Abstract
Fat transplants increase body fat mass without changing the energy status of an animal and provide a tool for investigating control of total body fat. Early transplant studies found that small pieces of transplanted fat took on the morphology of the transplant recipient. Experiments described here tested whether this response was dependent upon expression of leptin receptors in either transplanted fat or the recipient mouse. Fat from leptin receptor deficient db/db mice or wild-type mice was placed subcutaneously in db/db mice. After 12 wk, cell size distribution in the transplant was the same as in endogenous fat of the recipient. Thus, wild-type fat cells, which express leptin receptors, were enlarged in a hyperleptinemic environment, indicating that leptin does not directly control adipocyte size. By contrast, db/db or wild-type fat transplanted into wild-type mice decreased in size, suggesting that a functional leptin system in the recipient is required for body fat mass to be controlled. In the final experiment, wild-type fat was transplanted into a db/db mouse parabiosed to either another db/db mouse to an ob/ob mouse or in control pairs in which both parabionts were ob/ob mice. Transplants increased in size in db/db–db/db pairs, decreased in db/db–ob/ob pairs and did not change in ob/ob-ob/ob pairs. We propose that leptin from db/db parabionts activated leptin receptors in their ob/ob partners. This, in turn, stimulated release of unidentified circulating factors, which travelled back to the db/db partner and acted on the transplant to reduce fat cell size.
Keywords: fat transplant, db/db mice, parabiosis, fat cell size
parabiosis studies by hervey (23, 24), in which two rats were joined surgically to establish chronic blood exchange between the members of the pair, demonstrated the presence of a circulating factor in the feedback control of body fat mass. Later studies in which one partner was made obese by overfeeding confirmed that normal parabiotic partners of obese rats lost a majority of their body fat (17, 18) and that this occurred in the presence of a small, but essential reduction in food intake (19). Studies with genetically obese ob/ob mice and db/db mice indicated that ob/ob mice were deficient in the hypothesized circulating factor, whereas the db/db mice were able to produce the factor, but were unable to respond to its effects on food intake or body composition (7, 8). Subsequently, Zhang et al. (47) identified the mutant protein in ob/ob mice as the adipose-derived cytokine leptin, and Tartaglia et al. (42) demonstrated that leptin receptors were mutated in db/db mice, consistent with the results of parabiosis studies.
These studies implied that leptin was the long sought after parabiosis “signal”, and there is now a large body of literature demonstrating that leptin administration in normal-weight animals inhibits food intake and specifically depletes body fat stores (6, 11, 20). Blood exchange between parabiotic partners is relatively slow, and this excludes many nutrients and hormones, such as glucose and insulin as potential feedback signals because they are cleared from the circulation faster than they exchange between parabiotic partners (18, 19, 37). The half-life of leptin is such that it does exchange effectively in parabiosed mice, but concentrations do not equalize in the members of pairs containing one hyperleptinemic db/db mouse and one leptin-deficient ob/ob mouse (21).
Thus, there is an abundance of evidence to support the notion that leptin is a circulating feedback signal in the regulation of energy balance, but in vitro, studies show little evidence of a direct effect of leptin on adipocyte metabolism (15). Although central leptin has been shown to inhibit adipocyte lipogenesis (6), other studies have shown that peripheral leptin administration reduces the size of denervated white fat depots (40, 46), and Park et al. (38) reported that both central and peripheral leptin receptors are required for control of body fat by leptin. We have previously reported (45) that pharmacological doses of leptin are required to inhibit preadipocyte proliferation in vitro, whereas low concentrations (0.25% by volume) of serum from rats that had received a 7-day peripheral infusion of 100 μg leptin/24 h significantly inhibit preadipocyte proliferation. These data suggest that there is a leptin-dependent release of one, or more, circulating factors that act directly on adipose tissue to inhibit growth.
A procedure that allows for investigation of the control of body fat in vivo is to surgically transplant fat from a donor and determine what happens to the transplanted fat, in addition to the impact the transplant has on endogenous fat in the transplant recipient. Early studies by Ashwell (2) and Meade et al. (34) demonstrated that fat took on the anatomic characteristics of the host mouse when it was transplanted under the kidney capsule of lean or obese mice, which implies that fat mass is determined by environment rather than any intrinsic property of the fat cells. The experiments described here follow up on these earlier studies (2, 34) and test whether leptin receptors are required for transplanted fat to adapt to its environment by transplanting fat from wild-type or leptin receptor-deficient db/db mice into db/db or wild-type recipient mice. The final experiment used parabiosed db/db and ob/ob mice to test whether transplanted fat responds to a circulating factor that is released in response to activation of leptin receptors.
METHODS
All wild-type and db/db mice used in these studies were obtained from breeding colonies maintained at the University of Georgia or Georgia Regents University. Male ob/ob mice used in experiment 4 were purchased from Jackson Laboratories (Bar Harbor, ME). Animal procedures were approved by the University of Georgia and/or Georgia Regents University Institutional Animal Care and Use Committees. The mice were housed in temperature-controlled rooms maintained at 21–23°C and ∼50% humidity. Lights were on for 12 h each day from 7:00 AM. The mice were housed in shoe-box cages on Tek-Fresh bedding (Harlan Teklad, Madison, WI) and had continuous free access to mouse chow (Mouse diet 5015, PMI Nutrition International, Brentwood, MO) and water. Two genotypes of db/db mice were used. The first was C57BL/6J dbLepr/dbLepr, which expresses the short and circulating isoforms of the leptin receptor, but not the long-form leptin receptor (ObRb) (32). A different point mutation in the gene causes a frame shift that results in animals that express only the circulating isoform of the leptin receptor, ObRe (31). This mutation, C57BL/6J Leprdb3J/Leprdb3J, will be referred to here as db3J/db3J and served as a negative control for the potential activation of short-form leptin receptors in dbLepr/dbLepr mice.
Surgeries were performed on 40-day-old animals; transplant donors and recipients were matched for sex and genotype for Experiment 1 or were littermates of the same sex as the recipient if fat were being transplanted between wild-type and db/db mice. Fat from donor mice was dissected, rinsed in sterile saline, weighed, and cut into pieces; two pieces were placed subcutaneously on the ventral surface of each recipient mouse. A small midline incision was made below the rib cage of the recipient mouse, and a piece of fat was placed subcutaneously on either side of the incision. This location was selected because it is one of the few locations on a db/db mouse that has only a small amount of subcutaneous fat. Sham mice were anesthetized and skin incisions were made, but no fat was transplanted. Mice were housed individually, and body weights were recorded daily. In Experiments 1, 2, and 3, single mice were euthanized 12 wk after placement of the transplant. Food was removed from the cages at 7:00 AM, and blood and tissue collection started 4 h later.
Experiment 1: transplant of db/db fat into db/db mice.
Two strains of leptin receptor-deficient db/db mice were used in this experiment. dbLepr/dbLepr mice do not express ObRb, whereas db3J/db3J mice do not express any membrane-bound leptin receptors. The objective of this experiment was to determine whether db/db fat transplanted into db/db mice retained its morphological phenotype and whether the recipient mouse responded to an increase in body fat mass in the absence of leptin receptors. Both male and female dbLepr/dbLepr and db3J/db3J mice were used in this experiment. Inguinal (ING) fat was transplanted between female mice, and epididymal (EPI) fat was transplanted between males. Each transplant weighed 450-1,500 mg. At the end of the study, the transplants were dissected and weighed, and a piece ∼50 mg was weighed and fixed in osmium tetroxide for determination of cell size and number using a particle counter (Multisizer III, Beckman Coulter, Brea, CA), as described previously (14). Transplants were easily visualized because they appeared as a small independent depot, even if they touched endogenous fat in the recipient. ING, EPI, parametrial, retroperitoneal, perirenal, and mesenteric fat pads were weighed, and a piece of ING fat from female mice or EPI fat from male mice was fixed for determination of fat cell size and number. Trunk blood was collected for measurement of serum leptin (mouse leptin RIA kit; EMD Millipore, Billerica, MA), insulin (mouse insulin RIA, EMD Millipore), glucose (glucose oxidase assay kit; Sigma Chemical, St. Louis, MO), and free fatty acids (NEFA-C kit, WAKO Chemicals, Richmond, VA). The carcasses were analyzed as described previously (17). Carcasses of recipient mice were analyzed without the transplants. Briefly, each carcass without the gastrointestinal tract, was autoclaved in a sealed container for 20 min. Subsequently an equal weight of water was added, and the carcass was homogenized with a polytron. Triplicate 8-g aliquots of homogenate were extracted for total lipid using chloroform-methanol extraction for determination of carcass fat. A second set or triplicates were placed in crucibles and dried to constant weight at 60°C in a convection oven for determination of carcass water. The crucibles were then held at 500°C for 8 h for measurement of ash content. Carcass protein was calculated by the difference.
Experiment 2: transplant of wild-type fat into db/db mice.
