Abstract
The calcium and calmodulin-dependent kinase II (CaMKII) translates increases in intracellular Ca2+ into downstream signaling events. Its function in pulmonary pathologies remains largely unknown. CaMKII is a well-known mediator of apoptosis and regulator of endoplasmic reticulum (ER) Ca2+. ER stress and apoptosis of type II pneumocytes lead to aberrant tissue repair and progressive collagen deposition in pulmonary fibrosis. Thus we hypothesized that CaMKII inhibition alleviates fibrosis in response to bleomycin by attenuating apoptosis and ER stress of type II pneumocytes. We first established that CaMKII was strongly expressed in the distal respiratory epithelium, in particular in surfactant protein-C-positive type II pneumocytes, and activated after bleomycin instillation. We generated a novel transgenic model of inducible expression of the CaMKII inhibitor peptide AC3-I limited to type II pneumocytes (Tg SPC-AC3-I). Tg SPC-AC3-I mice were protected from development of pulmonary fibrosis after bleomycin exposure compared with wild-type mice. CaMKII inhibition also provided protection from apoptosis in type II pneumocytes in vitro and in vivo. Moreover, intracellular Ca2+ levels and ER stress were increased by bleomycin and significantly blunted with CaMKII inhibition in vitro. These data demonstrate that CaMKII inhibition prevents type II pneumocyte apoptosis and development of pulmonary fibrosis in response to bleomycin. CaMKII inhibition may therefore be a promising approach to prevent or ameliorate the progression of pulmonary fibrosis.
Keywords: apoptosis, CaMKII, ER stress, bleomycin, pulmonary fibrosis
pulmonary fibrosis occurs as an idiopathic disorder or as a result of inflammatory states or injuries, such as radiation or bleomycin administration. There are currently limited treatment strategies that prevent or slow pulmonary fibrosis progression (34), suggesting that an improved understanding of disease mechanisms will be necessary to identify new therapeutic targets. The current concept of its pathogenesis encompasses an initial alveolar epithelial injury that leads to abnormal wound healing, which is associated with the formation of patchy fibroblast-myofibroblast foci that ultimately evolve to fibrosis (41).
Numerous cell types have been implicated in fibrosis formation, including macrophages and respiratory epithelium. Several studies point to type II pneumocytes as critical mediators of fibrosis development (2, 16). Patients with pulmonary fibrosis display increased endoplasmic reticulum (ER) stress (19) and apoptosis of type II pneumocytes (1), which is thought to lead to increased collagen deposition. Consistent with observations in patients, bleomycin rapidly induces apoptosis of respiratory epithelium in murine models (12). Consistently, the number of type II pneumocytes is strongly decreased within 7 days after instillation of bleomycin (13, 50). In addition, selective genetic deletion of type II pneumocytes has proven sufficient to induce fibrosis formation (43). The potential mechanisms by which compromised type II pneumocyte function lead to fibrosis include impaired homeostatic ability of the lung to replace type I cells and the loss of important signals that suppress proliferation and collagen synthesis by lung fibroblasts and production of profibrotic factors such as TGF-β by residual injured type II epithelium (50).
The Ca2+ and calmodulin-dependent kinase II (CaMKII) is an attractive candidate signal for promoting diseases where elevated intracellular Ca2+ or oxidative stress contributes to disease initiation or progression (7, 38). Under resting conditions, CaMKII is held in an inactive state. Numerous signaling events increase levels of calcified calmodulin (Ca2+/CaM), which then binds to and thereby activates CaMKII (10). Once activated by Ca2+/CaM, CaMKII can remain active even in the absence of Ca2+/CaM binding by oxidation or autophosphorylation (8). Active CaMKII catalyzes the phosphorylation of proteins that increase inflammatory signaling (42), apoptosis (8, 52), and cell proliferation (23). While CaMKII function is well defined in cardiac myocytes and neurons, we recently found that excessive levels of active CaMKII induces mechanisms that drive key features of allergic asthma, suggesting that CaMKII could participate in other pulmonary disease processes.
Here we show that CaMKII is robustly expressed in type II pneumocytes and activated after bleomycin exposure. In a new transgenic mouse model of type II pneumocyte-targeted CaMKII inhibition, pulmonary fibrosis was significantly reduced compared with wild-type (WT) littermates after bleomycin exposure. Conversely, CaMKII inhibition abolished apoptosis in response to bleomycin in vitro and in vivo. As a potential mechanism, we ascertained that bleomycin administration increased intracellular Ca2+ concentrations and ER stress. CaMKII inhibition significantly reduced ER Ca2+ levels and ER stress. Our data define CaMKII as a mediator of cellular mechanisms that are crucial for the development of lung fibrosis.
MATERIALS AND METHODS
Transgenic model of CaMKII inhibition in type II pneumocytes.
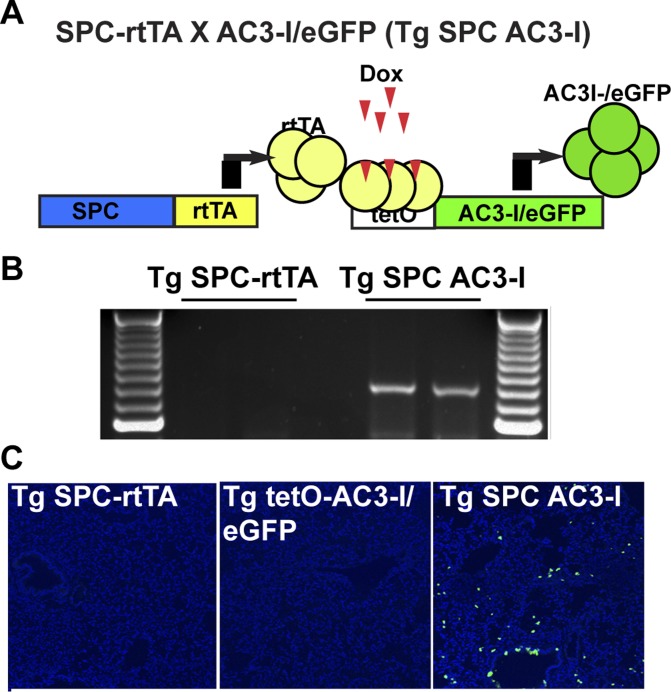
All animal care and housing requirements of the National Institutes of Health Committee on Care and Use of Laboratory Animals were followed. All protocols were reviewed and approved by the University of Iowa Animal Care and Use Committee. To test if CaMKII activity in type II pneumocytes affects pulmonary fibrosis in vivo, we interbred mice with tetracycline-induced (tetO) transgenic expression of a CaMKII inhibitory peptide (AC3-I) fused with eGFP (Tg tetO-AC3-I/eGFP, a kind gift of Dr. Daniel Winder, Vanderbilt University) with mice expressing the tetracycline reverse transcriptional activator (rtTA) under the control of the human surfactant, pulmonary-associated protein C [SFTPC, B6.Cg-Tg(SFTPC-rtTA)5Jaw/J, Jackson No. 06235, referred to as Tg SPC-rtTA] (30). In the double transgenic SFTPC-rtTA X tetO-AC3-I/eGFP mice (referred to as Tg SPC AC3-I mice), AC3-I/eGFP expression restricted to lung peripheral epithelial cells was induced by feeding a doxycycline-supplemented chow (625 mg/kg). AC3I transgene expression was verified by GFP fluorescence in 10-μm frozen lung sections. For all in vivo experiments, littermate Tg SPC-rtTA mice served as WT controls. Male and female mice were used in equal proportions.
