Abstract
Podoplanin/gp38+ stromal cells present in lymphoid organs play a central role in the formation and reorganization of the extracellular matrix and in the functional regulation of immune responses. Gp38+ cells are present during embryogenesis and in human livers of primary biliary cirrhosis. Since little is known about their function, we studied gp38+ cells during chronic liver inflammation in models of biliary and parenchymal liver fibrosis and steatohepatitis. Gp38+ cells were analyzed using flow cytometry and confocal microscopy, and the expression of their steady state and inflammation-associated genes was evaluated from healthy and inflamed livers. Gp38+ cells significantly expanded in all three models of liver injury and returned to baseline levels during regression of inflammation. Based on CD133 and gp38 expression in the CD45−CD31−Asgpr1− liver cell fraction, numerous subsets could be identified that were negative for CD133 (gp38hiCD133−, gp38lowCD133−, and gp38−CD133−). Moreover, among the CD133+ cells, previously identified as progenitor population in injured liver, two subpopulations could be distinguished based on their gp38 expression (gp38−CD133+ and CD133+gp38+). Importantly, the distribution of the identified subsets in inflammation illustrated injury-specific changes. Moreover, the gp38+CD133+ cells exhibited liver progenitor cell characteristics similar to the gp38−CD133+ population, thus representing a novel subset within the classical progenitor cell niche. Additionally, these cells expressed distinct sets of inflammatory genes during liver injury. Our study illuminates a novel classification of the stromal/progenitor cell compartment in the liver and pinpoints a hitherto unrecognized injury-related alteration in progenitor subset composition in chronic liver inflammation and fibrosis.
Keywords: fibrosis, NASH, inflammation, oval cells
chronic liver diseases often lead to extensive cellular damage, fibrosis, and ultimately to cirrhosis and organ failure. While hepatocytes provide the major source for liver regeneration, liver damage is accompanied by extensive progenitor cell expansion (7). These cells termed oval cells are quiescent at steady state and emerge in the portal or periportal area during injury (7, 27, 30, 38, 49). They exhibit bipotential differentiation capacity and are able to give rise to both hepatocytes and cholangiocytes in vitro (7, 9, 38, 39) and in vivo (39, 42). However, genetic tracing of Foxl1+ (39) and Sox9+ (42) oval cells indicated that these cells do not contribute significantly to hepatocyte regeneration in vivo, demonstrating an undetermined role of progenitor cell expansion during liver injury. Multiple studies have tried to identify the unequivocal cellular and molecular characteristic of oval cells (6–9, 27), and a variety of markers such as CD45, CD133, CD90, CD34, and CD326 were associated with these cells. Many of these markers are corresponding to hematopetic stem cells or mesenchymal progenitor cells suggesting a heterogeneous population of precursors identified as oval cells within the liver. Despite of extensive studies, using primarily histology and PCR analyses, the characteristic marker combinations and molecular signatures remain elusive (7).
Gp38/podoplanin+ nonhematopoetic cells called stromal cells are present in secondary lymphoid organs (SLOs) and play central role in the formation and reorganization of the extracellular matrix providing the three-dimensional scaffold for immune responses(44). Additionally, they produce cytokines/survival factors for immune cell homeostasis and guide the migration and cellular interaction of immune cells within lymphoid organs (24). Moreover, gp38+ stromal cells are crucial in the functional regulation of T- and B-cell responses during immunity (4, 23). Importantly, gp38+ cells similar to the ones present in SLOs have been identified in nonlymphoid organs associated with chronic inflammation where they are building elements of tertiary lymphoid structures (TLS) (29, 45). In the liver, such a CCL21+ reticular network of TLS built by gp38+ stromal cells has been recently identified in human primary biliary cirrhosis (21). Additionally, gp38+ cells emerge during liver embryogenesis (16, 17) and in adult liver as part of the lymphatics and the mesothelium and in low abundance in the luminal part of bile duct (19, 22). Nevertheless, little is known about the function of these cells, especially in the adult liver.
Thus we aimed to study the phenotypic and functional characteristics of gp38+ cells at steady state and during chronic liver inflammation. Using a novel isolation technique and analysis of gp38+ cells, we determined multiple subsets of nonhematopoetic/stromal cells of the liver that represent components of the progenitor cell response during liver injury. Moreover, we could identify differences in the responding subsets dependent on the type of injury and in the expression of inflammation associated genes, suggesting a hitherto unrecognized division of labor within the hepatic progenitor cell compartment.
MATERIALS AND METHODS
Mice.
Mice were obtained from Charles River (Sulzfeld, Germany) or Jackson Laboratories (Bar Harbor, ME). MDR2−/− animals were maintained under specific pathogen free conditions at the Helmholtz Centre for Infection Research (Braunschweig, Germany) or at the Translational Animal Research Center (Mainz, Germany). Animals were transported to Homburg and housed in pathogen free conditions in an assigned mouse cabinet (Bioscape, Castrop-Rauxel, Germany) in the Institute of Internal Medicine II (Saarland University, Homburg, Germany). All experimental procedures were conducted with the approval of the ethics and animal care committees of Homburg and Mainz University Medical Centers. Mice received CCl4 as described previously (35) for 3 or 6 wk (fibrosis progression), or CCl4 was stopped after 6 wk and mice were killed 1 wk later (regression phase). Seven- to eight-week-old C57Bl/6 male mice were fed methionine and choline-deficient (MCD) (catalog no. A02082002B) or control (CD) (catalog no. A02082003B) diet for 5 wk (Research Diets, New Brunswick, NJ). Control diet contained identical ingredients as the MCD diet, except it was methionine and choline sufficient (Research Diets). Subgroup of animals received the CD for 1 wk after termination of the MCD diet (regression phase).
Statistics.
For statistical analyses Prism 5 (Graphpad Software) was used. Data were compared using an unpaired two-tailed t-test or one-way ANOVA using Bonferoni posttest (*P < 0.05, **P < 0.005, and ***P < 0.0001).
Preparation of liver single cell suspension.
The liver was perfused (5 ml/min) through the portal vein with digestion buffer [RPMI containing 0.1 mg/ml DNase-I (Life Technologies, Darmstadt, Germany), 0.2 mg/ml collagenase P (Roche, Mannheim, Germany), and 0.8 mg/ml Dispase (Roche)] until the liver turned light. The liver was removed, cut into small pieces, and digested for 60–80 min at 37°C. During this time period the digestion was interrupted as follows: at 5–10 and 15 min, samples were mixed by agitating the tubes; at 20 and at 30 min, samples were mixed using 1,000-μl pipette tip where the tip was cut to allow larger pieces to pass through. At 45 min, noncut pipette tips were used. (For fibrotic samples additional mixing step was performed using noncut pipette tips.) Then, samples were mixed every 5 min using uncut pipette tips until the liver was completely digested. After each mixing step, organ pieces were allowed to settle down and supernatant, containing dissociated cells were collected, centrifuged and resuspended in RPMI with 2 mM EDTA 1% FCS, and filtered (100-μm mesh), and red blood cells were lysed using ACK lysis buffer (Life Technologies).
Flow cytometry of liver cells.
