LPS causes a conducible environment in the mesentery that decreases the neutrophils and shifts the balance toward a M2 macrophage polarization on and/or near the vicinity of lymphatics. LPS-TLR4-mediated regulation of NF-κB, pAKT, pERK, and pMLC20 in lymphatic muscle cells promotes inflammation and significant impairment in mesenteric lymphatic function.
Keywords: inflammation, lymphatic function, neutrophils, M1 and M2 macrophages, lipopolysaccharide
Abstract
Impairment of the lymphatic system is apparent in multiple inflammatory pathologies connected to elevated endotoxins such as LPS. However, the direct mechanisms by which LPS influences the lymphatic contractility are not well understood. We hypothesized that a dynamic modulation of innate immune cell populations in mesentery under inflammatory conditions perturbs tissue cytokine/chemokine homeostasis and subsequently influences lymphatic function. We used rats that were intraperitoneally injected with LPS (10 mg/kg) to determine the changes in the profiles of innate immune cells in the mesentery and in the stretch-mediated contractile responses of isolated lymphatic preparations. Results demonstrated a reduction in the phasic contractile activity of mesenteric lymphatic vessels from LPS-injected rats and a severe impairment of lymphatic pump function and flow. There was a significant reduction in the number of neutrophils and an increase in monocytes/macrophages present on the lymphatic vessels and in the clear mesentery of the LPS group. This population of monocytes and macrophages established a robust M2 phenotype, with the majority showing high expression of CD163 and CD206. Several cytokines and chemoattractants for neutrophils and macrophages were significantly changed in the mesentery of LPS-injected rats. Treatment of lymphatic muscle cells (LMCs) with LPS showed significant changes in the expression of adhesion molecules, VCAM1, ICAM1, CXCR2, and galectin-9. LPS-TLR4-mediated regulation of pAKT, pERK pI-κB, and pMLC20 in LMCs promoted both contractile and inflammatory pathways. Thus, our data provide the first evidence connecting the dynamic changes in innate immune cells on or near the lymphatics and complex cytokine milieu during inflammation with lymphatic dysfunction.
NEW & NOTEWORTHY
LPS causes a conducible environment in the mesentery that decreases the neutrophils and shifts the balance toward a M2 macrophage polarization on and/or near the vicinity of lymphatics. LPS-TLR4-mediated regulation of NF-κB, pAKT, pERK, and pMLC20 in lymphatic muscle cells promotes inflammation and significant impairment in mesenteric lymphatic function.
the lymphatics have emerged as a central player in the process of inflammation and play active roles in both resolution and progression of inflammation (40, 76). Mesenteric lymphatics, in particular, are directly exposed to both the inflammatory activation and dyslipidemia resulting from the aberrant elevated postprandial chylomicron production in metabolic syndrome, as well as the dietary endotoxins, such as LPS associated with them (32, 37, 74). LPS, a critical cell wall component of most gram-negative bacteria, has been identified as the major effector of conditions, such as peritonitis and endotoxemia, that result in septic shock and multiple organ failure (6, 58, 60, 70). As lymphatic pumping is significantly affected by mechanical and chemical stimuli, including inflammatory mediators and an increased fluid load, these stimuli significantly affect lymphatic pumping during edemagenic conditions (30). Previous studies in sheep and guinea pig have shown that mesenteric lymph flow increases rapidly on LPS injection, which may be mainly due to microvascular hyperpermeability and plasma albumin leakage, but later affects contractility (9, 28, 56). In mouse models of LPS-induced peritonitis, which closely replicates many features of endotoxemia and sepsis, LPS has been shown to increase lymphatic density and lymphangiogenesis in the mouse diaphragm. These changes appeared to be mediated by investiture of myeloid-derived CD11b+ cells on the peritoneal side of the diaphragmatic lymphatic vessels that exhibited a profibrotic phenotype (47). Another study has shown that lymph propulsion is interrupted in mouse in acute response to LPS (3). However, no direct link has been established between LPS-induced changes in surrounding inflammatory lymphatic microenvironment, specifically the surrounding immune cells and lymphatic contractile function.
We have previously documented that a novel population of antigen-presenting cells are resident within the walls of muscular, prenodal lymphatics in the normal rats (13). Chatterjee et al. (20) and Nagai et al. (55) have shown that in older rats, representing a chronic inflammatory condition, an increase in the number of preactivated mast cells is associated with mesenteric lymphatics that could be one of the underlying factors for the lymphatic contractile dysfunction observed in aged rats. In the present study, we have raised the following questions: 1) What are the effects of LPS on lymphatic contractile parameters and its pump function? 2) How does the profile of the immune cells found around the lymphatics in the mesentery change in acute inflammation? and 3) What are the molecular mechanisms of LPS-induced changes in lymphatic contractions? To address these questions, we designed a series of experiments to specifically delineate the association of two key innate immune cell populations, neutrophils and macrophages, with lymphatic function in a rat model of LPS-induced inflammation (42, 45).
Neutrophils, as the body's first line of defense, dominate the early stages of inflammation and set the stage for repair by macrophages of tissue damage (14). Macrophages on the other hand, play an essential role in homeostasis and defense and can be polarized by the microenvironment to mount specific M1 or M2 functional programs (35, 54). Classical macrophage polarization (M1) is driven in response to microbial products or Th1 cytokines, such as IFN-γ, and is characterized by an enhanced capacity to kill intracellular microorganisms and produce generous amounts of proinflammatory mediators. Conversely, alternative macrophage polarization (M2) can be generated in response to a variety of stimuli, such as Th2 cytokines (IL-4, IL-13, and IL-10), glucocorticoids, or a mixture of Ig complexes and TLR ligands, producing an anti-inflammatory phenotype (33, 69). Although the role of the lymphatic network in the recruitment and transport of activated dendritic cells has been well established (5, 41, 65, 66), its regulation of the acute phase of inflammatory insult, and coordination of the primary innate responders (macrophages, monocytes, and neutrophils) within the tissue are not clearly understood. We hypothesize that in the presence of an inflammatory stimulus, such as LPS, there is an impairment of lymphatic contractility that may be associated with distinct shifts in neutrophil populations and alterations in macrophage polarization on or near the mesenteric lymphatics. These changes would alter the milieu surrounding the mesenteric lymphatics and affect their physiological function. To test our hypothesis, we examined lymphatic contractile parameters in isolated vessel preparations from normal and LPS-injected rats and the effects of LPS on the recruitment of neutrophils and macrophage polarization in the vicinity of the collecting lymphatics. We also determined the direct molecular mechanisms by which LPS mediates the contractile and inflammatory signaling in the lymphatics and the effects of key cytokines associated with neutrophils and macrophages on lymphatics using the rat mesenteric lymphatic muscle cell (LMC) culture model.
MATERIALS AND METHODS
LPS rat model.
Male Sprague-Dawley rats weighing ∼150 g were intraperitoneally injected with LPS (10 mg/kg body wt) (LPS from Escherichia coli 0127:B8, L3129; Sigma-Aldrich, St. Louis, MO). All animals were housed in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and were maintained in accordance with the policies defined by the Public Health Service Policy for the Humane Care and Use of Laboratory Animals and the U.S. Department of Agriculture's Animal Welfare Regulations, and all of the protocols were approved by the Texas A&M University Laboratory Animal Care Committee. Rats were treated with PBS (control group) or LPS for 6 h, 24 h, or 3 days (LPS injections were administered every 24 h).
Isolated vessel preparation and functional analyses.
Control and LPS-treated rats (6 h and 72 h) were anesthetized with a combination of a droperidol-fentanyl (0.3 ml·kg−1·1−1 im of a solution of droperidol, 20 mg/ml fentanyl, 0.4 mg/ml), and diazepam (2.5 mg/kg im). A midline excision was made, and a loop of intestine 3–4 cm long was carefully exteriorized. A section of the mesentery was gently positioned over a semicircular viewing pedestal on a vessel preparation board. Mesenteric collecting lymphatic vessels were carefully cleaned of surrounding adipose and connective tissues. Vessels were maintained in albumin-supplemented physiological saline solution (APSS; in mM: 145.0 NaCl, 4.7 KCl, 2 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 sodium pyruvate, 0.02 EDTA, and 3.0 MOPS and 1% wt/vol BSA) at pH 7.4 at 38°C, as described earlier (29). The isolated lymphatic was cannulated and tied on to two glass pipettes (tip diameter: 80–100 μm). All of the isolated lymphatic length between the two glass tips (for all the experiments) was ∼1.2–1.5 mm containing one valve. Only vessels that did not have apparent constriction sites due to damage were used. Vessels were then allowed to equilibrate at a transmural pressure of 1 cmH2O for ∼30 min. After the equilibration period, contractions of each vessel were recorded for 5 min at pressures 1 cm, 3 cm, and 5 cmH2O. Finally, the passive diameter of the vessel at each transmural pressure was measured after the vessel was exposed to nominally Ca2+-free APSS (0 mM added Ca2+ and EDTA, 3.0 mM) for 15 min. Experimental data were acquired for the last 3 min of each 5-min interval at the different transmural pressures tested (1, 3, and 5 cmH2O). To determine the effect of LPS on lymphatic vessel contractility, isolated and cleaned mesenteric lymphatic vessels were immediately deposited into 3.5-mm sterile petri dishes filled with DMEM/F12 solution without (n = 12) or with LPS (10 μg/ml) (n = 13). The dishes were then placed in an incubator (5% CO2, 37°C) for a period of 24 h. Vessels were then subsequently cannulated with two glass pipettes, pressurized, and prepared for contractile activity measurements, as described above.
Isolated vessel video analysis.
Experiments were dynamically monitored by a microscope charge-coupled device video camera, and the video data were recorded to a video DVD for the functional analyses of lymphatic contractions after the experiment. Lymphatic diameter was traced for each 5 min of video capture with a vessel wall-tracking program developed and provided by Dr. Michael J. Davis (University of Missouri, Columbia, MO) (25). Outer lymphatic vessel diameters were tracked 30 times/s, providing a tracing of diameter changes throughout the periods of lymphatic systole and diastole. The following analogies to the cardiac pump parameters were derived: lymphatic tonic index, contraction amplitude, ejection fraction, contraction frequency, fractional/lymph pump flow, and systolic/diastolic diameters, as previously described (9, 29). Briefly, tonic index is the difference between passive outer lymphatic diameter in Ca2+-free APSS (normalized to 100%) and outer end-diastolic diameter, expressed as a percentage of the passive outer lymphatic diameter, reflecting the sustained tonic contraction of the lymphatics; fractional pump flow = ejection fraction [(end-diastolic volume − end-systolic volume)/end-diastolic volume] × contraction frequency, showing the practical capacity of the lymphatic pumping in lymph transportation and lymph pump flow = stroke volume (end-diastolic diameter − end-systolic diameter) × contraction frequency, showing the comprehensive capacity of the lymphatic pumping in lymph transportation.
