Abstract
In 1998 several groups reported the feasibility of functional magnetic resonance imaging (fMRI) experiments in monkeys, with the goal to bridge the gap between invasive nonhuman primate studies and human functional imaging. These studies yielded critical insights in the neuronal underpinnings of the BOLD signal. Furthermore, the technology has been successful in guiding electrophysiological recordings and identifying focal perturbation targets. Finally, invaluable information was obtained concerning human brain evolution.
We here provide a comprehensive overview of awake monkey fMRI studies mainly confined to the visual system. We review the latest insights about the topographic organization of monkey visual cortex and discuss the spatial relationships between retinotopy and category and feature selective clusters. We briefly discuss the functional layout of parietal and frontal cortex and continue with a summary of some fascinating functional and effective connectivity studies. Finally, we review recent comparative fMRI experiments and speculate about the future of nonhuman primate imaging.
Following the introduction of human functional magnetic resonance imaging (fMRI) in the early nineties, four groups independently reported the feasibility of monkey fMRI in 1998 (Dubowitz et al., 1998;Logothetis et al., 1998;Stefanacci et al., 1998;Vanduffel et al., 1998). Initially, disparate strategies were used, including high field (4.7 T) Blood Oxygen Level Dependent (BOLD) imaging with dedicated vertical scanner bores (Logothetis et al., 2001), BOLD imaging using clinical, low field (1.5 T) MR scanners (Stefanacci et al., 1998), and Cerebral Blood Volume fMRI using exogenous contrast agents at low field (Vanduffel et al., 2001). Since high field MR scanning is more susceptible to motion-induced imaging artifacts, the initial studies in vertical scanners were primarily performed in anesthetized animals (Hayashi et al., 1999;Logothetis et al., 2001;Sereno et al., 2002). This motion issue could be largely mitigated by using contrast agents at low field, increasing the contrast-to-noise ratio by ~5 at 1.5 T and ~3 at 3 T (Vanduffel et al., 2001). Later improvements included developments of implanted focal single loop coils (Logothetis et al., 2002), external phased array coils (Ekstrom et al., 2008), spin-echo imaging (Ku et al., 2011), and implanted phased array coils (Janssens et al., 2012).
Here we provide an overview of what has been achieved using fMRI in alert monkeys over the past 15 years, focusing on the visual system, by far the most investigated modality. We shall argue that functional imaging in animals is highly complementary to traditional invasive methods and may vastly increase the ‘yield’ of such methods in future investigations by guiding large-scale electrophysiological recordings and focal reversible perturbation experiments. The role of nonhuman primate fMRI in in systems neuroscience will only increase as sensitivity and spatio-temporal resolution is further refined. Moreover, comparative imaging will provide crucial insights into brain evolution and will prove essential for integrating the wealth of invasive animal results with the ever-expanding human imaging data sets.
In this review, we will first describe how fMRI provides a parcellation of visual cortex in individual, living subjects, using retinotopic mapping, paving the way for further characterization of cortical areas and functional networks. We will also show how monkey fMRI reconciled human imaging with single cell studies by mapping category-selective regions, including cortical patches preferentially processing faces, bodies or places, and by guiding recordings from these patches. Next, we will briefly describe the efforts to use fMRI in active subjects performing motor and cognitive tasks, which reveal the functional properties of parietal and (pre)frontal monkey cortex. Following a brief evaluation of a burgeoning human imaging field, resting state fMRI, we will emphasize a distinctive contribution of monkey fMRI: the possibility to combine refined perturbations of cortical areas with imaging giving, allowing us to make causal instead of correlational-based inferences about the participation of a specific brain regions in cognitive or perceptual processing. Finally, we will review comparative monkey-human fMRI studies providing unique insights into cortical primate evolution, and we will conclude with studies directly linking electrophysiology, monkey imaging, and human fMRI.
1. Topographic organization and parcellation of visual cortex
Primates rely heavily on vision for interacting with the environment and with their (non)conspecifics. This is reflected in the extent of cortical surface specialized for processing visual information. Using myeloarchitectonics, connectivity and receptive field mapping data, involving large cohorts of animals in dozens of laboratories, more than 30 visual areas have been identified in nonhuman primates, spanning half of cerebral cortex (Felleman and Van Essen, 1991). Considerable information regarding the topography and function of visual areas has been gleaned from such electrophysiological and tractography-based mapping studies. However, even at relatively early stages of the visual system the number and exact definition of visual areas remains heavily debated because of the sequential nature of the recordings, their finite sampling size, problems inherent to reconstructing electrode tracts in 3D space, and interpolation issues across subjects and experiments. After more than half a century of such efforts, functional imaging in nonhuman primates has finally allowed investigators to obtain detailed topographic information in a non-invasive manner across the entire brain of individual living subjects, obviating most of the issues raised above.
Retinotopic organization of occipitotemporal cortex
Initially, fMRI-based retinotopic mapping in monkeys, using either anesthetized high-field BOLD (Brewer et al., 2002), or awake low-field cerebral blood volume paradigms (Fize et al., 2003;Vanduffel et al., 2002), confirmed the alternating representations of vertical (VM), and horizontal meridians (HM) between V1-V2, V2-V3, and V3-V4, respectively, with a horizontal meridian as the anterior border of V4 (Fig. 1). Each area contains a representation of the entire contralateral hemifield, with segregated upper and lower quadrants (split-field representation). Evidence for additional visual field maps anterior to ventral V4 was scant in these original studies, except that a foveal and lower field representation in ventral cortex was attributed to TEO.
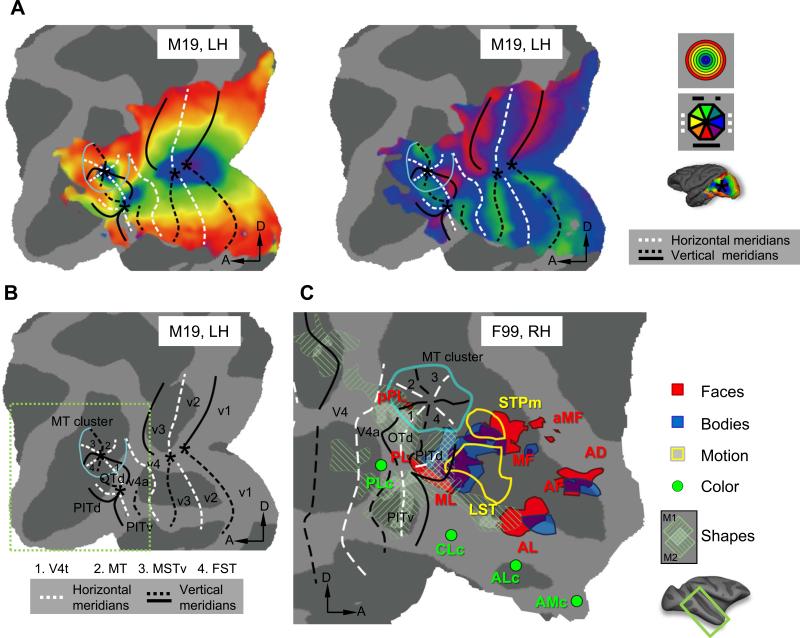
Figure 1.
Eccentricity and polar angle maps (A) defining retinotopic borders (B) in macaque occipital cortex. Real data from a representative subject (M19) is shown. Asterisks indicate the positions of the central visual field representations. Light blue line indicates the eccentricity ridge around the MT cluster. (C) Functional clustering of visual categories in occipitotemporal cortex displayed on F99 (RH) flattened surface. Face patches: probabilistic map from Janssens et al. (2014), real group data (N=3), cut-off 66%. Body patches: fixed-effect real group data (N=3) from Popivanov et al. (2012), p<10−5. Shape sensitive regions: real individual data (indicated by left and right hatching, respectively) (N=2) from Denys et al. (2004a). Motion sensitivity anterior to the MT cluster: real group data (N=5) from Nelissen et al. (2006). Color sensitivity data: approximate locations from Lafer-Sousa and Conway (2013). Retinotopy areas: real probabilistic data (N=4) from Janssens et al. (2014). A, anterior; D, dorsal; LH, left hemisphere; RH, right hemisphere.
Area V3A is located on the anectant gyrus at the junction between the parieto-occipital and lunate sulci. Within the visual hierarchy, this is the first region located exclusively in dorsal visual cortex containing a representation of both the upper and lower quadrants, split by a horizontal meridian (complete hemifield). The foveal representation of area V3A is separated from the central foveal confluence of areas V1 through V4 and a vertical meridian representation borders V3A posteriorly and anteriorly. However, unlike V1-V4, area V3A could not be identified consistently in all subjects, and the definitions of V3A differ slightly in the Brewer et al. (2002) and Fize et al. (2003) studies. This may reflect the complex folding pattern within parts of the lunate sulcus or variability in retinotopic organization of V3A across subjects.
An organization similar to that of V3A was initially ascribed to area MT (middle temporal area) in those initial monkey fMRI studies: A horizontal meridian split a ventral upper-quadrant representation from a dorsally-located lower field. Moreover, a vertical meridian separated this area from its neighbors, including FST (fundus superior temporal area), although these were not described in detail. A more recent study investigated MT and its surrounding areas in more detail using high-resolution (7 Tesla, 0.75 mm isotropic voxels) phase-encoded retinotopic mapping (Kolster et al., 2009). Results showed that MT and its immediate neighbors are organized as a visual-field map cluster (Wandell et al., 2005) with a shared foveal representation surrounded by a semicircular representation of isoeccentric contours (Fig. 1). This foveal representation, distinct from the central foveal confluence of V1-V4, is located in the posterior bank of the superior temporal sulcus (STS). When phase reversals along isoeccentricity lines were plotted, clear evidence emerged for a lower vertical meridian at the V4t-MT border, and an upper vertical meridian between MT and MSTv (ventral middle superior temporal area). There is again a lower vertical meridian between the latter areas and FST, and an upper vertical meridian at the anterior border of FST. Thus, MT, MSTv and FST each contain a complete contralateral visual field, while in V4t only the upper quadrant is represented, although the lower visual field representation may have remained undetected in V4t (Fig. 1). In addition, Kolster et al. (2009) described another full hemifield ventral to the MT cluster with a foveal representation distinct from that of the MT cluster, which they assigned to PITd (dorsal posterior inferior temporal area) in accordance with Felleman and Van Essen (1991).
Subsequent studies by Kolster et al. (2014) and Janssens et al. (2014) described the retinotopic organization of cortex rostroventral to V4 in greater detail, using checkerboard and dynamic biologically-relevant phase-encoded stimuli, respectively. Both studies show clear evidence for a split hemifield anterior to the horizontal meridian of V4, with a contralateral lower and upper visual field rostral to dorsal and ventral V4, respectively, fitting exactly the description of V4A in earlier studies (Zeki, 1971). Rostral to V4A and distinct from the MT cluster, 3 additional hemifields were found. The first, termed OTd (dorsal occipitotemporal area), was interposed between V4A and V4t of the MT cluster sharing a lower vertical meridian with V4A and an upper vertical meridian with PITd. The latter area extended more rostrally to a lower vertical meridian that bordered, at least partially, a more caudoventral field map, PITv (ventral PIT). PITv shared an upper vertical meridian with ventral V4A, more caudally. The Janssens et al. (2014) study showed that V4A, PITd, PITv and OTd shared a common foveal representation, clearly distinct from the central foveal confluence (of V1-V4) or the MT cluster (V4t, MT, MSTv, FST) (Fig. 1). Hence in monkey occcipitotemporal cortex, at least 3 separate visual field map clusters can be identified, indicating an evolutionarily preserved and fundamental organizational principle of primate visual cortex (Kolster et al., 2009). As Wandell et al. (2005) proposed, such clustering may be the most efficient manner for minimizing neural distance between processing modules requiring strong functional interactions. The Janssens et al. (2014) study also hinted at other regions anterior to the MT and PIT clusters showing foveal biases. These regions, located in the anterior bank of the STS, corresponding to STP (superior temporal polysensory area), and in the middle temporal gyrus, do not necessarily overlap with regions showing face selectivity, as postulated for human visual cortex (Hasson et al., 2002).
Parietal cortex and frontal eye fields (FEF)
Fize et al. (2003) reported a central visual field representation within the lateral bank of the IPS (intraparietal sulcus) in the majority of the animals tested, but without clear polar angle organization, consistent with published electrophysiology (Ben Hamed et al., 2001). Recent retinotopic mapping in the monkey focusing on IPS (Arcaro et al., 2011), reported three parietal regions showing a foveal bias and crude polar angle organization. Two were located in the posterior end of the lateral bank and were named CIP1 and CIP2 (caudal intraparietal area 1 and 2). The authors showed convincing evidence for 2 upper vertical meridian representations designated as the borders between V3A and CIP1, and CIP2 and LIP (lateral intraparietal ara), respectively, but their evidence for a lower visual field representations in CIP1/2 is rather marginal. Higher resolution and/or more complex stimuli better suited for driving IPS neurons (instead of colored checkerboard stimuli) may eventually clarify the organization of these two higher-order areas (e.g. Janssens et al., 2013). LIP was defined in the Arcaro study (2011) as a complete contralateral hemifield, with an upper vertical meridian at its caudal boundary with CIP2, and a lower vertical meridian as its rostral border. This region fits well with LIPv and LIPd as described in earlier electrophysiological and imaging studies (Baker et al., 2006;Ben Hamed et al., 2001). In frontal cortex, several studies have indicated a crude topographic organization in the anterior bank of the arcuate sulcus (Bruce and Goldberg, 1985). The small and large saccade zones of the FEF, located caudo-laterally and rostro-medially, respectively, receive visual afferents from foveal versus more peripheral visual field representations in extrastriate cortex, respectively (Schall et al., 1995). The receptive fields of the visual neurons are in register with the movement fields of the (visuo-) motor neurons in the FEF. Consistent with these neuronal properties, Wardak et al (2010) observed eccentricity biases in FEF, with larger eccentricities represented most medially. Higher-resolution imaging (Janssen et al., 2014) confirmed this finding: a foveal visual field in FEF is surrounded by more peripheral visual field representations stretching antero-medially, as expected, but also ventro-laterally.