Cells were counted using a MacsQuant Analyzer (Miltenyi Biotec, Bergisch Gladbach, Germany). Cell debris and doublets were gated out using FSC-A vs. SSC-A and FSC-A vs. FSC-H gates, respectively. Dead cells were distinguished using DAPI (Life Technologies) or propidium iodide (PI; Miltenyi Biotec). Then, 5 × 105 living, single cells were stained in FACS buffer (MacsQuant Running solution; Miltenyi Biotec). First, cells were resuspended in 50 μl FACS buffer containing murine Fc-block solution (10 μl/staining; Miltenyi Biotec) and anti-CD64 antibody (clone: X57-5/7.1, 1:100; Biolegend, San Diego, CA) and incubated for 5 min on ice, followed by the addition of antibodies to various surface markers in 50 μl buffer and incubation for further 20 min on ice. Samples were washed and measured using a MacsQuant Analyzer (Miltenyi) or FACS Aria III (BD Biosciences, Heidelberg, Germany). For intracellular labeling, cells were fixed, permeabilized, and stained using the Cytofix/Cytoperm kit (BD Biosciences), following the manufacturer's guidelines. Data were analyzed using FlowJo 10.0.7 software (FlowJo, Ashland, OR).
Flow cytometry sorting of stromal cell subsets.
Liver single cell suspensions were prepared as described above. Progenitor cells were enriched using magnetic beads as follows: 2 × 107 cells excluding debris via FSC-A vs. SSC-A gate were resuspended in 400 μl MACS buffer (Automacs Running buffer containing 0.1% BSA; Miltenyi Biotec) and incubated with 10 μl CD133 microbeads (Miltenyi Biotec) at 4°C for 15 min. In some cases, cells were resuspended in 400 μl MACS buffer containing mouse Fc block solution and anti-CD64 antibody and incubated on ice for 5 min, followed by surface staining of gp38 (clone: 8.1.1; Biolegend). Than samples were washed and resuspended in 400 μl MACS buffer containing 10 μl anti-APC microbeads (Miltenyi Biotec) and incubated at 4°C for 15 min. Respective fractions were enriched by an AutoMACS Cell Separator (Miltenyi Biotec) using the possel(s) program, according to the manufacturer's instructions. Cells were counted, stained, and sorted using FACSAria III (BD Biosciences) fitted with an 85-μm nozzle and at a pressure not exceeding 45 psi. Two healthy and one or two diseased livers were pooled for sorting 4,000–7,000 cells of each progenitor subset first in DMEM (Life Technologies) containing 20% FBS and than directly in RLT or RLT plus buffer (Qiagen, Hilden, Germany) and stored at −80°C until further use. Purity of sorted populations routinely exceeded 85–90%.
Antibodies and reagents.
Cells were stained using the following antibodies: CD45 (30-F11), gp38 (8.1.1), CD31 (MEC13.3), Epcam-1 (G8.8), CD90.2 (53-2.1), CD117 (2B8), CD105 (YN1/1.7.4), CD106 (429), CD26 (H194-112), CD133 (315-2C11), and CD157 (BP-3) were purchased from Biolegend (San Diego). CD133 (MB9-3G8), CD90.1 (His51), and Sca-1 (D7) were acquired from Miltenyi Biotec. CD34 (RAM34) and G-SCF (9B4CSF) were obtained from eBioscience (Frankfurt, Germany) while M6a (321) from MBL (Woburn, MA). Additionally, desmin was from Abcam (Cambridge, UK), and CCL21, CCL19, Asgpr1, TSG-6, and Pref-1 were from Bio-Techne (Minneapolis, MN). All secondary antibodies (anti-rabbit/anti-goat/anti-hamster/anti-rat labeled with either Alexa-488, Alexa-647 or Alexa-555) were obtained from Life Technologies.
Quantitative PCR analyses.
Cells were lysed in RLT or RLT plus buffer (Qiagen), snap-frozen, and stored at −80°C. RNA extraction, RNA amplification, and custom qPCR array (array ID: CAPM13093) analyzes were performed at Qiagen. Raw data provided by Qiagen were analyzed in house. For some sorted samples RNA was prepared using Qiagen RNeasy/plus kit and first-strand cDNA synthesis were performed using QuantiTect reverse transcription kit (Qiagen) according to the manufacturer guidelines. qPCR was carried out with validated primers in 10-ul reactions using the Fast SYBR Green qPCR reagent (Life Technologies) and the following program: 50°C for 2 min, 95°C for 2 min, and 40 cycles of amplification at 95°C for 15 s and 60°C for 45 s. Relative levels of target mRNA were compared with β2-microglobulin using the 2−ΔΔCT method. Primers were purchased from Bio-Rad (München, Germany) or from Qiagen.
Immunofluorescent staining of frozen tissue sections.
Livers were fixed in 4% PFA in PBS for 4 h followed by a 4-h incubation in PBS containing 15% sucrose and overnight incubation in PBS containing 30% sucrose at 4°C. Tissue was snap frozen in OCT media (Thermo Fischer Scientific, Waltham MA) on dry ice. Seven-micrometer-thick cryosections were air-dried and fixed with 2% PFA for 10 min, followed by a 30-min incubation with Image-iT FX signal enhancer (Life Technologies). Unspecific binding was blocked by PBS containing 3% BSA or BlockAid blocking solution (Life Technologies) for 1–2 h. In some cases, endogenous biotin was blocked by using the biotin-blocking system (Dako, Hamburg, Germany) before the blocking step according to manufacturer guidelines. Samples were stained with primary antibodies and secondary antibodies in 3% BSA in PBS or in BlockAid blocking solution for 1 h and then were washed three times with 0.01% Tween in PBS. For staining of cell nuclei, cells were incubated with PBS containing DAPI (1:1,000; Life Technologies) for 20 min. Samples were washed three times with PBS and covered with Fluorescence mounting medium (Dako, Hamburg Germany). Samples were imaged using LSM 780 Zeiss laser scanning confocal microscope. Images were analyzed using Zen (Zeiss) or ImageJ 1.46r software (National Institutes of Health, Bethesda, MD).
RESULTS
Gp38+ stromal cells are present in healthy liver and expand during chronic liver injury.