Bleomycin treatment.
Intratracheal administration of bleomycin (Hospira, Lake Forest, IL) was performed in 8- to 12-wk-old male and female mice under isoflurane anesthesia. Bleomycin was instilled at a dose of 2.5 U/kg in normal saline at a final volume of 50 μl. The operator was blinded to genetic status of mice.
Histology.
On days 1, 3, or 14 after bleomycin instillation, left lungs were fixed with 4% paraformaldehyde, submerged in a vacuum overnight, and then processed by paraffin embedding as we previously described (14, 38). To assess collagen deposition, 5-μm tissue sections were cut and stained with Masson's trichrome. Images were acquired on an Olympus BX-61 light microscope.
Immunofluorescence.
Lung sections from WT mice at 24 h after saline or bleomycin instillation were probed for phosphorylated or total CaMKII using commercial anti-phospho-CaMKII (3361; Cell Signaling) and anti-total-CaMKII antibodies (LS-c100735; LS Bio). Some sections were colabeled with an anti-prosurfactant protein C antibody (AB3786; EMD Milipore). Alexa-Fluor secondary antibodies (Invitrogen, CA) were used to visualize staining. Nuclei were counterstained with TO-PRO-3 (Invitrogen). Sections were mounted with VectaShield mounting media (VectaShield). Images were acquired with a Zeiss 710 Confocal Microscope at ×40 or ×63 magnification. All images were taken at the same time and using the same imaging settings.
Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining was performed with the In Situ Cell Death Detection Kit, TMR Red Kit as recommended by the manufacturer (12156792910; Roche).
Hydroxyproline assay.
Lung tissue was homogenized, dried to a stable weight, and then acidified with 6 N HCl and hydrolyzed by heating at 120°C for 24 h. Hydroxyproline measurements were determined as described previously (14) and normalized to dry lung weight.
ELISA assays.
With the use of a sandwich ELISA, tumor necrosis factor-α (TNF-α; cat no. DY410), interleukin-1β (IL-1β; cat no. DY401), and macrophage inflammatory protein-2 (MIP-2; cat no. DY452; R&D Systems) were quantified in duplicate in lung homogenates as specified by the manufacturer.
Cell culture.
MLE-12 cells, a cell line derived from mouse alveolar epithelial cells that express surfactant protein C, were purchased from ATCC. Cells in passages 6–12 were used.
Cells were seeded onto collagen-coated glass coverslips or cell culture plates. MLE-12 cells were grown to 70% confluence and infected with adenovirus containing control or the CaMKII peptide inhibitor CaMKIIN for 48 h (22). Similarly to AC3-I, CaMKIIN is a CaMKII inhibitor peptide that inhibits all isoforms and splice variants of CaMKII with great sensitivity and specificity (3). MLE-12 cells were exposed to 50 mU/ml of bleomycin for indicated times.
Apoptosis assays.
Cell death was assayed by TUNEL staining. Twenty-four hours after exposure to 50 mU/ml bleomycin, MLE-12 cells were fixed in 4% paraformaldehyde and processed as recommended by the manufacturer (In Situ Cell Death Detection Kit; Roche). The nuclei were counterstained with DAPI. Images were taken at ×20 and the number of apoptotic to total number of nuclei was determined in five visual fields per condition. Three independent experiments were performed. Additional assays were performed in MLE-12 cells treated with 100 nM thapsigargin for 24 h using the annexin-based apoptosis assay for the MUSE cell analyzer (Milipore).
Cytoplasmic Ca2+ measurements.
MLE-12 cells were infected with adenovirus as indicated. The cells were loaded with fluo-4 AM (Life Technologies) in normal Hanks' buffered saline solution with Ca2+ and Mg2+ at room temperature for 20 min. Baseline signal recordings were performed for 5 min. Then, bleomycin (50 mU/ml) was added and fluorescence at 520 nm recorded every 30 or 60 s for the next 30 min. Additional measurements were performed with thapsigargin (100 nM), histamine (10 μM), and thapsigargin (100 nM) or in Ca2+-free solution. Measurements in cells treated with Hanks' buffered saline solution alone served as negative controls. The negative control data were subtracted from the measurements with agonists. The results are displayed as fold change.
Moreover, cytoplasmic Ca2+ responses were assessed by confocal microscopy in Rhod-2-loaded cells (1 μmol/l; Life Technologies) after 10 min of bleomycin treatment, using with a Zeiss LSM 510 confocal microscope. Images were taken at ×20 and five visual fields were analyzed using ImageJ.
Immunoblotting.
Fifty-microgram aliquots of whole tissue lysate from MLE-12 cells were resolved by SDS-polyacrylamide electrophoresis and transferred to polyvinylidene difluoride membrane. After blocking with 5% nonfat milk, the membranes were incubated with primary antibodies for p-CaMKII (3361S; Cell Signaling) CaMKII (#LS-c100735; LS Bio), transcription factor C/EBP homologous protein (CHOP; ABC 955; EMD Millipore,), the molecular chaperone BiP (3138; Cell Signaling), and GAPDH (5174; Cell Signaling) followed by horseradish peroxidase-conjugated secondary antibody. The proteins were visualized with the ECL chemiluminescence system (Amersham). Densitometry was performed using National Institutes of Health ImageJ software.
RT-PCR.
Total RNA was isolated from mouse lungs per manufacturer's recommendation (Qiagen) followed by digestion with proteinase K and DNase I to eliminate genomic DNA contamination. cDNA was transcribed from 1 μg RNA using Superscript III enzyme (Invitrogen) and random nanomer primers, followed by PCR amplification and visualization by agarose gel electrophoresis. The primers for AC3-I are as follows: AC3-I forward: 5′-GCA AGG CAG TCA ACT GCC TCC TGG-3′ and AC3-I reverse: 5′-ATG GTG AGC AAG GGC GAG GAG CTG-3′.
Statistical analysis.
All values in the text and figures are presented as means ± SE. Statistical significance of multiple treatments was determined using GraphPad Prism Software Version 6 by Student's t-test or ANOVA followed by Sidak or Tukey multiple comparison test when appropriate. P < 0.05 was considered significant.
RESULTS
CaMKII is expressed in type II pneumocytes and activated in response to bleomycin.
