Abstract
Mechanical stretch can activate muscle and myotube protein synthesis through mammalian target of rapamycin complex 1 (mTORC1) signaling. While it has been established that tumor-derived cachectic factors can induce myotube wasting, the effect of this catabolic environment on myotube mechanical signaling has not been determined. We investigated whether media containing cachectic factors derived from Lewis lung carcinoma (LLC) can regulate the stretch induction of myotube protein synthesis. C2C12 myotubes preincubated in control or LLC-derived media were chronically stretched. Protein synthesis regulation by anabolic and catabolic signaling was then examined. In the control condition, stretch increased mTORC1 activity and protein synthesis. The LLC treatment decreased basal mTORC1 activity and protein synthesis and attenuated the stretch induction of protein synthesis. LLC media increased STAT3 and AMP-activated protein kinase phosphorylation in myotubes, independent of stretch. Both stretch and LLC independently increased ERK1/2, p38, and NF-κB phosphorylation. In LLC-treated myotubes, the inhibition of ERK1/2 and p38 rescued the stretch induction of protein synthesis. Interestingly, either leukemia inhibitory factor or glycoprotein 130 antibody administration caused further inhibition of mTORC1 signaling and protein synthesis in stretched myotubes. AMP-activated protein kinase inhibition increased basal mTORC1 signaling activity and protein synthesis in LLC-treated myotubes, but did not restore the stretch induction of protein synthesis. These results demonstrate that LLC-derived cachectic factors can dissociate stretch-induced signaling from protein synthesis through ERK1/2 and p38 signaling, and that glycoprotein 130 signaling is associated with the basal stretch response in myotubes.
Keywords: cachexia, mTORC1, gp130, MAP kinase, AMPK
cachexia, a condition that involves the unintentional loss of body weight, including muscle and fat mass, is associated with many types of cancer (16). Fundamentally, cachexia-associated skeletal muscle loss includes the activation of skeletal muscle protein degradation and the suppression of muscle protein synthesis (66). Implantation of Lewis lung carcinoma (LLC) cells into mice has been used widely to study mechanisms of cancer-induced muscle wasting (46). Studies with LLC cells have implicated cellular signaling involving TNF-α (33), NF-κB (9), glycoprotein 130 (gp130)/STAT3 (7, 49), and p38 in muscle wasting (75, 76). LLC cells can induce cultured myotube wasting directly in vitro by disrupting the regulation of protein degradation and protein synthesis (49, 75). Whereas LLC-induced gp130/STAT3, p38, and CCAAT enhancer-binding protein-β signaling pathways have been identified as important regulators of protein degradation (49, 75, 76), gaps remain in our understanding of the mechanisms of protein synthesis suppression by LLC-derived cachectic factors.
Mechanical stimuli such as loading or stretch induce an anabolic response in skeletal muscle that is important for the maintenance of mass and function (65). Therefore, it is plausible that disease-induced disruptions to mechanical signaling could contribute to muscle wasting. Cultured myotubes respond to chronic or intermittent stretch through increased protein synthesis, morphological maturation, and development of the contractile apparatus (13, 43, 67). Overload and stretch can stimulate skeletal muscle hypertrophy by inducing mammalian target of rapamycin (mTOR) complex 1 (mTORC1) (27, 74). The subsequent phosphorylation of p70 ribosomal protein S6 kinase (p70S6K), S6 ribosomal protein (S6RP), and eukaryotic initiation factor-4E binding protein 1 (4EBP1) results in ribosomal RNA and protein transcription, ribosomal biogenesis, translation initiation, and the induction of protein synthesis (35). Activation of mTOR signaling by mechanical stimulation can occur independent of Akt, and there is evidence that signaling involving phospholipase D (25, 73) and extracellular signal-regulated kinases 1/2 (ERK1/2) (34, 38) is involved in the process. Mechanical stimuli can activate muscle ERK1/2 and p38 signaling (37, 44), which can both induce mTORC1 (38, 42, 72). Chronic systemic inflammation has the potential to inhibit muscle protein synthesis activation. The muscle inflammatory response can attenuate IGF-I activation of mTOR (14, 62) and leucine induction of protein synthesis (29). Interestingly, muscle pathways associated with cachexia-induced skeletal muscle wasting also can be activated by mechanical stimuli to produce growth (31, 47, 49, 75). While muscle basal mTOR activity and protein synthesis are suppressed in several mouse models of cancer cachexia (49, 68), the ability of the cachectic environment to interact with muscle mechanical signaling is in need of further investigation. Further work is needed to determine whether cachexia-induced inflammatory signaling can negatively regulate the mechanical induction of protein synthesis.
The functional receptor complexes for the IL-6 family of cytokines involve gp130, which can induce several intracellular signal pathways, including STAT3 and MAPK (63). The IL-6 family of cytokines has a documented role in skeletal muscle mass regulation (39). Skeletal muscle expression of IL-6 and leukemia inhibitory factor (LIF) are induced by exercise (8, 45), and both IL-6 and LIF can regulate overload-induced muscle hypertrophy (5, 59). A role of gp130-initiated signaling for mechanical regulation of muscle protein synthesis has not been established. Cancer-induced systemic elevation of the IL-6 family of cytokines is also associated with muscle wasting (10). We have demonstrated that gp130 is necessary for LLC to induce muscle STAT3, p38 MAPK, and NF-κB signaling pathways, muscle protein degradation, and muscle wasting (49). Interestingly, IL-6 suppression had no effect on LLC-induced gp130 signaling (49). Additionally, IL-6 or gp130 antibody administration could not rescue LLC suppression of protein synthesis (49). Further work is needed to determine gp130’s role in LLC suppression of muscle protein synthesis.
AMP-activated protein kinase (AMPK) is an intracellular sensor of energy stress that can negatively regulate muscle protein synthesis in response to nutrient deficiency (54). AMPK can inhibit mTORC1 through the phosphorylation of tuberous sclerosis complex 2 and raptor, resulting in suppressed protein synthesis (22). Furthermore, AMPK activation is sufficient to prevent mTORC1 induction by anabolic signaling involving IGF-I, leucine, glucose, and electrically stimulated muscle contractions (41, 53, 64). In the mouse muscle, AMPK signaling is activated as cachexia progresses (49, 68). IL-6, an important inducer of muscle wasting in some cancer cachexia models, can activate AMPK (52). Additionally, AMPK inhibition rescues IL-6 suppression of mTOR signaling in myotubes (69), which suggests a role for AMPK in the basal suppression of muscle protein synthesis with some types of cancer. LLC-derived cachectic factors, independent of IL-6 or gp130 signaling, also induce myotube AMPK signaling (49). However, it is not known if LLC-induced AMPK signaling can alter the mechanical induction of protein synthesis.
Suppressed mechanical signaling in muscle has the potential to contribute to the overall rate of muscle wasting in cancer patients by disrupting anabolic signaling related to daily activity. It has not been established if intracellular signaling induced by tumor-derived cachectic factors can regulate stretch induction of myotube protein synthesis. The purpose of this study was to examine if the stretch induction of myotube protein synthesis could be inhibited by the administration of LLC media that induces wasting. We hypothesized that LLC-secreted factors would attenuate the mechanical stretch induction of skeletal muscle protein synthesis through chronic activation of MAPK, gp130/STAT3, and AMPK signaling. We examined the effect of chronic stretch on C2C12 myotube protein synthesis in the presence or absence of LLC media. The role of LLC-induced gp130-dependent STAT3, NF-κB, MAPK, and gp130 independent AMPK signaling was investigated with the administration of specific inhibitors.
METHODS
C2C12 myotube culture.
C2C12 myoblasts and LLC (American Type Culture Collection, Manassas, VA) were cultured in DMEM, supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin. To induce C2C12 myoblast differentiation, C2C12 myoblasts were incubated in DMEM supplemented with 2% heat-inactivated horse serum, 50 U/ml penicillin, and 50 μg/ml streptomycin for 72 h after they reached ∼95% confluence.
Cell stretch.
