line) based on healthy men from the general population show only a moderate decline from the age of 20–60 years. Superimposed (dotted line) is an illustration of the consequence of applying the average rate of decline (−1.6%/year) observed in a longitudinal study of individual testosterone levels (115) from the age of 22 (year of peak testosterone levels in the cross-sectional material). [From Andersson et al. (15).]
In older men, however, declining testosterone is not always accompanied by a reciprocal rise in LH. This reflects the fact that the attenuation of GnRH-LH signaling might influence the ability to compensate for an impairment of the Leydig cells inferred by aging through expected increased LH signaling. This blunting of the HPG axis might further lead to declining testosterone levels (442). Cross-sectional studies on age-related male testosterone levels generally show relatively modest changes in serum testosterone levels between different age groups with estimated changes per year of 0 to −0.8%, while steeper declines in individual total testosterone (e.g., −1.6%/year) have been observed in longitudinal studies (Figure 9) (115). The discrepancy in the rate of age-related decline in testosterone levels observed in cross-sectional studies compared with longitudinal studies could be due to a selection bias. Cross-sectional studies might be more likely to include the healthier segment of elderly men while longitudinal studies might be more prone to followup on participants irrespective of health status. However, we and others have suggested that a “true” age-related rate of decline in testosterone in cross-sectional study material could be blunted by the occurrence of a birth cohort related decline in testosterone levels over the generations of men included in the cross-sectional material (15, 115).
2. Secular trends
In 2007, a paper reported a population-level decline in male testosterone levels over time (427). The paper was based on a US study of more than 1,300 men, some of whom were examined for up to three times over an 18-yr period. The age-independent secular change in total testosterone levels and bioavailable testosterone corresponded to, respectively, −1.0%/yr and −1.3%/yr over the period 1987–2004 (427). A Danish study, which was published shortly after, also reported the observation of a secular decline in male testosterone levels in a study of more than 5,300 men from the general population in four studies conducted at different time points between 1982 and 2001 (15). In the Danish study, the secular decline was significant for total testosterone and SHBG levels but not for free testosterone and the decline could partly, but not exclusively, be explained by a secular increase in BMI. A Swedish study comparing reproductive hormone levels in comparable age groups of men examined in 1995 (n = 430) and in 2008 (n = 149) found that free testosterone was significantly lower in the men examined in 2008 (430). A trend of lower total testosterone was also observed in the Swedish men examined in 2008 compared with 1995, although this trend did not reach statistical significance. The difference between free testosterone levels in the Swedish men examined in 1995 versus 2008 remained after adjustment for a difference in weight in the oldest age group (430). More recently, secular declines in total testosterone, free testosterone, and SHBG were reported in Finnish men (>3,000) from the general population (329). These trends remained significant following adjustment for BMI. In the Finnish study, they also observed a secular decline in gonadotropins, indicating that while decline in testosterone might be due to detrimental changes at the gonad level, the hypothalamus-pituitary-axis did not seem to respond appropriately to this change in the examined men (329).
In studies on secular trends, as those described above, time period and birth year are completely confounded when adjusted for age because time period, age, and birth year are completely linearly dependent on each other. Thus, while adjusting for the effect of age on reproductive hormone levels, it is impossible to discern whether the remaining differences are due to a time period effect or a birth cohort effect. Irrespectively, it is perturbing that this secular decline is observed at the population level in several industrialized countries over the same period/birth years. While a concurrent increase in obesity and metabolic disturbances and a decrease in number of smokers among men in the same countries might contribute to the declining testosterone levels, these health and lifestyle changes do not seem to fully explain the observed trends (268, 329, 427) (see Figure 10).
Figure 10.

Secular trends in mean total testosterone levels of normal men by age. A: stratified by time period of study. B: stratified by 5-yr birth cohort. Please note the different scales of the y-axes in A and B. [Modified from Travison et al. (427).]
It is tempting to speculate that there is a link between trends of declining male testosterone and increased male reproductive health problems in general. Normal testicular function is dependent on paracrine communication between cells of the different compartments in the testis. Testosterone from the Leydig cells acts on the Sertoli cells and is crucial for Sertoli cell differentiation during sexual maturation and for Sertoli cell supported sperm production in the adult male. Likewise, paracrine factors from the seminiferous tubules and the peritubular cells influence the function of the Leydig cells. Thus the hormones inhibin B and anti-Müllerian hormone (AMH) produced by the Sertoli cells both seem to contribute to the regulation of LH-stimulated steroid production of the Leydig cells, with AMH having a suppressive (428, 429) and inhibin B having a putative stimulatory effect (167) on testosterone production, the latter presumably by reversing an inhibiting effect of activin on testosterone production by the Leydig cells. It is therefore not surprising that a compromised function in one of the compartments of the testis is reflected in the function of other compartments of the testis. Accordingly, while individual men with poor sperm concentration might have testosterone levels within the normal range, as a group, subfertile men have lower serum testosterone levels than fertile men (16). They also have, in general, higher serum LH levels resulting in an even lower testosterone-to-LH ratio compared with fertile men. This indicates that testosterone levels of subfertile men often are sustained on the basis of a more intense stimulation by gonadotropin (16), indicating that impaired Leydig cell function is more common among men with poor semen quality.
F. Sperm Concentration and Other Measures of Semen Quality
The publication of Carlsen et al. in 1992 (64), which concluded that sperm concentration had declined 50% over the previous 50 years, remains controversial (193, 383). As has been summarized elsewhere (411), this controversy centers on three primary concerns. Some authors suggested that poor or highly variable data invalidated any inference about trends in sperm counts (232, 316). Others questioned the validity of the statistical methods used in this analysis (52, 113, 316). Bias due to changing study populations (53) or confounded by factors such as age and abstinence time (time between sample collection and last ejaculation) was also suggested (316, 441).
Following the 1992 publication of Carlsen et al. (64), considerable research activity was initiated to address these concerns resulting in numerous studies. Some of these used retrospectively collected and others newly collected data, which we discuss below. Studies relying on retrospectively collected data differed in study design and methods including: 1) semen parameters examined (sperm concentration, semen volume, total sperm count, percent motile sperm, percent morphologically normal sperm); 2) semen collection and analysis methods (sperm counting methods, motility criteria, morphology criteria, abstinence time, season of collection, participation in an external quality control program); 3) variables used to assess temporal and spatial variability (study time period, geographical area); 4) study population/recruitment methods (partners of infertile women undergoing ART procedure, potential semen donors, male partners of subfertile couples, and young men with unknown fertility); and 5) potential confounders controlled in analysis (age, year of birth, abstinence time, sociodemographic variables, lifestyle factors, medical conditions) and sample size.
1. Reanalyses of historical semen quality data
A detailed reanalysis in 1997 of data from the 61 studies included in the systematic review by Carlsen et al. in 1992 (64) used multivariate linear models to control for potential sources of bias and confounding factors in those studies (410). The reanalysis showed significant declines in sperm concentration in the United States and Europe/Australia after controlling for abstinence time, age, percent of men with proven fertility, and specimen collection method. Declines in sperm concentration in the United States (∼1.5%/yr) and Europe/Australia (∼3%/yr) were greater than the average decline reported by Carlsen et al. (∼1%/yr). However, there was no evidence of a decline in non-Western countries, for which data were very limited. In 2000, an additional independent literature review and updated analysis was performed (411). In this, 47 English language studies published from 1934 to 1996 were added to those analyzed previously. Results of that analysis were consistent with those of the Carlsen study and the 1997 reanalysis by Swan et al. (410). The authors concluded that the trends in sperm concentration previously reported for 1938–1990 were also seen in data from 1934 to 1996.
2. Retrospective studies of temporal trends in semen quality within countries
Since 1992, many studies have examined trends in sperm counts within individual countries (both developed and developing countries) using historical data. A large recent study reported a significant decline in sperm concentration and morphology in 26,609 men from the French general population who provided samples between 1989 and 2005 (363). In line with this, another French study showed a decline in total sperm count and morphology among semen donor candidates 1976–2009 (400). Declines in sperm concentration have also been observed in Israel 1995–2009 (150), Tunisia 1996–2007 (114), and Scotland 1994–2005 (401). However, no decline was observed in sperm concentration in South Sweden from 1985 to 1995 (41), nor in North-Eastern Spain between 1960 and 1996 (20).
3. Prospectively designed cross-sectional studies of semen quality in partners of pregnant women and unselected young men
To overcome some of the problems of historical and cross-sectional data, several standardized and coordinated cross-sectional studies of semen quality have been undertaken. Two large multicenter studies designed to examine geographical variation of semen quality were conducted in partners of pregnant women. Each of these studies used consistent methods of recruitment and semen analysis and careful quality control to minimize between-center differences. The Study For Future Families (SFF) measured semen parameters in 763 partners of pregnant women in Los Angeles, CA; Minneapolis, MN; Columbia, MO; New York City, NY; and Iowa City, IA (351, 409). This was the first US study to compare semen parameters among study centers using standardized methods and strict quality control. These data suggested that sperm concentration, total count, and motility are reduced in semirural and agricultural areas relative to more urban and less agriculturally exposed areas. A multicenter European study collected semen samples from 1,082 fertile men from four European cities (Copenhagen, Denmark; Paris, France; Edinburgh, Scotland; and Turku, Finland) and demonstrated significant geographical differences in sperm counts, most notably between men living in Turku, Finland and Copenhagen, Denmark (186).
Population-based studies of semen parameters in young men conducted in a consistent manner have been ongoing in several European countries, the US, and Japan since the late 1990s (117, 187, 189, 274, 275, 320, 340). These studies provide information about both geographical differences in semen parameters over the past 20 years as well as about temporal trends in the countries that include cohorts across time (189, 191). The temporal trends available to date exhibit considerable geographical variation. Decreases of more than 20% in total sperm counts and sperm concentration were detected among Finnish men between 1998 and 2006 (191), and a decrease of ∼15% was seen in young Spanish men during the most recent decade (274). Conversely, a Swedish study that was not coordinated in the above studies but basically using the same methods found no significant changes in semen parameters among young Swedes between 2000 and 2010 (31). Increases in total sperm count and sperm concentration of ∼14 and 12%, respectively, were observed among Danish men between 1996 and 2010 (189). It should be noted that despite the increase in median sperm concentration during this time (from 43 to 48 × 106/ml), sperm concentration in these healthy young men was still markedly lower in 2010 than in Danish men in infertile couples in the 1940s (median above 60 × 106/ml) (See Figure 11). It is notable that most recent studies also show a very high frequency of morphologically abnormal spermatozoa. As an example, the recent study of young men from the general Danish population showed that the median percent of morphologically normal spermatozoa is ∼7%, a number that remained substantially unchanged throughout the 15-yr study period (189).
Figure 11.
Distributions of sperm counts in Danish men from the general population, examined from 1996 to 2010 and Danish men examined in an infertility clinic in the 1940s. All men had durations of ejaculation abstinence greater than 48 h. Sperm concentration (A) and total sperm counts (B) are shown. [From Jørgensen et al. (189).]
4. Possible etiological factors underlying geographical and temporal variability in semen quality
As discussed in the section on origin of testicular germ cell cancer (Figure 3), the hypothesis of the testicular dysgenesis syndrome, proposed in 1993, suggests that reduced spermatogenesis in adulthood can be a consequence of exposure in fetal life to environmental chemicals (381). Environmental chemicals, including endocrine disrupting chemicals such as dioxins and perfluorinated compounds (PFCs), as well as complex mixtures such as those in combustion products, appear to affect negatively both the perinatal and adult testes, emphasizing the importance of environmental and lifestyle factors that have impacts throughout life (380). Western lifestyle (sedentary work/lifestyle, obesity) is also potentially damaging to sperm production (103, 131, 175) as are other lifestyle factors (stress, sleep, smoking, maternal smoking, nutrition) (4, 130, 138, 173, 234). Data on the effects of environmental chemicals, such as pesticides, food additives, DDT, PCBs, or plasticizers, on spermatogenesis both in the perinatal period and in adult men are limited, lacking, or inconsistent (87, 160, 261). However, reports on reduced sperm counts and azoospermia in men exposed to dibromochloropropane (DBCP) (137, 335, 460) and dioxin (280, 281) provide proof of principle that exogenous chemicals during adult life can disturb human spermatogenesis as has been shown in numerous rodent studies (119, 140, 159, 444). In addition, recent studies have indicated that some endocrine disrupters, including ultraviolet filters, might have a direct effect on human sperm functions (289, 371, 414), including effects on CatSper, the calcium ion channel, which is crucial for sperm movements and acrosome reaction (239, 405).
Observations from wildlife and animal experiments lend support to the idea that environmental factors can adversely affect male reproduction. An example is the finding that cryptorchidism and other of the symptoms associated with TDS in humans, have also been reported in large numbers among populations of Sitka black-tailed deer in Alaska (56). Similar findings from studies of wildlife were also reported by other authors (cf. Ref. 459).
5. Semen quality and fecundity
Since 1980, the World Health Organization (WHO) manual for the examination of human semen has served as a standardized protocol for measurement methods and reference values for sperm parameters (464–467). In the WHO 2010 manual, the lower reference limit for sperm concentration was decreased from 20 × 106/ml to 15 × 106/ml, the value that had been in use since 1987. This value reflects the fifth centile for fertile men (defined as time to pregnancy <12 mo) and reflects the semen characteristics of recent fathers. It is notable that these cut-off points are appreciably below the value of 60 × 106/ml, considered to have been a “normal” sperm count in the 1940s (153, 255). Furthermore, other studies have shown reduced monthly probability of conception for sperm concentration below 40–50 × 106/ml (50, 147, 389). Bonde et al. (50) examined the monthly probability of conception in relation to semen parameters in couples attempting pregnancy and found that when sperm concentration was below 40 × 106/ml or the number of motile sperm <70%, the monthly probability of conception decreased. This is consistent with results from a large network study of fertile and infertile couples which found that semen samples with concentration below 48 × 106/ml, motility below 63%, and percent of sperm with normal morphology <9% (using strict criteria methods) were outside the fertile range (147). From an investigation of fertile men, Slama et al. (389) detected decreasing probability of conception with sperm concentrations below 55 × 106/ml and a total sperm count below 145 mill. Despite these population-level results, the ability of single semen parameters to predict fecundity on an individual level is limited, as was concluded by the United States National Cooperative Medicine Network in a large multicenter study comparing sperm parameters in 756 infertile and 696 fertile men (147). This national study concluded that although threshold values for sperm concentration, motility, and morphology can be used to classify men as subfertile or infertile, none of the parameters measured individually was diagnostic of fertility (147).
Although a healthy mature man has tens of millions of spermatozoa per ejaculate, it has been estimated that only 1 per million will succeed in contacting the egg within the fallopian tube (100). If these estimates are confirmed, an average semen sample with a total sperm count of ∼150 million, as currently often seen among young men in Denmark (189), might have as few as 150 spermatozoa capable of fertilization.
6. Conclusion
Over the past 25 years, abundant literature has identified important geographical differences in semen parameters between and within countries. While there is considerable variability in trends in sperm counts over the past 20 years, several recent studies report that 20–30% of young men today have sperm concentration below 40 × 106/ml, which is associated with reduced fecundity (50, 147, 389). We therefore estimate that 20–30% of men in the examined cohorts might be at risk of prolonged waiting time to pregnancy if they want to become fathers, and 10–15% have a sperm count so low that they might require fertility treatment (see sect. III).
G. Sex Ratio
Sex ratio of offspring may act as in indicator of male reproductive problems. For example, men who were exposed to the pesticide DBCP at their workplace (137) and men who were exposed to dioxin at the Seveso accident had an excess of girls (278). However, several factors may influence sex ratio.
It is generally assumed that ∼105 male births occur for each 100 female births leading to 51.5% of births being male (324). The sex ratio is important as it might reflect important demographic shifts as well as impact economic conditions (143, 264). In the first half of the 20th century, the sex ratio increased in most Western countries due to improved obstetrical care which led to relatively more live births of males. But while thought to be constant over time in the absence of health advances, recent data suggest a decline of the sex ratio in many Western countries (91).
An analysis from US birth data demonstrates a decline in the sex ratio beginning around 1940 (91, 264; see Figures 12 and 13). Over that period of time, the proportion of male births declined from 51.4 to 51.2%, or ∼2 fewer males per 1,000 births. In Denmark, the percentage of male births decreased from 51.5 in the 1950s to 51.3 in 1995, while in the Netherlands it declined from 51.6 to 51.3 over this same time period (283, 438). Canada also showed a similar decline in recent decades from 51.5 to 51.3 from 1970 to 1990 (10). However, an examination of 29 countries from a WHO database identified some countries where the sex ratio seemed to increase over time including several in southern Europe such as Italy and Spain (324). However, while a universal decline in the sex ratio was not reported for all countries, a majority (16 of the 29) did show a decline, while six showed an increase and seven showed no change. While certain regions and countries might not reflect the recent downward trend in sex ratio, for the remainder of the countries, this concerning index has been explored as a sentinel health indicator.
Figure 12.
Sex ratio at birth and joinpoint segments, 1940–2002, all mothers. [From Mathews and Hamilton (264).]
Figure 13.
Sex ratio at birth and joinpoint segments for births to white mothers, 1970–2002. [From Mathews and Hamilton (264).]
As data suggest a possible decline in male fertility over the past half century, investigators have explored whether a relationship exists between infertility and sex ratio. Hypothesizing that infertile men might have an impaired ability to sire male heirs, Weijin and Olsen (453) found that couples with a longer time to pregnancy had a lower sex ratio (453). However, other investigations have cast doubt on this relationship (105, 171, 180, 394). A Dutch group found that the proportion of male births increases with a longer time to pregnancy (394). As female influences are thought to be a powerful mechanism for gender selection, examining postgestational outcome such as live birth sex might be inadequate to assess the role of the male contribution to the sex ratio. A US group examined the proportion of Y bearing sperm and identified an inverse relationship between the production of Y chromosome bearing sperm and semen quality, suggesting an impaired ability for infertile men to sire male heirs (104).
In addition to the fertility of the parents, the health of the parents has also been examined as a factor that might impact sex ratio. Diabetes, non-Hodgkin's lymphoma, hepatitis B, and testicular cancer are among the diseases thought to lower sex ratio (70, 171, 318, 357). The impact that environmental exposure can have on sex ratio has prompted concern regarding the recent declines in the sex ratio in several countries. An explosion at a chemical plant in Seveso, Italy in 1976 exposed the local population to high levels of dioxin, a known endocrine disruptor. A subsequent generation of children sired by parents with high exposure levels displayed a lowered sex ratio (278, 279). Workers exposed to the gonadotoxic nematocide DBCP demonstrated reduced sex ratios compared with children born prior to paternal exposure (137, 336). Other exposures including boron and those from aluminium manufacture have also demonstrated decreases in the sex ratio or sperm Y:X ratio (277, 359). These data demonstrating an influence of chemical exposure on sex ratio has laid the foundation for many to hypothesize that environmental exposures might be the driver behind the declining sex ratio.
In addition to chemical exposure, environmental stressors in the form of catastrophic events have also been shown to alter the sex ratio. The Kobe earthquake, September 11 attack in New York, economic downturns, and war have all been shown to lower the sex ratio (67, 68, 126, 482). The authors speculated that alterations in semen quality or spontaneous abortion might have contributed, although the definitive etiology remains uncertain. While the preconception and adult environment might play a role in sex ratio, social factors might also impact on sex ratio. Sex-selective abortion in some countries have increased in prevalence based on availability of abortion and early identification of the sex of a fetus (124, 178, 476). Such practices might have a significant impact on the sex ratio of an entire population (29).
III. INFERTILITY
Given the reported changes in male reproductive health, an immediate question is whether they are associated with an increased prevalence of infertility. This is a challenging question to answer given the absence of population-based monitoring data suitable for assessing temporal patterns of infertility, and the methodological nuances associated with measuring infertility as briefly noted below.
A. Definition
Infertility has been defined as the inability of a couple to conceive after 1 yr of sexual intercourse without contraception (110). This broad definition does not reflect the considerable heterogeneity of infertility, which comprises both couples without and with prior pregnancies, or so-called primary and secondary infertility, respectively. Approximately half of infertility with an identifiable diagnostic finding is attributed to female factors (e.g., endocrine, tubal, uterine, cervical, and oocyte factors) and another half to male factors (e.g., poor spermatogenesis, cryptorchidism, poor semen quality, cancer, genetic syndromes). Such diagnostic categorization will be dependent on clinical norms and practices, the extent of diagnostic testing that couples undergo, and the sensitivity/specificity of such testing. For example, 66% of fertile couples undergoing standardized infertility evaluations for research purposes were observed to have one or more infertility factors (146). Another consideration with regard to infertility terminology is the uncertain percentage of couples that might have both male and female factors identified, while many others will have unknown or idiopathic infertility. Considerable misclassification of diagnostic subtypes of infertility arises when based on self-reported information (94).
Also, an unknown percentage of infertile couples will resolve their infertility either spontaneously or with medical treatment, while others will have unresolved infertility. This observation has prompted authors to define infertility as a continuum of fecundity ending with an absolute inability to conceive (145).
