This study is the first to simultaneously and directly investigate myocardial performance and metabolic exercise response by using state-of-the-art echocardiographic techniques (2-D speckle tracking) in healthy adolescents. It uncovers several relationships between cardiac and overall exercise performance that can enhance the role of exercise testing in cardiac disease.
Keywords: exercise echocardiography, exercise testing, myocardial exercise performance, myocardial reserve, ventricular function
Abstract
Background left ventricular (LV) and right ventricular (RV) myocardial reserve during exercise in adolescents has not been directly characterized. The aim of this study was to quantify myocardial performance response to exercise by using two-dimensional (2-D) speckle tracking echocardiography and describe the relationship between myocardial reserve, respiratory, and metabolic exercise parameters. A total of 23 healthy boys and girls (mean age 13.2 ± 2.7 yr; stature 159.1 ± 16.4 cm; body mass 49.5 ± 16.6 kg; BSA 1.47 ± 0.33 m2) completed an incremental cardiopulmonary exercise test (25 W·3 min increments) with simultaneous acquisition of 2-D transthoracic echocardiography at rest, each exercise stage up to 100 W, and in recovery at 2 min and 10 min. Two-dimensional LV (LV Sl) and RV (RV Sl) longitudinal strain and LV circumferential strain (LV Sc) were analyzed to define the relationship between myocardial performance reserve and metabolic exercise parameters. Participants achieved a peak oxygen uptake (V̇o2peak) of 40.6 ± 8.9 ml·kg−1·min−1 and a work rate of 154 ± 42 W. LV Sl and LV Sc and RV Sl increased significantly across work rates (P < 0.05). LV Sl during exercise was significantly correlated to resting strain, V̇o2peak, oxygen pulse, and work rate (0.530 ≤ r ≤ 0.784, P < 0.05). This study identifies a positive and moderate relationship between LV and RV myocardial performance and metabolic parameters during exercise by using a novel methodology. Relationships detected present novel data directly describing myocardial adaptation at different stages of exercise and recovery that in the future can help directly assess cardiac reserve in patients with cardiac pathology.
NEW & NOTEWORTHY
This study is the first to simultaneously and directly investigate myocardial performance and metabolic exercise response by using state-of-the-art echocardiographic techniques (2-D speckle tracking) in healthy adolescents. It uncovers several relationships between cardiac and overall exercise performance that can enhance the role of exercise testing in cardiac disease.
cardiovascular exercise response is a complex interplay between respiratory, metabolic, cardiac, and muscular adaptations and is traditionally assessed in children by using cardiopulmonary exercise testing (CPET) (1, 34). CPET has been used in diagnosis and risk stratification in children with heart disease (38) as it can impose physiological stress on the cardiovascular system to determine submaximal and maximal capacity, while numerous studies in adults with cardiovascular disease have shown CPET is a strong predictor of clinical outcome (2). Two methodological limitations of CPET are, however, the inability to provide data on myocardial function in response to the imposed exercise stress, the dominant process being to increase cardiac output to enhance oxygen delivery (3), and its limited and indirect inferences of cardiac reserve.
Over the last decade these methodological constraints have led to several pilot studies directly investigating cardiac function during exercise in the healthy pediatric population by using echocardiography (5, 37, 42, 43). A limitation, however, of these studies is that the investigated echocardiographic parameters, for example, fractional shortening or ejection fraction, do not measure myocardial performance and show a significant cardiac load dependency, which is augmented by the significant before and after load changes during exercise. Therefore, most echocardiographic parameters are unsuitable to describe myocardial performance and cardiac reserve during exercise.
The gold standard measure of load-independent contractility is end-systolic elastance (19). This parameter has been used to invasively investigate contractility responses to exercise in the animal model (32) and in adults by altering the force-frequency relationship by using either inotropy, exercise, or pacing in several classical studies (9, 16, 17). These studies have also highlighted that investigating the force-frequency relationship under stress as a measure of contractility can differentiate between cardiac adaptive and maladaptive processes (16, 17).
The use of invasive techniques such as inotropy or pacing in the pediatric population is, however, not ethically viable because of its invasive nature and only used occasionally in the clinical setting. To overcome these issues, noninvasive assessment of isovolumic acceleration time (IVA) as a load-independent measurement of contractility (45) has recently been successfully used to investigate contractility exercise response in pediatric patients (40, 41). IVA is, however, imaging plane dependent, is unsuitable to assess regional myocardial function (27), and has been primarily used for research purposes rather than in a clinical setting. Myocardial deformation imaging has emerged as a potentially suitable imaging modality to describe myocardial performance during exercise stress echocardiography, as it is less load dependent (50) than classic parameters of cardiac function. In particular, two-dimensional (2-D) speckle tracking also shows angle independency, paramount when acquiring echocardiographic images during exercise with significant translational heart movement. Currently, myocardial strain measurement by 2-D speckle tracking has only been used in children in one previous pilot study to assess myocardial function during exercise (4).
Two-dimensional strain measurement has been validated in clinical pediatric echocardiography for the assessment of left ventricular (LV) and right ventricular (RV) function (12), at rest in normative healthy pediatric volunteers (28), and has been validated against magnetic resonance imaging (20). The correlation of 2-D strain to invasive contractility measurements has been shown in the animal model (21). Additionally, 2-D strain was shown to be more sensitive to mild functional impairment than traditional echocardiographic functional parameters, can also assess regional myocardial function, and image acquisitions of 2-D strain data are time efficient and easy to obtain (7). Therefore, 2-D strain measurement could prove to be a very suitable tool to investigate myocardial performance response to exercise stress.
Furthermore, used simultaneously, 2-D strain echocardiography and CPET can provide a comprehensive and direct description of cardiopulmonary exercise responses. Therefore, the aim of this study is to describe the comprehensive assessment of cardiopulmonary response to incremental exercise by using 2-D strain to assess myocardial performance in conjunction with simultaneous assessment of metabolic gas analysis by CPET. This study presents normative healthy volunteer data in adolescents and the relationship between myocardial exercise performance and metabolic exercise parameters.
MATERIALS AND METHODS
Participants
Twenty-three healthy children, 17 boys and 6 girls (mean age 13.2 ± 2.7 yr), volunteered to participate in this study. All participants underwent a full structural and functional resting echocardiogram to exclude any underlying cardiac disease following pediatric guidelines (25, 26). Participants then underwent a maximal CPET with simultaneous echocardiographic assessment on a semirecumbent bicycle. The study was conducted at the Children's Health and Research Centre, University of Exeter. Ethical approval for the study was obtained from the UK National Research Ethics Service (NRES). All participants signed a NRES-approved assent form and parents or guardians signed a NRES-approved consent form prior to participation.