We first established that CaMKII is expressed in the lung parenchyma by immunofluorescence. In addition to robust expression in respiratory endothelium as we described in the past (38), CaMKII protein was present in alveolar epithelium (Fig. 1A). Its presence in type II pneumocytes was confirmed through colocalization with the prosurfactant protein C (Fig. 1B).
Fig. 1.
calcium and calmodulin-dependent kinase II (CaMKII) expression in distal respiratory epithelium. A: immunofluorescence for CaMKII (green) in murine lung sections. Nuclei, TO-PRO-3 (blue). No primary antibody serves as negative control (×20). B: prosurfactant protein C and CaMKII colocalize in distal respiratory epithelium. Left and middle: prosurfactant protein C (red) or CaMKII (green), TO-PRO-3 (blue), and bright field image. Right: merged image of prosurfactant protein C, CaMKII, and TO-PRO-3. Arrows denote cells with positive staining for CaMKII and prosurfactant protein C (×63). C: immunofluorescence for active phosphorylated CaMKII (p-CaMKII: green; nuclei TO-PRO-3: blue) in murine lung sections at baseline and 24 h after bleomycin instillation (×63).
Next, we investigated whether CaMKII is activated in pneumocytes after bleomycin instillation. At baseline, low levels of active phosphorylated CaMKII were detected (Fig. 1C). After exposure to bleomycin for 24 h, labeling for active phosphorylated CaMKII was strongly increased, suggesting that CaMKII is activated after bleomycin treatment through Ca2+/calmodulin-dependent CaMKII autophosphorylation. No differences in CaMKII protein levels were seen after bleomycin treatment (data not shown).
Transgenic model of CaMKII inhibition in type II pneumocytes.
To assess whether CaMKII in type II pneumocytes contributes to apoptosis and bleomycin-induced fibrosis, we developed a transgenic model that expresses the CaMKII inhibitor peptide AC3-I in type II pneumocytes when doxycycline is supplemented in the chow (Fig. 2A). As in most tissues outside the nervous system, CaMKII isoforms-γ and -δ are expressed in the lung (47). AC3-I is a peptide inhibitor that serves as a pseudosubstrate and inhibits all isoforms of CaMKII with >100-fold selectivity relative to PKC, PKA, and CaMKIV (30). Similar models of CaMKII inhibition by expression of AC3-I as a fusion protein with eGFP have been validated in other tissues and cell types (38, 51). We ascertained that the transgene RNA is only expressed in lungs of double transgenic Tg SPC-AC3-I mice compared with littermate controls after treatment with doxycycline-containing chow (Fig. 2B). Accordingly, in WT littermate mice, no significant eGFP fluorescence was detected in TG SPC-rtTA or in TG AC3-I/eGFP littermate control mice after administration of doxycycline-containing chow (Fig. 2C). In contrast, eGFP-positive cells were seen throughout the alveoli in double transgenic mice Tg SPC-AC3-I mice (Fig. 2C).
Fig. 2.
Transgenic model of CaMKII inhibition in type II pneumocytes. A: schematic overview of AC3-I expression in SPC-rtTA X AC3-I/eGFP mice (Tg SPC AC3-I). B: RT-PCR for AC3-I reveals induction of transgene expression in Tg SPC AC3-I mice only after administration of doxycycline-containing chow for 7 days. C: immunofluorescence for eGFP (green) and nuclei (DAPI) demonstrates AC3-I/eGFP expression only in Tg SPC AC3-I mice but not in Tg SPC-rtTA or Tg AC3-I/eGFP control mice after administration of Doxycycline-containing chow for 7 days (×10).
Pulmonary fibrosis is decreased after CaMKII inhibition in type II pneumocytes.
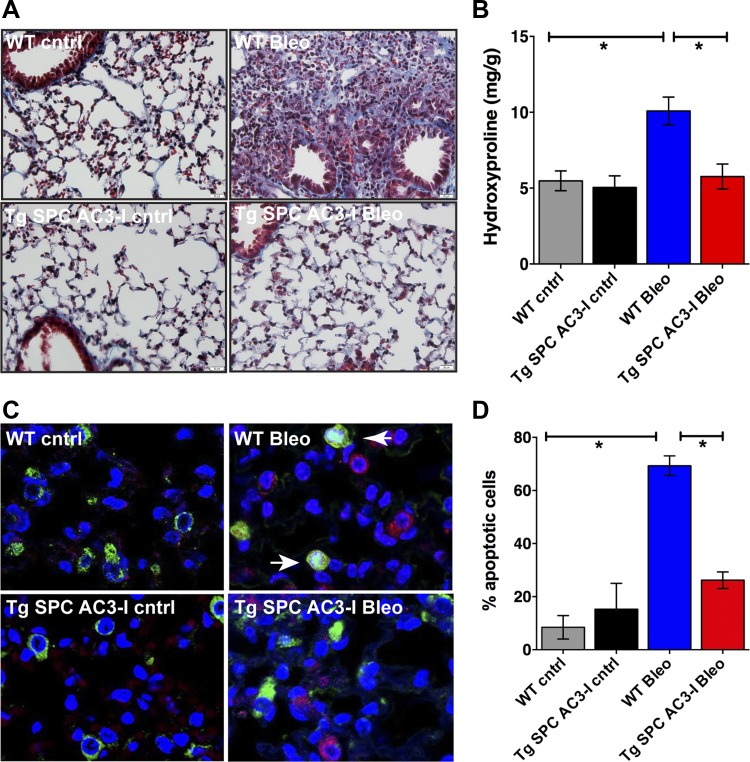
Because apoptosis of type II pneumocytes ultimately leads to lung fibrosis (43) and CaMKII controls apoptosis in other cell types and tissues, we assessed whether mice with CaMKII inhibition in type II pneumocytes are protected from bleomycin-induced fibrosis. We performed Masson's trichrome staining of lung sections from WT and Tg SPC-AC3-I mice at baseline and on day 14 after bleomycin exposure (Fig. 3A). In WT mice, bleomycin induced dense collagen deposition and pronounced destruction of lung architecture, whereas Tg SPC-AC3-I mice had essentially normal lung parenchyma.
Fig. 3.
CaMKII inhibition in type II pneumocytes abrogates bleomycin-induced pulmonary fibrosis. A: representative Masson's trichrome staining in lung sections from wild-type (WT) and Tg SPC-AC3-I mice at baseline and on day 14 after bleomycin administration (×20). B: hydroxyproline assay in lungs in untreated littermate WT (Tg SPC-rtTA) and Tg SPC-AC3-I mice on day 14 after bleomycin administration. (n = 3–8 per group). C: terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining (red), immunofluorescence for prosurfactant protein C (green), and nuclear counterstain (DAPI: blue) in lungs on day 3 after exposure to bleomycin or saline (cntrl). Arrows indicate apoptotic type II pneumocytes. D: quantification of apoptotic type II pneumocytes (positive for TUNEL and prosurfactant protein C staining) (n = 3 visual fields, 3 samples per group). *P < 0.05.