In conclusion, a clear-cut retinotopic organization as found in occipitotemporal cortex is largely lacking within major parts of parietal and frontal cortex. While smoothly progressing eccentricity representations along the cortex are preserved to some extent, polar angle representations are much more variable. This overview demonstrates that retinotopy has proven useful to parcel visual cortex and to define visual areas. Initially, it was proposed that functional properties could also be used to define cortical areas, with V4 and MT being labeled the color and motion specific areas (Zeki et al., 1991). The latter strategy has received little support as many cortical areas forming functional networks proved motion or color selective as discussed below.
Motion selectivity of visual areas
Over a decade ago, monkey fMRI confirmed and extended earlier electrophysiological studies demonstrating visual motion sensitivity for translating dot fields in areas V2, V3, MT, MSTv, FST, regions within the IPS (then labeled VIP) and the arcuate sulcus (FEF). When moving lines were presented, motion sensitivity was also found in V4, TE, LIP, and CIP (Vanduffel et al., 2001). The somewhat surprising motion sensitivity in area V4 was also confirmed by Tolias et al. (2001). In contrast to human V3A (Tootell et al., 1997), but again consistent with monkey electrophysiology, Vanduffel et al. (2001) showed a lack of motion sensitivity in monkey V3A.
A year later, the same group showed sensitivity for three-dimensional-structure-from-motion using paperclip stimuli in areas V2, V3, V4, and area MT along with its neighbors. Unlike observations in humans, very little three-dimensional-structure-from-motion sensitivity was observed in parietal cortex (see below) (Vanduffel et al., 2002). A more exhaustive mapping of motion sensitivity within the STS using a wide variety of motion stimuli was performed by Nelissen et al. (2006). This study confirmed fMRI-defined motion sensitivity in MT, MSTv, MSTd, FST and STPm (middle STP) (Fig. 1C). However, profound motion sensitivity was also observed in an area immediately rostro-ventral to FST deemed LST (lower super temporal area). This region responded to a broad range of speeds, moving images of objects, patterns defined by opponent motion, and to actions. The recently-described field maps anterior to V4 and caudo-ventral to the MT cluster (i.e. V4A, PITd, PITv and OTd) displayed little motion sensitivity unlike components of the MT cluster itself (Kolster et al., 2014), supporting the notion that fieldmap clusters comprise functionally similar areas.
Color sensitivity of occipitotemporal areas
Color processing is another instance where fMRI revised the original view of ‘centers’, such as V4, once considered the ‘color area’ (Zeki, 1973). Confirming earlier maps obtained with 2-deoxyglucose (Tootell et al., 2004) and PET (Takechi et al., 1997), several color-selective fMRI-defined patches have been discovered in monkey ventral stream beyond V4 (Conway et al., 2007;Harada et al., 2009). More recently, (Lafer-Sousa and Conway, 2013) showed color-biased patches in IT that were anatomically paired with the face patches (Fig. 1C).
2. Functional clustering of object-category selectivity in occipitotemporal cortex
Detecting and recognizing objects in a visual scene is one of the primary tasks of the visual system. Neural representations of objects are critical for object categorization (e.g. a dog versus a cat), identification (e.g. my dog ‘Gust’ versus my neighbor's ‘Max’), and the assessment of object characteristics (e.g. ’Max’ is aggressive). Categorization must generalize across physically disparate objects (e.g. a Rhodesian ridgeback versus a Chihuahua) while identification has to discriminate between very similar objects (e.g. two white Labrador puppies). To accomplish these tasks one must match the visual representation of objects with items stored in memory. Lesion and electrophysiological studies have demonstrated the crucial role of the ventral stream in accomplishing these computationally challenging tasks. For more than forty years it has been known that IT neurons are selective for (2D) object shape (Gross et al., 1972). Area V4, moreover, shows selectivity for parts of objects (Pasupathy and Connor, 2001). Inspired by such electrophysiological findings, human fMRI revealed that a large cortical region, designated the lateral occipital complex, was activated by viewing intact versus scrambled pictures of objects (Malach et al., 1995). To link the electrophysiological evidence for shape selectivity in monkey IT with results in human lateral occipital complex, Denys et al. (2004a) used monkey fMRI to show that human lateral occipital complex is indeed the human equivalent of a substantial portion of monkey IT cortex (Fig. 1C). More recently, Komatsu's group also showed selective fMRI responses to surface properties of objects in monkey IT (Goda et al., 2014;Okazawa et al., 2012). Specific pathologies, such as prosopagnosia and achromatopsia, hinted at a modular organization of ventral cortex for processing specific objects (e.g. faces) or visual features (e.g color), although such lesion evidence is not conclusive (Palmeri and Gauthier, 2004). Extensive evidence (e.g. Kanwisher et al., 1997) showed that in human occipitotemporal cortex specific regions preferentially process specific object classes, in particular faces, bodies and places. This evidence does not imply, however, that such areas exclusively process the respective object classes, nor that cortex outside these regions is irrelevant for representing those objects (Haxby et al., 2001). This debate regarding distributed versus modular representation of objects, while still persisting, may ultimately be resolved by fMRI-guided invasive studies in monkeys. Indeed, over the last decade similar domain-specific patches have been mapped using monkey fMRI and, using these maps as guide, it has been shown that face and body selectivity are more clustered at the neuronal level than initial single cell studies had indicated.
Faces
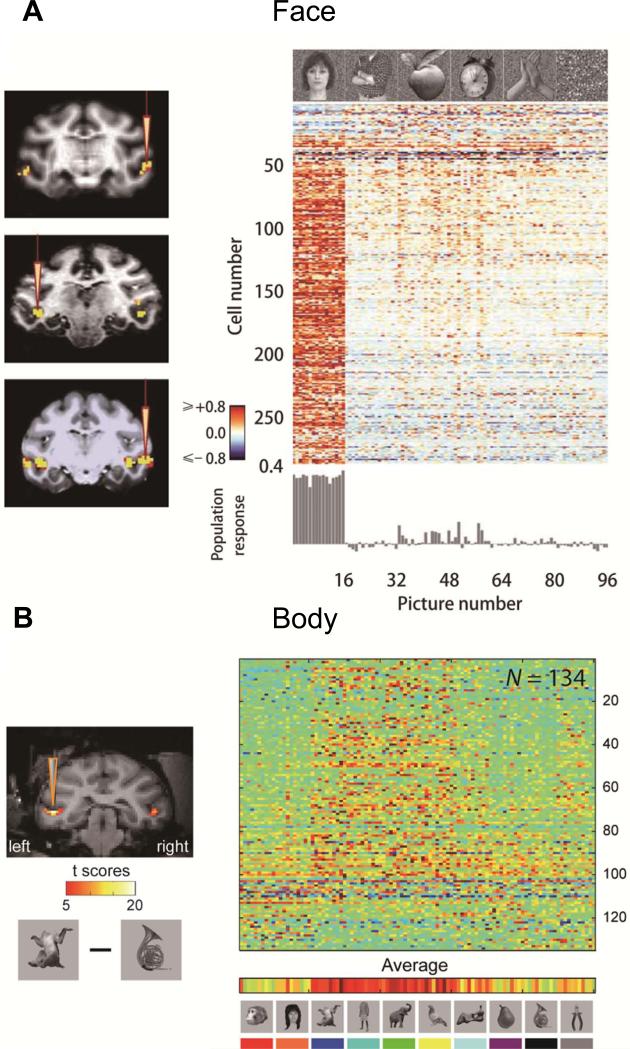
Although selectivity of IT neurons for faces (Gross et al., 1972;Perrett et al., 1982) has long been established, the systematic grouping of face-selective neurons had evaded neurophysiological investigation (Tanaka et al., 1991). The presence of the purported face areas found with human fMRI (Kanwisher et al., 1997) seemed difficult to interpret, until similar face ‘patches’ were described in monkeys (Tsao et al., 2001). Several groups (Pinsk et al., 2009;Popivanov et al., 2012;Tsao et al., 2006) have since described a set of regions in monkey extrastriate cortex more strongly activated by faces than non-face objects. Initial reports defined 6 patches: PL, ML, MF, AL, AF and AD (with P = posterior, M = medial, A = anterior, L = lateral, F = fundus and D = dorsal) (Fig. 1 C). The combination of AF and AD (Pinsk et al., 2009) may correspond to AM described by Tsao et al. (2010). In the original fMRI-guided single-unit study (Tsao et al., 2006), 97% of the ML neurons were categorized as face selective, defined as twice as responsive to face than non-face stimuli (face-selectivity index > 0.3, Fig. 2A). Subsequent studies confirmed this finding, though the proportions reported were slightly smaller. Freiwald and Tsao (2010) found 90 % face-selective neurons (face selectivity index > 0.3) in ML/MF, 62% in AL, and 79% in AM. More recently, Issa et al. (2013) reported 83% face-selective neurons in PL, 75% in ML and 88% in AM/AL.
Figure 2.
Single cell selectivity in fMRI defined face patch ML (A) and body patch mSTS (B). Population data adapted with permission from Freiwald et al. (2009) and Popivanov et al. (2014).
In addition, using concurrent microstimulation and fMRI, Tsao's group showed that the face patches in monkey extrastriate cortex formed an interconnected functional network (Moeller et al., 2008). Recently, Janssens et al. (2014) described the relationship between the face patches and the topographic organization of extrastriate cortex. They showed that PL is located within retinotopic area PITd while ML shows a consistent foveal bias. In contrast, MF, anterior temporal and prefrontal monkey face patches lack any form of topographic organization. These authors also showed evidence for additional, smaller face patches in occipital cortex, one posterior to PL within retinotopic area V4t (pPL, posterior PL), and a second immediately anterior to MF (aMF) (Fig. 1C, 3A). Additional patches have been reported in ventral cortex (Janssens et al., 2014;Ku et al., 2011), but await confirmation. Three face patches were identified in frontal cortex: PO (prefrontal orbital), PL (prefrontal lateral), and PA (prefrontal arcuate) (Tsao et al., 2008b) (Fig. 4). They have been re-labeled PAf, PLf, POf, with ‘f’ indicating their frontal location, to avoid the ambiguity of the label ‘PL’ (Janssens et al., 2014). Interestingly, most functional imaging investigations of face patches used static, emotionally neutral stimuli. With emotional (Hadj-Bouziane et al., 2008), or dynamic faces (Zhu et al., 2012), cortex lateral to PL and ML was selectively activated (Fig. 3A). This was recently confirmed by Polosecki et al. (2013) and suggests that some aspects of face processing take place outside classically fMRI-defined face patches. Future studies will have to clarify the functional roles of face-processing neurons within and outside these face patches.
Figure 3.
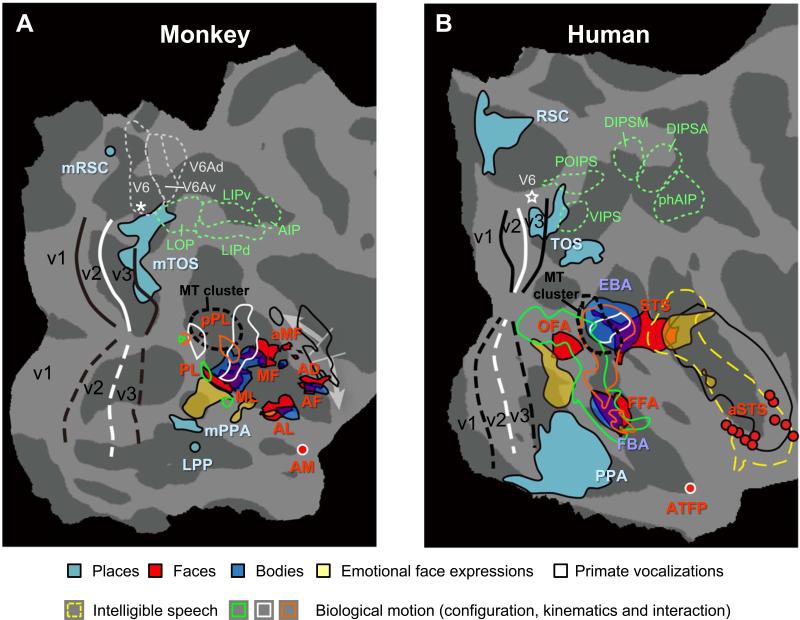
Comparison of face, place and body patches in monkey and human posterior occipital cortex. (A) Monkey: Activations (real data combined with approximate locations of local maxima) from different studies are projected onto the flattened right hemisphere of F99 atlas. Place-selective patches: real data from Nasr et al. (2011) and approximate data (LPP and mRSC) from Kornblith et al. (2013). Faces-selective regions: real probabilistic data from Janssens et al. (2014); AM: approximate data from Tsao et al. (2008a). Dynamic facial expressions (fear vs. chewing controlled for scrambles): real data from Zhu et al. (2012). Body-selective regions: real group data from Popivanov et al. (2012) (same as in Fig. 1C). Biological motion sensitivity (main effect of configuration and kinematics, as well as the interaction): approximate data from Jastorff et al. (2012). Regions sensitive for primate vocalizations (black outlines): real data from Joly et al. (2012). Retinotopy: real probabilistic data from Janssens et al. (2014). LOP, LIP and AIP from Lewis and Van Essen (2000). V6, V6A and V6Ad are approximate data from Fattori et al. (2009) and Pitzalis et al. (2013). The white arrows indicate regions in the monkey STS which are vastly expanded in the human for auditory processing (including speech).
