Abstract
This manuscript summarizes and discusses adaptations of skeletal muscle vasculature induced by physical activity and applies this understanding to benefits of exercise in prevention and treatment of type 2 diabetes (T2D). Arteriolar trees of skeletal muscle are heterogeneous. Exercise training increases capillary exchange and blood flow capacities. The distribution of vascular adaptation to different types of exercise training are influenced by muscle fiber type composition and fiber recruitment patterns that produce different modes of exercise. Thus training-induced adaptations in vascular structure and vascular control in skeletal muscle are not homogeneously distributed throughout skeletal muscle or along the arteriolar tree within a muscle. Results summarized indicate that similar principles apply to vascular adaptation in skeletal muscle in T2D. It is concluded that exercise training-induced changes in vascular gene expression differ along the arteriolar tree and by skeletal muscle fiber type composition. Results suggest that it is unlikely that hemodynamic forces are the only exercise-induced signals mediating the regulation of vascular gene expression. In patients with T2D, exercise training is perhaps the most effective treatment of the many related symptoms. Training-induced changes in the vasculature and in insulin signaling in the muscle fibers and vasculature augment glucose and insulin delivery as well as glucose uptake. If these adaptations occur in a sufficient amount of muscle mass, exposure to hyperglycemia and hyperinsulinemia will decrease along with the risk of microvascular complications throughout the body. It is postulated that exercise sessions in programs of sufficient duration, that engage as much skeletal muscle mass as possible, and that recruit as many muscle fibers within each muscle as possible will produce the greatest benefit. The added benefit of combined resistance and aerobic training programs and of high-intensity exercise programs is not simply “more exercise is better”.
Keywords: angiogenesis, arteriogenesis, capillary, diabetes, muscle fibers
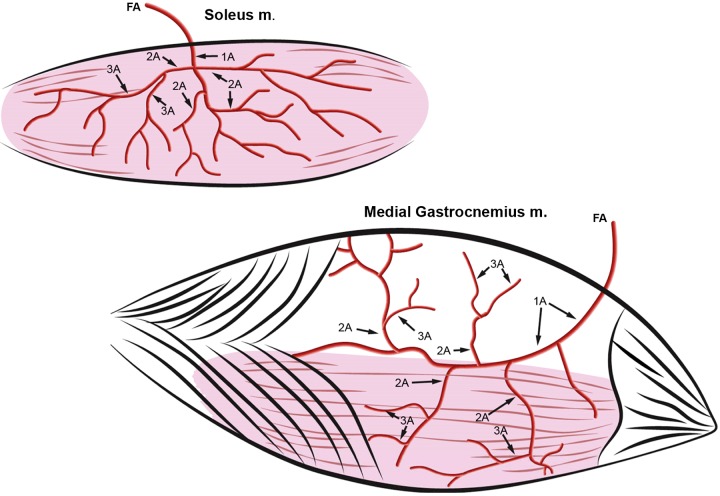
the purpose of this manuscript is to summarize and discuss the adaptations to skeletal muscle vasculature induced by physical activity and to apply this understanding to the use of exercise in prevention and treatment of type 2 diabetes (T2D). An important concept concerning physical activity-induced adaptation in skeletal muscle is that adaptations do not occur uniformly along the arteriolar tree in skeletal muscle. Figure 1 (top) illustrates the typical branching pattern observed in skeletal muscle, in this case the soleus muscle of the rat. As the feed artery (FA) of the soleus (Fig. 1) enters the epimysium of the muscle it is designated the 1A arteriole. The 1A arteriole branches off into 2A arterioles, and 2A arterioles branch off into 3A arterioles, and so on, out 10 to 15 branches to the capillaries. As discussed in detail below, current literature indicates that physical activity does not uniformly influence the arterioles of a skeletal muscle arteriolar tree. This is illustrated in Figure 2 where decreased physical activity produced by hindlimb unloading resulted in decreased dilation in response to ACh in the soleus FA and 1A arteriole but not in the 2A arterioles. As is discussed below, an additional source of nonuniformity of adaptation is that the effects of changes in physical activity are also not the same between muscles. For example, in Fig. 1, effects of exercise training on the 2A arterioles of the soleus may not be observed in the 2A arterioles of the red or white portions of the gastrocnemius muscle (GR and GW, respectively). The key concept then is that within skeletal muscle arteriolar networks the relative vasomotor responsiveness of each segment is tuned, through control of vascular cell gene expression, to the predominant signals present that control vascular structure and smooth muscle tone in those arterioles and resistance arteries. Current literature indicates that exercise training shifts these control factors and signaling mechanisms in vascular cells in a nonuniform pattern throughout the arteriolar tree in a muscle and that the signals differ with fiber type composition and recruitment among muscles. The goal of the discussion below is to focus on recent work related to exercise-induced adaptations in skeletal muscle arteriolar trees of subjects with T2D, but first we will review research in normal subjects to provide a foundation of understanding vascular adaptation in skeletal muscle as established by data in the current literature.
Fig. 1.
Drawings of arteriolar trees of the soleus muscle and gastrocnemius (medial head) muscles. In both muscles, the feed artery (FA) is the last artery entering the muscle prior to the epimysium and becomes the 1A arteriole under the epimysium; the 1A arteriole gives rise to second-order 2A arterioles, which give rise to the third-order arterioles (3A). In the gastrocnemius muscle, RG2A are the 2A arterioles that provide blood flow to the red portion of the muscle and WG2a are the 2A arterioles that provide blood to the superficial, white portion of the muscle (top of the gastrocnemius muscle). [Published with permission (71).]
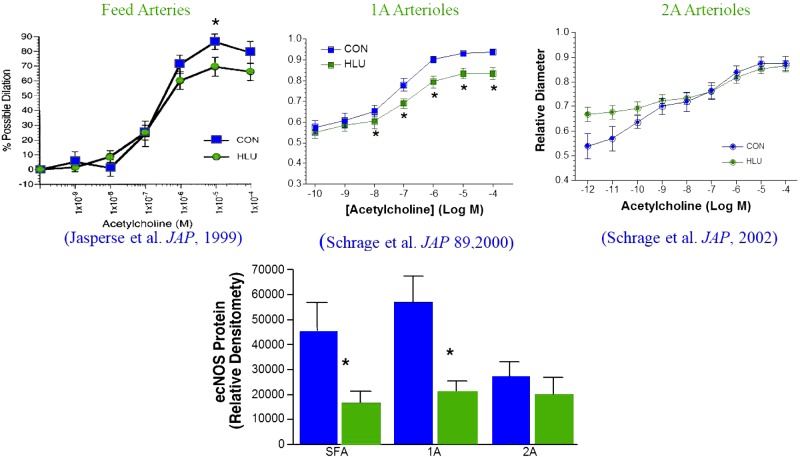
Fig. 2.
Effects of hindlimb unloading (HLU) on ACh-induced endothelium-dependent vasodilation of rat soleus feed arteries and arterioles. *Significantly different from control (CON). [Published with permission (51, 117, 118).]
EXERCISE TRAINING INCREASES MAXIMAL OXYGEN CONSUMPTION
Dr. Edward F. Adolph, who according to the American Physiological Society web site “. . . is best known for his research in environmental physiology, particularly in adaptation in hot and cold environments” defined adaptations as “modifications of organisms that occur in the presence of particular environments or circumstances. Physiological adaptations appear within the single individual, and constitute changes in its functions. The term ‘adaptations’ as here used includes phenomena sometimes labeled acclimatization and/or acclimations” (4). One key adaptation induced by chronic physical activity/exercise training is an increase in the maximal ability of an animal to consume oxygen (maximal oxygen consumption) (45, 127, 131).
The maximal oxygen consumption of an animal or person is best determined by measuring oxygen consumption with increasing exercise intensity (45, 113, 114, 131). Oxygen consumption increases with increasing exercise intensity until maximum is reached. At this point oxygen consumption no longer increases with increasing exercise intensity. Prolonged aerobic exercise training can increase an individual's maximal oxygen consumption, although the relationship between oxygen consumption and exercise intensity below maximal oxygen consumption is largely unaffected by training. Substantial literature is available that examines where along the “pathway for oxygen” maximal oxygen consumption is determined or limited between outside air and skeletal muscle mitochondria (127, 133). There are examples of each step in the transport pathway in various disease states that provide a limit to maximal oxygen consumption (113, 114). That is, maximal oxygen consumption can be limited by the ability of the respiratory system to maintain alveolar oxygen levels, by limited maximal cardiac output, limited capacity of the blood to carry oxygen (i.e., anemia), limited skeletal muscle blood flow capacity, limited skeletal muscle capillary exchange capacity, limited skeletal muscle oxidative capacity, or a combination of these (127, 132, 133). It appears that the step that constitutes the major limitation for oxygen transport may differ among normal mammals. In all animals examined, exercise training-induced increases in maximal oxygen consumption could result from increases in any one of these steps in the pathway for oxygen and/or from increased oxidative capacity of the skeletal muscle (45, 127, 131, 133). Over the years our research has focused on exercise training-induced adaptations in the skeletal muscle vasculature that increase skeletal muscle blood flow capacity and capillary exchange capacity and thereby contribute to increased maximal oxygen consumption.
EXERCISE TRAINING INCREASES SKELETAL MUSCLE VASCULAR TRANSPORT CAPACITY; BOTH CAPILLARY EXCHANGE CAPACITY AND BLOOD FLOW CAPACITY ARE INCREASED
Convective transfer of blood to (blood flow) and distribution of blood flow among skeletal muscle capillaries represents the first step in the process of transport in skeletal muscle vasculature because it brings nutrients into the exchange vessels. Diffusional transcapillary exchange from blood to tissue is the final step in oxygen transport from air to muscle mitochondria. Current literature establishes that exercise training increases skeletal muscle blood flow capacity in both young and old men and women (82, 124), and both capillary exchange capacity and blood flow capacity in various other mammals (66, 67). Transcapillary exchange in microvascular exchange vessels and delivery of matched blood flow are equally important to the supply of oxygen for muscle tissue. Studies in several animal models reveal that training increases skeletal muscle capillary diffusion capacity (63, 111, 121, 123). In addition to these findings from nonhuman species, Roca et al. (112) reported that 9 wk of endurance exercise training of human subjects increased oxygen-diffusing capacity in skeletal muscle by 33.5% during maximal exercise. Roca et al. (111) used the ratio of maximal oxygen consumption divided by mean capillary PO2 as an index of tissue diffusion capacity and determined that exercise training reduces diffusional limitations to oxygen transport. Thus tissue diffusion capacities in exercise-trained individuals who have higher maximal oxygen consumptions were nearly twice the values of sedentary individuals (111). Our results from exercise-trained rodents indicate that exercise training increases capillary exchange capacity because both capillary filtration coefficient for water and capillary diffusion capacity for lipid insoluble substances are increased (68, 74, 121, 122).
