This study offers novel insights into the role of circulating microRNA in exercise-induced adaptations in chronic kidney disease, which can eventually lead to the identification of targets for effective preventive strategies.
Keywords: chronic kidney disease, microRNA, acute exercise, exercise training
Abstract
Exercise training is an effective way to improve exercise capacity in chronic kidney disease (CKD), but the underlying mechanisms are only partly understood. In healthy subjects (HS), microRNA (miRNA or miR) are dynamically regulated following exercise and have, therefore, been suggested as regulators of cardiovascular adaptation to exercise. However, these effects were not studied in CKD before. The effect of acute exercise (i.e., an acute exercise bout) was assessed in 32 patients with CKD and 12 age- and sex-matched HS (study 1). miRNA expression in response to chronic exercise (i.e., a 3-mo exercise training program) was evaluated in 40 CKD patients (study 2). In a subgroup of study 2, the acute-exercise induced effect was evaluated at baseline and at follow-up. Plasma levels of a preselected panel miRNA, involved in exercise adaptation processes such as angiogenesis (miR-126, miR-210), inflammation (miR-21, miR-146a), hypoxia/ischemia (miR-21, miR-210), and progenitor cells (miR-150), were quantified by RT-PCR. Additionally, seven miRNA involved in similar biological processes were quantified in the subgroup of study 2. Baseline, studied miRNA were comparable in CKD and HS. Following acute exercise, miR-150 levels increased in both CKD (fold change 2.12 ± 0.39, P = 0.002; and HS: fold change 2.41 ± 0.48 P = 0.018, P for interaction > 0.05). miR-146a acutely decreased in CKD (fold change 0.92 ± 0.13, P = 0.024), whereas it remained unchanged in HS. Levels of miR-21, miR-126, and miR-210 remained unaltered. Chronic exercise did not elicit a significant change in the studied miRNA levels. However, an acute exercise-induced decrease in miR-210 was observed in CKD patients, only after training (fold change 0.76 ± 0.15). The differential expression in circulating miRNA in response to acute and chronic exercise may point toward a physiological role in cardiovascular adaptation to exercise, also in CKD.
NEW & NOTEWORTHY
This study offers novel insights into the role of circulating microRNA in exercise-induced adaptations in chronic kidney disease, which can eventually lead to the identification of targets for effective preventive strategies.
microRNAs (miRNA or miR) are short, endogenous, noncoding RNAs that negatively regulate gene expression at the posttranscriptional level. By targeting multiple genes, often involved in related signaling pathways, they are important for various aspects of homeostasis and disease, including cardiovascular and kidney disease (10, 31, 37). Previously, it has been shown that their presence in plasma could be of great diagnostic and even prognostic value (9). Apart from being released following cell death or injury, miRNAs can be selectively packaged inside intracellular exosomes, actively secreted into the circulation, and transferred to other cells (39). Therefore, it occurs that miRNAs are important for intercellular cross talk, modulating protein expression of the recipient cells (13). Moreover, miRNAs may represent novel therapeutic targets, since there is now ample evidence that miRNA levels in vivo can be regulated by the use of anti-miRNAs (43).
miRNAs contribute to both development and progression of chronic kidney disease (CKD) and might add to the increased cardiovascular risk in these patients (24, 37). The high prevalence of cardiovascular disease in CKD is the result of a complex interplay of several mechanisms, including endothelial dysfunction and vascular calcification (11). Exercise training is an effective way to reduce this cardiovascular risk and to improve exercise capacity, also in CKD patients (15). The analysis of miRNAs can improve our understanding of adaptations to exercise and training at the gene expression level in CKD.
In this regard, previous work has already demonstrated that both brief exercise and exercise training transiently or adaptively change several circulating miRNAs in athletes and healthy volunteers (2, 6). However, little is known about baseline values and variation in miRNA levels following exercise in CKD patients. We tested the hypothesis that plasma profiles of specific miRNAs involved in angiogenesis (miR-126, miR-210), inflammation (miR-21, miR-146a), hypoxia/ischemia adaptation (miR-21, miR-210), and progenitor cell biology (miR-150) are affected following both acute and chronic exercise in CKD.
The aims of the present study were 1) to examine the effects of acute exercise (i.e., an acute exercise bout) on plasma levels of miR-21, miR-126, miR-146a, miR-150, and miR-210 in patients with CKD compared with healthy controls; and 2) to evaluate whether plasma levels of miR-21, miR-126, miR-146a, miR-150, and miR-210 at rest and after acute exercise are influenced by chronic exercise (i.e., an aerobic exercise training program of 3 mo). Additionally, the effect of chronic exercise on the acute exercise-induced response of seven other miRNAs involved in angiogenesis (miR-17, miR-92a, miR-130, miR-24), hypoxia/ischemia adaptation (miR-24), and vascular calcification in CKD (miR-125b, miR-145, and miR-155) was studied in a subgroup of patients.
METHODS
Study population and design.
The effect of acute exercise on plasma miRNA levels (study 1) was studied in 32 ambulatory patients with CKD stages 1–5. Twelve healthy subjects (HS), without relevant medical history or pharmacological treatment, served as controls. All participants were called in for a cardiopulmonary exercise test (CPET), and refrained from excessive physical exertion 24 h before the test. Venous blood was sampled immediately before and 10 min after peak exercise.
