The dietary compound carvacrol activates high-amplitude TRPV3 sparklets in endothelial cells from cerebral pial arteries and parenchymal arterioles. This is the first study to characterize TRPV3 sparklets. This is the first report to show that activation of a TRP channel causes endothelium-dependent dilation of cerebral parenchymal arterioles.
Keywords: endothelium-dependent hyperpolarization, parenchymal arterioles, vanilloid transient receptor potential 3, transient receptor potential channel, transient receptor potential sparklet
Abstract
Cerebral parenchymal arterioles (PA) regulate blood flow between pial arteries on the surface of the brain and the deeper microcirculation. Regulation of PA contractility differs from that of pial arteries and is not completely understood. Here, we investigated the hypothesis that the Ca2+ permeable vanilloid transient receptor potential (TRPV) channel TRPV3 can mediate endothelium-dependent dilation of cerebral PA. Using total internal reflection fluorescence microscopy (TIRFM), we found that carvacrol, a monoterpenoid compound derived from oregano, increased the frequency of unitary Ca2+ influx events through TRPV3 channels (TRPV3 sparklets) in endothelial cells from pial arteries and PAs. Carvacrol-induced TRPV3 sparklets were inhibited by the selective TRPV3 blocker isopentenyl pyrophosphate (IPP). TRPV3 sparklets have a greater unitary amplitude (ΔF/F0 = 0.20) than previously characterized TRPV4 (ΔF/F0 = 0.06) or TRPA1 (ΔF/F0 = 0.13) sparklets, suggesting that TRPV3-mediated Ca2+ influx could have a robust influence on cerebrovascular tone. In pressure myography experiments, carvacrol caused dilation of cerebral PA that was blocked by IPP. Carvacrol-induced dilation was nearly abolished by removal of the endothelium and block of intermediate (IK) and small-conductance Ca2+-activated K+ (SK) channels. Together, these data suggest that TRPV3 sparklets cause dilation of cerebral parenchymal arterioles by activating IK and SK channels in the endothelium.
NEW & NOTEWORTHY
The dietary compound carvacrol activates high-amplitude TRPV3 sparklets in endothelial cells from cerebral pial arteries and parenchymal arterioles. This is the first study to characterize TRPV3 sparklets. This is the first report to show that activation of a TRP channel causes endothelium-dependent dilation of cerebral parenchymal arterioles.
cerebral parenchymal arterioles (PA) are small-diameter, high-resistance vessels that regulate blood flow between pial arteries on the surface of the brain and the subsurface microvasculature. PAs serve specific capillary beds with little or no collateral circulation (20, 29), and damage or blockage of a single vessel causes cortical microinfarctions and subsequent cognitive decline (22). Thus, preservation of blood flow through PAs may be a useful treatment for small vessel disease associated with vascular cognitive impairment. However, compared with other vascular segments, little is presently known about the regulation of PA contractility.
The walls of all blood vessels, including PAs, are made up of contractile smooth muscle cells that directly control arterial diameter and endothelial cells that primarily exert a vasodilatory influence through the production of nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factors (EDHFs). Hyperpolarization of the endothelial cell plasma membrane resulting from the activation of small- and intermediate-conductance Ca2+-activated K+ channels (SK and IK) can also be communicated to underlying smooth muscle cells via myoendothelial gap junctions to elicit arterial dilation. This later mechanism is called endothelium-dependent hyperpolarization (EDH) to distinguish it from vasodilatory pathways involving diffusible EDHFs (3, 7, 12, 13). Prior studies show that IK channel current density is greater in endothelial cells from PAs compared with other vascular beds (14, 32), and SK and IK channel activity have a major effect on the regulation of PA diameter and cerebral blood flow (6, 14). However, the Ca2+-signaling pathways regulating SK and IK channel activity in PAs are not known. Ca2+-permeable members of the transient receptor potential (TRP) superfamily of cation channels contribute to endothelium-dependent vasodilation in cerebral pial, mesenteric, coronary, and other arteries (9), but no prior studies have investigated TRP channels in the endothelium of cerebral PAs (9).
Ca2+ influx through single ion channels present on the plasma membrane can be optically recorded using rapid chemical or genetically encoded Ca2+ indicators and total internal reflection fluorescence (TIRF) (26) or confocal microscopy (25). Such unitary Ca2+ influx events are called “sparklets.” Prior studies show that vanilloid transient receptor potential (TRPV) 4 sparklets (25) and ankyrin TRPA1 sparklets (28) underlie EDH in mesenteric and cerebral pial arteries, respectively. Another member of the TRPV subfamily, TRPV3, is present in the endothelium of cerebral pial arteries and causes EDH in response to carvacrol, a monoterpenoid compound found in oregano (11). TRPV3 channels have a large unitary conductance (∼150–200 pS) (5) and are highly permeable to Ca2+ [relative permeability of Ca2+ versus Na+ (PCa:PNa)] (∼12:1) (5). These properties predict that the amplitude of a TRPV3 sparklet is greater than that of a TRPV4 sparklet. However, no prior studies have reported the presence and biophysical characteristics of TRPV3 sparklets in the endothelium or have linked TRPV3 sparklet activity with endothelium-dependent vasodilation. Thus, the goals of the present study were to 1) determine whether TRPV3 channel activation causes endothelium-dependent dilation of cerebral PA and 2) to record and characterize TRPV3 sparklets in endothelial cells from pial arteries and PAs.
Here, we show that TRPV3 channels are present in the endothelium of cerebral PAs and that Ca2+ influx through these channels stimulates SK and IK channels to cause EDH. In addition, we describe and characterize TRPV3 sparklets recorded from primary endothelial cells isolated from pial arteries and PAs. Our data show that the amount of Ca2+ influx during the opening of a single TRPV3 channel is greater than that of a single TRPV4 or TRPA1 channel, indicating that TRPV3 is a major Ca2+ influx pathway in endothelial cells. These findings suggest that TRPV3 channel activity could have significant influence on cerebral microvascular function.
MATERIALS & METHODS
Animals.
Male Sprague-Dawley rats (300–400 g; Harlan) were deeply anesthetized with pentobarbital sodium (50 mg ip) and euthanized by exsanguination. Brains were isolated and placed in ice-cold MOPS-buffered saline (in mM): 3 MOPS (pH 7.4), 145 NaCl, 5 KCl, 1 MgSO4, 2.5 CaCl2, 1 KH2PO4, 0.02 EDTA, 2 sodium pyruvate, 5 glucose, and 1% bovine serum albumin. Cerebral and cerebellar pial arteries were isolated from the brain, cleaned of connective tissue, and stored in MOPS-buffered saline.
To isolate PAs, a 5 × 3-mm section of brain tissue containing the middle cerebral artery (MCA) was removed and placed in freshly-made Ca2+-free MOPS solution (in mM: 3 MOPS, 145 NaCl, 5 KCl, 1 MgSO4, 5.5 d-glucose, and 2 sodium pyruvate) with 1% bovine serum albumin at 4°C. The pia with the MCA was gently separated from the tissue, and PAs branching from the MCA were dissected. The Institutional Animal Care and Use Committees of the University of Nevada Reno and Colorado State University approved all animal procedures.