B. Prevalence/Incidence of Infertility
One of the earliest prevalence estimates of infertility estimated that 16% of couples in the United Kingdom were affected (169) followed by estimates that varied by place and time. For example, the prevalence of infertility ranged from 8 to 12% in Asian and Latin American studies (458), with similar ranges reported in parts of sub-Saharan Africa. Specifically, prevalence was 9% in Gambia (406), although 20–30% in Nigeria (229, 315). An overall prevalence in Europe was estimated to be 16%, ranging from a low of 10% in Southern Italy to a high of 24% in East Germany (196). Prevalence varied from ∼15 to 33% in five population-based samples (Denmark, Germany, Italy, Poland, and Spain) of women aged 25–44 yr reporting for their first pregnancy attempt (205). In a random sample of Scottish women aged 31–50 yr, ∼19% reported having experienced infertility with more primary than secondary infertility reported (43). Most recently, prevalence in Canada was reported to range from ∼12 to 16% when varying the assumptions about factors associated with conception (62). Geographical variation in prevalence is also observed among developing countries, ranging from ∼4 to 17% in 25 population-based surveys comprising 172,413 women, with lifetime infertility ranging from 12 to 26% (49). Of note is the preponderance of prevalence data based on female rather than male reporting. A recent systematic review revealed wide fluctuations in prevalence depending on definition, choice of referent population, and specification of numerators/denominators underscoring the inability to derive a single prevalence estimate across studies (145). Figure 14 illustrates some of the considerations that are needed when defining infertility (numerator) and selecting the population at risk (denominator), which might affect prevalence estimates. In addition, global prevalence of lifetime infertility has been reported to vary from 6.6% in Norway (364) to 32.6% in the US (372).
Figure 14.
Roles of definitions, populations, numerators, and denominators of calculated prevalence of infertility.
These estimates of the prevalence of infertility were obtained from cross-sectional surveys, but ideally incidence data are needed. Such data must be derived from prospective cohort studies that recruit couples prior to or upon becoming at risk for pregnancy, such as when discontinuing contraception for purposes of becoming pregnant. Couples are then followed daily through 12 menstrual cycles or months at risk for pregnancy. Three such studies have been conducted (59, 170, 480), while another four preconception cohort studies have followed only female partners for 12 cycles or months (58, 109, 120, 418). Collectively, these prospective cohort studies with preconception enrollment of couples or women suggest that the incidence of infertility ranges between 12 and 18%. Irrespective of study design, it is important to keep in mind that human fertility is dynamic in nature, as infertility does not necessarily imply sterility. For example, while fecund couples have pregnancies resulting in births, many couples with fecundity impairments, such as those experiencing pregnancy losses or 12-mo infertility, will become pregnant and have births either with or without medical assistance as illustrated in Figure 15.
Figure 15.
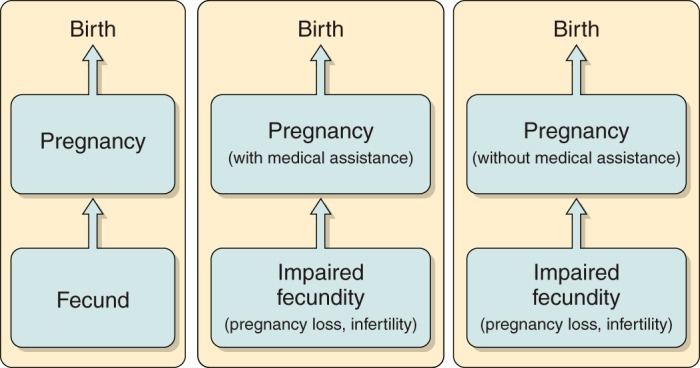
Dynamic nature of fecundity and fertility.
Another approach for estimating the prevalence of infertility utilizes time-to-pregnancy (TTP) data usually obtained from pregnant women or through record linkages or registries data. TTP is easily obtained from questionnaires asking how long the couple had regular unprotected intercourse before pregnancy occurred and allows for the categorization of couples not only with regard to infertility (TTP >12 cycles/mo) but also in relation to conception delay or so-called impaired fecundity (TTP >6 cycles/mo). Reliance on retrospectively reported TTP requires some caution with regard to interpretation, given its uncertain validity that has only been empirically assessed in two studies using the gold standard of prospectively measured TTP. Specifically, the validity of self-reported TTP was reported to be good for shorter periods of recall (477), but poor for longer periods of recall given notable bidirectional errors in reporting (80). However, reliability of retrospectively reported TTP has been reported to be good (182).
Recently, the current duration approach has been developed and offers a novel method for identifying women currently at risk for pregnancy. This cross-sectional approach queries women (or men) regarding the time since stopping contraception or attempting to become pregnant at the time of interview. This method allows for the estimation of a TTP-like distribution that accounts for left censoring (207). Applying this approach in France using a household-based sampling framework yielded a 12-mo infertility prevalence of 24% and a 24-mo prevalence of 11% (390). Recently, the current duration approach was used to estimate infertility prevalence among respondents in the 2002 National Survey of Family Growth (NSFG) conducted in the US. Prevalence was estimated to be 15.5% based on female reporting (416) and 12.0% for male reporting (251).
C. Temporal Patterns of Infertility
It is exceedingly difficult with available data to accurately estimate the temporal pattern of infertility as illustrated in a recent systematic review (145). Doing so would require longitudinal assessments using similar methods that are responsive to the nuances underlying pregnancy intentions, periods at risk, and other methodological issues including sources of sampling biases. While several authors have estimated infertility prevalence in various countries for particular time periods as noted above, few attempts have been undertaken to assess temporal patterns. Perhaps the closest available “temporal” data are derived from the NSFG. This cross-sectional survey was conducted at specific time periods (1982, 1988, 1995, 2002) until continual enrollment began in 2006. The NSFG survey interviews a representative sample of US women aged 18–44 yr (and men commencing in 2006) about many aspects of reproductive health. Infertility is not directly queried but is derived from respondents' answers to a series of conditional questions on relationship status, sexual activity, contraceptive use, and pregnancy attempts within the past 12 mo. In 2002, this construct estimated a US prevalence of 7.4% (72), which is half the estimate (15.5%) for this same time period using the current duration approach, as noted above. This might be a function of the survey's continued reliance on a construct measure of infertility rather than direct querying of men/women. Based on repeated cross-sectional NSFG data, the 12-mo infertility prevalence for married women has steadily declined in the US from 8.5% in 1982 to 6.0% in 2006–2010 (71); however, a growing percentage (41% in 2011) of US births are to unmarried women (262). We are unaware of data on temporal patterns of infertility for other geographical locations.
To our knowledge, there is no population-based monitoring of infertility in any country. This critical data gap is in the context of considerable reported variations in human fecundability, as measured by TTP (176) or semen quality (186, 409), and despite earlier calls for monitoring human fecundity via surveys (179) or more purposeful research initiatives inclusive of the couple (317).
Attempts to assess temporal patterns of human fecundity include the following four initiatives presented in chronological order. First, temporal patterns of self-reported TTP, defined as the number of years of involuntary childlessness, were assessed for 832,000 primiparous women aged 20 yr and older with births between 1983 and 2002 as identified in the Swedish Medical Birth Registry (370). This unique investigation accounted for two important sources of bias, namely, truncation and age and calendar time at the initiation of trying. Subfertility was estimated to have decreased for more recent cohorts, although it is important to note that the sampling framework comprised only fertile women giving birth, which underrepresents women with impaired fecundity who either cannot conceive or carry a pregnancy to live birth. Another important consideration is the increased awareness of the fertile window over this time period and the availability of in-home tests aimed at timing intercourse to maximize chances of conception or for identifying pregnancy, which were not as readily available in earlier cohorts.
A second initiative assessed temporal patterns in the rates of natural conceptions for 803,435 Danish women born between 1960 and 1984, allowing for an indirect assessment of infertility (230). A gradual decline in both observed and projected rates of natural conception was observed. Other important observed trends included declining abortion rates and a slight increase in the percentage of childless women regardless of use of assisted technologies (14.5 to 15.6%). Another investigation assessed trends in childlessness among birth cohorts of males, which is unique in that most research focuses on females. Specifically, 1,359,975 Danish men born between 1945 and 1980 were linked with their children using national birth and ART registries (338). The percentage of childless men at age 45 yr increased from 14.8 to 21.9%.
While infertility was not estimated specifically, investigators pooled five European databases to assess fertility time trends for 8,532 combined pregnancies whose mothers were aged 20–34 yr and for whom “valid” self-reported TTP was available along with 715 contraception failures occurring between 1953 and 1993 (181). An increasing fertility trend was reported, and attributed to a male cohort effect for TTP and contraception failure. While the authors undertook several analyses, there are noteworthy limitations that might impact on temporal patterns including restricting pregnancies: 1) to those resulting in a live birth, which systematically excludes women unable to conceive or carry a pregnancy to birth, and 2) to the first trying attempt rather than including all such attempts. This latter practice assumes that the first pregnancy attempt is representative of all subsequent trying attempts, which might or might not be true (250).
D. Use of ART
Indirect information on trends in infertility might be obtained from statistics on assisted reproduction. In Denmark, nationwide activities on ART are registered every year. As seen from Figure 16, there has been a significant increase in the use of ART during the past 13 years. While the level of assisted reproduction might seem to have levelled out in recent years, there has at the same time been a 13% drop in the number of Danish women aged 25–40 yr, which is the age range of the majority of women seeking help to reproduce. Consequently, the proportion of couples/women seeking treatment has continued to increase.
Figure 16.
Assisted reproduction (ART) in Denmark, 2001–2014. IUI-D, insemination with donor semen; IUI-H, husband semen; OD, oocyte donation; FER, frozen embryo replacement; IVF, in vitro fertilization; ICSI, intracytoplasmatic sperm injection. From The Danish Fertility Society: http://www.fertilitetsselskab.dk/images/2015_dok/dfs2014.pdf.
While it might appear that there has been a huge increase in the usage of donor semen, it is important to note several possible reasons for this increase. As a consequence of legislative changes, treatment of lesbian and single women was introduced during the time period, thus increasing the number of women seeking this treatment option. Furthermore, complete registration on the number of treatments using donor sperm have only been achieved in recent years.
It is important also to recognize that data on birth cohorts always include children conceived through assisted reproduction, and this could mask the “real” fertility potential in a population.
IV. ROLE OF GENETIC BACKGROUND FOR THE RISK OF MALE REPRODUCTIVE DISORDERS
It is clear that the quick pace of incidence trends of the above reviewed disorders of male reproductive health is consistent with the relatively recent changes in the environment and lifestyle-related factors, rather than accumulation of inherited genetic aberrations. However, the available data demonstrate that there have been quite striking geographical and ethnic differences in most of these trends.
A. Genetic Polymorphisms Explaining Ethnic Differences
The most clear-cut evidence of ethnic differences in disease incidence has been provided by epidemiological studies of testicular cancer, which are briefly reviewed above. The incidence of TGCC worldwide is by far greatest among white people of northern European ancestry and lowest among African men. These prevalence differences are not primarily related to environment, as convincingly illustrated by ethnic differences among the populations in the US (133, 269).
Men of African ancestry seem to be “protected” from testicular cancer, and possibly also have a lower incidence of cryptorchidism. An explanation has been sought in several studies. Because of the association of TGCC with inborn disorders, especially those linked to insufficient masculinization, including cryptorchidism, it was hypothesized that the steroid hormone levels might differ between black and white people. Although no marked differences have been noted in serum testosterone between white and black men, there might be some differences in women. Significantly higher (by 48%) levels of serum testosterone were identified in black women during the first trimester of pregnancy (164). These data are uncertain, because the studied groups were very small, and the genetic polymorphisms responsible for the observed differences have not been identified.
It is known that androgen action is modulated to a small extent by the number of trinucleotide CAG or GGC/GGN repeats in exon 1 of the androgen receptor (AR) gene. A highly expanded AR(CAG)>40 is a cause of a serious neurodegenerative disorder (spinal bulbar muscular atrophy, or Kennedy syndrome), which is also associated with progressive failure of spermatogenesis and with hypogonadism (226). A subtle decrease in the transactivation of the AR has been reported in men with either long or very short (CAG)n stretches (303). Conversely, both long and short stretches (CAG)n repeats have been associated with infertility (304), but many other studies did not show any such association (457). On the other hand, the AR polymorphisms might modulate sperm production in normal men (448). The current consensus based on meta-analytic studies is that longer CAG repeats are associated with male subfertility at a cohort level and are considered a contributing factor rather than a cause of infertility (93, 304, 434). In general, no significant associations of the AR(CAG)n repeats polymorphism and testicular cancer have been detected among white men (346), but some weak associations with some TGCC histologies or with certain combinations of the repeats have been reported (93, 128, 135). Shorter AR(CAG)n repeats have also been associated with cryptorchidism in white males (92). Taken together, these data show that the AR polymorphisms might have a minor modulating effect on testis function and possibly also on the ethnic variability in the risk of reproductive disorders, but cannot explain the huge difference in the TGCC incidence between blacks and whites.
However, GWAS of testicular cancer performed in recent years in multi-ethnic populations have identified a robust genetic risk factor, a polymorphic SNP rs995030 in the KITLG locus (203, 349). The frequency of the KITLG risk allele differs significantly between populations, with a sizable majority of Caucasians (81%) carrying it, compared with <25% in African populations (203, 240). This skewed distribution of the polymorphic alleles makes biological sense, as, in addition to germ cell migration and survival, the KITLG/KIT signaling pathway is involved in differentiation of melanocytes and regulation of skin pigmentation; hence, the KITLG has likely undergone positive selection in the European population during adaptation to changed light and temperature conditions (203).
B. Genetic Causes of Male Infertility
As far as the genetic risk for male subfertility or infertility is concerned, it is clear that sex chromosome aneuploidy, deleterious gene mutations or copy number variations (CNV, especially deletions) that negatively affect testis development, germ cell development, or sperm maturation will cause these phenotypes. Numerous genetic aberrations can be mentioned here, including XXY, XX-male, deletions and rearrangements of the Y-chromosome, mutations in SRY, AR, CFTR, NR5A1, etc. A detailed description exceeds the scope of this review, so the reader should consult recent papers devoted specifically to genetics of male infertility (28, 36, 166, 216, 245, 273). Some of the Y-chromosome deletions often have significant impact on spermatogenesis, but the phenotypes are variable depending on the copy number, other rearrangements, or a constellation of inherited common gene variants. Partial AZFc deletions (e.g., gr/gr), removing some but not all copies of DAZ, CDY, and other coding and noncoding genes are a typical example (245, 352, 366). Genome-wide CNV array studies using modern versions of the comparative genomic hybridization (CGH) technique began to uncover additional CNV linked to male infertility (73, 217, 249, 403, 435 471). Interestingly, a generally increased CNV burden seems to be associated with male infertility (28, 216). Several such aberrations, inculuding mutations within TEX11 gene, have been mapped to the X-chromosome, which houses many germ cell-specific genes, and in analogy to the Y contains palindromic regions that facilitate rearrangements (73, 217, 471).
However, knowledge on more subtle regulation of human spermatogenesis by genetic variability remains rather limited, and many single-gene studies based on educated guess, including the above-summarized AR story, did not contribute much (434). One polymorphic pathway is a notable exception: gene variants of FSHB and FSHR have been confirmed as biologically relevant by independently performed robust studies (142). Two polymorphisms in this pathway, FSHB −211G>T and FSHR 2039A>G, have been shown to be associated with serum FSH and testicular volume, and the carriers of a combination of the two less favorable genotypes had a greater risk of oligozoospermia (433).
After the appearance of the genome-wide SNP microarrays, several GWA studies and numerous replication attempts assessing single SNPs failed to associate such variants with male infertility. Most of those studies have given inconclusive results due to heterogeneity of patient phenotypes and often insufficient power of studies. It required much larger study populations such as of azoospermic men from China (168, 474) or selecting a genetically related cohort (214) to identify a quite modest number of informative SNPs, which are still of limited clinical relevance (28). To what extent these common variants contribute to modulation of reproductive function remains to be established.
In summary, it is clear that the genetic variability between ethnically different populations, e.g., Africans versus Europeans, has a profound effect on the risk of reproductive disorders, which is well documented for TGCC. However, genetic background cannot explain temporary trends within the same ethnic group, which are predominantly environmentally determined. Importantly, common genetic variants can modulate the individual susceptibility within the same population, because not all exposed men develop the same phenotypes. Another important aspect is that inherited traits are not always genetic. Fascinating new research provides evidence that the predominant way of individual adaptation to the changing environment is likely epigenetic, as summarized below.
V. ENVIRONMENTAL MODULATION OF EPIGENETIC GERM CELL PROFILE: A POSSIBLE EXPLANATION FOR SOME OF THE REPRODUCTIVE HEALTH TRENDS?
Epigenetic processes affect gene expression without changing the gene sequence. There are numerous mechanisms of epigenetic regulation, and only those best described in the literature with regard to reproduction are mentioned here. Since the topic of this review is human fertility, the emphasis is on germ cells, which are remarkably different from the somatic cells in terms of epigenetic regulation during development and maturation.
A. DNA Methylation in Germ Cells
The best known and probably most common mechanism of gene silencing is DNA methylation, which involves direct alteration of DNA by methylation of cytosine/CG dinucleotides. This process is fundamental for developmental programming and cell differentiation and is best known from parental imprinting and X-chromosome inactivation. DNA methylation is partly determined by DNA sequence because of the nonrandom localization of the CpG islands, which are especially frequent in promoter regions or repetitive sequences (376).
Studies in mice demonstrated that soon after fertilization the genome is demethylated to remove paternal marks, and the process of erasure of DNA methylation is repeated again only in primordial germ cells (PGC) (152). The demethylation process requires a set of specialized enzymes (e.g., APOBEC1, TETs) and the base excision repair proteins (MBD4, APEX1, PARP1) (152, 198). Subsequently, the genome is progressively remethylated according to the preprogrammed pattern, including restoration of the imprinting. In human testes, this remethylation process begins when fetal gonocytes gradually mature to prespermatogonia (136, 456). In pathological situations, where the gonocytes fail to mature and become pre-GCNIS cells due to gonadal dysgenesis and TDS (described earlier in this review), the genome remains essentially completely demethylated (12, 306, 456), suggesting the maintenance of this status might be an active process inherent to germ cells (220), similar to the one described in mice. In normal male germ cells, the DNA remethylation process continues and is considered final in spermatocytes just before they enter meiosis (312). The remethylation of DNA requires both maintenance and de novo DNA methyltransferases (DNMTs). Inactivation of these genes in mice causes disturbance of maternal and paternal imprinting, loss of spermatogonia, and meiotic catastrophe (51, 202, 225).
B. Changes in DNA Methylation in TGCC
The regulation of the DNA methylation process in human germ cells has not yet been well described, and even less information is available regarding what causes disturbances of this process. We know, however, what happens if germ cells turn malignant, but interestingly, different types of TGCC show strikingly different patterns of genome methylation (reviewed in Ref. 221). Classical seminoma, which resembles GCNIS, is also characterized by low DNA methylation, whereas the genome of non-seminomas is methylated in a nonrandom manner: highly methylated at Alu repeats, but hypomethylated at LINE1 transposons and imprinted genes, likely due to the secondary genomic changes and vast reprogramming (12, 306, 393, 436, 456). In contrast, spermatocytic tumor, a rare TGCC predominantly of older men that originates from clonally expanding mature spermatogonia with gain-of-function mutations (139), is characterized by completely chaotic and dysregulated DNA methylation (219).
Numerous studies have documented abnormal DNA methylation in spermatozoa of patients with infertility, especially oligo-astheno-zoospermia, but also in some forms of idiopathic azoospermia (reviewed in Ref. 48). These methylation aberrations might occur both at imprinted sites, e.g., IGF2/H19 and promoter regions as well as genome-wide, so it is beyond doubt that the system is essential for sperm function and might fail at many different points. Whether these abnormalities are genetically determined, acquired during development, or caused by environmental factors specifically disturbing spermiogenesis remains to be established in most cases.
C. Does Environment Affect DNA Methylation?
A direct effect of some environmental factors on DNA methylation has been demonstrated in experimental studies in animal models. Human data are scarce, especially concerning the prenatal development. In a recent study of human fetal tissues matched for maternal smoking, changes of DNA methylation at the imprinted gene IGF2 and the glucocorticoid receptor gene (GR/NR3C1) were found, likely due to alterations in methyl donor availability and changes in 1-carbon metabolism (98). This is of relevance in view of clinical studies reporting an increased risk of cryptorchidism (see sect. IIB), changes in reproductive hormones, earlier puberty, and impaired semen quality in the males exposed in utero to maternal smoking (350).
D. Histone Modifications
Another main mechanism of gene expression regulation involves posttranslational modifications of histones, which are proteins building the cell's chromatin. The histone tails can be modified by acetylation, methylation, phosphorylation, ubiquitination, crotonylation, and other chemical additions, which change the chromatin structure allowing or prohibiting binding of transcription factors to DNA and thus regulating gene transcription (365). Some of these modifications, such as crotonylation, seem to be specific for haploid germ cells and preferentially positioned in nonrandom chromosomal regions, e.g., sex chromosomes (286). These modifications require the action of specific enzymes, such as histone acetyltransferases (HAT), histone deacetylases (HDAC), histone methyl-transferases (HMT), or histone demethylases (HDM), with the latter encoded by a family of Jumonji genes, expression of which is developmentally regulated and differs among cell types (121). Again, the current knowledge on the dynamics of histone modifications in germ cells is mainly based on rodent studies (151), with few human studies exploring this field. Only a few studies, which analyzed histone modifications in GCNIS cells and TGCC, also examined a few samples of normal fetal testes, and found some differences between mice and men, including high levels of H2A.Z, HP1γ, H3K9ac, and H4/H2AR3me2, but low levels of H3K9me2/3 and H3K27me3 (12, 35, 99). The pattern observed in GCNIS cells was characterized by an open structure of chromatin, with high levels of H2A.Z, H3K4me1/2/3, H3K9ac, H3K27ac, and H4/H2AR3me2, and the absence of the restrictive H3K9me2 and H3K27me3, but surprisingly high levels of H3K9me3 (12, 35). Combined with a very low DNA methylation level, the absence of DNA damage response and a high proliferation rate, this “permissive” chromatin of GCNIS cells may render them vulnerable to exogenous factors, possibly causing chromosomal instability and secondary genomic aberrations (35, 221).
