Abstract
Health care-associated bacterial pneumonias due to multiple-drug resistant (MDR) pathogens are an important public health problem and are major causes of morbidity and mortality worldwide. In addition to antimicrobial resistance, these organisms have adapted to the milieu of the human airway and have acquired resistance to the innate immune clearance mechanisms that normally prevent pneumonia. Given the limited efficacy of antibiotics, bacterial clearance from the airway requires an effective immune response. Understanding how specific airway pathogens initiate and regulate innate immune signaling, and whether this response is excessive, leading to host-induced pathology may guide future immunomodulatory therapy. We will focus on three of the most important causes of health care-associated pneumonia, Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae, and review the mechanisms through which an inappropriate or damaging innate immune response is stimulated, as well as describe how airway pathogens cause persistent infection by evading immune activation.
I. INTRODUCTION
Bacterial pneumonia is a major complication of hospitalization, associated with substantial morbidity and mortality (102, 289). Many of these pneumonias are caused by airway opportunists, the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebiella pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa, and Enterobacter species) (366). In this review, we explore how three of the most common causes of health-care associated bacterial pneumonias (S. aureus, P.aeruginosa, and K. pneumoniae) interact with the innate immune system in the respiratory tract (329). While each of these is a distinct species with a diverse phenotype, these organisms share the ability to evolve in response to antimicrobial pressure without a loss in fitness and proliferate in health care-associated settings. We briefly review the common components of innate immune signaling in the airway, how they are activated by conserved microbial pathogen-associated patterns (PAMPs), the coordinated interactions of “nontraditional” immune cells (i.e., epithelial cells) as well as professional immune cells, and then describe the mechanisms and types of immune cells that each of these pathogens targets to cause pneumonia. While there are a finite number of immune components that are activated in the early stages of airway infection, it will become evident in this review that the mechanisms through which each of these major pathogens interacts with the host result in very different pathophysiology. The ultimate result is death of the host through respiratory failure and/or sepsis, but the immunopathological events leading up to respiratory compromise by each of these organisms are distinct.
The pathogens that we have targeted each possesses a tremendous genetic capability of adaptation to human airways and cannot only persist but flourish in this environmental niche. Their acquisition of novel genetic elements, such as the uptake of antibiotic resistance genes, is readily apparent and has made effective treatment of infections due to these organisms difficult. In addition, phenotypic adaptation may be evident, with selection of pathogens with exuberant extracellular polysaccharide production. Less obvious, but central to pathogenesis, are the innumerable adaptations to the human immune system. The co-evolution of these bacteria in response to immune pressure imposed by both innate and adaptive immunity has made them especially formidable pathogens. As will be apparent in the sections to follow, each of these organisms has developed its own mechanism(s) for thwarting, evading, or exploiting innate immune clearance.
A. Pathogenesis of Airway Infection
Airway infection occurs primarily through the aspiration of organisms colonizing the upper respiratory tract. Through the selection of mutants that can evade immune clearance, a subpopulation of the colonizing strains is selected, then aspirated into the lower airways where inflammation mediated by local “nontraditional” immune cells as well as professional immune cells is initiated. Pneumonia results from: disruption of epithelial tight junctions, neutrophil recruitment, release of proteases and reactive oxygen intermediates, generation of alveolar edema, and impairment of normal physiological gas exchange, while bacterial invasion across the capillary or epithelial barrier, or lymphatic system, leads to bacteremia. In a hospital setting, the selection of highly antibiotic-resistant pathogens is recognized as a major clinical problem. Less apparent is the simultaneous selection of bacterial mutants resistant to the various components of host innate immunity that contribute to airway immune clearance. The genetic flexibility of these organisms enables the overall population to proliferate through selection of mutants that thrive despite the presence of phagocytes and their bactericidal capabilities.
B. Surface Airway Defenses
The respiratory mucosa is exceptionally well defended from bacterial infection through its multiple mechanisms of airway clearance that include expression of antimicrobial proteins, mucociliary clearance, and active surveillance of airway macrophages (1). Remarkably few organisms ever contact airway epithelial cells, a process which stimulates organism virulence factor expression (393) and induces innate immune signaling through the epithelial cells. Bacterial appendages such as flagella bind to mucin components and provide a physical link to enhance mucociliary clearance. Selection of P. aeruginosa mutants that lack flagella is observed in chronic airway infection as a response not only to their evasion of clearance, but also to their lack of immunostimulation (22, 246). Organisms that are enmeshed in mucin are subject to destruction by the multiple antimicrobial peptides constitutively expressed by airway mucosal cells, and further expressed as a component of immune activation. The “mix” of antimicrobial peptides includes cathelicidins and lipocalins that compete with microorganisms for iron, providing a selective milieu that enables relatively resistant organisms to flourish while eliminating proliferation of the more susceptible species. Factors such as pH and NaCl concentrations affect the potency of antimicrobial peptides and contribute to the selection of the relatively resistant species (1). In cystic fibrosis (CF), a disease affecting the pH of airway secretions as a consequence of chloride channel dysfunction, pH effects may contribute to the bacterial flora that persist within the airway (319). Induction of lipocalins suppresses proliferation of organisms unable to compete for iron and enhances the proliferation of opportunists such as P. aeruginosa and K. pneumoniae that express multiple siderophores and are able to effectively compete for iron. When these mechanical and antimicrobial defenses fail, the host must then recruit phagocytes to eliminate the infection. Thus it is only when an especially flexible pathogen, such as those we will review, establishes residence in the airway that such opportunistic pneumonias occur, as the vast majority of organisms would be readily cleared by typical innate immune defenses.
C. Epithelial Signaling
The function and distribution of the numerous germline encoded pattern recognition receptors, TLRs, NLRs, and related proteins in both nontraditional and professional immune cells have been well recognized. Their profound importance in host defense is perhaps best exemplified by the susceptibility of individuals with mutations in components of innate immune signaling, such as IRAK4, to specific infections (321). CF provides an example of the importance of epithelial cell function in normal airway clearance mechanisms, as well as the contribution of excess proinflammatory signaling in causing lung damage. The clinical data accrued from CF patients has enabled investigators to follow the evolution and selection of bacterial mutants in response to innate immune clearance over time. This process is likely similar to what occurs in an intensive care unit (ICU) setting, in which pathogens are acquired from the environment and through selective proliferation specific clones infect the susceptible host to cause pneumonia.
Epithelial signaling is initiated by TLRs distributed on the apical surface of airway cells as well as through intracellular receptors that can respond to internalized bacterial gene products including cell wall fragments, DNA, and lipopolysaccharide (LPS) (3, 182, 196, 283, 305, 306, 308). Surface signaling is generally mediated by TLR/MyD88/NF-κB and mitogen-activated protein kinase (MAPK) activation resulting in the production of chemokines such as interleukin (IL)-8 and proinflammatory cytokines. TLR initiated responses can be propagated through Ca2+ fluxes, transmitted via connexins that transiently activate adjacent epithelial cells and amplify the local response to infection (460). This provides a rapid induction of proinflammatory cytokine and chemokine expression to recruit phagocytes. Signaling is promptly regulated through phosphorylation of the connexins. The airway epithelium produces granulocyte-macrophage colony stimulating factor (GM-CSF), a major macrophage cytokine that stimulates the polarization of airway macrophages to the M1 or more phagocytic and inflammatory phenotype, as opposed to the more anti-inflammatory M2 phenotype usually associated with the resting resident population (179, 423).
TLRs expressed intracellularly can also be activated by airway pathogens (136). While the major airway pathogens S. aureus, P. aeruginosa, and K. pneumoniae are categorized as “extracellular pathogens,” they are nonetheless able to initiate signaling mediated through intracellular receptors, TLRs expressed in the endosome, cytosolic receptors, and other intracellular targets that are endocytosed by mucosal as well as immune cells. In the airways organisms proliferate, shedding components such as LPS, surface lipoproteins, flagella, and pili/fimbriae as they grow. Bacteria are lysed through the activities of host defense mechanisms, releasing bacterial DNA fragments and cell wall components, all highly immunostimulatory and recognized by specific intracellular receptors, such as endosomal expressed TLR4 along with TRIF, TLR9, and NOD-like receptors. TLR4/TRIF signaling gives rise to the type I and III interferons, best known for their participation in antiviral defenses but also critically important in host defense against acute bacterial infection.
Bacteria that have breached the mechanical defenses of the airway then gain access to the epithelium. This may be considered the initial step in “invasion” as it represents the failure of the normal antimicrobial defenses of the airway, leading the host to induce the recruitment of phagocytes, a potentially damaging but necessary immune response. Mice lacking epithelial cell expression of MyD88, a critical adapter between multiple TLR-NF-κB signaling pathways, have profound defects in pathogen clearance (268), illustrating the critical role of the epithelium in initiating innate clearance.
1. Alveolar epithelial responses
Bacterial stimulation of the polarized cells of the upper airway has been extensively characterized and is clearly a critical component of mucosal defenses. Inhaled bacteria that gain access to the lower airway stimulate signaling by alveolar epithelial cells. Signaling by the type I alveolar epithelial cells, which function primarily in air exchange and comprise the majority of the cells in the lung, can be activated by bacterial components, but responses are limited to specific cytokines, CCL20 and CXCL5 (473). The participation of type II alveolar epithelial cells that produce surfactant and have more biosynthetic functions is more robust (259). Epithelial STAT-3-mediated signaling, which includes production of the IL-6 family of cytokines, is important in the overall antibacterial defenses in the airway (332). Models of bacterial infection in the airways of epithelial-specific RelA mutant mice convincingly demonstrate the importance of epithelial signaling for the efficient recruitment and activation of neutrophils into the infected lung (472). Similar studies using MyD88 mouse chimeras documented the importance of epithelial as opposed to immune cell proinflammatory responses to P. aeruginosa (268). The interaction between the alveolar epithelium and local immune cells is also an important component of the host response to airway pathogens. In a model of LPS or S. aureus airway infection, the alveolar macrophage was demonstrated to regulate epithelial proinflammatory cytokine production through the movement of Ca2+ fluxes through connexin-43 channels to suppress further alveolar epithelial signaling (460).
D. Immune Cell Signaling in Response to Airway Infection
The respiratory mucosa, like other mucosal surfaces, regulates the interface between the environment and constant exposure to “non-self” antigens that include innumerable benign particles as well as the very occasional potential pathogen. From numerous studies using 16S rRNA sequencing to sample the respiratory microbiome, it has become clear that there is a native, predominantly anaerobic flora in the airways and lung (177). This flora presumably serves to induce a state of microbial tolerance (143) as observed in the gut (110, 353, 388, 468). Changes in the gut microbiota can also have major effects not only on intestinal inflammation but on macrophage polarization in the lung as well (208). The major surveillance cells of the airway, the alveolar macrophages, express a predominantly “anti-inflammatory” repertoire of surface proteins, such as CD200R and CD206 in their resting state (179, 258). To protect the respiratory tract's major function in gas exchange, the resting state of the respiratory mucosa eliminates potential pathogens through clearance mechanisms that do not involve the recruitment of neutrophils and their damaging products.
Alveolar macrophages are the major pathogen surveillance cells in the airway. A substantial portion of the resident macrophage population is sessile, adherent to the epithelium and able to communicate with epithelial cells through connexins (460). Endocytosis of bacteria by immune cells, particularly macrophages, provides a mechanism for the bacterial PAMPs to activate both surface displayed as well as intracellular TLRs, NLRs, and NLR-inflammasome signaling.
E. Activation of the Inflammasome
The most potent inflammatory response initiated by bacterial invasion involves the activation of the inflammasome and release of IL-1β and IL-18, powerful proinflammatory cytokines (389). As the components of the inflammasome are cytosolic, this pathway involves the escape of bacteria or their products from the endosomal compartment, and thus requires the ability to escape endosomal clearance mechanisms, properties of both S. aureus and P. aeruginosa (437). The inflammasome consists of a scaffold that combines and coordinates the activation of caspase-1, which then targets pro-IL-1β and pro-IL-18, facilitating their cleavage to biologically active products. Induction of inflammasome activity is thought to require two signals, a TLR-induced expression of the pro-forms of the cytokines and a stimulus for NLR-specific activation (440). Stimuli for well-characterized NLRs, such as NLRP3 and NLRC4, include pore-forming toxins resulting in the cytosolic release of K+ or ATP. This second signal can also be provided by flagellin, the needle protein of the type III secretion system (474), or cytosolic DNA (174). In the setting of acute pneumonia, the exaggerated immunostimulation provided by the NLRC4 inflammasome has been shown to contribute to lung pathology in murine models (77, 218). Less apparent is whether inflammasome activation is uniformly associated with pathology, as Nlrp3−/− mice have only a minimal phenotype in S. aureus pneumonia (201). As a consequence, bacterial pathogens have evolved multiple mechanisms to avoid activation of the inflammasome (437).
F. Dendritic Cells and Types I and III Interferons
Bacteria and their components in the airway are sampled by dendritic cells (DCs), which are thought to intercalate between the tight junctions of the epithelium. Bacterial antigens are processed and presented to T cells. DCs are a major source of the types I and III interferons (IFNs). The type I IFNs (α, β, ε, and ω) are signaled through the ubiquitous IFNAR, which stimulates expression of over 300 genes, the interferome (364). While best characterized as a major component of host defense against viral infection, type I IFN signaling is a major component of the host response to LPS, as signaled through endosomal TLR4/TRIF that activate Jak/STAT-mediated gene transcription. Importantly, the type I IFN response serves to counterbalance the proinflammatory signaling initiated through the activation of inflammasomes. Studies with Francisella tularensis, Listeria monocytogenes, and Streptococcus pneumoniae have identified that type I IFN signaling is required for inflammasome activation (115, 167). In the case of S. pneumoniae, it has been shown that type I IFN signaling upregulates the AIM2 inflammasome, known for sensing DNA (115). A more recent study contradicts these observations whereby type I IFN signaling via the STAT1 transcription factor repressed the NLRP1 and NLRP3 inflammasomes. Type I IFN signaling further suppressed the inflammasome response by reducing expression of pro-IL-1α and pro-IL-1β via production of IL-10 and STAT3 (153). Given the conflicting reports, the connection between type I IFN signaling and inflammasome activation is present, but the outcome is dependent on the biological system studied. Diminished type I IFN signaling in cells with a CFTR mutation can contribute to their exaggerated expression of IL-1β and inflammatory airway damage (305). Type III IFN or IFN-λ signaling is also activated in DCs, but the type III IFNs signal through the composite IL-28R-IL-10R that is predominantly expressed on mucosal and hepatic cells (109). Thus IFNs generated by immune cells target mucosal surfaces to coordinate and fine tune the overall mucosal response to airway infection.
G. Lymphocytes
Several different types of lymphocytes actively participate in innate immune defenses, primarily by producing a number of cytokines with major antibacterial activity. Gamma, delta, and Th17 T cells produce IL-17A and IL-17F, important in the recruitment and activation of neutrophils in response to infection by extracellular bacteria, such as these major airway pathogens (69). NK cells are known to also have important innate immune defense functions, participating in bacterial pathogen clearance (46). Among the larger family of innate lymphoid cells (ILCs), the ILC3 group of lymphocytes are major producers of IL-22, a cytokine of the IL-10 family that contributes to expression of several antimicrobial peptides, including RegIIIγ, S100A8, and S100A9, and enhances the barrier function of the airway epithelium (323). Production of IL-22 by ILCs could promote the expression of antimicrobial peptides in response to S. aureus and to enhance barrier function (216, 323, 349), although this has yet to be tested. In addition, the expression of superantigens by many pathogens, especially S. aureus, provides a potent stimulus for cytokine expression by T cells present in the airways (311).
II. INNATE IMMUNE RESPONSES TO STAPHYLOCOCCUS AUREUS IN THE AIRWAY
A. Epidemiology
S. aureus, a component of the commensal flora found in upwards of 30% of individuals (457), has the potential to become an airway pathogen in the appropriate setting. It has the extraordinary ability of thwarting immune clearance mechanisms, enabling them to spread through populations worldwide (412). Genetic analyses demonstrate the dominance of a single clone of S. aureus spread throughout Europe, demonstrating the co-evolution and horizontal gene acquisitions of both antimicrobial resistance and virulence genes within the epidemic strains of S. aureus (30). Much of the virulence of S. aureus is due to gene products that interact with components of the immune system, as illustrated in the very different susceptibilities of inbred strains of mice to infection (448). Staphylococcal gene products interfere with host clearance mechanisms by thwarting phagocytosis and opsonization and through expression of multiple, somewhat functionally redundant leukotoxins. These leukotoxins target human macrophages and neutrophils through the recognition and exploitation of numerous human receptors (125). The ability of S. aureus to initiate excessive inflammatory responses is a major factor in the severity of the pulmonary damage it generates. In murine models of infection, mice lacking components of innate immune clearance, such as the receptors for the types I or III IFN cascade, NOD2 which is important for inflammasome activation, or TNFR1 all have improved outcomes, such as reduced mortality, decreased leukocyte infiltration, proinflammatory cytokine production, and alveolar leakage (78, 144, 307). Over the past decade, an epidemic methicillin resistant S. aureus (MRSA) clone, USA300, has been associated with the majority of severe infections in the United States (93). The distinction between hospital- versus community-acquired strains of MRSA has become blurred. A rapid expansion of community-acquired, virulent, and widely disseminated strains of S. aureus is evident and suggests rapid evolution of single clones with enhanced virulence and transmissibility (64). Although the expression of the mecA gene that confers β-lactam resistance to MRSA strains does not in itself enhance virulence, the acquisition of the SCCII element that includes mecA, genes that enhance skin colonization as well as toxin production, as part of the same evolutionary process (322) indicates co-evolution of antimicrobial resistance and pathogenicity.
B. Colonization
As a ubiquitous colonizer of the human nasopharynx, S. aureus expresses surface proteins that mediate attachment to mucosal tissues and resist the microbiocidal activity of skin polyamines (322). These adhesins, the microbial surface component recognizing adhesive matrix molecules (MSCRAMMs), originally defined by their binding of substrates such as fibrinogen and collagen, share structural similarities and have a common mechanism of ligand binding (125, 126).
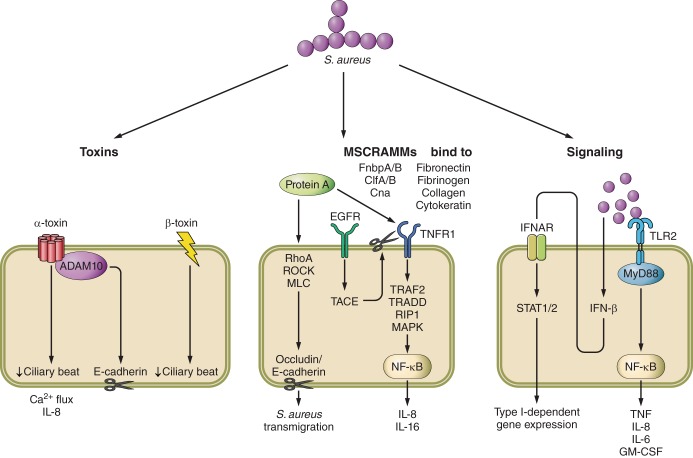
Clumping factor A (ClfA) and especially B (ClfB) play prominent roles in respiratory infection by binding to the cytokeratins on nasal epithelial cells (Figure 1) (449). S. aureus strains lacking ClfB have reduced adherence and in a human colonization model were more rapidly cleared than wild-type strains of S. aureus (293, 372, 459). In a murine colonization model, ClfB antibodies or immunization with ClfB reduced the ability of S. aureus to persist (372). Human clinical pneumonia isolates express clumping factor, but a direct contribution to pathogenesis in the lung is undetermined (367). ClfA has been shown to be antiphagocytic towards neutrophils and macrophages (170, 301) and, while important in some animal models, has not shown a role in pulmonary infection (189, 279, 443, 456). Additional MSCRAMMs include the collagen binding protein (cna gene) that binds collagen substrates and tissues (169, 346), and fibronectin binding proteins (FnbpA, FnbpB) (273, 372). These surface proteins may facilitate stromal cell uptake through fibronectin interactions with human integrins. Strains lacking fibronectin binding proteins actually lead to more damage in the lung using a pneumonia model in rats (262).
FIGURE 1.
S. aureus virulence factors that influence epithelial cell function and signaling.
C. Surface Proteins
Surface protein A (SpA) is an abundant surface protein that is present on the majority of S. aureus isolates with many interactions important in immune evasion and pathogenesis. SpA has several domains including a signal peptide, five repeated domains that bind IgG, and a variable region (Xr; containing 24 bp repeats) at the carboxy end (271, 436). The IgG binding domains bind human IgG (145), widely considered an important factor in its resistance to phagocytosis, while the Xr region is the basis for many epidemiological studies (213, 387). This IgG binding region also mediates attachment to tumor necrosis factor receptor 1 (TNFR1) and epidermal growth factor receptor (EGFR) (145, 147). The number of repeats in the Xr region has been associated with increased induction of an inflammatory response (134) and is a site for mutation in the context of human pulmonary infection in CF patients (191). SpA is anchored to the cell surface through the action of the sortase enzyme and its LPXTG motif (261, 375), released from cells via the action of the LytN murein hydrolase and LytM pentaglycyl-endopeptidase (28).
The IgG binding domain of SpA has many important immune interactions, especially induction of the expansion of B cells. Interaction with the Fab protein of V(H)3-type B cell receptors blocks the generation of adaptive immune responses and results in nonspecific polyclonal B cell expansion (161, 206). Mutations in SpA that make it unable to interact with B cells have shown promise as vaccine candidates in murine models of infection (205). SpA further contributes to immune evasion by binding Fcγ portion of immunoglobulin preventing opsonization (314), with the immunoglobulin binding domain interfering with efficient uptake of these organisms. SpA mutants that are unable to bind to IgG elicit an adaptive immune response that is protective against recurrent infection (114). Immunization with mutated protein A protects mice from infection by raising antibodies that promote opsonophagocytic clearance (205, 424). S. aureus strains lacking SpA have significantly reduced virulence (50, 144), with decreased bacterial burden in the lung and spleen, less inflammatory signaling and neutrophil recruitment (144), and decreased mortality (50). SpA also facilitates invasion across the epithelial tight junctions (Figure 1), which is accomplished through activation of a RhoA/ROCK/MLC cascade that along with calpain activity leads to contraction of the cytoskeleton and cleavage of tight junction proteins, occludin, and E-cadherin (404). This facilitates invasion of these organisms that themselves are not motile.
Expression of SpA is increased upon initial in vivo infection (228) and acts as an agonist for the TNF cascade through its recognition of TNFR1 (145). Interaction with SpA leads to increased levels of TNFR1 at the cell surface and subsequent shedding of soluble TNFR1 into the milieu via the action of TNF-α converting enzyme (TACE) also known as ADAM17 (144). SpA-TNFR1 signaling occurs through TRAF2/TRADD/RIP1/MAPK and NF-κB to produce IL-8 in airway epithelial cells (144, 145, 147). SpA also induces shedding of TNFR1 in macrophages (147). The cleavage of TNFR1 is mediated by interaction with EGFR. SpA interaction with EGFR leads to activation of TACE, which is activated via phosphorylation to then cleave TNFR1 (147). Mice lacking TNFR1 have reduced severity of pneumonia analogous to wild-type mice infected with the spa null strain (144). The magnitude of TNFR1 signaling in the host response to S. aureus in the lung is perhaps best illustrated by the observation that MyD88 null mice, which are substantially impaired in TLR-NF-κB activated gene expression, are nonetheless able to readily clear the infection (394).
