Abstract
The electrogenic sodium bicarbonate cotransporter (NBCe2) is encoded by SLC4A5, variants of which have been associated with salt sensitivity of blood pressure, which affects 25% of the adult population. NBCe2 is thought to mediate sodium bicarbonate cotransport primarily in the renal collecting duct, but NBCe2 mRNA is also found in the rodent renal proximal tubule (RPT). The protein expression or function of NBCe2 has not been demonstrated in the human RPT. We validated an NBCe2 antibody by shRNA and Western blot analysis, as well as overexpression of an epitope-tagged NBCe2 construct in both RPT cells (RPTCs) and human embryonic kidney 293 (HEK293) cells. Using this validated NBCe2 antibody, we found NBCe2 protein expression in the RPT of fresh and frozen human kidney slices, RPTCs isolated from human urine, and isolated RPTC apical membrane. Under basal conditions, NBCe2 was primarily found in the Golgi, while NBCe1 was primarily found at the basolateral membrane. Following an acute short-term increase in intracellular sodium, NBCe2 expression was increased at the apical membrane in cultured slices of human kidney and polarized, immortalized RPTCs. Sodium bicarbonate transport was increased by monensin and overexpression of NBCe2, decreased by NBCe2 shRNA, but not by NBCe1 shRNA, and blocked by 2,2′-(1,2-ethenediyl)bis[5-isothiocyanato-benzenesulfonic acid]. NBCe2 could be important in apical sodium and bicarbonate cotransport under high-salt conditions; the implication of the ex vivo studies to the in vivo situation when salt intake is increased remains unclear. Therefore, future studies will examine the role of NBCe2 in mediating increased renal sodium transport in humans whose blood pressures are elevated by an increase in sodium intake.
Keywords: human renal proximal tubule, NBC4, NBCE2, SLC4A5, sodium bicarbonate cotransporter
salt sensitivity (ss), which affects over 25% of the adult population, is found in 14% of normotensive individuals and confers cardiovascular morbidity and mortality similar to hypertension. (36) A locus in chromosome 2 (2p14-2p13.1) (27, 28) contains the SLC4A5 gene, which has been linked to increased blood pressure (BP). (6, 29) SLC4A5 encodes the electrogenic sodium bicarbonate cotransporter 4 (NBCe2, formerly known as NBC4) (30), which has been significantly associated with high BP and/or SS (4, 5, 19, 32, 33). A related sodium bicarbonate cotransporter, NBCe1, encoded by SLC4A4, is located in the human renal proximal tubule (RPT) (21), medullary thick ascending limb (mTAL), and collecting duct (7). However, little is known about the normal renal cellular expression and function of NBCe2 compared with NBCe1.
The rat kidney expresses NBCe2 in the mTAL, cortical TAL (cTAL), and proximal straight tubule (38). The localization of NBCe2 in either the apical or basolateral membrane in the human RPT is not known, but Good (17) and Xu et al. (38) hypothesized that NBCe2 should be located at the basolateral membrane of the mTAL and cTAL, since there was no measurable sodium-dependent bicarbonate transport activity in the lumen of these nephron segments under basal conditions. There are two isoforms of NBCe2 (∼130 kDa and 119 kDa) (13). The 119-kDa isoform is thought to derive from an alternate transcriptional start site. We did not consistently find the 119-kDa isoform in our human kidney preparations; thus, we focused these studies only on the 140-kDa isoform. The 140-kDa band that we found consistently is the principal isoform found in skeletal muscle (∼130 kDa) (20). In the choroid plexus (13), the 119-kDa isoform is the only one expressed and is apically localized. We hypothesized that NBCe2 is localized to the brush-border membrane in the RPT because it may be responsible for the increase in renal proximal sodium transport in salt-sensitive hypertension (1). The increased activity of NBCe2 in the RPT in hypertension (5) may be one mechanism to increase sodium transport, but the decreased activity of NBCe1 (25) results in a decrease in overall HCO3− reabsorption; low serum HCO3− and high anion gap are associated with hypertension (2, 9–11). Mice with germline deletion of SLC4A5 on SV129/C57 background were acidotic and had elevated BP that was thought to be due to increased distal tubule bicarbonate transport via other bicarbonate sodium transporters, e.g., SLC26A4 and SLC4A7 (18). Interestingly, increased bicarbonate consumption elevated the BP of wild-type SV129/C57 mice to the levels seen in SLC4A5 knockout mice. An increase in sodium bicarbonate consumption can also increase BP in salt-sensitive Blacks (31). Mice with germline deletion of SLC4A5 on C57BL/6 background have normal BP on a normal diet, but an acid diet caused hypertension that was due to increased epithelial sodium channel-mediated sodium reabsorption (37). Therefore, to provide a basis for understanding the role of NBCe2 and NBCe1 in normal sodium homeostasis and on the pathophysiology of SS, we characterized the expression of these transporters in the human kidney, human RPT cells (RPTC) in culture derived from fresh kidney tissue, and human RPTC isolated from freshly voided urine. NBCe2-mediated pH recovery from cell acidification was also used as a measure of NBCe2 activity.
METHODS
RNA In Situ Hybridization
Four-micrometer sections were cut from formalin-fixed, paraffin-embedded (FFPE) kidney tissue blocks obtained from the University of Virginia Biorepository and Tissue Research Facility (BTRF) under an institutional review board-approved protocol, according to the Declaration of Helsinki, Title 45, Part 46, U.S. Code of Federal Regulations. In situ hybridization for SLC4A4 and SLC4A5 was performed using the RNAscope 2-Plex chromogenic detection kit (Advanced Cell Diagnostics). The 2-Plex assay (cat. no. 320494) was carried out following FFPE pretreatment (cat. no. 320511).
Human Renal Proximal Tubule Cell Cultures
Normal tissues at the opposite pole of kidneys removed due to advanced noninvasive renal cell carcinoma were obtained from BTRF. Renal tissue was used as is, or cultured for renal cell studies. Urine-derived renal proximal tubule cell (RPTCs) were collected by our laboratory under an approved Institutional Review Board protocol. The dietary sodium intake of the subjects who underwent nephrectomy could not be determined; sodium intake could affect the renal expression pattern of NBCe2.
Primary and immortalized RPTC cultures from kidney tissue.
We generated human RPTC cell lines isolated from human kidney specimens as previously described (14). The cell lines have been extensively characterized using RPTC-specific markers (16). Primary (preimmortalization) and immortalized RPTCs were used (23, 41). All cell lines have been DNA-fingerprinted to validate their origin and continued expression of genes of interest. The RPTCs were grown at 37°C in full humidity with 5% CO2. The cells were fed DMEM-F12 media (Invitrogen) supplemented with 2% FCS, 5 μg/ml plasmocin (InvivoGen), 10 ng/ml epidermal growth factor (Sigma), 36 ng/ml dexamethasone (Sigma), 2 ng/ml triiodothyronine (Sigma), 1 × insulin/transferrin/selenium (Invitrogen), 1 × penicillin/streptomycin (Invitrogen), and 0.2 mg/ml G418 sulfate (EMD Chemicals).
The RPTCs were cultured for 24 h before sodium or monensin treatment; each experiment was performed in triplicate.
Primary RPTC cultures from freshly voided urine.
Cells were collected from a fresh urine void, washed with Dulbecco's PBS, resuspended in medium and then transferred to a 12-well plate. The cells were fed every other day and in 1 wk, colonies were seen, and were characterized as RPTC by staining for CD-13 (also known as aminopeptidase-N, a specific RPTC membrane marker) (35).
Antibody Specificity Validation
NBCe1 has been well characterized in the RPT (24), and we confirmed protein expression using the Sigma WH0008671M1 antibody to NBCe1. The NBCe2 antibody (Sigma HPA036621) used in the current studies was characterized extensively in the Human Protein Atlas (http://www.proteinatlas.org/ENSG00000188687-SLC4A5/tissue). We further verified its specificity using confocal imaging with dual staining for RPT-specific markers CD-13 (APN, BD 347837; 1:500) and Lotus tetragonobulus agglutinin (LTA; Vector Laboratories) in cells treated with mock and SLC4A5 short hairpin inhibitory RNA (shRNA). This was performed in human tissue and cultured primary and immortalized RPTC, including V5-tagged NBCe2 overexpressed in RPTC and HEK293 cell lines. The V5 tag antibody used in the overexpressed cells was from Life Technologies (Invitrogen, 46-0705, 1:100). We also preadsorbed the Sigma antibody with the immunizing peptide (Atlas Antibodies) prior to staining human renal tissue and cultured RPTCs.
NBCe2-overexpressed cell lines.
A lentiviral construct (CCSB-Broad Lentiviral Expression Human SLC4A5 Clone; Clone ID:ccsbBroad304_12409) was purchased from Thermo Scientific. The plasmid was packaged into the virus with compatible packaging plasmids using HEK293 cells (Clontech Laboratories). The lentivirus was added to RPTCs and HEK293 cells at 30–40% confluence for 18–20 h, then removed and replaced with regular growth medium. After 48 h, the medium was changed to selection medium containing Blasticidin S (InvivoGen; 5 μg/ml).
Western Blot Analysis of Human Kidney Tissue
Human kidney homogenates and plasma membrane preparation and Western blot analysis.