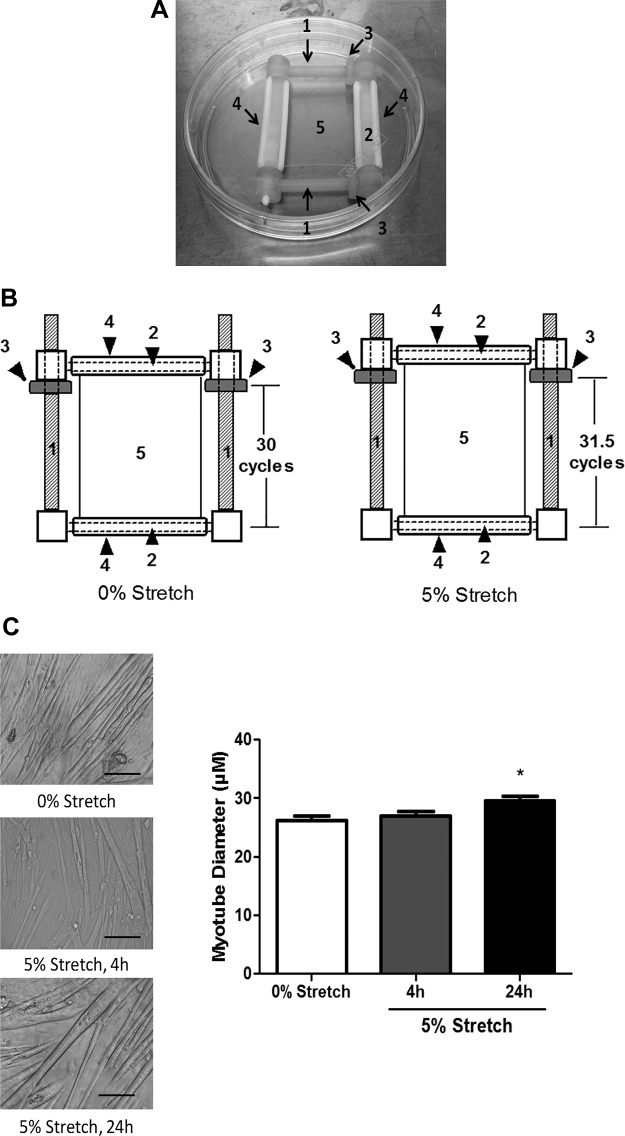
Cell stretch experiments were conducted using methods previously described (11, 58). Static stretching device consists of a small frame with two axles (part 1 in Fig. 1, A and B) separated by lateral supports (part 2 in Fig. 1, A and B). The axles were threaded to accept screw nuts (part 3 in Fig. 1, A and B). Silastic membranes (GLOSS/GLOSS, 0.02 in., Speciality Manufacturing, Saginaw, MI, part 5 in Fig. 1, A and B) were mounted onto the devices by two friction-fit C-clamps (part 4 in Fig. 1, A and B). The assembled stretching device was then immersed into distilled H2O, autoclaved, and transferred into a sterile 100-mm petri dish (Fig. 1A). The screw nuts were set to 30 cycles from baseline when the stretching device was assembled (Fig. 1B, left). Approximately 175 μl type I collagen solution (Advanced Biomatrix, San Diego, CA; diluted into 1 mg/ml by serum-free DMEM) was applied onto one edge of Silastic membrane on each stretching device. The collagen solution was then drawn across the substrate with a sterile cell scraper. Excess collagen was aspirated, and the culture dish containing the stretching device was transferred to a 37°C incubator for 1 h. After the incubation period, stretching device was washed by sterile distilled H2O twice and air dried under UV overnight. C2C12 myoblasts were suspended (∼1 × 106 cells/ml) and plated onto Silastic membrane mounted in a stretching device (∼1.5–2 × 105 cells/stretching device). The cells were grown to ∼95% confluence and differentiated into myotubes. To induced 5% stretch, screw nuts on both axles were rotated by 1.5 cycles using sterile forceps (Fig. 1B, right). Myotubes were constantly stretched by for 4 or 24 h.
Fig. 1.
Cell stretch regulation on myotube hypertrophy. A: cell stretch device. Static stretching device is a small frame with two threaded axles (part 1) and two lateral supports (part 2). The distance between two lateral supports was adjusted by rotating screw nuts (part 3) on both axles. The Silastic membrane where cells were grown (part 5) was fixed on lateral supported by two friction-fit C-clamps (part 4). B: representative image of cell stretch. The screw nuts were set to 30 cycles from the baseline when stretch device was assembled (left) and were rotated by another 1.5 cycles when cells were stretched (right). C: pictures (left, bar: 100 μM) and mean diameter of 0% stretch control and 4 h/24 h stretched myotubes (right). All values are means ± SE. *P < 0.05 vs. control, one-way ANOVA.
Cell stretch in LLC conditioned media.
C2C12 myotubes were treated with control or LLC media for 72 h, as previously described (75). Briefly, ∼2 × 105 LLC cells were plated into 10-cm-diameter culture dish. LLC cells were grown for 48 h, and the final density of LLC cells was 0.8–11 × 106 cells per culture dish. The LLC cell culture media was then collected by centrifuge (3,000 rpm for 5 min). One volume of LLC cell culture media was mixed with three volumes of serum-free DMEM to form 25% LLC media. Seventy-two hour differentiated myotubes were incubated in LLC media for 72 h. LLC media was refreshed every 24 h. DMEM media supplemented with 2.5% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin was used as control media. Myotubes were stretched by 5% in last 4 or 24 h of control/LLC media incubation.
gp130 signaling and ERK1/2 signaling inhibition.
To inhibit LLC-induced gp130 dependent signaling, myotubes were incubated in LLC media supplemented with gp130 antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA; dialyzed in PBS at 4°C overnight) for 72 h. To inhibit gp130 downstream targets, myotubes were incubated in LLC media supplemented with NF-κB and STAT3 inhibitor ammonium pyrrolidinecarbodithioate (PDTC) (50 μM; Sigma-Aldrich, St. Louis, MO) and STAT3 specific inhibitor, LLL12 (100 nM, BioVision, Milpitas, CA) for 72 h. To inhibit LLC-induced ERK1/2 signaling, myotubes were incubated in LLC media supplemented with PD-98059 (20 μM, Cell Signaling Technology, Danvers, MA) for 72 h.
AMPK and p38 MAPK signaling inhibition.
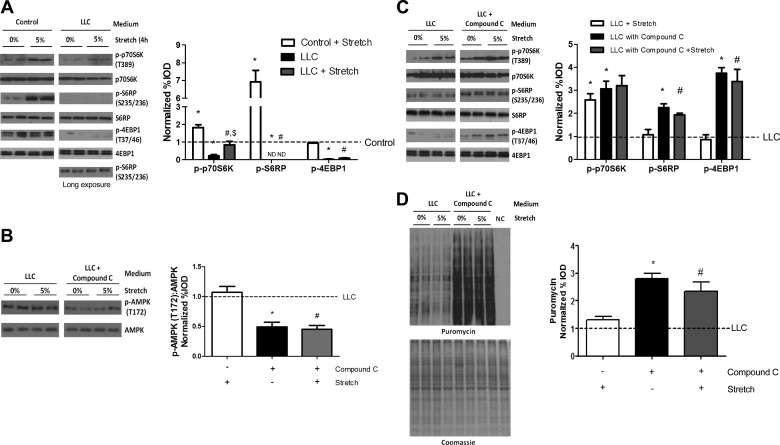
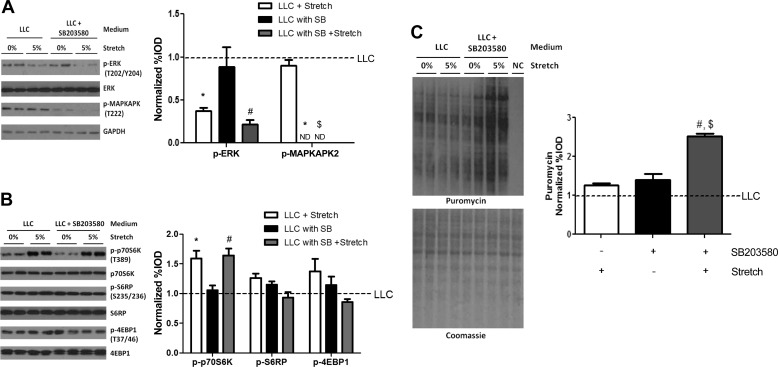
To inhibit LLC-induced AMPK and p38 MAPK signaling, myotubes were incubated in LLC media for 72 h. AMPK-specific inhibitor, compound C (20 μM, Sigma-Aldrich), or p38 MAPK-specific inhibitor, SB-203580 (10 μM, Cell Signaling Technology), was added into LLC media, and myotubes were stretched by 5% in the last 4 h of LLC media incubation.
Cytokine analysis of LLC media.
The level of 10 inflammatory cytokines [IL-1β, IL-6, IL-10, IL-17, IFN-γ, monocyte chemoattractant protein (MCP)-1, regulated on activation normal T-expressed and presumably secreted (RANTES), TNF-α, LIF, and macrophage colony-stimulating factor (M-CSF)] in culture media from three independent LLC cell cultures was measured by Bio-Plex multiplex analysis kit (Mouse Cytokine Th17 Panel A 6-Plex Group l and 2, Bio-Rad, Hercules, CA), following the manufacturer's instructions. The beads in a 96-well filter plate were analyzed by Bio-Plex 200 system (Bio-Rad). The growth media (DMEM supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin) used in those three LLC cell cultures were used as controls.
LIF signaling inhibition.
To inhibit the LLC-induced LIF/gp130 signaling, myotubes were incubated in LLC media supplemented with LIF antibody (1.5 μg/ml, Novus Biologicals, Littleton, CO) for 72 h. Myotubes were stretched by 5% in last 24 h. The dosage of LIF antibody was determined by treating myotubes with 10 ng/ml mouse recombinant LIF (Thermo Fisher Scientific, Waltham, MA) and different doses of LIF antibody for 30 min.
Western blots.
Western blot analysis was performed as previously described (24, 69). Briefly, cells were scraped into ice-cold RIPA buffer [50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM NaF, 1 mM NaVO4, 1 mM β-glycerophosphate, and 1% protease inhibitor cocktail (Sigma-Aldrich)]. Cell lysates were homogenized on ice and centrifuged at 4°C, and supernatants were collected. Protein concentrations were determined by the Bradford method. Homogenates were fractionated in SDS-polyacrylamide gels and transferred to polyvinylidene fluoride membranes. After the membranes were blocked, antibodies for phosphorylated/total ERK1/2, STAT3, NF-κB p65, AMPK, MAPK-activated protein kinase 2 (MAPKAPK-2), p70S6K, S6RP, 4EBP1, GAPDH (Cell Signaling Technology), p38 MAPK (Santa Cruz Biotechnology), and puromycin (Millipore, Billerica, MA) were incubated at dilutions from 1:2,000 to 1:8,000 overnight at 4°C in 2% Tris-buffered saline-Tween 20 milk. Anti-rabbit or anti-mouse IgG-conjugated secondary antibodies (Cell Signaling Technology) were incubated with the membranes at 1:2,000 to 1:5,000 dilutions for 2 h in 2% Tris-buffered saline-Tween 20 milk. Enhanced chemiluminescence (Advansta, Menlo Park, CA) developed by autoradiography was used to visualize the antibody-antigen interactions. Blots were analyzed by measuring the integrated optical density (IOD) of each band with ImageJ software (National Institutes of Health, Bethesda, MD).