Fat from wild-type mice expresses all of the subtypes of leptin receptors, whereas obese dbLepr/dbLepr and db3J/db3J mice are leptin receptor deficient, obese, and hyperleptinemic. Therefore, the objective of this study was to determine whether fat that expressed leptin receptors took on the phenotype of the obese transplant recipient even in the presence of high concentrations of leptin. ING fat from male or female donor wild-type mice was used for transplants (100–200 mg). Recipient mice were male and female db3J/db3J and dbLepr/dbLepr mice. At the end of the study, the transplants were recovered and weighed, and a piece was fixed for determination of cell size and number, as described above. The remainder of the transplant was snap frozen. Samples of the transplant and of ING fat from the db3J/db3J mice were used for Western blot determination (33) of PPARγ (abcam ab191407) as a marker of adipocyte differentiation and Ki67 (abcam ab15580) as a marker of cell proliferation. Fat depots were dissected and weighed, as described for Experiment 1. Carcass composition was determined, and trunk blood was collected for measurement of serum leptin, insulin, glucose, and free fatty acids. At the same time as the experimental animals were killed, ING fat was collected from six female wild-type mice that were 124 days old. This fat served as a control for the cell size distribution of ING fat that had been transplanted into db/db mice. A portion of the transplant and ING fat from 6 male dbLepr/dbLepr mice with transplants was fixed in 4% paraformaldehyde solution for 48 h, transferred to 70% ethanol, and then embedded in paraffin for sectioning and H&E staining.
Experiment 3: transplant of wild-type and db3J/db3J fat into wild-type mice.
The previous experiments showed that fat that was transplanted into db/db mice that either do not express ObRb or do not express any membrane-bound leptin receptors took on the characteristics of the endogenous fat of the obese recipient. This occurred irrespective of whether the transplant expressed leptin receptors. This experiment tested whether the absence of leptin receptors on db/db transplanted fat influenced the response of the transplant or the reciepient in wild-type mice that expressed leptin receptors. Each recipient mouse received one transplant from a wild-type mouse and one from a db3J/db3J mouse. This allowed us to compare the response of transplants that did and did not express leptin receptors in an identical environment. Transplant recipients were wild-type male or female C57BL3J mice that were littermates of db3J/db3J mice. ING fat from littermate wild-type or db3J/db3J mice was used for transplants (120–150 mg). The size of transplants in this study was limited by the size of wild-type ING fat depots. At the end of the study, fat depots and the transplanted fat were dissected and weighed as described above, and serum leptin was measured. Samples of ING fat from the transplant recipients and controls were used to measure PPARγ and Ki-67 by Western blot. Groups of male and female mice that were the same age as the transplant recipients were euthanized at the same time to determine whether the transplanted fat had influenced endogenous fat pad mass.
Experiment 4: transplant of wild-type fat into parabiosed obese mice.
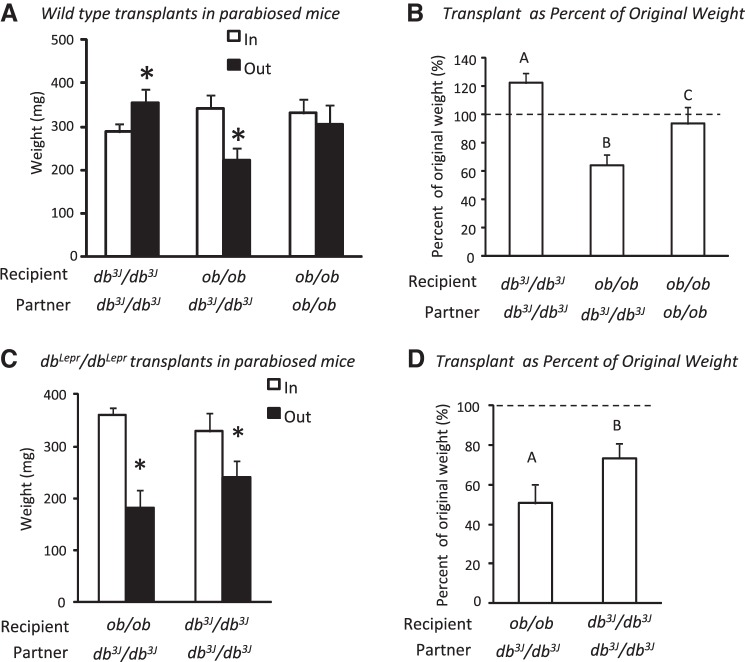
Experiment 2 showed that wild-type fat, which expressed leptin receptors, transplanted into db3J/db3J mice, which were hyperleptinemic, enlarged rather than decreased in size, whereas Experiment 3 showed that db3J/db3J fat and wild-type fat placed in a wild-type mouse decreased in size. These data implied that leptin did not act directly on adipose tissue to reduce fat depot size, but that the presence of leptin receptors in the wild-type recipient resulted in a decrease in the size of the transplant. This study tested whether leptin receptor activation was required to initiate release of a second circulating factor, which then contributed to a reduction in the size of the fat transplant. Transplants were placed in db3J/db3J members of parabiotic pairs. The partners were either another db3J/db3J mouse or a leptin-deficient, but leptin-responsive ob/ob mouse (47). If leptin induced release of a second circulating factor, then this factor would be released in ob/ob mice exchanging blood with a db3J/db3J mouse. It would then return via the circulation to the db3J/db3J partner and reduce the size of the transplant. By contrast, a transplant in pairs of two db3J/db3J mice would be expected to increase in size, as it had in the single mice. For a majority of the pairs the donor mice were wild-type males. For some pairs, the transplant was ING fat from male dbLepr/dbLepr mice. These pairs were included to test whether different results were obtained if the transplanted fat expressed short-, but not long-form leptin receptors. Control pairs were pairs in which neither partner received a transplant. Additional pairs of two ob/ob mice transplanted with wild-type fat were included in the experiment to test for the requirement for leptin in the transplant recipient mouse.
Individual mice that were to be joined in parabiotic pairs were housed together for 3 or 4 days before surgery. Parabiosis surgery was performed when the animals were 40 days of age, as described previously (12). The animals were anesthetized with isoflurane and were treated with analgesic (Ketofen: 2 mg/kg ip) before surgery and again 24 and 48 h after surgery. At the same time as the mice were parabiosed, a transplant of ING fat was placed subcutaneously on the ventral surface of the recipient partner. Transplants weighed 200–350 mg. Four ob/ob pairs did not receive a transplant and controlled for the possibility that leptin released from transplanted fat would reduce endogenous fat mass in ob/ob mice that received leptin-expressing transplants. Following surgery, the pairs were housed in cages with food scattered in the bedding to facilitate access, and the cages were placed on heating pads to help the mice maintain body temperature. Six of twenty-two pairs of db3J/db3J–db3J/db3J, five out of eighteen ob/ob–db3J/db3J pairs, and two of eleven pairs of ob/ob–ob/ob mice died within a week of surgery. The cause of death was not determined, but we have previously found that parabiosed obese mice are highly susceptible to hypothermia (12, 13). The pairs were weighed daily and were killed 17 days after surgery. This early time point was chosen because ob/ob partners of db/db mice lose 50–60% of their body weight and body fat within this time period (13).
Mice were killed by decapitation, and trunk blood was collected from individual members of a pair for measurement of serum leptin. The two carcasses were separated along the union, fat pads and liver of each mouse were weighed, and the transplanted fat was dissected out and weighed. A small piece of the transplant was fixed in osmium tetroxide for determination of cell size distribution. Body composition was determined for individual members of a pair.
Data analysis.
Statistically significant differences between treatment groups were determined using Statistica software version 9.0 (StatSoft, Tulsa, OK). Differences were considered significant at P < 0.05. Single end-point measures were compared by unpaired t-test, one-way ANOVA, or two-way ANOVA depending upon experimental design. Daily measures of food intake or body weight were compared by repeated-measures ANOVA. Post hoc differences were determined using Duncan's multiple-range test.
RESULTS
Experiment 1: transplant of db/db fat into db/db mice.