E. Sperm Protamination
A mechanism specific for haploid germ cells and spermiogenesis is the gradual exchange of histones by protamines, P1 and P2, in preparation for DNA compaction and inactivation in late spermatids. This process is not complete, and a fraction of the compacted chromatin in human spermatozoa retains classical histones. These low-protaminated foci are predominantly associated with genome regions with regulatory functions essential for the early development of the embryo (25, 156). Despite a very marked compaction of chromatin in spermatozoa, the regions that retain histones contain many active RNAs, including protamine transcripts, but also various small RNAs (see below), which are thought to play a role immediately after fertilization (227). Dysregulation of the histone-protamine transition, resulting in increased retention of protamine transcripts (23), low protamination, or an abnormal P1/P2 ratio in sperm, has been described in patients with infertility. Abnormalities of sperm transcriptome (129) and histone retention (155) have also been detected in sperm of infertile men. Whether these abnormalities of sperm protamination in subfertile men are caused by genetic or environmental factors requires more research. So far, only cigarette smoking has been implicated in one study (472).
F. Role of Noncoding RNAs
A previously unknown mechanism of epigenetic regulation that has emerged more recently is the direct inactivation of transcripts by small noncoding RNAs (sncRNAs), comprising microRNA (miRNA), PIWI-interacting RNA (piRNA), endogenous small interfering RNA (endo-siRNA), circular RNA (circRNA), and others. piRNAs are of special relevance in the field of male reproduction, because this well-conserved class of RNA is preferentially present in germ cells (236, 470), and although piRNAs have been detected both in male and female germlines, in mammals they seem to be active mainly in testes and during spermatogenesis. Their function has not been completely elucidated, but it is thought that piRNAs together with PIWI and other similar proteins (Mili/Miwi etc) evolved to silence transposon sequences in germ cells (24, 437) and might also be involved in parental imprinting. Mouse knockout models have revealed that small RNAs and the associated proteins are essential for spermatogenesis, but human studies have only recently begun. One study reported changes in the expression profile of PIWIL2, PIWIL4, MOV10L1, and TDRD9 in testes of cryptorchid boys (149), but these data need to be confirmed in additional studies. In addition to piRNAs, male germ cells express yet another small RNA type of unclear biological significance, endo-siRNAs, which require DICER1 but no DROSHA/DGCR8, hence they differ from miRNAs (395, 478).
More robust human data exist on miRNAs, which are particularly abundant in mammalian testes and germ cells (470). Mature miRNAs are incorporated into RNA-silencing complexes, called RISC, which direct silencing of messenger RNAs (mRNAs). It has been hypothesized that miRNAs can act as a novel class of hormones, because they are often enriched in exosomes which are transported in circulation to remote organs, and this mechanism might be particularly active in pathological conditions, such as cancer (475). Indeed, very important recent studies profiled miRNAs in patients with TGCC revealed the presence of embryonic miRNAs and provided evidence that serum miRNAs are very specific and robust markers for this malignancy regardless of the age of the patient harboring the tumor (294, 322, 355, 449). Importantly, these miRNA are already present in the preinvasive GCNIS cells, thus opening possibilities of an early diagnosis of this disease (311). Specific miRNAS are enriched in mammalian testes (358), so an important role for miRNAs in regulation of human spermatogenesis is expected, but the data so far are scarce. Several human studies have shown that the miRNA profile changes in infertile men, depending on the testis histopathology, especially the presence of germ cells in seminiferous tubules (2, 85, 235, 431), but more studies are needed to dissect the role of these miRNAs and the target transcripts.
Interestingly, recent mouse studies suggest that the miRNA profile of sperm can be affected by paternal stress or trauma, even if sustained early in life, and these changes might be transmitted to the next generation (127, 362).
G. Transgenerational Environmental Effects
Since it became evident that some epigenetic modifications present in sperm are being transferred to the embryo during conception, and some of these changes are not erased, a long-suspected phenomenon of nongenomic inheritance has become a hot topic of intense research. Transgenerational effects of some environmental exposures or lifestyle habits have been observed in humans. For example, a lower incidence of heart disease and obesity was observed among grandsons of men who experienced famine in childhood (197). These observations have been confirmed and extended in transgenerational animal studies. The field is, however, not without controversy, because some of ground-breaking studies that reported such effects in the second generation of rats treated with the commonly used pesticides vinclozolin or metoxychlor (21, 22) were challenged with lack of reproducibility and other problems, which had to be clarified in an erratum, and even required withdrawal of one paper. However, since then, the group has produced very robust data confirming the transgenerational effects on male reproduction (sperm epigenome) and obesity of several endocrine disrupters used as pesticides or components of plastics, including DDT, metoxychlor, bisphenol A (BPA), and other compounds (78, 259, 260, 388). If these important data can be extrapolated to humans, some of the currently observed trends in male reproductive health might be explained by exposures to chemicals in previous generations.
In conclusion, the evidence is growing that numerous endocrine disrupters and probably other lifestyle-related factors, such as smoking, and possibly also diet and stress, are able to exert a direct effect on the human epigenome, both in utero and in adulthood, with germ cells apparently among the most sensitive cells. These effects might be aggravated by the existence of genetic variants predisposing to less optimal function of some endocrine pathways, e.g., FSHR/FSHB or AR polymorphisms, which might result in pathology, including germ cell cancer and reduced semen quality.
VI. POSSIBLE ROLE OF MALE REPRODUCTIVE DISORDERS IN DECREASING PREGNANCY RATES
The ultimate end point of normal male reproductive function is conception and delivery of a normal child without use of assisted reproductive techniques. As seen from Figure 17, there has been a remarkable decline in fertility rates in most parts of the world during the past 50–60 years, although African and some Asian and South American countries and Mexico still have TFR substantially above replacement level.
Figure 17.
Fertility rates, 1960–2013, across North America, South America, Africa, Asia, and Europe. From the World Bank: http://databank.worldbank.org/data/views/variableselection/selectvariables.aspx?source=world-development-indicators.
A decline in TFR is also noticeable in recently industrialized developing countries such as Brazil and Chile. These changes can be due to changes in social and economic factors, well described by demographers in their studies on the transition from high-fertility to low-fertility societies. Several countries have had public health policies encouraging couples to use contraception (419), although only China has had a one-child policy (122, 476). However, the early period of the declining TFR started long before the introduction of the pill, which occurred in the late 1960s. In some European countries such as Denmark, the drop in TFR even started 100 years ago (Figure 2).
There is no doubt that socioeconomic factors are important for fertility rates in modern industrialized countries (254). However, it is a crucial question whether they can fully explain the current fertility rates, which in many countries have constantly been below replacement level for 30–40 years (Figure 17). Importantly, the decline of TFR does not seem to be caused by increasing rates of induced abortions during the past 40 years. On the contrary, a decline in abortion rates has been noticed for decades in countries where legal abortions are registered (82) (see Figure 18).
Figure 18.

Total pregnancy rate of the total Danish population according to year of birth (1960–1980) of the pregnant women. ART pregnancies not included. Note the declining pregnancy rate. [From Jensen et al. (177).]
Thus we seem to be witnessing a true trend with lower pregnancy rates (82). Interestingly, in a study based on the entire Danish population, we found a significant birth cohort effect in the trend in pregnancy rates: women born in 1970 had lower rates of pregnancies (including both births and abortions) than those born in 1960 (177, 230). We also found a birth cohort trend in childlessness: Danish men born in 1960 were significantly more often childless than those born in 1945 (22 and 15%, respectively) (338). It has been speculated that increasing age of women at first pregnancy could explain the decreasing fertility rate. However, data from Statistics Denmark clearly show that the average age of delivering women was in fact higher in 1901 than today (Figure 19; Blomberg Jensen et al., unpublished data).
Figure 19.
Mean ages of Danish women delivering from 1901–2014. From Statistics Denmark: http://www.statistikbanken.dk/statbank5a/default.asp?w=1600.
A crucial question is whether reduced fecundity plays a role for the lower number of pregnancies in the more recently born cohorts. As reviewed above, recent studies have shown clear adverse trends in several aspects of male reproductive health, including an increase in the incidence of TGCC (231, 269) and lower and decreasing serum testosterone levels (15, 329, 427). Also, the incidence of congenital genital abnormalities have become more common (83, 258), and semen quality has deteriorated in many countries (30, 64, 363). Thus there is substantial evidence that a significant proportion of young men from Europe (13), Japan, and the US (212) have semen quality compatible with some degree of subfertility or even infertility. Fortunately, the reproductive capacity of normal, healthy men is very high, as normal semen specimens contain excesses of sperm. However, the evidence presented above suggests that semen quality of a significant proportion of young men in developed countries might be at or below a tipping point, where fecundity might in fact be affected (17). The situation might not yet lead to widespread infertility as moderately lower fecundity might just lead to longer waiting time to pregnancy and not be affecting family sizes of modern couples (391), most of whom only wish for two or three children. However, moderately lower fecundity might still result in a lower chance of unplanned pregnancies among couples in the general population and thereby influence pregnancy rate. Theoretically, this might have a significant effect on TFR, as unplanned (but still accepted) pregnancies without abortion occur very often (291). However, severely reduced male fecundity due to poor semen quality might cause “clinical” infertility necessitating ART, particularly if the female partner is also subfertile, for example, due to age.
Unfortunately, as discussed above, there are big gaps in our knowledge concerning trends and extent of human infertility. In addition, it is often difficult or impossible to elucidate whether a female or a male factor is the main problem of an infertile couple. Quite often combined female and male problems exist. However, the reported decrease in semen quality in some countries and widespread poor semen quality reported from other countries combined with increasing use of intracytoplasmatic sperm injection (ICSI), which is particularly useful in cases of poor semen quality, are in line with the assumption that male factor infertility has become more frequent.
In Denmark, ∼8% of all children are now born after ART, indicating that infertility has become a major health issue. As seen from Figure 20, the ART activity has for several years contributed significantly to the total number of children born in Denmark.
Figure 20.
Total number of births in Denmark, 1940–2012 (blue curve), and total number of births minus births after ART, 2003–2012 (red curve). Note that the numbers of births conceived without the use of ART in 2012 were similar to the low point reached in 1983, before ART was introduced. From Statistics Denmark and The Danish Fertility Society: http://www.statistikbanken.dk/statbank5a/default.asp?w=1600 and http://www.fertilitetsselskab.dk.
VII. AFTERWORD: RESEARCH CHALLENGES
Reproductive health is fundamental for a society and its culture. Both high and low fertility rates can be problematic for economy, social structures, and health. Overpopulation has for several decades been considered a global threat, and World Health Organization and other international bodies have focussed on contraceptive programs worldwide (96) and fertility rates are still high in several parts of the world (Figure 17). However, as illustrated above (463), we are now seeing clear downward trends towards TFR being persistently below replacement level, not only in the “old” industrialised countries (Figures 17 AND 18), but also newly industrialized societies like Brazil and Chile have below-replacement birth rates. And these shifts in fertility occur despite increasing use of ART. Hitherto, low fertility has caught public attention mainly because of the economic and social effects, including decreasing work force and increased economic burden that comes from care of relatively more elderly people (450). In contrast, remarkably little attention has been given to the possibility that decreasing fertility rates could represent a public health problem due to widespread decreased fertility among couples in modern societies (112).
The epidemiology of infertility continues to be an under-studied end point despite increasing evidence that it has implications for health and disease across the lifespan and, possibly, generations. A longer TTP, or requiring more than 12 mo for conception, has been associated with a higher risk of adverse pregnancy outcomes (276, 343) and gravid diseases (37). Infertility is a reported risk factor for both ovarian and testicular cancer (33, 305) among other later onset adult diseases. Despite the importance of infertility for human health, few risk factors have been identified other than partners' ages. While many socio-demographic or lifestyle factors have been associated with infertility, much of the available data rely on retrospectively measured TTP and exposure data. The few prospective cohort studies conducted to date underscore the absence of longitudinal data for this important outcome, with even fewer data on risk factors for diagnostic subtypes of infertility (60). Paradigms for further study of human fecundity, its impairments, and health across the lifespans or generations have been advanced and might help guide the synthesis of available data and the design of future work to fill critical data gaps (57, 387). To do so will require standardized methodologies and surveillance, as recently called for by the US National Public Health Action Plan for the Detection, Prevention, and Management of Infertility (69).
If indeed poor reproductive health plays a role for the declining pregnancy trends, we might not see any upward trends in fertility for the foreseeable future, as the populations of women of reproductive ages and future generations of children will undoubtedly decrease substantially in the coming years, considering that the current cohorts of children below 18 years are substantially smaller than a generation ago. As a matter of fact, in Demark where the average TFR has been ∼1.7 for 40 years, the population of women in reproductive age already seems to have declined by 20% (Figure 21), although the population as a whole has not declined, due to the fact that people live longer and the number of immigrants has increased. In countries with significantly lower TFR like Japan, a decrease in the total population is already visible and prone to further decreases in the years to come (314).
Figure 21.
Examples of longitudinal changes in population sizes with different total fertility rates (TFR). A population is sustained if TFR is 2.1. However, a constant TFR of 1.5 (which is the current average in EU) will result in a 60% reduction of the population of young people over three generations, excluding immigration.
A. Pertinent Research Needs
The persistently extremely low fertility rates we have described for European and some Asian countries call for strategic research initiatives focusing on the role of both female and male infertility factors. In this review we have limited ourselves to male reproductive problems. Our analysis of these male disorders points to several pertinent research needs.
We need to clarify to what extent the lower pregnancy rates reported from many countries during the past decades are due to socio-economic factors (availability of more efficient contraceptive methods, delayed family initiation, less frequent sex, less desire/opportunity to raise children, etc.) or to biological causes resulting in a generally lower fecundity of the population. This might involve collaboration between researchers in reproductive medicine and social sciences to:
Establish methods to monitor trends in fertility in a meaningful way, including methods to distinguish between voluntary and nonvoluntary childlessness. This could include, for example, monitoring of frequency of unintended pregnancies or changes in sex ratios as potential surrogates for fecundity in analyses of fertility.
Develop epidemiological tools to distinguish between the role of male and female factors for infertility.
Initiate national prospective surveillance programs on infertility and other reproductive health problems.
Gender differences in reproductive health issues need to be explored, including the role of gender differences in regulation of sex development:
Can a sex difference with regard to effects of environmental exposures on the reproductive system explain that there are many more reports on environmental effects on the male than the female reproductive system? Is this biology or is there simply a publication bias?
Is it possible that current exposures to industrial chemicals have stronger effects on males than on females due to anti-androgenic and estrogenic properties of the chemicals?
What is the role of prenatal and childhood factors for male infertility and decreased testosterone levels in adulthood?
Why do healthy humans in general have poorer spermatogenesis than most other mammals?
Why is testicular cancer increasing all over the world and are the trends inversely related to trends in fecundity?
We need a better understanding of gene-environment interactions to disentangle the environmental impact on reproductive health from biological variation:
What is the role of genetic polymorphisms for the racial differences in male reproductive health, such as the relatively high incidences of testicular cancer among Caucasians and low incidences among Africans and Asians?
What is the contribution of environment versus genetic polymorphisms for congenital disorders of male reproductive tract, including cryptorchidism and hypospadias?
What is the role of epigenetics for male reproductive disorders and male infertility?
This long list of questions, which is far from complete, illustrates our ignorance regarding several important aspects of biology and pathophysiology related to human reproduction. A reason why the lack of focus on research in reproductive biology in countries with low fertility has persisted for decades might be the fact that effects of low fertility rates in the beginning are silent. Paradoxically, the total number of people might still be increasing for a couple of decades in spite of birth rates below replacement level, because elderly people who now live longer more than compensate for several years for the fewer children. Therefore, large-scale population effects will not be manifest until many years later. In addition, immigration might to some extent compensate for the fewer births. Therefore, people might see the current demographic development as something that can relatively easily be managed by socioeconomic initiatives. This might also have been the case if low fertility rates would persist for only a couple of decades. However, the current trends we see with regard to Europe, Japan, and Singapore do not suggest any change in fertility rate in the foreseeable future. On the contrary, it seems a more likely scenario that fertility rates significantly below replacement level have become “chronic” in those areas of the world, where they have virtually been unchanged for more than a generation. If they persist at the current level, our grandchildren and their children will face a different world.
GRANTS
Work in the laboratories of N. E. Skakkebaek, E. Rajpert-De Meyts, A. Juul, A.-M. Andersson, N. Jørgensen, L. Priskorn, and T. K. Jensen was generously supported by the Danish Medical Research Council, the Danish Cancer Society, the Danish Innovation Fund, and Kirsten and Freddy Johansen's Fund. J. Toppari has received grant support from Academy of Finland and Sigrid Juselius Foundation. G. M. Buck Louis and K. J. Sapra are supported by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Acknowledgments
N. E. Skakkebaek framed the concept of the paper and made the first outline. E. Rajpert-De Meyts was responsible for the genetics and epigenetics sections. All other authors contributed to the content of various other sections of the work.
Address for reprint requests and other correspondence: N. E. Skakkebaek, University Department of Growth and Reproduction and EDMaRC, Rigshospitalet, Copenhagen, Denmark (e-mail: nes@rh.dk).
