Abstract
Unloading or disuse rapidly results in skeletal muscle atrophy, switching to fast-type fibers, and decreased resistance to fatigue. The recovery process is of major importance in rehabilitation for various clinical conditions. Here we studied mouse soleus muscle during 60 days of reloading after 4 wk of hindlimb suspension. Unloading produced significant atrophy of soleus muscle with decreased contractile force and fatigue resistance, accompanied by switches of myosin isoforms from IIa to IIx and IIb and fast troponin T to more low-molecular-weight splice forms. The total mass, fiber size, and contractile force of soleus muscle recovered to control levels after 15 days of reloading. However, the fatigue resistance showed a trend of worsening during this period with significant infiltration of inflammatory cells at days 3 and 7, indicating reloading injuries that were accompanied by active regeneration with upregulations of filamin-C, αB-crystallin, and desmin. The fatigue resistance partially recovered after 30–60 days of reloading. The expression of peroxisome proliferator-activated receptor γ coactivator 1α and mitofusin-2 showed changes parallel to that of fatigue resistance after unloading and during reloading, suggesting a causal role of decreased mitochondrial function. Slow fiber contents in the soleus muscle were increased after 30–60 days of reloading to become significantly higher than the normal level, indicating a secondary adaption to compensate for the slow recovery of fatigue resistance.
Keywords: skeletal muscle unloading, reloading injury and adaptation, in situ fatigue resistance, peroxisome proliferator-activated receptor γ coactivator 1α, type I slow fiber
the primary function of skeletal muscle is in animal locomotion and posture maintenance against load and gravity. Skeletal muscle is a highly plastic tissue and rapidly changes mass and fiber type in response to load and activity demand (95). Under various physiological and disease conditions, such as aging, disuse, and unloading, skeletal muscle decreases mass and contractile force, leading to physical disability (41, 49, 57, 74). Promoting recovery from these conditions has major medical and socioeconomic importance.
Fiber type contents determine the performance of skeletal muscle (16, 42). Slow- and fast-twitch fibers have different metabolic and contractile properties and are classified by the expression of specific isoforms of myosin heavy chain (MHC) (13, 24) that confer different ATPase activities (18). Fiber type composition of skeletal muscle dynamically changes in adaptation to changes in functional demands, neurohumoral signaling, and metabolic conditions (8). In type 2 diabetes, the number of slow oxidative fibers is reduced along with an increase of fast glycolytic fibers (77, 80). Patients with obesity (93) or chronic heart failure (11, 21, 84, 88, 92) also showed a shift from oxidative fibers to glycolytic fibers. Being bedridden for 5–7 days rapidly produced muscle atrophy and switch to more type II fibers with muscle weakness and increased fatigability (6, 44, 48). Extensive studies in human (7, 28, 38, 79) and rodent models (10, 55, 60) of muscle disuse demonstrated imbalances of protein synthesis and degradation, leading to a net loss of myofilament proteins. Several laboratories including ours have reported that unloading of rat soleus muscle by hindlimb suspension reproduced such progressive skeletal muscle atrophy and slow-to-fast fiber type switch (13, 75, 91, 104).
No effective approach is currently available for the treatment and prevention of the loss of muscle mass and contractile function resulting from unloading or disuse. The muscle atrophy and fiber type switch are reversible phenotypes. The reloading-induced muscle injury and its impact on functional recovery (30, 62, 86, 101) impose a major challenge in physical rehabilitation. Facilitating the recovery process is of practical medical importance, for which mice and rats have been extensively studied in various experimental conditions with different lengths of unloading and reloading (19, 32, 54, 76, 78).
Muscle fatigability is a key factor that determines muscle quality and physical abilities. Together with fiber type switch, mitochondrial dysfunction has been shown to decrease the tolerance of skeletal muscle to exercise (1, 102). In the present study, we investigated the chronic recovery of adult mouse soleus muscle during 60 days of reloading after 4 wk of hindlimb suspension. We employed in situ contractility measurement for the first time to study muscle reloading to avoid isolation and hypoxia injuries accompanying the commonly used superfusion muscle strip preparations. The results showed that, whereas the muscle mass, fiber size, and contractile force completely returned to control levels by 15 days of reloading, fatigue resistance had a trend of worsening during the early phase of recovery with significant inflammation at days 3 and 7, indicating reloading injuries. The slow recovery of fatigue resistance corresponded to parallel changes in the expression of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and mitofusin-2, suggesting a causal relationship. The expression of type I myosin and the number of type I fibers increased after 30–60 days of reloading to a level significantly higher than that in normal adult mouse soleus muscle, indicating a secondary adaptation to compensate for the slow recovery of fatigue resistance.
MATERIALS AND METHODS
Mouse model of hindlimb unloading.
All animal protocols were approved by the Institutional Animal Care and Use Committee of Wayne State University.
Four-month-old male C57BL/6 mice obtained from Harlan Laboratories (Indianapolis, IN) were randomly assigned to control, 28-day hindlimb-unloading, and 3-, 7-, 15-, 30-, 45-, and 60-day reloading groups. The control mice were maintained free in single cages for 14 days or 2 mo. The hindlimb-unloading paradigm here was similar to the protocol of Ferreira et al. (29) as used by others (12, 69, 103). Briefly, animals were anesthetized with 2% isoflurane, and the fifth and sixth intervertebral spaces of the tail were located. A pilot hole was made with a 25-G needle between the two vertebrae into the intervertebral space. A sterile steel wire suture (Steelex Monofilament; Jorgensen Laboratory, Loveland, CO) was inserted into the space and twisted to form a loop. A smaller second loop was then formed above the first loop. After the surgery, animals were given 7 days to recover (29).
On the first day of hindlimb suspension, animals were placed in a large rodent cage with a slotted steel bar extending the full length of the cage. The second steel wire loop on the tail was connected to a fishing swivel connected to a sewing machine bobbin. This approach allows the animal full rotational movement in a stationary location. The animal's hindlimb was raised so as not to make any contact with the bottom of the cage. The tail was covered with sterile gauze and secured with tape to prevent dropping downward or becoming necrotic (29).
The mice had access to water and food ad libitum during the suspension period. At the end of suspension, the mice were used for muscle function measurements or unfastened from the loop wire and maintained free in the single cage for reloading and recovery studies.
In situ measurement of soleus muscle contractions.
In situ soleus muscle contractility was measured using a protocol modified from a method described previously (26). The mouse was anesthetized with isoflurane delivered through a nose cone using a small animal anesthesia system (SomnoSuite; Kent Scientific, Torrington, CT). For induction, 3% of isoflurane was used, and for maintenance, 2% was used during the experiment.
A small incision was made at the ankle to expose the Achilles tendon. Because gastrocnemius muscle and soleus muscle share the same tendon, the distal part of gastrocnemius muscle was isolated from soleus muscle and cut from the tendon. Care was taken to avoid damaging the soleus-tendon junction. While avoiding stretch injury, the tendon of soleus muscle was dissected from surrounding connective tissues and cut at the bone attachment. Another incision was made at the thigh to expose sciatic nerve.
The mouse was then placed on a platform maintained at 37°C with circulating warm water, and the knee of one leg was immobilized by being mounted between a pair of screws instrumented on the platform (Aurora Scientific, Aurora, Ontario, Canada). The foot was taped and secured on the surface of the platform. The end of soleus tendon was tied with 5-0 silk suture to securely attach to the lever arm of a force transducer (model 300B-LR; Aurora Scientific). A pair of custom-modified platinum wire electrodes was placed around the sciatic nerve for electronic stimulation. The open surgical sites were kept under moisture with dripping Krebs solution (118.00 mM NaCl, 4.70 mM KCl, 2.25 mM MgSO4, 1.20 mM KH2PO4, 2.25 mM CaCl2, 11.00 mM glucose, and 21.00 mM NaHCO3) gassed with 5% CO2-95% O2, pH 7.4 at 37°C. Anal temperature was monitored by an electronic thermometer to confirm that the body temperature was maintained at 37.0 ± 0.5°C.
Twitch contractions of soleus muscle were induced by electrical stimulation of the sciatic nerve with 0.1-ms square pulses at 2–4 V adjusted as 1.5-fold of the threshold voltage. An optimum length of soleus muscle was determined by reaching the maximum isometric twitch force during step increases of the muscle length. The muscle was then equilibrated with tetanic contractions induced by stimulation at 200 Hz for 500 ms at 1-min intervals for 20 min, before being tested by stimulations at 80–260 Hz to find the optimum frequency that generates the maximum tetanic force (Po), typically at 200 Hz. The optimum length and stimulating frequency were then used in a fatigue protocol consisting of 300 repeats of 500 ms tetanic contraction every 1.5 s. After the fatigue protocol, the muscle was allowed to recover under 500 ms of 200-Hz stimulation per minute for 20 min when a plateau of recovery in tetanic force development was reached.
The contractility data were collected using ASI 600A_4CH software (Aurora Scientific) for later analysis. At the end of each experiment, the tested soleus muscle was removed and frozen in liquid nitrogen for later analysis of proteins.
Histology and immunohistochemistry.
After the contractility measurement, gastrocnemius muscle of the other leg was removed to expose entire soleus muscle from tendon to tendon. Two 30-G needles were inserted into the proximal and distal tendon, respectively. The distance between two needles was measured when the ankle was kept at a 90° angle. The muscle was then removed and placed in optimal cutting temperature compound in a cryostat tissue holder with the tendons pinned down onto a cork at the in situ muscle length between the two needles. Frozen soleus muscle tissue blocks were chilled in liquid nitrogen-cooled isopentane for 1 min before being completely submerged into liquid nitrogen. Ten-micron cryo-sections were cut, processed for hematoxylin and eosin staining (27), and imaged using a Zeiss Axio Observer A1 microscope attached to a digital camera (ProGres C3; Jenoptik, Jena, Germany).