Next, we performed hydroxyproline assays in samples from Tg SPC-AC3-I mice and WT littermate at baseline and on day 14 after bleomycin instillation. Hydroxyproline levels were similar in Tg SPC-AC3-I mice and WT littermates at baseline (Fig. 3B). In contrast, the concentration of hydroxyproline was significantly increased in WT mice on day 14 after treatment, whereas collagen production was almost near the control concentration in Tg SPC-AC3-I mice. Since early apoptosis of type II pneumocytes initiates the disease process that leads to fibrosis, we tested whether CaMKII inhibition protects from apoptosis in vivo. In WT mice, increased apoptosis of type II pneumocytes was detected, whereas, as predicted, type II pneumocytes in Tg SPC-AC3-I mice were protected (Fig. 3, C and D). These data strongly suggest that CaMKII has a key role in the development of a fibrotic phenotype, possibly by decreasing bleomycin-induced apoptosis of type II pneumocytes.
CaMKII inhibition in type II pneumocytes decreases the inflammatory response to bleomycin.
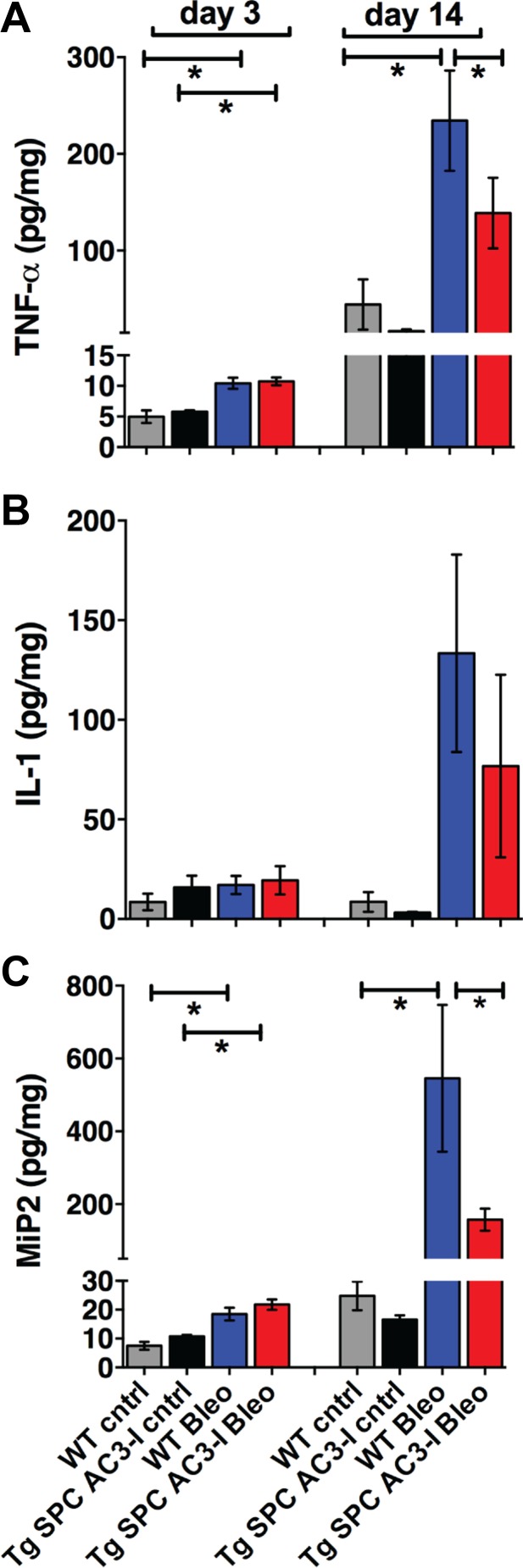
Because bleomycin-induced lung injury is, in part, mediated by a proinflammatory state, we tested whether CaMKII inhibition in type II pneumocytes is sufficient to block the inflammatory response of lung tissue to bleomycin injury. For this purpose, we performed ELISA assays in whole lung lysates from Tg SPC-AC3-I mice and WT littermates on days 3 or 14 after instillation of bleomycin or normal saline. While increases in cytokines were present in bleomycin- compared with saline-treated control samples on day 3, no differences between the genotypes were detected. In contrast, on day 14, the overall concentrations of MIP-2 (17), IL-1β (32), and TNF-α (29, 31) were greatly increased in bleomycin-treated lungs compared with day 3 and a reduction, especially in TNF-α and MIP-2, was seen in Tg SPC AC3-I mice compared with WT mice (Fig. 4, A–C).
Fig. 4.
CaMKII inhibition in type II pneumocytes decreases the inflammatory response to bleomycin. ELISA assays for TNF-α (A), IL-1β (B), and macrophage inflammatory protein-2 (MIP-2; C) in homogenates of lungs from WT or TG SPC AC3-I mice on days 3 and 14 after treatment with bleomycin or normal saline (cntrl) [n = 7 (WT cntrl), 4 (Tg SPC AC3-I cntrl), 13 (WT Bleo), and 8 (Tg SPC AC3-I Bleo)]. * P < 0.05.
CaMKII inhibition blocks bleomycin-induced apoptosis and ER stress in MLE-12 cells.
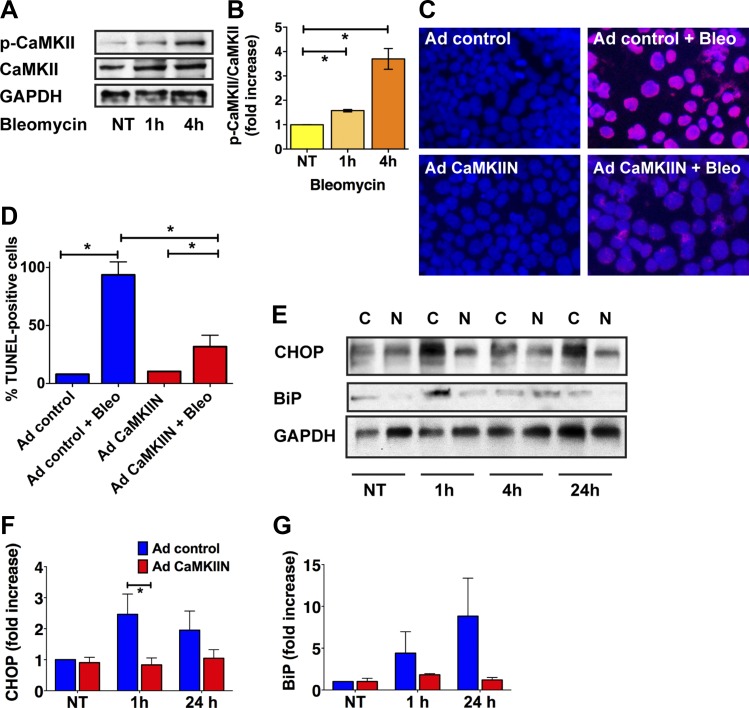
To understand the mechanisms by which CaMKII inhibition in type II pneumocytes reduces pulmonary fibrosis, we conducted in vitro experiments in MLE-12 cells, an immortalized murine mouse epithelial cell line with type II characteristics. First, we tested whether bleomycin induces CaMKII activity by performing immunoblots for activated CaMKII. Similarly to our findings in vivo (Fig. 1C), the level of p-CaMKII was increased at 1 and 4 h (Fig. 5, A and B). Since CaMKII autophosphorylation depends on binding of calcified calmodulin, these findings suggest that the intracellular Ca2+ levels are increased after bleomycin application. Next, we examined whether CaMKII inhibition protects from bleomycin-induced apoptosis. For this purpose, we performed TUNEL staining in cells that were infected with control adenovirus or an adenovirus that expresses the potent and specific CaMKII inhibitor CaMKIIN (22). In untreated MLE-12 cells, very few TUNEL-positive cells were detected regardless of the adenovirus used (Fig. 5, C and D). Incubation with bleomycin for 24 h significantly increased the number of TUNEL-positive cells infected with control adenovirus. In contrast, significantly fewer apoptotic cells were detected after infection with CaMKIIN (Fig. 5, C and D). ER stress is believed to mediate type II pneumocyte apoptosis and thereby predispose to the development of lung fibrosis after treatment with bleomycin (45). To assess whether CaMKIIN protected from ER stress, we performed immunoblots for the ER stress markers CHOP in bleomycin-treated MLE-12 cells. While the protein levels of CHOP and BiP were strongly increased with bleomycin treatment, the response was abolished with CaMKII inhibition (Fig. 5, E–G).