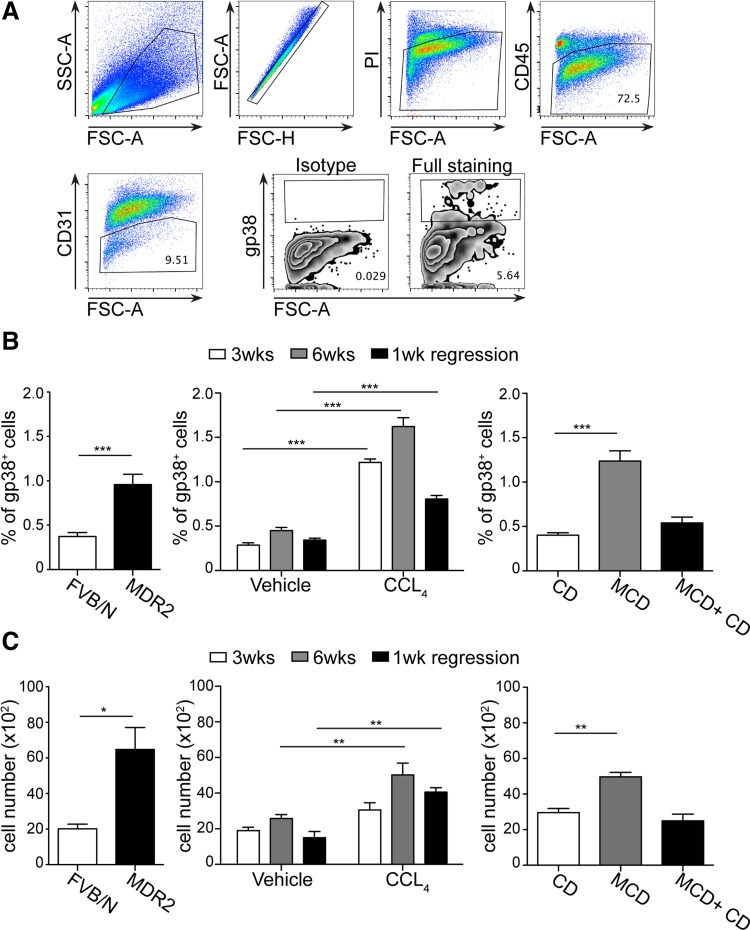
In stromal biology, nonhematopoetic (CD45−) cells are collectively referred to as stromal cells (23). In the article, we refer to stromal cells as nonhematopetic cells where endothelial cells (CD31−) and hepatocytes (asialoglycoprotein receptor 1 negative, Asgpr1−) are additionally excluded. To study podoplanin positive (gp38+) stromal cells in the liver, we established a novel digestion method for liver similar to the one used for lymph node stromal cells (23). Importantly, gp38+ cells were readily detectable in healthy livers and represented up to 0.3–0.5% of CD45− living cells (Fig. 1, A and B). To assess how gp38+ cells are altered during liver inflammation, their presence was analyzed in various chronic liver injury models, i.e., spontaneously progressive biliary fibrosis, CCl4-induced fibrosis and a model of nonalcoholic steatohepatitis (MCD diet). The percentage and absolute number of gp38+ cells increased significantly in all three injury models (Fig. 1, B and C). Moreover, when liver inflammation ceased during resolution, the amount of gp38+ cells decreased but was still increased in the CCl4 model and comparable to healthy values in MCD model (Fig. 1, B and C). The reduction of gp38+ cells in the CCl4 model during regression has been associated with early inflammatory response that decreases after 1 wk while scar resolution extends for a longer period of time (15). These data overall indicate a strong association between the presence of gp38+ cells and liver inflammation.
Fig. 1.
Gp38+ stromal cells are present in healthy liver and expand during chronic liver injury. A: single cell suspension was prepared from healthy livers and subsequently stained with a panel of surface markers: CD45, CD31, and gp38. For dead cell exclusion propidium iodide (PI) was utilized. Representative dot plots with gating strategy are depicted. B: percentages of gp38+ stromal cells present among the CD45-negative cells in control animals and in chronic liver injuries. C: absolute numbers of gp38+ cells were calculated within 106 living cells. FSC, forward scatter; SSC, scatter. Data are means ± SE. Data in A–C represent 2–3 independent experiments with n = 3–4 per experiment. *P < 0.05, **P < 0.005, and ***P < 0.0001.
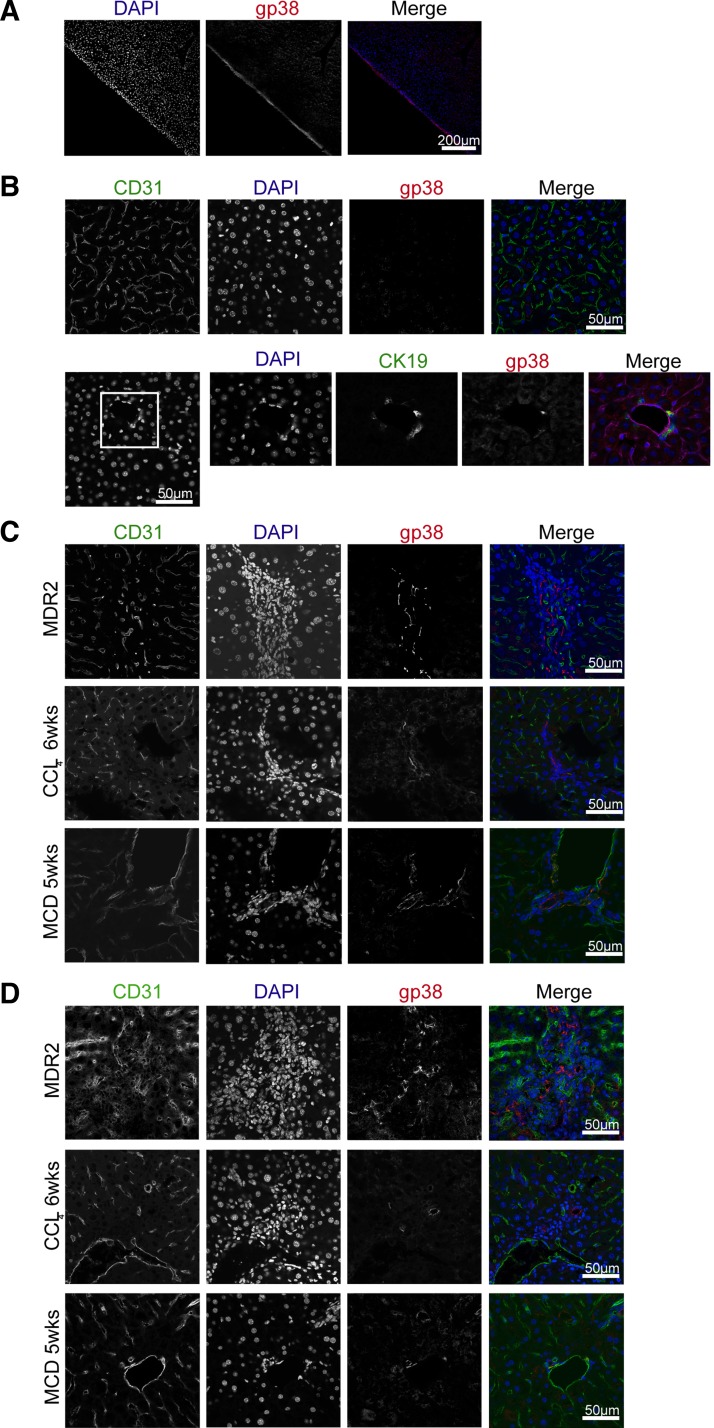
Next, we sought to understand where gp38+ cells localize during liver injury. As previously demonstrated, healthy liver exhibited strong podoplanin expression in the capsular area that was previously identified as mesothelium (19, 22) (Fig. 2A). Within the liver parenchyma, gp38+ cells in healthy liver could rarely be identified (Fig. 2B) but were detected during liver injury in the liver parenchyma where they typically localized in cell dense areas defined by closely packed DAPI+ cells (Fig. 2C). Such cell-dense regions were mostly close to the portal or periportal area (Fig. 2C) and contained various inflammatory cells (data not shown). This localization was prevalent in all three liver injury models but most prominent in MDR2-deficient animals (Fig. 2C). Moreover, within these structures CD31+gp38+ lymphatic endothelial cells could be identified in the absence of high endothelial venules (data not shown), suggesting that these cell-dense areas are not associated with classical tertiary lymphoid structures as identified in other organs (45). Furthermore, occasionally, gp38+ cells appeared as ductular structures within the parenchyma in all liver injury models investigated (Fig. 2D).
Fig. 2.