Exercise Testing
An incremental CPET on a recumbent cycle ergometer (45° inclination) (Lode and GE Healthcare eBike EL Stress Echo Couch) to volitional exhaustion (25 W∙3 min increments) was performed by all participants. The pedaling frequency was kept constant at 60 ± 5 rpm, and the test was stopped when subjects reached volitional physical exhaustion or when they were unable to maintain the required pedaling frequency (11). Subjective exercise intensity was assessed by using a standard modified Borg scale at the end of each stage and end exercise.
Ventilation volume and expired gas composition were measured breath-by-breath with a metabolic cart (Metalyzer II, Cortex, Leipzig, Germany). The gas analyzer was calibrated for barometric pressure, flow volume (3-liter syringe, Hans Rudolph Kansas City, MO), and two-point gas composition (atmospheric air and a standard gas cylinder containing 15% O2 and 5% CO2) before testing. The subjects were fitted with size-appropriate face masks and breathed through low dead space (73–114 ml) and low-resistance oronasal mask and breathing valve assemblies (V-mask, 7400 series, Hans Rudolph). Heart rate (HR) at submaximal and maximal exercise stages was recorded from the electrocardiographic (ECG) leads attached to the echocardiographic machine. The gas exchange parameters captured by the metabolic parameters were analyzed and displayed by online software in the computer (Metasoft, Cortex). After the completion of the exercise test, the breath-by-breath gas exchange data were averaged over consecutive 10-s periods and exported as Excel spreadsheets (Microsoft Office) for further analyses.
The gas exchange parameters were then averaged over the last 30 s of each stage. The peak work rate (Wpeak) for the unfinished stage of the step protocol was computed by the following formula (22): Wpeak = POf + (t/T × D), where POf is power output of the last stage, t is time in seconds of the last uncompleted stage, T is duration in seconds of each completed stage, and D is power output difference in watts between consecutive work rates.
Echocardiography
Echocardiographic studies were performed with a 4–6 Mhz transducer Vivid Q GE ultrasound system (GE Healthcare, Little Chalfont, UK). A standardized supine resting echocardiogram was performed prior to exercise testing following published guidelines (25, 26). Images were obtained in 2-D, M-Mode, spectral, and tissue Doppler; 2-D images were optimized for off-line 2-D speckle tracking assessment. Chamber diameters were measured in parasternal short-axis view on M-Mode images and fractional shortening calculated. Spectral pulse wave Doppler interrogation of the transmitral inflow and aortic outflow was performed from the four-chamber view; mitral peak E and A waves were measured and E/A ratio calculated. Pulse wave and continuous tissue Doppler imaging were recorded with peak velocities of ventricular wall excursion (S′, E′, and A′ waves) at the base of the LV and RV just below the level of the mitral and tricuspid valves, respectively. All resting echocardiographic parameters were measured on the same day prior to exercise testing.
Strain Analysis
All strain analysis was performed using manufacturer-specific software platform (ECHOPAC version 112, GE Vingmed Ultrasound AS, Horten, Norway). Two-dimensional images were obtained at rates of 40–90 frames per second (fps) in apical four-chamber view, or modified apical four-chamber view for RV assessment, parasternal short axis at the level of the base of the LV. Three cardiac cycles were acquired in raw DICOM format, and analysis was performed on one manually selected cardiac cycle. The endocardial borders were manually contoured at end systole with the range of interest adjusted to include the whole myocardium. Peak systolic global longitudinal (Sl) and circumferential (Sc) strain was defined as the maximal deformation of a segment in systole and is represented as a percentage (%) of the original size. Peak systolic global strain rate (SRc) was defined as the rate of maximal deformation of a segment in systole over time and is expressed in 1/s (46). Standard nomenclature was used to describe LV and RV 2-D strain (46). RV longitudinal peak systolic strain was measured in three lateral segments. Circumferential peak systolic strain was measured at the base of the LV. Mean or global values for circumferential and longitudinal strain were calculated for each level only if good tracking was obtained in a minimum of four segments. Image acquisition and off-line analysis was performed by two physicians experienced in pediatric echocardiography and strain analysis.
Exercise Echocardiography
Focused echocardiography was performed for 2-D speckle tracking strain analysis during free breathing exercise 60 s into each exercise stage starting at baseline (unloaded pedaling), at 25, 50, 75, 100 W, and during recovery at 2 min and 10 min after end exercise. Myocardial performance response to exercise was assessed by 2-D speckle tracking echocardiography for the LV up to a work rate of 100 W and for the RV up to a work rate of 50 W and at 2- and 10-min recovery in both ventricles. Images were acquired over three continuous cardiac cycles in LV and modified RV apical four-chamber view for longitudinal strain analysis and for the LV in parasternal short-axis view at the base of the LV for circumferential strain by using frame rates of 40–90 fps. A minimum of six cardiac cycles were recorded to capture at least one cardiac cycle in expiration. Strain analysis was performed as described above. Images were rejected if software indicated poor tracking quality or by analyst's judgement of insufficient image quality. Strain analysis was not performed at stages where image quality was insufficient. LV circumferential peak systolic strain was measured with the same echo settings. Intraobserver and interobserver evaluation of exercise strain measurements was performed on two independent occasions 3 mo apart with the same selected images in a subset of subjects.
Statistics
Descriptive statistics (means ± SD) of measured and derived variables were used to characterize the sample. Prior to analyses, diagnostic plots were created to provide checks for heteroscedasticity, normality, and influential observations. Outliers were identified as being >1.5 times the interquartile range by using box and whisker plots.
Relationships between strain parameters and CPET variables were determined by scatterplots and linear regression analysis. Investigation of the force-frequency relationship during exercise was assessed by locally weighted scatterplot smoothers (LOESS) and t-test analysis. Paired t-tests and one-way analysis of variance ANOVA were used to test for differences in strain and strain rate measures.
All statistical analyses were performed by R (R Core Team 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria) and by SPSS Statistics Software (version 20.0, IBM, Armonk, NY) and Graphpad Prism (version 5.04 for Windows, GraphPad Software, La Jolla, CA). Sample sizes did vary because of two main reasons: the image quality was deemed not sufficient to analyze the selected parameter or the participant did not reach the selected power output. A probability level of P < 0.05 was accepted to indicate statistical significance.
For reliability analysis, intraobserver and interobserver reliability were tested by using a two-way mixed effects model intraclass correlation coefficient during all analyzed exercise stages and at 2- and 10-min recovery. This was completed in a total of 24 strain measurement data sets for LV longitudinal and circumferential strain, respectively, and 18 strain measurement data sets for RV longitudinal strain.