(B) Human: activations are projected onto flattened right hemisphere of fsaverage atlas. Faces- and body selective regions: approximate probabilistic data of Engell and McCarthy (2013); Dynamic facial expressions: real data from Zhu et al. (2012); ATFP: approximate data from Rajimehr et al. (2009); aSTS: approximate data from Pitcher et al., (2011). Biological motion sensitivity: approximate data from Jastorff and Orban (2009). Regions sensitive for primate vocalizations (black outlines) and intelligible speech (dashed yellow outlines): real data from Joly et al. (2012). Retinotopy: approximate probabilistic data from PALS-B12 atlas; VIPS, POIPS, DIPSM, DIPSA and phAIP: approximate locations from Jastorff et al. (2010). White stars are foveal representations in V6
Figure 4.
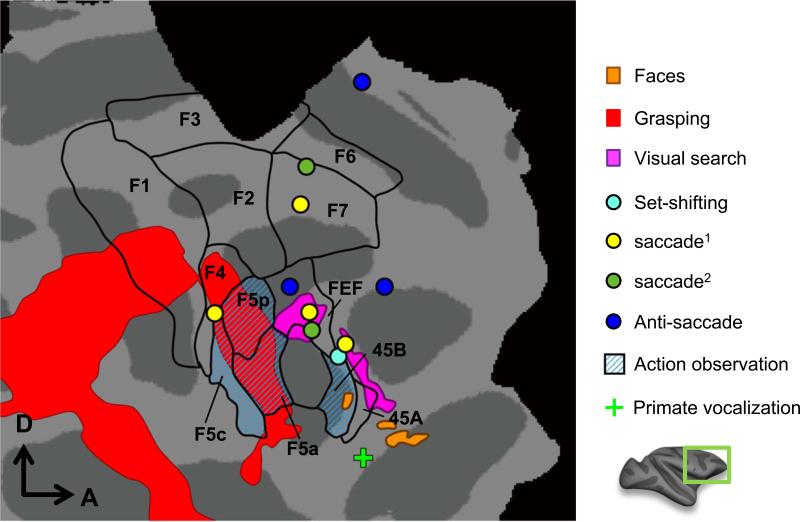
Saccade, search, set-shifting, grasping, face-selective, action observation and primate vocalization sensitive regions (all approximate data except face patches) in monkey frontal cortex (F99). Face-selective regions: probabilistic real data from Janssens et al. (2014). Grasping: from Nelissen and Vanduffel (2011). Action observation: from Nelissen et al. (2005). Search: from Wardak et al. (2010). Primate vocalizations: (data from opposite hemisphere) from Joly et al. (2012). Task-set shifting: from Nakahara et al. (2002). Visually-guided saccades: from Koyama et al. (2004) (yellow dots) and Baker et al. (2006) (green dots). Anti-saccades: from Ford et al. (2009). Black outlines indicate anatomically-defined ROIs of seven motor areas (F1-F7), FEF and areas 45A/B as defined by Belmalih et al. (2007).
Monkey fMRI has transformed the field of visual object processing with the ability to quickly examine many neurons of similar selectivity in a hierarchical network of interconnected, category-selective areas (Moeller et al., 2008). fMRI-guided recordings taught us that face-selective neurons in PL are driven by the barest elements of a face: a single eye region in the context of a face-like outline (Issa and DiCarlo, 2012). Neurons in ML, on the other hand, encode the entire face according to geometrical relationships between small numbers (typically 3) of face components in the context of an upright facial outline (Freiwald et al., 2009). Most ML neurons are tuned for both geometry and contrast polarity of facial features (Ohayon et al., 2012). The face category is thus extracted at the level of the ML patch. ML neurons, however, respond predominantly to one view of the face (identity information), generally a frontal view. Neurons at the presumptive next stage, in the AL face patch, are selective for the viewpoint from which the face is seen, one population preferring vertical frontal views, the other population side views. Neurons in AM become viewpoint-invariant and encode individual faces. Face patches thus differ in their representations of facial identity: ML and MF neurons are view-specific, confusing identity and viewpoint; AL neurons encode face identity in a mirror-symmetrical manner, while AM neurons achieve full invariance. This patchwork system first extracts the category in two steps and then the individual faces in two additional steps. Hence, 10 years of MRI-guided single neuron studies have yielded greater progress in understanding face processing in IT cortex than did the previous 30 years of unguided single cell work. An important remaining issue concerns the functional role of IT cortex lying outside face-selective regions and the regions receiving the output from the face patches, such as prefrontal cortex.
Bodies
A number of imaging studies have also shown occipitotemporal areas responding more strongly to images of bodies and body components than to faces and other object categories (Bell et al., 2009;Pinsk et al., 2005;Popivanov et al., 2012;Popivanov et al., 2014;Tsao et al., 2003a). These body-selective patches are located adjacent to the middle and anterior face patches (Popivanov et al., 2012): the midSTS patch lies between the ML and MF face patches while parts of the antSTS patch abut AL medially and AF more laterally (Fig. 1C, 3A). As with most face patches, the body patches are located within non-retinotopic cortex, except for the most posterior part of the midSTS patch.
Popivanov et al. (2014) quantified the selectivity of the midSTS body patch and reported an average body selectivity index (same metric as face selectivity index) of 0.53. Individual neurons were selective for a relatively small number of body stimuli, hence they exhibited high within-category selectivity (Fig. 2B). Selectivity for other non-body stimuli was variable across individual body patch neurons, but was robust across the population, as reflected in the fMRI responses. Given this mixed selectivity it is unsurprising that most types of stimuli (including non-body stimuli) could be encoded by the neuronal population in the body patches (Popivanov et al., 2014). Using a second set of body-part stimuli, Popivanov et al. (2014) was able to show that body patch neurons displayed surprisingly strong orientation selectivity for individual body parts (such as upright male genitals), perhaps partially explaining the heterogeneous responses to whole-body stimuli. Interestingly, within the body patches, multivoxel pattern analysis primarily differentiated between faces and other object classes (including bodies) instead of the expected distinction between animate and inanimate objects (Popivanov et al., 2012).
Scenes
Alongside face and body patches, fMRI has also revealed evidence for three specialized scene-processing modules in human visual cortex: the parahippocampal place area (PPA), transverse occipital sulcus (TOS), and retrosplenial cortex (RSC) (Fig. 3B). In the monkey, Tootell's group described scene-selective regions in dorsal occipital cortex: mTOS in a region partially overlapping V3A, V4d or DP (dorsal prelunate area), mRSC on the medial wall near peripheral V1, and mPPA lying lateral to the ML face patch (Nasr et al., 2011). Kornblith et al. (2013) also described a scene patch located more latero-ventrally within the OTS (occipitotemporal sulcus) which they call the lateral place patch (LPP) and which possibly corresponds to mPPA (Fig. 3A). These authors recorded from LPP, reporting 46% scene-selective neurons with a scene selectivity index (SSI) > 0.3, and responding twice as strongly to scene than non-scene stimuli (objects, textures, scrambled scenes).
Degree of neuronal category selectivity across fMRI-defined category-selective patches
A comparison of category selectivity within different category selective patches reveals that proportions of category selective neurons are the highest and lowest in the face and place patches, respectively. This has been confirmed by Bell et al. (2011), who recorded from three types of category-selective patches and intervening IT cortex in the same animals. They observed that 60% of the neurons in the anterior face patch and 42% in the posterior face patch were face selective (face selectivity index > 0.3), compared to 50% of the neurons in body patches and 32% in the scene patch displaying corresponding selectivity. The authors explain their lower proportions compared to those of Tsao et al. (2006) as sampling discrepancies in the center versus margins of the patches.
Issa et al. (2013) also explored large regions of IT cortex, taking an interesting alternative approach, comparing neuronal responses over an extensive area of IT cortex with fMRI data from that region. In contrast to the more traditional fMRI-guided recordings, many sites in the single cell recordings were only sparsely sampled. The authors correlated neurophysiological ‘maps’ with fMRI maps obtained using identical contrasts. They observed high correlations between multiunit activity and fMRI maps, averaging 0.6, provided that the multiunit maps were smoothed with kernels > 3.5 mm. This correlation is as strong as that obtained with LFP maps, supporting earlier observations in V1 of anesthetized monkeys (Logothetis et al 2001). These results indicate that MRI effectively captures low-frequency variations in neuronal response, but not necessarily the higher ones. This means that an fMRI-defined patch could either contain relatively few highly category-selective neurons (Tsao et al., 2006), or many neurons with mixed selectivities but a modest average bias for a given category (Popivanov et al., 2014). In any event, it is impossible to make conclusive inferences about underlying neuronal selectivities from fMRI data alone, hence, the need for fMRI-guided electrophysiology in regions where functional correspondence can be established using parallel imaging experiments.
fMRI studies have unambiguously shown that neurons selective for some categories are grouped together in IT cortex, a strategy to reduce anatomical connection distance between interacting neurons and to enhance computational efficiency. Hence for processing object categories, similar principles apply as with those governing columnar (Bonhoeffer and Grinvald, 1991), or cluster organizations in retinotopically-organized cortex (Kolster et al., 2009;Wandell et al., 2005). In considering all published category- and feature-selective (i.c. color selectivity) monkey fMRI data, an intriguing functional organization emerges beyond retinotopic cortex, whereby multiple processing clusters (super-modules) are repeated 2 to 3 times along the caudo-rostral axis of IT. In addition, if one considers the functional organization perpendicular to this caudo-rostral axis, a mirror-symmetric organization of category or feature-selective clusters (or supra-modules) within each super-module becomes apparent. The cortex encountered from ventral to more dorsal locations within a super-module responds successively to places, emotional face expressions, faces, bodies, faces, and possibly again emotional facial expressions. Such a mirror-symmetric organization is reminiscent of clustered field maps in retinotopically organized cortex and suggests that similar ontogenetic and developmental forces may govern the formation of retinotopic and non-retinotopic primate visual cortex (Fig. 1C). An intriguing recent finding, supporting this view, showed that intensive training with artificial symbols may cause the emergence of category-selective supra-modules within IT's super-modules (Srihasam et al., 2012). Hence, in the lingering nature-versus-nurture debate surrounding the formation of category-selective regions, it appears that both experience (Srihasam et al., 2012) and genetics, as evidenced by remarkably stable patterns across individuals, are co-existing driving forces.
3. Parietal and frontal cortex: task related fMRI
While occipitotemporal cortex can be explored, at least in the first instance, by using ‘passive’ subjects who only fixate, exploration of parietal and certainly frontal cortex usually requires the subjects to perform tasks. Progress has been slower because training animals to perform complex cognitive tasks takes considerable time.