D. Secreted Toxins and Their Targets
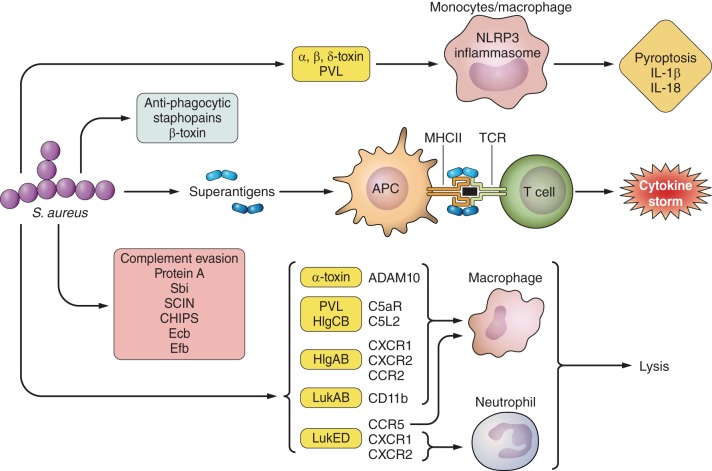
Once adherent to the airway mucosa, S. aureus sense this environment (106) and express a host of gene products (“virulence factors”) that support their proliferation. The production of numerous toxins, some with specificity for human immune cells, indicates their longstanding adaptation as human pathogens (Figure 2).
FIGURE 2.
Effect of S. aureus factors on the immune system. Shown are toxins that target specific immune cells, leading to lysis and inflammation as well as factors that aid in immune evasion.
1. Hemolysin-α-toxin-Hla
A major secreted virulence factor of S. aureus is the α-toxin or α-hemolysin (encoded by hla), a pore-forming toxin that assembles into heptamers at the cell membrane leading to loss of small ions and cytotoxicity (402). Cell death is mediated by activation of the inflammasome (82, 285), as discussed below, and cell damage-associated loss of epithelial barrier function. The production of α-toxin is mediated by the global agr regulatory system (281), with higher α-toxin expression in the epidemic USA300 isolates, and correlative virulence among S. aureus strains (51). Hla production is also increased upon interaction with epithelial cells (53, 84, 234, 274), leading to altered ciliary beat frequency, calcium fluxes, and proinflammatory signaling (352, 478). S. aureus mutants lacking α-toxin are significantly attenuated for virulence in murine pneumonia models, associated with less mortality, reduced pulmonary inflammation, and increased clearance from the lung (24, 49, 50). The α-toxin binds the metalloproteinase ADAM10 which leads to epithelial barrier disruption and E-cadherin cleavage (180, 463). This was demonstrated in an epithelial-specific ADAM10 knockout mouse (180) that was resistant to lethal pneumonia, had improved alveolar structure after infection, and had reduced leukocyte influx. Direct application of purified α-toxin to perfused lungs also decreased epithelial barrier function and caused vascular leakage (27, 53, 263, 320, 382).
Due to its important role in pathogenesis, α-toxin has been an active focus of vaccine development. Passive transfer of α-toxin antibodies to naive mice (4, 51) and rabbits (124, 409) confers protection in models of pneumonia. Chemicals with structural similarity to α-toxin (β-cyclodextrin compounds) reduce epithelial cell injury and pneumonia (336, 337). In vaccine trials, mice immunized with α-toxin mutants lacking pore formation have improved clearance, decreased vascular permeability, and reduced mortality when challenged with wild-type strains (51, 176).
2. Phenol soluble modulins
The phenol soluble modulins (PSMs) are small peptides referred to as modulins due to their ability to induce cytokine release. They are cytolytic, forming pores in host membranes. There are several PSMs so far identified in MRSA USA300 (453). USA300 expresses greater quantities of PSMs than other strains, and these include four short (alpha; ∼20 amino acids) and two longer (beta; ∼40 amino acids) types (234). MRSA produce mec-PSM encoded by the SCC-mec locus, likely to also contribute to pathogenesis (331). The PSMs (psmα, psmβ) are regulated by the agr system independent of the RNAIII regulatory RNA (330) and influence Hla expression (32). Their secretion is via the Pmt transport system that is essential for bacterial growth and facilitates immunity to PSM-producing strains (68). Sensing of PSMs by the host is via the ubiquitously expressed formyl peptide 2 receptor, which leads to activation and lysis of neutrophils and DCs, and contributes to virulence in skin models of infection (215, 331). In models of pneumonia, mutants lacking the PSMs induce less pulmonary pathology, injury, and bacterial burden compared with wild-type strains (32).
3. S. aureus toxins that are specific for human immune cells
There are numerous staphylococcal toxins that are specific for human leukocytes. Thus the data generated over the past two decades using animal models of infection may not accurately reflect what occurs in human pneumonias. The best studied of these toxins is Panton-Valentine leukocidin (PVL).
4. PVL
PVL is a toxin that forms octomeric pores at the cell membrane of neutrophils, monocytes, and macrophages, encoded by the cotranscribed genes lukF-PV and lukS-PV integrated into the genome by a bacteriophage (327). PVL has significant host specificity, limited to humans and rabbits (103, 119, 135, 142, 233, 239, 269), targeting neutrophils and macrophages via the C5a complement receptors (20, 103, 239, 407). PVL can directly alter Ca2+ stores (422). Due to its host specificity, there has been some dispute regarding the in vivo role of PVL in pneumonia, based on studies performed in animal models (48, 49, 103, 220, 244, 441, 444). Direct instillation of the toxin induces acute lung injury in rabbits leading to necrotizing pneumonia and is associated with robust expression of IL-6, IL-8, TNF, and IL-10. Much of this inflammation and damage is a result of neutrophil lysis attributed to apoptosis via caspases 3 and 9 (138). Neutropenic rabbits have significantly reduced inflammation, NF-κB, and cytokine production (244). Other markers of lung injury in the rabbit model of pneumonia indicate that strains lacking PVL cause less mortality.
The γ-hemolysin HlgCB targets the same human C5a receptors (C5aR and C5L2) as PVL to cause toxicity in human neutrophils and macrophages (318, 408). HlgCB requires intracellular acidic calcium stores to induce a rise in free Ca2+ (422). The other γ-hemolysin, HlgAB, utilizes CXCR1, CXCR2, and CCR2 as target receptor sites (408).
LukAB/GH targets the human CD11b subunit of the integrin Mac-1 resulting in cytotoxicity of neutrophils, macrophages, and monocytes (108, 249, 442). LukAB causes neutrophils to release extracellular DNA nets that aid in capture of S. aureus, but does not lead to bacterial killing (250). As a pore-forming toxin, it is likely that LukAB contributes to S. aureus virulence, possibly through activation of the inflammasome and through targeting critical populations of immune cells.
LukED is an additional bicomponent toxin, not limited to human targets. It interacts with CCR5 on macrophages, T cells, and dendritic cells, while it targets neutrophils and monocytes via the chemokine receptors CXCR1 and CXCR2 (7, 345). LukED is able to lyse both human and murine neutrophils, macrophages, T cells, and monocytes (6, 7, 345) and is likely to contribute to the pathogenicity of S. aureus in many of the models studied.
E. Contribution of Host Signaling to S. aureus-Induced Pathology
The ability of S. aureus to activate excessive proinflammatory signaling is a major mechanism of pathogenesis, via the stimulation of redundant mechanisms of cytokine production and phagocyte recruitment. This leads to airway damage that interferes with the efficient clearing of staphylococcal infection (Figure 2).
1. Inflammasome signaling
Many of the toxins described above contribute to the activation of the NLRP3 inflammasome; a cytosolic structure that responds to two separate stimuli, bacterial PAMPs via TLR and pore formation. This leads to caspase-1-mediated release of the potent proinflammatory cytokines IL-1β and IL-18 and cell death via pyroptosis (31). Pyroptosis may or may not result in death of S. aureus by activating the inflammasome but, nonetheless, is a major stimulus for neutrophil recruitment, a critical component for staphylococcal clearance (229, 270).
Detection of S. aureus peptidoglycan and lipoteichoic acid (82, 285) is often the initial stimulus, induced by lysozyme-based degradation releasing cell wall fragments (384) with toxin-induced pore formation providing the second stimulus. Initially the α-toxin was demonstrated to function as the essential pore, but subsequent studies have demonstrated that β- and γ-toxin as well as PVL all can activate the NLRP3 inflammasome (82, 173, 285, 318, 384).
The role of the inflammasome in S. aureus infection has been best studied in the skin and the lung. In the skin IL-1β production is required for neutrophil recruitment to clear the infection (270). Whether the activation of NLRP3 signaling contributes to lung damage through excessive inflammation, or whether this response is critical to control S. aureus infection remains to be established. IL-1β drives the production of IL-17 by γδ T cells to help clear infection (247). In airway epithelial cells, IL-1β induces IL-8 and MCP-1, both major chemokines (318). The IL-1β-induced responses may be excessive as mice lacking NLRP3 exhibit less severe pneumonia in response to S. aureus pulmonary infection and reduced mortality compared with wild-type mice (201). In this model lung injury was not strictly dependent on IL-1β as IL-1R null mice were observed to have higher bacterial burdens (349). However, blocking of IL-1 signaling with the receptor antagonist Anakinra reduces S. aureus-induced pulmonary pathology (77). The inflammasome-mediated immune responses are clearly an important element of innate immune signaling, stimulated by many staphylococcal components, and may be a useful target for immunomodulation. It appears likely that the regulation of this response is critical in whether this is beneficial in the lung, or if the resultant inflammation contributes to loss of pulmonary function and mortality.
2. Type I IFN signaling
S. aureus airway infection activates type I IFNs in both in vivo and in vitro models (255) and results in induction of transcription factors, STAT1, STAT2, and STAT3 (255), through several different IFN regulator factor (IRF) transcriptional activators (217, 310). The type I IFN family consists of 13 IFN-α subtypes, IFN-β, IFN-ε, and IRF-ω (90). Interaction of a type I IFN, such as IFN-β, with its heterodimeric receptor (interferon alpha/beta receptor, IFNAR) results in dimerization and phosphorylation of STAT1/2 via Jak1 and Tyk2 leading to the downstream transcription of hundreds of genes including Lif, Mx-1, and Ifnb (98, 158). Many bacterial pathogens or their PAMPs, including S. aureus, readily gain access to both endosomal and cytosolic receptors to induce a type I IFN response via recognition of PAMPs and messengers such as DNA, RNA, peptidoglycan, LPS, and cyclic diadenosine monophosphate (c-diAMP) (148, 184, 467). TLR3, 4, 7, 8, 9, NOD, RNA polymerase III, and stimulator of IFN genes (STING) are among the many sensors involved in activating the type I IFN response (184, 417). Type I IFNs exert their influence on a variety of cell types influencing cell function and antimicrobial production (148, 429). The type I IFN response to MRSA triggers lung damage through excessive inflammation, as mice lacking the IFNAR receptor are protected from severe pulmonary infection in contrast to the high mortality exhibited by wild-type controls (255, 307, 309) or by exogenous stimulation of this pathway (426). Lung damage activated by different strains of S. aureus has been attributed to their overall ability to induce IFN-β (307) and differing mechanisms of activation through TLR9-IRF1 (309) or the NOD2-RIP2-IRF5 pathway.
3. Type III IFN (IFN-λ) signaling
S. aureus also activates the type III IFN pathway to contribute to the excessive inflammation characteristic of staphylococcal pneumonia. Type III IFN signaling shares many common effectors as the better studied type I IFN cascade (231, 299), but differs importantly in the distribution of its receptors, which are expressed predominantly on mucosal and hepatic cells (401). The IFN-λ members [IL-28A/B and IL-29 (only in humans)] signal through the IL-28 receptor (IL-28R) that is primarily located on epithelial cells in contrast to IFNAR that is ubiquitous (401). What differs in the transcriptome of type I and III IFN is the intensity and duration of the response, with type I activated early and strongly and type III induced over time at lower levels (38, 251). IFN-λ is especially important in the immune responses to mucosal pathogens such as S. aureus (36, 78). Mice lacking IL-28R show resistance to S. aureus pneumonia with improved bacterial clearance, decreased cytokine production (KC, GM-CSF, IL-1β), and decreased pulmonary pathology (78). Downstream of the IL-28R stimulation, miR-21 suppression of PDCD4 is reduced via STAT3, resulting in the induction of cytokine production. Outcomes from S. aureus pneumonia were improved in mice lacking PDCD4 (78), consistent with the observation that redundancies in proinflammatory signaling contribute to lung damage elicited by S. aureus, and especially the USA300 MRSA strains.
F. T Cell Activation by S. aureus
One of the best-studied mechanisms of pathological inflammation induced by S. aureus is that associated with the production of superantigens (232, 253, 272). The staphylococcal superantigen TSST-1 was the first to be analyzed in detail. TSST-1 causes systemic cytokine storm through activating T cells via crosslinking of the T cell receptor (TCR) [through recognition of Vβ chain types unique to each superantigen (118)] with MHC class II molecules on antigen presenting cells (232, 253, 272). This causes widespread T cell proliferation, cytokine production, and apoptosis (338). Several additional superantigens have been well characterized such as SEA and SEB (16, 107, 130, 257, 291) and have been associated with pathological inflammatory responses in the lung. The USA300 strain of S. aureus encodes three characterized superantigens: SelX, SEK, and SEQ (104, 465). Thus several S. aureus gene products are capable of activating CD4 T cells in the setting of pulmonary infection (311). Mice lacking Rag2 or Cd4 had improved clearance of USA300 MRSA and preserved lung architecture. The Cd28−/− mice lacking the critical T cell costimulatory receptor were protected from pathology due to toxic shock and similarly had increased staphylococcal clearance with decreased amounts of proinflammatory cytokine expression (10, 11, 339, 358, 420). Further evidence for a pathological role for T cell activation in the early innate immune response to S. aureus airway infection was provided by studies blocking the effects of the T cell cytokine and chemoattractant IL-16 (466), which was induced not by a superantigen, but by SpA (5).
G. Natural Killer Cells
Natural killer (NK) cells (14, 83) are important in innate responses to S. aureus, and their activation is determined by the environment in the lung (451). In response to staphylococcal infection, NK cells are rapidly recruited to the airway, and depletion of NK cells increases the susceptibility of mice to S. aureus infection (397). A similar result is observed in IL-15 knockout mice, which have naturally lower levels of NK cells. Production of TNF is important to the function of NK cells as mice expressing NK cells deficient in TNF production are highly susceptible to infection (398). In addition, NK cells enhance the ability of alveolar macrophages to phagocytose S. aureus through an unknown mechanism (397, 481).
H. Neutrophils
Neutrophils have long been recognized as critical for host defense against S. aureus infection, as documented by the genetic data linking humans with defective neutrophil function and recurrent severe staphylococcal infection (229). Neutrophils are readily recruited to sites of staphylococcal infection by the chemokines IL-8, MIP-2, and MCP-1 and are activated by the S. aureus surface components, peptidoglycan, lipoteichoic acid, and polysaccharide as well as several of the toxins made by S. aureus (146, 214, 374, 453). Once activated, neutrophils phagocytose and destroy bacteria through reactive oxygen species, superoxide production, and phagolysosome degradation. Generation of damaging oxygen radicals can be detrimental to the host as well as the staphylococci, as can be the release of cytotoxic granule contents from toxin-induced lysis of neutrophils (103). Neutrophils also produce extracellular traps containing chromatin, histones, and granule proteins to catch and destroy bacteria, and delayed removal of these neutrophil extracellular traps (NETs) can lead to proinflammatory cytokine production and tissue injury (44, 242, 295). Depletion of neutrophils increases the susceptibility of mice to S. aureus infection, and neutropenic mice show increased bacterial loads in the lung and decreased survival in response to S. aureus (348, 349). Having ingested S. aureus, neutrophils may undergo programmed cell death such as apoptosis and the RIP-1 kinase-dependent process necroptosis (151, 211). S. aureus also influences efferocytosis, the clearance of neutrophils by macrophages. Furthermore, neutrophils containing S. aureus increase expression of CD47 that prevents macrophage phagocytosis (151).
I. Macrophages
Macrophages are critically important in regulating immune responses through their selective display of surface proteins that promote or suppress inflammatory signaling by many other types of lymphocytes. They are targets for many of the staphylococcal toxins outlined above. Neutrophils, which may be highly susceptible to several staphylococcal toxins, are rapidly replenished from the bone marrow. In contrast, pulmonary macrophages are generated in the local tissues and may not be replaced as readily or by cells with appropriate regulatory phenotypes (155). Transient depletion of alveolar macrophages using clodronate results in excessive mortality in murine models of pneumonia, whereas loss of DCs did not increase morbidity (256). While macrophages participate in phagocytosis, they are not as efficient as neutrophils in pathogen uptake. The resting anti-inflammatory state of airway macrophages may also be important in controlling lung damage associated with inflammation. The presence of M2 anti-inflammatory macrophages during influenza insult curtails inflammatory damage by production of the anti-inflammatory cytokines IL-10 and TGF-β (450). Various forms of cell death contribute to the inflammatory cytokines released by macrophages. In humans, caspase-4 mediates an apoptotic death that leads to the release of IL-1α, a potent inflammatory cytokine (61). IL-1α is also produced through another form of cell death, necroptosis. Necroptosis is a highly inflammatory form of cell death mediated by receptor-interacting serine-threonine kinase 1 (RIP1) and RIP3 through mixed lineage kinase domain-like (MLKL) that forms a pore leading to loss of membrane integrity. S. aureus induces necroptosis via several of its pore-forming toxins, α-toxin, LukAB, and PSMs (210). Mice deficient in the ability to undergo necroptosis have decreased proinflammatory cytokine production and improved lung architecture.
J. Immune Evasion
A major feature of S. aureus pathogenesis is clearly the initiation of robust proinflammatory immune signaling and the recruitment and activation of the phagocytes that ensues. Not surprisingly, these organisms have potent mechanisms to avoid phagocytosis, by either impeding uptake or resisting intracellular killing. While S. aureus is, strictly speaking, an extracellular pathogen, an extensive literature details its ability to persist within host cells, both immune and stromal, and in some cases to proliferate (26, 84, 152, 193, 266, 357, 431). S. aureus also uses quorum sensing (9, 117, 212) to coordinate gene expression, promoting the formation of biofilms that protects many members of the bacterial community from exposure to phagocytes, antibody, and complement.
To prevent phagocytosis, S. aureus has several mechanisms to block complement and antibody mediated opsonization (the effects of proteins A are described above). However, despite major efforts to identify the correlates of protective immunity against S. aureus infection, vaccines developed to generate specific anti-staphylococcal proteins have been unsuccessful (127, 207, 378). Thus the ability of these organisms to thwart opsonization and phagocytosis by both neutrophils and macrophages has been highly successful.
1. Complement
S. aureus expresses several proteins that protect it against the complement system. Two proteins, SpA and Sbi (S. aureus IgG binding protein), can both bind IgG in addition to components of the complement cascade. Sbi promotes survival in the blood, while SpA has proven important in models of pneumonia (50, 144, 400). Four other complement evasion proteins are staphylococcal complement inhibitor (SCIN), chemotaxis inhibitory protein of staphylococci (CHIPS), extracellular complement binding protein (Ecb), and its homolog extracellular fibrinogen binding protein (Efb). The presence of SCIN aids in protection against phagocytosis and neutrophil killing via preventing the formation of C3 convertases (351), and CHIPS inhibits neutrophil and monocyte chemotaxis via binding of C5a receptors (89, 326). Ecb and Efb have both been studied in vivo. In a model of pneumonia, mutants in either gene are cleared more effectively than wild-type strains, possibly due to their ability to block neutrophil recruitment (188).
K. Staphylococcal Superinfection Following Influenza
Viral infections are a major source of increased susceptibility to S. aureus pneumonia, and their ability to perturb the innate immune response is a factor in the ability to clear S. aureus infection. A prime example is influenza. The ability of influenza to increase susceptibility to S. aureus pneumonia has been documented during the major influenza pandemics. The influenza pandemic of 1918 had high mortality and affected many young, otherwise healthy individuals. Many of these deaths were subsequently attributed to S. aureus pneumonia (71, 280). This observation can also be replicated in mice. Mice infected with influenza then S. aureus are more susceptible to infection, leading to more destructive lung damage and subsequent death (185, 216, 228, 398). Synergism exists between S. aureus, influenza, and host signaling pathways, as infection with either the staphylococci or influenza virus alone was readily cleared.
Type I IFN signaling is likely to play a role in the susceptibility to S. aureus infection. As type I IFN signaling increases susceptibility to S. aureus and is rapidly induced upon influenza infection, this combination of factors is likely to increase pathology and inflammation (228, 255). Another pathway by which type I IFN contributes to susceptibility is its effect on the Th17 response. Type I IFNs have been associated with reductions in the Th17 response, which is not observed in Ifnar−/− mice, and leading to diminished clearance of S. aureus in the airway (216).
Th17 signaling is critical in anti-staphylococcal defenses and has been well characterized and highlighted in the setting of influenza. The γδ T cell is a main producer of IL-17 (216), and these cells rapidly accumulate in the lung after S. aureus infection. Mice lacking γδ T cells exhibit reduced cytokines, immune cell recruitment, and ability to clear infection (70). Influenza infection suppresses Th17 cytokines (IL-17, -22, and -23) (216) attributed at least in part due to increased type I interferons. Removal of Th17 cells leads to reduced clearance of S. aureus. Improved clearance of S. aureus, in the context of influenza co-infection, is improved if IL-23 is overexpressed (216). These studies provide examples of how induction of innate immune signaling by viral infections (namely influenza) can influence host signaling and the innate immune system, which can increase the susceptibility of individuals to secondary pneumonia.
L. Conclusions
As a human commensal, S. aureus illustrates the ability of bacterial pathogens to retain genetic elements that favor their proliferation in the setting of human infection. This adaptation includes genes that result in antimicrobial resistance as well as immune evasion, specifically targeting multiple components of innate immunity through several complementary mechanisms. This highly successful pathogen expresses surface proteins to colonize the airway as a commensal, failing to elicit a protective immune response. When aspirated into the lower airways, it elicits a robust inflammatory response, associated with substantial pulmonary damage, through activation of inflammasomes and multiple redundant proinflammatory cascades. However, through its expression of toxins and antiphagocytic surface proteins, it can evade host phagocytic clearance and exploit the benefits of inflammation; the disruption of normal mucociliary clearance, the generation of pulmonary edema, and increased availability of carbon and iron sources released from destruction of host tissues.
III. PSEUDOMONAS AERUGINOSA AN AIRWAY OPPORTUNIST
In contrast to S. aureus that is a common component of the commensal airway flora, P. aeruginosa is an environmental opportunist that normally inhabits moist environments such as streams and puddles. With its large genome and many accessory elements (300), P. aeruginosa has the genetic flexibility to rapidly adapt to and flourish in diverse environments. P. aeruginosa display intrinsic resistance to many classes of antimicrobial agents and the ability to acquire resistance in response to antimicrobial exposure. Thus, when P. aeruginosa contaminates the airways of a susceptible patient, it rapidly adapts to the host immune pressures imposed and is among the most common causes of ventilator-associated pneumonia (369). With minimal nutritional requirements, it flourishes in the airway and especially in hospitalized patients, outcompeting commensal organisms that cause airway contamination.
The adaptation of P. aeruginosa to the human lung as a component of the pulmonary disease in patients with CF has been exceptionally well characterized from a phenotypic and genotypic basis. There are clearly differences in the chronic colonization of this pathogen to human airways versus the acute adaptation to the lung, as seen in the setting of ventilator-associated pneumonia. While therapeutic trials to target specific P. aeruginosa virulence factors have been attempted, the genetic flexibility of this organism and diverse repertoire of toxins make it likely that mutants resistant to a single drug will arise (462).
A. Attachment and Colonization
As with the other ESKAPE pathogens, P. aeruginosa colonize the airway, are aspirated, and proliferate locally in the absence of an effective innate immune response. Attachment to components of mucin, as mediated by flagella, facilitates clearance (29, 99), whereas pilin-mediated attachment is associated with the generation of local proinflammatory signaling. Organisms that are not cleared shed PAMPs, interact with epithelial receptors, and initiate immune signaling. The adherence of P. aeruginosa to airway epithelial cells has been extensively characterized in vitro, particularly those addressing potential effects of CFTR dysfunction on the binding capabilities of the organism (363).
In the airway, apical receptors for pilin-mediated attachment such as TLR2-linked asialoGM1 (362, 406) are displayed and initiate TLR-mediated proinflammatory gene expression, via MyD88 and NF-κB. However, given the abundant mucin layer, few organisms directly interact with epithelial surfaces in the absence of damage (87, 88). Exposed asialylated glycolipids provide receptors for pili, which in turn initiate the tight binding necessary for the function of the type three secretion system and its injected toxins (419), which mediate much of the acute damage to the airway. Pili encoded by the gene pilA are modified posttranscriptionally by PilO-mediated addition of glucose (399). Pili attachment to the apical epithelial surface induces actin-mediated cytoskeletal changes resulting in the loss of host cell polarity (428). Relocalization of basal cell components to the apical surface also results in accumulation of NF-κB signaling components, increasing the immune response to colonizing P. aeruginosa.