Slices of human kidney cortex or medulla (∼125–150 mg per slice) were homogenized in detergent-free lysis buffer (1.5 ml per mg of tissue) with protease inhibitor cocktail (Sigma) containing PMSF and then centrifuged at 3,100 rpm for 10 min at 4°C to remove cellular debris. An aliquot of the supernatant, representing whole cell membrane, was saved. The remaining supernatant was transferred to Beckman tubes and spun at 35,000 rpm for 75 min at 4°C. The supernatant was discarded, and 0.75 ml of solubilization buffer containing 0.5% Triton X with protease inhibitor cocktail and PMSF was added to each tube. A pipette was used to break up the pellet, and then the tubes were shaken for 1 h on ice. The tubes were spun at 18,000 rpm for 10 min. The final supernatant represented total plasma membranes. Total protein was quantified using a bicinchoninic acid (BCA) assay. SDS samples were prepared, separated by SDS-PAGE (10% Tris·HCl polyacrylamide gels; 40 μg of protein loaded per lane), and transferred onto a nitrocellulose membrane by electroblotting. The membranes were incubated in Odyssey blocking buffer (LI-COR Biosciences) for 1 h at room temperature and then further incubated overnight at 4°C with the following primary antibodies in Odyssey blocking buffer: NBCe2 (Sigma, 1:500) and villin (Millipore, 1:1,000), a brush-border membrane-specific marker. After several washes in TBS with 0.1% Tween (TBST), the membranes were incubated with their respective infrared secondary antibodies (anti-rabbit IRDye 800 or anti-mouse IRDye 680, LI-COR, both at 1:15,000) in Odyssey blocking buffer for 1 h at room temperature. After several washes in TBST, immunoreactivity was quantified using the Odyssey infrared imaging system. The blots were then stripped using LI-COR nitrocellulose stripping buffer and reprobed with CD-13 (APN; Santa Cruz Biotechnology, 1:200) primary antibody, to verify the presence of RPT plasma membranes.
LTA apical membrane isolation and Western blot analysis.
After the human kidney cortex was homogenized, 1 mg of total protein, quantified by the BCA assay, was resuspended in 10 ml of detergent-free lysis buffer and incubated with 20 μg of biotinylated LTA on a 360° rocker for 2 h at room temperature, as previously published. (22) A 50% vol/vol slurry (20 μl) of Ultralink Neutravidin beads (Pierce) was then added and incubated on a 360° rocker for 30 min at room temperature. The beads were pelleted and thoroughly washed using a microcentrifuge spin cup filter. The LTA affinity-attached membranes were eluted by incubating the beads in the spin cup filter with 125 μl of 70°C 2× sample buffer for 10 min and underwent Western blot analysis as described above. The membranes were incubated with the following primary antibodies in Odyssey blocking buffer: NBCe2 (Sigma; 1:500) and villin (Millipore; 1:1,000) followed by their respective infrared secondary antibodies, as mentioned previously. Immunoreactivity was quantified using the Odyssey infrared imaging system. The blots were then stripped using LI-COR nitrocellulose stripping buffer, reprobed with CD-13 (APN, Santa Cruz Biotechnology; 1:200) primary antibody, and processed as previously stated.
Magnesium precipitation for brush-border membrane isolation and Western blot analysis.
An alternative method was used to isolate brush-border membranes (26). Slices of human kidney cortex (∼200 mg per slice) were homogenized in 0.5 M sucrose (6 ml/g of tissue) with polytron and Dounce homogenizers. MgCl2 (10 mmol/l final concentration) was added to the supernatant and shaken on ice for 15 min. The supernatant was spun at 1,500 g for 20 min, and the pellet was discarded. The supernatant was then spun at 15,000 g for 10 min. The pellet was resuspended in 100 μl of a solution containing 300 mmol/l mannitol, 12 mmol/l Tris·HCl, and 10 mmol/l MgCl2. The supernatant was spun at 2,200 g for 12 min, and the pellet was discarded. The supernatant was spun again at 15,000 g for 12 min. The final pellet was resuspended in 200 μl of 2× sample buffer. Samples in SDS were prepared, separated by SDS-PAGE (10% Tris·HCl polyacrylamide gels; 20 μl of protein loaded per lane), transferred onto a nitrocellulose membrane by electroblotting, and underwent Western blot analysis, as described above. The membranes were incubated with the following primary antibodies in Odyssey blocking buffer: NBCe2 (Sigma, 1:500) and villin (Millipore, 1:1,000), followed by their respective infrared antibodies. Immunoreactivity was quantified using the Odyssey Infrared Imaging System. The blots were then stripped using LI-COR nitrocellulose stripping buffer and reprobed with CD-13 (APN, Santa Cruz; 1:200) and cis-Golgi (GM130, Becton Dickinson; 1:250) primary antibodies.
Raising Intracellular Sodium
Intracellular sodium was raised by increasing the extracellular sodium chloride concentration from 120 mmol/l (the normal sodium chloride concentration in the medium) to 170 mmol/l for 30 min, which raised intracellular sodium by 5.2 ± 1.1 mmol/l similar to that shown in other studies (12, 34, 40) by a previously published method (8). For control cells, mannitol was used as the osmolyte, and osmolar equivalency was achieved by measuring freezing point osmometry (Osmette A, Fisher). Raising intracellular sodium was also achieved by using the sodium ionophore monensin (Sigma) at 10 μmol/l based on monensin titration and intracellular sodium calibration (8). Monensin was used at 1-h time point.
NBCe1 and NBCe2 Immunofluorescence Staining and Confocal Microscopy
Localization in human kidney cortical tissue sections.
FIXED HUMAN KIDNEY CORTICAL TISSUE SECTIONS.
Surgically removed human kidneys were decapsulated. The outer 1 mm of cortical tissue was excised and washed three times in PBS. The tissues were fixed with 4% paraformaldehyde in PBS for 1 h at room temperature and washed with PBS. The fixative was inactivated by a 10-min wash with Tris·HCl (100 mmol/l; pH 8.0). The tissues were embedded in optimal cutting temperature compound (OTC) and snap frozen in liquid nitrogen. Eight-micrometer sections on slides were rehydrated in PBS with 1% Triton X-100 for 10 min and washed three times in PBS with 0.02% Tween-20 (PBST). The tissues were blocked overnight using Odyssey blocking buffer at 4°C. The following primary antibodies in Odyssey blocking buffer were added for 1 h at room temperature: NBCe1 (Sigma, 1:100) and NBCe2 (Sigma, 1:100). Biotinylated LTA (1:200) and biotinylated Dolichos biflorus agglutinin (DBA, Vector Laboratories; 1:200) were also used for the identification of the RPT and distal convoluted tubule + connecting tubule + collecting duct (24), respectively. Each tissue slice was stained for either LTA or DBA, which contained both NBCe1 and NBCe2 primary antibodies. Following two washes with PBS, the following secondary antibodies in Odyssey blocking buffer were added for 30 min at room temperature: Alexa Fluor 488 (Invitrogen, 1:500) for NBCe2; Alexa Fluor 594 (Invitrogen, 1:500) for NBCe1; and streptavidin 647 (Invitrogen; 1:500) for DBA or LTA. The slides were washed twice with PBS and then postfixed for 5 min with 4% paraformaldehyde in PBS. Finally, 100 mmol/l Tris·HCl was added for 10 min to inactivate the fixative.
LOCALIZATION IN EX VIVO HUMAN KIDNEY SLICE CULTURE.
Live human renal cortical tissue slices (1-mm-thick coronal sections) were cultured in 12-well plates in a 37°C, 5% CO2 incubator with rocking for 30 min in prewarmed and CO2-equilibrated media. The culture medium contained sodium at a concentration of 120 or 170 mmol/l, as described above. In separate experiments, the kidney slices were incubated for 1 h with monensin, as an alternative method to raise intracellular sodium. The slices were then fixed, 8-μm frozen sections were cut, and staining was performed as described above.
Localization in cultured primary and immortalized human RPTCs.
RPTCs grown on collagen IV-coated glass bottom 96-well plates to 50% confluence were serum-starved overnight (see isolation method for urinary RPTCs). They were stimulated with monensin (1 or 10 μmol/l) or vehicle for 3 h and washed twice in PBS. They were then fixed for 5 min in PBS containing 4% paraformaldehyde and 1% Triton-X 100 and then washed three times for 5 min with TBS. Immunofluorescence staining was performed as previously reported (16). The fixed cells were blocked in Odyssey blocking buffer at room temperature for 1 h. The following primary antibodies in Odyssey blocking buffer were added for 1 h with gentle rocking at room temperature: NBCe2 (Sigma; 1:250) NBCe1 (Sigma; 1:500), or GM130 (Becton Dickinson; 1:500). Following three washes in PBS, the following secondary antibodies, fluorescent stains, or directly labeled primary antibodies in Odyssey blocking buffer were added for 1 h with gentle rocking at room temperature: Alexa Fluor 488 (Invitrogen, 2 μg/ml) for NBCe2, Alexa Fluor 594 (Invitrogen, 2 μg/ml) for NBCe1, Alexa Fluor 594 phalloidin (Invitrogen A12381; 1: 200) to stain filamentous actin (f-actin), CD-13-PE antibody (BD 347837; 1:50) to identify RPTCs, and Hoechst 33342 (Invitrogen 735969; 1:2,000) to stain nuclei. The cells were washed three times in PBST and imaged using an Olympus IX81 automated multiwell spinning disk confocal microscope.
Confocal microscopy.
The confocal microscope is an IX81, 6D (linear encoded x, and y axis, piezo z axis, time, wavelength, positions) spinning disk confocal with both mercury and xenon light sources and Semrock hard-coated filters in a Sedat configuration. Images were acquired using a 60× UPlanSApo water immersion objective with NA 1.2 for tissue section imaging, and a 100 × UPlanSApo oil immersion objective with NA 1.4 for cell imaging. The microscope is controlled by Olympus Slidebook 5.5 software.
Localization using total internal reflection fluorescence microscopy.
The effect of monensin (10 μmol/l, 3 h) on NBCe2 apical expression was measured in polarized RPTCs grown on GEM three-dimensional (3D) microcarriers, as described previously. (16) Two-color immunofluorescence total internal reflection fluorescence (TIRF) microscopy with an imaging depth set at 70 nm from the coverslip surface was used. We used 70 nm as the imaging depth chosen on the basis of previous work done on α-Na/K/ATPase, that showed a decrease in TIRF when this transporter underwent endocytosis inside the cell (17).
Localization by immunoelectron microscopy.