Whole-mount mesenteric preparation and imaging.
Rat mesenteric tissue was removed post mortem and pinned out into loops in GIBCO Dulbecco's phosphate buffered saline (DPBS) on a Sylgard-coated dish. These loops were fixed and permeabilized for 1 h with prechilled methanol at −20°C and blocked in DPBS supplemented with 5% goat serum. Following this, mesenteric arcades were cut from the intestinal wall, and the two mesenteric bundles were incubated in block solution (DPBS supplemented with 5% goat serum) for 2 h at room temperature. The tissue arcades were then incubated overnight with primary antibodies against neutrophil elastase (NE; 1:100), CD11b (1:100), smooth muscle α-actin (1:500), CD206 (1:100), MHCII (1:200), CD163 (1:100), substance P, and SP (1:200) overnight. Controls were prepared with normal mouse, rabbit, or hamster IgG. Goat anti-mouse/anti-rabbit Alexa Fluor 488 or anti-mouse/anti rabbit Alexa Fluor 647 (Life Technologies, Carlsbad CA) was applied for 2 h. Images were taken using confocal microscope (Leica) at 488 nm or 647 nm. Tissues were then mounted between two coverslips with antifade mounting reagent (Life Technologies). Images were collected at ×20, ×40 and ×63 magnifications using a Leica scanning confocal microscope with lymphatic vessels centered in the field of view (FOV). At multiple sites within each sample, 0.5-μm z-axis steps were taken. One to three lymphatic vessels were “tracked” from the intestinal wall toward the root of the mesentery per staining combination. For each animal, around five to eight separate ×20, ×40, or ×63 magnification image stacks were acquired and quantified, and average cell counts were determined. The images were focused around mesenteric collecting lymphatics and used to determine the changes in the number of immune cells and lymphatic vessel association. Lymphatic vessels were determined by their morphology, and the presence of bulbar valve regions after smooth muscle actin-positive vessels were traced within the mesentery. Image reconstruction and orthogonal viewing on the image stacks were performed using the Leica confocal software and ImageJ. The negative controls for all experiments were produced and analyzed via the same instrumental and image-processing procedures. Average intensity projections representative of the data are shown. All experiments were repeated at minimum in triplicates.
Immunohistochemistry of liver and lymph node sections.
Liver and mesenteric lymph node cross sections were cut using a cryostat at 10 μm at −20°C. Sections were air-dried for 30 min at room temperature and then fixed overnight in Bouin's solution at room temperature. Masson Trichrome staining to detect fibrosis was then carried out as described earlier (67). For immunohistochemical analysis of liver and lymph node, the sections were fixed in cooled acetone for 60 min at −20°C. Samples were blocked with 10% serum from the host of the secondary antibody in TBS (100 μl) for 30 min. The appropriate primary antibodies, [NE, SP, myeloperoxidase (MPO)] were then applied in blocking buffer, and sections were incubated for 1 h at room temperature. After three washes with PBS, the secondary antibodies Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen) were placed on the sections (1:200 dilution in PBS), which were incubated for 30 min. A cover slip was placed over the sections and fixed with ProLong DAPI mounting medium (Life Technologies). Images were acquired by Zeiss 510 META confocal microscope at ×40 magnification and quantified using the NIH ImageJ analysis program.
Immunofluorescence analysis of lymphatic muscle cells and isolated mesenteric vessels.
Immunofluorescence experiments were carried out using cultured LMCs and stained with NF-κB antibody, as described earlier (19). Briefly, the LMCs were grown to about 70% confluence and plated onto coverslips. They were treated with LPS (20 ng/ml) for 6 or 24 h. Cells were then fixed with 2% paraformaldehyde, permeabilized with ice-cold methanol and incubated with NF-κB primary antibody for 1 h. Goat anti-mouse IgG-Alexa Fluor was used as a secondary. Images were scanned using the Leica AOBS SP2 confocal microscope (Wetzlar, Germany). For isolated, paraformaldehyde-fixed rat mesenteric collecting lymphatics, immunofluorescence was also performed as previously described (78). Briefly, fixed lymphatic vessels were incubated in blocking solution (1% BSA, 5% normal goat serum in PBS) for 1 h at RT and then divided into two pieces. The two sections were incubated in blocking solution overnight at 4°C in the presence of intralumenal and extralumenal primary NF-κB or the corresponding normal mouse Ig (negative control), respectively. Both segments were then incubated with the secondary goat anti-mouse IgG Alexa Fluor 488. The vessels were then cannulated and tied onto two glass pipettes, pressurized at 2 cmH2O and secured to the stage of a multiphoton/confocal microscope (Leica AOBS SP2) for immediate observation.
Protein isolation and Western blot analysis.
Rat mesenteric LMCs from mesenteric vessel explants were used as described earlier (18). LMCs were treated with LPS (0–200 ng/ml) for 0–72 h. Similarly treatments were also carried out with IL-8, (100 nM) in the absence or presence of LPS. Cells were also treated with the TLR4 inhibitor, polymixin B (10 μg/ml; InvivoGen, San Diego, CA), ERK inhibitor, PD98059 (10 nM), and AKT inhibitor LY294002 (20 μg/ml) in the presence or absence of LPS for 24 h. Western blot analysis was then carried out as previously described (18). LMCs were lysed in 1× SDS buffer supplemented with protease and phosphatase inhibitor and run on a 4–20% precast gradient SDS-polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA). The proteins were transferred to a nitrocellulose membrane with a Bio-Rad transblot apparatus. The transfer was verified with Ponceau-S staining. The membrane was blocked in 5% milk in TBS/TBST and then incubated with a primary antibody followed by incubation with the corresponding HRP-conjugated secondary antibody. The following dilutions of the primary antibodies in TBS were used: p-MLC20 (1:500), MLC20 and GAPDH (1:5,000), phospho-ERK1/2, phospho-ERK1/2, AKT, p-AKT (1:1,000), VCAM1 (1:2,000), ICAM1 (1:1,000), CXCR2 (1:1,000), galectin 9 (1:500), pI-κBα and I-κBα (1:1,000), and β-actin (1:10,000). The immunoreactive bands were visualized using the Pierce detection system (SuperSignal West Dura Extended Duration Substrate, Thermo Scientific, Rockford, IL). Densitometry analyses on the resulting bands were performed using Quantity One Multi-Analyst Software (Bio-Rad Laboratories). The membranes were stripped using ImmunoPure IgG Elution Buffer (Pierce, Rockford, IL) and then reprobed. GAPDH was used as the loading control. All experiments were done in triplicate, and data are presented as means ± SE.
Protein cytokine array analysis.
Rat proteome profiler cytokine arrays were obtained from R&D Systems (Minneapolis, MN). For parallel detection of a panel of 29 proinflammatory cytokines and chemokines, protein cytokine array profiling was carried out according to manufacturer's instructions. Briefly, lymphatic mesenteric tissue arcades were isolated from untreated control and LPS-treated rats (10 mg/kg body wt) for 24 h. Proteins were isolated from these tissue arcades and mixed with a cocktail of biotinylated detection antibodies and streptavidin-HRP, and chemiluminescent detection was carried out as per the manufacturer's instructions. The images were captured on Fuji ImageQuantLAS 4000 detection (GE Healthcare, Piscataway, NJ) and processed by National Institutes of Health's ImageJ software.
Statistical analyses.
Statistical significance in the pressurized vessel experiments was determined through two-way ANOVA with Fisher's post hoc analysis by the Statplus (Analystsoft) statistical software package. All other data were analyzed by Student's t-test or one-way or two-way ANOVA, as applicable. Data are expressed as means ± SE, and P values of ≤0.05 were considered as significant. All experiments were carried out at minimum in triplicate.
RESULTS
LPS induces inflammation and recruits large numbers of CD11b+ cells in mesenteric bed and near the lymphatic collecting vessels.
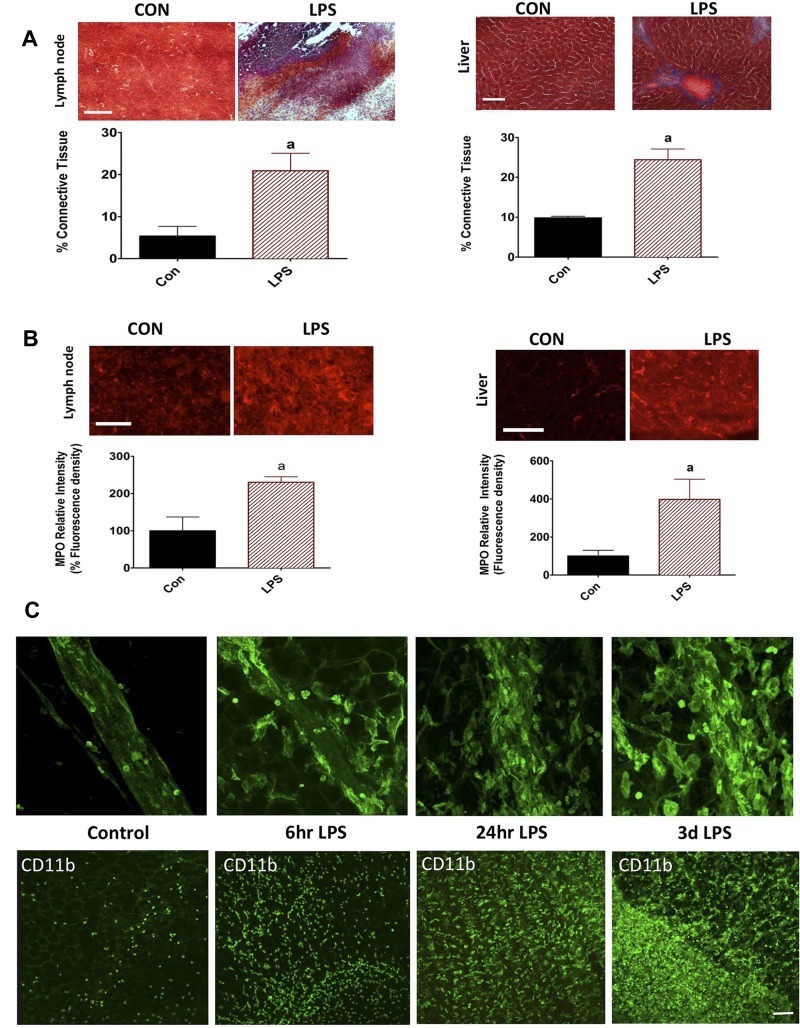
Masson Trichrome and MPO staining was significantly increased in the lymph node and liver sections from the LPS-injected rats, indicating fibrosis and onset of acute-phase inflammatory cascades compared with the sections from untreated controls (Fig. 1, A and B). In addition, LPS recruited significantly large numbers of CD11b+ cells to the vicinity of and on the walls of the lymphatics and in the clear mesenteric beds, which is indicative of an inflammatory response. As shown in the representative images in Fig. 1C, by 6 h, there is an increase in CD11b+ cells after LPS treatment. By 24 h and 72 h, the mesenteric bed and the lymphatic vessels show a very robust infiltration of CD11b+ cells into the mesenteric plexus and neurovascular bundles.