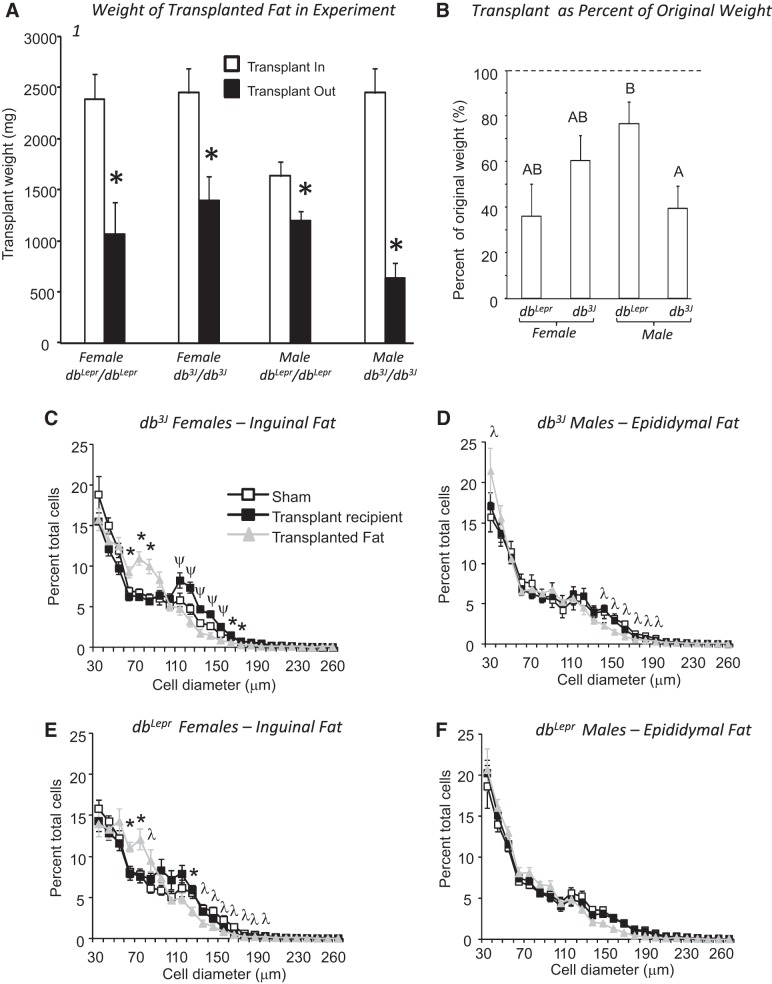
In this experiment, fat from db/db mice was transplanted into db/db mice matched for sex and genotype to test whether fat transplanted retained its morphological phenotype and whether the recipient mouse responded to an increase in body fat mass in the absence of leptin receptors. There was no difference in the weights of dbLepr/dbLepr and db3J/db3J mice at the start of the study, and there was no effect of gene, sex, or transplant on weight gain during the 12 wk of the experiment (Table 1). At the end of the study, the weight of all transplants was significantly lower than when it had been placed in recipient mice (Fig. 1A). Because the weight of fat transplanted into different mice varied significantly, the weight of each transplant removed was expressed as a percentage of its original weight (Fig. 1B). Transplants removed from male dbLepr/dbLepr mice were significantly larger as a percentage of their original weight than those in male db3J/db3J mice (gene × sex: P < 0.01: Fig. 1B). There was no effect of genotype on percent cell size distribution in the transplant for either male or female mice (Fig. 1, C–F). For both genotypes of females, there was no significant effect of treatment, but a significant effect of size (P < 0.0001) and a significant interaction (P < 0.0001) for the distribution. Transplanted fat in females contained fewer cells in the 120–160-μm cell size range and more in the 70–100-μm size range than in endogenous ING of sham or transplant recipient mice. Repeated-measures analysis showed a significant effect of treatment (P < 0.03), a significant effect of size (P < 0.0001) for both genotypes of male mice and a significant interaction (P < 0.001) in db3J/db3J, but not dbLepr/dbLepr males. There were fewer cells in the 150–200-μm size range for transplants in db3J/db3J compared with endogenous EPI fat of the shams and transplant recipient mice.
Table 1.
Body weights and body composition of db/db mice in Experiment 1 that received db/db fat transplants
|
dbLepr/dbLepr Female |
db3J/db3J Female |
dbLepr/dbLepr Male |
db3J/db3J Male |
|||||
|---|---|---|---|---|---|---|---|---|
| Sham | Transplant | Sham | Transplant | Sham | Transplant | Sham | Transplant | |
| n | 7 | 6 | 8 | 7 | 9 | 9 | 7 | 6 |
| Start weight, g | 33.9 ± 0.6 | 32.3 ± 1.2 | 33.3 ± 1.5 | 36.5 ± 1.4 | 33.1 ± 0.9 | 34.2 ± 1.4 | 38.6 ± 1.5 | 36.4 ± 1.2 |
| Weight gain, g | 22.2 ± 1.9 | 26.0 ± 2.6 | 26.6 ± 2.7 | 23.9 ± 26.6 | 20.9 ± 1.3 | 22.8 ± 1.2 | 22.7 ± 1.4 | 26.6 ± 1.3 |
| Carcass composition (no transplant) | ||||||||
| Fat, % | 53.0 ± 1.7A | 53.3 ± 2.9A | 57.5 ± 1.6A | 56.3 ± 2.0A,C | 45.6 ± 1.6B | 45.4 ± 1.7B | 51.2 ± 1.1B,C | 47.5 ± 1.2B |
| Fat, g | 26.5 ± 2.0A,B | 28.3 ± 3.2A,C | 31.1 ± 2.6A | 30.4 ± 2.3A | 21.9 ± 1.1B | 23.3 ± 1.7B,C | 27.8 ± 1.0A,B | 26.8 ± 0.9A,B |
| Lean mass, g | 22.3 ± 1.1A | 23.1 ± 0.9A,C | 21.5 ± 1.1A | 22.2 ± 1.0A | 25.1 ± 1.1C | 26.0 ± 1.0A,C | 25.6 ± 1.2A,C | 28.7 ± 1.0B,C |
Data are expressed as means ± SE. Data from db/db mice that received transplants of sex and genotype-matched fat transplants 12 wk before the end of the study. Values for a specific parameter that do not share a common superscripted letter are significantly different at P < 0.05.
Fig. 1.
Data from Experiment 1 in which db/db fat was transplanted into db/db mice of the same genotype. Data are expressed as means ± SE for groups of 5–7 mice. A: *Significant decrease in the size of the transplanted fat at the end of the 12-wk experiment, compared with the weight at the start of the experiment. B: superscripted letters indicate significant differences between groups. C–F: compare the cell size distribution for transplanted fat and the equivalent endogenous fat depot for the different groups of mice. *Significant difference between cells in transplants compared with endogenous fat in sham and transplant recipient mice. λ indicates a significant difference in the number of cells in transplants compared with endogenous fat in sham mice. Ψ indicates a difference in the number of cells from endogenous fat in transplant recipients compared with transplants and endogenous fat from sham mice. Differences were considered significant at P < 0.05.
Transplants did not influence the body fat or lean tissue mass of the recipient mice, compared with their appropriate sham control (Table 1). Statistical analysis indicated a significant effect of genotype on carcass fat content expressed either as a percentage or as grams per mouse [Table 1: gene: P < 0.01, sex: P < 0.0001, transplant: not significant (NS), interactions: NS], but post hoc analysis did not show any difference in carcass fat content between genotypes. Female db/db mice were fatter than the males. Although statistical analysis showed some genotype effects on the weight of individual fat depots, none of these differences were consistent between genotype, and none of them was associated with differences between sham and transplant recipient mice of the same sex and genotype (data not shown). Male mice had a significantly greater lean mass than females (Table 1: P < 0.0001). There were no significant differences in leptin, serum glucose, insulin, or free fatty acids (FFA) at the end of the study (data not shown).
Experiment 2: transplant of wild-type fat into db/db mice.