RPTCs were grown on a 1-mm thick pad of cross-linked gelatin in a 10-cm cell culture dish. The gelatin pad was prepared by pouring and incubating 10% gelatin (300 bloom; Sigma) and 1% microbial transglutaminase (Activa-TI, Ajinomoto, Japan) for 24 h in a humidified cell culture incubator. After washing and equilibration in culture media for 30 min, RPTCs were grown for 5 days, 3 days after reaching complete confluence. Using a 1-cm cork boring tool and a scalpel, pads of cells on gelatin were transferred to six-well plates, incubated and treated identically to the tissue slice culture method above. The tissues were fixed, and primary antibody incubations identical to that for tissue confocal immunofluorescence microscopy except that Alexa Fluor 594-labeled FluoroNanogold (Invitrogen) was used for the secondary antibody followed by silver enhancement (Invitrogen) before fixing overnight in 2.5% glutaraldehyde. The samples were then handed over to the advanced microscopy facility. Tissues were washed in distilled water, postfixed in osmium-tetroxide, dehydrated through an ethanol series and then an acetone series before embedding in epoxy resin (EPON 812, EMS). Ultrathin 70-μm sections were cut (Diatome, EMS) on a Leica Ultracut UCT ultramicrotome, collected on 200-μm copper mesh and contrast-stained by double lead procedure (Daddow). Sections were examined in a JEOL 1230 transmission electron microscope and imaged with a SIA 12-C slow-scan 16.8-megapixel camera (Scientific Instruments and Applications). Four different sections were imaged for each condition and positive apical membrane-immunoreactive granules were counted as dots per micrometer surface length using ImageJ.
Sodium Accumulation Assay
RPTCs were cultured in 96-well glass bottom collagen-coated Matrical plates (Spokane, WA) at 37°C until they reached 50% confluence. The cells were serum-starved overnight prior to loading with a sodium ion indicator, sodium benzofuran isophthalate (5 μmol/l; Molecular Probes) with 0.04% Pluronic F-127 for 2 h in PBS with calcium (0.9 mmol/l) and magnesium (0.49 mmol/l). The cells were washed twice and allowed to recover at 37°C in serum-free media for 30 min. They were washed two more times with PBS and incubated at room temperature. The cells were then placed in the fluorescent plate reader (PherastarFS) and ratiometric readings (340-nm excitation and 510-nm emission/380-nm excitation and 510-nm emission) were recorded every 30 s for 30 min. A 5-min baseline reading was acquired prior to automatic injection of monensin (10 μmol/l). The change in sodium accumulation was measured as the change in 340/380 ratio at the 30-min time point minus the 5-min average reading prior to monensin injection.
NBCe2 knock-down cell lines.
Validated mission shRNAi lentiviral constructs for knocking down the SLC4A4 (clone ID: NM_003759.1-598s1c1, NM_003759.1-1955s1c1, and NM_003759.1-3183s1c1), or SLC4A5 gene (clone ID: NM_021196.3-676s21c1; NM_021196.3-1161s21c1, and NM_021196.2-1989s1c1), as well as a negative shRNA control (mission nontarget shRNA control vector; SHC002) were purchased from Sigma and packaged into virus with compatible packaging plasmids using HEK293T cells (Clontech Laboratories). After 48 h, the selection agent puromycin (Sigma, P7255; 2 μg/ml) was added to the media. Knock-down of protein expression was verified by Western blot analysis using the NBCe2 antibody (Sigma; 1:500) and analyzed using Odyssey software. Both isoforms of NBCe2 (140 kDa and 119 kDa) are both knocked down by shRNA because the region used to target the shRNA is in a shared region of the two different mRNA transcripts.
Bicarbonate-Dependent pH Recovery
Confocal ratiometric time-lapse imaging.
RPTCs were cultured for 30 min in serum-free media with monensin (10 μmol/l). The plate was loaded similarly to the sodium accumulation assay described above, with the exception that BCECF AM, Invitrogen, 5 μmol/l) was the dye used and loaded without Pluronic for 30 min instead of 2 h. The RPTCs were then acidified by incubating the cells with 20 mmol/l ammonium chloride for 10 min. Sodium- and bicarbonate-free buffer was then used in the presence of EIPA [5-(N-ethyl-N-isopropyl)-amiloride, Sigma, 10 μmol/l] to inhibit NHE3 activity. Buffer containing both sodium and bicarbonate was then used, and intracellular pH was measured by time-lapse ratiometric imaging on a spinning disk confocal microscope every 30 s (485 nm excitation, 530 nm emission/430 nm excitation, 530 nm emission), and the initial rate of pH recovery was recorded. Ratiometric images were acquired and analyzed using the ratio imaging module of the Slidebook software version 5.5. The entire assay was performed within CO2 and humidified incubator surrounding the confocal microscope that contains a software-controlled eight-channel pressurized perfusion system, and up to 20 wells can be imaged every 30 s using the automated xyz stage insert.
Kinetic ratiometric fluorescent microplate assay.
An alternate, higher-throughput 96-well bicarbonate-dependent pH recovery assay was carried out as mentioned previously, but instead, kinetic measurements using a fluorescence plate reader (PherastarFS) were performed. Kinetic measurements of the entire plate could be completed within 30 s, and sodium and bicarbonate were added by automated injection to final concentrations of 60 and 12.5 mmol/l, respectively, by injecting a volume equal to that already in each well. The pH was internally calibrated using the nigericin high-potassium calibration method (8). Two negative controls were used: 1) no sodium and 2) 2,2′-(1,2-ethenediyl)bis[5-isothiocyanato-benzenesulfonic acid] (DIDS, Cayman Chemical; 500 μmol/l) added along with the EIPA as controls for each cell type. The pH recovery rate was measured as the slope between the last sodium and bicarbonate-free time point and the first 2 min after injection by linear regression.
Statistical Analysis
Data are expressed as means ± SE. Comparisons within and among groups (>2) were made by repeated-measures or factorial ANOVA, respectively, followed by Holm-Sidak post hoc test. Student's t-test was used for two-group comparisons.
RESULTS
Expression of SLC4A4 and SLC4A5 mRNA in Human Renal Cortical Tissue Sections
RNAscope 2-Plex chromogenic detection kit (Fig. 1) confirmed the presence of both SLC4A4 and SLC4A5 mRNA in RPTs in FFPE kidney tissue.
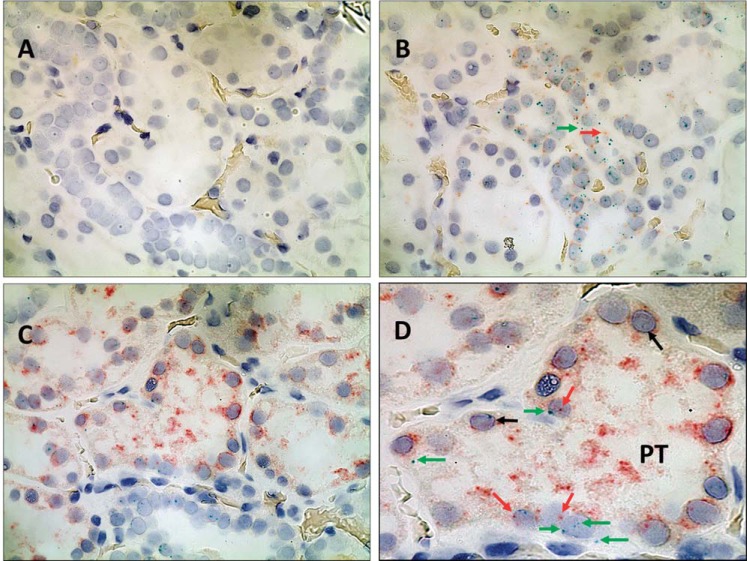
Fig. 1.
In situ hybridization of SLC4A4 and SLC4A5 in human renal cortical formalin-fixed paraffin-embedded sections using the RNAscope 2-Plex chromogenic assay. Cell nuclei are stained gray (black arrows) using Gill's hematoxylin no. 1. A: negative control shows no binding of DapB (no red or green; ×600 magnification). B: positive control shows binding of both control probes, POLR2A (green) and PPIB (red) (×600 magnification). C: proximal tubules (PT) with binding of both SLC4A4 (red) and SLC4A5 (green) (×600 magnification). D: close up of a PT from C, demonstrating the presence of both SLC4A4 (red) and SLC4A5 (green, see arrows).
Validation of NBCe2 Antibody for Immunostaining
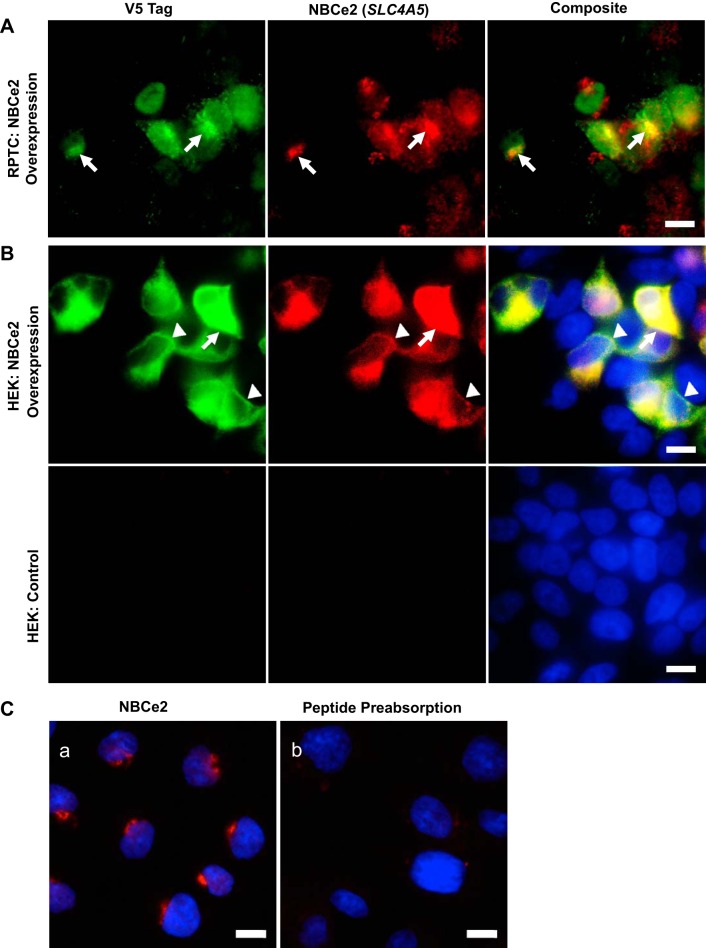
The colocalization of V5 epitope-tagged NBCe2 OE in RPTCs (Fig. 2A) and HEK293 cells (Fig. 2B) and NBCe2 immunostained with the NBCe2 antibody (Sigma HPA036621) demonstrated antibody specificity; NBCe2 OE was validated by the presence of the V5 epitope-tagged transgene. No NBCe2 or V5 staining was seen in nontransfected HEK293 cells (Fig. 2B, bottom). This negative control further validates the specificity of the NBCe2 antibody. Moreover, almost no NBCe2 immunostaining was observed using the NBCe2 antibody that was preadsorbed with the NBCe2-immunizing peptide (Fig. 2C).