Fig. 1.
Induction of inflammation in response to LPS. Lymph nodes and liver cryostat sections were made from both control and LPS-injected animals. A: Mason trichrome staining was carried out on the liver and lymph node sections. Blue color indicates fibrotic areas, and red indicates normal cellular area. B: lymph node and liver sections were also stained with myeloperoxidase to ascertain the degree of inflammation. The percentage of fluorescent intensity was calculated as detailed in the methods section and plotted. A minimum of four to six sections was quantified per animal. Data are represented as means ± SE. aP < 0.05; n = 3. C: whole-mount mesenteric arcades harvested at 6 h, 24 h, and 72 h LPS postinjection were stained for the CD11b along with the control. Top: recruitment of CD11b+ cells in response to LPS on the mesenteric lymphatic vessel. Confocal images were acquired at ×40. Bottom: time-dependent increases in infiltration of CD11b+ cells at 6 h, 24 h, and 72 h in the clear field mesentery. Confocal images were acquired at ×20. A common scale bar for all figure panels is indicated. Scale bar: 150 μm.
LPS decreases the number of neutrophils on and in the vicinity of the lymphatics.
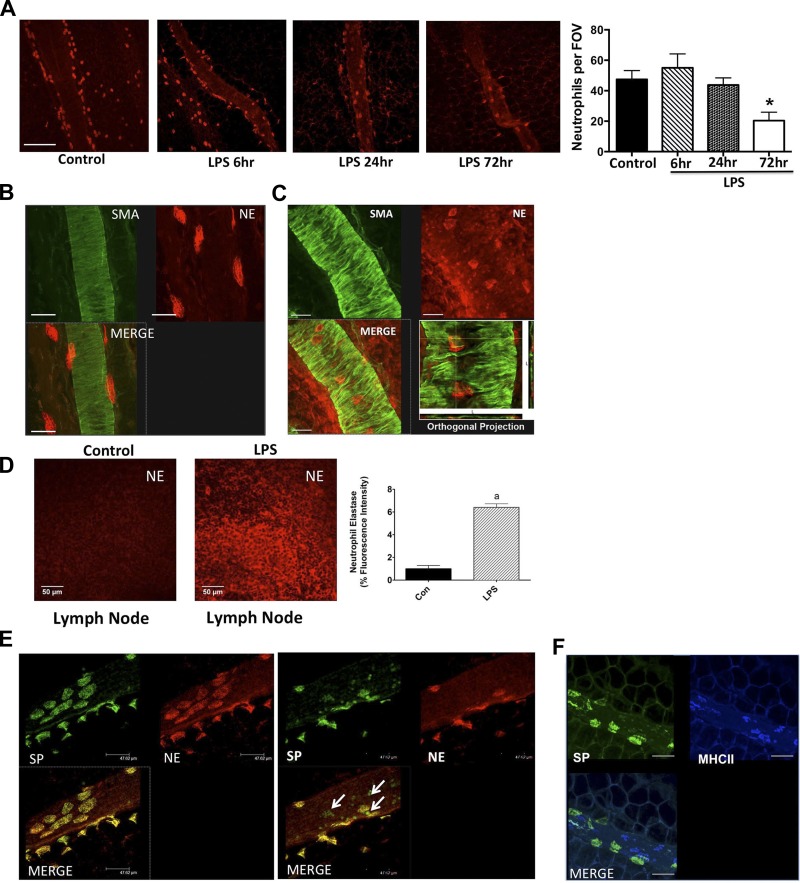
Mesenteric whole mounts from normal and LPS-injected animals were stained for a neutrophil-specific marker, NE. In control animals, a large number of NE-positive cells were found associated with the lymphatic vessel, in the close vicinity of the vessels, as well as in the surrounding clear mesentery (Fig. 2). The number of neutrophils near the lymphatic vessels did not show a significant change after 6 h or 24 h of LPS treatment (Fig. 2A). However, a significant decrease in the numbers of neutrophils was found after 3 days in the LPS-injected group compared with the control mesentery (49.67 ± 5.24% decrease in LPS-injected group compared with control mesentery) (Fig. 2A). To ascertain whether the neutrophils were on the walls of lymphatic vessels, we costained some of these vessels with the lymphatic muscle marker, α-smooth muscle actin. As shown in control lymphatic vessels Fig. 2, B and C, a number of the NE-positive cells directly adhered to the vessel wall of the collecting lymphatics; three-dimensional orthogonal confocal projections revealed that some of the neutrophils were also present beneath the muscle cell fibrils. In addition, a significantly increased number of NE-positive cells were found in the mesenteric lymph nodes from the LPS-treated animals (Fig. 2D). The NE-positive cells did not costain with CD11b, indicating that these are two distinct populations of cells (data not shown). The morphology of the neutrophils identified by NE staining was found to be similar to those reported previously in other studies (38, 79).
Fig. 2.
LPS causes significant decreases in the neutrophil population in the vicinity of the lymphatics. A: whole-mount mesenteric arcades from control and LPS-injected animals were stained for the neutrophil-specific serine protease NE at 6 h, 24 h, and 72 h postinjection. Confocal images were acquired at ×20. Scale bar: 150 μm. A single scale bar for entire panel is indicated. Left: neutrophil elastase (NE) staining near the lymphatics and on the lymphatic vessel wall. Right: quantification of the neutrophils on or near the lymphatic vessel in response to LPS at 6 h, 24 h, and 72 h compared with the untreated control. A minimum of five ×20 fields were counted and averaged per animal (n = 6). Data are presented as means ± SE. *Significant difference, P < 0.05. B: confocal images of whole-mount mesenteric preparations from LPS-treated rats showing that neutrophils (red) adhere to the lymphatic vessel muscle layer stained by α-smooth muscle actin (SMA; green). Magnification: ×63; scale bar: 50 μm. C: orthogonal projection of a lymphatic vessel, i.e., cross sections through the vessel wall in the x–z and y–z directions on the zoomed rotated and cropped part of the “Merge” image. The yellow lines depicting locations of the cross sections that show the neutrophils between and under lymphatic muscle cells (LMC) fibrils. Scale bar: 50 μm. D: significant increase in numbers of neutrophils were observed in lymph node sections from control and LPS-treated animals stained for NE. The percentage of fluorescent intensity was calculated and plotted. Minimum of four to six sections were quantified per animal. Data are represented as means ± SE; aP < 0.05; n = 3. A common scale bar for all figure panels is indicated. Scale bar: 50 μm. E: confocal images of whole mount mesentery showing that neutrophils also express substance P (SP). Many other immune cells on the mesenteric lymphatics apart from neutrophils also express SP. Magnification: ×20; scale bar: 150 μm. F: SP and MHCII costain was also carried out. MHCII and SP stain separate populations of cells. Magnification: ×20; scale bar: 75 μm.
Neutrophils in the vicinity of the lymphatics and in the lymph node stain positively for substance P.
Substance P (SP) is a known modulator of lymphatic contractility, and it has been shown that during inflammatory conditions, various immune cells, including neutrophils secrete SP (4, 26, 49, 75). Hence, we costained the lymphatic mesenteric tissue arcades with NE and SP to determine whether the neutrophils associated with the lymphatics also expressed SP. As seen in Fig. 2E, most of the NE-positive cells express SP. We also found that a number of cells that do not stain for NE, but positively stain for SP, are present on the lymphatics. Further, we costained SP with MHCII to show whether the SP-expressing cells were dendritic cells or macrophages. SP-MHCII coexpressing cells were not found on the wall of lymphatics and/or in the mesentery (Fig. 2F).
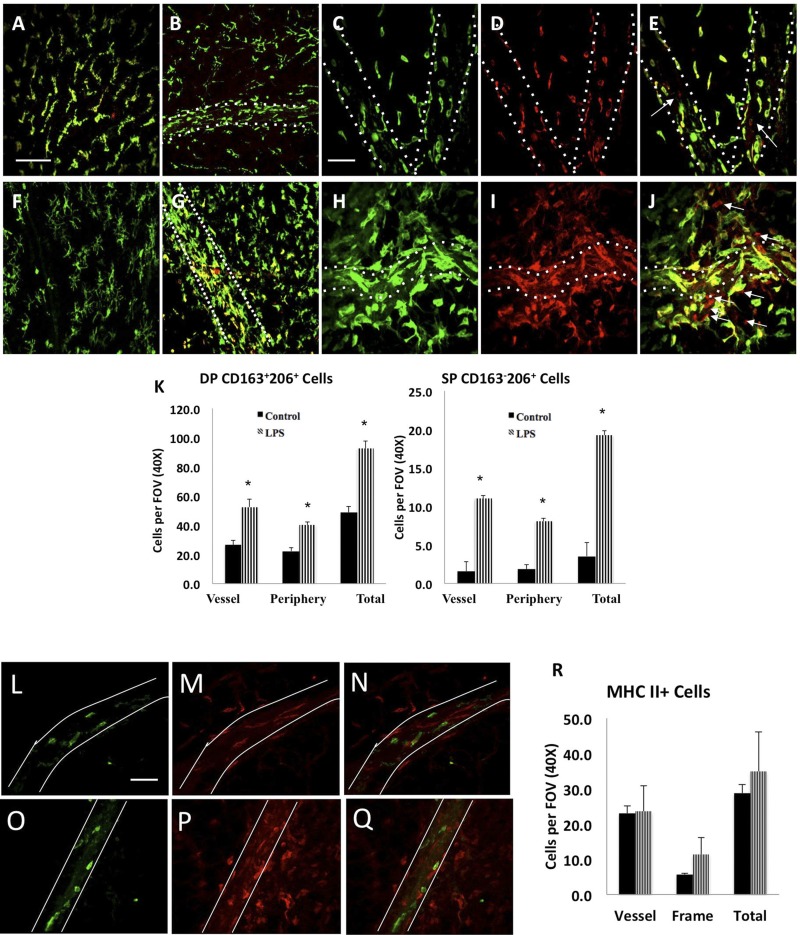
Characterization of macrophages associated with lymphatic collecting vessels.