RESULTS
The participant's mean anthropometric data were stature 159.1 ± 16.4 cm, body mass 49.5 ± 16.6 kg, BMI 19.0 ± 3.3 kg/m2, lean body mass 40.0 ± 11.5 kg, and BSA 1.47 ± 0.33 m2 (Table 1). The completed standard physical activity questionnaire (6) classified our participants as moderately active. Mean (SD) cardiopulmonary exercise data were peak power output 154 ± 42 W, peak oxygen uptake (V̇o2peak) 40.6 ± 8.9 ml·kg−1·min−1, gaseous exchange threshold (GET, as a percent of V̇o2peak) 52.2 ± 5.1% and end exercise duration 18:45 ± 4:56 (min:s) (Table 2).
Table 1.
Anthropometrics and resting echocardiographic parameters of participants
| N = 23 | Mean ± SD |
|---|---|
| Anthropometrics | |
| Age, yr | 13.2 ± 2.7 |
| Standing stature, cm | 159.1 ± 16.4 |
| Sitting stature, cm | 82.2 ± 8.9 |
| Body mass, kg | 49.5 ± 16.6 |
| Body mass index, kg/m2 | 19.0 ± 3.3 |
| Body surface area, m2 | 1.47 ± 0.33 |
| Lean body mass, kg | 40.0 ± 11.5 |
| Gas exchange threshold, % V̇o2peak | 52.2 ± 5.1 |
| 2-D echocardiogram and systolic parameters | |
| IVSd, mm | 7.5 ± 1.3 |
| LVIDd, mm | 44.1 ± 3.8 |
| LVPWd, mm | 7.3 ± 1.0 |
| IVSs, mm | 9.5 ± 4.1 |
| LVIDs, mm | 28.7 ± 5.8 |
| LVPWs, mm | 9.5 ± 1.4 |
| FS, % | 32.4 ± 3.6 |
| MAPSE, mm | 14.1 ± 2.5 |
| TAPSE, mm | 20.8 ± 2.5 |
| Spectral and tissue Doppler parameters | |
| MV E, cm/s | 99.5 ± 16.0 |
| MV A, cm/s | 52.4 ± 13.7 |
| E/A ratio | 1.9 ± 0.3 |
| LV lat S′, cm/s | 10.2 ± 2.1 |
| LV lat E′, cm/s | 17.2 ± 3.1 |
| LV lat A′, cm/s | 5.3 ± 1.4 |
| RV S′, cm/s | 13.1 ± 2.4 |
| RV E′, cm/s | 16.4 ± 3.7 |
| RV A′, cm/s | 9.2 ± 3.1 |
IVSd, interventricular septum in systole; LVIDd, left ventricle internal dimension in diastole; LVPWd, left ventricular posterior wall in diastole; IVSs, interventricular septum in systole; LVIDs, left ventricle internal dimension in systole; FS, fractional shortening; MAPSE, mitral annular plane systolic excursion; TAPSE, tricuspid annular systolic excursion; MV E, transmitral inflow in early diastole; MV A, transmitral inflow with atrial contraction; LV lat S′, left ventricular lateral wall systolic velocity; LV lat E′, left ventricular early diastolic velocity; LV lat A′, left ventricular late diastolic velocity; RV S′, right ventricular free wall systolic velocity; RV E′, right ventricular early diastolic velocity; RV A′, right ventricular late diastolic velocity.
Table 2.
Participants resting, exercise, and recovery cardiopulmonary exercise testing parameters
| Stage of Test | Absolute V̇o2, liters/min−1 | Relative V̇o2, ml·kg−1·min−1 | Heart Rate, b/min−1 | Oxygen Pulse, ml/beat−1 |
|---|---|---|---|---|
| Rest | 0.04 ± 0.01 | 1.00 ± 0.00 | 70 ± 12 | 0.4 ± 16.6 |
| 0 W | 0.52 ± 0.03 | 11.8 ± 0.84 | 92 ± 14 | 5.7 ± 1.7 |
| 25 W | 0.68 ± 0.04 | 15.6 ± 1.2 | 108 ± 15 | 5.7 ± 1.3 |
| 50 W | 0.91 ± 0.02 | 21.4 ± 1.7 | 117 ± 30 | 7.5 ± 1.3 |
| 75 W | 1.20 ± 0.02 | 26.6 ± 2.1 | 134 ± 34 | 8.5 ± 1.2 |
| 100 W | 1.40 ± 0.03 | 30.7 ± 3.0 | 159 ± 18 | 9.0 ± 1.5 |
| Maximum end exercise | 1.93 ± 0.71 | 40.6 ± 8.9 | 177 ± 15 | 11.2 ± 3.4 |
| 2 Min recovery | 0.54 ± 0.05 | 11.7 ± 0.69 | 107 ± 20 | 6.4 ± 3.0 |
| 10 Min recovery | 0.35 ± 0.02 | 7.8 ± 0.65 | 98 ± 18 | 3.7 ± 0.75 |
Values are means ± SD, n = 18.
Standard resting echocardiographic measurements for all participants are presented in Table 1 with resting echocardiography parameters confirming normal cardiac anatomy with normal systolic and diastolic function. Morphological and functional measurements are in line with previously published values (8, 18, 35).
Myocardial Performance During Exercise
LV Sl and LV Sc increased significantly during exercise between rest and a work rate of 100 W (P < 0.001). LV Sl was significantly increased between rest and each exercise stage (P < 0.05) after the first stage. LV Sc was significantly increased between rest and each exercise stage (P < 0.05) after the second stage. LV SRc increased between rest and exercise from −1.3 ± 0.3 (rest) to −1.8 ± 0.3 (0 W), −2.3 ± 0.9 (25 W), −2.3 ± 0.6 (50 W), −3.1 ± 0.9 (75 W), and −3.2 ± 1.0 (100 W), with statistically significant differences found between rest and work rates from 50 to 100 W (P < 0.05). RV Sl increased significantly between rest and a work rate of 50 W (P < 0.05). Exercise stage-specific strain values are listed in Table 3. For LV strain measurements, no participants had to be excluded from the analysis; for RV strain measurements, two were excluded because of insufficient image quality at the majority of exercise stages. Representative images series for LV Sl, LV Sc, and RV Sl analysis at rest, exercise, and recovery are shown in Figs. 1, 2, and 3.
Table 3.