Gp38+ stromal cells localizes at cell dense structures of the injured liver. Healthy (A and B) or injured (C and D) livers were removed, preprocessed, and snap frozen. Cryosections were prepared and stained for gp38, CD31, and DAPI. Images were recorded using ×20 (B) or × 40 (A, C, and D) objectives. Scale bar = 200 μm (B) or 50 μm (A, C, and D). B, bottom: white square pinpoint area that were zoomed in. Data are representative of 2 independent experiments, n = 3 mice per group and 1 mold per organ per mouse. From each mold multiple sections were analyzed in 100- to 300-μm distances within the tissue. MCD, methionine and choline-deficient diet.
Podoplanin and CD133 define a heterogeneous population of nonhematopoetic stromal cells.
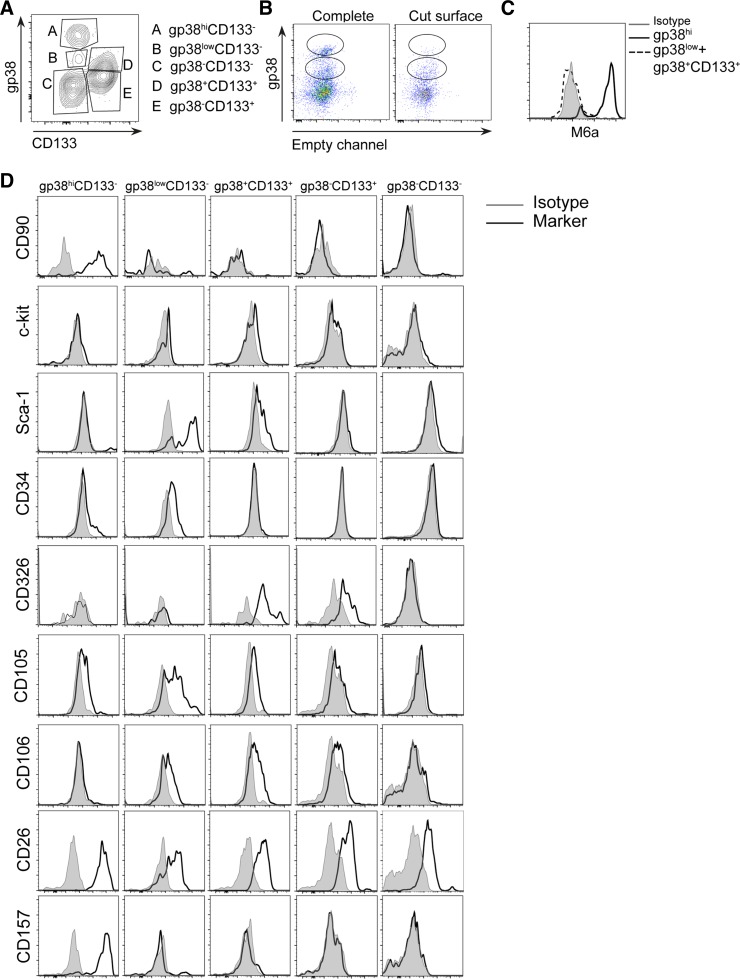
Closer evaluation of gp38 expression detected via flow cytometry demonstrated two different levels of signal intensity determining a population with low and another population with high expression level of gp38 in healthy liver (Fig. 1A). To further establish whether cells with different podoplanin expression indicate phenotypically divergent subsets, additional surface markers were evaluated. With the use of gp38 together with anti-CD133, a marker associated with progenitor cells in chronic inflammation (38), multiple subsets could be distinguished among CD45−CD31−Asgpr1− living cells: gp38hiCD133−, gp38lowCD133−, and gp38+CD133+ (Fig. 3A). Moreover, a fourth population of cells expressing CD133 but not gp38 (gp38−CD133+) and a fifth population that was negative for both markers (gp38−CD133−) (Fig. 3A) were identified. Thus the flow cytometry analyses indicated that among the CD133+ cells, previously identified as progenitor/oval cells, a novel subpopulation could be separated expressing gp38+ (Fig. 3A) (38).
Fig. 3.
Podoplanin and CD133 define a heterogeneous cell population consisting of multiple subsets. A: liver single cell suspension was stained with PI, CD45, CD31, Asgpr1, gp38, and CD133. Representative dot plot is displayed of PI−CD45−CD31-Asgpr1− cells. B: total liver and liver without the external capsule (cut surface) were digested and stained for PI and gp38. Dot plot depicts PI− cells. C: liver cells were stained with PI, gp38, and M6a. Representative histograms are shown. D: liver was stained as in A and with 1 of the depicted surface markers. Representative histograms are shown. Data in A–D represent 3 independent experiments with n = 3–4 per experiment.
Since the strongest podoplanin expression in microscopy images could be detected in the capsular area of the healthy liver (Fig. 2A), we studied whether the gp38hi cells (gp38hiCD133−) in the flow cytometry analyses could represent this cell population. Indeed, gp38hi cells could not be detected when liver was digested without the capsule (Fig. 3B). Additionally, only these cells were positive for the mesothelial marker glycoprotein M6a(19, 22) (Fig. 3C). Thus the gp38hiCD133− cells represent the mesothelium in the single cell suspension.
To further ascertain phenotypical differences among podoplanin positive cells and their relation to the CD133+ oval cells, liver samples were tested for the expression of various surface molecules associated with mesenchymal stem and liver precursor cells under steady state. Evidently, all subsets expressed the mesenchymal maker CD26 while mesothelial cells, appearing as gp38hi cells, exclusively expressed CD90 and CD157 (Fig. 3D and Table 1). Moreover, gp38lowCD133− cells were the only subset that expressed low levels of CD34 (hematopoetic stem cell marker) and was associated with the highest expression of Sca-1 (Fig. 3D and Table 1). The gp38+CD133+ population was negative or expressed lower levels of all above-mentioned markers (Fig. 3D and Table 1) but represented the highest CD326 (EpCAM) expressing cell population. As expected the gp38−CD133+ cells were similar to gp38+CD133+ but showed a lower expression of CD326 and were negative for the mesenchymal marker CD105 and for CD106 (VCAM; Fig. 3D and Table 1). Except for CD26, gp38−CD133− cells did not express any of the mesenchymal or progenitor-related surface markers investigated, thus representing the least characterized stromal population. In summary, gp38hiCD133− cells are mesothelial cells, the gp38lowCD133− cells represent cells expressing mesenchymal and precursor-associated molecules, and gp38+CD133+ cells seem to be a subpopulation of the classical oval cell compartment previously identified as CD133+ cells (7).
Table 1.
Summary of surface markers
| Markers | gp38hiCD133− | gp38lowCD133− | gp38+CD133+ | gp38−CD133+ | gp38−CD133− |
|---|---|---|---|---|---|
| CD90 | +++ | − | − | − | − |
| CD117 | − | − | − | − | − |
| Sca−1 | − | +++ | + | − | − |
| CD34 | − | + | − | − | − |
| CD326 | − | − | +++ | ++ | − |
| CD105 | + | ++ | + | − | − |
| CD106 | − | + | + | − | − |
| CD26 | ++ | ++ | ++ | ++ | ++ |
| CD157 | +++ | − | − | − | − |
+++: High expression level; ++: moderate expression level; +: low expression level; −: negative expression.
Subset distribution during liver inflammation reveals injury specific differences.