As shown in Fig. 1, there was a significant population of CD11b+ cells that were recruited in abundance to the mesenteric lymphatic collecting vessels in response to LPS in a time-dependent manner (Fig. 1C). To further characterize the polarization of these cells, we performed immunofluorescence for the M1 marker MHCII and the common M2 markers CD163 and CD206 in 72-h LPS-treated animals and their corresponding controls. Under control physiological conditions, we observed at a minimum two distinct and prominent macrophage populations invested within the mesenteric neurovascular bundles that were identified as CD163+CD206+ or MHCII+ (Fig. 3). The CD163+CD206+M2 macrophages were the most abundant cell population within the tissue under normal conditions and bear similarity to adipose tissue macrophages (Fig. 3, A–E). These CD163+CD206+ cells reside both on the lymphatic collecting vessels and within the peripheral adipose (Fig. 3B). In response to 72-h LPS, the number of these CD163+CD206+ macrophages increases twofold (Fig. 3, F–J) and demonstrate a tendency toward residing on or within the lymphatic vessel wall (Fig. 3H). Additionally, LPS injections resulted in the recruitment of CD163−CD206+ macrophages with ∼19 per FOV that were preferentially found in tight association with lymphatic vessels, as opposed to the surrounding mesentery/adipose tissue (Fig. 3I). Macrophage polarization is described as a dynamic spectrum, and the single-positive (CD163−CD206+) cells represent a different M2 phenotype than the double-positive (CD163+CD206+) cells. The summary of quantification of CD163+CD206+ and CD163−CD206+ cells on the lymphatic vessel, periphery, and total counts for normal and LPS groups are shown in Fig. 3K. Under control conditions, the MHCII+ macrophages demonstrate a significant spatial tropism to reside along or within the mesenteric lymphatic vessels or other microvasculature structures (Fig. 3, L–Q). In contrast to the CD163+CD206+ macrophages, the number of MHCII+ cells per FOV does not dramatically increase in response to LPS (Fig. 3, L–Q and summary of quantification in Fig. 3R).
Fig. 3.
Macrophage accumulation and M2 polarization in the LPS-treated rat mesentery. Top: mesenteric arcades from control (A–E) and intraperitoneal LPS-injected (F–J) were stained for CD163 (green) and CD206 (red). Arrows indicate the single-positive CD163-CD206+ cells. Mesenteric lymphatic collecting vessels are outlined with dashed white lines. K: quantification of the M2 macrophage phenotypes in control and LPS-injected rats; *P < 0.05. DP denotes double-positive (CD163+206+) cells; SP denotes single-positive cells (CD163−206+). A minimum of five ×40 fields were quantified and averaged per animal (n = 4). Within the FOV for every image stack analyzed, cells that were directly in contact with the vessel were counted and represented as “vessel”, and the remaining cells were considered to be in the “periphery”. Magnification in A, B, F, G: ×20 magnification; scale bar = 150 μm. A common scale bar for all figure panels is indicated. Magnification in C, D, E, H, I, J: ×40; scale bar = 75 μm. A common scale bar for all figure panels is indicated. Data are represented as means ± SE. *Significant difference, P < 0.05. L–R: LPS treatment does not promote M1 but M2 macrophage phenotype on and around the mesenteric lymphatics. Control (L–N) and LPS (O–Q)-treated animals were stained for MHCII (blue) and CD206 (red). Magnification in L and O: ×40; scale bar: 75 μm. Magnification in M and P: ×20 magnification; scale bar: 150 μm. A common scale bar for all figure panels is indicated. A minimum of five ×40 fields were quantified and averaged per animal (n = 4).
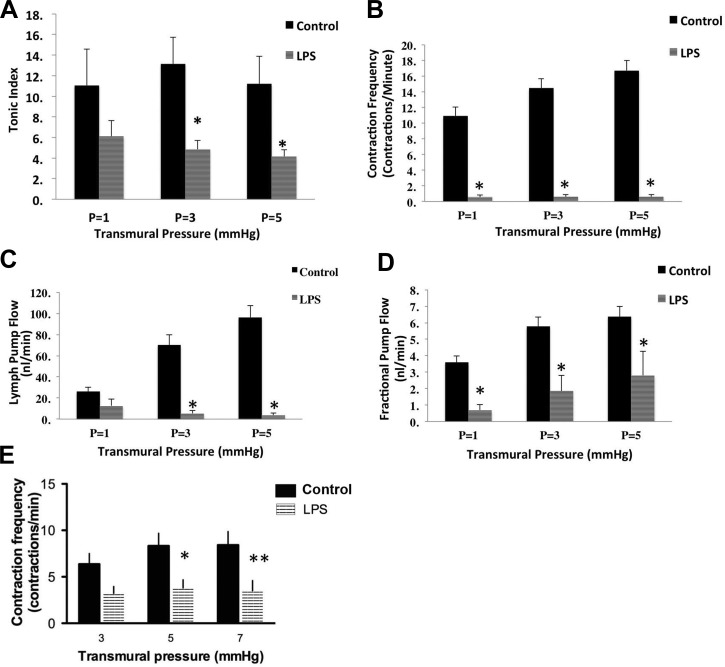
Impairment of lymphatic contractility in response to LPS induced inflammation.
Mesenteric lymphatic vessels were isolated from rats at various times after vehicle or LPS injection and studied. Exposure to LPS resulted in significant impairment of mesenteric lymphatic vessel contractile activity in regard to both tonic and phasic contractile parameters. Mesenteric lymphatic vessels isolated from LPS injected rats after 72 h had significantly reduced vessel tone (Fig. 4A) at transmural pressures of 3 and 5 cmH2O compared with their control counterparts [values for LPS vessels (n = 7) were 4.8 ± 0.86 and 4.2 ± 0.66 vs. control vessels (n = 13) 13.2 ± 2.6 and 11.2 ± 2.6 at 3 and 5 cmH2O, respectively]. (Here, n represents the number of animals used). Four out of seven rats injected with LPS examined at 6 and 72 h completely lacked spontaneous mesenteric lymphatic contractions at all tested transmural pressures. In addition to the disruption in vessel tone, LPS injections resulted in reduction in the phasic contraction frequency, which was significant at 6 h after injection (1.50 ± 0.98 in LPS compared with 6.25 ± 1.05 in control at pressure 5 cmH2O), as well as at 72 h (0.57 ± 0.29, 0.59 ± 0.30, 0.59 ± 0.31 at pressures 1, 3, and 5 cmH2O, respectively) compared with the control vessels (10.913 ± 1.14, 14.499 ± 1.17, and 16.74 ± 1.27 at 1, 3, and 5 cmH2O, respectively) (Fig. 4B). Lymph pump flow (Fig. 4C) was also significantly reduced in the 72-h LPS-injected rats at transmural pressure of 3 and 5 cmH2O (54.1 ± 2.75 and 3.7 ± 1.98 nl/min LPS; 26.1 ± 4.1 nl and 96.3 ± 11 nl/min controls, respectively). As shown in Fig. 4D, fractional pump flow was significantly reduced in the 72-h LPS-injected animals at all pressures examined (0.69 ± 0.33, 1.86 ± 0.94, and 2.8 ± 1.46 at 1, 3, and 5 cmH2O, respectively) compared with its controls (3.6 ± 0.39, 5.77 ± 0.58, and 6.38 ± 0.61 at 1, 3, and 5 cmH2O, respectively). Despite changes in both vessel tone and phasic contraction frequency, there were no significant differences in the stroke volume, ejection fraction, and diastolic diameter in the 72-h LPS-treated animals (data not shown). However, both the diastolic diameter and stroke volume were significantly reduced in the 6-h LPS animals (diameter: 82.40 ± 20.74 in LPS vs. 144.33 ± 12.01 in controls; and stroke volume: 1.88 ± 0.86 in LPS vs. 7.98 ± 2.55 in controls).
Fig. 4.
LPS causes significant impairment of collecting lymphatic vessels. Pressurized isolated lymphatic vessels from control, and 72-h LPS-treated animals were subjected to transmural pressures of 1, 3, and 5 cmH2O. Lymphatic tonic index (A), frequency (B), lymph pump flow (C), and fractional pump flow (D) were measured as described in the materials and methods. E: contraction frequency measured in vessels mounted on a pressure myograph after a 24-h incubation in media without (Sham) and with 10 μg/ml LPS. Data are presented as means ± SE. Two-way ANOVA was carried out. * and ** denote significance at P < 0.05 and P < 0.01 vs. sham, respectively. Dark bars denote control, and striped bars denote LPS-treated.
To further determine whether LPS would directly influence the lymphatic contractile activity, the isolated mesenteric lymphatic vessels were treated with LPS as described in materials and methods. As shown in Fig. 4E, the vessels that were treated directly with LPS showed a significant decrease in phasic contraction frequency at both 5 and 7 cmH2O compared with the sham controls.
Differential expression of key adhesion molecules on LMCs in response to LPS.
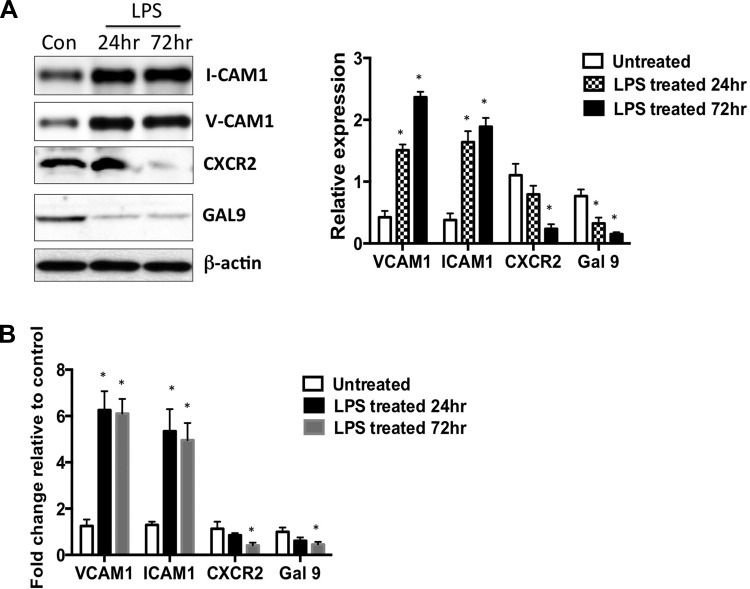
As we found distinct shifts in the recruitment of immune cells in the vicinity of the lymphatics in response to LPS, we analyzed the expression of key cell adhesion molecules that are known to regulate this process in cultured LMCs. LPS significantly increased the expression of VCAM1 and ICAM1 at 24 h and 72 h compared with the untreated controls. Expression of CXCR2, a key receptor for the neutrophil-associated cytokine IL-8 showed a slight decrease at 24 h but was significantly reduced in LPS-treated LMCs at 72 h. Galectin 9, another neutrophil chemoattractant was significantly reduced at both 24 h and 72 h in LPS-treated cells (Fig. 5A). Our real-time PCR data further corroborated these findings in LPS-treated LMCs (Fig. 5B).
Fig. 5.
LPS modulates the levels of key adhesion molecules and chemoattractants on LMCs. A, left: representative Western blot showing the expression of VCAM1 ICAM1, CXCR2, and Gal-9 in LMCs in the absence and presence of LPS in a time-dependent manner. Right: quantitative values from three independent experiments are plotted as a ratio vs. housekeeping control β-actin. Data are represented as means ± SE; *P < 0.05; n = 3. B: quantitative real-time PCR showing the expression of VCAM1 ICAM1, CXCR2, and Gal-9. Real-time quantification was carried out, and the relative fold change over the untreated control was calculated. RPL19 was used as the housekeeping control. Experiments were done in triplicate, and the values are presented as means ± SE. *P < 0.05.