Fig. 2.
Immunostaining of renal proximal tubule cells (RPTCs) and HEK 293 cells overexpressing NBCe2 protein. A and B: both an RPTC line (A) and HEK 293 cell line (1st row in B, which has no measurable NBCe2) were stably infected with lentivirus to overexpress NBCe2 protein. Overexpression of NBCe2 protein is shown on this same field of cells stained with both the V5 tag antibody (green), which detects the V5 tag in the SLC4A5 construct used, and the NBCe2 antibody (red). These results show that in cells in which NBCe2 was overexpressed (V5 epitope tagged transgene), there were costaining with NBCe2 (stained by the NBCe2 antibody), as indicated by the yellow area in the composite image. B, bottom row: nontransfected HEK 293 cells and composite image show nuclear staining (Hoechst). Arrows indicate Golgi. Arrowheads indicate cell membrane. Scale bar is 10 μm, and all of the images were taken at ×600 magnification. C: human renal proximal tubule cells were stained by NBCe2 before and after preadsorption with the immunizing peptide. a: NBCe2 is stained tight and close to nucleus indicating the location of NBCe2 in the Golgi apparatus (shown in red). b: preadsorption of the antibody with the immunizing peptide almost completely prevented NBCe2 staining. Nuclei were stained in blue. Scale bar = 10 μm.
Expression of NBCe2 Protein in Human Kidney Tissue Using Western Blot Analysis
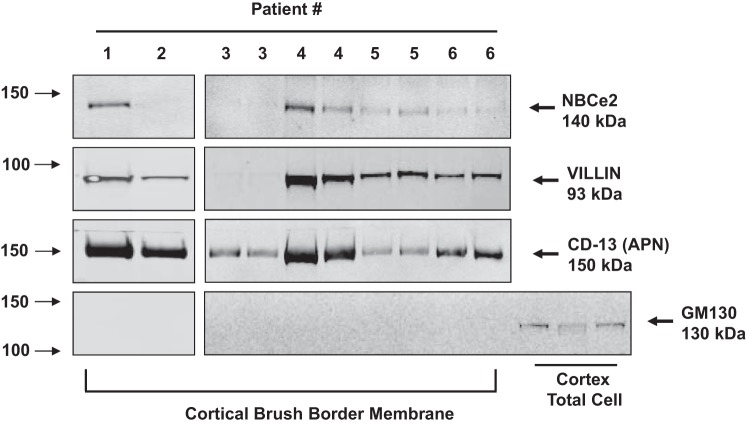
Western blot analysis confirmed the presence of NBCe2 protein in three separate patient kidney specimens (Fig. 3). NBCe2 expression was seen in total homogenates (Fig. 3A) and plasma membranes (Fig. 3B) from cortical and medullary (includes outer stripe that contains the S3 segment of the RPT). In the LTA apical membrane fraction from RPTs, NBCe2 was expressed along with the brush-border markers villin and RPT-specific marker CD-13 (Fig. 3C). NBCe2 protein was also observed in renal cortical brush-border membranes obtained by the magnesium precipitation method (Fig. 4) in four of six specimens, providing further support of its presence in RPTs. Total cortical cell lysates served as a positive control for Golgi staining, using GM130. In cortical brush-border fractions, there was negative staining for Golgi, which suggests that there was no cytosolic contamination of the brush-border membranes. The expression levels in the patient samples varied considerably, suggesting that environmental or nutritional conditions may have led to the different levels of expression and/or localization of NBCe2 in proximal tubule brush-border membranes.
Fig. 3.
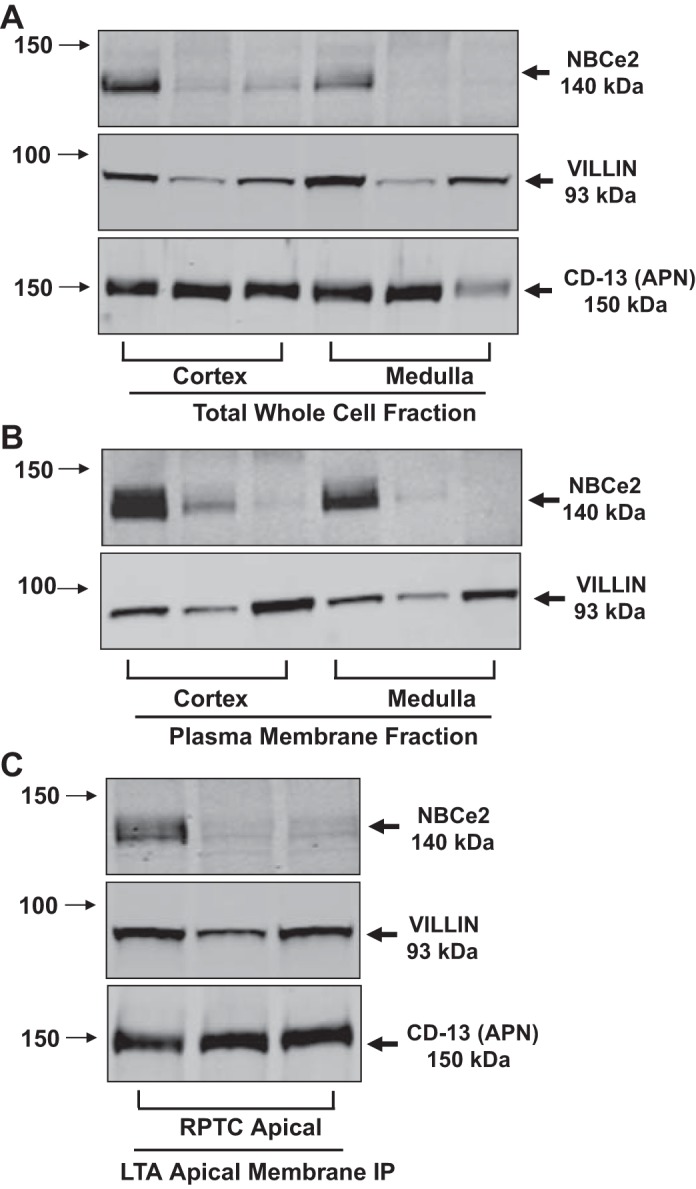
Western Blot analysis of total whole cell homogenates and membrane fractions from human kidney tissue. A–C: results from three separate patient samples. Arrows on the left side of the blots indicate the molecular sizes, while the arrows on the right side indicate the bands of the proteins of interest. Villin was used as a RPTC microvilli marker. CD-13 (APN) was also used in A and C as another RPTC-specific, brush-border membrane marker. A: Western blot analysis of total whole cell fractions from cortex and medulla. The blots were probed with NBCe2, villin, and CD-13 antibodies. B: Western blot analysis of plasma membrane fractions from the cortex and medulla: The blots were probed with NBCe2 and villin antibodies. C: Western blot analysis of LTA apical membrane fractions from cortex. The blots were probed with NBCe2, villin, and CD-13 antibodies. A clear signal in the Lotus tetragonobulus agglutinin (LTA)-immunoprecipitated (IP) apical membrane fraction, along with two different RPTC apical membrane markers, suggests that NBCe2 is expressed in the RPTC apical membrane, including brush border in human kidneys.
Fig. 4.
Western blot analysis of brush border membrane fractions from human kidney tissue. Results from six separate patient samples processed at two different times to isolate brush-border membranes. Patient tissue samples 3–6 were processed in duplicate experiments to demonstrate reproducibility. Arrows on the left side of the blots depict the molecular sizes, while the arrows on the right side depict the bands of the proteins of interest. The blots were probed with NBCe2, villin, CD13 (APN), and GM130 antibodies. Villin and CD-13 were used as RPTC microvilli-localized proteins while GM130 was used to stain the Golgi apparatus. Positive staining for NBCe2 in cortical brush-border membranes combined with positive bands for two different RPTC apical membrane markers and negative staining for the Golgi apparatus, indicate that NBCe2 is expressed in RPTC brush-border membranes.