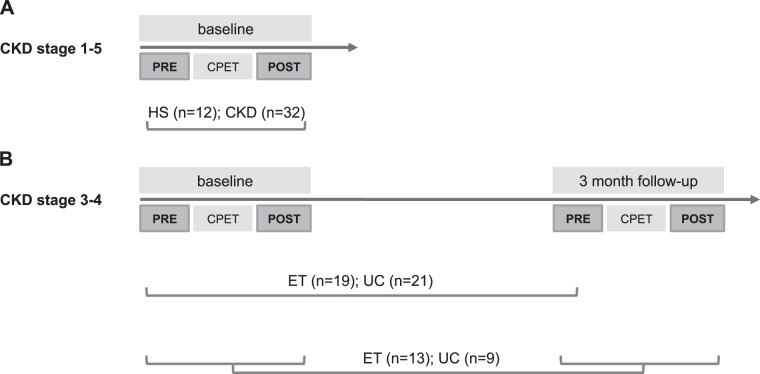
Next, the effect of chronic exercise on plasma miRNA levels (study 2) was studied in 40 ambulatory patients with CKD stages 3 and 4. They were randomized into a 12-wk aerobic training program [exercise training (ET); n = 19] or usual care (UC; n = 21) as part of a single center randomized controlled trial (41). A CPET was performed at baseline and after 12 wk, and blood was sampled before the CPET. In a subgroup of patients (ET: n = 13, UC: n = 9), venous blood was sampled both at rest and 10 min after peak exercise, baseline, and at 12 wk follow-up (Fig. 1).
Fig. 1.
Study design and sampling moments. A: in study 1, the effect of acute exercise [cardiopulmonary exercise test (CPET)] on plasma microRNA (miRNA) levels was studied in 32 chronic kidney disease (CKD) patients and 12 healthy subjects (HS). B: in study 2, the effect of chronic exercise on plasma miRNA levels was studied as part of a single center randomized controlled trial. In total, 40 patients with CKD stages 3 and 4 were involved, and measurements took place at randomization and after 12 wk of aerobic training [exercise training (ET); n = 19] or usual care (UC; n = 21). In a substudy of patients (ET n = 13; UC n = 9), the effect of chronic exercise on the acute exercise-induced response on plasma miRNA was evaluated to unravel mechanisms for adaptation to exercise. PRE, pre-CPET; POST, post-CPET.
For both studies, diagnosis of CKD was based on the estimated glomerular filtration rate (eGFR) using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula (17) and/or the presence of kidney injury, as recommended by the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative guidelines (22a). The following exclusion criteria were applied: history of overt cardiovascular disease, pregnancy, renal replacement therapy, age <18 yr, treatment with immunosuppressive or oral anticoagulation therapy, and active malignant disease. The studies were approved by the ethics committee of the Antwerp University Hospital and conformed to the principles outlined in the declaration of Helsinki. Inclusion took place between April 2012 and July 2014. Written, informed consent was obtained from all participants.
Acute exercise: CPET.
A maximal symptom-limited CPET was performed on a bicycle ergometer (Cardiovit CS-200 Ergo-Spiro, Schiller AG, Baar, Switzerland). An individualized ramp protocol, starting with either 20 or 40 W and an incremental load of 10 or 20 W/min was chosen to ensure an optimal exercise duration of 8–10 min. Twelve-lead ECG was recorded continuously, and blood pressure was measured every 2 min. Breath-by-breath gas exchange measurements allowed online determination of ventilation, oxygen uptake, and carbon dioxide production. Peak oxygen consumption (V̇o2peak) was determined as the highest attained oxygen uptake during the final 30 s of exercise. V̇o2peak and maximal workload were also expressed as a percentage of the predicted value (%predicted V̇o2peak, %predicted Watt maximum), according to the nomogram of Hansen et al. (12). Subjects were encouraged to exercise upon exhaustion, according to the respiratory exchange ratio and identification of the anaerobic threshold (V-slope method).
Chronic exercise: ET.
Patients in the ET group underwent a 12-wk home-based aerobic training next to their standard therapy (maintenance medication). The program consisted of four daily cycling sessions of 10 min on magnetically-braked home trainers (DKN Mag410B, Belgium) using heart rate transmitters (Polar FT7) to obtain the target heart rate (90% of the heart rate at anaerobic threshold). Adherence was monitored monthly by reviewing heart rates during training and by detailed training logs. Patients in the UC group were given standard therapy, without specific instructions on physical activity. In both groups, medical therapy was unchanged during the study period.
Selection of the miRNA panel.
Based on the literature, we initially selected five miRNAs previously implicated in cellular processes underlying exercise adaptation: miR-21, miR-126, miR-146a, miR-150, and miR-210. Additionally, seven miRNAs involved in the same relevant processes were selected (Table 1).
Table 1.
Overview of the studied miRNAs
| miRNA | Biological Process | Selected Validated Target Genes | Reference Nos. |
|---|---|---|---|
| mi-21 | Inflammation | PTEN, PDC4, BCL-2 | 2, 6, 36 |
| Apoptosis | |||
| Hypoxia/ischemia adaptation | |||
| miR-126 | Angiogenesis | Spred-1, PI3KR2, SDF-1 | 8, 38, 48 |
| miR-146a | Inflammation | IRAK-1, TRAF6, CXCR4, TLR4 | 2, 29, 30, 34, 48 |
| miR-150 | Hematopoiesis | CXCR4, MYB, FLT3, CBL, EGR2, AKT2, DKC | 21, 47, 50 |
| Progenitor cell mobilization and migration | |||
| miR-210 | Angiogenesis | HIF-1a | 2, 6, 44 |
| Hypoxia | |||
| Proliferation | |||
| miR-17 | Angiogenesis | E2F1 | 4, 5, 33 |
| Apoptosis | |||
| miR-24 | Hypoxia | H2A histone family, member X, heme oxygenase 1 | 19 |
| Apoptosis | |||
| miR-92a | Angiogenesis | Integrin subunit α5 | 3, 5, 33 |
| miR-125b | Vascular calcification | NFκB | 7 |
| miR-130a | Angiogenesis | GAX, HoxA5 | 32 |
| miR-145 | Vascular calcification | Myocardin | 7 |
| miR-155 | Vascular calcification | AT1R | 7, 33 |
miRNA, microRNA.
RNA extraction.