Fig. 5.
CaMKII inhibition prevents apoptosis and endoplasmic reticulum (ER) stress in type II pneumocytes in vitro. A: immunoblots for CaMKII, active phosphorylated p-CaMKII in MLE-12 cells after bleomycin treatment as indicated. NT, no treatment. B: quantification of 3 independent experiments. The relative increase in signal intensity compared with untreated conditions was determined by densitometry and is indicated as fold increase. C: representative TUNEL staining in MLE-12 cells that were transduced with control adenovirus or adenovirus expressing CaMKIIN for 48 h before bleomycin or sham treatment for additional 24 h. D: quantification of TUNEL-positive cells in 3 independent experiments; 5 visual fields per conditions were analyzed. E: representative immunoblot for CHOP and BiP in MLE-12 cells after bleomycin treatment as indicated. MLE-12 cells were infected with control adenovirus (C) or adenovirus expressing CaMKIIN (N) for 48 h before bleomycin was added. Quantification of CHOP (F) and BiP immunoblots (G), adjusted to GAPDH. Data are the average of 5 (CHOP) and 4 (BiP) independent experiments. *P < 0.05.
CaMKII controls intracellular Ca2+ release and ER Ca2+ content in MLE-12 cells.
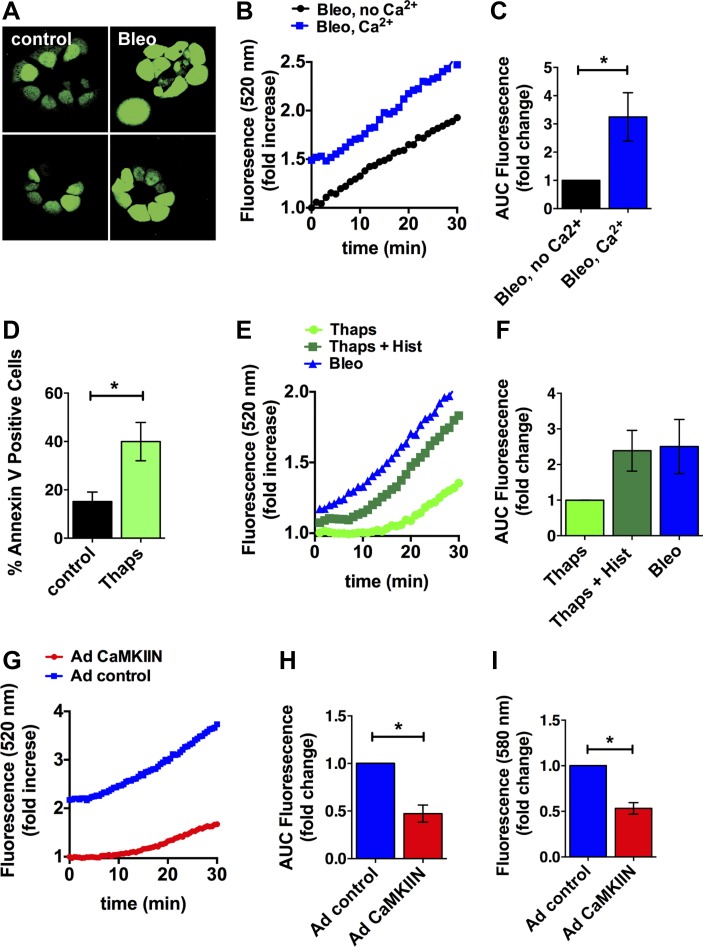
Since CaMKII promotes apoptosis, in part through regulation of ER Ca2+ uptake (36), we next assessed whether bleomycin increased intracellular Ca2+ levels in MLE-12 cells by fluo-4 fluorescence. Bleomycin induced a robust increase in intracellular Ca2+ that persisted over 30 min (Fig. 6, A and B). The response was blunted when the experiment was performed under nominally Ca2+-free conditions (Fig. 6, B and C), suggesting that an influx of Ca2+ from the extracellular space contributes to the bleomycin-dependent increase in cytoplasmic Ca2+. The sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) inhibitor thapsigargin induces apoptosis in many cell types through depletion of ER Ca2+ stores (28). Therefore, as a positive control, we tested the response of MLE-12 cells to thapsigargin treatment. As expected, thapsigargin induced MLE-12 cell apoptosis (Fig. 6D). The addition of histamine to thapsigargin is predicted to maximize the Ca2+ release from the ER, and as expected, it further increased intracellular Ca2+ levels. In direct comparison, the cytoplasmic Ca2+ levels were insignificantly greater after bleomycin treatment compared with thapsigargin (Fig. 6, E and F). This finding implies that the increase in intracellular Ca2+ after bleomycin treatment is sufficient to induce apoptosis. Lastly, we tested the effect of CaMKII inhibition on cytoplasmic Ca2+ levels. CaMKII inhibition significantly reduced the bleomycin-induced increase in Ca2+ (Fig. 6, G and H). These findings were confirmed in separate experiments by confocal microscopy with the fluorescent dye Rhod-2 (Fig. 6I). Our data demonstrate that bleomycin increases cytoplasmic Ca2+ to a level comparable to thapsigargin and that CaMKII inhibition blocks the Ca2+ response to bleomycin. Since thapsigargin induces apoptosis through ER Ca2+ release and ER stress (37), it is very feasible that the reduced ER Ca2+ release under CaMKII inhibition diminishes the ER stress response and blunts apoptosis.
Fig. 6.