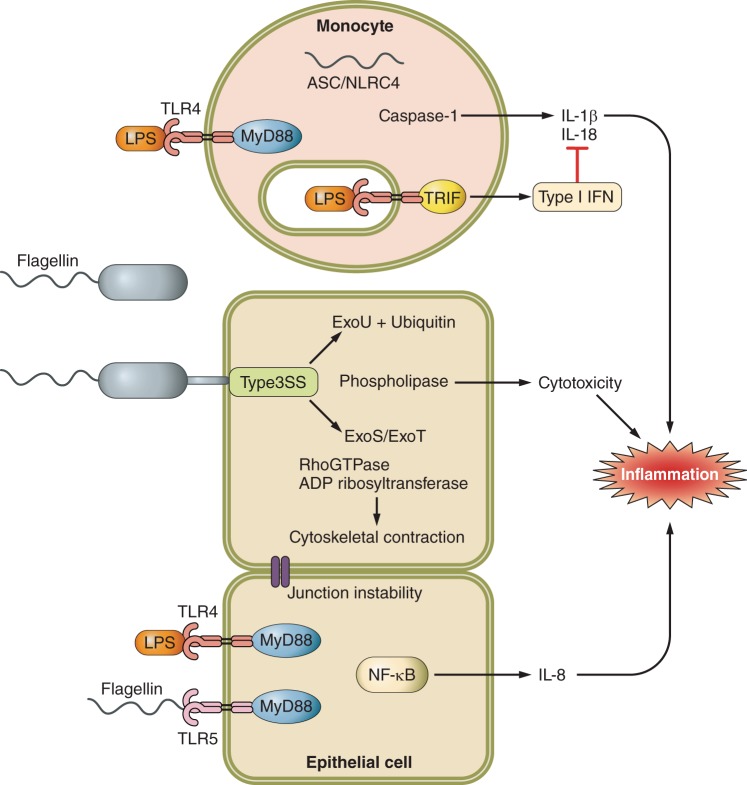
The ability to colonize the airways is greatly facilitated by P. aeruginosa motility, mediated by flagella (414). Flagella extend out through the bacterial cell wall utilizing components of the type III secretion system and are anchored by a motor complex at the internal face of the cell membrane. P. aeruginosa flagellin is a potent immunostimulant interacting with surface displayed TLR5 as well as the cytosolic NLRC4 inflammasome (Figure 3) (128, 267, 282, 287, 421, 477), generating cleavage of caspase-1, IL-1β, and IL-18. In vivo there is selection for nonmotile mutants, as these have an impaired ability to bind mucin and are resistant to macrophage engulfment (165, 267, 282, 361, 362). Flagellin and pilin synthesis are regulated by the alternative sigma factor σ54/RpoN, and many clinical isolates from long-term colonized patients are rpoN negative (245). The presence of flagella, a highly inflammatory virulence factor, is not required for long-term survival within the host, and a selective advantage exists for bacteria that lose flagellin expression.
FIGURE 3.
Influence of P. aeruginosa surface proteins and type III secreted toxins on monocytes and epithelial cell function.
1. Biofilm expression
P. aeruginosa, like S. aureus, readily adapts to the airway through the generation of bacterial communities or biofilms (86, 172, 350, 391). Biofilm formation is the result of coordinated gene expression of the microbial population through secretion of highly soluble quorum sensors that serve as biomarkers of advanced infection (415). In P. aeruginosa biofilms, homoserine lactones and quinolones act in concert with specific transcriptional activators to regulate gene expression (156). The Las system produces the homoserine lactone N-3-oxo-dodecanoyl that activates the transcriptional regulator LasR, influencing expression of elastases and proteases (313, 315), which are important virulence factors. A second lactone, N-butanoyl, activates an alternate transcriptional regulator RhlR, which represses expression of the type III secretion system (TTSS) and has a major role in the pathogenesis of respiratory infection. These signaling pathways regulate each other as LasR upregulates expression of RhlR and RhlR can control expression of LasR dependent factors in lasR mutant bacteria (91). The Pseudomonas quinolone signal (PQS) activates its receptor PqsR that increases production of PQS, phenazine, and pyocyanin (121), both of which participate in adaptation to the host, potentially due to a relationship between PQS production and expression of inflammatory LPS (156). Organisms within the biofilm produce the expoplysaccharides Psl and Pel that firmly attach bacteria to the epithelial surface (79, 80, 186, 243, 260). As bacteria congregate, recognition of deposited Psl upregulates production of cyclic diGMP (c-diGMP) which in turn increases their Psl production and further propagates biofilm formation (183).
c-diGMP participates in expression of many P. aeruginosa gene products that affect airway infection. Besides inducing biofilm formation, c-diGMP represses flagellar biosynthesis (21). Once the biofilm is established, c-diGMP is suppressed by quorum sensing pathways that turn off genes in the Pel locus (434). c-diGMP levels have been correlated with antimicrobial resistance (74, 157), indicating that for P. aeruginosa genetic mechanisms of adaptation to the human lung also contribute to antimicrobial resistance phenotypes.
Host factors enhance biofilm formation. Mucus from barrier cells within the epithelium induces mucA mutations, which are strongly associated with biofilm formation and production of the exopolysaccharide alginate (62, 73). Alginate contributes to the structural development of the bacterial community (85, 168, 292). Along with Pel and Psl, alginate protects bacteria within the biofilm from immune cell clearance and may restrict antimicrobial access (65, 219). During the transition to a biofilm growth mode, spontaneous selection of mucA mutants that promote alginate production allow the organisms to elude phagocytosis (37, 254, 390). Host proteins are also found within the matrix of the biofilm. Neutrophils release F-actin as part of extracellular trap formation that serve as a scaffold for biofilm growth. Bacterial DNA binds F-actin allowing bacterial colonization and along with alginate provides stimulation for inflammatory signaling and recruitment of inflammatory monocytes while not necessarily resulting in bacterial clearance (131, 290, 312, 316, 373, 461). The formation of a dynamic bacterial community in the airway composed of both sessile and planktonic organisms provides a source of diverse immunostimulants that are ineffective in eradicating the infecting organisms.
B. Activation of Host Signaling by P. aeruginosa PAMPs
P. aeruginosa produces an array of surface-bound and secreted PAMPs such as LPS, lipoproteins, and flagella as well as toxins that are injected into target cells via the type III secretion needle (Figure 3). These include both structural components of the bacteria (lipoprotein and flagella) as well as bacterial DNA and RNA. These PAMPs are recognized by diverse receptors displayed on epithelial and immune cell surfaces, within endosomes and the cytosol. Despite its characterization as an extracellular pathogen, P. aeruginosa gene products are readily internalized by both epithelial and immune cells and stimulate the entire panoply of innate immune receptors. Downstream of these receptors, signaling cascades involving proteins MyD88, TRIF, and caspases mediate inflammatory responses to bacteria in the airway recruiting phagocytes to the site of infection. Also initiated is anti-inflammatory signaling, critical to localize and regulate the extent of the inflammatory response. P. aeruginosa has evolved to evade innate immune clearance through alterations in the expression of various gene products. P. aeruginosa has been observed to modify its appendages such as flagella, as well as its LPS and extracellular polysaccharide biosynthesis, express siderophores to scavenge iron, secretion of toxic compounds including antimicrobial agents, as well as the induction of multiple efflux systems to control access to required nutrients.
1. LPS
P. aeruginosa LPS is composed of a core region, the extracellular O-antigens, and lipid A, which is buried in the cell wall. Signaling activity of LPS is dependent on lipid A, specifically the acylation state of its side chains (132, 483). A cell wall component of intact bacteria, LPS is also shed from growing organisms and taken up by immune and stromal cells. Lipid A complexes with adaptor proteins MD2 and CD14 and is recognized by TLR4, resulting in activation of MyD88 and TRIF dependent signaling, which is usually activated by endocytosed TLR4 (190, 354, 446). Recognition of LPS by TLR4 is essential for survival and clearance of P. aeruginosa from the airway, likely leading to both pro- and anti-inflammatory signaling (395). Downstream of MyD88, inflammatory cytokine/chemokine production recruits neutrophils to the site of infection to phagocytose and kill bacteria. TRIF-dependent signaling generated from endocytosed or cytosolic ligands induces production of type I interferons. Type I IFNs inhibit MyD88-dependent inflammatory responses by limiting inflammasome activation and protecting the lung from inflammatory damage (76, 154). Additionally, type I IFNs have been linked to activation of macrophage and dendritic cells in the airway (305). Both arms of the TLR4 signaling pathway are required for regulating the innate immune response, and P. aeruginosa has evolved mechanisms for modifying the structure of LPS such that it can alter the nature of TLR4 signaling activation (111, 112, 160).
During acute infection, lipid A directly influences the degree of inflammatory cytokines produced by host cells, although this specificity is restricted to human compared with murine TLR4. The addition of palmitate to lipid A correlates with an increased IL-8 response from host cells; this modification is often found in isolates obtained from nonchronic infections (112). Palmitate along with aminoarabinose confer protection against cationic antimicrobial peptides (CAMPs) giving isolates containing these alterations to their lipid A a significant survival advantage. Lipid A alterations are controlled via transcriptional regulation (PhoP and PhoQ) of pagP, pagL, and lpxO genes that are responsible for acylation, deacylation, and hydroxylation of lipid A, respectively, and whose expression is determined by environmental conditions (140, 200). A long-term isolate from a cystic fibrosis patient correlated with a mutation in the pagL gene that is responsible for deacylation, which was also observed in an independently published study of early and chronic infection in cystic fibrosis (75, 112).
2. P. aeruginosa activation of types I and III IFNs
P. aeruginosa LPS signals via TLR4/TRIF resulting the activation of STAT transcription factors expression of IFN-β. Pretreatment of macrophages with IFN-β significantly reduces production of IL-1 and IL-18 (77). Defects in the type I IFN pathway have been observed in cells with CFTR dysfunction and may contribute to the excessive inflammation associated with this CF lung disease (77, 305). Poly(I:C) induction of the type I IFN pathway significantly improves clearance of P. aeruginosa in a murine model of infection; however, its exact contribution to defense against P. aeruginosa remains unclear as the type I IFN receptor (IFNAR) null mouse does not have a defect in bacterial clearance (78). While numerous other genes are induced in response to signaling initiated by the IFNAR, the net result of this cascade in the context of P. aeruginosa infection appears to be to regulate proinflammatory signaling.
Somewhat different immune mechanisms appear to be involved in the induction of type III IFN-λ signaling by P. aeruginosa (78). P. aeruginosa stimulate production of IFN-λ in murine airways by 4 h postinfection, and IL-28R null mice have significantly improved P. aeruginosa clearance and decreased pathology than the wild-type strains. Although lack of IFN-λ signaling did not significantly affect levels of IL-1β during P. aeruginosa infection, the IL-28R null mice did have significantly increased production of IL-10, suggesting the type III IFN signaling is enhancing inflammation. While IFN-λ is produced by all cells in the lung, its effects are mediated by mucosal receptors (401). Downstream of IL-28R, the activation of mIR-21 and suppression of PDCD4 results in decreased IL-8 production in airway epithelial cells as well (78). The complexities of these signaling cascades and numerous downstream consequences may limit the therapeutic potential of targeting IFN signaling in an attempt to suppress exaggerated proinflammatory signaling induced by P. aeruginosa infection in the lung.
C. Effects of Secreted Toxins
1. Type III secreted toxins
Following attachment to epithelial surfaces, most P. aeruginosa express components of the TTSS. The TTSS is a complex apparatus, and its secreted toxins are a major component of P. aeruginosa virulence in the lung. Expression of type III secreted toxin genes is induced upon contact with the host cell and initiates profound changes in the polarization and metabolism of the eukaroytic targets (164). Injected toxins modulate signaling pathways responsible for cytoskeletal organization and inflammasome signaling as well as having cytotoxic effects on host cells. The TTSS apparatus is composed of numerous proteins including PscC, which is necessary for activating the NLRC4 inflammasome, independent of effector protein secretion (267). Novel antibody therapies targeting the TTSS needle have shown some promise in protecting against P. aeruginosa lung damage (105, 454). For pore formation in the host cell membrane to occur, P. aeruginosa pili must bind asialoGM1 on the host cell membrane (192, 362, 419). Once the pore has formed, the bacteria inject a cocktail of proteins made up of combinations of ExoU, ExoY, ExoS, and ExoT. Individual bacterial isolates do not necessarily express each effector, but they have major effects on the host. This is a tightly regulated system that is suppressed under metabolically unfavorable conditions but contributes significantly to lung damage associated with acute P. aeruginosa infection (164).
The patatin-like exotoxin ExoU is a target for host ubiquitination, which activates phospholipase, a characteristic shared by many other bacterial species (368). This phospholipase targets eukaryotic membranes, but is inactive against prokaryotic cells lacking ubiquitin (8), providing target specificity (368). Expression of ExoU has been correlated with increased virulence of clinical isolates especially in the ICU setting, corresponding to its substantial induction of host cytotoxicity (175).
ExoS is a bicomponent toxin that has separate GTPase activating and an ADP ribosylating activity (416). It shares substantial homology and activity with ExoT, and both type III toxins perturb the actin cytoskeleton. ExoT affects numerous host targets including Rac1 and cell division (97). Infections due to organisms expressing ExoS/ExoT are associated with increased rates of bacteremia in murine models of pneumonia (405). ExoS and ExoT can impair phagocytic killing by neutrophils (164) contributing to bacterial persistence in the lung (341). Therefore, through either direct killing of host cells, or by modifying host signaling pathways, the TTSS effector proteins enable P. aeruginosa to avoid clearance by the innate immune system.
While important for bacterial dissemination during acute infection, expression of TTSS components is reduced in colonizing strains of P. aeruginosa (187). The bacterium is able to sense the host and regulates expression and activation of the TTSS through the RsmAYZ regulatory cascade (294). Furthermore, mucA is part of the regulatory pathway upstream of TTSS expression, and selection of mucA mutants also selects for bacteria lacking TTSS expression (469). P. aeruginosa isolates from CF patients that have adapted to the airways through mutations in mucA suppress the expression of the type III secretion genetic network (181). Therefore, as with other virulence factors such as flagella, expression of TTSS needle and effector proteins is repressed facilitating the selection of clones that evade detection by the innate immune system and eradication by the host.
D. P. aeruginosa Induction of Epithelial Signaling
Polarized epithelial cells form the primary barrier that maintains the air-liquid interface at the mucosal surface. They maintain the barrier with transmembrane junctional proteins anchored to the actin cytoskeleton by a complex of intracellular proteins. Human airway epithelial cells express an array of TLRs (13, 284) whose participation in bacterial recognition depends on their localization. While TLR2 is surface expressed and upregulated by exposure to P. aeruginosa (406), TLR4 is present within endosomes and mediates signaling through TRIF to induce anti-inflammatory responses (190). Flagellin is sensed by TLR5; however, in polarized epithelial cells this receptor is restricted to the basal surface (52), conferring responses to invasive organisms or functioning in disrupted epithelia. Upon stimulation with P. aeruginosa, the airway epithelium produces a number of cytokines including IL-8, IL-6, and GM-CSF (57, 222, 308). Cytokine production is dependent on multiple TLRs including TLR2, TLR4, and TLR5 as well as expression of MyD88. These cytokines are essential for innate defense against Pseudomonas, as mice lacking epithelial MyD88 are unable to control P. aeruginosa infection (268). However, in diseases such as cystic fibrosis, epithelial signaling abnormalities have been suggested as a primary mechanism of immune dysfunction. Excessive epithelial proinflammatory cytokine production can result in tissue damage through effects on recruited immune cells. GM-CSF, for example, increases macrophage production of TNF and promotes survival of neutrophils, adding to the inflammatory cycle (77, 356, 392).
The epithelium responds to numerous Pseudomonas products including flagellin and pyocyanin by upregulating mucin genes (29, 162, 235, 333). Specific mucins are essential in the regulation of epithelial inflammatory responses, functioning to inhibit TLR5 interaction with MyD88 (197) and PPARγ-dependent cytokine production (304, 435). Mucin can also potentiate infection. Mucin can influence the innate immune response (241), in addition to mediating attachment of P. aeruginosa to the epithelium (195), and promoting alginate and biofilm production as previously discussed.
Pyocyanin, regulated by transcriptional regulators LasR and OxyR, is produced by all P. aeruginosa strains, but at increased levels in biofilms (371, 445). It can directly interact with host cells, inducing inflammatory signaling and reducing the function of antioxidants GSH and N-acetylcysteine by blocking the dual oxidase-based antimicrobial system (240, 334, 335). In the epithelium, pyocyanin inhibits V-ATPase activity preventing endosomal acidification and perhaps inhibiting degradation of endocytosed bacteria. Pyocyanin may also negatively affect the function of the chloride channel CFTR, perhaps enhancing disease progression in cystic fibrosis (377). Levels of pyocyanin have been correlated with decreased lung function in clinical studies, and in vivo experiments have documented decreased virulence of P. aeruginosa pyocyanin mutant strains (121, 178, 223, 342).
E. Evasion of Host Defenses
1. Neutrophils
The central role of neutrophils in the clearance of P. aeruginosa from the airways is best illustrated by the high prevalence of these infections in neutropenic patients, usually as a consequence of chemotherapy (209). Neutrophils are rapidly recruited to the airway/lung following infection with P. aeruginosa and are essential for the elimination of the infection. Models of P. aeruginosa pneumonia demonstrate significant (>6 logs) increases in bacterial burden in mice lacking neutrophils, demonstrating that neutrophil function is key for the host response to this organism (72, 105, 381). Subsequently, P. aeruginosa has evolved mechanisms for evading neutrophil-mediated clearance.
Biofilm formation protects the bacterial population from neutrophil uptake. Encased by extracellular proteins, alginate, and DNA P. aeruginosa is protected from surveying neutrophils (248, 317, 403). Planktonic organisms also thwart neutrophil function. Upon neutrophil contact P. aeruginosa binds siglec-9 on the neutrophil surface, decreasing elastase, reactive oxygen species (ROS), and neutrophil extracellular trap (NET) production (204). In vivo, P. aeruginosa secretes multiple factors that can inhibit neutrophil function in a contact-independent manner. Alkaline phosphatase (AP) and elastase inhibit chemotaxis (425) and myeloperoxidase-based killing as well as influence oxidative burst and superoxide production (202, 203). At physiological concentrations, AP and elastase also inhibit phagocytosis, possibly by cleaving surface receptors required for uptake (202). The presence of pyocanin within a cell inhibits endosomal acidification by V-ATPases and neutrophil death through a caspase-3-mediated mechanism (438). Neutrophil death can also be caused by the ADP ribosyltransferase activity of type III toxins (ExoS, ExoT, and ExoU) (101, 418).
F. Macrophages and Inflammasome Activation
The primary immune cell present in the airway in healthy individuals is the alveolar macrophage. It communicates with the epithelium to coordinate innate responses to shed LPS through gap junction channels (460), producing proinflammatory cytokines that recruit other immune cells and regulate antimicrobial peptide production. While macrophages provide a major function in antigen presentation and stimulation of T cell signaling, they are especially critical in maintaining the anti-inflammatory tone of the resting human airway, preventing excessive inflammatory signaling that compromises normal lung physiology.
In addition to activating macrophages via the surface and endosomal display of TLRs, several P. aeruginosa products activate NLRC4 inflammasome activity through endocytosed flagellin or via PcrV components of the type III secretion apparatus (267, 421). P. aeruginosa activation of the inflammasome increases lung inflammation due to release of IL-1β, IL-18, and HMGB1 (77). In addition, IL-18 suppresses IL-17-dependent antimicrobial peptide production from innate lymphoid cells, increasing bacterial survival (77, 116). Given the multiple sources of proinflammatory cytokine production in the airway, activation of the inflammasome in murine models appears to contribute to lung pathology. Depletion of alveolar macrophages significantly improved bacterial clearance and survival in a murine model of P. aeruginosa pneumonia, and mice lacking IL-1R had significant preservation of normal lung architecture in the setting of P. aeruginosa pneumonia (77). A recently described virulence protein RhsT expressed by some P. aeruginosa strains is also capable of activating inflammasome-mediated signaling (218), indicating that multiple P. aeruginosa gene products contribute to this excessive inflammatory response. While macrophages participate in phagocytosis, it appears that their role in proinflammatory signaling can be excessively stimulated and thus could be a target for immunomodulatory therapy.
G. T Lymphocytes
The participation of T cells in P. aeruginosa pneumonia has been examined in mouse models, in the context of cystic fibrosis lung disease and in vaccine development. In contrast to S. aureus, there does not appear to be pathological T cell cytokine induction in response to P. aeruginosa. Infection, especially chronic infection, stimulates production of T cell-associated cytokines, and Th1-, Th2-, and Th17-associated cytokines are all significantly increased in the airways of CF patients (427). The importance of IL-17 production and associated neutrophil recruitment has been well established in the effective clearance of Gram-negative pneumonias. Anti-P. aeruginosa vaccines have been designed to include gene products that specifically upregulate Th17 signaling, including components of the TTSS (470). Innate lymphoid cells (ILCs) participate in the response to P. aeruginosa through their production of IL-22 (265). As IL-22 also contributes to the integrity of the epithelial barrier in the lung, this may also help to limit system infection (323). IL-17 production also helps to generate the organization of bronchus-associated lymphoid tissue (BALT), which may be important in orchestrating an effective immune response to P. aeruginosa infection (122). Of note, there are no data to date suggesting that P. aeruginosa targets human immune cell receptors, in contrast to S. aureus.
H. Conclusions
P. aeruginosa remains a common and much-feared cause of health care-associated pneumonia. In contrast to S. aureus that has multiple human-specific adaptations, P. aeruginosa is more of an opportunist, and its adaptation to the host, as in the porin OprD mutation to resist carbapenems and many other antibiotics, may also facilitate survival in human infection (396). Its specific interactions with the components of the innate immune system result in a highly proinflammatory host response. Yet these ubiquitous organisms can also evade immunostimulation through growth within a biofilm and suppression of genes that contribute to immunoactivation. The components of the proinflammatory response evoked by P. aeruginosa differ from the signaling initiated by S. aureus. Although both organisms, through different PAMPs and toxins, induce inflammasome activation, the ultimate consequences of the immune response activated are distinct. The production of type III toxins, and particularly the phospholipase ExoU, leads to airway inflammation and loss of barrier function that all contribute to pulmonary pathology, invasive infection, and sepsis. The efficacy of inhibitors of the IL-1 pathway to limit but not totally suppress P. aeruginosa-induced excessive inflammation may provide ancillary therapy, especially for the P. aeruginosa isolates that are resistant to antimicrobial agents.
IV. KPC: KLEBSIELLA PNEUMONIAE AN EVOLVING PATHOGEN
Klebsiella pneumoniae producing carbapenemase (KPC), included as a member of the ESKAPE pathogens, is increasing in prevalence worldwide (40, 324). These highly antibiotic-resistant strains are associated with close to 50% mortality (286, 365, 432) and have achieved recognition by the CDC and World Health Organization as emerging major public health problems (63, 467a). K. pneumoniae have been well recognized as major respiratory pathogens, originally associated with aspiration pneumonias in compromised patients, especially alcoholics (60, 141). The alarming increase in their prevalence has spurred substantial interest in understanding how these organisms are handled by innate immune clearance mechanisms and has raised the question of whether mutants selected for antimicrobial resistance are also selected for their ability to proliferate in the human airway. In contrast to either S. aureus or P. aeruginosa, which have been the focus of pathogenesis studies using isogenic mutants in vitro and in vivo, there are many fewer studies detailing exactly how K. pneumoniae and especially the KPC strains are handled in the lung. However, there are numerous epidemiological analyses that follow the patterns of disease caused by specific KPC clones that have been correlated with pathogenesis in mouse models of infection.