Immunohistochemistry staining was performed to identify type I fibers using an anti-type I MHC mouse monoclonal antibody (mAb) FA2 (52) as previous described (27). Fixed with 25% ethanol and 75% acetone at room temperature for 5 min, muscle sections were blocked with 1% BSA in PBS containing 0.05% Tween-20 (PBS-T) for 30 min. The sections were then incubated in 1% H2O2 at room temperature for 10 min to inactivate endogenous peroxidases. Washed three times with PBS-T for 5 min each, the slides were incubated with mAb FA2 in PBS-T at 4°C overnight. Washed again five times with PBS-T for 10 min each, the sections were incubated with horseradish peroxidase-labeled goat-anti-mouse secondary antibody (1,200 dilution in PBS-T containing 0.1% BSA) at room temperature for 1 h. After final washes as above, the slides were developed in 0.05% 3,3′-diaminobenzidine and 0.03% H2O2 substrate solution at room temperature for 30 s when brown color was visible. The development was stopped by being washed five times with 20 mM Tris·HCl (pH 7.6) for 5 min each. Nuclei were counterstained using Mayer's hematoxylin solution for 5 min. After being washed with running tap water for 3 min, the slides were mounted in 50% glycerol in PBS with a #1 cover slip and sealed with Permount for microscopic imaging.
SDS-PAGE and Western blotting.
After rapidly measuring the weight, we cut the frozen soleus and extensor digitorum longus (EDL) muscles into small pieces in a tube on dry ice. SDS-PAGE sample buffer containing 2% SDS and 1% β-mercaptoethanol, pH 8.8, was added to the tube at 40-fold of the muscle weight (μl/mg) to homogenize the muscle tissue using a high-speed mechanical homogenizer. The SDS-PAGE samples were then heated at 80°C for 5 min and centrifuged at 14,000 g in a microcentrifuge for 5 min to remove insoluble debris. The supernatant was resolved on SDS gels with 14% acrylamide/bisacrylamide at the ratio of 180:1 or 12% acrylamide/bisacrylamide at the ratio of 29:1 prepared in a modified Laemmli buffer system, in which both stacking and resolving gels were at pH 8.8, or ∼2–12% gradient gel with acrylamide/bisacrylamide at the ratio of 180:1. The resolved protein bands were visualized by Coomassie blue R-250 staining. The actin bands were quantified using ImageJ software (National Institutes of Health, Bethesda, MD) to normalize sample loading.
Duplicate SDS gels were transferred to nitrocellulose membranes using a Bio-Rad (Hercules, CA) semidry electrical transfer device at a constant current of 5 mA/cm2 for 15 min. The blotted membranes were blocked with 1% BSA in Tris-buffered saline (TBS, 150 mM NaCl, 50 mM Tris·HCl, pH 7.5) at room temperature with shaking for 30 min. The blocked membranes were probed at 4°C overnight with anti-TnI mAb TnI-1 (53), anti-slow TnT mAb CT3, anti-fast TnT mAb T12 (51), anti-tropomyosin mAb CH1 (67), anti-desmin mAb D1033 (Sigma, St. Louis, MO), rabbit anti-mitofusin-2 mAb (NIAR164; Abcam, Cambridge, MA), rabbit anti-voltage-dependent anion channel (VDAC) polyclonal antibody (4866S; Cell Signaling Technology, Beverly, MA), or rabbit anti-PGC-1α polyclonal antibody (ab54481, Abcam) diluted in TBS containing 0.1% BSA. The membranes were then washed three times for 7 min each with TBS containing 0.5% Triton X-100 and 0.05% SDS and three times for 3 min each with TBS before incubation with alkaline phosphatase-labeled goat anti-mouse IgG second antibody (Santa Cruz Biotechnology, Dallas, TX) at room temperature for 1 h. The membranes were washed again and developed in 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate solution to visualize the protein bands recognized by each of the antibodies.
MHC isoforms were examined as described previously (27). An SDS gel sample equal to 5 μg of muscle tissue each was resolved on SDS-PAGE with 8% acrylamide/bisacrylamide at the ratio of 50:1 containing 30% glycerol in an icebox for 24 h. The gels were stained with Coomassie blue R250, and the relative amounts of MHC isoforms were quantified by two-dimensional densitometry using ImageJ software (National Institutes of Health).
Mass spectrometry identification of protein bands isolated from SDS gel.
Protein bands of interest were excised from Coomassie blue R-250 stained SDS gel for in-gel protein digestion. The gel slices were washed with 50 mM ammonium bicarbonate, reduced in 10 mM DTT at 37°C for 45 min, and then alkylated with 55 mM iodoacetamide at room temperature for 30 min. Trypsin was added to the tubes at 10 ng/μl, and the digestion was incubated at 37°C overnight. The resulting peptides were analyzed by nano LC-MS/MS.
The peptides were first separated on a reverse-phase C18 column with a 90-min gradient using a Dionex Ultimate HPLC system. MS and MS/MS spectra were then acquired on an Applied Biosystems (Foster City, CA) QSTAR XL mass analyzer using information-dependent acquisition mode. MS scan was performed from m/z 400-1,500 for 1 s followed by product ion scans on the two most intense multiply charged ions. Peak lists were submitted to a Mascot server to search against the NCBInr database for all entries with carbamidomethyl used as a fixed modification and oxidation, N-acetylation (protein NH2 terminus) as variable modifications.
Data analysis and statistics.
Quantitative data are presented as means ± SE, and statistical analysis was performed using one-way or two-way ANOVA as noted in the figure legends. A Tukey post hoc follow-up test was conducted if the overall F ratio was significant.
RESULTS
The mass and force of mouse soleus muscle recovered from unloading caused atrophy after 15 days of reloading.
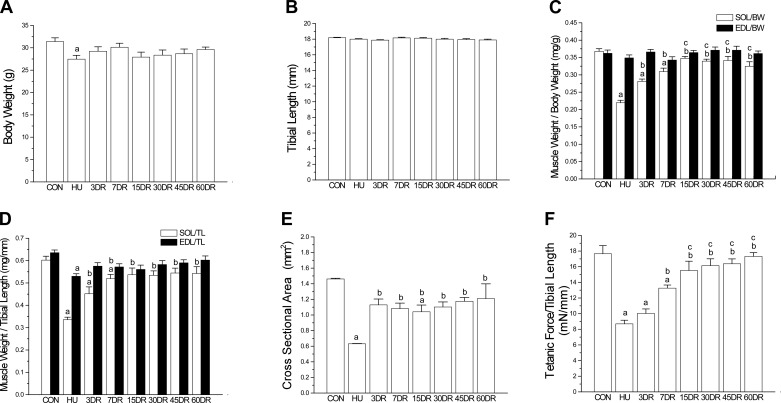
Four weeks of hindlimb suspension produced unloading phenotypes in soleus muscle of adult male C57BL/6 mice as that in previous studies of mice (91) and rats (104). The body weight of the 4-wk unloading group was slightly lower than controls (Fig. 1A). However, tibial lengths were similar in all groups (Fig. 1B), indicating unaffected development and growth. Therefore, the decrease in body weight after 4-wk tail suspension may reflect a stress effect, which was also shown by a slight decrease in the weight of non-weight-bearing muscle EDL (normalized to tibial length) (Fig. 1C).
Fig. 1.
Atrophy and decreased contractile force in mouse soleus muscle after unloading and the recovery during reloading. A: 4 wk of hindlimb unloading (HU) resulted in a slight decrease of body weight, reflecting the stress effect of tail suspension. B: unloading and reloading did not produce any change in the tibial length, indicating no effect on overall growth and development. C: normalized to body weight (BW), 4 wk of HU resulted in a significant loss of soleus (SOL) muscle mass compared with that of control (CON) mice. The weight of soleus muscle recovered during reloading and reached the normal level after 15 days (15DR). EDL, extensor digitorum longus. D: same trend was shown by normalization to tibial length (TL). E: quantification of cross-sectional area (CSA) of soleus muscle confirms the atrophy after 4-wk unloading and incomplete recovery after 60 days of reloading. F: tetanic force normalized to tibial length decreased in HU soleus muscle and recovered during reloading to reach the control level after 15 days. Values are presented as means ± SE. A, B, D, and F: n = 11 in CON, n = 9 in HU, n = 9 in 3DR, n = 8 in 7DR, n = 4 in 15DR, n = 8 in 30DR, n = 7 in 45DR, and n = 7 in 60DR. C and E: n = 9 in CON, n = 4 in HU, n = 7 in 3DR, n = 6 in 7DR, n = 4 in 15DR, n = 8 in 30DR, n = 7 in 45DR, and n = 5 in 60DR. aP < 0.05 vs. CON; bP < 0.05 vs. HU; cP < 0.05 vs. 3DR. Statistical analysis was performed using 1-way ANOVA with mean comparison in Tukey's test.
Significant atrophy occurred in soleus muscle after 4 wk of unloading, as shown by muscle weight normalized to tibial length or to body weight (Fig. 1, C and D), as well as the muscle cross-sectional area (Fig. 1E). Correspondingly, tetanic force of soleus muscle was significantly decreased after 4 wk of unloading (Fig. 1F). The weight (Fig. 1, C and D) and force production (Fig. 1F) of soleus muscle recovered during early reloading and returned to the control level at 15 days.