REFERENCES
- 1.Abduljabbar M, Taheini K, Picard JY, Cate RL, Josso N. Mutations of the AMH type II receptor in two extended families with persistent Mullerian duct syndrome: lack of phenotype/genotype correlation. Horm Res Paediatr 77: 291–297, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Halima M, Hammadeh M, Backes C, Fischer U, Leidinger P, Lubbad AM, Keller A, Meese E. Panel of five microRNAs as potential biomarkers for the diagnosis and assessment of male infertility. Fertil Steril 102: 989–997, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Acerini CL, Miles HL, Dunger DB, Ong KK, Hughes IA. The descriptive epidemiology of congenital and acquired cryptorchidism in a UK infant cohort. Arch Dis Child 94: 868–872, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Afeiche MC, Bridges ND, Williams PL, Gaskins AJ, Tanrikut C, Petrozza JC, Hauser R, Chavarro JE. Dairy intake and semen quality among men attending a fertility clinic. Fertil Steril 101: 1280–1287, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aho M, Koivisto AM, Tammela TL, Auvinen A. Is the incidence of hypospadias increasing? Analysis of Finnish hospital discharge data 1970–1994. Environ Health Perspect 108: 463–465, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akre O, Boyd HA, Ahlgren M, Wilbrand K, Westergaard T, Hjalgrim H, Nordenskjold A, Ekbom A, Melbye M. Maternal and gestational risk factors for hypospadias. Environ Health Perspect 116: 1071–1076, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akre O, Lipworth L, Cnattingius S, Sparén P, Ekbom A. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology 10: 364–369, 1999. [PubMed] [Google Scholar]
- 8.Aksglæde L, Olsen LW, Sorensen TI, Juul A. Forty years trends in timing of pubertal growth spurt in 157,000 Danish school children. PLoS One 3: e2728, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Abbadi K, Smadi SA. Genital abnormalities and groin hernias in elementary-school children in Aqaba: an epidemiological study. East Mediterr Health J 6: 293–298, 2000. [PubMed] [Google Scholar]
- 10.Allan BB, Brant R, Seidel JE, Jarrel JF. Declining sex ratios in Canada. Can Med Assoc J 156: 37–41, 1997. [PMC free article] [PubMed] [Google Scholar]
- 11.Almstrup K, Høi-Hansen CE, Wirkner U, Blake J, Schwager C, Ansorge W, Nielsen JE, Skakkebæk NE, Rajpert-De Meyts E, Leffers H. Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res 64: 4736–4743, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Almstrup K, Nielsen JE, Mlynarska O, Jansen MT, Jørgensen A, Skakkebæk NE, Rajpert-De Meyts E. Carcinoma in situ testis displays permissive chromatin modifications similar to immature foetal germ cells. Br J Cancer 103: 1269–1276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen AG. Semen Quality and Reproductive Hormones in Normal Young Men and in Partners of Pregnant Women (PhD thesis) Copenhagen: Univ. of Copenhagen, 2001. [Google Scholar]
- 14.Andersen HR, Schmidt IM, Grandjean P, Jensen TK, Budtz-Jorgensen E, Kjaerstad MB, Baelum J, Nielsen JB, Skakkebæk NE, Main KM. Impaired reproductive development in sons of women occupationally exposed to pesticides during pregnancy. Environ Health Perspect 116: 566–572, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson AM, Jensen TK, Juul A, Petersen JH, Jørgensen T, Skakkebæk NE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab 92: 4696–4705, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Andersson AM, Jørgensen N, Larsen LF, Rajpert-De Meyts E, Skakkebæk NE. Impaired Leydig cell function in infertile men: a study of 357 idiopathic infertile men and 318 proven fertile controls. J Clin Endocrinol Metab 89: 3161–3167, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Andersson AM, Jørgensen N, Main KM, Toppari J, Rajpert-De Meyts E, Leffers H, Juul A, Jensen TK, Skakkebæk NE. Adverse trends in male reproductive health: we may have reached a crucial “tipping point.” Int J Androl 31: 74–80, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson AM, Juul A, Petersen JH, Müller J, Groome NP, Skakkebæk NE. Serum inhibin B in healthy pubertal and adolescent boys: relation to age, stage of puberty, and follicle-stimulating hormone, luteinizing hormone, testosterone and estradiol levels. J Clin Endocrinol Metab 82: 3976–3982, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Andersson AM, Toppari J, Haavisto AM, Petersen JH, Simell T, Simell O, Skakkebæk NE. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab 83: 675–681, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Andolz P, Bielsa MA, Villa J. Evolution of semen quality in North-eastern Spain: a study in 22,759 infertile men over a 36 year period. Hum Reprod 14: 731–735, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308: 1466–1469, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology 147: S43–S49, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Aoki VW, Liu L, Carrell DT. A novel mechanism of protamine expression deregulation highlighted by abnormal protamine transcript retention in infertile human males with sperm protamine deficiency. Mol Hum Reprod 12: 41–50, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316: 744–747, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Arpanahi A, Brinkworth M, Iles D, Krawetz SA, Paradowska A, Platts AE, Saida M, Steger K, Tedder P, Miller D. Endonuclease-sensitive regions of human spermatozoal chromatin are highly enriched in promoter and CTCF binding sequences. Genome Res 19: 1338–1349, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aschim EL, Haugen TB, Tretli S, Daltveit AK, Grotmol T. Risk factors for hypospadias in Norwegian boys: association with testicular dysgenesis syndrome? Int J Androl 27: 213–221, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Aschim EL, Nordenskjold A, Giwercman A, Lundin KB, Ruhayel Y, Haugen TB, Grotmol T, Giwercman YL. Linkage between cryptorchidism, hypospadias, and GGN repeat length in the androgen receptor gene. J Clin Endocrinol Metab 89: 5105–5109, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Aston KI. Genetic susceptibility to male infertility: news from genome-wide association studies. Andrology 2: 315–321, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Attane I. China's family planning policy: an overview of its past and future. Stud Fam Plann 33: 103–113, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Auger J, Czyglik F, Kunstmann JM, Jouannet P. Significant decrease of semen characteristics of fertile men from the Paris area during the last 20 years. Hum Reprod 9, Suppl 4: 72, 1994. [Google Scholar]
- 31.Axelsson J, Rylander L, Rignell-Hydbom A, Giwercman A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum Reprod 26: 1012–1016, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Azevedo MF, Horvath A, Bornstein ER, Almeida MQ, Xekouki P, Faucz FR, Gourgari E, Nadella K, Remmers EF, Quezado M, de Alexandre RB, Kratz CP, Nesterova M, Greene MH, Stratakis CA. Cyclic AMP and c-KIT signaling in familial testicular germ cell tumor predisposition. J Clin Endocrinol Metab 98: E1393–E1400, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker JA, Buck GM, Vena JE, Moysich KB. Fertility patterns prior to testicular cancer diagnosis. Cancer Causes Contr 16: 295–299, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Barthold JS, Wang Y, Kolon TF, Kollin C, Nordenskjold A, Olivant FA, Figueroa TE, BaniHani AH, Hagerty JA, Gonzalez R, Noh PH, Chiavacci RM, Harden KR, Abrams DJ, Kim CE, Mateson AB, Robbins AK, Li J, Akins RE Jr, Hakonarson H, Devoto M. Phenotype-specific association of the TGFBR3 locus with nonsyndromic cryptorchidism. J Urol 193: 1637–1645, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartkova J, Moudry P, Hodny Z, Lukas J, Rajpert-De Meyts E, Bartek J. Heterochromatin marks HP1gamma, HP1alpha and H3K9me3, and DNA damage response activation in human testis development and germ cell tumours. Int J Androl 34: e103–e113, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Bashamboo A, Ferraz-de-Souza B, Lourenco D, Lin L, Sebire NJ, Montjean D, Bignon-Topalovic J, Mandelbaum J, Siffroi JP, Christin-Maitre S, Radhakrishna U, Rouba H, Ravel C, Seeler J, Achermann JC, McElreavey K. Human male infertility associated with mutations in NR5A1 encoding steroidogenic factor 1. Am J Hum Genet 87: 505–512, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basso O, Weinberg CR, Baird DD, Wilcox AJ, Olsen J. Subfecundity as a correlate of preeclampsia: a study within the Danish National Birth Cohort. Am J Epidemiol 157: 195–202, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Bay K, Main KM, Toppari J, Skakkebæk NE. Testicular descent: INSL 3, testosterone, genes and the intrauterine milieu. Nature Rev Urol 8: 187–196, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Beleza-Meireles A, Omrani D, Kockum I, Frisen L, Lagerstedt K, Nordenskjold A. Polymorphisms of estrogen receptor beta gene are associated with hypospadias. J Endocrinol Invest 29: 5–10, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Bergström R, Adami HO, Möhner M, Zatonski W, Storm H, Ekbom A, Tretli S, Teppo L, Akre O, Hakulinen T. Increase in testicular cancer incidence in six european countries: a birth cohort phenomenon. J Natl Cancer Inst 88: 727–733, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Berling S, Wölner-Hanssen P. No evidence of deteriorating semen quality among men in infertile relationships during the last decade: a study of males from Southern Sweden. Hum Reprod 12: 1002–1005, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Bhatia R, Shiau R, Petreas M, Weintraub JM, Farhang L, Eskenazi B. Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ Health Perspect 113: 220–224, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhattacharya S, Porter M, Amalraj E, Templeton A, Hamilton M, Lee AJ, Kurinczuk JJ. The epidemiology of infertility in the North East of Scotland. Hum Reprod 24: 3096–3107, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Bogatcheva NV, Ferlin A, Feng S, Truong A, Gianesello L, Foresta C, Agoulnik AI. T222P mutation of the insulin-like 3 hormone receptor LGR8 is associated with testicular maldescent and hinders receptor expression on the cell surface membrane. Am J Physiol Endocrinol Metab 292: E138–E144, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Boisen KA. Prevalence of Cryptorchidism and Hypospadias: 1072 Danish Boys Followed From Birth to Three Years of Age. (Dissertation) Copenhagen: Univ. of Copenhagen, 2005. [Google Scholar]
- 46.Boisen KA, Chellakooty M, Schmidt IM, Kai CM, Damgaard IN, Suomi AM, Toppari J, Skakkebæk NE, Main KM. Hypospadias in a cohort of 1072 Danish newborn boys: Prevalence and relationship to placental weight, anthropometrical measurements at birth, and reproductive hormone levels at 3 months of age. J Clin Endocrinol Metab 90: 4041–4046, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Boisen KA, Kaleva M, Main KM, Virtanen HE, Haavisto AM, Schmidt IM, Chellakooty M, Damgaard IN, Mau C, Reunanen M, Skakkebæk NE, Toppari J. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet 363: 1264–1269, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Boissonnas CC, Jouannet P, Jammes H. Epigenetic disorders and male subfertility. Fertil Steril 99: 624–631, 2013. [DOI] [PubMed] [Google Scholar]
- 49.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 22: 1506–1512, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Bonde JPE, Ernst E, Jensen TK, Hjollund NHI, Kolstad H, Henriksen TB, Scheike T, Giwercman A, Olsen J, Skakkebæk NE. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 352: 1172–1177, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Bourc'his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 431: 96–99, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Brake A, Krause W. Decreasing quality of semen. Br Med J 305: 1498, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bromwich P, Cohen J, Stewart I, Walker A. Decline in sperm counts: an artefact of changed reference range of “normal”? Br Med J 309: 19–22, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, Roeleveld N. Hypospadias: a transgenerational effect of diethylstilbestrol? Hum Reprod 21: 666–669, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Brucker-Davis F, Wagner-Mahler K, Delattre I, Ducot B, Ferrari P, Bongain A, Kurzenne JY, Mas JC, Fenichel P. Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Hum Reprod 23: 1708–1718, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Bubenik GA, Jacobson JP. Testicular histology of cryptorchild black-tailed deer (Odocoileus hemionus sitkensis) of Kodiak island, Alaska. Zeitschrift fur Jagdwissenschaft 48: 234–243, 2002. [Google Scholar]
- 57.Buck Louis GM, Cooney MA, Peterson CM. The ovarian dysgenesis syndrome. J Dev Origins Health Dis 2: 25–35, 2011. [Google Scholar]
- 58.Buck Louis GM, Dmochowski J, Lynch C, Kostyniak P, McGuinness BM, Vena JE. Polychlorinated biphenyl serum concentrations, lifestyle and time-to-pregnancy. Hum Reprod 24: 451–458, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Chen Z, Kim S, Caldwell KL, Barr DB. Heavy metals and couple fecundity, the LIFE Study. Chemosphere 87: 1201–1207, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buck Louis GM, Sever LE, Batt RE, Mendola P. Life-style factors and female infertility. Epidemiology 8: 435–441, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Buemann B, Henriksen H, Villumsen ÅL, Westh Å, Zachau-Christiansen B. Incidence of undescended testis in the newborn. Acta Chir Scan Suppl 283: 289–293, 1961. [PubMed] [Google Scholar]
- 62.Bushnik T, Cook JL, Yuzpe AA, Tough S, Collins J. Estimating the prevalence of infertility in Canada. Hum Reprod 27: 738–746, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calzolari E, Contiero MR, Roncarati E, Mattiuz PL, Volpato S. Aetiological factors in hypospadias. J Med Genet 23: 333–337, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carlsen E, Giwercman A, Keiding N, Skakkebæk NE. Evidence for decreasing quality of semen during past 50 years. BMJ 305: 609–613, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carmichael SL, Cogswell ME, Ma C, Gonzalez-Feliciano A, Olney RS, Correa A, Shaw GM. Hypospadias and maternal intake of phytoestrogens. Am J Epidemiol 178: 434–440, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carmichael SL, Shaw GM, Nelson V, Selvin S, Torfs CP, Curry CJ. Hypospadias in California: trends and descriptive epidemiology. Epidemiology 14: 701–706, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Catalano R, Bruckner T, Marks AR, Eskenazi B. Exogenous shocks to the human sex ratio: the case of September 11, 2001 in New York City. Hum Reprod 21: 3127–3131, 2006. [DOI] [PubMed] [Google Scholar]
- 68.Catalano R, Margerison-Zilko C, Goldman-Mellor S, Pearl M, Anderson E, Saxton K, Bruckner T, Subbaraman M, Goodman J, Epstein M, Currier R, Kharrazi M. Natural selection in utero induced by mass layoffs: the hCG evidence. Evol Appl 5: 796–805, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention.2014 Infertility and public health: www.cdc.gov/reproductivehealth/Infertility/PublicHealth.htm. [Google Scholar]
- 70.Chahnazarian A, Blumberg BS, London WT. Hepatitis B and the sex ratio at birth: a comparative analysis of four populations. J Biosoc Sci 20: 357–370, 1988. [DOI] [PubMed] [Google Scholar]
- 71.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: Data from the National Survey of Familiy Growth. Hyattsville, MD: National Center for Health Statistics, 2013. (National Health Statistics Reports 67) [PubMed] [Google Scholar]
- 72.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat 23: 1–160, 2005. [PubMed] [Google Scholar]
- 73.Chianese C, Gunning AC, Giachini C, Daguin F, Balercia G, Ars E, Lo Giacco D, Ruiz-Castane E, Forti G, Krausz C. X chromosome-linked CNVs in male infertility: discovery of overall duplication load and recurrent, patient-specific gains with potential clinical relevance. PLoS One 9: e97746, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi H, Kim KM, Koh SK, Kim KS, Woo YN, Yoon JB, Choi SK, Kim SW. A survey of externally recognizable genitourinary anomalies in Korean newborns. J Kor Med Sci 4: 13–21, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christensen JS, Asklund C, Skakkebæk NE, Jørgensen N, Andersen HR, Jørgensen TM, Olsen LH, Høyer AP, Moesgaard J, Thorup J, Jensen TK. Association between organic dietary choice during pregnancy and hypospadias in offspring: a study of mothers of 306 boys operated on for hypospadias. J Urol 189: 1077–1082, 2013. [DOI] [PubMed] [Google Scholar]
- 76.Christiansen S, Scholze M, Axelstad M, Boberg J, Kortenkamp A, Hass U. Combined exposure to anti-androgens causes markedly increased frequencies of hypospadias in the rat. Int J Androl 31: 241–248, 2008. [DOI] [PubMed] [Google Scholar]
- 77.Chung CC, Kanetsky PA, Wang Z, Hildebrandt MA, Koster R, Skotheim RI, Kratz CP, Turnbull C, Cortessis VK, Bakken AC, Bishop DT, Cook MB, Erickson RL, Fossa SD, Jacobs KB, Korde LA, Kraggerud SM, Lothe RA, Loud JT, Rahman N, Skinner EC, Thomas DC, Wu X, Yeager M, Schumacher FR, Greene MH, Schwartz SM, McGlynn KA, Chanock SJ, Nathanson KL. Meta-analysis identifies four new loci associated with testicular germ cell tumor. Nat Genet 45: 680–685, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clement TM, Savenkova MI, Settles M, Anway MD, Skinner MK. Alterations in the developing testis transcriptome following embryonic vinclozolin exposure. Reprod Toxicol 30: 353–364, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clemmesen J. Testis cancer incidence: suggestion of a world pattern. Int J Androl Suppl 4: 111–122, 1981. [DOI] [PubMed] [Google Scholar]
- 80.Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology 20: 56–59, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cortes D, Kjellberg EM, Breddam M, Thorup J. The true incidence of cryptorchidism in Denmark. J Urol 179: 314–318, 2008. [DOI] [PubMed] [Google Scholar]
- 82.Curtin SC, Abma JC, Ventura SJ, Henshaw SK. Pregnancy rates for U. S. women continue to drop. NCHS Data Brief 1–8, 2013. [PubMed] [Google Scholar]
- 83.Czeizel A. Increasing trends in congenital malformations of male external genitalia. Lancet i: 462–463, 1985. [DOI] [PubMed] [Google Scholar]
- 84.Czeizel A, Toth J, Erodi E. Aetiological studies of hypospadias in Hungary. Hum Hered 29: 166–171, 1979. [DOI] [PubMed] [Google Scholar]
- 85.Dabaja AA, Mielnik A, Robinson BD, Wosnitzer MS, Schlegel PN, Paduch DA. Possible germ cell-Sertoli cell interactions are critical for establishing appropriate expression levels for the Sertoli cell-specific MicroRNA, miR-202-5p, in human testis. Basic Clin Androl 25: 2, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dalgaard MD, Weinhold N, Edsgard D, Silver JD, Pers TH, Nielsen JE, Jorgensen N, Juul A, Gerds TA, Giwercman A, Giwercman YL, Cohn-Cedermark G, Virtanen HE, Toppari J, Daugaard G, Jensen TS, Brunak S, Rajpert-De Meyts, Skakkebaek NE, Leffers H, Gupta R. A genome-wide association study of men with symptoms of testicular dysgenesis syndrome and its network biology interpretation. J Med Genet 49: 58–65, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dallinga JW, Moonen EJ, Dumoulin JC, Evers JL, Geraedts JP, Kleinjans JC. Decreased human semen quality and organochlorine compounds in blood. Hum Reprod 17: 1973–1979, 2002. [DOI] [PubMed] [Google Scholar]
- 88.Damgaard IN, Jensen TK, Petersen JH, Skakkebæk NE, Toppari J, Main KM. Risk factors for congenital cryptorchidism in a prospective birth cohort study. PLoS One 3: e3051, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Damgaard IN, Skakkebæk NE, Toppari J, Virtanen HE, Shen H, Schramm KW, Petersen JH, Jensen TK, The Nordic Cryptorchidism Study Group, Main KM. Persistent pesticides in human breast milk and cryptorchidism. Environ Health Perspect 114: 1133–1138, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Damgaard I, Jensen TK, The Nordic Cryptorchidism Study Group, Petersen JH, Skakkebæk NE, Toppari J, Main KM. Cryptorchidism and maternal alcohol consumption during pregnancy. Environ Health Perspect 115: 272–277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davis DL, Gottlieb MB, Stampnitzky JR. Reduced ratio of male to female births in several industrial countries. A sentinel health indicator? JAMA 279: 1018–1023, 1998. [DOI] [PubMed] [Google Scholar]
- 92.Davis-Dao C, Koh CJ, Hardy BE, Chang A, Kim SS, De FR, Hwang A, Pike MC, Carroll JD, Coetzee GA, Vandenberg D, Siegmund K, Cortessis VK. Shorter androgen receptor CAG repeat lengths associated with cryptorchidism risk among Hispanic white boys. J Clin Endocrinol Metab 97: 393–399, 2012. [DOI] [PubMed] [Google Scholar]
- 93.Davis-Dao CA, Siegmund KD, Vandenberg DJ, Skinner EC, Coetzee GA, Thomas DC, Pike MC, Cortessis VK. Heterogenous effect of androgen receptor CAG tract length on testicular germ cell tumor risk: shorter repeats associated with seminoma but not other histologic types. Carcinogenesis 32: 1238–1243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Boer EJ, den Tonkelaar I, Burger CW, van Leeuwen FE. Validity of self-reported causes of subfertility. Am J Epidemiol 161: 978–986, 2005. [DOI] [PubMed] [Google Scholar]