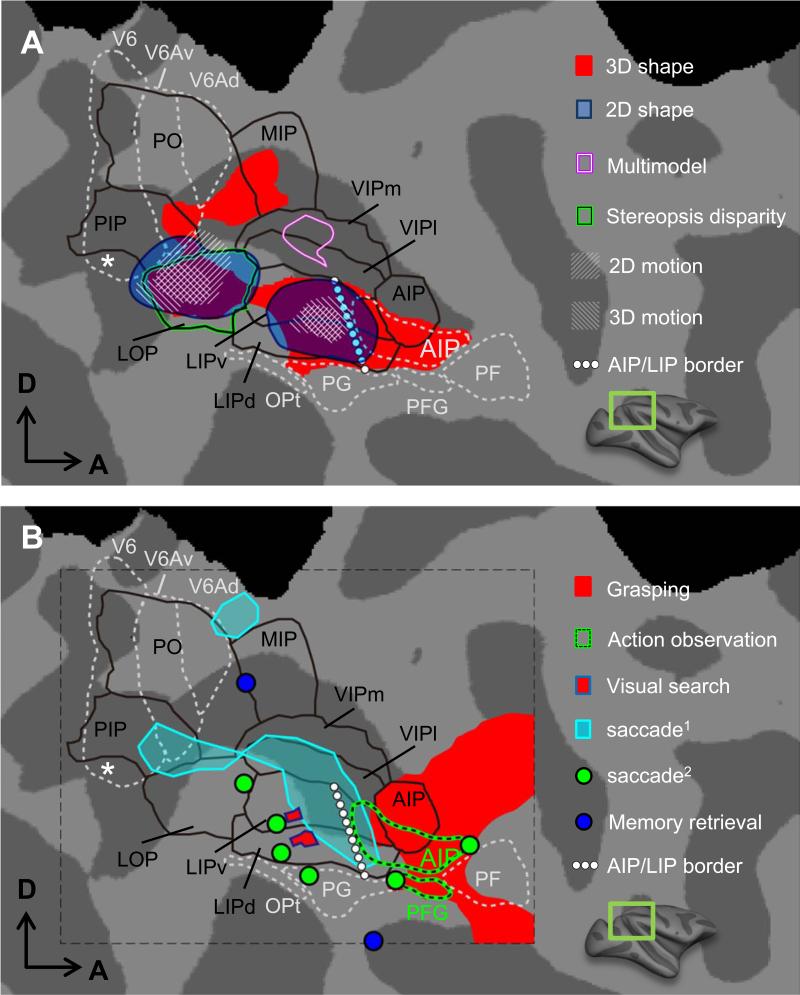
Parietal cortex is situated at the end of the dorsal ‘where’ stream, being involved in attention, decision formation, spatial and sensori-motor functions with the ultimate goal to prepare for actions. It consists of highly specialized areas in humans and monkeys, for which only scant evidence existed that they may be homologous, hence the need for comparative fMRI experiments. As a framework for describing fMRI activations in monkey parietal cortex, we use regions defined by myelination (Lewis and Van Essen, 2000) in the intraparietal sulcus (IPS) (black outlines in Fig. 5). These are supplemented with the four subdivisions of the inferior parietal lobule (IPL) based on cyto-architectonics, and with AIP (anterior intraparietal area), V6 and V6A based on connections and physiology (Borra et al., 2008;Gregoriou et al., 2006;Luppino et al., 2005). LOP (lateral occipital parietal area) is located adjacent to the central representation of V6 and is synonymous with CIP (Durand et al., 2007) and with CPDR (caudal parietal disparity region) of Tsao et al. (2003b). As described above, this region contains two retinotopic areas, CIP1 and CIP2 (Arcaro et al., 2011).
Figure 5.
Activation to 2D & 3D shape, 2D & 3D motion, action observation and execution, visually-guided saccade, memory retrieval and multimodal stimuli (all approximate group data) in monkey parietal cortex (F99). (A) 3D shape selectivity from disparity: from Durand et al. (2009) (red) and from Tsao et al. (2003b) (green outline). 2D shape selectivity and 2D & 3D motion: from Orban et al. (2006). Visual-auditory-tactile conjunction: from Guipponi et al. (2013). (B) Grasping: from Nelissen and Vanduffel (2011). Visual search: from Wardak et al. (2010). Memory retrieval: from Miyamoto et al. (2013). Saccade-related activity: from Baker et al. (2006) (cyan) and Koyama et al. (2004) (green dots). AIP/LIP border: from Durand et al. (2007), note that the AIP definition of Lewis &Van Essen is more restrictive (mainly anterior part projecting to SII). Action observation in AIP and PFG: from Nelissen et al. (2011). V6, V6A and V6Ad: same as in Figure 3. Black outlines indicate regions defined by myelination Lewis and Van Essen (2000) in the intraparietal sulcus (IPS). PF, PFG, PG and Opt: from Gregoriou et al. (2006).
As frontal framework we use the anatomically-defined ROIs of seven motor areas (F1-F7), FEF and areas 45A/B as defined by Gerbella et al. (2007) and Luppino and Rizzolatti (2000) (Fig. 4).
Visual fMRI responses in IPS
The passive visual fMRI paradigms revealed several distinct rostro-caudally oriented strips in posterior parietal cortex. Medial IPS and IPL responded weakly to visual stimuli, in contrast to lateral IPS (Fig. 5). This is consistent with visual inputs from MT and V4 mainly avoiding medial IPS (Felleman and Van Essen, 1991). 2D shape sensitivity, captured by intact vs scrambled images (Kourtzi and Kanwisher, 2000) and visual motion activate LOP/CIP and rostral LIP, consistent with single cell data (Sereno and Maunsell, 1998). 3D structure-from-motion sensitivity, though much weaker in monkey than in human IPS (Vanduffel et al., 2002), activates the same regions. 3D structure-from-stereo, and to a lesser degree, 3D structure-from-texture stimuli (Durand et al., 2007) activate a wider portion of the IPS, including AIP and a region in medial IPS rostral to V6A. The 3D shape stereo activation extends further rostrally than that evoked by disparity-defined skyscraper stimuli activating chiefly LOP/CIP (Tsao et al., 2003b). Wide-field optic flow activates the ventral intraparietal area (VIP), a multimodal area also driven by auditory and tactile stimuli (Guipponi et al., 2013), exactly as predicted from electrophysiology (Schlack et al., 2005).
(Oculo)motor- and cognitive-driven parietal activations
The oculomotor and cognitive paradigms used so far in monkey fMRI yielded activations restricted mainly to lateral IPS. Saccades activated LIP in several fMRI studies, including those using paradigms specifically controlling for visual stimulation (Baker et al., 2006;Durand et al., 2007;Ford et al., 2009;Kagan et al., 2010;Koyama et al., 2004), consistent with numerous single cell studies (Goldberg et al., 2006). Available evidence suggests that ventral and dorsal LIP, and perhaps PG, is activated by saccades. Consistent with the role of LIP in attention (Bisley and Goldberg, 2003), this area was activated during visual search (Atabaki et al., 2014;Wardak et al., 2010). LIP activation depends on the serial nature of the search (Atabaki et al., 2014), diminishing when the monkeys are strongly over-trained on the task (Wardak et al., 2010). Again in agreement with single cell studies (Murata et al., 2000), AIP and PFG were activated during execution of grasping, compared to touching objects (Nelissen and Vanduffel, 2011). The same two areas are also activated by observation of others’ actions (Nelissen et al., 2011), a necessary but not sufficient condition for the presence of mirror neurons, as documented by single neuron studies (Fogassi et al., 2005;Murata et al., 2000).
During a memory retrieval task Miyamoto et al. (2013) showed a parietal region in the vicinity of PG activated for first-item retrieval (Fig. 5B). This region showed fMRI-defined functional connectivity with the hippocampus, with which it is anatomically connected disynaptically. A medial IPS region near stereo-activated sites was engaged during retrieval of the last item.
(Oculo)motor-driven frontal activations
Studies by Baker et al. (2006) and Koyama et al. (2004) agree that saccades activate FEF and SEF (supplementary eye fields), as predicted from single cell studies (Schall et al., 1993). Ford et al. (2009) also showed that anti-saccades activate not only FEF but also two prefrontal regions in the dorsal principal sulcus and cingulate cortex (Fig. 4). Execution of grasping, contrasted to simple touching of the same object, activates ventral premotor cortex, F4 and the three subdivisions of F5. The latter are also engaged during observation of grasping, which also activates 45B, an area driven by viewing intact versus scrambled objects (Denys et al., 2004b;Nelissen et al., 2005). Presentation of socially relevant visual (faces) (Tsao et al., 2008b) and auditory stimuli (monkey calls) (Joly et al., 2012) activate orbitofrontal cortex, but presumably slightly different positions.
Cognitive frontal fMRI activations
Cognitive tasks with an executive component activate prefrontal regions. However, with the limited number of tasks used in fMRI, mainly regions posterior and lateral to the principal sulcus have been activated. Visual search engaged areas 46v and 45A/B, supposedly involved in spatial attention control and rule definition, respectively (Wardak et al., 2010). Area 45 was also activated by a task involving set-shifting, as in the Wisconsin test (Nakahara et al., 2002), supporting the interpretation that this area is involved in rule definition and rule change (Wardak et al., 2010).
4. Functional connectivity: resting state networks
Compared to human studies, monkey fMRI experiments can be time consuming because monkeys must be trained to fixate or perform motor and cognitive tasks. The ability to observe fMRI activation patterns in resting or anesthetized monkeys offered a considerable advantage, especially since the so-called resting state networks showed some similarity with anatomically connected regions (Vincent et al., 2007). Further studies have shown that functional connectivity underlying resting state networks relies only partially upon anatomical connections (Scholvinck et al., 2013). Monkey fMRI provides the opportunity to evaluate this rapidly expanding field of human imaging, increasingly used in patient populations. The full evaluation is beyond the scope of the present review (see Hutchison and Everling, 2012), but two aspects are worth emphasizing. First, combining functional connectivity investigations with other functional tests can be surprisingly informative. This was nicely illustrated by the aforementioned memory study in parietal cortex (Miyamoto et al., 2013), where resting state networks were used to show that the two parietal regions involved in item retrieval belonged to different functional circuits, with the PG region functionally connected to the hippocampus. Another illustration is the study of Babapoor-Farrokhran et al. (2013) showing that medial and lateral parts of FEF, which control large and small saccades, are also parts of separate functional circuits. Medial FEF is functionally connected to reaching and executive areas, lateral FEF to grasping and manipulation areas. Similarly, the fact that resting state networks routinely include both a central and peripheral visual cortex network (Mantini et al 2012, Moeller et al 2009) can be seen as an indication that early visual cortex devoted to these two visual fields belongs to different functional circuits.
Secondly, resting state studies are ideal for comparative purposes, especially between human and non-human primates (Hutchison et al., 2013). Again these studies benefit from combining resting state analysis with other functional or anatomical determinations. This is illustrated by Mantini et al. (2013) who compared resting state networks in humans and monkeys, warping them to the same template (Van Essen and Dierker, 2007) and examining temporal correlations during natural vision. They described nine cortical networks common to both species, including a heavily-modified language network, dorsal and ventral attention networks, but also three human-specific networks: cingulo-insular, and left and right frontoparietal networks.
5. Causal experiments: effective connectivity
Another area where monkey fMRI proves exquisitely useful is the study of effective connectivity. Such studies, so far mainly restricted to animals, attempt to assess causal functional interactions across remote brain regions, i.e. how a specific brain region influences activity in remotely connected regions.
Electrical microstimulation fMRI (EM-fMRI)
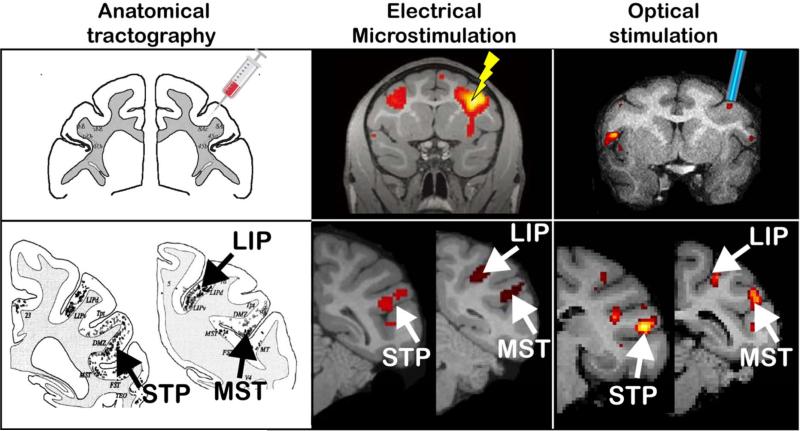
Logothetis’ group (Tolias et al., 2005) showed the feasibility of the EM-fMRI approach in anesthetized animals, using relatively high currents delivered to V1 and tracing causal microstimulation -induced effects within occipital cortex (i.e. mainly V2 and MT). Following this landmark paper, Ekstrom et al. (2008) extended the EM-fMRI approach to alert animals, stimulating an area at the other end of the visual system (FEF), while exploring the functional consequences throughout the brain. The authors specifically investigated interactions between microstimulation and sensory-driven activity by presenting visual stimuli within the stimulated FEF movement fields (Ekstrom et al., 2008;Ekstrom et al., 2009), while employing behaviorally relevant current levels (i.e. 50% of that evoking saccadic eye movements, typically < 50μA). Results demonstrated that most regions monosynaptically connected with FEF showed increased fMRI activity. Moreover, spatially-specific heterogeneous modulation of visual processing was observed in occipital cortex with regions exhibiting either enhanced or decreased activity (Ekstrom et al., 2008). The processing of low-contrast stimuli was enhanced, whereas that of high contrast stimuli was unaltered or even suppressed, resulting in a contrast-gain effect in visual cortex (Ekstrom et al., 2009). Unlike results reported by Logothetis and colleagues, the Ekstrom studies (Ekstrom et al., 2008) and a recent report from Miyashita's group (Matsui et al., 2012) showed clear evidence for polysynaptic propagation of the microstimulation signals. Indeed FEF-EM (Ekstrom et al., 2008) as well as S1-EM (Matsui et al., 2012) resulted in increased cerebellar activity. Moreover, Everling's group (Field et al., 2008) observed substantial contralateral positive fMRI signals after unilateral stimulation of the superior colliculus in an anesthetized preparation, despite the virtual absence of anatomical connections between superior colliculus and the contralateral cortex. In contrast, LGN-EM (Logothetis et al., 2010) and STS-EM (Sultan et al., 2011) elicited positive fMRI signals only in monosynaptically-connected regions and negative signals in regions polysynaptically connected to the stimulation site. Although the distinction between brain regions stimulated may explain observed differences in EM signal propagation, discrepancies may originate from the typically high (Logothetis et al., 2010) versus low stimulation currents employed (Ekstrom et al., 2008;Matsui et al., 2012).