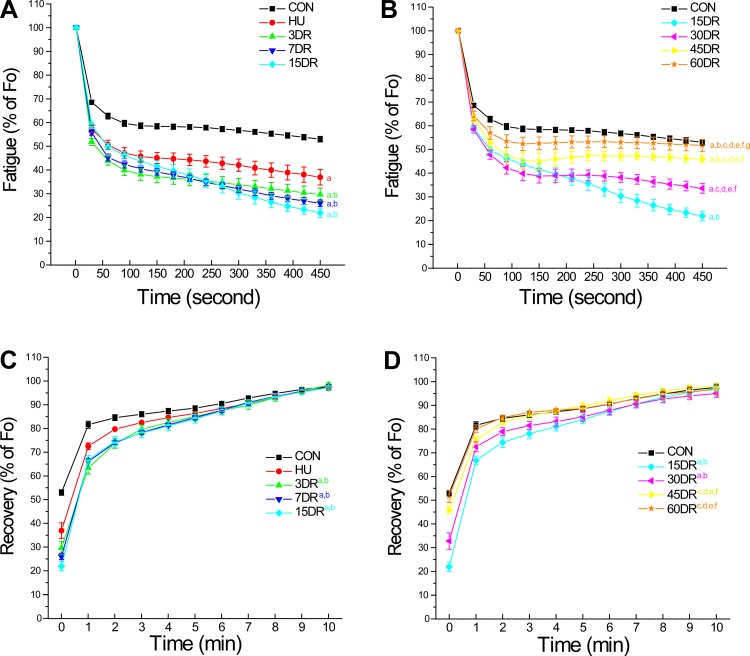
The unloading caused by decrease in fatigue resistance of soleus muscle exhibited a slow two-phase partial recovery during 60 days of reloading.
In situ intermittent fatigability studies showed that 4 wk of unloading significantly decreased fatigue resistance of mouse soleus muscle (Fig. 2A). Despite the recovered muscle mass and force (Fig. 1), the fatigue resistance did not recover but worsened during early reloading and reached the lowest level at 15 days (Fig. 2A). Fatigue resistance of soleus muscle began to improve after 30 days of reloading but remained lower than normal after 60 days of reloading (Fig. 2B). The postfatigue regaining of force showed a similar trend (Fig. 2, C and D).
Fig. 2.
Decreased fatigue resistance of soleus muscle after unloading and slow recovery during reloading. A: plotted at 30-s intervals, in situ intermittent fatigue protocol demonstrated that tetanic force normalized to tibial length dropped rapidly during fatigue compared with the prefatigue force (Fo). 4-wk HU significantly decreased fatigue resistance of soleus muscle, which was further worsened during the early phase of reloading (3–15 days). B: compared with the fatigability curves of HU and 15DR, the fatigue resistance of soleus muscle started to recover after 30 days of reloading. The recovery was slow and did not reach the control level after 60 days of reloading. C and D: postfatigue force development showed the same trends. Values are presented as means ± SE; n = 9 in CON, n = 4 in HU, n = 7 in 3DR, n = 6 in 7DR, n = 4 in 15DR, n = 8 in 30DR, n = 7 in 45DR, and n = 5 in 60DR. aP < 0.05 vs. CON; bP < 0.05 vs. HU; cP < 0.05 vs. 3DR; dP < 0.05 vs. 7DR; eP < 0.05 vs. 15DR; fP < 0.05 vs. 30DR, and gP < 0.05 vs. 45DR. Statistical analysis was performed using 2-way ANOVA with mean comparison with Tukey's test.
The fatigability results are summarized in Table 1 with the decreases in contractile force at 450 s of fatigue treatment and the regaining of force at the first 1 min after fatigue. The data demonstrate two phases of reloading effects on the unloading-treated mouse soleus muscle. The early phase of reloading (3–15 days) showed recovery of muscle mass and force but worsening of fatigue resistance, whereas the later phase demonstrated a very slow course of the recovery of fatigue resistance.
Table 1.
Fatigability in force reduction of mouse soleus muscle after unloading and during postfatigue recovery
| Fatigue (% of Fo) at 450 s | Recovery (% of Fo) at 1 min | |
|---|---|---|
| CON | 53.00 ± 1.19 | 81.57 ± 1.49 |
| HU | 36.96 ± 3.31a | 72.52 ± 1.38a |
| 3DR | 29.66 ± 2.75a | 63.41 ± 2.64ab |
| 7DR | 26.06 ± 1.35ab | 66.05 ± 1.14a |
| 15DR | 21.96 ± 1.96ab | 66.78 ± 1.84a |
| 30DR | 33.59 ± 2.05a | 72.51 ± 2.09ac |
| 45DR | 45.87 ± 1.60cdef | 75.37 ± 1.65ede |
| 60DR | 51.67 ± 2.58cdefg | 80.18 ± 1.56ede |
Values are presented as means ± SE. Soleus muscle fatigue and postfatigue force recovery were calculated as the percentage of peak tetanic force at optimal muscle length (Fo). The force reduction at the end of fatigue treatment and the 1st minute after fatigue force recovery were compared in control mice (CON), mice with 4 wk of hindlimb unloading (HU), and those during 3–60 days of reloading (DR). The results showed that the fatigability and postfatigue force recovery were worsened to become lower than HU level at 3–15 days of reloading and gradually improved to the control level at 30–60 days reloading. n = 9 in CON, n = 4 in HU, n = 7 in 3DR, n = 6 in 7DR, n = 4 in 15DR, n = 8 in 30DR, n = 7 in 45DR, and n = 5 in 60DR.
P < 0.05 vs. CON,
P < 0.05 vs. HU,
P < 0.05 vs. 3DR,
P < 0.05 vs. 7DR,
P < 0.05 vs. 15DR,
P < 0.05 vs. 30DR,
P < 0.05 vs. 45DR. Statistical analysis was performed using 1-way ANOVA with mean comparison with Tukey's test.
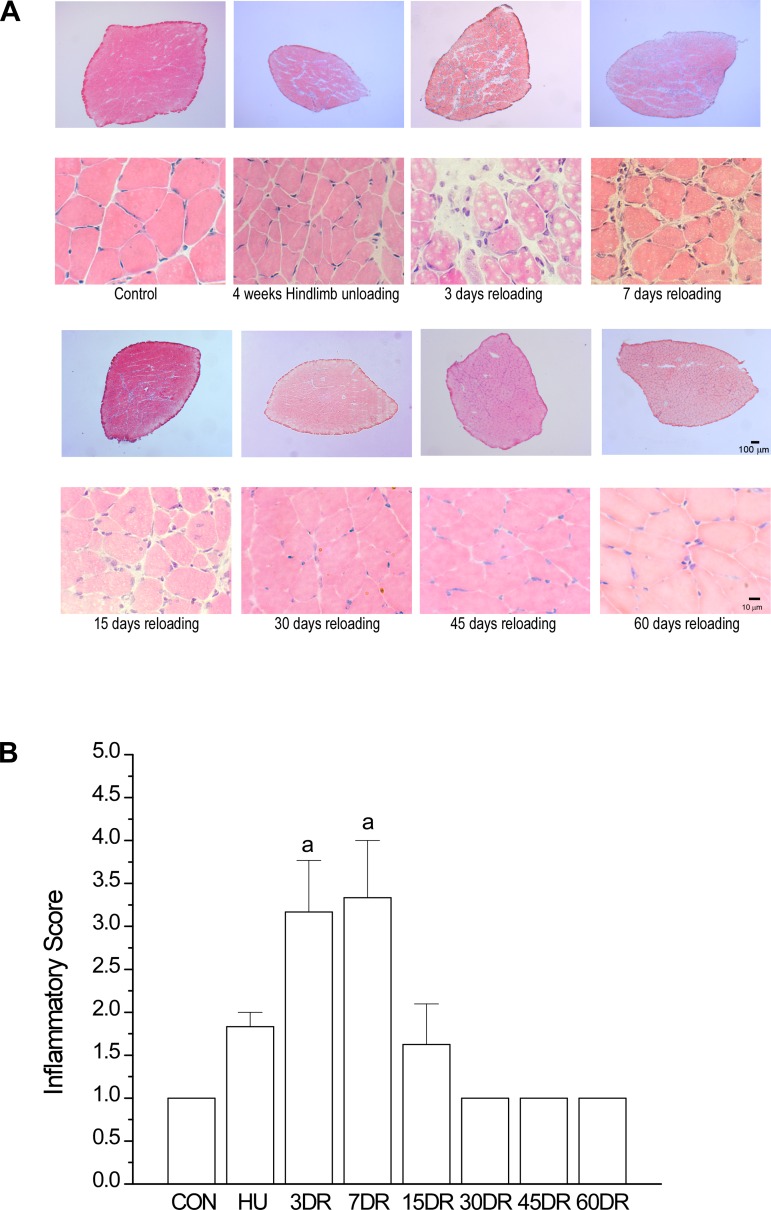
Injury and regeneration of soleus muscle during reloading.
Despite the early recovery of muscle mass and force by 15 days of reloading (Fig. 1, C–F), fatigue resistance of soleus muscle became lower than that at the end of 4 wk of unloading (Fig. 2A). This phenotype was concurrent with muscle tissue swelling and infiltration of inflammatory cells, evident at 3, 7, and 15 days of reloading (Fig. 3), indicating a reloading-mediated injury of the atrophic and weakened soleus muscle.
Fig. 3.
Inflammatory response in mouse soleus muscle during the early phase of reloading. A: hematoxylin and eosin staining detected infiltration of inflammatory cells in soleus muscle after 3, 7, and 15 days of reloading, indicating reloading injury of the atrophic and weakened muscle. The increased interstitial space in soleus muscle after 3 and 7 days of reloading indicates inflammatory swelling. B: using a published method (47, 68), the inflammation response to reloading injury was quantified in a blinded manner as scores of 1–4, where 1 = a few scattered inflammatory cells, 2 = clusters of inflammatory cells, 3 = diffuse infiltrate of inflammatory cells, and 4 = dense sheets of inflammatory cells, including lymphoid follicles. Values are presented as means ± SE; n = 3 in CON, n = 3 in HU, n = 3 in 3DR, n = 3 in 7DR, n = 4 in 15DR, n = 4 in 30DR, n = 4 in 45DR; and n = 3 in 60DR. aP < 0.01 vs. CON. Statistical analysis was performed using 1-way ANOVA with mean comparison in Tukey's test.