Primary endothelial cell culture.
For culture of pial artery endothelial cells, cerebral arteries were cut into segments, pinned to silgard blocks, and cut open longitudinally. Vessel segments were placed intima side down onto 35-mm MatTek dishes (14-mm microwell; Fisher Scientific) coated with Matrigel (BD Biosciences) containing one drop of endothelial cell growth medium [Dulbecco's minimum essential medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 0.075 mg/ml endothelial cell growth supplement (Sigma), 0.5% penicillin-streptomycin (Invitrogen), 1% l-glutamine (Invitrogen), and 1% minimal essential amino acids (Invitrogen)]. Tissue was incubated at 37°C with 6% CO2 for 4–5 h to allow adherence, and additional growth medium was added. Medium was changed every 2–3 days, and after 1 wk, tissue was removed from culture and migrated endothelial cells were allowed to proliferate. RT-PCR and immunolabeling were used to confirm the presence endothelial nitric oxide synthase (eNOS) and TRPV3 mRNA and protein in the isolated cells.
A similar protocol was followed to culture primary endothelial cells from PAs. Vessels were isolated as described and placed on 35-mm MatTek dishes (14-mm microwell, Fisher Scientific) coated with Matrigel (BD Biosciences) containing one drop of endothelial cell growth medium. The tissue was incubated at 37°C with 6% CO2 for 4–5 h to allow adherence, and additional growth medium was added. Medium was changed every 2–3 days, and after 1 wk, tissue was removed from culture and migrated endothelial cells were allowed to proliferate. Cells were allowed to grow for an additional 2 wk and then used for total internal reflection fluorescence microscopy (TIRFM) experiments. Culture purity was confirmed by immunolabeling using an anti-platelet endothelial cell adhesion molecule-1 (PECAM-1) primary antibody (a marker of endothelial cells, 1:250, Abcam ab28364).
Enzymatic dissociation of smooth muscle cells and endothelial cell tubes from PAs.
Freshly dissociated endothelial cell tubes from PAs were used for immunofluorescence labeling of TRPV3 and PECAM-1 (a marker of endothelial cells). Briefly, PAs were isolated as described below and placed in a 1.7-ml Eppendorf tube containing Ca2+-free artificial cerebrospinal fluid (aCSF) supplemented with 10 mg/ml BSA + 1 mg/ml papain + 1 mg/ml dl-dithiothreitol and incubated in a water bath at 37°C for 15 min. PAs were then washed three times with Ca2+-free aCSF and incubated in Ca2+-free aCSF + 1 mg/ml collagenase type II (Worthington Biochemical, Lakewood, NJ) for 15 min at 37°C. PAs were carefully washed three times with Ca2+-free aCSF and then placed in 300 μl of aCSF containing 1.8 mM Ca2+. Dissociation was completed by triturating five times with a fire-polished glass Pasteur pipette. Cells were then added to a MatTek dish previously coated with poly-l-lysine and allowed to adhere to the dish for 1 h at room temperature prior to immunolabeling.
Immunocytochemistry.
The presence of TRPV3 in endothelial cells of PAs was assessed by immunofluorescence labeling of TRPV3. PAs were isolated as described below, cannulated in a pressure myography chamber, and fixed with 4% formaldehyde in phosphate-buffered saline (PBS). Vessels were then washed three times with PBS, and PAs were incubated with 10% BSA + 1% Triton-X in PBS for 2 h. PAs were incubated overnight at 4°C with a polyclonal rabbit anti-rat TRPV3 antibody (1:250; Abcam, Cambridge, MA). Vessels were washed and incubated with a secondary antibody conjugated with Alexa Fluor 594 (Life Technologies, Grand Island, NY) for 2 h at room temperature. PAs were washed and incubated with 5 μM DAPI nuclear staining in PBS, washed, and placed on a coverslip for imaging. Fluorescence images were obtained using an Olympus IX71 confocal microscope coupled to a CSU22 Confocal Scanning Unit (Yokogawa Electric, Tokyo, Japan) and a 60×, 1.4 numerical aperture oil immersion objective with the pinhole diameter set for 1 airy unit. Excitation of Alexa Fluor 594 was by illumination with the 561-nm line set at 40% transmission, and emission was collected using a variable band pass filter set to 607–636 nm. All images were acquired at 512 × 512 pixels at 400 ms/frame and analyzed using Andor iQ2 software (version 2.9.1; Andor Technology, Belfast, UK). The primary antibody was omitted from some PAs to assess specificity.
Immunocytochemistry was used to confirm the presence of TRPV3 and eNOS protein in primary pial artery endothelial cells, PECAM-1 in endothelial cells from PAs, and myosin heavy chain in PA smooth muscle cells. Endothelial cells were isolated from Matrigel by treatment with dispase (BD Biosciences) for 30 min at 37°C. Cells were washed in PBS, suspended in media, and allowed to adhere to a glass coverslip overnight at 37°C with 6% CO2. Cell medium was removed, and cells were fixed with 4% formaldehyde for 10 min, permeabilized with methanol (−80°C), blocked with 2% BSA (in PBS), and incubated with a primary rabbit antibody specific to TRPV3 (1:250, sc-50414; Santa Cruz Biotechnology) or a primary rabbit antibody specific for eNOS (1:250, Abcam ab66127); endothelial cell tubes from PAs were incubated with a goat primary antibody against TRPV3 (1:300, Abcam ab85022) and a rabbit primary antibody against PECAM-1 (1:250, Abcam ab28364) overnight at 4°C. PA smooth muscle cells were incubated with the TRPV3 antibody (1:300, Abcam ab85022) and a rabbit primary antibody against smooth muscle myosin heavy chain (1:300, Alfa Aesar J64817; Alfa Aesar, Ward Hill, MA). Cells were washed and incubated with a fluorescent secondary antibody conjugated with Alexa Fluor 488 (goat anti-rabbit 1:500, A-11008; Invitrogen) and Alexa Fluor 594 (goat anti-mouse, 1:500, A-11032; Invitrogen) or a Texas Red fluorophore (goat anti-rabbit, 1:1,000, sc-2780; Santa Cruz Biotechnology) for 2 h at room temperature in the dark. Prior to imaging, pial artery endothelial cells were washed and mounted with UltraCruz Mounting Medium (sc-24941; Santa Cruz Biotechnology), whereas PA endothelial cell tubes and smooth muscle cells were mounted with ProLong Gold Antifade Mountant with DAPI (P36935; Invitrogen). Fluorescence images for pial artery endothelial cells were obtained using a FluoView 1000 laser-scanning confocal microscope (Olympus) and a 60×, 1.4 numerical aperture oil immersion objective with the pinhole diameter set for 1 airy unit. All images were acquired at 1,024 × 1,024 pixels at 4.0 μs/pixel and analyzed using the Volocity software package (version 6.0; Perkin-Elmer). Fluorescence images of PA smooth muscle cells and endothelial cell tubes were acquired using an Olympus IX71 confocal microscope coupled to a CSU22 Confocal Scanning Unit (Yokogawa Electric, Tokyo, Japan) and a 100×, 1.4 numerical aperture oil immersion objective with the pinhole diameter set for 1 airy unit. Excitation of Alexa Fluor 488 was achieved by illuminating the fluorophore with a 488 nm laser, and emission was captured after the light was filtered through a 525- to 530-nm band pass filter. Similarly excitation of Alexa Fluor 594 was achieved by illumination with the 561-nm line, and emission was collected using a variable band pass filter set to 607 to 636 nm.