The Miyashita group directly compared resting-state measures of functional connectivity with EM-derived measures of effective connectivity, targeting somatosensory cortex. The reported mismatch between functional connectivity and EM-derived connectivities in somatosensory cortex was predictable (Matsui et al., 2011), since EM-fMRI mainly reveals foci anatomically connected with the site of stimulation, which are predominantly ipsilateral (Ekstrom et al., 2008;Moeller et al., 2008;Tolias et al., 2005) whereas resting state-based functional connectivity typically reveals bilateral networks, underscoring the loose relationship between resting- state networks and anatomical connections.
Optogenetic stimulation and fMRI: OS-fMRI
Optogenetics is a recently-developed tool allowing control of cell-type-specific neuronal activity on a millisecond time-scale. Optogenetics, combined with fMRI (OS-fMRI), was pioneered in rodents and showed network-wide modulation of fMRI activity induced by focal optogenetic modulation (for review see Gerits and Vanduffel, 2013). Only two primate studies, so far, have combined fMRI with optogenetics (Gerits et al., 2012;Ohayon et al., 2013). Although both targeted the FEF, only the former study revealed fMRI activation patterns consistent with the anatomical connectivity of FEF, as established by traditional tract-tracing experiments and fMRI activations induced by EM (Gerits and Vanduffel, 2013) (Fig. 6). In both studies, a viral vector was used where the AAV5 serotype was combined with depolarizing opsins (ChR2). These studies differed in optical stimulation frequencies (40Hz vs 80 Hz), and promotors (neuron-type aspecific CAG versus a neuron-type a-specific hSyn or a CaMKII specific for excitatory cells). It is noteworthy that the Gerits et al. (2012) study revealed optogenetic-induced changes in saccade latencies while the Ohayon et al. (2013) study revealed behavioral changes only when OS was combined with EM. The latter authors concluded that optogenetics induces subthreshold activity in neurons too weak to evoke motor responses. Yet several primate studies find clear optogenetic-induced behavioral effects (for review see Gerits and Vanduffel, 2013), underscoring the importance of choosing experimental parameters (type, tropism, titer, and promotor of the viral vector, stimulation parameters) to sufficiently drive the neurons and to produce measurable behavioral effects and network-wide neuronal responses.
Figure 6.
Comparison of functional connectivity obtained with electrical EM-fMRI (Ekstrom et al., 2008) and OS-fMRI (Gerits et al., 2012), and anatomical connectivity with traditional tract tracing (Schall et al., 1995). FEF Injection, EM or OS resulted in labeled cells or enhanced activations in the lateral intraparietal area (LIP), the media superior temporal area (MST) and the superior temporal polysensory area (STP). Adapted with permission from Gerits and Vanduffel (2013).
With the advent of novel increasingly more powerful genetic tools, we predict that fMRI combined with (opto)genetics will become indispensable to unravel brain-wide network interactions in nonhuman primates.
Reversible inactivations and fMRI
Smirnakis et al. (2005) used fMRI to investigate cortical plasticity induced by retinal lesions. Unlike previous reports (e.g. Heinen and Skavenski, 1991), these authors observed surprisingly little functional reorganization in V1 following retinal injury in adult monkeys. More recently, however, the same group showed modest changes in population receptive fields in V1 of a monkey afflicted with macular degeneration, in which the largest functional changes were observed in area MT, suggesting that functional reorganization after retinal damage might primarily affect higher-order cortex (Shao et al., 2013). When V1 was lesioned, Schmid et al. (2009) found persistent, mildly-reduced fMRI activity but largely preserved retinotopy in the lesion-projection zone in V2 and V3, suggesting that the activation in the V2/V3 lesion-projection zone depends little upon V1 input. A later fMRI study confirmed that these responses in the lesion-projection zone in higher-order areas depend on LGN input, bypassing V1 (Schmid et al., 2010).
Ungerleider's group investigated the effect of presumptive feedback connections onto IT cortex by reversible lesions of the amygdala while monkeys viewed images of emotional and neutral faces. They elegantly demonstrated that valence effects in IT are selectively disrupted after amygdala inactivation, but not face responses as such (Hadj-Bouziane et al., 2012). Finally, Wilke et al. (2012) and Gerits et al. (2009) investigated the effects of reversible LIP lesions on brain-wide fMRI activity and performance during a memory-guided saccade and search task, respectively. While the first study reported both reduced and increased fMRI activity at distant sites, the latter exclusively found increased activity in functional networks activated during the search task. Hence, both studies show rapid functional network adaptations after lesions.
6. Comparative monkey and human fMRI
Retinotopic regions
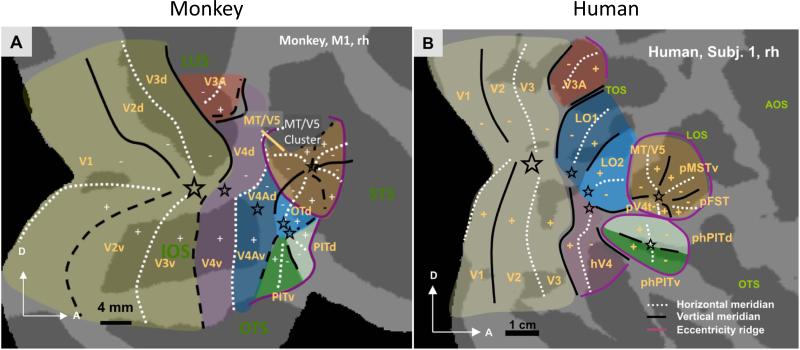
Correspondences between retinotopic areas in humans and monkeys are straightforward for areas V1, V2 and V3. The topography of human hV4, however, differs significantly from monkey V4 as it covers a complete hemifield confined to the ventral occipital surface, with the V3v-hV4 border comprising the upper vertical meridian and its anterior border the lower vertical meridian (Fig. 7). Monkey V4, in contrast, is a split-field representation, spanning portions of the dorsal and ventral occipital surfaces. Initially, discrepancies in the organizations of V4 and hV4 hampered direct comparisons of monkey and human retinotopy in much of anterior extrastriate visual cortex. However, more recently a picture has begun to emerge linking extrastriate visual field maps in the two species. For example, monkey PITd/PITv and human phPITd/phPITv, as well as the MT clusters in the two species, show similarities in their topographies, response properties and population receptive fields, suggesting a homology (Kolster et al., 2010). The latter authors showed that the so-called human motion area in occipitotemporal cortex (hMT+) includes the whole MT cluster plus more rostral motion regions, perhaps homologous to LST and STPm. Finally, similar population receptive fields and functional criteria imply correspondences between V4A and human LO1, and OTd and human LO2 (Kolster et al., 2014).
Figure 7.
Comparison of retinotopic layout of human and monkey visual cortex. Adapted with permission from Kolster et al. (2010) and Kolster et al. (2014). Stars indicate foveal representations.
Non-retinotopic regions
Several comparative studies have used the warping technique of Van Essen and colleagues (Van Essen and Dierker, 2007) to correlate fMRI activations in human and monkey non-retinotopic visual cortex. Using such methods, Denys et al. (2004a) compared the lateral occipital cortex in humans (Malach et al., 1995) with the corresponding part of monkey IT. In monkeys, cortex lateral to the STS mainly displayed shape sensitivity with this lateral occipital complex localizer (hatched region in Fig. 1C). Homologies of the body and face-processing modules of humans and monkeys have also been inferred primarily from their topographic locations (Rajimehr et al., 2009;Tsao et al., 2008a). Such topological criteria suggest correspondences between the midSTS body patch and human EBA (extrastriate body area), and between the antSTS body patches and FBA (fusiform body area) (Fig. 3).
Homologies of face patches are less obvious. In monkeys the face patches are all found near (or within) the STS while in the human this holds true only for the posterior and anterior STS face areas (Fig. 3), supporting the idea that STS is functionally fairly dissimilar in both species. Based on biological motion sensitivities, Jastorff et al. (2012) and Jastorff and Orban (2009) suggested a possible homology between the anterior two-thirds of the lower bank of macaque STS and human posterior inferior temporal and middle fusiform gyri. They suggested that the upper bank of macaque STS corresponds with the cortex swathing the posterior human MTG (middle temporal gyrus), STS and STG (superior temporal gyrus) (Fig. 3). We propose a refinement whereby the most anterior part of human STS, selective for faces (aSTS in Fig. 3), also corresponds to anterior monkey STS (probably corresponding to AF and AD). The drive for these changes (arrows in Fig 3) is the expansion of auditory cortex into the anterior part of human STS starting from the STG. Indeed, the region between the posterior and anterior face patches in human STS has evolved much greater sensitive for auditory stimuli, such as vocalizations (black lines) and intelligible speech (dashed yellow outlines, Fig. 3B) without having a clear counterpart in monkey STS (black outline in STG, Fig. 3B) (Joly et al., 2012). These evolutionary changes of the STS may suggest homologies for the MF and AF/AD patches but do not speak to the homologies of the lateral patches. Instead, Janssens et al. (2014) showed that monkey PL is located within monkey PITd. The vicinity of published coordinates of human PITd and OFA (occipital face area), provides corroborating evidence of homologies between human and monkey PITd/v, and between monkey PL and human OFA (Fig. 3). Using Van Essen's warping strategy to match the face patches of humans and monkeys, Tsao et al. (2008a) suggested that FFA may correspond to ML. Using identical criteria, Rajimehr et al. (2009) matched the anterior monkey face (deemed ATFP but probably corresponding to AL) with a human anterior temporal fusiform face patch (ATFP in Fig. 3). Alternatively the topological relation with the body patches may suggest that ML and AL correspond to OFA and FFA respectively. Neural representations for face views were also found similar between FFA and AL (invariant for mirror-symmetric views), and between OFA and ML (viewpoint specific) (Axelrod and Yovel, 2012; Freiwald and Tsao, 2010). Hence further work is needed to solve the issue of homology of face patches, especially using alternative analytical strategies quantifying similarity in the time domain without spatial assumptions, e.g. (Mantini et al., 2012).
Parietal & frontal cortex
Initially, imaging of human and monkey cortex using identical paradigms suggested pronounced interspecies differences in posterior parietal cortex. Vanduffel et al. (2002) reported that 3D-structure-from-motion stimuli drove many more regions in human than in monkey posterior parietal cortex, and human activations were invariably stronger. A comparison of simple translation to static stimuli revealed similar cross-species differences in parietal cortex. Such differences imply that the monkey would be a relatively poor animal model for human posterior parietal cortex. However, this may be only partially true. Using a series of 7 functional criteria, Durand et al. (2009) showed that motion-sensitive regions DIPSM (dorsal IPS medial) and DIPSA (dorsal IPS anterior) (Sunaert et al., 1999) were the likely human counterparts of anterior LIP and posterior AIP, respectively. One of the most striking interspecies similarities was the activation by saccades in LIP/DIPSM and its absence in AIP/DIPSA. DIPSA, located posterior to phAIP (Fig. 3B), is considered the human homologue of monkey AIP based on motor and multisensory activation criteria. As in the monkey, the somatosensory input also increases rostrally in AIP (Borra et al., 2008). Therefore, Orban et al. (2006) proposed that DIPSA and phAIP together represent the equivalent of monkey AIP, with DIPSA corresponding to its more visual, posterior part and phAIP to its more somatosensory and motor, anterior part. These results relay an important message concerning any strategy for ascertaining homologies across primates: the more functional the criteria used in the comparison, the more accurate the assessment (Orban et al., 2004). For the AIP/LIP homologies only 1/7 criteria, 3D structure from motion, differed, with the majority supporting homology. It is worth mentioning that the proposed homology fits the general view of Grefkes and Fink (2005) that monkey lateral IPS regions have migrated to the medial wall of human IPS consequent to IPL expansion in humans.
More caudally in posterior parietal cortex, Durand et al. (2009) and Georgieva et al. (2009) speculated that VIPS (ventral IPS), which overlaps area V7, and another area (tentatively called V7a), sharing its central representation, corresponds to CIP. This possible homology is further supported by the finding that monkey CIP also contains two areas sharing a central representation (Arcaro et al., 2011;Janssens et al., 2013) and that CIP, like VIPS, is located between the central representation of V6 and a large cortical expanse representing the peripheral visual field.
To re-evaluate these homologies within the IPS0-5 framework using polar angle analyses to differentiate areas (Silver and Kastner, 2009), we propose that V7 and V7A correspond to IPS0/1, while DIPSA may correspond to IPS4/5 and DIPSM to IPS3/4 (Durand et al., 2009). Thus comparative imaging studies suggest homologies for several lateral IPS monkey areas, yet also provide evidence for species differences in parietal cortex. In humans, the anterior left supramarginal gyrus is driven by observation of tool actions but not hand actions with similar goals. This characteristic pattern is not observed in monkey IPL, even after extensive training in tool use, suggesting that this functional difference might underlie behavioral differences between human and monkey tool use (Peeters et al., 2009). Human tool use, unlike that of monkeys, is frequent and spontaneous, and based on causal understanding. The view that the human anterior supramarginal gyrus is uniquely specialized rests on a single functional test and a comparison of two species. Nonetheless, the lateralization of this area and its location in the IPL which is greatly expanded in humans (Van Essen and Dierker, 2007), makes it plausible that the anterior supramarginal gyrus is a typical human area.