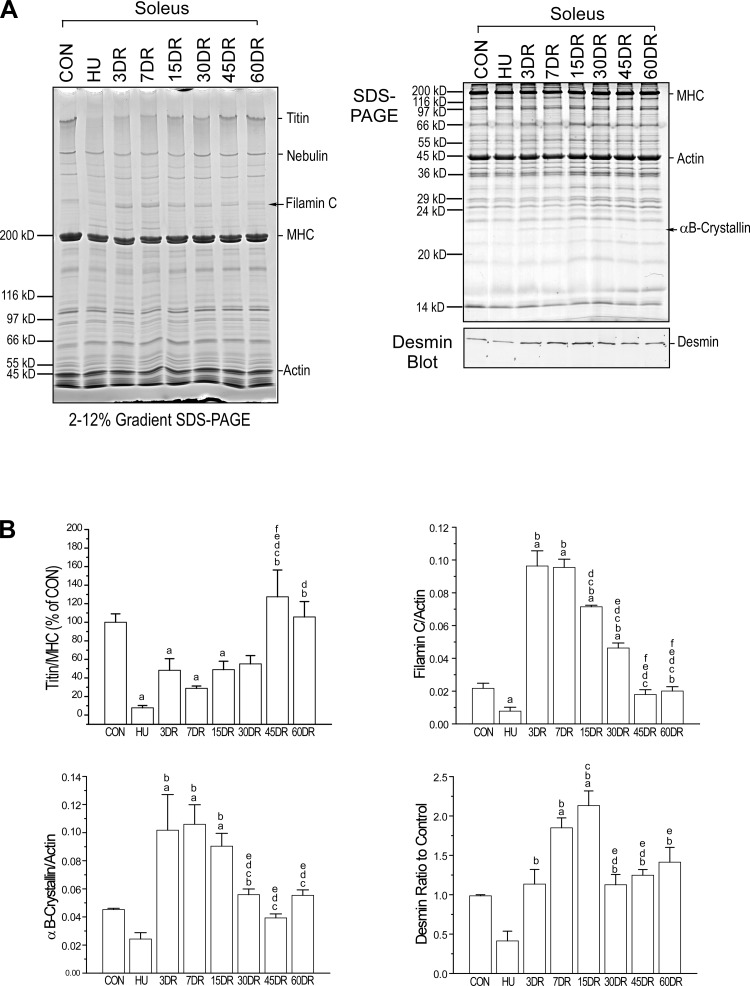
SDS-PAGE revealed that two protein bands in soleus muscle were decreased after 4 wk of unloading and increased at 3, 7, and 15 days of reloading (Fig. 4A). Mass spectrometry identified them to be filamin C and αB-crystallin.
Fig. 4.
Changes in titin and increases of filamin C, αB-crystallin, and desmin in mouse soleus muscle after unloading and during reloading. Gradient and uniform SDS gels and Western blot (A) and densitometry quantification (B) showed that titin level was decreased after 4 wk of unloading and gradually recovered to the control level after 45 days of reloading. Muscle regeneration markers filamin C, αB-crystallin (identified using mass spectrometry), and desmin were decreased after 4 wk of unloading, rebounded to higher than normal levels early during reloading, and returned to normal level after 30 days of reloading. Values are presented as means ± SE; n = 3 in CON, HU, 3DR, and 7DR; n = 4 in 15 DR, n = 4 in 30DR; n = 4 in 45DR; and n = 3 in 60DR. aP < 0.05 vs. CON; bP < 0.05 vs. HU; cP < 0.05 vs. 3DR; dP < 0.05 vs. 7DR; eP < 0.05 vs. 15DR; fP < 0.05 vs. 30DR. Statistical analysis was performed using 1-way ANOVA with mean comparison in Tukey's test. MHC, myosin heavy chain.
Filamin C is a Z-disc protein in myofibrils and plays an essential role in the maintenance of structural integrity of striated muscle (17, 36, 39). Increase of filamin C has been observed as an indicator of muscle regeneration (39). The increase of filamin C in soleus muscle during the early phase of reloading (Fig. 4A), therefore, reflects a molecular response to the reloading-mediated muscle injury.
αB-crystallin is a small heat shock protein and is also localized in the Z-line of striated muscles (22). As a chaperone protein, αB-crystallin binds unfolded proteins to prevent their denaturation and aggregation under stress conditions (82). The increase in αB-crystallin in soleus muscle during the early phase of reloading (Fig. 4A) may indicate a compensatory response to reloading injury.
The Western blot in Fig. 4A further showed similar changes in desmin that was downregulated in soleus muscle after 4 wk of unloading, upregulated after 7 and 15 days of reloading to higher-than-control level, and returned to control level after 30 days of reloading. Desmin is another striated muscle Z-line protein (40) and plays a role in myofibrillogenesis (98). It was reported that desmin mRNA was significantly reduced in rat soleus muscle after 7 days of unloading and increased after 7 days of reloading (35). Previous studies also found increases of desmin in the cytoplasm of regenerating and partially damaged skeletal muscle fibers (43, 85). The increased expression of desmin in soleus muscle during the early phase of reloading but slightly lagging that of filamin C and αB-crystallin indicates an activation of regeneration in response to the reloading-mediated muscle injury.
Gradient SDS gel revealed that titin was significantly diminished in soleus muscle after 4 wk of unloading and slowly recovered to the control level after 45 days of reloading (Fig. 4A). Decrease of titin in unloaded rat soleus muscle was reported to depress muscle performance via abnormal sarcomeric organization (97). Eccentric exercise induced fragmentation of titin (71), suggesting that titin may be involved in stretch injury during skeletal muscle reloading. The unloading that caused loss of titin may be an indicator of muscle atrophy, and its slow recovery during reloading may reflect the slow course of reconstruction of sarcomeres. The impact of the changes in titin on muscle contractility and fatigability remains to be investigated. The changes in filamin C, αB-crystallin, desmin, and titin are quantified, and the results are shown in Fig. 4B.
Decrease and slow recovery of PGC-1α and mitofusin-2 concurrent with the change in fatigue resistance.
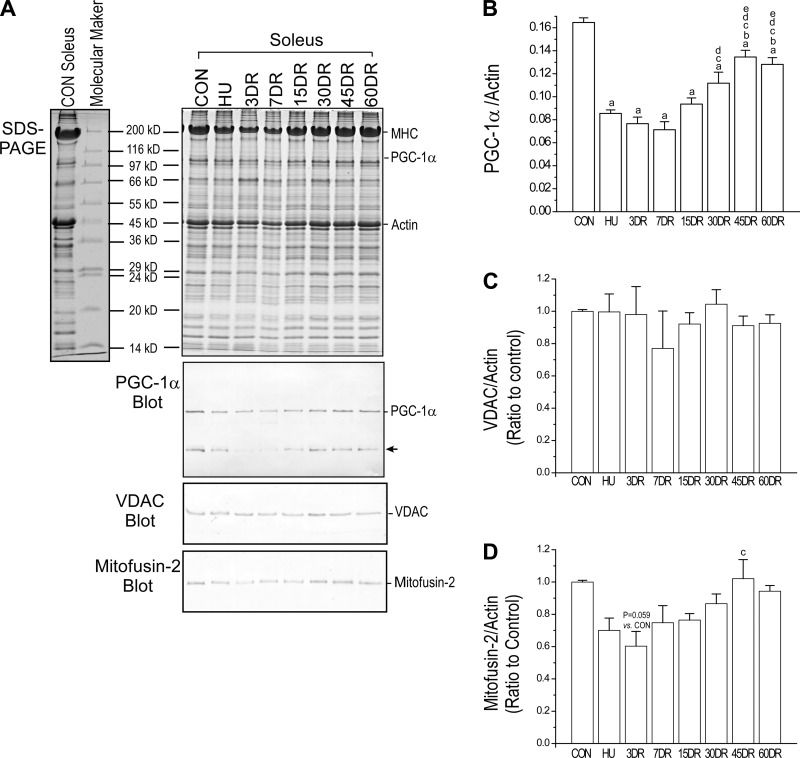
The Western blot and densitometry quantification in Fig. 5 showed that the level of PGC-1α was downregulated in soleus muscle after 4 wk of unloading and slowly recovered during reloading. Similar to the time course of fatigue-resistance changes, the level of PGC-1α was only partially recovered after 60 days of reloading (Fig. 5B). Western blot further showed that total mitochondrial contents indicated by the level of VDAC were not different in control, 4-wk-unloaded, and reloaded soleus muscle (Fig. 5C). However, mitofusin-2, an indicator of mitochondrial fusion, showed a similar trend of change to that of PGC-1α (Fig. 5D). These findings implicated a parallel relationship between the impairment and slow recovery of mitochondrial function and the slow recovery of soleus muscle fatigue resistance.
Fig. 5.
Changes of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), voltage-dependent anion channel (VDAC), and mitofusin-2 in mouse soleus muscles corresponding to decreased fatigue resistance after unloading and during reloading. A: Western blot using rabbit anti-PGC-1α polyclonal antibody (ab54481, Abcam) showed decreased expression of PGC-1α in mouse soleus muscle after 4 wk of unloading. B: its partial recovery during 60 days of reloading was parallel to the resistance to fatigue (a nonspecific protein band, indicated by the arrow, was also recognized by the anti-PGC-1α antibody). C: total mitochondrial content measured using a rabbit polyclonal antibody against mitochondrial VDAC was unchanged. D: however, the expression of mitochondrial fusion protein mitofusin-2 detected using a rabbit polyclonal antibody showed a similar trend to that of PGC-1α. Values are presented as means ± SE; n = 3 in CON, HU, 3DR, and 7DR; n = 4 in 15 DR; n = 4 in 30DR; n = 4 in 45DR; and n = 3 in 60DR. aP < 0.05 vs. CON; bP < 0.05 vs. HU; cP < 0.05 vs. 3DR; dP < 0.05 vs. 7DR; eP < 0.05 vs. 15DR. Statistical analysis was performed using 1-way ANOVA with mean comparison in Tukey's test.
Switching of fiber type-specific myofilament protein isoforms in soleus muscle after unloading and during reloading.