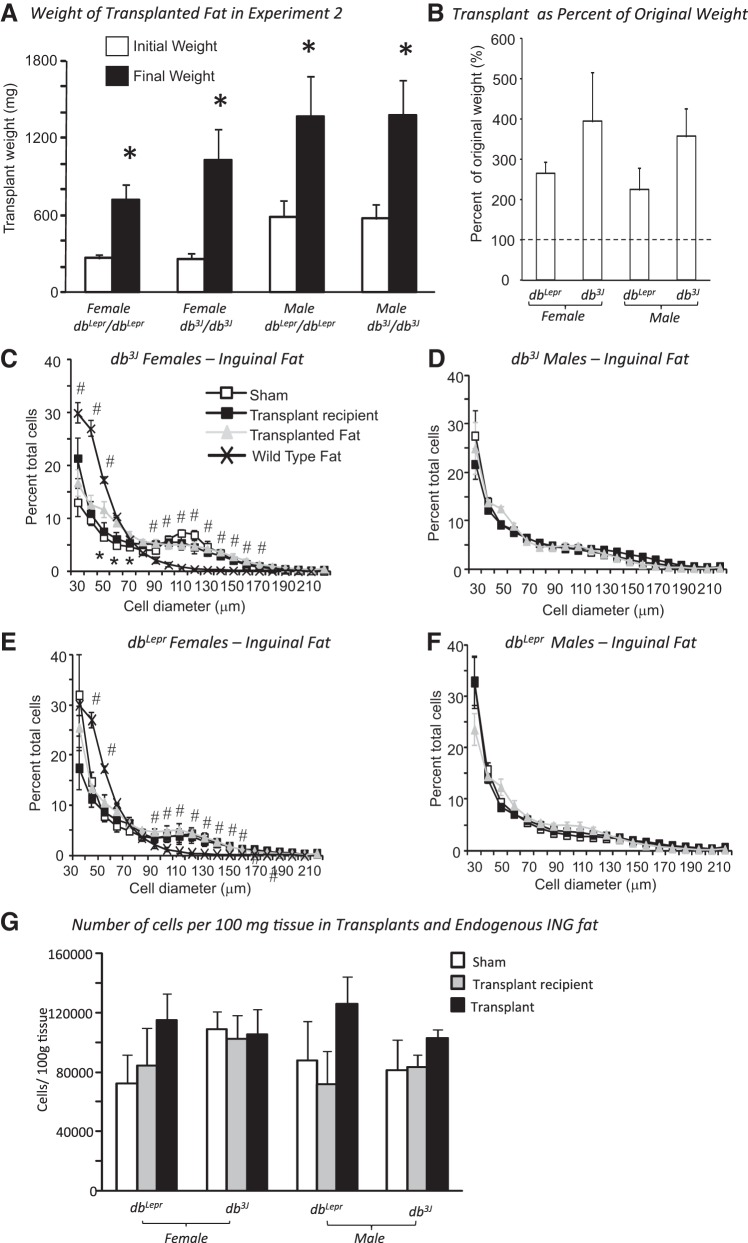
This study tested whether fat that expressed leptin receptors took on the phenotype of the obese transplant recipient, even in the presence of high concentrations of leptin. Similar to Experiment 1, placement of transplants of ING fat from wild-type mice had no effect on weight gain of db/db mice during the 12 wk of the study (Table 2). By contrast to the results of Experiment 1, all of the transplants increased 3- to 4-fold in size when placed in db/db mice, but there was no effect of sex or genotype of the host on the percent increase in weight of the transplants (Fig. 2, A and B). Transplanted wild-type ING fat had fewer cells in the 30–50 μm range and more in the 90–200 μm range than ING fat from age-matched wild-type mice (Fig. 2, C and E). In female db3J/db3J mice, there were more cells in the 50–70-μm size range for the transplant compared with ING fat in the recipient and sham mice (Fig. 2C), but there were no differences between transplants and endogenous fat of the other recipient db/db mice (Fig. 2, D–F). There were no differences in the number of cells in transplants vs. endogenous fat when expressed per unit weight of tissue (Fig. 2G). hematoxylin-and-eosin (H&E) staining confirmed the similar morphology of transplanted wild-type and endogenous db/db ING fat (Fig. 3).
Table 2.
Body weight, fat depot weight, body composition, and serum leptin in db/db mice that received wild-type transplants in Experiment 2
|
dbLepr/dbLepr Female |
db3J/db3J Female |
dbLepr/dbLepr Male |
db3J/db3J Male |
|||||
|---|---|---|---|---|---|---|---|---|
| Sham | Transplant | Sham | Transplant | Sham | Transplant | Sham | Transplant | |
| n | 7 | 7 | 6 | 8 | 6 | 8 | 6 | 5 |
| Start weight, g | 36 ± 1 | 36 ± 2 | 37 ± 1 | 37 ± 1 | 38 ± 1 | 37 ± 2 | 38 ± 2 | 38 ± 2 |
| Weight gain, g | 24 ± 1 | 24 ± 2 | 22 ± 2 | 24 ± 1 | 24 ± 1 | 23 ± 2 | 20 ± 2 | 20 ± 1 |
| Fat pad weights, mg | ||||||||
| ING | 6312 ± 267A,C | 5150 ± 734B | 5738 ± 395B,C | 5650 ± 300B | 5563 ± 193B,C | 4733 ± 353B,C | 4475 ± 409B | 4542 ± 440B |
| EPI/PM | 5733 ± 317A | 5828 ± 367A | 4535 ± 511B,C | 5425 ± 632A,B | 3112 ± 342C | 2830 ± 342C | 2616 ± 213C | 2931 ± 420C |
| Carcass composition (no transplant) | ||||||||
| Fat, % | 57.3 ± 1.2A,B | 55.0 ± 2.2A,B | 51.6 ± 1.2A,B | 58.1 ± 0.7A | 53.2 ± 1.2A,B | 47.1 ± 2.2B | 49.6 ± 0.1A,B | 49.5 ± 0.2B |
| Fat, g | 28.6 ± 2.5 | 28.2 ± 3.4 | 28.3 ± 1.7 | 31.6 ± 1.0 | 29.9 ± 1.1 | 25.7 ± 1.7 | 26.3 ± 2.4 | 27.1 ± 1.6 |
| Lean mass, g | 26.0 ± 3.2A,B | 22.1 ± 2.0B | 25.6 ± 0.7A,B | 22.0 ± 0.6B | 25.5 ± 0.7A,B | 28.7 ± 1.7A | 25.8 ± 2.8A,B | 26.6 ± 0.4A,B |
| Serum Assays | ||||||||
| Leptin, ng/ml | 159 ± 17A | 164 ± 22A | 154 ± 32A | 177 ± 17A | 149 ± 18A | 184 ± 13A | 79 ± 20B | 129 ± 19A,B |
Data are expressed as means ± SE. Data from db/db mice that received transplants from sex matched wild-type mice 12 wk before the end of the study. ING, inguinal; EPI, epididymal; PM, parametrial. Values for a specific parameter that do not share a common superscripted letter are significantly different at P < 0.05.
Fig. 2.
Data from Experiment 2 in which wild-type fat was transplanted into db/db mice. Data are expressed as means ± SE for groups of 5–7 mice. A: *Significant increase in the size of the transplanted fat at the end of the 12-wk experiment, compared with the weight at the start of the experiment. B: weight of the transplants at the end of the experiment expressed as a percentage of weight at the start of the experiment. C–F: compare the cell size distribution for transplanted fat and the equivalent endogenous fat depot for the different groups of mice. G: number of cells per 100 mg of tissue for transplants and endogenous inguinal (ING) fat from sham and recipient mice. *Significant difference between cell in transplants compared with endogenous fat in sham and transplant recipient mice. #Significant difference in the number of cells in ING fat from age-matched sham mice compared with transplants and endogenous fat in sham and recipient mice. Differences were considered significant at P < 0.05.
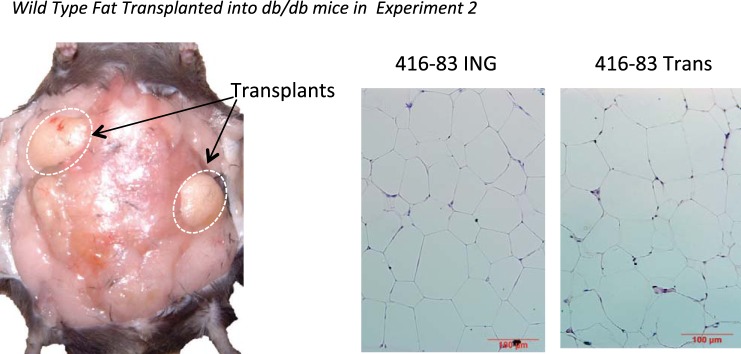
Fig. 3.
Left: illustration of transplanted fat placed dorsally in a db/db mouse. The experiment ended 12 wk after transplant placement. Right: hematoxylin-and-eosin staining of sections of endogenous ING fat and transplanted wild-type fat in a db3J/db3J mouse.
Transplants reduced the size of ING fat depots in dbLepr/dbLepr, but not db3J/db3J mice. This difference reached significance in female, but not male dbLepr/dbLepr mice (P < 0.1) (Table 2: gene: NS, sex: P < 0.02, transplant: NS, gene × transplant: P < 0.04). Parametrial fat in female mice was larger than the EPI fat in male mice and tended to be larger in dbLepr/dbLepr than db3J/db3J mice, but there was no effect of transplant on either fat depot. (Table 2: gene: P < 0.04, sex: P < 0.0001, transplant: NS, interactions: NS). There were no significant differences in the size of retroperitoneal or mesenteric fat depots of any of the groups of mice (data not shown). Percent carcass fat was greater in female than in male mice, but there was no effect of transplanting fat on the endogenous fat content of any of the groups of mice (Table 2: gene: NS, sex: P < 0.04, transplant: NS, gene × transplant: P < 0.01, sex × transplant: P < 0.01). Lean tissue tended to be greater in male than female mice, but there was no impact of fat transplants on lean body mass of any group of mice (Table 2: gene: NS, sex: P < 0.04, transplant: NS, sex × transplant: P < 0.03). All of the db/db mice were hyper-leptinemic, but leptin was lower in male db3J/db3J mice than the other groups (Table 2: gene: P < 0.04, sex: P < 0.05, transplant: NS, sex × gene: P < 0.025).
Experiment 3: transplant of wild-type and db3J/db3J fat into wild-type mice.