EDTA-treated blood samples were centrifuged within 30 min after collection, and plasma was immediately stored at −80°C. To avoid technical variability, all samples from a given individual were processed and analyzed in a single batch. Stored plasma was thawed on ice and centrifuged at 4°C for 10 min (16,000 g). Total RNA, including miRNA, was isolated from 200-μl plasma with the miRNeasy serum/plasma kit (Qiagen, Venlo, the Netherlands). A fixed amount of the synthetic Caenorhabditis elegans-miR-39 was added to the standard volume of 200-μl plasma, immediately after lysis with Qiazol, to test for sample-to-sample variation in RNA isolation. Total RNA was extracted using chloroform, ethanol, and spin column and eluted in 15-μl RNase-free water.
Targeted quantification of miRNA.
Isolated RNA was used for multiplexed reverse transcription of mature miR-21, miR-126, miR-146a, miR-150, and miR-210 into cDNA using specific stem-loop primers (Applied Biosystems). Plasma levels of selected miRNA were quantified using real-time PCR via TaqMan probes (Applied Biosystems) in a Bio-Rad CFX96 Real-Time PCR system. Exogenously added Caenorhabditis elegans-miR-39 was used as a spike-in normalization control. Additional miRNAs were quantified in exactly the same way. All reactions and analyses were performed in duplicate. The coefficient of variation accepted for intra-assay replicates was set at 4%. Threshold cycle values were used for relative miRNA quantification using the delta threshold cycle method. Relative miRNA levels were expressed as log (2−ΔCT × 100). Fold change of the respective miRNAs was calculated as relative expression postintervention/relative expression preintervention.
Statistical analysis.
Continuous data are expressed as means ± SD or SE (miRNA data). Skewed data were logarithmically transformed. Baseline comparisons were performed using independent sample T-test or χ2 test. For correlations, Pearson (r) or Spearman's (ρ) correlation coefficients were used.
The response to acute and chronic exercise was assessed by linear mixed-model analysis with the miRNA level as dependent variable and time and group as fixed-effect independent variables. Individual identification was entered as a random intercept, to account for the relatedness between observations within the same individual. To test whether the groups show a different response over time, the significance of the interaction term between time and group was calculated.
All statistical analysis was performed with R, version 3.1.2 (R core team 2014). Linear mixed models were fitted using the add-on package lme4.
RESULTS
Demographic and clinical variables.
Demographic and clinical characteristics for study 1 are shown in Table 2. Patients and HS were matched for age and sex. Severity of CKD ranged from stages 1–5, with the majority in stage 3 (6, 22, 38, 35, 9%, respectively, for CKD stages 1–5). Both HS and CKD patients performed a maximal exercise test, as was objectified by a respiratory exchange ratio value > 1.15. Aerobic exercise capacity and maximal workload were significantly lower in CKD compared with HS.
Table 2.
Baseline demographic and clinical variables of participants in study 1
| CKD | HS | P Value | |
|---|---|---|---|
| n | 32 | 12 | |
| Age, yr | 49.6 ± 15.3 | 43.4 ± 4.7 | 0.179 |
| Sex (F/M) | 18/14 | 5/7 | 0.388 |
| BMI, kg/m2 | 26.7 ± 5.3 | 23.7 ± 2.2 | 0.012 |
| eGFR, ml·min−1·1.73 m−2 | 46.2 ± 23.8 | 101.4 ± 9.7 | <0.001 |
| Systolic blood pressure, mmHg | 125 ± 15 | 125 ± 13 | 0.935 |
| Diastolic blood pressure, mmHg | 81 ± 12 | 79 ± 9 | 0.520 |
| V̇o2peak, ml·kg−1·min−1 | 26.1 ± 8.0 | 38.5 ± 9.8 | 0.002 |
| %Predicted V̇o2peak | 85.2 ± 24.3 | 109.3 ± 22.9 | 0.008 |
| V̇o2 at anaerobic threshold, ml·kg−1·min−1 | 24.3 ± 7.1 | 32.2 ± 8.9 | 0.027 |
| Maximal workload, W | 150.4 ± 53.2 | 259.6 ± 89.2 | <0.001 |
| Exercise duration, s | 398 ± 117 | 790 ± 238 | <0.001 |
| Respiratory exchange ratio | 1.36 ± 0.11 | 1.27 ± 0.09 | 0.021 |
| Resting heart rate, beats/min | 74 ± 12 | 75 ± 13 | 0.826 |
| Peak heart rate, beats/min | 154 ± 23 | 175 ± 11 | <0.001 |
| Medication, no. (%) | |||
| ACE-I | 13 (41) | 0 | 0.007 |
| ARB | 6 (18) | 0 | 0.128 |
| Statin | 13 (41) | 0 | 0.007 |
Values are means ± SD or no. (%); n, no. of subjects. CKD, chronic kidney disease; HS, healthy subjects; F, female; M, male; BMI, body mass index; eGFR, estimated glomerular filtration rate; V̇o2peak, peak oxygen uptake; V̇o2, oxygen uptake; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Demographic and clinical characteristics for study 2 are shown in Table 3. Groups were comparable at baseline, including variables of exercise capacity.
Table 3.
Baseline demographic and clinical variables of participants in study 2
| UC | ET | P Value | |
|---|---|---|---|
| n | 21 | 19 | |
| Age, yr | 54.7 ± 14.1 | 51.5 ± 11.8 | 0.441 |
| Sex (F/M) | 10/11 | 8/11 | 0.726 |
| BMI, kg/m2 | 28.3 ± 5.8 | 28.3 ± 6.2 | 0.965 |
| eGFR, ml·min−1·1.73 m−2 | 42.2 ± 14.9 | 40.2 ± 15.2 | 0.665 |
| Systolic blood pressure, mmHg | 123 ± 16 | 129 ± 17 | 0.259 |
| Diastolic blood pressure, mmHg | 79 ± 11 | 81 ± 13 | 0.740 |
| V̇o2peak, ml·kg−1·min−1 | 24.4 ± 6.6 | 26.4 ± 5.4 | 0.287 |
| Medication, no. (%) | |||
| ACE-I | 8 (38) | 12 (63) | 0.102 |
| ARB | 9 (43) | 4 (21) | 0.129 |
| Statin | 10 (48) | 12 (63) | 0.252 |
Values are means ± SD or no. (%); n, no. of subjects. UC, usual care; ET, exercise training.