Protein synthesis measurement.
The rate of protein synthesis rate in myotubes was determined by puromycin, as previously described (21). In brief, puromycin (EMD Chemicals, San Diego, CA) was added into cell culture media (1 μM final concentration) 30 min before protein collection. The amount of puromycin incorporated into newly synthesized protein was determined by Western blots.
Myotube diameter measurement.
C2C12 myotube diameter was quantified as previously published (69). Briefly, the diameter of each myotube was determined by the average value of diameters at three points randomly distributed on whole myotube. For each condition, all myotubes in 15 randomly chosen fields were measured. All measurements were conducted in a blinded fashion.
Statistical analysis.
Student's t-test, one-way ANOVA, and two-way ANOVA (indicated in figure legends) were used to examine the effects of stretch and culture conditions. Post hoc analyses were performed with Student-Newman-Keuls method. For all comparisons, six replicates from two independent experiments were included. P values < 0.05 were considered significant.
RESULTS
Mechanical stretch induces C2C12 myotube protein synthesis and hypertrophy.
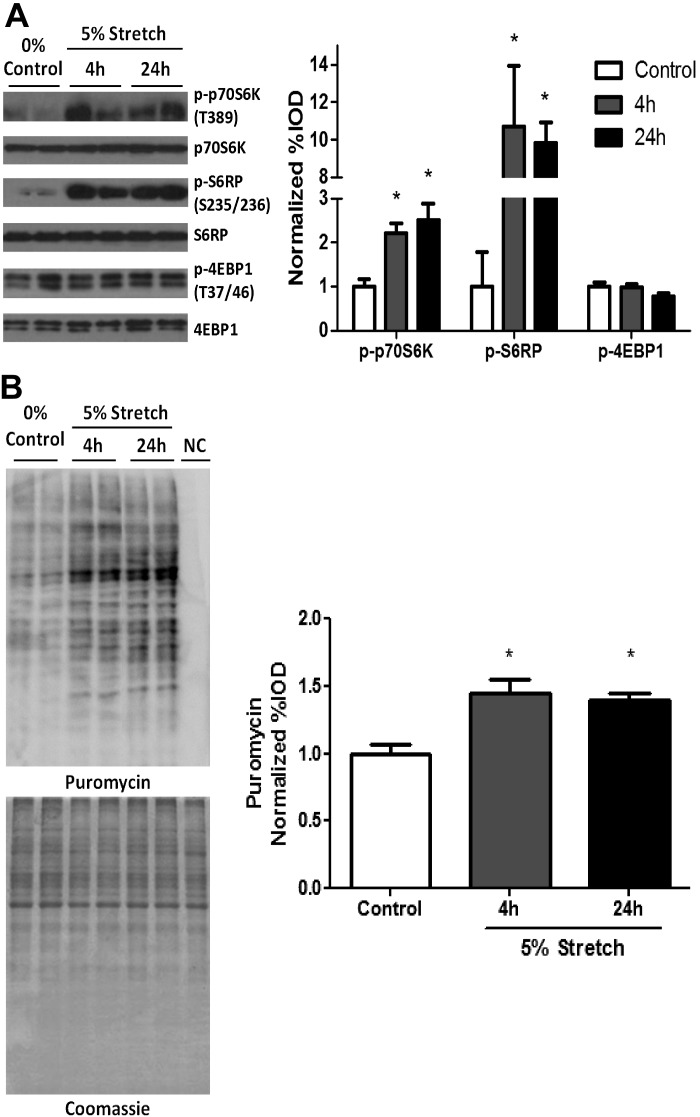
Differentiated C2C12 myotubes were subjected to 5% stretch for either 4 or 24 h. Stretch for 24 h significantly increased C2C12 myotube mean diameter (Fig. 1C). Related to growth signaling, both 4- and 24-h stretch induced p70S6K and S6RP phosphorylation (Fig. 2A). However, neither stretch time point demonstrated an increase 4EBP1 phosphorylation (Fig. 2A). Myotube protein synthesis rate was also significantly increased by both 4- and 24-h stretch (Fig. 2B). These results demonstrate stretch can induce a strong anabolic stimulus to cultured myotubes.
Fig. 2.
Effect of mechanical stretch on myotube mammalian target of rapamycin (mTOR) signaling pathway and protein synthesis. A: phosphorylation [ratio between phosphorylated (p) and total] of p70 ribosomal protein S6 kinase (p70S6K), S6 ribosomal protein (S6RP), and eukaryotic initiation factor-4E binding protein 1 (4EBP1) was measured by Western blot. B: protein synthesis rate was measured by Western blot against puromycin. NC is the negative control, the protein sample from C2C12 myotubes without puromycin treatment. IOD, integrated optical density. Bar graphs represent fold changes relative to control group. All values are means ± SE. *P < 0.05 vs. control, one-way ANOVA.
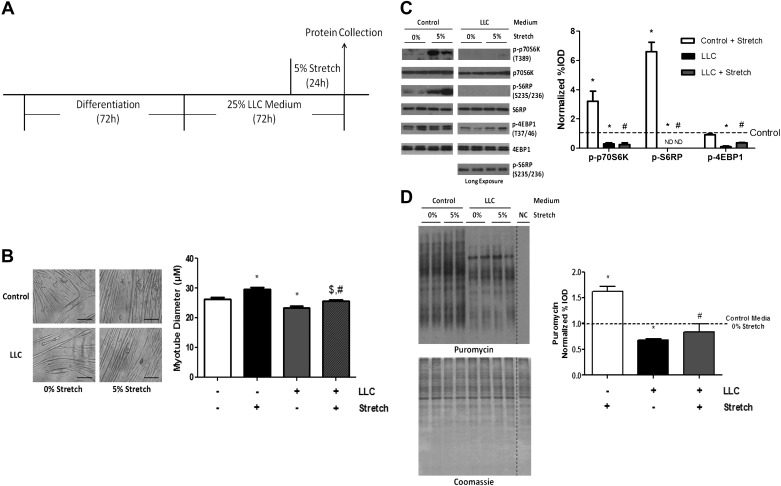
LLC attenuates stretch induction of C2C12 myotube protein synthesis.
Previously, we have demonstrated that LLC secreted factors can suppress myotube protein synthesis and mTOR signaling (49). To investigate whether LLC secreted factors can suppress the stretch induction of protein synthesis, we compared stretch effect on protein synthesis signaling in C2C12 myotubes preincubated with control and LLC media (Fig. 3A). As previously reported (49), LLC media resulted in myotube atrophy (Fig. 3B). Stretch was able to increase myotube diameter in LLC media, but stretched myotubes in LLC media had a decreased mean diameter compared with stretched myotubes in control media (Fig. 3B). Stretch for 24 h increased the phosphorylation of p70S6K and S6RP, but not the phosphorylation of 4EBP1, in myotubes that were incubated in control media (Fig. 3C). LLC media decreased the basal expression level of p70S6K, S6RP, and 4EBP1 phosphorylation and prevented the stretch induction of p70S6K and S6RP phosphorylation (Fig. 3C). Stretch significantly increased myotube protein synthesis in control media. In addition to the LLC media suppression of basal protein synthesis, the stretch induction of protein synthesis was prevented (Fig. 3D). These results demonstrate LLC-derived cachectic factors can dissociate stretch-induced mechanical signaling from mTORC1 signaling and protein synthesis induction.
Fig. 3.
Lewis lung carcinoma (LLC) regulation on stretch-induced myotube protein synthesis activation and hypertrophy. A: experiment design. C2C12 myotubes were incubated in control or 25% LLC media for 72 h and stretched (5%) in last 24-h period. B: diameter of C2C12 myotubes after 24 h of 0% or 5% stretch in control or LLC media. C: phosphorylation of p70S6K, S6RP, and 4EBP1 was measured by Western blot. Blots shown on separate panels are from different areas of the same gel. D: protein synthesis was measured by Western blot against puromycin. Dashed lines indicate different areas on the same gel. Bar graphs represent fold changes relative to control group (B–D). All values are means ± SE. *P < 0.05 vs. control; $P < 0.05 vs. LLC; #P < 0.05 vs. control + stretch: two-way ANOVA.