LPS activates parallel inflammatory and contractile pathways in LMCs in a p-AKT and p-ERK dependent manner.
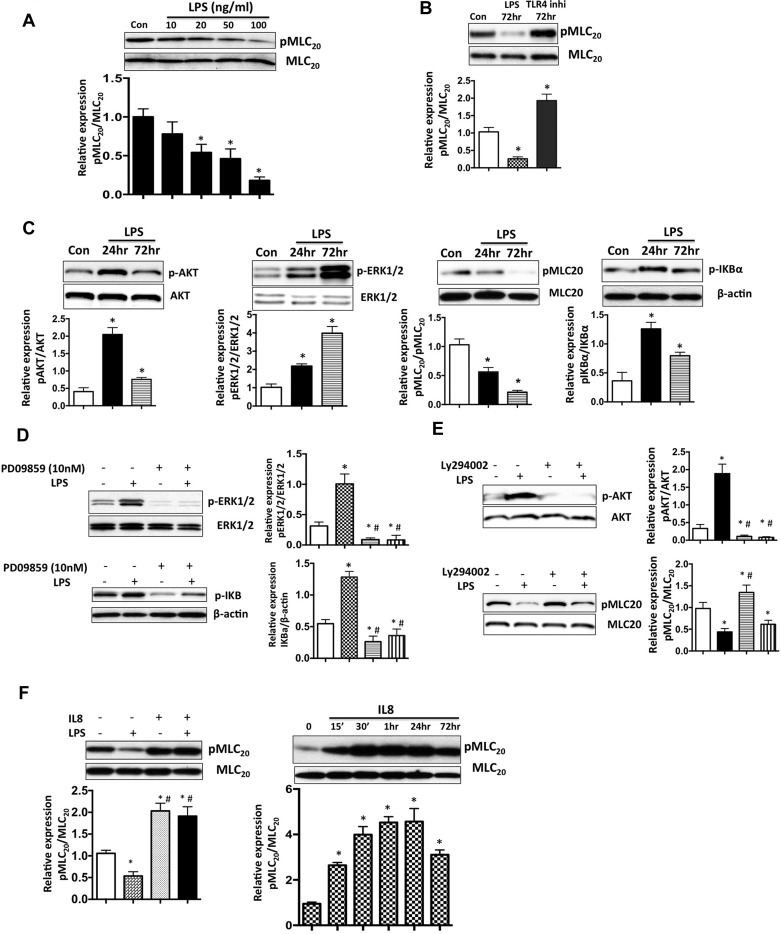
To further investigate the detailed molecular mechanisms underlying LPS-induced lymphatic contractile impairment, we analyzed the effects of LPS treatment on LMCs at varying concentrations and times. LPS treatment (20–100 ng/ml) significantly inhibited MLC20 phosphorylation (Fig. 6A). Further, to ascertain that LPS acts through Toll-like receptor (TLR), we used the TLR4 inhibitor (LPS-RS) and evaluated its effects on pMLC20, both in the presence and absence of LPS (Fig. 6B). In the presence of TLR4 inhibitor, MLC20 in 24-h LPS-treated LMCs, reverted to normal levels, but MLC20 phosphorylation in 72-h LPS-treated LMCs was significantly increased. Further, LPS was found to significantly increase p-AKT, p-ERK, and pI-κBα levels in LMCs at both 24 h and 72 h (Fig. 6C). Further, to elucidate the roles of p-AKT and p-ERK in modulating downstream signaling by LPS, we used p-AKT inhibitor (LY294002) or p-ERK inhibitor (PD98059) in conjunction with LPS on LMCs. Inhibition of p-ERK was found to significantly reduce pERK1/2 and IκBα levels, both in the presence or absence of LPS (Fig. 6D). Similarly, LY294002 caused a significant reduction of p-AKT levels. Interestingly pMLC20 was found to increase when p-AKT was suppressed both in the presence or absence of LPS (Fig. 6E). These results suggest that two parallel pathways may be activated in LMCs by LPS signaling through TLR4, one that depresses MLC20 phosphorylation levels in LMCs via activation of AKT phosphorylation and the second that increases the downstream inflammatory pathways regulated by pI-κBα by its effects on ERK phosphorylation. Because cytokines released by immune cells have been shown to affect muscle tone and function, we used LMCs to evaluate the effects of IL-8, one of the key cytokines released by neutrophils, as well as a prominent neutrophil chemoattractant. As shown in Fig. 6F, IL-8 treatment increased pMLC20 significantly in LMCs at different time points, and IL-8 was shown to induce levels of pMLC20, even in the presence of LPS.
Fig. 6.
LPS activates contractile and inflammatory pathways in a pAKT and p-ERK-dependent manner. A: representative Western blot showing the expression of pMLC20 in LMCs in the absence or presence of LPS in a dose-dependent manner. B: representative Western blot showing that LPS-mediated inhibition of pMLC20 is TLR4 dependent. LMCs were treated with LPS and polymixin B (TLR4 inhibitor) for 72 h, and the effects on pMLC20 was determined. Bottom: relative expression of pMLC20/MLC20 was quantified and plotted. Data are represented as means ± SE. *P < 0.05. C, top: representative Western blots showing LPS-mediated activation of pAKT, pERK, p-I-κB, and inhibition of pMLC20 at 24 h and 72 h after treatment. Bottom: relative expression of pAKT/AKT, pERK/ERK, p-I-κB/I-κB, and pMLC20/MLC20 was quantified and plotted. Data are represented as means ± SE. *P < 0.05. D, left: representative Western blots showing the expression of pERK and p-I-κB in the presence of ERK inhibitor, PD98059. Right: quantification from triplicate experiments. *P < 0.05 vs. control; #P < 0.05 vs. LPS. E, left: representative Western blots showing that the expression of pAKT and MLC20 in the presence of AKT inhibitor, LY294002. Right: quantification from triplicate experiments. *P < 0.05 vs. control; #P < 0.05 vs. LPS. F, top: representative Western blots showing effects of IL-8 on pMLC20 in LMCs in the absence or presence of LPS. Bottom: relative expression of pMLC20/MLC20 and pAKT/AKT was quantified and plotted. Data are represented as means ± SE. *P < 0.05; n = 3.
LPS activates NF-κB in LMCs.
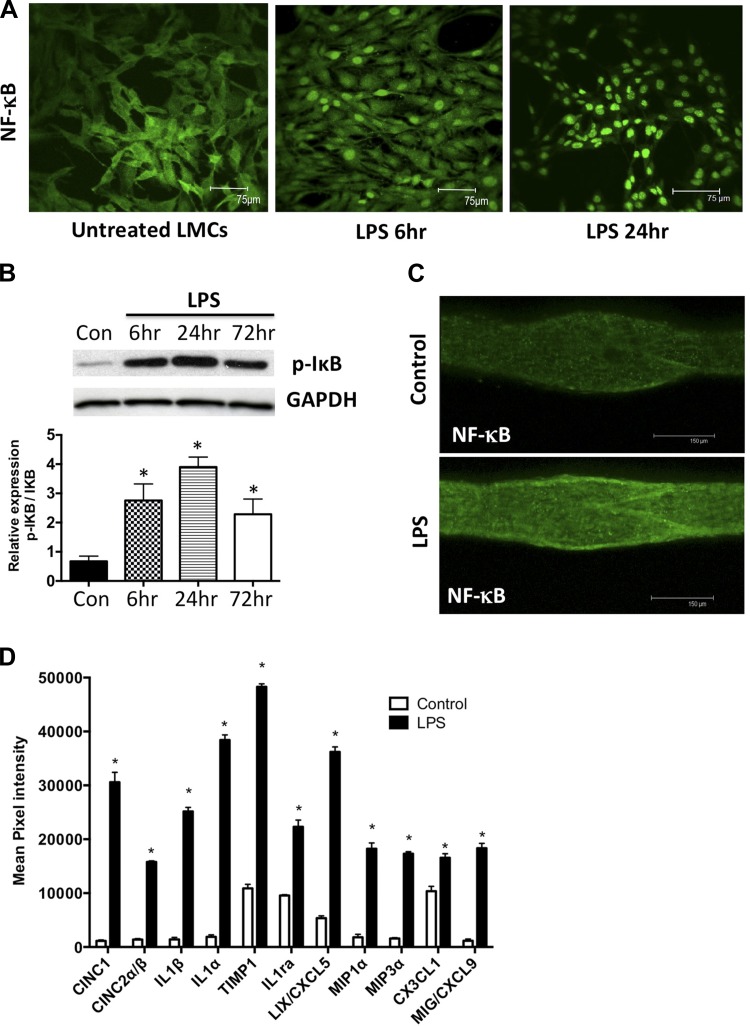
LMCs treated for 6 h and 24 h with LPS showed a robust increase in NF-κB nuclear translocation in a time-dependent manner (Fig. 7A). This was also associated with increases in the levels of p-IκB in LMCs on treatment with LPS (Fig. 7B). Similar response was also seen in mesenteric lymphatic vessels isolated from control and 6 h LPS-treated animals with a rapid increase in nuclear NF-κB levels in lymphatic vessels from LPS animals (Fig. 7C).
Fig. 7.
LPS causes activation of the NF-κB and proinflammatory cytokines and chemokines in the lymphatics. A: LPS activates NF-κB in cultured mesenteric LMCs. LMCs were treated with LPS (20 ng/ml) for 6 h and 24 h, and nuclear translocation of NF-κB was assessed using immunofluorescence. Magnification is ×20. B: LPS activates I-κB in cultured mesenteric LMCs. LMCs were treated with LPS (20 ng/ml) for 6, 24, and 72 h, and Western blot analysis was carried out. Magnification is ×20. C: LPS activates NF-κB in mesenteric lymphatic vessels. Mesenteric lymphatic vessels isolated from a 6-h LPS-treated rat were assessed for nuclear translocation of NF-κB using immunofluorescence. Magnification is ×20. Scale bar: 150 μm. D: LPS induces the expression of several cytokines and chemokines in lymphatic mesenteric tissue arcades. Proteins isolated from mesenteric tissue arcades from untreated and LPS-treated animals were analyzed by inflammatory cytokine array. Mean pixel density of each analyte was quantified and plotted and represented as fold change over control. Data are represented as means ± SE. B and D: *P < 0.05; n = 3.
Inflammatory cytokines and chemokines are induced in the mesenteric lymphatic bed in response to LPS.