Baseline levels of miRNA in relation to eGFR.
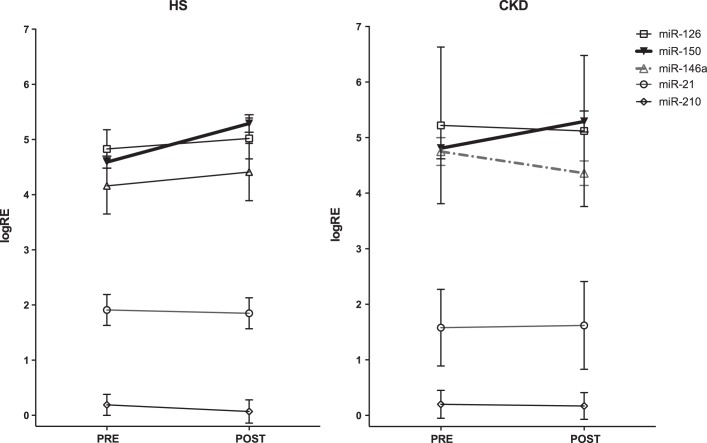
All miRNAs were at a detectable level; baseline plasma levels of all miRNA were comparable between CKD patients and HS in study 1 (Fig. 2). In univariate analysis, a significant correlation was found between miR-146a levels and eGFR, with higher expression levels in more severe renal disease (ρ = −0.252, P = 0.050).
Fig. 2.
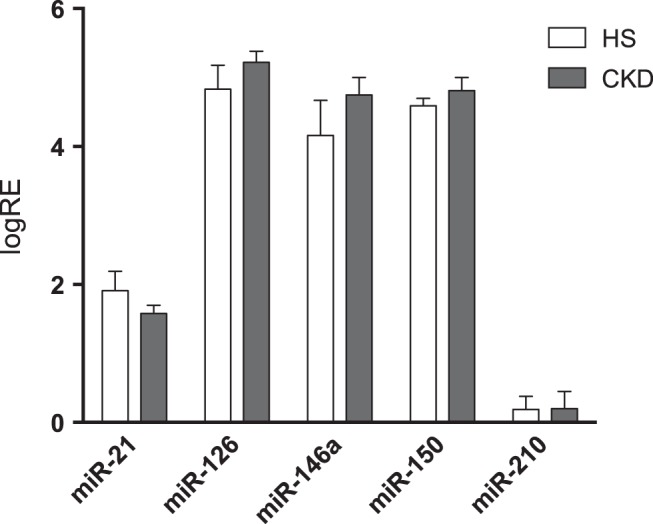
Circulating miRNA at steady state in CKD and HS (study 1). Values are the mean logarithm of the relative expression (logRE) of the respective miRNA ± SE. Baseline plasma levels of all studied miRNAs were comparable between the studied CKD patients (n = 32) and HS (n = 12).
Effect of acute exercise.
Following a single exercise bout, the plasma expression level of miR-150 was significantly upregulated in both CKD patients (fold change 2.12 ± 0.39, P = 0.002) and in HS (fold change 2.41 ± 0.48, P = 0.018). On the other hand, the expression of miR-146a decreased significantly in CKD patients (fold change 0.92 ± 0.13, P = 0.024), whereas it remained unchanged in HS (P = 0.83) (Fig. 3). Plasma levels of miR-21, miR-126, and miR-210 remained unaltered in both groups.
Fig. 3.
Effect of acute exercise on plasma levels of miRNA (study 1). Values are the logRE of the respective miRNA ± SE. Plasma levels of miRNA were quantified immediately before and 10 min after peak exercise in CKD patients (n = 32) and HS (n = 12). In both CKD patients and HS, plasma levels of miR-150 were significantly upregulated following acute exercise (solid line). The expression of miR-146a decreased significantly in CKD patients (shaded dotted-dashed line), but remained unchanged in HS. Plasma levels of miR-21, miR-126, and miR-210 remained unaltered in both groups.
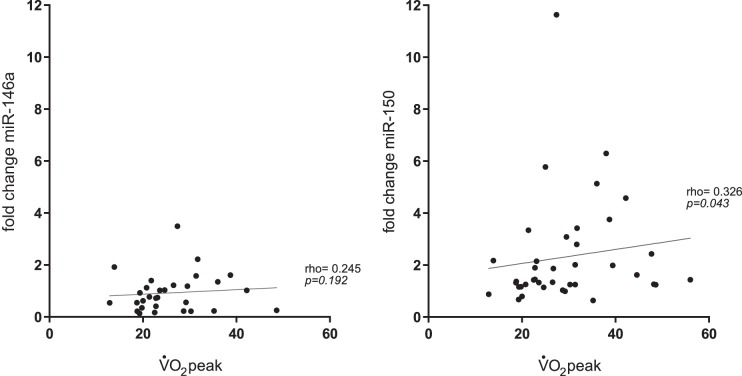
The acute exercise-induced change in miR-150 levels was correlated with V̇o2peak (ρ = 0.326, P = 0.043). No correlation was found between V̇o2peak and changes in miR-146a (ρ = 0.245, P = 0.192) (Fig. 4).
Fig. 4.
Relation between peak oxygen consumption (V̇o2peak) and acute exercise-induced changes in miR-146a and miR-150 (study 1). The acute exercise-induced change in miR-150, but not miR-146a levels was significantly correlated with V̇o2peak.
Effect of chronic exercise in CKD patients.
After a formal exercise training program, V̇o2peak (+5.82 ml·kg−1·min−1) and maximal workload (+37 W) improved significantly in the ET group compared with the UC group (P for interaction < 0.001) (41). However, training did not result in a significant change of any of the studied miRNAs compared with the UC group (P for interaction > 0.05, Table 4).
Table 4.