A. Epidemiology
As a member of the Enterobacteriaciae family, K. pneumoniae readily exchange genomic material with other Gram-negative organisms and have the genetic flexibility to adapt to their environment, especially in a health-care related setting. Like MRSA and P. aeruginosa, K. pneumoniae readily adapt through genomic mutation as well as through the acquisition and maintenance of plasmids that mediate expression of carbapenemases among other antibiotic resistance genes. Analysis of the pathogenicity of the newly emerging KPC strains has been determined through whole genome comparisons, identification of expected virulence determinants, and in some cases confirmation of expected phenotypes through the generation and complementation of mutants (34). Whole genome analysis of K. pneumoniae serotype 2 (ATCC 43816) compared with clinical isolates shows high levels of genome conservation between strains (>96%) and a lack of plasmids (123). With the acquisition of plasmids and genomic mutations, the K. pneumoniae producing carbapenemases (KPCs) have developed exceptional plasticity. Clinical isolates variably express the chromosomal class A β-lactamase, a penicillinase, the broad-spectrum carbapenemases, SHV variants, and many additional plasmid-mediated β-lactamases (blaTEM-1, blaOXA-9, blaCTX-M-2, blaKPC-2, blaKPC-3) (340). The efficiency of the β lactamases is potentiated by expression of efflux pumps, alterations in porins, LPS, and PhoP/PhoQ mutations as well as numerous SNPs. There is tremendous genetic diversity in these strains and substantial variability in plasmid acquisition, as reflected in the differing antimicrobial susceptibility patterns and capsular composition within the most common KPC sequence type 258 (ST258) clades (100). Recent reports emphasize the high transmissibility of the antibiotic resistance plasmids in these clinical isolates between hospitalized patients (230) and high colonization rates even in healthy children, within communities with unrestricted antibiotic use (413). KPC strains lead to poorer outcome from infection (39), which is associated with host susceptibility as well as the pathogenicity of these organisms. Relatively little is known about the virulence factors that these KPC organisms have acquired, but their fitness in the human reservoir suggests evolutionary advantage (35, 225).
B. Colonization
Most of the available data detailing the pathogenesis of pulmonary infection due to K. pneumoniae are based on studies using a common laboratory strain (serotype 2). A comprehensive signature tagged mutagenesis screen of K. pneumoniae identified a relatively small number of genes required for pulmonary or bloodstream infection (226), in contrast with either P. aeruginosa or S. aureus. Identified loci associated with pathogenesis were largely limited to the expression of surface polysaccharides. Moreover, while mutants with reduced survival in vivo were identified such as the capsule mutant cpsB, traditional competitive index experiments were unsuccessful. This suggests that wild-type organisms may transcomplement mutants, as has been suggested for other opportunistic pathogens (452). The cpsB mutants did not evoke a neutrophil response and were able to persist, if not proliferate within the lung, a phenotype that may be relevant to human infection.
1. Fimbriae
Fimbrial gene clusters expected to be important in cell attachment are located in regions of the genome with increased plasticity (288). KPCs express multiple fimbrial genes and types 1 and 3 have been associated with pathogenesis with the identification of fimA and mrkD as the genetic loci, respectively (139, 288). Type 3 fimbrial genes play a major role in the adhesion to multiple surfaces such as collagen IV on respiratory and urinary epithelium (379, 380) and have been associated with colonization of abiotic surfaces such as plastic catheters (297). Protective immunity is conferred by purified fimbrial proteins (224), supporting the potential for vaccine development (236). However, K. pneumoniae exhibits high variability in the type 3 fimbrial genes, with horizontal transmission to other Enterobacteriaceae family members such as E. coli (410).
2. Capsular polysaccharide
The best described virulence factor of K. pneumoniae is the thick capsule of the organism, which contributes to its success as a murine pathogen (226) and has been associated with community acquired infections (430). Over 77 capsular polysaccharide types have been described and as suggested by the many genomic studies, KPC strains exhibit diversity at these loci as well (12, 45). These extracellular polysaccharides also contribute to biofilm formation, which enhances the persistence of the microbial community by limiting access to phagocytes, antibodies, and antibiotics. Analysis of the capsular polysaccharide (CPS) of clinical isolates of KPCs, including the ST258 strains, revealed diversity in composition and biofilm formation, but little effect of CPS phenotypes on growth. CPS increased resistance to killing by human serum in some strains, but did not consistently affect uptake and killing by a J744.2 macrophage cell line (100). Virulence in the G. mellonella model (moth) did not correlate with either biofilm formation, macrophage killing in vitro, or with clinical outcomes in patients, suggesting the importance of host factors in overcoming infection (264).
In animal models, infection by cpsB mutants was not completely attenuated, suggesting alternative mechanisms of virulence (226), a finding that was confirmed in other mouse models of infection. Of note, a KPC strain associated with over 40% mortality in human bloodstream infection was found to be entirely avirulent in a murine model of sepsis, suggesting that either the model does not reflect what occurs in humans, or that antimicrobial resistance and overwhelming infection are the major causes of death (433). Capsular polysaccharide interferes with airway clearance, an effect that may be associated cps downregulation of human β-defensins, with improved bacterial killing by antimicrobial peptides in the absence of the capsular proteins (58, 278).
The correlation between capsular polysaccharide and virulence is more clearly illustrated in the so-called hypervirulent K. pneumoniae (K1), associated primarily with systemic infection (386). These hypervirulent strains have a hypermucoviscous phenotype caused by rmpA mutations resulting in increased expression of CPS (221, 386) and increased virulence in a murine model of systemic infection (237). However, the hypervirulent, RmpA+ phenotype and the expression of carbapenemases thus far do not appear to be linked epidemiologically. No direct correlation between antimicrobial resistant KPC strains, CPS expression, and virulence was observed in an extensive comparison of clinical isolates that included hypervirulent and KPC strains (238).
As KPCs demonstrate such genetic plasticity in adapting to environmental pressures, there is concern that the development of antimicrobial resistance could also result in increased resistance to innate immune clearance. This may be clinically relevant as subtherapeutic concentrations of antibiotics actually increase production of capsular proteins (166), an observation that has been made in other pathogens of the carbapenemase resistant Enterobacteriaceae (CRE) family (137). Furthermore, upregulation of interferons has long been known to be increased in response to capsular proteins (198, 199), which have been used as an adjuvant to help clear airway infection with influenza virus (347).
C. Iron Acquisition
In contrast to the variable association of CPS production and pathogenicity, there is a well-characterized association between the genes important in iron acquisition and K. pneumoniae virulence, similar to other gram-negative pathogens (120). Analysis of the hypervirulent strains demonstrated 100% mortality in a murine model of intraperitoneal infection even at a low dose, an effect that was associated with the expression of siderophores, specifically aerobactin (355). The K. pneumoniae siderophores enterobactin (Ent+) and yersinobactin (Ybt+) are also important in pathogenesis. Ent+Ybt+ isolates are over-represented among respiratory isolates, and specifically in the epidemic ST258 clones (18). Both of these siderophores are outcompeted by lipocalin 2 (lcn2) in the airways, which contributes to host defenses locally (17, 18, 66). Of note, K. pneumoniae induction of IL-22 in turn upregulates lipocalin 2 and enhances host defenses in the airway (15).
D. K. pneumoniae Activation of Immune Responses in the Lung
Relatively few specific virulence factors have been shown to contribute to K. pneumoniae infection in contrast to other ESKAPE pathogens. Most published studies of K. pneumoniae pulmonary infection have focused on the components of innate immunity that are involved in the host response to airway infection. The major factors surrounding the ability of K. pneumoniae to persist within the airway and to resist phagocytosis and killing is their relative lack of immunogenicity and failure to stimulate the robust proinflammatory response associated with clearance of other airway pathogens. Whether this is due to structural differences in PAMPs, induction of anti-inflammatory responses, or inhibition of host signaling pathways are all topics under investigation.
The distinct functions and interplay of individual cytokines during K. pneumoniae infection have been carefully dissected (163). The shared and independent components of IL-23 and IL-12 are important to host defenses, with IL-23 being critical for Th17 signaling and subsequent neutrophil recruitment. IL-17 signaling is also augemented with IL-12-dependent production of IFN-γ. These observations confirmed studies initiated nearly two decades ago demonstrating that IFN-γ null mice had significantly increased mortality due to pulmonary infection (276, 476) and that IL-12 overexpression improves outcome, in an IFN-γ dependent manner (149). Several T-cell populations produce IFN-γ in response to IL-12 including γδ, naïve, and memory T cells (149, 275). Consistent with the participation of STAT4 and IL-12 in host defense, STAT4 knockout mice had a greater bacterial burden in the lung with decreased expression of proinflammatory cytokines, but no differences in numbers of phagocytes in the bronchoalveolar lavage fluid (BALF) of the K. pneumoniae-infected mice (96).
Proinflammatory signaling is important to K. pneumoniae clearance as demonstrated by the increased mortality in TNFR1-deficient mice (277), as well as MyD88/TRIF double knockout (56). Strategies to increase proinflammatory signaling in the airway during K. pneumoniae infection have been proven successful by CCL3 (MIP-1α) intrapulmonary expression (479) or intratrachael CpG oligodeoxynucleotides (ODN) instillation (95). TLR9 signaling by CpG led to enhanced cytokine expression (TNF, IL-12, and CXCR3 chemokines) improving K. pneumoniae clearance. CpG ODN also recruited various inflammatory cells into the lungs (γδ T cells, NK cells, and neutrophils), with TLR9-dependent recruitment and maturation of DCs (129). Cooperative roles of TLR4 and TLR9 signaling are involved in the activation of macrophages and induction of adaptive immunity (IL-23 and IL-17) (33), with TLR4 playing a central role in host defense (376). From the published literature it is unclear why K. pneumoniae in general, and specifically the KPC organisms fail to elicit a robust and protective immune response from an immunocompetent host.
Little is known about the mechanisms of cellular death in response to K. pneumoniae, but a small group of studies suggest that pyroptosis likely plays a vital role in bacterial clearance (455). For example, IL-1β production has been suggested to be associated with NLRC4 (55), ROS (67), and NLRP3/ASC-dependent (464) responses associated with pyroptosis. Bacterial clearance and survival from K. pneumoniae infection were worse in mice lacking components of this cascade. However, the toxins and specific K. pneumoniae PAMPs involved have not yet been defined.
E. K. pneumoniae Interactions With Leukocytes
Historically, the expression of capsular polysaccharides has been associated with K. pneumoniae resistance to phagocytosis, although this may vary from strain to strain (Figure 4) (81). The abundance of sialic acid in the capsular polysaccharides of the hypervirulent K. pneumoniae was recently recognized, sialic acid being a common modification of eukaryotic surfaces and, hence, not immunogenic. Moreover, the presence of multiple sialic acid moieties and their negative charge could also contribute to neutrophil repulsion (227). Several studies have identified factors that inhibit opsonization of K. pneumoniae by phagocytes including wzm (LPS O-antigen transporter), clpx (protease subunit), and outer membrane proteins (252, 302).
FIGURE 4.
Mechanisms of innate immunity to K. pneumoniae pulmonary infection. Neutrophils are critical to combating infection with K. pneumoniae, with recruitment dependent on Th17 cell signaling. Phagocytosis and intracellular killing by neutrophils is directly impaired by capsular polysaccharide (CPS). CPS is the best described virulence factor in infection, contributing to the evasion of typical host mechanisms of pathogen eradication. Other innate immune cells such as natural killer (NK) T cells and antigen presenting cells (APCs) such as dendritic cells also participate in bacterial clearance.
1. Macrophages
The activation of macrophages is an important factor in the ability of the host to efficiently clear K. pneumoniae from the airway, as first demonstrated in a model of infection in alcoholic mice (296). Few publications detail the interactions of this pathogen with fully characterized alveolar macrophage populations. These cell populations are clearly important to host defense as mice lacking resident macrophages were more likely to die from pulmonary infection, due to impaired neutrophil recruitment (47). Pulmonary expression of CXCL10, which acts as a chemokine for several cells types including macrophages/monocytes and T cells, was important in mediating K. pneumoniae clearance (480). Additionally, treatment of mice with c-diGMP, which stimulated the recruitment of T cells, NK cells, neutrophils, and specifically activated macrophages also enhanced the clearance of K. pneumoniae from the airway (194). Consistent with these findings, treating mice with GM-CSF to stimulate the polarization of macrophages to the phagocytic inflammatory phenotype also enhanced K. pneumoniae clearance (411). Less clear from the published literature is the reason why K. pneumoniae compared with either S. aureus or the more closely related gram-negative P. aeruginosa fail to elicit a more robust activation of macrophages on their own.
2. Neutrophils
The recruitment of neutrophils and their subsequent cytokine signaling are critical to the clearance of K. pneumoniae with the activation of the expected proinflammatory signaling cascades are activated in response to K. pneumoniae. For example, TLR4-mediated responses to K. pneumoniae LPS activate cytokine and chemokine expression including production of IL-1β, IL-17, and CXCL1, critical to the early host response to infection (55, 376). CXCL1 then propagates proinflammatory signaling through NF-κB, MAPKs, and the induction of CXCL2 and CXCL5 (54, 150). Independent studies demonstrate that K. pneumoniae infection stimulates CCL2 signaling from resident or recruited monocytes, promoting recruitment of more neutrophils, upregulation of CCR2 expression, and increasing G-CSF production (19).
Factors that inhibit neutrophil function contribute to K. pneumoniae pathogenesis. For example, deficiency in adenosine 2B receptor attenuates inflammation by neutrophils, with deletion of the gene thereby enhancing extracellular bacterial killing and NET formation (23). The importance of NET formation in K. pneumoniae clearance has been observed (383, 455), likely acting through a neutrophil elastase-dependent digestion of nucleosomal digestion, chromatin decondensation (303), and upregulation of cytoplasmic proteins (2). However, the in vivo role of NET formation in the clearance of gram-negative pathogens such as E. coli or K. pneumoniae has been questioned, particularly compared with larger organisms such as yeast (42).
3. DCs
Participation of DCs in host defenses against K. pneumoniae pulmonary infection was shown to involve the induction of IL-12, IL-23, and IL-17, which are critical for host defenses against this organism (163). The specific role for DCs themselves in the clearance of K. pneumoniae infection has not been fully defined as it has for other ESKAPE pathogens (256), but numerous subsets of DCs are stimulated by these organisms (159). K. pneumoniae activated DCs also participate in stimulating NK cells (439), which are important in K. pneumoniae clearance by virtue of their expression of IL-22 (471). However, the presence of K. pneumoniae CPS interferes with DC activation. The shared O-antigens of CPS and LPS have been shown to interfere with DC maturation and pro-Th1 cytokine production (113). CPS expression in fact impeded opsonization by DCs, as well as their maturation through decreased expression Th1 cytokines, CD83 and CD86 (41), as well as TLR4 expression (343) compared with capsular mutants.
The more recently described myeloid-derived suppressor cells (MDSCs) also participate in K. pneumoniae clearance. Recombinant G-CSF administration improves survival from K. pneumoniae pneumonia through STAT3, a key regulator in the expansion of MDSCs (133). MDSCs play a vital role in the efferocytosis of K. pneumoniae infected and apoptotic neutrophils, through the production of IL-10 that is important for the resolution of inflammation (325).
4. T cells
The participation of Th17 cells is critical in K. pneumoniae host defense in the lung detailed in studies dissecting the participation of individual elements of this cascade using mice with targeted deletions in IL-12, IL-23, and IL-17 (163). Additional studies document the importance of IL-17 or IL-17RA in K. pneumoniae clearance, as mice lacking IL-17RA have decreased production of G-CSF and decreased neutrophil recruitment compared with controls. Exogenous treatment with IL-17 in mice lacking the upstream mediator IL-23p19 results in recruitment of neutrophils and improved clearance of the organisms (163, 475). Thus, in contrast to the pathological responses activated by staphylococcal superantigens, the participation of Th17 cells is a major factor in the successful eradication of the organisms in the murine model of pneumonia.
5. Intracellular killing
Once phagocytosed, not all airway pathogens are killed through identical mechanisms or with equal efficiency. Neutrophil killing of K. pneumoniae is dependent on myeloperoxidase, through direct killing and inactivation of elastase (171). Ingested K. pneumoniae induce CXCL1 expression that upregulates NADPH oxidase and inducible nitric oxide synthase, as well as production of leukotriene B4, effectors which act as chemokines for neutrophil mobilization and in promoting bacterial death (25). The composition of the capsular polysaccharide affects intracellular killing by neutrophils, with an enhanced respiratory burst in response to the presence of di-Man/Rha, likely through early and late complement activation (359). The success of KPC multidrug resistant clinical isolates has been accompanied by increased resistance to neutrophil killing (92). KPC strains have been found to have alterations in the induction of the respiratory burst and release of reactive oxygen species compared with antibiotic-sensitive strains (94, 359). Exactly how this phenotype contributes to in vivo pathogenesis remains to be established. In contrast, despite increasing antibiotic resistance, multidrug resistant KPC appear to remain susceptible to human AMPs (360).
F. Epithelial Inflammatory Signaling
Signaling by the airway epithelium is an important component of the innate immune response to airway pathogens and is an important source of proinflammatory cytokine production, which is well illustrated in the studies of S. aureus and P. aeruginosa infection in cystic fibrosis (76). K. pneumoniae CPS can increase TLR2 and TLR4 expression in human airway epithelial cells (343). Cytotoxicity of airway epithelial cells was suggested to be due to CPS, consistent with studies demonstrating a lack of virulence associated with infection by a cps mutant (59). In agreement with these observations, TLR4 activation by K. pneumoniae has been shown to upregulate GM-CSF, protecting the airway epithelium from injury (411).
However, the presence of CPS interferes with the induction of epithelial IL-8 and intracellular adhesion molecule, suppressing recruitment of the phagocytes (343, 344). This downregulation of NFκB-dependent gene expression has been attributed to upregulation of CYLD, a deubiquitinase that limits MAPK phosphorylation via MAPK phosphatase-1 (MKP-1). CYLD activity is also a consequence of EGFR signaling, a potential ligand for CPS components (129). Exactly how K. pneumoniae interaction with deubiquitinating enzymes contributes to pathogenesis remains to be fully explained, but is a major virulence mechanism exploited by other pathogens (482).
G. Conclusions
As should be evident from this review the interactions of K. pneumoniae, specifically the KPCs, and the components of host innate immune clearance are not as clearly defined as for other major human pathogens. Substantial data confirm the importance of activating innate immunity, especially through induction of IL-12/IL-23/IL-17 signaling and the recruitment of neutrophils into the lung. In contrast to S. aureus, it appears that the lack of immunostimulatory activities, and especially the impaired activation of macrophages, facilitates pathogenesis. In addition, septicemia appears to be a common complication of pneumonia, both in humans and in rodent models, further contributing to mortality. Therapeutic strategies to improve innate immune clearance, perhaps through proinflammatory agents, may enhance clearance of these organisms. Given the genetic flexibility of these organisms, it would appear that strategies to target the major virulence factors such as the capsular polysaccharide, are less likely to be successful given the likelihood of selection of mutants able to evade therapy which is focused upon a single or limited number of gene products.
V. OVERALL CONCLUSIONS
The ESKAPE organisms, grouped as a common and formidable cause of severe and often fatal hospital-associated pneumonias, share tremendous success as human pathogens. These organisms display impressive adaptability to what should be a hostile environment, with selected clones proliferating within the human lung through rapid evolution in response to environmental pressures imposed by antimicrobial agents and immune clearance mechanisms. Each of these pathogens has made major adaptations to the human airway. Despite their epidemiologic commonalities, each of the organisms detailed has distinct biological features, and each has species-specific interactions with the host, which may require targeted enhancement of immune defenses.
S. aureus and the epidemic USA300 MRSA strains are a component of the commensal flora that express an exceptional repertoire of adhesins and toxins to accommodate colonization and proliferation within the lung. In response to appropriate metabolic signals, these virulence factors are expressed facilitating the transition from commensal to pathogen. Several staphylococcal toxins are human specific, targeting common leukocyte receptors, CXCR1, CCR5, CD11b and the complement receptors C5aR and C5R2. Their contribution to pathogenesis may not be readily assessed in conventional murine models of infection. Toxin-induced loss of specific populations of immune cells includes airway macrophages, T cells, and DCs and their immunoregulatory functions as well as the more numerous neutrophils. Loss of immunoregulatory populations of leukocytes likely contributes to the dysregulated inflammation associated with MRSA pneumonia. Toxin-associated activation of inflammasomes, IL-1β, and IL-18 release may further exacerbate airways inflamed through the epithelial production of proinflammatory cytokines and neutrophil recruitment. Toxin-induced loss of epithelial barrier function and airway edema further compromises pulmonary function, facilitating invasive infection by this nonmotile pathogen. As the correlates of immune protection against repeated staphylococcal infection are not known, devising strategies to protect the airways from pathological inflammation induced by these organisms seems a reasonable goal.
P. aeruginosa, from a somewhat different environmental niche and metabolic capabilities, often is found in the same health care-associated setting as the gram-positive pathogen S. aureus. Induction of airway inflammation initiated by both epithelial and immune cells is an important component of innate signaling in response to P. aeruginosa. Excessive inflammation, as activated by the inflammasome, is a major cause of airway damage, alveolar leakage, and respiratory compromise. Inhibition of inflammasome activation may be a reasonable target for P. aeruginosa airway infection (76, 77). Given the redundancies in proinflammatory signaling in the lung, P. aeruginosa toxins, like those of S. aureus, target immune cells, but are especially damaging to the epithelium. The P. aeruginosa toxin ExoU usurps host ubiquitin, targeting membranes to cause a highly destructive, hemorrhagic pneumonia.
Likely as important in pathogenesis as toxin production, P. aeruginosa rapidly adapts to the airway mucosa forming dynamic biofilms that serve to protect the bacterial community from phagocytic and antimicrobial clearance. As a consequence of its exceptional genetic flexibility, P. aeruginosa efficiently adapts to the milieu of the airway, altering its LPS composition, secreted polysaccharides, and upregulating the activity of broadly active efflux pumps. In combination with its minimal metabolic requirements, P. aeruginosa is the consummate opportunist.
Innate immune clearance of the carbapenemase-producing K. pneumoniae presents significantly different challenges than the better-studied P. aeruginosa or MRSA. Although the benefits of whole genome sequencing have greatly enhanced the understanding of the clonality, diversity, and epidemiology of KPC infections, there are limited data detailing how this seemingly inert organism persists within the airway. In contrast to MRSA or P. aeruginosa, relatively few KPC virulence adhesins or toxins have been described. These organisms regulate the composition and amount of extracellular polysaccharide as their major mechanism to facilitate proliferation in the human host. Based on the studies of the laboratory strain serotype 2, it is likely that KPCs function as “stealth” pathogens, which fail to stimulate the robust immune response associated with MRSA or P. aeruginosa. Lack of activation of macrophages in KPC clearance seems likely and may provide a target for immunotherapy. In contrast to the staphylococci, there may be an association between the antibiotic resistance of the KPCs and their adaptation to innate immune clearance within the lung, primarily through changes in capsular polysaccharide. There appears to be a relatively indolent pace of KPC infection, until the bacterial load becomes excessive and bloodstream invasion and sepsis occur. Thus there may be a window between colonization and severe infection that would provide an opportunity for targeted immunotherapy to enhance clearance of these organisms. However, given the exceptional genetic flexibility of KPCs, as well as the other organisms that we have reviewed, it is likely that they will continue to evolve to maintain their niche as major hospital-associated pathogens.
VI. FUTURE DIRECTIONS
The historical approach to understanding microbial pathogenesis, i.e., constructing bacterial mutants and studying “virulence” in animal models, has produced a formidable body of literature that we have attempted to review. A tremendous amount about each of these MDR pathogens is known as well as the genetics of their adaptation to the host. In the past for organisms such as S. pneumoniae, this was sufficient for the development of highly effective vaccines. However, the development of effective vaccines against the ESKAPE pathogens has been unsuccessful for many reasons. The reliance upon mouse models of infection is problematic for pathogens like S. aureus that express toxins with human specificity, as evidenced by the recent human trial of an anti-staphylococcal vaccine that was terminated due to lack of efficacy, despite promising results in murine models (127). The use of humanized mice that express human hematopoietic cells susceptible to staphylococcal toxins may be useful for anti-staphylococcal vaccine development (43), even though exactly what constitutes protective immunity against these commensals is not well defined. Vaccines targeting P. aeruginosa have also failed (328) in part due to the selection of mutants lacking the vaccine targets. However, recognition of the specific P. aeruginosa toxins that are associated with lung damage may facilitate therapy with monoclonal antibodies directed at neutralizing these toxins (370, 385), if such patients can be identified early in the course of infection.