RNA isolation and RT-CR.
Total RNA was isolated from primary cerebral artery endothelial cells using a Qiagen RNeasy Protect Mini Kit according to the manufacturer's instructions. First-strand cDNA was synthesized using an Omniscript Reverse Transcriptase kit (Qiagen, Valencia, CA). PCR was performed using primer sets specific for rat TRPV3 (QT01616503, 160-bp amplicon; Qiagen) or rat eNOS (QT01570618, 117-bp amplicon; Qiagen). PCR products were resolved on a 2% agarose gel. PCR reactions always included a template-free negative control. The PCR products were sequenced to confirm identity.
Total internal reflection fluorescence microscopy.
TIRFM recordings (3 ms of exposure time) were acquired using a through-the-lens TIRF system built around an inverted Olympus IX71 microscope equipped with an Olympus PlanApo 100× oil immersion lens (numeral aperture = 1.45) and an Andor iXON charge-coupled device camera. Cells were loaded with Fluo-4 AM (4 μM) for 20 min at 37°C with 6% CO2 in the dark. Cells were washed with and imaged in a physiological HEPES-buffered solution (in mM): 2.5 CaCl2, 146 NaCl, 4.7 KCl, 0.6 MgSO4, 0.15 NaH2PO4, 0.1 ascorbic acid, 8 glucose, and 10 HEPES (pH 7.4). All experiments were performed at room temperature (22–25°C). Each recording was 1,500 frames and ∼30–60 s in length for endothelial cells isolated from pial arteries and 750 frames (∼15–30 s in length) for cells isolated from PAs.
LC_Pro data analysis.
Data were processed using a custom algorithm implemented as a plugin (LC_Pro) for ImageJ software essentially as described previously (27). LC_Pro is specifically designed to 1) detect statistically significant fluorescent signals within background noise, 2) automatically define circular regions of interest (ROIs; 15-pixel diameter) centered at active sites containing statistically significant fluorescent signals, and 3) calculate mean fluorescence intensities within ROIs to determine specific event parameters. Mean intensity within each ROI was calculated using a modified version of the multimeasure plugin for ImageJ. Events are defined as fluorescent signals that meet several criteria: 1) a spatial restriction of ≥12.56 pixels/frame, 2) a temporal restriction of ≥2 frames, and 3) a signal that falls within P < 0.01 for Gaussian variation.
Location (x,y), amplitude, duration, attack time, decay time, and spatial spread were then calculated for each event. Fluorescence is expressed as F/F0, with the peak fluorescence (F) normalized to basal fluorescence (F0). Duration is defined as the time interval at 50% maximum peak fluorescence. Spatial spread is calculated as the area of the maximum best-fit ellipse at 95% of the peak fluorescence of an event.
PA pressure myography experiments.
Isolated PAs were transferred to a pressure myography chamber (Living Systems, Burlington, VT) and were cannulated, pressurized to 50 mmHg, and bathed in warmed (37°C), aerated aCSF (composition in mM: 124 NaCl, 3 KCl, 1.3 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 1.8 CaCl2, and 10 glucose, pH 7.4) until stable myogenic tone was generated. PA diameter was monitored continuously using video microscopy and edge detection software (Ionoptix). Passive diameter was determined by superfusing vessels with Ca2+-free aCSF supplemented with 3 mM EGTA, 0.01 mM diltiazem, and 0.1 mM sodium nitroprusside. Myogenic tone (%) was calculated as the difference between active and passive diameter at 50 mmHg divided by the passive diameter multiplied by 100. The mechanisms underlying carvacrol-induced dilation of PAs were assessed by endothelium removal after an air bubble was passed through the lumen and pharmacological inhibition of the generation of NO, prostacyclins, or EDH. NO and prostacyclin production were concomitantly inhibited by preincubation for 15 min with Nω-nitro-l-arginine methyl ester hydrochloride (100 μM), a NO synthase inhibitor, and indomethacin (10 μM), a cyclooxygenases inhibitor. EDH-dependent mechanisms were assessed by inhibition of intermediate and small-conductance Ca2+-activated K+ channels (IK and SK, respectively) with TRAM-34 (1 μM) and apamin (1 μM).
Data analysis and statistics.
All data are represented as means ± SE. Values of n refer to the number of cells or vessels used for each experiment. Each experiment was repeated using tissue from at least three different animals. Statistical analysis was performed, and graphs were constructed using GraphPad Prism version 6. Student's unpaired t-tests were used to determine statistical differences between two groups (see Fig. 3B). Paired t-tests were used to determine statistical difference before and after treatments (see Fig. 3, C–E). Two-way repeated-measures analysis of variance (ANOVA) was used to test for statistical significance within a data set with two different treatments (Fig. 4A). One-way ANOVA was used to determine statistical differences among various treatments, as in Fig. 2C. A Student-Newman-Keuls post hoc test was used to compare groups and ascertain statistical differences. A value of P ≤ 0.05 was considered statistically significant for all experiments. Histograms were constructed and fit to multiple Gaussian functions using OriginPro versio 8.5, and GraphPad Prism version 6 was used to create Figs. 4, B and C, and 5B. Concentration-response curves were made by fitting data to a four-parameter logistic equation using GraphPad Prism version 6 (Fig. 2B).
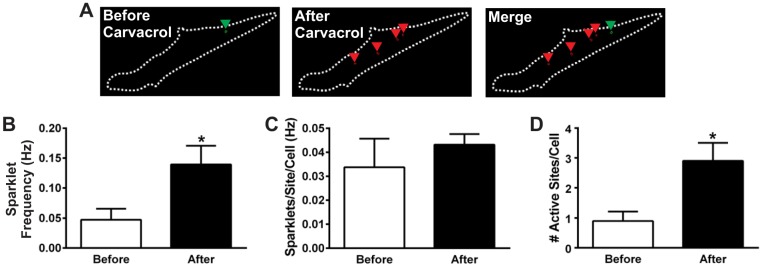
Fig. 3.