CaMKII regulates ER Ca2+ release in response to bleomycin. A: intracellular Ca2+ levels by fluo-4 fluorescence after bleomycin treatment for 10 min in MLE-12 cells. B: cytoplasmic Ca2+ levels were determined in MLE-12 cells during treatment with 50 mU/ml bleomycin in Hanks' buffered saline solution with and without Ca2+. Negative control data were subtracted from the measurements with agonists. A representative experiment is shown. C: area under the curve (AUC) was determined in 4 independent experiments and plotted as fold increase. D: apoptosis in MLE-12 cells after treatment with 100 nM thapsigargin for 24 h. E: cytoplasmic Ca2+ levels in MLE-12 cells during treatment with 50 mU/ml bleomycin, 100 nM thapsigargin, and 100 nM thapsigargin with 10 μM histamine. A representative experiment is shown. F: AUC was determined for each condition in 4 independent experiments and plotted as fold increase. G: cytoplasmic Ca2+ levels in MLE-12 cells that were infected with adenovirus expressing the CaMKII inhibitor peptide CaMKIIN or control for 48 h during treatment with 50 mU/ml bleomycin in Hanks' buffered saline solution with Ca2+. A representative experiment is shown. H: AUC was determined in 5 independent experiments and plotted as fold increase. I: Rhod-2 fluorescence in MLE-12 cells infected with adenovirus expressing the CaMKII inhibitor peptide CaMKIIN or control after bleomycin treatment for 10 min. *P < 0.05.
DISCUSSION
Apoptosis of type II pneumocytes plays an important role in the development of pulmonary fibrosis, but specific molecular mediators that regulate this process have not been identified. Here, we demonstrate that CaMKII is robustly expressed in type II pneumocytes and activated by bleomycin. Bleomycin-induced pulmonary fibrosis is abrogated in a novel transgenic model of CaMKII inhibition in type II pneumocytes. Consistently, hydroxyproline levels and inflammatory cytokines are significantly lower in lungs of mice with CaMKII inhibition. After bleomycin instillation, apoptosis of type II pneumocytes is greatly reduced in mice with CaMKII inhibition. In vitro experiments in MLE-12 cells revealed that bleomycin-induced apoptosis and ER stress are suppressed with CaMKII inhibition. Numerous apoptotic pathways including ER stress are induced by a rise in intracellular Ca2+ levels (28). Bleomycin increased intracellular Ca2+ levels in part through Ca2+ release from the ER. CaMKII inhibition significantly reduced the ER Ca2+ loading and release in MLE-12 cells. Thus we propose that bleomycin induces apoptosis and ER stress of type II pneumocytes at least in part through increasing cytoplasmic Ca2+ levels and that CaMKII controls apoptosis through regulating Ca2+ levels. Since apoptosis of type II pneumocytes impairs the homeostatic ability of the lung to replace type I cells, CaMKII inhibition in type II pneumocytes is sufficient to prevent pulmonary fibrosis. Thus CaMKII inhibition may be a promising novel approach to prevent or halt the progression of pulmonary fibrosis.
While intensively studied as a decoder of Ca2+-dependent signals in neurons and cardiac myocytes, CaMKII expression and function in the lung have remained largely unknown. A single report recently demonstrated that CaMKII is expressed in nonsmall cell lung cancer in vivo (21) where it promotes proliferation. In vitro, CaMKII function was studied in an adenocarcinoma human alveolar basal cell line (21) and respiratory smooth muscle (39) in the context of proliferation and inflammation. Moreover, CaMKII regulates surfactant secretion in rat alveolar type II cells by modulating lamellar body calcium (4). We recently developed a model of CaMKII inhibition in Clara cells and reported that CaMKII promotes mucus production, expression of inflammatory cytokines, eosinophil invasion, and airway hyperreactivity in vivo and in vitro models of allergic asthma (38). In this project, we concentrated on CaMKII expression and function in alveolar epithelium where we detected robust expression in surfactant C-positive type II pneumocytes. Our data suggest that CaMKII is an important yet widely unrecognized regulator of pulmonary pathophysiology.
Type II pneumocytes play various roles in alveolar fluid balance, coagulation/fibrinolysis, and host defense. Type II pneumocytes proliferate, differentiate into type I cells, and remove apoptotic type II pneumocytes cells by phagocytosis, thus contributing to epithelial repair. Deletion of type II pneumocytes is sufficient to induce pulmonary fibrosis (43). The bleomycin model is widely used to understand the various cellular and molecular pathways that contribute to pulmonary fibrosis, including apoptosis of type II pneumocytes (50). However, the mechanism by which bleomycin induces apoptosis is controversial since conflicting data providing evidence for activation of the intrinsic (48) and extrinsic (27) pathways have been reported. Oxidative stress-dependent mechanisms are widely accepted as upstream activators of bleomycin-induced apoptosis (48), although a few studies have directly implicated Ca2+ (18, 26). Interestingly, a recent study demonstrated that the administration of the Ca2+ channel blocker nifedipine significantly decreases extracellular matrix deposition after bleomycin exposure (26). Nifedipine administration had no effect on total inflammatory cell counts, indicating that its protective effect on lung fibrosis was due to the profibrotic rather than the inflammatory response to bleomycin (26). While initially believed to be a specific inhibitor of the L-type Ca2+ channel, nifedipine also blocks store-operated Ca2+ entry (SOCE) (5). Our data demonstrate that the Ca2+ response to bleomycin is dependent on influx of Ca2+ from the extracellular space. Ca2+ entry in type II pneumocytes is mainly dependent on transient receptor potential (TRP) channels (9) and SOCE via its molecular components stromal interaction molecule 1 (STIM1) and Orai1 (6a). In addition, the L-Type Ca2+ channel has been identified in type II pneumocyte-derived cell lines (6). Several lines of evidence link these channels to CaMKII. CaMKII was recently reported to promote TRP channel activity in endothelial cells (44). Conversely, knockdown of STIM1/Orai1 inhibits CaMKII activation. Moreover, CaMKII is known to phosphorylate the β-subunit of the L-Type Ca2+ channel, leading to increased opening probability of the channel (11, 33). Thus we speculate that CaMKII regulates Ca2+ channels in type II pneumocytes and that decreased influx of Ca2+ from the extracellular space drives the reduction in ER Ca2+ content under CaMKII inhibition.