LV peak systolic longitudinal, LV peak systolic circumferential, and RV peak systolic longitudinal strain development during exercise
| Stage | Mean Peak Strain | SD | Lower CI | Upper CI | Mean Strain Reserve | P Value | Participants (n) |
|---|---|---|---|---|---|---|---|
| LV peak systolic longitudinal strain | |||||||
| Rest | −17.6 | 2.9 | −18.9 | −16.4 | — | — | 23 |
| 0 W | −19.7 | 3.2 | −21.1 | −18.3 | −1.2 ± 5.0 | 0.016* | 22 |
| 25 W | −21.4 | 3.6 | −21.4 | −17.6 | −2.2 ± 4.3 | 0.062* | 16 |
| 50 W | −21.9 | 3.8 | −21.9 | −18.5 | −2.8 ± 3.8 | 0.002* | 22 |
| 75 W | −23.6 | 4.9 | −23.6 | −18.9 | −3.8 ± 4.2 | 0.001* | 19 |
| 100 W | −23.0 | 2.7 | −23.0 | −20.5 | −4.4 ± 8.5 | 0.000* | 19 |
| 2 Min recovery | −16.9 | 3.7 | −18.5 | −15.3 | 0.7 ± 4.0 | 0.422 | 23 |
| 10 Min recovery | −16.4 | 4.5 | −18.3 | −14.4 | 1.2 ± 3.8 | 0.127 | 23 |
| LV peak systolic circumferential strain | |||||||
| Rest | −25.3 | 3.8 | −27.5 | −23.0 | — | — | 23 |
| 0 W | −29.2 | 6.6 | −32.2 | −26.3 | −2.9 ± 6.5 | 0.342 | 21 |
| 25 W | −30.2 | 5.1 | −33.1 | −27.2 | −3.5 ± 5.5 | 0.150 | 14 |
| 50 W | −31.6 | 5.8 | −34.2 | −29.0 | −5.9 ± 5.8 | 0.011* | 21 |
| 75 W | −32.7 | 7.4 | −36.6 | −28.8 | −6.9 ± 7.8 | 0.009* | 16 |
| 100 W | −35.5 | 6.3 | −39.3 | −31.7 | −10.2 ± 6.0 | 0.000* | 13 |
| 2 Min recovery | −24.7 | 5.6 | −27.3 | −22.2 | 1.2 ± 5.2 | 0.279 | 21 |
| 10 Min recovery | −24.3 | 4.3 | −26.2 | −22.0 | 1.7 ± 4.0 | 0.234 | 21 |
| RV peak systolic longitudinal strain | |||||||
| Rest | −25.1 | 6.0 | −27.7 | −22.4 | — | — | 23 |
| 0 W | −26.1 | 4.8 | −28.4 | −23.7 | −0.9 ± 5.0 | 0.442 | 19 |
| 25 W | −28.6 | 4.4 | −31.2 | −26.1 | −2.9 ± 4.5 | 0.030* | 14 |
| 50 W | −27.9 | 6.0 | −30.9 | −25.0 | −2.6 ± 5.2 | 0.047* | 18 |
| 2 Min recovery | −24.6 | 8.2 | −28.5 | −20.8 | 1.0 ± 7.4 | 0.534 | 20 |
| 10 Min recovery | −23.8 | 5.6 | −26.6 | −21.0 | 1.6 ± 5.9 | 0.287 | 18 |
P value represents difference from resting strain values. LV longitudinal strain, LV circumferential strain, and RV longitudinal strain in %. Lower n at 25 W is due to participants >15 yr of age omitting this stage as per protocol. Images of insufficient quality were excluded from analysis resulting in n < 23.
Significant at the 0.05 level.
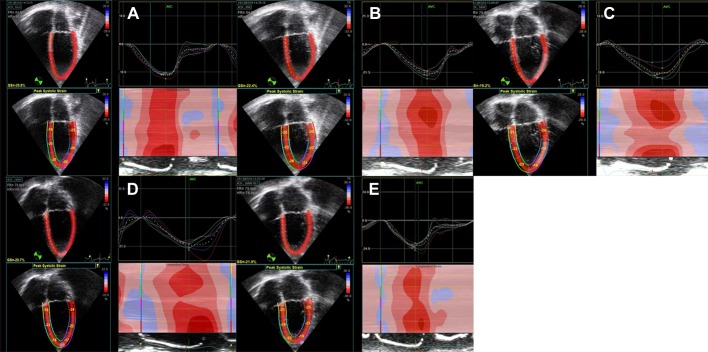
Fig. 1.
Representative 2-D image sequence during exercise for left ventricular (LV) longitudinal strain (Sl). A: rest at heart rate (HR) = 86 bpm; B: 0 W at HR = 100 bpm; C: 50 W at HR = 108 bpm; D: 100 W at HR = 148 bpm; E: 2-min recovery at HR = 74 bpm. GS = global LV Sl.
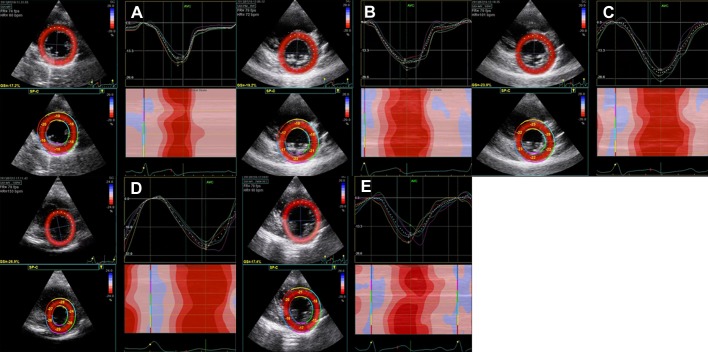
Fig. 2.
Representative 2-D image sequence during exercise for LV circumferential strain (Sc). A: rest at HR = 60 bpm; B: 0 W at HR = 72 bpm; C: 50 W at HR = 101 bpm; D: 100 W at HR = 153 bpm; E: 2-min recovery at HR = 90 bpm. GS = global LV Sc.
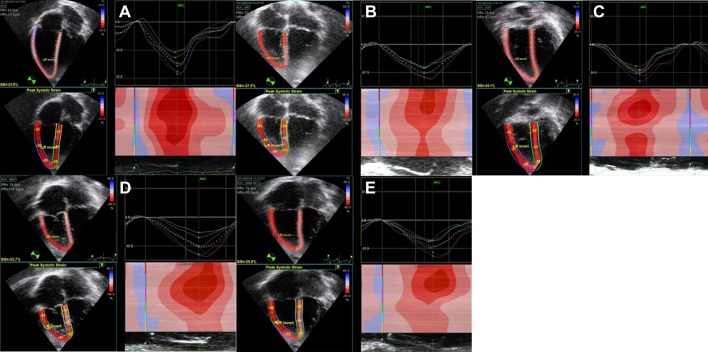
Fig. 3.
Representative 2-D image sequence during exercise for RV Sl. A: rest at HR = 75 bpm; B: 0 W at HR = 80 bpm; C: 25 W at HR = 83 bpm; D: 50 W at HR = 110 bpm; E: 2-min recovery at HR = 93 bpm. GS = global RV Sl.
Myocardial Performance During Recovery
LV Sl returned to resting values at 2- and 10-min recovery without a significant difference for LV Sl (P = 0.304 and 0.082, respectively) and LV Sc between resting and recovery values (P = 0.422 and 0.127, respectively). RV Sl returned to resting values at 2- and 10-min recovery without a significant difference for RV Sl (P = 0.534 and 0.287, respectively) (Table 3).