Carvacrol recruits previously inactive TRPV3 channels. A: outlines of a total internal reflection fluorescence image of a primary pial artery EC showing active sparklet sites before (green; left) and after (red; middle) the addition of carvacrol (30 μM). The merged image (right) indicates recruitment of new sparklet sites upon carvacrol addition. B: summary data of the frequency of sparklets per cell before and after the addition of carvacrol (30 μM); n = 10 cells. C: summary data for the frequency of sparklets per active site before and after the addition of carvacrol (30 μM); n = 10 cells. D: total number of active sparklet sites per cell before and after treatment with carvacrol (30 μM); n = 10 cells. *P ≤ 0.05 vs. before carvacrol.
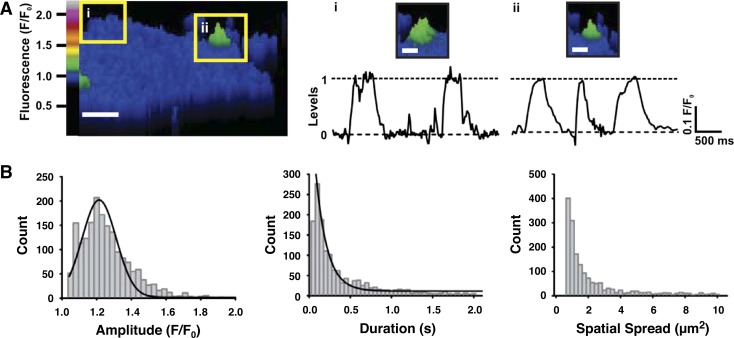
Fig. 4.
Characteristics of TRPV3 sparklets in pial artery EC. A: representative traces of fluorescence intensity (F/F0) over time for 2 active sites on a CA EC (insets i and ii, left). The majority of carvacrol-induced TRPV3 sparklets (30 μM) had an amplitude of 1.20 F/F0. Scale bar, 4 μm; inset i and ii scale bars (right), 2 μm. B: histograms of TRPV3 sparklet amplitudes (left), durations (middle), and spatial spreads (right). One prominent peak was observed at an amplitude of F/F0 = 1.20, as seen in the traces of fluorescence in A; n = 1,641 total events.
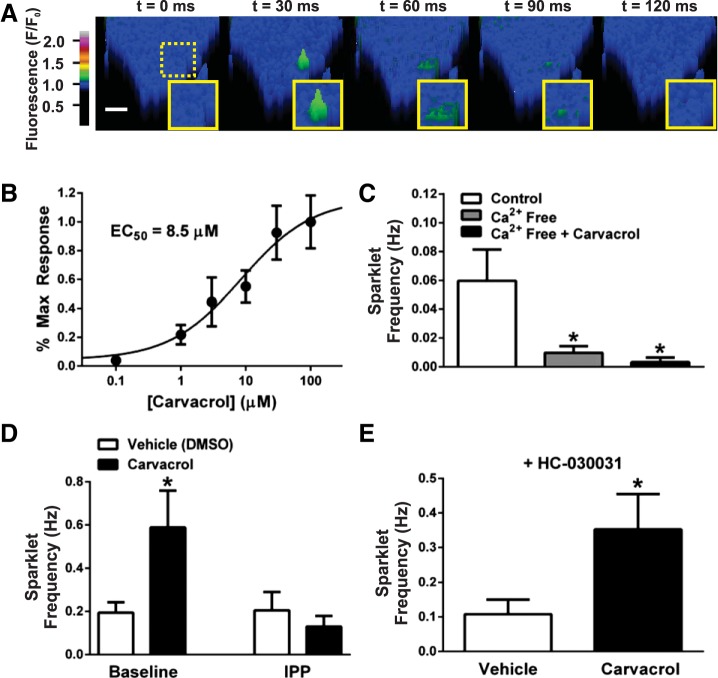
Fig. 2.
TRPV3 sparklets in primary pial artery EC. A: pseudocolor time lapse of a TRPV3 sparklet (green) in a primary pial artery EC recorded using total internal reflection fluorescence microscopy. Scale bar, 4 μm. B: TRPV3 agonist carvacrol induces a concentration-dependent increase in the frequency of sparklets (EC50 = 8.5 μM); n = 5–12 cells/concentration. C: summary data indicating that carvacrol-induced (30 μM) TRPV3 sparklets are dependent on extracellular Ca2+; n = 9–16 cells/group. *P ≤ 0.05 vs. control (not exposed to carvacrol). D: summary data indicating that the carvacrol-induced increase (30 μM) in TRPV3 sparklet frequency is attenuated by IPP (10 μM); n = 12–19 cells/group, *P ≤ 0.05 vs. baseline, vehicle. E: the TRPA1 blocker HC-030031 (10 μM) has no effect on the carvacrol-induced increase (30 μM) in TRPV3 sparklet frequency; n = 9–15 cells/group, *P ≤ 0.05 vs. vehicle.
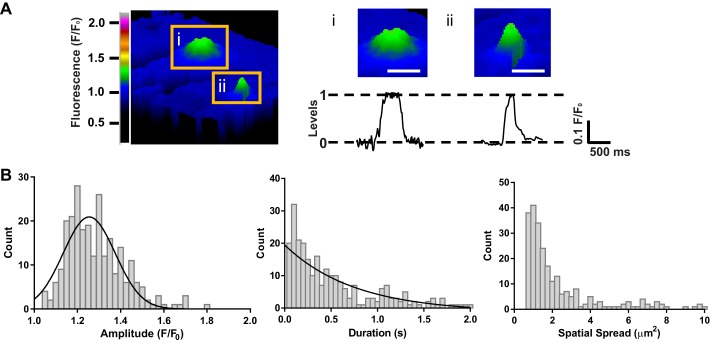
Fig. 5.
Characteristics of TRPV3 sparklets in primary PA EC. A, left: pseudocolored image (F/F0) of 2 distinct sparklet sites (insets i and ii) in the same EC after addition of carvacrol (30 μM). Scale bar, 5 μm. A, right: representative traces of fluorescence intensity (F/F0) over time for the sparklets shown in images i and ii above trace. The majority of carvacrol-induced TRPV3 sparklets (30 μM) had an amplitude of 1.20 F/F0. Scale bar, 3 μm. B: histograms of TRPV3 sparklet amplitudes (left), durations (middle), and spatial spreads (right). One prominent peak was observed at an amplitude of F/F0 = 1.20, as seen in the traces of fluorescence in A; n = 256 total events.
RESULTS
TRPV3 channels are present in the PA endothelium.