Effect of chronic exercise on plasma levels of miRNA
| ET (n = 19) |
UC (n = 21) |
||||||
|---|---|---|---|---|---|---|---|
| Baseline | 3 mo Follow-up | P value | Baseline | 3 mo Follow-up | P value | P value for interaction | |
| miR-21 | 2.15 ± 0.22 | 2.04 ± 0.3 | 0.538 | 2.28 ± 0.25 | 2.18 ± 0.18 | 0.477 | 0.960 |
| miR-126 | 5.97 ± 0.33 | 5.72 ± 0.33 | 0.472 | 6.32 ± 0.30 | 6.06 ± 0.26 | 0.212 | 0.904 |
| miR-146a | 5.53 ± 0.36 | 4.94 ± 0.41 | 0.047 | 5.66 ± 0.33 | 5.27 ± 0.27 | 0.113 | 0.661 |
| miR-150 | 5.40 ± 0.25 | 5.26 ± 0.32 | 0.552 | 5.44 ± 0.23 | 5.49 ± 0.24 | 0.842 | 0.557 |
| miR-210 | 0.90 ± 0.39 | 0.61 ± 0.29 | 0.222 | 0.82 ± 0.32 | 0.49 ± 0.26 | 0.233 | 0.086 |
Values are expressed as the logarithm of the relative expression of the respective miRNA ± SE; n, no. of subjects.
Response to acute exercise following exercise training.
To evaluate whether the acute exercise-induced changes in miRNA are comparable before and after a 12-wk training period, a linear mixed-model analysis was performed in a subgroup of patients of study 2 (ET, n = 13; UC, n = 9). In a second analysis, the response of miR-17, miR-24, miR-92a, miR-125b, miR-130a, miR-145, and miR-155 was also studied in these patients.
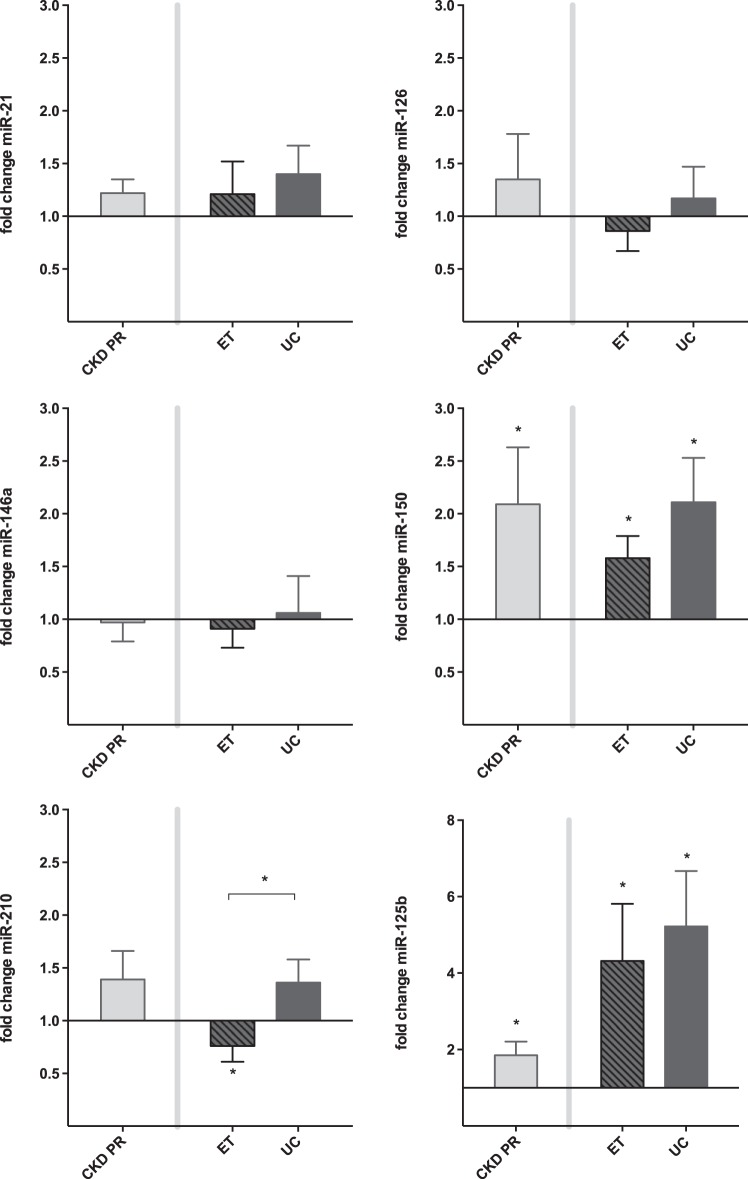
Figure 5 presents the fold change in response to acute exercise of miR-21, miR-126, miR-146a, miR-150, as well as miR-125b. Only for miR-210, exercise training resulted in a different response to an acute exercise bout. Whereas an acute exercise bout did not elicit an effect in the UC group, a significant decrease in miR-210 levels following acute exercise was observed in the ET group after the training program was completed (fold change 0.76 ± 0.15, p for interaction = 0.045). Moreover, this acute exercise-induced decrease in miR-210 levels correlated with an increase in V̇o2peak (ρ = −0.236, P < 0.05).
Fig. 5.
Response to acute exercise: effect of ET (study 2). Values are mean fold change ± SE. The effect of chronic exercise on the response to an acute exercise bout was evaluated in 22 CKD patients (ET n = 13; UC n = 9). Only for miR-210, ET resulted in a different response to an acute exercise bout (*P for interaction < 0.05). Indeed, a significant decrease in miR-210 was seen only in the trained patients. Next, the figure shows a significant increase following acute exercise of circulating miR-150 and miR-125b in the studied population. PR, prerandomization.
In the studied population, plasma levels of miR-150 and miR-125b significantly increase following an acute exercise bout, both at baseline and follow-up. In contrast with the findings of study 1, miR-146a did not decrease following acute exercise in the studied subjects. Plasma levels of miR-17, miR-24, miR-92a, miR-130a, miR-145, and miR-155 remained unaffected by both acute and chronic exercise (data not shown).