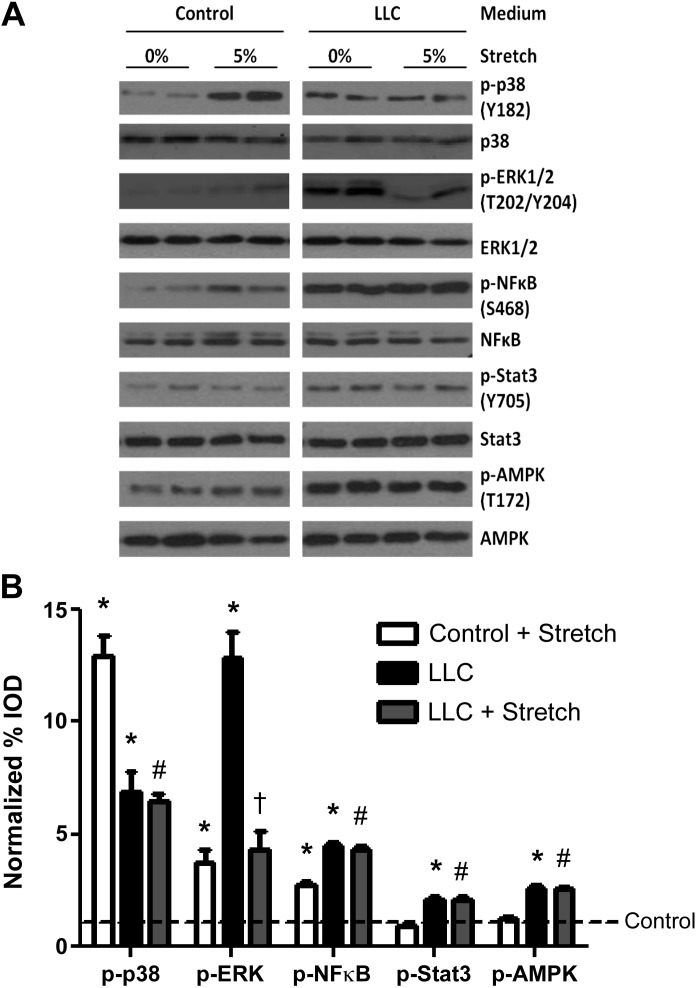
Stretch- and LLC-induced C2C12 myotube signaling pathways.
To understand the potential mechanisms underlying the LLC suppression on stretch induction of myotube protein synthesis, we investigated the effects of stretch and LLC media on stress and inflammatory signaling pathways involved in muscle protein turnover, including STAT3 (49), NF-κB (49), ERK1/2 (38), p38 MAPK (17), and AMPK (22). Both mechanical stretch alone and LLC alone increased p38, ERK1/2, and NF-κB p65 phosphorylation (Fig. 4). However, stretch in the presence of LLC media significantly attenuated the LLC induction of p38 and ERK1/2 phosphorylation (Fig. 4). LLC increased AMPK and STAT3 phosphorylation, independent of stretch, while stretch alone or in the presences of LLC media did not increase AMPK or STAT3 phosphorylation (Fig. 4).
Fig. 4.
Effect of stretch and LLC on stress and inflammatory signaling pathways in myotubes. C2C12 myotubes were incubated in control or 25% LLC media for 72 h and stretched (5%) in last 24-h period. Phosphorylation of p38 MAPK, ERK1/2, NF-κB p65, STAT3, and AMP-activated protein kinase (AMPK) was measured by Western blot. A: blots shown on separate panels are from different areas of the same gel. B: bar graphs represent fold changes relative to control group. All values are means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. control + stretch; †P < 0.05 vs. LLC: two-way ANOVA.
Inflammatory cytokines in LLC media.
The levels of 10 well-described inflammatory cytokines (IL-1β, IL-17, IL-6, IFN-γ, IL-10, MCP-1, TNF-α, LIF, M-CSF, and RANTES) were measured in media collected from LLC cell culture. M-CSF, LIF, and RANTES were only detected in media collected from LLC cell cultures (M-CSF: 12.40 ± 3.34 pg/ml; LIF: 9.93 ± 2.55 pg/ml, RANTES: 0.58 ± 0.33 pg/ml) (Table. 1). MCP-1 was significantly elevated in LLC media (353.90 ± 179.60 pg/ml) compared to control media (6.83 ± 5.10 pg/ml). No significant differences in IL-1β, IL-6, IL-10, or TNF-α levels were found between control and LLC media. IL-17 and IFN-γ were not detected in either control or LLC media.
Table 1.
Concentration of cytokines in control and LLC media
| Cytokine | Control | LLC |
|---|---|---|
| Increased | ||
| MCP-1 | 6.83 ± 5.10 | 353.90 ± 179.60* |
| RANTES | ND | 0.58 ± 0.33* |
| LIF | ND | 9.93 ± 2.55* |
| M-CSF | ND | 12.40 ± 3.34* |
| No change | ||
| IL-1β | 94.25 ± 1.88 | 103.30 ± 1.90 |
| IL-6 | 3.38 ± 0.48 | 2.60 ± 0.65 |
| IL-10 | 8.07 ± 0.18 | 6.28 ± 0.54 |
| TNF-α | 42.97 ± 1.46 | 45.86 ± 1.33 |
| Not detected | ||
| IL-17 | ND | ND |
| IFN-γ | ND | ND |
Values are means ± SE in pg/ml. The levels of 10 inflammatory cytokines in control medium (DMEM with 10% FBS) and culture medium from 3 independent LLC cell cultures were measured by Bio-Plex multiplex analysis.
LLC, Lewis lung carcinoma; MCP-1, monocyte chemoattractant protein; RANTES, regulated on activation normal T-expressed and presumably secreted; LIF, leukemia inhibitory factor; M-CSF, macrophage colony-stimulating factor; ND, not detected. Student's t-test was performed to determine the difference between levels of cytokines.
Statistical significance was set at P < 0.05.
The role of LIF in LLC regulation of myotube protein turnover/stretch-induced protein synthesis.
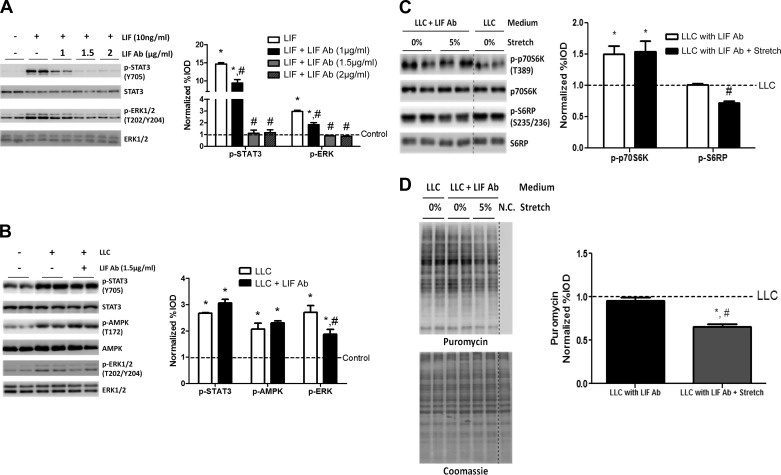
LLC media can activate myotube gp130/STAT3 signaling pathway through IL-6-independent mechanisms (49). We found no significant difference in IL-6 level between control and LLC media, while another gp130-dependent cytokine, LIF, was only detected in LLC media (Table 1). LIF/STAT3 signaling is required for colon-26-induced cancer cachexia (57). Administration of a mouse LIF antibody was used to inhibit LIF signaling in LLC media (57). Myotubes were treated with recombinant mouse LIF (10 ng/ml) and different doses of LIF antibody for 30 min to examine LIF antibody dose effects. As previously reported (57), LIF administration induced STAT3 and ERK1/2 phosphorylation (Fig. 5A). LIF did not induce STAT3 or ERK1/2 phosphorylation in the presence of 1.5 μg/ml LIF antibody, and 2 μg/ml LIF antibody showed no further inhibitory effects on STAT3 or ERK1/2 phosphorylation (Fig. 5A). These results demonstrate 1.5 μg/ml LIF antibody was sufficient to attenuate STAT3 and ERK1/2 induction by 10 ng/ml LIF, which was ∼1,000-fold higher than LIF level in LLC media. However, LIF antibody (1.5 μg/ml) with LLC media did not attenuate the induction of STAT3 or AMPK, whereas ERK1/2 phosphorylation was attenuated (Fig. 5B). LIF antibody administration in LLC media increased p70S6K phosphorylation (Fig. 5C), but did not change S6RP phosphorylation (Fig. 5C) or protein synthesis (Fig. 5D). Stretch in LLC media with LIF antibody had no effect on p70S6K phosphorylation (Fig. 5C), but decreased S6RP phosphorylation (Fig. 5C) and protein synthesis (Fig. 5D).
Fig. 5.
Leukemia inhibitory factor (LIF) role in LLC regulation of stretch-induced myotube protein synthesis. A: myotubes were administrated with LIF (10 ng/ml) and different doses of LIF antibody (Ab). Phosphorylation of STAT3 and ERK1/2 were determined by Western blot. *P < 0.05 vs. control; #P < 0.05 vs. LIF: one-way ANOVA. B: myotubes were administrated with LLC media and 1.5 μg/ml LIF Ab for 72 h. Phosphorylation of STAT3, AMPK, and ERK1/2 was measured by Western blot. *P < 0.05 vs. control; #P < 0.05 vs. LLC: one-way ANOVA. C: mytoubes were stretched for 24 h in LLC media with LIF Ab. Phosphorylation of p70S6K and S6RP was measured by Western blot. D: protein synthesis was measured by Western blot against puromycin. Bar graphs represent fold changes relative to control group (A and B) and LLC group (C and D). All values are means ± SE. *P < 0.05 vs. LLC; #P < 0.05 vs. LLC with LIF Ab: one-way ANOVA.
The role of gp130 in LLC regulation of stretch-induced myotube protein synthesis.