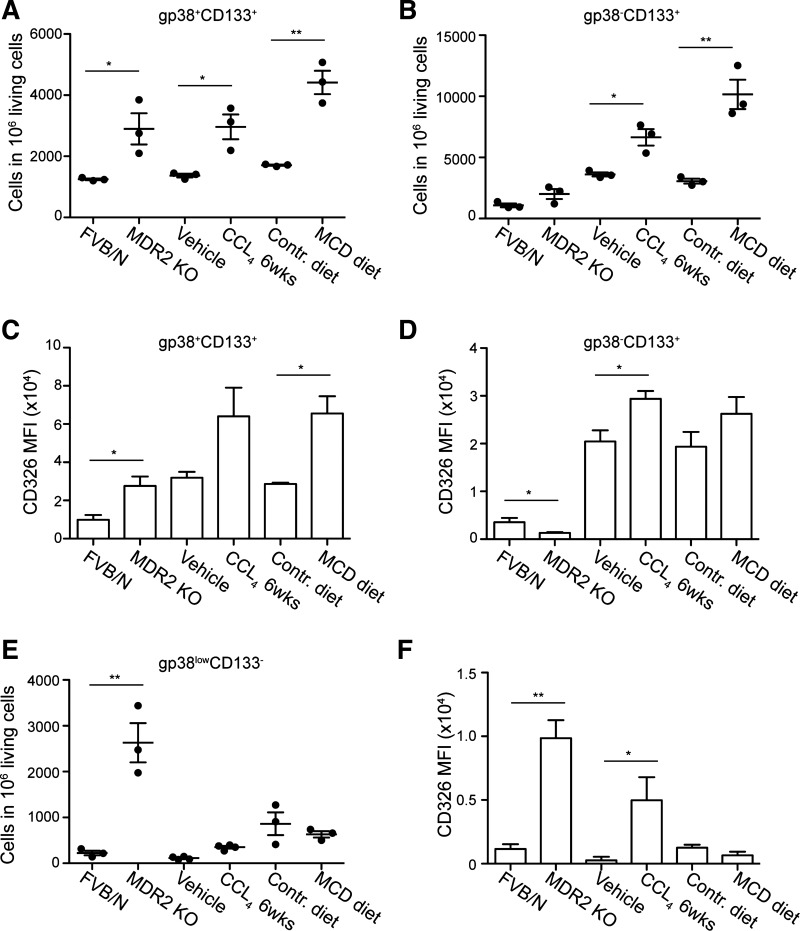
To assess the contribution of the above-described subsets in the overall increase of the gp38+ population found in liver injury (Fig. 1, B and C), we determined the individual changes in each gp38+ group. The subset that increased in all three liver injury models was the gp38+CD113+ population (Fig. 4A). These cells seemed to be a subpopulation of CD133+ cells (Fig. 3, A and D) previously identified as liver progenitors (38). Notably, the gp38−CD113+ population increased in liver injury albeit only significantly in the CCl4 and MCD models (Fig. 4, A and B). The increased frequencies of both cell populations during CCl4 treatment were accompanied by elevated expression of CD326 (Fig. 4, C and D). In contrast, MDR2 KO mice did not show a significant increase in the frequency of gp38−CD113+ cells and rather demonstrated a decrease in CD326 (EpCAM) expression in this cell population compared with wild-type controls (Fig. 4D). Additionally, the frequency of gp38lowCD113− cells and their CD326 expression were highly elevated, suggesting the presence of a specific inflammatory milieu selectively favoring the expansion of this subpopulation in this type of biliary fibrosis (Fig. 4, E and F). In accordance with this, the frequency of the gp38lowCD113− population was largely unaltered in the other two models of liver inflammation despite an elevated CD326 expression in CCl4-mediated liver injury (Fig. 4F). In accordance with analyses in healthy animals (Fig. 3D), the expanding gp38lowCD113− population in MDR2-deficient animals represented Sca-1+CD34+ cells and additionally contained cells positive for the cholangiocytes/progenitor differentiation marker CK19 (data not shown).
Fig. 4.
Subset distribution during liver inflammation reveals injury specific differences. Liver from healthy animals was digested and single cell suspension was prepared. Cells were stained with PI, CD45, CD31, gp38, and CD133. The absolute numbers of the gp38+CD133+(A), gp38−CD133+ (B), and gp38lowCD133− (E) are depicted within 106 living cells present in the single cell suspension of control and injured livers. The median fluorescence intensity of CD326 expression is depicted for the gp38+CD133+ subset (C), for the gp38−CD133+ cells (D), and for the gp38lowCD133− cells (F). Data are means ± SE. Data in A–F represent 2–3 independent experiments with n = 3–4 per experiment. *P < 0.05 and **P < 0.005.
Gp38+CD113+ cells exhibit oval cell characteristics.
Since gp38+ cells have been identified in the CD133+ cell compartment that were identified as progenitor/oval cells in injured liver, we next investigated whether the gp38+CD113+ subpopulation exhibit similarities to CD133+ oval/progenitor cells. To this end, gp38+CD113+ and gp38−CD113+ cell populations were sorted from healthy liver and further characterized by qPCR analyses of oval cell-related genes. Accordingly, gp38 was expressed only in the gp38+CD113+ cell and was missing in the gp38−CD113+ population, while CD133 (Prom1) was present in both populations underlining the purity of the samples on the genetic level (Fig. 5A). Interestingly, transcription factors associated with oval cells such as FoxJ1 and Sox9 could be detected in both cell types. Additional genes associated in previous studies with bipotential progenitor/oval cells such as albumin (Alb), alpha-fetoprotein (Afp), and cytokeratin 7 and 19 (Krt7, Krt19) were also equally present in both group of cells (Fig. 5A). Moreover, aldehyde dehydrogenase (Aldh1a1), an enzyme that activity enriched progenitor cells in healthy liver (6), and all tested genes involved in multiple pathways associated with stem/progenitor cell differentiation or maintenance such as members of the Notch, Wnt, hedgehog, and Hippo pathways (11, 47) could be detected equally in both cell types in healthy liver (Fig. 5, A and B), indicating that both population belongs to the oval/progenitor compartment previously identified as CD133+ cells in the liver (7, 8, 38).
Fig. 5.
gp38+CD133+ cells exhibit oval cell characteristics. gp38+CD133+ and gp38−CD133+ subsets were sorted with high purity, RNA and cDNA were prepared and qPCR was performed. Graph depicts the ΔCT values of each subset compared with its housekeeping gene (β2-microglobulin) ± SE for the listed genes. A: progenitor-related genes. B: various signaling pathway-associated genes. From each subset 3 independent samples were sorted and processed.
As gp38+ stromal cells in secondary lymphoid organs and in nonlymphoid organs express a high level of homeostatic chemokines (24), we sought to determine whether their presence would be associated with the expression of gp38+ stromal cell-related chemokines CCL19 and CCL21 (24) also in the liver. Notably, none of the populations expressed these two chemokines in the liver, while as expected CCL21 expression was detected in lymphatic endothelial cells of normal livers (data not shown). Thus gp38+CD113+ and gp38−CD113+ display oval/progenitor characteristic and differ from lymph node stromal cells.
The gp38+CD113+ subpopulations demonstrate differences in genes related to inflammation.