We sought to determine the expression of key immunomodulatory and inflammatory cytokines within the specific tissue space in the mesentery in response to LPS-induced inflammation. Mesenteric arcades from untreated and 24-h LPS-treated rats were subjected to protein cytokine array analysis. Among the 29 inflammatory cytokines analyzed, LPS stimulation significantly upregulated the expression of 10 cytokines by more than twofold compared with the untreated controls (Fig. 7D), indicating a complex cytokine signaling in the mesenteric milieu in response to an inflammatory signal. As expected for acute inflammation, six of those were found to be chemoattractants for neutrophils, macrophages, and monocytes. These included cytokine-induced neutrophil chemoattractant 1 (CINC1), cytokine-induced neutrophil chemoattractant 2, or macrophage inflammatory protein 2α (CINC2α also known as MIP2α), macrophage inflammatory protein 1α (MIP1α also known as CCL3), macrophage inflammatory protein 3α (MIP3α also known as CCL20), LPS-inducible CXC chemokine or chemokine (C-X-C motif) ligand 5 (LIX also known as CXCL5) and chemokine (C-X3-C motif) ligand 1 (CX3CL1 also known as fractalkine). In addition, proinflammatory interleukins such as IL-1α, IL-1β, and anti-inflammatory IL-1ra were also induced in the mesenteric beds. Further, elevated levels of, tissue inhibitor of metalloproteases (TIMP1) and monokine induced by IFN-γ (MIG) were also observed (Fig. 7D).
DISCUSSION
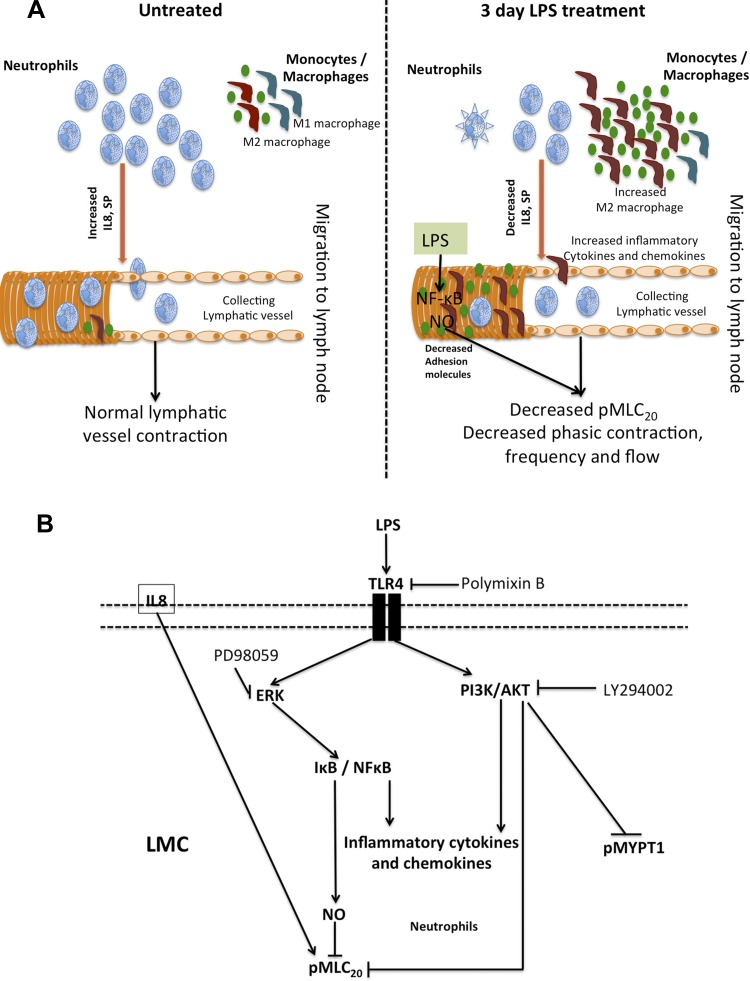
The data presented in this study are the first to identify the effects of LPS on temporal and spatial dynamics of immune cells (specifically neutrophils and macrophages) associated with lymphatics and their impact on lymphatic contractility and function. Using a rat model of LPS-induced peritonitis, we focus on the lymphatic mesenteric plexus, which is subject to the most dynamic physiological changes in fluid flow (associated with feeding patterns) and alterations in the surrounding microenvironment due to interactions between inflammatory mediators, cytokines, chemokines, and immune cells. In this study, we provide clear evidence that LPS causes significant impairment of lymphatic contractility in both tonic and phasic contractile parameters and, as a result, inhibits lymphatic pump flow. Further, we demonstrate that exposure to exogenous LPS causes significant changes in the recruitment of neutrophils and macrophages, and favors a shift to a M2 macrophage polarized state that acts in parallel with activation of a number of inflammatory cytokines and chemokines in the mesenteric beds (Fig. 8).
Fig. 8.
A: schematic representation of LPS mediated immune cell modulation and impairment of lymphatic function. Neutrophils and macrophages are present in the lymphatic mesenteric bed in normal physiological conditions. After 3-day LPS challenge, a significant decrease in neutrophils and increase in M2 polarized macrophages is noted in the vicinity of the lymphatics. LPS significantly reduces key neutrophil-associated cell adhesion molecules as CXCR2 and Gal-9 on the LMCs, while activating ICAM1 and VCAM1 that attract monocytes and macrophages. LPS further causes a significant impairment of lymphatic contractility by directly activating NO-mediated inhibitory signaling by activation of NF-κB. The alterations in the immune cell population on/near the mesenteric lymphatics also cause changes in the complex cytokine-mediated signaling that affect lymphatic vessel function. B: schematic representation of LPS-TLR4 pathways in LMCs. In the LMCs, LPS signals through the TLR4 receptor and activates parallel contractile and inflammatory pathways by activation of pAKT and pERK, respectively. Inhibition of ERK inhibits I-κB-mediated induction of inflammatory cytokines, NO and NF-κB activation. On the other hand, induction of pAKT represses pMLC20. IL-8 may also directly activate pMLC20.
The rat model of LPS used in this study does cause a systemic inflammation, as shown in the immunohistochemical staining of the liver and lymph node sections (Fig. 1) and, in fact, very closely resembles a peritonitis model, as demonstrated by Kim et al. (47). In addition, this model has pronounced global effects immediately after administration of LPS, as rats exhibited raised body temperature, huddled posture, slow gait, and symptoms of uveitis. Further, molecular data suggest that systemic responses are created in the mesenteric bed in response to LPS as a number of inflammatory cytokines and chemokines, known mediators of inflammatory responses, appeared dysregulated in the mesenteric tissue arcades (Fig. 7D).
LPS causes endotoxin tolerance in the mesentery.
Our data clearly indicate that there are many neutrophils adhered to and/or present beneath the muscle cells of the afferent collecting lymphatic vessels (Fig. 2, B and C), and 72 h after initiation of the LPS challenge, there is a dramatic decrease in the number of neutrophils in the vicinity of the lymphatics (Fig. 2A). Furthermore, immunohistochemical analyses of the mesenteric lymph nodes from the same animals indicate a significant upregulation in the numbers of neutrophils in the lymph node (Fig. 2D). Hence, we speculate that in response to LPS, neutrophils migrate through the mesenteric lymphatic vessels to reach the lymph nodes, where they may be important players in eliciting an immune response. Indeed, the ability of neutrophils to migrate to lymph nodes has been previously documented. Abadie et al. (1) provided in vivo evidence that neutrophils migrate via afferent lymphatics to lymphoid tissues and can shuttle live microorganisms there. It has also been shown that neutrophils are recruited to sites of inflammation and can then coordinate lymph node lymphangiogenesis and modulate immune responses (22, 71). The confocal imaging data, together with the finding that mesenteric lymphatic arcades from LPS-injected animals show a significant induction of several neutrophil chemoattractants such as CINC1, CINC2, MIP1α (Fig. 7D), support our speculation of inflammation-mediated neutrophil migration through collecting lymphatics. However, additional experiments using fluorescence-tagged neutrophils studying their migration in the mesenteric region are warranted.
As noted earlier, we observed significant populations of CD11b+ cells in and around the lymphatic vessels and surrounding mesenteric tissue beds, which based solely on CD11b+ staining could be monocytes, macrophages, or even activated neutrophils (47). However, the CD11b+ cells identified in this population of immune cells in our studies did not costain with the neutrophil-specific marker, NE (data not shown), indicating that these cells were either macrophages or monocytes, as previously described by Kim et al. (47). Previous microarray data collected on the mesenteric collecting vessels demonstrated a large immune fingerprint, particularly that of antigen-presenting cells (13). The data presented in this study corroborate those findings, as a strong contingent of MHCII+ macrophages were seen not only in the adipose tissue surrounding the mesenteric lymphatics but also invested within and along the walls of the mesenteric collecting vessels. These MHCII+ macrophages appear to represent M1 skewed cells, and their tropism for the vessel wall suggests that specific chemoattractants would be mediating this effect. The chemokine CX3CL1, or fractalkine, that we found to be significantly induced in the lymphatic beds (Fig. 7) is actively involved in the recruitment of monocytes/macrophages to inflamed tissue (73). Although LPS has been associated with the activation of M1 inflammation, the CD163+CD206+ M2 phenotype expanded significantly in the LPS-injected animals and was closely associated with lymphatic tissues. In our study, we found an increase in the number of CD163−CD206+ single-positive cells near the lymphatic vessels as well. These CD163−CD206+ cells may be similar to the M2b phenotype that are also induced by TLR stimulation and are involved in inflammation resolution. These M2 macrophages are found within the adipose tissue and along the mesenteric vessels under physiological conditions, and they are likely of the fibrotic/tissue repair phenotype. In response to TLR stimulation M2b macrophages that often lack CD163 express CD206 in addition to producing high levels of TNFα, IL-10, and nitric oxide (NO) (8). Macrophages, particularly the M2 subpopulation, can mediate tissue repair and growth by the release of a number of growth-promoting factors and cytokines, including the anti-inflammatory IL-10.
Our finding that neutrophils are significantly decreased in the vicinity of the lymphatics coupled with the propagation of an M2 anti-inflammatory macrophage phenotype after repeated injections of LPS may be indicative of endotoxin tolerance, a phenomenon where cells show reduced responsiveness toward repeated endotoxin or TLR4 stimulation. Recent evidence suggests that both proinflammatory and anti-inflammatory processes occur early and simultaneously in endotoxin-mediated sepsis and that immunosuppression is the predominant driving force for mortality and morbidity in sepsis (39). This is also in keeping with the recently discovered immunosuppressive roles of neutrophils in inflammation progression (10). Because of this characteristic desensitization response, endotoxin tolerance is considered a regulatory mechanism to balance inflammation (7). Pena et al. (61) have shown that substantial similarities exist between M2 polarization and endotoxin tolerance and suggest that this phenomenon can be considered another form of alternative activation triggered by bacterial signatures such as LPS. We surmise that the alterations in the lymphatic vessel-associated neutrophils and predominant M2 phenotype that we observe as an acute response to LPS set the stage for a more profound systemic inflammatory response of the host.