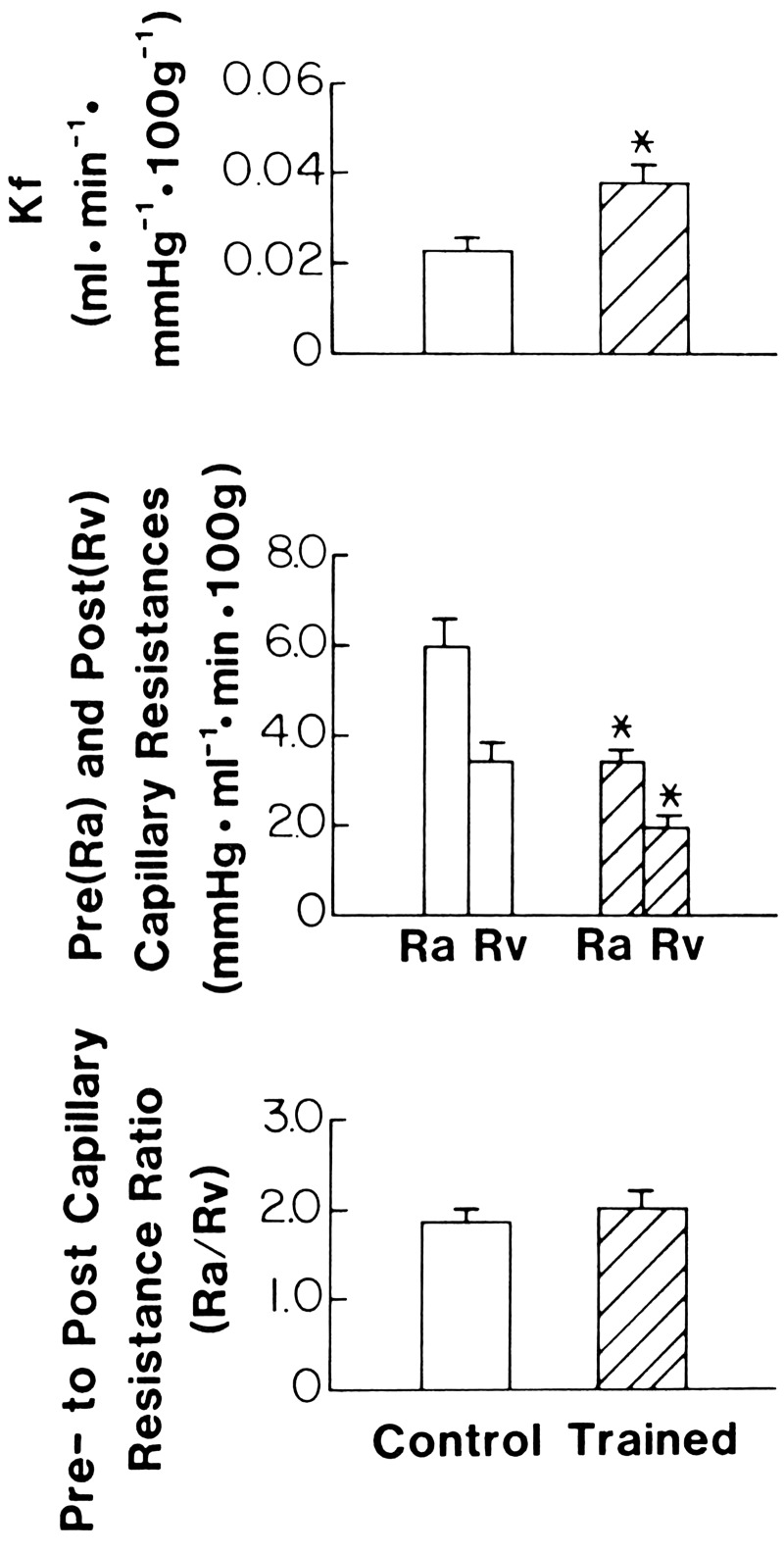
Skeletal muscle consists of three general phenotypes of muscle fibers classified according to both their contractile and metabolic properties (115): slow-twitch oxidative; fast-twitch glycolytic; and fast-twitch, oxidative, glycolytic (FOG). Skeletal muscle fiber type composition has a major influence on vascularization, capillary exchange capacity, vascular structure, and mechanisms of vasomotor control within and among muscles (5–9, 12, 48, 65–67). We have examined adaptation of vascular transport capacity in rat skeletal muscle using three training programs that we hypothesized would induce different spatial patterns of adaptation among skeletal muscles: low-intensity training (1 h/day, 5 days/wk, for 15 wk at a treadmill speed of 30 m/min, 0 incline) (8, 74); interval sprint training (6 bouts of 60 m/min up a 15% incline for 2.5 min each followed by 4.5 min rest, 5 days/wk, for 9 wks) (68, 121); and high-intensity endurance training (32 m/min, 15% incline, 90 min/day, 5 days/wk, 12 wk) (122). We evaluated vascular transport capacity in these models of exercise training with isolated perfused hindquarters and conscious rats running on a treadmill. Capillary exchange capacity and total and regional blood flows were measured in isolated perfused hindquarters (74, 121, 122), and total and regional skeletal muscle blood flows were measured in rats during treadmill exercise (8, 64, 68). Capillary exchange capacity was measured with two techniques: 1) the single-injection indicator dilution technique to measure the capillary diffusion capacity for 51Cr-labeled EDTA; and 2) gravimetric techniques to measure capillary filtration coefficients, isogravimetric capillary pressures, and pre- and postcapillary resistances (121). Low-intensity training resulted in 1) increased blood flow capacity, primarily in the red high-oxidative muscle tissue; 2) no significant changes in maximal capillary filtration coefficients or isogravimetric capillary pressures; and 3) increased capillary diffusion capacity for EDTA (67). We conclude that low-intensity training increased blood flow capacity specifically in the skeletal muscle tissue that had the greatest relative increase in activity during training bouts. Results of the interval sprint training study demonstrate that blood flow capacity is increased and that primary changes in blood flow capacity occur in FOG muscle tissue (68, 121). These results agree with those reported by Mackie and Terjung (79) who used a similar training program. As shown in Fig. 3, we also found that high-intensity exercise-trained rats had increased maximal capillary filtration coefficients, normal isogravimetric capillary pressures, and decreased pre- and postcapillary resistances (121). These physiologic results indicate that sprint training alters all three components of the microvascular tree: precapillary resistance vessels, postcapillary resistance vessels, and exchange vessels.
Fig. 3.
Effects of high-intensity exercise training on capillary filtration coefficient (Kf), precapillary resistance (Ra), postcapillary resistance (Rv), and the ration of pre- to postcapillary resistance (Ra/Rv) of rat hindquarters. Values are means ± SE. *Significant difference from control at P < 0.05. [Published with permission (121).]
We also examined high-intensity endurance training to further test the hypothesis of regional specificity of training-induced adaptations. We reasoned that if this hypothesis was correct, then this training program would produce increases in vascular transport capacity throughout the extensor muscles. This seemed appropriate because all fibers in extensor muscles should have increased activity at some point during training bouts of this intensity and duration (35). Results demonstrate that high-intensity endurance training produced increases in blood flow capacity of total hindquarters, increases in maximal capillary filtration coefficients, normal isogravimetric capillary pressures, and decreases in pre- and postcapillary resistances (Fig. 3) (121). Our hypothesis also predicted that blood flow capacity would increase in muscles of all fiber type compositions. In contrast, regional blood flow data indicated that the primary changes in blood flow capacity occurred in mixed muscle tissue because the relative training-induced change in blood flow capacity was linearly related to the % FOG composition of the muscles (123).
Thus overall results from our studies of regional blood flow capacity indicate that exercise training-induced adaptations are localized to the areas within skeletal muscles that have the greatest relative increase in muscle fiber activity during exercise training bouts (66, 67). This specificity of training-induced changes suggests that adaptations of muscle oxidative capacity and blood flow capacity are linked in skeletal muscle (3).
Optimal capillary exchange requires that regional capillary perfusion be matched to exchange capacity. A recent review by Poole et al. (100) clearly summarizes how important it is to recognize that most capillaries in resting skeletal muscle have moving red blood cells (RBCs) and that during exercise the increase in RBC flux is the result of a number of changes including increased RBC velocity, capillary hematocrit, and altered flow path through the capillaries that increases the “effectiveness” of the exchange area of the capillaries. More recent observations from those researchers (46, 47) confirm that exercise training increases capillary diffusion capacity through a number of adaptations in the microcirculation that improve matching of oxygen transport and oxygen utilization. These exercise training-induced adaptations cause improved muscle microvascular partial pressure of oxygen (PO2mv) kinetics in normal active muscle partially through increased nitric oxide (NO)-mediated dilation (46). Of interest, in animals with congestive heart failure when increased non-RBC flowing capillaries compromise oxygen transport, exercise training has similar effects on muscle PO2mv kinetics, but the adaptation does not appear to be NO-mediated (47).
There is growing appreciation of the importance of heterogeneities of blood flow and oxygen uptake in skeletal muscle because it is clear that if blood flow and capillary exchange area are not matched, oxygen transport will be limited due to poor diffusion (56, 66, 67, 69). Importantly, there is solid evidence that these heterogeneities are conserved across humans and animals (55, 101). We must keep in mind that variations in capillary blood flow not matched to exchange area can produce limitation of oxygen transport (108, 109). However, the presence of microvascular blood flow heterogeneity is not necessarily a sign of poor vascular function (56, 69). Indeed, because oxygen consumption of muscle tissue is not uniform during exercise, a heterogeneous distribution of blood flow is the most efficient mechanism to fulfill oxygen transport to a heterogeneous mass of muscle. Also, exercise training induces vascular adaptation heterogeneously within and among skeletal muscles (66, 67). This nonuniform vascular adaption in skeletal muscle results from a number of mechanisms including the heterogeneity of fiber type composition of skeletal muscle and the heterogeneous muscle fiber recruitment patterns generated by different modes and intensities of exercise. Also, as discussed below, muscle fiber recruitment patterns during exercise have a major influence on the regional distribution of adaptations in vascularization, capillary exchange capacity, vascular structure, mechanisms of vasomotor control, and regional distribution of blood flow within and among muscles during exercise (12, 48, 65–67, 83). Importantly, relationships among muscle fiber type, recruitment patterns, and blood flow are altered by exercise training (8, 68, 123) through changes in vascular structure as well as functional changes in endothelium (31–33, 57, 66, 67, 73, 84, 85, 93, 98, 120, 130, 134) and vascular smooth muscle of skeletal muscle arteries and arterioles (14–21, 59–62, 66, 67, 70, 92, 128, 129). Our results in rodents indicate that although many exercise training-induced vascular adaptations are concentrated in the muscle tissues that have the greatest increase in activity during training sessions (8, 11, 25, 42, 43, 68, 75, 78, 114, 121, 123), the relative amount of adaptation is not distributed uniformly for any of these parameters (66, 75) and these adaptations are not the same for different types of exercise training (8, 66–68, 94, 123). Thus different intensities and types of exercise activities require different fiber recruitment patterns, which subsequently influence the spatial distribution of exercise training-induced adaptations of muscle fibers and the associated microvasculature.