- 95.Depue RH, Pike MC, Henderson BE. Cryptorchidism and testicular cancer. J Natl Cancer Inst 77: 830–832, 1986. [DOI] [PubMed] [Google Scholar]
- 96.Diczfalusy E. The third epoch, the third world and the third millennium. Contraception 53: 1–7, 1996. [DOI] [PubMed] [Google Scholar]
- 97.Dolk H, Vrijheid M, Scott JE, Addor MC, Botting B, De Vigan C, De Walle H, Garne E, Loane M, Pierini A, Garcia-Minaur S, Physick N, Tenconi R, Wiesel A, Calzolari E, Stone D. Toward the effective surveillance of hypospadias. Environ Health Perspect 112: 398–402, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Drake AJ, O'Shaughnessy PJ, Bhattacharya S, Monteiro A, Kerrigan D, Goetz S, Raab A, Rhind SM, Sinclair KD, Meharg AA, Feldmann J, Fowler PA. In utero exposure to cigarette chemicals induces sex-specific disruption of one-carbon metabolism and DNA methylation in the human fetal liver. BMC Med 13: 18, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eckert D, Biermann K, Nettersheim D, Gillis AJ, Steger K, Jack HM, Muller AM, Looijenga LH, Schorle H. Expression of BLIMP1/PRMT5 and concurrent histone H2A/H4 arginine 3 dimethylation in fetal germ cells, CIS/IGCNU and germ cell tumors. BMC Dev Biol 8: 106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eisenbach M, Giojalas LC. Sperm guidance in mammals: an unpaved road to the egg. Nat Rev Mol Cell Biol 7: 276–285, 2006. [DOI] [PubMed] [Google Scholar]
- 101.Eisenberg ML, Hsieh MH, Walters RC, Krasnow R, Lipshultz LI. The relationship between anogenital distance, fatherhood, and fertility in adult men. PLoS One 6: e18973, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eisenberg ML, Jensen TK, Walters RC, Skakkebæk NE, Lipshultz LI. The relationship between anogenital distance and reproductive hormone levels in adult men. J Urol 187: 594–598, 2012. [DOI] [PubMed] [Google Scholar]
- 103.Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod 29: 193–200, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eisenberg ML, Murthy L, Hwang K, Lamb DJ, Lipshultz LI. Sperm counts and sperm sex ratio in male infertility patients. Asian J Androl 14: 683–686, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eisenberg ML, Schembri M, Croughan MS, Walsh TJ. Fecundity and sex ratio of offspring in an infertile cohort. Fertil Steril 96: 833–836, 2011. [DOI] [PubMed] [Google Scholar]
- 106.Eisenberg ML, Shy M, Walters RC, Lipshultz LI. The relationship between anogenital distance and azoospermia in adult men. Int J Androl 35: 726–730, 2012. [DOI] [PubMed] [Google Scholar]
- 107.Ekbom A, Akre O. Increasing incidence of testicular cancer-birth cohort effects. APMIS 106: 225–231, 1998. [DOI] [PubMed] [Google Scholar]
- 108.El Houate B, Rouba H, Sibai H, Barakat A, Chafik A, Chadli el B, Imken L, Bogatcheva NV, Feng S, Agoulnik AI, McElreavey K. Novel mutations involving the INSL3 gene associated with cryptorchidism. J Urol 18: 1947–1951, 2007. [DOI] [PubMed] [Google Scholar]
- 109.Ellish NJ, Saboda K, O'Connor J, Nasca PC, Stanek EJ, Boyle C. A prospective study of early pregnancy loss. Hum Reprod 11: 406–412, 1996. [DOI] [PubMed] [Google Scholar]
- 110.Eshre Capri Workshop Group. Europe the continent with the lowest fertility. Hum Reprod Update 16: 590–602, 2010. [DOI] [PubMed] [Google Scholar]
- 111.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sørensen TIA, Dunkel L, Himes JH, Teilmann G, Swan SH. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics 121: 172–191, 2008. [DOI] [PubMed] [Google Scholar]
- 112.European Science Foundation. 2010 Science Policy Briefing no. 40: http://www.esf.org/publications/science-policy-briefings.html. [Google Scholar]
- 113.Farrow S. Falling sperm quality: fact or fiction? Br Med J 309: 1–2, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Feki NC, Abid N, Rebai A, Sellami A, Ayed BB, Guermazi M, Bahloul A, Rebai T, Ammar LK. Semen quality decline among men in infertile relationships: experience over 12 years in the South of Tunisia. J Androl 30: 541–547, 2009. [DOI] [PubMed] [Google Scholar]
- 115.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviella AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged man: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 87: 589–598, 2002. [DOI] [PubMed] [Google Scholar]
- 116.Ferlin A, Pengo M, Pizzol D, Carraro U, Frigo AC, Foresta C. Variants in KITLG predispose to testicular germ cell cancer independently from spermatogenic function. Endocr Relat Cancer 24: 101–108, 2012. [DOI] [PubMed] [Google Scholar]
- 117.Fernandez MF, Duran I, Olea N, Avivar C, Vierula M, Toppari J, Skakkebæk NE, Jørgensen N. Semen quality and reproductive hormone levels in men from southern Spain. Int J Androl 35: 1–10, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fisch H, Lambert SM, Hensle TW, Hyun G. Hypospadias rates in new york state are not increasing. J Urol 181: 2291–2294, 2009. [DOI] [PubMed] [Google Scholar]
- 119.Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction 127: 305–315, 2004. [DOI] [PubMed] [Google Scholar]
- 120.Florack EIM, Zielhuis GA, Rolland R. Cigarette smoking, alcohol consumption, and caffeine intake and fecundability. Prev Med 23: 175–180, 1994. [DOI] [PubMed] [Google Scholar]
- 121.Franci G, Ciotta A, Altucci L. The Jumonji family: past, present and future of histone demethylases in cancer. Biomol Concepts 5: 209–224, 2014. [DOI] [PubMed] [Google Scholar]
- 122.Frejka T, Jones GW, Sardon JP. East Asian childbearing patterns and policy developments. Popul Dev Rev 36: 579–606, 2010. [DOI] [PubMed] [Google Scholar]
- 123.Frisen L, Lagerstedt K, Tapper-Persson M, Kockum I, Nordenskjold A. A novel duplication in the HOXA13 gene in a family with atypical hand-foot-genital syndrome. J Med Genet 40: e49, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frost MD, Puri M, Hinde PR. Falling sex ratios and emerging evidence of sex-selective abortion in Nepal: evidence from nationally representative survey data. BMJ Open 3: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fukami M, Wada Y, Miyabayashi K, Nishino I, Hasegawa T, Nordenskjold A, Camerino G, Kretz C, Buj-Bello A, Laporte J, Yamada G, Morohashi K, Ogata T. CXorf6 is a causative gene for hypospadias. Nat Genet 38: 1369–1371, 2006. [DOI] [PubMed] [Google Scholar]
- 126.Fukuda M, Fukuda K, Shimizu T, Moller H. Decline in sex ratio at birth after Kobe earthquake. Hum Reprod 13: 2321–2322, 1998. [DOI] [PubMed] [Google Scholar]
- 127.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 17: 667–669, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garolla A, Ferlin A, Vinanzi C, Roverato A, Sotti G, Artibani W, Foresta C. Molecular analysis of the androgen receptor gene in testicular cancer. Endocr Relat Cancer 12: 645–655, 2005. [DOI] [PubMed] [Google Scholar]
- 129.Garrido N, Martinez-Conejero JA, Jauregui J, Horcajadas JA, Simon C, Remohi J, Meseguer M. Microarray analysis in sperm from fertile and infertile men without basic sperm analysis abnormalities reveals a significantly different transcriptome. Fertil Steril 91: 1307–1310, 2009. [DOI] [PubMed] [Google Scholar]
- 130.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod 27: 2899–2907, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gaskins AJ, Mendiola J, Afeiche M, Jørgensen N, Swan SH, Chavarro JE. Physical activity and television watching in relation to semen quality in young men. Br J Sports Med 4–2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Geller F, Feenstra B, Carstensen L, Pers TH, van Rooij IA, Korberg IB, Choudhry S, Karjalainen JM, Schnack TH, Hollegaard MV, Feitz WF, Roeleveld N, Hougaard DM, Hirschhorn JN, Franke L, Baskin LS, Nordenskjold A, van der Zanden LF, Melbye M. Genome-wide association analyses identify variants in developmental genes associated with hypospadias. Nat Genet 46: 957–963, 2014. [DOI] [PubMed] [Google Scholar]
- 133.Ghazarian AA, Trabert B, Devesa SS, McGlynn KA. Recent trends in the incidence of testicular germ cell tumors in the United States. Andrology 3: 13–18, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Giordano F, Abballe A, De FE, di DA, Ferro F, Grammatico P, Ingelido AM, Marra V, Marrocco G, Vallasciani S, Figa-Talamanca I. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res A Clin Mol Teratol 88: 241–250, 2010. [DOI] [PubMed] [Google Scholar]
- 135.Giwercman A, Lundin KB, Eberhard J, Stahl O, Cwikiel M, Cavallin-Stahl E, Giwercman YL. Linkage between androgen receptor gene CAG trinucleotide repeat length and testicular germ cell cancer histological type and clinical stage. Eur J Cancer 40: 2152–2158, 2004. [DOI] [PubMed] [Google Scholar]
- 136.Gkountela S, Li Z, Vincent JJ, Zhang KX, Chen A, Pellegrini M, Clark AT. The ontogeny of cKIT+ human primordial germ cells proves to be a resource for human germ line reprogramming, imprint erasure and in vitro differentiation. Nat Cell Biol 15: 113–122, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goldsmith JR, Potashnik G, Israeli R. Reproductive outcomes in families of DBCP-exposed men. Arch Environ Health 39: 85–89, 1984. [DOI] [PubMed] [Google Scholar]
- 138.Gollenberg AL, Liu F, Brazil C, Drobnis EZ, Guzick D, Overstreet JW, Redmon JB, Sparks A, Wang C, Swan SH. Semen quality in fertile men in relation to psychosocial stress. Fertil Steril 93: 1104–1111, 2010. [DOI] [PubMed] [Google Scholar]
- 139.Goriely A, Hansen RM, Taylor IB, Olesen IA, Jacobsen GK, McGowan SJ, Pfeifer SP, McVean GA, Rajpert-De Meyts E, Wilkie AO. Activating mutations in FGFR3 and HRAS reveal a shared genetic origin for congenital disorders and testicular tumors. Nat Genet 41: 1247–1252, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gray LE Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58: 350–365, 2000. [DOI] [PubMed] [Google Scholar]
- 141.Gray LE Jr, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update 7: 248–264, 2001. [DOI] [PubMed] [Google Scholar]
- 142.Grigorova M, Punab M, Zilaitiene B, Erenpreiss J, Ausmees K, Matulevicius V, Tsarev I, Jørgensen N, Laan M. Genetically determined dosage of follicle-stimulating hormone (FSH) affects male reproductive parameters. J Clin Endocrinol Metab 96: E1534–E1541, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Griskevicius V, Tybur JM, Ackerman JM, Delton AW, Robertson TE, White AE. The financial consequences of too many men: sex ratio effects on saving, borrowing, and spending. J Perspect Soc Psychol 102: 69–80, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, MacIsaac RJ, Clarke S, Zajac JD, Jerums G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 93: 1834–1840, 2008. [DOI] [PubMed] [Google Scholar]
- 145.Gurunath S, Pandian Z, Anderson RA, Bhattacharya S. Defining infertility–a systematic review of prevalence studies. Hum Reprod Update 17: 575–588, 2011. [DOI] [PubMed] [Google Scholar]
- 146.Guzick DS, Grefenstette I, Baffone K, Berga SL, Krasnow JS, Stovall DW, Naus GJ. Infertility evaluation in fertile women: a model for assessing the efficacy of infertility testing. Hum Reprod 9: 2306–2310, 1994. [DOI] [PubMed] [Google Scholar]
- 147.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA, Xu D, Vogel DL. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 345: 1388–1393, 2001. [DOI] [PubMed] [Google Scholar]
- 148.Habert R, Muczynski V, Grisin T, Moison D, Messiaen S, Frydman R, Benachi A, Delbes G, Lambrot R, Lehraiki A, N'tumba-Byn T, Guerquin MJ, Levacher C, Rouiller-Fabre V, Livera G. Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors. Reproduction 147: R119–R129, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hadziselimovic F, Hadziselimovic NO, Demougin P, Krey G, Oakeley EJ. Deficient expression of genes involved in the endogenous defense system against transposons in cryptorchid boys with impaired mini-puberty. Sex Dev 5: 287–293, 2011. [DOI] [PubMed] [Google Scholar]
- 150.Haimov-Kochman R, Har-Nir R, Ein-Mor E, Ben-Shoshan V, Greenfield C, Eldar I, Bdolah Y, Hurwitz A. Is the quality of donated semen deteriorating? Findings from a 15 year longitudinal analysis of weekly sperm samples. Isr Med Assoc J 14: 372–377, 2012. [PubMed] [Google Scholar]
- 151.Hajkova P, Ancelin K, Waldmann T, Lacoste N, Lange UC, Cesari F, Lee C, Almouzni G, Schneider R, Surani MA. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 452: 877–881, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hajkova P, Jeffries SJ, Lee C, Miller N, Jackson SP, Surani MA. Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway. Science 329: 78–82, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hammen R. Studies on Impaired Fertility in Man With Special Reference to the Male. Koebenhavn: Einar Munksgaard, 1944, p. 1–206. [Google Scholar]
- 154.Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod 85: 431–441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hammoud SS, Nix DA, Hammoud AO, Gibson M, Cairns BR, Carrell DT. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum Reprod 26: 2558–2569, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature 460: 473–478, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hardell L, van Bavel B, Lindstrom G, Carlberg M, Dreifaldt AC, Wijkstrom H, Starkhammar H, Eriksson M, Hallquist A, Kolmert T. Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ Health Perspect 111: 930–934, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Harris LE, Steinberg AG. Abnormalities observed during the first six days of life in 8,716 live-born infants. Pediatrics 14: 314–326, 1954. [PubMed] [Google Scholar]
- 159.Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, Metzdorff SB, Kortenkamp A. Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ Health Perspect 115 Suppl 1: 122–128, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology 17: 682–691, 2006. [DOI] [PubMed] [Google Scholar]
- 161.Hemminki E, Merilainen J, Teperi J. Reporting of malformations in routine health registers. Teratology 48: 227–231, 1993. [DOI] [PubMed] [Google Scholar]
- 162.Hemminki K, Chen B. Familial risks in testicular cancer as aetiological clues. Int J Androl 29: 205–210, 2006. [DOI] [PubMed] [Google Scholar]
- 163.Hemminki K, Li X. Cancer risks in Nordic immigrants and their offspring in Sweden. Eur J Cancer 38: 2428–2434, 2002. [DOI] [PubMed] [Google Scholar]
- 164.Henderson BE, Bernstein L, Ross RK, Depue RH, Judd HL. The early in utero oestrogen and testosterone environment of blacks and whites: Potential effects on male offspring. Br J Cancer 57: 216–218, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, Wasserman R, Serwint JR, Smitherman L, Reiter EO. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics 130: e1058–e1068, 2012. [DOI] [PubMed] [Google Scholar]
- 166.Hotaling J, Carrell DT. Clinical genetic testing for male factor infertility: current applications and future directions. Andrology 2: 339–350, 2014. [DOI] [PubMed] [Google Scholar]
- 167.Hsueh AJ, Dahl KD, Vaughan J, Tucker E, Rivier J, Bardin CW, Vale W. Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc Natl Acad Sci USA 84: 5082–5086, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hu Z, Li Z, Yu J, Tong C, Lin Y, Guo X, Lu F, Dong J, Xia Y, Wen Y, Wu H, Li H, Zhu Y, Ping P, Chen X, Dai J, Jiang Y, Pan S, Xu P, Luo K, Du Q, Yao B, Liang M, Gui Y, Weng N, Lu H, Wang Z, Zhang F, Zhu X, Yang X, Zhang Z, Zhao H, Xiong C, Ma H, Jin G, Chen F, Xu J, Wang X, Zhou Z, Chen ZJ, Liu J, Shen H, Sha J. Association analysis identifies new risk loci for non-obstructive azoospermia in Chinese men. Nat Commun 5: 3857, 2014. [DOI] [PubMed] [Google Scholar]
- 169.Hull MGR, Glazener CMA, Kelly NJ, Conway DI, Foster PA, Hinton RA, Coulson C, Lambert PA, Watt EM, Desai KM. Population study of causes, treatment, and outcome of infertility. Br Med J 291: 1693–1697, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Issa Y, Sallmen M, Nijem K, Bjertness E, Kristensen P. Fecundability among newly married couples in agricultural villages in Palestine: a prospective study. Hum Reprod 25: 2132–2138, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jacobsen R, Bostofte E, Engholm G, Hansen J, Skakkebæk NE, Møller H. Fertility and offspring sex ratio of men who develop testicular cancer: a record linkage study. Hum Reprod 15: 1958–1961, 2000. [DOI] [PubMed] [Google Scholar]
- 172.Jensen MS, Anand-Ivell R, Norgaard-Pedersen B, Jonsson BA, Bonde JP, Hougaard DM, Cohen A, Lindh CH, Ivell R, Toft G. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology 26: 91–99, 2015. [DOI] [PubMed] [Google Scholar]
- 173.Jensen TK, Andersson AM, Skakkebæk NE, Joensen UN, Blomberg Jensen M, Lassen TH, Nordkap L, Olesen IA, Hansen AM, Rod NH, Jørgensen N. Association of sleep disturbances with reduced semen quality: a cross-sectional study among 953 healthy young Danish men. Am J Epidemiol 177: 1027–1037, 2013. [DOI] [PubMed] [Google Scholar]
- 174.Jensen TK, Andersson AM, Jørgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebæk NE. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril 82: 863–870, 2004. [DOI] [PubMed] [Google Scholar]
- 175.Jensen TK, Jørgensen N, Punab M, Haugen TB, Suominen J, Zilaitiene B, Horte A, Andersen AG, Carlsen E, Magnus Ø, Matulevicius V, Nermoen I, Vierula M, Keiding N, Toppari J, Skakkebæk NE. Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1,770 young men from the general population in five European countries. Am J Epidemiol 159: 49–58, 2004. [DOI] [PubMed] [Google Scholar]
- 176.Jensen TK, Slama R, Ducot B, Suominen J, Cawood EH, Andersen AG, Eustache F, Irvine S, Auger S, Jouannet P, Vierula M, Jørgensen N, Toppari J, Skakkebæk NE, Keiding N, Spira A. Regional differences in waiting time to pregnancy among fertile couples from four European cities. Hum Reprod 16: 2697–2704, 2001. [DOI] [PubMed] [Google Scholar]
- 177.Jensen TK, Sobotka T, Hansen MA, Pedersen AT, Lutz W, Skakkebæk NE. Declining trends in conception rates in recent birth cohorts of native Danish women: a possible role of deteriorating male reproductive health. Int J Androl 31: 81–92, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jha P, Kesler MA, Kumar R, Ram F, Ram U, Aleksandrowicz L, Bassani DG, Chandra S, Banthia JK. Trends in selective abortions of girls in India: analysis of nationally representative birth histories from 1990 to 2005 and census data from 1991 to 2011. Lancet 377: 1921–1928, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Joffe M. Invited commentary: the potential for monitoring of fecundity and the remaining challenges. Am J Epidemiol 157: 89–93, 2003. [DOI] [PubMed] [Google Scholar]
- 180.Joffe M, Bennett J, Best N, Jensen TK. Sex ratio and time to pregnancy: analysis of four large European population surveys. Br Med J 334: 524, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Joffe M, Holmes J, Jensen TK, Keiding N, Best N. Time trends in biological fertility in Western Europe. Am J Epidemiol 178: 722–730, 2013. [DOI] [PubMed] [Google Scholar]
- 182.Joffe M, Villard L, Li Z, Plowman R, Vessey M. A time to pregnancy questionnaire designed for long term recall: validity in Oxford, England. J Epidemiol Community Health 49: 314–319, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Johansen ML, Arnand-Ivell R, Mouritsen A, Hagen CP, Mieritz MG, Søeborg T, Johannsen TH, Main KM, Andersson AM, Ivell R, Juul A. Serum levels of insulin-like factor 3, anti-Mullerian hormone, inhibin B, and testosterone during pubertal transition in healthy boys: a longitudinal pilot study. Reproduction 147: 529–535, 2014. [DOI] [PubMed] [Google Scholar]
- 184.John Radcliffe Hospital Cryptorchidism Study Group. Cryptorchidism: a prospective study of 7500 consecutive male births, 1984-8. Arch Dis Child 67: 892–899, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jørgensen A, Nielsen JE, Blomberg Jensen M, Græm N, Rajpert-De Meyts E. Analysis of meiosis regulators in human gonads: a sexually dimorphic spatio-temporal expression pattern suggests involvement of DMRT1 in meiotic entry. Mol Hum Reprod 18: 523–534, 2012. [DOI] [PubMed] [Google Scholar]
- 186.Jørgensen N, Andersen AG, Eustache F, Irvine DS, Suominen J, Petersen JH, Andersen AN, Auger J, Cawood EHH, Horte A, Jensen TK, Jouannet P, Keiding N, Vierula M, Toppari J, Skakkebæk NE. Regional differences in semen quality in Europe. Hum Reprod 16: 1012–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 187.Jørgensen N, Carlsen E, Nermoen I, Punab M, Suominen J, Andersen AG, Andersson AM, Haugen TB, Horte A, Jensen TK, Magnus Ø, Petersen JH, Vierula M, Toppari J, Skakkebæk NE. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod 17: 2199–2208, 2002. [DOI] [PubMed] [Google Scholar]
- 188.Jørgensen N, Giwercman A, Müller J, Skakkebæk NE. Immunohistochemical markers of carcinoma in situ of the testis also expressed in normal infantile germ cells. Histopathology 22: 373–378, 1993. [DOI] [PubMed] [Google Scholar]
- 189.Jørgensen N, Joensen UN, Jensen TK, Blomberg Jensen M, Almstrup K, Olesen IA, Juul A, Andersson AM, Carlsen E, Petersen JH, Toppari J, Skakkebæk NE. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2: e000990, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jørgensen N, Rajpert-De Meyts E, Græm N, Müller J, Giwercman A, Skakkebæk NE. Expression of immunohistochemical markers for testicular carcinoma in situ by normal human fetal germ cells. Lab Invest 72: 223–231, 1995. [PubMed] [Google Scholar]