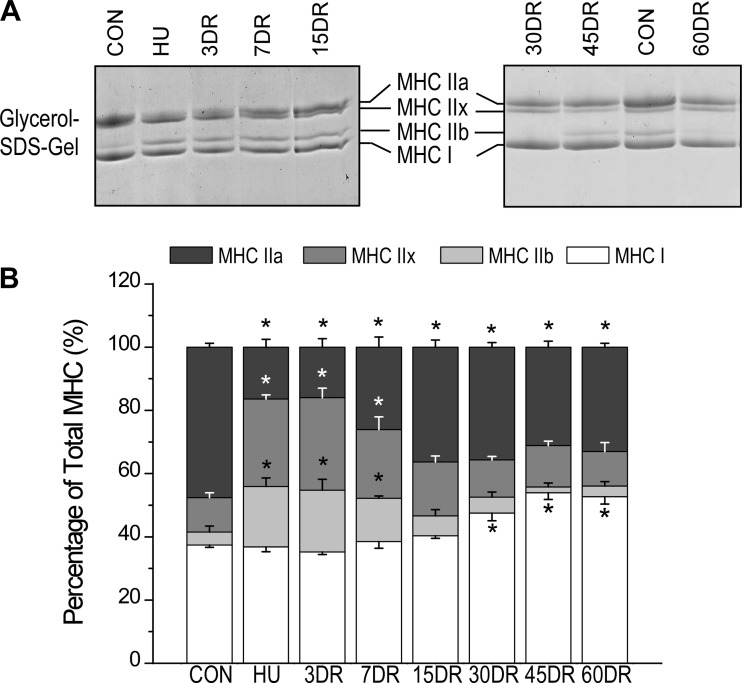
Four weeks of unloading caused a decrease in MHC IIa and increases in MHC IIx and IIb in mouse soleus muscle with no change in MHC I (Fig. 6). MHC IIx and IIb returned to control level after 15 days of reloading (Fig. 6). MHC IIa increased after 15 days of reloading but remained lower than control level throughout the 60 days of reloading (Fig. 6). An interesting finding was that MHC I increased after 30 days of reloading to a level higher than the level in normal soleus muscle at 45 days and remained high at 60 days (Fig. 6), indicating a secondary adaption to the slow recovery of fatigue resistance during reloading.
Fig. 6.
Switching of MHC isoforms in mouse soleus muscle after unloading and during reloading. A: glycerol SDS-PAGE revealed changes in MHC isoform expression in soleus muscle after 4 wk of unloading and recoveries during early (3–15 days) and later (30–60 days) phases of reloading. B: densitometry quantification determined that 4 wk of unloading of soleus muscle decreased MHC IIa and increased MHC IIx and IIb, whereas no change was seen in MHC I. MHC IIx and IIb returned to control level after 15 days of reloading; MHC IIa started recovering after 7 days of reloading but stayed below normal level at 15–60 days of reloading. Although not affected by unloading, MHC I showed an increase after 30 days of reloading and reached a level higher than normal at 45 and 60 days. Values are presented as means ± SE; n = 4 in CON, n = 5 in HU, n = 4 in 3DR, n = 3 in 7DR, n = 3 in 15DR, n = 8 in 30DR, n = 8 in 45DR, and n = 6 in 60DR. *P < 0.05 vs. CON. Statistics was performed using 1-way ANOVA with mean comparison in Tukey's test.
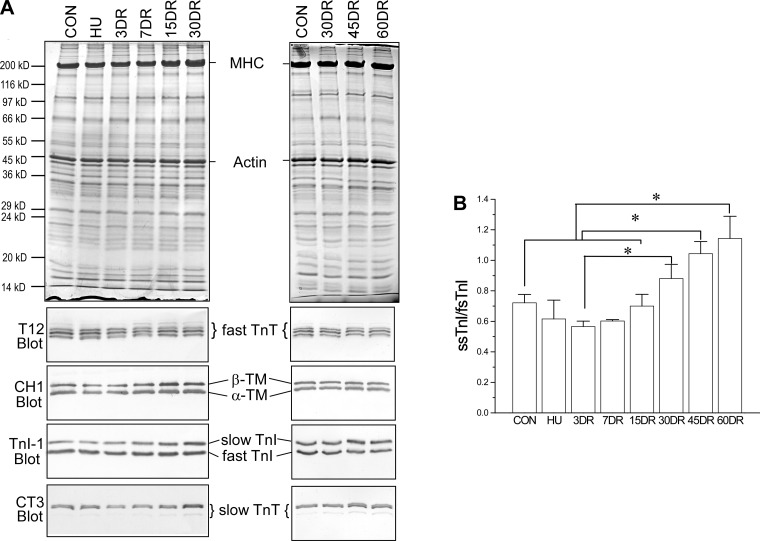
The results further showed that 4 wk of unloading did not induce significant change in the expression of thin filament regulatory proteins tropomyosin, TnT, and TnI in mouse soleus muscle (Fig. 7). During the course of reloading, the level of slow TnT and the ratio of slow to fast isoforms of TnI were increased, becoming higher than the controls after 45–60 days of reloading (Fig. 7B). This change was accompanied by an increase in the number of type I fibers (Fig. 8), further indicating the secondary adaption to the slow recovery of fatigue resistance.
Fig. 7.
Expression of thin-filament regulatory protein isoforms in mouse soleus muscle after unloading and during reloading. A: Western blots using mAbs CH1 recognizing α and β tropomyosins, TnI-1 recognizing all TnI isoforms, T12 recognizing fast skeletal muscle TnT, and CT3 recognizing cardiac and slow skeletal muscle TnT showed that 4 wk of unloading did not produce significant change in the expression of tropomyosin, TnI, and slow TnT in mouse soleus muscle, whereas the expression of fast TnT switched to more low-molecular-weight splice forms, which returned to the control pattern after 7 days of reloading. During the later phase of reloading, the expressions of slow TnT and slow TnI were increased, most clearly at 45 and 60 days. B: densitometry quantification further demonstrated an increase in the ratio of slow vs. fast TnI (ssTnI and fsTnI), which became higher than that in normal soleus muscle after 45 and 60 days of reloading, indicating an increase in slow fiber contents as a secondary adaptation possibly to the decreased fatigue resistance. Values are presented as means ± SE; n = 3 in CON, n = 3 in HU, n = 3 in 3DR, n = 3 in 7DR, n = 3 in 15DR, n = 4 in 30DR, n = 7 in 45DR, and n = 4 in 60DR. *P < 0.05 in 1-way ANOVA with mean comparison in Tukey's test.
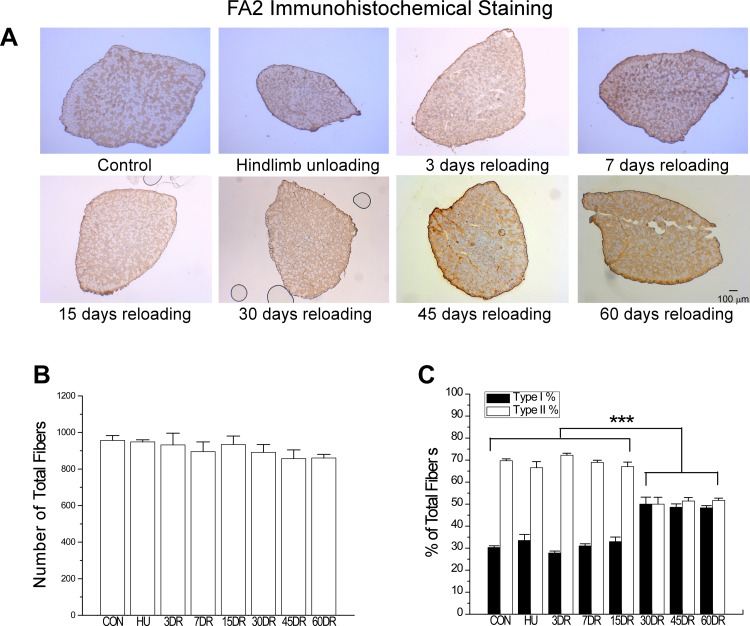
Fig. 8.
Increased number of MHC I fibers in mouse soleus muscle after 30 days of reloading. A: immunohistochemistry using mAb FA2 recognizing type I MHC determined the type I slow fiber contents of the mouse soleus muscles studied. B: total number of fibers in soleus muscle was similar in all groups, suggesting no fiber loss or hyperplasia. C: numbers of type I fibers increased, whereas type II fiber decreased after 30 days of reloading compared with the 4-wk unloading or normal control groups. This trend remained at 60 days of reloading. Values are presented as means ± SE; n = 3 in CON, n = 3 in HU, n = 3 in 3DR, n = 3 in 7DR, n = 4 in 15DR, n = 4 in 30DR, n = 4 in 45DR, and n = 3 in 60DR. ***P < 0.001 compared with the same type of fibers at different time points. Statistical analysis was performed using 1-way ANOVA with mean comparison in Tukey's test.
DISCUSSION
Whereas the structural and functional effects of unloading on skeletal muscle have been extensively documented (9, 34), the recovery and adaptation during reloading are much less understood. Our present study investigated for the first time the recovery process during 60 days of reloading using a mouse hindlimb suspension model. Muscle histology, in vivo muscle contractility, fatigue resistance, fiber type, and myofilament protein isoform changes were examined for early and later phase adaptations. In addition to demonstrating the beneficial effect of reloading on the ability of atrophic muscles to regain mass and contractile force, novel findings of this investigation are the significant worsening of fatigue resistance during the early phase of reloading due to reloading-induced muscle injury, the very slow recovery of fatigue resistance concurrent with decreased PGC-1α and mitofusin 2, and the late-phase adaptive increase of type I slow fibers.
The use of a mouse model to study skeletal muscle unloading and reloading.