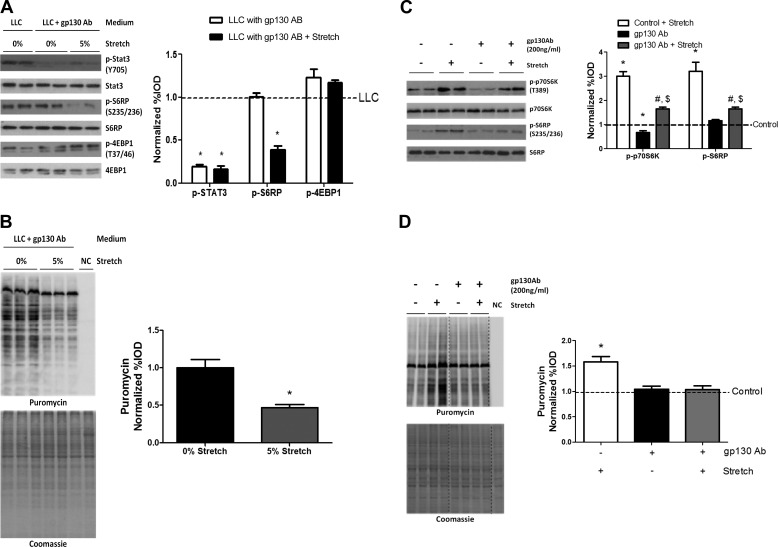
We investigated the role of gp130 receptor-dependent signaling in the LLC suppression of stretch-induced myotube protein synthesis. Inhibition of gp130 signaling was achieved by administration of a gp130 antibody to the media. We have reported that gp130 is required for LLC-induced STAT3, NF-κB, and p38 signaling, but gp130 signaling inhibition is not sufficient to rescue LLC suppression of mTORC1 signaling or protein synthesis in LLC tumor-bearing mice or LLC media-treated myotubes (49). Compared with LLC-treated myotubes, gp130 antibody administration decreased STAT3 phosphorylation, which was independent of stretch (Fig. 6A). Stretch in LLC media with the gp130 antibody decreased S6RP phosphorylation, but had no influence on 4EBP1 phosphorylation (Fig. 6A). Consistent with the change in S6RP phosphorylation, stretch in LLC media with gp130 antibody further reduced myotube protein synthesis (Fig. 6B). To further investigate the gp130 role in stretch induction of myotube protein synthesis, we stretched myotubes in control media supplemented with gp130 antibody. Stretch in control media with gp130 antibody significantly increased p70S6K and S6RP phosphorylation (Fig. 6C). Basal p70S6K phosphorylation was decreased by gp130 antibody administration in control media, but there was no effect on S6RP phosphorylation (Fig. 6C). gp130 antibody administration decreased p70S6K and S6RP phosphorylation in stretched myotubes (Fig. 6C). Basal myotube protein synthesis was not affected by gp130 antibody administration, but did abrogate the stretch induction of protein synthesis in control media (Fig. 6D).
Fig. 6.
Glycoprotein 130 (gp130) role in LLC regulation of stretch-induced myotube protein synthesis. A: phosphorylation of STAT3, S6RP, and 4EBP1 in myotubes stretched in LLC media with gp130 Ab. *P < 0.05 vs. LLC, one-way ANOVA. B: protein synthesis was measured by Western blot against puromycin. *P < 0.05, Student's t-test. C: phosphorylation of p70S6K and S6RP in myotubes stretched in control media with/without gp130 Ab. D: protein synthesis was measured by Western blot against puromycin. Bar graphs represent fold changes relative to LLC group (A), 0% stretch group (B), and control group (C and D). All values are means ± SE. *P < 0.05 vs. control; $P < 0.05 vs. gp130 Ab; #P < 0.05 vs. control + stretch: two-way ANOVA.
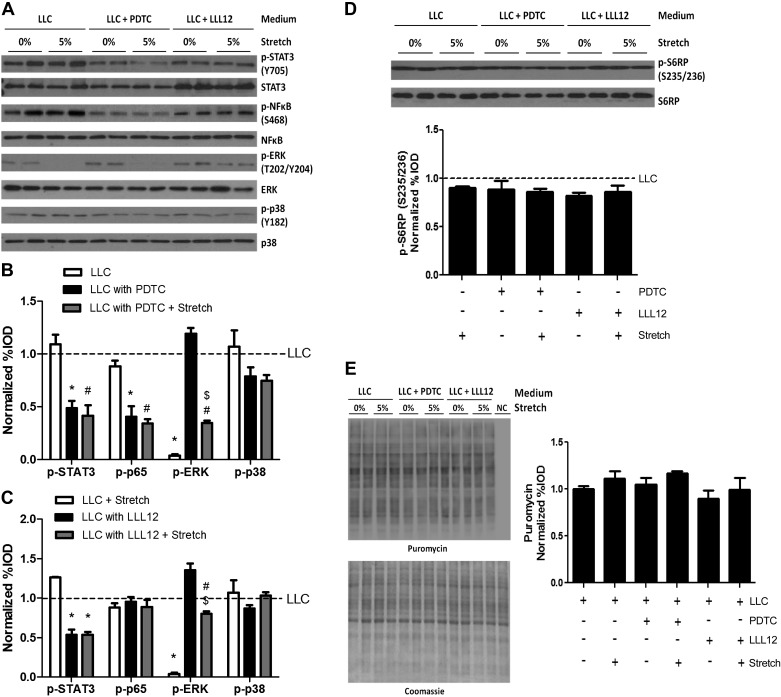
We used PDTC (a NF-κB and STAT3 inhibitor) and LLL12 (a STAT3-specific inhibitor) to exam the role of gp130 downstream targets, STAT3 and NF-κB. As previously reported (49), PDTC administration decreased both NF-κB and STAT3 phosphorylation, whereas LLL12 administration specifically decreased STAT3 phosphorylation (Fig. 7, A–C). The PDTC and LLL12 suppression of STAT3 and NF-κB p65 phosphorylation was independent of stretch (Fig. 7, A–C). Interestingly, stretch did not downregulate LLC-induced ERK1/2 phosphorylation in the presence of LLL12 (Fig. 7, A and C). Stretch in LLC media containing PDTC or LLL12 did not increase myotube S6RP phosphorylation (Fig. 7D) or protein synthesis (Fig. 7E). These results demonstrate gp130-dependent signaling is involved in stretch induction of myotube protein synthesis. However, it is not involved in LLC suppression effect on stretch induction of myotube protein synthesis.
Fig. 7.
STAT3 and NF-κB role in LLC regulation of stretch-induced myotube protein synthesis. A: representative blots. B and C: phosphorylation of STAT3, NF-κB p65, ERK, and p38 MAPK in myotubes stretched in LLC media with/without inhibitors [ammonium pyrrolidinecarbodithioate (PDTC; B); LLL12 (C)]. *P < 0.05 vs. LLC; #P < 0.05 vs. LLC + stretch; $P < 0.05 vs. LLC with PDTC/LLL12: two-way ANOVA. D: phosphorylation of S6RP in myotubes stretched in LLC media with/without inhibitors. No significant difference was detected. P < 0.05, two-way ANOVA. E: protein synthesis was measured by Western blot against puromycin. Bar graphs represent fold changes relative to LLC group (B–E). All values are means ± SE. No significant difference was detected in D or E. P < 0.05, two-way ANOVA.
The role of ERK1/2 in LLC regulation of stretch-induced myotube protein synthesis.
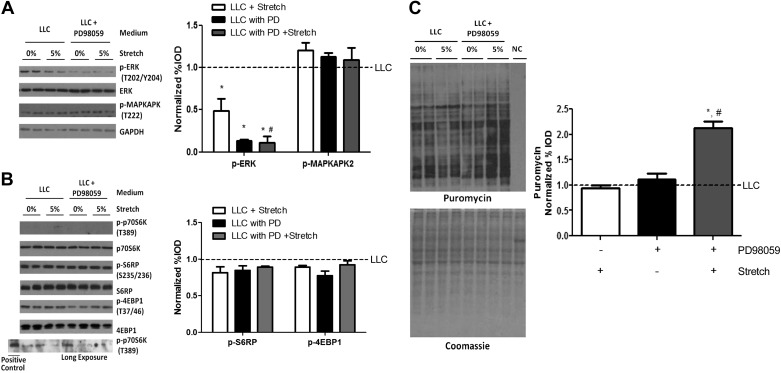
To investigate the role of ERK1/2 signaling in LLC suppression of stretch-induced myotube protein synthesis, we used PD-98059 to inhibit LLC-induced ERK1/2 phosphorylation. Myotubes were incubated in 25% LLC media for 72 h in the presence of PD-98059 and were stretched during last 24 h. PD-98059 administration in LLC media decreased ERK1/2 phosphorylation but had no influence on MAPKAPK-2 phosphorylation, a marker of p38 MAPK activity (Fig. 8A). PD98059 administration in LLC media did not change phosphorylation of p70S6K, S6RP, and 4EBP1 in nonstretched myotubes (Fig. 8B). Stretch in either LLC media or LLC media with PD-98059 did not increase p70S6K or 4EBP1 phosphorylation (Fig. 8B). However, stretch significantly increased protein synthesis in LLC media supplemented with PD-98059 (Fig. 8C). These results demonstrate a novel role of ERK1/2 in the mechanical regulation of protein synthesis in LLC-induced atrophic myotubes, which may be independent of p70S6K or 4EBP1.
Fig. 8.
ERK1/2 role in LLC regulation of stretch-induced myotube protein synthesis. A: phosphorylation of ERK1/2 and MAPK-activated protein kinase 2 (MAPKAPK-2) was measured by Western blot. Blots shown on separate panels are from different areas of the same gel. *P < 0.05 vs. LLC; #P < 0.05 vs. LLC + stretch: two-way ANOVA. B: phosphorylation of p70S6K, S6RP, and 4EBP1 was measured by Western blot. Protein sample from nonstretched myotubes in control media was used for p-p70S6K positive control. Blots shown on separate panels are from different areas of the same gel. C: protein synthesis was measured by Western blot against puromycin. Bar graphs represent fold changes relative to LLC group. All values are means ± SE. *P < 0.05 vs. LLC + stretch; #P < 0.05 vs. LLC with PD-98059 (PD): two-way ANOVA.