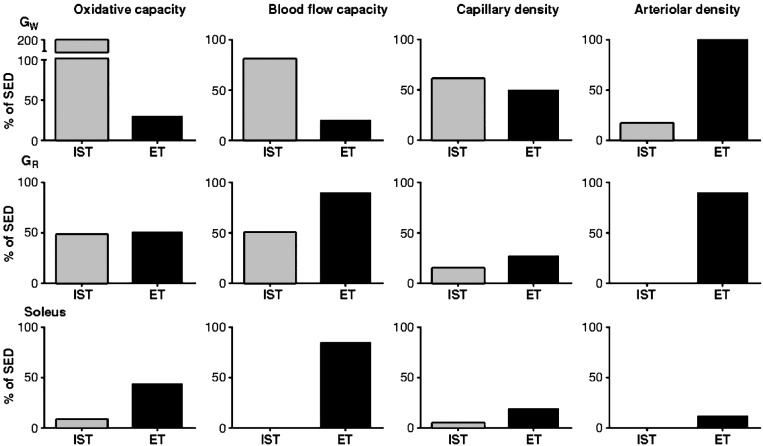
EXERCISE TRAINING-INDUCED STRUCTURAL VASCULAR ADAPTATION IN SKELETAL MUSCLE
Exercise training induces vascular adaptation in skeletal muscle through two general mechanisms: 1) vascular structural adaptation (angiogenesis of capillaries, remodeling and enlargement of arteries and arterioles and arteriogenesis), and 2) vascular function adaptation (i.e., altered control of vascular resistance) (66, 67). As shown in Fig. 4, endurance training produced increases in blood flow capacity in FOG (the red portion of the gastrocnemius; GR) and soleus muscle, whereas interval sprint training (IST) produced increases in blood flow capacity throughout the gastrocnemius muscle but not the soleus, and these changes in blood flow capacity seemed to be linked with changes in muscle oxidative capacity. In contrast, both types of training produced increased capillary density in the white (GW) and red (GR) portions of the gastrocnemius muscle (Fig. 4), but not in the soleus. Finally, arteriolar density was increased throughout the gastrocnemius muscle but it was not altered in the soleus by endurance training (ET) (Fig. 4). ET resulted in greater capillary and arteriolar densities in GW, but blood flow capacity was not altered. On the other hand, ET increased the blood flow capacity of soleus muscle but it did not alter capillary or arteriolar density. Thus as shown in Fig. 4, neither changes in capillary density nor arteriolar density explain the increases in skeletal muscle blood flow capacity. Our work has revealed that it is important to think of the arteriolar networks within the skeletal muscle, not just arteriolar density. Furthermore, model analysis indicates that changes in the arteriolar network of the gastrocnemius muscle induced by IST could explain the increases in blood flow capacity we measured in this muscle of interval sprint-trained rats (12). So the results summarized above and the results of others indicate that adaptations in the structure of the vascular bed of skeletal muscle induced by exercise training include greater capillary density and arteriolar density (network analysis indicates that this is focused in larger arterioles) (12), and that these adaptations are not homogeneous within or among skeletal muscles. Muscle fiber type composition and muscle fiber recruitment patterns influence the spatial distribution of adaptations in capillary and arteriolar density. Importantly, structural adaptation of the skeletal muscle arteriolar networks only partially explains the increases in blood flow capacity (75), suggesting that exercise training induces an additional adaptation that may be vasomotor reactivity, another determinant of vascular resistance and control of blood flow.
Fig. 4.
Effects of interval sprint training (IST) (10 wk of 6 training bouts/day, 5 days/wk, with each rat running 60 m/min up a 15% incline for 2.5 min with 4.5 min of rest between bouts) and endurance training (ET) (10 to 12 wk of treadmill running at 30 m/min, 60 min/day, 5 days/wk) on oxidative capacity (cytochrome-c concentrations), blood flow capacity, capillary density, and arteriolar density in the white (GW) and red (GR) portions of the gastrocnemius and soleus muscles of rats. These results demonstrate that training-induced changes in arteriolar density do not explain changes in blood flow capacity. Data are expressed as a percent increase above respective values of sedentary rats. Results demonstrate that IST increases GW oxidative capacity and blood flow capacity the most, and that these changes are correlated with increases in capillary density and small increases in arteriolar density. Results indicate that ET increased oxidative capacity and blood flow capacity the most in soleus and GR muscles. In contrast to IST, changes in oxidative and blood flow capacity were not correlated with changes in capillary or arteriolar density in the soleus muscle. [Published with permission (34, 42, 43, 65, 66, 68, 74).]
Available evidence suggests that exercise training increases capillary exchange capacity and/or microvascular oxygenation by adaptations beyond structural increases in capillary density/angiogenesis (66, 67). Thus Hirai and colleagues (46, 47) have shown that exercise training can reverse the derangements in skeletal muscle oxygen transport resulting in greater peak oxygen uptake in skeletal muscle of rats with congestive heart failure. The improvement in oxygen transport is the result of modification of spatial and temporal heterogeneities of blood flow and oxygen uptake that are remodeled by exercise training. A better understanding of the matching of oxygen transport (convective and diffusive) with oxygen consumption of the muscle is an important addition to our understanding of the mechanisms of vascular adaptation in skeletal muscle (37, 44, 100). The improved matching of blood flow distribution with capillary exchange capacity and with regional muscle oxygen consumption after exercise training appears to result in better microvascular oxygenation in contracting skeletal muscle, and suggests that exercise training induces changes in vascular control.
To summarize, in normal mammals, exercise training increases vascular transport capacity (total and regional blood flow capacity and capillary exchange capacity) and causes structural vascular adaptation in skeletal muscle. Exercise training-induced increases in regional blood flow capacity are associated with increased capillary density and increased arteriolar density. Importantly, results indicate that vascular adaptations are not homogeneous throughout skeletal muscle or along the arteriolar tree. Equally important, results indicate that muscle fiber type composition and muscle fiber recruitment patterns that produce various exercise performances influence the spatial distribution of adaptations in vascular structure. Because increases in vascular transport capacity cannot be explained from measured changes in vascular structure, our discussion will next examine the novel hypothesis that adaptations in the total and/or regional control of vascular resistance in skeletal muscle contribute to the increases in transport capacity.
EXERCISE TRAINING-INDUCED ADAPTATION IN CONTROL OF VASCULAR RESISTANCE
A vast array of mechanisms is believed to be involved in the control of vascular resistance and responsible for exercise hyperemia in striated muscle that may be adapted by exercise training (66, 67). Our approach to testing the hypothesis that exercise training alters control of blood flow and vascular resistance in skeletal muscle vascular beds has been to evaluate the hypothesis that exercise training induces adaptive changes in the function of endothelial cells and/or smooth muscle of resistance arteries and/or arterioles in skeletal muscle (50). To allow separation of the effects of skeletal muscle fiber metabolism and neurohumeral control factors we examined vasomotor function of cannulated arterioles and resistance arteries isolated from skeletal muscle of sedentary and trained animals (1, 2, 49, 50, 76, 83, 117, 118, 136, 137). In a major resistance artery of the soleus muscle, the soleus feed artery (FA), we were initially surprised to discover that endurance exercise training did not alter endothelium-dependent dilation (EDD) induced by ACh or the EDD signaled with intraluminal flow (flow-induced dilation) (50). Jasperse et al. (51) proposed that soleus FAs do not adapt to endurance exercise because soleus blood flow is only modestly increased during endurance exercise training bouts. Specifically, the soleus resistance vasculature is already adapted because the soleus muscle has a high level of activity in just maintaining posture even in untrained subjects, so endurance exercise does not generate a sufficient signal to induce adaptations in the soleus or its arteriolar tree. Jasperse et al. (50) also proposed that if this concept were correct, then decreased activity of the soleus muscle caused by chronic hindlimb unloading would result in decreased EDD in the soleus FA. As shown in Fig. 2, results of his experiments supported this hypothesis and further demonstrated that endothelial nitric oxide synthase (eNOS) expression was decreased in the soleus FA of hindlimb unloaded rats. Similar changes in vasomotor function and eNOS expression were found in soleus 1A arterioles of hindlimb unloaded rats (117). In contrast, soleus 2A arterioles did not exhibit adaptation of EDD behavior or eNOS expression in response to hindlimb unloading (117, 118). It is also of interest that hindlimb unloading decreased the response of vascular smooth muscle to the vasodilator actions of adenosine in the gastrocnemius 1A arteriole but did not alter the response of soleus 1A arterioles to adenosine (87).
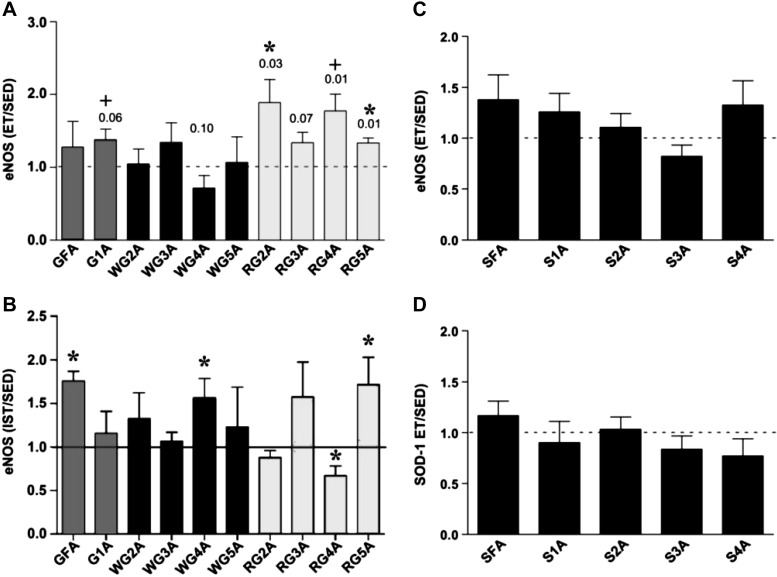
We used a similar strategy to study the arteriolar network of the gastrocnemius muscle. The data are not summarized here, but suffice it to say that we found that exercise training-induced functional vascular adaptation differed along the arteriolar tree within skeletal muscle as well as between skeletal muscles of the same animal (76, 83). As illustrated in Figure 5 A and B, endurance exercise training produced changes in eNOS expression along the arteriolar tree of the gastrocnemius muscle that were different from the changes induced by IST and, as mentioned above, neither ET nor IST altered arteriolar eNOS expression (Fig. 5, C and D) or vasomotor function of soleus arterioles.