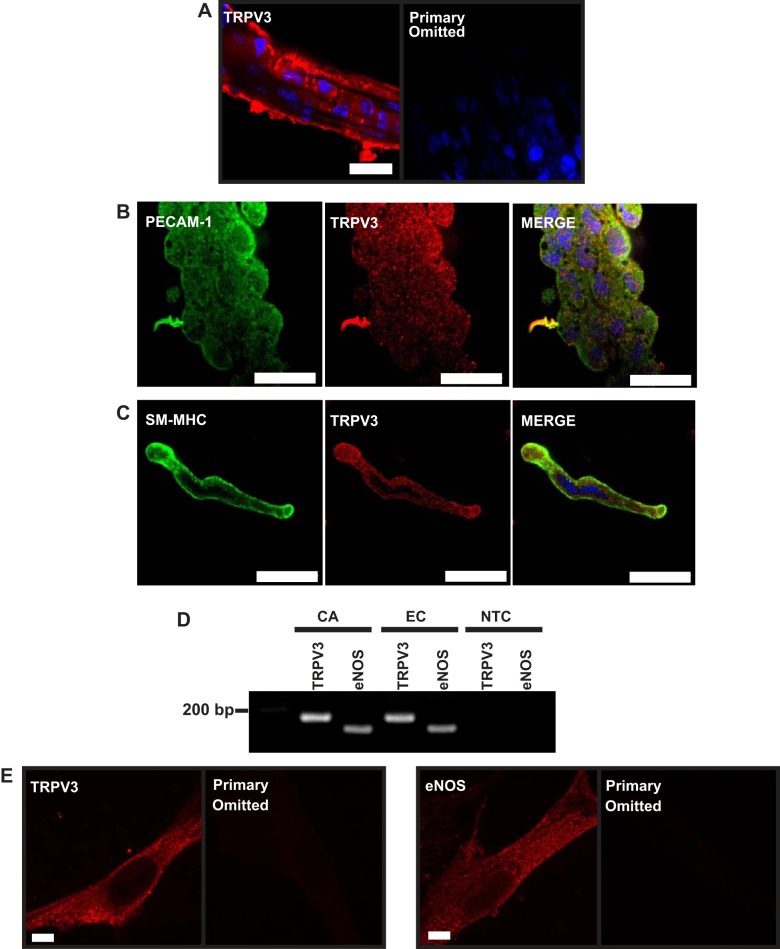
Our prior study demonstrated that TRPV3 channels are present in the endothelium of cerebral pial arteries (11). We confirmed that TRPV3 is present in PA endothelial cells through immunofluorescence labeling using an anti-TRPV3 primary antibody. Endothelial cells in intact PAs were identified as cells running parallel to the direction of blood flow visualized by labeling the nuclei with DAPI (Fig. 1A). TRPV3 immunolabeling was detected in the endothelium (Fig. 1A, left) but was absent when the primary antibody was omitted (Fig. 1A, right). To provide additional evidence for the presence of TRPV3 in the endothelium, freshly dissociated endothelial cell tubes from PAs were coimmunolabeled with antibodies against PECAM-1 and TRPV3. Figure 1B shows that both PECAM-1 (green) and TRPV3 (red) are present in these acutely isolated endothelial cell tubes.
Fig. 1.
Vanilloid transient receptor potential 3 (TRPV3) channels are present in parenchymal arterioles (PAs) and primary pial artery endothelial cells. A, left: representative image showing TRPV3 immunolabeling in the endothelium of a cerebral PA (red). A, right: no signal was detected when the primary antibody was omitted. Scale bar, 16 μm. Images are representative of 3 independent experiments. B: platelet endothelial cell adhesion molecule-1 (PECAM-1) (green; left), a marker of endothelial cells, and TRPV3 (red; middle) coimmunofluorescence in freshly isolated endothelial cell tubes from PAs. Note the colocalization in the merged image (right). Scale bar, 16 μm. Images are representative of 3 independent experiments. C: freshly dissociated smooth muscle cells from PAs positive for smooth muscle myosin heavy chain (SM-MHC) (green; left) and TRPV3 (red; middle). Scale bar, 16 μm. D: RT-PCR for TRPV3 (160 bp) and endothelial nitric oxide synthase (eNOS; 117 bp) in cerebral arteries (CA) and primary CA endothelial cells (EC). Images are representative of 3 independent experiments. E: immunocytochemistry for TRPV3 (left) and eNOS (right) in primary pial artery EC. No signal was detected when the primary antibody was omitted. Scale bar, 4 μm. Images are representative of 3 independent experiments. NTC, no cDNA template control.
Previously, we reported that TRPV3 was not present in pial artery smooth muscle cells (11). Immunolabeling for TRPV3 in PAs was present in the arteriolar wall as well as the endothelium (Fig. 1A), suggesting that the channel was present in the smooth muscle layer in this vascular segment. To further investigate this possibility, smooth muscle cells were enzymatically dissociated from PAs and coimmunolabeled for smooth muscle myosin heavy chain (a smooth muscle cell marker; green) and TRPV3 (red). These experiments demonstrate the presence of TRPV3 in smooth muscle cells in the PA wall (Fig. 1C).
Expression of eNOS and TRPV3 mRNA (Fig. 1D) and protein (Fig. 1E) was also present in primary pial artery endothelial cells, demonstrating that these cells retain an endothelial cell phenotypic marker (eNOS) after isolation and continue to express TRPV3 channels.
TRPV3 sparklets in pial artery endothelial cells.
Primary cerebral artery endothelial cells were loaded with the fast Ca2+ indicator dye Fluo-4 AM and were imaged at 488 nm using TIRF microscopy, as described previously (27, 33). Rapid, subcellular changes in Ca2+ at the cell surface (i.e., sparklets) were present but rare in unstimulated cells (0.06 ± 0.02 Hz; n = 10). Cells were treated with carvacrol to activate TRPV3 activity. Carvacrol induced a concentration-dependent increase in sparklet frequency, with a half-maximal response (EC50) at 8.5 μM and maximal stimulation at ∼30 μM (Fig. 2, A and B). Carvacrol (30 μM) did not increase sparklet frequency in the absence of extracellular Ca2, indicating that carvacrol-induced sparklets are generated by influx of Ca2+ (Fig. 2C). The selective TRPV3 inhibitor isopentenyl pyrophosphate (IPP; 10 μM) had no effect on sparklet frequency under basal conditions but blocked carvacrol-induced increases in TRPV3 sparklet frequency (Fig. 2D).
Prior studies indicate that carvacrol can also activate TRPA1 channels (30) and that high concentrations of IPP inhibit TRPA1 channel activity in human embryonic kidney-293 cell expression systems (2). Therefore, we examined the effect of the TRPA1-selective antagonist HC-030031 (10, 18) on carvacrol-induced sparklets. We found that carvacrol induced a threefold increase in sparklet frequency over baseline in the presence of HC-030031 (10 μM; Fig. 2E) that was identical to the magnitude of carvacrol-induced sparklet frequency increase in the absence of HC-030031 (Fig. 2D), indicating that Ca2+ influx events stimulated by carvacrol in these cells are the first recordings of bona fide TRPV3 sparklets.
Carvacrol recruits previously inactive TRPV3 channels.