Relationship Between Resting and Exercise Myocardial Performance
A significant and positive moderate relationship was found between LV Sl at rest and 100 W (r = 0.530, P = 0.019). The relationship between LV Sc at rest and 100 W was not found to be significantly correlated (r = 0.368, P = 0.216). Analysis of the relationship of LV SRc between rest and exercise revealed a positive relationship between LV SRc at rest and 75 W (P = 0.004) with a moderate positive correlation (r = 0.656), but not at 100 W (r = 0.294, P = 0.287).
Relationship Between Resting and Recovery Myocardial Performance
Moderate, positive, and significant relationships between resting and 10-min posttest strain values were observed for LV Sl by linear modeling (r = 0.566, P = 0.005) and for LV Sc (r = 0.503, P = 0.028), demonstrating resting strain is proportional to strain at 10-min recovery in individual subjects. A moderate positive and significant relationship between resting and both 2-and 10-min recovery RV Sl values was observed on linear modeling (P = 0.027 and 0.043, respectively) alongside moderate positive correlations (r = 0.494 and 0.481, respectively), showing RV Sl at rest correlates to RV Sl at both 2- and 10-min recovery in participants.
Force-Frequency Relationship
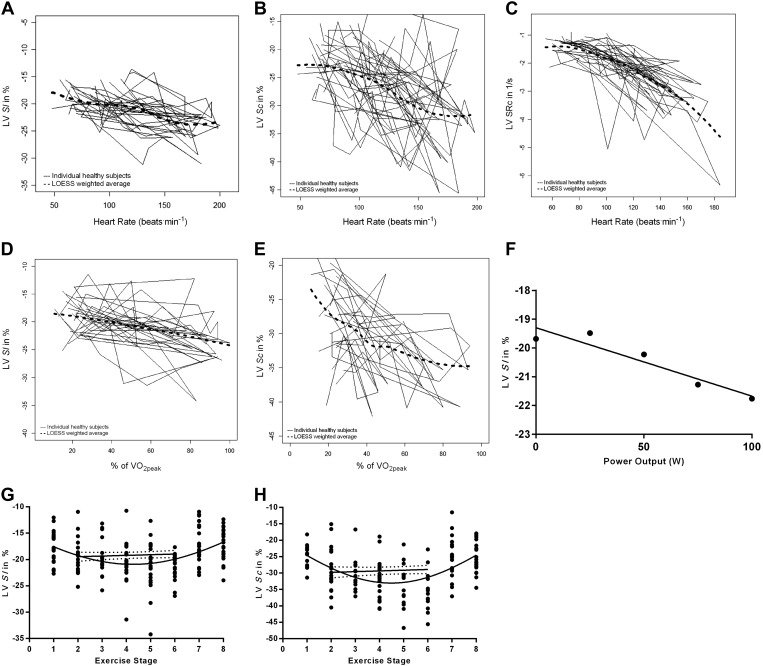
A significant and linear correlation for LV Sl and HR (r = 0.525, P = 0.021) was found. The relationship between LV Sc and HR (r = 0.261, P = 0.390) was not significant at 100 W. The force-frequency relationship was also assessed by using strain rate as a more load-independent measure of contractility. A weak linear correlation for LV SRc and HR was identified and nearing significance (r = 0.489, P = 0.064, n = 15) (Fig. 4). A significant relationship between LV SRc and HR was present at 50 W (r = 0.510, P = 0.018).
Fig. 4.
A–C: variability of LV Sl, LV Sc, and LV SRc with heart rate during exercise. The individual curves for each study participant are shown together with a locally weighted scatterplot smoothing (LOESS) weighted average. D–E: variability of LV Sl and LV Sc with percentage of V̇o2peak during exercise. The individual curves for each study participant are shown together with LOESS-weighted average. F: linear regression analysis of the relationship between LV longitudinal global systolic strain and power output; Gaussian distribution and linear regression analysis of the relationship between LV Sl (G) and LV Sc (H) and exercise stage. Exercise stages: 1–8 as rest, 0, 25, 50, 75, 100 W, 2-min recovery, and 10-min recovery, respectively.
Relationship Between Myocardial Performance and Metabolic Exercise Response
Myocardial performance and V̇o2peak.
We assessed the relationship between myocardial performance and metabolic exercise response by using LOESS plots and t-tests and found a very significant positive correlation between percentage of V̇o2peak and LV Sl (r = 0.729, P = 0.005, n = 13). For LV Sc the relationship was not significant (r = 0.687, P = 0.088, n = 16) (Fig. 4).
More specifically, we assessed the relationship between LV 2-D strain and different stages of exercise metabolism, and we calculated LV strain reserve at 70 ± 13% of V̇o2peak for a subset of 10 subjects. No significant correlations between LV strain reserve at 70 ± 13% V̇o2peak and percent of gas exchange threshold (%GET) for both LV Sl reserve (r = 0.152, P = 0.604, n = 14) and LV Sc reserve (r = 0.036, P = 0.921, n = 10) were found. LV SRc at 70 ± 13% V̇o2peak and %GET were not significantly correlated (r = 0.118, P = 0.781). The LV longitudinal maximal strain reserve and V̇o2peak relative to body mass was found to be significantly and positively correlated (r = 0.562, P = 0.046, n = 13).
Myocardial performance and oxygen pulse.
Oxygen pulse at 100 W showed a strong positive significant relationship to LV Sl (r = 0.650, P = 0.016), LV peak Sc (r = 0.784, P = 0.037), and at 75 W with LV SRc (r = 0.626, P = 0.03) (Fig. 4).
Myocardial performance and work rate.
LV Sl correlated also to work rate and showed a direct relationship to power output throughout the different exercise stages between 25 and 100 W (r = 0.234, P = 0.018) (Fig. 4). Linear regression analysis of LV Sl and LV Sc to work rate between 0 and 100 W demonstrated a significant relationship (P = 0.016 and 0.004, respectively) (Fig. 4). Univariate analysis demonstrated that HR had no significant effect (P = 0.636) on the relationship of LV Sl and work rate.
The intra- and interobserver average variance for resting, exercise, and recovery strain values ranged from 0.6 to 8% with and intraclass correlation coefficient (ICC) between r = 0.78 to 0.98 for LV Sl; from 0.9 to 9.1% with ICC between r = 0.87 and 0.98 for LV Sc and from 0.8 to 6.6% with ICC between r = 0.75 and 0.93 for RV Sl.
DISCUSSION
In this study we have defined LV and RV myocardial response to exercise and its relationship to resting and recovery myocardial performance. To the best of our knowledge, we report for the first time data on simultaneous assessment of myocardial performance by 2-D strain and metabolic exercise parameters in healthy adolescents. We have demonstrated feasibility of using 2-D strain analysis to describe myocardial performance reserve and force-frequency relationship during exercise and recovery and have uncovered several relationships between myocardial exercise performance and metabolic exercise parameters. We have used exercise stress and not inotropic stimulation, as it is noninvasive and more importantly mimics physical activity in its effect on cardiac performance. This is advantageous over inotropy or pacing, as adult data have shown that the relationship between HR and contractility is different for pacing, inotrope, and exercise stress (16).