Fig. 5.
Effects of IST (10 wk of 6 training bouts/day, 5 days/wk, with each rat running 60 m/min up a 15% incline for 2.5 min with 4.5 min of rest between bouts) and ET (10 to 12 wk of treadmill running at 30 m/min, 60 min/day, 5 days/wk) on endothelial nitric oxide synthase (eNOS) protein content in arterioles of rat skeletal muscle. Average eNOS protein content in arteries and arterioles of rats in ET and IST groups expressed relative to the eNOS content of paired samples from sedentary (Sed) animals. eNOS protein content was quantified by scanning densitometry with ImageJ (National Institutes of Health) software. Values are means ± SE. A: ET n = 4 groups of pooled vessels each. GFA, gastrocnemius feed artery; G1A, first order; 2A, second order; 3A, third order; 4A, fourth order; 5A, fifth order. *Differences between Sed and ET are significant (P < 0.05). +Significant differences (0.05 < P < 0.10). [Published with permission (83).] B: effects of IST on eNOS protein content of gastrocnemius muscle arteries. GFA data are combined from two sets of IST and Sed animals. Gastrocnemius 1A-5A data are from four different groups of Sed and IST rats, thus means and SE are presented. Each group of Sed and IST animals consisted of 5–10 rats, so the data are from 10 to 30 different rats. *IST value is different from that of Sed, P < 0.05 (by one-way Student's t-test). [Published with permission (76).] C: effects of ET on eNOS protein content in soleus (S) arterioles. SFA, soleus feed artery. D: SOD-1 protein content in soleus resistance arterioles. [Published with permission (83).]
Aging-associated reductions in EDD have been shown to be different in skeletal muscles of differing fiber type composition, wherein Ach-induced EDD is blunted in arterioles and feed arteries from highly oxidative muscle but not from low-oxidative muscle, whereas in contrast, flow-induced EDD is impaired in both gastrocnemius and soleus muscle arterioles (91, 125, 126). Metabolic syndrome/T2D and heart failure have also been reported to result in muscle-specific decreases in EDD and other changes in skeletal muscle vascular structure/function that differ along the arteriolar tree within skeletal muscle as well as between different muscles (80, 81, 89, 99). In these disease states, exercise training has been shown to reverse blunted EDD (81, 99) and restore vascular structure, and to restore myogenic constrictor responses in skeletal muscle arterioles (40).
The fact that exercise training-induced functional vascular adaptations differ along the arteriolar tree within skeletal muscle is interesting given the intrinsic variation in functional phenotype of vascular cells observed along the arteriolar vascular tree (30). These observations indicate that within the arteriolar network the relative responsiveness of each segment is tuned to the predominant signals present that control smooth muscle tone in those arterioles and resistance arteries. Available results indicate that exercise training shifts these control factors and signaling mechanisms in vascular cells in a nonuniform pattern throughout the arteriolar tree. As a result, there does not appear to be any one branch arteriole that can be sampled to reflect the adaptive changes induced by exercise training throughout the arteriolar network of any skeletal muscle so far examined. Analysis of what is known about exercise training-induced adaptations in vasomotor function indicates that the nonuniform adaptations along the arteriolar tree are the result of differential adaptation of gene expression in vascular cells.
DO THESE CONCEPTS RELATING TO INTERACTIONS OF EXERCISE, ADAPTATIONS OF VASCULAR STRUCTURE AND FUNCTION IN STRIATED MUSCLE APPLY IN T2D?
The importance of this question is established by the ∼30 million Americans age 20 yr and older who have diabetes (nearly 95% T2D), and the estimated additional 86 million Americans 20 yr and older who have prediabetes (116). Diabetes is associated with micro- and macrovascular complications resulting in adult-onset blindness, end-stage renal failure, nontraumatic limb amputation, coronary artery disease, stroke, and peripheral vascular disease (22, 29, 44, 95, 110). Inactivity is a recognized risk factor in the development of T2D (110), and participation in no or insufficient physical activity is associated with an increased incidence of cardiovascular events, microvascular complications, and all-cause mortality (13), and a decreased aerobic capacity is associated with the presence of neuropathy, retinopathy, or nephropathy among patients with T2D (36).
There is evidence that the beneficial effects of exercise training are at least partially related to changes in skeletal muscle vascular beds. For example, T2D is associated with attenuated skeletal muscle blood flow responses to exercise and to glucose intolerance (54, 135). Studies performed in humans with insulin resistance or T2D and in animal models of disease demonstrate that exercise training mediates local and systemic improvements in endothelial (10, 27, 28, 80, 81, 88–90) and smooth muscle (10, 27, 81) function indicated by improved vasodilator signaling. Exercise training also appears to attenuate capillary rarefaction in skeletal muscle associated with insulin resistance (26, 39, 77, 99, 102). Therefore, collectively, these exercise training-induced adaptations may play important roles in optimal treatment of T2D.
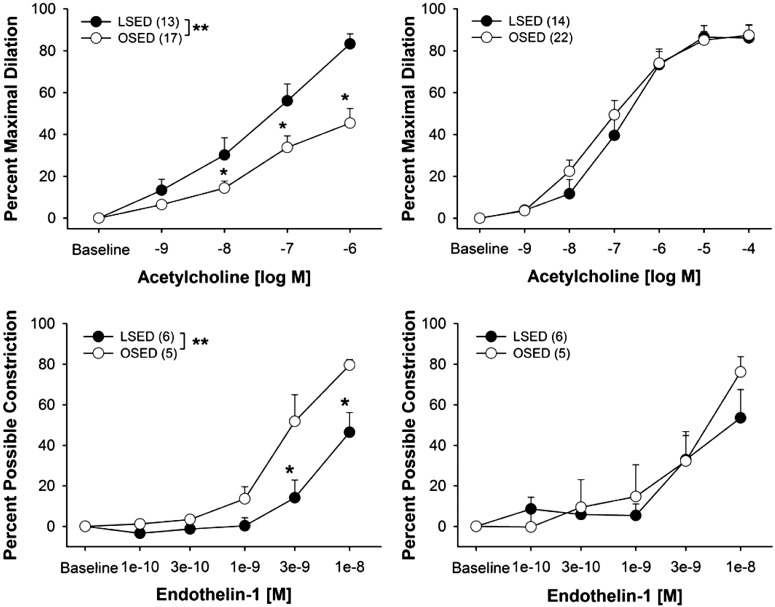
Using the same strategy outlined above for normal rats, we examined the functional vascular adaptations along the arteriolar tree of skeletal muscle of differing fiber type in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. OLETF rats are hyperphagic and develop obesity and T2D, and they have been established as a model of obesity and T2D (103–107). We focused on vasomotor function because recent results make it clear that vascular cells are insulin resistant in T2D subjects and that insulin can contribute importantly to control of blood flow in muscle by signaling to vascular cells (24). In our first experiment with this animal model we found dysfunction of endothelial-dependent vasomotor reactivity in the FAs of gastrocnemius, but not soleus FAs of OLETF rats (Fig. 6, top) (10). When OLETF rats were physically active using voluntary running wheels in their cages we found that the endothelial dysfunction of the gastrocnemius FAs was prevented and, as has been true in normal animals, wheel running exercise did not alter soleus FA vasomotor function (10). The improved endothelial function in gastrocnemius FA was associated with increased phospho-eNOS (10). There is now a substantial body of evidence that T2D is associated with decreased EDD and vascular rarefaction in skeletal muscle microcirculation (54, 58, 81, 88, 89, 110), which suggests that exercise training restores both vascular structure and function through reversing the effects of T2D on EDD and perhaps due to changes in NO availability.
Fig. 6.
Concentration-response curves to ACh and endothelin-1 GFA (left) and soleus feed arteries (right). Data are presented as percent maximal dilation (ACh) and percent possible constriction (endothelin-1). Values are means ± SE; sample size appears in parentheses. OSED, Otsuka Long-Evans Tokushima Fatty rats that are sedentary; LSED, Long-Evans Tokushima Otsuka rats. *P < 0.05. [Published with permission (10).]