NBCe2 Localization Changes Following Short-Term Exposure to High Extracellular Sodium or the Ionophore Monensin
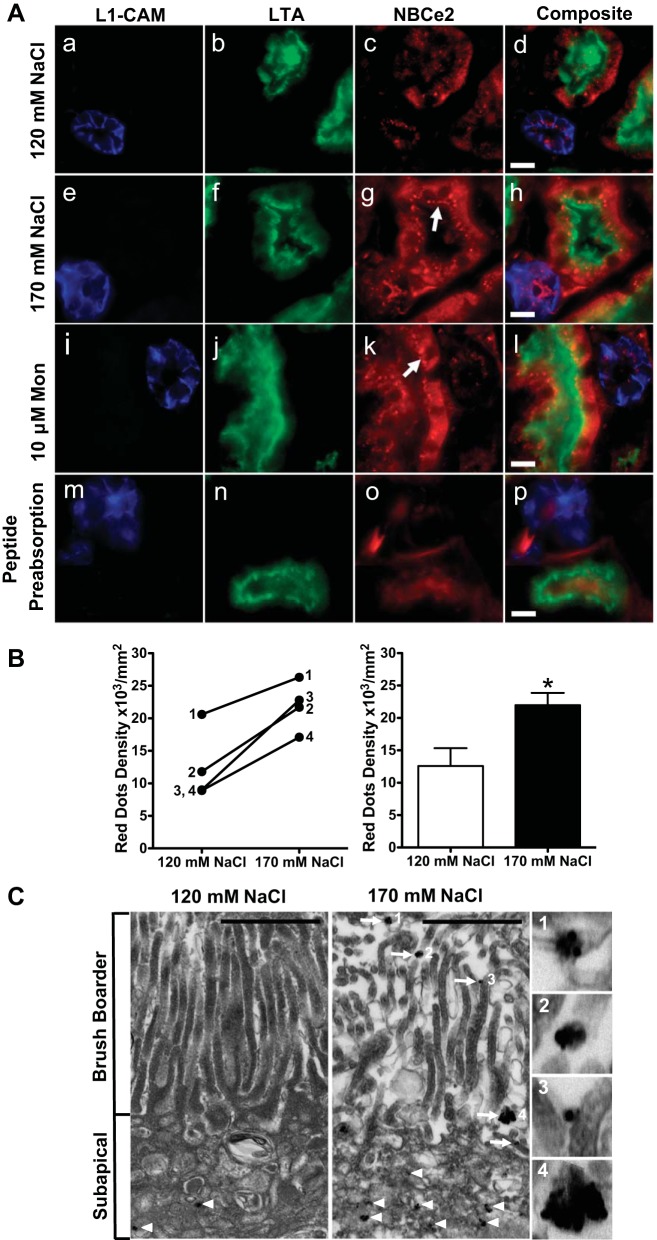
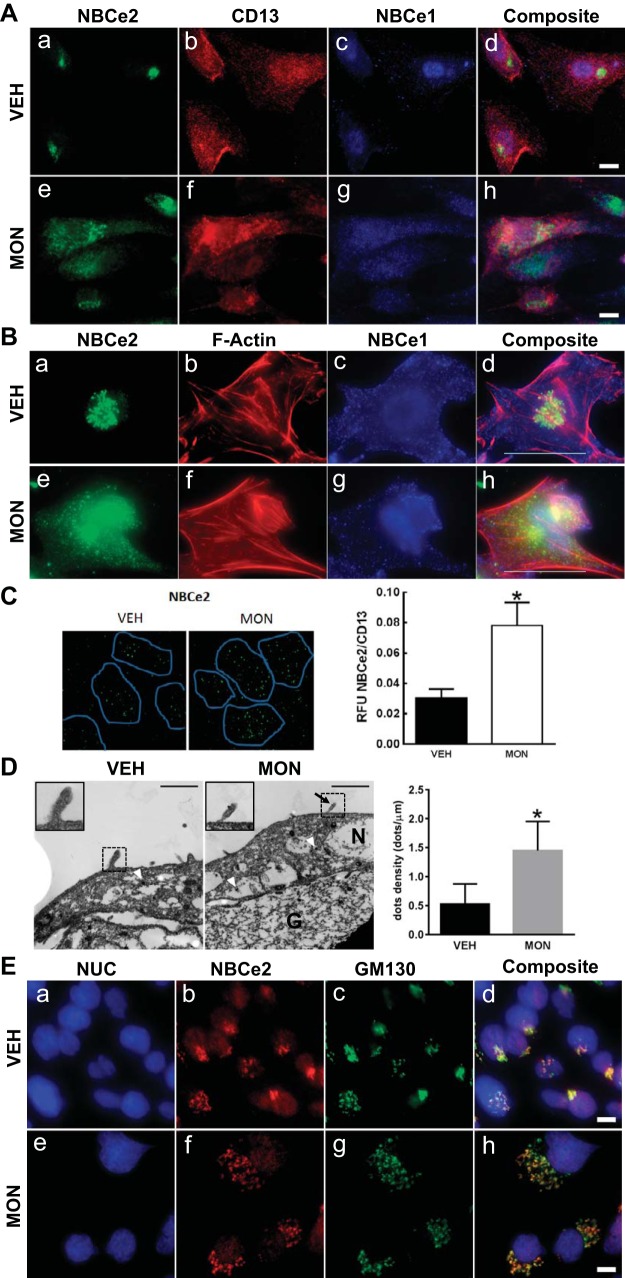
Human kidney cortical tissue slices cultured in media with two different extracellular sodium concentrations were stained for NBCe2, and costained with the lectins L1-CAM (cortical collecting duct marker) or LTA (RPT brush-border marker), confirmed the presence of NBCe2 in both the RPT and cortical collecting duct. NBCe2 staining in the cortical collecting duct is consistent with earlier reports of its expression in principal cells (7) (Fig. 5A). There was a 1.75-fold increase (n = 4, P < 0.05) in RPT NBCe2 subapical dot staining when the sodium concentration in the culture medium was increased from 120 to 170 mmol/l NaCl (Fig. 5A, g vs. c, and B). Slices that were incubated with ionophore monensin (10 μmol/l) also showed an increase in NBCe2 staining in the RPT (Fig. 5A, k vs. c). As the intracellular sodium is increased in either treatment, the NBCe2 staining shifted from predominantly cytoplasmic to a more punctate subapical localization, with some colocalization with the brush-border marker LTA (see arrows). However, after preadsorption of the NBCe2-immunizing peptide, the punctate pattern was no longer observed. Quantification of the data is shown in Fig. 5B. Immunogold-labeled NBCe2 antibodies were used for electron microscopy, which demonstrated deposited gold particles in the RPTC subapical cytoplasm in what appear to be vesicular bodies after exposure to 120 mmol/l ex vivo sodium (Fig. 5C). Under elevated sodium conditions (170 mmol/l sodium), there was a significant increase in brush-border localized immunoreactive electron dense puncta at the microvilli of the proximal tubule, indicated by arrows. Enlarged photos are shown in Fig. 5C, 1–4, (scale bar = 1 μm).
Fig. 5.
Change in localization of NBCe2 following an increase in extracellular sodium in kidney tissue slice culture. A: effect of an increase in extracellular sodium on NBCe2 localization. Fresh human renal cortical tissue slices (1-mm thickness) were cultured for 30 min in media containing low (120 mmol/l NaCl; top), or high (170 mmol/l NaCl, second row) extracellular sodium. They were also incubated for 1 h in media containing 10 mmol/l monensin (third row). Slices were fixed, and 8-mm frozen sections were made. The cortical collecting duct (CCD) marker, L1-CAM (blue; a, e, i, and m), the proximal tubule brush border marker, LTA lectin (green; b, f, g, and n), and NBCe2 antibody (red; c, g, k, and o) confirmed NBCe2 staining in both proximal tubule and CCD. In 120 mmol/l extracellular NaCl concentration, NBCe2 localization is predominantly cytoplasmic (C), while in increased (170 mmol/l) NaCl concentration or in the presence of monensin, NBCe2 is also localized in punctate subapical structures (g and k, white arrow), with some colocalizing with LTA (yellow orange). After peptide preadsorption (m–p), NBCe2 immunostaining is markedly decreased. The tissue for peptide preadsorption was incubated in 170 mmol/L NaCl. Scale bar = 10 μm. B: quantification of images from the low and high extracellular sodium experiments shown in A. Kidney slices from four separate patient samples were analyzed by counting the red dots from all the proximal tubules and measuring the area (dots/mm2). Changes following the increase in extracellular sodium from 120 to 170 mmol/l NaCl are shown for each individual patient on the left graph, and the summary is shown on the right graph. A significant increase in NBCe2 staining in the subapical area of proximal tubules (as identified by LTA) is shown (n = 4, *P < 0.05). C: immuno-electron micrograph of NBCe2 in human kidney cortex. Ex vivo incubation of fresh human kidney cortex under low or elevated NaCl concentrations produced an increase in brush border-localized immune-reactive electron dense puncta on the microvilli of the proximal tubule, indicated by arrows. C: enlarged photos in panels 1–4 to demonstrate that NBCe2-immunoreactive particles appear in clusters. A notable increase in puncta in the microvilli is found under elevated sodium conditions. Scale bar = 1 μm.
Expressions of NBCe1 and NBCe2 protein were also seen in primary cultures of RPTCs from freshly voided urine (Fig. 6A). CD-13 costaining identified the cells as RPTC. NBCe2 and NBCe1 were present in different subcellular perinuclear compartments; intranuclear localization was ruled out by TIRF imaging (data not shown). Increasing intracellular sodium by incubating cells with monensin caused the NBCe2 to change from a punctate intracellular localization to a more diffuse staining throughout the cell, (Fig. 6A, a and e).
Fig. 6.