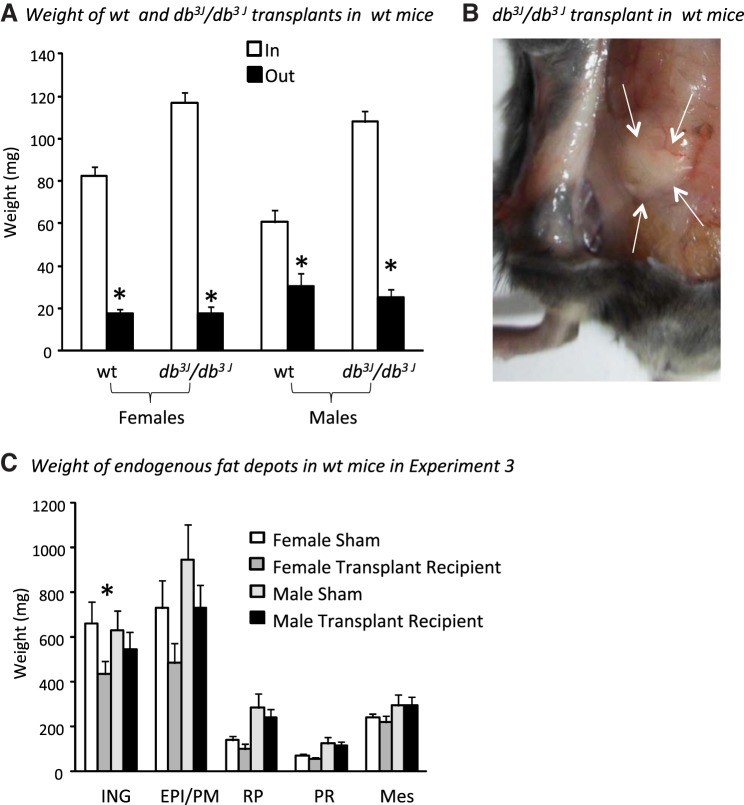
In this experiment, each recipient wild-type mouse received one transplant from a wild-type mouse and one from a db3J/db3J mouse. This allowed us to compare the response of transplants that did and did not express leptin receptors in identical environments. All transplanted fat was reduced in size by the end of the experiment in both male and female mice, irrespective of whether it was obtained from a wild-type or a db3J/db3J mouse (Fig. 4A). Even though larger pieces of db3J/db3J fat than wild-type fat were transplanted into the mice, the final weights of the two transplants were not different. This was true for both males and females, even though the final weight of the transplants was higher in males than females (Fig. 3; sex: P < 0.01, depot transplanted: NS, interaction: NS). The total weight of dissected endogenous fat pads in female transplant recipient mice was 1,378 ± 202 mg, compared with 1,664 ± 227 mg (P < 0.05) in age-matched sham controls. Endogenous fat depots dissected from male transplant recipients weighed 1,930 ± 208 mg compared with 2,287 ± 374 (P < 0.2) in their age and weight-matched controls. Comparison of individual fat depots (Fig. 4C) indicated that ING, gonadal, and retroperitoneal fat tended to be smaller in transplant recipient than control mice, but this difference reached significance only for the ING fat in female mice. There were no differences in serum leptin or in PPARγ or Ki-67 protein expression in ING fat from male or female transplant recipient and control mice (data not shown).
Fig. 4.
Data from Experiment 3 in which wild-type and db3J/db3J fat was transplanted into wild-type mice. Data are means ± SE for groups of 8 or 9 mice. A: *Significant decrease (P < 0.05) in the size of the transplanted fat at the end of the 12-wk experiment, compared with the weight at the start of the experiment. B: db3J/db3J transplant at the end of the 12-wk study. C: weights of endogenous fat depots in recipient and sham male and female mice at the end of the experiment.
Experiment 4: transplant of wild-type fat into parabiosed db3J/db3J mice.
This study used parabiosed mice to test whether leptin receptor activation was required to initiate release of a second circulating factor, which then contributed to a reduction in the size of the fat transplant. This experiment was shorter than those with single mice due to the rapid weight loss experienced by ob/ob partners of db3J/db3J mice. The weights of wild-type transplants are shown in Fig. 5A. As expected, transplants in pairs of two db3J/db3J mice increased in size, whereas transplants in pairs of db3J/db3J–ob/ob mice decreased in size and those in pairs of two ob/ob mice did not change during the 17 days of the study. This was true whether the size of the transplant was expressed as an actual weight or as percent original weight (Fig. 5B). There were no significant differences in cell size distribution for the transplants from the different pairs (data not shown). All dbLepr/dbLepr transplants decreased in size, but the percent decrease in weight was greater for those in db3J/db3J–ob/ob pairs than pairs of two db3J/db3J mice (Fig. 5, C and D).
Fig. 5.
Data from Experiment 4 in which fat was transplanted in members of parabiotic pairs. A: weights of wild-type fat transplants at the start and at the end of the 17-day experiment. B: same data shown with the weight of the transplant at the end of the study expressed as a percentage of starting weight. C: start and end weight of dbLepr/dbLepr fat transplants are shown. D: transplant weight at the end of the study expressed as a percentage of start weight. *Significant difference (P < 0.05) in weight of transplants at the start of a study compared with the end of the study. Superscripts indicate significant differences in transplant weight expressed as a percentage of their original weight.
The ob/ob partners of db3J/db3J mice lost body weight and body fat during the study (Tables 3 and 4). The ob/ob partners in pairs that received db3J/db3J transplants (Table 4) also had a reduced lean body mass compared with the members of control ob/ob–ob/ob pairs (Table 3), whereas ob/ob partners in pairs that received wild-type transplants did not. In pairs with two ob/ob mice, the transplant recipients lost weight and body fat, but to a lesser degree than the partners of db3J/db3J mice. The ob/ob partners that did not receive the transplant did not lose any weight or fat mass. The fat depot weights of db3J/db3J mice that were partners of ob/ob mice were larger than those of members of db3J/db3J-db3J/db3J pairs, although the difference was not statistically significant for mice that received wild-type transplants.
Table 3.
Body composition of parabiosed mice with wild-type transplants in Experiment 4
|
db3J/db3J–db3J/db3J Pairs |
db3J/db3J–ob/ob Pairs |
ob/ob–ob/ob Pairs |
ob/ob Control Pairs: No Transplant |
||||
|---|---|---|---|---|---|---|---|
| db3J/db3J Recipient | db3J/db3J Partner | db3J/db3J Recipient | ob/ob Partner | ob/ob Recipient | ob/ob Partner | ob/ob Partner | |
| Carcass weight, g | 31 ± 2 | 32 ± 1 | 34 ± 3 | 26 ± 3A | 28 ± 3A,B | 37 ± 1B | 38 ± 1B |
| Fat, g | 14 ± 1 | 15 ± 1 | 17 ± 2 | 9 ± 2A | 14 ± 2A,B | 18 ± 1B | 21 ± 1B |
| Protein, g | 4.9 ± 0.5 | 4.8 ± 0.4 | 4.2 ± 0.5 | 6.6 ± 1.3 | 4.4 ± 0.1 | 6.9 ± 0.9 | 5.0 ± 0.4 |
| Ash, g | 0.63 ± 0.04 | 0.61 ± 0.05 | 0.64 ± 0.04 | 0.62 ± 0.04A | 0.64 ± 0.03A,B | 0.77 ± 0.02B | 0.65 ± 0.04A,B |
| Lean mass, g | 16 ± 1 | 16 ± 1 | 15 ± 1 | 16 ± 1 | 14 ± 1 | 19 ± 1 | 17 ± 1 |
| Fat depot weights, mg | |||||||
| ING, mg | 2671 ± 191a | 2157 ± 132a | 2964 ± 188b | 1638 ± 390A | 2165 ± 248A | 3240 ± 215A,B | 3453 ± 161B |
| EPI, mg | 2722 ± 139a | 2856 ± 196a,b | 3302 ± 267b | 2322 ± 430A | 2976 ± 167A,B | 3470 ± 180B | 3493 ± 171B |
| RP, mg | 595 ± 47 | 576 ± 53 | 668 ± 109 | 371 ± 70 | 525 ± 87 | 752 ± 59 | 898 ± 45 |
| PR, mg | 422 ± 25a | 397 ± 37a | 545 ± 64b | 281 ± 113A | 300 ± 57A,B | 590 ± 35A,B | 648 ± 65B |
| Mes, mg | 789 ± 60a | 801 ± 52a,b | 981 ± 160b | 430 ± 128A | 591 ± 124A,B | 1029 ± 79B | 981 ± 39B |
Data are expressed as means ± SE for groups of 5–8 mice. A transplant of wt fat was placed at the same time as mice were parabiosed. Tissues were collected 17 days later. Carcass fat content does not include the transplanted fat. RP, retroperitoneal; PR, perirenal; Mes, mesenteric. Values for a specific parameter that do not share a common superscripted letter are significantly different at P < 0.05.
Table 4.