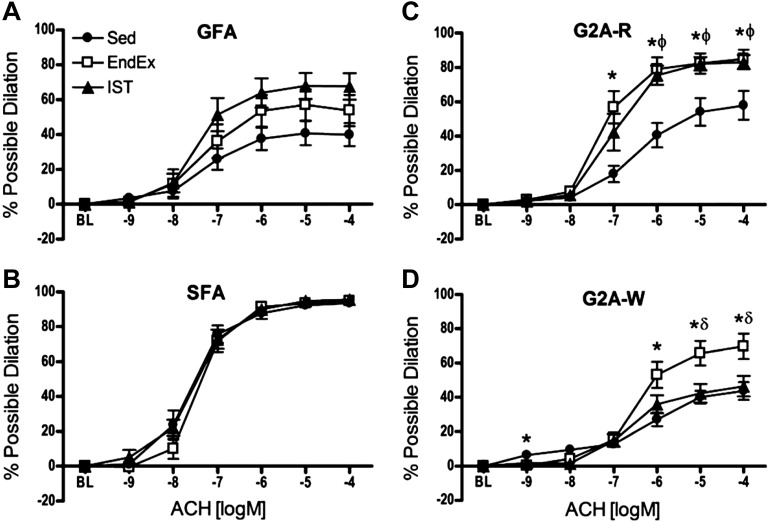
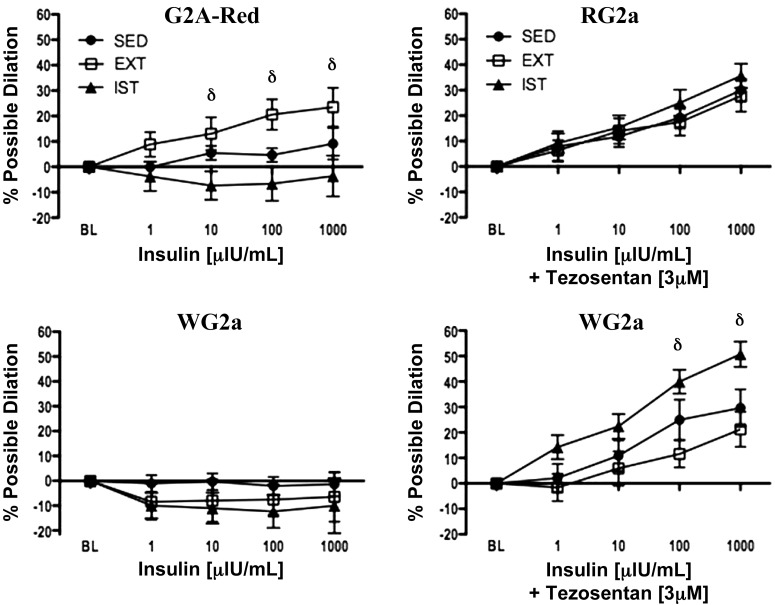
In our most recent studies we isolated FAs and arterioles from the soleus and gastrocnemius muscles of OLETF rats after two different exercise training programs. We studied OLETF rats assigned to one of three groups: endurance exercise-trained (EX; n = 13), interval sprint-trained (SPRINT; n = 14), and sedentary (Sed; n = 12). The experiments were designed to measure vasomotor function in gastrocnemius feed arteries (GFAs) and of 2A arterioles isolated from the red (RG2a; G2A-R in Fig. 7) and white (WG2a; G2A-W in Fig. 7) portion of the gastrocnemius muscle and the soleus FA (81). Importantly, we demonstrated that EDD is blunted by T2D differentially in muscle with different muscle fiber type composition, and that exercise training restores EDD in a fiber type-dependent manner (10, 81, 88, 89) because exercise training improves EDD nonuniformly in the arterial tree of skeletal muscle (10, 52, 81, 88, 89). As shown in Figure 7, both SPRINT and EX rats produced an increase in ACh-induced EDD in the GFA and the RG2a, but improved vasodilation of the WG2a occurred only in EX rats. Neither training program altered responses of the soleus FA. As shown in Figure 8, insulin produced vasodilation of the RG2a in EX animals only. When ET-1 receptors were blocked with tezosentan (a nonselective endothelin-1 receptor antagonist), RG2a from all three groups exhibited vasodilation to insulin. Similar results were observed in WG2a. Thus these results led us to conclude that EDD is blunted in T2D skeletal muscle arterioles in a muscle fiber type-dependent manner. EX and SPRINT rat exhibited increased EDD in some arterioles but not all. Results also indicate that insulin signaling in arteriolar endothelium differs among types of skeletal muscle and among different branch orders in skeletal muscle of arteriolar trees (81, 88, 89). Also, in EX rats, insulin-induced EDD was improved nonuniformly in the arterial tree of skeletal muscle. Thus results indicate that the blunting of EDD induced by T2D differs with muscle fiber type composition of skeletal muscle and that different exercise training programs reverse this dysfunction differently in arterioles from skeletal muscles of differing fiber type composition (10, 81, 88). Indeed, it is striking that exercise training improves EDD nonuniformly even within the arteriolar tree of a given muscle, the gastrocnemius (81).
Fig. 7.
Concentration-response curves to ACh in GFAs (A), soleus feed arteries (SFA) (B), red gastrocnemius 2A (G2A-R) arterioles (C), and white gastrocnemius 2A (G2A-W) (D) from Otsuka Long-Evans Tokushima Fatty rats that were cage-confined (Sed), or underwent endurance exercise training (EndEx) or IST. Data are presented as percent possible dilation. Values are means ± SE. *Sed vs. EndEx (P < 0.0167). ϕSed vs. IST (P < 0.0167). δEndEx vs. IST (P < 0.0167). [Published with permission (81).]
Fig. 8.
Vascular reactivity to insulin alone (left) and to insulin in the presence of the nonselective endothelin-1 receptor antagonist tezosentan (right) for red gastrocnemius 2A arterioles (G2A-Red and RG2a) (top) and white gastrocnemius 2A arterioles (WG2a) (bottom) across all doses of insulin in 32-wk-old Sed, EndEx, and IST OLETF rats. Values are expressed as mean percent possible dilation ± SE. Within each group and vessel, n = 9–12. δEndEx vs. IST (P < 0.0167). [Published with permission (81).]
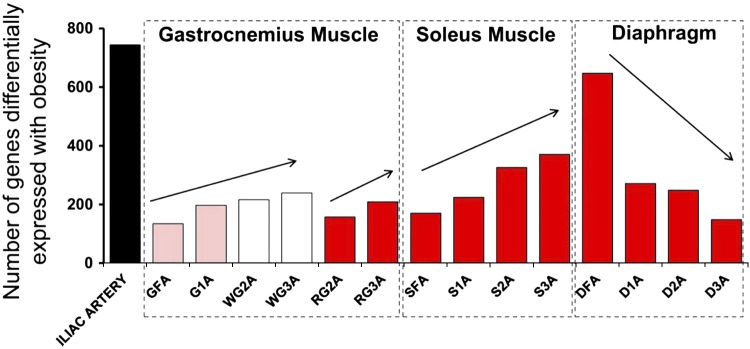
Applying techniques described previously (53, 97), we determined transcriptional profiles for samples of arterioles/arteries from the same rats we used for the function experiments described above. We harvested aorta; iliac; GFA, G1a, RG2a, RG3a, WG2a, and WG3a; soleus FA, S1a, S2a, and S3a; and diaphragm feed artery (DFA) diaphragm 1a (D1a), D2a, and D3a for isolation of total RNA for RNA sequence analysis of gene expression (53, 96) from the same rats that were used by Martin et al. (81) for vasomotor function experiments. We used transcriptome-wide RNA sequencing (RNA-Seq) analysis to provide a better understanding of the molecular events involved in exercise-training induced skeletal muscle vascular adaptations in rats with obesity and T2D. One group of OLETF rats underwent an endurance exercise training program (EX), a second group underwent an IST program (SPRINT), and a third group was restricted to cage activity (Sed). Our hypothesis was that the greatest effects of exercise training on the transcriptome would be in the gastrocnemius arterioles compared with soleus arterioles. Furthermore we reasoned that rats in the SPRINT group would produce greater vascular transcriptional changes compared with rats in the EX group in arterioles isolated from GW muscle because of greater increases in skeletal muscle fiber recruitment of this muscle during sprinting (7, 8, 10, 38, 64, 81, 83, 86, 88, 89). We analyzed arterioles of similar branch order (FA through 3A) from both muscles from the same rats used for the vasomotor function experiments (81). In the initial analysis of these RNA-Seq results we found that with increasing branch order of arterioles, the number of genes differentially expressed with obesity decreased in the diaphragm, whereas we found the opposite pattern of alterations in obesity genes in the two limb skeletal muscles (Fig. 9) (96). Because we observed nearly opposite effects of obesity/T2D on vascular gene expression in limb muscles and diaphragm, we proposed that the effects of exercise training on gene expression in diaphragm arteries/arterioles may also be different than that observed in the soleus and gastrocnemius muscles. We contrasted the EX- and SPRINT-induced changes observed in diaphragm arterioles to those observed in soleus and gastrocnemius. Because soleus blood flow increases during exercise to a similar extent as in diaphragm, we proposed that exercise training would have similar effects on gene expression in the arterioles of these two muscles. The basis of this hypothesis was the assumption that shear stress is a primary signal for the effects of exercise on gene expression in arterioles of both muscles, so if blood flow changes during exercise are similar, then alterations in gene expression should also be similar.
Fig. 9.
Illustration showing the effects of obesity on gene expression in 15 arteries. DFA, diaphragm feed artery; D1A, D2A, and D3A, diaphragm first-, second, and third-order branch arterioles, respectively. [Published with permission (96).]
The results summarized in Figure 9 demonstrate that the number of genes whose expression was altered by obesity increased with increasing branch order in the arteriolar trees of gastrocnemius and soleus muscles, whereas the opposite effect of branch order was observed in the diaphragm. This figure also illustrates an important principle: a systemic intervention such as obesity/T2D can have differential effects on gene expression along/throughout the arterial/arteriolar tree. Furthermore, these effects may not be the same from one muscle to the next. Our training study was designed with a translational focus. Because of the cost of carrying out RNA-Seq on this number of samples per animal, we chose to study only the effects of EX and SPRINT on gene expression of the arterioles in OLETF rats (obese/T2D), not in normal animals. However, in a subset of arteries we examined the interaction of obesity/T2D and exercise training (97). As shown in Figure 10, expression of a small number of genes was altered by obesity/T2D, whereas expression was partially or entirely restored by EX and/or SPRINT training.
Fig. 10.
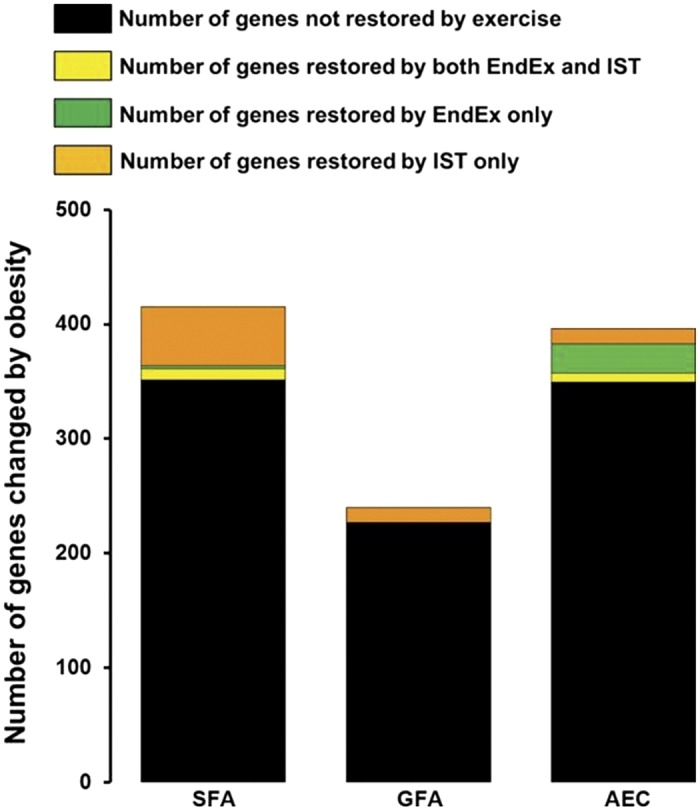
Numbers of genes whose expression was altered by obesity that were partially or entirely restored by exercise training. [Published with permission (53, 97).]