Hindlimb suspension of rodents has been used extensively as a model to study muscle unloading and reloading (3, 9). Various lengths of hindlimb suspension and reloading have been previously studied using rats and mice. In our present study, the atrophy, weakened force production, and decreased fatigue resistance of mouse soleus muscle after 4 wk of unloading were similar to that found in rat soleus muscle in previous studies (72, 104). However, different outcomes are seen in these two rodent models. The expression of fiber type-specific myofilament protein isoforms has less profound change in mouse than that in rat (104). Four weeks of hindlimb suspension of rats resulted in significant reductions in MHC I in soleus muscle (104). This was not seen in the mouse model that, on the other hand, showed a decrease in MHC IIa and increases of MHC IIx and IIb (Fig. 6). These findings are in agreement with previous studies using the same mouse strain (91). Four weeks of unloading resulted in early and significant decreases in slow isoforms of TnT and TnI in rat soleus muscle (104), which were not seen in the mouse model (Fig. 7).
Although the mouse model does not represent the human muscle unloading and reloading as well as the rat model does, it allows the study of many genetically modified mouse models of human myopathy. Therefore, better understanding of the mouse as an experimental system to study skeletal muscle adaptation to unloading and reloading is of particular value by empowering investigators to take advantage of the increasing availability of genetically modified mouse lines for the study of skeletal muscle contractility, fatigue resistance, growth, regeneration, and neuromuscular diseases.
In situ measurement of muscle contractility and fatigue resistance.
A novel approach in our present studies is the in situ measurement of contractility and fatigability of mouse soleus muscle. The in situ measurement of muscle contraction is via nerve stimulation, maintaining in vivo blood circulation, normal oxygenation, and temperature. Therefore, it is more physiologically relevant than most of the previous muscle fatigue studies that used isolated ex vivo muscle strips under superfusion, in which dissection injury, hypoxia, limited energy supply, and artificial effects of field electrical pacing were intrinsic drawbacks (4, 105). The methodology and functional data for the in situ mouse soleus muscles reported here laid a foundation for broader applications in testing genetically modified mouse models of human skeletal muscle diseases and adaptation.
Injury of deconditioned soleus muscle during the early phase of reloading.
Our fatigue-resistance studies demonstrated two phases of adaptation in mouse soleus muscle during reloading. The early reloading resulted in a continuing worsening of fatigue resistance, reflecting the impact of reloading-caused injury of the atrophic postunloading soleus muscle, whereas the later phase of reloading exhibited a significantly lagging recovery of fatigue resistance (Fig. 2) after the full recovery of muscle mass, cross-sectional area, and force (Fig. 1, C–F).
Reloading-caused muscle injury was reported as an acute response when atrophic muscle returns to weight-bearing conditions (33). Our present study demonstrated that, whereas the mass and force of the unloaded mouse soleus muscle recovered by 15 days of reloading (Fig. 1), the resistance to fatigue did not recover but, in contrast, worsened (Fig. 2). An explanation was the reloading-induced injury, as indicated by muscle tissue swelling and the infiltration of inflammatory cells (Fig. 3), which were absent after 4 wk of unloading but became prominent at 3, 7, and 15 days of reloading.
Reloading-caused muscle injury has been reported to reduce force production, shortening velocity, and power in rat soleus muscle after 15 days of hindlimb unloading and 9 days of reloading (101). The atrophic rat soleus muscle after unloading showed a reduction of fiber length that was overextended upon the resumption of weight bearing, suggesting a mechanism for reloading to cause muscle injury (101). Consistent with this notion, a previous electron microscopy study showed disordered sarcomere arrangement with the Z-line interrupted by mitochondria in soleus muscle after 3 wk of unloading and 2 wk of reloading (62).
Injury-induced necrosis is followed by the release of cytokines to trigger inflammatory response. Infiltration of neutrophils in eccentric contraction-injured muscle can remain elevated for as long as 5 days (30), with activities such as phagocytosis (70) and the releases of proteases and high concentrations of cytolytic and cytotoxic molecules that can further damage the muscle cells (96) and negatively impact on contractile function (86). The inflammatory responses to reloading injury may contribute to the decreased fatigue resistance of soleus muscle during the early phase of reloading (Fig. 2). This hypothesis is worth further investigation.
Atrophy- and reloading injury-induced regeneration of soleus muscle and functional impact.
Z-line-related intermediate filament proteins filamin C, αB-crystallin, and desmin were decreased after 4 wk of hindlimb unloading and then rapidly upregulated during the early phase of reloading (Fig. 4), indicating reloading injury and active muscle regeneration in response to functional demands. We also observed regeneration in soleus muscle, as indicated by centralized nuclei at 15 days of reloading (Fig. 2A). The mechanism and detailed functional role of the Z-line-related proteins in postatrophy reloading remain to be further investigated.
Skeletal muscle regeneration in response to muscle injury mimics the developmental program involving reexpression of embryonic, neonatal genes. For example, developmental isoforms of voltage-gated Ca2+ channel (α1C) and of ryanodine receptor type 3 are known to express in regenerating rat soleus muscle, relating to immature excitation-contraction coupling (23, 81). Cardiac TnT was found in both developing and regenerating skeletal muscles (87). Because the regenerating fibers represented only a small proportion of the muscle mass, no expression of cardiac TnT was detectable in the Western blots of whole muscle protein extracts (Fig. 7). Nonetheless, the reexpression of developmental isoforms of excitation-contraction coupling proteins may have contributed to the decreased function of soleus muscle during the early phase of reloading, a hypothesis that remains to be investigated.
Decreased mitochondrial function in soleus muscle may be responsible for the slow recovery of fatigue resistance during reloading.
After 30 days of reloading, the muscle mass and force returned to the control level with recovered sarcomere protein contents. However, the fatigue resistance remained lower than normal (Fig. 2). Slow recovery of rat soleus muscle during reloading or remobilization was reported in previous studies (19, 32, 54, 76, 78). Consistent with our results from 60 days of reloading, the recovery was slow, and the force generation did not return to control level until 60 (soleus) or 90 (EDL) days in rats (32).
One of the mechanisms that may affect muscle fatigability is the function of mitochondria (31). Altered distribution of mitochondria was found in rat soleus muscle after space flight (5). PGC-1α is a member of transcriptional coactivators, which interacts with multiple DNA-binding transcription factors to coordinate the regulation of multiple mitochondrial genes (45) and plays a central role in mitochondrial function (66). Consistent with its function, PGC-1α significantly decreased in mouse soleus muscle after unloading and showed a slow and incomplete recovery during 60 days of reloading, corresponding to the slow recovery of fatigue resistance (Fig. 5). Whereas the total mitochondria contents were not changed, the mitochondrial fusion marker, mitofusin 2 showed similar changes to that of PGC-1α (Fig. 5). The strikingly concurring decreases and partial recoveries of PGC-1α and mitofusin 2 levels and fatigue resistance suggest a causal relationship in which a slow recovery of mitochondrial function may be a key determinant. Supporting this notion, muscle-specific overexpression of PGC-1α in skeletal muscle was reported to prevent activation of catabolic systems and disuse muscle atrophy in mouse soleus muscles subjected to 3 and 7 days of hindlimb suspension (14).
Mitochondria dynamically fuse and divide, and the fusion and fission govern mitochondrial function (20, 46). Imbalance of these two processes negatively impacts on mitochondrial morphology and activity (15). Mitofusins in the mitochondrial outer membrane participate in the early steps in membrane fusion (89). Mitofusin-2 is a target of PGC-1α. Maintenance of a normal expression of mitofusin-2 is critical for the stimulatory effect of PGC-1α on mitochondrial membrane potential, and PGC-1α may regulate mitochondrial fusion/fission events and hence mitochondrial function (90). Therefore, impaired mitochondrial fusion may contribute to the decreased fatigue resistance and delayed recovery of soleus muscle after unloading and during reloading.
Recent studies suggested that PGC-1α also plays a regulatory role in the expression of endogenous antioxidant proteins. Decreased mRNA levels of superoxide dismutase 1 (SOD1) (CuZn-SOD), SOD2 (Mn-SOD) (64), as well as SOD2 protein content (37, 63) were observed in skeletal muscle from PGC-1α knockout mice, whereas mice overexpressing PGC-1α showed upregulated SOD2 in skeletal muscle (100). The increase in reactive oxygen species that occurs during intense exercise has been proposed to be one of the major causes of muscle fatigue. Muscle-specific SOD2-deficient mice showed severe disturbances in exercise activity without atrophy of skeletal muscles (59), suggesting that PGC-1α-mediated regulation of SOD2 may be a mechanism to affect muscle fatigue resistance during the late phase of reloading recovery even when the slow fiber content increased to a level higher than normal (Fig. 8).
Secondary adaptation by increases of type I slow fibers in soleus muscle to compensate for the slow recovery of fatigue resistance during reloading.
Type I slow and type IIa relatively slow fibers (83) play important roles in muscle fatigue resistance (56, 73). In the early phase of 3–15 days of reloading of mouse soleus muscle, MHC IIa increased, whereas MHC IIb and IIx decreased. The increase of type IIa MHC appeared unable to overcome the worsening of fatigue resistance during the early phase of recovery, probably attributable to the dominant negative impact of reloading injury. Nonetheless, the change in MHC IIa instead of MHC I in mouse soleus unloading indicated a functional exchangeability of the two slow MHC isoforms in skeletal muscle function and adaption. The presumably complementary increase of MHC IIa for MHC I is consistent with the “nearest neighbor” rule (I ↔ IIa ↔ IIx ↔ IIb) of myosin isoform expression in mammalian limb and trunk muscles (2, 94).
The recovery of MHC IIa stopped at a level lower than normal throughout the later phase of reloading (Fig. 6). In the meantime, although MHC I was not decreased after 4 wk of unloading in the mouse model of hindlimb suspension, it was elevated at 30–60 days of reloading to a level higher than that in the control muscle (Fig. 6). This trend was accompanied by corresponding increases in slow isoforms of TnT and TnI (Fig. 7) and increases in the number of slow fibers (Fig. 8, A and C). It is known that type I slow fibers are responsible for the fatigue resistance of skeletal muscle (58). Therefore, the increase in slow fibers indicated a secondary adaptation to compensate for the slow recovery of decreased fatigue resistance during reloading.