Body composition of parabiosed mice with dLepr/dbLepr transplants in Experiment 4
|
db3J/db3J–db3J/db3J Pairs |
db3J/db3J–ob/ob Pairs |
|||
|---|---|---|---|---|
| db3J/db3J Recipient | db3J/db3J Partner | db3J/db3J Recipient | ob/ob Partner | |
| Carcass weight | 29 ± 2 | 28 ± 3 | 36 ± 1 | 24 ± 1* |
| Fat, g | 12 ± 3A | 14 ± 1A | 19 ± 1B | 9 ± 1* |
| Protein, g | 4.6 ± 0.5 | 4.5 ± 0.5 | 5.2 ± 0.4 | 4.5 ± 0.2 |
| Ash, g | 0.63 ± 0.05 | 0.62 ± 0.06 | 0.73 ± 0.02 | 0.70 ± 0.02 |
| Lean mass, g | 16 ± 1 | 14 ± 2 | 16 ± 1 | 14 ± 1* |
| Fat depot weights, mg | ||||
| ING, mg | 2037 ± 308a | 1908 ± 199a | 2867 ± 198B | 1553 ± 89* |
| EPI, mg | 2543 ± 309A,B | 2313 ± 248A | 3282 ± 236B | 2313 ± 144* |
| RP, mg | 433 ± 93A | 530 ± 69A,B | 886 ± 205B | 334 ± 24 |
| PR, mg | 383 ± 73 | 358 ± 41 | 510 ± 61 | 191 ± 21* |
| Mes, mg | 686 ± 166 | 716 ± 109 | 989 ± 30 | 385 ± 22* |
Data are expressed as means ± SE for groups of six mice. A transplant of dbLepr/dbLepr fat was placed at the same time as mice were parabiosed. Tissues were collected 17 days later. Carcass fat content does not include the transplanted fat. Values for a specific parameter that do not share a common superscripted letter are significantly different at P < 0.05. An asterisk indicates a significant difference (P < 0.05) from members of ob/ob–ob/ob control pairs included in Table 3.
As expected, serum leptin was very high in the db3J/db3J parabionts, and even though ob/ob mice are leptin-deficient, the assay detected very low concentrations in members of ob/ob control pairs. Leptin exchanged between parabiosed mice, but more slowly than it was removed from the circulation so that serum leptin in ob/ob partners of db3J/db3Jmice was approximately one-third of that in their partners (Table 5). There was no effect of parabiosis or fat transplant on serum glucose or insulin of the db3J/db3J mice, but partners of ob/ob mice had higher serum FFA levels than other db3J/db3J mice. There was no effect of parabiosis or transplants on serum FFA or glucose in ob/ob mice, but serum insulin was significantly reduced in partners of db3J/db3J mice, ob/ob mice that received transplants, and partners of ob/ob mice that received transplants (Table 5).
Table 5.
Serum hormones and metabolites in parabiosed mice with wild-type transplants from Experiment 4
| Role | Leptin, ng/ml | FFA, mmole/l | Glucose, mg/dl | Insulin, ng/ml | |
|---|---|---|---|---|---|
| db3J/db3J- db3J/db3J wt transplant | Recipient | 157 ± 16 | 0.86 ± 0.03a,b | 173 ± 30 | 24 ± 5 |
| Partner | 124 ± 25 | 1.15 ± 0.11a | 177 ± 36 | 26 ± 6 | |
| db3J/db3J -ob/ob wt transplant | db3J/db3J Recipient | 122 ± 45 | 0.78 ± 0.02b | 99 ± 35 | 30 ± 10 |
| ob/ob Partner | 32 ± 4A | 0.86 ± 0.12 | 82 ± 41 | 12 ± 8A | |
| ob/ob-ob/ob wt transplant | Recipient | 1.3 ± 0.05B | 1.00 ± 0.23 | 82 ± 48 | 18 ± 11A,B |
| Partner | 1.8 ± 0.39B | 0.90 ± 0.15 | 79 ± 6 | 20 ± 11A,B | |
| ob/ob No transplant | 1.1 ± 0.18B | 0.68 ± 0.02 | 102 ± 24 | 40 ± 2B |
Data are expressed as means ± SE for groups of 5 to 8 mice. Blood was collected 17 days after parabiosis and transplant surgery. Values for a specific parameter that do not share a common superscripted letter are significantly different at P < 0.05.
DISCUSSION
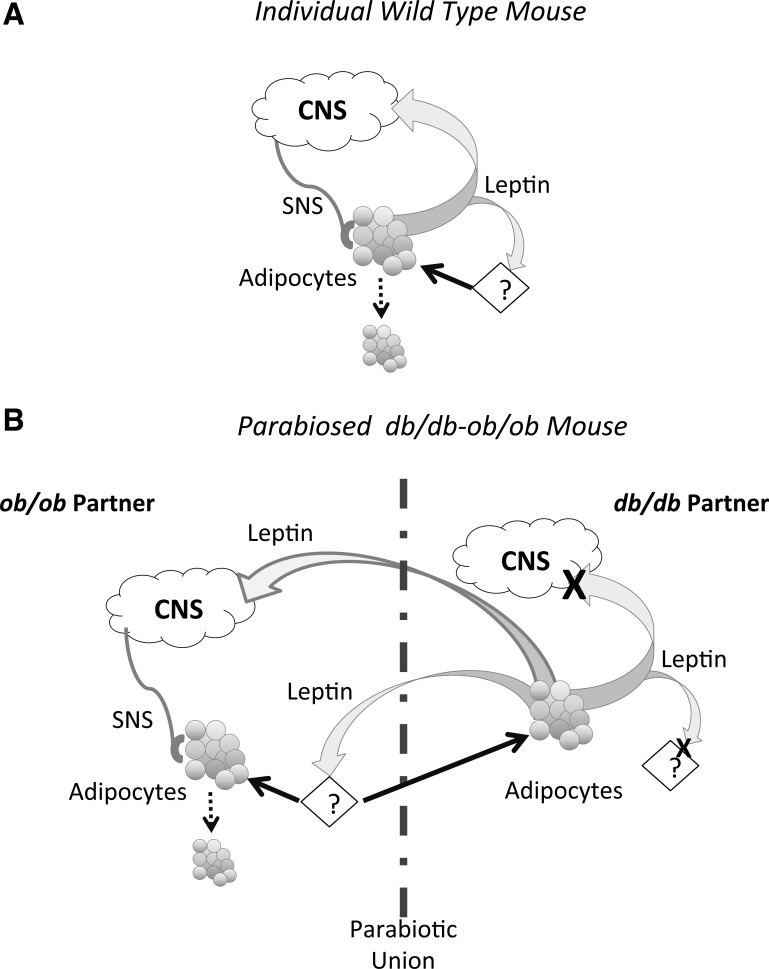
The objective of these studies was to determine the importance of adipocyte leptin receptors in mediating the response to a surgical increase in body fat mass caused by a fat transplant. We tested the importance of leptin receptors both in the fat that was transplanted and in the recipient of the transplant. Overall, the results support the conclusion that leptin does not act directly on adipose tissue to control body fat mass, but that it may promote release of other circulating factors that influence the size of white adipose tissue (see Fig. 6A). Therefore, leptin receptors are involved in the control of white fat mass, but only as part of a cascade that may be initiated in a tissue other than white fat. These results are consistent with our previous observations (45) that serum from leptin-treated rats, but not leptin alone, inhibits cell proliferation in primary adipocyte culture. We have also shown that serum from overfed obese, but not Zucker fatty, rats inhibits adipocyte lipogenesis (16), and it remains to be determined whether this “antilipogenic” factor is the same factor that limits preadipocyte proliferation or transplant size.
Fig. 6.
Schematic of the proposed, leptin-dependent release of unidentified circulating factor in the control of white fat mass. Adipocytes in this figure represent cells present in endogenous fat depots in addition to transplanted fat. A: depiction of the situation in a normal mouse. B: illustration of the potential mechanism by which this factor could influence fat mass in parabiosed ob/ob–db/db mice.
In the first two experiments, where fat was transplanted into db/db mice that were hyperleptinemic, but leptin receptor-deficient, the final size of the transplant was similar irrespective of whether the transplant had been donated by wild-type or by db3J/db3J mice. In order for this to occur, the wild-type transplant increased in size, whereas the obese transplant decreased during the experiment. There were fewer large-diameter cells and more small-diameter cells in the obese transplant compared with endogenous fat. Whereas the cell diameter was increased in the wild-type transplant, compared with inguinal fat from a wild-type mouse. These results are very similar to those reported by Ashwell and Meade (4), who transplanted fat into mice that became obese following gold thioglucose (GTG) injection. Fat from a lean mouse was transplanted under one kidney capsule, and fat from an obese GTG-mouse was placed under the other. The recipient obese GTG-mice were then food restricted for 3 wk, and at the end of this time, fat cell size distribution was the same for both of the transplants as for the endogenous fat of the recipient. Cell diameter in endogenous fat and in the obese transplant was reduced by food restriction, whereas cell diameter in the lean transplant had increased during the same time period. These observations, together with those reported here, indicate that transplanted fat takes on the morphology of the host primarily by controlling fat cell size. Our data show that this occurs irrespective of whether the fat expresses leptin receptors. We did not investigate systems that might control cell size, but Fig. 4B clearly shows that transplants had an established blood supply. It is possible that the transplants also were innervated, but Lacy and Bartness (29) suggested that the low levels of norepinephrine found in fat transplants in hamsters represented sympathetic innervation of the blood vessels, rather than of white fat cells. Some of the decrease in size of the db/db transplants may have been due to tissue necrosis, which may explain why there were fewer large cells in the db/db transplants than endogenous db/db fat in females. This effect had to be small because the transplants in female db/db mice were decreased by proportionally the same amount as those in male dbLepr/dbLepr mice in which the cell size distribution of the transplant was identical to that of endogenous fat.