Carvacrol-induced increases in whole cell TRPV3 sparklet frequency could result from increased generation of sparklets at basally active sites or from an increase in the total number of active sites. To distinguish between these possibilities, primary pial artery endothelial cells were imaged by TIRF microscopy before and after the addition of carvacrol (30 μM). Basal sparklets were rarely observed (0.05 ± 0.02 Hz; Fig. 3, A and B). After carvacrol addition, the frequency of sparklets increased by nearly threefold (0.14 ± 0.03 Hz, n = 10 cells; Fig. 3, A and B). Although carvacrol increased the total frequency of sparklets per cell, the frequency of sparklets at each site did not change [0.03 ± 0.01 (before) vs. 0.04 ± 0.003 (after), n = 10 cells; Fig. 3C]. In contrast, the total number of active sparklet sites per cell increased following agonist treatment [0.9 ± 0.3 (before) vs. 2.9 ± 0.6 (after), n = 10 cells; Fig. 5D]. These data suggest that carvacrol acts by recruiting previously inactive TRPV3 channels rather than modifying the kinetics of basally active channels. In addition, our findings indicate that on average only about three TRPV3 channels per cell are active during maximal agonist stimulation.
The properties of TRPV3 sparklets in endothelial cells from pial arteries and PAs are similar.
Plots of fluorescence (F/F0) as a function of time within ROIs containing active sparklet sites in endothelial cells treated with carvacrol (30 μM) revealed single channel-like events of uniform amplitude (Fig. 4A). A histogram of TRPV3 sparklet amplitudes fitted to a Gaussian curve indicated a single distinct peak at F/F0 = 1.20, suggesting that the unitary TRPV3 sparklet amplitude is ΔF/F0 = 0.20 (Fig. 4, A and B). The modal amplitude of TRPV3 sparklets was also F/F0 = 1.20 (Table 1), indicating that the majority of TRPV3 sparklets resulted from the opening of a single TRPV3 channel. A histogram of TRPV3 sparklet durations was fit to a single exponential function (τ = 170 ms) and indicates that the most frequently occurring TRPV3 sparklets are ∼70 ms in duration (Fig. 4B and Table 1). The distribution of TRPV3 sparklet area suggests that most TRPV3 sparklets had a maximum spread of 1–2 μm2 (mode spatial spread = 0.7 μm2; Fig. 4B and Table 1). Carvacrol (30 μM) also stimulated TRPV3 sparklets in endothelial cells isolated from PAs (Fig. 5, A and B). The properties of TRPV3 sparklets recorded from PA endothelial cells were similar to those recorded from pial artery endothelial cells, with the exception of the spatial spread, which is slightly larger in sparklets from PA endothelial cells (mode spatial spread = 1 μm2; Fig. 5, A and B, and Table 1). Similar unitary amplitude but increased spatial spread in endothelial cells from PAs may reflect differences in cytoskeletal architecture or endogenous Ca2+ buffering compared with pial artery endothelial cells.
Table 1.
Characteristics of transient receptor potential sparklets recorded from cerebral artery endothelial cells
TRP sparklets recorded using TIRFM exhibit distinctive amplitudes. Here, we show that the unitary amplitude of TRPV3 sparklets (ΔF/F0 = 0.20) is greater than that of TRPV4 (ΔF/F0 = 0.06) and TRPA1 (ΔF/F0 = 0.13) sparklets recorded under identical conditions (Table 1).
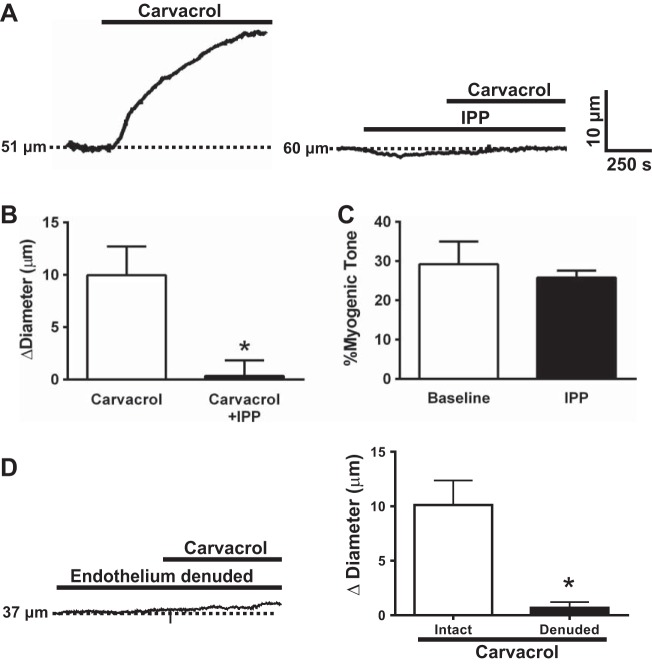
Endothelial cell TRPV3 channel activity dilates cerebral PAs.
Our prior study showed that carvacrol causes dilation of cerebral pial arteries (11). Here, we show that carvacrol also induced dilation of cerebral PAs with spontaneous myogenic tone (Fig. 6A). Blockade of TRPV3 channels with IPP nearly abolished carvacrol-induced dilation (Fig. 6, A and B), indicating that this response is dependent on TRPV3 channel activity. These data also show that IPP did not alter resting myogenic tone [myogenic tone: 29.2 ± 5.8 vs. 25.8 ± 1.8%, baseline tone vs. myogenic tone after IPP (3 μM), n = 5; Fig. 6C], suggesting that TRPV3 channel activity is very low and does not influence PA contractility under basal conditions.
Fig. 6.
TRPV3 channel activation dilates cerebral PA. A: representative recording of a pressurized PA after exposure to carvacrol (10 μM) or IPP (3 μM) + carvacrol. B: summary data of the change in PA diameter (Δdiameter) after exposure to carvacrol in the absence and presence of isopentenyl pyrophosphate (IPP; n = 5). C: IPP had no effect on basal myogenic tone of cerebral PA (n = 5). D: removal of the endothelium almost completely abolished carvacrol-induced dilation of PAs (n = 6). *P ≤ 0.05 vs. carvacrol.
TRPV3 channels are present in both smooth muscle and endothelial cells of PAs. To determine the relative involvement of each cell type in carvacrol-induced vasodilation, experiments were performed using PAs following removal of the endothelium by passage of air through the lumen. Carvacrol-induced dilation was essentially abolished by this procedure, indicating that the response is dependent on intact endothelial cell function (Fig. 6D).