Change in localization of NBCe2 and NBCe1 following monensin treatment of RPTCs. A: immunofluorescent localization in primary cultures of human RPTCs obtained from freshly voided urine. NBCe2 staining is shown in green (a and e), while NBCe1 staining is shown in blue (c and g). RPTCs have a generalized red membrane staining using an antibody to CD-13, a specific membrane-bound ectopeptidase present in RPT but not in other nephron segments (b and f). All of the images were merged into a composite image (d and h); there is no overlap of NBCe2 and NBCe1. With monensin treatment, NBCe2 expression expanded and became more diffuse (e). Scale bar = 10 μm (×600 magnification). B: immunofluorescent localization of NBCe2 and NBCe1 in cultured immortalized human RPTCs. NBCe2 staining is shown in green (a and e), while NBCe1 staining is in blue (c and g). b and f: filamentous-actin staining with phalloidin is shown in red. All of the images were merged into a composite image (d and h). Upon the addition of monensin (MON; 3 h, 1 μmol/l), a marked change in NBCe2 localization occurred, changing from a perinuclear localization to a diffuse punctate pattern throughout the cell (compare a and e; yellow in d and h indicates NBCe2 colocalization with F-actin). Some NBCe2 remains in the perinuclear location, which is possibly stored NBCe2. By contrast, MON induced a more subtle change in the intracellular localization of NBCe1 in both the cytosol and submembranous vesicular bodies (purple in d and h indicates colocalization of NBCe1 and F-actin). In c, NBCe1 shows a uniform membranous staining pattern. After the addition of MON, g shows less uniform membranous staining with a more concentrated intracellular localization. The presence of cyan or white dots in the composite images indicates that NBCe2 and NBCe1 are present in some cellular compartments. Scale bar = 10 μm, ×600 magnification. C: apical TIRF on GEMs. The effect of monensin (MON; 10 μmol/l, 3 h) on NBCe2 apical expression was measured in polarized RPTCs grown on GEM three-dimensional microcarriers, by TIRF microscopy. TIRF imaging depth was set at 70 nm from the coverslip surface. CD-13 staining was used to indicate where the brush border contacted the coverslip (blue outline). CD-13 expression did not change with MON. The apical expression of NBCe2 (green; Alexa Fluor 488) increased when incubated with MON. The ratio of NBCe2/CD-13 RFU was measured, and MON was found to increase the apical expression of NBCe2 (*P < 0.05 vs VEH; n = 5). D: immuno-electron micrograph of polarized RPTCs. Cells were grown on gelatin-coated Transwells to form a polarized epithelium. Localization of NBCe2 was measured by immuno-electron microscopy with and without increasing intracellular sodium with MON (1 h). Positive electron-dense granules along the extreme apical membrane were counted. Intracellular positive staining granules were found in all cells, with no difference in the number of granules; however, there was an increase in the number of positive staining granules in the apical membrane of cells that had increased intracellular sodium (P < 0.05; n = 3–5). NBCe2 was found more often within microvilli under elevated sodium conditions, see inset. Arrowheads indicate NBCe2-associated electron-dense granules on the apical membrane within microvilli. N denotes the nucleus, while G denotes gelatin. Scale bar = 1 μm. E: staining of RPTCs using markers for subcellular compartments. Immunofluorescence staining is shown for NBCe2 (b and f) and GM130 (Golgi-specific marker; c and g), and nucleus (NUC) (a and e). The composite image (d and h) shows a high degree of colocalization between NBCe2 and GM130 under both vehicle and monensin conditions. Magnification was ×600; scale bar = 10 μm.
Monensin treatment of immortalized RPTCs in culture also changed NBCe2 localization from the perinuclear area to a diffuse punctate pattern throughout the cell (Fig. 6B, a and e). By contrast, monensin changed the NBCe1 localization from a membrane location to an intracellular location, similar to that seen in monensin-treated parotid acinar cells (26) (Fig. 6B, c and g). The presence of cyan or white dots in the composite images indicates that NBCe2 and NBCe1 are both present in some cellular compartments. TIRF microscopy showed a 2.6-fold increase in expression of NBCe2 in the apical membrane following monensin treatment of RPTCs grown on GEM 3D microcarriers (Fig. 6C; n = 5, P < 0.05). Similar to the GEM 3D microcarriers, RPTCs form a polarized epithelium when grown on a thick cross-linked gelatin pad. Immunoelectron microscopy was performed on the RPTCs grown on gelatin pads (Fig. 6D), because the magnetic particle containing GEMs might damage the diamond knife used for making ultrathin sections for TEM. Equal numbers of intracellular electron-dense granules were found between the VEH and MON-treated cells, consistent with the confocal microscopy results. Incubation with MON (10 μmol/l, 1 h), however, led to a significant increase in apical cell surface immunoreactive NBCe2, as measured by counting silver gold-enhanced electron-dense granules by transmission electron microscopy (P < 0.05, n = 4). The subcellular localization seen under basal conditions in Fig. 6, A and B was identified using a subcellular localization panel, and the only structure that colocalized with NBCe2 was GM130, a specific Golgi marker under both control and monensin treatments (Fig. 6E). An increase in intracellular sodium did not cause any translocation of NBCe2 into early endosomes, caveosomes, multivesicular bodies, gap junctions, adherins, focal adhesions, endoplasmic reticula, lysosomes, actin fibers, or microtubules (data not shown).
Bicarbonate-Dependent pH Recovery in RPTC Lines
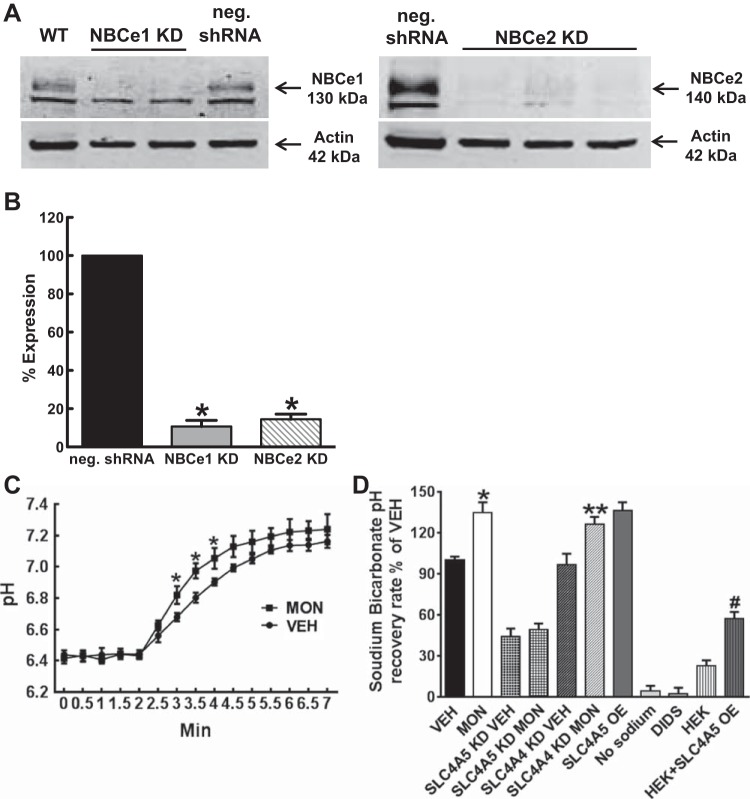
To test whether there is an increase in NBCe2 activity when intracellular sodium is increased, studies were performed in RPTC lines endogenously expressing NBCe1 and NBCe2, as well as RPTCs, in which SLC4A5 (NBCe2) was OE or in RPTCs, in which either SLC4A5 or SLC4A4 was silenced [knocked down (KD)]. Western blot analysis confirmed the marked decrease in NBCe2 and NBCe1 protein in RPTCs treated with their respective shRNA (Fig. 7, A and B). These results also reconfirm the specificity of the antibodies for NBCe1 and NBCe2 used in these studies.
Fig. 7.
NBCe1 and NBCe2 antibody validation using Western blot analysis of RPTCs stably expressing mock or shRNA and use in functional assays. A: representative Western blots showing knockdown (KD) of NBCe1 and NBCe2; WT, wild-type RPTC; NBCe1 KD, RPTC infected with Mission shRNA NBCe1 lentivirus; NBCe2 KD, RPTC infected with Mission shRNA NBCe2 lentivirus; negative (neg.); shRNA, RPTC-infected with Mission negative control shRNA lentivirus. B: Western blots were analyzed using the LI-COR Odyssey software. The expression of NBCe1 was knocked down by 91 ± 3.2% (P < 0.005; n = 4), relative to the neg. shRNA control, and the expression of NBCe2 was knocked down by 85.5 ± 2.7% (P < 0.005; n = 3), relative to the neg. shRNA control. Results were normalized by actin. C: bicarbonate-dependent pH recovery was measured as pH recovery after ammonium chloride prepulse (20 mmol/l, 5 min) in RPTCs from three individuals treated with vehicle (VEH; 0.01% ethanol, 30 min) or MON (10 μmol/l, 30 min). The sodium bicarbonate recovery assay was carried out by ratiometric confocal microscopy. The cells were loaded with BCECF and then excited at 485 nm, and then 440 nm. Light and the fluorescence emission ratio at 525 nm was recorded every 30 s. Buffers containing sodium (130 mmol/l) and bicarbonate (25 mmol/l) were rapidly perfused at the fourth time point, and the ratio was measured for 5 min. Because this assay was performed with EIPA to inhibit NHE3 activity, the increase in pH recovery was due to increased bicarbonate-dependent pH recovery at the 3, 3.5, and 4-min time points after MON treatment (*P < 0.05 MON vs. CON; n = 9). D: bicarbonate-dependent pH recovery was measured in RPTCs treated with vehicle (VEH; 0.01% ethanol, 30 min), MON (10 μmol/l, 30 min) in vector control, overexpressing (OE) SLC4A5, KD SLC4A5, and KD SLC4A4. MON in vector control induced an increase in pH recovery (*P < 0.01 MON vs VEH, n = 9). Bicarbonate-dependent pH recovery was slower in SLC4A5 KD and faster in SLC4A5 OE, relative to vector control (VEH) RPTCs, (n = 9, *P < 0.01 vs. VEH). By contrast, bicarbonate-dependent pH was not altered in SLC4A4 KD. SLC4A5 KD decreased pH recovery, but knockdown of SLC4A4 did not affect the increased pH recovery in MON-treated RPTCs. These data indicate that the increased bicarbonate-dependent pH recovery in MON-treated RPTCs is caused by SLC4A5. Bicarbonate-dependent pH recovery was completely blocked in sodium-free buffer (no sodium) or buffer containing DIDS (500 μmol/l). *MON vs. VEH, P < 0.01; **SLC4A4 KD MON vs. VEH, P < 0.01; # HEK+SLC4A5 OE vs. HEK, P < 0.01.