DISCUSSION
This study describes the effect of acute (single exercise bout) and chronic exercise (exercise training) on plasma levels of specific vascular-related miRNAs in CKD. In addition, the effect of exercise training on the acute exercise-induced response is studied to unravel mechanisms for adaptation to exercise.
Several new findings emerge from this study: at baseline, plasma levels of miR-21, miR-126, miR-146a, miR-150, and miR-210 are comparable between CKD patients and HS. For miR-146a, an inverse correlation was found between plasma levels of miR-146a and eGFR in univariate analysis.
Acute exercise significantly upregulates plasma levels of miR-150, and this both in CKD and in HS. Next, in CKD patients only, acute exercise results in a significant decrease in miR-146a. Plasma levels of miR-125b significantly increase following acute exercise, both in sedentary and CKD patients.
In CKD patients, chronic exercise results in a significant decrease of miR-210 in response to an acute exercise bout.
Dysregulation of miRNA in CKD.
To date, limited information is available on plasma levels of miRNA potentially implicated in the pathophysiology of impaired exercise capacity in patients with CKD.
The group of Neal and coworkers (23) described reduced levels of total circulating miRNA and five specific miRNAs (including miR-21 and miR-210) in renal failure, at least for patients with end-stage renal disease (eGFR < 15 ml·min−1·1.73 m−2) and for those with CKD stage 4 (eGFR 15–30 ml·min−1·1.73 m−2). Other evidence of lower circulating miRNA levels in CKD came from the study of Chen et al. (7), who found decreased circulating levels of miR-125b, miR-145, and miR-155 (all affecting vascular smooth muscle cell proliferation) with deterioration of renal function in patients with CKD stage 3 and 4.
In the present study, the circulating levels of miR-21, miR-126, miR-146a, miR-150, and miR-210 were comparable between CKD patients (stages 1–5) and healthy controls. Differences in the studied populations (disease severity and comorbidities) could contribute to this finding. While the group of Neal (23) included a considerable amount of end-stage renal disease patients, the majority of patients in the present study suffered from CKD stages 3 and 4. Moreover, they report that the decrease in levels of miR-21 and miR-210 takes place at CKD stage 4 at the earliest. Whereas the study of Chen concentrates on the issue of vascular disease in CKD and, therefore, describes a population with objective CV disease, the present study only investigated patients without cardiovascular disease, which is only a small and very selective subgroup of CKD patients.
In the present study, we focused on vascular-related miRNA, since endothelial dysfunction and arterial stiffness are characteristic features of CKD inferring adverse prognosis. We found a relation between worse renal function and higher plasma levels of miR-146a. miR-146a is an inflammation-associated miRNA and can be induced by different proinflammatory stimuli, such as IL-1β, TNF-α, and Toll-like receptors (TLR) (30, 34). IL-1 receptor-associated kinase-1 and TNF receptor-associated factor-6 have been identified as target genes of miR-146a posttranslational repression, proposed as a negative feedback mechanism of TLR and cytokine receptor signaling (34). As such, the expression of miR-146a might be part of a mechanism for restraining the excessive production of proinflammatory cyto- or chemokines in inflammatory states, such as CKD. Tissue miR-146a is upregulated in various inflammatory human diseases, such as rheumatoid arthritis (18, 22, 25) and atherosclerosis (27). In a murine model of CKD, miR-146a expression in the kidneys has been associated with the development of interstitial lesions and correlated with inflammatory cell infiltration (14). As such, the potential of miR-146a as disease biomarker in CKD is promising, but should be validated in larger studies.
Effects of acute exercise.
The dynamic regulation of circulating miRNA in response to a single exercise bout could offer insights into the beneficial effects of exercise training. Originating from endothelial, blood, and muscle cells, miRNAs can be released into the circulation, packed into microvesicles or being part of protein complexes, in response to exercise to carry information from one cell to another (1, 26, 46, 49). As demonstrated in HS, miRNAs are differentially regulated in response to exercise, which suggests a role for miRNAs in (vascular) adaptation to exercise. The prompt regulation of miRNAs within 10 min of maximal exercise might be due to an accelerated posttranscriptional processing of premature miRNA, to an expedited secretion into the circulation or to an aspecific leakage through cell damage (38, 45). On the other hand, downregulation can be due to uptake of the specific miRNA into recipient cells (e.g., elicited by exercise-induced leukocytosis) or reduced secretion.
miR-146a is an inflammation-associated miRNA and can be induced by different proinflammatory stimuli (30, 34). We showed that there was an inverse correlation between eGFR and miR-146a levels, with higher levels in more advanced renal disease. Interestingly, acute exercise resulted in a normalization of miR-146a levels, at least in study 1. It is possible that this decrease reflects a swift uptake of miR-146a by monocytes with subsequent downregulation of TLR-4 in these recipient cells, but it is clear that this hypothesis needs further exploration (35, 40). In study 2, the acute exercise-induced decrease in miR-146a could not be confirmed, possibly due to the lower basal plasma levels of these studied patients.
miR-150 is involved in endothelial progenitor cell (EPC) biology, and exercise training is known to beneficially affect the mobilization and migration of EPCs (28, 42). We observed a significant upregulation of miR-150 after acute exercise, both in HS and patients with CKD. It has been shown that microvesicles isolated from plasma of patients with atherosclerosis contain higher levels of miR-150, and that these microvesicles more effectively promote the migration of human dermal microvascular endothelial cell-1 than microvesicles from healthy donors (50). In line, miR-150 was found to support the migration and tube formation ability of EPC in vitro and to enhance their homing ability in vivo in a c-Myb-dependent manner (47). Recently, miR-150 was also found to be a modulator of physiological cardiac hypertrophy in response to exercise (21). Whether increase of plasma miR-150 levels relates to cardiovascular exercise-induced adaptation, i.e., left ventricular hypertrophy or the increased mobilization of EPCs, remains to be experimentally validated in an in vivo animal model of physical exercise.