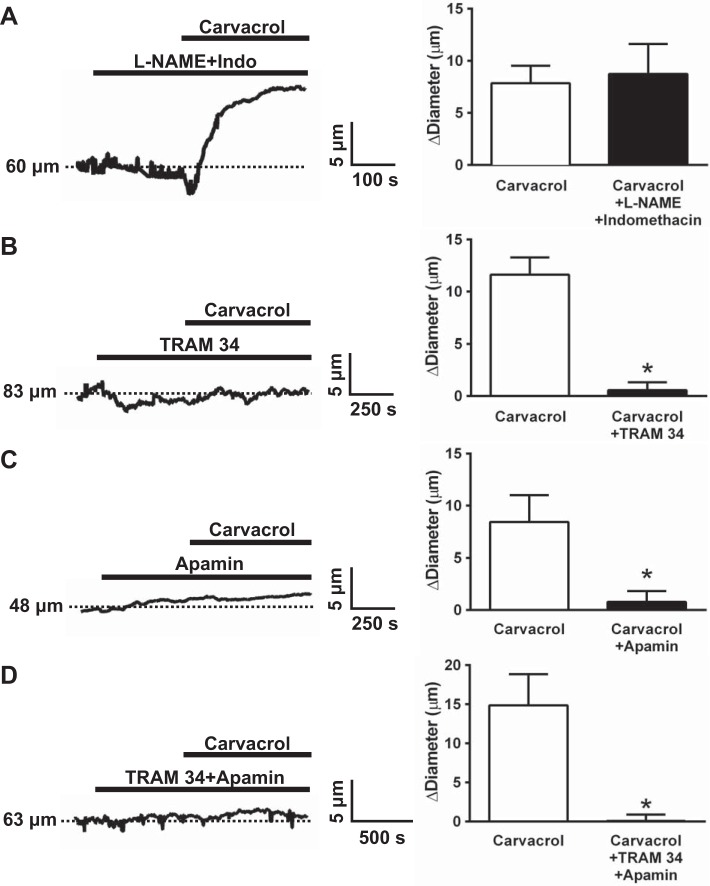
TRPV3 sparklets induce PA dilation by activating IK and SK channels.
Our previous study showed that TRPV3-mediated dilation of pial arteries is independent of NO synthase and cyclooxygenase activity and occurs via EDH involving Ca2+-activated K+ channels (11). Consistently, inhibition of NOS and cyclooxygenases had no effect on carvacrol-induced dilation of PA (Fig. 7A). In contrast, inhibition of IK channels with TRAM 34 (Fig. 7B) or inhibition of SK channels with apamin (Fig. 7C) blunted carvacrol-induced vasodilation. Concomitant inhibition of IK and SK channels completely abolished PA dilation to carvacrol (Fig. 7D). These data suggest that Ca2+ influx through endothelial cell TRPV3 channels stimulates the activity of IK and SK channels, leading to EDH and PA dilation.
Fig. 7.
Intermediate (IK) and small-conductance Ca2+-activated K+ (SK) channel inhibition abolishes carvacrol-induced dilation of cerebral PAs. A: representative recording and summary data of carvacrol-induced (10 μM) PA dilation after NO synthase and cyclooxygenase inhibition by preincubation with Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME; 100 μM) and indomethacin (10 μM), respectively (n = 6). B and C: representative recordings and summary data demonstrating that blockade of either IK inhibition with TRAM-34 (1 μM) or SK inhibition with apamin (1 μM) attenuates carvacrol-induced PA dilation (n = 6). D: representative trace and summary indicating that dual inhibition of both SK and IK channels abolishes carvacrol-induced dilation (n = 6). *P ≤ 0.05 vs. carvacrol.
DISCUSSION
Single cerebral PAs serve specific capillary beds within the brain with little or no collateral circulation, rendering the perfused tissues uniquely vulnerable to vascular dysfunction (22, 23). Regulation of PA contractility differs from that of cerebral pial vessels (4, 6, 8, 14) and is incompletely understood. In the present study, we show that TRPV3 channels are present in the endothelium of cerebral PAs, and their activity mediates endothelium-dependent dilation in response to carvacrol. In addition, we report the first characterization of TRPV3 sparklets in endothelial cells from pial arteries and PAs. The major findings of this study are that 1) functional TRPV3 channels are present in the endothelium of cerebral PAs; 2) TRPV3 sparklets occur rarely under basal conditions, but their frequency is increased by carvacrol; 3) IPP is efficacious in preventing the carvacrol-induced increase in TRPV3 sparklet frequency but does not alter non-TRPV3 sparklets in unstimulated endothelial cells; 4) TRPV3 sparklets have the largest unitary amplitude of any sparklet reported to date; and 5) carvacrol causes dilation of cerebral PA through an endothelium-dependent mechanism that requires the activation of IK and SK channels. These data suggest that carvacrol-induced TRPV3 sparklets stimulate Ca2+-activated K+ channels in the endothelium to cause dilation of cerebral PAs.
Recent groundbreaking studies demonstrate that TRPV4 and TRPA1 sparklets mediate EDH in mesenteric and cerebral pial arteries (25, 27, 28). The present data expand this concept by demonstrating that TRPV3 sparklets underlie endothelium-dependent vasodilation of cerebral PAs in response to carvacrol. We report here that carvacrol stimulated an increase in the frequency of TRPV3 sparklets (EC50 = 8.5 μM) with approximately the same potency as it caused vasodilation of cerebral pial arteries (EC50 = 4.1 μM) in our prior study (11). The amount of carvacrol needed to increase the frequency of TRPV3 sparklets and dilate cerebral arteries is nearly an order of magnitude less than that required to evoke increases in the global intracellular [Ca2+] of cerebral artery endothelial cells (EC50 = 34 μM) (11), suggesting that localized TRPV3 sparklet activity is sufficient to cause vasodilation. Our data also show that TRPV3 sparklets have the largest unitary amplitude of any Ca2+ influx event described to date, approximately three times greater than that of TRPV4 sparklets (27) and ∼1.5 times greater than the TRPA1 sparklets that were reported in our prior studies (Table 1) (28). The amount of Ca2+ entering a cell during the opening of a single TRPV4 channel is ∼100 times greater than that of an L-type Ca2+ channel (25), suggesting that a single TRPV3 sparklet allows at least ∼300 times more Ca2+ to enter compared with an L-type Ca2+ channel. Our findings further suggest that these large-amplitude, albeit relatively rare, Ca2+ signals (∼3–5/cell during maximal carvacrol-induced stimulation) are sufficient to activate SK and IK channels, leading to endothelium-dependent PA dilation. Our data are consistent with prior reports indicating an important role for SK and IK channels in the regulation of PA tone (6, 14, 32) and suggest that TRPV3 channels may serve as an important Ca2+ influx pathway to regulate the activity of these channels in PAs. Other TRP channels with high Ca2+ permeability, such as TRPV4 and TRPA1, could also be involved in the regulation of endothelial cell Ca2+ influx and PA tone by a similar mechanism.
Our data show that inhibition of either IK or SK alone is sufficient to virtually eliminate vasodilation in response to carvacrol-induced TRPV3 activity. These findings suggest that the EDH response does not result from a simple summation of SK and IK currents. We have not investigated this topic directly, but we speculate that this response could be due to differences in Ca2+ affinity between IK and SK channels. A prior study demonstrated that in native aortic endothelial cells, IK channels are more sensitive to Ca2+ compared with SK channel by nearly an order of magnitude in terms of molar [Ca2+] (17). It is possible that TRPV3-mediated Ca2+ influx initially activates outward IK channel currents. The ensuing membrane hyperpolarization increases the electrochemical driving force for Ca2+ influx, allowing Ca2+ levels to reach a magnitude sufficient to activate SK channels and cause EDH. This scheme is consistent with a recent study showing that EDH in cerebral arteries occurs through initial hyperpolarization of the endothelial cell plasma membrane initiated by IK currents that is sustained by subsequent SK activation (16) and plausibly explains our data, but rigorous study is needed test to this hypothesis.