The intracellular pH recovery after intracellular acidification of RPTCs was measured by confocal ratiometric imaging (Fig. 7C). Raising the intracellular sodium by incubating with monensin (10 μmol/l, 30 min) resulted in an increase in sodium bicarbonate-dependent pH recovery rate (P < 0.05 MON vs. CON; n = 9). RPTC lines with vector control (VEH and MON), OE SLC4A5, KD SLC4A5, and KD SLC4A4 were then studied (10 μmol/l, 30 min) using microplate fluorometry. Bicarbonate-dependent pH recovery was faster in SLC4A5 OE and slower in SLC4A5 KD VEH, relative to vector control RPTCs treated with vehicle (VEH, 0.002% EtOH), (Fig. 7D) (n = 9, P < 0.05 vs. VEH). By contrast, bicarbonate-dependent pH was not altered in SLC4A4 KD VEH, relative to vector control RPTCs with vehicle (VEH), indicating that either NBCe1 does not affect pH recovery rate in this assay, or the effect of the KD is masked by compensation by another transporter. Monensin-treated vector control RPTCs (MON) showed faster pH recovery than (P < 0.05 MON vs. VEH; n = 9). As aforementioned, SLC4A5 KD decreased pH recovery (SLC4A5 KD VEH) and was not responsive to MON (SLC4A5 KD MON). When the assay was performed under sodium-free conditions (no sodium) or in the presence of DIDS, pH recovery was not changed. A final set of controls confirmed that NBCe2 mediates an increase in pH recovery in HEK293T cells overexpressing SLC4A5 (HEK293+SLC4A5 OE) and HEK293 cells that do not endogenously express SLC4A5. HEK293T cells that overexpress NBCe2 (HEK293+SLC4A5 OE) had a higher rate of pH recovery than HEK293T cells (P < 0.01 HEK293+SLC4A5 OE vs. HEK, n = 9). These data suggest that NBCe2 mediates the increased pH recovery rate when intracellular sodium is increased and tested in the presence of the NHE3 inhibitor EIPA.
DISCUSSION
While there is agreement that NBCe1 is present in the human proximal tubule (21–23), there is no report on the location and role of NBCe2 in the human proximal tubule. Our studies in the human kidney demonstrate that NBCe2 mRNA is found in the RPT, in agreement with previous studies in rodents (38). Our immunofluorescence studies demonstrate NBCe2 protein in the RPT of flash-frozen human kidney, primary cultures of RPTCs freshly exfoliated into human urine, primary cultures of RPTCs from fresh human kidneys, and immortalized human RPTCs. We localized NBCe2 expression to the RPT subapical membrane in flash-frozen human kidney tissue, as well as in fresh renal cortical tissue, particularly when intracellular sodium was increased by increasing the sodium concentration in the incubation medium or adding the ionophore monensin. These data support our hypothesis that NBCe2 expression is increased and recruited to the RPTC apical membrane by an increase in intracellular sodium. We also demonstrated that bicarbonate-dependent pH recovery in RPTCs was due, in part, to NBCe2 at the apical membrane, the exact location of which is the subject of future studies.
Previous attempts to localize NBCe2 in human RPTs may have been thwarted by its low level of expression in the basal state, similar to that shown for rodents (38). In addition, until relatively recently, there was a lack of good antibodies to NBCe2 (7). We tested various commercial antibodies and validated the Sigma NBCe2 antibody by preadsorption of NBCe2 immunoreactivity with the immunizing peptide. We then used the Sigma antibody to study NBCe2 expression in empty vector- and NBCe2-shRNA-treated cells by Western blot and immunofluorescence microscopy of RPTCs and HEK293 cells expressing an epitope-tagged NBCe2 lentiviral expression construct. When used to stain fresh or frozen human kidney sections, NBCe2 immunofluorescence was also found in cortical collecting duct, but not in the distal convoluted tubule, in agreement with a previous report (7).
The subcellular localization of NBCe2 is of interest because we have hypothesized that NBCe2 increases the cotransport of sodium and bicarbonate across the apical membrane under the condition of a high sodium load. In the current study, under basal conditions, we demonstrated that NBCe2 was localized primarily in RPTC Golgi bodies with some diffuse staining throughout the cell. However, when intracellular sodium was increased by two methods (either by increasing extracellular sodium or adding the ionophore monensin), there was a distinct recruitment of NBCe2 to punctate structures subjacent to the apical membrane of the RPT. Current resolution of light microscopy did not allow us to determine the anatomical structure that was the destination NBCe2 following an increase in intracellular sodium. Therefore, we performed electron microscopy that localized NBCe2 in the basal state (low-sodium exposure) in the subapical compartment of the apical membrane. After increasing the salt concentration, electron microscopy revealed that NBCe2 was present in higher amounts in the microvilli. Upon closer examination, we saw NBCe2 in vesicle-like structures which may be either transport-related or functional buffering vesicles, or both. This assumption would be consistent with the punctate nature of NBCe2 expression in the TIRF microscopy of the apical membrane in polarized RPTCs. This location is similar to that shown for NHE3 after the induction of hypertension where NHE3-mediated sodium transport is still functional, albeit at a lower level, even after its movement in the microvilli or in the intermicrovilliary cleft. (39) Apical RPT localization of NBCe2 was also confirmed in the Western blot studies of apical membranes isolated by two different methods.
We believe that the variability in the apical expression of NBCe2 in kidneys removed from patients has an important implication. The amount of sodium that the person was consuming when the kidney tissue was procured may have determined the amount and localization of NBCe2. Although we lysed the same amount of human kidney cortex, the yield of brush border through either LTA or magnesium precipitation procedure needed to be normalized. In these studies, the expression of villin, a component of brush borders, was used for normalization and a marker of RPTs. Our brush-border preparations did not contain a measurable amount of intracellular membranes, as demonstrated by the absence of GM130 by Western blot analysis. There are two isoforms of NBCe2: 119 kDa and 140 kDa. The 119-kDa isoform is thought to derive from an alternate transcriptional start site. This is also the only isoform in the choroid plexus where it is found to be apically localized (13). An ∼119-kDa band was shown in a published Western blot from neutrophils, although it was described as 130 kDa (14). We occasionally found the 119-kDa isoform in the RPT, but more consistently found the 140-kDa band. Our various membrane isolation techniques might differentially isolate these isoforms. One technique (MgCl2) uses biophysical characteristics of the brush border to precipitate these protein complexes differentially, while the LTA pull-down uses an extracellular exposed affinity ligand to isolate the brush border. ShRNA to NBCe2 reduced the expression of both isoforms, making the study of the individual isoforms of NBCe2 difficult. One possible explanation for the differential expressions that we found might also be the absence of the NH2-terminal protein domain of NBCe2 in the shorter (119 kDa) isoform. We hypothesize that the human donor kidney may have been exposed to different amounts of dietary sodium prior to the nephrectomy. The differences in sodium load may have differentially regulated the expression of NBCe2, and the two bands were not present in equal proportion from subject to subject. Future studies will focus on the effects of dietary sodium on NBCe2 isoform expression.
We also demonstrated functional NBCe2 bicarbonate transport in RPTCs using a well-established pH recovery assay (25). This method has been used to demonstrate NBCe1 activity in rat RPTs (3) because the expression of NBCe2 in this nephron segment was not yet recognized. We eliminated the contribution of NBCe1 in bicarbonate-dependent pH recovery in the RPTCs with shRNA treatment. DIDS-inhibitable pH recovery in acid-loaded cells, in the absence of NBCe1, can be attributed to NBCe2. KD of SLC4A4 (NBCe1) did not affect basal or monensin-stimulated bicarbonate-dependent pH recovery. By contrast, KD of SLC4A5 (NBCe2) decreased not only basal, but also monensin-stimulated bicarbonate-dependent pH recovery. Moreover, basal bicarbonate-dependent pH recovery was increased in RPTCs overexpressing SLC4A5. These studies conclusively demonstrated a function of NBCe2 independent of NBCe1. We conclude that functional NBCe2 is present in luminal membranes of human RPT and in microvilli after high sodium exposure.
Cultured human RPTCs are convenient for studying NBCe2- and NBCe1-mediated sodium transport. Thus, to rule out the possibility that cultured cells had dedifferentiated and altered their NBCe2 expression, we compared fresh tissue slice culture with cultured immortalized human RPTCs. Similar results were obtained, i.e., the increase in intracellular sodium recruited NBCe2 to the apical membrane, paving the way for future studies on receptor-mediated regulation of sodium and bicarbonate transport. In addition, our ability to culture freshly voided RPTCs from human urine will allow us to measure NBCe2 and NBCe1 activity following various dietary perturbations in human subjects (15).
Perspectives and Significance
Salt sensitivity, defined as a significant increase in blood pressure (BP) in response to an increase in sodium intake, affects 25% of the adult population. The presence of salt sensitivity is clearly associated with an increased prevalence of cardiovascular events and mortality, irrespective of blood pressure levels. Normotensive salt-sensitive individuals have a cumulative mortality rate similar to that of hypertensive patients, yet little is known about the regulation of blood pressure and its relationship to sodium transport in the kidney, especially in individuals with salt sensitivity. Although gene variants in the electrogenic sodium bicarbonate cotransporter (NBCe2) have been associated with salt sensitivity of blood pressure, NBCe2 has not been described in the human kidney. The fact that we found NBCe2 protein expression in the RPT of fresh and frozen human kidney slices, RPTCs isolated from human urine, and isolated RPTC apical membrane paves the way to understand its role in the etiology of salt sensitivity. Furthermore, functional studies demonstrating that sodium bicarbonate transport was increased by increasing extracellular sodium suggest that NBCe2 appears to be important in apical sodium and bicarbonate cotransport under high-salt conditions. Future studies will examine the role of NBCe2 in mediating the increased renal sodium transport in salt-sensitive humans.