A. Prevention/Early Targeted Intervention
The concept of targeted therapy for specific MDR pathogens is increasingly plausible with the availability of RT-PCR-based methods for rapid pathogen detection. These vastly improved culture-independent detection systems rapidly identify patients at risk, those colonized or even infected with MDR organisms, and can be used to direct appropriate therapy. Screening and decolonization of MRSA colonized patients has been tremendously effective in decreasing the prevalence of infection with these organisms (447, 457, 458). Influenza-infected patients, with increased risk of severe bacterial pneumonias, could be routinely decolonized to diminish susceptibility to subsequent MRSA infection. As culture-independent diagnostics improve, this information can be used to optimize immunomodulatory therapy. It is evident that it is often the host response, or lack thereof, which is directly associated with morbidity and mortality in the pneumonias. The availability of drugs and biologics that target inappropriate immune signaling, excessive IL-1β for example in the setting of P. aeruginosa pneumonia, could be promptly initiated to prevent irreversible lung damage. Identification of KPC producing ST258 or similar strains of K. pneumoniae in the airway might generate therapeutic approaches to activate macrophages and enhance the inflammatory clearance mechanisms in the lungs. While the concept of tailoring antibiotic therapy to the specific infecting pathogen is widely accepted, it should also be possible to individualize anti- or proinflammatory modulators to similarly direct a more effective immune response against specific MDR pathogens.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1 HL079395 and RO1 HL073989.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
Address for reprint requests and other correspondence: A. Prince, Dept. of Pediatrics, 650 West 168th St. Black, Building 416, New York, NY 10032 (e-mail: asp7@columbia.edu).
REFERENCES
- 1.Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, Zabner J, Welsh MJ. pH modulates the activity and synergism of the airway surface liquid antimicrobials beta-defensin-3 and LL-37. Proc Natl Acad Sci USA 111: 18703–18708, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achouiti A, Vogl T, Urban CF, Röhm M, Hommes TJ, van Zoelen MAD, Florquin S, Roth J, van't Veer C, de Vos AF, van der Poll T. Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog 8: e1002987, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol 30: 627–634, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Devi VS, Shulenin S, Warfield KL, Aman MJ. Novel structurally designed vaccine for S. aureus alpha-hemolysin: protection against bacteremia and pneumonia. PLoS One 7: e38567, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn DS, Parker D, Planet PJ, Nieto PA, Bueno SM, Prince A. Secretion of IL-16 through TNFR1 and calpain-caspase signaling contributes to MRSA pneumonia. Mucosal Immunol 7: 1366–1374, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonzo F 3rd, Benson MA, Chen J, Novick RP, Shopsin B, Torres VJ. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol Microbiol 83: 423–435, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonzo F 3rd, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493: 51–55, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson DM, Sato H, Dirck AT, Feix JB, Frank DW. Ubiquitin activates patatin-like phospholipases from multiple bacterial species. J Bacteriol 197: 529–541, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antunes LC, Ferreira RB, Buckner MM, Finlay BB. Quorum sensing in bacterial virulence. Microbiology 156: 2271–2282, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Arad G, Levy R, Hillman D, Kaempfer R. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat Med 6: 414–421, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, Supper E, Shpilka T, Minis A, Kaempfer R. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol 9: e1001149, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol 177: 1788–1796, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong L, Medford AR, Uppington KM, Robertson J, Witherden IR, Tetley TD, Millar AB. Expression of functional toll-like receptor-2 and -4 on alveolar epithelial cells. Am J Respir Cell Mol Biol 31: 241–245, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Artis D, Spits H. The biology of innate lymphoid cells. Nature 517: 293–301, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Aujla SJ, Aujla SJ, Chan YR, Chan YR, Zheng M, Zheng M, Fei M, Fei M, Askew DJ, Askew DJ, Pociask DA, Pociask DA, Reinhart TA, Reinhart TA, McAllister F, McAllister F, Edeal J, Edeal J, Gaus K, Gaus K, Husain S, Husain S, Kreindler JL, Kreindler JL, Dubin PJ, Dubin PJ, Pilewski JM, Pilewski JM, Myerburg MM, Myerburg MM, Mason CA, Mason CA, Iwakura Y, Iwakura Y, Kolls JK, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14: 275–281, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachert C, Gevaert P, van Cauwenberge P. Staphylococcus aureus enterotoxins: a key in airway disease? Allergy 57: 480–487, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Bachman MA, Lenio S, Schmidt L, Oyler JE, Weiser JN. Interaction of lipocalin 2, transferrin, and siderophores determines the replicative niche of Klebsiella pneumoniae during pneumonia. mBio 3: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachman MA, Oyler JE, Burns SH, Caza M, Lépine F, Dozois CM, Weiser JN. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect Immun 79: 3309–3316, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balamayooran G, Batra S, Theivanthiran B, Cai S, Pacher P, Jeyaseelan S. Intrapulmonary G-CSF rescues neutrophil recruitment to the lung and neutrophil release to blood in Gram-negative bacterial infection in MCP-1−/− mice. J Immunol 189: 5849–5859, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem 285: 7633–7644, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baraquet C, Harwood CS. Cyclic diguanosine monophosphate represses bacterial flagella synthesis by interacting with the Walker A motif of the enhancer-binding protein FleQ. Proc Natl Acad Sci USA 110: 18478–18483, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardoel BW, van der Ent S, Pel MJ, Tommassen J, Pieterse CM, van Kessel KP, van Strijp JA. Pseudomonas evades immune recognition of flagellin in both mammals and plants. PLoS Pathog 7: e1002206, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barletta KE, Cagnina RE, Burdick MD, Linden J, Mehrad B. Adenosine A(2B) receptor deficiency promotes host defenses against gram-negative bacterial pneumonia. Am J Respir Crit Care Med 186: 1044–1050, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartlett AH, Foster TJ, Hayashida A, Park PW. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J Infect Dis 198: 1529–1535, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batra S, Cai S, Balamayooran G, Jeyaseelan S. Intrapulmonary administration of leukotriene B4 augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. J Immunol 188: 3458–3468, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayles KW, Wesson CA, Liou LE, Fox LK, Bohach GA, Trumble WR. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun 66: 336–342, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker RE, Berube BJ, Sampedro GR, DeDent AC, Bubeck Wardenburg J. Tissue-specific patterning of host innate immune responses by Staphylococcus aureus alpha-toxin. J Innate Immun 6: 619–631, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker S, Frankel MB, Schneewind O, Missiakas D. Release of protein A from the cell wall of Staphylococcus aureus. Proc Natl Acad Sci USA 111: 1574–1579, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben Mohamed F, Garcia-Verdugo I, Medina M, Balloy V, Chignard M, Ramphal R, Touqui L. A crucial role of Flagellin in the induction of airway mucus production by Pseudomonas aeruginosa. PLoS One 7: e39888, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benson MA, Ohneck EA, Ryan C, Alonzo F 3rd, Smith H, Narechania A, Kolokotronis SO, Satola SW, Uhlemann AC, Sebra R, Deikus G, Shopsin B, Planet PJ, Torres VJ. Evolution of hypervirulence by a MRSA clone through acquisition of a transposable element. Mol Microbiol 93: 664–681, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7: 99–109, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berube BJ, Sampedro GR, Otto M, Bubeck Wardenburg J. The psmalpha locus regulates production of Staphylococcus aureus alpha-toxin during infection. Infect Immun 82: 3350–3358, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhan U, Ballinger MN, Zeng X, Newstead MJ, Cornicelli MD, Standiford TJ. Cooperative interactions between TLR4 and TLR9 regulate interleukin 23 and 17 production in a murine model of gram negative bacterial pneumonia. PLoS One 5: e9896, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bialek-Davenet S, Criscuolo A, Ailloud F, Passet V, Jones L, Delannoy-Vieillard AS, Garin B, Le Hello S, Arlet G, Nicolas-Chanoine MH, Decré D, Brisse S. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerging Infect Dis 20: 1812–1820, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bialek S, Lavigne JP, Chevalier J, Marcon E, Leflon-Guibout V, Davin A, Moreau R, Pagès JM, Nicolas-Chanoine M-H. Membrane efflux and influx modulate both multidrug resistance and virulence of Klebsiella pneumoniae in a Caenorhabditis elegans model. Antimicrob Agents Chemother 54: 4373–4378, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bierne H, Travier L, Mahlakoiv T, Tailleux L, Subtil A, Lebreton A, Paliwal A, Gicquel B, Staeheli P, Lecuit M, Cossart P. Activation of type III interferon genes by pathogenic bacteria in infected epithelial cells and mouse placenta. PLoS One 7: e39080, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjarnsholt T, Jensen PO, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Hoiby N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol 44: 547–558, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Bolen CR, Ding S, Robek MD, Kleinstein SH. Dynamic expression profiling of type I and type III interferon-stimulated hepatocytes reveals a stable hierarchy of gene expression. Hepatology 59: 1262–1272, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, Schlaeffer F, Sherf M. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 30: 972–976, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48: 1–12, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Braat H, de Jong EC, van den Brande JMH, Kapsenberg ML, Peppelenbosch MP, van Tol EAF, van Deventer SJH. Dichotomy between Lactobacillus rhamnosus and Klebsiella pneumoniae on dendritic cell phenotype and function. J Mol Med 82: 197–205, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 15: 1017–1025, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brehm MA, Jouvet N, Greiner DL, Shultz LD. Humanized mice for the study of infectious diseases. Curr Opin Immunol 25: 428–435, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Brisse S, Issenhuth-Jeanjean S, Grimont PAD. Molecular serotyping of Klebsiella species isolates by restriction of the amplified capsular antigen gene cluster. J Clin Microbiol 42: 3388–3398, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broquet A, Roquilly A, Jacqueline C, Potel G, Caillon J, Asehnoune K. Depletion of natural killer cells increases mice susceptibility in a Pseudomonas aeruginosa pneumonia model. Crit Care Med 42: e441–450, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Broug-Holub E, Broug-Holub E, Toews GB, Toews GB, van Iwaarden JF, van Iwaarden JF, Strieter RM, Strieter RM, Kunkel SL, Kunkel SL, Paine R, Paine R, Standiford TJ, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun 65: 1139–1146, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, Vazquez V, Etienne J, Lina G, Vandenesch F, Bowden MG. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect 15: 156–164, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13: 1405–1406, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 75: 1040–1044, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 205: 287–294, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bucior I, Pielage JF, Engel JN. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathogens 8: e1002616, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burlak C, Hammer CH, Robinson MA, Whitney AR, McGavin MJ, Kreiswirth BN, Deleo FR. Global analysis of community-associated methicillin-resistant Staphylococcus aureus exoproteins reveals molecules produced in vitro and during infection. Cell Microbiol 9: 1172–1190, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol 185: 6214–6225, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. NLRC4 inflammasome-mediated production of IL-1β modulates mucosal immunity in the lung against gram-negative bacterial infection. J Immunol 188: 5623–5635, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai S, Cai S, Batra S, Batra S, Shen L, Shen L, Wakamatsu N, Wakamatsu N, Jeyaseelan S, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol 183: 6629–6638, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campodonico VL, Gadjeva M, Paradis-Bleau C, Uluer A, Pier GB. Airway epithelial control of Pseudomonas aeruginosa infection in cystic fibrosis. Trends Mol Med 14: 120–133, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campos MA, Vargas MA, Regueiro V, Llompart CM, Albertí S, Bengoechea JA. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect Immun 72: 7107–7114, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cano V, Moranta D, Llobet-Brossa E, Bengoechea JA, Garmendia J. Klebsiella pneumoniae triggers a cytotoxic effect on airway epithelial cells. BMC Microbiol 9: 156, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carpenter JL. Klebsiella pulmonary infections: occurrence at one medical center and review. Rev Infect Dis 12: 672–682, 1990. [DOI] [PubMed] [Google Scholar]
- 61.Casson CN, Yu J, Reyes VM, Taschuk FO, Yadav A, Copenhaver AM, Nguyen HT, Collman RG, Shin S. Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc Natl Acad Sci USA 112: 6688–6693, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cattoir V, Narasimhan G, Skurnik D, Aschard H, Roux D, Ramphal R, Jyot J, Lory S. Transcriptional response of mucoid Pseudomonas aeruginosa to human respiratory mucus. mBio 3: e00410–00412, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Center for Disease Control. Antibiotic Resistance Threats in the United States. Washington, DC: CDC, 2013. [Google Scholar]
- 64.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7: 629–641, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan C, Burrows LL, Deber CM. Alginate as an auxiliary bacterial membrane: binding of membrane-active peptides by polysaccharides. J Peptide Res 65: 343–351, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Chan YR, Liu JS, Pociask DA, Zheng M, Mietzner TA, Berger T, Mak TW, Clifton MC, Strong RK, Ray P, Kolls JK. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol 182: 4947–4956, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chao LK, Lin CH, Chiu HW, Wong WT, Chiu HW, Tasi YL, Kuo YH, Chiu YC, Liu ML, Ho CL, Hua KF. Peroxyauraptenol inhibits inflammation and NLRP3 inflammasome activation by inhibiting reactive oxygen species generation and preserving mitochondrial integrity. J Agric Food Chem. In press. [DOI] [PubMed]
- 68.Chatterjee SS, Joo HS, Duong AC, Dieringer TD, Tan VY, Song Y, Fischer ER, Cheung GY, Li M, Otto M. Essential Staphylococcus aureus toxin export system. Nat Med 19: 364–367, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, Alcorn JF, Weaver CT, Kolls JK. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity 35: 997–1009, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng P, Liu T, Zhou WY, Zhuang Y, Peng LS, Zhang JY, Yin ZN, Mao XH, Guo G, Shi Y, Zou QM. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol 13: 38, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chien YW, Klugman KP, Morens DM. Bacterial pathogens and death during the 1918 influenza pandemic. N Engl J Med 361: 2582–2583, 2009. [DOI] [PubMed] [Google Scholar]
- 72.Choi SM, McAleer JP, Zheng M, Pociask DA, Kaplan MH, Qin S, Reinhart TA, Kolls JK. Innate Stat3-mediated induction of the antimicrobial protein Reg3gamma is required for host defense against MRSA pneumonia. J Exp Med 210: 551–561, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chotirmall SH, Smith SG, Gunaratnam C, Cosgrove S, Dimitrov BD, O'Neill SJ, Harvey BJ, Greene CM, McElvaney NG. Effect of estrogen on pseudomonas mucoidy and exacerbations in cystic fibrosis. N Engl J Med 366: 1978–1986, 2012. [DOI] [PubMed] [Google Scholar]
- 74.Chua SL, Tan SY, Rybtke MT, Chen Y, Rice SA, Kjelleberg S, Tolker-Nielsen T, Yang L, Givskov M. Bis-(3′-5′)-cyclic dimeric GMP regulates antimicrobial peptide resistance in Pseudomonas aeruginosa. Antimicrobial Agents Chemother 57: 2066–2075, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cigana C, Curcuru L, Leone MR, Ierano T, Lore NI, Bianconi I, Silipo A, Cozzolino F, Lanzetta R, Molinaro A, Bernardini ML, Bragonzi A. Pseudomonas aeruginosa exploits lipid A and muropeptides modification as a strategy to lower innate immunity during cystic fibrosis lung infection. PLoS One 4: e8439, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med 18: 509–519, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest 123: 1630–1637, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen TS, Prince AS. Bacterial pathogens activate a common inflammatory pathway through IFNlambda regulation of PDCD4. PLoS Pathogens 9: e1003682, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GC, Parsek MR. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathogens 7: e1001264, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colvin KM, Irie Y, Tart CS, Urbano R, Whitney JC, Ryder C, Howell PL, Wozniak DJ, Parsek MR. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ Microbiol 14: 1913–1928, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cortés G, Borrell N, de Astorza B, Gómez C, Sauleda J, Albertí S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun 70: 2583–2590, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4: e7446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology 128: 151–163, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Da Silva MC, Zahm JM, Gras D, Bajolet O, Abely M, Hinnrasky J, Milliot M, de Assis MC, Hologne C, Bonnet N, Merten M, Plotkowski MC, Puchelle E. Dynamic interaction between airway epithelial cells and Staphylococcus aureus. Am J Physiol Lung Cell Mol Physiol 287: L543–L551, 2004. [DOI] [PubMed] [Google Scholar]
- 85.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280: 295–298, 1998. [DOI] [PubMed] [Google Scholar]
- 86.Davies JC, Stern M, Dewar A, Caplen NJ, Munkonge FM, Pitt T, Sorgi F, Huang L, Bush A, Geddes DM, Alton EW. CFTR gene transfer reduces the binding of Pseudomonas aeruginosa to cystic fibrosis respiratory epithelium. Am J Respir Cell Mol Biol 16: 657–663, 1997. [DOI] [PubMed] [Google Scholar]
- 87.De Bentzmann S, Plotkowski C, Puchelle E. Receptors in the Pseudomonas aeruginosa adherence to injured and repairing airway epithelium. Am J Respir Crit Care Med 154: S155–162, 1996. [DOI] [PubMed] [Google Scholar]
- 88.De Bentzmann S, Roger P, Dupuit F, Bajolet-Laudinat O, Fuchey C, Plotkowski MC, Puchelle E. Asialo GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect Immun 64: 1582–1588, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ, Heezius EC, Poppelier MJ, Van Kessel KP, van Strijp JA. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199: 687–695, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Chem 282: 20053–20057, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Dekimpe V, Deziel E. Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: the transcriptional regulator RhlR regulates LasR-specific factors. Microbiology 155: 712–723, 2009. [DOI] [PubMed] [Google Scholar]
- 92.Deleo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, Porter AR, Chavda KD, Jacobs MR, Mathema B, Olsen RJ, Bonomo RA, Musser JM, Kreiswirth BN. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci USA 111: 4988–4993, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deleo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375: 1557–1568, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Demirel I, Kinnunen A, Onnberg A, Söderquist B, Persson K. Comparison of host response mechanisms evoked by extended spectrum beta lactamase (ESBL)–and non-ESBL-producing uropathogenic E. coli. BMC Microbiol 13: 181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deng JC, Moore TA, Newstead MW, Zeng X, Krieg AM, Standiford TJ. CpG oligodeoxynucleotides stimulate protective innate immunity against pulmonary Klebsiella infection. J Immunol 173: 5148–5155, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deng JC, Zeng X, Newstead M, Moore TA, Tsai WC, Thannickal VJ, Standiford TJ. STAT4 is a critical mediator of early innate immune responses against pulmonary Klebsiella infection. J Immunol 173: 4075–4083, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng Q, Barbieri JT. Modulation of host cell endocytosis by the type III cytotoxin, Pseudomonas ExoS. Traffic 9: 1948–1957, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci USA 95: 15623–15628, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Descamps D, Le Gars M, Balloy V, Barbier D, Maschalidi S, Tohme M, Chignard M, Ramphal R, Manoury B, Sallenave JM. Toll-like receptor 5 (TLR5), IL-1beta secretion, and asparagine endopeptidase are critical factors for alveolar macrophage phagocytosis and bacterial killing. Proc Natl Acad Sci USA 109: 1619–1624, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diago-Navarro E, Diago-Navarro E, Chen L, Chen L, Passet V, Passet V, Burack S, Burack S, Ulacia-Hernando A, Ulacia-Hernando A, Kodiyanplakkal RP, Kodiyanplakkal RP, Levi MH, Levi MH, Brisse S, Brisse S, Kreiswirth BN, Kreiswirth BN, Fries BC, Fries BC. Carbapenem-Resistant Klebsiella pneumoniae exhibit variability in capsular polysaccharide and capsule associated virulence traits. J Infect Dis 210: 803–813, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diaz MH, Shaver CM, King JD, Musunuri S, Kazzaz JA, Hauser AR. Pseudomonas aeruginosa induces localized immunosuppression during pneumonia. Infect Immun 76: 4414–4421, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dick AW, Perencevich EN, Pogorzelska-Maziarz M, Zwanziger J, Larson EL, Stone PW. A decade of investment in infection prevention: a cost-effectiveness analysis. Am J Infect Control 43: 4–9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci USA 107: 5587–5592, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367: 731–739, 2006. [DOI] [PubMed] [Google Scholar]
- 105.DiGiandomenico A, Keller AE, Gao C, Rainey GJ, Warrener P, Camara MM, Bonnell J, Fleming R, Bezabeh B, Dimasi N, Sellman BR, Hilliard J, Guenther CM, Datta V, Zhao W, Gao C, Yu XQ, Suzich JA, Stover CK. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Science Transl Med 6: 262ra155, 2014. [DOI] [PubMed] [Google Scholar]
- 106.Ding Y, Liu X, Chen F, Di H, Xu B, Zhou L, Deng X, Wu M, Yang CG, Lan L. Metabolic sensor governing bacterial virulence in Staphylococcus aureus. Proc Natl Acad Sci USA 111: E4981–4990, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13: 16–34, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, Shopsin B, Unutmaz D, Voyich JM, Torres VJ. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol 79: 814–825, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Durbin RK, Kotenko SV, Durbin JE. Interferon induction and function at the mucosal surface. Immunol Rev 255: 25–39, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 355: 2226–2235, 2006. [DOI] [PubMed] [Google Scholar]
- 111.Ernst RK, Moskowitz SM, Emerson JC, Kraig GM, Adams KN, Harvey MD, Ramsey B, Speert DP, Burns JL, Miller SI. Unique lipid a modifications in Pseudomonas aeruginosa isolated from the airways of patients with cystic fibrosis. J Infect Dis 196: 1088–1092, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ernst RK, Yi EC, Guo L, Lim KB, Burns JL, Hackett M, Miller SI. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286: 1561–1565, 1999. [DOI] [PubMed] [Google Scholar]
- 113.Evrard B, Balestrino D, Dosgilbert A, Bouya-Gachancard JLJ, Charbonnel N, Forestier C, Tridon A. Roles of capsule and lipopolysaccharide O antigen in interactions of human monocyte-derived dendritic cells and Klebsiella pneumoniae. Infect Immun 78: 210–219, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Falugi F, Kim HK, Missiakas DM, Schneewind O. Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. mBio 4: e00575–00513, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fang R, Hara H, Sakai S, Hernandez-Cuellar E, Mitsuyama M, Kawamura I, Tsuchiya K. Type I interferon signaling regulates activation of the absent in melanoma 2 inflammasome during Streptococcus pneumoniae infection. Infect Immun 82: 2310–2317, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Faure E, Mear JB, Faure K, Normand S, Couturier-Maillard A, Grandjean T, Balloy V, Ryffel B, Dessein R, Chignard M, Uyttenhove C, Guery B, Gosset P, Chamaillard M, Kipnis E. Pseudomonas aeruginosa type-3 secretion system dampens host defense by exploiting the NLRC4-coupled inflammasome. Am J Respir Crit Care Med 189: 799–811, 2014. [DOI] [PubMed] [Google Scholar]