Next, we observed a significant increase in miR-125b levels in CKD patients. Low expression levels of miR-125b (both at the plasma and vascular tissue level) have been indirectly linked to the process of osteoblastic differentiation of the vascular smooth muscle cells, resulting in vascular calcification in patients with CKD (7). miR-125b is known to inhibit the Runt-related transcription factor-2-mediated osteoblastic differentiation of mesenchymal cells, possibly through regulation of nuclear factor-κB (16). Whether this exercise-induced increase witnesses or mediates the beneficial effect of exercise still needs to be established.
Unlike the observations of Baggish et al. (2) (1.89 ± 0.28-fold increase in miR-21 in 10 male athletes immediately postexercise) and Uhlemann et al. (38) (2.0 ± 0.2-fold increase in miR-126 in 13 healthy volunteers 5 min after exercise), miR-21 and miR-126 were unresponsive to acute exercise in our cohort of 12 HS and 32 CKD patients. Differences in the exercise protocols, sampling time, and participants' characteristics could possibly account for this discrepancy. Following acute exercise, the level of miR-210 did not change in plasma, which is in accordance with the findings of Baggish et al. (2).
Effects of chronic exercise.
Chronic exercise did not result in a significant change in plasma levels of any of the studied miRNAs. However, the miR-210 response to acute exercise was altered in CKD patients who had completed a 3-mo exercise training program. Hypoxia-inducible factor-1α (HIF-1α) has been identified both as inductor and target of miR-210, suggesting a key role for miR-210 in a negative feedback loop (44). The HIF signaling cascade mediates the effects of hypoxia at the cellular and tissue level by upregulation of glycolysis enzymes and promotion of VEGF-induced angiogenesis, respectively. At the level of the skeletal muscle, an increase in HIF-1α levels is seen after acute exercise, and this effect is blunted after exercise training (20). The mirror image happens in the circulation, with an absent response to acute exercise of miR-210 and a significant decrease elicited by an acute exercise bout following training.
It is plausible that miR-210 has an essential role in the blunted effect on HIF-1 following training, as the adaptation process could imply rapid uptake by muscle cells of miR-210, leading to a persistent downregulation of HIF-1, also when minimal hypoxic state would be present.
In healthy individuals, higher miR-210 levels are directly related to a lower V̇o2peak (6). This relation was confirmed in our CKD patients: the higher the acute exercise-induced decrease in miR-210 levels, the higher the improvement in V̇o2peak after training. Therefore, adaptive mechanisms to a hypoxic state seem to contribute to the beneficial exercise-induced adaptations, which underlie the increase in aerobic capacity posttraining.
Study limitations.
In the present study, the effects of acute exercise were assessed 10 min after peak exercise. Future studies evaluating the time course of miRNA responses to acute as well as chronic exercise might shed more light on the beneficial effects of exercise training and point toward novel therapeutic targets in CKD. However, before we can expect implementation on this level, underlying mechanisms have to be clarified, for example, by loss-of-function models (anti-miRNA) in a rodent model of physical exercise.
Conclusion.
The findings of this study extend the previous observation in healthy volunteers and athletes by showing that circulating miRNA may also play a role in exercise-induced adaptations in the context of CKD. Our data demonstrate that the expression levels of miR-125b, miR-146a, miR-150, and miR-210 change following acute and chronic exercise, albeit with a differential profile. miR-150 shows similar changes in CKD patients and healthy controls, whereas the change in miR-146a is disease specific. Moreover, we suggest a role for miR-210 in favorable exercise-induced cardiovascular adaptations. Whether these changes in miRNA expression patterns underlie a true mechanistic explanation or may serve as a biomarker of cell damage remains to be elucidated.
GRANTS
E. M. Van Craenenbroeck is supported by the Research Foundation-Flanders (FWO) as a senior clinical investigator.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.H.V.C., K.J.L., V.Y.H., V.A., G.A.V., C.J.V., and E.M.V.C. conception and design of research; A.H.V.C., K.V.A., and A.J. performed experiments; A.H.V.C., V.Y.H., E.F., and E.M.V.C. analyzed data; A.H.V.C., K.J.L., V.Y.H., B.Y.D.W., G.A.V., C.J.V., M.M.C., and E.M.V.C. interpreted results of experiments; A.H.V.C. prepared figures; A.H.V.C. and E.M.V.C. drafted manuscript; A.H.V.C., K.J.L., V.Y.H., E.F., V.A., B.Y.D.W., M.M.C., and E.M.V.C. edited and revised manuscript; A.H.V.C., K.J.L., K.V.A., A.J., V.Y.H., E.F., V.A., B.Y.D.W., G.A.V., C.J.V., M.M.C., and E.M.V.C. approved final version of manuscript.