LPS causes conducive environment in LMCs and mesentery for regulating neutrophils and macrophages.
IL-8 acts as a chemoattractant through CXCR1 and CXCR2 G protein-coupled receptors. During septicemia, neutrophil responsiveness to IL-8 and other CXC chemokines is reduced via TNF-α mediated downregulation of CXCRl and CXCR2 (68, 72). Supporting the existence of a similar mechanism in lymphatics, we also find that LPS significantly represses CXCR2 in LMCs (Fig. 5). Further LPS significantly reduced the expression of Gal-9, another neutrophil chemoattractant, in the LMCs. On the other hand, LPS significantly increased the expression of ICAM1 and VCAM1, which are known monocyte and macrophage adhesion molecules (21, 46). Hence, LMCs seemed to be primed by LPS challenge to reduce the recruitment of neutrophils and to increase macrophage recruitment by reduction of some of its key receptors and adhesion molecules. In addition, the neutrophils that we identified as adhering to the mesenteric lymphatics also strongly expressed SP. We have previously shown that SP is a strong modulator of lymphatic contractility and activates contractile pathways in the lymphatics (4, 18, 26). Although SP is released by C-sensory fibers in the vicinity of the lymphatics, changes in SP levels from surrounding immune cells, such as the neutrophils, may also affect lymphatic contractile functions. Thus, our data suggest that cytokines directly expressed by immune cells in the vicinity of the lymphatics could be potentially involved in modulating the lymphatic contractile function in response to various immune and inflammatory stimuli.
Corroborating these findings, our cytokine array analysis provides novel insights into the complex crosstalk of cytokines and chemokines in the mesenteric bed in response to LPS that could play a critical role in determining the balance between inflammation progression and resolution. Several key neutrophil and macrophage/monocyte chemoattractants (CINC1, CINC2α, MIP1α, MIP3α, CXCL5, and CX3CL1) were induced in mesenteric lymphatic beds in response to LPS (Fig. 7D). This is interesting as CINC1 is a member of the IL-8 family and is a major neutrophil chemoattractant released in response to tissue injury (15). Similarly, members of the MIP1 family of proteins play a critical role in leukocyte chemotaxis, as well as the production of proinflammatory cytokines, and they are fundamental components of the acute phase response to sepsis (59). Proinflammatory interleukins such as IL-1α, IL-1β, as well as anti-inflammatory IL-1ra were also induced in the lymphatic mesenteric beds, indicating that the lymphatics may actively participate both in the progression and resolution of inflammation. Furthermore, TIMP1 that is known to be associated with tissue degradation and remodeling is elevated in inflamed lymphatic tissue beds. CX3CL1, or fractalkine, is directly involved in trafficking of antigen-loaded dendritic cells from the periphery via afferent lymphatics to draining lymph node during tissue inflammation and has been significantly correlated with the severity of inflammatory diseases (41, 73). As it was significantly induced in mesenteric lymphatic arcades from the LPS-treated group, this could be setting the stage for a more widespread systemic inflammatory response due to LPS. It is not clear whether the production of cytokines and chemokines is a cause or consequence of the immune cell infiltration or, more likely, a result of the systemic inflammatory mechanisms activated by LPS on the mesenteric tissue bed.
LPS-TLR4 pathway activates both contractile and inflammatory pathways in LMCs.
We have previously demonstrated that the lymphatic pump is regulated by intrinsic forces, pressure/stretch, and flow/shear, as well as extrinsic mechanisms such as humoral/neural actions (reviewed in Refs. 17, 30, 81). One of the most common mechanisms mediating lymphatic contractile function is the regulation of the phosphorylation status of the contractile regulatory protein MLC20 (57, 78). We conjectured that LPS would have a direct role on the lymphatic contractile machinery by regulating levels of pMLC20, as well as the cytokines potentially released by the immune cells, or lack of their availability by changes in immune cell dynamics would affect the LMC contractile apparatus. Our data show that LPS directly represses phosphorylation of MLC20 and activates parallel pathways mediated by the phosphorylation of AKT and ERK to modulate lymphatic contraction and inflammation, respectively (Fig. 6C). Interestingly, LPS increased p-AKT levels while decreasing pMLC20. It has been shown that p-AKT inhibits pMLC20 by inhibiting pMYPT1 (51). We have previously shown that increased p-MYPT1 directly correlates with increased pMLC20 in the LMCs in response to inflammatory agonist (18). Hence, our data provide the basis for a novel mechanism of pMLC20 inhibition in LMCs by activation of p-AKT (Fig. 8). This was further corroborated, as inhibition of p-AKT increased pMLC20 levels (Fig. 6E). Previously, we have also demonstrated that p-ERK is required for MLC20 phosphorylation (18). Hence, we concluded that p-ERK was not playing a direct role in MLC20 phosphorylation in response to LPS, as effects of LPS elicited opposite responses (Fig. 6C). However, p-ERK inhibition by pharmacological inhibitors in the presence of LPS directly affected pI-κB and, hence, was mediating the inflammatory signaling downstream of LPS (Fig. 6D).
In addition to activation of various immune cells, LPS directly stimulates inflammatory cellular responses in LMCs through TLR4, which subsequently activates the downstream signaling mechanisms and activation of NF-κB (83). Previous studies in sheep show that although endotoxin impaired lymphatic pumping, it did not have a direct effect on the lymphatic vessels; the LPS-mediated effects were believed to have occurred indirectly through interactions with cellular and humoral factors in blood and lymph (27, 28). A number of recent studies have demonstrated that inflammatory cytokines significantly affect lymphatic contractile functions, as well as endothelial permeability (3, 24, 43). However, except for one study in bovine mesenteric lymphatic vessels, the direct effects of LPS on LMCs have not been previously investigated (53). Our data are, however, the first to clearly delineate different molecular pathways activated by LPS in LMCs and show that it initiates proinflammatory pathways in LMCs by causing nuclear translocation of NF-κB and upregulation of pI-κB levels. NF-κB induction can be directly correlated with induction of iNOS and NO, which have been documented as a crucial regulator of lymphatic contractile activity, and high NO levels leads to strong pumping inhibition (20). Liao et al. (52) have shown that bone marrow-derived Cd11b+ myeloid cells expressing iNOS infiltrate the tissue surrounding the contractile lymphatics during inflammation and inhibit autonomous lymphatic contraction. This is significant, as we have demonstrated the recruitment of large numbers of Cd11b+ cells on the collecting lymphatics in response to LPS, and our data support a direct induction of NF-κB pathways that may mediate downstream NO signaling. It must also be noted that in addition to the initiation of inflammatory cascades, the activation of NF-κB is also involved in resolution of inflammation and M2 polarization of tumor-associated macrophages (36, 50, 63).
Taken together, our data suggest that the mesenteric lymphatic vessels are highly involved in regulating the inflammatory process, and their contractility is subject to control by immune activation in the vicinity of the vessel, as well as inflammatory signaling cascades activated within the tissue. Immune cell trafficking and its relation with lymphatic dysfunction constitute a relatively underexplored area of lymphatic research. We have previously demonstrated that during inflammatory conditions, there is a significant loss of lymphatic vessel function in TNBS-treated guinea pigs (80). Corroborating these findings, we have recently shown that lymph transport significantly decreased after TNBS treatment, and these changes were preceded by increased numbers of MHCII+ cells surrounding mesenteric lymphatics leading to an altered lymphatic environment favoring dysfunction (23). These recent findings coupled with the results of this study underscore the direct link between inflammation-induced activation of immune cell dynamics and alterations in the lymphatic microenvironment with impairment of lymphatic function. While some cytokines have been individually reported to have an effect on lymphatic contractility (2, 3), as shown in Fig. 8, our study is the first to demonstrate how inflammation alters the surrounding immune cells near the lymphatics in vivo and also activates global inflammatory pathways within the lymphatic cells. A combination of these events influences the immune cells' recruitments and trafficking through the lymphatics. We can speculate that the alterations in cytokines and chemokines that we observed in response to LPS is an effect of these dynamic shifts in the immune populations in response to an inflammatory insult and that these changes, in turn, affect lymphatic function. It is also possible that these changes are key in determining whether there will be a progression or resolution of inflammation by the initial triggering of a systemic response to a pathogenic insult. We know that lymph flow moves not only fluid and macromolecules but also many immune cells and, thus, impairment of pump function could well relate to temporal changes in immune cell numbers. Indeed, recent work by our lab group and our collaborators has shown that these cells then traffic to the lymph nodes via the afferent lymph flow (48, 62). So although we did not directly measure this in this study, we can infer that the impaired lymph pump flow will impede the removal of fluid, macromolecules, and immune cells from the tissues, thereby precipitating further inflammatory insult, as we observed recently (23). Further studies are also warranted to carefully evaluate the role of the specific chemokines and cytokines and cell adhesion molecules that were identified to be dysregulated in the mesenteric lymphatics on lymphatic contractility. However, our study sets the stage for further investigation in this area aimed at identifying other immune cell-mediated alterations in inflammatory pathways that directly affect lymphatic contractility and pump function and are key determinants of progression or resolution of inflammation.
GRANTS
This work was supported by National Institutes of Health RO1-DK-99221 to M. Muthuchamy and D. Zawieja.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.C., S.D.Z., P.-Y.v.d.W., D.C.Z., and M.M. conception and design of research; S.C., S.D.Z., W.W., Y.L., and Y.J.W. performed experiments; S.C., S.D.Z., W.W., Y.L., Y.J.W., P.-Y.v.d.W., D.C.Z., and M.M. analyzed data; S.C., S.D.Z., W.W., Y.L., P.-Y.v.d.W., D.C.Z., and M.M. interpreted results of experiments; S.C., S.D.Z., and M.M. prepared figures; S.C., S.D.Z., and M.M. drafted manuscript; S.C., S.D.Z., P.-Y.v.d.W., D.C.Z., and M.M. edited and revised manuscript; S.C., S.D.Z., W.W., Y.L., Y.J.W., P.-Y.v.d.W., D.C.Z., and M.M. approved final version of manuscript.