- 117.Fechter P, Caldelari I, Lioliou E, Romby P. Novel aspects of RNA regulation in Staphylococcus aureus. FEBS Lett 588: 2523–2529, 2014. [DOI] [PubMed] [Google Scholar]
- 118.Ferry T, Thomas D, Genestier AL, Bes M, Lina G, Vandenesch F, Etienne J. Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin Infect Dis 41: 771–777, 2005. [DOI] [PubMed] [Google Scholar]
- 119.Finck-Barbancon V, Duportail G, Meunier O, Colin DA. Pore formation by a two-component leukocidin from Staphylococcus aureus within the membrane of human polymorphonuclear leukocytes. Biochim Biophys Acta 1182: 275–282, 1993. [DOI] [PubMed] [Google Scholar]
- 120.Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, Aderem A, Smith KD. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci USA 103: 16502–16507, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fito-Boncompte L, Chapalain A, Bouffartigues E, Chaker H, Lesouhaitier O, Gicquel G, Bazire A, Madi A, Connil N, Veron W, Taupin L, Toussaint B, Cornelis P, Wei Q, Shioya K, Deziel E, Feuilloley MG, Orange N, Dufour A, Chevalier S. Full virulence of Pseudomonas aeruginosa requires OprF. Infect Immun 79: 1176–1186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fleige H, Ravens S, Moschovakis GL, Bolter J, Willenzon S, Sutter G, Haussler S, Kalinke U, Prinz I, Forster R. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med 211: 643–651, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fodah RA, Scott JB, Tam HH, Yan P, Pfeffer TL, Bundschuh R, Warawa JM. Correlation of Klebsiella pneumoniae comparative genetic analyses with virulence profiles in a murine respiratory disease model. PLoS One 9: e107394, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Foletti D, Strop P, Shaughnessy L, Hasa-Moreno A, Casas MG, Russell M, Bee C, Wu S, Pham A, Zeng Z, Pons J, Rajpal A, Shelton D. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus alpha-hemolysin. J Mol Biol 425: 1641–1654, 2013. [DOI] [PubMed] [Google Scholar]
- 125.Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12: 49–62, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Foster TJ, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol 6: 484–488, 1998. [DOI] [PubMed] [Google Scholar]
- 127.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, Chan IS, McNeely TB, Kartsonis NA, Guris D, Onorato MT, Smugar SS, DiNubile MJ, Sobanjo-ter Meulen A. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309: 1368–1378, 2013. [DOI] [PubMed] [Google Scholar]
- 128.Franchi L, Kamada N, Nakamura Y, Burberry A, Kuffa P, Suzuki S, Shaw MH, Kim YG, Nunez G. NLRC4-driven production of IL-1beta discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nature Immunol 13: 449–456, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Frank CG, Reguerio V, Rother M, Moranta D, Maeurer AP, Garmendia J, Meyer TF, Bengoechea JA. Klebsiella pneumoniae targets an EGF receptor-dependent pathway to subvert inflammation. Cell Microbiol 15: 1212–1233, 2013. [DOI] [PubMed] [Google Scholar]
- 130.Fujiki M, Shinbori T, Suga M, Miyakawa H, Mizobe T, Ando M. Bacterial superantigen staphylococcal enterotoxin B induces interstitial pneumonia in SCID mice reconstituted with peripheral blood mononuclear cells from collagen vascular disease patients. Clin Immunol 96: 38–43, 2000. [DOI] [PubMed] [Google Scholar]
- 131.Fuxman Bass JI, Russo DM, Gabelloni ML, Geffner JR, Giordano M, Catalano M, Zorreguieta A, Trevani AS. Extracellular DNA: a major proinflammatory component of Pseudomonas aeruginosa biofilms. J Immunol 184: 6386–6395, 2010. [DOI] [PubMed] [Google Scholar]
- 132.Gangloff SC, Hijiya N, Haziot A, Goyert SM. Lipopolysaccharide structure influences the macrophage response via CD14-independent and CD14-dependent pathways. Clin Infect Dis 28: 491–496, 1999. [DOI] [PubMed] [Google Scholar]
- 133.Gardner JC, Noel JG, Nikolaidis NM, Karns R, Aronow BJ, Ogle CK, McCormack FX. G-CSF drives a posttraumatic immune program that protects the host from infection. J Immunol 192: 2405–2417, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garofalo A, Giai C, Lattar S, Gardella N, Mollerach M, Kahl BC, Becker K, Prince AS, Sordelli DO, Gomez MI. The length of the Staphylococcus aureus protein A polymorphic region regulates inflammation: impact on acute and chronic infection. J Infect Dis 206: 81–90, 2012. [DOI] [PubMed] [Google Scholar]
- 135.Gauduchon V, Werner S, Prevost G, Monteil H, Colin DA. Flow cytometric determination of Panton-Valentine leucocidin S component binding. Infect Immun 69: 2390–2395, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol 14: 546–558, 2014. [DOI] [PubMed] [Google Scholar]
- 137.Geisinger E, Isberg RR. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11: e1004691, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Genestier AL, Michallet MC, Prevost G, Bellot G, Chalabreysse L, Peyrol S, Thivolet F, Etienne J, Lina G, Vallette FM, Vandenesch F, Genestier L. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest 115: 3117–3127, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gerlach GF, Clegg S, Allen BL. Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol 171: 1262–1270, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Geurtsen J, Steeghs L, Hamstra HJ, Ten Hove J, de Haan A, Kuipers B, Tommassen J, van der Ley P. Expression of the lipopolysaccharide-modifying enzymes PagP and PagL modulates the endotoxic activity of Bordetella pertussis. Infect Immun 74: 5574–5585, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gill RJ. Treatment of Friedlander's pneumonia. Am J Med Sci 221: 5–9, 1951. [DOI] [PubMed] [Google Scholar]
- 142.Gladstone GP, Van Heyningen WE. Staphylococcal leucocidins. Br J Exp Pathol 38: 123–137, 1957. [PMC free article] [PubMed] [Google Scholar]
- 143.Gollwitzer ES, Marsland BJ. Microbiota abnormalities in inflammatory airway diseases: potential for therapy. Pharmacol Ther 141: 32–39, 2014. [DOI] [PubMed] [Google Scholar]
- 144.Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med 10: 842–848, 2004. [DOI] [PubMed] [Google Scholar]
- 145.Gomez MI, O'Seaghdha M, Magargee M, Foster TJ, Prince AS. Staphylococcus aureus protein A activates TNFR1 signaling through conserved IgG binding domains. J Biol Chem 281: 20190–20196, 2006. [DOI] [PubMed] [Google Scholar]
- 146.Gomez MI, Prince A. Airway epithelial cell signaling in response to bacterial pathogens. Pediatr Pulmonol 43: 11–19, 2008. [DOI] [PubMed] [Google Scholar]
- 147.Gomez MI, Seaghdha MO, Prince AS. Staphylococcus aureus protein A activates TACE through EGFR-dependent signaling. EMBO J 26: 701–709, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol 12: 125–135, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Greenberger MJ, Kunkel SL, Strieter RM, Lukacs NW, Bramson J, Gauldie J, Graham FL, Hitt M, Danforth JM, Standiford TJ. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol 157: 3006–3012, 1996. [PubMed] [Google Scholar]
- 150.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Laichalk LL, McGillicuddy DC, Standiford TJ. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia: J Infect Dis 173: 159–165, 1996. [DOI] [PubMed] [Google Scholar]
- 151.Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J Immunol 192: 4709–4717, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol 164: 3713–3722, 2000. [DOI] [PubMed] [Google Scholar]
- 153.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34: 213–223, 2011. [DOI] [PubMed] [Google Scholar]
- 154.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34: 213–223, 2011. [DOI] [PubMed] [Google Scholar]
- 155.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210: 1977–1992, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guina T, Purvine SO, Yi EC, Eng J, Goodlett DR, Aebersold R, Miller SI. Quantitative proteomic analysis indicates increased synthesis of a quinolone by Pseudomonas aeruginosa isolates from cystic fibrosis airways. Proc Natl Acad Sci USA 100: 2771–2776, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gupta K, Liao J, Petrova OE, Cherny KE, Sauer K. Elevated levels of the second messenger c-di-GMP contribute to antimicrobial resistance of Pseudomonas aeruginosa. Mol Microbiol 92: 488–506, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gupta S, Yan H, Wong LH, Ralph S, Krolewski J, Schindler C. The SH2 domains of Stat1 and Stat2 mediate multiple interactions in the transduction of IFN-alpha signals. EMBO J 15: 1075–1084, 1996. [PMC free article] [PubMed] [Google Scholar]
- 159.Hackstein H, Kranz S, Lippitsch A, Wachtendorf A, Kershaw O, Gruber AD, Michel G, Lohmeyer J, Bein G, Baal N, Herold S. Modulation of respiratory dendritic cells during Klebsiella pneumonia infection. Respir Res 14: 91, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nature Immunol 3: 354–359, 2002. [DOI] [PubMed] [Google Scholar]
- 161.Hakoda M, Hayashimoto S, Yamanaka H, Terai C, Kamatani N, Kashiwazaki S. Molecular basis for the interaction between human IgM and staphylococcal protein A. Clin Immunol Immunopathol 72: 394–401, 1994. [DOI] [PubMed] [Google Scholar]
- 162.Hao Y, Kuang Z, Xu Y, Walling BE, Lau GW. Pyocyanin-induced mucin production is associated with redox modification of FOXA2. Respir Res 14: 9921–9982, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med 202: 761–769, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 7: 654–665, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hazlett LD, Moon MM, Singh A, Berk RS, Rudner XL. Analysis of adhesion, piliation, protease production and ocular infectivity of several P. aeruginosa strains. Curr Eye Res 10: 351–362, 1991. [DOI] [PubMed] [Google Scholar]
- 166.Held TK, Adamczik C, Trautmann M, Cross AS. Effects of MICs and sub-MICs of antibiotics on production of capsular polysaccharide of Klebsiella pneumoniae. Antimicrob Agents Chemother 39: 1093–1096, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med 204: 987–994, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol 183: 5395–5401, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hienz SA, Schennings T, Heimdahl A, Flock JI. Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J Infect Dis 174: 83–88, 1996. [DOI] [PubMed] [Google Scholar]
- 170.Higgins J, Loughman A, van Kessel KP, van Strijp JA, Foster TJ. Clumping factor A of Staphylococcus aureus inhibits phagocytosis by human polymorphonuclear leucocytes. FEMS Microbiol Lett 258: 290–296, 2006. [DOI] [PubMed] [Google Scholar]
- 171.Hirche TO, Gaut JP, Heinecke JW, Belaaouaj A. Myeloperoxidase plays critical roles in killing Klebsiella pneumoniae and inactivating neutrophil elastase: effects on host defense. J Immunol 174: 1557–1565, 2005. [DOI] [PubMed] [Google Scholar]
- 172.Hoboth C, Hoffmann R, Eichner A, Henke C, Schmoldt S, Imhof A, Heesemann J, Hogardt M. Dynamics of adaptive microevolution of hypermutable Pseudomonas aeruginosa during chronic pulmonary infection in patients with cystic fibrosis. J Infect Dis 200: 118–130, 2009. [DOI] [PubMed] [Google Scholar]
- 173.Holzinger D, Gieldon L, Mysore V, Nippe N, Taxman DJ, Duncan JA, Broglie PM, Marketon K, Austermann J, Vogl T, Foell D, Niemann S, Peters G, Roth J, Loffler B. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J Leukoc Biol 92: 1069–1081, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458: 514–518, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Howell HA, Logan LK, Hauser AR. Type III secretion of ExoU is critical during early Pseudomonas aeruginosa pneumonia. mBio 4: e00032–00013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, Keller A, O'Day T, Du Q, Cheng L, Damschroder M, Robbie G, Suzich J, Stover CK, Sellman BR. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 58: 1108–1117, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol 135: 25–30, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hunter RC, Klepac-Ceraj V, Lorenzi MM, Grotzinger H, Martin TR, Newman DK. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am J Respir Cell Mol Biol 47: 738–745, 2012. [DOI] [PubMed] [Google Scholar]
- 179.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 14: 81–93, 2014. [DOI] [PubMed] [Google Scholar]
- 180.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg J. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med 17: 1310–1314, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Intile PJ, Diaz MR, Urbanowski ML, Wolfgang MC, Yahr TL. The AlgZR two-component system recalibrates the RsmAYZ posttranscriptional regulatory system to inhibit expression of the Pseudomonas aeruginosa type III secretion system. J Bacteriol 196: 357–366, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ioannidis I, Ye F, McNally B, Willette M, Flano E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J Virol 87: 3261–3270, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Irie Y, Borlee BR, O'Connor JR, Hill PJ, Harwood CS, Wozniak DJ, Parsek MR. Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 109: 20632–20636, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol 14: 36–49, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Iverson AR, Boyd KL, McAuley JL, Plano LR, Hart ME, McCullers JA. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis 203: 880–888, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jackson KD, Starkey M, Kremer S, Parsek MR, Wozniak DJ. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J Bacteriol 186: 4466–4475, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jain M, Ramirez D, Seshadri R, Cullina JF, Powers CA, Schulert GS, Bar-Meir M, Sullivan CL, McColley SA, Hauser AR. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J Clin Microbiol 42: 5229–5237, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jongerius I, von Kockritz-Blickwede M, Horsburgh MJ, Ruyken M, Nizet V, Rooijakkers SH. Staphylococcus aureus virulence is enhanced by secreted factors that block innate immune defenses. J Innate Immun 4: 301–311, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Josefsson E, Hartford O, O'Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis 184: 1572–1580, 2001. [DOI] [PubMed] [Google Scholar]
- 190.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nature Immunol 9: 361–368, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kahl BC, Mellmann A, Deiwick S, Peters G, Harmsen D. Variation of the polymorphic region X of the protein A gene during persistent airway infection of cystic fibrosis patients reflects two independent mechanisms of genetic change in Staphylococcus aureus. J Clin Microbiol 43: 502–505, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kang PJ, Hauser AR, Apodaca G, Fleiszig SM, Wiener-Kronish J, Mostov K, Engel JN. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol Microbiol 24: 1249–1262, 1997. [DOI] [PubMed] [Google Scholar]
- 193.Kapral FA, Shayegani MG. Intracellular survival of staphylococci. J Exp Med 110: 123–138, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Karaolis DKR, Newstead MW, Zeng X, Hyodo M, Hayakawa Y, Bhan U, Liang H, Standiford TJ. Cyclic di-GMP stimulates protective innate immunity in bacterial pneumonia. Infect Immun 75: 4942–4950, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kato K, Lillehoj EP, Kai H, Kim KC. MUC1 expression by human airway epithelial cells mediates Pseudomonas aeruginosa adhesion. Front Biosci 2: 68–77, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kato K, Lillehoj EP, Kim KC. MUC1 regulates epithelial inflammation and apoptosis by PolyI:C through inhibition of Toll/IL-1 receptor-domain-containing adapter-inducing IFN-beta (TRIF) recruitment to Toll-like receptor 3. Am J Respir Cell Mol Biol 51: 446–454, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kato K, Lillehoj EP, Park YS, Umehara T, Hoffman NE, Madesh M, Kim KC. Membrane-tethered MUC1 mucin is phosphorylated by epidermal growth factor receptor in airway epithelial cells and associates with TLR5 to inhibit recruitment of MyD88. J Immunol 188: 2014–2022, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kato N, Nakashima I, Ohta M, Naito S, Kojima T. Interferon and cytotoxic factor (cytotoxin) released in the blood of mice infected with Mycobacterium bovis BCG. I. Enhanced production of interferon and appearance of cytotoxin stimulated by capsular polysaccharide of Klebsiella pneumoniae or bacterial lipopolysaccharide. Microbiol Immunol 23: 383–394, 1979. [DOI] [PubMed] [Google Scholar]
- 199.Kato N, Nakashima I, Ota M. Interferon production in mice by the capsular polysaccharide of Klebsiella pneumoniae. Infect Immun 12: 1–6, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kawasaki K, Ernst RK, Miller SI. 3-O-deacylation of lipid A by PagL, a PhoP/PhoQ-regulated deacylase of Salmonella typhimurium, modulates signaling through Toll-like receptor 4. J Biol Chem 279: 20044–20048, 2004. [DOI] [PubMed] [Google Scholar]
- 201.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 205: 807–817, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kharazmi A, Eriksen HO, Doring G, Goldstein W, Hoiby N. Effect of Pseudomonas aeruginosa proteases on human leukocyte phagocytosis and bactericidal activity. Acta Pathol Microbiol Immunol Scand 94: 175–179, 1986. [DOI] [PubMed] [Google Scholar]
- 203.Kharazmi A, Hoiby N, Doring G, Valerius NH. Pseudomonas aeruginosa exoproteases inhibit human neutrophil chemiluminescence. Infect Immun 44: 587–591, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Khatua B, Bhattacharya K, Mandal C. Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec-9. J Leukoc Biol 91: 641–655, 2012. [DOI] [PubMed] [Google Scholar]
- 205.Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med 207: 1863–1870, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kim HK, Falugi F, Thomer L, Missiakas DM, Schneewind O. Protein A suppresses immune responses during Staphylococcus aureus bloodstream infection in guinea pigs. mBio 6: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim HK, Missiakas D, Schneewind O. Mouse models for infectious diseases caused by Staphylococcus aureus. J Immunol Methods 410: 88–99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kim YG, Udayanga KG, Totsuka N, Weinberg JB, Nunez G, Shibuya A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE2. Cell Host Microbe 15: 95–102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kim YJ, Jun YH, Kim YR, Park KG, Park YJ, Kang JY, Kim SI. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia; retrospective study of impact of combination antimicrobial therapy. BMC Infect Dis 14: 161, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog 11: e1004820, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kobayashi SD, Braughton KR, Palazzolo-Ballance AM, Kennedy AD, Sampaio E, Kristosturyan E, Whitney AR, Sturdevant DE, Dorward DW, Holland SM, Kreiswirth BN, Musser JM, DeLeo FR. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun 2: 560–575, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kong KF, Vuong C, Otto M. Staphylococcus quorum sensing in biofilm formation and infection. Int J Med Microbiol 296: 133–139, 2006. [DOI] [PubMed] [Google Scholar]
- 213.Koreen L, Ramaswamy SV, Graviss EA, Naidich S, Musser JM, Kreiswirth BN. Spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J Clin Microbiol 42: 792–799, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Krakauer T. Interleukin-8 production by human monocytic cells in response to staphylococcal exotoxins is direct and independent of interleukin-1 and tumor necrosis factor-alpha. J Infect Dis 178: 573–577, 1998. [DOI] [PubMed] [Google Scholar]
- 215.Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, Boulay F, Klebanoff SJ, van Kessel KA, van Strijp JA, Otto M, Peschel A. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7: 463–473, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 186: 1666–1674, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol 30: 16–34, 2011. [DOI] [PubMed] [Google Scholar]
- 218.Kung VL, Khare S, Stehlik C, Bacon EM, Hughes AJ, Hauser AR. An rhs gene of Pseudomonas aeruginosa encodes a virulence protein that activates the inflammasome. Proc Natl Acad Sci USA 109: 1275–1280, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kuo HH, Chan C, Burrows LL, Deber CM. Hydrophobic interactions in complexes of antimicrobial peptides with bacterial polysaccharides. Chem Biol Drug Design 69: 405–412, 2007. [DOI] [PubMed] [Google Scholar]
- 220.Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Hook M, Etienne J, Vandenesch F, Bowden MG. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315: 1130–1133, 2007. [DOI] [PubMed] [Google Scholar]
- 221.Lai YC, Peng HL, Chang HY. RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional level. J Bacteriol 185: 788–800, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lau GW, Hassett DJ, Britigan BE. Modulation of lung epithelial functions by Pseudomonas aeruginosa. Trends Microbiol 13: 389–397, 2005. [DOI] [PubMed] [Google Scholar]
- 223.Lau GW, Ran H, Kong F, Hassett DJ, Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect Immun 72: 4275–4278, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lavender H, Jagnow JJ, Clegg S. Klebsiella pneumoniae type 3 fimbria-mediated immunity to infection in the murine model of respiratory disease. Int J Med Microbiol 295: 153–159, 2005. [DOI] [PubMed] [Google Scholar]
- 225.Lavigne JP, Cuzon G, Combescure C, Bourg G, Sotto A, Nordmann P. Virulence of Klebsiella pneumoniae isolates harboring blaKPC-2 carbapenemase Ggene in a Caenorhabditis elegans model. PLoS One 8: e67847, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lawlor MS, Hsu J, Rick PD, Miller VL. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol Microbiol 58: 1054–1073, 2005. [DOI] [PubMed] [Google Scholar]
- 227.Lee CH, Chang CC, Liu JW, Chen RF, Yang KD. Sialic acid involved in hypermucoviscosity phenotype of Klebsiella pneumoniae and associated with resistance to neutrophil phagocytosis. Virulence 5: 673–679, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee MH, Arrecubieta C, Martin FJ, Prince A, Borczuk AC, Lowy FD. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis 201: 508–515, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med 343: 1703–1714, 2000. [DOI] [PubMed] [Google Scholar]
- 230.Lerner A, Adler A, Abu-Hanna J, Cohen Percia S, Kazma Matalon M, Carmeli Y. Spread of KPC-producing carbapenem-resistant Enterobacteriaceae: the importance of super-spreaders and rectal KPC concentration. Clin Microbiol Infect 21: 470.e1–7, 2015. [DOI] [PubMed] [Google Scholar]
- 231.Levy DE, Marie IJ, Durbin JE. Induction and function of type I and III interferon in response to viral infection. Curr Opin Virol 1: 476–486, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Annu Rev Immunol 17: 435–466, 1999. [DOI] [PubMed] [Google Scholar]
- 233.Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, Otto M. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis 202: 1866–1876, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci USA 106: 5883–5888, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li W, Yan F, Zhou H, Lin X, Wu Y, Chen C, Zhou N, Chen Z, Li JD, Shen H. P. aeruginosa lipopolysaccharide-induced MUC5AC and CLCA3 expression is partly through Duox1 in vitro and in vivo. PLoS One 8: e63945, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li Y, Li Zj Han Wy Lei Lc Sun Cj Feng X, Du CT, Du TF, Gu JM. Identification and characterization of Th cell epitopes in MrkD adhesin of Klebsiella pneumoniae. Microb Pathog 49: 8–13, 2010. [DOI] [PubMed] [Google Scholar]
- 237.Lin HA, Huang YL, Yeh KM, Siu LK, Lin JC, Chang FY. Regulator of the mucoid phenotype A gene increases the virulent ability of extended-spectrum beta-lactamase-producing serotype non-K1/K2 Klebsiella pneumonia. J Microbiol Immunol Infect 2014. [DOI] [PubMed]
- 238.Liu YM, Li BB, Zhang YY, Zhang W, Shen H, Li H, Cao B. Clinical and molecular characteristics of emerging hypervirulent Klebsiella pneumoniae bloodstream infections in mainland China. Antimicrob Agents Chemother 58: 5379–5385, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 6: e1000715, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Look DC, Stoll LL, Romig SA, Humlicek A, Britigan BE, Denning GM. Pyocyanin and its precursor phenazine-1-carboxylic acid increase IL-8 and intercellular adhesion molecule-1 expression in human airway epithelial cells by oxidant-dependent mechanisms. J Immunol 175: 4017–4023, 2005. [DOI] [PubMed] [Google Scholar]
- 241.Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, Chen W, Cross AS, Gendler SJ, Gewirtz AT, Kim KC. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol 176: 3890–3894, 2006. [DOI] [PubMed] [Google Scholar]
- 242.Luo L, Zhang S, Wang Y, Rahman M, Syk I, Zhang E, Thorlacius H. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am J Physiol Lung Cell Mol Physiol 307: L586–L596, 2014. [DOI] [PubMed] [Google Scholar]