Far less progress has been made in frontal cortex, for reasons indicated above. It is worth mentioning that monkey, but not human, prefrontal cortex, or at least its ventro-lateral part, is readily activated by visual (Denys et al., 2004b;Nelissen et al., 2005) or auditory (Joly et al., 2012) stimuli. Regarding specific areas, evidence for homology is still scant, with the notable exception of FEF, the human counterpart of which has tentatively identified on the anterior bank of the precentral sulcus, at its junction with the superior frontal sulcus (Luna et al., 1998).
From single units via monkey fMRI to human imaging: integration of techniques
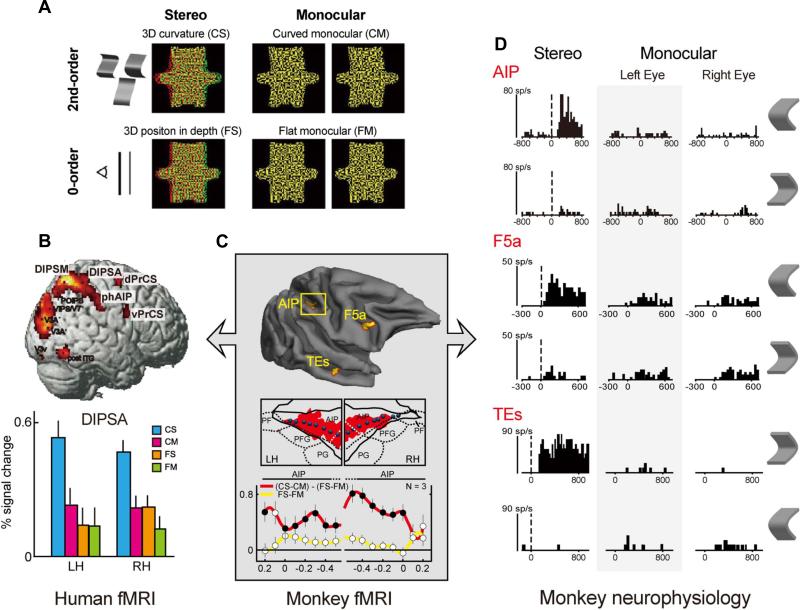
So far we have discussed monkey fMRI combined with single cell recordings, and parallel functional imaging comparing human and monkey. Yet these combinations are only two aspects of a three-pronged strategy linking single cell recordings in monkeys with human fMRI. Unquestionably, direct comparison between the extremes is difficult because monkey single cell recordings and human fMRI differ in species and technique. These confounds can be disentangled by comparing single cells with fMRI in monkeys, addressing the effect of technique, and then fMRI in humans and monkeys, to investigate species differences. Hitherto, few studies have attempted to close this circle. For 3-dimensional structure-from-disparity, this is illustrated in Fig. 8. The interaction between factors stereo and curvature reaches significance in three areas (TEs, AIP and F5a) in the monkey (Fig. 8C), all hosting higher-order disparity-selective neurons (Fig. 8D), i.e. neurons selective for the 3D shape specified by disparity (Janssen et al., 2000;Srivastava et al., 2009;Theys et al., 2013). Predictably, based on homologies described above, the interaction was also significant in human DIPSA, phAIP and a ventral premotor region, possibly corresponding to F5a (Fig. 8B). Thus, this series of studies suggests that DIPSA, phAIP and ventral premotor cortex host higher-order disparity-selective cells, much like those described in the monkey.
Figure 8.
3D shape from disparity: comparison of single unit selectivity with human and monkey imaging (real data). (A) Stimuli used in the 3 types of experiments. (B) Human fMRI activation in right hemisphere and activity profile for DIPSA showing the interaction effect (see text): based on Durand et al. (2009). (C) Monkey 3D-shape selectivity (interaction effect) with activity profiles along a path within the IPS: based on Durand et al. (2007). (D) Example of single cell 3D-shape selectivity in AIP (Srivastava et al., 2009), F5a (Theys et al., 2013) and TEs (Janssen et al., 2000).
Secondly, monkey MT and FST neurons are selective for 3D shape-from-motion that can be captured by comparing random lines rotating in depth to the same lines translating in the fronto-parallel plane (Mysore et al., 2010). With monkey fMRI, this subtraction activates MT and FST (Vanduffel et al., 2002) and in humans the same subtraction also drives human MT (Kolster et al., 2010), suggesting that 3D shape-from-motion selective neurons are present in human MT. Finally, the homology between monkey and human face patches proposed above, combined with physiological studies of these patches (see above) suggest that human OFA and FFA contain face-selective neurons. These results indicate that monkey fMRI is a unique tool enhancing the value of the monkey model for human brain function, as it indicates its validity at the neuronal and area levels.
Prospects
Monkey imaging is a growing field, now attracting interest from hardcore electrophysiologists acknowledging that fMRI-guided recordings can vastly increase productivity by mitigating the needle-in-a-haystack problem. To learn how information is transformed in each processing node within a given functional network, one needs high-band multi-site electrophysiological recordings. Unlike unguided electrophysiological strategies covering substantial parts of the cortex (Bosman et al., 2012), imaging could restrict these parallel recordings precisely to the regions of interest in the same subjects. We predict that improving imaging capabilities (e.g. using implanted phased array coils, micro-coils, novel (non)genetic fast MRI sensors (e.g. Jasanoff, 2012)), thereby increasing spatial and temporal resolution up to columnar and laminar levels, will allow parallel targeting of many sites of interest with increasing precision and accuracy. In addition, non-classical analytical approaches, such as multivoxel pattern analysis (Haxby et al., 2001) and inter-species activity correlation (Mantini et al., 2012), or using simultaneously acquired physiological data as regressors as meticulously pioneered by Logothetis et al. (2012), will increase the quantity and quality of information obtained by imaging, raising novel questions to be addressed with electrophysiology or comparative human imaging experiments. Furthermore, it will become imperative to obtain multiple, independent lines of evidence, such as combined retinotopic, functional, connectional and possibly detailed anatomical (e.g. myelin density) information, in formulating conclusions about specific parcellation schemes (Janssens et al., 2013) or homologies across species. To link activity in micro and macro circuitries to behavior, we also predict an increasing use of genetically tractable primate species, such as marmosets, in imaging experiments (Liu et al., 2013). Finally, one very promising avenue combines monkey imaging with state-of-the-art causal perturbation techniques such as pharmacology (Arsenault et al., 2013;Nelissen et al., 2012), optogenetics (Gerits and Vanduffel, 2013), designer receptors exclusively activated by designer drugs (Lee et al., 2013) or other genetic tools such as the double-infection techniques (Kinoshita et al., 2012), potentially unraveling network interactions at an unprecedented scale in an animal model crucial to understand the human brain. It should be emphasized that for ethical reasons these very precise reversible perturbation methods will never be applicable in normal human subjects, hence the prodigious need for an animal model close to the human.
Acknowledgements
We thank C. Galletti, Y. Miyashita, K. Miyamoto, T. Osada, Y. Adachi, R Tootell and S. Nasr for sharing their published data, and S. Raiguel for his comments on the manuscript. This work received support from Inter-University Attraction Pole 7/11, Odysseus G0007.12, Programme Financing PFV/10/008, Impulsfinanciering Zware Apparatuur and Hercules funding of the KU Leuven, Fonds Wetenschappelijk Onderzoek–Vlaanderen G0A5613N, G043912N, G062208N10, G059309N10, G083111N10, and K714811N. QZ is post-doctoral fellow of FWO-Vlaanderen. The Martinos Center for Biomedical Imaging is supported by National Center for Research Resources grant P41RR14075.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arcaro MJ, Pinsk MA, Li X, Kastner S. Visuotopic organization of macaque posterior parietal cortex: a functional magnetic resonance imaging study. J. Neurosci. 2011;31:2064–2078. doi: 10.1523/JNEUROSCI.3334-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault JT, Nelissen K, Jarraya B, Vanduffel W. Dopaminergic reward signals selectively decrease fMRI activity in primate visual cortex. Neuron. 2013;77:1174–1186. doi: 10.1016/j.neuron.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabaki A, Marciniak K, Dicke PW, Karnath HO, Thier P. Parietal blood oxygenation level-dependent response evoked by covert visual search reflects set-size effect in monkeys. Eur. J. Neurosci. 2014;39(5):832–40. doi: 10.1111/ejn.12427. [DOI] [PubMed] [Google Scholar]
- Babapoor-Farrokhran S, Hutchison RM, Gati JS, Menon RS, Everling S. Functional connectivity patterns of medial and lateral macaque frontal eye fields reveal distinct visuomotor networks. J. Neurophysiol. 2013;109:2560–2570. doi: 10.1152/jn.01000.2012. [DOI] [PubMed] [Google Scholar]
- Baker JT, Patel GH, Corbetta M, Snyder LH. Distribution of activity across the monkey cerebral cortical surface, thalamus and midbrain during rapid, visually guided saccades. Cereb. Cortex. 2006;16:447–459. doi: 10.1093/cercor/bhi124. [DOI] [PubMed] [Google Scholar]
- Bell AH, Hadj-Bouziane F, Frihauf JB, Tootell RB, Ungerleider LG. Object representations in the temporal cortex of monkeys and humans as revealed by functional magnetic resonance imaging. J. Neurophysiol. 2009;101:688–700. doi: 10.1152/jn.90657.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AH, Malecek NJ, Morin EL, Hadj-Bouziane F, Tootell RB, Ungerleider LG. Relationship between functional magnetic resonance imaging-identified regions and neuronal category selectivity. J. Neurosci. 2011;31:12229–12240. doi: 10.1523/JNEUROSCI.5865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmalih A, Borra E, Contini M, Gerbella M, Rozzi S, Luppino G. A multiarchitectonic approach for the definition of functionally distinct areas and domains in the monkey frontal lobe. J. Anat. 2007;211:199–211. doi: 10.1111/j.1469-7580.2007.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: a quantitative receptive field analysis. Exp. Brain Res. 2001;140:127–144. doi: 10.1007/s002210100785. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. The role of the parietal cortex in the neural processing of saccadic eye movements. Adv. Neurol. 2003;93:141–157. [PubMed] [Google Scholar]
- Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991;353:429–431. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
- Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb. Cortex. 2008;18:1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De WP, Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75:875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer AA, Press WA, Logothetis NK, Wandell BA. Visual areas in macaque cortex measured using functional magnetic resonance imaging. J. Neurosci. 2002;22:10416–10426. doi: 10.1523/JNEUROSCI.22-23-10416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J. Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Conway BR, Moeller S, Tsao DY. Specialized color modules in macaque extrastriate cortex. Neuron. 2007;56:560–573. doi: 10.1016/j.neuron.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys K, Vanduffel W, Fize D, Nelissen K, Peuskens H, Van Essen D, Orban GA. The processing of visual shape in the cerebral cortex of human and nonhuman primates: a functional magnetic resonance imaging study. J. Neurosci. 2004a;24:2551–2565. doi: 10.1523/JNEUROSCI.3569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys K, Vanduffel W, Fize D, Nelissen K, Sawamura H, Georgieva S, Vogels R, Van Essen D, Orban GA. Visual activation in prefrontal cortex is stronger in monkeys than in humans. J. Cogn Neurosci. 2004b;16:1505–1516. doi: 10.1162/0898929042568505. [DOI] [PubMed] [Google Scholar]
- Dubowitz DJ, Chen DY, Atkinson DJ, Grieve KL, Gillikin B, Bradley WG, Jr., Andersen RA. Functional magnetic resonance imaging in macaque cortex. Neuroreport. 1998;9:2213–2218. doi: 10.1097/00001756-199807130-00012. [DOI] [PubMed] [Google Scholar]
- Durand JB, Nelissen K, Joly O, Wardak C, Todd JT, Norman JF, Janssen P, Vanduffel W, Orban GA. Anterior regions of monkey parietal cortex process visual 3D shape. Neuron. 2007;55:493–505. doi: 10.1016/j.neuron.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand JB, Peeters R, Norman JF, Todd JT, Orban GA. Parietal regions processing visual 3D shape extracted from disparity. Neuroimage. 2009;46:1114–1126. doi: 10.1016/j.neuroimage.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Ekstrom LB, Roelfsema PR, Arsenault JT, Bonmassar G, Vanduffel W. Bottom-up dependent gating of frontal signals in early visual cortex. Science. 2008;321:414–417. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom LB, Roelfsema PR, Arsenault JT, Kolster H, Vanduffel W. Modulation of the contrast response function by electrical microstimulation of the macaque frontal eye field. J. Neurosci. 2009;29:10683–10694. doi: 10.1523/JNEUROSCI.0673-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell AD, McCarthy G. Probabilistic atlases for face and biological motion perception: an analysis of their reliability and overlap. Neuroimage. 2013;74:140–151. doi: 10.1016/j.neuroimage.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattori P, Pitzalis S, Galletti C. The cortical visual area V6 in macaque and human brains. J. Physiol Paris. 2009;103:88–97. doi: 10.1016/j.jphysparis.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebr. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Field CB, Johnston K, Gati JS, Menon RS, Everling S. Connectivity of the primate superior colliculus mapped by concurrent microstimulation and event-related FMRI. PLoS. One. 2008;3:e3928. doi: 10.1371/journal.pone.0003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fize D, Vanduffel W, Nelissen K, Denys K, Chef d'Hotel C, Faugeras O, Orban GA. The retinotopic organization of primate dorsal V4 and surrounding areas: A functional magnetic resonance imaging study in awake monkeys. J. Neurosci. 2003;23:7395–7406. doi: 10.1523/JNEUROSCI.23-19-07395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Ford KA, Gati JS, Menon RS, Everling S. BOLD fMRI activation for anti-saccades in nonhuman primates. Neuroimage. 2009;45:470–476. doi: 10.1016/j.neuroimage.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Freiwald WA, Tsao DY. Functional compartmentalization and viewpoint generalization within the macaque face-processing system. Science. 2010;330:845–851. doi: 10.1126/science.1194908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiwald WA, Tsao DY, Livingstone MS. A face feature space in the macaque temporal lobe. Nat. Neurosci. 2009;12:1187–1196. doi: 10.1038/nn.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva S, Peeters R, Kolster H, Todd JT, Orban GA. The processing of three-dimensional shape from disparity in the human brain. J. Neurosci. 2009;29:727–742. doi: 10.1523/JNEUROSCI.4753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G. Multimodal architectonic subdivision of the caudal ventrolateral prefrontal cortex of the macaque monkey. Brain Struct. Funct. 2007;212:269–301. doi: 10.1007/s00429-007-0158-9. [DOI] [PubMed] [Google Scholar]