- 191.Jørgensen N, Vierula M, Jacobsen R, Pukkala E, Perheentupa A, Virtanen HE, Skakkebæk NE, Toppari J. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. Int J Androl 34: e37–e48, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Josso N, Picard JY, Imbeaud S, Carré ED, Zeller J, Adamsbaum C. The persistent müllerian duct syndrome: a rare cause of cryptorchidism. Eur J Pediatr 152 Suppl 2: S76–S78, 1993. [DOI] [PubMed] [Google Scholar]
- 193.Jouannet P, Wang C, Eustache F, Jensen TK, Auger J. Semen quality and male reproductive health: the controversy about human sperm concentration decline. APMIS 109: 333–344, 2001. [DOI] [PubMed] [Google Scholar]
- 194.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Hormone IGF Res 13: 113–170, 2003. [DOI] [PubMed] [Google Scholar]
- 195.Juul A, Magnusdottir S, Scheike T, Prytz S, Skakkebaek NE. Age at voice break in Danish boys: effects of pre-pubertal body mass index and secular trend. Int J Androl 30: 537–542, 2007. [DOI] [PubMed] [Google Scholar]
- 196.Juul S, Karmaus W, Olsen J. Regional differences in waiting time to pregnancy: pregnancy-based surveys from Denmark, France, Germany, Italy and Sweden. The European Infertility and Subfecundity Study Group. Hum Reprod 14: 1250–1254, 1999. [DOI] [PubMed] [Google Scholar]
- 197.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents' and grandparents' slow growth period. Eur J Hum Genet 10: 682–688, 2002. [DOI] [PubMed] [Google Scholar]
- 198.Kagiwada S, Kurimoto K, Hirota T, Yamaji M, Saitou M. Replication-coupled passive DNA demethylation for the erasure of genome imprints in mice. EMBO J 32: 340–353, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kalfa N, Philibert P, Sultan C. Is hypospadias a genetic, endocrine or environmental disease, or still an unexplained malformation? Int J Androl 32: 187–197, 2009. [DOI] [PubMed] [Google Scholar]
- 200.Kallen B, Bertollini R, Castilla E, Czeizel A, Knudsen LB, Martinez Frias ML, Mastroiacovo P, Mutchinick O. A joint international study on the epidemiology of hypospadias. Acta Paediatr Scand Suppl 324: 1986. [DOI] [PubMed] [Google Scholar]
- 201.Kallen B, Finnstrom O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: risk for congenital malformations after different IVF methods. Birth Defects Res A Clin Mol Teratol 73: 162–169, 2005. [DOI] [PubMed] [Google Scholar]
- 202.Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, Sasaki H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 429: 900–903, 2004. [DOI] [PubMed] [Google Scholar]
- 203.Kanetsky PA, Mitra N, Vardhanabhuti S, Li M, Vaughn DJ, Letrero R, Ciosek SL, Doody DR, Smith LM, Weaver J, Albano A, Chen C, Starr JR, Rader DJ, Godwin AK, Reilly MP, Hakonarson H, Schwartz SM, Nathanson KL. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat Genet 41: 811–815, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kanetsky PA, Mitra N, Vardhanabhuti S, Vaughn DJ, Li M, Ciosek SL, Letrero R, D'Andrea K, Vaddi M, Doody DR, Weaver J, Chen C, Starr JR, Hakonarson H, Rader DJ, Godwin AK, Reilly MP, Schwartz SM, Nathanson KL. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum Mol Genet 20: 3109–3117, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Karmaus W, Juul S. Infertility and subfecundity in population-based samples from Denmark, Germany, Italy, Poland and Spain. Eur J Pub Health 9: 229–235, 1999. [Google Scholar]
- 206.Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 30: 222–225, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Keiding N, Kvist K, Hartvig H, Tvede M, Juul S. Estimating time to pregnancy from current durations in a cross-sectional sample. Biostatistics 3: 565–578, 2002. [DOI] [PubMed] [Google Scholar]
- 208.Kelce WR, Lambright CR, Gray LE Jr, Roberts KP. Vinclozolin and p,p'-DDE alter androgen-dependent gene expression: in vivo confirmation of an androgen receptor-mediated mechanism. Toxicol Appl Pharmacol 142: 192–200, 1997. [DOI] [PubMed] [Google Scholar]
- 209.Klemetti R, Gissler M, Sevon T, Koivurova S, Ritvanen A, Hemminki E. Children born after assisted fertilization have an increased rate of major congenital anomalies. Fertil Steril 84: 1300–1307, 2005. [DOI] [PubMed] [Google Scholar]
- 210.Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, van Leeuwen FE. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet 359: 1102–1107, 2002. [DOI] [PubMed] [Google Scholar]
- 211.Kollin C, Granholm T, Nordenskjold A, Ritzen EM. Growth of spontaneously descended and surgically treated testes during early childhood. Pediatrics 131: e1174–e1180, 2013. [DOI] [PubMed] [Google Scholar]
- 212.Kollin C, Karpe B, Hesser U, Granholm T, Ritzen EM. Surgical treatment of unilaterally undescended testes: testicular growth after randomization to orchiopexy at age 9 months or 3 years. J Urol 178: 1589–1593, 2007. [DOI] [PubMed] [Google Scholar]
- 213.Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect 115 Suppl 1: 98–105, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kosova G, Scott NM, Niederberger C, Prins GS, Ober C. Genome-wide association study identifies candidate genes for male fertility traits in humans. Am J Hum Genet 90: 950–961, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kratz CP, Han SS, Rosenberg PS, Berndt SI, Burdett L, Yeager M, Korde LA, Mai PL, Pfeiffer R, Greene MH. Variants in or near KITLG, BAK1, DMRT1, and TERT-CLPTM1L predispose to familial testicular germ cell tumour. J Med Genet 48: 473–476, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Krausz C, Chianese C. Genetic testing and counselling for male infertility. Curr Opin Endocrinol Diabetes Obes 21: 244–250, 2014. [DOI] [PubMed] [Google Scholar]
- 217.Krausz C, Giachini C, Lo Giacco D, Daguin F, Chianese C, Ars E, Ruiz-Castane E, Forti G, Rossi E. High resolution X chromosome-specific array-CGH detects new CNVs in infertile males. PLoS One 7: e44887, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Krentz AD, Murphy MW, Kim S, Cook MS, Capel B, Zhu R, Matin A, Sarver AL, Parker KL, Griswold MD, Looijenga LH, Bardwell VJ, Zarkower D. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc Natl Acad Sci USA 106: 22323–22328, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kristensen DG, Mlynarska O, Nielsen JE, Jacobsen GK, Rajpert-De Meyts E, Almstrup K. Heterogeneity of chromatin modifications in testicular spermatocytic seminoma point toward an epigenetically unstable phenotype. Cancer Genet 205: 425–431, 2012. [DOI] [PubMed] [Google Scholar]
- 220.Kristensen DG, Nielsen JE, Jorgensen A, Skakkebaek NE, Rajpert-De Meyts, Almstrup K. Evidence that active demethylation mechanisms maintain the genome of carcinoma in situ cells hypomethylated in the adult testis. Br J Cancer 110: 668–678, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kristensen DG, Skakkebæk NE, Rajpert-De Meyts E, Almstrup K. Epigenetic features of testicular germ cell tumours in relation to epigenetic characteristics of foetal germ cells. Int J Dev Biol 57: 309–317, 2013. [DOI] [PubMed] [Google Scholar]
- 222.Krysiak-Baltyn K, Toppari J, Skakkebæk NE, Jensen TS, Virtanen HE, Schramm KW, Shen H, Vartiainen T, Kiviranta H, Taboureau O, Audouze K, Brunak S, Main KM. Association between chemical pattern in breast milk and congenital cryptorchidism: modelling of complex human exposures. Int J Androl 35: 294–302, 2012. [DOI] [PubMed] [Google Scholar]
- 223.Krysiak-Baltyn K, Toppari J, Skakkebæk NE, Jensen TS, Virtanen HE, Schramm KW, Shen H, Vartiainen T, Kiviranta H, Taboureau O, Brunak S, Main KM. Country-specific chemical signatures of persistent environmental compounds in breast milk. Int J Androl 33: 270–278, 2010. [DOI] [PubMed] [Google Scholar]
- 224.Kumanov P, Robeva R, Tomova A, Hubaveshki S. Prevalence of the hypospadias among Bulgarian boys: a prospective study. Eur J Pediatr 166: 987–988, 2007. [DOI] [PubMed] [Google Scholar]
- 225.La Salle S, Oakes CC, Neaga OR, Bourc'his D, Bestor TH, Trasler JM. Loss of spermatogonia and wide-spread DNA methylation defects in newborn male mice deficient in DNMT3L. BMC Dev Biol 7: 104, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 4: 77–79, 1991. [DOI] [PubMed] [Google Scholar]
- 227.Lalancette C, Miller D, Li Y, Krawetz SA. Paternal contributions: new functional insights for spermatozoal RNA. J Cell Biochem 104: 1570–1579, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lambrot R, Muczynski V, Lecureuil C, Angenard G, Coffigny H, Pairault C, Moison D, Frydman R, Habert R, Rouiller-Fabre V. Phthalates impair germ cell development in the human fetal testis in vitro without change in testosterone production. Environ Health Perspect 117: 32–37, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Larsen U. Primary and secondary infertility in sub-Saharan Africa. Int J Epidemiol 29: 285–291, 2000. [DOI] [PubMed] [Google Scholar]
- 230.Lassen TH, Sobotka T, Jensen TK, Jacobsen R, Erb K, Skakkebæk NE. Trends in rates of natural conceptions among Danish women born during 1960–1984. Hum Reprod 27: 2815–2822, 2012. [DOI] [PubMed] [Google Scholar]
- 231.Le Cornet C, Lortet-Tieulent J, Forman D, Beranger R, Flechon A, Fervers B, Schuz J, Bray F. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur J Cancer 50: 831–839, 2014. [DOI] [PubMed] [Google Scholar]
- 232.Lerchl A, Nieschlag E. Decreasing sperm counts? A critical (re)view. Exp Clin Endocrinol Diabetes 104: 301–307, 1996. [DOI] [PubMed] [Google Scholar]
- 233.Lessel D, Gamulin M, Kulis T, Toliat MR, Grgic M, Friedrich K, Zunec R, Balija M, Nurnberg P, Kastelan Z, Hogel J, Kubisch C. Replication of genetic susceptibility loci for testicular germ cell cancer in the Croatian population. Carcinogenesis 33: 1548–1552, 2012. [DOI] [PubMed] [Google Scholar]
- 234.Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril 95: 116–123, 2011. [DOI] [PubMed] [Google Scholar]
- 235.Lian J, Tian H, Liu L, Zhang XS, Li WQ, Deng YM, Yao GD, Yin MM, Sun F. Downregulation of microRNA-383 is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation by targeting IRF1. Cell Death Dis 1: e94, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lin H. piRNAs in the germ line. Science 316: 397, 2007. [DOI] [PubMed] [Google Scholar]
- 237.Lin Y, Gill ME, Koubova J, Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science 17: 1685–1687, 2008. [DOI] [PubMed] [Google Scholar]
- 238.Lindhardt Johansen M, Hagen CP, Rajpert-De Meyts E, Kjærgaard S, Petersen BL, Skakkebæk NE, Main KM, Juul A. 45,X/46,XY mosaicism: phenotypic characteristics, growth, and reproductive function–a retrospective longitudinal study. J Clin Endocrinol Metab 97: E1540–E1549, 2012. [DOI] [PubMed] [Google Scholar]
- 239.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 471: 387–391, 2011. [DOI] [PubMed] [Google Scholar]
- 240.Litchfield K, Shipley J, Turnbull C. Common variants identified in genome-wide association studies of testicular germ cell tumour: an update, biological insights and clinical application. Andrology 3: 34–46, 2015. [DOI] [PubMed] [Google Scholar]
- 241.Liu B, Agras K, Willingham E, Vilela ML, Baskin LS. Activating transcription factor 3 is estrogen-responsive in utero and upregulated during sexual differentiation. Horm Res 65: 217–222, 2006. [DOI] [PubMed] [Google Scholar]
- 242.Liu B, Wang Z, Lin G, Agras K, Ebbers M, Willingham E, Baskin LS. Activating transcription factor 3 is up-regulated in patients with hypospadias. Pediatr Res 58: 1280–1283, 2005. [DOI] [PubMed] [Google Scholar]
- 243.Liu PY, Takahashi PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Aging in healthy men impairs recombinant human luteinizing hormone (LH)-stimulated testosterone secretion monitored under a two-day intravenous pulsatile LH clamp. J Clin Endocrinol Metab 90: 5544–5550, 2005. [DOI] [PubMed] [Google Scholar]
- 244.Livadas S, Mavrou A, Sofocleous C, van Vliet-Constantinidou C, Dracopoulou M, Dacou-Voutetakis C. Gonadoblastoma in a patient with del(9)(p22) and sex reversal: report of a case and review of the literature. Cancer Genet Cytogenet 143: 174–177, 2003. [DOI] [PubMed] [Google Scholar]
- 245.Lo Giacco D, Chianese C, Sanchez-Curbelo J, Bassas L, Ruiz P, Rajmil O, Sarquella J, Vives A, Ruiz-Castane E, Oliva R, Ars E, Krausz C. Clinical relevance of Y-linked CNV screening in male infertility: new insights based on the 8-year experience of a diagnostic genetic laboratory. Eur J Hum Genet 22: 754–761, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Longnecker MP, Klebanoff MA, Brock JW, Zhou H, Gray KA, Needham LL, Wilcox AJ. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. Am J Epidemiol 155: 313–322, 2002. [DOI] [PubMed] [Google Scholar]
- 247.Looijenga LH, Hersmus R, Gillis AJ, Pfundt R, Stoop HJ, van Gurp RJ, Veltman J, Beverloo HB, van DE, van Kessel AG, Pera RR, Schneider DT, Summersgill B, Shipley J, McIntyre A, van der Spek P, Schoenmakers E, Oosterhuis JW. Genomic and expression profiling of human spermatocytic seminomas: primary spermatocyte as tumorigenic precursor and DMRT1 as candidate chromosome 9 gene. Cancer Res 66: 290–302, 2006. [DOI] [PubMed] [Google Scholar]
- 248.Looijenga LH, Stoop H, de Leeuw HP, Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, Perlman EJ, Schneider DT, Kononen J, Sauter G, Oosterhuis JW. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res 63: 2244–2250, 2003. [PubMed] [Google Scholar]
- 249.Lopes AM, Aston KI, Thompson E, Carvalho F, Goncalves J, Huang N, Matthiesen R, Noordam MJ, Quintela I, Ramu A, Seabra C, Wilfert AB, Dai J, Downie JM, Fernandes S, Guo X, Sha J, Amorim A, Barros A, Carracedo A, Hu Z, Hurles ME, Moskovtsev S, Ober C, Paduch DA, Schiffman JD, Schlegel PN, Sousa M, Carrell DT, Conrad DF. Human spermatogenic failure purges deleterious mutation load from the autosomes and both sex chromosomes, including the gene DMRT1. PLoS Genet 9: e1003349, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Louis GB, Dukic V, Heagerty PJ, Louis TA, Lynch CD, Ryan LM, Schisterman EF, Trumble A. Analysis of repeated pregnancy outcomes. Stat Methods Med Res 15: 103–126, 2006. [DOI] [PubMed] [Google Scholar]
- 251.Louis JF, Thoma ME, Sorensen DN, McLain AC, King RB, Sundaram R, Keiding N, Buck Louis GM. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology 1: 741–748, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lund L, Engebjerg MC, Pedersen L, Ehrenstein V, Norgaard M, Sorensen HT. Prevalence of hypospadias in Danish boys: a longitudinal study, 1977–2005. Eur Urol 55: 1022–1026, 2009. [DOI] [PubMed] [Google Scholar]
- 253.Lund L, Engebjerg MC, Pedersen L, Ehrenstein V, Norgaard M, Sorensen HT. Prevalence of Hypospadias in Danish Boys: A Longitudinal Study, 1977–2005. Eur Urol 55: 1022–1026, 2009. [DOI] [PubMed] [Google Scholar]
- 254.Lutz W, O'Neill BC, Scherbov S. Demographics: Europe's population at a turning point. Science 299: 1991–1992, 2003. [DOI] [PubMed] [Google Scholar]
- 255.MacLeod J, Heim LM. Characteristics and variations in semen specimens in 100 normal young men. J Urol 54: 474–482, 1945. [DOI] [PubMed] [Google Scholar]
- 256.Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, Vartiainen T, Skakkebæk NE, Toppari J. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspec 115: 1519–1526, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Main KM, Mortensen GK, Kaleva M, Boisen K, Damgaard I, Chellakooty M, Schmidt IM, Suomi AM, Virtanen H, Petersen JH, Andersson AM, Toppari J, Skakkebæk NE. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in three months old infants. Environ Health Perspect 114: 270–276, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Main KM, Skakkebæk NE, Virtanen HE, Toppari J. Genital anomalies in boys and the environment. Best Pract Res Clin Endocrinol Metab 24: 279–289, 2010. [DOI] [PubMed] [Google Scholar]
- 259.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One 7: e46249, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One 8: e55387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Martenies SE, Perry MJ. Environmental and occupational pesticide exposure and human sperm parameters: a systematic review. Toxicology 307: 66–73, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Matthews TJ. Births: Data for 2011. Hyattsville, MD: National Center for Health Statistics, 2013. (National Vital Statistics Reports) [Google Scholar]
- 263.Massart F, Saggese G. Morphogenetic targets and genetics of undescended testis. Sex Dev 4: 326–335, 2010. [DOI] [PubMed] [Google Scholar]
- 264.Mathews TJ, Hamilton BE. Trend analysis of the sex ratio at birth in the United States. Natl Vital Stat Rep 53: 1–17, 2005. [PubMed] [Google Scholar]
- 265.Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476: 101–104, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mavrogenis S, Czeizel AE. Trends in the prevalence of recorded isolated hypospadias in Hungarian newborn infants during the last 50 years: a population-based study. Reprod Toxicol 42: 251–255, 2013. [DOI] [PubMed] [Google Scholar]
- 267.Mazaud-Guittot S, Nicolaz CN, Desdoits-Lethimonier C, Coiffec I, Maamar MB, Balaguer P, Kristensen DM, Chevrier C, Lavoue V, Poulain P, Dejucq-Rainsford N, Jegou B. Paracetamol, aspirin, and indomethacin induce endocrine disturbances in the human fetal testis capable of interfering with testicular descent. J Clin Endocrinol Metab 98: E1757–E1767, 2013. [DOI] [PubMed] [Google Scholar]
- 268.Mazur A, Westerman R, Mueller U. Is rising obesity causing a secular (age-independent) decline in testosterone among American men? PLoS One 8: e76178, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer 97: 63–70, 2003. [DOI] [PubMed] [Google Scholar]
- 270.McGlynn KA, Guo X, Graubard BI, Brock JW, Klebanoff MA, Longnecker MP. Maternal pregnancy levels of polychlorinated biphenyls and risk of hypospadias and cryptorchidism in male offspring. Environ Health Perspect 117: 1472–1476, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.McIntosh R, Merritt KK, Richards MR, Samuels MH, Bellows MT. The incidence of congenital malformations: a study of 5,964 pregnancies. Pediatrics 14: 505–521, 1954. [PubMed] [Google Scholar]
- 272.McLachlan JA, Newbold RR, Burow ME, Li SF. From malformations to molecular mechanisms in the male: three decades of research on endocrine disrupters. APMIS 109: 263–272, 2001. [DOI] [PubMed] [Google Scholar]
- 273.McLachlan RI, O'Bryan MK. Clinical Review: state of the art for genetic testing of infertile men. J Clin Endocrinol Metab 22: 1013–1024, 2010. [DOI] [PubMed] [Google Scholar]
- 274.Mendiola J, Jørgensen N, Minguez-Alarcon L, Sarabia-Cos L, Lopez-Espin JJ, Vivero-Salmeron G, Ruiz-Ruiz KJ, Fernandez MF, Olea N, Swan SH, Torres-Cantero AM. Sperm counts may have declined in young university students in Southern Spain. Andrology 1: 408–413, 2013. [DOI] [PubMed] [Google Scholar]
- 275.Mendiola J, Stahlhut RW, Jørgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect 119: 958–963, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Messerlian C, Maclagan L, Basso O. Infertility and the risk of adverse pregnancy outcomes: a systematic review and meta-analysis. Hum Reprod 28: 125–137, 2013. [DOI] [PubMed] [Google Scholar]
- 277.Milham S., Jr Unusual sex ratio of births to carbon setter fathers. Am J Ind Med 23: 829–831, 1993. [DOI] [PubMed] [Google Scholar]
- 278.Mocarelli P, Brambilla P, Gerthoux PM, Patterson DG Jr, Needham LL. Change in sex ratio with exposure to dioxin. Lancet 348: 409, 1996. [DOI] [PubMed] [Google Scholar]
- 279.Mocarelli P, Gerthoux PM, Ferrari E, Patterson DG Jr, Kieszak SM, Brambilla P, Vincoli N, Signorini S, Tramacere P, Carreri V, Sampson EJ, Turner WE, Needham LL. Paternal concentrations of dioxin and sex ratio of offspring. Lancet 355: 1858–1863, 2000. [DOI] [PubMed] [Google Scholar]
- 280.Mocarelli P, Gerthoux PM, Needham LL, Patterson DG Jr, Limonta G, Falbo R, Signorini S, Bertona M, Crespi C, Sarto C, Scott PK, Turner WE, Brambilla P. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect 119: 713–718, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mocarelli P, Gerthoux PM, Patterson DG Jr, Milani S, Limonta G, Bertona M, Signorini S, Tramacere P, Colombo L, Crespi C, Brambilla P, Sarto C, Carreri V, Sampson EJ, Turner WE, Needham LL. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect 116: 70–77, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Møller H. Clues to the aetiology of testicular germ cell tumours from descriptive epidemiology. Eur Urol 23: 8–15, 1993. [DOI] [PubMed] [Google Scholar]
- 283.Moller H. Change in male:female ratio among newborn infants in Denmark. Lancet 348: 828–829, 1996. [DOI] [PubMed] [Google Scholar]
- 284.Moniot B, Farhat A, Aritake K, Declosmenil F, Nef S, Eguchi N, Urade Y, Poulat F, Boizet-Bonhoure B. Hematopoietic prostaglandin D synthase (H-Pgds) is expressed in the early embryonic gonad and participates to the initial nuclear translocation of the SOX9 protein. Dev Dyn 240: 2335–2343, 2011. [DOI] [PubMed] [Google Scholar]
- 285.Monteilh C, Kieszak S, Flanders WD, Maisonet M, Rubin C, Holmes AK, Heron J, Golding J, McGeehin MA, Marcus M. Timing of maturation and predictors of Tanner stage transitions in boys enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol 25: 75–87, 2011. [DOI] [PubMed] [Google Scholar]