REFERENCES
- 1.Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood 106: 1843–1850, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Al-Kofahi M, Becker F, Gavins FN, Woolard MD, Tsunoda I, Wang Y, Ostanin D, Zawieja DC, Muthuchamy M, von der Weid PY, Alexander JS. IL-1β reduces tonic contraction of mesenteric lymphatic muscle cells, with the involvement of cycloxygenase-2 and prostaglandin E2. Br J Pharmacol 172: 4038–4051, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldrich MB, Sevick-Muraca EM. Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine 64: 362–369, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amerini S, Ziche M, Greiner ST, Zawieja DC. Effects of substance P on mesenteric lymphatic contractility in the rat. Lymph Res Biol 2: 2–10, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21: 561–574, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Azuma K, Akiyama M, Ebata T, Totsuka M, Hayasaka H. Endogenous endotoxin absorption and the role of intestinal lymphatics. Jpn J Surg 13: 535–539, 1983. [DOI] [PubMed] [Google Scholar]
- 7.Bain CC, Mowat AM. The monocyte-macrophage axis in the intestine. Cell Immunol 291: 41–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6: 498–510, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol 2: 120134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol 301: H1897–H1906, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol 297: H1319–H1328, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridenbaugh EA, Wang W, Srimushnam M, Cromer WE, Zawieja SD, Schmidt SE, Jupiter DC, Huang HC, Van Buren V, Zawieja DC. An immunological fingerprint differentiates muscular lymphatics from arteries and veins. Lymph Res Biol 11: 155–171, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train 41: 457–465, 2006. [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell SJ, Hughes PM, Iredale JP, Wilcockson DC, Waters S, Docagne F, Perry VH, Anthony DC. CINC-1 is an acute-phase protein induced by focal brain injury causing leukocyte mobilization and liver injury. FASEB J 17: 1168–1170, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty S, Davis MJ, Muthuchamy M. Emerging trends in the pathophysiology of lymphatic contractile function. Sem Cell Dev Biol 38: 55–66, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty S, Nepiyushchikh Z, Davis MJ, Zawieja DC, Muthuchamy M. Substance P activates both contractile and inflammatory pathways in lymphatics through the neurokinin receptors NK1R and NK3R. Microcirculation 18: 24–35, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakraborty S, Zawieja DC, Davis MJ, Muthuchamy M. MicroRNA signature of inflamed lymphatic endothelium and role of miR-9 in lymphangiogenesis and inflammation. Am J Physiol Cell Physiol 309: C680–C692, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol 303: H693–H702, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20: 538–549, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chtanova T, Schaeffer M, Han SJ, van Dooren GG, Nollmann M, Herzmark P, Chan SW, Satija H, Camfield K, Aaron H, Striepen B, Robey EA. Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29: 487–496, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cromer W, Wang W, Zawieja SD, von der Weid PY, Newell-Rogers M K, Zawieja DC. Colonic insult impairs lymph flow, increases cellular content of the lymph, alters local lymphatic microenvironment, and leads to sustained inflammation in the rat ileum. Inflamm Bowel Dis 21: 1553–1563, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cromer WE, Zawieja SD, Tharakan B, Childs EW, Newell MK, Zawieja DC. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis 17: 395–406, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis MJ. An improved, computer-based method to automatically track internal and external diameter of isolated microvessels. Microcirculation 12: 361–372, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, Muthuchamy M, Gashev AA. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol 295: H587–H597, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elias RM, Johnston MG. Modulation of lymphatic pumping by lymph-borne factors after endotoxin administration in sheep. J Appl Physiol 68: 199–208, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Elias RM, Johnston MG, Hayashi A, Nelson W. Decreased lymphatic pumping after intravenous endotoxin administration in sheep. Am J Physiol Heart Circ Physiol 253: H1349–H1357, 1987. [DOI] [PubMed] [Google Scholar]
- 29.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gashev AA, Zawieja DC. Hydrodynamic regulation of lymphatic transport and the impact of aging. Pathophysiology 17: 277–287, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasheva OY, Gashev AA, Zawieja DC. Cyclic guanosine monophosphate and the dependent protein kinase regulate lymphatic contractility in rat thoracic duct. J Physiol 591: 4549–4565, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res 50: 90–97, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Gordon S, Pluddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev 262: 36–55, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-κB. J Exp Med 205: 1261–1268, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haidari M, Leung N, Mahbub F, Uffelman KD, Kohen-Avramoglu R, Lewis GF, Adeli K. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J Biol Chem 277: 31,646–31,655, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nat Rev Immunol 9: 364–375, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13: 260–268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, Werner S, Alitalo K, Detmar M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 117: 4667–4678, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson LA, Jackson DG. The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. J Cell Sci 126: 5259–5270, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juskewitch JE, Knudsen BE, Platt JL, Nath KA, Knutson KL, Brunn GJ, Grande JP. LPS-induced murine systemic inflammation is driven by parenchymal cell activation and exclusively predicted by early MCP-1 plasma levels. Am J Pathol 180: 32–40, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kakei Y, Akashi M, Shigeta T, Hasegawa T, Komori T. Alteration of cell-cell junctions in cultured human lymphatic endothelial cells with inflammatory cytokine stimulation. Lymph Res Biol 12: 136–143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kavanagh K, Wylie AT, Tucker KL, Hamp TJ, Gharaibeh RZ, Fodor AA, Cullen JM. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am J Clin Nutr 98: 349–357, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly JL, O'Sullivan C, O'Riordain M, O'Riordain D, Lyons A, Doherty J, Mannick JA, Rodrick ML. Is circulating endotoxin the trigger for the systemic inflammatory response syndrome seen after injury? Ann Surg 225: 530–541; discussion 541-533, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kevil CG, Patel RP, Bullard DC. Essential role of ICAM-1 in mediating monocyte adhesion to aortic endothelial cells. Am J Physiol Cell Physiol 281: C1442–C1447, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Kim KE, Koh YJ, Jeon BH, Jang C, Han J, Kataru RP, Schwendener RA, Kim JM, Koh GY. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol 175: 1733–1745, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC, Randolph GJ. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol 194: 5200–5210, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai JP, Douglas SD, Shaheen F, Pleasure DE, Ho WZ. Quantification of substance P mRNA in human immune cells by real-time reverse transcriptase PCR assay. Clin Diagn Lab Immunol 9: 138–143, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harbor Perspect Biol 1: a001651, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JH, Palaia T, Ragolia L. Impaired insulin-mediated vasorelaxation in diabetic Goto-Kakizaki rats is caused by impaired Akt phosphorylation. Am J Physiol Cell Physiol 296: C327–C338, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D, Padera TP. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA 108: : 18,784–18,789, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lobov GI, Kubyshkina NA. Mechanisms underlying the effect of E. coli endotoxin on contractile function of lymphatic vessels. Bull Exp Biol Med 137: 114–116, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation 18: 463–473, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nemoto K, Sato H, Tanuma K, Okamura T. Mesenteric lymph flow in endotoxemic guinea pigs. Lymph Res Biol 9: 129–134, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Nepiyushchikh ZV, Chakraborty S, Wang W, Davis MJ, Zawieja DC, Muthuchamy M. Differential effects of myosin light chain kinase inhibition on contractility, force development and myosin light chain 20 phosphorylation of rat cervical and thoracic duct lymphatics. J Physiol 589: 5415–5429, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niu CY, Hou YL, Zhao ZG, Zhang YF, Ji JJ, Qiao HX, Zhang J, Yao YM. [Role of intestinal lymphatic pathway in pathogenesis of intestine-derived bacteria/endotoxin translocation in rats in shock]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 19: 266–269, 2007. [PubMed] [Google Scholar]
- 59.O'Grady NP, Tropea M, Preas HL 2nd, Reda D, Vandivier RW, Banks SM, Suffredini AF. Detection of macrophage inflammatory protein (MIP)-1α and MIP-1β during experimental endotoxemia and human sepsis. J Infect Dis 179: 136–141, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Olofsson P, Nylander G, Olsson P. Endotoxin: routes of transport in experimental peritonitis. Am J Surg 151: 443–446, 1986. [DOI] [PubMed] [Google Scholar]
- 61.Pena OM, Pistolic J, Raj D, Fjell CD, Hancock RE. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol 186: 7243–7254, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, Skobe M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol 183: 1767–1779, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, Sica A. Cellular and molecular pathways linking inflammation and cancer. Immunobiology 214: 761–777, 2009. [DOI] [PubMed] [Google Scholar]
- 64.Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P, Mantovani A, Sica A. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor κB. Proc Natl Acad Sci USA 106: 14,978–14,983, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 5: 617–628, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Randolph GJ, Sanchez-Schmitz G, Angeli V. Factors and signals that govern the migration of dendritic cells via lymphatics: recent advances. Springer Sem Immunopathol 26: 273–287, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Rizwan A, Bulte C, Kalaichelvan A, Cheng M, Krishnamachary B, Bhujwalla ZM, Jiang L, Glunde K. Metastatic breast cancer cells in lymph nodes increase nodal collagen density. Sci Rep 5: 10002, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol 280: L1094–L1103, 2001. [DOI] [PubMed] [Google Scholar]
- 69.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinmuller M, Srivastava M, Kuziel WA, Christman JW, Seeger W, Welte T, Lohmeyer J, Maus UA. Endotoxin induced peritonitis elicits monocyte immigration into the lung: implications on alveolar space inflammatory responsiveness. Respir Res 7: 30, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan KW, Chong SZ, Wong FH, Evrard M, Tan SM, Keeble J, Kemeny DM, Ng LG, Abastado JP, Angeli V. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood 122: 3666–3677, 2013. [DOI] [PubMed] [Google Scholar]
- 72.Tikhonov I, Doroshenko T, Chaly Y, Smolnikova V, Pauza CD, Voitenok N. Down-regulation of CXCR1 and CXCR2 expression on human neutrophils upon activation of whole blood by S. aureus is mediated by TNF-α. Clin Exp Immunol 125: 414–422, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, Graham G, Combadiere C, Gregory CD. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 112: 5026–5036, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol Gastrointest Liver Physiol 250: G715–G726, 1986. [DOI] [PubMed] [Google Scholar]
- 75.Tuncer LI, Alacam T, Oral B. Substance P expression is elevated in inflamed human periradicular tissue. J Endod 30: 329–332, 2004. [DOI] [PubMed] [Google Scholar]
- 76.von der Weid PY, Muthuchamy M. Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology 17: 263–276, 2010. [DOI] [PubMed] [Google Scholar]
- 77.Wang H. Activated macrophage-mediated endogenous prostaglandin and nitric oxide-dependent relaxation of lymphatic smooth muscles. Jpn J Physiol 47: 93–100, 1997. [DOI] [PubMed] [Google Scholar]
- 78.Wang W, Nepiyushchikh Z, Zawieja DC, Chakraborty S, Zawieja SD, Gashev AA, Davis MJ, Muthuchamy M. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol 297: H726–H734, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Witko-Sarsat V, Mocek J, Bouayad D, Tamassia N, Ribeil JA, Candalh C, Davezac N, Reuter N, Mouthon L, Hermine O, Pederzoli-Ribeil M, Cassatella MA. Proliferating cell nuclear antigen acts as a cytoplasmic platform controlling human neutrophil survival. J Exp Med 207: 2631–2645, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu TF, Carati CJ, Macnaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol 291: G566–G574, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Zawieja DC. Contractile physiology of lymphatics. Lymph Res Biol 7: 87–96, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 302: H643–H653, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang G, Ghosh S. Toll-like receptor-mediated NF-κB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest 107: 13–19, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]