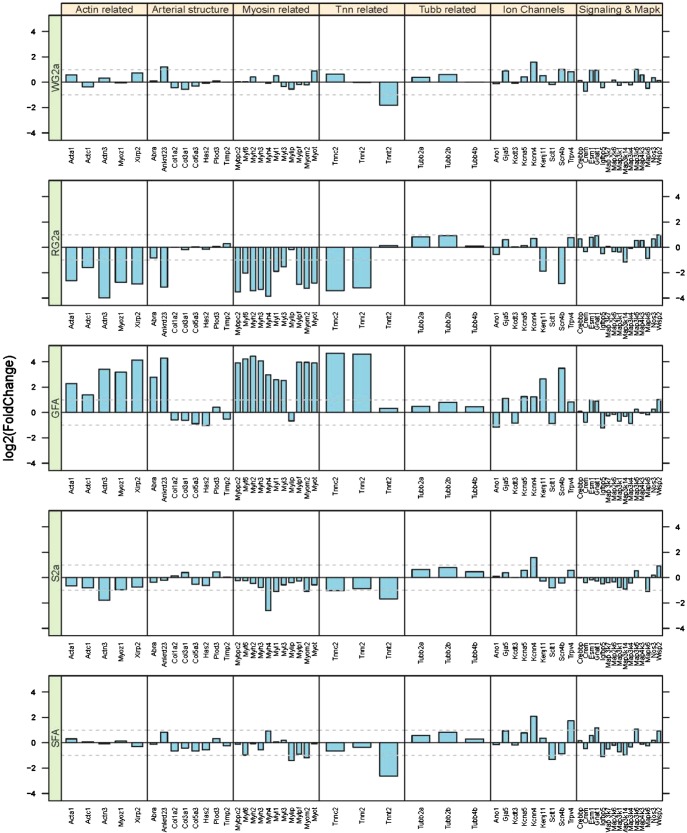
Further analysis of results indicated that whereas rats in the EX group exhibited few to no changes in gene expression in the GFA, but there were many gene expression changes in the S2a and WG2a arterioles. The effects of exercise in the SPRINT and EX groups were much different; we observed substantial changes in soleus FA and GFA gene expression. Overall, we were surprised that SPRINT exercise caused so few changes in gene expression in the WG2a because our hypothesis predicted the largest changes in these arterioles. The contrasts between SPRINT training-induced changes in gene expression among WG2a, RG2a, and GFA can be appreciated by examination of the data summarized in Figure 11. First note that for genes related to contractile proteins and arterial structure that (Actin related, Arterial structure, Myosin related, Tnn related, and Tubb related; Fig. 11) RG2a indicates changes that are nearly the opposite of those in GFA, whereas WG2a shows only modest changes in these genes. Similar observations are true of the changes in gene expression produced by EX in the soleus and gastrocnemius arteriolar trees (71). S2a and RG2a exhibit similar changes in gene expression caused by EX, but the magnitude of changes is different among the other arteries/arterioles, and which branches of the soleus and gastrocnemius arteriolar trees exhibit the largest changes in gene expression are not the same between EX and SPRINT exercise training (71).
Fig. 11.
Illustration of the effects of IST (10 wk of 6 training bouts/day, 5 days/wk, with each rat running 60 m/min up a 15% incline for 2.5 min with 4.5 min of rest between bouts) on gene expression in gastrocnemius and soleus arterioles of OLETF rats. Bar graphs present changes in gene expression as fold changes in gene expression relative to the expression level of OLETF rats confined to cage activity. Categories of genes are listed across the top, specific genes are listed across the bottom. From top to bottom: WG2a, RG2a, GFA, S2a, and SFA. Notice that for Actin related, Myosin related, and troponin (Tnn) genes of the GFA (middle) how gene expression is increased, whereas expression of these same genes is decreased in S2a and RG2a and relatively unchanged in WG2a and SFA. [Published with permission (71).]
As one might expect from the results shown in Figures 6 and 7, SPRINT training and EX did not induce the same adaptive changes in gene expression among the 2A arterioles of the diaphragm, soleus, and gastrocnemius muscles (71, 72). Among the three FAs, SPRINT training induced similar increased expression of two genes (Wisp2 and Tubb2b), but there were no genes whose expression was altered similarly by EX in all FAs (71, 72).
CONCLUSIONS
On the basis of current literature we draw the following conclusions. First, exercise training-induced changes in vascular gene expression differ along the arteriolar tree and by muscle fiber type composition of the muscle in which the arteriolar tree is located. Second, vascular cell gene expression changes signaled by exercise training appear to be relatively unrelated to the spatial location of skeletal muscle adaptations. Given the complex nature of changes in vascular gene expression reported in obesity/T2D (53, 96) and with EX and SPRINT training (71, 97), it seems unlikely that hemodynamic forces are the only exercise-induced signals mediating the regulation of vascular gene expression. Also, I conclude that neither EX nor SPRINT have similar effects on the transcriptome of diaphragm and soleus arteries/arterioles, even though these muscles have similar blood flows at rest and during exercise.
On the basis of these results we propose that exercise prescription for patients with T2D should be designed to cause adaptations throughout the skeletal musculature (all fiber types) to produce the greatest benefit systemically and on vascular health. Both aerobic training and resistance training have beneficial effects on health and fitness (23). A meta-analysis of randomized controlled trials comparing aerobic training, resistance training, and combined aerobic and resistance training revealed that aerobic training was more effective in reducing HbA1c and fasting glucose than was resistance training (119). Furthermore, the analysis indicated that combined aerobic and resistance training interventions are the most efficacious exercise training prescription for improvement of glycemic control and blood lipids (119). Equally important, Gordon et al. (41) concluded that vigorous-intensity exercise is associated with more favorable T2D risk profiles and greater insulin sensitivity in both youth and adults than is low-intensity exercise training (physical activity). We consider that both combined aerobic and resistance training and higher-intensity aerobic training may be most advantageous because they induce adaptations in a larger number of muscle fibers than does moderate-intensity aerobic training. Moderate-intensity training is clearly beneficial for cardiovascular health. Although cardiovascular health is important in T2D, exercise training can also restore metabolic health, we believe, through its ability to induce phenotypic changes in skeletal muscle fibers.
On the basis of these observations and considerations, we postulate that an exercise training program that engages the most skeletal muscle and the most muscle fibers within each skeletal muscle during training sessions (i.e., greatest increase in fiber recruitment from rest to exercise) will generate the most widespread adaptations leading to greater improvements in microvascular function and insulin sensitivity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-036088.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.H.L. conception and design of research; M.H.L. performed experiments; M.H.L. analyzed data; M.H.L. interpreted results of experiments; M.H.L. prepared figures; M.H.L. drafted manuscript; M.H.L. edited and revised manuscript; M.H.L. approved final version of manuscript.
REFERENCES
- 1.Aaker A, Laughlin MH. Diaphragm arterioles are less responsive to alpha-1-adrenergic constriction than gastrocnemius arterioles. J Appl Physiol 92: 1808–1816, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Aaker A, Laughlin MH. Differential adenosine sensitivity of diaphragm and skeletal muscle arterioles. J Appl Physiol 93: 848–856, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Adair TH, Gay WJ, Montani JP. Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am J Physiol Regul Integr Comp Physiol 259: R393–R404, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Adolph EF. General and specific characteristics of physiological adaptations. Am J Physiol 184: 18–28, 1956. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Distribution of blood flow in muscles of miniature swine during exercise. J Appl Physiol 62: 1285–1298, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Progressive elevations in muscle blood flow during prolonged exercise in swine. J Appl Physiol 63: 285–291, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 344: 187–208, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. Am J Physiol Heart Circ Physiol 246: H59–H68, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. J Appl Physiol 59: 1322–1328, 1985. [DOI] [PubMed] [Google Scholar]
- 10.Bender SB, Newcomer SC, Harold Laughlin M. Differential vulnerability of skeletal muscle feed arteries to dysfunction in insulin resistance: impact of fiber type and daily activity. Am J Physiol Heart Circ Physiol 300: H1434–H1441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bevegard BS, Shepherd JT. Reaction in man of resistance and capacity vessels in forearm and hand to leg exercise. J Appl Physiol 21: 123–132, 1966. [DOI] [PubMed] [Google Scholar]
- 12.Binder KW, Murfee WL, Song J, Laughlin MH, Price RJ. Computational network model prediction of hemodynamic alterations due to arteriolar remodeling in interval sprint trained skeletal muscle. Microcirculation 14: 181–192, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blomster JI, Chow CK, Zoungas S, Woodward M, Patel A, Poulter NR, Marre M, Harrap S, Chalmers J, Hillis GS. The influence of physical activity on vascular complications and mortality in patients with type 2 diabetes mellitus. Diabetes Obes Metab 15: 1008–1012, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Bowles DK. Adaptation of ion channels in the microcirculation to exercise training. Microcirculation 7: 25–40, 2000. [PubMed] [Google Scholar]
- 15.Bowles DK. Gender influences coronary L-type Ca2+ current and adaptation to exercise training in miniature swine. J Appl Physiol 91: 2503–2510, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Bowles DK, Heaps CL, Turk JR, Maddali KK, Price EM. Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J Appl Physiol 96: 2240–2248, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Bowles DK, Hu Q, Laughlin MH, Sturek M. Exercise training increases L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol 275: H2159–H2169, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Bowles DK, Hu Q, Laughlin MH, Sturek M. Heterogeneity of L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol 273: H2083–H2089, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Bowles DK, Laughlin MH, Sturek M. Exercise training alters the Ca2+ and contractile responses of coronary arteries to endothelin. J Appl Physiol 78: 1079–1087, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Bowles DK, Laughlin MH, Sturek M. Exercise training increases K+-channel contribution to regulation of coronary arterial tone. J Appl Physiol 84: 1225–1233, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Bowles DK, Woodman CR, Laughlin MH. Coronary smooth muscle and endothelial adaptations to exercise training. Exerc Sport Sci Rev 28: 57–62, 2000. [PubMed] [Google Scholar]
- 22.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics 8: 1–12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation 113: 2642–2650, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Clarkson P, Celermajer DS, Donald AE, Sampson M, Sorensen KE, Adams M, Yue DK, Betteridge DJ, Deanfield JE. Impaired vascular reactivity in insulin-dependent diabetes mellitus is related to disease duration and low density lipoprotein cholesterol levels. J Am Coll Cardiol 28: 573–579, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Clausen JP, Trap-Jensen J. Effects of training on the distribution of cardiac output in patients with coronary artery disease. Circulation 42: 611–624, 1970. [DOI] [PubMed] [Google Scholar]