The role of AMPK in LLC regulation of stretch-induced myotube protein synthesis.
Previously, we have demonstrated that LLC secreted factors can induce myotube AMPK signaling (49), which is an upstream suppressor of mTOR and is involved in muscle anabolic resistance (22, 53). To investigate the role of AMPK signaling in the LLC inhibition of stretch-induced protein synthesis, we stretched myotubes in LLC media containing AMPK pharmaceutical inhibitor, compound C. Because 24 h or more of compound C treatment resulted in myotube death (data not shown), we did stretch and compound C administration on myotubes in last 4 h of LLC media incubation. First, we compared 4-h stretch effect on mTORC1 signaling pathway in control and LLC media incubated myotubes. Four-hour stretch induced p70S6K phosphorylation in both control and LLC media incubated myotubes (Fig. 9A). However, stretched myotubes in LLC media showed less p70S6K phosphorylation than stretched myotubes in control media (Fig. 9A). Furthermore, 4-h stretch was not sufficient to increase S6RP, 4EBP1 phosphorylation (Fig. 9A), or protein synthesis in LLC media incubated myotubes (Fig. 9D). The administration of compound C decreased AMPK phosphorylation independent of stretch (Fig. 9B) and increased phosphorylation of p70S6K, S6RP, and 4EBP1 in both nonstretched and stretched myotubes (Fig. 9C). However, the increase in p70S6K, S6RP, or 4EBP1 phosphorylation due to compound C was not further increased by stretch (Fig. 9C). Compound C administration in LLC media increased protein synthesis in both nonstretched and stretched myotubes. However, stretch did not further increase the compound C-induced protein synthesis (Fig. 9D). These results demonstrate LLC-induced AMPK signaling is an upstream suppressor of myotube mTORC1 signaling. However, AMPK signaling is not involved in LLC suppression of stretch induction of myotube mTORC1 signaling and protein synthesis.
Fig. 9.
AMPK role in LLC regulation of stretch-induced myotube protein synthesis. A: 4-h stretch effect on control or LLC-treated myotubes p70S6K, S6RP, and 4EBP1 phosphorylation were measured by Western blot. Blots shown on separate panels are from different areas of the same gel. *P < 0.05 vs. control; #P < 0.05 vs. control + stretch; $P < 0.05 vs. LLC: two-way ANOVA. B: phosphorylation of AMPK in myotubes stretched in LLC media with or without compound C. Blots shown on separate panels are from different areas of the same gel. C: phosphorylation of p70S6K, S6RP, and 4EBP1 in myotubes stretched in LLC media with or without compound C. Blots shown on separate panels are from different areas of the same gel. D: protein synthesis was measured by Western blot against puromycin. Bar graphs represent fold changes relative to control group (A) and LLC group (B–D). All values are means ± SE. *P < 0.05 vs. LLC; #P < 0.05 vs. LLC + stretch: two-way ANOVA.
The role of p38 MAPK in LLC regulation of stretch-induced myotube protein synthesis.
p38 MAPK plays both an anabolic and catabolic role in skeletal muscle protein turnover regulation (17, 76) and was upregulated by both stretch and LLC media (Fig. 4). To investigate the role of p38 MAPK signaling in LLC suppression of stretch-induced myotube protein synthesis, we used p38 MAPK-specific inhibitor SB-203580, which blocks downstream signaling initiated by p38 without affecting p38 phosphorylation. Chronic (24 h or more) SB-203580 administration in LLC media resulted in myotube death (data not shown). Therefore, we stretched myotubes for 4 h in LLC media containing SB-203580. SB-203580 administration in LLC media decreased phosphorylation of p38 MAPK downstream target, MAPKAPK-2, in both nonstretched and stretched myotubes, whereas SB-203580 did not influence the downregulation of stretch on ERK1/2 phosphorylation in LLC media (Fig. 10A). SB-203580 administration in LLC media did not change phosphorylation of p70S6K, S6RP, and 4EBP1 induced by LLC alone and did not influence 4-h stretch induction of p70S6K phosphorylation (Fig. 10B). However, stretch significantly increased protein synthesis in LLC media supplemented with SB-203580 (Fig. 10C). These results demonstrate that LLC-induced p38 MAPK signaling is a suppressor of stretch induction of myotube protein synthesis through mTORC1-independent mechanisms.
Fig. 10.
p38 MAPK role in LLC regulation of stretch-induced myotube protein synthesis. A: phosphorylation of ERK1/2 and MAPKAPK-2 was measured by Western blot. B: phosphorylation of p70S6K, S6RP, and 4EBP1 was measured by Western blot. C: relative protein synthesis was measured by Western blot against puromycin. ND, not detected. Bar graphs represent fold changes relative to LLC group. All values are means ± SE. *P < 0.05 vs. LLC; #P < 0.05 vs. LLC with SB-203580 (SB); $P < 0.05 vs. LLC + stretch: two-way ANOVA.
DISCUSSION
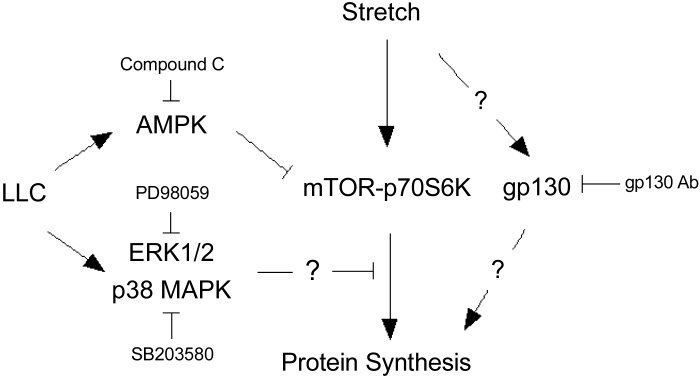
Increased mechanical signaling, such as overload, has been theorized to have potential for attenuating cancer-induced muscle mass loss. However, it is not clear if the mechanical induction of muscle protein synthesis can be regulated by cachectic factors. Disrupted mechanical signaling in cachectic skeletal muscle would have the potential to accelerate the wasting process and negatively impact the therapeutic potential of exercise and physical activity interventions. We report the important and novel finding that media derived from LLC cells can negatively affect the stretch induction of mTORC1 signaling and protein synthesis in myotubes. While both STAT3 and NF-κB signaling have been implicated in the regulation of myotube and muscle catabolism, we report that these pathways are not necessary for the LLC suppression of stretch-regulated protein synthesis in myotubes. LLC-induced AMPK signaling, an established suppressor of basal myotube protein synthesis, is also not involved in the LLC attenuation of the stretch response. However, the inhibition of either ERK1/2 or p38 MAPK in LLC-derived media restored myotube protein synthesis stretch responsiveness. LLC media expressed high levels of LIF, which is involved in LLC induction of ERK1/2 in myotubes. Interestingly, we report the novel finding that gp130-initiated signaling plays a role in the mechanical induction of myotube protein synthesis. In untreated myotubes, the stretch induction of protein synthesis was inhibited by gp130 antibody administration, while stretch in the presence of LLC media caused a further suppression of myotube protein synthesis suppression. Overall, these results demonstrate that LLC-derived cachectic factors can dissociate mechanical signaling from myotube protein synthesis, possibly through the hyperactivation of ERK1/2 and p38, and that the mechanical stretch-induced signaling involves gp130 in both control and LLC-treated myotubes (Fig. 11).
Fig. 11.
Speculated pathways of LLC regulation on stretch-induced myotube protein synthesis in myotubes. Mechanical stretch induces mTOR-p70S6K signaling activation and subsequent protein synthesis increase. gp130 also mediated stretch-induced protein synthesis, which is independent of mTOR-p70S6K. LLC media suppress stretch induction of protein synthesis through the hyperactivation of ERK1/2 and p38 MAPK signaling, which is independent of mTOR-p70S6K. LLC media also induces AMPK signaling activation, which is an additional suppressor of mTOR-p70S6K signaling, independent of stretch.
While cancer-induced skeletal muscle wasting involves the disrupted homeostatic regulation of muscle protein turnover (66), our understanding of protein degradation regulation during the wasting process exceeds our understanding of protein synthesis. The integration of muscle anabolic processes through mTOR signaling is an acknowledged control point of skeletal muscle mass (6), which also can be targeted by the cancer environment (55). Paradoxically, we have reported that, during cachexia, mTOR signaling and protein synthesis suppression are accompanied by increased Akt phosphorylation (49, 68, 69), suggesting a disconnection between Akt and mTOR in cancer cachexia. Mechanical signaling can activate muscle mTOR signaling and protein synthesis through Akt-independent mechanisms (26). We report that LLC-derived cachectic factors are sufficient to attenuate stretch-induced mTOR signaling involving the phosphorylation of p70S6K and S6RP. Interestingly, mTOR inhibition occurred while ERK1/2 and p38 MAPK, mediators of stretch-induced protein synthesis, were activated. MAPKs, including ERK1/2 and p38, are part of an important skeletal muscle signal transduction nexus that mediates a muscle's response to a variety of physiological and pathological stressors (70). Several studies provide support for ERK1/2’s role in the mechanical induction of skeletal muscle mTOR signaling (38, 71). Additionally, mechanical activation of p38 signaling has been associated with peroxisome proliferator-activated receptor-α coactivator-transcription (2), glucose uptake (12), and mTOR signaling activation (17, 72). However, chronic inflammatory diseases also activate muscle MAPK signaling, resulting in increased protein degradation and muscle wasting (51, 76). LLC-released factors can induce a strong inflammatory response in skeletal muscle (49), and our results are consistent with the current understanding of mechanical stimuli and inflammation regulation on MAPK signaling (4, 32). Interestingly, stretch attenuated LLC-induced ERK1/2 phosphorylation, which suggests that mechanical stimuli can counter the LLC-induced stress response. However, the paradoxical activation of MAPK signaling during both wasting and growth conditions requires further examination.