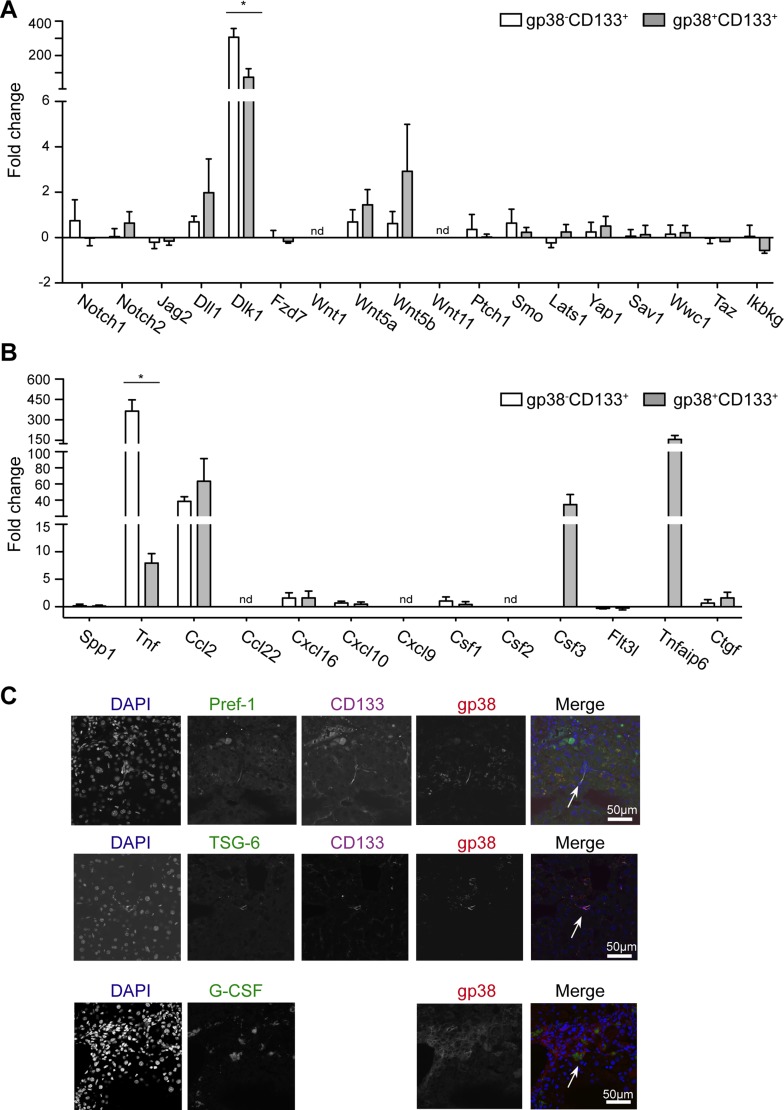
Gp38 separated the CD133+ population into two subsets (Fig. 3A). Accordingly, the gp38+CD113+ cells showed alterations in their frequency in all injury models investigated and seemed to change together with the gp38−CD113+ cells (Fig. 4, A–D). To determine whether the new subpopulation (gp38+CD133+) functionally differs from the main oval/progenitor population (gp38−CD133+) (7, 8, 38), high purity sort of these populations was performed from healthy and CCl4-treated animals. Importantly, not short-term injection of CCl4, but chronic CCl4 treatment-induced liver fibrosis demonstrated the presence of oval/progenitor cells in previous studies (18, 36). Indeed, in the two cell populations genes related to oval cells remained similar with and without CCl4 treatment, and only Afp levels were upregulated in both populations (data not shown). The expression levels of genes related to the Notch, Hh, or Wnt pathways were largely unchanged between cells from control vs. fibrotic mice except Wnt5a, Wnt5b, and Dll1, which showed a modest upregulation in both cell populations, while in DPs Ikbkg (Nemo) was slightly repressed (Fig. 6A). Dlk1 (Pref-1), previously associated with hepatocyte differentiation in embryonic liver (41), was markedly upregulated in gp38−CD113+ but only moderately increased in gp38+CD113+ cells (Fig. 6A). When histology sections were investigated, Pref-1 protein was detected in cell-dense structures during CCl4 injury in line with the mRNA data (Fig. 6C).
Fig. 6.
gp38+CD133+ and gp38−CD133+ cells demonstrate differences in inflammatory genes. qPCR was performed from sorted cells. Graph depicts mean fold change ± SE compared with healthy animals. Three independent sorts/subset were performed. A: genes are related to Notch, Wnt, hedgehog, and Hippo pathway. B: genes are involved in inflammation and myeloid-lymphoid cell interaction. C: cryosections from CCl4-treated liver stained for gp38, (CD133), DAPI, and either Pref-1, TSG-6, or G-SCF (×40 objectives; scale bar = 50 μm). Data are representative of 2 independent experiments, n = 3 mice/group and 1 mold/organ/mouse. *P < 0.05.
Since oval cell proliferation is associated with the activation of multiple inflammatory signaling pathways (11) and the direction of their differentiation is greatly influenced by myeloid cells (1), inflammation-related cytokines and growth factors were evaluated. Both groups expressed Csf1, Cxcl10, Spp1, and Flt3L and modestly upregulated Ctgf and CXCL16 in injury (Fig. 6B), all of which are molecules involved in cellular growth, in differentiation, and in the recruitment of immune cells (24, 44). Notably, upon injury CCL2 mRNA was nearly 60-fold increased in both cell types suggesting a strong association of these progenitor cells with monocyte and macrophage recruitment (Fig. 6B). In addition to this, TNF, an important inflammatory mediator was largely upregulated by gp38−CD113+ cells (∼300 fold) and showed just an ∼10-fold increase on RNA level in the gp38+CD113+ cell population (Fig. 6B). As opposite to this, TNFα-induced protein-6 (TSG-6), an anti-inflammatory molecule(3), and Csf3 (G-CSF) were selectively upregulated in gp38+CD113+ cells and were not expressed by the other population, indicating its selective role in inflammation. Consequently, at the protein level, G-CSF and TSG-6 could be detected within the liver parenchyma (Fig. 6C).
DISCUSSION
Emerging evidence suggests that gp38+ stromal cells are not only present in SLOs but are abundant in nonlymphoid organs during inflammation (29, 45). Additionally, gp38+ cells display multiple functions besides providing the structural framework for immune cells (4, 23, 24, 44). Here we show that the number of gp38+ cells expands during liver injury and decreases upon resolution, providing the first evidence for a close association of these cells with the status of liver inflammation. Importantly, these stromal cells were not components of classical TLSs in the three models of liver inflammation investigated. Thus the formation of TLS is associated with specific pathological processes in the liver as observed in human primary biliary cirrhosis (21) and murine granulomatous hepatitis (48). Additionally, multiple subsets of stromal cells could be identified within the healthy and injured liver: mesothelial cells (gp38hiCD133−), cells associated with multiple mesenchymal/progenitor surface markers (gp38lowCD133−), and a novel subpopulation (gp38+CD113+) within CD133+ progenitor compartment displaying oval cell characteristics. Except for DPPIV (CD26) the gp38−CD113− population did not express any of the additional surface makers used in this study requiring further characterization.