- Gerits A, Farivar R, Rosen BR, Wald LL, Boyden ES, Vanduffel W. Optogenetically induced behavioral and functional network changes in primates. Curr. Biol. 2012;22:1722–1726. doi: 10.1016/j.cub.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerits A, Vanduffel W. Optogenetics in primates: a shining future? Trends Genet. 2013;29:403–411. doi: 10.1016/j.tig.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Gerits A, Wardak C, Kolster H, Arsenault J, Orban GA, Vanduffel W. Behavioral and brain-wide functional consequences of reversible LIP inactivation during visual search. Soc.Neurosci. 2009 Abstr.. [39], 803.13. [Google Scholar]
- Goda N, Tachibana A, Okazawa G, Komatsu H. Representation of the material properties of objects in the visual cortex of nonhuman primates. J. Neurosci. 2014;34:2660–2673. doi: 10.1523/JNEUROSCI.2593-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Bisley JW, Powell KD, Gottlieb J. Saccades, salience and attention: the role of the lateral intraparietal area in visual behavior. Prog. Brain Res. 2006;155:157–175. doi: 10.1016/S0079-6123(06)55010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J. Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Borra E, Matelli M, Luppino G. Architectonic organization of the inferior parietal convexity of the macaque monkey. J. Comp Neurol. 2006;496:422–451. doi: 10.1002/cne.20933. [DOI] [PubMed] [Google Scholar]
- Gross CG, Rocha-Miranda CE, Bender DB. Visual properties of neurons in inferotemporal cortex of the Macaque. J. Neurophysiol. 1972;35:96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- Guipponi O, Wardak C, Ibarrola D, Comte JC, Sappey-Marinier D, Pinede S, Ben Hamed S. Multimodal convergence within the intraparietal sulcus of the macaque monkey. J. Neurosci. 2013;33:4128–4139. doi: 10.1523/JNEUROSCI.1421-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj-Bouziane F, Bell AH, Knusten TA, Ungerleider LG, Tootell RB. Perception of emotional expressions is independent of face selectivity in monkey inferior temporal cortex. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5591–5596. doi: 10.1073/pnas.0800489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj-Bouziane F, Liu N, Bell AH, Gothard KM, Luh WM, Tootell RB, Murray EA, Ungerleider LG. Amygdala lesions disrupt modulation of functional MRI activity evoked by facial expression in the monkey inferior temporal cortex. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E3640–E3648. doi: 10.1073/pnas.1218406109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Goda N, Ogawa T, Ito M, Toyoda H, Sadato N, Komatsu H. Distribution of colour-selective activity in the monkey inferior temporal cortex revealed by functional magnetic resonance imaging. Eur. J. Neurosci. 2009;30:1960–1970. doi: 10.1111/j.1460-9568.2009.06995.x. [DOI] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–490. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Konishi S, Hasegawa I, Miyashita Y. Mapping of somatosensory cortices with functional magnetic resonance imaging in anaesthetized macaque monkeys. Eur. J. Neurosci. 1999;11:4451–4456. doi: 10.1046/j.1460-9568.1999.00892.x. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Skavenski AA. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Exp. Brain Res. 1991;83:670–674. doi: 10.1007/BF00229845. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Everling S. Monkey in the middle: why non-human primates are needed to bridge the gap in resting-state investigations. Front Neuroanat. 2012;6:29. doi: 10.3389/fnana.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum. Brain Mapp. 2013;34:2154–2177. doi: 10.1002/hbm.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa EB, DiCarlo JJ. Precedence of the eye region in neural processing of faces. J. Neurosci. 2012;32:16666–16682. doi: 10.1523/JNEUROSCI.2391-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa EB, Papanastassiou AM, DiCarlo JJ. Large-scale, high-resolution neurophysiological maps underlying FMRI of macaque temporal lobe. J. Neurosci. 2013;33:15207–15219. doi: 10.1523/JNEUROSCI.1248-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P, Vogels R, Orban GA. Selectivity for 3D shape that reveals distinct areas within macaque inferior temporal cortex. Science. 2000;288:2054–2056. doi: 10.1126/science.288.5473.2054. [DOI] [PubMed] [Google Scholar]
- Janssens T, Arsenault J, Polimeni JR, Vanduffel W. Definition of the macaque posterior parietal regions using MRI-based measures of retinotopy, connectivity, myelination, and function. Soc.Neurosci. 2013 Abstr. 64.04. [Google Scholar]
- Janssens T, Keil B, Farivar R, McNab JA, Polimeni JR, Gerits A, Arsenault JT, Wald LL, Vanduffel W. An implanted 8-channel array coil for high-resolution macaque MRI at 3T. Neuroimage. 2012;62:1529–1536. doi: 10.1016/j.neuroimage.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens T, Zhu Q, Popivanov ID, Vanduffel W. Probalistic and single-subject retinotopic maps reveal the topographic organization of face patches in the macaque cortex. J. Neurosci. 2014 doi: 10.1523/JNEUROSCI.2914-13.2013. In press. DOI:10.1523/JNEUROSCI.2914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasanoff A. Adventures in neurobioengineering. ACS Chem. Neurosci. 2012;3:575. doi: 10.1021/cn300102p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastorff J, Begliomini C, Fabbri-Destro M, Rizzolatti G, Orban GA. Coding observed motor acts: different organizational principles in the parietal and premotor cortex of humans. J. Neurophysiol. 2010;104:128–140. doi: 10.1152/jn.00254.2010. [DOI] [PubMed] [Google Scholar]
- Jastorff J, Orban GA. Human functional magnetic resonance imaging reveals separation and integration of shape and motion cues in biological motion processing. J. Neurosci. 2009;29:7315–7329. doi: 10.1523/JNEUROSCI.4870-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastorff J, Popivanov ID, Vogels R, Vanduffel W, Orban GA. Integration of shape and motion cues in biological motion processing in the monkey STS. Neuroimage. 2012;60:911–921. doi: 10.1016/j.neuroimage.2011.12.087. [DOI] [PubMed] [Google Scholar]
- Joly O, Pallier C, Ramus F, Pressnitzer D, Vanduffel W, Orban GA. Processing of vocalizations in humans and monkeys: a comparative fMRI study. Neuroimage. 2012;62:1376–1389. doi: 10.1016/j.neuroimage.2012.05.070. [DOI] [PubMed] [Google Scholar]
- Kagan I, Iyer A, Lindner A, Andersen RA. Space representation for eye movements is more contralateral in monkeys than in humans. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7933–7938. doi: 10.1073/pnas.1002825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, Watanabe D, Kobayashi K, Isa T. Genetic dissection of the circuit for hand dexterity in primates. Nature. 2012;487:235–238. doi: 10.1038/nature11206. [DOI] [PubMed] [Google Scholar]
- Kolster H, Janssens T, Orban GA, Vanduffel W. The retinotopic organization of macaque occipitotemporal cortex anterior to V4 and caudo-ventral to the MT cluster. J. Neurosci. 2014 doi: 10.1523/JNEUROSCI.3288-13.2014. In Press. DOI:10.1523/JNEUROSCI.3288-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolster H, Mandeville JB, Arsenault JT, Ekstrom LB, Wald LL, Vanduffel W. Visual field map clusters in macaque extrastriate visual cortex. J. Neurosci. 2009;29:7031–7039. doi: 10.1523/JNEUROSCI.0518-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolster H, Peeters R, Orban GA. The retinotopic organization of the human middle temporal area MT/V5 and its cortical neighbors. J. Neurosci. 2010;30:9801–9820. doi: 10.1523/JNEUROSCI.2069-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblith S, Cheng X, Ohayon S, Tsao DY. A network for scene processing in the macaque temporal lobe. Neuron. 2013;79:766–781. doi: 10.1016/j.neuron.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Cortical regions involved in perceiving object shape. J. Neurosci. 2000;20:3310–3318. doi: 10.1523/JNEUROSCI.20-09-03310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- Ku SP, Tolias AS, Logothetis NK, Goense J. fMRI of the face-processing network in the ventral temporal lobe of awake and anesthetized macaques. Neuron. 2011;70:352–362. doi: 10.1016/j.neuron.2011.02.048. [DOI] [PubMed] [Google Scholar]
- Lafer-Sousa R, Conway BR. Parallel, multi-stage processing of colors, faces and shapes in macaque inferior temporal cortex. Nat. Neurosci. 2013;16:1870–1878. doi: 10.1038/nn.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Giguere PM, Roth BL. DREADDs: novel tools for drug discovery and development. Drug Discov. Today. 2013 doi: 10.1016/j.drudis.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J. Comp Neurol. 2000;428:79–111. doi: 10.1002/1096-9861(20001204)428:1<79::aid-cne7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Liu JV, Hirano Y, Nascimento GC, Stefanovic B, Leopold DA, Silva AC. fMRI in the awake marmoset: somatosensory-evoked responses, functional connectivity, and comparison with propofol anesthesia. Neuroimage. 2013;78:186–195. doi: 10.1016/j.neuroimage.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N, Merkle H, Augath M, Trinath T, Ugurbil K. Ultra high-resolution fMRI in monkeys with implanted RF coils. Neuron. 2002;35:227–242. doi: 10.1016/s0896-6273(02)00775-4. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Augath M, Murayama Y, Rauch A, Sultan F, Goense J, Oeltermann A, Merkle H. The effects of electrical microstimulation on cortical signal propagation. Nat. Neurosci. 2010;13:1283–1291. doi: 10.1038/nn.2631. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard HC, Besserve M, Oeltermann A. Hippocampal-cortical interaction during periods of subcortical silence. Nature. 2012;491:547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Peled S, Pauls J. Development and Application of fMRI for Visual Studies in Monkeys. Soc.Neurosci. 1998 Abstr.USA, 28. [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA. Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb. Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Luppino G, Ben Hamed S, Gamberini M, Matelli M, Galletti C. Occipital (V6) and parietal (V6A) areas in the anterior wall of the parieto-occipital sulcus of the macaque: a cytoarchitectonic study. Eur. J. Neurosci. 2005;21:3056–3076. doi: 10.1111/j.1460-9568.2005.04149.x. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rizzolatti G. The Organization of the Frontal Motor Cortex. News Physiol Sci. 2000;15:219–224. doi: 10.1152/physiologyonline.2000.15.5.219. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Romani GL, Orban GA, Vanduffel W. Evolutionarily Novel Functional Networks in the Human Brain? J. Neurosci. 2013;33:3259–3275. doi: 10.1523/JNEUROSCI.4392-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Hasson U, Betti V, Perrucci MG, Romani GL, Corbetta M, Orban GA, Vanduffel W. Interspecies activity correlations reveal functional correspondence between monkey and human brain areas. Nat. Methods. 2012;9:277–282. doi: 10.1038/nmeth.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Koyano KW, Tamura K, Osada T, Adachi Y, Miyamoto K, Chikazoe J, Kamigaki T, Miyashita Y. FMRI activity in the macaque cerebellum evoked by intracortical microstimulation of the primary somatosensory cortex: evidence for polysynaptic propagation. PLoS. One. 2012;7:e47515. doi: 10.1371/journal.pone.0047515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Tamura K, Koyano KW, Takeuchi D, Adachi Y, Osada T, Miyashita Y. Direct comparison of spontaneous functional connectivity and effective connectivity measured by intracortical microstimulation: an fMRI study in macaque monkeys. Cereb. Cortex. 2011;21:2348–2356. doi: 10.1093/cercor/bhr019. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Osada T, Adachi Y, Matsui T, Kimura HM, Miyashita Y. Functional differentiation of memory retrieval network in macaque posterior parietal cortex. Neuron. 2013;77:787–799. doi: 10.1016/j.neuron.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Moeller S, Freiwald WA, Tsao DY. Patches with links: a unified system for processing faces in the macaque temporal lobe. Science. 