Data from various other cell types have identified CaMKII as a regulator of cell proliferation and migration. Moreover, CaMKII has been reported to promote apoptosis (8, 36, 52). Salas et al. (36) demonstrated that CaMKII controls apoptosis through regulation of phosphorylation of ER proteins. In particular, CaMKII phosphorylates the SERCA inhibitor phospholamban at Thr-17 and thereby reverses the inhibition of SERCA (24). The de-inhibition of SERCA facilitates ER Ca2+ loading. Apoptosis is then induced upon ER Ca2+ release in response to proapoptotic stimuli such as ischemia-reperfusion (36). Several mechanisms for Ca2+ regulation of apoptosis have been reported in the last several decades (25). For example, cytochrome c is released from mitochondria after an initial insult and binds to and activates the IP3 receptor. As a result, Ca2+ is released from the ER. The rise in cytoplasmic Ca2+ induces a mass exodus of cytochrome c from mitochondria, which then elicit a robust apoptotic response (25). Since we detected decreased ER Ca2+ loading in MLE-12 cells with CaMKII inhibition and were able to induce apoptosis with thapsigargin through ER Ca2+ depletion, we propose that bleomycin induces apoptosis in part through ER Ca2+ release and ER stress by a mechanism that includes CaMKII. These data are in line with other reports that induction of ER stress in type II pneumocytes predisposes to enhanced pulmonary fibrosis after bleomycin, at least in part by increased alveolar epithelial cell apoptosis (20, 45). Of note, exaggerated ER stress responses in type II pneumocytes have been reported in patients with idiopathic pulmonary fibrosis (19). Moreover, studies in a variety of cell types have provided evidence that CaMKII induces ER stress (46).
Apart from its role in type II pneumocytes and ER Ca2+ loading, CaMKII may contribute to pulmonary fibrosis through other mechanisms, for example, via impaired secretion of surfactant (4). In addition, CaMKII was recently identified in mitochondria in cardiac myocytes where it regulates ischemia-induced apoptosis (15). Thus CaMKII may selectively control mitochondria-dependent apoptotic pathways. While we concentrated on the role of type II pneumocytes in pulmonary fibrosis, numerous other cell types contribute to its pathogenesis, including macrophages. Recent studies have demonstrated that Ca2+-dependent ER stress in macrophages contributes to pulmonary fibrosis in the chrysotile asbestos model (35). Since CaMKII is expressed in macrophages (40), we cannot rule out that CaMKII also regulates Ca2+-dependent ER stress leading to pulmonary fibrosis in this cell type. Regardless, we found that selective inhibition of CaMKII in type II pneumocytes was sufficient to protect against collagen deposition and the development of pulmonary fibrosis. It is possible that global CaMKII inhibition may provide a greater protection against fibrosis than selective blockade in type II pneumocytes. Future studies that examine the therapeutic benefit of CaMKII inhibition in the lung following injury are warranted.
GRANTS
This work was supported by a grant from the Spear Family Pulmonary Research Fund (to O. A. Jaffer), National Institutes of Health Grants R01-HL-108932 (to I. M. Grumbach) and 2R01-ES-015981-06 (to A. B. Carter) and a fellowship from the Iowa Center for Research By Undergraduates (to C. J. Winters). This work was also supported by Department of Veterans Affairs, Office of Research and Development, Biomedical Laboratory Research and Development Grant 1 BX001135-03 (to A. B. Carter).
DISCLAIMERS
The content of this manuscript is solely the responsibility of the authors and do not necessarily represent the views of the granting agencies.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.J.W., O.M.K., S.C.S., O.A.J., A.B.C., and I.M.G. conception and design of research; C.J.W., O.M.K., S.M., C.A., J.D.P., and O.A.J. performed experiments; C.J.W., O.M.K., S.M., C.A., S.C.S., J.D.P., O.A.J., A.B.C., and I.M.G. analyzed data; C.J.W., O.M.K., S.M., S.C.S., O.A.J., A.B.C., and I.M.G. interpreted results of experiments; C.J.W., O.M.K., S.C.S., and I.M.G. prepared figures; C.J.W., O.M.K., S.M., C.A., O.A.J., A.B.C., and I.M.G. edited and revised manuscript; C.J.W., O.M.K., S.M., C.A., S.C.S., O.A.J., A.B.C., and I.M.G. approved final version of manuscript; I.M.G. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kristina W. Thiel, University of Iowa, for assistance in the preparation of the manuscript.
REFERENCES
- 1.Barbas-Filho JV, Ferreira MA, Sesso A, Kairalla RA, Carvalho CR, Capelozzi VL. Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IFP)/usual interstitial pneumonia (UIP). J Clin Pathol 54: 132–138, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camelo A, Dunmore R, Sleeman MA, Clarke DL. The epithelium in idiopathic pulmonary fibrosis: breaking the barrier. Front Pharmacol 4: 173, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci USA 95: 10890–10895, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chintagari NR, Mishra A, Su L, Wang Y, Ayalew S, Hartson SD, Liu L. Vacuolar ATPase regulates surfactant secretion in rat alveolar type II cells by modulating lamellar body calcium. PLoS One 5: e9228, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis TM, Scholfield CN. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J Physiol 532: 609–623, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietl P, Haller T, Wirleitner B, Volkl H, Friedrich F, Striessnig J. Activation of L-type Ca2+ channels after purinoceptor stimulation by ATP in an alveolar epithelial cell (L2). Am J Physiol Lung Cell Mol Physiol 269: L873–L883, 1995. [DOI] [PubMed] [Google Scholar]
- 6a.Dietl P, Haller T, Frick M. Spatio-temporal aspects, pathways and actions of Ca2+ in surfactant secreting pulmonary alveolar type II pneumocytes. Cell Calcium 52: 296–302, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev 91: 889–915, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fois G, Wittekindt O, Zheng X, Felder ET, Miklavc P, Frick M, Dietl P, Felder E. An ultra fast detection method reveals strain-induced Ca(2+) entry via TRPV2 in alveolar type II cells. Biomech Model Mechanobiol 11: 959–971, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Griffith LC. Regulation of calcium/calmodulin-dependent protein kinase II activation by intramolecular and intermolecular interactions. J Neurosci 24: 8394–8398, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grueter CE, Abiria SA, Wu Y, Anderson ME, Colbran RJ. Differential regulated interactions of calcium/calmodulin-dependent protein kinase II with isoforms of voltage-gated calcium channel beta subunits. Biochemistry 47: 1760–1767, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagimoto N, Kuwano K, Nomoto Y, Kunitake R, Hara N. Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 16: 91–101, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol 294: L24–L33, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Jaffer OA, Carter AB, Sanders PN, Dibbern ME, Winters CJ, Murthy S, Ryan AJ, Rokita AG, Prasad AM, Zabner J, Kline JN, Grumbach IM, Anderson ME. Mitochondrial-targeted antioxidant therapy decreases TGFbeta mediated collagen production in a murine asthma model. Am J Respir Cell Mol Biol 52: 106–115, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME. CaMKII determines mitochondrial stress responses in heart. Nature 491: 269–273, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasper M, Haroske G. Alterations in the alveolar epithelium after injury leading to pulmonary fibrosis. Histol Histopathol 11: 463–483, 1996. [PubMed] [Google Scholar]