CD90 expression is associated with progenitor cells in multiple studies (16, 17, 43), most of which used in vitro expansion techniques to study oval cells. We identified CD90 as a selective marker associated with gp38hiCD113− mesothelial cells. Notably, in vitro expansion of the liver stromal fraction allows the proliferation of mesothelial cells (data not shown) possibly accounting for the presence of CD90 in prior studies. In the present study, mesothelial cells were also exclusively expressing CD157, also known as bone marrow stromal cell antigen-1. This molecule has been associated with immune regulation and inflammation (e.g., in rheumatoid arthritis) (5). This is especially intriguing as mesothelial cells express other immunoregulatory molecules such as CD200 and can trans-differentiate towards hepatic stellate cells and myofibroblasts (19, 22). Such a trans-differentiation process, however, only occurs within 150-μm distance close to the liver surface (19, 22). Importantly, gp38hiCD133− cells exhibited slightly increased frequencies during inflammation that could indicate their contribution to mesothelial-mesenchymal transition close to the liver surface or might reflect surface changes accompanying liver diseases. How the identified immunomodulatory CD157 might modulate the function of these cells remains to be determined.
Two surface molecules, Sca-1 and CD34, could unequivocally identify the gp38lowCD133− cells. CD34, a hematopoetic stem cell marker, has also been identified in bone marrow-mesenchymal stem cells and adipose tissue-derived stem cells (20). Importantly, the frequency of this progenitor population showed selective expansion in liver injury associated with Mdr2 deficiency. The absence of the hepatobiliary transporter Mdr2 biliary fibrosis resembles of primary sclerosing cholangitis in humans (13, 34). It remains to be elucidated whether the expanding population of gp38lowCD133− cells contributes to the pathogenesis of this liver disease. Although these cells did not change in CCl4-induced liver injury, their CD326 expression increased significantly. This may be due to the portal fibrotic component in both disease models (31). CD326 (EpCAM) has a dual role as cell adhesion molecule and receptor involved in the regulation of gene transcription and cell proliferation (26). Additionally, full-length EpCAM is a precursor for its signaling moiety that after cleavage translocates to the nucleus and promotes gene transcription (26). Thus the observed CD326 (EpCAM) upregulation could alter signaling events or adhesion properties in gp38lowCD133− progenitor cells that needs further clarification.
The gp38−CD133+ and the gp38+CD133+ subsets showed alterations either in frequency or in CD326 expression in all three liver injury models. This is in accordance with the identification of the CD133+ cells (involving both subsets) as progenitor/oval cells in previous studies (7, 8, 38). Additionally, this is in line with evidence that certain amounts of oval cells are present in fibrosis and in steatohepatitis not only in mouse models but in humans as well (14). Additionally, the gp38+CD133+ subset within the CD133+ compartment exhibited great similarities to the gp38−CD133+ cells in multiple progenitor-associated genes under steady state and in inflammation. Notably, the mRNA level did not differ in these populations between steady state and inflammation, despite the increase in CD326 expression on the protein level. The similarities in their gene expression profile may suggest two different developmental stages present of the same cell type that remain to be elucidated.
Upon CCl4 treatment, both cell subsets (gp38+CD133+, gp38−CD133+) showed similar changes in various pathway components related to hedgehog, Wnt, and Notch signaling. All of these pathways have been previously linked to oval cell function and liver regeneration (11). Specifically, the presence of Dlk1/Pref-1 in both populations on the mRNA level could be confirmed in CCl4-treated liver sections. Pref-1 is a noncanonical Notch ligand that acts as an inhibitor of Notch signaling in vitro (12), but its in vivo role is less well understood. Nevertheless, Pref-1 is strongly upregulated in regenerating liver and a neutralizing antibody decreased progenitor cell proliferation during regeneration (50). Moreover, Pref-1 promotes liver fibrosis progression via activating hepatic stellate cells (28), suggesting a direct profibrotic activity. This is in line with emerging evidence that hepatic progenitor activation is linked to a fibrogenic response (18, 37). How the expression of Pref-1 by the different progenitor subsets contributes to this process remains to be determined. During liver injury inflammatory mediators are released and various cellular interactions ensure progenitor cell activation and expansion (7, 11), a cellular process that is not completely understood in detail. Lymphocytes support progenitor cell activation (40) and macrophages influence the activation and the direction of the differentiation process (1, 10). According to this, gp38+CD133+ and gp38−CD133+ cells express multiple cytokines such as CCL2, Flt3l, and CXCL10 that recruit and influence various immune cells. This indicates a strong interplay between immune and stromal cells during liver injury and stresses further the complex role of progenitor cells in liver inflammation.
TSG-6, an anti-inflammatory protein, promotes liver regeneration in acute liver injury (25, 46). Although the molecular mechanism is not completely understood, the selective expression of TGS-6 in gp38+CD133+ pinpoints a progenitor subset specific role in this process. Besides TSG-6, DPs were selectively enriched in G-CSF during liver inflammation. Based on genetic analyses, CSF signaling occurs together with liver regeneration and progenitor expansion (2, 33) and has been recently identified in human livers (32), stressing its relevance in this process. Notably, G-CSF expression was not exclusively restricted to gp38+CD133+ cells but could be found in liver parenchymal cells (data not shown).
Taken together, we have delineated a close correlation between the abundance of gp38+ stromal cells and liver injury. With the use of various surface markers, multiple subsets of stromal cells were identified. Importantly, injury specific distribution of the identified subsets could be observed, outlining the complexity of inflammatory response and progenitor cell expansion accompanying liver diseases. This subset differences might reflect the phenotypic complexity and variance of ductular reaction observed in human liver diseases (14). In addition to this, the presented subset distribution is especially relevant, as previous studies mostly used a single surface marker to identify progenitor cells (6, 8, 9, 16, 27, 38), barely reflective of the true composition or function of this cellular compartment. This is especially true for markers that are exclusively associated with one subset such as CD90, CD34, and Sca-1. In multiple studies A6 antibody targeting an unknown epitope has been used as a classical oval cell marker to identify these cells in mice (8, 9, 30). It remains to be determined how the novel CD133+gp38+ subset relates to the distribution of this marker in situ. Additional studies are required delineating the plasticity and the cell specific contribution of the described subpopulations to liver inflammation, hepatocyte regeneration and fibrosis. Moreover, understanding the expanding stromal subpopulations and their interactions with immune cells could provide a better understanding of the pathomechanisms underlying these pathologies and yield novel therapeutic targets in chronic liver disease.
GRANTS
This work was supported by Collaborative Research Center 894 Grant (to E. Krause), Deutsche Krebshilfe Grant 111184 (to M. Kornek), a European Research Council Advanced Grant (to D. Schuppan), and the Alexander von Humboldt Foundation, Sofja Kovalevskaja Award (to V. Lukacs-Kornek).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.E., Y.O.K., H.J., E.-C.H., N.K., and V.L.-K. performed experiments; C.E., H.J., and V.L.-K. analyzed data; Y.O.K., M.K., D.S., and V.L.-K. interpreted results of experiments; E.K., T.T., M.K., F.L., D.S., and V.L.-K. approved final version of manuscript; F.L., D.S., and V.L.-K. edited and revised manuscript; V.L.-K. conception and design of research; V.L.-K. prepared figures; V.L.-K. drafted manuscript.
ACKNOWLEDGMENTS
We thank Marion Schwarz for excellent technical assistance with histology samples at the Institute of Anatomy and Cell Biology, University of Saarland.