In Experiment 2, the size of wild-type transplants, which express leptin receptors (27), increased in a hyperleptinemic environment. These results are also consistent with those from studies by Ashwell and colleagues (3, 5, 34), which showed that fat transplanted from a lean to an obese mouse takes on the morphological characteristics of the recipient and change in size, according to endogenous fat mass. The same results were obtained whether the obese mouse was an ob/ob, db/db, or obese yellow (Ay) mouse (5, 34). In our studies, the response was not influenced by the sex of the recipient mice, whether or not the recipient expressed short-form leptin receptors or the anatomic origin of the fat depot that was transplanted. Therefore, we have confirmed that differences in adipose tissue morphology from lean and obese mice are due to extrinsic and not intrinsic factors (3).
The recipient mice in Experiments 1 and 2 were deficient in both peripheral and central leptin receptors (42). GTG-mice lose leptin sensitivity in hypothalamic tissue (1), and once they are obese, it is likely that they are also resistant to peripheral leptin administration; ob/ob mice are leptin deficient (47); and Ay mice are resistant to peripheral leptin, but very responsive to central leptin (11). Therefore, in all experiments that show lean fat taking on an obese morphology, the recipient mouse had some degree of leptin resistance. By contrast, in Experiment 3, when fat lacking leptin receptors, but expressing leptin, was transplanted into a mouse that expressed leptin and leptin receptors, the transplants decreased in size. The parabiosis study tested whether a functional leptin system in the recipient mouse explained the decrease in size of transplants in wild-type mice (Experiment 3 and Ref. 39). In this experiment, the transplants decreased only when there were both leptin and leptin receptors present in the recipient parabiotic pair (db3J/db3J–ob/ob pairs). We hypothesize that leptin induces release of additional circulating factors and that these factors then act on white adipocytes to reduce cell size. In a lean mouse, leptin would act on endogenous leptin receptors, and the released, but unidentified, factors would control the size of the transplant. In parabiosed mice, leptin from the db/db partner would activate leptin receptors in an ob/ob partner which, in turn, would release the hypothesized factors. These factors would then travel back into the db/db partner and reduce the size of the transplant. The change in size of transplanted fat in all of the experiments described here was consistent with this hypothesis.
The parabiosis experiment was very short because the relatively high levels of leptin that develop in ob/ob partners of db/db mice cause a dramatic inhibition of food intake and body weight (13). The exaggerated leptin sensitivity of ob/ob mice was illustrated in Experiment 4 by the observations that leptin released by a 300-mg transplant of wild-type fat was enough to reduce the size of some fat depots compared with those in pairs of ob/ob mice that did not receive fat transplants. Even though the presence of the transplant reduced the fat content of the recipient ob/ob mice, there was no change in the size of the fat transplant during the experiment, which suggests that the small amount of leptin that was released, which was not detectable as a change in serum leptin concentration, was not enough to induce release of significant quantities of the unidentified circulating factors. These results also indicate that leptin modified whole animal body composition through a mechanism that did not influence the transplant. It is possible that the leptin released from the transplant acted centrally to decrease food intake and increase sympathetic nervous system activity, which would have promoted thermogenesis of the recipient mouse (9). Increased sympathetic drive to adipose tissue could also decrease endogenous body fat; however, we and others have found that peripherally administered leptin can reduce the size of white fat depots independent of an increase in norepinephrine turnover (40, 46).
By contrast to the results from ob/ob–ob/ob pairs, transplanted fat from wild-type mice decreased in the db/db partners of ob/ob mice. In these pairs of mice, leptin was transported from the db/db mouse into the ob/ob partner that expressed leptin receptors. The exchange was not 100% efficient, but the amount of leptin present in the circulation of ob/ob mice was as high as would be found in a diet-induced obese wild-type mouse (41). We propose that activation of leptin receptors in ob/ob mice led to the release of additional circulating factors that were then transported back into the db/db mouse and were able to reduce the size of the transplanted fat (see Fig. 6B). We did not determine which tissue is responsible for the release of the hypothesized circulating factors because leptin receptors are widely distributed in peripheral tissue of mice. The identity of the factor or factors is also unknown. Any protein that is functional in parabiosed mice must be relatively stable in the circulation because hormones with a short half-life, such as insulin, will be cleared from the circulation faster than they can exchange between members of a pair (19). Leptin has a half-life in the circulation of ∼25 min and still does not reach equilibrium in parabiosed mice (21). Leptin is known to influence the release of other hormones, such as corticosterone (22) and glucagon (44), but the half-life of these proteins is short and they are unlikely to exchange between parabionts in significant amounts. The reduction in size of db/db transplants placed in wild-type mice was associated with a decrease in cell size, which implies net mobilization of stored lipid. We have previously reported that serum from overfed obese rats contains a factor that inhibits adipocyte lipogenesis. This factor is a protein of molecular weight greater than 30 kDa and may be glycosylated (16). Further experiments are needed to determine whether the “antilipogenic” factor is responsible for the changes in transplant cell size that were observed in experiments described here.
Others have reported that fat transplants influence whole body glucose metabolism of the recipient (10, 25, 28, 43), although there is some disagreement on which type of fat is most beneficial. Tran et al. (43) compared the effect of transplanting subcutaneous or intra-abdominal fat either subcutaneously or in the peritoneal cavity of mice. Transplants originating from subcutaneous (ING) fat inhibited growth, reduced body fat, and improved insulin sensitivity in the recipient, whereas transplants originating from intra-abdominal (EPI) fat had none of these beneficial effects. The beneficial effects of subcutaneous vs. intra-abdominal fat has been attributed to the type of immune cells present in each fat depot with subcutaneous fat being anti-inflammatory and intra-abdominal fat being proinflammatory (26). In experiments 1 and 2 described here, we transplanted ING fat in female mice and EPI fat in male mice. There was no change in glucose or insulin of either male or female transplant recipients; therefore, it did not appear that the source of the transplanted fat had any impact in db/db mice. The difference between this study and that of Tran et al. (43) could be explained by the obesity of the recipient mice and the relatively small size of the fat transplants in our study. Tran et al. (43) used transplants that were at least five times larger, and they were placed in lean, insulin-responsive recipients. In addition, obesity of the db/db mice is associated with a proinflammatory state, and although their inguinal fat contains less macrophages than their intra-abdominal fat, the infiltration is still much higher than in fat from lean animals (36). Therefore, ING transplants originating from a db/db mouse may not have the anti-inflammatory profile found in fat from a lean mouse (35), and the anti-inflammatory benefit conferred by an ING transplant donated by a lean mouse would likely be too small to influence the metabolic state of a db/db mouse.
There was no effect of the transplanted fat on the body composition of the recipient db/db mice in either Experiment 1 or 2. This differs from results reported for fat transplants in lean mice (39), but it is possible that even if the mice had compensated for the increase in body fat, it was too small for a change to be detected. The final size of the transplants was ∼1 g in mice that had a total body fat mass of 25–30 g and a standard error for a group of up to 3 g. By contrast, lean mice that received transplants in Experiment 3, especially females, tended to compensate for the increase in body fat by reducing the size of their endogenous fat depots, consistent with previous reports that lean mice compensate for the increase in body fat caused by a fat transplant (39). In our experiment, endogenous inguinal fat appeared to be the most responsive to the addition of fat as a transplant, but this was the only subcutaneous depot measured, and it is possible that other subcutaneous fat depots were also responsive. Other investigators have failed to find compensation for increased fat mass in animals receiving transplants. Lacy and Bartness (30) reported that Siberian hamsters do not reduce body fat to compensate for dorsal subcutaneous fat transplants, and Foster et al. (10) reported no compensation for intra-abdominal fat transplants in rats. Hocking et al. (25) found that transplants of subcutaneous fat into the intra-abdominal space reduced the size of endogenous fat depots in male mice, but transplanting intra-abdominal fat subcutaneously did not. Therefore, there appears to be a complex interaction between the source of the transplant, the site at which the transplant is placed, and the species or strain of the animal that determines whether or not a recipient compensates for transplanted fat. These results do not detract from evidence that leptin is involved in the control of body fat, because in the experiments reported here, transplants increased fat mass without a simultaneous measureable increase in circulating leptin, except in ob/ob transplant recipients. By contrast, enlargement of endogenous fat depots would be expected to be accompanied by an increase in circulating leptin.