- 26.Cocks M, Shaw CS, Shepherd SO, Fisher JP, Ranasinghe A, Barker AA, Wagenmakers AJ. Sprint interval and moderate-intensity continuous training have equal benefits on aerobic capacity, insulin sensitivity, muscle capillarisation and endothelial eNOS/NAD(P)Hoxidase protein ratio in obese men. J Physiol. First published January 23, 2015; doi: 10.1113/jphysiol.2014.285254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohn ND, Dunstan DW, Robinson C, Vulikh E, Zimmet PZ, Shaw JE. Improved endothelial function following a 14-month resistance exercise training program in adults with type 2 diabetes. Diabetes Res Clin Pract 79: 405–411, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care 33: 2692–2706, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dagogo-Jack S. Preventing diabetes-related morbidity and mortality in the primary care setting. J Natl Med Assoc 94: 549–560, 2002. [PMC free article] [PubMed] [Google Scholar]
- 30.Davis MJ, Hill MA, Kuo L. Local regulation of microvascular perfusion. In: Handbook of physiology: Microcirculation II, Regulation of Microvascular Blood Flow, edited by Tuma R, Duran W, and Ley K. New York: Elsevier, 2008, p. 1–127. [Google Scholar]
- 31.Delp MD, Laughlin MH. Time course of enhanced endothelium-mediated dilation in aorta of trained rats. Med Sci Sports Exerc 29: 1454–1461, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Delp MD, McAllister RM, Laughlin MH. Exercise training alters aortic vascular reactivity in hypothyroid rats. Am J Physiol Heart Circ Physiol 268: H1428–H1435, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol 75: 1354–1363, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol 53: 844–850, 1982. [DOI] [PubMed] [Google Scholar]
- 35.Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol 53: 844–850, 1982. [DOI] [PubMed] [Google Scholar]
- 36.Estacio RO, Regensteiner JG, Wolfel EE, Jeffers B, Dickerson M, Shchrier RW. The association between diabetic complications and exercise capacity in NIDDM patients. Diabetes Care 21: 291–295, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson SK, Holdsworth CT, Wright JL, Fees AJ, Allen JD, Jones AM, Musch T, Poole DC. Microvascular oxygen pressures in muscles comprised of different fiber types: impact of dietary nitrate supplementation. Nitric Oxide 48: 38–43, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fogarty JA, Muller-Delp JM, Delp MD, Mattox ML, Laughlin MH, Parker JL. Exercise training enhances vasodilation responses to vascular endothelial growth factor in porcine coronary arterioles exposed to chronic coronary occlusion. Circulation 109: 664–670, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol 291: H2483–H2492, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh P, Solis FR, Dominquez JM 2nd, Spier SA, Donato AJ, Delp MD, Muller-Delp JM. Exercise training reverses aging-induced impairment of myogenic constriction in skeletal muscle arterioles. J Appl Physiol 118: 904–911, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon JW, Dolinsky VW, Mugal W, Gordon GR, McGavock J. Targeting skeletal muscle mitochondria to prevent type 2 diabetes in youth. Biochem Cell Biol 27: 1–14, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Gute D, Fraga C, Laughlin MH, Amann JF. Regional changes in capillary supply in skeletal muscle of high-intensity endurance-trained rats. J Appl Physiol 81: 619–626, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Gute D, Laughlin MH, Amann JF. Regional changes in capillary supply in skeletal muscle of interval-sprint and low intensity, endurance trained rats. Microcirculation 1: 183–193, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Heinonen I, Koga S, Kalliokoski KK, Musch T, Poole DC. Heterogeneity of muscle blood flow and metabolism: Influence of exercise, aging, and disease sates. Exerc Sport Sci Rev 43: 117–124, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill AV, Lupton H. Muscular exercise, lactic acid, and the supply and utilisation of oxygen. Q J Med 16: 135–171, 1923. [Google Scholar]
- 46.Hirai DM, Copp SW, Ferguson SK, Holdsworth CT, McCullough DJ, Behnke BJ, Musch T, Poole DC. Exercise training and muscle microvascular oxygenation: functional role of nitric oxide. J Appl Physiol 113: 557–565, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirai DM, Copp SW, Holdsworth CT, Ferguson SK, McCullough DJ, Behnke BJ, Musch TI, Poole DC. Skeletal muscle microvascular oxygenation dynamics in heart failure: exercise training and nitric oxide-mediated function. Am J Physiol Heart Circ Physiol 306: H690–H698, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jasperse JJ, Laughlin MH. Exercise and skeletal muscle microcirculation. In: Microvascular Research: Biology and Pathology, edited by Shepro D. San Diego, CA: Elsevier, 2005, p. 527–535. [Google Scholar]
- 49.Jasperse JL, Laughlin MH. Flow-induced dilation of rat soleus feed arteries. Am J Physiol Heart Circ Physiol 273: H2423–H2427, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Jasperse JL, Laughlin MH. Vasomotor responses of soleus feed arteries from sedentary and exercise trained rats. J Appl Physiol 86: 441–449, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Jasperse JL, Woodman CR, Price EM, Hasser EM, Laughlin MH. Hindlimb unweighting decreases ecNOS gene expression and endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol 87: 1476–1482, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Jenkins NT, Padilla J, Martin JS, Crissey JM, Thyfault JP, Rector RS, Laughlin MH. Differential vasomotor effects of insulin on gastrocnemius and soleus feed arteries in the OLETF rat model: role of endothelin-1. Exp Physiol 99: 262–271, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenkins NT, Padilla J, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. Transcriptome-wide RNA sequencing analysis of rat skeletal muscle feed arteries. Part I: impact of obesity. J Appl Physiol 116: 1017–1032, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 26: 899–904, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol 103: 2049–2056, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc 46: 860–876, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Koller A, Huang A, Sun D, Kaley G. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles. Role of endothelial nitric oxide and prostaglandins. Circ Res 76: 544–550, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Lalande S, Gusso S, Hofman PL, Baldi JC. Reduced leg blood flow during submaximal exercise in type 2 diabetes. Med Sci Sports Exerc 40: 612–617, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Lash JM. Exercise training enhances adrenergic constriction and dilation in the rat spinotrapezius muscle. J Appl Physiol 85: 168–174, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Lash JM, Bohlen HG. Functional adaptations of rat skeletal muscle arterioles to aerobic exercise training. J Appl Physiol 72, 2052–2062, 1992. [DOI] [PubMed] [Google Scholar]
- 61.Lash JM, Bohlen HG. Time-and order-dependent changes in functional and NO-mediated dilation during exercise training. J Appl Physiol 82: 460–468, 1997. [DOI] [PubMed] [Google Scholar]
- 62.Lash JM, Nixon JC, Unthank JL. Exercise training effects on collateral and microvascular resistance in rat model of arterial insufficiency. Am J Physiol Heart Circ Physiol 268: H125–H137, 1995. [DOI] [PubMed] [Google Scholar]
- 63.Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol Heart Circ Physiol 253: H993–H1004, 1987. [DOI] [PubMed] [Google Scholar]
- 64.Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol 243: H296–H306, 1982. [DOI] [PubMed] [Google Scholar]
- 65.Laughlin MH, Cook J, Tremble R, Ingram D, Colleran PN, Turk JR. Exercise training produces nonuniform increases in arteriolar density of rat soleus and gastrocnemius muscle. Microcirculation 13: 175–186, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2012. [DOI] [PubMed] [Google Scholar]
- 67.Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. Compr Physiol. Suppl 29: Handbook of Physiology, Exercise Regulation and Integration of Multiple Systems: 705–769. First published in print 1996. doi: 10.1002/cphy.cp120116. [DOI] [Google Scholar]
- 68.Laughlin MH, Korthuis RJ, Sexton WL, Armstrong RB. Regional muscle blood flow capacity and exercise hyperemia in high-intensity trained rats. J Appl Physiol 64: 2420–2427, 1988. [DOI] [PubMed] [Google Scholar]
- 69.Laughlin MH, McAllister RM, Delp MD. Heterogeneity of blood flow in striated muscle. In: The Lung (Crystal RG, West JB, Barnes PJ, Weibel ER, ). Philadelphia: Raven Publishers, 1997, p. 1945–1955. [Google Scholar]
- 70.Laughlin MH, Muller JM. Vasoconstrictor responses of coronary resistance arteries in exercise-trained pigs. J Appl Physiol 84: 884–889, 1998. [DOI] [PubMed] [Google Scholar]
- 71.Laughlin MH, Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Akter S, Davis JW. Exercise-induced differential changes in gene expression among arterioles of skeletal muscles of obese rats. J Appl Physiol 119: 583–603, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laughlin MH, Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Akter S, Davis JW. Exercise training causes differential changes in gene expression in diaphragm arteries and 2A arterioles of obese rats. J Appl Physiol 119: 604–616, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol 90: 501–510, 2001. [DOI] [PubMed] [Google Scholar]
- 74.Laughlin MH, Ripperger J. Vascular transport capacity of hindlimb muscles of exercise-trained rats. J Appl Physiol 62: 438–443, 1987. [DOI] [PubMed] [Google Scholar]
- 75.Laughlin MH, Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. J Physiol Pharmacol 59: 71–88, 2008. [PMC free article] [PubMed] [Google Scholar]
- 76.Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol 96: 233–244, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Järvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80: 414–424, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mackie BG, Terjung RL. Blood flow to different skeletal muscle fiber types during contraction. Am J Physiol Heart Circ Physiol 245: H265–H275, 1983. [DOI] [PubMed] [Google Scholar]
- 79.Mackie BG, Terjung RL. Influence of training on blood flow to different skeletal muscle fiber types. J Appl Physiol 55: 1072–1078, 1983. [DOI] [PubMed] [Google Scholar]