Our results demonstrate that the suppression of mechanical signaling by LLC can be rescued. Inhibition of either ERK1/2 or p38 during LLC treatment restored the stretch induction of myotube protein synthesis. Unexpectedly, ERK1/2 or p38 MAPK inhibition did not restore the stretch regulation to mTOR signaling. Stretch for 24 h in the presence of LLC did not increase the phosphorylation of p70S6K, S6RP, or 4EBP1. In fact, some studies have demonstrated that mTORC1 is not the only mediator of mechanical stimuli-induced muscle protein synthesis. For example, synergistic ablation-induced muscle rRNA accumulation is only partially attenuated by mTORC1 suppression (20). In denervated skeletal muscles, protein synthesis is also decreased due to the downregulation of ribosomal biogenesis, even though mTORC1 signaling is activated (36). MAPKs, such as ERK1/2, can directly activate rRNA transcription and ribosomal biogenesis through the phosphorylation of rRNA transcriptional factor, transportation infrastructure financing and innovation act and upstream binding factor, independent of mTORC1 signaling (61, 78). Interestingly, hyperphosphorylation of the ERK1/2 phosphorylation site on upstream binding factor can decrease rRNA transcriptional activity (61), suggesting a dual role for ERK1/2 signaling in ribosomal biogenesis and protein synthesis regulation.
The IL-6 family of cytokines regulates intracellular signal transducers, such as STAT3 and MAPK, through interaction with their specific cytokine receptors and the gp130 transmembrane protein (63). The IL-6 cytokines play an enigmatic role in skeletal mass regulation (39). They are secreted by muscle fibers during exercise or overload and are involved in muscle hypertrophy and regeneration by stimulating satellite cell proliferation (5, 45, 56, 60). However, they are involved in muscle wasting during cancer cachexia. Inhibition of IL-6/STAT3 signaling can attenuate muscle wasting in different cachexia models, including ApcMin/+ mice and C26 tumor-bearing mice (7, 68). Interestingly, we did not find elevated IL-6 levels in our LLC media, but another member of this cytokine family, LIF, was significantly increased. It was recently reported that LIF signaling is required for C26-induced muscle atrophy (57). We have reported that LLC-induced muscle protein degradation involves signaling though the muscle gp130, both in vivo and in vitro (49). Additionally, LLC activation of NF-κB, STAT3, and the ubiquitin protein degradation pathway requires the muscle gp130 receptor (49). Our present results demonstrate that LIF is highly expressed in LLC media and plays a role in the induction of ERK1/2 by LLC, while LLC induction of STAT3 appears LIF independent. However, LLC signaling through the gp130 receptor is not required for basal mTOR suppression in either wasting myotubes or skeletal muscle (49).
Mechanical stimulation can induce the muscle expression of some members of the IL-6 cytokine family, and these cytokines play a role in muscle mass regulation related to both hypertrophy and atrophy (39). However, despite significant interest in these cytokines, relatively little is known about gp130-dependent signaling in mechanical induction of muscle protein synthesis. We report that inhibition of gp130 signaling prevented the stretch induction of protein synthesis in control condition. Furthermore, inhibition of gp130 or LIF, but not STAT3 or NF-κB, in stretched myotubes resulted in further LLC suppression of mTOR signaling and protein synthesis. These findings suggest that gp130-dependent cytokines play an important role in mechanical stretch regulation of protein synthesis. Additional study is needed to determine whether LIF/gp130 signaling has a permissive role in maintaining mechanical signaling. However, our results support previous research demonstrating that IL-6 and LIF are required for overload-induced muscle hypertrophy (56, 59). We have previously reported that IL-6 administration can cause a dose-dependent increase in myotube p70S6K phosphorylation, paradoxically being most effective at low doses (69). Taken together, these results suggest that gp130 may play a dual role in the regulation of muscle protein turnover that is based on the muscle's microenvironment, which involves expression of circulating and local cytokines. However, future research is still needed to investigate the downstream targets of gp130 signaling that play a role in protein synthesis regulation by mechanical and cachectic signaling.
NF-κB and STAT3 are important mediators of muscle catabolism. The activation of NF-κB during cachexia increases muscle RING finger-1 transcription, inducible nitric oxide synthase expression, and ubiquitin-proteasome-mediated protein degradation and suppresses MyoD mRNA expression (9, 15). STAT3 signaling is also an important regulator of muscle wasting and increases atrogin-1 (7, 49) and myostatin expression (77). Furthermore, NF-κB and STAT3 have the potential to disrupt anabolic activation of muscle protein synthesis (19). For example, anabolic resistance to amino acid and resistance exercise caused by either sarcopenia or sepsis is associated with NF-κB activation (23). Muscle contraction-induced mTORC1 signaling is attenuated in severely cachectic ApcMin/+ mice, which exhibit chronic activation of muscle STAT3 (50). Interestingly, PDTC administration to these cachectic mice can rescue contraction-induced mTORC1 activation (50). We found that both anabolic and catabolic stimuli can activate muscle NF-κB signaling, which may be related to differential functions of NF-κB in muscle as it relates to inflammatory and mechanical signals (28). NF-κB and STAT3 signaling pathways are acutely activated by exercise, but these signaling pathways have not been clearly linked to the mechanical induction of protein synthesis (30). We did not observe any effect of stretch on STAT3 phosphorylation, and stretch did not alter LLC induction of STAT3. The inhibition of STAT3 or NF-κB does not appear sufficient to restore the stretch induction of myotube protein synthesis in LLC media.
An energy deficiency or cachectic environment can induce a shift toward muscle catabolism. AMPK signaling plays a role in this shift through mTORC1 inhibition via raptor and tuberous sclerosis complex 2 phosphorylation (22), induction of FOXO-dependent E3 ubiquitin ligase transcription (40), and increased autophagy processes (3). Muscle AMPK signaling is chronically activated in cachectic ApcMIN/+ mice (49, 68), but it remains to be determined whether energy status, metabolic regulation related to oxidative metabolism, or other inflammatory signaling is responsible for the induction. Exercise and repetitive muscle contractions also induce a transient activation of AMPK signaling (18). Interestingly, contracted cachectic muscle has an induction of AMPK signaling, which goes above an already elevated baseline level (50). Here, we report that the inhibition of myotube AMPK activity during LLC treatment can rescue suppressed mTORC1 signaling and protein synthesis. Clearly AMPK signaling has a role in the LLC suppression of basal protein synthesis. However, chronic stretch does not increase AMPK phosphorylation or alter LLC activation of AMPK. Furthermore, AMPK inhibition in myotubes treated with LLC media does not rescue the stretch regulation of myotube protein synthesis. These results suggest limited cross talk between AMPK and mechanical signaling in myotubes. The relationship between the AMPK and mechanical signaling on the regulation of muscle protein synthesis needs to be further defined.
In summary, we examined whether tumor-derived cachectic factors could disrupt mechanical stretch regulation of protein synthesis in cultured myotubes. Chronic stretch in basal conditions induced myotube protein synthesis and hypertrophy, while the addition of LLC-derived media attenuated the stretch induction of protein synthesis. LLC-induced p38 and ERK1/2 signaling appear critical for disrupted mechanical signaling, as their inhibition restored stretch induction of protein synthesis in the presence of LLC media. Interestingly, we demonstrated that the stretch induction of myotube protein synthesis involves gp130-initiated signaling in myotubes cultured in both untreated and LLC media. AMPK signaling plays a regulatory role in the inhibition of basal myotube protein synthesis by LLC, but inhibition did not restore stretch sensitivity. Our work provides a new insight into the mechanisms by which cancer cachexia suppresses muscle protein synthesis. Further work is necessary to determine whether anti-inflammatory therapies combined with mechanical stimulation are useful to alleviate muscle protein synthesis suppression in cancer cachexia.
GRANTS
The research described in this report was supported by research grant R01 CA121249 to J. A. Carson from the National Cancer Institute, and National Center for Research Resources Grant P20 RR-017698 to J. A. Carson.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.G. and J.A.C. conception and design of research; S.G. performed experiments; S.G. analyzed data; S.G. and J.A.C. interpreted results of experiments; S.G. and J.A.C. prepared figures; S.G. and J.A.C. drafted manuscript; S.G. and J.A.C. approved final version of manuscript; J.A.C. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Gaye Christmas, Justin P. Hardee, and Kimbell L. Hetzler for editorial review of the manuscript.