We have determined that LV and RV myocardial performance increased significantly and incrementally through different exercise stages well beyond the GET without reaching a plateau, and this has not been directly documented before. Specifically, we have found a significant relationship of 2-D strain values between rest and exercise for LV Sl at rest and 100 W, which was moderately and positively correlated (r = 0.534, P = 0.019). However, this relationship for LV Sc between rest and 100 W was not significantly correlated (r = 0.368, P = 0.216), which could be due to the smaller sample size analyzed for LV Sc at 100 W, but will need to be tested in larger cohorts. The smaller sample size for LV Sc is the result of exclusion secondary to increased translational movement artefacts at higher work rates in the transverse plane affecting LV Sc measurement in some participants. We also investigated LV peak systolic strain rate as a relatively load-independent parameter (10) in the circumferential plane as the dominant plane of force generation in the LV and found a significant increase during exercise and a moderate positive correlation between resting and exercise LV SRc. We have also described an accentuated force-frequency relationship during exercise. In this respect, our data is in concordance with the only previous study investigating the force-frequency relationship during exercise in children, but by using IVA (41). The exercise force-frequency relationship has, however, not been demonstrated by 2-D strain during exercise—a more clinically useful and practical tool than IVA to measure myocardial performance. Direct measurement of the force-frequency relationship during exercise stress could particularly be of importance to discover early ventricular dysfunction in pediatric patients with normal resting ejection fraction as recently shown (39, 41).
A positive correlation and dependency between absolute resting and exercise strain was found in our study; for example, LV Sl is directly proportional to LV Sl at 100 W exercise work rate in participants. This observation will need to be evaluated in a larger population and in cardiac pathology, but could make resting 2-D strain a predictor of exercise myocardial performance.
In our study we found a linear and significant relationship between maximal strain reserve of LV Sl and peak oxygen consumption, and this relationship can be inferred to be weakened in pathological conditions and become an important marker for early ventricular dysfunction. Strain reserve reflects the ability of the ventricles to increase contractile function (cardiac reserve) during exercise regardless of resting values. The classic concept of cardiac reserve has in the past been used to help define recoverability of LV in heart failure (33), and 2-D strain reserve might be suitable to further validate and extend this approach.
Interestingly, detailed analysis showed that 2-D strain during exercise increased minimally in the initial exercise stages, particularly for LV Sc, but that an increase was more pronounced after the initial stages. This observation is very likely a reflection of the previously observed early cardiac output increase by HR and filling rather than systolic contractility in healthy nonathlete adults (15).
We have additionally found a significant linear relationship between resting and recovery strain values for LV and RV Sl. Two-dimensional strain data during recovery showed normalization within 2 min of recovery time. Of note is a trend to subresting 2-D strain values during recovery, albeit not statistically significant in our sample. This could reflect a myocardial debt as a reflection of altered or even impaired myocardial recovery metabolism and will need to be explored further. Overall, our data indicate that myocardial performance assessment by 2-D strain is a sensitive and responsive tool for the quantification of cardiac reserve during exercise and in recovery.
This first time assessment of correlating myocardial performance response to exercise with simultaneously acquired metabolic exercise parameters revealed several important relationships. LV myocardial exercise performance is closely linked to parameters of metabolic capacity during exercise such as V̇o2peak and O2 pulse, but also work rate (Fig. 4). In particular, the linear relationship between both LV Sl and V̇o2peak has not been described previously and provides direct evidence that myocardial performance plays a distinctive part in defining overall exercise capacity. Our study also showed that O2 pulse correlates to myocardial performance of the LV as measured by 2-D strain during exercise and, to our knowledge, directly confirms for the first time that O2 pulse is therefore an adequate indirect assessment parameter for cardiac function response as used in clinical CPET (2).
This study also adds to the scarce data on RV function and describes myocardial reserve using 2-D strain for the RV for the first time in adolescent children. The RV has been implicated as a cause for exercise dysfunction in adults with congenital heart disease (14), as well as in the healthy adult population (24). Besides, RV pathology and LV-RV interaction play a dominant role in the pathophysiology of children with congenital heart disease, where assessment of RV function at rest is used in risk stratification in coronary heart disease (31, 49), and RV function assessment during exercise could further enhance this evaluation.
Methodology Limitations
Our imaging protocol was robust in acquiring echocardiographic data to work rates up to 100 W for the LV. Higher work rates are associated with significant movement artefacts due to whole upper body muscle utilization and increased respiratory efforts, and this limits high-fidelity image acquisition in particular of the RV. We therefore only included for analysis RV strain for work rates up to 50 W. Additionally, tachycardia at maximal exercise efforts does not allow for recording of sufficient frame rates per cardiac cycle for 2-D strain analysis (under sampling) and led to exclusion of some participant data at highest recorded work rates of 100 W for LV (Table 3). Intra- and interobserver variability analysis does, however, show an acceptable intraclass correlation. Furthermore, 2-D strain analysis was performed up to 70% of V̇o2 max, which was significantly beyond GET (Table 1) and correlated to a work rate of ∼100 W in our cohort (Table 2). This allowed for modeling of all metabolic relationships beyond GET. The ability to model these relationships at submaximal exercise levels makes our methodology suitable for patient cohorts with significantly reduced exercise capacity incapable of reaching maximal exercise levels.
We have included LV SRc into our analysis as it correlates with the load-independent gold standard measures of contractility change in pressure over time and end-systolic elastance (13). Two-dimensional strain assessment was chosen as at rest is more sensitive to picking up regional myocardial dysfunction in children than traditional parameters such as ejection fraction as, for example, shown in children with Duchenne muscular dystrophy (30). This in combination with exercise imaging, which also is more sensitive to mild dysfunction, could help make 2-D strain become a useful clinical tool. It needs to be noted that 2-D strain assesses only unidirectional myocardial deformation forces and cannot therefore capture the complex multidimensional and directional cardiac myofiber deformation (10). We have attempted to address the multidimensional LV myocardial deformation by analyzing the two most widely used deformation planes, longitudinal and circumferential strain analysis. Longitudinal and circumferential strain show lower intra- and interobserver variability in children compared with the third deformation plane, for example, radial strain (20). A disadvantage of 2-D strain analysis is the acquisition at low frame rates, which makes measurements at higher HR and work rates less reliable because of undersampling, in particular, for strain rate. In contrast, tissue Doppler-derived strain with acquisition of much higher frame rates has some advantages in children (48), but is inferior during exercise because of its angle dependency, in particular, at higher work rates with more significant translational chest movement.