There are presently no known endogenously produced substances that regulate the activity of native TRPV3 channels (19). TRPV3 is activated by carvacrol (oregano) (30), thymol (thyme) (30), eugenol (cloves) (30), and vanillin (vanilla) (30), but it is unlikely that these dietary molecules reach circulating levels of sufficient concentration to modulate TRPV3 activity in the cerebral endothelium. In patch clamp experiments, TRPV3 activity is stimulated by innocuous heat (i.e., between 30 and 40°C), suggesting that the channel could be basally active at physiological temperatures (21, 24, 31). However, we found that blockade of TRPV3 channels in pressure myograph studies had little effect on the diameter of isolated cerebral PAs, arguing against significant tonic activity under these conditions. TRPV3 is also activated by farnesyl pyrophosphate (FPP), an endogenous lipid required for the synthesis of cholesterol that is generated by the fusion of IPP with dimethylallyl pyrophosphate by the enzyme farnesyl pyrophosphate synthase (FPPS) (1). A recent review proposes that FPPS indirectly modulates TRPV3 channel activity in the endothelium by altering the intracellular ratio of IPP (TRPV3 antagonist) to FPP (TRPV3 agonists) (19). This scenario predicts that increased FPPS activity leads to elevated levels of FPP and diminished levels of IPP, favoring TRPV3 activation, but there is no direct experimental evidence supporting this pathway. Another potential activating mechanism is suggested by data showing that the oxygen-dependent asparaginyl hydroxylase factor inhibiting hypoxia-inducible factor (FIH) hydroxylates TRPV3 channels at asparagine 242, a residue located within the cytosolic ankyrin repeat domain (15). In heterologous expression systems, hypoxia or pharmacological inhibition of FIH activity reversed this modification and potentiated TRPV3 currents stimulated by 2-aminoethoxyphenyl borate, leading the authors of this study to propose that TRPV3 activity is regulated by hypoxia (15). Hypoxia-dependent activation of TRPV3 channels leading to endothelium-dependent dilation of PAs could have a significant impact on functional hyperemic responses in the brain, but in vivo studies are needed to investigate this hypothesis.
In summary, our data show that activation of TRPV3 channels in the endothelium of cerebral PAs causes EDH. In addition, we show that high-amplitude TRPV3 sparklets can be recorded from endothelial cells from pial arteries and PAs and underlie the vasodilatory response. Further studies are needed to understand the endogenous regulation of TRPV3 channels in PA and the impact of these mechanisms on cerebral blood flow regulation. It is possible that targeted activation of TRPV3 may emerge as a potential treatment for neurological disorders where vascular dysfunction leads to local perfusion deficits, including vascular cognitive impairment and ischemic stroke.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-091905 to S. Earley and American Heart Association Grants 15POST24720002 to P. W. Pires and 15PRE22670024 to M. N. Sullivan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.W.P., M.N.S., and S.E. conception and design of research; P.W.P., M.N.S., H.A.T.P., and J.J.R. performed experiments; P.W.P., M.N.S., H.A.T.P., and S.E. analyzed data; P.W.P., M.N.S., and S.E. interpreted results of experiments; P.W.P., M.N.S., and S.E. prepared figures; P.W.P., M.N.S., and S.E. drafted manuscript; P.W.P., M.N.S., and S.E. edited and revised manuscript; P.W.P., M.N.S., H.A.T.P., J.J.R., and S.E. approved final version of manuscript.
REFERENCES
- 1.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J Biol Chem 285: 19362–19371, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain 152: 1156–1164, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Bolton TB, Lang RJ, Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol 351: 549–572, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brayden JE, Li Y, Tavares MJ. Purinergic receptors regulate myogenic tone in cerebral parenchymal arterioles. J Cereb Blood Flow Metab 33: 293–299, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci 24: 5177–5182, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke 40: 1451–1457, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol 31: 641–649, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Dabertrand F, Nelson MT, Brayden JE. Ryanodine receptors, calcium signaling, and regulation of vascular tone in the cerebral parenchymal microcirculation. Microcirculation 20: 307–316, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev 95: 645–690, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ Res 104: 987–994, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earley S, Gonzales AL, Garcia ZI. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol 77: 612–620, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch 459: 863–879, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci 16: 23–30, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31: 1175–1186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karttunen S, Duffield M, Scrimgeour NR, Squires L, Lim WL, Dallas ML, Scragg JL, Chicher J, Dave KA, Whitelaw ML, Peers C, Gorman JJ, Gleadle JM, Rychkov GY, Peet DJ. Oxygen-dependent hydroxylation by FIH regulates the TRPV3 ion channel. J Cell Sci 128: 225–231, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Kochukov MY, Balasubramanian A, Abramowitz J, Birnbaumer L, Marrelli SP. Activation of endothelial transient receptor potential C3 channel is required for small conductance calcium-activated potassium channel activation and sustained endothelial hyperpolarization and vasodilation of cerebral artery. J Am Heart Assoc 3: e000913, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchenko SM, Sage SO. Calcium-activated potassium channels in the endothelium of intact rat aorta. J Physiol 492: 53–60, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 104: 13525–13530, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilius B, Biro T, Owsianik G. TRPV3: time to decipher a poorly understood family member! J Physiol 592: 295–304, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci USA 104: 365–370, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science 296: 2046–2049, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci 16: 55–63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih AY, Nishimura N, Nguyen J, Friedman B, Lyden PD, Schaffer CB, Kleinfeld D. Optically induced occlusion of single blood vessels in rodent neocortex. Cold Spring Harb Protoc 2013: 1153–1160, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418: 186–190, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan MN, Earley S. TRP channel Ca2+ sparklets: fundamental signals underlying endothelium-dependent hyperpolarization. Am J Physiol Cell Physiol 305: C999–C1008, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan MN, Francis M, Pitts NL, Taylor MS, Earley S. Optical recording reveals novel properties of GSK1016790A-induced vanilloid transient receptor potential channel TRPV4 activity in primary human endothelial cells. Mol Pharmacol 82: 464–472, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, Oulidi A, Boop FA, Feng Y, Jaggar JH, Welsh DG, Earley S. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci Signal 8: ra2, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woolsey TA, Rovainen CM, Cox SB, Henegar MH, Liang GE, Liu D, Moskalenko YE, Sui J, Wei L. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex 6: 647–660, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 9: 628–635, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418: 181–186, 2002. [DOI] [PubMed] [Google Scholar]
- 32.You J, Johnson TD, Marrelli SP, Bryan RM Jr. Functional heterogeneity of endothelial P2 purinoceptors in the cerebrovascular tree of the rat. Am J Physiol Heart Circ Physiol 277: H893–H900, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, Sullivan MN, Chase M, Gonzales AL, Earley S. Calcineurin/nuclear factor of activated T cells-coupled vanilliod transient receptor potential channel 4 ca2+ sparklets stimulate airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol 50: 1064–1075, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]