- 243.Ma L, Conover M, Lu H, Parsek MR, Bayles K, Wozniak DJ. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathogens 5: e1000354, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ma X, Chang W, Zhang C, Zhou X, Yu F. Staphylococcal Panton-Valentine leukocidin induces pro-inflammatory cytokine production and nuclear factor-kappa B activation in neutrophils. PLoS One 7: e34970, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mahenthiralingam E, Campbell ME, Speert DP. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun 62: 596–605, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mahenthiralingam E, Speert DP. Nonopsonic phagocytosis of Pseudomonas aeruginosa by macrophages and polymorphonuclear leukocytes requires the presence of the bacterial flagellum. Infect Immun 63: 4519–4523, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Maher BM, Mulcahy ME, Murphy AG, Wilk M, O'Keeffe KM, Geoghegan JA, Lavelle EC, McLoughlin RM. Nlrp-3-driven interleukin 17 production by gammadeltaT cells controls infection outcomes during Staphylococcus aureus surgical site infection. Infect Immun 81: 4478–4489, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mai GT, Seow WK, Pier GB, McCormack JG, Thong YH. Suppression of lymphocyte and neutrophil functions by Pseudomonas aeruginosa mucoid exopolysaccharide (alginate): reversal by physicochemical, alginase, and specific monoclonal antibody treatments. Infect Immun 61: 559–564, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, Deleo FR. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis 206: 1185–1193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Malachowa N, Kobayashi SD, Freedman B, Dorward DW, DeLeo FR. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J Immunol 191: 6022–6029, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, Rice CM. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 131: 1887–1898, 2006. [DOI] [PubMed] [Google Scholar]
- 252.March C, Cano V, Moranta D, Llobet E, Pérez-Gutiérrez C, Tomás JM, Suárez T, Garmendia J, Bengoechea JA. Role of bacterial surface structures on the interaction of Klebsiella pneumoniae with phagocytes. PLoS One 8: e56847, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science 248: 705–711, 1990. [DOI] [PubMed] [Google Scholar]
- 254.Martin DW, Schurr MJ, Mudd MH, Govan JR, Holloway BW, Deretic V. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc Natl Acad Sci USA 90: 8377–8381, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Martin FJ, Gomez MI, Wetzel DM, Memmi G, O'Seaghdha M, Soong G, Schindler C, Prince A. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J Clin Invest 119: 1931–1939, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Martin FJ, Parker D, Harfenist BS, Soong G, Prince A. Participation of CD11c(+) leukocytes in methicillin-resistant Staphylococcus aureus clearance from the lung. Infect Immun 79: 1898–1904, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Martin WJ, Marcus S. Relation of pyrogenic and emetic properties of enterobacteriaceal endotoxin and of staphylococcal enterotoxin. J Bacteriol 87: 1019–1026, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6: 13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mason RJ. Biology of alveolar type II cells. Respirology 11 Suppl: S12–15, 2006. [DOI] [PubMed] [Google Scholar]
- 260.Matsukawa M, Greenberg EP. Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J Bacteriol 186: 4449–4456, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mazmanian SK, Liu G, Ton-That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285: 760–763, 1999. [DOI] [PubMed] [Google Scholar]
- 262.McElroy MC, Cain DJ, Tyrrell C, Foster TJ, Haslett C. Increased virulence of a fibronectin-binding protein mutant of Staphylococcus aureus in a rat model of pneumonia. Infect Immun 70: 3865–3873, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.McElroy MC, Harty HR, Hosford GE, Boylan GM, Pittet JF, Foster TJ. Alpha-toxin damages the air-blood barrier of the lung in a rat model of Staphylococcus aureus-induced pneumonia. Infect Immun 67: 5541–5544, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.McLaughlin MM, Advincula MR, Malczynski M, Barajas G, Qi C, Scheetz MH. Quantifying the clinical virulence of Klebsiella pneumoniae producing carbapenemase Klebsiella pneumoniae with a Galleria mellonella model and a pilot study to translate to patient outcomes. BMC Infect Dis 14: 31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mear JB, Gosset P, Kipnis E, Faure E, Dessein R, Jawhara S, Fradin C, Faure K, Poulain D, Sendid B, Guery B. Candida albicans airway exposure primes the lung innate immune response against Pseudomonas aeruginosa infection through innate lymphoid cell recruitment and interleukin-22-associated mucosal response. Infect Immun 82: 306–315, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Melly MA, Thomison JB, Rogers DE. Fate of staphylococci within human leukocytes. J Exp Med 112: 1121–1130, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA 107: 3076–3080, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mijares LA, Wangdi T, Sokol C, Homer R, Medzhitov R, Kazmierczak BI. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J Immunol 186: 7080–7088, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Miles G, Movileanu L, Bayley H. Subunit composition of a bicomponent toxin: staphylococcal leukocidin forms an octameric transmembrane pore. Protein Sci 11: 894–902, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O'Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol 179: 6933–6942, 2007. [DOI] [PubMed] [Google Scholar]
- 271.Moks T, Abrahmsen L, Nilsson B, Hellman U, Sjoquist J, Uhlen M. Staphylococcal protein A consists of five IgG-binding domains. Eur J Biochem 156: 637–643, 1986. [DOI] [PubMed] [Google Scholar]
- 272.Mollick JA, McMasters RL, Grossman D, Rich RR. Localization of a site on bacterial superantigens that determines T cell receptor beta chain specificity. J Exp Med 177: 283–293, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mongodin E, Bajolet O, Cutrona J, Bonnet N, Dupuit F, Puchelle E, de Bentzmann S. Fibronectin-binding proteins of Staphylococcus aureus are involved in adherence to human airway epithelium. Infect Immun 70: 620–630, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Montgomery CP, Boyle-Vavra S, Adem PV, Lee JC, Husain AN, Clasen J, Daum RS. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis 198: 561–570, 2008. [DOI] [PubMed] [Google Scholar]
- 275.Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol 165: 2643–2650, 2000. [DOI] [PubMed] [Google Scholar]
- 276.Moore TA, Moore TA, Perry ML, Perry ML, Getsoian AG, Getsoian AG, Newstead MW, Newstead MW, Standiford TJ, Standiford TJ. Divergent role of gamma interferon in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infect Immun 70: 6310–6318, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Moore TA, Perry ML, Getsoian AG, Monteleon CL, Cogen AL, Standiford TJ. Increased mortality and dysregulated cytokine production in tumor necrosis factor receptor 1-deficient mice following systemic Klebsiella pneumoniae infection. Infect Immun 71: 4891–4900, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Moranta D, Moranta D, Regueiro V, Regueiro V, March C, March C, Llobet E, Llobet E, Margareto J, Margareto J, Larrarte E, Larrarte E, Larrate E, Larrate E, Garmendia J, Garmendia J, Bengoechea JA, Bengoechea JA. Klebsiella pneumoniae capsule polysaccharide impedes the expression of beta-defensins by airway epithelial cells. Infect Immun 78: 1135–1146, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Moreillon P, Entenza JM, Francioli P, McDevitt D, Foster TJ, Francois P, Vaudaux P. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun 63: 4738–4743, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 198: 962–970, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Morfeldt E, Taylor D, von Gabain A, Arvidson S. Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J 14: 4569–4577, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Morris AE, Liggitt HD, Hawn TR, Skerrett SJ. Role of Toll-like receptor 5 in the innate immune response to acute P. aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol 297: L1112–L1119, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 30: 777–783, 2004. [DOI] [PubMed] [Google Scholar]
- 284.Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 30: 777–783, 2004. [DOI] [PubMed] [Google Scholar]
- 285.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol 183: 3942–3948, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13: 785–796, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Muramatsu S, Tamada T, Nara M, Murakami K, Kikuchi T, Kanehira M, Maruyama Y, Ebina M, Nukiwa T, Ichinose M. Flagellin/TLR5 signaling potentiates airway serous secretion from swine tracheal submucosal glands. Am J Physiol Lung Cell Mol Physiol 305: L819–L830, 2013. [DOI] [PubMed] [Google Scholar]
- 288.Murphy CN, Clegg S. Klebsiella pneumoniae and type 3 fimbriae: nosocomial infection, regulation and biofilm formation. Future Microbiol 7: 991–1002, 2012. [DOI] [PubMed] [Google Scholar]
- 289.Nair GB, Niederman MS. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med 41: 34–48, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Nemoto K, Hirota K, Murakami K, Taniguti K, Murata H, Viducic D, Miyake Y. Effect of Varidase (streptodornase) on biofilm formed by Pseudomonas aeruginosa. Chemotherapy 49: 121–125, 2003. [DOI] [PubMed] [Google Scholar]
- 291.Neumann B, Engelhardt B, Wagner H, Holzmann B. Induction of acute inflammatory lung injury by staphylococcal enterotoxin B. J Immunol 158: 1862–1871, 1997. [PubMed] [Google Scholar]
- 292.Nivens DE, Ohman DE, Williams J, Franklin MJ. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J Bacteriol 183: 1047–1057, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.O'Brien LM, Walsh EJ, Massey RC, Peacock SJ, Foster TJ. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell Microbiol 4: 759–770, 2002. [DOI] [PubMed] [Google Scholar]
- 294.O'Callaghan J, Reen FJ, Adams C, Casey PG, Gahan CG, O'Gara F. A novel host-responsive sensor mediates virulence and type III secretion during Pseudomonas aeruginosa-host cell interactions. Microbiology 158: 1057–1070, 2012. [DOI] [PubMed] [Google Scholar]
- 295.Obermayer A, Stoiber W, Krautgartner WD, Klappacher M, Kofler B, Steinbacher P, Vitkov L, Grabcanovic-Musija F, Studnicka M. New aspects on the structure of neutrophil extracellular traps from chronic obstructive pulmonary disease and in vitro generation. PLoS One 9: e97784, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ohama H, Asai A, Ito I, Suzuki S, Kobayashi M, Higuchi K, Suzuki F. M2b macrophage elimination and improved resistance of mice with chronic alcohol consumption to opportunistic infections. Am J Pathol 185: 420–431, 2015. [DOI] [PubMed] [Google Scholar]
- 297.Ong CLY, Beatson SA, Totsika M, Forestier C, McEwan AG, Schembri MA. Molecular analysis of type 3 fimbrial genes from Escherichia coli, Klebsiella and Citrobacter species. BMC Microbiol 10: 183, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Osterlund PI, Pietila TE, Veckman V, Kotenko SV, Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol 179: 3434–3442, 2007. [DOI] [PubMed] [Google Scholar]
- 300.Ozer EA, Allen JP, Hauser AR. Characterization of the core and accessory genomes of Pseudomonas aeruginosa using bioinformatic tools Spine and AGEnt. BMC Genomics 15: 737, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Palmqvist N, Patti JM, Tarkowski A, Josefsson E. Expression of staphylococcal clumping factor A impedes macrophage phagocytosis. Microbes Infect 6: 188–195, 2004. [DOI] [PubMed] [Google Scholar]
- 302.Pan YJ, Lin TL, Hsu CR, Wang JT. Use of a Dictyostelium model for isolation of genetic loci associated with phagocytosis and virulence in Klebsiella pneumoniae. Infect Immun 79: 997–1006, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191: 677–691, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Park YS, Lillehoj EP, Kato K, Park CS, Kim KC. PPARgamma inhibits airway epithelial cell inflammatory response through a MUC1-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 302: L679–L687, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Parker D, Cohen TS, Alhede M, Harfenist BS, Martin FJ, Prince A. Induction of type I interferon signaling by Pseudomonas aeruginosa is diminished in cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 46: 6–13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Parker D, Martin FJ, Soong G, Harfenist BS, Aguilar JL, Ratner AJ, Fitzgerald KA, Schindler C, Prince A. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. mBio 2: e00016–00011, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Parker D, Planet PJ, Soong G, Narechania A, Prince A. Induction of type I interferon signaling determines the relative pathogenicity of Staphylococcus aureus strains. PLoS Pathog 10: e1003951, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 45: 189–201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Parker D, Prince A. Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9. J Immunol 189: 4040–4046, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Parker D, Prince A. Type I interferon response to extracellular bacteria in the airway epithelium. Trends Immunol 32: 582–588, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Parker D, Ryan CL, Alonzo F, Torres VJ 3rd, Planet PJ, Prince AS. CD4+ T cells promote the pathogenesis of Staphylococcus aureus pneumonia. J Infect Dis 211: 835–845, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Parks QM, Young RL, Poch KR, Malcolm KC, Vasil ML, Nick JA. Neutrophil enhancement of Pseudomonas aeruginosa biofilm development: human F-actin and DNA as targets for therapy. J Med Microbiol 58: 492–502, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Passador L, Cook JM, Gambello MJ, Rust L, Iglewski BH. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260: 1127–1130, 1993. [DOI] [PubMed] [Google Scholar]
- 314.Pauli NT, Kim HK, Falugi F, Huang M, Dulac J, Henry Dunand C, Zheng NY, Kaur K, Andrews SF, Huang Y, DeDent A, Frank KM, Charnot-Katsikas A, Schneewind O, Wilson PC. Staphylococcus aureus infection induces protein A-mediated immune evasion in humans. J Exp Med 211: 2331–2339, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, Greenberg EP. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA 91: 197–201, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Pedersen SS. Lung infection with alginate-producing, mucoid Pseudomonas aeruginosa in cystic fibrosis. APMIS Suppl 28: 1–79, 1992. [PubMed] [Google Scholar]
- 317.Pedersen SS, Kharazmi A, Espersen F, Hoiby N. Pseudomonas aeruginosa alginate in cystic fibrosis sputum and the inflammatory response. Infect Immun 58: 3363–3368, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Perret M, Badiou C, Lina G, Burbaud S, Benito Y, Bes M, Cottin V, Couzon F, Juruj C, Dauwalder O, Goutagny N, Diep BA, Vandenesch F, Henry T. Cross-talk between Staphylococcus aureus leukocidins-intoxicated macrophages and lung epithelial cells triggers chemokine secretion in an inflammasome-dependent manner. Cell Microbiol 14: 1019–1036, 2012. [DOI] [PubMed] [Google Scholar]
- 319.Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Banfi B, Horswill AR, Stoltz DA, McCray PB Jr, Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487: 109–113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Phillips JR, Tripp TJ, Regelmann WE, Schlievert PM, Wangensteen OD. Staphylococcal alpha-toxin causes increased tracheal epithelial permeability. Pediatr Pulmonol 41: 1146–1152, 2006. [DOI] [PubMed] [Google Scholar]
- 321.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clin Microbiol Rev 24: 490–497, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Planet PJ, LaRussa SJ, Dana A, Smith H, Xu A, Ryan C, Uhlemann AC, Boundy S, Goldberg J, Narechania A, Kulkarni R, Ratner AJ, Geoghegan JA, Kolokotronis SO, Prince A. Emergence of the epidemic methicillin-resistant Staphylococcus aureus strain USA300 coincides with horizontal transfer of the arginine catabolic mobile element and speG-mediated adaptations for survival on skin. mBio 4: e00889–00813, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn JF. IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol 182: 1286–1296, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Podschun R, Ullmann U. Klebsiella spp as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11: 589–603, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Poe SL, Poe SL, Arora M, Arora M, Oriss TB, Oriss TB, Yarlagadda M, Yarlagadda M, Isse K, Isse K, Khare A, Khare A, Levy DE, Levy DE, Lee JS, Lee JS, Mallampalli RK, Mallampalli RK, Chan YR, Chan YR, Ray A, Ray A, Ray P, Ray P. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol 6: 189–199, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Postma B, Poppelier MJ, van Galen JC, Prossnitz ER, van Strijp JA, de Haas CJ, van Kessel KP. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J Immunol 172: 6994–7001, 2004. [DOI] [PubMed] [Google Scholar]
- 327.Prevost G, Cribier B, Couppie P, Petiau P, Supersac G, Finck-Barbancon V, Monteil H, Piemont Y. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect Immun 63: 4121–4129, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Priebe GP, Goldberg JB. Vaccines for Pseudomonas aeruginosa: a long and winding road. Expert Rev Vaccines 13: 507–519, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Quartin AA, Scerpella EG, Puttagunta S, Kett DH. A comparison of microbiology and demographics among patients with healthcare-associated, hospital-acquired, and ventilator-associated pneumonia: a retrospective analysis of 1184 patients from a large, international study. BMC Infect Dis 13: 561, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell 32: 150–158, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Queck SY, Khan BA, Wang R, Bach TH, Kretschmer D, Chen L, Kreiswirth BN, Peschel A, Deleo FR, Otto M. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog 5: e1000533, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol 38: 699–706, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Rada B, Gardina P, Myers TG, Leto TL. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol 4: 158–171, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol 181: 4883–4893, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Rada B, Leto TL. Pyocyanin effects on respiratory epithelium: relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol 21: 73–81, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun 77: 2712–2718, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ragle BE, Karginov VA, Bubeck Wardenburg J. Prevention and treatment of Staphylococcus aureus pneumonia with a beta-cyclodextrin derivative. Antimicrob Agents Chemother 54: 298–304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rajagopalan G, Sen MM, Singh M, Murali NS, Nath KA, Iijima K, Kita H, Leontovich AA, Gopinathan U, Patel R, David CS. Intranasal exposure to staphylococcal enterotoxin B elicits an acute systemic inflammatory response. Shock 25: 647–656, 2006. [DOI] [PubMed] [Google Scholar]
- 339.Ramachandran G, Tulapurkar ME, Harris KM, Arad G, Shirvan A, Shemesh R, Detolla LJ, Benazzi C, Opal SM, Kaempfer R, Cross AS. A peptide antagonist of CD28 signaling attenuates toxic shock and necrotizing soft-tissue infection induced by Streptococcus pyogenes. J Infect Dis 207: 1869–1877, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ramos PIP, Picão RC, Almeida LGPd Lima NCB, Girardello R, Vivan ACP, Xavier DE, Barcellos FG, Pelisson M, Vespero EC, Médigue C, Vasconcelos ATRd Gales AC, Nicolás MF. Comparative analysis of the complete genome of KPC-2-producing Klebsiella pneumoniae Kp13 reveals remarkable genome plasticity and a wide repertoire of virulence and resistance mechanisms. BMC G 15: 54, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rangel SM, Logan LK, Hauser AR. The ADP-ribosyltransferase domain of the effector protein ExoS inhibits phagocytosis of Pseudomonas aeruginosa during pneumonia. mBio 5: e01080–01014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Recinos DA, Sekedat MD, Hernandez A, Cohen TS, Sakhtah H, Prince AS, Price-Whelan A, Dietrich LE. Redundant phenazine operons in Pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. Proc Natl Acad Sci USA 109: 19420–19425, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Regueiro V, Moranta D, Campos MA, Margareto J, Garmendia J, Bengoechea JA. Klebsiella pneumoniae increases the levels of Toll-like receptors 2 and 4 in human airway epithelial cells. Infect Immun 77: 714–724, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Regueiro V, Regueiro V, Moranta D, Moranta D, Frank CG, Frank CG, Larrarte E, Larrarte E, Margareto J, Margareto J, March C, March C, Garmendia J, Garmendia J, Bengoechea JA, Bengoechea JA. Klebsiella pneumoniae subverts the activation of inflammatory responses in a NOD1-dependent manner. Cell Microbiol 13: 135–153, 2011. [DOI] [PubMed] [Google Scholar]
- 345.Reyes-Robles T, Alonzo F, Kozhaya L 3rd, Lacy DB, Unutmaz D, Torres VJ. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 14: 453–459, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Rhem MN, Lech EM, Patti JM, McDevitt D, Hook M, Jones DB, Wilhelmus KR. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect Immun 68: 3776–3779, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Robert D, Quillon JP, Ivanoff B, Beaudry Y, Fontanges R, Normier G, Pinel AM, D'hinterland LD. Role of interferon in mice in protection against influenza A virus by bacterial ribosomes together with membranal glycoproteins of Klebsiella pneumoniae as adjuvant. Infect Immun 26: 515–519, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Robertson CM, Perrone EE, McConnell KW, Dunne WM, Boody B, Brahmbhatt T, Diacovo MJ, Van Rooijen N, Hogue LA, Cannon CL, Buchman TG, Hotchkiss RS, Coopersmith CM. Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance. J Surg Res 150: 278–285, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Robinson KM, McHugh KJ, Mandalapu S, Clay ME, Lee B, Scheller EV, Enelow RI, Chan YR, Kolls JK, Alcorn JF. Influenza A virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production. J Infect Dis 209: 865–875, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Rodriguez-Rojas A, Oliver A, Blazquez J. Intrinsic and environmental mutagenesis drive diversification and persistence of Pseudomonas aeruginosa in chronic lung infections. J Infect Dis 205: 121–127, 2012. [DOI] [PubMed] [Google Scholar]
- 351.Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJ, van Kessel KP, van Strijp JA. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol 6: 920–927, 2005. [DOI] [PubMed] [Google Scholar]
- 352.Rose F, Dahlem G, Guthmann B, Grimminger F, Maus U, Hanze J, Duemmer N, Grandel U, Seeger W, Ghofrani HA. Mediator generation and signaling events in alveolar epithelial cells attacked by S. aureus alpha-toxin. Am J Physiol Lung Cell Mol Physiol 282: L207–L214, 2002. [DOI] [PubMed] [Google Scholar]
- 353.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9: 313–323, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Rowe DC, McGettrick AF, Latz E, Monks BG, Gay NJ, Yamamoto M, Akira S, O'Neill LA, Fitzgerald KA, Golenbock DT. The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci USA 103: 6299–6304, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Russo TA, Olson R, Macdonald U, Metzger D, Maltese LM, Drake EJ, Gulick AM. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun 82: 2356–2367, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Saba S, Soong G, Greenberg S, Prince A. Bacterial stimulation of epithelial G-CSF and GM-CSF expression promotes PMN survival in CF airways. Am J Respir Cell Mol Biol 27: 561–567, 2002. [DOI] [PubMed] [Google Scholar]
- 357.Sachse F, Becker K, von Eiff C, Metze D, Rudack C. Staphylococcus aureus invades the epithelium in nasal polyposis and induces IL-6 in nasal epithelial cells in vitro. Allergy 65: 1430–1437, 2010. [DOI] [PubMed] [Google Scholar]
- 358.Saha B, Harlan DM, Lee KP, June CH, Abe R. Protection against lethal toxic shock by targeted disruption of the CD28 gene. J Exp Med 183: 2675–2680, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Sahly H, Aucken H, Benedi VJ, Forestier C, Fussing V, Hansen DS, Ofek I, Podschun R, Sirot D, Sandvang D, Tomás JM, Ullmann U. Impairment of respiratory burst in polymorphonuclear leukocytes by extended-spectrum beta-lactamase-producing strains of Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis 23: 20–26, 2004. [DOI] [PubMed] [Google Scholar]
- 360.Sahly H, Schubert S, Harder J, Kleine M, Sandvang D, Ullmann U, Schröder JM, Podschun R. Activity of human beta-defensins 2 and 3 against ESBL-producing Klebsiella strains. J Antimicrob Chemother 57: 562–565, 2006. [DOI] [PubMed] [Google Scholar]
- 361.Saiman L, Ishimoto K, Lory S, Prince A. The effect of piliation and exoproduct expression on the adherence of Pseudomonas aeruginosa to respiratory epithelial monolayers. J Infect Dis 161: 541–548, 1990. [DOI] [PubMed] [Google Scholar]
- 362.Saiman L, Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Invest 92: 1875–1880, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Sajjan US, Corey M, Karmali MA, Forstner JF. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest 89: 648–656, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Samarajiwa SA, Forster S, Auchettl K, Hertzog PJ. INTERFEROME: the database of interferon regulated genes. Nucleic Acids Res 37: D852–857, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Sanchez GV, Sanchez GV, Master RN, Master RN, Clark RB, Clark RB, Fyyaz M, Fyyaz M, Duvvuri P, Duvvuri P, Ekta G, Ekta G, Bordon J, Bordon J. Klebsiella pneumoniae antimicrobial drug resistance, United States, 1998–2010. Emerging Infect Dis 19: 133–136, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Sandiumenge A, Rello J. Ventilator-associated pneumonia caused by ESKAPE organisms: cause, clinical features, and management. Curr Opin Pulm Med 18: 187–193, 2012. [DOI] [PubMed] [Google Scholar]