Exercise was performed on a semisupine cycle ergometer, and this will lead to an altered before and after load response during exercise, changing the differential contribution of HR and stroke volume to enhance cardiac output (47). However, our group and others have shown significant stroke volume increase also during supine exercise by using cardiac magnetic resonance imaging (23, 36), and a comparative study in children between upright and supine CPET has additionally found good correlation of parameters (29).
To minimize the effects of body movement and heart rate, only exercise stages up to 100 W were assessed in this study, and we were therefore not able to describe myocardial reserve at maximal exercise intensity. And the much debated question as to whether myocardial deformation reaches a plateau or continues to rise linearly with HR (44) could be investigated in future studies by using our methodology.
Simultaneous assessment of biventricular myocardial performance and metabolic exercise and recovery response uncovered several novel relationships. Direct assessment of ventricular function parameters during exercise can be effectively utilized if cardiac and metabolic responses are simultaneously assessed. This protocol can overcome the limited predictive value of exercise capacity on cardiac function. Direct investigation of myocardial performance response to exercise has contributed to the still limited understanding on how myocardial performance facilitates stroke volume increase at different exercise stages. In the clinical setting, this protocol could serve as a tool to better quantify myocardial reserve, which is an important concept in risk stratification of ventricular dysfunction.
GRANTS
The study was supported by the University of Exeter and the Bristol NIHR Biomedical Research Unit for Cardiovascular Disease. It was funded by a David Telling and an Above & Beyond project grant. G. Pieles holds a National Institute for Health Research Academic Clinical Lectureship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.E.P., P.R., F.M., A.G.S., and C.A.W. conception and design of research; G.E.P., P.R., F.M., and C.A.W. performed experiments; G.E.P., L.G., J.F., P.R., and F.M. analyzed data; G.E.P. and C.A.W. interpreted results of experiments; G.E.P. and L.G. prepared figures; G.E.P. and C.A.W. drafted manuscript; G.E.P., L.G., J.F., A.G.S., and C.A.W. edited and revised manuscript; G.E.P., L.G., J.F., P.R., F.M., A.G.S., and C.A.W. approved final version of manuscript.
REFERENCES
- 1.Astrand PO. Methods of ergometry in children. Definitions, testing procedures, accuracy and reproduceability. Acta Paediatr Scand Suppl 217: 9–12, 1971. [PubMed] [Google Scholar]
- 2.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122: 191–225, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Bassett DR Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 32: 70–84, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Boissiere J, Maufrais C, Baquet G, Schuster I, Dauzat M, Doucende G, Obert P, Berthoin S, Nottin S. Specific left ventricular twist-untwist mechanics during exercise in children. J Am Soc Echocardiogr 26: 1298–1305, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Cifra B, Dragulescu A, Border WL, Mertens L. Stress echocardiography in paediatric cardiology. Eur Heart J Cardiovasc Imaging 16: 1051–1059, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exerc 29: 1344–1349, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Dragulescu A, Mertens LL. Developments in echocardiographic techniques for the evaluation of ventricular function in children. Arch Cardiovasc Dis 103: 603–614, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Eidem BW, McMahon CJ, Cohen RR, Wu J, Finkelshteyn I, Kovalchin JP, Ayres NA, Bezold LI, O'Brian Smith E, Pignatelli RH. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr 17: 212–221, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Feldman MD, Alderman JD, Aroesty JM, Royal HD, Ferguson JJ, Owen RM, Grossman W, McKay RG. Depression of systolic and diastolic myocardial reserve during atrial pacing tachycardia in patients with dilated cardiomyopathy. J Clin Invest 82: 1661–1669, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferferieva V, Van den Bergh A, Claus P, Jasaityte R, Veulemans P, Pellens M, La Gerche A, Rademakers F, Herijgers P, D'Hooge J. The relative value of strain and strain rate for defining intrinsic myocardial function. Am J Physiol Heart Circ Physiol 302: H188–H195, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira LF, Townsend DK, Lutjemeier BJ, Barstow TJ. Muscle capillary blood flow kinetics estimated from pulmonary O2 uptake and near-infrared spectroscopy. J Appl Physiol 98: 1820–1828, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Friedberg MK, Mertens L. Deformation imaging in selected congenital heart disease: is it evolving to clinical use? J Am Soc Echocardiogr 25: 919–931, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, Drinko JK, Rodriguez LL, Thomas JD, Garcia MJ. Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation 105: 99–105, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hasan BS, Lunze FI, McElhinney DB, Stantcheva E, Brown DW, Rhodes J, Chen MH. Exercise stress echocardiographic assessment of outflow tract and ventricular function in patients with an obstructed right ventricular-to-pulmonary artery conduit after repair of conotruncal heart defects. Am J Cardiol 110, 1527–1533, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res 58: 281–291, 1986. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki M, Yokota M, Izawa H, Ishiki R, Nagata K, Iwase M, Yamada Y, Koide M, Sobue T. Impaired force-frequency relations in patients with hypertensive left ventricular hypertrophy. A possible physiological marker of the transition from physiological to pathological hypertrophy. Circulation 99: 1822–1830, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Izawa H, Yokota M, Nagata K, Iwase M, Sobue T. Impaired response of left ventricular relaxation to exercise-induced adrenergic stimulation in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 28: 1738–1745, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Kampmann C, Wiethoff CM, Wenzel A, Stolz G, Betancor M, Wippermann CF, Huth RG, Habermehl P, Knuf M, Emschermann T, Stopfkuchen H. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart 83: 667–672, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz AM. Influence of altered inotropy and lusitropy on ventricular pressure-volume loops. J Am Coll Cardiol 11: 438–445, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Koopman LP, Slorach C, Hui W, Manlhiot C, McCrindle BW, Friedberg MK, Jaeggi ET, Mertens L. Comparison between different speckle tracking and color tissue Doppler techniques to measure global and regional myocardial deformation in children. J Am Soc Echocardiogr 23: 919–928, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs A, Olah A, Lux A, Matyas C, Nemeth BT, Kellermayer D, Ruppert M, Torok M, Szabo L, Meltzer A, Assabiny A, Birtalan E, Merkely B, Radovits T. Strain and strain rate by speckle tracking echocardiography correlate with pressure-volume loop derived contractility indices in a rat model of athlete's heart. Am J Physiol Heart Circ Physiol 308: H743–H748, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Kuipers H, Verstappen FT, Keizer HA, Geurten P, van Kranenburg G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med 6: 197–201, 1985. [DOI] [PubMed] [Google Scholar]