2008;320:1355–1359. doi: 10.1126/science.1157436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J. Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Mysore SG, Vogels R, Raiguel SE, Todd JT, Orban GA. The selectivity of neurons in the macaque fundus of the superior temporal area for three-dimensional structure from motion. J. Neurosci. 2010;30:15491–15508. doi: 10.1523/JNEUROSCI.0820-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara K, Hayashi T, Konishi S, Miyashita Y. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- Nasr S, Liu N, Devaney KJ, Yue X, Rajimehr R, Ungerleider LG, Tootell RB. Scene-selective cortical regions in human and nonhuman primates. J. Neurosci. 2011;31:13771–13785. doi: 10.1523/JNEUROSCI.2792-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen K, Borra E, Gerbella M, Rozzi S, Luppino G, Vanduffel W, Rizzolatti G, Orban GA. Action observation circuits in the macaque monkey cortex. J. Neurosci. 2011;31:3743–3756. doi: 10.1523/JNEUROSCI.4803-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen K, Jarraya B, Arsenault JT, Rosen BR, Wald LL, Mandeville JB, Marota JJ, Vanduffel W. Neural correlates of the formation and retention of cocaine-induced stimulus-reward associations. Biol. Psychiatry. 2012;72:422–428. doi: 10.1016/j.biopsych.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Luppino G, Vanduffel W, Rizzolatti G, Orban GA. Observing others: multiple action representation in the frontal lobe. Science. 2005;310:332–336. doi: 10.1126/science.1115593. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Vanduffel W. Grasping-related functional magnetic resonance imaging brain responses in the macaque monkey. J. Neurosci. 2011;31:8220–8229. doi: 10.1523/JNEUROSCI.0623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen K, Vanduffel W, Orban GA. Charting the lower superior temporal region, a new motion-sensitive region in monkey superior temporal sulcus. J. Neurosci. 2006;26:5929–5947. doi: 10.1523/JNEUROSCI.0824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon S, Freiwald WA, Tsao DY. What makes a cell face selective? The importance of contrast. Neuron. 2012;74:567–581. doi: 10.1016/j.neuron.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon S, Grimaldi P, Schweers N, Tsao DY. Saccade modulation by optical and electrical stimulation in the macaque frontal eye field. J. Neurosci. 2013;33:16684–16697. doi: 10.1523/JNEUROSCI.2675-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa G, Goda N, Komatsu H. Selective responses to specular surfaces in the macaque visual cortex revealed by fMRI. Neuroimage. 2012;63:1321–1333. doi: 10.1016/j.neuroimage.2012.07.052. [DOI] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Palmeri TJ, Gauthier I. Visual object understanding. Nat. Rev. Neurosci. 2004;5:291–303. doi: 10.1038/nrn1364. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Shape representation in area V4: position-specific tuning for boundary conformation. J. Neurophysiol. 2001;86:2505–2519. doi: 10.1152/jn.2001.86.5.2505. [DOI] [PubMed] [Google Scholar]
- Peeters R, Simone L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, Orban GA. The representation of tool use in humans and monkeys: common and uniquely human features. J. Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Exp. Brain Res. 1982;47:329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Arcaro M, Weiner KS, Kalkus JF, Inati SJ, Gross CG, Kastner S. Neural representations of faces and body parts in macaque and human cortex: a comparative FMRI study. J. Neurophysiol. 2009;101:2581–2600. doi: 10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsk MA, Moore T, Richter MC, Gross CG, Kastner S. Methods for functional magnetic resonance imaging in normal and lesioned behaving monkeys. J. Neurosci Methods. 2005;143:179–195. doi: 10.1016/j.jneumeth.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Dilks DD, Saxe RR, Triantafyllou C, Kanwisher N. Differential selectivity for dynamic versus static information in face-selective cortical regions. Neuroimage. 2011;56:2356–2363. doi: 10.1016/j.neuroimage.2011.03.067. [DOI] [PubMed] [Google Scholar]
- Pitzalis S, Sereno MI, Committeri G, Fattori P, Galati G, Tosoni A, Galletti C. The human homologue of macaque area V6A. Neuroimage. 2013;82:517–530. doi: 10.1016/j.neuroimage.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosecki P, Moeller S, Schweers N, Romanski LM, Tsao DY, Freiwald WA. Faces in motion: selectivity of macaque and human face processing areas for dynamic stimuli. J. Neurosci. 2013;33:11768–11773. doi: 10.1523/JNEUROSCI.5402-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popivanov ID, Jastorff J, Vanduffel W, Vogels R. Stimulus representations in body-selective regions of the macaque cortex assessed with event-related fMRI. Neuroimage. 2012;63:723–741. doi: 10.1016/j.neuroimage.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Popivanov ID, Jastorff J, Vanduffel W, Vogels R. Heterogeneous Single-Unit Selectivity in an fMRI-Defined Body-Selective Patch. J. Neurosci. 2014;34:95–111. doi: 10.1523/JNEUROSCI.2748-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajimehr R, Young JC, Tootell RB. An anterior temporal face patch in human cortex, predicted by macaque maps. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1995–2000. doi: 10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Morel A, Kaas JH. Topography of supplementary eye field afferents to frontal eye field in macaque: implications for mapping between saccade coordinate systems. Vis. Neurosci. 1993;10:385–393. doi: 10.1017/s0952523800003771. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J. Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlack A, Sterbing-D'Angelo SJ, Hartung K, Hoffmann KP, Bremmer F. Multisensory space representations in the macaque ventral intraparietal area. J. Neurosci. 2005;25:4616–4625. doi: 10.1523/JNEUROSCI.0455-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MC, Mrowka SW, Turchi J, Saunders RC, Wilke M, Peters AJ, Ye FQ, Leopold DA. Blindsight depends on the lateral geniculate nucleus. Nature. 2010;466:373–377. doi: 10.1038/nature09179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MC, Panagiotaropoulos T, Augath MA, Logothetis NK, Smirnakis SM. Visually driven activation in macaque areas V2 and V3 without input from the primary visual cortex. PLoS. One. 2009;4:e5527. doi: 10.1371/journal.pone.0005527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Leopold DA, Brookes MJ, Khader PH. The contribution of electrophysiology to functional connectivity mapping. Neuroimage. 2013;80:297–306. doi: 10.1016/j.neuroimage.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno AB, Maunsell JH. Shape selectivity in primate lateral intraparietal cortex. Nature. 1998;395:500–503. doi: 10.1038/26752. [DOI] [PubMed] [Google Scholar]
- Sereno ME, Trinath T, Augath M, Logothetis NK. Three-dimensional shape representation in monkey cortex. Neuron. 2002;33:635–652. doi: 10.1016/s0896-6273(02)00598-6. [DOI] [PubMed] [Google Scholar]
- Shao Y, Keliris GA, Papanikolaou A, Fischer MD, Zobor D, Jagle H, Logothetis NK, Smirnakis SM. Visual cortex organisation in a macaque monkey with macular degeneration. Eur. J. Neurosci. 2013;38:3456–3464. doi: 10.1111/ejn.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnakis SM, Brewer AA, Schmid MC, Tolias AS, Schuz A, Augath M, Inhoffen W, Wandell BA, Logothetis NK. Lack of long-term cortical reorganization after macaque retinal lesions. Nature. 2005;435:300–307. doi: 10.1038/nature03495. [DOI] [PubMed] [Google Scholar]
- Srihasam K, Mandeville JB, Morocz IA, Sullivan KJ, Livingstone MS. Behavioral and anatomical consequences of early versus late symbol training in macaques. Neuron. 2012;73:608–619. doi: 10.1016/j.neuron.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Orban GA, De Maziere PA, Janssen P. A distinct representation of three-dimensional shape in macaque anterior intraparietal area: fast, metric, and coarse. J. Neurosci. 2009;29:10613–10626. doi: 10.1523/JNEUROSCI.6016-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Reber P, Costanza J, Wong E, Buxton R, Zola S, Squire L, Albright T. fMRI of monkey visual cortex. Neuron. 1998;20:1051–1057. doi: 10.1016/s0896-6273(00)80485-7. [DOI] [PubMed] [Google Scholar]
- Sultan F, Augath M, Murayama Y, Tolias AS, Logothetis N. esfMRI of the upper STS: further evidence for the lack of electrically induced polysynaptic propagation of activity in the neocortex. Magn Reson. Imaging. 2011;29:1374–1381. doi: 10.1016/j.mri.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Sunaert S, Van HP, Marchal G, Orban GA. Motion-responsive regions of the human brain. Exp. Brain Res. 1999;127:355–370. doi: 10.1007/s002210050804. [DOI] [PubMed] [Google Scholar]
- Takechi H, Onoe H, Shizuno H, Yoshikawa E, Sadato N, Tsukada H, Watanabe Y. Mapping of cortical areas involved in color vision in non-human primates. Neurosci Lett. 1997;230:17–20. doi: 10.1016/s0304-3940(97)00461-8. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Saito H, Fukada Y, Moriya M. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. J. Neurophysiol. 1991;66:170–189. doi: 10.1152/jn.1991.66.1.170. [DOI] [PubMed] [Google Scholar]
- Theys T, Pani P, van LJ, Goffin J, Janssen P. Three-dimensional shape coding in grasping circuits: a comparison between the anterior intraparietal area and ventral premotor area F5a. J. Cogn Neurosci. 2013;25:352–364. doi: 10.1162/jocn_a_00332. [DOI] [PubMed] [Google Scholar]
- Tolias AS, Smirnakis SM, Augath MA, Trinath T, Logothetis NK. Motion processing in the macaque: revisited with functional magnetic resonance imaging. J. Neurosci. 2001;21:8594–8601. doi: 10.1523/JNEUROSCI.21-21-08594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias AS, Sultan F, Augath M, Oeltermann A, Tehovnik EJ, Schiller PH, Logothetis NK. Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron. 2005;48:901–911. doi: 10.1016/j.neuron.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM. Functional analysis of V3A and related areas in human visual cortex. J. Neurosci. 1997;17:7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Nelissen K, Vanduffel W, Orban GA. Search for color ‘center(s)’ in macaque visual cortex. Cereb. Cortex. 2004;14:353–363. doi: 10.1093/cercor/bhh001. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald D, Knutsen T, Sasaky Y, Mandeville JB, Leite F, Dale AM, Rosen BR, Vanduffel W, Orban GA, Tootell RB. fMRI reveals face selective activity in awake behaving macque. Soc. Neurosci. Abstr. U. S. A. 2001;27:320. [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat. Neurosci. 2003a;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc. Natl. Acad. Sci. U. S. A. 2008a;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Schweers N, Moeller S, Freiwald WA. Patches of face-selective cortex in the macaque frontal lobe. Nat. Neurosci. 2008b;11:877–879. doi: 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Vanduffel W, Sasaki Y, Fize D, Knutsen TA, Mandeville JB, Wald LL, Dale AM, Rosen BR, Van Essen DC, Livingstone MS, Orban GA, Tootell RB. Stereopsis activates V3A and caudal intraparietal areas in macaques and humans. Neuron. 2003b;39:555–568. doi: 10.1016/s0896-6273(03)00459-8. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Beatse E, Sunaert S, Van Hecke P, Tootell RBH, Orban GA. Functional magnetic resonance imaging in an awake rhesus monkey. Soc. Neurosci. Abstr. 1998;24:11. [Google Scholar]
- Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RB, Orban GA. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Peuskens H, Denys K, Sunaert S, Todd JT, Orban GA. Extracting the Third Dimension from Motion: Differences in Human and Monkey intraparietal Cortex. Science. 2002;298:413–415. doi: 10.1126/science.1073574. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Brewer AA, Dougherty RF. Visual field map clusters in human cortex. Philos. Trans. R. Soc. Lond B Biol. Sci. 2005;360:693–707. doi: 10.1098/rstb.2005.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardak C, Vanduffel W, Orban GA. Searching for a salient target involves frontal regions. Cereb. Cortex. 2010;20:2464–2477. doi: 10.1093/cercor/bhp315. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kagan I, Andersen RA. Functional imaging reveals rapid reorganization of cortical activity after parietal inactivation in monkeys. Proc. Natl. Acad. Sci. U. S. A. 2012;109:8274–8279. doi: 10.1073/pnas.1204789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. Cortical projections from two prestriate areas in the monkey. Brain Res. 1971;34:19–35. doi: 10.1016/0006-8993(71)90348-9. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Colour coding in rhesus monkey prestriate cortex. Brain Res. 1973;53:422–427. doi: 10.1016/0006-8993(73)90227-8. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Nelissen K, van den Stock JV, De Winter FL, Pauwels K, de Gelder B, Vanduffel W, Vandenbulcke M. Dissimilar processing of emotional facial expressions in human and monkey temporal cortex. Neuroimage. 2012;66C:402–411. doi: 10.1016/j.neuroimage.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]