- 80.Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 38: 860–866, 2001. [DOI] [PubMed] [Google Scholar]
- 81.Martin JS, Padilla J, Jenkins NT, Crissey JM, Bender SB, Rector RS, Thyfault JP, Laughlin MH. Functional adaptations in the skeletal muscle microvasculature to endurance and interval sprint training in the type 2 diabetic OLETF rat. J Appl Physiol 113: 1223–1232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin WH 3rd, Ogawa T, Kohrt WM, Malley MT, Korte E, Kieffer PS, Schechtman KB. Effects of aging, gender, and physical training on peripheral vascular function. Circulation 84: 654–664, 1991. [DOI] [PubMed] [Google Scholar]
- 83.McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol 98: 753–761, 2005. [DOI] [PubMed] [Google Scholar]
- 84.McAllister RM, Kimani JK, Webster JL, Parker JL, Laughlin MH. Effects of exercise training on responses of peripheral and visceral arteries in swine. J Appl Physiol 80: 216–225, 1996. [DOI] [PubMed] [Google Scholar]
- 85.McAllister RM, Laughlin MH. Short-term exercise training alters responses of porcine femoral and brachial arteries. J Appl Physiol 82: 1438–1444, 1997. [DOI] [PubMed] [Google Scholar]
- 86.McAllister RM, Newcomer SC, Laughlin MH. Vascular nitric oxide: effects of exercise training in animals. Appl Physiol Nutr Metab 33: 173–178, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCurdy MR, Colleran PN, Muller-Delp J, Delp MD. Effects of fiber composition and hindlimb unloading on the vasodilator properties of skeletal muscle arterioles. J Appl Physiol 89: 398–405, 2000. [DOI] [PubMed] [Google Scholar]
- 88.Mikus CR, Rector RS, Arce-Esquivel AA, Libla JL, Booth FW, Ibdah JA, Laughlin MH, Thyfault JP. Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol 109: 1203–1210, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mikus CR, Roseguini BT, Uptergrove GM, Matthew Morris E, Scott Rector R, Libla JL, Oberlin DJ, Borengasser SJ, Taylor AM, Ibdah JA, Laughlin MH, Thyfault JP. Voluntary wheel running selectively augments insulin-stimulated vasodilation in arterioles from white skeletal muscle of insulin resistant rats. Microcirculation 19: 729–738, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitranun W, Deerochanawong C, Tanaka H, Suksom D. Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand J Med Sci Sports 24: e69–e76, 2014. [DOI] [PubMed] [Google Scholar]
- 91.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002. [DOI] [PubMed] [Google Scholar]
- 92.Muller JM, Myers PR, Laughlin MH. Exercise training alters myogenic responses in porcine coronary resistance arteries. J Appl Physiol 75: 2677–2682, 1993. [DOI] [PubMed] [Google Scholar]
- 93.Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation 89: 2308–2314, 1994. [DOI] [PubMed] [Google Scholar]
- 94.Musch TI, Haidet GC, Ordway GA, Longhurst JC, Mitchell JH. Training effects on regional blood flow response to maximal exercise in foxhounds. J Appl Physiol 62: 1724–1732, 1987. [DOI] [PubMed] [Google Scholar]
- 95.Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hagmeman KS, Musch T, Poole DC. Effects of Type II diabetes on capillary hemodynamics in skeletal muscle. Am J Physiol Heart Circ Physiol 291: H2439–H2444, 2006. [DOI] [PubMed] [Google Scholar]
- 96.Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. Identification of genes whose expression is altered by obesity throughout the arterial tree. Physiol Genomics 46: 821–832, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. Transcriptome-wide RNA sequencing analysis of rat skeletal muscle feed arteries. II. Impact of exercise training in obesity. J Appl Physiol 116: 1033–1047, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parker JL, Mattox ML, Laughlin MH. Contractile responsiveness of coronary arteries from exercise-trained rats. J Appl Physiol 83: 434–443, 1997. [DOI] [PubMed] [Google Scholar]
- 99.Phillips SA, Mahmoud AM, Brown MD, Haus JM. Exercise interventions and peripheral arterial function: implications for cardio-metabolic disease. Prog Cardiovasc Disease 57: 521–534, 2015. [DOI] [PubMed] [Google Scholar]
- 100.Poole DC, Copp SW, Ferguson SK, Musch TI. Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol 98: 1645–1658, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol 2: 933–996, 2012. [DOI] [PubMed] [Google Scholar]
- 102.Prior SJ, Blumenthal JB, Katzel LI, Goldberg AP, Ryan AS. Increased skeletal muscle capillarization after aerobic exercise training and weight loss improves insulin sensitivity in adults with IGT. Diabetes Care 37: 1469–1475, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. [DOI] [PubMed] [Google Scholar]
- 104.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab 298: E1179–E1187, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol 300: G874–G883, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rector RS, Warner SO, Liu Y, Hinton PS, Sun GY, Cox RH, Stump CS, Laughlin MH, Dellsperger KC, Thomas TR. Exercise and diet induced weight loss improves measures of oxidative stress and insulin sensitivity in adults with characteristics of the metabolic syndrome. Am J Physiol Endocrinol Metab 293: E500–E506, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Renkin EM. Transcapillary exchange in relation to capillary circulation. J Gen Physiol 52: 96S–108S, 1968. [PubMed] [Google Scholar]
- 109.Renkin EM, Michel CC. Control of microcirculation and blood-tissue exchange. Handbook of Physiology. The Cardiovascular System. Bethesda, MD: Am Physiol Soc, 1984, sect. 2, vol. IV, p. 626–687. [Google Scholar]
- 110.Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord 14: 77–86, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at V̇o2 max. J Appl Physiol 73: 1067–1076, 1992. [DOI] [PubMed] [Google Scholar]
- 112.Roca J, Hogan MC, Story D, Debout DE, Haab P, Gonzalez R, Ueno O, Wagner PD. Evidence for tissue diffusion limitation of V̇o2 max in normal humans. J Appl Physiol 67: 291–299, 1989. [DOI] [PubMed] [Google Scholar]
- 113.Rowell L. Human Circulation. New York: Oxford University Press, 1986. [Google Scholar]
- 114.Rowell LB. Human Cardiovascular Control. Oxford, UK: Oxford University Press, 1993. [Google Scholar]
- 115.Saltin B, Gollnick PD. Skeletal muscle adaptability: significance for metabolism and performance. Handbook of Physiology: Skeletal Muscle. Bethesda, MD: Am Physiol Soc, 1977, sect. 10, p. 555–631. [Google Scholar]
- 116.Saydah S, Imperatore G, Geiss L, Gregg E. Diabetes death rates among youths aged ≤19 years–United States, 1968–2009. MMWR Morb Mortal Wkly Rep 61: 869–872, 2012. [PubMed] [Google Scholar]
- 117.Schrage WG, Woodman CR, Laughlin MH. Hindlimb unweighting alters endothelium-dependent vasodilation and ecNOS expression in soleus arterioles. J Appl Physiol 89: 1483–1490, 2000. [DOI] [PubMed] [Google Scholar]
- 118.Schrage WG, Woodman CR, Laughlin MH. Mechanisms of flow and ACh-induced dilation in rat soleus arterioles are altered by hindlimb unweighting. J Appl Physiol 92: 901–911, 2002. [DOI] [PubMed] [Google Scholar]
- 119.Schwingshackl L, Missbach B, Dias S, König J, Hoffmann G. Impact of different training modalities on glycemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia 57: 1789–1797, 2014. [DOI] [PubMed] [Google Scholar]
- 120.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res 74: 349–353, 1994. [DOI] [PubMed] [Google Scholar]
- 121.Sexton WL, Korthuis RJ, Laughlin MH. High-intensity exercise training increases vascular transport capacity of rat hindquarters. Am J Physiol Heart Circ Physiol 254: H274–H278, 1988. [DOI] [PubMed] [Google Scholar]
- 122.Sexton WL, Laughlin MH. Influence of endurance exercise training on distribution of vascular adaptations in rat skeletal muscle. Am J Physiol Heart Circ Physiol 266: H483–H490, 1994. [DOI] [PubMed] [Google Scholar]
- 123.Sexton WL, Laughlin MH. Influence of endurance exercise training on distribution of vascular adaptations in rat skeletal muscle. Am J Physiol Heart Circ Physiol 266: H483–H490, 1994. [DOI] [PubMed] [Google Scholar]
- 124.Snell PG, Martin WH, Buckey JC, Blomqvist CG. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol 62: 606–610, 1987. [DOI] [PubMed] [Google Scholar]
- 125.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spier SA, Delp MD, Stallone JN, Dominguez JM 2nd, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292: H3119–H3127, 2007. [DOI] [PubMed] [Google Scholar]
- 127.Spurway NC, Ekblom B, Noakes TD, Wagner PD. What limits V̇o2 max? A symposium held at the BASES conference, 6 September 2010. J Sports Sci 30: 517–531, 2012. [DOI] [PubMed] [Google Scholar]
- 128.Stehno-Bittel L, Laughlin MH, Sturek M. Exercise training alters Ca release from coronary smooth muscle sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol 259: H643–H647, 1990. [DOI] [PubMed] [Google Scholar]
- 129.Stehno-Bittel L, Laughlin MH, Sturek M. Exercise training depletes sarcoplasmic reticulum calcium in coronary smooth muscle. J Appl Physiol 71: 1764–1773, 1991. [DOI] [PubMed] [Google Scholar]
- 130.Sun D, Huang A, Koller A, Kaley G. Adaptation of flow-induced dilation of arterioles to daily exercise. Microvasc Res 56: 54–61, 1998. [DOI] [PubMed] [Google Scholar]
- 131.Tipton CM. Determinants of V̇o2 max: insights gained from non-human species. Acta Physiol Scand 556: 33–43, 1986. [PubMed] [Google Scholar]
- 132.Wagner PD. Modeling O2 transport as an integrated system limiting V̇o2 max. Comput Methods Programs Biomed 101: 109–114, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wagner PD. New ideas on limitations to V̇o2 max. Exerc Sport Sci Rev 28: 10–14, 2000. [PubMed] [Google Scholar]
- 134.Wang J, Wolin MS, Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res 73: 829–838, 1993. [DOI] [PubMed] [Google Scholar]
- 135.Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol 53: 2175–2183, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Woodman CR, Schrage WG, Rush JW, Ray CA, Price EM, Hasser EM, Laughlin MH. Hindlimb unweighting decreases endothelium-dependent dilation and eNOS expression in soleus not gastrocnemius. J Appl Physiol 91: 1091–1098, 2001. [DOI] [PubMed] [Google Scholar]
- 137.Woodman CR, Trott DW, Laughlin MH. Short-term increases in intraluminal pressure reverse age-related decrements in endothelium-dependent dilation in soleus muscle feed arteries. J Appl Physiol 103: 1172–1179, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]