REFERENCES
- 1.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 108: 5003–5008, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggish AL, Hale A, Weiner RB, Lewis GD, Systrom D, Wang F, Wang TJ, Chan SY. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol 589: 3983–3994, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 324: 1710–1713, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Bonauer A, Dimmeler S. The microRNA-17-92 cluster: still a miRacle? Cell Cycle 8: 3866–3873, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Bye A, Rosjo H, Aspenes ST, Condorelli G, Omland T, Wisloff U. Circulating microRNAs and aerobic fitness–the HUNT-Study. PLoS One 8: e57496, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen NX, Kiattisunthorn K, O'Neill KD, Chen X, Moorthi RN, Gattone VH 2nd, Allen MR, Moe SM. Decreased microRNA is involved in the vascular remodeling abnormalities in chronic kidney disease (CKD). PLoS One 8: e64558, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Da Silva ND Jr, Fernandes T, Soci UP, Monteiro AW, Phillips MI, Oliveira EM. Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med Sci Sports Exerc 44: 1453–1462, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res 107: 677–684, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Fichtlscherer S, Zeiher AM, Dimmeler S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol 31: 2383–2390, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis 129: S49–S55, 1984. [DOI] [PubMed] [Google Scholar]
- 13.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14: 249–256, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Ichii O, Otsuka S, Sasaki N, Namiki Y, Hashimoto Y, Kon Y. Altered expression of microRNA miR-146a correlates with the development of chronic renal inflammation. Kidney Int 81: 280–292, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Johansen KL, Painter P. Exercise in individuals with CKD. Am J Kidney Dis 59: 126–134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SW, Ramasamy K, Bouamar H, Lin AP, Jiang D, Aguiar RC. MicroRNAs miR-125a and miR-125b constitutively activate the NF-kappaB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proc Natl Acad Sci U S A 109: 7865–7870, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Wan Y, Guo Q, Zou L, Zhang J, Fang Y, Zhang J, Zhang J, Fu X, Liu H, Lu L, Wu Y. Altered microRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res Ther 12: R81, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenzen JM, Kaucsar T, Schauerte C, Schmitt R, Rong S, Hubner A, Scherf K, Fiedler J, Martino F, Kumarswamy R, Kolling M, Sorensen I, Hinz H, Heineke J, van Rooij E, Haller H, Thum T. MicroRNA-24 antagonism prevents renal ischemia reperfusion injury. J Am Soc Nephrol 25: 2717–2729, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundby C, Gassmann M, Pilegaard H. Regular endurance training reduces the exercise induced HIF-1alpha and HIF-2alpha mRNA expression in human skeletal muscle in normoxic conditions. Eur J Appl Physiol 96: 363–369, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Martinelli NC, Cohen CR, Santos KG, Castro MA, Biolo A, Frick L, Silvello D, Lopes A, Schneider S, Andrades ME, Clausell N, Matte U, Rohde LE. An analysis of the global expression of microRNAs in an experimental model of physiological left ventricular hypertrophy. PLoS One 9: e93271, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakasa T, Miyaki S, Okubo A, Hashimoto M, Nishida K, Ochi M, Asahara H. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum 58: 1284–1292, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39, Suppl 1: S1–S266, 2002. [PubMed] [Google Scholar]
- 23.Neal CS, Michael MZ, Pimlott LK, Yong TY, Li JY, Gleadle JM. Circulating microRNA expression is reduced in chronic kidney disease. Nephrol Dial Transplant 26: 3794–3802, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Paloian NJ, Giachelli CM. A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol 307: F891–F900, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther 10: R101, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A 107: 6328–6333, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raitoharju E, Lyytikainen LP, Levula M, Oksala N, Mennander A, Tarkka M, Klopp N, Illig T, Kahonen M, Karhunen PJ, Laaksonen R, Lehtimaki T. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 219: 211–217, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro F, Ribeiro IP, Alves AJ, do Ceu Monteiro M, Oliveira NL, Oliveira J, Amado F, Remiao F, Duarte JA. Effects of exercise training on endothelial progenitor cells in cardiovascular disease: a systematic review. Am J Phys Med Rehabil 92: 1020–1030, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Sawada S, Kon M, Wada S, Ushida T, Suzuki K, Akimoto T. Profiling of circulating microRNAs after a bout of acute resistance exercise in humans. PLoS One 8: e70823, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheedy FJ, O'Neill LA. Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann Rheum Dis 67, Suppl 3: iii50–iii55, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 469: 336–342, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res 100: 1164–1173, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Suarez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res 104: 442–454, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103: 12481–12486, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol 84: 1271–1278, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Tonevitsky AG, Maltseva DV, Abbasi A, Samatov TR, Sakharov DA, Shkurnikov MU, Lebedev AE, Galatenko VV, Grigoriev AI, Northoff H. Dynamically regulated miRNA-mRNA networks revealed by exercise. BMC Physiol 13: 9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trionfini P, Benigni A, Remuzzi G. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 11: 23–33, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Uhlemann M, Mobius-Winkler S, Fikenzer S, Adam J, Redlich M, Mohlenkamp S, Hilberg T, Schuler GC, Adams V. Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. Eur J Prev Cardiol 21: 484–491, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Van Craenenbroeck AH, Van Ackeren K, Hoymans VY, Roeykens J, Verpooten GA, Vrints CJ, Couttenye MM, Van Craenenbroeck EM. Acute exercise-induced response of monocyte subtypes in chronic heart and renal failure. Mediators Inflamm 2014: 216534, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Craenenbroeck AH, Van Craenenbroeck EM, Van Ackeren K, Vrints CJ, Conraads VM, Verpooten GA, Kouidi E, Couttenye MM. Effect of moderate aerobic exercise training on endothelial function and arterial stiffness in CKD stages 3–4: A randomized controlled trial. Am J Kidney Dis 66: 285–296, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Van Craenenbroeck EM, Beckers PJ, Possemiers NM, Wuyts K, Frederix G, Hoymans VY, Wuyts F, Paelinck BP, Vrints CJ, Conraads VM. Exercise acutely reverses dysfunction of circulating angiogenic cells in chronic heart failure. Eur Heart J 31: 1924–1934, 2010. [DOI] [PubMed] [Google Scholar]
- 43.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res 110: 496–507, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Flach H, Onizawa M, Wei L, McManus MT, Weiss A. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol 15: 393–401, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A 106: 4402–4407, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res 38: 7248–7259, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Li C, Li W, Kong L, Qian A, Hu N, Meng Q, Li X. MiR-150 enhances the motility of EPCs in vitro and promotes EPCs homing and thrombus resolving in vivo. Thromb Res 133: 590–598, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Wu XD, Zeng K, Liu WL, Gao YG, Gong CS, Zhang CX, Chen YQ. Effect of aerobic exercise on miRNA-TLR4 signaling in atherosclerosis. Int J Sports Med 35: 344–350, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Koppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal 2: ra81, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 39: 133–144, 2010. [DOI] [PubMed] [Google Scholar]