- 286.Montellier E, Rousseaux S, Zhao Y, Khochbin S. Histone crotonylation specifically marks the haploid male germ cell gene expression program: post-meiotic male-specific gene expression. Bioessays 34: 187–193, 2012. [DOI] [PubMed] [Google Scholar]
- 287.Morales-Suarez-Varela MM, Toft GV, Jensen MS, Ramlau-Hansen C, Kaerlev L, Thulstrup AM, Llopis-Gonzalez A, Olsen J, Bonde JP. Parental occupational exposure to endocrine disrupting chemicals and male genital malformations: a study in the Danish National Birth Cohort study. Environ Health 10: 3, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Morgan EA, Nguyen SB, Scott V, Stadler HS. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development 130: 3095–3109, 2003. [DOI] [PubMed] [Google Scholar]
- 289.Mortimer D, Barratt CL, Bjorndahl L, de JC, Jequier AM, Muller CH. What should it take to describe a substance or product as “sperm-safe.” Hum Reprod Update 19 Suppl 1: i1–45, 2013. [DOI] [PubMed] [Google Scholar]
- 290.Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet 15: 179–180, 1997. [DOI] [PubMed] [Google Scholar]
- 291.Mosher WD, Jones J, Abma JC. Intended and unintended births in the United States: 1982–2010. Natl Health Stat Report 1–28, 2012. [PubMed] [Google Scholar]
- 292.Mouriquand PD, Mure PY. Current concepts in hypospadiology. BJU Int 93 Suppl 3: 26–34, 2004. [DOI] [PubMed] [Google Scholar]
- 293.Mouritsen A, Aksglæde L, Sørensen K, Hagen CP, Petersen JH, Main KM, Juul A. The pubertal transition in 179 healthy Danish children: associations between pubarche, adrenarche, gonadarche, and body composition. Eur J Endocrinol 168: 129–136, 2013. [DOI] [PubMed] [Google Scholar]
- 294.Murray MJ, Halsall DJ, Hook CE, Williams DM, Nicholson JC, Coleman N. Identification of microRNAs From the miR-371∼373 and miR-302 clusters as potential serum biomarkers of malignant germ cell tumors. Am J Clin Pathol 135: 119–125, 2011. [DOI] [PubMed] [Google Scholar]
- 295.Myrianthopoulos NC, Chung CS. Congenital malformations in singletons: epidemiologic survey. Report from the Collaborative Perinatal project. Birth Defects Orig Artic Ser 10: 1–58, 1974. [PubMed] [Google Scholar]
- 296.Myrup C, Schnack TH, Wohlfahrt J. Correction of cryptorchidism and testicular cancer. N Engl J Med 357: 825–827, 2007. [DOI] [PubMed] [Google Scholar]
- 297.Myrup C, Westergaard T, Schnack T, Oudin A, Ritz C, Wohlfahrt J, Melbye M. Testicular cancer risk in first- and second-generation immigrants to Denmark. J Natl Cancer Inst 100: 41–47, 2008. [DOI] [PubMed] [Google Scholar]
- 298.Nassar N, Abeywardana P, Barker A, Bower C. Parental occupational exposure to potential endocrine disrupting chemicals and risk of hypospadias in infants. Occup Environ Med 67: 585–589, 2010. [DOI] [PubMed] [Google Scholar]
- 299.Nassar N, Bower C, Barker A. Increasing prevalence of hypospadias in Western Australia, 1980–2000. Arch Dis Child 92: 580–584, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Nathanson KL, Kanetsky PA, Hawes R, Vaughn DJ, Letrero R, Tucker K, Friedlander M, Phillips KA, Hogg D, Jewett MA, Lohynska R, Daugaard G, Richard S, Chompret A, Bonaiti-Pellie C, Heidenreich A, Olah E, Geczi L, Bodrogi I, Ormiston WJ, Daly PA, Oosterhuis JW, Gillis AJ, Looijenga LH, Guilford P, Fossa SD, Heimdal K, Tjulandin SA, Liubchenko L, Stoll H, Weber W, Rudd M, Huddart R, Crockford GP, Forman D, Oliver DT, Einhorn L, Weber BL, Kramer J, McMaster M, Greene MH, Pike M, Cortessis V, Chen C, Schwartz SM, Bishop DT, Easton DF, Stratton MR, Rapley EA. The Y deletion gr/gr and susceptibility to testicular germ cell tumor. Am J Hum Genet 77: 1034–1043, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Navarro-Costa P, Nogueira P, Carvalho M, Leal F, Cordeiro I, Calhaz-Jorge C, Goncalves J, Plancha CE. Incorrect DNA methylation of the DAZL promoter CpG island associates with defective human sperm. Hum Reprod 25: 2647–2654, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Nef S, Parada LF. Cryptorchidism in mice mutant for Insl3. Nat Genet 22: 295–299, 1999. [DOI] [PubMed] [Google Scholar]
- 303.Nenonen H, Bjork C, Skjaerpe PA, Giwercman A, Rylander L, Svartberg J, Giwercman YL. CAG repeat number is not inversely associated with androgen receptor activity in vitro. Mol Hum Reprod 16: 153–157, 2010. [DOI] [PubMed] [Google Scholar]
- 304.Nenonen HA, Giwercman A, Hallengren E, Giwercman YL. Non-linear association between androgen receptor CAG repeat length and risk of male subfertility–a meta-analysis. Int J Androl 29: 327–332, 2011. [DOI] [PubMed] [Google Scholar]
- 305.Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, Purdie DM, Risch HA, Vergona R, Wu AH. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol 155: 217–224, 2002. [DOI] [PubMed] [Google Scholar]
- 306.Netto GJ, Nakai Y, Nakayama M, Jadallah S, Toubaji A, Nonomura N, Albadine R, Hicks JL, Epstein JI, Yegnasubramanian S, Nelson WG, De Marzo AM. Global DNA hypomethylation in intratubular germ cell neoplasia and seminoma, but not in nonseminomatous male germ cell tumors. Mod Pathol 21: 1337–1344, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nielsen CT, Skakkebæk NE, Richardson DW, Darling JAB, Hunter WM, Jørgensen M, Nielsen Aa Ingerslev O, Keiding N, Müller J. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair, and height. J Clin Endocrinol Metab 62: 532–535, 1986. [DOI] [PubMed] [Google Scholar]
- 308.Nissen KB, Udesen A, Garne E. Hypospadias: prevalence, birthweight and associated major congenital anomalies. Congenit Anom 55: 37–41, 2015. [DOI] [PubMed] [Google Scholar]
- 309.Nordenvall AS, Frisen L, Nordenstrom A, Lichtenstein P, Nordenskjold A. Population based nationwide study of hypospadias in Sweden, 1973 to 2009: incidence and risk factors. J Urol 191: 783–789, 2014. [DOI] [PubMed] [Google Scholar]
- 310.North K, Golding J. A maternal vegetarian diet in pregnancy is associated with hypospadias. The ALSPAC Study Team Avon Longitudinal Study of Pregnancy and Childhood. Br J Urol 85: 107–113, 2000. [DOI] [PubMed] [Google Scholar]
- 311.Novotny GW, Belling K, Bramsen JB, Nielsen JE, Bork-Jensen J, Almstrup K, Sonne SB, Kjems J, Rajpert-De Meyts E, Leffers H. MicroRNA expression profiling of carcinoma in situ cells of the testis. Endocr Relat Cancer 19: 365–379, 2012. [DOI] [PubMed] [Google Scholar]
- 312.Oakes CC, La Salle S, Smiraglia DJ, Robaire B, Trasler JM. Developmental acquisition of genome-wide DNA methylation occurs prior to meiosis in male germ cells. Dev Biol 307: 368–379, 2007. [DOI] [PubMed] [Google Scholar]
- 313.Ogata T, Laporte J, Fukami M. MAMLD1 (CXorf6): a new gene involved in hypospadias. Horm Res 71: 245–252, 2009. [DOI] [PubMed] [Google Scholar]
- 314.Ogawa N, Shah IH. Low Fertility and Reproductive Health in East Asia. New York: Springer, 2015, p. 1–220. [Google Scholar]
- 315.Okonofua FE. The case against new reproductive technologies in developing countries. Br J Obstet Gynaecol 103: 957–962, 1996. [DOI] [PubMed] [Google Scholar]
- 316.Olsen GW, Bodner KM, Ramlow JM, Ross CE, Lipshultz LI. Have sperm counts been reduced 50 percent in 50 years? A statistical model revisited. Fertil Steril 63: 887–893, 1995. [DOI] [PubMed] [Google Scholar]
- 317.Olsen J, Rachootin P. Invited commentary: monitoring fecundity over time–if we do it, then let's do it right. Am J Epidemiol 157: 94–97, 2003. [DOI] [PubMed] [Google Scholar]
- 318.Olsson H, Brandt L. Sex ratio in offspring of patients with non-Hodgkin lymphoma. N Engl J Med 306: 367–368, 1982. [DOI] [PubMed] [Google Scholar]
- 319.Oosterhuis JW, Looijenga LHJ. The biology of human germ cell tumours: retrospective speculations and new prospectives. Eur Urol 23: 245–250, 1993. [DOI] [PubMed] [Google Scholar]
- 320.Paasch U, Salzbrunn A, Glander HJ, Salzbrunn H, Grunewald S, Stucke J, Skakkebæk NE, Jørgensen N. Semen quality in sub-fertile range for a significant proportion of young men from the general German population: a co-ordinated, controlled study of 791 men from Hamburg and Leipzig. Int J Androl 31: 93–102, 2008. [DOI] [PubMed] [Google Scholar]
- 321.Palmer JR, Herbst AL, Noller KL, Boggs DA, Troisi R, Titus-Ernstoff L, Hatch EE, Wise LA, Strohsnitter WC, Hoover RN. Urogenital abnormalities in men exposed to diethylstilbestrol in utero: a cohort study. Environ Health 8: 37, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Palmer RD, Murray MJ, Saini HK, van DS, Abreu-Goodger C, Muralidhar B, Pett MR, Thornton CM, Nicholson JC, Enright AJ, Coleman N. Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res 70: 2911–2923, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Papadimitriou A, Douros K, Kleanthous K, Papadimitriou DT, Attilakos A, Fretzayas A. Pubertal maturation of contemporary Greek boys: no evidence of a secular trend. J Adolesc Health 49: 434–436, 2011. [DOI] [PubMed] [Google Scholar]
- 324.Parazzini F, La VC, Levi F, Franceschi S. Trends in male:female ratio among newborn infants in 29 countries from five continents. Hum Reprod 13: 1394–1396, 1998. [DOI] [PubMed] [Google Scholar]
- 325.Parent AS, Teilmann G, Juul A, Skakkebæk NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends and changes after migration. Endocr Rev 24: 668–693, 2003. [DOI] [PubMed] [Google Scholar]
- 326.Paulozzi LJ. International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect 107: 297–302, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics 100: 831–834, 1997. [DOI] [PubMed] [Google Scholar]
- 328.Pedersen JF, Molsted-Pedersen L. Early fetal growth delay detected by ultrasound marks increased risk of congenital malformation in diabetic pregnancy. Br Med J 283: 269–271, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Perheentupa A, Makinen J, Laatikainen T, Vierula M, Skakkebæk NE, Andersson AM, Toppari J. A cohort effect on serum testosterone levels in finnish men. Eur J Endocrinol 168: 227–233, 2013. [DOI] [PubMed] [Google Scholar]
- 330.Pettersson A, Richiardi L, Nordenskjold A, Kaijser M, Akre O. Age at surgery for undescended testis and risk of testicular cancer. N Engl J Med 356: 1835–1841, 2007. [DOI] [PubMed] [Google Scholar]
- 331.Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RF. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environ Health Perspect 112: 1570–1576, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Pierik FH, Burdorf A, Nijman JM, de Muinck Keizer-Schrama SM, Juttmann RE, Weber RF. A high hypospadias rate in The Netherlands. Hum Reprod 17: 1112–1115, 2002. [DOI] [PubMed] [Google Scholar]
- 333.Pierik FH, Klebanoff MA, Brock JW, Longnecker MP. Maternal pregnancy serum level of heptachlor epoxide, hexachlorobenzene, and beta-hexachlorocyclohexane and risk of cryptorchidism in offspring. Environ Res 105: 364–369, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Porter MP, Faizan MK, Grady RW, Mueller BA. Hypospadias in Washington State: maternal risk factors and prevalence trends. Pediatrics 115: e495–e499, 2005. [DOI] [PubMed] [Google Scholar]
- 335.Potashnik G, Ben-Aderet N, Israeli R, Yanai-Inbar I, Sober I. Suppressive effect of 1,2-dibromo-3-chloropropane on human spermatogenesis. Fertil Steril 30: 444–447, 1978. [DOI] [PubMed] [Google Scholar]
- 336.Potashnik G, Goldsmith J, Insler V. Dibromochloropropane-induced reduction of the sex-ratio in man. Andrologia 16: 213–218, 1984. [DOI] [PubMed] [Google Scholar]
- 337.Poynter JN, Hooten AJ, Frazier AL, Ross JA. Associations between variants in KITLG, SPRY4, BAK1, and DMRT1 and pediatric germ cell tumors. Genes Chromosomes Cancer 51: 266–271, 2012. [DOI] [PubMed] [Google Scholar]
- 338.Priskorn L, Holmboe SA, Jacobsen R, Jensen TK, Lassen TH, Skakkebæk NE. Increasing trends in childlessness in recent birth cohorts: a registry-based study of the total Danish male population born from 1945 to 1980. Int J Androl 35: 449–455, 2012. [DOI] [PubMed] [Google Scholar]
- 339.Priskorn L, Holmboe SA, Jørgensen N, Andersson AM, Almstrup K, Toppari J, Skakkebæk NE. Adverse trends in male reproductive health and decreasing fertility rates. Anim Reprod 9: 760–771, 2012. [Google Scholar]
- 340.Punab M, Zilaitiene B, Jørgensen N, Horte A, Matulevicius V, Peetsalu A, Skakkebæk NE. Regional differences in semen qualities in the Baltic region. Int J Androl 25: 243–252, 2002. [DOI] [PubMed] [Google Scholar]
- 341.Purdue MP, Devesa SS, Sigurdson AJ, McGlynn KA. International patterns and trends in testis cancer incidence. Int J Cancer 115: 822–827, 2005. [DOI] [PubMed] [Google Scholar]
- 342.Quigley CA, DeBellis A, Marschke KB, El-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16: 271–321, 1995. [DOI] [PubMed] [Google Scholar]
- 343.Raatikainen K, Harju M, Hippelainen M, Heinonen S. Prolonged time to pregnancy is associated with a greater risk of adverse outcomes. Fertil Steril 94: 1148–1151, 2010. [DOI] [PubMed] [Google Scholar]
- 344.Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update 12: 303–323, 2006. [DOI] [PubMed] [Google Scholar]
- 345.Rajpert-De Meyts E, Hanstein R, Jørgensen N, Græm N, Vogt PH, Skakkebæk NE. Developmental expression of POU5F1(OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod 19: 1338–1344, 2004. [DOI] [PubMed] [Google Scholar]
- 346.Rajpert-De Meyts E, Leffers H, Daugaard G, Andersen CB, Petersen PM, Hinrichsen J, Pedersen LG, Skakkebæk NE. Analysis of the polymorphic CAG repeat length in the androgen receptor gene in patients with testicular germ cell cancer. Int J Cancer 102: 201–204, 2002. [DOI] [PubMed] [Google Scholar]
- 347.Rajpert-De Meyts E, Skakkebæk NE. Expression of the c-kit protein product in carcinoma-in-situ and invasive testicular germ cell tumours. Int J Androl 17: 85–92, 1994. [DOI] [PubMed] [Google Scholar]
- 348.Raman Wilms L, Tseng AL, Wighardt S, Einarson TR, Koren G. Fetal genital effects of first-trimester sex hormone exposure: a meta-analysis. Obstet Gynecol 85: 141–149, 1995. [DOI] [PubMed] [Google Scholar]
- 349.Rapley EA, Turnbull C, Al Olama AA, Dermitzakis ET, Linger R, Huddart RA, Renwick A, Hughes D, Hines S, Seal S, Morrison J, Nsengimana J, Deloukas P, Rahman N, Bishop DT, Easton DF, Stratton MR. A genome-wide association study of testicular germ cell tumor. Nat Genet 41: 807–810, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ravnborg TL, Jensen TK, Andersson AM, Toppari J, Skakkebæk NE, Jørgensen N. Prenatal and adult exposures to smoking are associated with adverse effects on reproductive hormones, semen quality, final height and body mass index. Hum Reprod 26: 1000–1011, 2011. [DOI] [PubMed] [Google Scholar]
- 351.Redmon JB, Thomas W, Ma W, Drobnis EZ, Sparks A, Wang C, Brazil C, Overstreet JW, Liu F, Swan SH. Semen parameters in fertile US men: the Study for Future Families. Andrology 1: 806–814, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Repping S, Skaletsky H, Brown L, van Daalen SK, Korver CM, Pyntikova T, Kuroda-Kawaguchi T, de Vries JW, Oates RD, Silber S, van der Veen F, Page DC, Rozen S. Polymorphism for a 1.6-Mb deletion of the human Y chromosome persists through balance between recurrent mutation and haploid selection. Nature Genet 7: 247–251, 2003. [DOI] [PubMed] [Google Scholar]
- 353.Rey RA, Codner E, Iniguez G, Bedecarras P, Trigo R, Okuma C, Gottlieb S, Bergada I, Campo SM, Cassorla FG. Low risk of impaired testicular Sertoli and Leydig cell functions in boys with isolated hypospadias. J Clin Endocrinol Metab 90: 6035–6040, 2005. [DOI] [PubMed] [Google Scholar]
- 354.Rider CV, Furr JR, Wilson VS, Gray LE Jr. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl 33: 443–462, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Rijlaarsdam MA, van AT, Gillis AJ, Patel S, Hayashibara K, Lee KY, Looijenga LH. Identification of known and novel germ cell cancer-specific (embryonic) miRs in serum by high-throughput profiling. Andrology 3: 85–91, 2015. [DOI] [PubMed] [Google Scholar]
- 356.Ritzen EM, Bergh A, Bjerknes R, Christiansen P, Cortes D, Haugen S, Jørgensen N, Kollin C, Lindahl S, Läckgren G, Main KM, Nordenskjöld A, Rajpert-De Meyts E, Söder O, Taskinen S, Thorsson A, Thorup J, Toppari J, Virtanen H. Nordic consensus on treatment of undescended testes. Acta Paediatr 96: 638–643, 2007. [DOI] [PubMed] [Google Scholar]
- 357.Rjasanowski I, Kloting I, Kovacs P. Altered sex ratio in offspring of mothers with insulin-dependent diabetes mellitus. Lancet 351: 497–498, 1998. [DOI] [PubMed] [Google Scholar]
- 358.Ro S, Park C, Sanders KM, McCarrey JR, Yan W. Cloning and expression profiling of testis-expressed microRNAs. Dev Biol 311: 592–602, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Robbins WA, Wei F, Elashoff DA, Wu G, Xun L, Jia J. Y:X sperm ratio in boron-exposed men. J Androl 29: 115–121, 2008. [DOI] [PubMed] [Google Scholar]
- 360.Rocheleau CM, Romitti PA, Dennis LK. Pesticides and hypospadias: a meta-analysis. J Pediatr Urol 5: 17–24, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Rocheleau CM, Romitti PA, Sanderson WT, Sun L, Lawson CC, Waters MA, Stewart PA, Olney RS, Reefhuis J. Maternal occupational pesticide exposure and risk of hypospadias in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol 91: 927–936, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33: 9003–9012, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Rolland M, Le MJ, Wagner V, Royere D, De MJ. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod 28: 462–470, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Rostad B, Schei B, Sundby J. Fertility in Norwegian women: results from a population-based health survey. Scand J Public Health 34: 5–10, 2006. [DOI] [PubMed] [Google Scholar]
- 365.Rothbart SB, Strahl BD. Interpreting the language of histone and DNA modifications. Biochim Biophys Acta 1839: 627–643, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Rozen SG, Marszalek JD, Irenze K, Skaletsky H, Brown LG, Oates RD, Silber SJ, Ardlie K, Page DC. AZFc deletions and spermatogenic failure: a population-based survey of 20,000 Y chromosomes. Am J Hum Genet 91: 890–896, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Ruark E, Seal S, McDonald H, Zhang F, Elliot A, Lau K, Perdeaux E, Rapley E, Eeles R, Peto J, Kote-Jarai Z, Muir K, Nsengimana J, Shipley J, Bishop DT, Stratton MR, Easton DF, Huddart RA, Rahman N, Turnbull C. Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nature Gen 15: 686–689, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Rutgers JL, Scully RE. Pathology of the testis in intersex syndromes. Semin Diagn Pathol 4: 275–291, 1987. [PubMed] [Google Scholar]
- 369.Sathyanarayana S, Beard L, Zhou C, Grady R. Measurement and correlates of ano-genital distance in healthy, newborn infants. Int J Androl 33: 317–323, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Scheike TH, Rylander L, Carstensen L, Keiding N, Jensen TK, Stromberg U, Joffe M, Akre O. Time trends in human fecundability in Sweden. Epidemiology 19: 191–196, 2008. [DOI] [PubMed] [Google Scholar]
- 371.Schiffer C, Muller A, Egeberg DL, Alvarez L, Brenker C, Rehfeld A, Frederiksen H, Waschle B, Kaupp UB, Balbach M, Wachten D, Skakkebæk NE, Almstrup K, Strunker T. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep 15: 758–765, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Schmidt L, Münster K. Infertility, involuntary infecundity, and the seeking of medical advice in industrialized countries 1970–1992: a review of concepts, measurements and results. Hum Reprod 10: 1407–1418, 1995. [DOI] [PubMed] [Google Scholar]
- 373.Schnack TH, Poulsen G, Myrup C, Wohlfahrt J, Melbye M. Familial coaggregation of cryptorchidism, hypospadias, and testicular germ cell cancer: a nationwide cohort study. J Natl Cancer Inst 102: 187–192, 2010. [DOI] [PubMed] [Google Scholar]
- 375.Schnack TH, Zdravkovic S, Myrup C, Westergaard T, Wohlfahrt J, Melbye M. Familial aggregation of cryptorchidism–a nationwide cohort study. Am J Epidemiol 167: 1453–1457, 2008. [DOI] [PubMed] [Google Scholar]
- 376.Schubeler D. Function and information content of DNA methylation. Nature 517: 321–326, 2015. [DOI] [PubMed] [Google Scholar]
- 377.Scorer CG. The descent of the testis. Arch Dis Child 39: 605–609, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Scully RE. Gonadoblastoma. A review of 74 cases. Cancer 25: 1340–1356, 1970. [DOI] [PubMed] [Google Scholar]
- 379.Serrano T, Chevrier C, Multigner L, Cordier S, Jegou B. International geographic correlation study of the prevalence of disorders of male reproductive health. Hum Reprod 28: 1974–1986, 2013. [DOI] [PubMed] [Google Scholar]
- 380.Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc Lond B Biol Sci 365: 1697–1712, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Sharpe RM, Skakkebæk NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341: 1392–1395, 1993. [DOI] [PubMed] [Google Scholar]
- 382.Skakkebæk NE. Possible carcinoma-in-situ of the testis. Lancet ii: 516–517, 1972. [DOI] [PubMed] [Google Scholar]
- 383.Skakkebæk NE, Andersson AM, Juul A, Jensen TK, Almstrup K, Toppari J, Jørgensen N. Sperm counts, data responsibility, and good scientific practice. Epidemiology 22: 620–621, 2011. [DOI] [PubMed] [Google Scholar]
- 384.Skakkebæk NE, Berthelsen JG, Giwercman A, Müller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl 10: 19–28, 1987. [DOI] [PubMed] [Google Scholar]