REFERENCES
- 1.Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab 5: 476–487, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280: 19587–19593, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32: 2–11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronson D, Violan MA, Dufresne SD, Zangen D, Fielding RA, Goodyear LJ. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J Clin Invest 99: 1251–1257, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begue G, Douillard A, Galbes O, Rossano B, Vernus B, Candau R, Py G. Early activation of rat skeletal muscle IL-6/STAT1/STAT3 dependent gene expression in resistance exercise linked to hypertrophy. PLoS One 8: e57141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab 303: E410–E421, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broholm C, Pedersen BK. Leukaemia inhibitory factor–an exercise-induced myokine. Exerc Immunol Rev 16: 77–85, 2010. [PubMed] [Google Scholar]
- 9.Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev 38: 168–176, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carson JA, Booth FW. Effect of serum and mechanical stretch on skeletal alpha-actin gene regulation in cultured primary muscle cells. Am J Physiol Cell Physiol 275: C1438–C1448, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Chambers MA, Moylan JS, Smith JD, Goodyear LJ, Reid MB. Stretch-stimulated glucose uptake in skeletal muscle is mediated by reactive oxygen species and p38 MAP-kinase. J Physiol 587: 3363–3373, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Deyne PG. Formation of sarcomeres in developing myotubes: role of mechanical stretch and contractile activation. Am J Physiol Cell Physiol 279: C1801–C1811, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Dehoux M, Gobier C, Lause P, Bertrand L, Ketelslegers JM, Thissen JP. IGF-I does not prevent myotube atrophy caused by proinflammatory cytokines despite activation of Akt/Foxo and GSK-3β pathways and inhibition of atrogin-1 mRNA. Am J Physiol Endocrinol Metab 292: E145–E150, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Di Marco S, Mazroui R, Dallaire P, Chittur S, Tenenbaum SA, Radzioch D, Marette A, Gallouzi IE. NF-kappa B-mediated MyoD decay during muscle wasting requires nitric oxide synthase mRNA stabilization, HuR protein, and nitric oxide release. Mol Cell Biol 25: 6533–6545, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 27: 793–799, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Frey JW, Jacobs BL, Goodman CA, Hornberger TA. A role for Raptor phosphorylation in the mechanical activation of mTOR signaling. Cell Signal 26: 313–322, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedrichsen M, Mortensen B, Pehmoller C, Birk JB, Wojtaszewski JF. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol 366: 204–214, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Frost RA, Lang CH. mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda) 26: 83–96, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol 589: 5485–5501, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J 25: 1028–1039, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haran PH, Rivas DA, Fielding RA. Role and potential mechanisms of anabolic resistance in sarcopenia. J Cachexia Sarcopenia Muscle 3: 157–162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardee JP, Puppa MJ, Fix DK, Gao S, Hetzler KL, Bateman TA, Carson JA. The effect of radiation dose on mouse skeletal muscle remodeling. Radiol Oncol 48: 247–256, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A 103: 4741–4746, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J 380: 795–804, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornberger TA, Sukhija KB, Chien S. Regulation of mTOR by mechanically induced signaling events in skeletal muscle. Cell Cycle 5: 1391–1396, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J 16: 529–538, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Kazi AA, Pruznak AM, Frost RA, Lang CH. Sepsis-induced alterations in protein-protein interactions within mTOR complex 1 and the modulating effect of leucine on muscle protein synthesis. Shock 35: 117–125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol (1985) 103: 388–395, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Mittal A, Paul PK, Kumar M, Srivastava DS, Tyagi SC, Kumar A. Tumor necrosis factor-related weak inducer of apoptosis augments matrix metalloproteinase 9 (MMP-9) production in skeletal muscle through the activation of nuclear factor-kappaB-inducing kinase and p38 mitogen-activated protein kinase: a potential role of MMP-9 in myopathy. J Biol Chem 284: 4439–4450, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19: 362–370, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llovera M, Garcia-Martinez C, Lopez-Soriano J, Carbo N, Agell N, Lopez-Soriano FJ, Argiles JM. Role of TNF receptor 1 in protein turnover during cancer cachexia using gene knockout mice. Mol Cell Endocrinol 142: 183–189, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Machida M, Takeda K, Yokono H, Ikemune S, Taniguchi Y, Kiyosawa H, Takemasa T. Reduction of ribosome biogenesis with activation of the mTOR pathway in denervated atrophic muscle. J Cell Physiol 227: 1569–1576, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Martineau LC, Gardiner PF. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J Appl Physiol (1985) 91: 693–702, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Miyazaki M, McCarthy JJ, Fedele MJ, Esser KA. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J Physiol 589: 1831–1846, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J 280: 4131–4148, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakashima K, Yakabe Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci Biotechnol Biochem 71: 1650–1656, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Ning J, Clemmons DR. AMP-activated protein kinase inhibits IGF-I signaling and protein synthesis in vascular smooth muscle cells via stimulation of insulin receptor substrate 1 S794 and tuberous sclerosis 2 S1345 phosphorylation. Mol Endocrinol 24: 1218–1229, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, Yamauchi-Takihara K. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem 273: 9703–9710, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Palmer RM, Reeds PJ, Atkinson T, Smith RH. The influence of changes in tension on protein synthesis and prostaglandin release in isolated rabbit muscles. Biochem J 214: 1011–1014, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, Takahashi T, Kato T, Ogawa S. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res 84: 1127–1136, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol 536: 329–337, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penna F, Busquets S, Argiles JM. Experimental cancer cachexia: evolving strategies for getting closer to the human scenario. Semin Cell Dev Biol. In press. [DOI] [PubMed] [Google Scholar]
- 47.Penna F, Costamagna D, Fanzani A, Bonelli G, Baccino FM, Costelli P. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS One 5: e13604, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pruznak AM, Kazi AA, Frost RA, Vary TC, Lang CH. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-d-ribonucleoside prevents leucine-stimulated protein synthesis in rat skeletal muscle. J Nutr 138: 1887–1894, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puppa MJ, Gao S, Narsale AA, Carson JA. Skeletal muscle glycoprotein 130’s role in Lewis lung carcinoma-induced cachexia. FASEB J 28: 998–1009, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puppa MJ, Murphy EA, Fayad R, Hand GA, Carson JA. Cachectic skeletal muscle response to a novel bout of low frequency stimulation. J Appl Physiol (1985) 116: 1078–1087, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riddoch-Contreras J, George T, Natanek SA, Marsh GS, Hopkinson NS, Tal-Singer R, Kemp P, Polkey MI. p38 Mitogen-activated protein kinase is not activated in the quadriceps of patients with stable chronic obstructive pulmonary disease. COPD 9: 142–150, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes 55, Suppl 2: S48–S54, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes 59: 2426–2434, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez AM, Candau RB, Csibi A, Pagano AF, Raibon A, Bernardi H. The role of AMP-activated protein kinase in the coordination of skeletal muscle turnover and energy homeostasis. Am J Physiol Cell Physiol 303: C475–C485, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280: 4294–4314, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Seto DN, Kandarian SC, Jackman RW. A key role for leukemia inhibitory factor in C26 cancer cachexia. J Biol Chem 290: 19976–19986, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simpson DG, Majeski M, Borg TK, Terracio L. Regulation of cardiac myocyte protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ Res 85: e59–e69, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Spangenburg EE, Booth FW. Leukemia inhibitory factor restores the hypertrophic response to increased loading in the LIF(−/−) mouse. Cytokine 34: 125–130, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Spangenburg EE, Booth FW. Multiple signaling pathways mediate LIF-induced skeletal muscle satellite cell proliferation. Am J Physiol Cell Physiol 283: C204–C211, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Stefanovsky VY, Pelletier G, Hannan R, Gagnon-Kugler T, Rothblum LI, Moss T. An immediate response of ribosomal transcription to growth factor stimulation in mammals is mediated by ERK phosphorylation of UBF. Mol Cell 8: 1063–1073, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology 145: 4592–4602, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 15: 797–819, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Thomson DM, Fick CA, Gordon SE. AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signaling responses to high-frequency electrically stimulated skeletal muscle contractions. J Appl Physiol (1985) 104: 625–632, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Tidball JG. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol (1985) 98: 1900–1908, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 89: 381–410, 2009. [DOI] [PubMed] [Google Scholar]
- 67.Vandenburgh H, Kaufman S. In vitro model for stretch-induced hypertrophy of skeletal muscle. Science 203: 265–268, 1979. [DOI] [PubMed] [Google Scholar]
- 68.White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS One 6: e24650, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White JP, Puppa MJ, Gao S, Sato S, Welle SL, Carson JA. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab 304: E1042–E1052, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Widegren U, Ryder JW, Zierath JR. Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol Scand 172: 227–238, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Williamson DL, Kubica N, Kimball SR, Jefferson LS. Exercise-induced alterations in extracellular signal-regulated kinase 1/2 and mammalian target of rapamycin (mTOR) signalling to regulatory mechanisms of mRNA translation in mouse muscle. J Physiol 573: 497–510, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu XN, Wang XK, Wu SQ, Lu J, Zheng M, Wang YH, Zhou H, Zhang H, Han J. Phosphorylation of Raptor by p38beta participates in arsenite-induced mammalian target of rapamycin complex 1 (mTORC1) activation. J Biol Chem 286: 31501–31511, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.You JS, Frey JW, Hornberger TA. Mechanical stimulation induces mTOR signaling via an ERK-independent mechanism: implications for a direct activation of mTOR by phosphatidic acid. PLoS One 7: e47258, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zanchi NE, Lancha AH Jr. Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis. Eur J Appl Physiol 102: 253–263, 2008. [DOI] [PubMed] [Google Scholar]
- 75.Zhang G, Jin B, Li YP. C/EBPβ mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting. EMBO J 30: 4323–4335, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang G, Li YP. p38β MAPK upregulates atrogin1/MAFbx by specific phosphorylation of C/EBPβ. Skelet Muscle 2: 20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L, Pan J, Dong Y, Tweardy DJ, Garibotto G, Mitch WE. Stat3 activation links a C/EBPdelta to myostatin pathway to stimulate loss of muscle mass. Cell Metab 18: 368–379, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao J, Yuan X, Frodin M, Grummt I. ERK-dependent phosphorylation of the transcription initiation factor TIF-IA is required for RNA polymerase I transcription and cell growth. Mol Cell 11: 405–413, 2003. [DOI] [PubMed] [Google Scholar]