REFERENCES
- 1.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18: 572–579, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen XG, Xu CS. Expression profiles uncover the relevance between colony stimulating factor-mediated signaling pathways in liver cells and partial hepatectomy-induced hepatic regeneration. Genet Mol Res 13: 6356–6366, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 118: 330–338, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, Chang J, Cremasco F, Harvey CJ, Wucherpfennig K, Ludewig B, Carroll MC, Turley SJ. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol 15: 973–981, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol 61: 301–332, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolle L, Best J, Empsen C, Mei J, Van Rossen E, Roelandt P, Snykers S, Najimi M, Al Battah F, Theise ND, Streetz K, Sokal E, Leclercq IA, Verfaillie C, Rogiers V, Geerts A, van Grunsven LA. Successful isolation of liver progenitor cells by aldehyde dehydrogenase activity in naive mice. Hepatology 55: 540–552, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Dolle L, Best J, Mei J, Al Battah F, Reynaert H, van Grunsven LA, Geerts A. The quest for liver progenitor cells: a practical point of view. J Hepatol 52: 117–129, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR, Grompe M. Surface markers for the murine oval cell response. Hepatology 48: 1282–1291, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, Smirnova O, Duncan AW, Finegold MJ, Sander M, Kaestner KH, Grompe M. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev 25: 1193–1203, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsegood CL, Chan CW, Degli-Esposti MA, Wikstrom ME, Domenichini A, Lazarus K, van Rooijen N, Ganss R, Olynyk JK, Yeoh GC. Kupffer cell-monocyte communication is essential for initiating murine liver progenitor cell-mediated liver regeneration. Hepatology 62: 1272–1284, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Erker L, Grompe M. Signaling networks in hepatic oval cell activation. Stem Cell Res 1: 90–102, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Falix FA, Aronson DC, Lamers WH, Gaemers IC. Possible roles of DLK1 in the Notch pathway during development and disease. Biochim Biophys Acta 1822: 988–995, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K, Marschall HU, Denk H, Trauner M. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 127: 261–274, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology 54: 1853–1863, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Jiao J, Sastre D, Fiel MI, Lee UE, Ghiassi-Nejad Z, Ginhoux F, Vivier E, Friedman SL, Merad M, Aloman C. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology 55: 244–255, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamo N, Yasuchika K, Fujii H, Hoppo T, Machimoto T, Ishii T, Fujita N, Tsuruo T, Yamashita JK, Kubo H, Ikai I. Two populations of Thy1-positive mesenchymal cells regulate in vitro maturation of hepatic progenitor cells. Am J Physiol Gastrointest Liver Physiol 292: G526–G534, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Konishi S, Yasuchika K, Ishii T, Fukumitsu K, Kamo N, Fujita N, Ikai I, Uemoto S. A transmembrane glycoprotein, gp38, is a novel marker for immature hepatic progenitor cells in fetal mouse livers. In Vitro Cell Dev Biol Anim 47: 45–53, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuramitsu K, Sverdlov DY, Liu SB, Csizmadia E, Burkly L, Schuppan D, Hanto DW, Otterbein LE, Popov Y. Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol 183: 182–194, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci USA 110: 2324–2329, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CS, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy 14: 1159–1163, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Link A, Hardie DL, Favre S, Britschgi MR, Adams DH, Sixt M, Cyster JG, Buckley CD, Luther SA. Association of T-zone reticular networks and conduits with ectopic lymphoid tissues in mice and humans. Am J Pathol 178: 1662–1675, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lua I, James D, Wang J, Wang KS, Asahina K. Mesodermal mesenchymal cells give rise to myofibroblasts, but not epithelial cells, in mouse liver injury. Hepatology 60: 311–322, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier A, Turley SJ. Regulated nitric oxide release by fibroblastic reticular cells and lymphatic endothelial cells controls the expansion of activated T cells within lymph nodes. Nat Immunol 12: 1096–1104, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev 251: 160–176, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci 116: 1863–1873, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res 69: 5627–5629, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, Nakamura K, Miyajima A. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development 136: 1951–1960, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Pan RL, Wang P, Xiang LX, Shao JZ. Delta-like 1 serves as a new target and contributor to liver fibrosis down-regulated by mesenchymal stem cell transplantation. J Biol Chem 286: 12340–12348, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peduto L, Dulauroy S, Lochner M, Spath GF, Morales MA, Cumano A, Eberl G. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J Immunol 182: 5789–5799, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Petersen BE, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology 37: 632–640, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Pinzani M, Macias-Barragan J. Update on the pathophysiology of liver fibrosis. Expert Rev Gastroenterol Hepatol 4: 459–472, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Piscaglia AC, Arena V, Passalacqua S, Gasbarrini A. A case of granulocyte-colony stimulating factor/plasmapheresis-induced activation of granulocyte-colony stimulating factor-positive hepatic progenitors in acute-on-chronic liver failure. Hepatology 62: 649–652, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Piscaglia AC, Shupe TD, Oh SH, Gasbarrini A, Petersen BE. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology 133: 619–631, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol 43: 1045–1054, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Popov Y, Sverdlov DY, Sharma AK, Bhaskar KR, Li S, Freitag TL, Lee J, Dieterich W, Melino G, Schuppan D. Tissue transglutaminase does not affect fibrotic matrix stability or regression of liver fibrosis in mice. Gastroenterology 140: 1642–1652, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pritchard MT, Nagy LE. Hepatic fibrosis is enhanced and accompanied by robust oval cell activation after chronic carbon tetrachloride administration to Egr-1-deficient mice. Am J Pathol 176: 2743–2752, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, Purdie DM, Demetris AJ, Clouston AD. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology 133: 80–90, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Rountree CB, Barsky L, Ge S, Zhu J, Senadheera S, Crooks GM. A CD133-expressing murine liver oval cell population with bilineage potential. Stem cells 25: 2419–2429, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Shin S, Walton G, Aoki R, Brondell K, Schug J, Fox A, Smirnova O, Dorrell C, Erker L, Chu AS, Wells RG, Grompe M, Greenbaum LE, Kaestner KH. Foxl1-Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev 25: 1185–1192, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strick-Marchand H, Masse GX, Weiss MC, Di Santo JP. Lymphocytes support oval cell-dependent liver regeneration. J Immunol 181: 2764–2771, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci 116: 1775–1786, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Tarlow BD, Finegold MJ, Grompe M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury. Hepatology 60: 278–289, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tirnitz-Parker JE, Tonkin JN, Knight B, Olynyk JK, Yeoh GC. Isolation, culture and immortalisation of hepatic oval cells from adult mice fed a choline-deficient, ethionine-supplemented diet. Int J Biochem Cell Biol 39: 2226–2239, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Turley SJ, Fletcher AL, Elpek KG. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat Rev Immunol 10: 813–825, 2010. [DOI] [PubMed] [Google Scholar]
- 45.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol 10: 664–674, 2010. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Lee JS, Hyun J, Kim J, Kim SU, Cha HJ, Jung Y. Tumor necrosis factor-inducible gene 6 promotes liver regeneration in mice with acute liver injury. Stem Cell Res Ther 6: 20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. Cell 157: 1324–1338, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoneyama H, Matsuno K, Zhang Y, Murai M, Itakura M, Ishikawa S, Hasegawa G, Naito M, Asakura H, Matsushima K. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med 193: 35–49, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yovchev MI, Zhang J, Neufeld DS, Grozdanov PN, Dabeva MD. Thymus cell antigen-1-expressing cells in the oval cell compartment. Hepatology 50: 601–611, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Zhu NL, Asahina K, Wang J, Ueno A, Lazaro R, Miyaoka Y, Miyajima A, Tsukamoto H. Hepatic stellate cell-derived delta-like homolog 1 (DLK1) protein in liver regeneration. J Biol Chem 287: 10355–10367, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]