In summary, the results from the experiments described here confirm previous reports that the morphology of white fat is determined by factors that are present in the environment, rather than endogenous to white fat cells. A transplant does not have to express leptin receptors to take on the same morphology as the endogenous fat of the transplant recipient because fat from db/db mice reduced in size when placed in a lean wild-type mouse. Conversely, fat from wild-type mice, which expressed leptin receptors, enlarged when placed in a hyperleptinemic, obese db/db mouse. The results from the parabiosis study suggest, however, that currently unidentified circulating factors may be responsible for the reduction in size of transplants that are placed in a wild-type mouse and that release of these factors is dependent upon a functional leptin system in the recipient mouse.
GRANTS
This work was supported by National Institutes of Health Grant DK-053903 awarded to R. B. S. Harris.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
Author contributions: R.B.H. conception and design of research; R.B.H. performed experiments; R.B.H. analyzed data; R.B.H. interpreted results of experiments; R.B.H. prepared figures; R.B.H. drafted manuscript; R.B.H. edited and revised manuscript; R.B.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Mary Shaw for her assistance with the Western blots.
REFERENCES
- 1.Anderson KD, Lambert PD, Corcoran TL, Murray JD, Thabet KE, Yancopoulos GD, Wiegand SJ. Activation of the hypothalamic arcuate nucleus predicts the anorectic actions of ciliary neurotrophic factor and leptin in intact and gold thioglucose-lesioned mice. J Neuroendocrinol 15: 649–660, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Ashwell M. The use of the adipose tissue transplantation technique to demonstrate that abnormalities in the adipose tissue metabolism of genetically obese mice are due to extrinsic rather than intrinsic factors. Int J Obes 9 Suppl 1: 77–82, 1985. [PubMed] [Google Scholar]
- 3.Ashwell M, Meade CJ. Obesity: Do fat cells from genetically obese mice (c57BL/6j ob/ob) have an innate capacity for increased fat storage? Diabetologia 15: 465–470, 1978. [DOI] [PubMed] [Google Scholar]
- 4.Ashwell M, Meade CJ. Obesity: Can some fat cells enlarge while others are shrinking? Lipids 16: 475–478, 1981. [DOI] [PubMed] [Google Scholar]
- 5.Ashwell M, Meade CJ, Medawar PM, Sowter C. Adipose tissue: Contributions of nature and nurture to the obesity of an obese mutant mouse (ob/ob). Proc R Soc Lond B 195: 343–353, 1977. [DOI] [PubMed] [Google Scholar]
- 6.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med 14: 667–675, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman DL. Effects of parabiosis of obese with diabetes, and normal mice. Diabetologia 9: 294–298, 1973. [DOI] [PubMed] [Google Scholar]
- 8.Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol 217: 1298–1304, 1969. [DOI] [PubMed] [Google Scholar]
- 9.Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci 31: 12,189–12,197, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster MT, Shi H, Seeley RJ, Woods SC. Transplantation or removal of intra-abdominal adipose tissue prevents age-induced glucose insensitivity. Physiol Behav 101: 282–288, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94: 8878–8883, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RB. Loss of body fat in lean parabiotic partners of ob/ob mice. Am J Physiol Regul Integr Comp Physiol 272: R1809–R1815, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Harris RB. Parabiosis between db/db, and ob/ob or db/+ mice. Endocrinology 140: 138–145, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Harris RB. Sympathetic denervation of one white fat depot changes norepinephrine content, and turnover in intact white and brown fat depots. Obesity (Silver Spring) 20: 1355–1364, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris RB. Direct, and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta 1842: 414–423, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RB, Bruch RC, Martin RJ. In vitro evidence for an inhibitor of lipogenesis in serum from overfed obese rats. Am J Physiol Regul Integr Comp Physiol 257: R326–R336, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Harris RB, Martin RJ. Specific depletion of body fat in parabiotic partners of tube-fed obese rats. Am J Physiol Regul Integr Comp Physiol 247: R380–R386, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Harris RB, Martin RJ. Metabolic response to a specific lipid-depleting factor in parabiotic rats. Am J Physiol Regul Integr Comp Physiol 250: R276–R286, 1986. [DOI] [PubMed] [Google Scholar]
- 19.Harris RB, Martin RJ. Site of action of putative lipostatic factor: Food intake and peripheral pentose shunt activity. Am J Physiol Regul Integr Comp Physiol 259: R45–R52, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Harris RB, Zhou J, Redmann SM Jr, Smagin GN, Smith SR, Rodgers E, Zachwieja JJ. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology 139: 8–19, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Harris RB, Zhou J, Weigle DS, Kuijper JL. Recombinant leptin exchanges between parabiosed mice but does not reach equilibrium. Am J Physiol Regul Integr Comp Physiol 272: R1800–R1808, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology 138: 3859–3863, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Hervey GR. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol 145: 336–352, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hervey GR, Parameswaran SV, Steffens AB. The effects of lateral hypothalamic stimulation in parabiotic rats J. Physiol 266: 64P–65P, 1977. [PubMed] [Google Scholar]
- 25.Hocking SL, Chisholm DJ, James DE. Studies of regional adipose transplantation reveal a unique and beneficial interaction between subcutaneous adipose tissue and the intra-abdominal compartment. Diabetologia 51: 900–902, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Hocking SL, Stewart RL, Brandon AE, Suryana E, Stuart E, Baldwin EM, Kolumam GA, Modrusan Z, Junutula JR, Gunton JE, Medynskyj M, Blaber SP, Karsten E, Herbert BR, James DE, Cooney GJ, Swarbrick MM. Subcutaneous fat transplantation alleviates diet-induced glucose intolerance and inflammation in mice. Diabetologia 58: 1587–1600, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Kielar D, Clark JS, Ciechanowicz A, Kurzawski G, Sulikowski T, Naruszewicz M. Leptin receptor isoforms expressed in human adipose tissue. Metabolism 47: 844–847, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Konrad D, Rudich A, Schoenle EJ. Improved glucose tolerance in mice receiving intraperitoneal transplantation of normal fat tissue. Diabetologia 50: 833–839, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Lacy EL, Bartness TJ. Autologous fat transplants influence compensatory white adipose tissue mass increases after lipectomy. Am J Physiol Regul Integr Comp Physiol 286: R61–R70, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Lacy EL, Bartness TJ. Effects of white adipose tissue grafts on total body fat and cellularity are dependent on graft type and location. Am J Physiol Regul Integr Comp Physiol 289: R380–R388, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Lee G, Li C, Montez J, Halaas J, Darvishzadeh J, Friedman JM. Leptin receptor mutations in 129 db3j/db3j mice and NIH facp/facp rats. Mamm Genome 8: 445–447, 1997. [PubMed] [Google Scholar]
- 32.Lee S, Rivier C. Gender differences in the effect of prenatal alcohol exposure on the hypothalamic-pituitary-adrenal axis response to immune signals. Psychoneuroendocrinology 21: 145–155, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Madiehe AM, Hebert S, Mitchell TD, Harris RB. Strain-dependent stimulation of growth in leptin-treated obese db/db mice. Endocrinology 143: 3875–3883, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Meade CJ, Ashwell M, Sowter C. Is genetically transmitted obesity due to an adipose tissue defect? Proc R Soc Lond B Biol Sci 205: 395–410, 1979. [DOI] [PubMed] [Google Scholar]
- 35.Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: Phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care 14: 341–346, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res 49: 1562–1568, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Parameswaran SV, Steffens AB, Hervey GR, de Ruiter L. Involvement of a humoral factor in regulation of body weight in parabiotic rats. Am J Physiol Regul Integr Comp Physiol 232: R150–R157, 1977. [DOI] [PubMed] [Google Scholar]
- 38.Park BH, Wang MY, Lee Y, Yu X, Ravazzola M, Orci L, Unger RH. Combined leptin actions on adipose tissue and hypothalamus are required to deplete adipocyte fat in lean rats: Implications for obesity treatment. J Biol Chem 281: 40,283–40,291, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Rooks C, Bennet T, Bartness TJ, Harris RB. Compensation for an increase in body fat caused by donor transplants into mice. Am J Physiol Regul Integr Comp Physiol 286: R1149–R1155, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Rooks CR, Penn DM, Kelso E, Bowers RR, Bartness TJ, Harris RB. Sympathetic denervation does not prevent a reduction in fat pad size of rats or mice treated with peripherally administered leptin. Am J Physiol Regul Integr Comp Physiol 289: R92–R102, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Surwit RS, Petro AE, Parekh P, Collins S. Low plasma leptin in response to dietary fat in diabetes- and obesity-prone mice. Diabetes 46: 1516–1520, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J. Identification and expression cloning of a leptin receptor, ob-r. Cell 83: 1263–1271, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7: 410–420, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 122: 4–12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagoner B, Hausman DB, Harris RB. Direct and indirect effects of leptin on preadipocyte proliferation and differentiation. Am J Physiol Regul Integr Comp Physiol 290: R1557–R1564, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Wang ZW, Zhou YT, Lee Y, Higa M, Kalra SP, Unger RH. Hyperleptinemia depletes fat from denervated fat tissue. Biochem Biophys Res Commun 260: 653–657, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]