Supporting a benefit of this secondary adaptation by increasing type I slow fibers, progressive losses of locomotor function and muscle mass occurring in aging with declined muscle strength (65) were accompanied by an increased number of type I fibers (61). A loss of slow TnT was shown to cause diminished slow fibers in human skeletal muscle in a lethal type of nemaline myopathy (50). The slow-to-fast fiber switch in mouse diaphragm and soleus muscles caused by knocking down or knocking out the slow TnT gene produced decreased fatigue resistance (27, 99). On the other hand, an adaptive fast-to-slow fiber type switch in Gs protein α-subunit-deficient mouse soleus muscle improved fatigue resistance (25).
In summary, our in situ contractility studies investigated for the first time the recovery of fatigue resistance in mouse soleus muscle during 60 days of reloading. Together with quantitative analyses of fiber composition, myofilament protein isoform regulation, and proteomic identification, the results demonstrated an early-phase reloading-caused muscle injury with a worsening of fatigue resistance. The changes in PGC-1α and mitofusin 2 concurrent with decreased fatigue resistance after unloading and slow recovery during reloading suggest a novel role of mitochondrial function in the resistance of skeletal muscle to fatigue. The secondary adaptation by increasing type I slow fibers demonstrates a mechanism to compensate for the loss of muscle fatigue resistance and an indicator for monitoring postatrophy recovery, a potential target to develop new rehabilitation therapies for aging individuals and bedridden patients.
GRANTS
This study was supported by National Institutes of Health grants AR048816 and HL098945 and a Multidisciplinary Incubator Grant from the Wayne State University Office of Vice President for Research to J.-P. Jin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.-Z.F., X.C., and M.H.M. performed experiments; H.-Z.F. and X.C. analyzed data; H.-Z.F. and J.-P.J. interpreted results of experiments; H.-Z.F. and J.-P.J. prepared figures; H.-Z.F., M.H.M. and J.-P.J. drafted manuscript; H.-Z.F., M.H.M. and J.-P.J. edited and revised manuscript; H.-Z.F., X.C., M.H.M. and J.-P.J. approved final version of manuscript; M.H.M. and J.-P.J. responsible for conception and design of research.
ACKNOWLEDGMENTS
We thank Hui Wang for technical assistance and Dr. Jim Lin, University of Iowa for the CH1 mAb.
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Ausoni S, Gorza L, Schiaffino S, Gundersen K, Lomo T. Expression of myosin heavy chain isoforms in stimulated fast and slow rat muscles. J Neurosci 10: 153–160, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin KM, Haddad F, Pandorf CE, Roy RR, Edgerton VR. Alterations in muscle mass and contractile phenotype in response to unloading models: role of transcriptional/pretranslational mechanisms. Front Physiol 4: 284, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barclay CJ. Modelling diffusive O(2) supply to isolated preparations of mammalian skeletal and cardiac muscle. J Muscle Res Cell Motil 26: 225–235, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bell GJ, Martin TP, Ilyina-Kakueva EI, Oganov VS, Edgerton VR. Altered distribution of mitochondria in rat soleus muscle fibers after spaceflight. J Appl Physiol 73: 493–497, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Bierbrauer J, Koch S, Olbricht C, Hamati J, Lodka D, Schneider J, Luther-Schroder A, Kleber C, Faust K, Wiesener S, Spies CD, Spranger J, Spuler S, Fielitz J, Weber-Carstens S. Early type II fiber atrophy in intensive care unit patients with nonexcitable muscle membrane. Crit Care Med 40: 647–650, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Biolo G, Ciocchi B, Lebenstedt M, Barazzoni R, Zanetti M, Platen P, Heer M, Guarnieri G. Short-term bed rest impairs amino acid-induced protein anabolism in humans. J Physiol 558: 381–388, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaauw B, Schiaffino S, Reggiani C. Mechanisms modulating skeletal muscle phenotype. Compr Physiol 3: 1645–1687, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol 45: 2200–2208, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth FW, Seider MJ. Early change in skeletal muscle protein synthesis after limb immobilization of rats. J Appl Physiol 47: 974–977, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Braith RW, Limacher MC, Leggett SH, Pollock ML. Skeletal muscle strength in heart transplant recipients. J Heart Lung Transplant 12: 1018–1023, 1993. [PubMed] [Google Scholar]
- 12.Brown M, Ferreira JA, Foley AM, Hemmann KM. A rehabilitation exercise program to remediate skeletal muscle atrophy in an estrogen-deficient organism may be ineffective. Eur J Appl Physiol 112: 91–104, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Caiozzo VJ, Baker MJ, Baldwin KM. Novel transitions in MHC isoforms: separate and combined effects of thyroid hormone and mechanical unloading. J Appl Physiol 85: 2237–2248, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Cannavino J, Brocca L, Sandri M, Bottinelli R, Pellegrino MA. PGC1-alpha over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J Physiol 592: 4575–4589, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet 14: R283–R289, 2005. [DOI] [PubMed] [Google Scholar]
- 16.D'Antona G, Lanfranconi F, Pellegrino MA, Brocca L, Adami R, Rossi R, Moro G, Miotti D, Canepari M, Bottinelli R. Skeletal muscle hypertrophy and structure and function of skeletal muscle fibres in male body builders. J Physiol 570: 611–627, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalkilic I, Schienda J, Thompson TG, Kunkel LM. Loss of FilaminC (FLNc) results in severe defects in myogenesis and myotube structure. Mol Cell Biol 26: 6522–6534, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Desaphy JF, Pierno S, Liantonio A, De Luca A, Didonna MP, Frigeri A, Nicchia GP, Svelto M, Camerino C, Zallone A, Camerino DC. Recovery of the soleus muscle after short- and long-term disuse induced by hindlimb unloading: effects on the electrical properties and myosin heavy chain profile. Neurobiol Dis 18: 356–365, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8: 870–879, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 85: 1751–1759, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Dubin RA, Ally AH, Chung S, Piatigorsky J. Human alpha B-crystallin gene and preferential promoter function in lens. Genomics 7: 594–601, 1990. [DOI] [PubMed] [Google Scholar]
- 23.Esposito A, Germinario E, Zanin M, Palade PT, Betto R, Danieli-Betto D. Isoform switching in myofibrillar and excitation-contraction coupling proteins contributes to diminished contractile function in regenerating rat soleus muscle. J Appl Physiol 102: 1640–1648, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Fauteck SP, Kandarian SC. Sensitive detection of myosin heavy chain composition in skeletal muscle under different loading conditions. Am J Physiol 268: C419–C424, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Feng HZ, Chen M, Weinstein LS, Jin JP. Improved fatigue resistance in Gsα-deficient and aging mouse skeletal muscles due to adaptive increases in slow fibers. J Appl Physiol 111: 834–843, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng HZ, Wang Q, Reiter RS, Lin JL, Lin JJ, Jin JP. Localization and function of Xinα in mouse skeletal muscle. Am J Physiol Cell Physiol 304: C1002–C1012, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng HZ, Wei B, Jin JP. Deletion of a genomic segment containing the cardiac troponin I gene knocks down expression of the slow troponin T gene and impairs fatigue tolerance of diaphragm muscle. J Biol Chem 284: 31798–31806, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol 270: E627–E633, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira JA, Crissey JM, Brown M. An alternant method to the traditional NASA hindlimb unloading model in mice. J Vis Exp 10: 2467, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG. Acute phase response in exercise. III. Neutrophil and IL-1β accumulation in skeletal muscle. Am J Physiol 265: R166–R172, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Filler K, Lyon D, Bennett J, McCain N, Elswick R, Lukkahatai N, Saligan LN. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin 1: 12–23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitts RH, Brimmer CJ. Recovery in skeletal muscle contractile function after prolonged hindlimb immobilization. J Appl Physiol 59: 916–923, 1985. [DOI] [PubMed] [Google Scholar]
- 33.Fitts RH, Riley DR, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 204: 3201–3208, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Fitts RH, Trappe SW, Costill DL, Gallagher PM, Creer AC, Colloton PA, Peters JR, Romatowski JG, Bain JL, Riley DA. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J Physiol 588: 3567–3592, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fluck M, Schmutz S, Wittwer M, Hoppeler H, Desplanches D. Transcriptional reprogramming during reloading of atrophied rat soleus muscle. Am J Physiol Regul Integr Comp Physiol 289: R4–R14, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Fujita M, Mitsuhashi H, Isogai S, Nakata T, Kawakami A, Nonaka I, Noguchi S, Hayashi YK, Nishino I, Kudo A. Filamin C plays an essential role in the maintenance of the structural integrity of cardiac and skeletal muscles, revealed by the medaka mutant zacro. Dev Biol 361: 79–89, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Geng T, Li P, Okutsu M, Yin X, Kwek J, Zhang M, Yan Z. PGC-1α plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol 298: C572–C579, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 586: 6049–6061, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goetsch SC, Martin CM, Embree LJ, Garry DJ. Myogenic progenitor cells express filamin C in developing and regenerating skeletal muscle. Stem Cells Dev 14: 181–187, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Goldfarb LG, Dalakas MC. Tragedy in a heartbeat: malfunctioning desmin causes skeletal and cardiac muscle disease. J Clin Invest 119: 1806–1813, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332: 556–561, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harridge SD, Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, Esbjornsson M, Saltin B. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflügers Arch 432: 913–920, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Helliwell TR, Gunhan O, Edwards RH. Lectin binding and desmin expression during necrosis, regeneration, and neurogenic atrophy of human skeletal muscle. J Pathol 159: 43–51, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS; Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348: 683–693, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 71: 177–203, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem 76: 751–780, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM 2nd, Tak PP, Haines GK 3rd, Nicchitta CV, Pope RM. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol 182: 4965–4973, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hudson LD, Lee CM. Neuromuscular sequelae of critical illness. N Engl J Med 348: 745–747, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50: 889–896, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Jin JP, Brotto MA, Hossain MM, Huang QQ, Brotto LS, Nosek TM, Morton DH, Crawford TO. Truncation by Glu180 nonsense mutation results in complete loss of slow skeletal muscle troponin T in a lethal nemaline myopathy. J Biol Chem 278: 26159–26165, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Jin JP, Chong SM. Localization of the two tropomyosin-binding sites of troponin T. Arch Biochem Biophys 500: 144–150, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin JP, Malik ML, Lin JJ. Monoclonal antibodies against cardiac myosin heavy chain. Hybridoma 9: 597–608, 1990. [DOI] [PubMed] [Google Scholar]