- 385.Skakkebæk NE, Berthelsen JG, Visfeldt J. Clinical aspects of testicular carcinoma-in-situ. Int J Androl Suppl 4: 153–162, 1981. [DOI] [PubMed] [Google Scholar]
- 386.Skakkebæk NE, Høi-Hansen CE, Holm M, Jørgensen N, Rajpert-De Meyts E. Association between testicular dysgenesis syndrome (TDS) and testicular neoplasia: evidence from 20 adult patients with signs of maldevelopment of the testis. APMIS 111: 1–11, 2003. [DOI] [PubMed] [Google Scholar]
- 387.Skakkebæk NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16: 972–978, 2001. [DOI] [PubMed] [Google Scholar]
- 388.Skinner MK, Manikkam M, Tracey R, Guerrero-Bosagna C, Haque M, Nilsson EE. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med 11: 228, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Slama R, Eustache F, Ducot B, Jensen TK, Jørgensen N, Horte A, Irvine S, Suominen J, Andersen AG, Auger J, Vierula M, Toppari J, Andersen AN, Keiding N, Skakkebæk NE, Spira A, Jouannet P. Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum Reprod 17: 503–515, 2002. [DOI] [PubMed] [Google Scholar]
- 390.Slama R, Hansen OK, Ducot B, Bohet A, Sorensen D, Giorgis AL, Eijkemans MJ, Rosetta L, Thalabard JC, Keiding N, Bouyer J. Estimation of the frequency of involuntary infertility on a nation-wide basis. Hum Reprod 27: 1489–1498, 2012. [DOI] [PubMed] [Google Scholar]
- 391.Slama R, Kold-Jensen T, Scheike T, Ducot B, Spira A, Keiding N. How would a decline in sperm concentration over time influence the probability of pregnancy? Epidemiology 15: 458–465, 2004. [DOI] [PubMed] [Google Scholar]
- 392.Small CM, DeCaro JJ, Terrell ML, Dominguez C, Cameron LL, Wirth J, Marcus M. Maternal exposure to a brominated flame retardant and genitourinary conditions in male offspring. Environ Health Perspect 117: 1175–1179, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Smiraglia DJ, Szymanska J, Kraggerud SM, Lothe RA, Peltomaki P, Plass C. Distinct epigenetic phenotypes in seminomatous and nonseminomatous testicular germ cell tumors. Oncogene 21: 3909–3916, 2002. [DOI] [PubMed] [Google Scholar]
- 394.Smits LJ, de Bie RA, Essed GG, van den Brandt PA. Time to pregnancy and sex of offspring: cohort study. Br Med J 331: 1437–1438, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Song R, Hennig GW, Wu Q, Jose C, Zheng H, Yan W. Male germ cells express abundant endogenous siRNAs. Proc Natl Acad Sci USA 108: 13159–13164, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Sonne SB, Almstrup K, Dalgaard M, Juncker AS, Edsgard D, Ruban L, Harrison NJ, Schwager C, Abdollahi A, Huber PE, Brunak S, Gjerdrum LM, Moore HD, Andrews PW, Skakkebæk NE, Rajpert-De Meyts E, Leffers H. Analysis of gene expression profiles of microdissected cell populations indicates that testicular carcinoma in situ is an arrested gonocyte. Cancer Res 69: 5241–5250, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Sørensen K, Aksglæde L, Munch-Andersen T, Aachmann-Andersen NJ, Leffers H, Helge JW, Hilsted L, Juul A. Impact of the growth hormone receptor exon 3 deletion gene polymorphism on glucose metabolism, lipids, and insulin-like growth factor-I levels during puberty. J Clin Endocrinol Metab 94: 2966–2969, 2009. [DOI] [PubMed] [Google Scholar]
- 398.Sørensen K, Aksglæde L, Munch-Andersen T, Aachmann-Andersen NJ, Petersen JH, Hilsted L, Helge JW, Juul A. Sex hormone-binding globulin levels predict insulin sensitivity, disposition index and cardiovascular risk during puberty. Diabetes Care 32: 909–914, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Sørensen K, Aksglæde L, Petersen JH, Juul A. Recent changes in pubertal timing in healthy danish boys: associations with body mass index. J Clin Endocrinol Metab 95: 263–270, 2010. [DOI] [PubMed] [Google Scholar]
- 400.Splingart C, Frapsauce C, Veau S, Barthelemy C, Royere D, Guerif F. Semen variation in a population of fertile donors: evaluation in a French centre over a 34-year period. Int J Androl 35: 467–474, 2012. [DOI] [PubMed] [Google Scholar]
- 401.Sripada S, Fonseca S, Lee A, Harrild K, Giannaris D, Mathers E, Bhattacharya S. Trends in semen parameters in the northeast of Scotland. J Androl 28: 313–319, 2007. [DOI] [PubMed] [Google Scholar]
- 402.Stoop H, Honecker F, van de Geijn GJ, Gillis AJ, Cools MC, de BM, Bokemeyer C, Wolffenbuttel KP, Drop SL, De Krijger RR, Dennis N, Summersgill B, McIntyre A, Shipley J, Oosterhuis JW, Looijenga LH. Stem cell factor as a novel diagnostic marker for early malignant germ cells. J Pathol 216: 43–54, 2008. [DOI] [PubMed] [Google Scholar]
- 403.Stouffs K, Vandermaelen D, Massart A, Menten B, Vergult S, Tournaye H, Lissens W. Array comparative genomic hybridization in male infertility. Hum Reprod 27: 921–929, 2012. [DOI] [PubMed] [Google Scholar]
- 404.Strandberg-Larsen K, Jensen MS, Ramlau-Hansen CH, Gronbaek M, Olsen J. Alcohol binge drinking during pregnancy and cryptorchidism. Hum Reprod 24: 3211–3219, 2009. [DOI] [PubMed] [Google Scholar]
- 405.Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471: 382–386, 2011. [DOI] [PubMed] [Google Scholar]
- 406.Sundby J, Mboge R, Sonko S. Infertility in the Gambia: frequency and health care seeking. Soc Sci Med 46: 891–899, 1998. [DOI] [PubMed] [Google Scholar]
- 407.Suzawa M, Ingraham HA. The herbicide atrazine activates endocrine gene networks via non-steroidal NR5A nuclear receptors in fish and mammalian cells. PLoS One 3: e2117, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Swan SH. Prenatal phthalate exposure and anogenital distance in male infants. Environ Health Perspect 114: A88–A89, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, Hatch M, Redmon JB, Wang C, Overstreet JW. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect 111: 414–420, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect 105: 1228–1232, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect 108: 961–966, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 113: 1056–1061, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Sweet RA, Schrott HG, Kurland R, Culp OS. Study of the incidence of hypospadias in Rochester, Minnesota, 1940–1970, and a case-contol comparison of possible etiologic factors. Mayo Clin Proc 49: 52–58, 1974. [PubMed] [Google Scholar]
- 414.Tavares RS, Mansell S, Barratt CL, Wilson SM, Publicover SJ, Ramalho-Santos J. p,p'-DDE activates CatSper and compromises human sperm function at environmentally relevant concentrations. Hum Reprod 28: 3167–3177, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Thankamony A, Lek N, Carroll D, Williams M, Dunger DB, Acerini CL, Ong KK, Hughes IA. Anogenital distance and penile length in infants with hypospadias or cryptorchidism: comparison with normative data. Environ Health Perspect 122: 207–211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 99: 1324–1331, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Thorup J, Cortes D, Petersen BL. The incidence of bilateral cryptorchidism is increased and the fertility potential is reduced in sons born to mothers who have smoked during pregnancy. J Urol 176: 734–737, 2006. [DOI] [PubMed] [Google Scholar]
- 418.Tietze C. Fertility after discontinuation of intrauterine and oral contraception. Int J Fertil 13: 385–389, 1968. [PubMed] [Google Scholar]
- 419.Tober DM, Taghdisi MH, Jalali M. “Fewer children, better life” or “as many as God wants”? Family planning among low-income Iranian and Afghan refugee families in Isfahan, Iran. Med Anthropol Q 20: 50–71, 2006. [DOI] [PubMed] [Google Scholar]
- 420.Tomova A, Lalabonova C, Robeva RN, Kumanov PT. Timing of pubertal maturation according to the age at first conscious ejaculation. Andrologia 43: 163–166, 2011. [DOI] [PubMed] [Google Scholar]
- 421.Toppari J, Kaleva M, Virtanen HE. Trends in the incidence of cryptorchidism and hypospadias, and methodological limitations of registry-based data. Hum Reprod Update 7: 282–286, 2001. [DOI] [PubMed] [Google Scholar]
- 422.Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJJr Jégou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Müller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebæk NE. Male reproductive health and environmental xenoestrogens. Environ Health Perspect 104: 741–803, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Toppari J, Virtanen HE, Main KM, Skakkebæk NE. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res A Clin Mol Teratol 88: 910–919, 2010. [DOI] [PubMed] [Google Scholar]
- 424.Trabert B, Chodick G, Shalev V, Sella T, Longnecker MP, McGlynn KA. Gestational diabetes and the risk of cryptorchidism and hypospadias. Epidemiology 25: 152–153, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Trabert B, Longnecker MP, Brock JW, Klebanoff MA, McGlynn KA. Maternal pregnancy levels of trans-nonachlor and oxychlordane and prevalence of cryptorchidism and hypospadias in boys. Environ Health Perspect 120: 478–482, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Travison TG, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 92: 549–555, 2007. [DOI] [PubMed] [Google Scholar]
- 427.Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab 92: 196–202, 2007. [DOI] [PubMed] [Google Scholar]
- 428.Trbovich AM, Martinelle N, O'Neill FH, Pearson EJ, Donahoe PK, Sluss PM, Teixeira J. Steroidogenic activities in MA-10 Leydig cells are differentially altered by cAMP and Mullerian inhibiting substance. J Steroid Biochem Mol Biol 92: 199–208, 2004. [DOI] [PubMed] [Google Scholar]
- 429.Trbovich AM, Sluss PM, Laurich VM, O'Neill FH, MacLaughlin DT, Donahoe PK, Teixeira J. Mullerian Inhibiting Substance lowers testosterone in luteinizing hormone-stimulated rodents. Proc Natl Acad Sci USA 98: 3393–3397, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Trimpou P, Lindahl A, Lindstedt G, Olerod G, Wilhelmsen L, Landin-Wilhelmsen K. Secular trends in sex hormones and fractures in men and women. Eur J Endocrinol 166: 887–895, 2012. [DOI] [PubMed] [Google Scholar]
- 431.Tsatsanis C, Bobjer J, Rastkhani H, Dermitzaki E, Katrinaki M, Margioris AN, Giwercman YL, Giwercman A. Serum miR-155 as a potential biomarker of male fertility. Hum Reprod 30: 853–860, 2015. [DOI] [PubMed] [Google Scholar]
- 432.Turnbull C, Rapley EA, Seal S, Pernet D, Renwick A, Hughes D, Ricketts M, Linger R, Nsengimana J, Deloukas P, Huddart RA, Bishop DT, Easton DF, Stratton MR, Rahman N. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. 42: 604–607, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Tuttelmann F, Laan M, Grigorova M, Punab M, Sober S, Gromoll J. Combined effects of the variants FSHB −211G>T and FSHR 2039A>G on male reproductive parameters. J Clin Endocrinol Metab 14: 3639–3647, 2012. [DOI] [PubMed] [Google Scholar]
- 434.Tüttelmann F, Rajpert-De Meyts E, Nieschlag E, Simoni M. Gene polymorphisms and male infertility: a meta-analysis and literature review. RBM Online 15: 643–658, 2007. [DOI] [PubMed] [Google Scholar]
- 435.Tuttelmann F, Simoni M, Kliesch S, Ledig S, Dworniczak B, Wieacker P, Ropke A. Copy number variants in patients with severe oligozoospermia and Sertoli-cell-only syndrome. PLoS One 6: e19426, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Ushida H, Kawakami T, Minami K, Chano T, Okabe H, Okada Y, Okamoto K. Methylation profile of DNA repetitive elements in human testicular germ cell tumor. Mol Carcinog 51: 711–722, 2012. [DOI] [PubMed] [Google Scholar]
- 437.Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD. A distinct small RNA pathway silences selfish genetic elements in the germline. Science 313: 320–324, 2006. [DOI] [PubMed] [Google Scholar]
- 438.Van der Pal-de Bruin KM, Verloove-Vanhorick SP, Roeleveld N. Change in male:female ratio among newborn babies in Netherlands. Lancet 349: 62, 1997. [DOI] [PubMed] [Google Scholar]
- 439.Van der Zanden LF, van Rooij I, Feitz WF, Knight J, Donders AR, Renkema KY, Bongers EM, Vermeulen SH, Kiemeney LA, Veltman JA, Rias-Vasquez A, Zhang X, Markljung E, Qiao L, Baskin LS, Nordenskjold A, Roeleveld N, Franke B, Knoers NV. Common variants in DGKK are strongly associated with risk of hypospadias. Nat Genet 43: 48–50, 2011. [DOI] [PubMed] [Google Scholar]
- 440.Van der Zanden LF, van Rooij I, Feitz WF, Vermeulen SH, Kiemeney LA, Knoers NV, Roeleveld N, Franke B. Genetics of hypospadias: are single-nucleotide polymorphisms in SRD5A2, ESR1, ESR2, and ATF3 really associated with the malformation? J Clin Endocrinol Metab 95: 2384–2390, 2010. [DOI] [PubMed] [Google Scholar]
- 441.Van Waeleghem K, De Clercq N, Vermeulen L, Schoonjans F, Comhaire F. Deterioration of sperm quality in young healthy Belgian men. Hum Reprod 11: 325–329, 1996. [DOI] [PubMed] [Google Scholar]
- 442.Veldhuis JD, Zwart A, Mulligan T, Iranmanesh A. Muting of androgen negative feedback unveils impoverished gonadotropin-releasing hormone/luteinizing hormone secretory reactivity in healthy older men. J Clin Endocrinol Metab 86: 529–535, 2001. [DOI] [PubMed] [Google Scholar]
- 443.Villumsen ÅL, Zachau-Christiansen B. Spontaneous alterations in position of the testes. Arch Dis Child 41: 198–200, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Vinggaard AM, Christiansen S, Laier P, Poulsen ME, Breinholt V, Jarfelt K, Jacobsen H, Dalgaard M, Nellemann C, Hass U. Perinatal exposure to the fungicide prochloraz feminizes the male rat offspring. Toxicol Sci 85: 886–897, 2005. [DOI] [PubMed] [Google Scholar]
- 445.Virtanen HE, Kaleva M, Haavisto AM, Schmidt IM, Chellakooty M, Main KM, Skakkebæk NE, Toppari J. The birth rate of hypospadias in the Turku area in Finland. APMIS 109: 96–100, 2001. [DOI] [PubMed] [Google Scholar]
- 446.Virtanen HE, Koskenniemi JJ, Sundqvist E, Main KM, Kiviranta H, Tuomisto JT, Tuomisto J, Viluksela M, Vartiainen T, Skakkebæk NE, Toppari J. Associations between congenital cryptorchidism in newborn boys and levels of dioxins and PCBs in placenta. Int J Androl 35: 283–293, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Virtanen HE, Tapanainen AE, Kaleva MM, Suomi AM, Main KM, Skakkebæk NE, Toppari J. Mild gestational diabetes as a risk factor for congenital cryptorchidism. J Clin Endocrinol Metab 91: 4862–4865, 2006. [DOI] [PubMed] [Google Scholar]
- 448.Von Eckardstein S, Syska A, Gromoll J, Kamischke A, Simoni M, Nieschlag E. Inverse correlation between sperm concentration and number of androgen receptor CAG repeats in normal men. J Clin Endocrinol Metab 86: 2585–2590, 2001. [DOI] [PubMed] [Google Scholar]
- 449.Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A, Zlotorynski E, Yabuta N, De Vita G, Nojima H, Looijenga LH, Agami R. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 124: 1169–1181, 2006. [DOI] [PubMed] [Google Scholar]
- 450.Walker A. Ageing in Europe–challenges and consequences. Z Gerontol Geriatr 32: 390–397, 1999. [DOI] [PubMed] [Google Scholar]
- 451.Watanabe M, Yoshida R, Ueoka K, Aoki K, Sasagawa I, Hasegawa T, Sueoka K, Kamatani N, Yoshimura Y, Ogata T. Haplotype analysis of the estrogen receptor 1 gene in male genital and reproductive abnormalities. Hum Reprod 22: 1279–1284, 2007. [DOI] [PubMed] [Google Scholar]
- 452.Weidner IS, Møller H, Jensen TK, Skakkebæk NE. Risk factors for cryptorchidism and hypospadias. J Urol 161: 1606–1609, 1999. [PubMed] [Google Scholar]
- 453.Weijin Z, Olsen J. Offspring sex ratio as an indicator of reproductive hazards. Occup Environ Med 53: 503–504, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest 118: 1479–1490, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Wennerholm UB, Bergh C, Hamberger L, Lundin K, Nilsson L, Wikland M, Kallen B. Incidence of congenital malformations in children born after ICSI. Hum Reprod 15: 944–948, 2000. [DOI] [PubMed] [Google Scholar]
- 456.Wermann H, Stoop H, Gillis AJ, Honecker F, van Gurp RJ, Ammerpohl O, Richter J, Oosterhuis JW, Bokemeyer C, Looijenga LH. Global DNA methylation in fetal human germ cells and germ cell tumours: association with differentiation and cisplatin resistance. J Pathol 221: 433–442, 2010. [DOI] [PubMed] [Google Scholar]
- 457.Westerveld H, Visser L, Tanck M, van der Veen F, Repping S. CAG repeat length variation in the androgen receptor gene is not associated with spermatogenic failure. Fertil Steril 89: 253–259, 2008. [DOI] [PubMed] [Google Scholar]
- 458.WHO. Infertility: A Tabulation of Available Data on Prevalence of Primary and Secondary Infertility. Programme on Maternal and Child Health and Family Planning, Division of Family Health. Geneva: World Health Organization, 1991. [Google Scholar]
- 459.WHO, UNEP. State Of The Science Of Endocrine Disrupting Chemicals 2012: An Assessment Of The State Of The Science Of Endocrine Disruptors Prepared By A Group Of Experts For The United Nations Environment Programme And World Health Organization. Geneva: WHO, 2013, p. 1–260. [Google Scholar]
- 460.Whorton D, Milby TH, Krauss RM, Stubbs HA. Testicular function in DBCP exposed pesticide workers. J Occup Med 21: 161–166, 1979. [PubMed] [Google Scholar]
- 461.Wilhelm D, Hiramatsu R, Mizusaki H, Widjaja L, Combes AN, Kanai Y, Koopman P. SOX9 regulates prostaglandin D synthase gene transcription in vivo to ensure testis development. J Biol Chem 282: 10553–10560, 2007. [DOI] [PubMed] [Google Scholar]
- 462.Willingham E, Baskin LS. Candidate genes and their response to environmental agents in the etiology of hypospadias. Nat Clin Pract Urol 270: 270–279, 2007. [DOI] [PubMed] [Google Scholar]
- 463.World Bank.www.worldbank.org, 2014. [Google Scholar]
- 464.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: World Health Organisation, 2010. [Google Scholar]
- 465.World Health Organization. Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction. Geneva: World Health Organization, 1980. [Google Scholar]
- 466.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction. New York: Cambridge Univ. Press, 1987, p. 1–67. [Google Scholar]
- 467.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Cambridge, UK: Cambridge Univ. Press, 1992. [Google Scholar]
- 468.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 93: 2737–2745, 2008. [DOI] [PubMed] [Google Scholar]
- 469.Yamaji M, Seki Y, Kurimoto K, Yabuta Y, Yuasa M, Shigeta M, Yamanaka K, Ohinata Y, Saitou M. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet 40: 1016–1022, 2008. [DOI] [PubMed] [Google Scholar]
- 470.Yang Q, Hua J, Wang L, Xu B, Zhang H, Ye N, Zhang Z, Yu D, Cooke HJ, Zhang Y, Shi Q. MicroRNA and piRNA profiles in normal human testis detected by next generation sequencing. PLoS One 8: e66809, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Yatsenko AN, Georgiadis AP, Röpke A, Berman AJ, Jaffe T, Okszewska M, Westernströer B, Sanfilippo J, Kurpisz M, Rajkovic A, Yatsenko SA, Kliesch S, Schlatt S, Tüttelmann F. X-linked TEX11 mutations, meiotic arrest, and azoospermia in infertile men. N Engl J Med 372: 2097–2107, 2015. (PMID: 259700010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Yu B, Qi Y, Liu D, Gao X, Chen H, Bai C, Huang Z. Cigarette smoking is associated with abnormal histone-to-protamine transition in human sperm. Fertil Steril 101: 51–57, 2014. [DOI] [PubMed] [Google Scholar]
- 473.Zhang S, Tang Q, Wu W, Yuan B, Lu C, Xia Y, Ding H, Hu L, Chen D, Sha J, Wang X. Association between DAZL polymorphisms and susceptibility to male infertility: systematic review with meta-analysis and trial sequential analysis. Sci Rep 4: 4642, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Zhao H, Xu J, Zhang H, Sun J, Sun Y, Wang Z, Liu J, Ding Q, Lu S, Shi R, You L, Qin Y, Zhao X, Lin X, Li X, Feng J, Wang L, Trent JM, Xu C, Gao Y, Zhang B, Gao X, Hu J, Chen H, Li G, Zhao J, Zou S, Jiang H, Hao C, Zhao Y, Ma J, Zheng SL, Chen ZJ. A genome-wide association study reveals that variants within the HLA region are associated with risk for nonobstructive azoospermia. Am J Hum Genet 90: 900–906, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Zhao L, Liu W, Xiao J, Cao B. The role of exosomes and “exosomal shuttle microRNA” in tumorigenesis and drug resistance. Cancer Lett 356 (2 pt B): 339–346, 2015. (PMID: 25449429) [DOI] [PubMed] [Google Scholar]
- 476.Zhu WX, Lu L, Hesketh T. China's excess males, sex selective abortion, and one child policy: analysis of data from 2005 national intercensus survey. Br Med J 338: b1211, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Zielhuis GA, Hulscher MEJL, Florack EIM. Validity and reliability of a questionnaire on fecundability. Int J Epidemiol 21: 1151–1156, 1992. [DOI] [PubMed] [Google Scholar]
- 478.Zimmermann C, Romero Y, Warnefors M, Bilican A, Borel C, Smith LB, Kotaja N, Kaessmann H, Nef S. Germ cell-specific targeting of DICER or DGCR8 reveals a novel role for endo-siRNAs in the progression of mammalian spermatogenesis and male fertility. PLoS ONE 9: e107023, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, Adham IM. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol 13: 681–691, 1999. [DOI] [PubMed] [Google Scholar]
- 480.Zinaman MJ, Clegg ED, Brown CC, O'Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril 65: 503–509, 1996. [PubMed] [Google Scholar]
- 481.Znaor A, Lortet-Tieulent J, Jemal A, Bray F. International variations and trends in testicular cancer incidence and mortality. Eur Urol 65: 1095–1106, 2014. [DOI] [PubMed] [Google Scholar]
- 482.Zorn B, Sucur V, Stare J, Meden-Vrtovec H. Decline in sex ratio at birth after 10-day war in Slovenia: brief communication. Hum Reprod 17: 3173–3177, 2002. [DOI] [PubMed] [Google Scholar]