GRANTS
This work was supported by National Institutes of Health Grants HL-074940 (to R. A. Felder, R. M. Carey and P. A. Jose) and DK-039308 (to P. A. Jose).
DISCLOSURES
The University of Virginia has submitted a patent on the use of NBCe2 for the diagnosis of salt sensitivity and named R. A. Felder, J. J. Gildea, P. A. Jose, and R. M. Carey as inventors.
AUTHOR CONTRIBUTIONS
Author contributions: J.J.G., J.M.C., R.M.C., P.A.J., and R.A.F. conception and design of research; J.J.G., P.X., J.M.C., R.T.G., D.B.W., B.A.K., and C.M.R. performed experiments; J.J.G., P.X., J.M.C., R.T.G., D.B.W., B.A.K., C.M.R., H.E.M., and R.A.F. analyzed data; J.J.G., P.X., J.M.C., R.T.G., D.B.W., B.A.K., H.E.M., R.M.C., P.A.J., and R.A.F. interpreted results of experiments; J.J.G., P.X., J.M.C., R.T.G., D.B.W., B.A.K., H.E.M., P.A.J., and R.A.F. prepared figures; J.J.G., J.M.C., B.A.K., H.E.M., P.A.J., and R.A.F. drafted manuscript; J.J.G., P.X., H.E.M., R.M.C., P.A.J., and R.A.F. edited and revised manuscript; J.J.G., P.X., H.E.M., R.M.C., P.A.J., and R.A.F. approved final version of manuscript.
REFERENCES
- 1.Aalkjaer C, Frische S, Leipziger J, Nielsen S, Praetorius J. Sodium coupled bicarbonate transporters in the kidney, an update. Acta Physiol Scand 181: 505–512, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Abramowitz MK, Hostetter TH, Melamed ML. Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney Int 81: 1033–1042, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amlal H, Chen Q, Greeley T, Pavelic L, Soleimani M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int 60: 1824–1836, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Barkley RA, Chakravarti A, Cooper RS, Ellison RC, Hunt SC, Province MA, Turner ST, Weder AB, Boerwinkle E. Positional identification of hypertension susceptibility genes on chromosome 2. Hypertension 43: 477–482, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, Park MJ, Sobota RS, Underwood PC, Williams J, Sun B, Raby B, Lasky-Su J, Hopkins PN, Adler GK, Williams SM, Jose PA, Felder RA. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension 60: 1359–1366, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper RS, Luke A, Zhu X, Kan D, Adeyemo A, Rotimi C, Bouzekri N, Ward R. Genome scan among Nigerians linking blood pressure to chromosomes 2, 3, and 19. Hypertension 40: 629–633, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol 293: R2136–R2146, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem 278: 28,719–28,726, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O'Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sõber S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjögren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimäki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O'Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kähönen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Köttgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grässler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stančáková A, Raffel LJ, Yao J, Kathiresan S, O'Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikäinen LP, Soininen P, Tukiainen T, Würtz P, Ong RT, Dörr M, Kroemer HK, Völker U, Völzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Järvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103–109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farwell WR, Taylor EN. Serum anion gap, bicarbonate and biomarkers of inflammation in healthy individuals in a national survey. CMAJ 182: 137–141, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman JP, Rifas-Shiman SL, Taylor EN, Lane K, Gillman MW. Association between the serum anion gap and blood pressure among patients at Harvard Vanguard Medical Associates. J Hum Hypertens 22: 122–125, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Friedman SM. The relation of cell volume, cell sodium and the transmembrane sodium gradient to blood pressure. J Hypertens 8: 67–73, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda H, Hirata T, Nakamura N, Kato A, Kawahara K, Wakabayashi S, Chang MH, Romero MF, Hirose S. Identification and properties of a novel variant of NBC4 (Na+/HCO3−- co-transporter 4) that is predominantly expressed in the choroid plexus. Biochem J 450: 179–187, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Giambelluca MS, Ciancio MC, Orlowski A, Gende OA, Pouliot M, Aiello EA. Characterization of the Na/HCO3− cotransport in human neutrophils. Cell Physiol Biochem 33: 982–990, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Gildea JJ, Lahiff DT, Van Sciver RE, Weiss RS, Shah N, McGrath HE, Schoeffel CD, Jose PA, Carey RM, Felder RA. A linear relationship between the ex-vivo sodium mediated expression of two sodium regulatory pathways as a surrogate marker of salt sensitivity of blood pressure in exfoliated human renal proximal tubule cells: the virtual renal biopsy. Clin Chim Acta 421: 236–242, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gildea JJ, McGrath HE, Van Sciver RE, Wang DB, Felder RA. Isolation, growth, and characterization of human renal epithelial cells using traditional and 3D methods. Methods Mol Biol 945: 329–345, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Good DW. The thick ascending limb as a site of renal bicarbonate reabsorption. Semin Nephrol 13: 225–235, 1993. [PubMed] [Google Scholar]
- 18.Gröger N, Vitzthum H, Fröhlich H, Krüger M, Ehmke H, Braun T, Boettger T. Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum Mol Genet 21: 1025–1036, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Hunt SC, Xin Y, Wu LL, Cawthon RM, Coon H, Hasstedt SJ, Hopkins PN. Sodium bicarbonate cotransporter polymorphisms are associated with baseline and 10-year follow-up blood pressures. Hypertension 47: 532–536, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen JM, Kristensen M, Juel C. Expression of Na+/HCO3− co-transporter proteins (NBCs) in rat and human skeletal muscle. Acta Physiol Scand 182: 69–76, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz I, Zhu Q. Proximal renal tubular acidosis mediated by mutations in NBCe1-A: unraveling the transporter's structure-functional properties. Front Physiol 4: 350, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz I, Zhu Q. Structure, function, and regulation of the SLC4 NBCe1 transporter and its role in causing proximal renal tubular acidosis. Curr Opin Nephrol Hypertens 22: 572–583, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura M, Shirai A, Yamazaki O, Satoh N, Suzuki M, Horita S, Yamada H, Seki G. Roles of renal proximal tubule transport in acid/base balance and blood pressure regulation. Biomed Res Int 2014: 504808, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Pedrosa R, Gonçalves N, Hopfer U, Jose PA, Soares-da-Silva P. Activity and regulation of Na+-HCO3− cotransporter in immortalized spontaneously hypertensive rat and Wistar-Kyoto rat proximal tubular epithelial cells. Hypertension 49: 1186–1193, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Perry C, Quissell DO, Reyland ME, Grichtchenko II. Electrogenic NBCe1 (SLC4A4), but not electroneutral NBCn1 (SLC4A7), cotransporter undergoes cholinergic-stimulated endocytosis in salivary ParC5 cells. Am J Physiol Cell Physiol 295: C1385–C1398, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao DC, Province MA, Leppert MF, Oberman A, Heiss G, Ellison RC, Arnett DK, Eckfeldt JH, Schwander K, Mockrin SC, Hunt SC, Network H. A genome-wide affected sibpair linkage analysis of hypertension: the HyperGEN network. Am J Hypertens 16: 148–150, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Rice T, Cooper RS, Wu X, Bouchard C, Rankinen T, Rao DC, Jaquish CE, Fabsitz RR, Province MA. Meta-analysis of genome-wide scans for blood pressure in African American and Nigerian samples. The National Heart, Lung, and Blood Institute GeneLink Project. Am J Hypertens 19: 270–274, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Rice T, Rankinen T, Chagnon YC, Province MA, Perusse L, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Genomewide linkage scan of resting blood pressure: HERITAGE Family Study. Health, Risk Factors, Exercise Training, and Genetics. Hypertension 39: 1037–1043, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO3−) transporters. Mol Aspects Med 34: 159–182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidlin O, Forman A, Sebastian A, Morris RC. Sodium-selective salt sensitivity: its occurrence in blacks. Hypertension 50: 1085–1092, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stutz AM, Teran-Garcia M, Rao DC, Rice T, Bouchard C, Rankinen T. Functional identification of the promoter of SLC4A5, a gene associated with cardiovascular and metabolic phenotypes in the HERITAGE Family Study. Eur J Hum Genet 17: 1481–1489, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor JY, Maddox R, Wu CY. Genetic and environmental risks for high blood pressure among African American mothers and daughters. Biol Res Nurs 11: 53–65, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tribe RM, Barton JR, Poston L, Burney PG. Dietary sodium intake, airway responsiveness, and cellular sodium transport. Am J Respir Crit Care Med 149: 1426–1433, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Van der Hauwaert C, Savary G, Gnemmi V, Glowacki F, Pottier N, Bouillez A, Maboudou P, Zini L, Leroy X, Cauffiez C, Perrais M, Aubert S. Isolation and characterization of a primary proximal tubular epithelial cell model from human kidney by CD10/CD13 double labeling. PLoS One 8: e66750, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Wen D, Yuan Y, Warner PC, Wang B, Cornelius RJ, Wang-France J, Li H, Boettger T, Sansom SC. Increased epithelial sodium channel activity contributes to hypertension caused by Na+-HCO3− cotransporter electrogenic 2 deficiency. Hypertension 66: 68–74, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Wang Z, Barone S, Petrovic M, Amlal H, Conforti L, Petrovic S, Soleimani M. Expression of the Na+-HCO3− cotransporter NBC4 in rat kidney and characterization of a novel NBC4 variant. Am J Physiol Renal Physiol 284: F41–F50, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Leong PK, Chen JO, Patel N, Hamm-Alvarez SF, McDonough AA. Acute hypertension provokes internalization of proximal tubule NHE3 without inhibition of transport activity. Am J Physiol Renal Physiol 282: F730–F740, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Zemel MB, Kraniak J, Standley PR, Sowers JR. Erythrocyte cation metabolism in salt-sensitive hypertensive blacks as affected by dietary sodium and calcium. Am J Hypertens 1: 386–392, 1988. [DOI] [PubMed] [Google Scholar]