- 23.La Gerche A, Claessen G, Van De Bruaene A, Pattyn N, Van Cleemput J, Gewillig M, Bogaert J, Dymarkowski S, Claus P, Heidbuchel H. Cardiac magnetic resonance imaging: A new gold standard for ventricular volume quantification during high-intensity exercise. Circ Cardiovasc Imaging 6: 329–338, 2012. [DOI] [PubMed] [Google Scholar]
- 24.La Gerche A, Connelly KA, Mooney DJ, MacIsaac AI, Prior DL. Biochemical and functional abnormalities of left and right ventricular function after ultra-endurance exercise. Heart 94: 860–866, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, Pignatelli RH, Rychik J; Task Force of the Pediatric Council of the American Society of Echocardiography; Pediatric Council of the American Society of Echocardiography. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 19: 1413–1430, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 23: 465–495; quiz 576-467, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Lyseggen E, Rabben SI, Skulstad H, Urheim S, Risoe C, Smiseth OA. Myocardial acceleration during isovolumic contraction: relationship to contractility. Circulation 111: 1362–1369, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Marcus KA, Mavinkurve-Groothuis AM, Barends M, van Dijk A, Feuth T, de Korte C, Kapusta L. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J Am Soc Echocardiogr 24: 625–636, 2011. [DOI] [PubMed] [Google Scholar]
- 29.May LJ, Punn R, Olson I, Kazmucha JA, Liu MY, Chin C. Supine cycling in pediatric exercise testing: disparity in performance measures. Pediatr Cardiol 35: 705–710, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Mertens L, Ganame J, Claus P, Goemans N, Thijs D, Eyskens B, Van Laere D, Bijnens B, D'Hooge J, Sutherland GR, Buyse G. Early regional myocardial dysfunction in young patients with Duchenne muscular dystrophy. J Am Soc Echocardiogr 21: 1049–1054, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Mertens LL, Friedberg MK. Imaging the right ventricle–current state of the art. Nat Rev Cardiol 7: 551–563, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Miura T, Miyazaki S, Guth BD, Kambayashi M, Ross J Jr. Influence of the force-frequency relation on left ventricular function during exercise in conscious dogs. Circulation 86: 563–571, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Naqvi TZ, Goel RK, Forrester JS, Siegel RJ. Myocardial contractile reserve on dobutamine echocardiography predicts late spontaneous improvement in cardiac function in patients with recent onset idiopathic dilated cardiomyopathy. J Am Coll Cardiol 34: 1537–1544, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Paridon SM, Alpert BS, Boas SR, Cabrera ME, Caldarera LL, Daniels SR, Kimball TR, Knilans TK, Nixon PA, Rhodes J, Yetman AT; American Heart Association Council on Cardiovascular Disease in the Young; Committee on Atherosclerosis, Hypertension, and Obesity in Youth. Clinical stress testing in the pediatric age group: a statement from the American Heart Association Council on Cardiovascular Disease in the Young, Committee on Atherosclerosis, Hypertension, and Obesity in Youth. Circulation 113: 1905–1920, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr 21: 922–934, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Pieles GE, Szantho G, Rodrigues JC, Lawton CB, Stuart AG, Bucciarelli-Ducci C, Turner MS, Williams CA, Tulloh RM, Hamilton MC. Adaptations of aortic and pulmonary artery flow parameters measured by phase-contrast magnetic resonance angiography during supine aerobic exercise. Eur J Appl Physiol 2014. [DOI] [PubMed] [Google Scholar]
- 37.Punn R, Obayashi DY, Olson I, Kazmucha JA, DePucci A, Hurley MP, Chin C. Supine exercise echocardiographic measures of systolic and diastolic function in children. J Am Soc Echocardiogr 25: 773–781, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes J, Ubeda Tikkanen A, Jenkins KJ. Exercise testing and training in children with congenital heart disease. Circulation 122: 1957–1967, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Roche SL, Grosse-Wortmann L, Friedberg MK, Redington AN, Stephens D, Kantor PF. Exercise echocardiography demonstrates biventricular systolic dysfunction and reveals decreased left ventricular contractile reserve in children after tetralogy of Fallot repair. J Am Soc Echocardiogr 28: 294–301, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Roche SL, Grosse-Wortmann L, Friedberg MK, Redington AN, Stephens D, Kantor PF. Exercise echocardiography demonstrates biventricular systolic dysfunction and reveals decreased left ventricular contractile reserve in children after tetralogy of Fallot repair. J Am Soc Echocardiogr 28: 294–301, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Roche SL, Vogel M, Pitkanen O, Grant B, Slorach C, Fackoury C, Stephens D, Smallhorn J, Benson LN, Kantor PF, Redington AN. Isovolumic acceleration at rest and during exercise in children normal values for the left ventricle and first noninvasive demonstration of exercise-induced force-frequency relationships. J Am Coll Cardiol 57: 1100–1107, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Rowland T. Echocardiography and circulatory response to progressive endurance exercise. Sports Med 38: 541–551, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Rowland T, Unnithan V, Roche D, Garrard M, Holloway K, Marwood S. Myocardial function and aerobic fitness in adolescent females. Eur J Appl Physiol 111: 1991–1997, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Vella CA, Robergs RA. A review of the stroke volume response to upright exercise in healthy subjects. Br J Sports Med 39: 190–195, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel M, Cheung MM, Li J, Kristiansen SB, Schmidt MR, White PA, Sorensen K, Redington AN. Noninvasive assessment of left ventricular force-frequency relationships using tissue Doppler-derived isovolumic acceleration: validation in an animal model. Circulation 107: 1647–1652, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d'Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 28: 183–193, 2015. [DOI] [PubMed] [Google Scholar]
- 47.Warburton DE, Haykowsky MJ, Quinney HA, Blackmore D, Teo KK, Humen DP. Myocardial response to incremental exercise in endurance-trained athletes: influence of heart rate, contractility and the Frank-Starling effect. Exp Physiol 87: 613–622, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Weidemann F, Eyskens B, Jamal F, Mertens L, Kowalski M, D'Hooge J, Bijnens B, Gewillig M, Rademakers F, Hatle L, Sutherland GR. Quantification of regional left and right ventricular radial and longitudinal function in healthy children using ultrasound-based strain rate and strain imaging. J Am Soc Echocardiogr 15: 20–28, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Weidemann F, Eyskens B, Mertens L, Dommke C, Kowalski M, Simmons L, Claus P, Bijnens B, Gewillig M, Hatle L, Sutherland GR. Quantification of regional right and left ventricular function by ultrasonic strain rate and strain indexes after surgical repair of tetralogy of Fallot. Am J Cardiol 90: 133–138, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Weidemann F, Jamal F, Kowalski M, Kukulski T, D'Hooge J, Bijnens B, Hatle L, De Scheerder I, Sutherland GR. Can strain rate and strain quantify changes in regional systolic function during dobutamine infusion, B-blockade, and atrial pacing–implications for quantitative stress echocardiography. J Am Soc Echocardiogr 15: 416–424, 2002. [DOI] [PubMed] [Google Scholar]