- 17.Keane MP, Belperio JA, Moore TA, Moore BB, Arenberg DA, Smith RE, Burdick MD, Kunkel SL, Strieter RM. Neutralization of the CXC chemokine, macrophage inflammatory protein-2, attenuates bleomycin-induced pulmonary fibrosis. J Immunol 162: 5511–5518, 1999. [PubMed] [Google Scholar]
- 18.Kong L, Gao YQ, Wang JF, Niu JZ, Zhang XX. [Apoptosis of type II alveolar epithelial cell induced by bleomycin in lung fibrotic rat]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 29: 782–786, 2007. [PubMed] [Google Scholar]
- 19.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 178: 838–846, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, Morrisey EE, Beers MF, Yull FE, Blackwell TS. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA 108: 10562–10567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee IT, Lin CC, Lin WN, Wu WL, Hsiao LD, Yang CM. Lung inflammation caused by adenosine-5′-triphosphate is mediated via Ca2+/PKCs-dependent COX-2/PGE2 induction. Int J Biochem Cell Biol 45: 1657–1668, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Li W, Gupta AK, Mohler PJ, Anderson ME, Grumbach IM. Calmodulin kinase II is required for angiotensin II-mediated vascular smooth muscle hypertrophy. Am J Physiol Heart Circ Physiol 298: H688–H698, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Li H, Sanders PN, Mohler PJ, Backs J, Olson EN, Anderson ME, Grumbach IM. The multifunctional Ca2+/calmodulin-dependent kinase II delta (CaMKIIdelta) controls neointima formation after carotid ligation and vascular smooth muscle cell proliferation through cell cycle regulation by p21. J Biol Chem 286: 7990–7999, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattiazzi A, Vittone L, Mundina-Weilenmann C, Said M. Phosphorylation of the Thr17 residue of phospholamban. New insights into the physiological role of the CaMK-II pathway of phospholamban phosphorylation. Ann NY Acad Sci 853: 280–283, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol 5: 1041–1043, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee S, Ayaub EA, Murphy J, Lu C, Kolb M, Ask K, Janssen LJ. Disruption of calcium signaling in fibroblasts and attenuation of bleomycin-induced fibrosis by nifedipine. Am J Respir Cell Mol Biol 53: 450–458, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Mungunsukh O, Griffin AJ, Lee YH, Day RM. Bleomycin induces the extrinsic apoptotic pathway in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 298: L696–L703, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz LA, Lasky J, Hamilton RF Jr, Holian A, Hoyle GW, Banks W, Peschon JJ, Brody AR, Lungarella G, Friedman M. Expression of TNF and the necessity of TNF receptors in bleomycin-induced lung injury in mice. Exp Lung Res 24: 721–743, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Pellicena P, Schulman H. CaMKII inhibitors: from research tools to therapeutic agents. Front Pharmacol 5: 21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med 170: 655–663, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piguet PF, Vesin C, Grau GE, Thompson RC. Interleukin 1 receptor antagonist (IL-1ra) prevents or cures pulmonary fibrosis elicited in mice by bleomycin or silica. Cytokine 5: 57–61, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Prasad AM, Nuno DW, Koval OM, Ketsawatsomkron P, Li W, Li H, Shen FY, Joiner ML, Kutschke W, Weiss RM, Sigmund CD, Anderson ME, Lamping KG, Grumbach IM. Differential control of calcium homeostasis and vascular reactivity by Ca2+/calmodulin-dependent kinase II. Hypertension 62: 434–441, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan AJ, Larson-Casey JL, He C, Murthy S, Carter AB. Asbestos-induced disruption of calcium homeostasis induces endoplasmic reticulum stress in macrophages. J Biol Chem 289: 33391–33403, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salas MA, Valverde CA, Sanchez G, Said M, Rodriguez JS, Portiansky EL, Kaetzel MA, Dedman JR, Donoso P, Kranias EG, Mattiazzi A. The signalling pathway of CaMKII-mediated apoptosis and necrosis in the ischemia/reperfusion injury. J Mol Cell Cardiol 48: 1298–1306, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samali A, Fitzgerald U, Deegan S, Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol 2010: 830307, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders PN, Koval OM, Jaffer OA, Prasad AM, Businga TR, Scott JA, Hayden PJ, Luczak ED, Dickey DD, Allamargot C, Olivier AK, Meyerholz DK, Robison AJ, Winder DG, Blackwell TS, Dworski R, Sammut D, Wagner BA, Buettner GR, Pope RM, Miller FJ Jr, Dibbern ME, Haitchi HM, Mohler PJ, Howarth PH, Zabner J, Kline JN, Grumbach IM, Anderson ME. CaMKII is essential for the proasthmatic effects of oxidation. Sci Transl Med 5: 195ra197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 297: L26–L34, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott JA, Klutho PJ, El Accaoui R, Nguyen E, Venema AN, Xie L, Jiang S, Dibbern M, Scroggins S, Prasad AM, Luczak ED, Davis MK, Li W, Guan X, Backs J, Schlueter AJ, Weiss RM, Miller FJ, Anderson ME, Grumbach IM. The multifunctional Ca(2+)/calmodulin-dependent kinase IIdelta (CaMKIIdelta) regulates arteriogenesis in a mouse model of flow-mediated remodeling. PLoS One 8: e71550, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selman M, King TE, Pardo A; American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134: 136–151, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Singh MV, Kapoun A, Higgins L, Kutschke W, Thurman JM, Zhang R, Singh M, Yang J, Guan X, Lowe JS, Weiss RM, Zimmermann K, Yull FE, Blackwell TS, Mohler PJ, Anderson ME. Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. J Clin Invest 119: 986–996, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181: 254–263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su KH, Lin SJ, Wei J, Lee KI, Zhao JF, Shyue SK, Lee TS. The essential role of transient receptor potential vanilloid 1 in simvastatin-induced activation of endothelial nitric oxide synthase and angiogenesis. Acta Physiol (Oxf) 212: 191–204, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Tanjore H, Blackwell TS, Lawson WE. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 302: L721–L729, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest 119: 2925–2941, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tobimatsu T, Kameshita I, Fujisawa H. Molecular cloning of the cDNA encoding the third polypeptide (gamma) of brain calmodulin-dependent protein kinase II. J Biol Chem 263: 16082–16086, 1988. [PubMed] [Google Scholar]
- 48.Wallach-Dayan SB, Izbicki G, Cohen PY, Gerstl-Golan R, Fine A, Breuer R. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am J Physiol Lung Cell Mol Physiol 290: L790–L796, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Yi ES, Salgado M, Williams S, Kim SJ, Masliah E, Yin S, Ulich TR. Keratinocyte growth factor decreases pulmonary edema, transforming growth factor-beta and platelet-derived growth factor-BB expression, and alveolar type II cell loss in bleomycin-induced lung injury. Inflammation 22: 315–325, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med 11: 409–417, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Zhu LJ, Klutho PJ, Scott JA, Xie L, Luczak ED, Dibbern ME, Prasad AM, Jaffer OA, Venema AN, Nguyen EK, Guan X, Anderson ME, Grumbach IM. Oxidative activation of the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) regulates vascular smooth muscle migration and apoptosis. Vascul Pharmacol 60: 75–83, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]