- 53.Jin JP, Yang FW, Yu ZB, Ruse CI, Bond M, Chen A. The highly conserved COOH terminus of troponin I forms a Ca2+-modulated allosteric domain in the troponin complex. Biochemistry 40: 2623–2631, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Kawano F, Nomura T, Ishihara A, Nonaka I, Ohira Y. Effects of chronic hindlimb suspension on landing performance in response to head-down drop in rats. Uchu Koku Kankyo Igaku 39: 21–29, 2002. [PubMed] [Google Scholar]
- 55.Kelleher AR, Kimball SR, Dennis MD, Schilder RJ, Jefferson LS. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am J Physiol Endocrinol Metab 304: E229–E236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knuth ST, Dave H, Peters JR, Fitts RH. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30 degrees C and 15 degrees C: implications for muscle fatigue. J Physiol 575: 887–899, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, Conger S, Lombeida J, Wolfe R, Evans WJ. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci 63: 1076–1081, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Kugelberg E. Histochemical composition, contraction speed and fatigability of rat soleus motor units. J Neurol Sci 20: 177–198, 1973. [DOI] [PubMed] [Google Scholar]
- 59.Kuwahara H, Horie T, Ishikawa S, Tsuda C, Kawakami S, Noda Y, Kaneko T, Tahara S, Tachibana T, Okabe M, Melki J, Takano R, Toda T, Morikawa D, Nojiri H, Kurosawa H, Shirasawa T, Shimizu T. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic Biol Med 48: 1252–1262, 2010. [DOI] [PubMed] [Google Scholar]
- 60.Lang SM, Kazi AA, Hong-Brown L, Lang CH. Delayed recovery of skeletal muscle mass following hindlimb immobilization in mTOR heterozygous mice. PLoS One 7: e38910, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand 117: 469–471, 1983. [DOI] [PubMed] [Google Scholar]
- 62.Lee K, Lee YS, Lee M, Yamashita M, Choi I. Mechanics and fatigability of the rat soleus muscle during early reloading. Yonsei Med J 45: 690–702, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Leick L, Lyngby SS, Wojtaszewski JF, Pilegaard H. PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp Gerontol 45: 336–342, 2010. [DOI] [PubMed] [Google Scholar]
- 64.Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1α is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab 294: E463–E474, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve 6: 588–595, 1983. [DOI] [PubMed] [Google Scholar]
- 66.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Lin JJ, Chou CS, Lin JL. Monoclonal antibodies against chicken tropomyosin isoforms: production, characterization, and application. Hybridoma 4: 223–242, 1985. [DOI] [PubMed] [Google Scholar]
- 68.Liu H, Eksarko P, Temkin V, Haines GK 3rd, Perlman H, Koch AE, Thimmapaya B, Pope RM. Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J Immunol 175: 8337–8345, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Peng Y, Cui Z, Wu Z, Qian A, Shang P, Qu L, Li Y, Long J. Depressed mitochondrial biogenesis and dynamic remodeling in mouse tibialis anterior and gastrocnemius induced by 4-week hindlimb unloading. IUBMB Life 64: 901–910, 2012. [DOI] [PubMed] [Google Scholar]
- 70.Lowe DA, Warren GL, Ingalls CP, Boorstein DB, Armstrong RB. Muscle function and protein metabolism after initiation of eccentric contraction-induced injury. J Appl Physiol 79: 1260–1270, 1995. [DOI] [PubMed] [Google Scholar]
- 71.Macaluso F, Isaacs AW, Di Felice V, Myburgh KH. Acute change of titin at mid-sarcomere remains despite 8 wk of plyometric training. J Appl Physiol 116: 1512–1519, 2014. [DOI] [PubMed] [Google Scholar]
- 72.McDonald KS, Delp MD, Fitts RH. Fatigability and blood flow in the rat gastrocnemius-plantaris-soleus after hindlimb suspension. J Appl Physiol 73: 1135–1140, 1992. [DOI] [PubMed] [Google Scholar]
- 73.Metzger JM, Moss RL. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol 393: 727–742, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol 5: 369, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol 92: 1367–1377, 2002. [DOI] [PubMed] [Google Scholar]
- 76.Mozdziak PE, Pulvermacher PM, Schultz E. Muscle regeneration during hindlimb unloading results in a reduction in muscle size after reloading. J Appl Physiol 91: 183–190, 2001. [DOI] [PubMed] [Google Scholar]
- 77.Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schon MR, Bluher M, Punkt K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29: 895–900, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Oishi Y, Taniguchi K, Matsumoto H, Kawano F, Ishihara A, Ohira Y. Upregulation of HSP72 in reloading rat soleus muscle after prolonged hindlimb unloading. Jpn J Physiol 53: 281–286, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab 91: 4836–4841, 2006. [DOI] [PubMed] [Google Scholar]
- 80.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pereon Y, Navarro J, Sorrentino V, Louboutin JP, Noireaud J, Palade P. Regulation of dihydropyridine receptor and ryanodine receptor gene expression in regenerating skeletal muscle. Pflügers Arch 433: 221–229, 1997. [DOI] [PubMed] [Google Scholar]
- 82.Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci 112: 2099–2112, 1999. [DOI] [PubMed] [Google Scholar]
- 83.Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol 115: 359–372, 2001. [DOI] [PubMed] [Google Scholar]
- 84.Ralston MA, Merola AJ, Leier CV. Depressed aerobic enzyme activity of skeletal muscle in severe chronic heart failure. J Lab Clin Med 117: 370–372, 1991. [PubMed] [Google Scholar]
- 85.Rantanen J, Hurme T, Lukka R, Heino J, Kalimo H. Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest 72: 341–347, 1995. [PubMed] [Google Scholar]
- 86.Reid MB, Andrade FH, Balke CW, Esser KA. Redox mechanisms of muscle dysfunction in inflammatory disease. Phys Med Rehabil Clin N Am 16: 925–949; ix, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Saggin L, Gorza L, Ausoni S, Schiaffino S. Cardiac troponin T in developing, regenerating and denervated rat skeletal muscle. Development 110: 547–554, 1990. [DOI] [PubMed] [Google Scholar]
- 88.Schaufelberger M, Eriksson BO, Grimby G, Held P, Swedberg K. Skeletal muscle alterations in patients with chronic heart failure. Eur Heart J 18: 971–980, 1997. [DOI] [PubMed] [Google Scholar]
- 89.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell 20: 3525–3532, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soriano FX, Liesa M, Bach D, Chan DC, Palacin M, Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 55: 1783–1791, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Stelzer JE, Widrick JJ. Effect of hindlimb suspension on the functional properties of slow and fast soleus fibers from three strains of mice. J Appl Physiol 95: 2425–2433, 2003. [DOI] [PubMed] [Google Scholar]
- 92.Sullivan MJ, Duscha BD, Klitgaard H, Kraus WE, Cobb FR, Saltin B. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exerc 29: 860–866, 1997. [DOI] [PubMed] [Google Scholar]
- 93.Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282: E1191–E1196, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Termin A, Staron RS, Pette D. Changes in myosin heavy chain isoforms during chronic low-frequency stimulation of rat fast hindlimb muscles. A single-fiber study. Eur J Biochem 186: 749–754, 1989. [DOI] [PubMed] [Google Scholar]
- 95.Thompson LV. Skeletal muscle adaptations with age, inactivity, and therapeutic exercise. J Orthop Sports Phys Ther 32: 44–57, 2002. [DOI] [PubMed] [Google Scholar]
- 96.Tiidus PM. Radical species in inflammation and overtraining. Can J Physiol Pharmacol 76: 533–538, 1998. [DOI] [PubMed] [Google Scholar]
- 97.Udaka J, Ohmori S, Terui T, Ohtsuki I, Ishiwata S, Kurihara S, Fukuda N. Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. J Gen Physiol 131: 33–41, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang SM, Huang YS, Wu JC, Tseng YZ. Role of desmin filaments in chicken cardiac myofibrillogenesis. J Cell Biochem 77: 635–644, 2000. [DOI] [PubMed] [Google Scholar]
- 99.Wei B, Lu Y, Jin JP. Deficiency of slow skeletal muscle troponin T causes atrophy of type I slow fibres and decreases tolerance to fatigue. J Physiol 592: 1367–1380, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci USA 106: 20405–20410, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Widrick JJ, Maddalozzo GF, Hu H, Herron JC, Iwaniec UT, Turner RT. Detrimental effects of reloading recovery on force, shortening velocity, and power of soleus muscles from hindlimb-unloaded rats. Am J Physiol Regul Integr Comp Physiol 295: R1585–R1592, 2008. [DOI] [PubMed] [Google Scholar]
- 102.Yamada T, Ivarsson N, Hernandez A, Fahlstrom A, Cheng AJ, Zhang SJ, Bruton JD, Ulfhake B, Westerblad H. Impaired mitochondrial respiration and decreased fatigue resistance followed by severe muscle weakness in skeletal muscle of mitochondrial DNA mutator mice. J Physiol 590: 6187–6197, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yarrow JF, McCoy SC, Ferreira JA, Pingel JE, Conrad BP, Wronski TJ, Williams AA, Borst SE, Brown M. A rehabilitation exercise program induces severe bone mineral deficits in estrogen-deficient rats after extended disuse. Menopause 19: 1267–1276, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu ZB, Gao F, Feng HZ, Jin JP. Differential regulation of myofilament protein isoforms underlying the contractility changes in skeletal muscle unloading. Am J Physiol Cell Physiol 292: C1192–C1203, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang SJ, Bruton JD, Katz A, Westerblad H. Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle. J Physiol 572: 551–559, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]