- 367.Sanford BA, Thomas VL, Ramsay MA, Jones TO. Characterization of clinical strains of Staphylococcus aureus associated with pneumonia. J Clin Microbiol 24: 131–136, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Sato H, Frank DW. Intoxication of host cells by the T3SS phospholipase ExoU: PI(4,5)P2-associated, cytoskeletal collapse and late phase membrane blebbing. PLoS One 9: e103127, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Sawa T, Shimizu M, Moriyama K, Wiener-Kronish JP. Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review. Crit Care 18: 668, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sawa T, Yahr TL, Ohara M, Kurahashi K, Gropper MA, Wiener-Kronish JP, Frank DW. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med 5: 392–398, 1999. [DOI] [PubMed] [Google Scholar]
- 371.Schaber JA, Carty NL, McDonald NA, Graham ED, Cheluvappa R, Griswold JA, Hamood AN. Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa. J Med Microbiol 53: 841–853, 2004. [DOI] [PubMed] [Google Scholar]
- 372.Schaffer AC, Solinga RM, Cocchiaro J, Portoles M, Kiser KB, Risley A, Randall SM, Valtulina V, Speziale P, Walsh E, Foster T, Lee JC. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect Immun 74: 2145–2153, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Schiotz PO, Nielsen H, Hoiby N, Glikmann G, Svehag SE. Immune complexes in the sputum of patients with cystic fibrosis suffering from chronic Pseudomonas aeruginosa lung infection. Acta Pathol Microbiol Scand 86: 37–40, 1978. [DOI] [PubMed] [Google Scholar]
- 374.Schmeling DJ, Peterson PK, Hammerschmidt DE, Kim Y, Verhoef J, Wilkinson BJ, Quie PG. Chemotaxigenesis by cell surface components of Staphylococcus aureus. Infect Immun 26: 57–63, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Schneewind O, Model P, Fischetti VA. Sorting of protein A to the staphylococcal cell wall. Cell 70: 267–281, 1992. [DOI] [PubMed] [Google Scholar]
- 376.Schurr JR, Young E, Byrne P, Steele C, Shellito JE, Kolls JK. Central role of toll-like receptor 4 signaling and host defense in experimental pneumonia caused by Gram-negative bacteria. Infect Immun 73: 532–545, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Schwarzer C, Fischer H, Kim EJ, Barber KJ, Mills AD, Kurth MJ, Gruenert DC, Suh JH, Machen TE, Illek B. Oxidative stress caused by pyocyanin impairs CFTR Cl− transport in human bronchial epithelial cells. Free Radic Biol Med 45: 1653–1662, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Scully IL, Liberator PA, Jansen KU, Anderson AS. Covering all the Bases: preclinical development of an effective Staphylococcus aureus vaccine. Front Immunol 5: 109, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Sebghati TA, Clegg S. Construction and characterization of mutations within the Klebsiella mrkD1P gene that affect binding to collagen type V. Infect Immun 67: 1672–1676, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Sebghati TA, Korhonen TK, Hornick DB, Clegg S. Characterization of the type 3 fimbrial adhesins of Klebsiella strains. Infect Immun 66: 2887–2894, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Secher T, Fas S, Fauconnier L, Mathieu M, Rutschi O, Ryffel B, Rudolf M. The anti-Pseudomonas aeruginosa antibody Panobacumab is efficacious on acute pneumonia in neutropenic mice and has additive effects with meropenem. PLoS One 8: e73396, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Seeger W, Birkemeyer RG, Ermert L, Suttorp N, Bhakdi S, Duncker HR. Staphylococcal alpha-toxin-induced vascular leakage in isolated perfused rabbit lungs. Lab Invest 63: 341–349, 1990. [PubMed] [Google Scholar]
- 383.Sharma A, Steichen AL, Jondle CN, Mishra BB, Sharma J. Protective role of Mincle in bacterial pneumonia by regulation of neutrophil mediated phagocytosis and extracellular trap formation. J Infect Dis 209: 1837–1846, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, Reyes CN, Miao EA, Aderem A, Gotz F, Liu GY, Underhill DM. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe 7: 38–49, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Shime N, Sawa T, Fujimoto J, Faure K, Allmond LR, Karaca T, Swanson BL, Spack EG, Wiener-Kronish JP. Therapeutic administration of anti-PcrV F(ab')(2) in sepsis associated with Pseudomonas aeruginosa. J Immunol 167: 5880–5886, 2001. [DOI] [PubMed] [Google Scholar]
- 386.Shon AS, Russo TA. Hypervirulent Klebsiella pneumoniae: the next superbug? Future Microbiol 7: 669–671, 2012. [DOI] [PubMed] [Google Scholar]
- 387.Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, Bost DA, Riehman M, Naidich S, Kreiswirth BN. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol 37: 3556–3563, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Shreiner A, Huffnagle GB, Noverr MC. The “Microflora Hypothesis” of allergic disease. Adv Exp Med Biol 635: 113–134, 2008. [DOI] [PubMed] [Google Scholar]
- 389.Siegemund S, Sauer K. Balancing pro- and anti-inflammatory TLR4 signaling. Nat Immunol 13: 1031–1033, 2012. [DOI] [PubMed] [Google Scholar]
- 390.Silo-Suh L, Suh SJ, Sokol PA, Ohman DE. A simple alfalfa seedling infection model for Pseudomonas aeruginosa strains associated with cystic fibrosis shows AlgT (sigma-22) and RhlR contribute to pathogenesis. Proc Natl Acad Sci USA 99: 15699–15704, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407: 762–764, 2000. [DOI] [PubMed] [Google Scholar]
- 392.Singh S, Barr H, Liu YC, Robins A, Heeb S, Williams P, Fogarty A, Camara M, Martinez-Pomares L. Granulocyte-macrophage colony stimulatory factor enhances the pro-inflammatory response of interferon-gamma-treated macrophages to Pseudomonas aeruginosa infection. PLoS One 10: e0117447, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Siryaporn A, Kuchma SL, O'Toole GA, Gitai Z. Surface attachment induces Pseudomonas aeruginosa virulence. Proc Natl Acad Sci USA 111: 16860–16865, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol 172: 3377–3381, 2004. [DOI] [PubMed] [Google Scholar]
- 395.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 292: L312–L322, 2007. [DOI] [PubMed] [Google Scholar]
- 396.Skurnik D, Roux D, Cattoir V, Danilchanka O, Lu X, Yoder-Himes DR, Han K, Guillard T, Jiang D, Gaultier C, Guerin F, Aschard H, Leclercq R, Mekalanos JJ, Lory S, Pier GB. Enhanced in vivo fitness of carbapenem-resistant oprD mutants of Pseudomonas aeruginosa revealed through high-throughput sequencing. Proc Natl Acad Sci USA 110: 20747–20752, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Small CL, McCormick S, Gill N, Kugathasan K, Santosuosso M, Donaldson N, Heinrichs DE, Ashkar A, Xing Z. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J Immunol 180: 5558–5568, 2008. [DOI] [PubMed] [Google Scholar]
- 398.Small CL, Shaler CR, McCormick S, Jeyanathan M, Damjanovic D, Brown EG, Arck P, Jordana M, Kaushic C, Ashkar AA, Xing Z. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol 184: 2048–2056, 2010. [DOI] [PubMed] [Google Scholar]
- 399.Smedley JG 3rd Jewell E, Roguskie J, Horzempa J, Syboldt A, Stolz DB, Castric P. Influence of pilin glycosylation on Pseudomonas aeruginosa 1244 pilus function. Infect Immun 73: 7922–7931, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Smith EJ, Visai L, Kerrigan SW, Speziale P, Foster TJ. The Sbi protein: a multifunctional immune evasion factor of Staphylococcus aureus. Infect Immun 79: 3801–3809, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog 4: e1000017, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 274: 1859–1866, 1996. [DOI] [PubMed] [Google Scholar]
- 403.Song Z, Wu H, Ciofu O, Kong KF, Hoiby N, Rygaard J, Kharazmi A, Mathee K. Pseudomonas aeruginosa alginate is refractory to Th1 immune response and impedes host immune clearance in a mouse model of acute lung infection. J Med Microbiol 52: 731–740, 2003. [DOI] [PubMed] [Google Scholar]
- 404.Soong G, Martin FJ, Chun J, Cohen TS, Ahn DS, Prince A. Staphylococcus aureus protein A mediates invasion across airway epithelial cells through activation of RhoA signaling and proteolytic activity. J Biol Chem 10.1074/jbc.M1111.295386, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Soong G, Parker D, Magargee M, Prince AS. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J Bacteriol 190: 2814–2821, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Soong G, Reddy B, Sokol S, Adamo R, Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest 113: 1482–1489, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJ, van Kessel KP, Vandenesch F, Lina G, van Strijp JA. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe 13: 584–594, 2013. [DOI] [PubMed] [Google Scholar]
- 408.Spaan AN, Vrieling M, Wallet P, Badiou C, Reyes-Robles T, Ohneck EA, Benito Y, de Haas CJ, Day CJ, Jennings MP, Lina G, Vandenesch F, van Kessel KP, Torres VJ, van Strijp JA, Henry T. The staphylococcal toxins gamma-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun 5: 5438, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Spaulding AR, Lin YC, Merriman JA, Brosnahan AJ, Peterson ML, Schlievert PM. Immunity to Staphylococcus aureus secreted proteins protects rabbits from serious illnesses. Vaccine 30: 5099–5109, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Stahlhut SG, Chattopadhyay S, Kisiela DI, Hvidtfeldt K, Clegg S, Struve C, Sokurenko EV, Krogfelt KA. Structural and population characterization of MrkD, the adhesive subunit of type 3 fimbriae. J Bacteriol 195: 5602–5613, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Standiford LR, Standiford TJ, Newstead MJ, Zeng X, Ballinger MN, Kovach MA, Reka AK, Bhan U. TLR4-dependent GM-CSF protects against lung injury in Gram-negative bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol 302: L447–L454, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Stegger M, Wirth T, Andersen PS, Skov RL, De Grassi A, Simoes PM, Tristan A, Petersen A, Aziz M, Kiil K, Cirkovic I, Udo EE, del Campo R, Vuopio-Varkila J, Ahmad N, Tokajian S, Peters G, Schaumburg F, Olsson-Liljequist B, Givskov M, Driebe EE, Vigh HE, Shittu A, Ramdani-Bougessa N, Rasigade JP, Price LB, Vandenesch F, Larsen AR, Laurent F. Origin and evolution of European community-acquired methicillin-resistant Staphylococcus aureus. mBio 5: e01044-01014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Stoesser N, Xayaheuang S, Vongsouvath M, Phommasone K, Elliott I, Del Ojo Elias C, Crook DW, Newton PN, Buisson Y, Lee SJ, Dance DAB Del Ojo Elias C, Crook DW, Newton PN, Buisson Y, Lee SJ, Dance DAB. Colonization with Enterobacteriaceae producing ESBLs in children attending pre-school childcare facilities in the Lao People's Democratic Republic. J Antimicrob Chemother. In press. [DOI] [PMC free article] [PubMed]
- 414.Strom MS, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol 47: 565–596, 1993. [DOI] [PubMed] [Google Scholar]
- 415.Struss AK, Nunes A, Waalen J, Lowery CA, Pullanikat P, Denery JR, Conrad DJ, Kaufmann GF, Janda KD. Toward implementation of quorum sensing autoinducers as biomarkers for infectious disease states. Anal Chem 85: 3355–3362, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Sun J, Barbieri JT. ExoS Rho GTPase-activating protein activity stimulates reorganization of the actin cytoskeleton through Rho GTPase guanine nucleotide disassociation inhibitor. J Biol Chem 279: 42936–42944, 2004. [DOI] [PubMed] [Google Scholar]
- 417.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339: 786–791, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Sun Y, Karmakar M, Taylor PR, Rietsch A, Pearlman E. ExoS and ExoT ADP ribosyltransferase activities mediate Pseudomonas aeruginosa keratitis by promoting neutrophil apoptosis and bacterial survival. J Immunol 188: 1884–1895, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Sundin C, Wolfgang MC, Lory S, Forsberg A, Frithz-Lindsten E. Type IV pili are not specifically required for contact dependent translocation of exoenzymes by Pseudomonas aeruginosa. Microbial pathogenesis 33: 265–277, 2002. [DOI] [PubMed] [Google Scholar]
- 420.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355: 1018–1028, 2006. [DOI] [PubMed] [Google Scholar]
- 421.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med 204: 3235–3245, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Tawk MY, Zimmermann-Meisse G, Bossu JL, Potrich C, Bourcier T, Dall Serra M, Poulain B, Prevost G, Jover E. Internalization of staphylococcal leukotoxins that bind and divert the C5a receptor is required for intracellular Ca2+ mobilization by human neutrophils. Cell Microbiol doi: 10.1111/cmi.12434, 2015. [DOI] [PubMed] [Google Scholar]
- 423.Tengroth L, Millrud CR, Kvarnhammar AM, Kumlien Georen S, Latif L, Cardell LO. Functional effects of Toll-like receptor (TLR) 3, 7, 9, RIG-I and MDA-5 stimulation in nasal epithelial cells. PLoS One 9: e98239, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Thammavongsa V, Rauch S, Kim HK, Missiakas DM, Schneewind O. Protein A-neutralizing monoclonal antibody protects neonatal mice against Staphylococcus aureus. Vaccine 33: 523–526, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Theander TG, Kharazmi A, Pedersen BK, Christensen LD, Tvede N, Poulsen LK, Odum N, Svenson M, Bendtzen K. Inhibition of human lymphocyte proliferation and cleavage of interleukin-2 by Pseudomonas aeruginosa proteases. Infect Immun 56: 1673–1677, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Tian X, Xu F, Lung WY, Meyerson C, Ghaffari AA, Cheng G, Deng JC. Poly I:C enhances susceptibility to secondary pulmonary infections by gram-positive bacteria. PLoS One 7: e41879, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Tiringer K, Treis A, Fucik P, Gona M, Gruber S, Renner S, Dehlink E, Nachbaur E, Horak F, Jaksch P, Doring G, Crameri R, Jung A, Rochat MK, Hormann M, Spittler A, Klepetko W, Akdis CA, Szepfalusi Z, Frischer T, Eiwegger T. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 187: 621–629, 2013. [DOI] [PubMed] [Google Scholar]
- 428.Tran CS, Eran Y, Ruch TR, Bryant DM, Datta A, Brakeman P, Kierbel A, Wittmann T, Metzger RJ, Mostov KE, Engel JN. Host cell polarity proteins participate in innate immunity to Pseudomonas aeruginosa infection. Cell Host Microbe 15: 636–643, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Trinchieri G. Type I interferon: friend or foe? J Exp Med 207: 2053–2063, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Tsay RW, Siu LK, Fung CP, Chang FY. Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch Intern Med 162: 1021–1027, 2002. [DOI] [PubMed] [Google Scholar]
- 431.Tuchscherr L, Medina E, Hussain M, Volker W, Heitmann V, Niemann S, Holzinger D, Roth J, Proctor RA, Becker K, Peters G, Loffler B. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med 3: 129–141, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25: 682–707, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Tzouvelekis LS, Tzouvelekis LS, Miriagou V, Miriagou V, Kotsakis SD, Kotsakis SD, Spyridopoulou K, Spyridopoulou K, Athanasiou E, Athanasiou E, Karagouni E, Karagouni E, Tzelepi E, Tzelepi E, Daikos GL, Daikos GL. KPC-producing, multidrug-resistant Klebsiella pneumoniae sequence type 258 as a typical opportunistic pathogen. Antimicrob Agents Chemother 57: 5144–5146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Ueda A, Wood TK. Connecting quorum sensing, c-di-GMP, pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA (PA3885). PLoS Pathogens 5: e1000483, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, Kim KC. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol 38: 263–268, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Uhlen M, Guss B, Nilsson B, Gatenbeck S, Philipson L, Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem 259: 1695–1702, 1984. [PubMed] [Google Scholar]
- 437.Ulland TK, Ferguson PJ, Sutterwala FS. Evasion of inflammasome activation by microbial pathogens. J Clin Invest 125: 469–477, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Usher LR, Lawson RA, Geary I, Taylor CJ, Bingle CD, Taylor GW, Whyte MK. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J Immunol 168: 1861–1868, 2002. [DOI] [PubMed] [Google Scholar]
- 439.Van Elssen CHMJ, Vanderlocht J, Frings PWH, Senden-Gijsbers BLMG, Schnijderberg MCA, van Gelder M, Meek B, Libon C, Ferlazzo G, Germeraad WTV, Bos GMJ. Klebsiella pneumoniae-triggered DC recruit human NK cells in a CCR5-dependent manner leading to increased CCL19-responsiveness and activation of NK cells. Eur J Immunol 40: 3138–3149, 2010. [DOI] [PubMed] [Google Scholar]
- 440.Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol 25: 308–315, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Vandenesch F, Couzon F, Boisset S, Benito Y, Brown EL, Lina G, Etienne J, Bowden MG. The Panton-Valentine leukocidin is a virulence factor in a murine model of necrotizing pneumonia. J Infect Dis 201: 967–969, 2010. [DOI] [PubMed] [Google Scholar]
- 442.Ventura CL, Malachowa N, Hammer CH, Nardone GA, Robinson MA, Kobayashi SD, DeLeo FR. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One 5: e11634, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Vernachio J, Bayer AS, Le T, Chai YL, Prater B, Schneider A, Ames B, Syribeys P, Robbins J, Patti JM. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob Agents Chemother 47: 3400–3406, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Villaruz AE, Bubeck Wardenburg J, Khan BA, Whitney AR, Sturdevant DE, Gardner DJ, DeLeo FR, Otto M. A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. J Infect Dis 200: 724–734, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Vinckx T, Wei Q, Matthijs S, Cornelis P. The Pseudomonas aeruginosa oxidative stress regulator OxyR influences production of pyocyanin and rhamnolipids: protective role of pyocyanin. Microbiology 156: 678–686, 2010. [DOI] [PubMed] [Google Scholar]
- 446.Visintin A, Mazzoni A, Spitzer JA, Segal DM. Secreted MD-2 is a large polymeric protein that efficiently confers lipopolysaccharide sensitivity to Toll-like receptor 4. Proc Natl Acad Sci USA 98: 12156–12161, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 344: 11–16, 2001. [DOI] [PubMed] [Google Scholar]
- 448.Von Kockritz-Blickwede M, Rohde M, Oehmcke S, Miller LS, Cheung AL, Herwald H, Foster S, Medina E. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am J Pathol 173: 1657–1668, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Walsh EJ, O'Brien LM, Liang X, Hook M, Foster TJ. Clumping factor B, a fibrinogen-binding MSCRAMM (microbial surface components recognizing adhesive matrix molecules) adhesin of Staphylococcus aureus, also binds to the tail region of type I cytokeratin 10. J Biol Chem 279: 50691–50699, 2004. [DOI] [PubMed] [Google Scholar]
- 450.Wang J, Li F, Sun R, Gao X, Wei H, Li LJ, Tian Z. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat Commun 4: 2106, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Wang J, Li F, Zheng M, Sun R, Wei H, Tian Z. Lung natural killer cells in mice: phenotype and response to respiratory infection. Immunology 137: 37–47, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Wang M, Schaefer AL, Dandekar AA, Greenberg EP. Quorum sensing and policing of Pseudomonas aeruginosa social cheaters. Proc Natl Acad Sci USA 112: 2187–2191, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13: 1510–1514, 2007. [DOI] [PubMed] [Google Scholar]
- 454.Warrener P, Varkey R, Bonnell JC, DiGiandomenico A, Camara M, Cook K, Peng L, Zha J, Chowdury P, Sellman B, Stover CK. A novel anti-PcrV antibody providing enhanced protection against Pseudomonas aeruginosa in multiple animal infection models. Antimicrob Agents Chemother 58: 4384–4391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Webster SJ, Daigneault M, Bewley MA, Preston JA, Marriott HM, Walmsley SR, Read RC, Whyte MKB, Dockrell DH. Distinct cell death programs in monocytes regulate innate responses following challenge with common causes of invasive bacterial disease. J Immunol 185: 2968–2979, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Weems JJ Jr, Steinberg JP, Filler S, Baddley JW, Corey GR, Sampathkumar P, Winston L, John JF, Kubin CJ, Talwani R, Moore T, Patti JM, Hetherington S, Texter M, Wenzel E, Kelley VA, Fowler VG Jr. Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 50: 2751–2755, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5: 751–762, 2005. [DOI] [PubMed] [Google Scholar]
- 458.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364: 703–705, 2004. [DOI] [PubMed] [Google Scholar]
- 459.Wertheim HF, Walsh E, Choudhurry R, Melles DC, Boelens HA, Miajlovic H, Verbrugh HA, Foster T, van Belkum A. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med 5: e17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 506: 503–506, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science 295: 1487, 2002. [DOI] [PubMed] [Google Scholar]
- 462.Wiener-Kronish JP, Pittet JF. Therapies against virulence products of Staphylococcus aureus and Pseudomonas aeruginosa. Semin Respir Crit Care Med 32: 228–235, 2011. [DOI] [PubMed] [Google Scholar]
- 463.Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci USA 107: 13473–13478, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, Ting JP. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol 183: 2008–2015, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Wilson GJ, Seo KS, Cartwright RA, Connelley T, Chuang-Smith ON, Merriman JA, Guinane CM, Park JY, Bohach GA, Schlievert PM, Morrison WI, Fitzgerald JR. A novel core genome-encoded superantigen contributes to lethality of community-associated MRSA necrotizing pneumonia. PLoS Pathog 7: e1002271, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Wilson KC, Center DM, Cruikshank WW. The effect of interleukin-16 and its precursor on T lymphocyte activation and growth. Growth Factors 22: 97–104, 2004. [DOI] [PubMed] [Google Scholar]
- 467.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328: 1703–1705, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467a.World Health Organization. Antimicrobial Resistance Global Report of Surveillance. Geneva: WHO, 2014. [Google Scholar]
- 468.Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3: 4–14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Wu W, Badrane H, Arora S, Baker HV, Jin S. MucA-mediated coordination of type III secretion and alginate synthesis in Pseudomonas aeruginosa. J Bacteriol 186: 7575–7585, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Wu W, Huang J, Duan B, Traficante DC, Hong H, Risech M, Lory S, Priebe GP. Th17-stimulating protein vaccines confer protection against Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 186: 420–427, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Xu X, Weiss ID, Zhang HH, Singh SP, Wynn TA, Wilson MS, Farber JM. Conventional NK Cells can produce IL-22 and promote host dDefense in Klebsiella pneumoniae pneumonia. J Immunol 192: 1778–1786, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Yamamoto K, Ahyi AN, Pepper-Cunningham ZA, Ferrari JD, Wilson AA, Jones MR, Quinton LJ, Mizgerd JP. Roles of lung epithelium in neutrophil recruitment during pneumococcal pneumonia. Am J Respir Cell Mol Biol 50: 253–262, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Yamamoto K, Ferrari JD, Cao Y, Ramirez MI, Jones MR, Quinton LJ, Mizgerd JP. Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia. J Immunol 189: 2450–2459, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Yang J, Zhao Y, Shi J, Shao F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci USA 110: 14408–14413, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194: 519–527, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Yoshida K, Matsumoto T, Tateda K, Uchida K, Tsujimoto S, Iwakurai Y, Yamaguchi K. Protection against pulmonary infection with Klebsiella pneumoniae in mice by interferon-gamma through activation of phagocytic cells and stimulation of production of other cytokines. J Med Microbiol 50: 959–964, 2001. [DOI] [PubMed] [Google Scholar]
- 477.Yu FS, Cornicelli MD, Kovach MA, Newstead MW, Zeng X, Kumar A, Gao N, Yoon SG, Gallo RL, Standiford TJ. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J Immunol 185: 1142–1149, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Yun YS, Min YG, Rhee CS, Jung IH, Koh YY, Jang TY, Jung DH. Effects of alpha-toxin of Staphylococcus aureus on the ciliary activity and ultrastructure of human nasal ciliated epithelial cells. Laryngoscope 109: 2021–2024, 1999. [DOI] [PubMed] [Google Scholar]
- 479.Zeng X, Moore TA, Newstead MW, Hernandez-Alcoceba R, Tsai WC, Standiford TJ. Intrapulmonary expression of macrophage inflammatory protein 1alpha (CCL3) induces neutrophil and NK cell accumulation and stimulates innate immunity in murine bacterial pneumonia. Infect Immun 71: 1306–1315, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Zeng X, Zeng X, Moore TA, Moore TA, Newstead MW, Newstead MW, Deng JC, Deng JC, Kunkel SL, Kunkel SL, Luster AD, Luster AD, Standiford TJ, Standiford TJ. Interferon-inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect Immun 73: 8226–8236, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Zhao H, Li W, Gao Y, Li J, Wang H. Exposure to particular matter increases susceptibility to respiratory Staphylococcus aureus infection in rats via reducing pulmonary natural killer cells. Toxicology 325: 180–188, 2014. [DOI] [PubMed] [Google Scholar]
- 482.Zhou Y, Zhu Y. Diversity of bacterial manipulation of the host ubiquitin pathways. Cell Microbiol 17: 26–34, 2015. [DOI] [PubMed] [Google Scholar]
- 483.Zughaier SM, Ryley HC, Jackson SK. Lipopolysaccharide (LPS) from Burkholderia cepacia is more active than LPS from Pseudomonas aeruginosa and Stenotrophomonas maltophilia in stimulating tumor necrosis factor alpha from human monocytes. Infect Immun 67: 1505–1507, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]